Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
171 results about "Orthopedic devices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
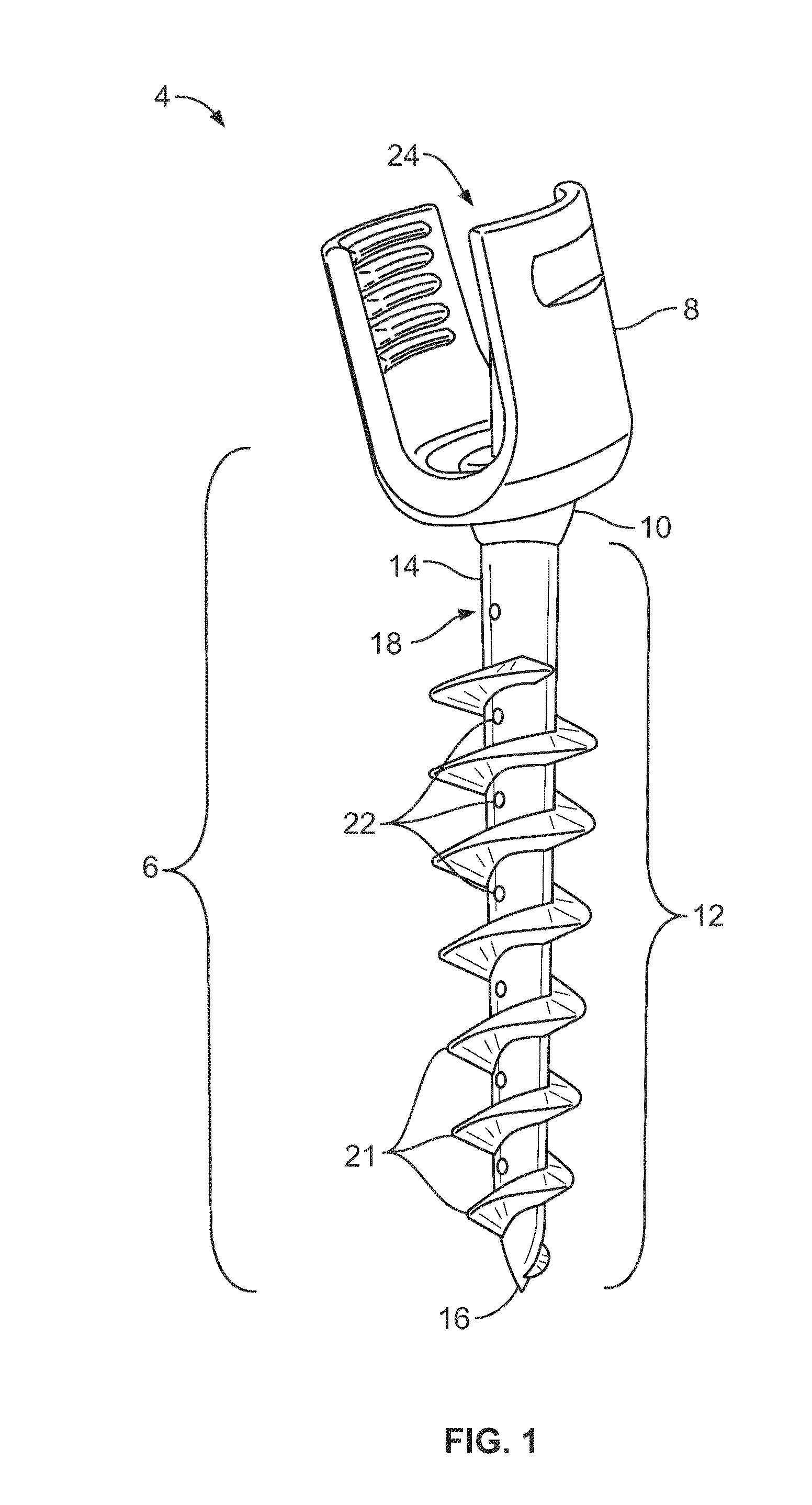
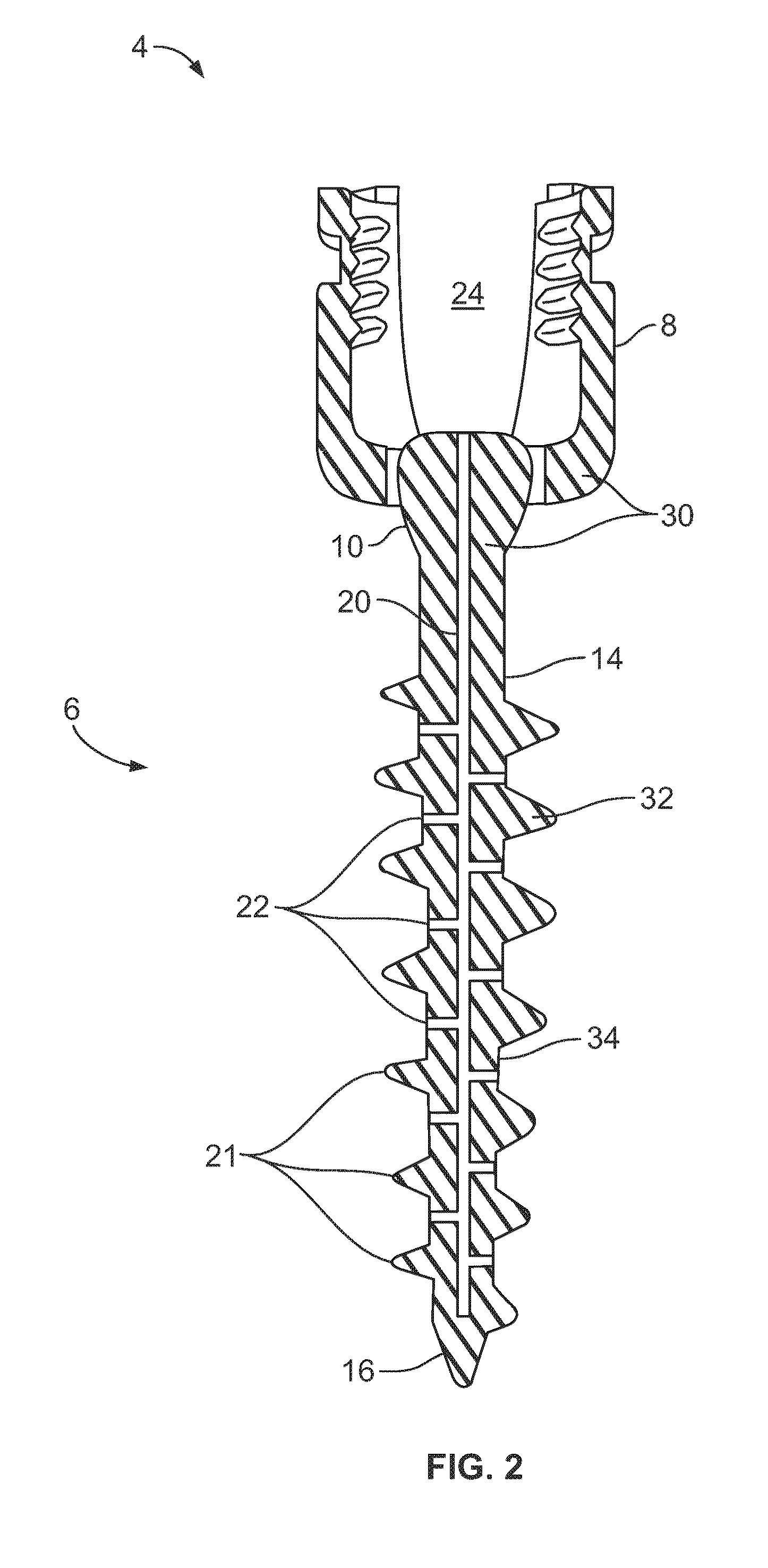
Devices for management of foot injuries and methods of use and manufacture thereof
The present invention provides orthotic devices for use in managing the treatment and prevention of lower extremity injuries, including foot ulcers. In various aspects, the present invention provides foot-worn orthotics which provide for improved compliance monitoring, and methods of their manufacture and use.
Owner:LASERCURE SCI
Orthotic trauma device
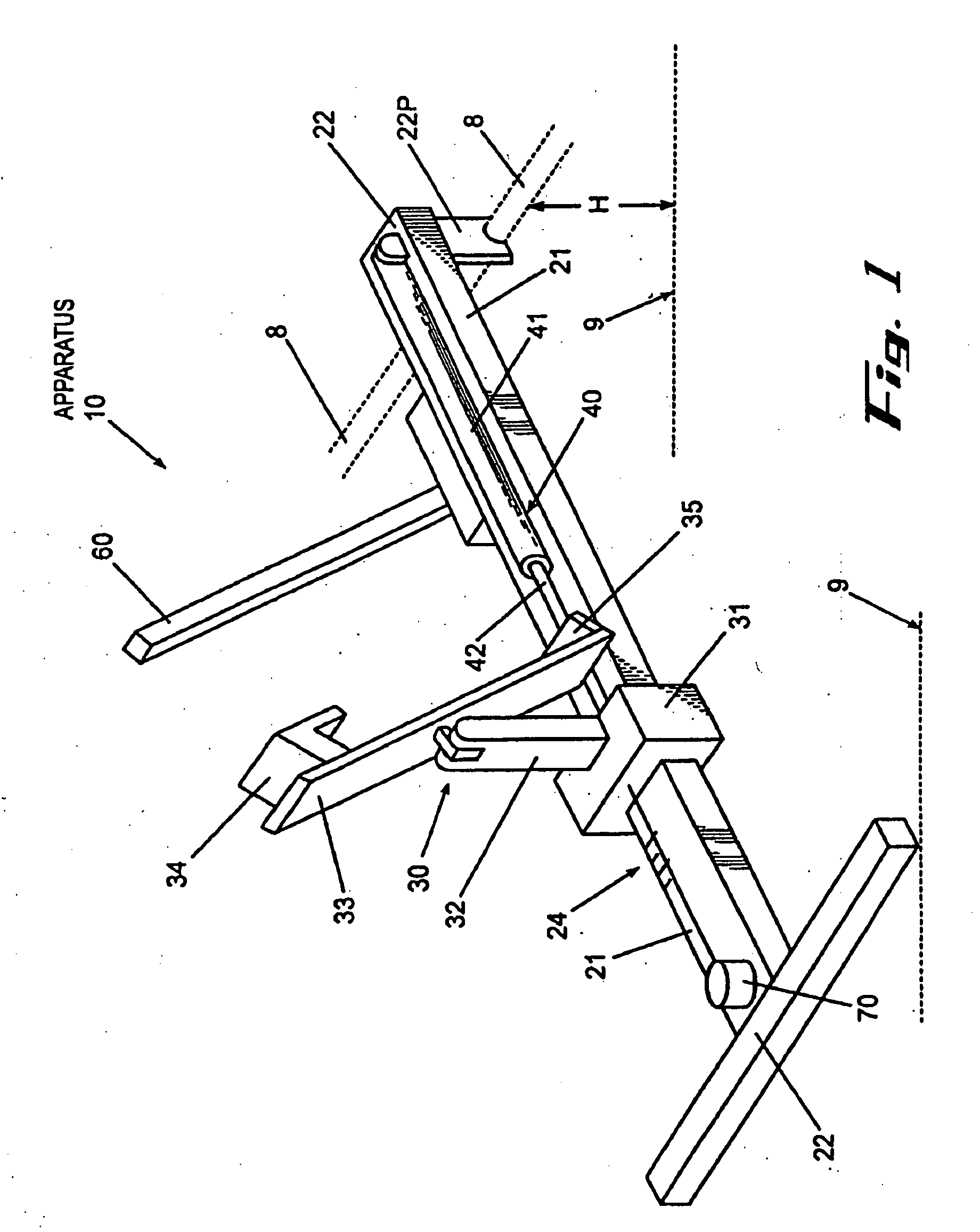
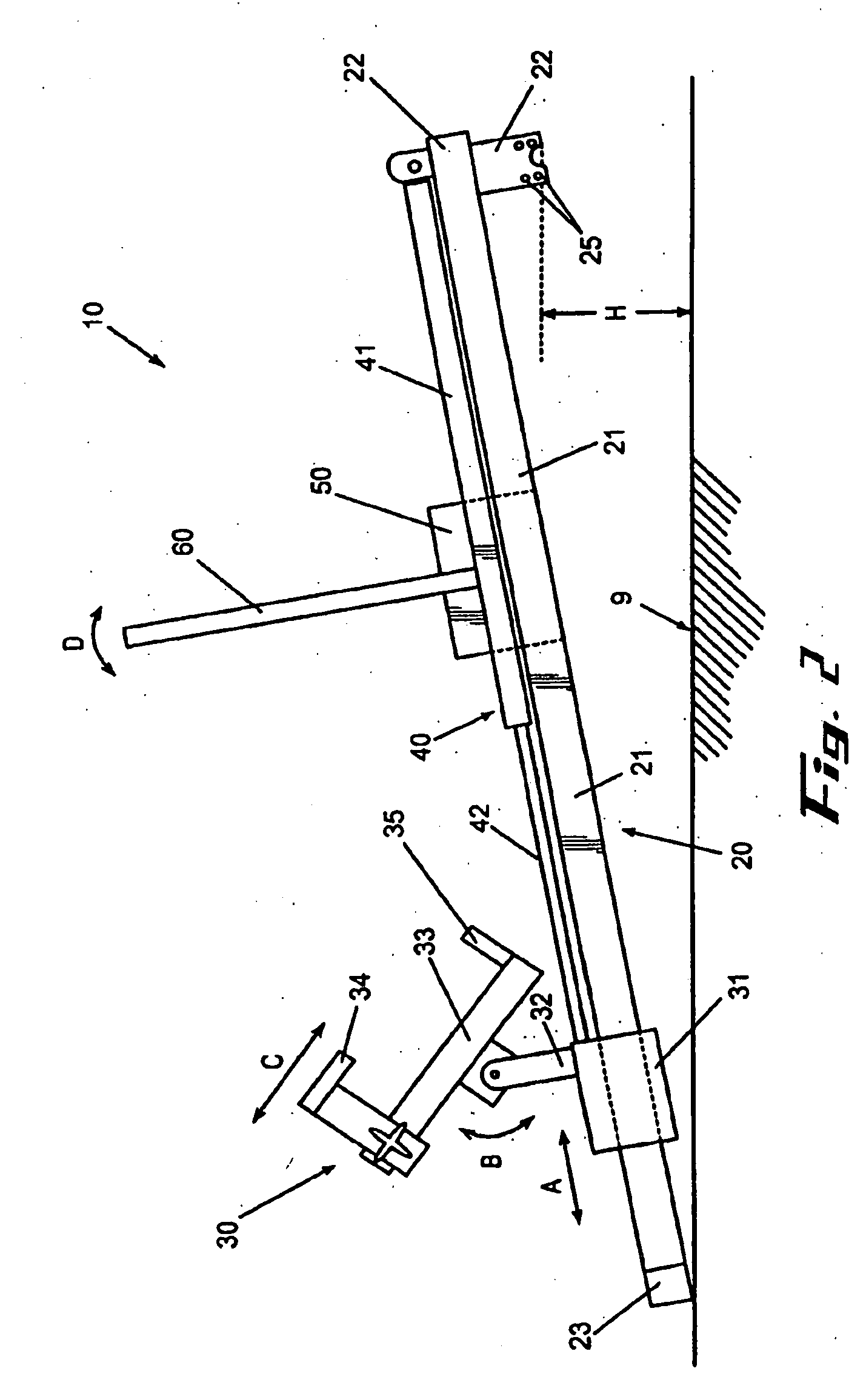
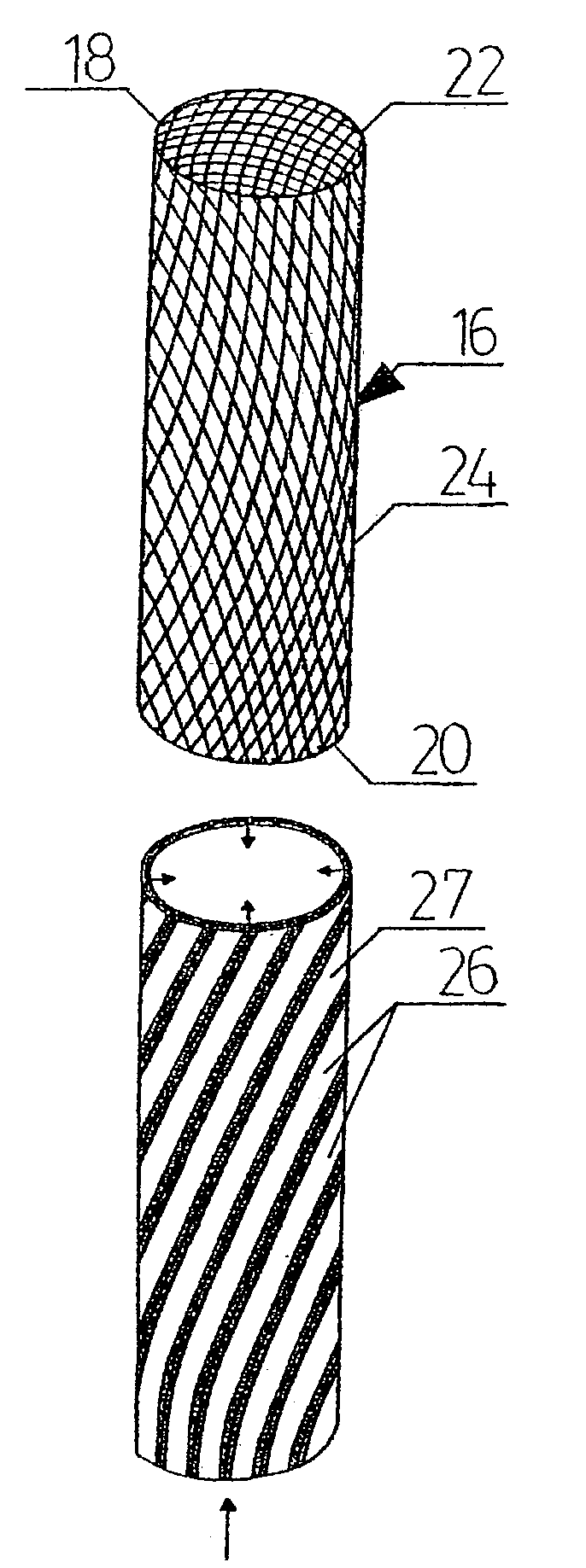
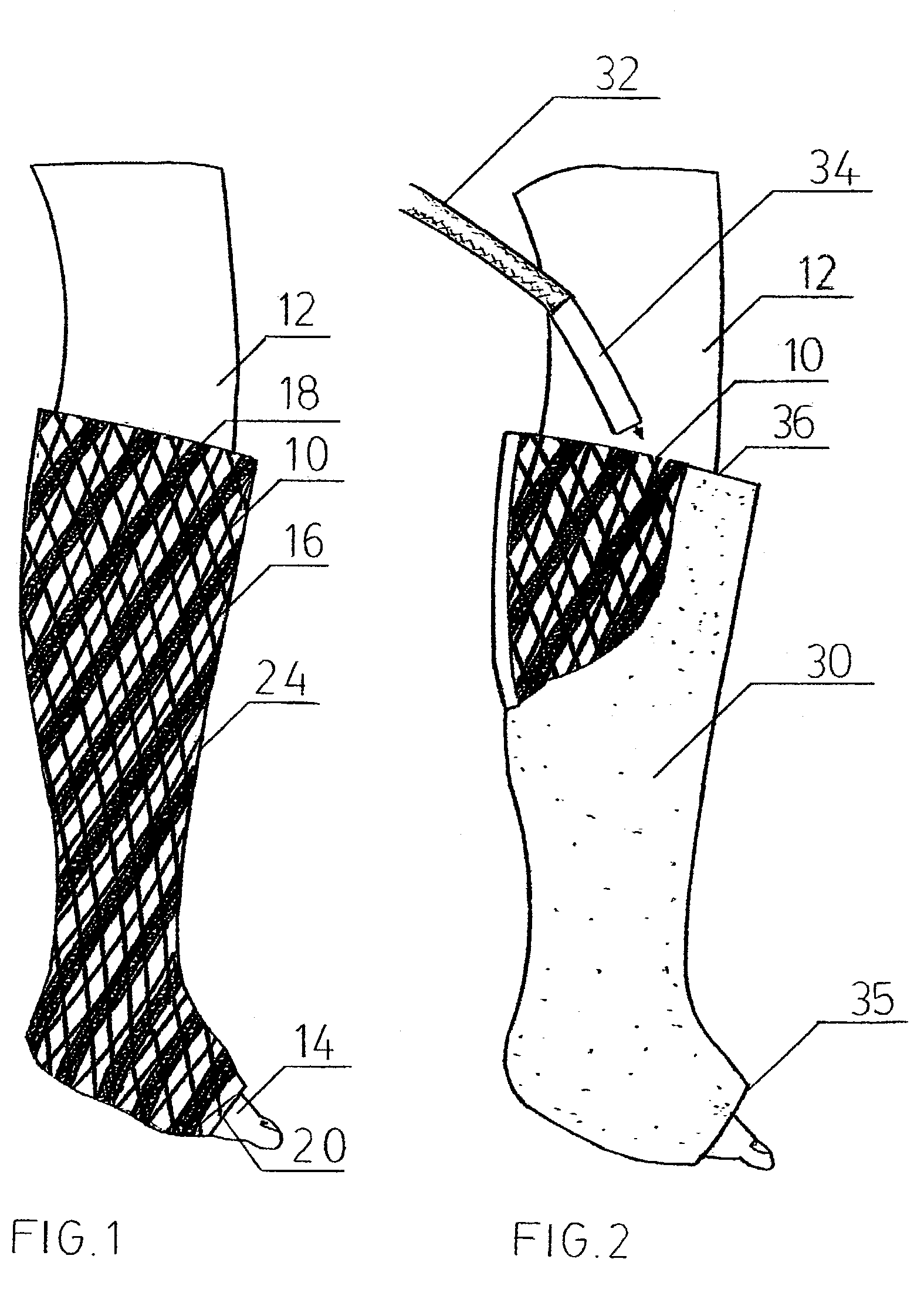
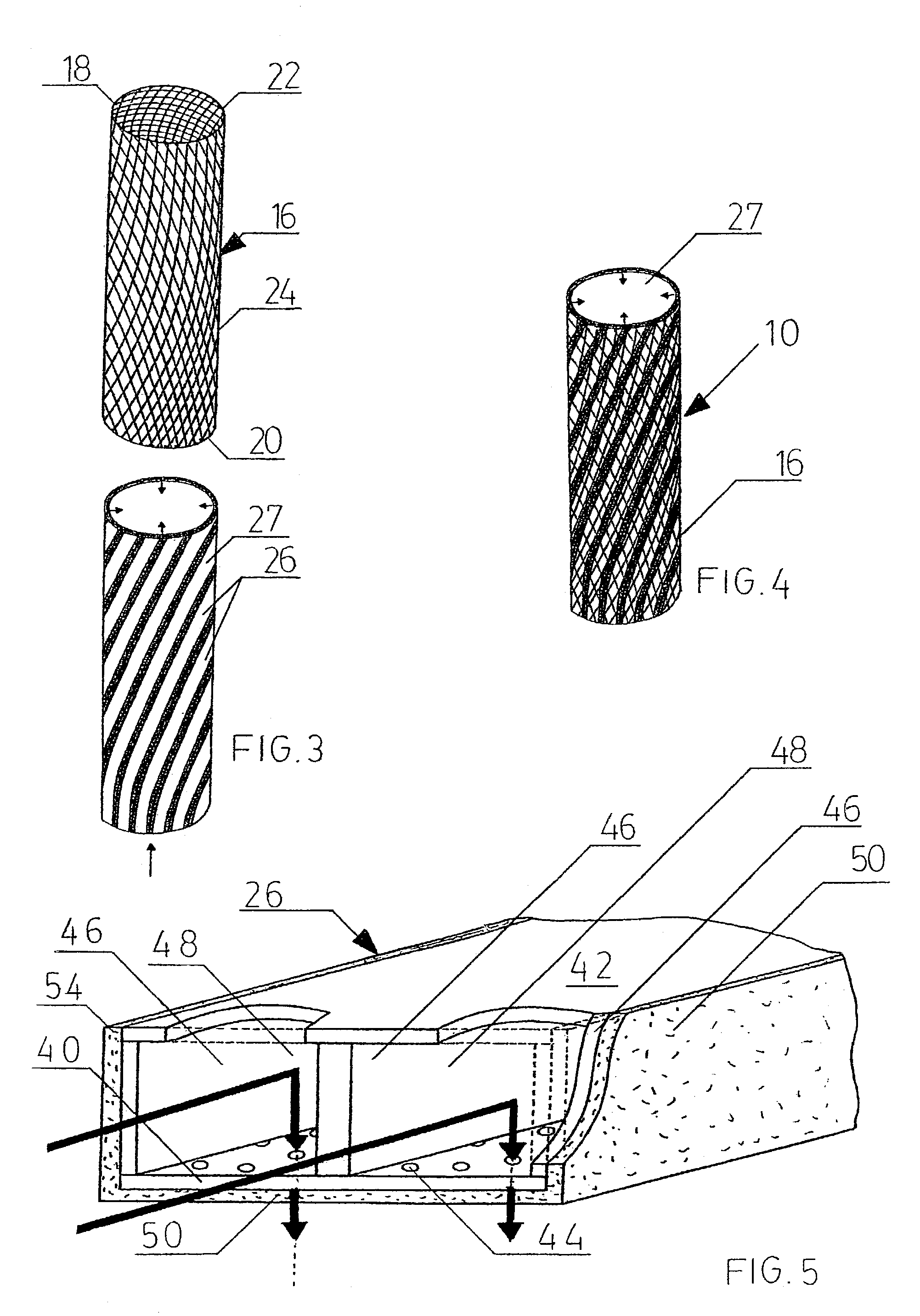
InactiveUS20020148461A1Stabilizes broken boneBlood lossNon-surgical orthopedic devicesKnee orthosisBlock and tackle
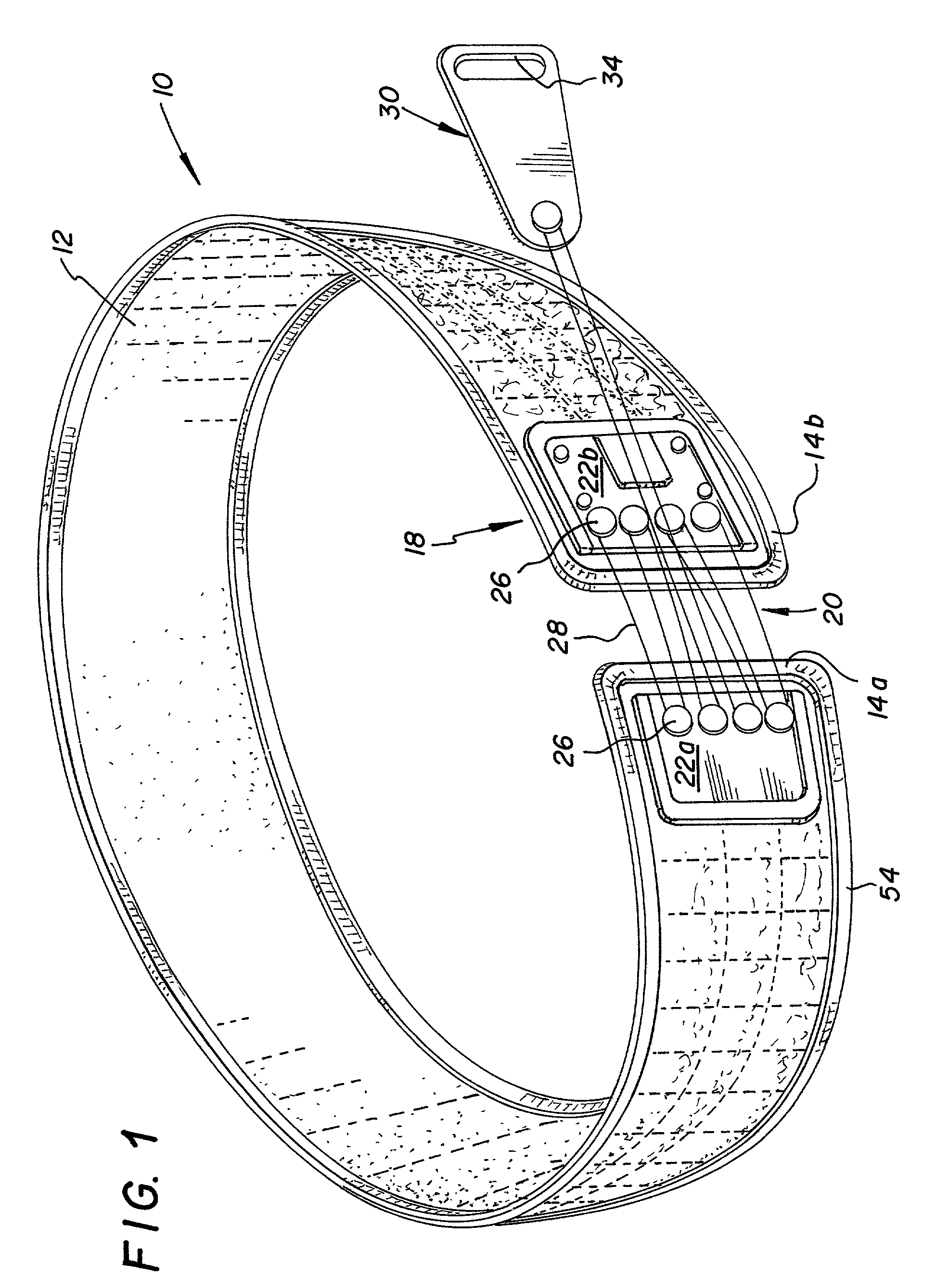
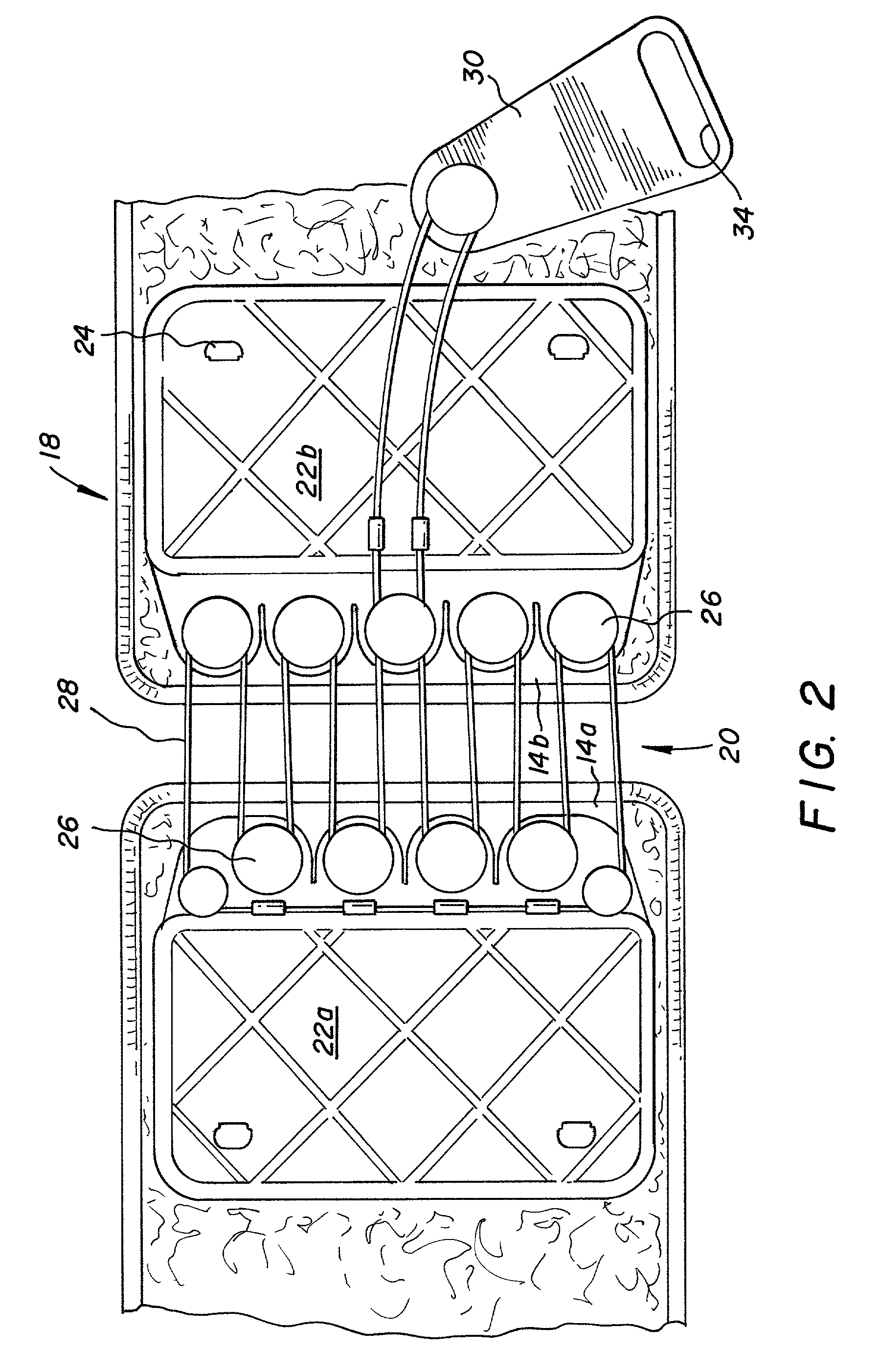
An orthotic trauma device is provided including an elongated orthosis body adapted to be wrapped around a portion of a body of a wearer of the device, the orthosis body being formed of a pliant material which is adapted to be easily cut with cloth-cutting scissors; a detachable fastening device provided at distal ends of the elongated orthosis body to releasably secure the distal ends to one another; and means for adjusting the tightness of the orthosis body operatively associated with the detachable fastening device. A pulley system for use in an orthotic device is also provided including a pair of pulley banks arranged in juxtaposed relationship, a first bank of which is adapted to be detachably disposed on a first distal end of an elongated orthosis body and a second bank of pulleys adapted to be detachably disposed on a second distal end of the elongated orthosis body; and a cable interconnecting the two pulley banks and running through a pulley on each of the pulley banks in alteration, such that shortening of the cable pulling the pulley banks together and tightening the orthotic device with the aid of a mechanical advantage dependent upon the number of pulleys mounted on each of the pulley banks.
Owner:PYNG MEDICAL CORP
Patient specific ankle-foot orthotic device
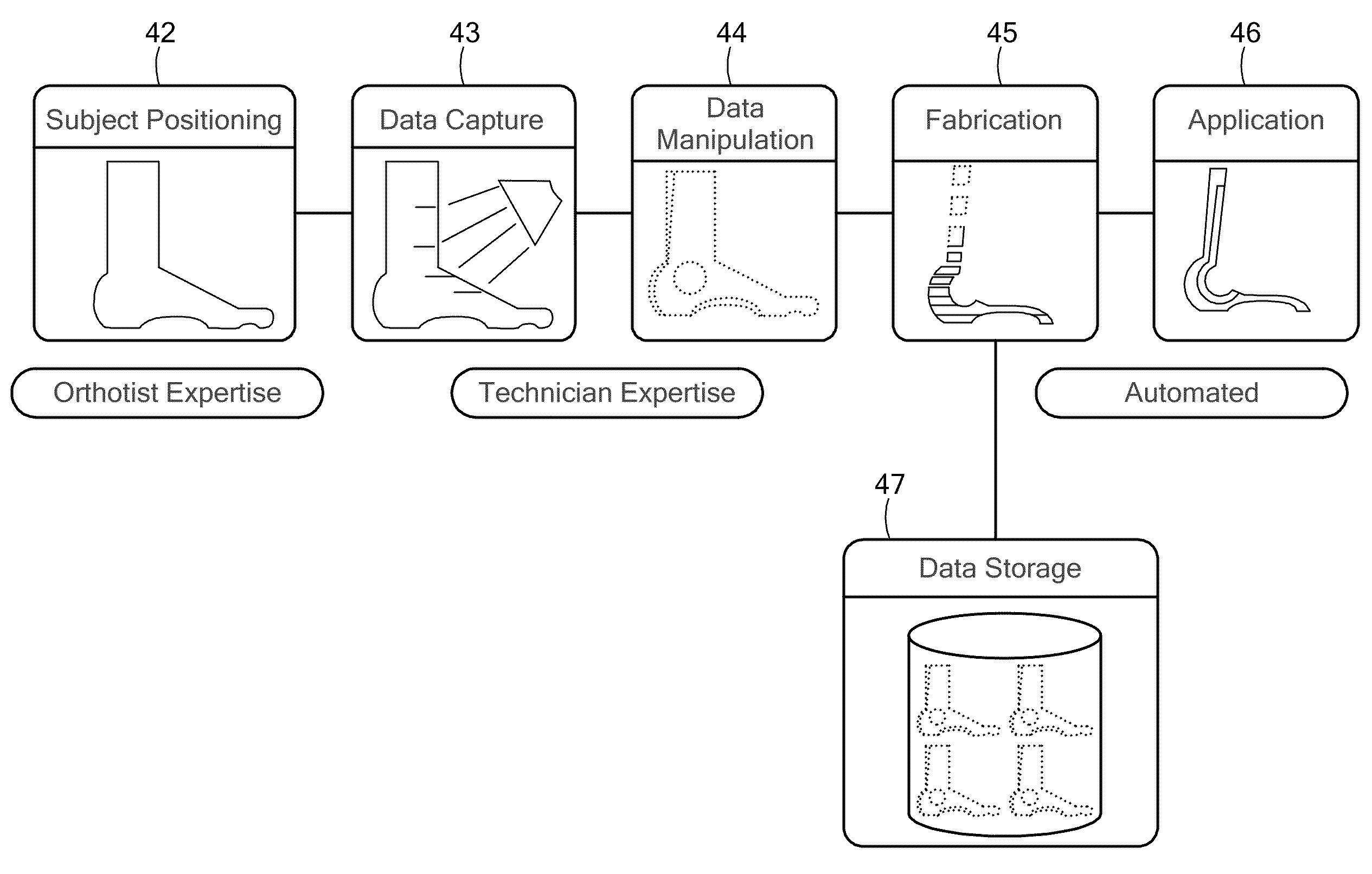
The unique advantages of computer-controlled fabrication of a patient-specific orthotic device using an automated fabrication machine capable of following computer instructions to create 3D surface contours and new developments in non-invasive three-dimensional (3D) scanning have made it possible to acquire digital models of freeform surfaces such as the surface anatomy of the human body and to then fabricate such a patient-specific device with high precision. Such a patient-specific device brings significant improvement in patient-specific fit, comfort, and function of medical devices (and, in particular, to orthoses that require a close fit to the wearer's body to act effectively). The combination of these two technologies is ideally suited for the development of patient-specific orthotic devices.A patient specific ankle-foot orthotic device using this technology is disclosed. This exemplary device is used to help stabilize the ankle-foot region, for example, in patients with impaired gait.
