Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Cartilage formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
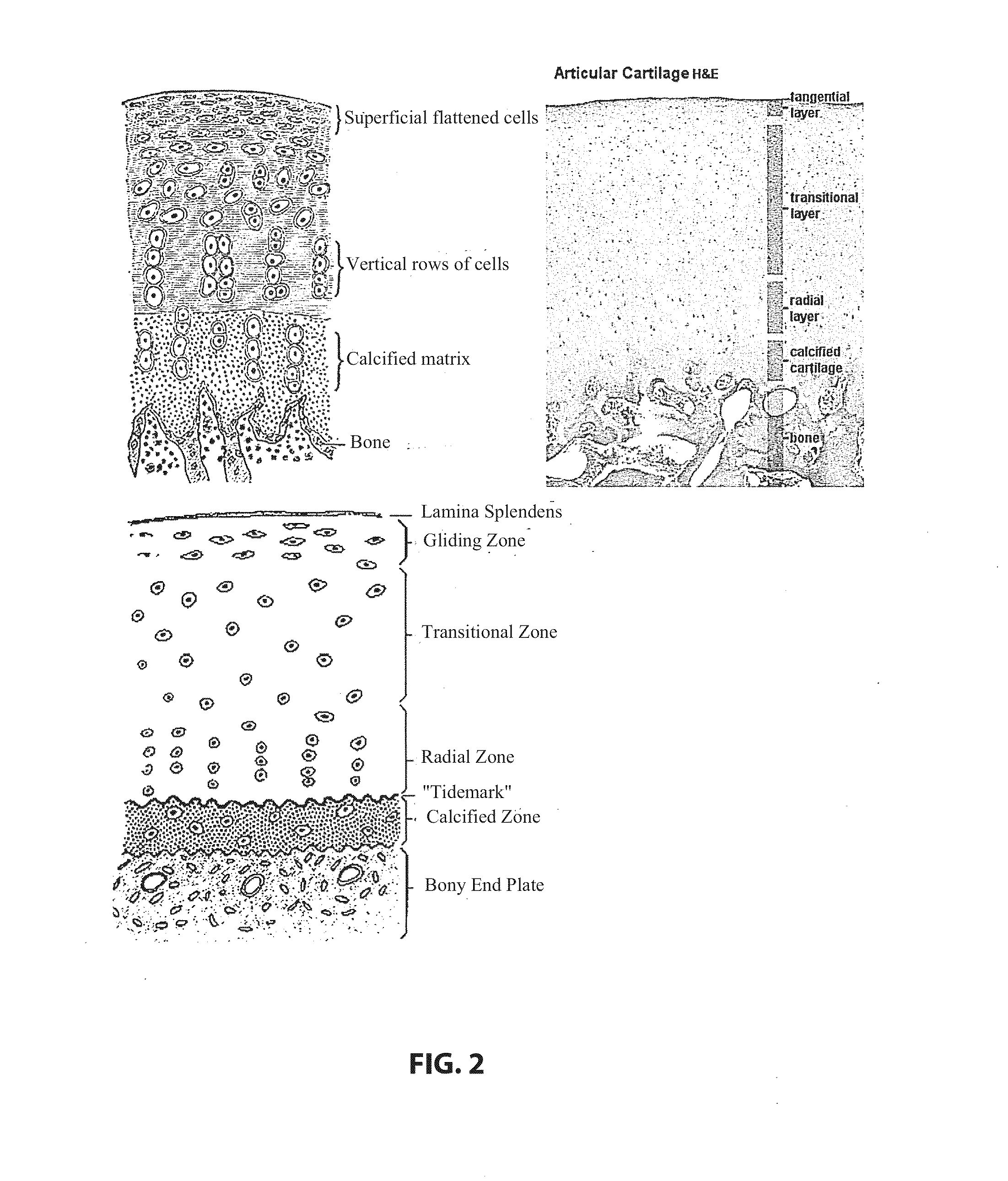
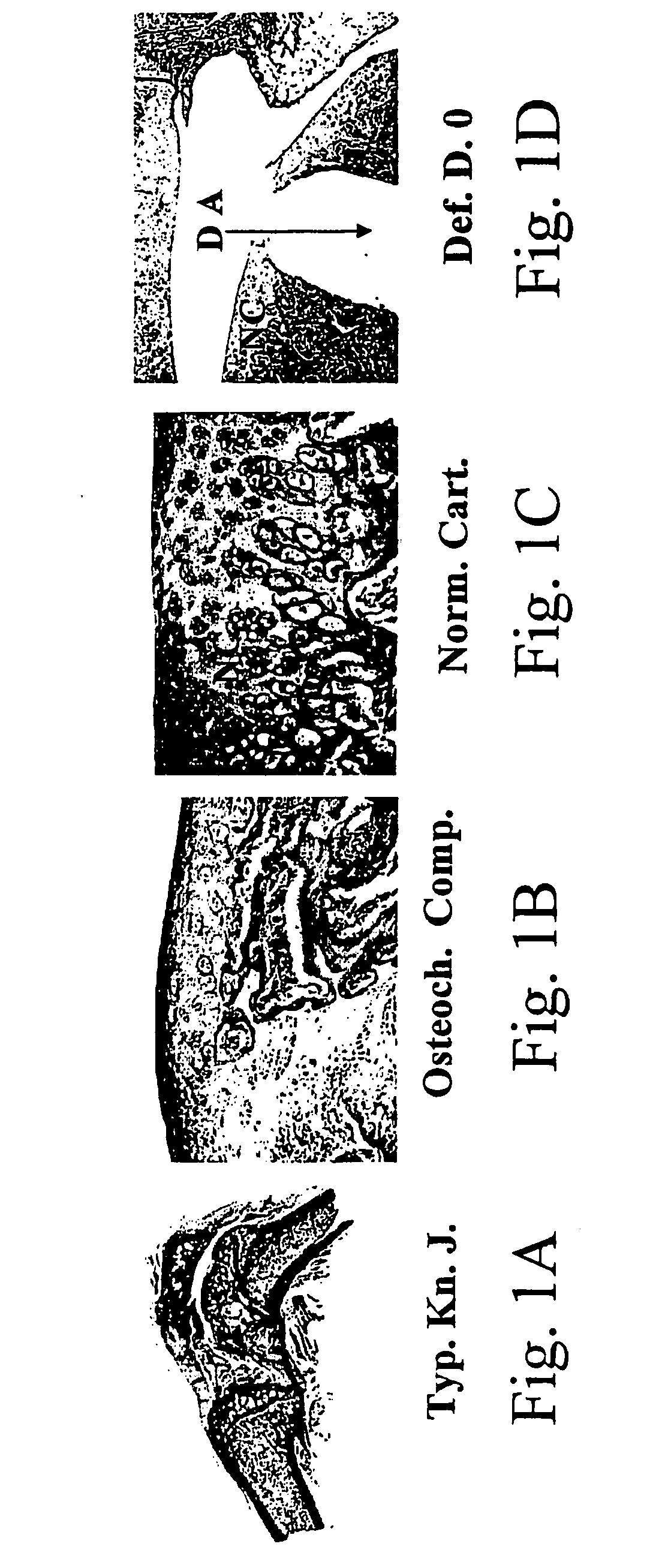
Chondrogenesis, or the formation of cartilage, is a dynamic cellular process involving mesenchymal cell recruitment and migration, condensation of progenitor cells, and chondrocyte differentiation which leads to the establishment of various types of cartilage, including hyaline, fibrous, and elastic cartilage.
Cartilage and bone repair and regeneration using postpartum-derived cells
Cells derived from postpartum tissue and methods for their isolation and induction to differentiate to cells of a chondrogenic or osteogenic phenotype are provided by the invention. The invention further provides cultures and compositions of the postpartum-derived cells and products related thereto. The postpartum-derived cells of the invention and products related thereto have a plethora of uses, including but not limited to research, diagnostic, and therapeutic applications, for example, in the treatment of bone and cartilage conditions.
Owner:DEPUY SYNTHES PROD INC
Cartilage and bone repair and regeneration using postpartum-derived cells
