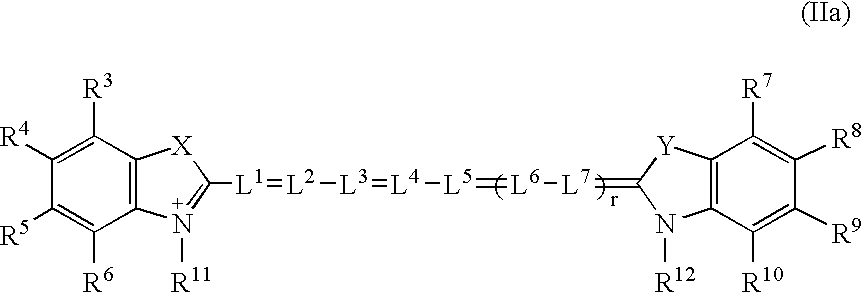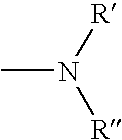Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
565results about "Luminescence/biological staining preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
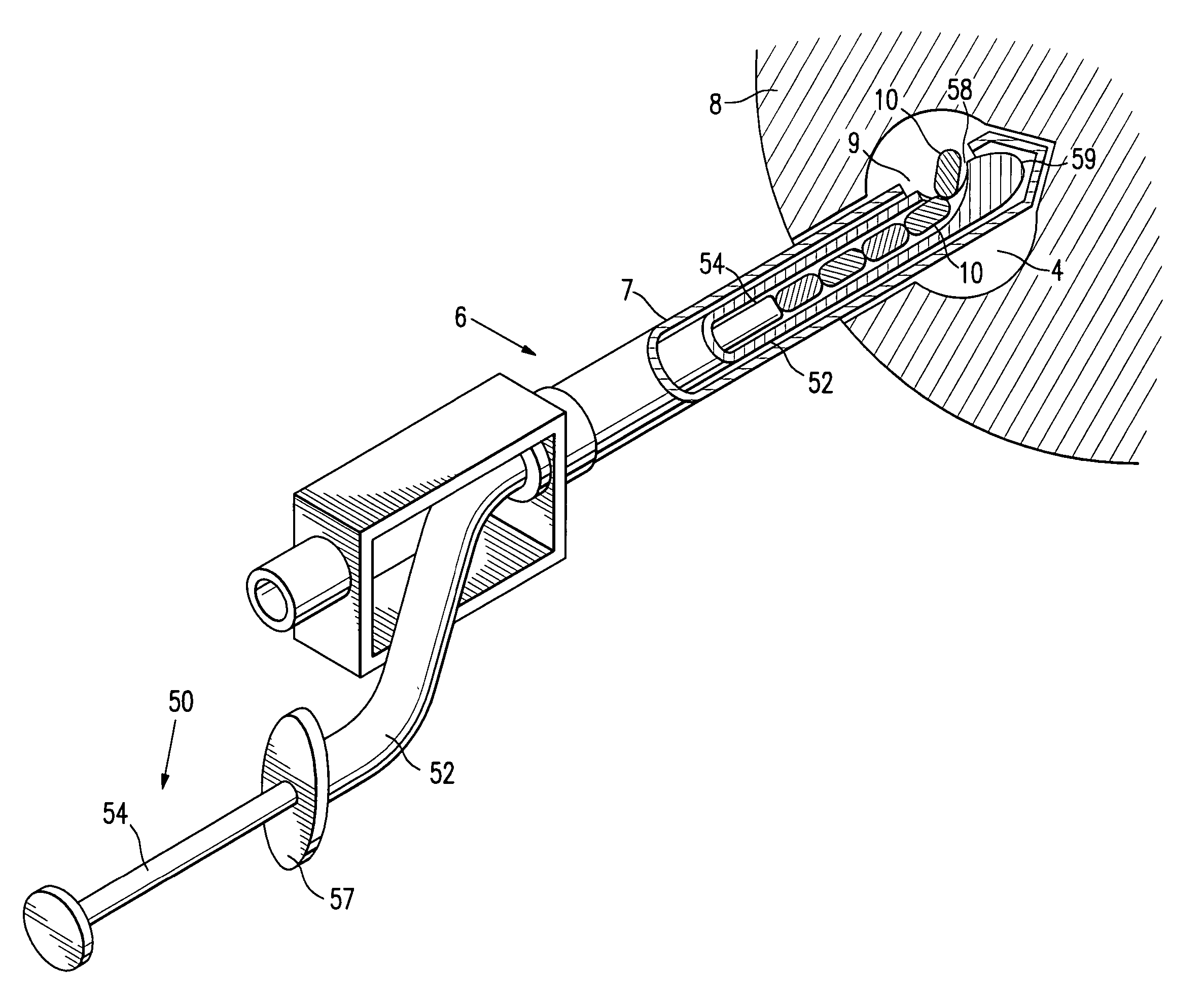
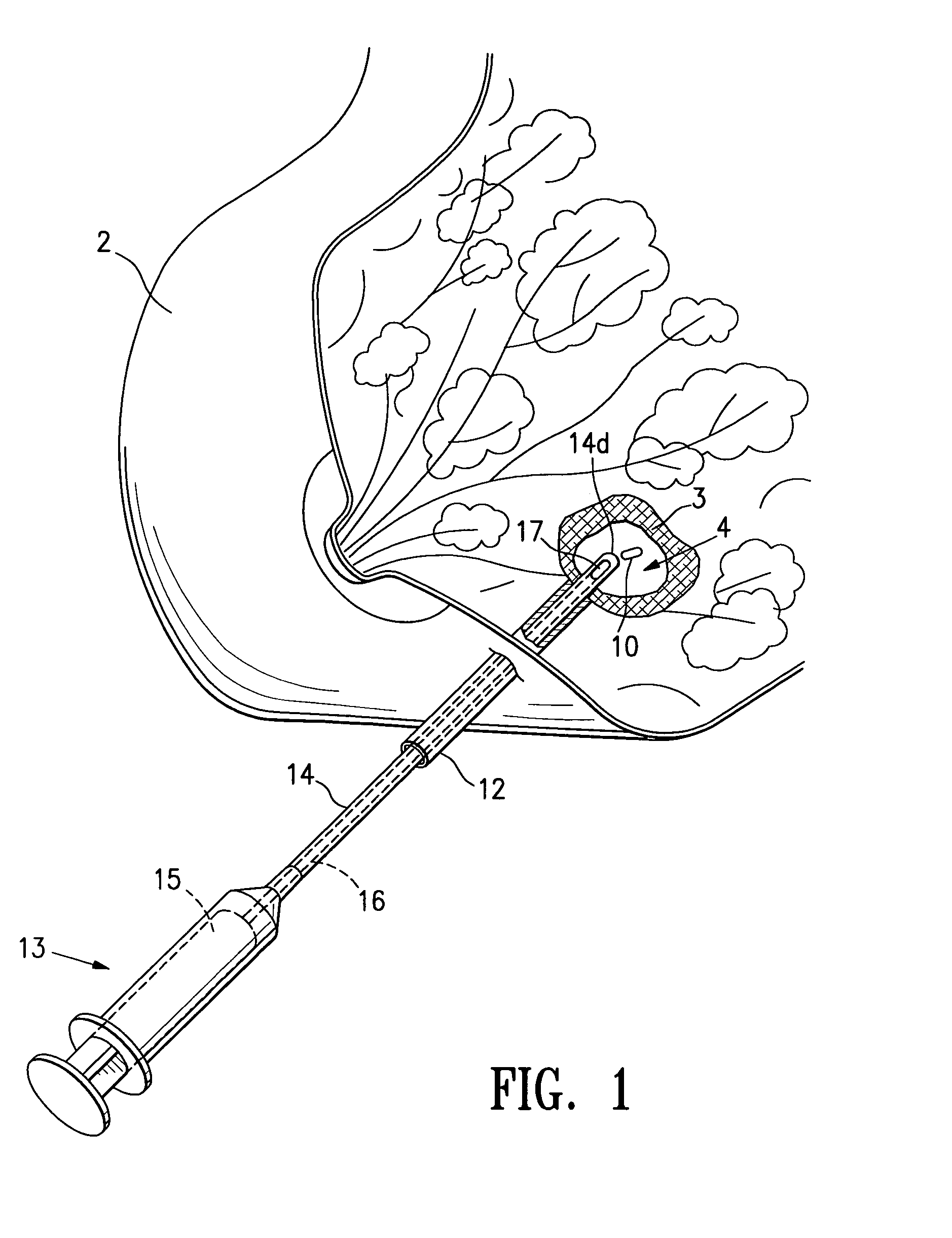
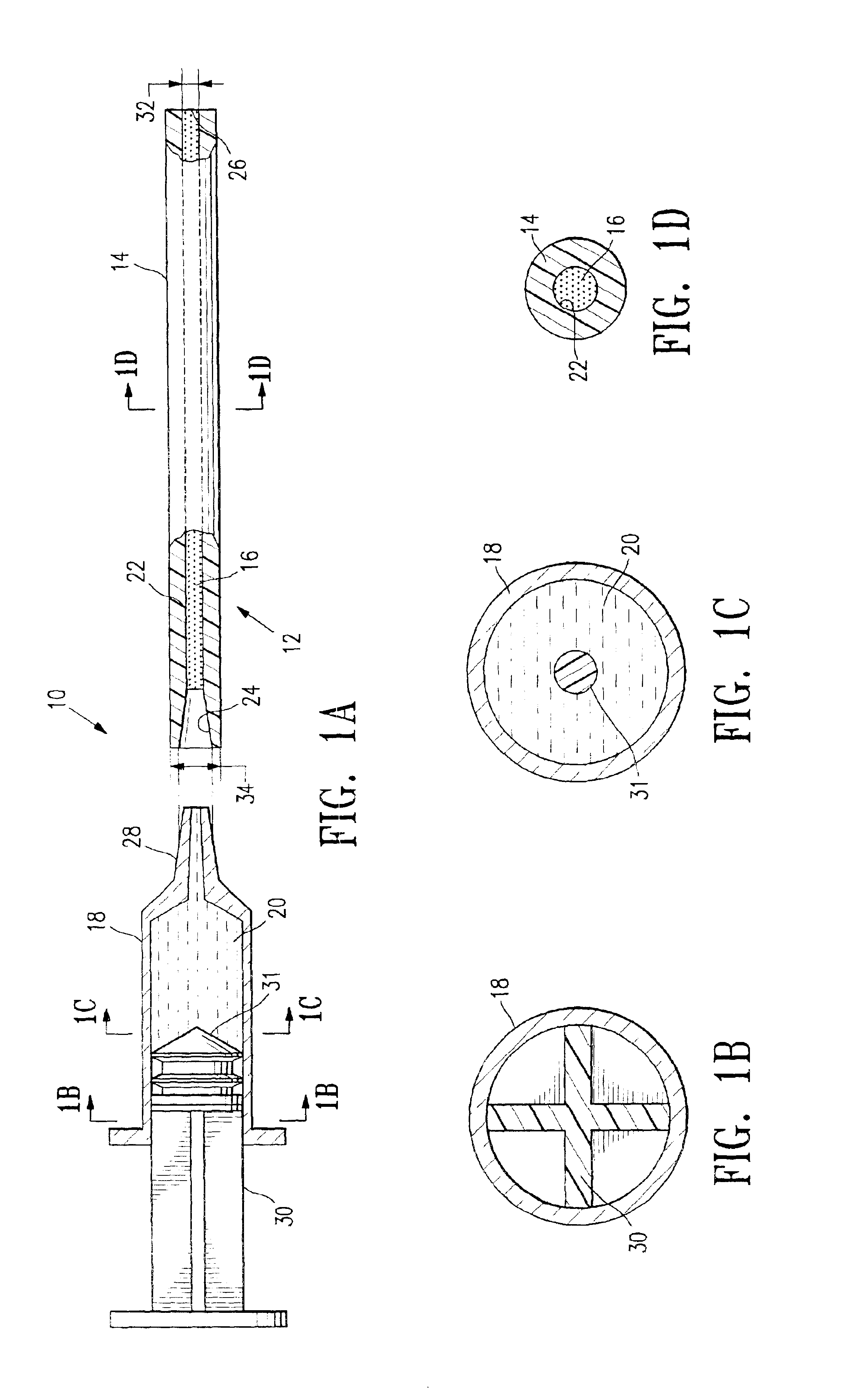
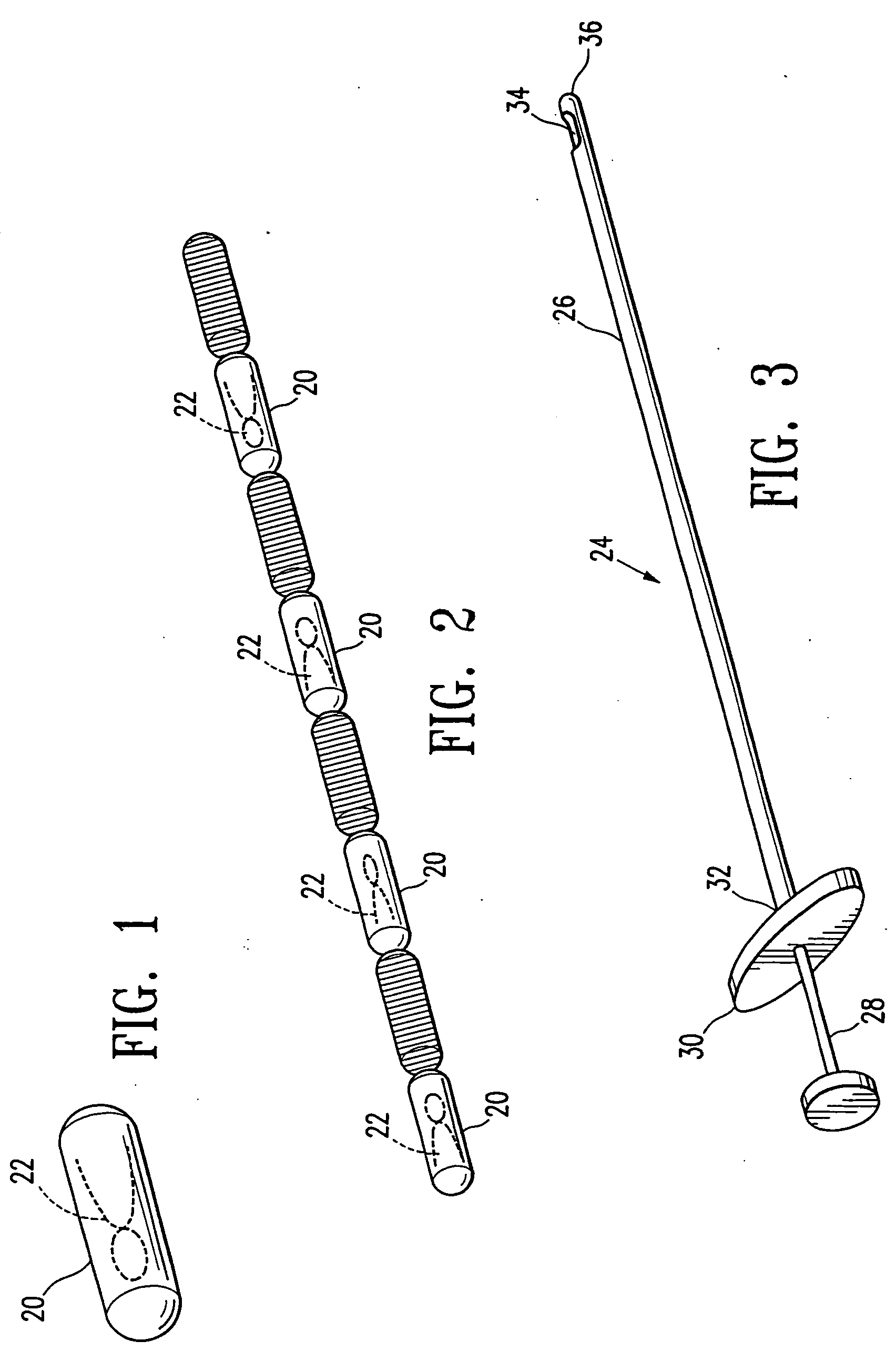
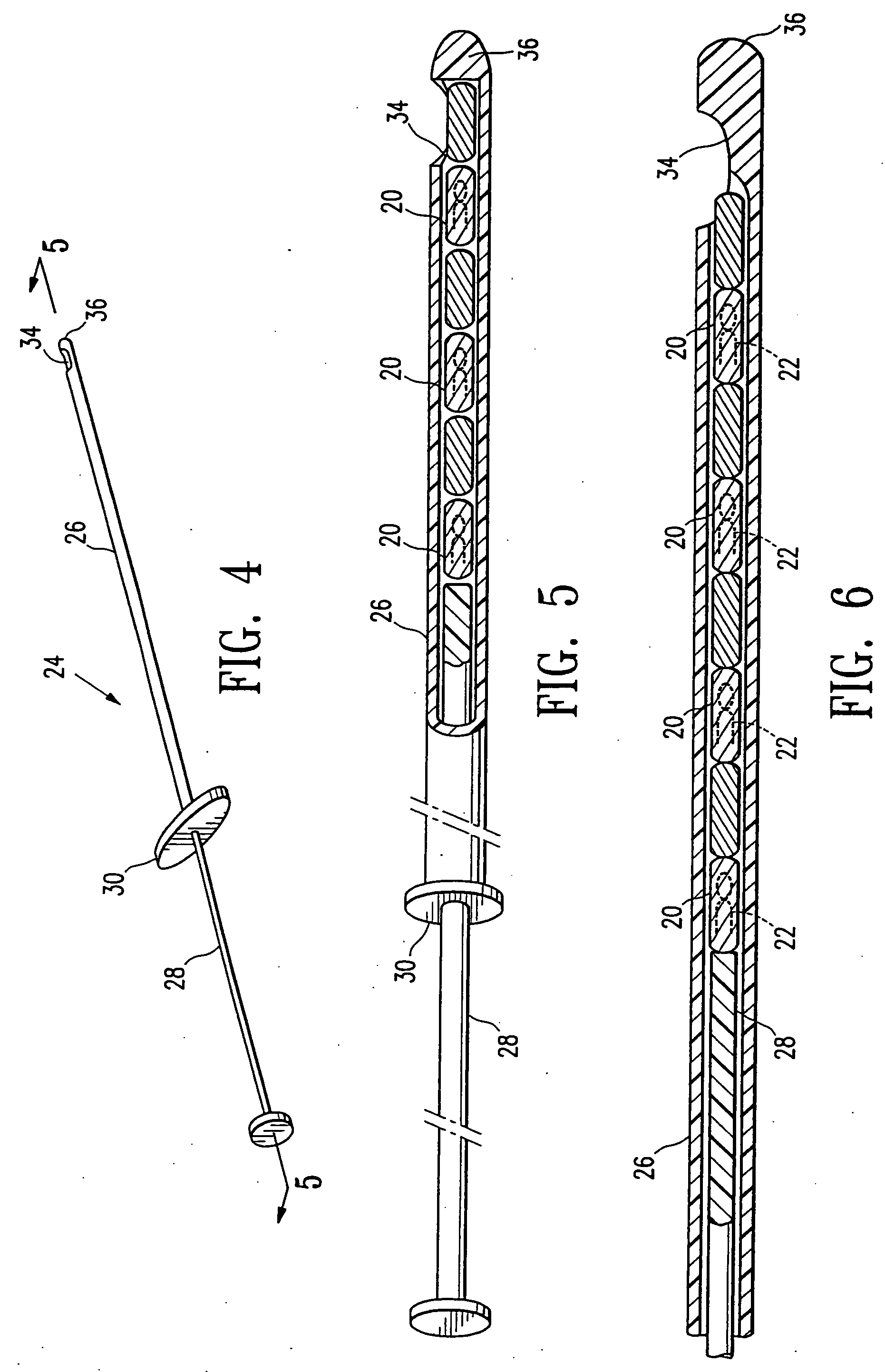
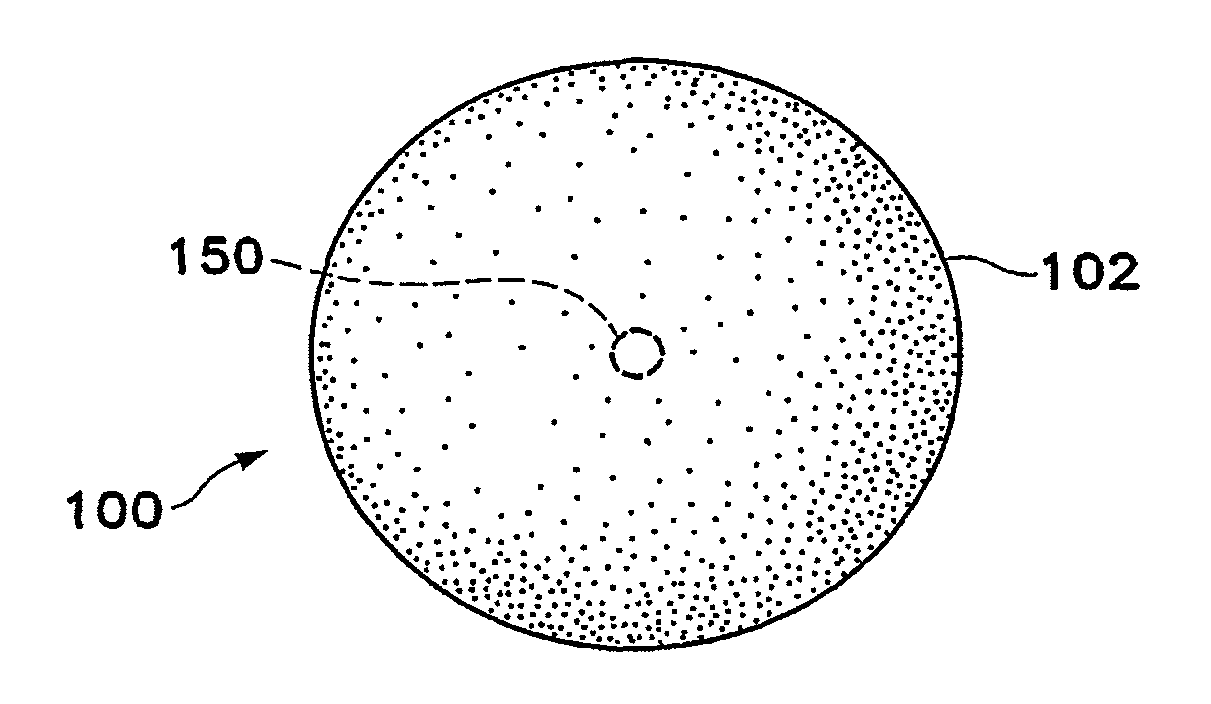
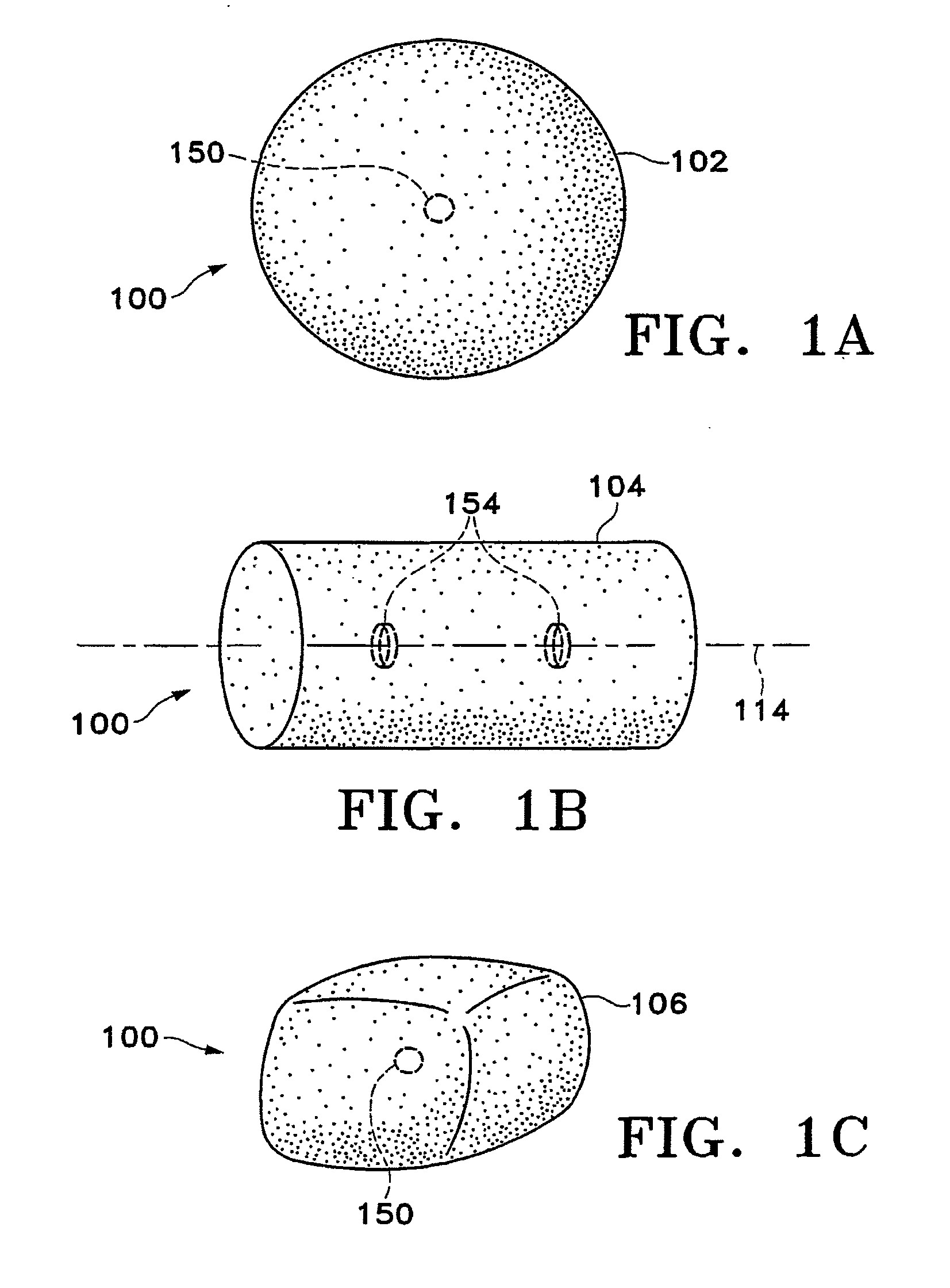
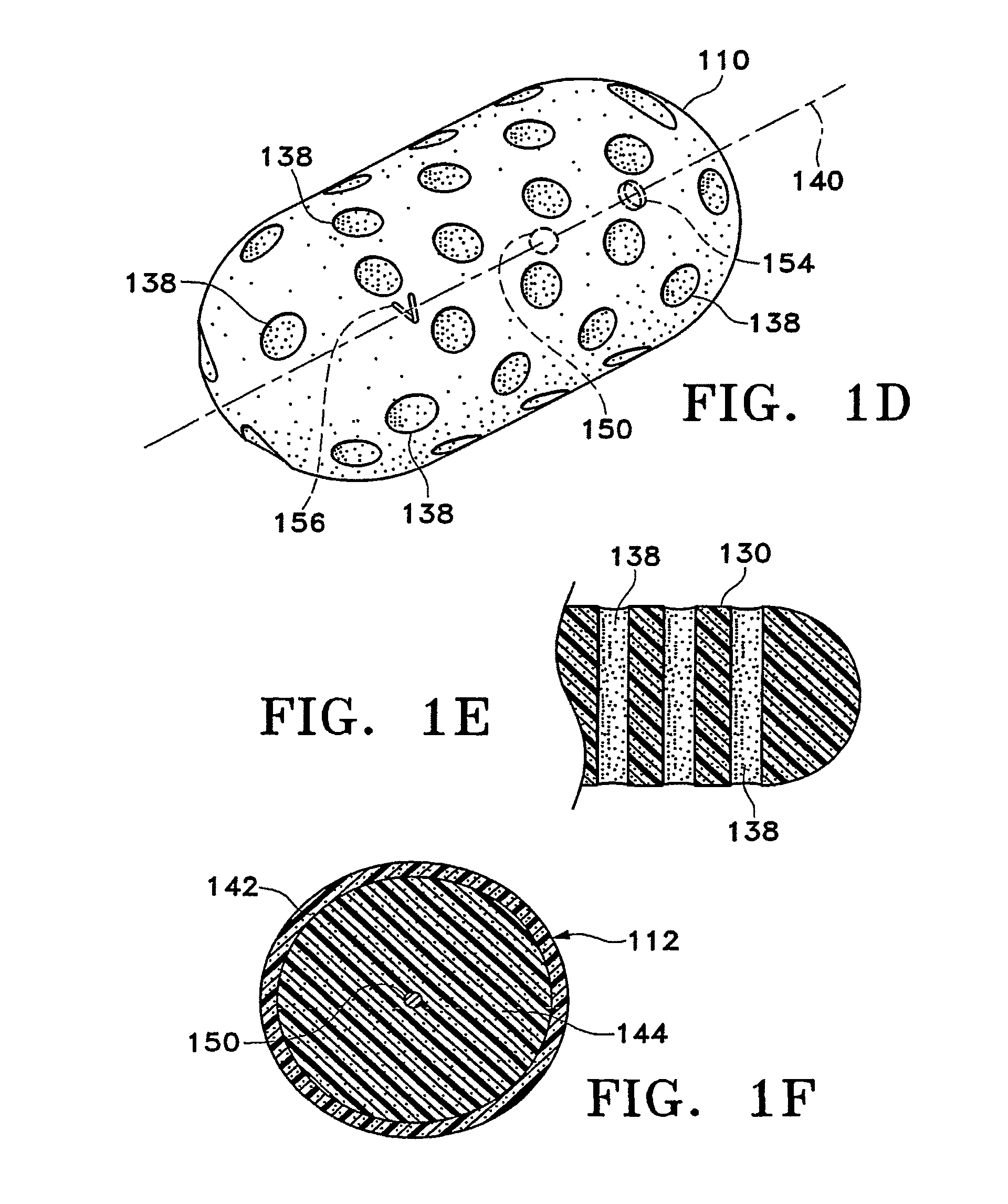
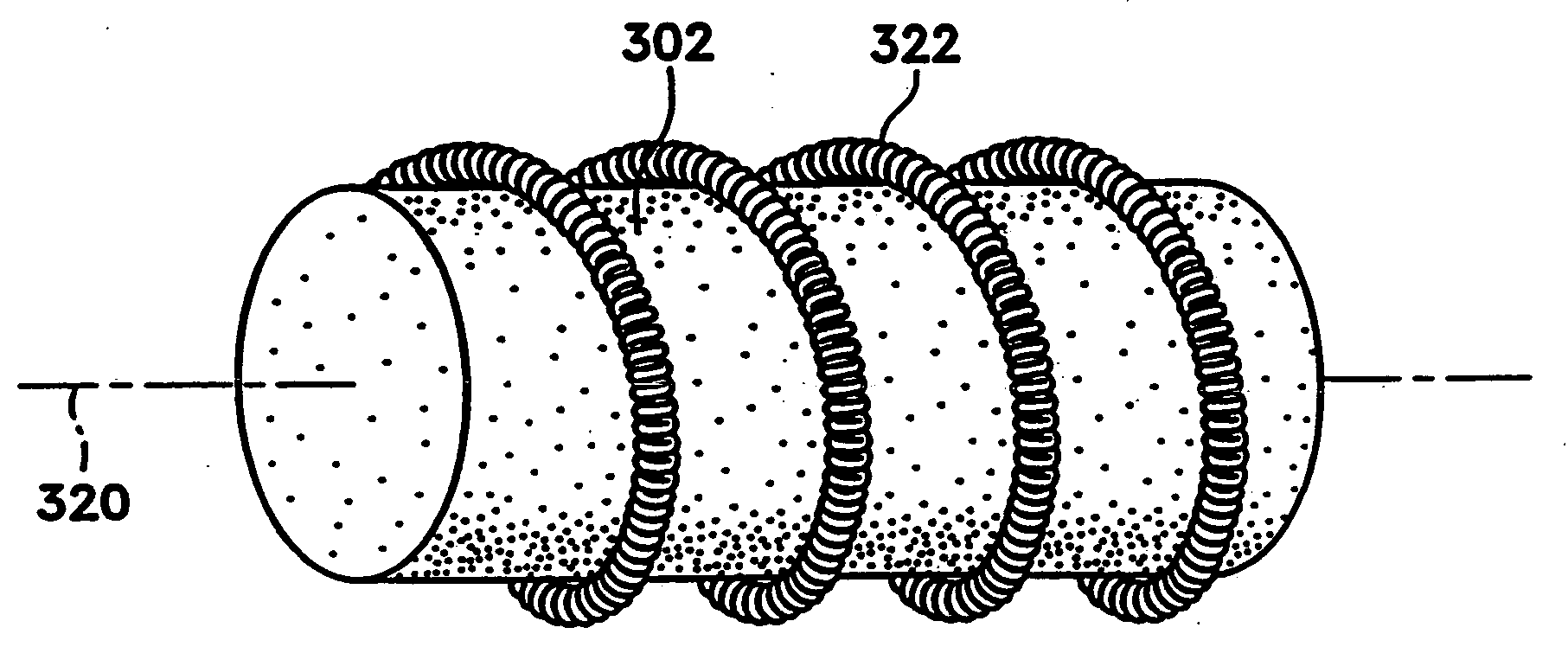
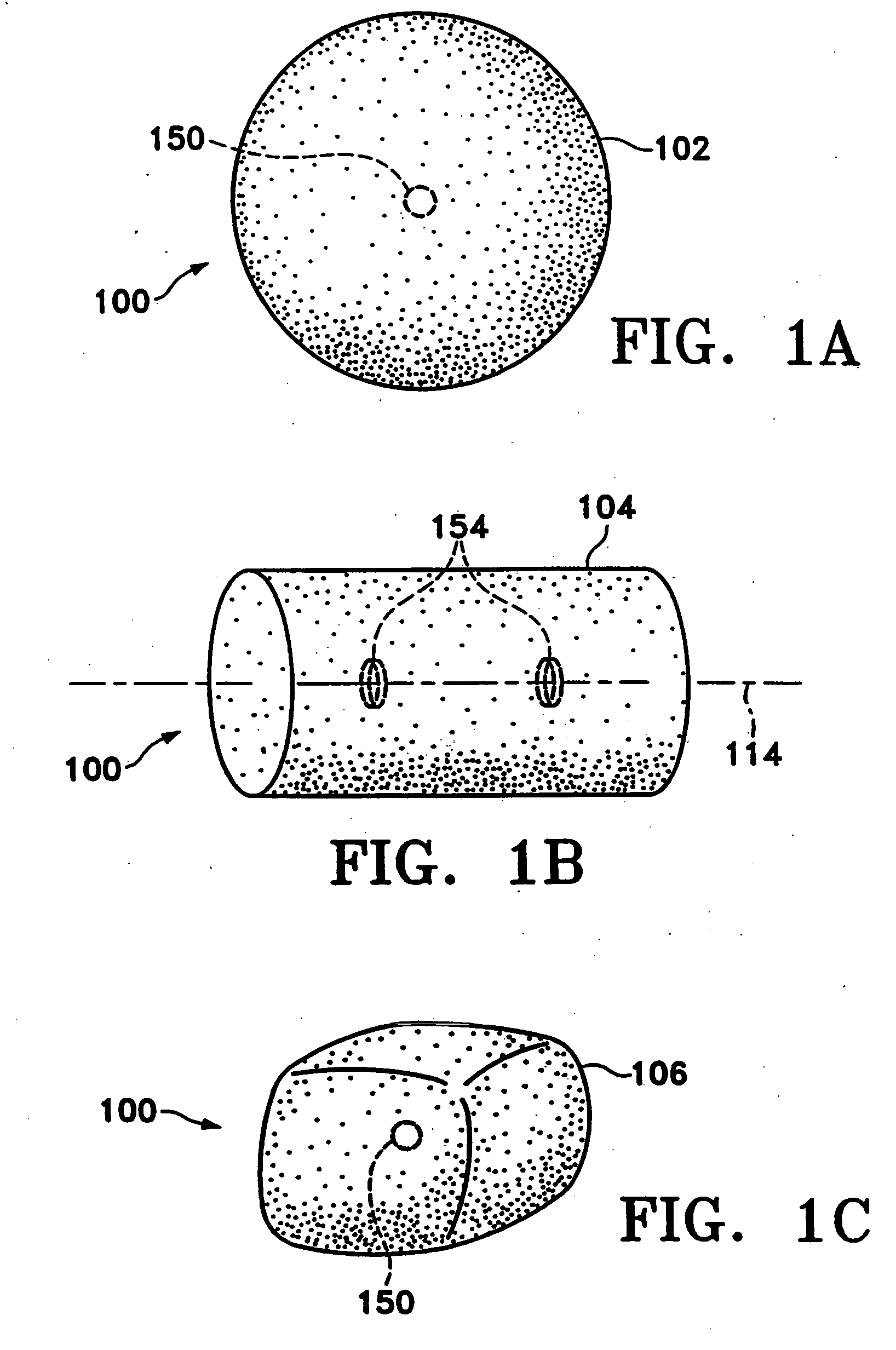
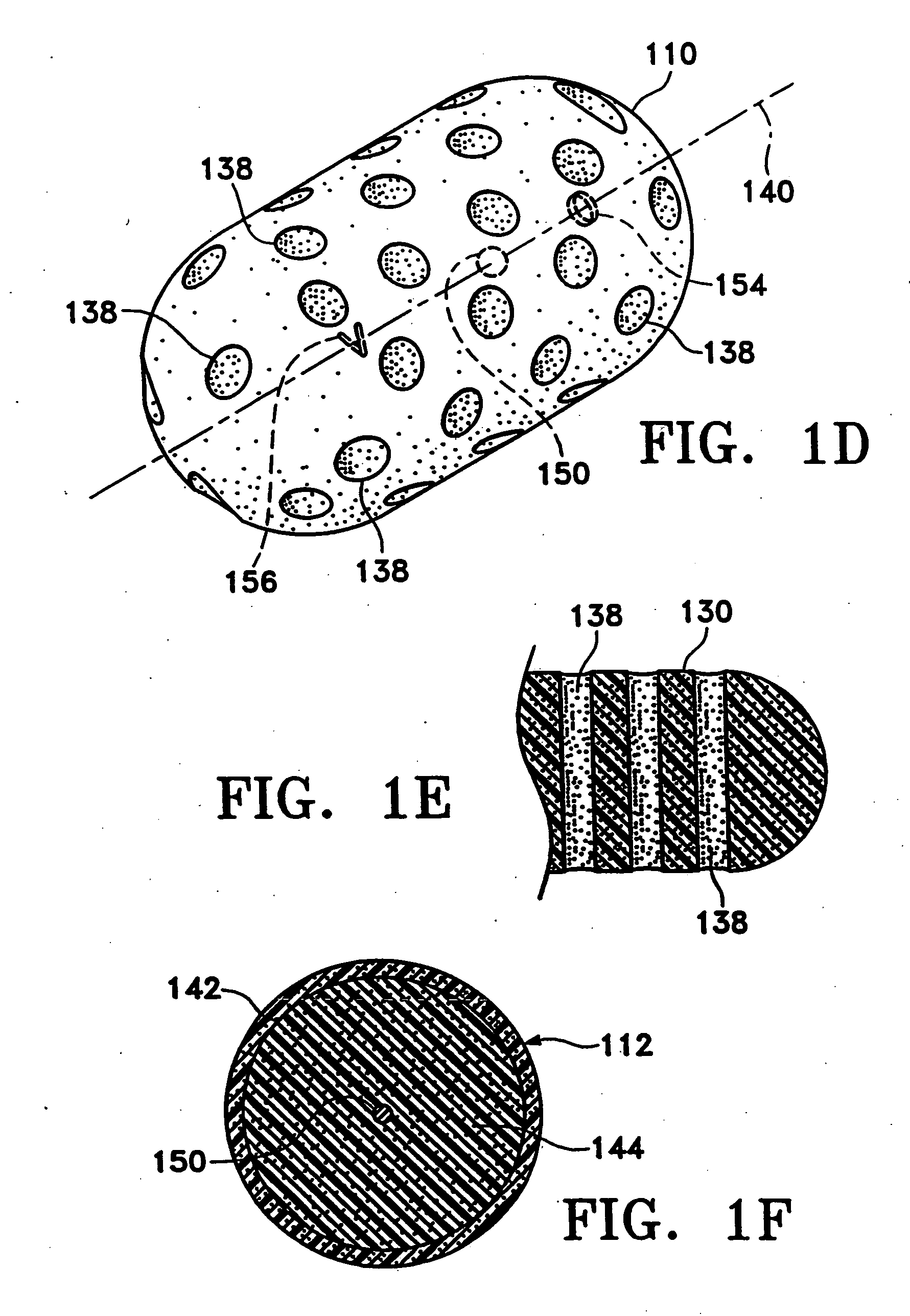
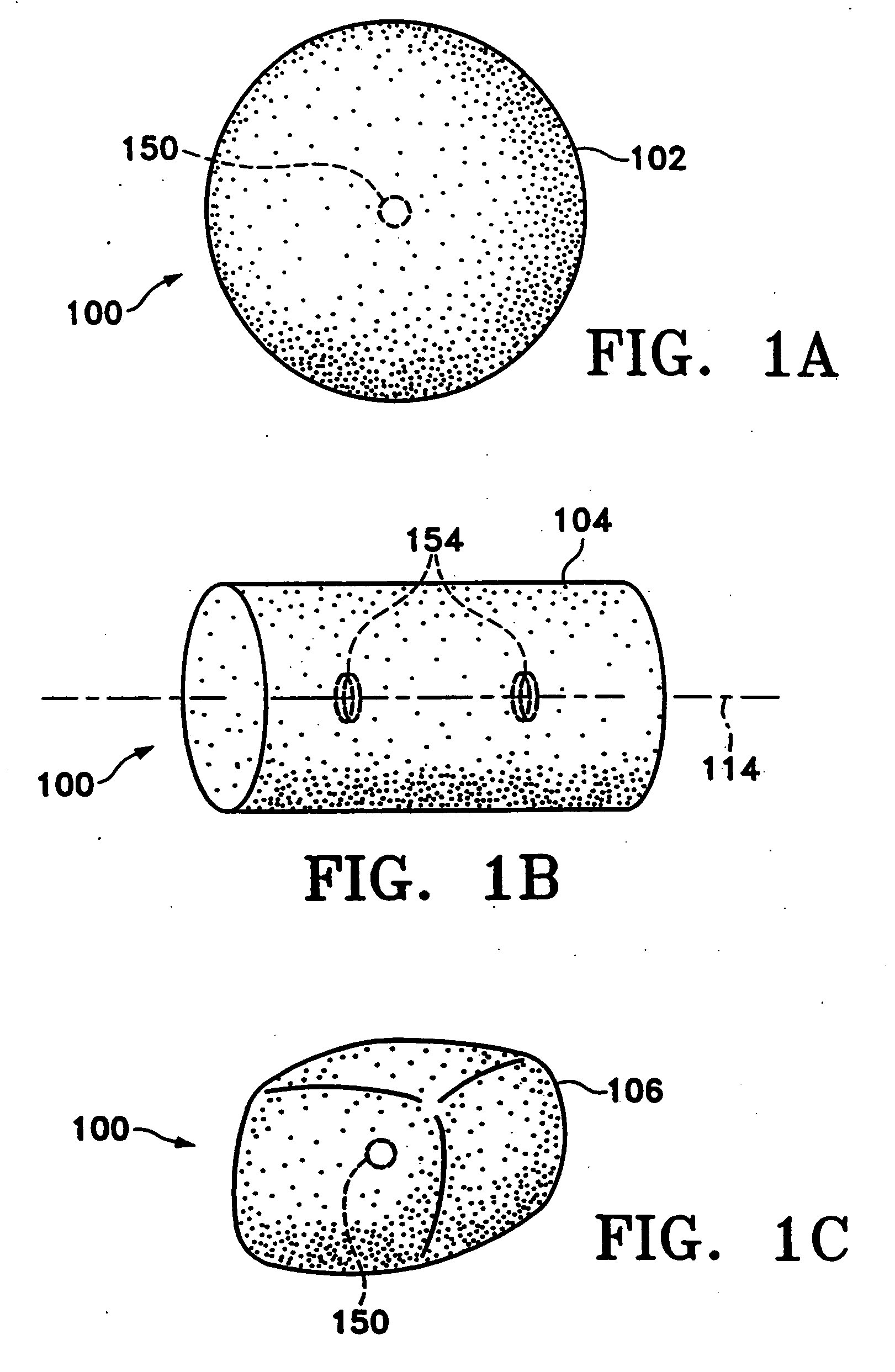
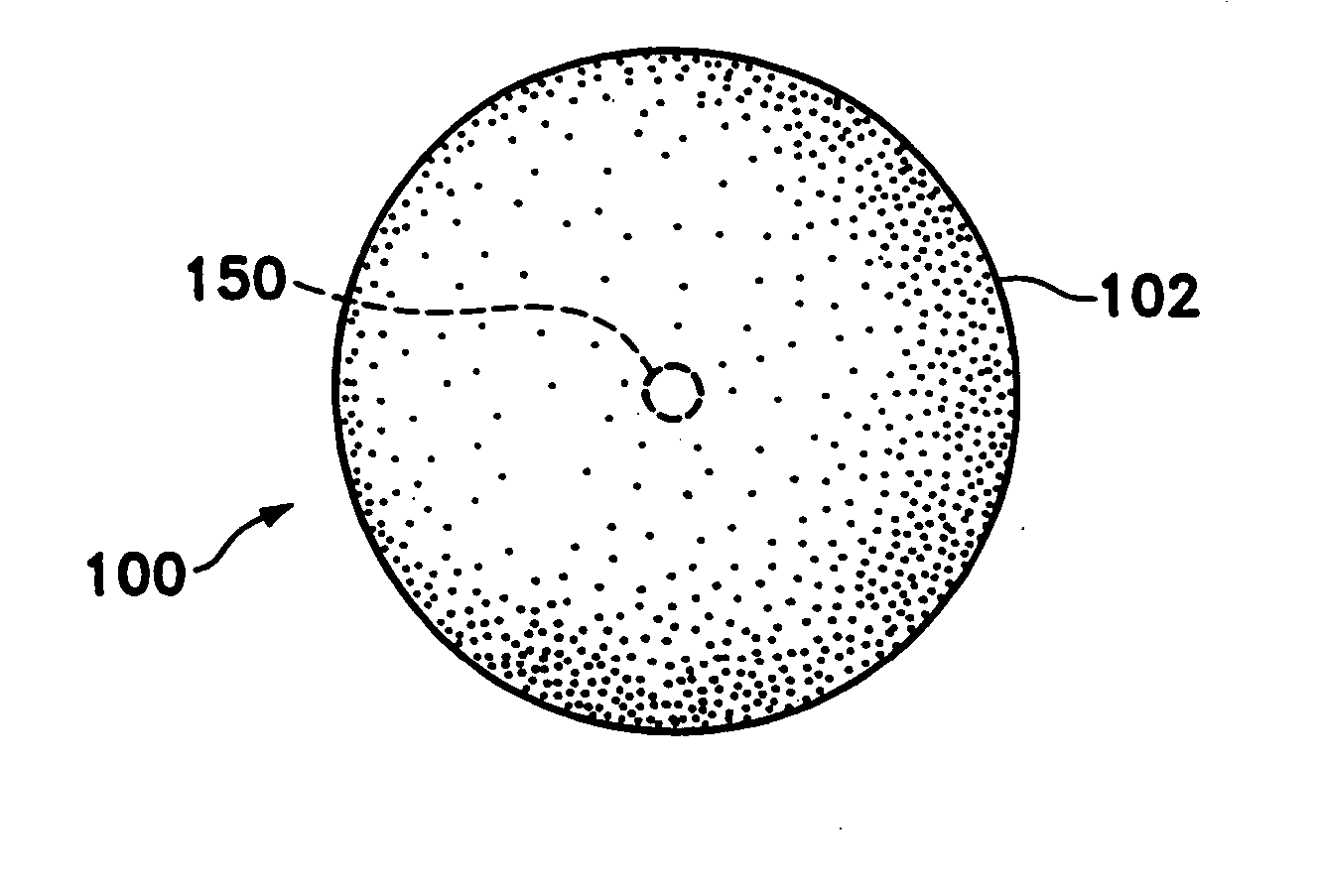
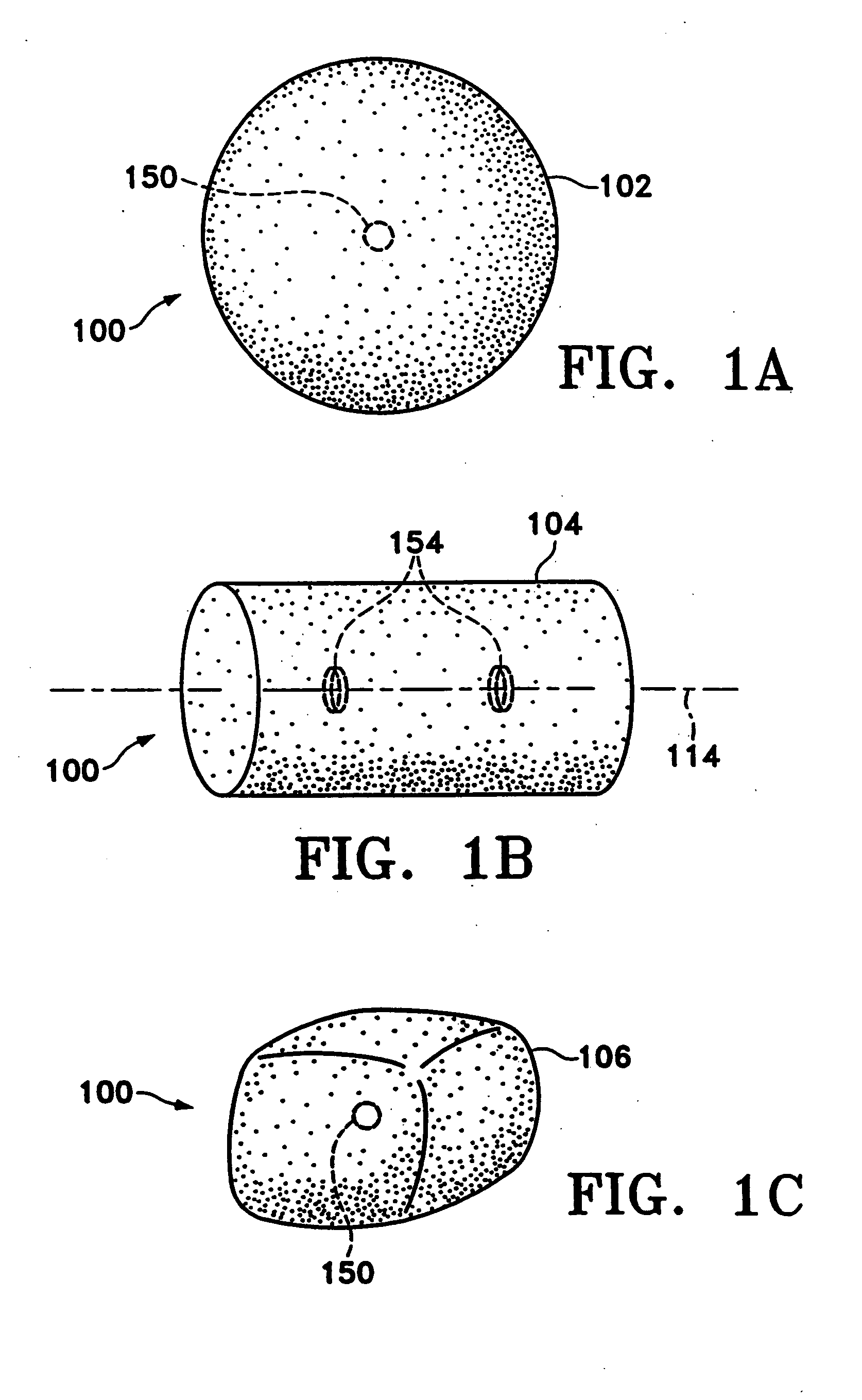
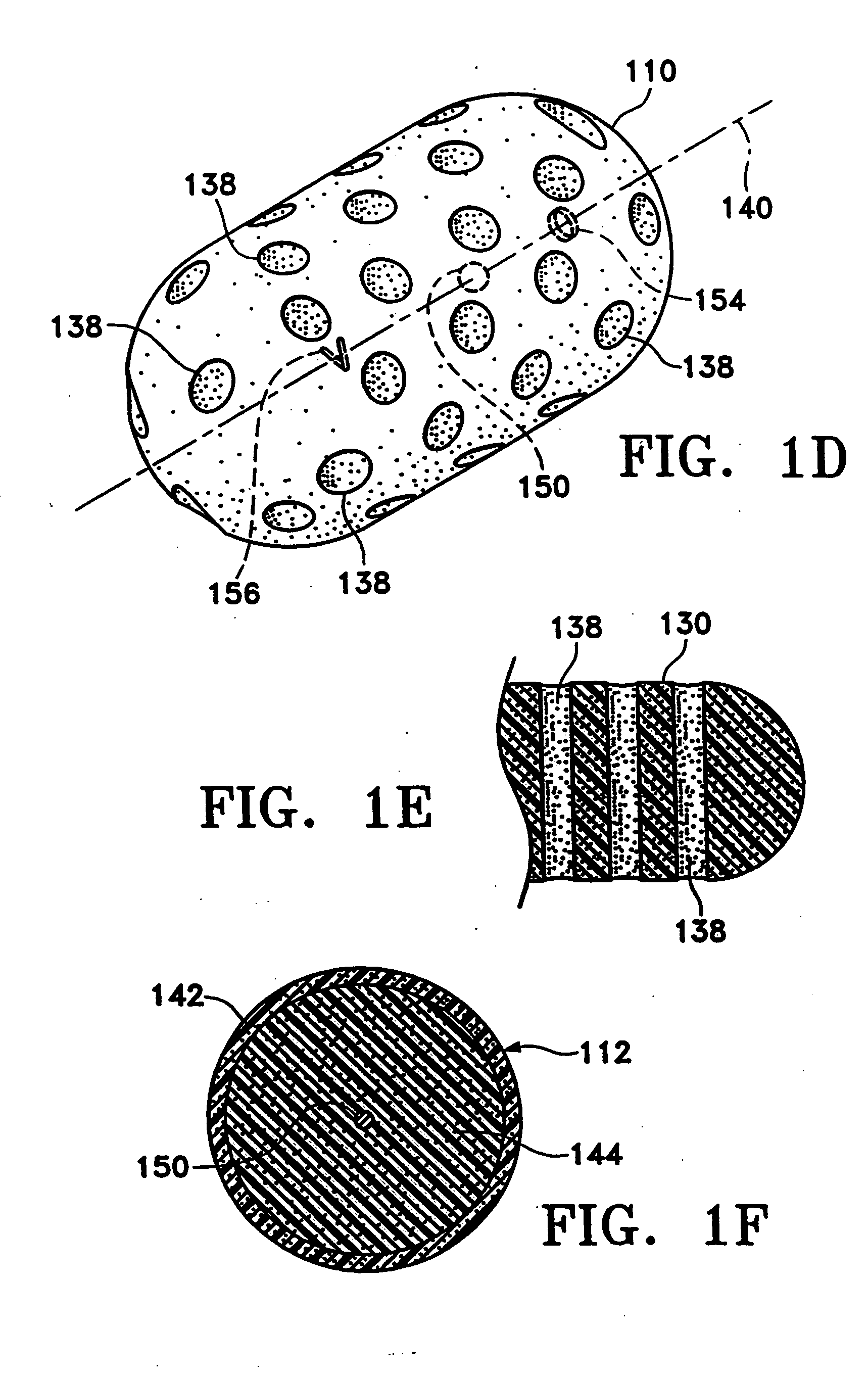
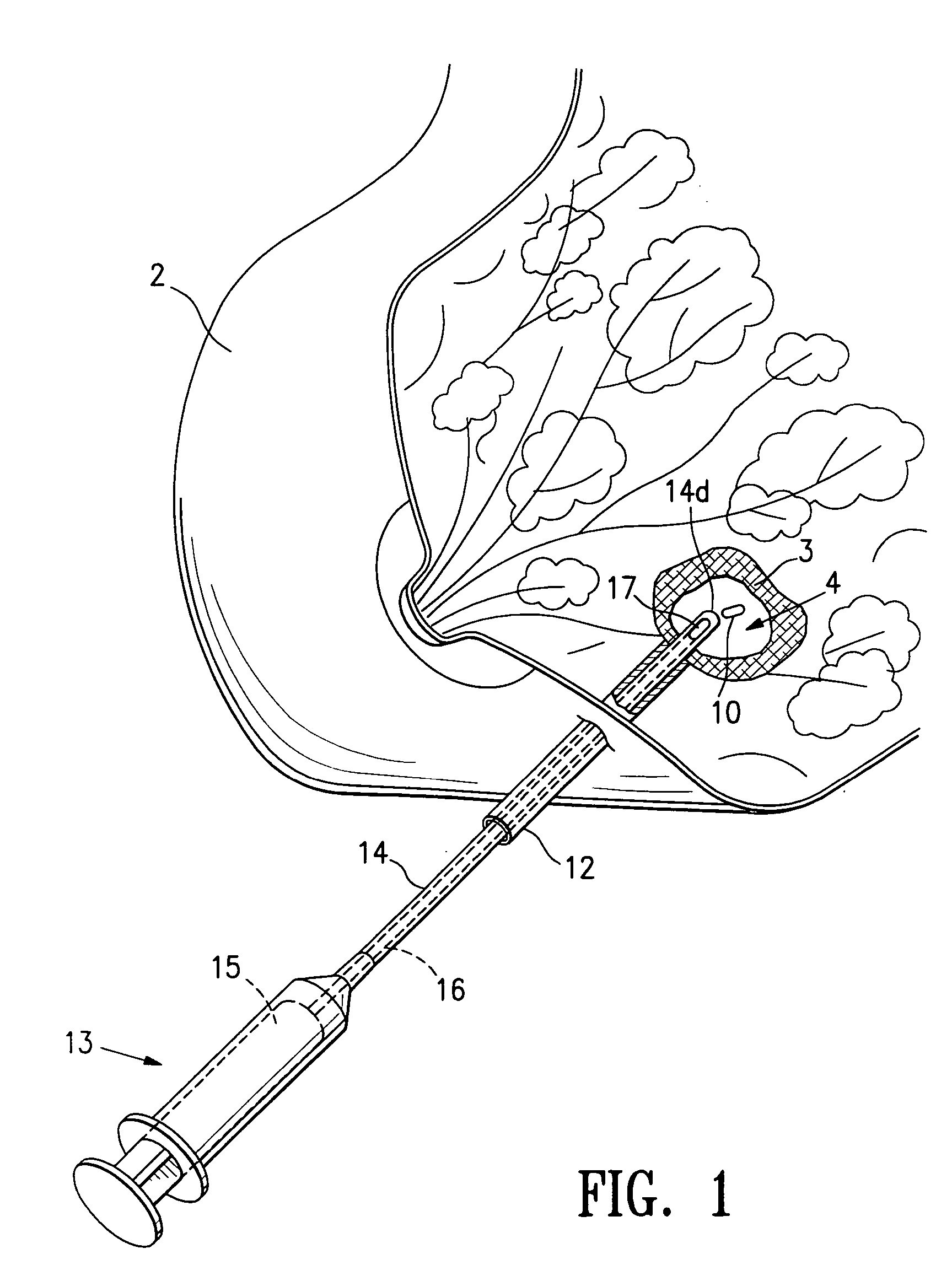
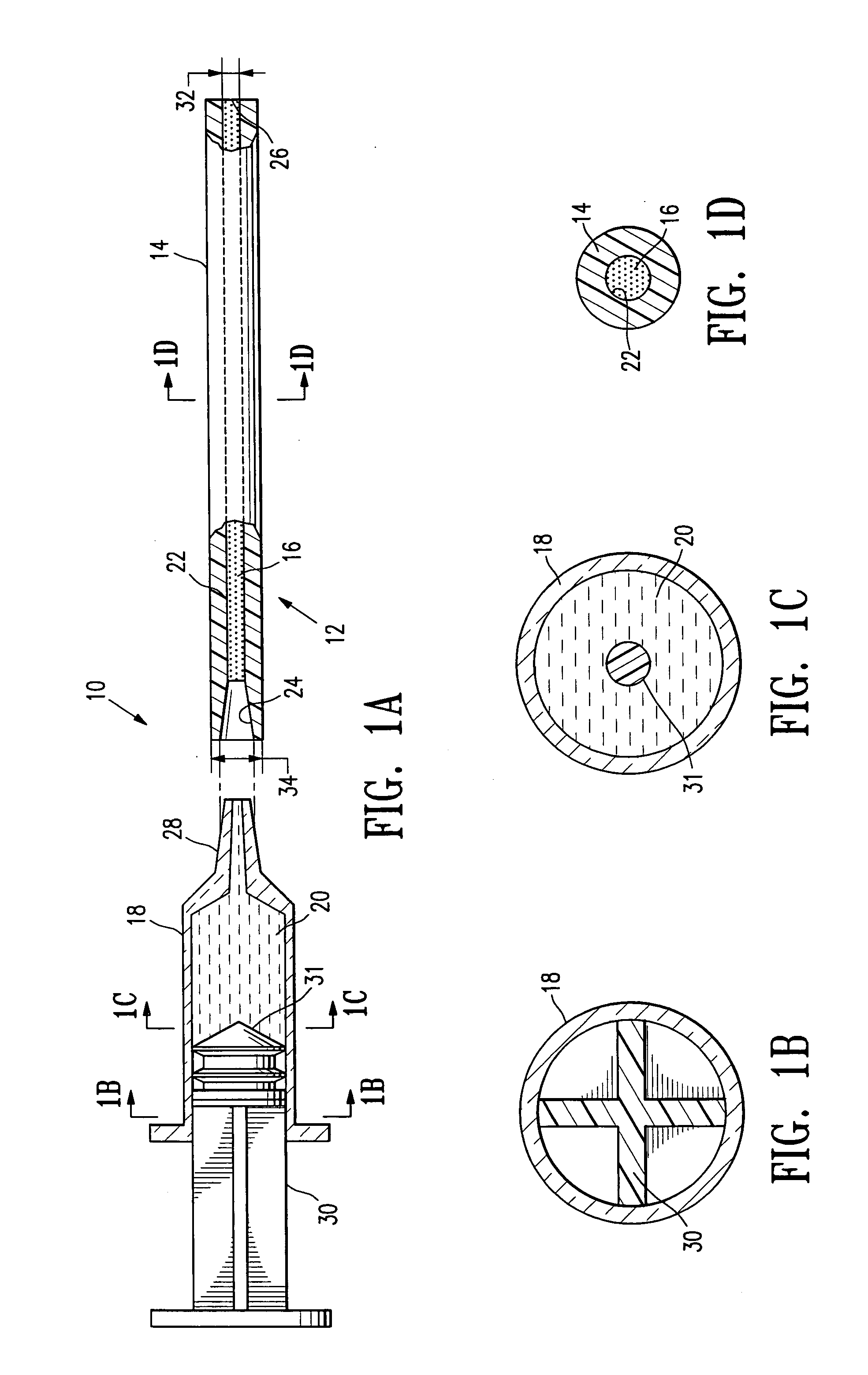
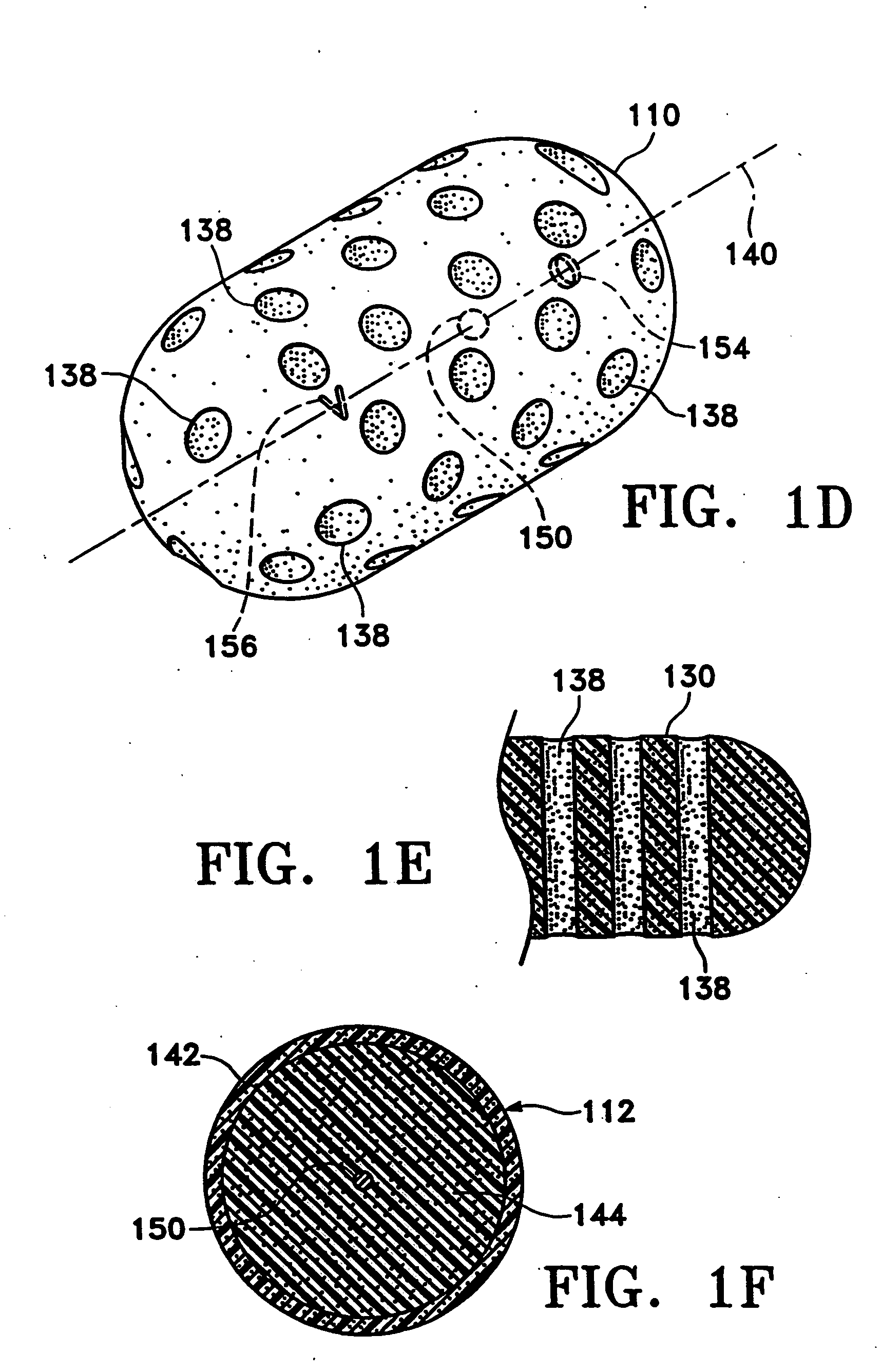