Owner:TECHNEST HLDG +2
Reflex fixation geometry revision and reconstruction system reverse articulation
An orthopedic device is disclosed for restoring the normal or natural joint mechanics in, for example, a hip joint. The device includes a first component, such as an acetabular component in a hip implant, that includes a convex articulation surface and a second component, such as a femoral component in a hip implant, that includes a concave articulation surface. It will be appreciated that the convex articulation surface of the device disclosed herein articulates with the concave articulation surface in a mating engagement that may be reversed with respect to the traditional hip implant, in which the concave articulation surface is part of the acetabular component and the convex articulation surface is part of the femoral component.
Owner:GLOBAL ORTHOPAEDIC TECH
In-situ formed intervertebral fusion device and method
InactiveUS20150173914A1MinimallyIncrease heightLuminescence/biological staining preparationBone implantOrthopedic devicesGonial angle
An orthopedic device for implanting between adjacent vertebrae comprising: an arcuate balloon and a hardenable material within said balloon.In some embodiments, the balloon has a footprint that substantially corresponds to a perimeter of a vertebral endplate. An inflatable device is inserted through a cannula into an intervertebral space and oriented so that, upon expansion, a natural angle between vertebrae will be at least partially restored. At least one component selected from the group consisting of a load-bearing component and an osteobiologic component is directed into the inflatable device through a fluid communication means.
Owner:DEPUY SYNTHES PROD INC
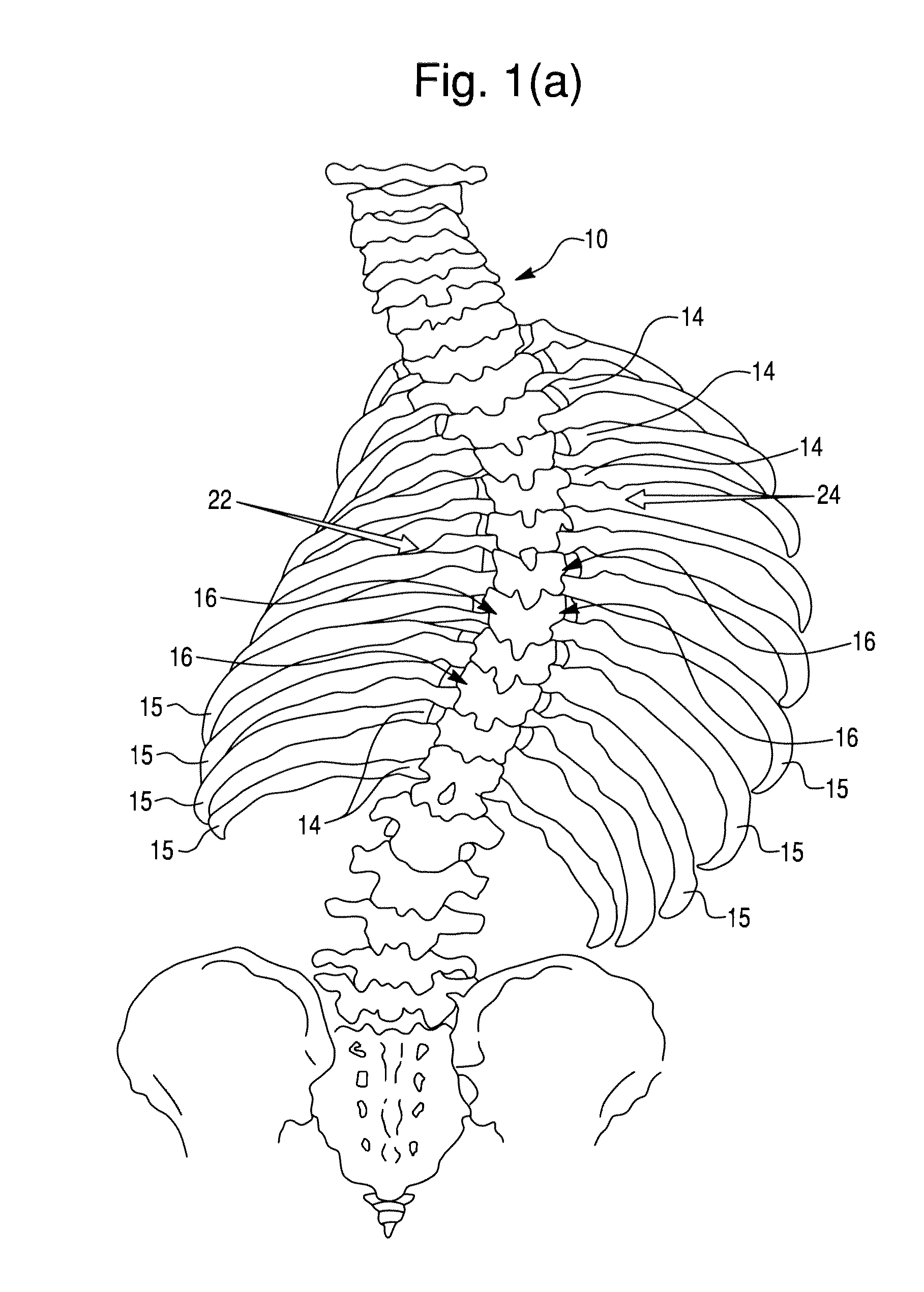
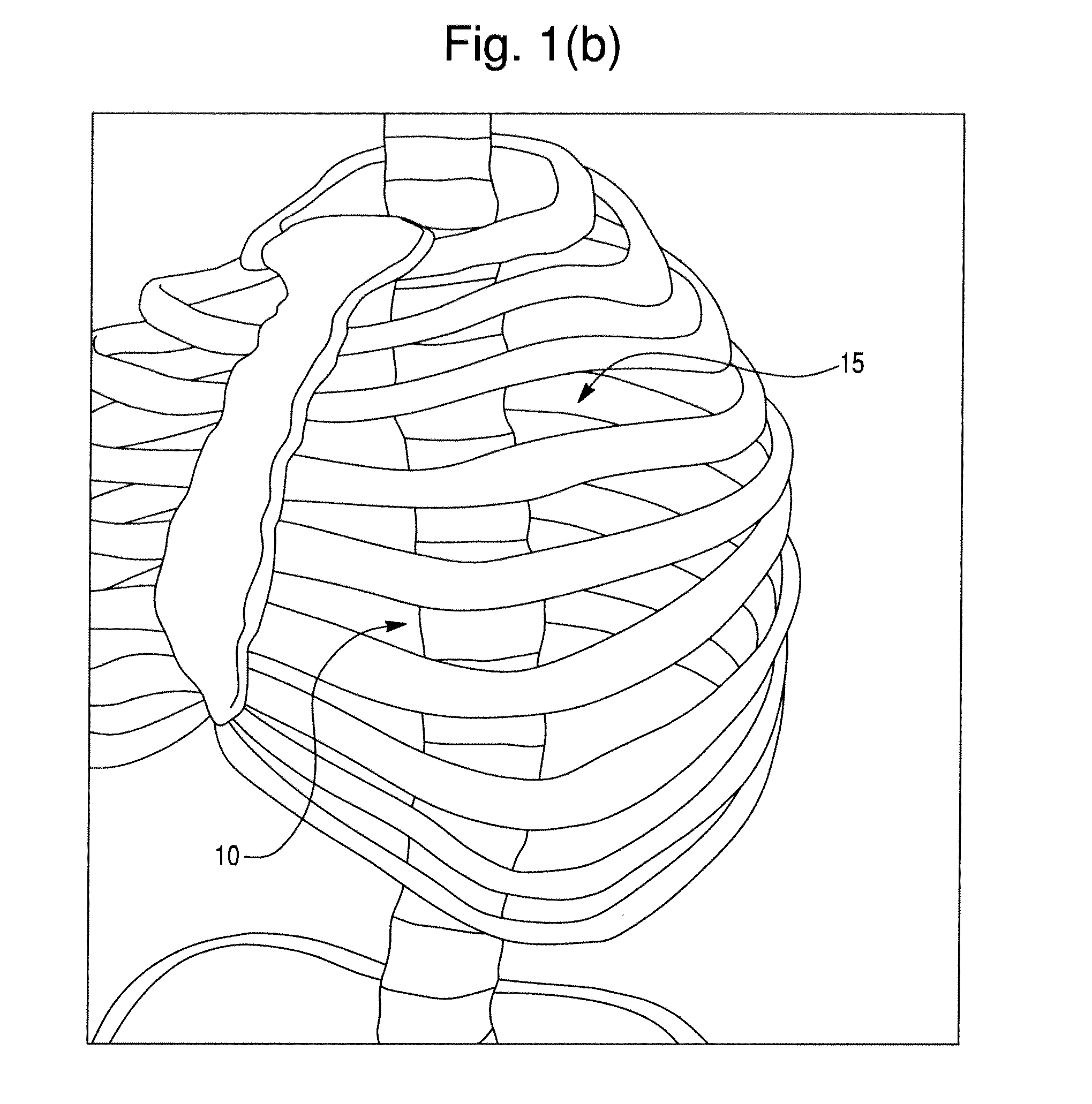
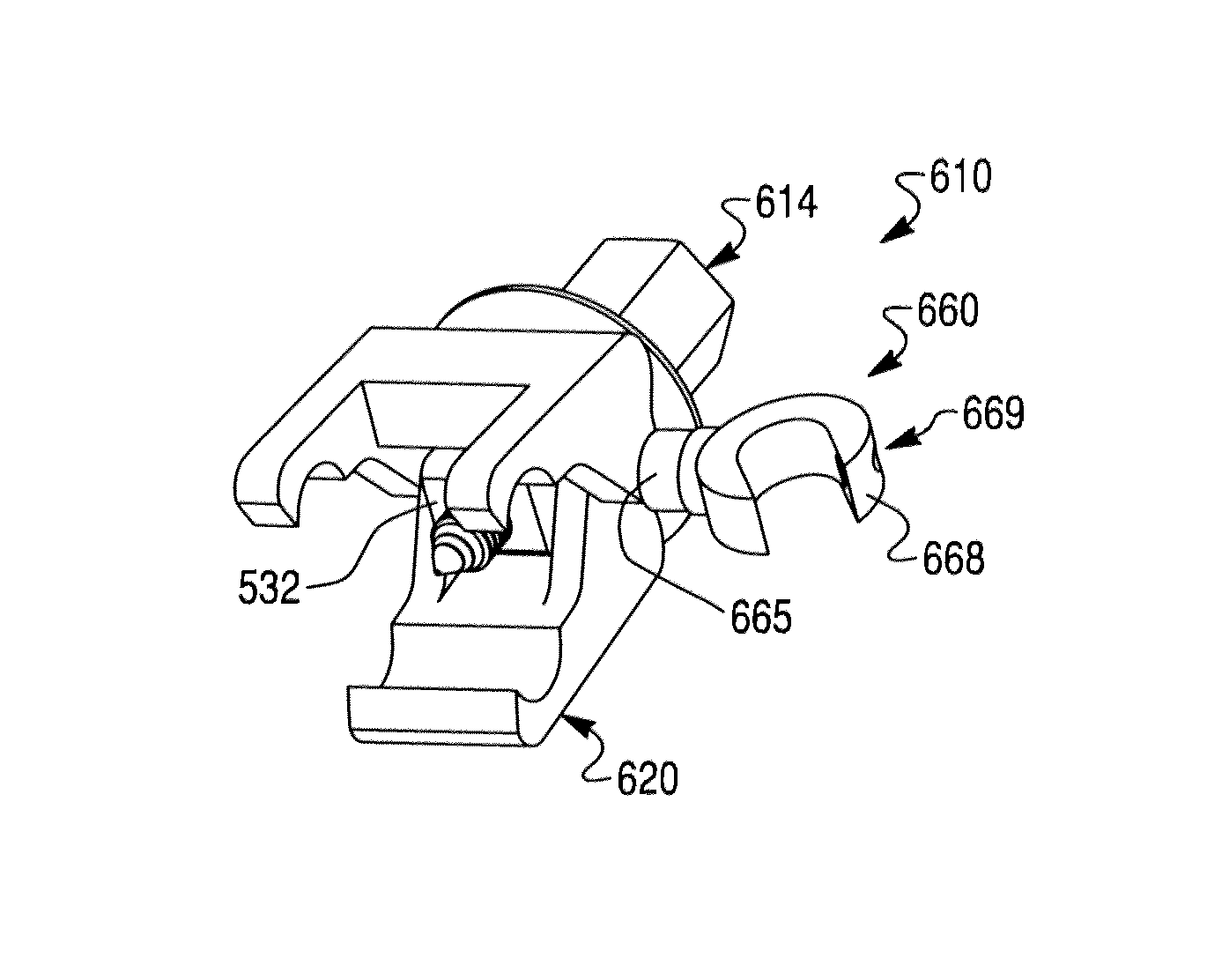
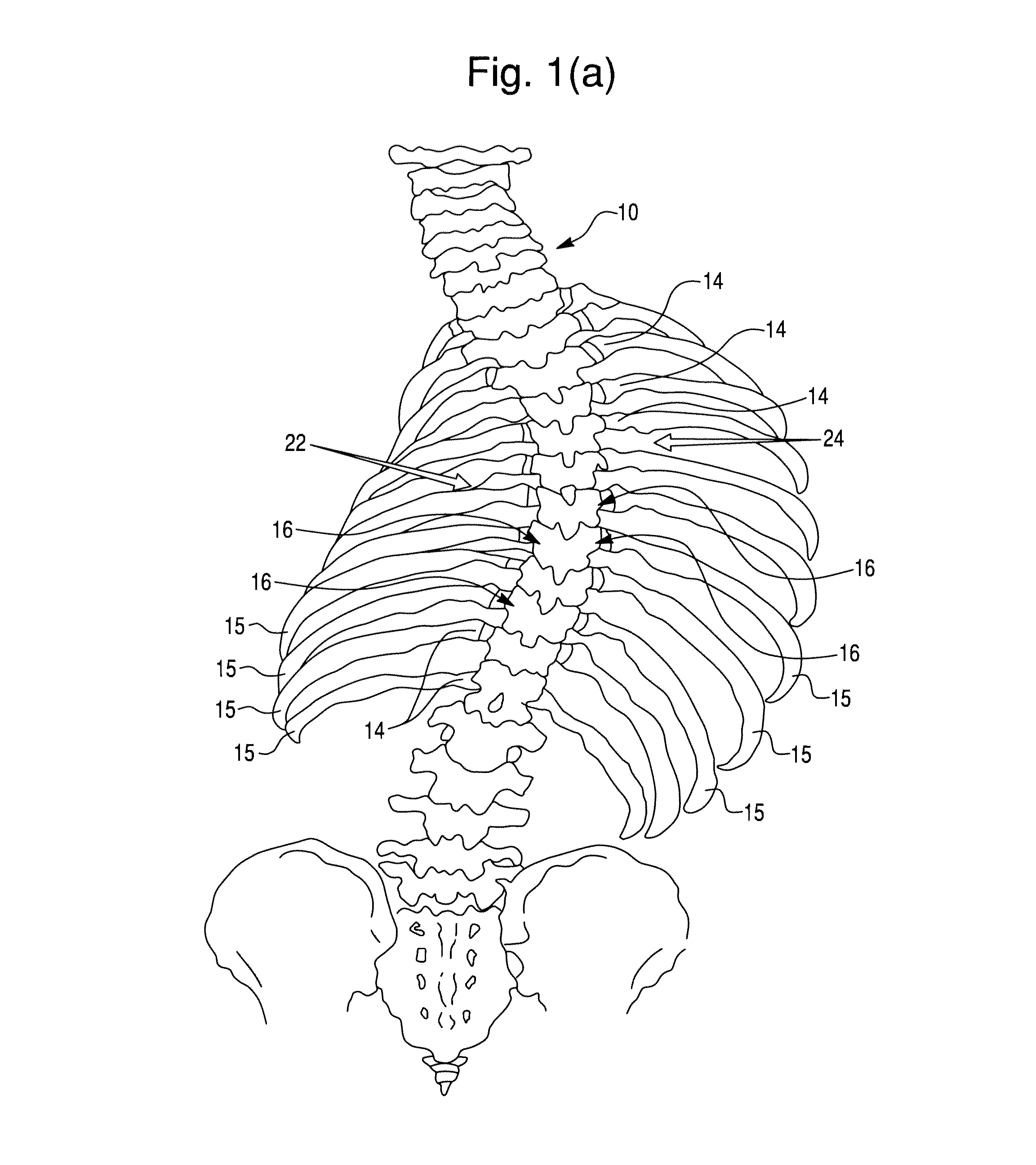
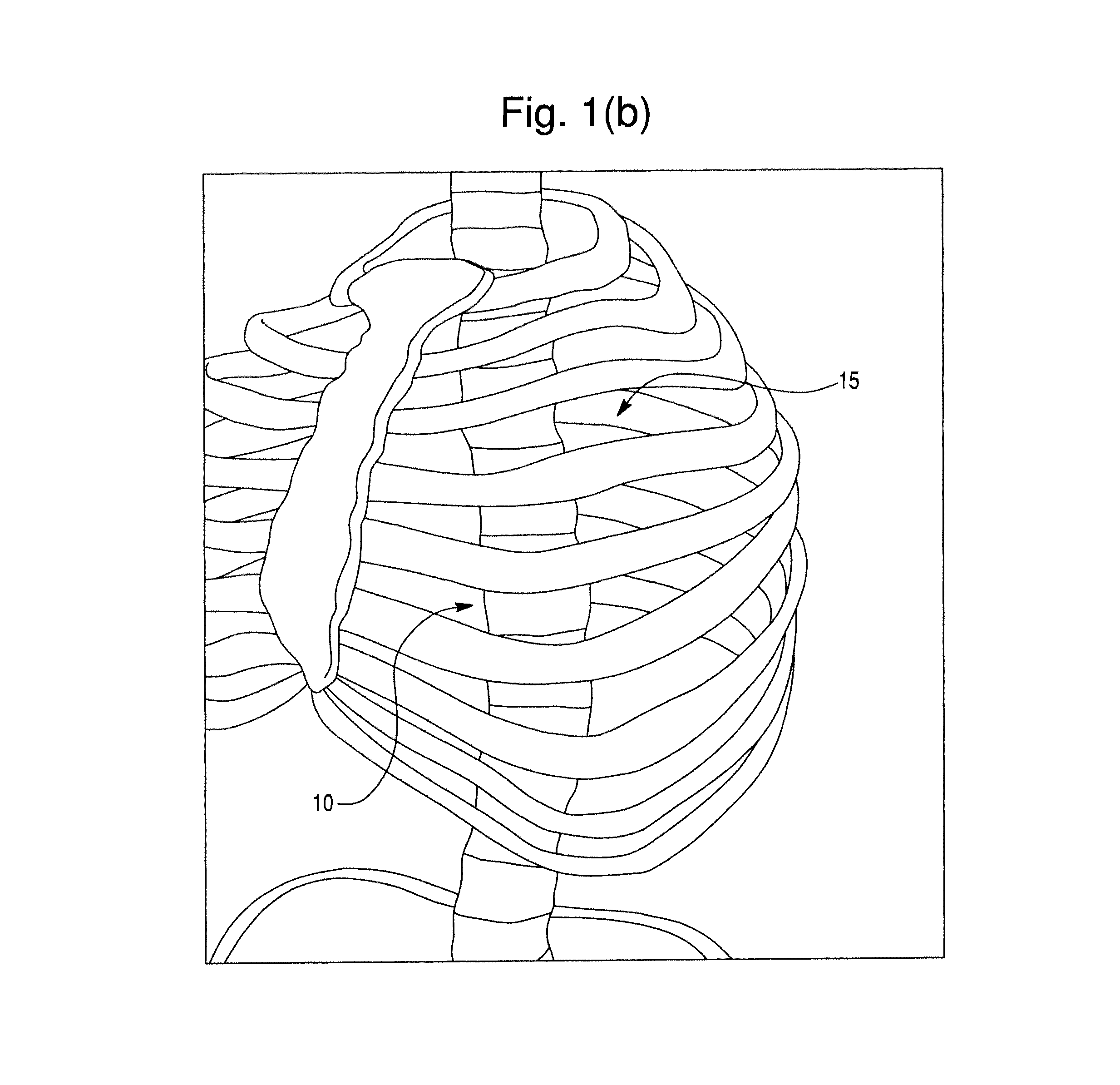
Segmental orthopedic device for spinal elongation and for treatment of scoliosis
ActiveUS20110137353A1Prevent movementSuture equipmentsInternal osteosythesisOrthopaedic deviceDistraction
An orthopaedic device to realign bone segments comprises first and second attachment members and a strut or spacer member. The first attachment member is attached to a first rib bone or first transverse process or first lamina of a vertebra. The second attachment member is attached to a second rib bone or second transverse process or second lamina of a vertebra. The strut is positioned between the first and second attachment members. The strut is fixedly or releasably connected to the first and second attachment members to couple the first attachment member to the second attachment member. The strut provides distraction between the first and second rib bones or transverse processes to realign the rib bones. The attachment members comprise a clamp and screw combination.
