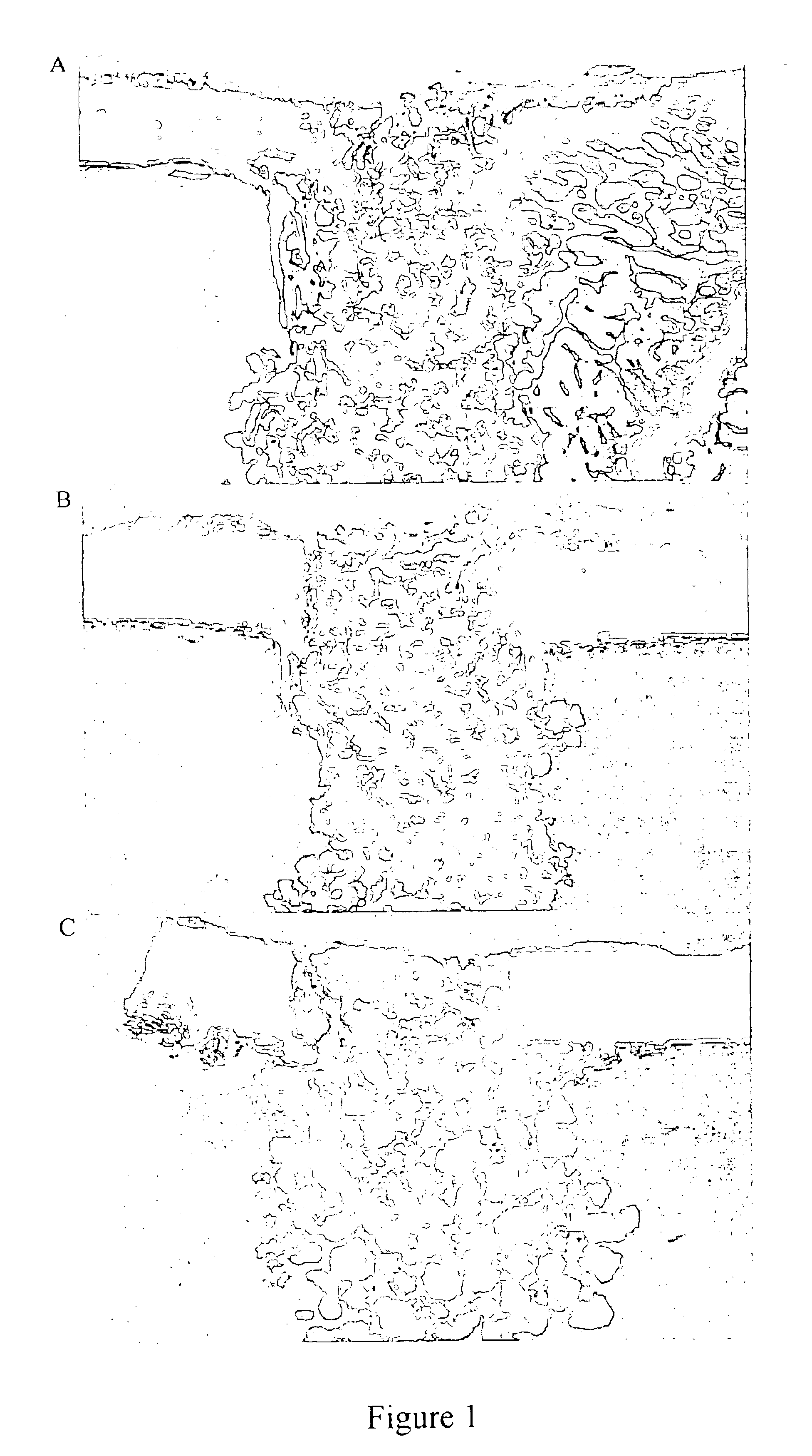
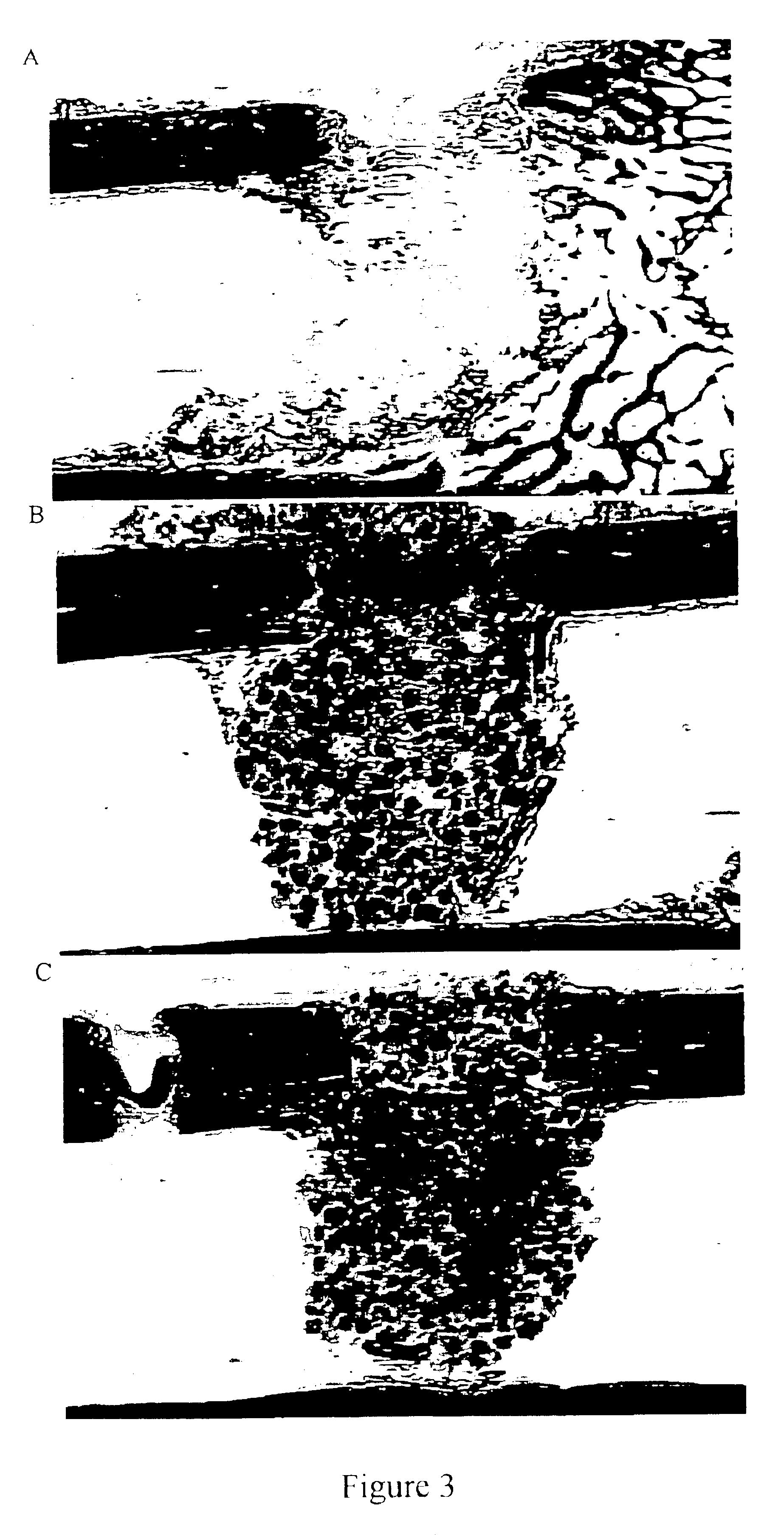
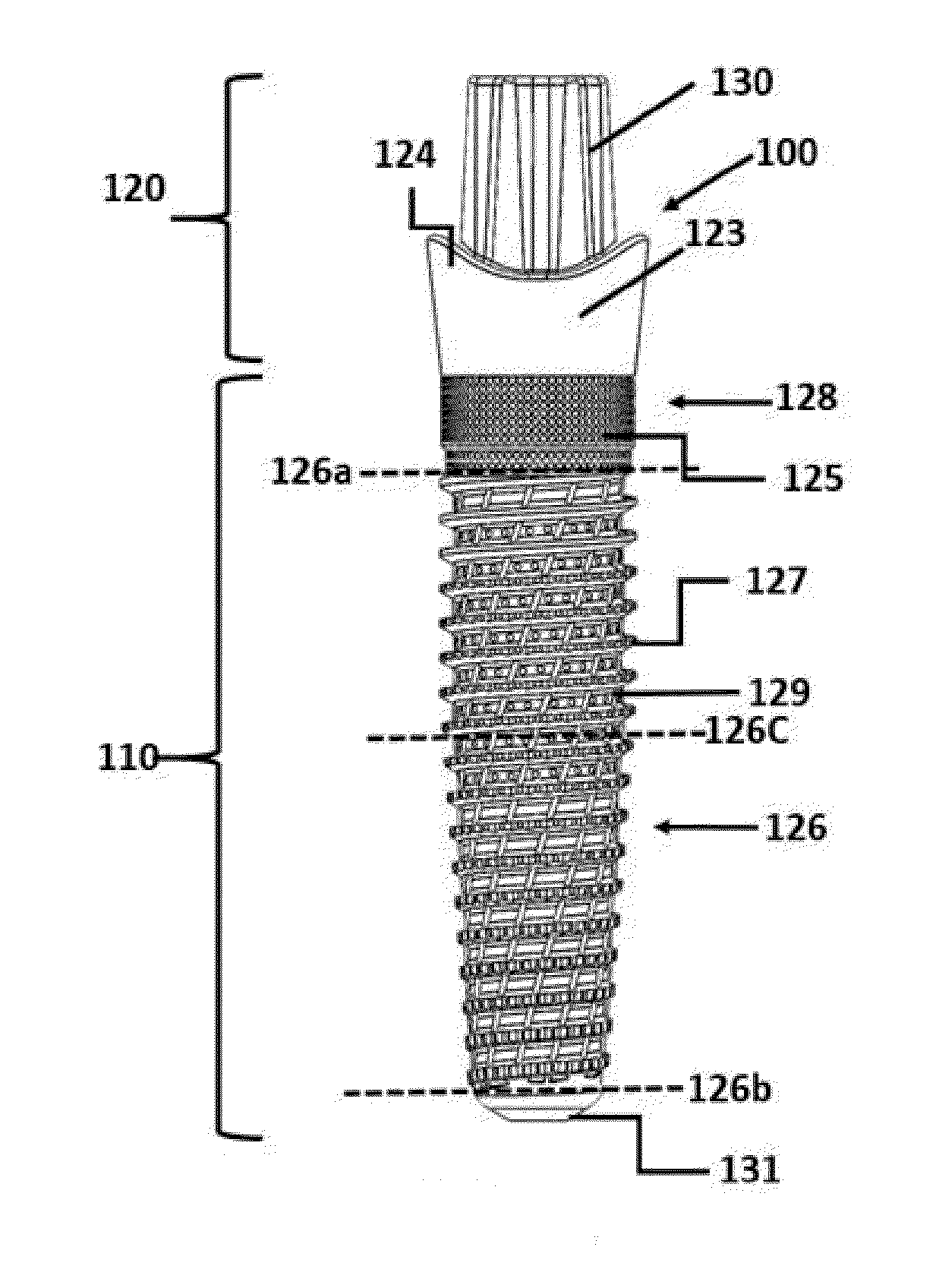
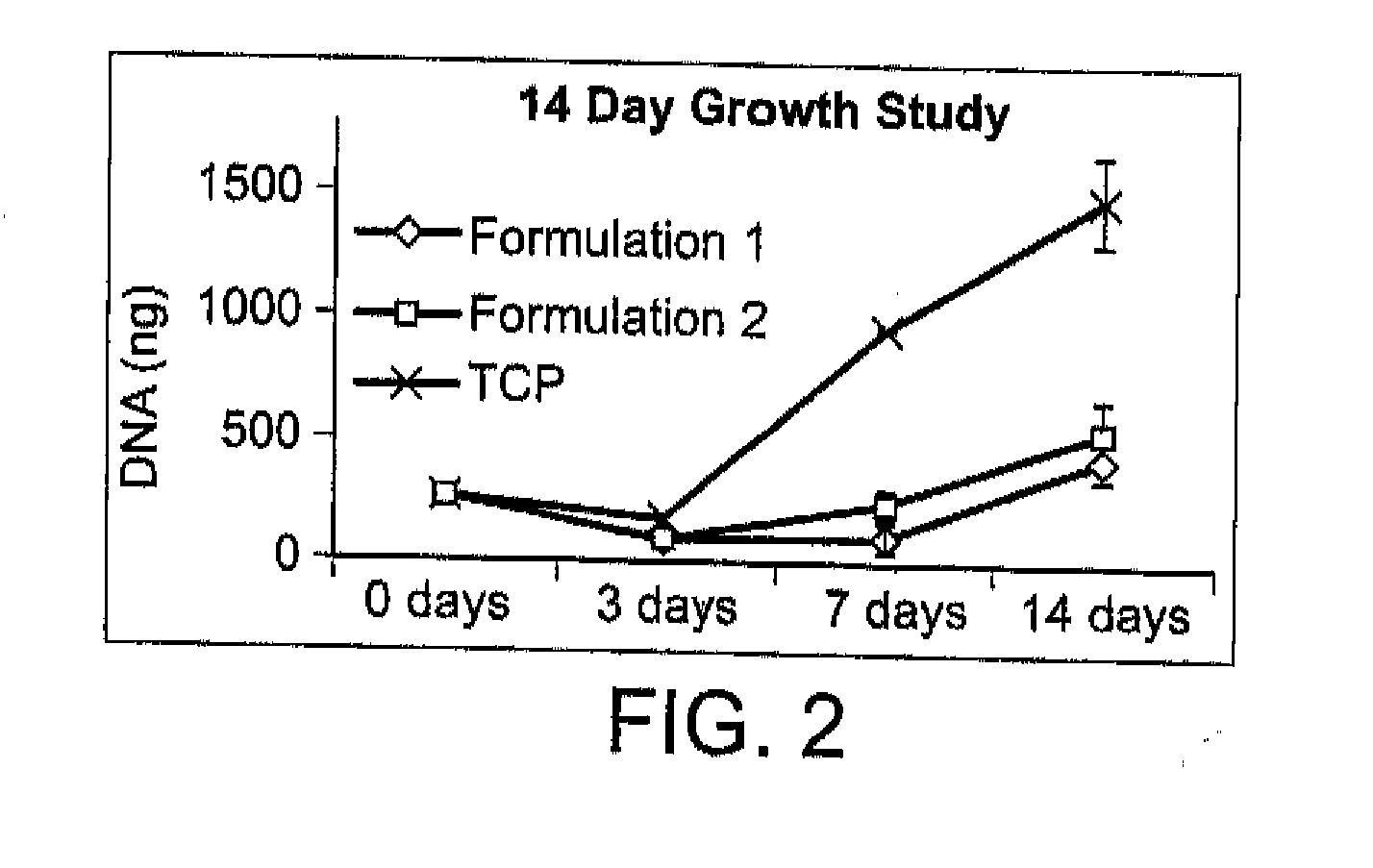
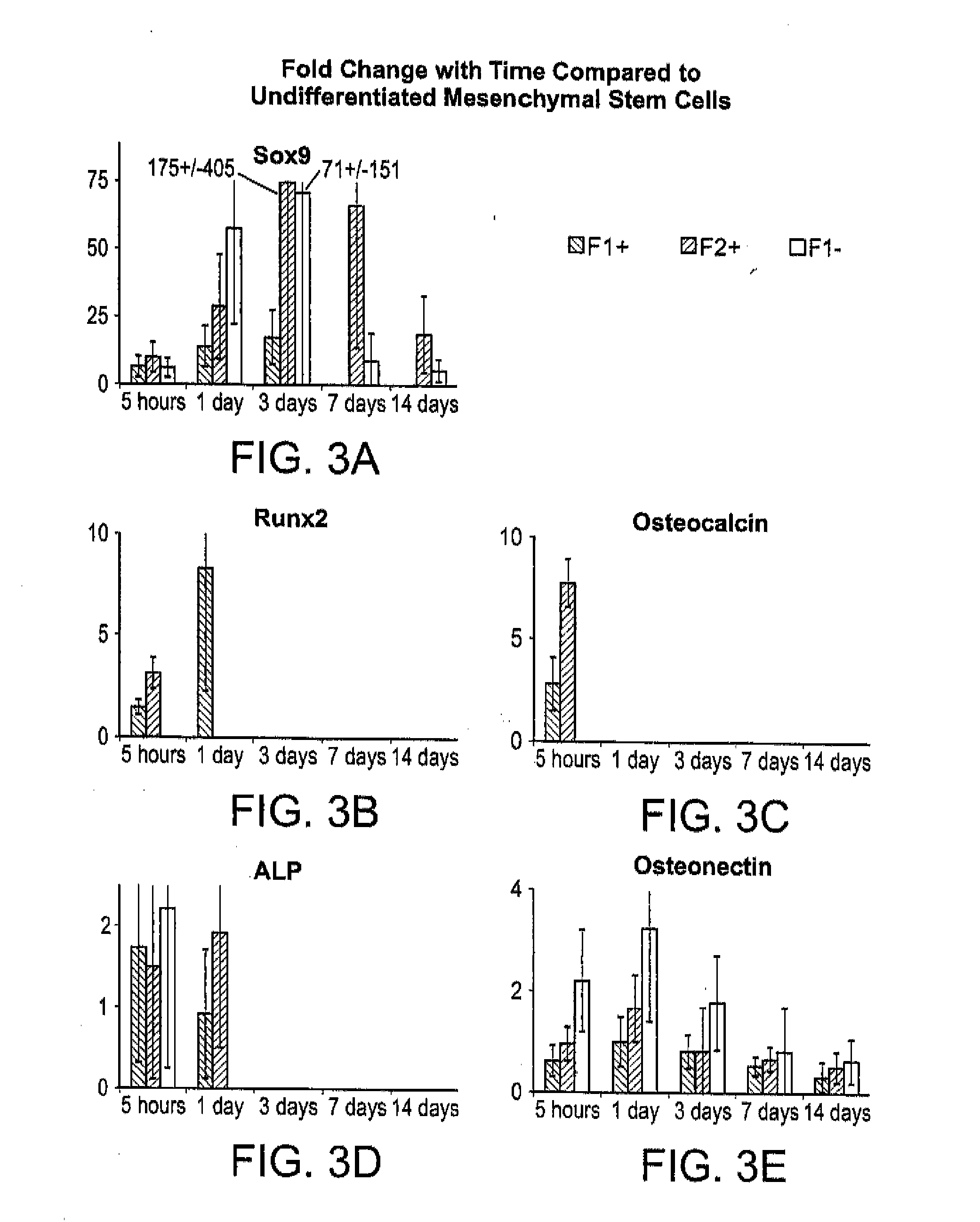
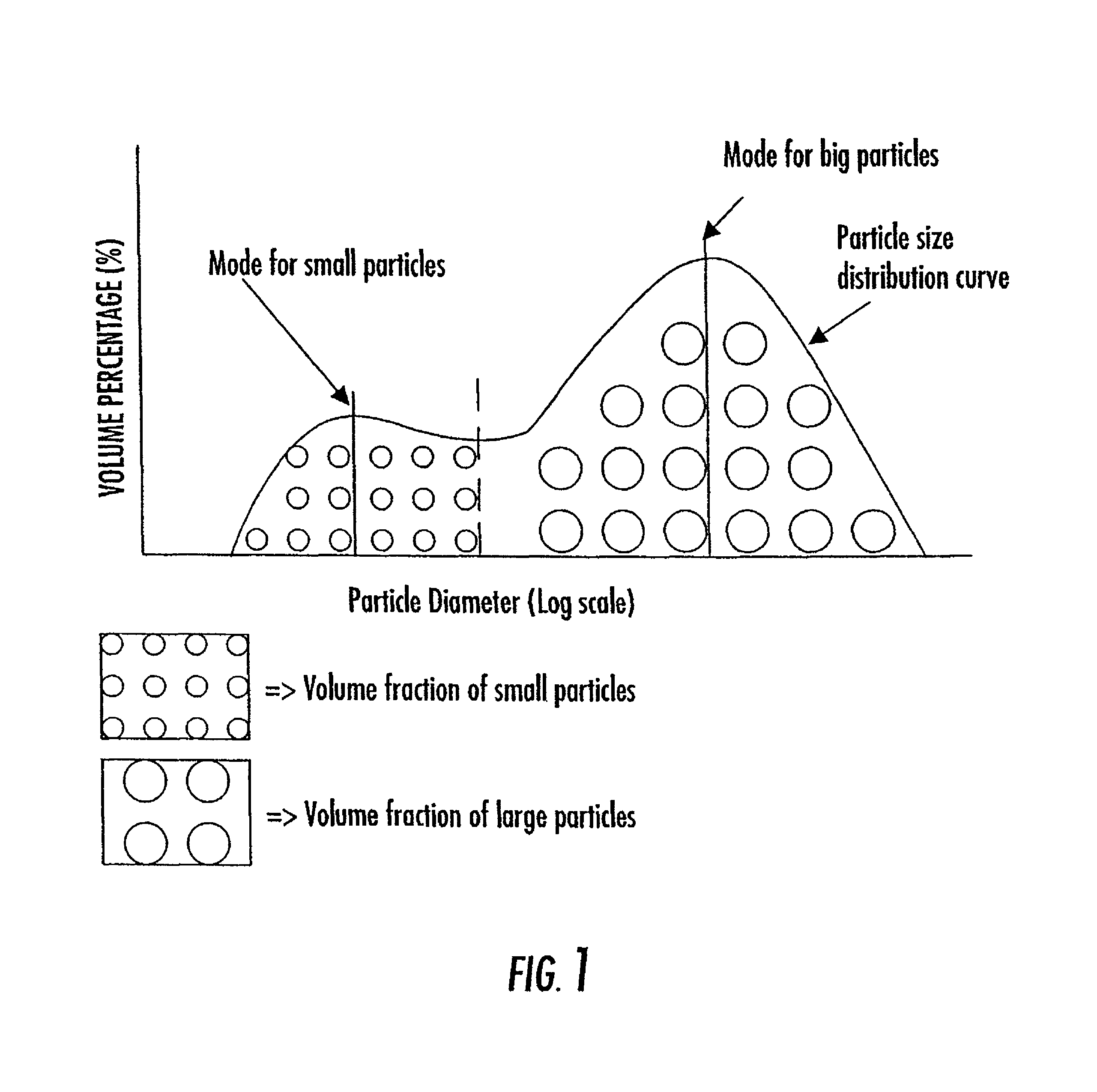
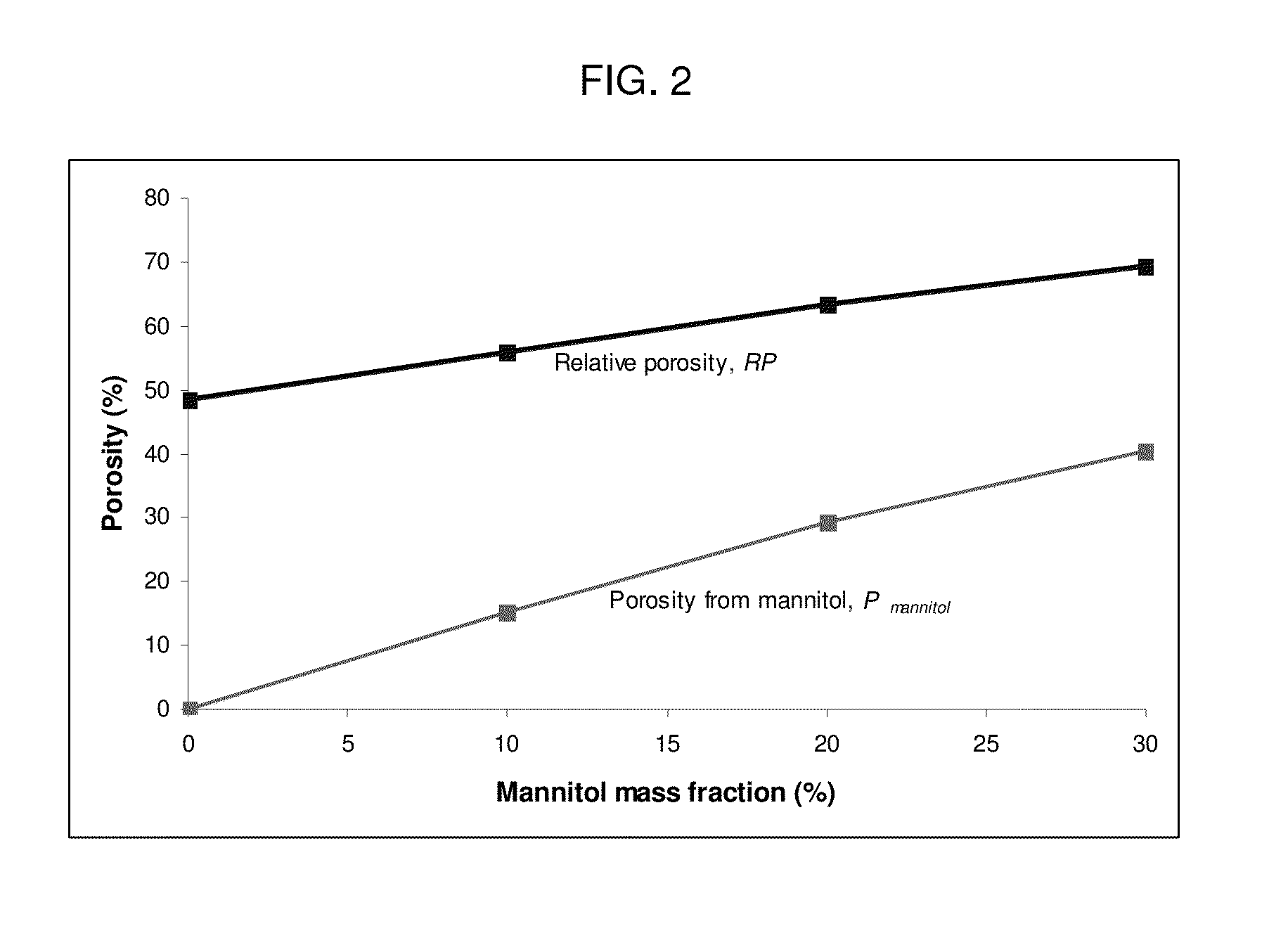
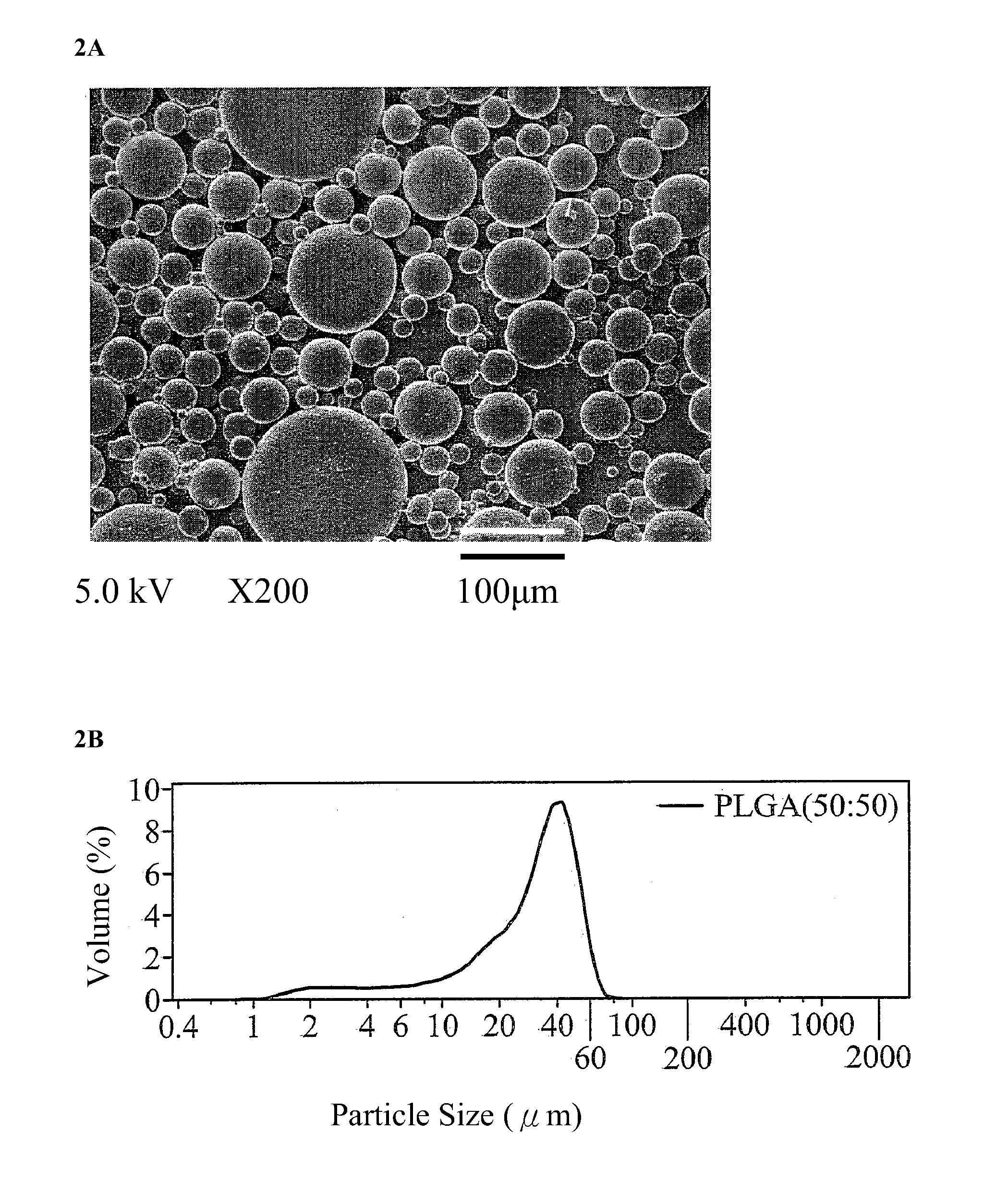
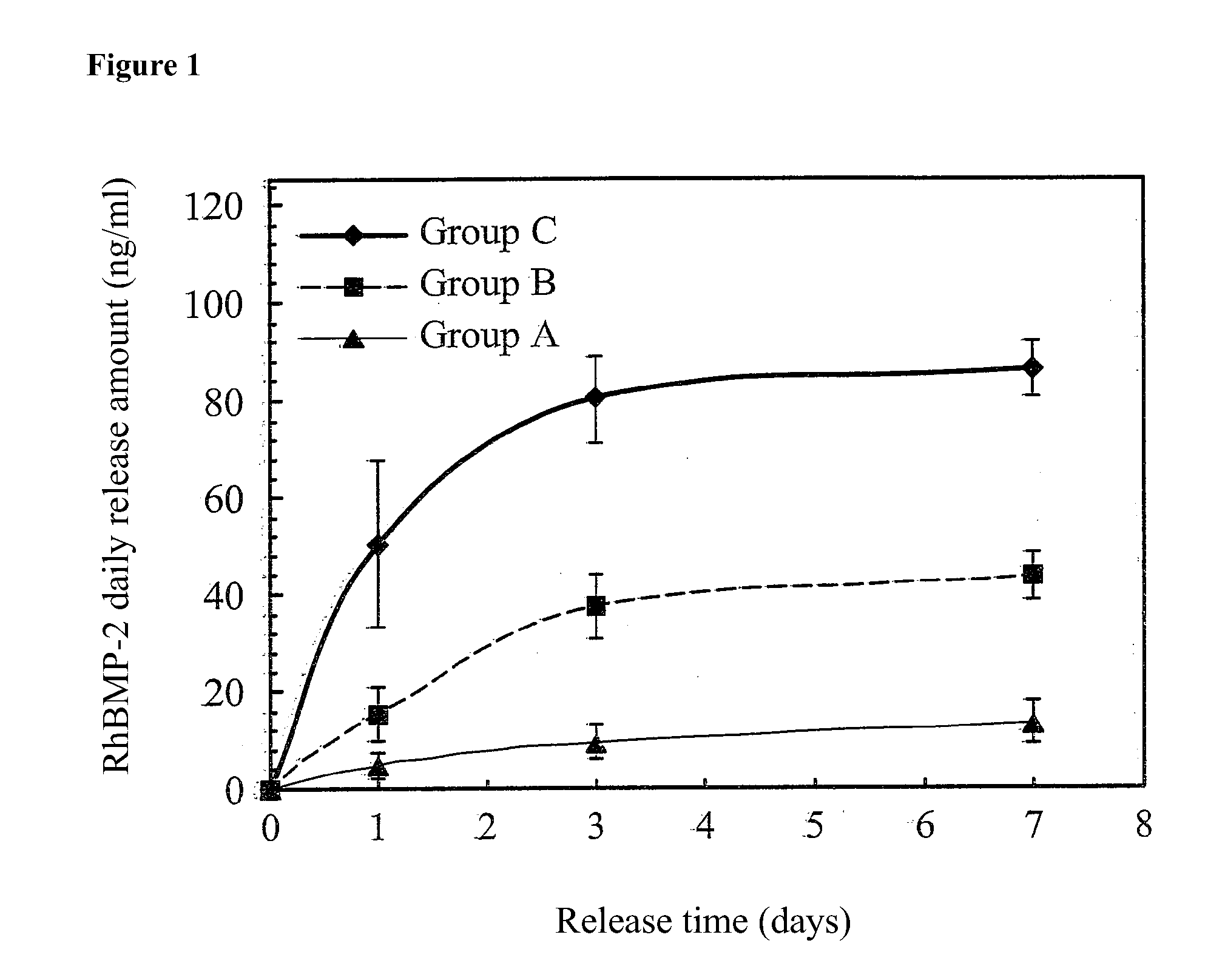
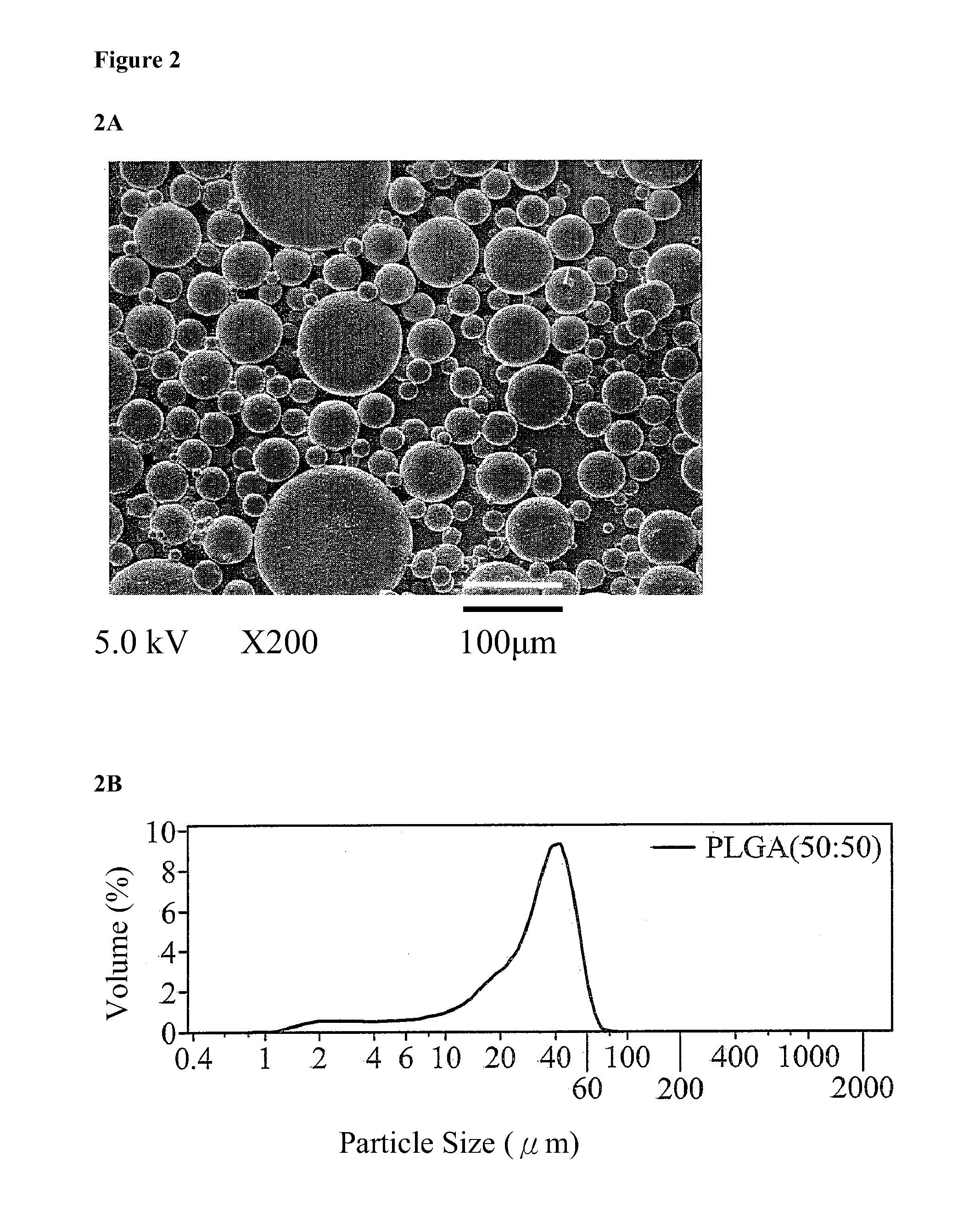
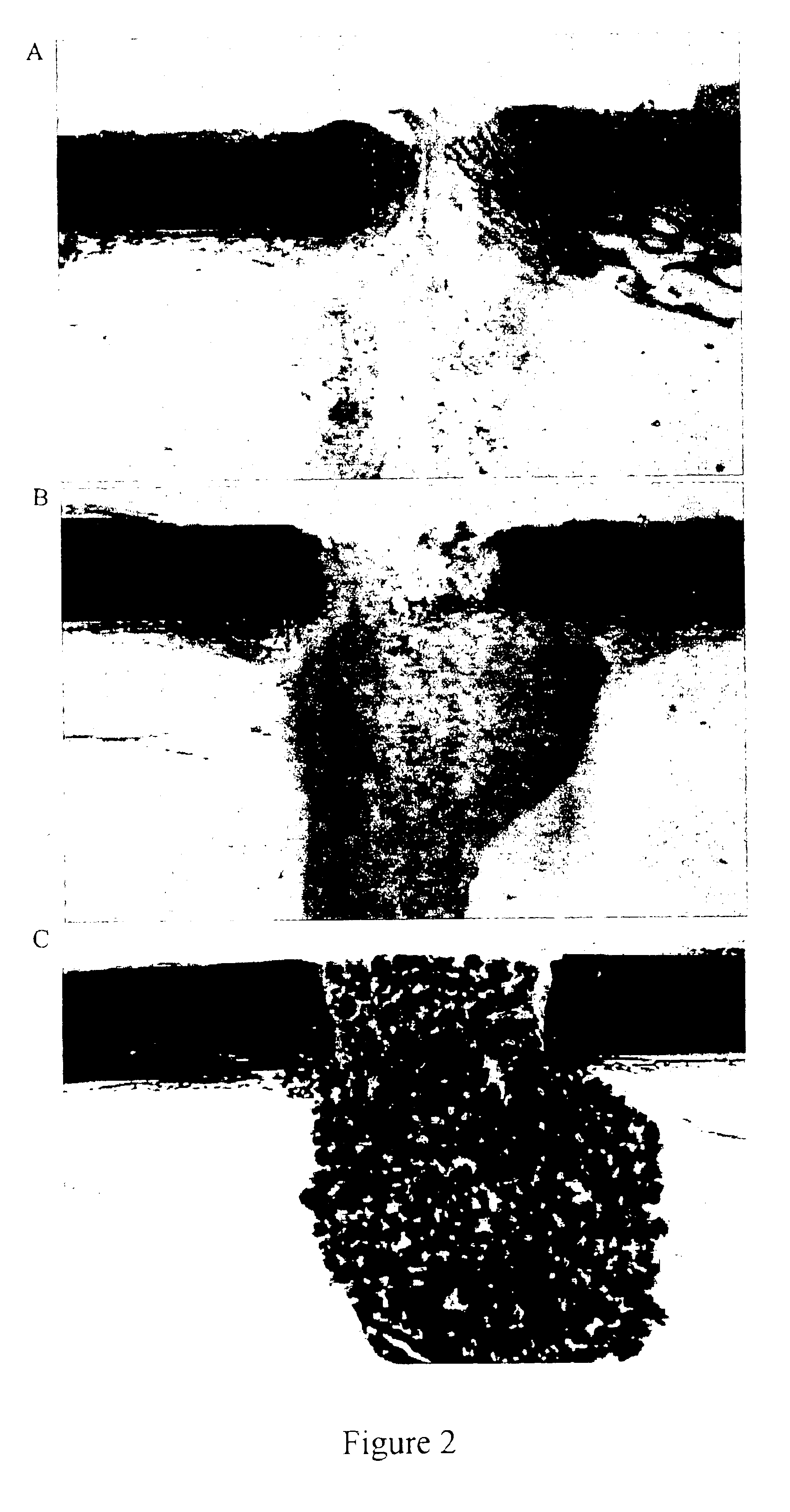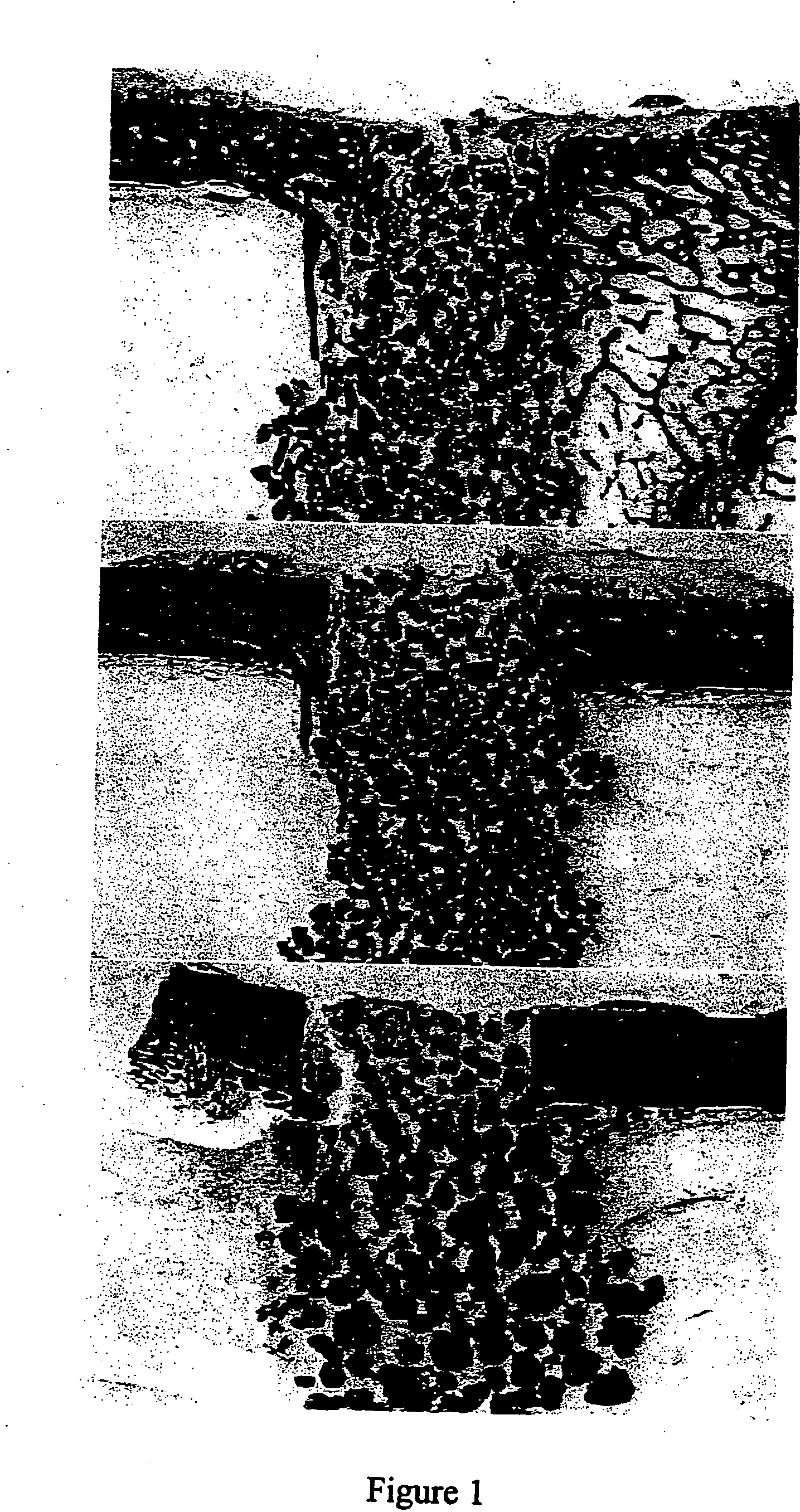Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Β tricalcium phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
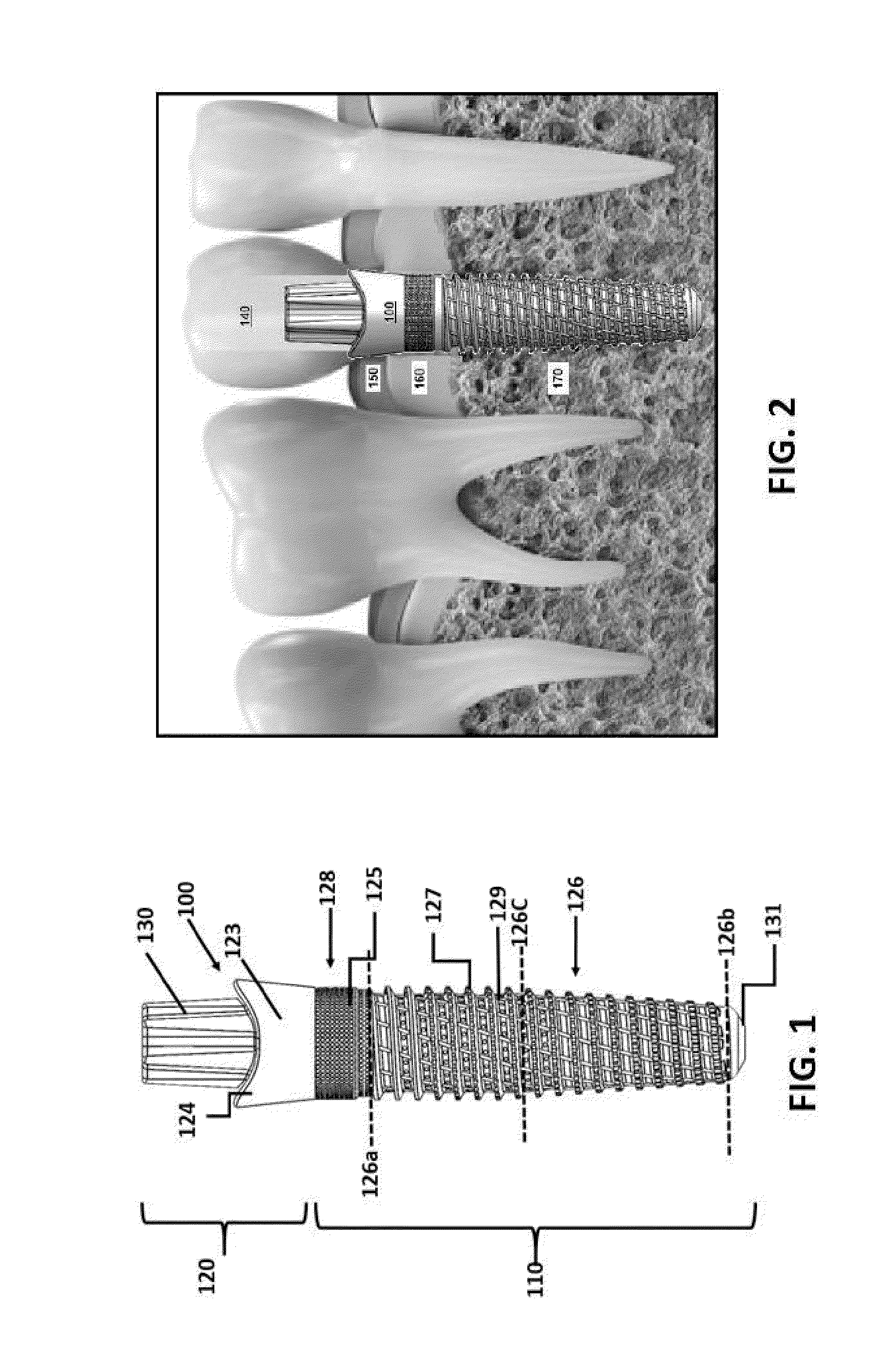
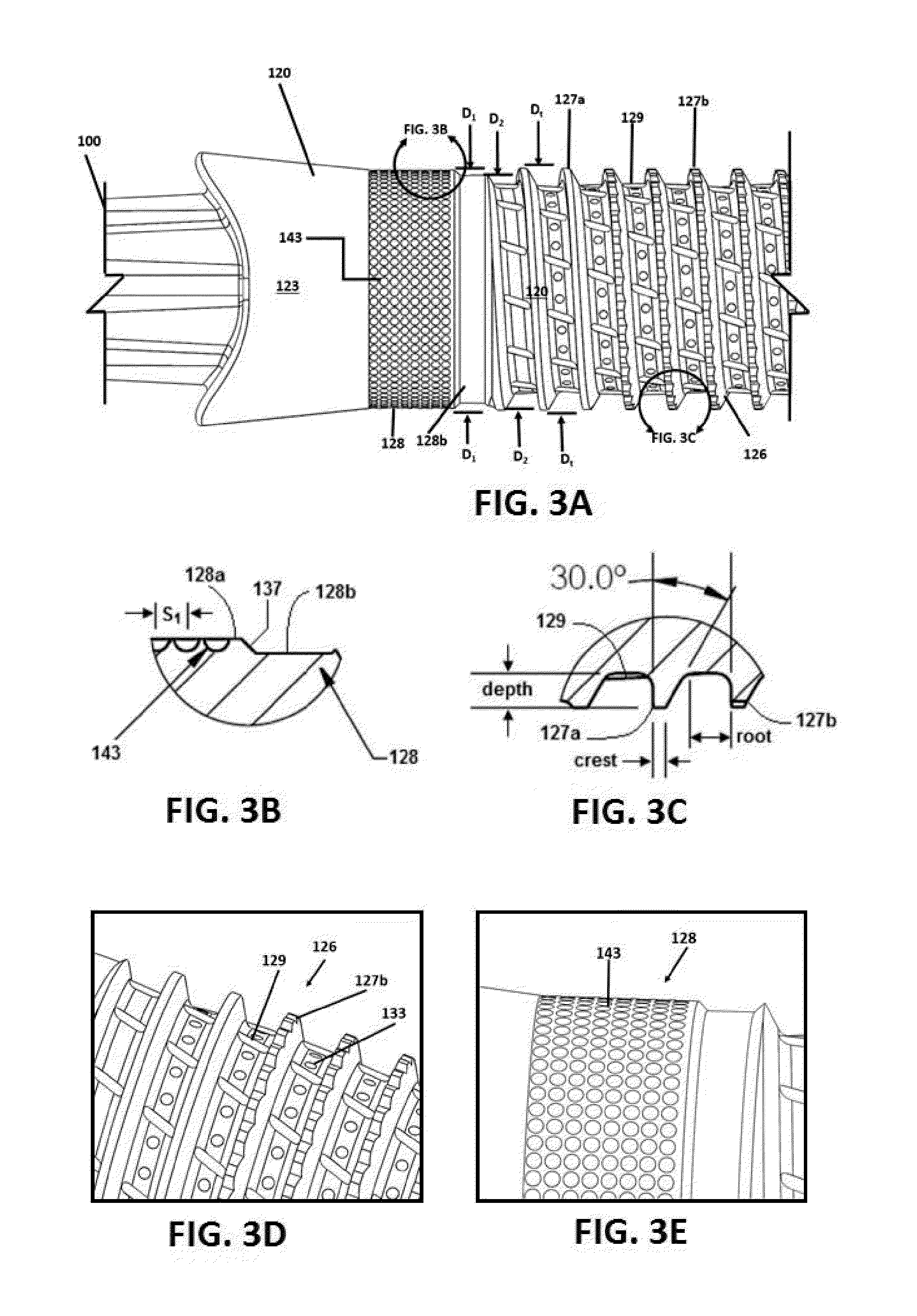
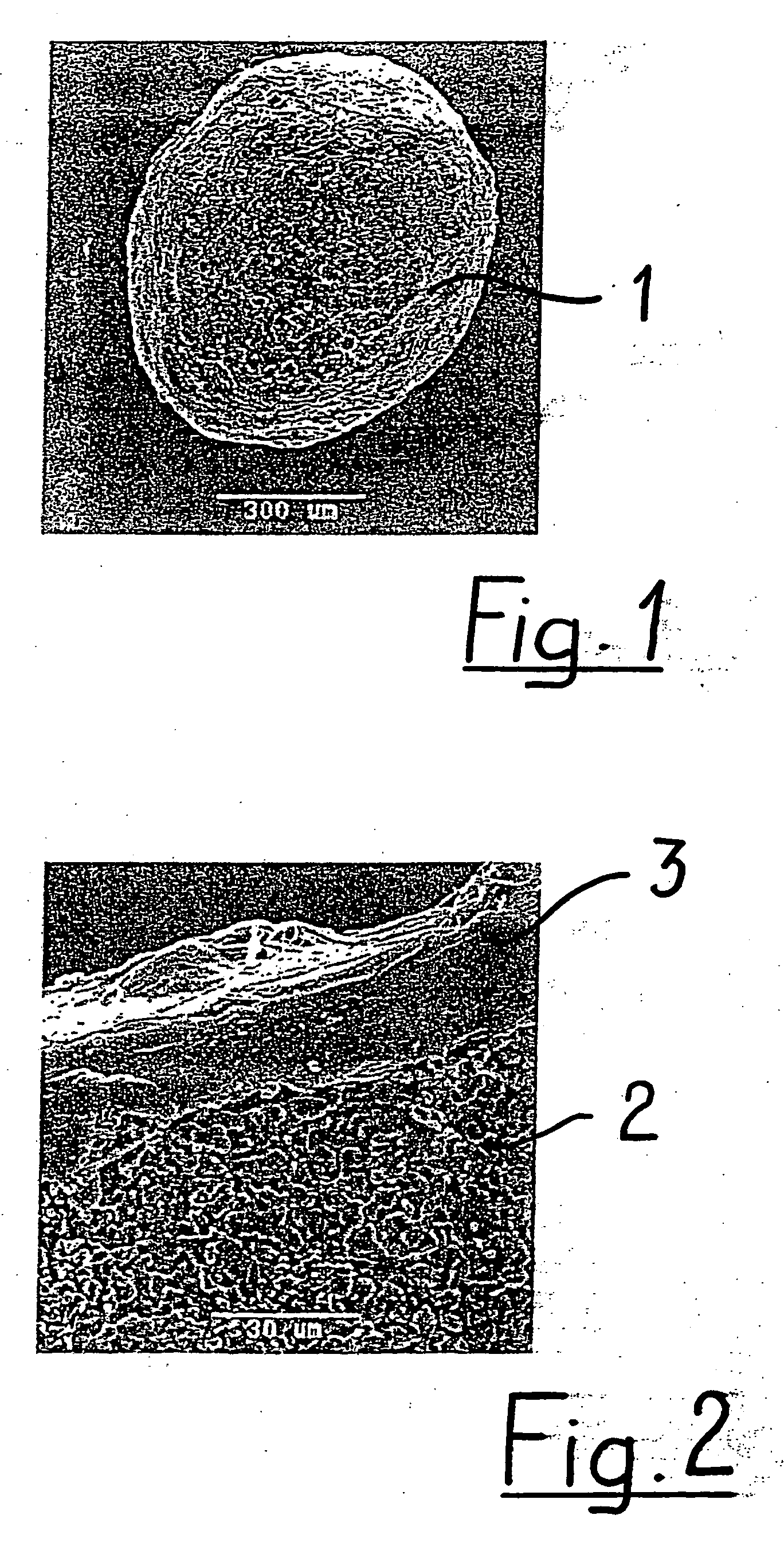
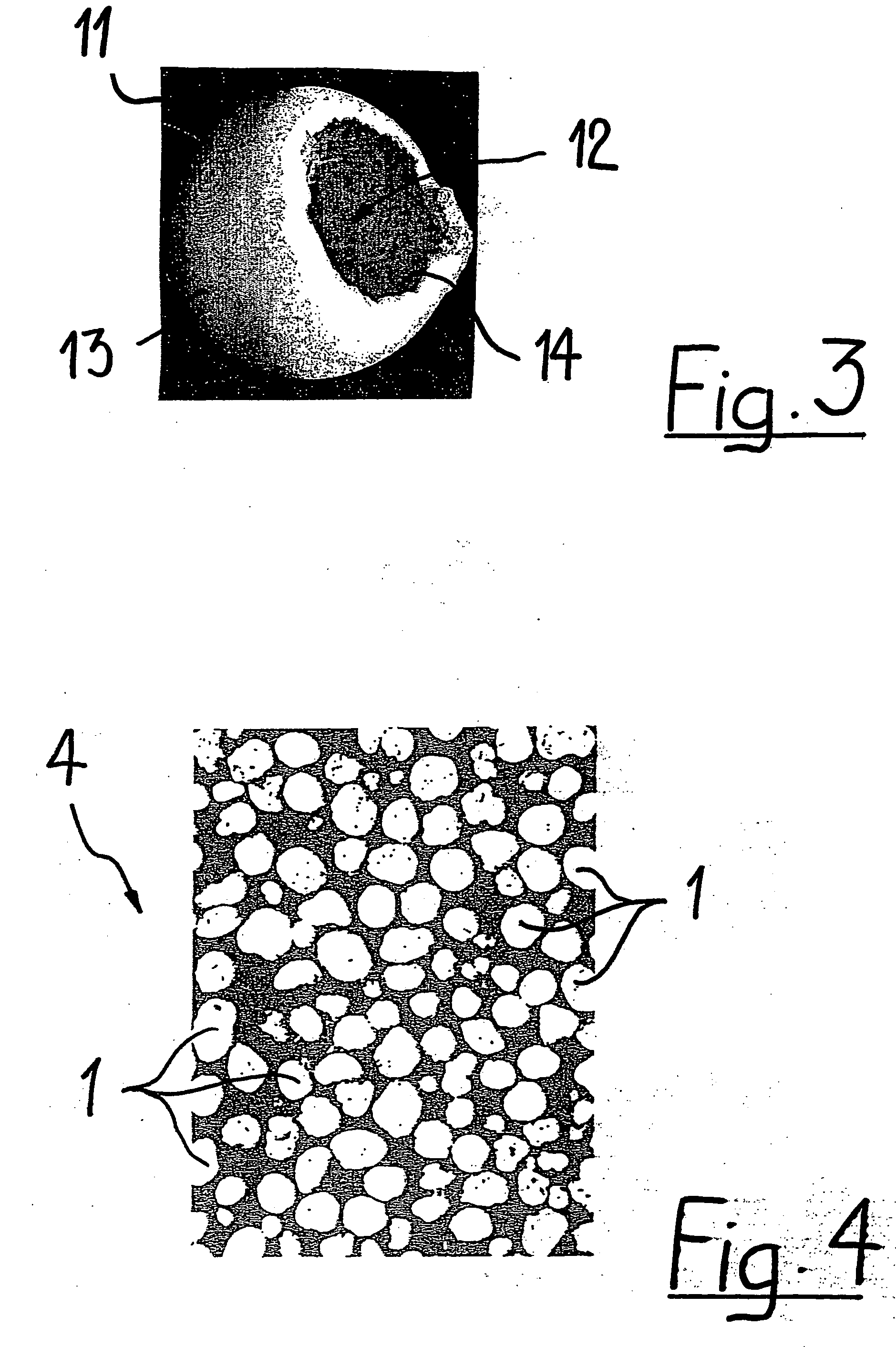
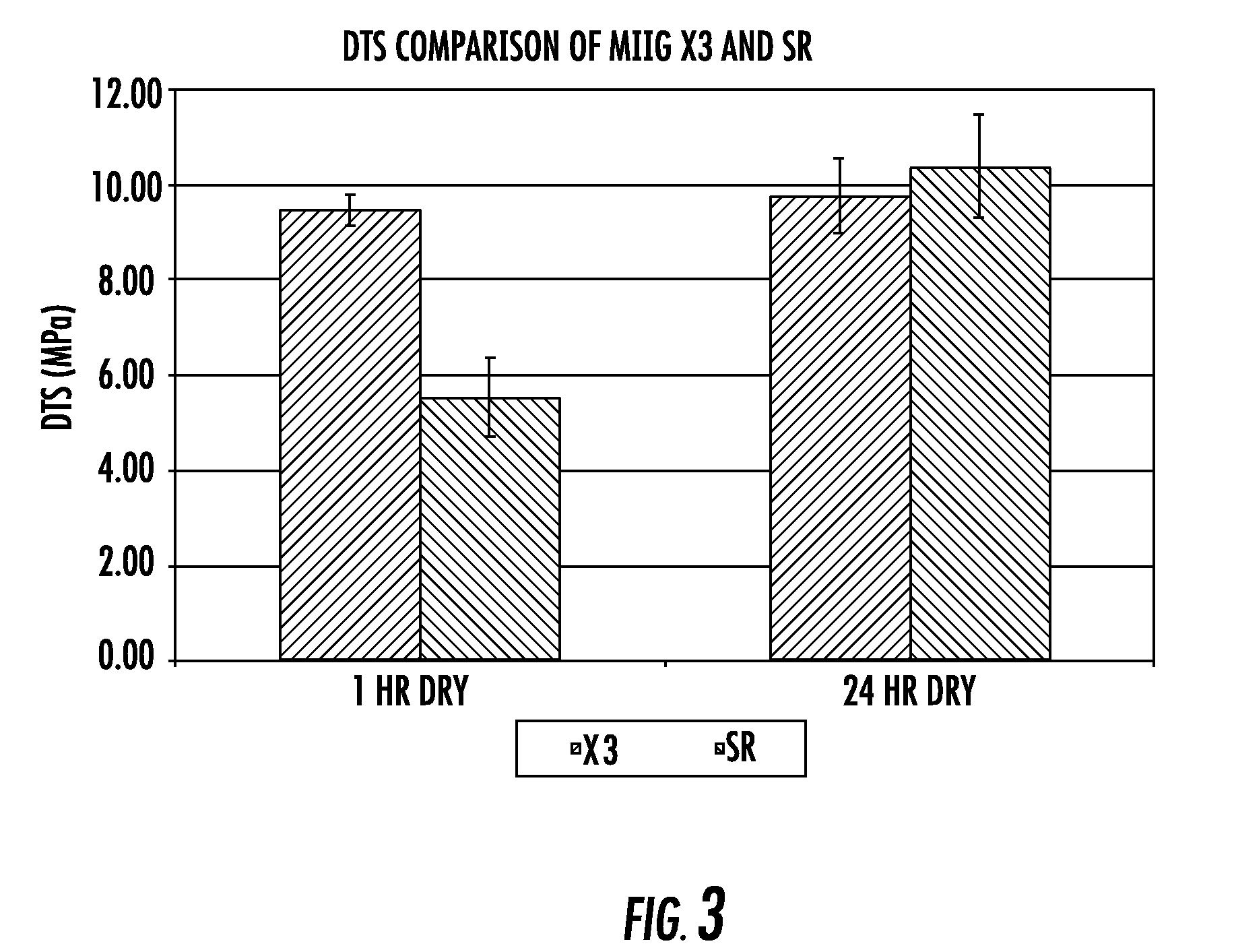
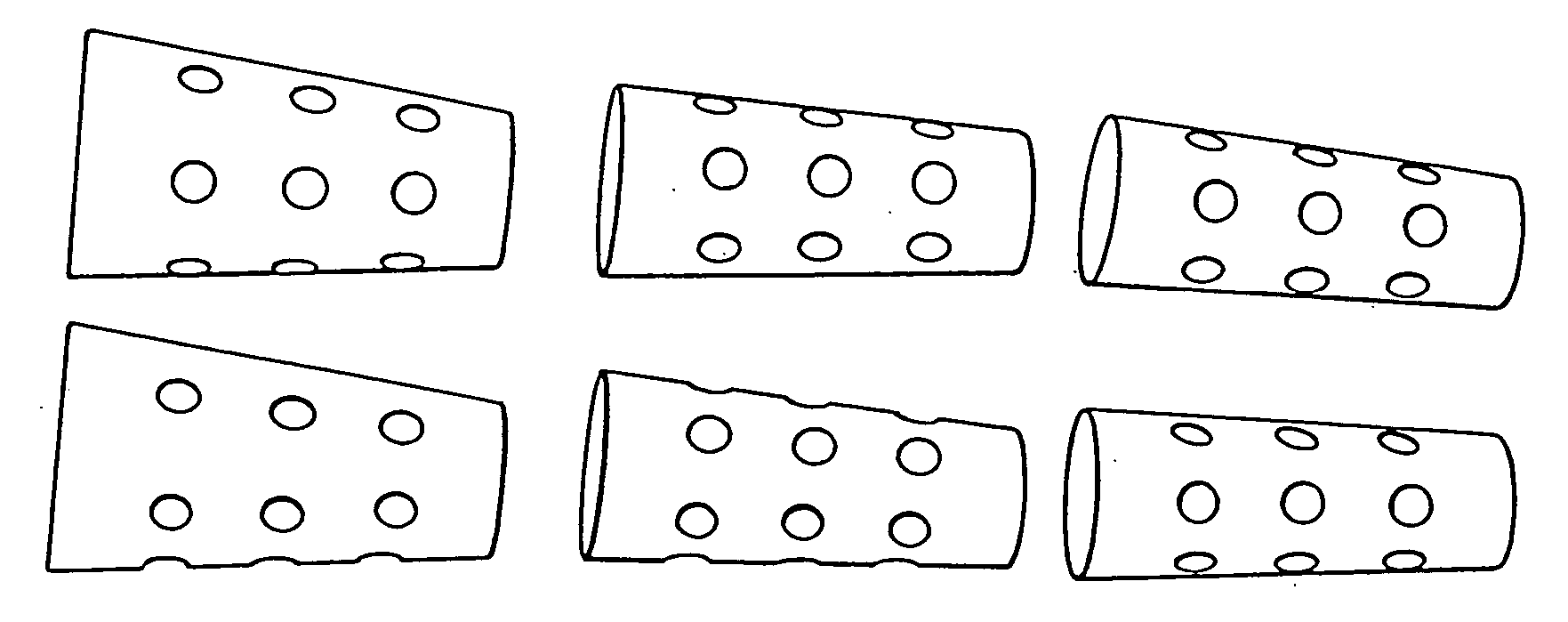
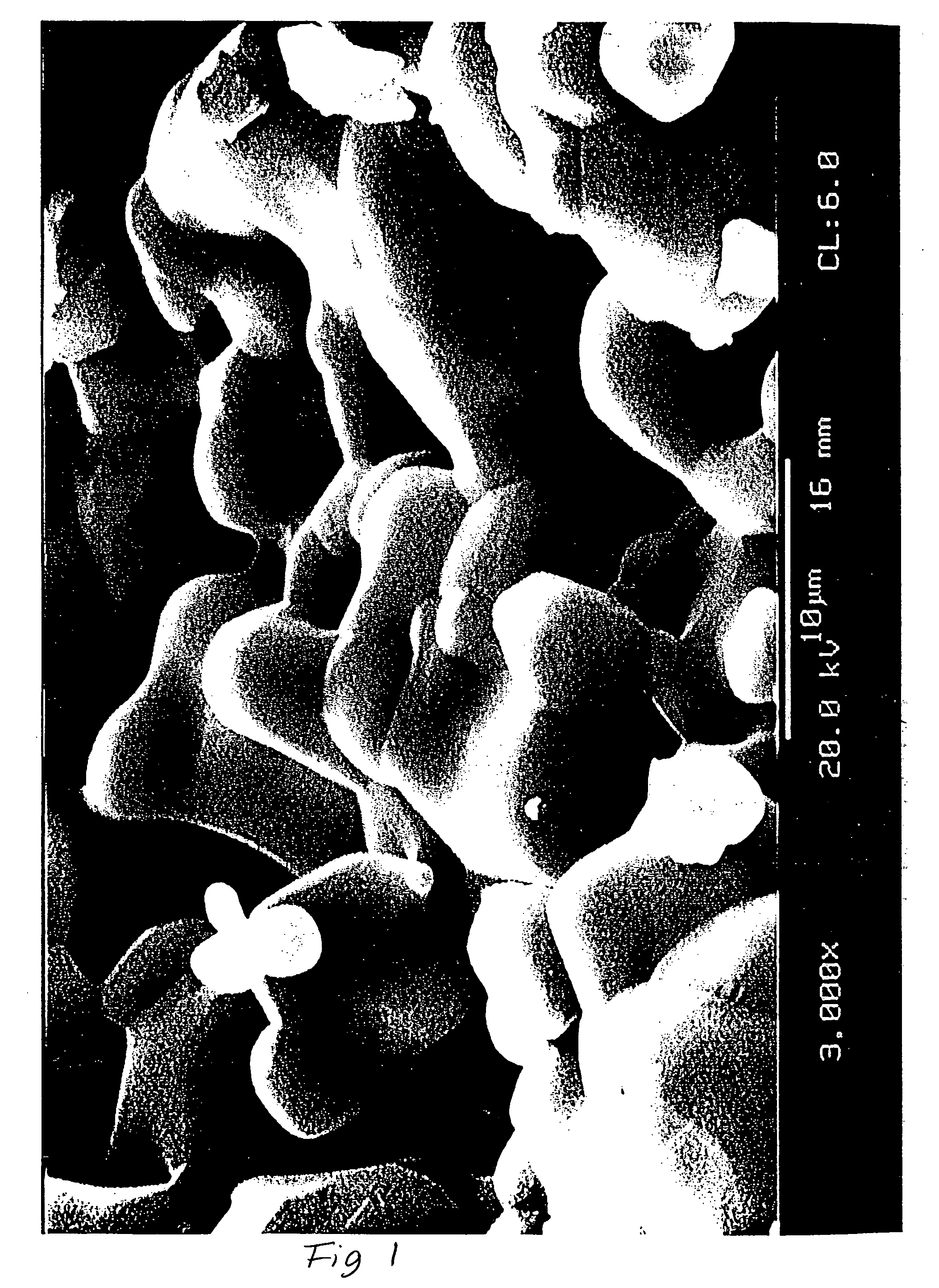
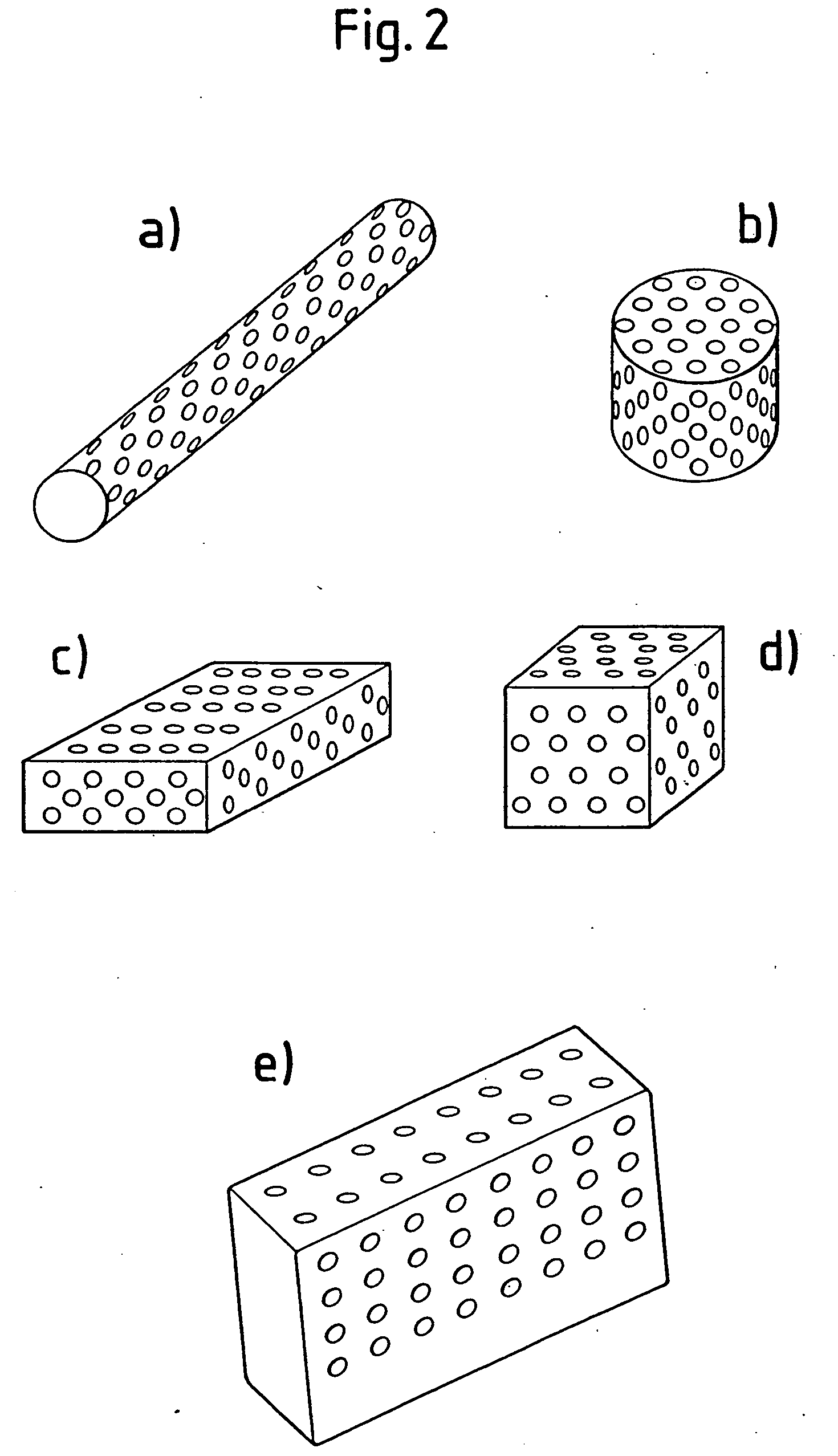
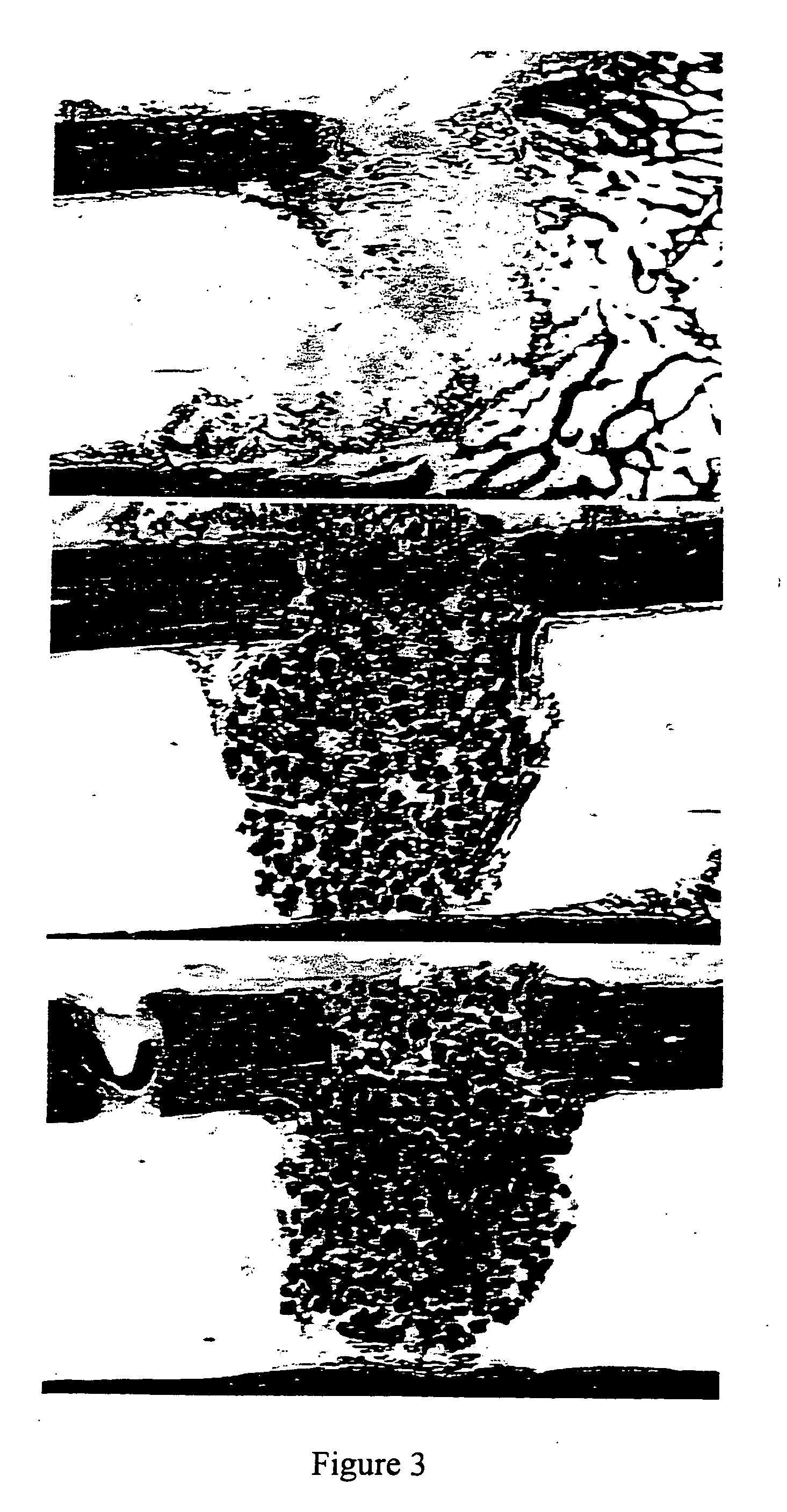
Porous β-tricalcium phosphate granules for regeneration of bone tissue