Tissue site markers for in vivo imaging
InactiveUS6993375B2Enhance acoustical reflective signature and signalEasy to detectLuminescence/biological staining preparationSurgical needlesContrast levelIn vivo
Owner:SENORX
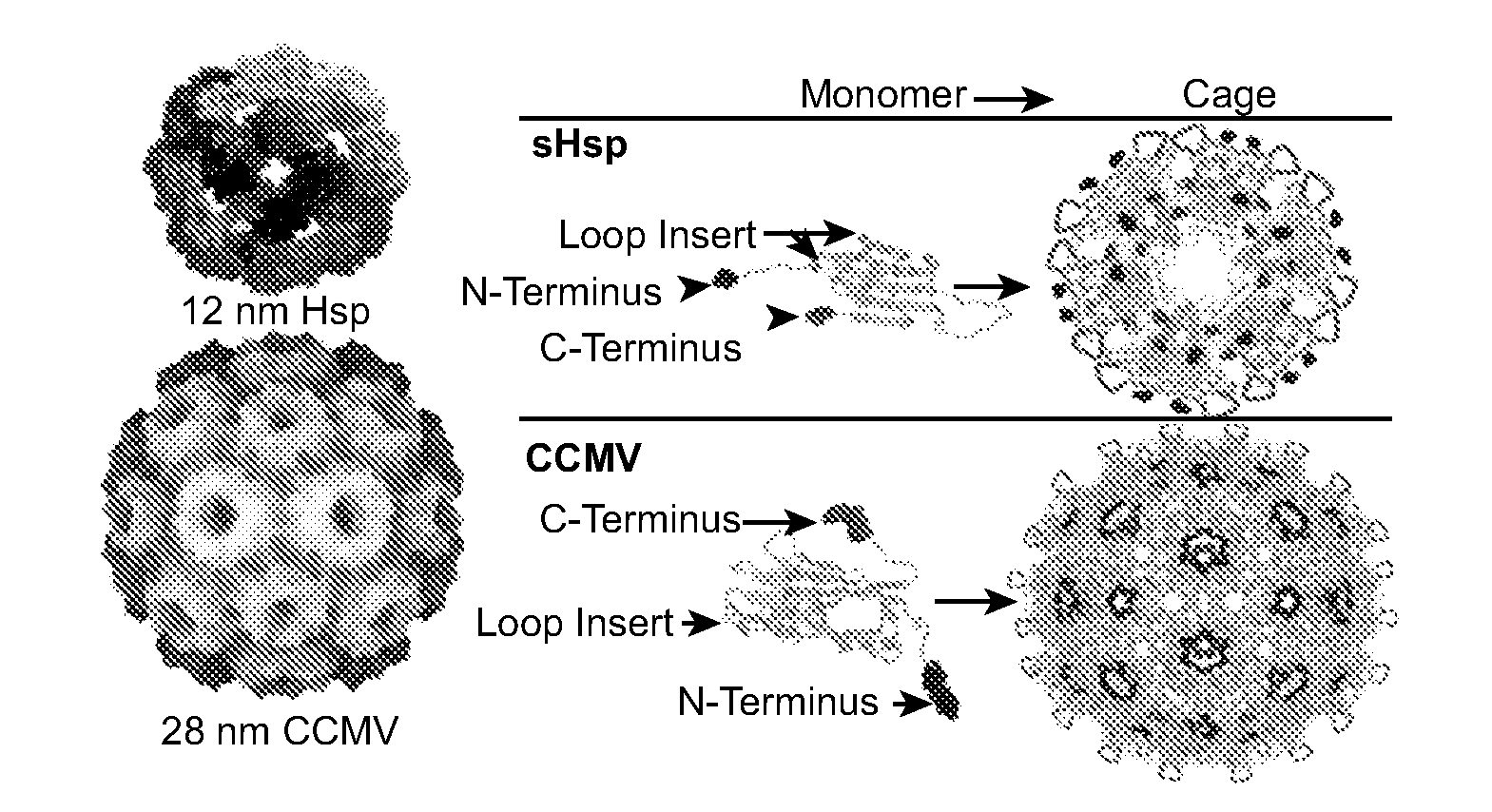
Novel nanoparticles and use thereof
InactiveUS20070258889A1Increase the number ofFacilitate aggregation and crystallizationPowder deliveryLuminescence/biological staining preparationImaging agentProtein cage
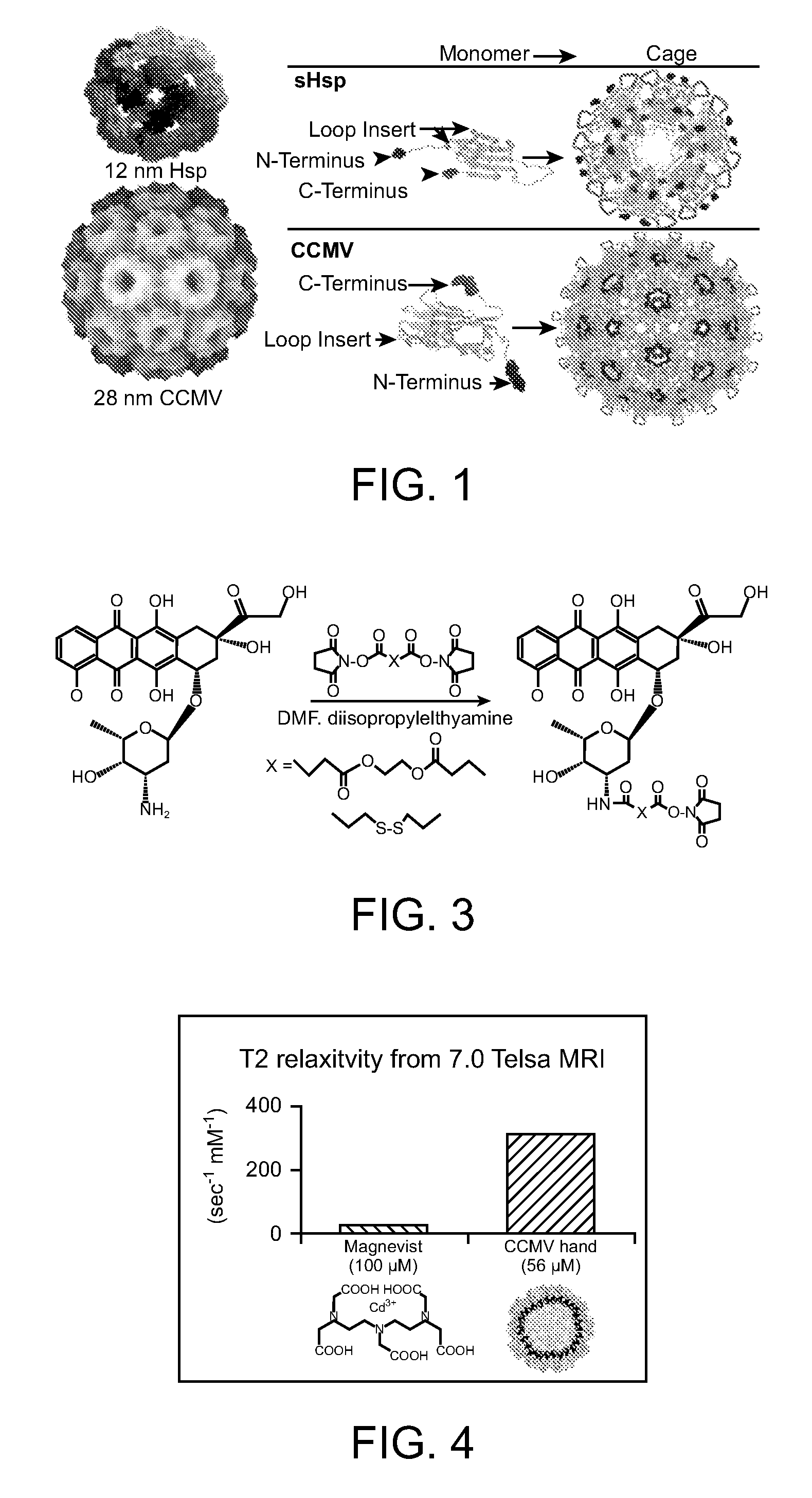
The present invention is directed to novel compositions and methods utilizing delivery agents or nanoparticles that include protein cages with modified and / or unmodified subunits and various agents, such as therapeutic and / or imaging agents located on the interior and / or exterior surface of the protein cages.
Owner:MONTANA STATE UNIVERSITY
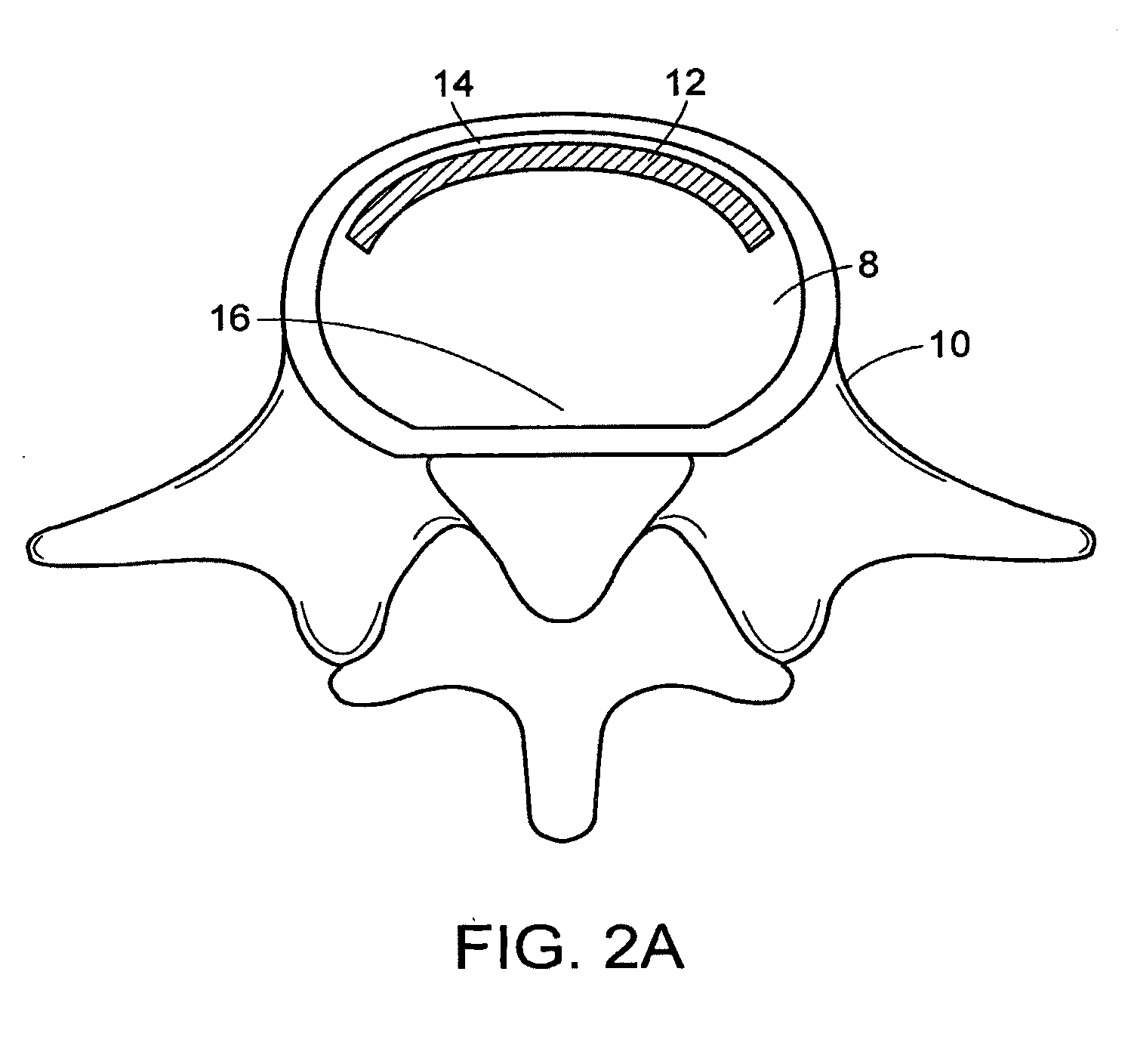
In-situ intervertebral fusion device and method