Cells derived from postpartum tissue and methods for their isolation and induction to differentiate to cells of a chondrogenic or osteogenic phenotype are provided by the invention. The invention further provides cultures and compositions of the postpartum-derived cells and products related thereto. The postpartum-derived cells of the invention and products related thereto have a plethora of uses, including but not limited to research, diagnostic, and therapeutic applications, for example, in the treatment of bone and cartilage conditions.
Owner:ETHICON INC
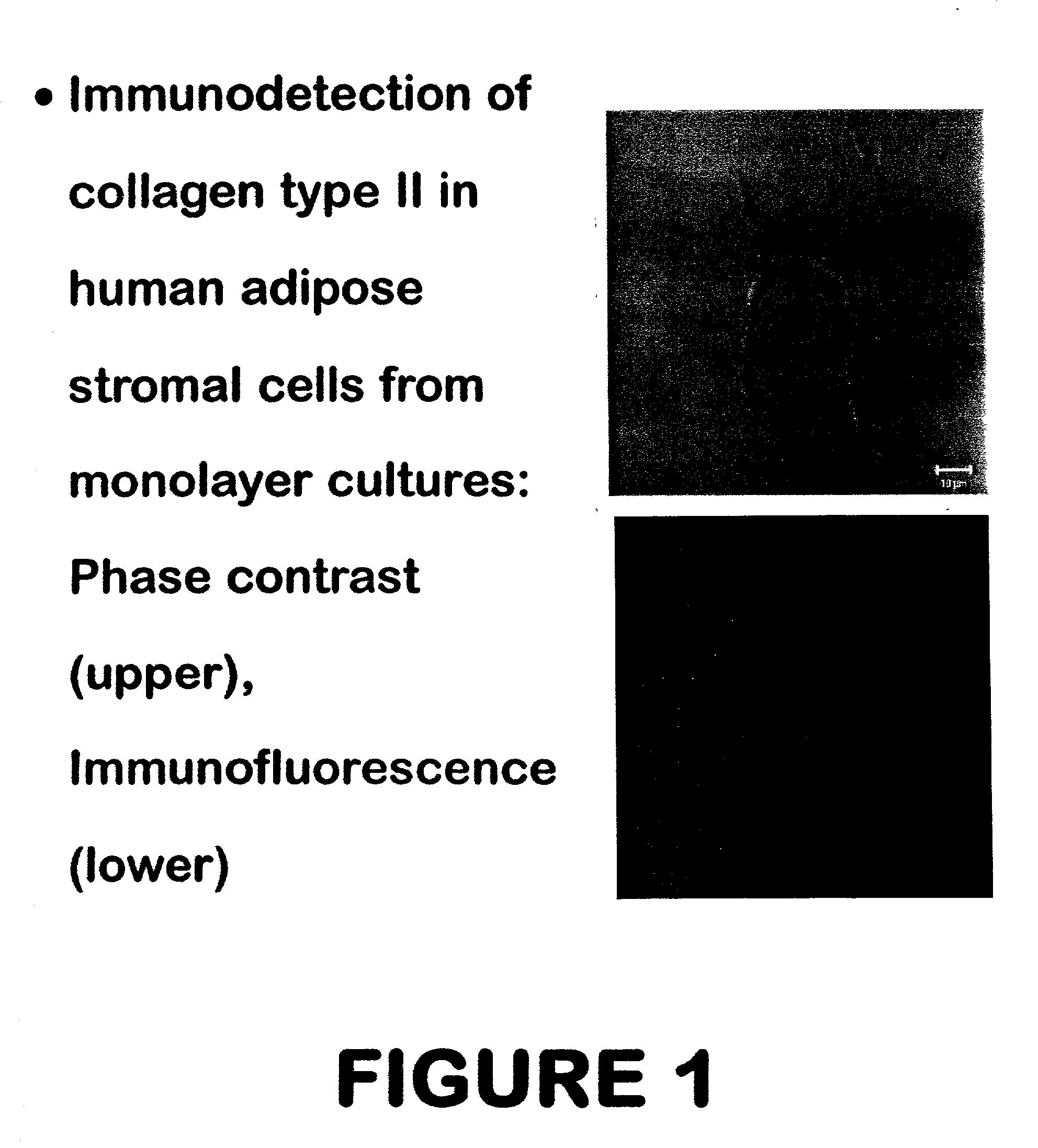
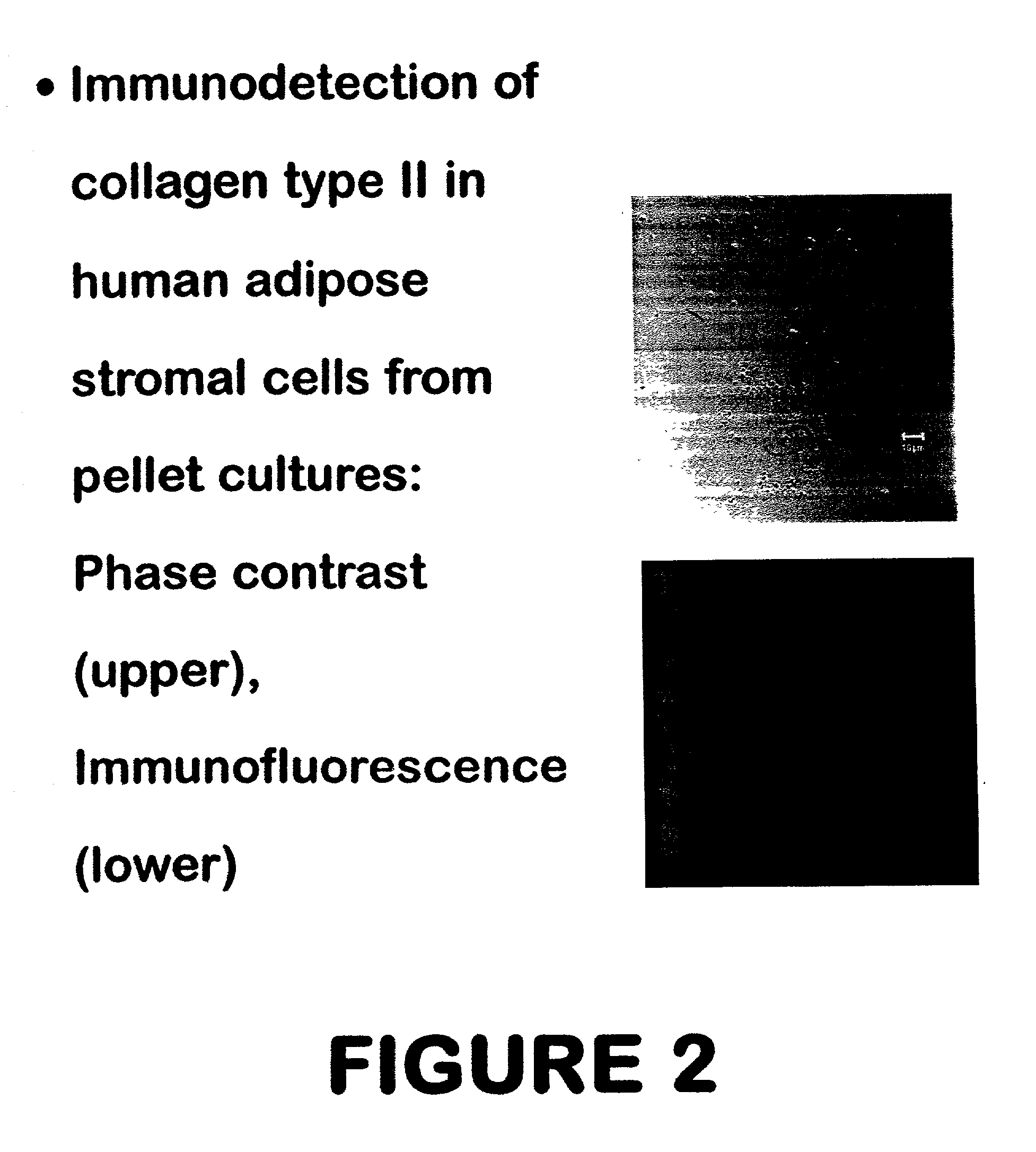
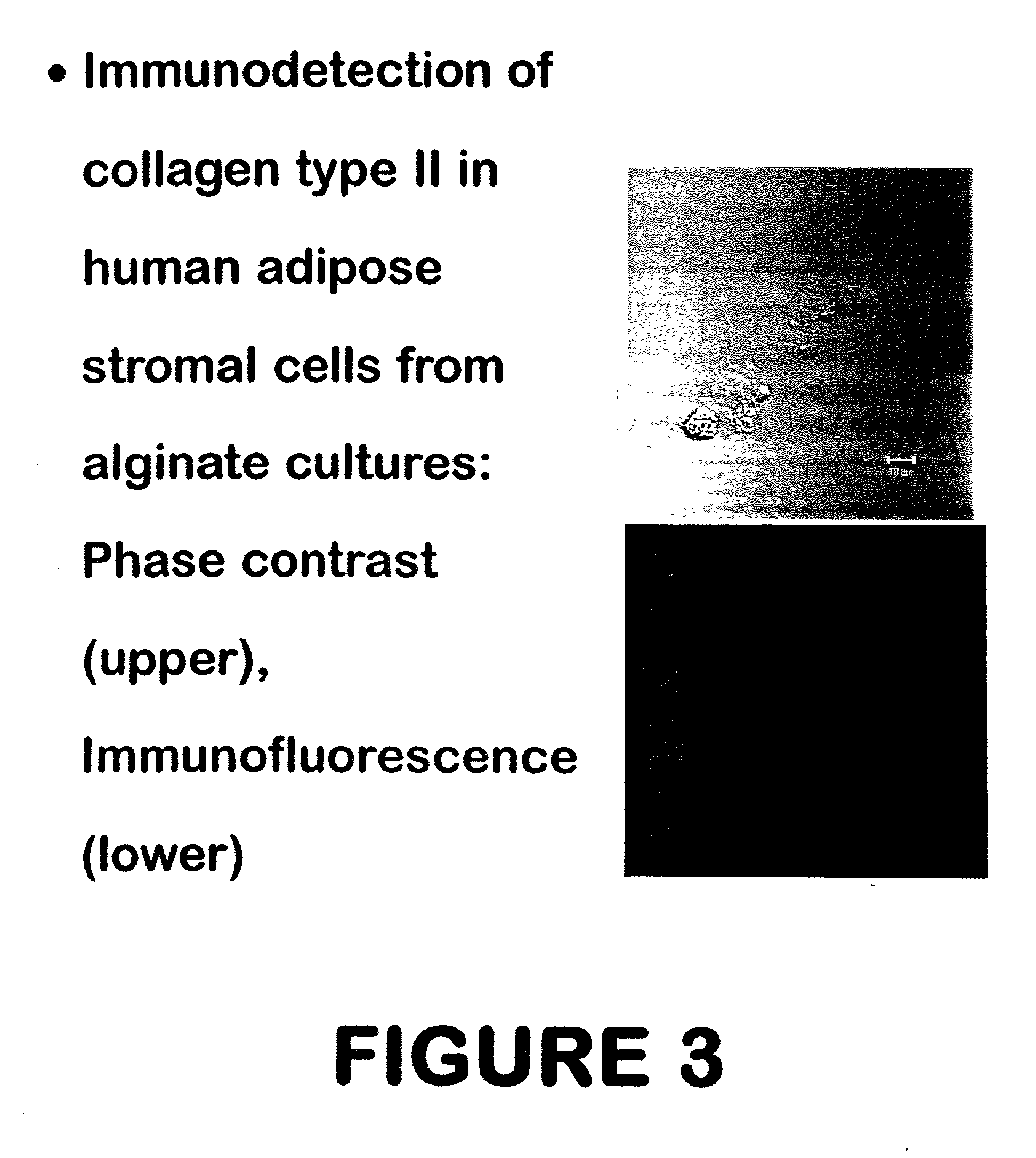
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Composition for cartilage
Owner:RGT UNIV OF CALIFORNIA
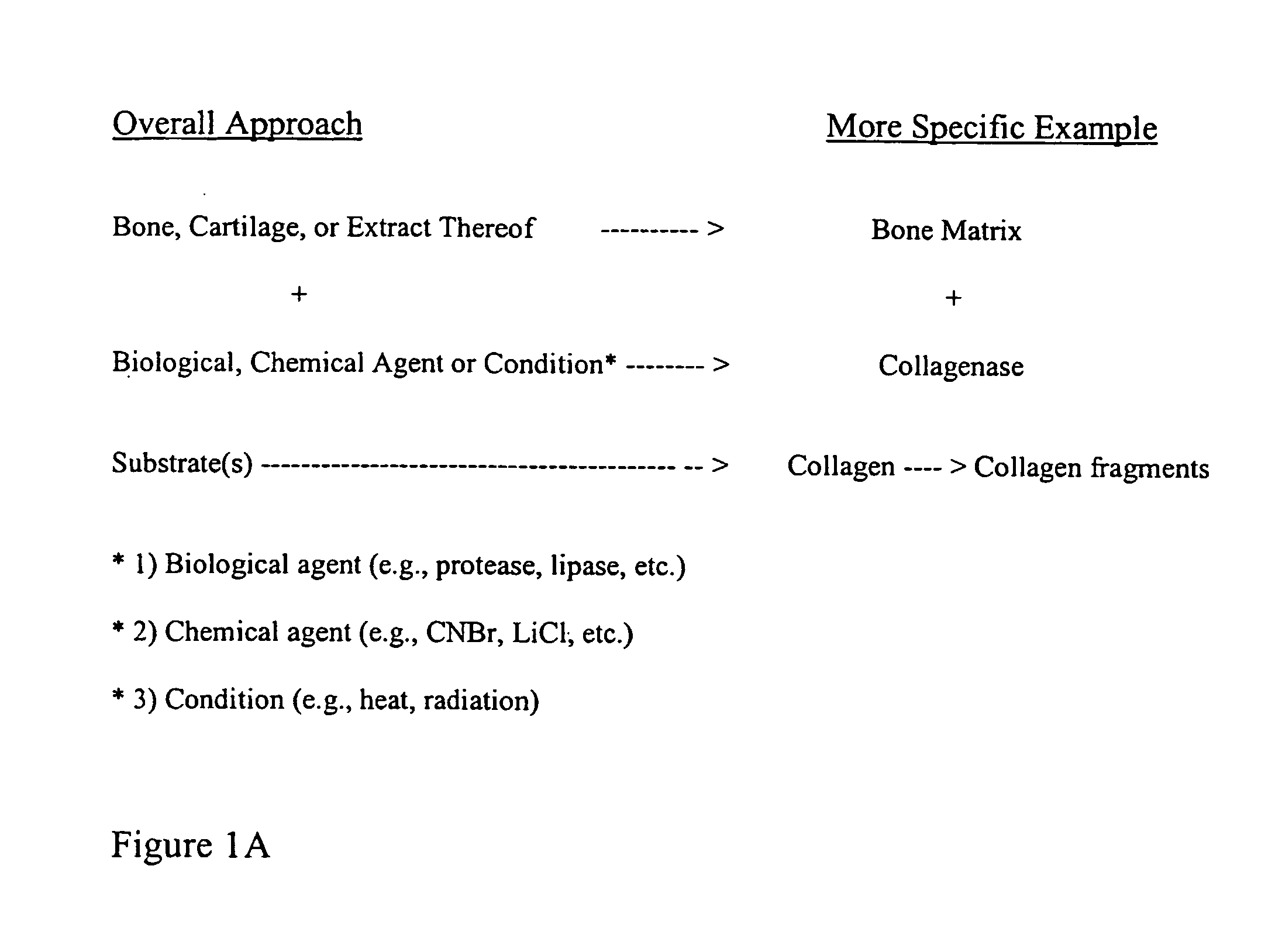
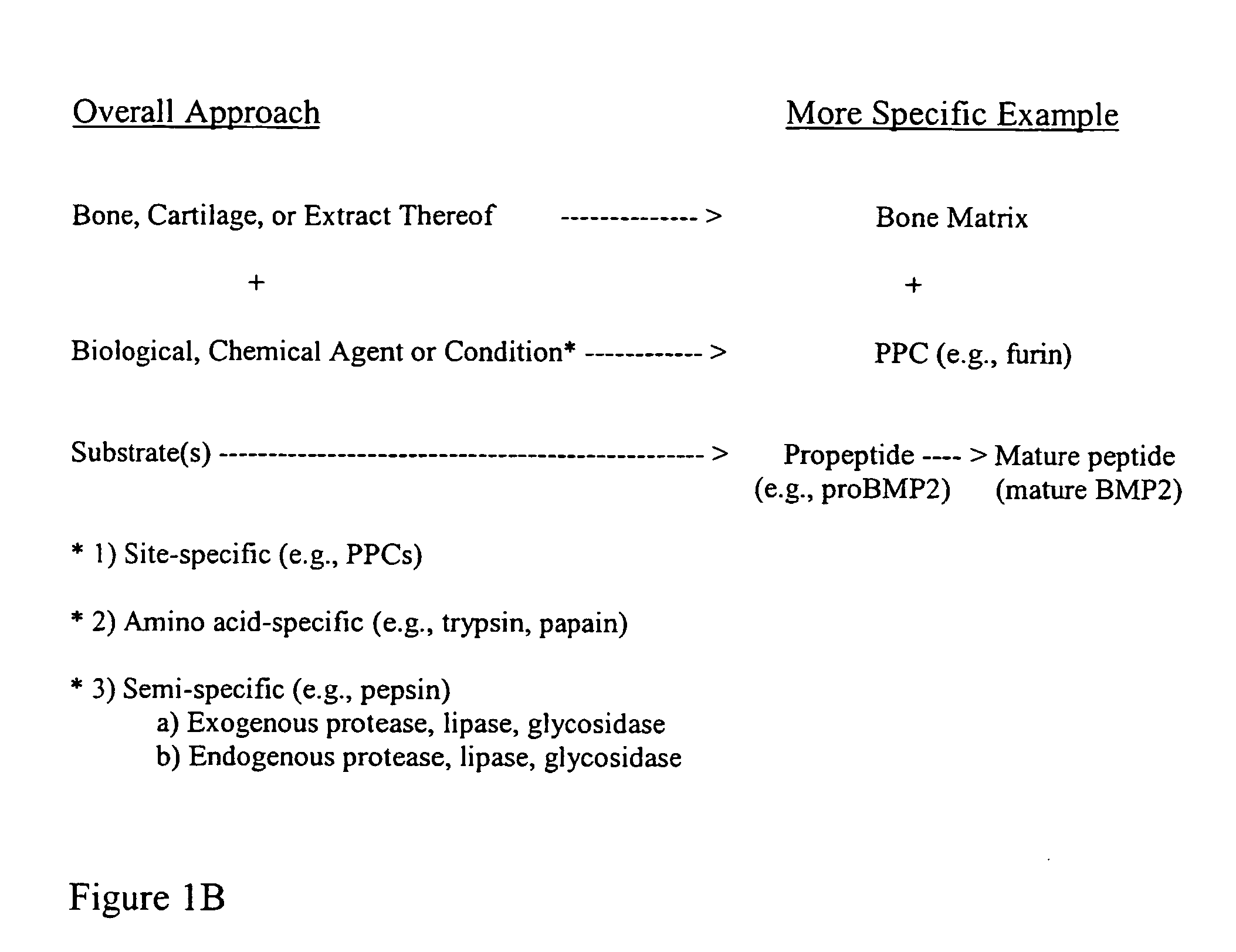
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
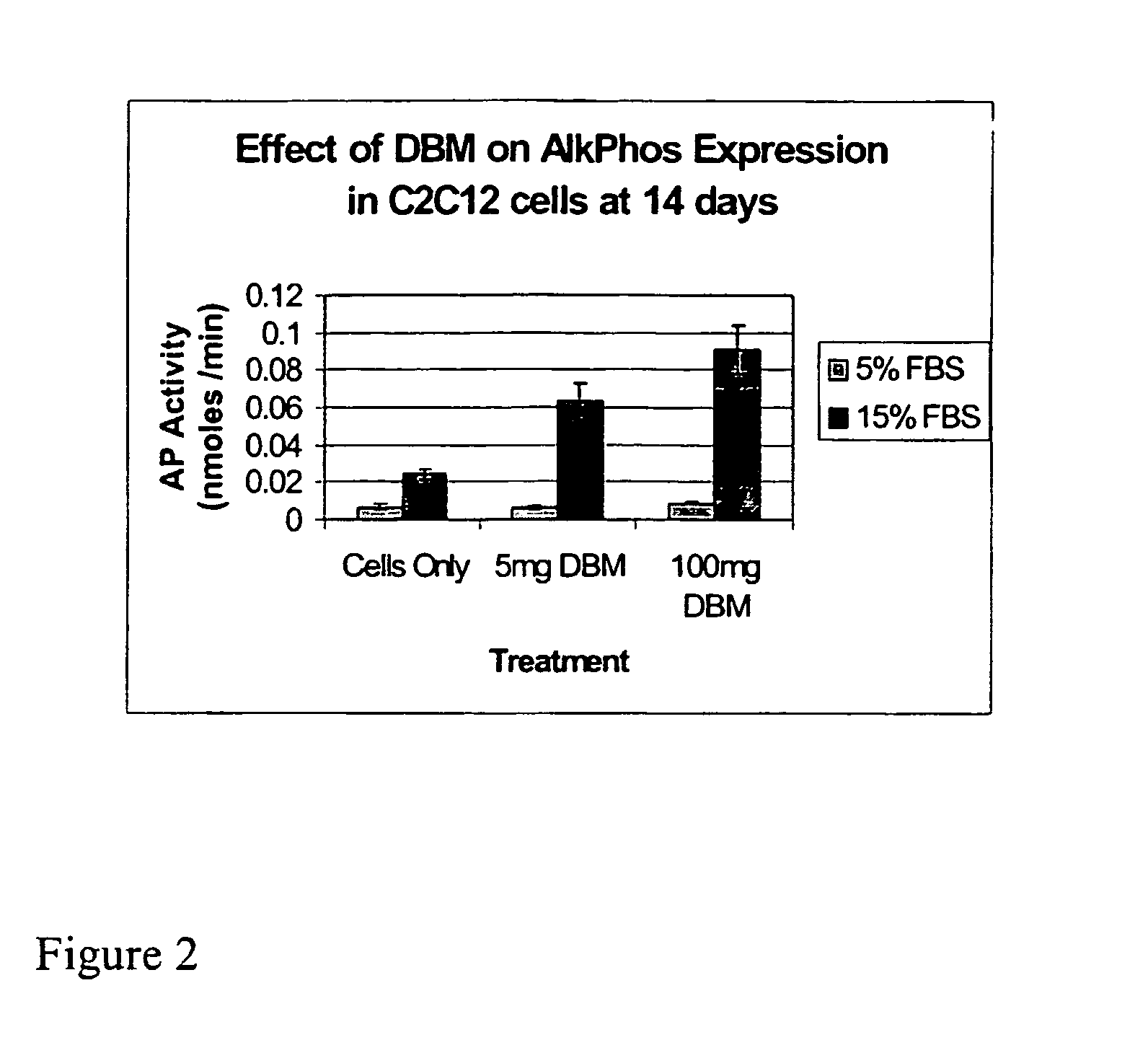
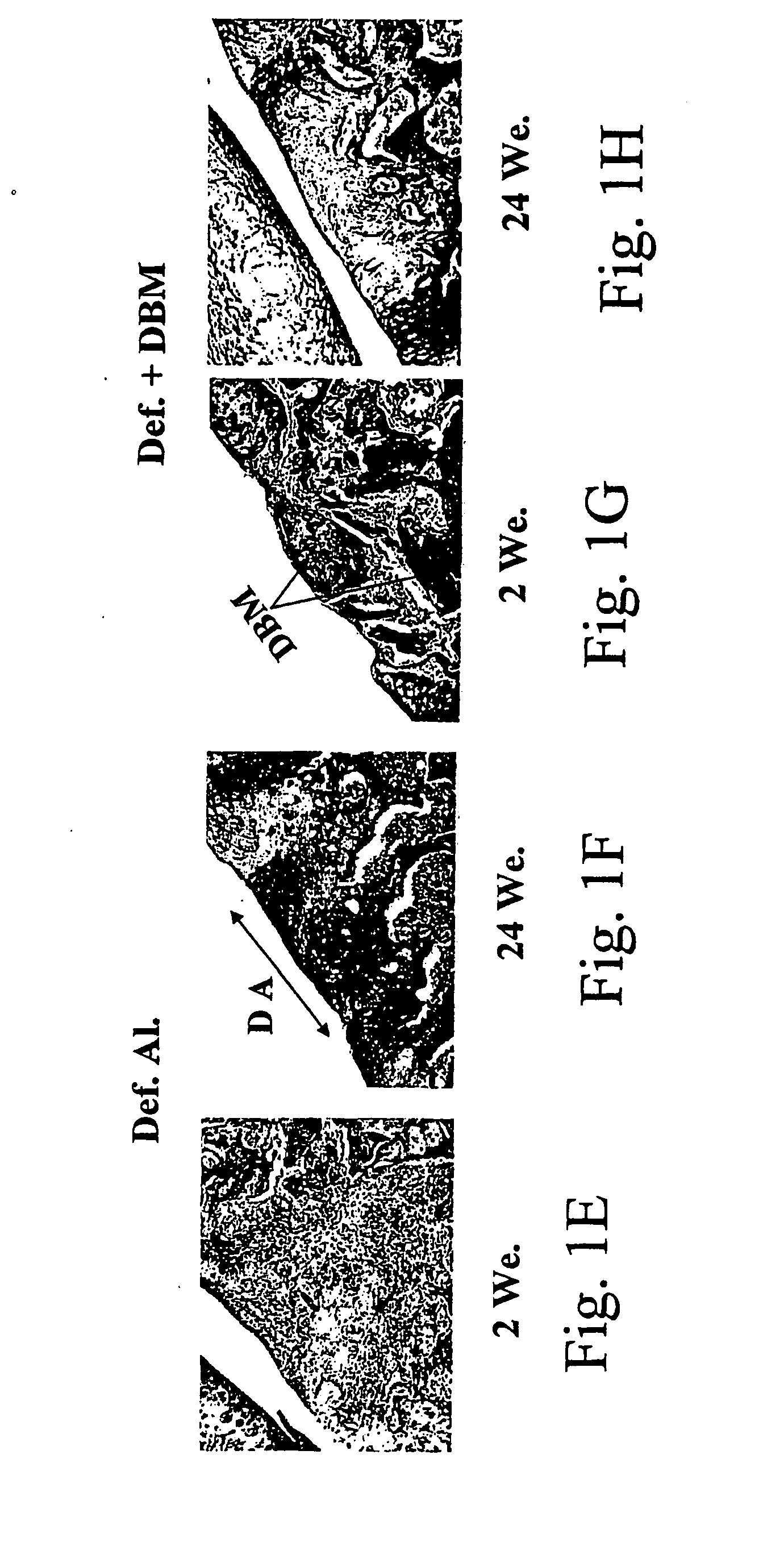
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
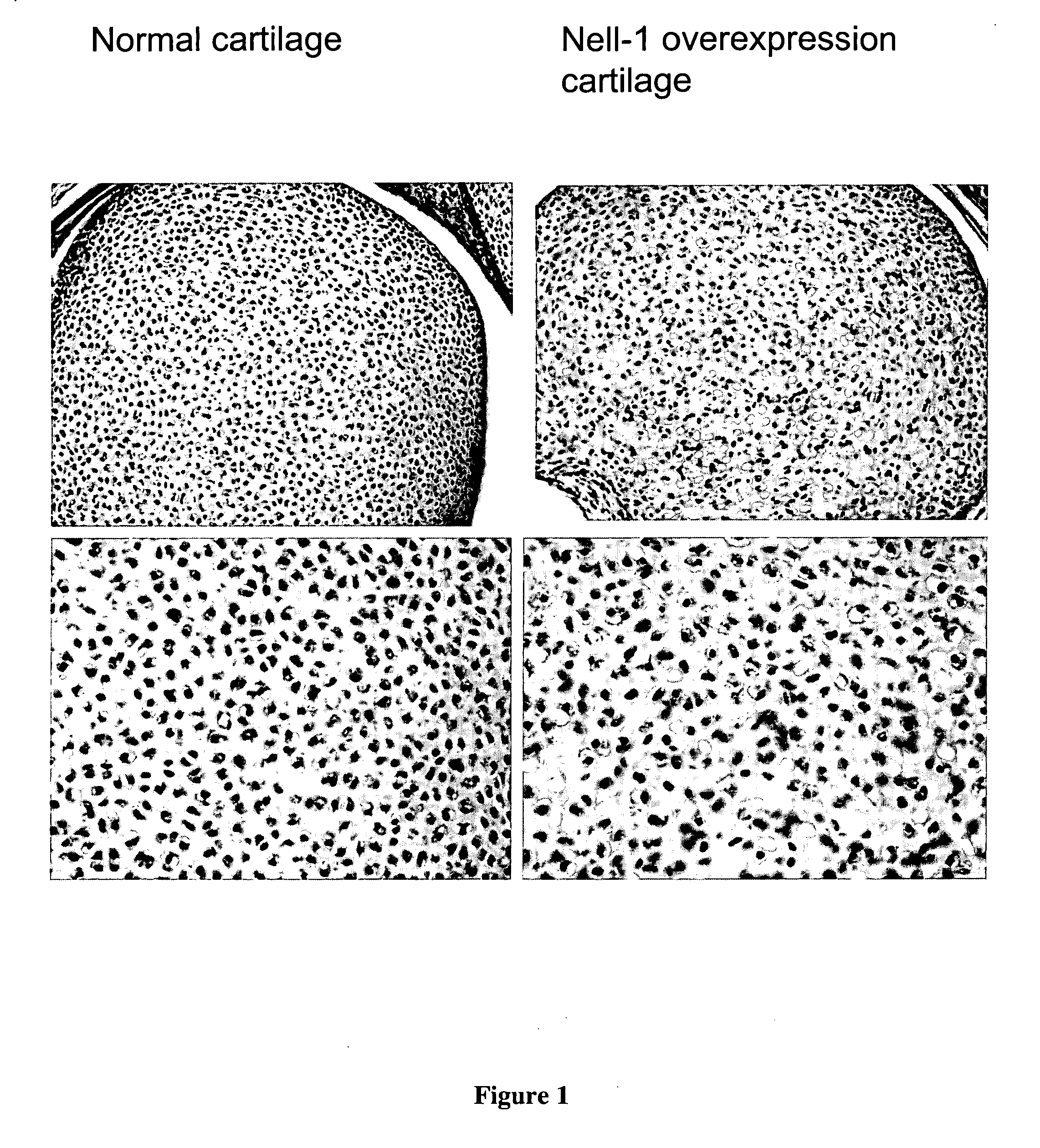
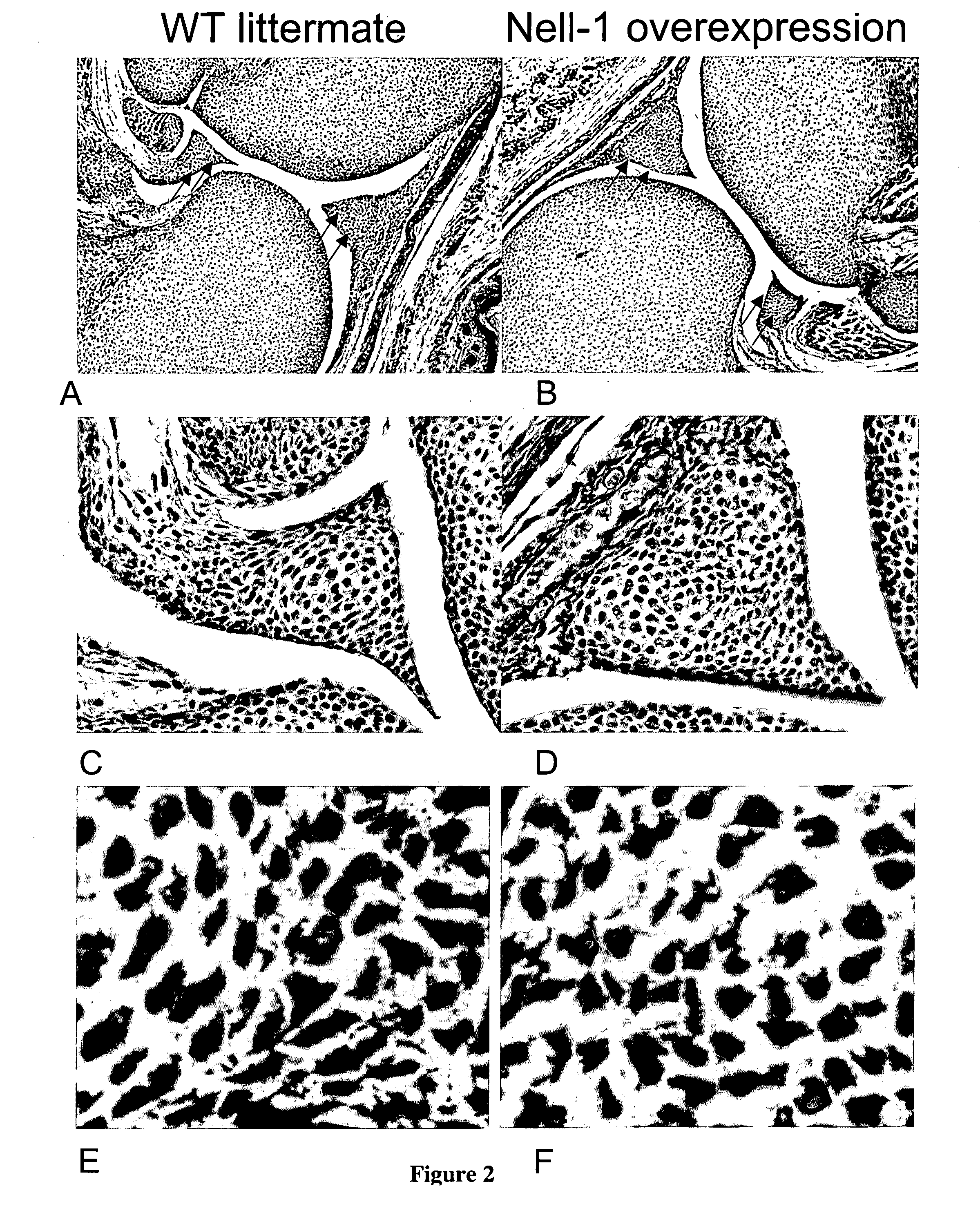
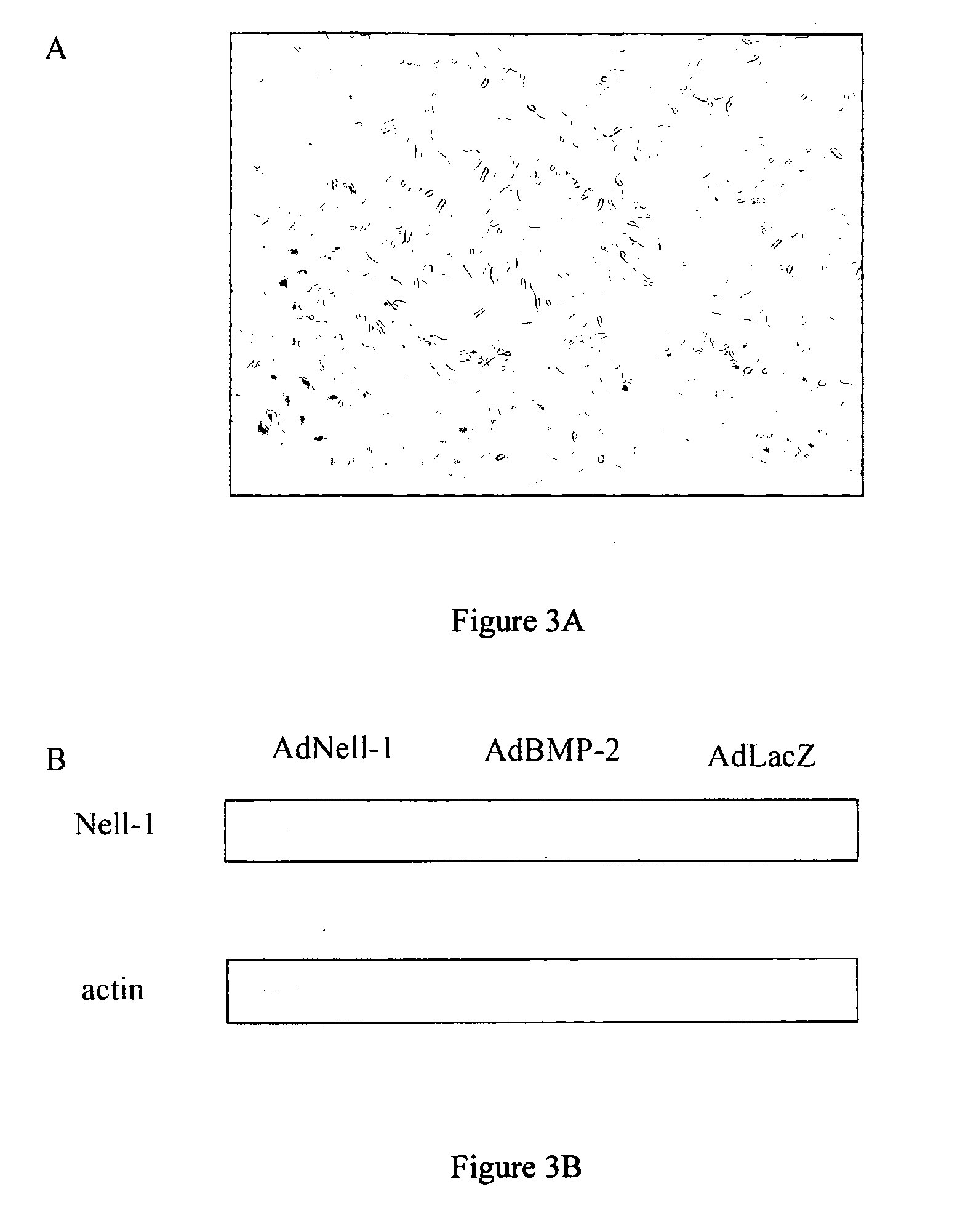
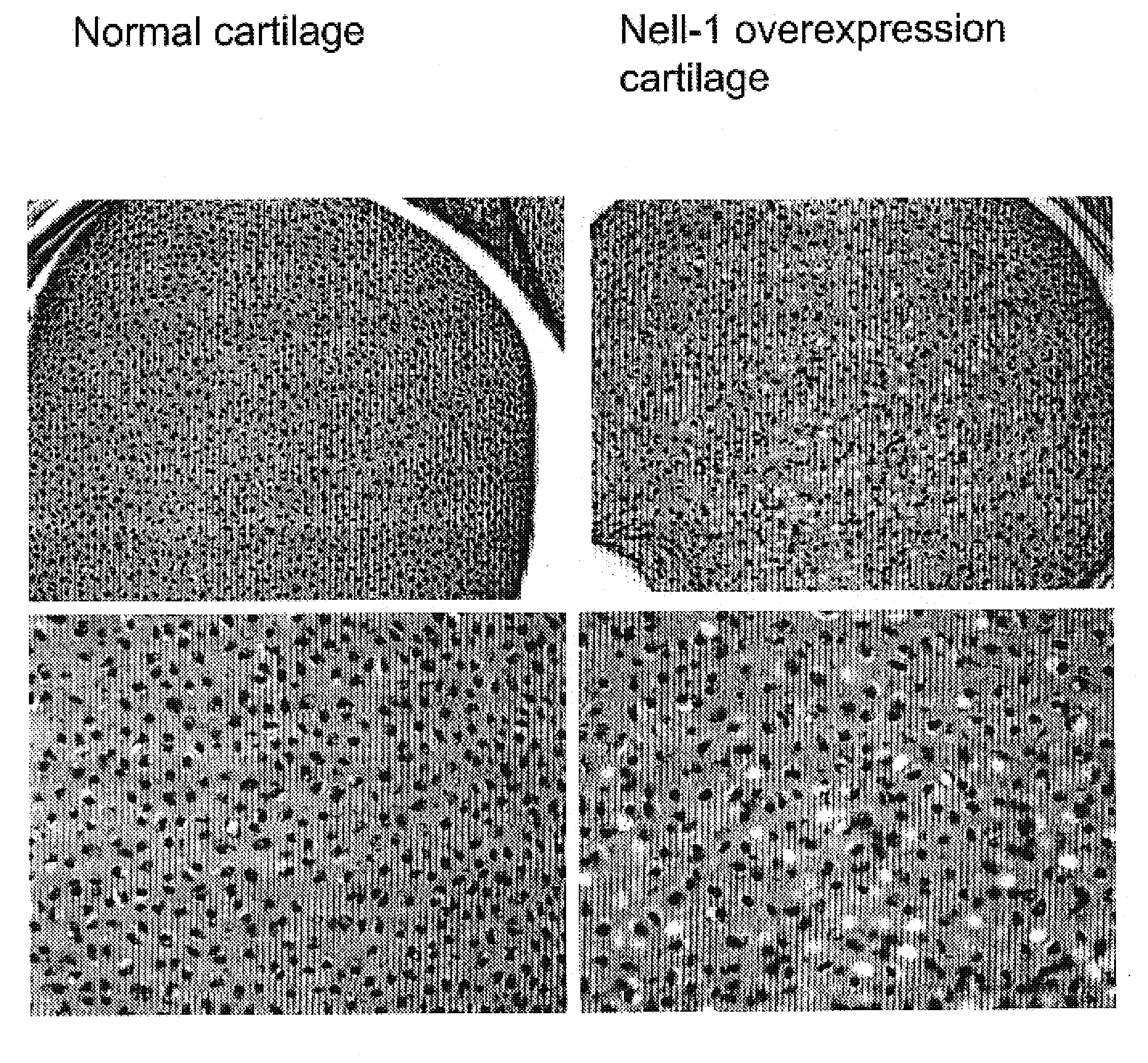
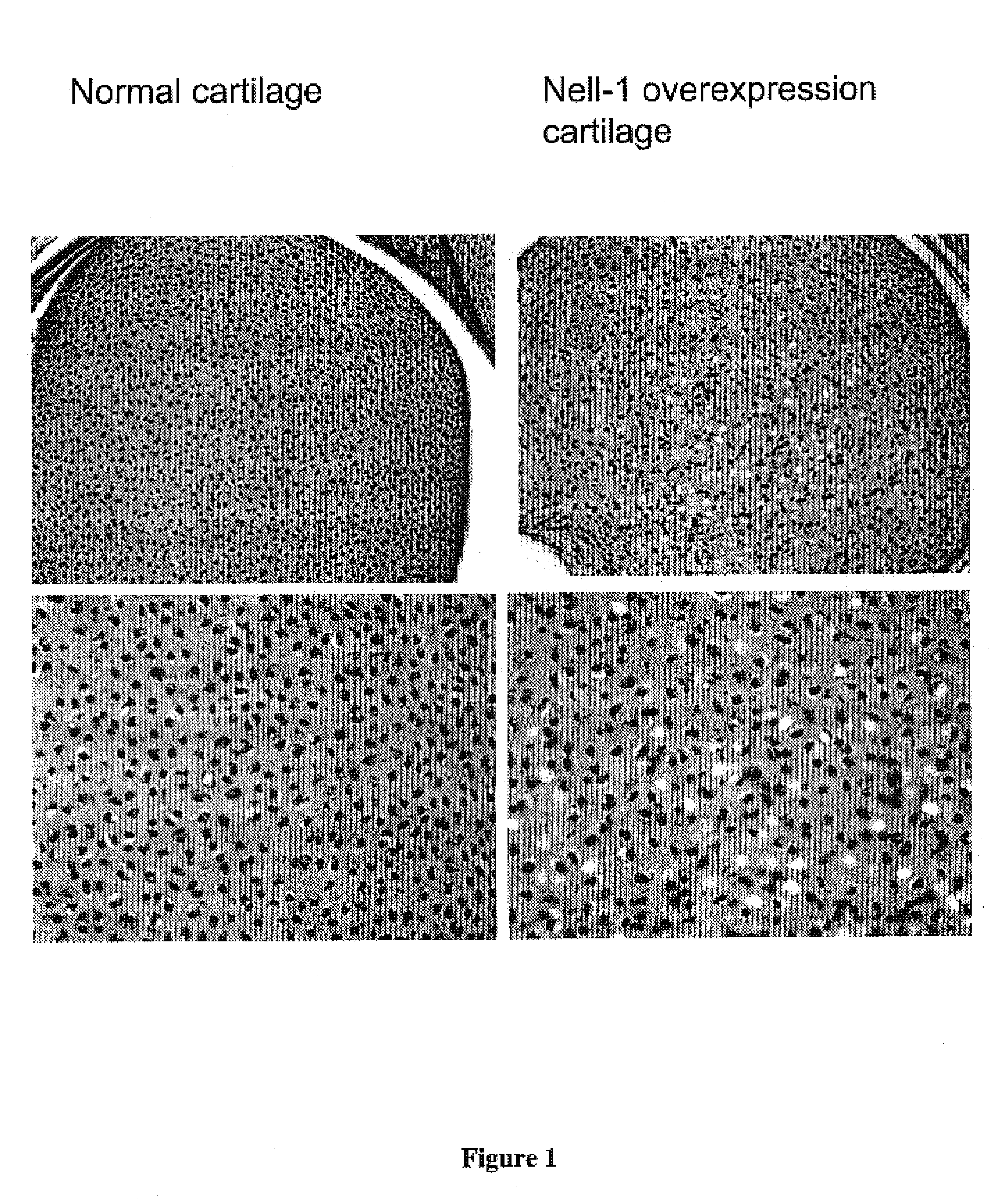
