Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
437 results about "Implant surface" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
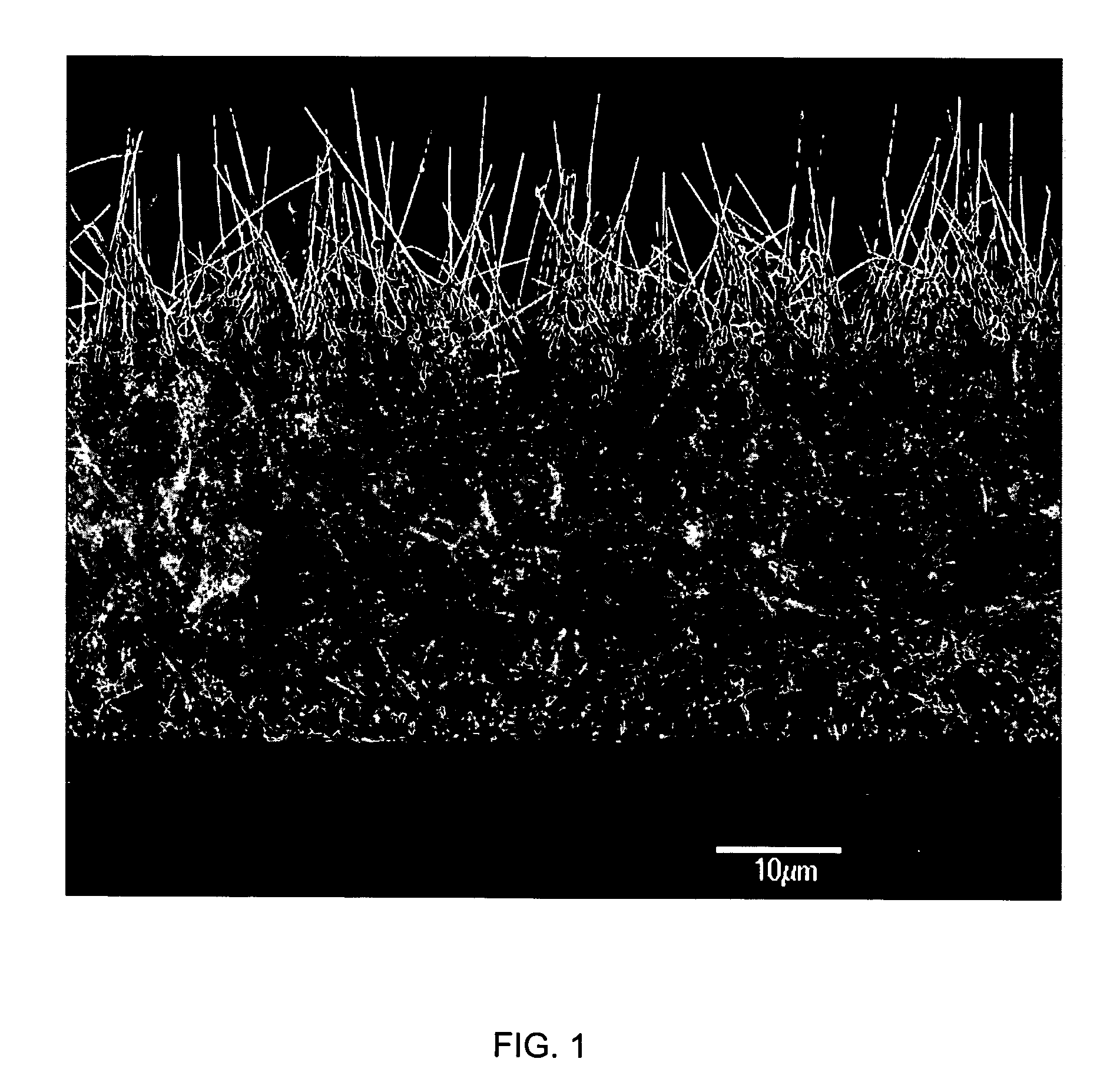
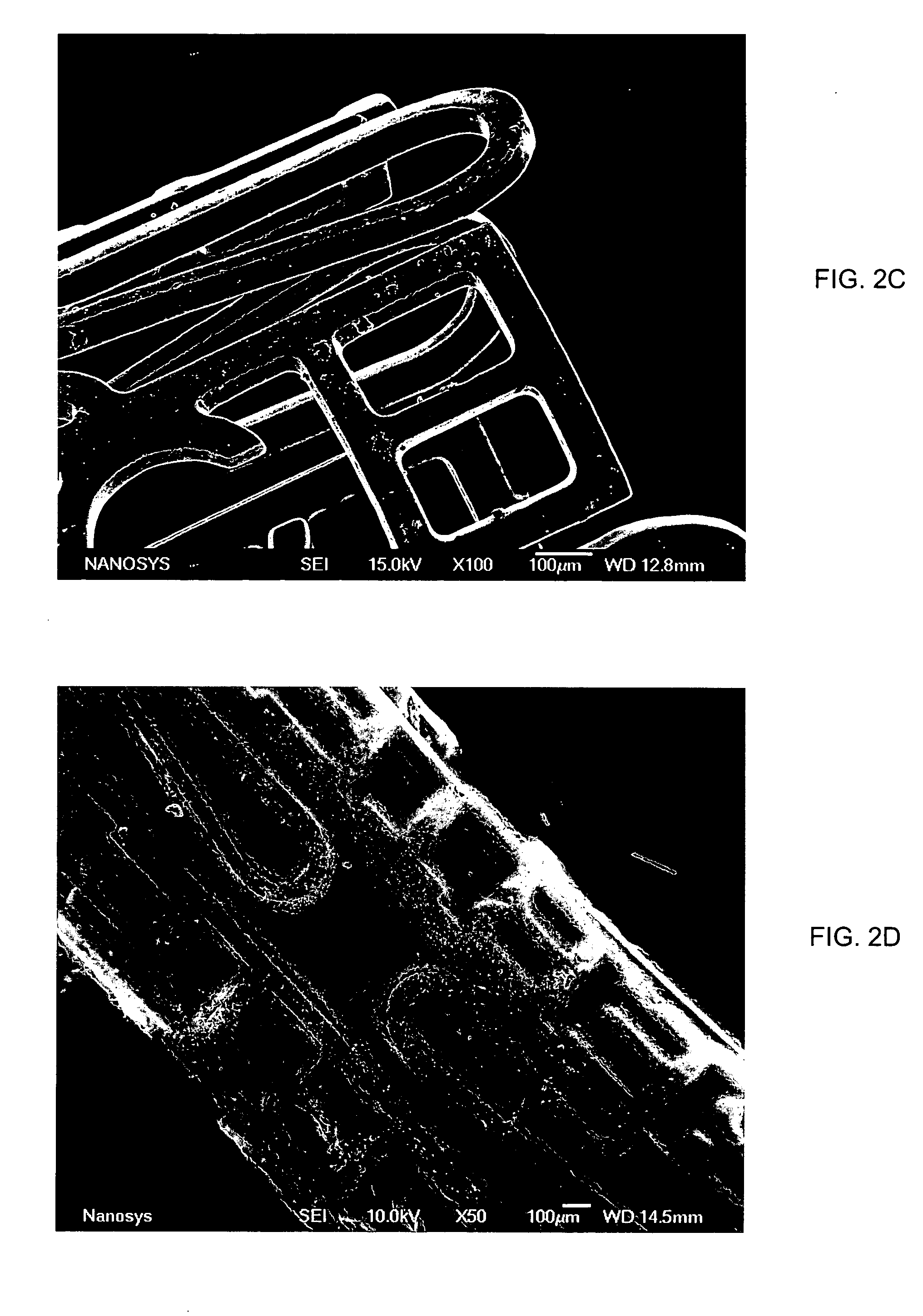
Medical device applications of nanostructured surfaces
InactiveUS20060204738A1Improve adhesionIncrease frictionBiocideMaterial nanotechnologyOsteoblastNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:GLO TECH LLC
Devices and methods for treating facet joints, uncovertebral joints, costovertebral joints and other joints
InactiveUS20070083266A1Improve functionalityPromote resultsLigamentsJoint implantsArticular surfacesArticular surface
The present invention describes methods, devices and instruments for resurfacing or replacing facet joints, uncovertebral joints and costovertebral joints. The joints can be prepared by smoothing the articular surface on one side, by distracting the joint and by implant insertion. Implants can be stabilized against a first articular surface by creating a high level of conformance with said first articular surface, while smoothing the second articular surface with a surgical instrument with a smooth mating implant surface.
Owner:VERTEGEN
Medical device applications of nanostructured surfaces
InactiveUS20050221072A1Fine surfacePrevent/reduce bio-foulingNanomedicinePharmaceutical delivery mechanismMedicineOsteoblast
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:NANOSYS INC
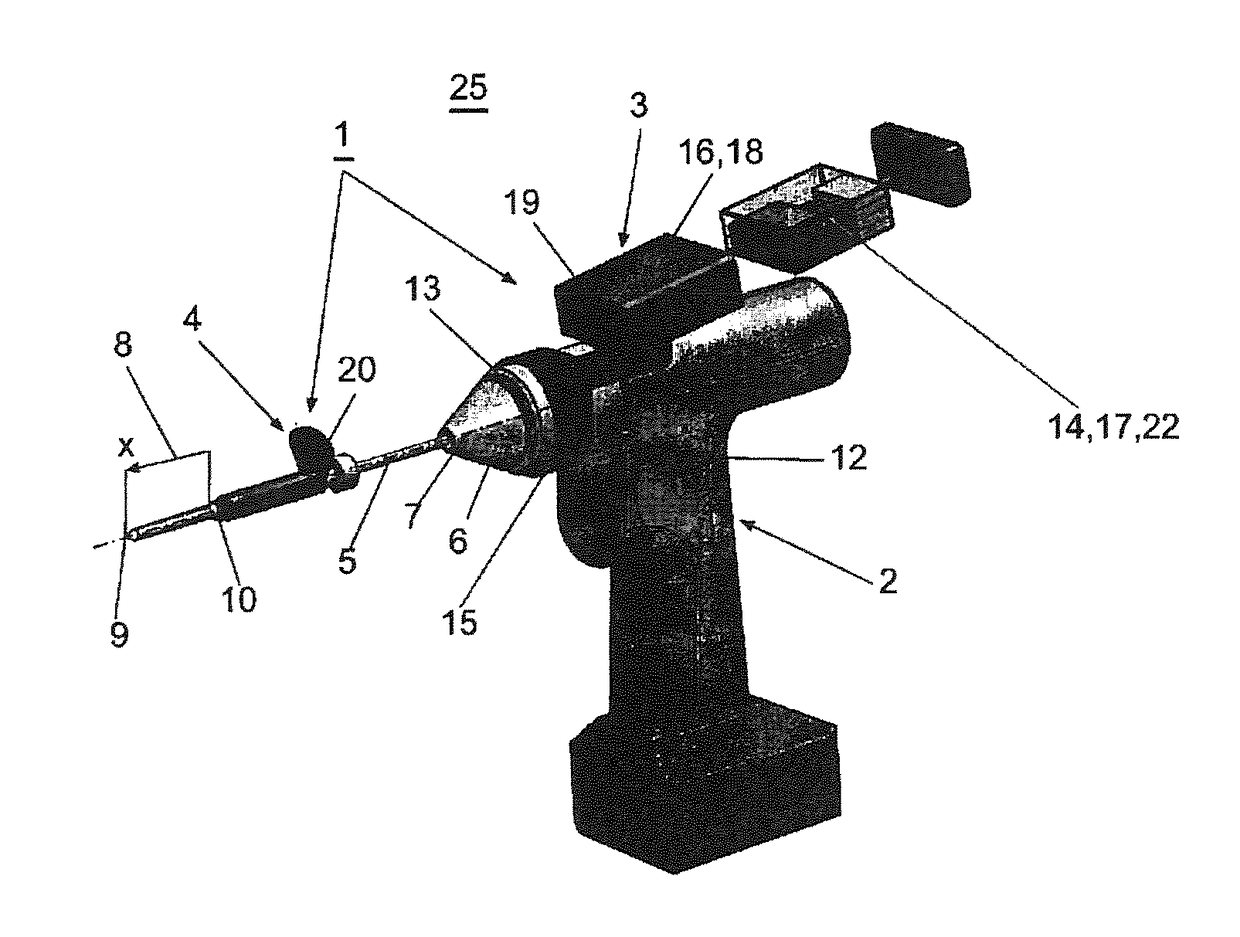
