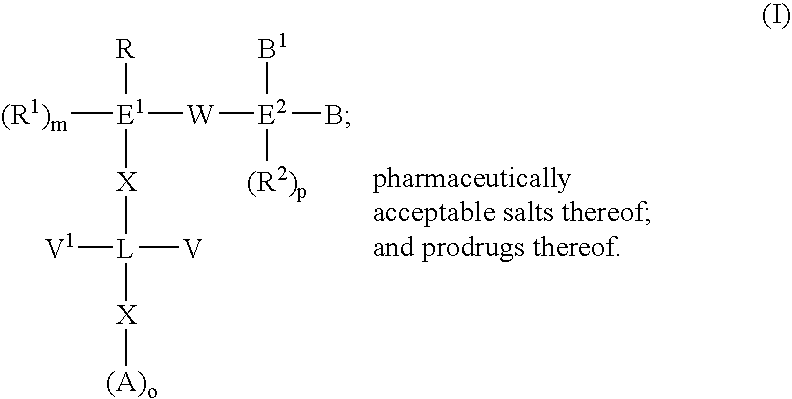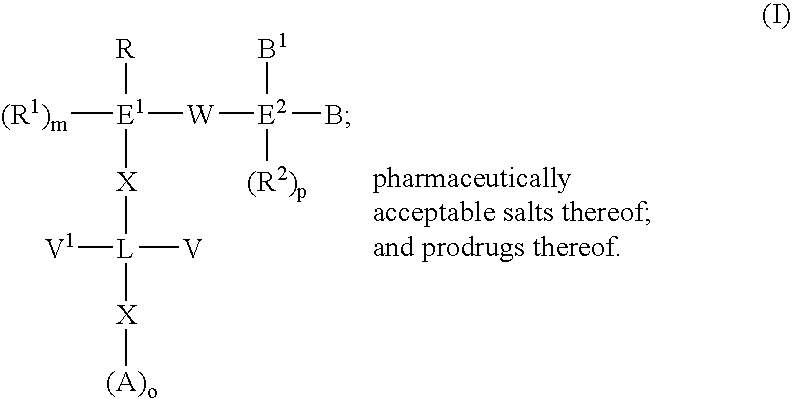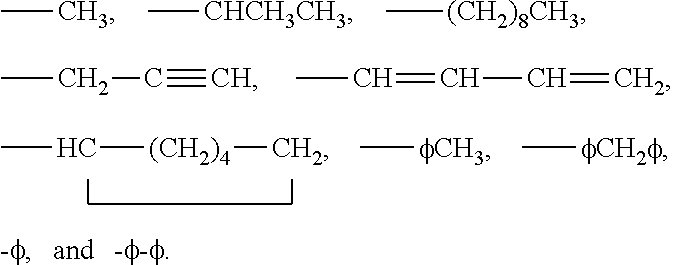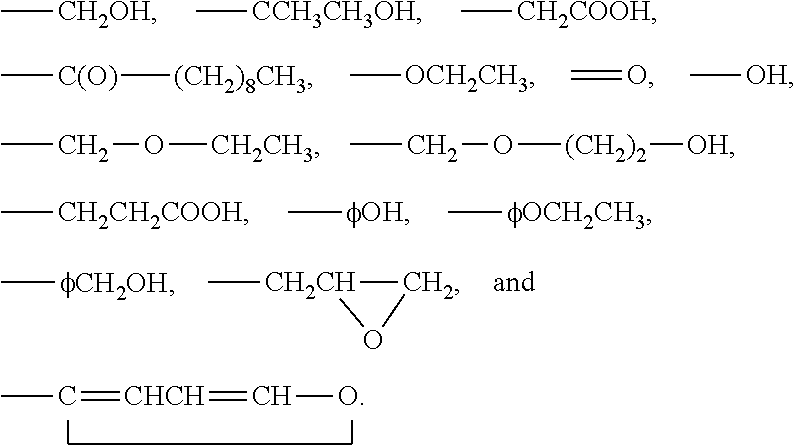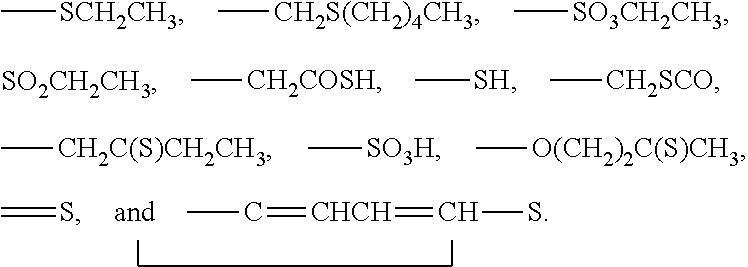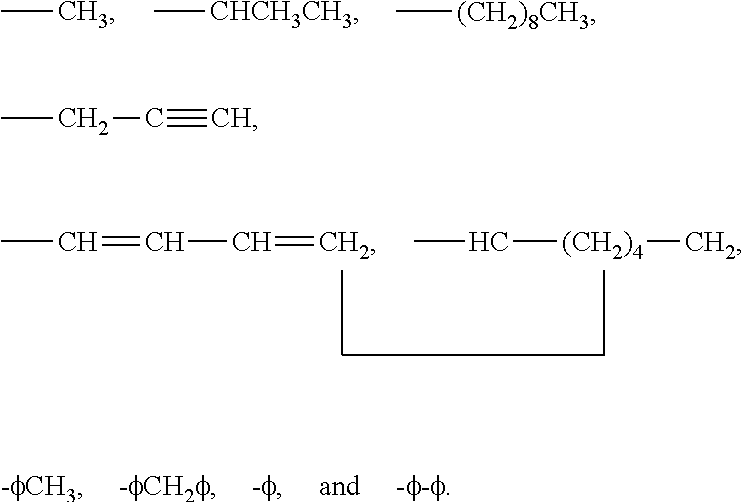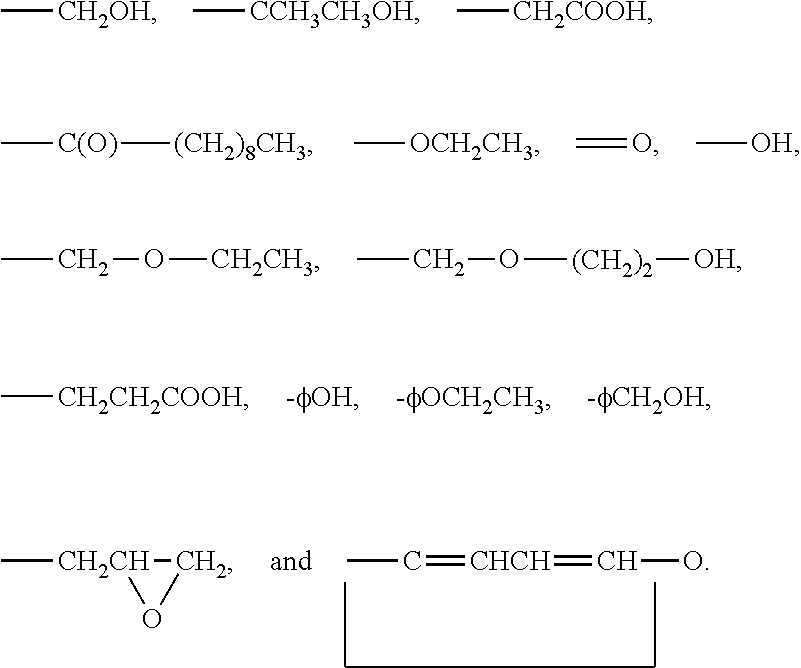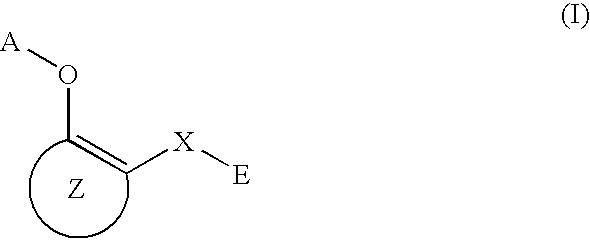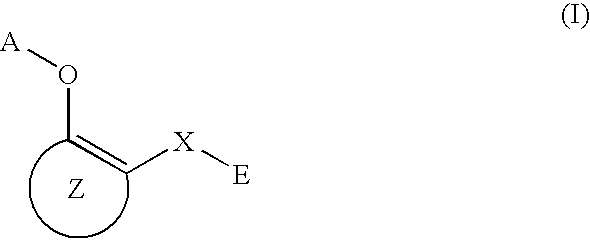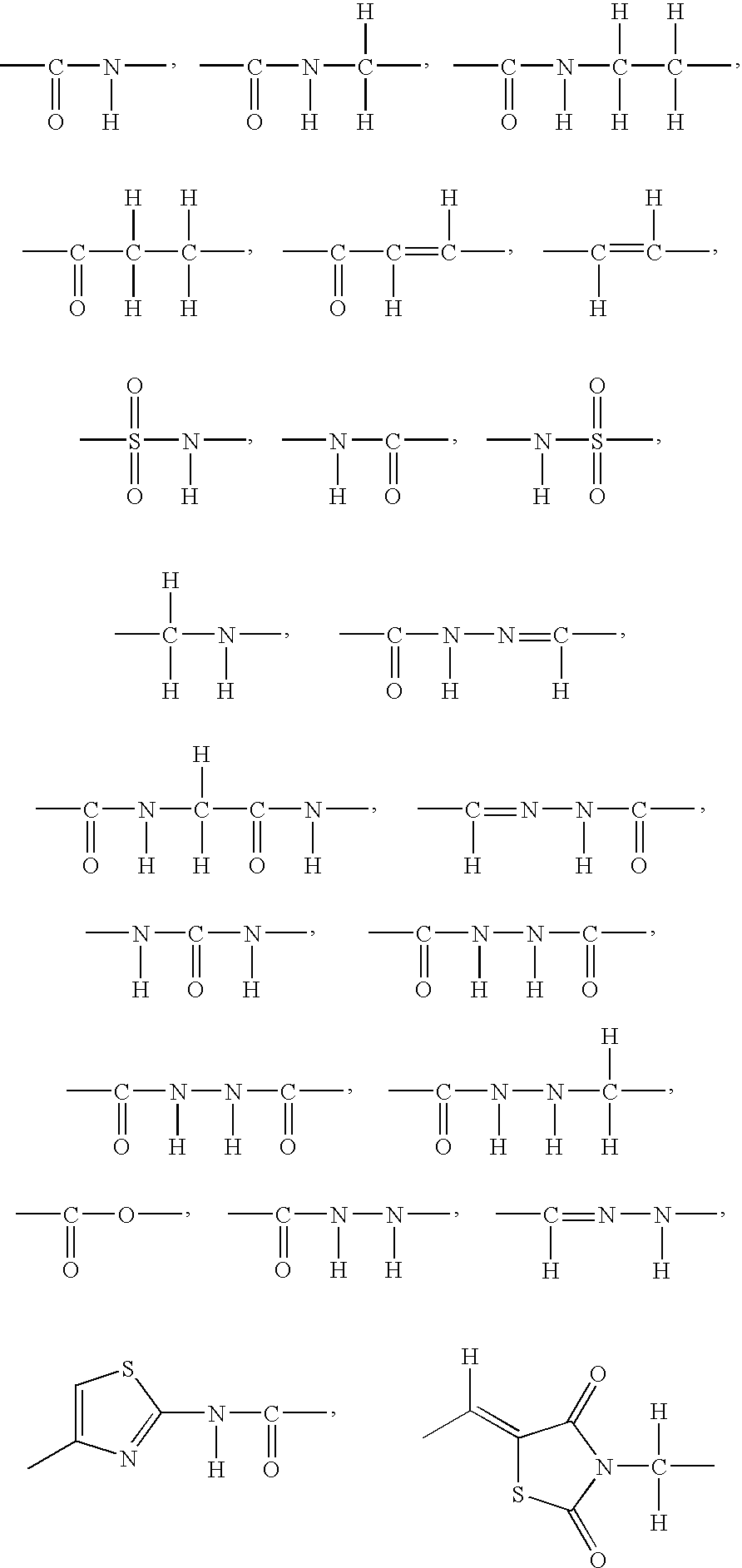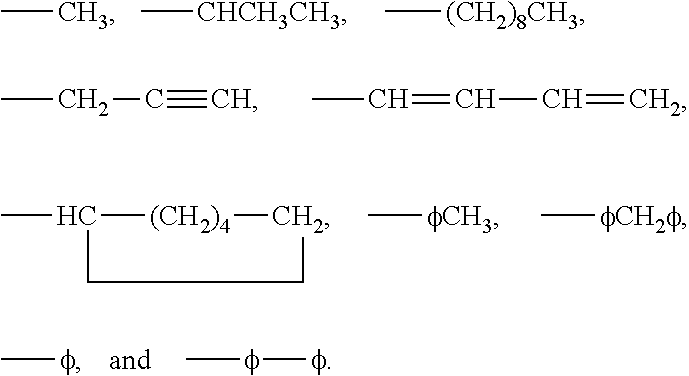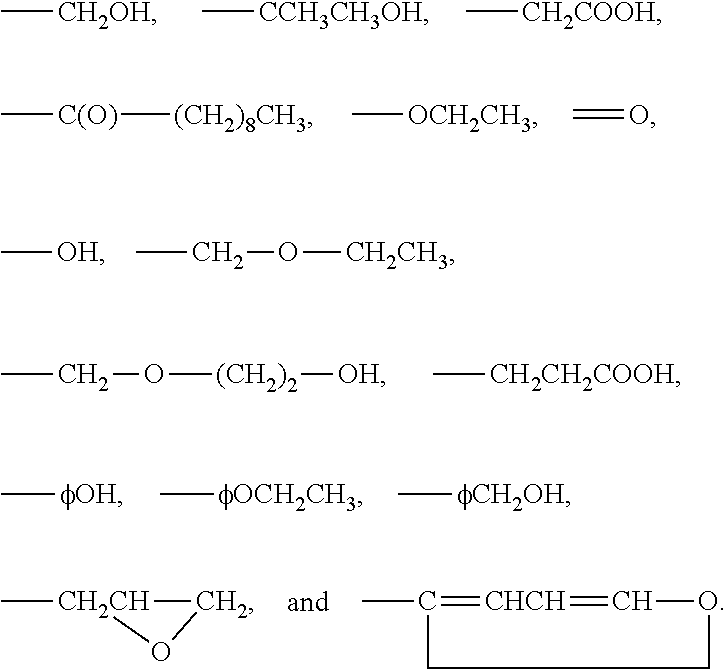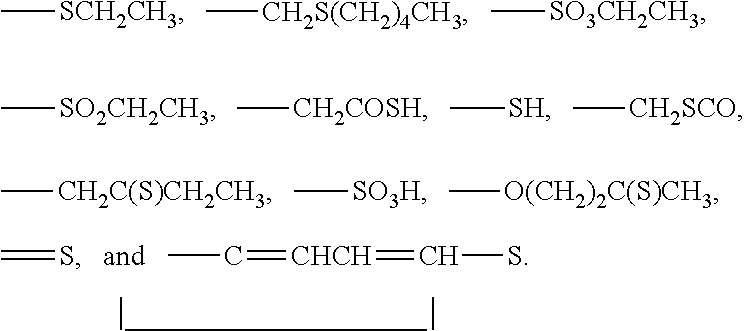Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1111results about "Silicon compound active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
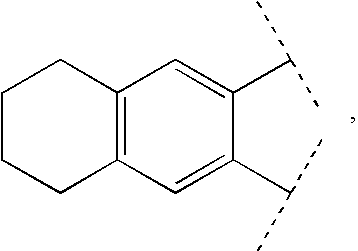
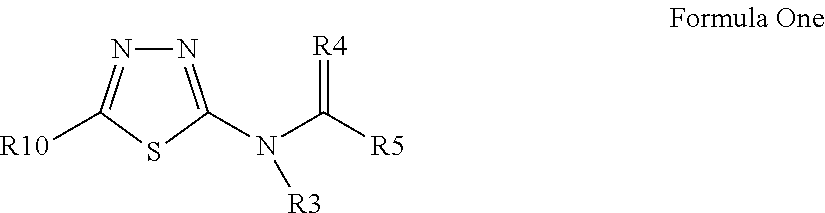
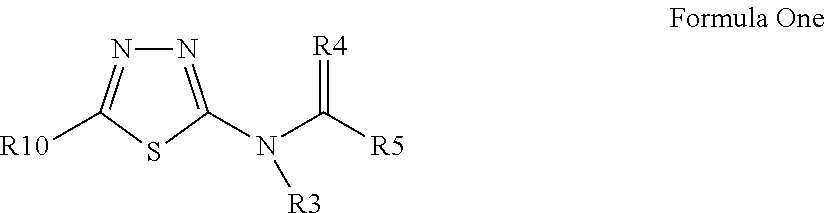
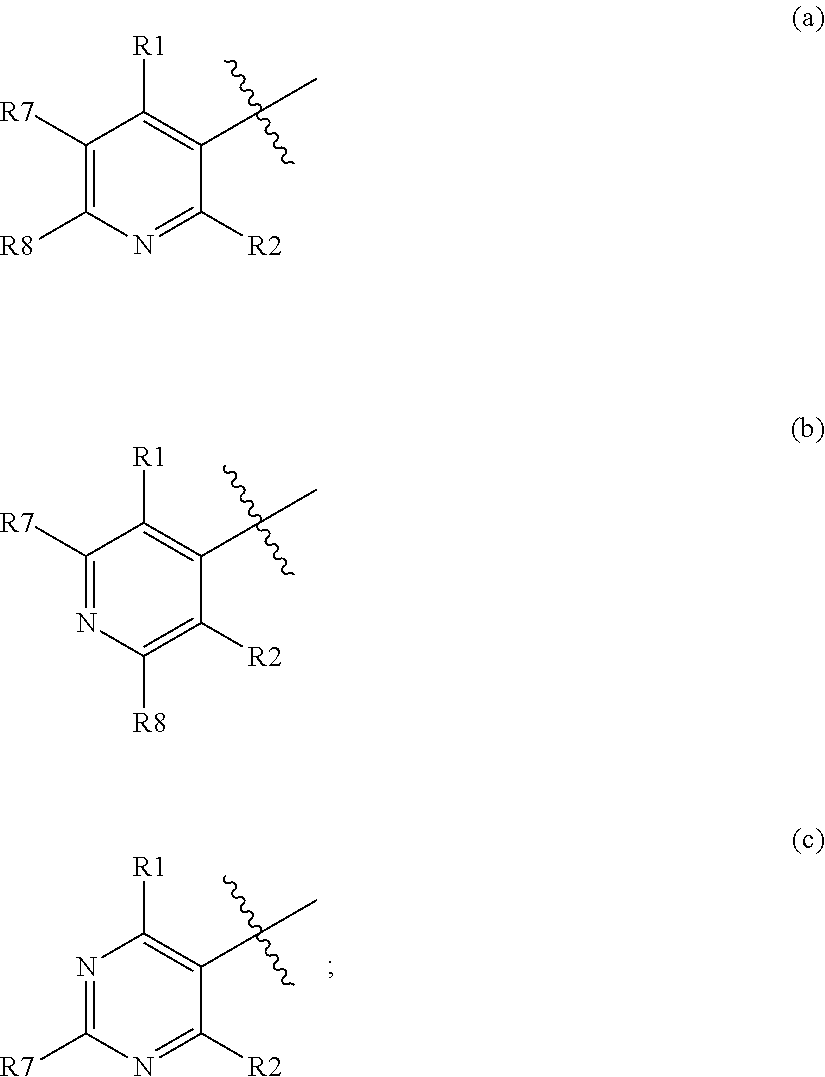
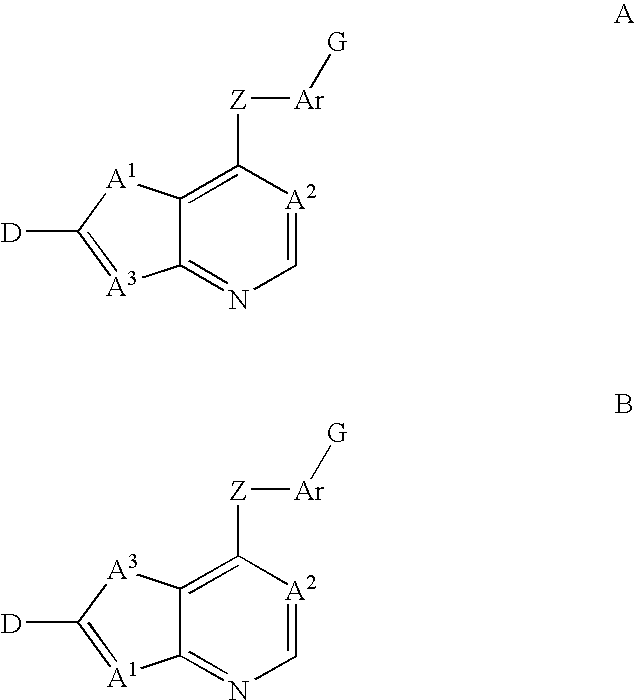
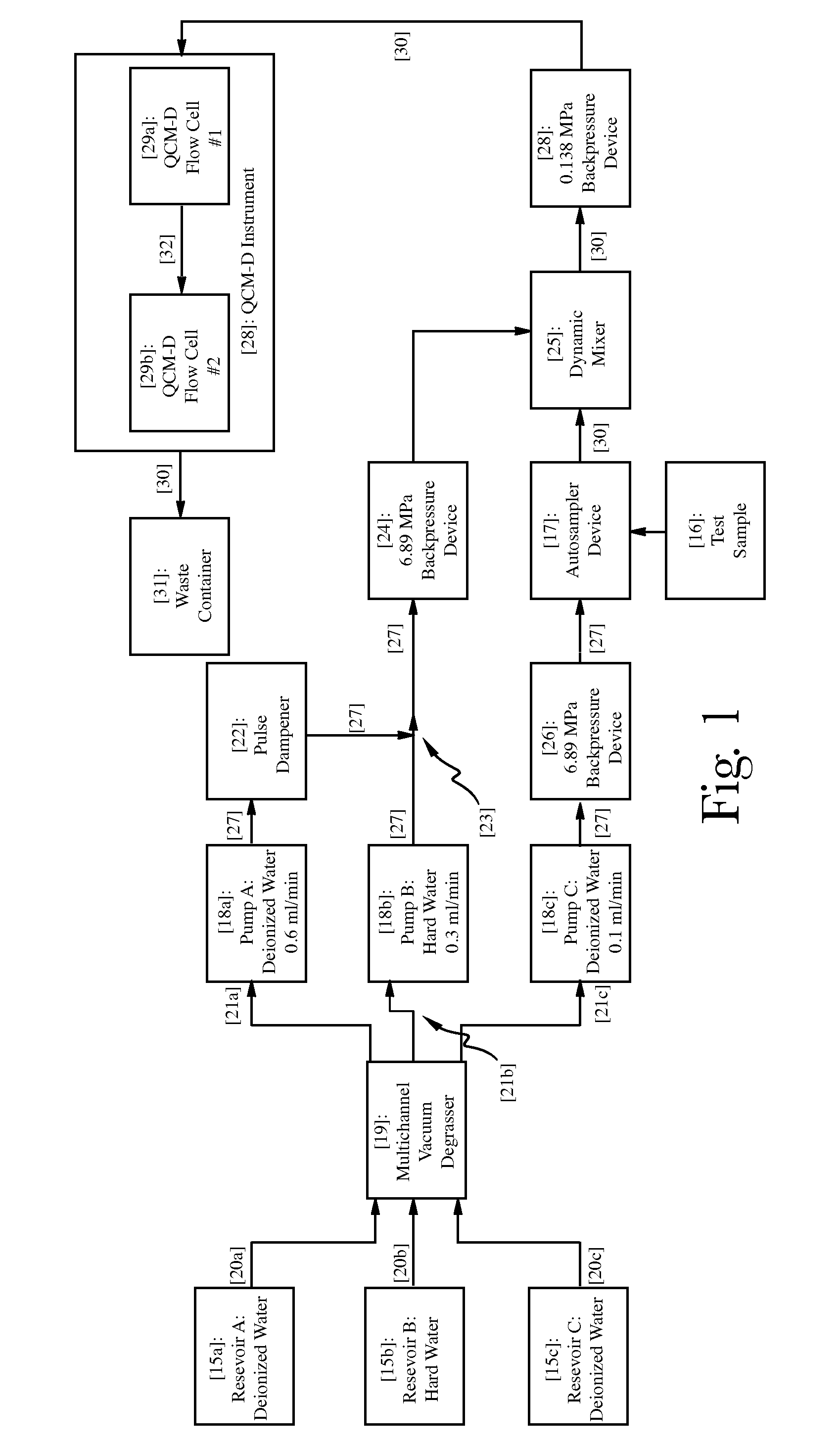
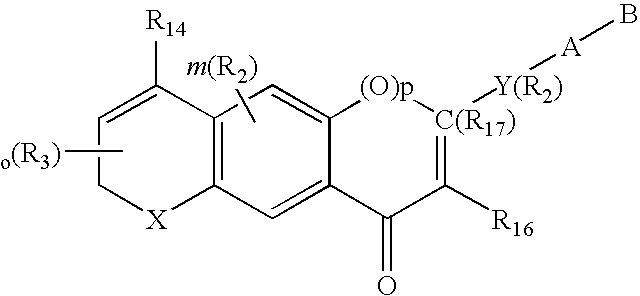
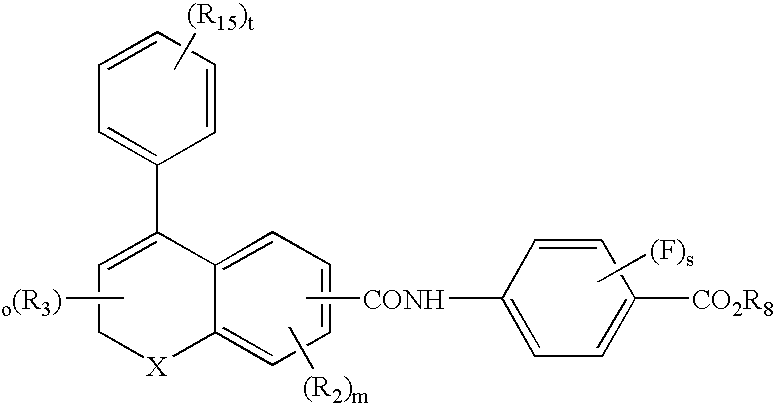
3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof
Owner:ANDADYS PHARMA INC
Antimicrobial compositions and methods
