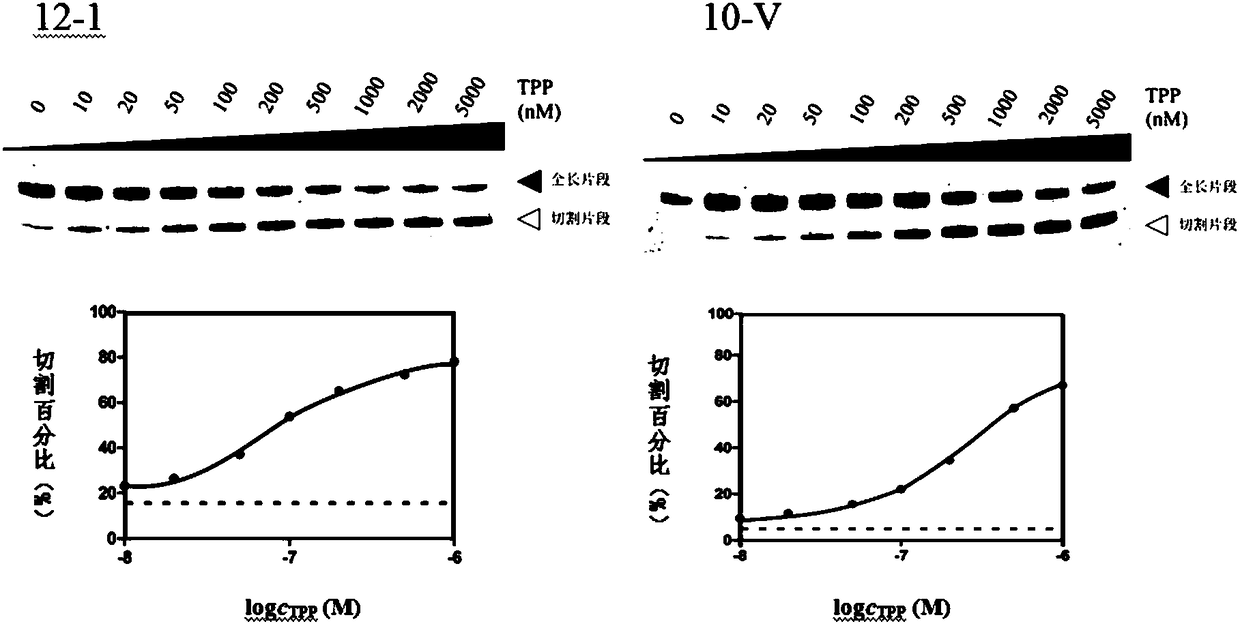
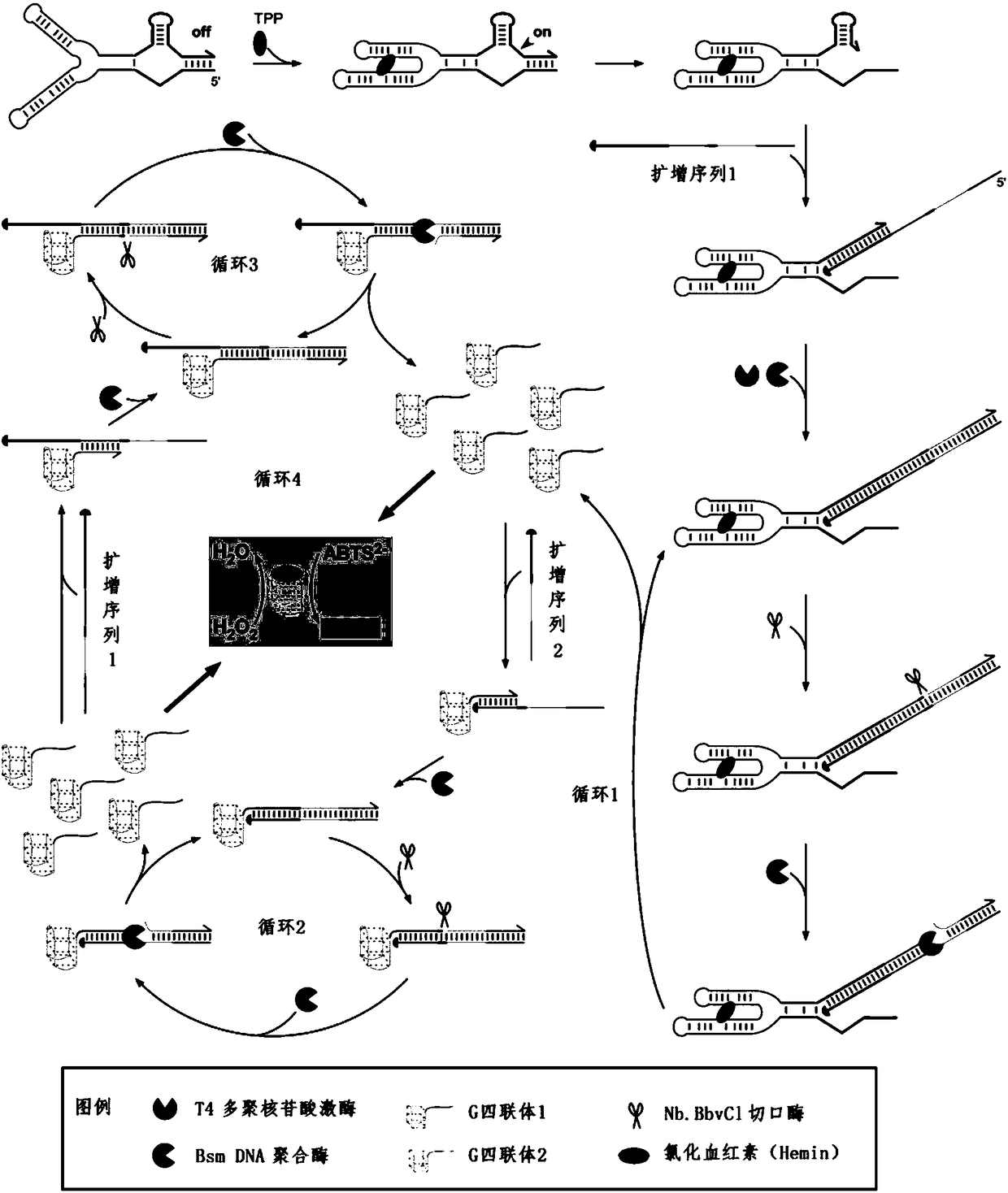
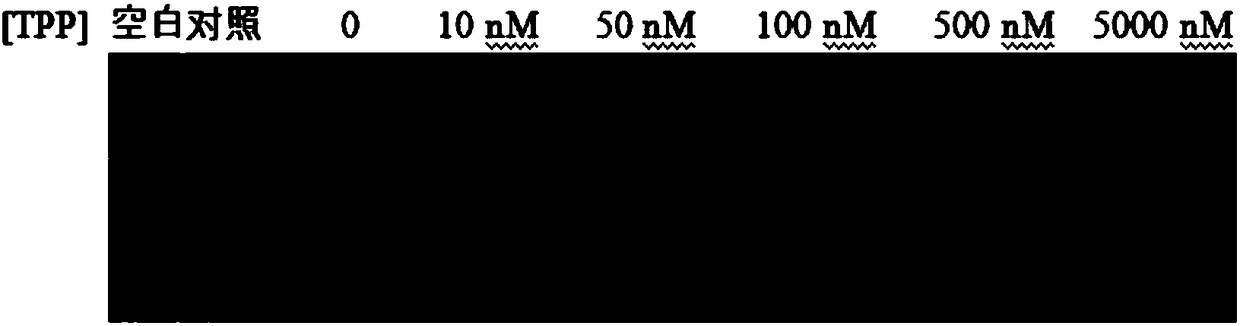
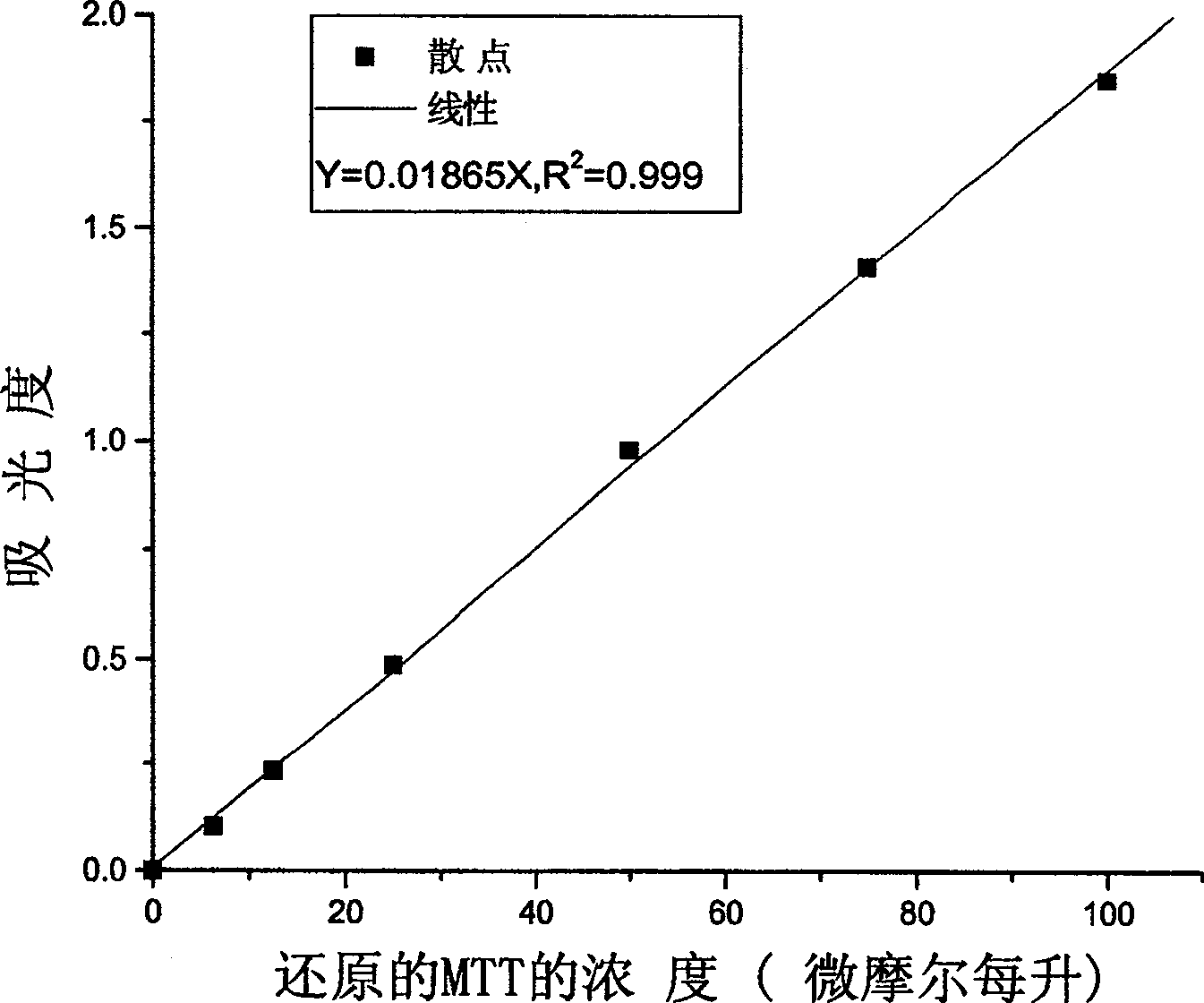
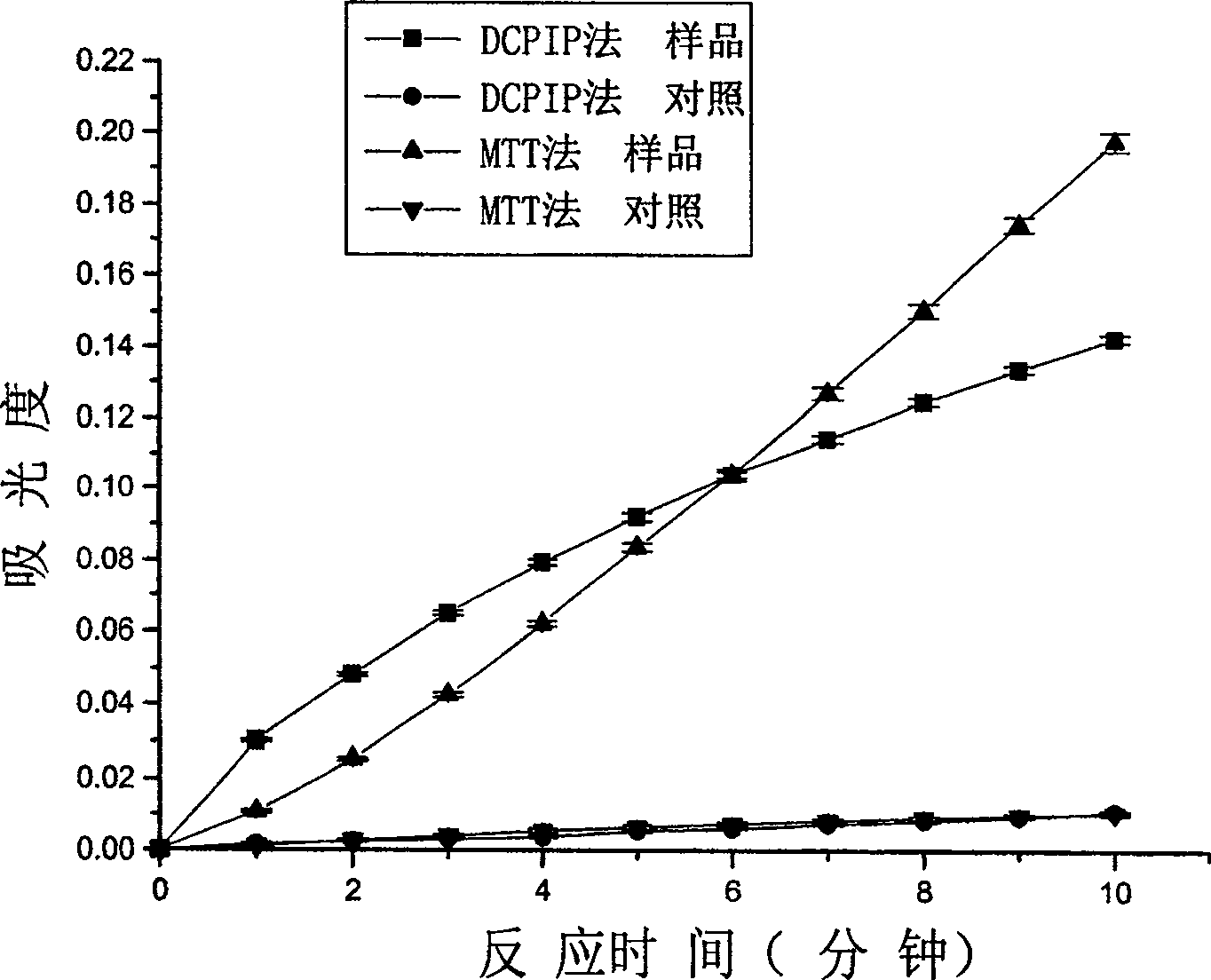
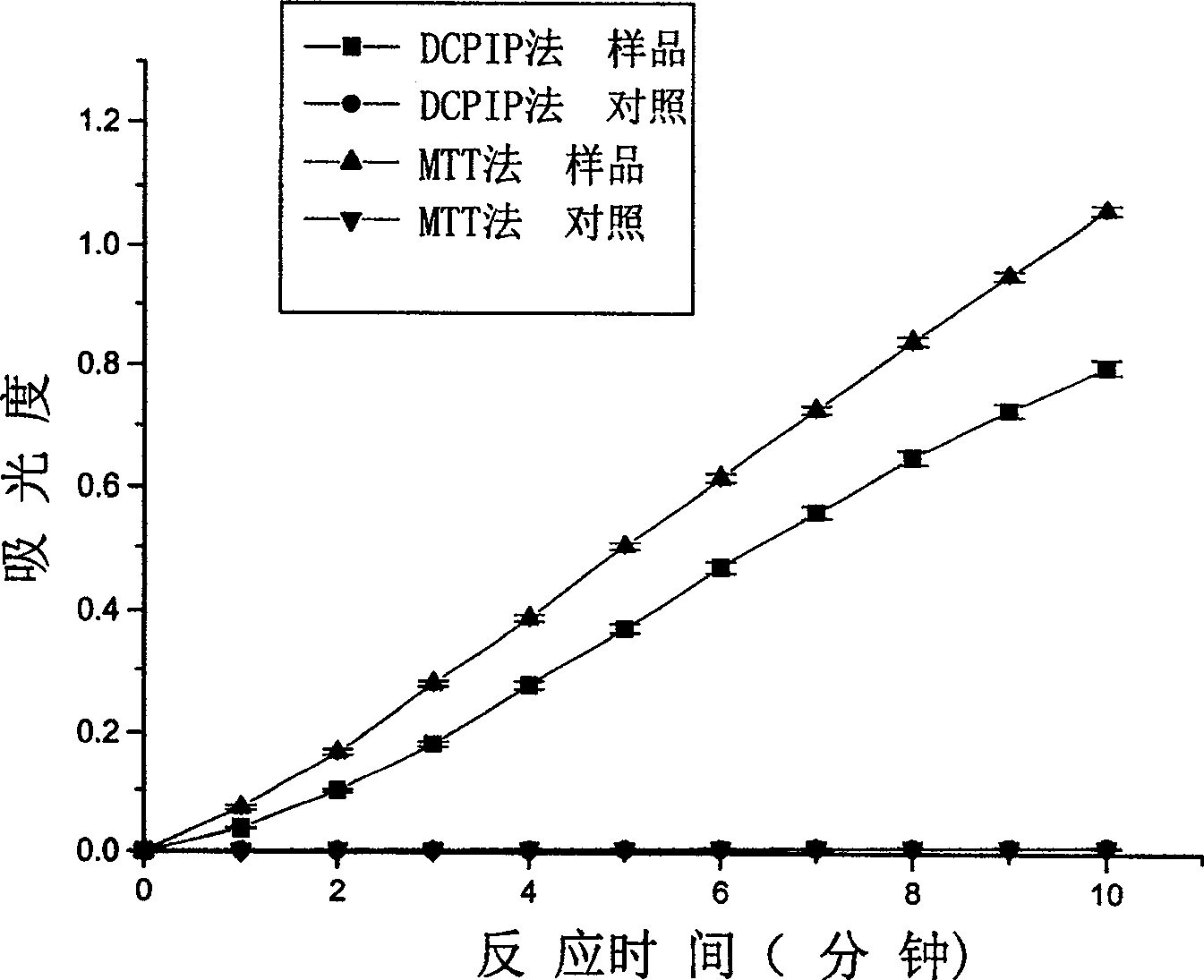
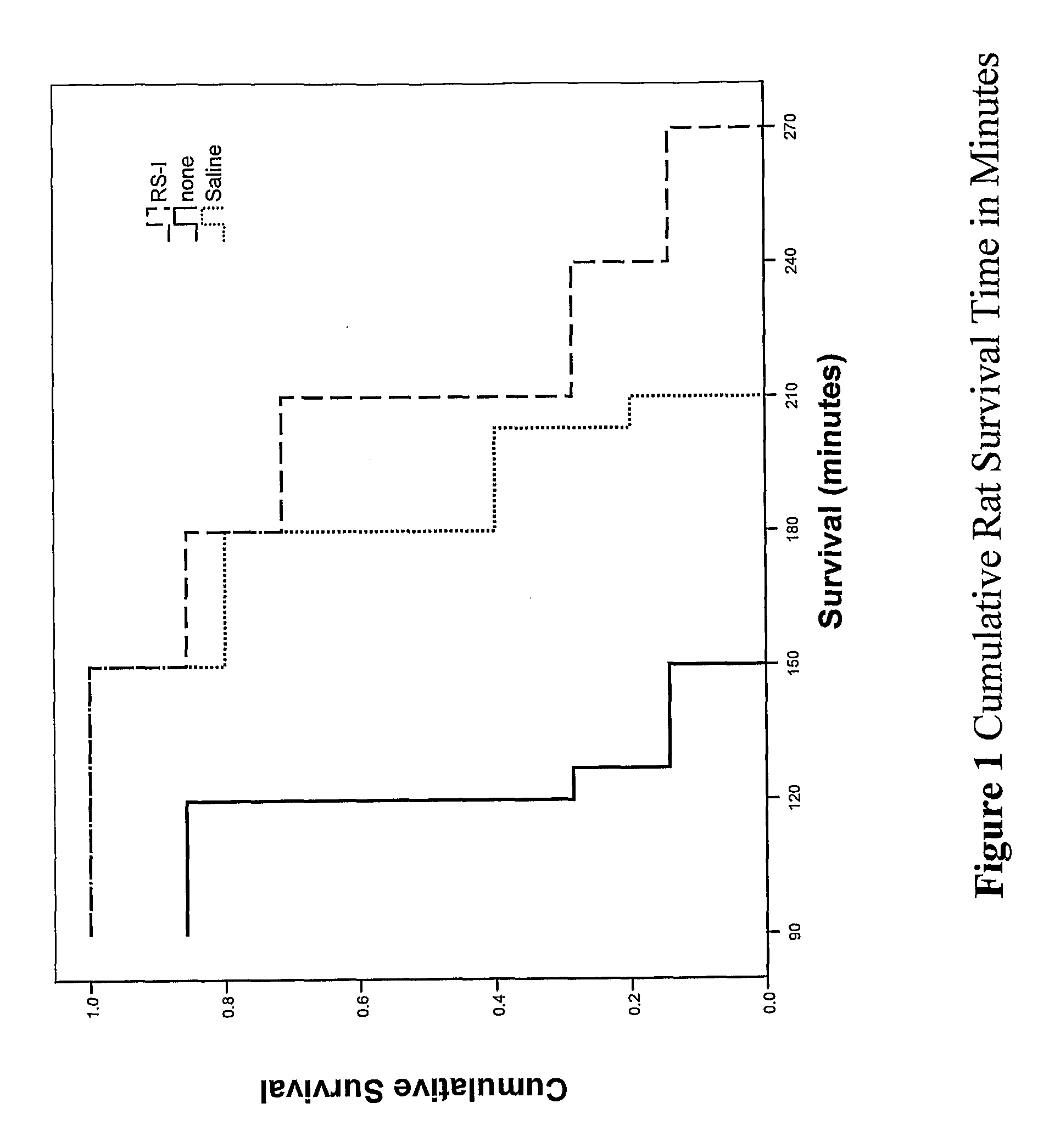
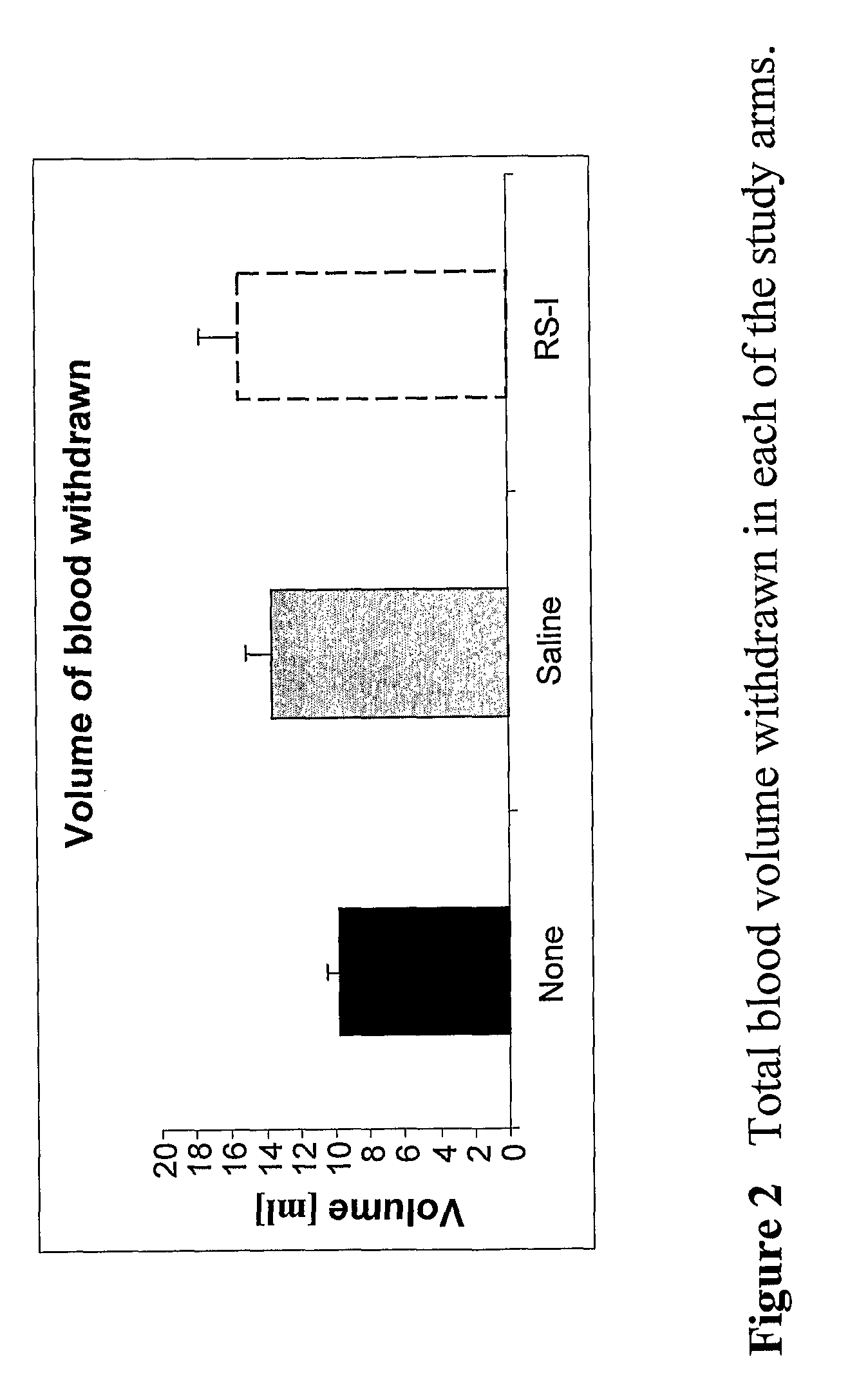
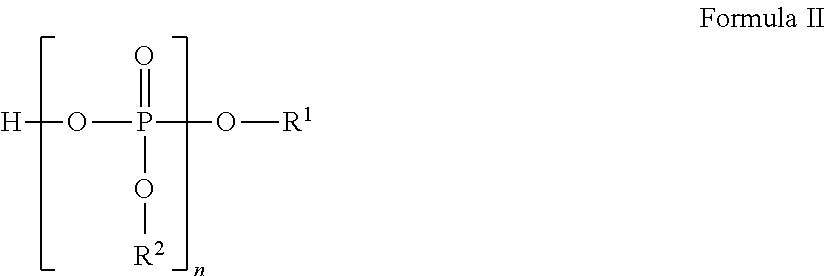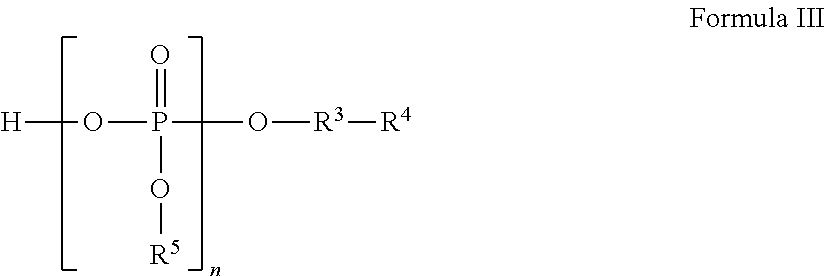Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Thiamine pyrophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thiamine pyrophosphate (TPP or ThPP), or thiamine diphosphate (ThDP), or cocarboxylase is a thiamine (vitamin B₁) derivative which is produced by the enzyme thiamine diphosphokinase. Thiamine pyrophosphate is a cofactor that is present in all living systems, in which it catalyzes several biochemical reactions.
