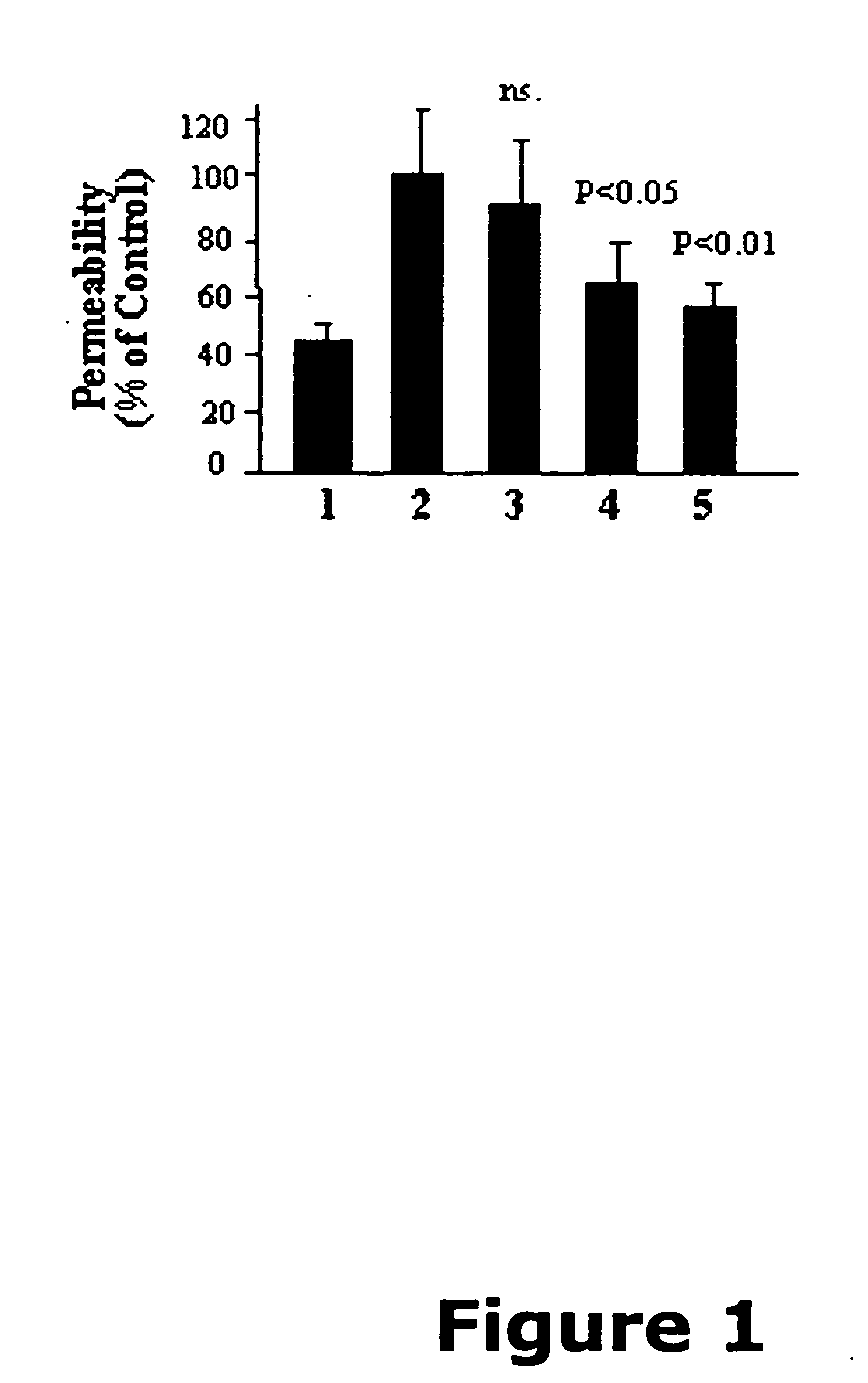
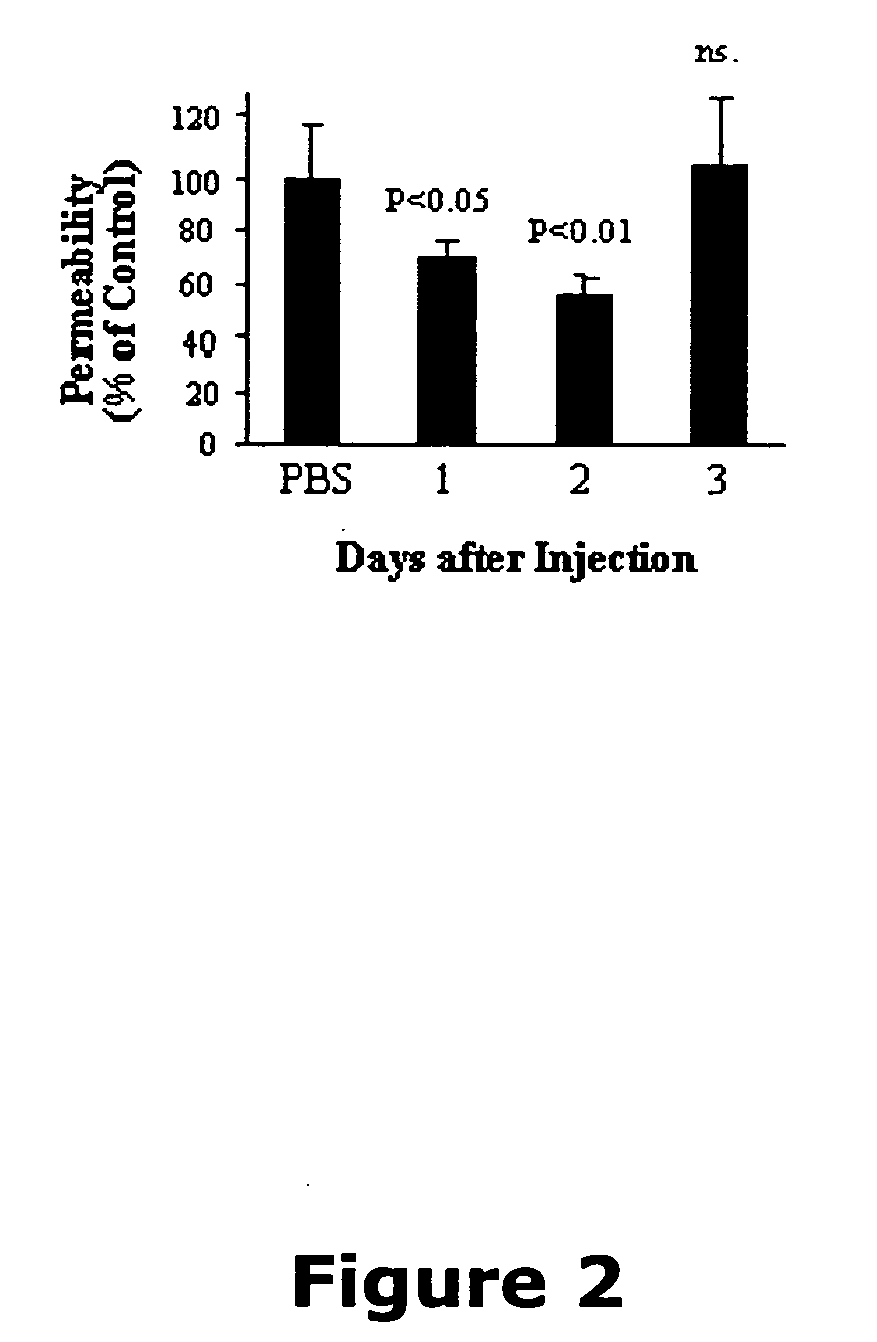
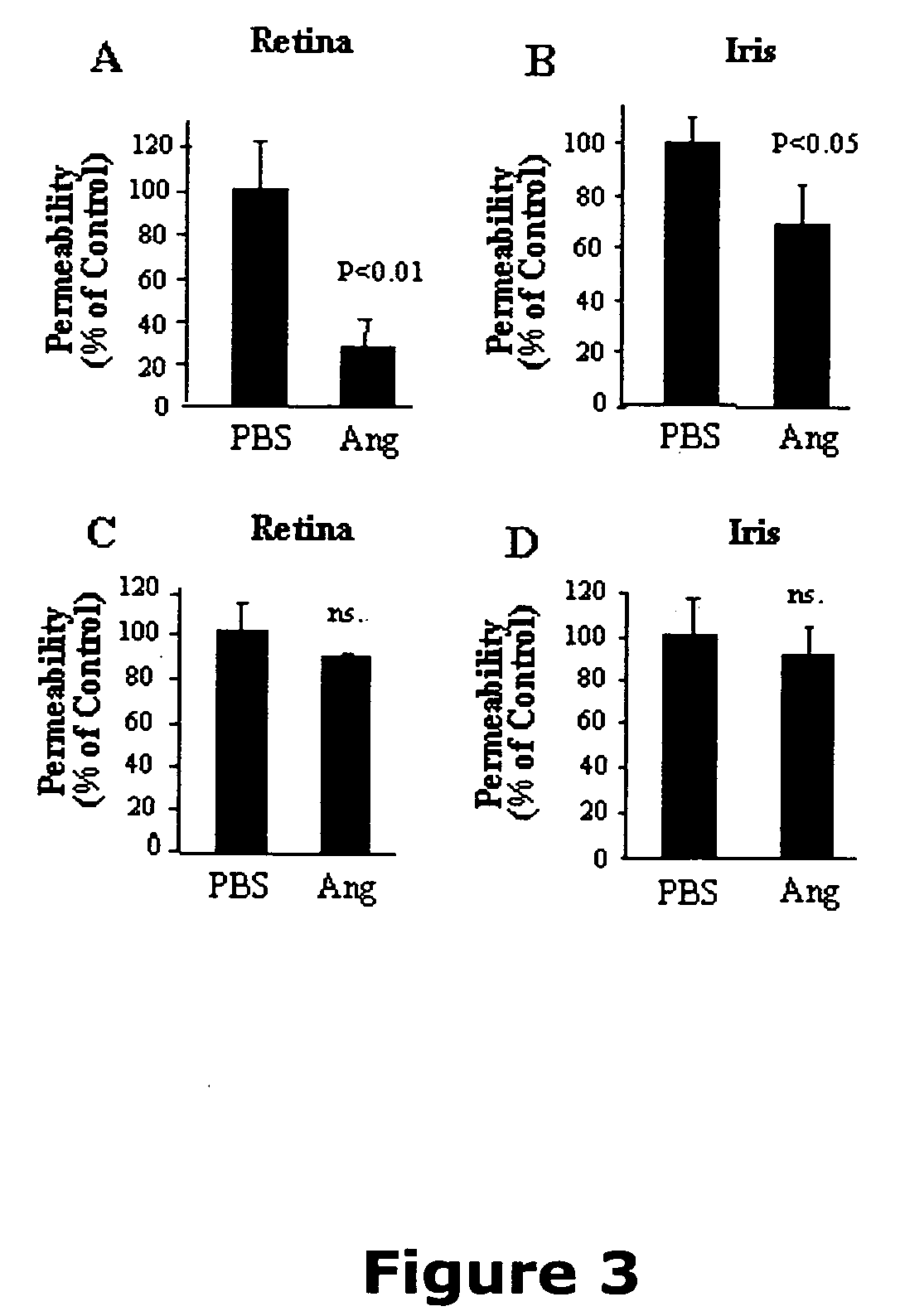
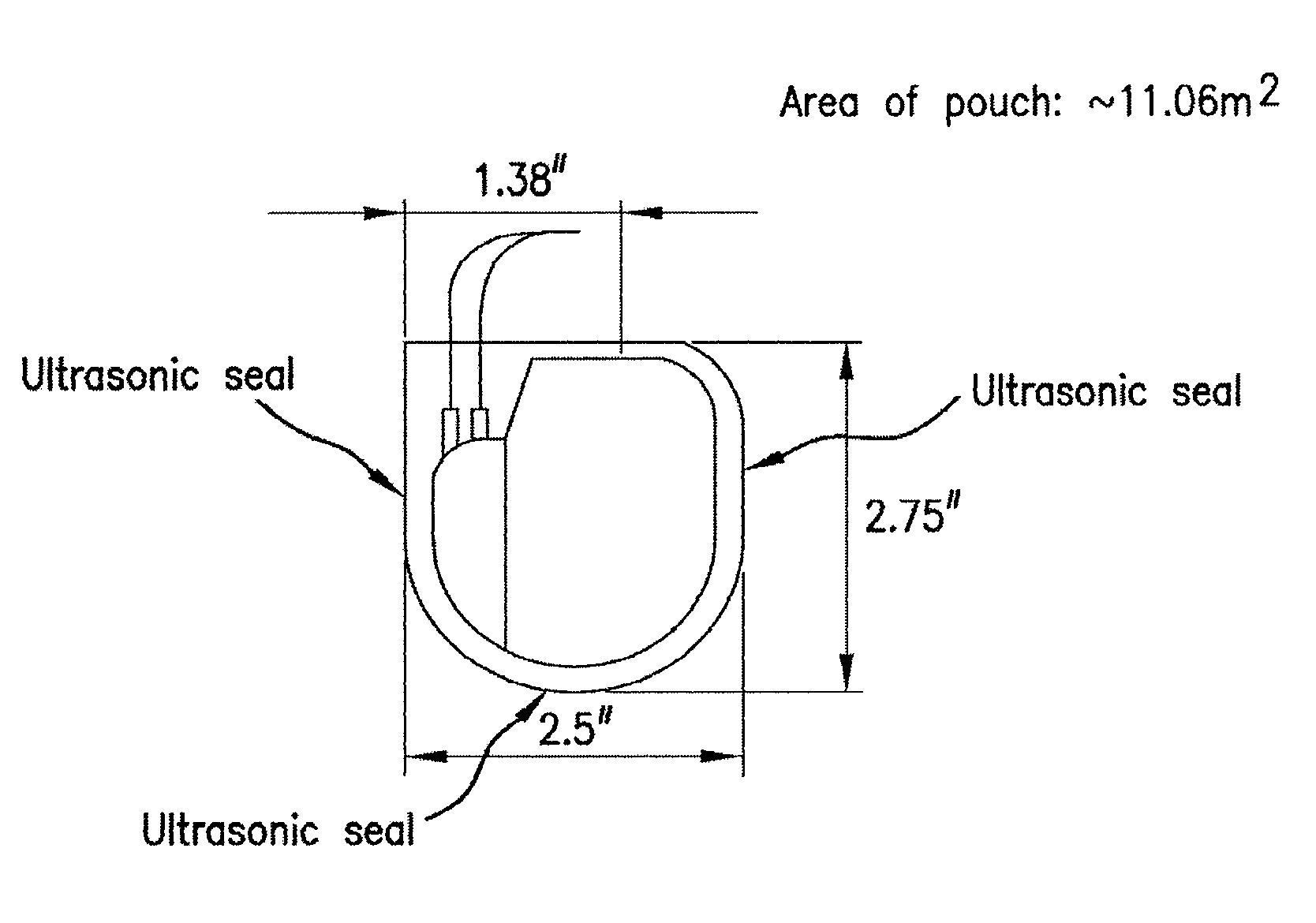
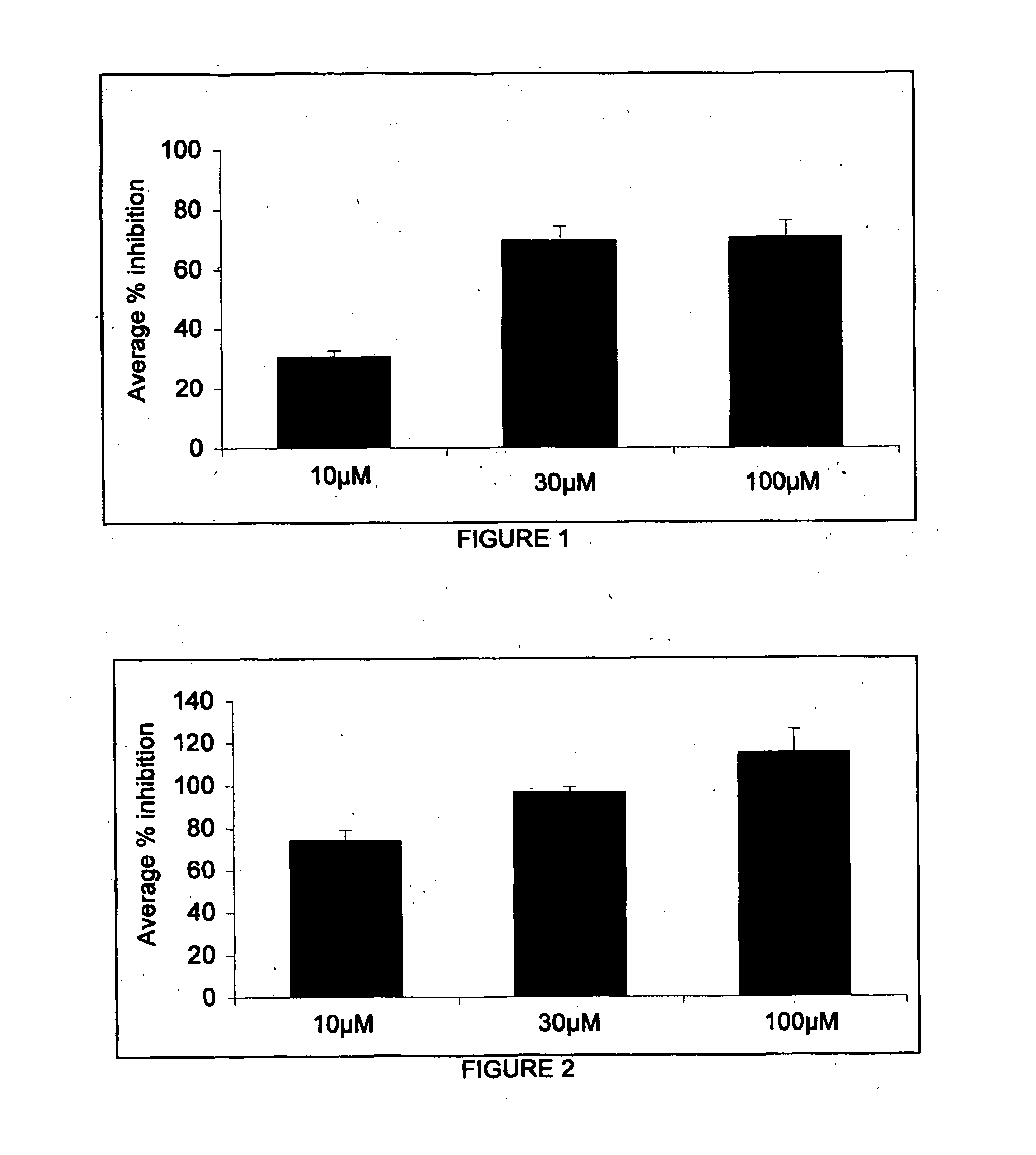
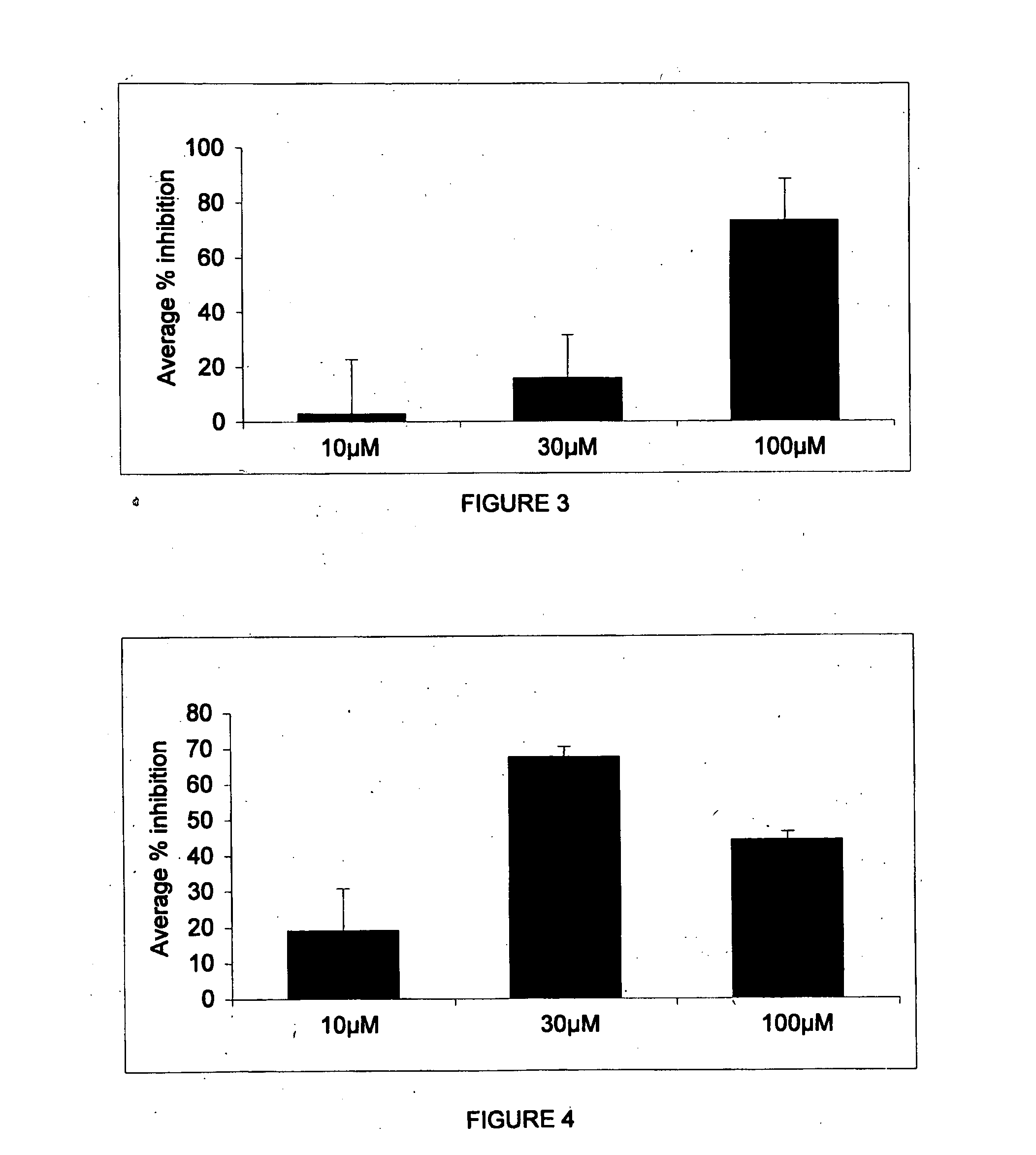
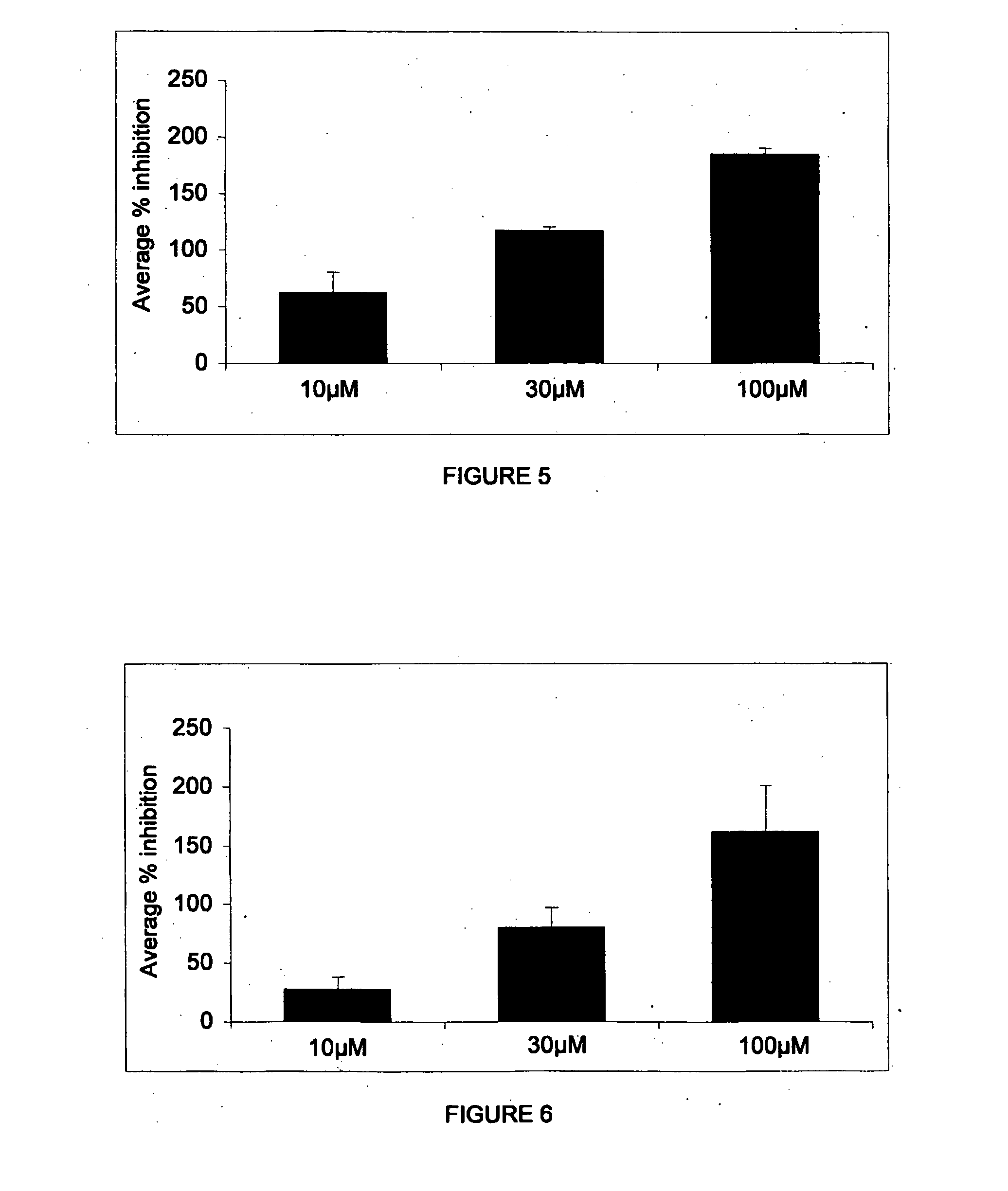
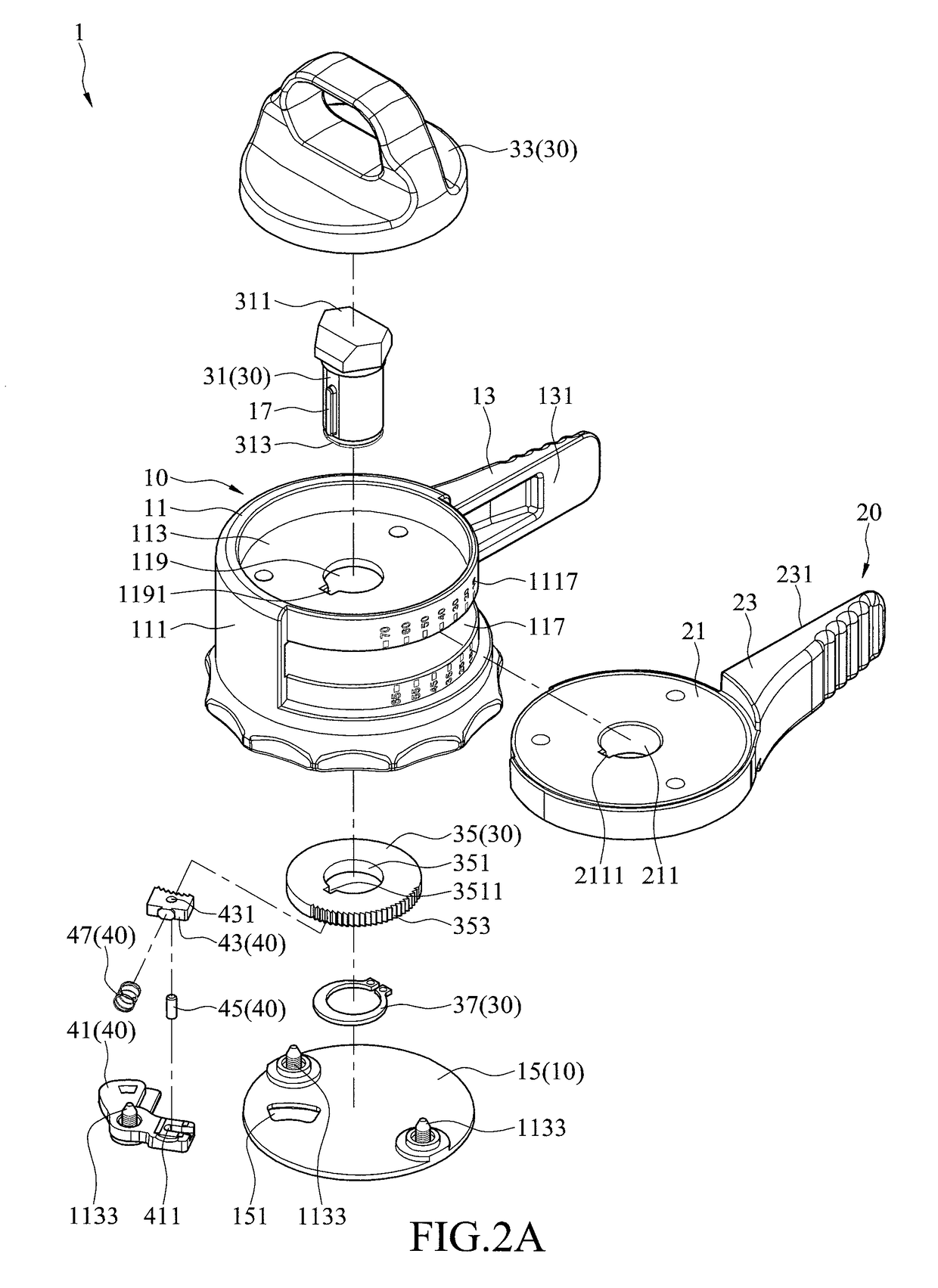
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
208results about How to "Prevent fibrosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Electrical devices and anti-scarring agents
