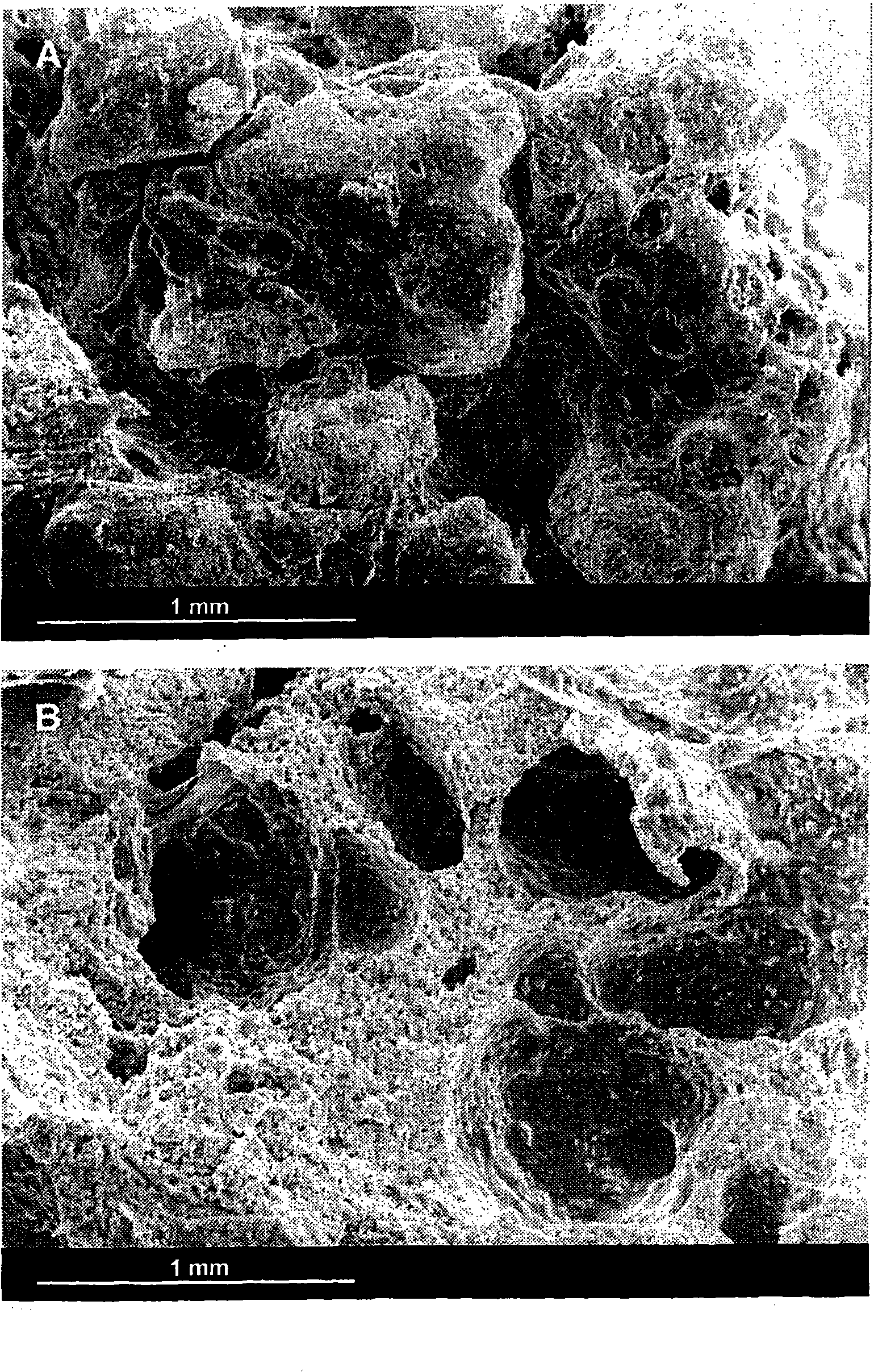
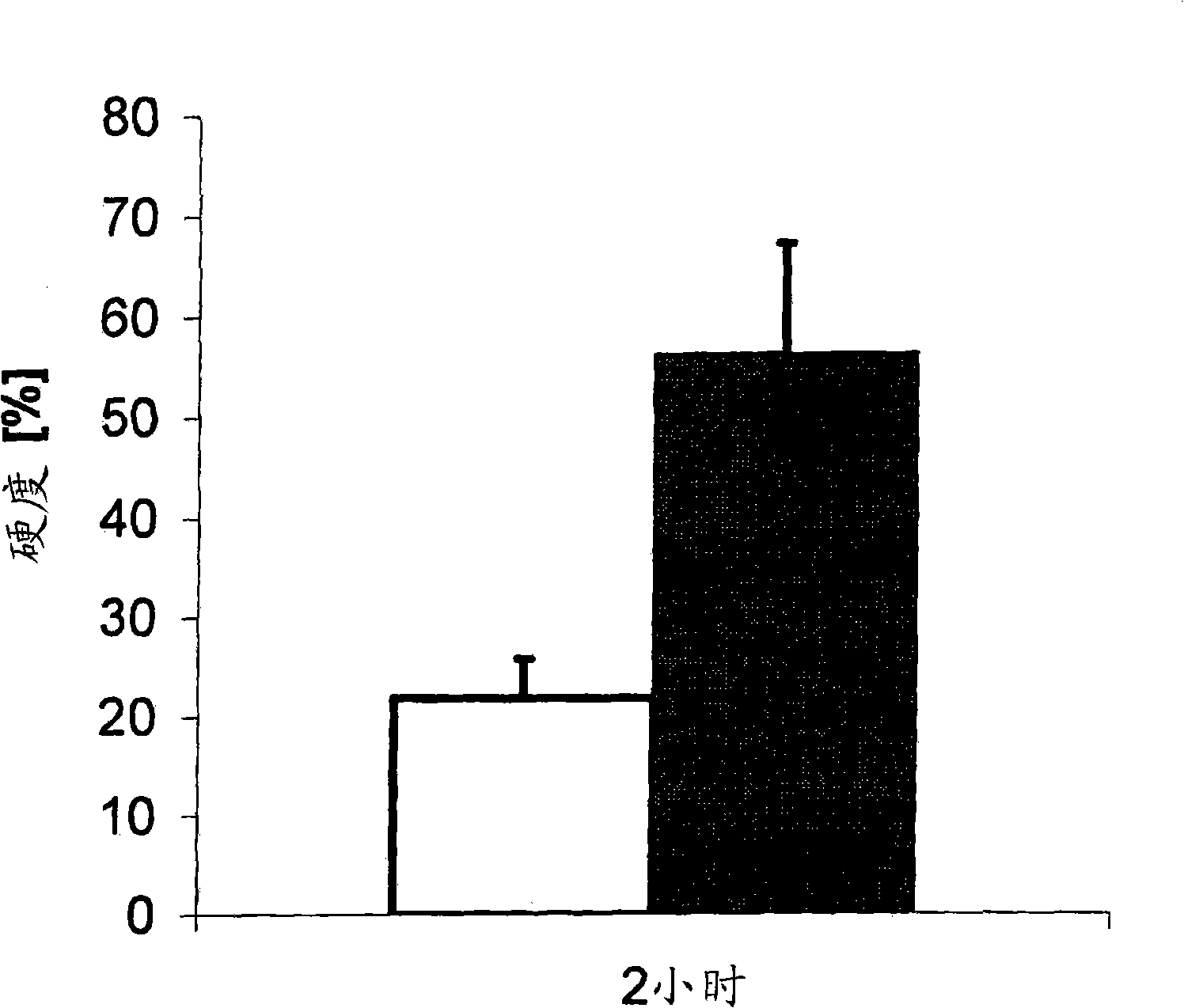
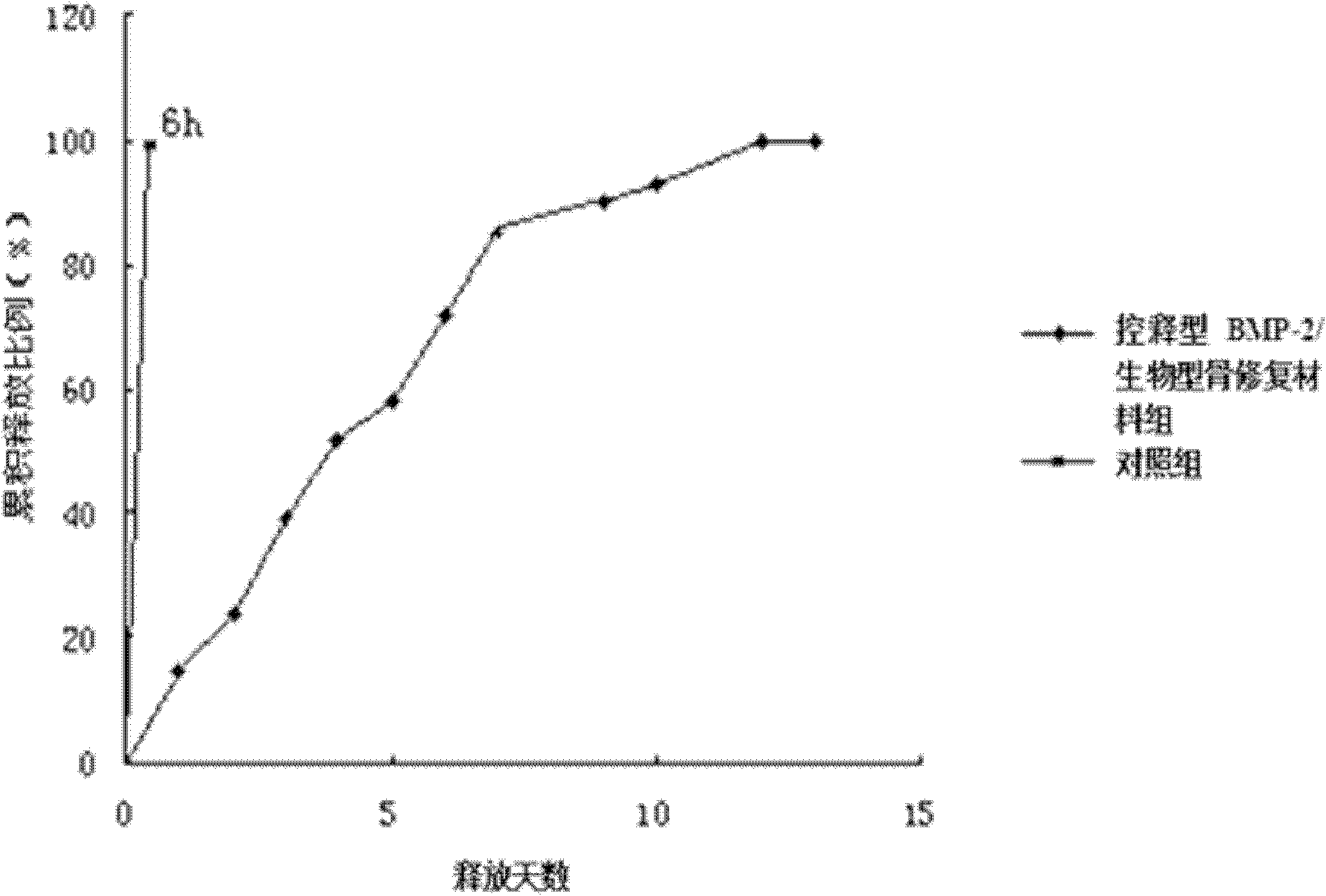
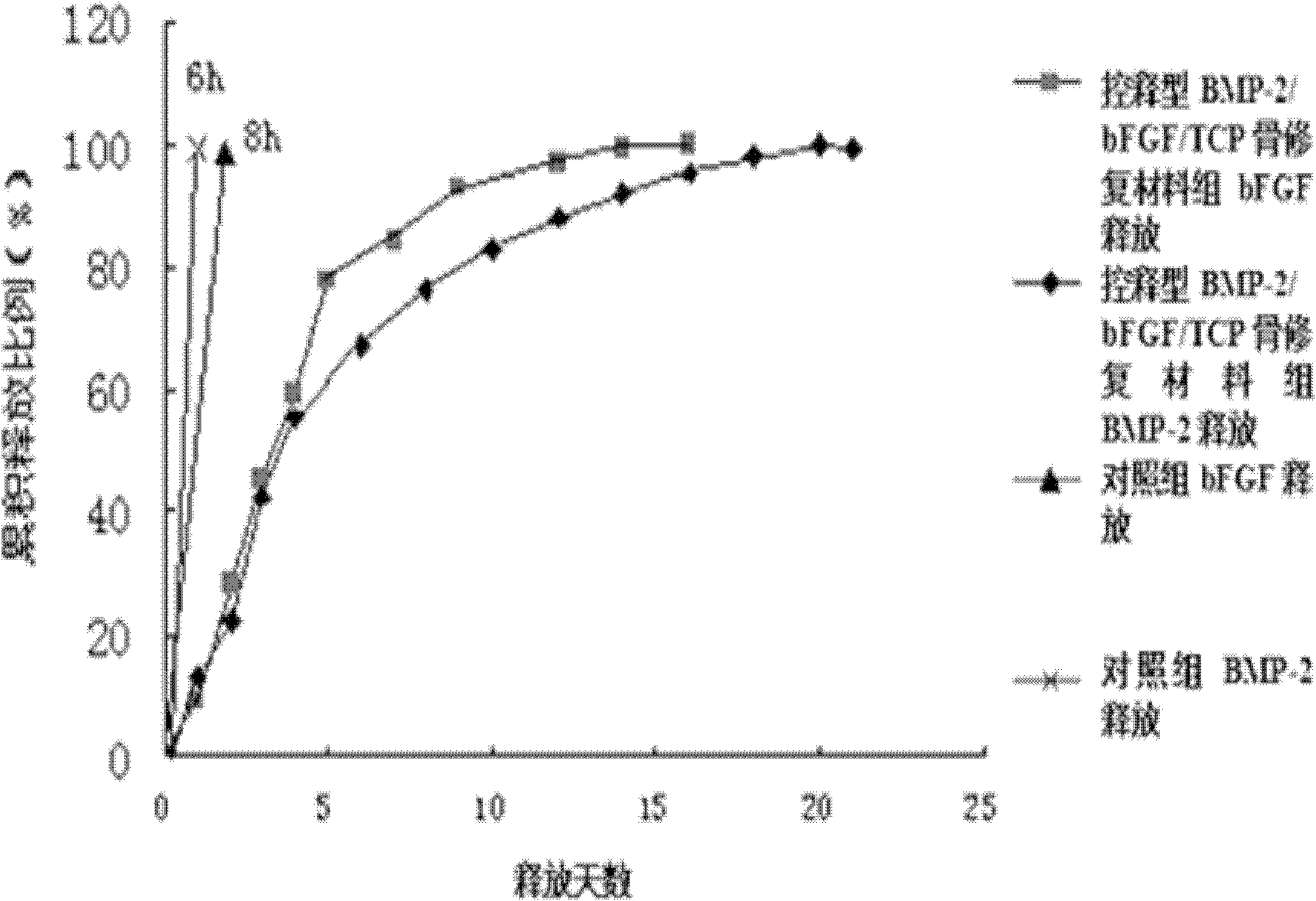
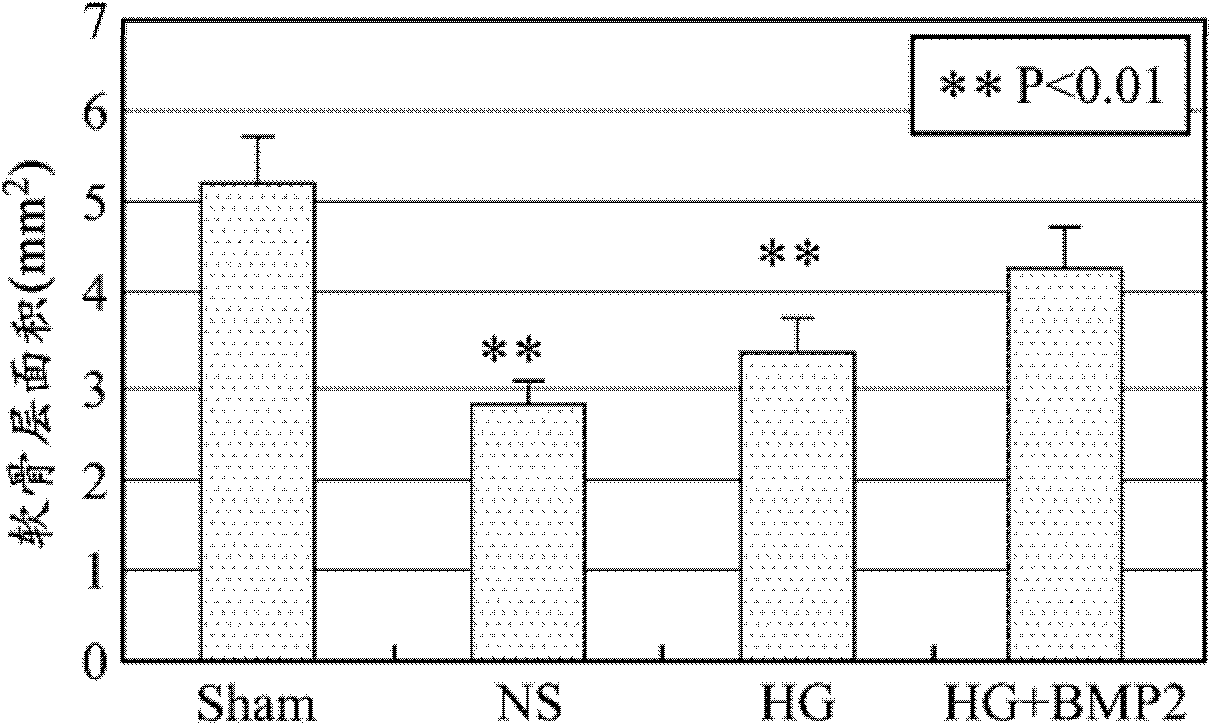
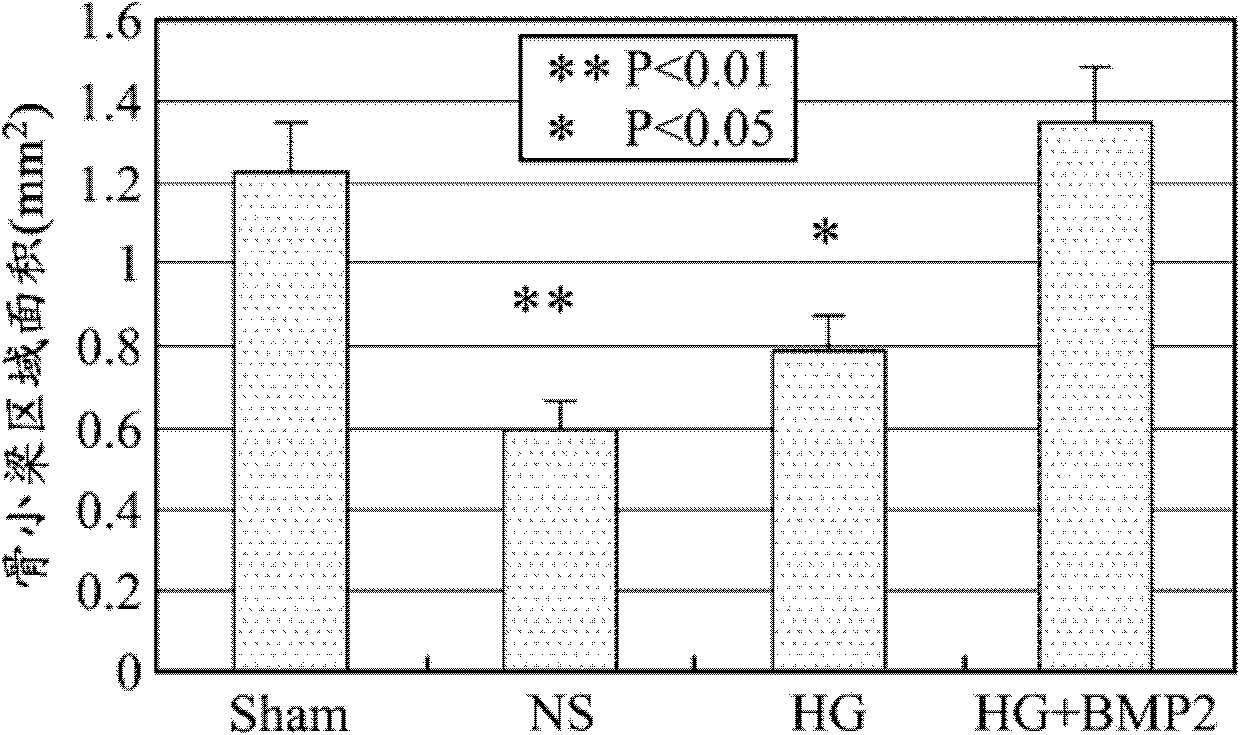
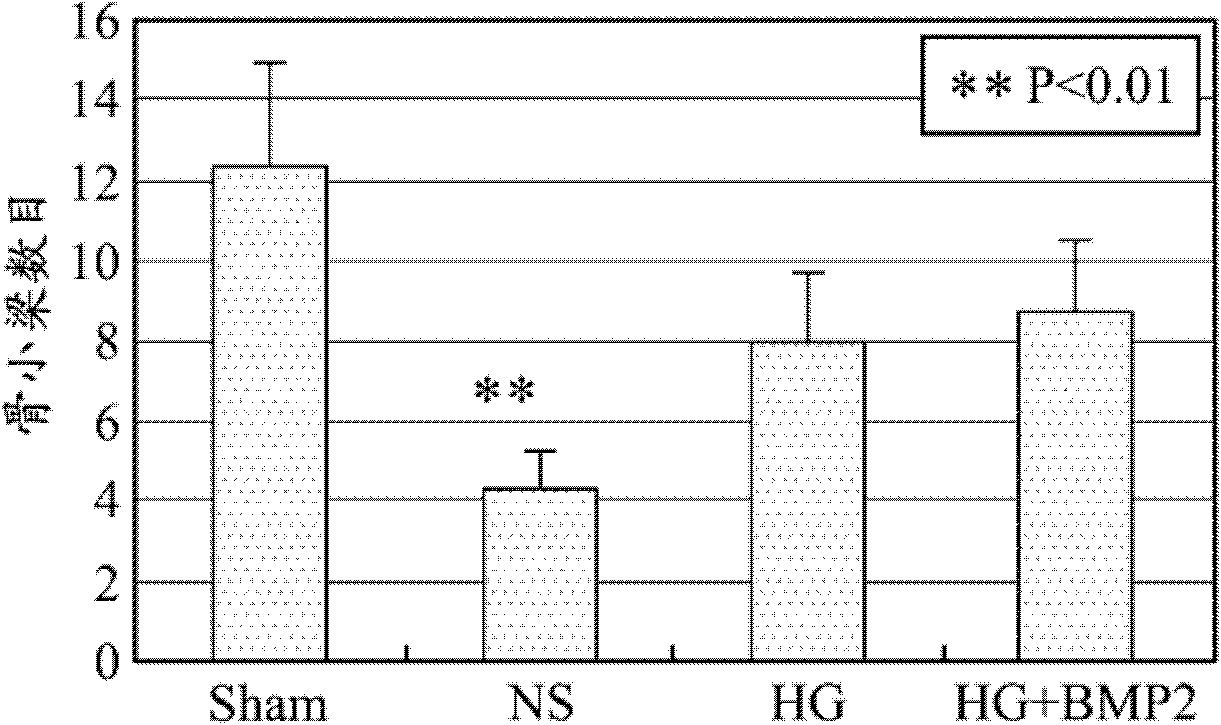
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
77 results about "Bone growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
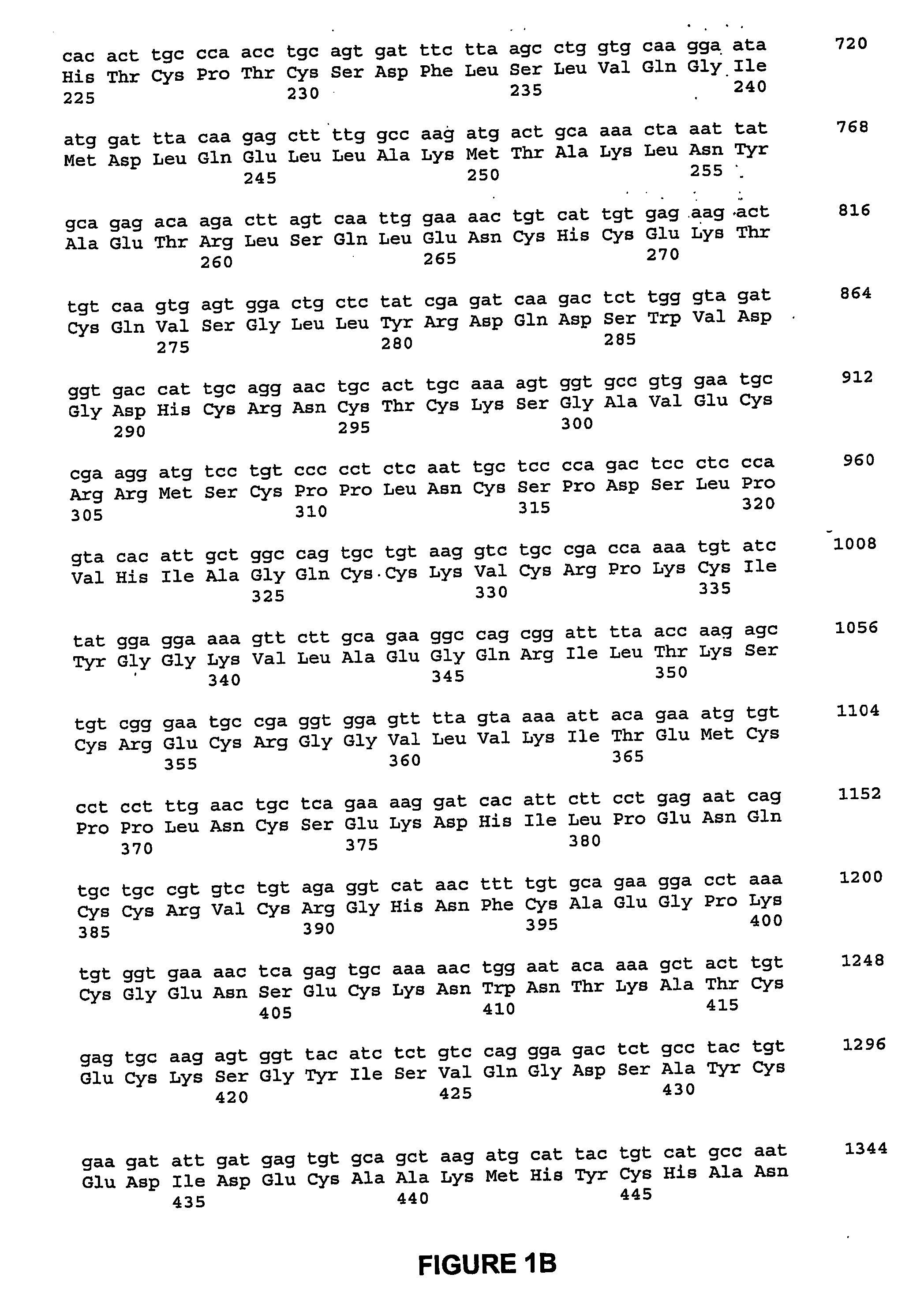
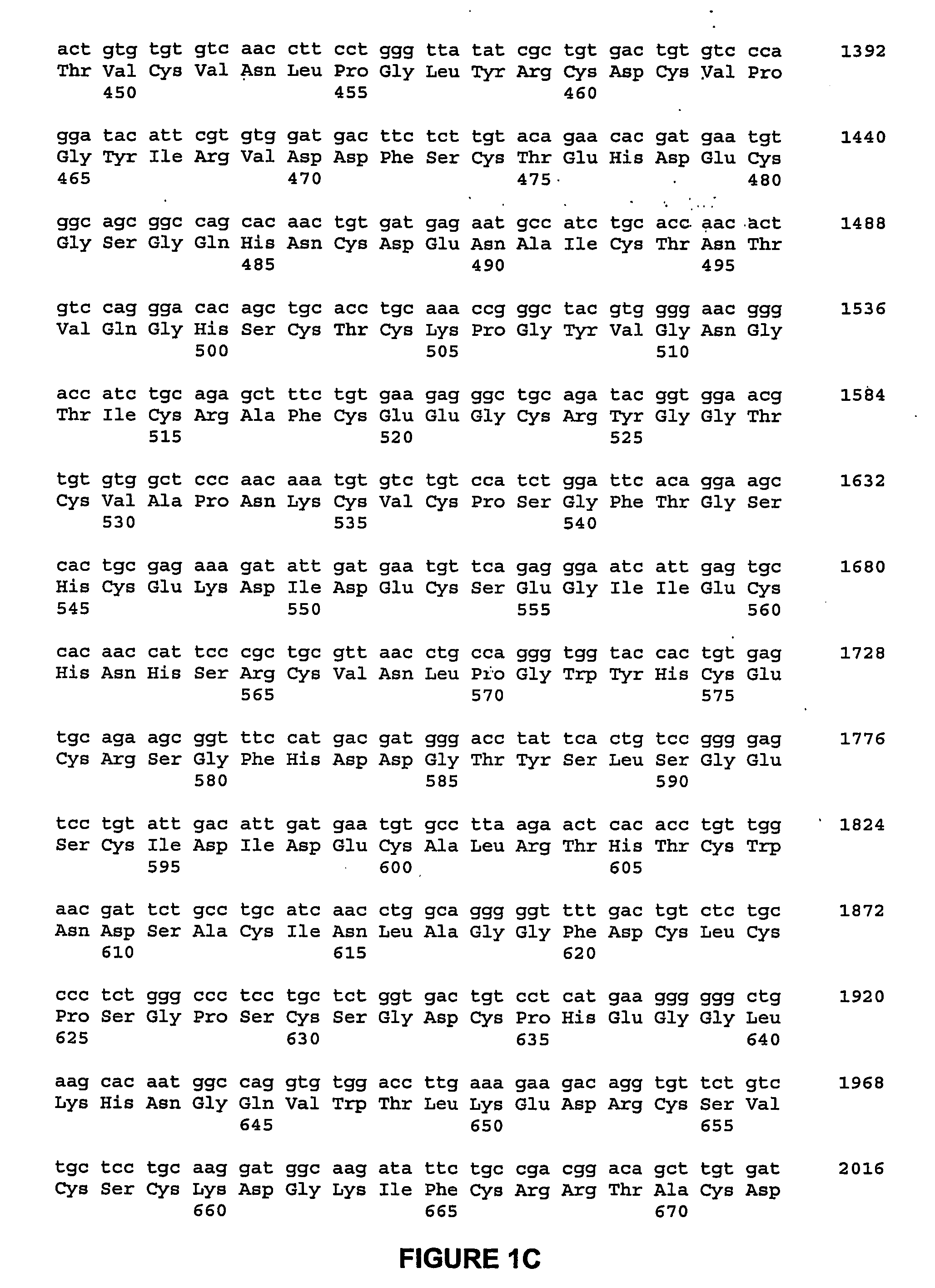
A Bone Growth Factor is a growth factor that stimulates the growth of bone tissue. Known bone growth factors include insulin-like growth factor-1 (IGF-1), insulin-like growth factor-2 (IGF-2), transforming growth factor beta (TGF-β), fibroblast growth factors (FGFs), platelet-derived growth factor (PDGF), parathyroid hormone-related peptide (PTHrP), bone morphogenetic proteins (BMPs), and certain members of the growth differentiation factor (GDF) group of proteins.
