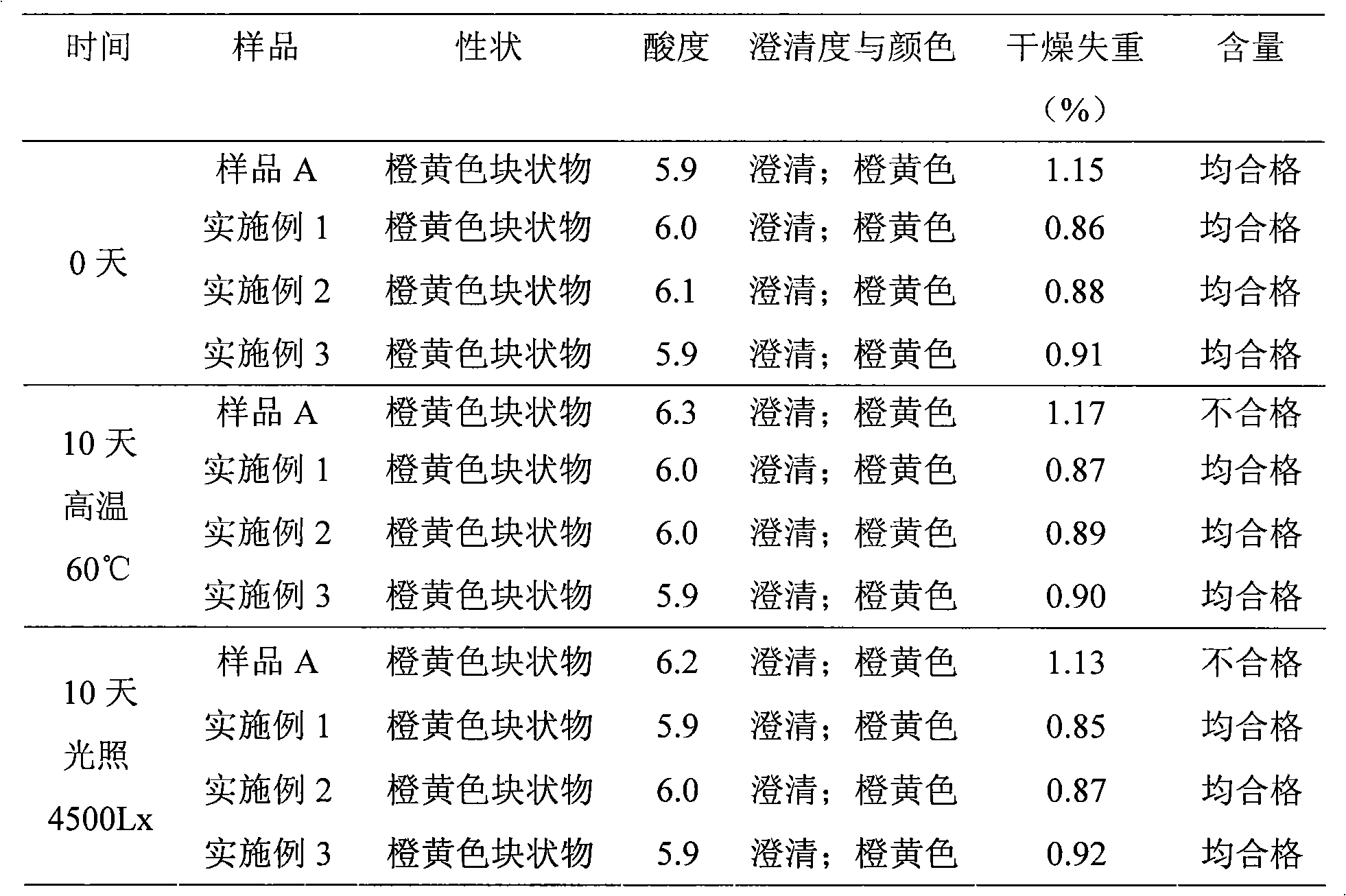
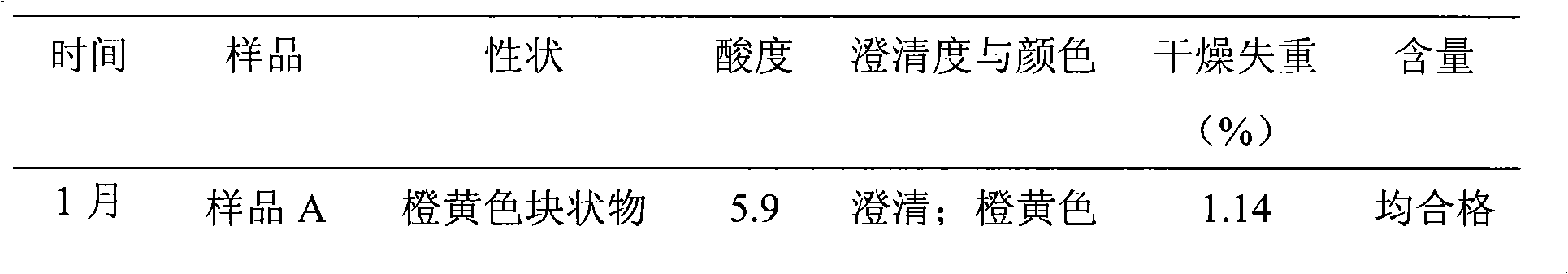
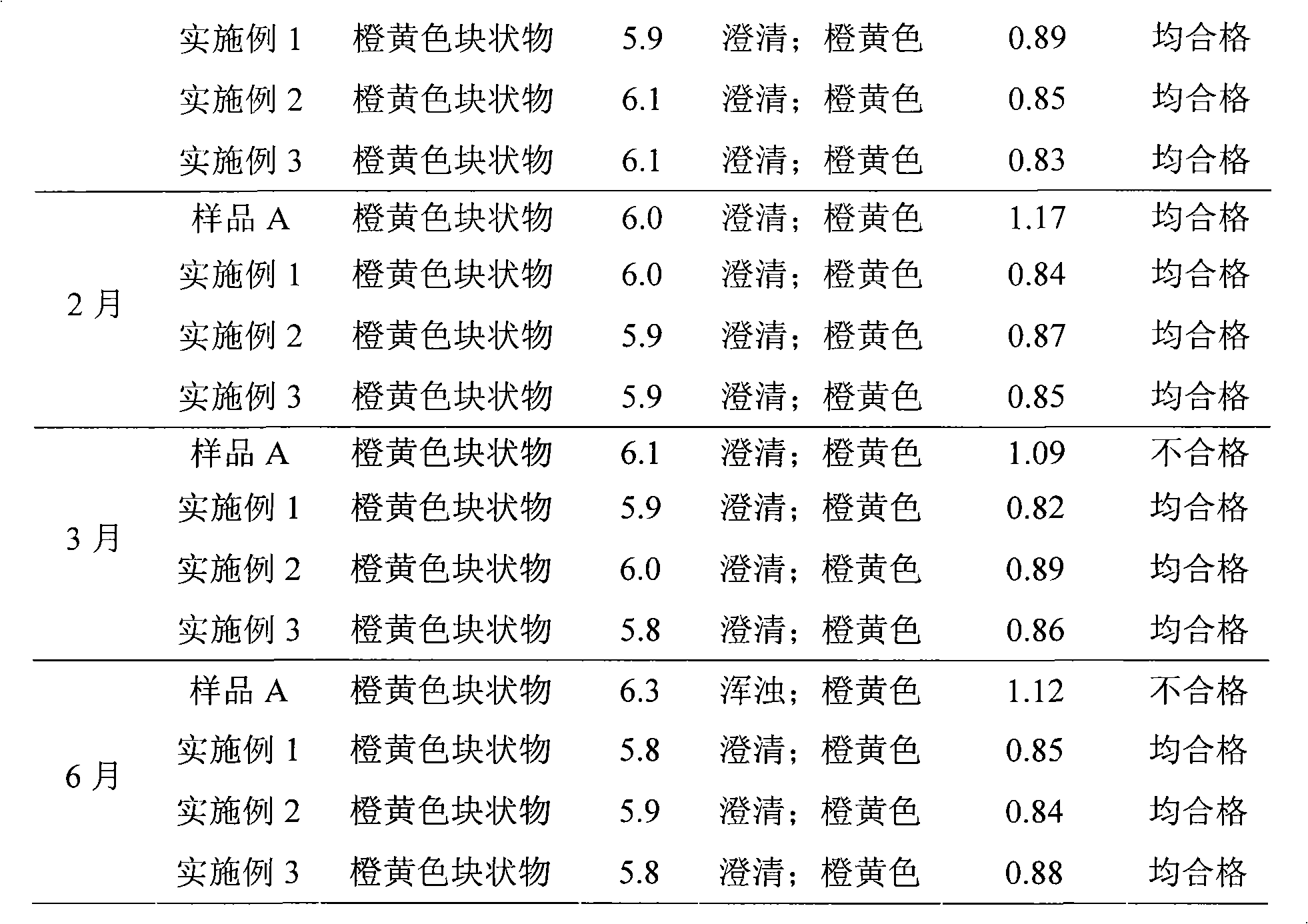
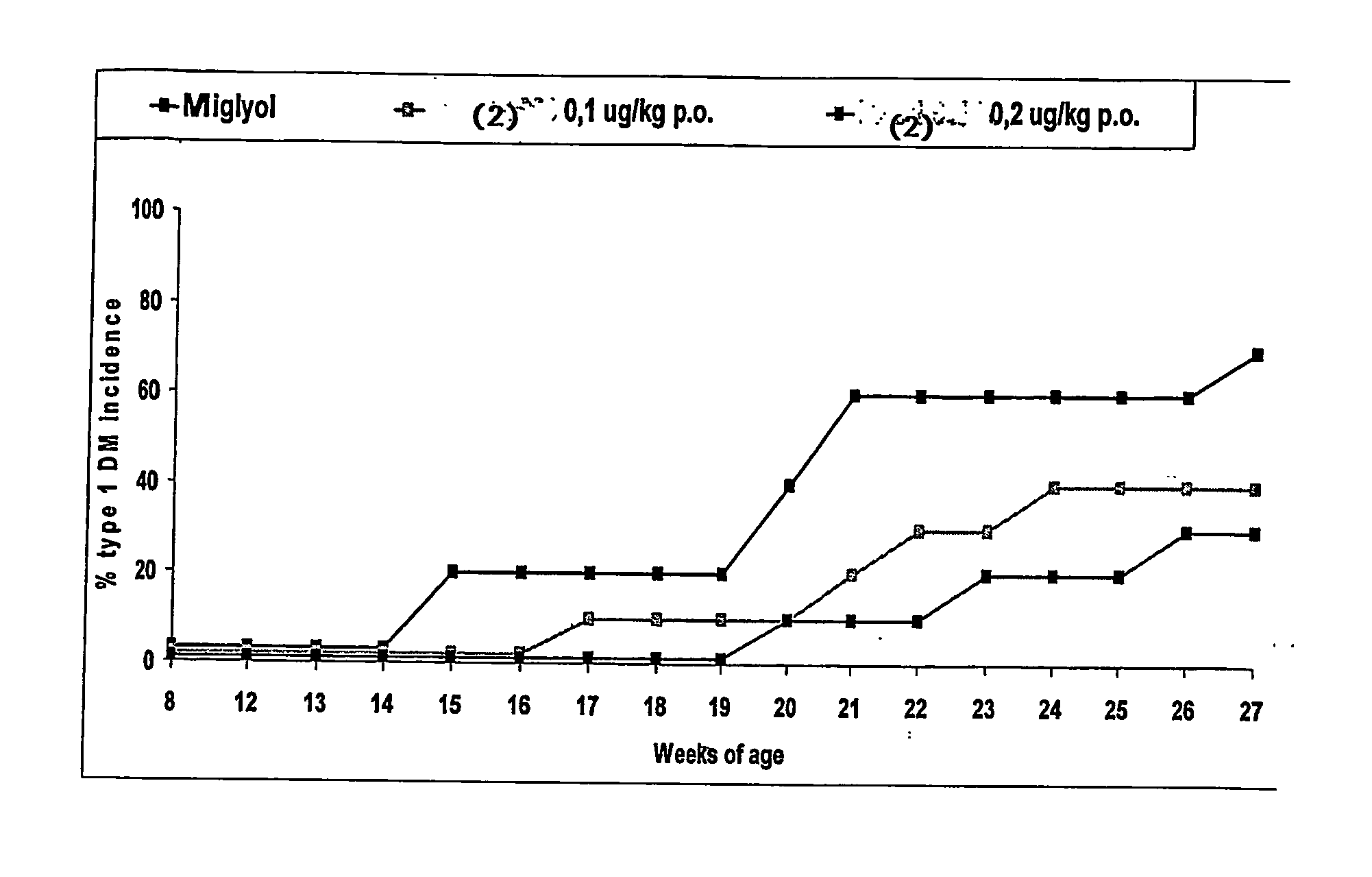
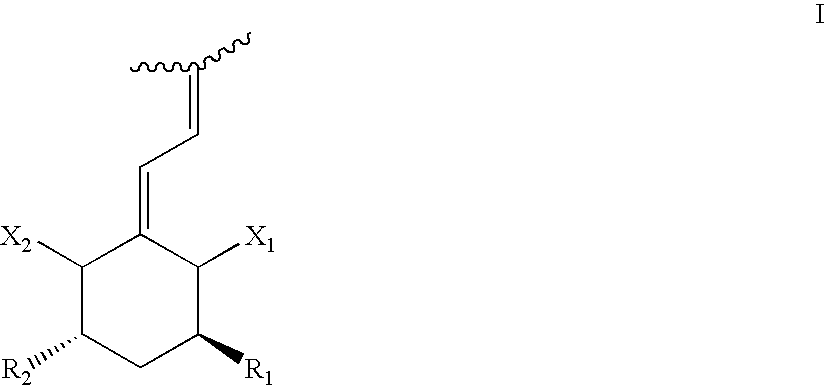Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "Cholecalciferol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
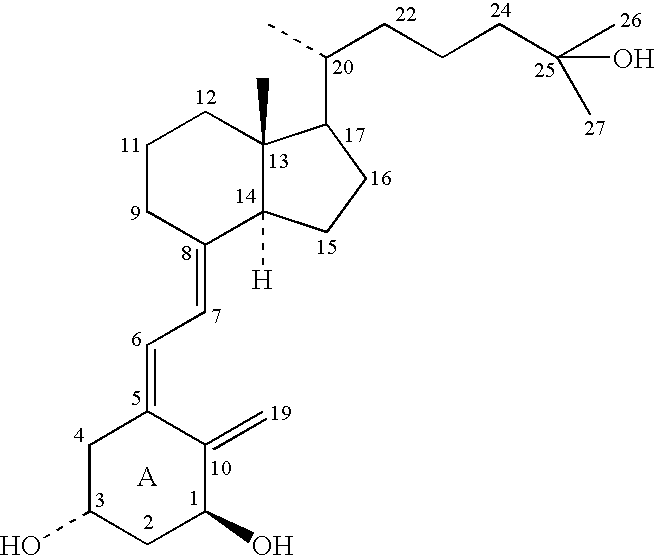
Cholecalciferol, also known as vitamin D₃ and colecalciferol, is a type of vitamin D which is made by the skin when exposed to sunlight; it is also found in some foods and can be taken as a dietary supplement. It is used to treat and prevent vitamin D deficiency and associated diseases, including rickets. It is also used for familial hypophosphatemia, hypoparathyroidism that is causing low blood calcium, and Fanconi syndrome. It is usually taken by mouth.
Composition for promoting healthy bone structure
InactiveUS6447809B1Increase bone densityPrevents radial bone lossBiocideHeavy metal active ingredientsVitamin CRegimen
A dietary supplement for benefitting human bone health includes a calcium source, a source of vitamin D activity, and an osteoblast stimulant. A preferred calcium source is microcrystalline hydroxyapatite, which also contains protein (mostly collagen), phosphorus, fat, and other minerals. A preferred source of vitamin D activity is cholecalciferol, and a preferred osteoblast stimulant is ipriflavone. In addition to these basic ingredients, the composition can further include various other minerals known to occur in bone, vitamin C, and glucosamine sulfate, all of which exert beneficial effects on growth and maintenance of healthy bone. A method for benefitting human bone health involves administering a daily regimen of the dietary supplement.
Owner:PHOENIX DICHTUNGSTECHN +1
Multiple antioxidant micronutrients
A method for administering an antioxidant composition to humans according to their age and sex is disclosed wherein the method comprises administering to said humans a daily dose of a multiple antioxidant micronutrient composition comprising vitamin A (palmitate), beta carotene (from natural d. salina), vitamin C (calcium ascorbate), vitamin D-3 (cholecalciferol), natural source vitamin E including both d-alpha tocopheryl and d-alpha tocopheryl acid succinate, thiamine mononitrate, riboflavin, niacinamide ascorbate, d-calcium pantothenate, pyridoxine hydrochloride, cyanocobalamin, folic acid (folacin), d-biotin, selenium (1-seleno methionine), chromium picolinate, zinc glycinate, calcium citrate, and magnesium citrate. For persons over the age of about 51, the composition preferably further comprises one or more of co-enzyme Q10, N-acetyl cysteine, and alpha lipoic acid. Preferably, also, vitamin D is added for women over the age of about 36.
Owner:NEW AGE HEALTH SCI INC
Composition for promoting healthy bone structure
InactiveUS20030059481A1Rate lossIncrease bone densityBiocideOrganic active ingredientsVitamin CRegimen
A dietary supplement for benefitting human bone health includes a calcium source, a source of vitamin D activity, and an osteoblast stimulant. A preferred calcium source is microcrystalline hydroxyapatite, which also contains protein (mostly collagen), phosphorus, fat, and other minerals. A preferred source of vitamin D activity is cholecalciferol, and a preferred osteoblast stimulant is ipriflavone. In addition to these basic ingredients, the composition can further include various other minerals known to occur in bone, vitamin C, and glucosamine sulfate, all of which exert beneficial effects on growth and maintenance of healthy bone. A method for benefitting human bone health involves administering a daily regimen of the dietary supplement.