InactiveUS6949251B2Improve regenerative abilitySurgical adhesivesSkeletal disorderActive agentBone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Osseointegrative surgical implant
InactiveUS20160015483A1Improve primary stabilityPromote healingSuture equipmentsDental implantsCeramic compositeSurgical implant
Embodiments of the present invention provide an osseointegrative implant and related tools, components and fabrication techniques for surgical bone fixation and dental restoration purposes. In one embodiment an all-ceramic single-stage threaded or press-fit implant is provided having finely detailed surface features formed by ceramic injection molding and / or spark plasma sintering of a powder compact or green body comprising finely powdered zirconia. In another embodiment a two-stage threaded implant is provided having an exterior shell or body formed substantially entirely of ceramic and / or CNT-reinforced ceramic composite material. The implant may include one or more frictionally anisotropic bone-engaging surfaces. In another embodiment a densely sintered ceramic implant is provided wherein, prior to sintering, the porous debound green body is exposed to ions and / or particles of silver, gold, titanium, zirconia, YSZ, α-tricalcium phosphate, hydroxyapatite, carbon, carbon nanotubes, and / or other particles which remain lodged in the implant surface after sintering. Optionally, at least the supragingival portions of an all-ceramic implant are configured to have high translucence in the visible light range. Optionally, at least the bone-engaging portions of an all-ceramic implant are coated with a fused layer of titanium oxide.
Owner:OSSEODYNE SURGICAL SOLUTIONS LLC
Porous biocompatible implant material and method for its fabrication
a biocompatible and biodegradable implant for a cavity in a bone of a living organism is made of a biocompatible and biodegradable granules which are selected from the group including biopolymers, bioglasses, bioceramics preferably calcium sulfate, calcium phosphate such as monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, tetracalcium phosphate, calcium orthophosphate phosphate, α-tricalcium phosphate, β-tricalcium phosphate, apatite such as hydroxyapatite, or a mixture