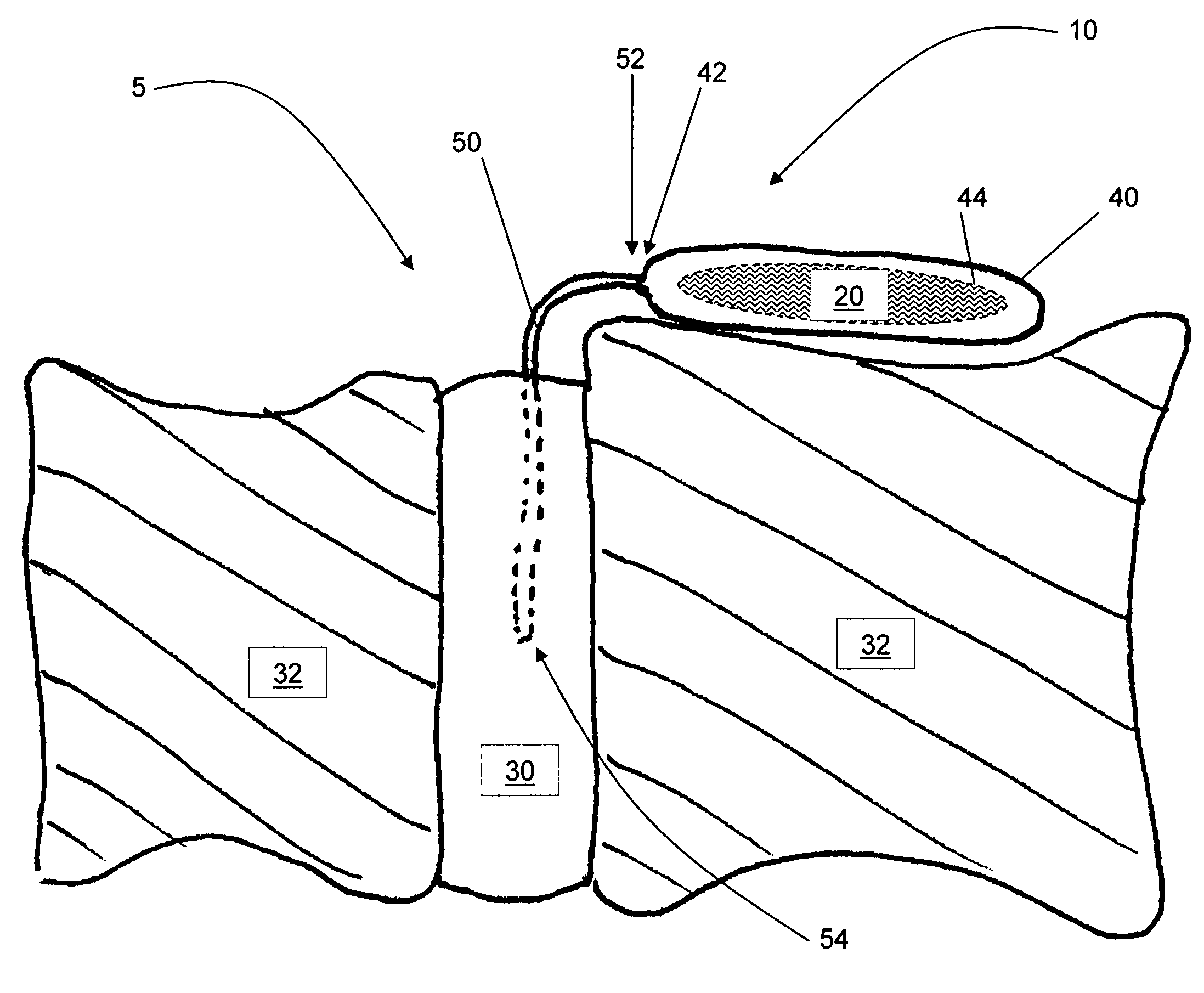
Composite metal and bone orthopedic fixation devices
ActiveUS9173692B1Promote healingImprove bio-integrationInternal osteosythesisBone implantFiberOrthopedic devices
Composite orthopedic devices that facilitate spine stabilization, such as: bone screws, rods, plates, interbodies, and corpectomy cages are disclosed. They are designed to provide both strength and load carrying capabilities, while increasing bio-integration of the devices with the surrounding bone tissue. They are constructed of composite layers of allograft and / or autograft bone and a structural material, such as titanium alloy or carbon / graphite fiber composite. Cannulations within the device are loaded with a mixture of stem cells, particles of allograft and / or autograft bone, and bone growth factors, such as BMP-2. The cannulations are connected to the surface of the device via multiple fenestrations that provide pathways to supply the bone / stem cell mixture to the surface, allowing living bone tissue to grow and insure bio-integration. The devices can also have radiofrequency (RF) stimulation implantation within the structure of the implanted device, capable of responding to external RF stimulation of enhanced bone growth.
Owner:STC UNM
Multiplex composite bone tissue engineering bracket material capable of degrading gradiently and preparation method thereof
The invention discloses a multiplex composite engineering scaffold material capable of gradually decomposing bone tissue and a preparation method thereof. The composite scaffold material consists of calcium phosphate bone cement, biological compatible degradable synthetic high polymer and biological compatible degradable natural high polymer, has better mechanical property and gradient degradation characteristic, and can achieve the aim of regenerating and repairing bone tissue defect by implanting a bone growth factor to induce in-vivo stem cells to be differentiated into bone cells, thereby obviously improving initial strength and toughness of the scaffold material, and ensuring enough strength and toughness of the scaffold material during operating and implanting. After compounded with the high polymer material, the scaffold has excellent flexibility, so that the scaffold can be subjected to certain machining, such as cutting and the like.
Owner:SOUTH CHINA UNIV OF TECH
Nell peptide expression systems and bone formation activity of nell peptide
ActiveUS20060292670A1Facilitate protein traffickingFacilitate post production modificationOrganic active ingredientsBacteriaBone formationBone growth factor
The invention generally relates to a bone growth factor, and more particularly to compositions including NELL1, articles of manufacture including NELL1 and methods of using NELL1 to induce bone formation. This invention also provides methods for the expression and purification of NELL1 and NELL2 peptides.
Owner:RGT UNIV OF CALIFORNIA
Release of BMP, bioactive agents and/or cells via a pump into a carrier matrix
ActiveUS7723291B2Efficient conversionMore controlled flowOrganic active ingredientsPeptide/protein ingredientsActive agentBone growth factor
A pump to deliver bone-growth factors to a carrier matrix within a patient. Pump can be internal or external. With external pumps, additional amounts of the same growth factor may be added, or the bioactive agent may be changed during the course of treatment. An external pump permits the use of cells to promote bone growth. The pump can have several reservoirs and the pump can itself be received in the carrier matrix with an outlet tube or other structure to defuse the growth factors into the carrier matrix. The pump protocol can be used for slow-to-heal fractures, such as closed fractures, and can be used for slow-to-heal patients.
Owner:WARSAW ORTHOPEDIC INC
Nell peptide expression systems and bone formation activity of nell peptide
ActiveUS7544486B2Facilitate protein trafficking and post production modificationPromote secretionOrganic active ingredientsBacteriaBone formationBone growth factor
The invention generally relates to a bone growth factor, and more particularly to compositions including NELL1, articles of manufacture including NELL1 and methods of using NELL1 to induce bone formation. This invention also provides methods for the expression and purification of NELL1 and NELL2 peptides.
Owner:RGT UNIV OF CALIFORNIA
Absorbable active tissue matter for repairing hard tissue and its prepn
The present invention relates to surgical material. The material includes biologically absorbable polymer 10-90 wt%, un-sintered calcium phosphate salt 5-80 wt%, and bone growth factor less than 10 wt%. The biologically absorbable polymer includes polyester, chitosan, chondroitin sulfate, collagen, alginate, etc.; and the calcium phosphate salt is un-sintered hydroxyapatite or tricalcium phosphate of granularity less than 100 microns. The hard tissue repairing material is prepared through mixing biologically absorbably polymer, un-sintered calcium phosphate salt and bone growth factor dispersed in solvent, molding, freeze drying to eliminate solvent and obtain the composition. The composition has the function of promoting bond healing and excellent biocompatibility.
Owner:SOUTHEAST UNIV
Moldable biomaterial for bone regeneration
InactiveUS20090148487A1Negative influenceHigh porosityPowder deliveryPeptide/protein ingredientsParticulatesBone growth factor
The present invention is directed to a moldable biomaterial comprising a particulate solid porous material and a biodegradable paste material. The paste material and the particulate solid porous material form a matrix usable for the replacement or augmentation of bone. In various embodiments the matrix has a high structural integrity, which does not immediately or shortly after implantation collapse into an amorphous non-porous mass, maintains its porosity after implantation, shows biphasic degradation after implantation and / or has a good resistance against being washed out when it is applied to a wet opened implant site. Active agents can be incorporated in the moldable biomaterial of the present invention, such as bone growth factors. Kits, implants, method of manufacturing as well as medicinal uses are also provided.
Owner:SCIL TECH GMBH
Injectable active bone repair material
The invention relates to an injectable active bone repair material. The material has good liquidity, can reach a bone defect part through injection and quickly solidify the defect part, and can obviously promote the healing of bone trauma along with gradual release of rear bone growth factors so as to shorten the healing period. Main components of the injectable active bone repair material consist of multiple calcium salts such as phosphoric acid calcium salt, calcium sulfate, calcium carbonate and the like, stowage factor microspheres and solidifying conditioner. The injectable active bone repair material has good injectability and collapse resistance. The solidifying time is 15 to 30 minutes, the temperature rise is not over 45 DEG C during solidifying, and the material does not cause adverse effect on tissues of an implanted part. After solidifying for half an hour, the material reaches the strength of spongy bone. All the components of the material are medicinal raw materials approved by the United States Food and Drug Administration, and can meet the safety of clinical use.
Owner:SHENZHEN LANDO BIOMATERIALS
Novel orthopaedics medicaments carrier system and preparation thereof
ActiveCN101259277ATo achieve a sustained release effectPromote degradationInorganic non-active ingredientsPharmaceutical delivery mechanismDiseaseAntituberculosis drug
The invention discloses a degradable orthopedics drug carrier system with osteo inductivity and a preparation method thereof. The drug carrier system essentially consists of the carrier material which is biological activity glass setting solid and the carried therapeutic drug. And the carrier material is the solid body carrier material with certain strength which is obtained through the fast curing after the reaction of highly reactive boron-containing biological activity glass and cured buffer liquid after the reaction. The carrier material can carry various drugs without thermal stability restriction, such as antibiotics, bone growth factors, antituberculosis drugs, antineoplastic drugs, which are used for the treatment of orthopedics diseases, thus composing the integrated drug carrier system. The drug carrier system can degrade automatically under the function of human tissue fluid in a human body with a porous structure left during the degradation which provides diffusion channels for the sustainable releasing of the drugs, thus achieving the effect that the drugs are chronically and uniformly released. The drug carrier system has good biocompatibility, biological activity and osteo inductivity and can be applied to the treatment of various diseases of orthopedics.
Owner:深圳市中科海世御生物科技有限公司
A moldable biomaterial for bone regeneration
InactiveCN101330934AHas a moldable adhesive consistencyEasy to acceptTissue regenerationProsthesisActive agentBone growth factor
The present invention is directed to a moldable biomaterial comprising a particulate solid porous material and a biodegradable paste material. The paste material and the particulate solid porous material form a matrix usable for the replacement or augmentation of bone. In various embodiments the matrix has a high structural integrity, which does not immediately or shortly after implantation collapse into an amorphous non-porous mass, maintains its porosity after implantation, shows biphasic degradation after implantation and / or has a good resistance against being washed out when it is appliedto a wet opened implant site. Active agents can be incorporated in the moldable biomaterial of the present invention, such as bone growth factors. Kits, implants, method of manufacturing as well as medicinal uses are also provided.
Owner:SCIL TECH GMBH
Bone growth factor controlled release type bone repairing material as well as preparation method and applications thereof
ActiveCN102160900AMeet the requirements for defect repairMaintain biological activityProsthesisMass ratioBiocompatibility Testing
The invention discloses a bone growth factor controlled release type bone repairing material as well as a preparation method and applications thereof. The bone repairing material provided by the invention is characterized in that the pore surface of a porous bone repairing inorganic material is coated with two layers of membranes from inside to outside, wherein the first-layer membrane is mainly composed of a bone growth factor and chitosan; and the second-layer membrane is formed by mixing a bone growth factor with biodegradability poly anions at a mass ratio of (0-0.001):1. The preparation method provided by the invention comprises the following steps: soaking the porous bone repairing inorganic material into a bone growth factor / chitosan solution, and airing; and soaking in a bone growth factor / biodegradability poly anion aqueous solution, and airing to obtain the bone growth factor controlled release type bone repairing material. The preparation method provided by the invention is simple; and the obtained bone repairing material has good mechanical property and biocompatibility, and ensures that the continuous slow united release or sequential release of various bone growth factors can be realized, and the repairing effect of a bone defect tissue is improved, thereby having a good clinical application prospect in bone repairing.
Owner:广州暨南大学科技园管理有限公司 +1
Bone growth factor injection and its preparation method
InactiveCN1443571AAvoid heterotopic ossificationGood compatibilityPeptide/protein ingredientsAerosol deliveryFracture zoneFreeze-drying
The present invention discloses a bone growth factor injection and its preparation method. It includes the following steps: firstly, preparing bone growth factor into solution or mixed suspension; then preparing heat-sensitive hydrogel carrier solution; combining above-mentioned two solutions and making them into injection liquor or freeze-dried product. After the injection is injected into the fracture zone, the coagulated gel can be quickly formed, the bone growth factor and gel composite can be retained in bone repairing zone so as to fully produce slowly-releasing effect-increasing and effect-prolonging action and can raise biological utilization rate of the bone growth factor can raise its bone-forming action.