Owner:METAGENICS INC
Therapeutic compositions
InactiveUS6946151B2Lower blood sugar levelsSlow onsetBiocideOrganic active ingredientsDiseaseAntioxidant
An oral composition, suitable as a hypoglycemic agent, includes an isolate from the leaves of Gymnema sylvestre, having a specified molecular weight. The isolate has a molecular weight at least about 3000 Daltons as determined by molecular weight cut-off filtration. Glucose metabolism in a human patient can be regulated by dosage forms that contain the aforesaid isolate from the leaves of Gymnema sylvestre, in combination with a non-metabolizable, water-swellable polysaccharide such as the exudate of Sterculia urens, and a water-soluble polysaccharide such as guar gum. Optionally, the present oral compositions can include a physiologically acceptable calcium source, a physiologically acceptable metal carbonate salt, a physiologically acceptable chromium salt, and / or a physiologically acceptable vanadium compound. In addition, antioxidants such as ascorbic acid, cholecalciferol, d-α-tocopherol, the carotenoids, lycopene, lutein, and the like, can be included as well. The present compositions are useful for amelioration of cholesterolemia, obesity, chronic complications of diabetes and prophylaxis for patients predisposed to the foregoing.
Owner:AYURVEDIC LIFE INT LLC
Treating hyperglycemia with 25-hydroxyvitamin d3
InactiveUS20110039811A1Reduce high blood glucoseMaintain blood glucoseBiocideOrganic active ingredientsCalciferolsPharmaceutical drug
We disclose treating hyperglycemia in a human with 25-hydroxyvitamin D3 (calcifediol). Blood glucose is reduced to a level which is closer to normal than baseline. Vitamin D3 (cholecalciferol) may optionally be used together with 25-hydroxy vitamin D3. Forms and dosages of a pharmaceutical composition, as well as processes for manufacturing medicaments, are also disclosed.
Owner:DSM IP ASSETS BV
Treating hypertension with 25-hydroxyvitamin d3
InactiveUS20110118218A1Maintain healthy blood pressureSuppress high blood pressureOrganic active ingredientsBiocideTreatment hypertensionCholecalciferol
We disclose the use of optionally in combination with vitamin D3 (cholecalciferol), 25-hydroxyvitamin D3 (cal-cifediol), to treat hypertension. Forms and dosages of a pharmaceutical composition, as well as processes for manufacturing medicaments, are also disclosed.
Owner:DSM IP ASSETS BV
Composite liposome for injection containing 12 vitamins and preparation method thereof
InactiveCN101491499ASafety proofMetabolism disorderAmide active ingredientsAlpha-TocopherolSodium phosphates
The invention relates to a liposome lyophilized preparation prepared from twelve compound vitamins. The lyophilized preparation is characterized in that the lyophilized preparation is prepared from a liposome which consists of soybean lecithin and glycocholic acid and is encapsulated with twelve vitamins of retinyl palmitate, cocarboxylase tetrahydrate, riboflavin sodium phosphate, pyridoxine hydrochloride, cyanocobalamin, cholecalciferol, ascorbic acid, racemization-alpha tocopherol, D-biotin, niacinamide, folacin and dexpanthenol.