Owner:DYNAMIC SPINE
Patient specific ankle-foot orthotic device
The unique advantages of computer-controlled fabrication of a patient-specific orthotic device using an automated fabrication machine capable of following computer instructions to create 3D surface contours and new developments in non-invasive three-dimensional (3D) scanning have made it possible to acquire digital models of freeform surfaces such as the surface anatomy of the human body and to then fabricate such a patient-specific device with high precision. Such a patient-specific device brings significant improvement in patient-specific fit, comfort, and function of medical devices (and, in particular, to orthoses that require a close fit to the wearer's body to act effectively). The combination of these two technologies is ideally suited for the development of patient-specific orthotic devices.A patient specific ankle-foot orthotic device using this technology is disclosed. This exemplary device is used to help stabilize the ankle-foot region, for example, in patients with impaired gait.
Owner:TECHNEST HLDG +2
Orthopedic Anchor Assembly
ActiveUS20100211116A1Connection securityQuick and easy lockingSuture equipmentsInternal osteosythesisOrthopedic devicesBone tissue
In an exemplary embodiment, the present invention provides an anchor assembly that can be used for the fixation or fastening of orthopedic devices or instruments to bone tissue. In particular, the present invention preferably provides a variable angle or fixed angle anchor assembly that is able to securely connect the orthopedic device to bone tissue even when there is a variance in the angle and position of the assembly with respect to the device. Furthermore, in an exemplary embodiment, the present invention provides an anchor assembly having a locking mechanism that will quickly and easily lock the anchor assembly with respect to the orthopedic device.
Owner:GLOBUS MEDICAL INC
Composite metal and bone orthopedic fixation devices
ActiveUS9173692B1Promote healingImprove bio-integrationInternal osteosythesisBone implantFiberOrthopedic devices
Composite orthopedic devices that facilitate spine stabilization, such as: bone screws, rods, plates, interbodies, and corpectomy cages are disclosed. They are designed to provide both strength and load carrying capabilities, while increasing bio-integration of the devices with the surrounding bone tissue. They are constructed of composite layers of allograft and / or autograft bone and a structural material, such as titanium alloy or carbon / graphite fiber composite. Cannulations within the device are loaded with a mixture of stem cells, particles of allograft and / or autograft bone, and bone growth factors, such as BMP-2. The cannulations are connected to the surface of the device via multiple fenestrations that provide pathways to supply the bone / stem cell mixture to the surface, allowing living bone tissue to grow and insure bio-integration. The devices can also have radiofrequency (RF) stimulation implantation within the structure of the implanted device, capable of responding to external RF stimulation of enhanced bone growth.
Owner:STC UNM
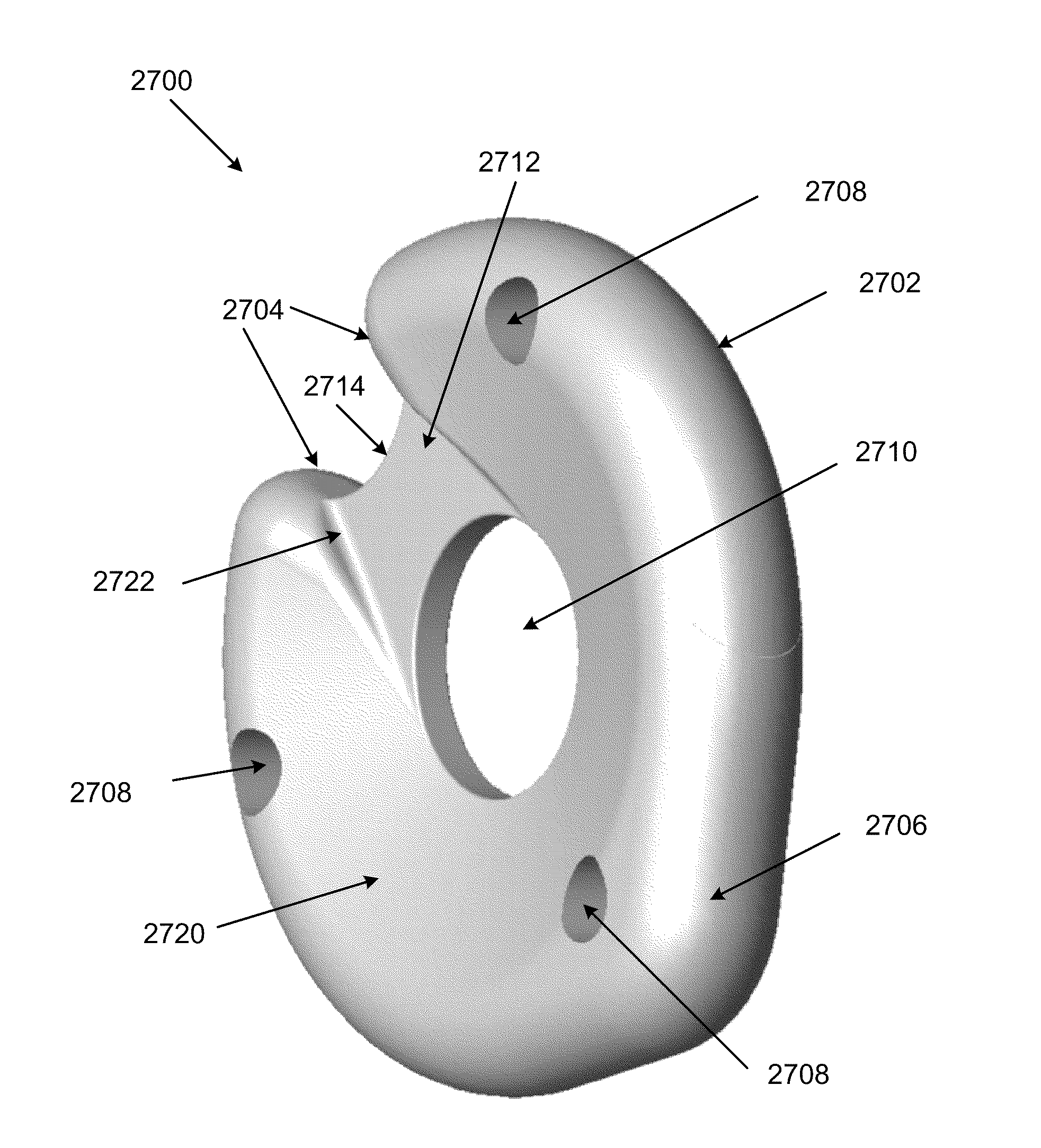
Disc-shaped orthopedic devices
InactiveUS20120022649A1Minimize impactPrevent implantationFinger jointsWrist jointsOrthopedic devicesSacroiliac joint
Methods and apparatuses for treatment of various joint conditions include a device inserted into a joint space. During delivery, the profile of the device is constrained in at least one dimension to minimize invasive impact on tissue and / or bone. The device may be restrained for implantation by a thread or a rigid elongate member. After insertion, the device may expand at the implantation site.
Owner:ARTICULINX
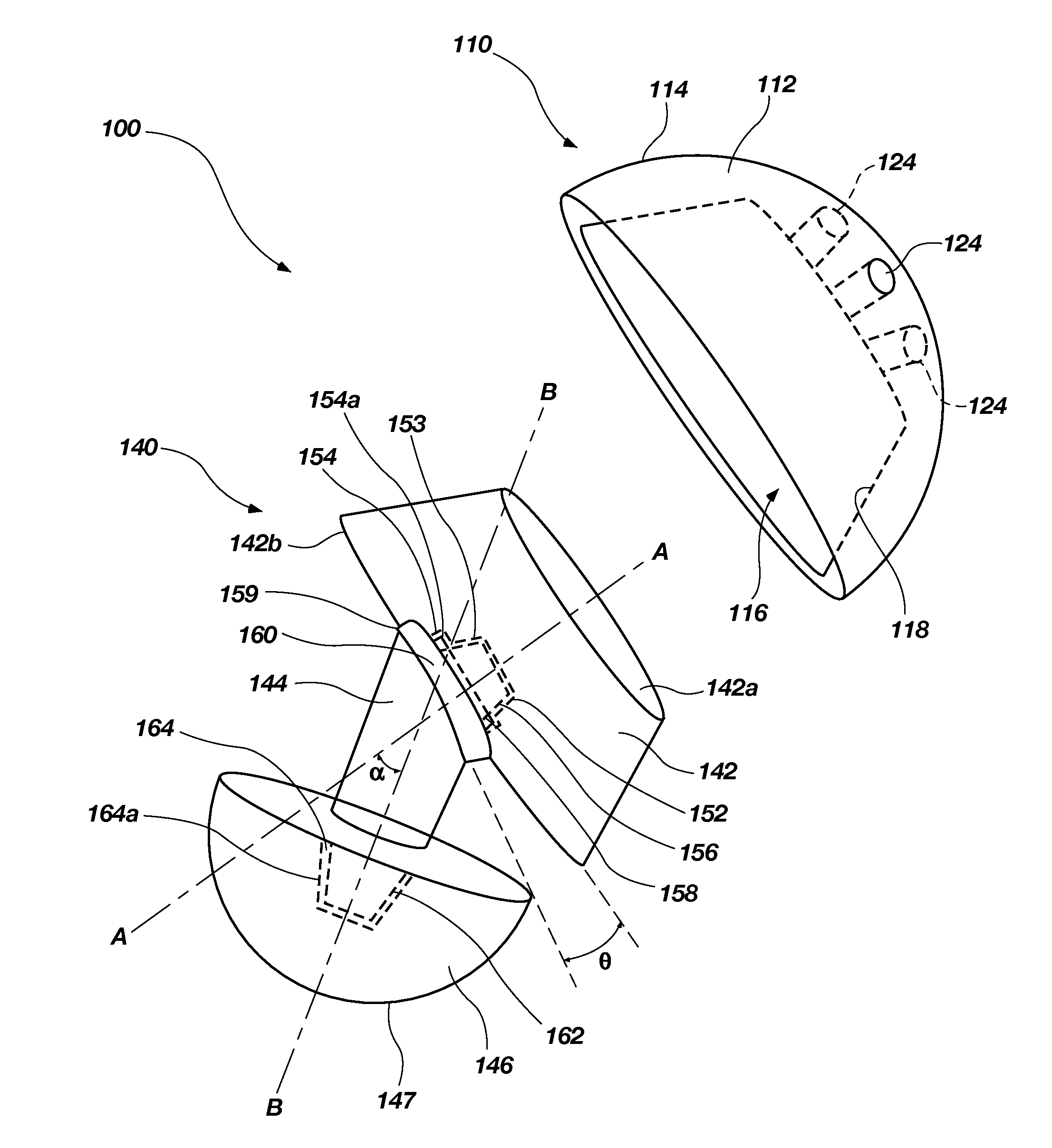
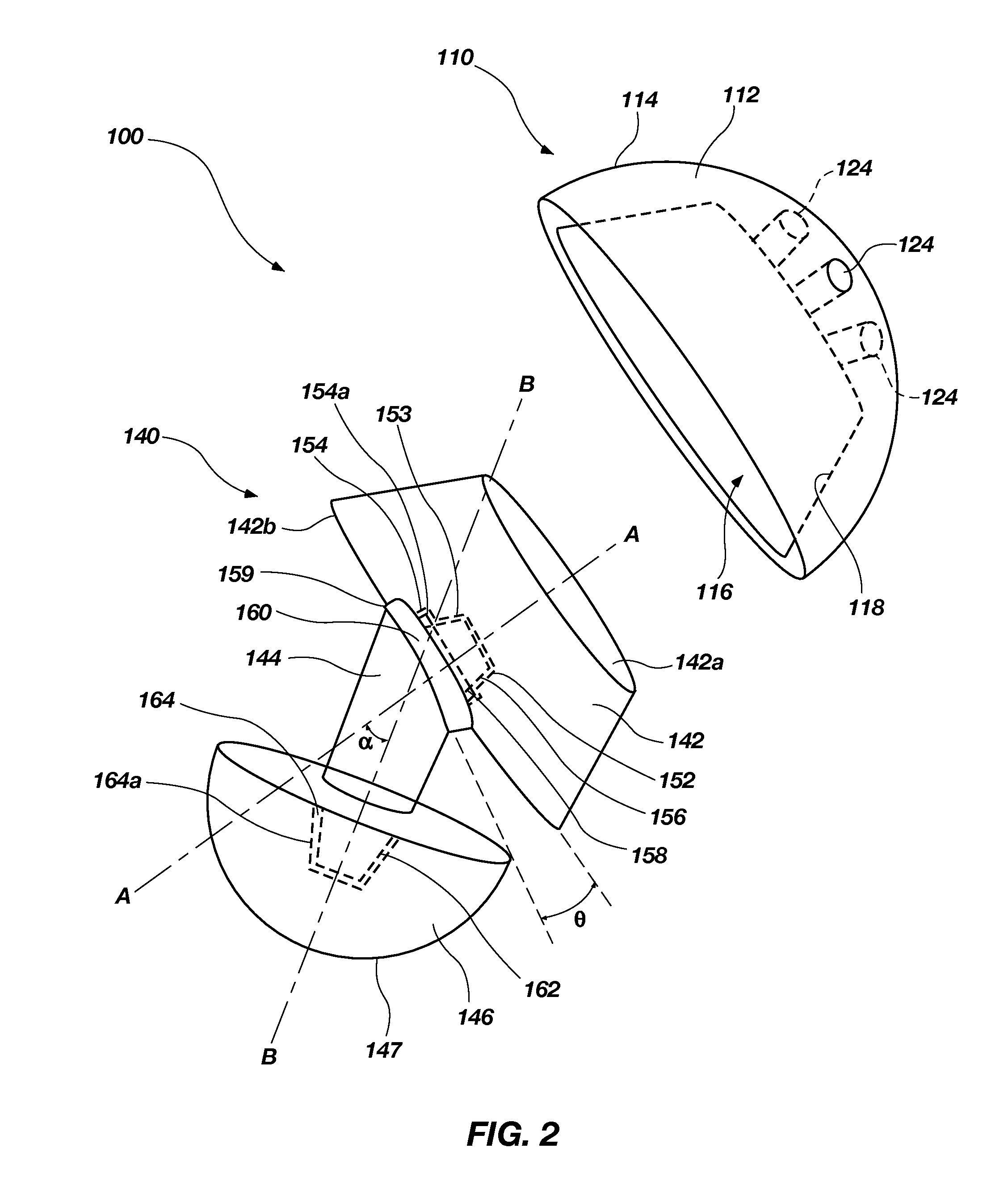
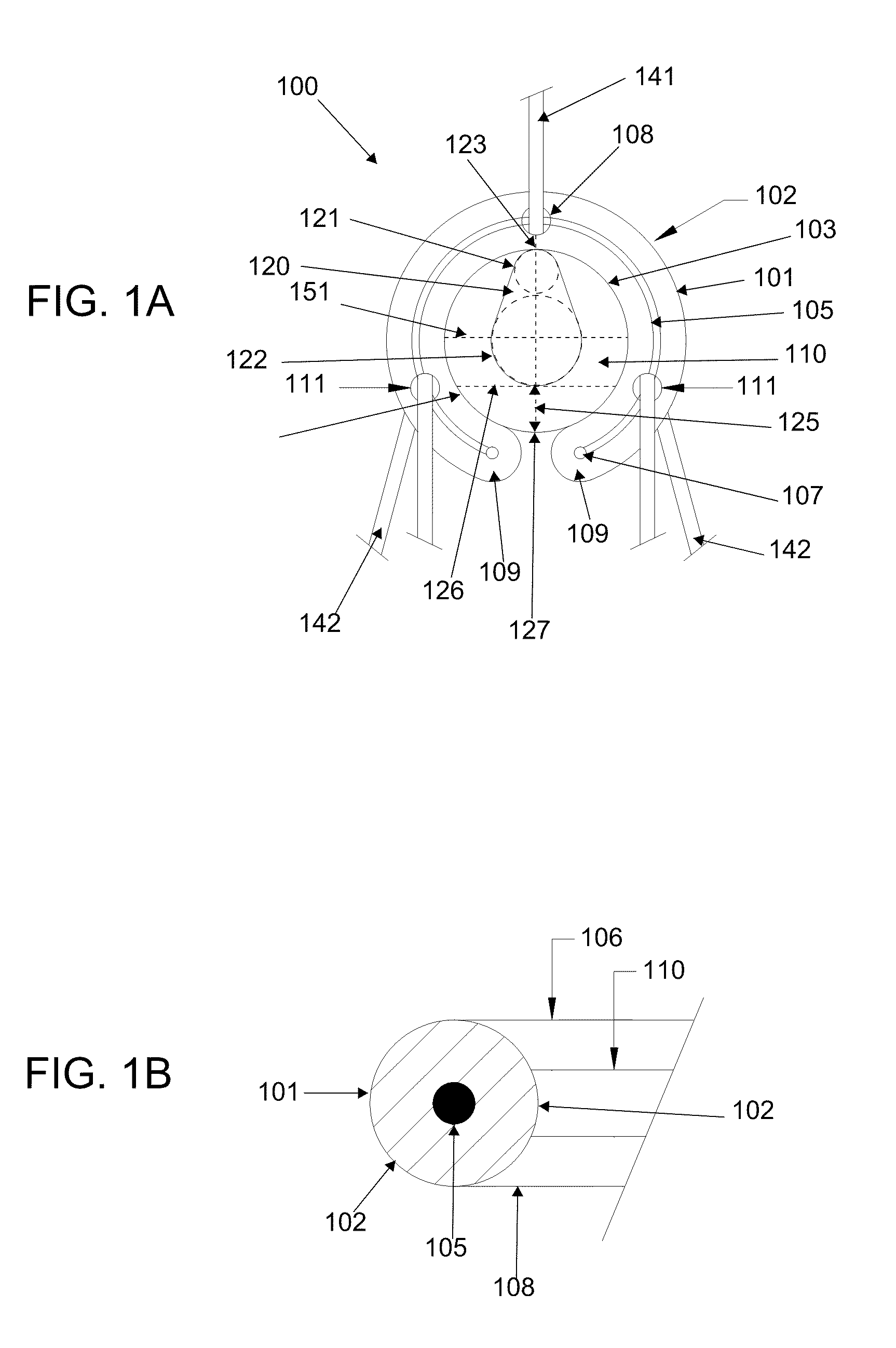
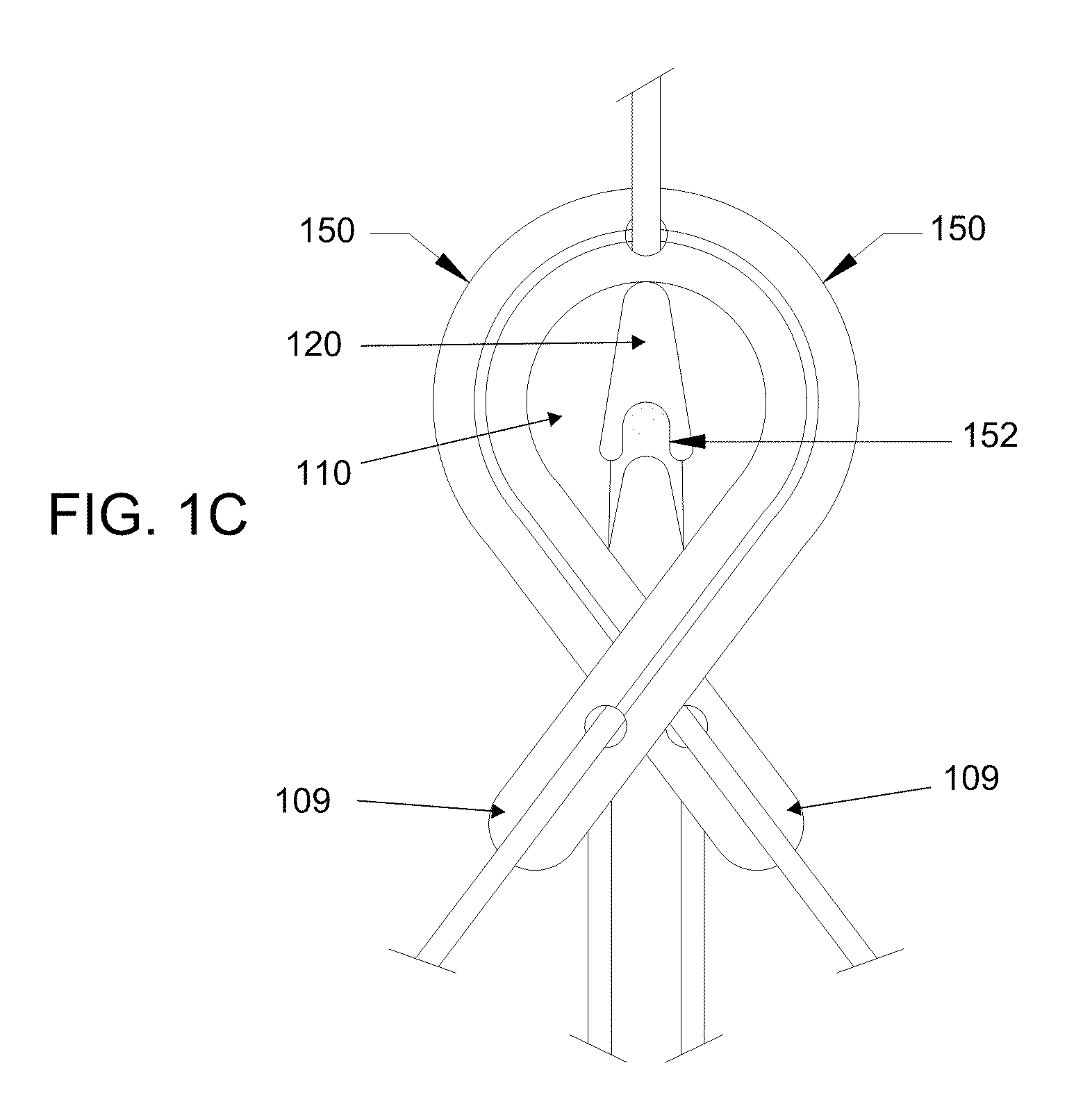
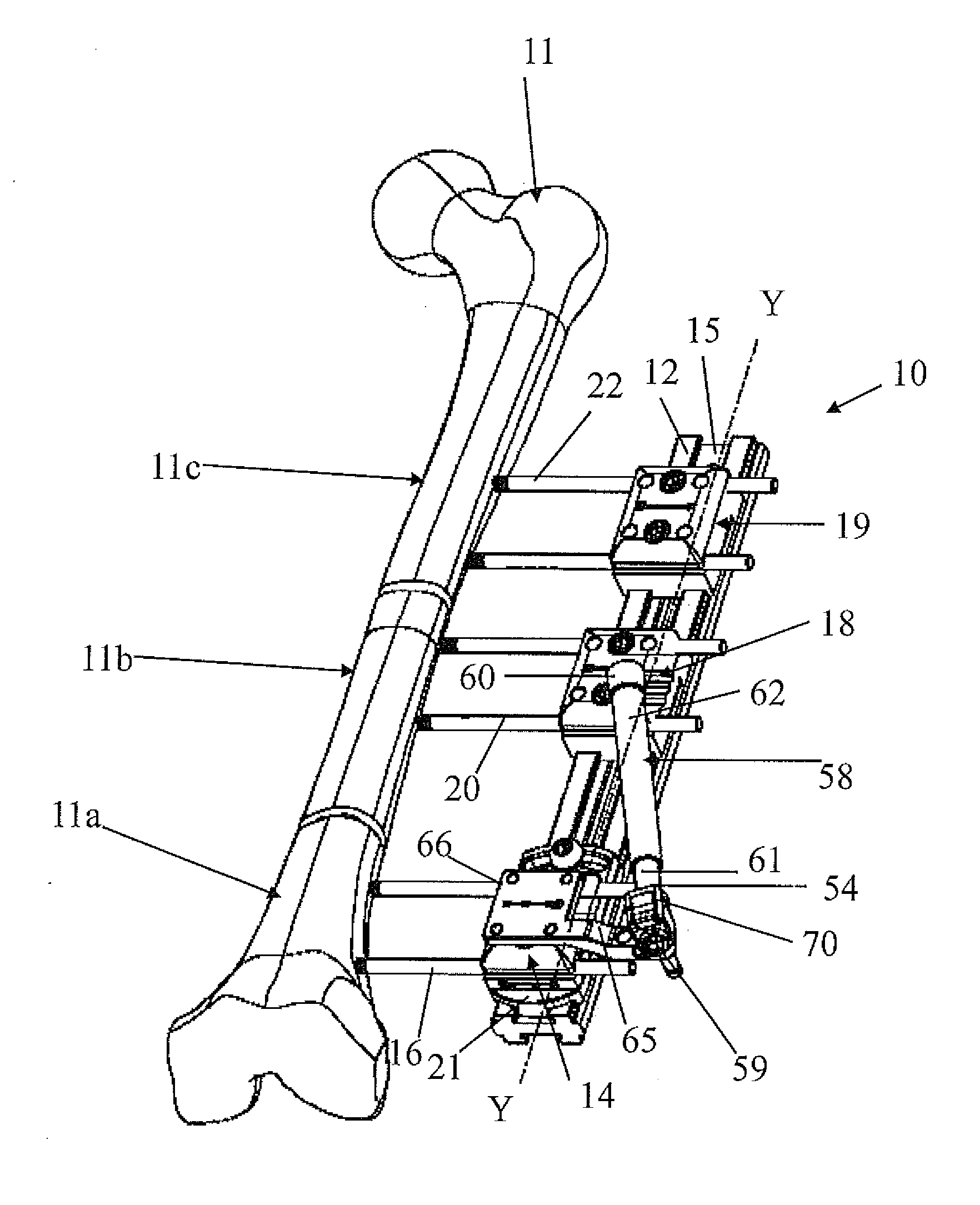
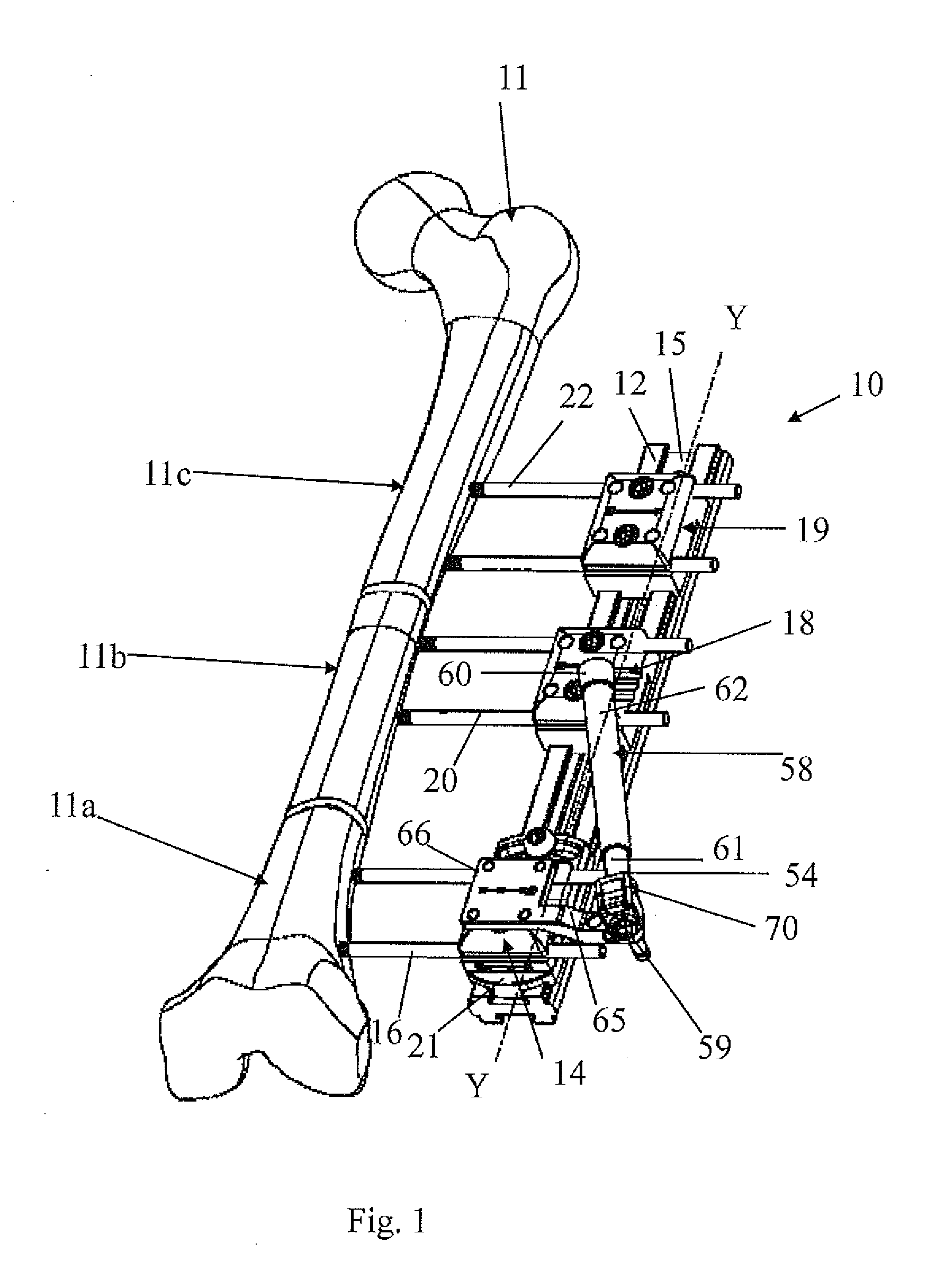
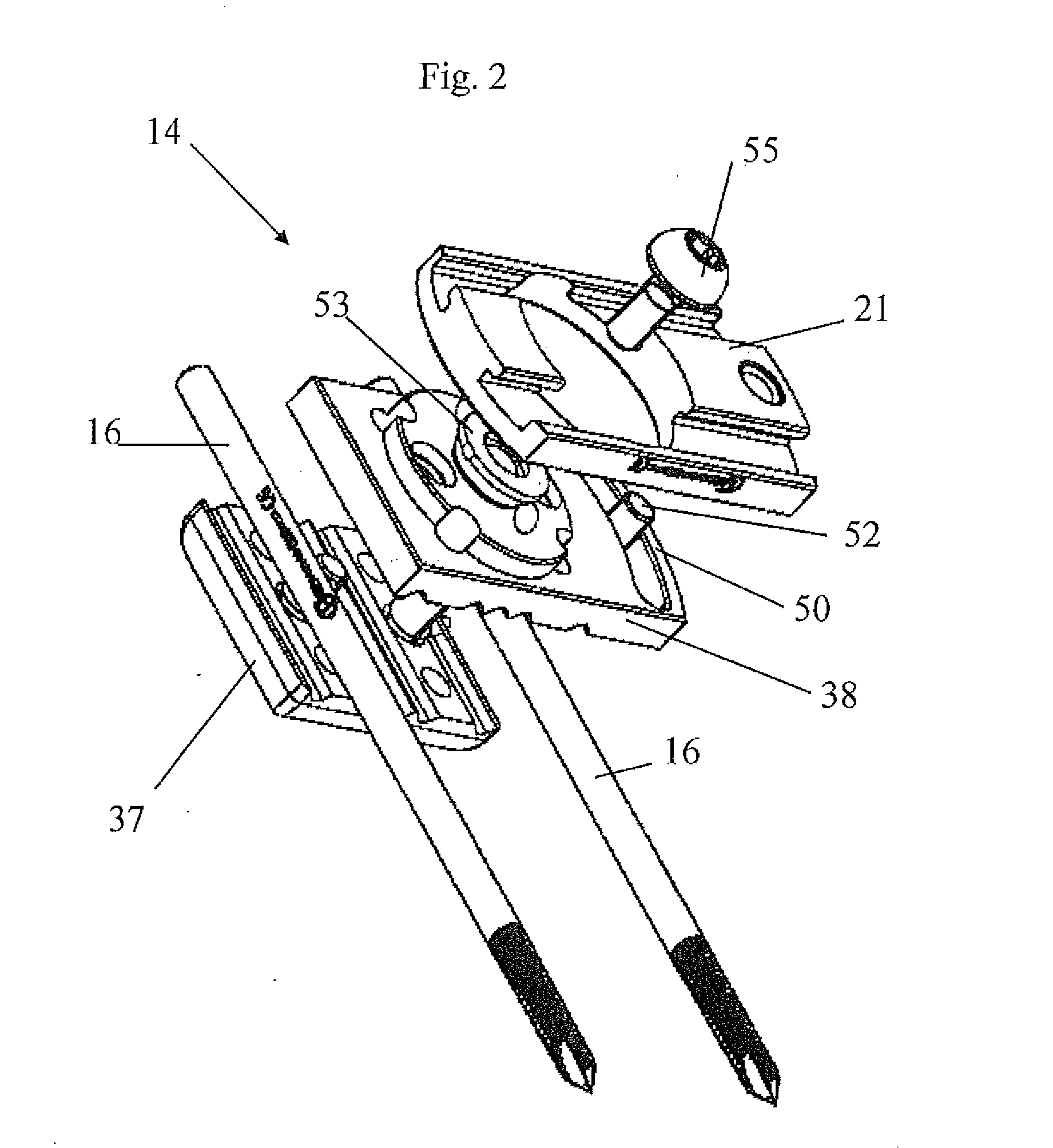
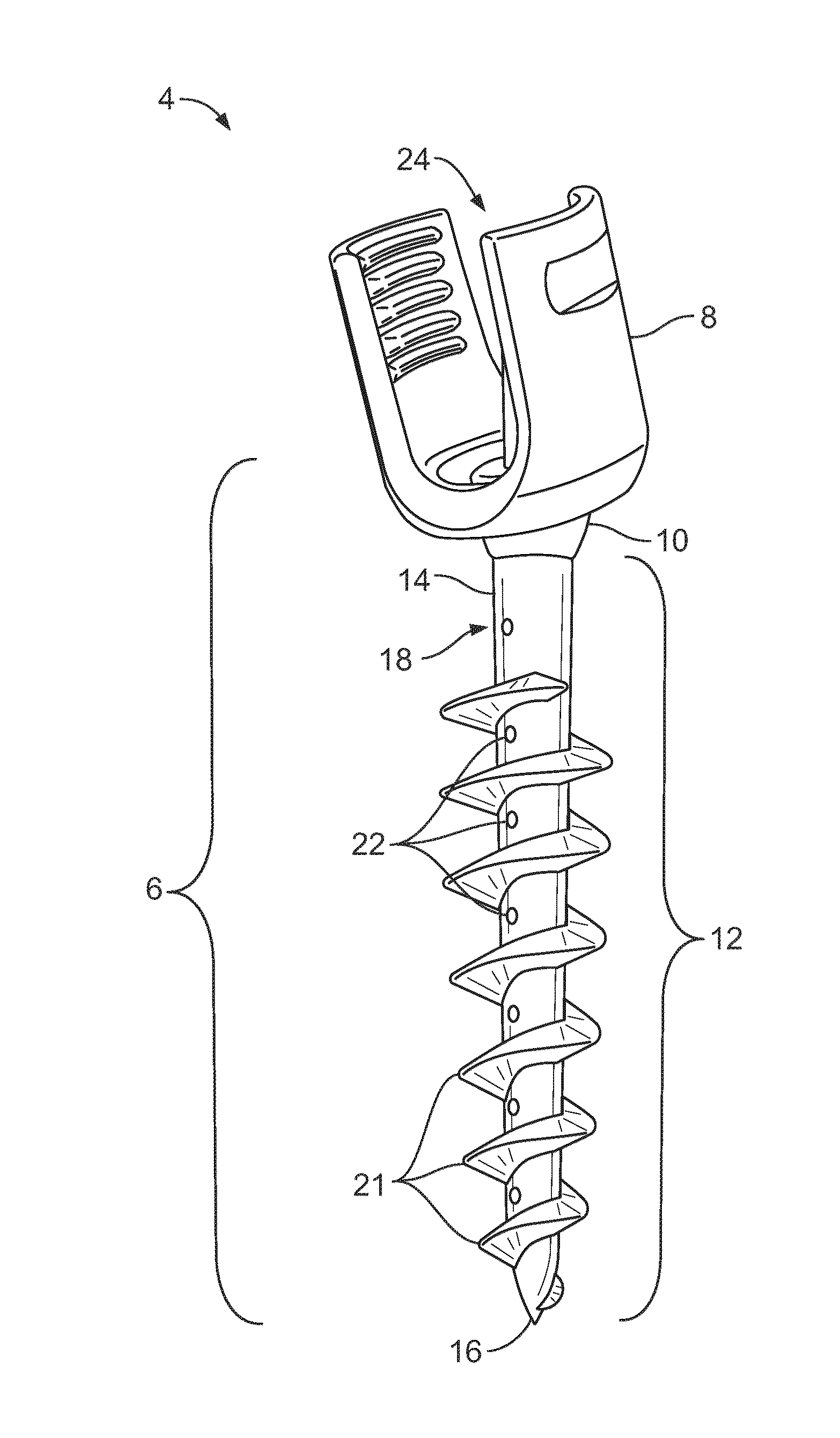