Owner:李亚非
Preparation method of novel injectable polypeptide hydrogel
InactiveCN102100925AGood compatibilityInduced growthSkeletal/connective tissue cellsProsthesisOsteoblastBone defect
The invention discloses a preparation method of a novel injectable polypeptide hydrogel. A polypeptide hydrogel bionic scaffold with osteogenic activity is formed by a polypeptide solution with a specific sequence under a condition with a temperature of 37 DEG C and a pH of 7 based on the self-assembly principle. The method has a simple preparation process; the hydrogel has simple and controllable components and high safety, is injectable before complete self-assembly, can perform in-situ self assembly at gaps with any form between bone defects; the hydrogel can be used as a bionic scaffold material to promote bone repair, and after bone growth factors are added, the hydrogel can be used as a carrier to control the release of the growth factors so as to shorten the treatment course. A culture medium can be added into the system as required, and the system can be used as a bionic scaffold for the three-dimensional culture of osteoblasts.
Owner:THE STOMATOLOGIAL HOSPITAL OF ZHEJIANG UNIV SCHOOL OF MEDICINE
Release of BMP, bioactive agents and/or cells via a pump into a carrier matrix
ActiveUS20080025987A1Efficient conversionMore controlled flowOrganic active ingredientsPeptide/protein ingredientsActive agentMedicine
A pump to deliver bone-growth factors to a carrier matrix within a patient. Pump can be internal or external. With external pumps, additional amounts of the same growth factor may be added, or the bioactive agent may be changed during the course of treatment. An external pump permits the use of cells to promote bone growth. The pump can have several reservoirs and the pump can itself be received in the carrier matrix with an outlet tube or other structure to defuse the growth factors into the carrier matrix. The pump protocol can be used for slow-to-heal fractures, such as closed fractures, and can be used for slow-to-heal patients.
Owner:WARSAW ORTHOPEDIC INC
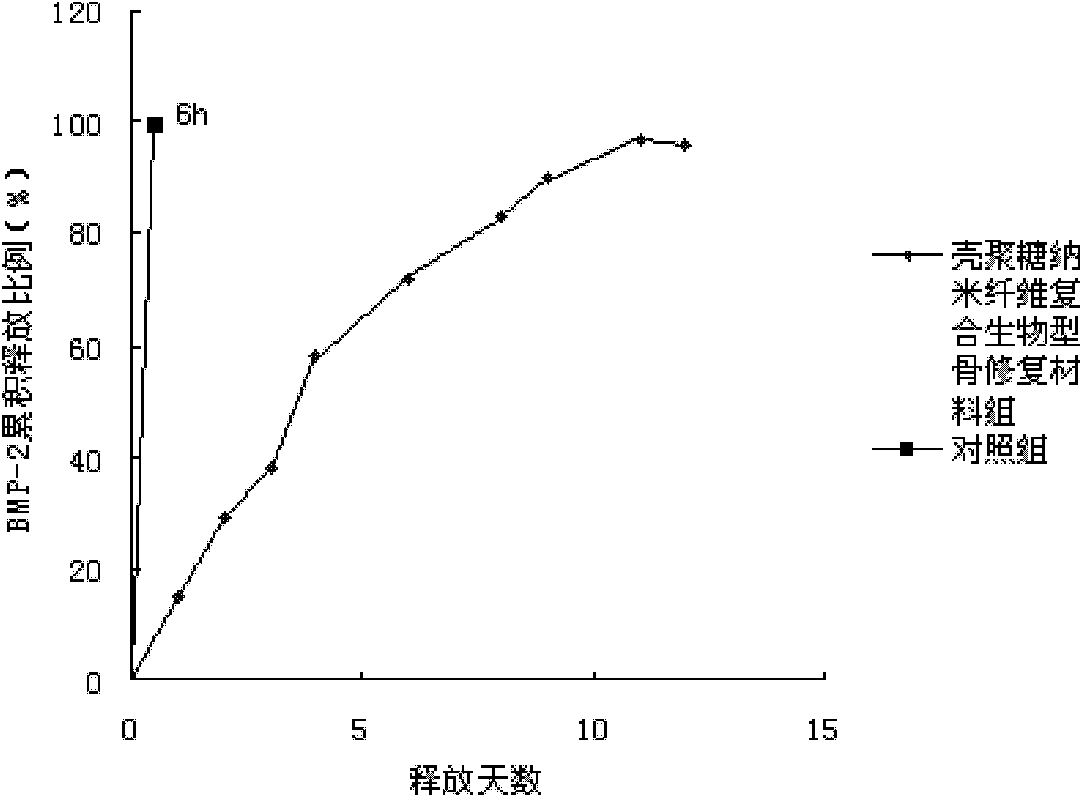
Growth-factor-containing nanofibre porous composite material capable of repairing bone and preparation method thereof
InactiveCN102139125ADoes not destroy biological activityThe preparation method is simple and environmentally friendlyProsthesisFiberCell-Extracellular Matrix
The invention discloses a growth-factor-containing nanofibre porous composite material capable of repairing a bone and a preparation method thereof. The preparation method comprises the following steps of: dissolving chitosan in acetic acid; adding bone growth factors into the solution, and uniformly mixing the solution; immersing a porous inorganic material capable of repairing the bone in the solution under a negative pressure for 1-2 hours, and taking out the material; and freezing the porous material capable of repairing the bone at a low temperature, lyophilizing the material, immersing the material in a stabilizer solution to stabilize the material, washing the material with water, lyophilizing the material again, and sterilizing the material to prepare the growth-factor-containing nanofibre porous composite material capable of repairing the bone. In the invention, the growth-factor-containing nanofibre porous composite material capable of repairing the bone simulates the nanofibre structure of an extracellular matrix of the bone; the bioactivity of the bone growth factors can be kept, and the bone growth factors can be released continuously and slowly; the growth-factor-containing nanofibre porous composite material is beneficial for the regeneration and functional reconstruction of bone tissues; and the defect that traditional slow-release systems of the bone growth factors cannot better simulate a physiological microenvironment required for the regeneration of the bone tissues is overcome.
Owner:JINAN UNIVERSITY
Thermal responsive composition for treating bone diseases
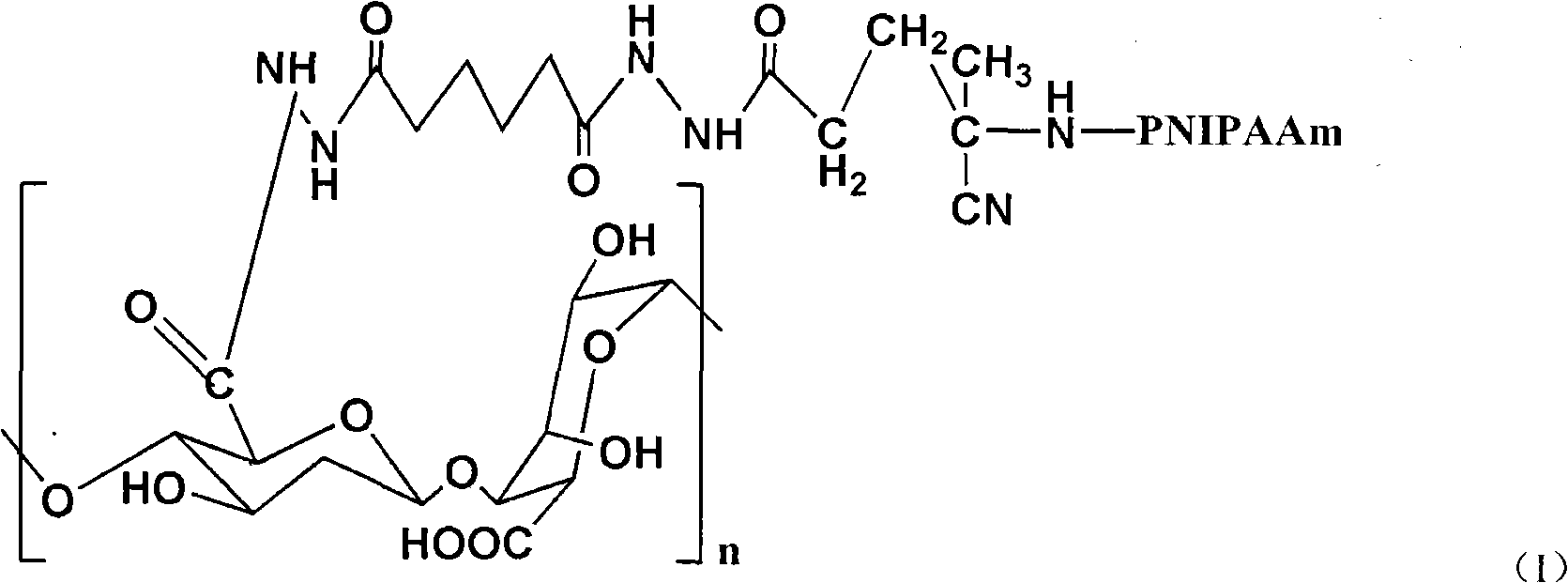
Thermal responsive compositions for treating bone diseases are provided. The thermal responsive composition for treating bone diseases includes a bone growth factor and a biodegradable copolymer, wherein the biodegradable copolymer is provided with an A high polymer and a B high polymer, and satisfies Formula (I) or Formula (II): A-B-BOX-B-A (Formula I) and B-A-B-(BOX-B-A-B)n-BOX-B-A-B Formula (II), wherein, A includes a hydrophilic polyethylene glycol polymer, B includes a hydrophobic polyester polymer, BOX is a bifunctional group monomer of 2,2'-Bis(2-oxazoline) and used for coupling the blocks A-B or B-A-B, and n is an integer and the same or more than 0.
Owner:IND TECH RES INST
High-calcium, low-fat, sucrose-free and solidification yoghurt and production method thereof
The invention relates to a high-calcium, low-fat, sucrose-free and solidification yoghurt and a production method thereof, and belongs to the technology field of dairy food. The production method comprises following steps of purifying milk, preparing materials, homogenizing, sterilizing, inoculating, filling, and fermenting to obtain the target product. The calcium content of the products is larger than 148 mg for each 100 gram of the product, the fat content is in a range of 0.8 to 2.0%, and the product is suitable for middle-aged and elderly people with hyperlipidemia or diabetes and improves the health state of the bones of the middle-aged and elderly people. The product comprises an absorption enhancer, which is composed of vitamin D3, bonepep and casein phosphopeptides, wherein the vitamin D3 can promote the absorption of calcium in small intestines; the bonepep can promote the generation of bone growth factors, inhibits the secretion of splitting factor of osteoclast, and promotes the health of the bones by promoting the bone growth and inhibiting the calcium loss; the casein phosphopeptides can weaken the actions of osteoclast, prevents the osteoclast from combining with the calcium, and inhibits the production of insoluble precipitate; and thus the calcium loss is avoided.
Owner:SHANDONG DEYI DAIRY IND
The controlled releasing method by using active pearl material instead of bone growth factors in the bone restoration
The invention belongs to the biomedical materials technology, especially relates to a controlled release method to replace bone growth factor with pearl active substances in bone repair. By means of the pure pearl active substance extraction contained within bone material and different dissolution rate with different grain size and content of pearl powder in the body, the material degradation in different stages in bone repair: at the early stage, rapidly activate the bone system as a start-up factor and accelerator in bone repair; subsequently, the pearl powder start to dissolve, but small particles pearl powder dissolve faster, consumed earlier, and the large particles of pearl powder dissolve slowly, consumed after that, in this way, pearl powder with different granularities degrade at different stages in bone repair, thus the pearl active substances can be continuous released in bone repair process, and can be control to release through active substance of pearl extraction and regulation of content and particle size of pearl powder. The screened and packed pearl powder and its combination are of simple production technology, easy to enlarge scale by the invention.
Owner:TSINGHUA UNIV +1
Human skull repairing scaffold and preparation method thereof
ActiveCN102302803APromote growthGood mechanical propertiesSpecial data processing applicationsProsthesisHuman bodySkull Injuries
The invention provides a human skull repairing scaffold which comprises a skull plate formed by a biodegradable polymer and bone growth factors adhered to the surface of the skull plate. The invention also provides a preparation method of the human skull repairing scaffold. After the human skull repairing scaffold provided by the invention is implanted into a human body, the self skull of a patient grows along the surface of the skull plate under the promotion of the bone growth factors, so that the skull injury part heals; because the self skull grows along the surface of the skull plate, the shape of the grown skull coincides with the shape of the original skull, so that malformation and other phenomena are not generated; and in addition, because the skull plate is formed by the biodegradable polymer, after the self skull grows to obtain a complete skull, the skull plate is slowly degraded and discharged out through human body absorption, metabolism and other means, so that the repaired skull injury part is completely formed by the growth of the self skull, no any other foreign bodies are left in the human body, and no any adverse effects are caused to the patient.
Owner:周强 +2
Injectable bone growth factor-carrying microspheres and preparation method thereof
InactiveCN101816780AEasy to prepareImprove stabilityPeptide/protein ingredientsSkeletal disorderInjectable boneMicrosphere
The invention discloses microspheres which are used for curing osteoporosis and have a bone growth factor slow release function and injectablity, and a preparation method thereof. The bone growth factor-carrying microspheres are prepared by adopting a solution emulation-solvent volatilization method, and taking biodegradable polylactic acid or polyglycolic acid or poly lactic-co-glycolic acid as a starting material of the microspheres, wherein embedded bone growth factors are BMP-2 or BMP-7 or BMP-1; the average grain diameters of the prepared slow release microspheres are between 1.1 and 20.4 microns; and the release of the bone growth factors has a controllable slow release characteristic, and the release period thereof is 20 to 45 days.
