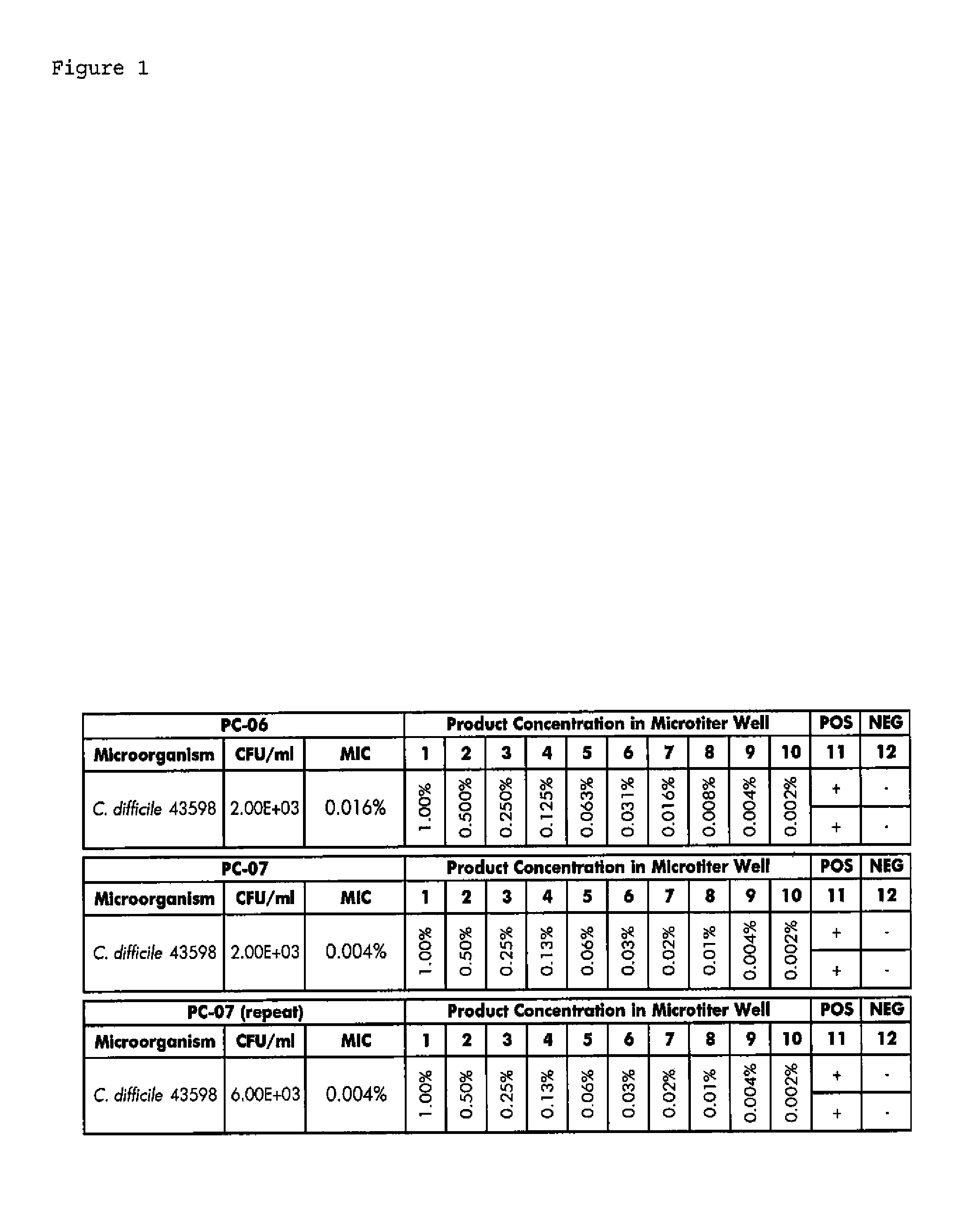
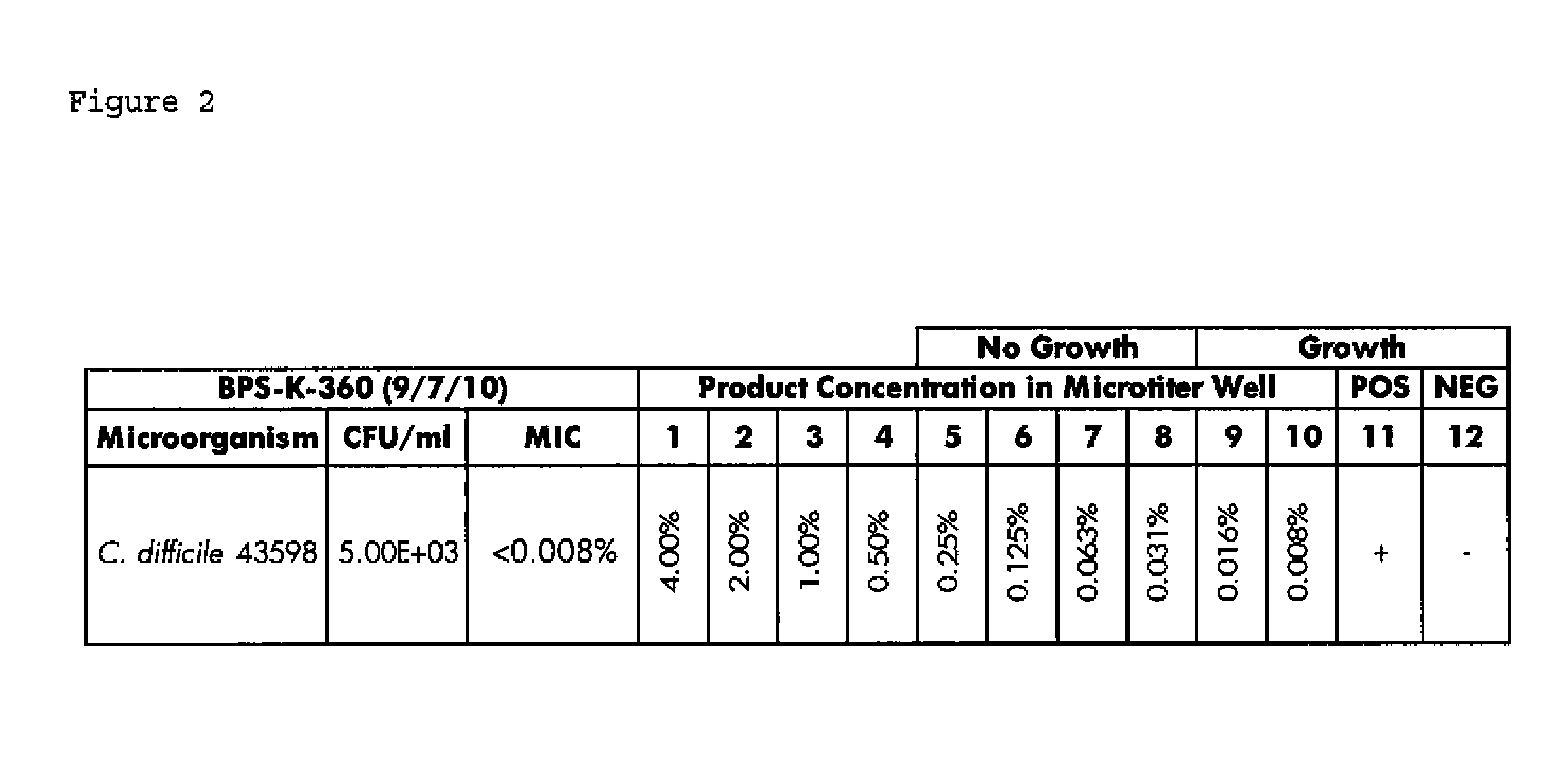
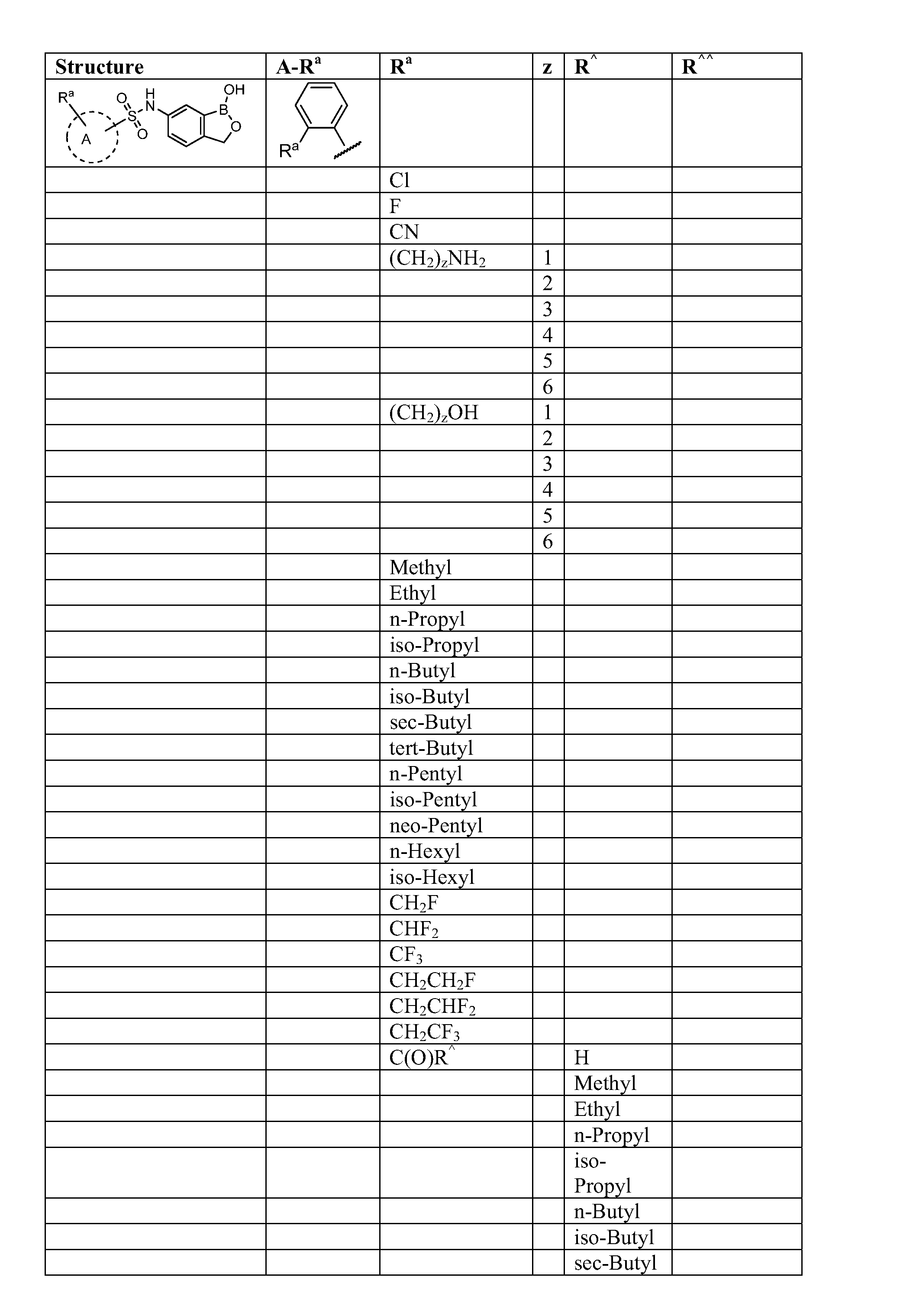
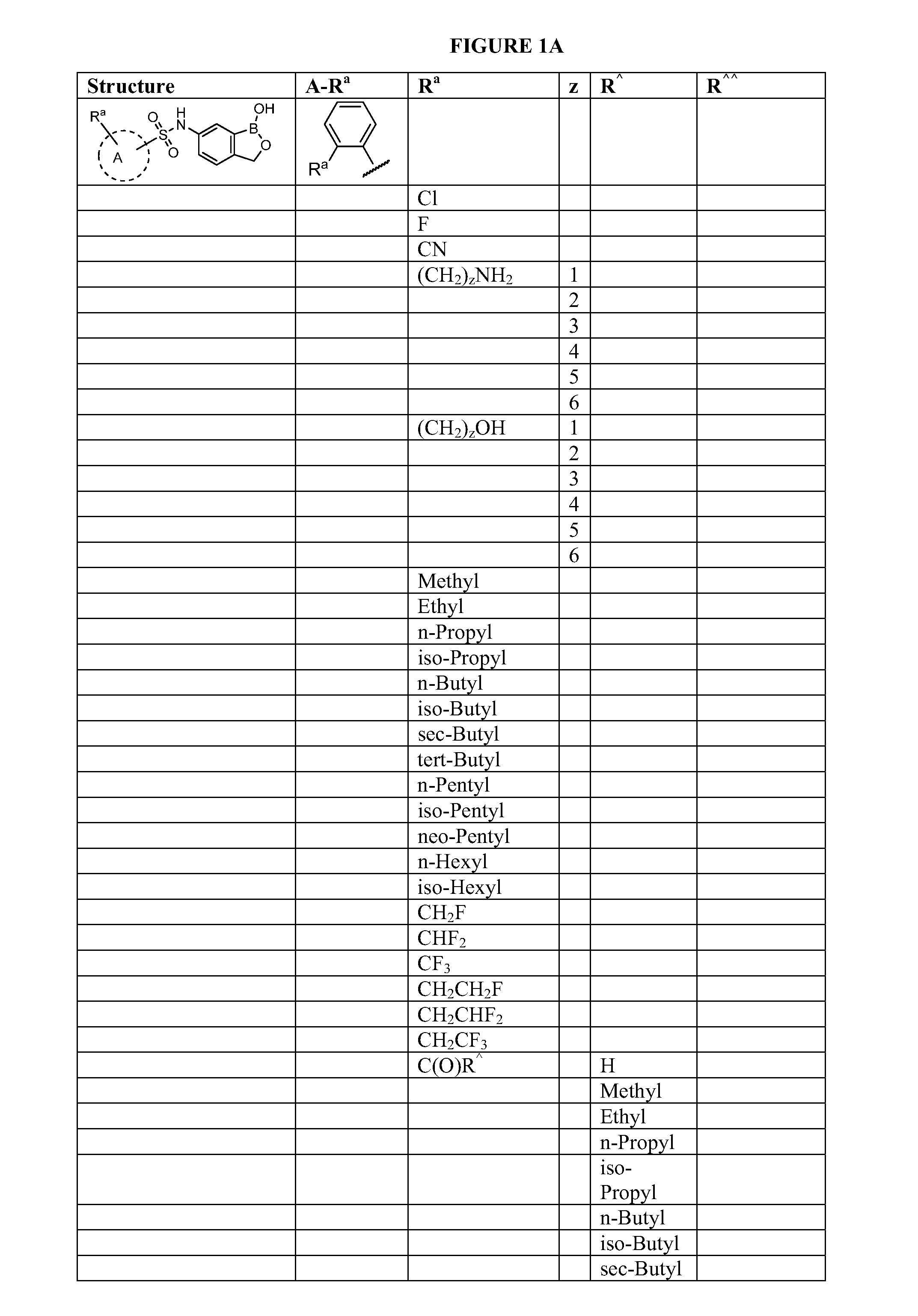
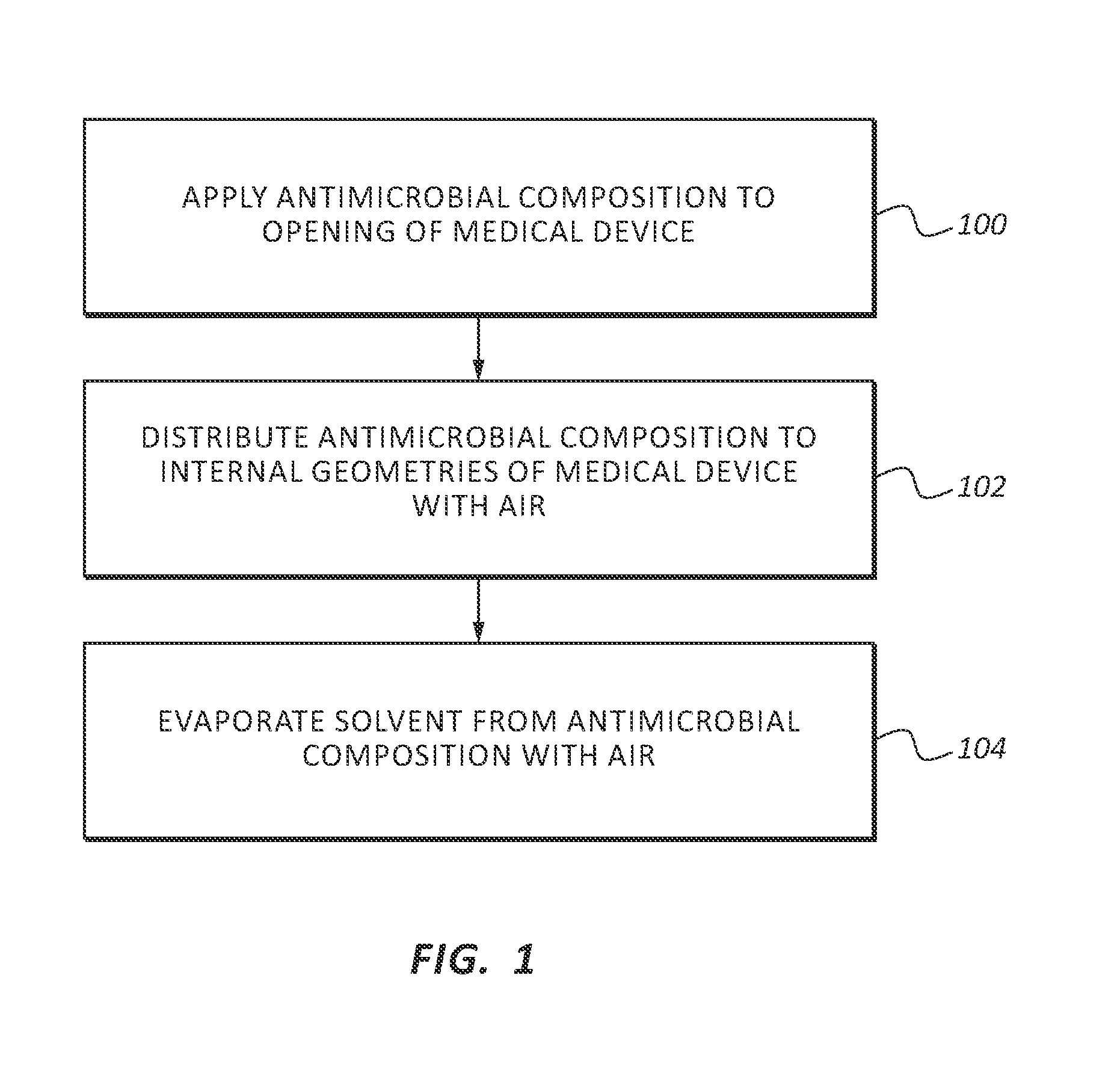
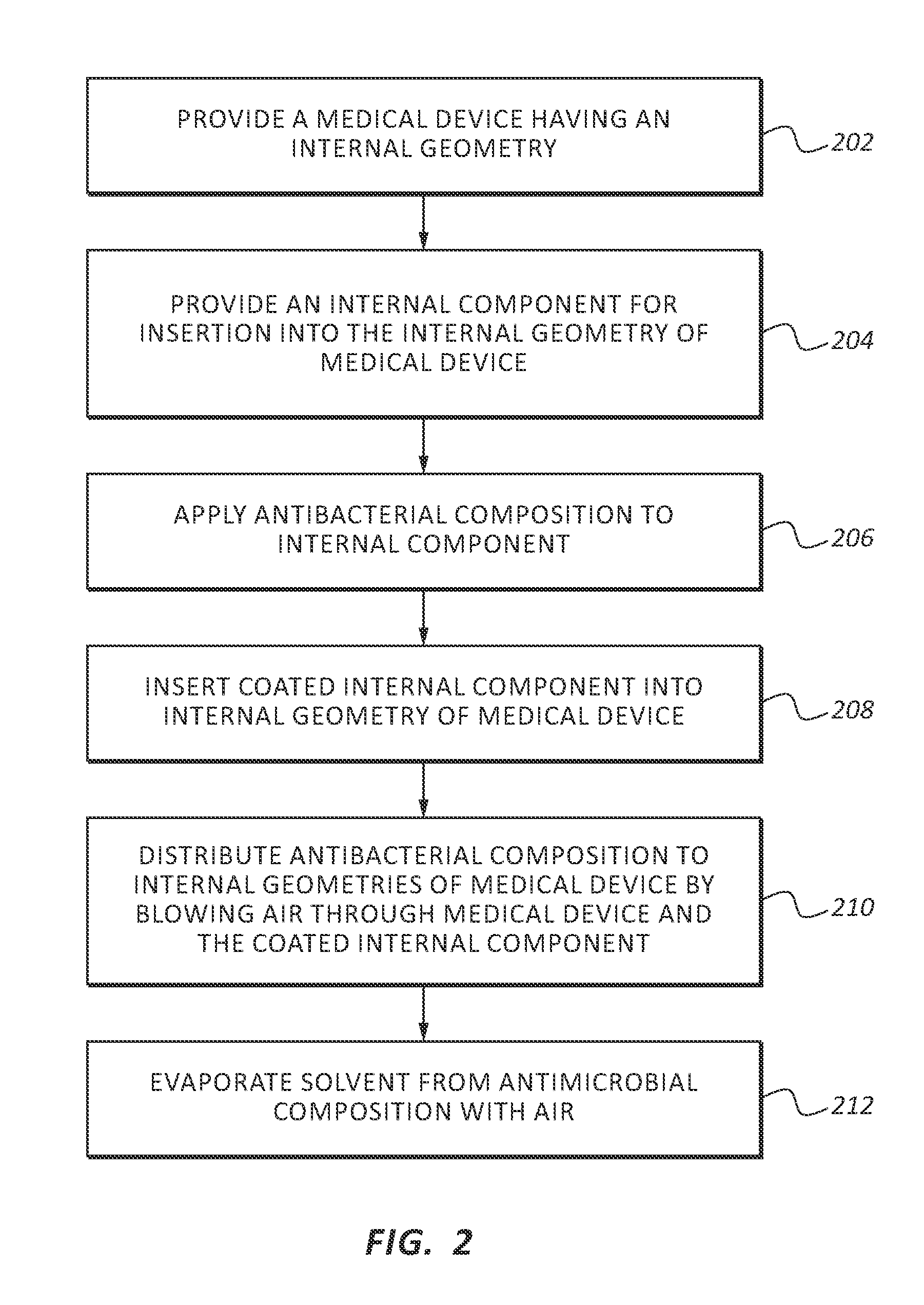
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
353results about How to "Prevent bacterial growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
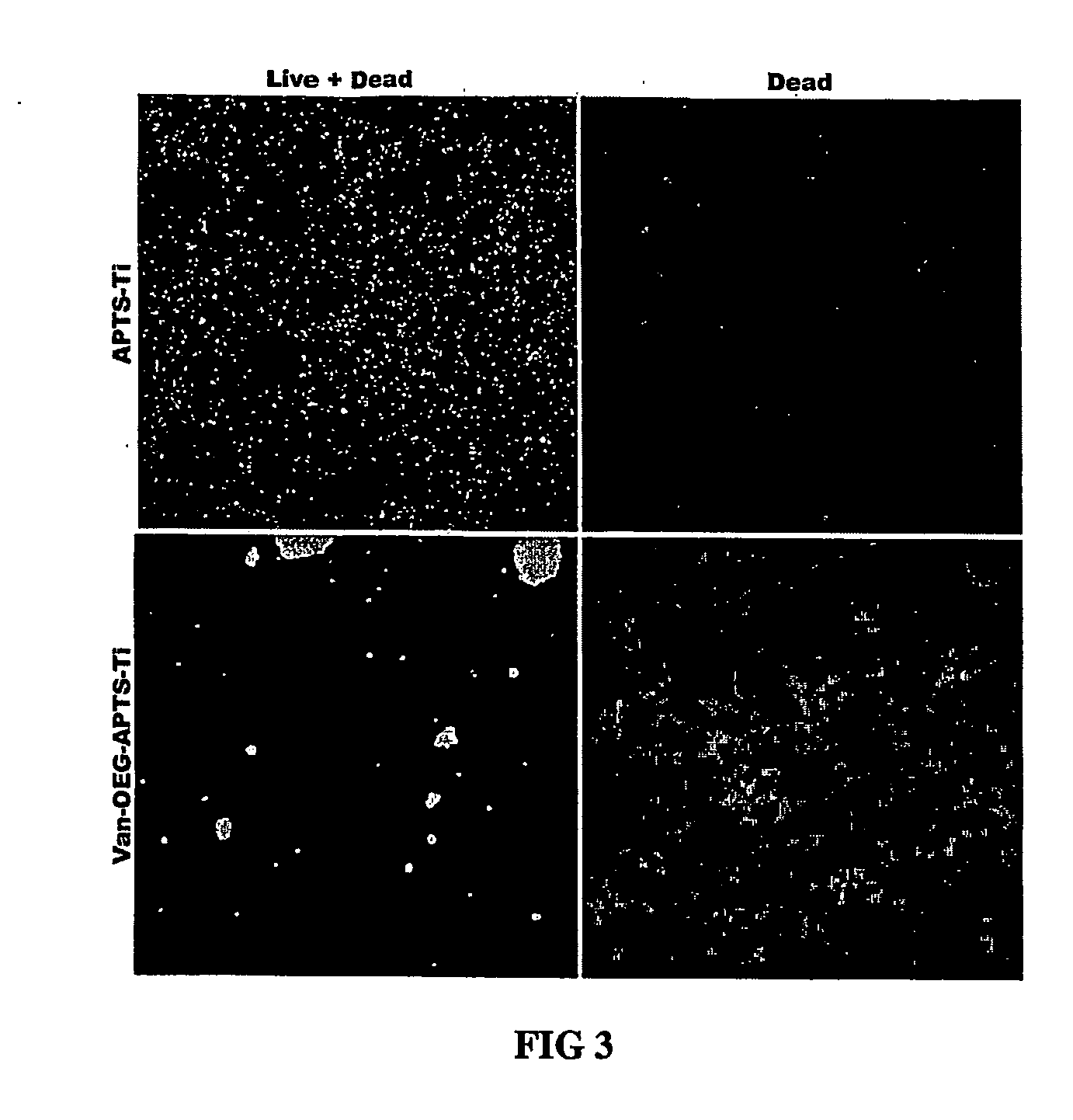
Systems for Efficient Staining and Sorting of Populations of Cells
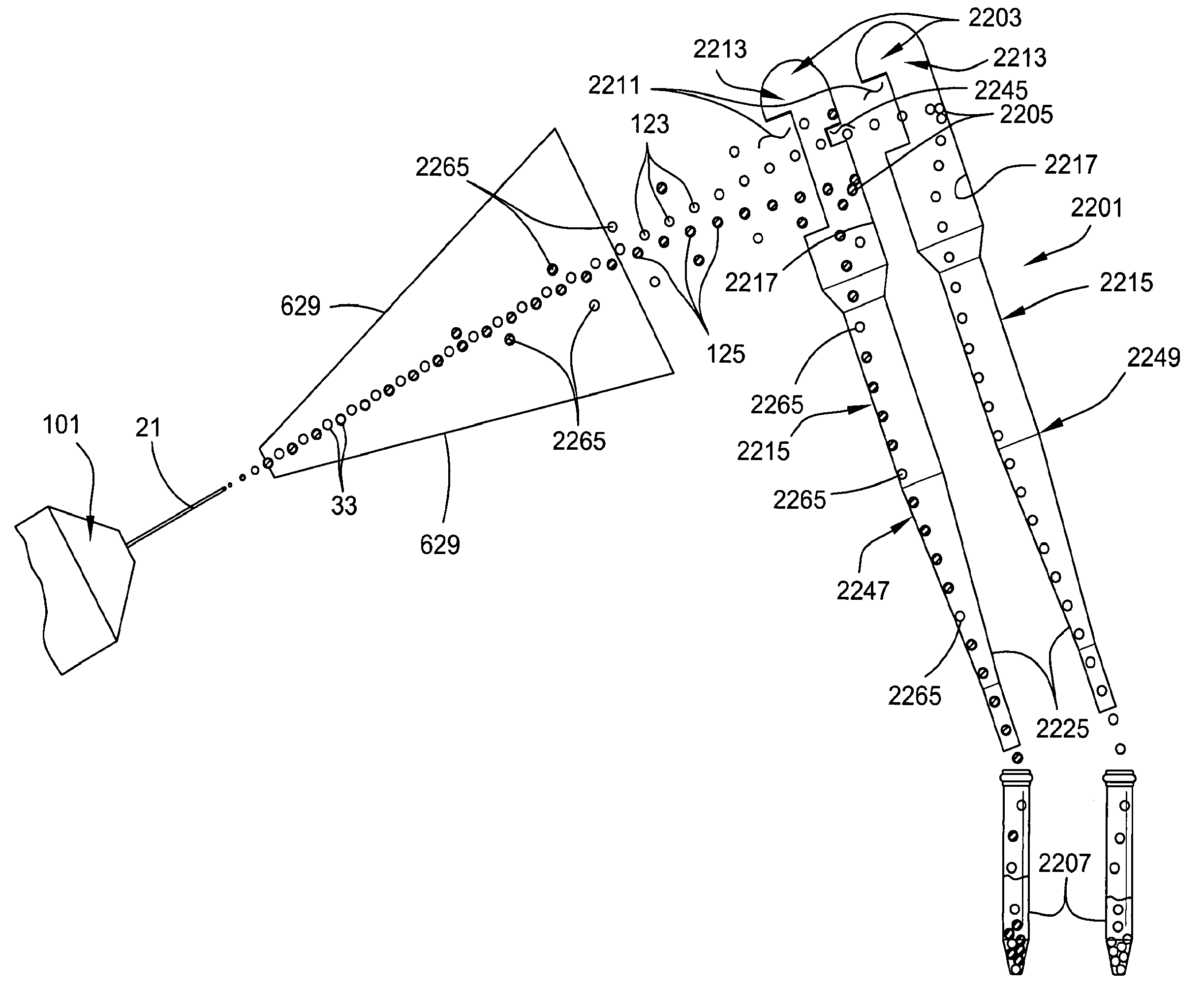
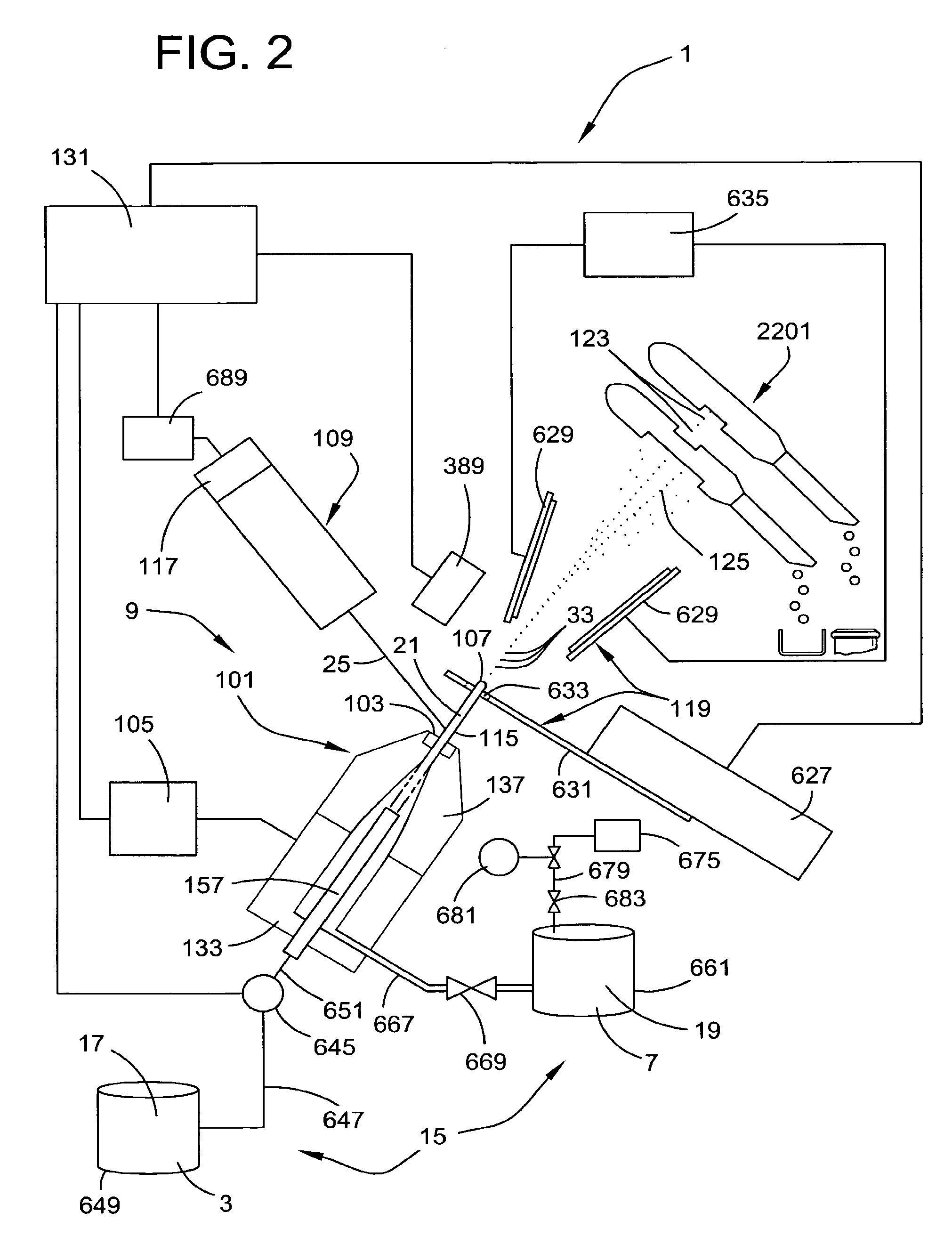
ActiveUS20090176271A1Preventing initiationLow viscosityBioreactor/fermenter combinationsBiological substance pretreatmentsStainingControl cell
A multi-channel apparatus for classifying particles according to one or more particle characteristics. The apparatus comprises a plurality of flow cytometry units, each of which is operable to classify particles in a mixture of particles by interrogating a stream of fluid containing the particles with a beam of electromagnetic radiation. The flow cytometry units share an integrated platform comprising at least one of the following: (1) a common supply of particles; (2) a common housing; (3) a common processor for controlling operation of the units; (4) a common processor for receiving and processing information from the units; and (5) a common fluid delivery system. The integrated platform can include a common source of electromagnetic radiation. A method of the invention comprises using a plurality of flow cytometry units sharing the integrated platform to perform a flow kilometric operation, such as analyzing or sorting particles.
Owner:INGURAN LLC
Composition and Method for Treating Infections and Promoting Intestinal Health
ActiveUS20110200570A1Improve floraPrevent bacterial growthAntibacterial agentsBiocideSuppositoryGlycerol
Compositions and methods for the treatment of intestinal infections. Compositions that include a liquid crystal mixture of an antimicrobial glycerol fatty acid ester and a polyhydric alcohol inhibit the growth of numerous deleterious intestinal pathogenic bacteria, including C. difficile. C. difficile is the causative agent in an increasing number of antibiotic-resistant bacterial infections. The formulations may be administered orally as capsules or soft gels, or alternatively as a enema, colonic, or rectal suppository. When combined with a probiotic supplement, the liquid crystal combinations reported here are able to treat an intestinal bacterial infection effectively and safely, thus promoting general intestinal health.