thereof. The biocompatible and biodegradable granules are provided with a coating, which comprises at least one layer of a biocompatible and biodegradable polymer which is selected from the group including poly((α-hydroxyesters), poly(orthoesters), polyanhydrides, poly(phosphazenes), poly(propylene fumarate), poly(ester amides), poly(ethylene fumarate), polylactide, polyglycolide, polycaprolactone, poly(glycolide-co-trimethylene carbonate), polydioxanone, co-polymers thereof and blends of those polymers. The biocompatible and biodegradable implants are obtained by fusing together the polymer-coated granules through polymer-linkage of the polymer coatings of neighboring granules.
Owner:COLLAGEN MATRIX
Porous beta-tricalcium phosphate granules for regeneration of bone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
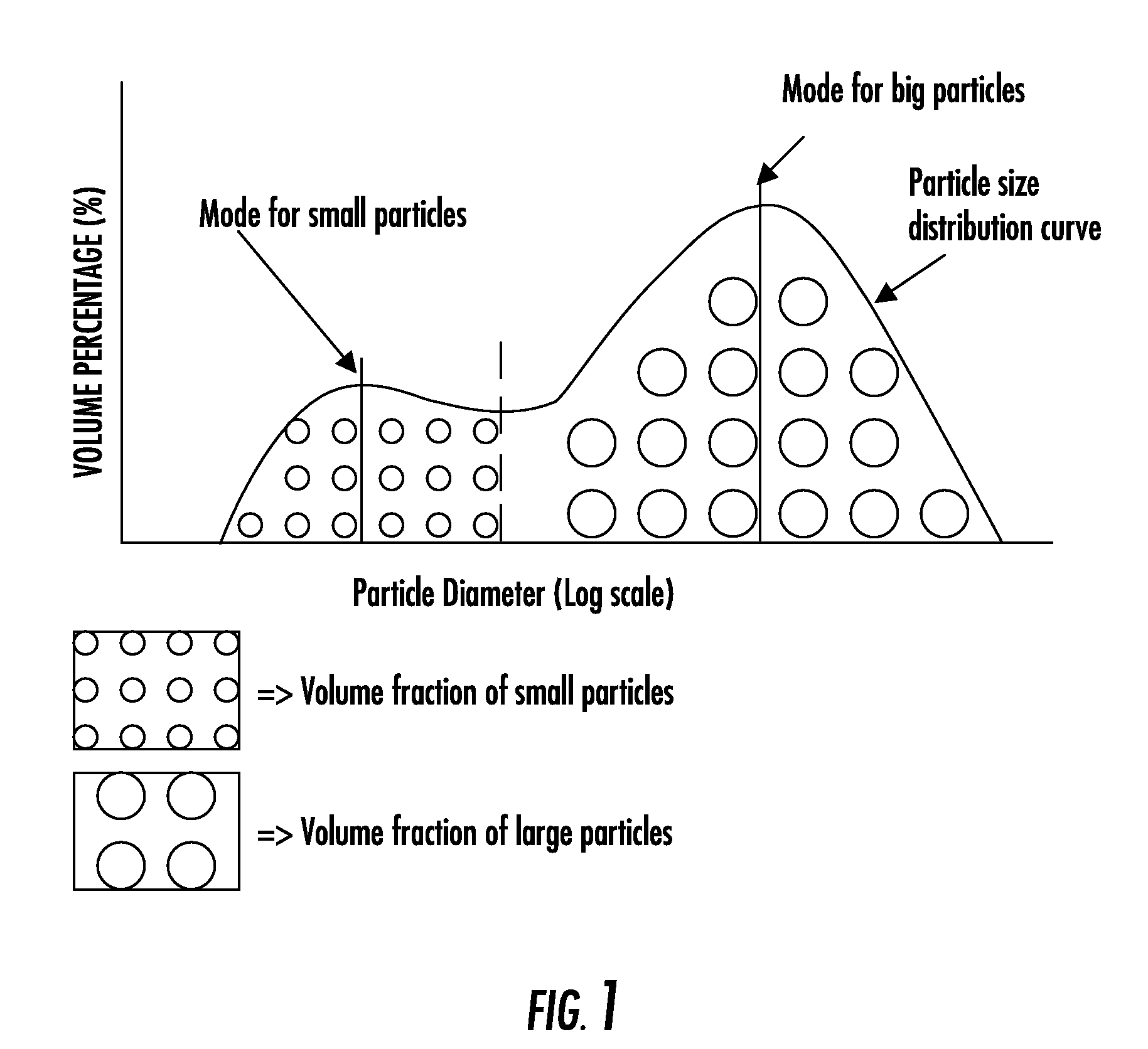
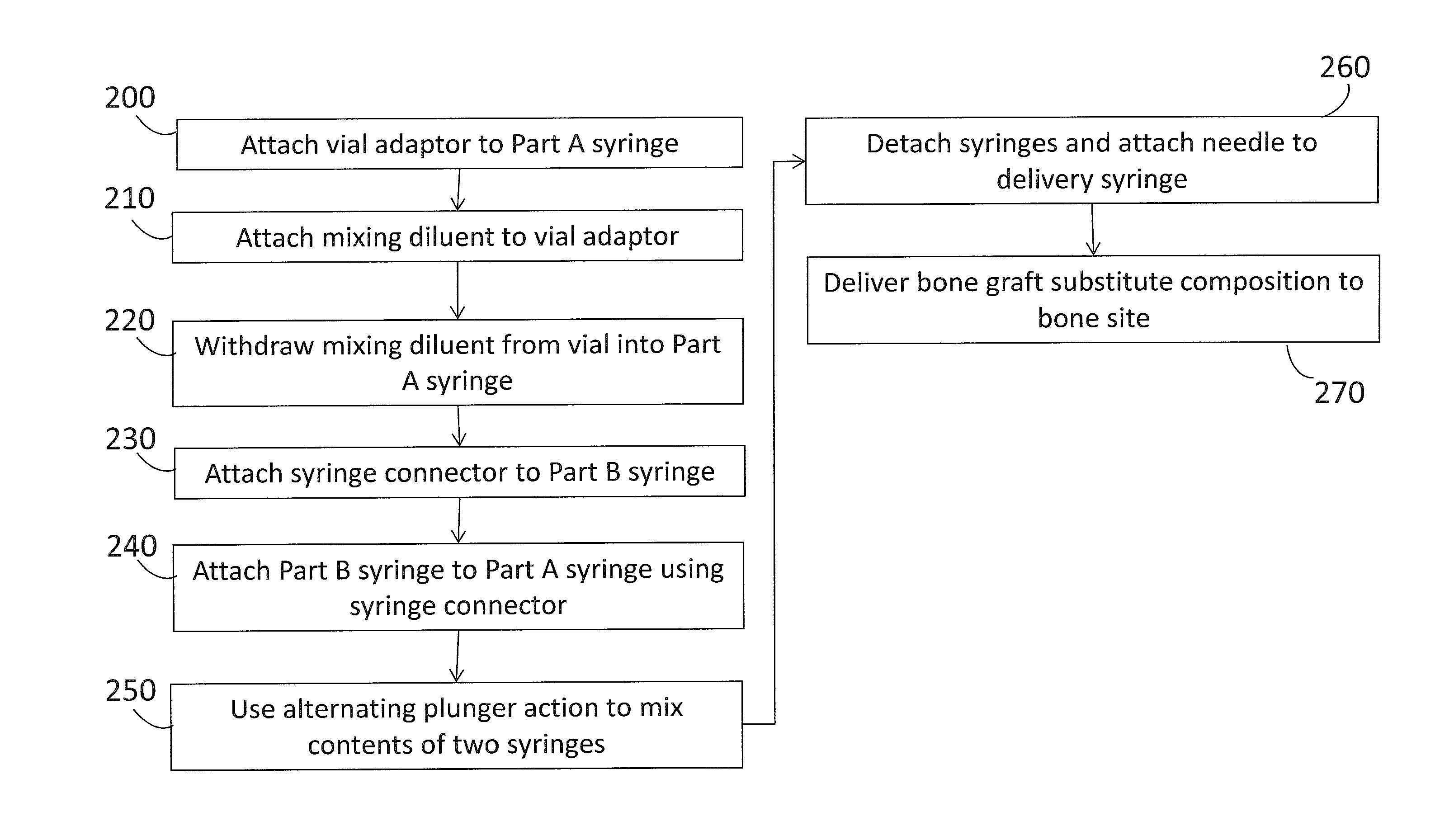
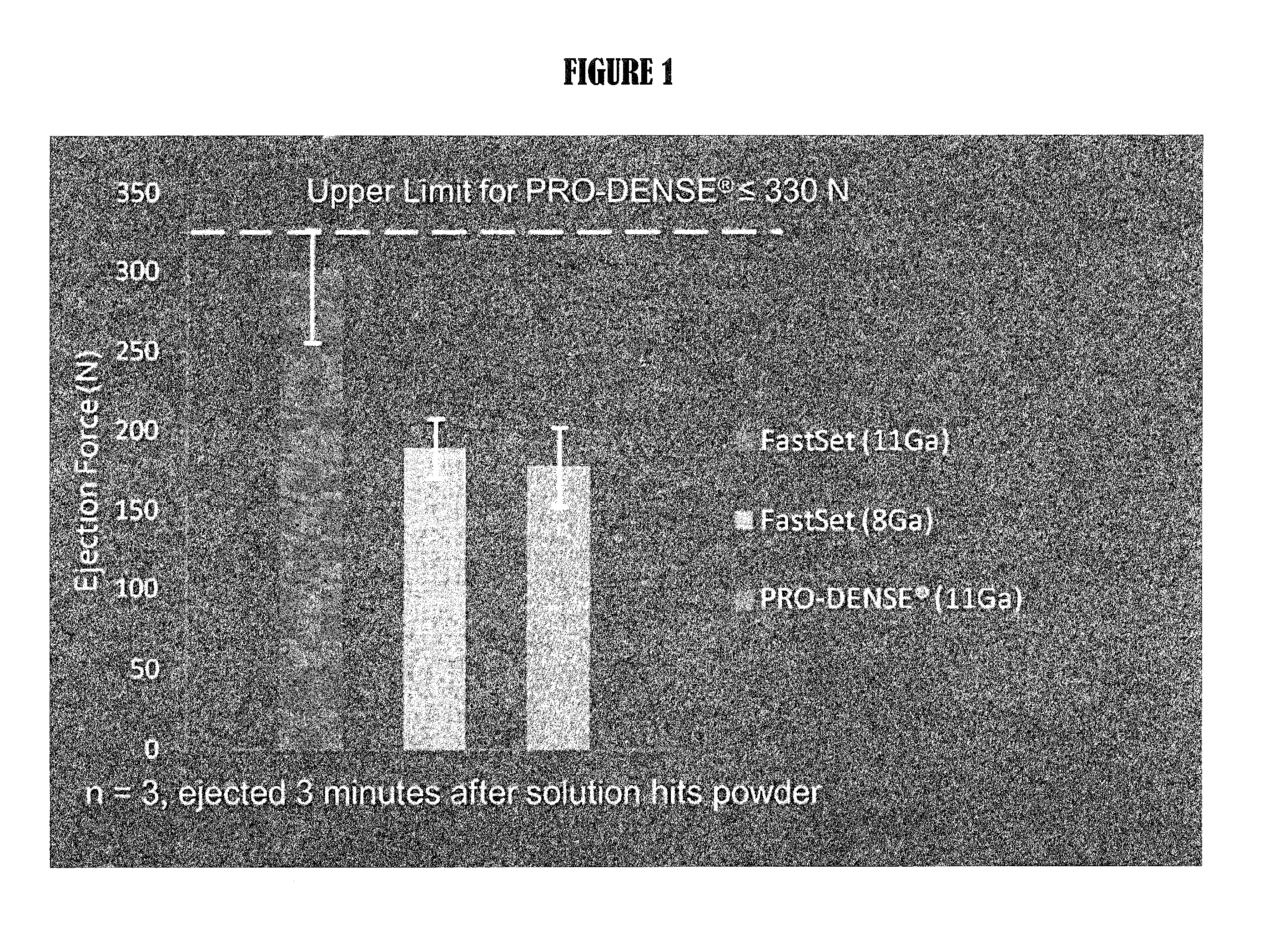
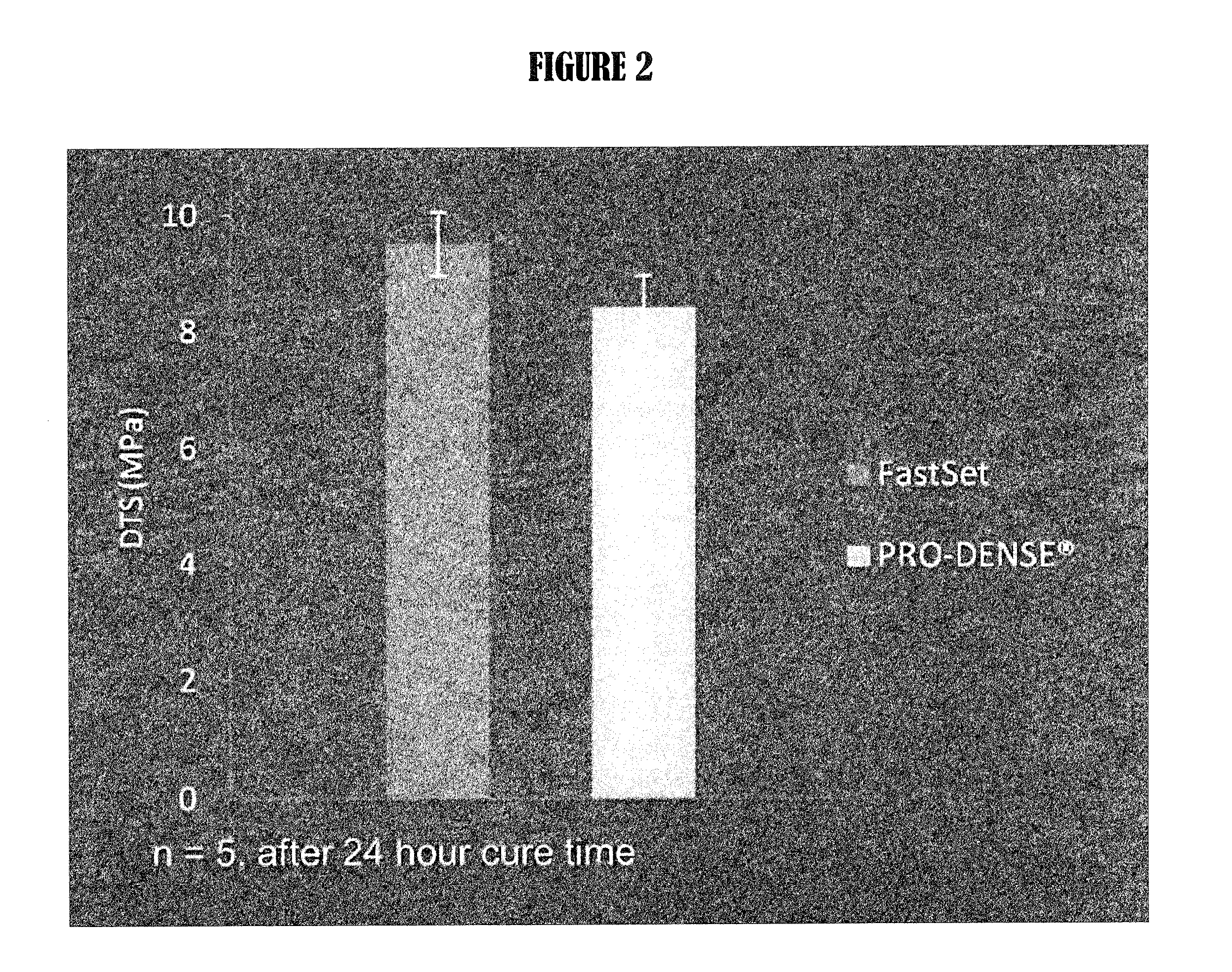
Composite bone graft substitute cement and articles produced therefrom