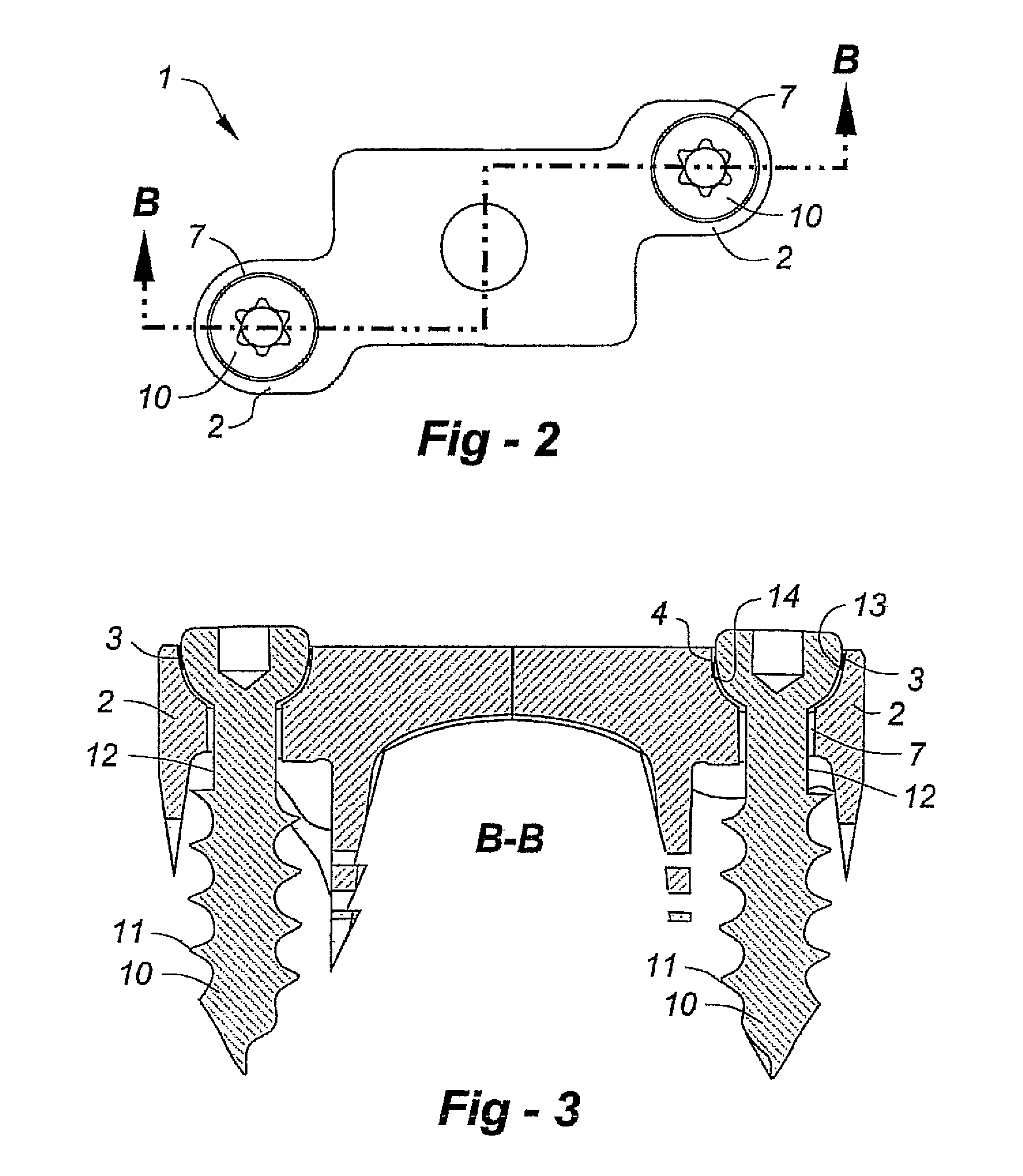
Orthopaedic device for correcting deformities of long bones
ActiveUS20110172664A1Wide angular displacement excursionOverall small sizeFractureInvalid friendly devicesOrthopaedic deviceLoose coupling
Orthopaedic device (10, 110, 210, 310) for correcting deformities of a long bone (11), comprising a bar (12), extended along an axis (Y-Y) and intended to be placed alongside the bone (11), at least a first clamp (14, 214, 314) for a first group of osseous screws (16) and a second clamp (18, 19, 118) for a second group of osseous screws (20, 22), removably mounted on said bar (12), wherein the first of said clamps (14, 214, 314) is placed onboard a support base (21, 221, 321) in turn mounted on said bar (12), and angularly movable in relation to the support base around a given rotation axis (X-X, X′-X′, Z-Z) by means of a rotary coupling, characterised in that said rotary coupling comprises a male element (35; 235, 236, 237, 238, 239; 333, 334) associated with the first clamp (14, 214, 314) and having a surface at least partially cylindrical, and a corresponding female element (36; 130; 250, 254, 255; 336, 337) associated with the support base (21, 221, 321) and having a surface at least partially cylindrical constituting a seat for the loose coupling of the male element (35; 235, 236, 237, 238, 239; 333, 334).
Owner:ORTHOFIX SRL
Knit elastic mesh loop pile fabric for orthopedic and other devices
InactiveUS20080072629A1Increase air circulationImprove moisture managementOrnamental textile articlesStraight-bar knitting machinesOrthopedic devicesMuscle tone
An improved fabric for orthopedic devices is provided, being a knitted fabric produced on either a warp or weft knit system and utilizing a filament yarn selected from nylon and polyester as well as spandex. The fabric enhances freedom of movement while stimulating blood flow and muscle tone. The fabric has an elasticity that enables closure of orthopedic and other devices by placing the hook component of a Velcro (®) strip at any convenient point rather than aligning it with the loop component of the Velcro (®). The fabric is more convenient, faster and easier for many incapacitated persons, enhancing wearing comfort. Its mesh structure makes it more porous, which enhances the air circulation and the moisture management in creating a cooling effect on the wearer's skin and ready evaporation of perspiration, while still providing therapeutic warmth without heat buildup.
Owner:GEHRING TEXTILES
Apparatus for enabling the movement of human limbs and method for using same
InactiveUS6872186B2Full normal flexionSufficient loadChiropractic devicesEye exercisersUser needsRange of motion
An orthotic apparatus for use in providing improved range of motion is provided which allows the amount of stretch to be hydraulically powered and measured by the device, but controlled by the user. Because the apparatus accurately calculates the amount of stretch, the user, together with the user's physician and therapist, can develop a rehabilitation plan based on accurate measurements. Progress is based on tangible results rather than the user's ability to tolerate pain. This knowledge provides the incentive the user needs to work toward and achieve the user's goal.
Owner:ERMI
Method and apparatus for enabling and monitoring the movement of human limbs
InactiveUS20090137369A1Full normal flexionSufficient loadChiropractic devicesNon-surgical orthopedic devicesUser needsRange of motion
An orthotic apparatus for use in providing improved range of motion is provided which allows the amount of stretch to be hydraulically powered and measured by the device, but controlled by the user. Because the apparatus accurately calculates the amount of stretch, the user, together with the user's physician and therapist, can develop a rehabilitation plan based on accurate measurements. Progress is based on tangible results rather than the user's ability to tolerate pain. This knowledge provides the incentive the user needs to work toward and achieve the user's goal.