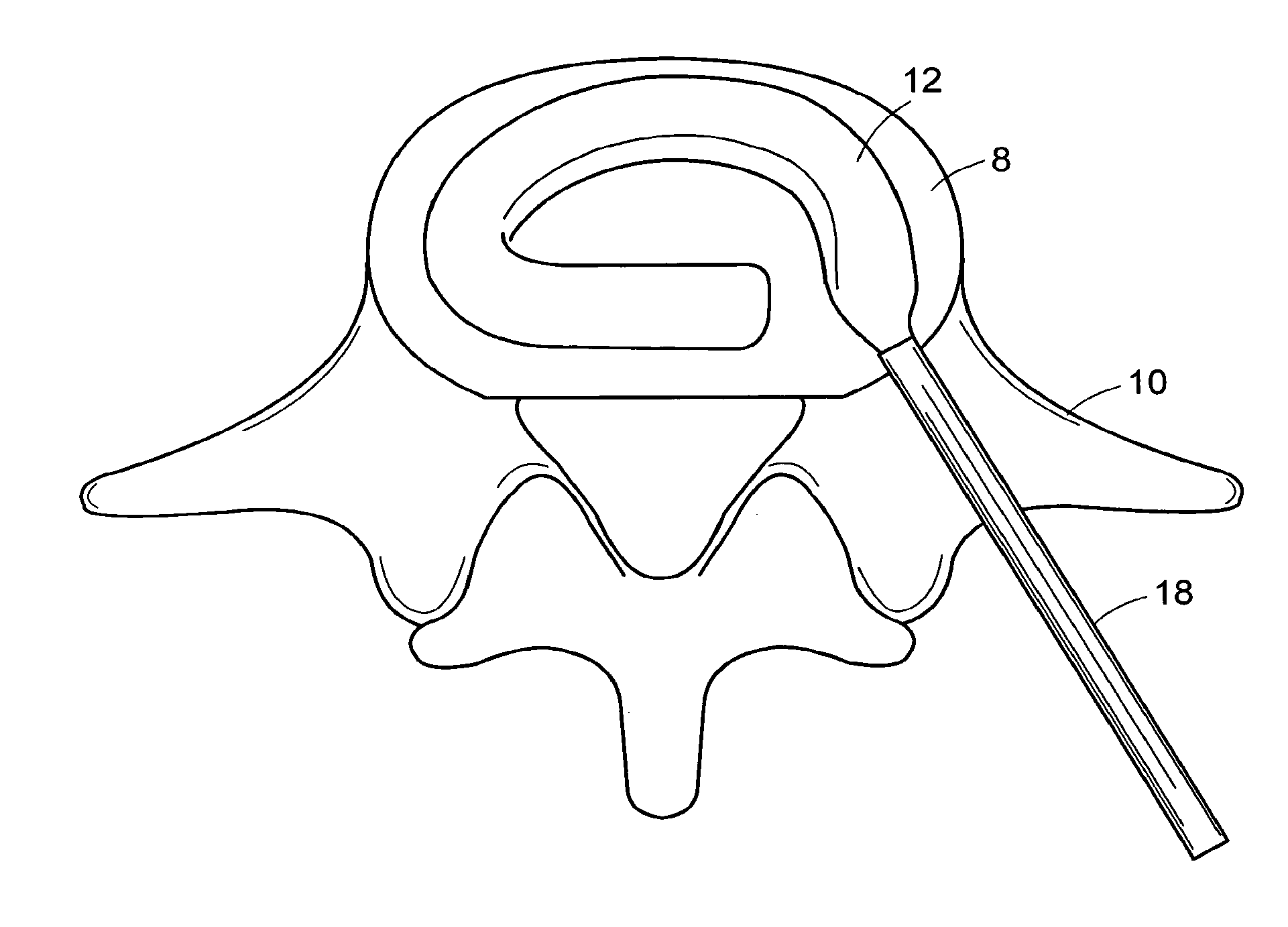
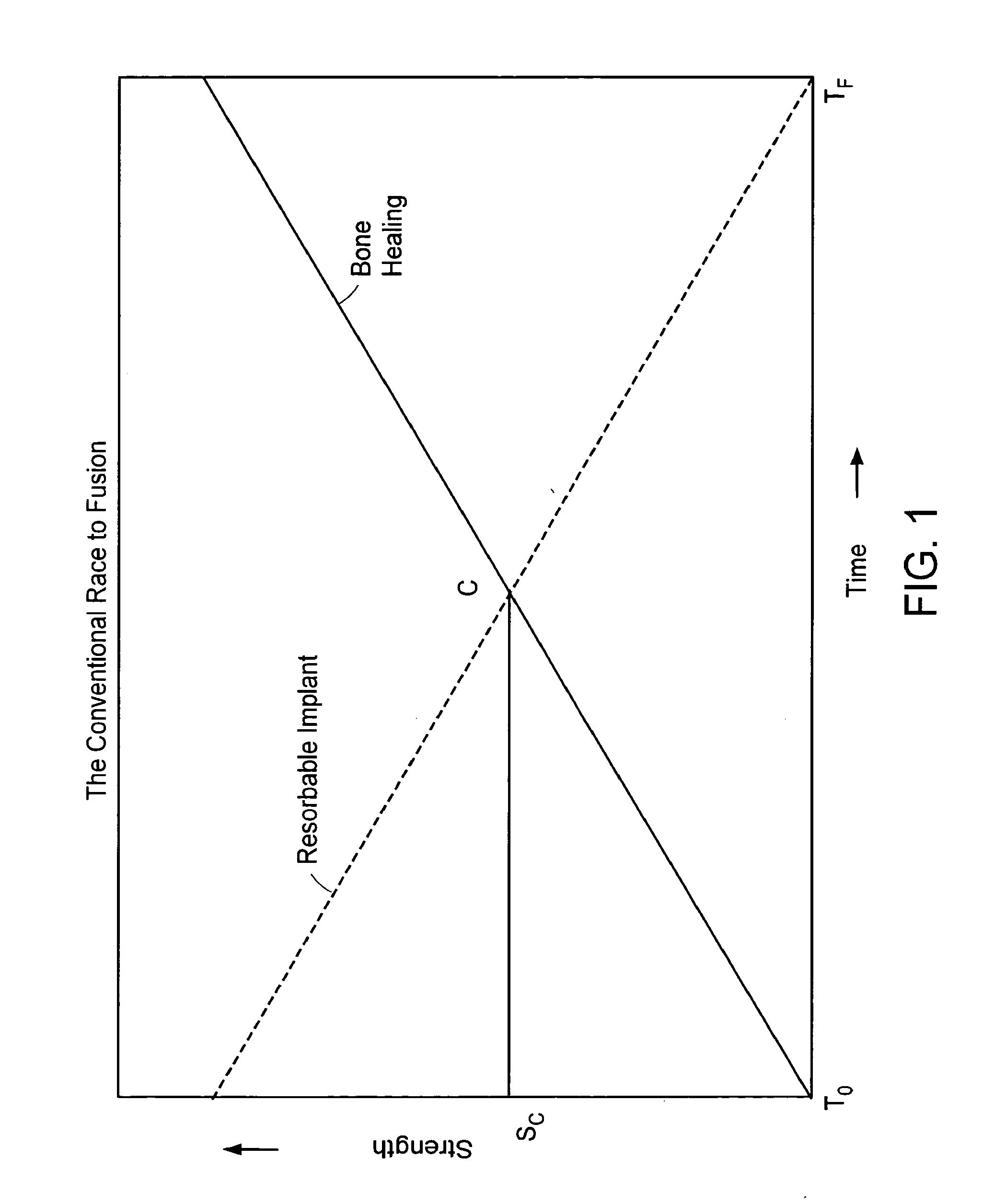
ActiveUS20120310352A1Increase disc heightMinimally invasiveLuminescence/biological staining preparationBone implantIntervertebral spaceVertebral bone
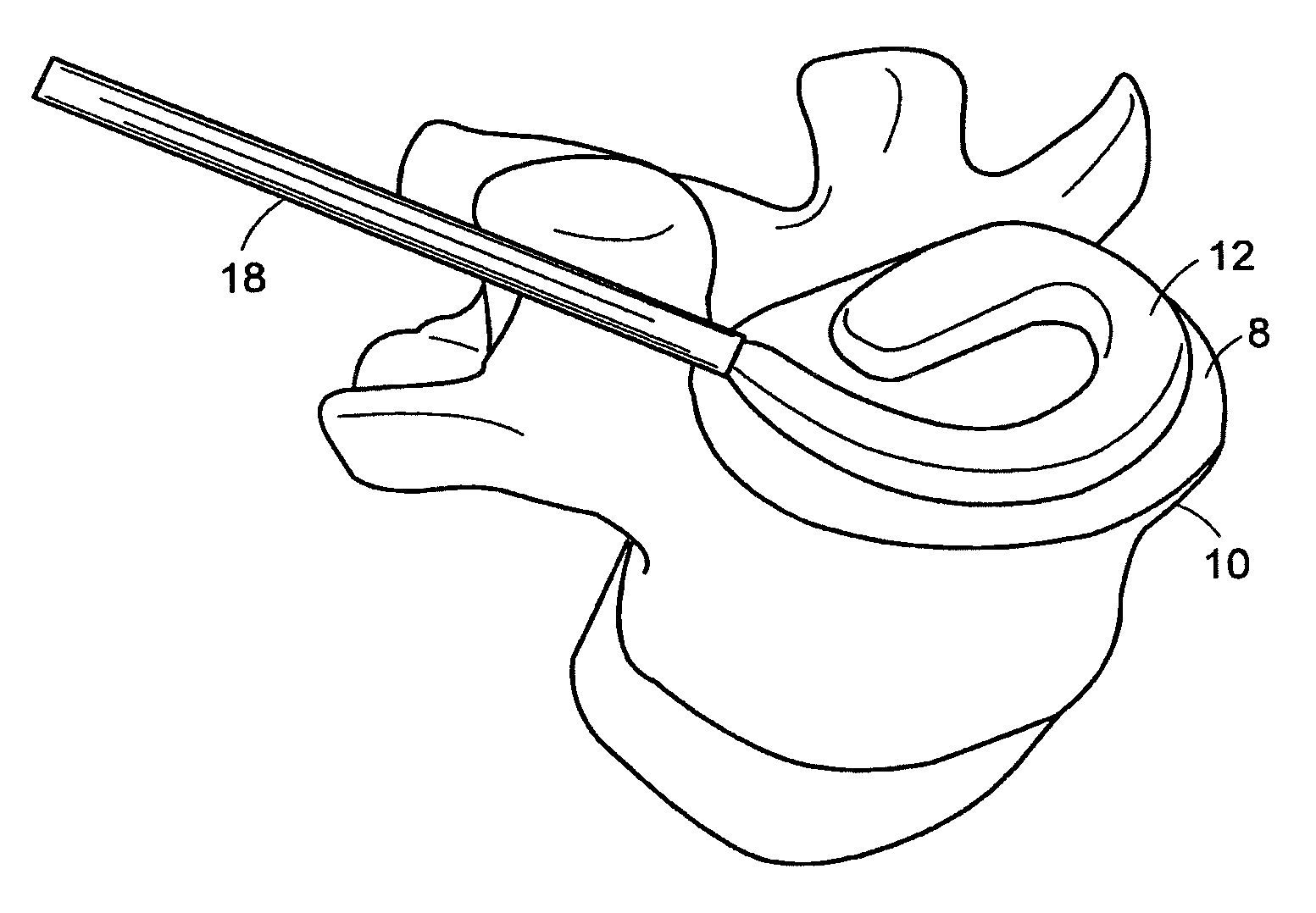
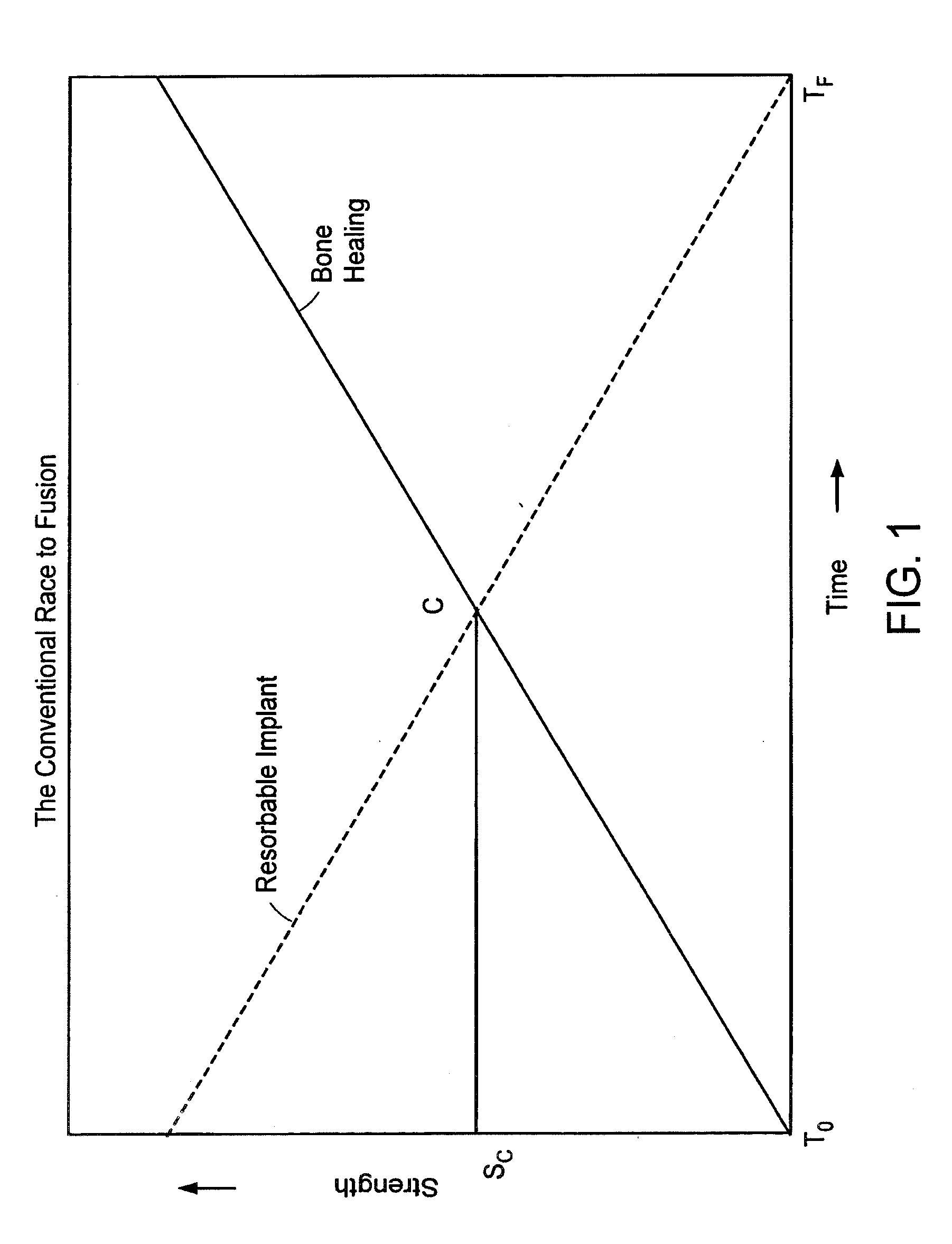
An orthopedic device for implanting between adjacent vertebrae comprising: an arcuate balloon and a hardenable material within said balloon.In some embodiments, the balloon has a footprint that substantially corresponds to a perimeter of a vertebral endplate. An inflatable device is inserted through a cannula into an intervertebral space and oriented so that, upon expansion, a natural angle between vertebrae will be at least partially restored. At least one component selected from the group consisting of a load-bearing component and an osteobiologic component is directed into the inflatable device through a fluid communication means.
