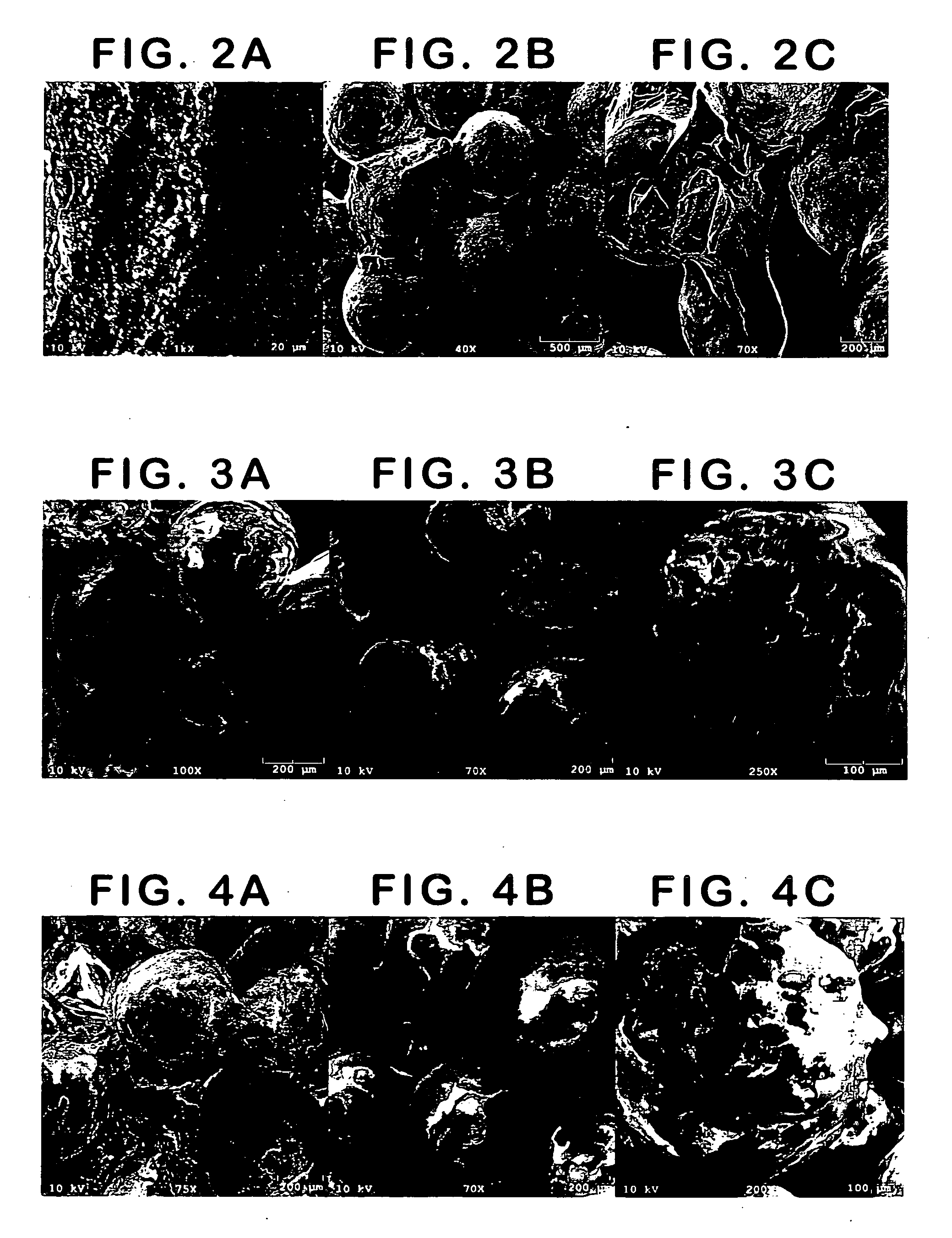
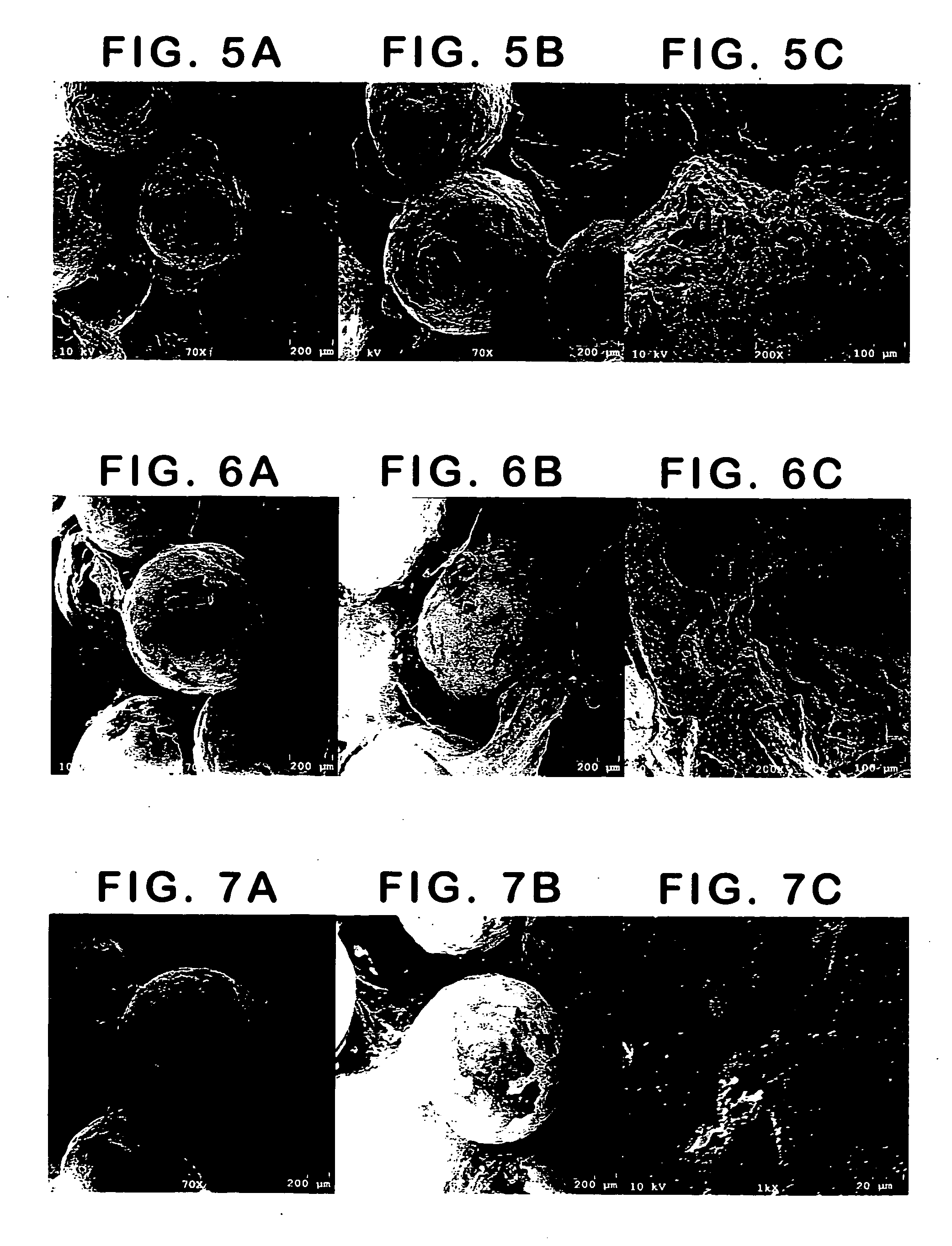
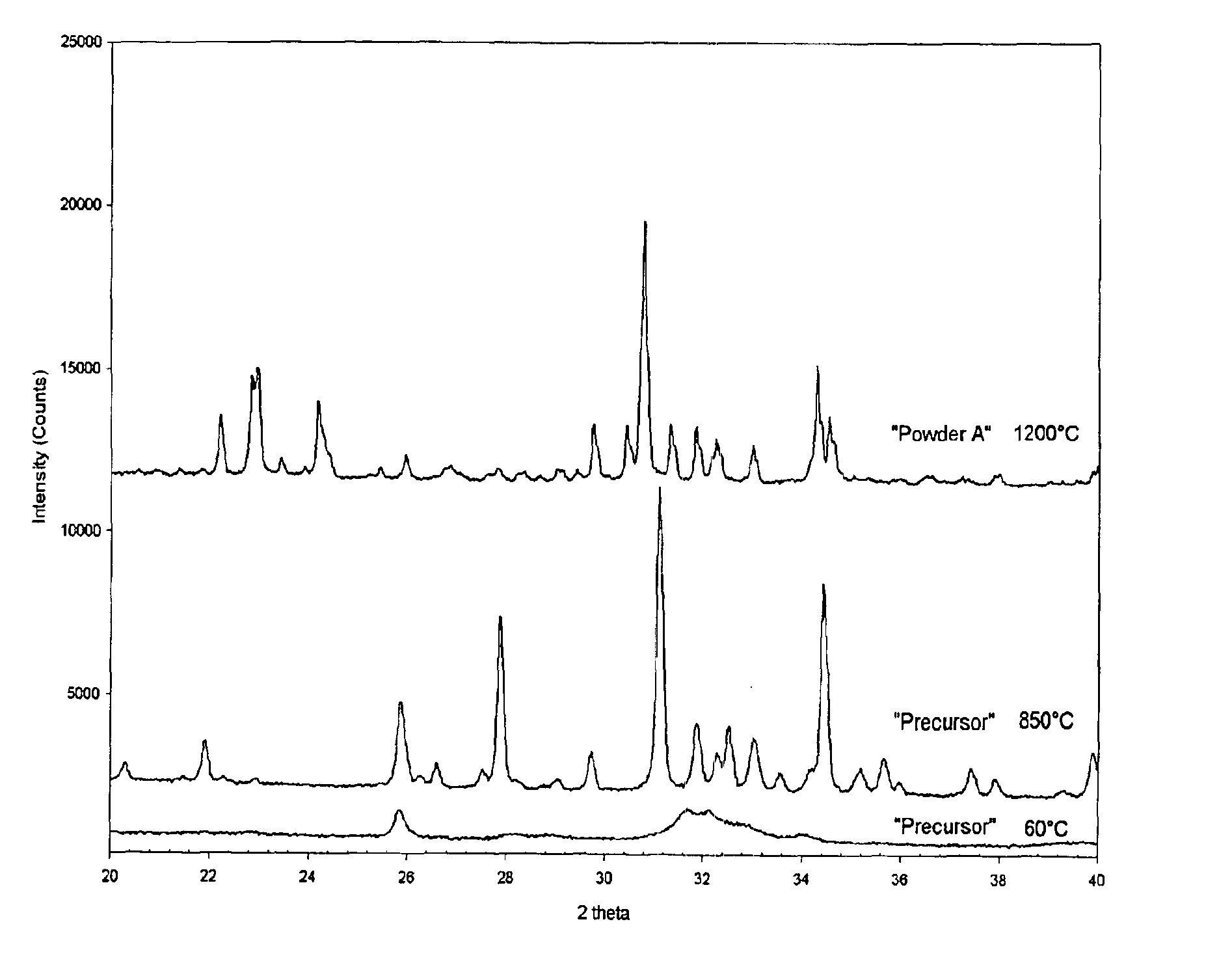
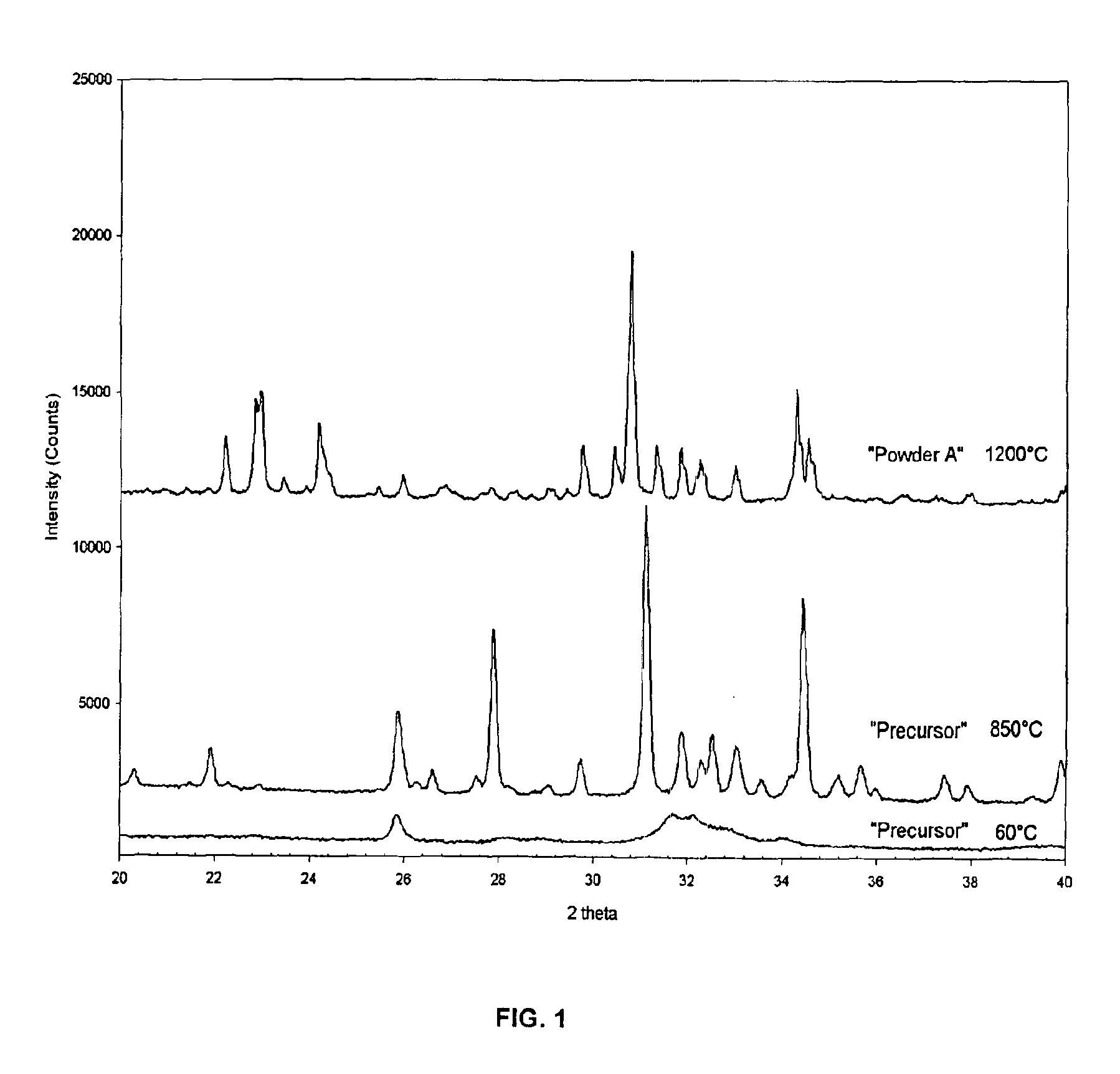
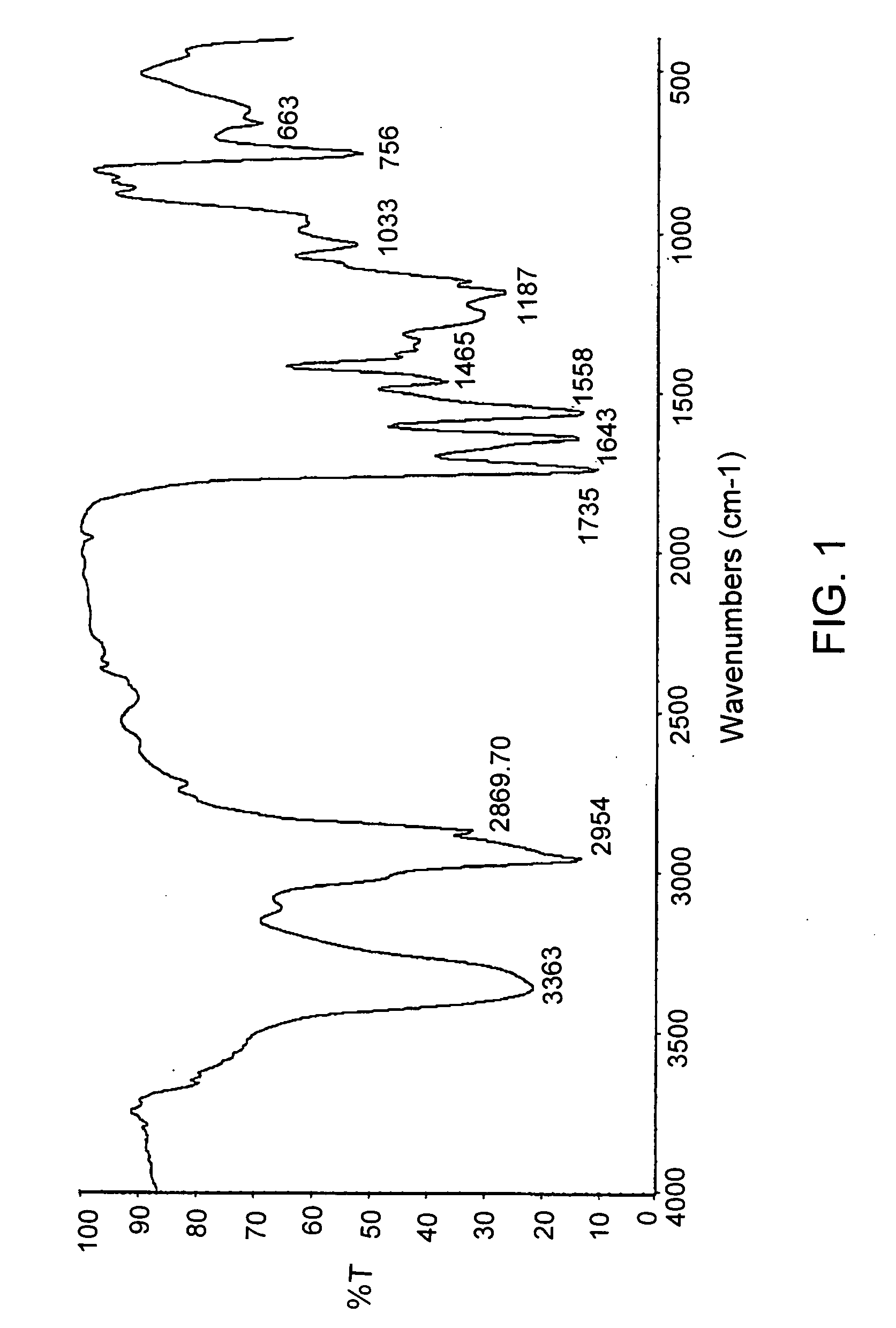
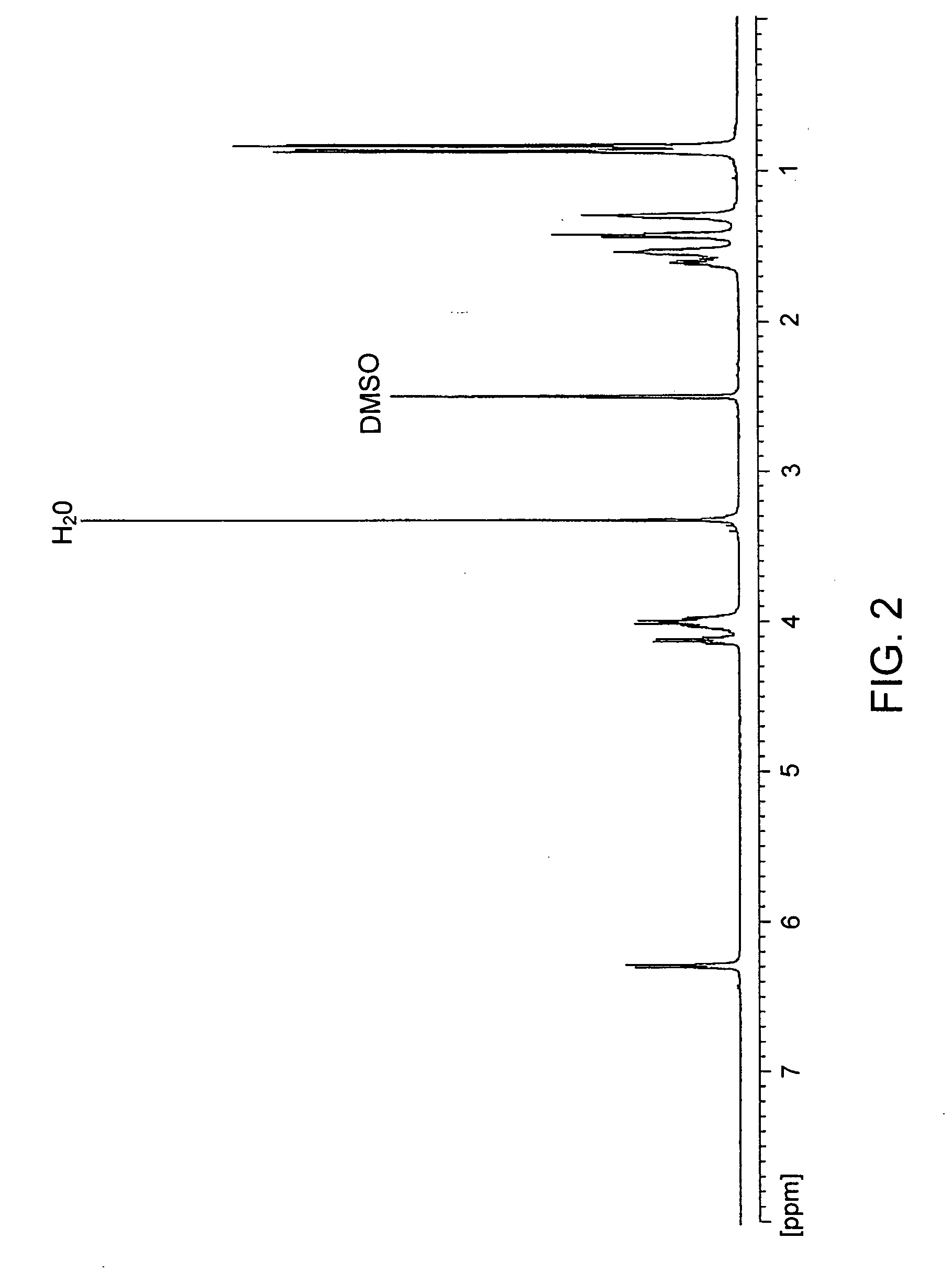
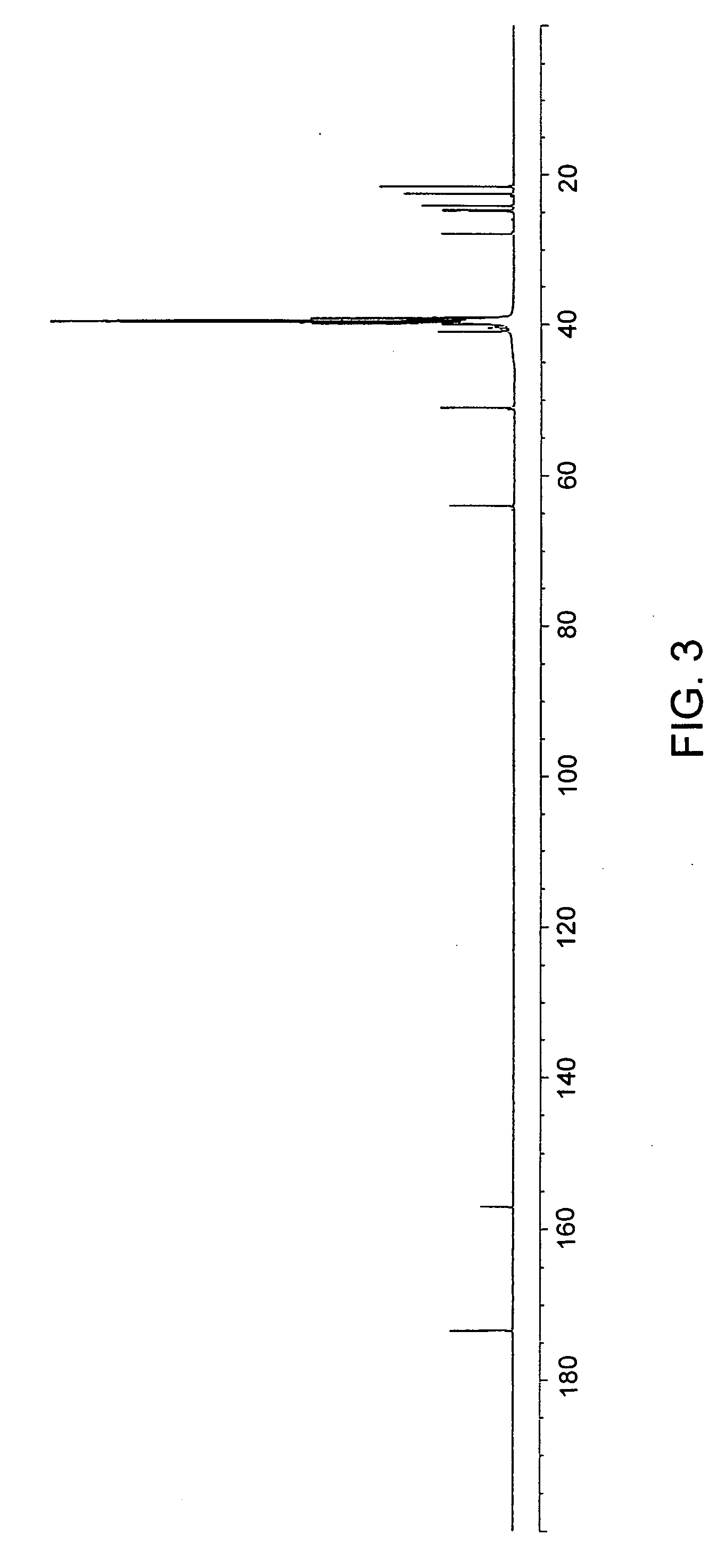
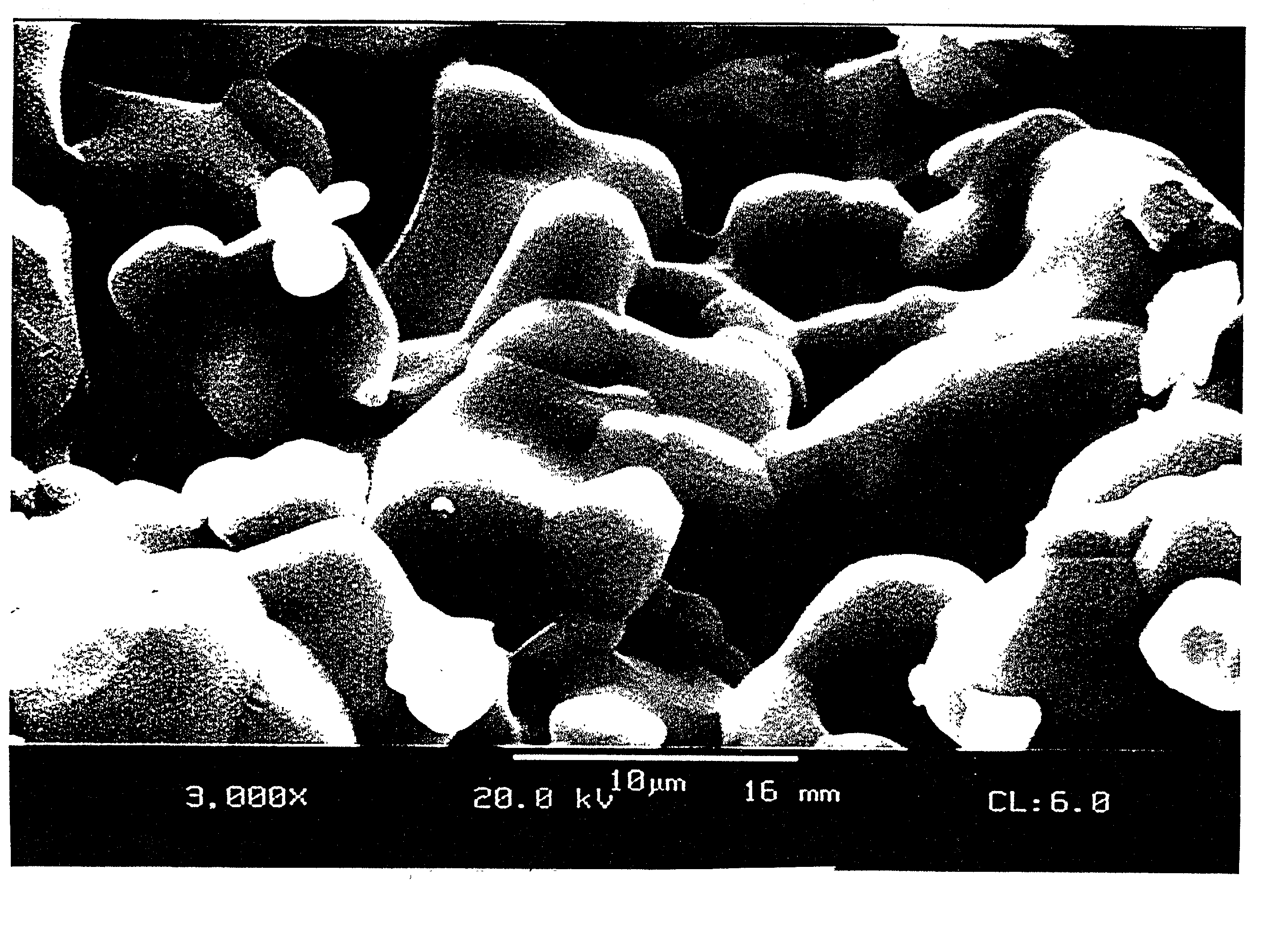
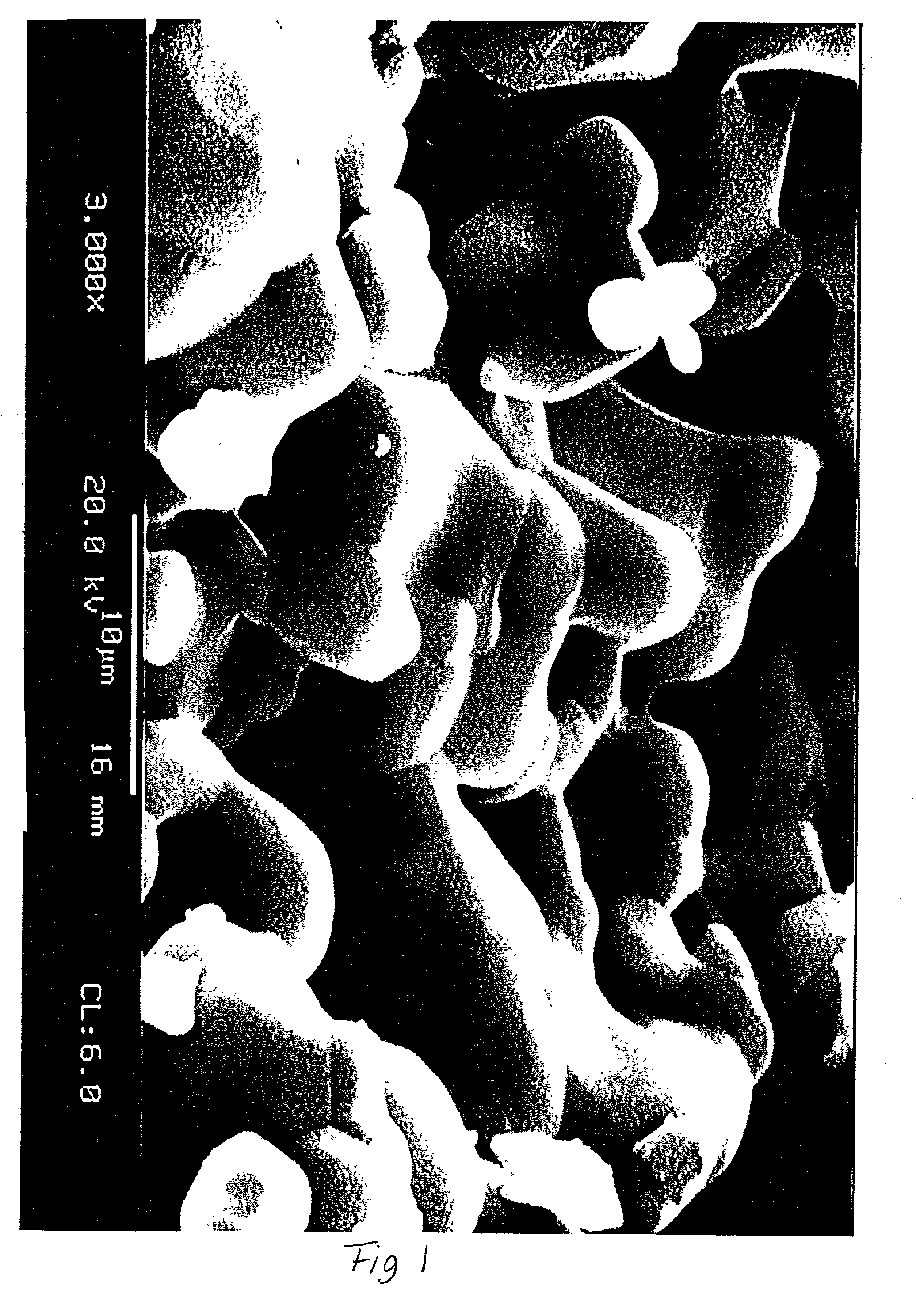
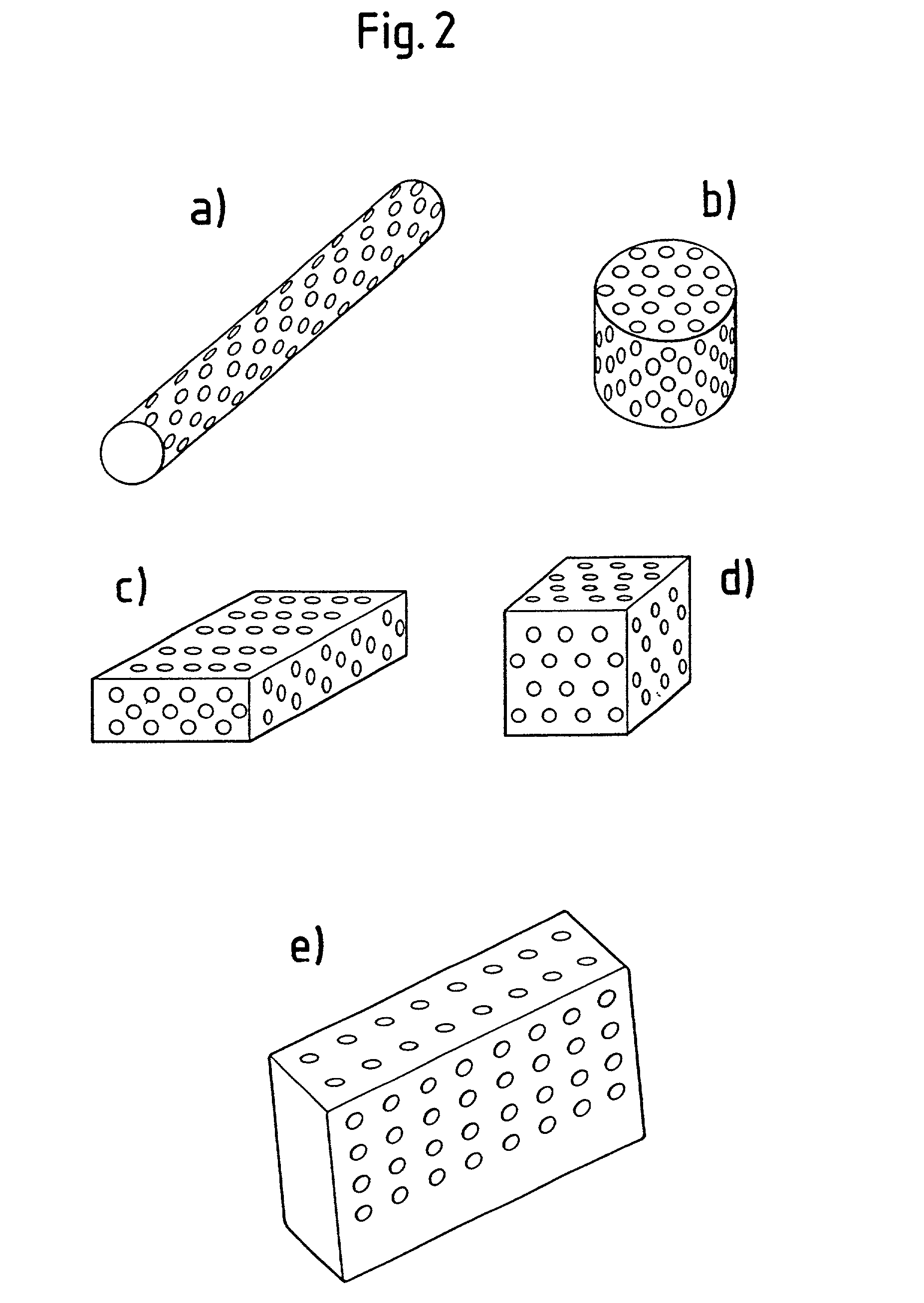
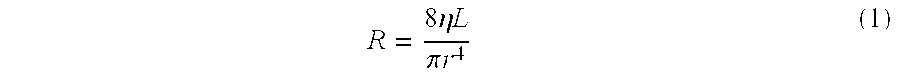Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
246 results about "Bone substitute" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