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including, in particular, an antimicrobial lipid component, such as a fatty acid ester, fatty ether, or alkoxide derivative thereof. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Aryl carbonyl derivatives as therapeutic agents
InactiveUS7384967B2Increase in number and sizeBiocideGroup 4/14 element organic compoundsArylBiochemistry
This invention relates to aryl carbonyl derivatives which are activators of glucokinase which may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
Composition for promoting healthy bone structure
InactiveUS6447809B1Increase bone densityPrevents radial bone lossBiocideHeavy metal active ingredientsVitamin CRegimen
A dietary supplement for benefitting human bone health includes a calcium source, a source of vitamin D activity, and an osteoblast stimulant. A preferred calcium source is microcrystalline hydroxyapatite, which also contains protein (mostly collagen), phosphorus, fat, and other minerals. A preferred source of vitamin D activity is cholecalciferol, and a preferred osteoblast stimulant is ipriflavone. In addition to these basic ingredients, the composition can further include various other minerals known to occur in bone, vitamin C, and glucosamine sulfate, all of which exert beneficial effects on growth and maintenance of healthy bone. A method for benefitting human bone health involves administering a daily regimen of the dietary supplement.
Owner:PHOENIX DICHTUNGSTECHN +1
Pesticidal compositions
Owner:DOW AGROSCIENCES LLC
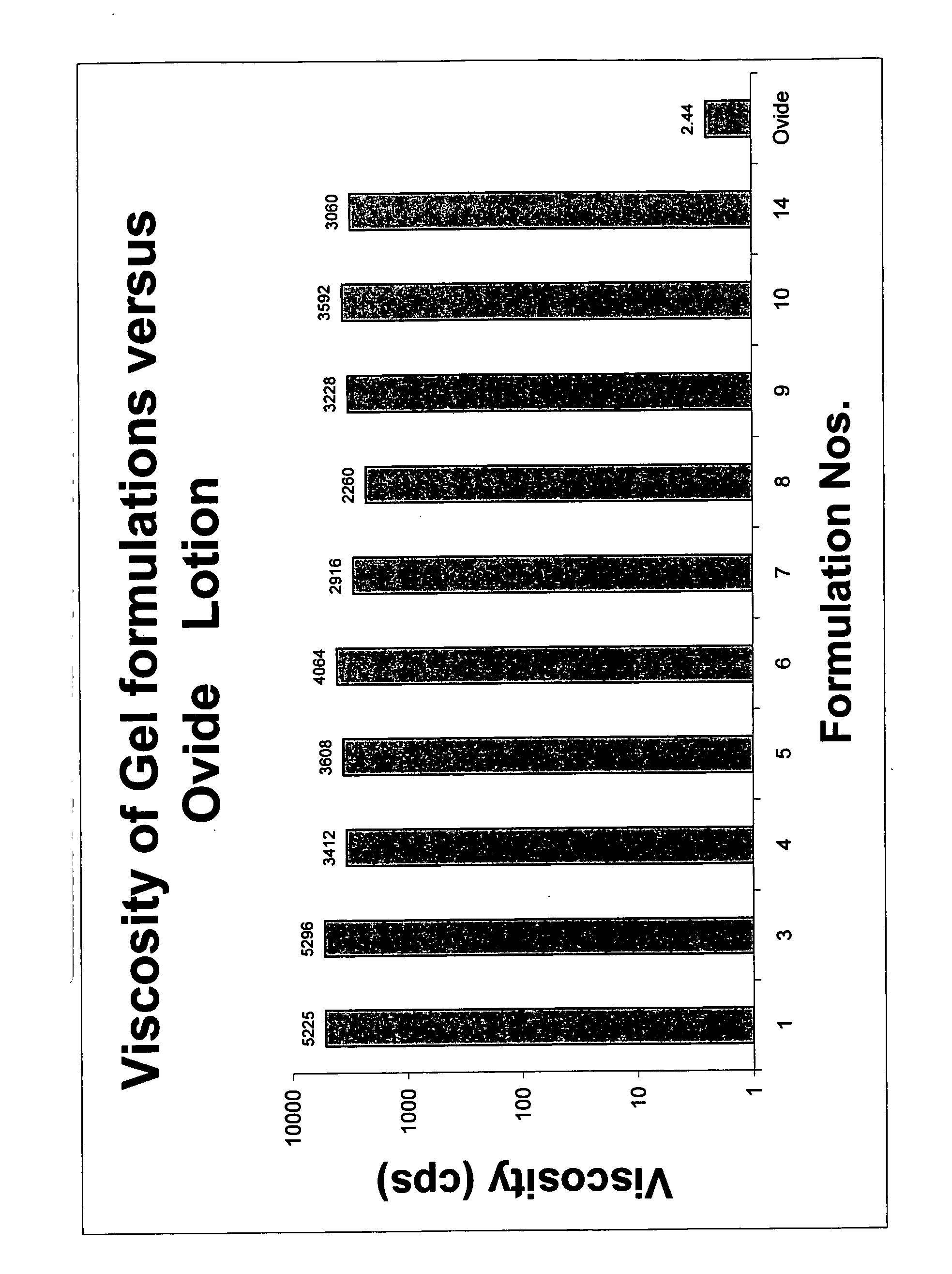
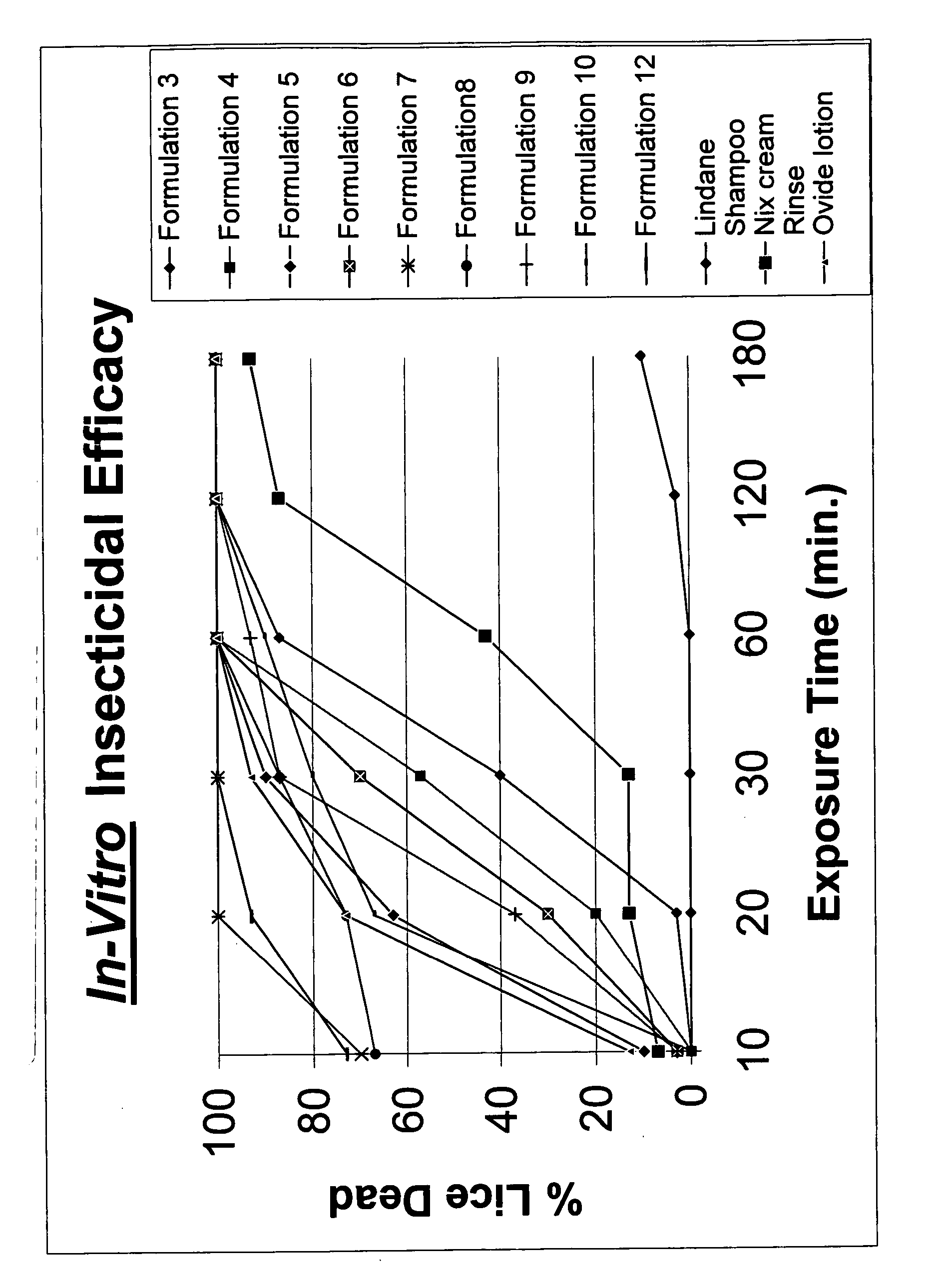
Topical gel formulation comprising insecticide and its preparation thereof
The present invention provides a topical gel pharmaceutical formulation of insecticide suitable for treating an ectoparasite in a mammal, comprising: a) about 0.1-10% by weight of an insecticide; b) at least about 75% by weight of an organic solvent selected from the group consisting of a lower alkyl alcohol, a ketone, a glycol and a mixture thereof, wherein the organic solvent contains at least about 40% by weight of the lower alkyl alcohol; and c) at least one polymer selected from the group consisting of a cellulosic polymer, acrylates, methacrylates, and polyvinyl pyrrolidone. The present invention further provides a process of preparing as well as a method of treating ectoparasites in a mammal using the same.