Owner:COPPERHEAD CHEM
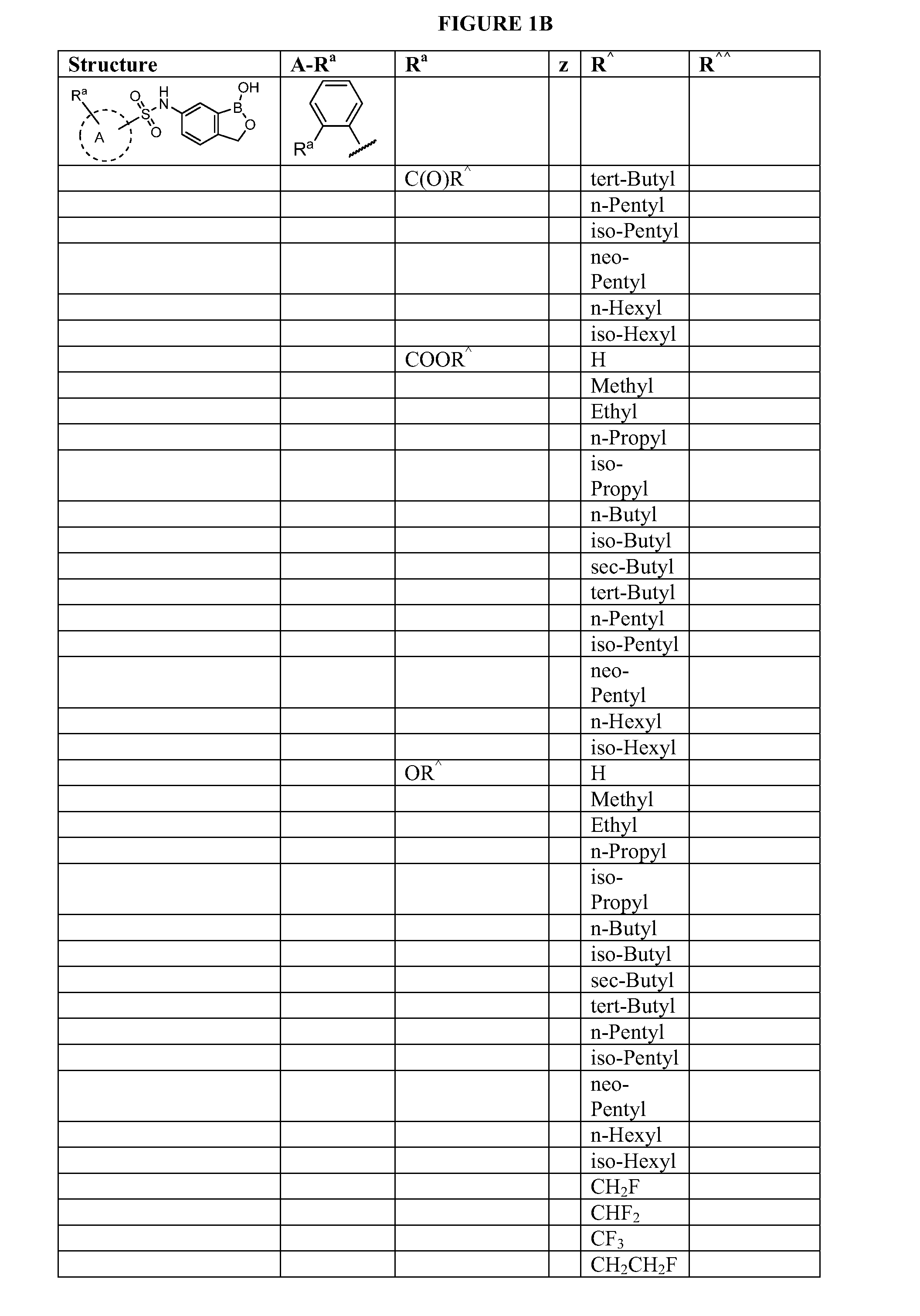
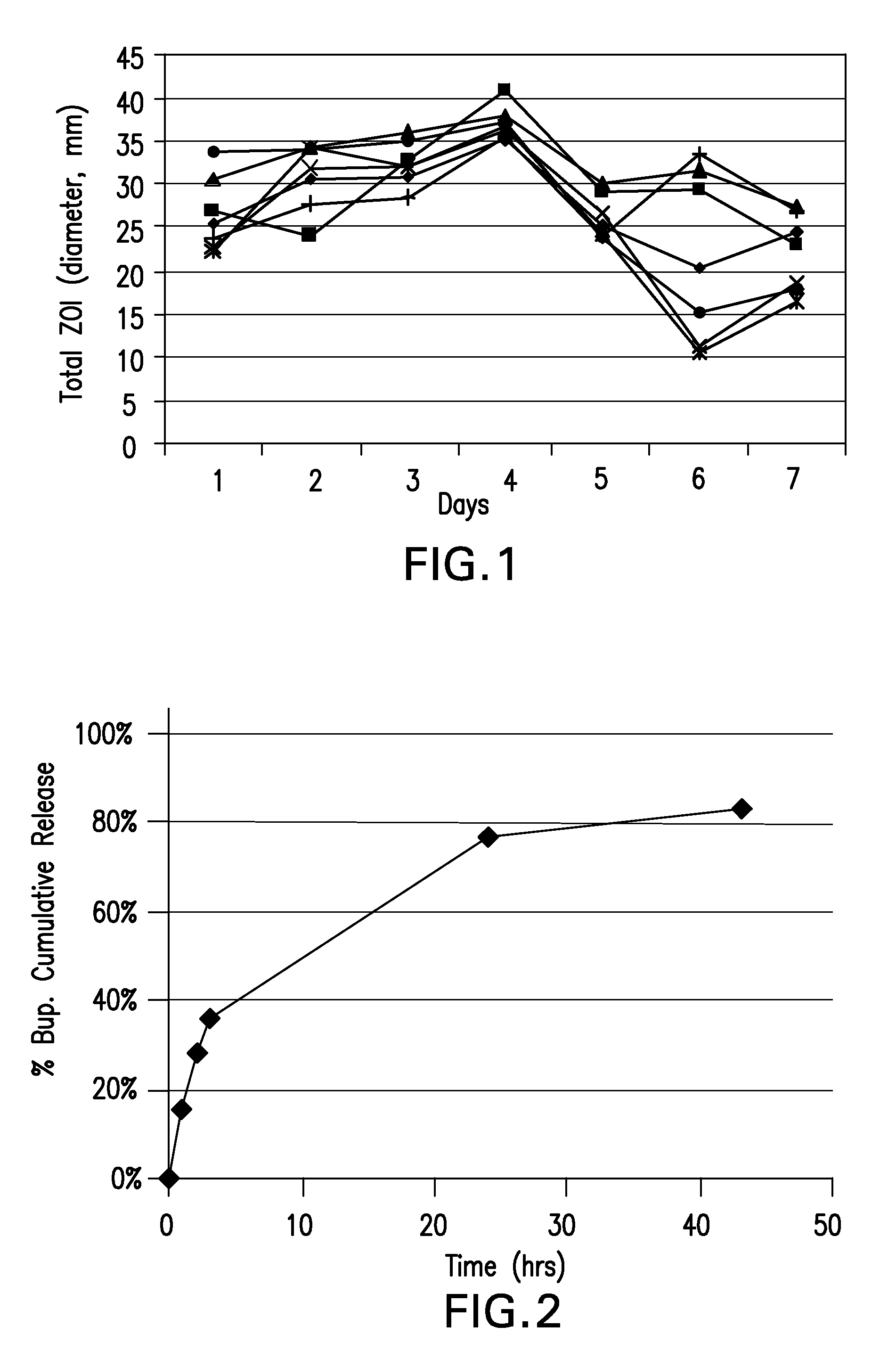
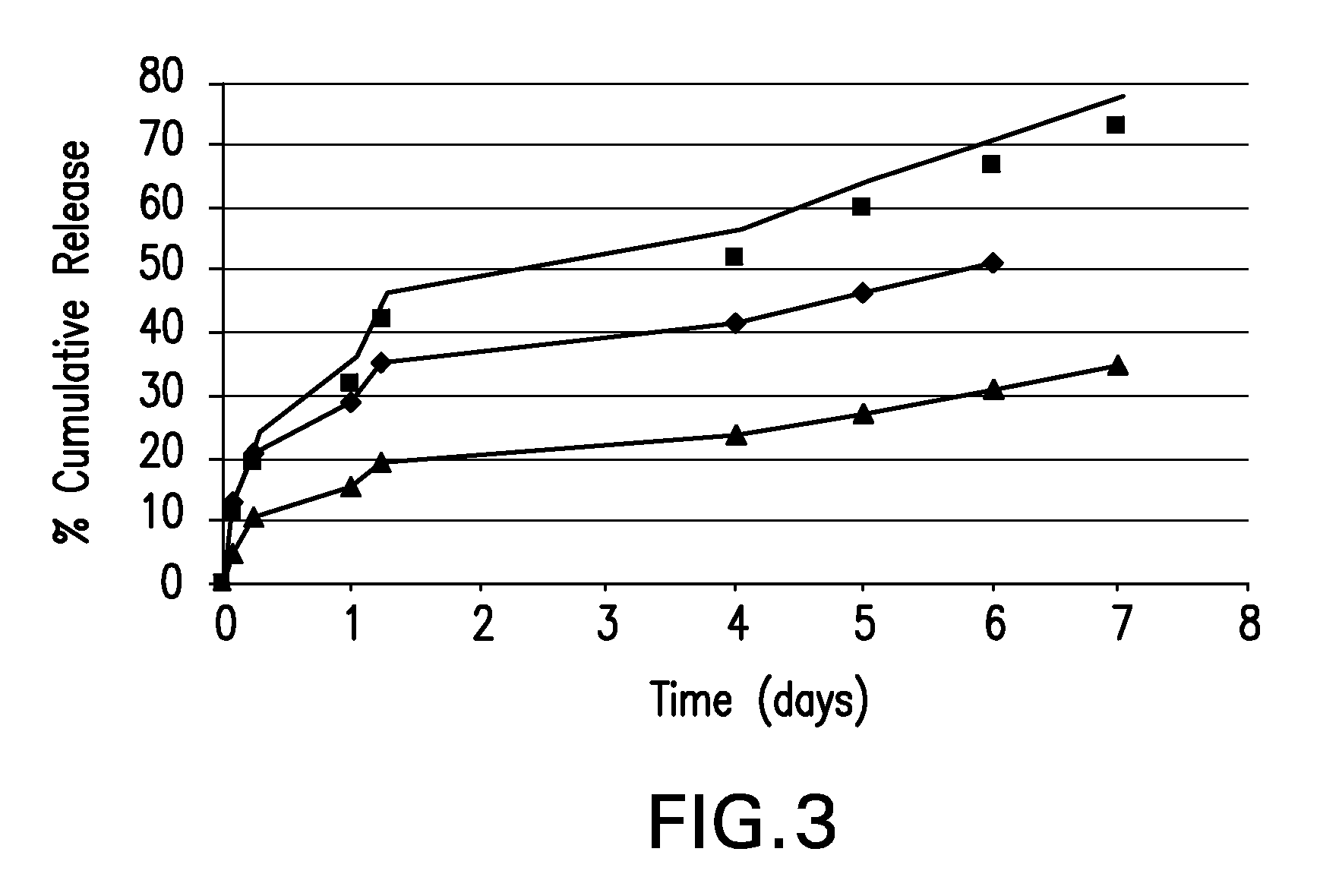
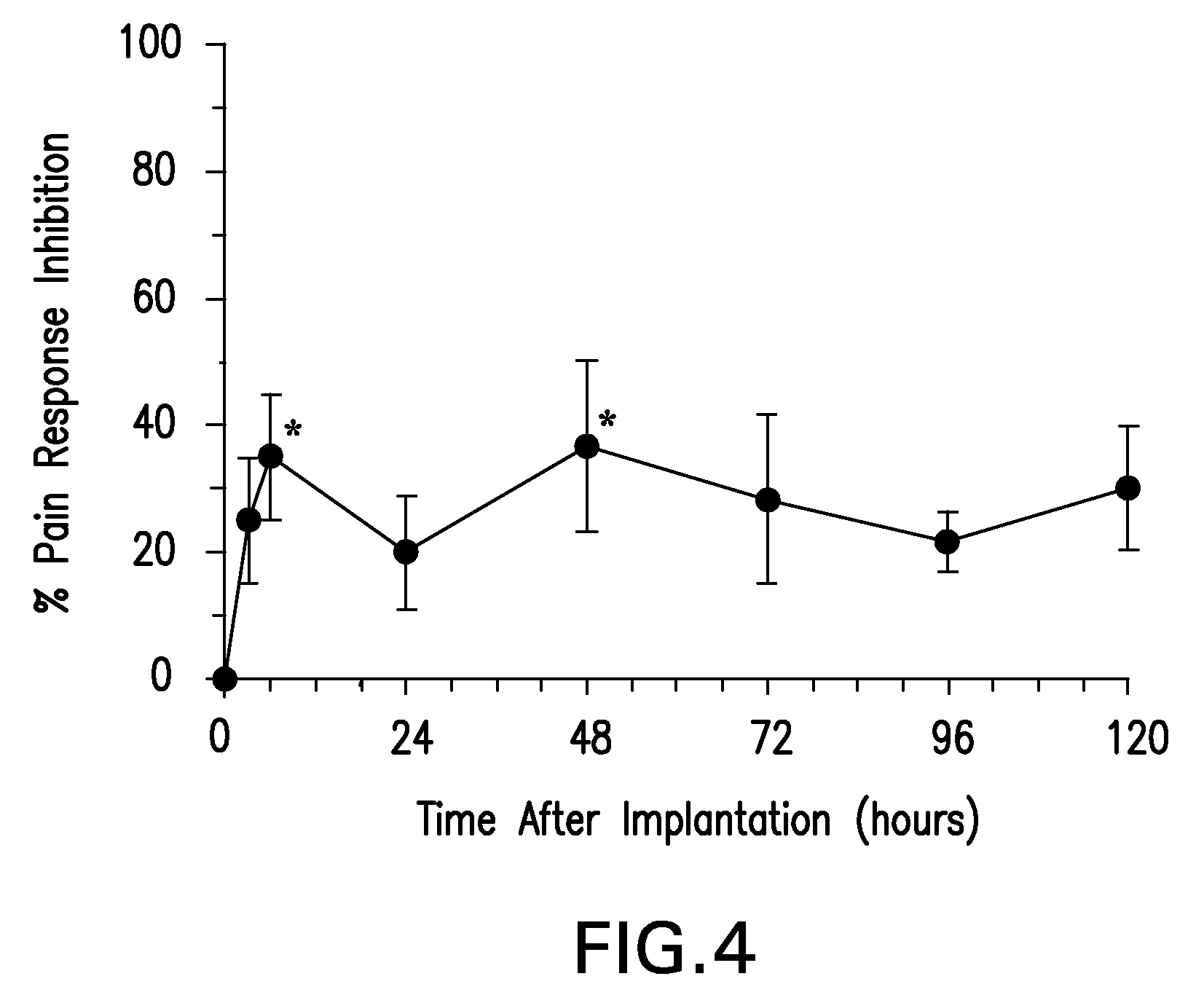
Boron-containing small molecules
InactiveUS20100256092A1Prevent bacterial growthInhibiting β-lactamaseAntibacterial agentsBiocideBoron containingSmall molecule
Owner:ANACOR PHARMA INC +1
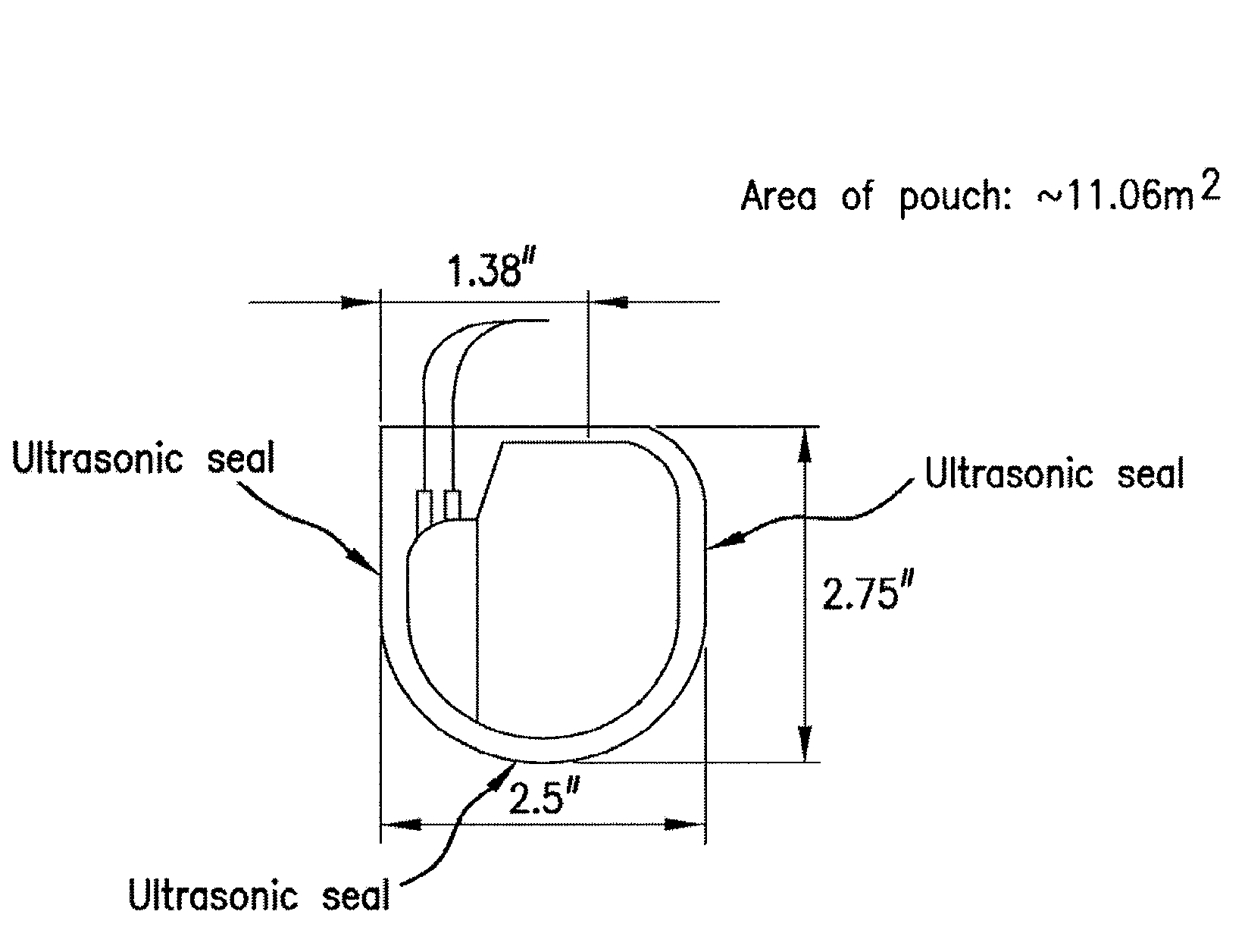
Mesh Pouches for Implantable Medical Devices
ActiveUS20080132922A1Reduces and prevents implantReduces and prevents and surgery-related complicationElectrotherapyDiagnosticsFiberSide effect
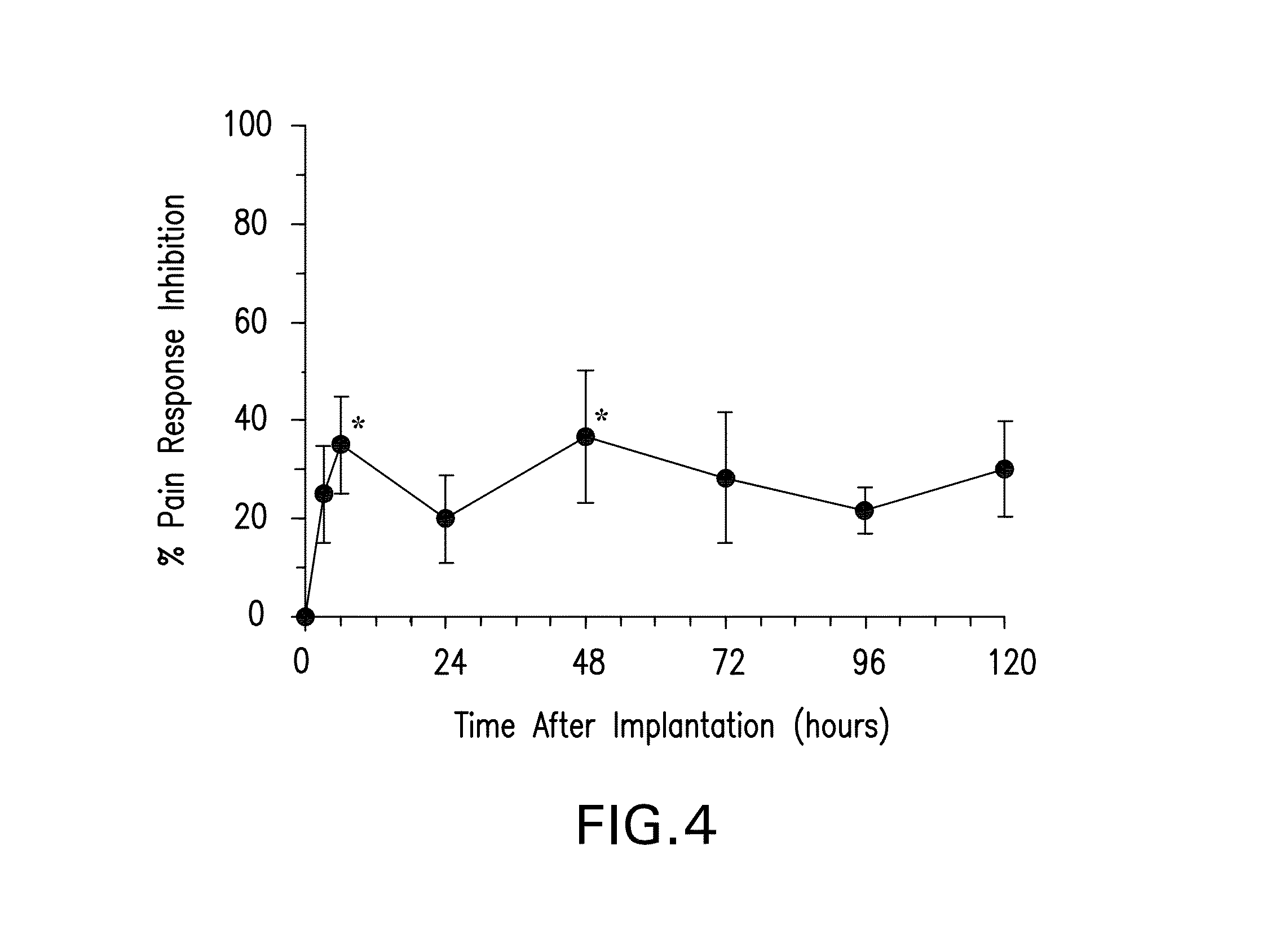
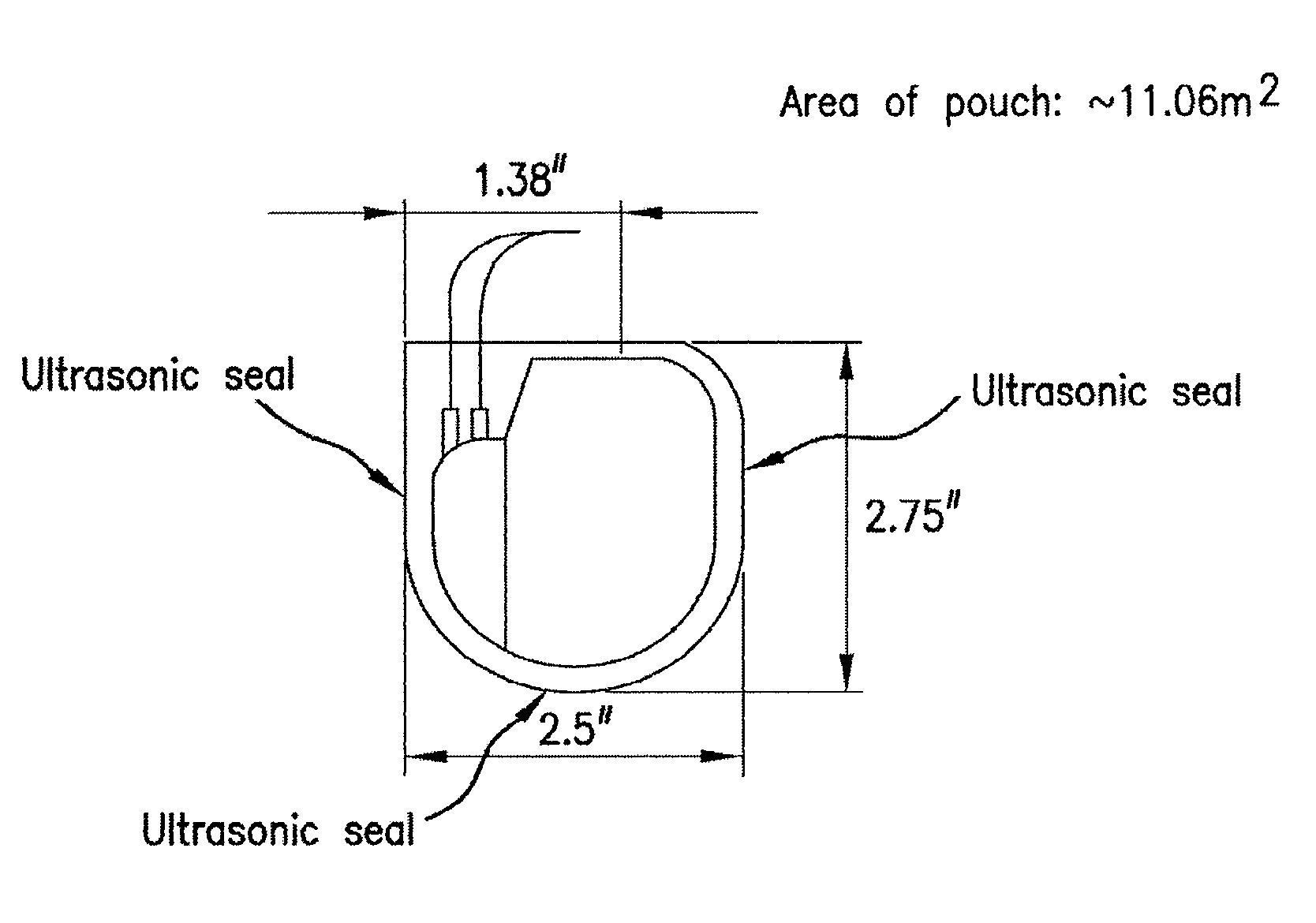
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:MEDTRONIC INC
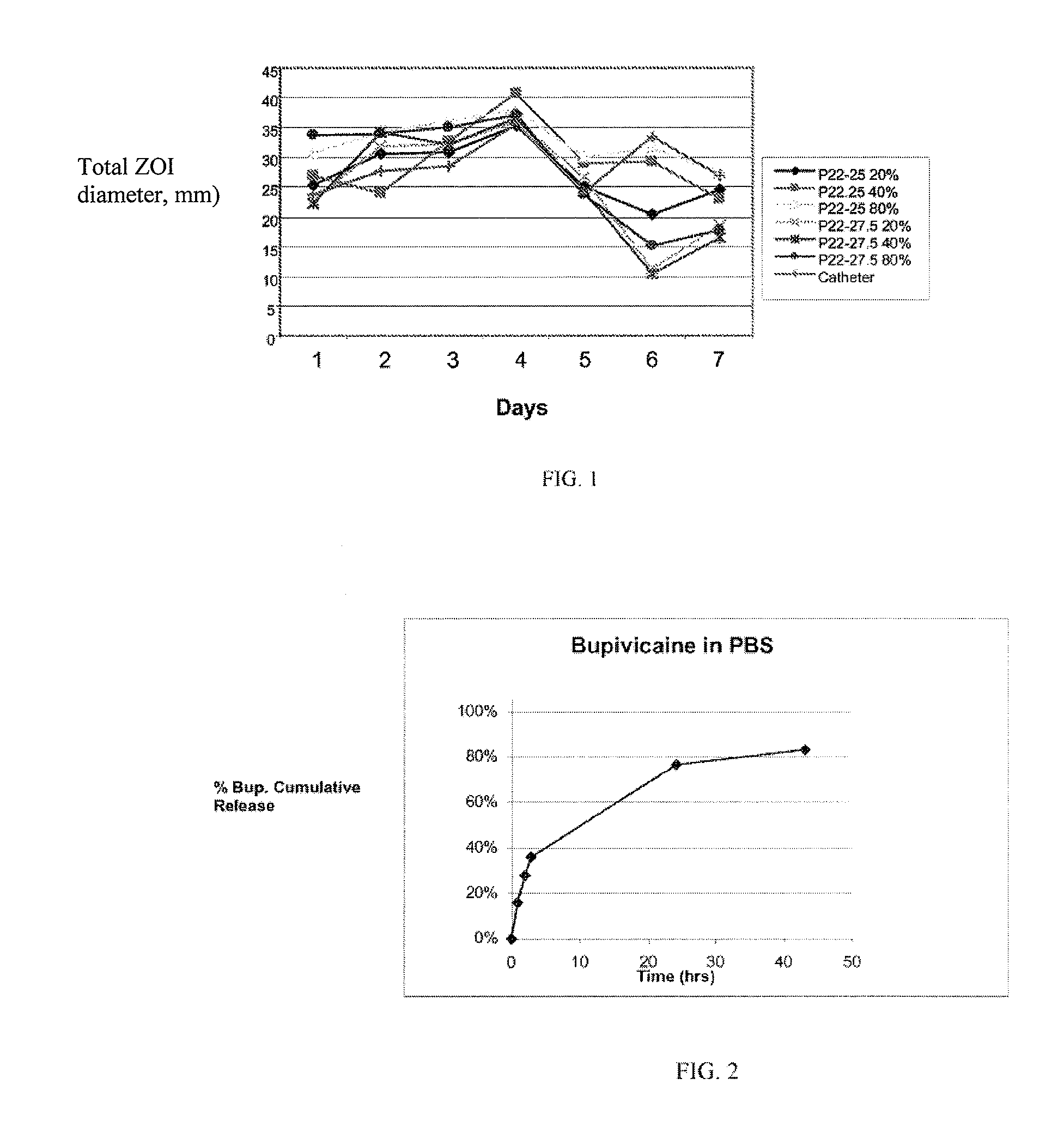
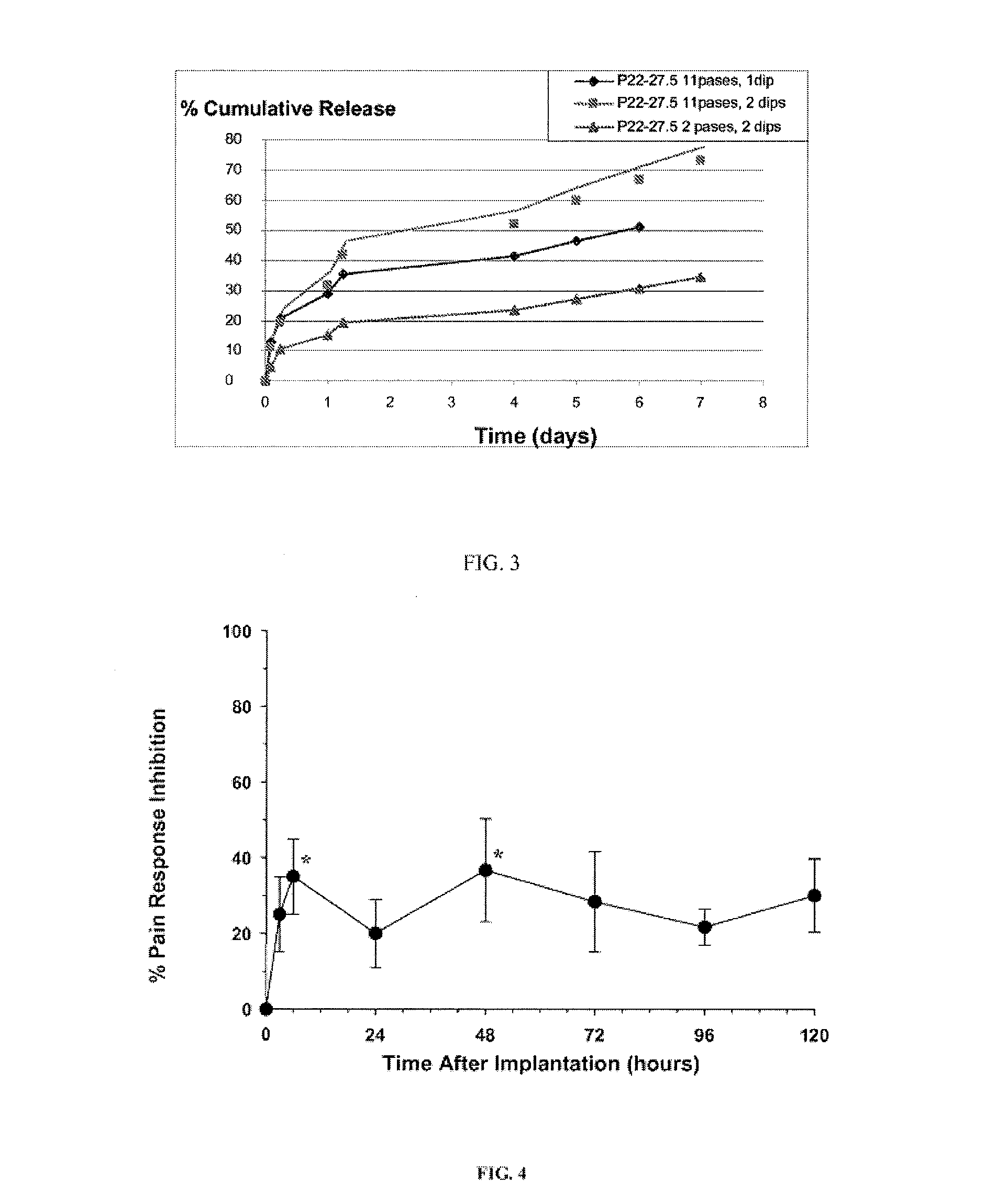
Preventing biofilm formation on implantable medical devices
ActiveUS20100168808A1Reduces and prevents implantReduces and prevents and surgery-related complicationMammary implantsDiagnosticsBiofilmMedical device
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device and preventing or retarding the formation of a biofilm.
Owner:MEDTRONIC INC
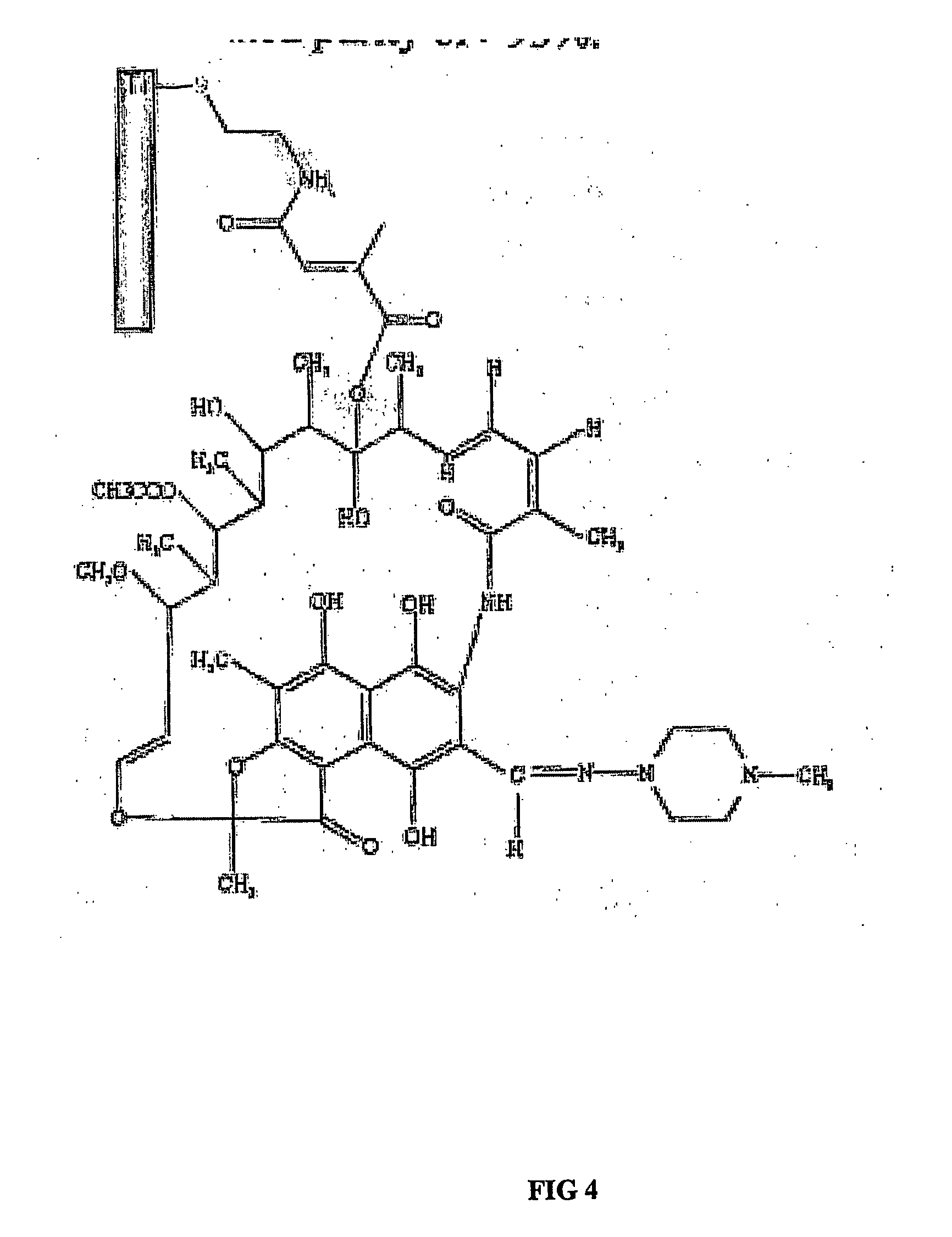
Implants with attached silylated therapeutic agents
InactiveUS20060286140A1Promote osseointegrationPrevent bacterial growthAntibacterial agentsBiocideBiofilmAntibiotic Y
The present invention is directed to implants that include therapeutic molecules bonded to their surfaces. The therapeutic molecules interact with cells that are adjacent, near, or adhering to the implant. The covalently-bonded therapeutic molecules may be released from the implant surface by changes in pH or enzymes characteristic of cells adjacent to the implant. Preferably the covalently-bonded agents include an antibiotics that are released from the implant surface by bacteria and in this way ensures that the antibiotic is released at sites on the implant that would serve as centers for both bacterial colonization and biofilm formation.
Owner:SMART TECH INC (CA)
Antimicrobial suture coating
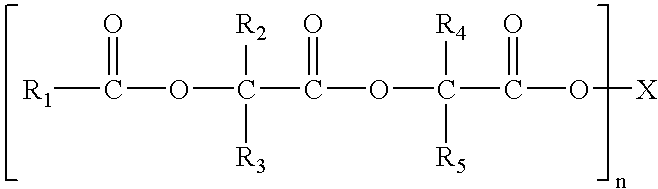
InactiveUS6878757B2Prevent bacterial growthPromote healingSuture equipmentsAntifouling/underwater paintsPolymer chemistryCopolymer
Compositions with antimicrobial properties contain a fatty acid ester salt mixed with a bioabsorbable copolymer. These compositions are useful in forming coatings for surgical articles, including multifilament sutures.
Owner:TYCO HEALTHCARE GRP LP
Sintered porous polymeric caps and vents for components of medical devices