Composition for gene delivery
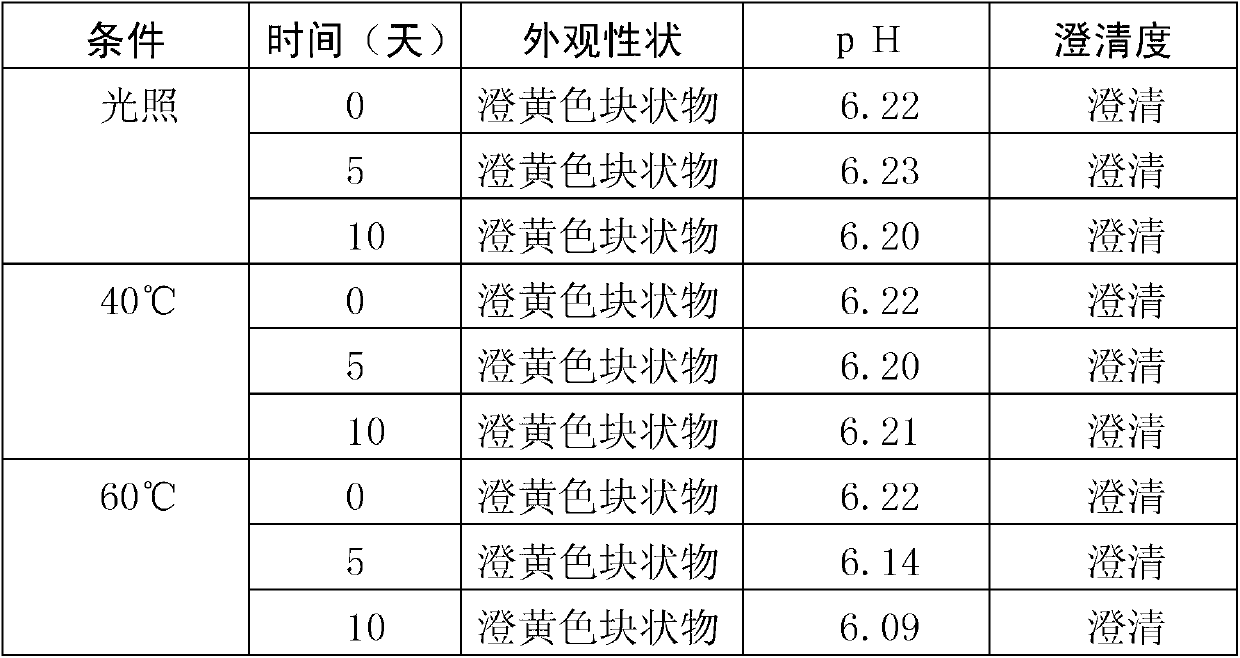
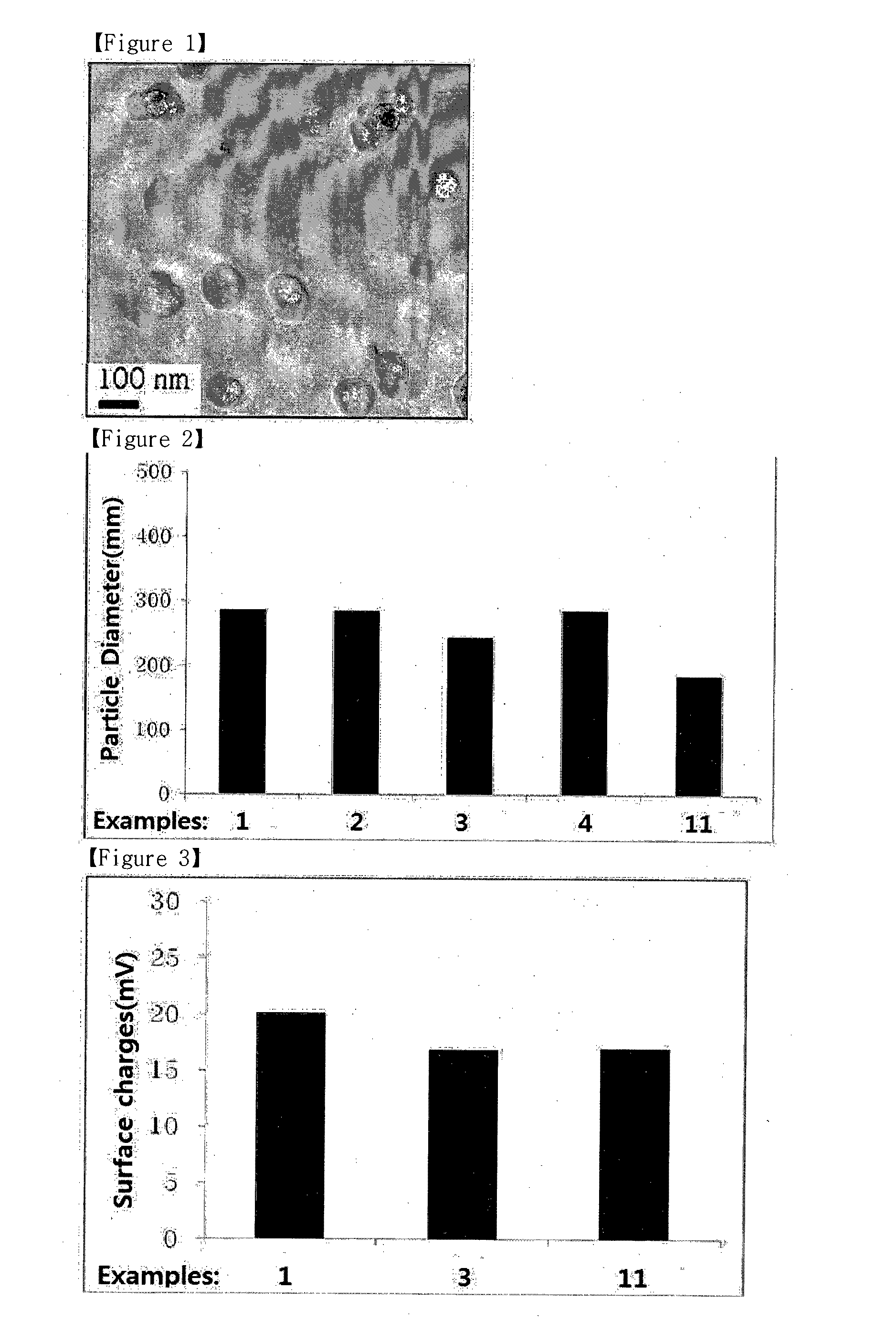
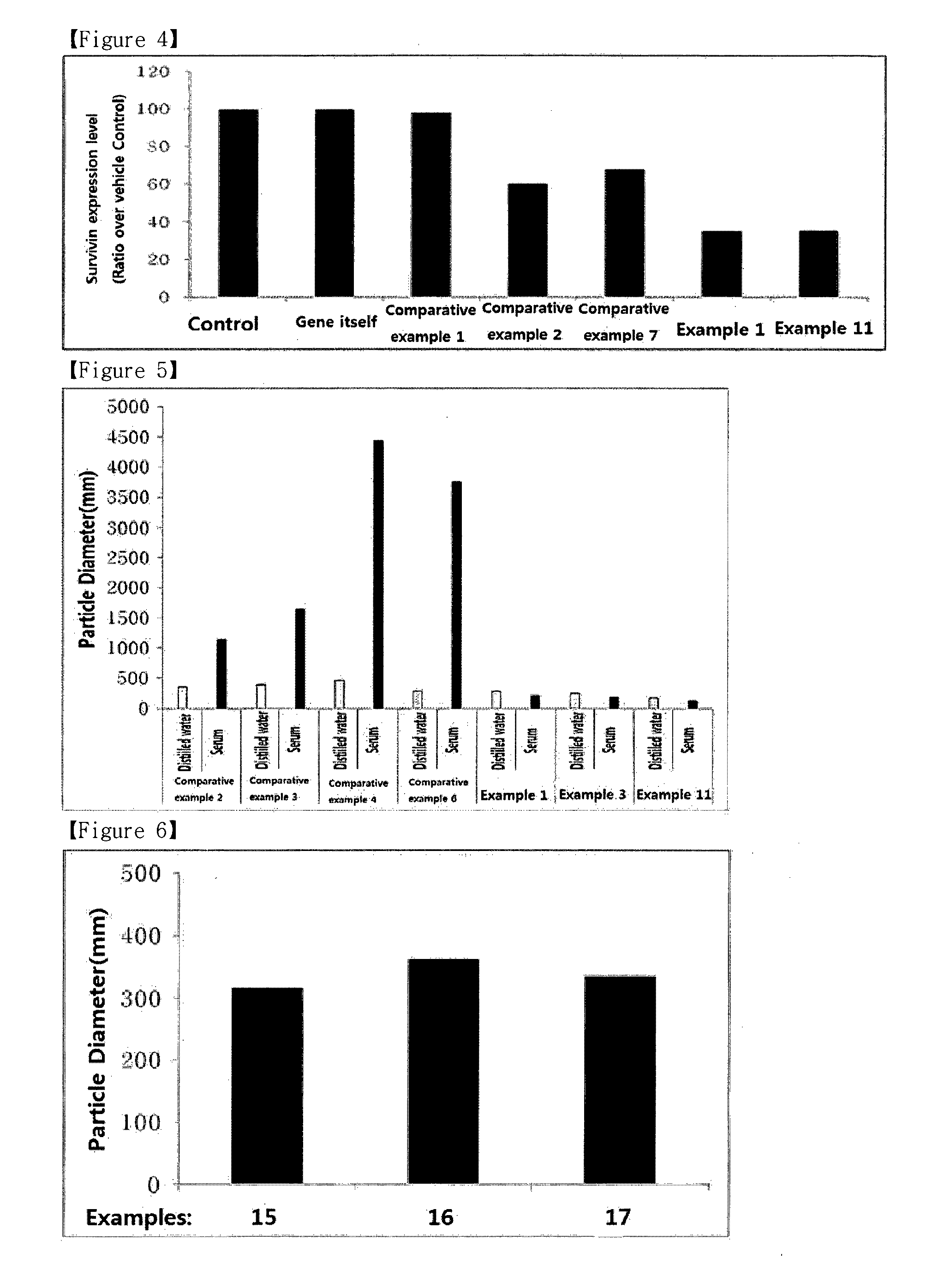
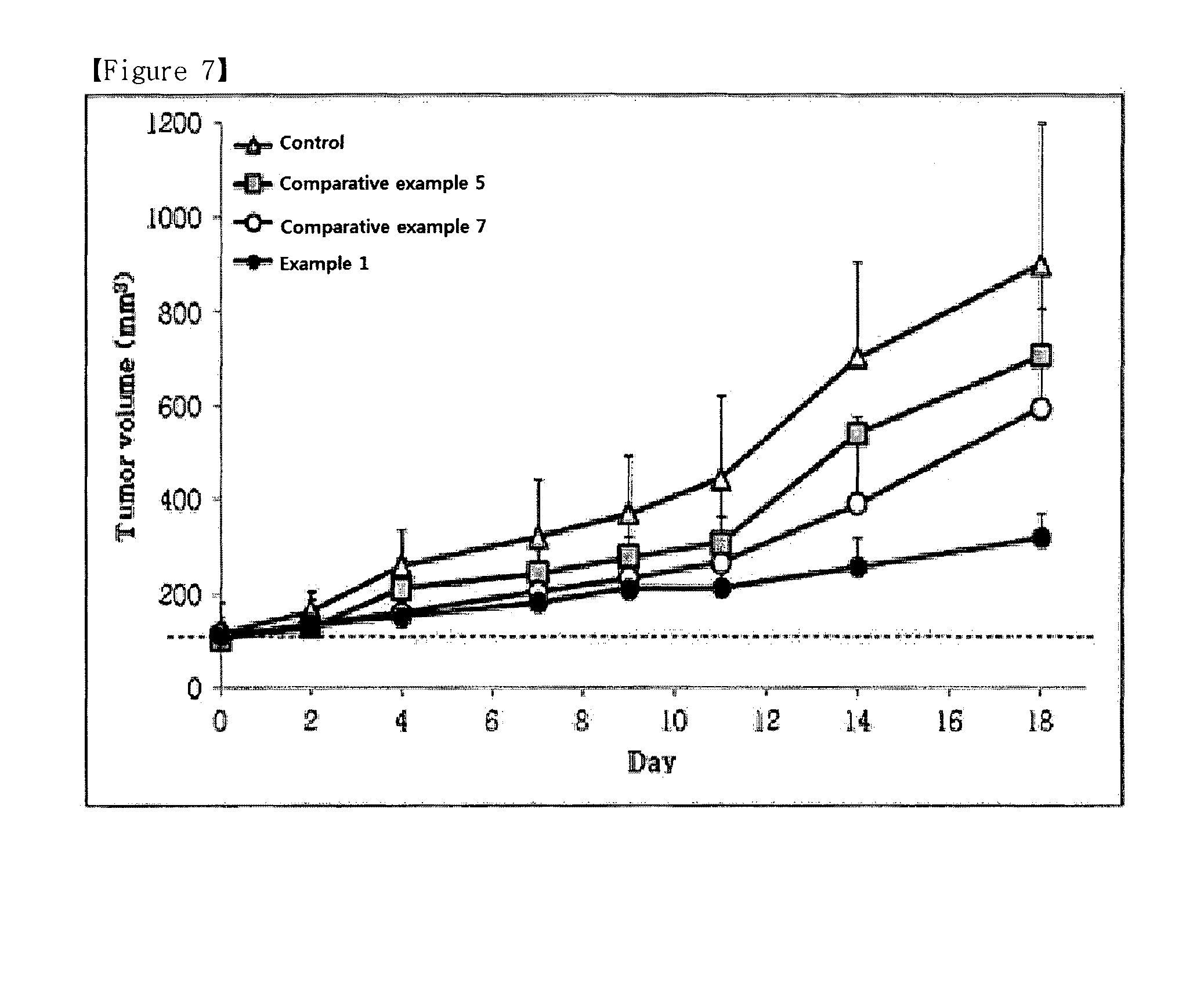
ActiveUS9173853B2Advantageously commercializedImprove efficiencyOrganic active ingredientsPowder deliveryGene deliveryThiamine pyrophosphate
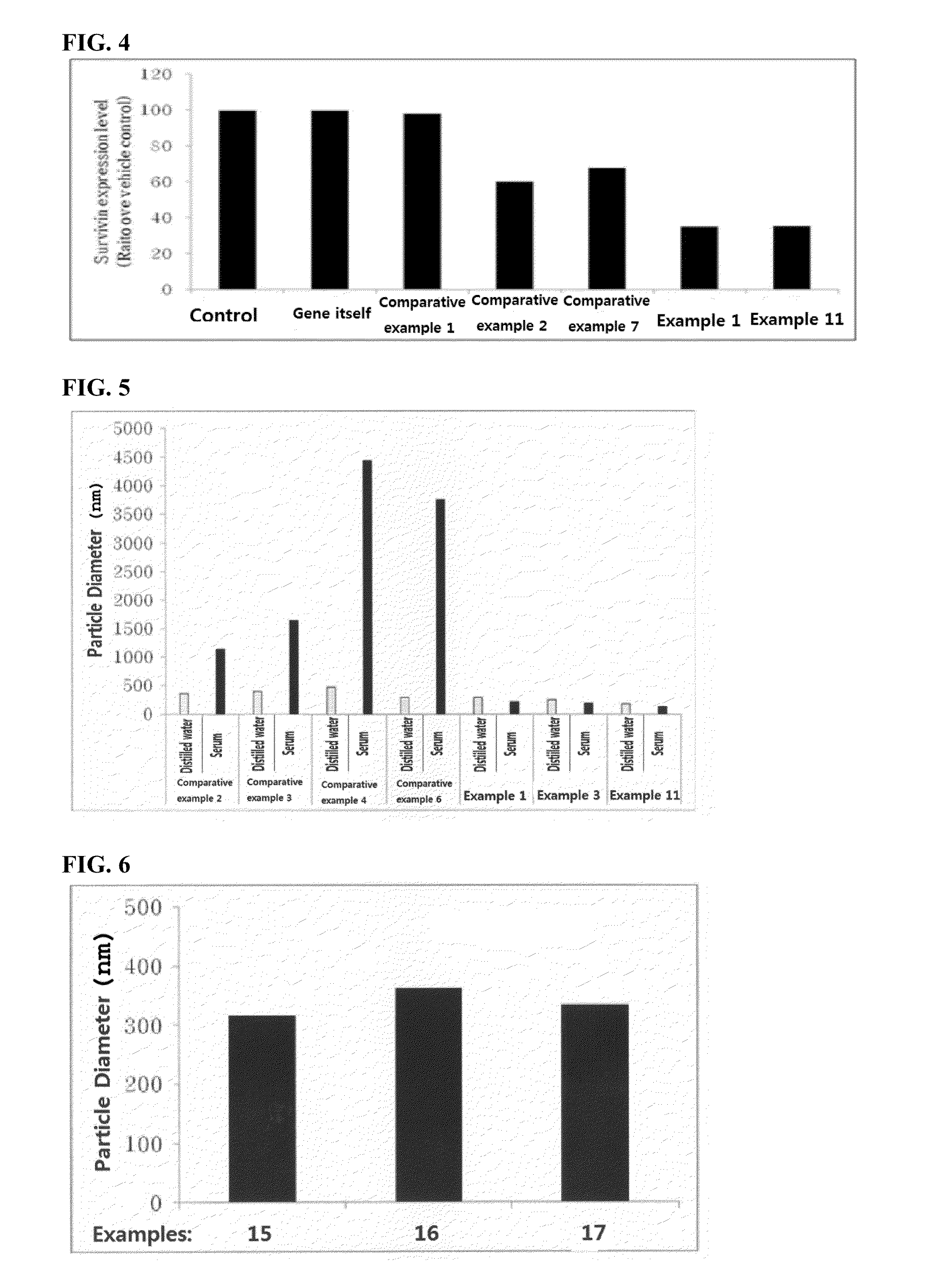
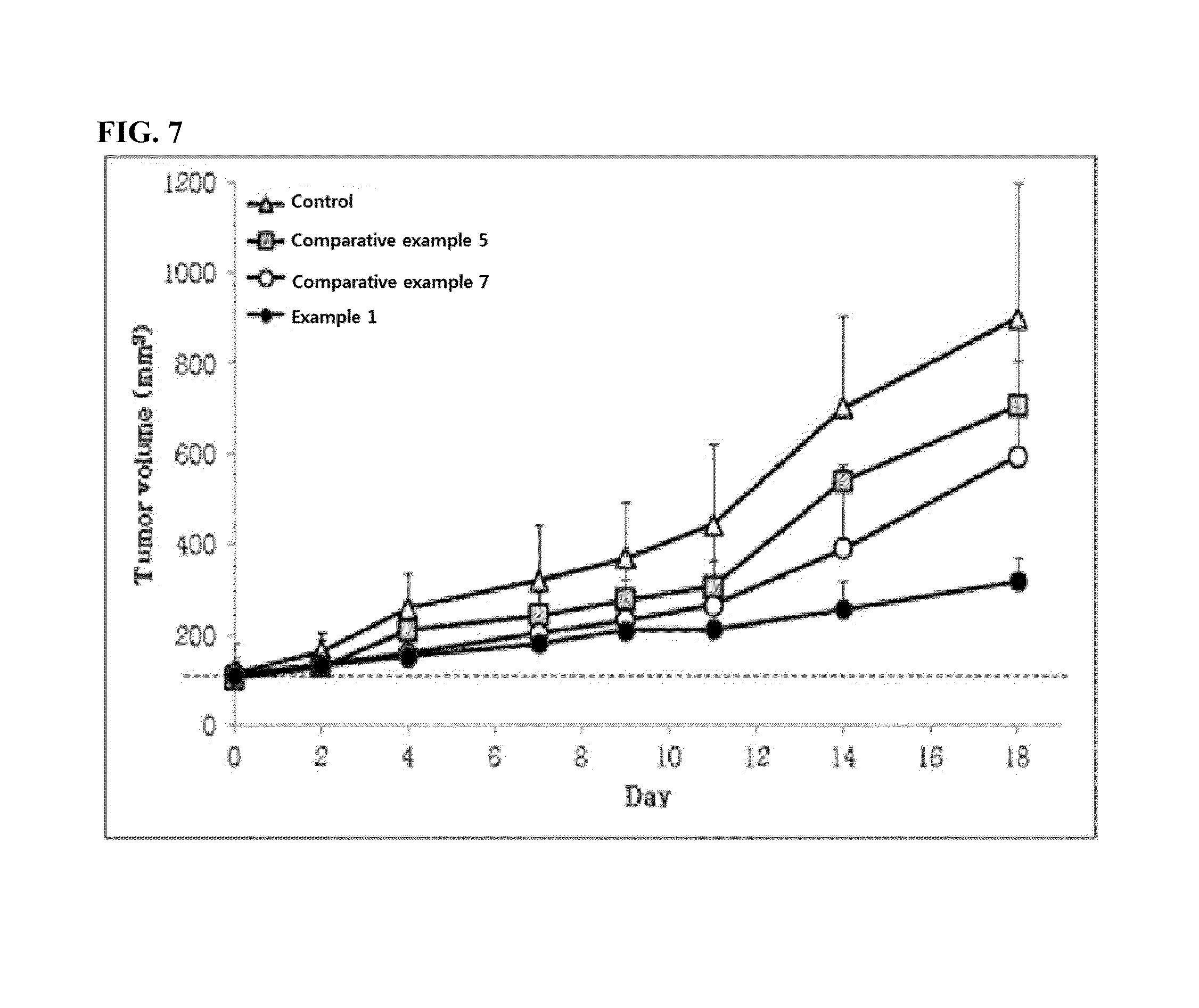
Disclosed is a pharmaceutical composition for gene delivery, comprising: (i) a gene; (ii) a water-soluble chitosan; (iii) a thiamine pyrophosphate or a pharmaceutically acceptable salt thereof; (iv) a protamine or a pharmaceutically acceptable salt thereof; and (v) a neutral or anionic phospholipid. The composition can introduce a gene into cells safely and effectively. Composed of non-toxic and injectable components, the composition is safe for the body and can be advantageously commercialized. Notably, it can deliver a gene at high efficiency in vivo as well as in vitro, and is stable in the blood.
Owner:CHONG KUN DANG PHARMA CORP
Inflaming retarding water-tolerant polyurethane tackiness agent and preparation method thereof
InactiveCN103059796AImprove adhesionImprove water resistanceNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesPrepolymerThiamine pyrophosphate
The invention discloses inflaming retarding water-tolerant polyurethane tackiness agent which is characterized by comprising following components by weight ratio: 88%-92% hydroxyl polyurethane resin, 5.5%-10% isocyanate group (NCO) enclosed type diisocyanate-trimethoprim (TMP), 1.5%-2.5% sodium dodecyl sulfate and 0.5%-1.5% phosphonolipid fire retardant thiamine pyrophosphate (TPP). The invention further discloses a preparation method of the inflaming retarding water-tolerant polyurethane tackiness agent. NCO enclosed type diisocyanate-TMP addition product aqueous dispersion is adopted, reaction of self de-enclosing NCO perssad with hydroxyl-terminated prepolymer is utilized to achieve solidification, and a strong chemical bond is formed to enable the tackiness agent to have the advantages of being strong in binding power, good in water tolerance, washable, free-falling, sun-proof, good in thermal endurance, endurable in chemical products and the like. Grade of products is greatly improved, simultaneously, the inflaming retarding water-tolerant polyurethane tackiness agent is simple in use, convenient to operate, environment-friendly, non-toxic, and environment-pollution-free, has inflaming retarding function and no volatile organic compounds (VOC) discharging, and is beneficial to protecting environment and health of using and production persons.
Owner:DONGGUAN SHENGBAO NEW MATERIAL TECH