Owner:DEPUY SYNTHES PROD INC +1
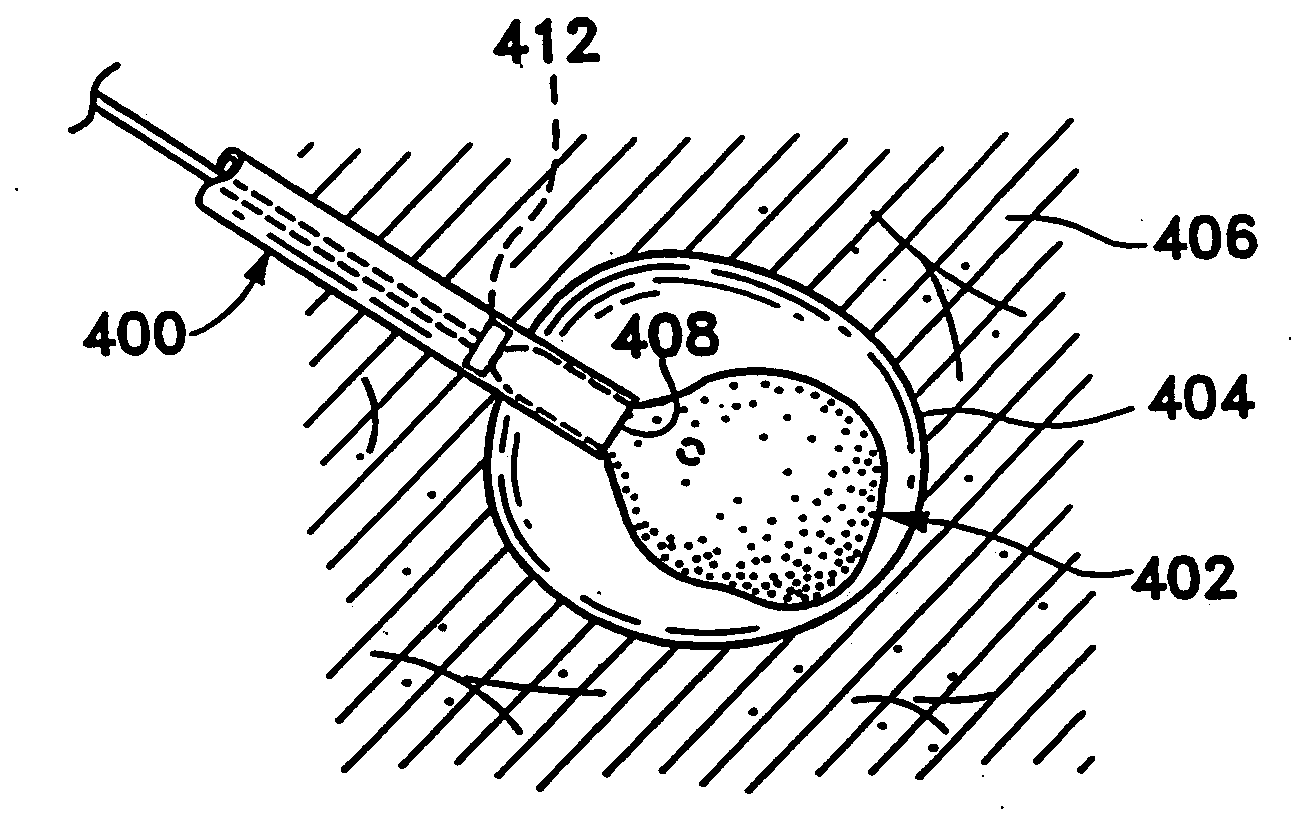
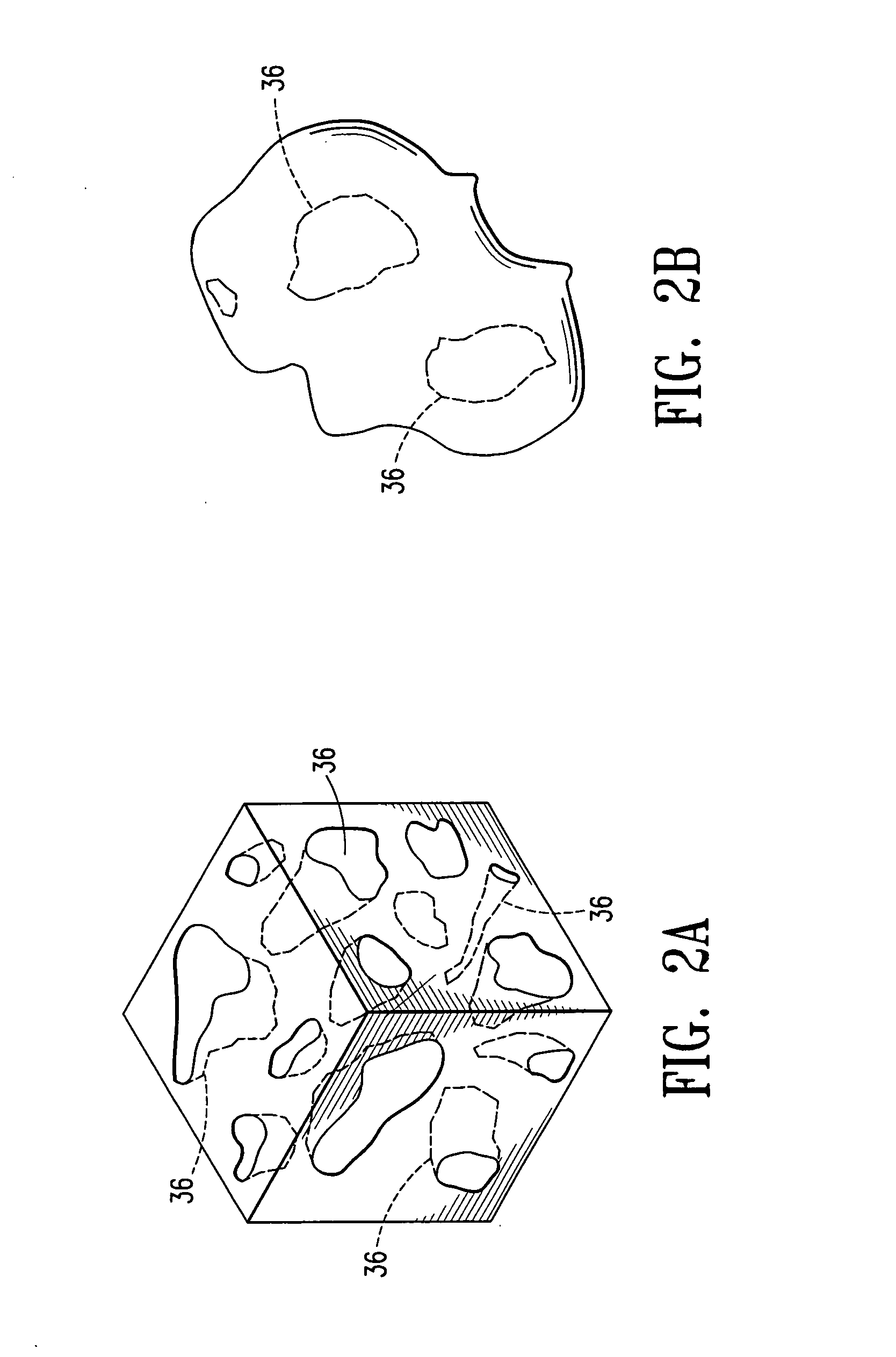
Cavity-filling biopsy site markers
InactiveUS6862470B2Easy to detectLuminescence/biological staining preparationSurgical needlesAnesthetic AgentMaximum dimension
The invention provides materials, devices and methods for marking biopsy sites for a limited time. The biopsy-marking materials are ultrasound-detectable bio-resorbable powders, with powder particles typically between about 20 microns and about 800 microns in maximum dimension, more preferably between about 300 microns and about 500 microns. The powders may be formed of polymeric materials containing cavities sized between about 10 microns and about 500 microns, and may also contain binding agents, anesthetic agents, hemostatic agents, and radiopaque markers. Devices for delivering the powders include tubes configured to contain the powders and to fit within a biopsy cannula, the powders being ejected by action of a syringe. Systems may include a tube containing powder, and a syringe containing sterile saline. The tube may be configured to fit within a biopsy cannula such as a Mammotome® or SenoCor 360™ cannula.
Owner:SENORX
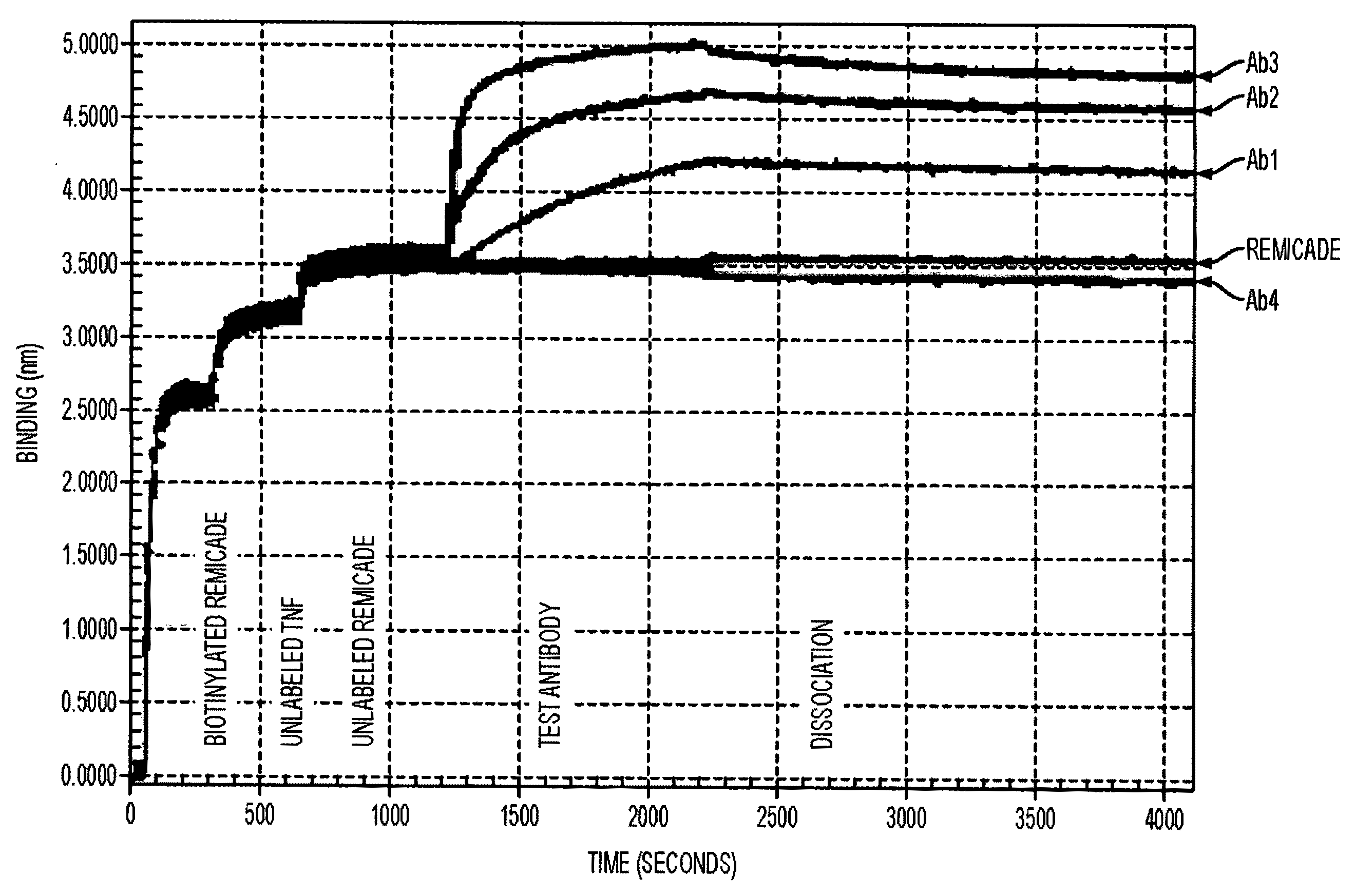
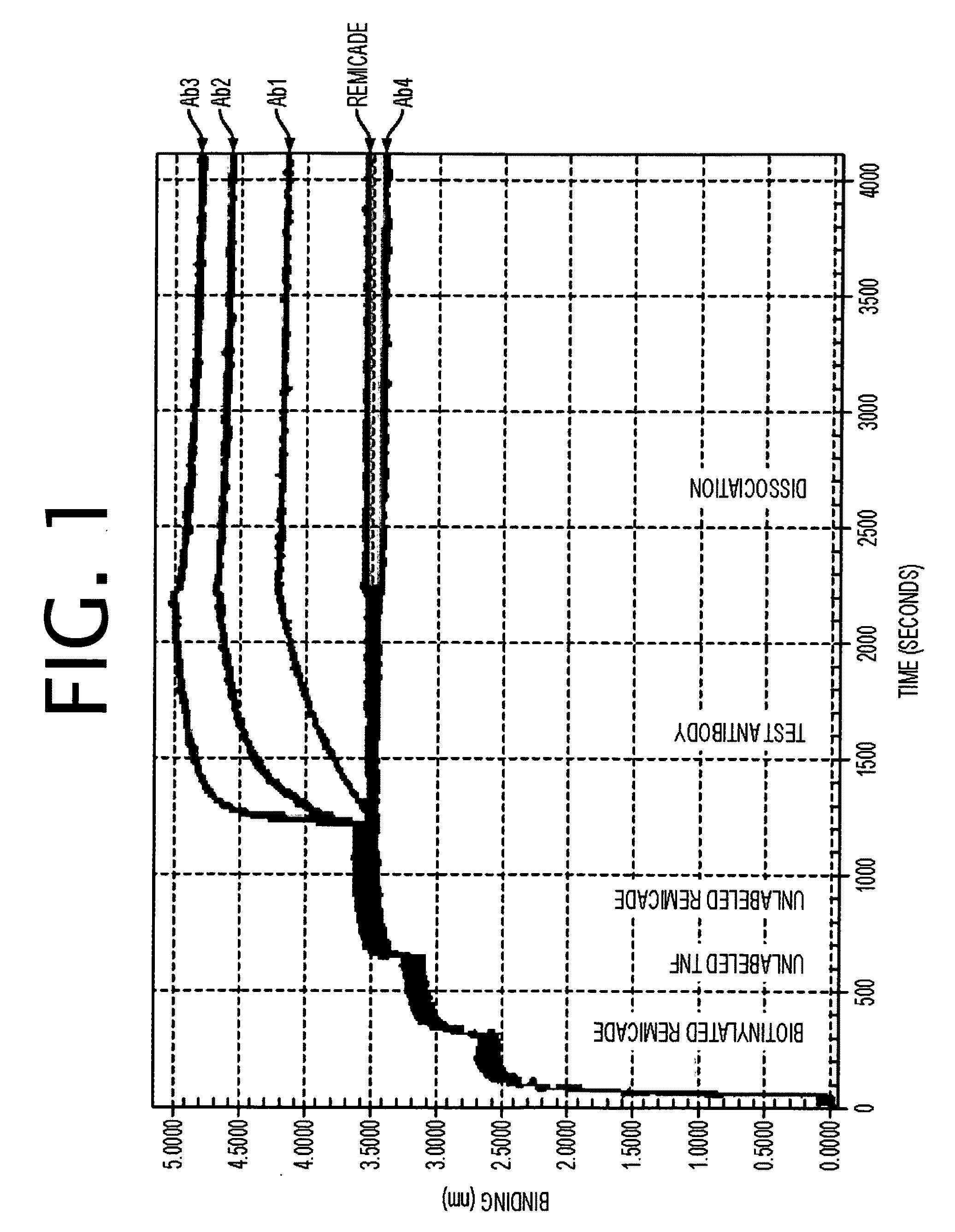
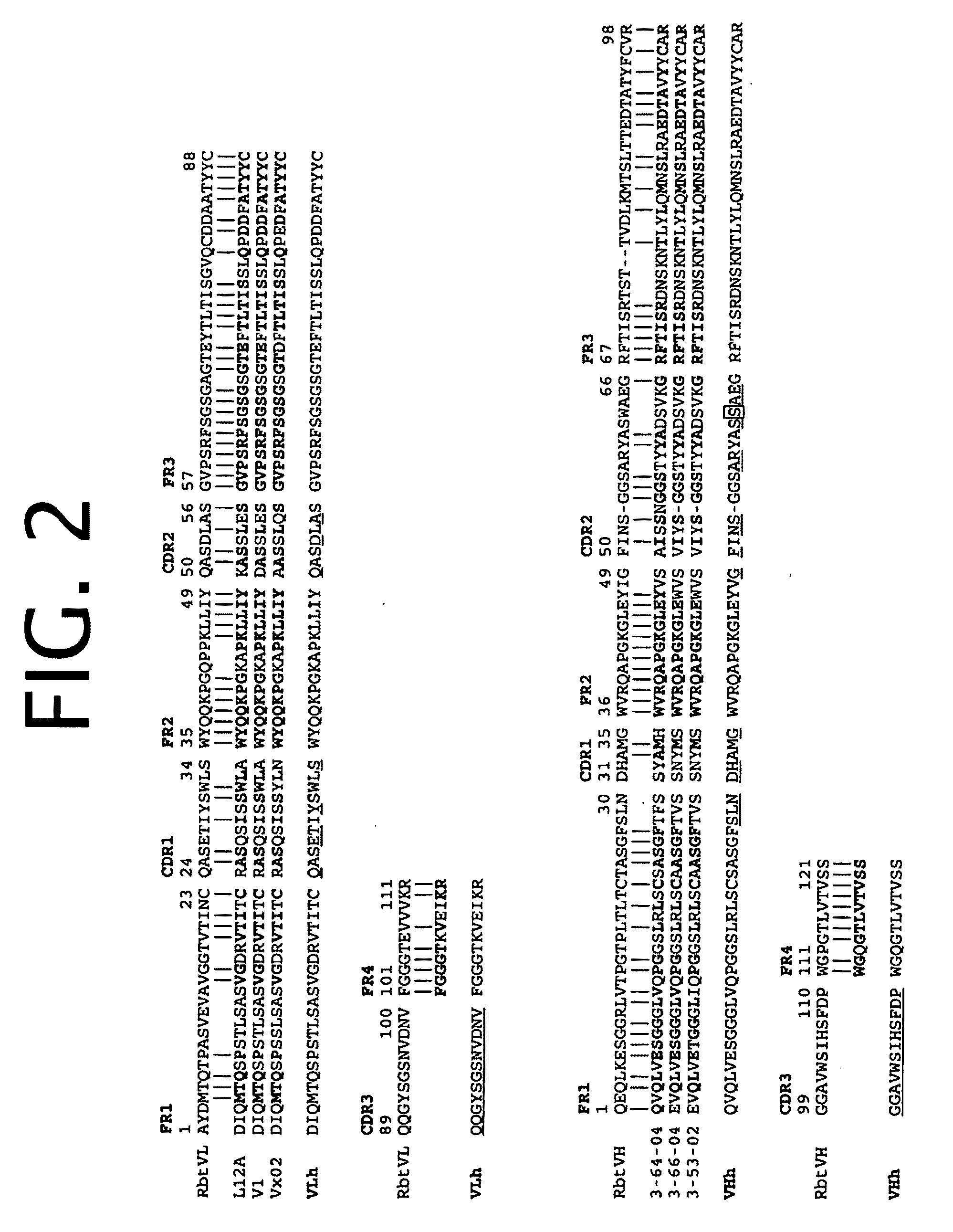
Antibodies to TNF alpha and use thereof
Owner:ALDERBIO HLDG LLC +1
Imageable biopsy site marker
InactiveUS20060122503A1Accurately excise and remove a quantityMark accuratelyLuminescence/biological staining preparationOrgan movement/changes detectionRadiologyPiston
A biopsy site marker having at least one small marker body or pellet of bioresorbable material such as gelatin, collagen, polylactic acid, polyglycolic acid which has a radiopaque object, preferably with a non-biological configuration. The at least one bioresorbable body or pellet with a radiopaque object is deposited into the biopsy site, by an delivery device that includes an elongated tubular body with a piston slidable within the tubular body. One end of the tube is placed into the biopsy site. At least one but preferably several marker bodies or pellets are deposited sequentially into the biopsy site through the tube. At least the bioresorbable materials of the detectable markers remain present in sufficient quantity to permit detection and location of the biopsy site at a first time point (e.g., 2 weeks) after introduction but clear from the biopsy site or otherwise do not interfere with imaging of tissues adjacent the biopsy site at a second time point (e.g., 5-7 months) after introduction.
Owner:SENORX
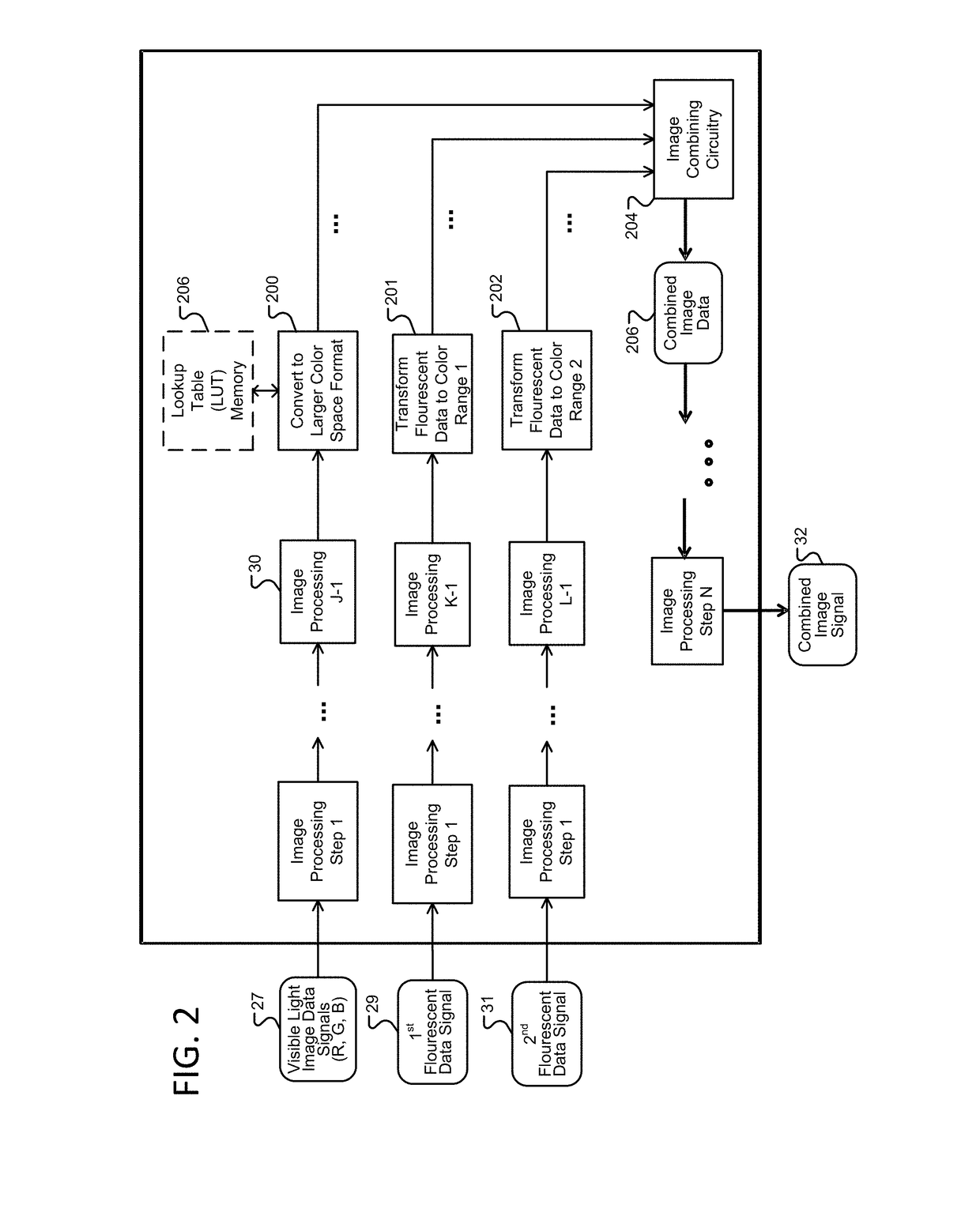
Image transformation and display for fluorescent and visible imaging
ActiveUS20170280029A1Improve the display effectImprove discriminationTelevision system detailsImage enhancementFluorescent imagingTime processing
Improved fluoresced imaging (FI) and other sensor data imaging processes, devices, and systems are provided to enhance display of different secondary types of images and reflected light images together. Reflected light images are converted to a larger color space in a manner that preserves the color information of the reflected light image. FI or secondary images are transformed to a color range within the larger color space, but outside the color area of the reflected light images, allowing the FI or secondary images to be combined with them in a manner with improved distinguishability of color. Hardware designs are provide to enable real-time processing of image streams from medical scopes. The combined images are encoded for an electronic display capable of displaying the larger color space.