ActiveUS7754246B2High mechanical strengthQuick changeAntibacterial agentsPeptide/protein ingredientsParticulatesTri calcium phosphate
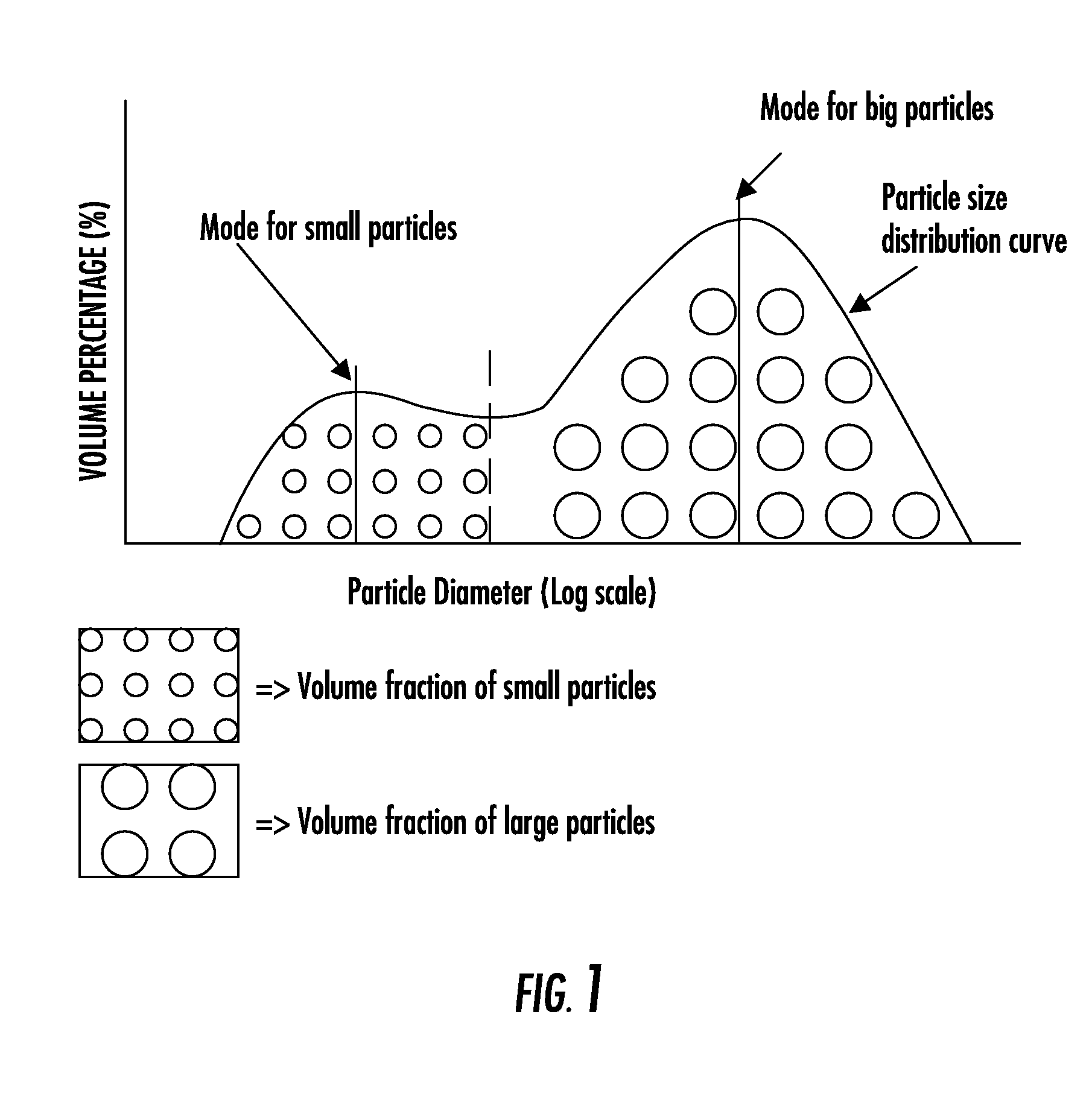
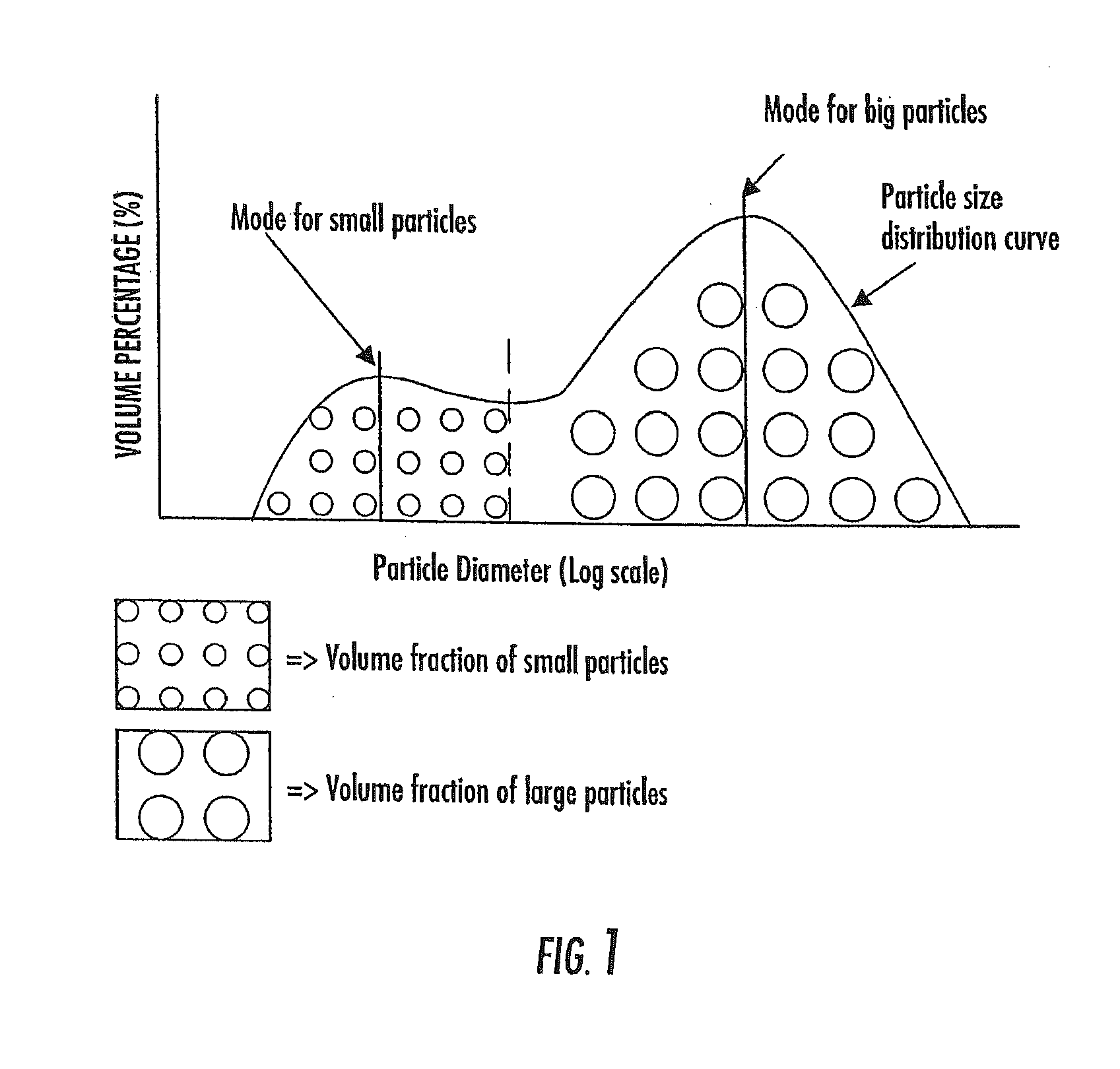
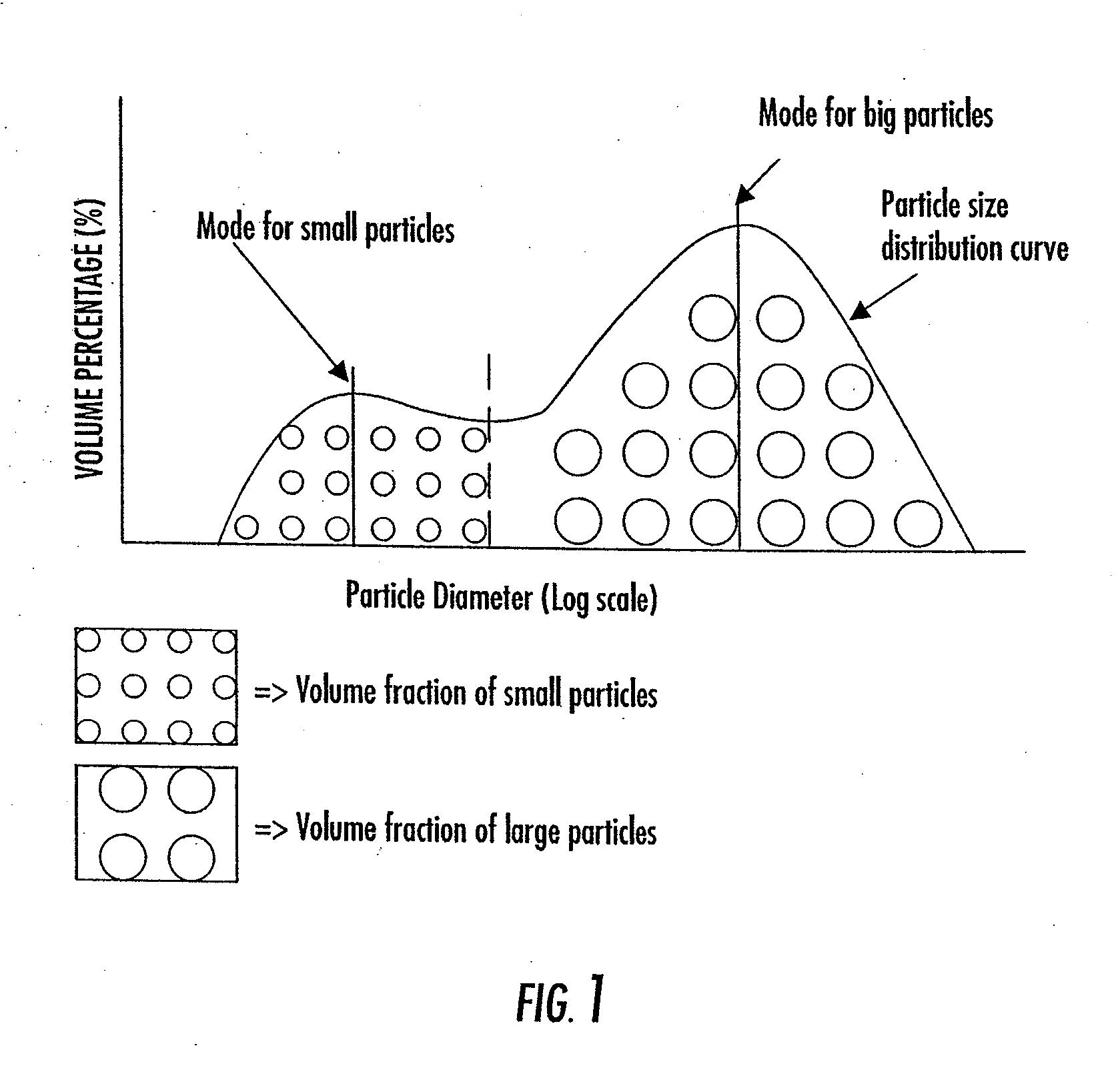
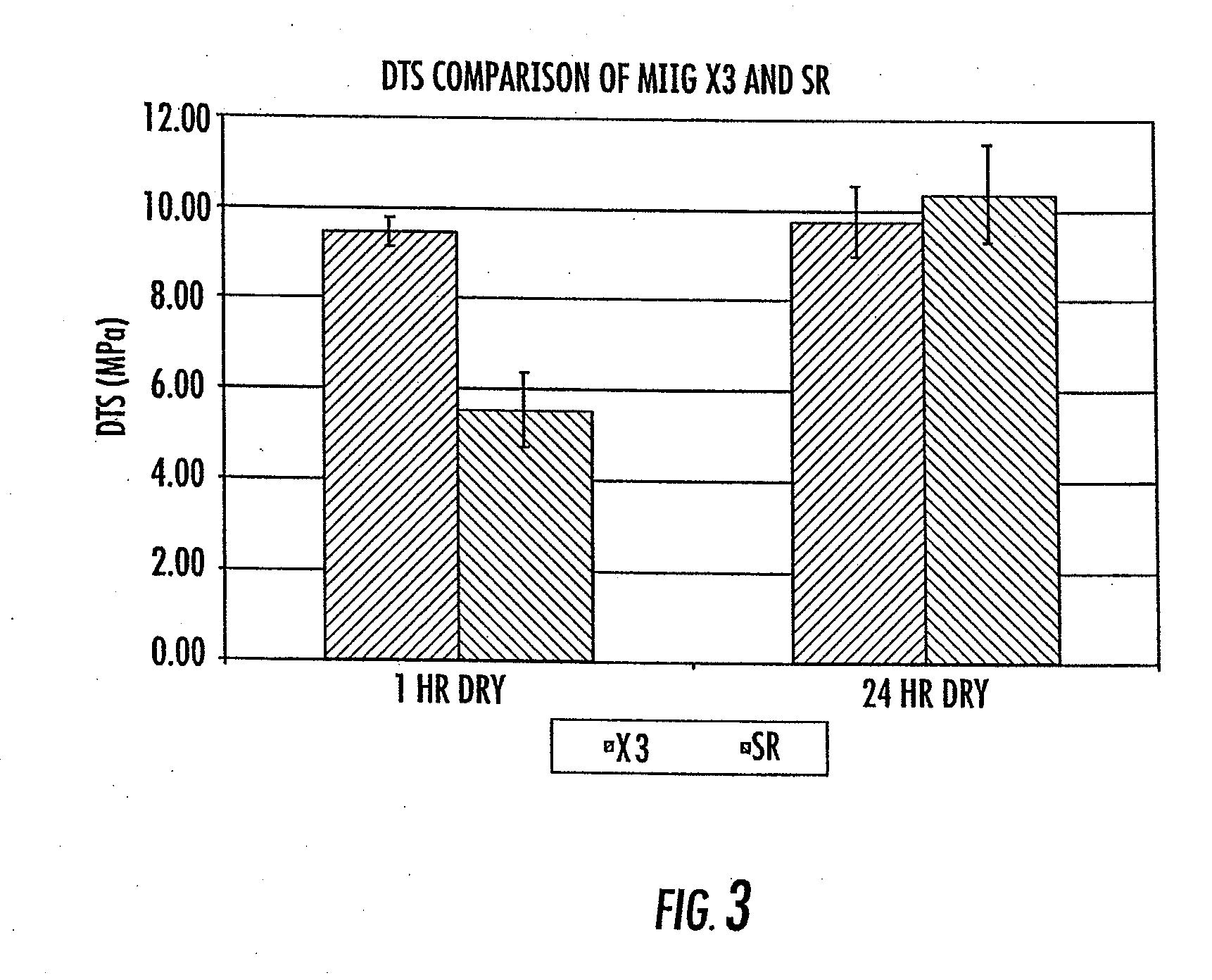
The invention provides a particulate composition adapted for forming a bone graft substitute cement upon mixing with an aqueous solution, including i) a calcium sulfate hemihydrate powder having a bimodal particle distribution and a median particle size of about 5 to about 20 microns, wherein the calcium sulfate hemihydrate is present at a concentration of at least about 70 weight percent based on the total weight of the particulate composition; ii) a monocalcium phosphate monohydrate powder; and iii) a β-tricalcium phosphate powder having a median particle size of less than about 20 microns. Bone graft substitute cements made therefrom, a bone graft substitute kit comprising the particulate composition, methods of making and using the particulate composition, and articles made from the bone graft substitute cement are also provided.