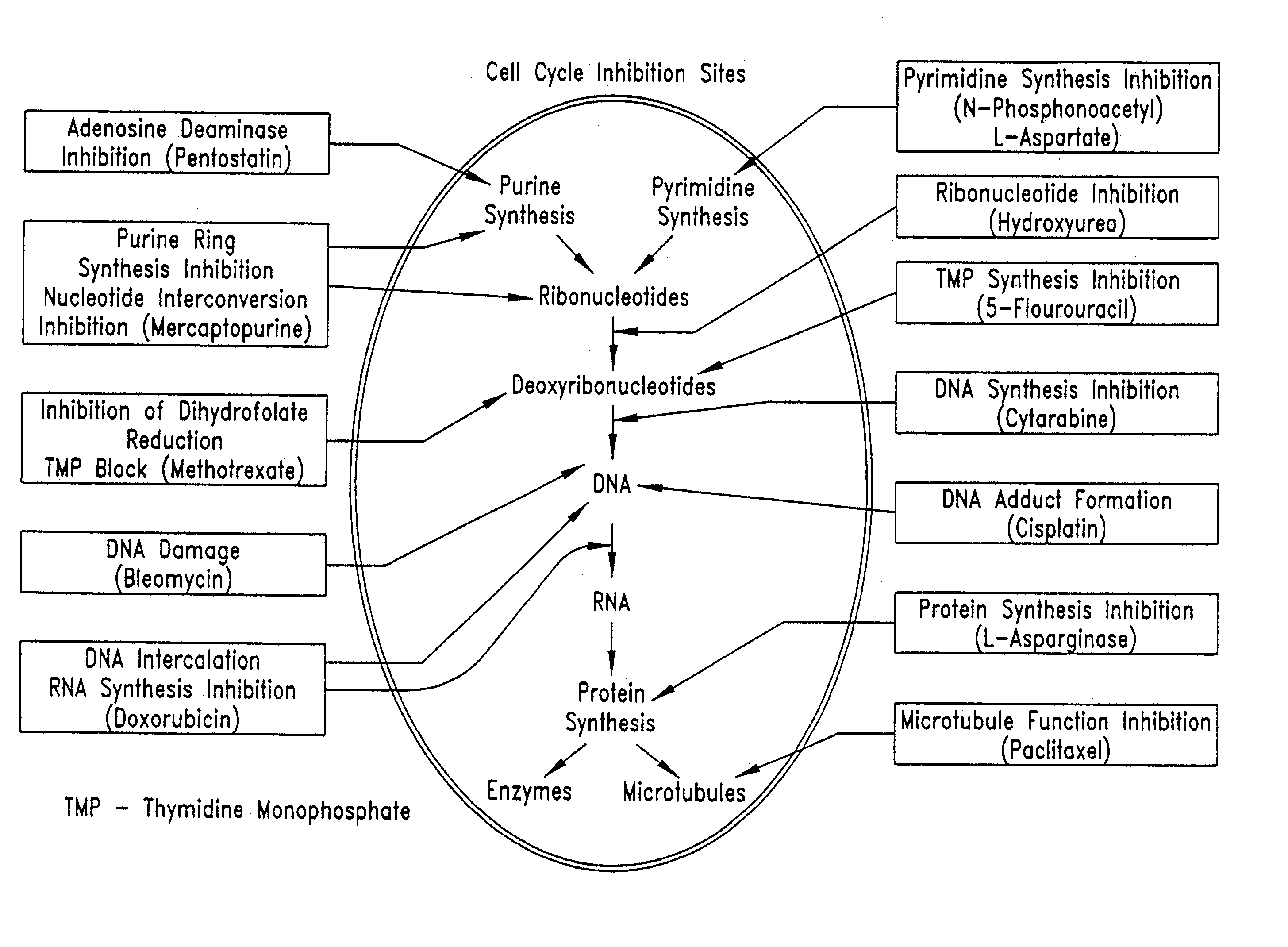
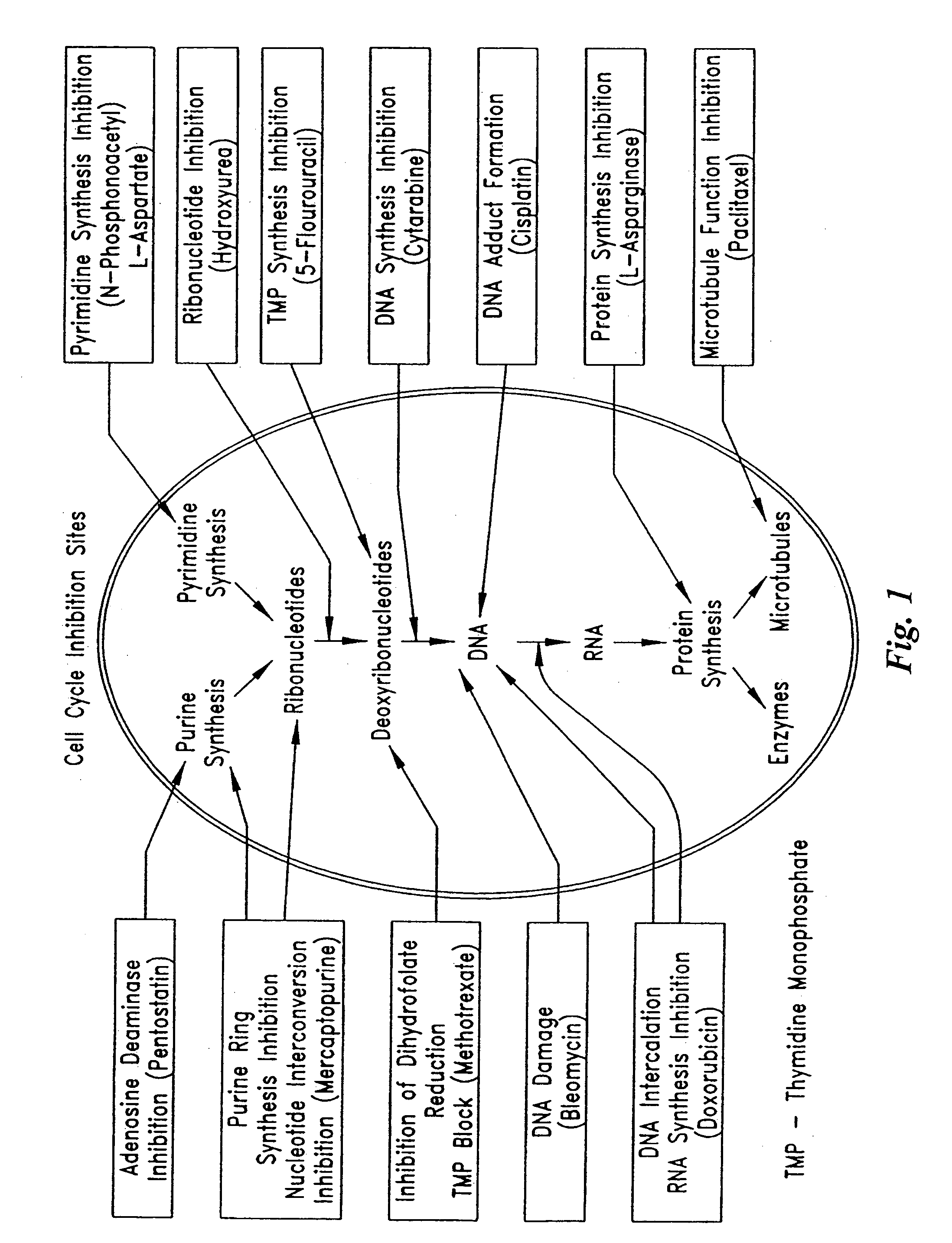
InactiveUS20050209664A1Prevent scar tissue overgrowthImprove conductivitySurgeryImplantable neurostimulatorsCell Cycle InhibitionElectrical devices
Electrical devices (e.g., cardiac rhythm management and neurostimulation devices) for contact with tissue are used in combination with an anti-scarring agent (e.g., a cell cycle inhibitor) in order to inhibit scarring that may otherwise occur when the devices are implanted within an animal.
Owner:ANGIOTECH INT AG (CH)
Intracapsular pseudophakic device
Intraocular devices for use in and attached to the natural lens capsule of an eye are provided. The lens capsule may be maintained in a configuration to avoid post-operative changes that are deleterious to vision. Single or dual optic systems are provided, which may be accommodating. Combinations of devices to obtain dual optic systems are disclosed.