Owner:GOYAL SANDHYA +4
Antimicrobial composition
An antimicrobial composition that involves a synergistic mixture in terms of active agents, of a primary antimicrobial agent, such as polyhexamethylene biguanide (PHMB), a secondary antimicrobial agent, and optionally an organic acid against various kinds of microbes is described. Various additional processing aids, such as alcohols and surfactants, may also be incorporated within the mixture. The composition allows one to use a significantly less concentration of individual constituent antimicrobial agents to achieve the same or a better degree of antimicrobial efficacy. The antimicrobial composition can be applied to the surface of almost any kind of substrate material, and can achieve a killing-efficacy of about 3 Log10 reduction in microbes within 30 minutes under ambient conditions.
Owner:KIMBERLY-CLARK WORLDWIDE INC
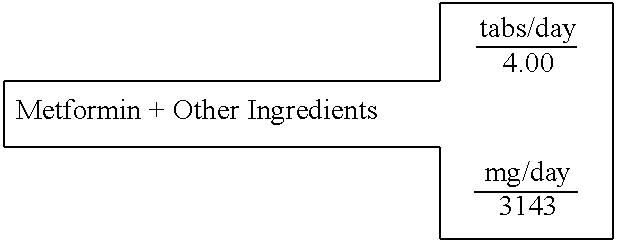
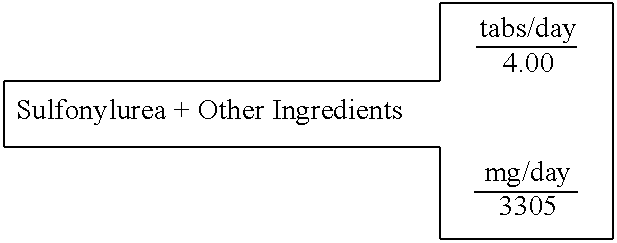
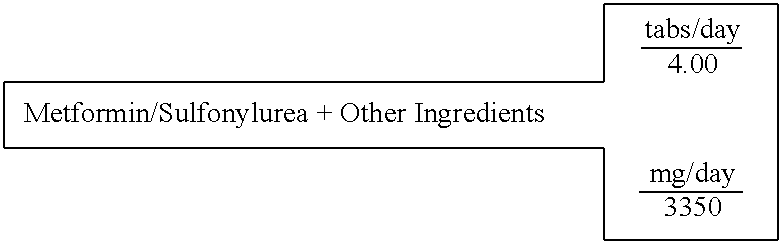
Biguanide and sulfonylurea formulations for the prevention and treatment of insulin resistance and type 2 diabetes mellitus
InactiveUS20030078269A1Maximum complementarityImprove effectivenessBiocidePeptide/protein ingredientsSulfonylureaTreatment level
The invention describes formulations that include either metformin, sulfonylurea or a biguanide-sulfonylurea combination as one active ingredient in addition to specific, other active ingredients. The compositions and dosage forms of the invention are clinically useful as methods for increasing the effectiveness, efficiency and safety of the included biguanide (metformin) and / or sulfonylurea in the prevention and treatment of insulin resistance and diabetes mellitus. The carefully chosen additional active ingredients of the invention are designed in a modular fashion to prevent and rectify adverse events associated with insulin resistance syndrome and diabetes mellitus, and those adverse incidences associated with the concurrent use of metformin and / or the sulfonylureas. When clinically administered, the invention will provide therapeutic levels of metformin and of a sulfonylurea, alone or in combination, and broaden their usefulness. The invention will retard the progression of insulin resistance to type 2 diabetes, and reduce the serious microvascular and macrovascular complications commonly associated with insulin resistance syndrome and diabetes mellitus.
Owner:CHRONORX
Shampoo compositions comprising and emulsified silicone an a microemulsified silicone
