Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
401 results about "Bone forming" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
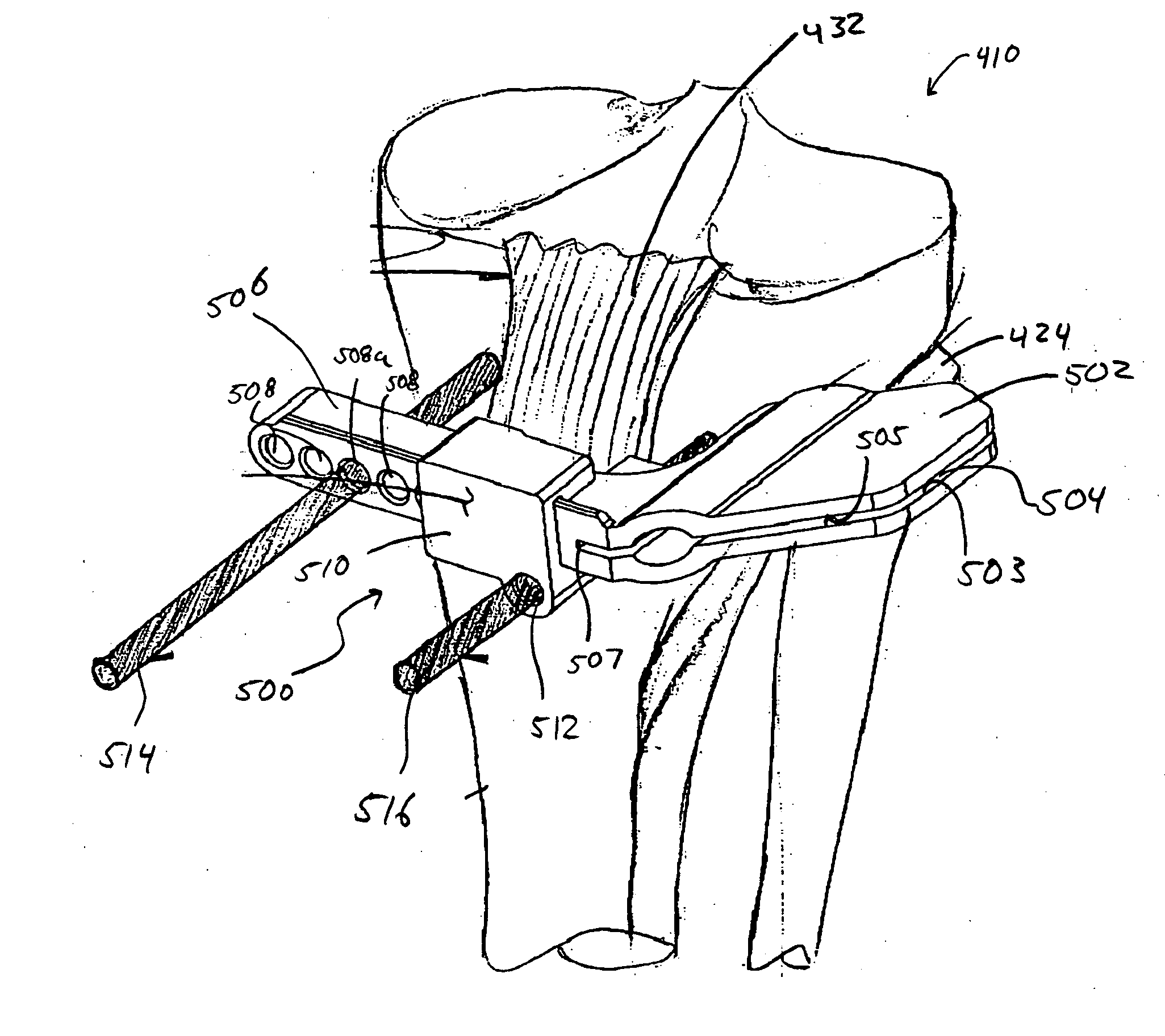
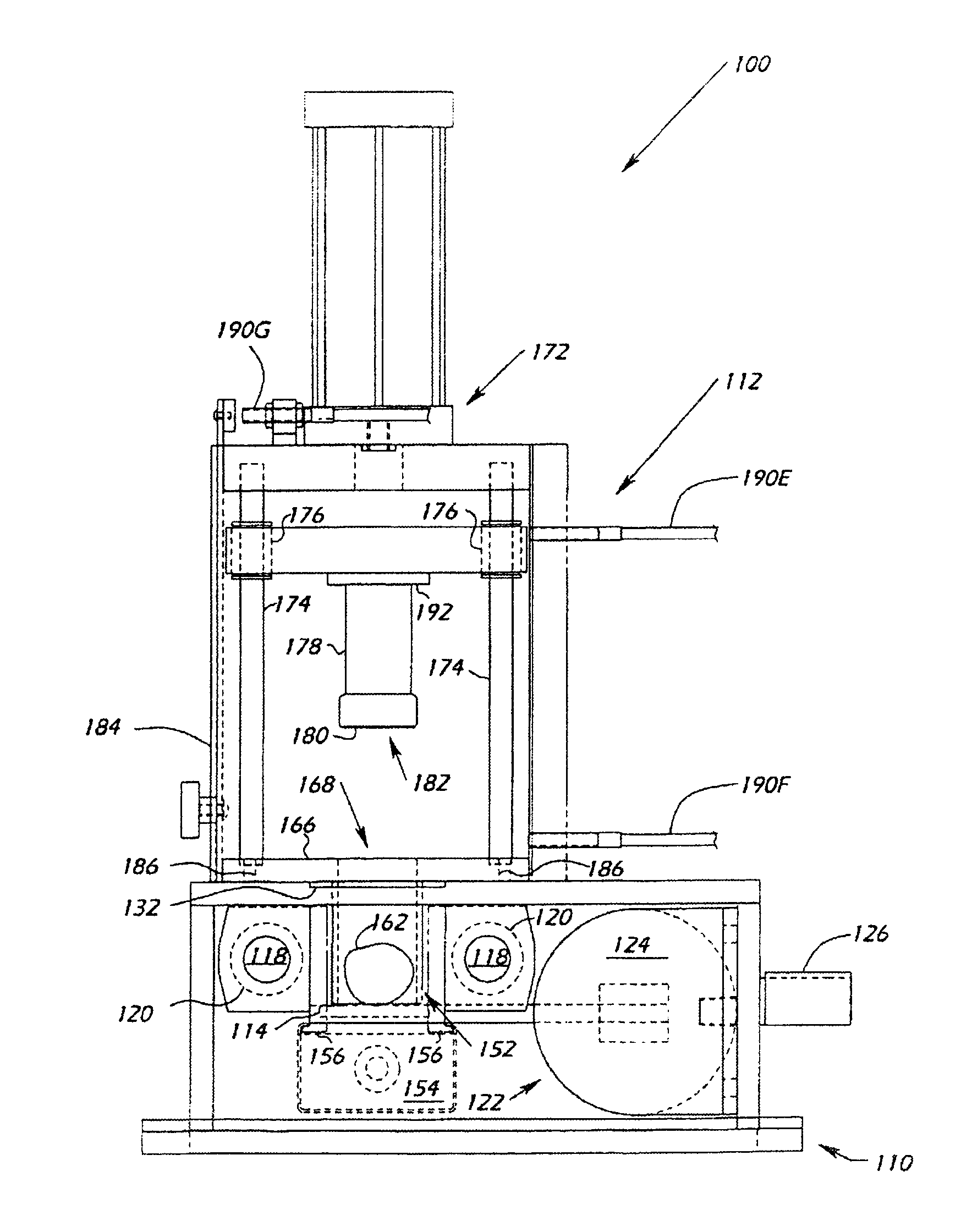
System and method for manufacturing arthroplasty jigs
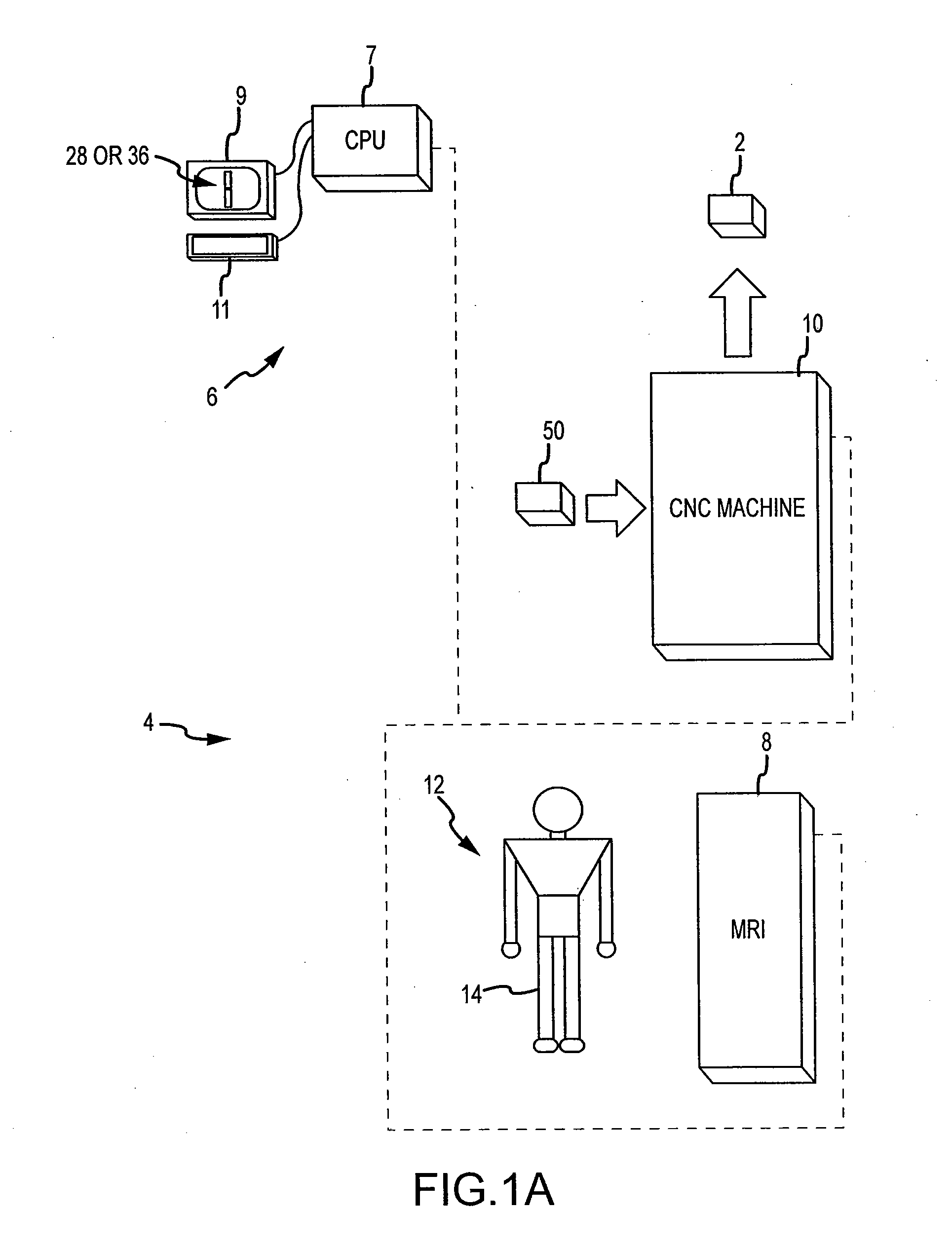
ActiveUS20090157083A1Facilitate arthroplasty implantsCharacter and pattern recognitionComputerised tomographsBone formingSacroiliac joint
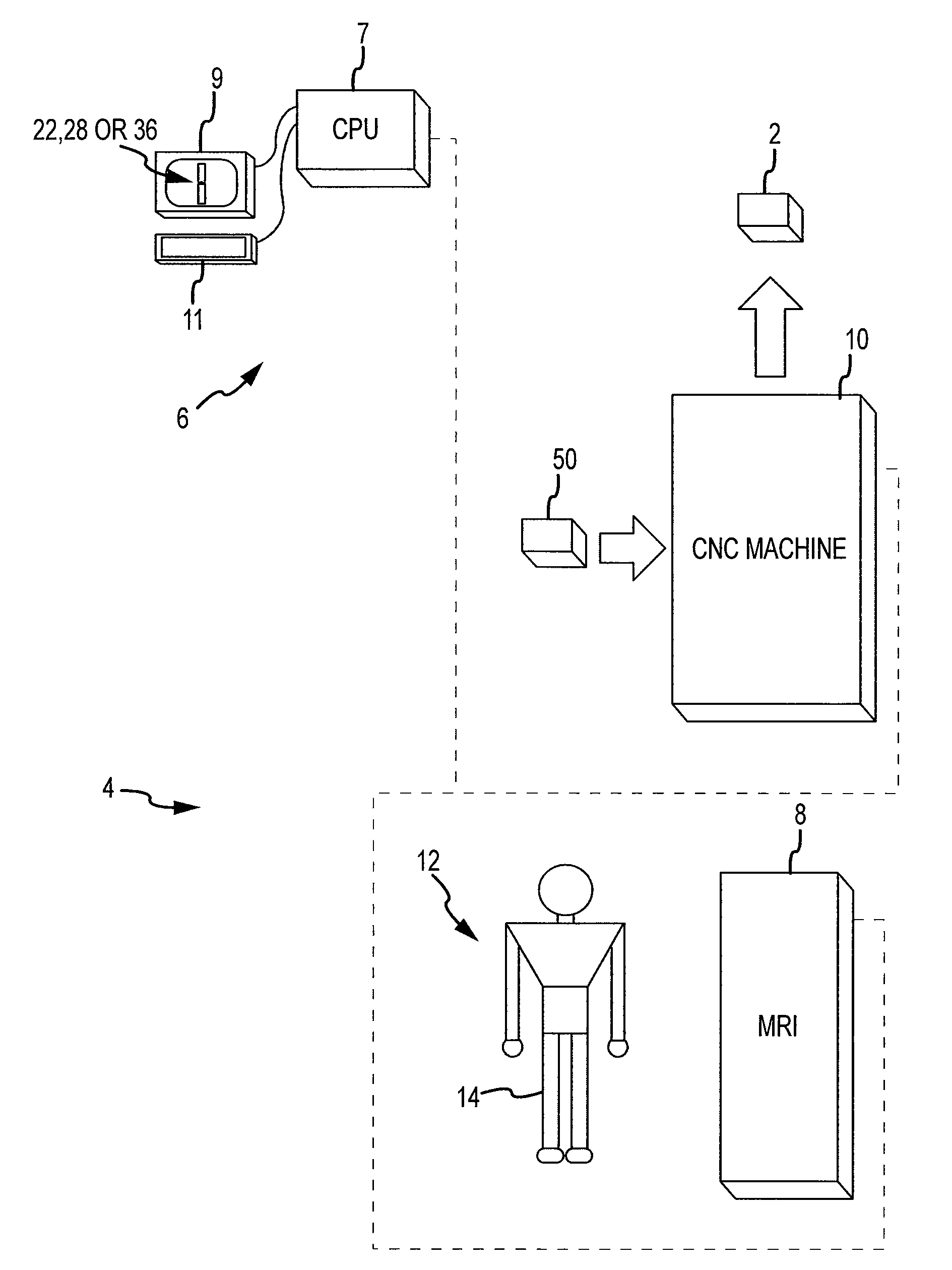
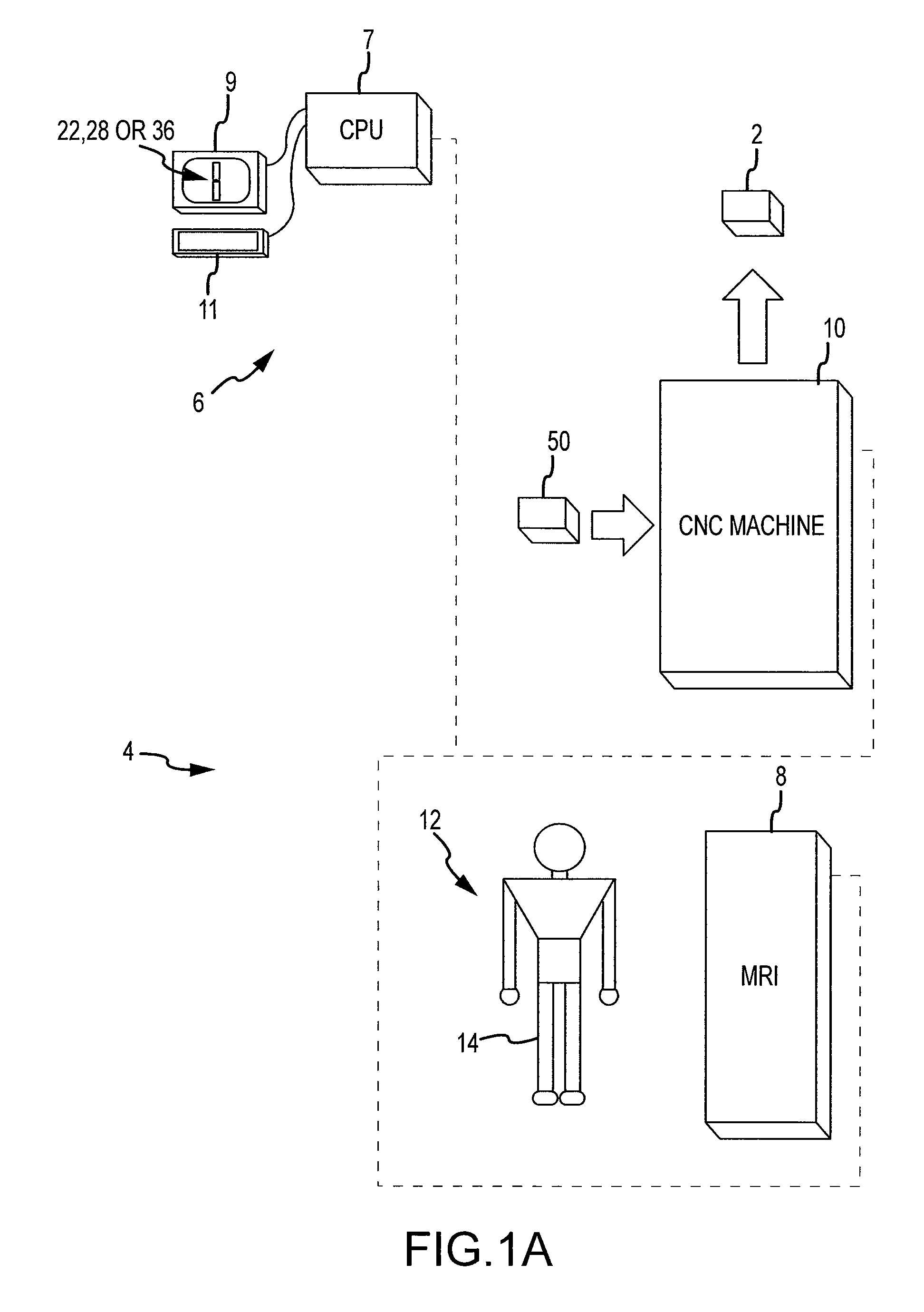
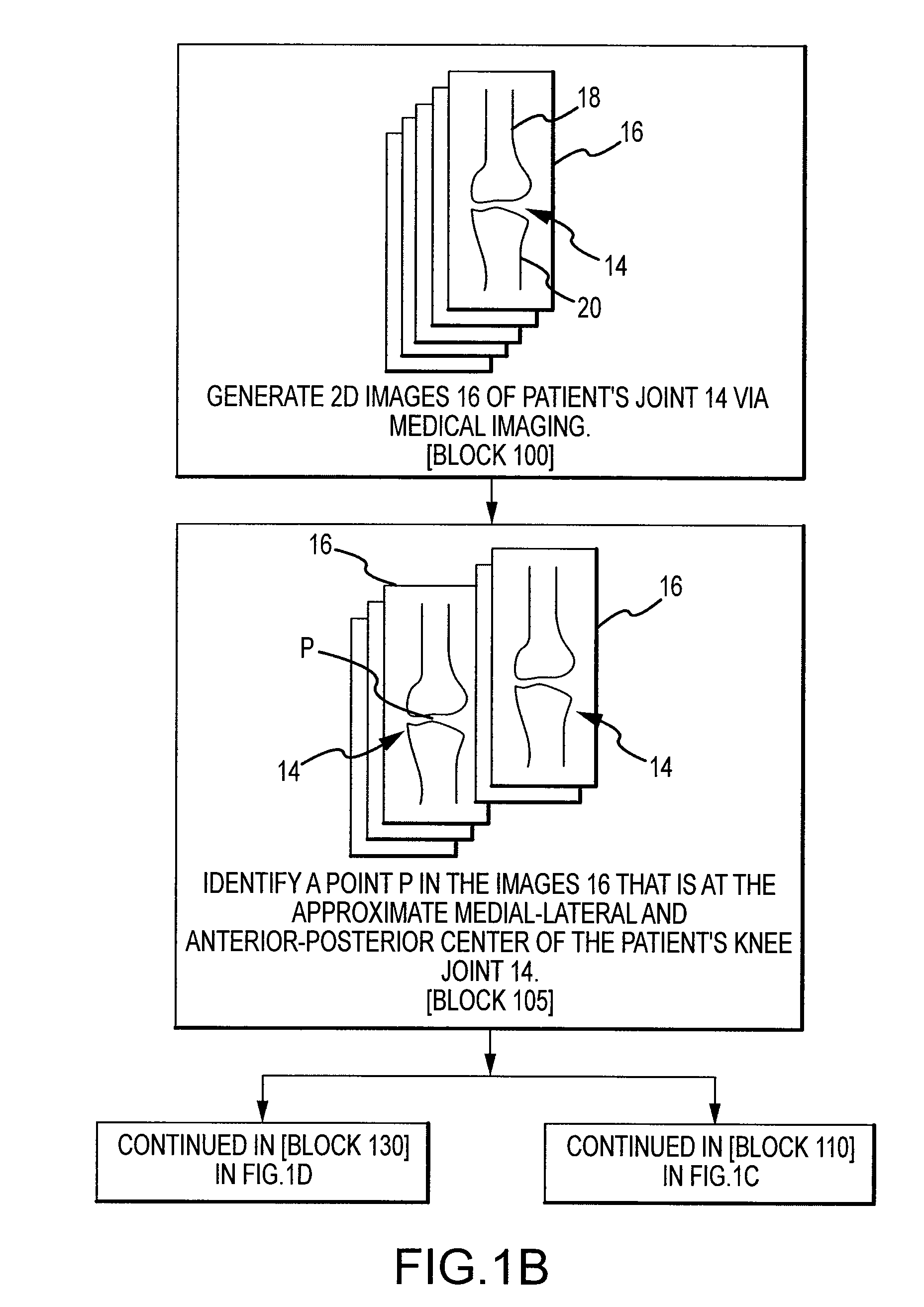
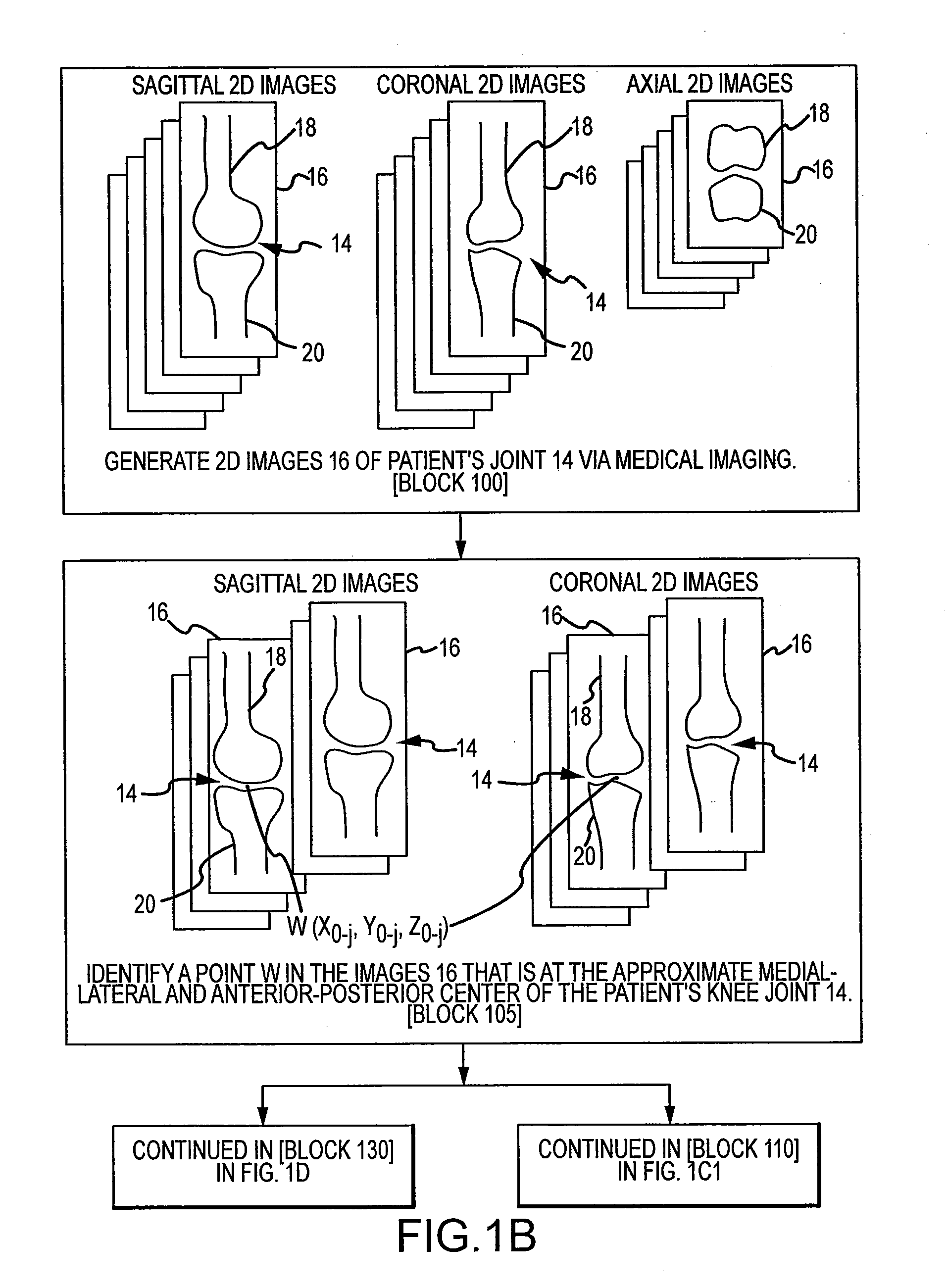
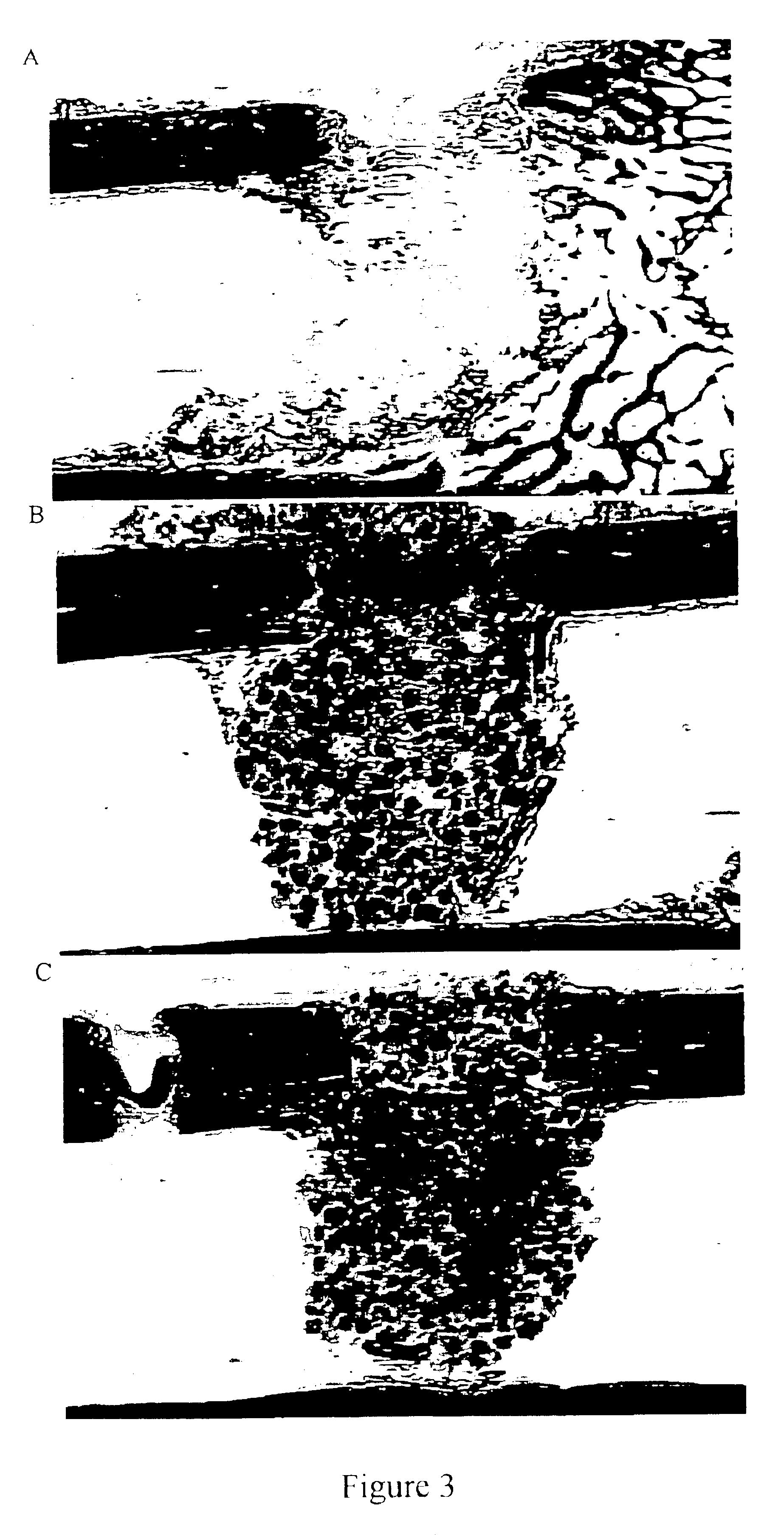
