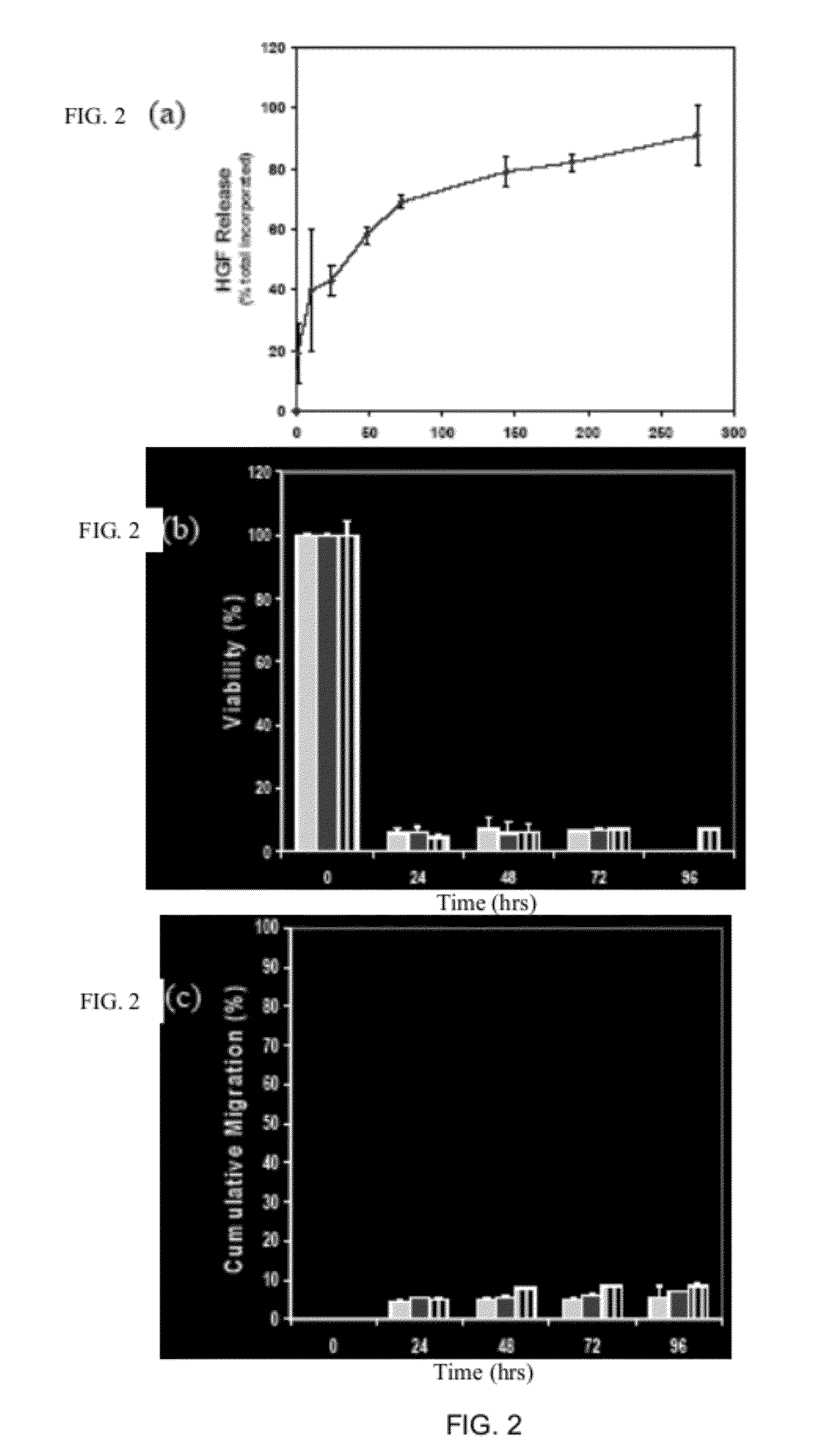
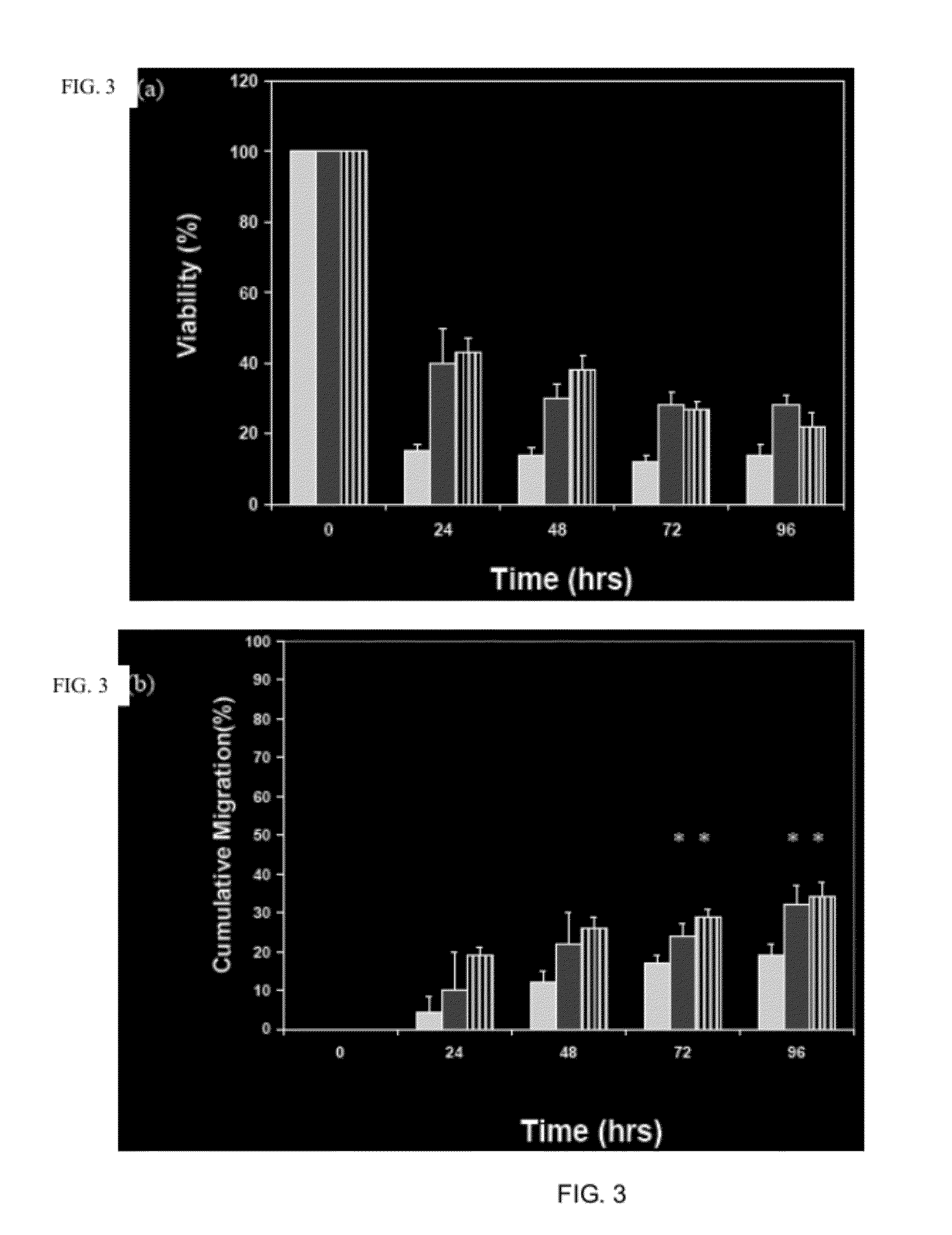
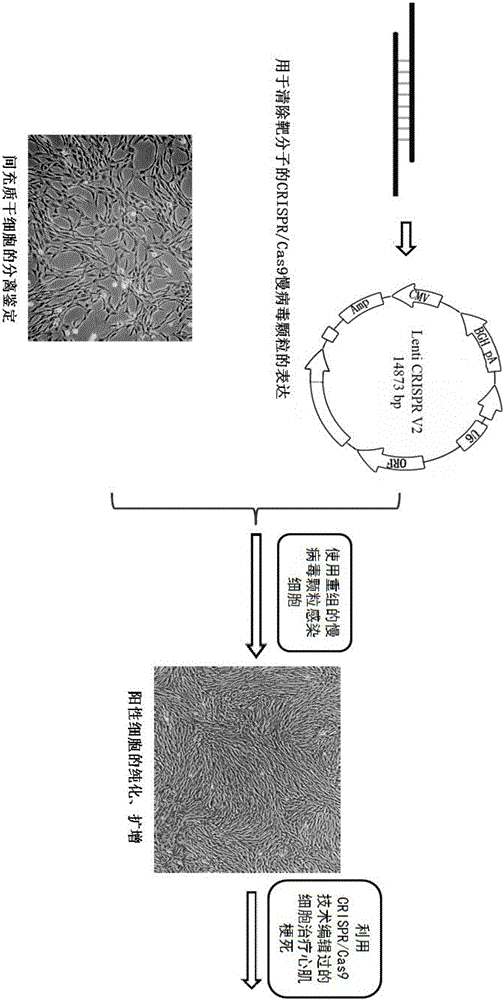
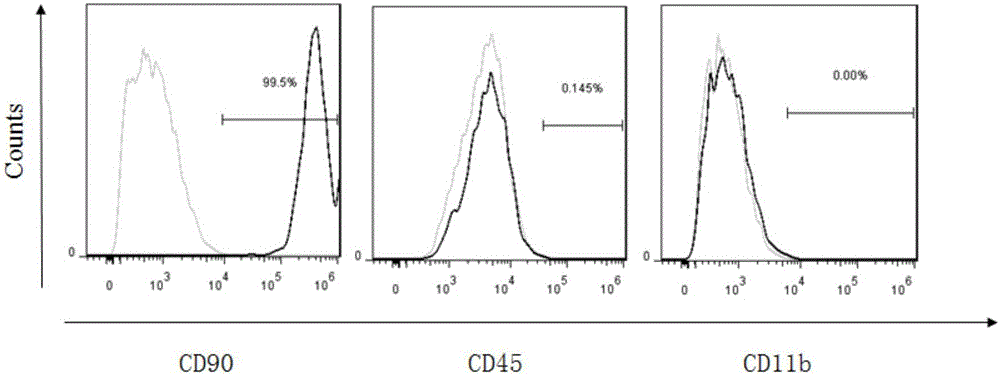
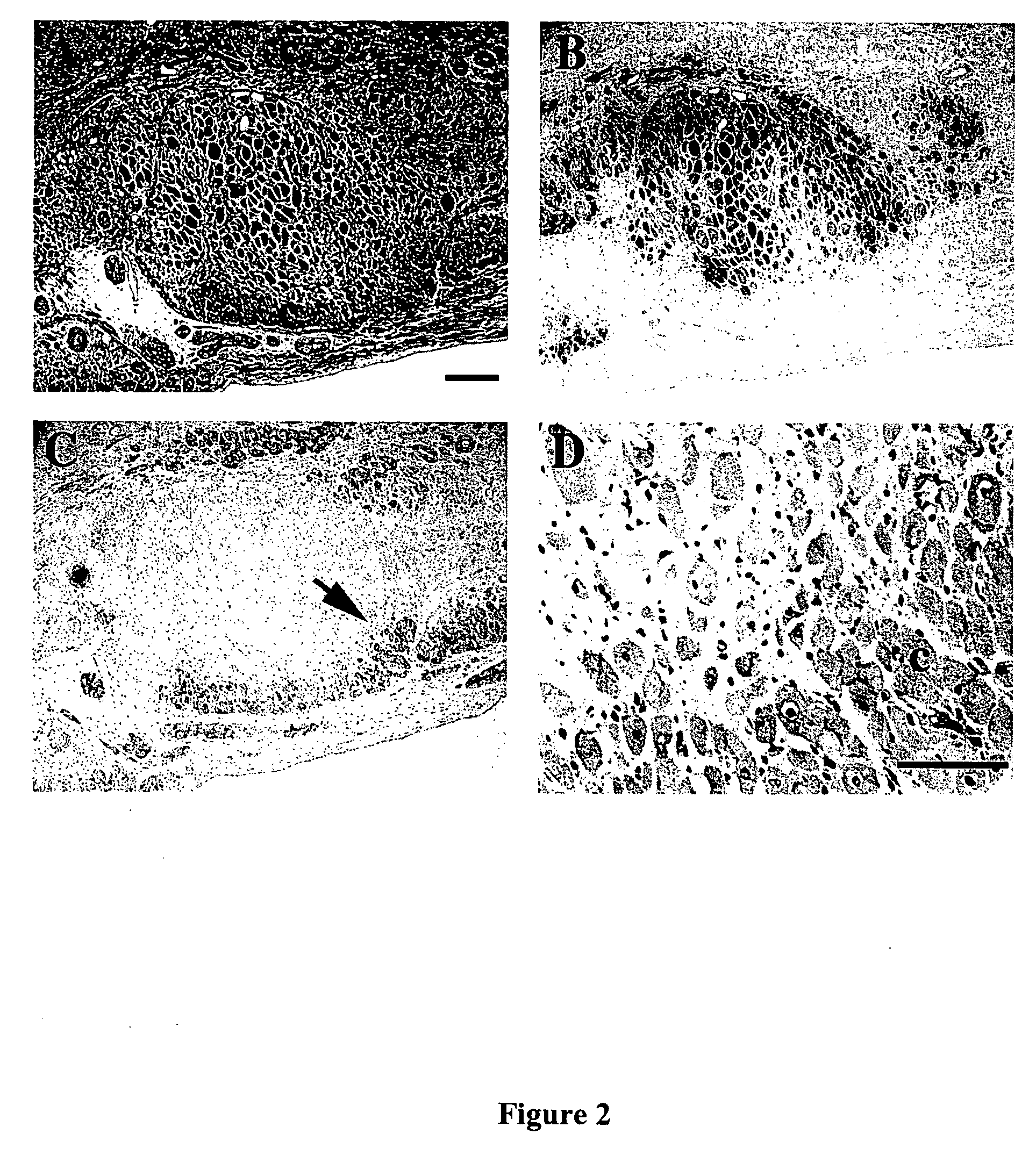
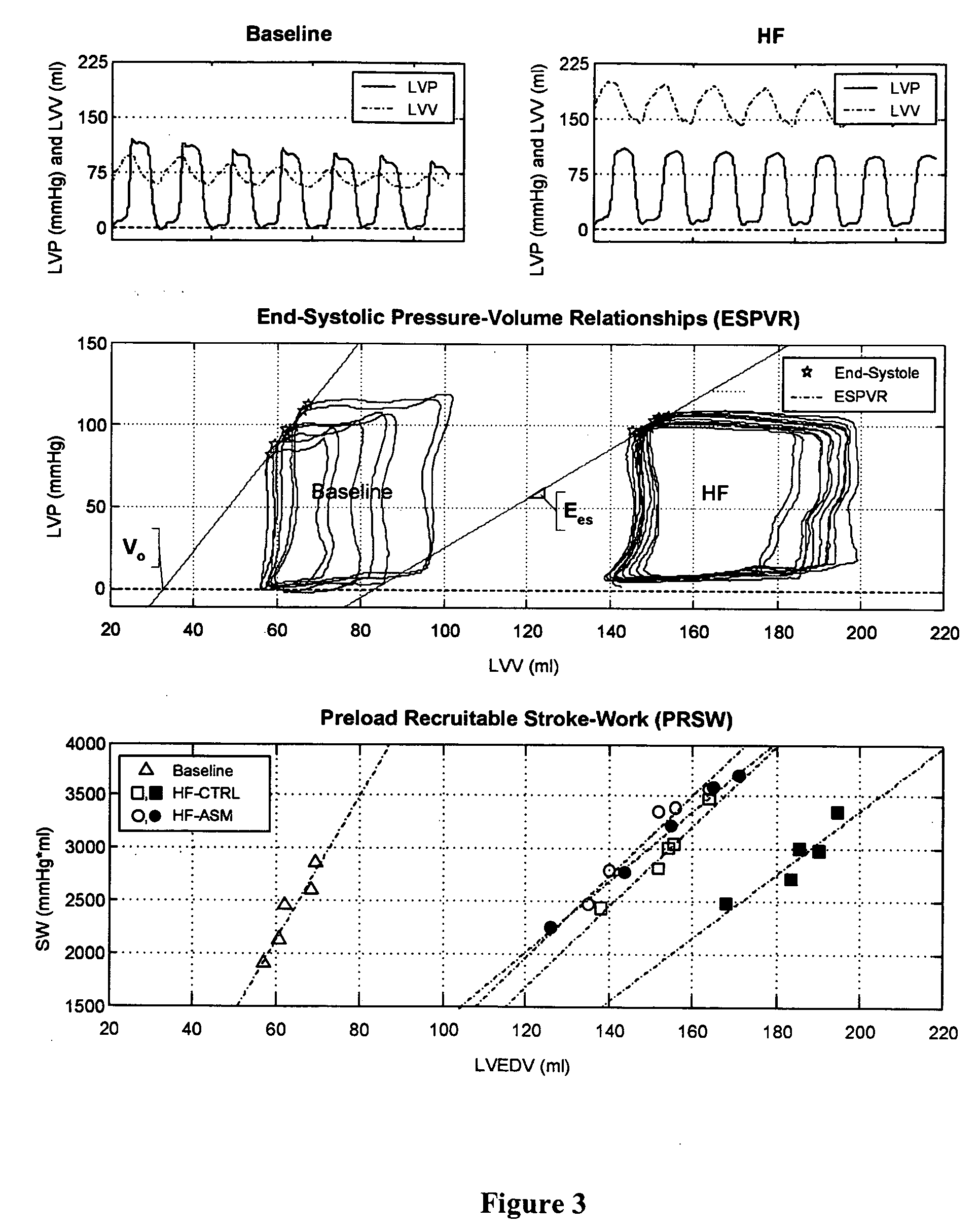