Owner:灵康药业集团股份有限公司
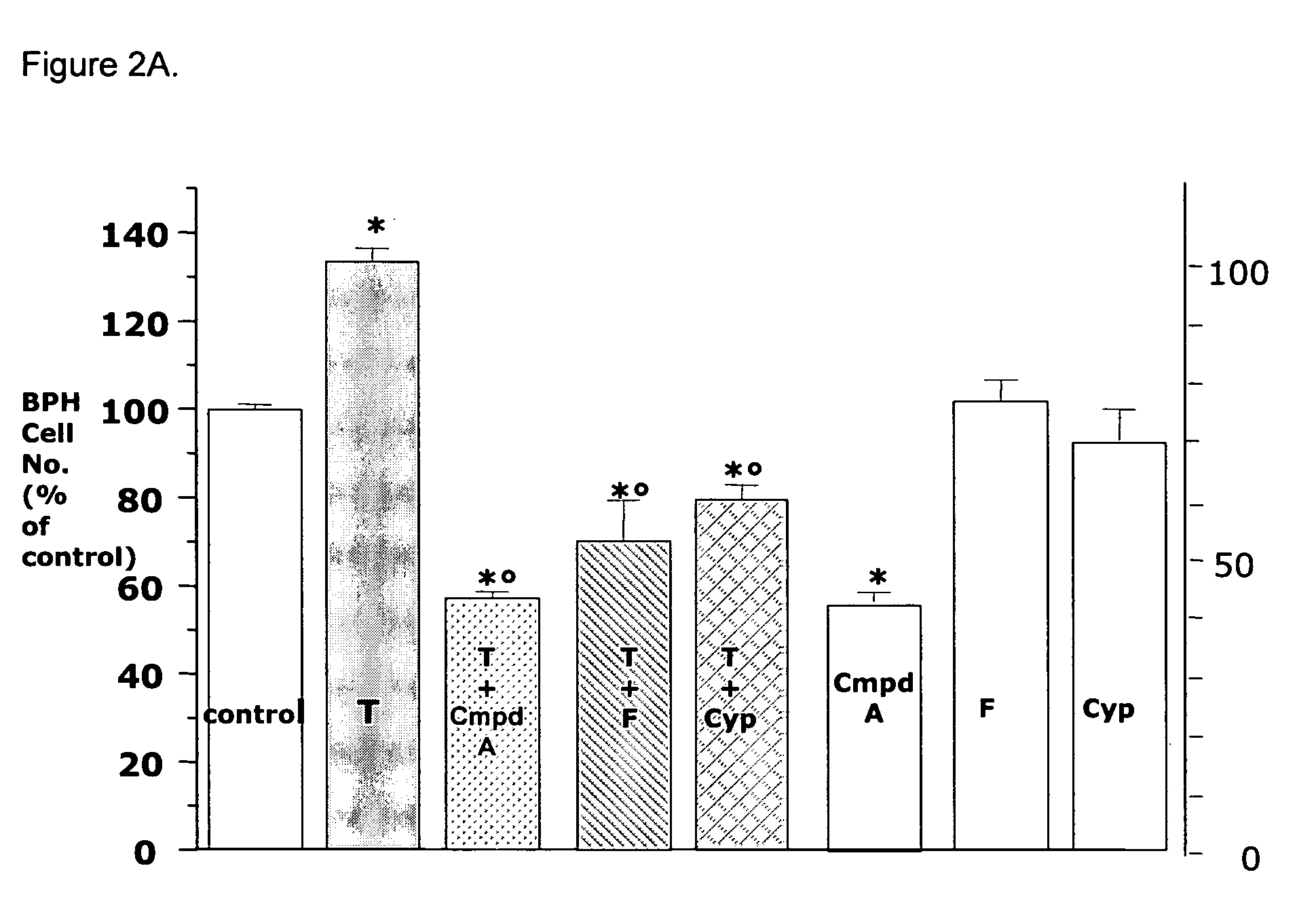
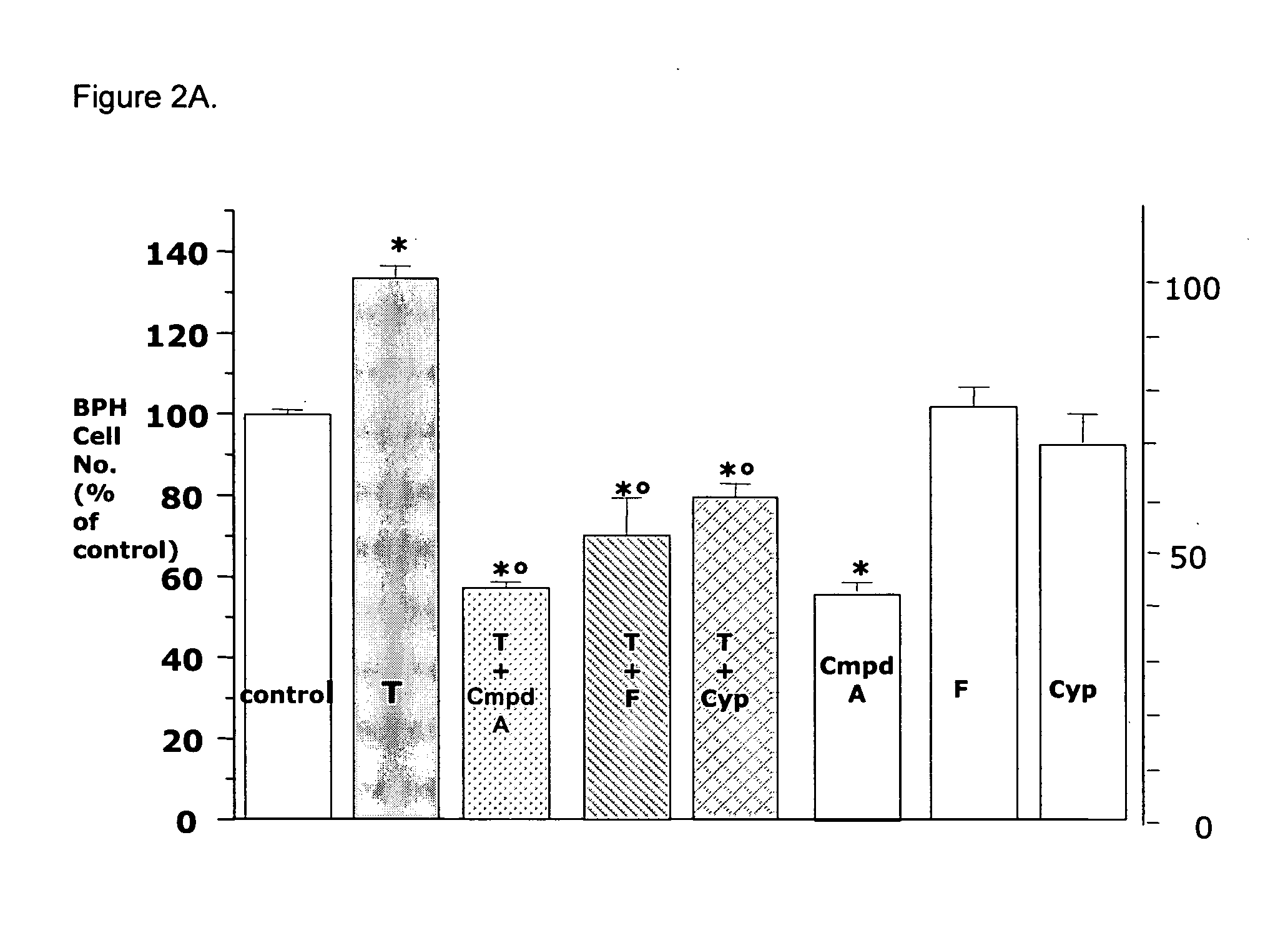
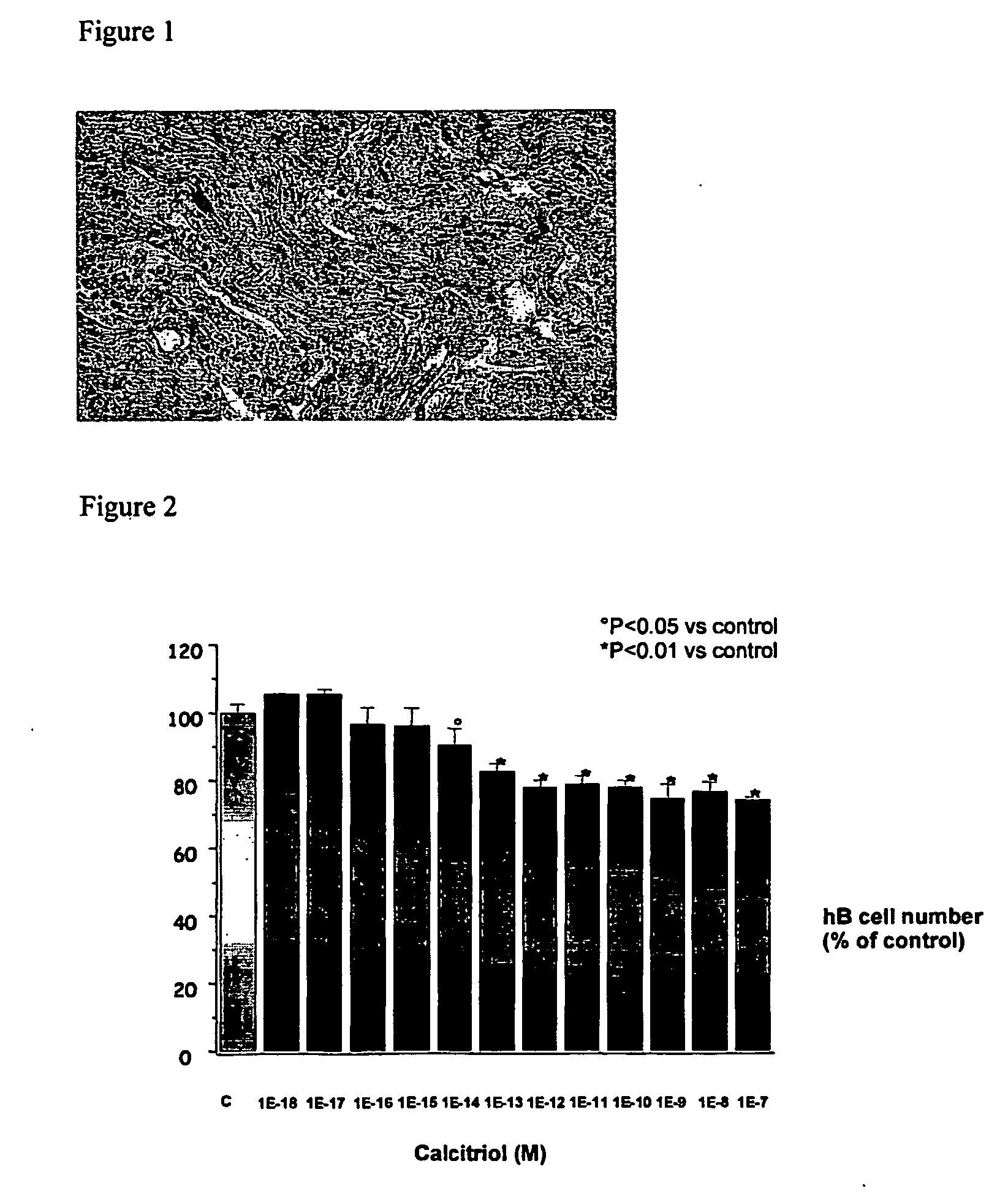
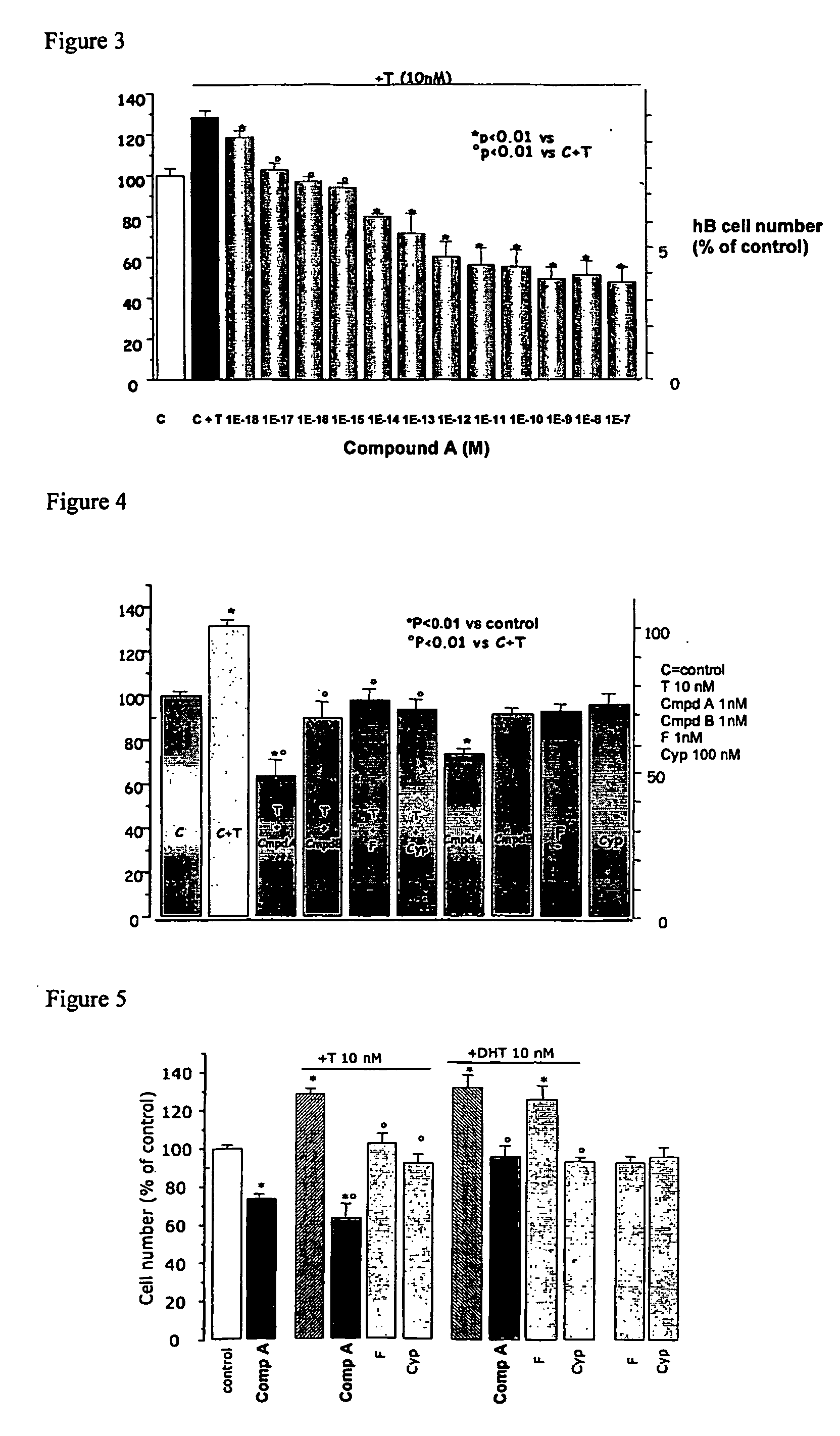
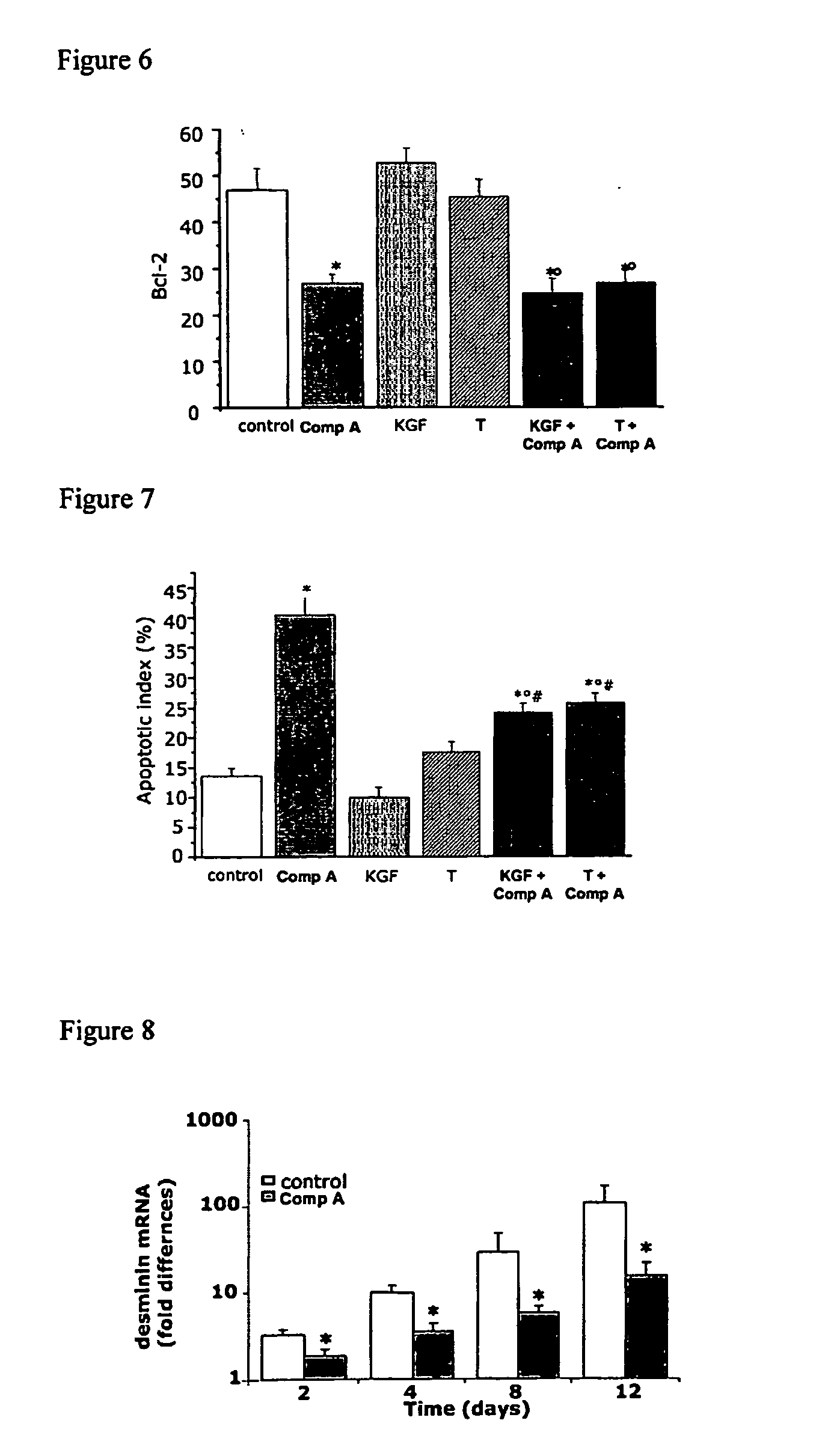
Method for treating benign prostatic hyperplasia
InactiveUS7332482B2Effective pharmacologic agentReduce the overall heightBiocideOrganic chemistryProstate hyperplasiaBenign prostatic hyperplasia (BPH)
Owner:BIOXELL
Extraction and quantification of vitamins a and d in fluid samples
The invention discloses monoclonal antibodies for vitamins A (retinol palmitate) and D3 (cholecalciferol); a method for using monoclonal antibodies, and the monoclonal antibodies disclosed herein, in particular, to quantitate these vitamins in fluids such as dairy products, and blood, and also raw or processed agri-food and beverage products. The method involves contacting the sample with a mixture of polar and non-polar organic solvents in combination with inorganic salts to remove fat molecules into an organic fraction, and assaying the organic fraction by immunoassay involving the monoclonal antibodies. The presence of a mixture of non-polar and polar organic solvents increases the separation of vitamins from fat molecules and enables the test samples to be quantified by immunoassay without any further treatment.
Owner:SCIMED TECH
Micronutrient formulations for hearing health
InactiveUS20080187526A1Prevent excess productionOrganic active ingredientsSenses disorderPhysiologyPalmitates
A hearing health micronutrient formulation is provided and the formulation comprises dietary antioxidants and endogenous antioxidants, and the dietary antioxidants are selected from a group consisting essentially of Vitamin A (Palmitate), Vitamin E, Vitamin C (Calcium Ascorbate), Vitamin D3 (Cholecalciferol), B Vitamins, Biotin, Pantothenic Acid (as D-Calcium Pantothenate), Calcium Citrate, Magnesium Citrate, Zinc Glycinate, Selenium (Seleno-L-Methionine), Chromium (as Chromium Picolinate), Mixed Carotenoids and mixtures thereof, and the endogenous antioxidants are selected from a group consisting essentially of N-Acetyl Cysteine (NAC), Coenzyme Q10, R-alpha Lipoic Acid, L-Carnitine and mixtures thereof,
Owner:NEW AGE HEALTH SCI INC
Oral Compositions, Products and Methods Of Use