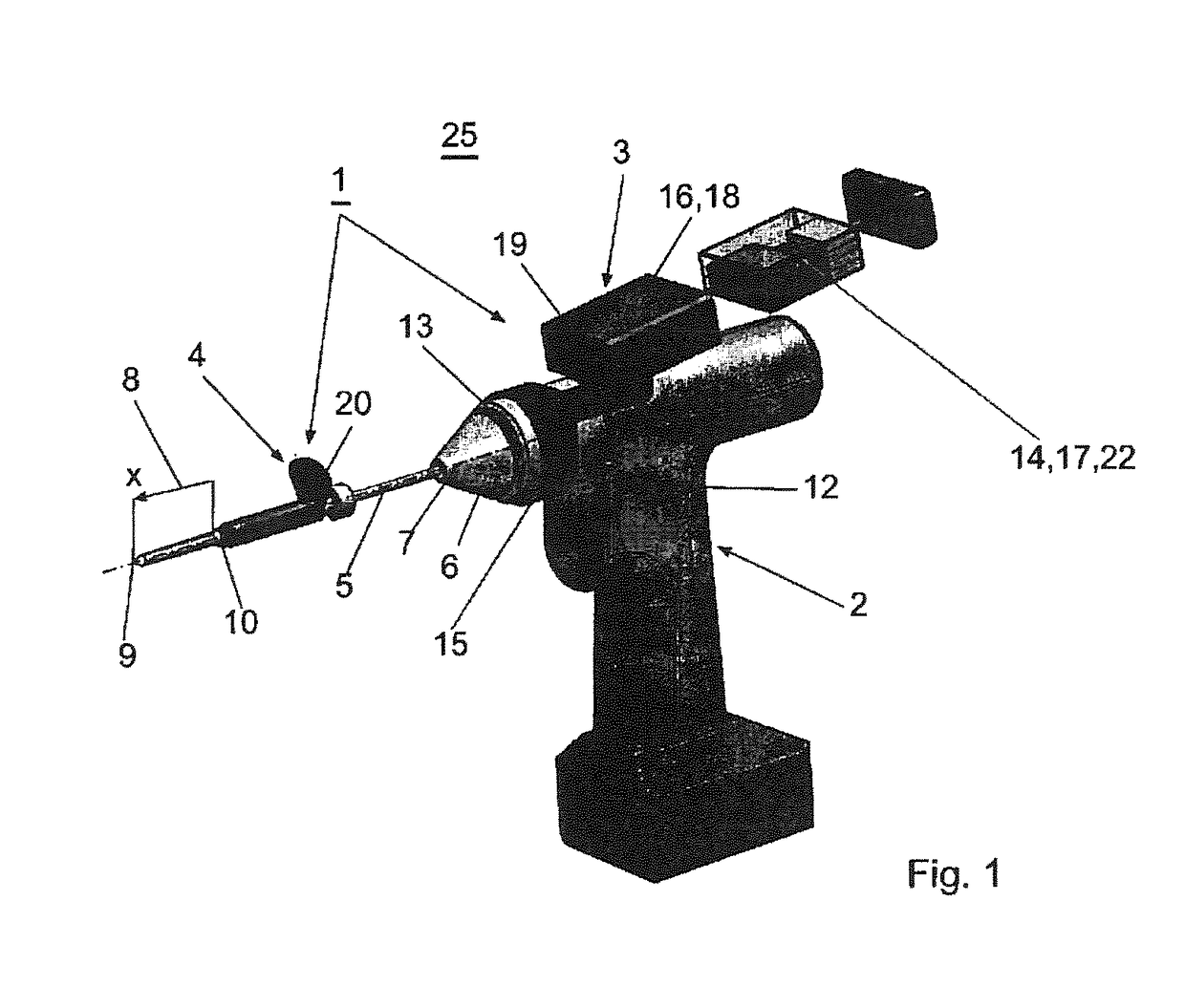
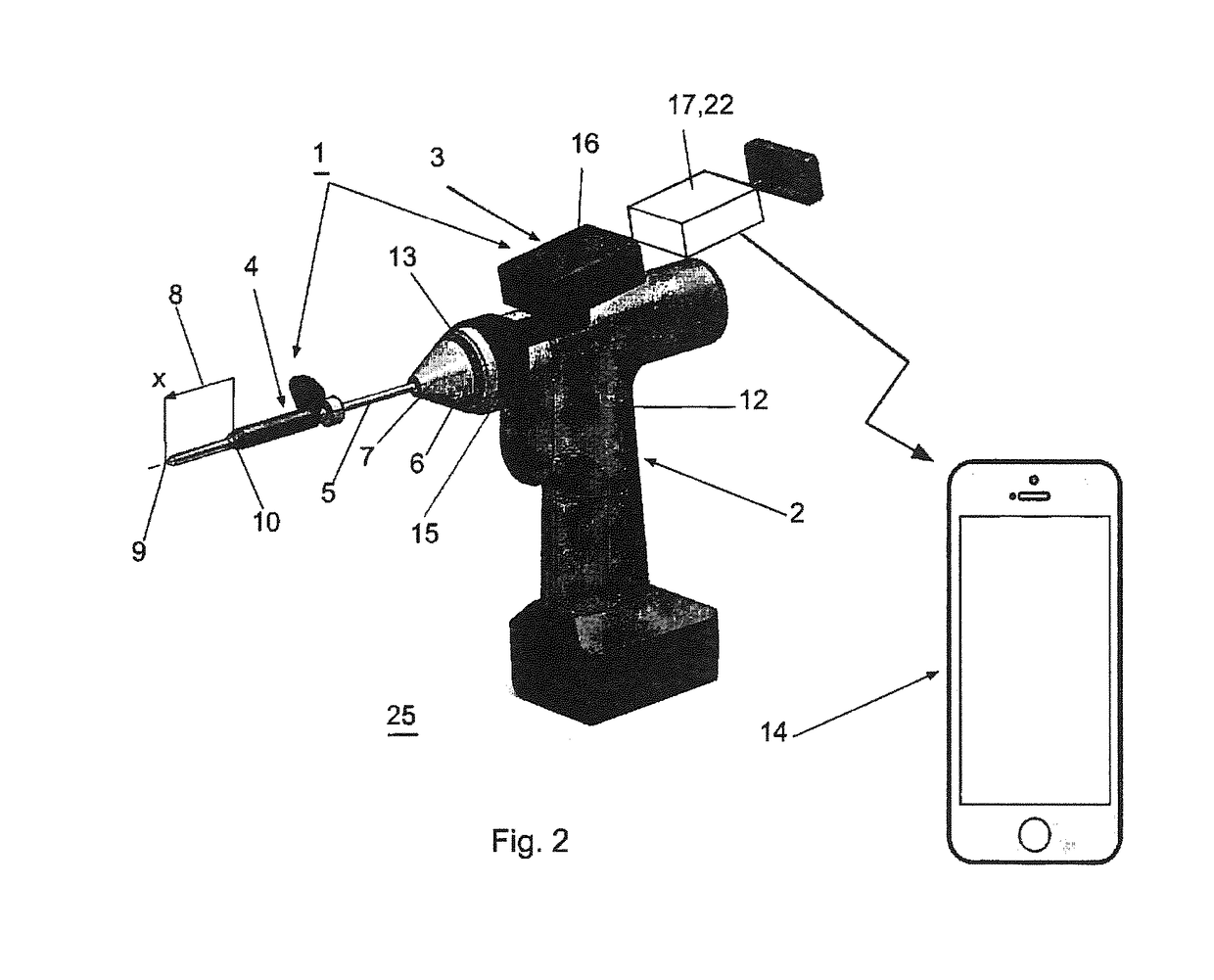
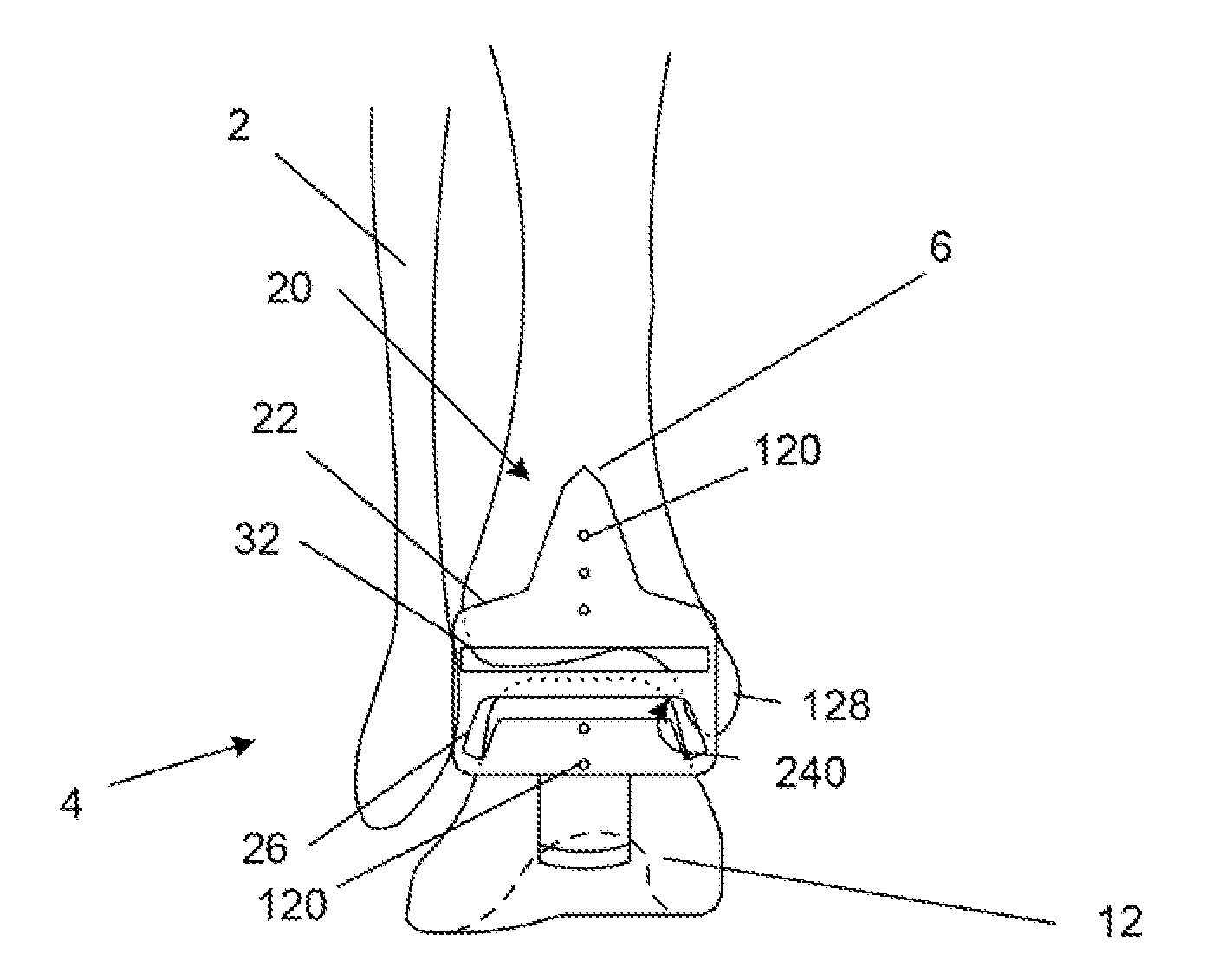
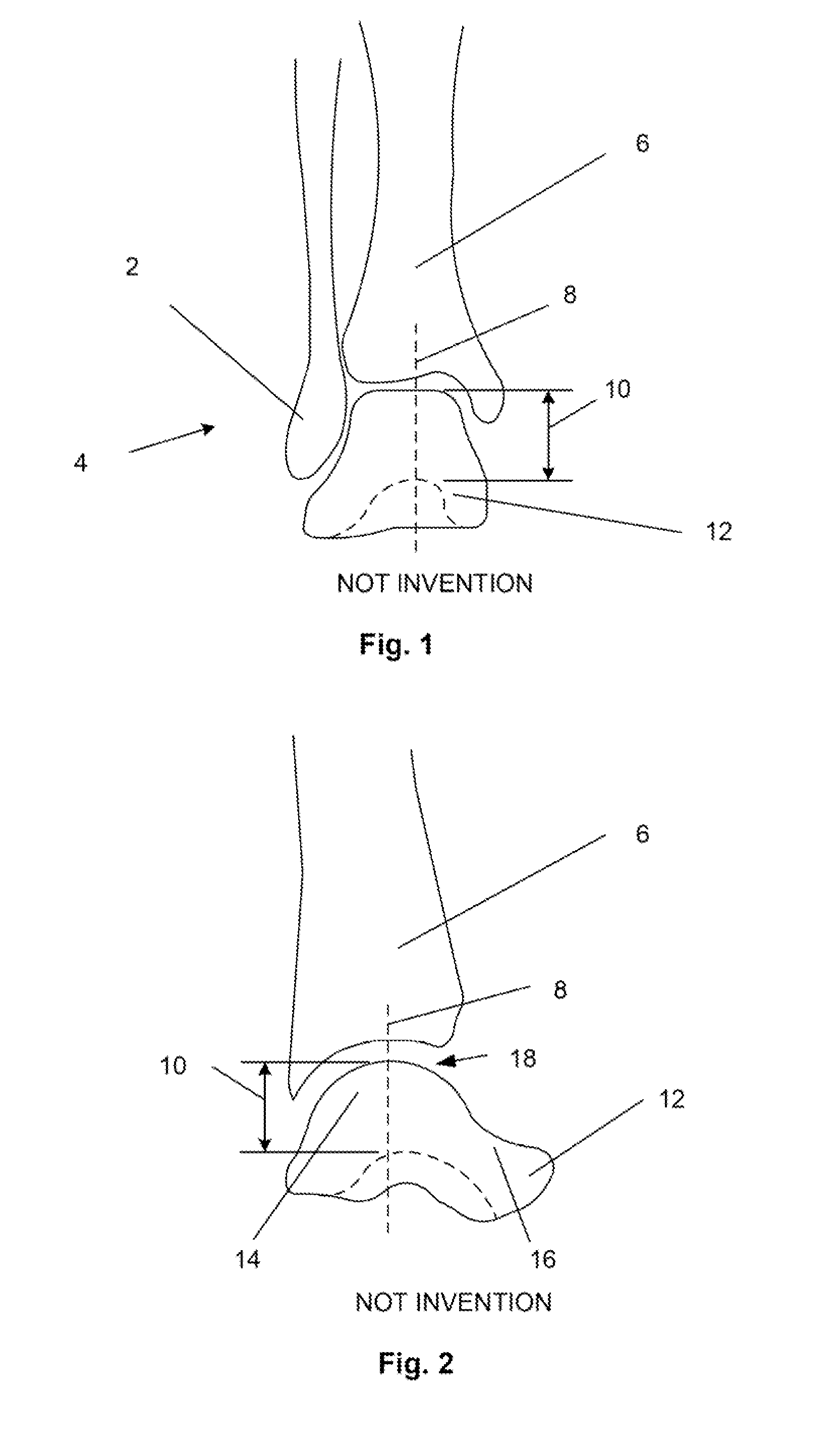
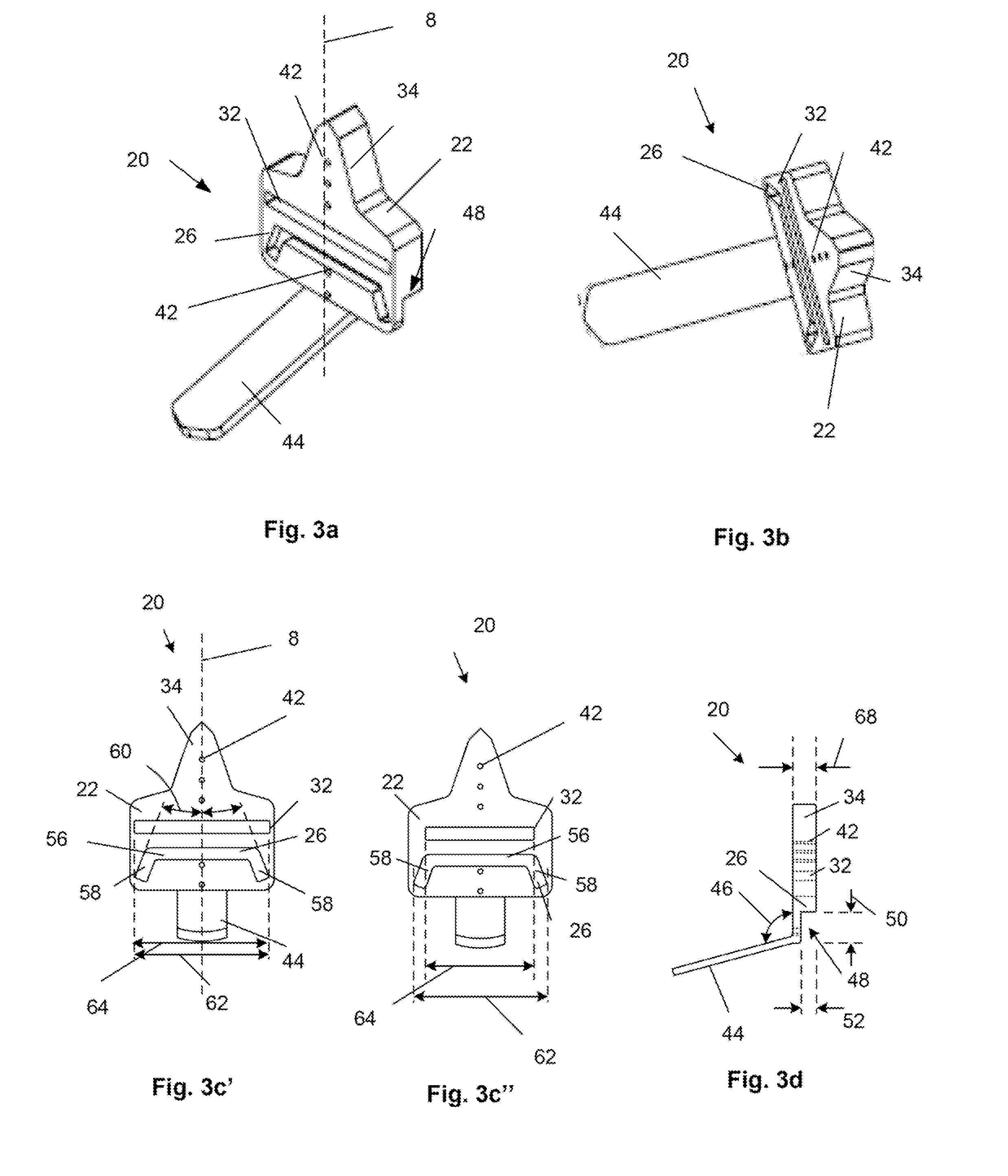
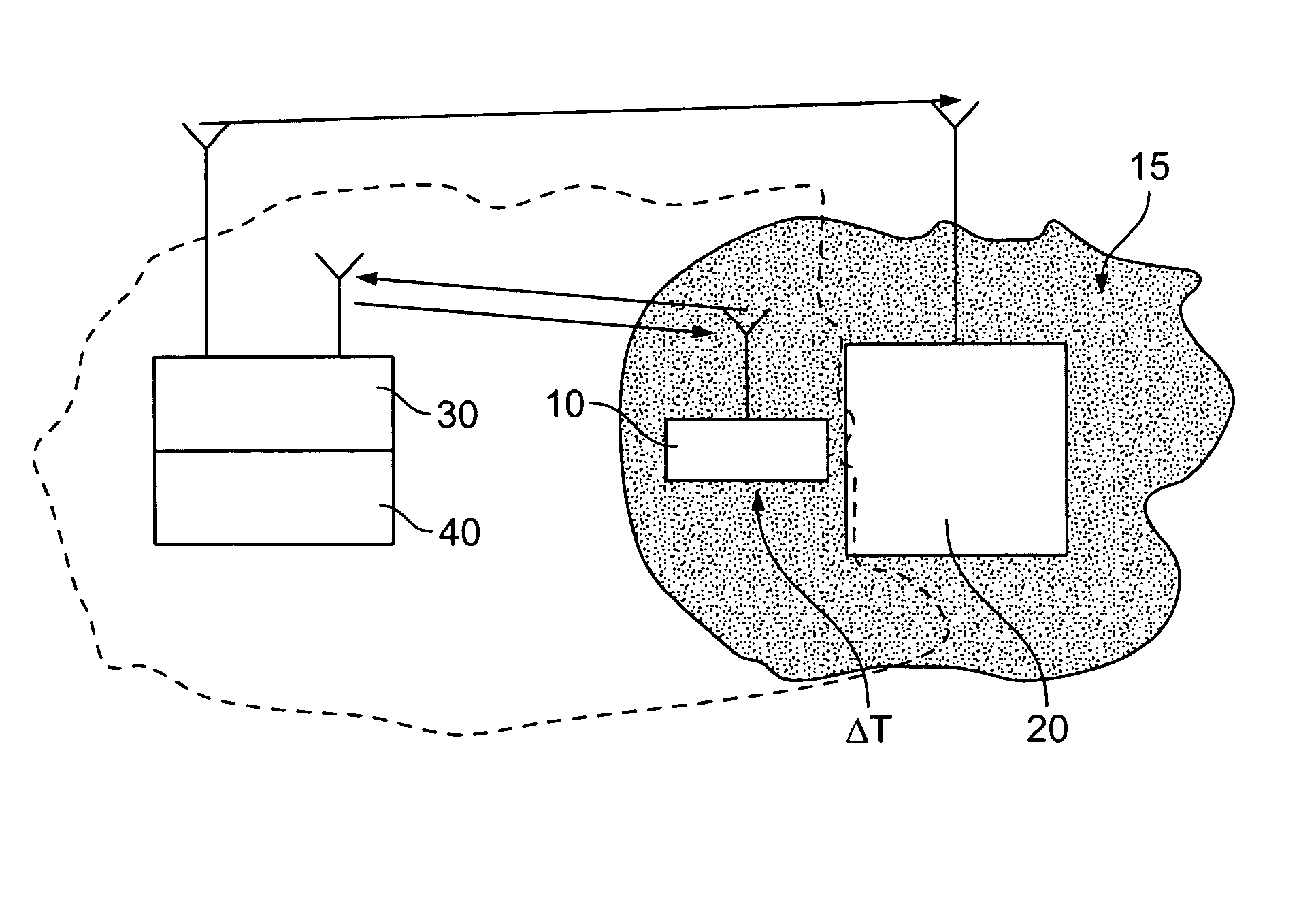
Surgical power drill including a measuring unit suitable for bone screw length determination
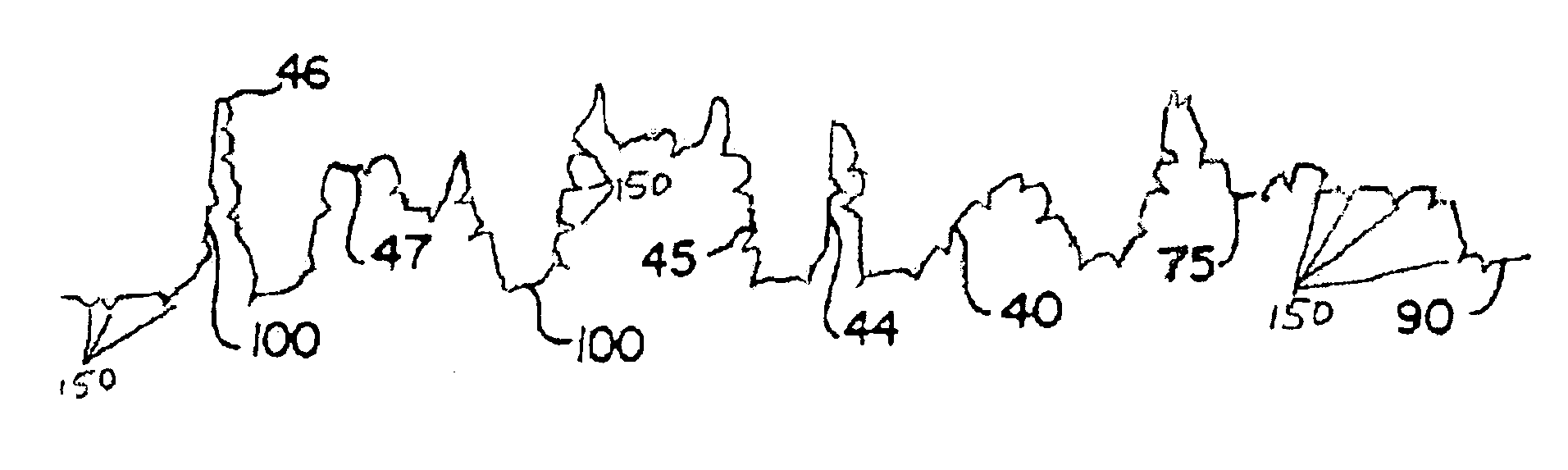
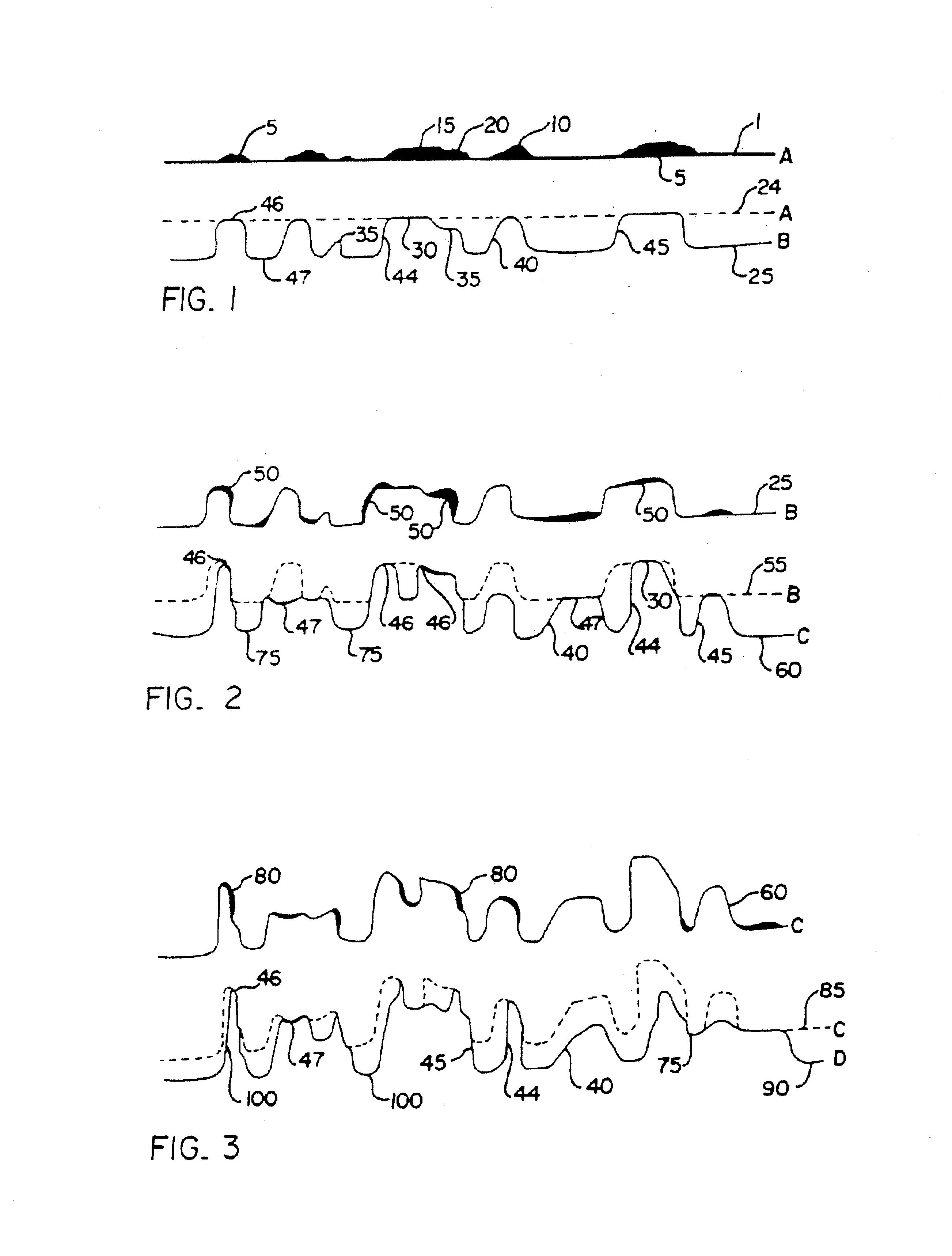
A device (25) for drilling holes in bone and configured to determine bone screw length, the device (25) including a surgical power drill (2) comprising: a) a housing (12) and; b) a measuring device (1) releasably attached or fixed to the housing (12), wherein the measuring device (1) is configured to measure the distance (x) covered by the housing (12) in the direction of the longitudinal axis (7) and relative to a surface of an implant (26) or a bone during a drilling process, wherein the measuring device (1) comprises a processing unit (14) to record the distance (x) covered with respect to time; the processing unit (14) comprises one or more differentiators to determine at least the first and second derivatives of the distance (x) covered with respect to time; and the processing unit (14) further comprises a peak detector to analyze one or more peaks occurring in the graph of the highest derivative with respect to time, and wherein the measuring device (1) comprises a laser device or an ultrasound position sensor for displacement assessment.