Owner:AGNOVOS HEALTHCARE
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous β-tricalcium phosphate (β-TCP).
Owner:CURASAN
Porous beta-tricalcium phosphate granules for regeneration of bone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous β-tricalcium phosphate (β-TCP).
Owner:CURASAN
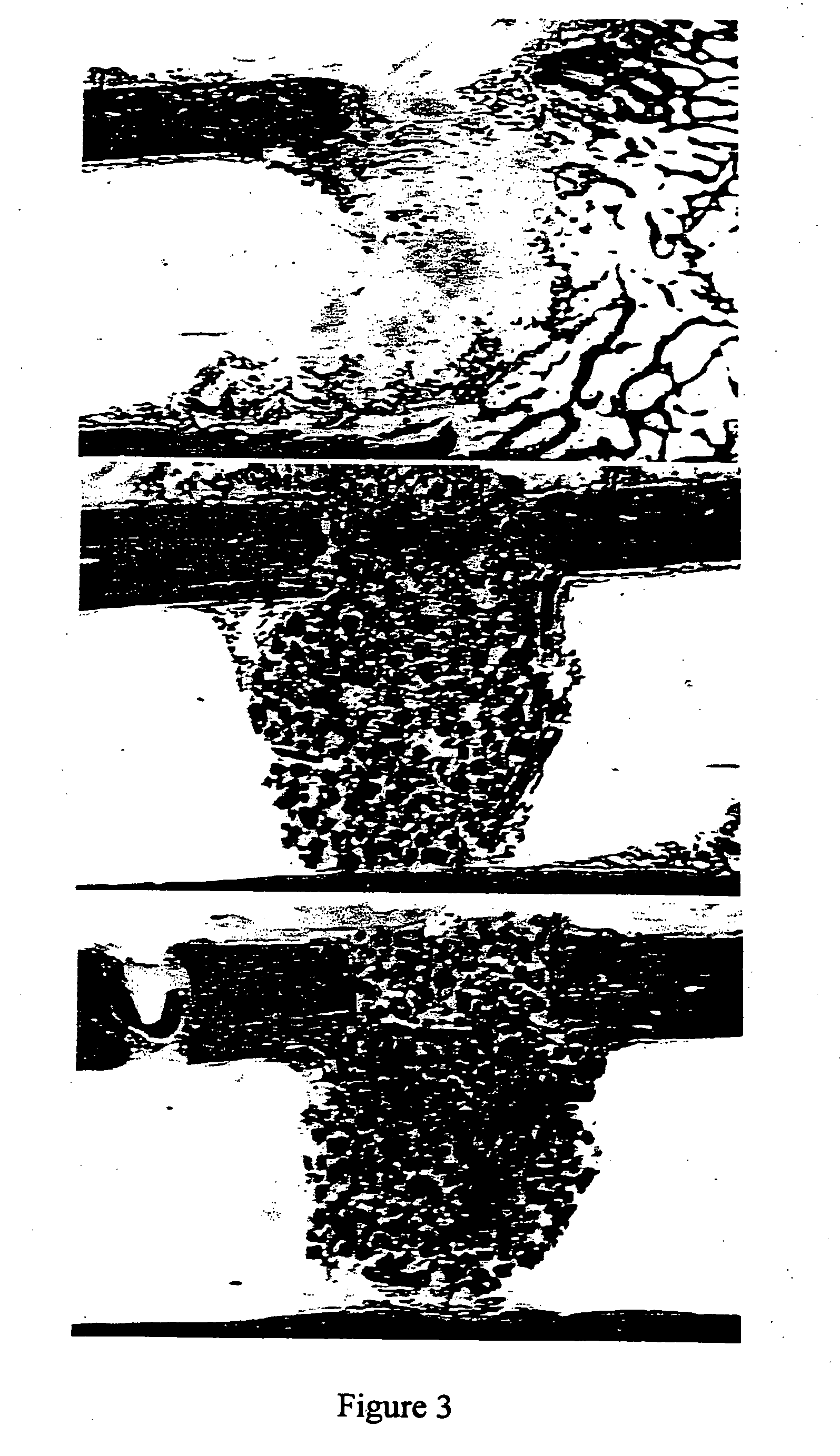
Porous beta-tricalcium phosphate and methods for producing the same
Methods are provided for producing porous β-Tricalcium Phosphate (β-TCP). In the subject methods, β-TCP is combined with graphite to produce an intermediate greenware product. The intermediate greenware product is then sintered to produce porous β-TCP. The subject methods and compositions produced thereby find use in a variety of applications.
Owner:SKELETAL KINETICS
Dentinal tubule sealant and method for producing the same
InactiveUS20130189337A1Impart caries resistanceSubstance may accumulateCosmetic preparationsImpression capsCalcium biphosphateDentinal Tubule
A dentinal tubule sealant comprises poorly-soluble calcium phosphate particles (A), a phosphorus-free calcium compound (B), and water (C), wherein the particles (A) are at least one member selected from the group consisting of dicalcium phosphate anhydrous [CaHPO4] particles, α-tricalcium phosphate [α-Ca3(PO4)2] particles, β-tricalcium phosphate [β-Ca3(PO4)2] particles, amorphous calcium phosphate [Ca3(PO4)2.nH2O] particles, calcium pyrophosphate [Ca2P2O7] particles, calcium pyrophosphate dihydrate [Ca2P2O7.2H2O] particles, octacalcium phosphate pentahydrate [Ca8H2(PO4)6.5H2O] particles, and dicalcium phosphate dihydrate [CaHPO4.2H2O] particles, and the dentinal tubule sealant contains 30 to 76% by weight of the particles (A), 0.001 to 4% by weight of the compound (B), and 23 to 69% by weight of the water (C). Thus, there is provided a dentinal tubule sealant capable of sealing dentinal tubules of an exposed dentin and also remineralizing the surrounding dentin after the sealing.
Owner:KURARAY NORITAKE DENTAL
Biomaterial composite composition and method of use
This invention relates to a process to facilitate osteochondral bone remodeling in a subject by inducing regeneration of this bone to a healthy, vascularized state capable of supporting the underlying hyaline cartilage of articular joints and spinal discs, both biomechanically and metabolically and to deliver a bioactive agent. This process involves the steps of: administering an effective amount of an injectable in situ curing biomaterial composite to a site. The biomaterial composite product is prepared by a process involving the steps: admixing an alginate solution with a nonporous aggregate of β-tricalcium phosphate, in a sufficient amount to initiate polymerization of the alginate solution, to form a hydrogel having from between 10 to 20 percent by volume of β-tricalcium phosphate.
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Composite bone graft substitute cement and articles produced therefrom
The invention provides a particulate composition adapted for forming a bone graft substitute cement upon mixing with an aqueous solution, including i) a calcium sulfate hemihydrate powder having a bimodal particle distribution and a median particle size of about 5 to about 20 microns, wherein the calcium sulfate hemihydrate is present at a concentration of at least about 70 weight percent based on the total weight of the particulate composition; ii) a monocalcium phosphate monohydrate powder; and iii) a β-tricalcium phosphate powder having a median particle size of less than about 20 microns. Bone graft substitute cements made therefrom, a bone graft substitute kit comprising the particulate composition, methods of making and using the particulate composition, and articles made from the bone graft substitute cement are also provided.
Owner:AGNOVOS HEALTHCARE
Preparation and Applications of 3D Bioprinting Bioinks for Repair of Bone Defects, Based on Cellulose Nanofibrils Hydrogels with Natural or Synthetic Calcium Phosphate Particles
ActiveUS20190307923A1Induce bone formationRepair large defectPharmaceutical delivery mechanismSkeletal/connective tissue cellsPorosityBiopolymer
The present invention relates to preparation of bioink composed of cellulose nanofibril hydrogel with native or synthetic Calcium containing particles. The concentration of the calcium containing particles can be between 1% and 40% w / v. Such bioink can be 3D Bioprinted with or without human or animal cells. Coaxial needle can be used where cellulose nanofibril hydrogel filled with Calcium particles can be used as shell and another hydrogel based bioink mixed with cells can be used as core or opposite. Such 3D Bioprinted constructs exhibit high porosity due to shear thinning properties of cellulose nanofibrils which provides excellent printing fidelity. They also have excellent mechanical properties and are easily handled as large constructs for patient-specific bone cavities which need to be repaired. The porosity promotes vascularization which is crucial for oxygen and nutrient supply. The porosity also makes it possible for further recruitment of cells which accelerate bone healing process. Calcium containing particles can be isolated from autologous bone, allogenic bone or xenogeneic bone but can be also isolated from minerals or be prepared by synthesis. Preferable Calcium containing particles consist of β-tricalcium phosphate which is resorbable or natural bone powder, preferably of human or porcine origin. The particles described in the present invention have particle size smaller than 400 microns, or more preferably smaller than 200 microns, to make it possible to handle in printing nozzle without clogging and to obtain a good resolution. Cellulose nanofibrils can be produced by bacteria orbe isolated from plants. They can be neutral, charged or oxidized to be biodegradable. The bioink can be additionally supplemented by other biopolymers which provide crosslinking. Such biopolymers can be alginates, chitosans, modified hyaluronic acid or modified collagen derived biopolymers.
Owner:CELLINK AB
Composite Bone Graft Substitute Cement and Articles Produced Therefrom
ActiveUS20070128248A1High mechanical strengthQuick changeBone implantSkeletal disorderParticulatesSulfate
The invention provides a particulate composition adapted for forming a bone graft substitute cement upon mixing with an aqueous solution, including i) a calcium sulfate hemihydrate powder having a bimodal particle distribution and a median particle size of about 5 to about 20 microns, wherein the calcium sulfate hemihydrate is present at a concentration of at least about 70 weight percent based on the total weight of the particulate composition; ii) a monocalcium phosphate monohydrate powder; and iii) a β-tricalcium phosphate powder having a median particle size of less than about 20 microns. Bone graft substitute cements made therefrom, a bone graft substitute kit comprising the particulate composition, methods of making and using the particulate composition, and articles made from the bone graft substitute cement are also provided.
Owner:AGNOVOS HEALTHCARE
Composite bone graft substitute cement and articles produced therefrom
ActiveUS20120061285A1High mechanical strengthQuick changeSurgical adhesivesInorganic phosphorous active ingredientsParticulatesSulfate
The invention provides a particulate composition adapted for forming a bone graft substitute cement upon mixing with an aqueous solution, including i) a calcium sulfate hemihydrate powder having a bimodal particle distribution and a median particle size of about 5 to about 20 microns, wherein the calcium sulfate hemihydrate is present at a concentration of at least about 70 weight percent based on the total weight of the particulate composition; ii) a monocalcium phosphate monohydrate powder; and iii) a β-tricalcium phosphate powder having a median particle size of less than about 20 microns. Bone graft substitute cements made therefrom, a bone graft substitute kit comprising the particulate composition, methods of making and using the particulate composition, and articles made from the bone graft substitute cement are also provided.
Owner:AGNOVOS HEALTHCARE
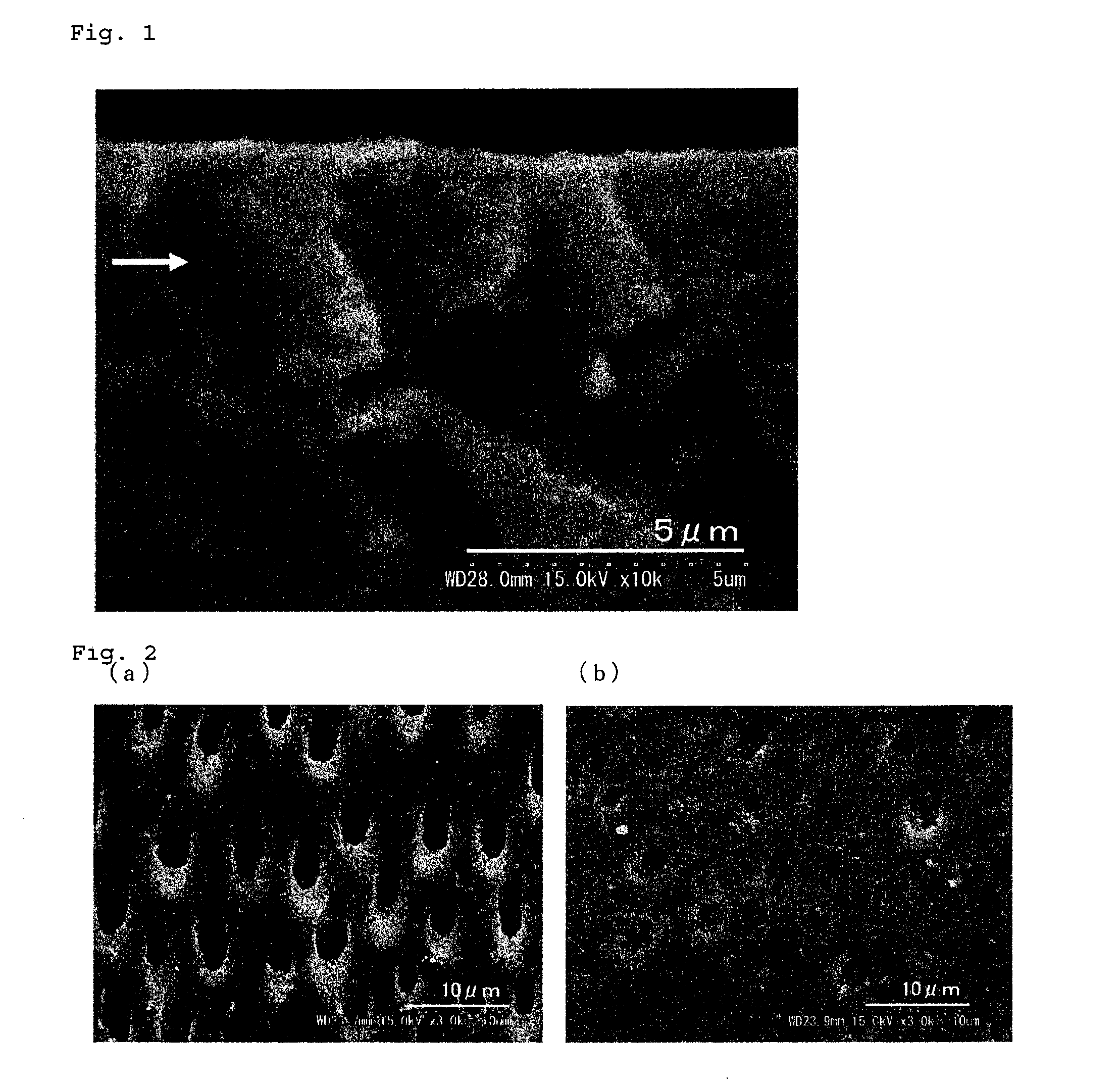
Porous composite comprising silicon-substituted hydroxyapatite and beta-tricalcium phosphate, and process for preparing the same
InactiveUS20100094419A1Good biocompatibilityInherent in structureBone implantTissue regenerationApatiteBiocompatibility Testing
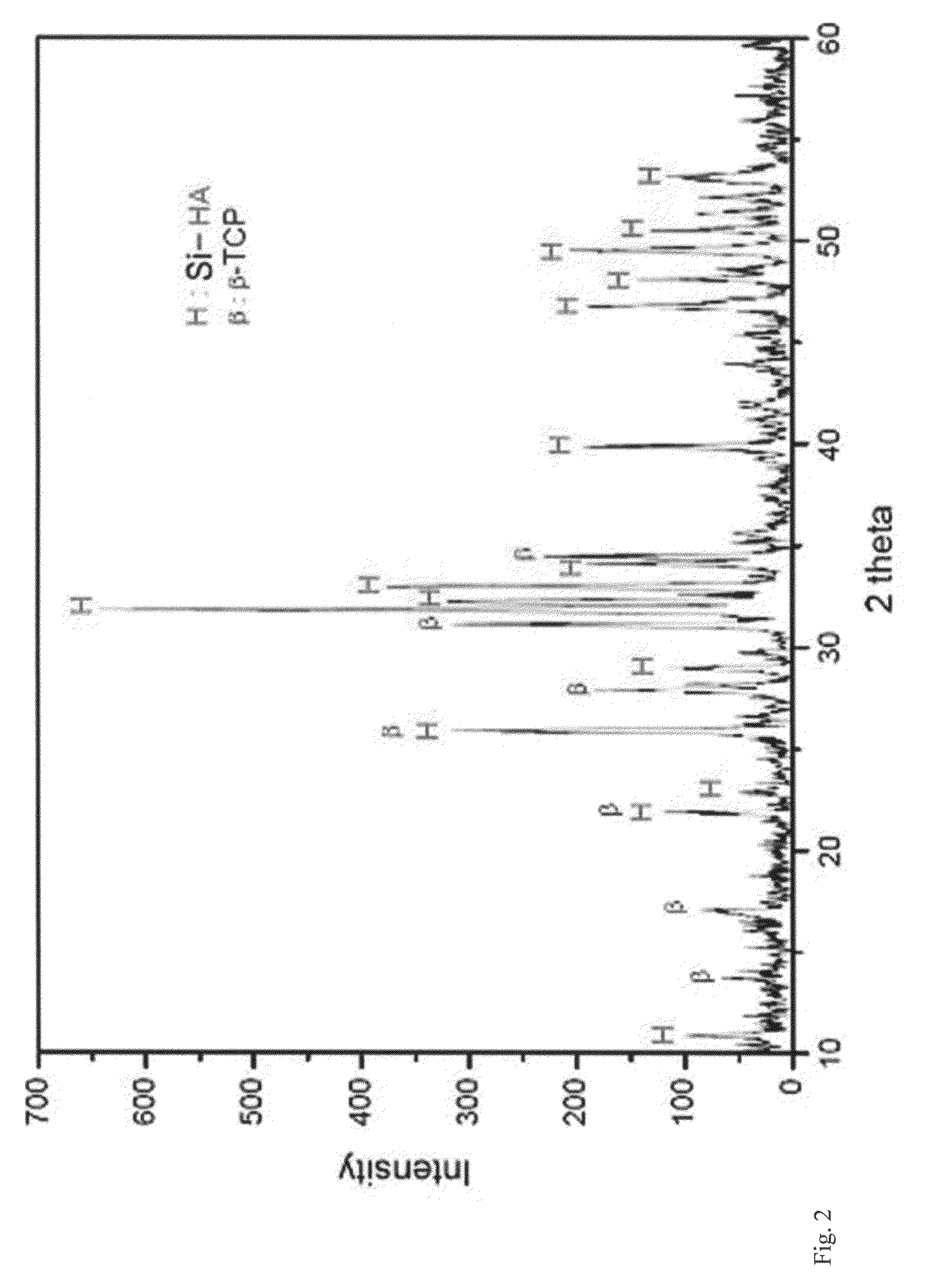
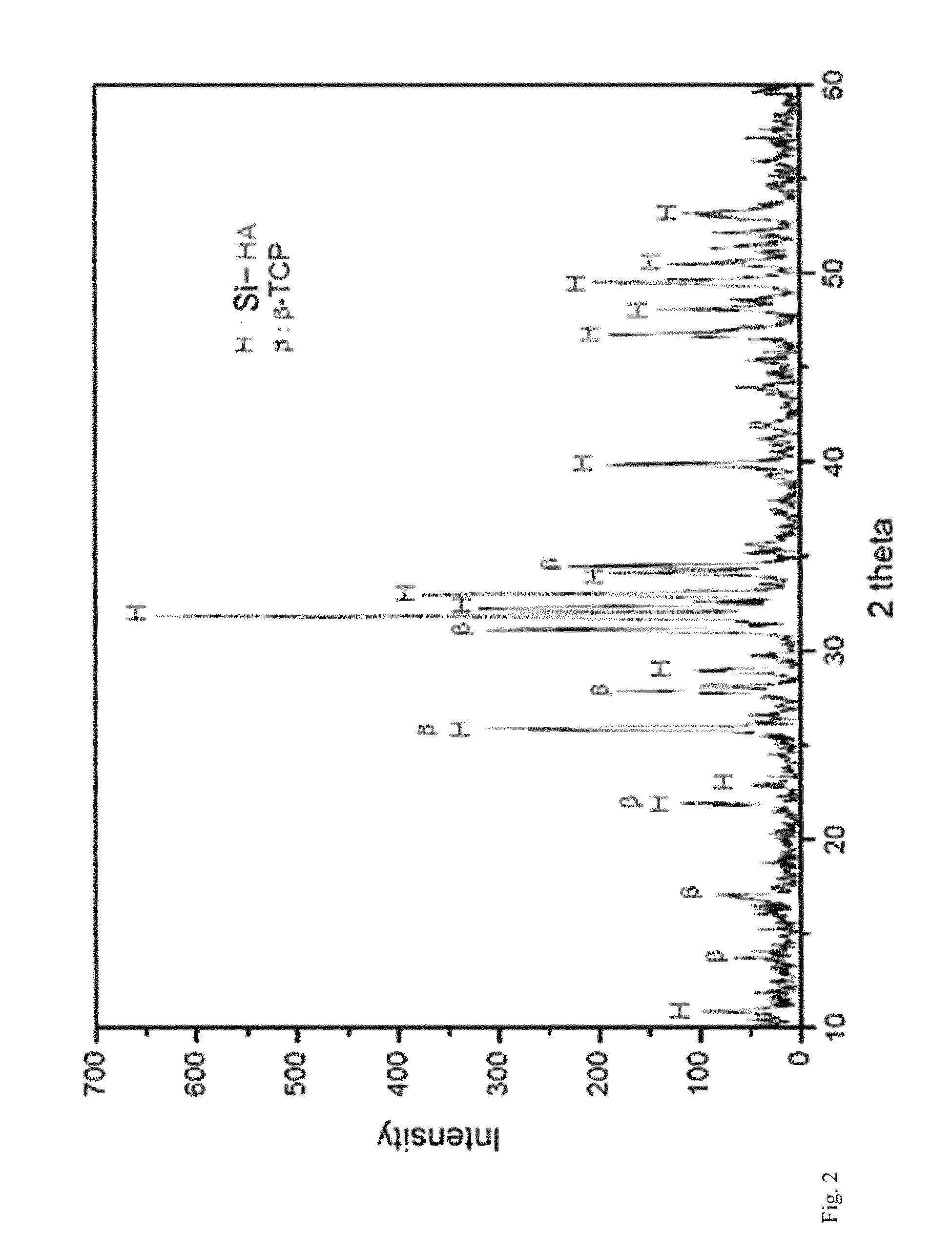
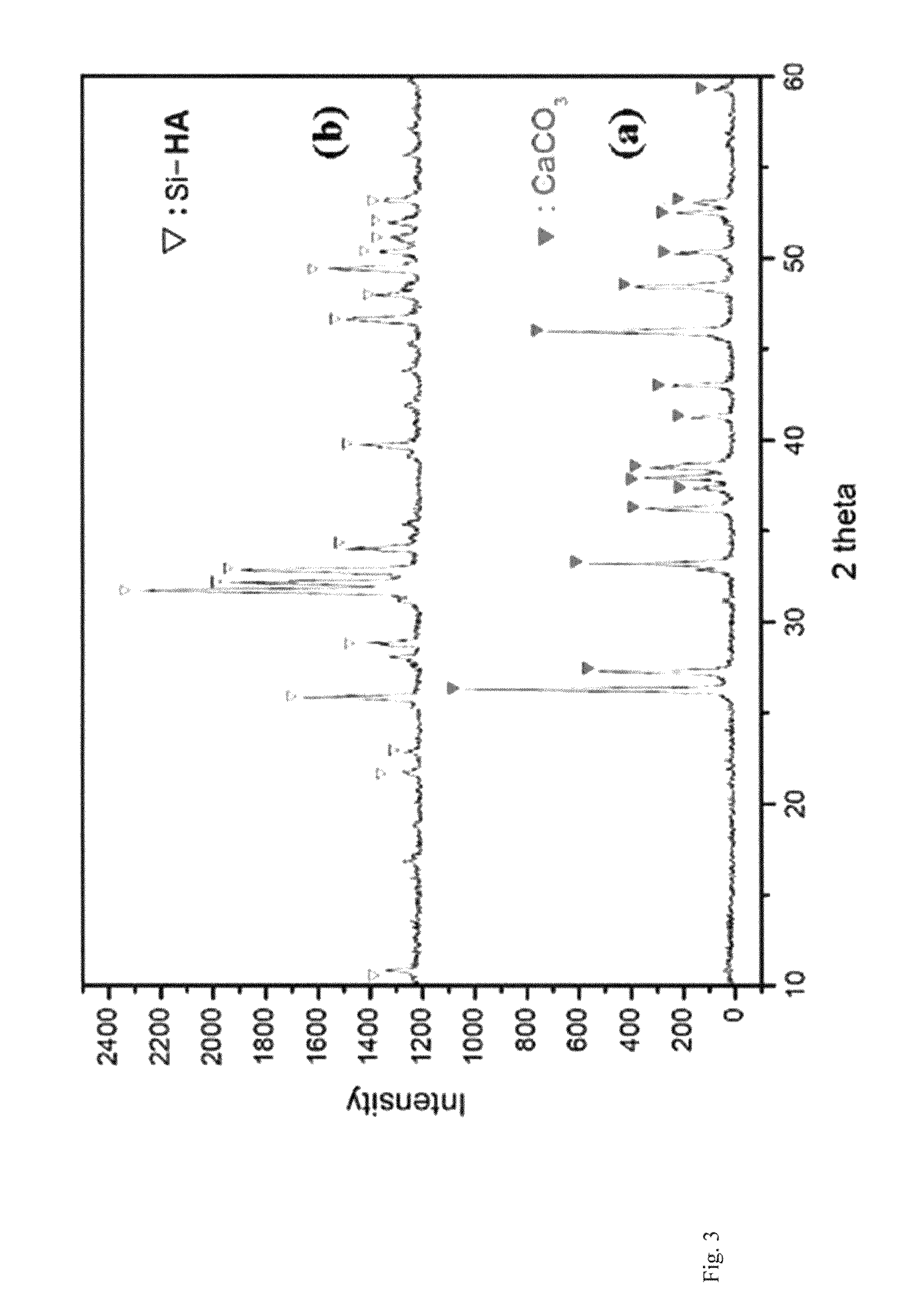
There are disclosed a porous composite comprising silicon-substituted hydroxyapatite and β-tricalcium phosphate (β-TCP), and a method for preparing the same. The porous composite is prepared by subjecting natural coral to hydrothermal and solvothermal reactions to prepare silicon-substituted hydroxyapatite (Si-HA) and subjecting the Si-HA to a heat treatment process. Thereafter, Si-HA and β-TCP may be mixed in the porous composite. As a result, the porous composite is excellent in biocompatibility and biodegradability. Also, the porous composite functions to maintain the microstructure of coral and is similar to natural bone in terms of composition and shape. Accordingly, the porous composite may be effectively used as a bone tissue repairing material and a bone graft material that can substitute for human hard tissue.