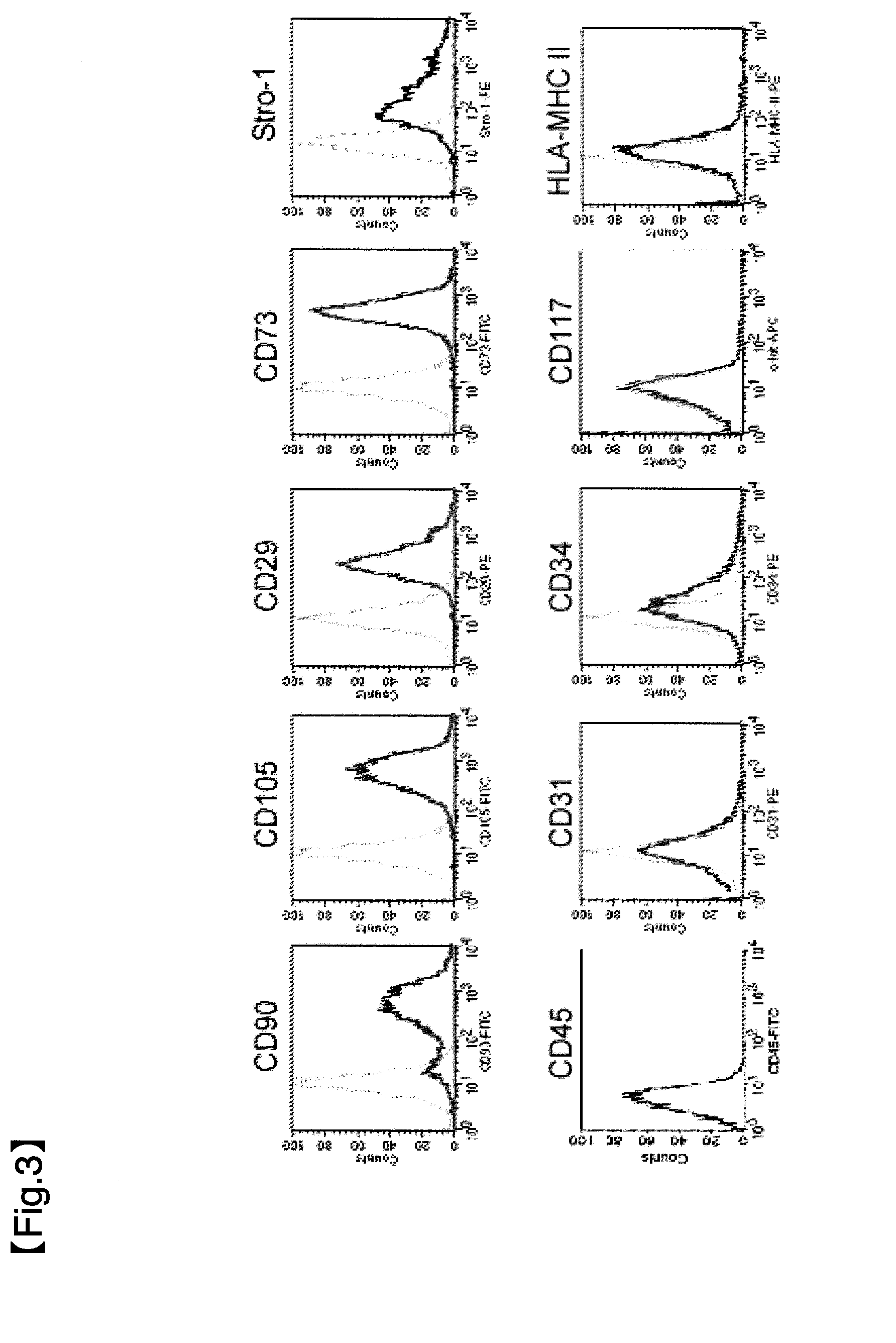
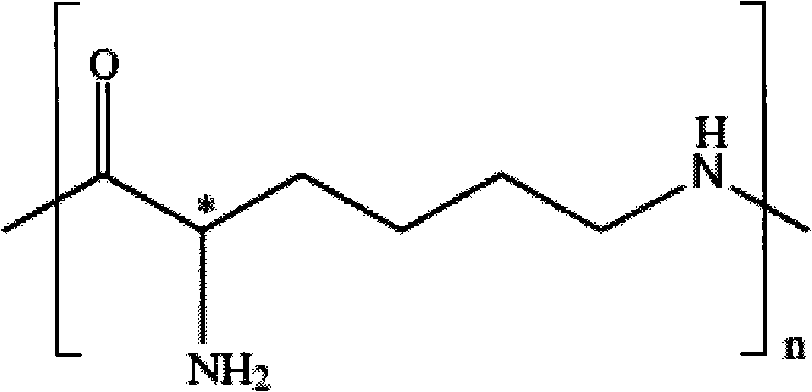
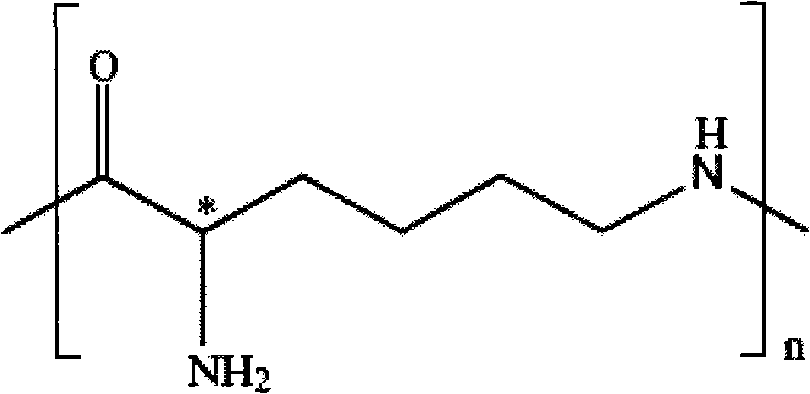
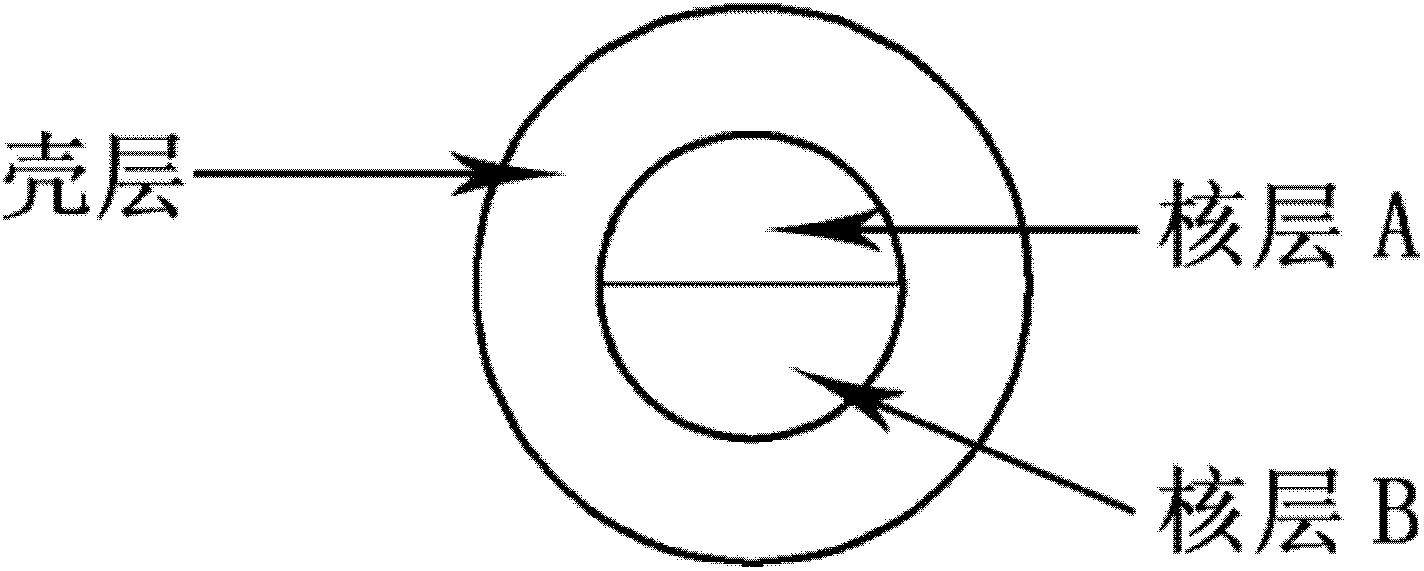
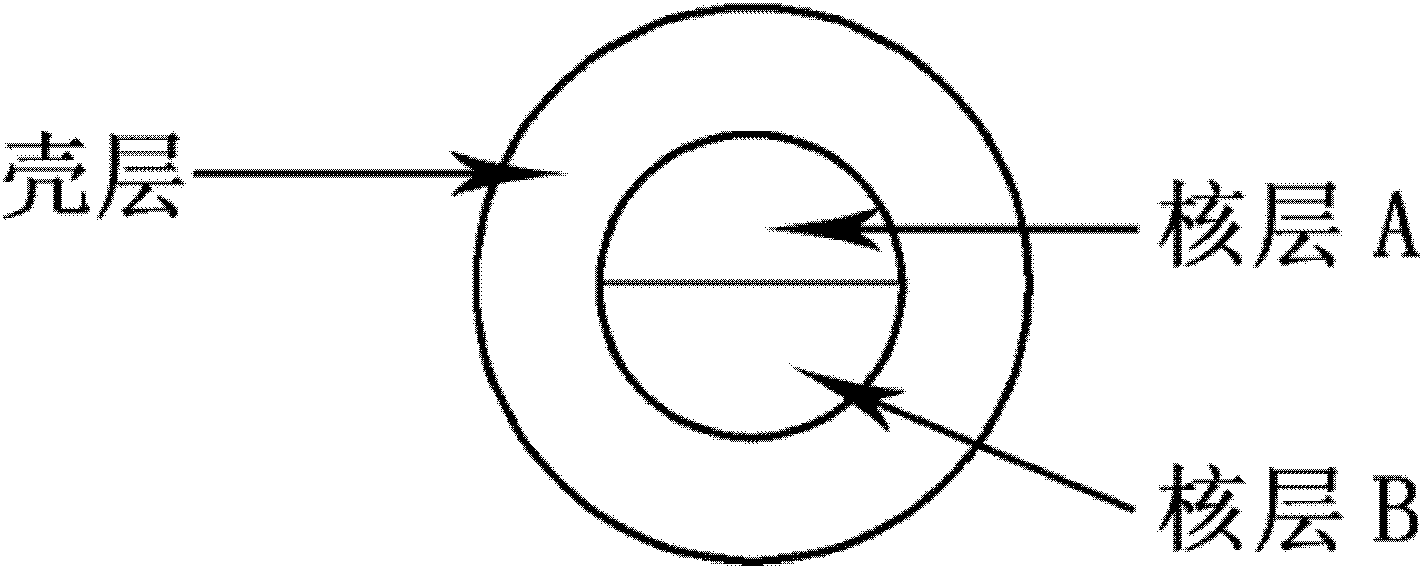
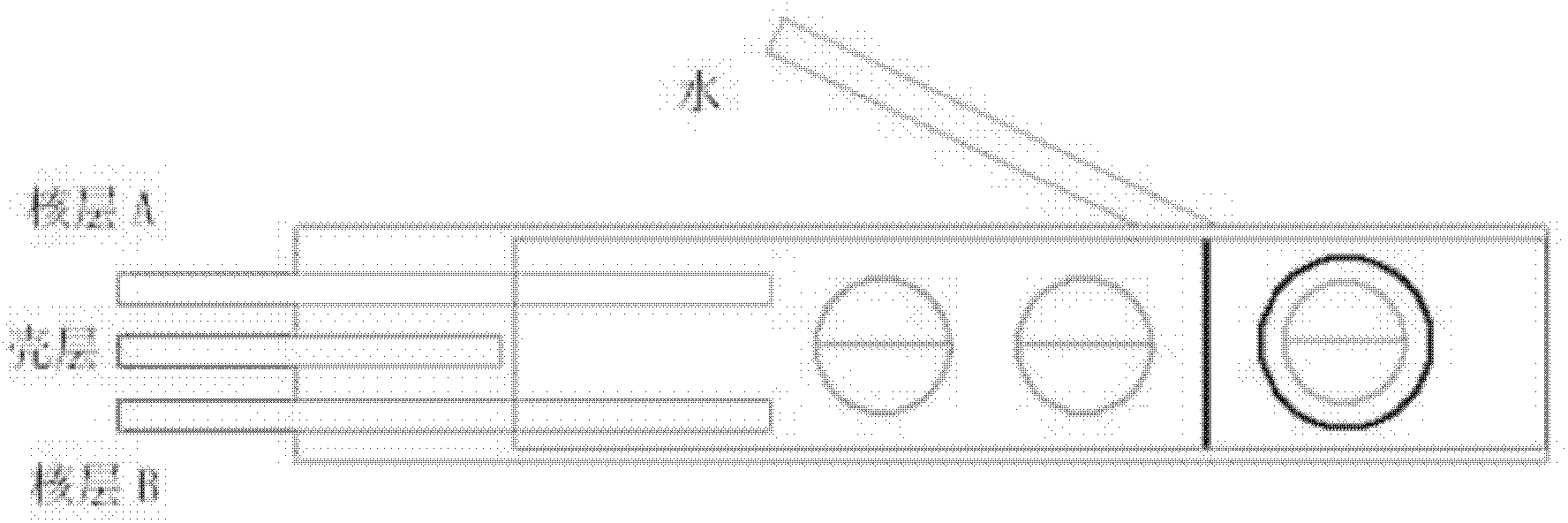Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