Owner:SYNTHES GMBH
Methods and devices for deploying biological implants
InactiveUS20100318088A1Reduce decreasePrevent yaw and twist and rotationWrist jointsAnkle jointsPlastic surgeryAnkle
Methods and devices for deploying biological implants are disclosed. The biological implants can include orthopedic, multi-component ankle implants. The target site can be prepared by fixing a rigid, alignable guide or jig with saw holes to the bone(s). Saws configured to fit through the saw holes can then be inserted through the saw holes to cut the bone(s). The jig can then be removed. Slidable implants can be positioned. Implants needing to be forced into place can be attached to elongated members to gently hold the implant and to provide a non-implant surface on which to apply the force.
Owner:TALUS MEDICAL
System and method for assisting with attachment of a stock implant to a patient tissue
ActiveUS20120109137A1Joint implantsComputer-aided planning/modellingProsthesisBiomedical engineering
A guide for assisting with attachment of a stock prosthetic implant to a patient tissue includes a lower guide surface configured to contact an upper implant surface of the stock prosthetic implant when a lower implant surface of the stock prosthetic implant contacts the patient tissue. An upper guide surface is accessible to a user when the lower guide surface is in contact with the upper implant surface. At least one guiding aperture extends through the guide body between the upper and lower guide surfaces at a predetermined aperture location with respect to the guide body and defines a predetermined target trajectory through the guide body. At least one of the target trajectory and the aperture location of each guiding aperture is preselected responsive to preoperative imaging of the patient tissue. A method of assisting with attachment of a stock prosthetic implant to a patient tissue is also provided.
Owner:THE CLEVELAND CLINIC FOUND
Devices and Methods for Treatment of Facet and Other Joints
InactiveUS20090276045A1Increase spacingReduce pressureJoint implantsLigamentsArticular surfacesArticular surface
Embodiments of the present invention describe methods, devices and instruments for resurfacing or replacing facet joints, uncovertebral joints and costovertebral joints. The joints can be prepared by smoothing the articular surface on one side, by distracting the joint and by implant insertion. Implants can be stabilized against a first articular surface by creating a high level of conformance with said first articular surface, while smoothing the second articular surface with a surgical instrument with a smooth mating implant surface.
Owner:CONFORMIS
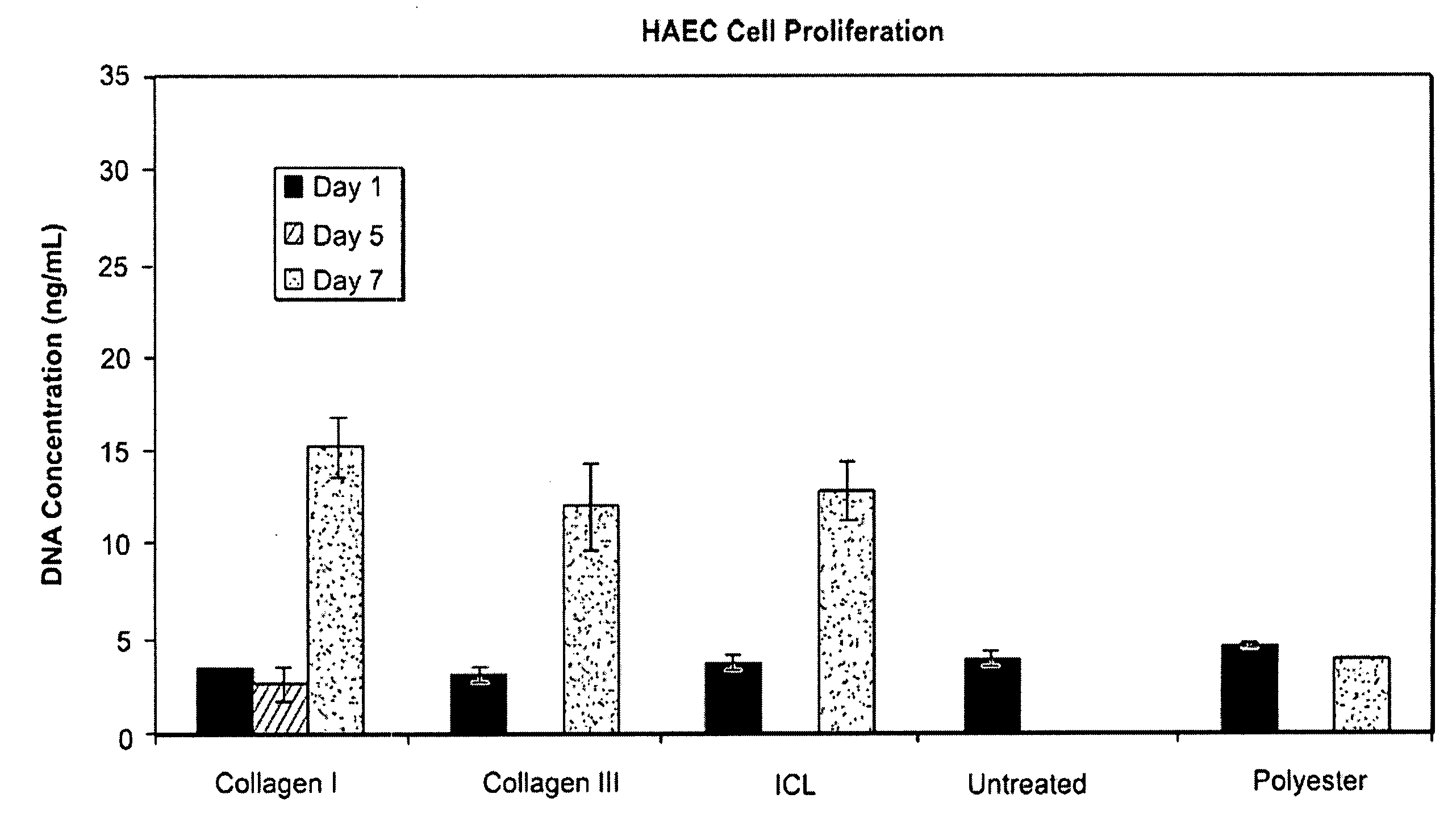
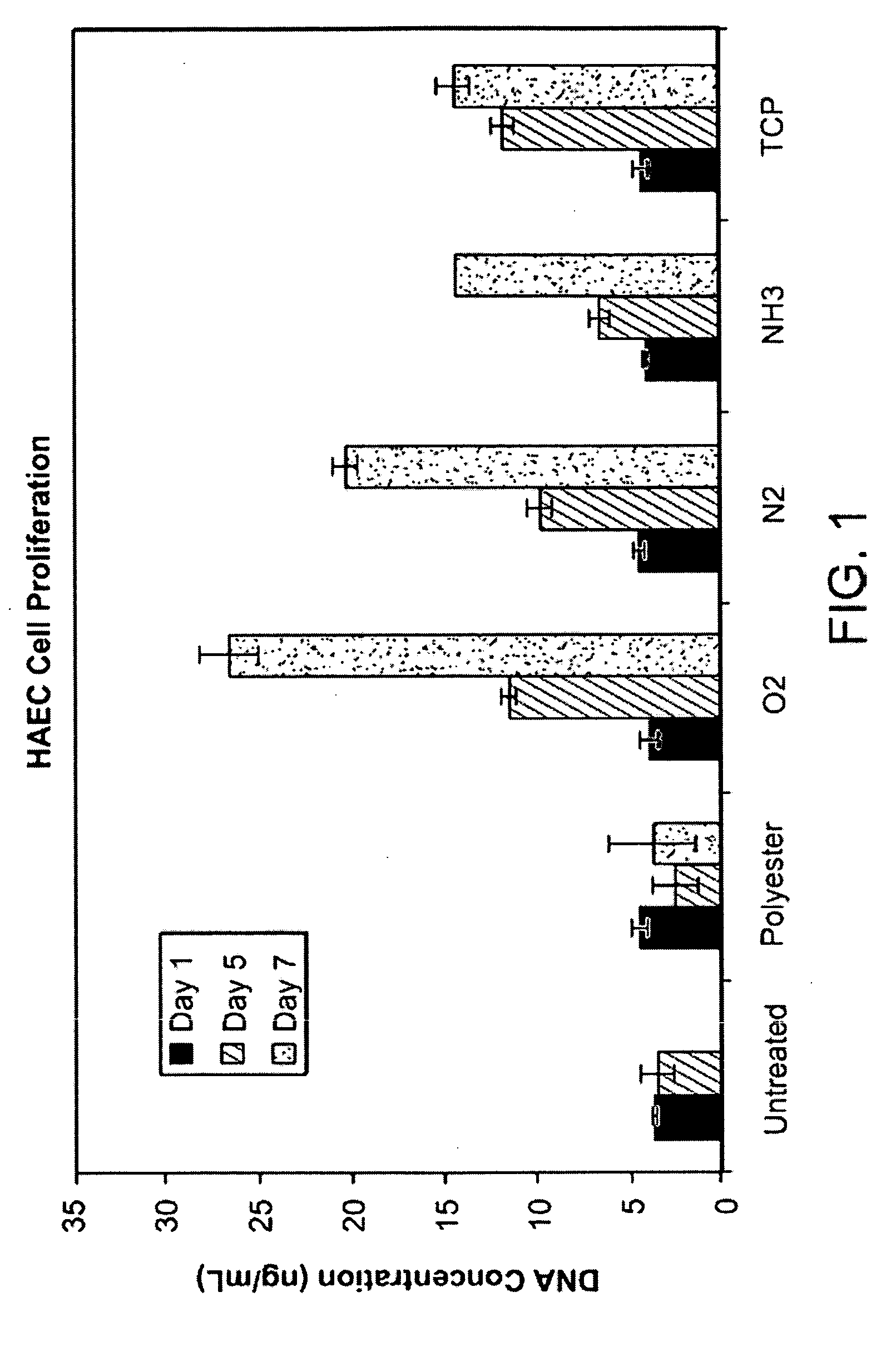
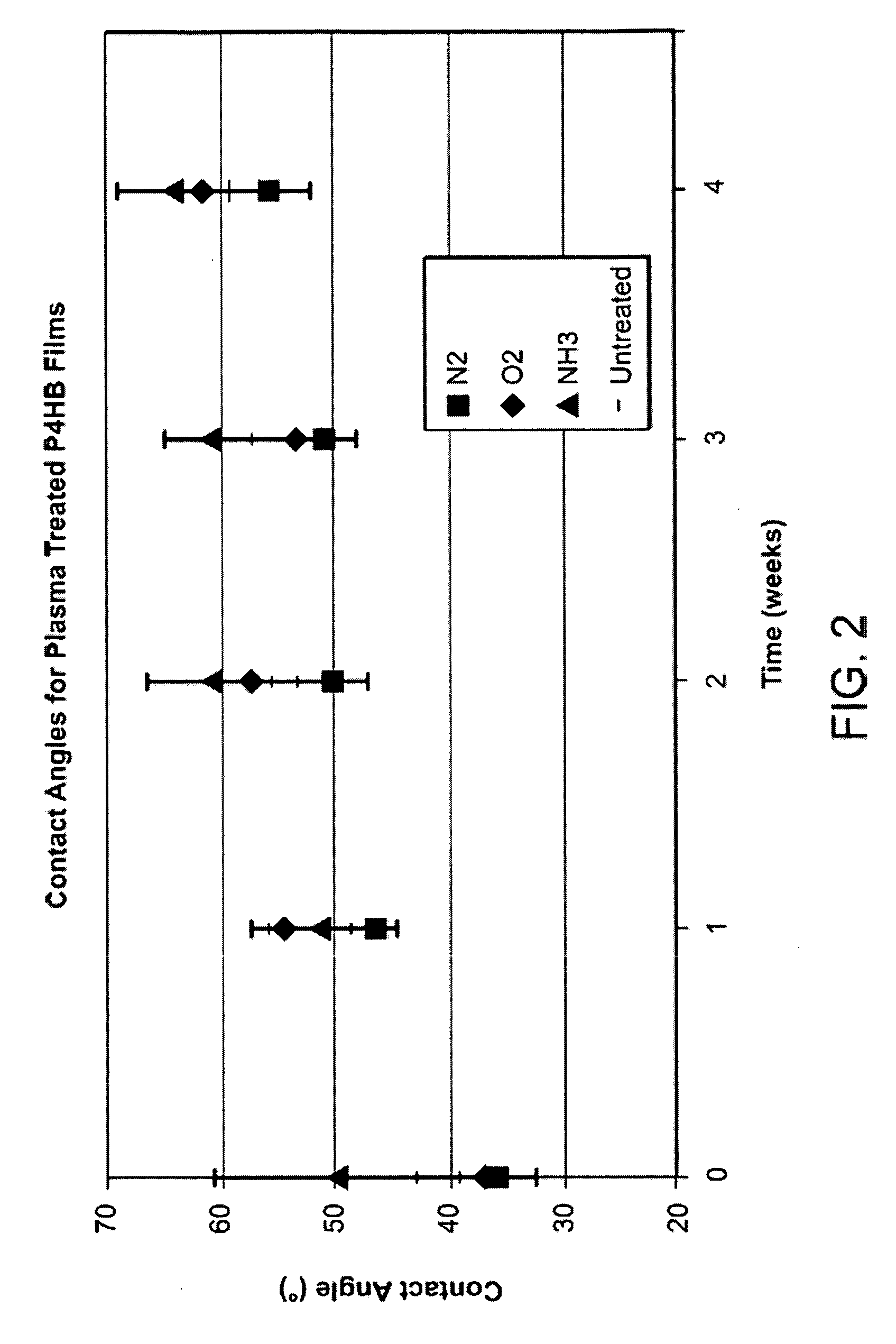
Stimulation of cell growth at implant surfaces
Owner:BOSTON SCI SCIMED INC
Functionally graded biocompatible coating and coated implant
ActiveUS20060210494A1Degree of reductionDental implantsCosmetic preparationsCalcium biphosphateCalcium phosphate coating
The present invention provides a biocompatible coating comprising calcium phosphate that is functionally graded across the thickness of the coating. The coating, which preferably includes hydroxyapatite, is particularly useful for coating implants, such as dental or orthopedic implants. The functionally graded coating is generally crystalline near the interface with the surface of the implant, with crystallinity and crystal diameter decreasing toward the outer layer of the coating. The invention further provides methods for preparing a coated implant comprising a functionally graded calcium phosphate coating thereon.