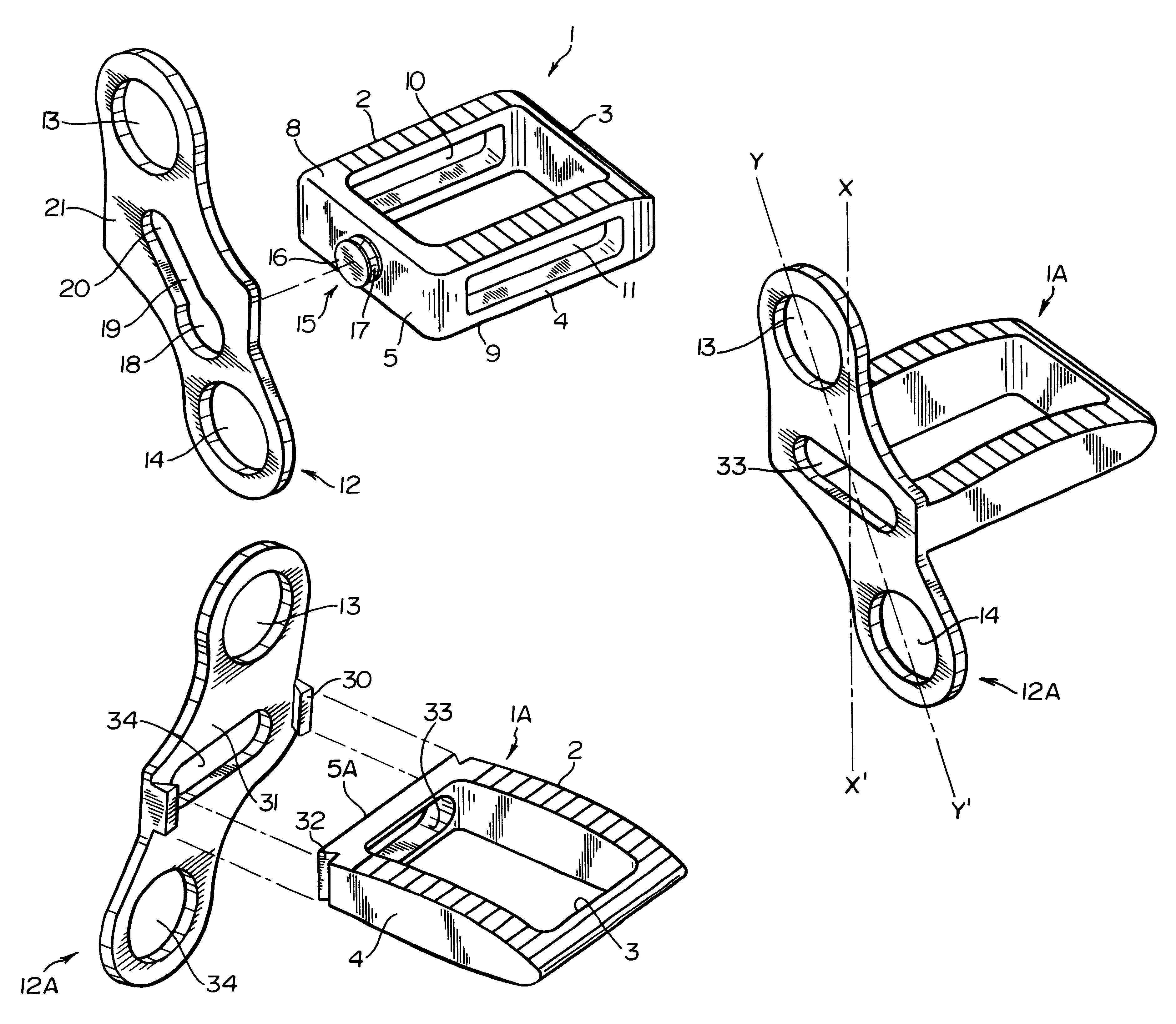
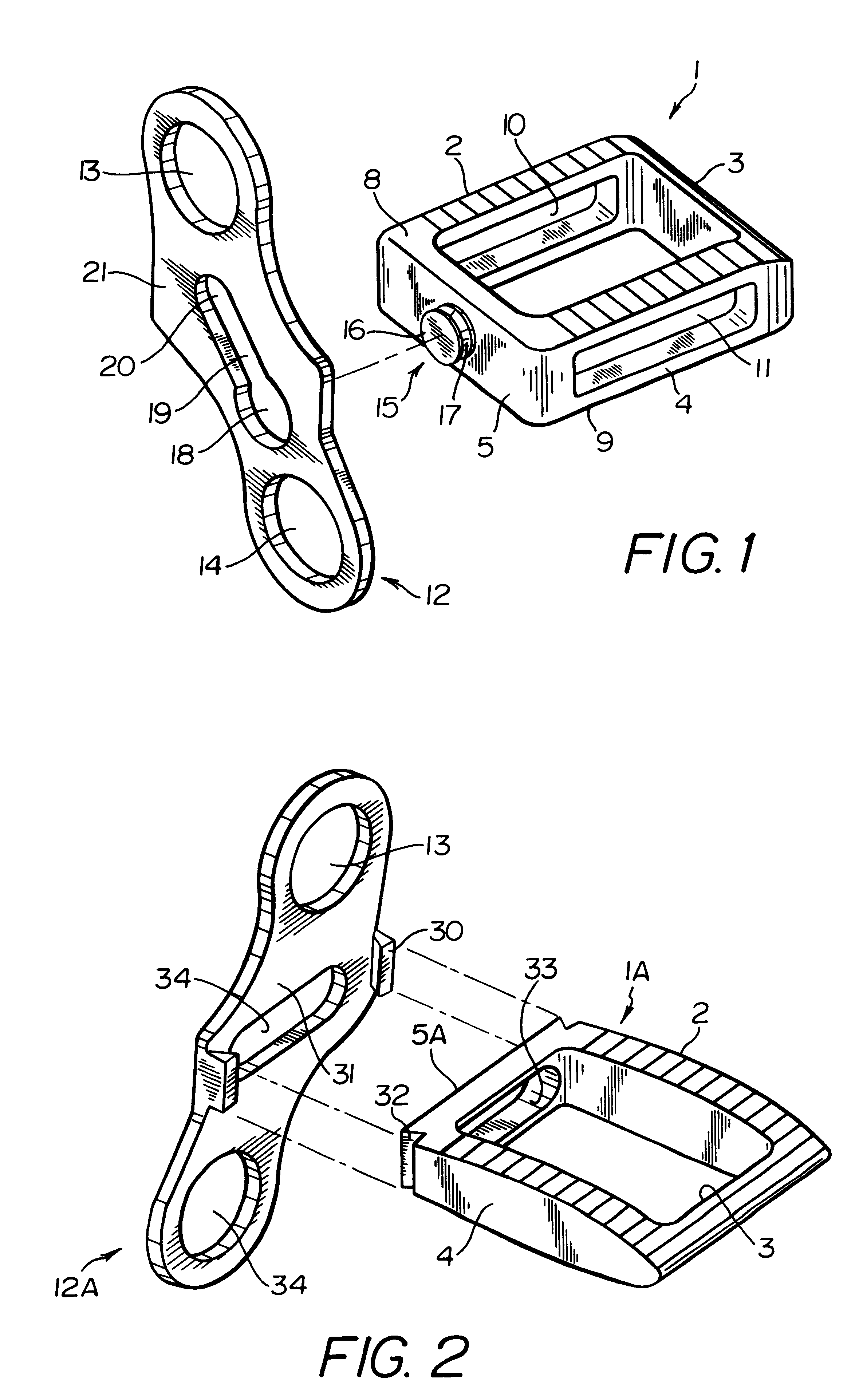
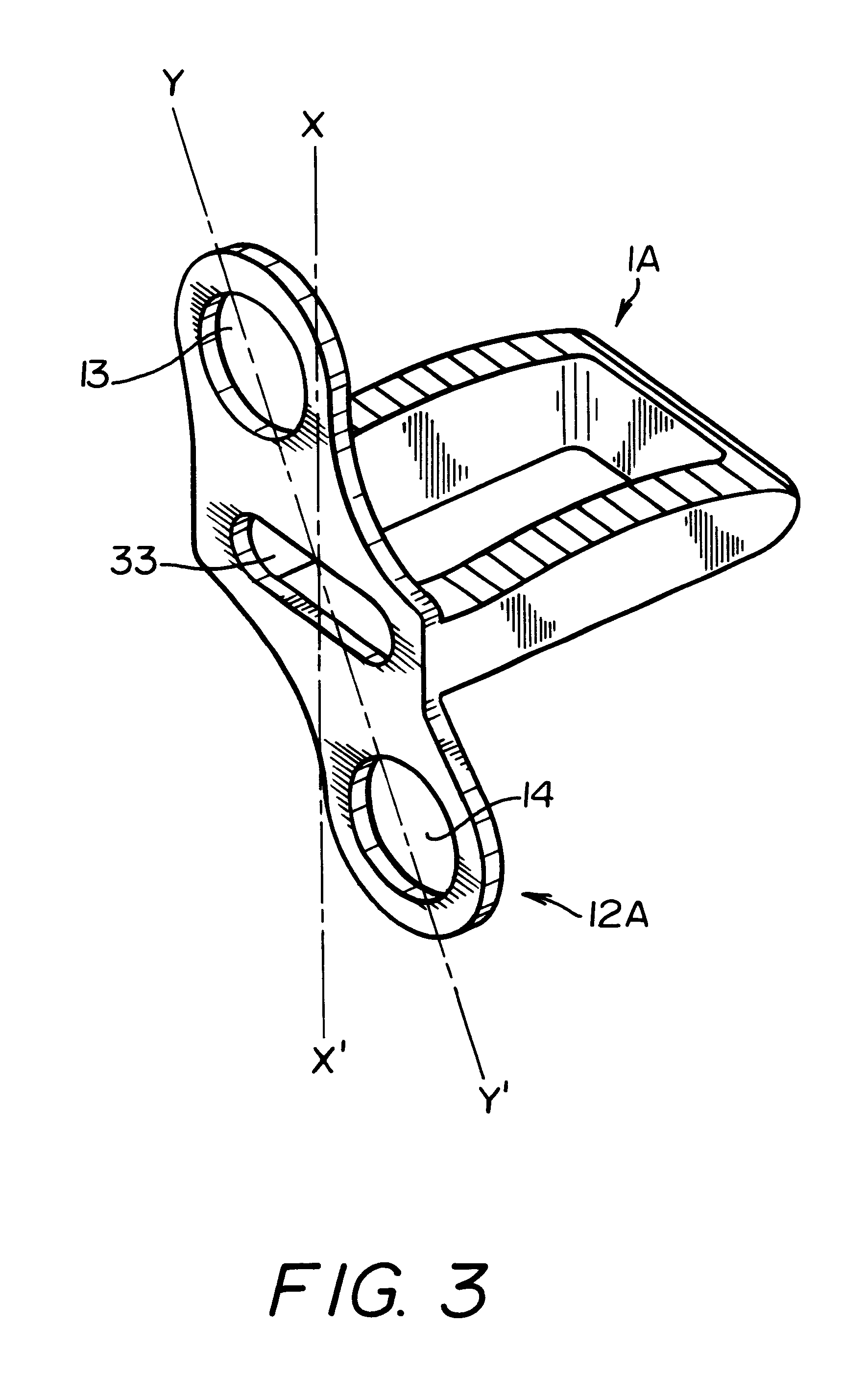
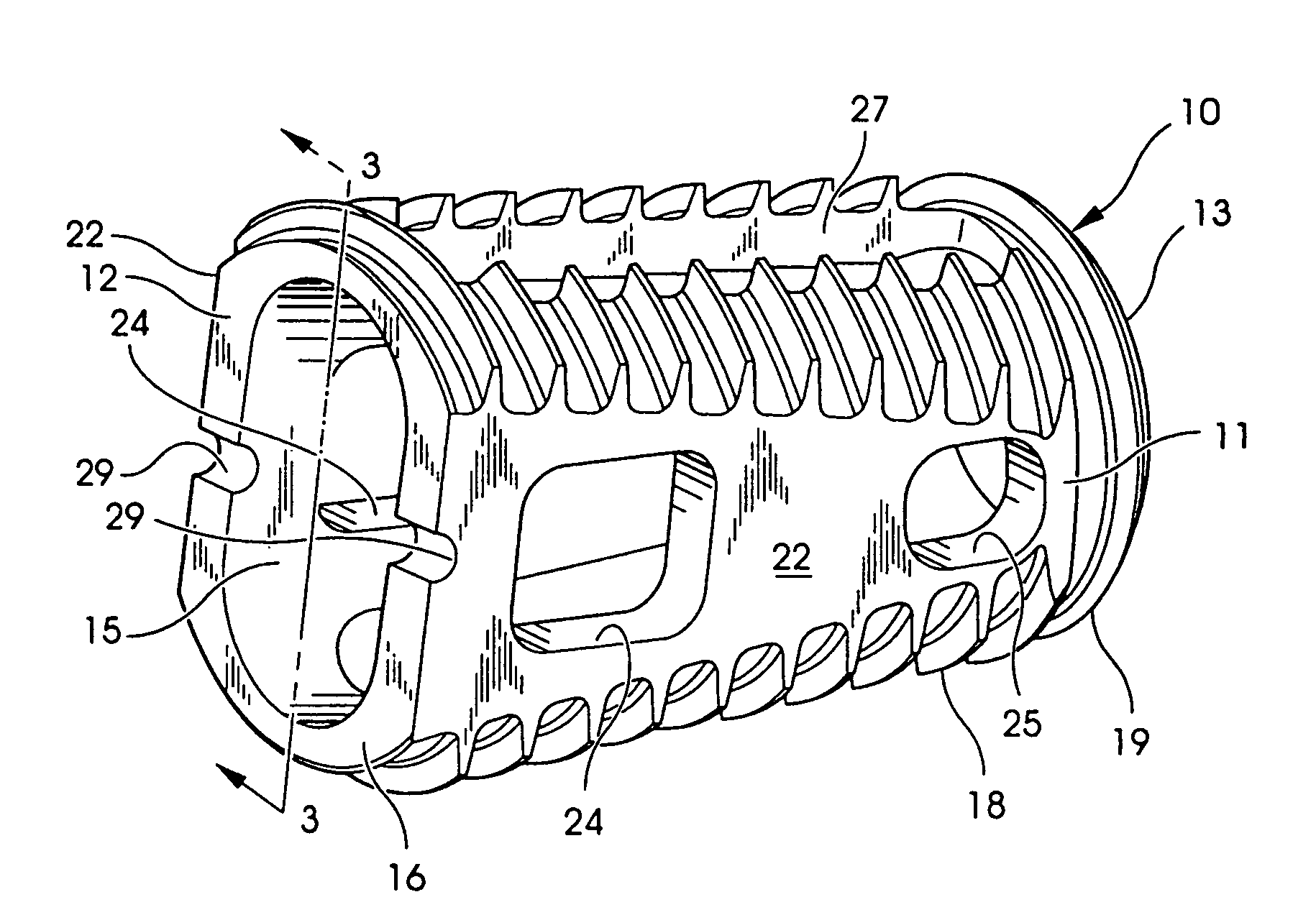
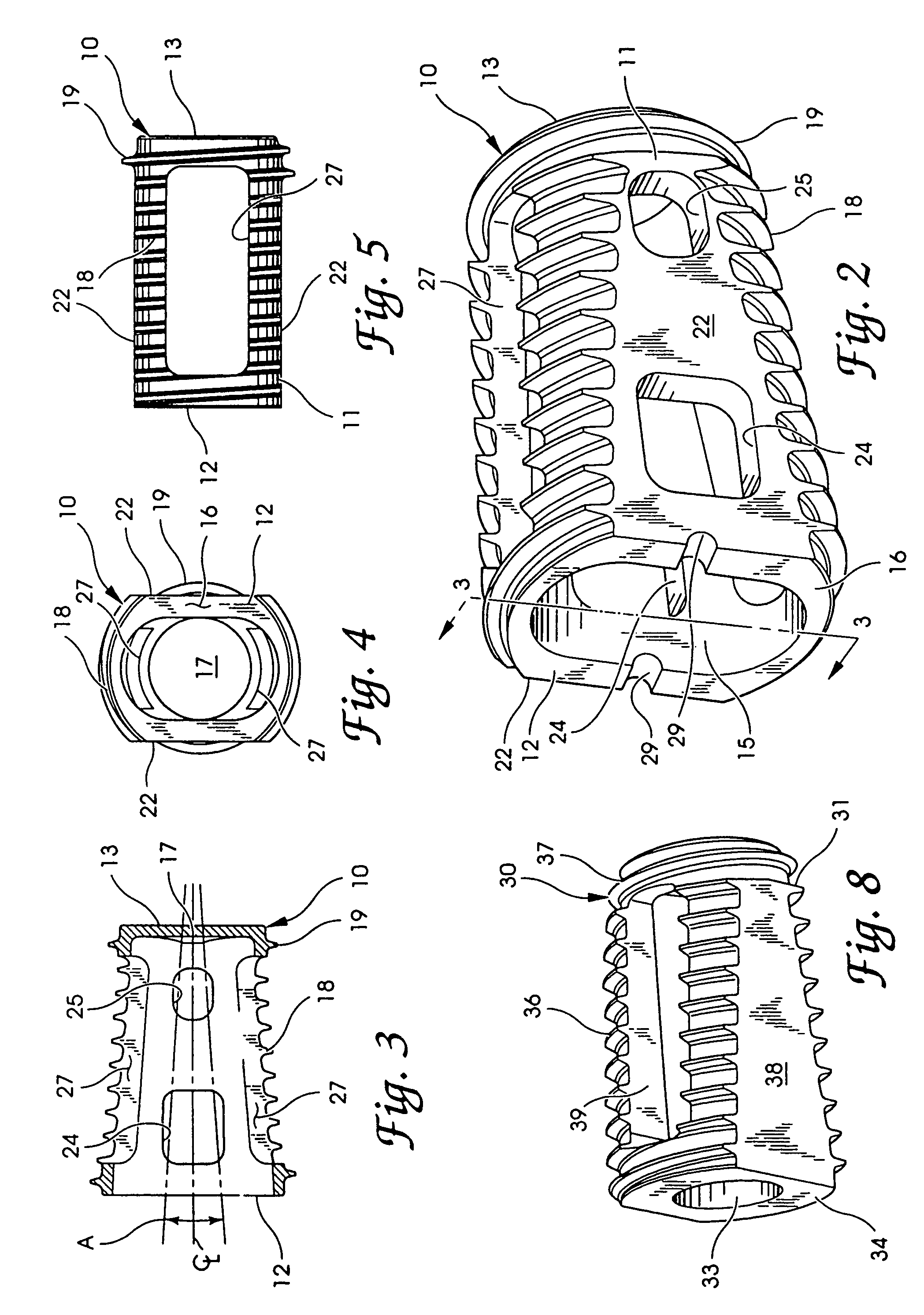
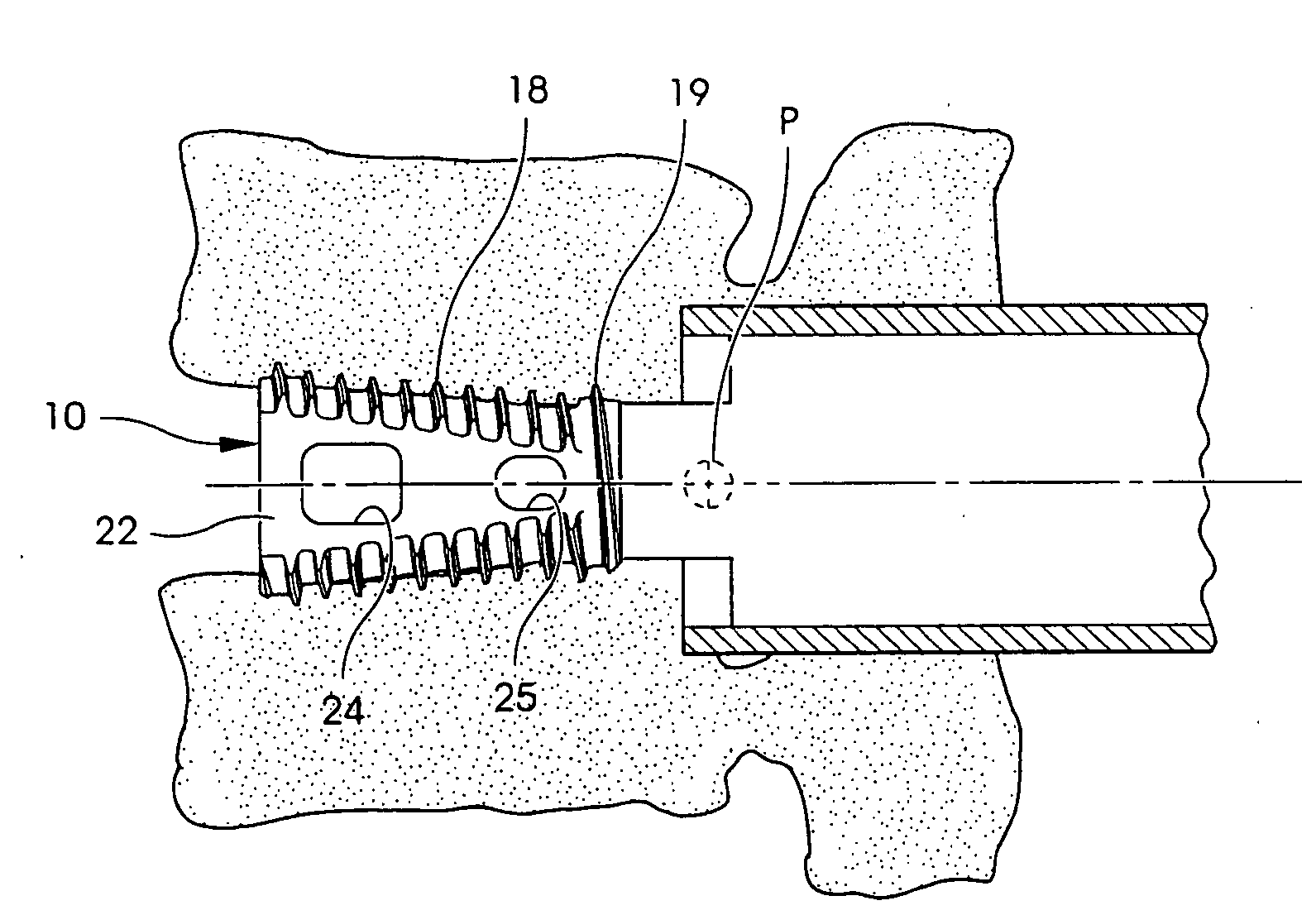
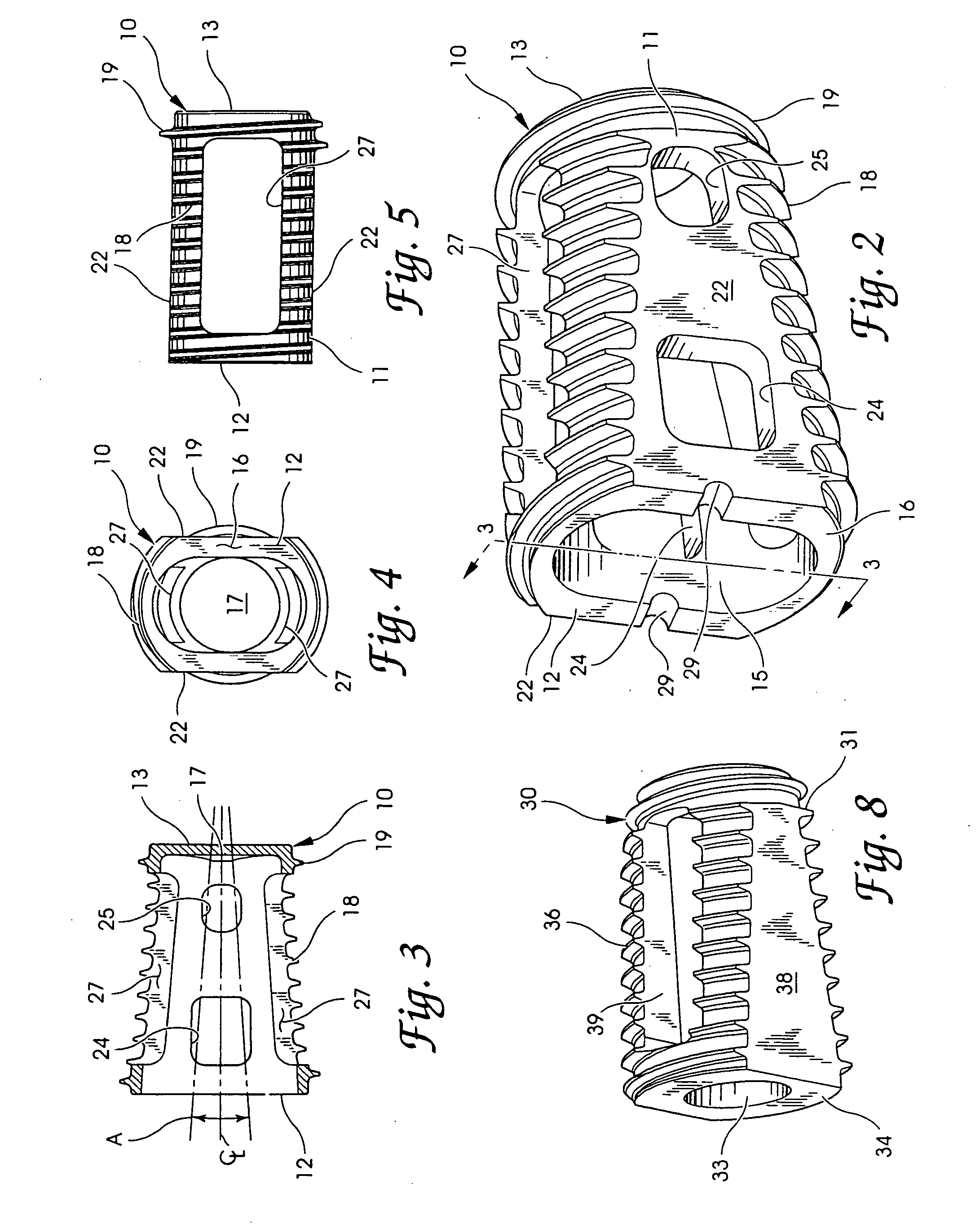
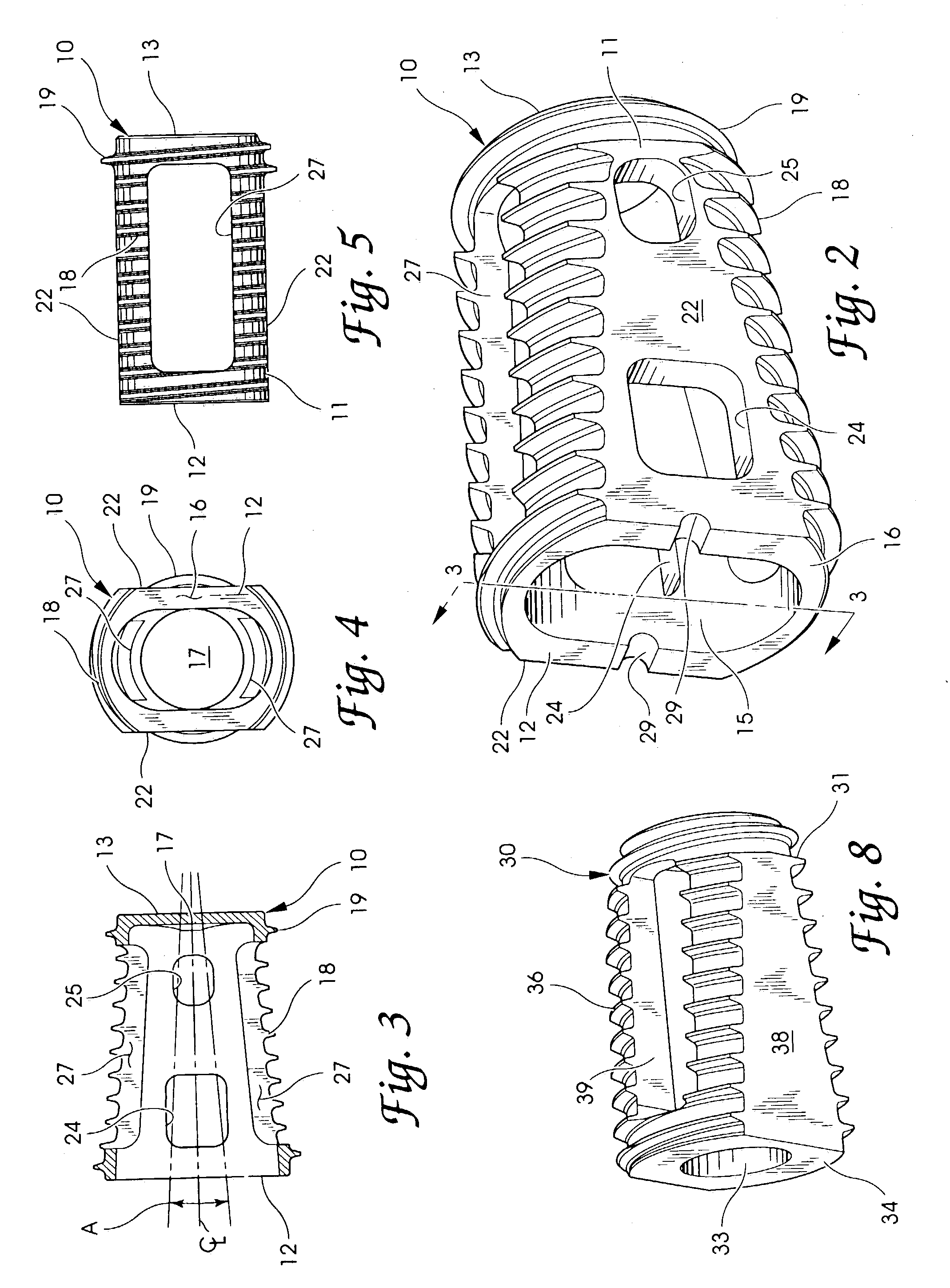
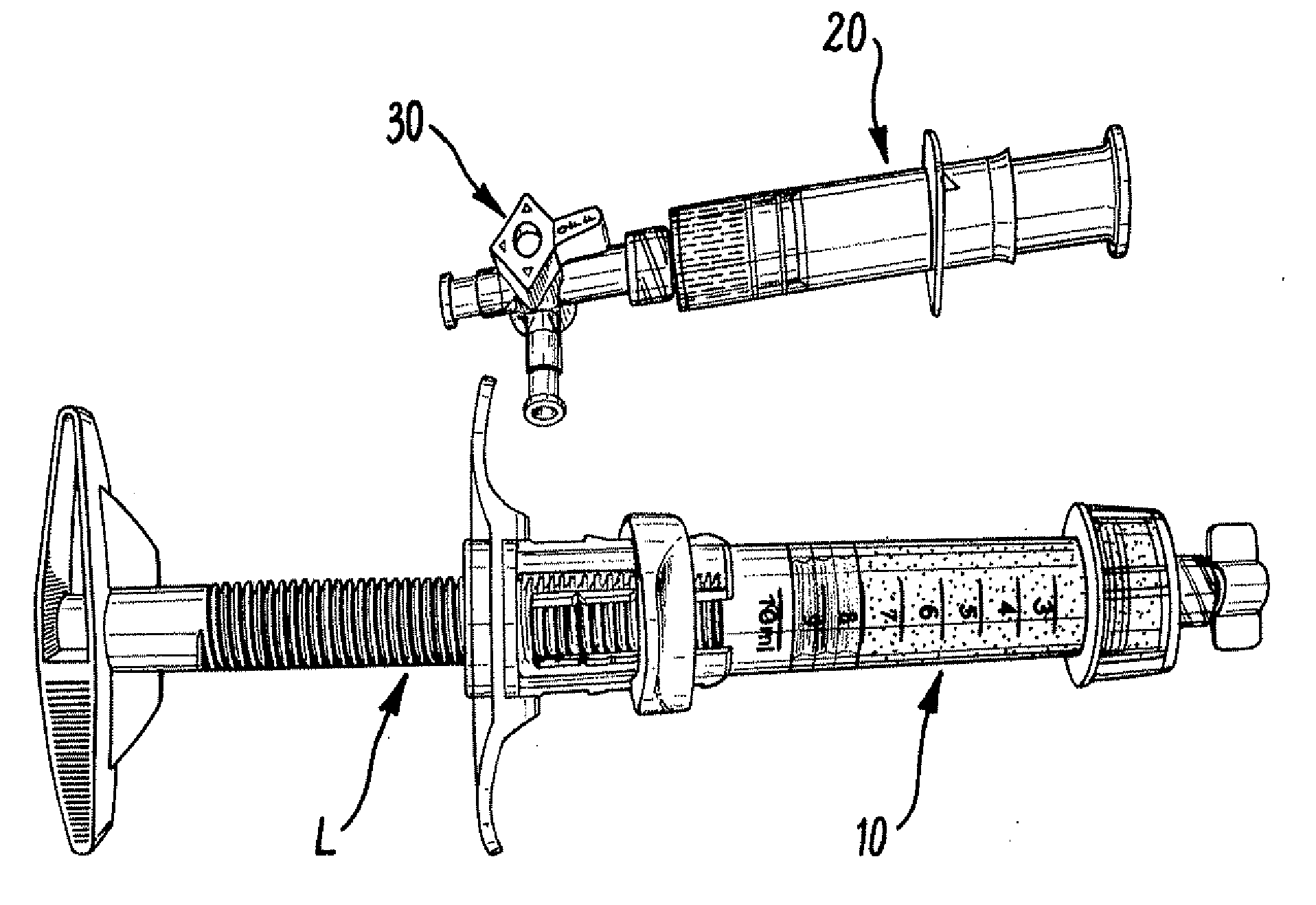
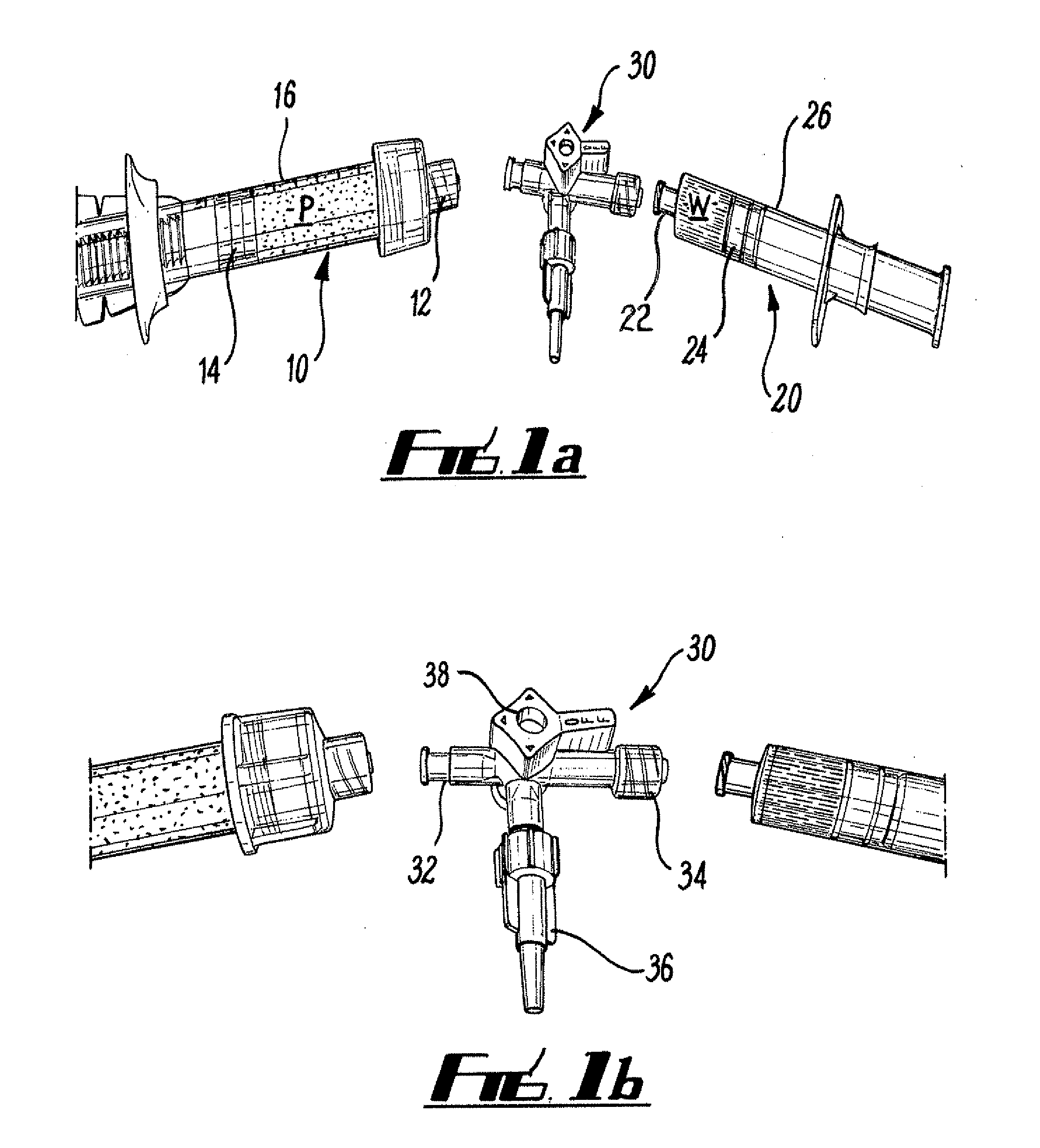
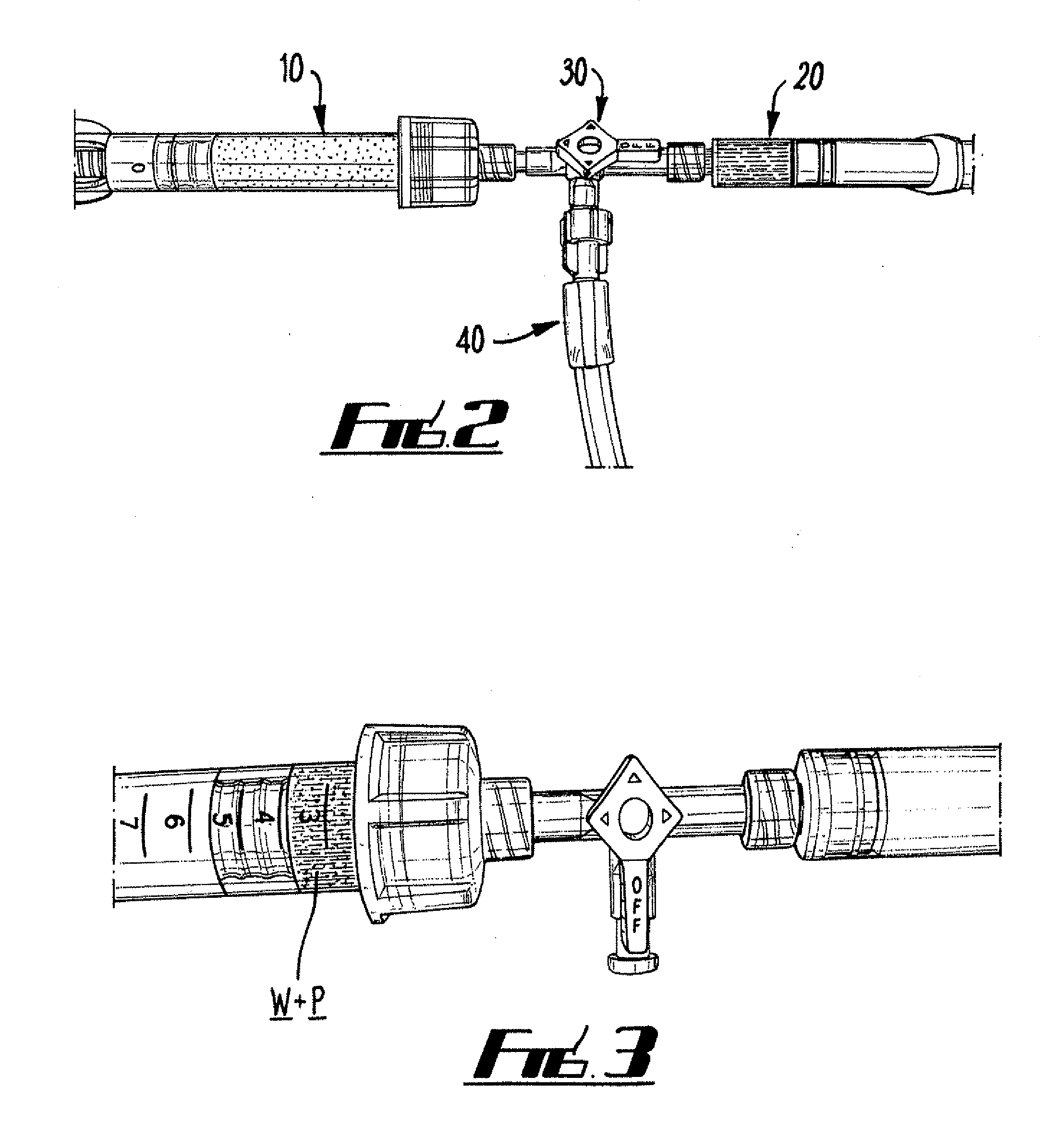
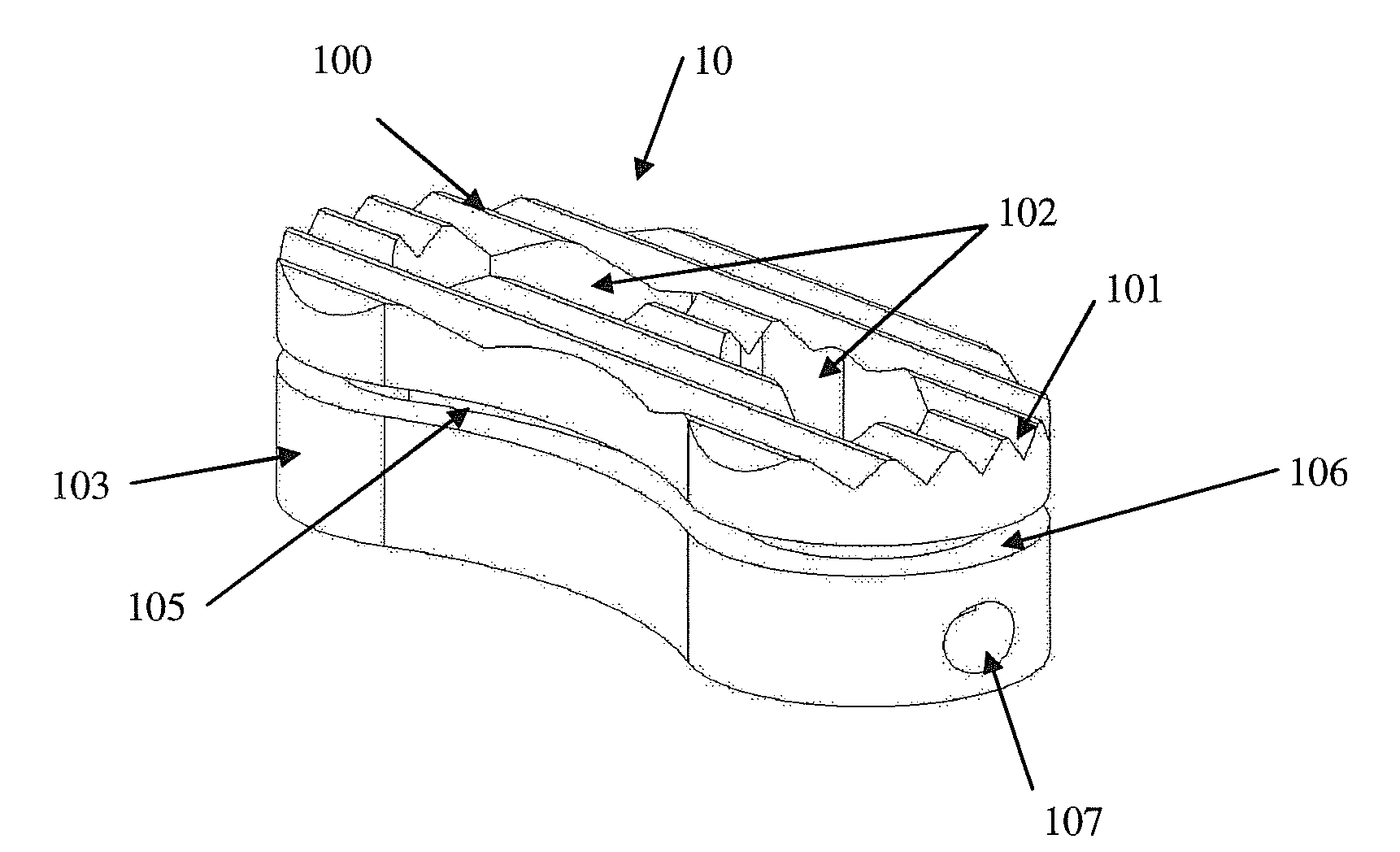
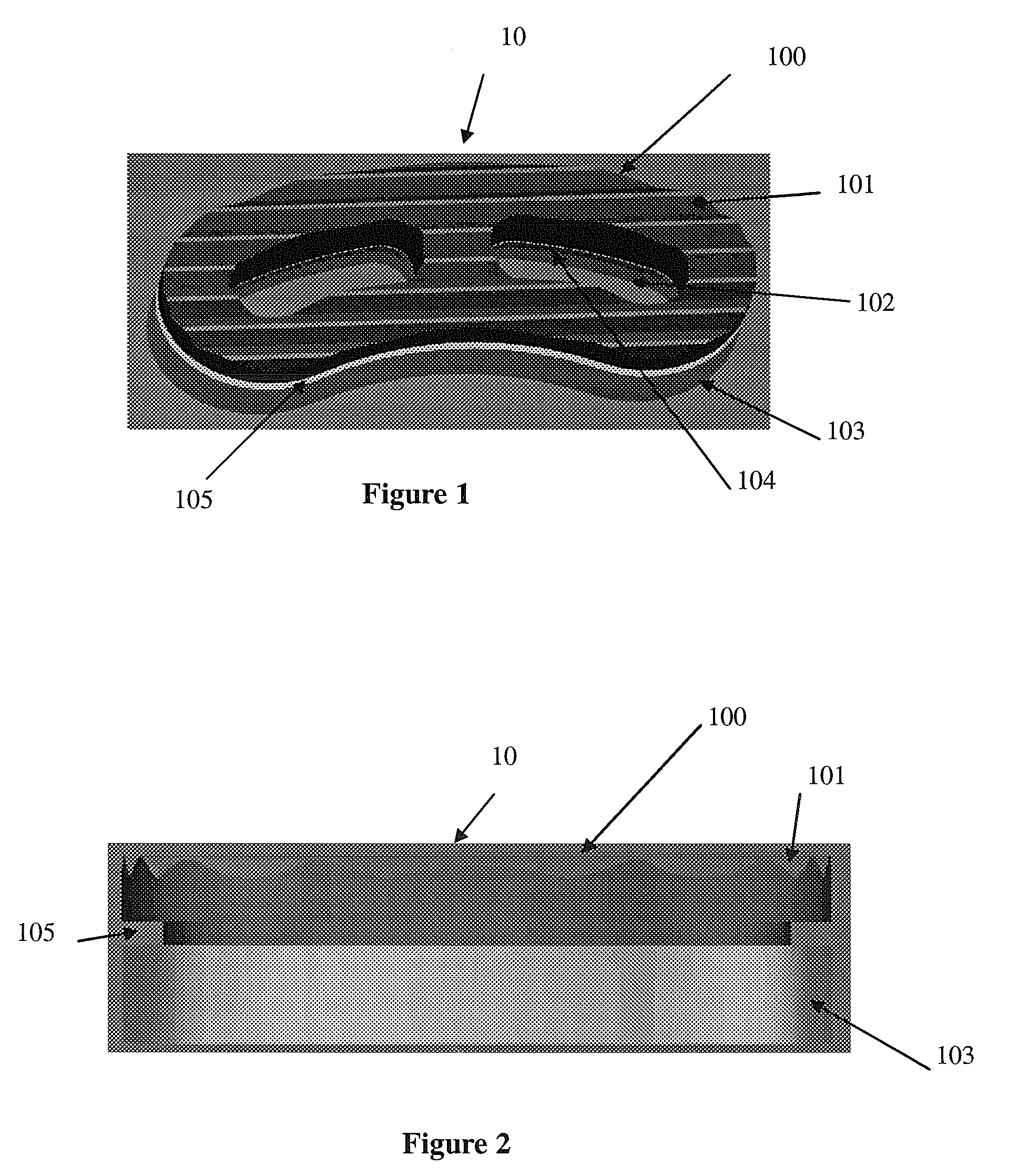
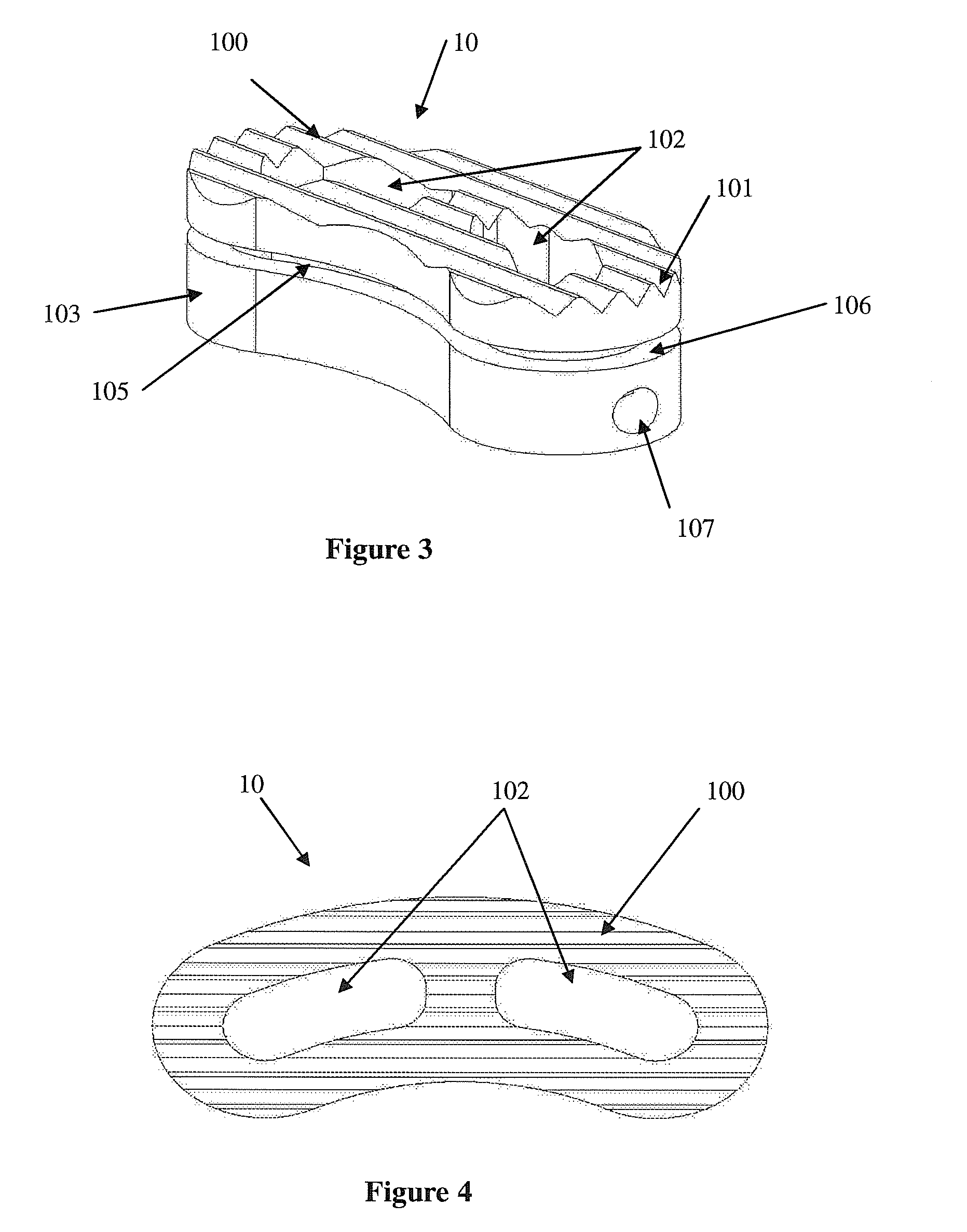