Detection reagent for alanine aminotransferase
ActiveCN103320497AHigh sensitivityImprove accuracyMicrobiological testing/measurementThiamine pyrophosphatePhosphoric acid
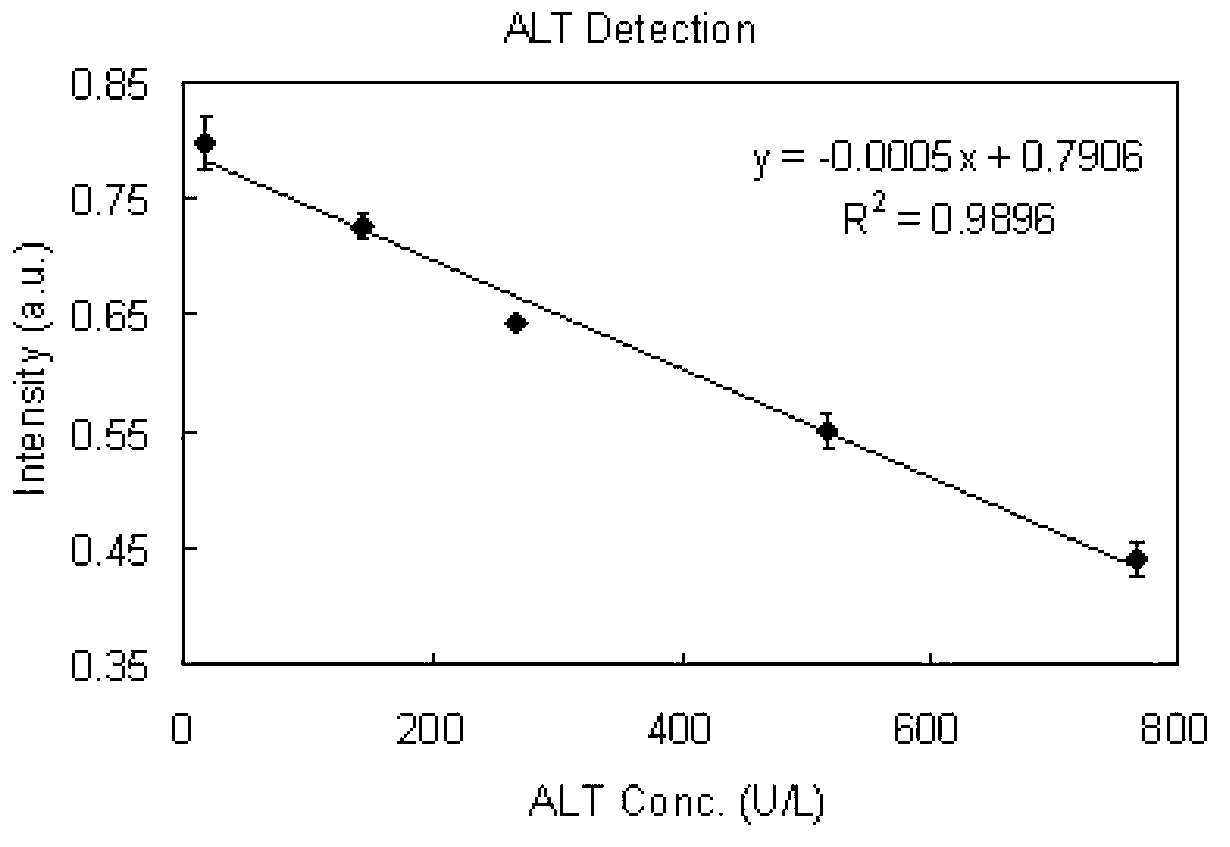
The invention discloses a detection reagent for alanine aminotransferase. The detection reagent comprises a diluent and a reaction reagent. The diluent comprises a buffer, a surfactant, an antiseptic, a bilirubin-interference removing reagent, and an ascorbic oxidase. The reaction reagent comprises a buffer, L-alanine, alpha-ketoglutaric acid, phosphoric acid, pyruvate oxidase, thiamine pyrophosphate, magnesium chloride, peroxidase, 4-aminoantipyrene, a chromogen, an antiseptic, and a freeze-drying protecting agent. The detection reagent has advantages of good sensitivity, accuracy, precision and linearity, and can completely meet the requirements of clinical examination.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Fermentation technology of 3Ec nano enzyme
Owner:EIN INTL
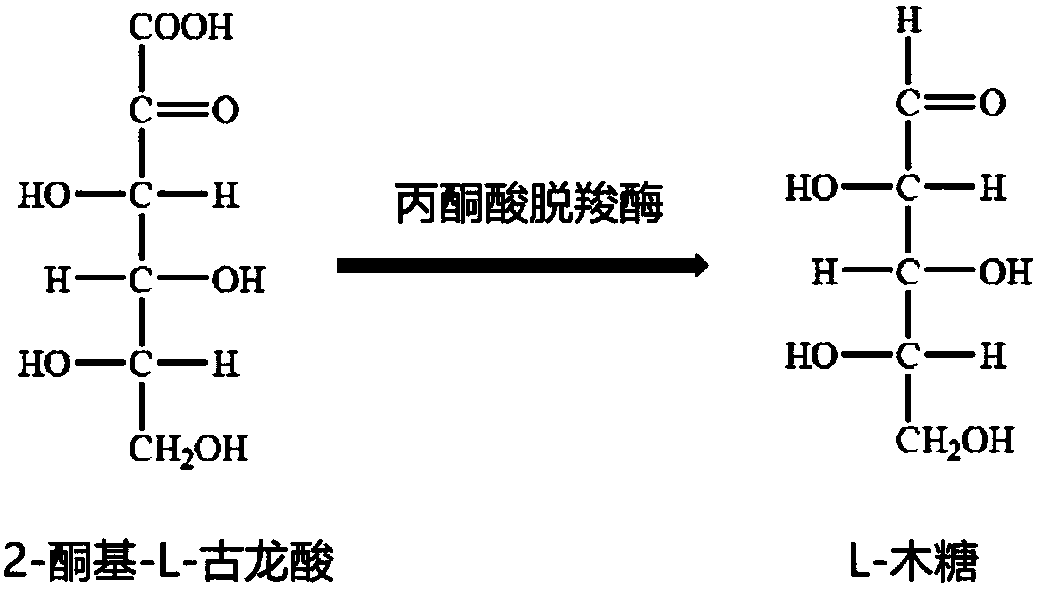
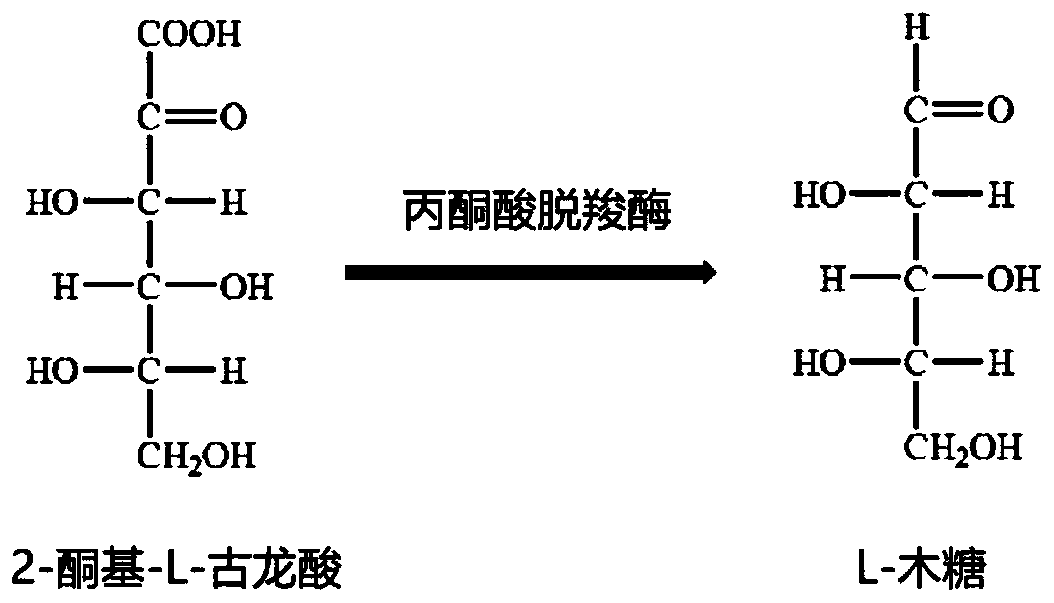
Enzymatic method for preparing L-xylose
The invention discloses an enzymatic method for preparing L-xylose and belongs to the field of bioengineering. In the method, 2-keto-L-gulonic acid is dissolved in a buffer containing magnesium ions and thiamine pyrophosphate, and pyruvate decarboxylase is added to the buffer for an enzyme-catalyzed reaction at a suitable temperature. After the end of the reaction, the production of the L-xylose is proved by a high performance liquid chromatograph. A new method for producing the L-xylose is found. The method is low in raw material cost, has no pollution to the environment, and has important economic and social benefits.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Pharmaceutical composition of 12 complex vitamins for injection and preparation method thereof
InactiveCN103006683AHydroxy compound active ingredientsMetabolism disorderThiamine pyrophosphateAlpha-Tocopherol
The invention provides a pharmaceutical composition of 12 complex vitamins for injection and a preparation method thereof. The pharmaceutical composition of 12 complex vitamins for injection provided by the invention comprises the active ingredients of vitamin A palmitate, cholecalciferol, racemic alpha-tocopherol, ascorbic acid, nicotinamide, dexpanthenol, pyridoxine hydrochloride, riboflavin sodium phosphate, tetrahydrate thiamine pyrophosphate, folic acid, D-biotin and cyanocobalamin, and auxiliary materials namely polysorbate 80 and mannitol. The prescription provided by the invention does not contain auxiliary material glycocholic acid, and can be clinically used for people with over-high glycocholic acid.