The present invention comprises an oral composition. More particularly to a method of treating and preventing a respiratory tract infection and providing energy, relieving stress, and mood enhancing benefits to a human comprising: the steps of administering to a human a composition comprising from about 450 IU to about 500,000 IU of cholecalciferol, per dose of the composition.
Owner:THE PROCTER & GAMBLE COMPANY
Oral Compositions, Products And Methods Of Use
The present invention comprises an oral composition. More particularly to a method of treating and preventing a respiratory tract infection and providing energy, relieving stress, and mood enhancing benefits to a human comprising: the steps of administering to a human a composition comprising from about 450 IU to about 500,000 IU of cholecalciferol, per dose of the composition.
Owner:THE PROCTER & GAMBLE COMPANY
High dosage Vitamin D
InactiveUS20090196862A1Useful in treatmentOrganic active ingredientsBiocideHypertension medicationsSeasonal Affective Disorders
A preparation containing vitamin D3 (cholecalciferol), preferably an edible oil, in an amount of 200 IU to 20,000 IU, preferably 1000 IU to 5000 IU, and most preferably about 1500 IU to 3500 IU, with about 2000 IU particularly preferred. Such a preparation is useful in providing adequate nutrition, in the prevention and / or treatment of Seasonal Affective Disorder (SAD), fall prevention, multiple sclerosis, chemoprevention of cancer, accelerated fracture healing, and metabolic syndrome. It acts as an anti-depressant, an anti-hypertensive for renovascular hypertension and high-renin hypertension; and is useful in hyperlipidemia treatment, as well as in the treatment of hypovitaminosis D in the renal failure population. The most important application to the present inventors is in treatment, prevention and also reversal of hypertension in renovascular and high-renin hypertension. The effective amount of vitamin D, based on blood serum content, is equal to or greater than 20 ng / mL, preferably equal to or greater than 40 ng / mL, and most preferably equal to or greater than 50 ng / mL, with no specific upper range, but preferably equal to or less than 200 ng / mL, and more preferably equal to or less than 100 ng / mL.