Owner:KARL STORZ IMAGING INC
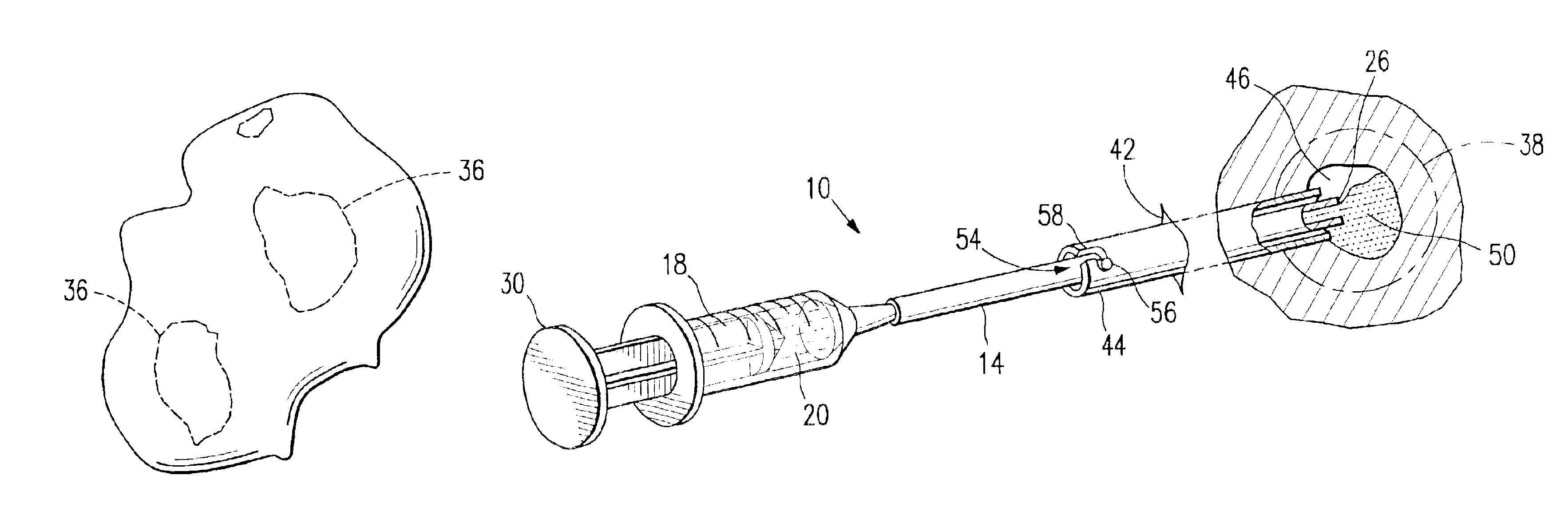
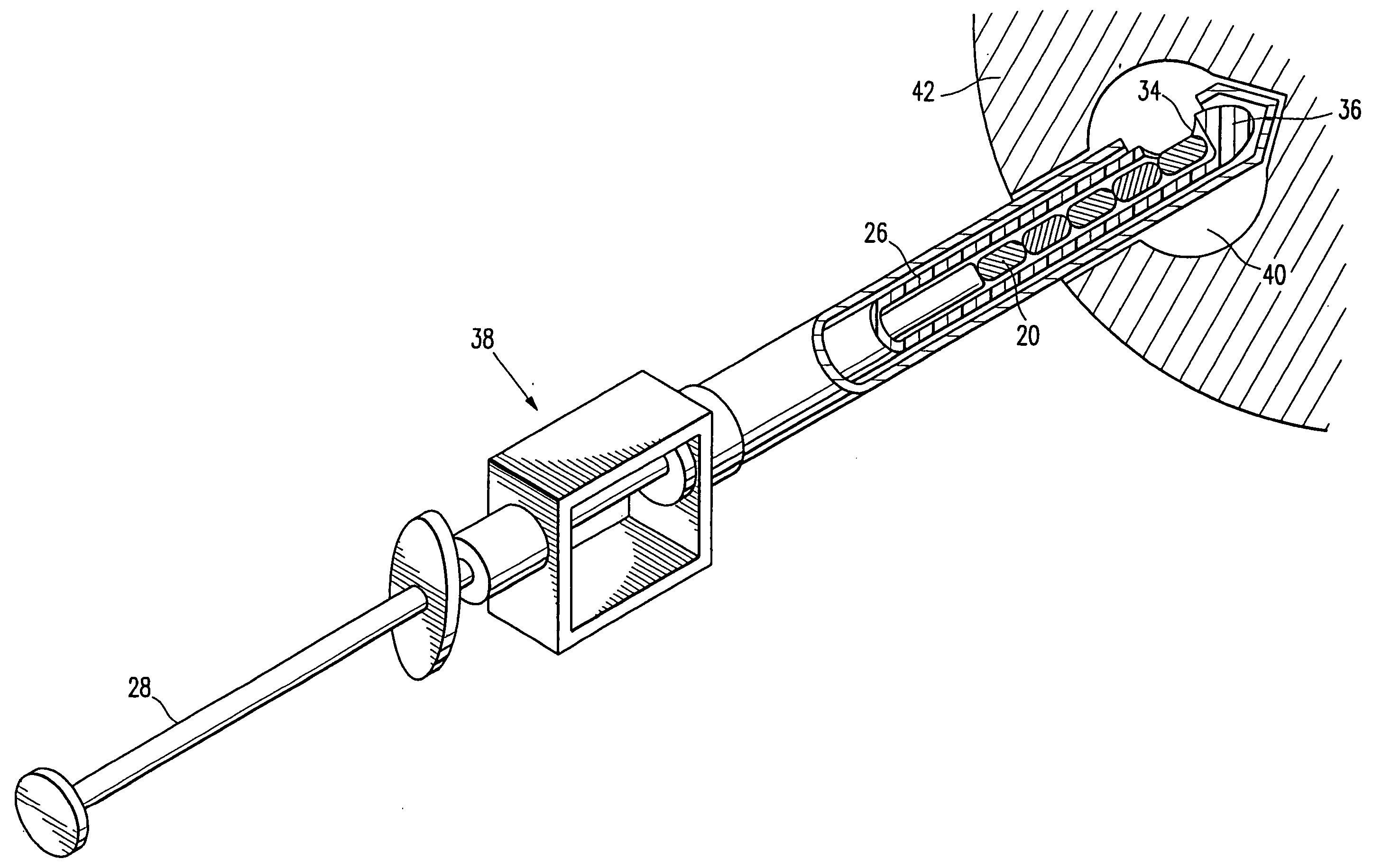
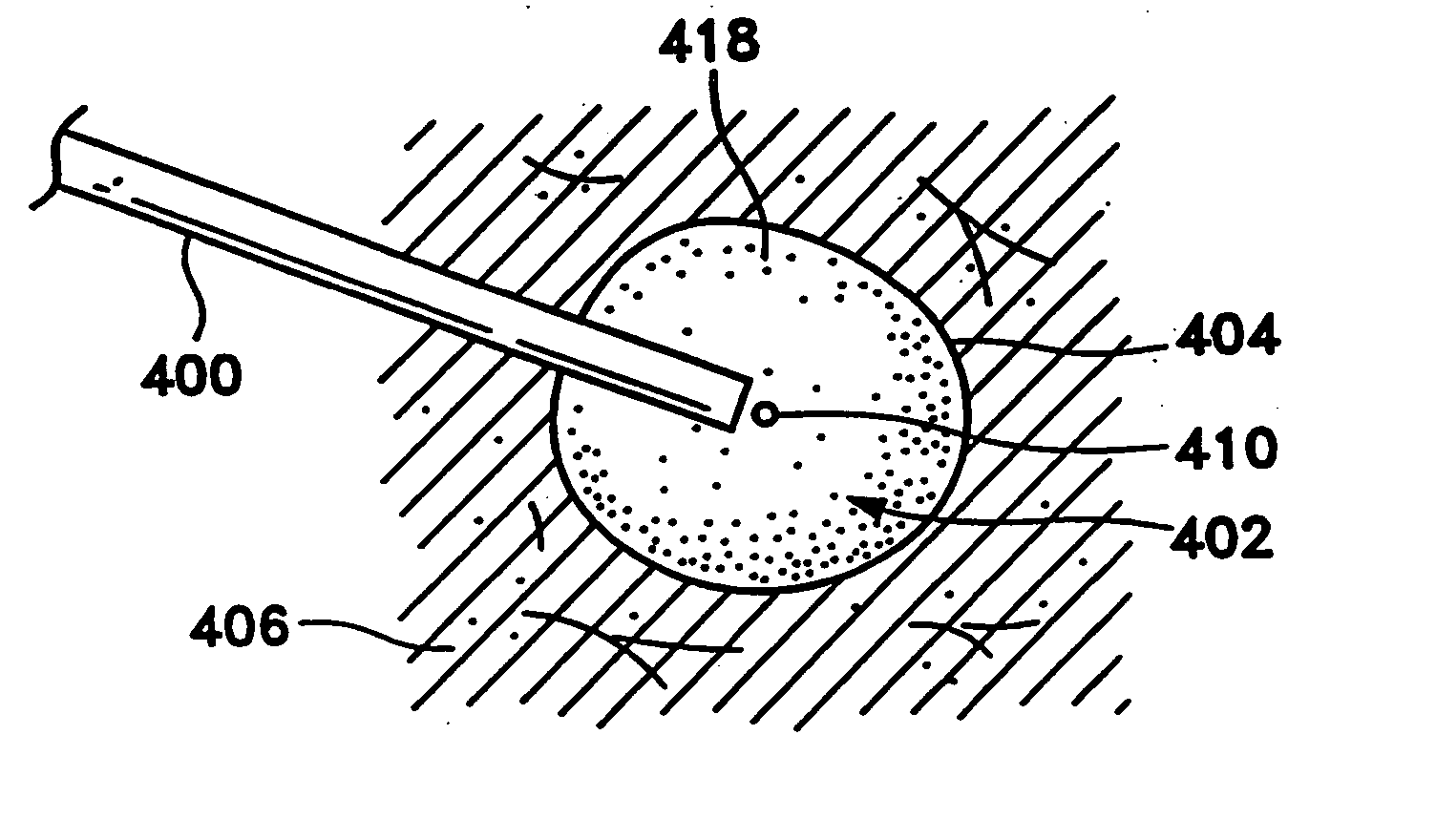
Device and method for safe location and marking of a biopsy cavity
InactiveUS20100234726A1Minimally invasiveEliminate needLuminescence/biological staining preparationSurgerySentinel nodeSentinel lymph node
Cavity and sentinel lymph node marking 412 devices, marker delivery devices, and methods are disclosed. More particularly, upon insertion into a body, the cavity marking device and method enable one to determine the center, orientation, and periphery of the cavity by radiographic, mammography, echogenic, or other noninvasive imaging techniques. A composition and method are disclosed for locating the sentinel lymph node in a mammalian body to determine if cancerous cells have spread thereto. The composition is preferably a fluid composition consisting of a carrier fluid and some type of contrast agent; alternatively, the contrast agent may itself be a fluid and therefore not need a separate carrier fluid. This composition is capable of (1) deposition in or around a lesion and migration to and accumulation in the associated sentinel node, and (2) remote detection via any number of noninvasive techniques. Also disclosed is a method for remotely detecting the location of a sentinel node by (1) depositing a remotely detectable fluid in or around a lesion for migration to and accumulation in the associated sentinel node and (2) remotely detecting the location of that node with a minimum of trauma and toxicity to the patient. The composition and method may serve to mark a biopsy cavity, as well as mark the sentinel lymph node. The marking methods also may combine any of the features as described with the marking device and delivery device.
Owner:DEVICOR MEDICAL PROD
Biopsy cavity marking device
InactiveUS20050080339A1Mark accuratelyUltrasonic/sonic/infrasonic diagnosticsLuminescence/biological staining preparationX-rayColloid bodies
Owner:DEVICOR MEDICAL PROD
Biopsy cavity marking device and method
InactiveUS20050059888A1Minimize impactMark accuratelyUltrasonic/sonic/infrasonic diagnosticsLuminescence/biological staining preparationGeneral surgery
These are biopsy cavity marking devices and methods. The devices and methods include a bioabsorbable filler body and a detectable marker attached to the filler body, where the detectable marker has a predetermined shape. The detectable marker may be mammographic, radiopaque, echogenic, or palpable. The predetermined shape may be non-spherical, a barb, a sphere, a ring, a wire, or a band. It may also be affixed to the interior of the body or to the surface of the body. In another embodiment, the device may include a bioabsorbable filler body and a detectable marker attached to the filler body, where the detectable marker is a wire. Methods of using these devices to mark a biopsy cavity are also included.
Owner:DEVICOR MEDICAL PROD
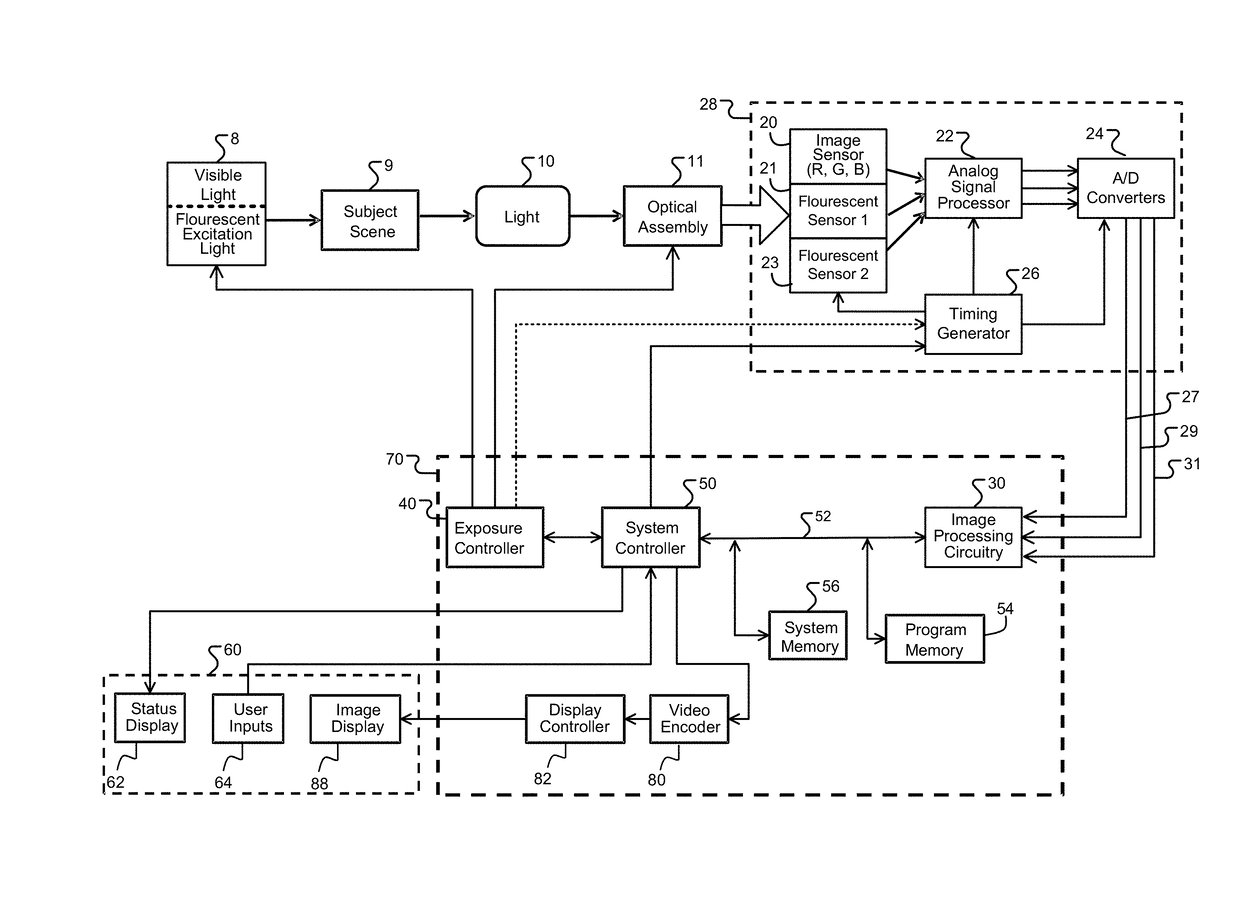
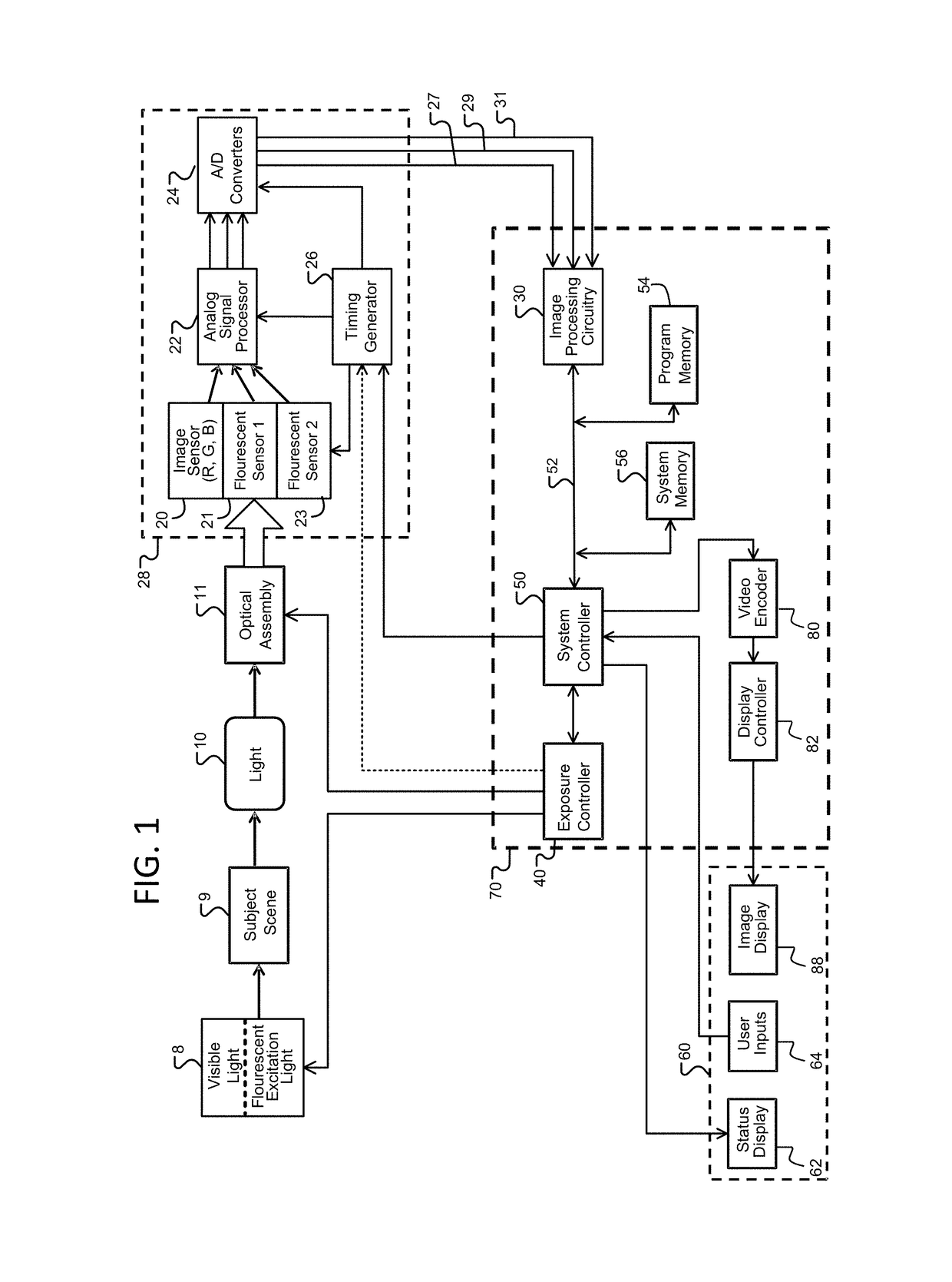
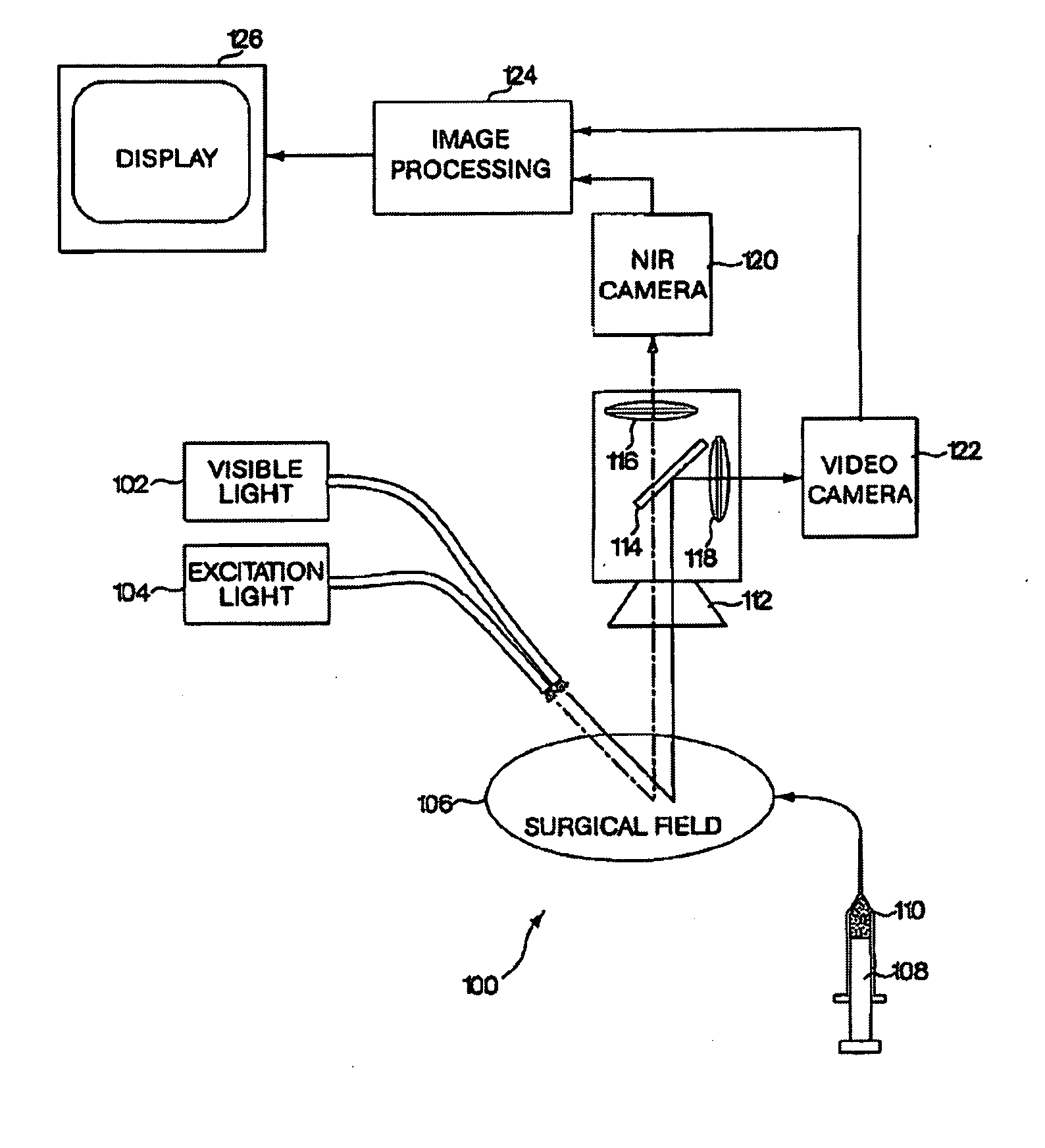
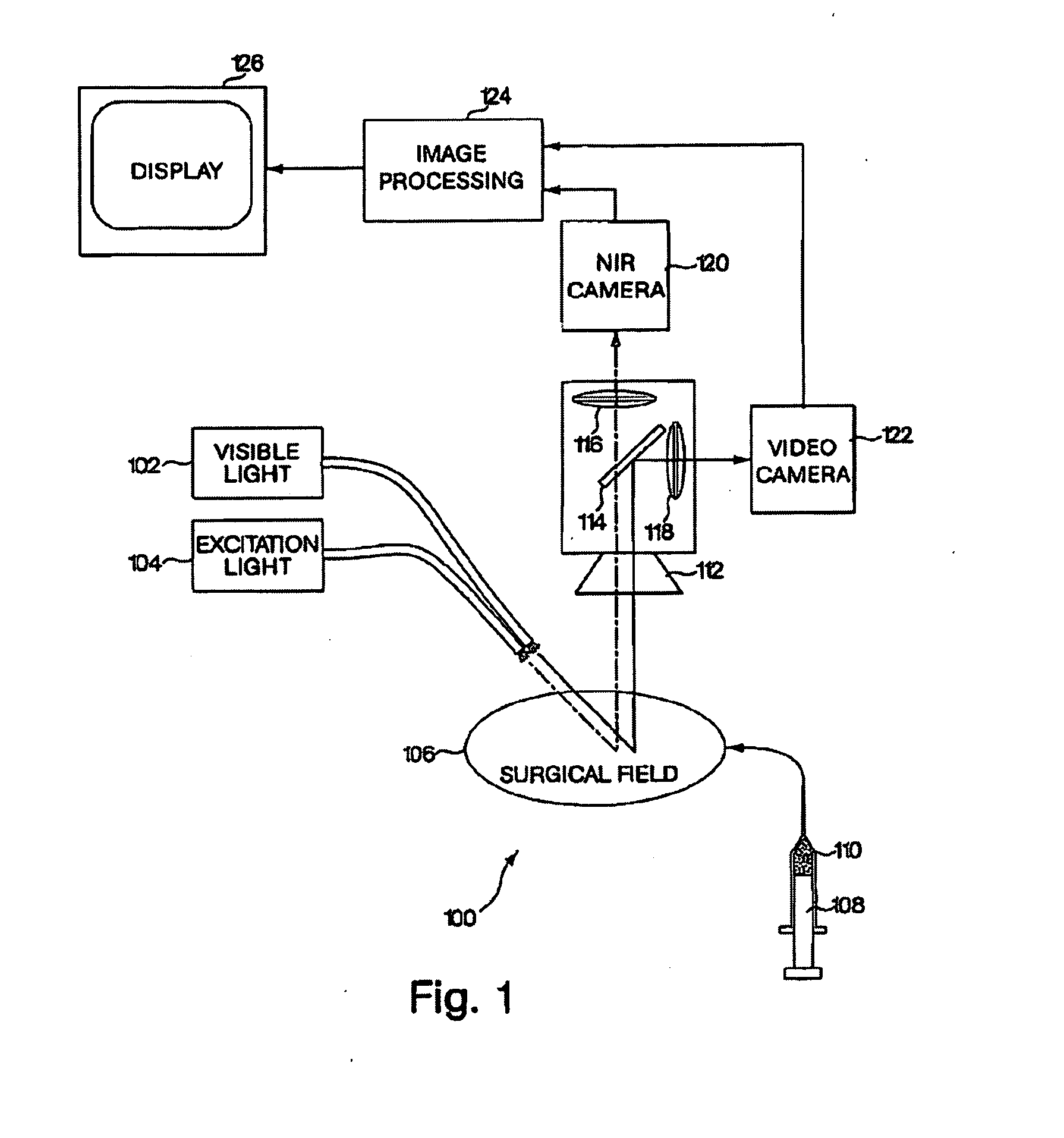
Multi-channel medical imaging system
ActiveUS20100262017A1Luminescence/biological staining preparationNanoinformaticsFluorescenceBlood stream
A medical imaging system provides simultaneous rendering of visible light and fluorescent images. The system may employ dyes in a small-molecule form that remain in a subject's blood stream for several minutes, allowing real-time imaging of the subject's circulatory system superimposed upon a conventional, visible light image of the subject. The system may provide an excitation light source to excite the fluorescent substance and a visible light source for general illumination within the same optical guide used to capture images. The system may be configured for use in open surgical procedures by providing an operating area that is closed to ambient light. The systems described herein provide two or more diagnostic imaging channels for capture of multiple, concurrent diagnostic images and may be used where a visible light image may be usefully supplemented by two or more images that are independently marked for functional interest.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
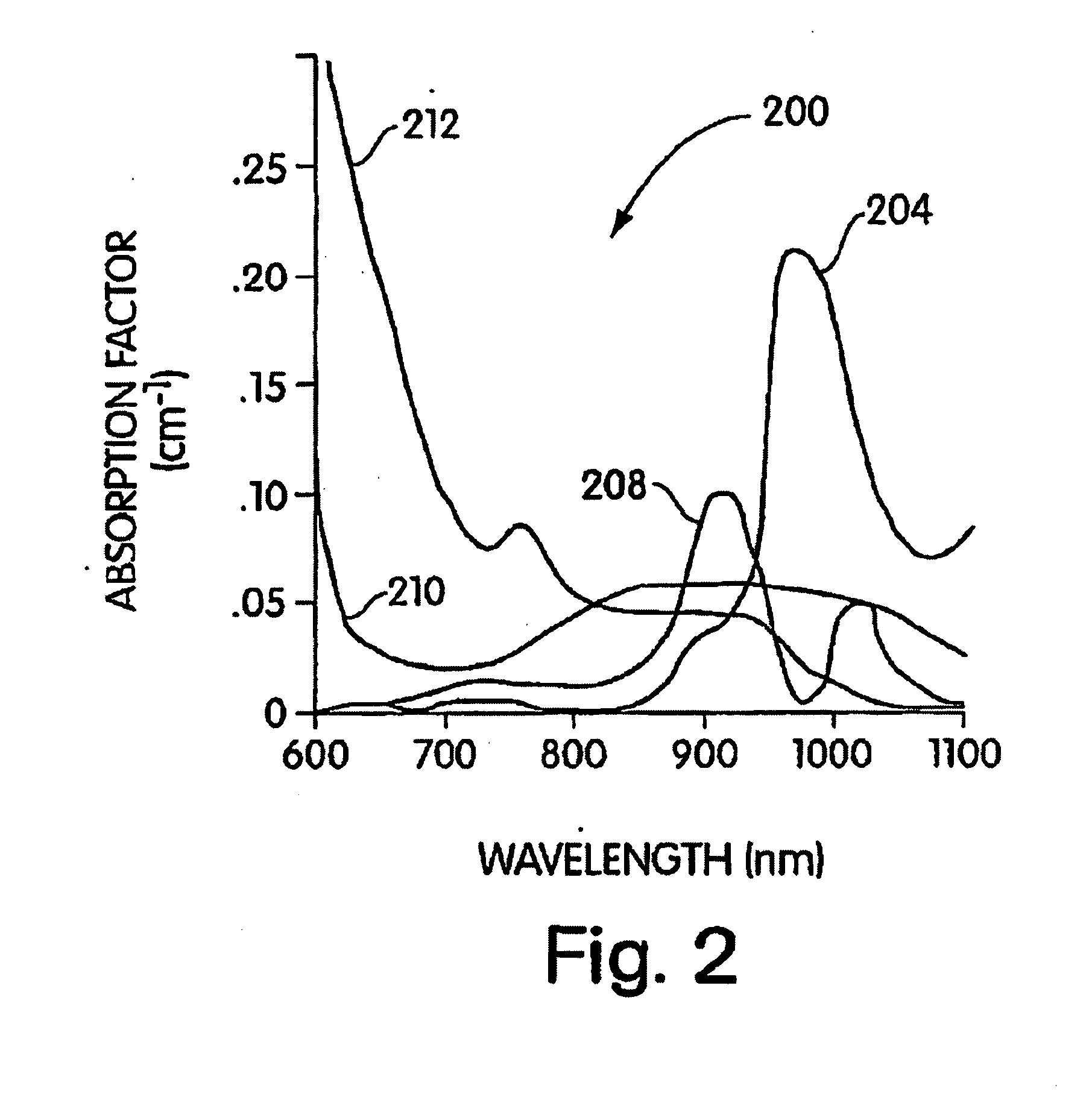
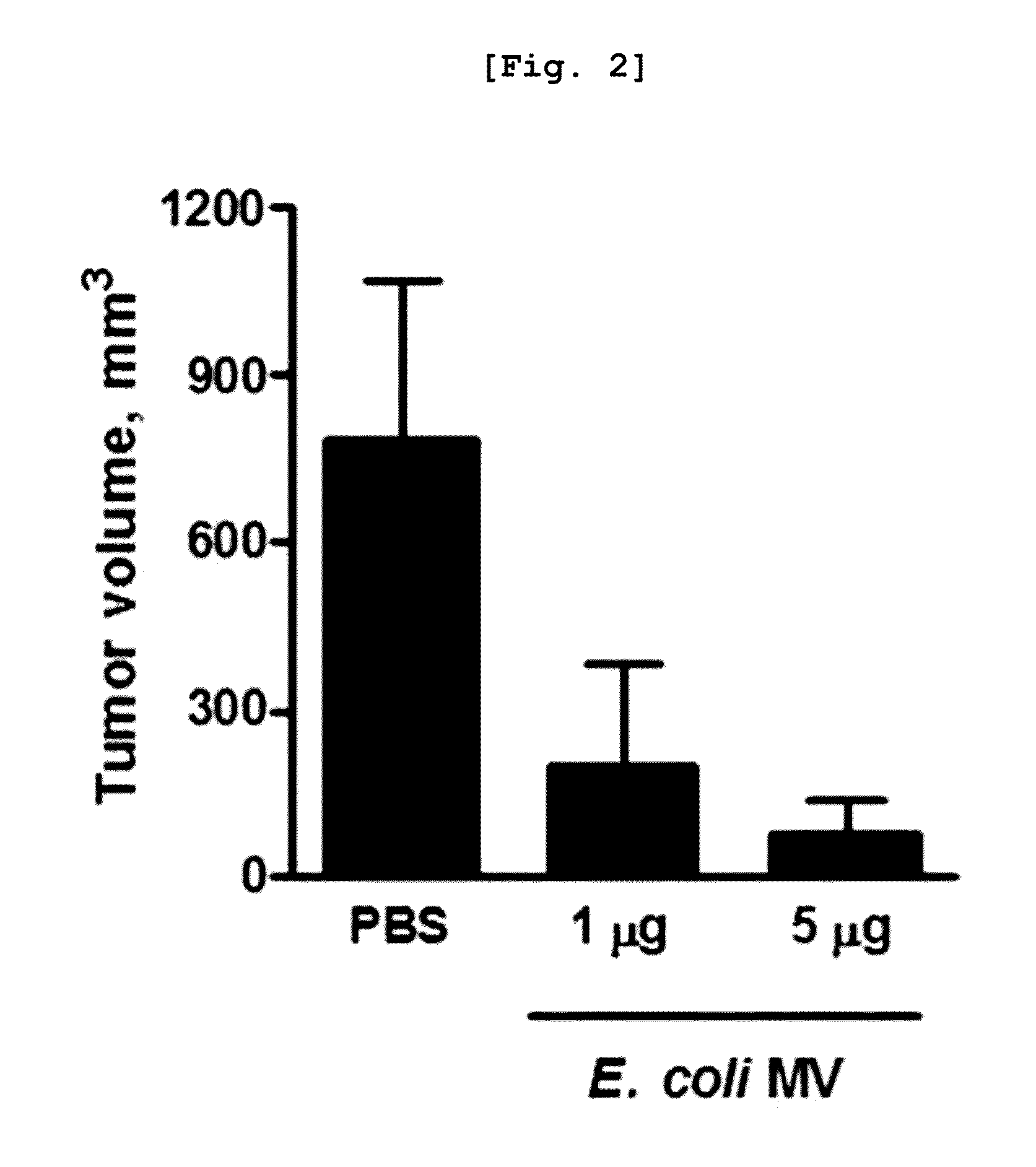
Method for treating and diagnosing cancer by using cell-derived microvesicles
ActiveUS20130195765A1Eliminate side effectsReduce inconvenienceUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsDiseaseSide effect
Disclosed is a method for the treatment and / or diagnosis of cancer, using bacterial cell-derived microvesicles, which is highly effective in cancer therapy with a significant reduction in side effects. Also, a method is provided for delivering of a drug therapeutic or diagnostic for a disease, using bacterial cell-derived microvesicles loaded with the drug, thereby treating and diagnosing the disease effectively and specifically.