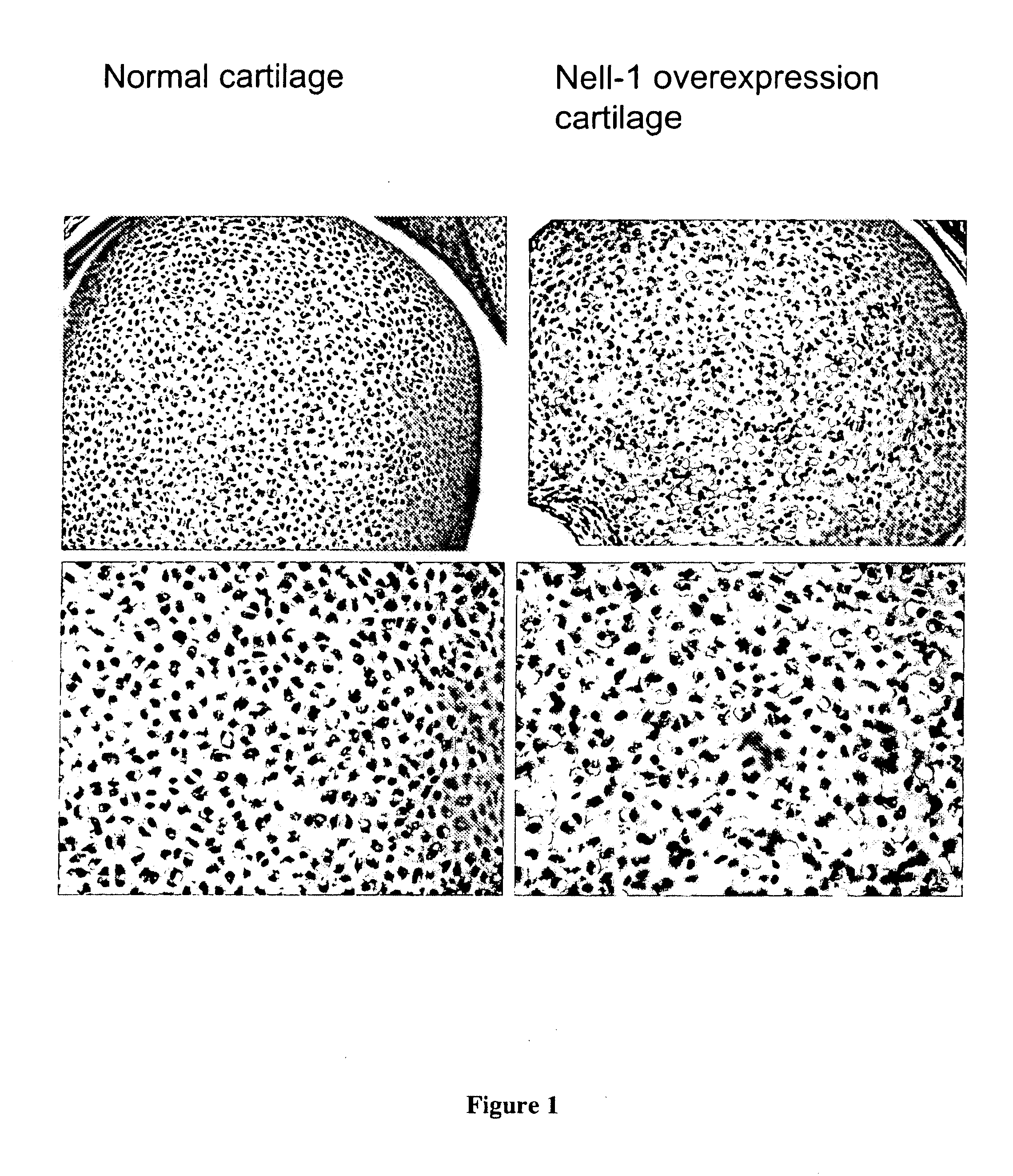
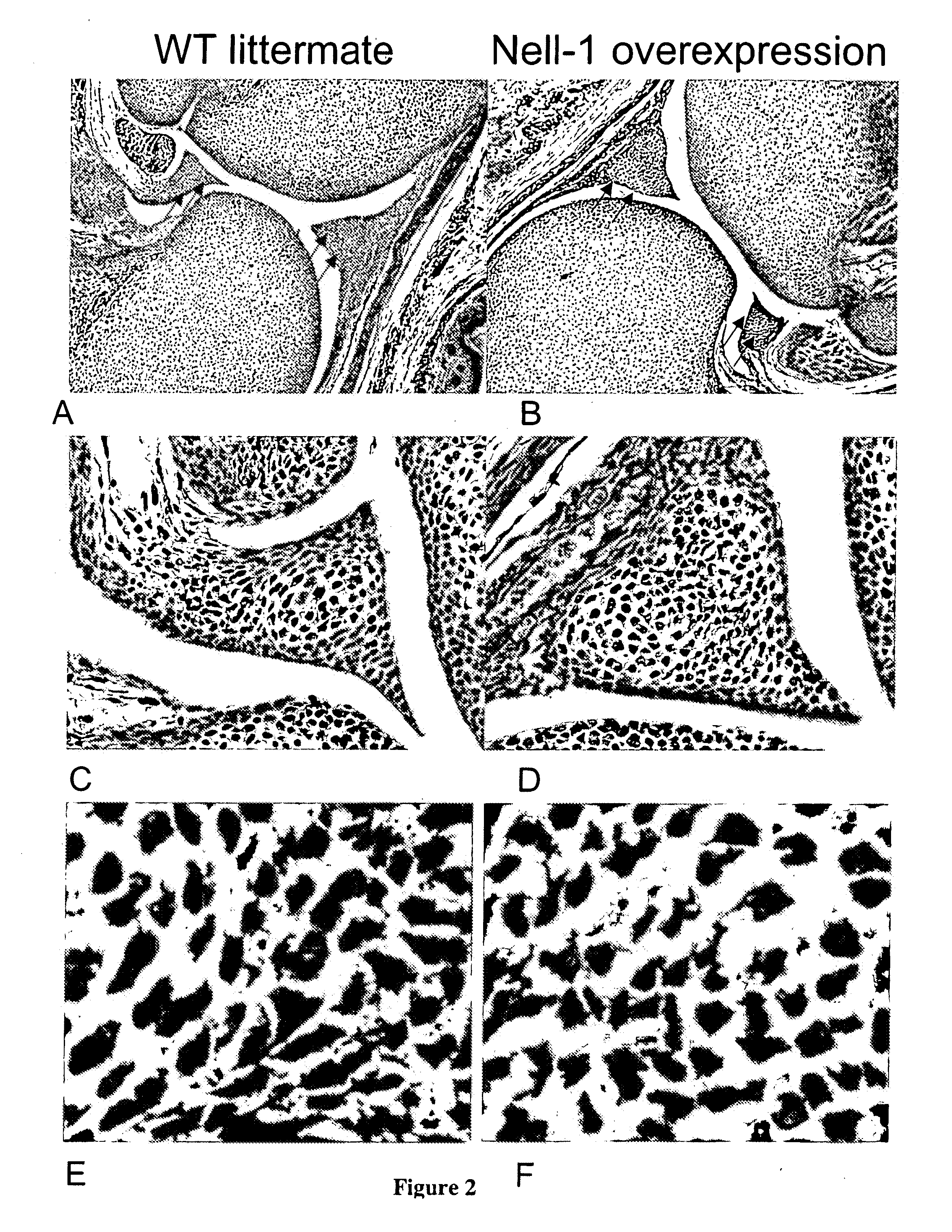
Composition for promoting cartilage formation or repair comprising a nell gene product and method of treating cartilage-related conditions using such composition
Owner:RGT UNIV OF CALIFORNIA
Method for chondrocyte expansion with phenotype retention
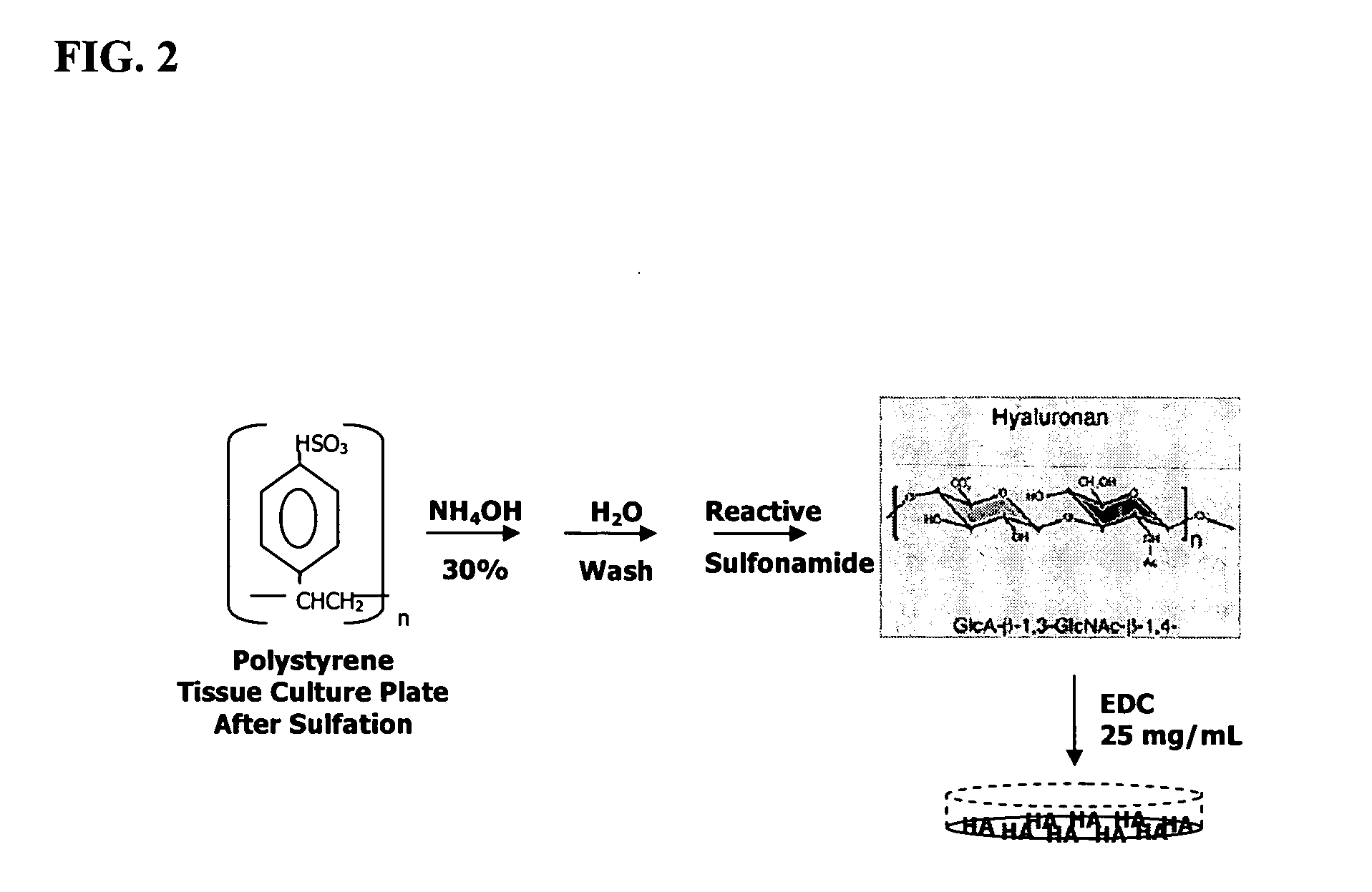
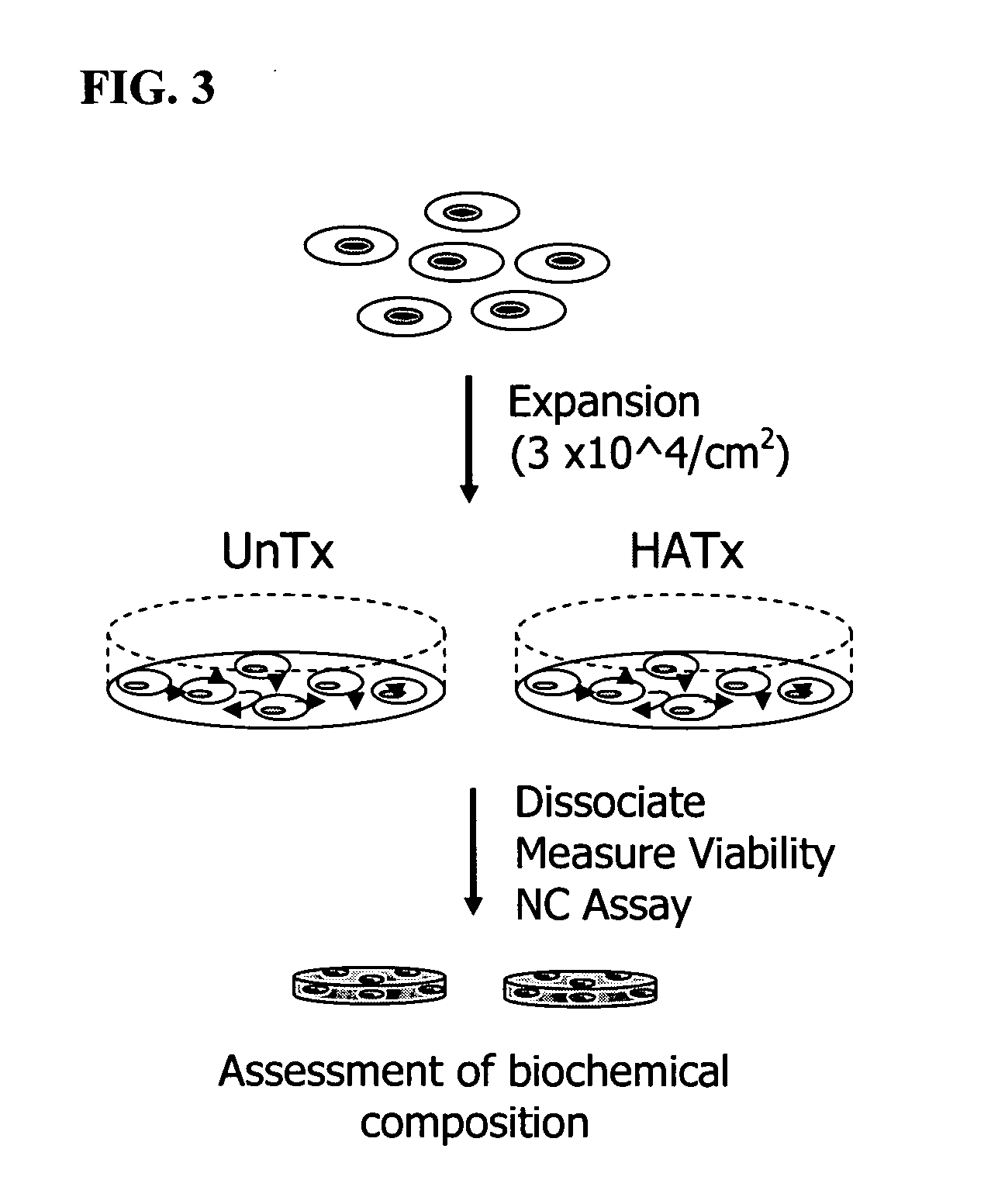
The present invention provides a method that maintains chondrocyte phenotype during serial expansion by culturing a population of chondrocytes in a defined serum-free culture medium containing cytokines and on a substrate that is modified by covalent attachment of hyaluronic acid. The underlying principle is to maintain native chondrocyte phenotype by growing the dissociated chondrocytes on a substrate modified by covalent attachment of hyaluronic acid to retain native chondrocyte morphology and function. Chondrocyte expanded in this manner can be used in various medical applications to repair cartilaginous tissues that have been injured by trauma or disease. This substratum provides a microenvironment that more closely mimics that of native articular cartilage, thereby promoting chondrogenesis in a predictable manner.
