A method for producing d-(-)-acetoin by enzymatic reaction in vitro
An acetoin and in vitro enzyme technology, which is applied in biochemical equipment and methods, botanical equipment and methods, and the introduction of foreign genetic material using vectors, which can solve the problems of low transformation rate and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Overexpression of α-acetolactate synthase (alsS) using commercialized protein expression vector pET28a
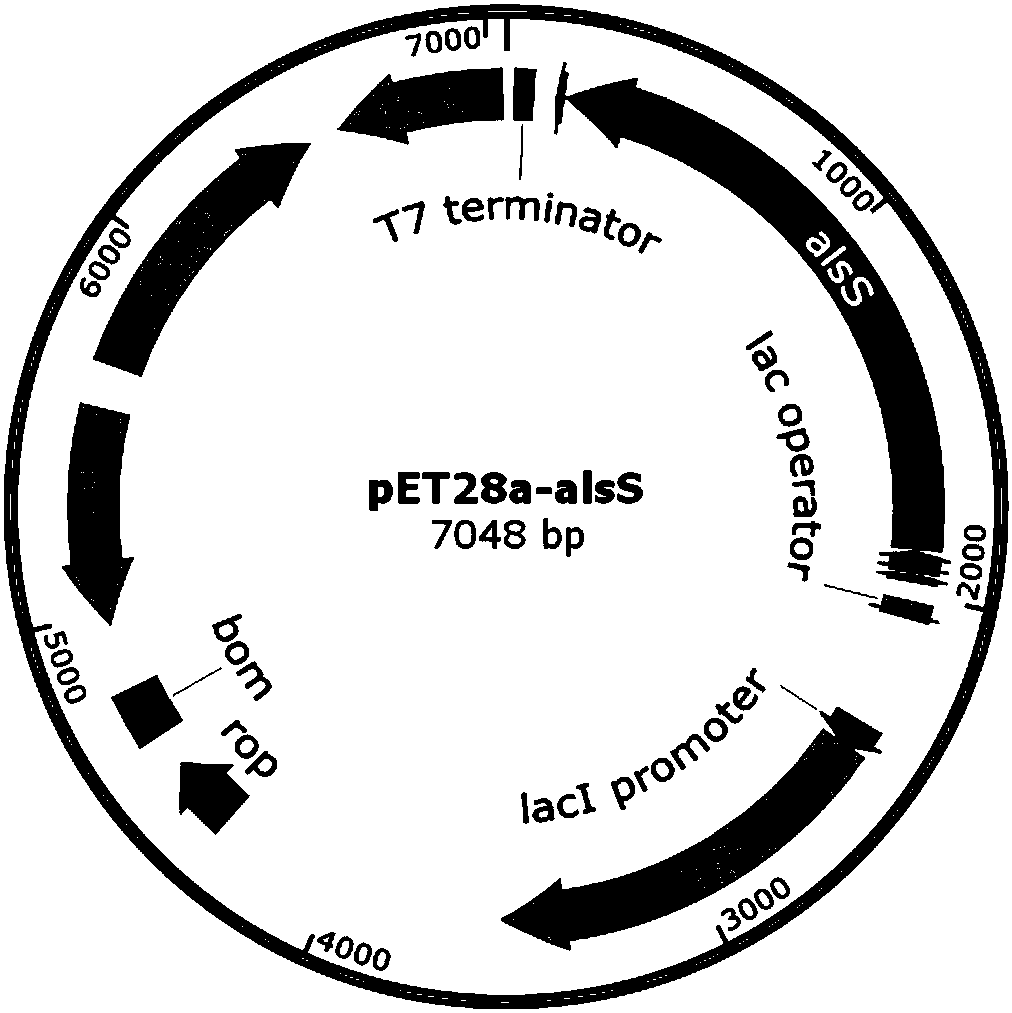
[0027] Using the Bacillus subtilis B. subtilis 168 genome as a template, primers p-alsS1 and p-alsS2 were used to amplify the gene alsS fragment (about 1.7kp). The alsS fragment and the pET28a plasmid were double digested with Thermo Fast digest XhoI / BamHI, and after ligation and transformation, the expression vector pET28a-alsS of the alsS gene was obtained (see figure 1 ), and the sequence detection was correct. The plasmid with the correct sequencing result was transformed into commercially competent E. coli BL21 (DE3) by the traditional calcium chloride method to obtain BL21-1 overexpressing α-acetolactate synthase (alsS).