Owner:SHANXI PUDE PHARMA CO LTD
Enzyme composition and use therefore
InactiveUS20100151554A1Improve combustion efficiencyTransferasesOxidoreductasesFlavin adenine dinucleotideThiamine pyrophosphate
The present invention provides an enzyme composition and a method for treating coal using the enzyme composition. The enzyme composition includes at least one enzyme, coenzyme and ammonium acetate, wherein the enzyme can be laccase-isozyme, pyruvate dehydrogenase, dihydrolipoyl transacetylase, and dihydrolipoyl dehydrogenase, and the coenzyme can be CoA, CoA-SH, thiamine pyrophosphate, lipoic acid, flavin adenine dinucleotide, and nicotinamide adenine dinucleotide. In addition, the method of the present invention includes treating the coal with the enzyme composition for more than 72 hours.
Owner:EIN INTL
High water resistance type water-soluble polyester material and preparation method thereof
InactiveCN107629201AImprove environmental adaptabilityLong effective timeThiamine pyrophosphateDimethyl terephthalate
The invention provides a high water resistance type water-soluble polyester material. The material is prepared from 55%-82% of terephthalic acid, 24%-41% of ethylene glycol, 2.1%-9% of isophthalic acid-5-sodium sulfonate, 5%-12% of polyether, 0.3%-1.5% of an alcohol-based hydrolysis agent, 0.02% of a catalyst and 0.3%-0.8% of a compound stabilizer, wherein terephthalic acid can be replaced with dimethyl terephthalate, the catalyst is antimonous oxide CPC, and the compound stabilizer comprises TPP (thiamine pyrophosphate) and an antioxidant 1010 mixed in the mass ratio being 1:2. A polyester main chain comprises the easy-to-hydrolyze functional group alcohol-based hydrolysis agent, the alcohol-based hydrolysis agent is hydrolyzed after being dissolved in water, a hydrophobic group is obtained, so that polyester has water resistance again and is prevented from being dissolved again, and the use performance of the polyester material in water, outdoors and humid environments is improved.
Owner:宜昌中盈科技发展有限公司
Porphyra zebra tps gene and its application in improving rice salt tolerance
InactiveCN102286494AImprove securityMorphologically normalMicrobiological testing/measurementFermentationBiotechnologyGenetically modified rice
The invention discloses a PyTPS (porphyra yezoensis ueda trehalose-6-phosphate synthase) gene which is cloned to construct a plant expression vector of the gene, and the plant expression vector is transferred into the agrobactrium tumefaciens strain, so that an engineering strain is obtained. A rice variety is transformed by utilizing agrobacterium-mediated transformation, so that genetically modified rice is obtained; after growing and acclimatization, a T0 individual plant is obtained, and a T1 seed is collected; a seedling germinated from the T1-generation genetically modified seed receives PCR (polymerase chain reaction) detection and a salt tolerance assay, so that a T1-generation genetically modified salt-resistant strain is obtained, a T2 generation receives a kanamycin resistance assay, PCR detection and Southern hybridization, and results indicate that the PyTPS gene is already integrated into the genome of the genetically modified strain. The PyTPS gene in the invention has a TPS domain as well as a TPP (thiamine pyrophosphate) domain; the PyTPS gene source is safe; the development of the plant morphology of the PyTPS genetically modified rice is normal; the PyTPS gene can be stably inherited in genetically modified plants; and moreover, the transformation system is highly effective.
Owner:QINGDAO AGRI UNIV
Novel composition for gene delivery
ActiveUS20150110876A1Advantageously commercializedImprove efficiencyPowder deliveryOrganic active ingredientsThiamine pyrophosphateGene delivery
Disclosed is a pharmaceutical composition for gene delivery, comprising: (i) a gene; (ii) a water-soluble chitosan; (iii) a thiamine pyrophosphate or a pharmaceutically acceptable salt thereof; (iv) a protamine or a pharmaceutically acceptable salt thereof; and (v) a neutral or anionic phospholipid. The composition can introduce a gene into cells safely and effectively. Composed of non-toxic and injectable components, the composition is safe for the body and can be advantageously commercialized. Notably, it can deliver a gene at high efficiency in vivo as well as in vitro, and is stable in the blood.
Owner:CHONG KUN DANG PHARMA CORP
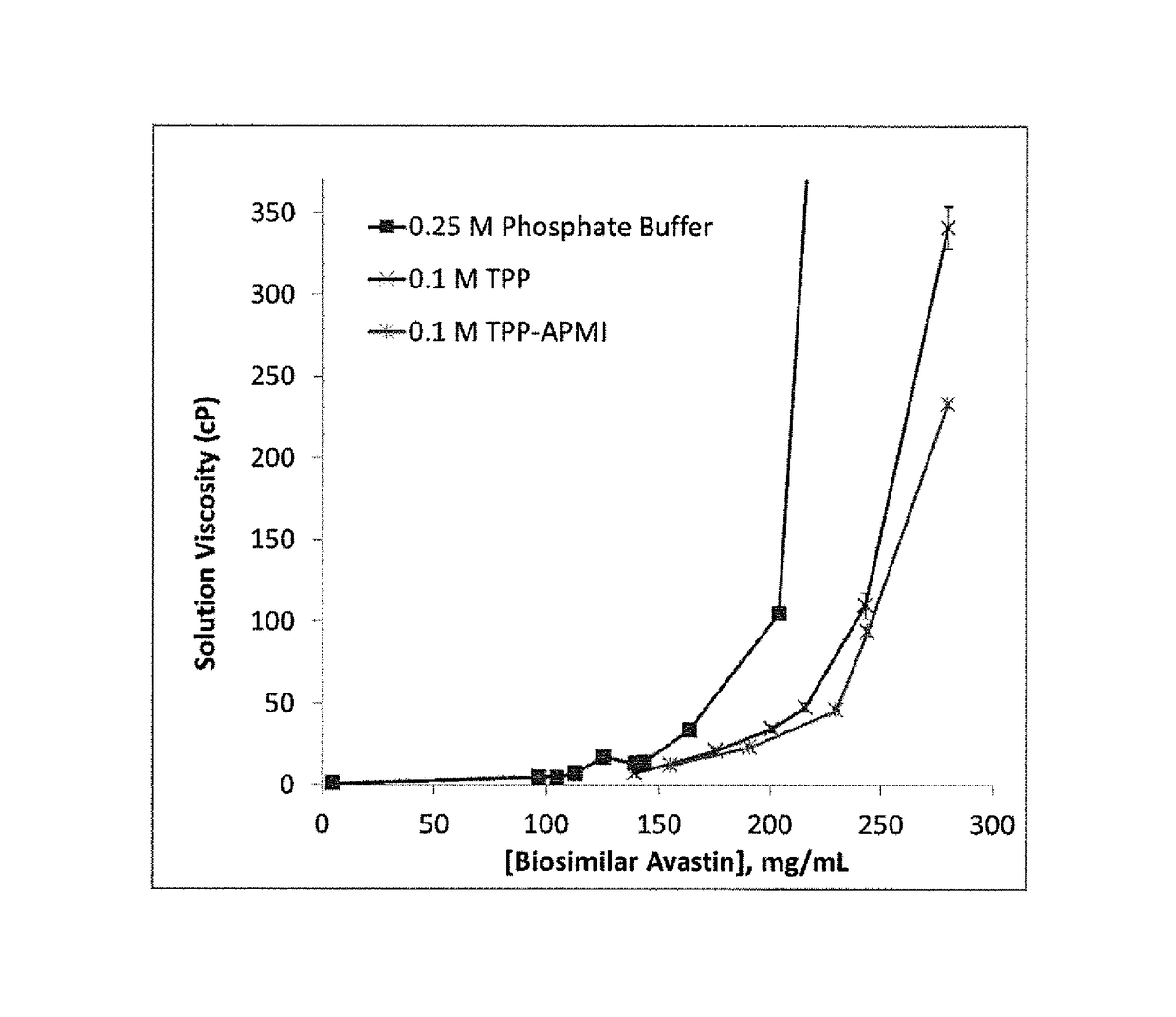
Liquid pharmaceutical formulations for injection comprising thiamine pyrophosphate 1-(3-aminopropyl)-2-methyl-1H-imidazole and uses thereof
ActiveUS9913905B2Rapidly and conveniently administeredFacilitates and accelerates reconstitutionPowder deliveryNervous disorderThiamine pyrophosphateHigh molecular mass
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous (SC) or intramuscular (IM) injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and organophosphates. The viscosity of the formulation is significantly reduced by the addition of one or more organophosphates.