Owner:ROSETTA EXOSOME CO LTD
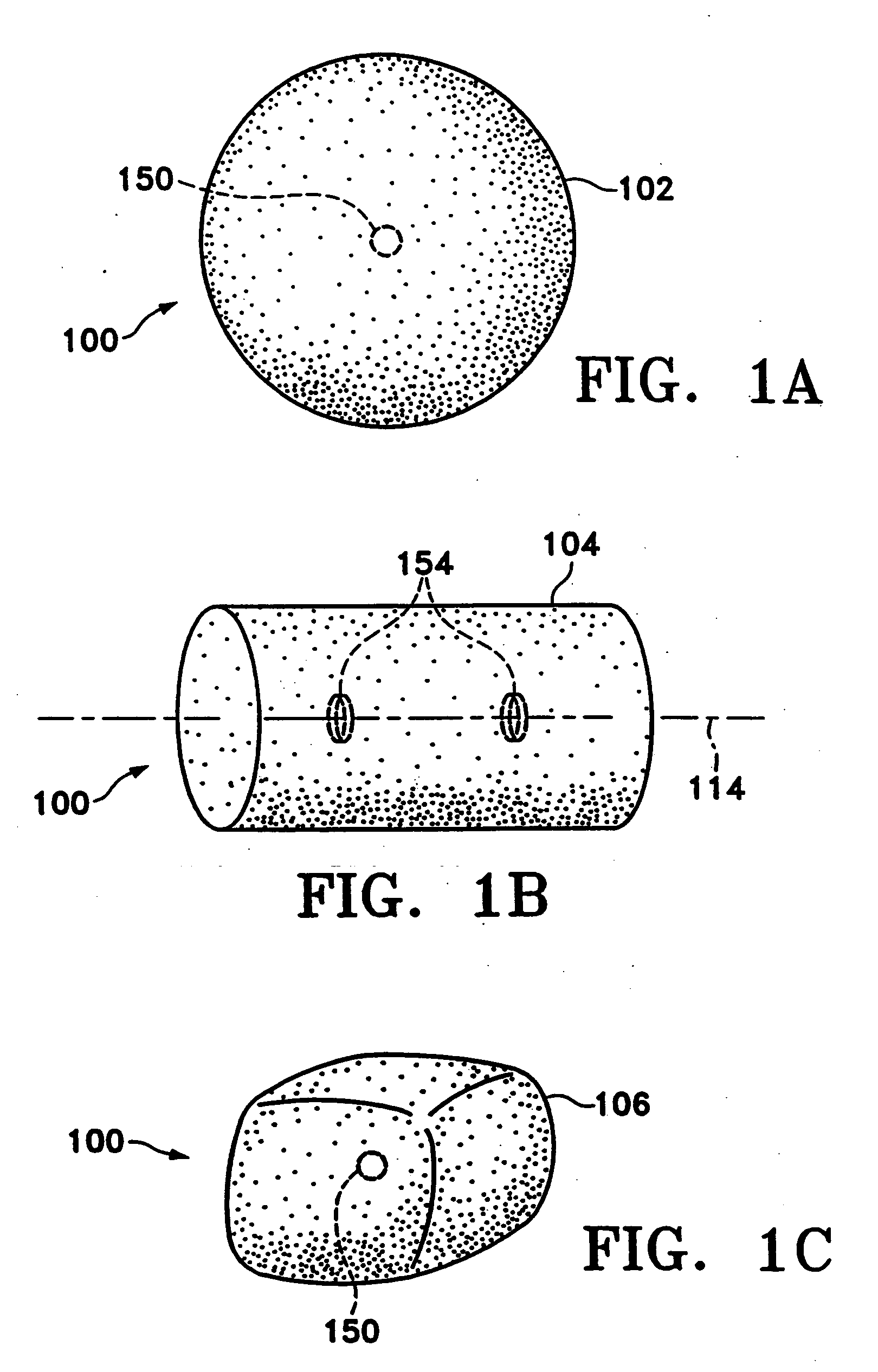
Biopsy site marker
InactiveUS20050080337A1Mark accuratelyUltrasonic/sonic/infrasonic diagnosticsLuminescence/biological staining preparationX-rayGelatin product
These are biopsy site marking devices. More particularly, the devices include a body of gelatin and an x-ray detectable body of a specific, predetermined non-biological configuration embedded in the body of gelatin. In one embodiment, the x-ray detectable body is made from metal. In alternative embodiments, the x-ray detectable body can be made from stainless steel or metal oxides.
Owner:DEVICOR MEDICAL PROD
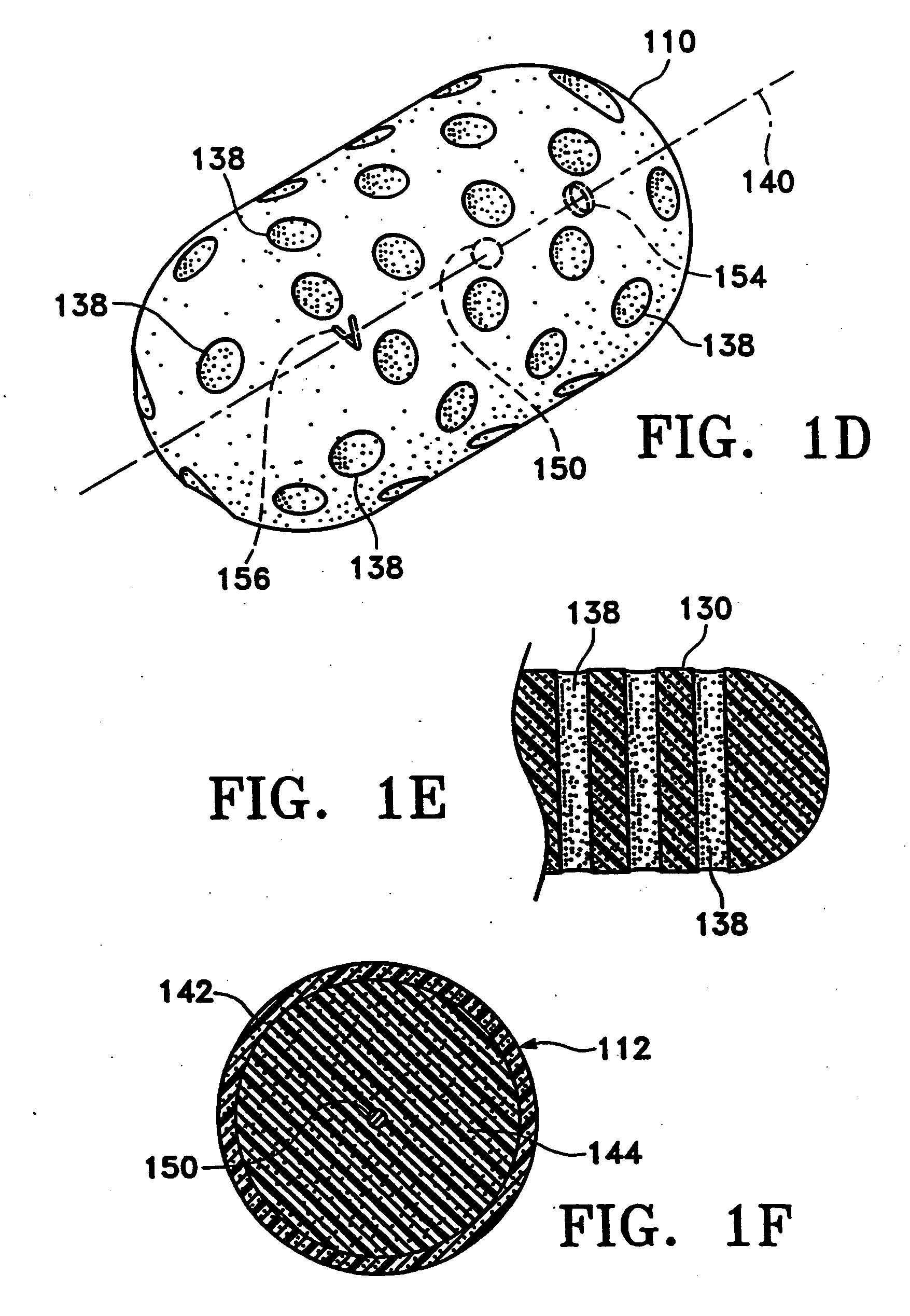
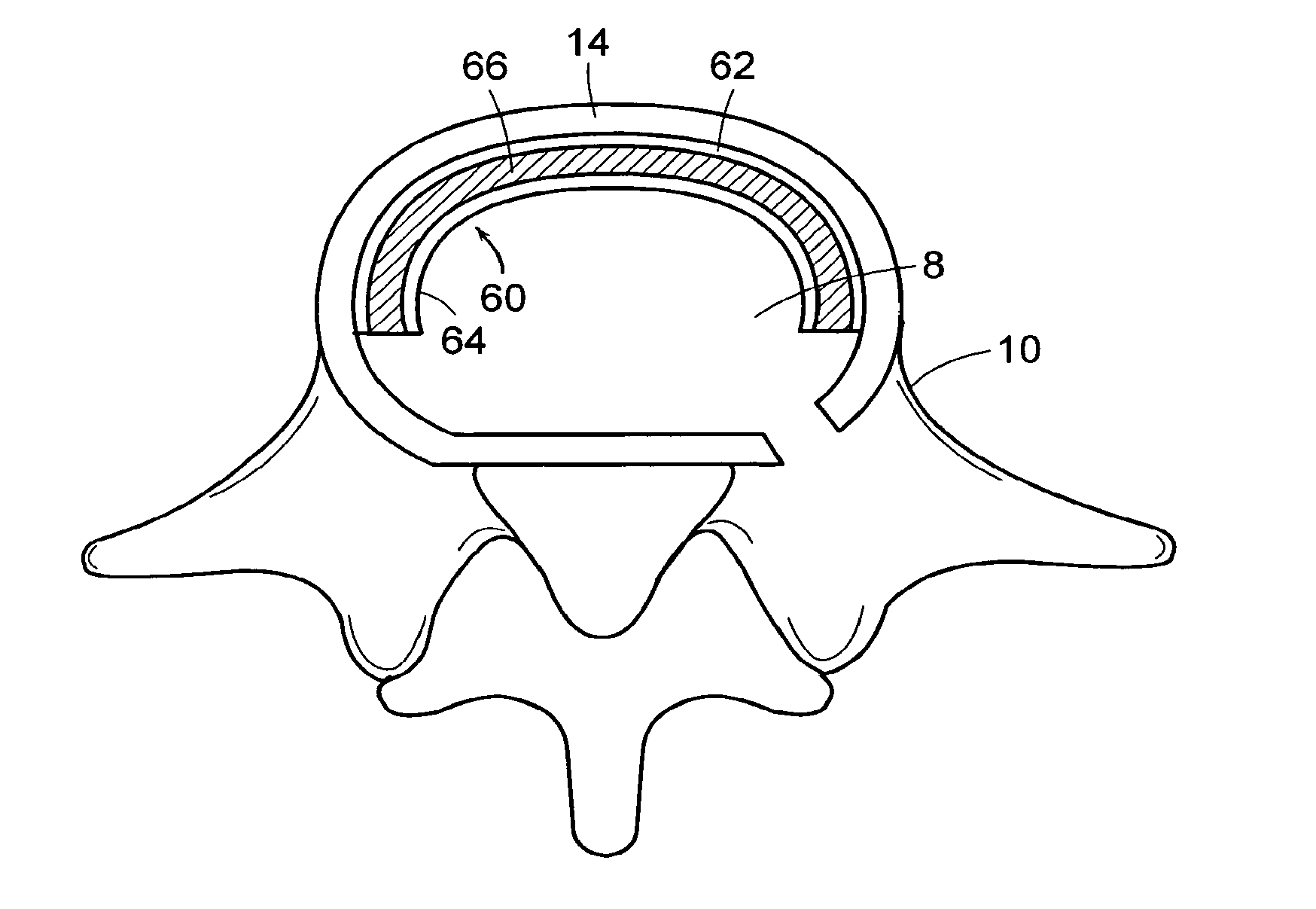
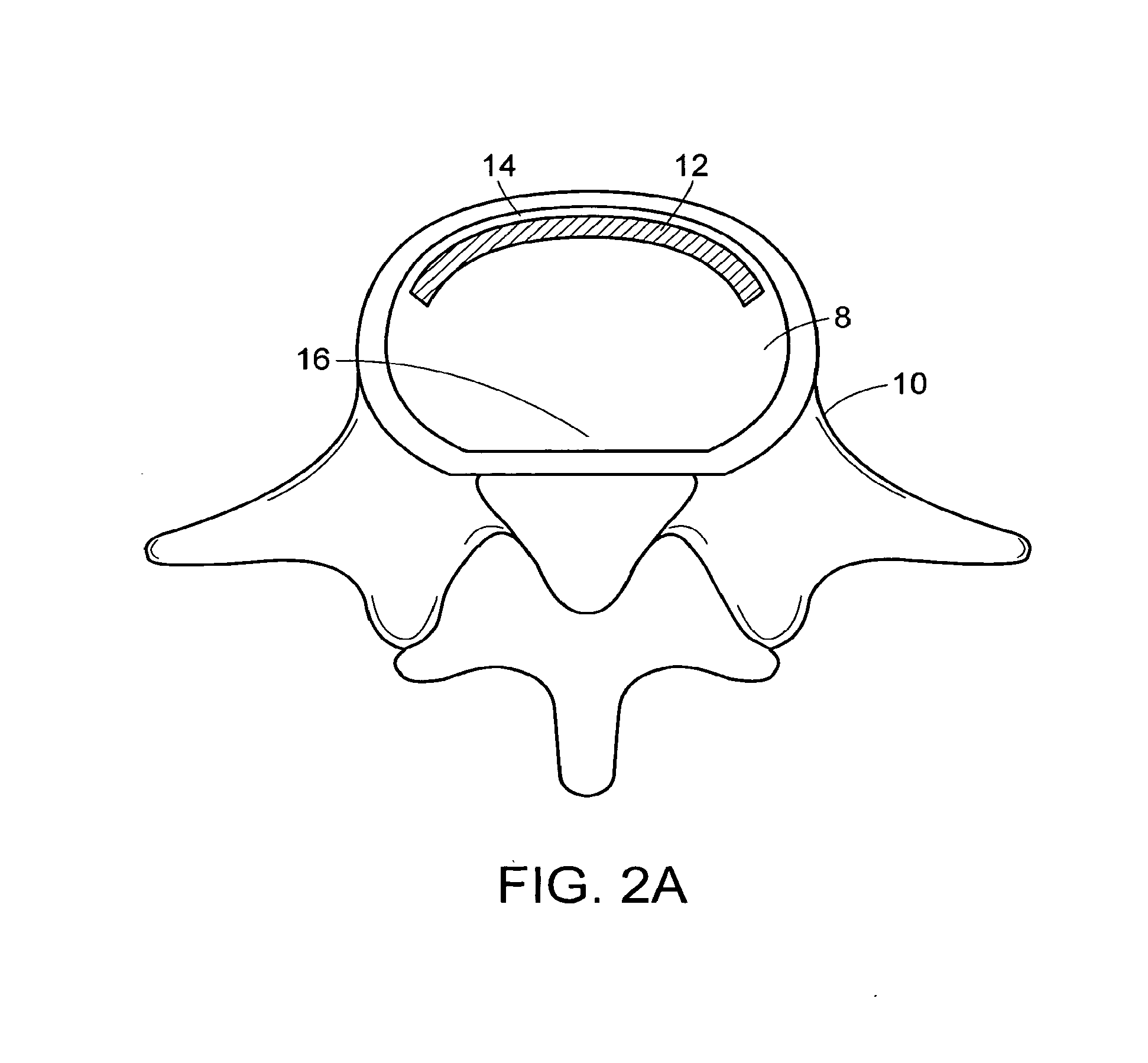
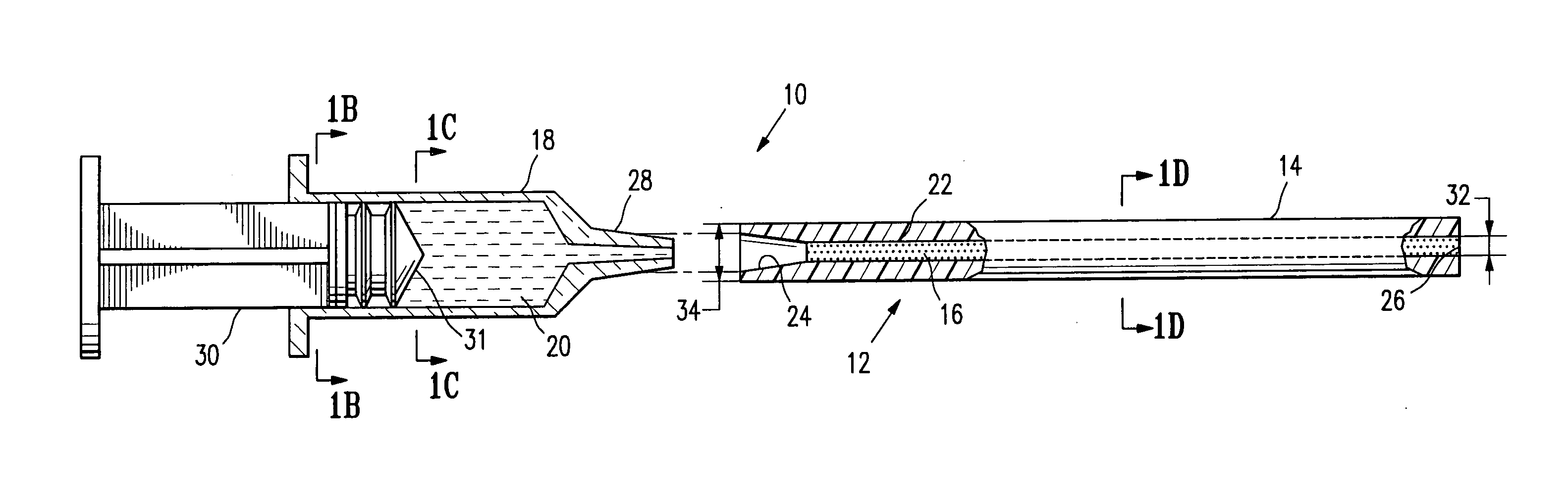
Tissue site markers for in vivo imaging
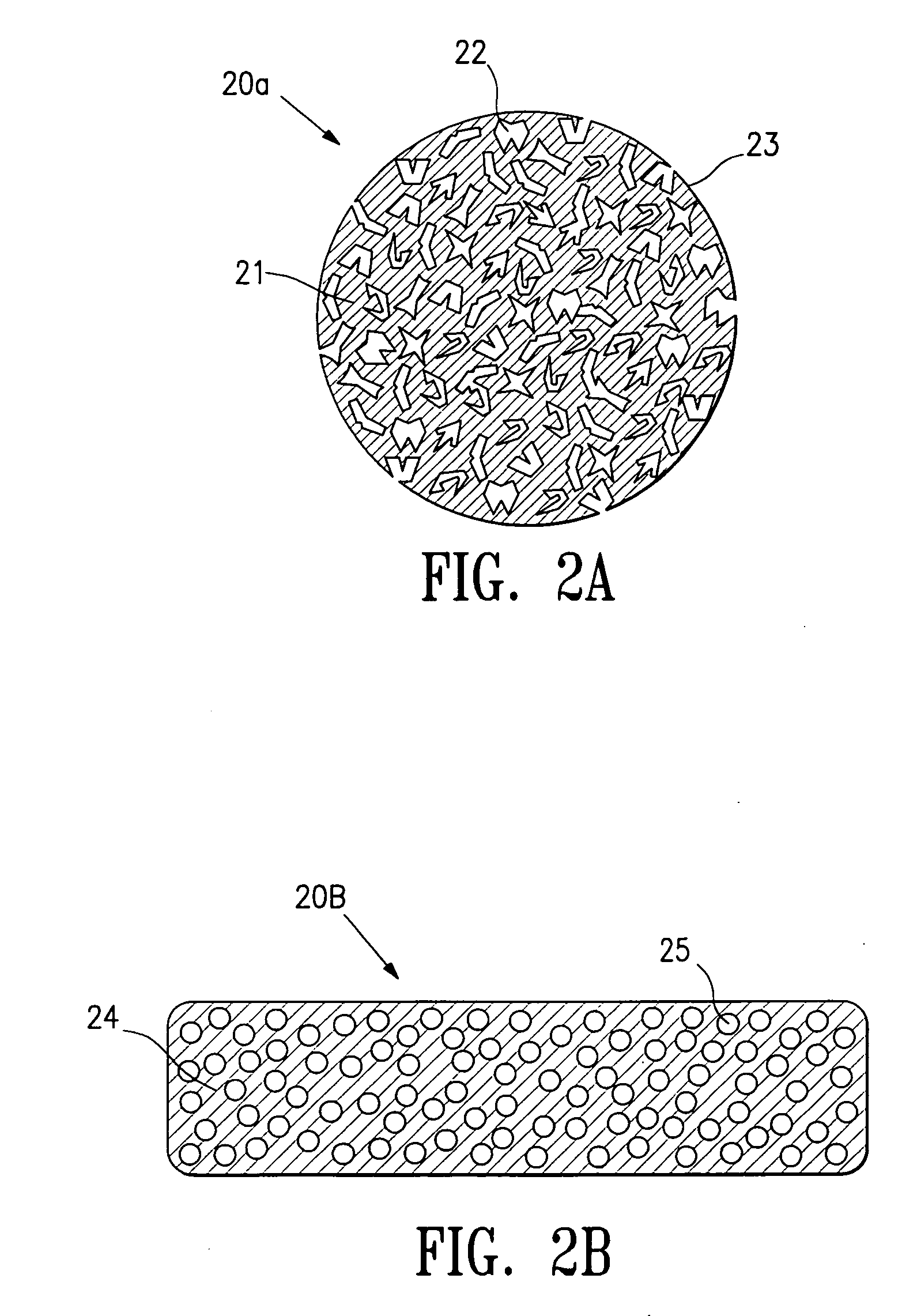
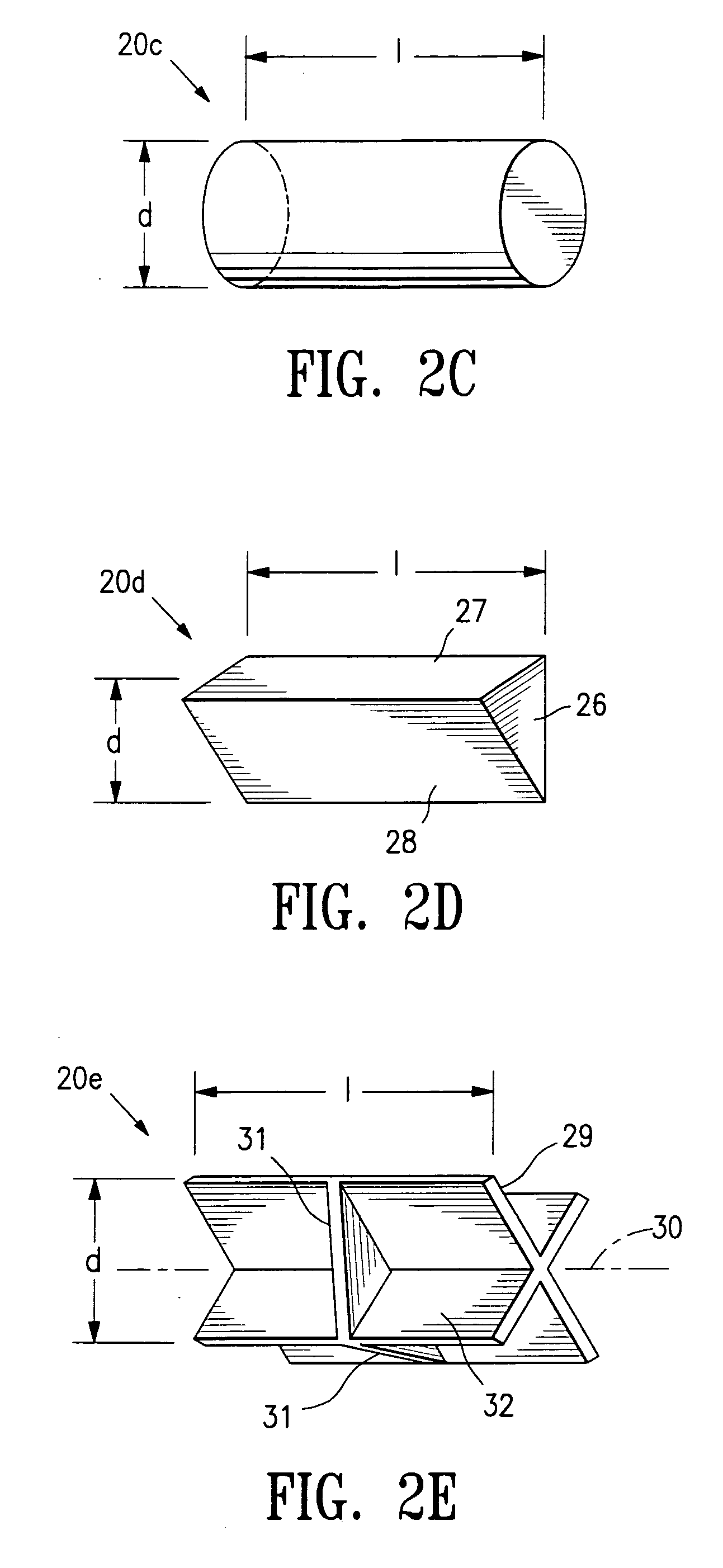
InactiveUS20050063908A1Small volumeImprove reflectivityLuminescence/biological staining preparationSurgical needlesContrast levelIn vivo
The invention is directed biopsy site markers and methods of marking a biopsy site, so that the location of the biopsy cavity is readily visible by conventional imaging methods, particularly by ultrasonic imaging. The biopsy site markers of the invention have high ultrasound reflectivity, presenting a substantial acoustic signature from a small marker, so as to avoid obscuring diagnostic tissue features in subsequent imaging studies, and can be readily distinguished from biological features. The several disclosed embodiments of the biopsy site marker of the invention have a high contrast of acoustic impedance as placed in a tissue site, so as to efficiently reflect and scatter ultrasonic energy, and preferably include gas-filled internal pores. The markers may have a non-uniform surface contour to enhance the acoustic signature. The markers have a characteristic form which is recognizably artificial during medical imaging. The biopsy site marker may be accurately fixed to the biopsy site so as to resist migration from the biopsy cavity when a placement instrument is withdrawn, and when the marked tissue is subsequently moved or manipulated.