Embodiment 2
[0028] Example 2 Overexpression of α-acetolactate decarboxylase (alsD) using commercialized protein expression vector pET28a
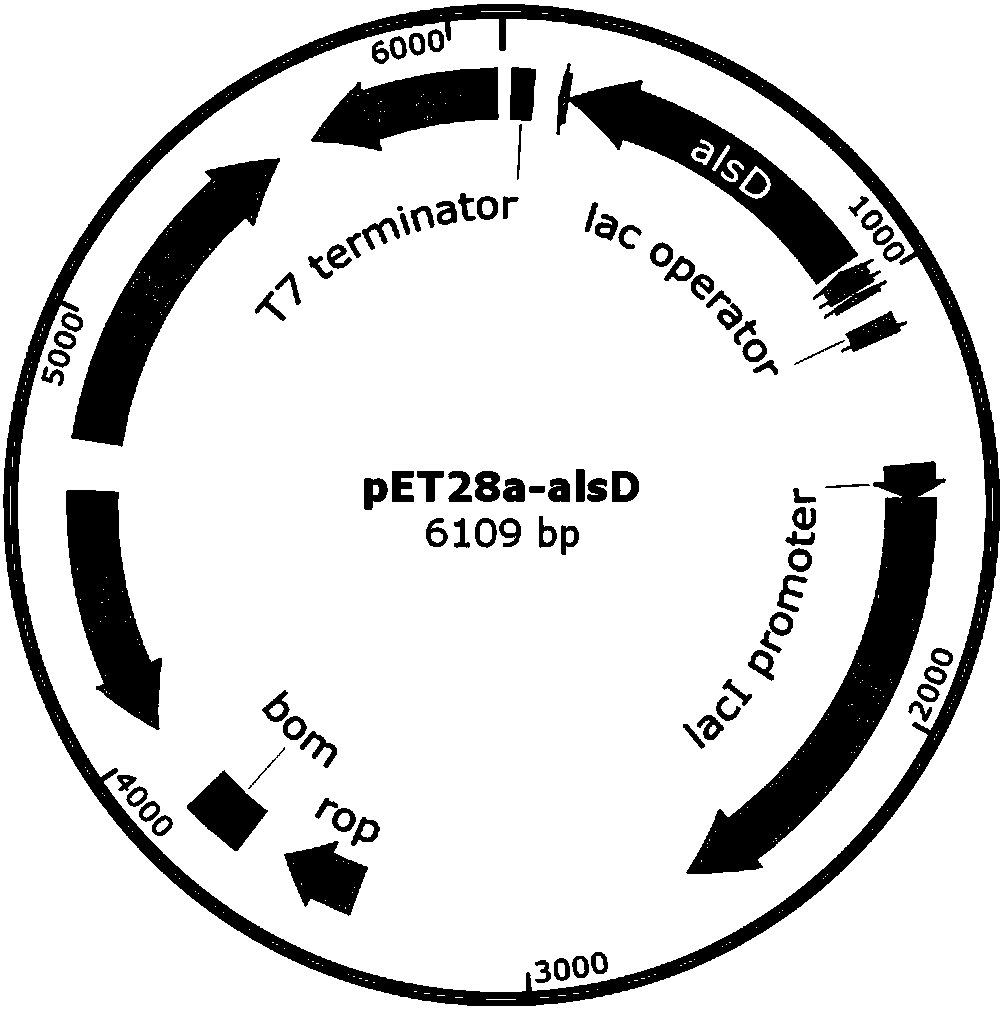
[0029] Using the genome of Bacillus subtilis B. subtilis 168 as a template, primers p-alsD1 and p-alsD2 were used to amplify the gene alsD fragment (768bp). Then the alsD fragment and the pET28a plasmid were digested with Thermo Fast digest XhoI / EcoRI, and after ligation and transformation, the expression vector pET28a-alsD of the alsD gene was obtained (see figure 2 ), and the sequence detection was correct. The plasmid with the correct sequencing result was transformed into commercially competent E. coli BL21 (DE3) by the traditional calcium chloride method to obtain BL21-2 overexpressing α-acetolactate decarboxylase (alsD).