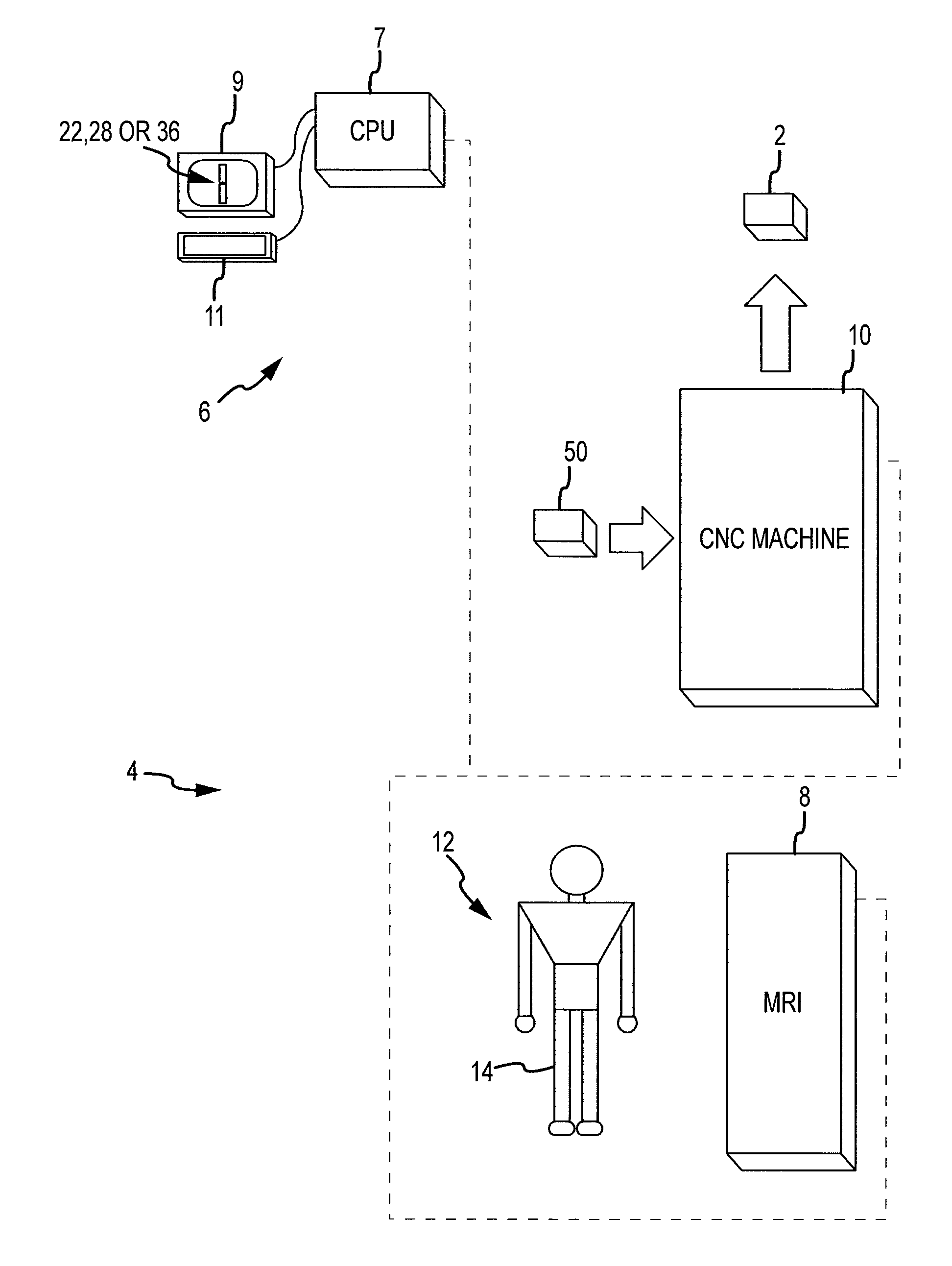
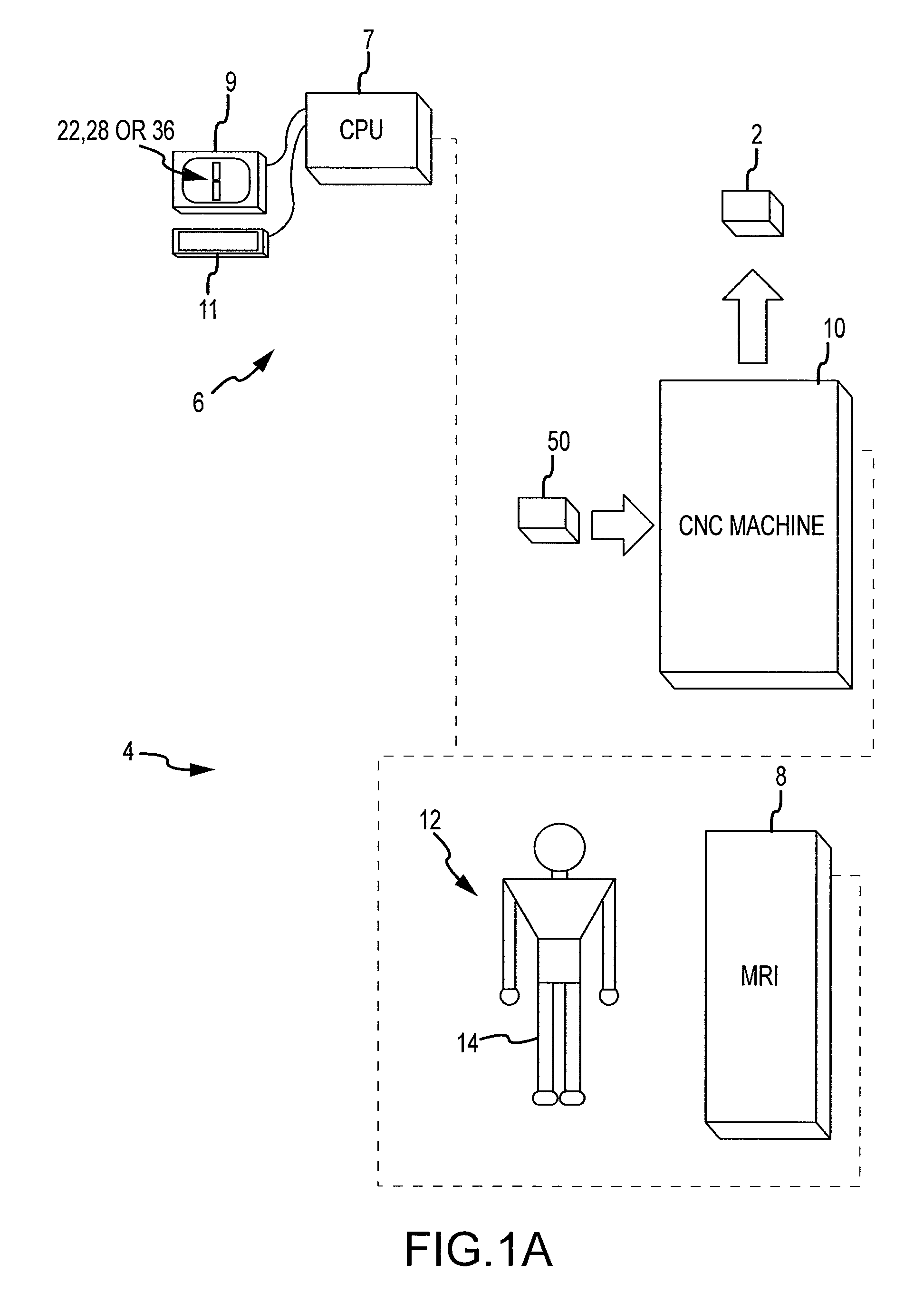
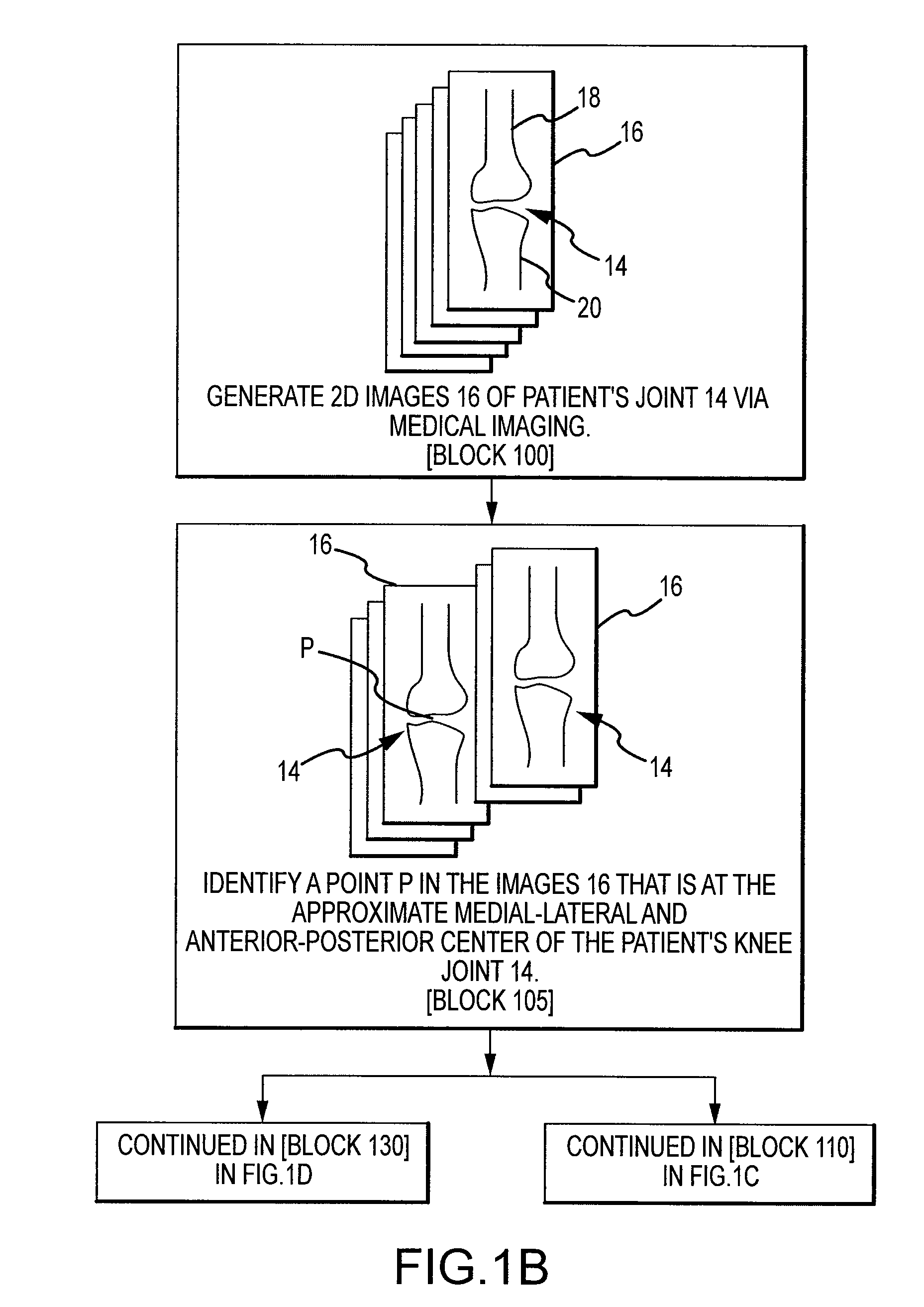
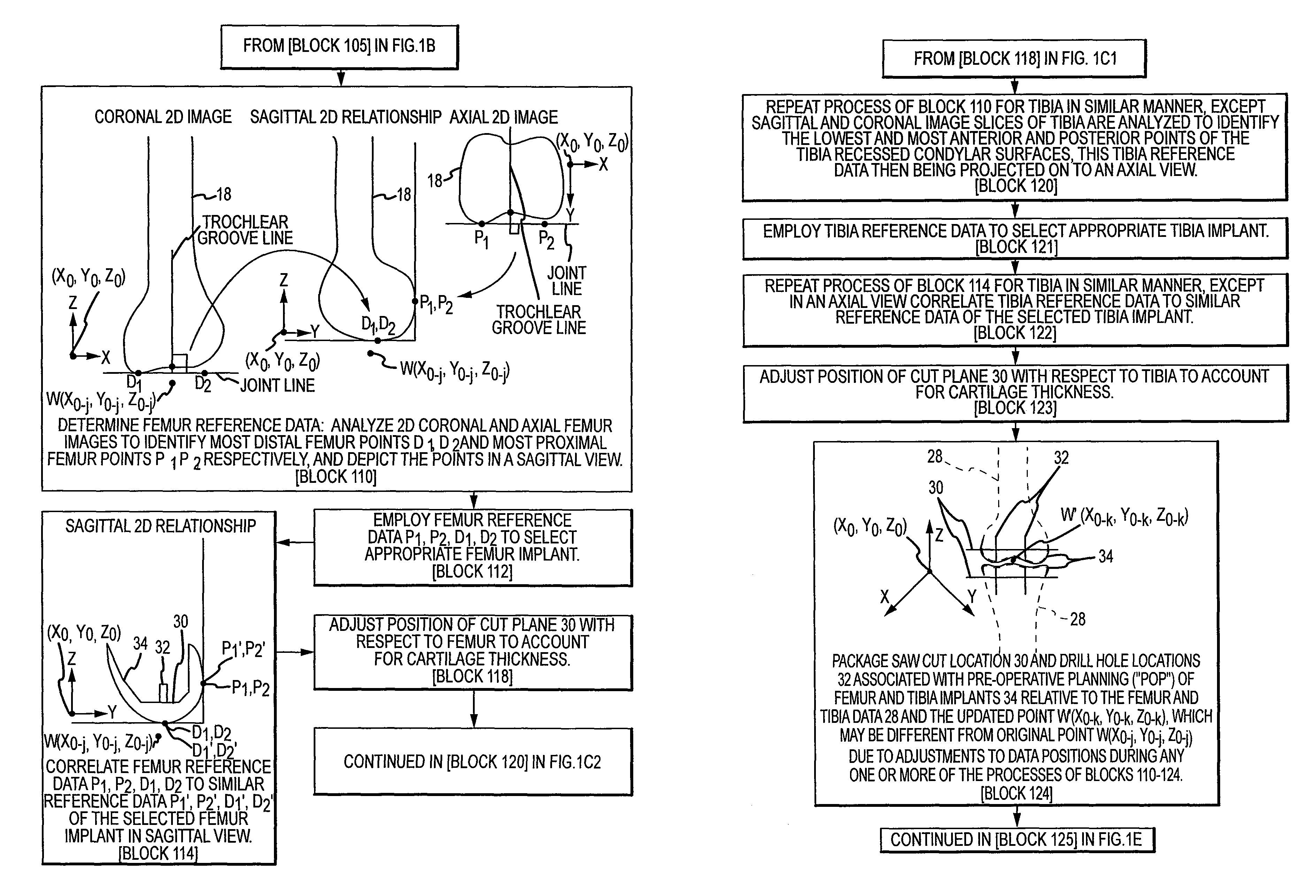
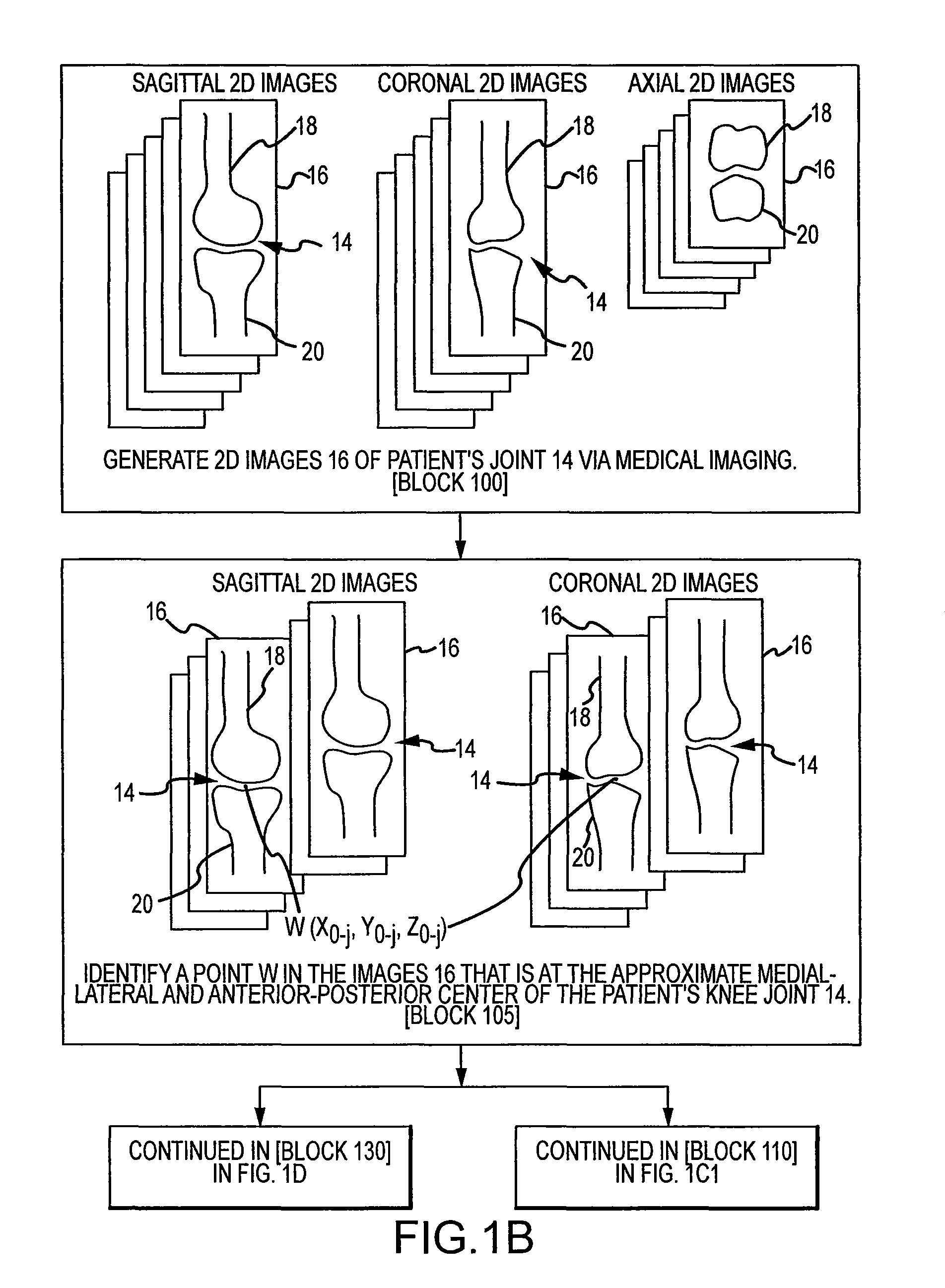
Disclosed herein is a method of computer generating a three-dimensional surface model of an arthroplasty target region of a bone forming a joint. The method may include: generating two-dimensional images of at least a portion of the bone; generating an open-loop contour line along the arthroplasty target region in at least some of the two-dimensional images; and generating the three-dimensional model of the arthroplasty target region from the open-loop contour lines.