ActiveUS20140188089A1Reduce and eliminate laborLiquid flow quicker and moreInfusion syringesInfusion devicesMedical deviceMaterials science
Owner:POREX CORP
Method of making an antimicrobial sintered porous plastic filter
InactiveUS6849214B2More hygienic conditionReduce concentrationUltrafiltrationTreatment involving filtrationThermoplastic
An improved sintered porous thermoplastic filter incorporating a second thermoplastic treated with a non-leaching antimicrobial agent for the purification of liquids. The method of making the filter is also disclosed.
Owner:MICROBAN PROD CO INC
Water reservoir pressure vessel
ActiveUS20080156015A1Fatigue failurePrevent bacterial growthOpening closed containersBottle/container closureHydrostatic pressureEngineering
Refrigeration devices that have a water dispensing system to dispense water to an exterior water dispenser from a water supply line have need of a water vessel for storing water under pressure within the refrigeration device until dispensed. The water vessel has an inlet for receiving water from the water supply line and an outlet for dispensing water to the exterior water dispenser. A plurality of pressure resistant walls are formed between the inlet and the outlet to form an internal volume to reservoir water within the water vessel. The water vessel is constructed to reservoir water within the vessel under hydrostatic pressure from the water supply line until dispensed through the outlet when requested by the exterior water dispenser and / or the icemaker.
Owner:WHIRLPOOL CORP
Apparatus and method for producing fuel ethanol from biomass
InactiveUS20080020437A1Improve performanceEliminate needBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismVegetable oil
Cellulosic biomass at the growing site is pulverized and hydrolyzed in collectors which transfer the comminuted biomass into pretreatment units. In the pretreatment units enzymes and / or other agents are added to the pretreatment units to disassociate the lignin from the cellulose and hemicellulose, and to further decompose the biomass to simple sugars. The sugar solution is transferred to a fermentation unit and fermenting microorganisms are added to produce ethanol. Preferably vegetable oil or other ethanol-miscible liquid is added to provide a medium to extract the ethanol from the solution for transfer to a device where heating or distillation removes the ethanol which is collected for use as a liquid fuel. All of the units are preferably mounted for easy deployment to various locations in the vicinity of the numerous growing sites which produce the biomass.