Owner:SENORX
In-situ formed intervertebral fusion device and method
InactiveUS20150173914A1MinimallyIncrease heightLuminescence/biological staining preparationBone implantOrthopedic devicesGonial angle
An orthopedic device for implanting between adjacent vertebrae comprising: an arcuate balloon and a hardenable material within said balloon.In some embodiments, the balloon has a footprint that substantially corresponds to a perimeter of a vertebral endplate. An inflatable device is inserted through a cannula into an intervertebral space and oriented so that, upon expansion, a natural angle between vertebrae will be at least partially restored. At least one component selected from the group consisting of a load-bearing component and an osteobiologic component is directed into the inflatable device through a fluid communication means.
Owner:DEPUY SYNTHES PROD INC
In-situ formed intervertebral fusion device and method
ActiveUS20150164655A1MinimallyIncrease heightLuminescence/biological staining preparationBone implantLamina terminalisIntervertebral space
An orthopedic device for implanting between adjacent vertebrae comprising: an arcuate balloon and a hardenable material within said balloon.In some embodiments, the balloon has a footprint that substantially corresponds to a perimeter of a vertebral endplate. An inflatable device is inserted through a cannula into an intervertebral space and oriented so that, upon expansion, a natural angle between vertebrae will be at least partially restored. At least one component selected from the group consisting of a load-bearing component and an osteobiologic component is directed into the inflatable device through a fluid communication means.
Owner:DEPUY SYNTHES PROD INC
Cavity-filling biopsy site markers
InactiveUS20050143656A1Easy to detectLuminescence/biological staining preparationSurgical needlesAnesthetic AgentMaximum dimension
The invention provides materials, devices and methods for marking biopsy sites for a limited time. The biopsy-marking materials are ultrasound-detectable bio-resorbable powders, with powder particles typically between about 20 microns and about 800 microns in maximum dimension, more preferably between about 300 microns and about 500 microns. The powders may be formed of polymeric materials containing cavities sized between about 10 microns and about 500 microns, and may also contain binding agents, anesthetic agents, hemostatic agents, and radiopaque markers. Devices for delivering the powders include tubes configured to contain the powders and to fit within a biopsy cannula, the powders being ejected by action of a syringe. Systems may include a tube containing powder, and a syringe containing sterile saline. The tube may be configured to fit within a biopsy cannula such as a Mammotome® or SenoCor 360™ cannula.
Owner:SENORX
Biopsy cavity marking device and method
InactiveUS20050080338A1Mark accuratelyUltrasonic/sonic/infrasonic diagnosticsMammary implantsFiberBreast implant
These are breast implant devices and methods of use. The breast implants are made of a matrix of collagen material having a porous structure for supporting surrounding tissue of a breast. The implants are also configured to provide a framework for the in-growth of fibrous tissue into the matrix. The matrix can be resilient and / or self-expanding. The methods of use include the steps of forming a cavity having surrounding breast tissue and forming a resorbable implant made up of collagen that is sized to occupy the cavity. The implant is then implanted into the cavity, thereby supporting the surrounding tissue and allowing for in-growth of fibrous tissue into and replacing the resorbable material. The resorbable material may also be elastically compressible, such that the step of implanting includes the step of compressing the resorbable material. The implants may also contain a medicinal, therapeutic, or diagnostic substance.
Owner:DEVICOR MEDICAL PROD
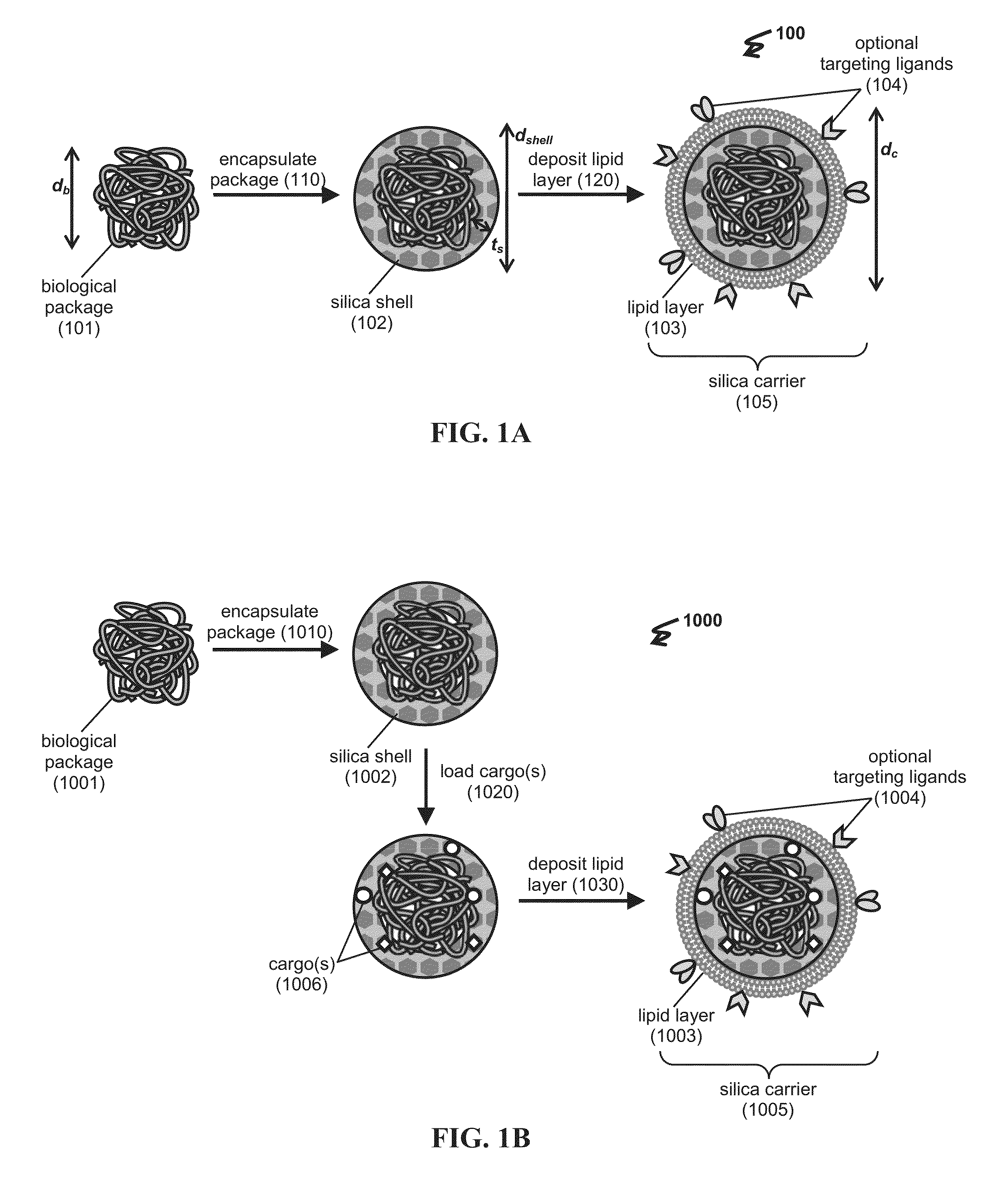
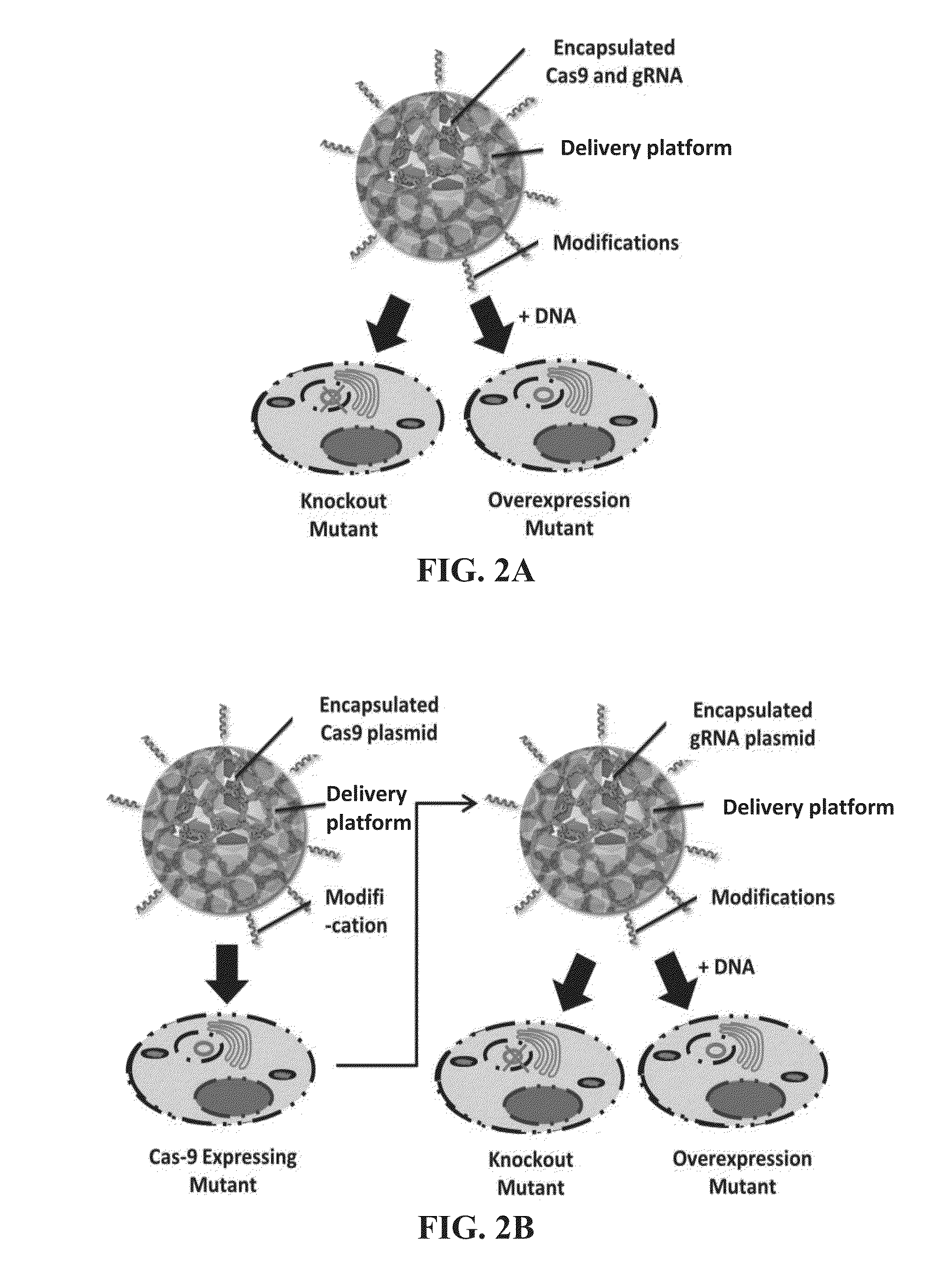
Delivery platforms for the domestication of algae and plants
InactiveUS20160090603A1Improve abilitiesHigh expressionAntibacterial agentsOrganic active ingredientsBiological activationPolynucleotide
The present invention relates to a delivery platform that can be used to genetically modify a target in a plant or an alga. In one instance, polypeptides and / or polynucleotides can be delivered using silica delivery platforms, e.g., silica carriers or protocells. Such platforms can be employed to control gene activation and repression in the plant or alga.
Owner:SANDIA
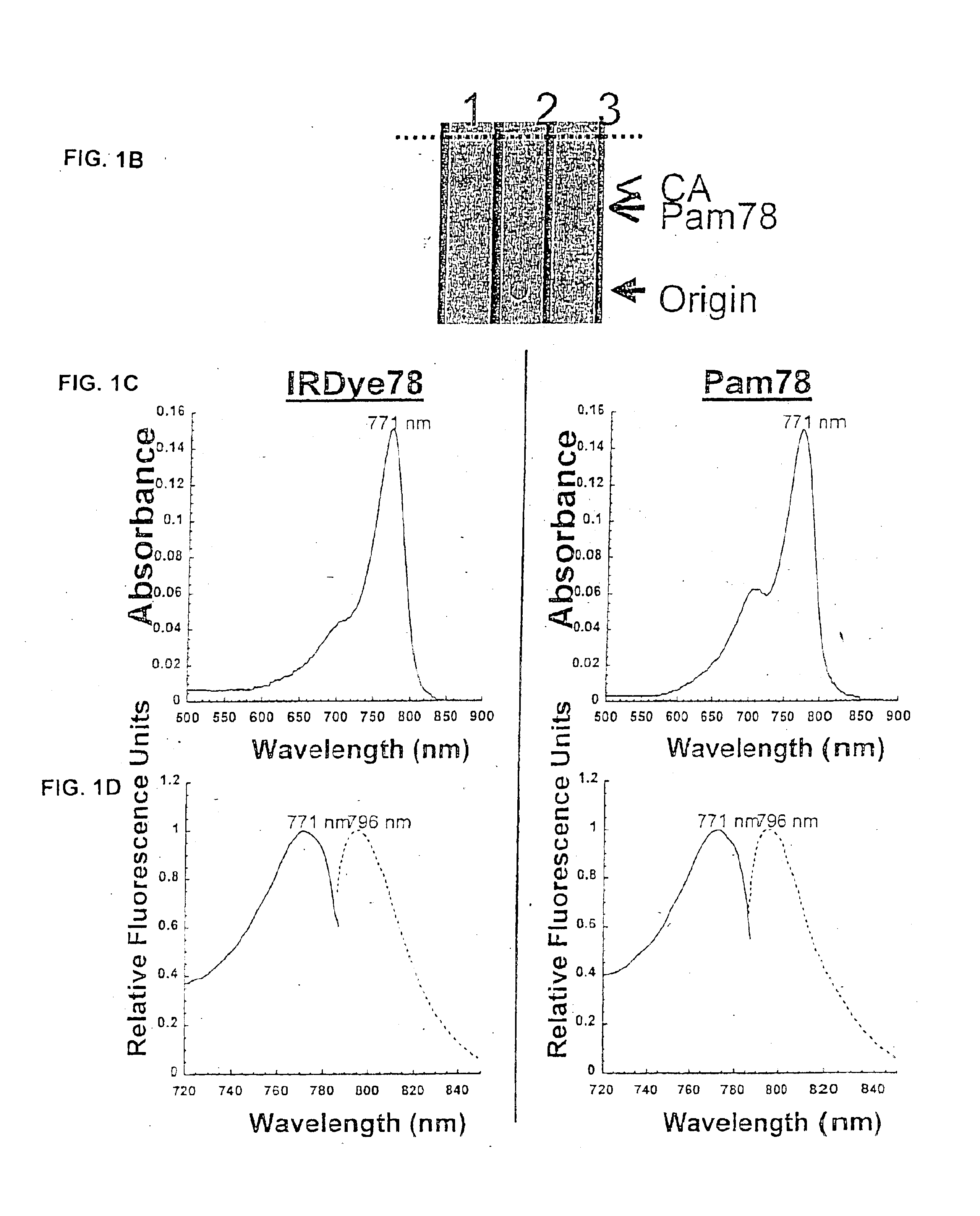
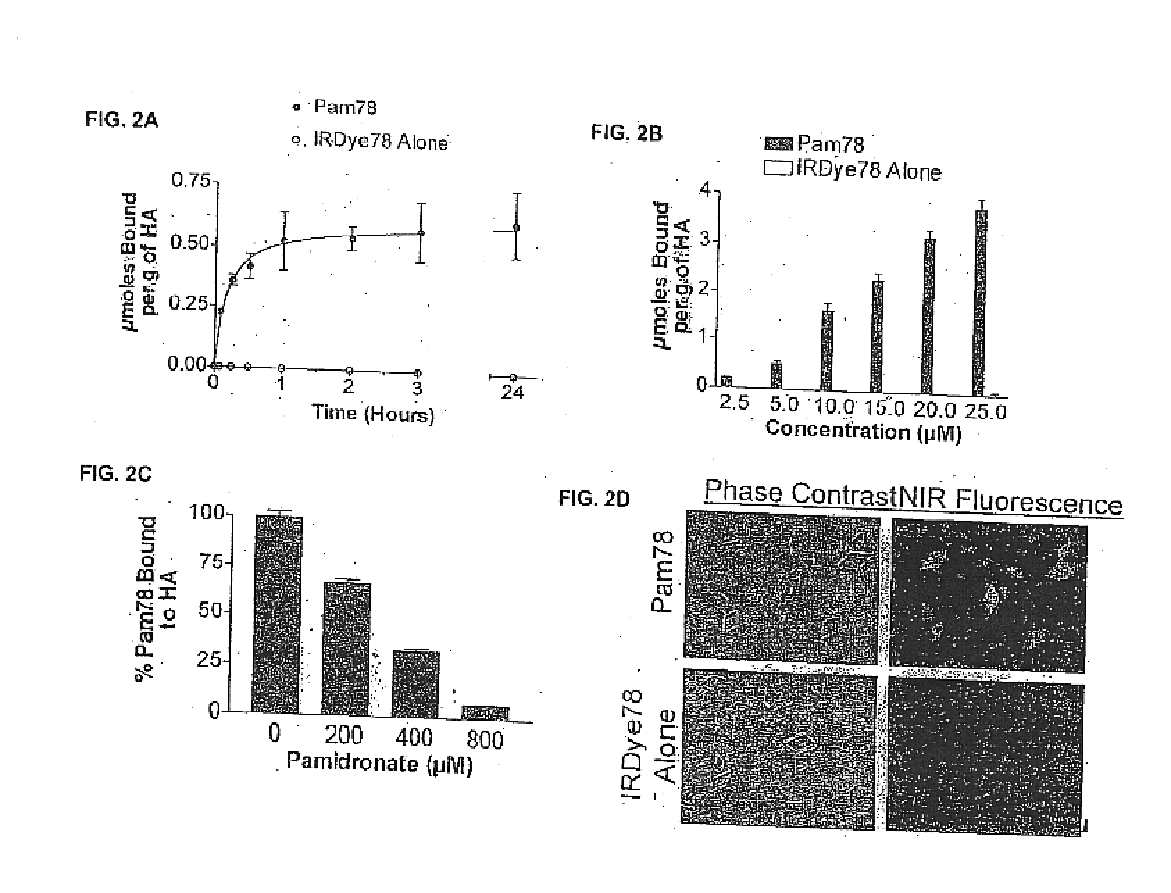
Near infrared imaging agent
InactiveUS6913743B2Ultrasonic/sonic/infrasonic diagnosticsOrganic chemistryFluorescenceNear infrared radiation
This invention relates to an in-vivo diagnostic method based on near infrared radiation (NIR radiation) that uses water-soluble dyes and their biomolecule adducts, each having specific photophysical and pharmaco-chemical properties, as a contrast medium for fluorescence and transillumination diagnostics in the NIR range, to new dyes and pharmaceuticals containing such dyes.
Owner:VISEN MEDICAL INC
Non-isotopic detection of osteoblastic activity in vivo using modified bisphosphonates
InactiveUS6869593B2Accurately cardiovascular riskUltrasonic/sonic/infrasonic diagnosticsLuminescence/biological staining preparationIn vivoOsteocyte
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
In-vivo diagnostic method by means of near infrared radiation
InactiveUS7025949B2Good water solubilityUltrasonic/sonic/infrasonic diagnosticsMethine/polymethine dyesFluorescenceNear infrared radiation
This invention relates to an in-vivo diagnostic method based on near infrared radiation (NIR radiation) that uses water-soluble dyes and their biomolecule adducts, each having specific photophysical and pharmaco-chemical properties, as a contrast medium for fluorescence and transillumination diagnostics in the NIR range, to new dyes and pharmaceuticals containing such dyes.
Owner:VISEN MEDICAL INC
Bio-nano-plasmonic elements and platforms
InactiveUS20120263793A1Improve artLow costOrganic active ingredientsMaterial nanotechnologyForms of energyProtein molecules
The invention relates generally to the field of plasmonics, and more specifically, in one embodiment, it relates to fabricating elements in whole or in part using one or more self-assembling elements comprised of purified, synthetic and or recombinant protein molecule elements and or their accessory elements, and in particular, composed of at least one or more Clathrin and or Coatomer I / II protein molecules, forming one or more self-assembling structure and framework elements of one or more molecular weights, dimensions, geometries, symmetries, configurations and combinations. In another aspect, the invention relates to a method using one or more nanoscale metal surface elements that, when one or more appropriate types or forms of energies are applied to one or more types of metal elements, emit one or more preferred types or forms of surface-plasmon-enhanced electromagnetic radiation and energy.
Owner:METAQOR
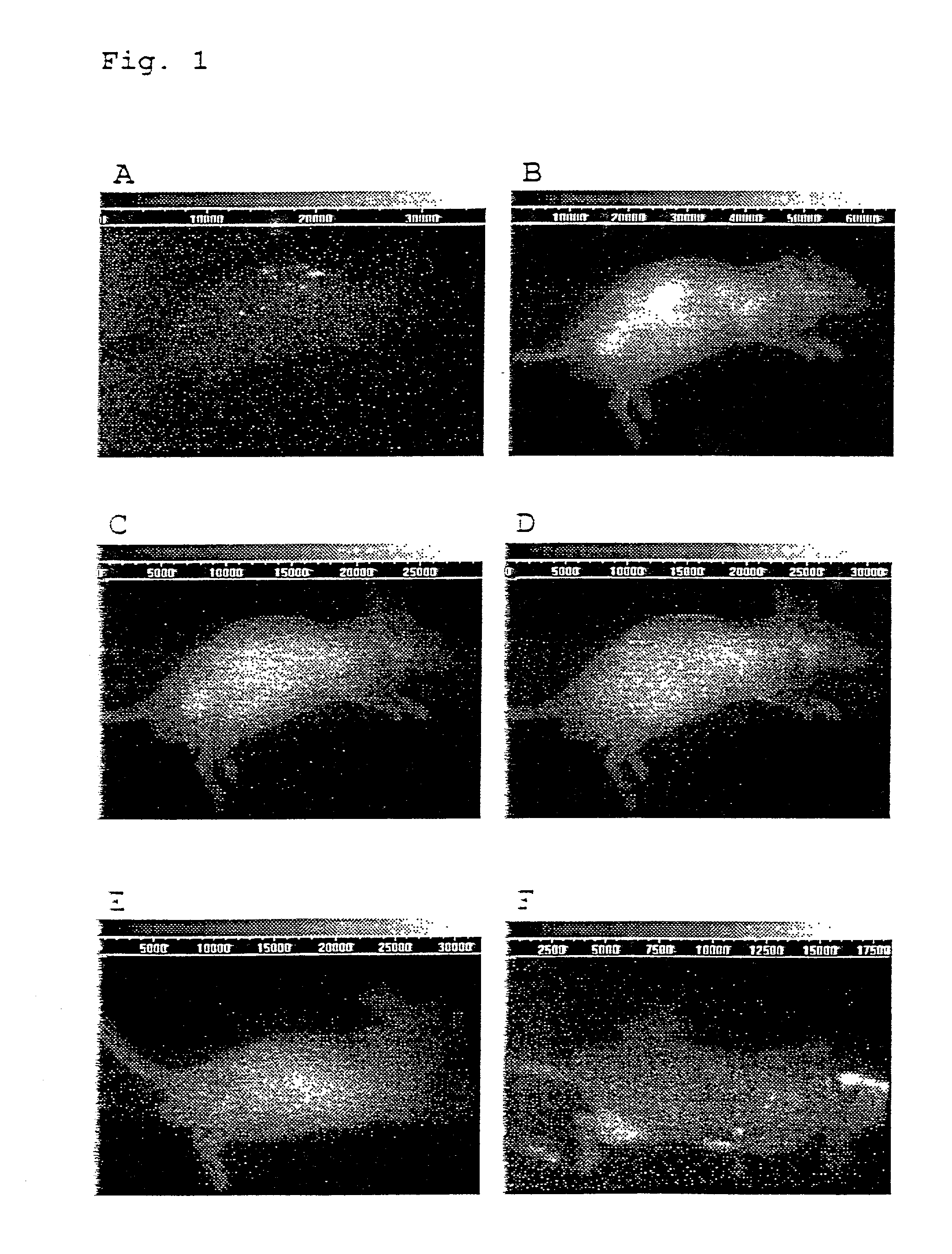
Molecular lymphatic mapping of sentinel lymph nodes
ActiveUS20050142556A1Luminescence/biological staining preparationSugar derivativesAbnormal tissue growthRadioactive tracer
The present invention describes a method for identification and labeling of sentinel lymph nodes (SLNs) and the presence or absence of lymph node metastases as an important diagnostic and prognostic factor in early stage cancers of all types. The method, know as Molecular Lymphatic Mapping, uses traditional dye / radioactive tracer based techniques in conjunction with a nucleic acid marker to identify and label the SLN, not only for current diagnostic methods, but for archival purposes. In addition, MLM can be used to deliver a therapeutic gene or genes to the SLN to activate tumor immunity to tumor cells, and / or to inhibit tumor metastases. The methods may be combined with therapeutic intervention including chemotherapy and radiotherapy.
Owner:JOHN WAYNE CANCER INST
PARP inhibitors
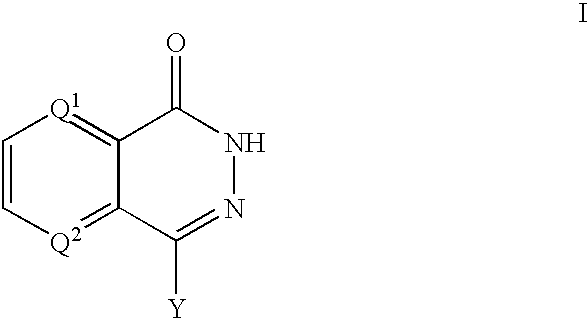
InactiveUS6924284B2None be use clinicallyReducing, arresting or ameliorating nervous insultBiocideNervous disorderArylPARP inhibitor
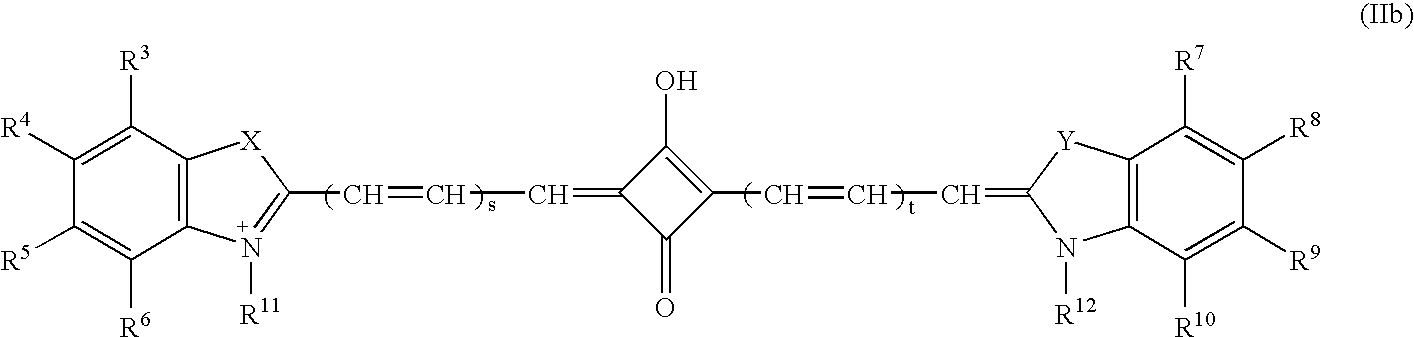
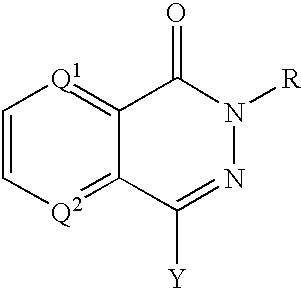
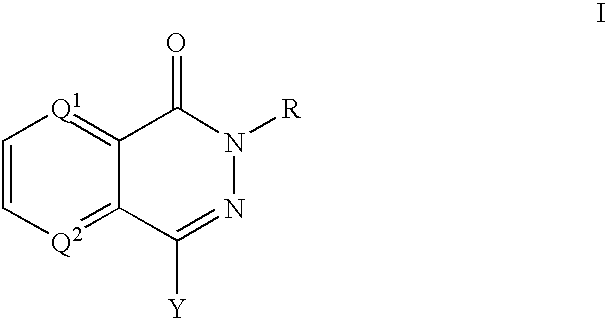
The present invention provides compounds comprising a bicyclic aryl moiety, such as 2H-phthalazin-1-one or derivatives thereof, compositions comprising the same, and methods for producing and using the same. In particular, the present invention provides compounds of the formula: or a pharmaceutically acceptable salt, a hydrate, a solvate, or a prodrug thereof; where Q1, Q2 and Y are those defined herein.