Owner:NORTH CAROLINA STATE UNIV
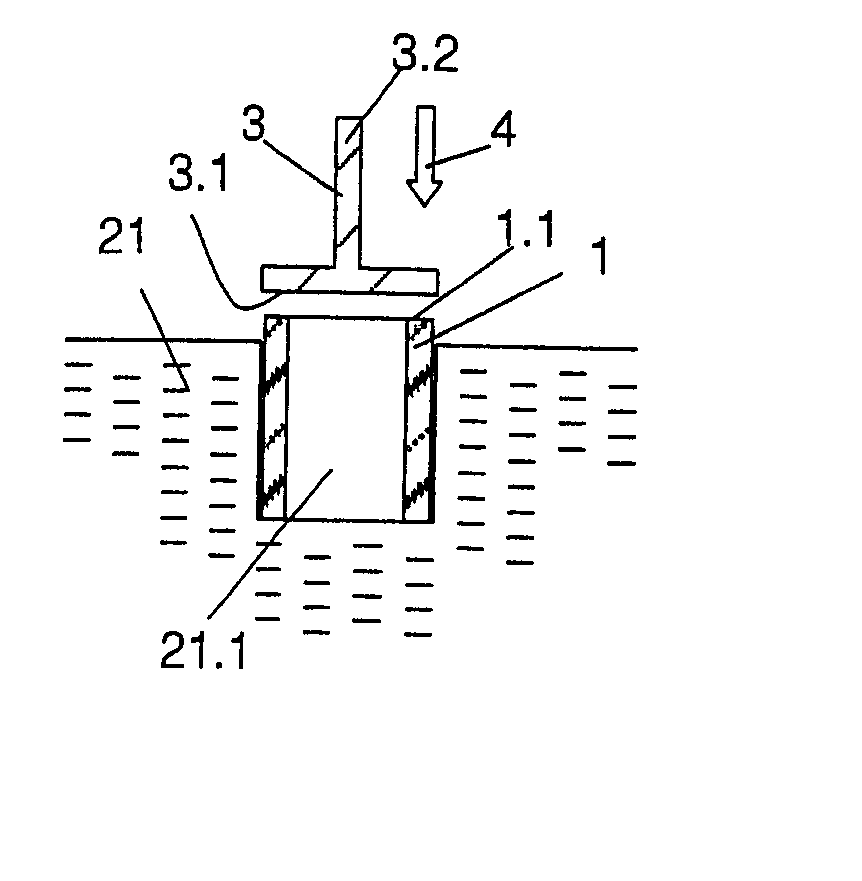
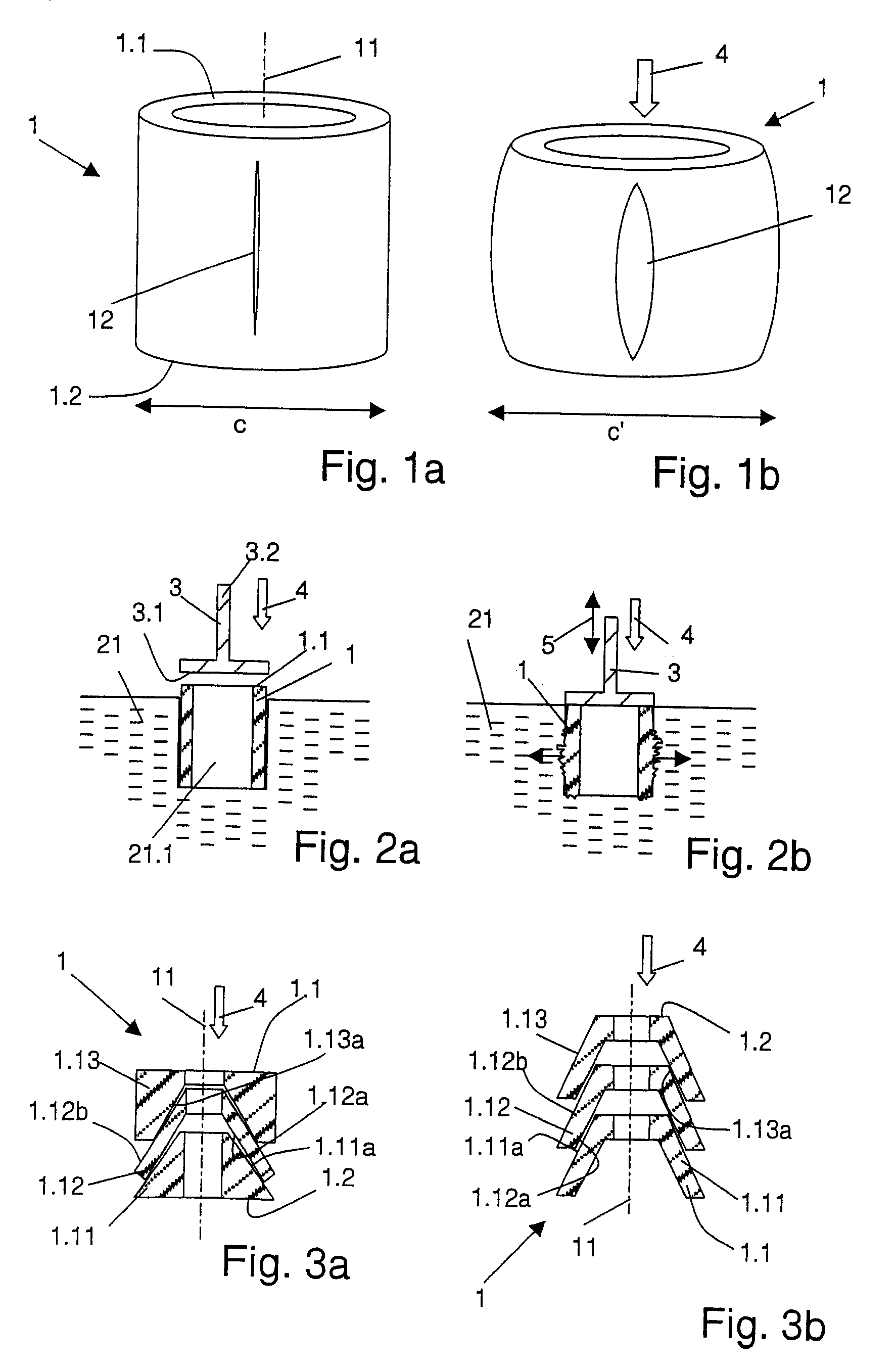
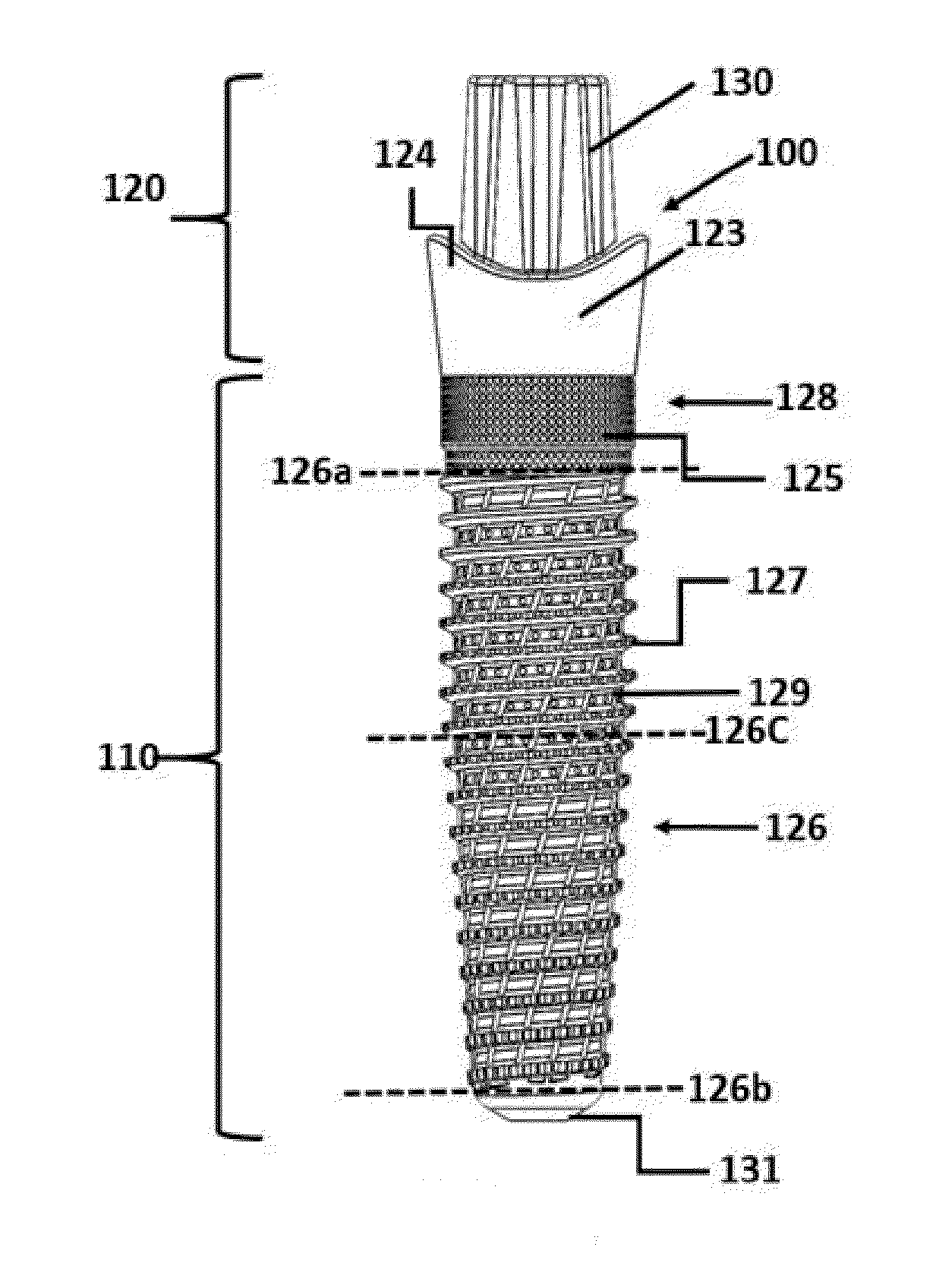
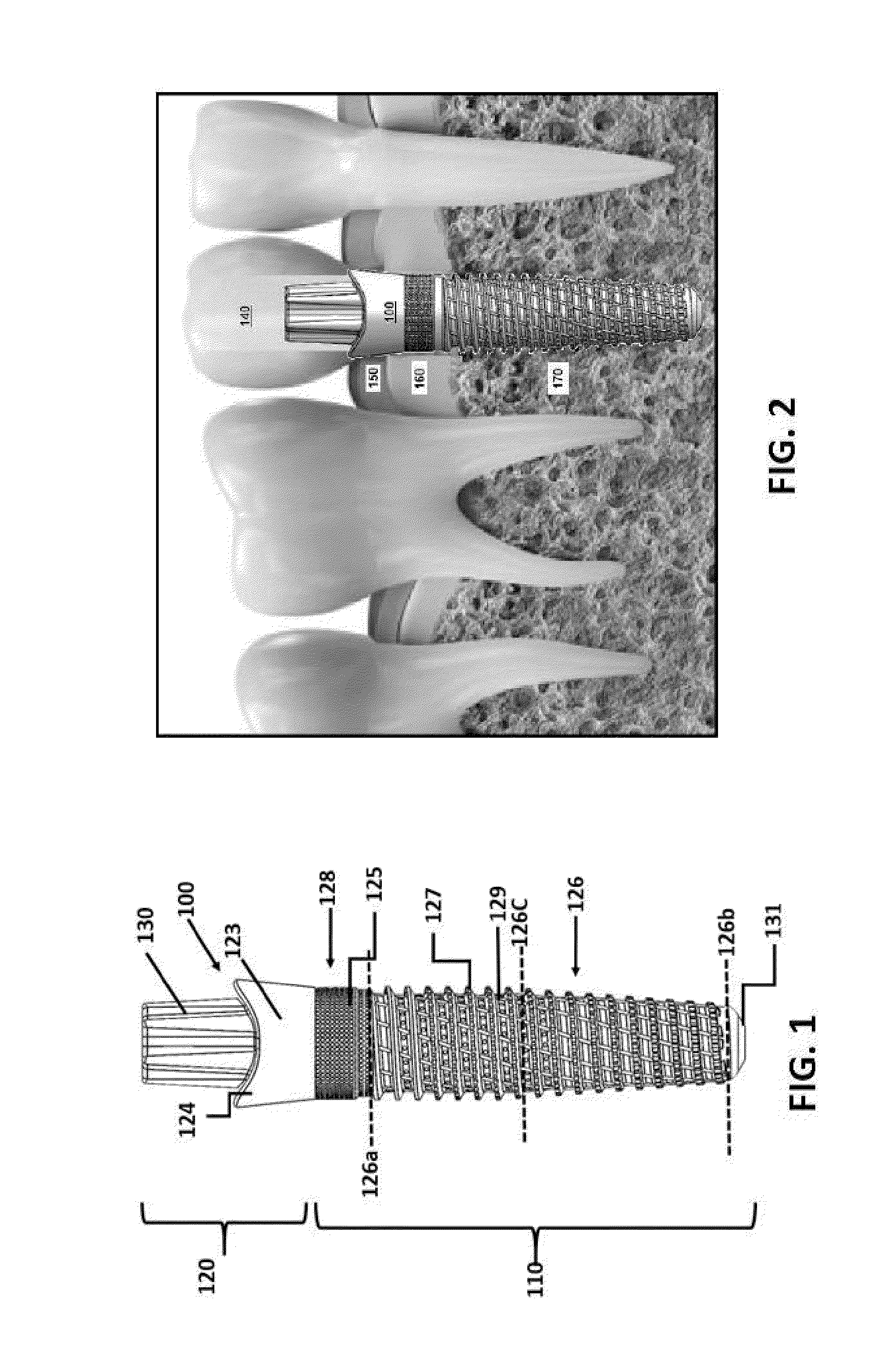
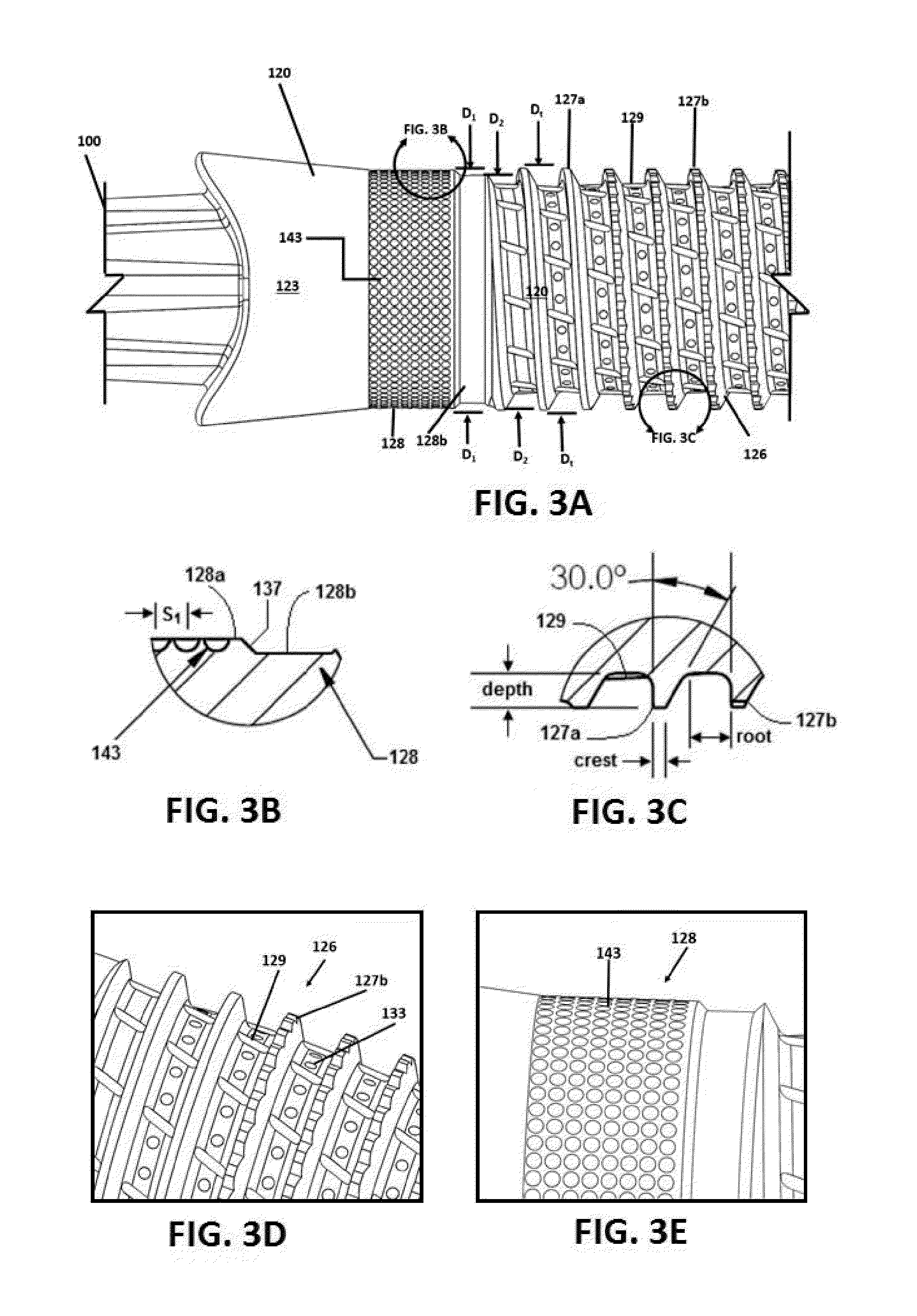
Implant, implantation device, implantation method
An implant suitable for being anchored with the aid of mechanical vibration in an opening provided in bone tissue. The implant is compressible in the direction of a compression axis under local enlargement of a distance between a peripheral implant surface and the compression axis. The implant includes a coupling-in face which serves for coupling a compressing force and the mechanical vibrations into the implant, which coupling-in face is not parallel to the compression axis. The implant also includes a thermoplastic material which, in areas of the local distance enlargement, forms at least a part of the peripheral surface of the implant.
Owner:WOODWELDING
Medical Device Applications of Nanostructured Surfaces
InactiveUS20110201984A1Improve adhesionIncrease frictionMaterial nanotechnologyInternal electrodesFiberOsteoblast
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:NANOSYS INC
Method for modifying a medical implant surface for promoting tissue growth
InactiveUS20080091234A1Without excessive fibrosisIncreased riskCoatingsSurgical veterinaryBioabsorbable polymerPolyhydroxybutyrate
Disclosed is an occluder for closing an intracardiac defect, such as a patent foramen ovale (PFO), and a method for making the same. The occluder includes a frame and at least one scaffold which are formed from a bioabsorbable polymer, such as poly-4-hydroxybutyrate. The surface of the frame and scaffold are textured to promote cell attachment. Texturing of the surface can be achieved by any number of mechanical or chemical procedures. The device is coated with collagen and heparin which are covalently bound to the surface of the device. The occluder provides improved defect closure compared to other septal occluders known in the art. In particular, the occluder described is specifically designed to improve host cell attachment to and tissue ingrowth over the device when implanted in a patient as compared to the level of host cell attachment and tissue ingrowth achieved with other implantable devices made of bioabsorbable polymers.
Owner:WL GORE & ASSOC INC
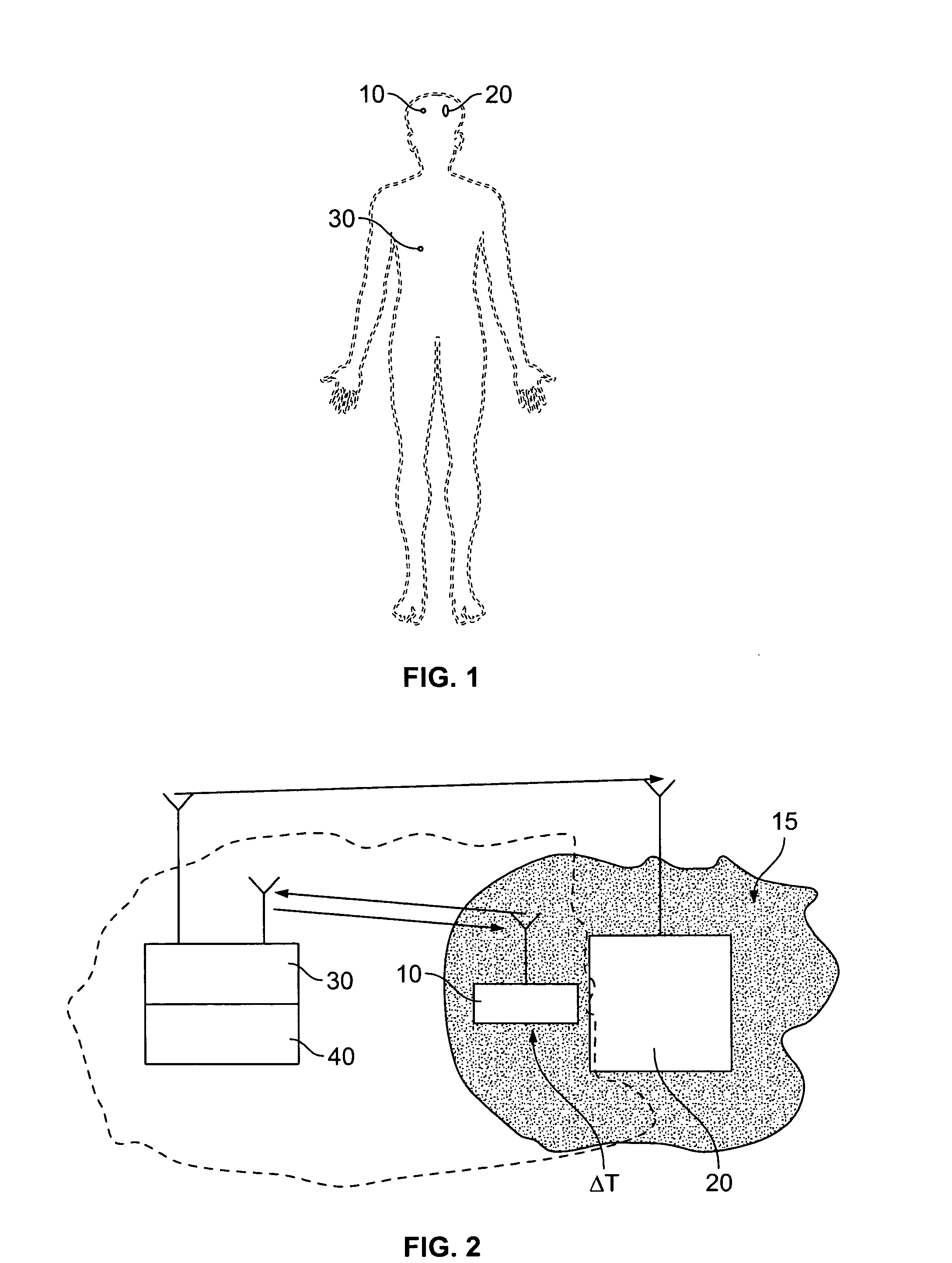
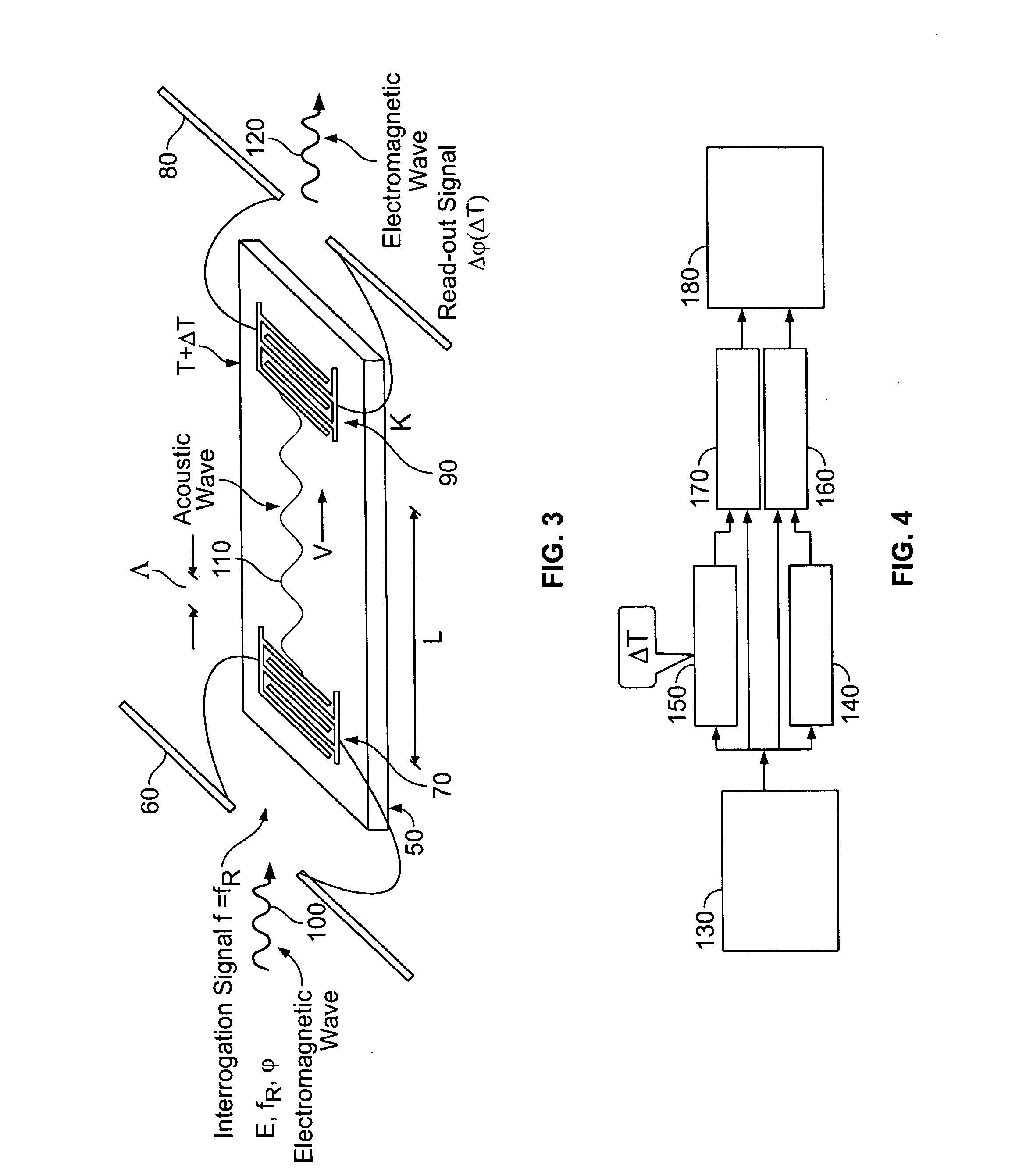
Surface acoustic wave probe implant for predicting epileptic seizures
InactiveUS20070073150A1Reliable detectionElectrotherapyOrgan movement/changes detectionAcoustic waveSurface acoustic wave
Owner:UCHICAGO ARGONNE LLC +1
Medical device applications of nanostructured surfaces
Owner:GLO TECH LLC
Osseointegrative surgical implant
InactiveUS20160015483A1Improve primary stabilityPromote healingSuture equipmentsDental implantsCeramic compositeSurgical implant
Embodiments of the present invention provide an osseointegrative implant and related tools, components and fabrication techniques for surgical bone fixation and dental restoration purposes. In one embodiment an all-ceramic single-stage threaded or press-fit implant is provided having finely detailed surface features formed by ceramic injection molding and / or spark plasma sintering of a powder compact or green body comprising finely powdered zirconia. In another embodiment a two-stage threaded implant is provided having an exterior shell or body formed substantially entirely of ceramic and / or CNT-reinforced ceramic composite material. The implant may include one or more frictionally anisotropic bone-engaging surfaces. In another embodiment a densely sintered ceramic implant is provided wherein, prior to sintering, the porous debound green body is exposed to ions and / or particles of silver, gold, titanium, zirconia, YSZ, α-tricalcium phosphate, hydroxyapatite, carbon, carbon nanotubes, and / or other particles which remain lodged in the implant surface after sintering. Optionally, at least the supragingival portions of an all-ceramic implant are configured to have high translucence in the visible light range. Optionally, at least the bone-engaging portions of an all-ceramic implant are coated with a fused layer of titanium oxide.