Owner:SAVARESE JOHN J
Mesh pouches for implantable medical devices
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:MEDTRONIC INC
Preventing biofilm formation on implantable medical devices
Owner:MEDTRONIC INC
Treatment of perishable products using aqueous chemical composition
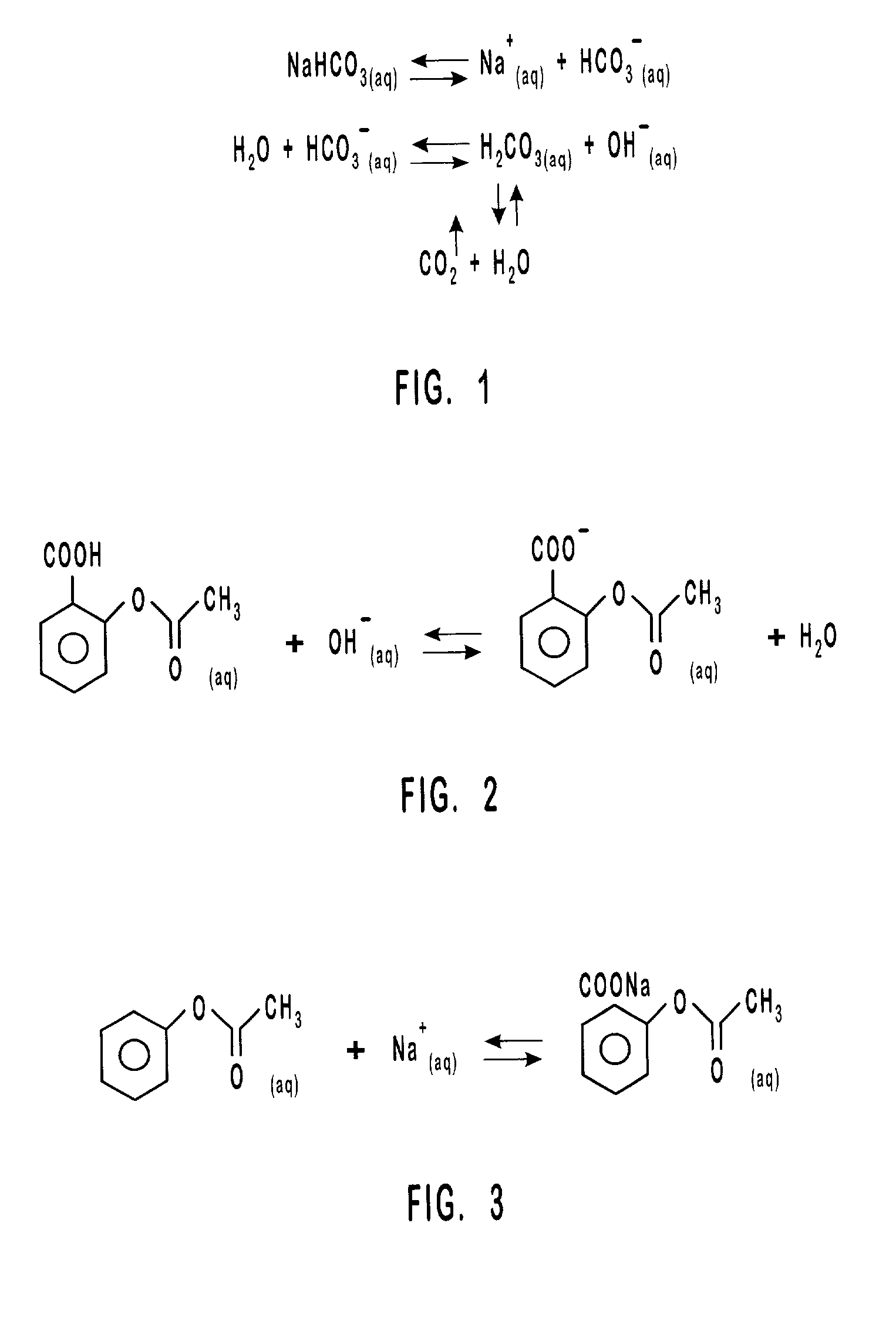
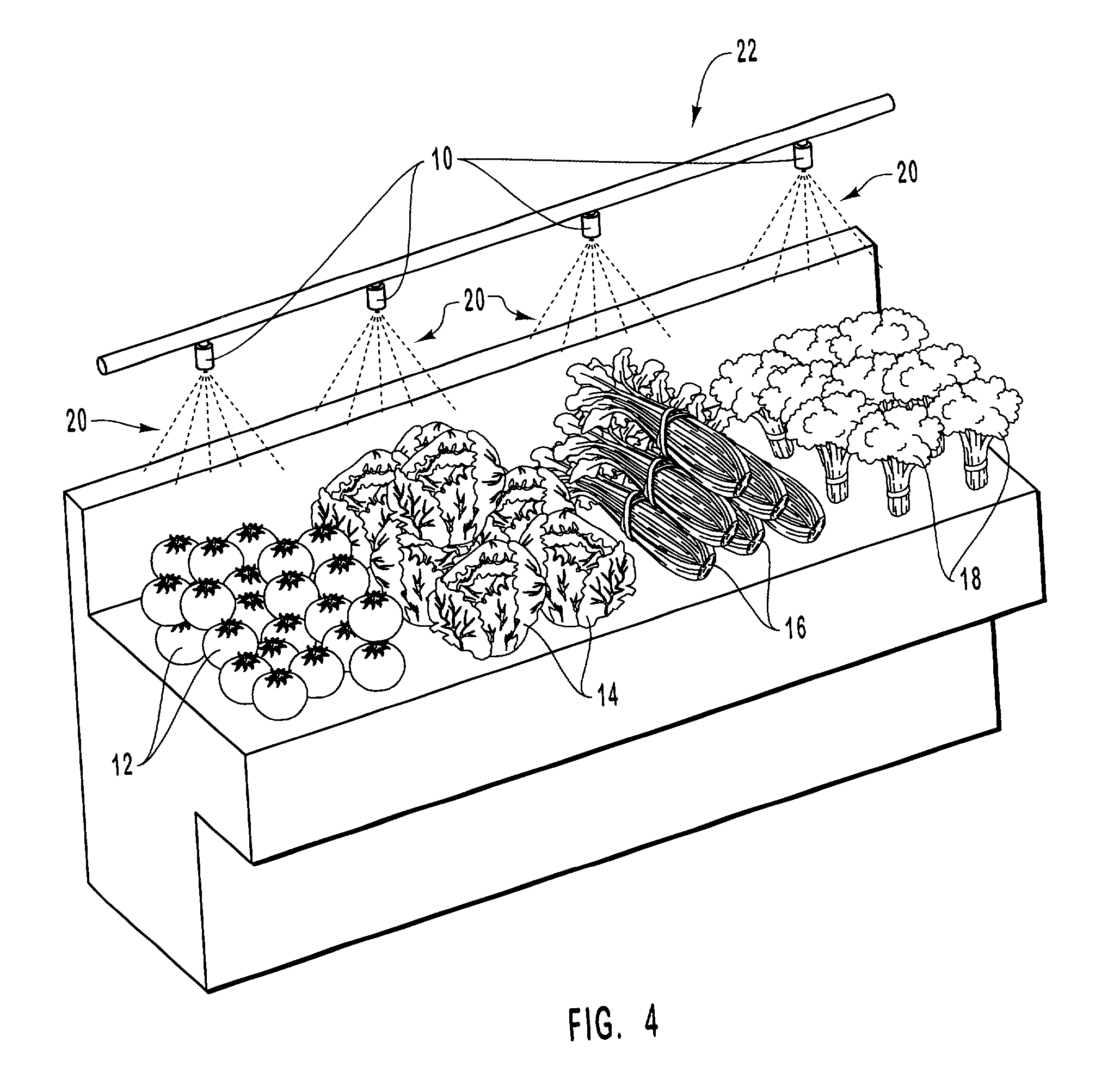
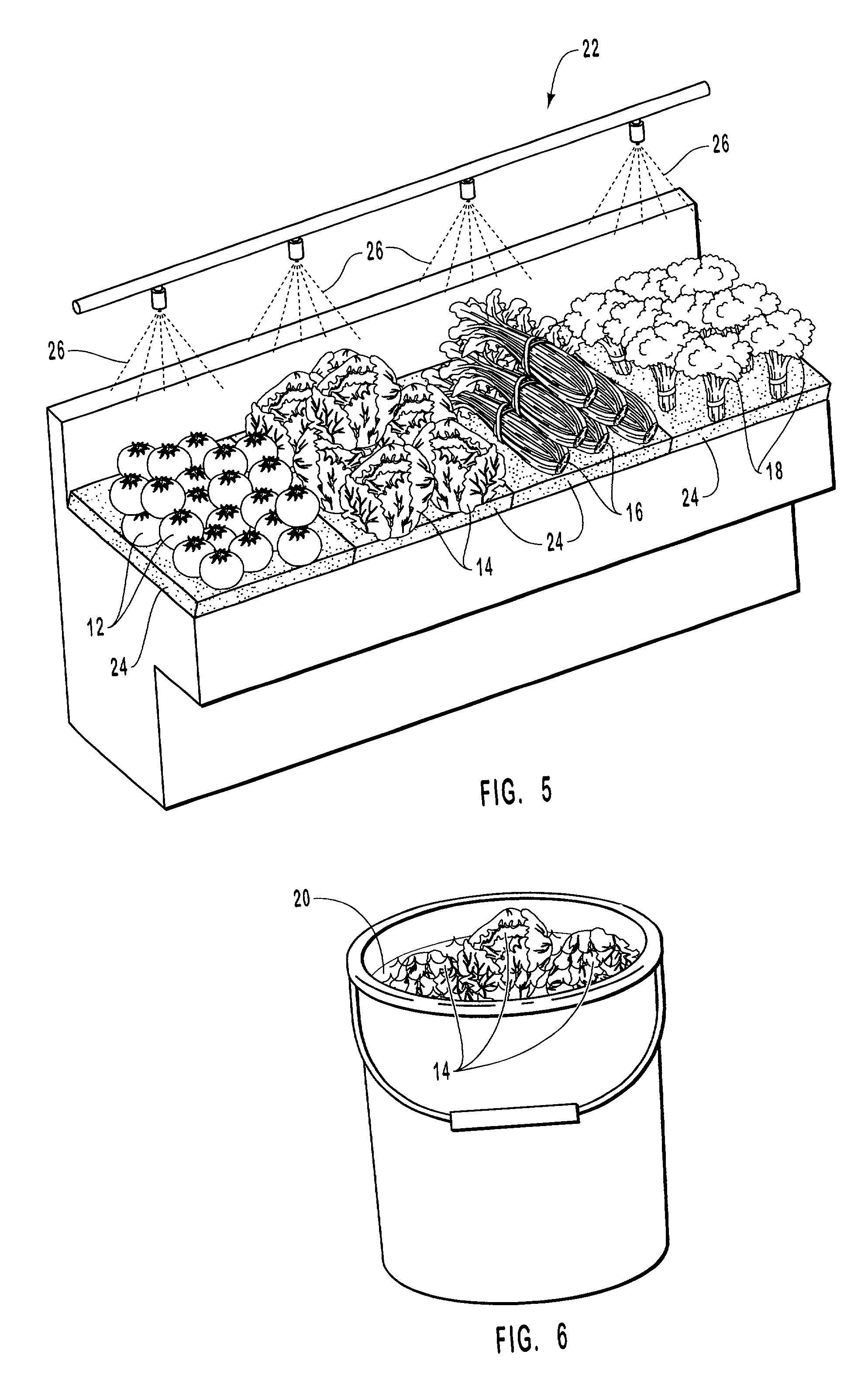
InactiveUS20010031298A1Prevent spoilagePrevent bacterial growthLighting and heating apparatusFood preservationSodium bicarbonateChemical composition
Aqueous compositions formed from combining water, and at least one solute such as sodium bicarbonate, acetylsalicylic acid or mixtures thereof, are used to introduce and maintain relatively high levels of carbon dioxide in the atmosphere of selected environments. The aqueous compositions benefit live plants and prolong the shelf life of various perishable foods and products, including vegetables, fruits, meats, fish, seafood, dairy products, and dry goods and ingredients. The aqueous compositions are applied directly by bathing or showering a perishable product in the aqueous compositions, or indirectly with absorption devices that carry the aqueous compositions, and which are placed in close proximity to a perishable product. An aqueous composition can be applied to or formed directly on an absorption device. An absorption device can also be recharged by reapplying or reforming the aqueous composition to the absorption device as needed.
Owner:CO2 TECH INC
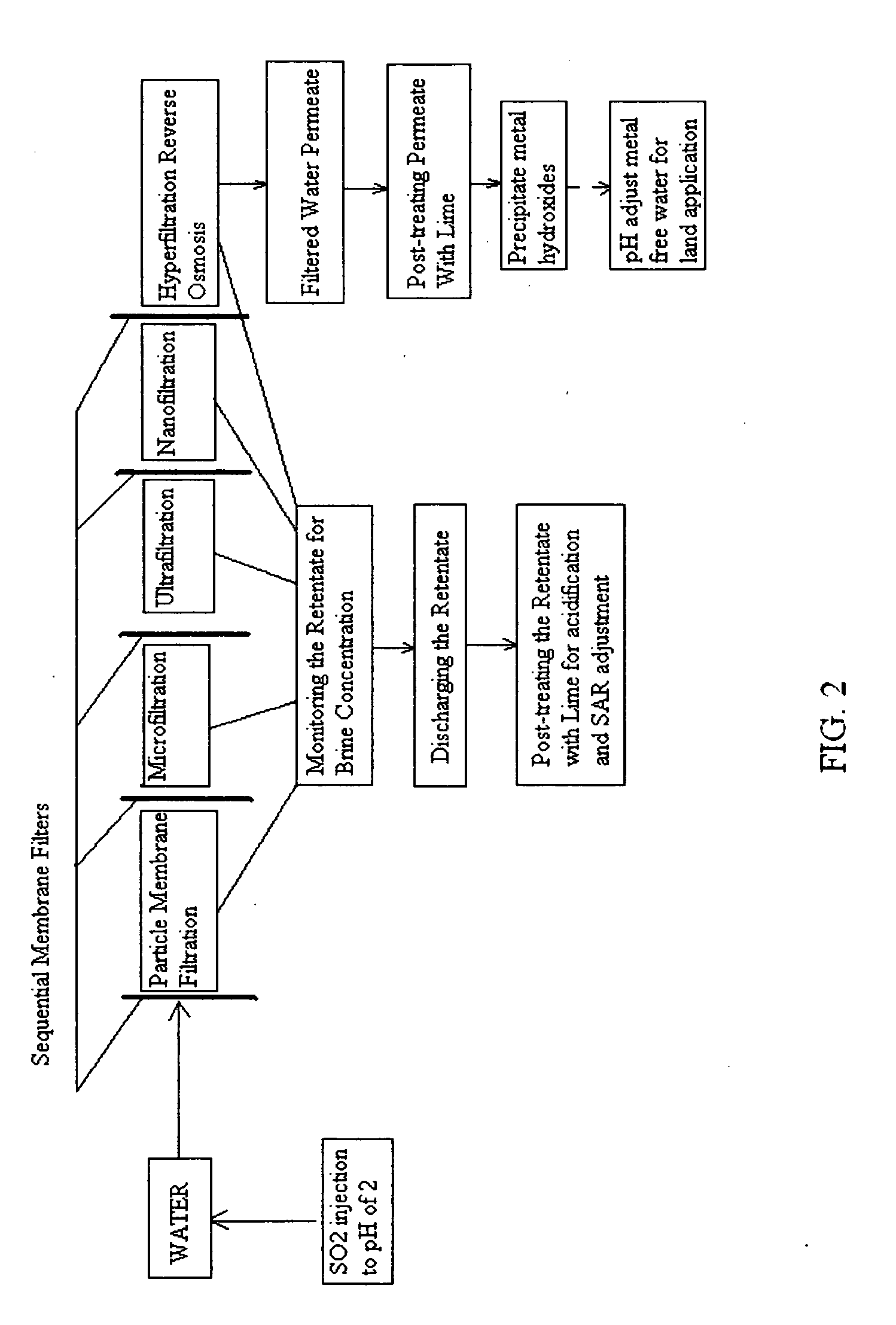
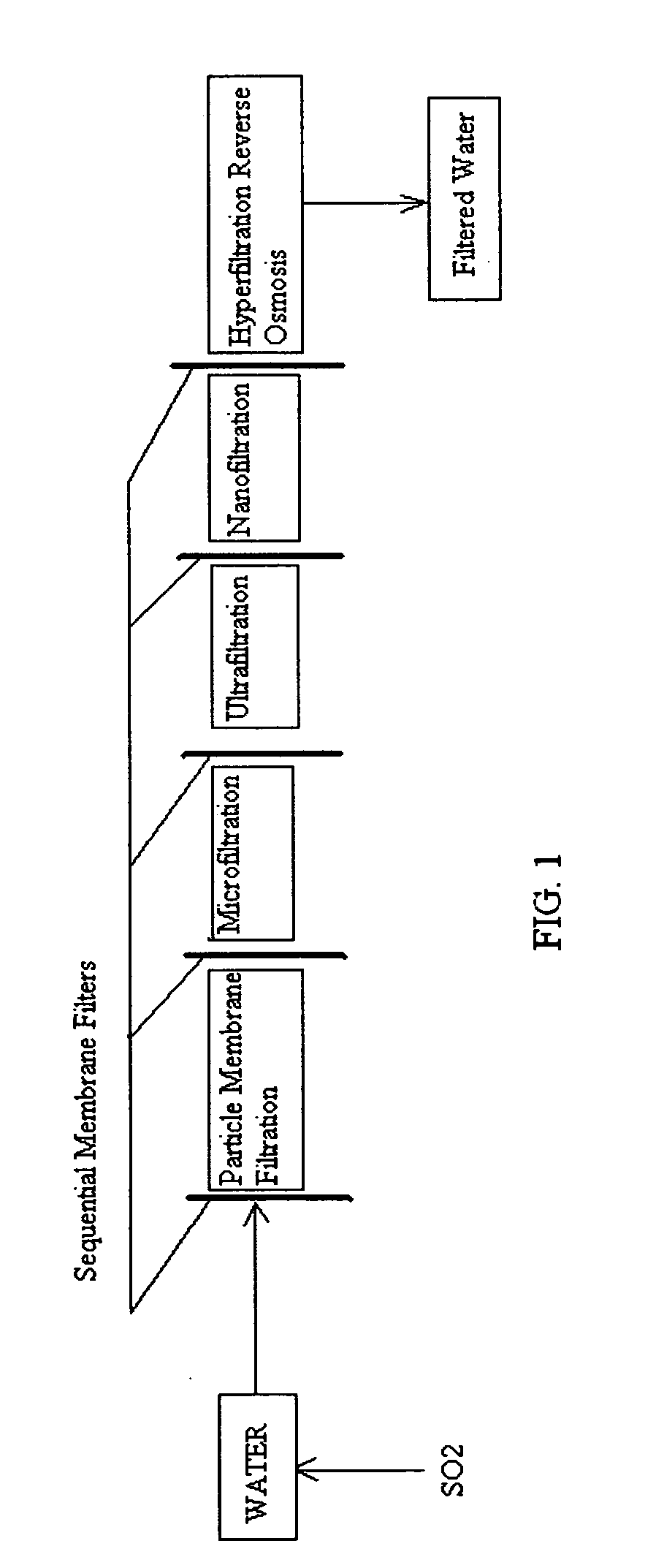
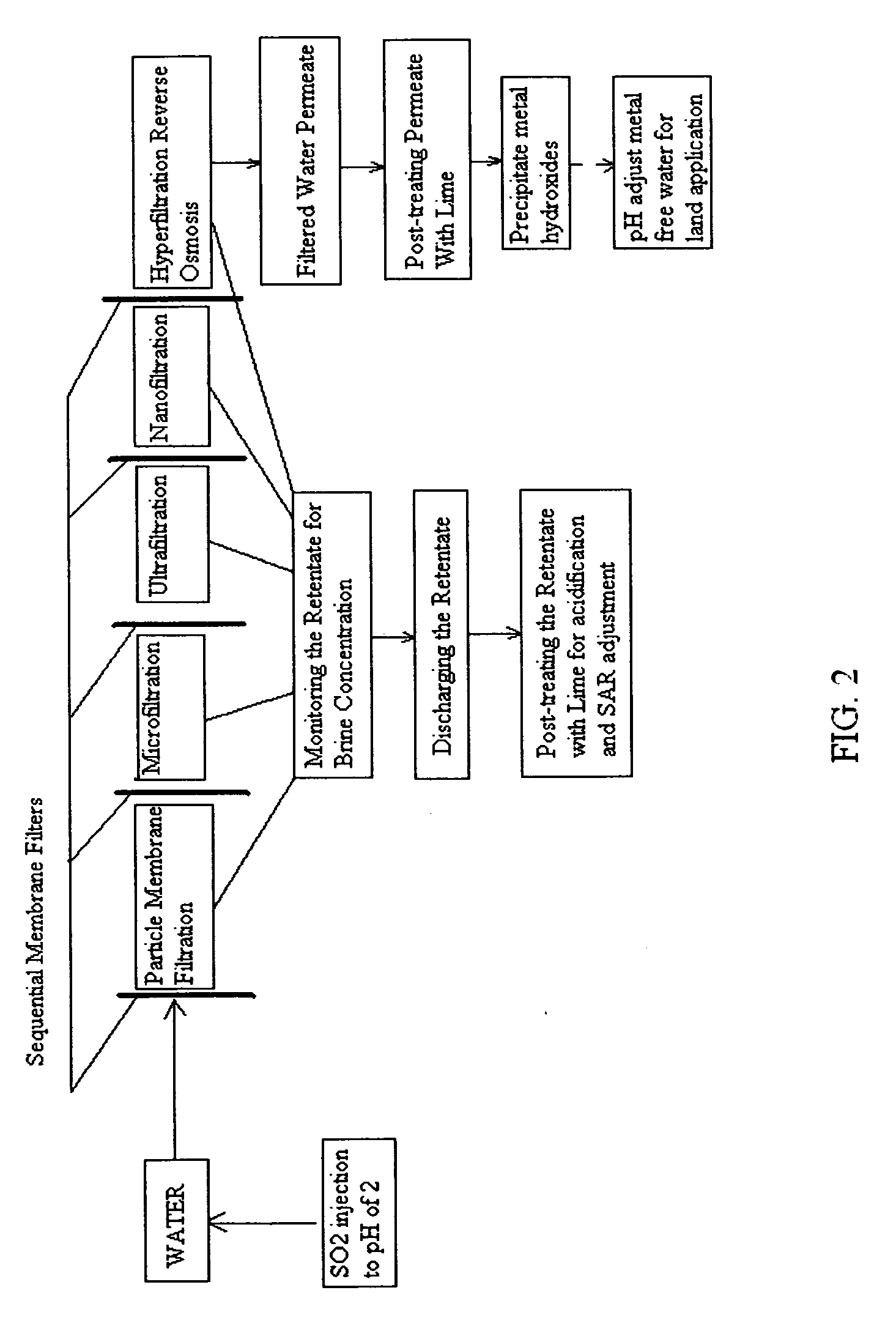
Pre-treatment reverse osmosis water recovery method for brine retentate metals removal
ActiveUS20100193436A1Minimize adhesionPrevent bacterial growthMembranesOther chemical processesRecovery methodPretreatment method
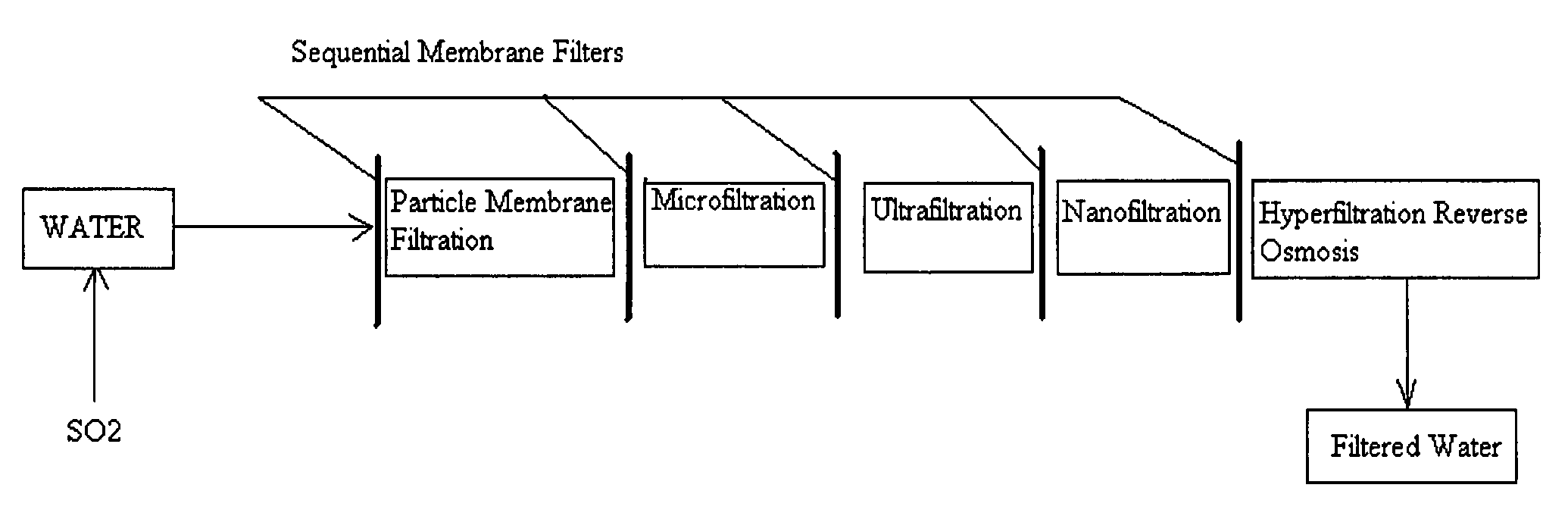
A pre-treatment method for cleaning and maintaining reverse osmosis membrane filters by injecting sulfurous acid into waters with suspended solids in a liquid fraction to be filtered to form sulfurous acid (H2SO3) to acid leach heavy metals into the liquid fraction, reduce alkalinity and mineral scaling, add sufficient SO2 as a biocide to attack bacteria and other micro organisms to prevent membrane fouling, reduce iron to prevent iron deposit build-up, scavenge and remove dissolved oxygen prior to filtration to prevent membrane oxidation, and then sequentially filtering the acidified water through membrane filters to create a metal free permeate and a brine retentate, which can be pH adjusted to remove the heavy metals as metal hydroxide precipitates.