Owner:DAVIS WILLIAM +1
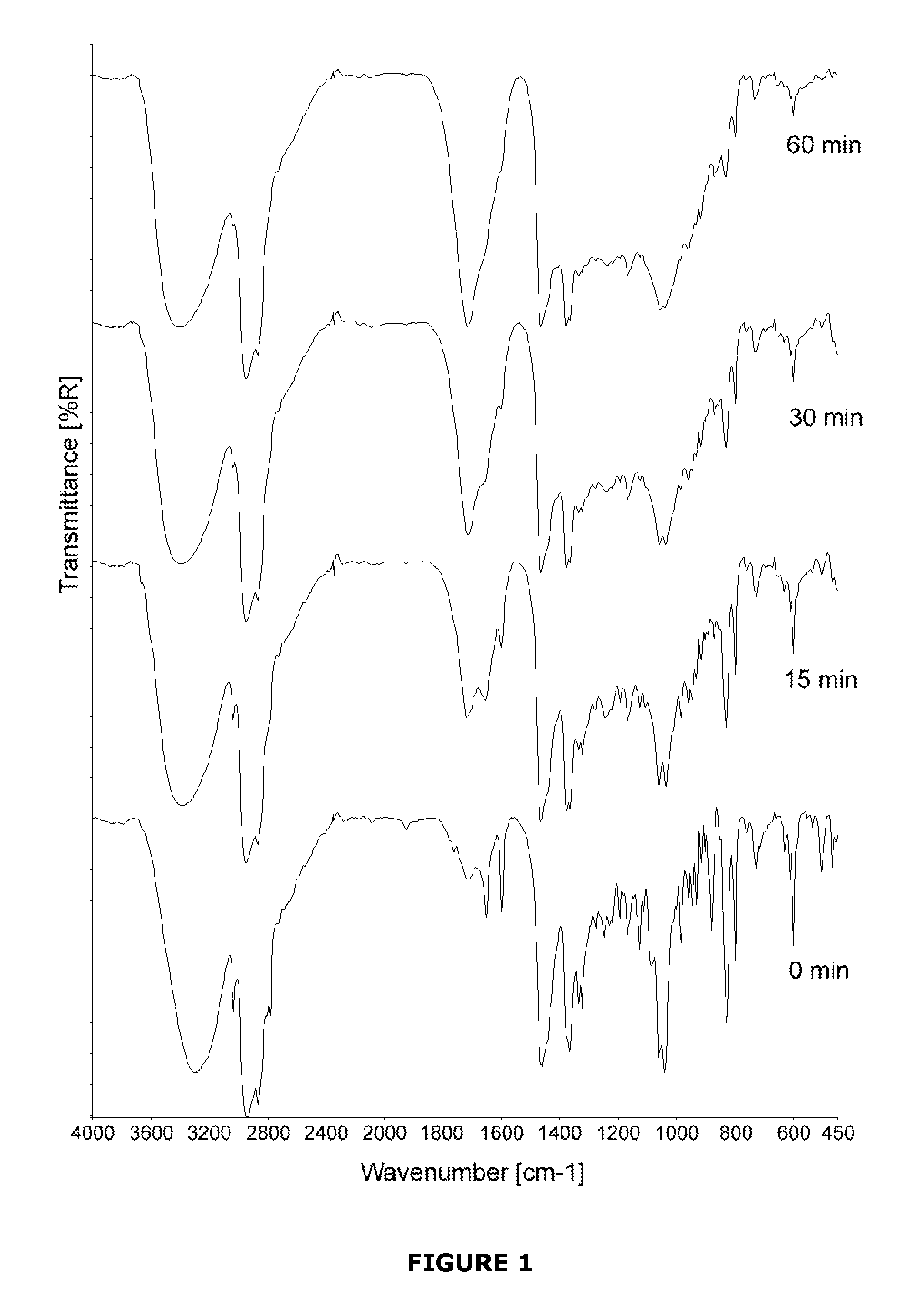
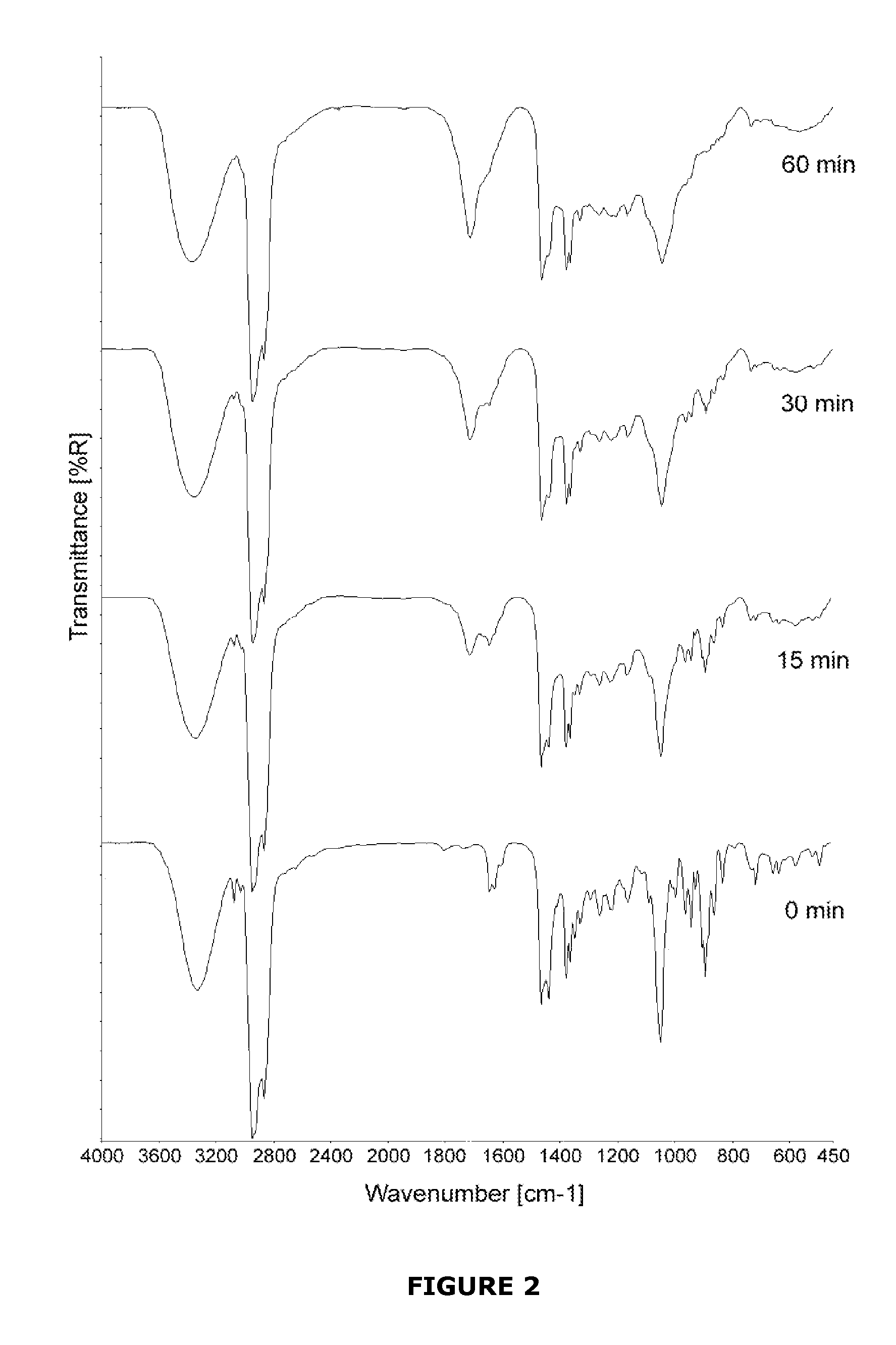
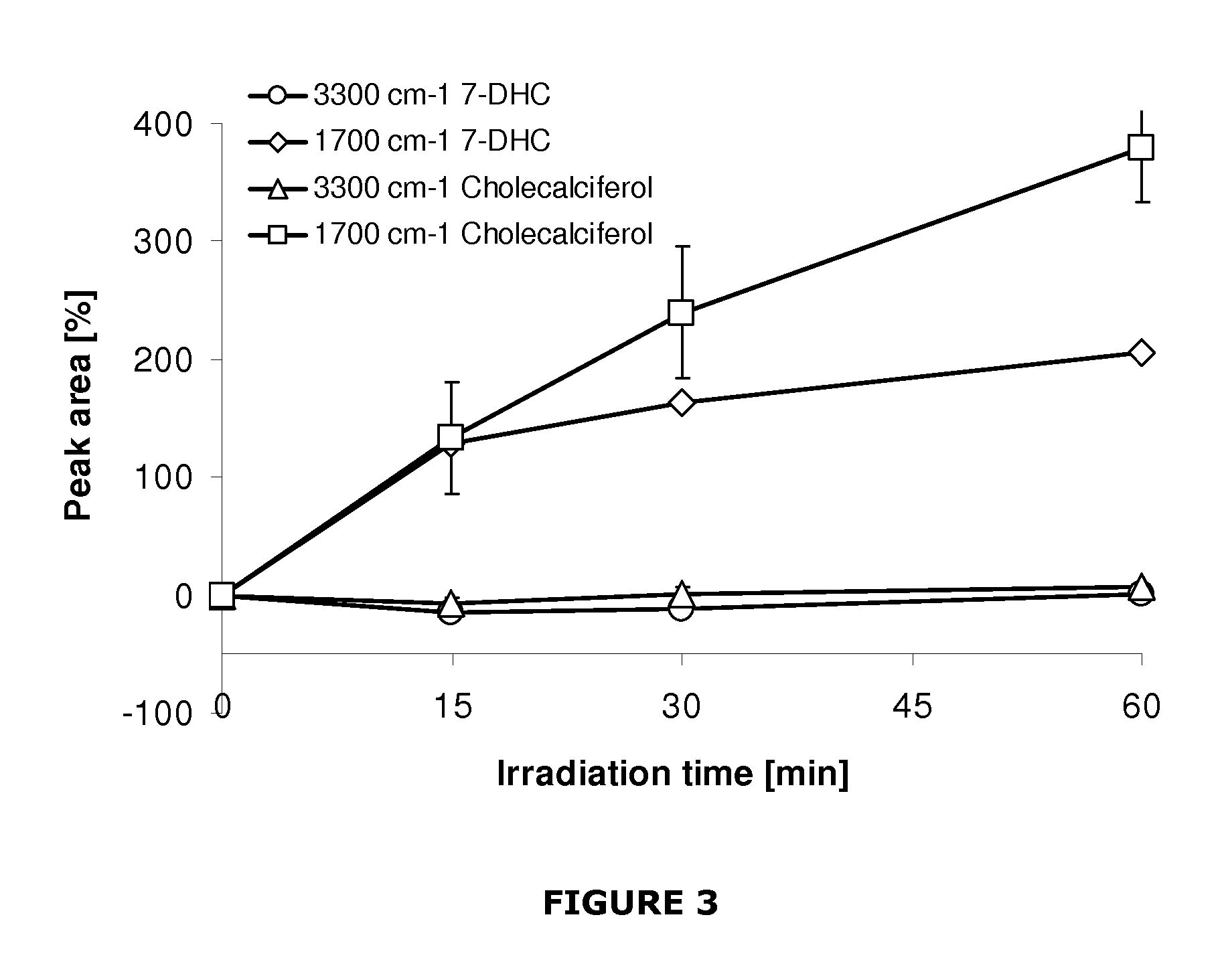
Implant modified with non-hydroxylated vitamin d precursors
InactiveUS20130017234A1Promote osseointegrationLow toxicityBiocideImpression capsAntioxidantHydroxylation
An implant to be used as medical or dental implant, comprising a metallic or polymeric base which is covered by the vitamin D precursor cholecalciferol. The implant can be obtained by direct covering of the polymeric or metallic base with a solution comprising cholecalciferol or also covering the base with the 7-dehydrocholesterol (7-DHC), and subsequently irradiated with UV light to induce the formation of cholecalciferol. Optionally, the coating of the implant may include an antioxidant such as vitamin E. This implant enhances osseointegration in compromised patients by means of the endogenous synthesis and activity of vitamin D in hard and mineralized tissue regeneration. Furthermore, a method to obtain these implants which comprises coating the surface of the implant directly with cholecalciferol or with a specific concentration of 7-DHC and irradiated with UV light to induce the formation of cholecalciferol.