Owner:ERMI
Orthopedic rehabilitation mechanism
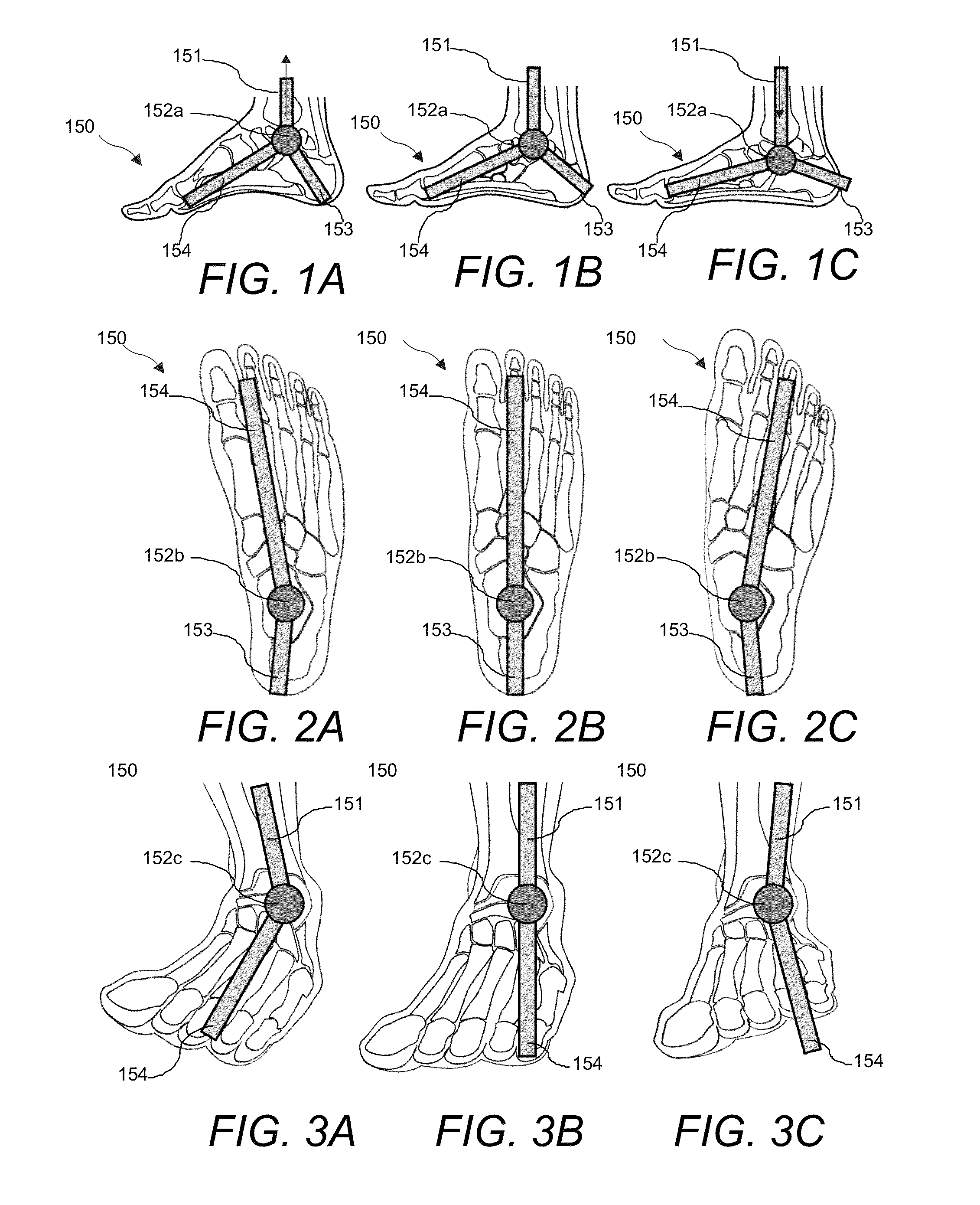
The present invention provides an orthopedic device for therapy and rehabilitation of joints to increase the range of motion of the joint, a method of manufacture therefor, and a method of operating the same. The orthopedic device, among other possible elements, may include a foot support element configured to substantially support a user's foot or ankle, at least two guide lines each having a first and a second end, wherein each of the first ends are coupleable to the foot support element. The orthopedic device, may further include a control member coupleable to each of the second ends, the control member configured to control the at least two guide lines to flex the user's foot or ankle in a plurality of directions.
Owner:RODGERS DARELL E
Segmental orthopedic device for spinal elongation and for treatment of scoliosis
ActiveUS9204908B2Prevent movementSuture equipmentsInternal osteosythesisOrthopaedic deviceSpinal column
An orthopaedic device to realign bone segments comprises first and second attachment members and a strut or spacer member. The first attachment member is attached to a first rib bone or first transverse process or first lamina of a vertebra. The second attachment member is attached to a second rib bone or second transverse process or second lamina of a vertebra. The strut is positioned between the first and second attachment members. The strut is fixedly or releasably connected to the first and second attachment members to couple the first attachment member to the second attachment member. The strut provides distraction between the first and second rib bones or transverse processes to realign the rib bones. The attachment members comprise a clamp and screw combination.
Owner:DYNAMIC SPINE
Orthopedic implant consisting of a support structure provided with at least an orifice for passing through a fixing screw associated with a nut
InactiveUS7410496B2Strengthen the mechanical connectionSimple structureSuture equipmentsLigamentsImplanted deviceEngineering
An orthopedic device having a support structure provided with at least an orifice for a fixing screw associated with a nut; the head of the screw to be pressed on one side of the support, and the nut adapted to be pressed on the other side of the support, in a housing enabling its being integrated at least partly, so as to enable the support structure to be clamped between the screw head and the nut when the screw body has been completely screwed in the receiving bone material. The implantable device includes elements for maintaining the nut in its housing opposite the orifice, and elements for locking the nut in rotation. The contours of the housing and of the nut are dimensioned to provide at least one degree of freedom to the nut in the housing enabling self-centering of the screw and nut.
Owner:DLP
Composite Metal and Bone Orthopedic Fixation Devices
InactiveUS20160000489A1Promote healingImprove bio-integrationSuture equipmentsInternal osteosythesisFiberOrthopedic devices
Composite orthopedic devices that facilitate spine stabilization, such as: bone screws, rods, plates, interbodies, and corpectomy cages are disclosed. They are designed to provide both strength and load carrying capabilities, while increasing bio-integration of the devices with the surrounding bone tissue. They are constructed of composite layers of allograft and / or autograft bone and a structural material, such as titanium alloy or carbon / graphite fiber composite. Cannulations within the device are loaded with a mixture of stem cells, particles of allograft and / or autograft bone, and bone growth factors, such as BMP-2. The cannulations are connected to the surface of the device via multiple fenestrations that provide pathways to supply the bone / stem cell mixture to the surface, allowing living bone tissue to grow and insure bio-integration. The devices can also have radiofrequency (RF) stimulation implantation within the structure of the implanted device, capable of responding to external RF stimulation of enhanced bone growth.
Owner:STC UNM
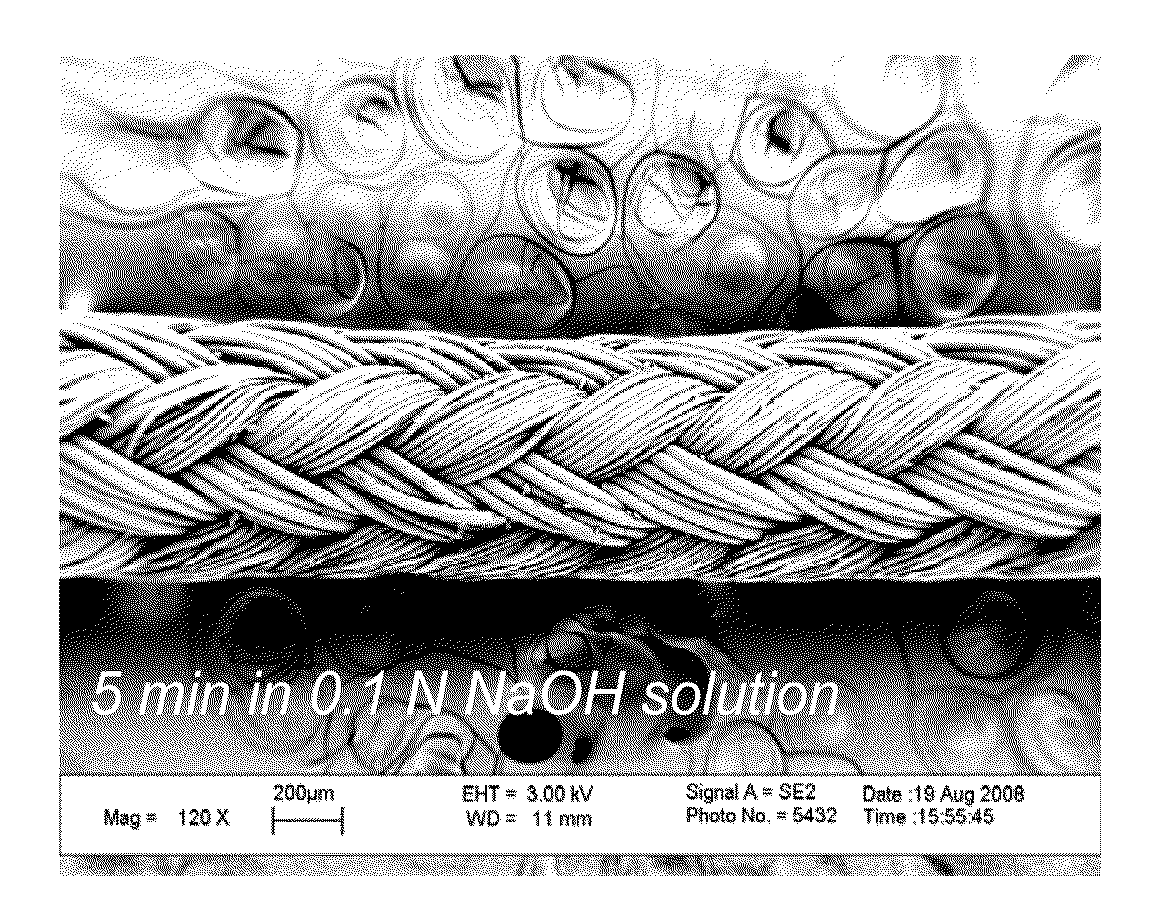
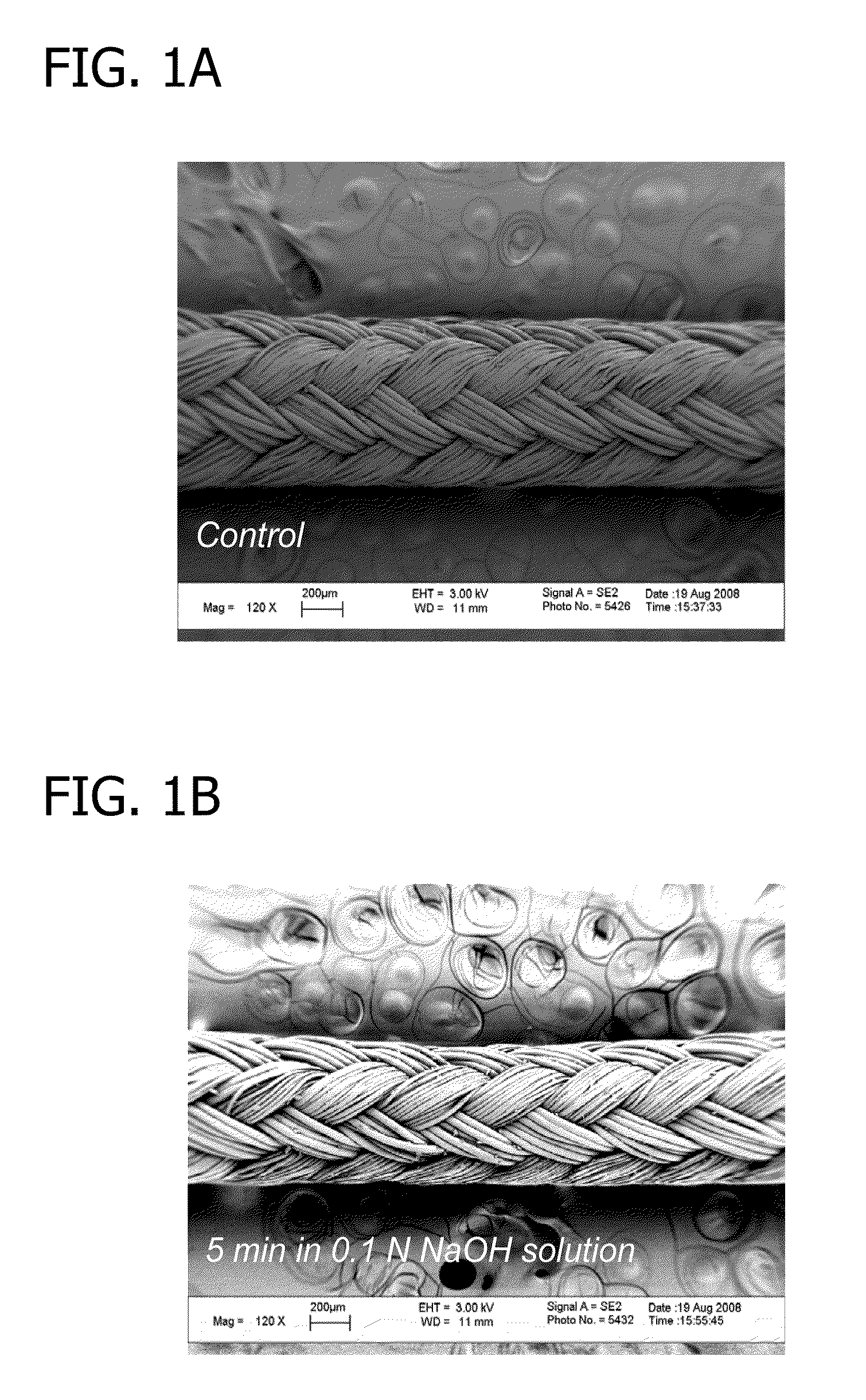
Biologically active sutures for regenerative medicine
The present disclosure generally relates to biodegradable and bioresorbable materials having a mineral layer on the surface of the material. More particularly, the disclosure relates to biodegradable and bioresorbable orthopedic devices having a degradable mineral layer on the surface thereof that can be used as a delivery vehicle for biological substances. Also provided are various methods of using the mineralized devices in tissue regeneration, including bone tissue engineering, and methods for producing the mineralized devices.
Owner:WISCONSIN ALUMNI RES FOUND
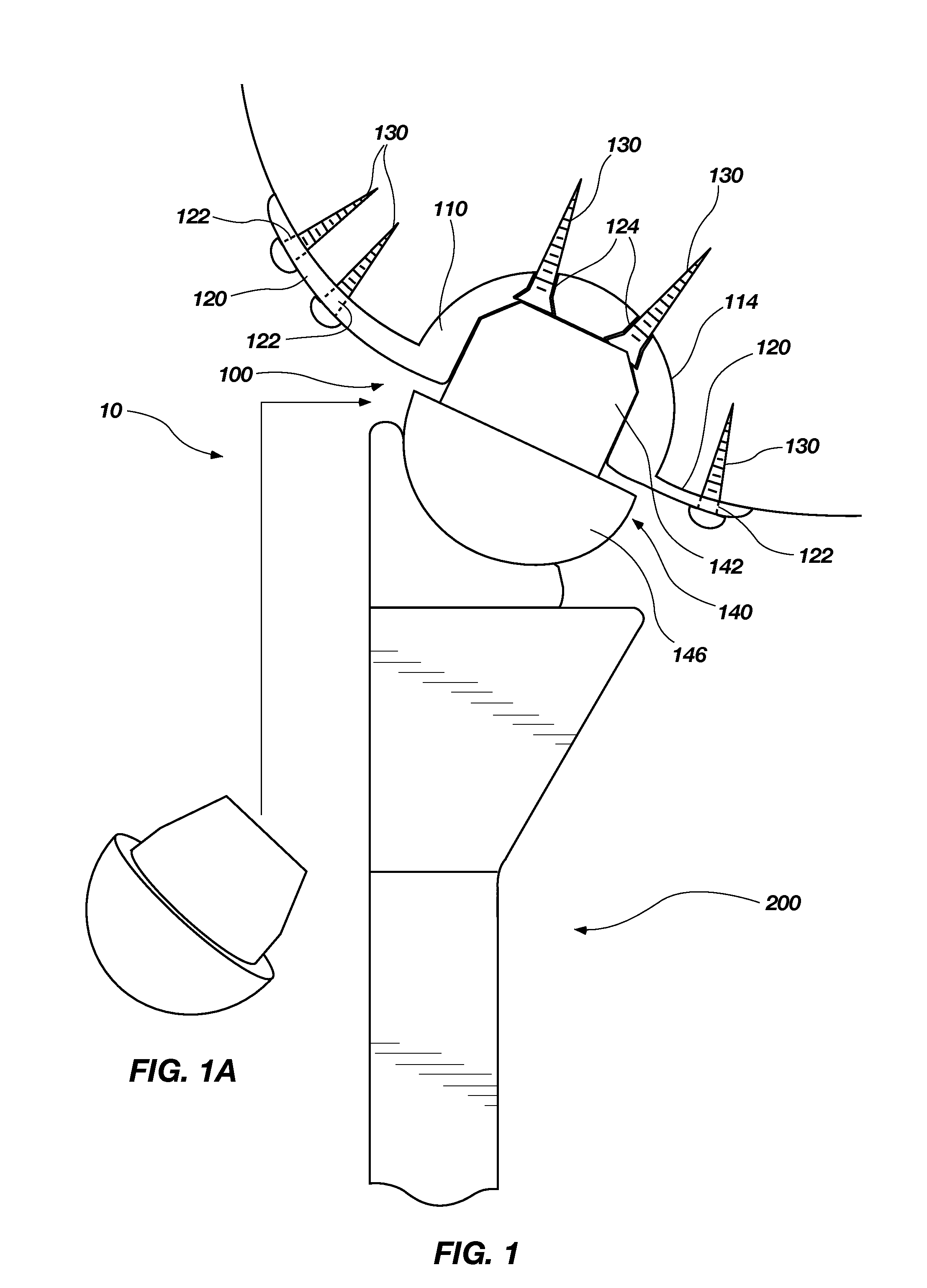
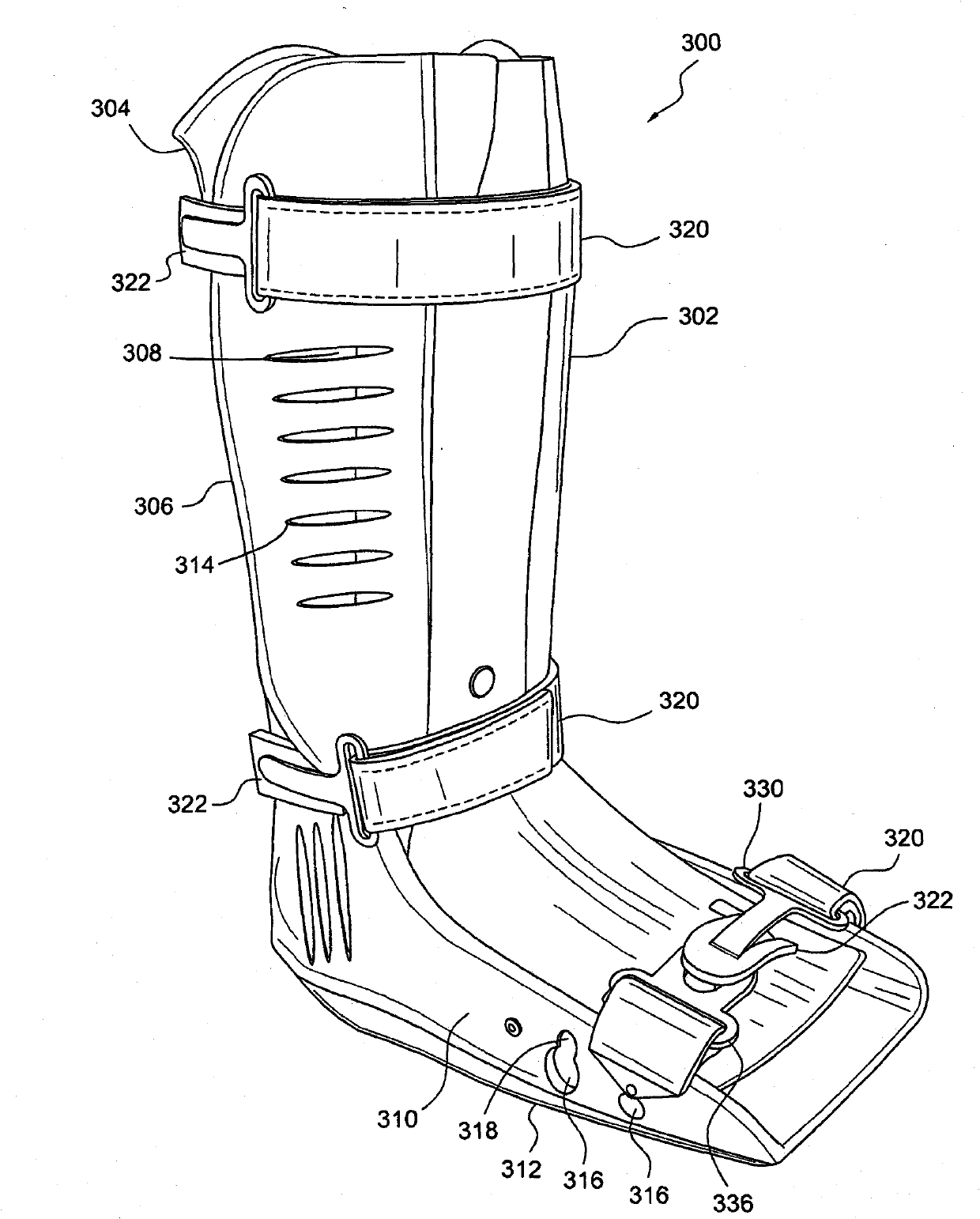
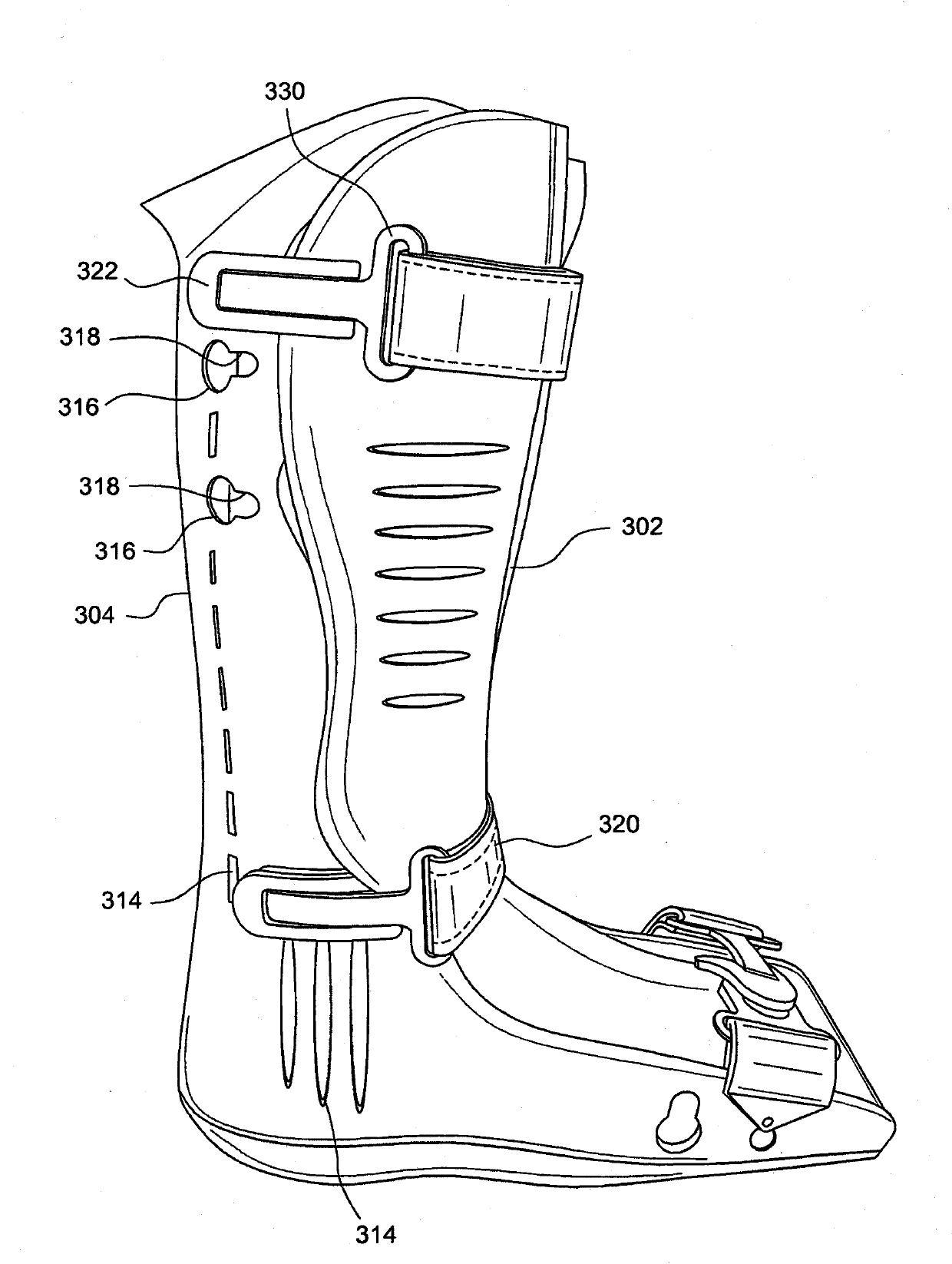
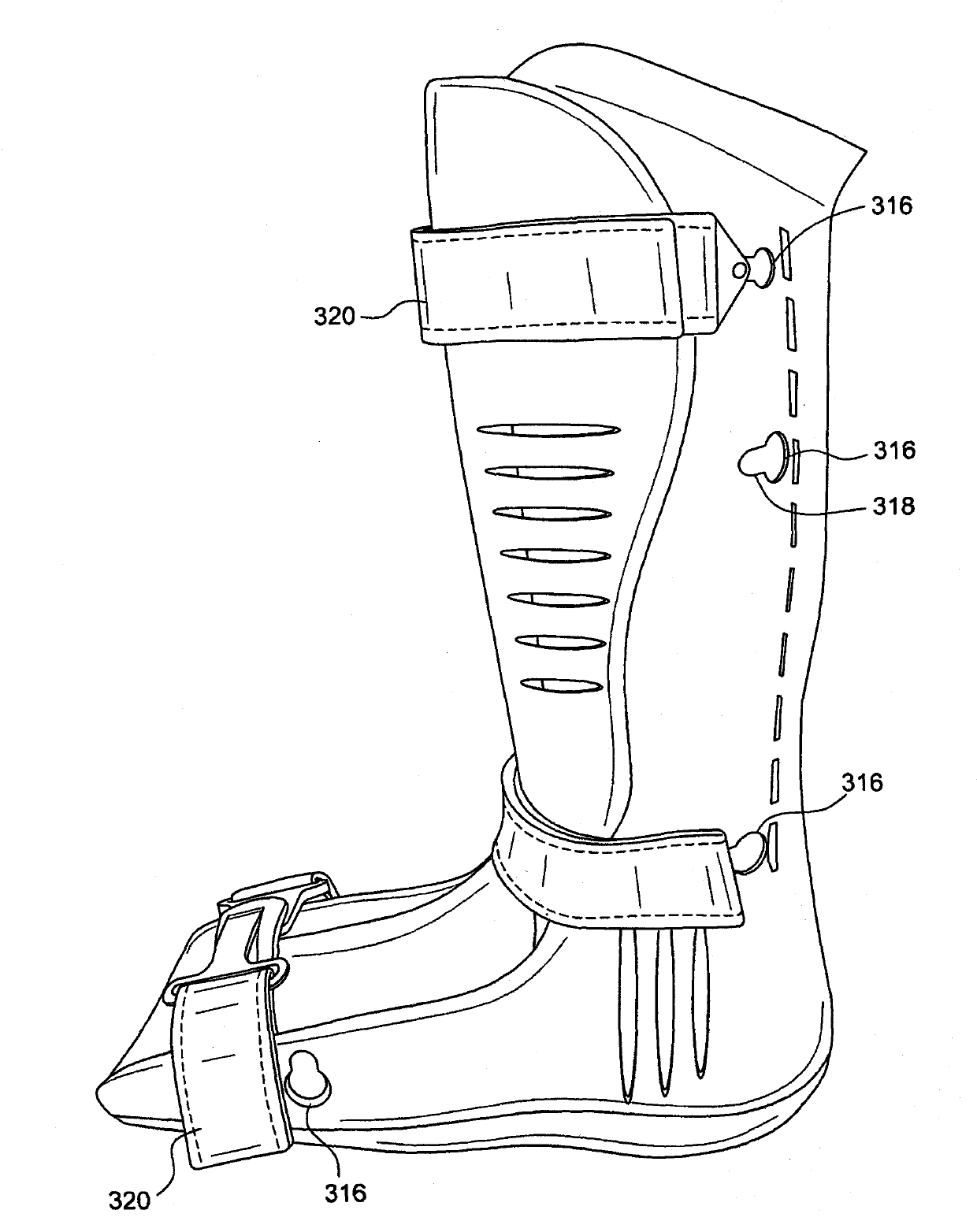
Circumferential walker
ActiveCN102026592ATime-consuming to eliminateReduce swellingNon-surgical orthopedic devicesPhysical medicine and rehabilitationExpansion joint
An orthopedic device in the form of a circumferential walker (2100) includes a first member (2120) (posterior shell) and a second member (2102) (dorsal shell) corresponding to the first member (2120). An outsole (2148) or plantar shell portion (2144) is attached to, or formed on or with the posterior shell (2120). An expansion joint (2126) is positioned in the posterior shell (2120) or dorsal shell (2102) to expand to accommodate larger sized lower legs. The dorsal and posterior shells (2102, 2120) may include flexible or resilient edge portions (1110, 2124) to reduce pressure points and to further accommodate users having larger sized lower legs. The dorsal shell (2102) also includes a flexible or resilient connecting portion (2114) to accommodate users having larger ankles and feet and to reduce or eliminate the formation of a pressure point along the dorsal shell (2102). Quick-connecting buckle assemblies (1148, 1250) can be provided to allow a user to quickly don and doff the orthopedic device.