236 results about "Cell transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
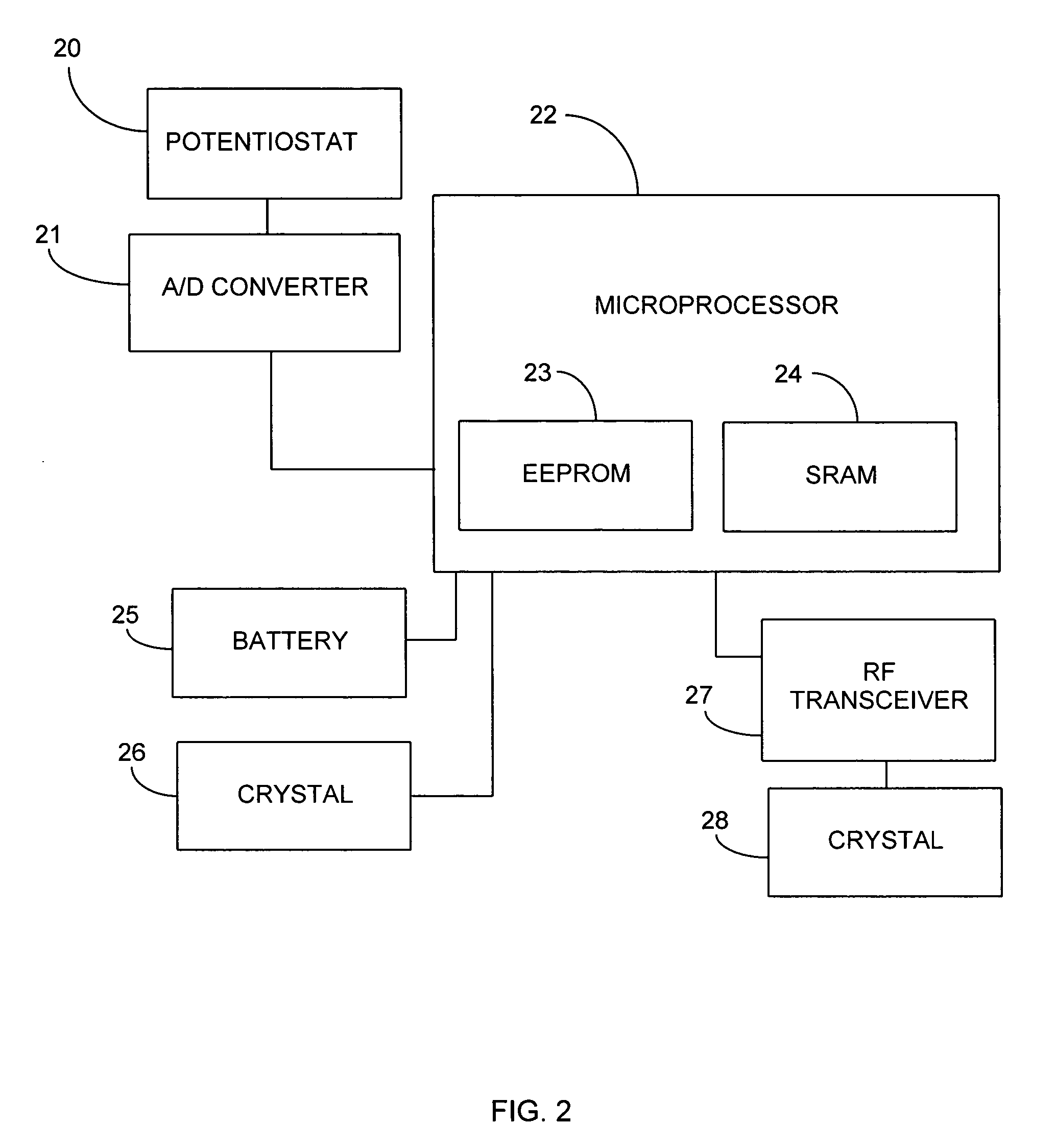
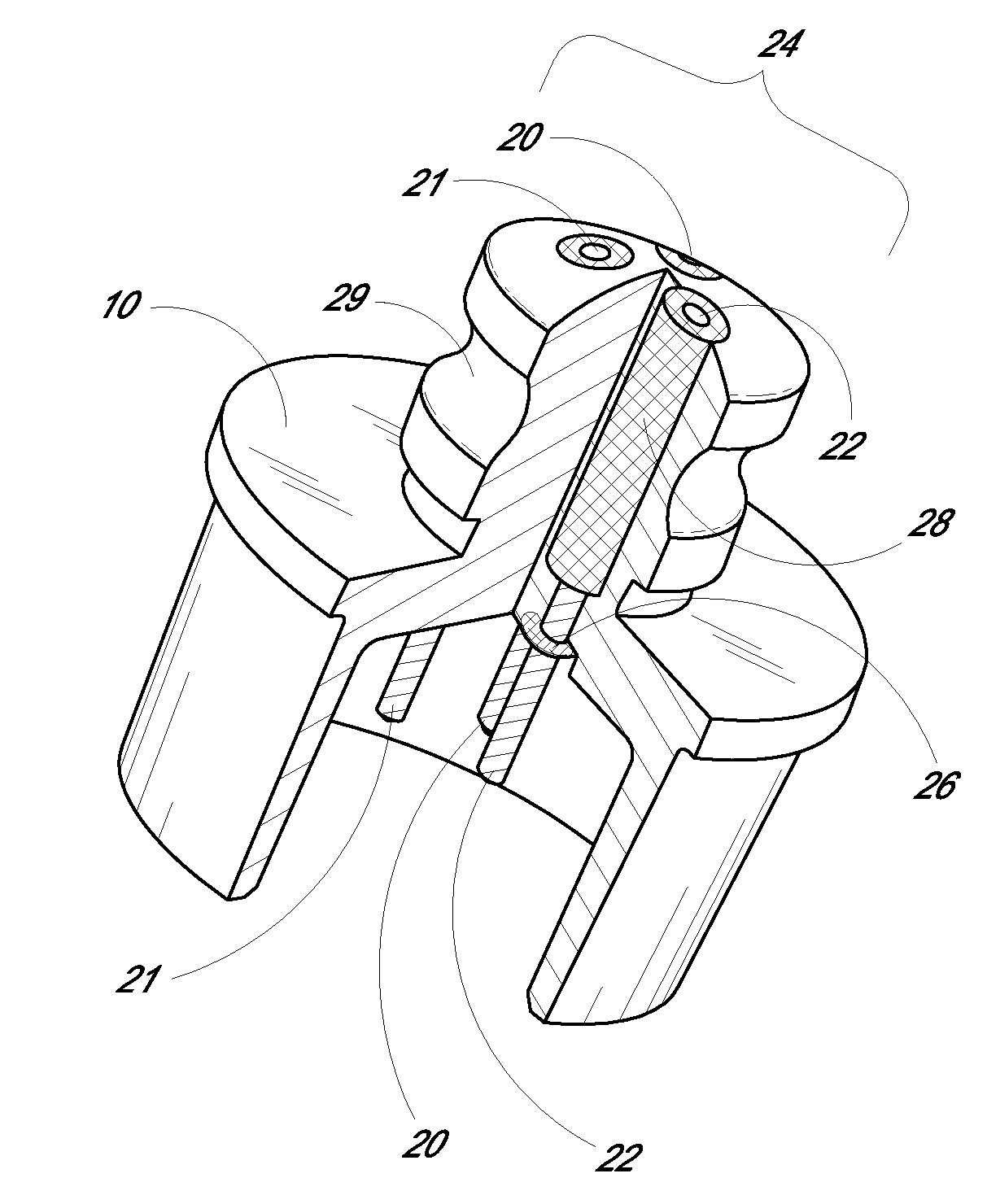
Porous membranes for use with implantable devices
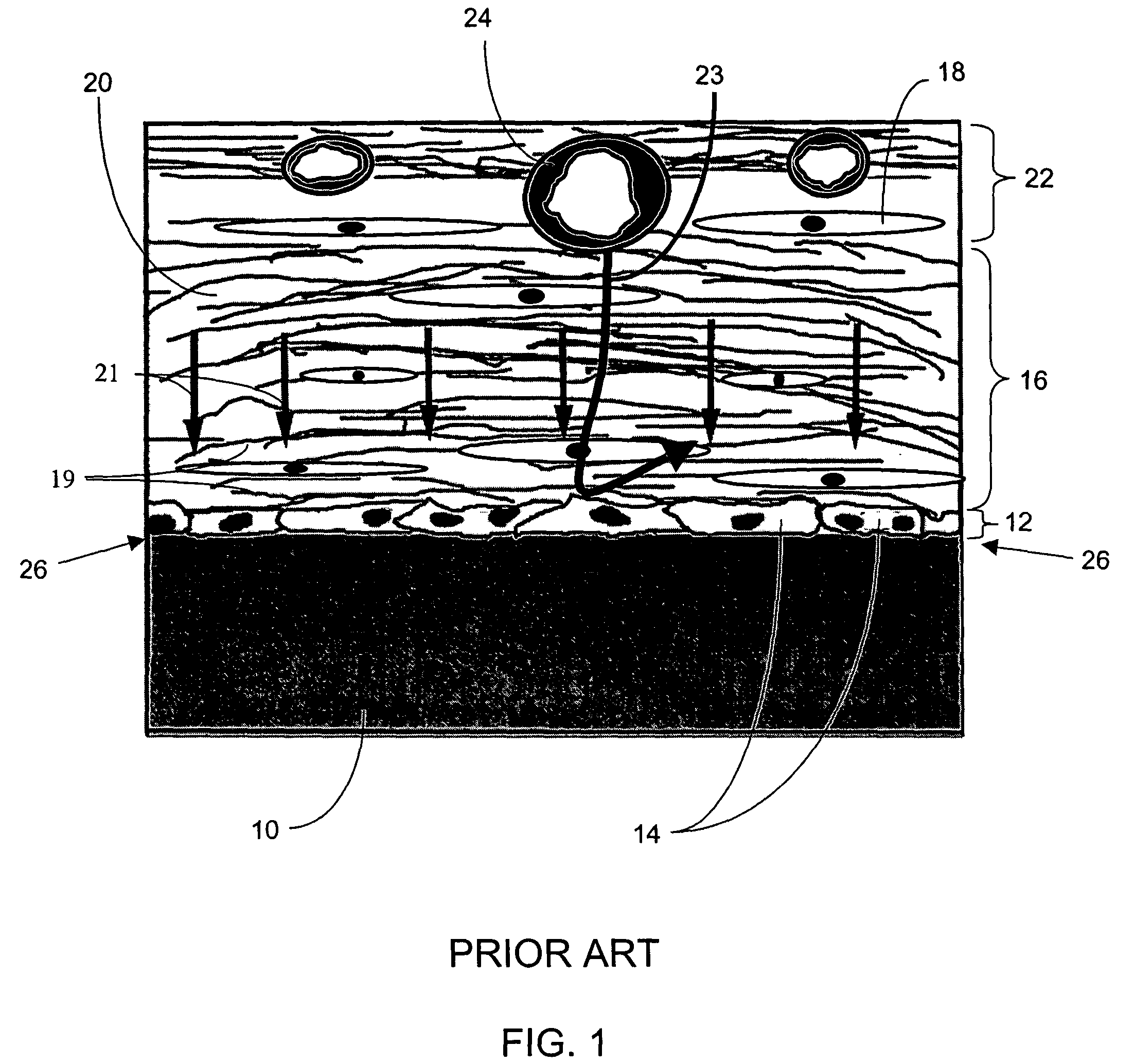