Intersomatic setting and fusion system
A system for intersomatic fusion and setting of vertebrae. The system includes at least one open internal cage arranged for receiving spongy bone or bone substitute and is designed to be interposed between two vertebrae during diskectomy. The cage (1) includes on its anterior face (5) an external element forming a plate (12) extending in a plane substantially perpendicular to the insertion plane of the cage (1), and has at each of its ends an anchor device (13,14) adapted for anchoring to at least two adjacent vertebrae to be secured to each other by the cage (1). The system can be separated into two parts, the cage and the plate.
Owner:SCIENTX
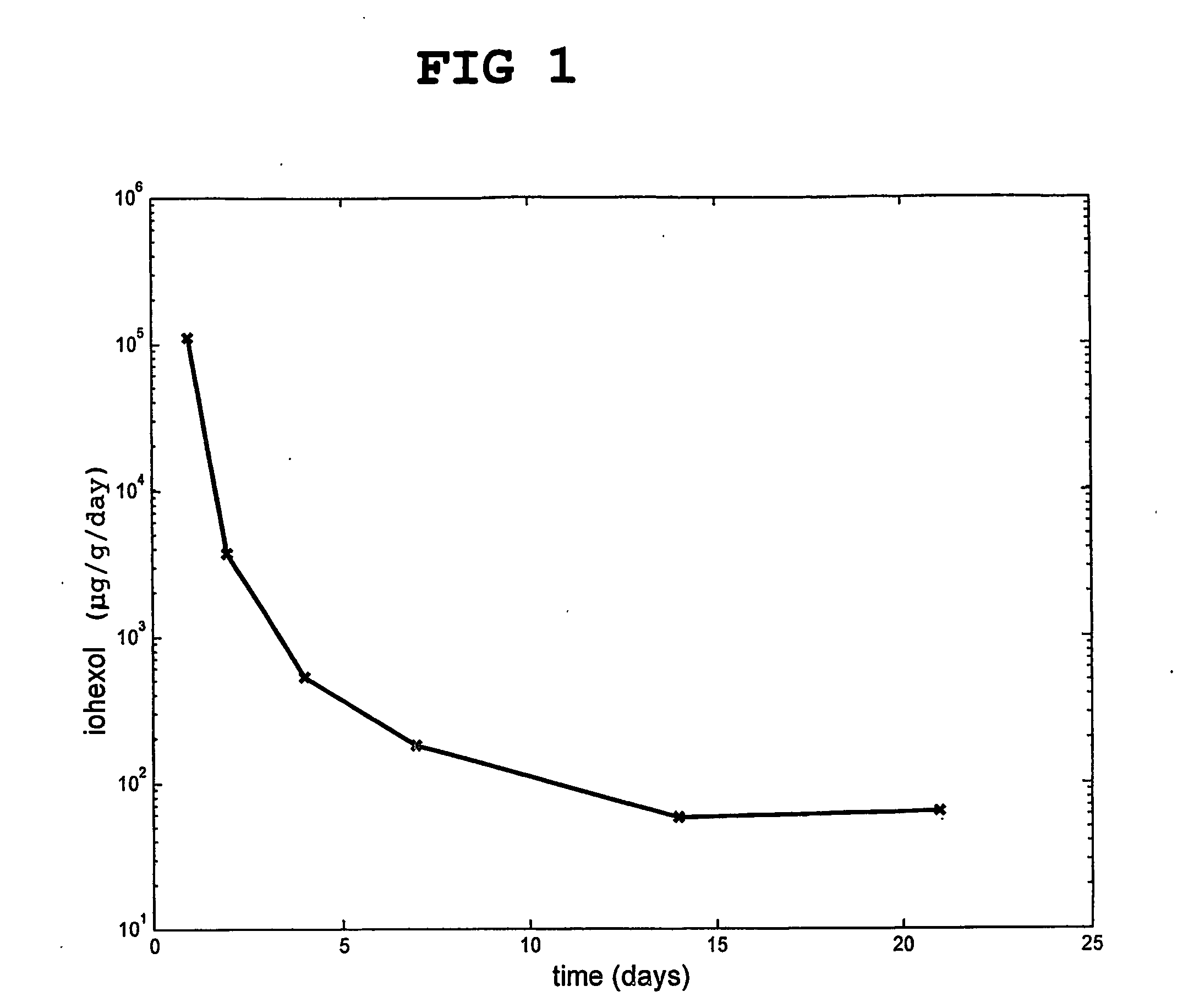
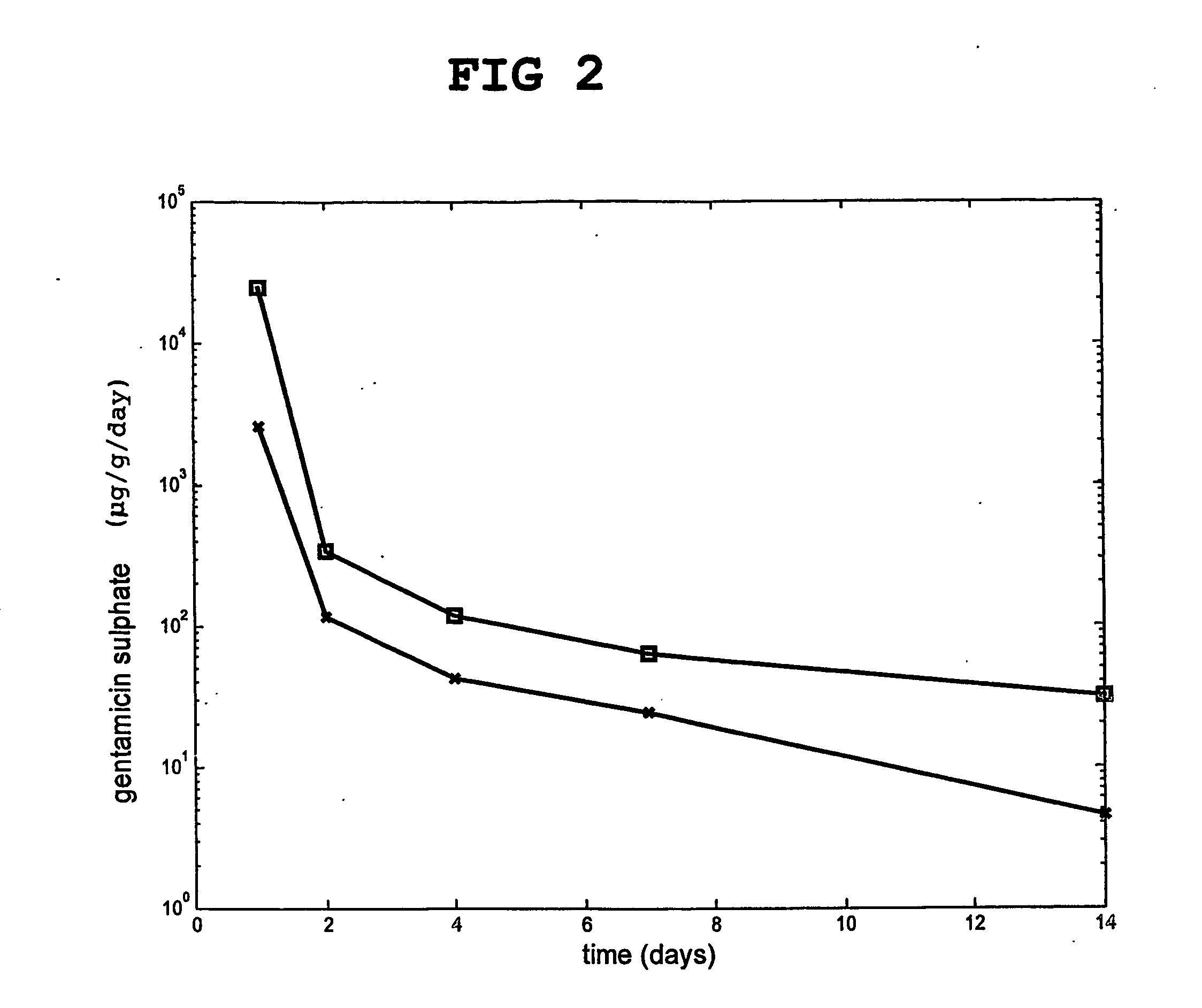
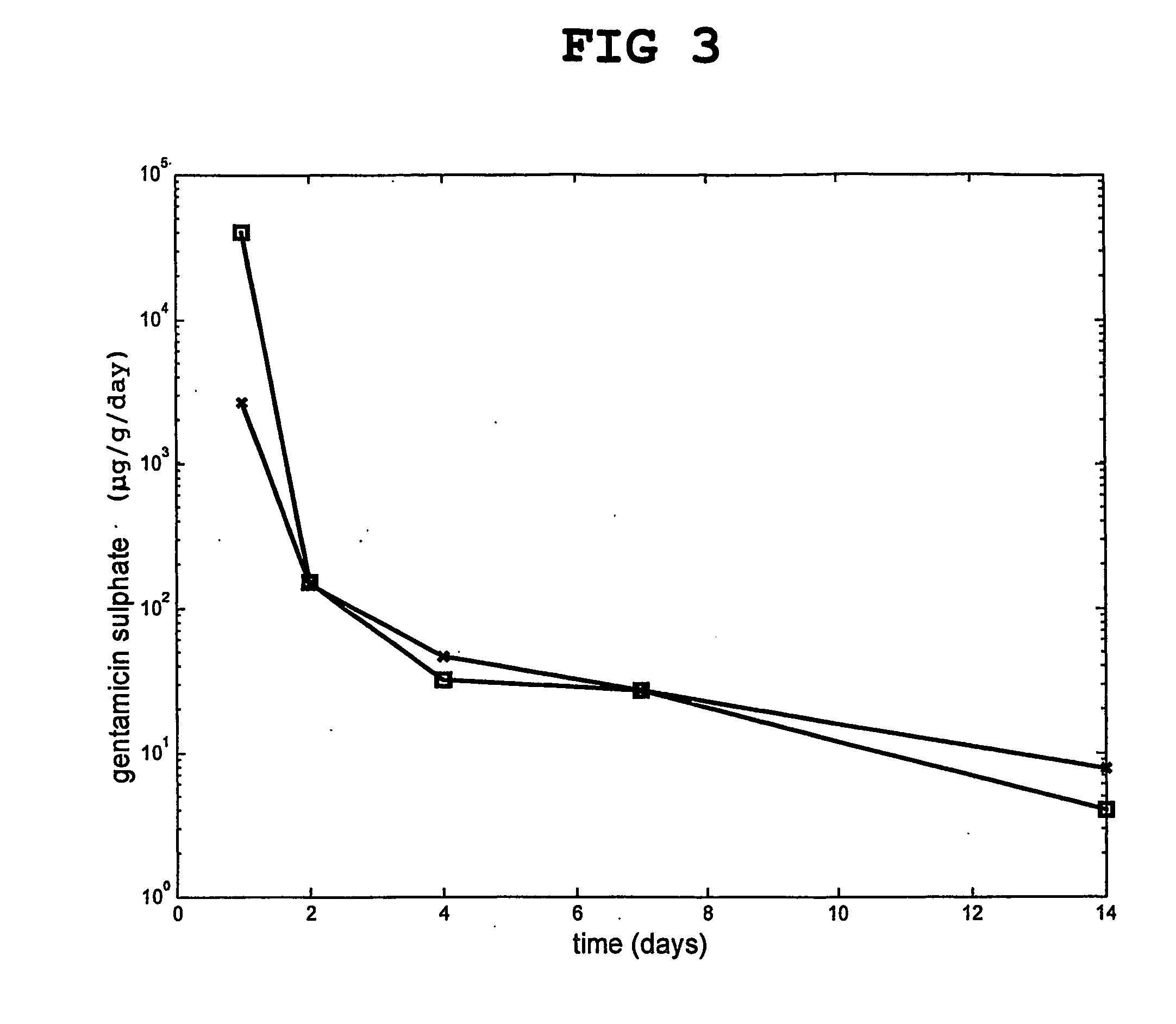
Injectable and moldable bone substitute materials
ActiveUS20070191963A1Reduce needEasy to manageBone implantPharmaceutical delivery mechanismBone implantFlowable Composite
An osteoimplant composite comprising a plurality of particles of an inorganic material, a bone substitute material, a bone-derived material, or any combination thereof; and a polymer material with which the particles are combined. The composite is either naturally moldable or flowable, or it can be made moldable or settable. After implantation, the composite may be set to provide mechanical strength to the implant. The inventive composite have the advantage of being able to fill irregularly shape implantation site while at the same time being settable to provide the mechanical strength required for most orthopedic applications. The invention also provides methods of using and preparing the moldable and flowable composites.