Owner:OSSUR HF
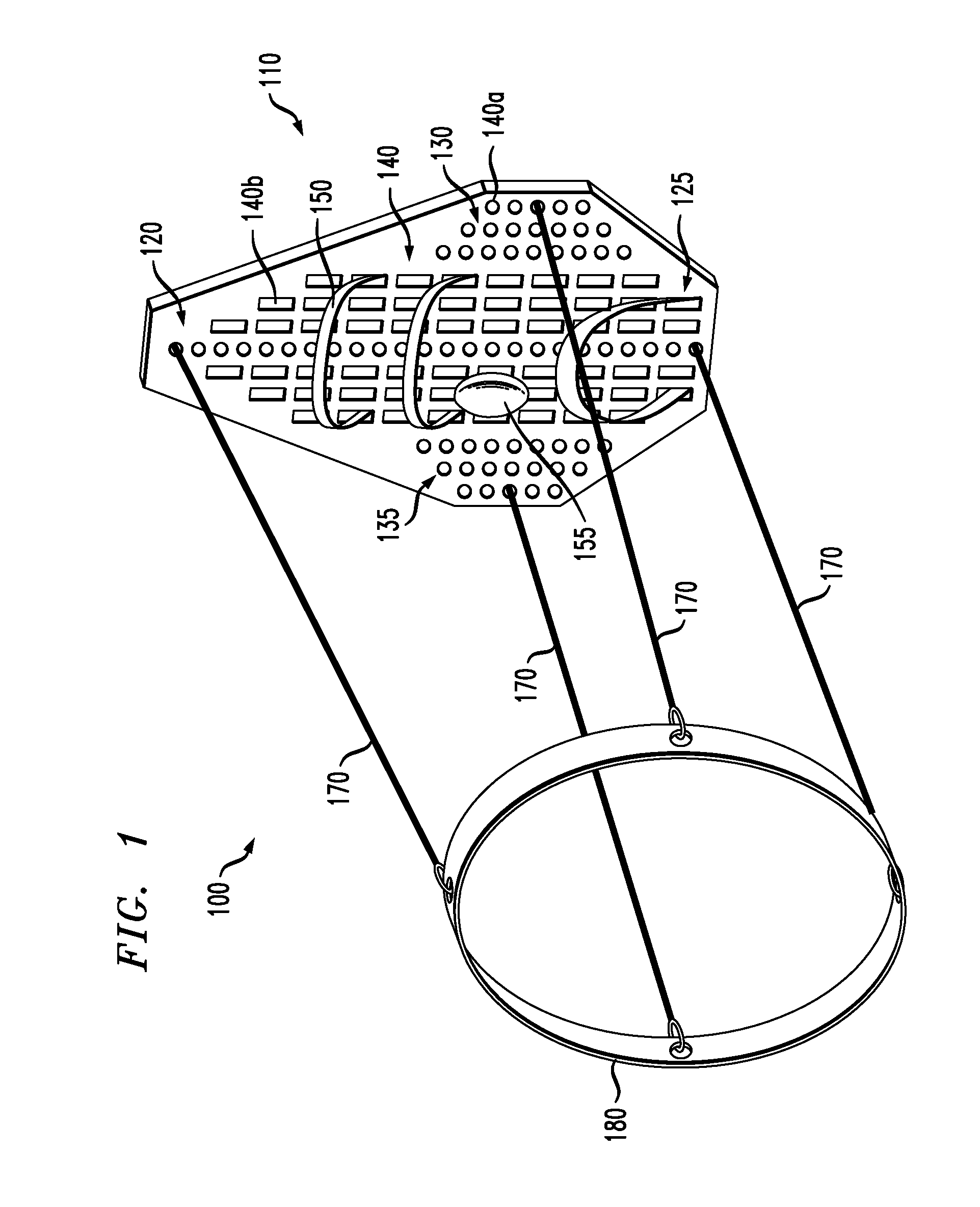
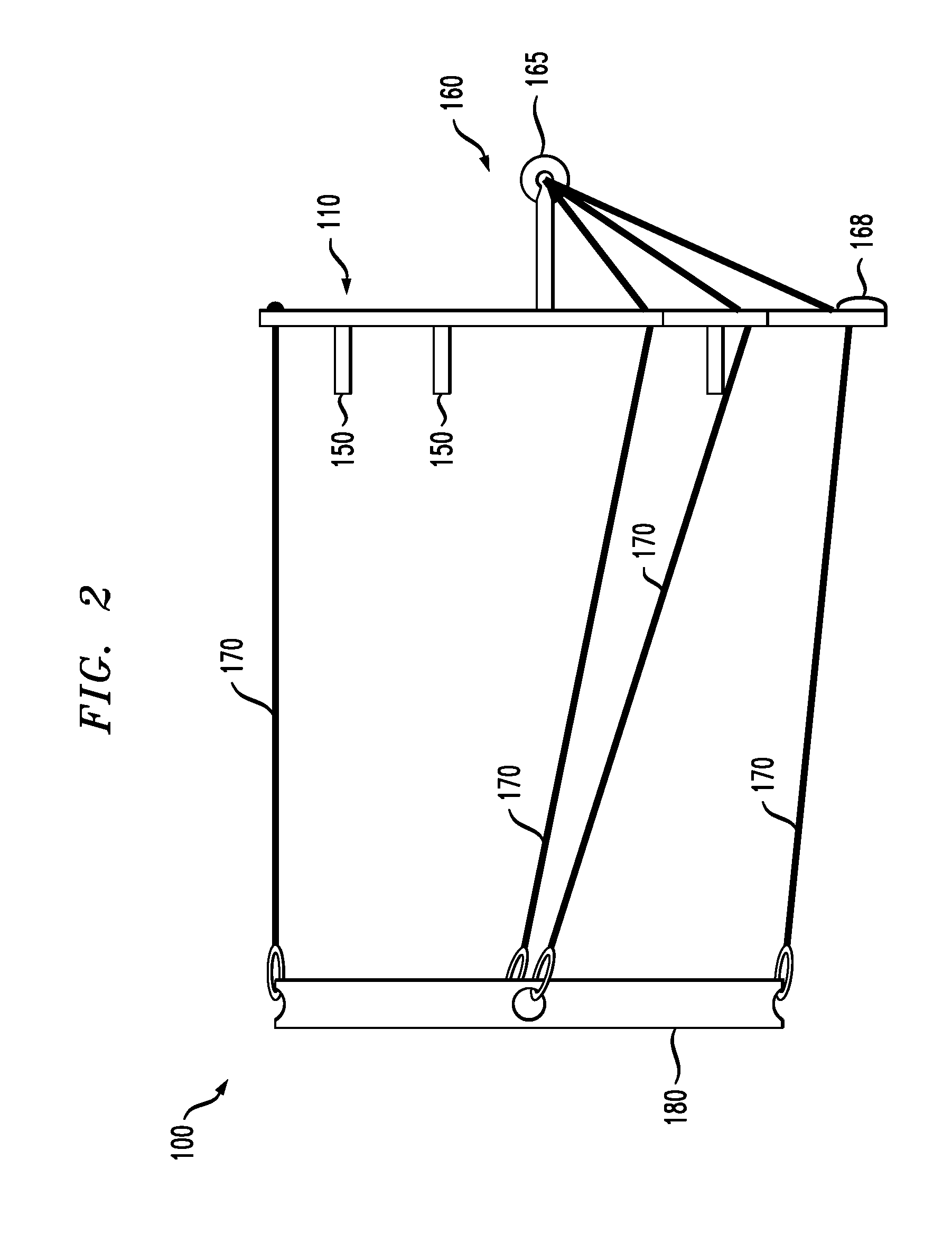
Venting devices for surgical casts and other orthopedic devices
A surgical cast venting device comprises two elongate strips or two layers of flexible material, at least one of which is porous. The strips or layers are spaced apart from each other and extend parallel to each other. Flexible, solid spacer members are arranged between the two strips or layers and connect them together. These members form air passageways between adjacent spacers so that air is normally free to pass along the passageways. A moisture-absorbent fabric layer extends along an outer side of the porous strip or layer opposite the spacers. In an alternate version, there is a first strip which is porous and a series of substantially flat, flexible outer members arranged in a row from one end of the strip to an opposite end. The outer members are spaced from the strip and opposite side edges of the strip and of the outer members are aligned with one another.
Owner:BARBERIO ALESSANDRO
Article of footwear with embedded orthotic devices
InactiveUS8640363B2Enhance comfort and fit and utilityMovement of footSolesNon-surgical orthopedic devicesCalcaneusFoot region
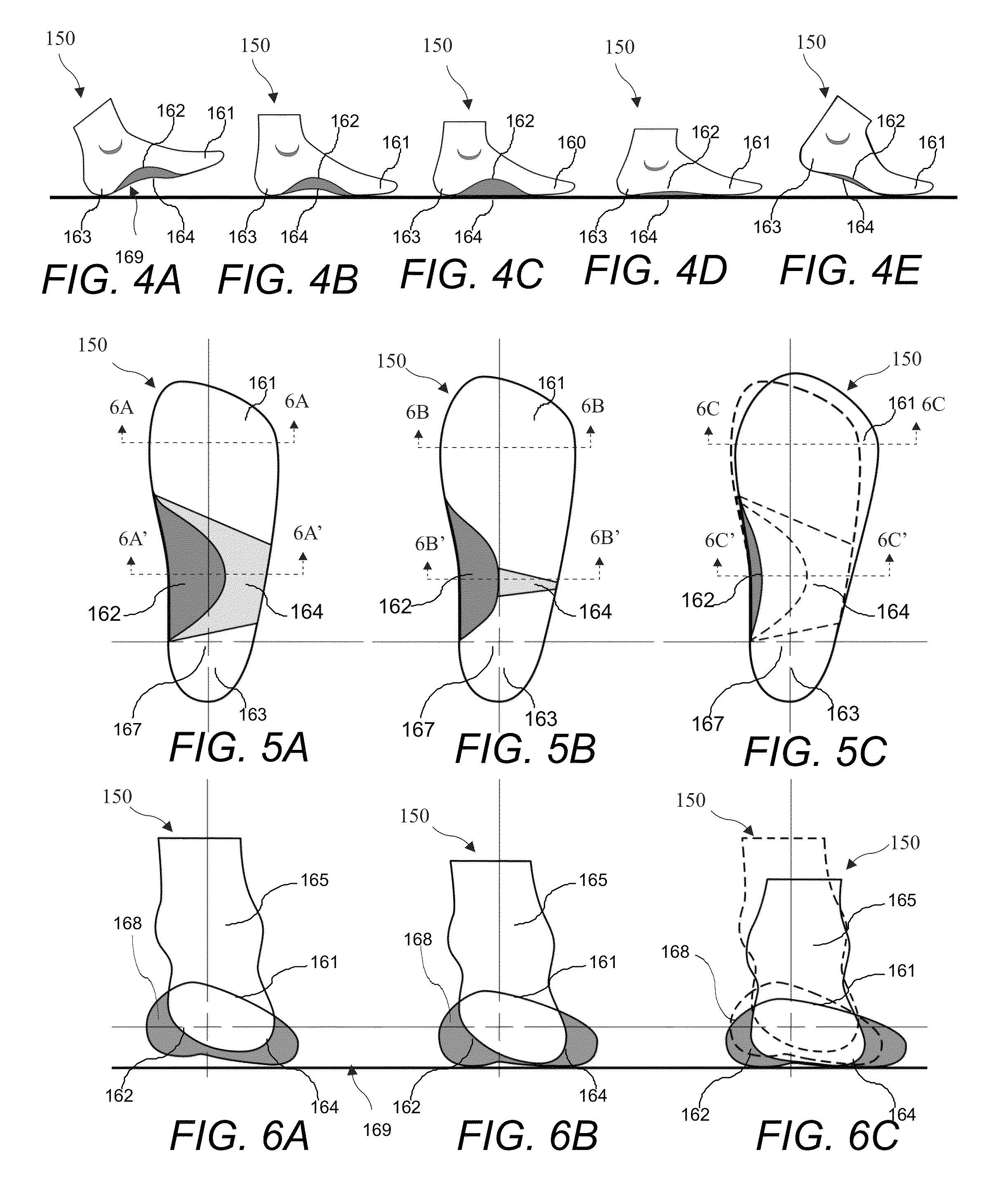
Footwear including an integrated orthotics system provides support with progressive resistance in pronation and supenation motions of the foot during a gait cycle. The orthotics system includes an orthotic device between the mid-sole and the outsole and extend from the rear foot's calcaneus region to the forefoot region where the phalanges and metatarsal joints meets. The orthotic device includes contours mimicking a foot sole shape in an unloaded state. The orthotic system may also include a secondary external orthotic device embedded underneath the outsole to provide control in the mid foot region to achieve progressive compression resistance in the mid foot arch zones. The secondary device may be customizable to achieve a wide range of resistance level needed in mid foot compression by the wearer. The orthotics combined with the midsole and outsole provide progressive mid foot compression resistance and directional flex associated with pronatory motions of the foot.
Owner:HSU HENRY
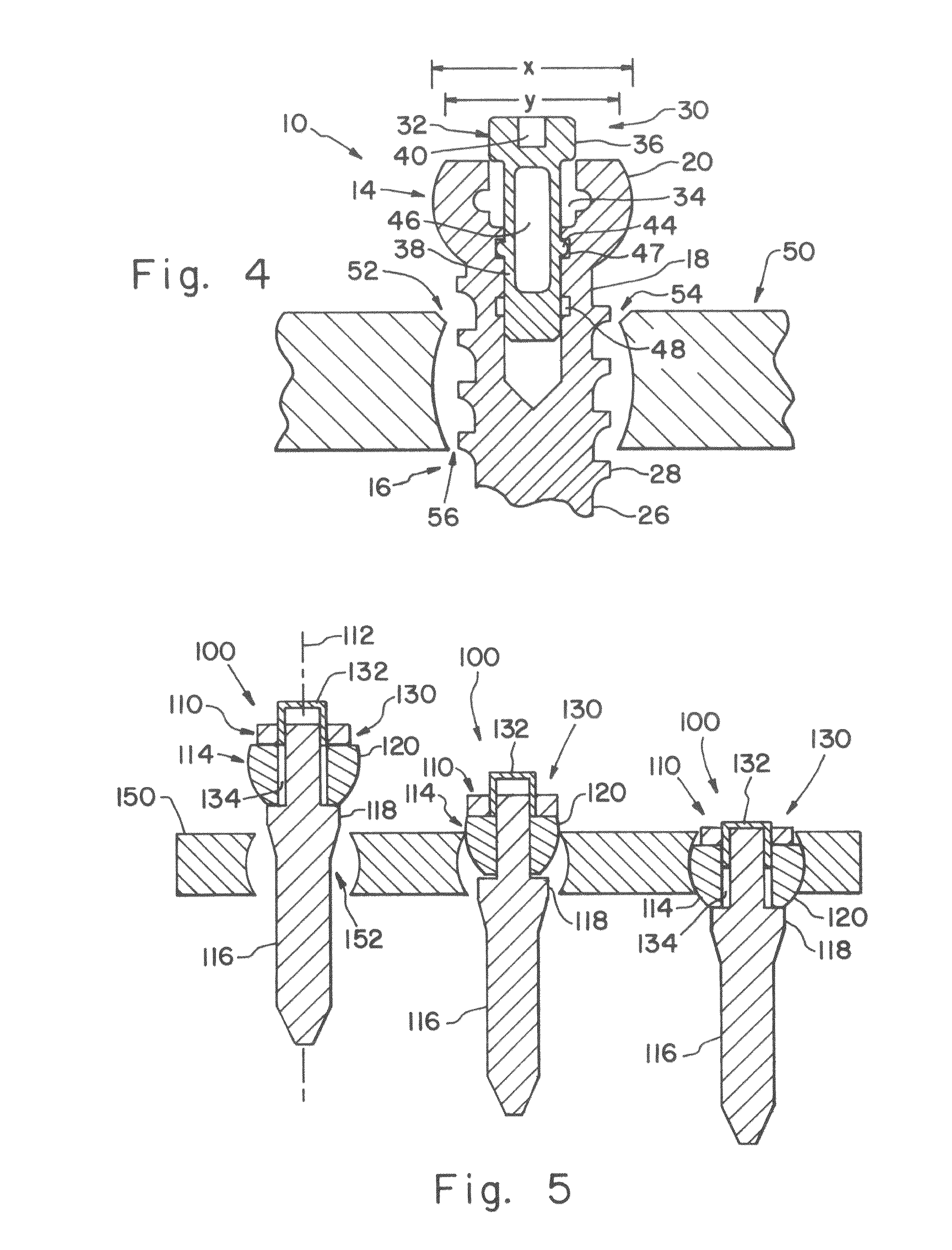
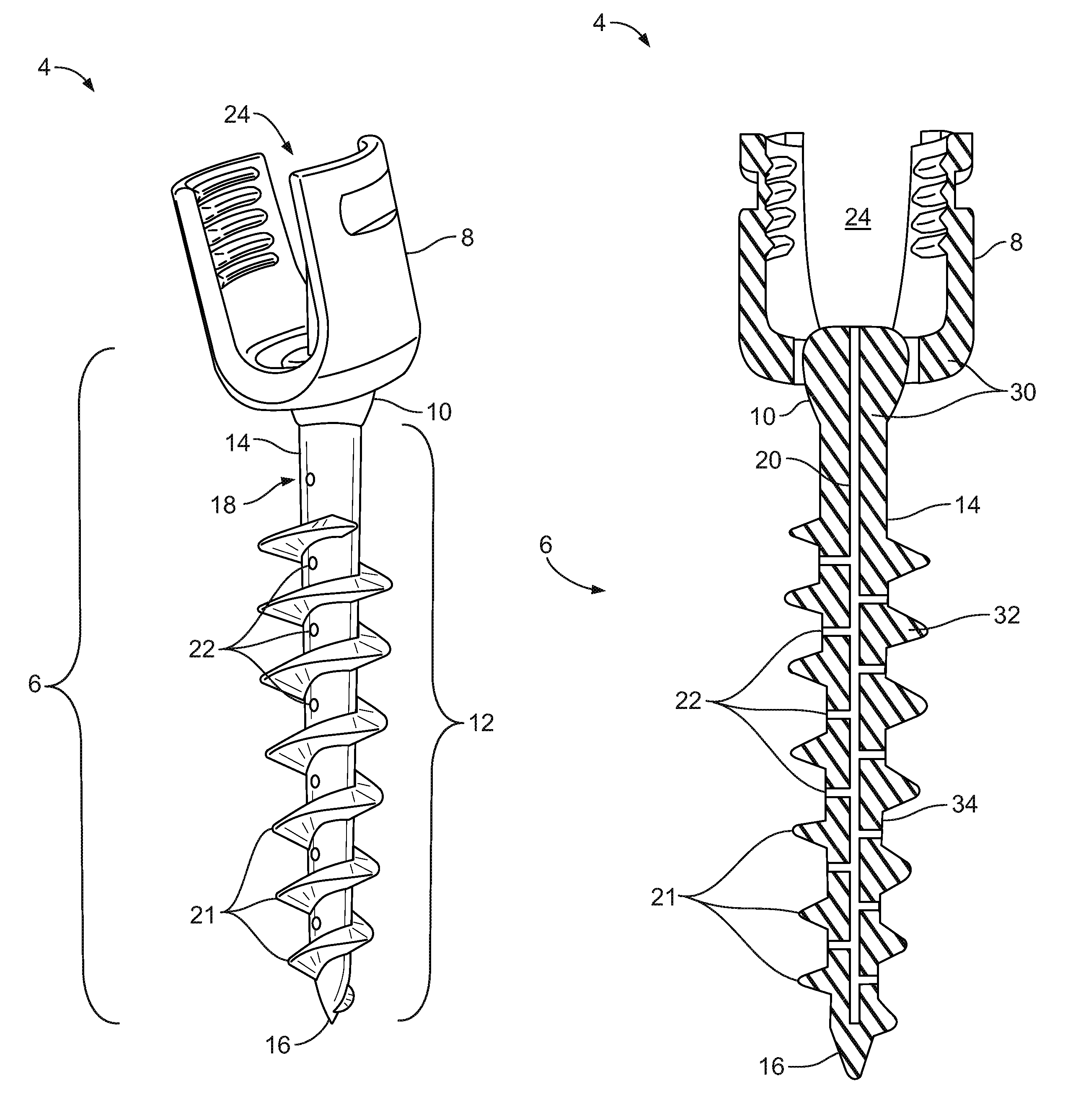
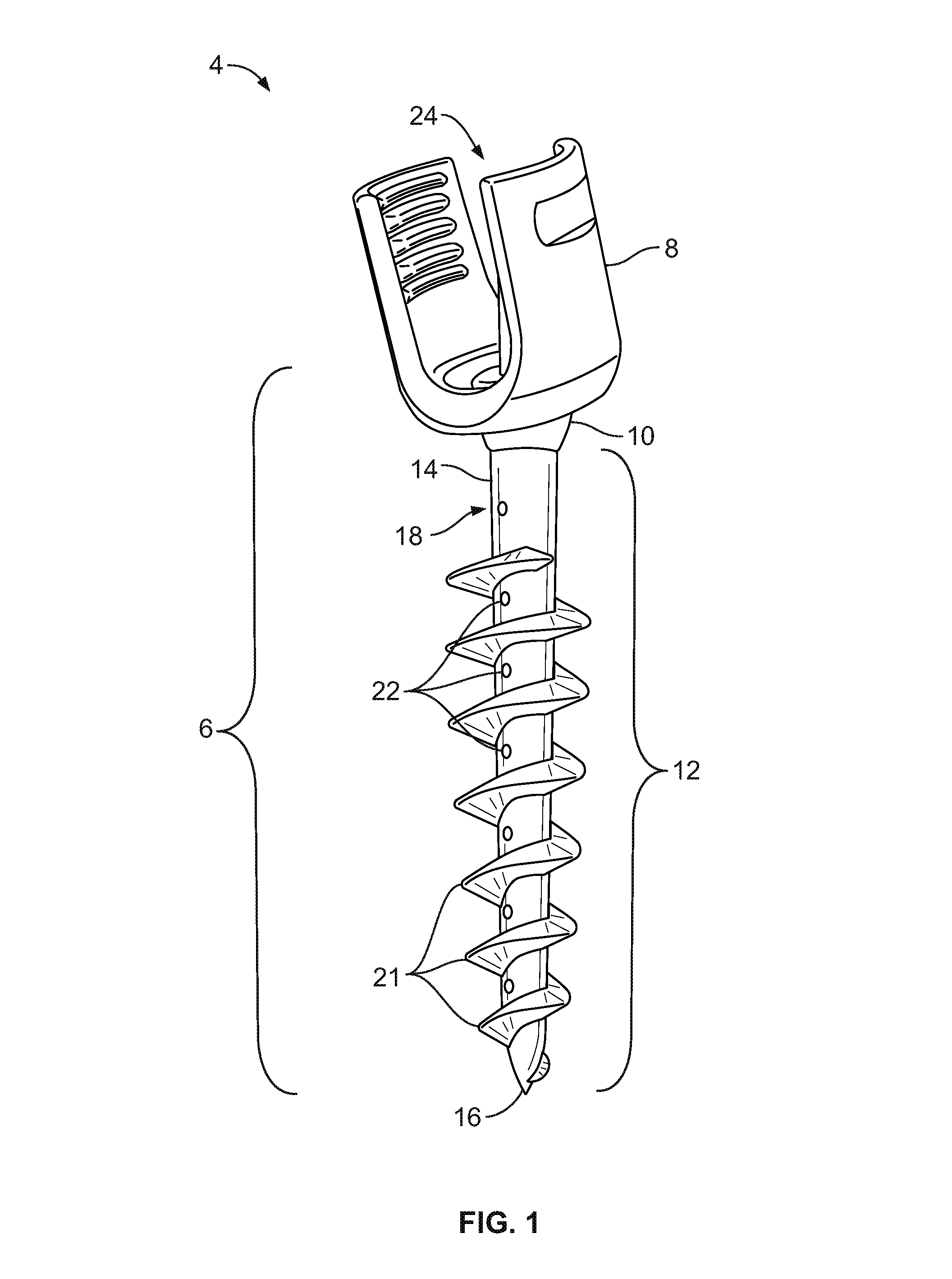