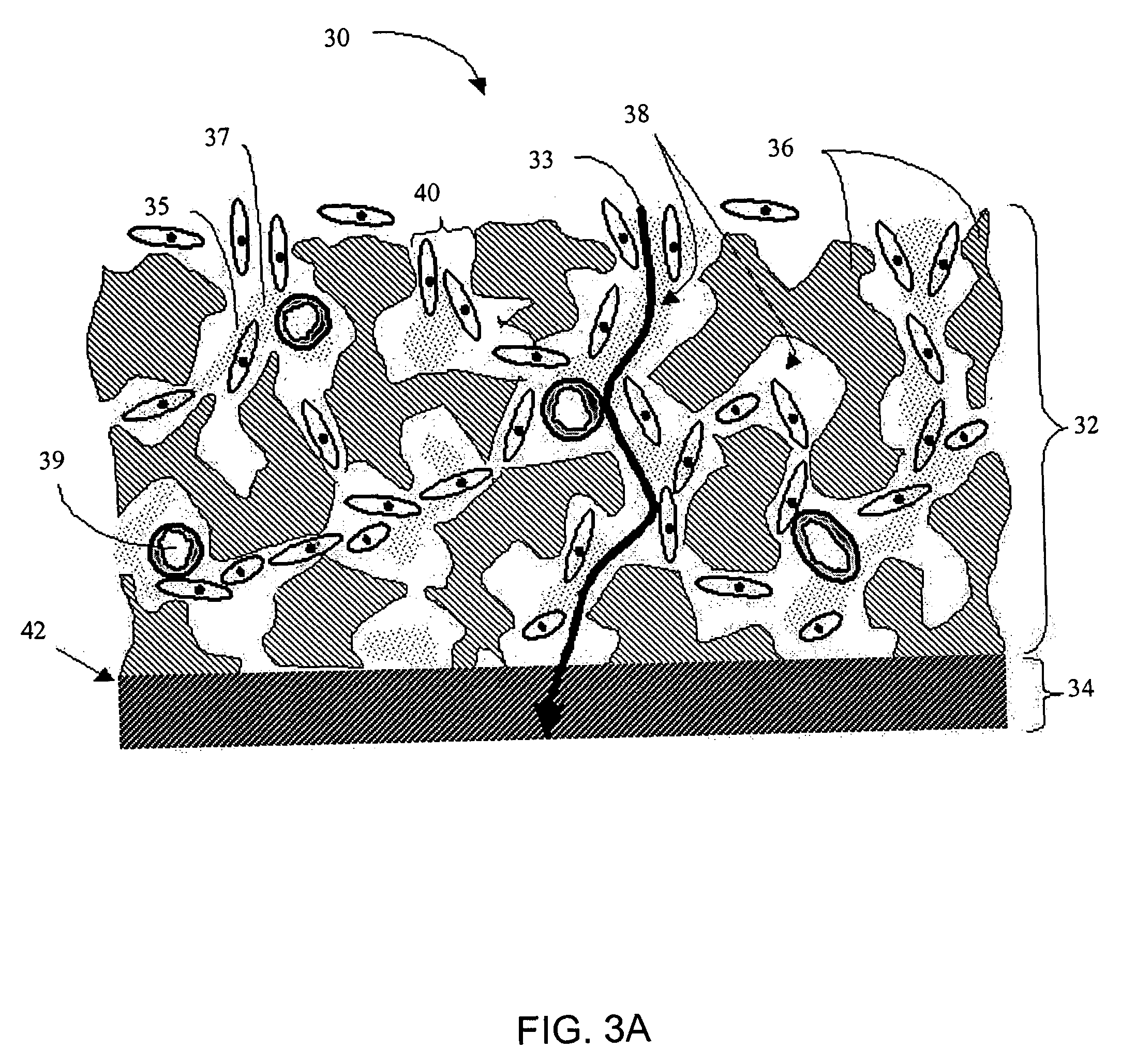
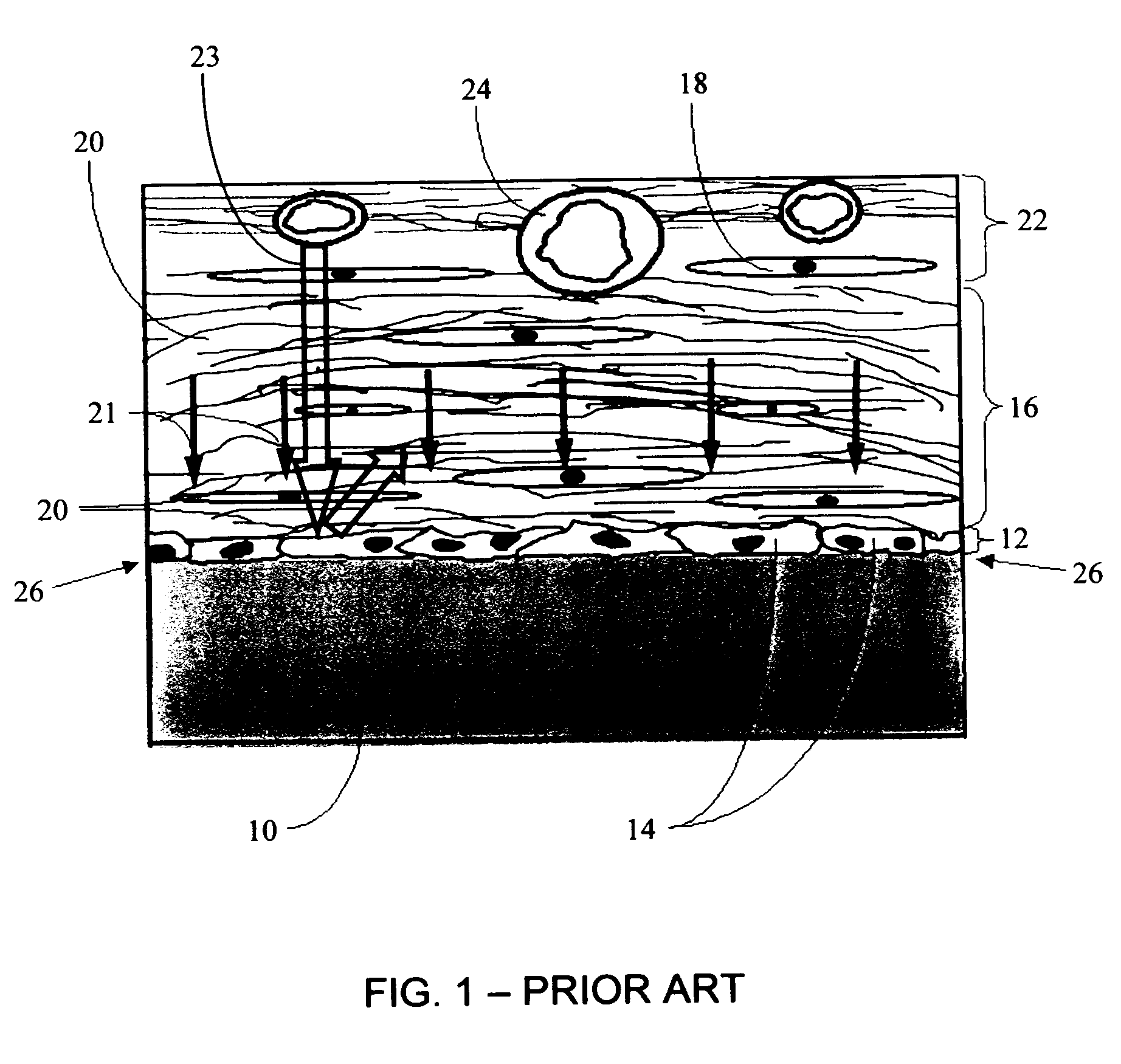
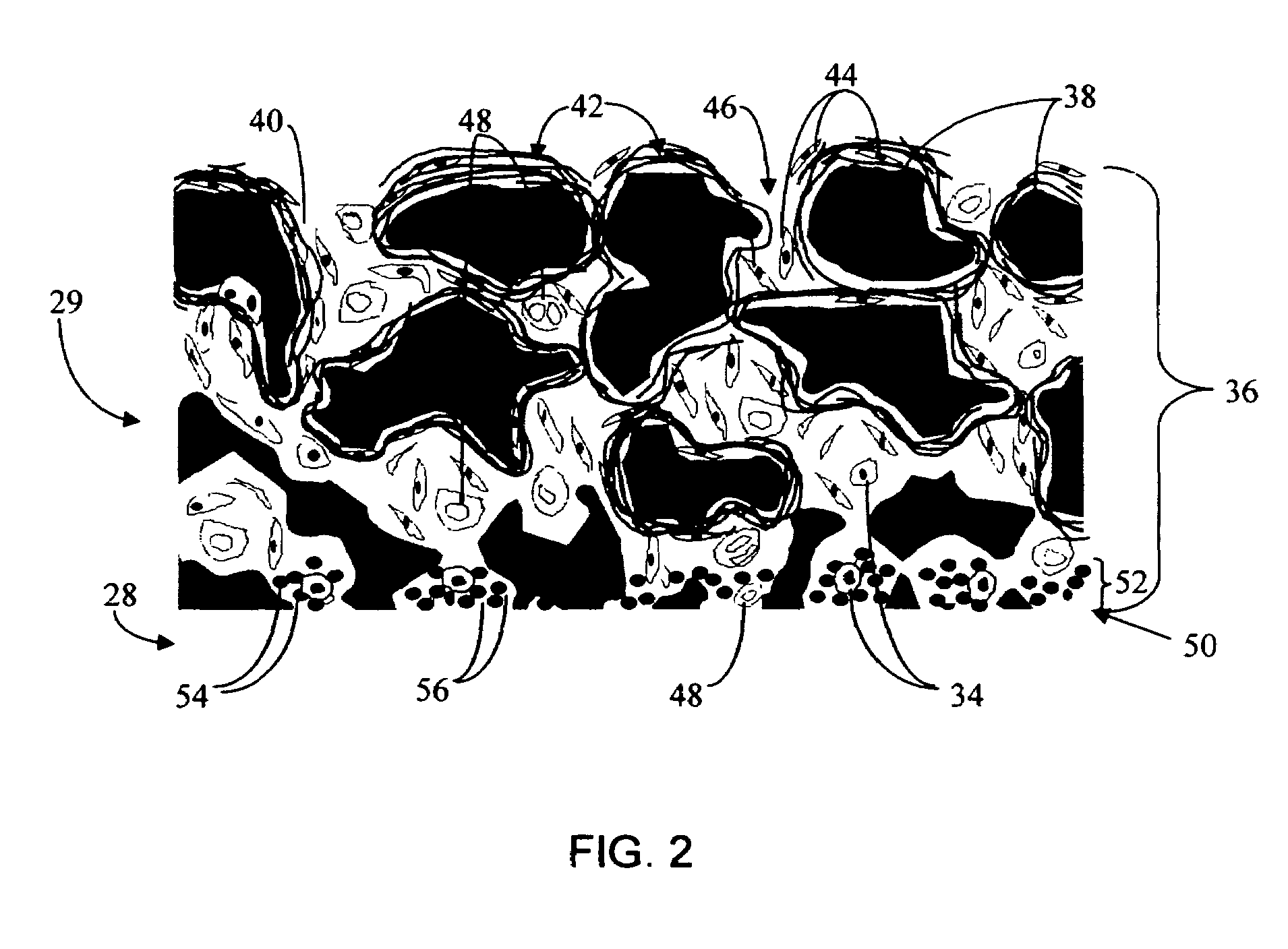
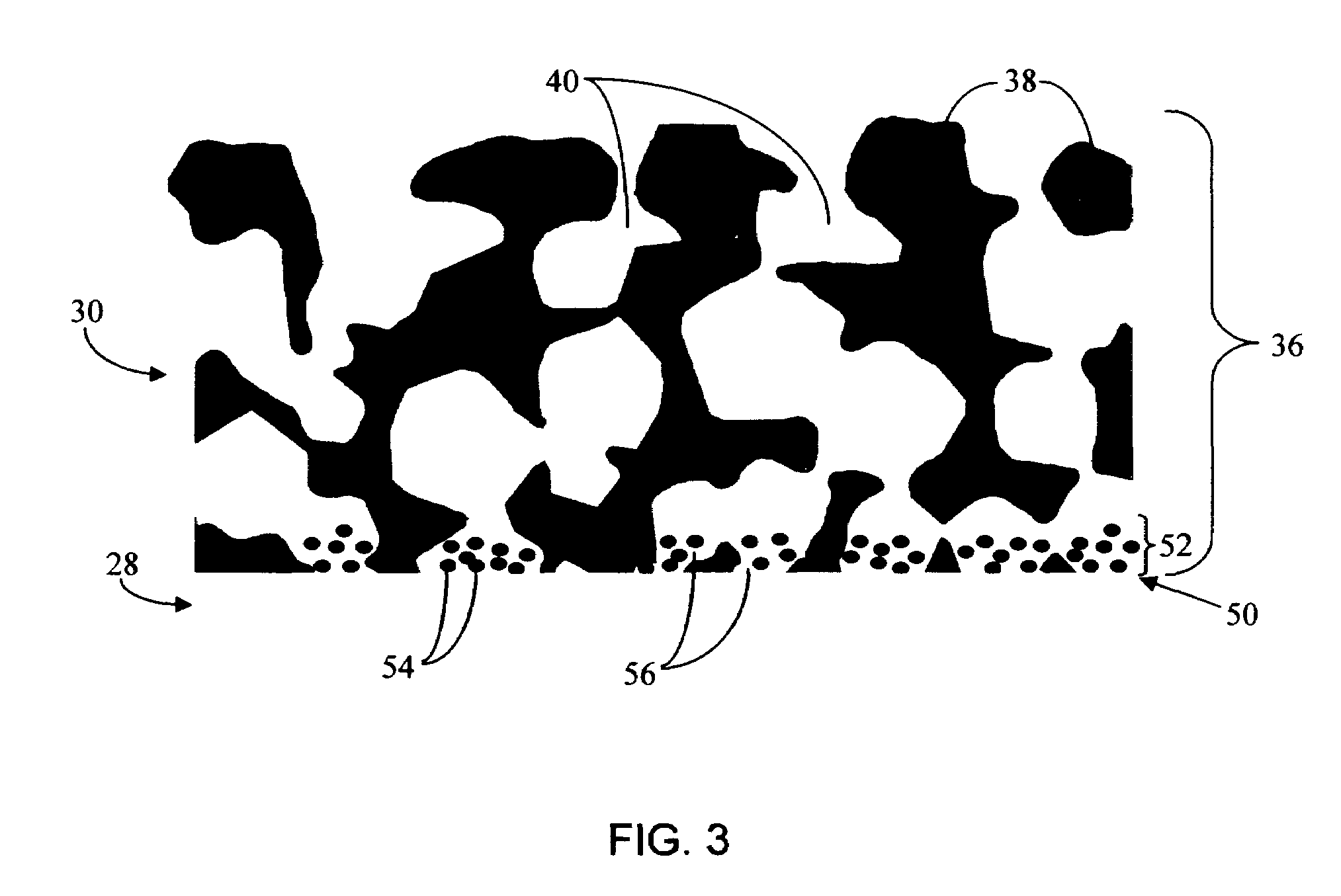
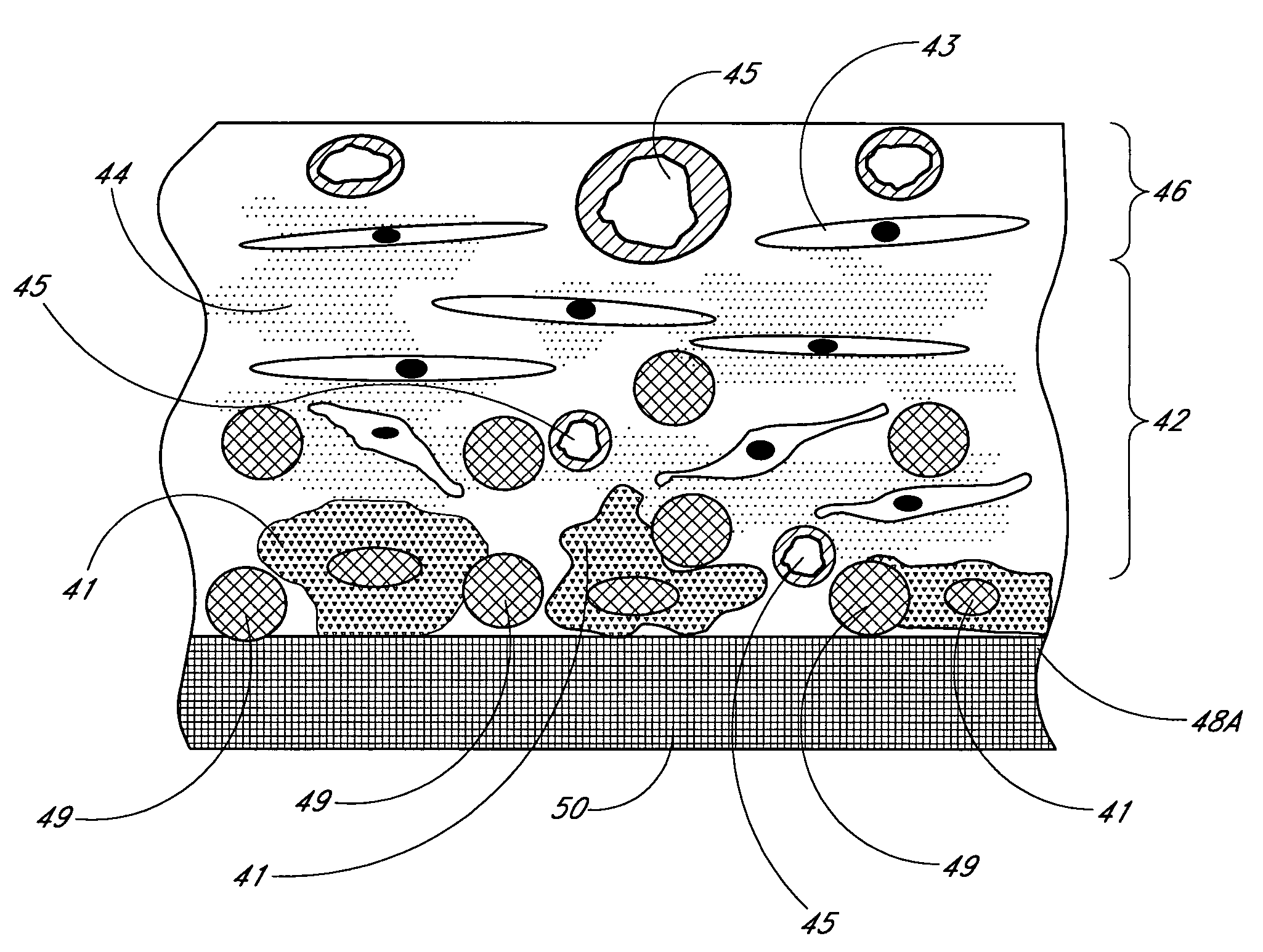
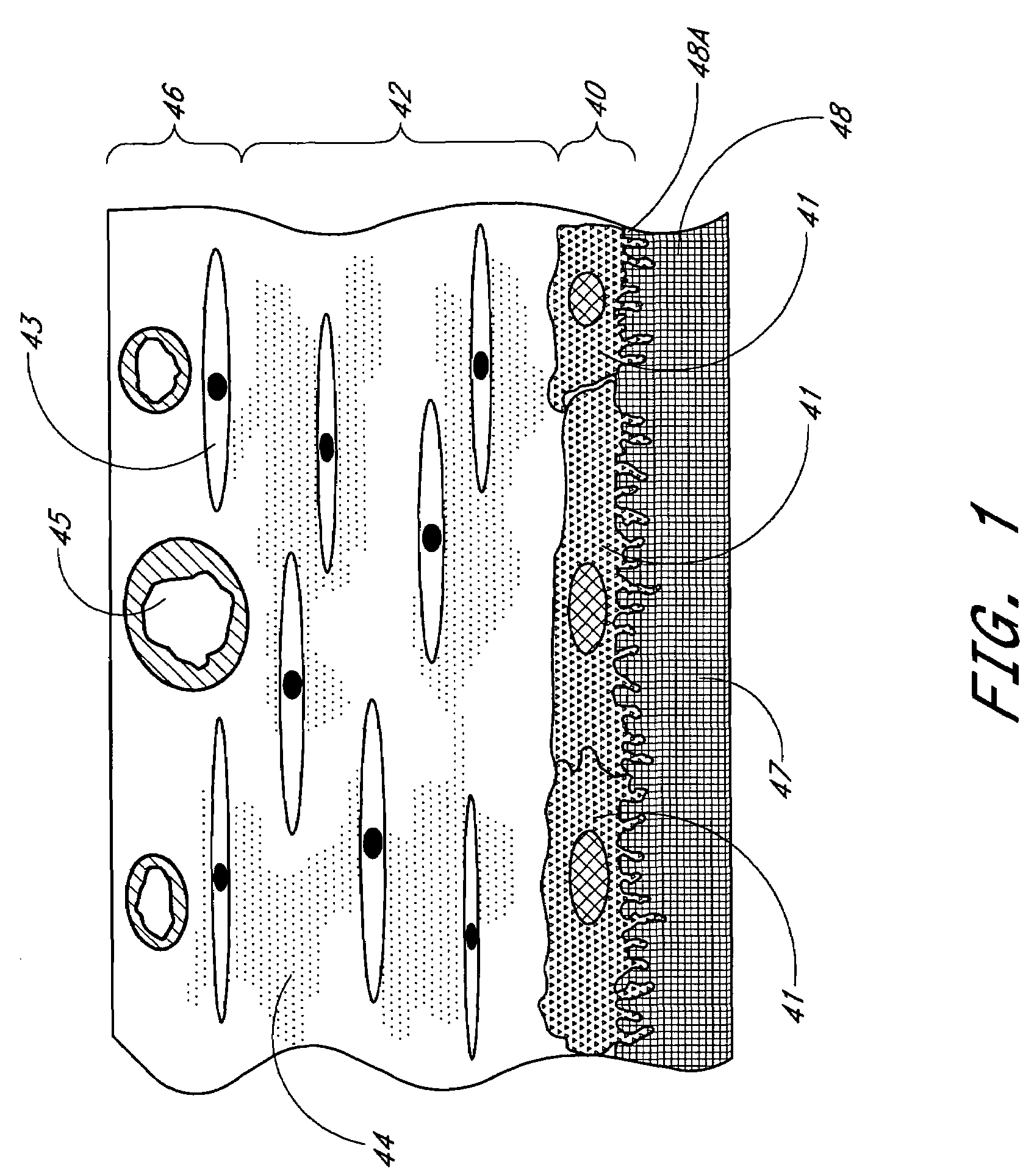