Owner:SHENZHEN LANDO BIOMATERIALS
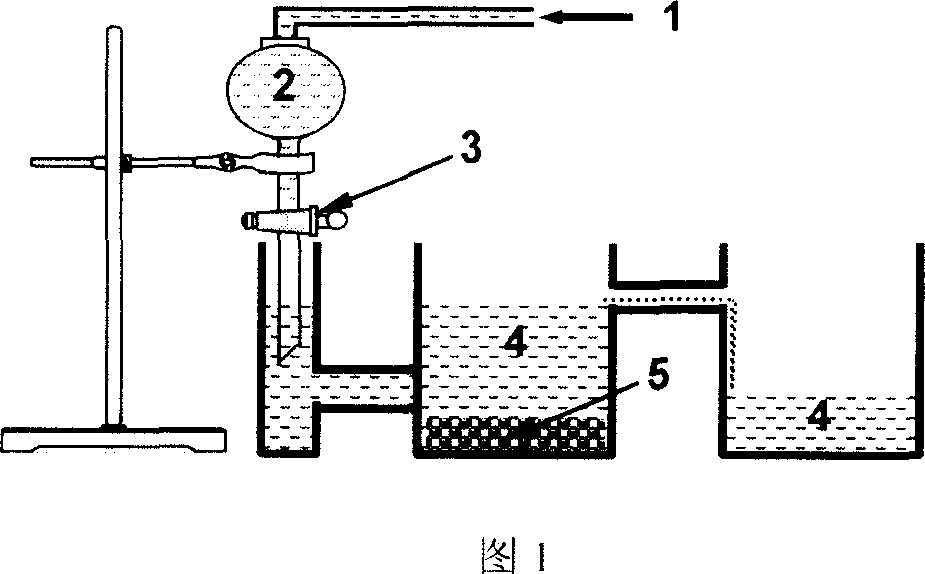
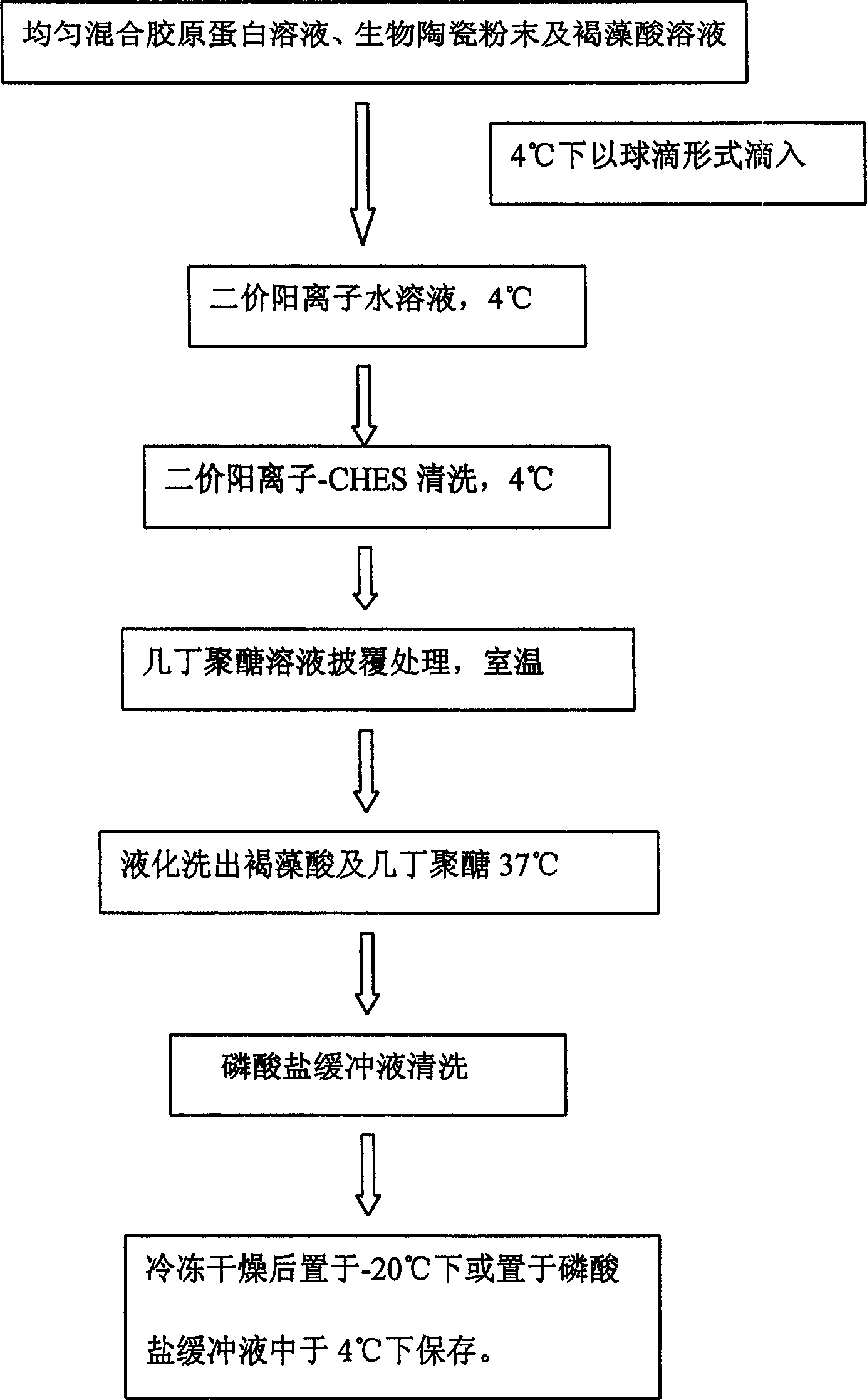
Method for preparing collagen and biological ceramic powder composite material microparticles
The present invention provides a method for preparing collagen and bioceramic powder composite material microparticles, said method includes the following steps: mixing collagen solution, bioceramic powder and alginic acid, adopting dropping mode to make the above-mentioned mixture drop into the bivalent cationic aqueous solution to peptize and form microparticle form, using chitosan solution to make treatment, liquifying to wash out internal alginic acid and chitosan from surface, at the same time making collagen implement recombination. Said invented microparticle composite material possesses the component similar to bont tissue and collagen fiber recticular formation, can provide cell growth environment similar in bone tissue, can be used as carrier to carry cell and cover and fix various bone growth factors, can be used for repairing bont tissue wound.
Owner:洪尧顺
Bone mineralization protein expression systems, and methods of studying intracellular signaling pathways induced thereby
The present invention is directed to methods of switching a differentiation of a cell from a non-osteogenic lineage into an osteogenic lineage. The present invention is also directed to methods of generating a model system for assessing the intracellular signaling pathways of bone growth factors.
Owner:EMORY UNIVERSITY
Formulations of peptides for periodontal and dental treatments
InactiveUS20060193916A1Reduce inflammationAvoid damageBiocideOrganic active ingredientsTooth fillingIrritation
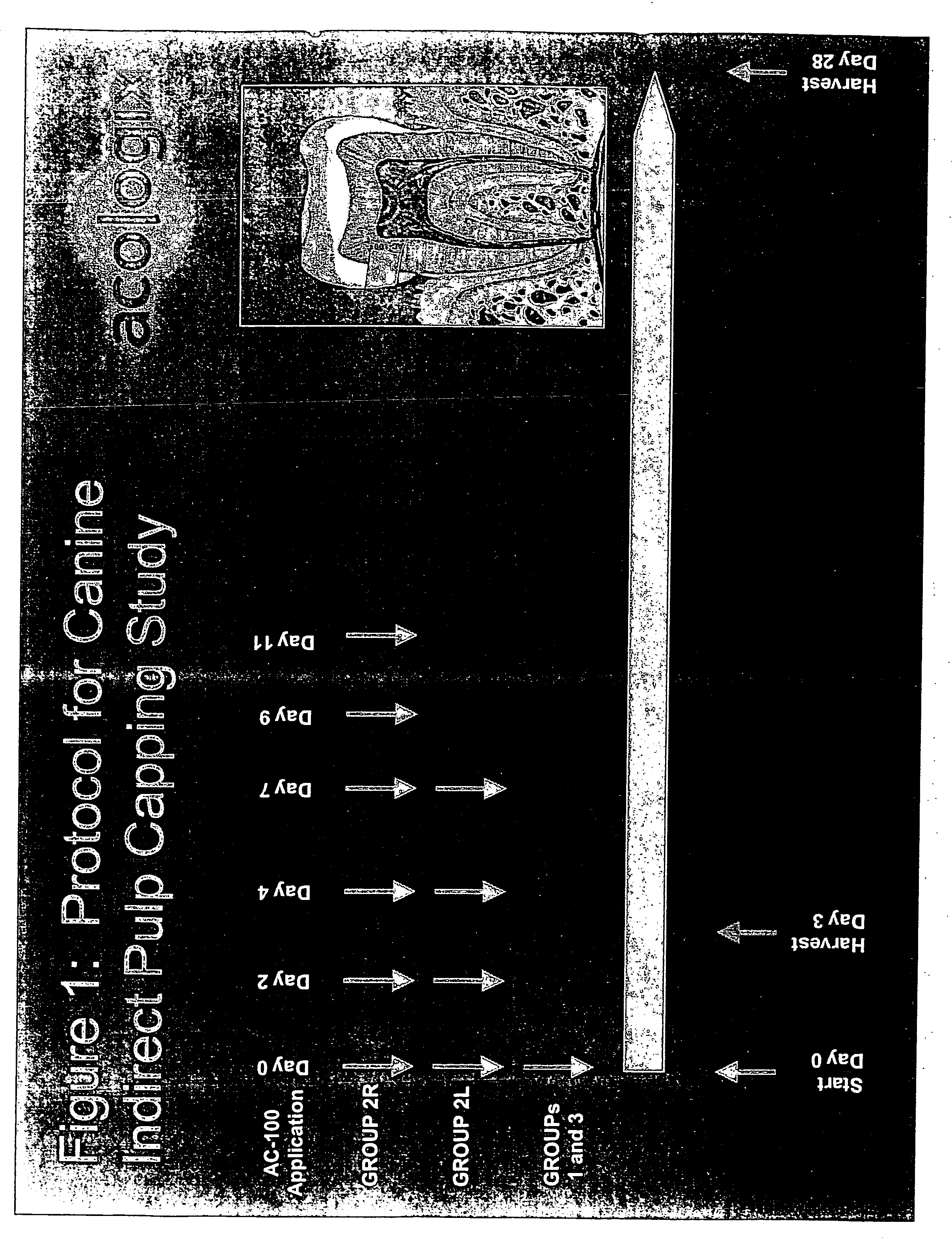
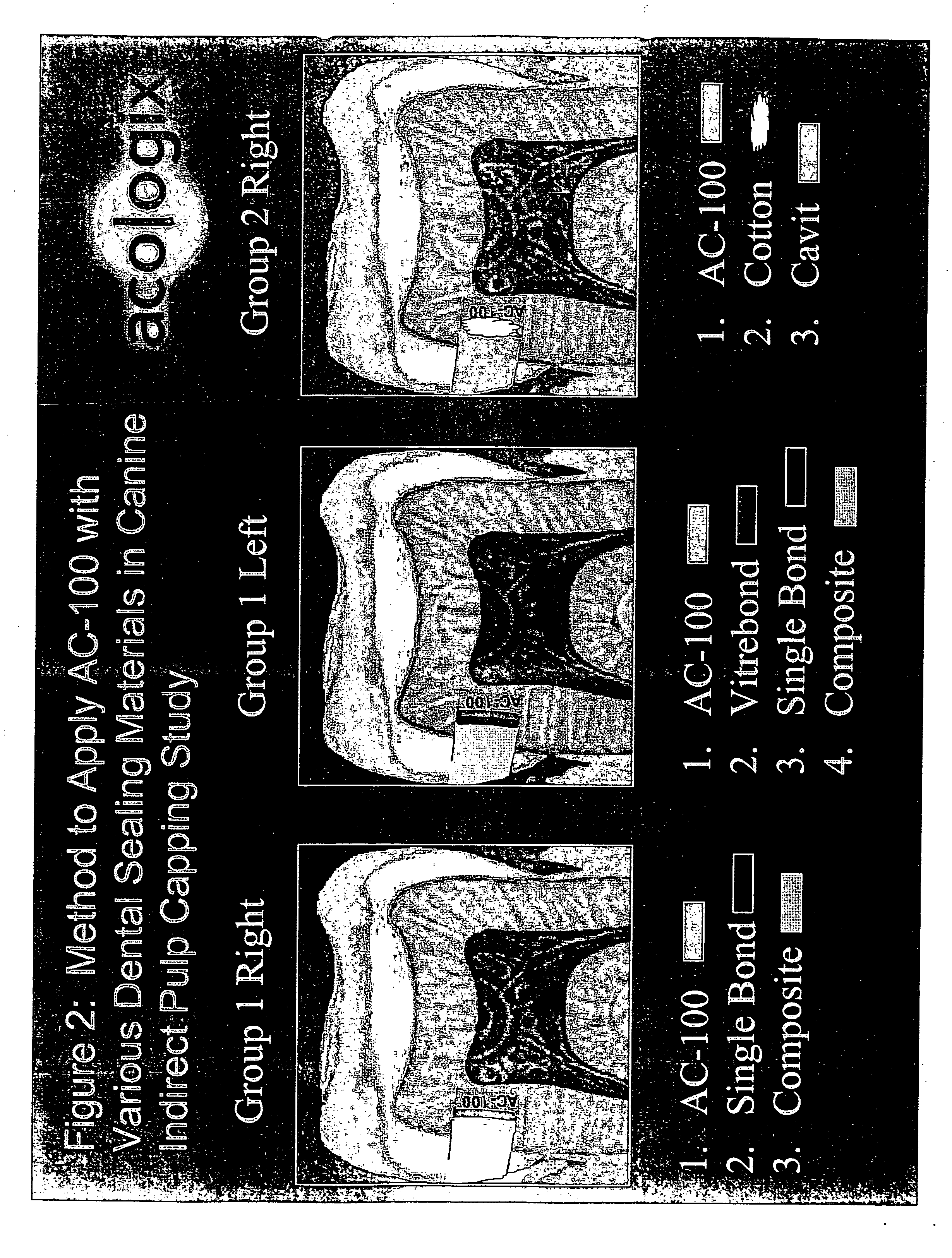
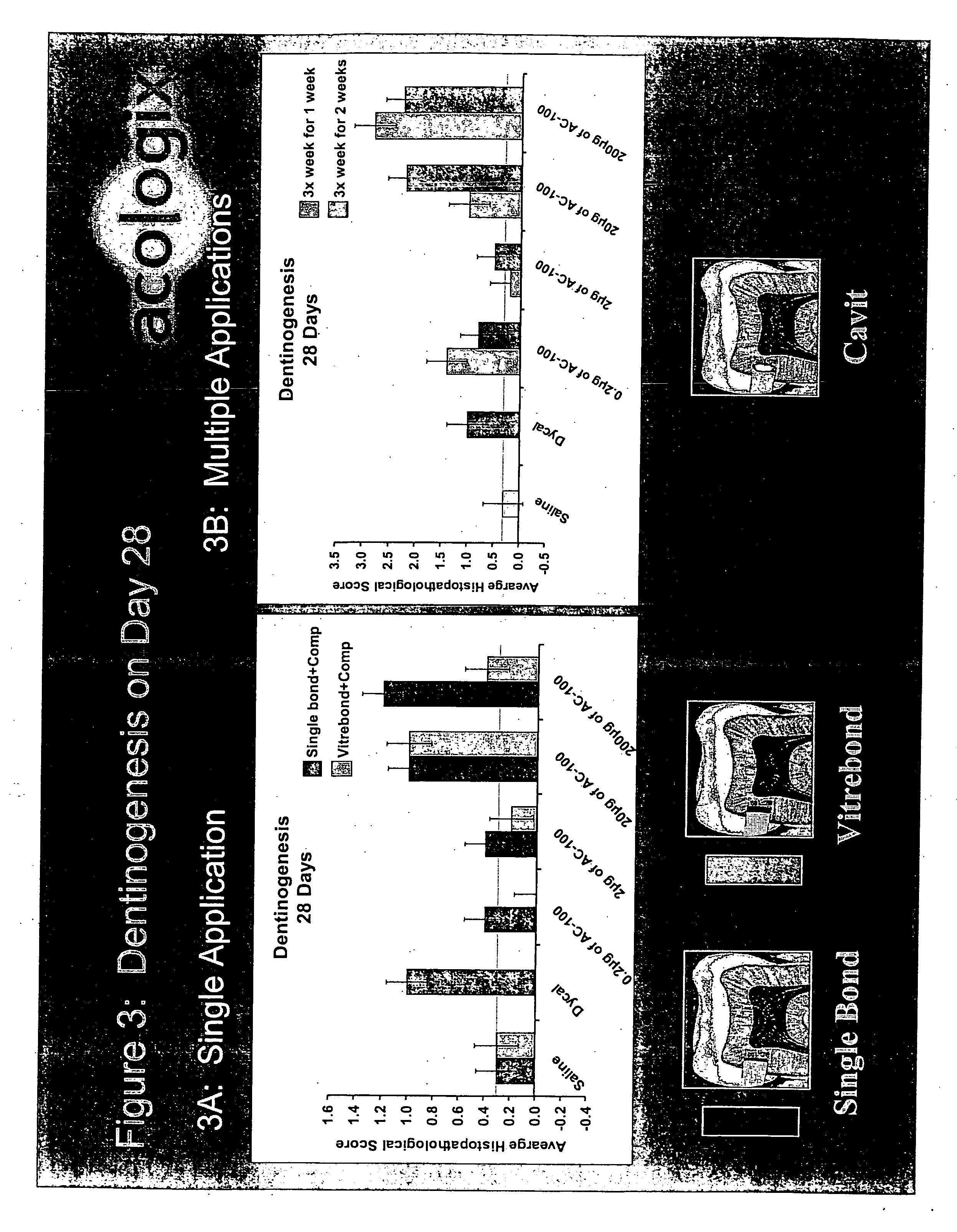
Methods to treat the defects in skeletal tissues characterized by protecting the marrow cells adjacent to the defects from apoptotic and / or necrotic cell death are disclosed. The methods provide additional benefits which are to reduce inflammation and irritation in the marrow tissues and assist promoting new skeletal tissue formation to an application of a simple skeletal formation or regeneration activity such as a bone growth factor. The methods may involve application of pharmacologically active peptidic compounds comprising about 15 to about 28 amino acids in their sequences characterized by containing at least one of an integrin binding motif such as an RGD sequence, a glycosaminoglycan attachment motif, and / or a calcium binding motif. The sequence may be a 23 amino acid sequence formulated for injection, topical application, or dispersed in a matrix such as collagen or a tooth filling composition or gum patch and administered to enhance bone / tooth growth or prevent loss.