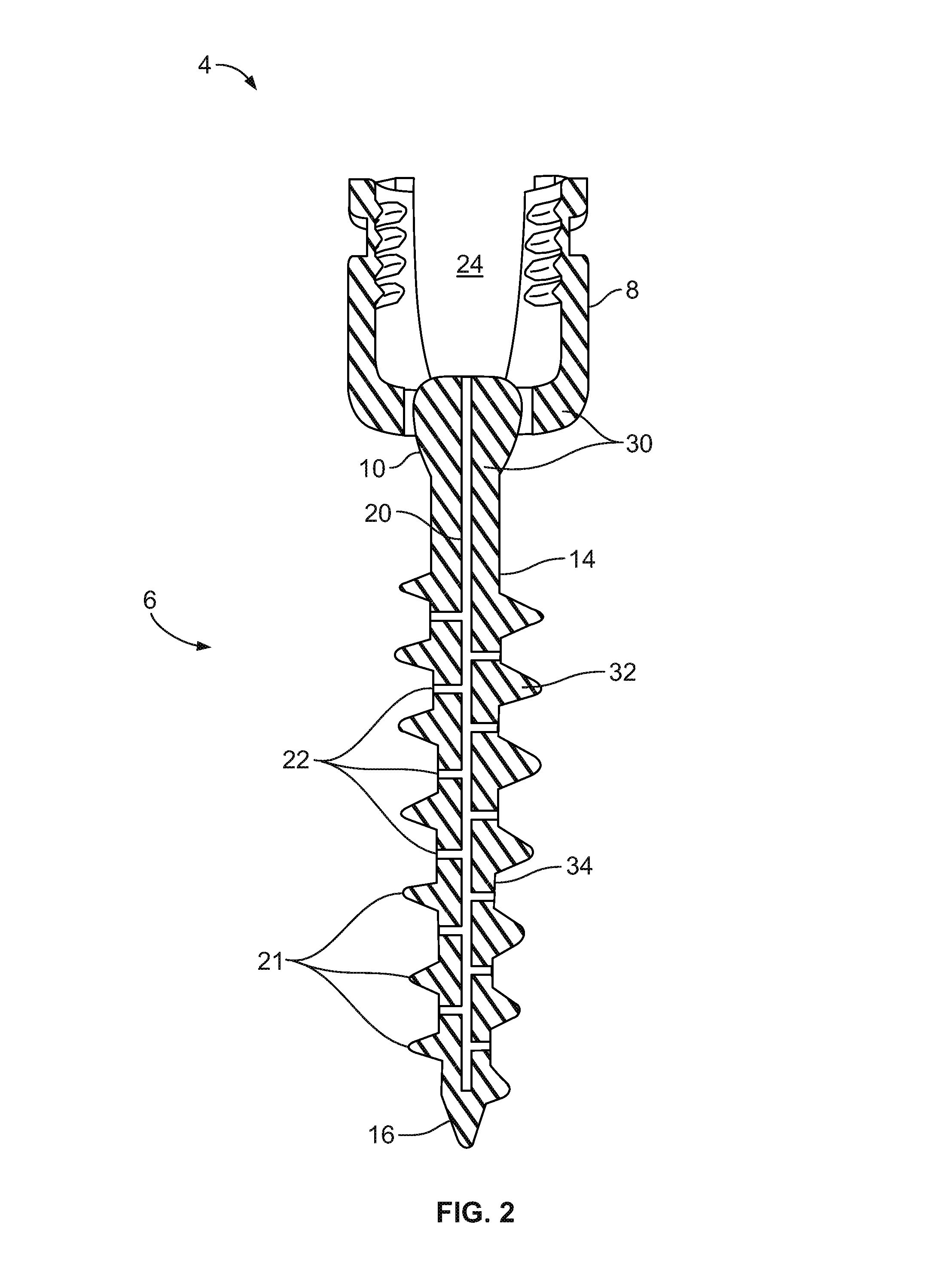
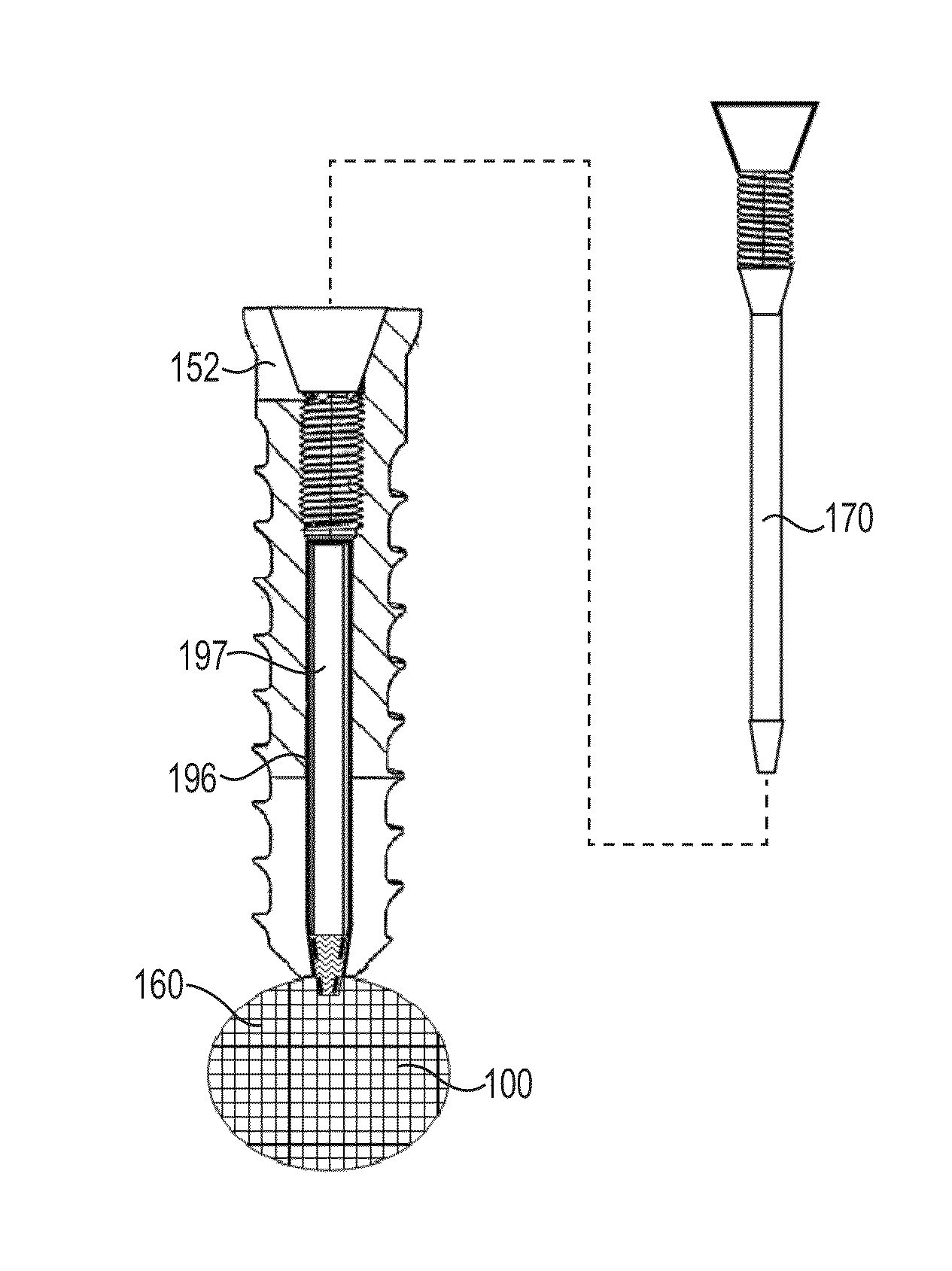
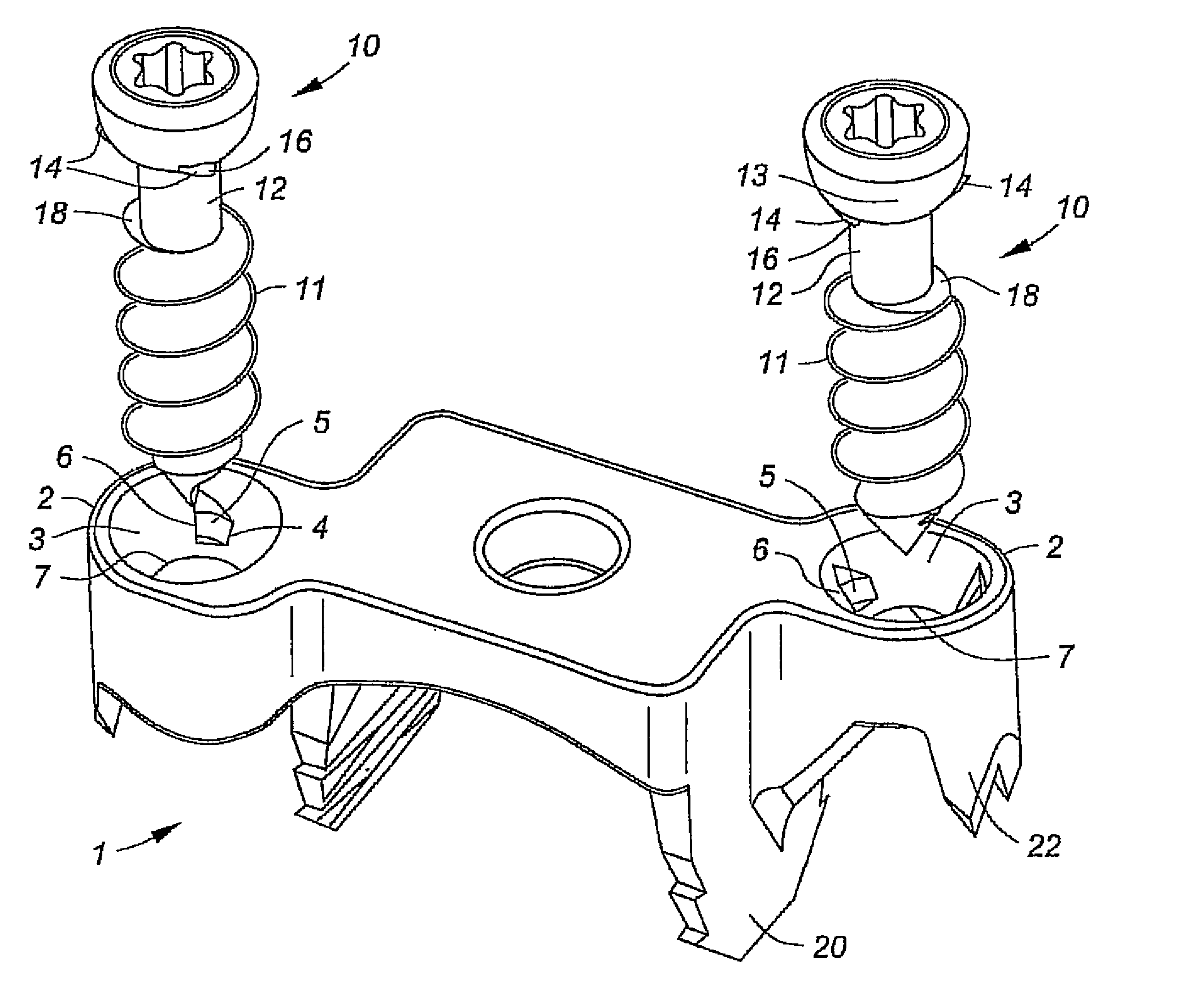
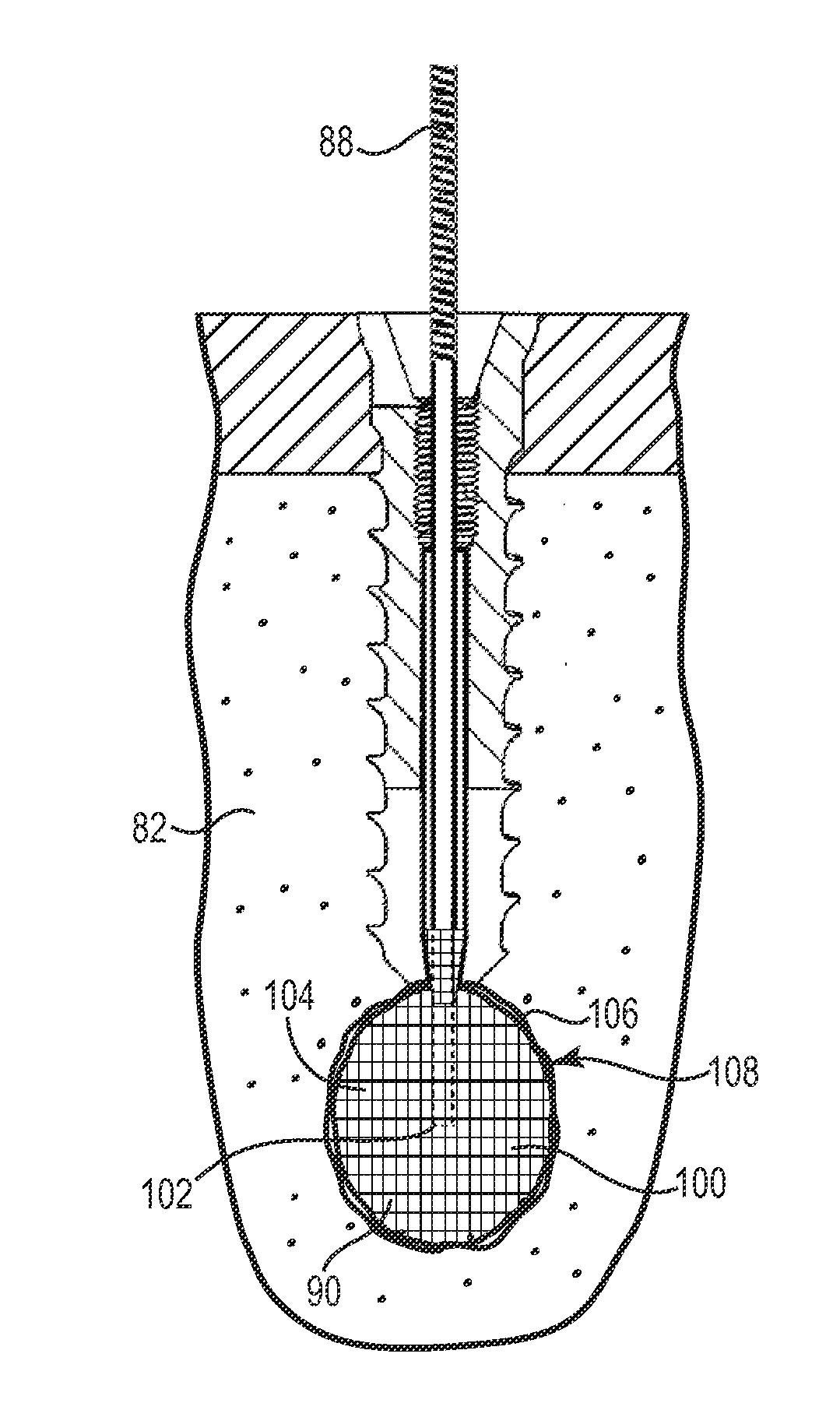
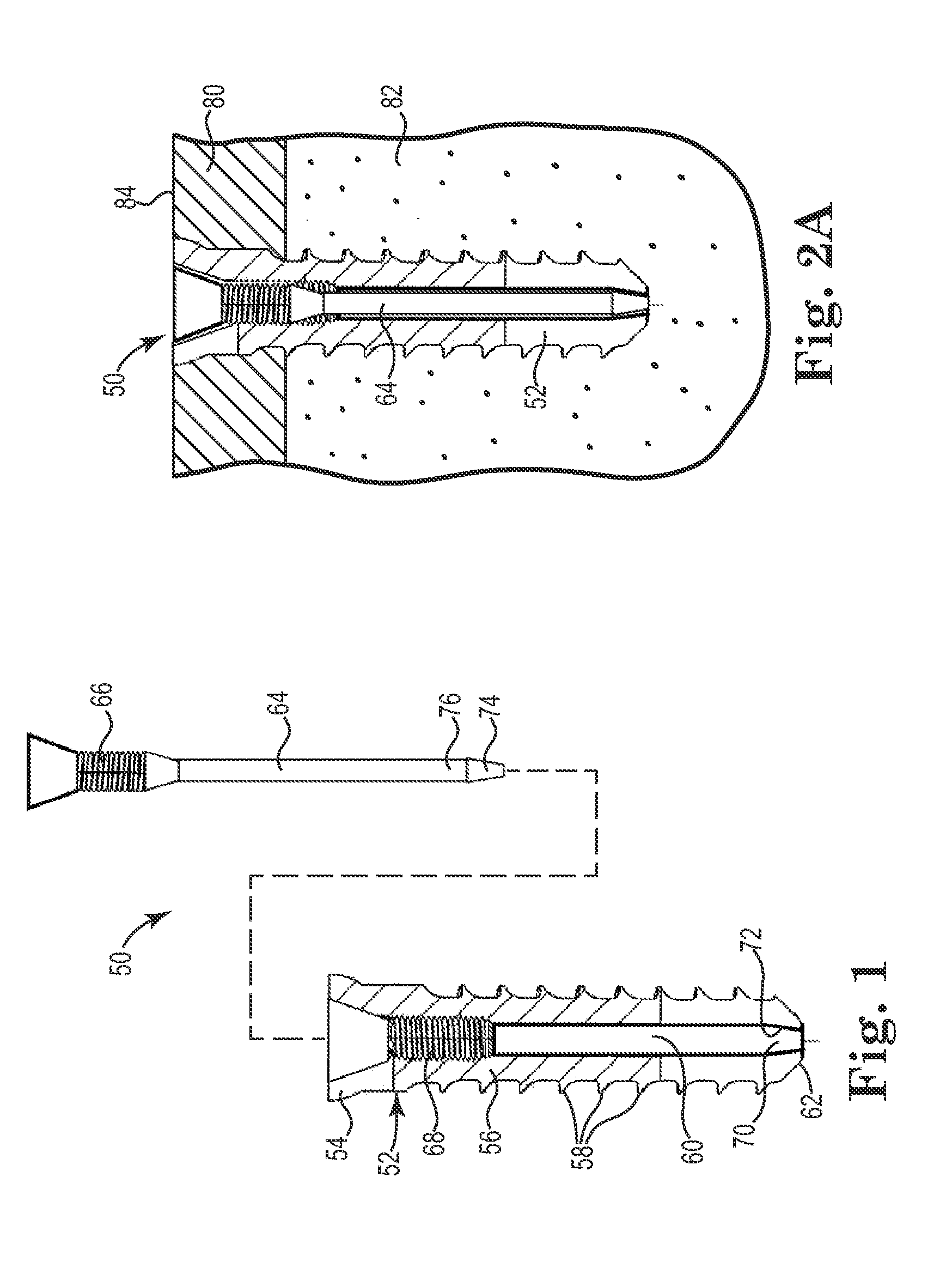
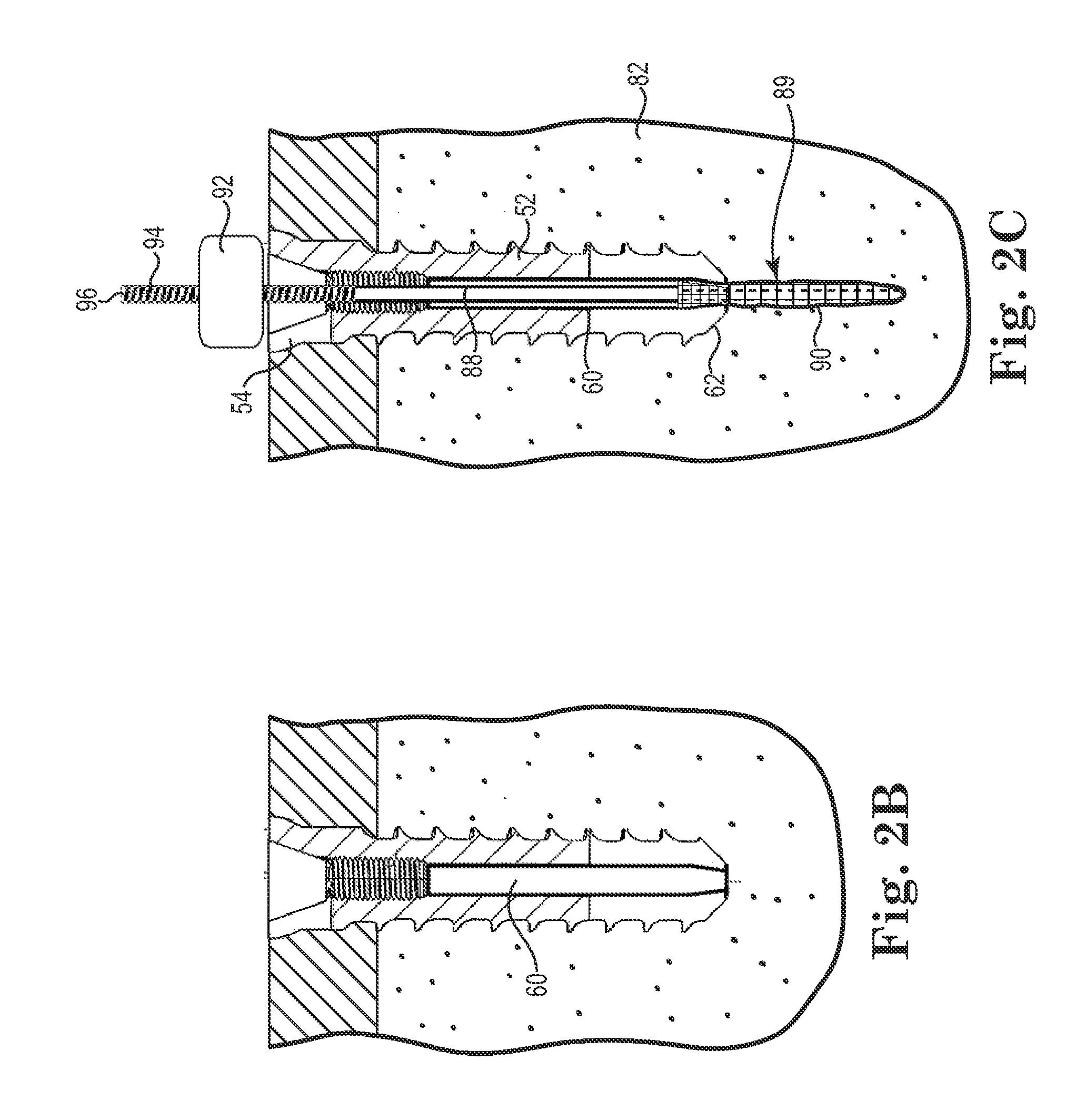
Fixation system for orthopedic devices
InactiveUS8998925B2Increase flexibilityIncreased pull-out strengthSuture equipmentsInternal osteosythesisOrthopaedic deviceOrthopedic devices
A fixation system configured to releasably secure an orthopedic implant to a bone. The orthopedic implant has at least one lumen extending from a proximal portion to a distal portion configured to extend through cortical portions and into cancellous portions of the bone. The fixation system includes a flowable biomaterial that flows through the lumen into the cancellous portion of the bone in an expanded configuration with at least one dimension greater than a corresponding dimension on the orthopedic implant located generally along a pull-out direction of the orthopedic implant. An insert is positioned in the lumen and in engagement with the flowable biomaterial located in the cancellous portion of the bone, such that the orthopedic implant is detachable from the biomaterial in the cancellous portion of the bone to facilitate subsequent removal of the orthopedic implant from the bone.
Owner:RDC HLDG
Article of Footwear with Embedded Orthotic Devices
Footwear including an integrated orthotics system provides support with progressive resistance in pronation and supenation motions of the foot during a gait cycle. The orthotics system includes an orthotic device between the mid-sole and the outsole and extend from the rear foot's calcaneus region to the forefoot region where the phalanges and metatarsal joints meets. The orthotic device includes contours mimicking a foot sole shape in an unloaded state. The orthotic system may also include a secondary external orthotic device embedded underneath the outsole to provide control in the mid foot region to achieve progressive compression resistance in the mid foot arch zones. The secondary device may be customizable to achieve a wide range of resistance level needed in mid foot compression by the wearer. The orthotics combined with the midsole and outsole provide progressive mid foot compression resistance and directional flex associated with pronatory motions of the foot.