Owner:ZIMMER INC +1
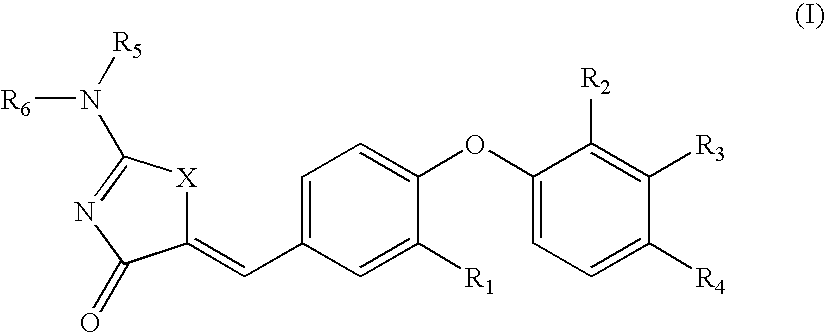
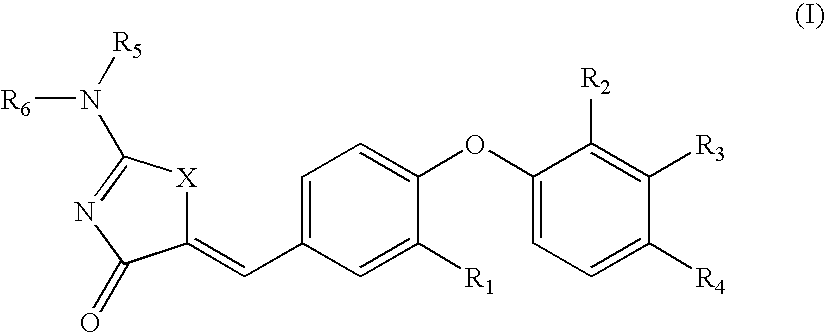
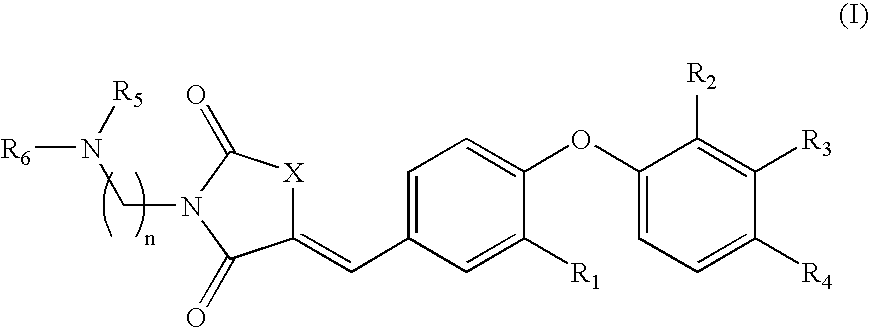
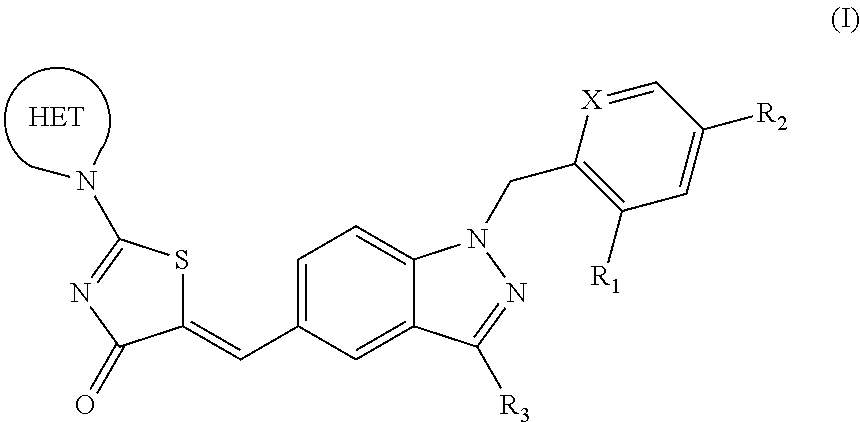
SUBSTITUTED PHENOXY AMINOTHIAZOLONES as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
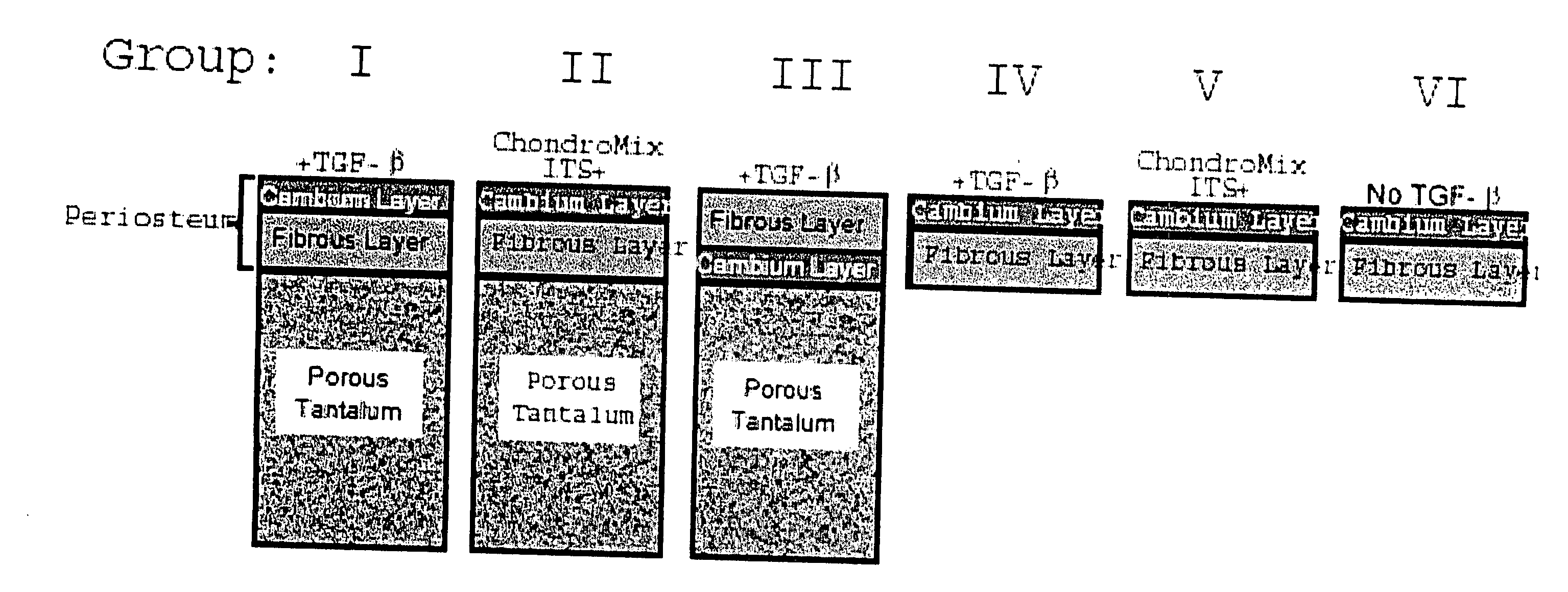
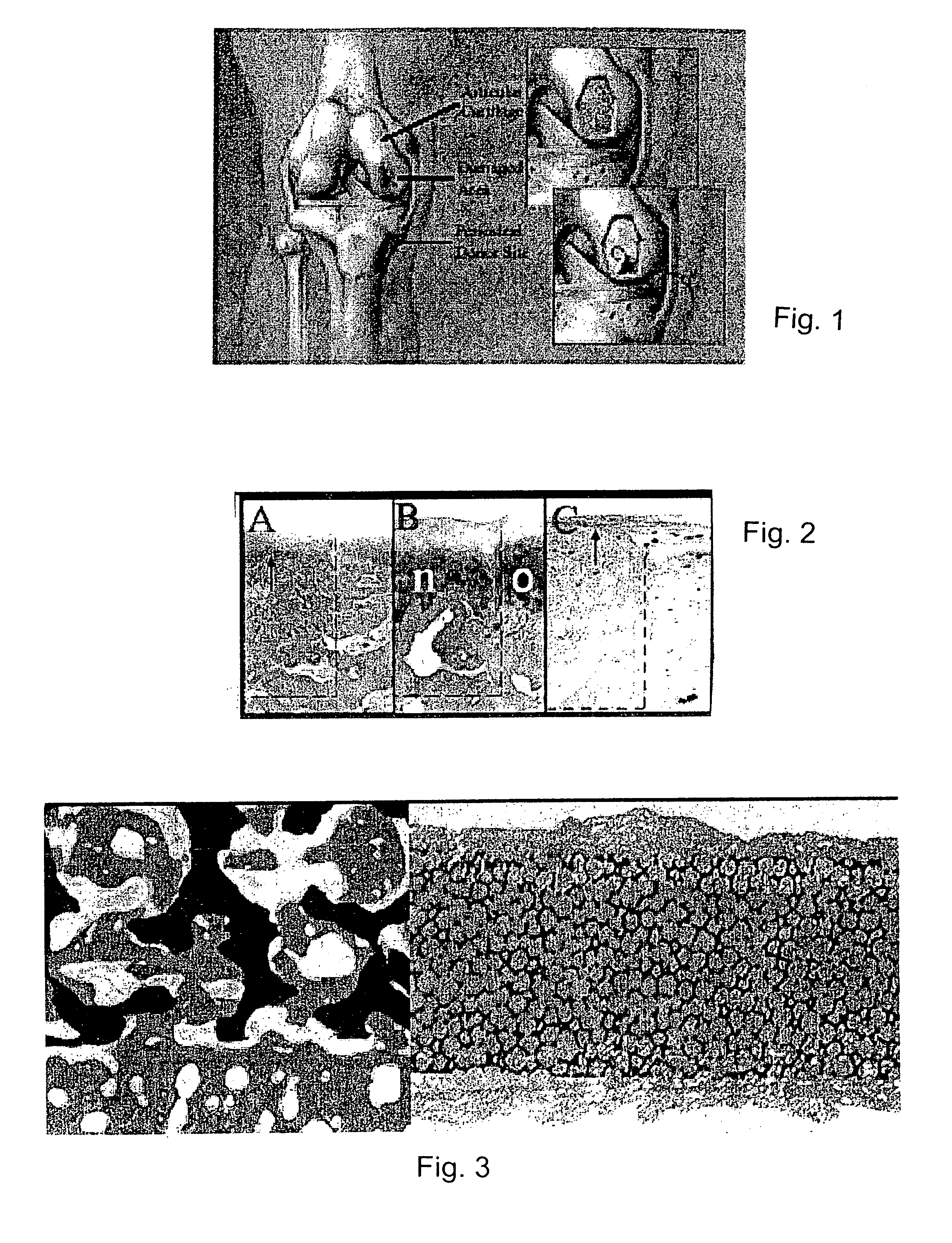
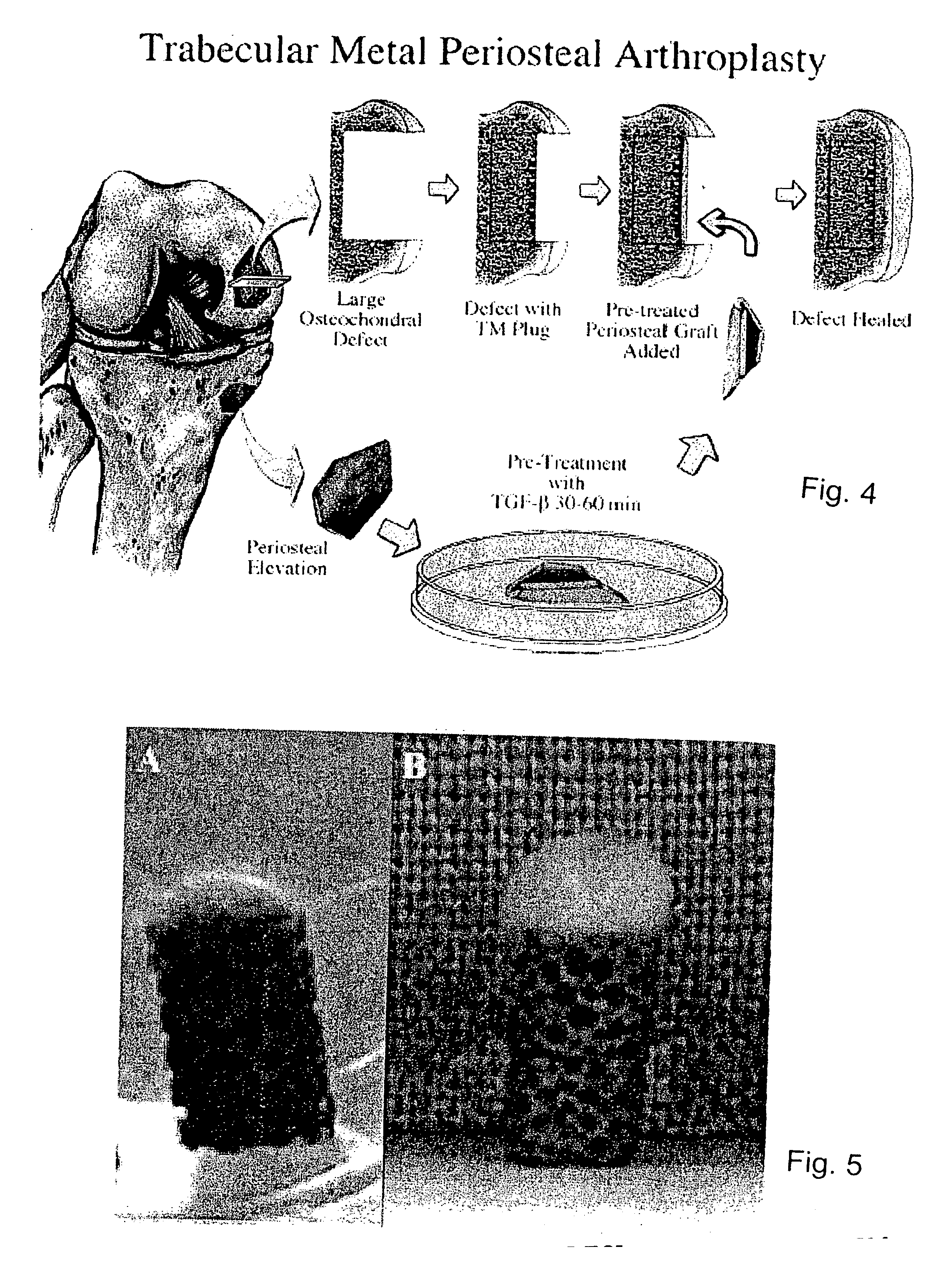
Biosynthetic composite for osteochondral defect repair
A composite for osteochondral defect repair includes a porous scaffold and a periosteal graft secured to a surface of the scaffold. The composite provides cartilage growth from autologous periosteum chondrogenesis. Biological resurfacing of large osteochondral defects, or a complete joint is feasible using the porous scaffold / autologous periosteal composite. The use of this composite eliminates the necessity of using normal cartilage surface as a donor site and its respective associated morbidity. In one form, the strong bone integration capacity of a porous metal (e.g., tantalum) scaffold and the high grade of integration observed from periosteal chondrogenesis into the normal cartilage eliminates the lack of chondral-chondral integration observed in the autologous osteochondral graft technique.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
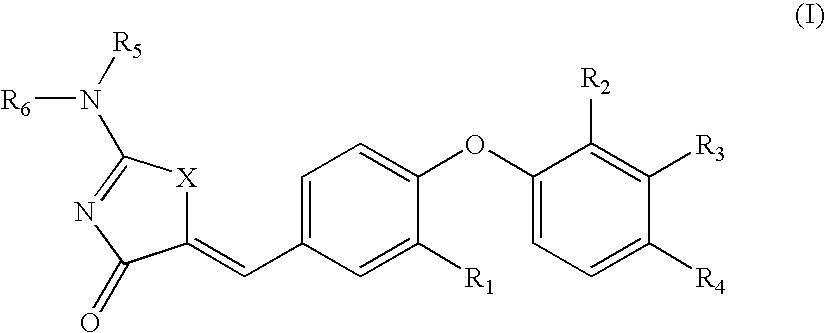
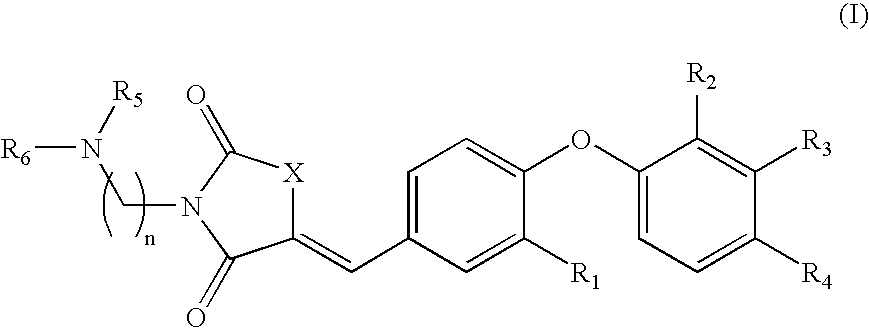
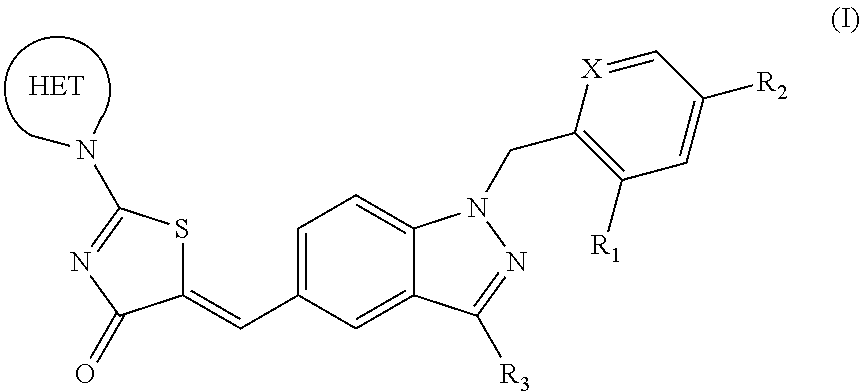
Substituted phenoxy n-alkylated thiazolidinediones as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Porated cartilage products
This invention provides porated cartilage products, methods of producing porated cartilage products, and methods of treating subjects by administering cartilage products. Optionally, the cartilage products are sized, porated, and digested to provide a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
Methods for administering fgf18
FGF18 is known to stimulate the proliferation of chondrocytes, resulting in increased cartilage formation. When hyaluronic acid is administered in addition to FGF18, the effects on chondrocyte proliferation and production of matrix were found to be greater than administration of FGF18 or hyaluronic acid alone.