Owner:HOWMEDICA OSTEONICS CORP
Arthroplasty system and related methods
A method of manufacturing an arthroplasty jig is disclosed herein. The method may include the following: generate two dimensional image data of a patient joint to undergo arthroplasty, identify in the two dimensional image data a first point corresponding to an articular surface of a bone forming the joint, identify a second point corresponding to an articular surface of an implant, identify a location of a resection plane when the first point is correlated with the second point, and create the arthroplasty jig with a resection guide located according to the identified location of the resection plane.
Owner:HOWMEDICA OSTEONICS CORP
Porous β-tricalcium phosphate granules for regeneration of bone tissue
InactiveUS6949251B2Improve regenerative abilitySurgical adhesivesSkeletal disorderActive agentBone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
System and method for manufacturing arthroplasty jigs
ActiveUS8221430B2Facilitate arthroplasty implantsCharacter and pattern recognitionComputerised tomographsBone formingSacroiliac joint
Disclosed herein is a method of computer generating a three-dimensional surface model of an arthroplasty target region of a bone forming a joint. The method may include: generating two-dimensional images of at least a portion of the bone; generating an open-loop contour line along the arthroplasty target region in at least some of the two-dimensional images; and generating the three-dimensional model of the arthroplasty target region from the open-loop contour lines.
Owner:HOWMEDICA OSTEONICS CORP
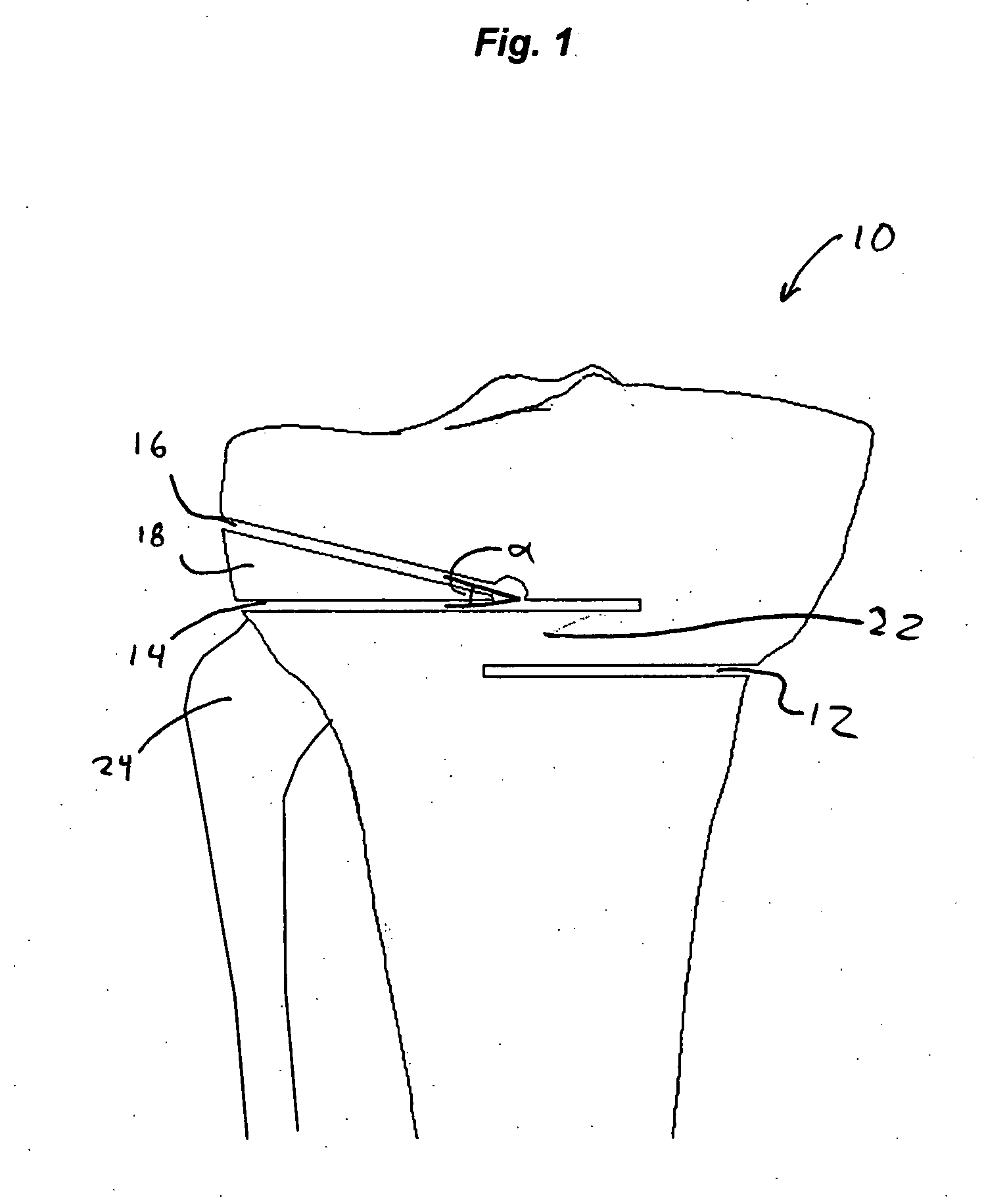
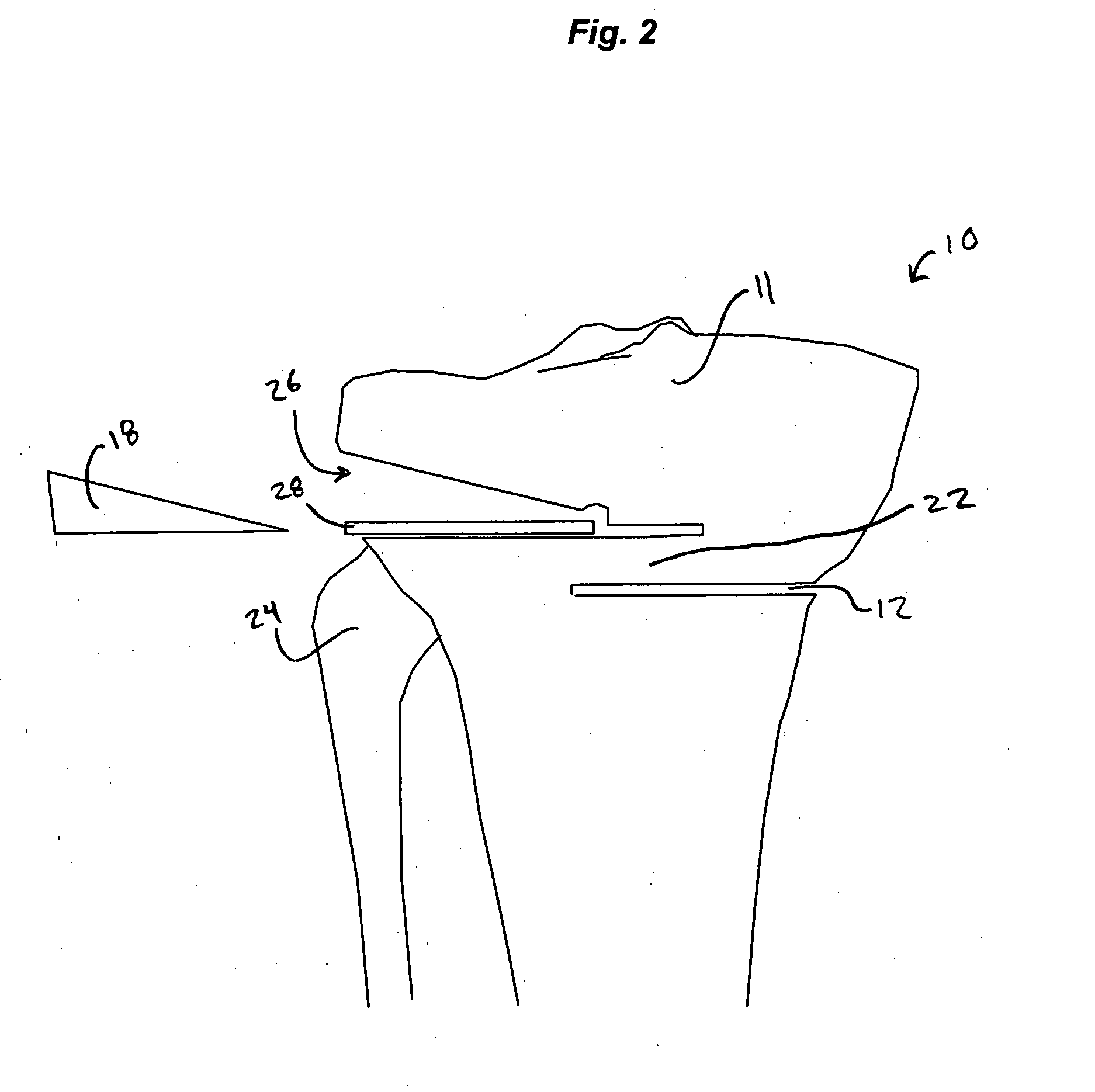
High tibial osteotomy system
A cutting block for use in a bone osteotomy procedure is disclosed, and includes a first cutting guide surface, a second cutting guide surface, and a third cutting guide surface. The first, second, and third cutting guide surfaces are adapted to be temporarily affixed to a bone having a first side and a second side such that the first cutting guide surface is disposed on the first side of the bone, and such that the second cutting guide surface and third cutting guide surface are disposed on the second side of the bone forming an angle therebetween.
Owner:HOWMEDICA OSTEONICS CORP
High tibial osteotomy guide