An aqueous shampoo composition comprising, in addition to water:i) at least one cleansing surfactant;ii) a cationic deposition polymer, andiii) a silicone component consisting of a blend of:(a) emulsified particles of an insoluble silicone, in which the emulsified particles of insoluble silicone are incorporated into the shampoo composition as a preformed aqueous emulsion having an average silicone particle size in the emulsion and in the shampoo composition of from 0.15 to 30 microns, and(b) microemulsified particles of an insoluble silicone, in which the microemulsified particles of insoluble silicone are incorporated into the shampoo composition as a preformed aqueous microemulsion having an average-silicone particle size in the microemulsion and in the shampoo composition of less than 0.10 microns.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Simethicone solid oral dosage form
The present invention provides a composition for forming a compressed solid dosage form that is a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22. Also included are solid dosage forms made from a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Organosilicones
The present application relates to organosilicones and compositions such as consumer products comprising such organosilicones, as well as processes for making and using such organosilicones and such compositions. Such compositions comprising such organosilicones are easier to formulate, and provide more economical and superior care benefits when compared to current silicone containing compositions.
Owner:THE PROCTER & GAMBLE COMPANY
Aryl carbonyl derivatives as therapeutic agents
InactiveUS20080119455A1Increase in number and sizeGroup 4/14 element organic compoundsBiocideArylBiochemistry
This invention relates to aryl carbonyl derivatives which are activators of glucokinase which may be useful for the management, treatment, control, or adjunct treatment of diseases, where increasing glucokinase activity is beneficial.
Owner:VTV THERAPEUTICS LLC
Formulation of a mixture of Free-B-ring flavonoids and flavans as a therapeutic agent
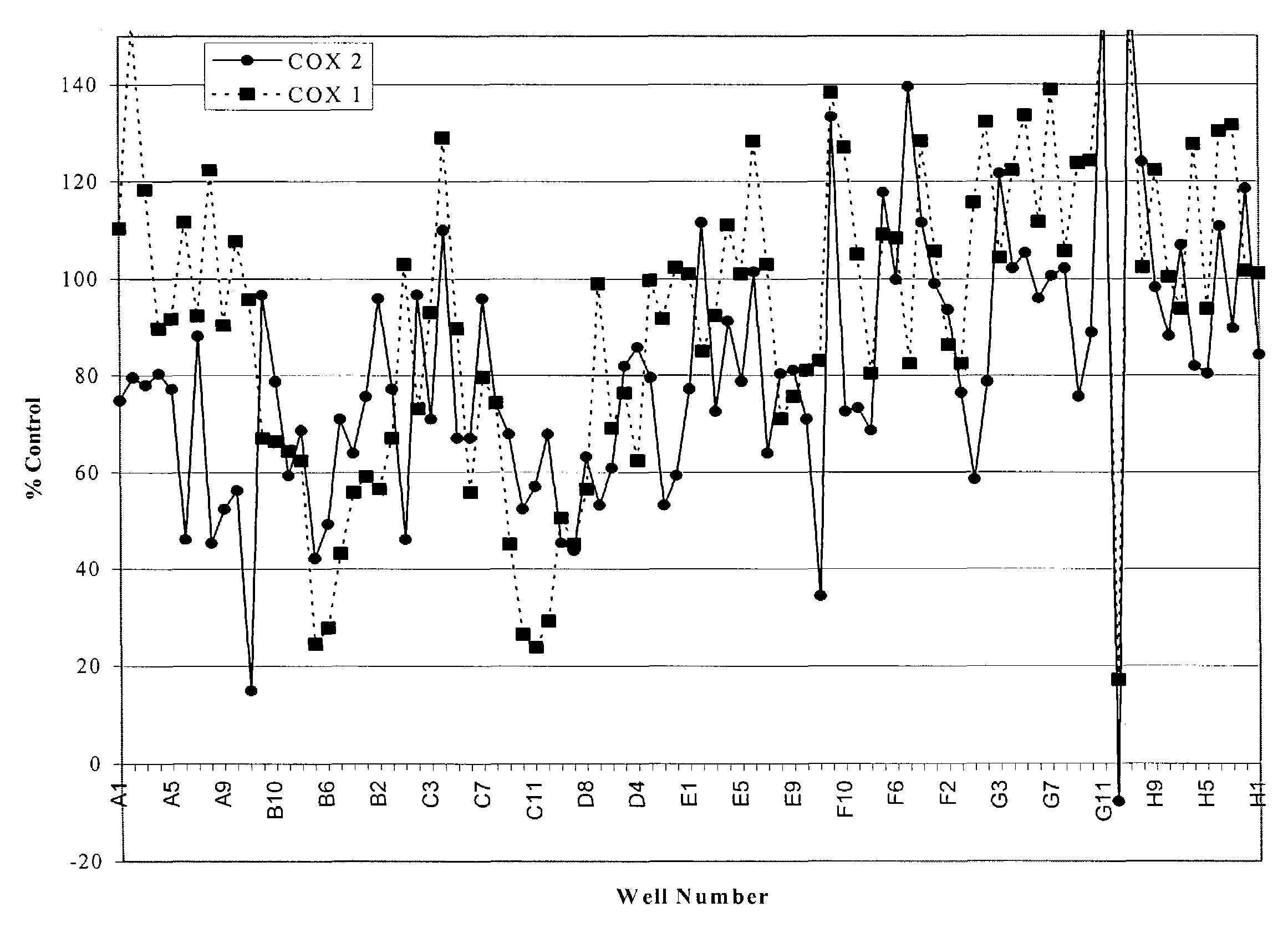
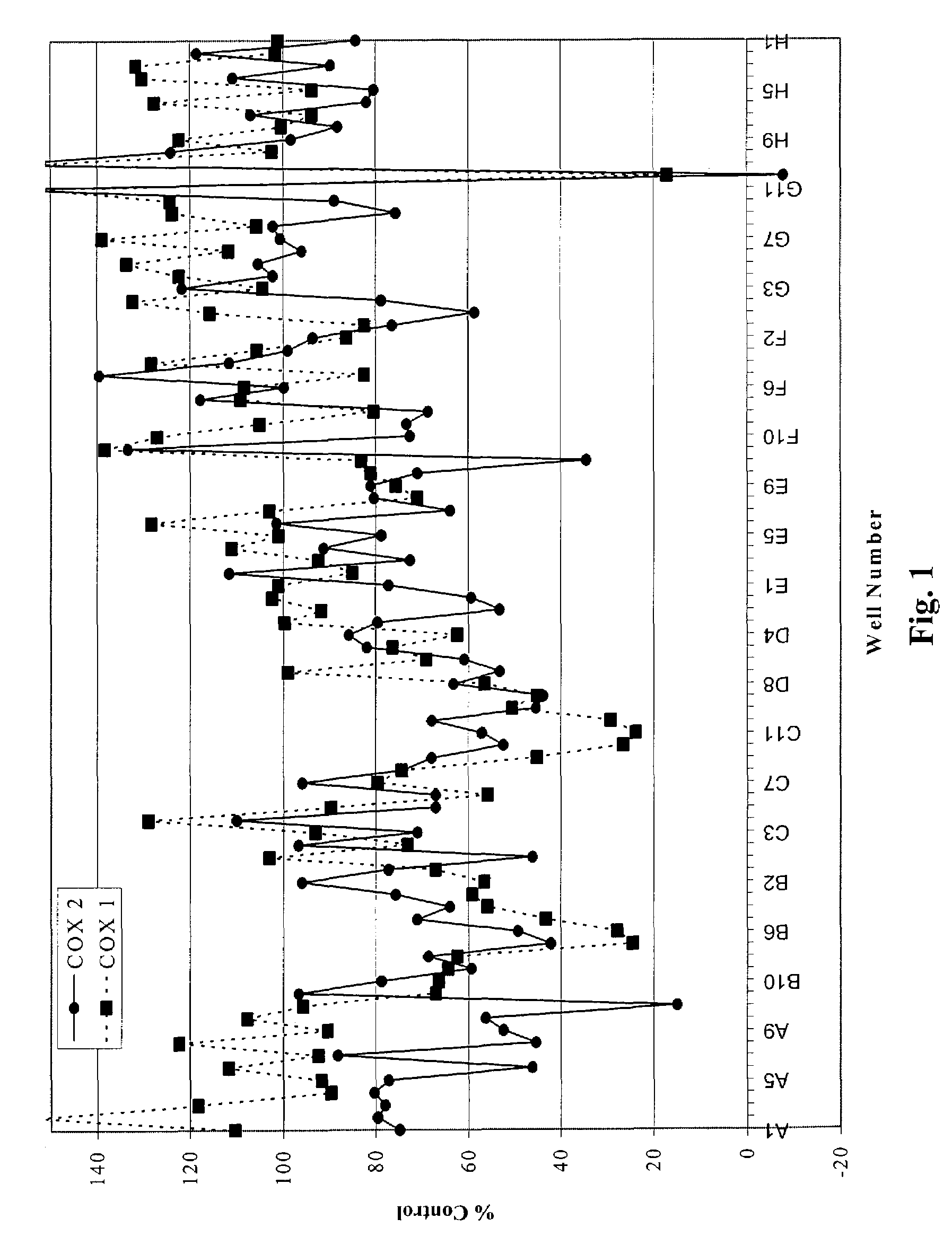
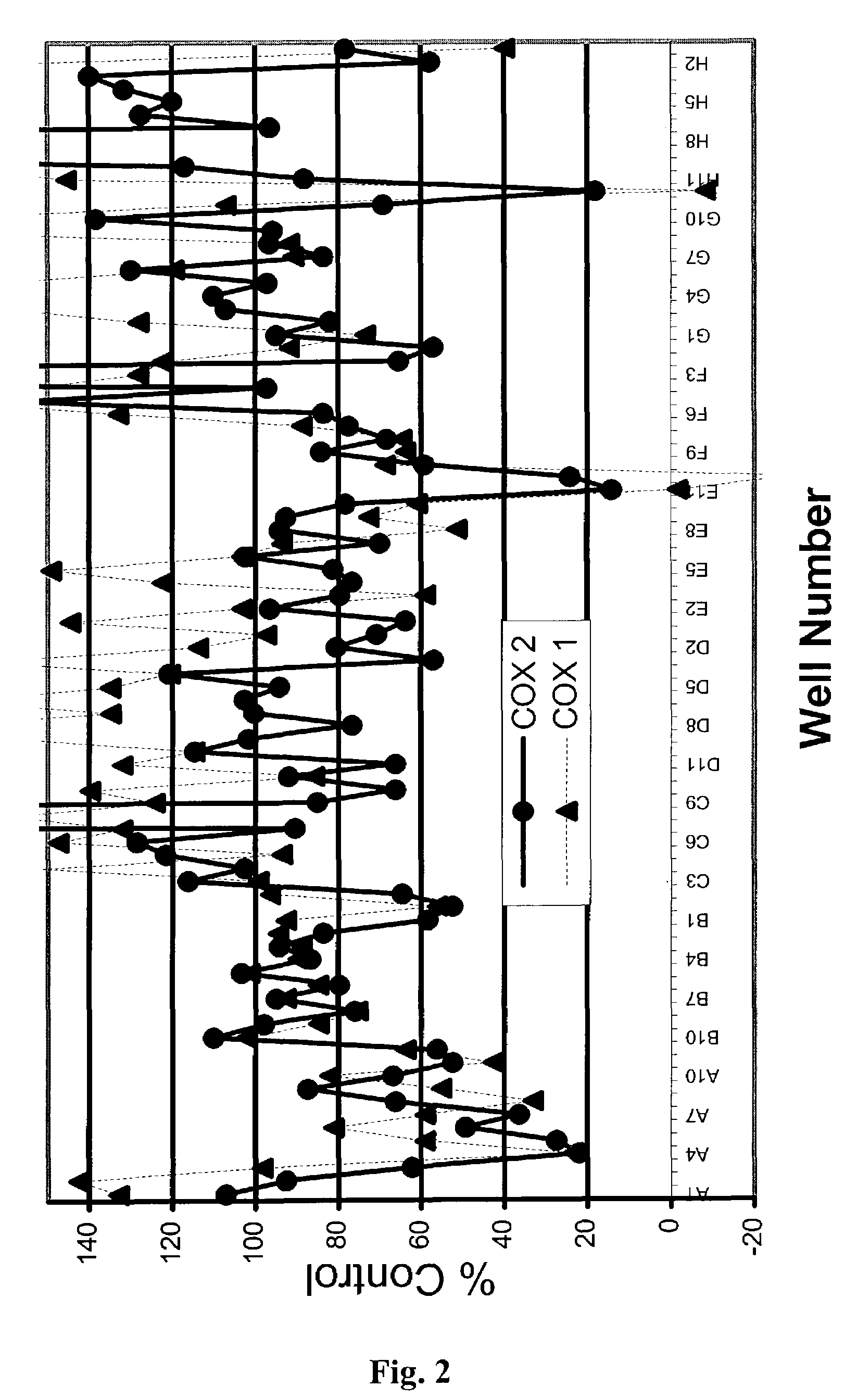
The present invention provides a novel composition of matter comprised of a mixture of two specific classes of compounds—Free-B-ring flavonoids and flavans—for use in the prevention and treatment of diseases and conditions mediated by the COX-2 and 5-LO pathways. The present invention further provides a novel method for simultaneously inhibiting the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) enzymes, and reducing cox-2 mRNA production. Finally, the present invention includes a method for weight loss and blood glucose control. The methods of this invention are comprised of administering to a host in need thereof an effective amount of the composition of this invention together with a pharmaceutically acceptable carrier. This invention relates generally to the prevention and treatment of diseases and conditions mediated by the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) pathways, including but not limited to the relief joint discomfort and pain associated with conditions such as osteoarthritis, rheumatoid arthritis, and other injuries that result from overuse.
Owner:UNIGEN
Artificial tear replacement solution