Owner:ZYMOGENETICS INC
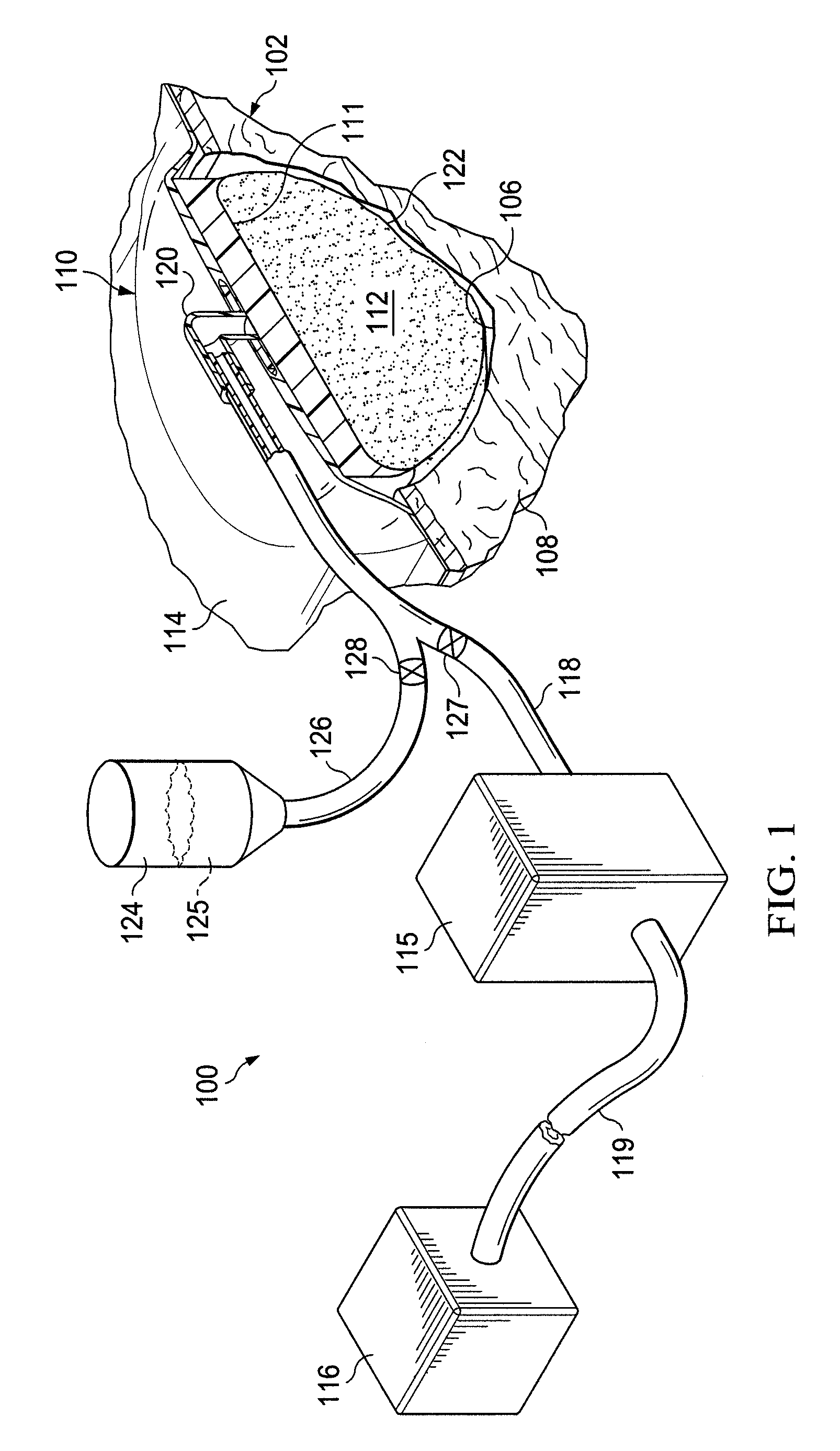
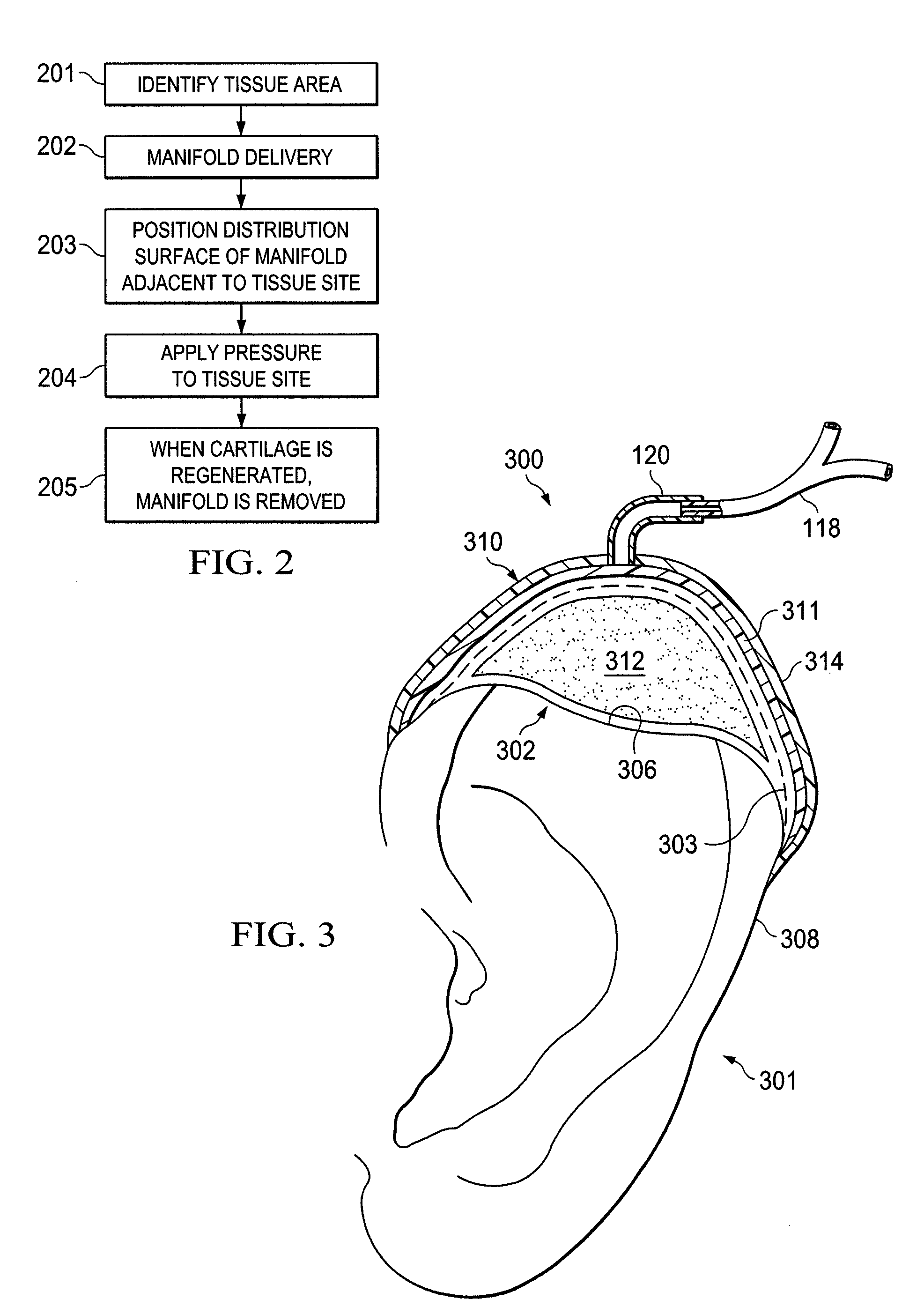
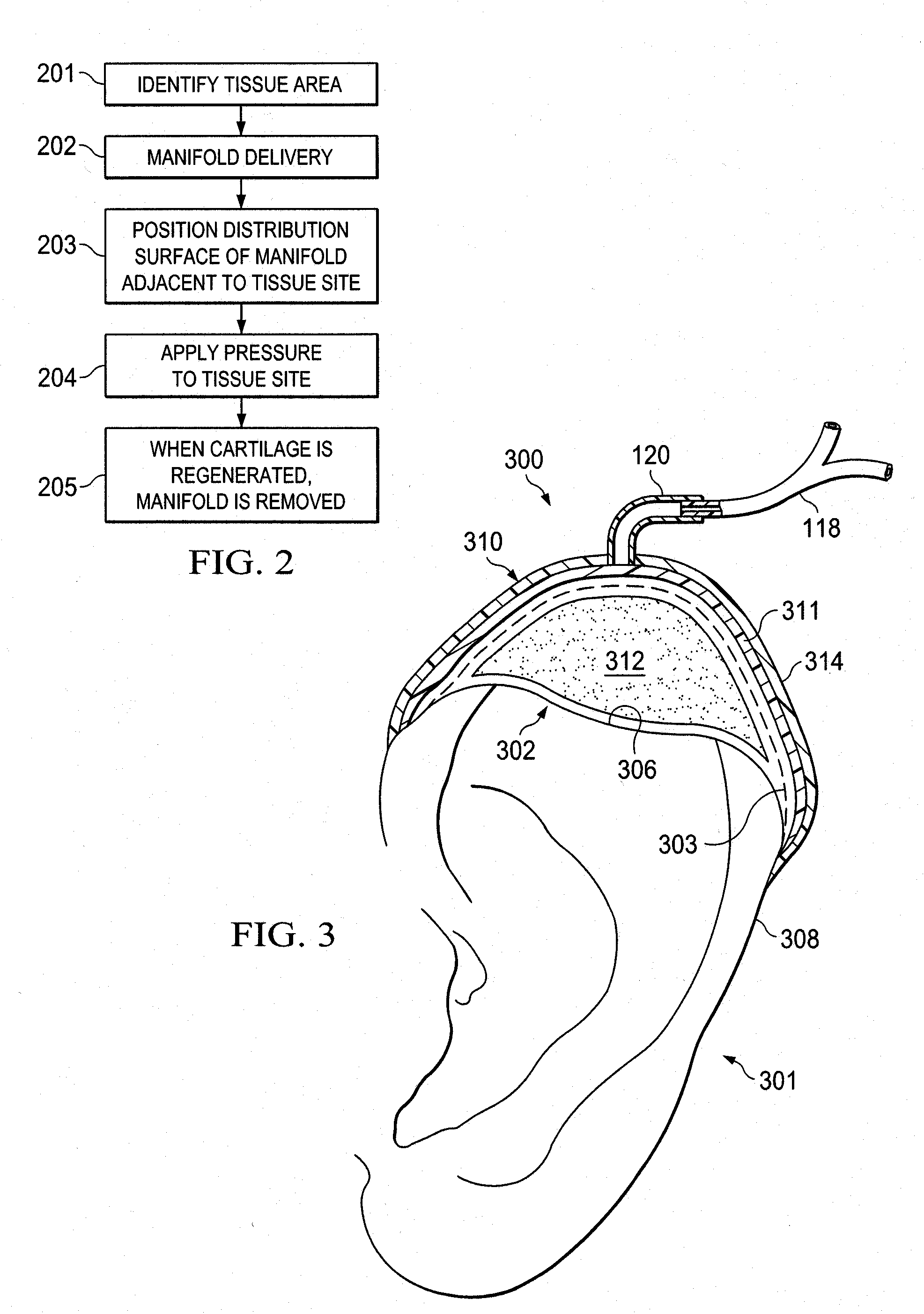
Stimulation of cartilage formation using reduced pressure treatment
ActiveUS8197806B2Promote growthBiocidePeptide/protein ingredientsBiomedical engineeringCartilage formation
Provided is a method of stimulating cartilage formation at a tissue site in a mammal. Also provided is a biocompatible scaffold. Additionally, a system for stimulating cartilage formation at a tissue site is provided. Further provided is the use of a manifold, a chondrocyte, and a reduced-pressure source to stimulate cartilage formation at a tissue site of a mammal.
Owner:SOLVENTUM INTPROP CO
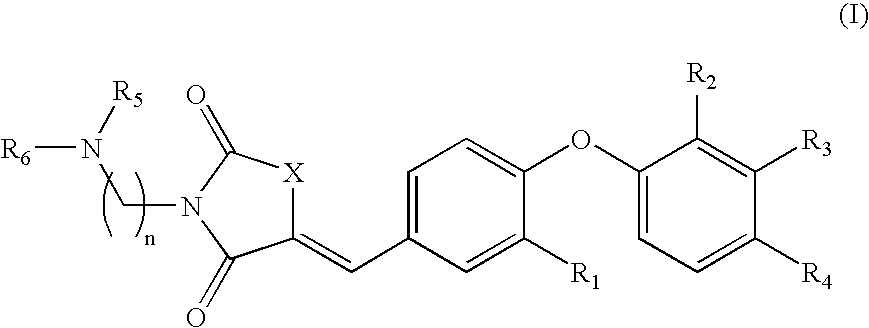
Substituted aminothiazolone indazoles as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
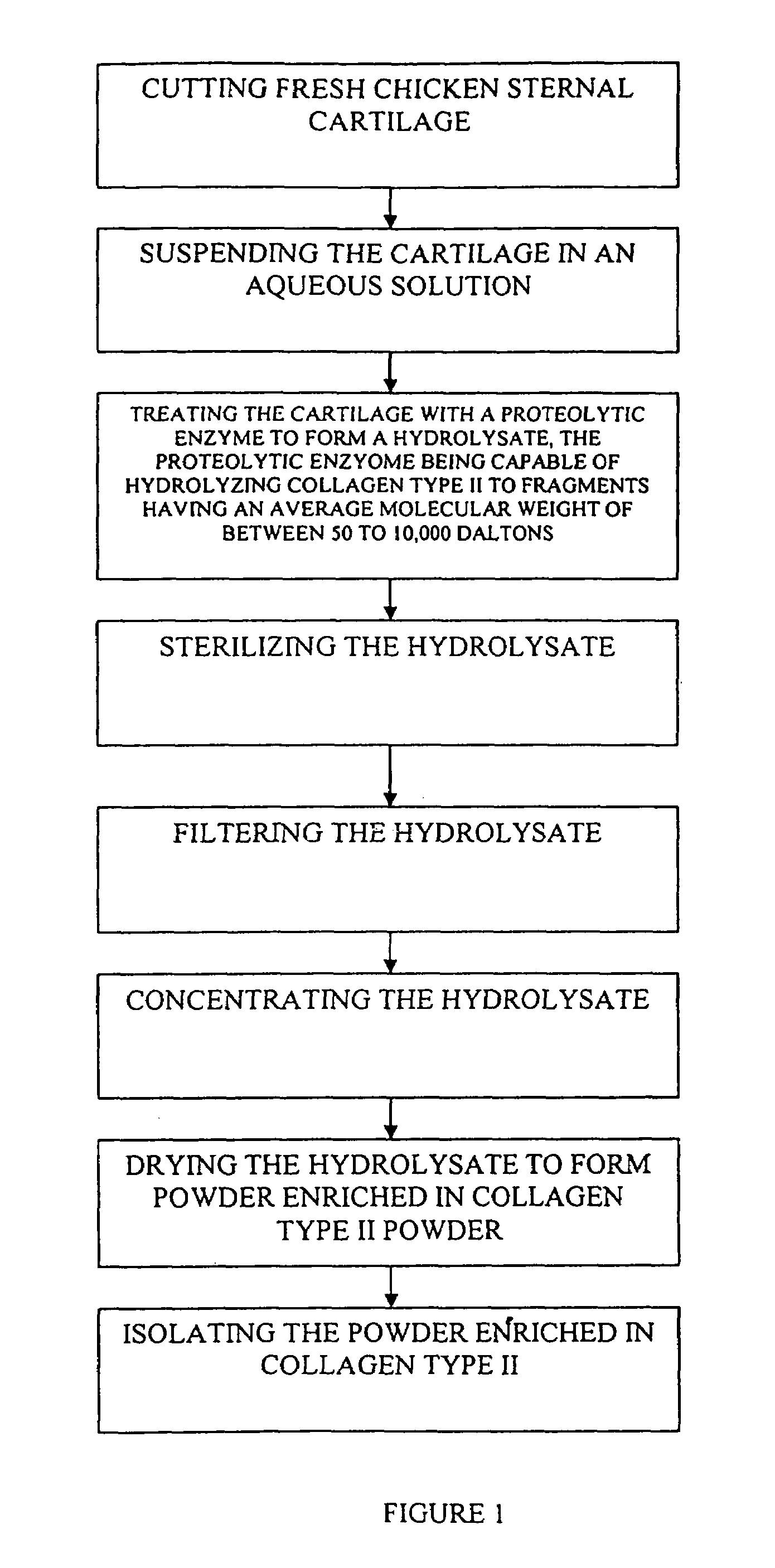
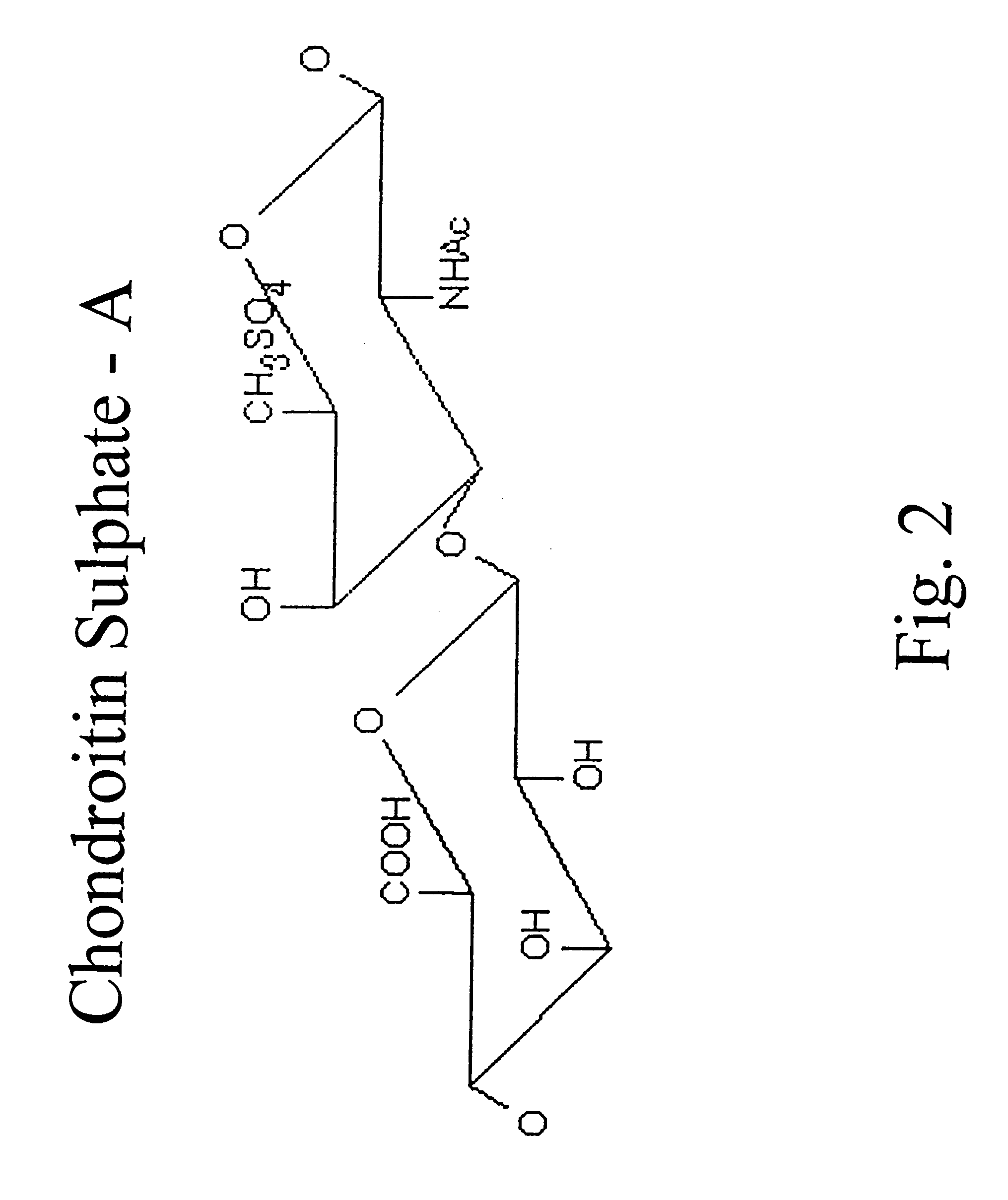
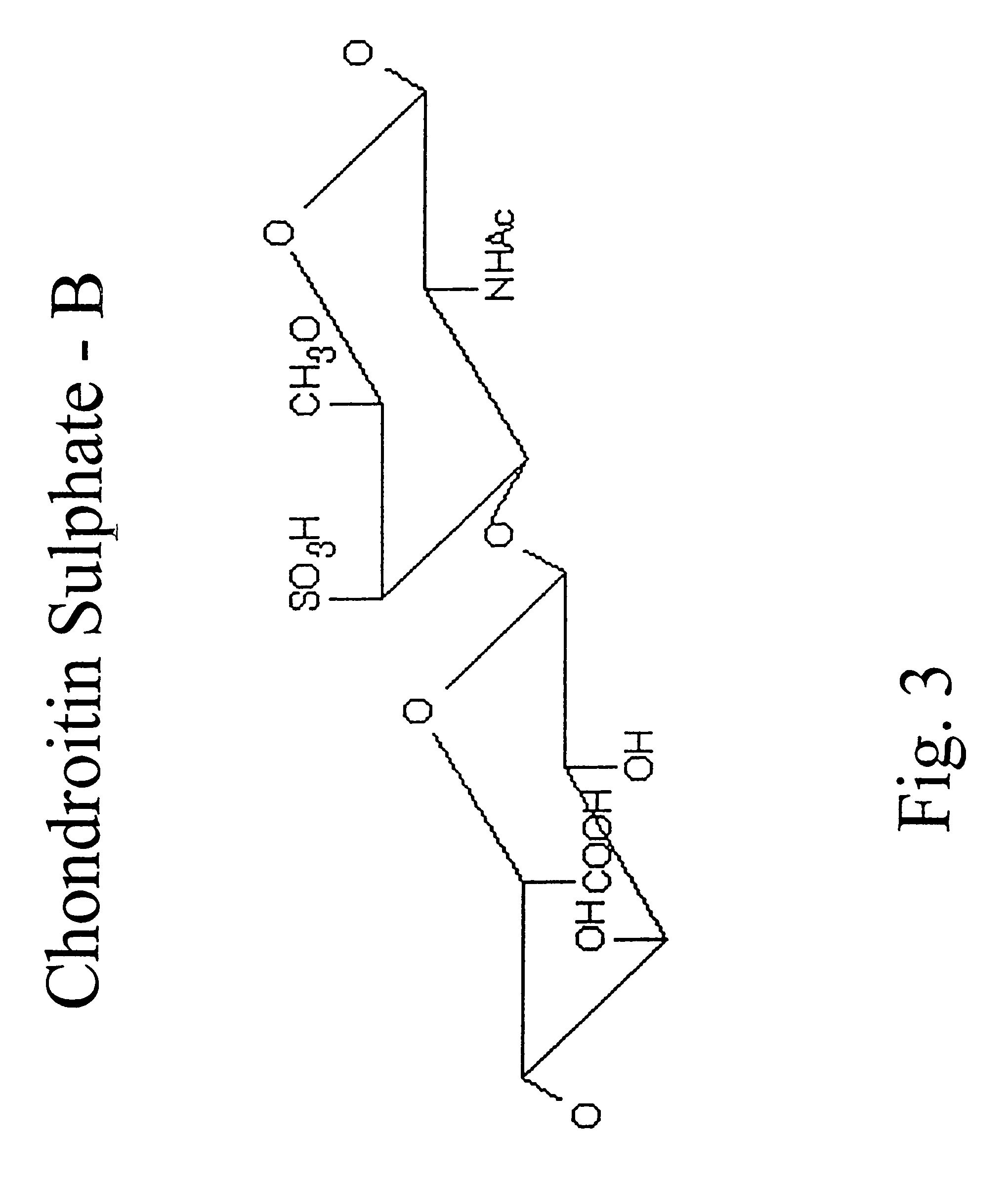
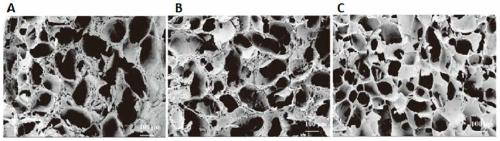
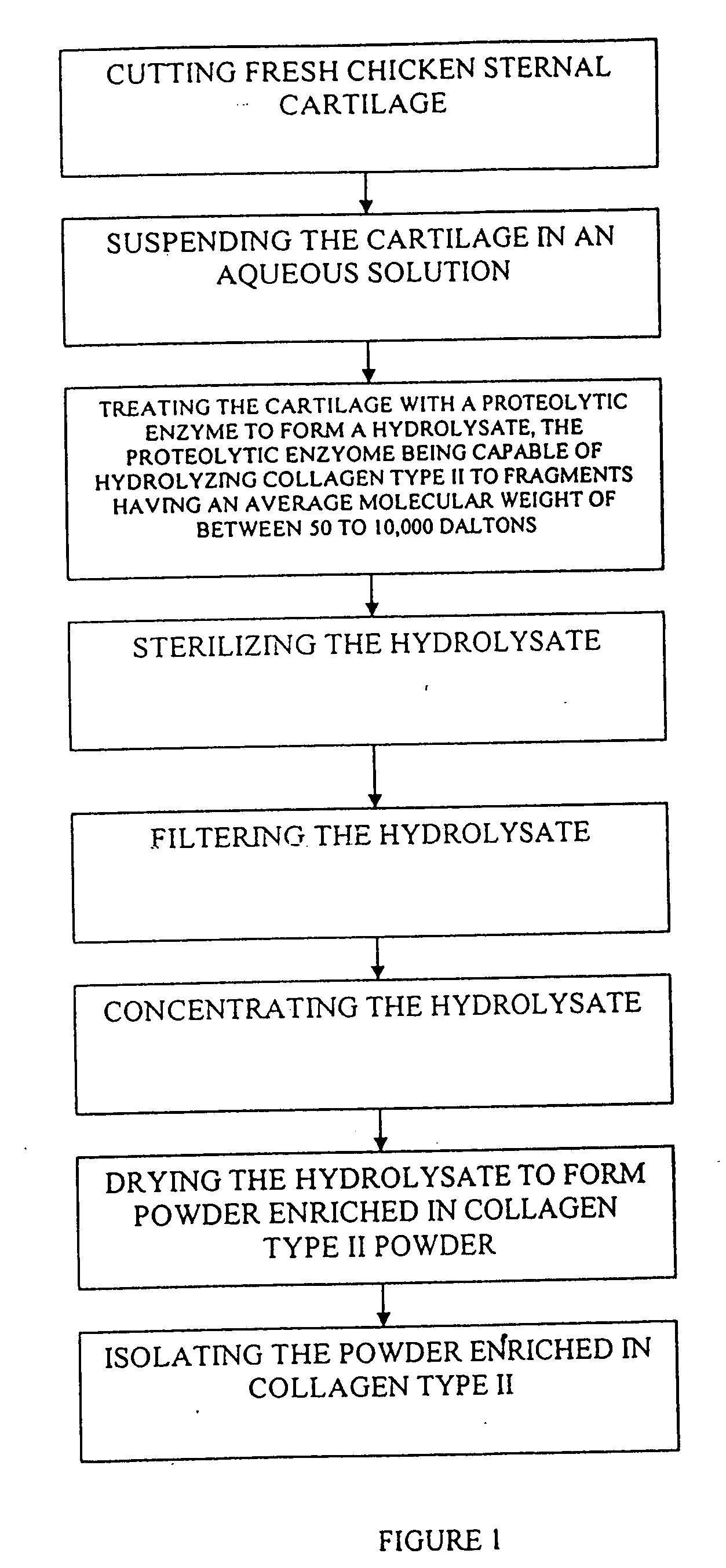
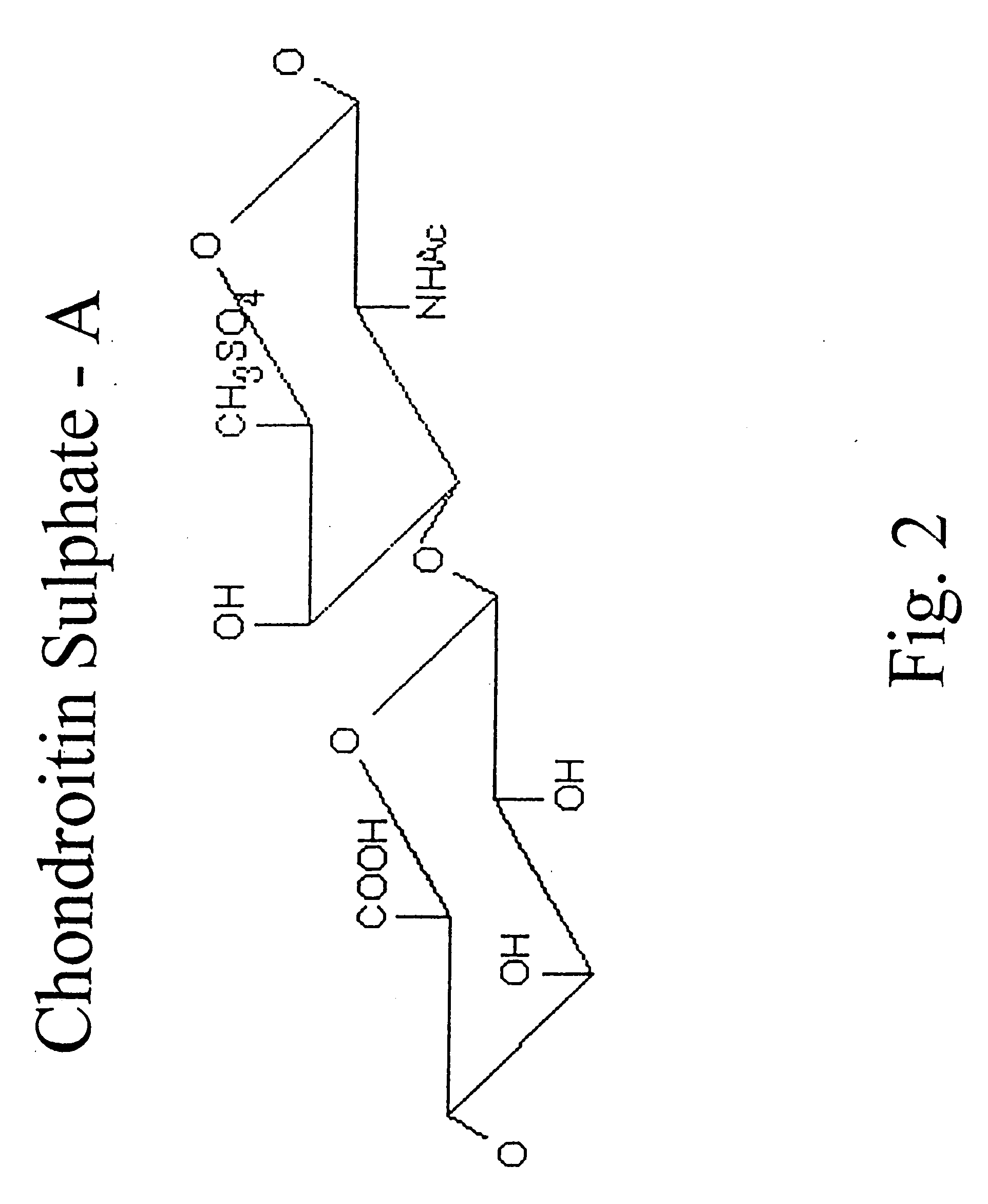
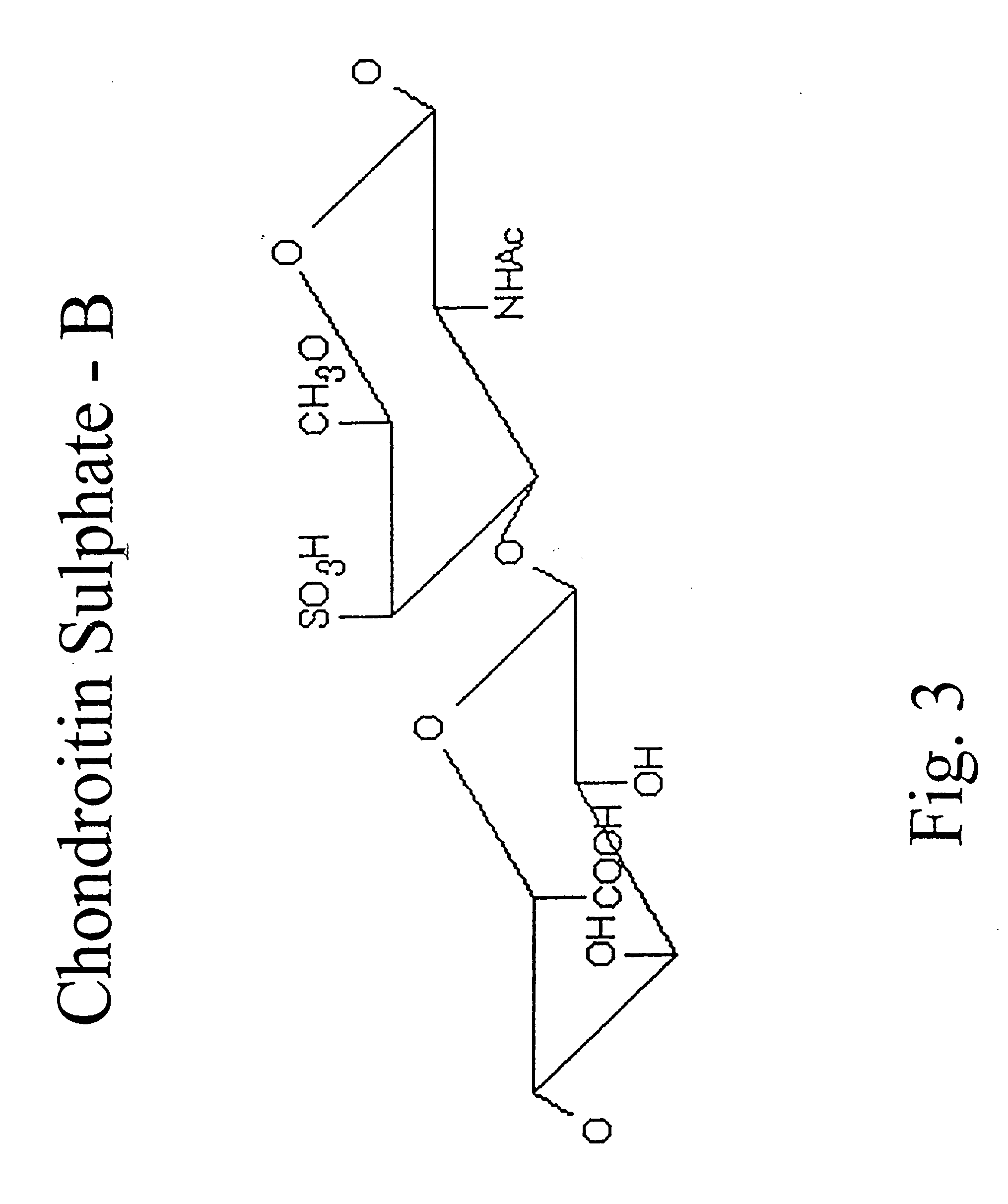
Hyaluronic acid and chondroitin sulfate based hydrolyzed collagen type II and method of making same
InactiveUS7091180B2Readily absorb into the gastrointestinal tract of an individualReduce molecular weightConnective tissue peptidesHydrolysed protein ingredientsConnective tissueHydrolyzed collagen
Hydrolyzed collagen type II powder compositions for inducing cartilage formation in an individual, method of preparing the compositions and use of the compositions in treating connective tissue disorder, replenishing skin viscoelasticity. The compositions are administered through an orally ingestible delivery medium for absorption into the gastrointestinal tract. The compositions are administered through a topical delivery medium for absorption into a dermis of the individual.
Owner:BIOCELL TECH
Human mesenchymal stem cell cartilage-formation induced differentiation culture medium and preparation method
InactiveCN108531448AEasy to useEasy to prepareCulture processSkeletal/connective tissue cellsUmbilical cordCulture mediums