Owner:NUMAT BIOMEDICAL
Extraction and quantification of vitamins A & D in fluid samples
The invention discloses monoclonal antibodies for vitamins A (retinol palmitate) and D3 (cholecalciferol); a method for using monoclonal antibodies, and the monoclonal antibodies disclosed herein, in particular, to quantitate these vitamins in fluids such as dairy products, and blood, and also raw or processed agri-food and beverage products. The method involves contacting the sample with a mixture of polar and non-polar organic solvents in combination with inorganic salts to remove fat molecules into an organic fraction, and assaying the organic fraction by immunoassay involving the monoclonal antibodies. The presence of a mixture of non-polar and polar organic solvents increases the separation of vitamins from fat molecules and enables the test samples to be quantified by immunoassay without any further treatment.
Owner:SCIMED TECH
Micronutrient formulations for pulmonary and heart health
A heart and pulmonry health micronutrient formulation is provided and the formulation comprises dietary antioxidants and endogenous antioxidants, and the formulation comprising dietary micronutrients and endogenous antioxidants, the dietary antioxidants are selected from a group consisting essentially of Vitamin A (Palmitate), Vitamin E, Vitamin C, Vitamin D3 (Cholecalciferol), B Vitamins, Biotin, Pantothenic Acid (as D-Calcium Pantothenate), Calcium Citrate, Magnesium Citrate, Zinc Glycinate, Selenium (Seleno-L-Methionine), Chromium (as Chromium Picolinate), Mixed Carotenoids and mixtures thereof, and the endogenous antioxidants are selected from a group consisting essentially of N-Acetyl Cysteine (NAC), Coenzyme Q10, R-alpha Lipoic Acid, Omega-3 fatty Acid, and L-Carnitine and mixtures thereof.
Owner:NEW AGE HEALTH SCI INC
Pharmaceutical composition of 12 complex vitamins for injection and preparation method thereof
InactiveCN103006683AHydroxy compound active ingredientsMetabolism disorderThiamine pyrophosphateAlpha-Tocopherol
The invention provides a pharmaceutical composition of 12 complex vitamins for injection and a preparation method thereof. The pharmaceutical composition of 12 complex vitamins for injection provided by the invention comprises the active ingredients of vitamin A palmitate, cholecalciferol, racemic alpha-tocopherol, ascorbic acid, nicotinamide, dexpanthenol, pyridoxine hydrochloride, riboflavin sodium phosphate, tetrahydrate thiamine pyrophosphate, folic acid, D-biotin and cyanocobalamin, and auxiliary materials namely polysorbate 80 and mannitol. The prescription provided by the invention does not contain auxiliary material glycocholic acid, and can be clinically used for people with over-high glycocholic acid.
Owner:SHANXI PUDE PHARMA CO LTD
Method for treating benign prostatic hyperplasia
InactiveUS20050065124A1Effective pharmacologic agentReduce the overall heightBiocideOrganic chemistryProstate hyperplasiaBenign prostatic hyperplasia (BPH)
The use of 1-alpha-fluoro-25-hydroxy-16,23E-diene-26,27-bishomo-20-epi-cholecalciferol, or a pharmaceutically acceptable salt or ester thereof, for the manufacture of a medicament for the prevention and / or treatment of benign prostatic hyperplasia (BPH) and associated symptoms.
Owner:BIOXELL
Methods for treating bladder dysfunction
InactiveUS20070054887A1Preventing and/or treatingEffective amountBiocideOrganic active ingredientsVitamin D+MetabolitesCholecalciferol
There is provided according to the invention the use of Vitamin D compounds such as 1-alpha-fluoro-25-hydroxy-16,23e-diene-26,27-bishomo-20-epi-cholecalciferol in the prevention or treatment of bladder dysfunction.
Owner:BIOXELL
Micronutrient formulations for hearing health
InactiveUS7635469B2Prevent excess productionOrganic active ingredientsSenses disorderPhysiologyPantothenic acid
A hearing health micronutrient formulation is provided and the formulation comprises dietary antioxidants and endogenous antioxidants, and the dietary antioxidants are selected from a group consisting essentially of Vitamin A (Palmitate), Vitamin E, Vitamin C (Calcium Ascorbate), Vitamin D3 (Cholecalciferol), B Vitamins, Biotin, Pantothenic Acid (as D-Calcium Pantothenate), Calcium Citrate, Magnesium Citrate, Zinc Glycinate, Selenium (Seleno-L-Methionine), Chromium (as Chromium Picolinate), Mixed Carotenoids and mixtures thereof, and the endogenous antioxidants are selected from a group consisting essentially of N-Acetyl Cysteine (NAC), Coenzyme Q10, R-alpha Lipoic Acid, L-Carnitine and mixtures thereof.