InactiveUS7001607B1Reduce wearGood film formingHalogenated hydrocarbon active ingredientsSenses disorderConjunctivaConjunctival sac
A tear replacement solution that contains at least one water-soluble fluorosurfactant, water and a non-polar component, preferably in gel form, and a method for the external treatment for the eye of an mammal by applying the tear replacement solution to the eye, preferably by placing in the conjunctival sac.
Owner:PHARMPUR
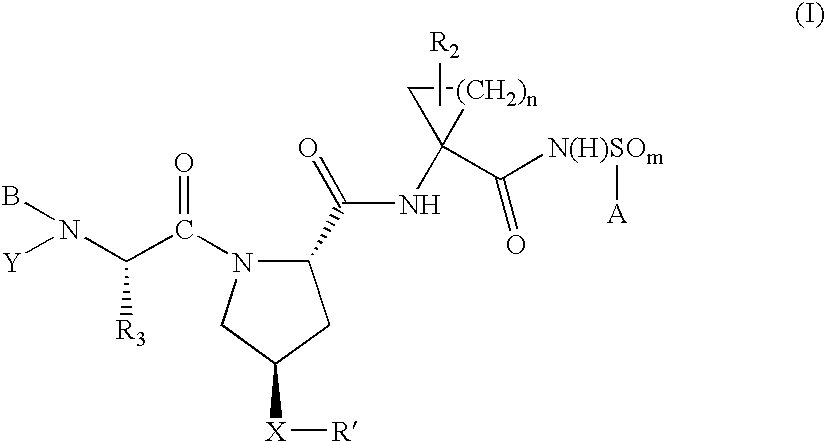
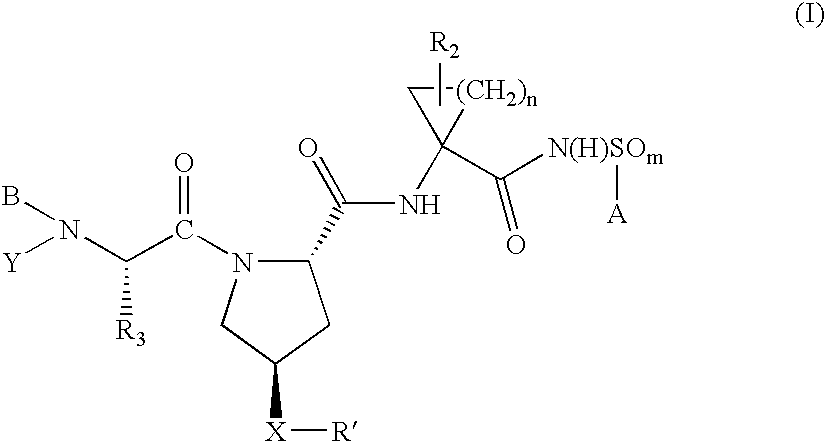
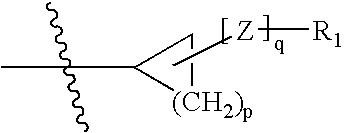
Hepatitis C virus inhibitors
ActiveUS7135462B2Inhibit functioningEffective treatmentBiocideDipeptide ingredientsHepatitis C Virus NS5A InhibitorHepatitis C virus
Owner:BRISTOL MYERS SQUIBB CO
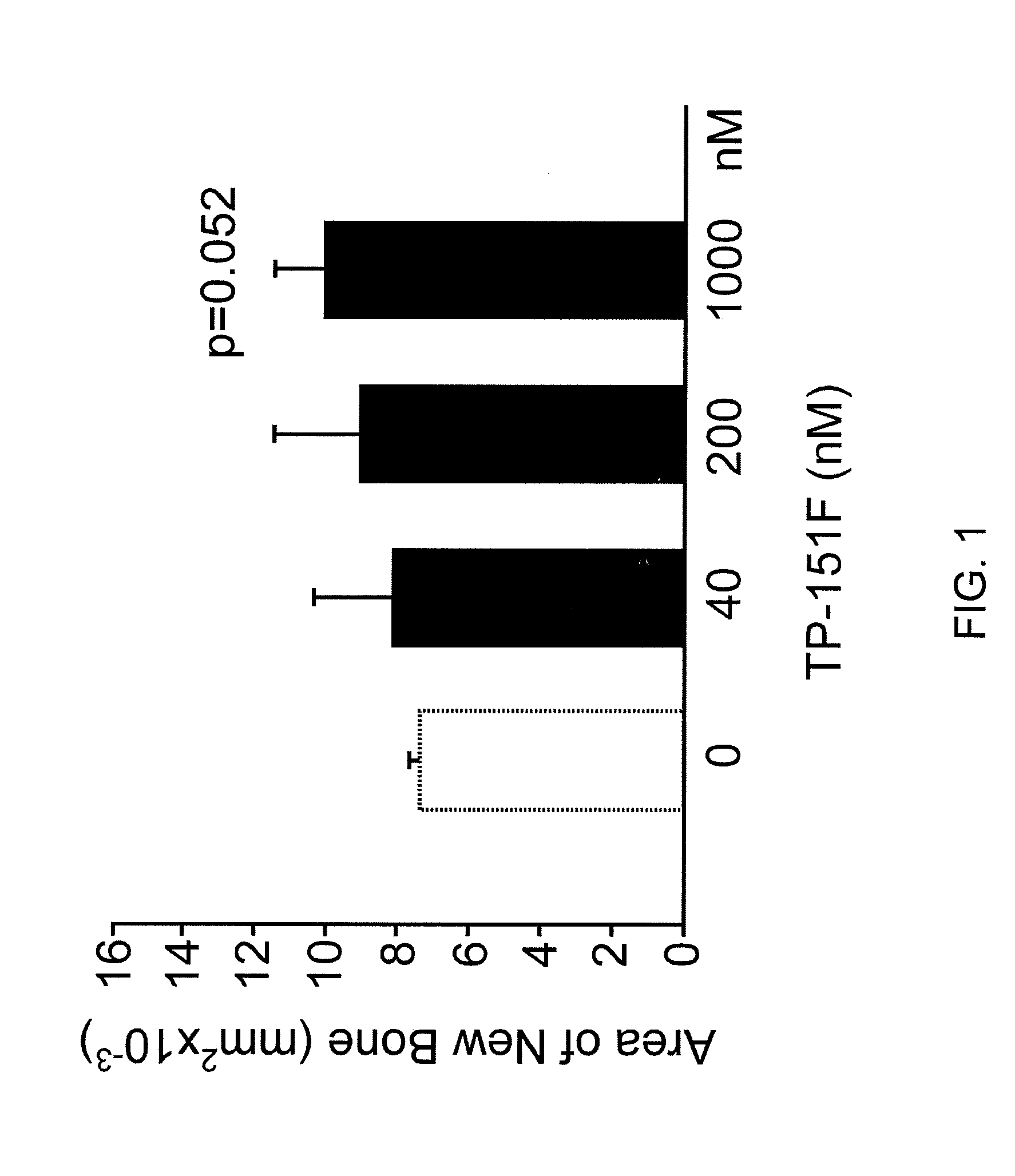
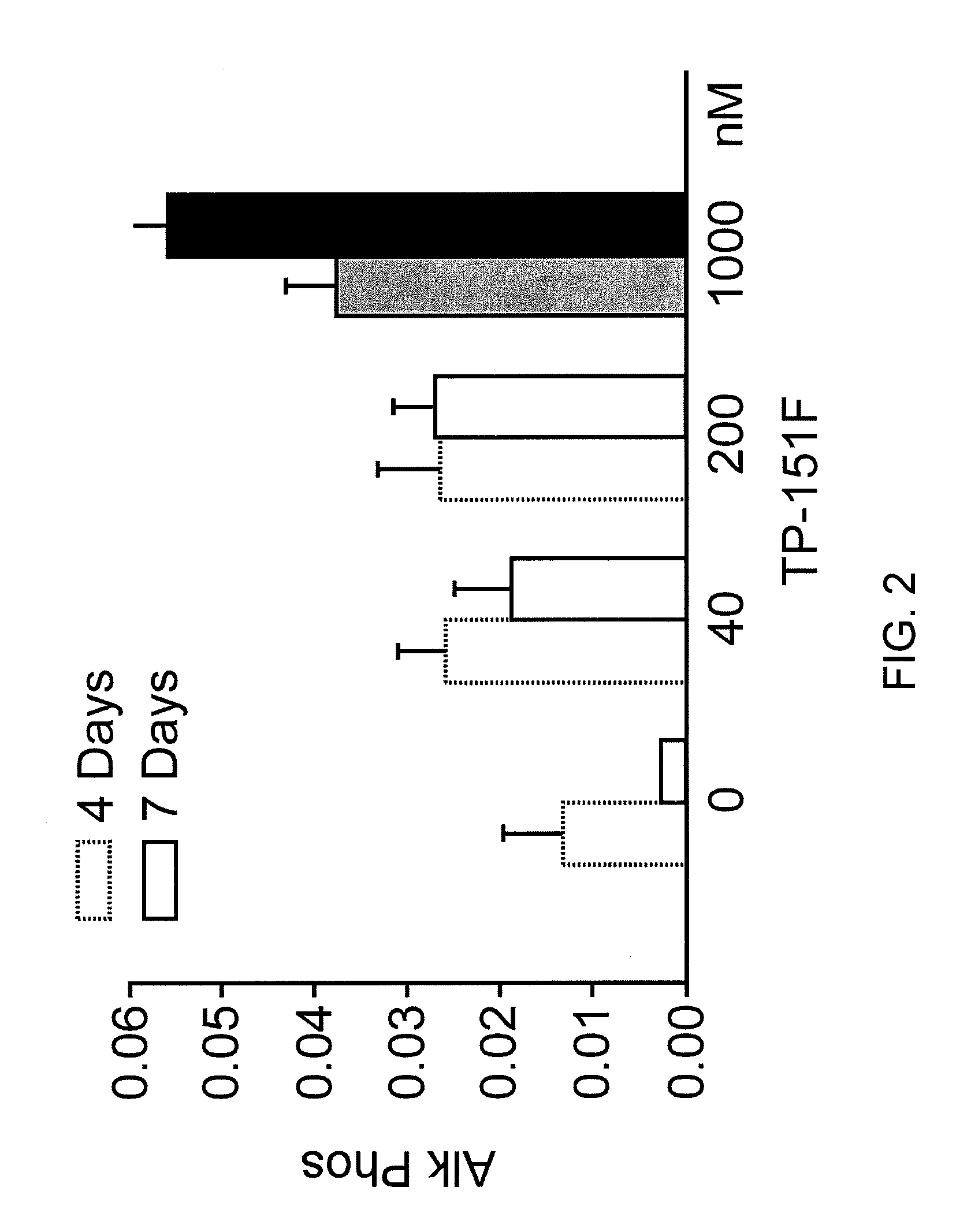
Synthetic triterpenoids and tricyclic-bis-enones for use in stimulating bone and cartilage growth
The present invention concerns methods for stimulating the growth and repair of bone and cartilage using synthetic triterpenoids and tricyclic-bis-enones. Examples of suitable triterpenoids include CDDO, CDDO-Me, CDDO-Im, and CDDO-Ethylamide. Examples of tricyclic-bis-enones include TBE-31 and TBE-34.
Owner:OSTEOSCREEN +2
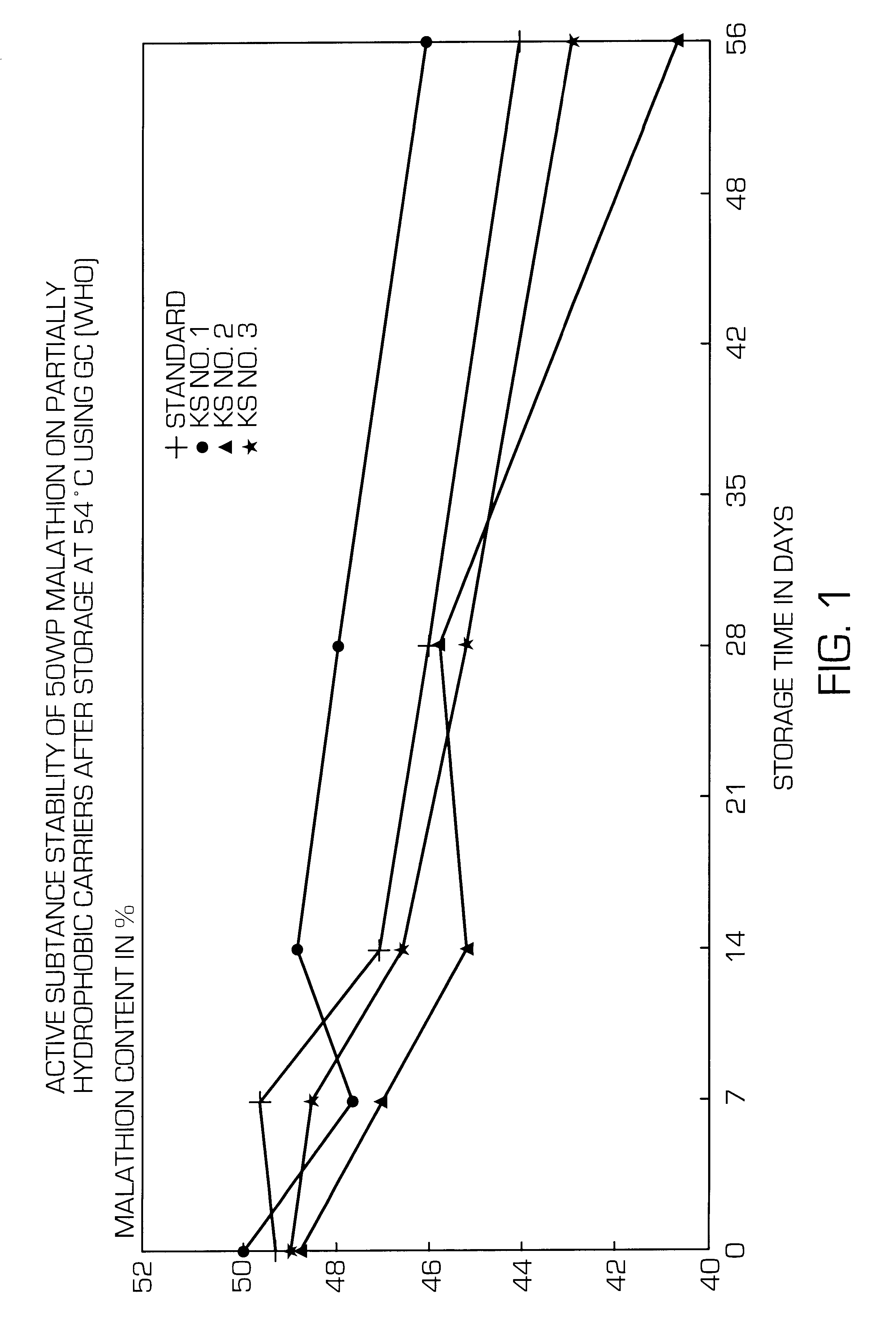
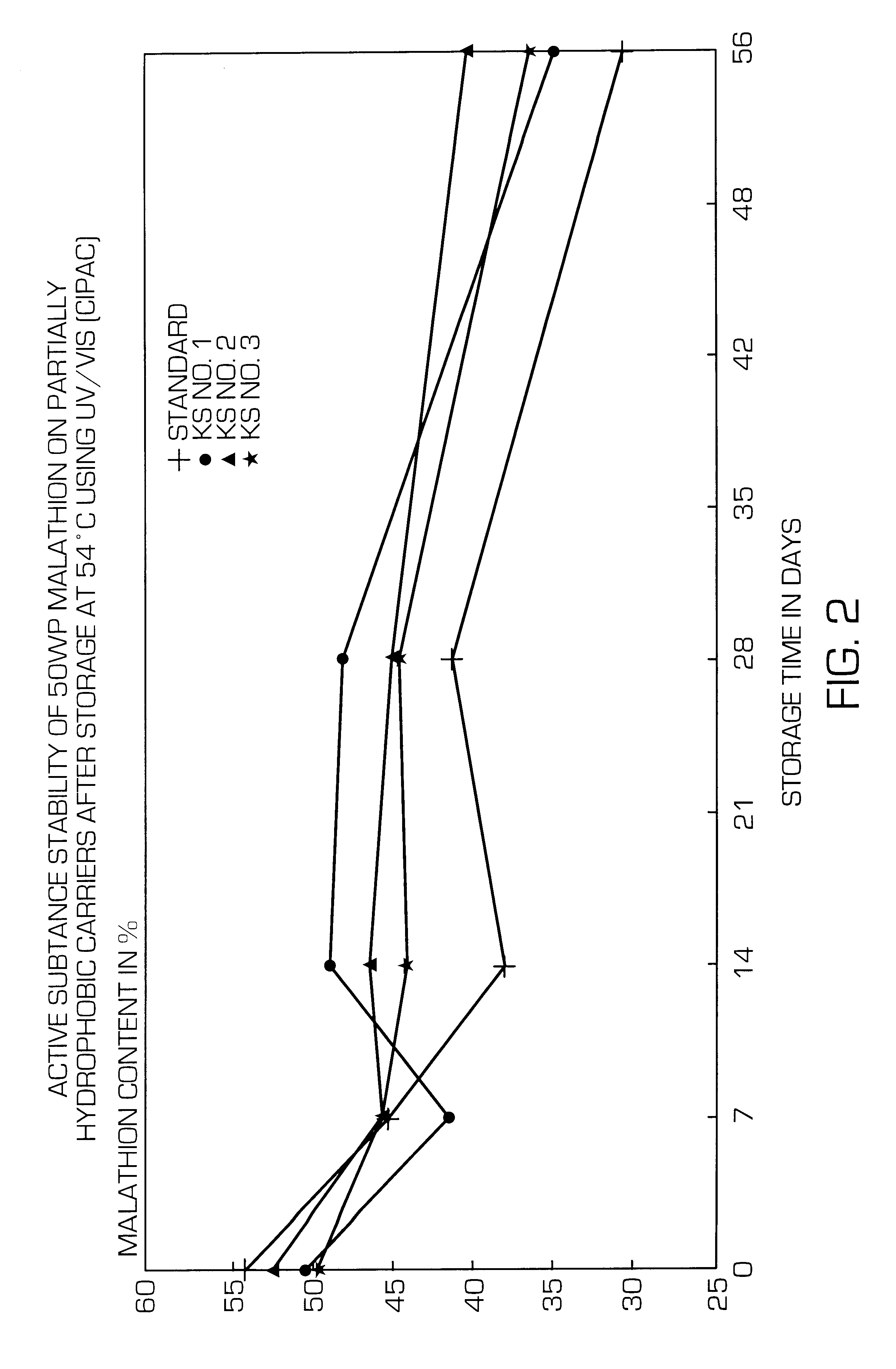
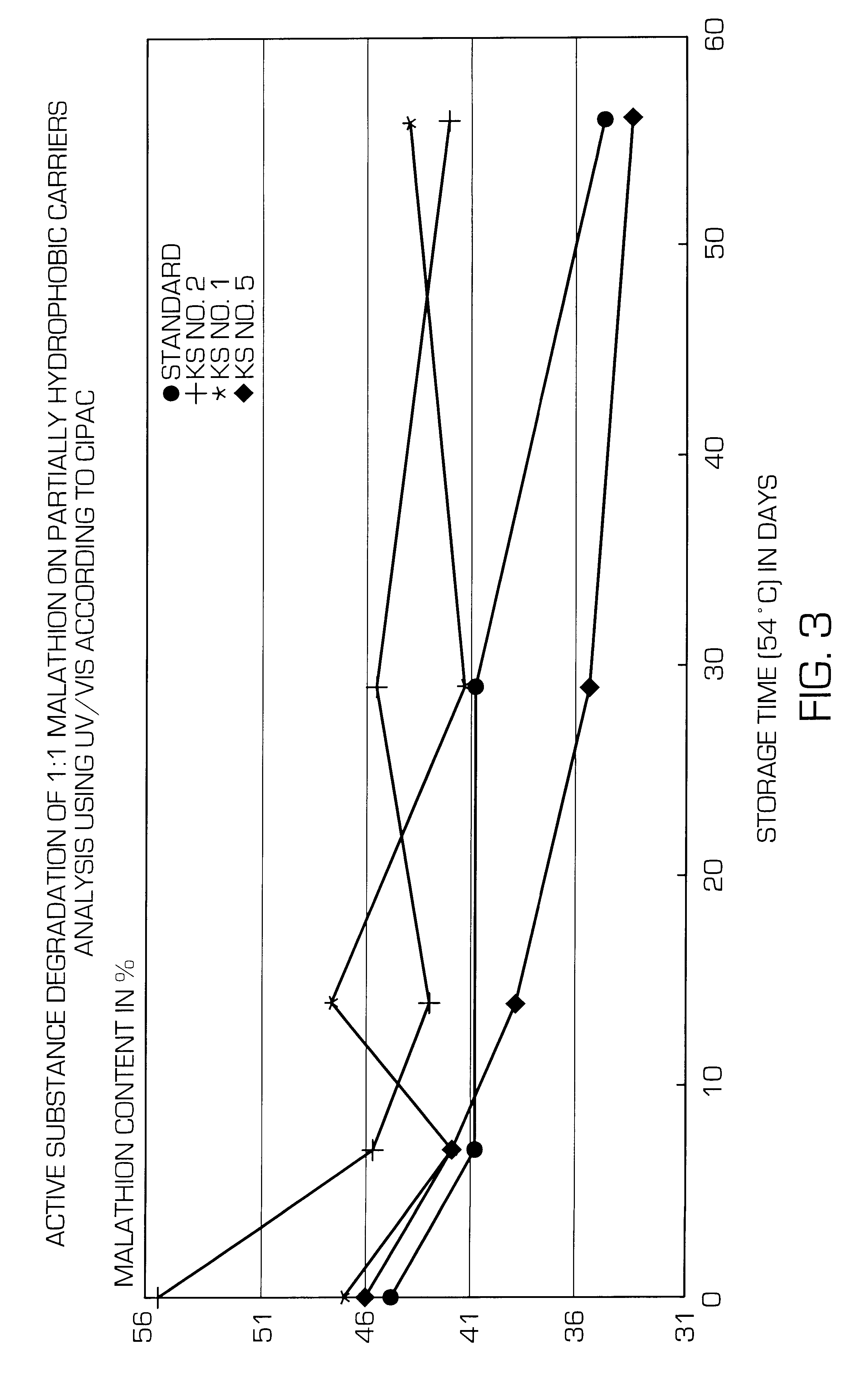
Partially hydrophobic precipitated silicas
A partially hydrophobic precipitated silica having a methanol wettability of 10 to 49%, in particular with a DBP uptake on a dry basis of greater than 250 g / 100 g and a mean particle size of 1 to 12 mum and / or a carbon content of 0.3 to 1.85% and / or a loss on drying of 2.6 to 10.0% and / or a pH value of 5.5 to 10.0, is prepared by mixing the amount of water-repellent agent with the precipitated silica suspension at very short residence time and low pH value, filtering off the solid substance, washing free of salt, drying, post-treating thermally and milling. The partially hydrophobic precipitated silica can be used in active substance formulations and active substance formulations of hydrolysis-sensitive substance and in defoaming agents.
Owner:EVONIK DEGUSSA GMBH
Biaryl compounds as serine protease inhibitors
InactiveUS6699994B1Organic chemistryPeptide/protein ingredientsSerine Protease InhibitorsFactor VIIa
Owner:BIOCRYST PHARM INC
Inhibitors of VEGF receptor and HGF receptor signaling
ActiveUS20070004675A1Promote motilityPromote invasionBiocideOrganic chemistryVEGF receptorsHGF Receptor
The invention relates to the inhibition of VEGF receptor signaling and HGF receptor signaling. The invention provides compounds and methods for inhibiting VEGF receptor signaling and HGF receptor signaling. The invention also provides compositions and methods for treating cell proliferative diseases and conditions
Owner:MIRATI THERAPEUTICS INC
Pharmaceutical treatments and compositions
InactiveUS20070129282A1High expressionStimulate immune responseBiocidePeptide/protein ingredientsRegimenSterol