The invention discloses a human mesenchymal stem cell cartilage-formation induced differentiation culture medium. The human mesenchymal stem cell cartilage-formation induced differentiation culture medium is characterized by being prepared from the following components: an alpha-MEM / HG-DMEM culture medium, fetal bovine serum (FBS) with the volume percent of 5 to 50 percent, sodium pyruvate with the concentration of 50 to 200mg / ml, ascorbic acid with the concentration of 20 to 200mu g / ml, proline with the concentration of 10 to 100mu g / ml, dexamethasone with the volume of 50 to 500nM, ITS withthe volume percent of 0.5 to 20 percent, TGF (Transforming Growth Factor)-beta with the concentration of 0.1 to 20ng / ml and an insulin growth factor-2 with the volume of 0.5 to 50nM. The human mesenchymal stem cell cartilage-formation induced differentiation culture medium is prepared through the following steps: disinfecting a culture dish; adding a source culture medium; mixing the components; adding FBS, a sodium pyruvate mother solution, an ascorbic acid mother solution, a proline mother solution, a dexamethasone mother solution, an ITS mother solution, a TGF-beta3 mother solution and an insulin growth factor-2 mother solution according to the concentration, and uniformly mixing; filtering and sterilizing; filtering and sterilizing a mixed culture solution by utilizing a 0.22mu m filtering membrane. The human mesenchymal stem cell cartilage-formation induced differentiation culture medium has a rapid induction speed, and has a better induced differentiation effect on differentiation raw materials with different sources, such as human bone mesenchymal stem cells, human umbilical cord mesenchymal stem cells and human adipose mesenchymal stem cells; a preparation method of the culture medium is simple, the culture medium is convenient to use and a process is stable.
Owner:安徽瑞杰赛尔生物科技有限公司
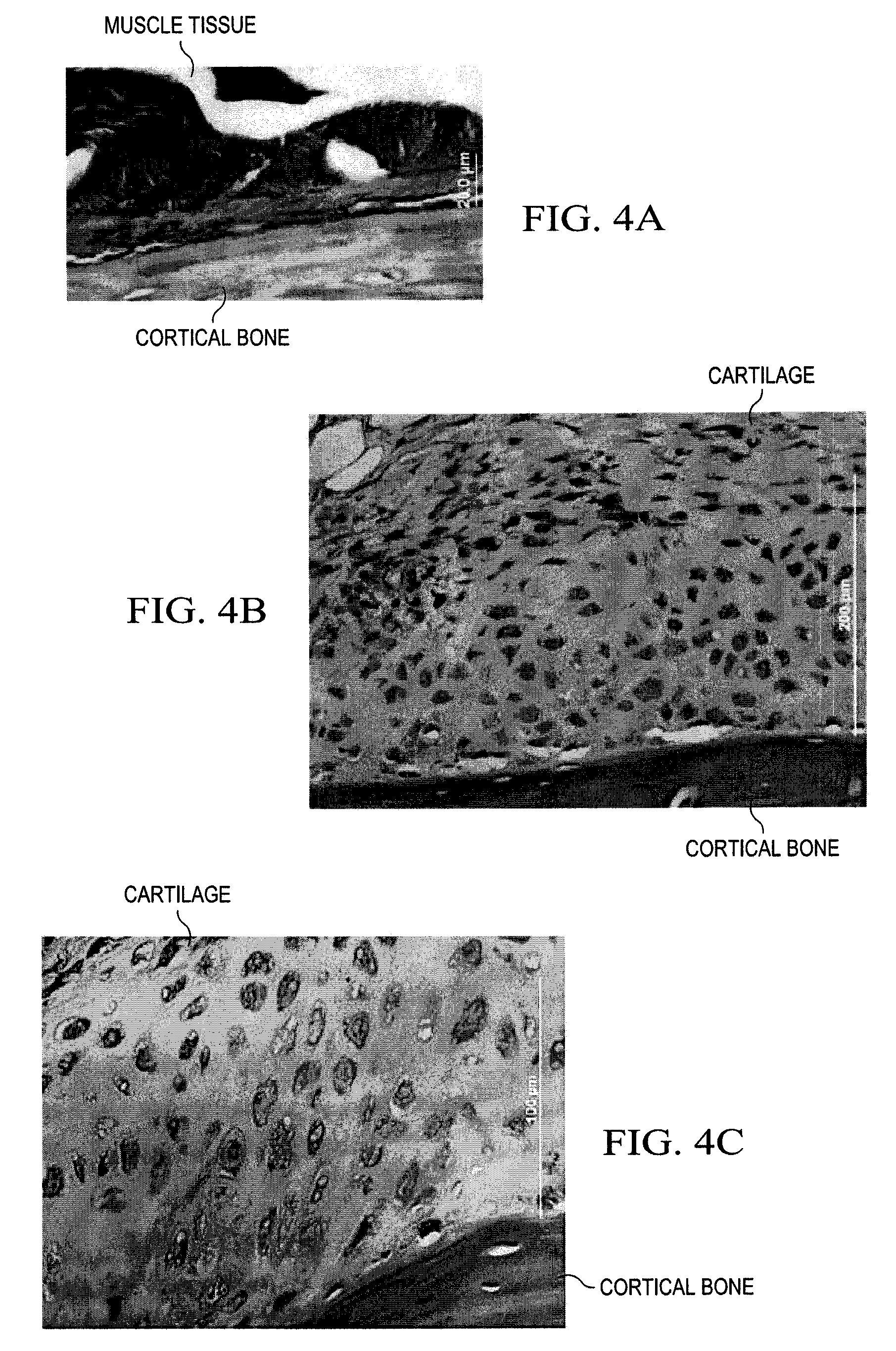
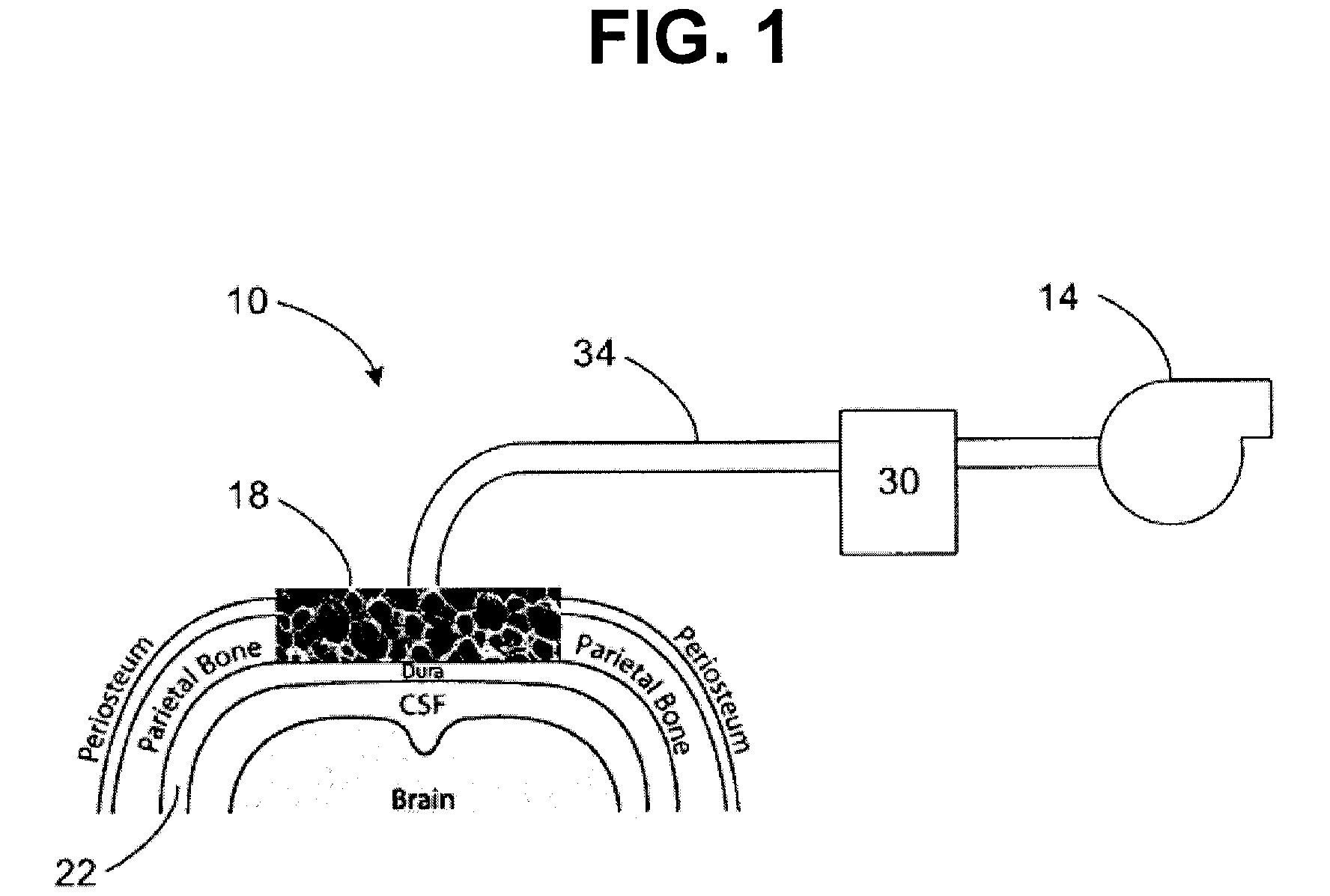
Activation of bone and cartilage formation
Provided is a method of activating osteogenic or chondrogenic activity at a site in a subject in need thereof. Also provided is a method of treating a bone or cartilage defect in a subject. Additionally, the use of a reduced pressure apparatus for treating a bone or cartilage defect adjacent to dura mater, periosteum, or endosteum is provided. Further provided is a composition for treating a bone or cartilage defect. Also, the use of a reduced pressure apparatus and a biocompatible scaffold for the manufacture of a composition for treating a bone or cartilage defect adjacent to dura mater, periosteum or endosteum is provided.