Owner:WARSAW ORTHOPEDIC INC
Implant to be implanted in bone tissue or in bone tissue supplemented with bone substitute material
InactiveUS6921264B2High softening temperatureLow softening temperatureDental implantsInternal osteosythesisBone tissueGrowth promoting
An implant (1) to be implanted in bone tissue, e.g. a dental implant or an implant for an orthopedic application, comprises surface regions (4) of a first type which have e.g. osseo-integrative, inflammation-inhibiting, infection-combating and / or growth-promoting properties, and surface regions (8) of a second type which consist of a material being liquefiable by mechanical oscillation. The implant is positioned in an opening of e.g. a jawbone and then mechanical oscillations, e.g. ultrasound is applied to it while it is pressed against the bone. The liquefiable material is such liquefied at least partly and is pressed into unevennesses and pores of the surrounding bone tissue where after resolidification it forms a positive-fit connection between the implant and the bone tissue. The surface regions of the two types are arranged and dimensioned such that, during implantation, the liquefied material does not flow or flows only to a clinically irrelevant degree over the surface regions of the first type such enabling the biologically integrative properties of these surface regions to start acting directly after implantation. The implant achieves with the help of the named positive fit a very good (primary) stability, i.e. it can be loaded immediately after implantation. By this, negative effects of non-loading are prevented and relative movements between implant and bone tissue are reduced to physiological measures and therefore have an osseo-integration promoting effect.
Owner:WOODWELDING
Bone substitute compositions and method of use
The present invention relates to novel bone substitute compositions and methods of use. It further encompasses the use of these novel bone substitute compositions for bone augmentation and the treatment of disease conditions. The invention also contemplates a kit including bone substitute compositions and a percutaneous delivery device.
Owner:KYPHON
Porous osteoimplant
ActiveUS20080069852A1Accelerate the remodeling processImprove permeabilityBone implantSkeletal disorderBone growthBone defect
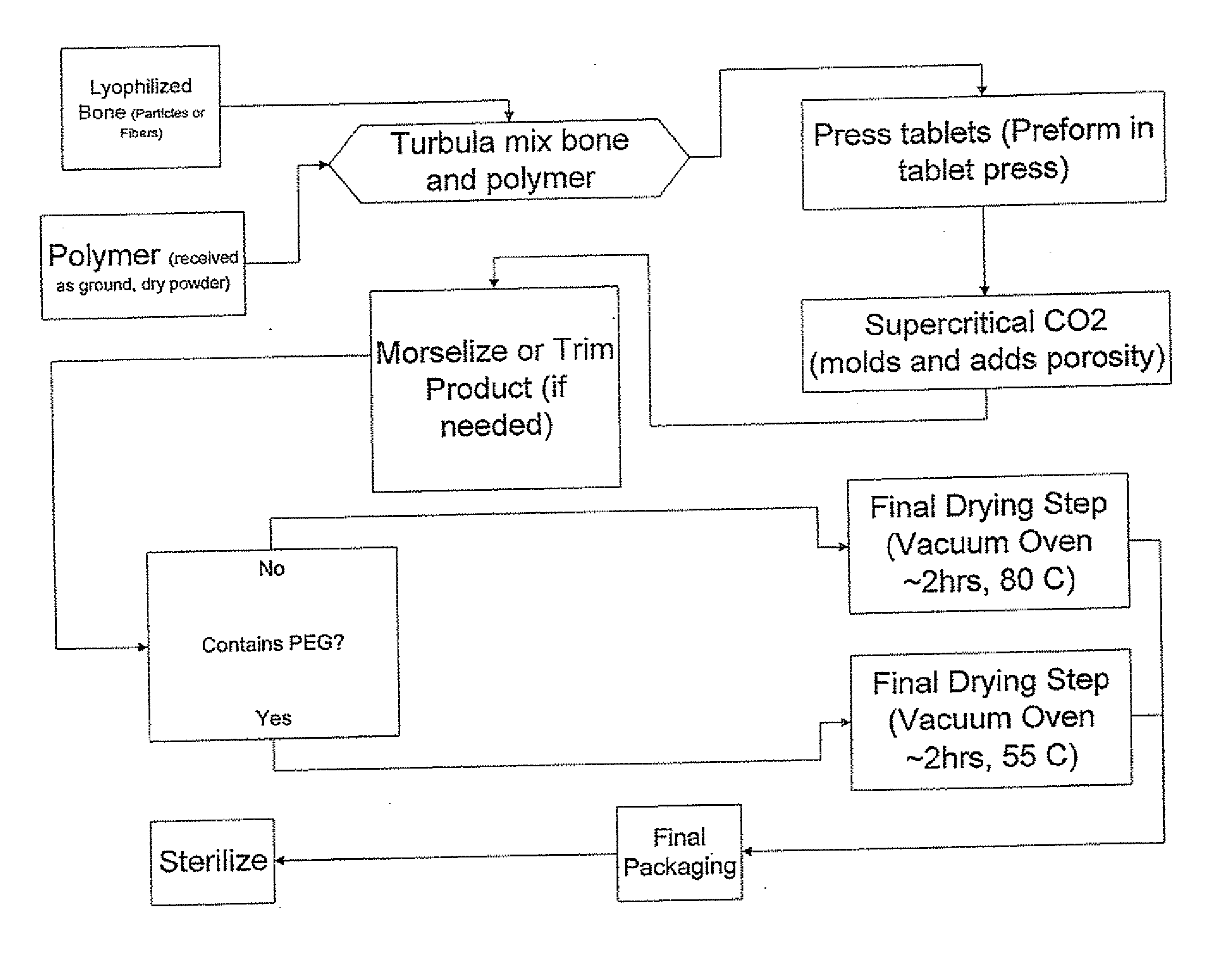
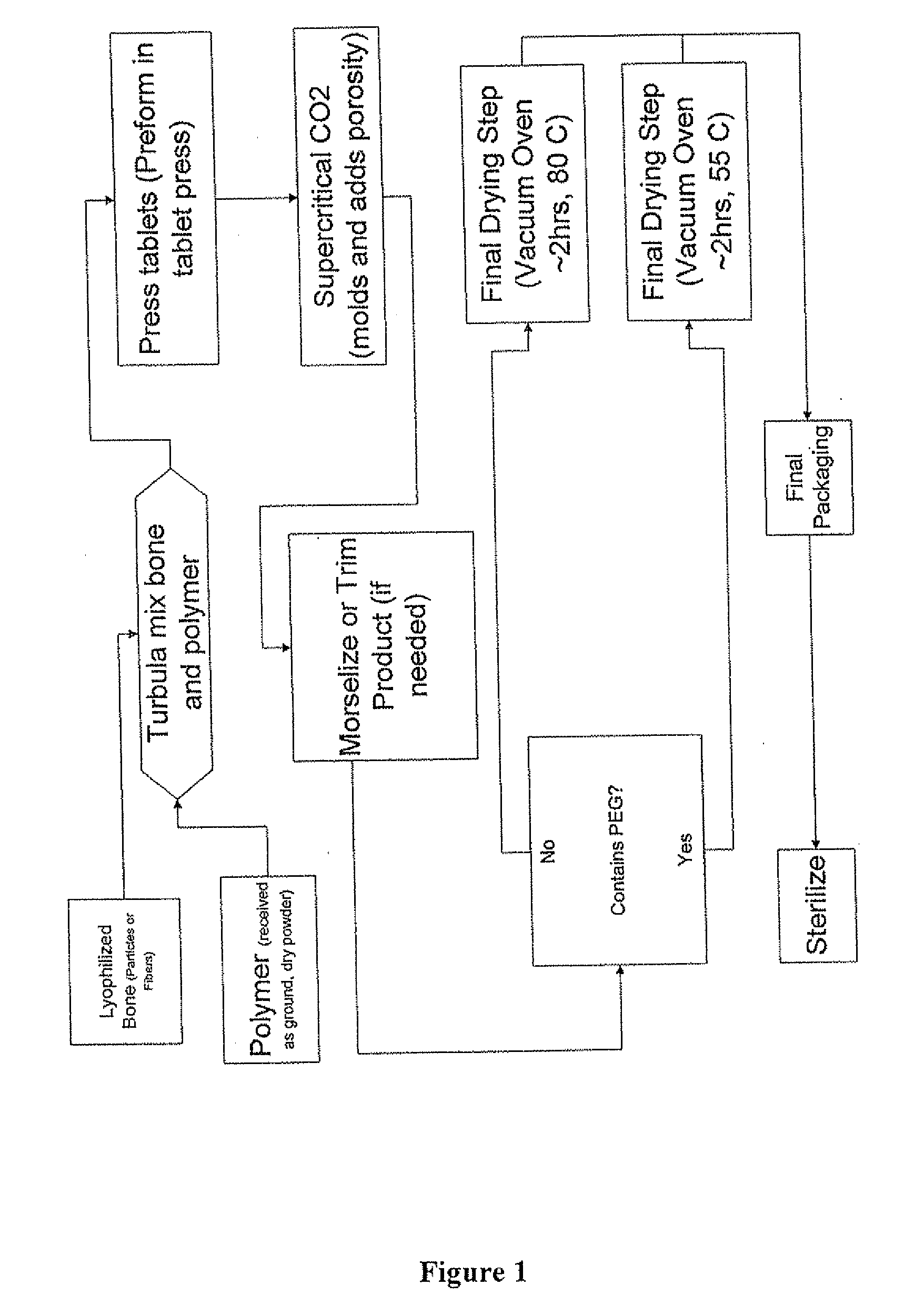
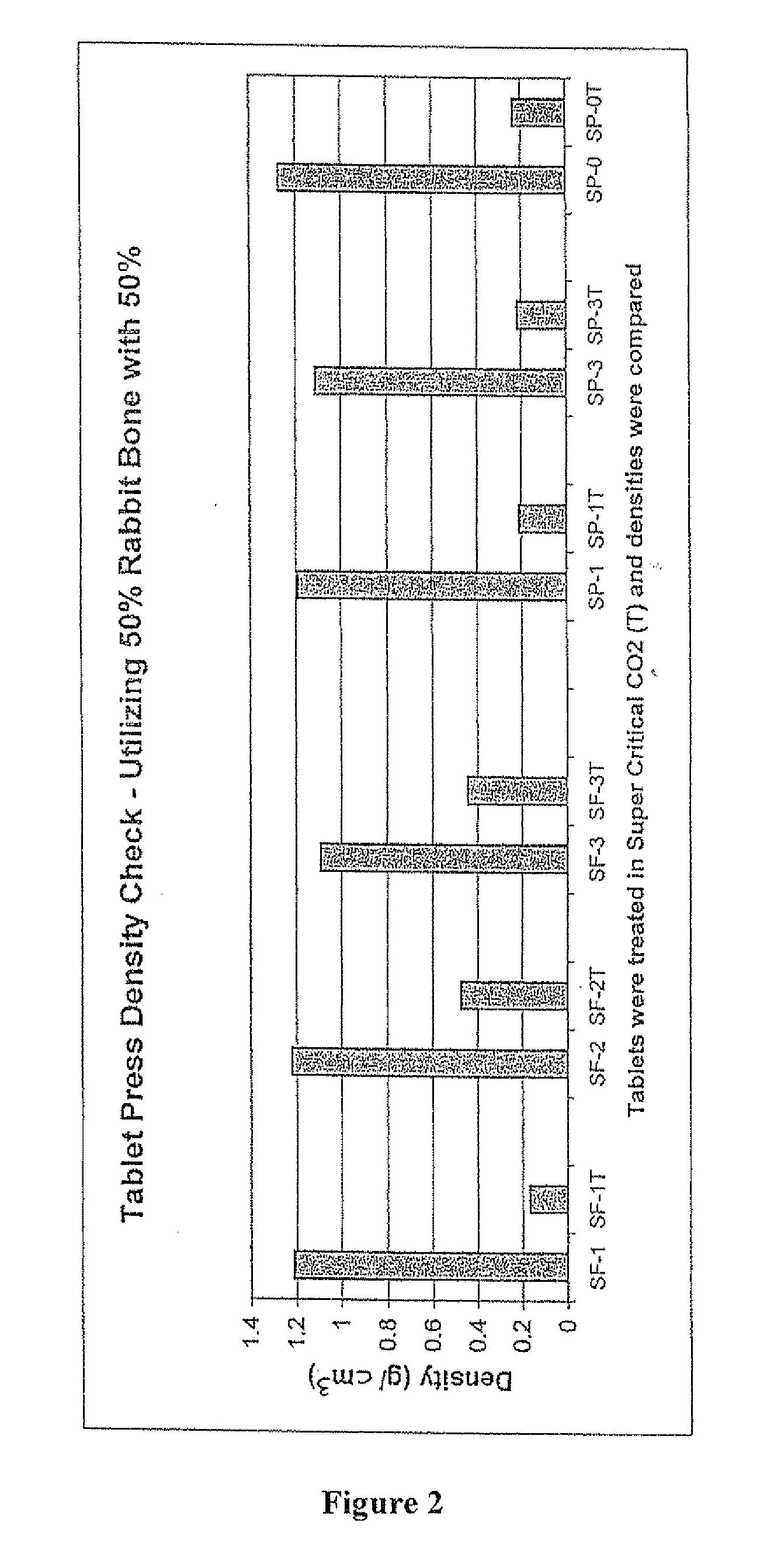
The invention is directed toward porous composites for application to a bone defect site to promote new bone growth. The inventive porous composites comprise a biocompatible polymer and a plurality of particles of bone-derived material, inorganic material, bone substitute material or composite material. In certain embodiments, the porous composites are prepared using a method that includes a supercritical fluid (e.g., supercritical carbon dioxide) treatment. The invention also discloses methods of using these composites as bone void fillers.
Owner:WARSAW ORTHOPEDIC INC
Interbody fusion device and method for restoration of normal spinal anatomy
InactiveUS7238186B2Maintain patency and stabilityRapid and stable arthrodesisInternal osteosythesisBone implantSpinal columnPorous tantalum
An interbody fusion device in one embodiment includes a tapered body defining a hollow interior for receiving bone graft or bone substitute material. The body defines exterior threads which are interrupted over portions of the outer surface of the device. The fusion device defines truncated side walls so that on end view the body takes on a cylindrical form. The side walls are provided with vascularization openings, and the body wall device includes opposite bone ingrowth slots extending through the interrupted thread portion of the body. In another embodiment, the tapered body is solid and formed of a porous biocompatible material having sufficient structural integrity to maintain the intradiscal space and normal curvature. The material is preferably a porous tantalum having fully interconnected pores to facilitate complete bone tissue ingrowth into the implant. An implant driver is provided which engages the truncated side walls to complete the cylindrical form of the implant at the root diameter of the interrupted threads, to thereby facilitate threaded insertion of the implant to the intra-discal space between adjacent vertebrae. Methods for posterior and anterior insertion of the fusion device are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Method and apparatus for engineered regenerative biostructures such as hydroxyapatite substrates for bone healing applications
An engineered regenerative biostructure (erb) for implantation into a human body as a bone substitute, which includes an internal microstructure, mesostructure and / or macrostructure to provide improved bone in-growth, and methods for making the erb. Under one aspect of the invention, the biostructure has resorbable and nonresorbable regions. Under another aspect of the invention, the biostructure is constructed of hydroxyapatite, tricalcium phosphate and / or demineralized bone. Under yet another aspect of the invention, the porous biostructure is partially or fully infused with a resorbable, nonresorbable or dissolvable material.
Owner:THEKEN SURGICAL LLC
Curable bone substitute
InactiveUS20070087031A1Promote bone growthInfection controlBone implantTissue regenerationCompound (substance)Bone substitute
A novel composition, kit, and method of using the composition as a bone substitute for dental, orthopedic and drug delivery purposes. Specifically, the bone substitute comprises a plurality of polymeric beads having a crosslinkable shell where the shell is cured by light and / or chemical curing.