[0030] Table 1 The sequences of primers used for strain construction
[0031]
Embodiment 3
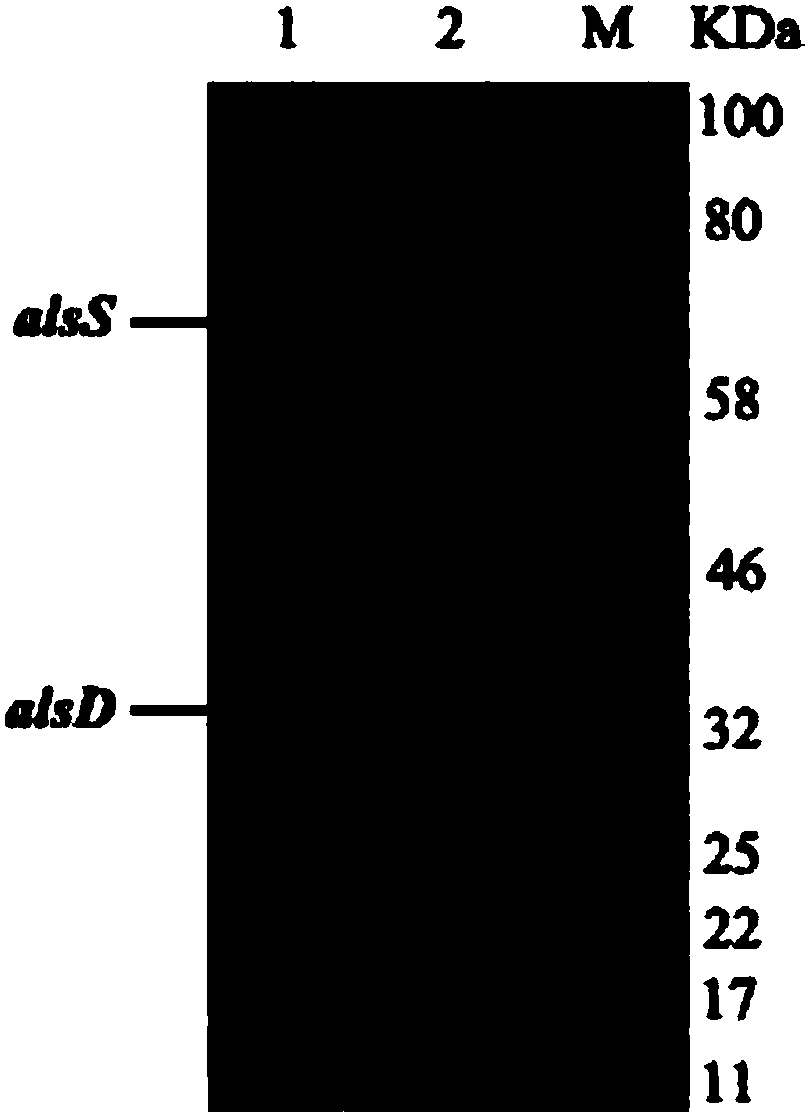
[0032] Example 3 Purification and Concentration of α-Acetolactate Synthase and α-Acetolactate Decarboxylase
[0033] 1. The specific steps for the purification and concentration of α-acetolactate synthase are:
[0034] 1) Escherichia coli BL21-1 was inoculated into 400mL LB medium, cultured on a shaker at 37°C and 220rpm until the OD600 was 0.6, and the inducer IPTG (isopropylthiogalactopyranoside) was added to a final concentration of 0.5mM, 16 Cultivate at ℃ for 12 hours, collect the bacterial cells by centrifuging at 4200 rpm for 20 minutes at 4℃, and suspend with 20 mL buffer A.
[0035]2) Collect the suspension of BL21-1 obtained in step 1), break the cells under the action of a high-pressure cell breaker, at 4°C, 1200bar, oil pressure 18Kg / cm 3 Treated 3 times under the same conditions, centrifuged at 4°C and 8000rpm for 30min after crushing, and collected the supernatant to obtain the crude enzyme solution.
[0036] 3) Using the crude enzyme solution obtained in step ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com