A cutting block for use in a bone osteotomy procedure is disclosed, and includes a first cutting guide surface, a second cutting guide surface, and a third cutting guide surface. The first, second, and third cutting guide surfaces are adapted to be temporarily affixed to a bone having a first side and a second side such that the first cutting guide surface is disposed on the first side of the bone, and such that the second cutting guide surface and third cutting guide surface are disposed on the second side of the bone forming an angle therebetween.
Owner:HOWMEDICA OSTEONICS CORP
High tibial osteotomy system
A cutting block for use in a bone osteotomy procedure includes a first cutting guide surface, a second cutting guide surface, and a third cutting guide surface. The first, second, and third cutting guide surfaces are adapted to be temporarily affixed to a bone having a first side and a second side such that the first cutting guide surface is disposed on the first side of the bone, and such that the second cutting guide surface and third cutting guide surface are disposed on the second side of the bone forming an angle therebetween.
Owner:HOWMEDICA OSTEONICS CORP
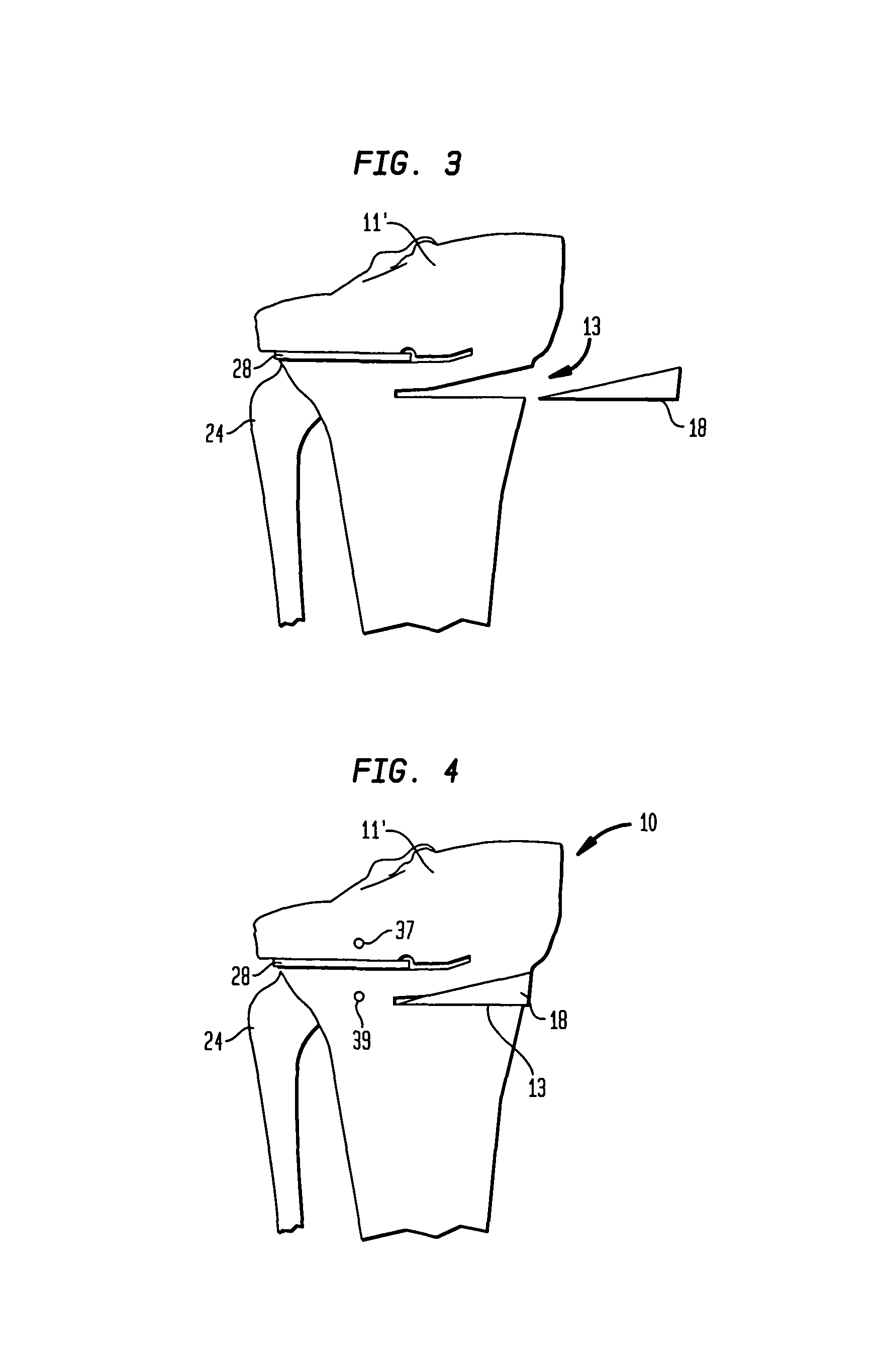
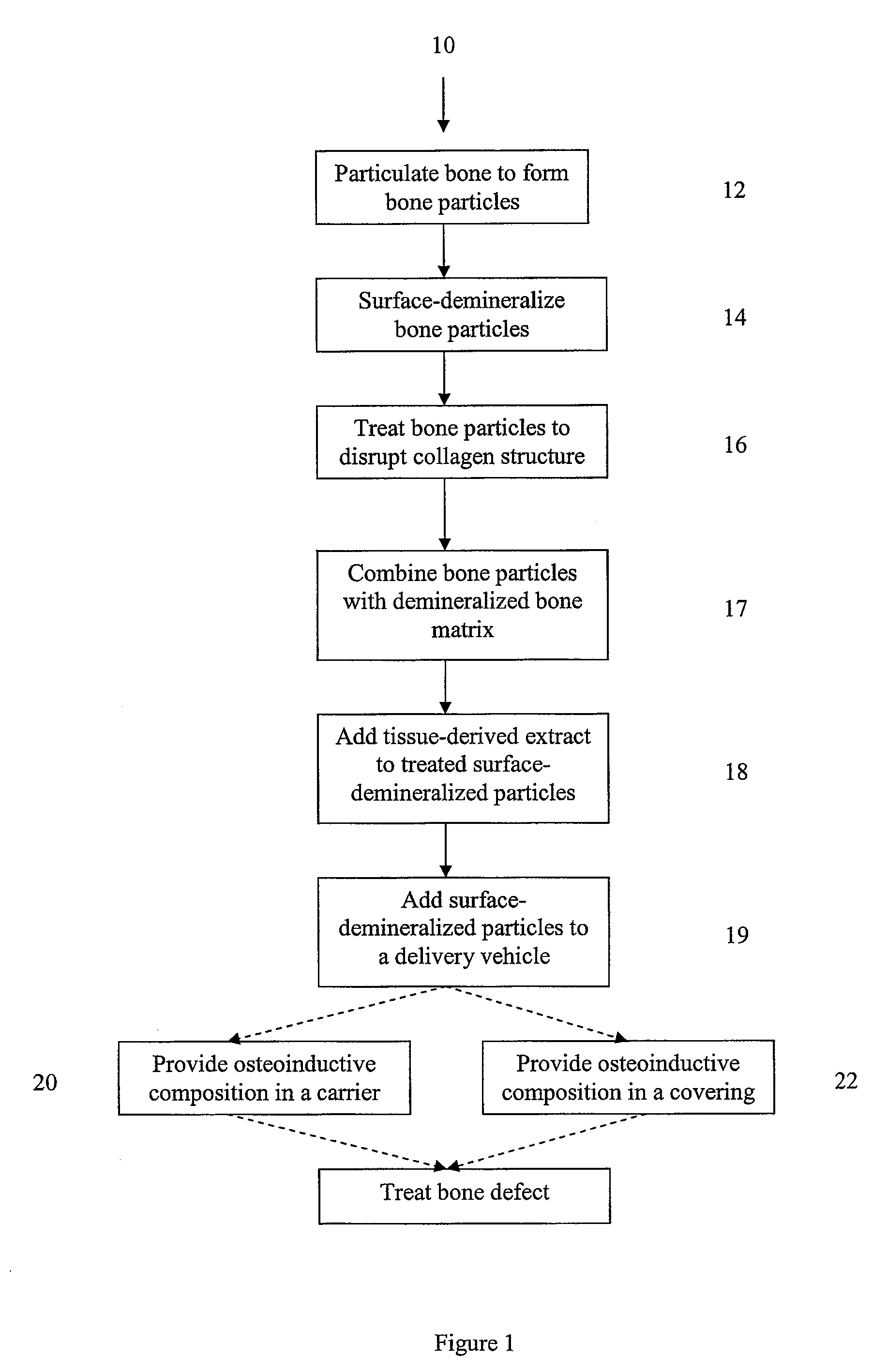
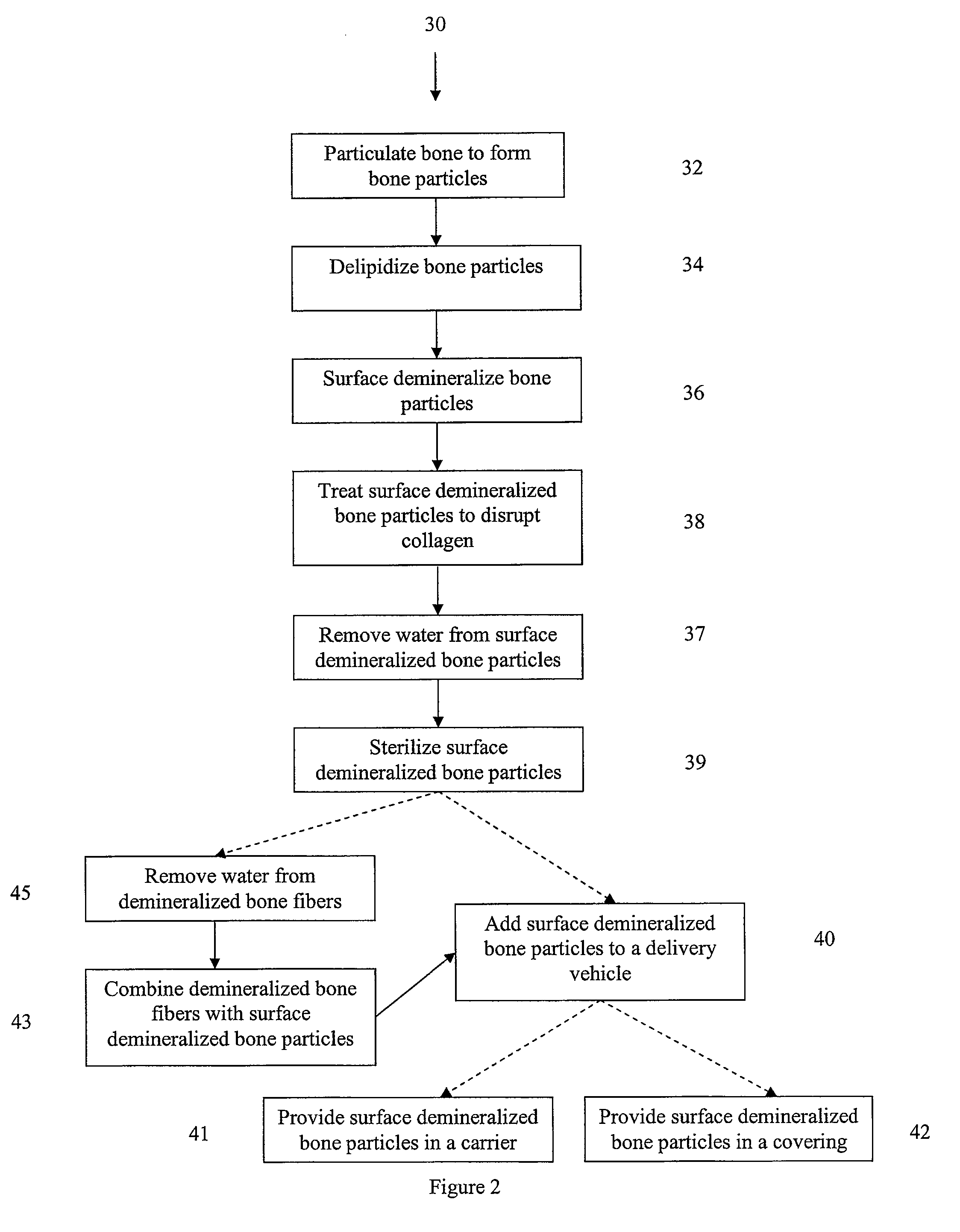
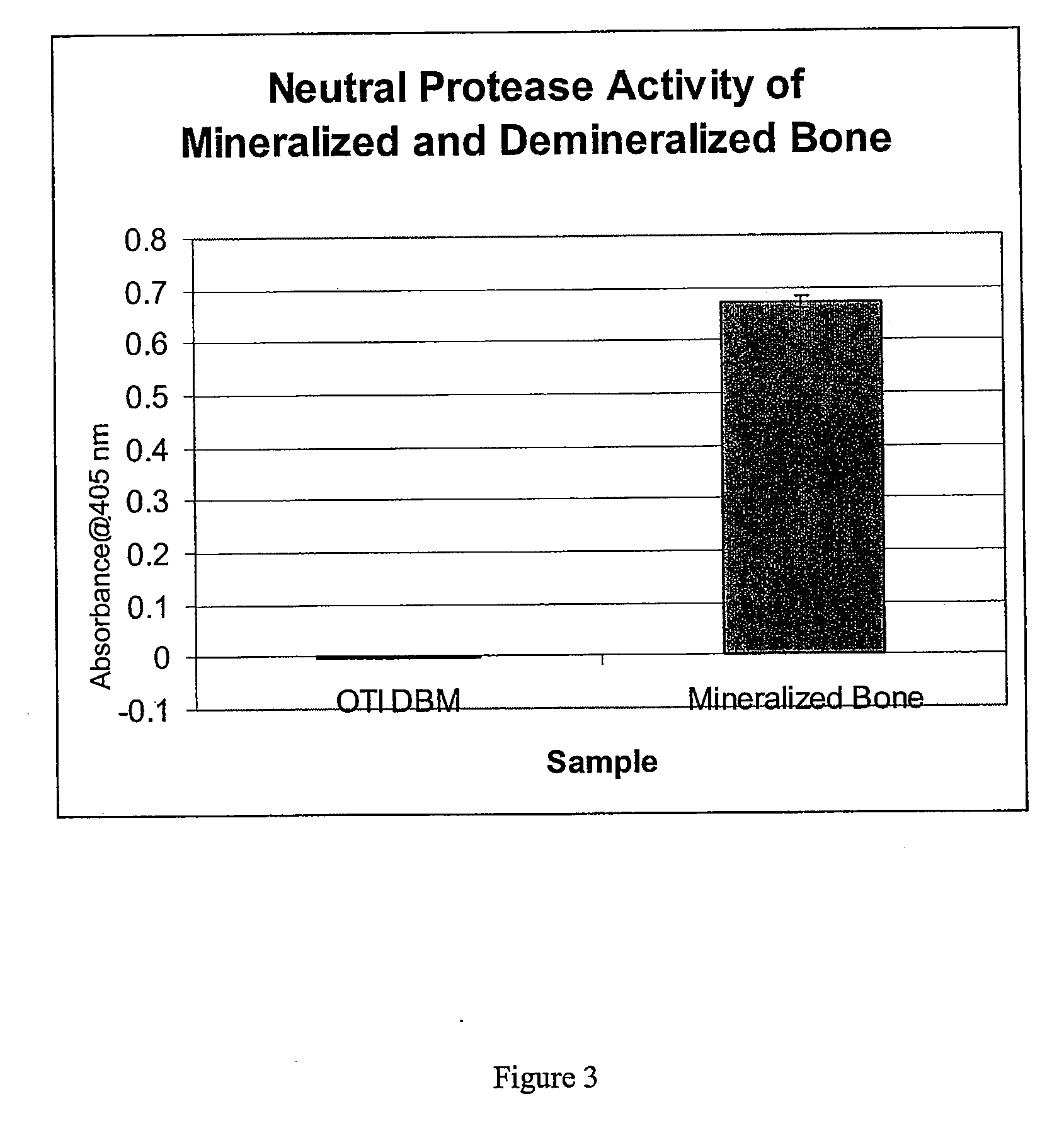
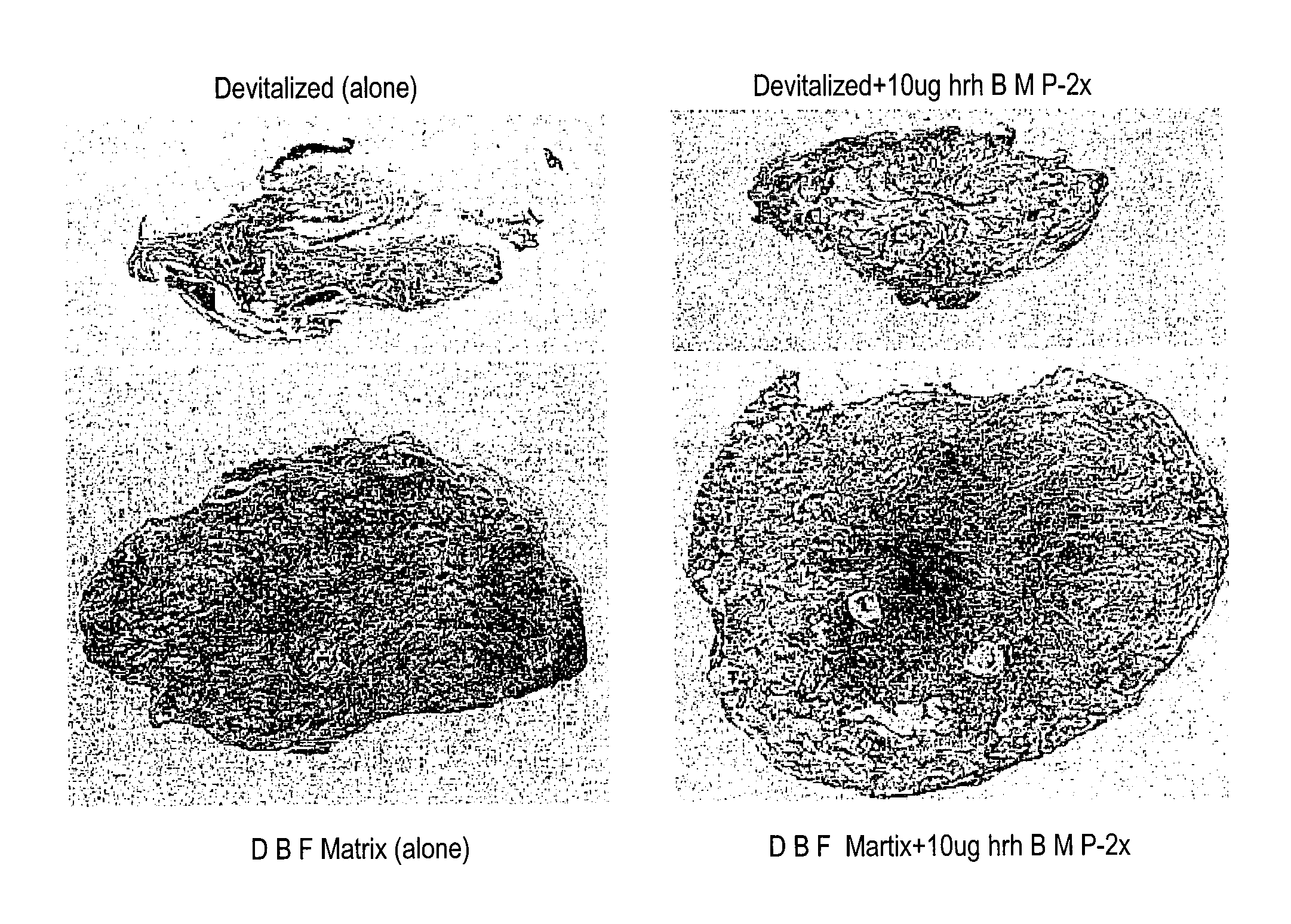
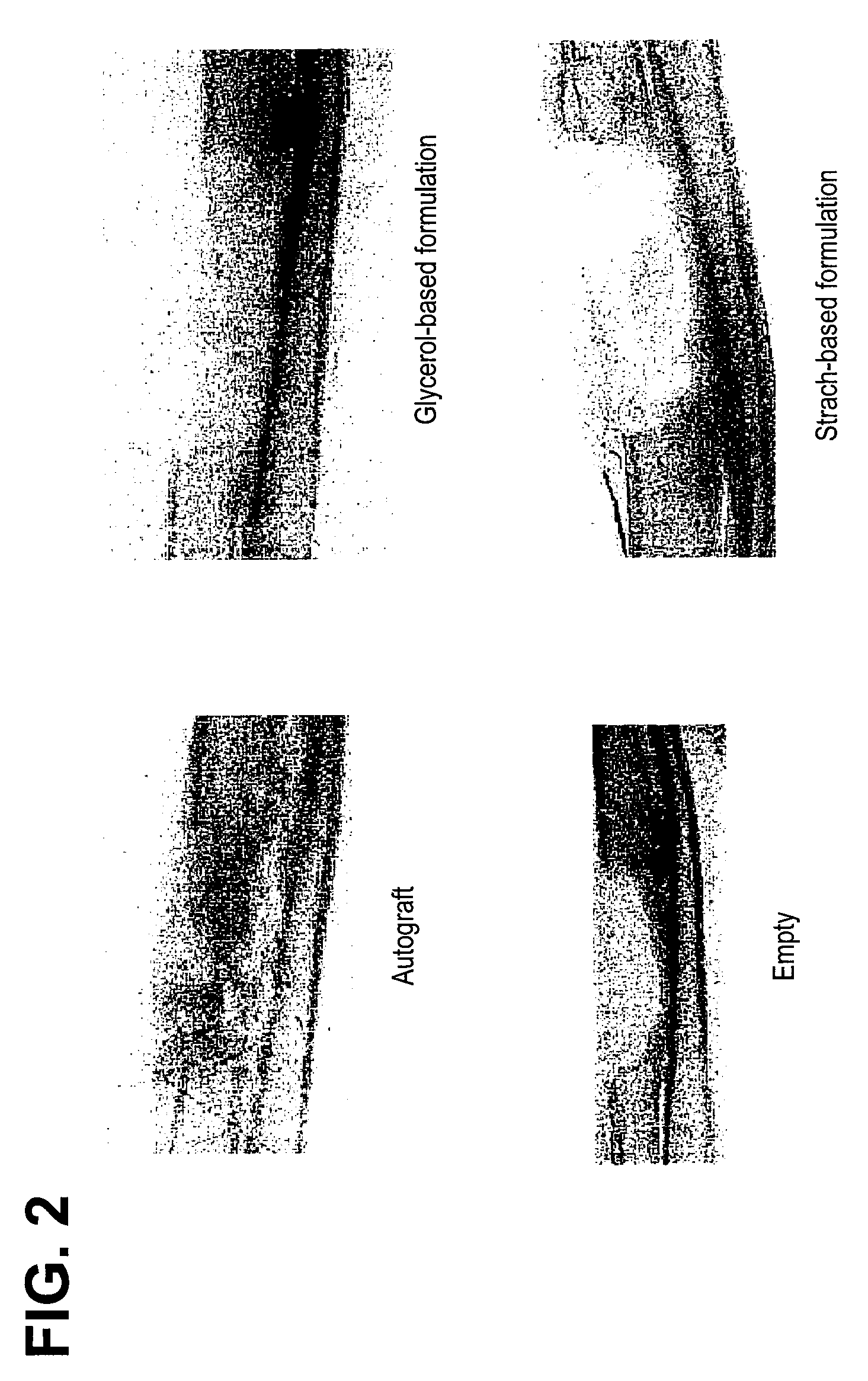
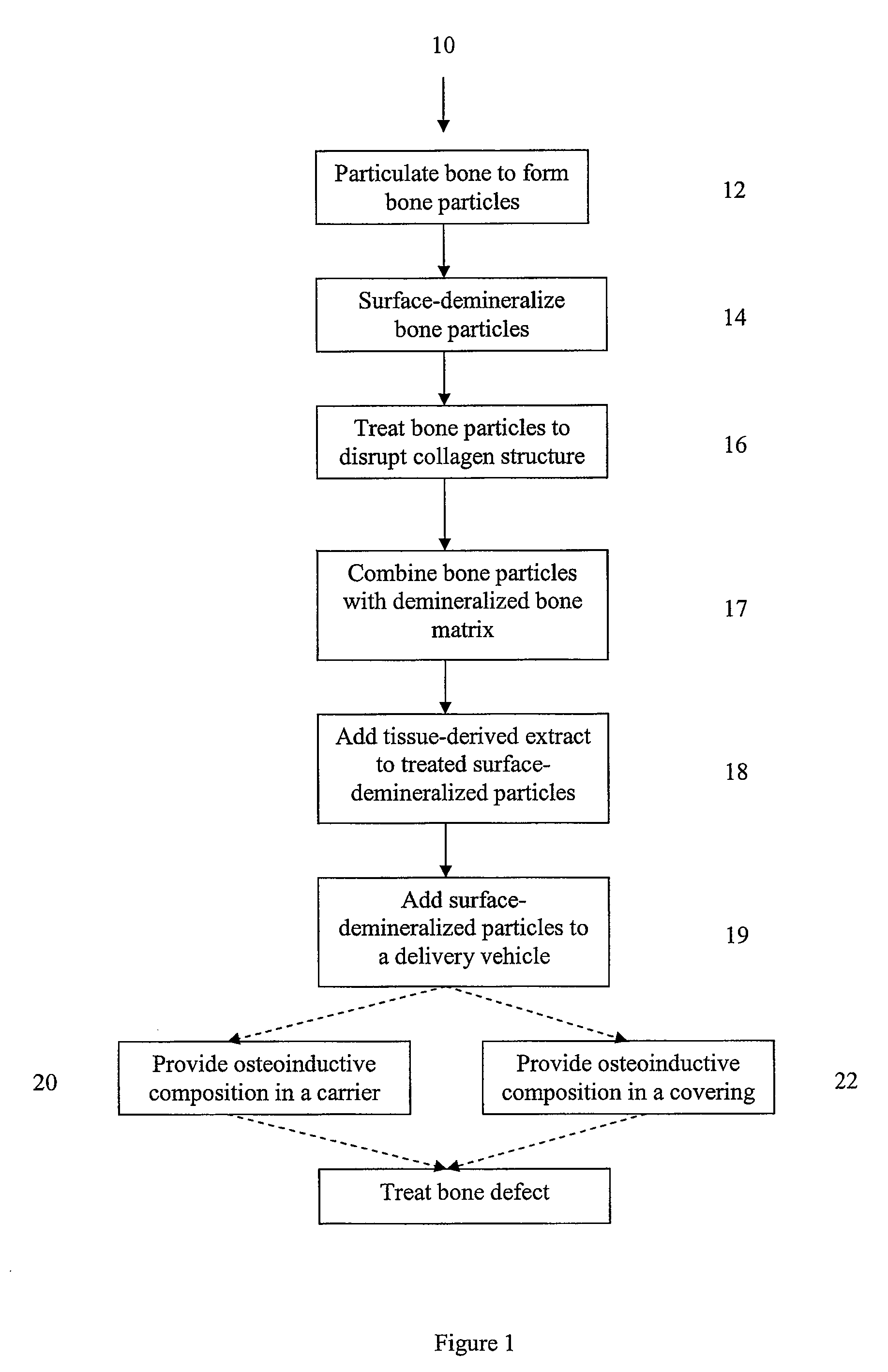
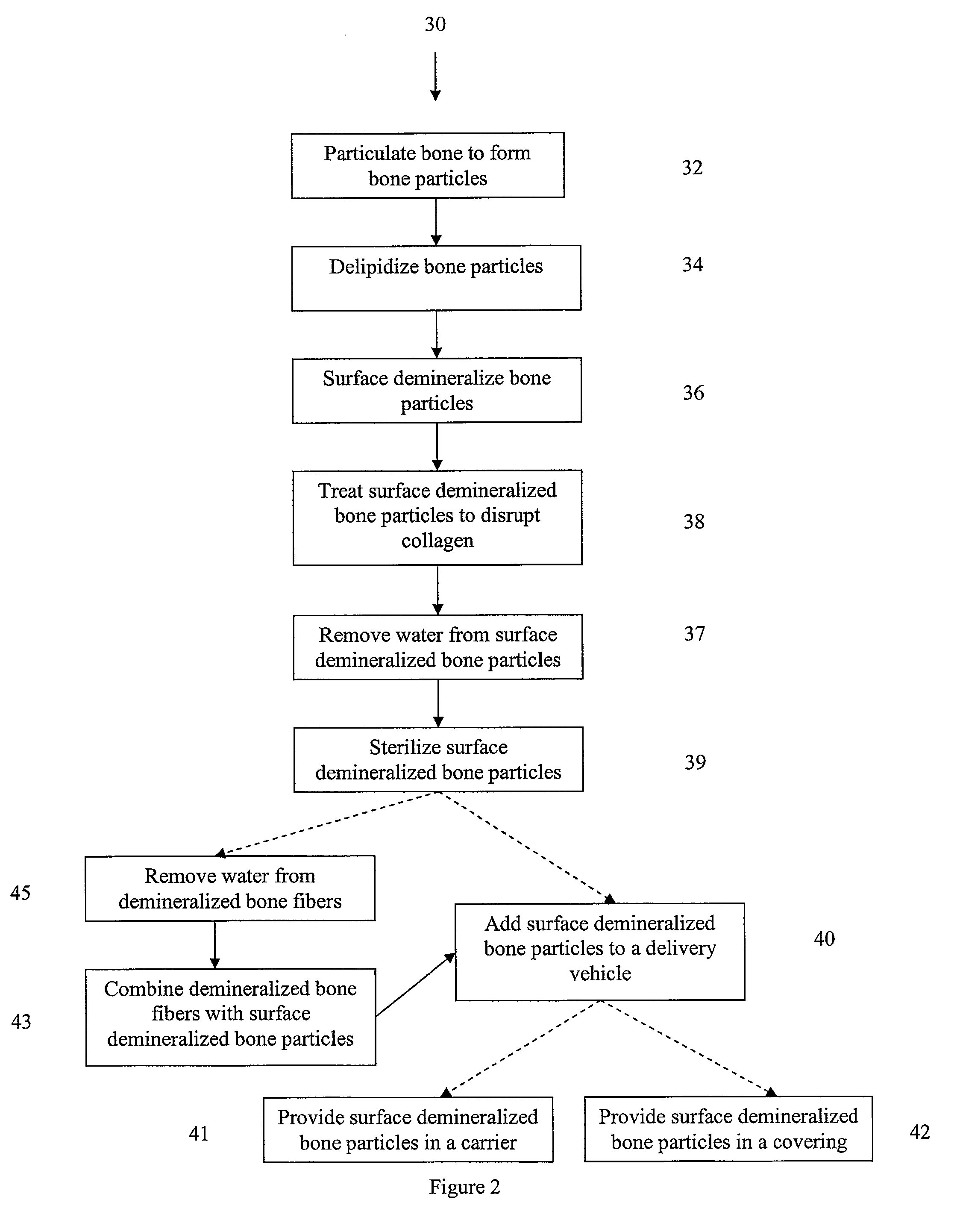
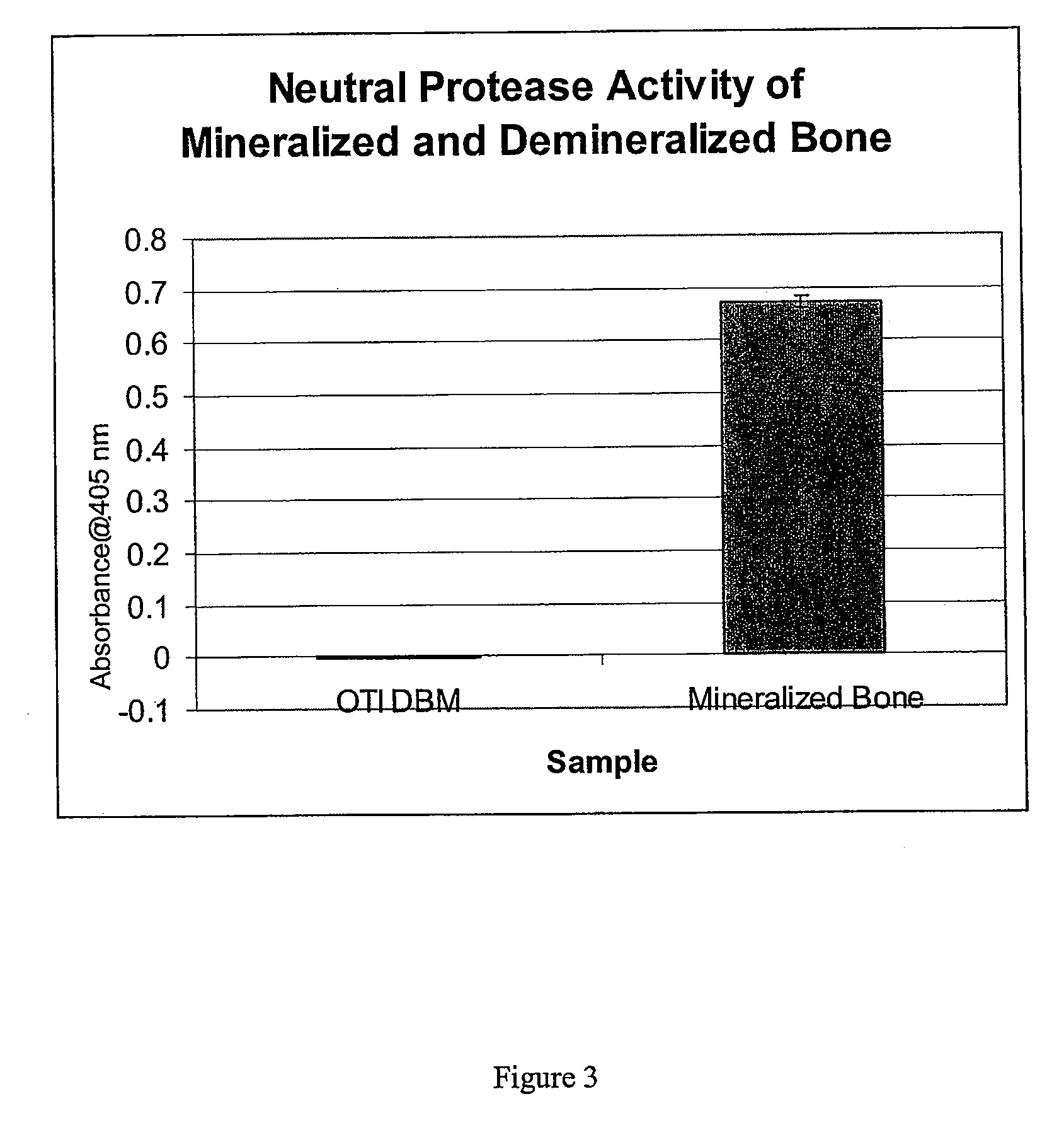
Bone matrix compositions and methods
Osteoinductive compositions and implants having increased biological activities, and methods for their production, are provided. The biological activities that may be increased include, but are not limited to, bone forming; bone healing; osteoinductive activity, osteogenic activity, chondrogenic activity, wound healing activity, neurogenic activity, contraction-inducing activity, mitosisinducing activity, differentiation-inducing activity, chemotactic activity, angiogenic or vasculogenic activity, and exocytosis or endocytosis-inducing activity. In one embodiment, a method for producing an osteoinductive composition comprises providing partially demineralized bone, treating the partially demineralized bone to disrupt the collagen structure of the bone, and optionally providing a tissue-derived extract and adding the tissue-derived extract to the partially demineralized bone. In another embodiment, an implantable osteoinductive and osteoconductive composition comprises partially demineralized bone, wherein the collagen structure of the bone has been disrupted, and, optionally, a tissue-derived extract.