Owner:ACOLOGIX
Pericardial collagen composite material and preparation method and application thereof
ActiveCN106890362ARemove completelyWeakened immunityTissue regenerationProsthesisWater soluble polysaccharidesWater immersion
The invention relates to a pericardial collagen composite material and a preparation method and application thereof. The pericardial collagen composite material contains at least one of chitosan, hyaluronic acid, chondroitin sulfate and other water-soluble polysaccharide or hydroxyapatites, BMP, PDGF and other bone replacement materials and bone growth factors. The preparation method of the pericardial collagen composite material comprises the steps that a pigpericardium is taken to remove chunk fat, and then water immersion, degreasing, alkali soaking, dealkalization, enzymolysis, compounding, crosslinking and disinfection. The pericardial collagen composite material can be used as a tooth implantation guided bone regeneration barrier isolating membrane, an oral cavity restorationmembrane and a bone repair material. The pericardial collagen composite materialhas good biocompatibility and compactness, high mechanical strength and excellent controllable degradation time.
Owner:SHANDONG UNIV
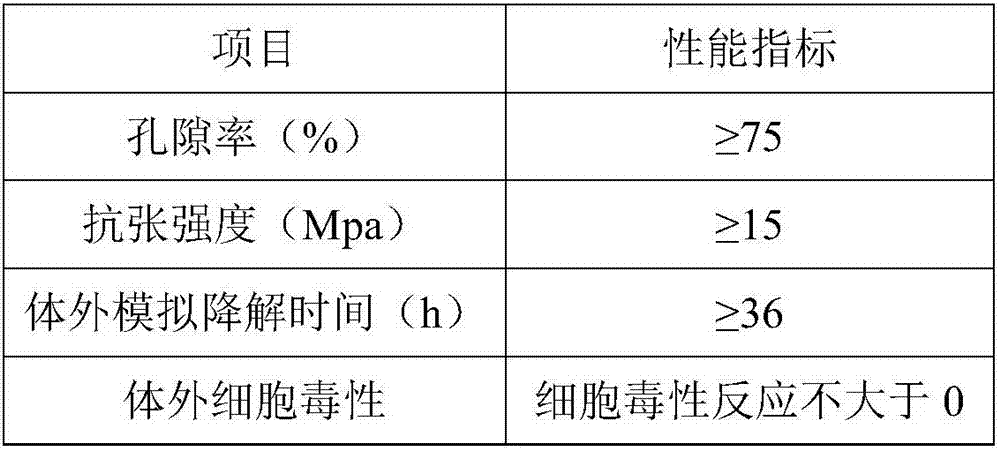
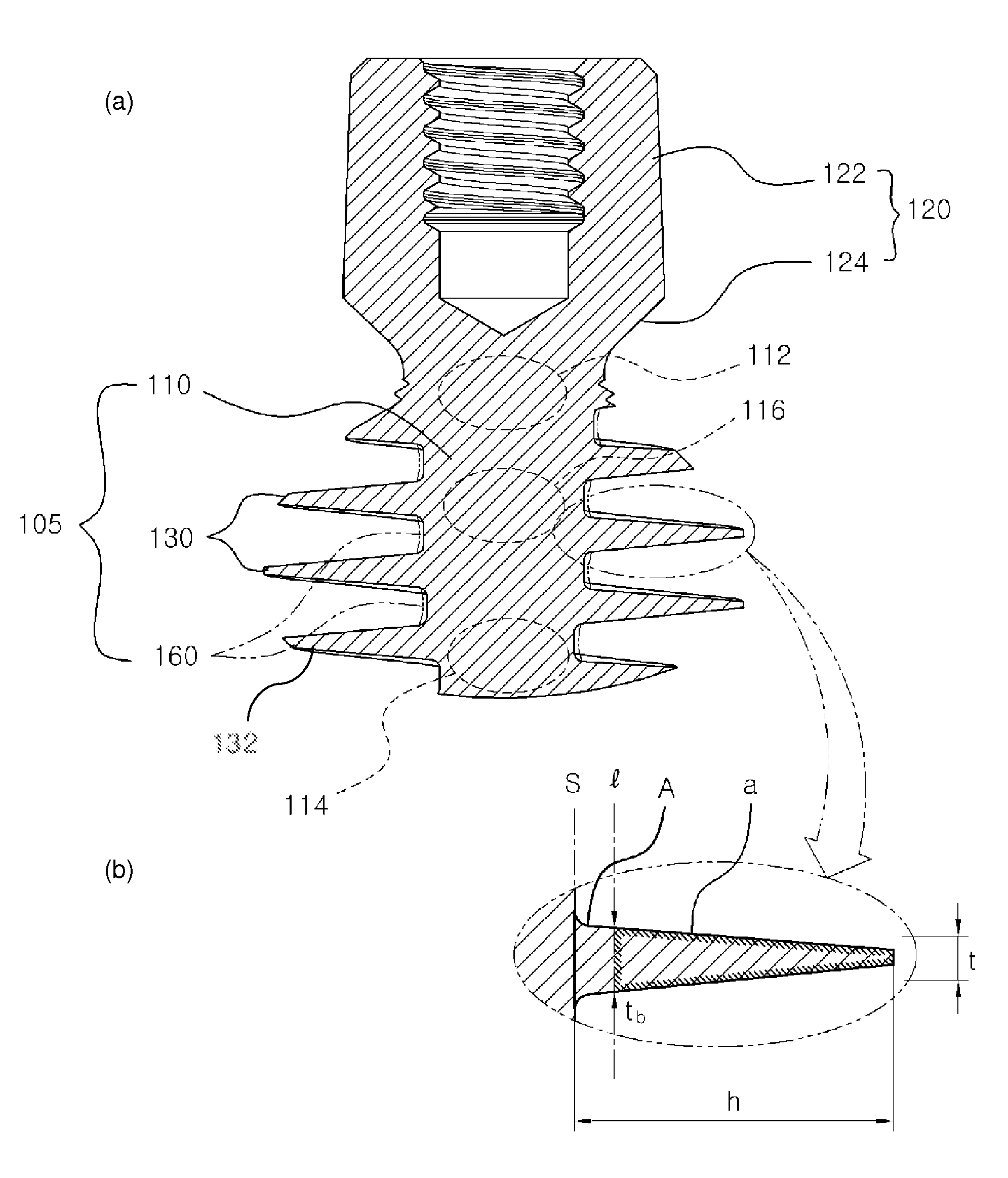
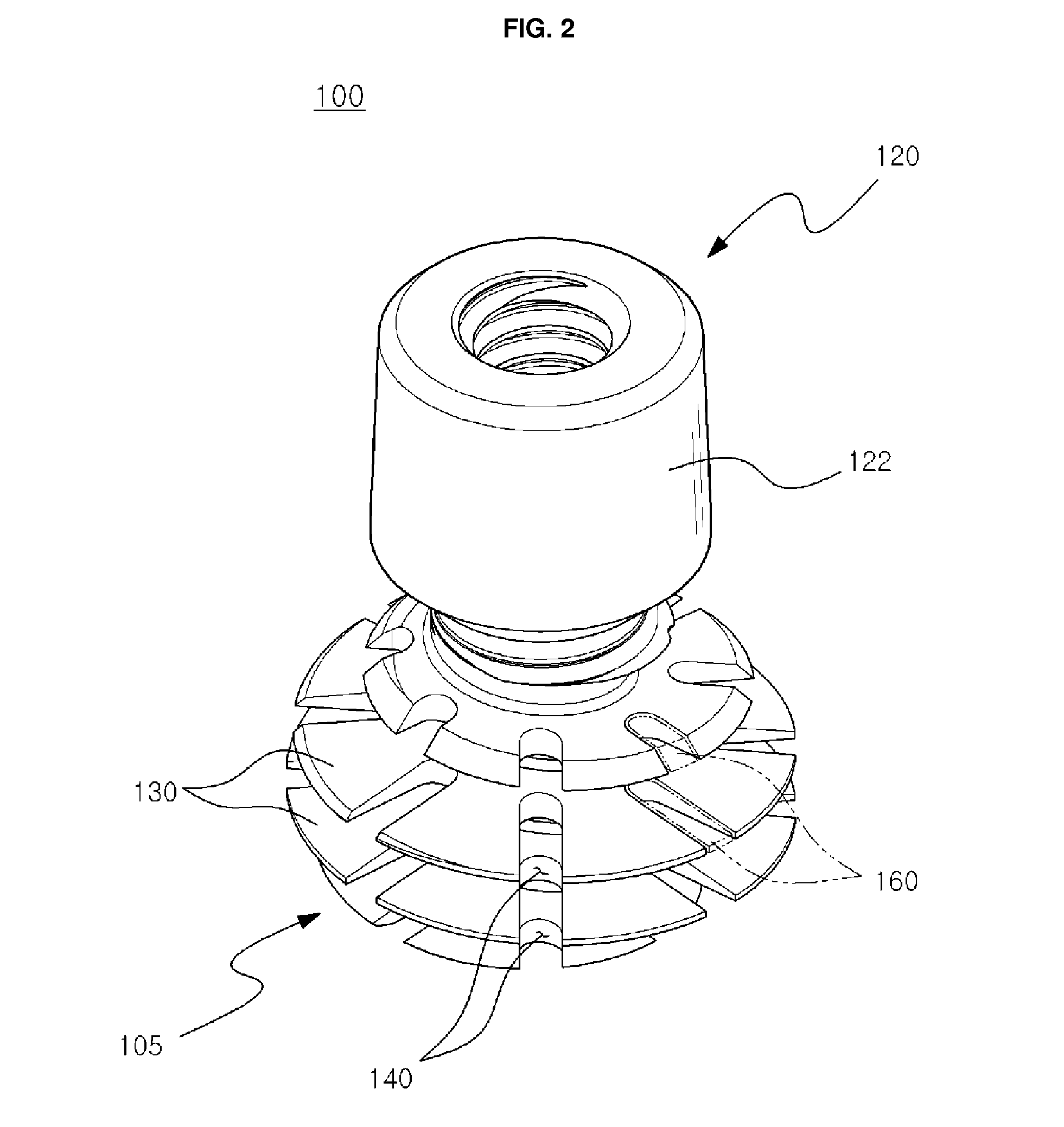
Dental implant
ActiveUS8523568B2Successfully implantedSufficient bearing capacityDental implantsBone structureHelical blade
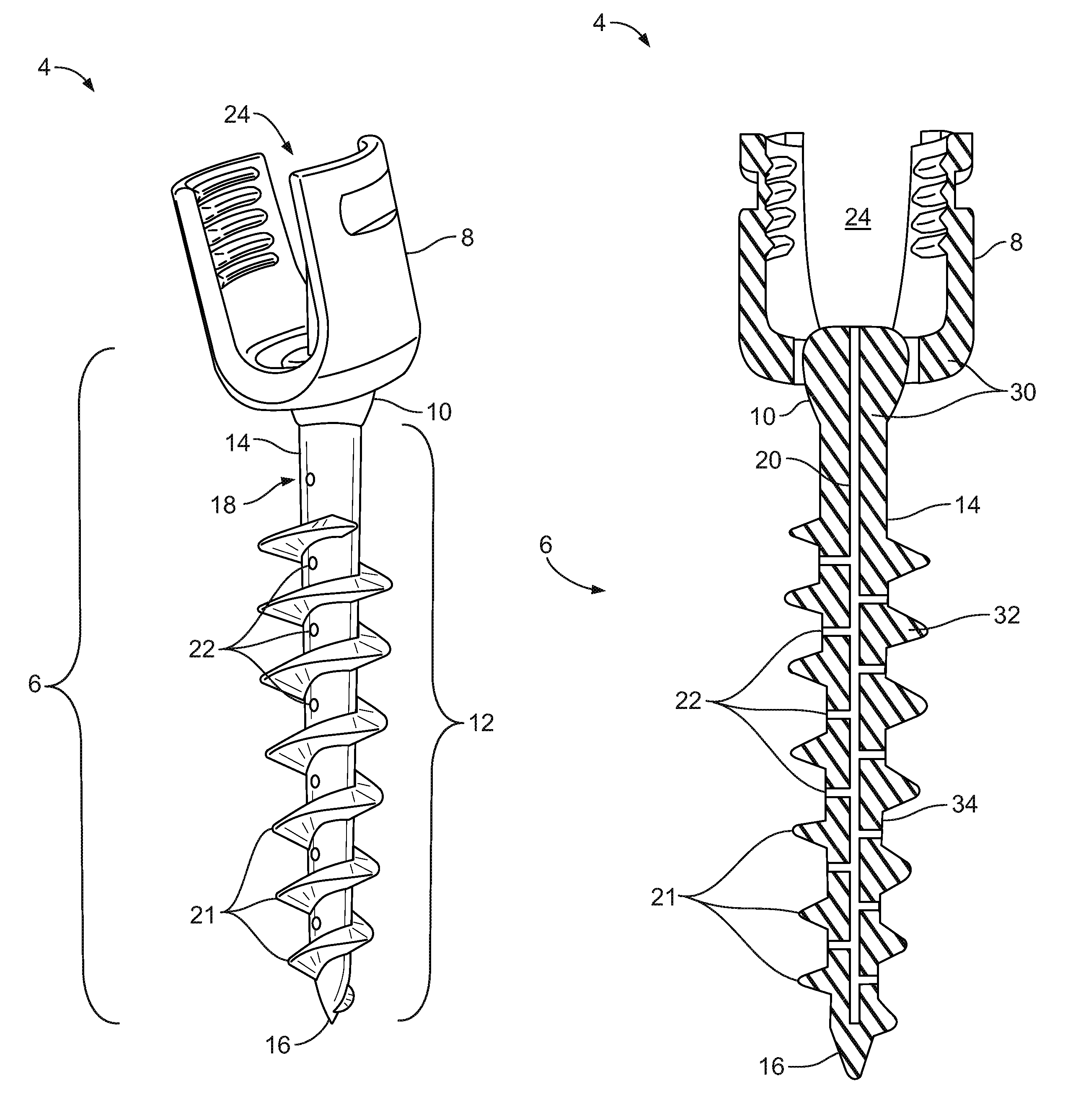
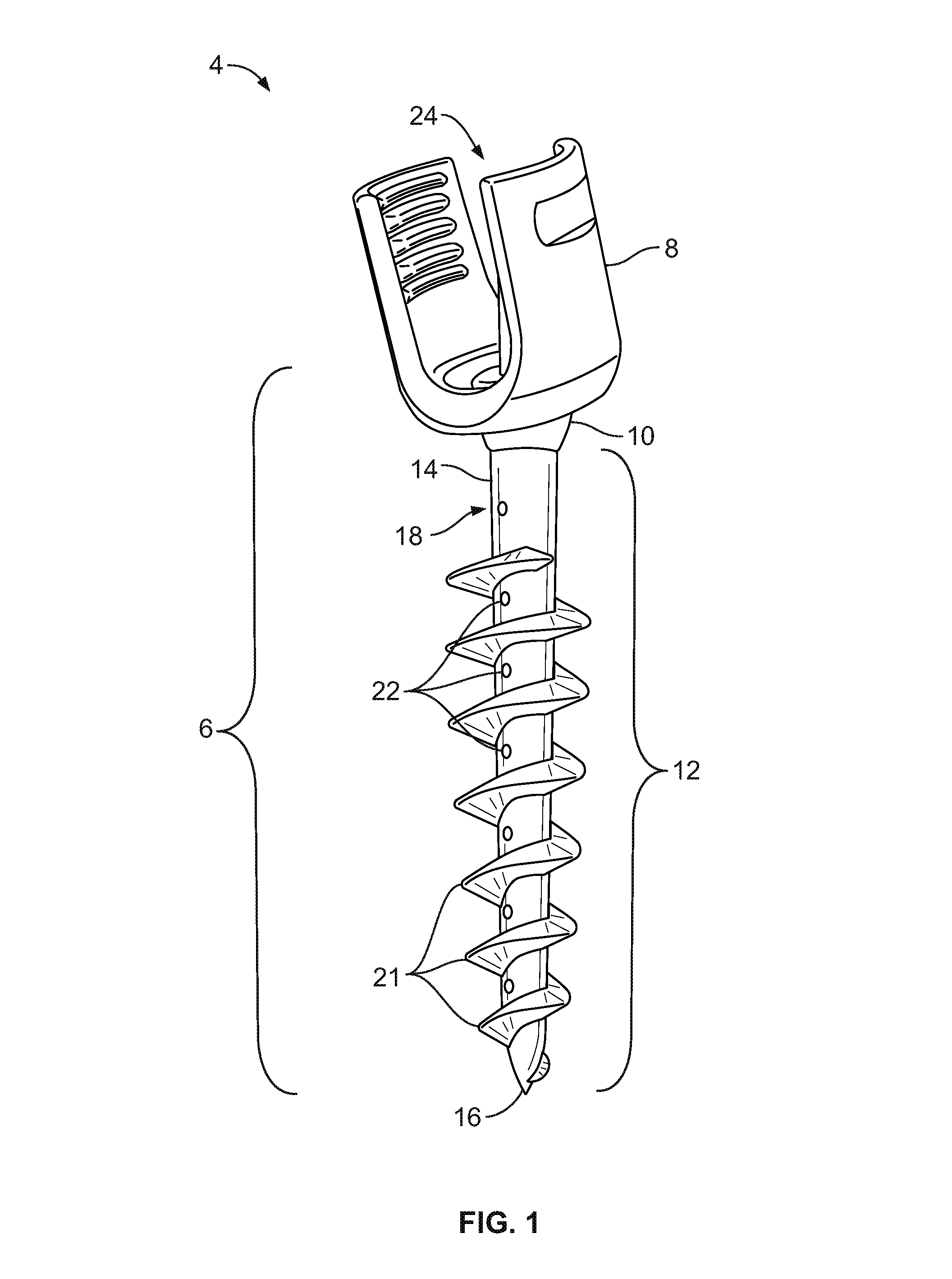
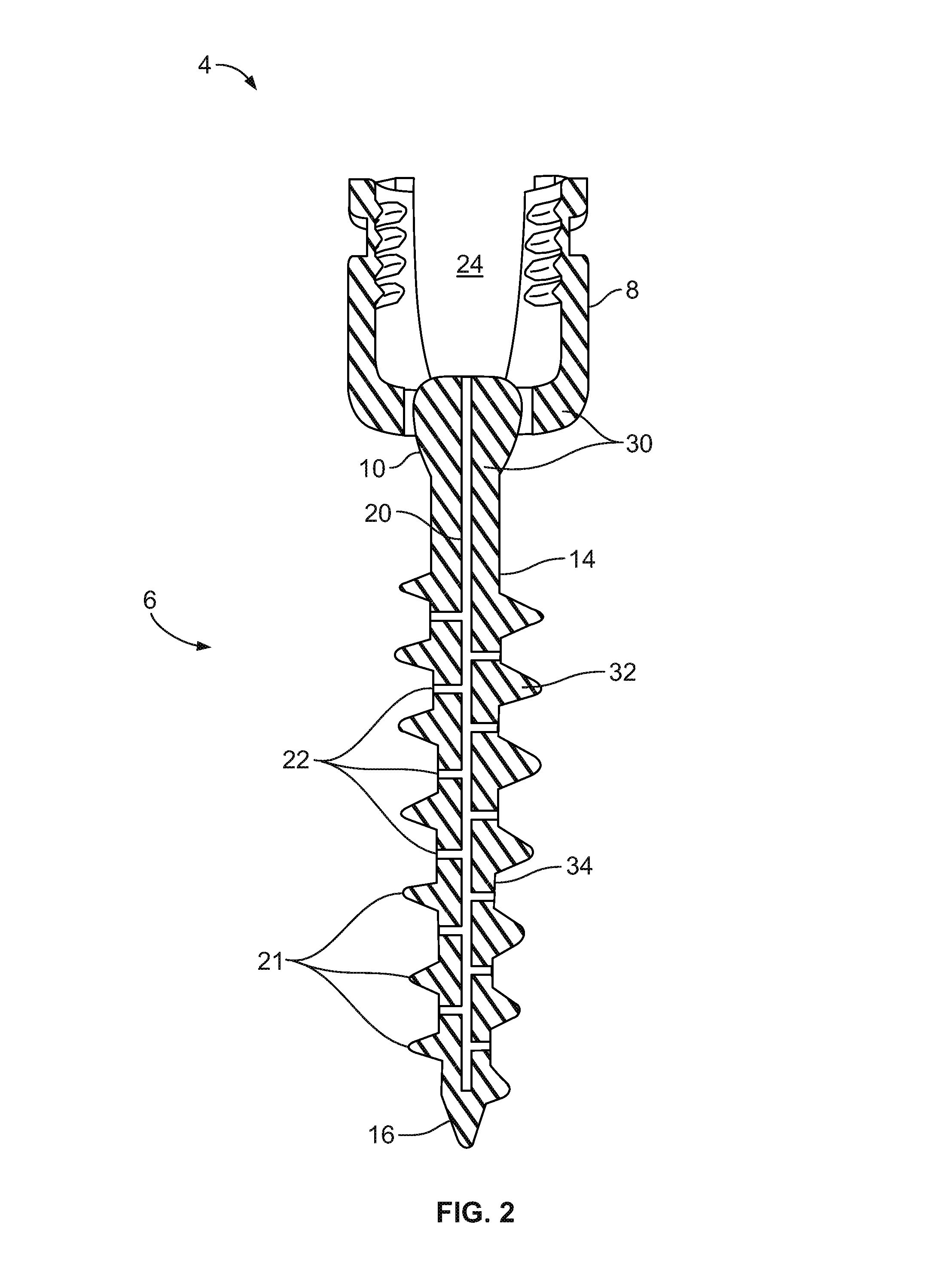
A dental implant of the invention allows an immediate masticatory function just after being implanted in a bone structure, does not need a secondary operation, and can be effectively applied even in thin and short alveolar bone. The dental implant comprises a body portion to be inserted in the bone structure and a mounting portion integrally formed on the body portion, the body portion including a thin and short core, screw blades formed along an outer peripheral surface of the core in the shape of a wide and deep screw, and a connecting portion formed in the shape of a plurality of vertical holes and grooves and capable of containing bone growth factors. The mounting portion has a gum adhering portion to protect bone tissues and a post to be mounted with an abutment or a prosthesis thereon.
Owner:NEOBIOTECH
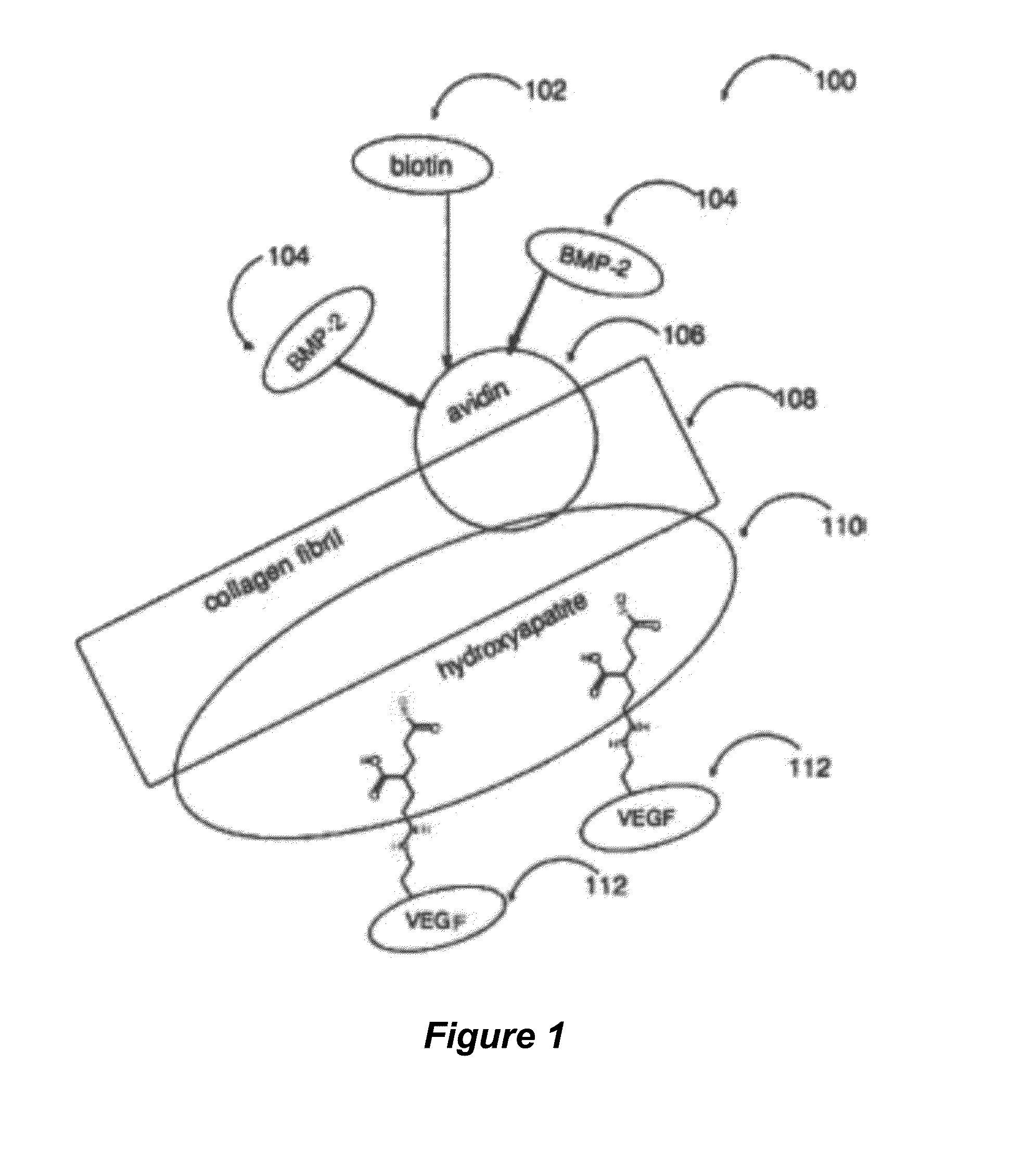
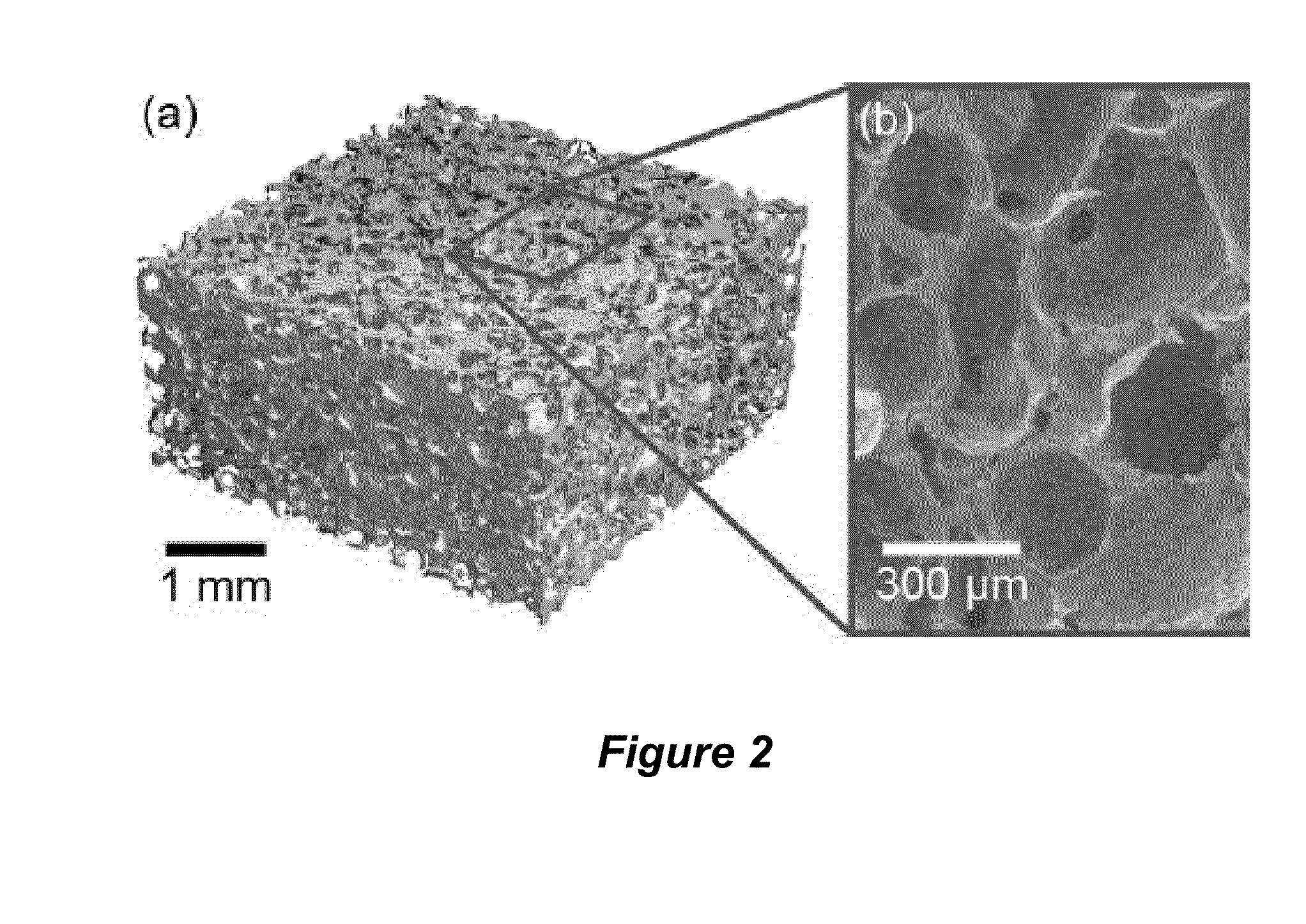
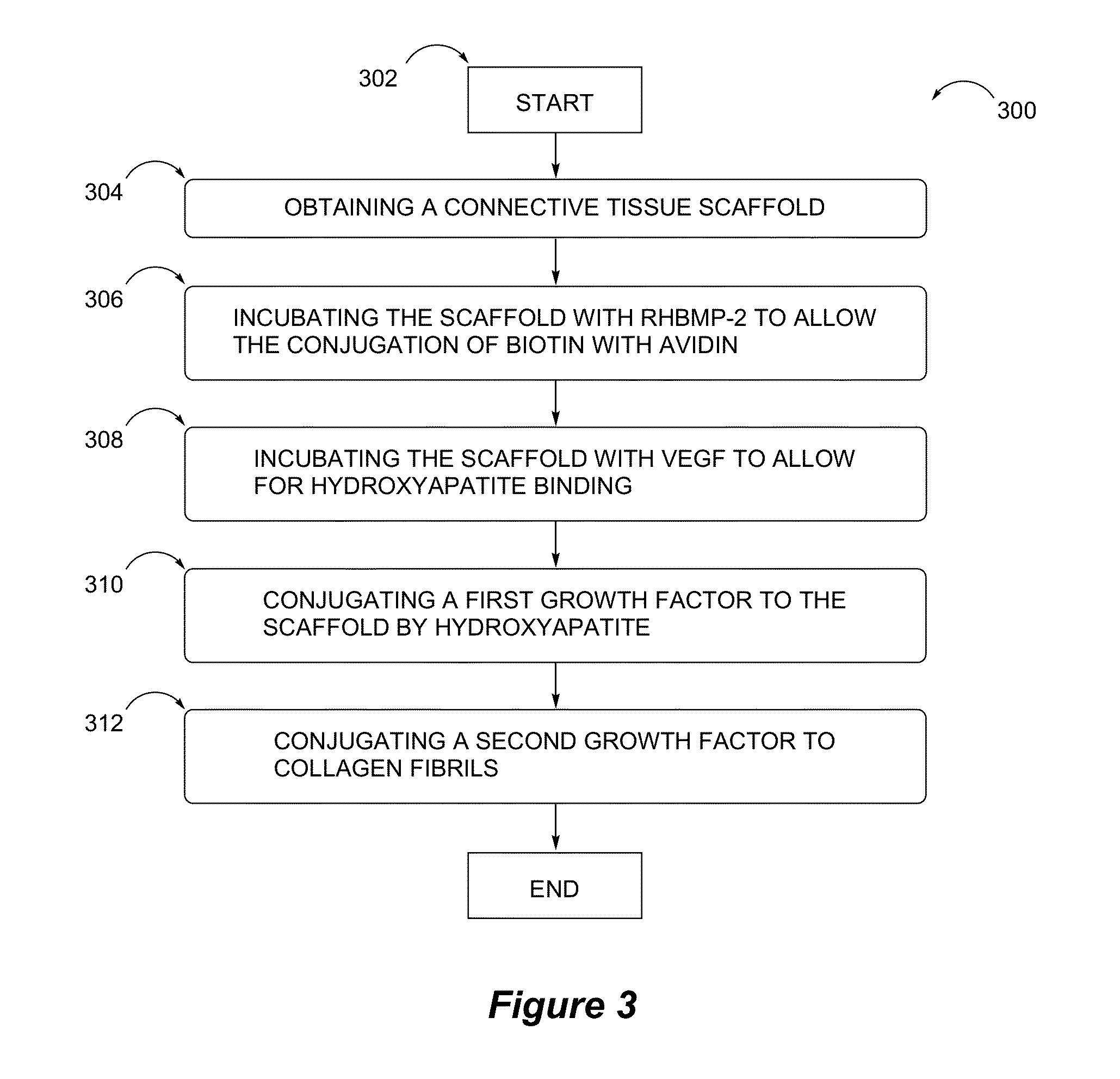
Tissue scaffolds having bone growth factors
ActiveUS20150132354A1High mechanical strengthEnhanced osteogenic propertiesPeptide/protein ingredientsSkeletal disorderCalcium biphosphateCollagen scaffold