Owner:EARTH RENAISSANCE TECH
Quick-connect coupler
InactiveUS20130181446A1Prevent bacterial growthEnsure hygieneSleeve/socket jointsFluid pressure sealed jointsEngineeringMechanical engineering
Owner:COMAP
Method of preparing blueberry leaf tea beverage
InactiveCN101129152APrevent rustPrevent bacterial growthTea substituesFood preparationNervous systemProanthocyanidin
The invention relates to a blueberry leaves drink with health function based on the blueberry leaves as main material and its making method, wherein the blueberry leaves contents a great amount of flavonoid with more than 40 physiologically activities of reducing blood sugar and blood fat and resisting arrhythmia, the resisting free radical oxidation ability of the blueberry is not compared with the other anti-oxidizing agent, meanwhile there is great amount proanthocyanidins contented in the blueberry with very good health function to the nervous system for children especially for the pregnant woman conspicuously. The making method is characterized by the following: picking the blueberry leaves with more than 90 days growth; using the technique process of washing, immersing, quick freezing, disintegrating, chilling, extracting with vacuum low-temperature, freezing, filtering, allocating, flash sterilizing and germ-free canned. The invention guarantees not to damage the nutrition and effective and active substances in the material almost, which makes the product have certain health function.
Owner:LIAONING TODAY AGRI
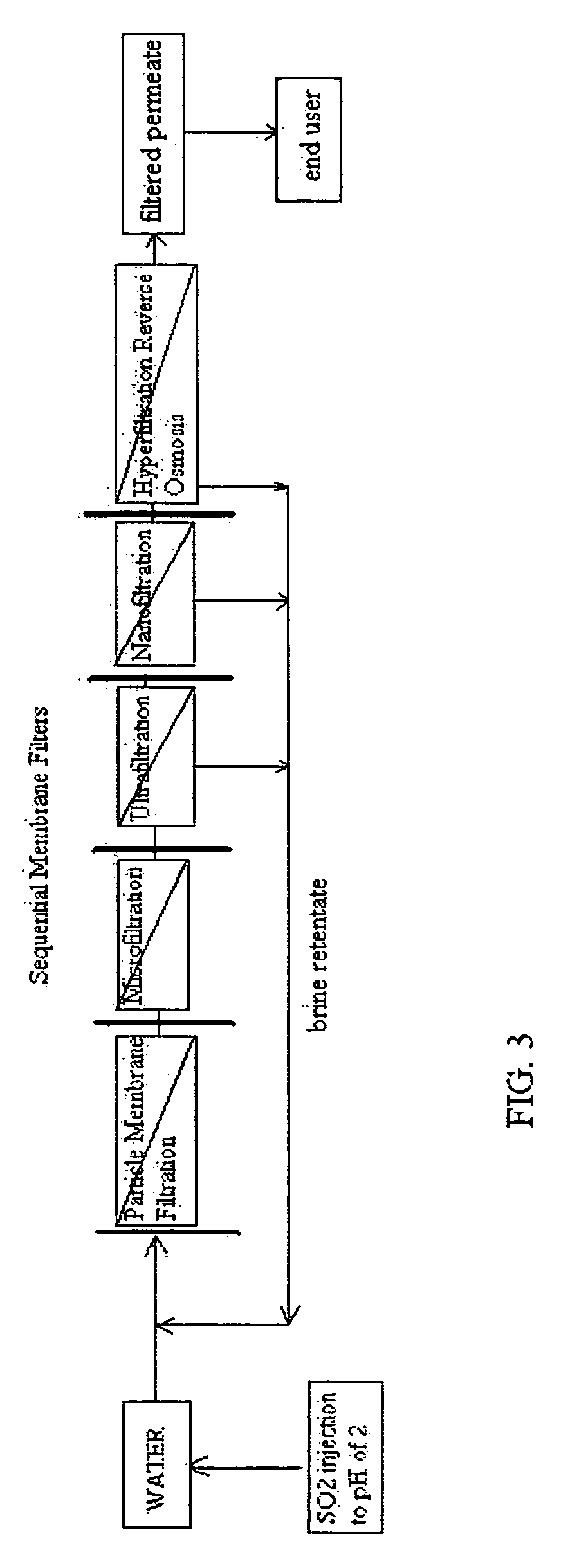
Reverse osmosis water recover method
ActiveUS20100018921A1Avoid applicationPrevent water infiltrationMembranesSedimentation separationMicroorganismFiltration
A method for cleaning and maintaining reverse osmosis membrane filters by injecting sulfurous acid into water to form sulfurous acid (H2SO3), and then sequentially filtering the acidified water through membrane filters to reduce alkalinity and mineral scaling, add sufficient SO2 as a biocide to attack bacteria and other micro organisms to prevent membrane fouling, reduce iron to prevent iron deposit build-up, scavenge and remove dissolved oxygen prior to filtration to prevent membrane oxidation, and prevent concentrated salts within the retentate from precipitating out of solution during transport for land application.
Owner:EARTH RENAISSSANCE TECH
Method for producing packaged drink
InactiveUS20090320415A1Simple equipmentImprove efficiencyPackage sterilisationSolid materialPositive pressureSterile water
It is intended to provide a process for producing a packaged drink whereby filling can be performed at room temperature without resorting to using a chemical or sterile water, the favorable taste and flavor of the content can be maintained while relieving the thermal degradation thereof, it becomes unnecessary to employ a heat-resistant container or to thermally sterilize or cool after sealing, and thus both of the equipment cost and the running cost can be largely reduced. After thermally sterilizing the content to give a definite sterilization value, it is quickly cooled to room temperature and then stored in a storage tank that has been preliminarily sterilized under such conditions as being equal to or exceeding the thermal sterilization conditions for the contents. While maintaining the storage tank under positive pressure with the use of a sterile gas, the content is fed into a filling machine that has been preliminarily sterilized under such conditions as being equal to or exceeding the thermal sterilization conditions for the contents. Thus, the liquid-feeding system ranging from the storage tank to the filling machine is made a closed liquid-feeding pathway free from the invasion of air from the outside. The drink is filled into a container having been sterilized with hot water in an environment-controlled space isolated from the outside wherein the surroundings have been thermally sterilized and washed with hot water at 65° C. to 100° C.
Owner:TOYO SEIKAN KAISHA LTD
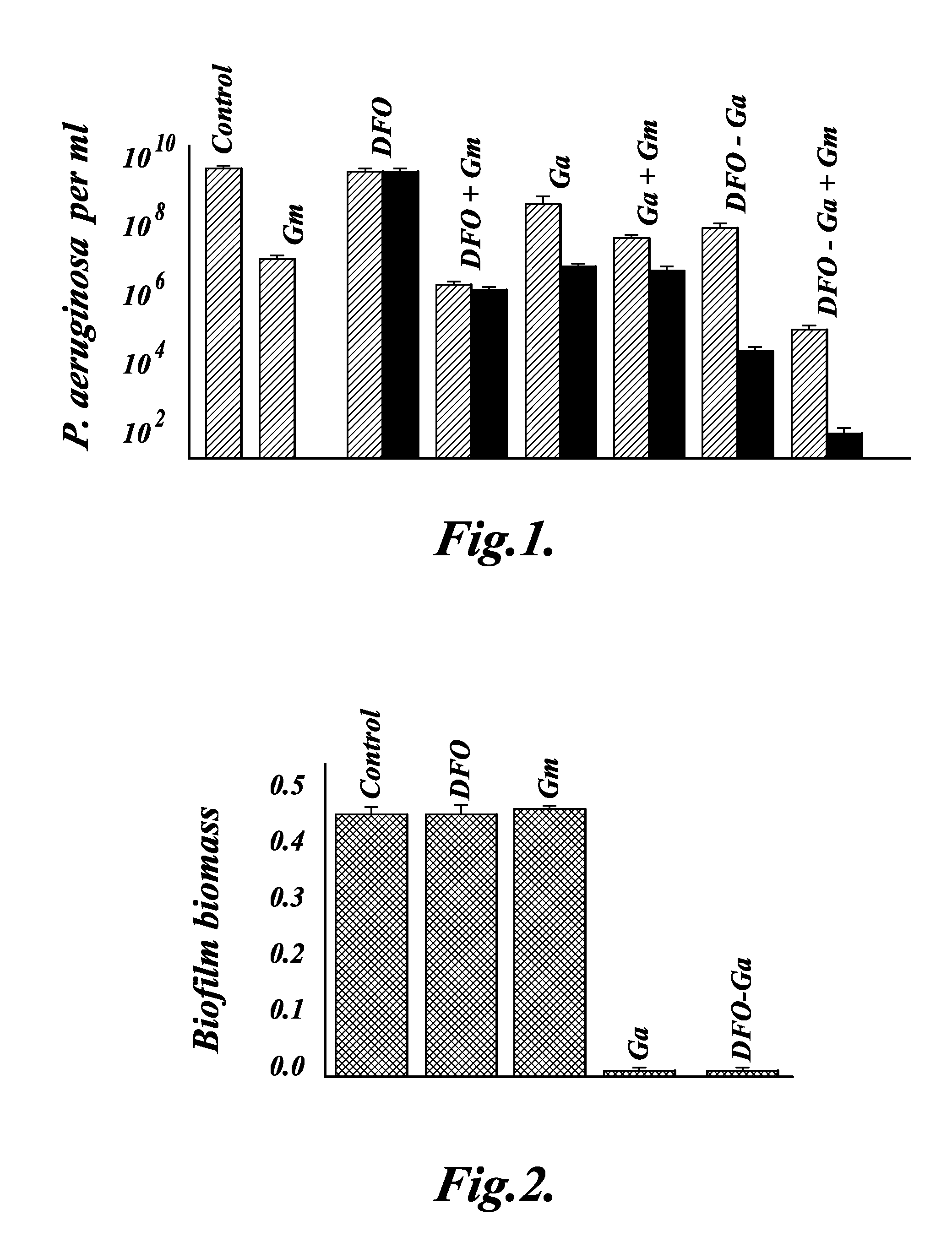
Metallo-desferrioxamine complexes and their use in the treatment of bacterial infections
InactiveUS20080085866A1Prevent bacterial growthAntibacterial agentsHuman health protectionMicrobiology
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +2
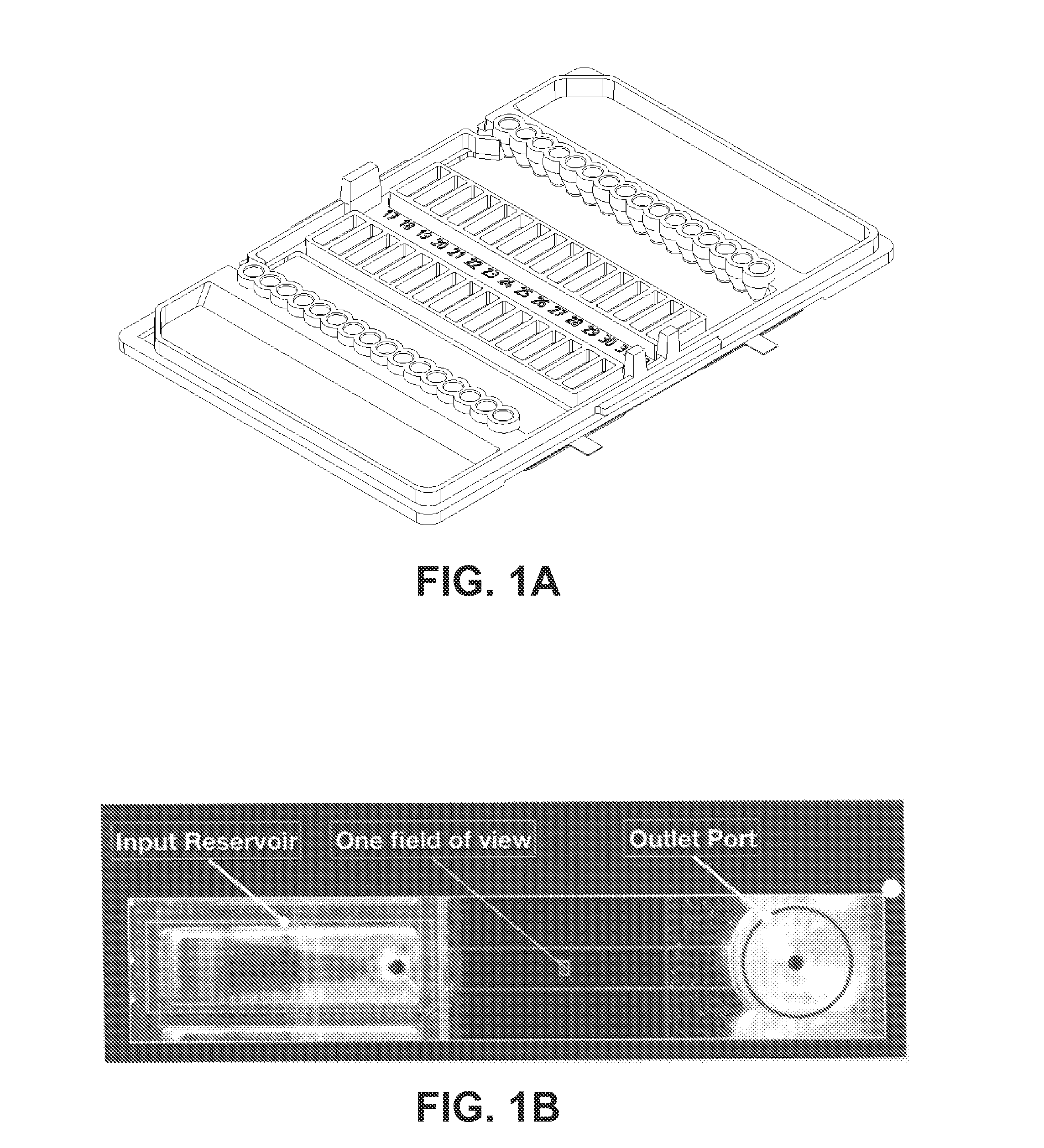
Same-day blood culture with digital microscopy
InactiveUS20150225762A1Inhibit microbial growthReduce and prevent depressionMicrobiological testing/measurementChemiluminescene/bioluminescenceCord blood cultureBiological cell
Generally provided are methods for rapid culture of microorganisms in a sample, including methods for growth and recovery of live microbial cells directly from a sample. Various features include enabling growth of microorganisms in a sample along with a reduction of sample debris that may interfere with microorganism detection, and reduction in toxicities that may inhibit microorganism growth. Further methods for selectively degrading non-viable microbial cells, are provided, for enhanced detection of viable microbial cells following a growth period.