Owner:BROWN DAVID C
Electrical devices and anti-scarring agents
InactiveUS20050149157A1Reduce fibrosisReduce gliosisPeptide/protein ingredientsAntipyreticCell Cycle InhibitionElectrical devices
Owner:ANGIOTECH INT AG (CH)
Mesh Pouches for Implantable Medical Devices
ActiveUS20080132922A1Reduces and prevents implantReduces and prevents and surgery-related complicationElectrotherapyDiagnosticsFiberSide effect
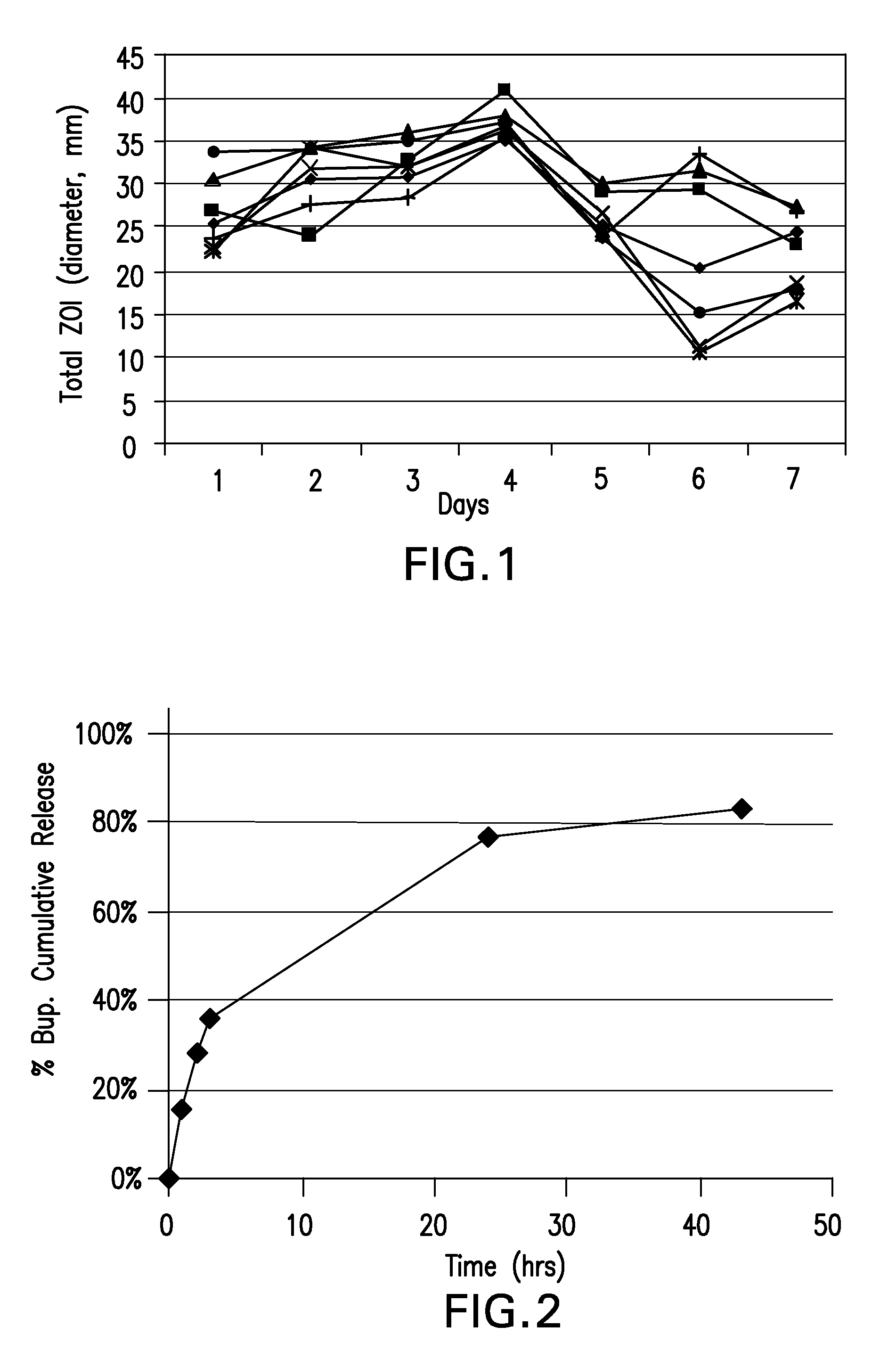
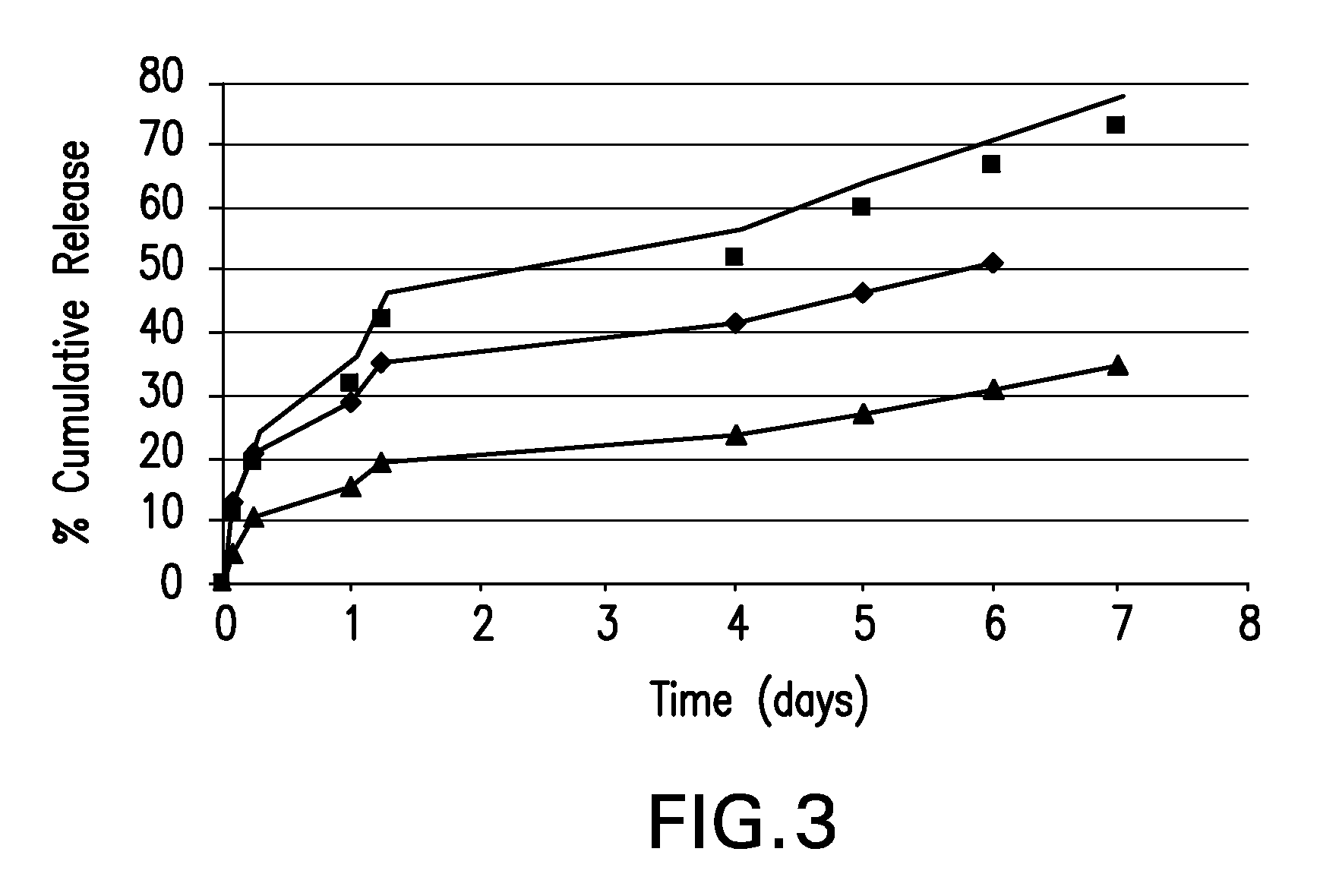
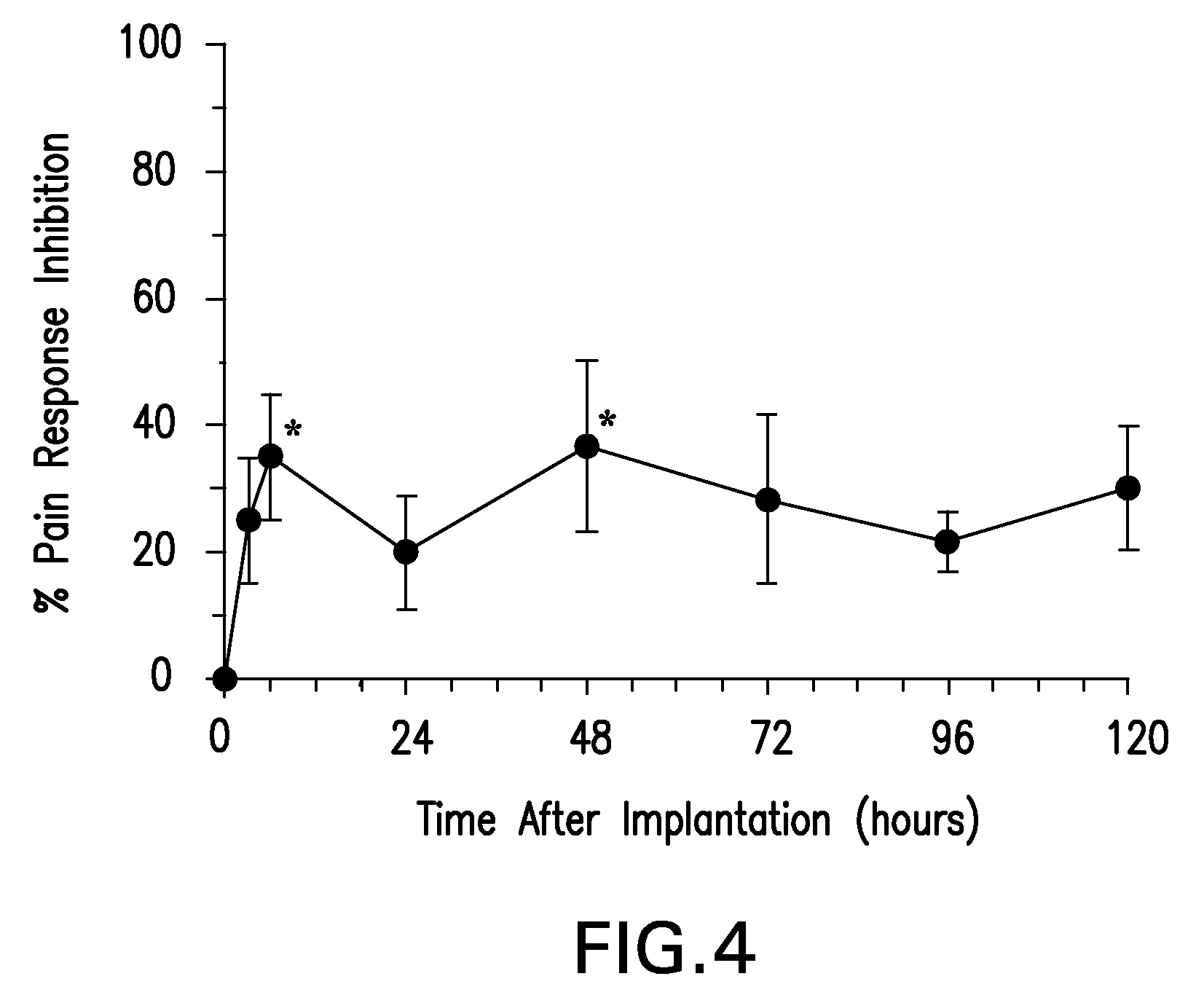
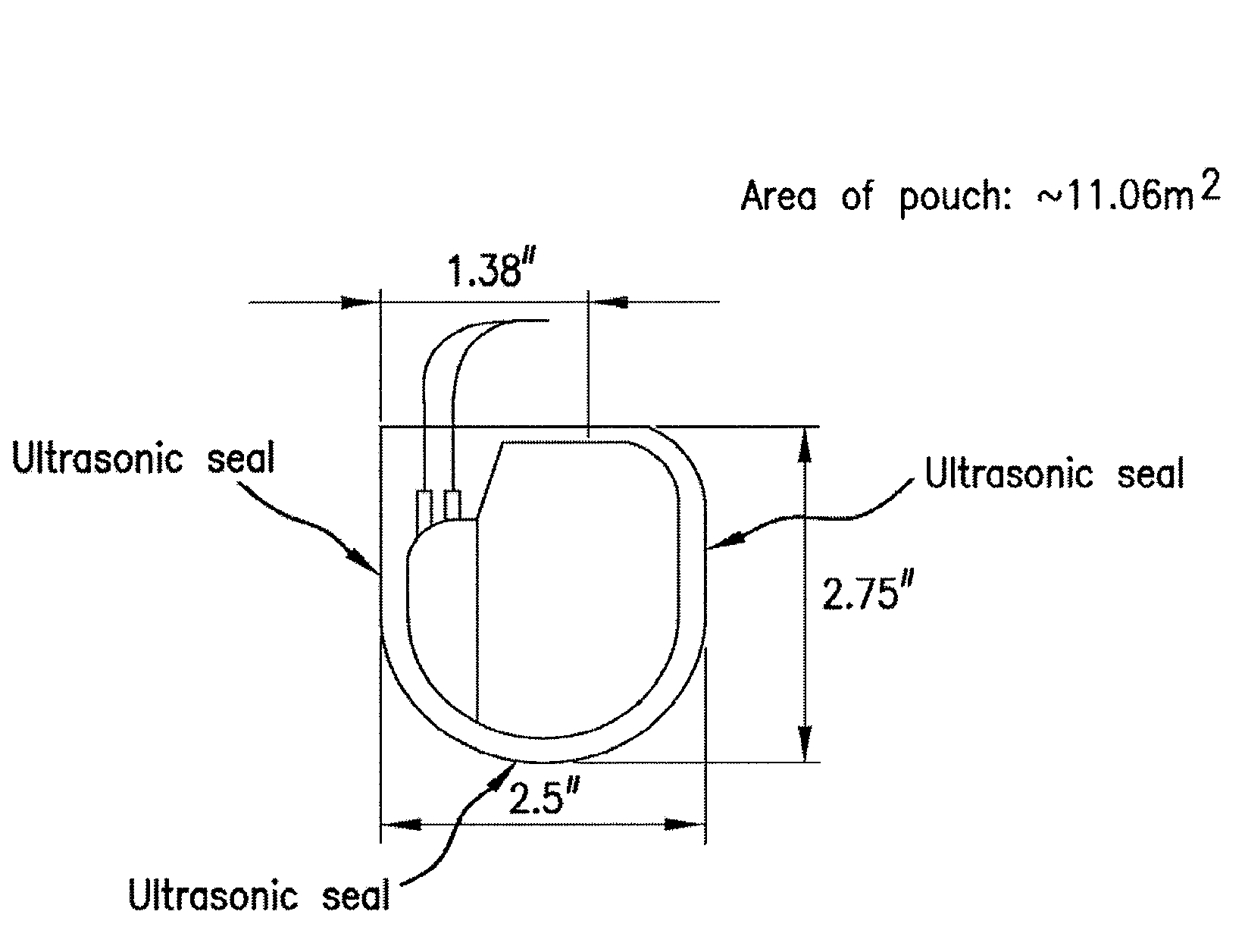
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:MEDTRONIC INC
Preventing biofilm formation on implantable medical devices
ActiveUS20100168808A1Reduces and prevents implantReduces and prevents and surgery-related complicationMammary implantsDiagnosticsBiofilmMedical device
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device and preventing or retarding the formation of a biofilm.