Owner:EAGLE BIOLOGICS INC
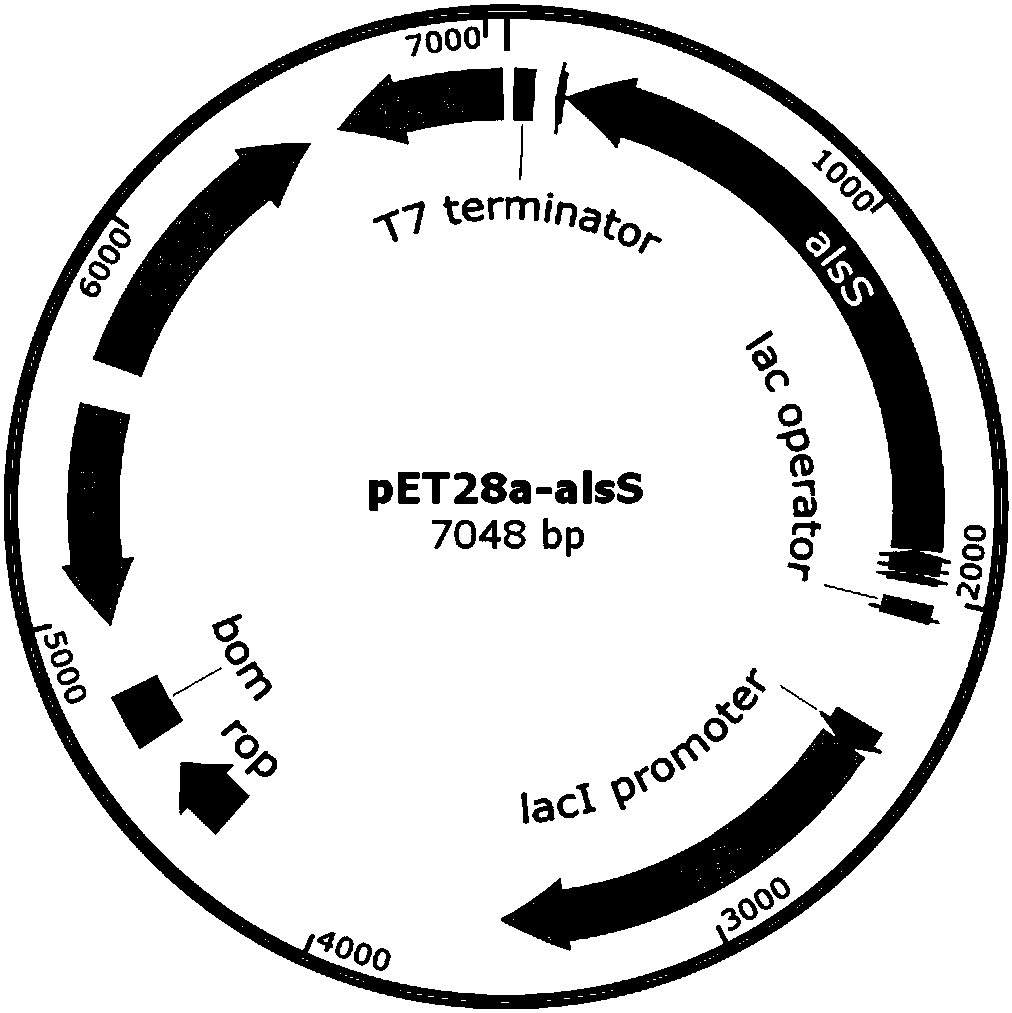
Method of producing D-(-)-acetoin by in-vitro enzyme reaction
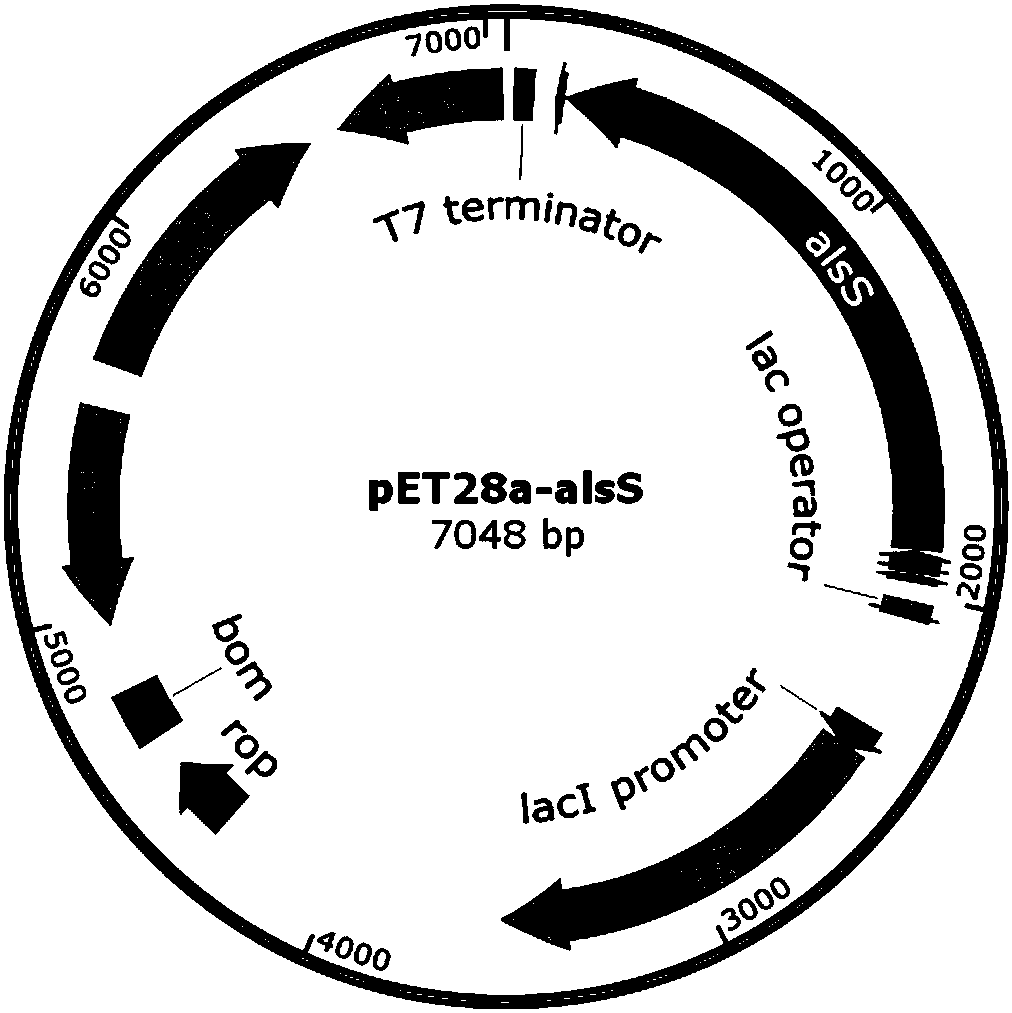
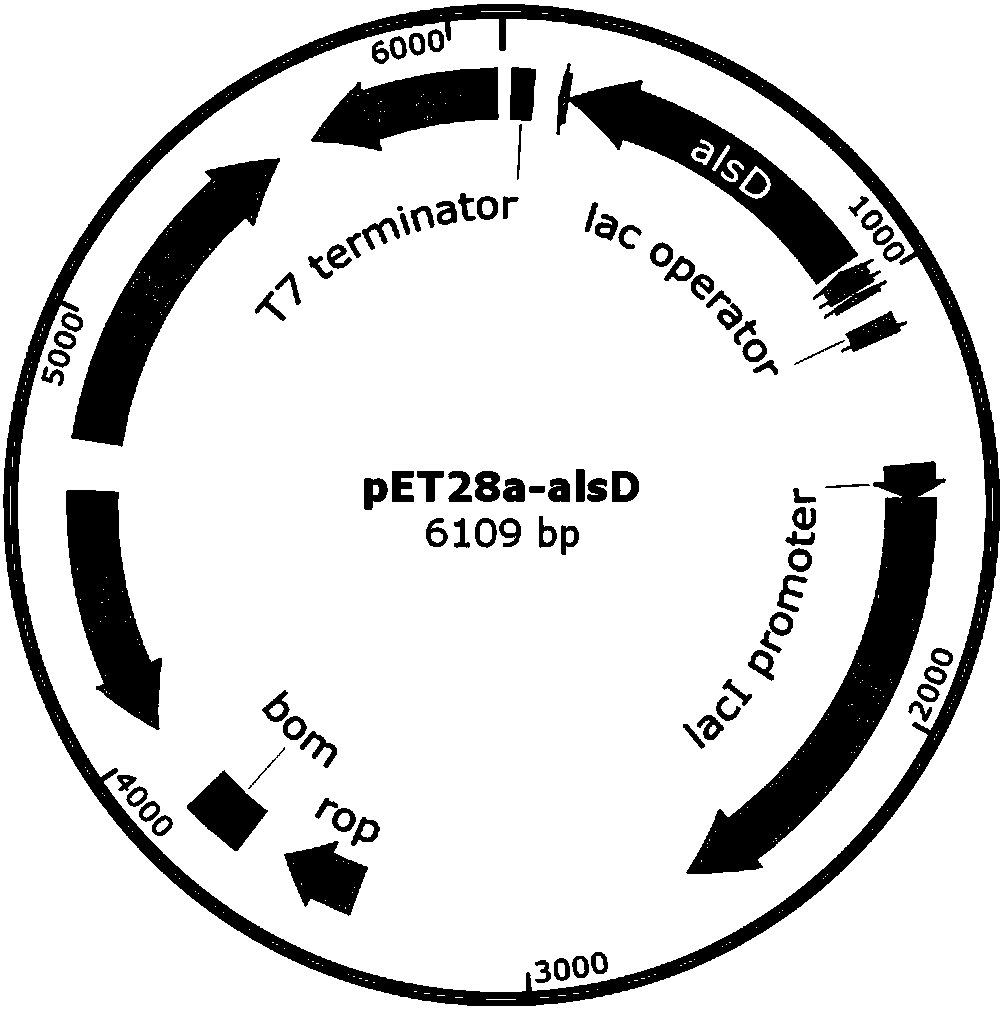
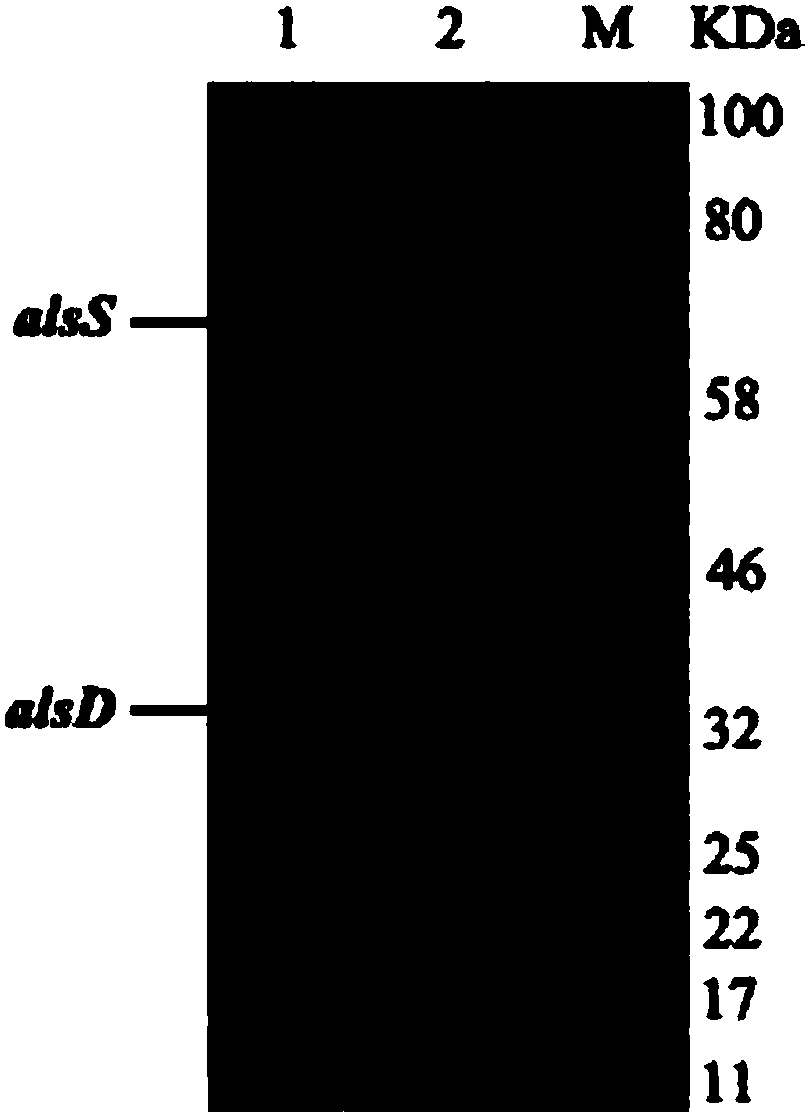
ActiveCN107653259AAchieve productionIncrease productionBacteriaTransferasesEscherichia coliThiamine pyrophosphate
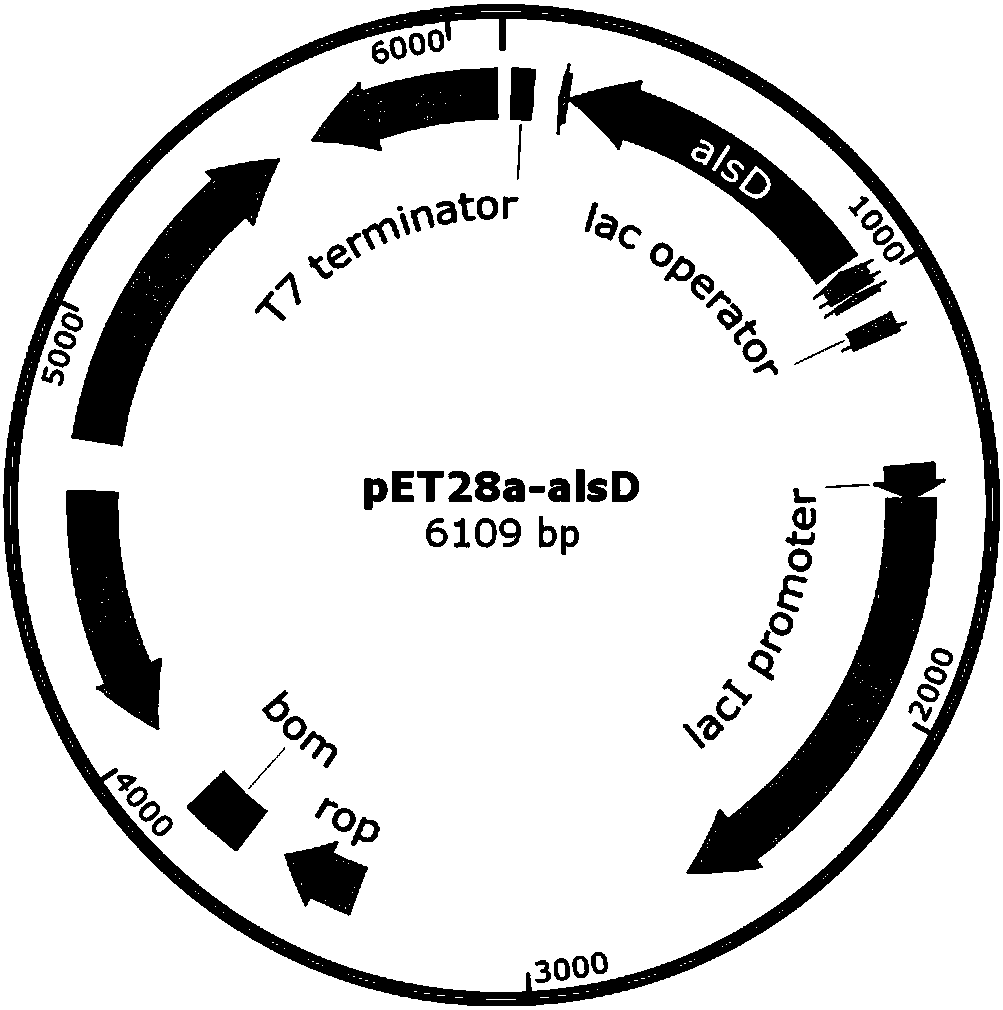
The invention discloses a method of producing D-(-)-acetoin by in-vitro enzyme reaction, comprising the steps of (1) linking the carrier pET28a and alpha-acetolactate synthase encoding gene alsS to obtain pET28a-alsS, introducing the pET28a-alsS into Escherichia coli, fermenting, purifying, and concentrating to obtain alpha-acetolactate synthase concentrate; linking the carrier pET28a and alpha-acetolactate decarboxylase encoding gene alsD to obtain pET29a-alsD, introducing the pET29a-alsD to Escherichia coli, fermenting, purifying, and concentrating to obtain alpha-acetolactate decarboxylaseconcentrate; (2) mixing well the two concentrates, sodium pyruvate, Mg2+ and thiamine pyrophosphate, and allowing reaction to obtain the D-(-)-acetoin. The sodium pyruvate low in cost is used as a substrate to provide in-vitro efficient production of high-added-value chiral D-(-)-acetoin, and the finished D-(-)-acetoin has high purity.
Owner:TIANJIN UNIV
Dry chemical reagent tablet for quantitatively measuring activity of alanine aminotransferase and preparation method of dry chemical reagent tablet
InactiveCN109916892ANo preparation requiredEasy to operateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsThiamine pyrophosphateHemolysis
The invention belongs to the technical field of clinical in vitro diagnostic reagents and relates to a dry chemical reagent tablet for quantitatively measuring the activity of alanine aminotransferaseand a preparation method of the dry chemical reagent tablet. The dry chemical reagent tablet comprises four laminations which are respectively a support layer, a color developing layer, a reagent layer and a diffusion layer distributed sequentially; reagents in the reagent layer include a buffer system, a hydrophilic colloid, a pyruvate oxidase, peroxidase, thiamine pyrophosphate, a chelating agent, an activator and a surfactant; and reagents in the color developing layer include a chromogen, a hydrophilic colloid, and a surfactant. The dry chemical reagent tablet and the preparation method thereof of the invention have the advantages of simple operation process, high stability, high good repeatability and high accuracy of detection results, quantitative detection, wide linear range, simple and convenient preparation process, easiness in operation, low risk and low pollution. According to the dry chemical reagent tablet for quantitatively measuring the activity of the alanine aminotransferase and the preparation method of the dry chemical reagent tablet of the invention, the reagents do not need to be prepared, and samples can be directly added dropwise; the reagent tablet is dryand is free of moisture; and the diffusion layer has a filtering function, so that hemolysis, bilirubin and ascorbic acid samples exert no significant interference on measurement results.