Owner:ACCELERATED MEDICAL DIAGNOSTICS INC
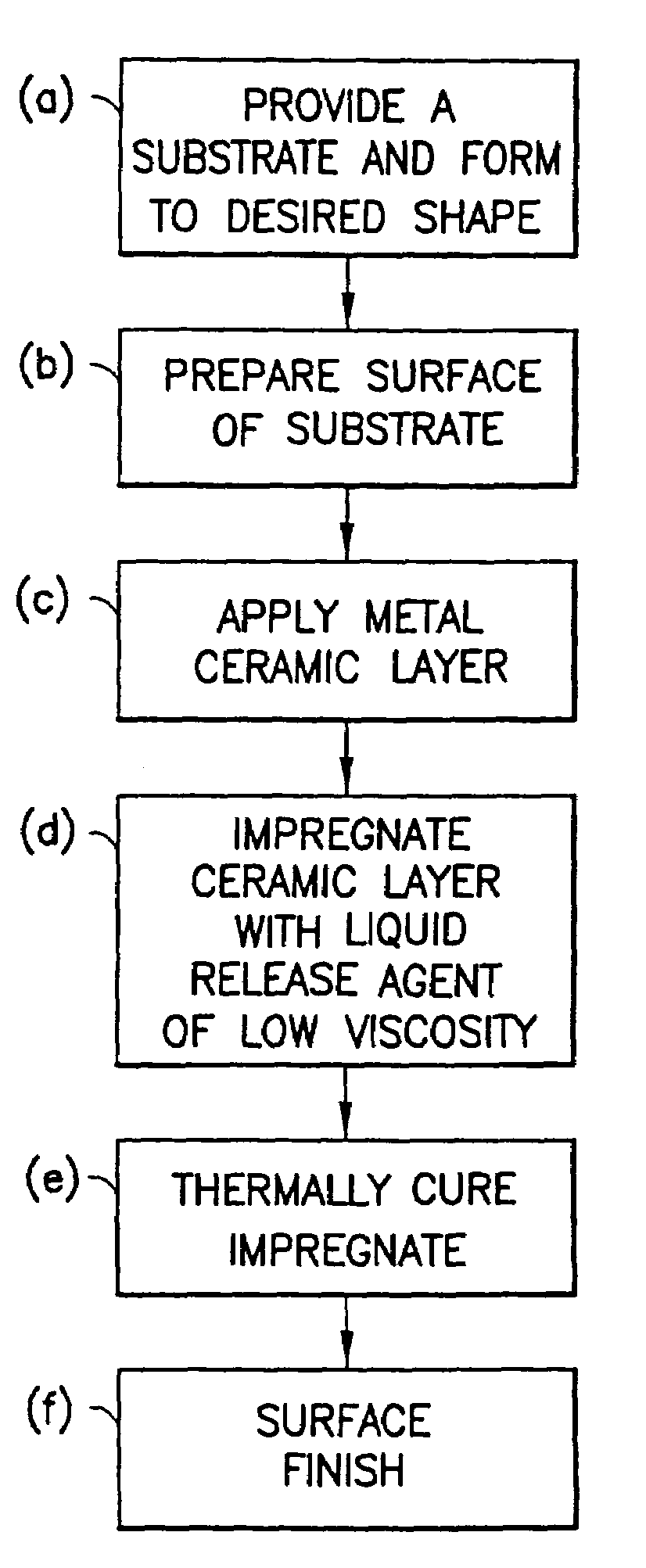
Method of making non-stick cookware
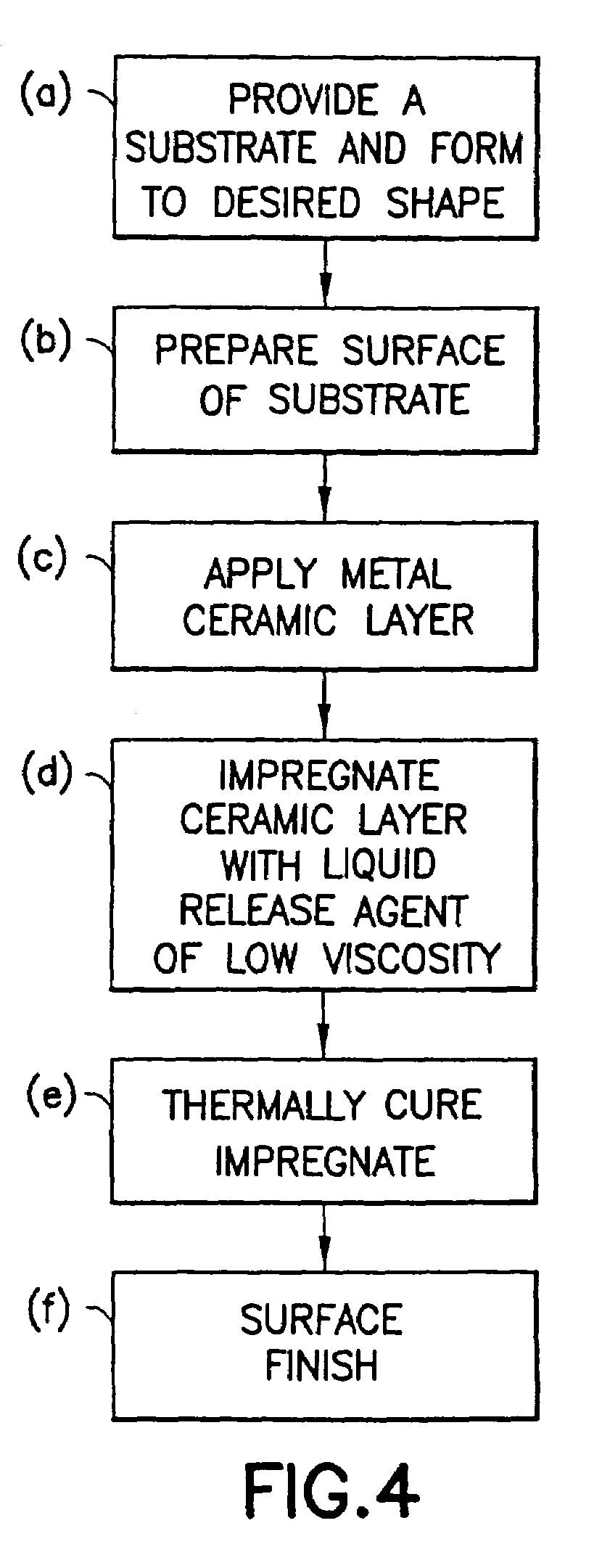
InactiveUS7488515B2Improve adhesionLow viscosityCooking-vessel materialsMolten spray coatingCeramic coatingHard metal
A non-stick surface comprising a porous, hard metal-ceramic coating such as chromium oxide applied to the cook surface of a cooking utensil by arc or plasma spraying. The pores of the coating are impregnated under vacuum with an inert release agent of low viscosity such as food grade liquid silicon resin. After vacuum impregnation, the impregnate is thermally cured and the cook surface is mechanically abraded / polished to remove the protruding peaks of metal-ceramic material to expose flattened bare metal portions interspersed between and substantially co-planar with the surfaces of the impregnated release agent residing in the valleys / pores.
Owner:CLAD METALS
Bacteria resistant coating for surgical instrument
InactiveUS20050058682A1Prevent bacterial growthReduce infectionBiocideSurgeryCoated surfaceSurgical site
A surgical instrument for use in a surgical site includes a first surface that is positionable within or near the surgical site and has an anti-bacterial coating disposed on the surface. The anti-microbial coating includes anti-microbial particles disposed in a polymer matrix wherein the anti-microbial particles are in sufficient concentration and are positioned to provide an anti-microbial effect at the surgical site. Bacterial growth is also inhibited on the coated surface of the instrument.
Owner:MINNESOTA SCI
Anti-bacterial medical waterproof material and sheet made of the same
A waterproof material of a circular knitted fabric woven from a first yarn and a second yarn. The first yarn is a nylon filament containing ultra-micro silver ions and the second yarn is a polyester yarn. The fabric is made of approximately 20 to 40% of the first yarn and 60-80% of the second yarn. An antibacterial waterproof sheet has a multilayer construction for controlling bacterial growth, eliminating odor and resisting wear and includes a polyurethane film having an upper and lower side forming an innermost layer. A layer of polyurethane adhesive is disposed on each upper and lower side of the film. A layer of knitted material is disposed on each layer of adhesive forming opposed outer layers of the sheet. The knitted material is woven from the first yarn and a second yarn. The silver powder ions are 1 micron or less.
Owner:TANIGUCHI YOSHIMICHI +2
False eyelash cleansing device
PendingUS20210030140A1Effective wayClean falseHair accessoriesToupeesEngineeringMechanical engineering
A false eyelash cleansing device having a compact, a false eyelash mold layer, and a false eyelash clamp layer is provided. The compact has a top and a tub, the top has an exterior surface and an interior surface and is adapted to cover the tub. The top is pivotally engaged to the tub. The false eyelash mold layer has an indentation to hold the false eyelashes and has a plurality of drainage holes adapted to let a solution drain. Furthermore, the false eyelash mold layer is adapted to be inserted into the tub. Additionally, the false eyelash clamp layer has a false eyelash clamp bar adapted to secure the false eyelash to the false eyelash mold layer. The false eyelash clamp layer is adapted to be inserted into the tub and cover the false eyelash mold layer.
Owner:CHICO SARAH
Lactation Suppression Garment
InactiveUS20120142253A1Infinite adjustabilityReduce the risk of infectionBrassieresProtective garmentBiomedical engineeringLactation suppression
Owner:JAVAID SUNBAL +1
Bacteriostatic filter cartridge
InactiveUS6854601B2Reduce bacteria countSafely and effectively and economically filterUltrafiltrationReverse osmosisYarnPolyester
A bacteriostatic filter cartridge having a porous core member about which is layered a yarn and / or a polyester membrane and / or melt blown web of polypropylene and / or a trilaminate polypropylene membrane, any or all of which may be impregnated with an antimicrobial agent. The filter cartridge is sized so as to fit tightly into a cartridge housing of a fluid filtration system. Fluid passing through the cartridge housing will be filtered by the filter cartridge to remove contaminants from the water and which prevents the growth of bacterial and other microorganisms on the filter media.