Owner:NEW AGE HEALTH SCI INC
Health food with efficacy for preventing osteoporosis and preparation method
InactiveCN101091720AGood for healthTwo-way regulation of bone balanceHydroxy compound active ingredientsInorganic phosphorous active ingredientsDecreased estrogenPhosphate
The present invention relates to a health-care food with function capable of preventing osteoporosis and its preparation method. Its composition includes 95-98% of tricalcium phosphate, 1.5-4.5% of astragalus triphenol and 1-5per mill of cholecalciferol. Besides, said invention also provides the concrete steps of its preparation method.
Owner:上海多普富生物技术有限公司
20-cycloalkyl,26,27-alkyl/haloalkyl vitamin D3 compounds and methods of use thereof
InactiveCN101106985AInhibition of basal growthOrganic active ingredientsSenses disorderVitamin d 3Vitamin D+Metabolites
The invention provides vitamin D3 analogs of cholecalciferol, substituted at carbon-20 with cycloalkyl, e.g., cyclopropyl, wherein carbon-16 is a double bond, and carbon-23 is a single, double, or triple bond. Various alkyl or haloalkyl substitutions are incorporated at carbon-25. The invention provides pharmaceutically acceptable esters, salts, and prodrugs thereof. Methods for using the compounds to treat vitamin D3 associated states, and pharmaceutical compositions containing the compounds are also disclosed.
Owner:BIOXELL
Anti-stress compound premix feed for laying fowls as well as preparation method and application of anti-stress compound premix feed
InactiveCN112568326AInhibition formationRepair and remove damageFood processingAnimal feeding stuffBiotechnologyPhytase
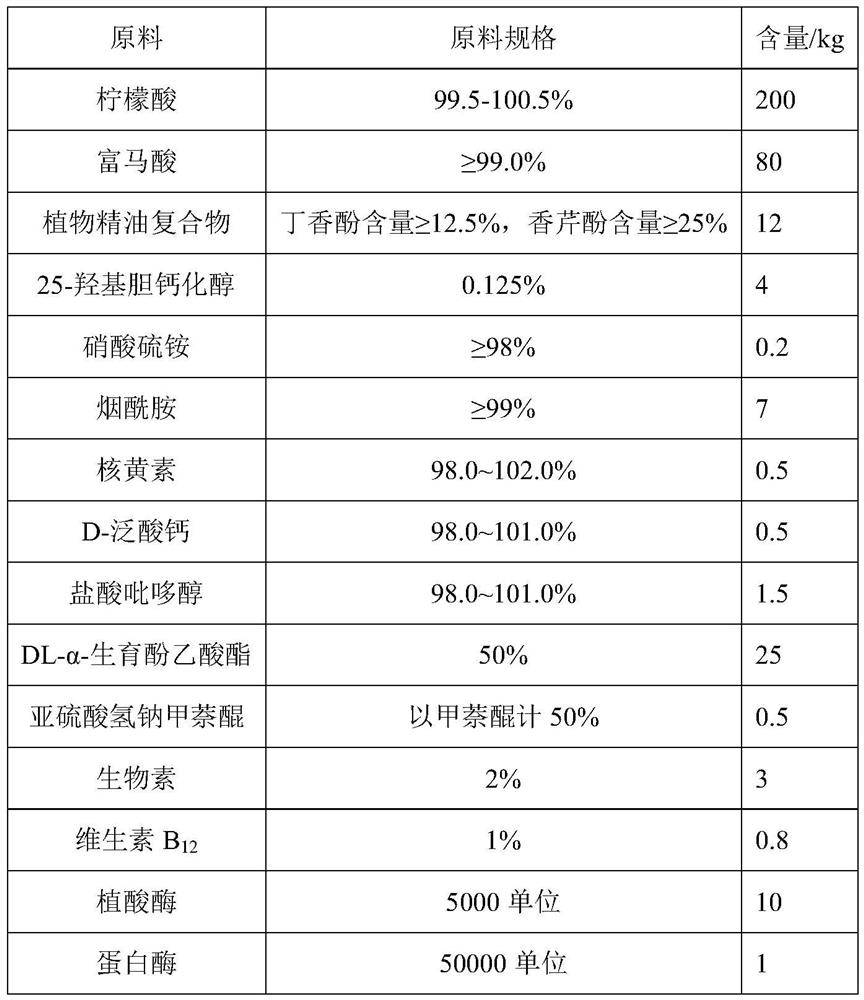
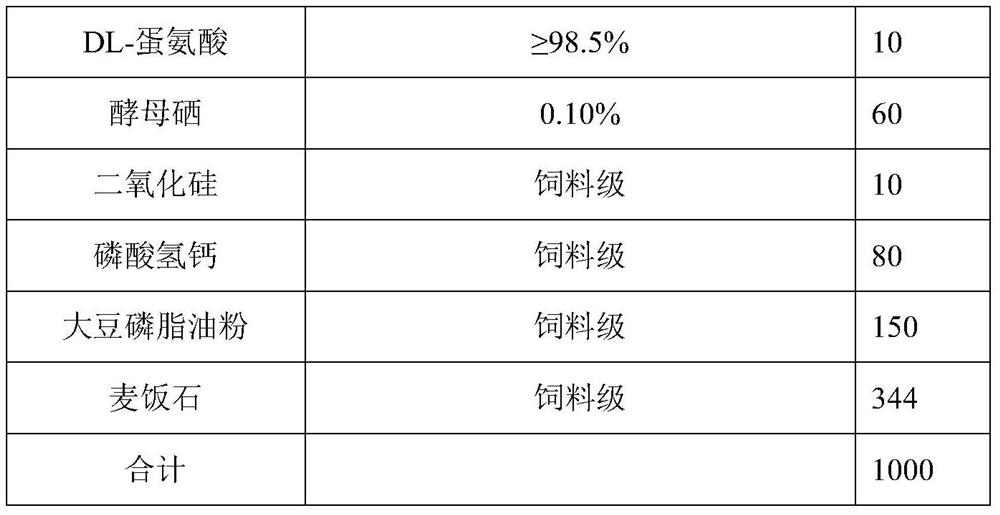
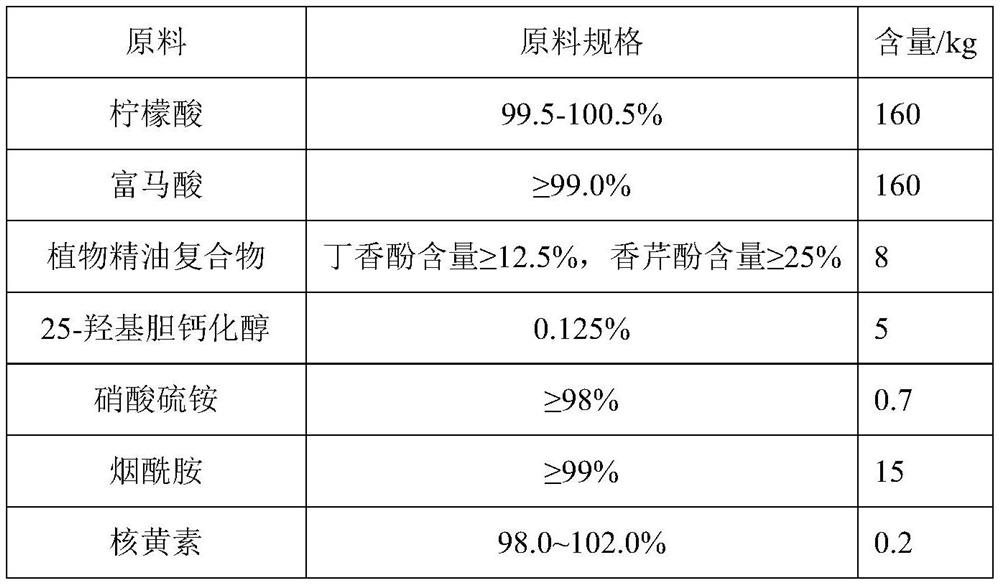
The invention relates to an anti-stress compound premix feed for laying fowls as well as a preparation method and application of the anti-stress compound premix feed. The premix feed is composed of the following raw materials in parts by weight: 160-240 parts of citric acid, 80-160 parts of fumaric acid, 8-15 parts of a plant essential oil compound, 2-5 parts of 25-hydroxy cholecalciferol, 0.15-0.75 part of ammonium sulfate nitrate, 6-15 parts of nicotinamide, 0.2-0.5 part of riboflavin, 0.2-0.5 part of D-calcium pantothenate, 1-2 parts of pyridoxine hydrochloride, 20-36 parts of DL-alpha-tocopheryl acetate, 0.4-0.7 part of menadione sodium bisulfite, 1-4 parts of biotin, 0.3-0.9 part of vitamin B12, 10-30 parts of phytase, 1-5 parts of protease, 2-10 parts of DL-methionine, 20-60 parts ofselenium yeast, 50-150 parts of calcium hydrophosphate and 200-600 parts of a carrier. The premix feed provided by the invention can significantly improve the stress intestinal injury of the laying fowls and improve the digestive absorption capacity, and finally achieves the effects of relieving the stress of the laying fowls, reducing the dirty egg rate, improving the egg laying rate in a stressstate, improving the egg quality and the breeding performance of breeding fowls, reducing the dirty egg rate, improving the fertilization rate and the hatching rate and increasing the economic benefits of farmers.
Owner:QILU ANIMAL HEALTH PROD +1
Micronutrient formulations for pulmonary and heart health
Owner:NEW AGE HEALTH SCI INC
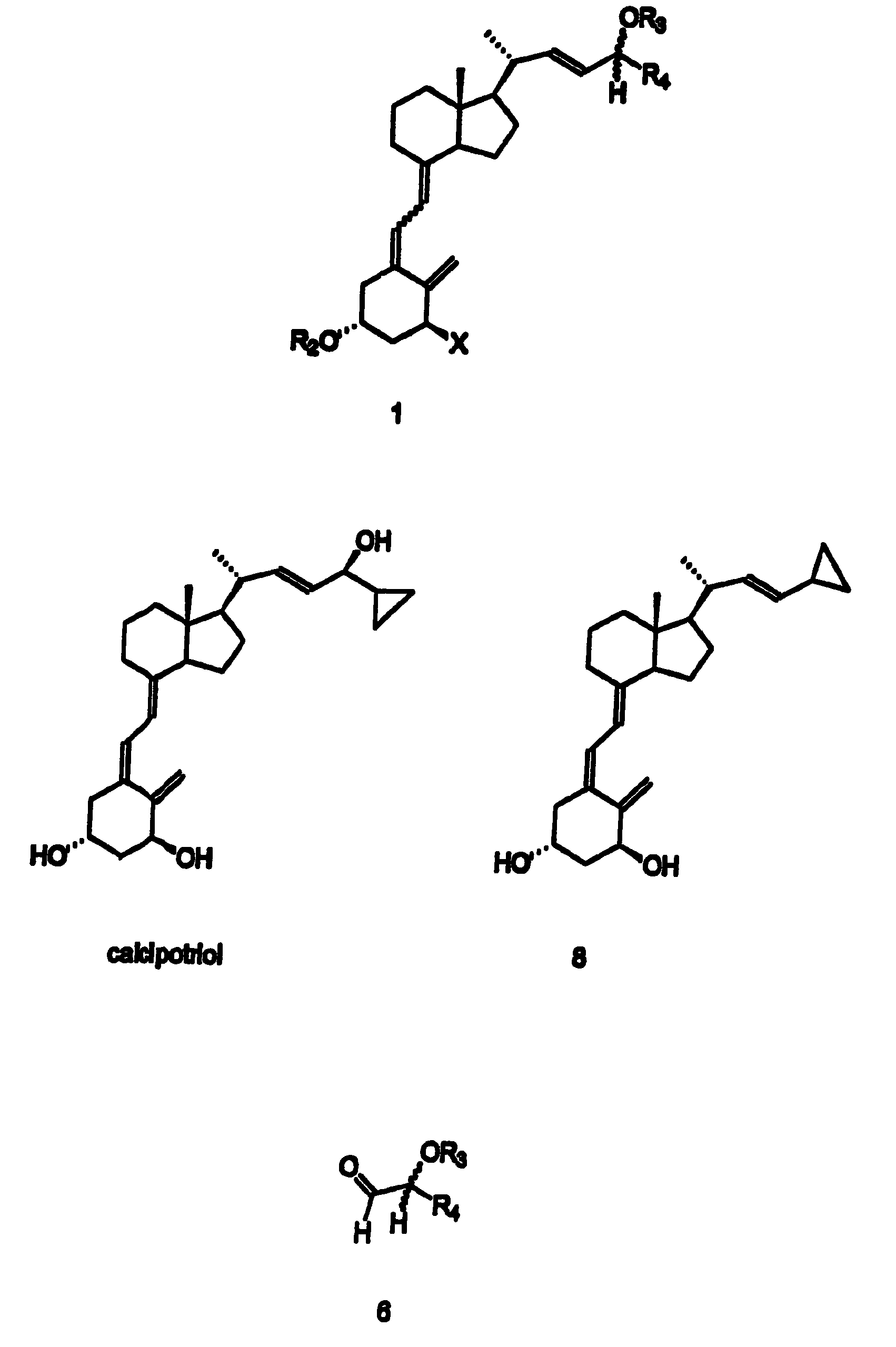
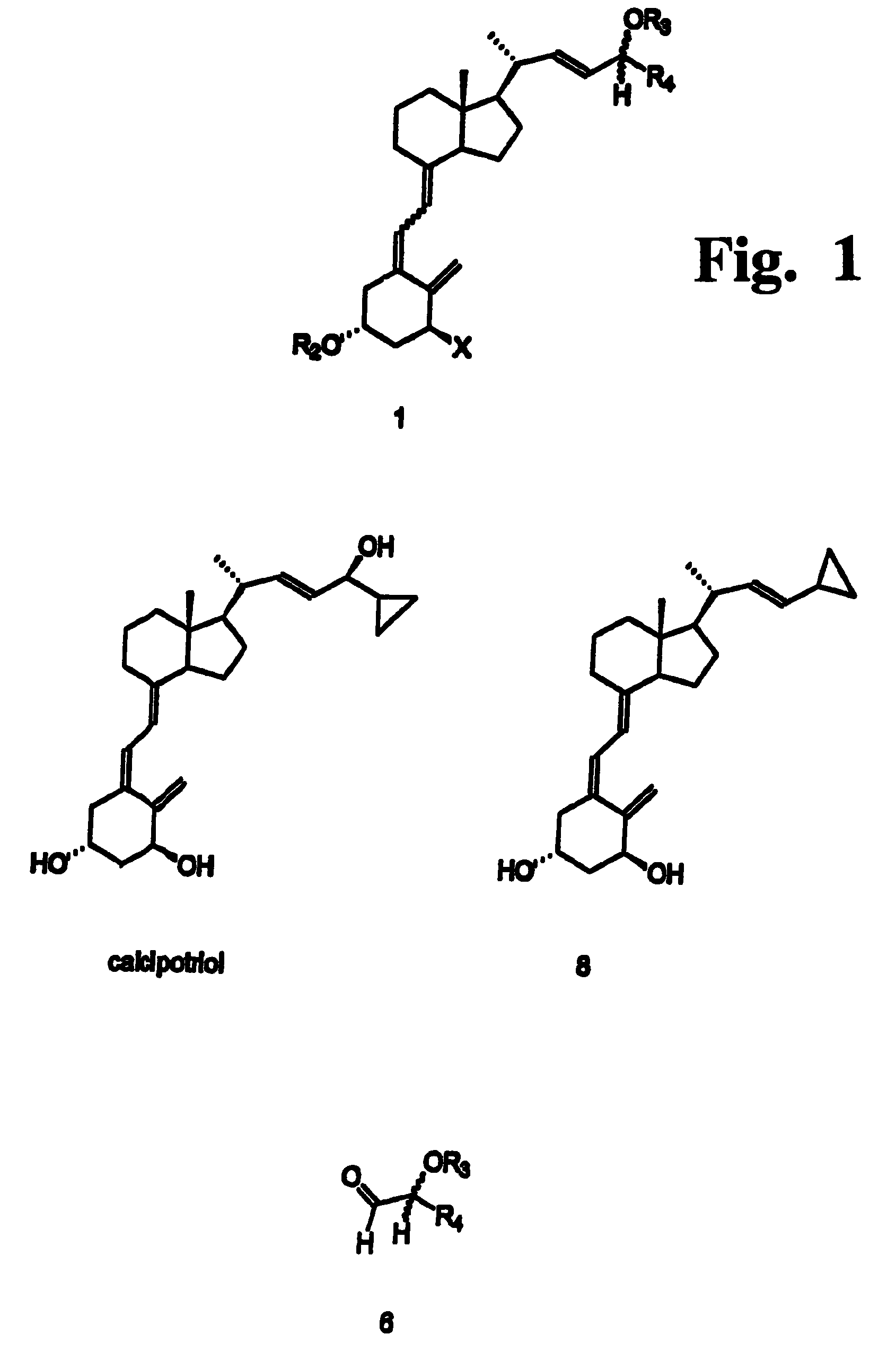
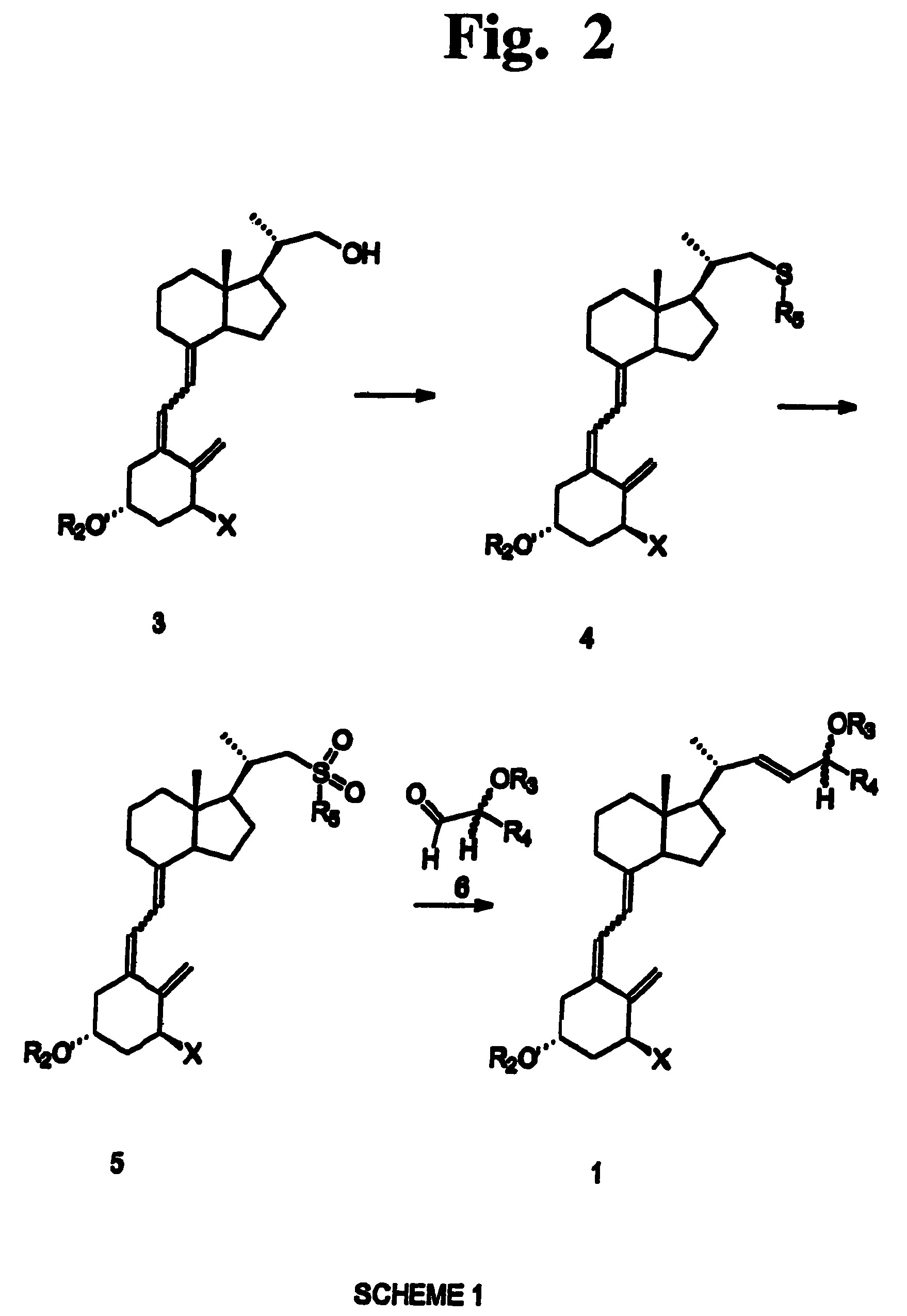
Preparation of 24 alkyl analogs of cholecalciferol and non-racemic compounds
Disclosed is a process for the preparation of 24-alkyl analogs of cholecalcyferol of Formula 1 having a (5E) or (5Z) configuration, wherein X represents a hydrogen atom, a hydroxy group or an OR1 group, where R1, R2 and R3 may be the same or different and represent groups suitable for hydroxyl protection, and R4 is a C1-6 alkyl chain or a C1-6 cykloalkyl group, optionally substituted with C1-3 alkyl groups, especially for calcipotriol.The invention also provides new intermediates and non-racemic compounds being valuable synthones for the synthesis of pharmacologically active substances.
Owner:INSTITUT FARMACEUTYCZNY
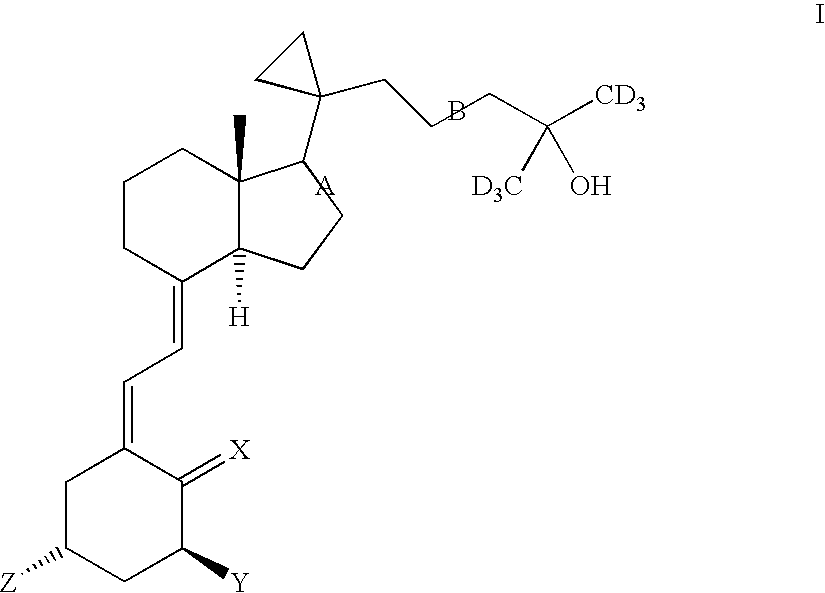
1,25-dihydroxy, 20-cyclopropyl,26-27-deuteroalkyl vitamin d3 compounds and methods of use thereof