Owner:METABIOMED
Composite Bone Graft Substitute Cement and Articles Produced Therefrom
ActiveUS20070178171A1High mechanical strengthQuick changeBiocideCapsParticulatesΒ tricalcium phosphate
The invention provides a particulate composition adapted for forming a bone graft substitute cement upon mixing with an aqueous solution, including i) a calcium sulfate hemihydrate powder having a bimodal particle distribution and a median particle size of about 5 to about 20 microns, wherein the calcium sulfate hemihydrate is present at a concentration of at least about 70 weight percent based on the total weight of the particulate composition; ii) a monocalcium phosphate monohydrate powder; and iii) a β-tricalcium phosphate powder having a median particle size of less than about 20 microns. Bone graft substitute cements made therefrom, a bone graft substitute kit comprising the particulate composition, methods of making and using the particulate composition, and articles made from the bone graft substitute cement are also provided.
Owner:AGNOVOS HEALTHCARE
RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) / β tricalcium phosphate composite material and preparation method thereof
InactiveUS7989532B2Good biocompatibilityHigh affinityConnective tissue peptidesFibrinogenArginineTyrosine
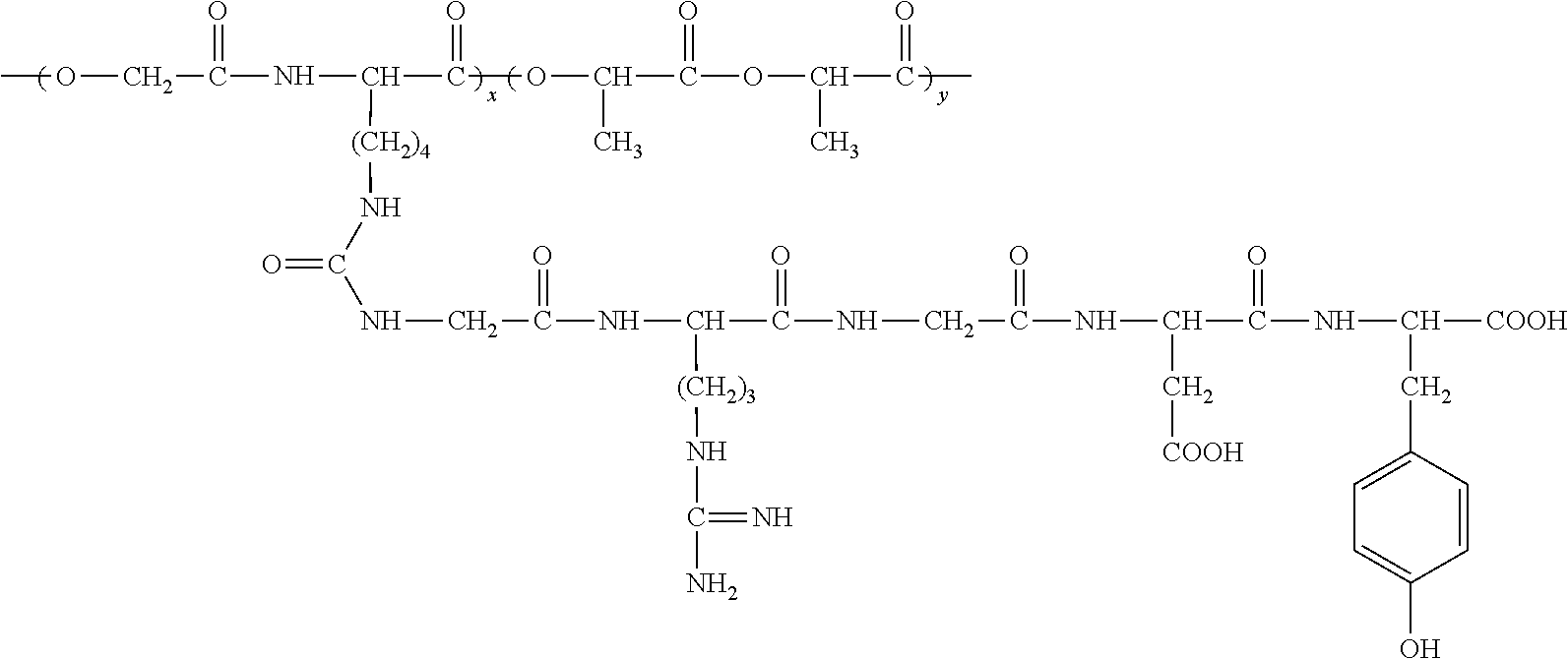
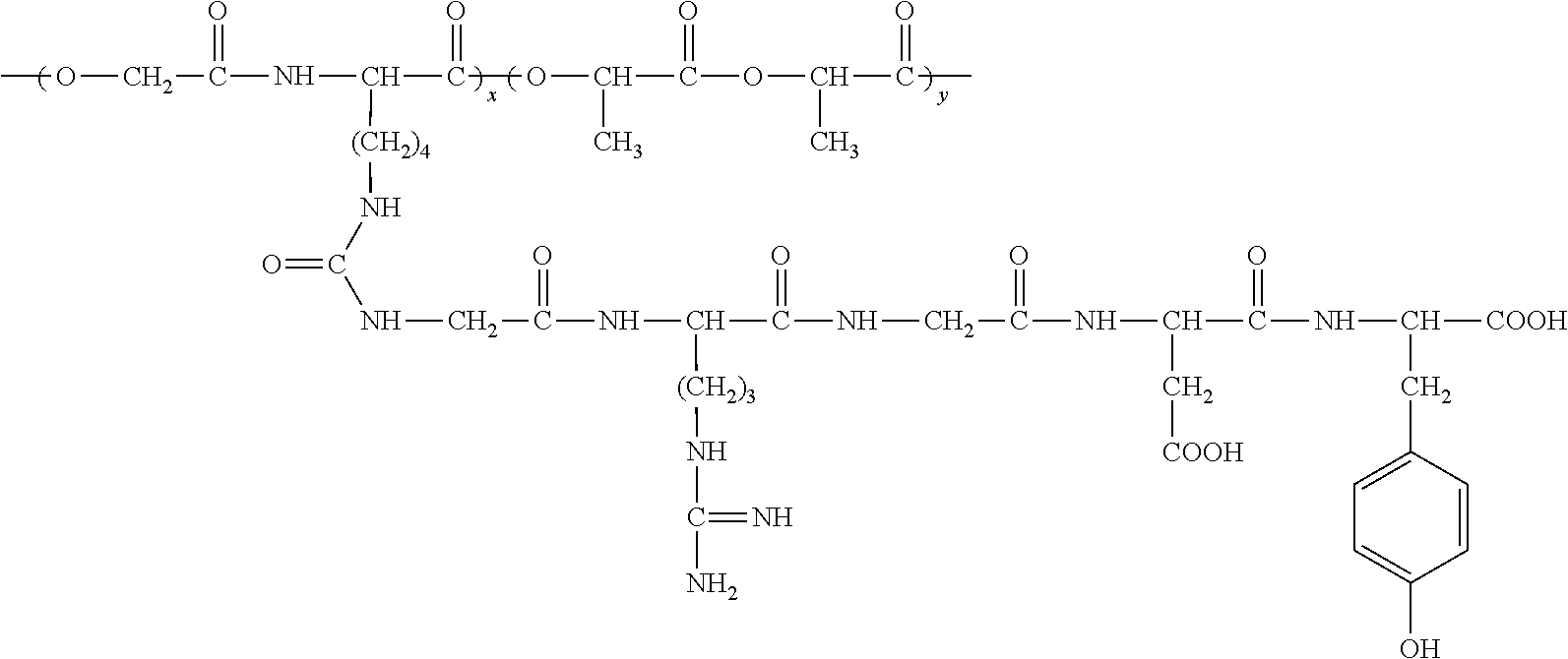
An RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) / β-tricalcium phosphate composite material is composed of β-tricalcium phosphate particles and RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) with mass ratio of 1:10-1:100, in which the β-tricalcium phosphate particles are uniformly dispersed in the RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) matrix. The preparation method includes that poly (glycolic acid-L-lysine-L-lactic acid) is polymerized with GRGDY short peptide (glycin-arginine-glycin-aspartic acid-tyrosine sequence) to obtain RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid), and then RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) is compounded with β-tricalcium phosphate particles. The composite material exhibits favorable biocompatibility, cellular affinity, biodegradability and mechanical behavior, and can avoid aseptic necrosis of tissues, which may be used as nerve guide or porous bone scaffold for repairing nerve tissue and bone tissue.
Owner:WUHAN UNIV OF TECH
Hydraulic Cements, Methods and Products
ActiveUS20120022023A1Easy and efficient deliveryWithout excessive material wasteBiocideAntipyreticCalcium biphosphateCalcium silicate
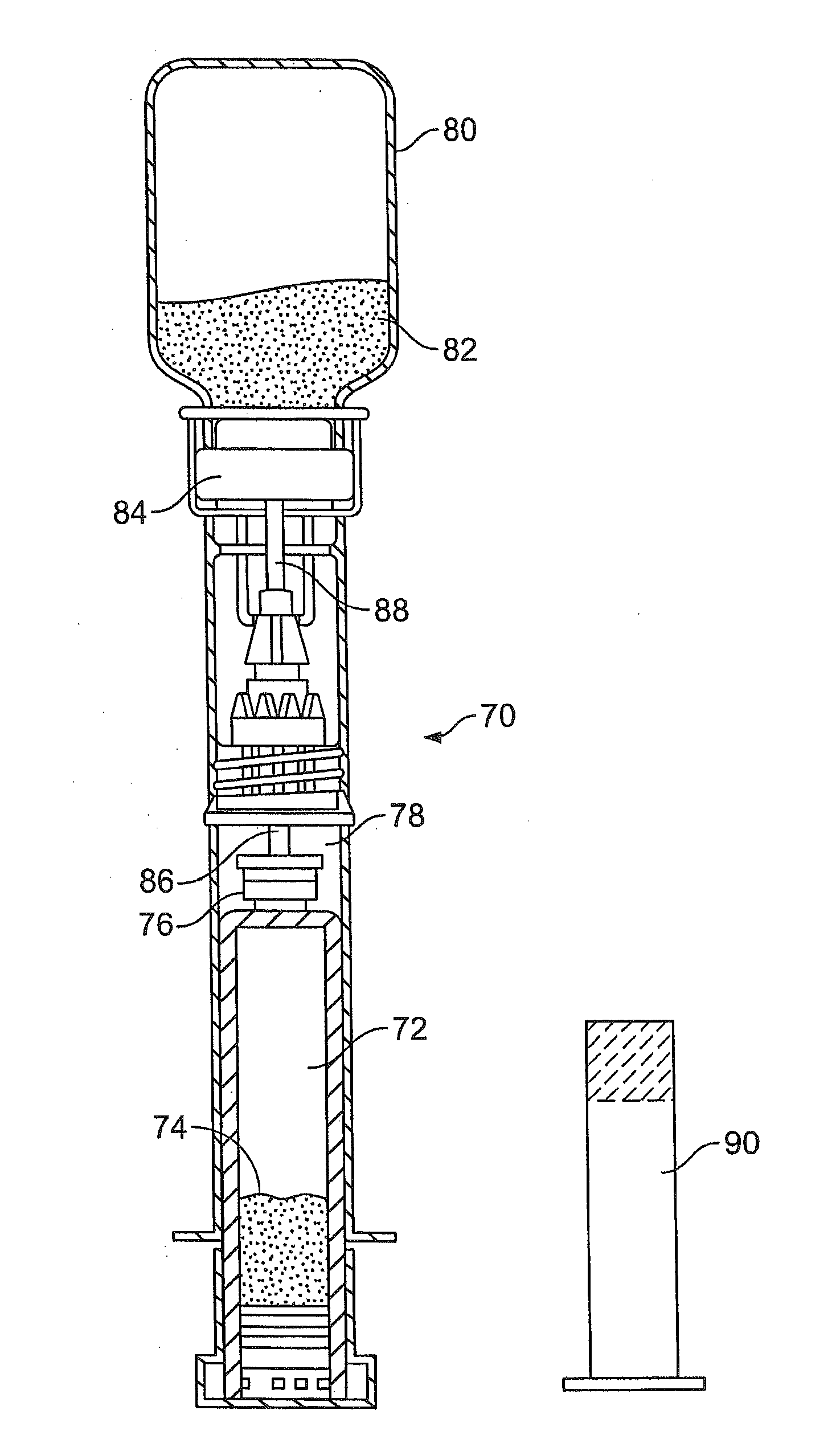
Non-aqueous hydraulic cement compositions comprise a non-aqueous mixture of a powder composition and a non-aqueous water-miscible liquid. In one embodiment, powder composition is a Brushite or Monetite-forming calcium phosphate powder composition. In another embodiment, the powder composition comprises porous β-tricalcium phosphate (β-TCP) granules and at least one additional calcium phosphate powder. In another embodiment, the powder composition comprises calcium silicate powder. In a further embodiment, the powder composition comprises calcium aluminate powder. In another embodiment, the powder composition is a cement composition and comprises nanopowders having a grain size of less than 1 micron. Hardened cements are formed from such hydraulic cement compositions, and methods of producing hardened cements, kits, and articles of manufacture employ such hydraulic cement compositions.
Owner:OSSDSIGN
Hydraulic cements with optimized grain size distribution, methods, articles and kits
ActiveUS20130066325A1Easy and efficient deliveryWithout excessive material wasteDental implantsOther chemical processesPhosphoric acidΒ tricalcium phosphate
A non-aqueous hydraulic cement composition comprises a non-aqueous mixture of (a) β-tricalcium phosphate powder, (b) monocalcium phosphate powder, and (c) non-aqueous water-miscible liquid, wherein (i) at least about 90% of the monocalcium phosphate powder has a grain size in a range of about 200-600 μm and the powder (weight) to liquid (volume) ratio is about 2.5-5.5, (ii) at least about 90% of the monocalcium phosphate powder has a grain size in a range of about 1-400 μm and the powder (weight) to liquid (volume) ratio is about 2-5, or (iii) at least about 90% of the monocalcium phosphate powder has a grain size in a range of about 1-600 μm and the powder (weight) to liquid (volume) ratio is about 2.5-5.5. Methods, hardened cements, articles of manufacture and kits employ such compositions.