Owner:吉林省汇酉生物技术股份有限公司
Detection reagent for aspartate aminotransferase
ActiveCN103320498BHigh sensitivityImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsThiamine pyrophosphateVitamin C
The invention discloses a detection reagent for aspartate aminotransferase. The detection reagent comprises a diluent and a reaction reagent. The diluent comprises a buffer, a surfactant, an antiseptic, a bilirubin-interference removing reagent, and an ascorbic oxidase. The reaction reagent comprises a buffer, aspartic acid, alpha-ketoglutaric acid, oxaloacetic decarboxylase, phosphoric acid, pyruvate oxidase, thiamine pyrophosphate, magnesium chloride, peroxidase, 4-aminoantipyrene, a chromogen, an antiseptic, and a stabilizer. The detection reagent has advantages of good sensitivity, accuracy, precision and linearity, and can completely meet the requirements of clinical examination.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Test card for detecting glutamic-pyruvic transaminase by photochemical method and preparation method of test card
PendingCN111197071AAvoid interferenceImprove matchMicrobiological testing/measurementThiamine pyrophosphateFormulary
The invention discloses a test card for detecting glutamic-pyruvic transaminase by a photochemical method and a preparation method of the test card. The test card comprises a test card upper shell, atest card lower shell, a main test hole, a contrast test hole, a sample adding hole, a diffusion film, a first blood filtering film, two second blood filtering films, a first reaction film, a second reaction film and a single-sided adhesive tape, wherein the formula of a first reaction solution comprises L-alanine, pyruvate oxidase, alpha-ketoglutaric acid, peroxidase, thiamine pyrophosphate, magnesium chloride, 4-aminoantipyrine, a color developing agent, a film forming agent, a stabilizer and a buffer solution; the formula of a second reaction solution comprises pyruvate oxidase, peroxidase,thiamine pyrophosphate, magnesium chloride, 4-aminoantipyrine, a color developing agent, a film forming agent, a stabilizer and a buffer solution; by using the test card and the preparation method provided by the invention, endogenous pyruvic acid interference can be avoided, and the detection result is well matched with the hospital inspection result.
Owner:杭州联晟生物科技有限公司
"thiamine pyrophosphate (TPP) riboswitch mutants producing vitamin b1 enriched food and feed crops"
InactiveUS20130198900A1Increase enzyme activityReduce accumulationBacteriaUnicellular algaeBiotechnologyThiamine pyrophosphate
The present invention provides bioengineered organisms producing elevated levels of thiamine and / or thiamine derivatives. Particularly, the present invention discloses that modifying TPP-responsive riboswitch results in accumulation of thiamine and / or its derivatives.
Owner:YEDA RES & DEV CO LTD +1
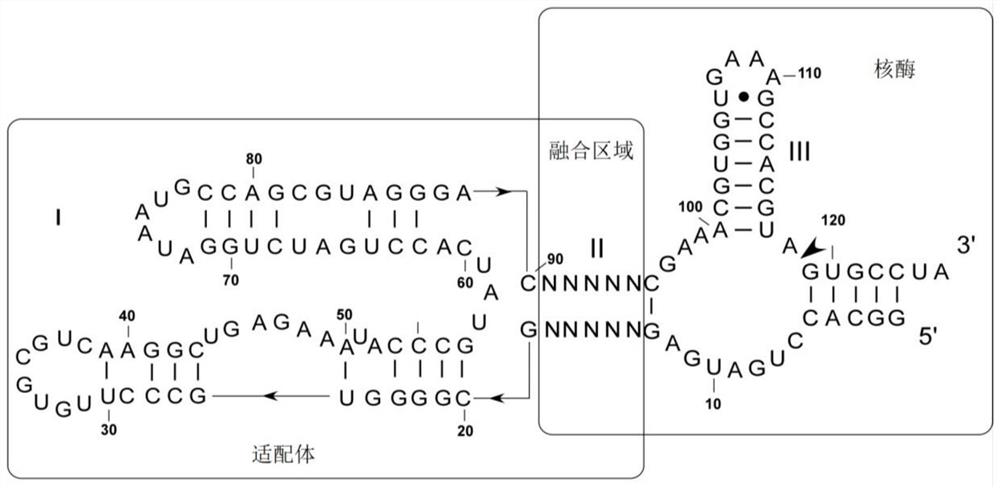
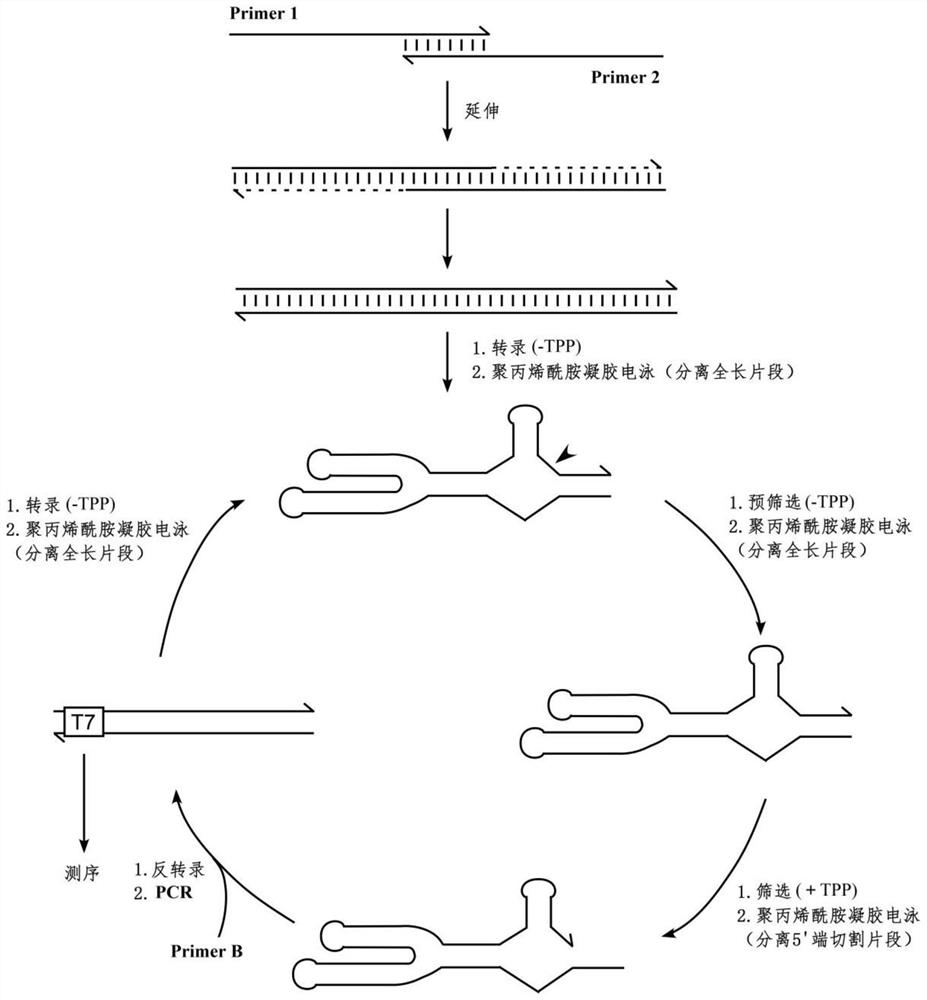
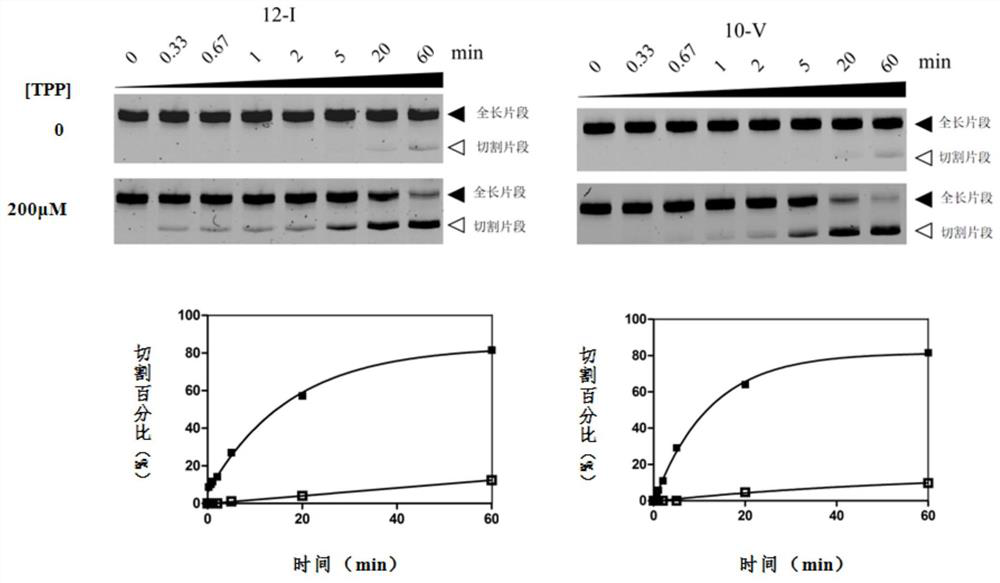
RNA sensor for detecting thiamine pyrophosphate and application thereof
ActiveCN108315332AEasy to detectHigh sensitivityMicrobiological testing/measurementDisease diagnosisThiamine pyrophosphatePyrophosphoric acid
The invention discloses an RNA sensor for detecting thiamine pyrophosphate. The sensor comprises an RNA aptamer for specifically sensing thiamine pyrophosphate, linkage segment RNA and hammerhead ribozyme, wherein the RNA aptamer is connected with the hammerhead ribozyme by virtue of the linkage segment RNA; when the RNA aptamer senses the thiamine pyrophosphate, self-cleavage of the hammerhead ribozyme is triggered. The invention further discloses a kit comprising the RNA sensor disclosed by the invention and a method for detecting the thiamine pyrophosphate. The RNA sensor disclosed by the invention is high in sensitivity of detecting the thiamine pyrophosphate and capable of performing qualitative and quantitative detection on the thiamine pyrophosphate.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Spectrophotometry for testing activity of pyruvic acid dehydrogenase system
InactiveCN100523784CLow priceSmall standard errorMicrobiological testing/measurementColor/spectral properties measurementsThiamine pyrophosphatePotassium ferricyanide
A spectrophotometric method for the determination of pyruvate dehydrogenase activity. This method uses a spectrophotometer, in the presence of substrate sodium pyruvate and cofactors magnesium chloride, thiamine pyrophosphate, using phenazine dimethyl sulfate as electron transporter, thiazole blue as electron acceptor, thiazole blue Reducted by pyruvate dehydrogenase (E1) product hydroxyethyl-pyrophosphate thiamine, the maximum absorption peak of thiazole blue changes from wavelength 400-430 nanometers to 540-640 nanometers, by measuring the wavelength 540-640 nanometers light absorption The amount of increase determines the reduction of thiazolium blue and defines the activity of the enzyme. The reagents used in this method are more economical, more stable, and more sensitive than the 2,6-dichloroindoxyl method and the potassium ferricyanide method. The influence of reagents is not conducive to the determination of E1 activity in the pyruvate dehydrogenase complex. This method is not only suitable for the determination of purified pyruvate dehydrogenation activity, but also for pyruvate dehydrogenation in the pyruvate dehydrogenase complex after mitochondria extraction. Hydrogenase activity assay.
Owner:HUAZHONG NORMAL UNIV
Body fluid expanders comprising n-substituted aminosulfonic acid buffers
InactiveUS20100183747A1Preventing and lowering incidenceLoss can be compensatedBiocideHydroxy compound active ingredientsThiamine pyrophosphateAcute toxicity testing
A buffered body fluid expander solution, in which the buffer is a physiologically acceptable buffer that is not an inorganic phosphate buffer, comprises calcium ions and magnesium ions at a concentration ratio of 5:1 to 1:1. The non-phosphate buffer may be a physiologically acceptable N-substituted aminosulfonic acid buffers, especially those having a pKa value in aqueous solution of from 7.1 to 7.5 at 2O0C, and most preferably N-tris(hydroxymethyl) methyl-2-aminoethanesulfonic acid (TES), 3-(N-morpholino) propanesulfonic acid (MOPS), N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES) and combinations thereof. Preferred components include from 100 to 150 (preferably about 135) mmoles / L sodium ions, from 2.5 to 6.2 (preferably about 5) mmoles / L potassium ions, from 0.1 to 2.5 (preferably about 1.25) mmoles / L calcium ions, from 0.4 to 25.0 (preferably about 0.45) mmoles / L magnesium ions, from 96 to 126 (preferably about 118) mmoles / L chloride ions, 2 to 11 mmoles / L (preferably about 10) glucose (preferably D-glucose), from 50 to 150 (preferably about 110) μmoles / L glycerol, from 7 to 15 (preferably about 10) μmoles / L choline, from 5 to 400 (preferably about 300) μmoles / L glutamate (preferably L-glutamate), from 5 to 200 (preferably about 20) μmoles / L aspartate (preferably L-aspartate), from 100 to 2000 (preferably about 400) μmoles / L glutamine (preferably L-glutamine), from 15 to 215 (preferably about 60) μmoles / L pyroglutamate, from 20 to 200 (preferably about 100) μmoles / L arginine (preferably L-arginine), from 1 to 120 (preferably about 40) nmoles / L thiamine pyrophosphate (TPP), from 40 to 70 (preferably about 50) μmoles / L D- or DL or L-carnitine (preferably L-carnitine), and from 5 to 200 (preferably about 28) ml.U. / L porcine or human insulin (preferably human insulin). The solutions are useful for the manufacture of medicaments and blood volume expanders, for treating hypovolemia or for treating the loss of extracellular and interstitial fluid in subjects suffering with burns, for treating respiratory and / or metabolic acidosis in a subject, for perfusion of the abdominal cavity during peritoneal dialysis of a subject with acute renal failure or an acute toxicity condition, for preventing and / or ameliorating reperfusion injury, and for delivering a therapeutic, test and / or synergistic agent to a subject, including a biological agent, such as at least one stem cell, peptide or genomic derived protein.