Owner:MICROBAN PROD CO INC
Systems and methods for applying a novel antimicrobial coating material to a medical device
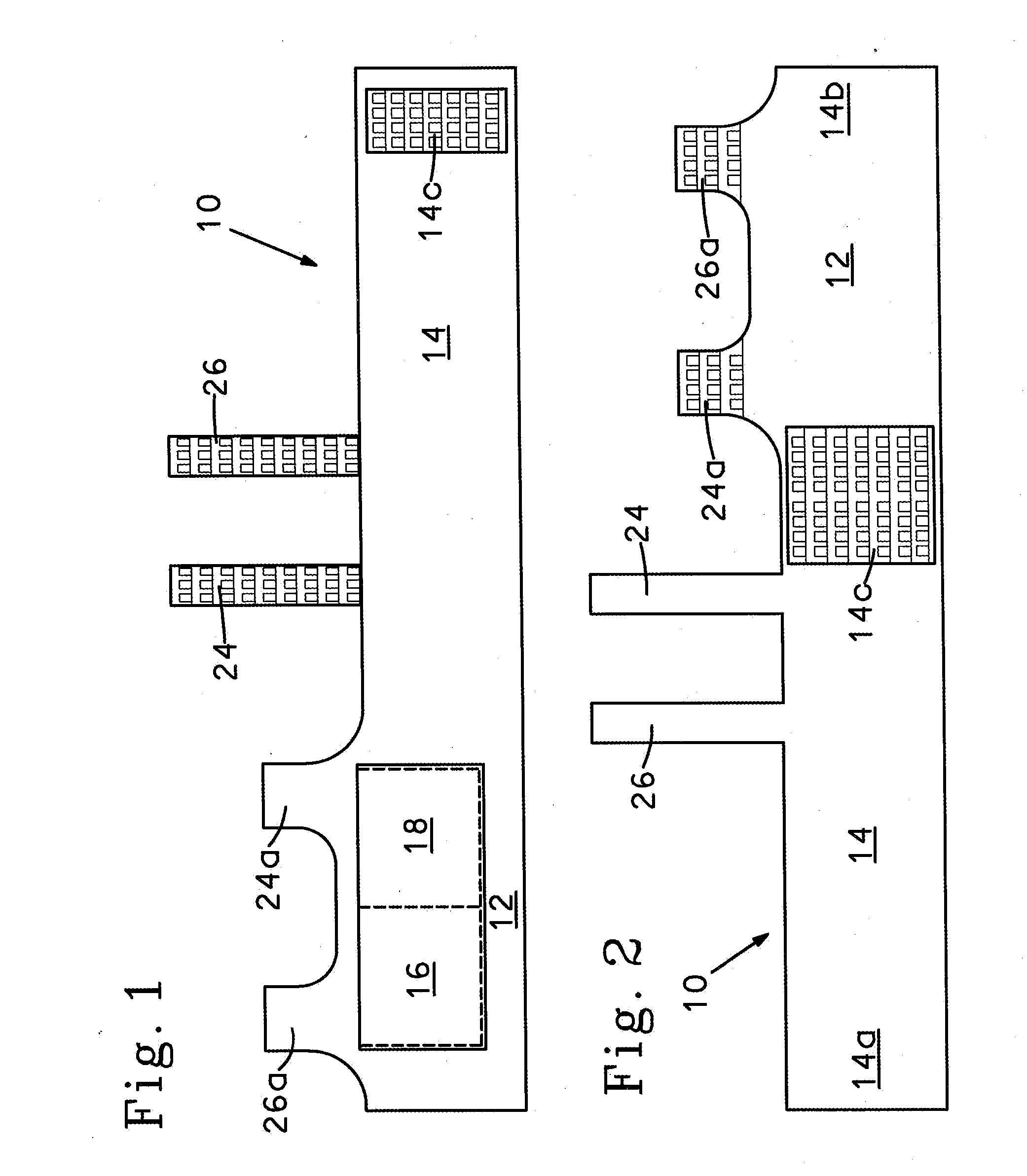
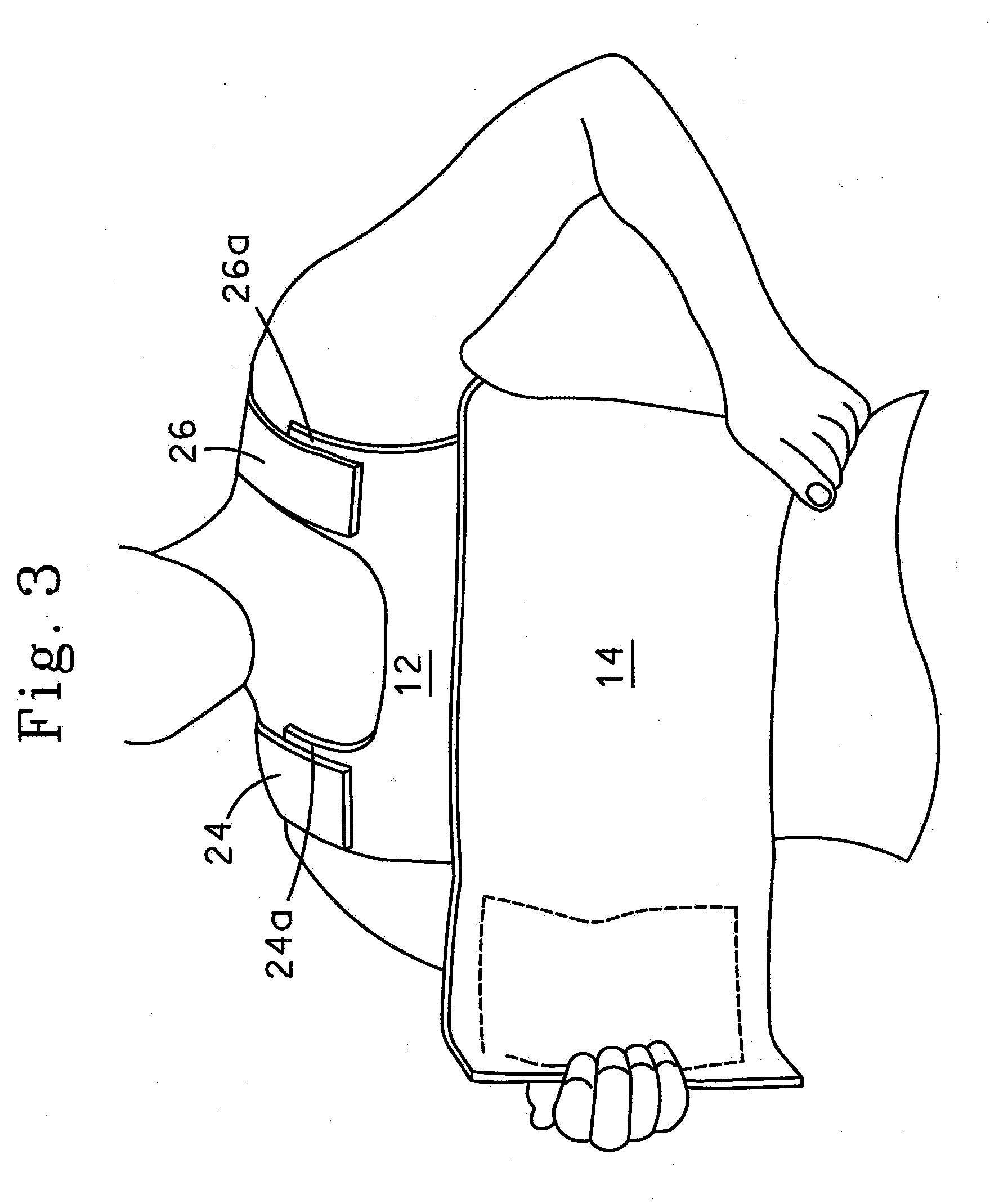
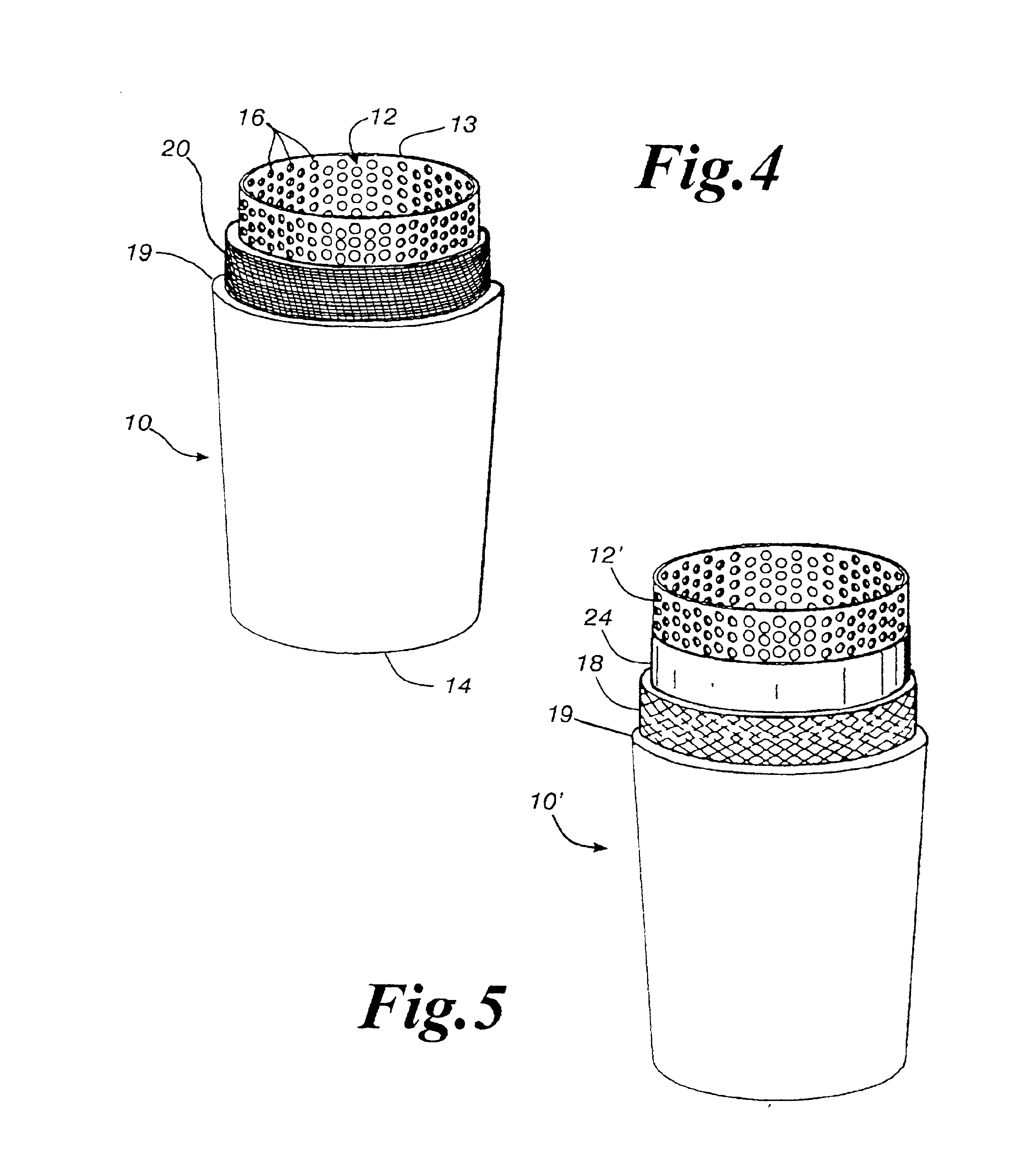
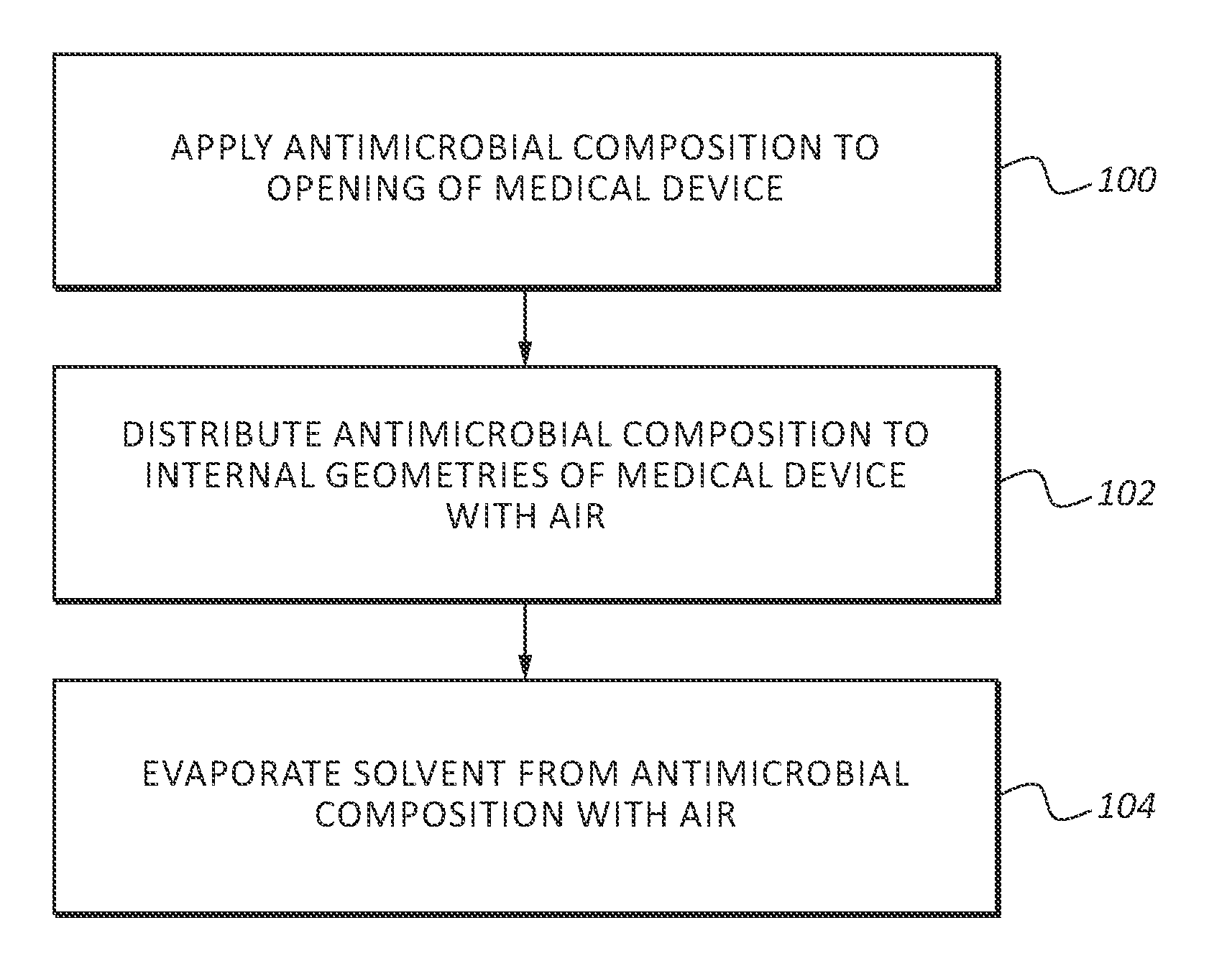
InactiveUS20130255061A1Reduce frictionPrevent bacterial growthBiocideOrganic chemistryAlcoholEvaporation
An antimicrobial composition, generally comprising a biocidal agent, such as chlorhexidine gluconate, a lubricant, such as a modified siloxane, and a solvent, such as an alcohol and / or water. The biocidal agent acts as a highly effective biocide while the lubricant reduces friction between various components of a medical device during assembly. The antimicrobial composition may be applied to an internal component of the medical device prior to assembly. The process of assembling the medical device results in antimicrobial composition being distributed to various internal structures and geometries of the medical device as the coated, internal component is assembled into the interior of the medical device. Air is then used to further distribute excess antimicrobial agent to the remaining surfaces downstream from the installed, internal component. In some embodiments, additional air is passed through the assembled medical device to assist in removing the carrier solvent from the medical device via evaporation.
Owner:BECTON DICKINSON & CO
Wipe Coated with a Botanical Composition having Antimicrobial Properties
A wipe that contains a fibrous web on which is coated an antimicrobial composition is provided. The composition includes a botanical oil derived from a plant (e.g., thymol, carvacrol, etc.). Because the botanical oil is volatile and tends to evaporate and lose efficacy during storage and prior to use, a protein is also employed in the composition to enhance long term stability of the oil and, in turn, its antimicrobial efficacy. The protein is “film-forming” in the sense that it tends to form a substantially continuous film when coated onto a surface of the fibrous web. Because such proteins are typically stiff and brittle in nature, a continuous film would restrict the ability of the fibers to move and bend, thereby reducing web flexibility and drape. Thus, it is typically desired that the antimicrobial composition form a discontinuous coating on the fibrous web. In this regard, the present inventors have surprisingly discovered that the addition of an organopolysiloxane can help achieve such a discontinuous coating without adversely impacting the ability of the protein to stabilize the botanical oil. The organopolysiloxane may also enhance the softness and overall handfeel of the wipe.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Antimicrobial nanocomposite compositions, fibers and films
ActiveUS9192625B1Prevent bacterial growthImprove thermal stabilityAluminium/calcium/magnesium active ingredientsNon-woven fabricsEscherichia coliFiber
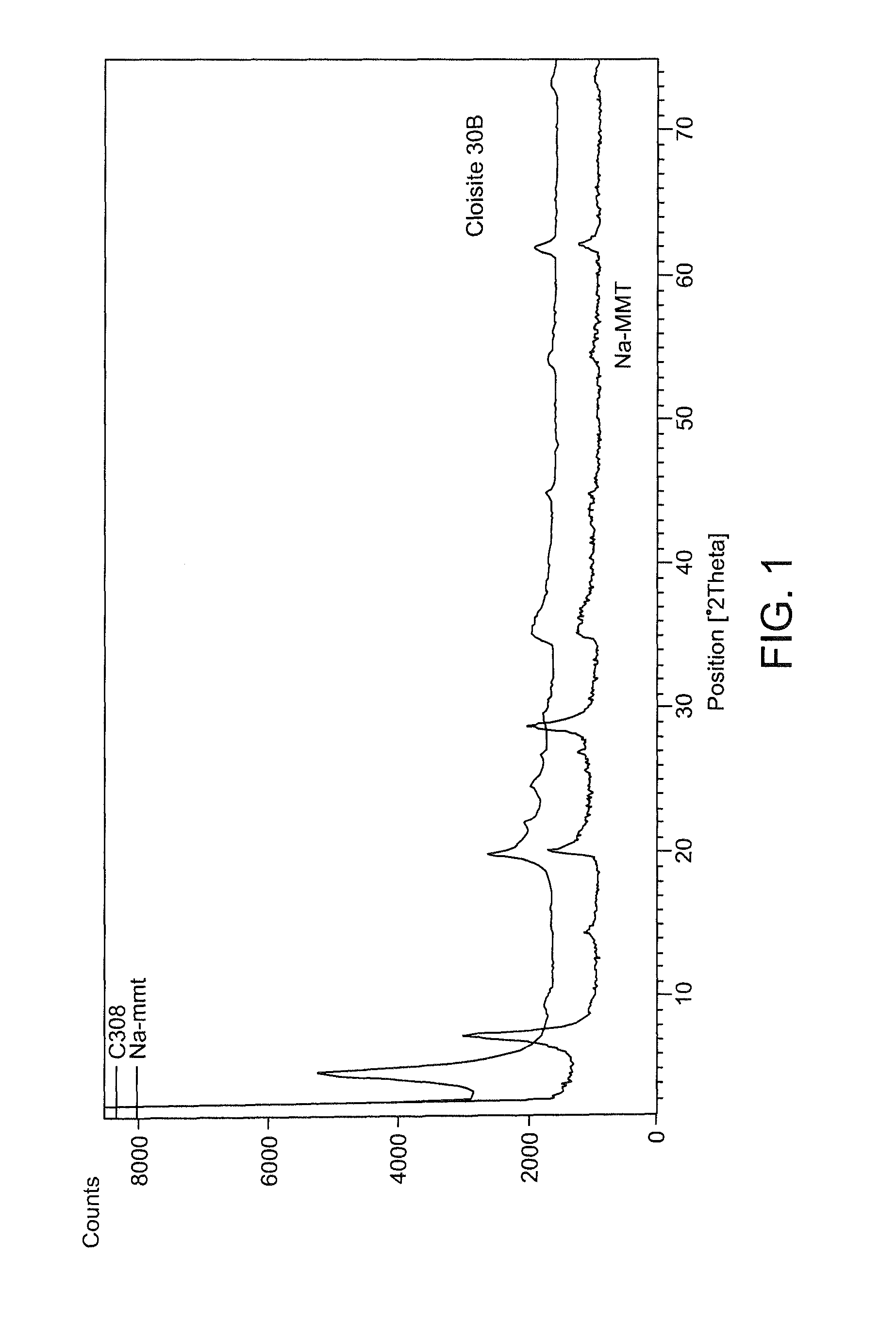
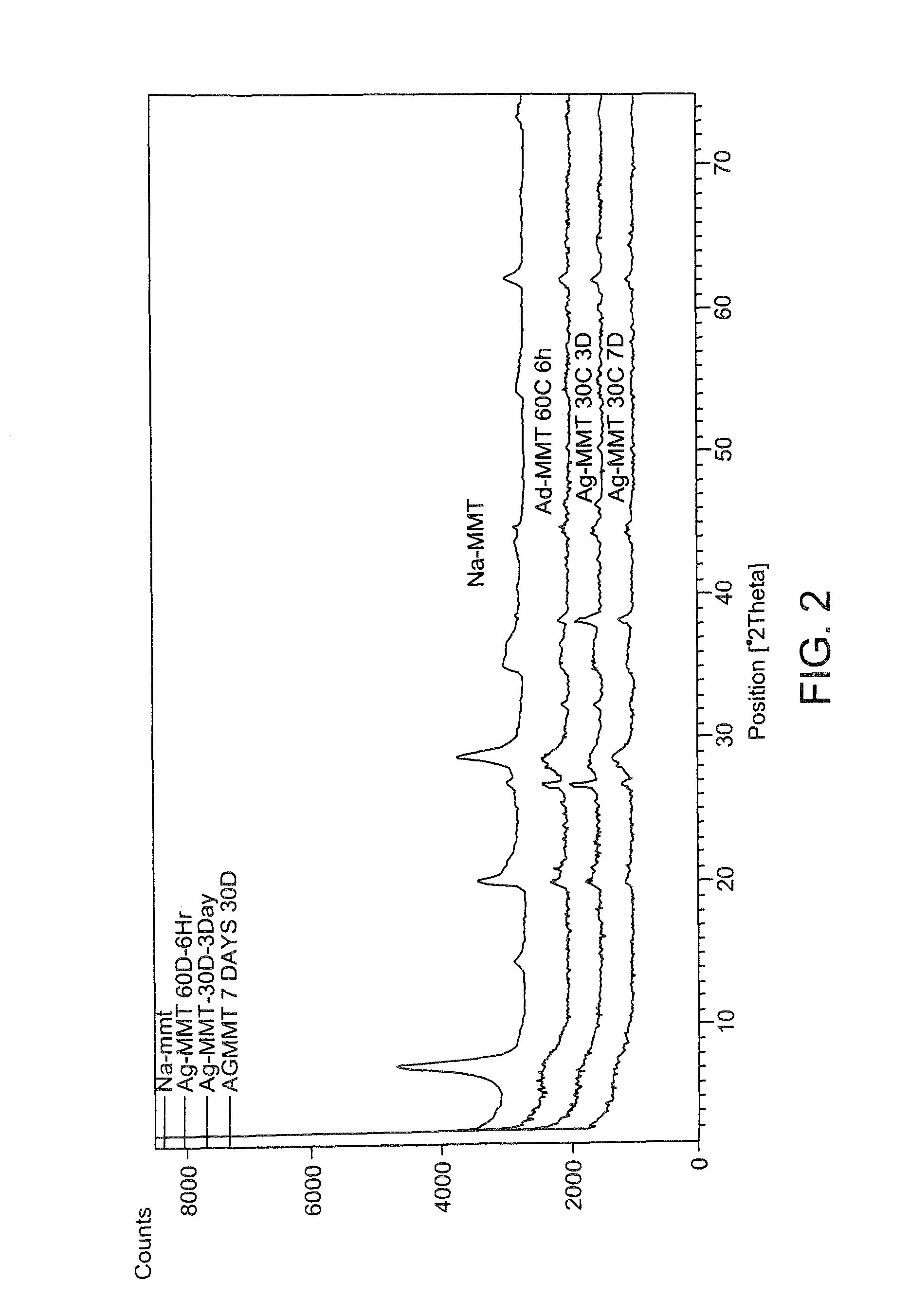
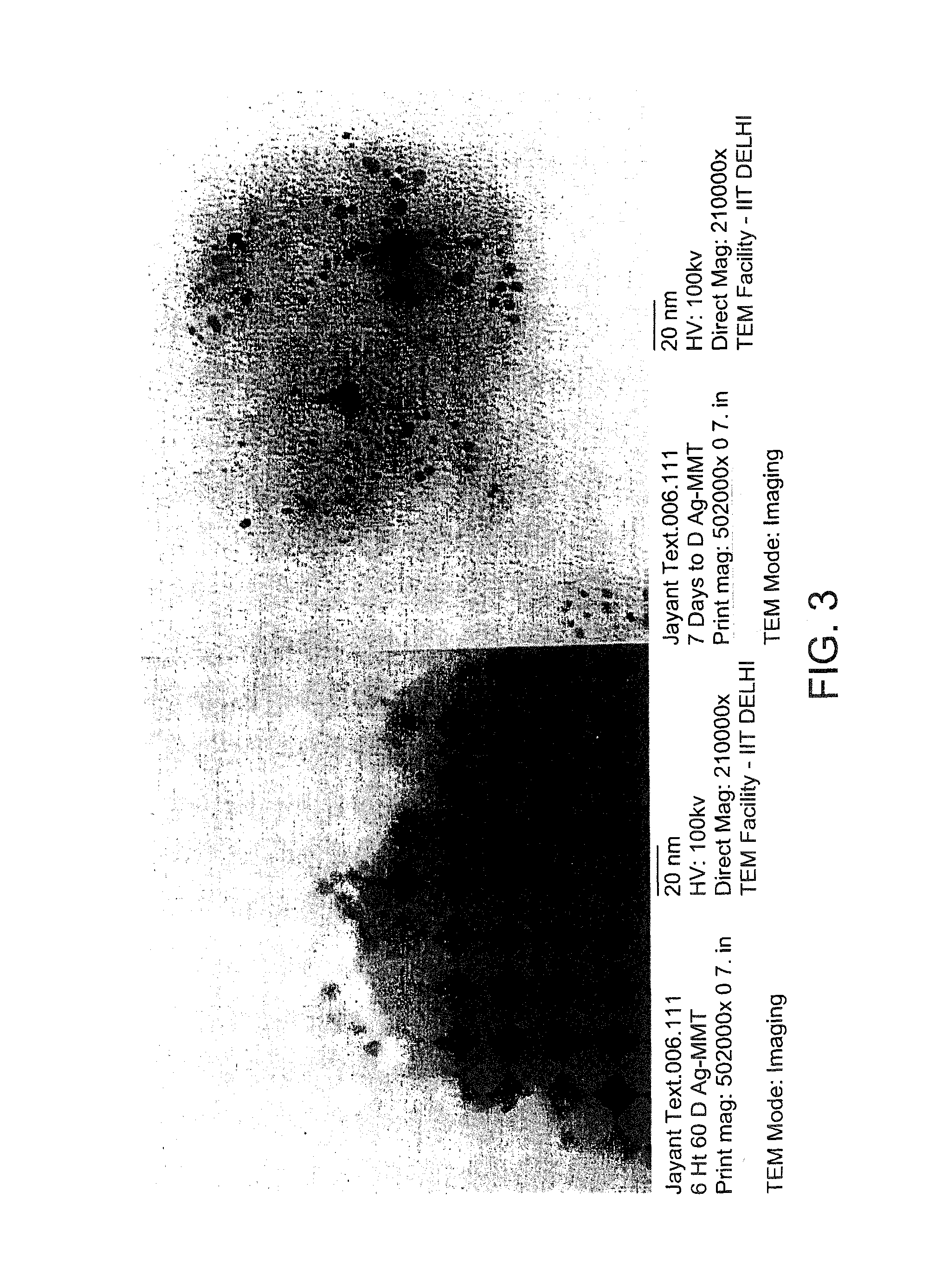
In the present disclosure, silver ions, copper ions, quaternary ammonium compounds and cationic drugs such as sulphanilamide, chlorhexidine acetate, etc., used as antibacterial agents are incorporated into fibers, filaments and films through nanoclays. The nanoclays serve as a carrier for the antimicrobial agents and are incorporated into the fiber-forming polymer. In one embodiment, the biocidal metals and organic compounds are incorporated into the clay structure via an ion exchange reaction. Nylon nanocomposite filaments and films based on copper and quaternary ammonium ion modified clays provide 100% antibacterial activity against Gram positive Staphylococcus aureus and Gram negative Escherichia coli bacteria at an optimum clay loading of 0.75% (by weight), and the activity is retained up to 50 washes. The resulting filaments show enhanced mechanical properties such as tensile strength and modulus, and find application in areas of medical textiles like sutures, wound dressings and health & hygiene textiles. In addition, they can be integrated into protective clothing, body garments, sportswear and upholstery.
Owner:INDIAN INST OF TECH DELHI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com