Owner:MEDTRONIC INC
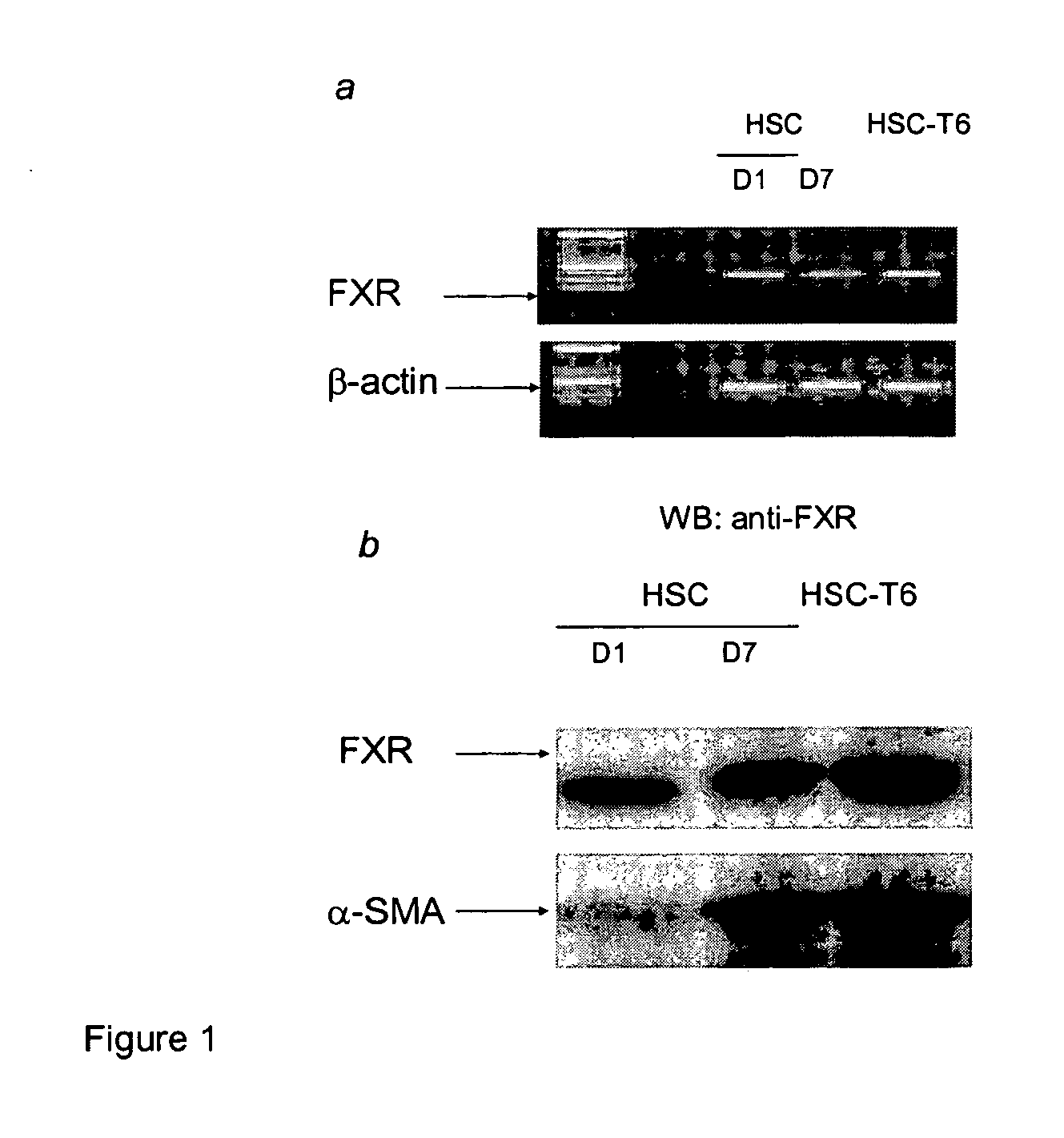
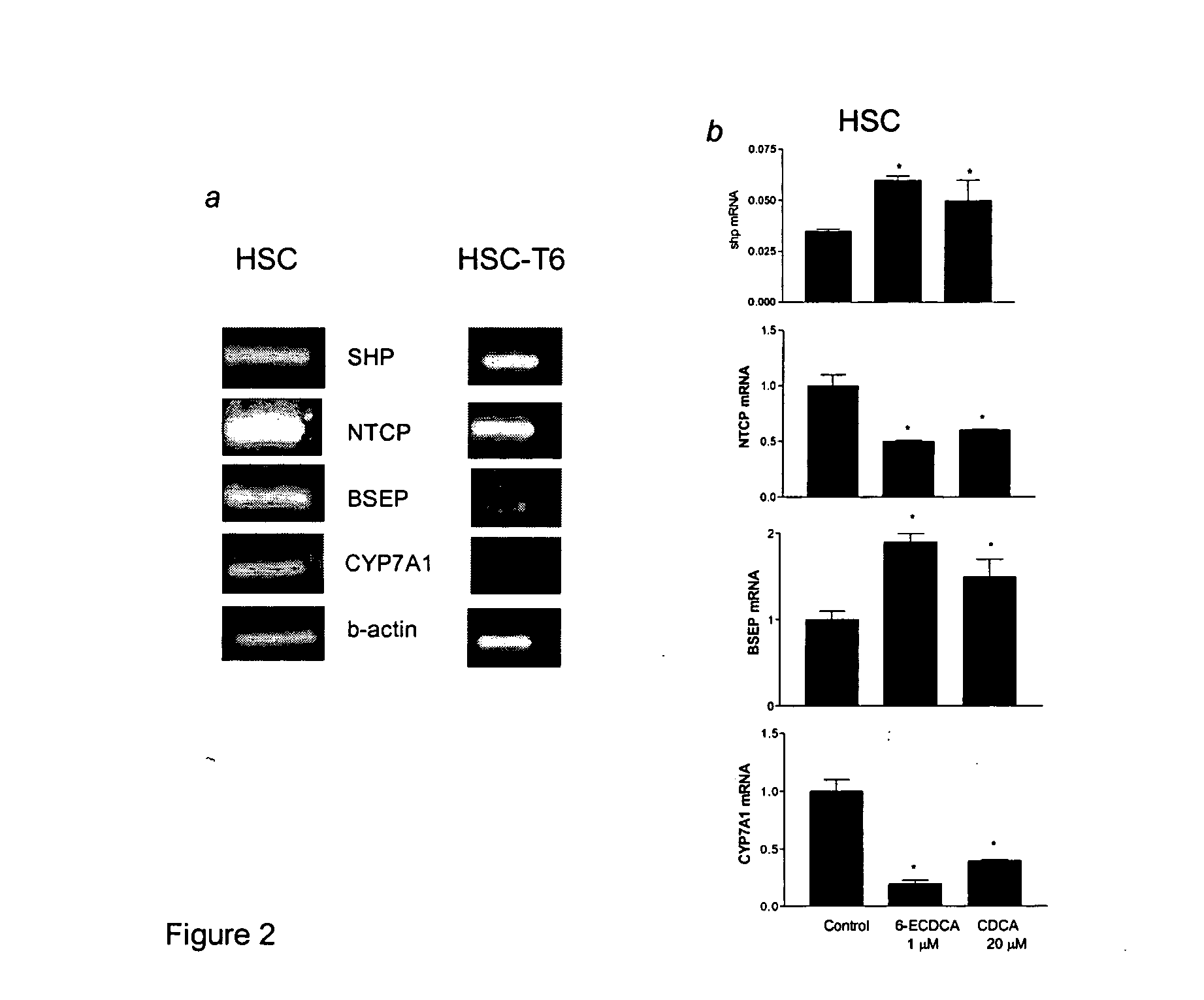
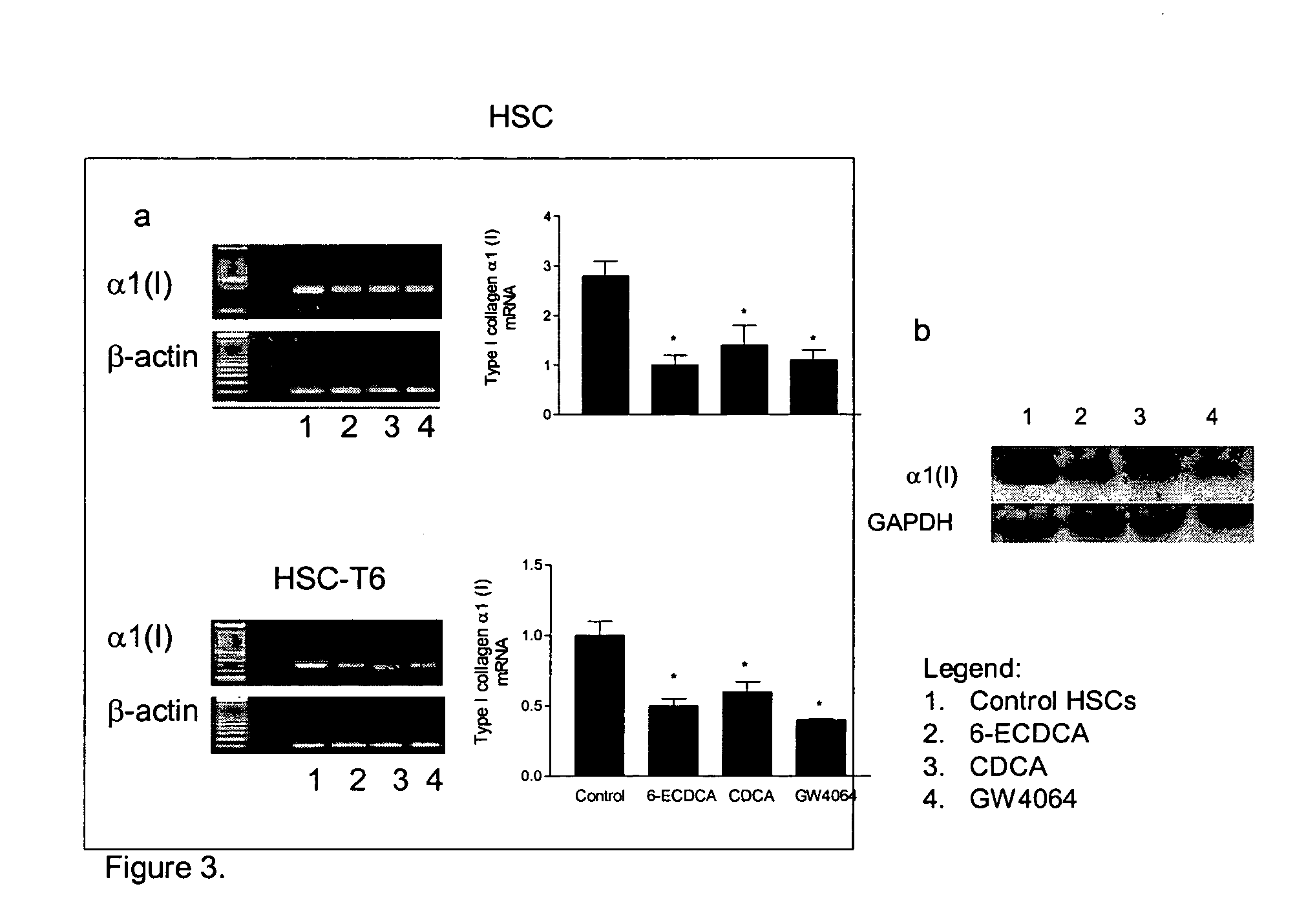
Treatment of fibrosis using FXR ligands
The present invention relates to a method for inhibiting fibrosis that occurs in an organ where the farnesoid X receptor (FXR) is expressed. This method involves the step of administering a high potency, activating ligand of FXR in an effective amount to a patient who is not suffering from a cholestatic condition. The invention also provides pharmaceutical compositions containing an effective amount of an FXR ligand and kits for dispensing the pharmaceutical compositions.