The invention provides compositions comprising formula 1 steroids, e.g., 16α-bromo-3β-hydroxy-5α-androstan-17-one hemihydrate and one or more excipients, including compositions that comprise a liquid formulation comprising less than about 3% v / v water. The compositions are useful to make improved pharmaceutical formulations. The invention also provides methods of intermittent dosing of steroid compounds such as analogs of 16α-bromo-3β-hydroxy-5α-androstan-17-one and compositions useful in such dosing regimens. The invention further provides compositions and methods to inhibit pathogen replication, ameliorate symptoms associated with immune dysregulation and to modulate immune responses in a subject using the compounds. The invention also provides methods to make and use these immunomodulatory compositions and formulations.
Owner:BIOVIE INC
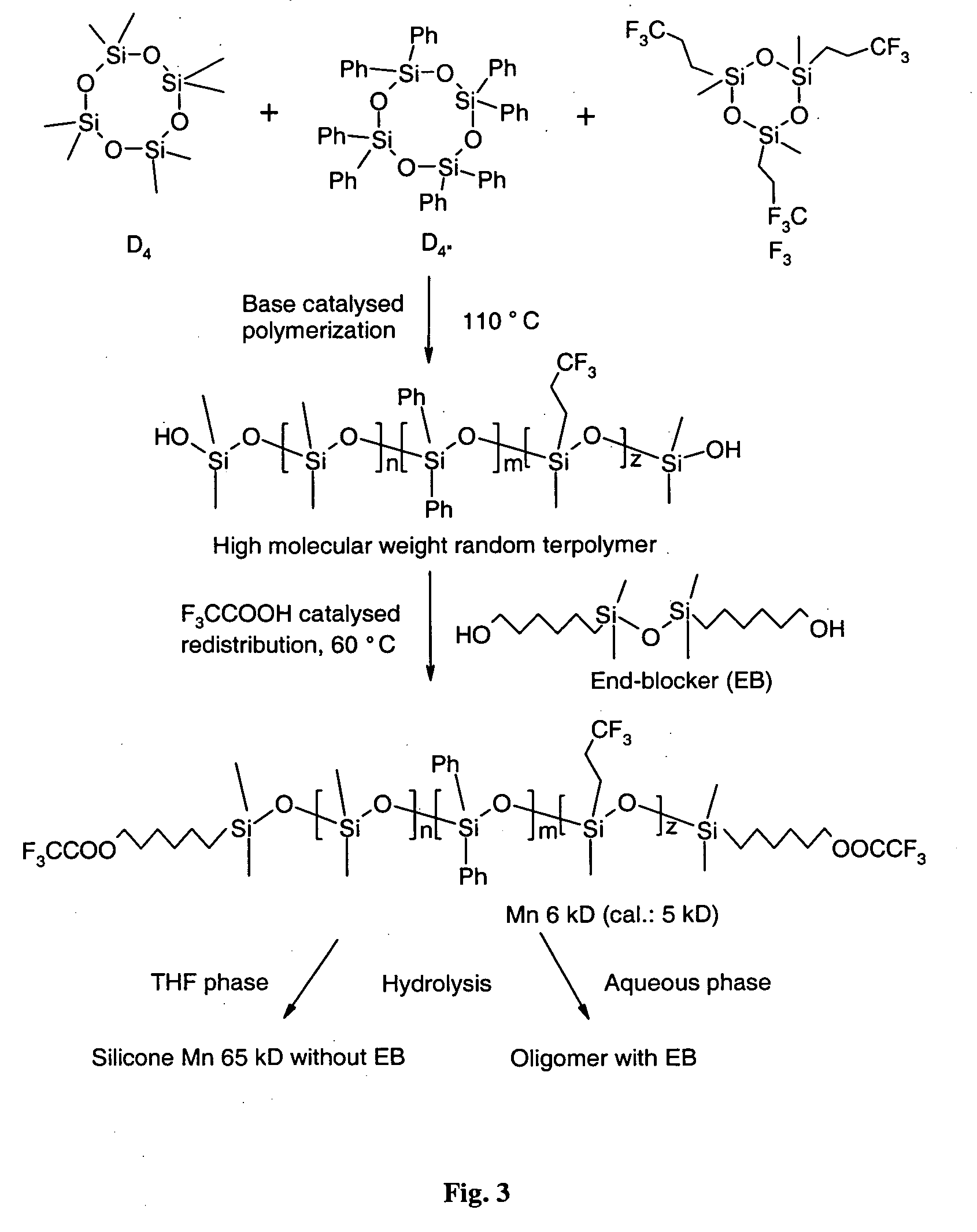
Polysiloxanes, method of synthesis and ophthalmic compositions
InactiveUS20060134173A1Stable and easily and rapidly photo-crosslinkedDesirable propertyBiocideSilicon organic compoundsIntraocular lensCopolymer
A linear polysiloxane copolymer has at least one terminal hydroxyalkyl group. The copolymer is preparable by a process comprising a combination of a base-catalysed polymerisation with an acid-catalysed redistribution, and is suitable for preparing ophthalmic compositions for forming an intraocular lens in situ.
Owner:AMO GRONINGEN
Targeted transscleral controlled release drug delivery to the retina and choroid
InactiveUS20050208103A1Good curative effectReduce impactSenses disorderPeptide/protein ingredientsDiagnostic agentMedicine
The invention provides methods for delivering a therapeutic or diagnostic agent to the eye of a mammal. The method involves contacting sclera with a therapeutic or diagnostic agent so as to permit its passage through the sclera into the choroidal and retinal tissues. The sclera may be contacted with a therapeutic or diagnostic agent together with a device for enhancing transport of the agent through the sclera.
Owner:ADAMIS ANTHONY P +2
Alcoholic pump foam
InactiveUS20060182690A1Disinfection safetyBiocideOrganic detergent compounding agentsAlcoholAdjuvant
An alcoholic foam composition, which can be dispensed as a foam via a pump-foam system contains a) at least 52 to ≦99 wt % of an alcohol or mixture of alcohols, b) a surfactant or a surfactant mixture, c) at least one polyalkylene glycol, d) optionally, at least one foam stabilizer, e) optionally, at least one member selected from the group consisting of cosmetic auxiliaries, adjuvants, active ingredients, and mixtures thereof, and f) optionally water. The surface tension of component b) lies in the range of ±15 dyn / cm of the surface tension of component a) or corresponds to the surface tension of component a), and the sum of components a) to f) is 100 wt % relative to the total quantity of the foam composition.