Owner:WARSAW ORTHOPEDIC INC
Production of tissue engineered digits and limbs
ActiveUS20060257377A1Enhance tissue maturationImprove functionalityBiocidePeptide/protein ingredientsBone formingTissue construct
The invention pertains to methods of producing artificial composite tissue constructs that permit coordinated motion. Biocompatable structural matrices having sufficient rigidity to provide structural support for cartilage-forming cells and bone-forming cells are used. Biocompatable flexible matrices seeded with muscle cells are joined to the structural matrices to produce artificial composite tissue constructs that are capable of coordinated motion.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Bone graft
ActiveUS7163691B2Fast reduction in osteoinductiveGood curative effectOrganic active ingredientsImpression capsOSTEOINDUCTIVE FACTORIn vivo
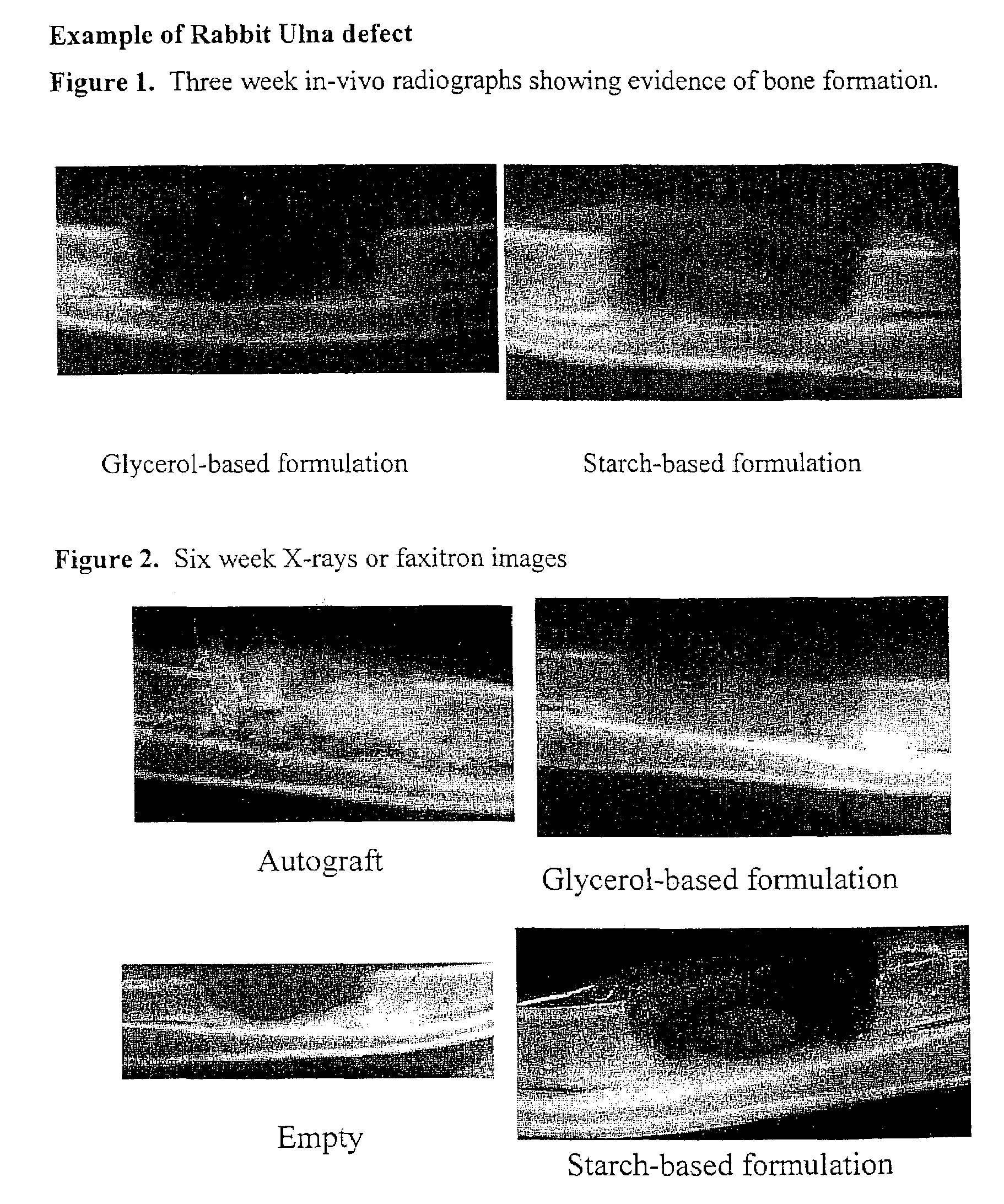
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-β, BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
Apparatus and methods for bone surgery
InactiveUS20050059978A1Joint implantsNon-surgical orthopedic devicesRight femoral headLeft femoral head
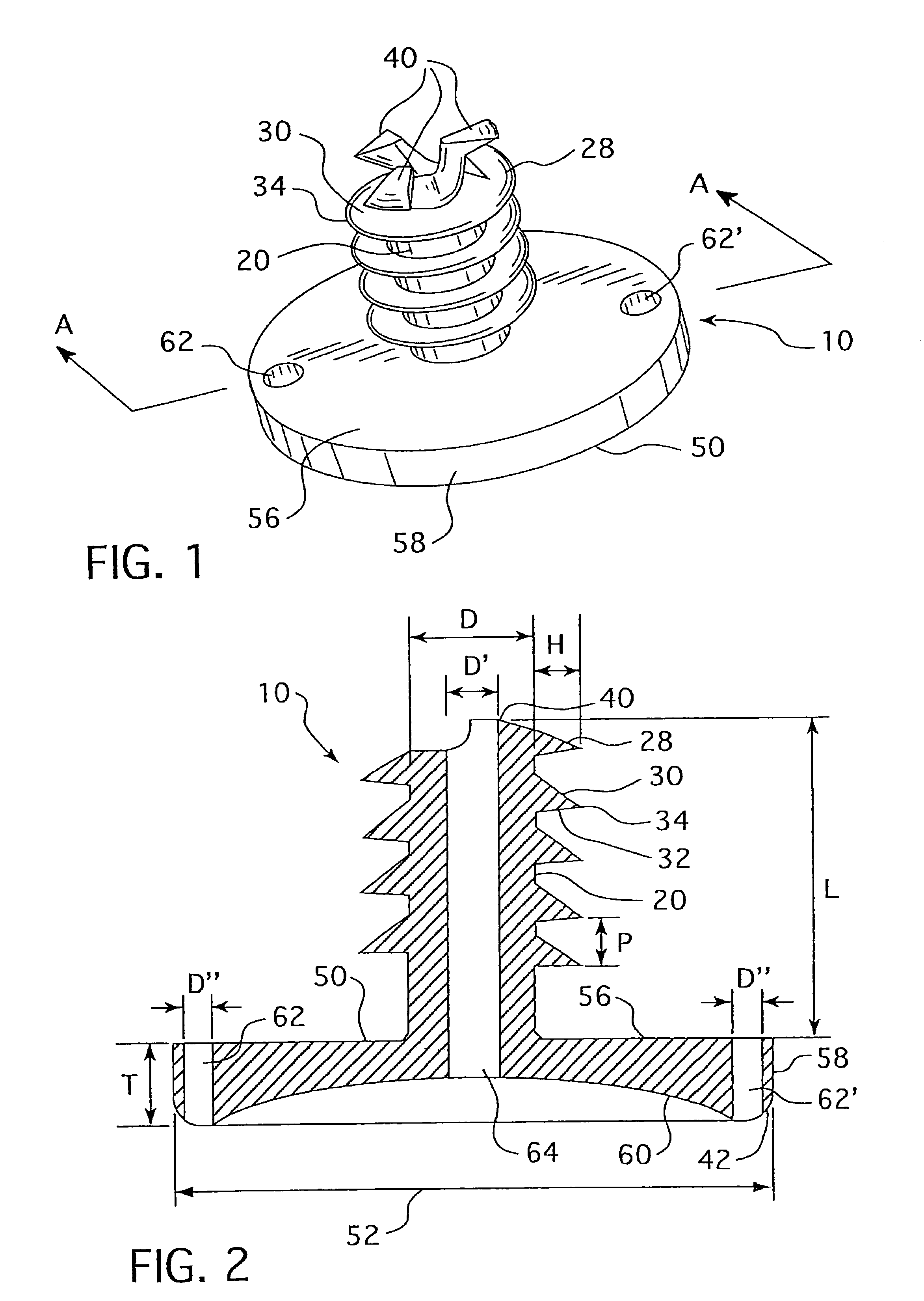
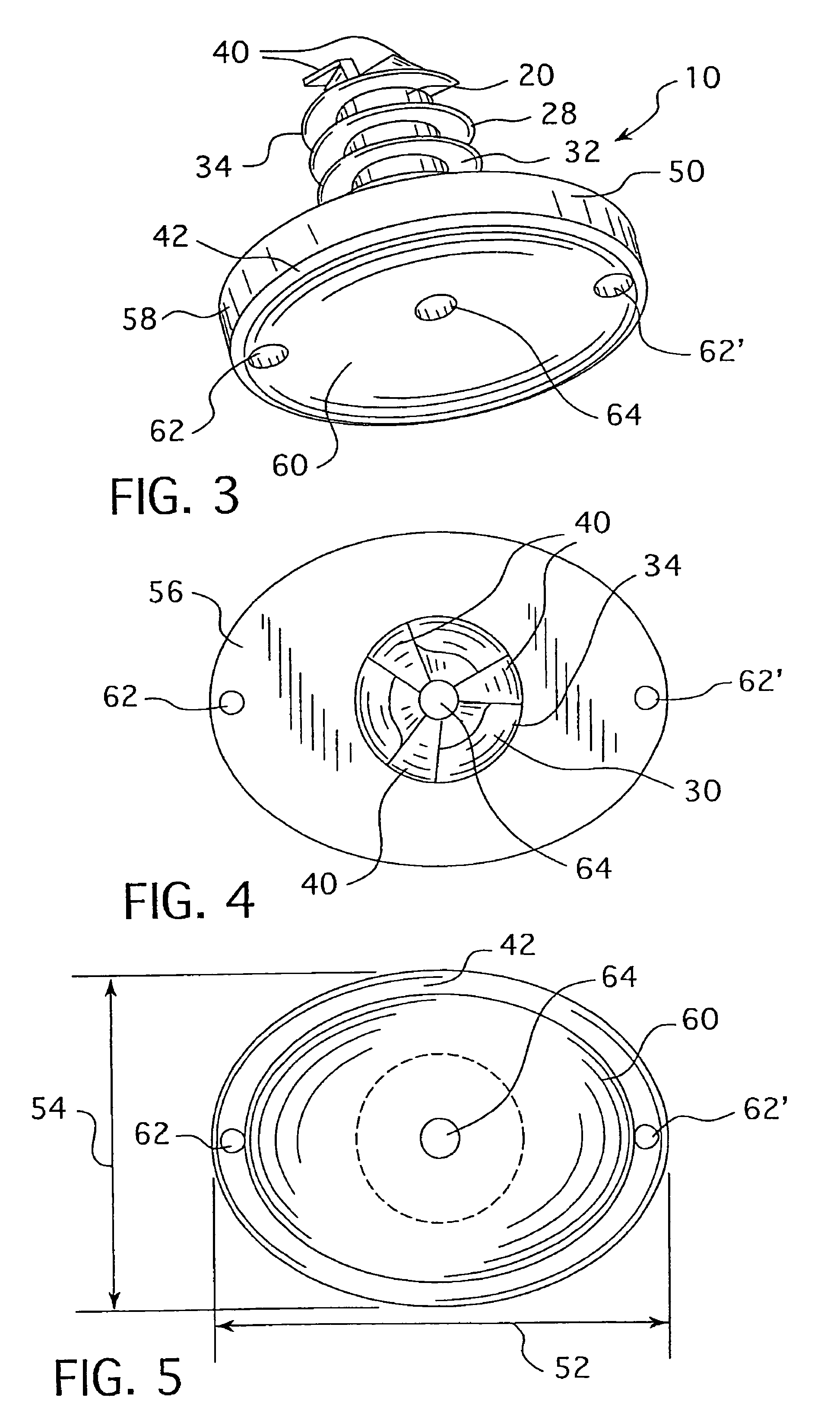
The surgeon grasps the jig by its handle and manipulates the head of the jig through the patient wound and onto the femoral neck. The head of the jig includes jig location means in the form of an elongate rod which acts as a spacer. The spacer has an end which abuts the trochanteric fossa so as to position the slot of the jig at the required position, which is between 5 mm and 25 mm, and most preferably 15 mm, from the trochantic fossa. Additional jig location means are provided by a surface adapted to receive a bone formation. This surface is provided by contours on the base of the head which are adapted to mate with contours of the femur. The slot is oriented generally perpendicularly to the elongate dimension of the rod. The slot functions as a surgical tool guide means which is positioned by the jig at the correct position for osteotomisation of the neck. Advantageously, osteotomisation takes place while the femoral head is still disposed within the acetabulum.
Owner:INT PATENT OWNERS CAYMAN
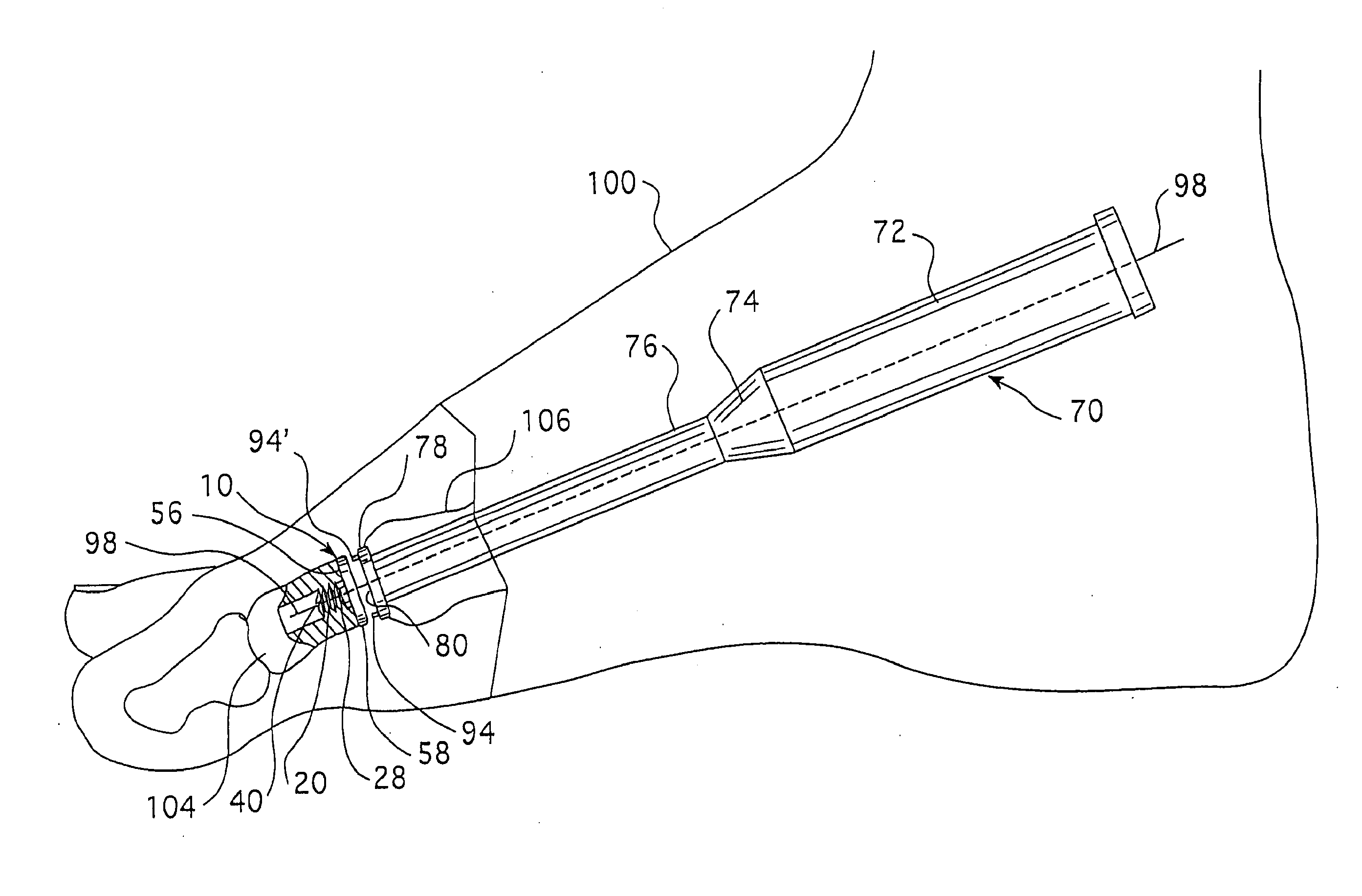
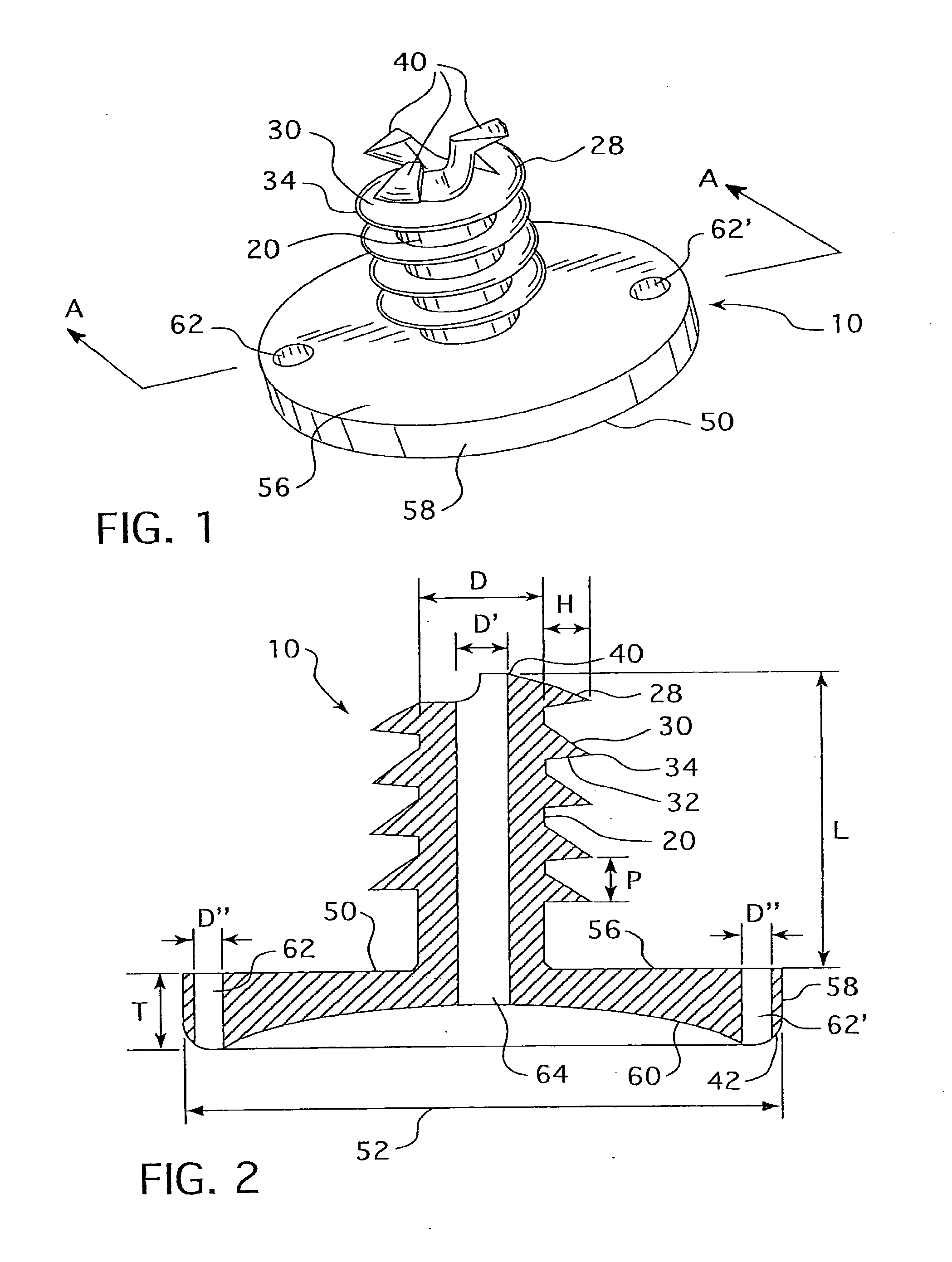
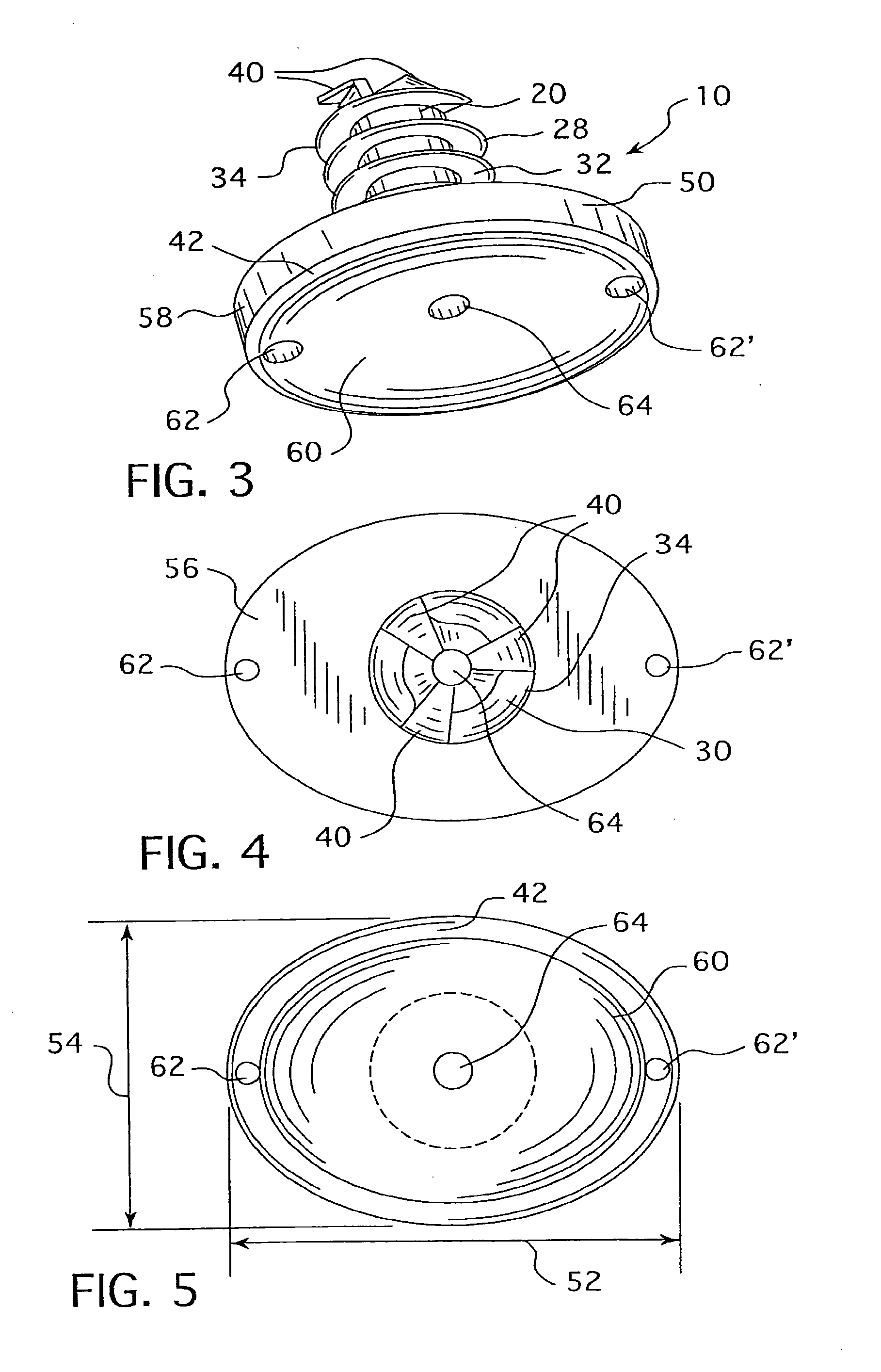
Cannulated hemi-implant and methods of use thereof
The present invention provides a cannulated hemi-implant with a base and a threaded stem for fixing the stem of the implant within a medullary canal of a phalanx or other bone in the foot. The implant includes a tri-part head capable of both cutting bone and self-threading, with bone forming the medullary canal of a phalanx of a human foot. The present invention also provides a hand tool which engages and rotates the implant within the medullary canal of the phalanx. The present invention further provides a method of surgically implanting the implant in the medullary canal of the phalanx or other bone.