Owner:A ENTERPRISES
Calcium phosphate cement composition and a method for the preparation thereof
ActiveUS6929692B2Good water solubilityIncreased formationOther chemical processesBone implantChemical synthesisCalcium biphosphate
The invention describes a new calcium phosphate cement powder, whose composition can best be described over the Ca / P molar ratio range of 1.35 to 1.40, most preferably 1.39, and whose two components were prepared by wet chemical synthesis procedures. One component is chemically-synthesized, bi-phasic alpha-TCP (Ca3(PO4)2, 95 wt %)+HA (Ca10(PO4)6(OH)2, 5 wt %) powder, while the second component is again a chemically-synthesized, single-phase DCPD (CaHPO4·2H2O) powder. A setting solution (Na2HPO4·2H2O) is used to form a self-setting calcium phosphate cement from the powder mixture. This cement can be used as bone filler or bone substitute in applications, which require higher rates of resorption.
Owner:DR AHMET CUNEYT TAS
Intervertebral spacer and insertion tool
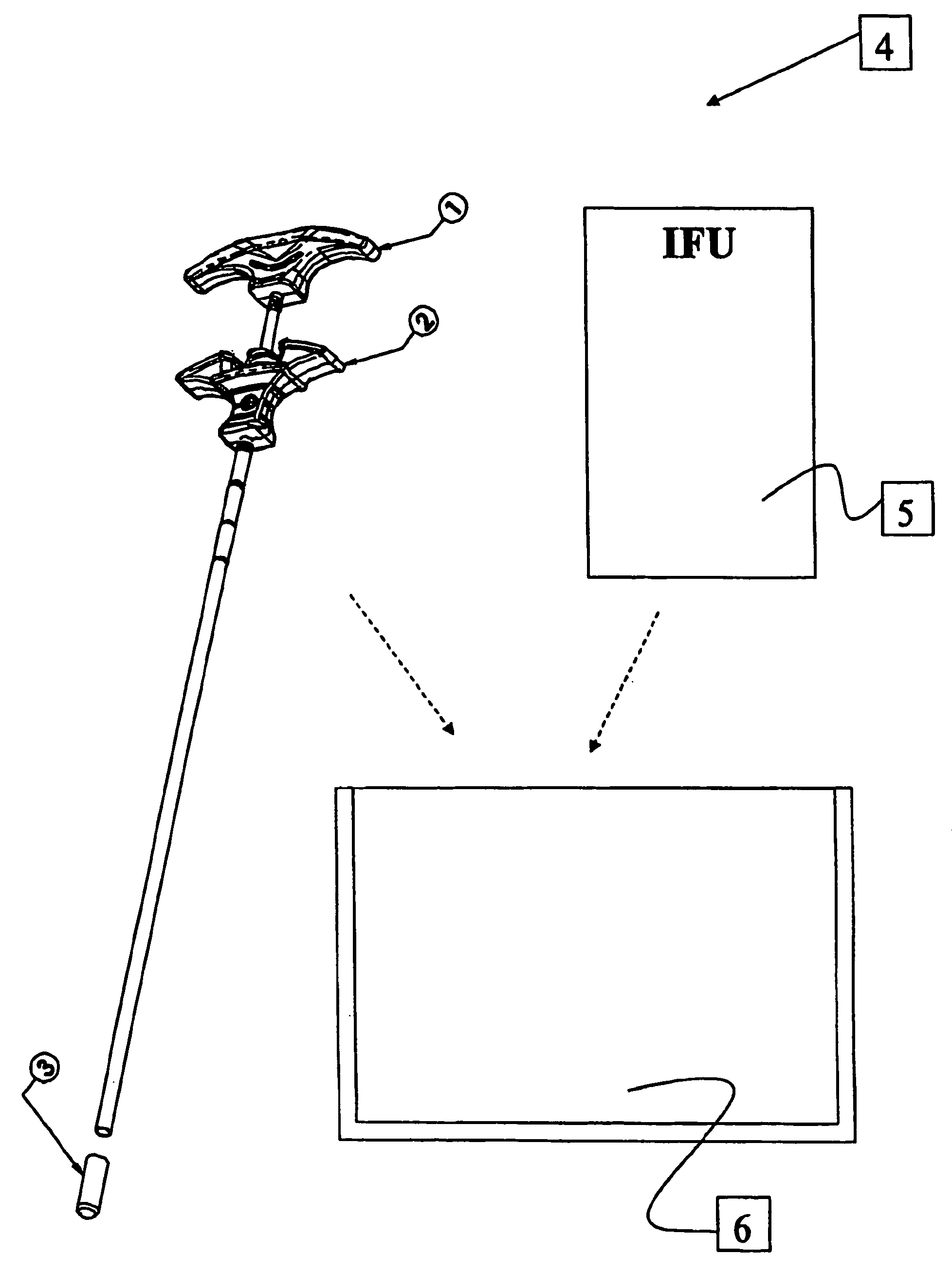
An implant for placing in the spine is disclosed. The implant has a tool engaging surface on its trailing end configured for intimate engagement with an insertion tool. The insertion tool configuration is particularly suited for implants made of brittle materials including bone and bone substitutes. Also disclosed is an insertion tool having a gripping end adapted for intimate engagement with an implant. In one embodiment, the insertion tool is operable by axial movement of one gripping arm with respect to the other.
Owner:WARSAW ORTHOPEDIC INC
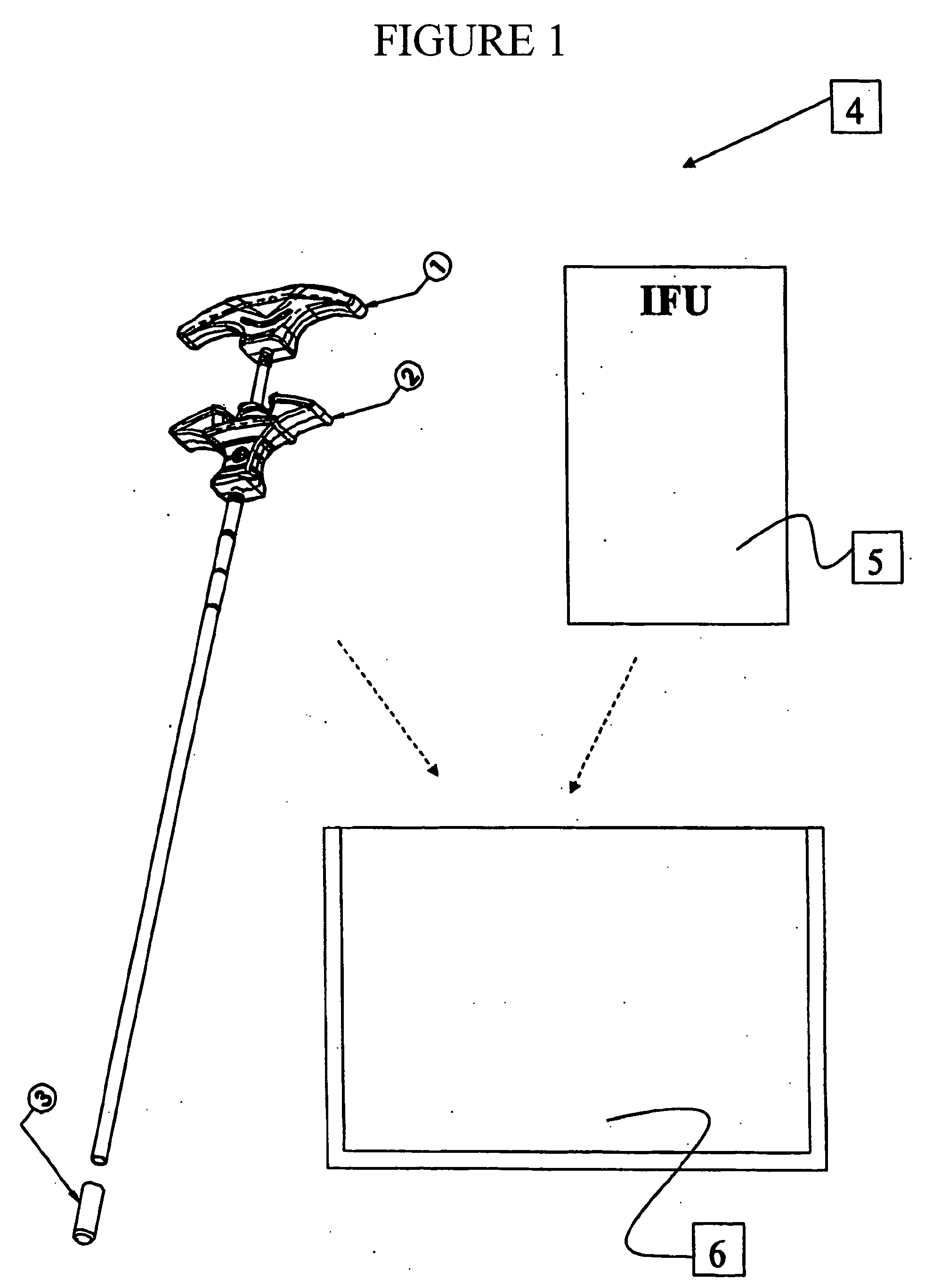
Intervertebral spacer and insertion tool
An implant for placing in the spine is disclosed. The implant has a tool engaging surface on its trailing end configured for intimate engagement with an insertion tool. The insertion tool configuration is particularly suited for implants made of brittle materials including bone and bone substitutes. Also disclosed is an insertion tool having a gripping end adapted for intimate engagement with an implant. In one embodiment, the insertion tool is operable by axial movement of one gripping arm with respect to the other.
Owner:WARSAW ORTHOPEDIC INC
Biocompatible polymer compositions for dual or multi staged curing
ActiveUS20050197422A1Increase ratingsHigh viscosityCosmetic preparationsImpression capsWound healingBioadhesive
The present invention provides a biocompatible polymer composition for use in biomedical applications comprising a base molecule, a linker molecule and at least one initiator compound, said base molecule having at least two differing functionalities, and said linker molecule having a functionality reactive with at least one functionality of said base molecule, the first of said at least two functionalities of said base molecule enabling a first curing stage of said polymer composition by reaction with said linker molecule, and the second and any further functionality of said base molecule enabling second and further curing stages of said polymer composition, said first, second and any further curing stages being capable of activation simultaneously or independently of each other as required. The invention further provides uses of the polymer compositions of the invention in biomedical applications such as tissue engineering, dry delivery, as a bioadhesive in wound healing, as bone substitutes or scaffolds, as cements in dental and periodontal applications and as anti-adhesives or protective barriers.
Owner:POLYNOVO BIOMATERIALS PTY LTD
Bone substitute compositions and method of use
The present invention relates to novel bone substitute compositions and methods of use. It further encompasses the use of these novel bone substitute compositions for bone augmentation and the treatment of disease conditions. The invention also contemplates a kit including bone substitute compositions and a percutaneous delivery device.
Owner:KYPHON
Porous ceramic composite bone grafts
ActiveUS20060198939A1Improve toughnessLimiting for fragmentationOrganic active ingredientsPeptide/protein ingredientsBiodegradable polymerPorous ceramics
The invention relates to porous ceramic composites incorporating biodegradable polymers for use as a bone substitute in the fields of orthopaedics and dentistry or as a scaffold for tissue engineering applications. The porous ceramic composite implant for connective tissue replacement comprises a porous ceramic matrix having a biodegradable polymer provided on internal and external surfaces of the ceramic matrix. The biodegradable polymer allows for the passage and / or delivery of a variety of agents throughout the porous ceramic matrix and improves mechanical properties of the implant in vivo.
Owner:WARSAW ORTHOPEDIC INC
Spinal fusion implants and tools for insertion and revision
InactiveUS20090043394A1Avoid expulsionInternal osteosythesisBone implantSpinal columnPorous tantalum
An interbody fusion device in one embodiment includes a tapered body defining a hollow interior or chamber for receiving bone graft or bone substitute material. The body defines exterior threads which are interrupted over portions of the outer surface of the device. The fusion device includes truncated side walls so that on end view the body takes on a cylindrical form. In another embodiment, the tapered body is solid and formed of a porous biocompatible material having sufficient structural integrity to maintain the intradiscal space and normal curvature. The material is preferably a porous tantalum composite having fully interconnected pores to facilitate complete bone tissue ingrowth into the implant. In further embodiments, the fusion devices are provided with osteogenic material to facilitate bone ingrowth. A cap is also provided to block the opening of hollow fusion devices. The cap includes an occlusion body and an elongated anchor. In some embodiments the anchor includes a lip which is engageable to openings in the body wall. Tools are also provided for manipulating caps for interbody fusion devices. In one embodiment the tool includes a pair of prongs each having facing engagement surfaces for engaging the fusion device, and a shaft slidably disposed between the prongs. The shaft has a first end defining a cap-engaging tip for engaging a tool hole in the cap. In one embodiment the cap engaging tip defines threads. In another embodiment the prongs include a pair of releasing members on each of the facing engagement surfaces. The releasing members have a height and a width for being insertable into apertures in a body wall in the fusion device to disengage the elongate anchors from the apertures.
Owner:WARSAW ORTHOPEDIC INC
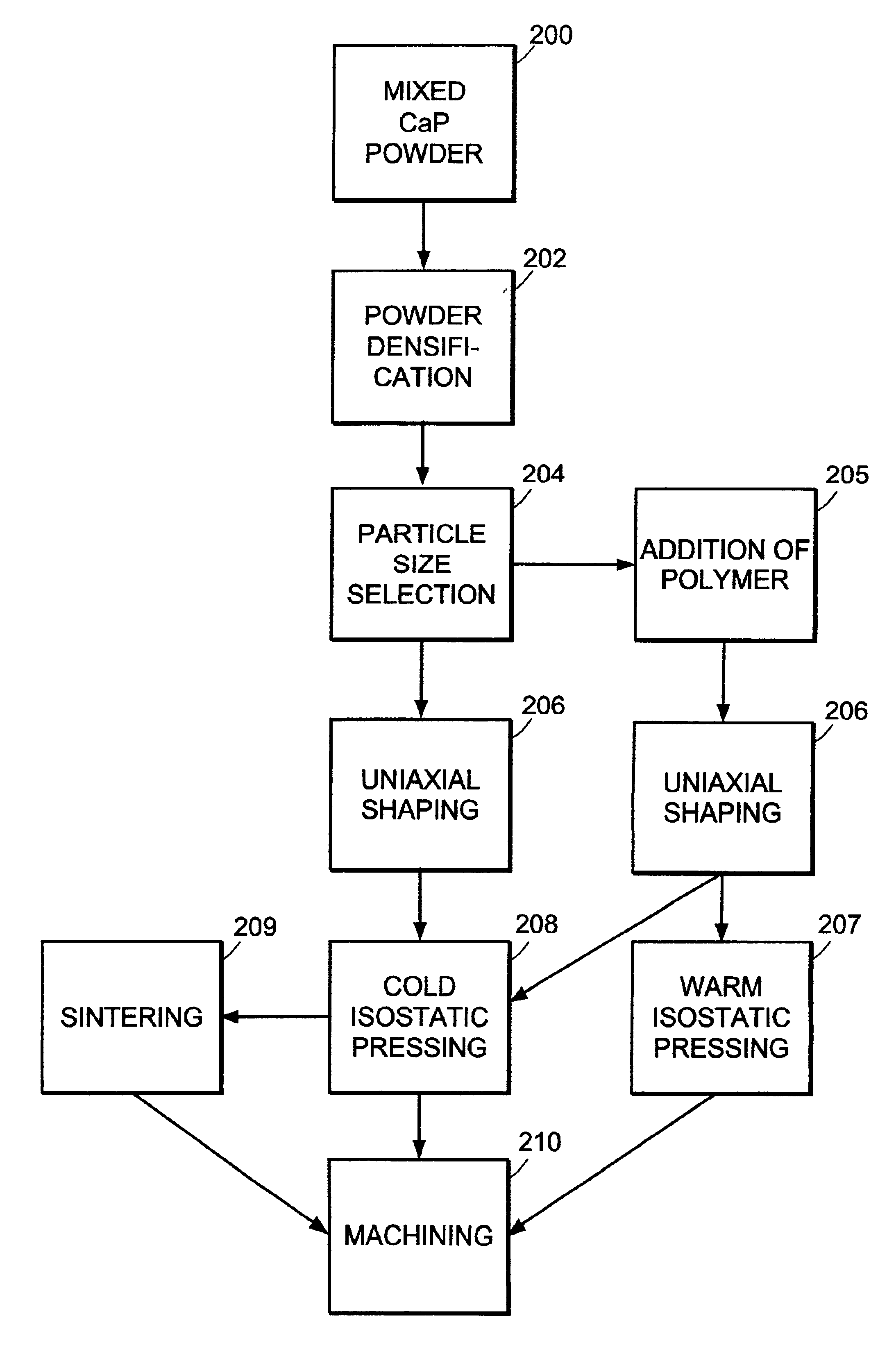
Machinable preformed calcium phosphate bone substitute material implants
InactiveUS6840961B2Process stabilitySlows natural bone growthBiocideSurgical adhesivesCalcium biphosphateNatural bone
The present invention provides machinable calcium phosphate bone substitute material implants having mechanical properties comparable to those of natural bone. The implants include intimately mixed solid precursor materials that react under physiological conditions to form poorly-crystalline hydroxyapatite and eventually are remodeled into bone in vivo. The implants can include a biocompatible polymer to increase density and strength and control resorbability.
Owner:ETEX
Spinal fusion procedure using an injectable bone substitute
InactiveUS20050101964A1Facilitate singleFacilitate multi level spinal fusionBone implantJoint implantsCalcium biphosphateInjectable bone
Methods for performing spinal fusions using an injectable calcium phosphate-based bone substitute are provided. The injectable bone substitute is injected into the anterior portion of an interbody space and allowed to solidify in vivo. The injectable bone substitute has a minimum compression strength of 10 MPa after setting for about 30 minutes and preferably solidifies to a compression strength of 25 MPa within 24 hours of injection. Optionally, the posterior portion of the interbody space is fixed using a metallic implant selected from rods and pedicle screws or plates and pedicle screws by attachment thereof to adjacent vertebrae.
Owner:CALCITEC
Spinal fusion implants and tools for insertion and revision
InactiveUS20050192669A1Avoid expulsionInternal osteosythesisBone implantSpinal columnPorous tantalum
An interbody fusion device in one embodiment includes a tapered body defining a hollow interior or chamber for receiving bone graft or bone substitute material. The body defines exterior threads which are interrupted over portions of the outer surface of the device. The fusion device includes truncated side walls so that on end view the body takes on a cylindrical form. In another embodiment, the tapered body is solid and formed of a porous biocompatible material having sufficient structural integrity to maintain the intradiscal space and normal curvature. The material is preferably a porous tantalum composite having fully interconnected pores to facilitate complete bone tissue ingrowth into the implant. In further embodiments, the fusion devices are provided with osteogenic material to facilitate bone ingrowth. A cap is also provided to block the opening of hollow fusion devices. The cap includes an occlusion body and an elongated anchor. In some embodiments the anchor includes a lip which is engageable to openings in the body wall. Tools are also provided for manipulating caps for interbody fusion devices. In one embodiment the tool includes a pair of prongs each having facing engagement surfaces for engaging the fusion device, and a shaft slidably disposed between the prongs. The shaft has a first end defining a cap-engaging tip for engaging a tool hole in the cap. In one embodiment the cap engaging tip defines threads. In another embodiment the prongs include a pair of releasing members on each of the facing engagement surfaces. The releasing members have a height and a width for being insertable into apertures in a body wall in the fusion device to disengage the elongate anchors from the apertures.
Owner:ZDEBLICK THOMAS +4
Bone substitute composition
InactiveUS20070041906A1High mechanical strengthFormation be controlledPowder deliveryBiocideCalcium biphosphateInjectable bone
An injectable bone mineral substitute material composition with the capability of being hardened in a body fluid in vivo, which comprises at least one calcium phosphate component and at least one calcium sulfate component as a dry mixture mixed with an aqueous liquid, and at least one accelerator, the at least one calcium sulfate component being particulate hardened calcium sulfate, which has a specified particle size that is in order to confer injectability to the composition. The invention also concerns the bone mineral substitute material produced from the composition as well as methods and uses thereof.