Owner:DEB IP
Care polymers
The present application relates to care agents, for example care polymers, and compositions such as consumer products comprising such care agents, as well as processes for making and using such care agents and such compositions. The performance of the care polymers that Applicants teach, can be further increased by following the emulsification teaching of the present specification and / or combining such care polymers with silicone materials.
Owner:THE PROCTER & GAMBLE COMPANY
Organosilicones
The present application relates to organosilicones and compositions such as consumer products comprising such organosilicones, as well as processes for making and using such organosilicones and such compositions. Such compositions comprising such organosilicones are easier to formulate, and provide more economical and superior care benefits when compared to current silicone containing compositions.
Owner:THE PROCTER & GAMBLE COMPANY
Medicament for treatment of dermal pigmentation
InactiveUS20070042997A1Inhibit dermal pigmentationLow toxicityBiocideOrganic chemistryPharmacometricsBackbone chain
Owner:INST OF MEDICINAL MOLECULAR DESIGN
Care polymers
The present application relates to care agents, for example care polymers, and compositions such as consumer products comprising such care agents, as well as processes for making and using such care agents and such compositions. The performance of the care polymers that Applicants teach, can be further increased by following the emulsification teaching of the present specification and / or combining such care polymers with silicone materials.
Owner:THE PROCTER & GAMBLE COMPANY
Fatty acid based compositions and methods for the control of plant infections and pests
InactiveUS6103768AColonization by desirable microbes can be even further enhancedPromote colonizationBiocideInorganic boron active ingredientsBiotechnologyFungicide
Owner:MYCOGEN CORP
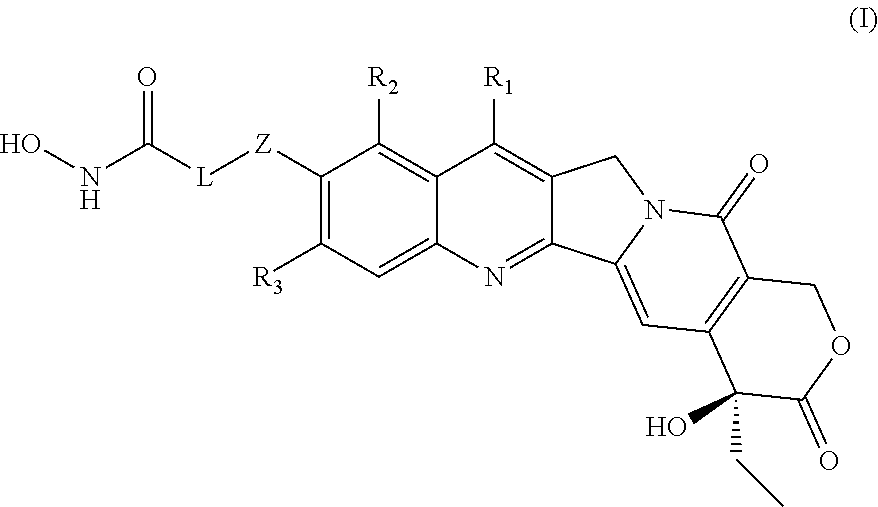
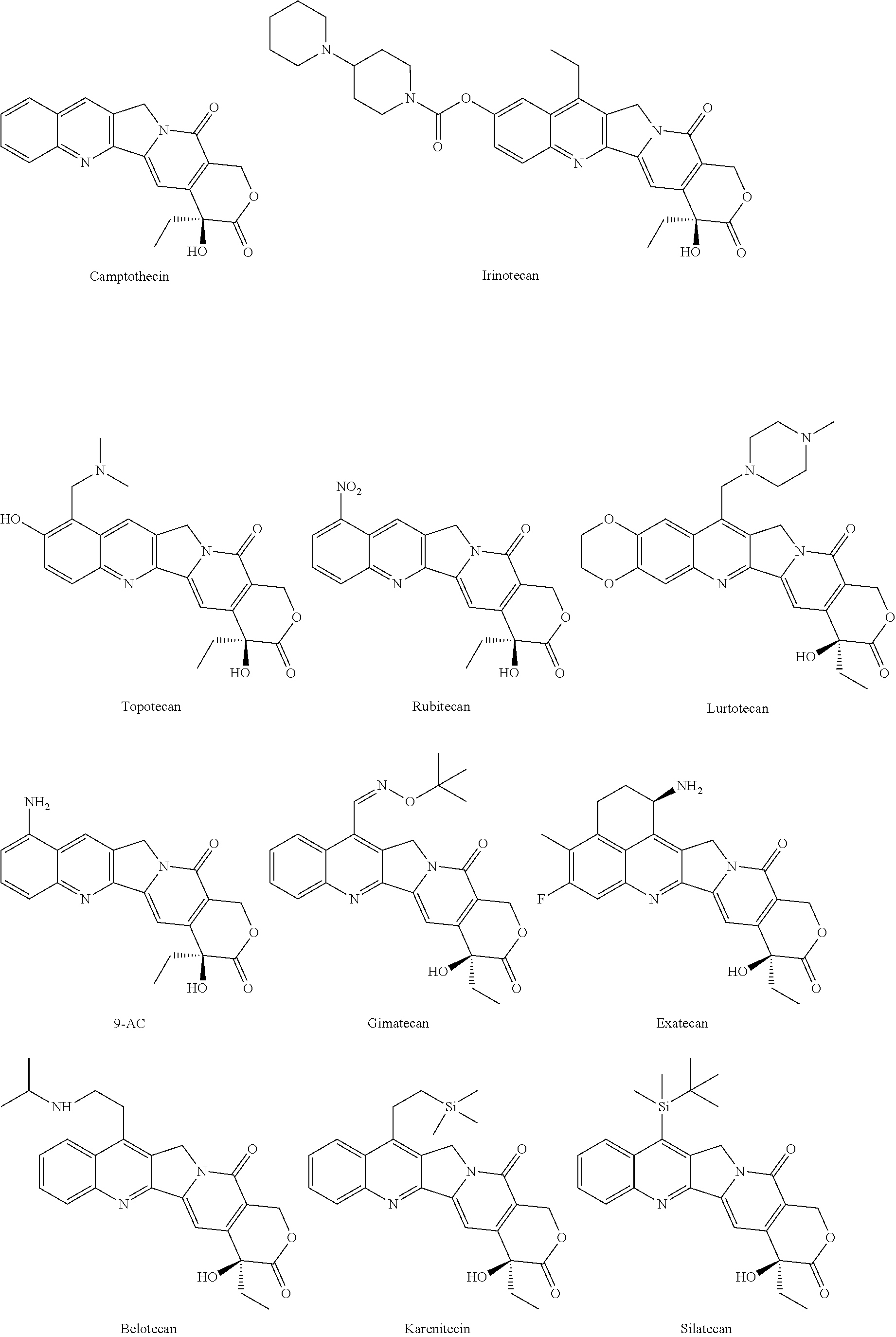
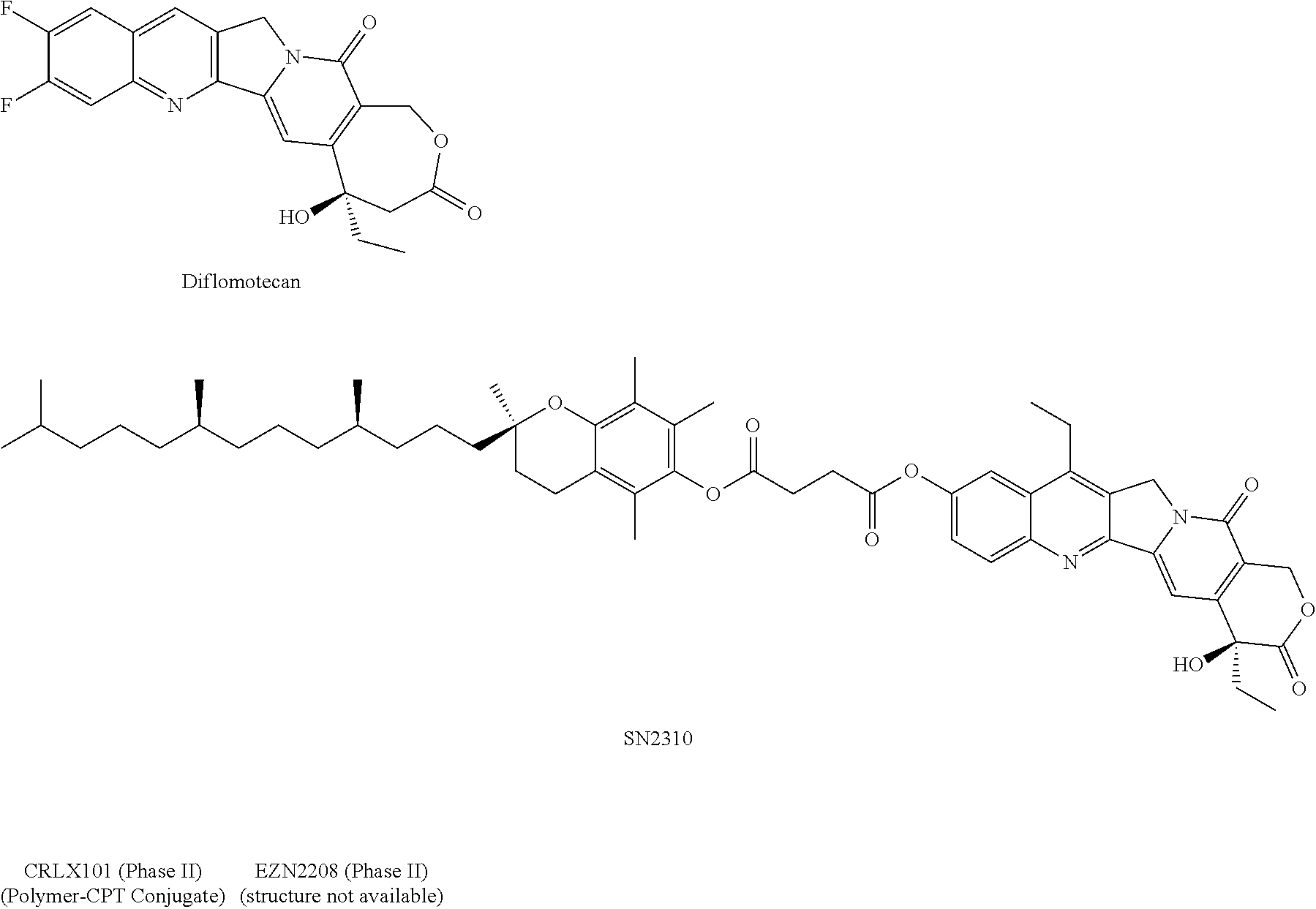
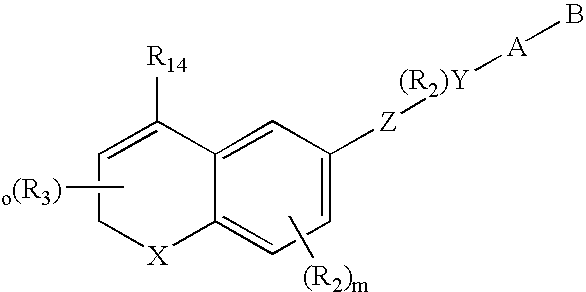
HDAC inhibiting derivatives of camptothecin
InactiveUS20130281402A1Improve efficacyIncreased toxicityBiocideSilicon organic compoundsDiseaseHydroxamic acid
The disclosure includes hydroxamic compounds of Formula I: (Formula I) wherein Z, L, R1, R2, and R3 are defined herein. Also disclosed is a method for treating a neoplastic disease or an immune disease with these compounds.
Owner:CRYSTAL BIOPHARML LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof 3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/aface2a3-2e71-42ae-936e-cb2a5aaaf846/US06924271-20050802-D00000.png)
![3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof 3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/aface2a3-2e71-42ae-936e-cb2a5aaaf846/US06924271-20050802-D00001.png)
![3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof 3-β-D-ribofuranosylthiazolo[4-5-d]pyridimine nucleosides and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/aface2a3-2e71-42ae-936e-cb2a5aaaf846/US06924271-20050802-C00001.png)