Owner:VILEX LLC
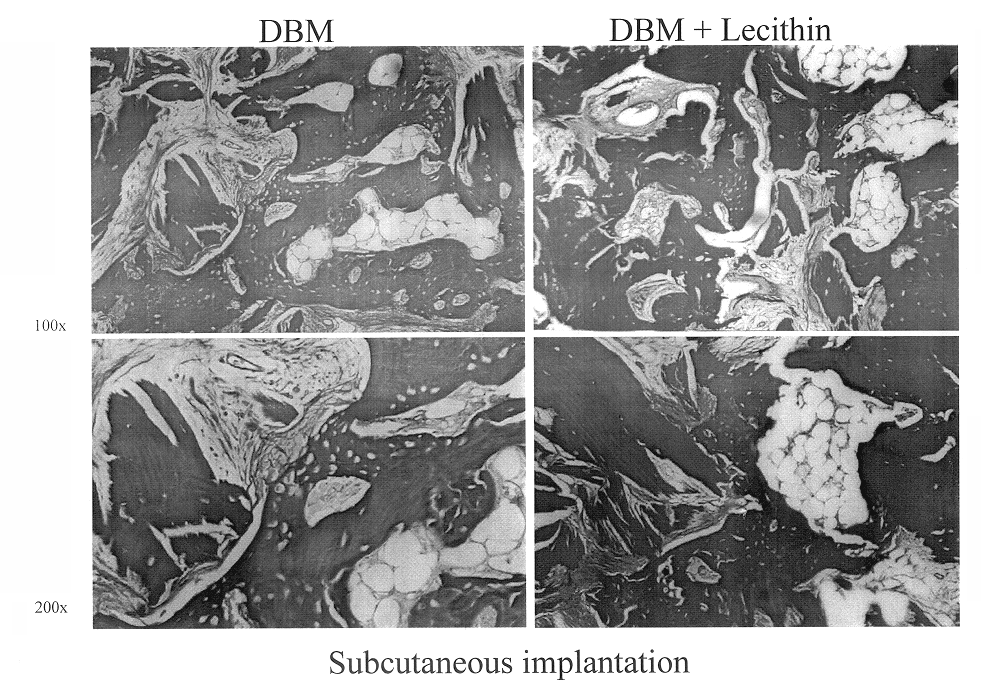
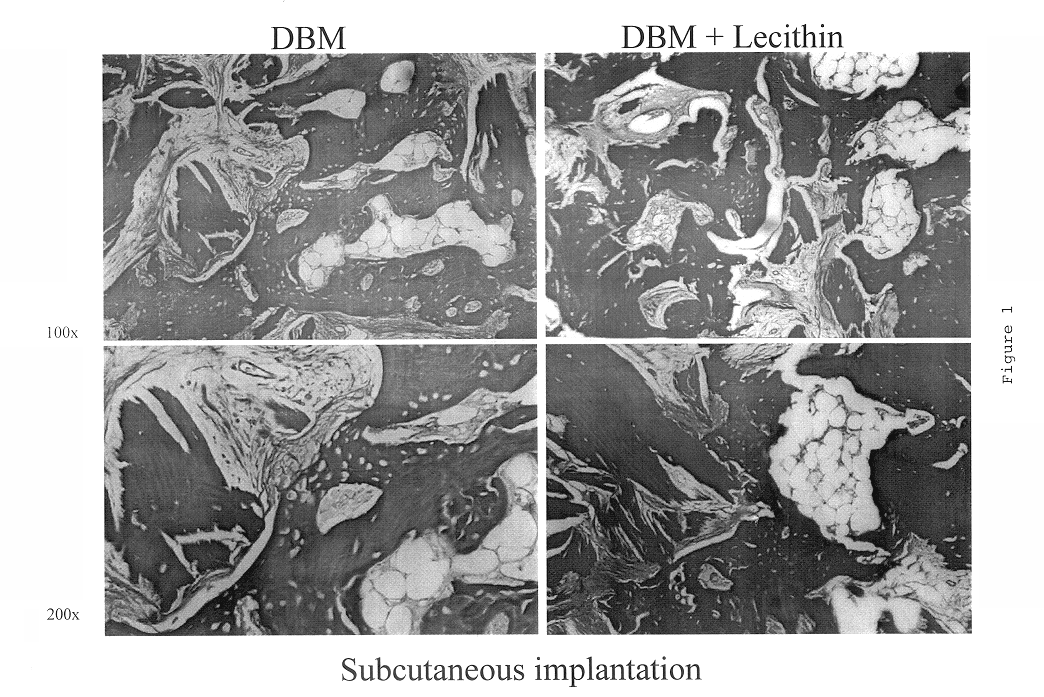
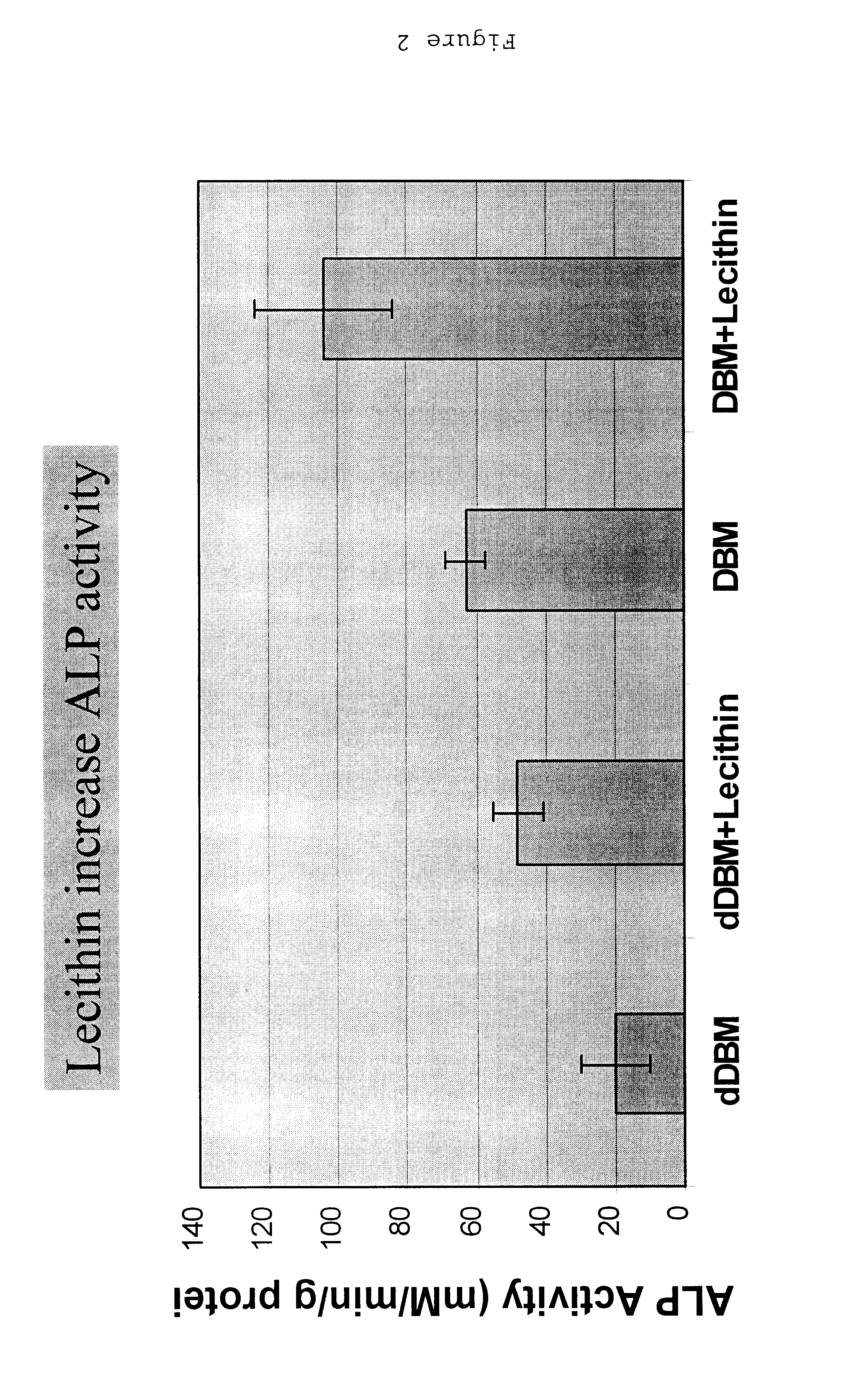
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Cannulated hemi-implant and methods of use thereof
The present invention provides a cannulated hemi-implant with a base and a threaded stem for fixing the stem of the implant within a medullary canal of a phalanx or other bone in the foot. The implant includes a tri-part head capable of both cutting bone and self-threading, with bone forming the medullary canal of a phalanx of a human foot. The present invention also provides a hand tool which engages and rotates the implant within the medullary canal of the phalanx. The present invention further provides a method of surgically implanting the implant in the medullary canal of the phalanx or other bone.
Owner:VILEX LLC
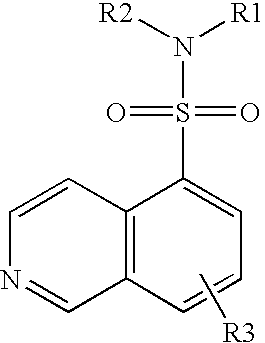
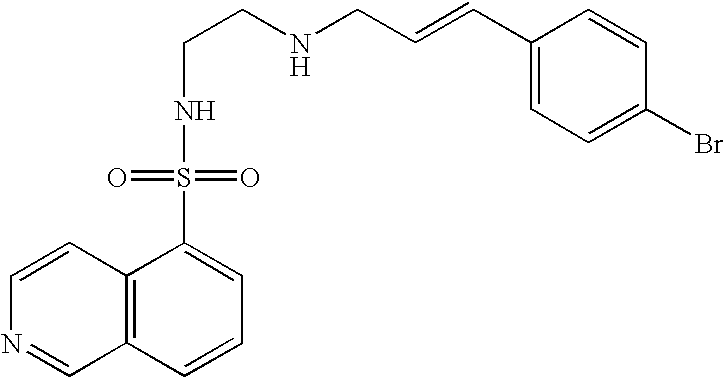
Nitrogen-containing heterocyclic compound
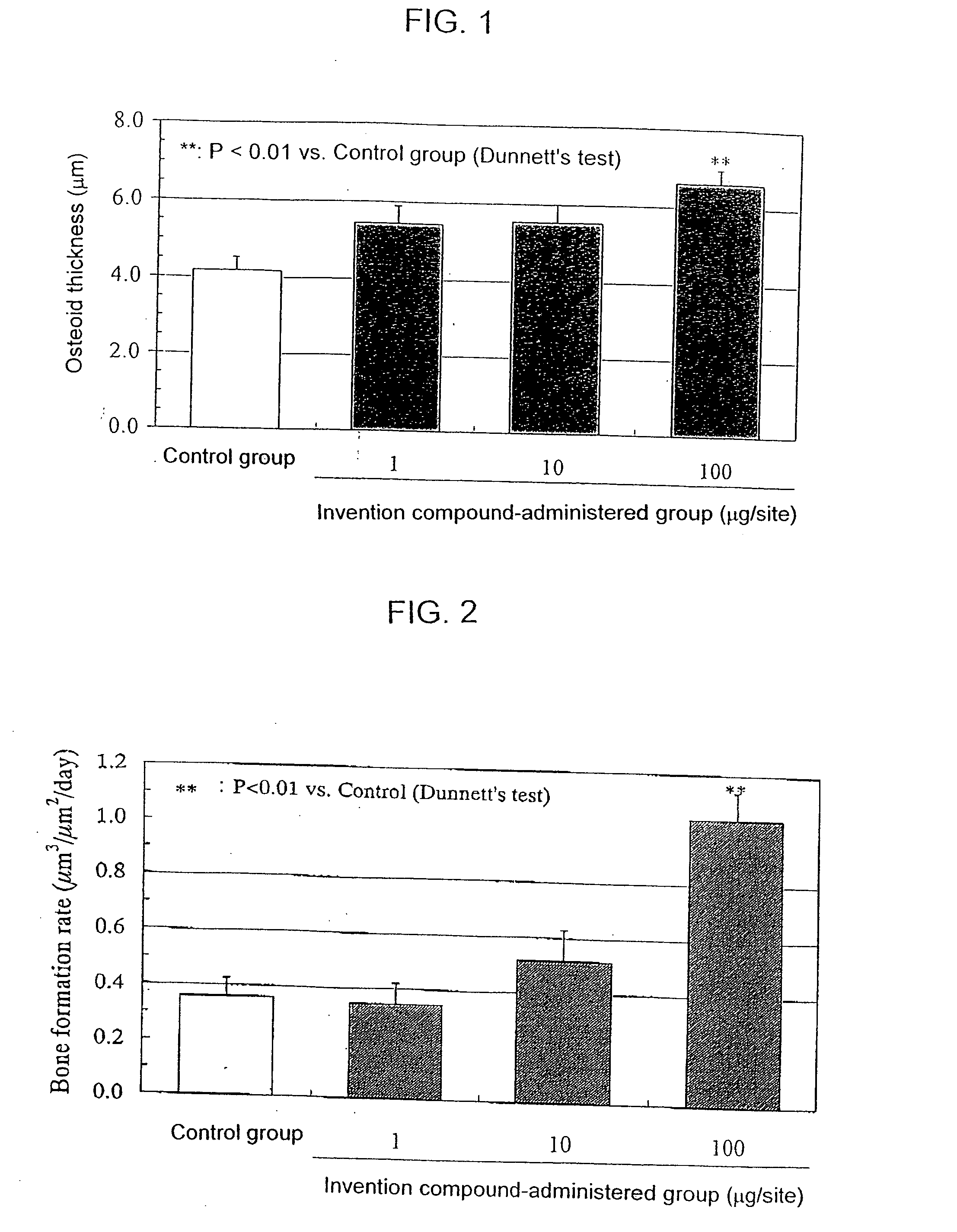
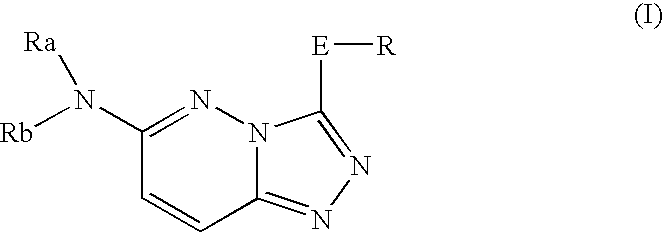
InactiveUS20050261297A1Excellent bone formation-stimulating effectIncrease ratingsBiocideOrganic chemistryPyridazineOsteoblast
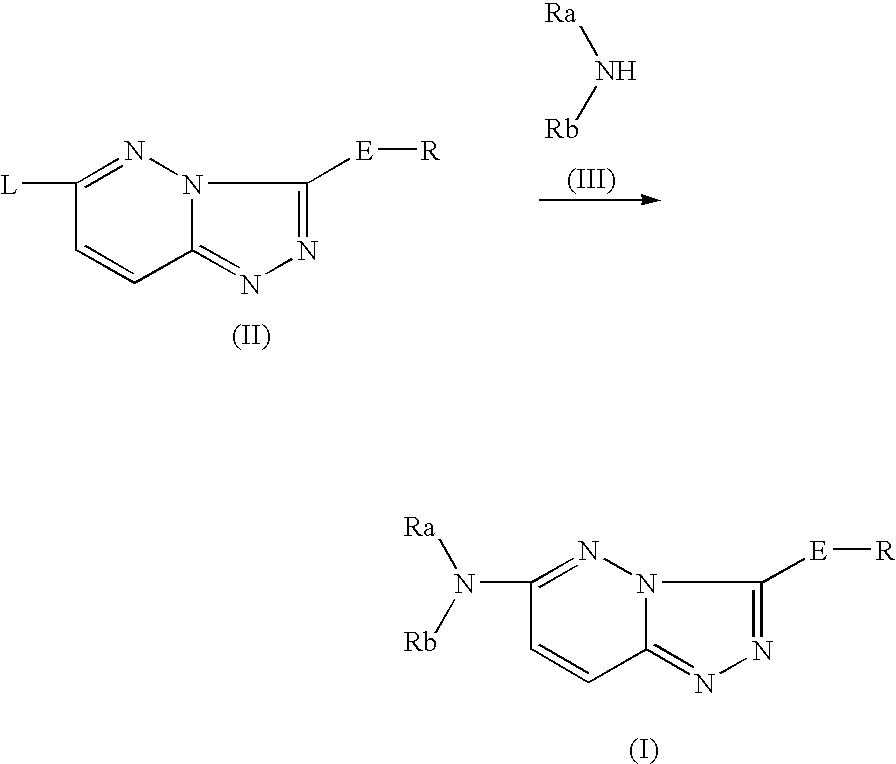
As a result of an effort made by us for the purpose of developing a therapeutic agent having a bone formation-stimulating effect by promoting the functions of osteoblasts, the present inventors discovered that a certain nitrogen-containing heterocyclic compound exhibits a potent bone formation-stimulating effect on the osteoblast and thus can serve as an excellent prophylactic or therapeutic agent against a metabolic bone disease, whereby establishing the present invention. Thus, the present invention provides a 3,6-disubstituted 1,2,4-triazolo[4,3-b]pyridazine compound or a pharmaceutically acceptable salt thereof as well as a pharmaceutical composition comprising such a compound and a pharmaceutically acceptable carrier, especially a bone-forming agent.
Owner:ASTELLAS PHARMA INC
Arthroplasty system and related methods
A method of manufacturing an arthroplasty jig is disclosed herein. The method may include the following: generate two dimensional image data of a patient joint to undergo arthroplasty, identify in the two dimensional image data a first point corresponding to an articular surface of a bone forming the joint, identify a second point corresponding to an articular surface of an implant, identify a location of a resection plane when the first point is correlated with the second point, and create the arthroplasty jig with a resection guide located according to the identified location of the resection plane.