Owner:BONE SUPPORT
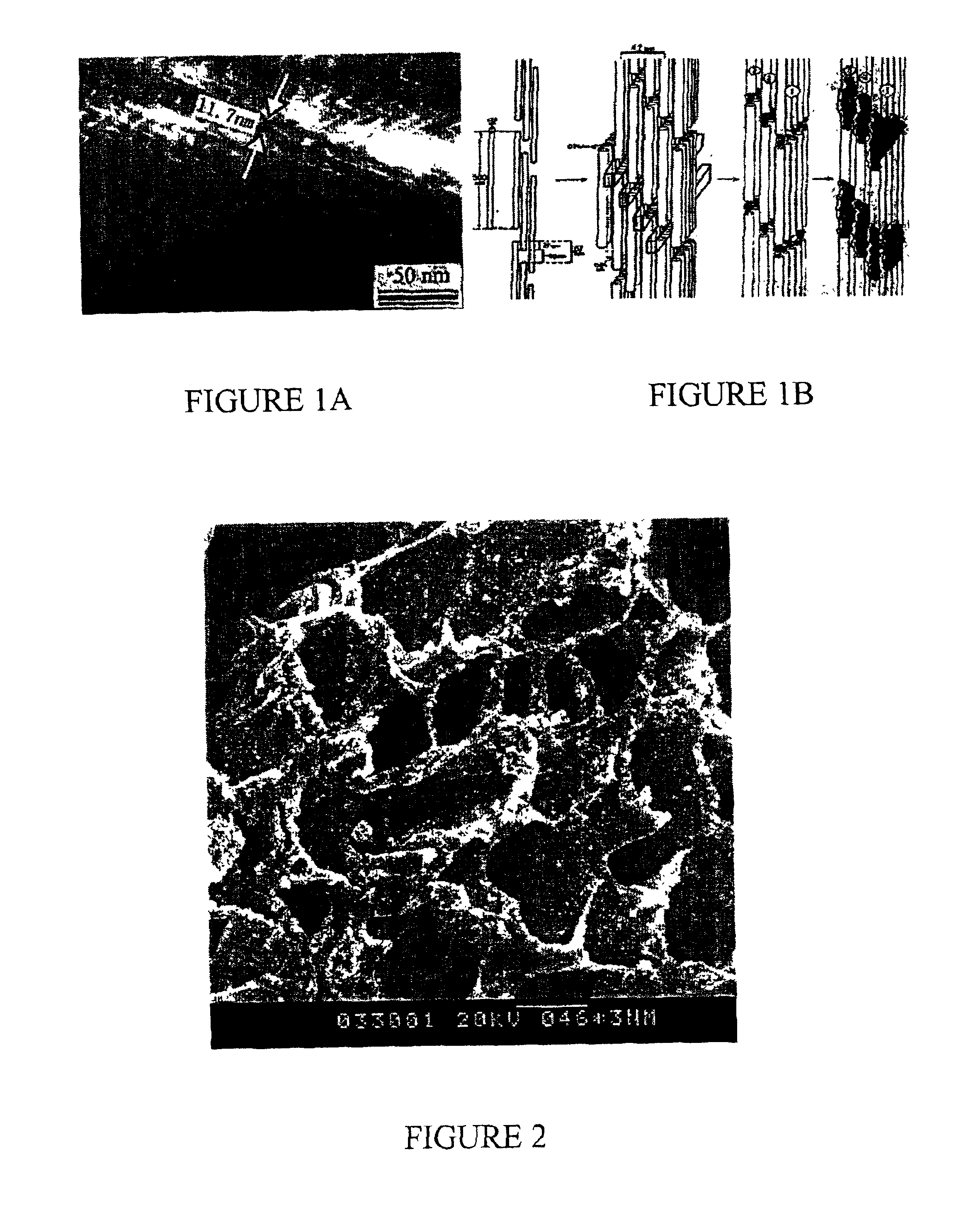
Nano-calcium phosphates/collagen based bone substitute materials
InactiveUS6887488B2Improve performanceExcellent bioactivityBiocideBone implantCalcium biphosphateNatural bone
The present invention relates to a nano-calcium phosphates / collagen composite that mimics the natural bone, both in composition and microstructure, as well as porous bone substitute and tissue engineering scaffolds made by a complex of said composite and poly(lactic acid)(PLA) or poly(lactic acid-co-glycolic acid)(PLGA). The invention also relates to the use of said scaffold in treating bone defect and bone fracture.
Owner:TSINGHUA UNIV
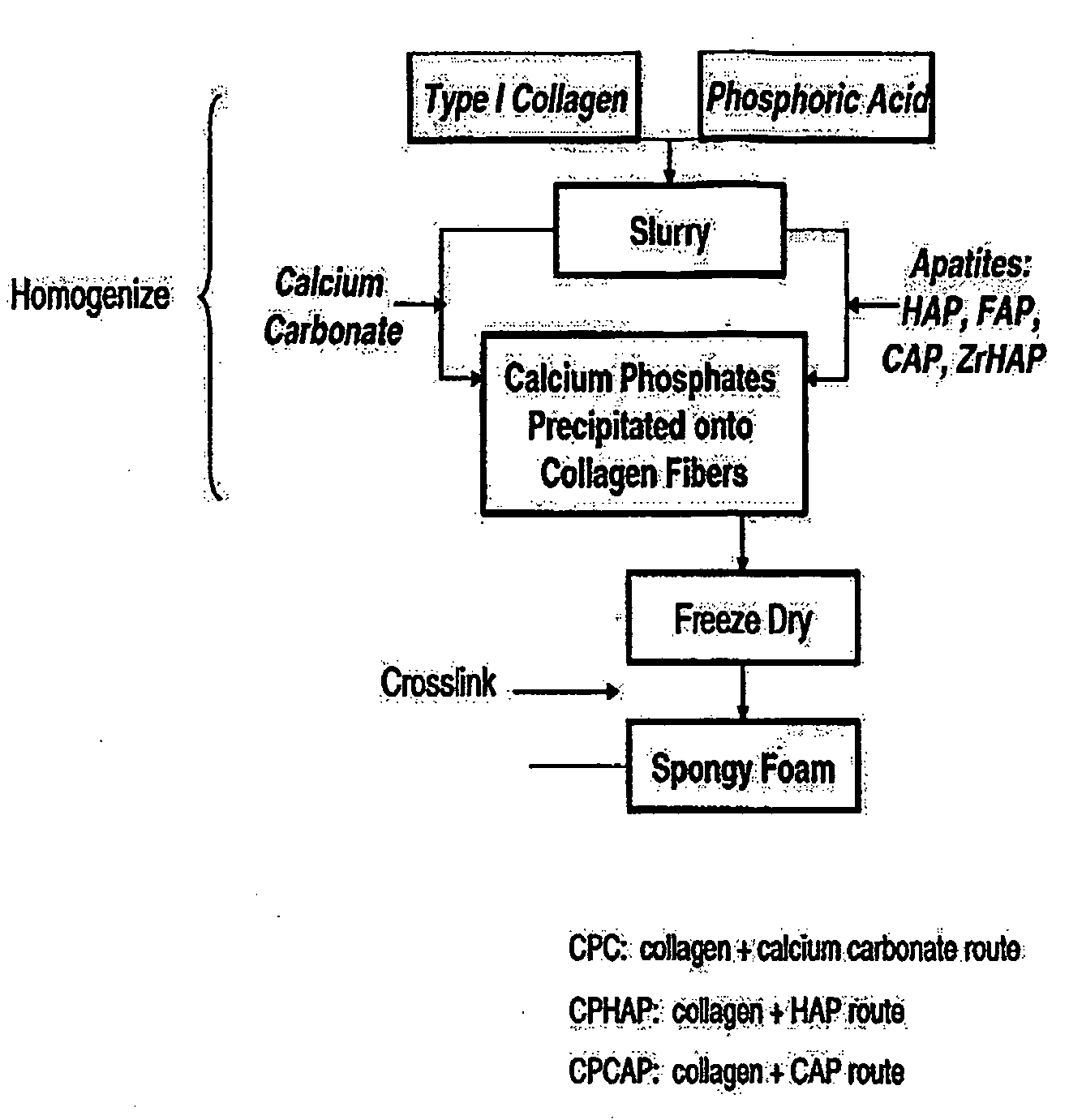
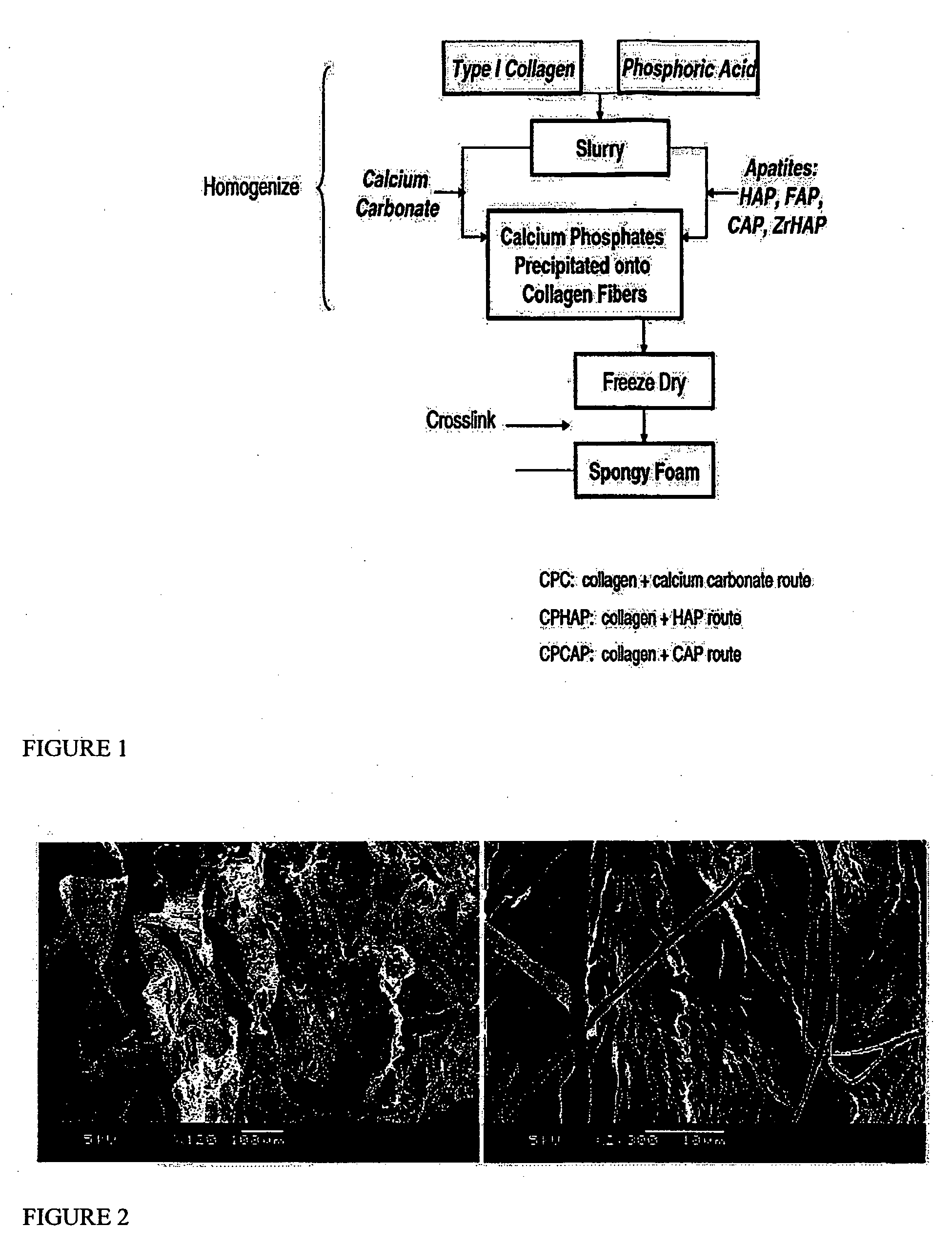
Porous biomaterial-filler composite and method for making the same
A porous biomaterial-filler composite comprising a biomaterial, such as collagen, interspersed with a calcium phosphate-type filler material. The porosity of the composite is similar to that of natural bone and can feature a pore size ranging from a few nanometres to greater than 100 microns. Scaffolds prepared from the biomaterial-filler composite are suitable for resorbable bone substitute materials.
Owner:YING JACKIE Y +4
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous β-tricalcium phosphate (β-TCP).
Owner:CURASAN
Synthetic cortical bone for ballistic testing
A bone substitute for use in impact testing of a structure simulating the human body which includes a member fabricated from epoxy resin and having a lengthwise dimension, and a fiberglass sheath embedded in an outer circumferential portion of the member, the sheath having glass fibers oriented along the length of the member.
Owner:ROBERTS JACK C +3
Poly(ester urea) polymers and methods of use
InactiveUS20070128250A1Easy to produceImprove mechanical propertiesOrganic active ingredientsSurgeryAntibiotic YHard tissue
The invention provides high molecular weight, crystalline or semi crystalline biodegradable and biocompatible poly(ester urea) (PEU) polymers useful for making vascular stents and hard tissue replacement implants, such as bone substitutes. The PEU polymers are based on α amino acids and are made by a polycondensation reaction. PEU polymer compositions can contain a therapeutic diol incorporated into the polymer backbone that is released from such an implant in situ. Bioactive agents, such as analgesics, antibiotics, and the like, can also be covalently attached to certain PEU polymers for release into tissue surrounding an implant during biodegradation of the polymer.
Owner:MEDIVAS LLC
Crosslinkable polymeric materials and their applications
InactiveUS20060148923A1Rapid lossSusceptible to water penetrationDental implantsImpression capsMedicinePrepolymer
The present invention relates to a novel composition for dental, orthopedic and drug delivery purpose. Specifically, it relates to composition comprising an admixture of a resorbable bone substitute and a crosslinkable prepolymer. It also relates to the composition formed by crosslinking the admixture.
Owner:A ENTERPRISES
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous beta-tricalcium phosphate (beta-TCP).
Owner:CURASAN
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous β-tricalcium phosphate (β-TCP).
Owner:CURASAN
Mixing and dispensing apparatus for bone void filler
InactiveUS20120071884A1High mechanical strengthHigh densityShaking/oscillating/vibrating mixersFlow mixersBone chamberBone cement
A method for preparing a de-aired hydraulic setting hardenable bone cement for filling a boney void or cavity by combining a powder bone substitute material and a aqueous liquid component, the method comprising the steps of: (a) supplying said powder bone substitute material in a first evacuable container; (b) withdrawing air from the first container to form a de-aired powder bone substitute material; and, then (c) mixing the de-aired powder bone substitute material and the aqueous liquid component together.
Owner:BIOCOMPOSITES
Load sharing interbody fusion device
InactiveUS20090157187A1Controlling reduction and heightReduce the overall heightBone implantSpinal implantsIntervertebral diskIntervertebral fusion
The present invention is a load sharing intervertebral fusion device that allows for reconstruction of the proper disc space between two vertebral bodies while allowing bone material packed within the fusion device to share loading and stress for enhanced healing. The device includes two sections, an upper and lower section, separated by a bioresorbable spacer. The upper section slides relative to the lower section with the bioresorbable material being placed in load therebetween. The upper section and lower sections are effectively held apart, creating an initial fixed spacer for implantation of the intervertebral disc space. Openings in the implant construct allow for bone graft and bone substitutes to be placed within the implant to allow for fusion through the implant construct. After implantation and over a period of time based on bone resorbtion and remodeling, the spacer resorbs, thereby maintaining load on the bone graft material.
Owner:INTELLIGENT IMPLANT SYST
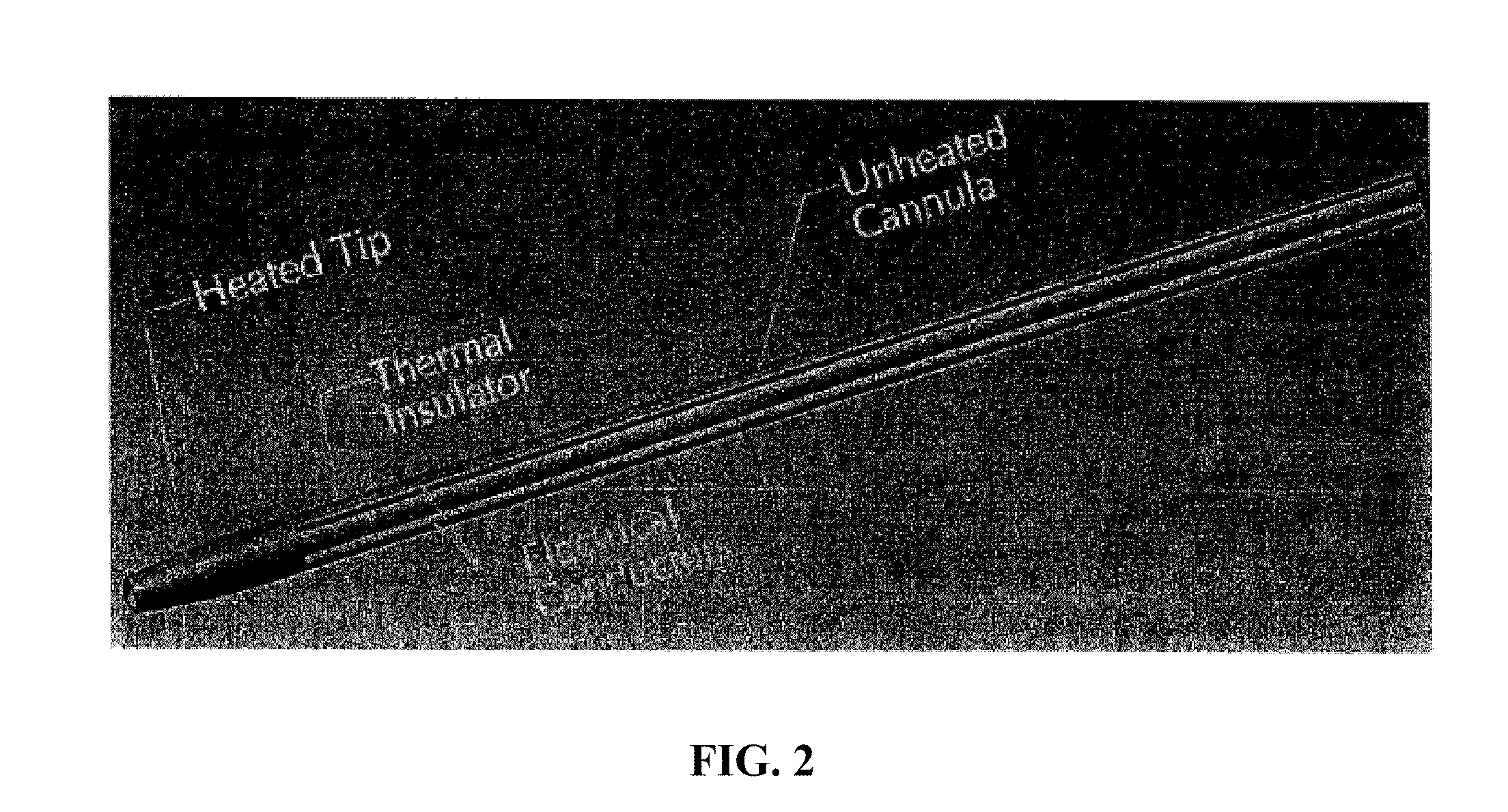
Heated tip implant delivery system
InactiveUS20100211058A1Short time spanShort timeSurgical instruments for heatingProsthesisHot meltHot-melt adhesive
Bone substitute materials have been developed that become flowable upon heating. In order to use these new materials in the special environment of an operating room, methods and devices have been developed to conveniently and sterilely heat samples of the material as the material is being implanted into a patient. Inventive heating devices include cannulas with a heated tip and devices similar to a hot melt glue gun. Therefore, the material is heated for only a short time preventing the degradation of biological components of the material.
Owner:WARSAW ORTHOPEDIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com