The invention provides a novel composite bone graft system utilizing a porous collagen scaffold having a matrix impregnated with calcium phosphate particles and more than one bioactive agent, one of which is conjugated to the matrix. The graft system exhibits increased mechanical strength and osteogenic properties by providing sites for tissue attachment and propagation. The bioactive agents are delivered to the scaffold via different mechanisms to enable sequential and sustained release of the bioactive agents over time.
Owner:UNIV OF NOTRE DAME DU LAC
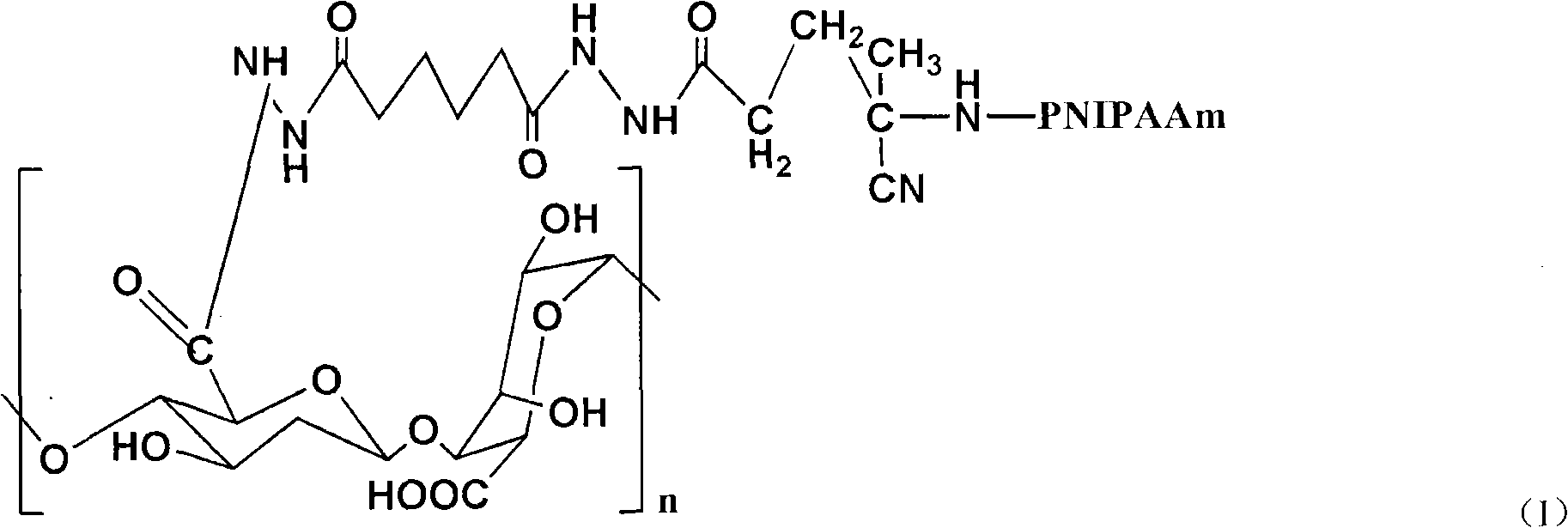
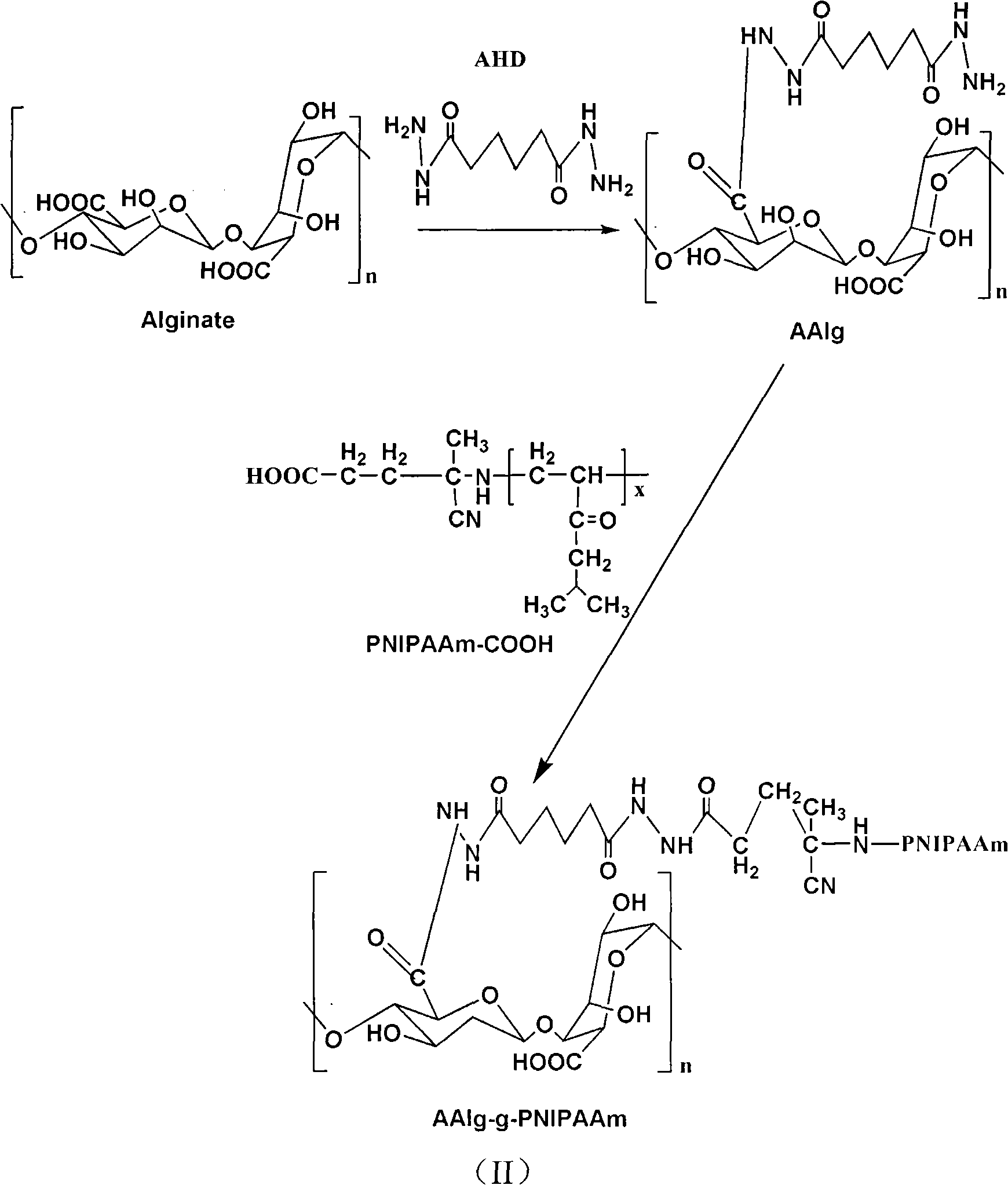
Injectable bone repairing material and preparation method thereof
The invention relates to an injectable bone repairing material and a preparation method thereof. The injectable bone repairing material mainly comprises poly(N-isopropylacrylamide)-grafted solution of aminated alginic acid. The method comprises the following steps of: preparing poly(N-isopropylacrylamide)-grafted aminated alginic acid; dissolving the poly(N-isopropylacrylamide)-grafted aminated alginic acid in buffer solution of phosphoric acid; adding one or more components selected from hydroxyapatite, beta-tricalcium phosphate, cells and bone growth factors into the prepared buffer solution of the phosphoric acid and uniformly stirring the mixture; and filling the obtained product into a syringe for later use. The injectable bone repairing material has the characteristics of body temperature sensitivity, high plasticity, high degradability, capability of promoting the regeneration of bone defect, high stability, high safety and the like.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Preparation method of bone repair material
InactiveCN112999422AImprove toughnessPlay a bactericidal effectPharmaceutical delivery mechanismTissue regenerationBone tissueBone growth factor
The invention discloses a preparation method of a bone repair material. The preparation method comprises the following steps: step 1, taking a fresh bovine bone, cutting into small blocks, degreasing, and removing antigens to obtain bovine bone particles; step 2, soaking the bovine bone particles in the step 1 into a bone growth factor-collagen mixture, taking out the soaked bovine bone particles, and freeze-drying the soaked bovine bone particles; step 3, crushing and calcining the bovine bone particles in the step 1, and crushing to obtain bovine bone powder; step 4, mixing the bovine bone particles in the step 1 and the bovine bone powder obtained in the step 3 with collagen and nano-silver, and then putting the mixture into a forming mold for freeze-drying and forming to obtain a scaffold material; and step 5, carrying out crosslinking modification