Owner:HSU HENRY
Orthopaedic device and method of use for treating bone fractures
InactiveUS20130310628A1Precise positioningElectrotherapyTourniquetsOrthopaedic devicePlastic surgery
Orthopaedic device and a method of use thereof are provided for treating a bone fracture. The orthopaedic device has at least one holder configured to operably attach to an external fixation device and at least one pressure applying element configured to apply pressure to a soft tissue adjacent to the bone fracture.
Owner:CHISENA ERNEST C +1
Modular head inserter
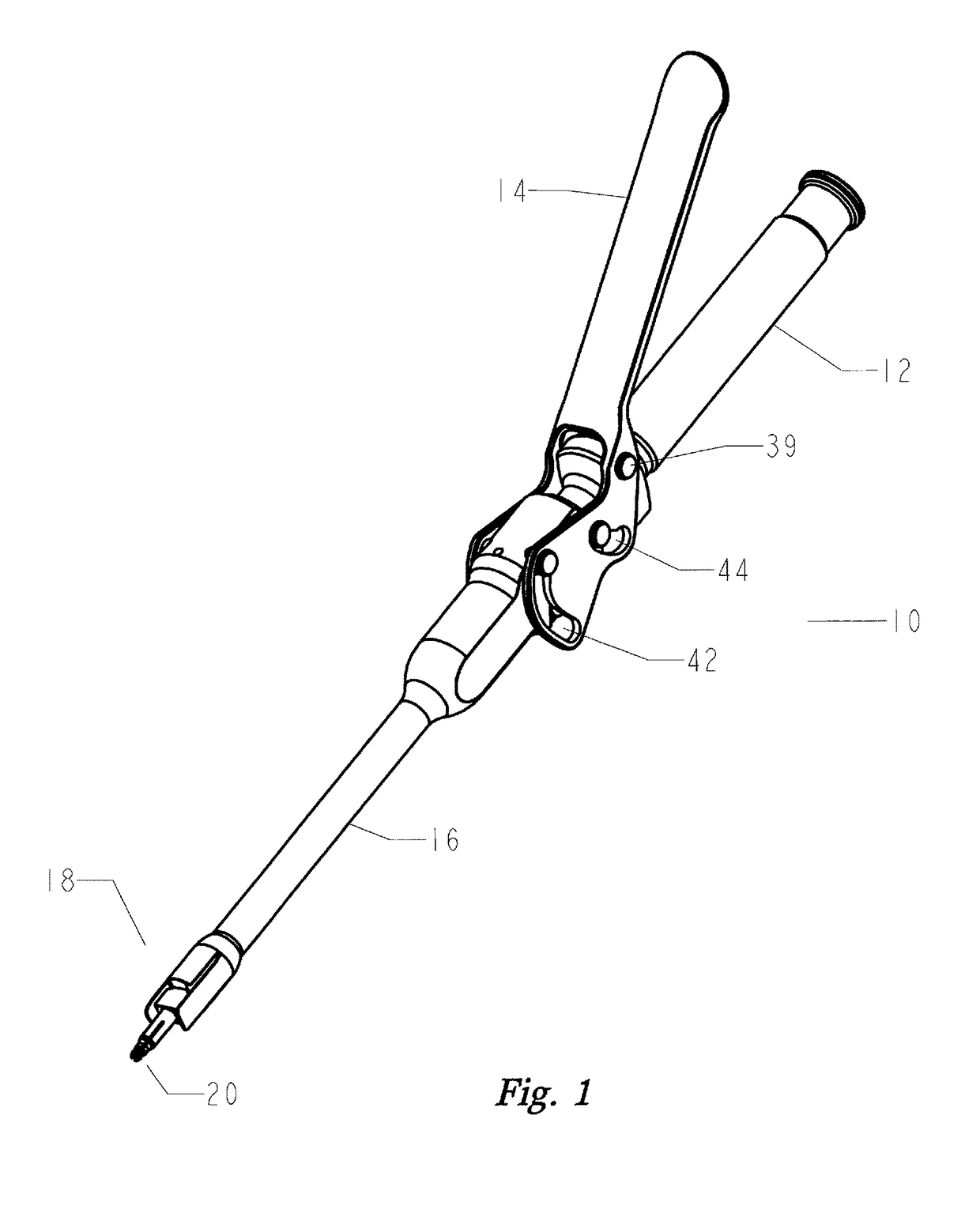
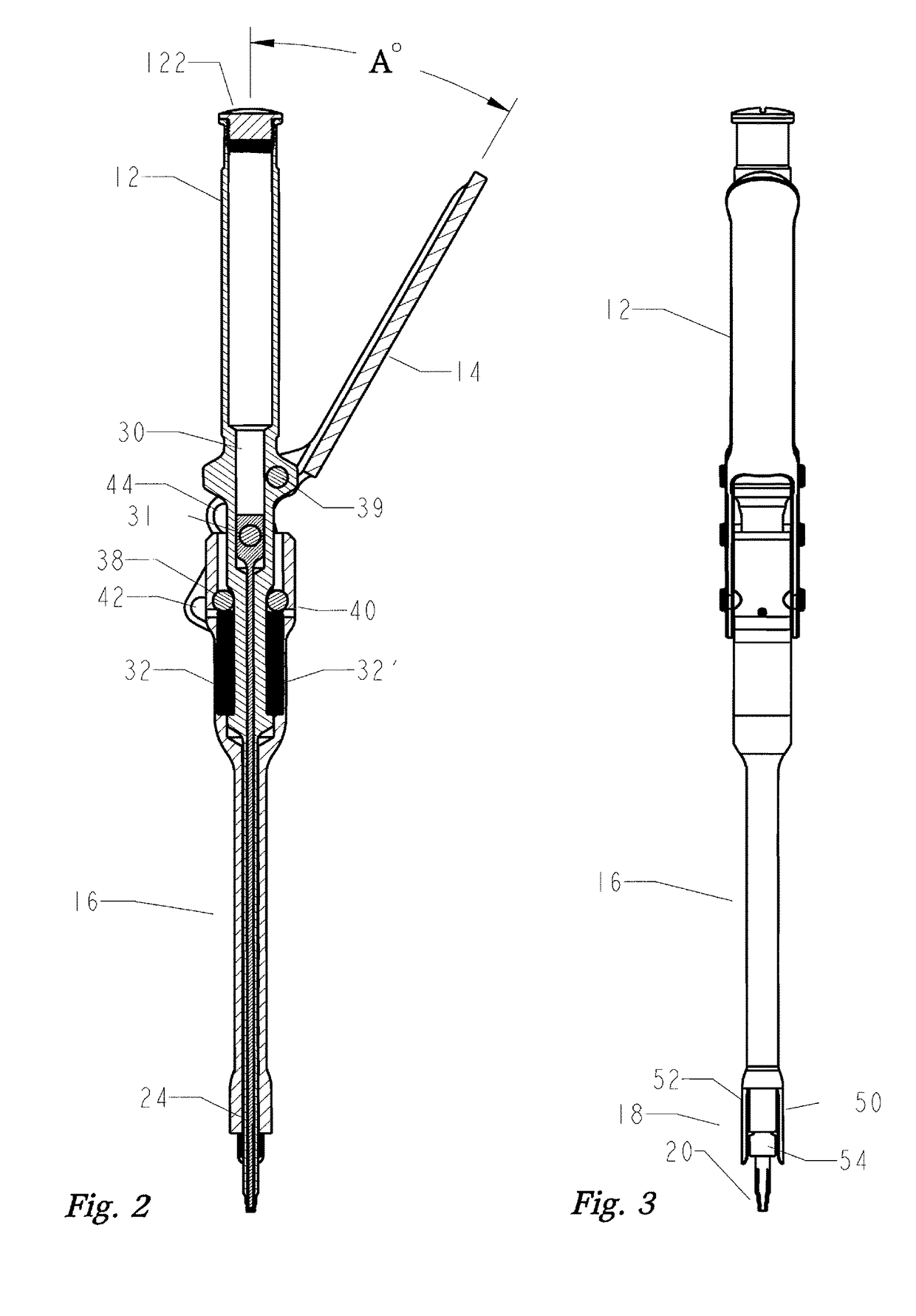
Disclosed is an instrument to assist in installing bottom loading orthopedic devices. The orthopedic devices are used to fix and stabilize bones to correct anomalies in skeletal structure occurring naturally or by trauma. Bone screws are anchored and tulips by the disclosed instrument wherein push / pull forces are equalized to eliminate the imposition of stress on the skeletal of individual.
Owner:SPINAL
Orthopedic rehabilitation mechanism employing a foot support having a first portion and a second portion configured to rotate with respect to one another
InactiveUS20110071441A1Gymnastic exercisingChiropractic devicesPhysical medicine and rehabilitationFoot supports
Provided is an orthopedic therapy and rehabilitation device. The orthopedic device, in one embodiment, includes a foot support element configured to substantially support a user's foot or ankle, the foot support element having a first portion and a second portion that are configured to rotate with respect to one another about a pivot point. The orthopedic device may further include a plurality of guide lines each having a first and a second end, wherein each of the first ends are coupleable to the foot support element. The orthopedic device may even further include a control member coupleable to each of the second ends, the control member configured to control the plurality of guide lines to flex the user's foot or ankle in a plurality of directions.
Owner:RODGERS DARELL E
Implant with integral fastener retention
InactiveUS8062294B2Move quicklySuture equipmentsJoint implantsAnatomical structuresOrthopedic devices
An orthopaedic implant provides a simple yet effective retention system requiring no additional components beyond the implant and the associated fastener(s). A plurality of anti-rotation protrusions on the fastener or screw head that match recesses in the implant. The protrusions engage the recesses to prevent the fastener from reversing direction, thereby assuring that the associated fastener does not backout during normal motion of the spine and other anatomical structures. A land on the fastener screw thread assures the fastener will not disengage from the implant should it strip out of the bone. Preventing the fastener from backing out assures it will not detach from the implant or staple, assuring that the implant will remain in place until all fasteners in the system have catastrophically failed. The anti-backout features of the invention provide feedback as they engage, allowing the surgeon to move quickly when placing a fastener without the concern for inadvertent over-tightening. Although ideally suited to a spinal correction system such as a cervical plate, the invention is applicable to other orthopedic devices, including plates and staples for other applications.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
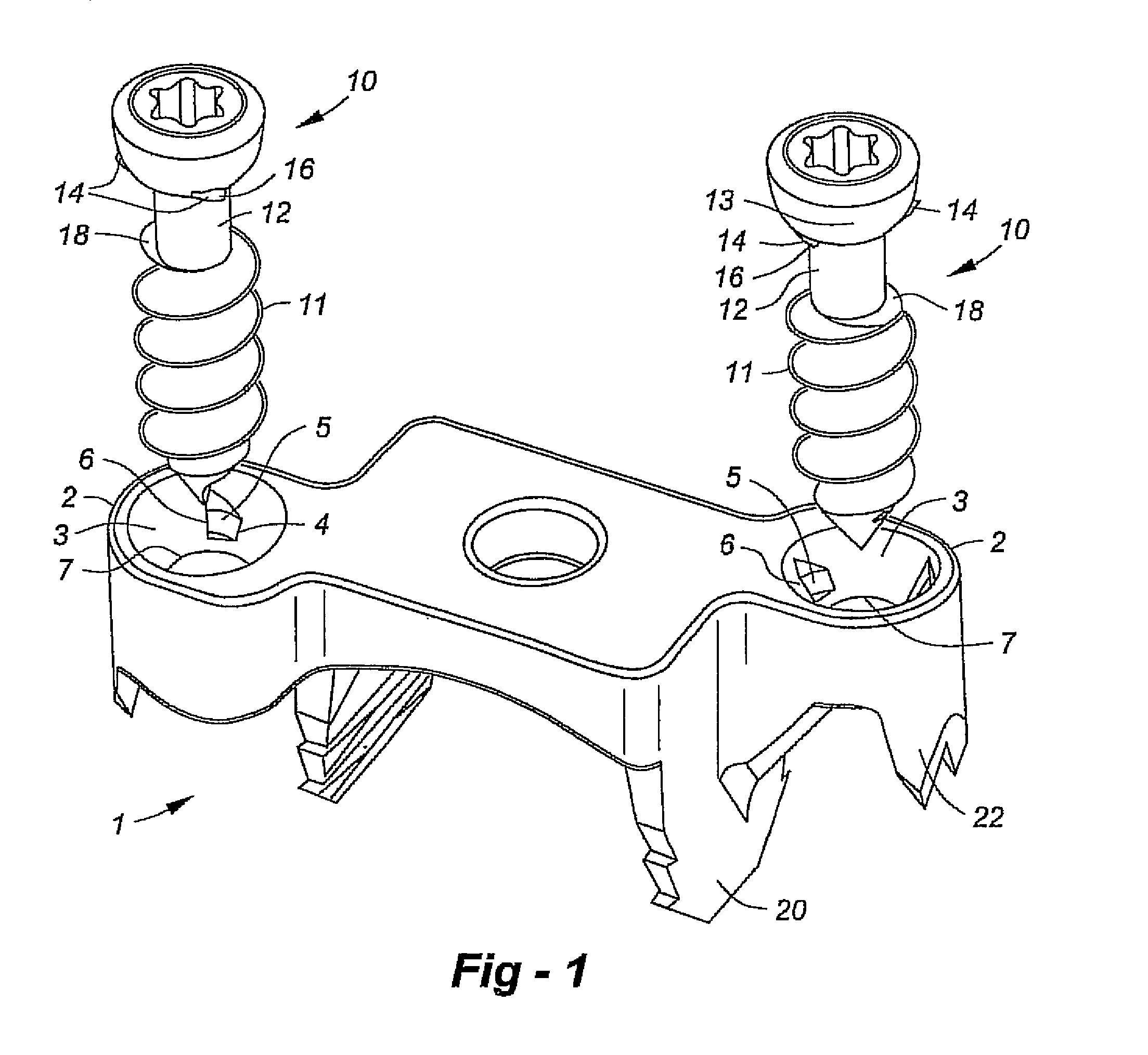
Fixation System for Orthopedic Devices
InactiveUS20130144348A1Increase flexibilityIncreased pull-out strengthSuture equipmentsLigamentsOrthopaedic deviceOrthopedic devices
A fixation system configured to releasably secure an orthopedic implant to a bone. The orthopedic implant has at least one lumen extending from a proximal portion to a distal portion configured to extend through cortical portions and into cancellous portions of the bone. The fixation system includes a flowable biomaterial that flows through the lumen into the cancellous portion of the bone in an expanded configuration with at least one dimension greater than a corresponding dimension on the orthopedic implant located generally along a pull-out direction of the orthopedic implant. An insert is positioned in the lumen and in engagement with the flowable biomaterial located in the cancellous portion of the bone, such that the orthopedic implant is detachable from the biomaterial in the cancellous portion of the bone to facilitate subsequent removal of the orthopedic implant from the bone.
Owner:RDC HLDG
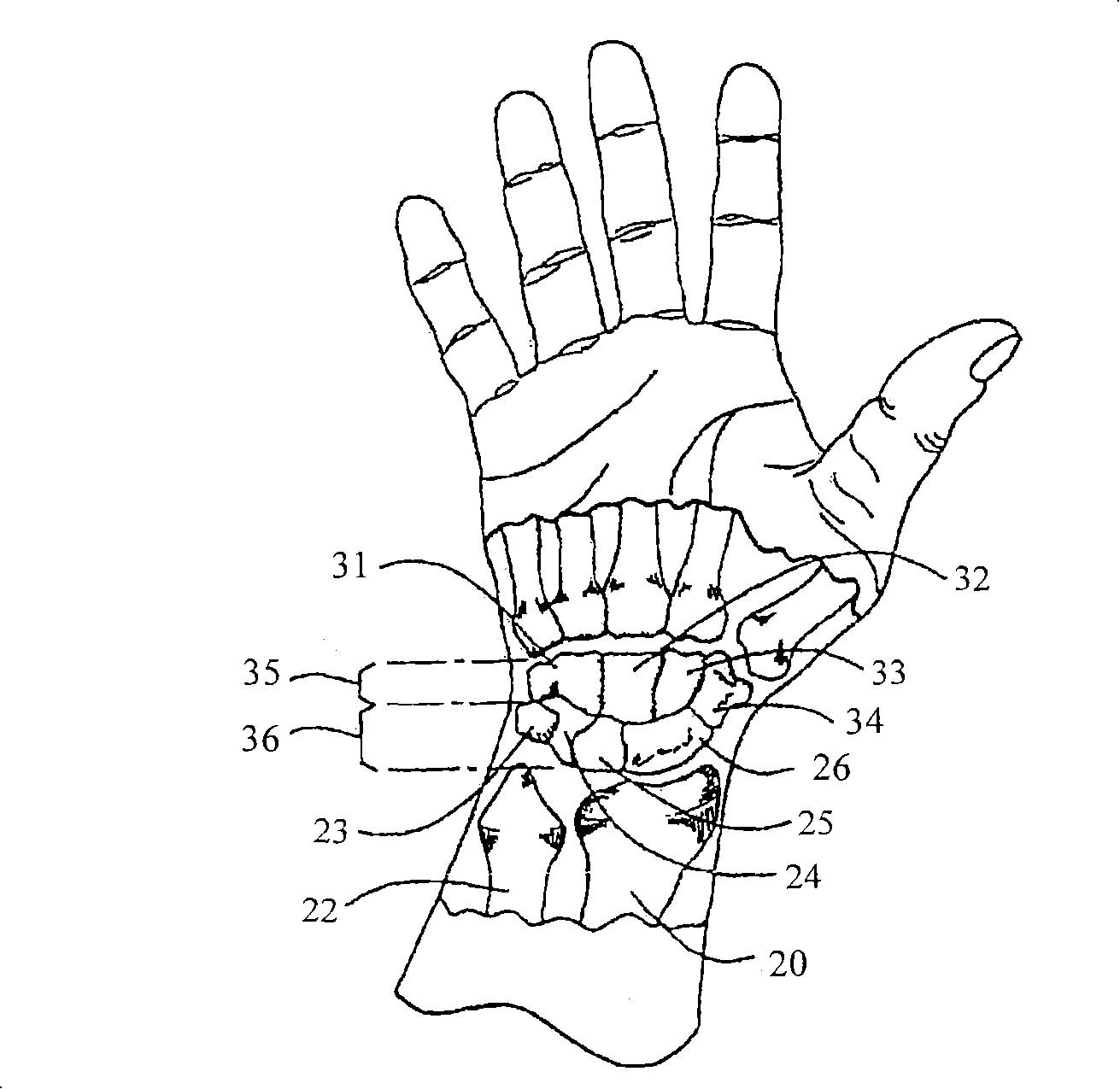
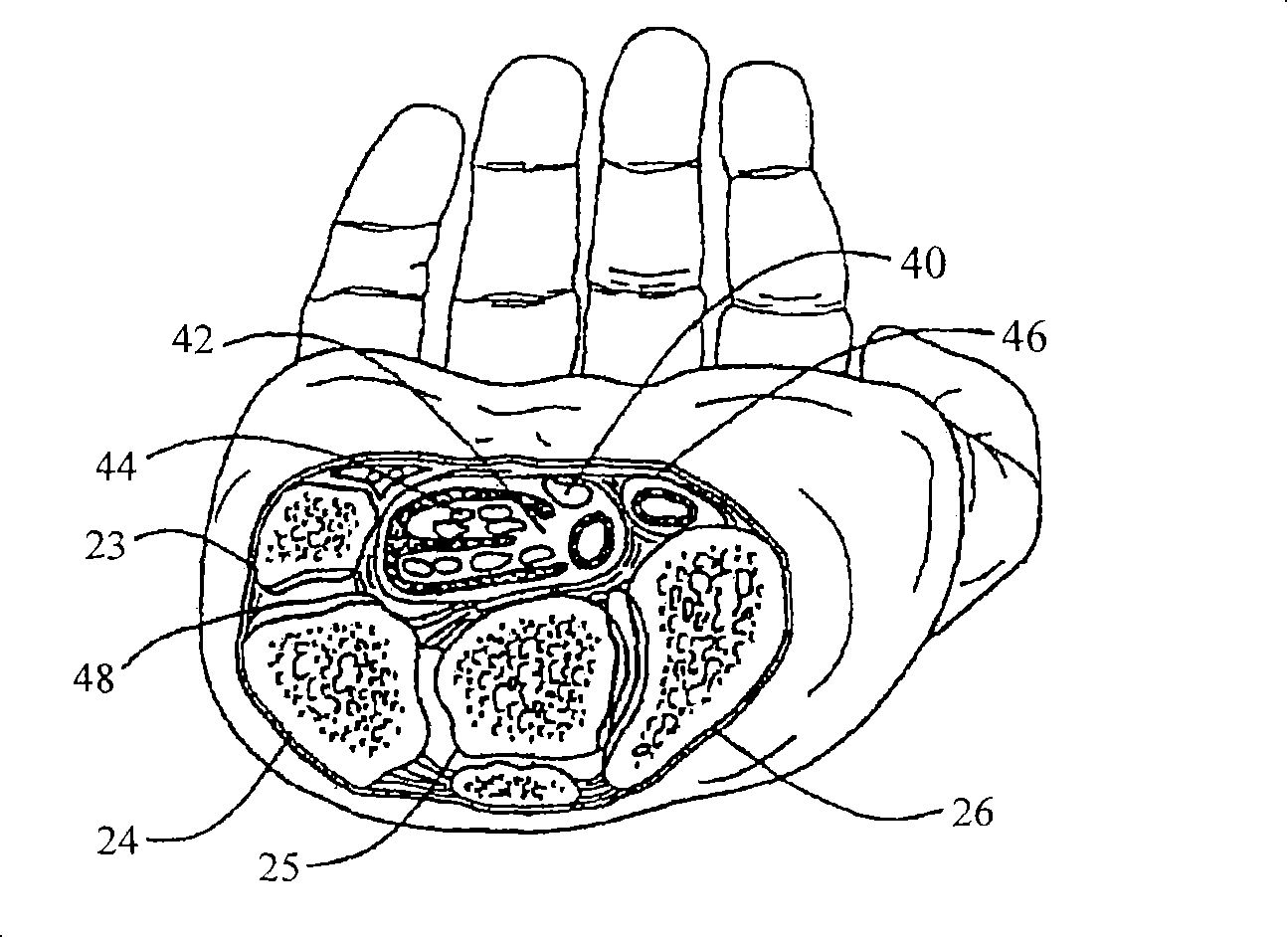
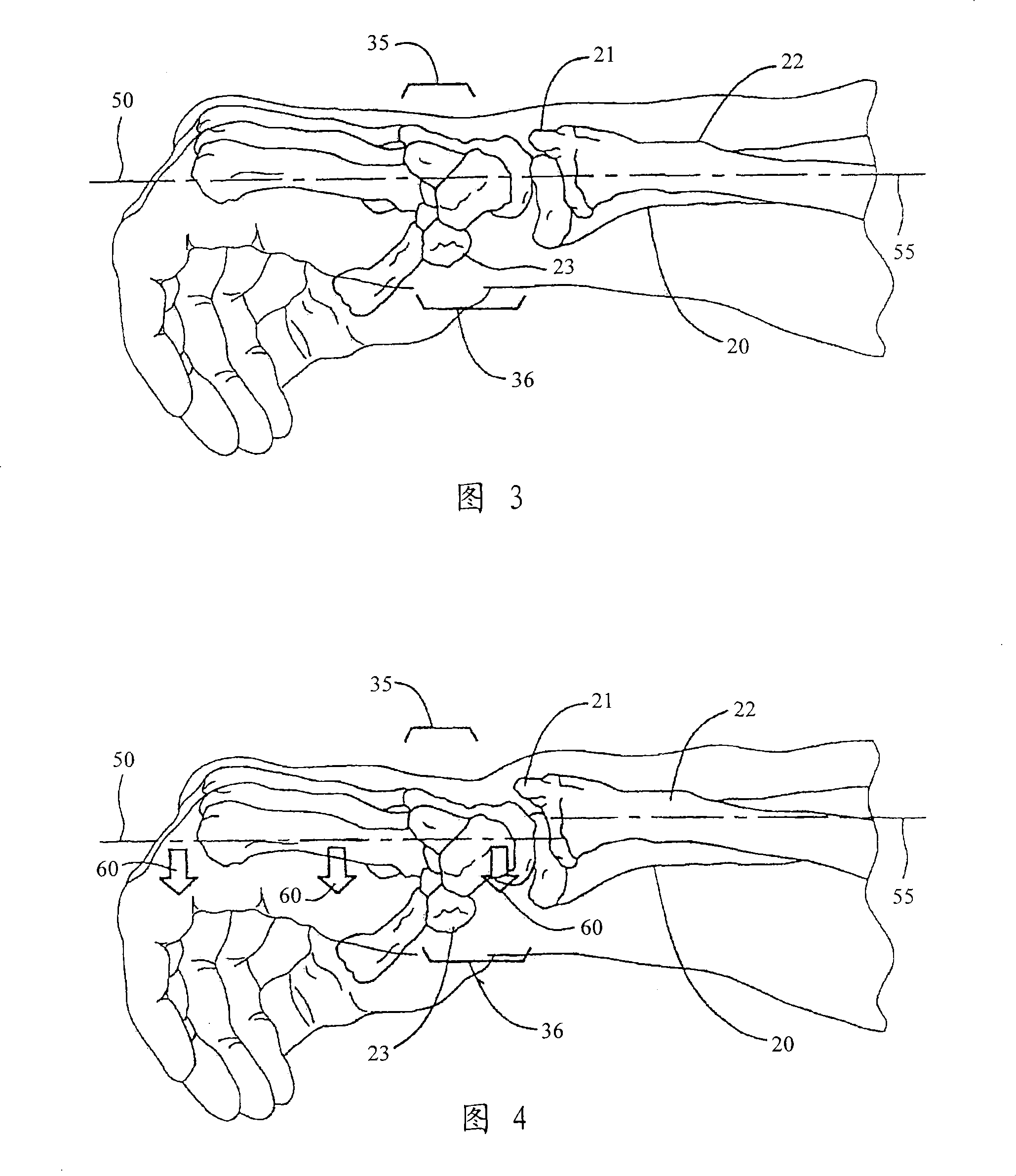
Improved orthotic appliance for carpal tunnel syndrome
Owner:乔治・R・威廉斯
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com