Owner:INTERCEPT PHARMA INC
Mesh pouches for implantable medical devices
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:MEDTRONIC INC
Compounds useful in inhibiting vascular leakage, inflammation and fibrosis and methods of making and using same
InactiveUS20050250694A1Prevent fibrosisInhibit angiogenesisPeptide/protein ingredientsDepsipeptidesPIGMENT EPITHELIUM-DERIVED FACTORAngiostatin
The present invention is directed to a method of inhibiting at least one of vascular leakage, inflammation and fibrosis in an animal by administering to the animal a vascular leakage inhibiting amount of a composition, wherein at a substantially higher amount the composition is effective in inhibiting angiogenesis, and wherein the anti-angiogenic activity of the composition is separate from the vascular leakage inhibiting activity of the composition. The animal experiencing at least one of vascular leakage, inflammation and fibrosis has a disease selected from the group consisting of diabetes, chronic inflammation, brain edema, arthritis, uvietis, macular edema, cancer, hyperglycemia, a kidney inflammatory disease, a disorder resulting in kidney fibrosis, a disorder of the kidney resulting in proteinuria, and combinations thereof. The composition capable of inhibiting at least one of vascular leakage, inflammation and fibrosis is selected from the group consisting of angiostatin, fragments of angiostatin, analogs or derivatives of angiostatin, kringle 5 of plasminogen, fragments of kringle 5 of plasminogen, analogs or derivatives of kringle 5 of plasminogen, pigment epithelium-derived factor, fragments of pigment epithelium-derived factor, analogs or derivatives of pigment epithelium-derived factor and combinations thereof.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Preventing biofilm formation on implantable medical devices
Owner:MEDTRONIC INC
Nerve conduit and preparation method thereof
ActiveCN102688076AAddress barriers to developmentModerate intensitySurgeryCatheterCatheterNerve repair
The invention discloses a nerve conduit and a preparation method thereof. The nerve conduit consists of an inner layer, an outer layer and at least one minitype cavity for storing a bioactive factor solution, wherein the inner layer is of a hydrophilic cell scaffold layer, and the outer layer is of a hydrophobic nerve conduit scaffold layer. The nerve conduit can further comprise a transition layer between the inner layer and the outer layer. The preparation method of the nerve conduit comprises the steps of preparing the inner layer by adopting an electrostatic spinning method, then adding the material for preparing the cavity, then preparing the outer layer, and taking out or dissolving the material for preparing the cavity, thus obtaining the nerve conduit. The nerve conduit has the minitype cavity, so that bioactive factors can be loaded by a manner of injection, soaking and the like as required before operation, thus not only leading the production quality to be easily controlled, but also being capable of greatly improving the survival rate of the bioactive factors, more efficiently promoting the regeneration of nerves, and enhancing the restoration effect of nerves.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Platelet Derived Growth Factor (PDGF) Nucleic Acid Ligand Complexes
InactiveUS20080207883A1Convenient treatmentInhibit angiogenesisOrganic active ingredientsPeptide/protein ingredientsImmunogenicityLipophilicity
This invention discloses a method for preparing a complex comprised of a PDGF Nucleic Acid Ligand and a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound by identifying a PDGF Nucleic Acid Ligand by SELEX methodology and associating the PDGF Nucleic Acid Ligand with a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound. The invention further discloses Complexes comprising one or more PDGF Nucleic Acid Ligands in association with a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound. The invention further includes a Lipid construct comprising a PDGF Nucleic Acid Ligand or Complex and methods for making the same.
Owner:GILEAD SCI INC
Functional layers of biomolecules and living cells, and a novel system to produce such
InactiveUS20110139617A1Maintain activityEliminate needImmobilised enzymesVolume/mass flow measurementCell layerEngineering
The present invention concerns a new process for depositing a thick compact layer of biomolecules for instance such a layer with thickness in the μm scale and, for depositing a thick compact layer of cells in the μm scale. The deposited layer is made by application of an unbalanced (asymmetrical) alternating voltage polarization between two electrodes to a dissolved biomolecule or cell from low conductivity solutions. The process allows the rapid manufacturing of sensors and the coating of devices with functional cells and biomolecules. Examples are provided on the preparation of functional sensors such as a glucose, a lactose sensor, a hydrogen peroxide sensor and a glutamate sensor. Examples are also provided on the deposition of eukaryoric cells such as saccharomyces cerevisiae. The examples demonstrate a process that can be applied to coat devices with biomolecules and biological cells.
Owner:KATHOLIEKE UNIV LEUVEN
Fused ring analogues of Anti-fibrotic agents
InactiveUS20120270863A1Prevent fibrosisGood treatment effectBiocideOrganic chemistryDiseaseAnti fibrotic
Owner:CERTA THERAPEUTICS PTY LTD