The invention provides vitamin D3 analogs of cholecalciferol, substituted at carbons-26 and 27 with deuterated alkyl groups, e.g., trideuteromethyl, wherein carbon-16 is a single or double bond, and carbon-23 is a single, double, or triple bond. The invention provides pharmaceutically acceptable esters, salts, and prodrugs thereof. Methods for using the compounds to treat vitamin D3 associated states, and pharmaceutical compositions containing the compounds are also disclosed.
Owner:BIOXELL
Micronutrient formulations for radiation applications
A radioactive protection micronutrient formulation system is provided and the system comprises: a formulation consisting essentially of antioxidants, the antioxidants are selected from the group consisting essentially of vitamin C, vitamin E, N-acetyl cysteine, natural mixed carotenoids, and alpha-lipoic acid, vitamin A (palmitate), vitamin D-3 (cholecalciferol), thiamine mononitrate, riboflavin, niacinamide ascorbate, d-calcium pantothenate, pyridoxine hydrochloride, cyanocobalamin, folic acid, D-Biotin, selenium (1-seleno-methionine), chromium picolinate, zinc glycinate, calcium citrate and magnesium citrate and mixtures thereof; and plus a booster formulation selected from a group consisting essentially of vitamin C, d-alpha tocopheryl acid succinate, alpha tocopherol, N-acetyl cysteine, natural mixed carotenoids and alpha lipoic acid, the formulation is designed to reduce the risk in humans exposed to doses of ionizing radiation of becoming subjected to at least one condition selected from the group consisting essentially of radiation-induced acute leukemia, breast cancer, thyroid cancer and other somatic and heritable mutations.
Owner:NEW AGE HEALTH SCI INC
Replacement-gilt feed additive and application thereof
InactiveCN104366148ACracked hoof reliefPromote absorption and metabolismAnimal feeding stuffCholecalciferolChemistry
The invention relates to a replacement-gilt feed additive and application thereof. The feed additive is prepared from components including, by weight, 0.3-0.5 part of 1, 25-dihydroxy cholecalciferol, 45-60 parts of calcium lactate, 2.5-5.0 parts of glycine manganese, 5.0-7.5 parts of glycine zinc, 1.0-3.0 parts of methionine copper, 20-25 parts of zeolite powder and 10-25 parts of corncobs. The application of the replacement-gilt feed additive includes adding the feed additive into basic ration during cultivation of replacement gilts, and feeding the replacement gilts with the feed additive and the basic ration, wherein the feed additive accounts for, by weight, 1% of the basic ration. Compared with the prior art, the feed additive has the advantage that various organic microelements and the 1, 25-dihydroxy cholecalciferol serve as main materials. When the feed additive is added into conventional feed, the replacement gilts can deposit more micro mineral elements, so that hoof growth is promoted, bone strength and density are enhanced and hoof cracking of the gilts is relieved.
Owner:FUJIAN AONONG BIOLOGICAL TECH GRP CO LTD
Age-related complementary food nutrition bag rich in hypoallergenic heterologous immunocompetence peptides and preparation method thereof
PendingCN112868794AImprove immune activityImprove stabilityProtein composition from fishMilk preparationBiotechnologyHeterologous
The invention relates to an age-related complementary food nutrition bag rich in hypoallergenic heterologous immunocompetence peptides and a preparation method thereof, and belongs to the technical field of age-related nutrition supplement food. Twelve nutrient supplement factors are formed on three low-sensitization protein-based carriers, namely sea cucumber protein peptide powder, terrestrial plant source-soybean protein peptide powder and terrestrial animal source high-quality protein-skimmed milk powder for the first time; I-level steady-state protection of nutrient supplementation factors, II-level steady-state protection formed on a low-sensitization protein-based carrier and a III-level steady-state protection technology of double quantitative precise filling are designed, and the senile complementary food nutrition bag rich in the low-sensitization heterologous immunocompetence peptide is created; and the symbolic characteristics of low sensitization, high immunocompetence and high stability of the compound are confirmed through inspection of three indexes including a BALB / C mouse sensitization evaluation model, a mouse macrophage RAW264.7 cell model and the decay rate of vitamin A acetate, cholecalciferol and cyanocobalamin, and technical support is provided for development of series products.
Owner:GANZHOU QUANBIAO BIOTECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com