Owner:HOWMEDICA OSTEONICS CORP
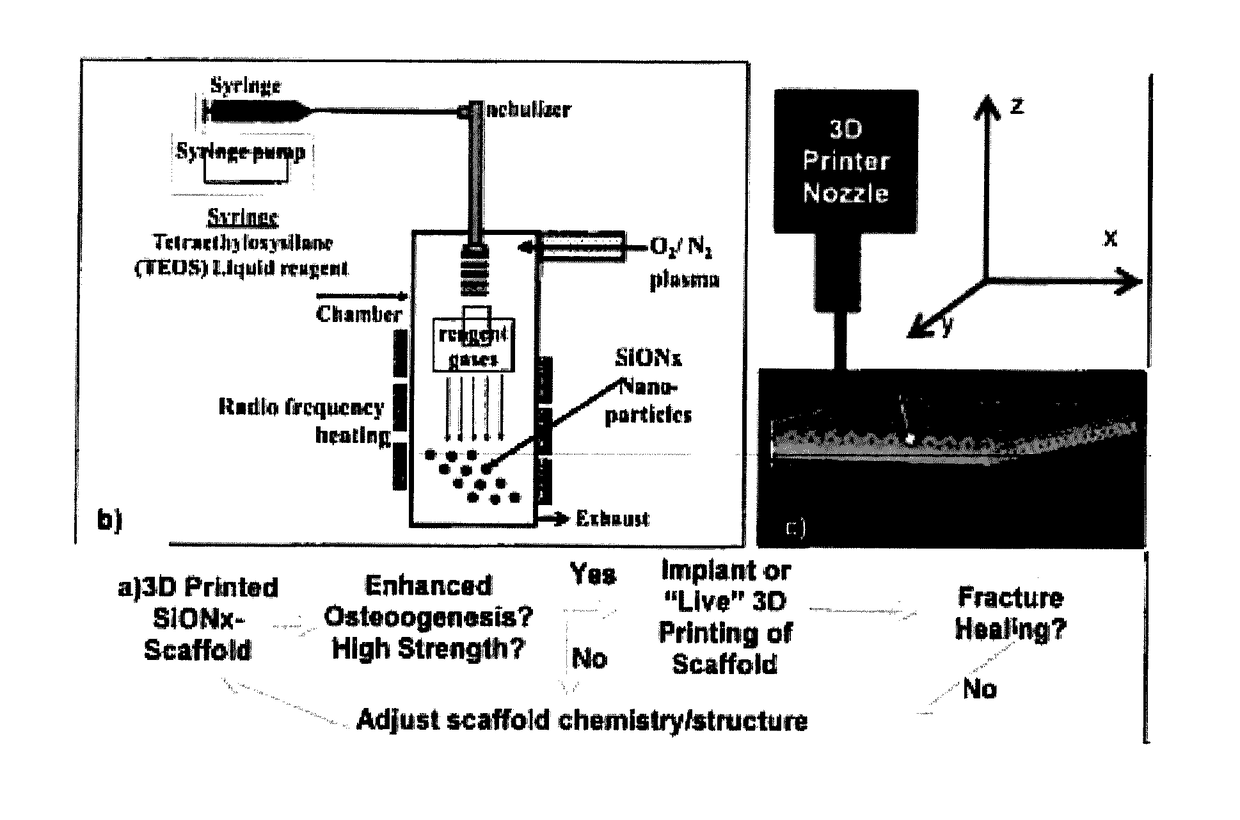
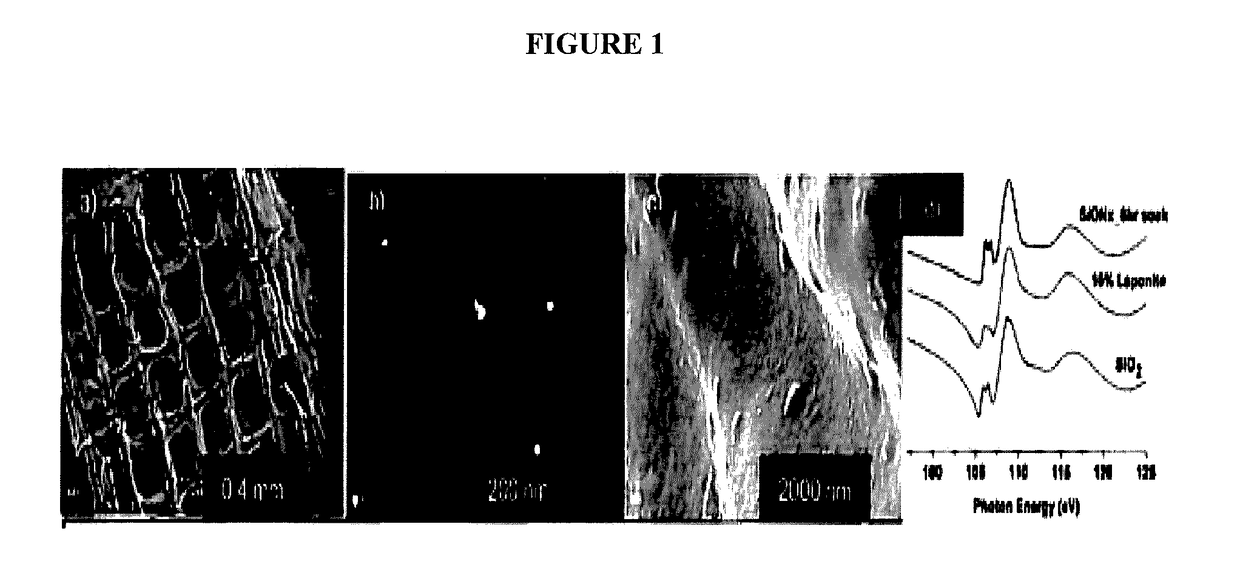
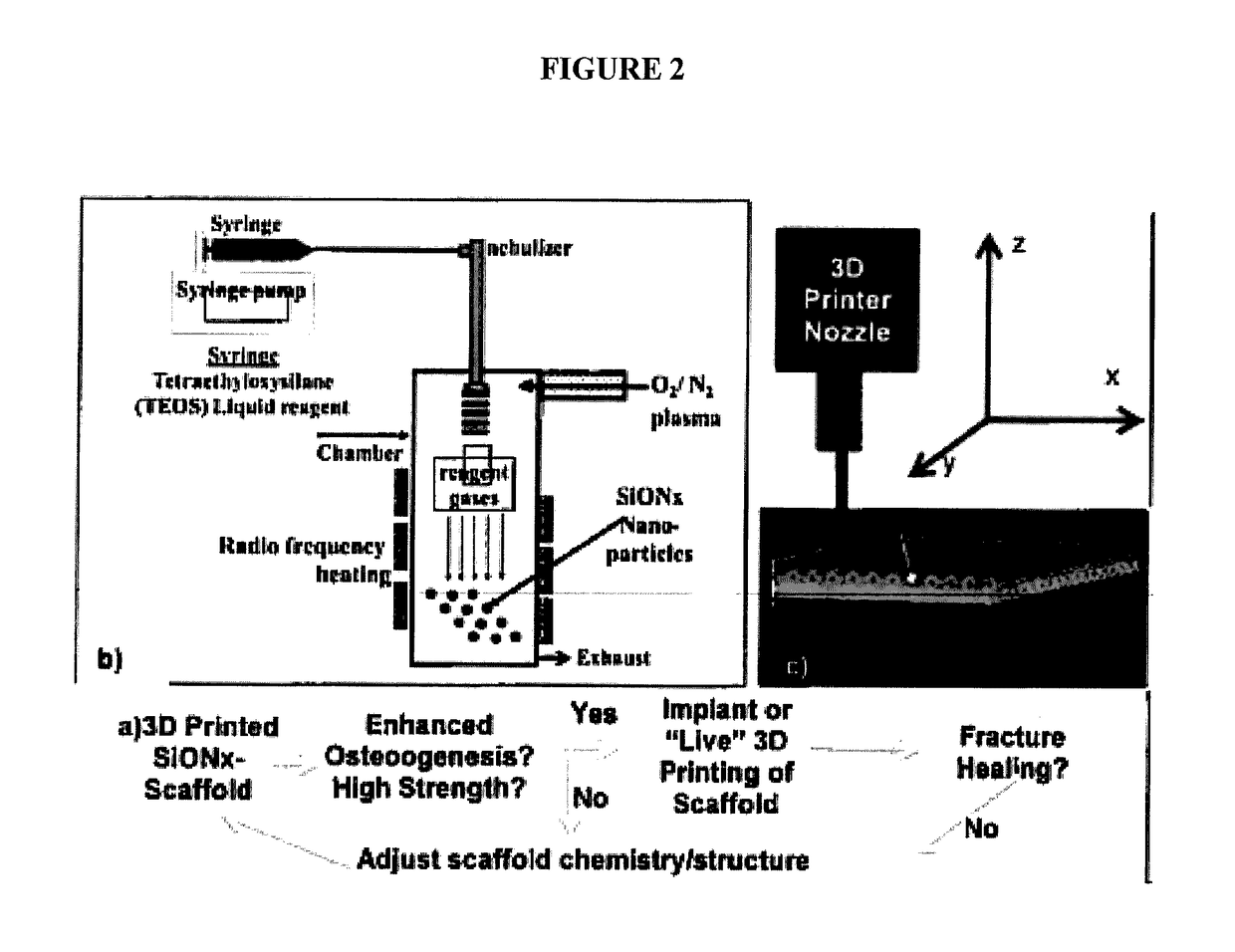
In vivo live 3D printing of regenerative bone healing scaffolds for rapid fracture healing
ActiveUS20170143831A1Promote degradationPowder deliveryOrganic active ingredientsCross-linkPoint of care
Bio-Inks and methods of using compositions comprising the bio-Inks are disclosed. 3-D tissue repair and regeneration through precise and specific formation of biodegradable tissue scaffolds in a tissue site using the bio-inks are also provided. Specific methylacrylated gelatin hydrogels (MAC) and methacrylated chitosan (MACh) preparations formulated with sucrose, a silicate-containing component (such as laponite), and / or a cross-linking agent (such as a photo-initiator or chemical initiator), as well as powdered preparations of these, are also disclosed. Kits containing these preparations are provided for point-of-care tissue repair in vivo. Superior, more complete (up to 99.85% tissue regeneration within 4 weeks applied in situ), and rapid in situ tissue repair and bone formation are also demonstrated.
Owner:TEXAS A&M UNIVERSITY +1
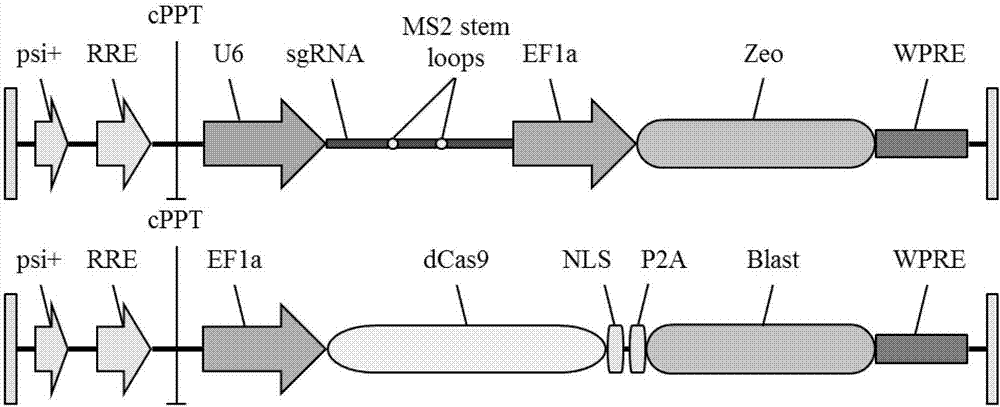
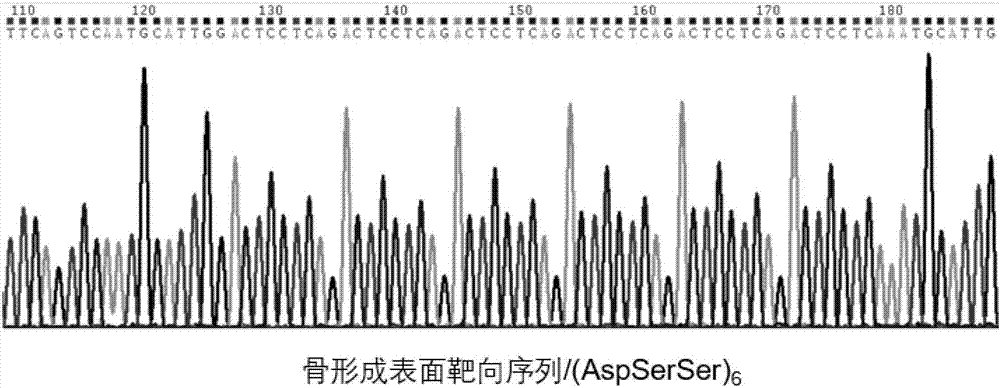
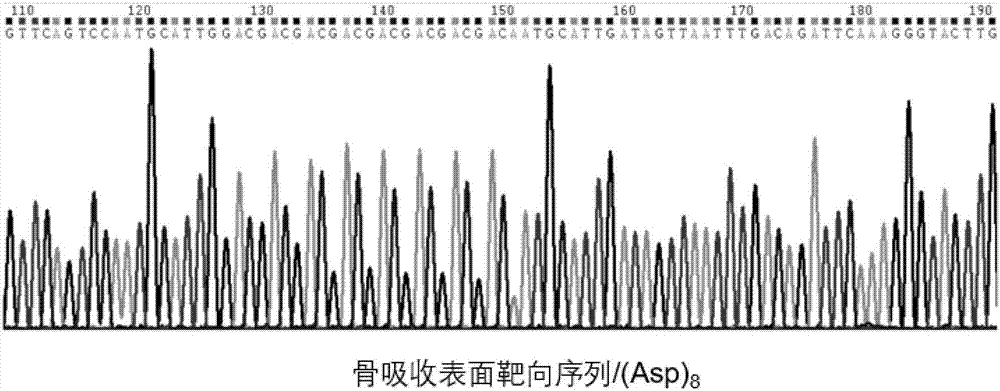
Exosome carrier of targeted bone, CRISPR/Cas9 gene editing system and application
ActiveCN107034188APlay gene editingFunctionHydrolasesStable introduction of DNATarget peptideExosome
The invention relates to an exosome carrier of a targeted bone, a CRISPR / Cas9 gene editing system and application. The exosome carrier comprises a targeted peptide of any one of a targeted bone forming surface, a bone absorbing surface and an endothelial cell. The exosome carrier can be fused with (AspSerSer)6, (Asp)8 and a CREDVW oligopeptide respectively by transforming an exosome surface membrane protein lamp2b, so that an exosome can be fused with the targeted bone forming surface, the bone absorbing surface and the endothelial cell. Therefore, the bone targeting exosome is developed as the carrier, a CRISPR / Cas9 system serves as a gene editing and controlling tool, and the two parts are combined to play the important significance on the treatment of various bone diseases.
Owner:HOSPITAL OF STOMATOLOGY SUN YAT SEN UNIV
Cannulated Hemi-Implant and Methods of Use Thereof
Owner:VILEX LLC
Device and Process for Producing Fiber Products and Fiber Products Produced Thereby
InactiveUS20100196333A1Relieve pressureDesired substrate propertiesBiocideBone implantFiberBone forming
The present invention is directed to a fiber, preferably bone fiber, having a textured surface, which acts as an effective binding substrate for bone-forming cells and for the induction or promotion of new bone growth by bone-forming cells, which bind to the fiber. Methods of using the bone fibers to induce or promote new bone growth and bone material compositions comprising the bone fibers are also described. The invention further relates to a substrate cutter device and cutter, which are effective in producing substrate fibers, such as bone fibers.
Owner:LIFENET HEALTH
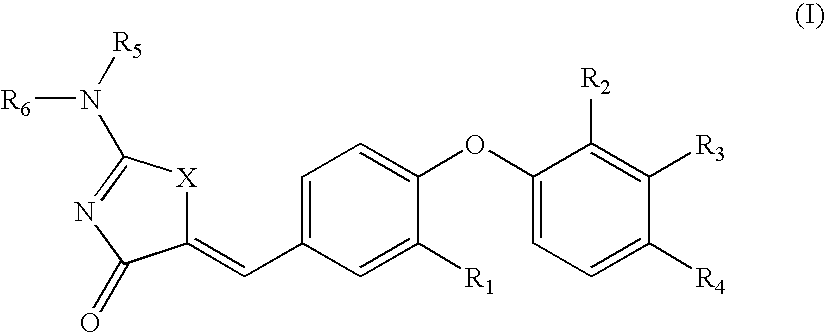
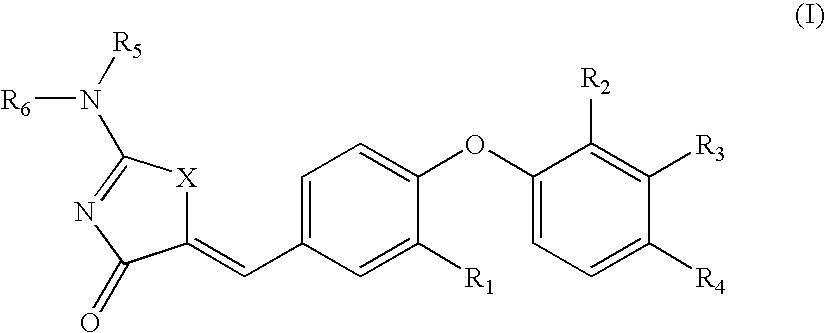
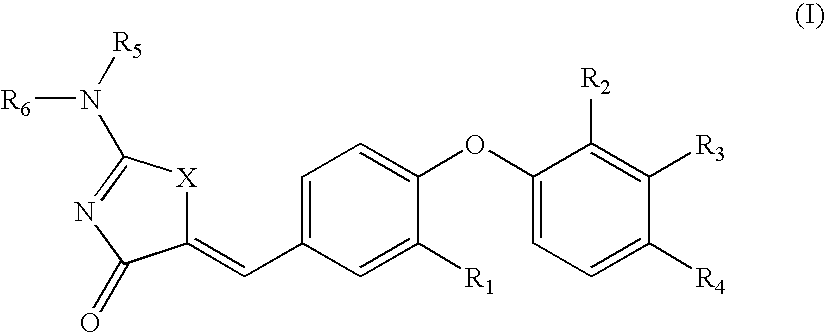
SUBSTITUTED PHENOXY AMINOTHIAZOLONES as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Nell peptide expression systems and bone formation activity of nell peptide
ActiveUS20060292670A1Facilitate protein traffickingFacilitate post production modificationOrganic active ingredientsBacteriaBone formationBone growth factor
The invention generally relates to a bone growth factor, and more particularly to compositions including NELL1, articles of manufacture including NELL1 and methods of using NELL1 to induce bone formation. This invention also provides methods for the expression and purification of NELL1 and NELL2 peptides.
Owner:RGT UNIV OF CALIFORNIA
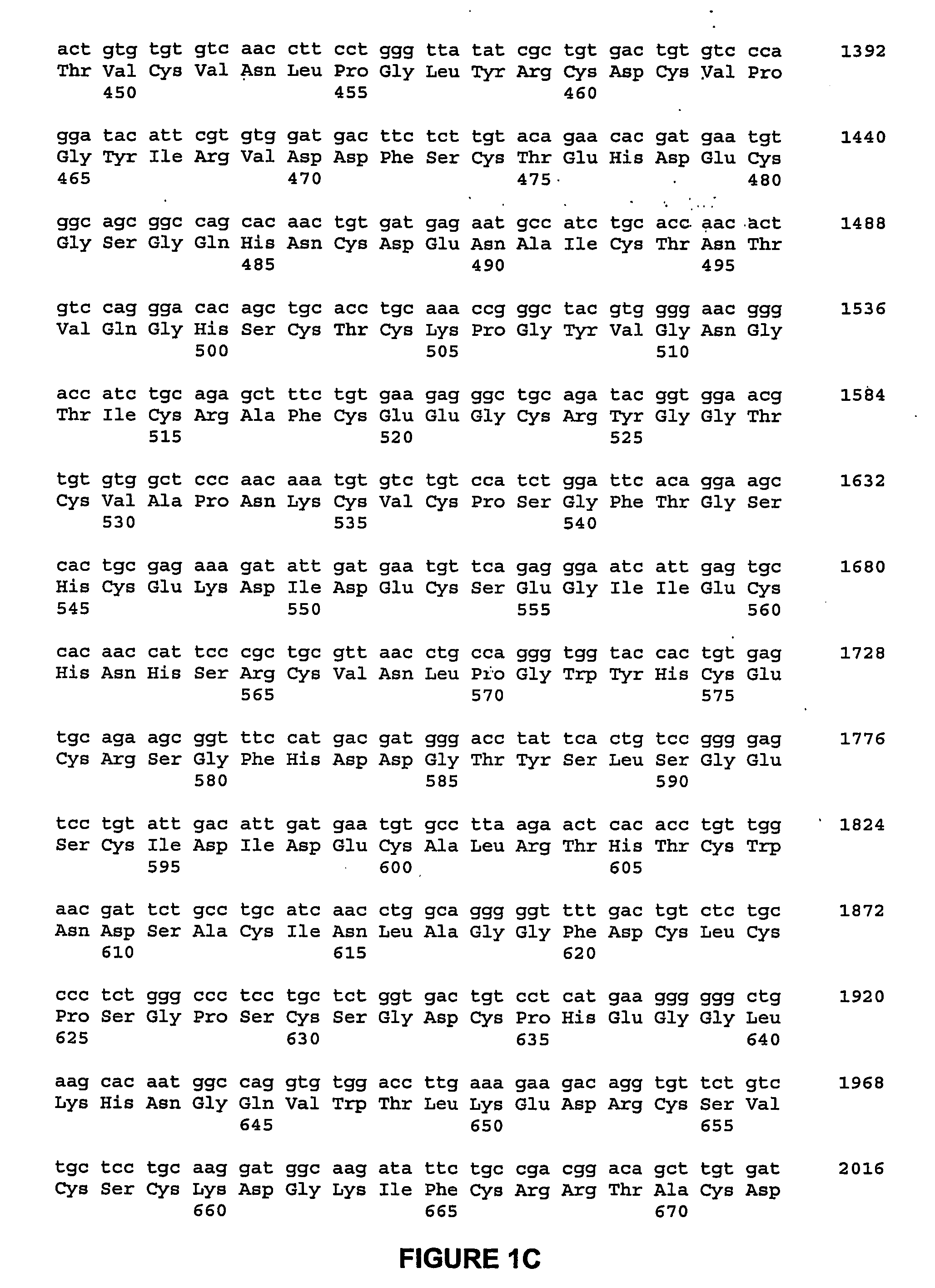
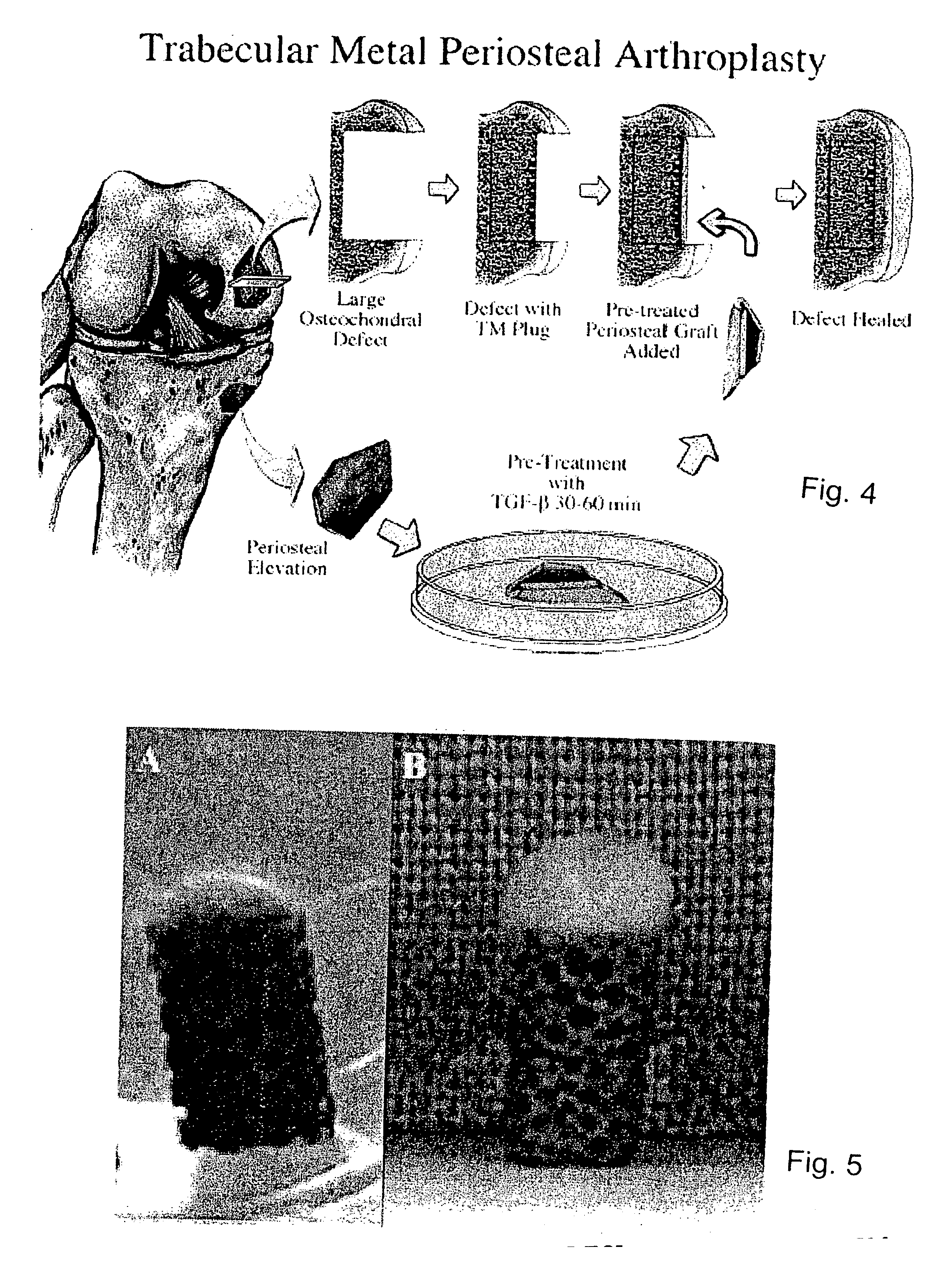
Biosynthetic composite for osteochondral defect repair
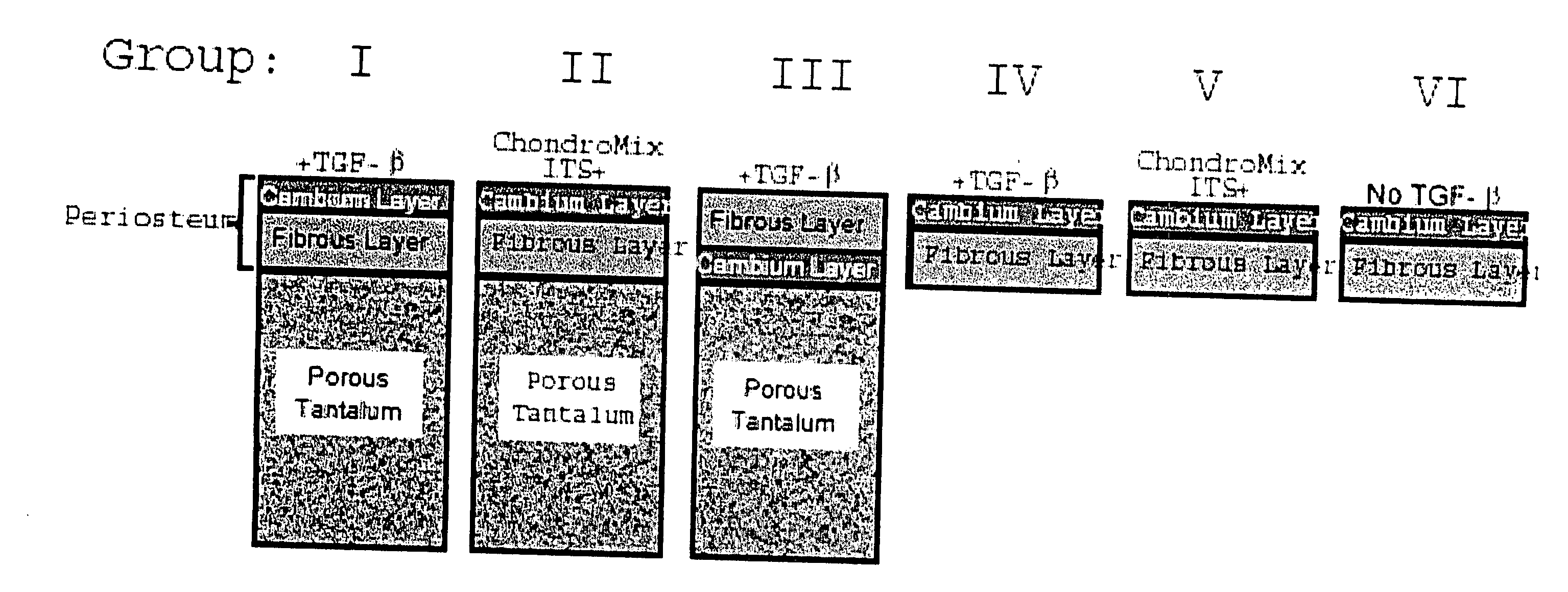
A composite for osteochondral defect repair includes a porous scaffold and a periosteal graft secured to a surface of the scaffold. The composite provides cartilage growth from autologous periosteum chondrogenesis. Biological resurfacing of large osteochondral defects, or a complete joint is feasible using the porous scaffold / autologous periosteal composite. The use of this composite eliminates the necessity of using normal cartilage surface as a donor site and its respective associated morbidity. In one form, the strong bone integration capacity of a porous metal (e.g., tantalum) scaffold and the high grade of integration observed from periosteal chondrogenesis into the normal cartilage eliminates the lack of chondral-chondral integration observed in the autologous osteochondral graft technique.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
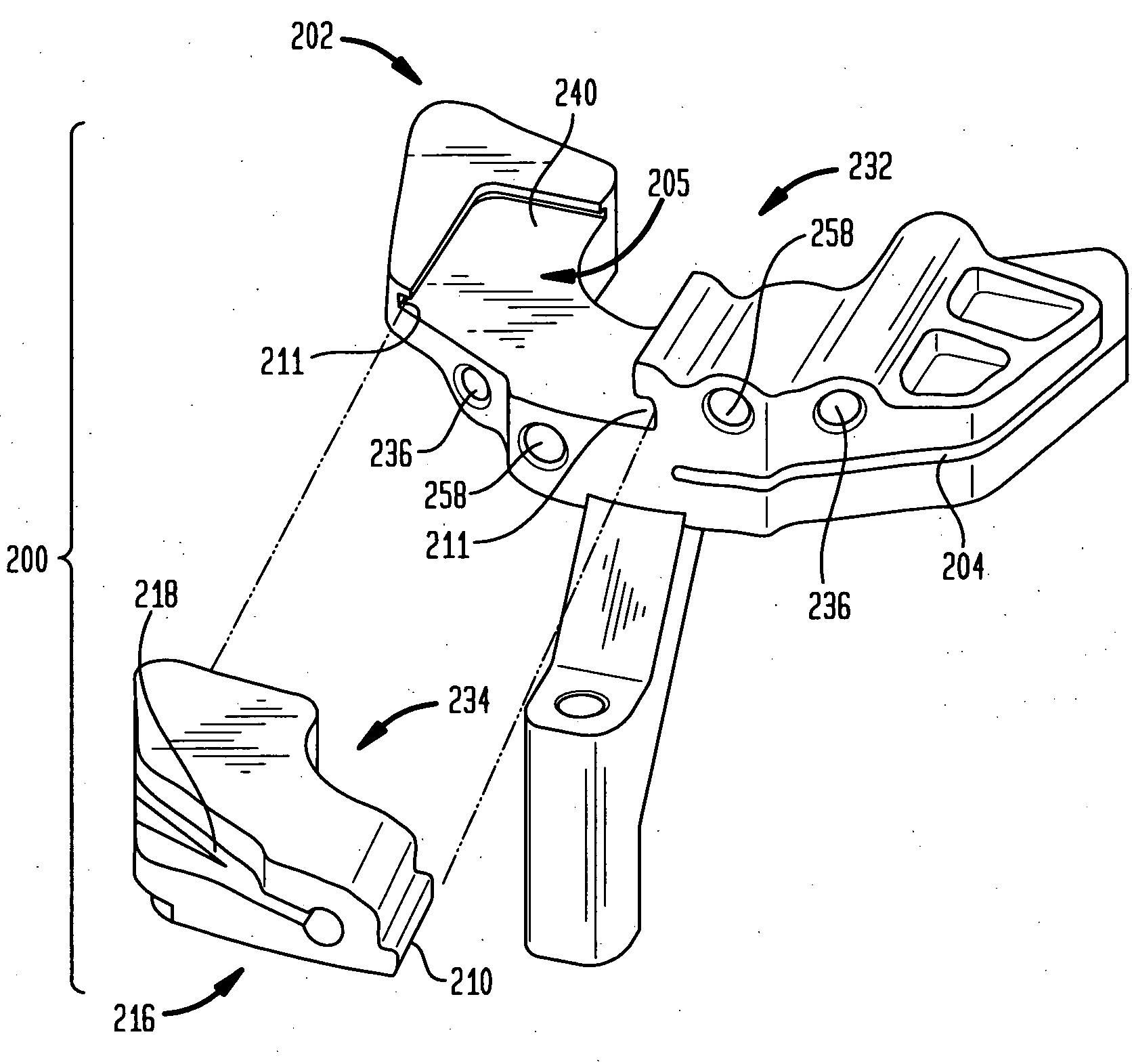
Prosthetic knee void filers with splined fixation
ActiveUS20120016482A1Lengthen the joint prosthesis systemRevision process easyJoint implantsKnee jointsKnee JointEngineering
Disclosed herein are systems and methods for filling bone voids which may be present at the time of surgery. The systems disclosed herein generally include a baseplate component, a spacer component, and void filler component. The spacer component is generally assembled to the baseplate component with a taper or press-fit, for example, in one of a plurality of selected axial positions. The void filler component is then generally assembled to the spacer component in one of a plurality of selected axial positions. The void filler component preferably has an outer surface with portions having varying diameters such that the outer surface thereof can be received within a canal of a bone and contact the bone forming the canal at different locations in order to aid in stabilizing the assembled components in the canal.