on the scaffold material obtained in the step 4. According to the bone repair material, the bovine bone particles serve as a skeleton of the bone repair material and play a role in enhancing strength, the mixture of the bovine bone powder and the collagen serves as filler, then cross-linking modification is conducted, high toughness is achieved, and bone growth factors can be slowly released in the bovine bone particles and play a role in promoting bone tissue regeneration and reconstruction; and the nano-silver in the filler has a sterilization effect.
Owner:WEIFANG AOJING MEDICAL RES CO LTD
Resorbable bone cement containing active agents
InactiveUS20040131678A1Easily release profileEasy to measureSurgical adhesivesPeptide/protein ingredientsMicrobial agentActive agent
Described is a water based bone substitute for in vivo implantation, promoting bone tissue growth in situ comprising bone substitute material, a slow release bone growth factor and a fast release antimicrobial agent. Further, a kit and a method for the preparation of said bone substitute is disclosed.
Owner:AM PHARMA
Preparation method of porous bioceramic microsphere bone scaffold grafted with bone cells on surface
InactiveCN104958787AReduced Chances of ContaminationGuaranteed one-time moldingAdditive manufacturing apparatusCoatingsMicrosphereOsteoblast
The invention discloses a preparation method of a porous bioceramic microsphere artificial bone scaffold grafted with bone cells on a surface, belonging to the technical field of biological tissue engineering. The preparation method comprises the following steps: firstly, establishing a CAD model according to the individual characteristics of a patient, importing the model into a 3D printer, and spraying a biological adhesive by the 3D printer to bond porous bioceramic microspheres, thereby realizing the preparation of an artificial bone scaffold substrate; next, performing multiple subculture on osteoblasts taken from the patient to reach the quantity demanded, and then mixing the osteoblasts with a DMEM cell culture medium, a bone growth factor and a biocompatible hydrogel into a specific mixture; and finally, printing the mixture on the prepared porous bioceramic artificial bone scaffold by use of a self-made biological forming machine, thereby obtaining the porous bioceramic microsphere artificial bone scaffold grafted with bone cells on the surface. The method is capable of realizing the customizability of the bone scaffold, the bone scaffold has better biocompatibility, and after the bone scaffold is transplanted into the body of the patient, the wound is easier to heal and the pain of the patient can be relieved.
Owner:宁波傲骨生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com