Owner:OSSEODYNE SURGICAL SOLUTIONS LLC
Method for producing nanostructures on a surface of a medical implant
InactiveUS20110125263A1Enhanced and increased in vivo chondrocyte functionalityImprove adhesionSurface reaction electrolytic coatingPharmaceutical delivery mechanismPorosityIn vivo
A method for treating a surface of a medical implant to create nanostructures on the surface that results in increased in-vivo chondrocyte adhesion to the surface. Further, disclosed is a method to fabricate a drug delivery system. The drug delivery system includes a medical implant that has undergone a surface treatment process that results in the modification of the surface configuration and topography. The modified surface acts as a depot or reservoir for loaded biological material, biologic agents or pharmaceutical products. Additionally, a device for delivering pharmaceutical products or other biological materials is disclosed. The device includes integrally attached nanostructures that retain or adsorb the loaded pharmaceutical products and / or biological materials. Further disclosed is a medical implant that includes a surface configured to allow for and regulate protein adsorption. The surface of the medical implant has a layer of nanostructures rigidly attached with varying porosity and orientation that allow for surface protein adsorption to be controlled.
Owner:BROWN UNIVERSITY
Method for manufacturing composite piezoelectric substrate and piezoelectric device
ActiveUS20110278993A1Improve featuresLess piezoelectric degradationPiezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostriction/magnetostriction machinesMetallurgyIon implantation
A piezoelectric device is manufactured in which the material of a supporting substrate can be selected from various alternative materials. Ions are implanted into a piezoelectric substrate to form an ion-implanted portion. A temporary supporting substrate is formed on the ion-implanted surface of the piezoelectric substrate. The temporary supporting substrate includes a layer to be etched and a temporary substrate. The piezoelectric substrate is then heated to be divided at the ion-implanted portion to form a piezoelectric thin film. A supporting substrate is then formed on the piezoelectric thin film. The supporting substrate includes a dielectric film and a base substrate. The temporary supporting substrate is made of a material that produces a thermal stress at the interface between the temporary supporting substrate and the piezoelectric thin film less than the thermal stress at the interface between the supporting substrate and the piezoelectric thin film.
Owner:MURATA MFG CO LTD
Laser based metal deposition (LBMD) of antimicrobials to implant surfaces
InactiveUS20070287027A1Improved bearing propertyImprove propertiesFinger jointsDental implantsWear resistantBearing surface
A method is provided for depositing a hard wear resistant surface onto a porous or non-porous base material of a medical implant. The wear resistant surface of the medical implant device may be formed by a Laser Based Metal Deposition (LBMD) method such as Laser Engineered Net Shaping (LENS). The wear resistant surface may include a blend of multiple different biocompatible materials. Further, functionally graded layers of biocompatible materials may be used to form the wear resistant surface. Usage of a porous material for the base may promote bone ingrowth to allow the implant to fuse strongly with the bone of a host patient. The hard wear resistant surface provides device longevity, particularly when applied to bearing surfaces such as artificial joint bearing surfaces or a dental implant bearing surfaces. An antimicrobial material such as silver may be deposited in combination with a metal to form an antimicrobial surface deposit.
Owner:MEDICINELODGE
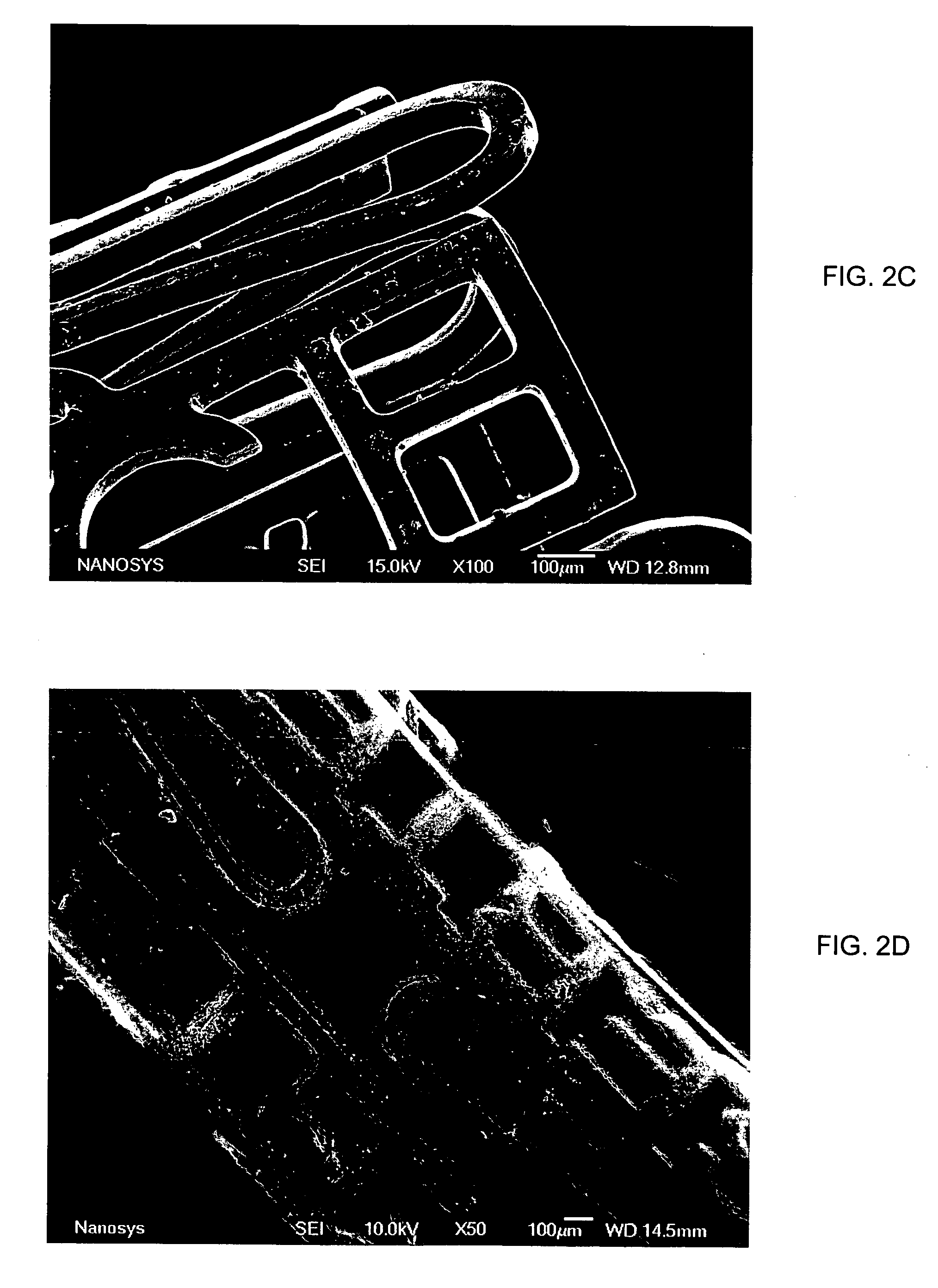
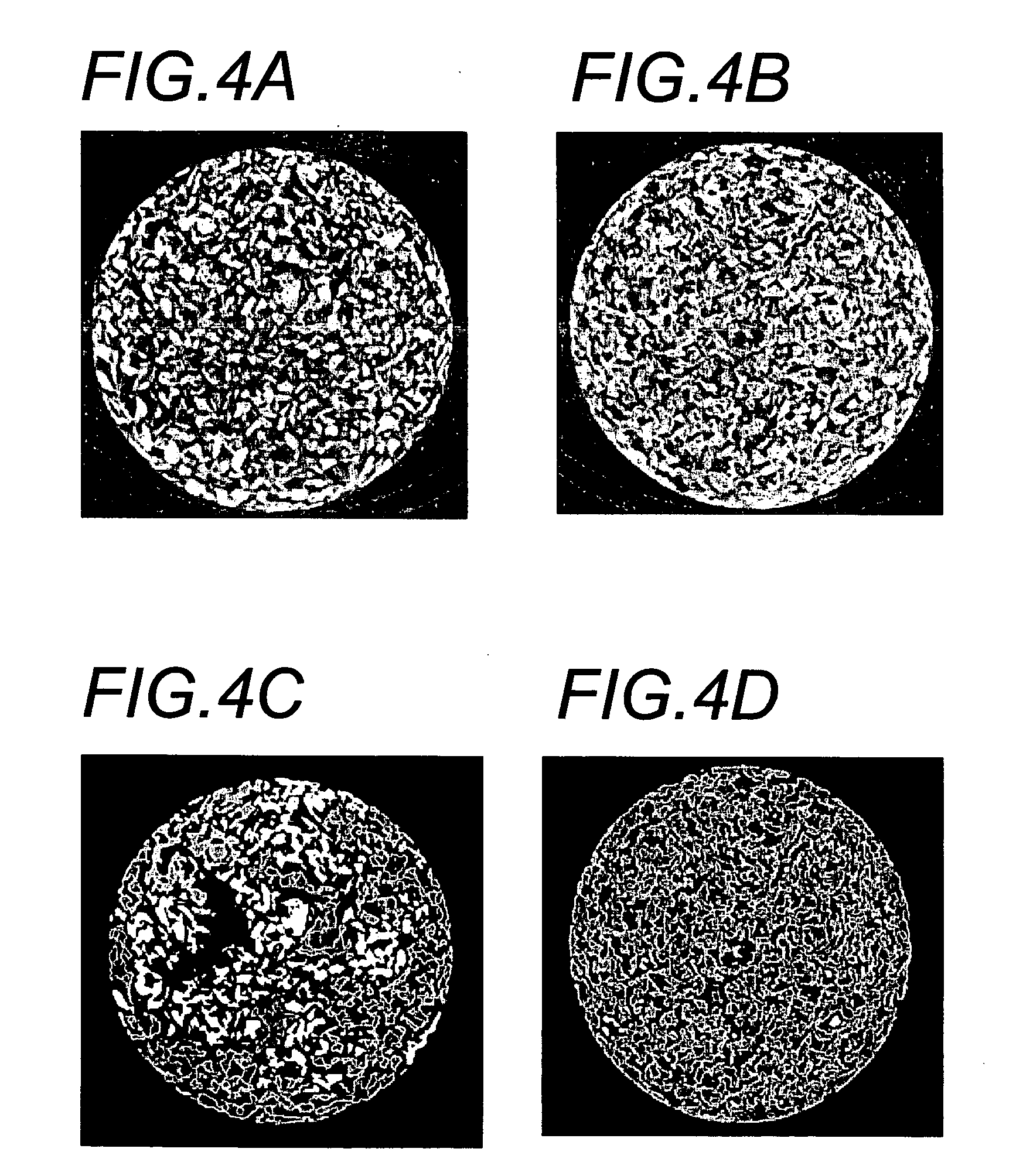
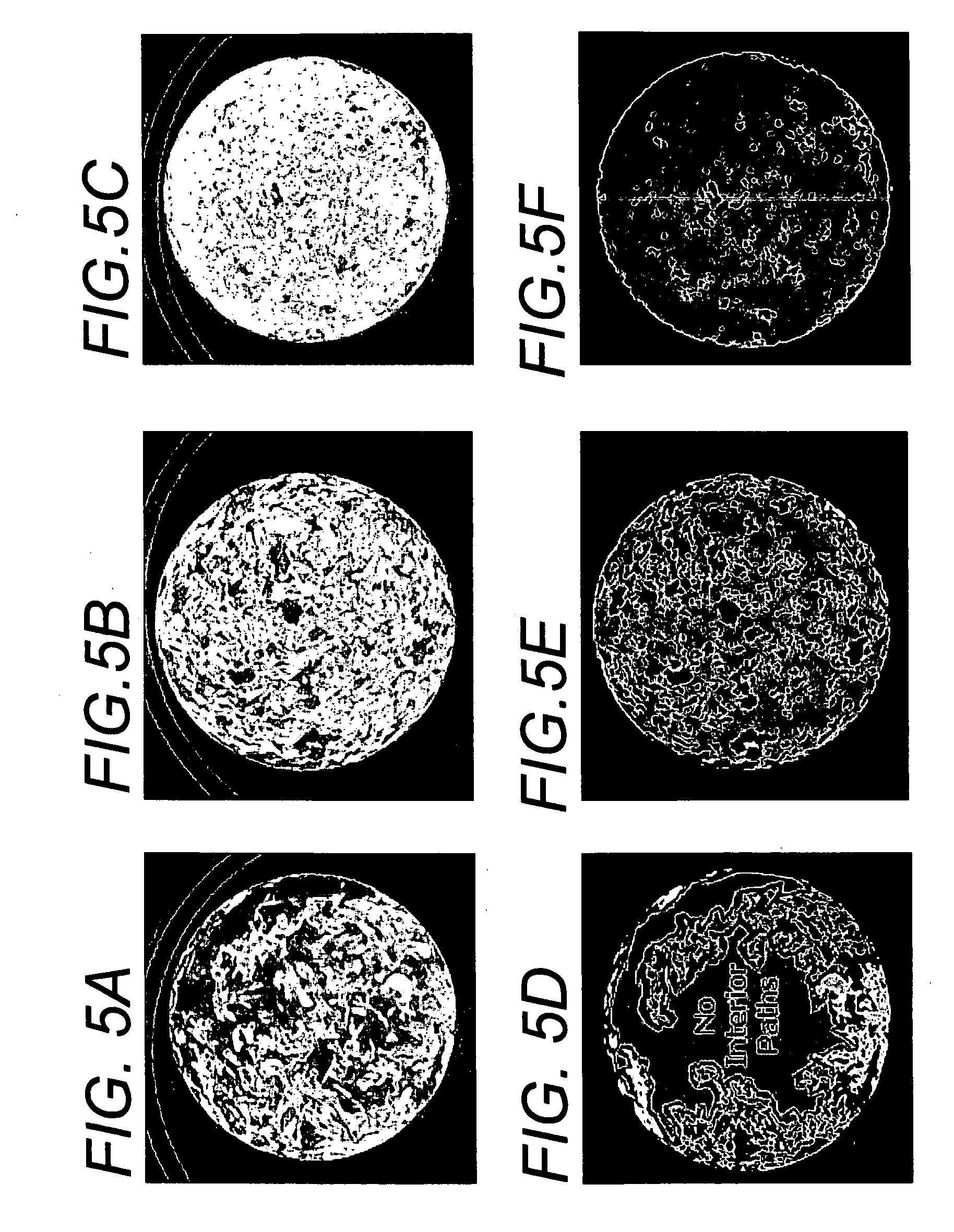
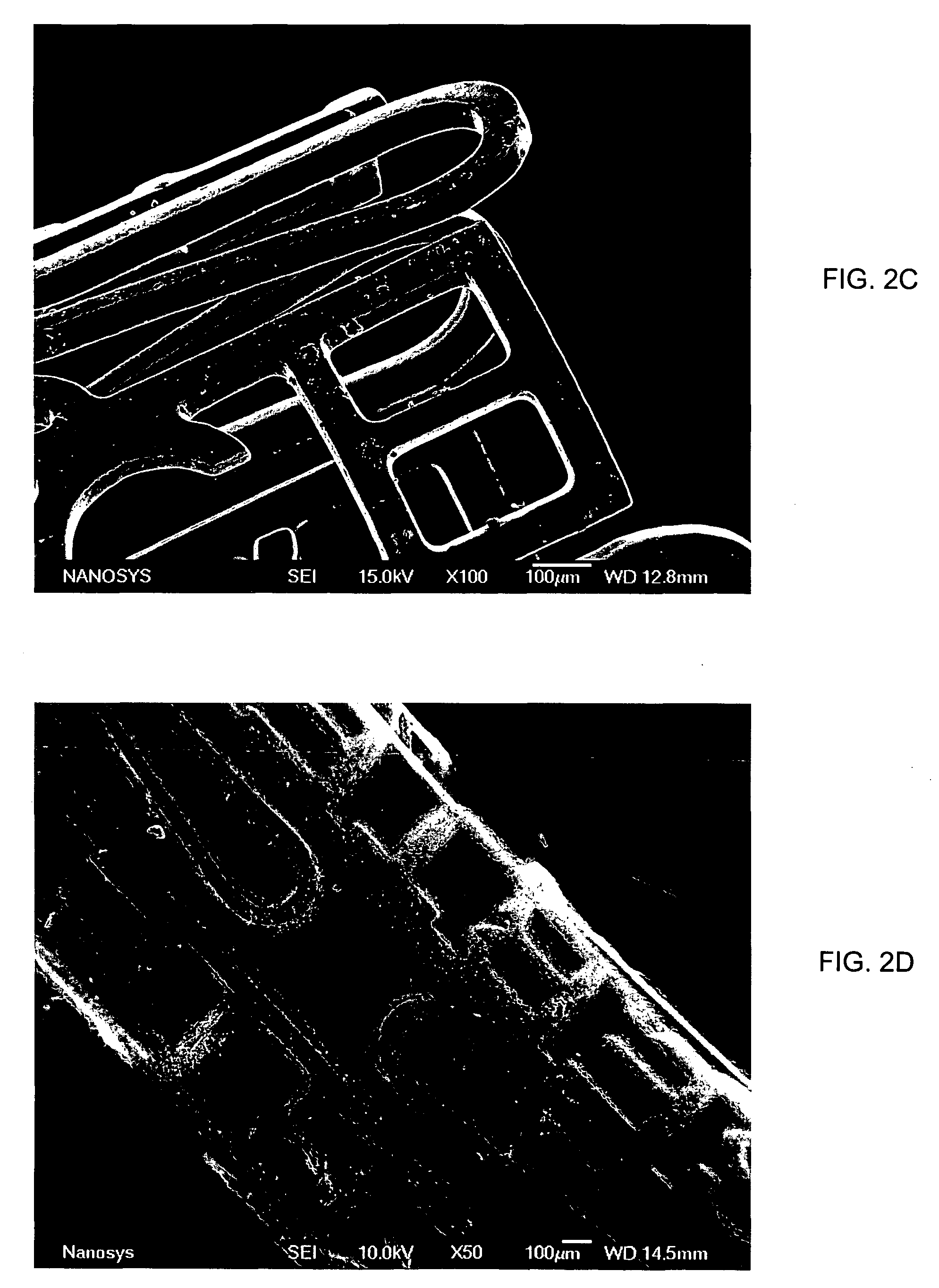
Microstructured implant surfaces
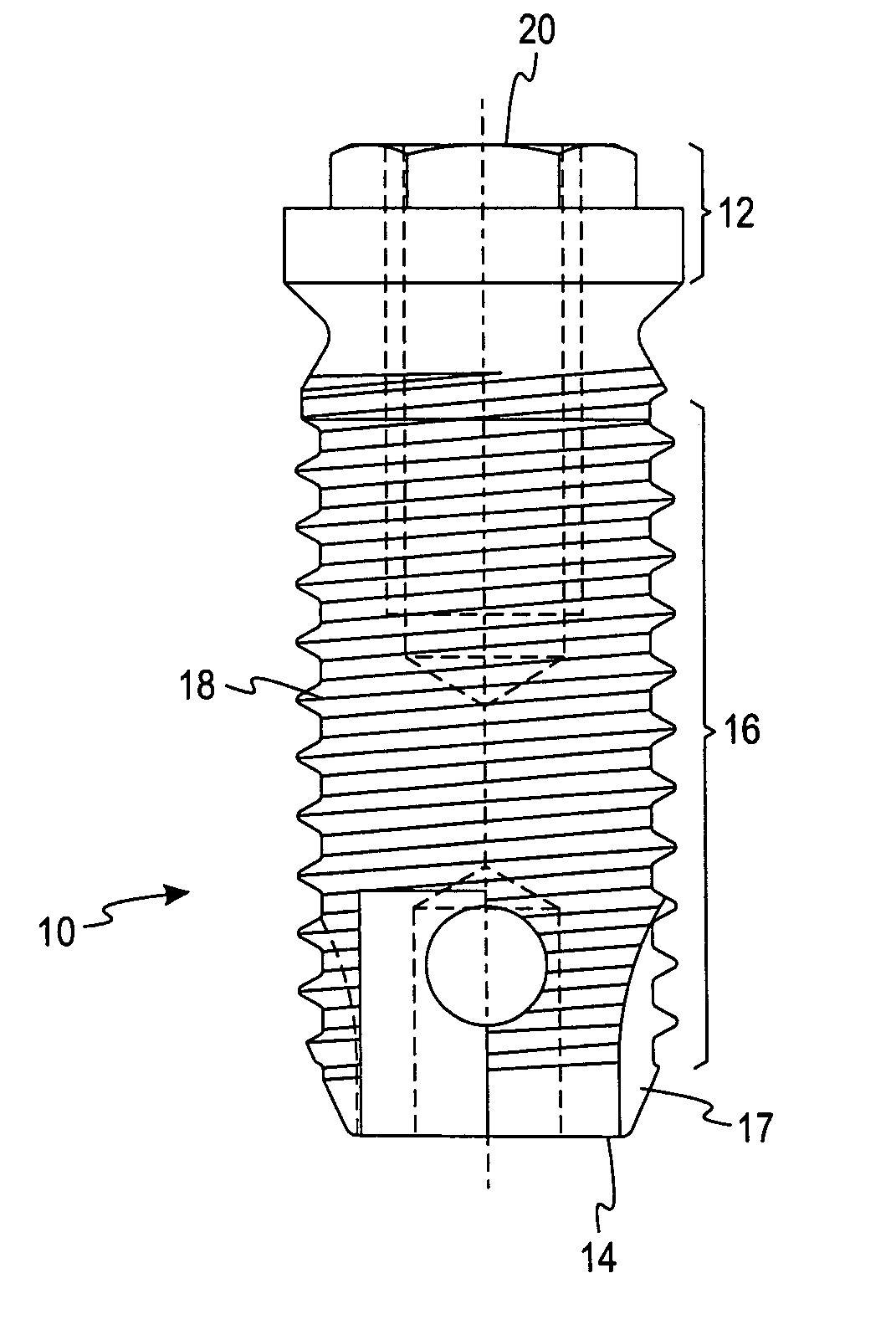
An implantable device for treating disc degenerative disease and arthritis of the spine. The implant is sized for placement into an intravertebral disc space. The implant has a body with a predetermined, defined, repeating, three-dimensional pattern at least partially on at least one of its surfaces. The pattern is adapted to create a surface area of bone-contacting features that enhance in-growth and biological attachment to a biocompatible material. Also disclosed are process steps for making the implant.