Owner:HOWMEDICA OSTEONICS CORP
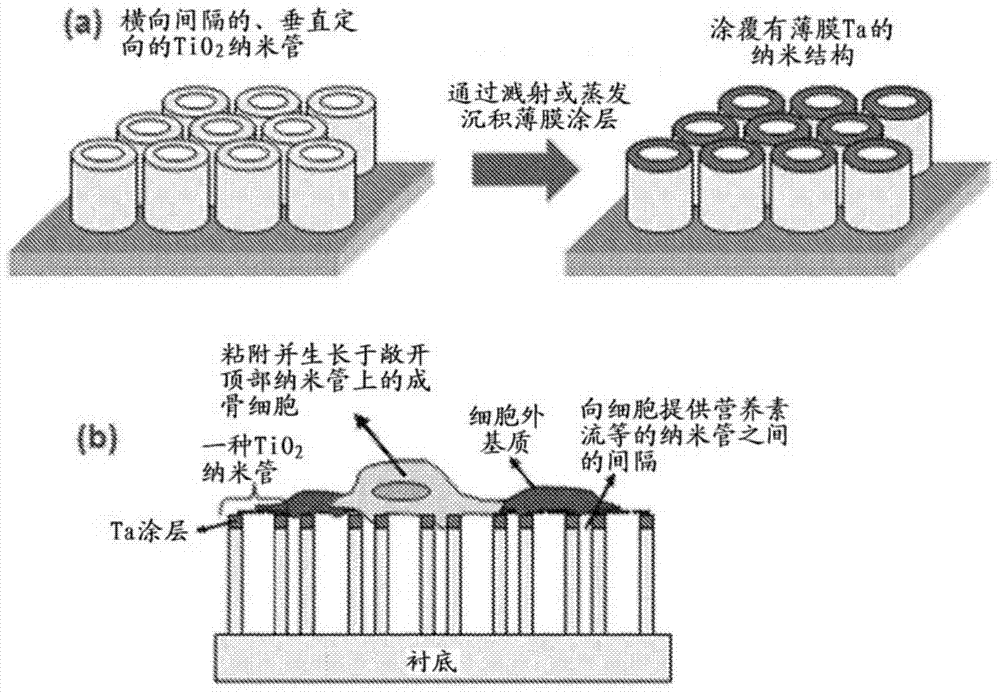
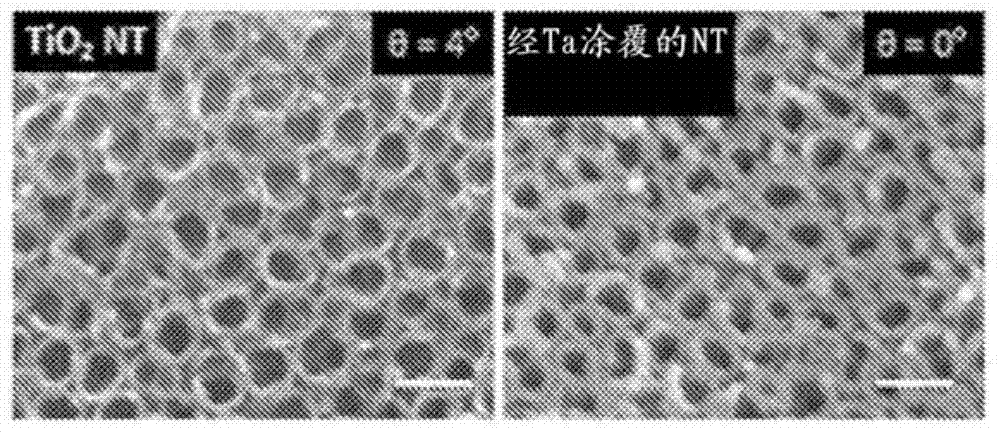
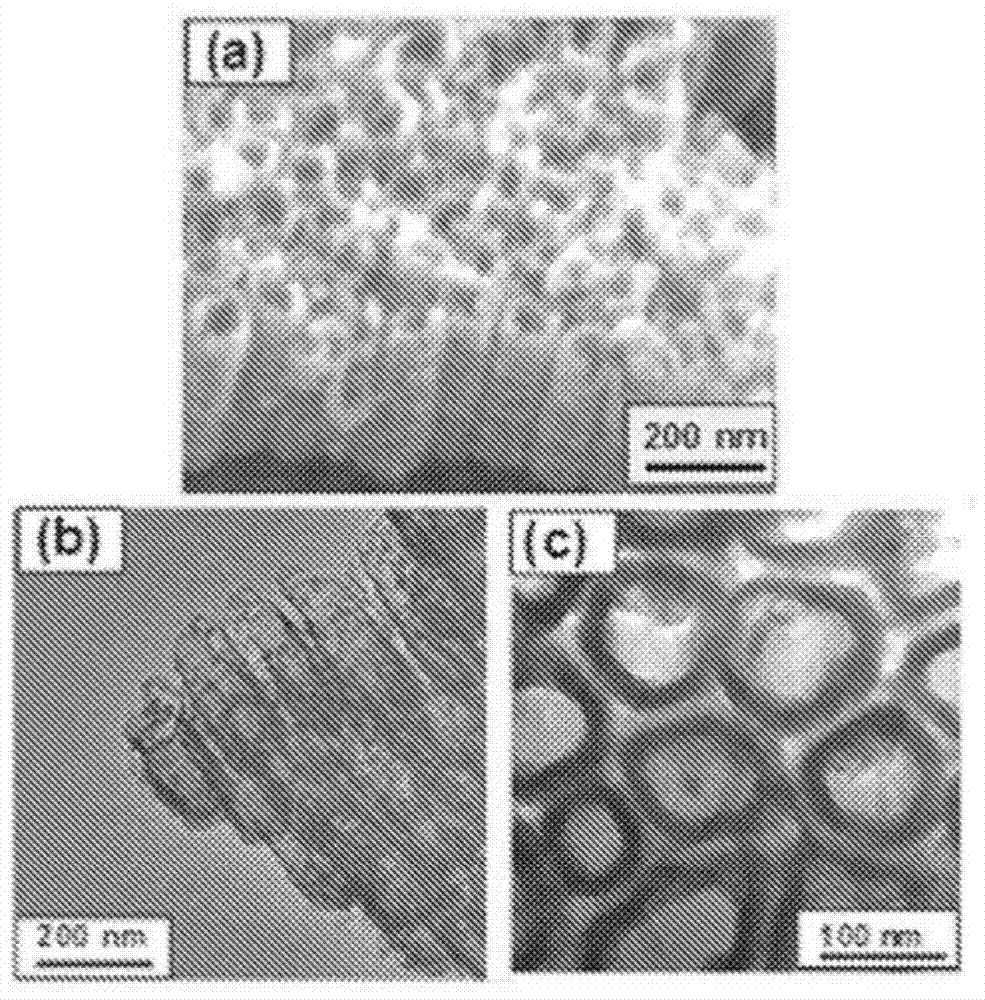
Products of manufacture having tantalum coated nanostructures, and methods of making and using them
In alternative embodiments, the invention provides products (articles) of manufacture comprising nanostructures such as nanotubes having a surface comprising tantalum. In alternative embodiments, products of manufacture of the invention include nanostructures, e.g., nanotubes, nanowire, nanopore, and the like comprising a surface layer of tantalum. In alternative embodiments, products or articles of manufacture of the invention are bioimplants, and the tantalum-surface-coated nanostructures of the invention provide increased bioactivity and bone forming ability. In alternative embodiments, products or articles of manufacture of the invention, e.g., bioimplants, comprising the tantalum-surface-coated nanostructures of the invention are used for in vitro, ex vivo and in vivo testing, implants, biomedical devices and therapeutics.
Owner:RGT UNIV OF CALIFORNIA
Bone Graft
ActiveUS20080145392A1Good curative effectSimple compositionAdditive manufacturing apparatusBone implantOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-.beta., BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions
Owner:WARSAW ORTHOPEDIC INC
Bone matrix compositions and methods
Owner:WARSAW ORTHOPEDIC INC
Device and process for producing fiber products and fiber products produced thereby
ActiveUS7744597B2Relieve pressureDesired substrate propertiesImpression capsBone implantFiberBone growth
The present invention is directed to a fiber, preferably bone fiber, having a textured surface, which acts as an effective binding substrate for bone-forming cells and for the induction or promotion of new bone growth by bone-forming cells, which bind to the fiber. Methods of using the bone fibers to induce or promote new bone growth and bone material compositions comprising the bone fibers are also described. The invention further relates to a substrate cutter device and cutter, which are effective in producing substrate fibers, such as bone fibers.
Owner:LIFENET HEALTH
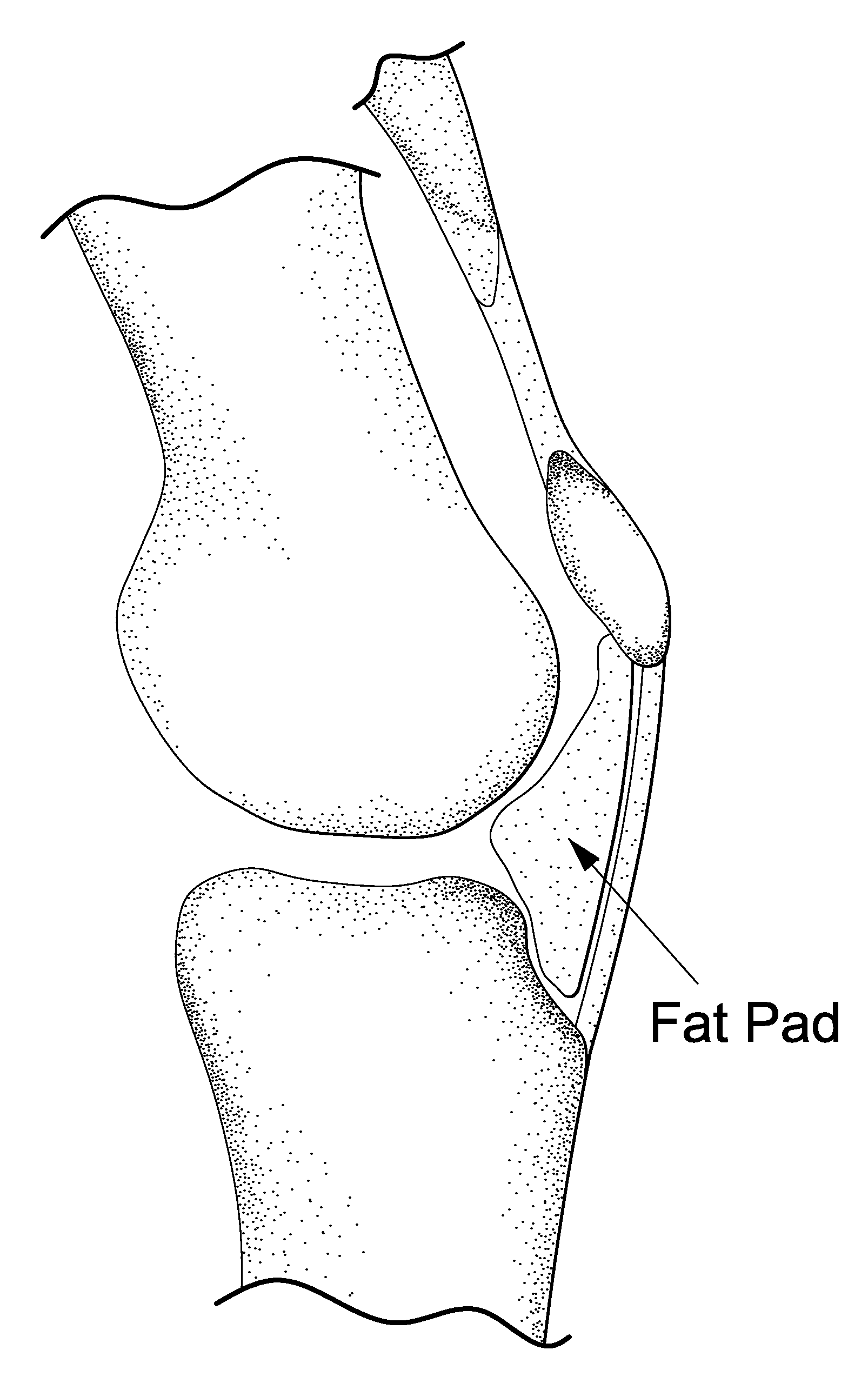
Method for improving cartilage repair and/or preventing cartilage degeneration in a joint
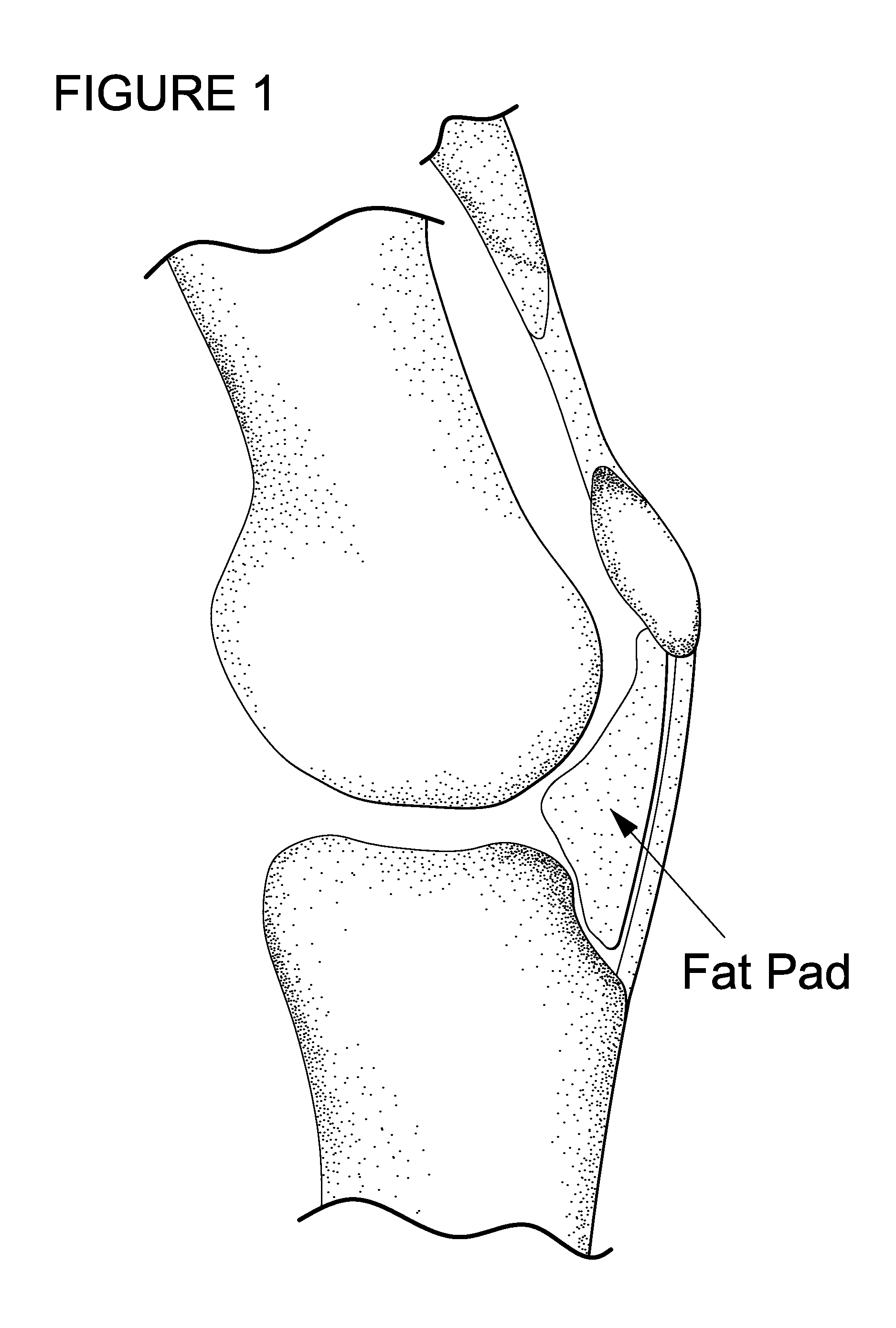
InactiveUS20100215731A1Convenient treatmentRelieve painPowder deliveryBiocideRepair tissueActive agent
The invention is in the field of methods for medical treatment. It provides an improved method for repairing damaged cartilage and / or preventing cartilage degeneration in tissue, in particular in a joint by administering a pharmaceutically active agent directly into the fat pad of a joint. The pharmaceutically active agent is preferably selected from the group consisting of agents that stimulate chondrogenic differentiation and / or cartilage matrix synthesis; agents that inhibit osteogenesis and / or hypertrophy, anti-inflammatory agents, agents that inhibit apoptosis of chondrocytes, agents that inhibit senescence of chondrocytes and agents that enhance lubrication of a joint.
Owner:ACADEMIC HOSPITAL MAASTRICHT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com