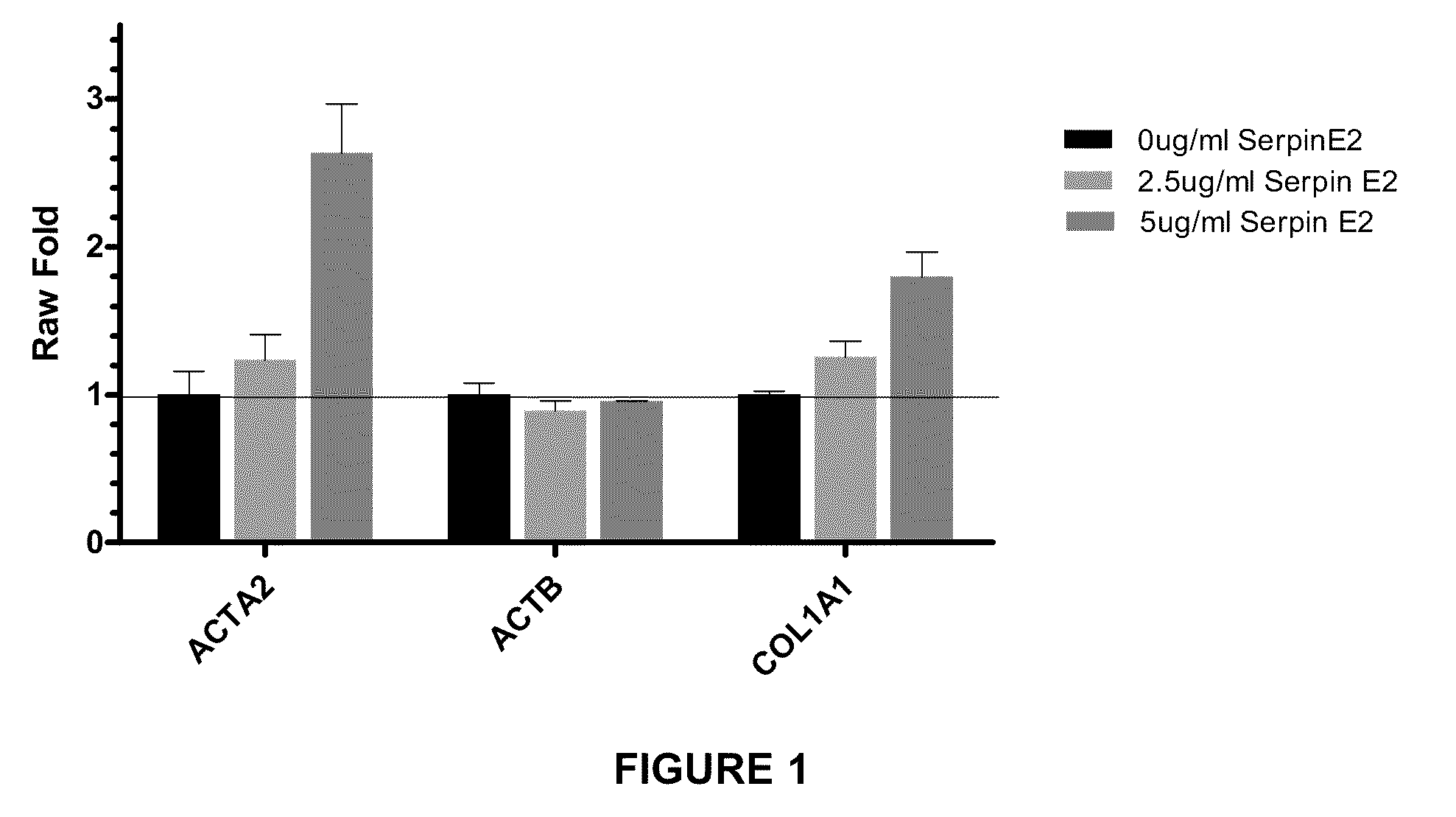
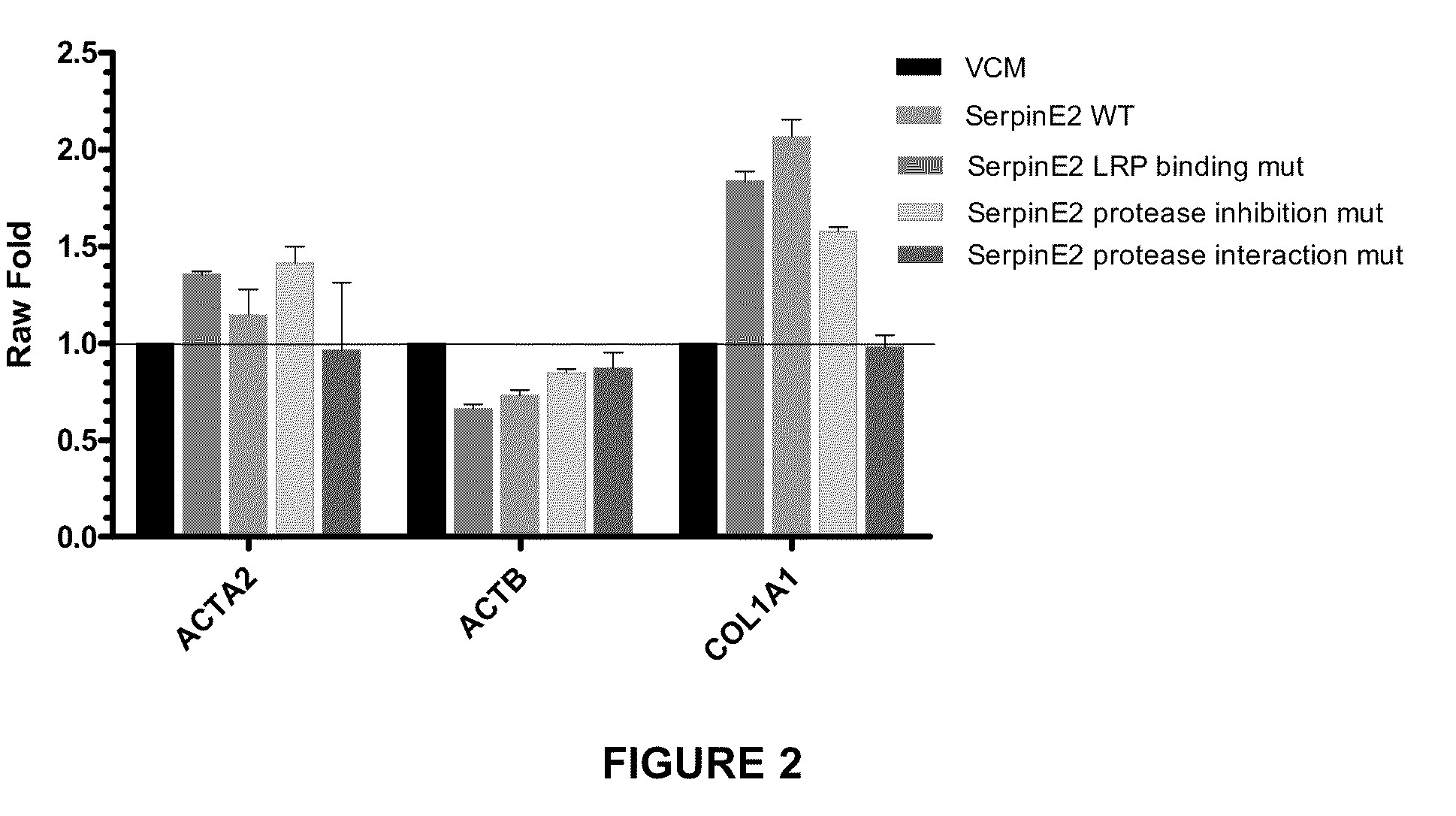
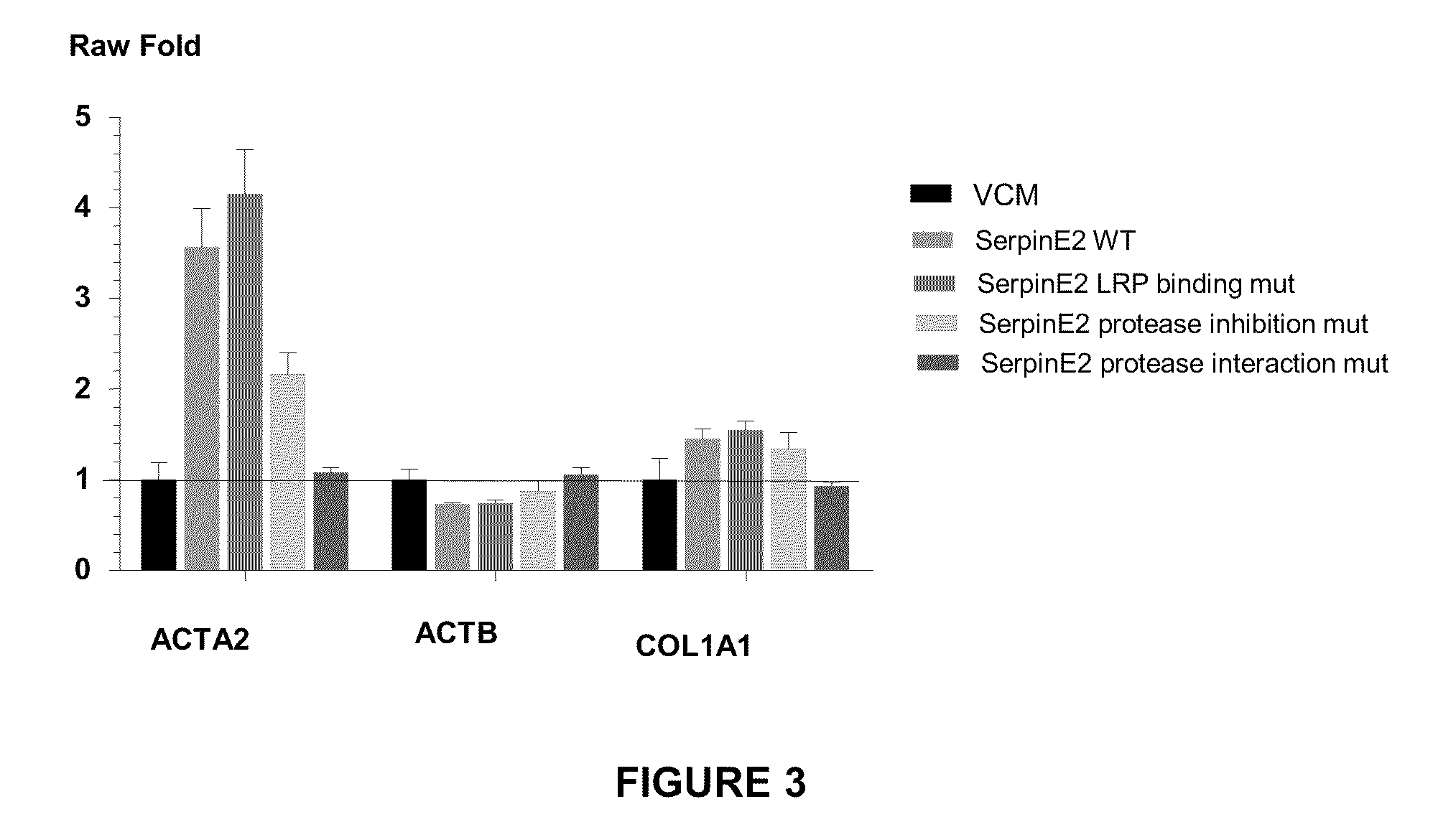
Compositions and methods for regulating collagen and smooth muscle actin expression by serpine2
InactiveUS20100183620A1Reduce expressionReduce formationOrganic active ingredientsPeptide/protein ingredientsObstructive Pulmonary DiseasesActin
The invention encompasses methods and compositions for increasing or decreasing collagen 1A1 expression and / or α-smooth muscle actin expression in lung fibroblasts using SERPINE2 and antagonists of SERPINE2. The invention also encompasses methods and compositions for increasing or decreasing the formation of myofibroblasts. The invention further provides methods and compositions for treatment of lung diseases, such as idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease.
Owner:JANSSEN BIOTECH INC +1
Nerve conduit and preparation method thereof
The invention discloses a nerve conduit and a preparation method thereof. The nerve conduit comprises an inner layer, a transition layer, an outer layer and at least one cavity used for storing a bioactive factor solution, wherein the outer surface of the inner layer is sequentially coated with the transition layer and the outer layer; the cavity is positioned between the inner surface of the outer layer and the outer surface of the transition layer and / or between the outer surface of the inner layer and the inner surface of the transition layer; the inner layer is a hydrophilic cytoskeleton layer made by adopting an electrostatic spinning method; and the outer layer is a hydrophobic nerve conduit scaffold layer made by adopting the electrostatic spinning method. The nerve conduit can be used for loading bioactive factors before an operation in a required mode, such as injection, soaking and the like due to the miniature cavity, so that the production quality of the product is easy to control, the survival rate of the bioactive factor can be greatly improved, the nerve regeneration can be promoted in high efficiency, and the nerve repairing effect can be reinforced.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Mesh Pouches for Implantable Medical Devices
ActiveUS20150086604A1Reduces and prevents implantReduces and prevents and surgery-related complicationBiocideElectrotherapyFiberSide effect
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:TYRX
Tissue engineering tendon and vitro construction method thereof
The invention discloses an organ engineering tendon graft which comprises (a) biodegradable materials which can be accepted in pharmacy; (b) seed cells which can be inoculated to the biodegradable materials, and the seed cells are selected from the following groups: (i) fibroblasts, (ii) adipose-derived cells, or (iii) the mixture of the dermal fibroblasts and the adipose-derived cells with the ratio of 1:10000 to 10000:1. The graft is gotten from the cultivation of a complex of the seed cells and the biodegradable materials in a bioreactor, and the complex is obtained through the mixing of the seed cells and the biodegradable materials which can be accepted in pharmacy. The graft can be used for restoring the tendon defect.
Owner:SHANGHAI TISSUE ENG LIFE SCI
Composition for preventing the formation of new scar comprising bmp-7
InactiveUS20060122109A1Avoid scaringPrevent fibrosisSenses disorderPeptide/protein ingredientsBiologyMyofibroblast
Composition for preventing the formation of new scar, e.g. myofibroblast, having BMP-7 polypeptide is disclosed. The composition for preventing the formation of scar includes an effective amount of BMP-7 (Bone Morphogenic Protein-7) polypeptide of sequence 1. The effective amount is 50 ng / ml-50 μg / ml or 0.1 ng-1 μg / kg by weight and the scar is a corneal scar.
Owner:EYEGENE INC +1
Fibrosis inhibitor for implanted organ
InactiveUS7696170B2Prevent fibrosisSuppress the fibrosis of a transplanted organPeptide/protein ingredientsGenetic material ingredientsFibrosisHepatocyte growth factor
A pharmaceutical preparation comprising a hepatocyte growth factor or a DNA molecule encoding the same and the like according to the present invention can suppress the fibrosis of a transplanted organ after organ transplantation. The present invention is useful in the fields of organ transplantation and regeneration therapy.
Owner:KRINGLE PHARMA INC
Application of miR-21 in preparation of medicine for treating intrauterine adhesion and/or thin inner membranes
ActiveCN107519194APrevent fibrosisOrganic active ingredientsMicrobiological testing/measurementDiseaseGynecology
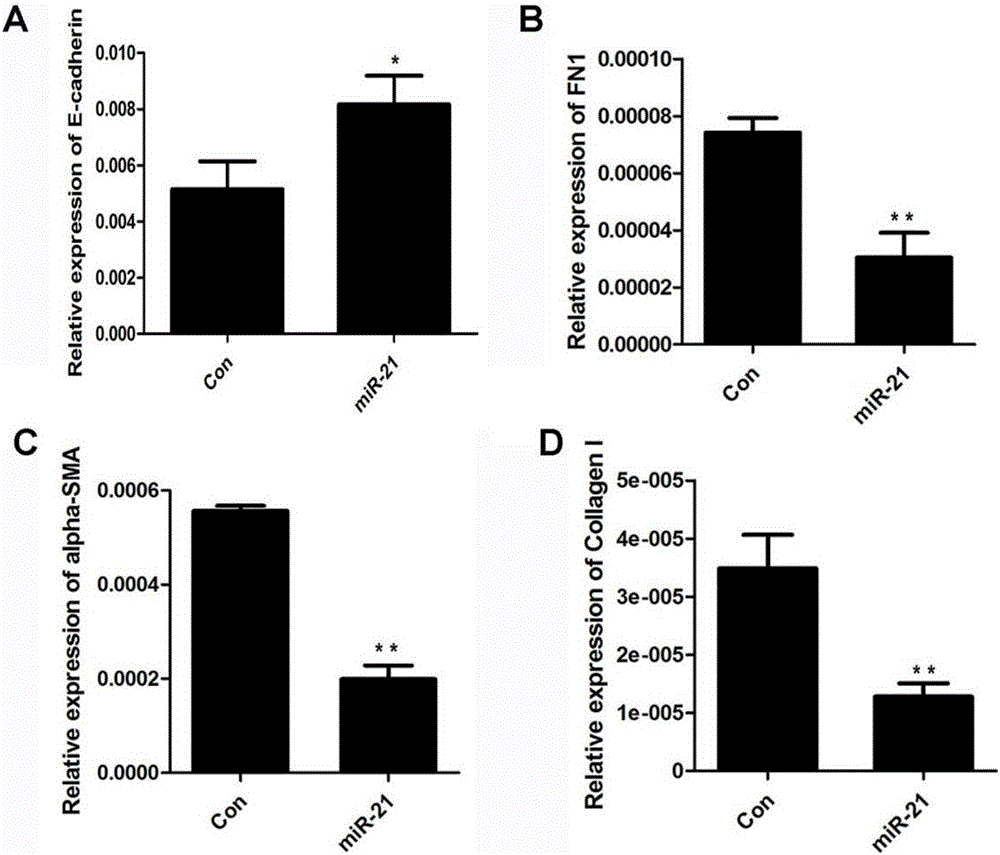
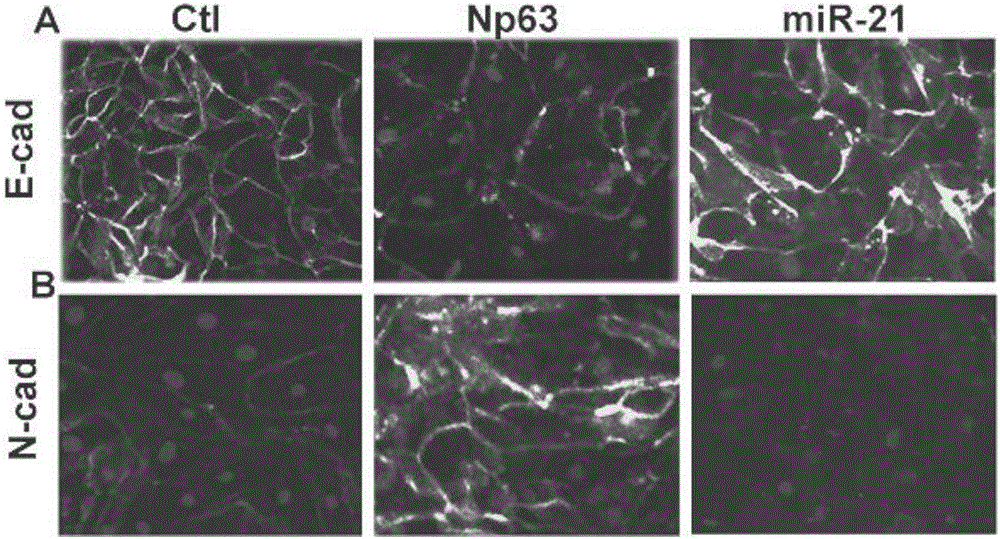
The invention discloses application of miR-21 in preparation of medicine for treating intrauterine adhesion and / or thin inner membranes. The study finds that the miR-21 expression for endometrial epithelium cells and mesenchymal cells is obviously reduced, and particularly the decrease for epithelial cells is most remarkable. Moreover, in vitro results indicate that the EMT of the endometrial epithelium cells is inverted through miR-21, and fibrosis is inhibited. Therefore, the miR-21 possibly plays important roles in diagnose and treatment aspects of intrauterine adhesion and / or thin inner membranes, and can be used for preparing medicine for diagnosing and treating intrauterine adhesion and / or thin inner membranes or preparing medicine for treating other diseases related to womb fibrosis.