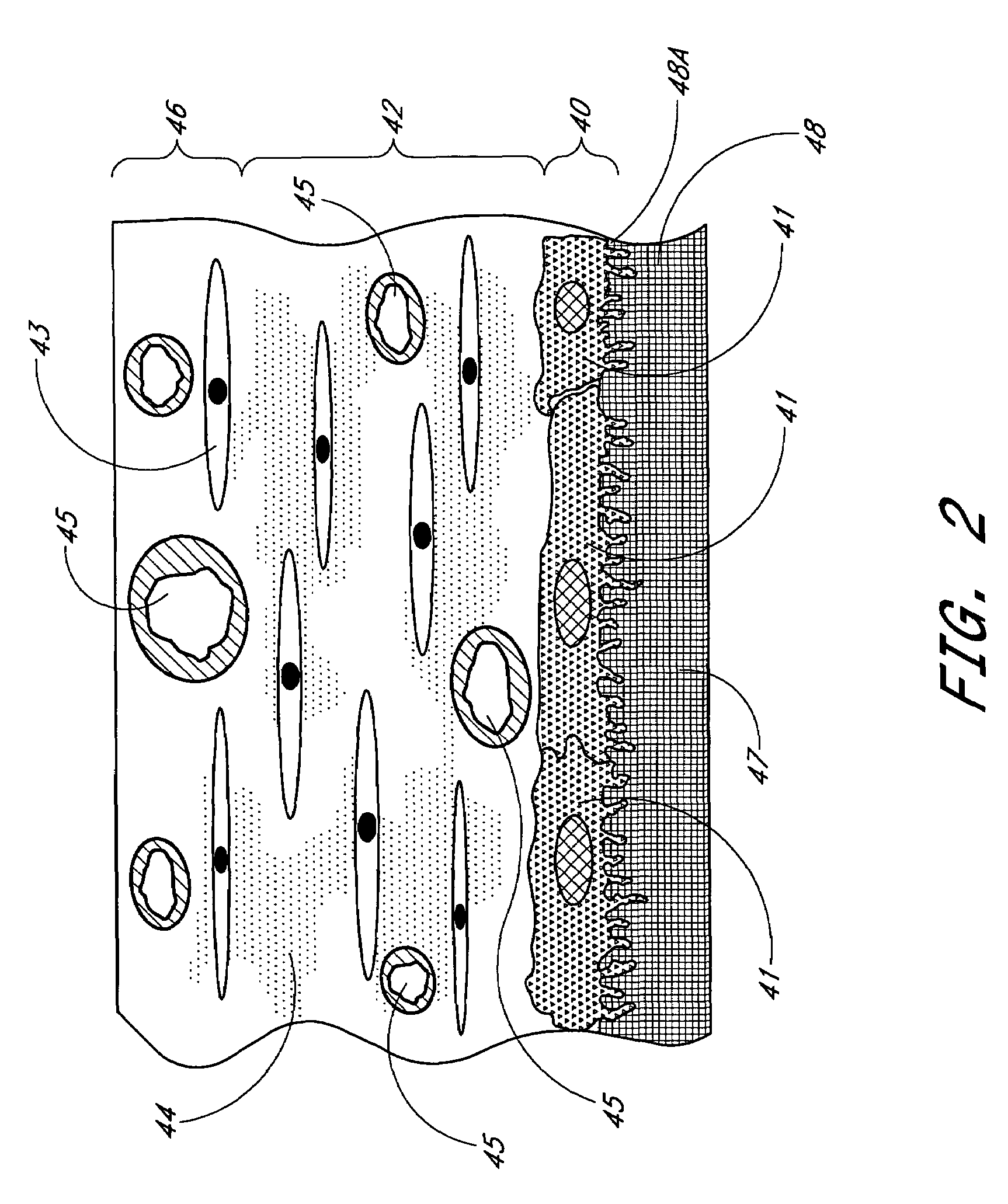
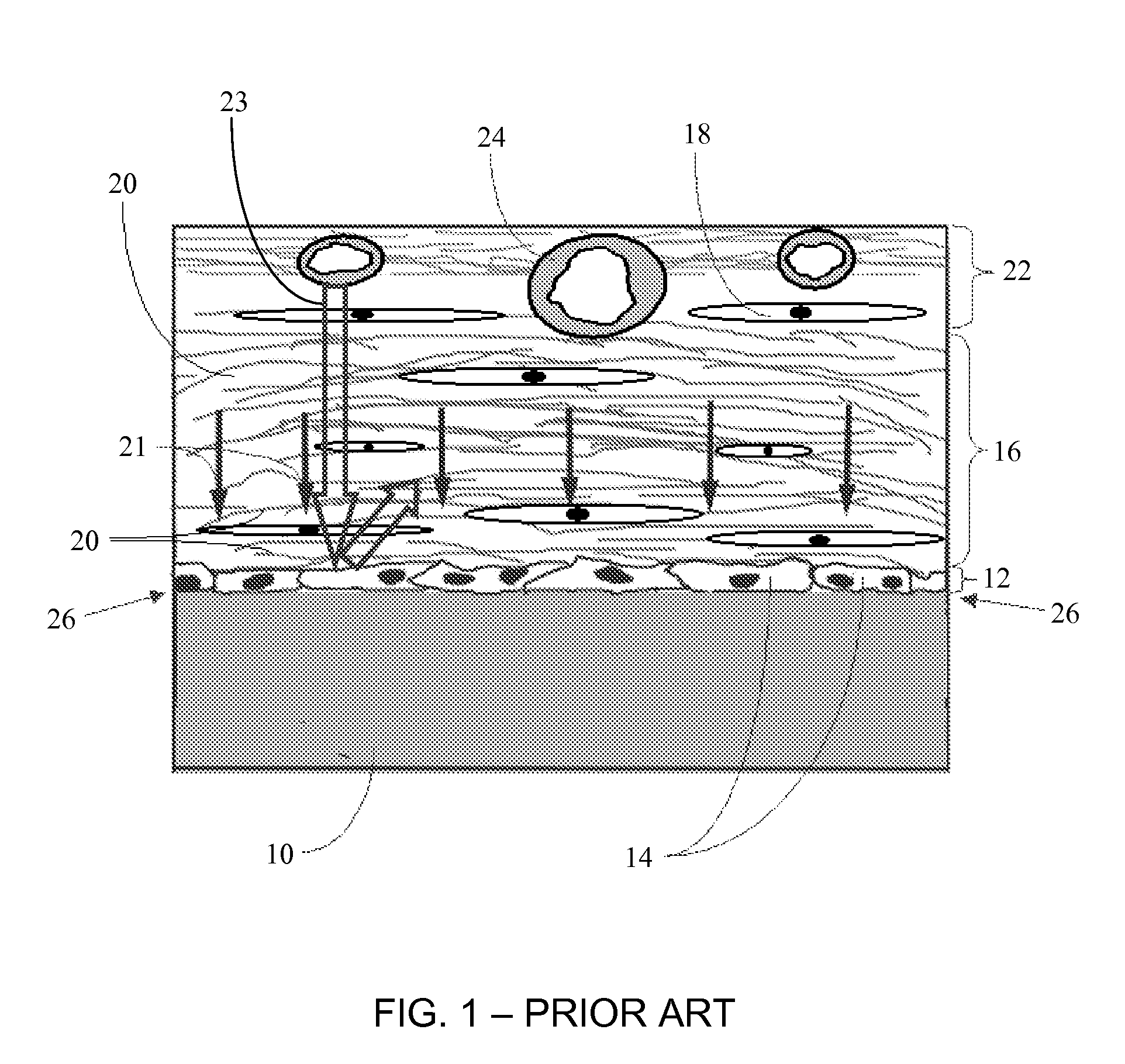
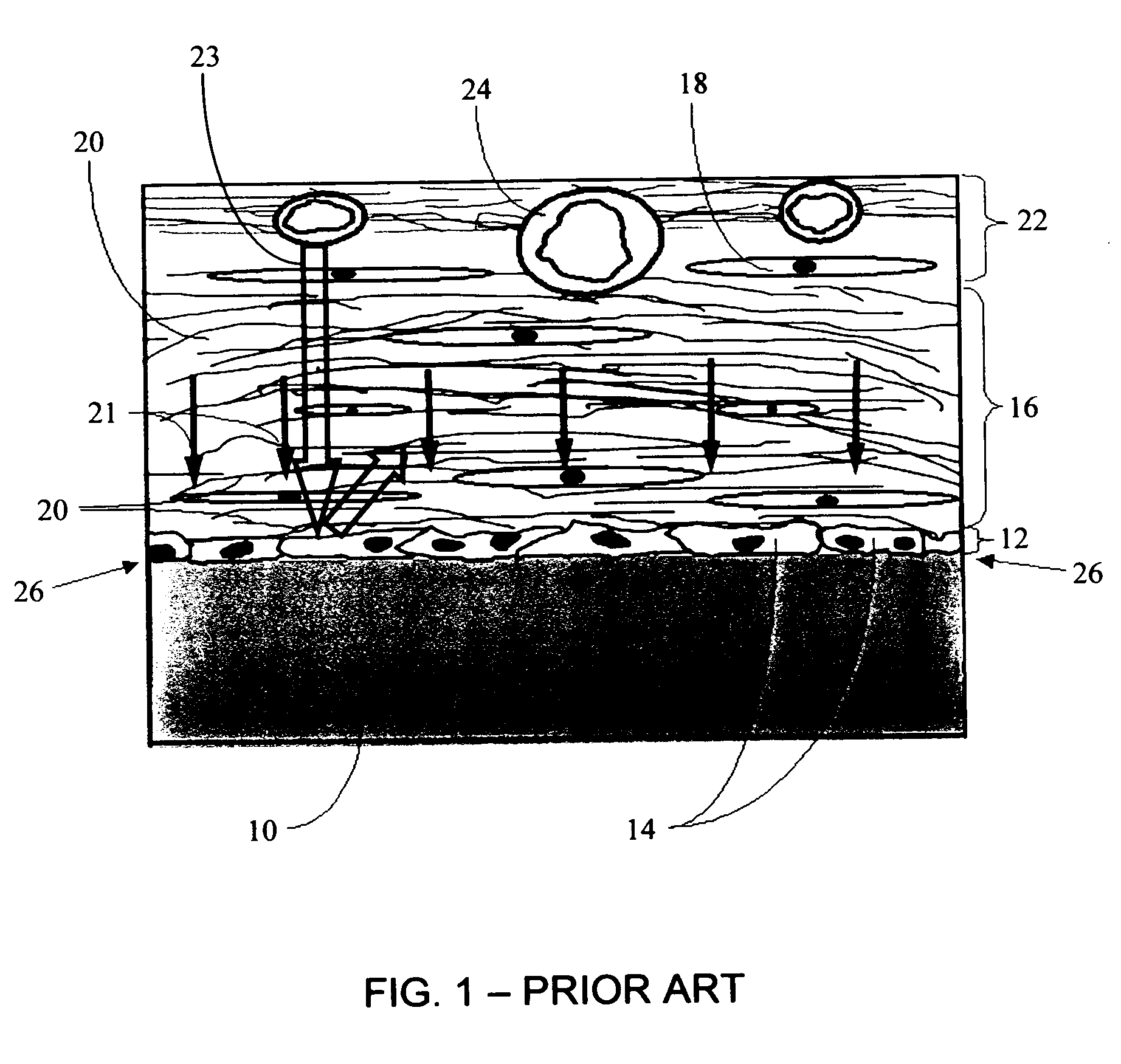
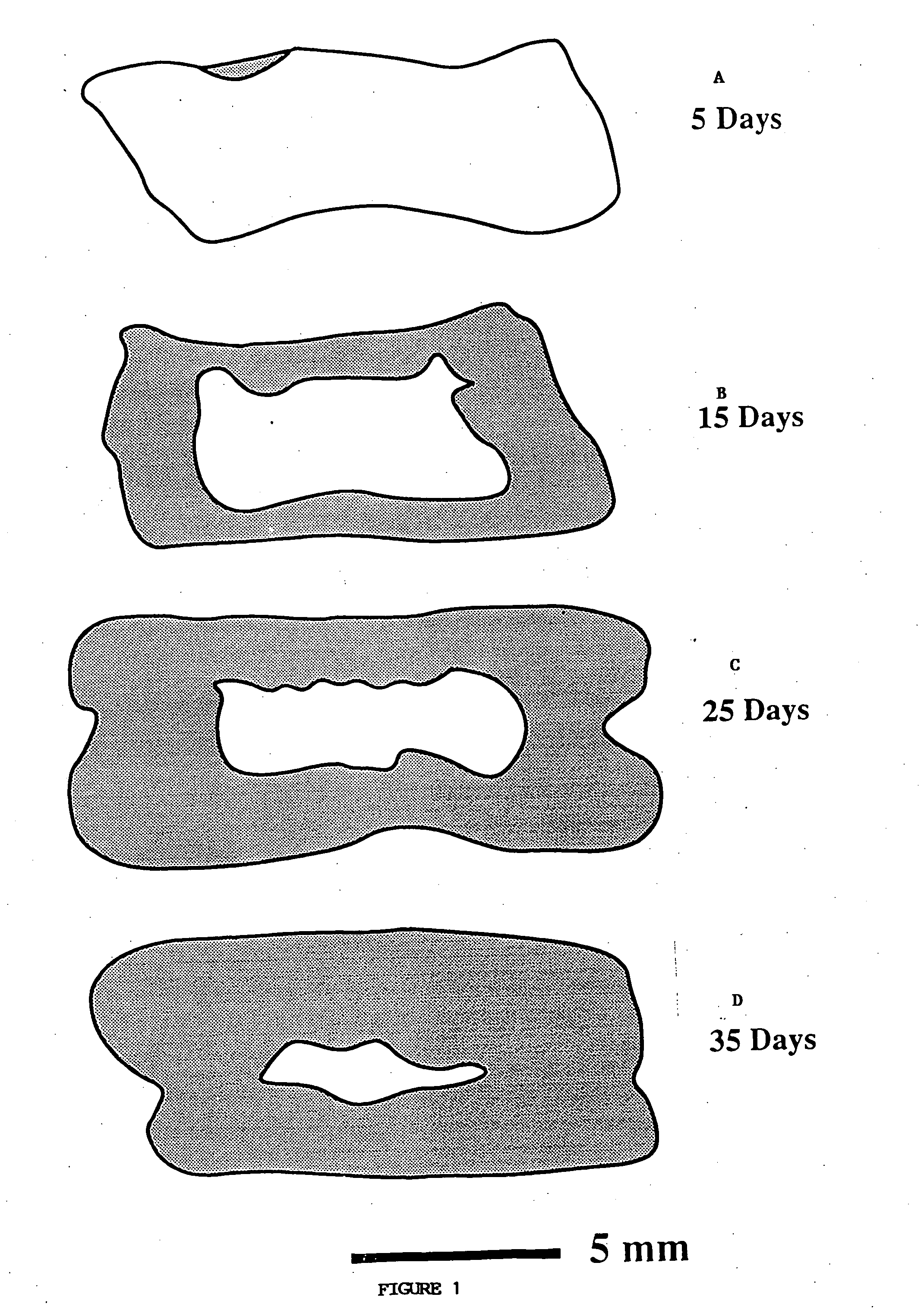
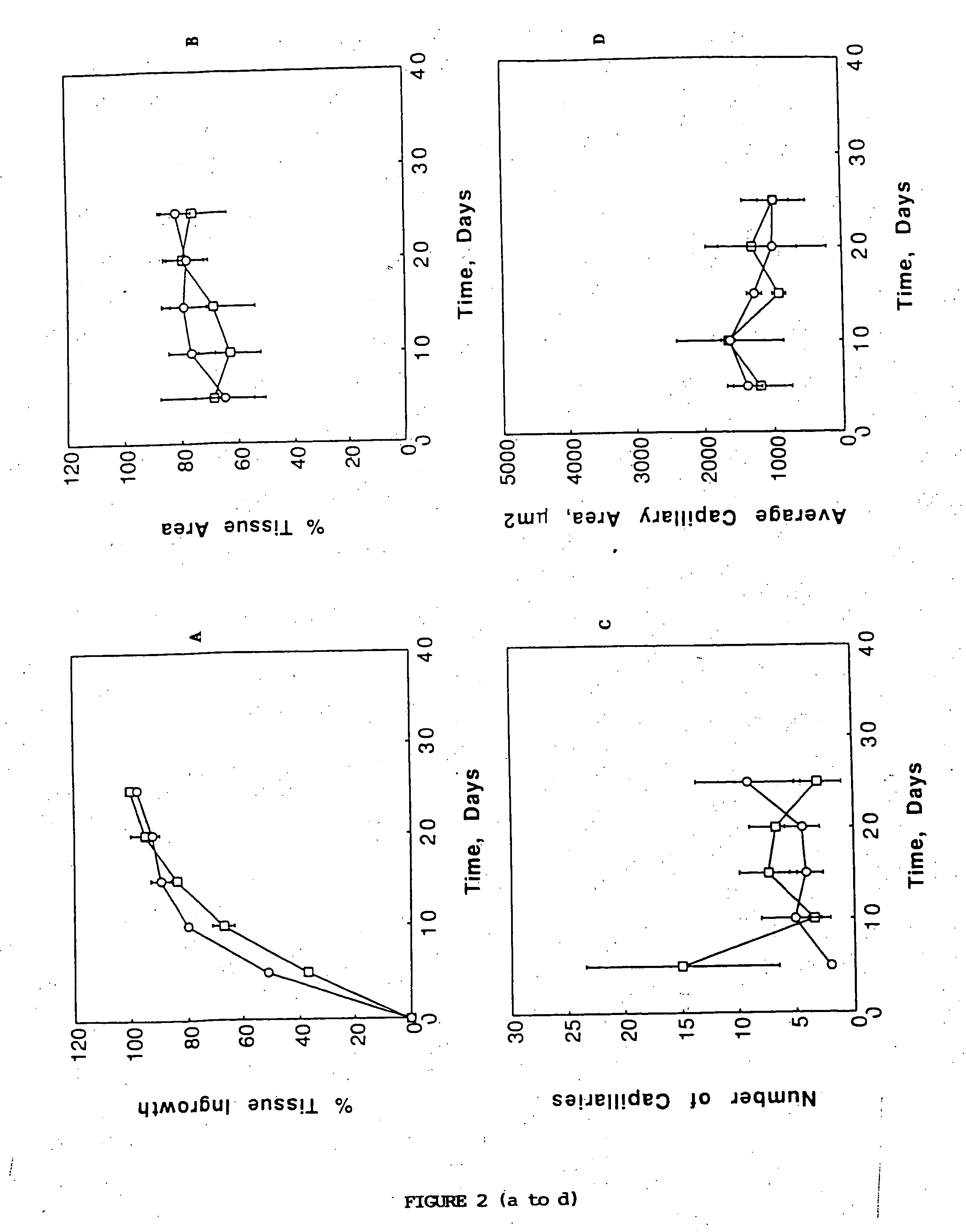
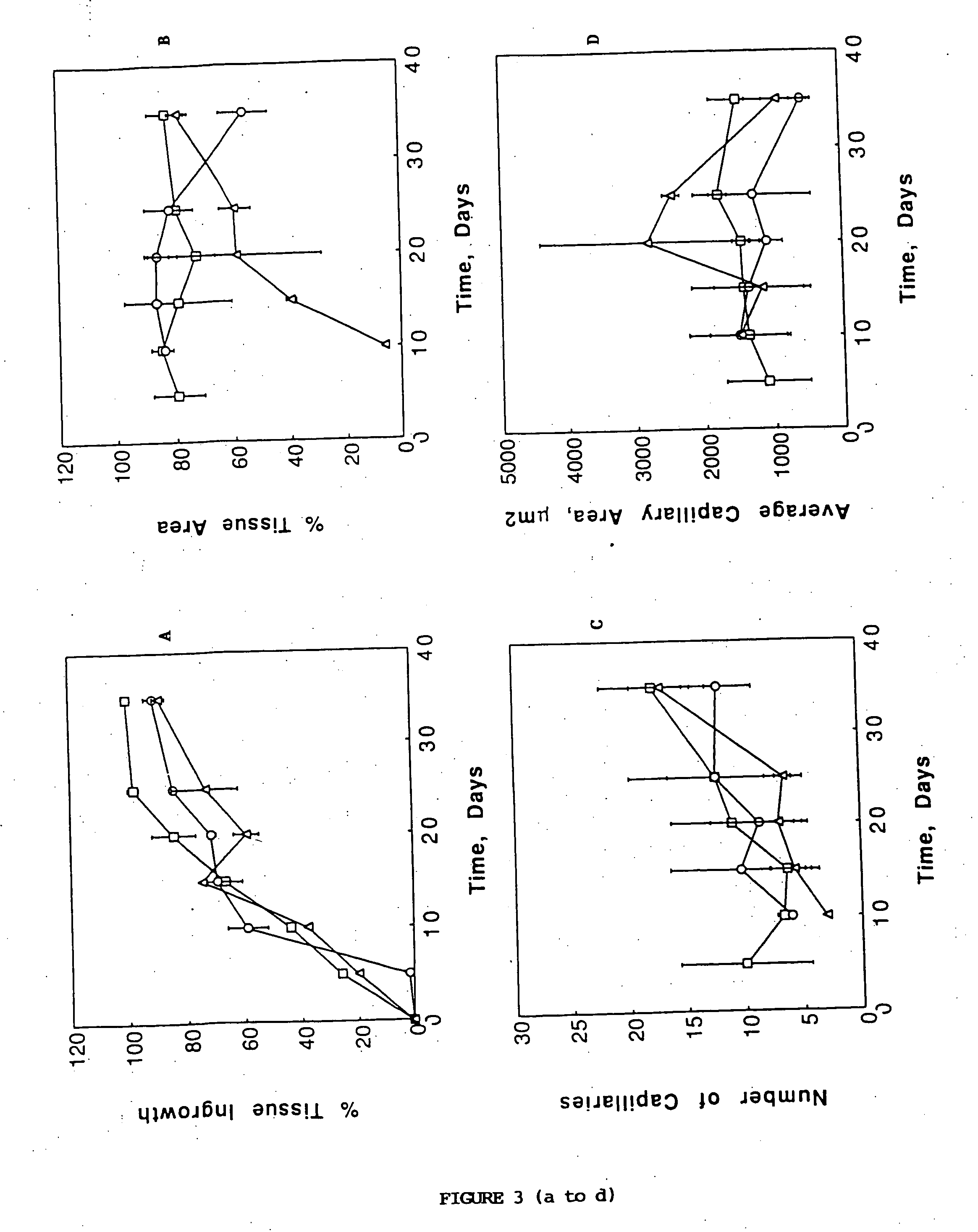