Owner:TITAN SPINE
Method for producing a thin membrane and resulting structure with membrane
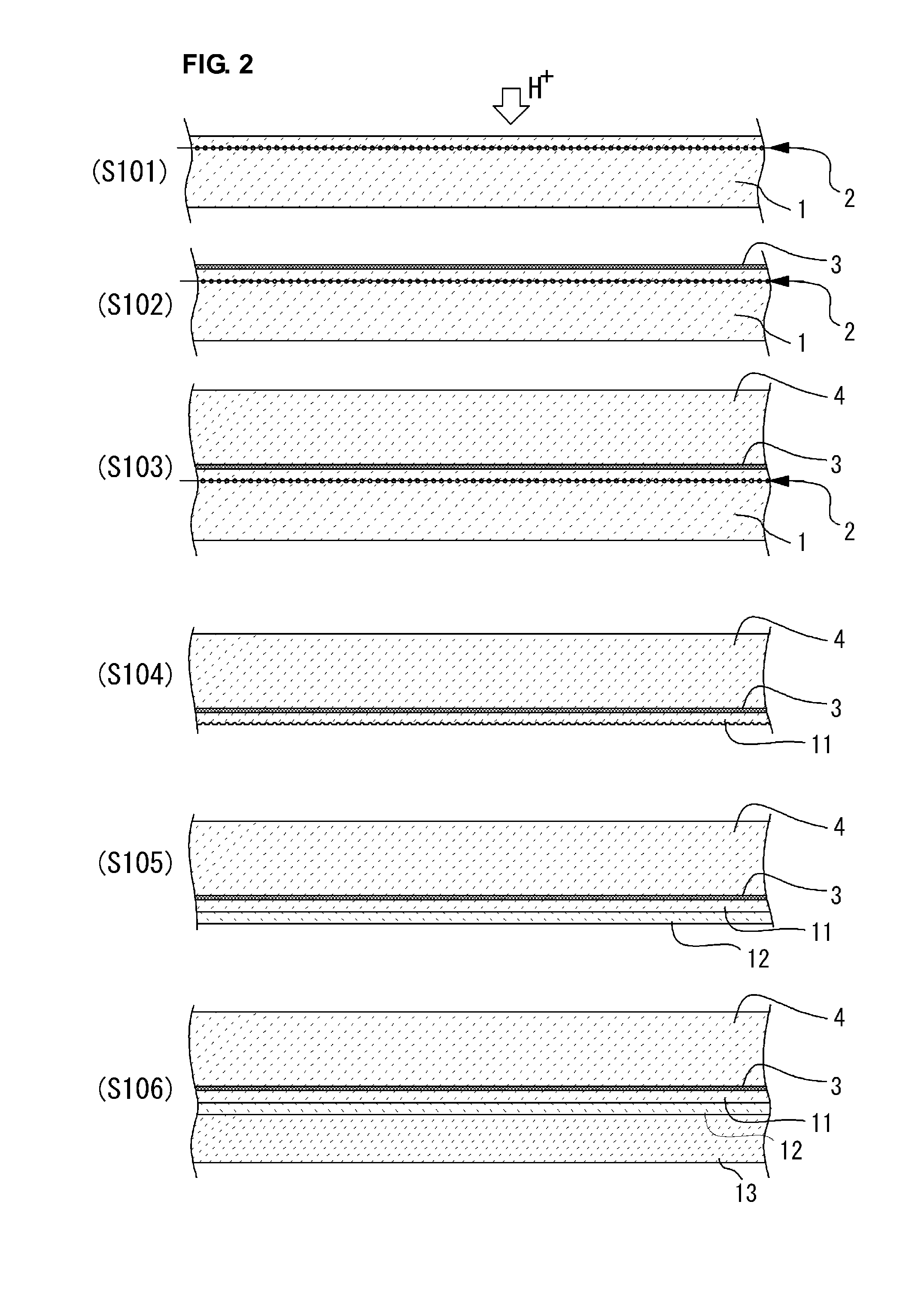
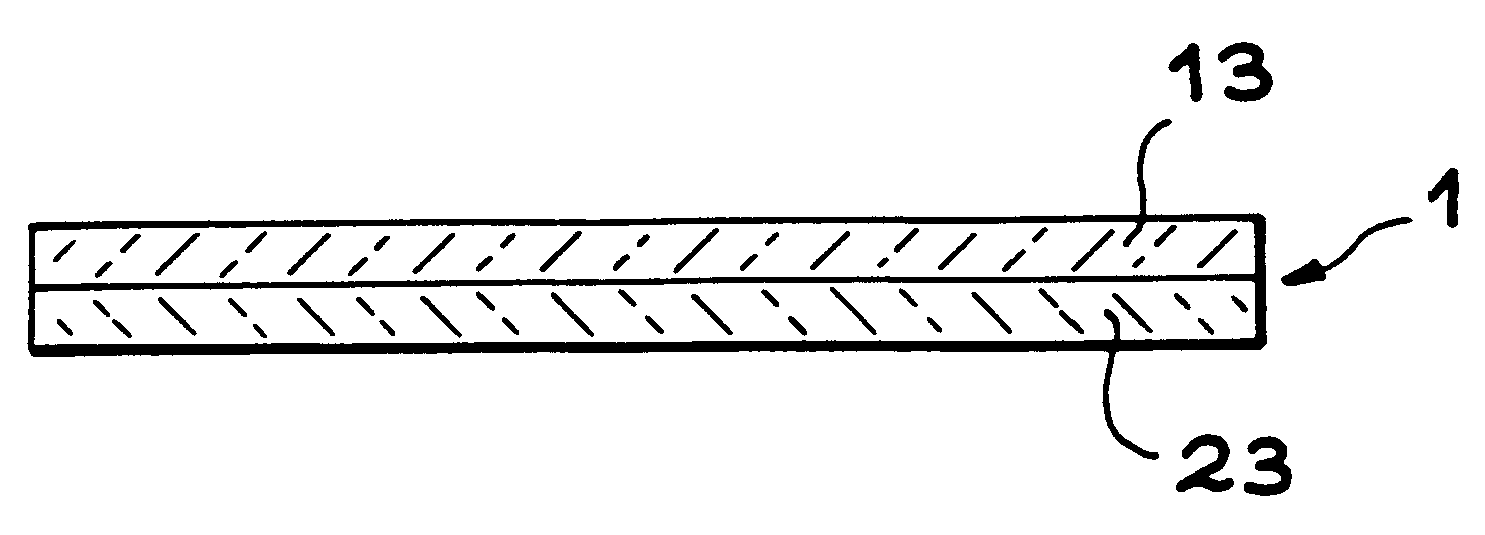
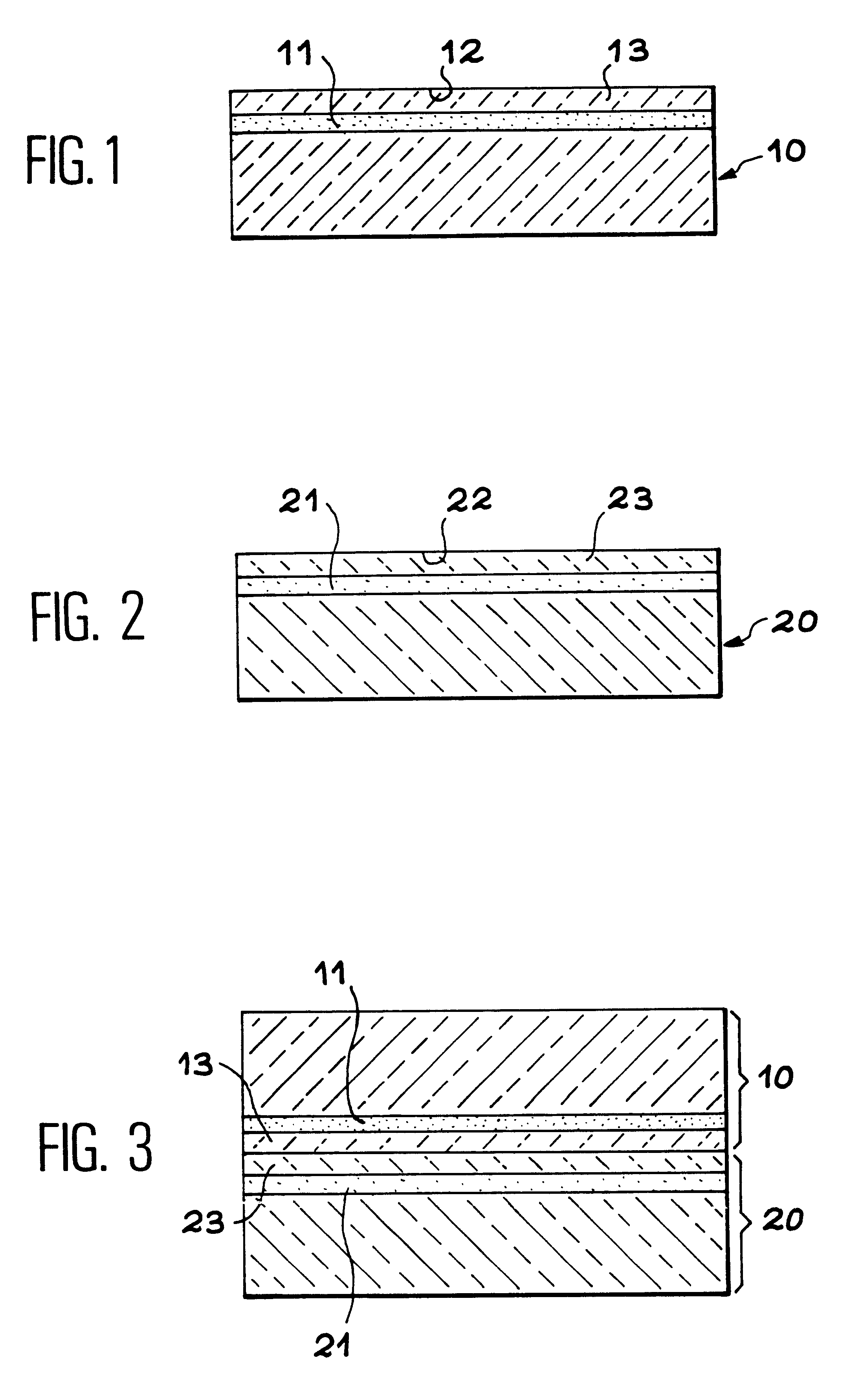
InactiveUS6465327B1Easy to separateSeparation can be delayedSolid-state devicesSemiconductor/solid-state device manufacturingThin membraneThin layer
The invention relates to a method for producing a thin membrane, comprising the following steps:implanting gas species, through one surface of a first substrate (10) and through one surface of a second substrate (20), which in said substrates are able to create microcavities (11, 21) delimiting, for each substrate, a thin layer (13, 23) lying between these microcavities and the implanted surface, the microcavities being able, after their implantation, to cause detachment of the thin layer from its substrate;assembly of the first substrate (10) onto the second substrate (20) such that their implanted surfaces face one another;detaching each thin layer (13, 23) from its substrate (10, 20), the thin layers remaining assembled together to form said thin membrane.The invention also concerns a thin membrane structure obtained with this method.
Owner:S O I TEC SILICON ON INSULATOR THECHNOLOGIES
Implants with attached silylated therapeutic agents
InactiveUS20060286140A1Promote osseointegrationPrevent bacterial growthAntibacterial agentsBiocideBiofilmAntibiotic Y
The present invention is directed to implants that include therapeutic molecules bonded to their surfaces. The therapeutic molecules interact with cells that are adjacent, near, or adhering to the implant. The covalently-bonded therapeutic molecules may be released from the implant surface by changes in pH or enzymes characteristic of cells adjacent to the implant. Preferably the covalently-bonded agents include an antibiotics that are released from the implant surface by bacteria and in this way ensures that the antibiotic is released at sites on the implant that would serve as centers for both bacterial colonization and biofilm formation.
Owner:SMART TECH INC (CA)
Surface for use on implantable device
InactiveUS6911249B2Reduce air resistancePromote absorptionLayered productsDecorative surface effectsImplanted deviceMaterials science
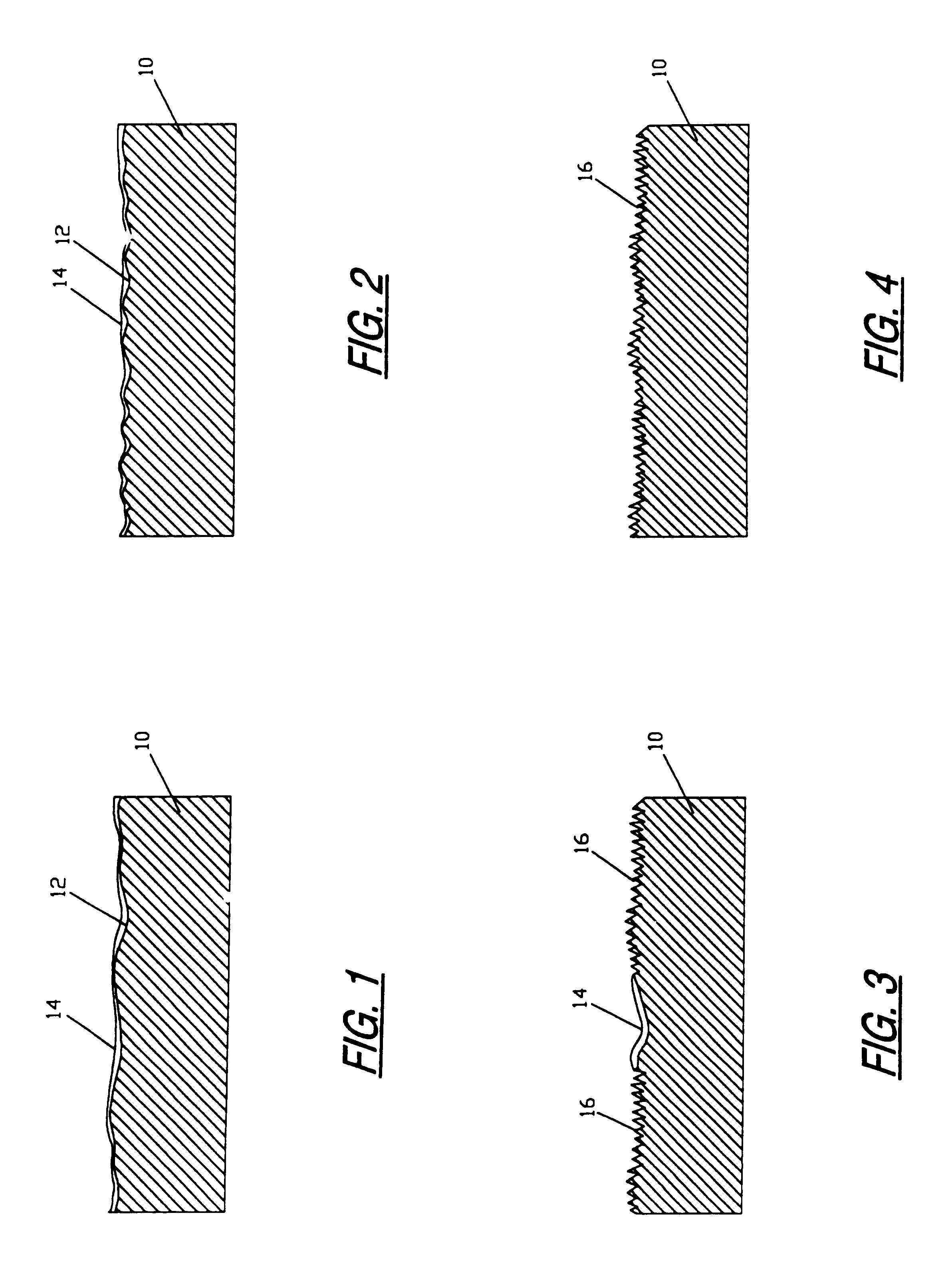
An attachment surface for an implantable device has an irregular pattern formed through a process including masking, chemical or electrochemical etching, blasting and debris removal steps. Surface material is removed from the implant surface without stress on the adjoining material and the process provides fully dimensional fillet radii at the base of the surface irregularities. This irregular surface is adapted to receive the ingrowth of bone material and to provide a strong anchor for that bone material which is resistant to cracking or breaking. The surface is prepared through an etching process which utilizes the random application of a maskant and subsequent etching in areas unprotected by the maskant. This chemical etching process is repeated a number of times as necessitated by the nature of the irregularities required in the surface. The blasting and debris removal steps produce microfeatures on the surface that enhance the ingrowth of bone material.