Owner:3M INNOVATIVE PROPERTIES CO
Activation of bone and cartilage formation
Provided is a method of activating osteogenic or chondrogenic activity at a site in a subject in need thereof. Also provided is a method of treating a bone or cartilage defect in a subject. Additionally, the use of a reduced pressure apparatus for treating a bone or cartilage defect adjacent to dura mater, periosteum, or endosteum is provided. Further provided is a composition for treating a bone or cartilage defect. Also, the use of a reduced pressure apparatus and a biocompatible scaffold for the manufacture of a composition for treating a bone or cartilage defect adjacent to dura mater, periosteum or endosteum is provided.
Owner:KCI LICENSING INC
Compositions comprising bone marrow cells, demineralized bone matrix and various site-reactive polymers for use in the induction of bone and cartilage formation
A composition comprising bone marrow cells (BMC) and demineralized bone matrix (DBM) or demineralized tooth matrix (DTM), together with a site-responsive polymer, optionally further comprising bone morphogenetic proteins (BMP) and / or other active agents, particularly for use in the transplantation of mesenchymal progenitor cells into a joint or a cranio-facial-maxillary bone, alveolar bone of maxilla and mandibula, spine, pelvis or long bones, or for construction or reconstruction of any extra skeletal bone, including for mechanical or biological support of artificial implants to the joint or of the joint or to the bone, for restoring and / or enhancing the formation of a new hyaline cartilage and subchondral bone structure. A kit is provided for performing transplantation of the composition into a joint, maxillary or mandibular alveolar bone or any bony structure of a mammal, including support of artificial implants.
Owner:SLAVIN SHIMON +4
Bone and cartilage integrated repair support and preparation method thereof
ActiveCN108992212AAvoid deformationHigh Compression ModulusBone implantJoint implantsCartilage cellsCartilage bone
The invention provides a bone and cartilage integrated repair support and a preparation method thereof. The bone and cartilage integrated repair support comprises a bone repair layer and a cartilage repair layer connected with the bone repair layer; the bone repair layer comprises a biodegradable synthetic polymeric compound and a powder component with osteogenic activity; and the cartilage repairlayer comprises a biodegradable synthetic polymeric compound and a powder component with a cartilage formation activity. The bone and cartilage integrated repair support can stimulate a defect part of cartilages and sub-cartilage bones in a targeted way and facilitates tissue regeneration. Further, the cartilage recovered layer of the support includes cartilage cells, so that cartilage repair canbe accelerated, and the cartilage repair quality is improved.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Injectable cartilage repair hydrogel and preparation method thereof
ActiveCN111184910AGood biocompatibilityFormation effect is goodPharmaceutical delivery mechanismTissue regenerationBenzoic acidMethacrylate
The invention relates to injectable cartilage repair hydrogel. The hydrogel is prepared from the following components in parts by weight: 5 to 15 parts of methacrylate gelatin, 1 to 10 parts of methacrylate hyaluronic acid, 1 to 2 parts of nano cellulose and 0.1 to 0.5 part of melanin nano particles loaded with 2-([1, 1-biphenyl]-4-ylcarbamyl) benzoic acid. According to the hydrogel disclosed by the invention, a catechol functionalized network is used as a paramagnetic metal ion chelating bracket, and the synthesized melanin base material has excellent biocompatibility and the capability of coordinating isolated paramagnetic metal centers. The base material is loaded with a cartilage repair promoting small molecular compound 2-([1, 1-biphenyl]-4-ylcarbamyl) benzoic acid. The nanoparticlesare dispersed into injectable hydrogel, so that the product can remarkably induce bone marrow mesenchymal stem cells to be differentiated into cartilage, has an extremely strong cartilage formation promoting effect, realizes detection of cartilage repair conditions in MRI, photoacoustic and photo-thermal multiple imaging modes, and enhancing application in medical clinics.
Owner:BEOGENE BIOTECH GUANGZHOU
Hyaluronic acid and chondroitin sulfate based hydrolyzed collagen type II and method of making same
InactiveUS20060045920A1Reduce molecular weightReadily absorb into the gastrointestinal tract of an individualHydrolysed protein ingredientsSkeletal disorderDiseaseViscoelasticity
Hydrolyzed collagen type II powder compositions for inducing cartilage formation in an individual, method of preparing the compositions and use of the compositions in treating connective tissue disorder, replenishing skin viscoelasticity. The compositions are administered through an orally ingestible delivery medium for absorption into the gastrointestinal tract. The compositions are administered through a topical delivery medium for absorption into a dermis of the individual.
Owner:BIOCELL TECH
Tissue- engineered cartilage graftimplant and preparation method thereof
InactiveCN103495208AImprove adhesionPromote vascularizationSkeletal/connective tissue cellsProsthesisCartilage cellsBiomechanics
The invention relates to a tissue tissue-engineered cartilage graftimplant and a preparation method thereof and belongs to the technical field of induced differentiation carried out on bone marrow mesenchymal stem cells (BMSCs) by utilizing a bioactive inducing factor to form a cartilage cell chondroblast composite scaffold material and so as to construct a tissue tissue-engineered cartilage by utilizing a biological activity inducing factor in biomedicine tissue engineering. The tissue tissue-engineered cartilage graftimplant is prepared by adopting the method comprising the following steps: (1) preparing a Nano-HA / PLLA (hyaluronic aciddroxyapatite / poly left L-lactic acid) cartilage scaffold material; (2) carrying out coculture on BMSCs and the Nano-HA / PLLA cartilage scaffold material, and carrying out induced differentiation on BMSCs to form cartilage cells by adopting a cartilage formation inducing solution, so that the tissue tissue-engineered cartilage graftimplant is obtained. The tissue tissue-engineered cartilage graftimplant improves flexibility and biodegradability of the cartilage scaffold material, improves biomechanical property and is more beneficial to adhesion, growth and vascularization of bone cells; an animal experiment proves that the tissue tissue-engineered cartilage graftimplant has a good cartilage defect repairing function.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Stimulation of cartilage formation using reduced pressure treatment