Owner:AQIX
Hericium erinaceus selenium-enriched instant grain powder and preparation method thereof
PendingCN113575845ASupplement activeImprove liquidityYeast food ingredientsFood ingredient functionsBiotechnologyThiamine pyrophosphate
The invention discloses hericium erinaceus selenium-enriched instant grain powder and a preparation method thereof. The hericium erinaceus selenium-enriched instant grain powder is prepared from the following raw materials in mass proportion: 70-80 g of fresh grains, 5-8 g of hericium erinaceus, 3-5 g of skimmed milk powder, 2-4 g of selenium-enriched yeast, 1-2 g of pericarpium citri reticulatae, 1-3 g of fructo-oligosaccharide, 300-400 mg of calcium carbonate, 30-50 mg of sodium ascorbate, 0.3-0.6 mg of riboflavin, 0.3-0.6 mg of thiamine hydrochloride and 1-3 mg of sodium pyrophosphate. The raw materials such as the grains, the hericium erinaceus and the selenium-rich components are scientifically matched, so that the hericium erinaceus selenium-rich powder is rich in carbohydrates, proteins, lipids, vitamins and mineral substances required by human bodies and has a positive promoting effect on supplement of human nutrition, and the production process is suitable for large-scale mechanical production in factories; and the obtained product is powder is easy to store and transport, is also easy to eat, can be simply brewed or cooked in a matched mode, conforms to the fast-paced life pattern of modern people, and is wider in market application range.
Owner:安徽过湾农业科技有限公司
A kind of alanine aminotransferase detection reagent
ActiveCN103320497BHigh sensitivityImprove accuracyMicrobiological testing/measurementThiamine pyrophosphateVitamin C
The invention discloses a detection reagent for alanine aminotransferase. The detection reagent comprises a diluent and a reaction reagent. The diluent comprises a buffer, a surfactant, an antiseptic, a bilirubin-interference removing reagent, and an ascorbic oxidase. The reaction reagent comprises a buffer, L-alanine, alpha-ketoglutaric acid, phosphoric acid, pyruvate oxidase, thiamine pyrophosphate, magnesium chloride, peroxidase, 4-aminoantipyrene, a chromogen, an antiseptic, and a freeze-drying protecting agent. The detection reagent has advantages of good sensitivity, accuracy, precision and linearity, and can completely meet the requirements of clinical examination.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
A method for producing d-(-)-acetoin by enzymatic reaction in vitro
ActiveCN107653259BAchieve productionIncrease productionBacteriaTransferasesThiamine pyrophosphateEscherichia coli
The invention discloses a method of producing D-(-)-acetoin by in-vitro enzyme reaction, comprising the steps of (1) linking the carrier pET28a and alpha-acetolactate synthase encoding gene alsS to obtain pET28a-alsS, introducing the pET28a-alsS into Escherichia coli, fermenting, purifying, and concentrating to obtain alpha-acetolactate synthase concentrate; linking the carrier pET28a and alpha-acetolactate decarboxylase encoding gene alsD to obtain pET29a-alsD, introducing the pET29a-alsD to Escherichia coli, fermenting, purifying, and concentrating to obtain alpha-acetolactate decarboxylaseconcentrate; (2) mixing well the two concentrates, sodium pyruvate, Mg2+ and thiamine pyrophosphate, and allowing reaction to obtain the D-(-)-acetoin. The sodium pyruvate low in cost is used as a substrate to provide in-vitro efficient production of high-added-value chiral D-(-)-acetoin, and the finished D-(-)-acetoin has high purity.
Owner:TIANJIN UNIV
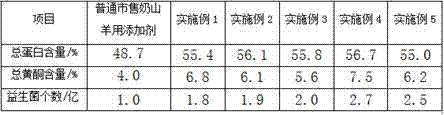
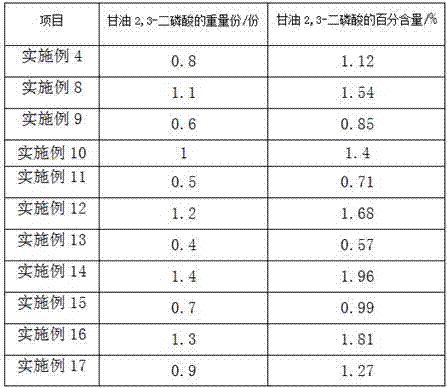
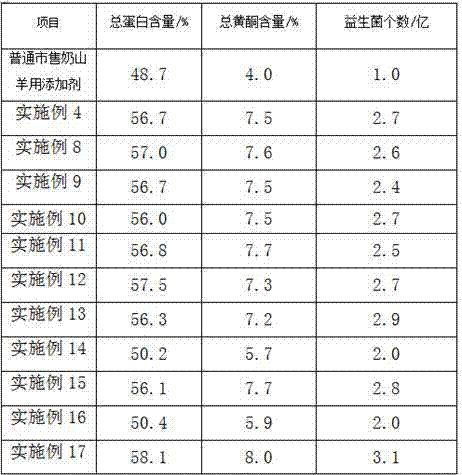
Additive for milk goat fodder and preparation method thereof
InactiveCN107319142AIncrease feed intakeIncrease appetiteAnimal feeding stuffAccessory food factorsThiamine pyrophosphateAdhesive
The invention provides an additive for a milk goat fodder. The additive is prepared from the following raw materials of gulcomannan, plant salt, magnesium stearate, B-sitosterol, antioxidant enzyme, cobalt chloride, thiamine pyrophosphate, proline, beta-glucanase, lecithin and glycerinum 2,3-diphosphonic acid. A preparation method of the additive disclosed by the invention comprises the step of granulating, wherein the granulating comprises the steps of adding olive oil, chitosan and an adhesive into a water-reduced mixture, evenly stirring and granulating. The adhesive is methyl cellulose and / or ethyl cellulose; the middle particle size of the additive for the fodder is 1.6mm, and evenness of the additive is 97.1%. The additive disclosed by the invention can reduce a death and culling rate of adult milk goats, and the death and culling rate of the adult milk goats is reduced to 4.0 to 5.8%.
Owner:SHANDONG YANGCHUN GOATS MILK DAIRY
An RNA sensor for detecting thiamine pyrophosphate and its application
ActiveCN108315332BEasy to detectHigh sensitivityMicrobiological testing/measurementDisease diagnosisThiamine pyrophosphateAptamer
The invention discloses an RNA sensor for detecting thiamine pyrophosphate, which comprises an RNA aptamer for specifically sensing thiamine pyrophosphate, a linker RNA and a hammerhead ribozyme, wherein the RNA aptamer passes the linker RNA and the hammerhead ribozyme. The head ribozyme is connected, and when the RNA aptamer senses thiamine pyrophosphate, it triggers the self-cleavage of the hammerhead ribozyme; the invention also discloses a kit comprising the RNA sensor of the invention and the detection of thiamine The method for thiamine pyrophosphate; the RNA sensor of the present invention has high sensitivity for detecting thiamine pyrophosphate, and can perform qualitative and quantitative detection of thiamine pyrophosphate.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Efficient plant growth medicament
InactiveCN103891763AGrow fastPromotes coordinated growthBiocidePlant growth regulatorsThiamine pyrophosphateGrowth plant
The invention discloses an efficient plant growth medicament, which mainly comprises 0.5-1.5 grams of gibberellin, 0.5-1.5 grams of thiamine pyrophosphate, 0.5-1.0 gram of daminozide, 0.5-1.5 kg of naphthyl acetic acid, 2-4 grams of ethephon and 1-2 grams of icosan-1-ol. The product provided by the invention can be applied to promote plant growth in overall coordination, and realize high survival rate and fast growth of plants. According to usage observation statistics, the efficient plant growth medicament can increase rooting rate of forest by 18-30% and accelerate forest growth cycle by 6.
Owner:许峰
A kind of flame-retardant water-resistant polyurethane adhesive and preparation method thereof
InactiveCN103059796BImprove adhesionImprove water resistanceNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesThiamine pyrophosphateEnvironmental resistance
The invention discloses inflaming retarding water-tolerant polyurethane tackiness agent which is characterized by comprising following components by weight ratio: 88%-92% hydroxyl polyurethane resin, 5.5%-10% isocyanate group (NCO) enclosed type diisocyanate-trimethoprim (TMP), 1.5%-2.5% sodium dodecyl sulfate and 0.5%-1.5% phosphonolipid fire retardant thiamine pyrophosphate (TPP). The invention further discloses a preparation method of the inflaming retarding water-tolerant polyurethane tackiness agent. NCO enclosed type diisocyanate-TMP addition product aqueous dispersion is adopted, reaction of self de-enclosing NCO perssad with hydroxyl-terminated prepolymer is utilized to achieve solidification, and a strong chemical bond is formed to enable the tackiness agent to have the advantages of being strong in binding power, good in water tolerance, washable, free-falling, sun-proof, good in thermal endurance, endurable in chemical products and the like. Grade of products is greatly improved, simultaneously, the inflaming retarding water-tolerant polyurethane tackiness agent is simple in use, convenient to operate, environment-friendly, non-toxic, and environment-pollution-free, has inflaming retarding function and no volatile organic compounds (VOC) discharging, and is beneficial to protecting environment and health of using and production persons.
Owner:DONGGUAN SHENGBAO NEW MATERIAL TECH
Production method of environment-friendly enzyme solution for coal
The invention provides a production method of an environment-friendly enzyme solution for coal. A solute of the environment-friendly enzyme solution for coal comprises an enzyme, a coenzyme and a stabilizer; a solvent is purified water. The enzyme is selected from one of the following substances: laccase isoenzyme and pyruvate dehydrogenase; the coenzyme is selected from one of the following substances: thiamine pyrophosphate and flavin adenine dinucleotide; the stabilizer is selected from one of the following substances: potassium benzoate and sodium benzoate. The storage temperature of the environment-friendly enzyme solution for coal ranges from 2 DEG C to 8 DEG C. In the environment at 0-26 DEG C, the environment-friendly enzyme solution is sprayed on coal, the coal sprayed with the solution is burned after being left to stand for 96 h, the pollution discharge of coal during burning can be decreased, and the burning efficiency of coal can be improved.
Owner:赛景波
A kind of enzymatic preparation method of l-xylose
The invention discloses an enzymatic method for preparing L-xylose and belongs to the field of bioengineering. In the method, 2-keto-L-gulonic acid is dissolved in a buffer containing magnesium ions and thiamine pyrophosphate, and pyruvate decarboxylase is added to the buffer for an enzyme-catalyzed reaction at a suitable temperature. After the end of the reaction, the production of the L-xylose is proved by a high performance liquid chromatograph. A new method for producing the L-xylose is found. The method is low in raw material cost, has no pollution to the environment, and has important economic and social benefits.
Owner:SHANGHAI LINKCHEM TECH CO LTD
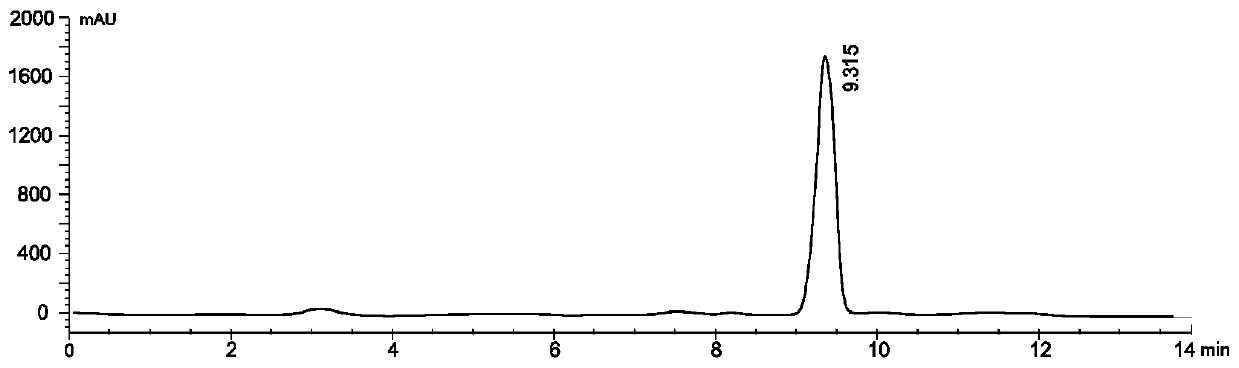
Preparation method of thiamine pyrophosphate tetrahydrate in 12 vitamin raw materials for injection
InactiveCN108191912AShorten the working cycleReduce manufacturing costGroup 5/15 element organic compoundsThiamine pyrophosphateDistilled water
The invention discloses a preparation method of thiamine pyrophosphate tetrahydrate in 12 vitamin raw materials for injection. The preparation method comprises the following steps: (1) acidizing of Amberlite resin to obtain acid-treated Amberlite resin; (2) activating the acid-treated Amberlite resin to obtain activated Amberlite resin; (3) packing the activated Amberlite resin; preparing a thiamine pyrophosphate hydrochloride solution, loading, reducing pressure and chromatographing by using distilled water as a mobile phase, and post-treating the eluate to obtain a crude product of thiaminepyrophosphate tetrahydrate; (4) dissolving the crude product of thiamine pyrophosphate tetrahydrate in water to form a thiamine pyrophosphate tetrahydrate solution; adding anhydrous ethanol to the solution until a turbid phenomenon appears, heating to complete dissolution, still standing, and filtering to obtain the thiamine pyrophosphate tetrahydrate.
Owner:SHANXI PUDE PHARMA CO LTD
Porphyra yezoensis ueda TPS (trehalose-6-phosphate synthase) gene and application thereof in enhancing salt tolerance of rice
InactiveCN102286494BImprove securityMorphologically normalMicrobiological testing/measurementFermentationGenetically modified riceThiamine pyrophosphate
The invention discloses a PyTPS (porphyra yezoensis ueda trehalose-6-phosphate synthase) gene which is cloned to construct a plant expression vector of the gene, and the plant expression vector is transferred into the agrobactrium tumefaciens strain, so that an engineering strain is obtained. A rice variety is transformed by utilizing agrobacterium-mediated transformation, so that genetically modified rice is obtained; after growing and acclimatization, a T0 individual plant is obtained, and a T1 seed is collected; a seedling germinated from the T1-generation genetically modified seed receives PCR (polymerase chain reaction) detection and a salt tolerance assay, so that a T1-generation genetically modified salt-resistant strain is obtained, a T2 generation receives a kanamycin resistance assay, PCR detection and Southern hybridization, and results indicate that the PyTPS gene is already integrated into the genome of the genetically modified strain. The PyTPS gene in the invention has a TPS domain as well as a TPP (thiamine pyrophosphate) domain; the PyTPS gene source is safe; the development of the plant morphology of the PyTPS genetically modified rice is normal; the PyTPS gene can be stably inherited in genetically modified plants; and moreover, the transformation system is highly effective.
Owner:QINGDAO AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com