Owner:TITAN SPINE
Reduction of adverse inflammation
InactiveUS20050025804A1Reduce the possibilityReduction of the flux of nutrientsBiocideNervous disorderPeroxynitriteIsomerization
Reduction of the likelihood of adverse inflammatory reaction to an implant or a transplant is achieved through several mechanisms including the catalysis of isomerization of peroxynitrite by a hydrogel-bound peroxynitrite isomerization catalysts. A second mechanism controls acceptable and unacceptable dimensions of surface features of implants, such as vascular stents. A third mechanism fabricates implants from materials which are substantially free from alloys transition metals which produce ions of which catalyze cell killing radical formation.
Owner:HELLER ADAM
Tooth Implant
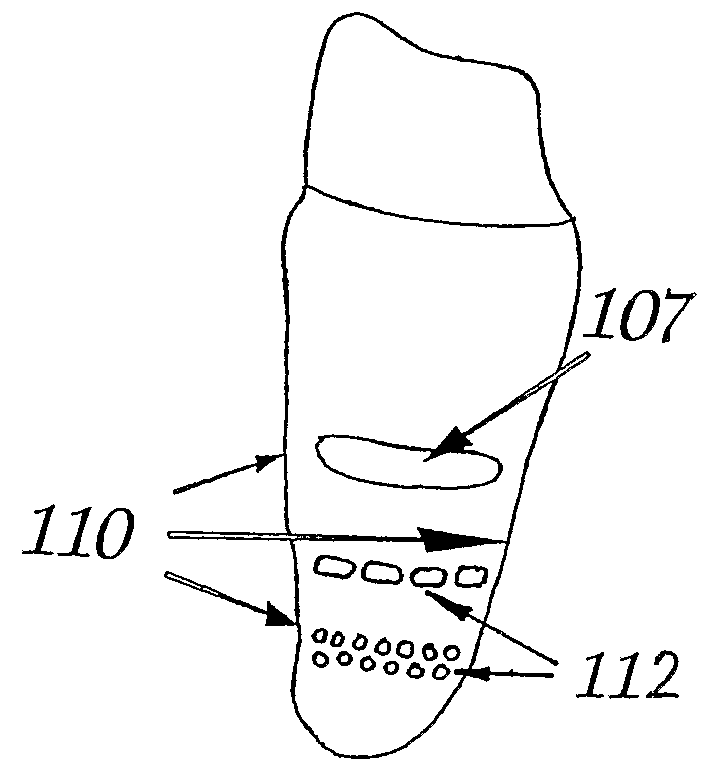
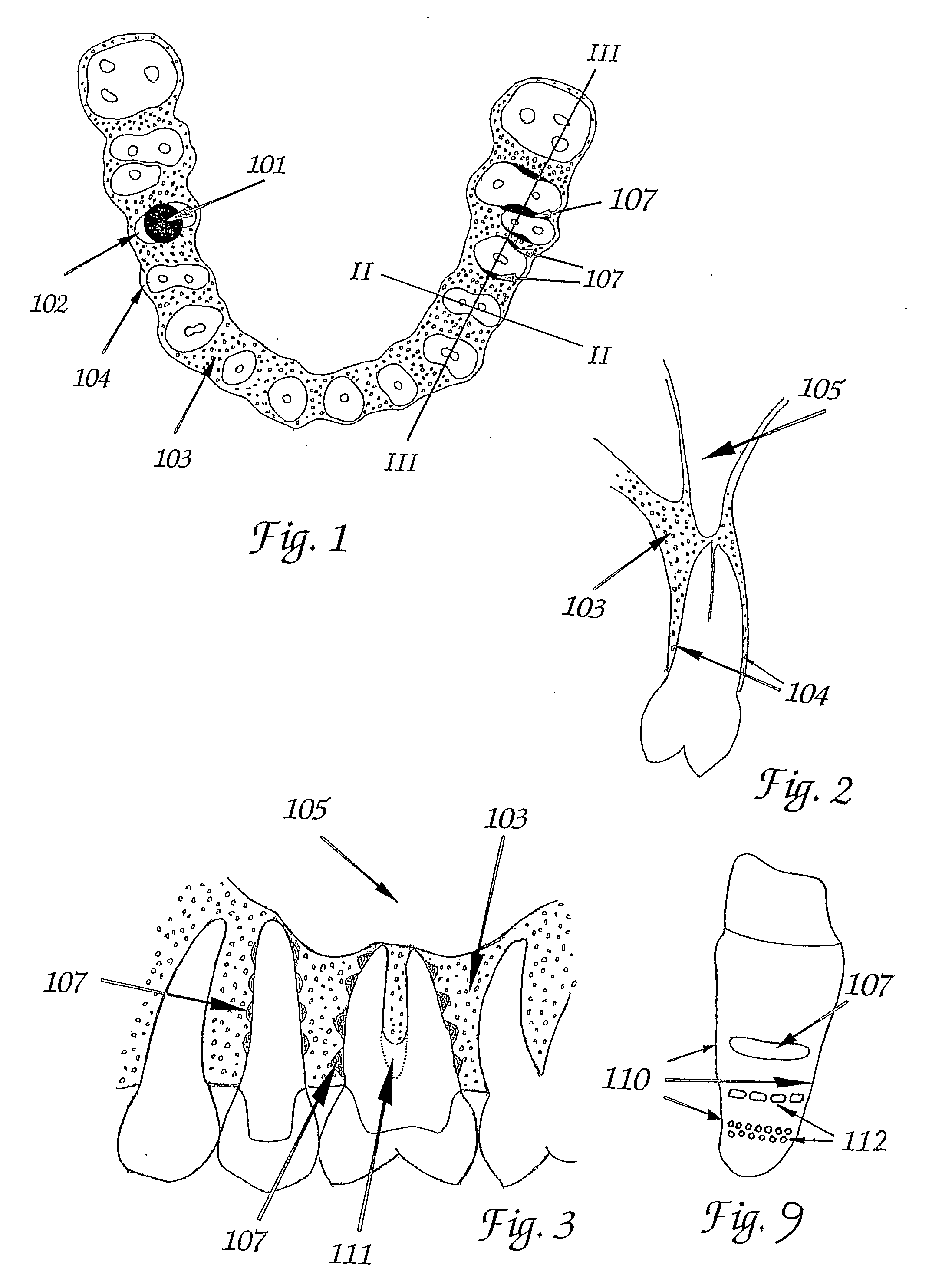
ActiveUS20090092944A1Short maintenance periodMinimal traumaDental implantsExtract toothThin cortical bone
The invention concerns a non rotation-symmetric but root-analogue or tooth socket-analogue dental implant of the same size and shape as the root of the extracted tooth with macro retentions protruding from the implant surface (107, 113, 116).Macro retentions (107, 113, 116) are strictly limited to surface areas of the implant in the interdental space next to spongy and thick bone and in case of the last molar, facing the bone at the end of the tooth row. The diameter of the dental implant in transverse direction next to the thin cortical bone buccal and lingual / palatinal is identical to the alveolar bone or preferably stands back to avoid any pressure induced resorption and fracture of the thin cortical bone layer, respectively, at any cost.
Owner:PIRKER WOLFGANG
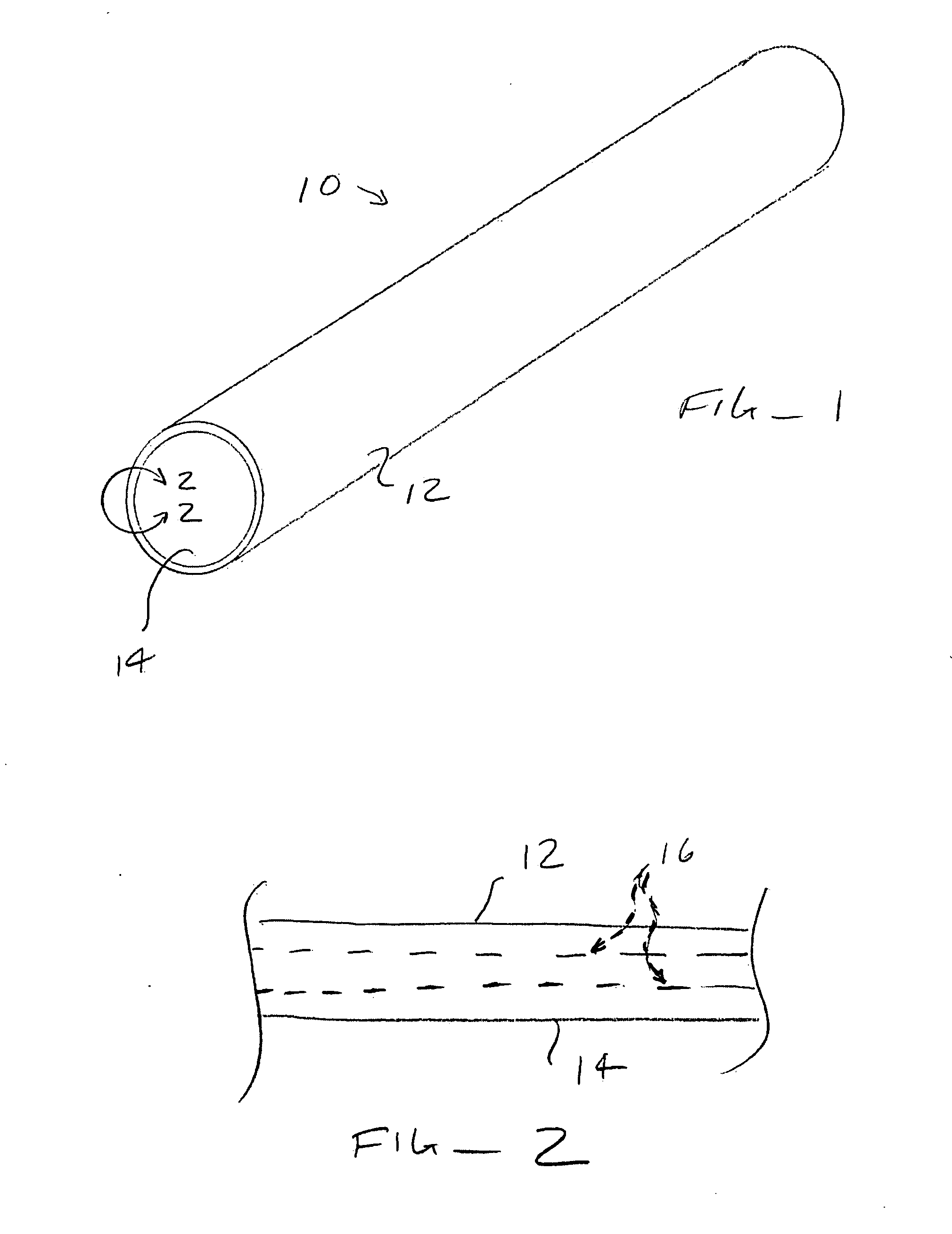
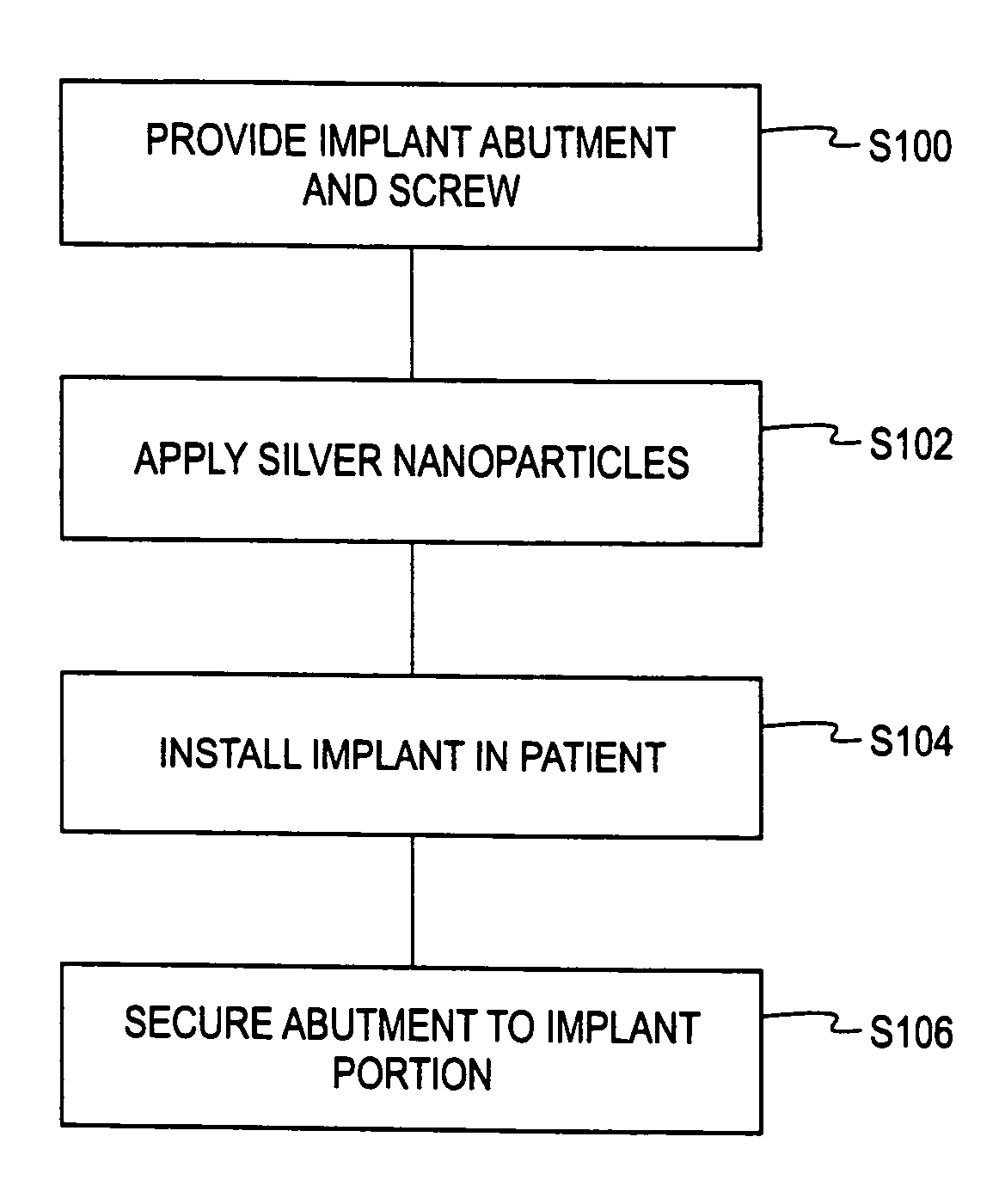
Deposition of silver particles on an implant surface
A dental implant assembly is disclosed. The dental implant assembly comprises an implant. The dental implant assembly further comprises an abutment coupled to a top portion of the implant. The dental implant assembly further comprises a screw for securing the abutment to the implant. The dental implant assembly further comprises silver nanoparticles positioned on at least one interior surface of at least one of the implant and the abutment.