Owner:ICOS CORP
In vivo stain composition, process of manufacture, and methods of use to identify dysplastic tissue
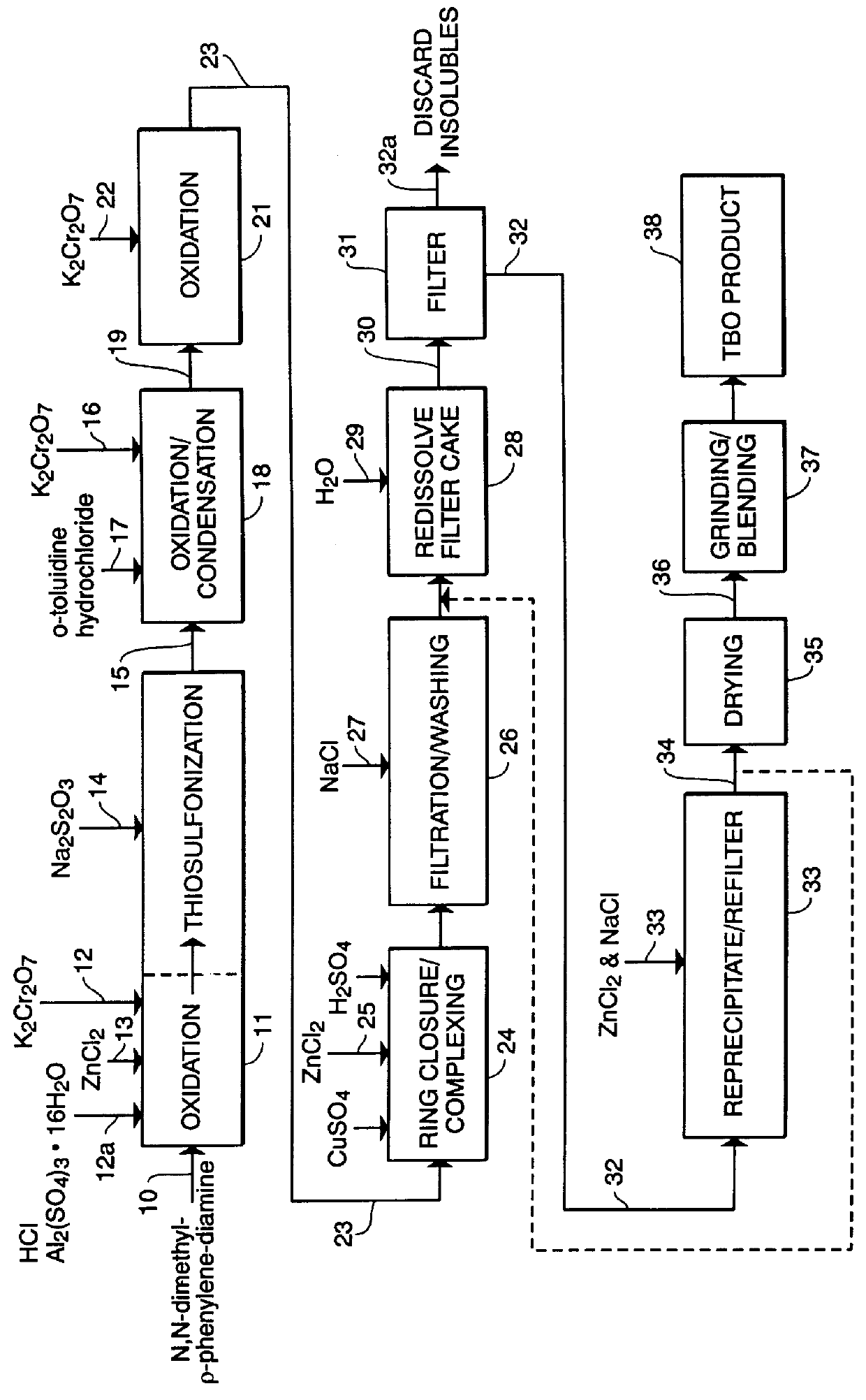
PCT No. PCT / US97 / 20981 Sec. 371 Date May 20, 1999 Sec. 102(e) Date May 20, 1999 PCT Filed Nov. 13, 1997 PCT Pub. No. WO99 / 25388 PCT Pub. Date May 27, 1999N-demethylated and N,N-demethylated derivatives of toluidine blue O and compositions which include these derivatives and the conformational isomers of toluidine blue O. Improved methods for the detection of dysplastic oral tissue using such compositions. Processes for synthesis of toluidine blue O products, in which a complexing agent is introduced prior to the last stage of oxidation of a three-step synthesis from N,N-dimethyl- rho -phenylenediamine. An HPLC method for characterizing toluidine blue O products in which the mobile phase is an aqueous solution of an organic acid.
Owner:DEN MAT HLDG
Diagnostic method for atherosclerosis
InactiveUS20050244336A1Enough timeUltrasonic/sonic/infrasonic diagnosticsIn-vivo radioactive preparationsChemical MoietySufficient time
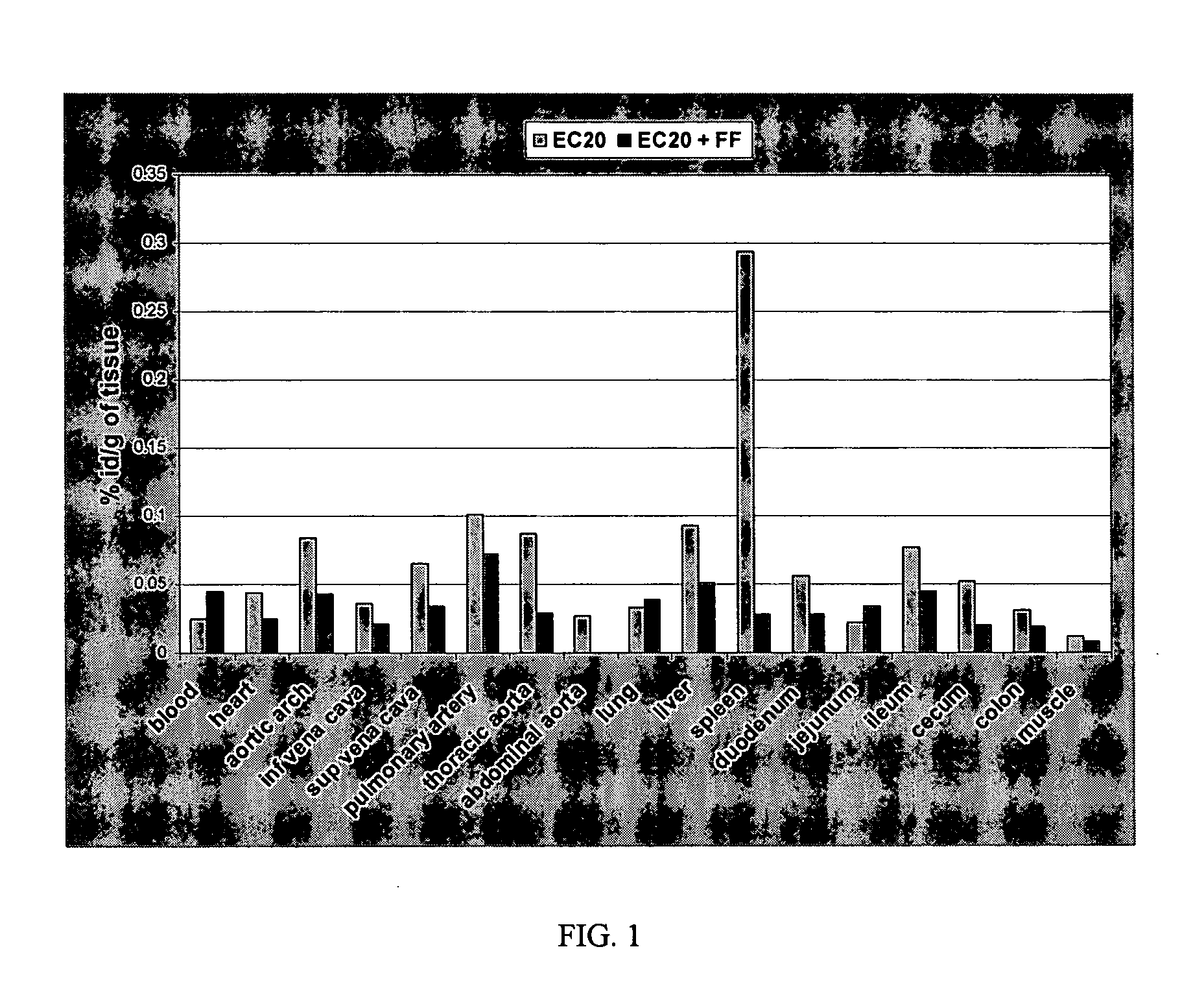
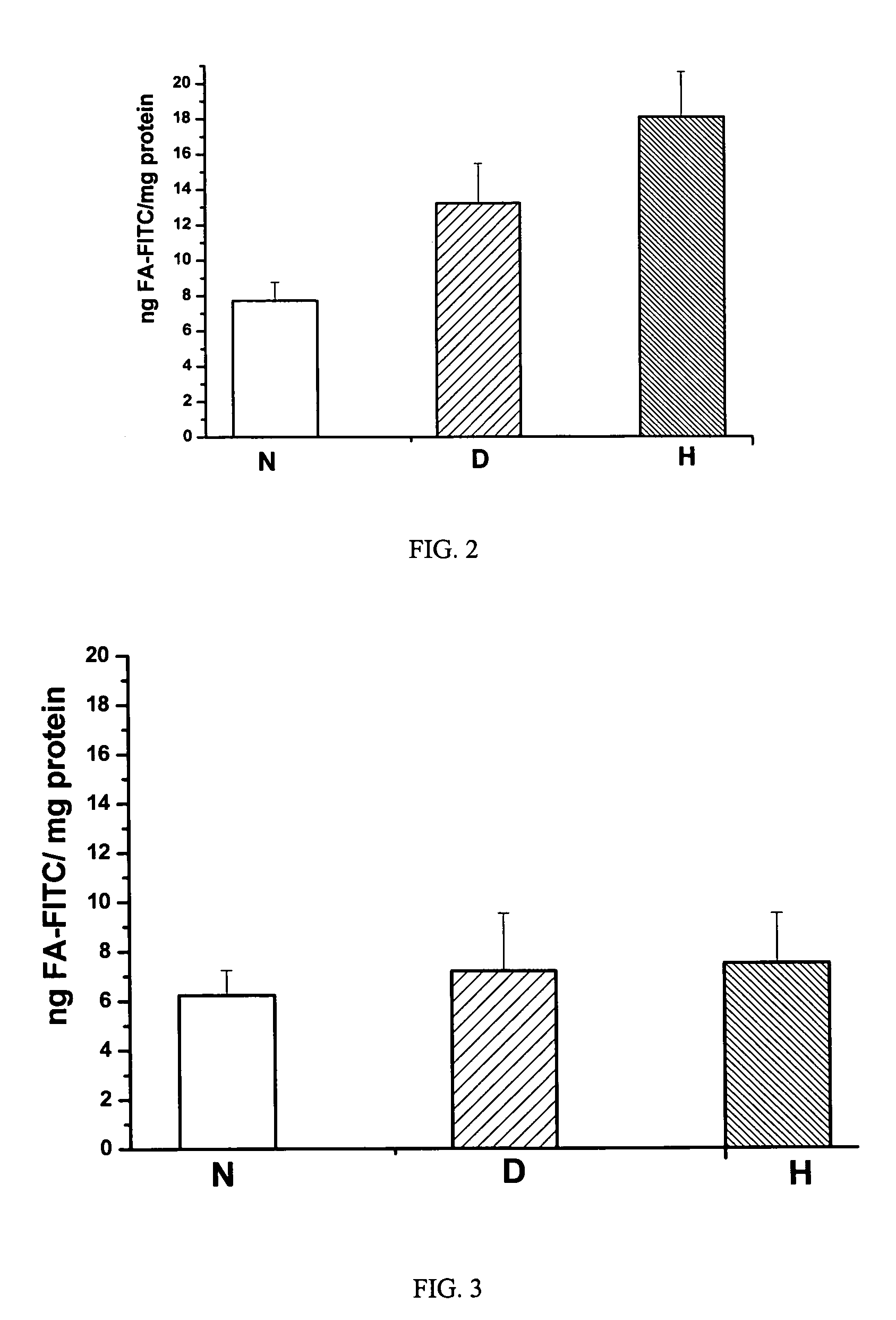
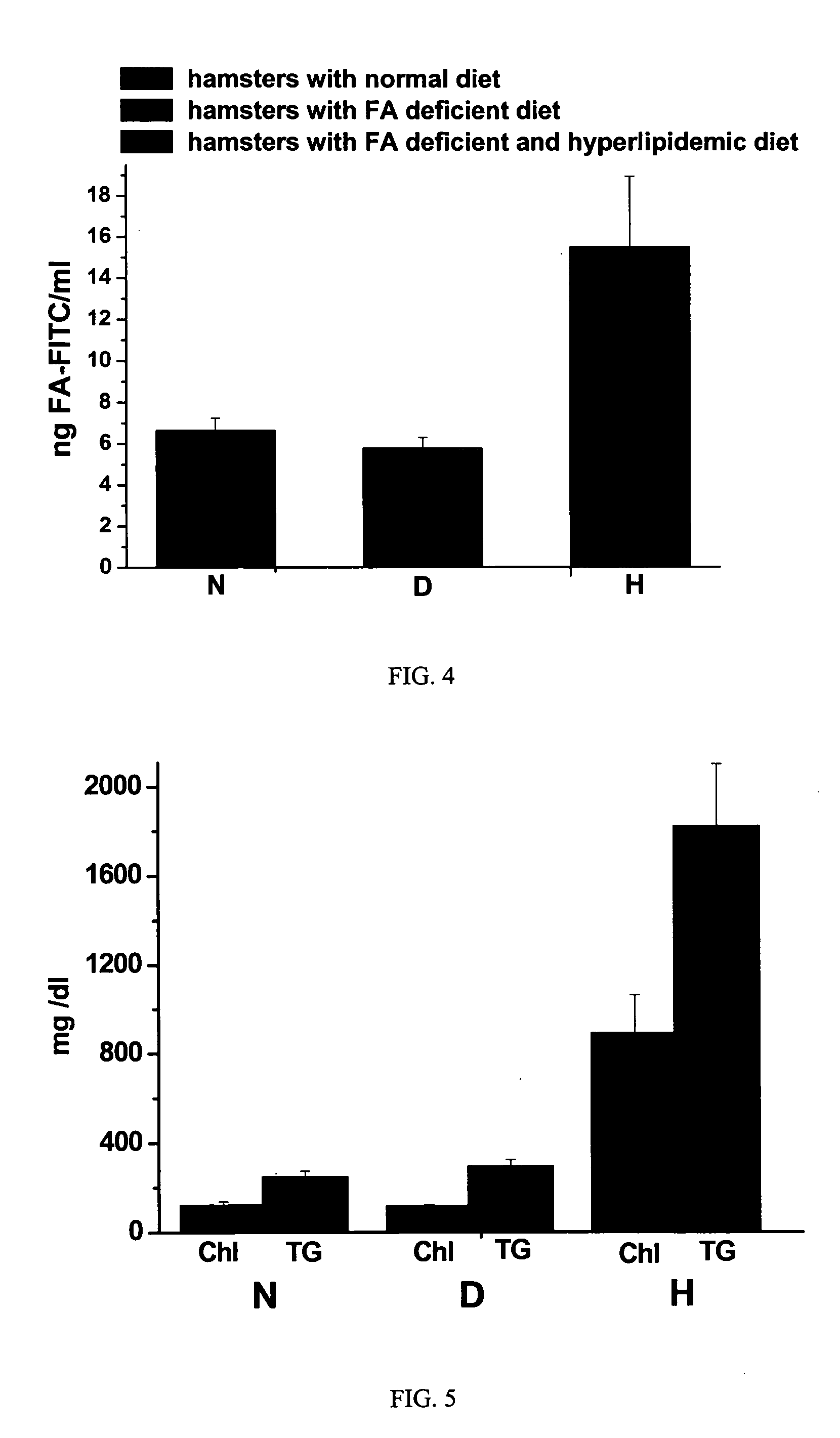
The invention relates to a method of identifying / monitoring active atherosclerotic plaques associated with blood vessel walls wherein the plaques comprise activated macrophages having accessible binding sites for a ligand. The method comprises the steps of administering to a patient being evaluated for atherosclerosis an effective amount of a composition comprising a conjugate of a ligand and a chromophore capable of emitting light under predetermined conditions, allowing sufficient time for the ligand conjugate to bind to the activated macrophages, subjecting the blood vessels to the predetermined conditions using a catheter-based device, and identifying active plaques by detecting light emitted by the chromophore using a catheter-based device or by using an external imaging technique. The invention also relates to a similar method wherein a chemical moiety capable of emitting radiation is conjugated to the ligand.
Owner:PURDUE RES FOUND INC
Biologically active methylene blue derivatives
InactiveUS20040147508A1Maintain long-termImprove stabilityAntibacterial agentsOrganic active ingredientsPhotodynamic therapyChemical compound
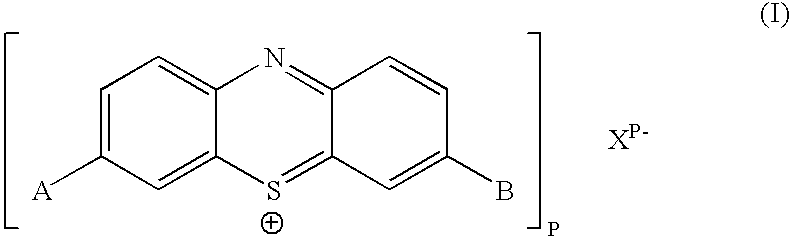
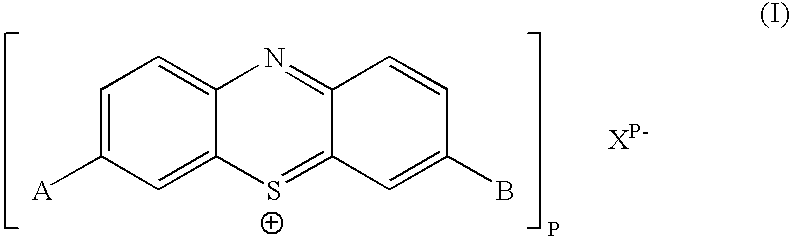
The present invention relates to a phenothiazinium compound of Formula (I): wherein: A and B each independently is in which R' and R'' each independently is a linear, branched or cyclic hydrocarbon group, or R' and R'' together with the N atom to which they are attached form an optionally substituted 5-, 6- or 7-membered ring; and where X<p-> is a counteranion and P is 1, 2 or 3; except for the compounds in which A and B are both either -N(CH3)2 or -N(CH2CH3)2 for use in a treatment that requires removal, deactivation or killing of unwanted tissues or cells. The invention also relates to compositions comprising the compounds of Formula I, to selected compounds of Formula I, use of the compounds of Formula I as medicaments and as a PDT agent or a photodiagnostic agent, a conjugate or composite formed between a compound of Formula I and a polymer; and to a method for sterilising fluids in which the fluid is passed over the conjugate or composite whilst it is illuminated. The compounds are biologically active photosensitisers which are strongly photocytotoxic and have application in the areas of photodynamic therapy (PDT), as well as for the diagnosis and detection of medical conditions and related uses in photochemical internalisation, in the production of cancer vaccines, in the treatment and prevention of microbial infections and in photodisinfection or photosterilisation.
Owner:PHOTOPHARMICA LTD
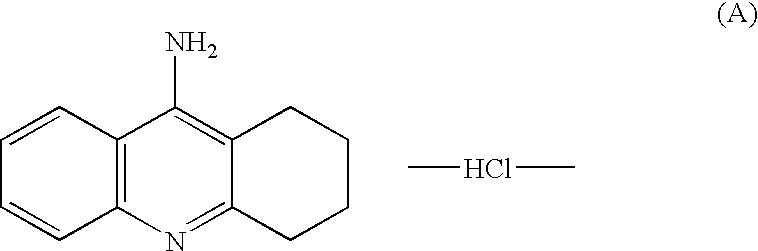
Agents for treating neurodegenerative disorders
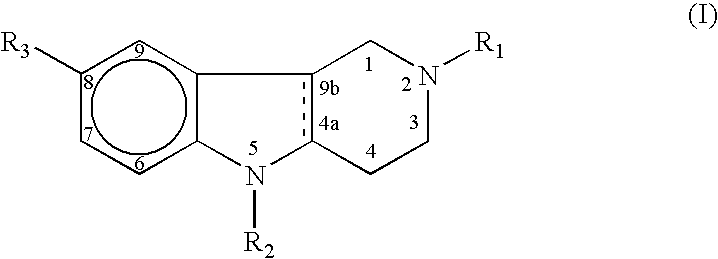
Compounds and methods of using these compounds to treat neurodegenerative diseases, especially Alzheimer's disease, are provided. The compounds that are provided for the treatment of neurodegenerative diseases can be represented by a general formula (I): wherein R1 is Me, Et or PhCH2; R2 is H, PhCH2 or 6-Me-3-Py-(CH2)2; and R3 is H, Me or Br. The solid line accompanied by the dotted line i.e. represents a single or double bond and salts thereof with pharmacologically acceptable acids and quaternary derivatives.
Owner:ZEFIROV N S +10
Porous nanoparticle supported lipid bilayer nanostructures
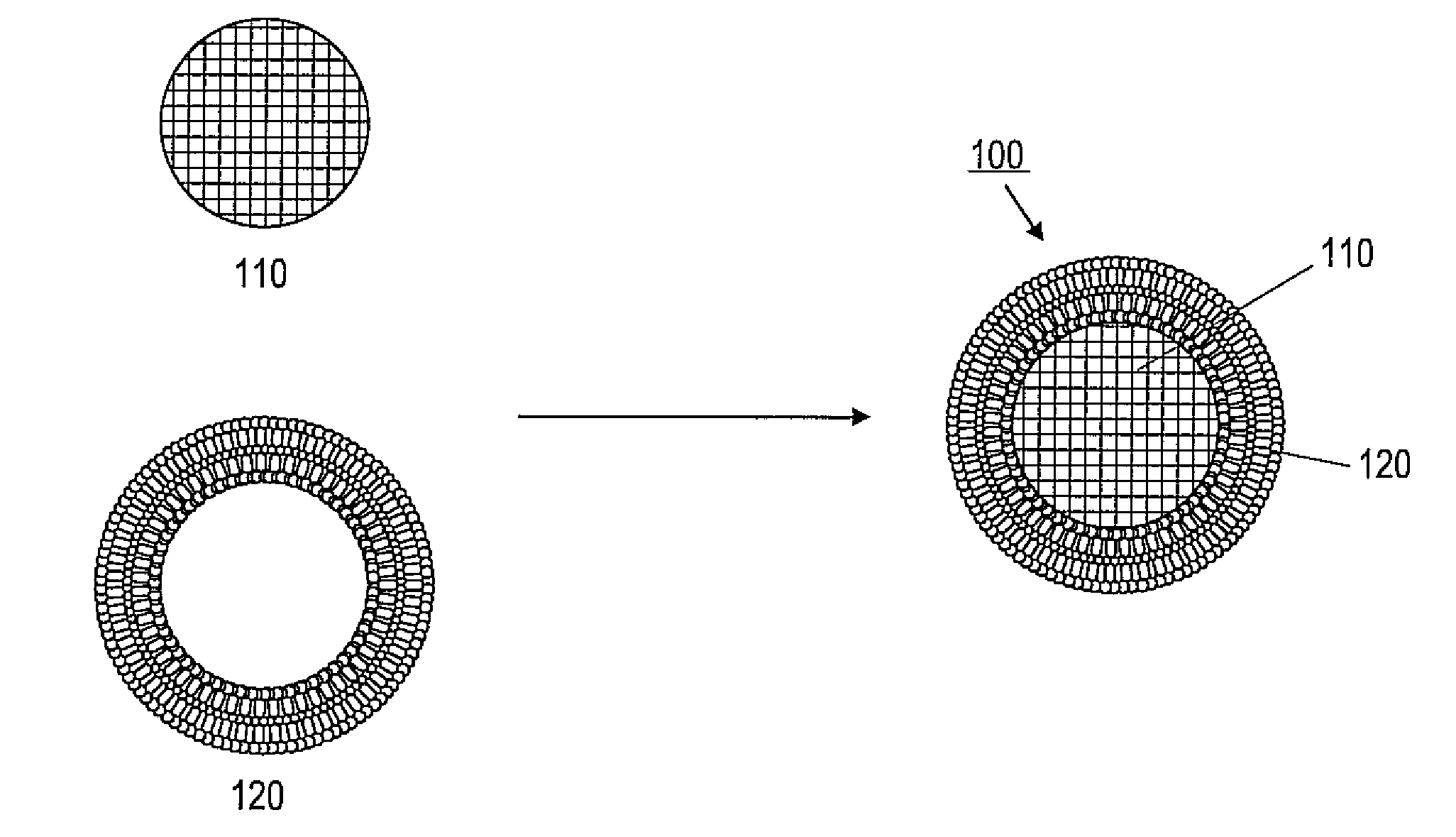
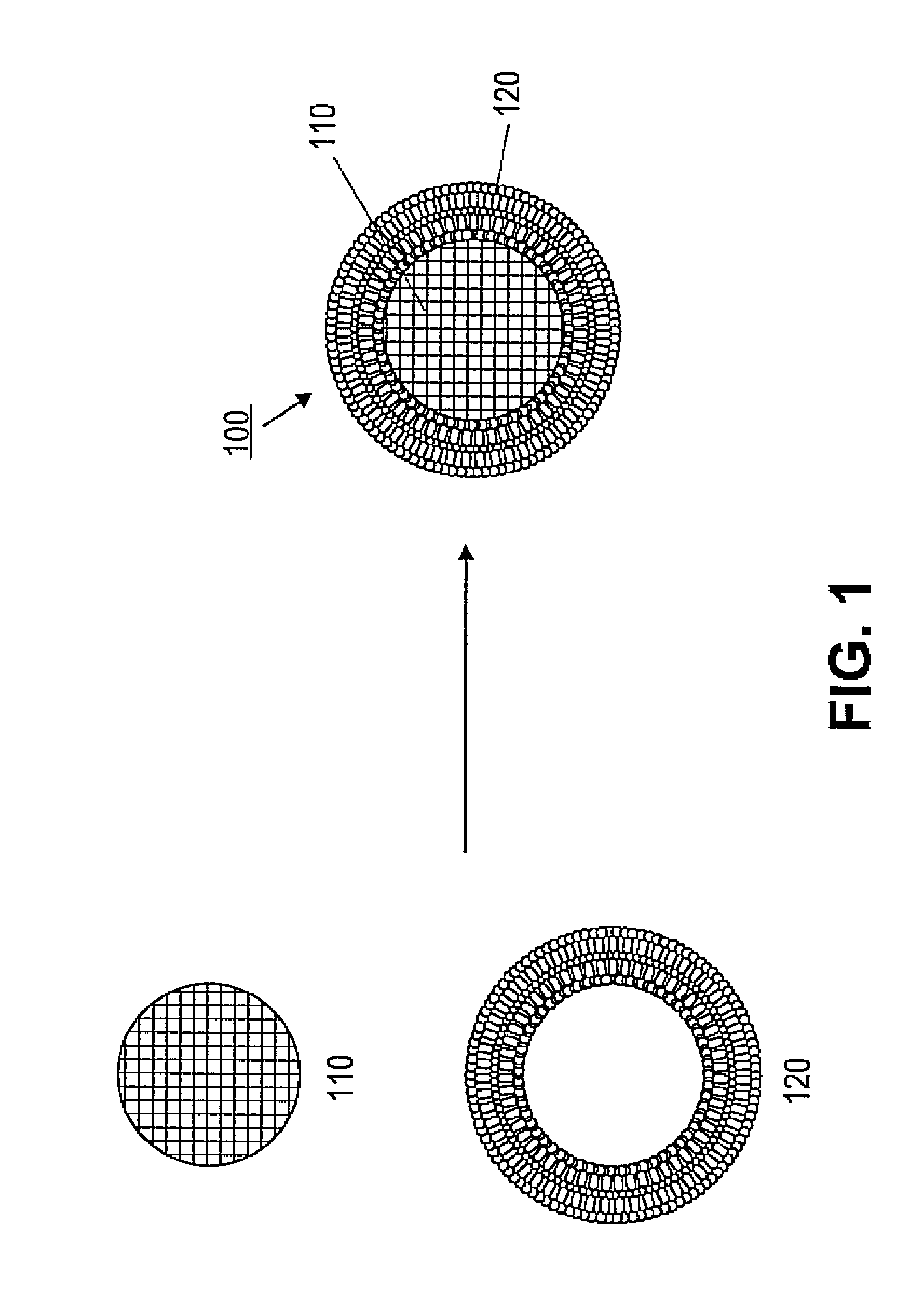
ActiveUS20110268791A1Low release rateFacilitated releaseOrganic active ingredientsHeavy metal active ingredientsNanoparticleLipid bilayer
Various exemplary embodiments provide protocell nanostructures and methods for constructing and using the protocell nanostructures. In one embodiment, the protocell nanostructures can include a core-shell structure including a porous particle core surrounded by a shell of lipid bilayer(s). The protocell can be internalized in a bioactive cell. Various cargo components, for example, drugs, can be loaded in and released from the porous particle core of the protocell(s) and then delivered within the bioactive cell.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com