Owner:NANJING DRUM TOWER HOSPITAL
Application of micromolecular polypeptide KP-6 in preparing drug for treating chronic kidney disease
ActiveCN106822865APrevent fibrosisNo obvious side effectsPeptide/protein ingredientsUrinary disorderSide effectTissue fibrosis
The invention belongs to the field of biological medicine, and relates to application of micromolecular polypeptide KP-6, in particular to application of micromolecular polypeptide KP-6 in preparing a drug for treating a chronic kidney disease (CKD). An amino acid sequence of the polypeptide KP-6 is shown as SEQ ID NO. 1. The micromolecular polypeptide KP-6 has a significant effect of inhibiting kidney tissue fibrosis and CKD development, has no obvious toxic or side effects, and therefore can be used for preparing the drug for effectively treating the chronic kidney disease.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
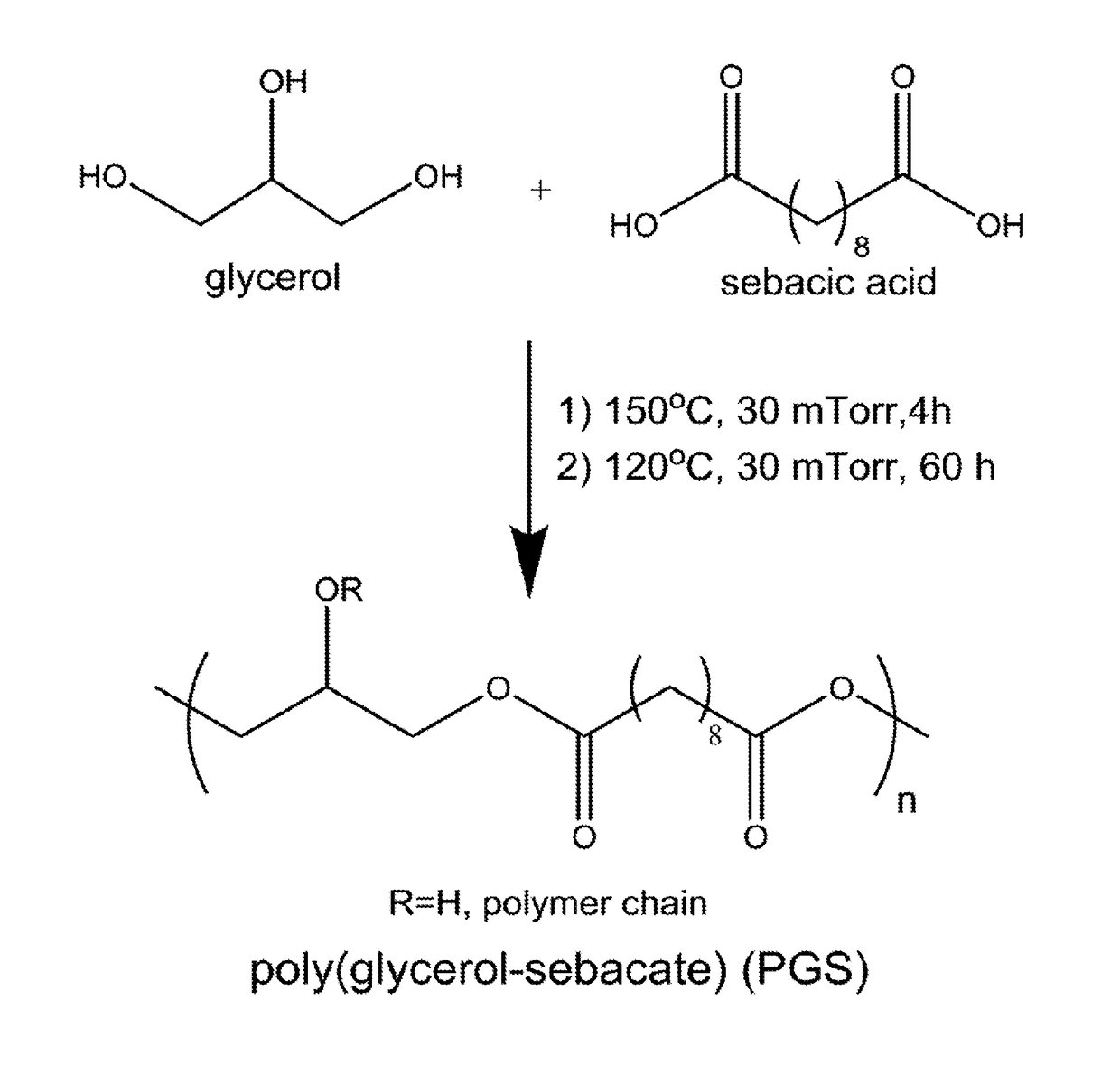
Synthesis and use of poly(glycerol-sebacate) films in fibroblast growth regulation
InactiveUS20170290950A1Prevent fibrosisReduce scarsOrganic active ingredientsPharmaceutical non-active ingredientsFiberMedicine
Owner:WAKE FOREST UNIV HEALTH SCI INC
Method for constructing cell transplantation slice by using human umbilical cord mesenchymal stem cells (hUC-MSCs) and amniotic membrane
InactiveCN105647859AInhibition formationWide variety of sourcesArtificial cell constructsSkeletal/connective tissue cellsHuc mscsWharton's jelly
The invention relates to a method for constructing a cell transplantation slice by using human umbilical cord mesenchymal stem cells (hUC-MSCs) and anthropogenic amniotic membrane. The method comprises the following steps: taking the freshly collected anthropogenic amniotic membrane and umbilical cord, separating chorion from the amniotic membrane, treating the separated amniotic membrane by normal saline, and then cryopreserving for later use; cutting the umbilical cord into small pieces, and tearing Wharton's jelly off; cutting the Wharton's jelly into pieces, putting the cut Wharton's jelly onto a sterile culture vessel, adding a culture solution into the sterile culture vessel, and putting the sterile culture vessel into an incubator for culturing to obtain primary generation hUC-MSCs; adding pancreatin into a culture medium of the primary generation hUC-MSCs, digesting, and continuously culturing in the incubator to obtain the hUC-MSCs; carrying out sterile hydration on the cryopreserved standby amniotic membrane, then flatly laying the amniotic membrane on an amniotic membrane carrier thimble, and then moving into the sterile culture vessel; inoculating the hUC-MSCs in the culture vessel, and then culturing in the incubator to obtain the cell transplantation slice with cell confluence reaching up to 80-90%. The cell transplantation slice prepared by the method is capable of carrying out damage repair at specific parts, so that the defects of using the hUC-MSCs or amniotic membrane alone for treatment are overcome; therefore, a new treatment strategy is provided for refractory diseases, and a new therapy approach is opened up for the clinical application of stem cells.
Owner:秦方园 +1
Oral dilator
ActiveUS20180085274A1Prevented from feelingImproving food-takingSurgeryChiropractic devicesEngineeringDilator
An oral dilator includes a first body, a second body, a rotary member, and a positioning member. The first body includes a housing and a first duckbilled element. The second body includes a second duckbilled element corresponding to the first duckbilled element. The rotary member drives the second body to rotate. The positioning member is on the first body and selectively locks or unlocks the relative position between the first duckbilled element and the second duckbilled element when the second duckbilled element is driven to rotate by the rotary member.
Owner:MEGAFORCE +1
Application of micro-molecule polypeptide KP-1 in preparing medicine for treating chronic kidney diseases
ActiveCN108042791APrevent fibrosisInhibit CKD progressionPeptide/protein ingredientsUrinary disorderSide effectCurative effect
The invention relates to a novel application of a micro-molecule polypeptide KP-1, and in particular to an application of the micro-molecule polypeptide KP-1 in preparing a medicine for treating chronic kidney diseases (CKD). The invention provides the application of the KP-1 in treating the CKD; and the KP-1 is obvious in curative effect and is free from obvious toxic and side effects. Therefore,the KP-1 provided by the invention can be used for preparing a pharmaceutical preparation for treating the CKD.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Therapeutics to facilitate cell transplantation for liver disease
InactiveUS20080181869A1Inhibition of differentiationDecreasing production of collagenBiocidePeptide/protein ingredientsLiver repairLiver fibrosis
The present invention provides compositions, formulations and methods for treating liver diseases related to tissue inflammation and progressive fibrosis, e.g., progressive liver fibrosis following either chronic or acute injury. The compositions of the invention provide the use of therapeutic agents as an adjunct therapy to transplantation of cell populations capable of effecting liver repair.
Owner:DEVORE DIANNA LOUISE
PPS (polyphenylene sulfide) and nano Al2O3 filled PTFE (polytetrafluoroethylene) composite material
InactiveCN108102261AEasing plastic deformationInhibit fibrosisPtfe compositeScanning electron microscope
The invention provides a PPS (polyphenylene sulfide) and nano Al2O3 filled PTFE (polytetrafluoroethylene) composite material. PPS and nano Al2O3 filled PTFE composite material samples are prepared with a method comprising steps of mechanical blending, cold press molding and sintering; frictional wear performance of the samples under the dry friction condition is tested by an MRH3 type ring-block tester; an SEM (scanning electron microscope) is adopted for observing and analyzing morphology of the wear surface and the transfer membrane surface of each sample. When the mass fraction of PPS is 5%, the friction coefficient and volume abrasion rate of the PPS / PTFE composite material both reach the minimum value; with addition of nano Al2O3, the tribological property of the PPS / PTFE composite material is further improved, when the mass fraction of nano Al2O3 is 5%, the volume abrasion rate of the nano Al2O3 / PPS / PTFE composite material is the minimum, and wear resistance is increased by 276 times as compared with that of pure PTFE. The main wear mechanism of PTFE / PPS / PTFE and the nano Al2O3 / PPS / PTFE composite material is adhesion wear at the room temperature, and the nano Al2O3 / PPS / PTFE composite material is accompanied with abrasive wear at 150 DEG C.
Owner:刘芳
Filling modified polytetrafluoroethylene and preparation method thereof
The invention belongs to the technical field of high polymer materials, and particularly relates to filling modified polytetrafluoroethylene and a preparation method thereof. The filling modified polytetrafluoroethylene provided by the invention is prepared from resonance mixed powder through curing, mould pressing extrusion, calendering, extrusion aid removal and stretching puffing; and the resonance mixed powder comprises the following components: polytetrafluoroethylene resin, carbon nanotubes, a fluorine-containing silane coupling agent and an extrusion aid. A resonance mixing technology is adopted to replace traditional high-speed mixing, so that fibration of polytetrafluoroethylene resin in the mixing process is avoided, and the puffing performance of the polytetrafluoroethylene resin-based material is improved; meanwhile, the fluorine-containing silane coupling agent is added, so that the interfacial compatibility of the carbon nanotubes and the polytetrafluoroethylene is remarkably improved, and the dispersion uniformity of the carbon nanotubes in the polytetrafluoroethylene is improved; and on the basis, a tensile expansion technology is combined to prepare the filling modified polytetrafluoroethylene material with high conductivity, high mechanical strength and low density.
Owner:成都希瑞方晓科技有限公司
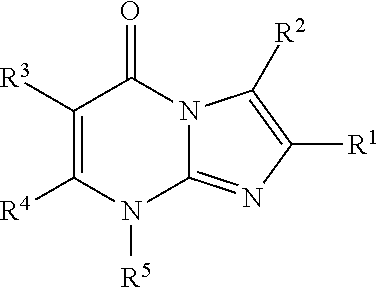
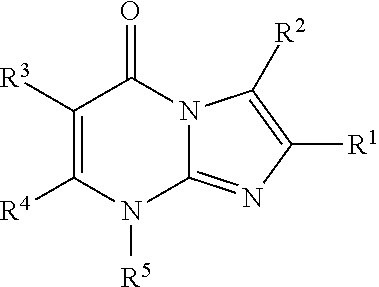
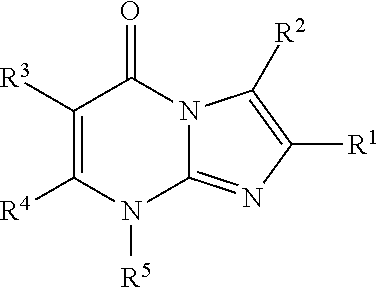
8-substituted imidazopyrimidinone derivative having autotaxin inhibitory activity
InactiveUS20160002247A1High activityPrevent fibrosisOrganic chemistryUrinary disorderAutotaxinPharmaceutical Substances
A compound of formula (I) wherein variables are as defined herein having an autotaxin inhibitory effect and a pharmaceutical composition comprising the same.
Owner:THE UNIV OF TOKYO +2
Sedum aizoon L. tea
InactiveCN101816357AEnhance physical fitnessAvoid absorptionTea substituesLiver tissueLiver fibrosis
The invention relates to Sedum aizoon L. tea, which is prepared by using the following raw materials in percentage by weight: 70 to 85 percent of Sedum aizoon L. leaves, 11 to 30 percent of aloe leaves, 3 to 15 percent of ginseng leaves and 3 to 15 percent of folium cortex eucommiae, optimally 76 percent of Sedum aizoon L. leaves, 18 percent of aloe leaves, 3 percent of ginseng leaves and 3 percent of folium cortex eucommiae. Sitosterol in the Sedum aizoon L. tea can prevent the cholesterol absorption of human bodies, reduce blood fat and prevent angiosclerosis; flavones in the Sedum aizoon L. tea can expand blood vessels, promote blood circulation and reduce myocardial oxygen consumption; and oleanolic acid in the Sedum aizoon L. tea can protect liver tissues and prevent liver fibrosis. The Sedum aizoon L. tea can be drunk for a long time for strengthening body, preventing arteriosclerosis and heart diseases, protecting the liver and delaying aging.
Owner:肖永英
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com