ActiveUS20090326423A1Promote growthBiocidePeptide/protein ingredientsBiomedical engineeringCartilage formation
Provided is a method of stimulating cartilage formation at a tissue site in a mammal. Also provided is a biocompatible scaffold. Additionally, a system for stimulating cartilage formation at a tissue site is provided. Further provided is the use of a manifold, a chondrocyte, and a reduced-pressure source to stimulate cartilage formation at a tissue site of a mammal.
Owner:KCI LICENSING INC
Composition for Promoting Cartilage Formation or Repair Comprising a NELL Gene Product and Method of Treating Cartilage-Related Conditions Using Such Composition
Owner:RGT UNIV OF CALIFORNIA
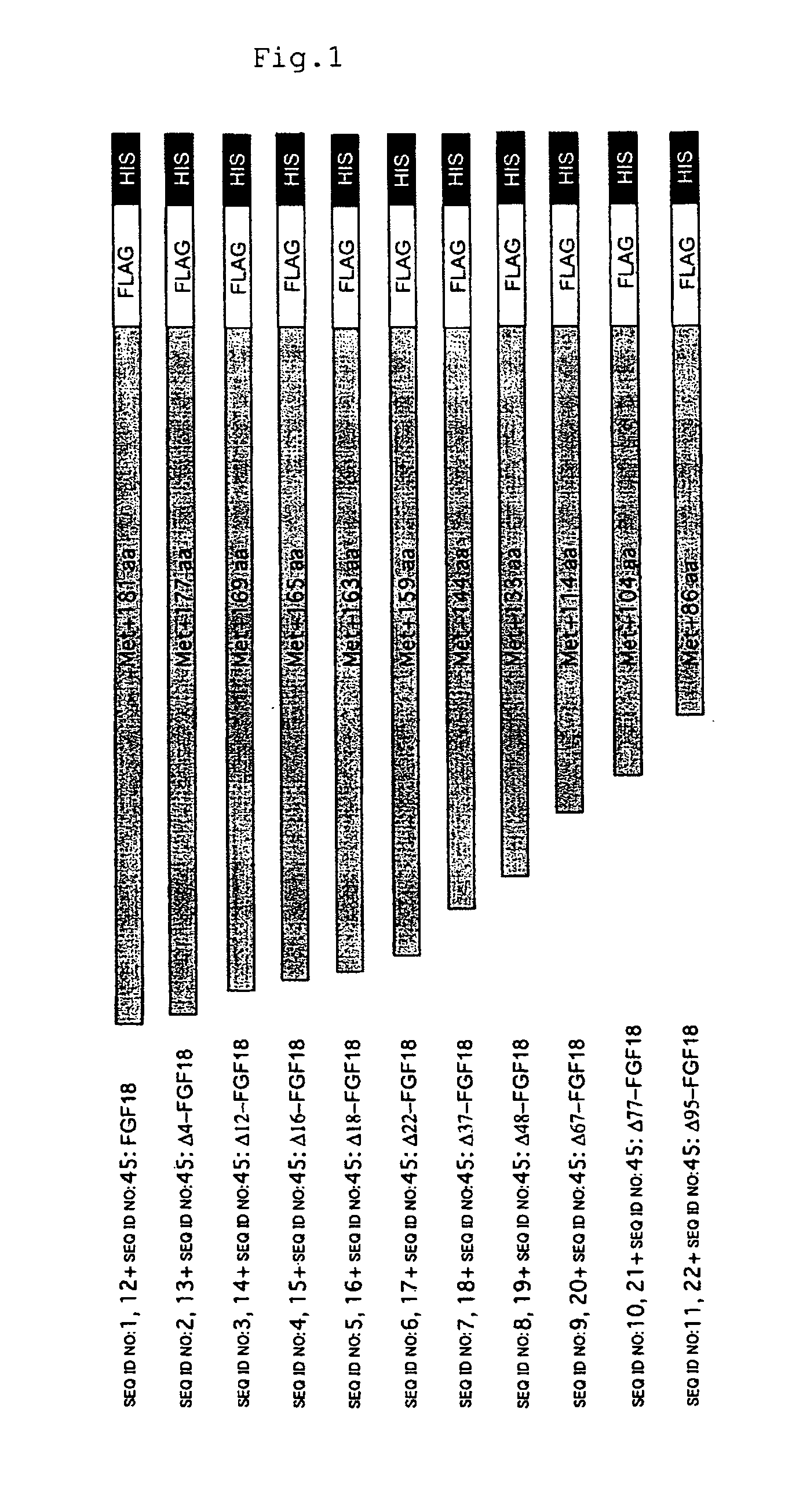
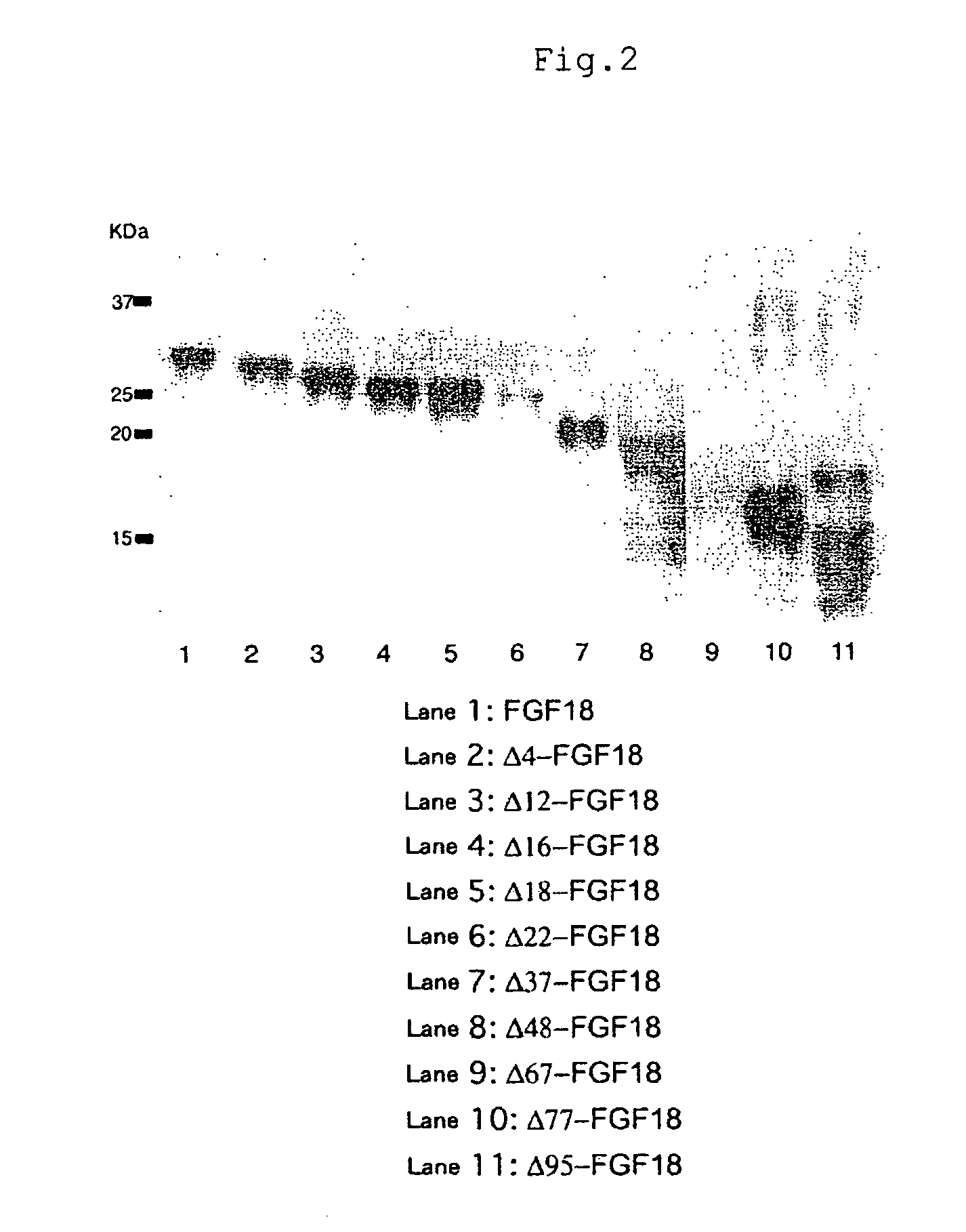
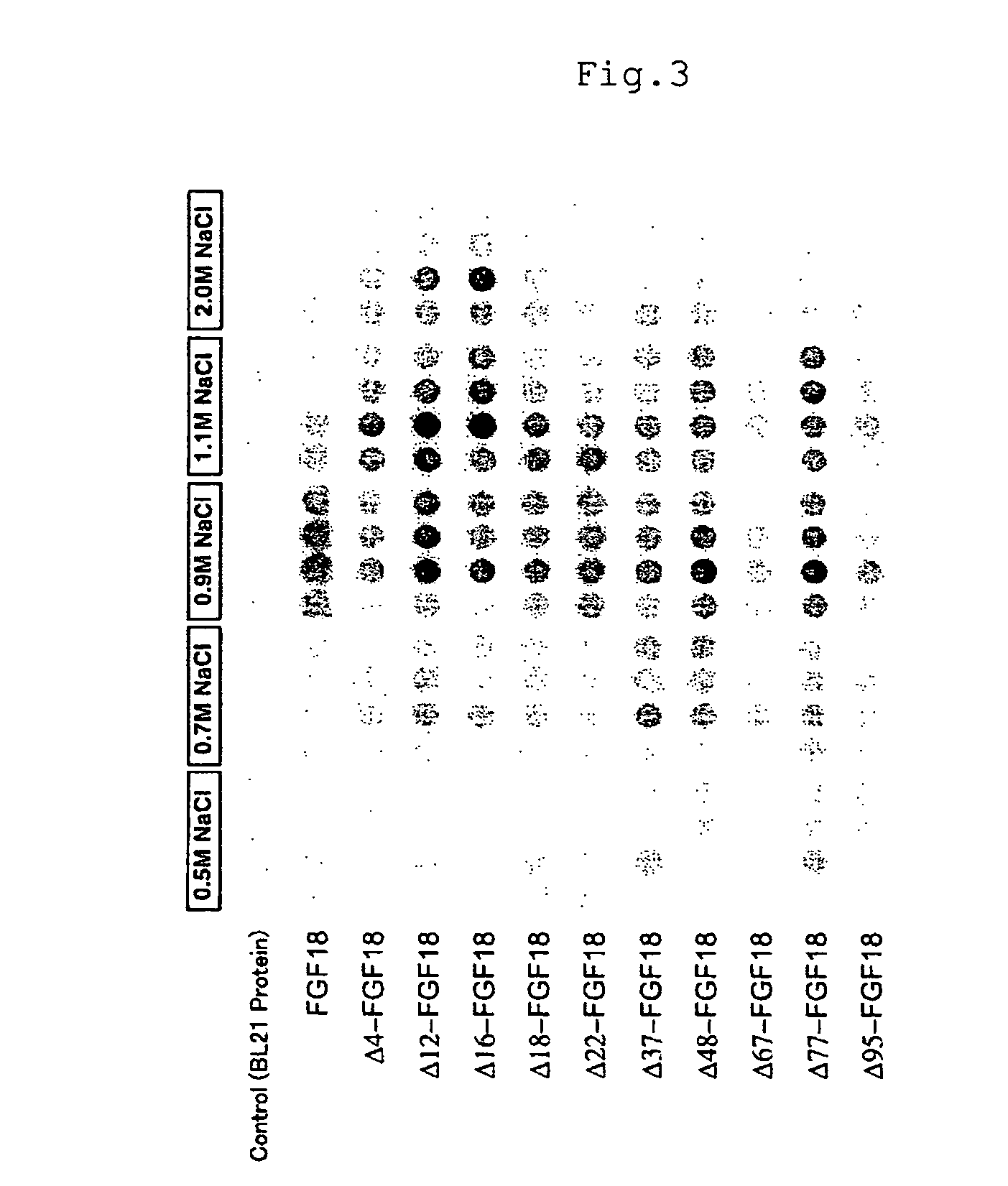
Mutant growth factors with altered receptor specificities and pharmaceutical composition comprising the same
InactiveUS20090286965A1Regulate a physiological activityCosmetic preparationsNervous disorderMutated proteinReceptor for activated C kinase 1
Disclosed is a mutant protein having an altered fibroblast growth factor receptor specificity, which is produced by deleting one or more amino acid residues from the N-terminus of the amino acid sequence of naturally secreted fibroblast growth factor 18. The protein mutant can be used in a pharmaceutical composition for regulating hair regeneration or growth or a pharmaceutical composition for regulating bone or cartilage formation.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Compositions comprising bone marrow cells together with demineralized and/or mineralized bone matrix and uses thereof in the induction of bone and cartilage formation
A composition comprising bone marrow cells (BMC) and demineralized bone matrix (DBM) and / or mineralized bone matrix (MBM) and optionally comprising bone morphogenetic protein / s (BMP) and / or other active agents, particularly for use in the transplantation of mesenchymal progenitor cells into a joint and / or a cranio-facial maxillary bone, for restoring and / or enhancing the formation of a new hyaline cartilage and subchondral bone structure. The composition of the invention and method of treatment employing the same may be used for the treatment of hereditary or acquired bone disorders, hereditary or acquired cartilage disorders, malignant bone or cartilage disorders, metabolic bone diseases, bone infections, conditions involving bone or cartilage deformities and Paget's disease. The composition and method may further be used for the correction of complex fractures, bone replacement and formation of new bone in plastic or sexual surgery, for support of implants of joints, cranio-facial-maxillary bones, or other musculoskeletal implants, including artificial implants. The method of the invention may further be used for treating damaged joints or degenerative arthropathy associated with malformation and / or dysfunction of cartilage and / or subchondral bone. A kit is provided for performing transplantation into a joint or a cranio-facial-maxillary bone of a mammal of the composition of the invention.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
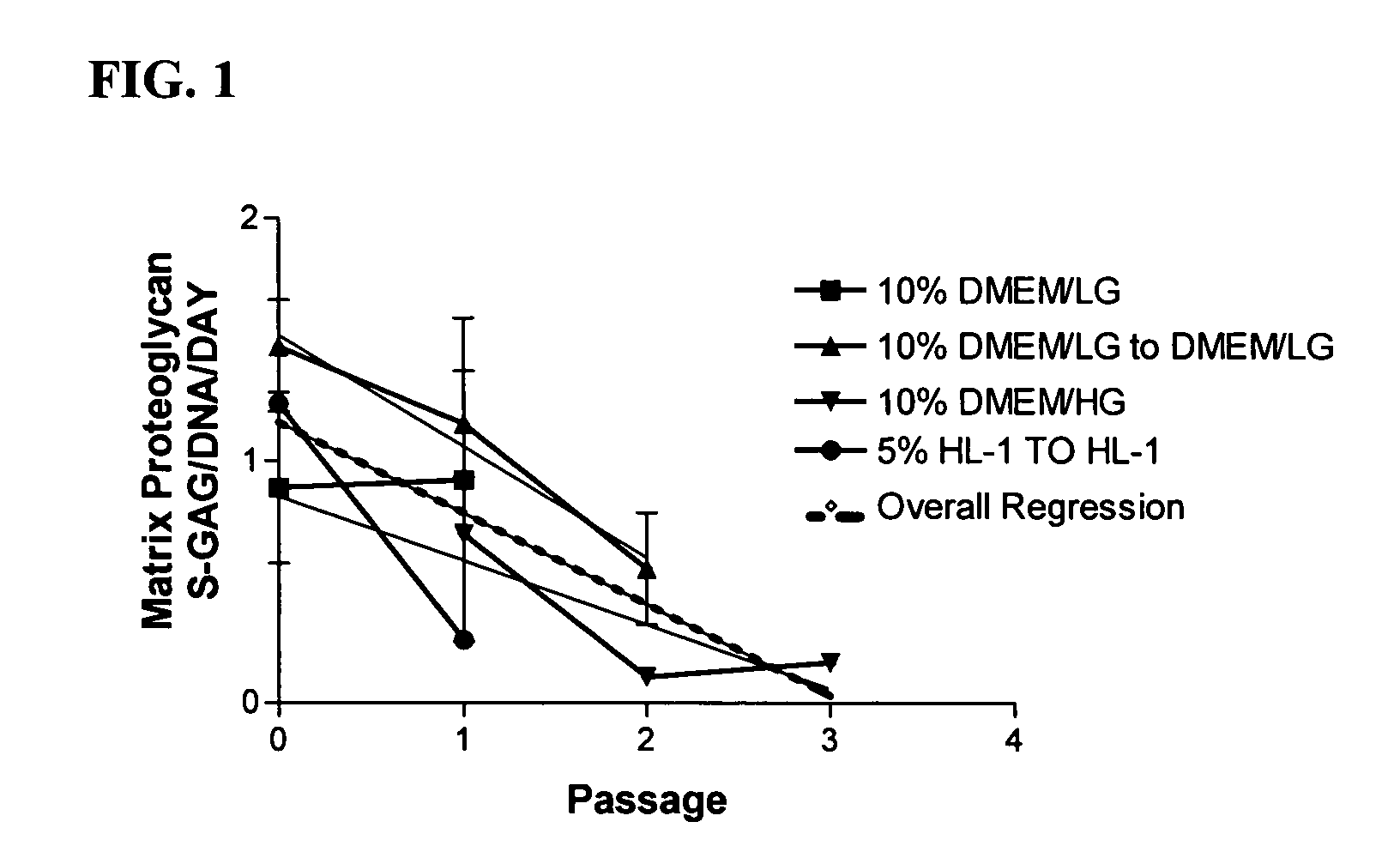
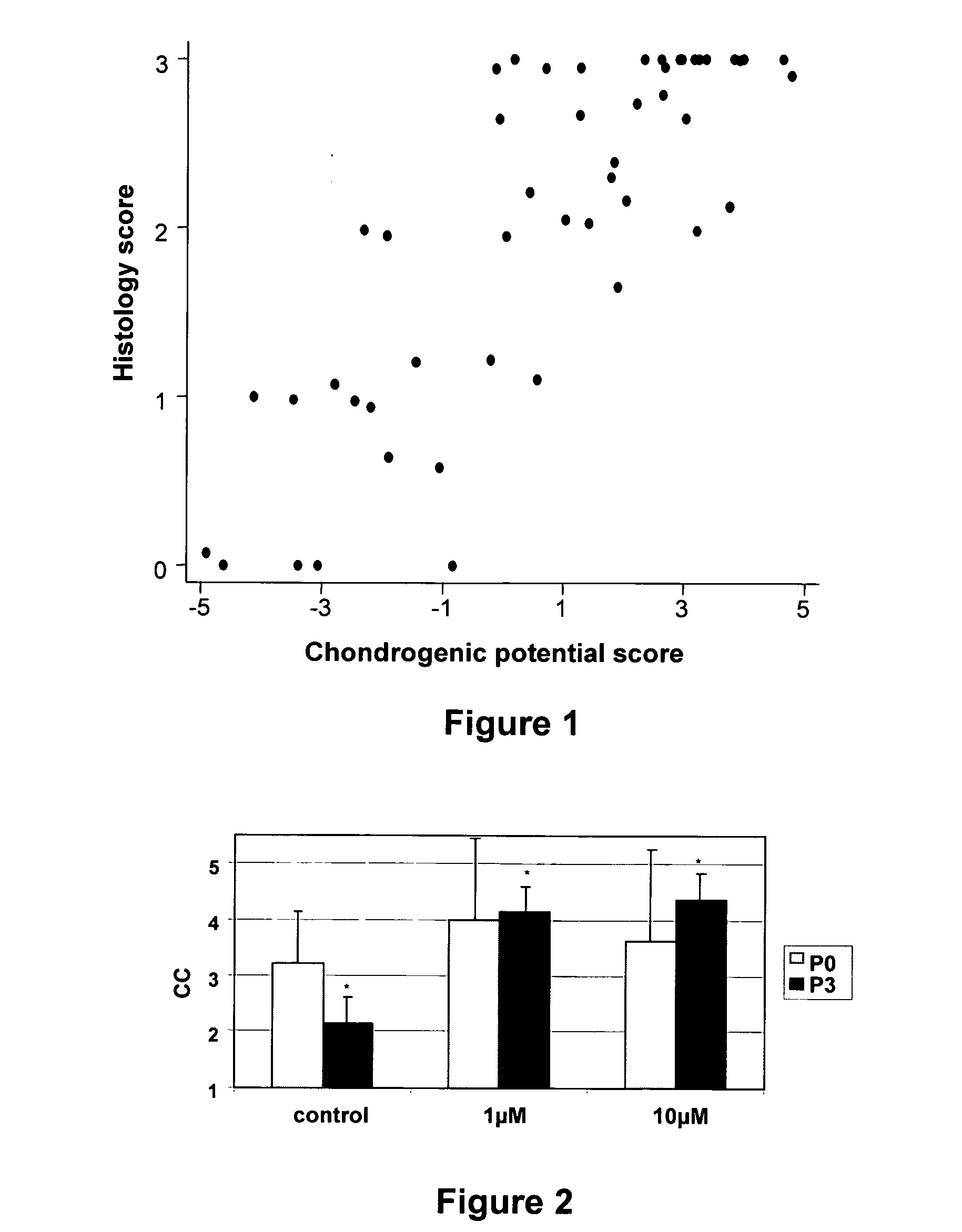
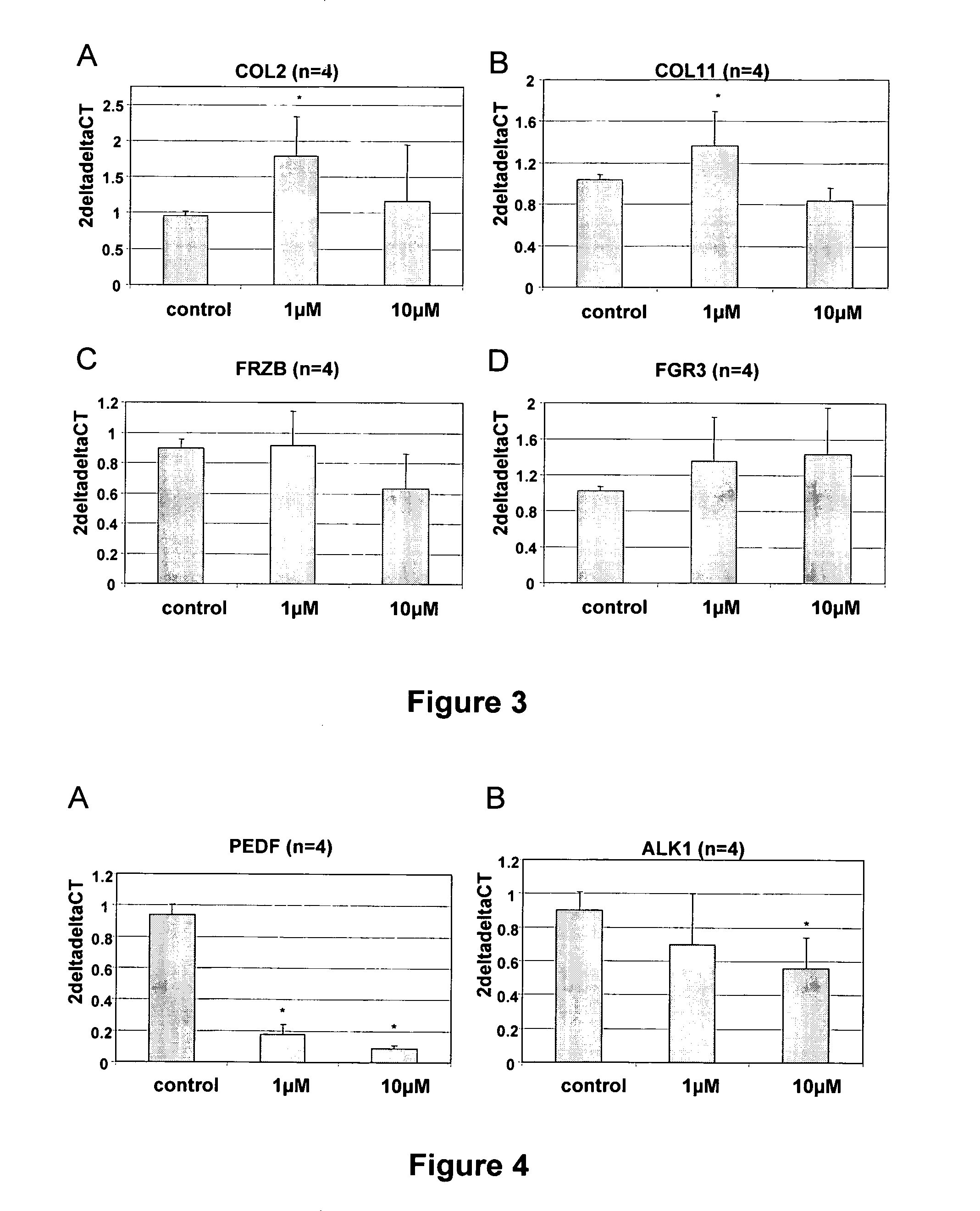
Marker genes for use in the identification of chondrocyte phenotypic stability and in the screening of factors influencing cartilage production
InactiveUS20100068715A1Affect stabilityReliable predictionBioreactor/fermenter combinationsBiological substance pretreatmentsAssayQuality control
The present invention relates to a set of genes which can be used to predict the potential of a cell population to form cartilage when implanted in vivo. The set of markers is used inter alia as a quality control of cells and in screening assays to evaluate the impact of compounds and conditions on the cartilage forming ability of cells.
Owner:TIGENIX NV
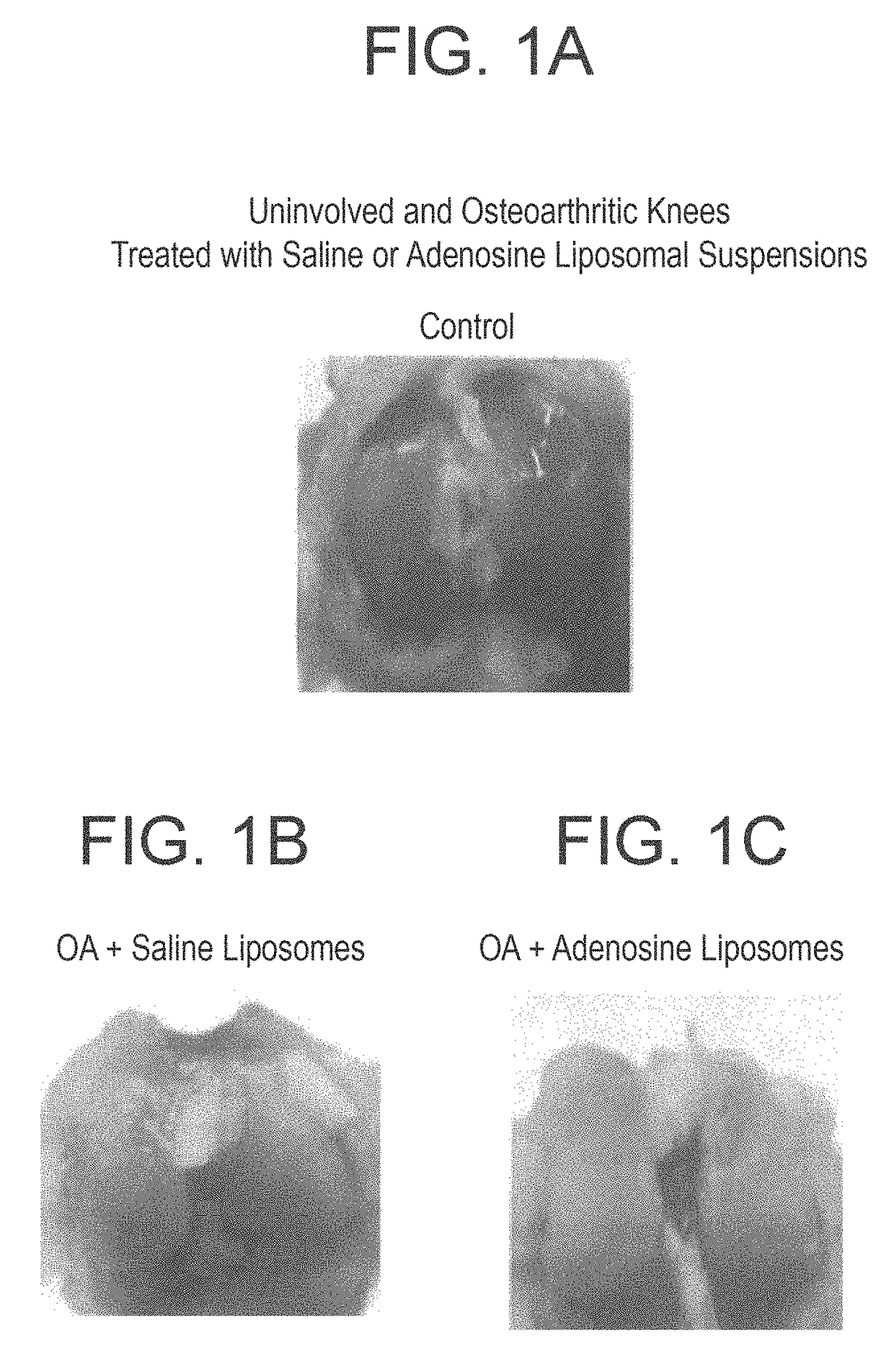
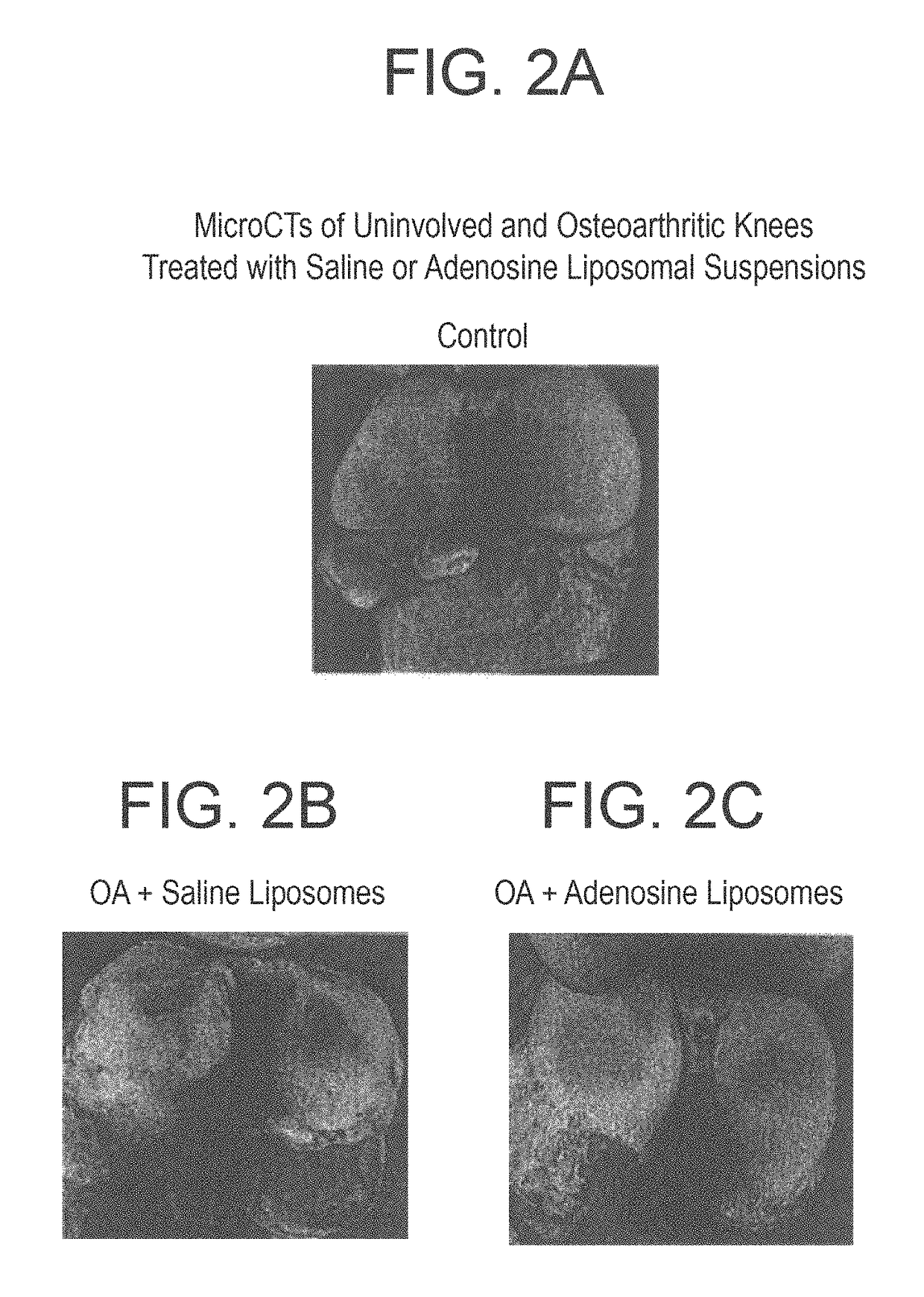
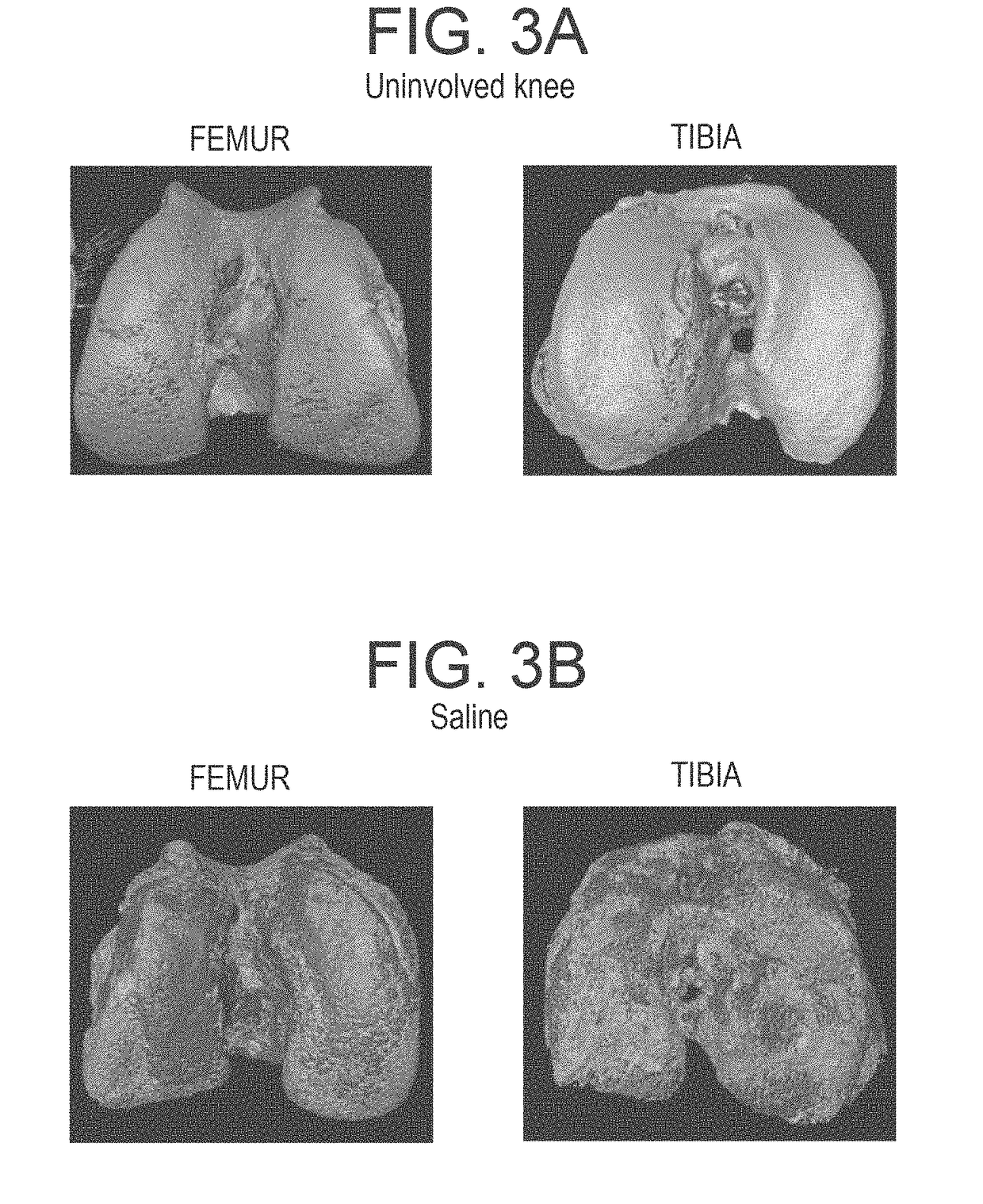
Methods and compositions for treating osteoarthritis and promoting cartilage formation
ActiveUS20180036238A1Minimize potential damageEliminate side effectsInorganic non-active ingredientsSkeletal disorderDipyridamoleTicagrelor
The invention provides methods and compositions for treating or inhibiting the development of osteoarthritis in a subject having osteoarthritis or at risk for developing osteoarthritis and for stimulating or increasing cartilage production or formation in a subject. The methods feature administering to the subject a therapeutically effective amount of a composition comprising one or more agent from among adenosine, an adenosine receptor agonist, and an agent that upregulates or increases the amount of or the biological activity of adenosine, or an analog or derivative thereof. The adenosine receptor may be an A1, A2A, A2B and A3 adenosine receptor. The agent that upregulates or increases the amount of or the biological activity of adenosine may be dipyridamole or ticagrelor. The composition may be administered via intraarticular injection such as injection into the synovial fluid of a joint. The composition may be effective to reduce or inhibit degeneration or damage to cartilage or to stimulate or increase cartilage production or formation. The composition may be a liposomal composition or contain a liposome.
Owner:NEW YORK UNIV
Biomatrix hydrogels and methods of use thereof
This invention is directed to aragonite and calcite hydrogel biomatrices, optionally seeded with precursor cells and uses thereof in tissue engineering, regeneration and repair, including in inducing or enhancing bone formation, cartilage formation or a combination thereof in a subject, and kits related thereto.
Owner:CARTIHEAL 2009
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com