Owner:OSSDSIGN
Sustained Release Systems and Preparation Method Thereof
The present invention relates to a hydrophilic drug and β-tricalcium phosphate (β-TCP) coating on a surface area of biopolymer matrix to form a sustained release system. The present invention also provides a method for preparing a sustained release system, comprising providing a surface are of biopolymer matrix coated with a hydrophilic drug and β-TCP.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
High strength synthetic bone for bone replacement for increasing ompressive strength and facilitating blood circulation, and manufacturing method therefor
InactiveUS20170065740A1Improve usabilityHigh compressive strengthSurgical adhesivesTissue regenerationSynthetic boneTri calcium phosphate
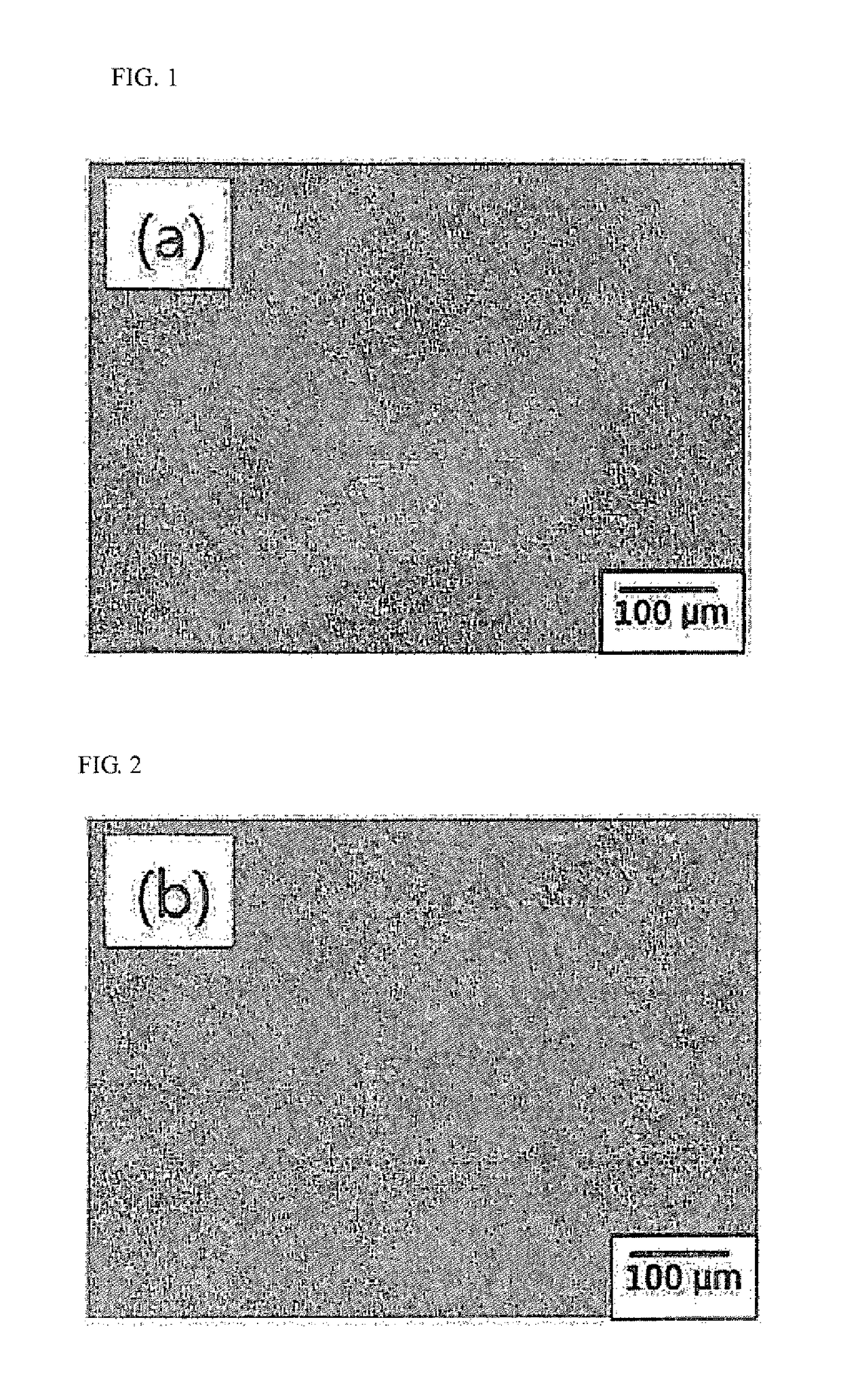
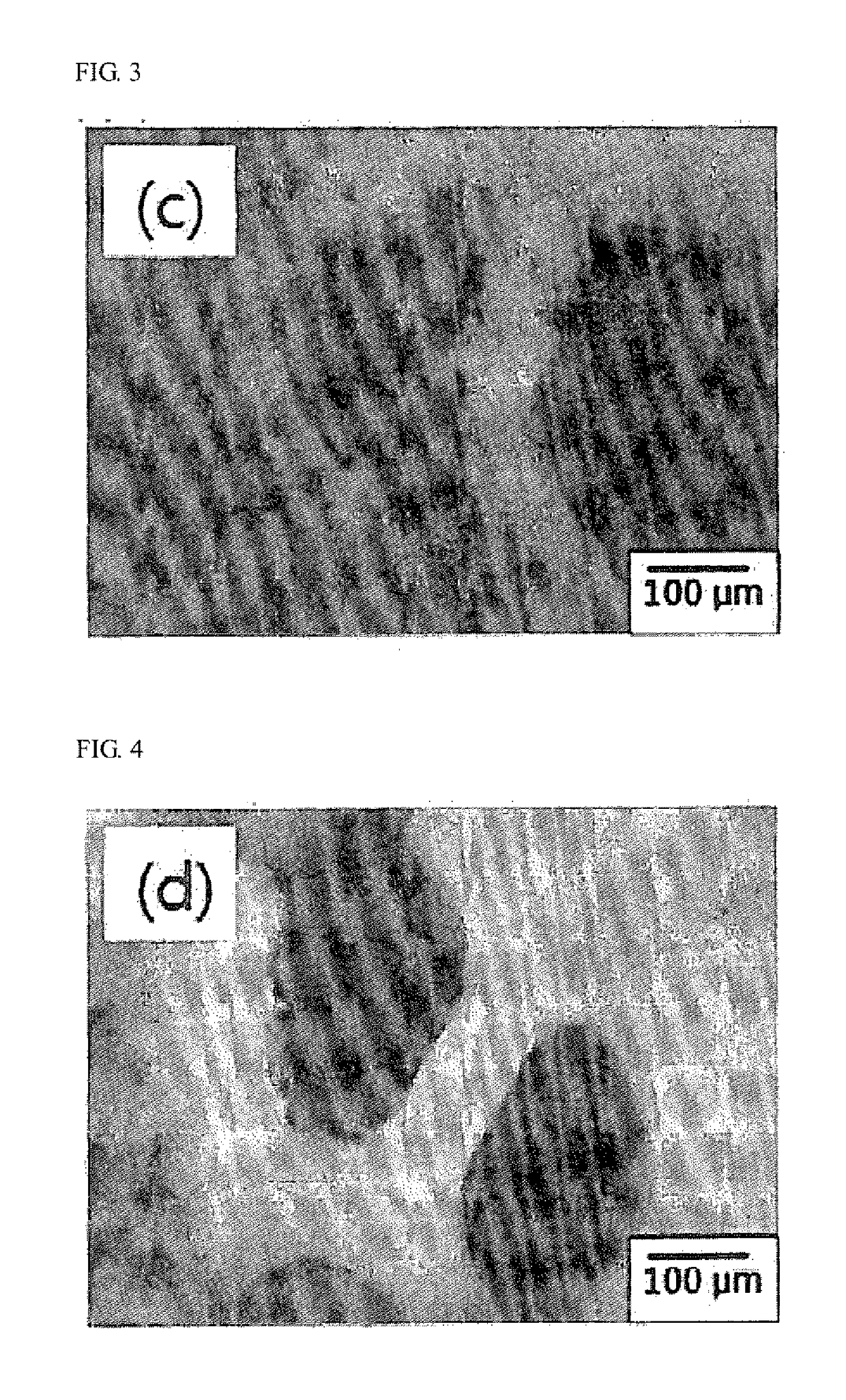
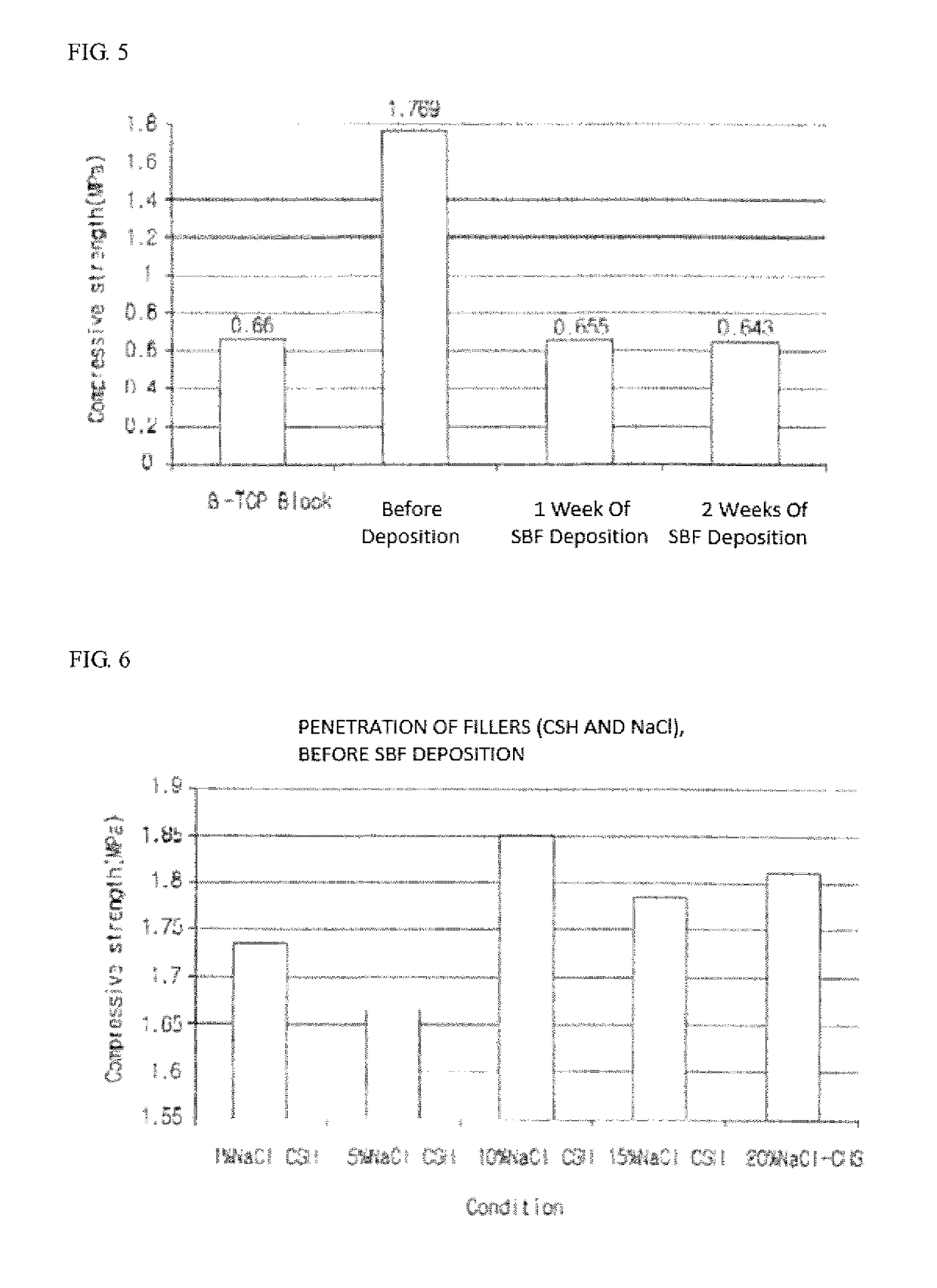
The present invention relates to a high strength synthetic bone for bone replacement for increasing compressive strength and facilitating blood circulation, and a manufacturing method therefor, and provides the high strength synthetic bone for bone replacement in which calcium sulfate hemihydrate (CSH) and NaCl, in a particle state, penetrate into the pores of a porous inorganic material such as β-tricalcium phosphate (β-TCP) and a wet treatment is performed on the same such that the CSH penetrated into the pores is combined with moisture so as to form a hydrated crystal of calcium sulfate dihydrate (CSD) to expand the volume thereof in the pores, thereby preventing the escape of a filler by physical force.
Owner:OSSEIN
Porous composite comprising silicon-substituted hydroxyapatite and ß- tricalcium phosphate, and process for preparing the same
InactiveUS20110185946A1Good biocompatibilityInherent in structureBone implantTissue regenerationApatiteBiocompatibility Testing
There are disclosed a porous composite comprising silicon-substituted hydroxyapatite and β-tricalcium phosphate (β-TCP), and a method for preparing the same. The porous composite is prepared by subjecting natural coral to hydrothermal and solvothermal reactions to prepare silicon-substituted hydroxyapatite (Si—HA) and subjecting the Si—HA to a heat treatment process. Thereafter, Si—HA and β-TCP may be mixed in the porous composite. As a result, the porous composite is excellent in biocompatibility and biodegradability. Also, the porous composite functions to maintain the microstructure of coral and is similar to natural bone in terms of composition and shape. Accordingly, the porous composite may be effectively used as a bone tissue repairing material and a bone graft material that can substitute for human hard tissue.
Owner:METABIOMED
Hydraulic cements, methods and products
ActiveUS8709149B2Easy and efficient deliveryWithout excessive material wasteBiocideImpression capsCalcium silicateCalcium biphosphate
Non-aqueous hydraulic cement compositions comprise a non-aqueous mixture of a powder composition and a non-aqueous water-miscible liquid. In one embodiment, powder composition is a Brushite or Monetite-forming calcium phosphate powder composition. In another embodiment, the powder composition comprises porous β-tricalcium phosphate (β-TCP) granules and at least one additional calcium phosphate powder. In another embodiment, the powder composition comprises calcium silicate powder. In a further embodiment, the powder composition comprises calcium aluminate powder. In another embodiment, the powder composition is a cement composition and comprises nanopowders having a grain size of less than 1 micron. Hardened cements are formed from such hydraulic cement compositions, and methods of producing hardened cements, kits, and articles of manufacture employ such hydraulic cement compositions.
Owner:OSSDSIGN
Hydraulic cements with optimized grain size distribution, methods, articles and kits
ActiveUS8591645B2Good injectabilityImprove mechanical propertiesDental implantsImpression capsPhosphoric acidΒ tricalcium phosphate
A non-aqueous hydraulic cement composition comprises a non-aqueous mixture of (a) β-tricalcium phosphate powder, (b) monocalcium phosphate powder, and (c) non-aqueous water-miscible liquid, wherein (i) at least about 90% of the monocalcium phosphate powder has a grain size in a range of about 200-600 μm and the powder (weight) to liquid (volume) ratio is about 2.5-5.5, (ii) at least about 90% of the monocalcium phosphate powder has a grain size in a range of about 1-400 μm and the powder (weight) to liquid (volume) ratio is about 2-5, or (iii) at least about 90% of the monocalcium phosphate powder has a grain size in a range of about 1-600 μm and the powder (weight) to liquid (volume) ratio is about 2.5-5.5. Methods, hardened cements, articles of manufacture and kits employ such compositions.
Owner:OSSDSIGN
Multiphasic bone graft substitute material
ActiveUS20150196689A1High mechanical strengthQuick changePowder deliveryBiocideParticulatesMonocalcium phosphate
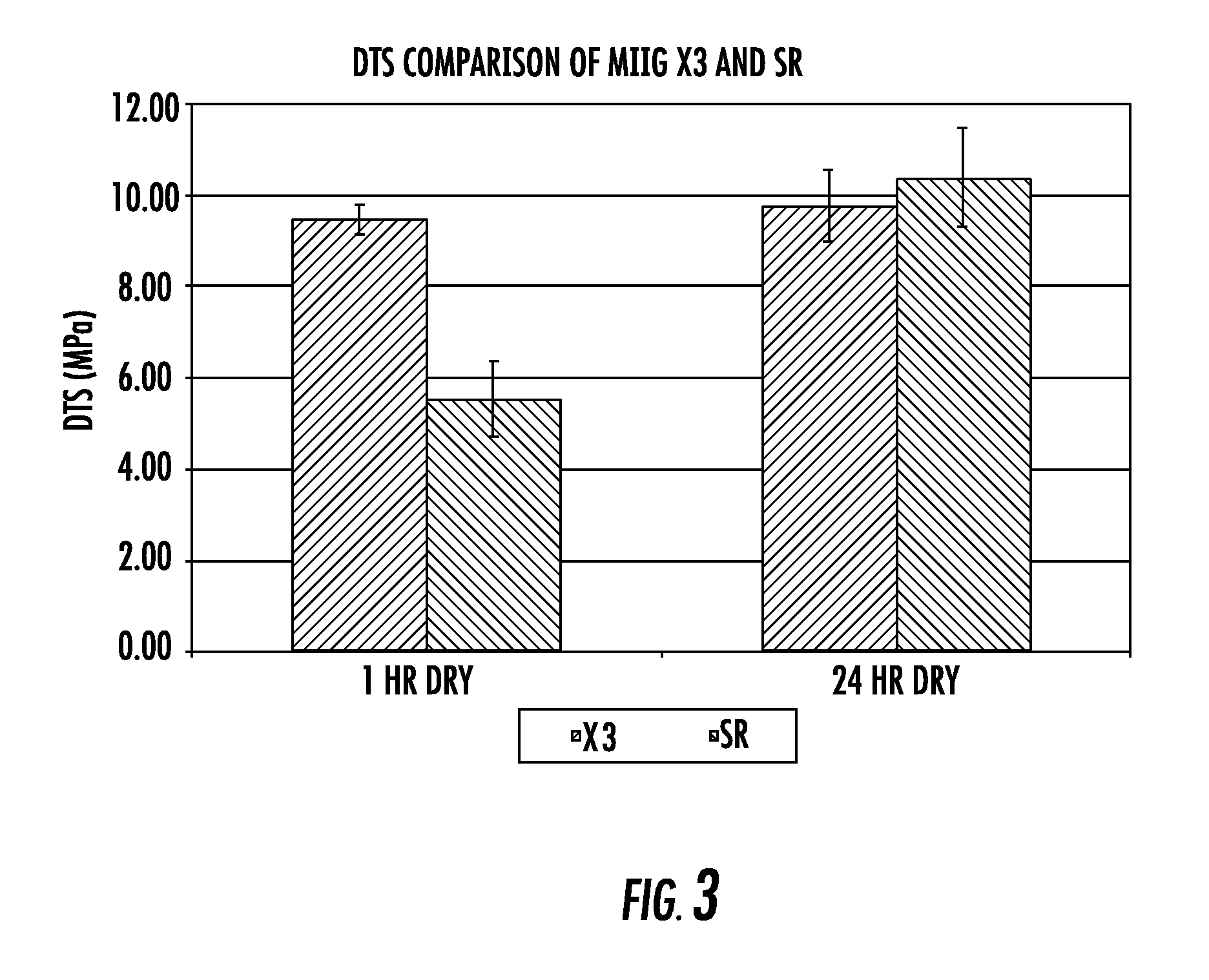
The invention provides a particulate composition adapted for forming a bone graft substitute cement upon mixing with an aqueous solution, comprising i) a calcium sulfate hemihydrate powder, wherein the calcium sulfate hemihydrate is present at a concentration of at least about 50 weight percent based on the total weight of the particulate composition; ii) a monocalcium phosphate monohydrate powder; iii) a non-porous β-tricalcium phosphate powder; and iv) a porous β-tricalcium phosphate powder. Bone graft substitute cements made therefrom, a bone graft substitute kit comprising the particulate composition, methods of making and using the particulate composition, and articles made from the bone graft substitute cement are also provided.
Owner:AGNOVOS HEALTHCARE
Rgd polypeptide grafted poly (glycolic acid-l-lysine-l-lactic acid) / beta tricalcium phosphate composite material and preparation method thereof
InactiveUS20080319114A1Good biocompatibilityHigh affinityConnective tissue peptidesFibrinogenArginineTyrosine
An RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) / β-tricalcium phosphate composite material is composed of β-tricalcium phosphate particles and RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) with mass ratio of 1:10-1:100, in which the β-tricalcium phosphate particles are uniformly dispersed in the RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) matrix. The preparation method includes that poly (glycolic acid-L-lysine-L-lactic acid) is polymerized with GRGDY short peptide (glycin-arginine-glycin-aspartic acid-tyrosine sequence) to obtain RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid), and then RGD polypeptide grafted poly (glycolic acid-L-lysine-L-lactic acid) is compounded with β-tricalcium phosphate particles. The composite material exhibits favorable biocompatibility, cellular affinity, biodegradability and mechanical behavior, and can avoid aseptic necrosis of tissues, which may be used as nerve guide or porous bone scaffold for repairing nerve tissue and bone tissue.
Owner:WUHAN UNIV OF TECH
Method for controlling work time for forming shape of biphasic self-setting calcium phosphate
ActiveUS20170072104A1Reduce physical painGood formabilityImpression capsPharmaceutical delivery mechanismWork periodCalcium biphosphate
[Problem] Biphasic self-setting calcium phosphate (SSCP) used for bone graft material and dental material applications having shape formability, shape retentivity, and bone replacement properties in addition to biocompatibility, safety, non-infectiousness, and absence of outflow, wherein the work time for forming the shape of a kneaded material obtained by kneading biphasic SSCP powder and biphasic SSCP liquid is controlled.[Solution] A method for controlling the work time for forming the shape of biphasic SSCP in which the moldable work time from the start of kneading to the setting of the kneaded material is adjusted to within a range of from 10 seconds to 600 seconds by kneading a biphasic SSCP powder and biphasic SSCP liquid, the biphasic SSCP powder comprising tetracalcium phosphate and α-tricalcium phosphate and the biphasic SSCP liquid comprising a phosphoric acid aqueous solution containing a calcium component.
Owner:FRONTIER SCI CORP
Porous beta-tricalcium phosphate and methods for producing the same
Methods are provided for producing porous β-Tricalcium Phosphate (β-TCP). In the subject methods, β-TCP is combined with graphite to produce an intermediate greenware product. The intermediate greenware product is then sintered to produce porous β-TCP. The subject methods and compositions produced thereby find use in a variety of applications.
Owner:SKELETAL KINETICS
Hydraulic Cements, Methods and Products
ActiveUS20140221303A1Easy and efficient deliveryWithout excessive material wasteBiocideImpression capsCalcium silicateCalcium biphosphate
Non-aqueous hydraulic cement compositions comprise a non-aqueous mixture of a powder composition and a non-aqueous water-miscible liquid. In one embodiment, powder composition is a Brushite or Monetite-forming calcium phosphate powder composition. In another embodiment, the powder composition comprises porous β-tricalcium phosphate (β-TCP) granules and at least one additional calcium phosphate powder. In another embodiment, the powder composition comprises calcium silicate powder. In a further embodiment, the powder composition comprises calcium aluminate powder. In another embodiment, the powder composition is a cement composition and comprises nanopowders having a grain size of less than 1 micron. Hardened cements are formed from such hydraulic cement compositions, and methods of producing hardened cements, kits, and articles of manufacture employ such hydraulic cement compositions.
Owner:OSSDSIGN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com