Owner:BIOMET 3I LLC
Devices and methods for treatment of facet and other joints
InactiveUS9308091B2Relieving pressure on a portion of a spineIncrease spacingLigamentsJoint implantsArticular surfacesArticular surface
Owner:CONFORMIS
Additive and Subtractive Manufacturing Process for Producing Implants with Homogeneous Body Substantially Free of Pores and Inclusions
ActiveUS20150335434A1Enhance bone bone fusionEnhance bone growth boneAdditive manufacturing apparatusDecorative surface effectsBone growthNanostructure
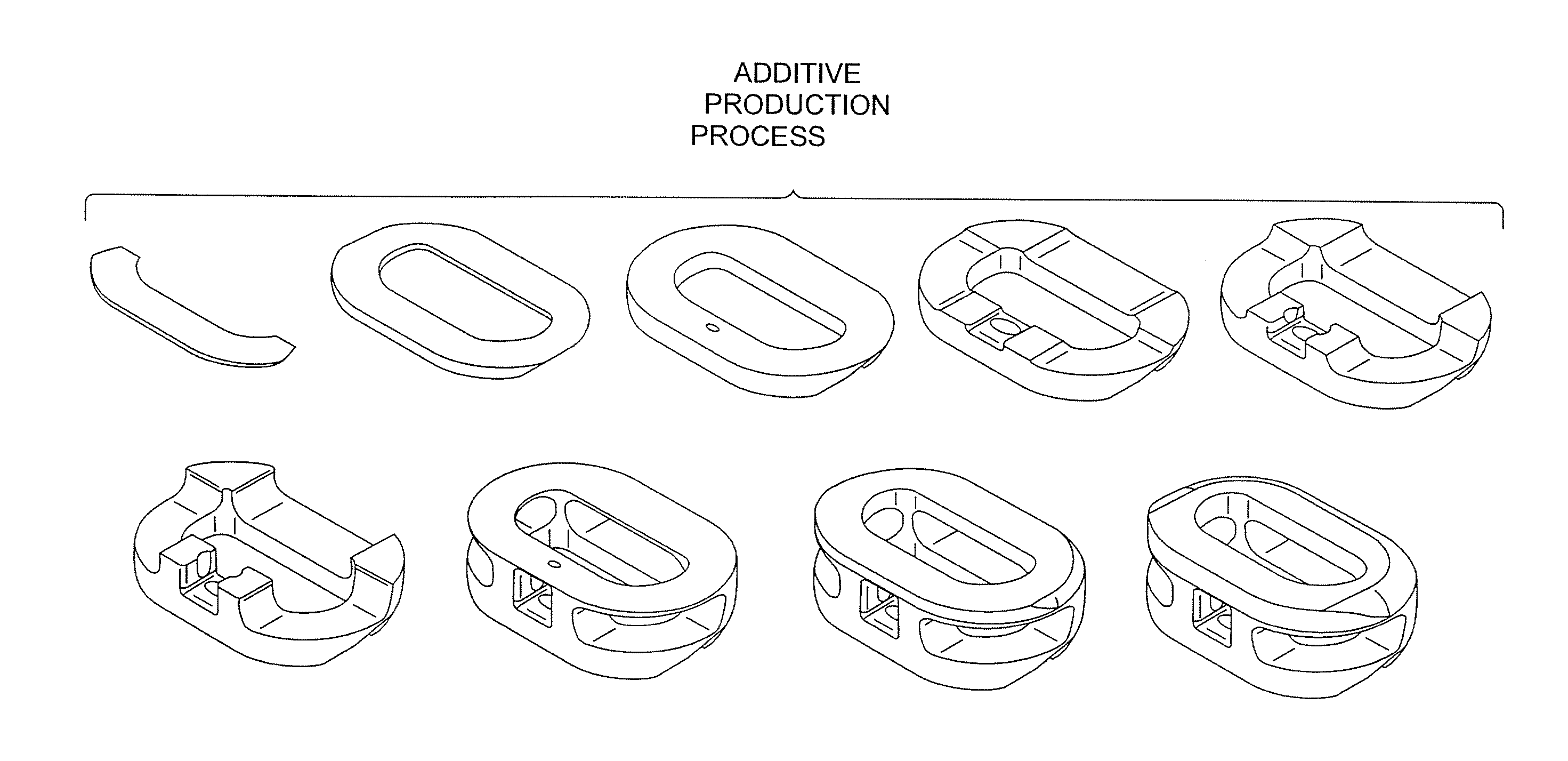
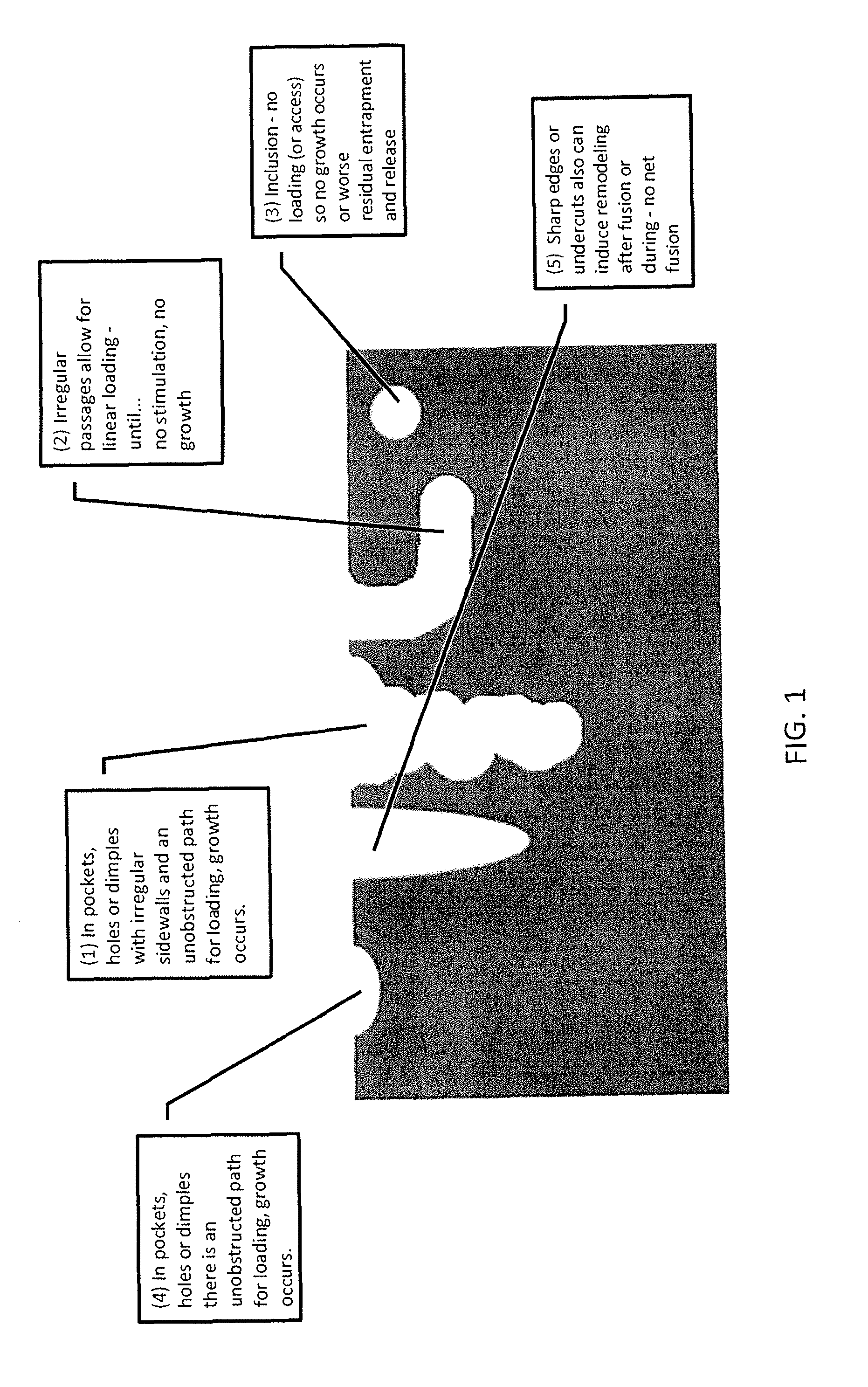
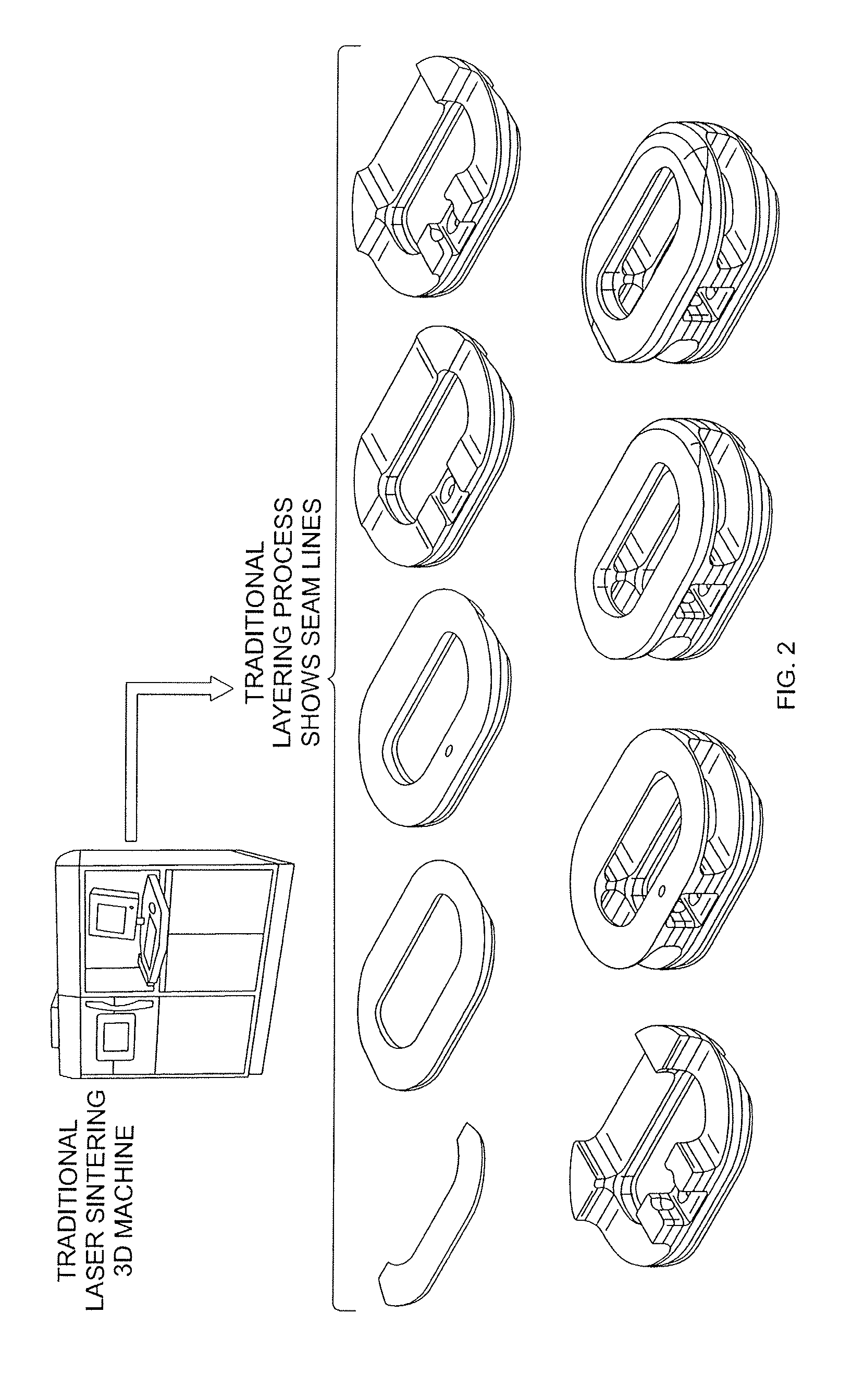
Implants are formed from a multiple staged process that combines both additive and subtractive techniques. Additive techniques melt powders and fragments of a desired material, then successively layer the molten material into the desired implant shape, without compressing or remelting for homogenization of the layers, thereby producing an implant that is substantially free of pores and inclusions. Subtractive techniques refine implant surfaces to produce a bioactive roughened surface comprised of macro, micro, and nano structural features that facilitate bone growth and fusion.
Owner:TITAN SPINE
Implant surface preparation
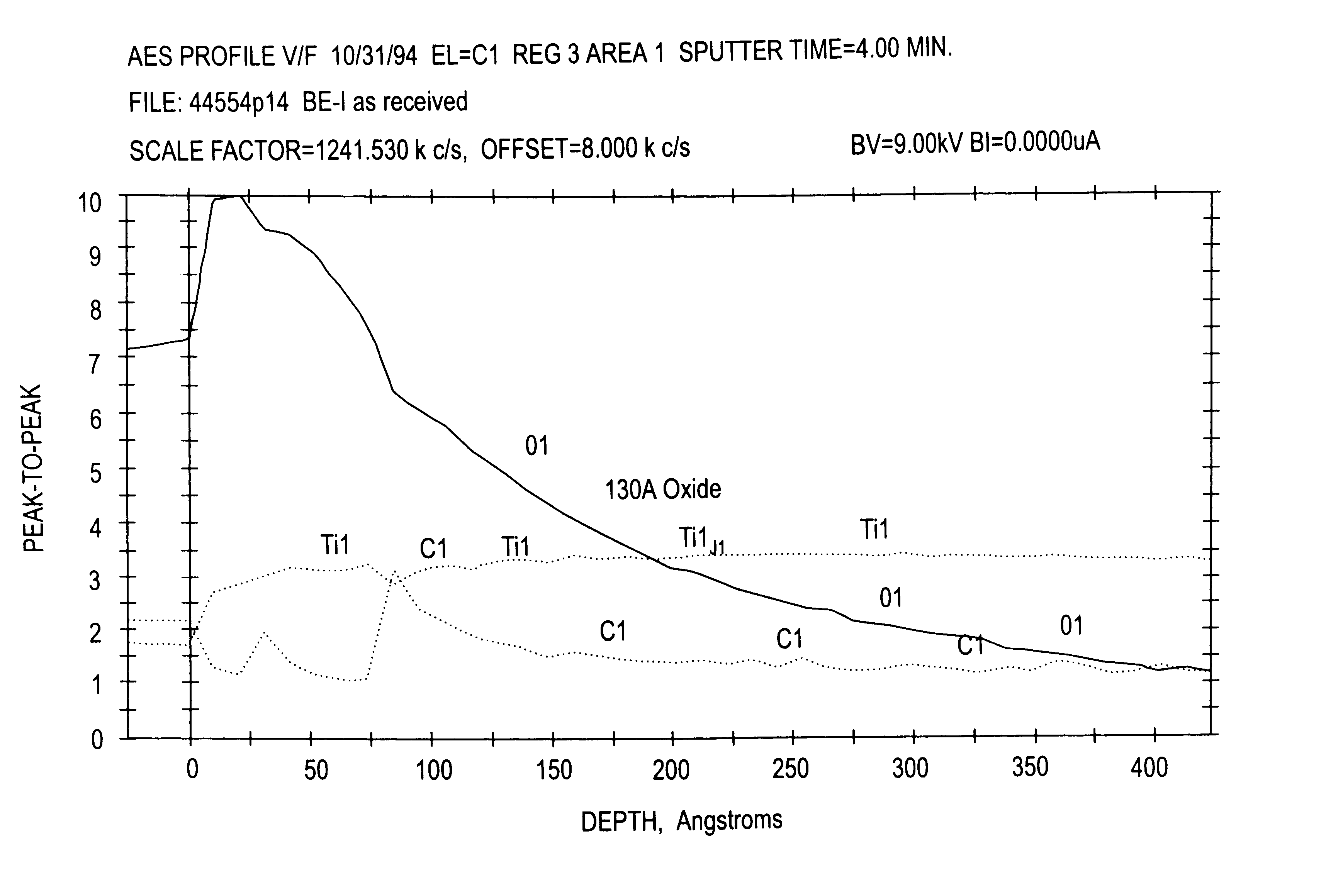
InactiveUS6969474B2Uniform roughnessUniform surface topographyDental implantsImpression capsTitaniumOxygen
The surface of a device that is surgically implantable in living bone is prepared. The device is made of titanium with a native oxide layer on the surface. The method of preparation comprises the steps of removing the native oxide layer from the surface of the device and performing further treatment of the surface substantially in the absence of unreacted oxygen.
Owner:BIOMET 3I LLC
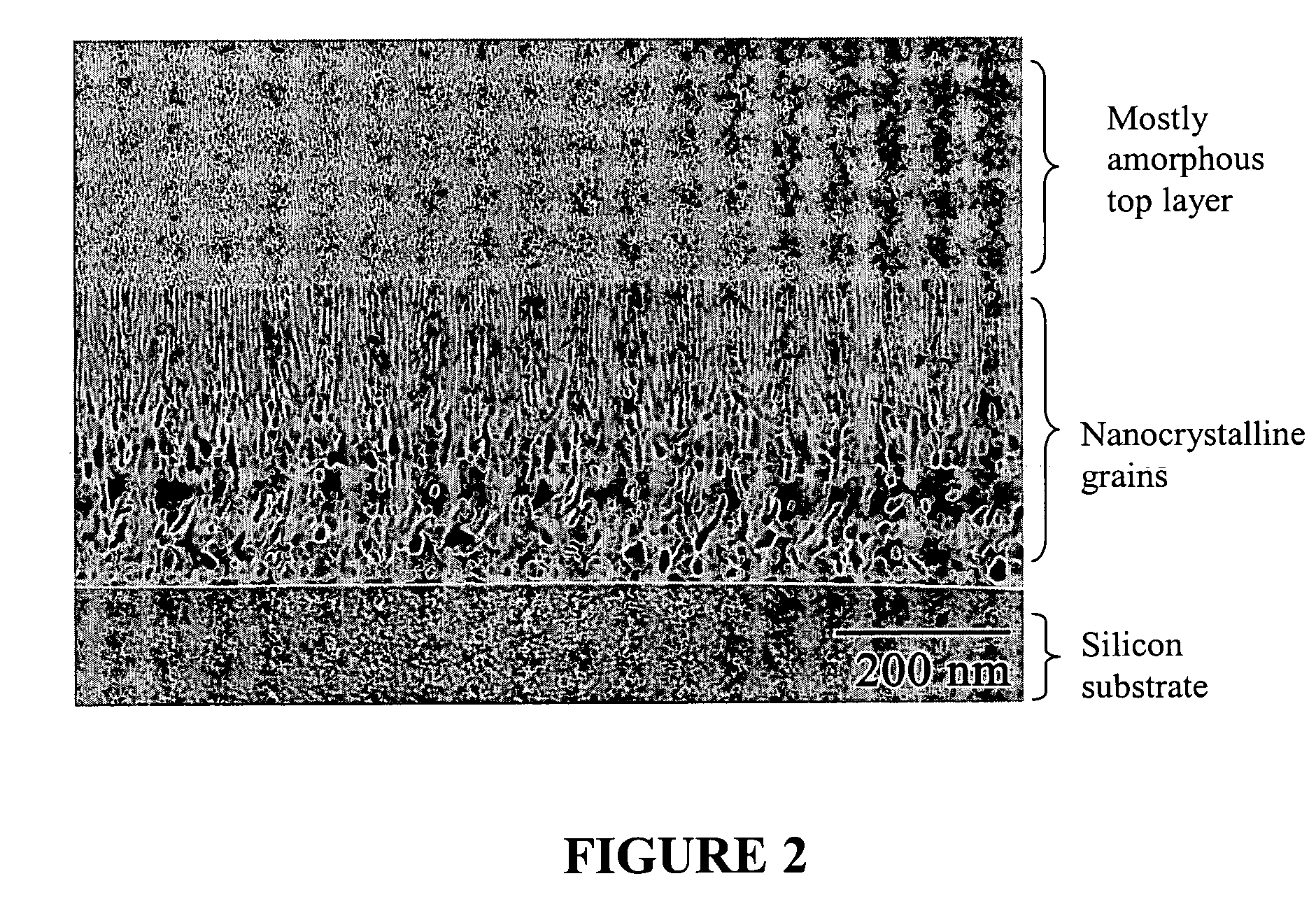
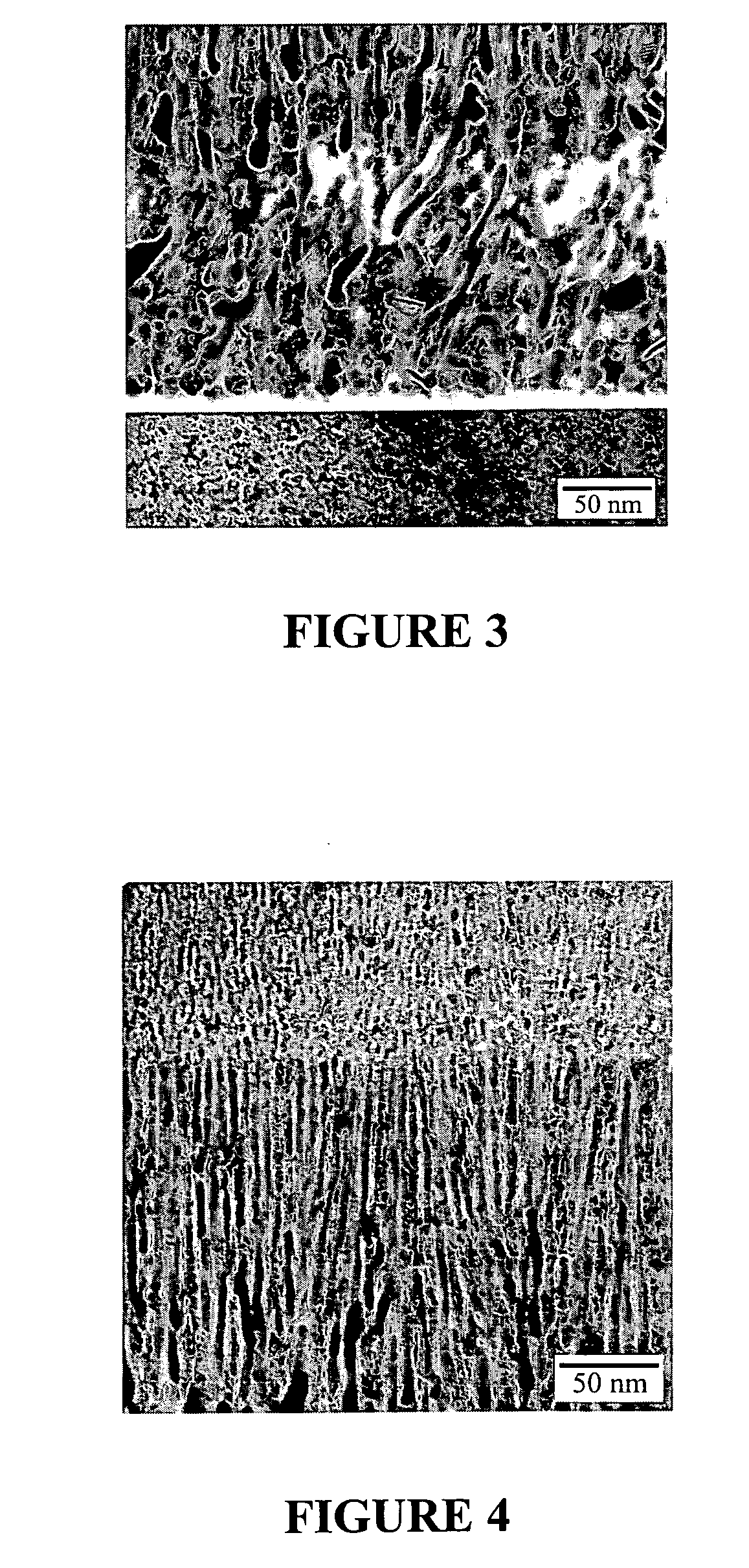
Deposition of discrete nanoparticles on an implant surface
A method of forming a nanocrystalline surface on an implant is disclosed. The method comprises the act of roughening at least a portion of the implant surface to form a roughened surface. The method further comprises the act of, without forming an alkoxide on the roughened surface, depositing nanocrystals on the roughened surface. The nanocrystals comprise a material having a property that promotes osseointegration.
Owner:BIOMET 3I LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com