A membrane for implantation in soft tissue comprising a first domain that supports tissue ingrowth, disrupts contractile forces typically found in a foreign body response, encourages vascularity, and interferes with barrier cell layer formation, and a second domain that is resistant to cellular attachment, is impermeable to cells and cell processes, and allows the passage of analytes. The membrane allows for long-term analyte transport in vivo and is suitable for use as a biointerface for implantable analyte sensors, cell transplantation devices, drug delivery devices, and / or electrical signal delivering or measuring devices. The membrane architecture, including cavity size, depth, and interconnectivity, provide long-term robust functionality of the membrane in vivo.
Owner:DEXCOM INC
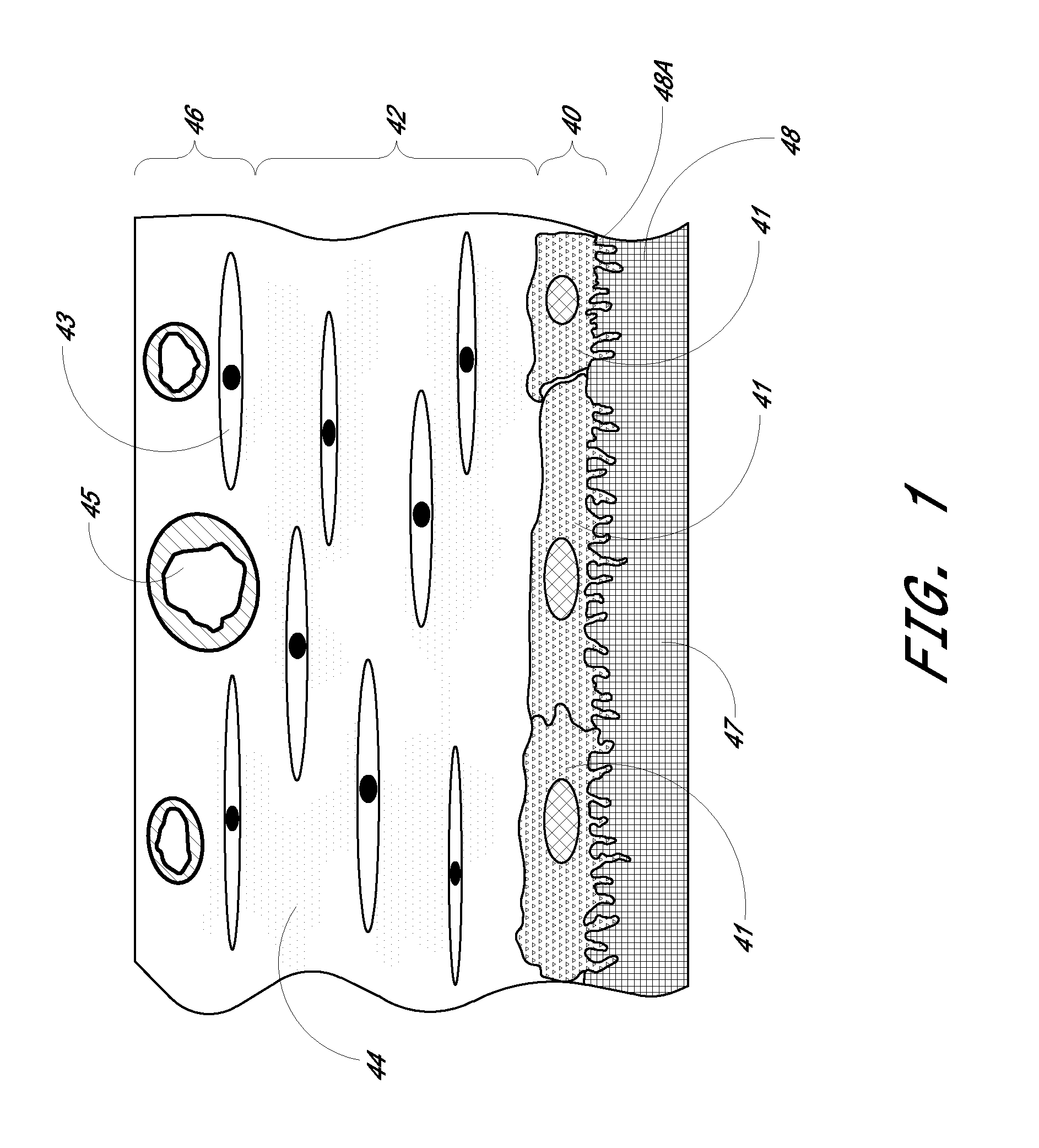
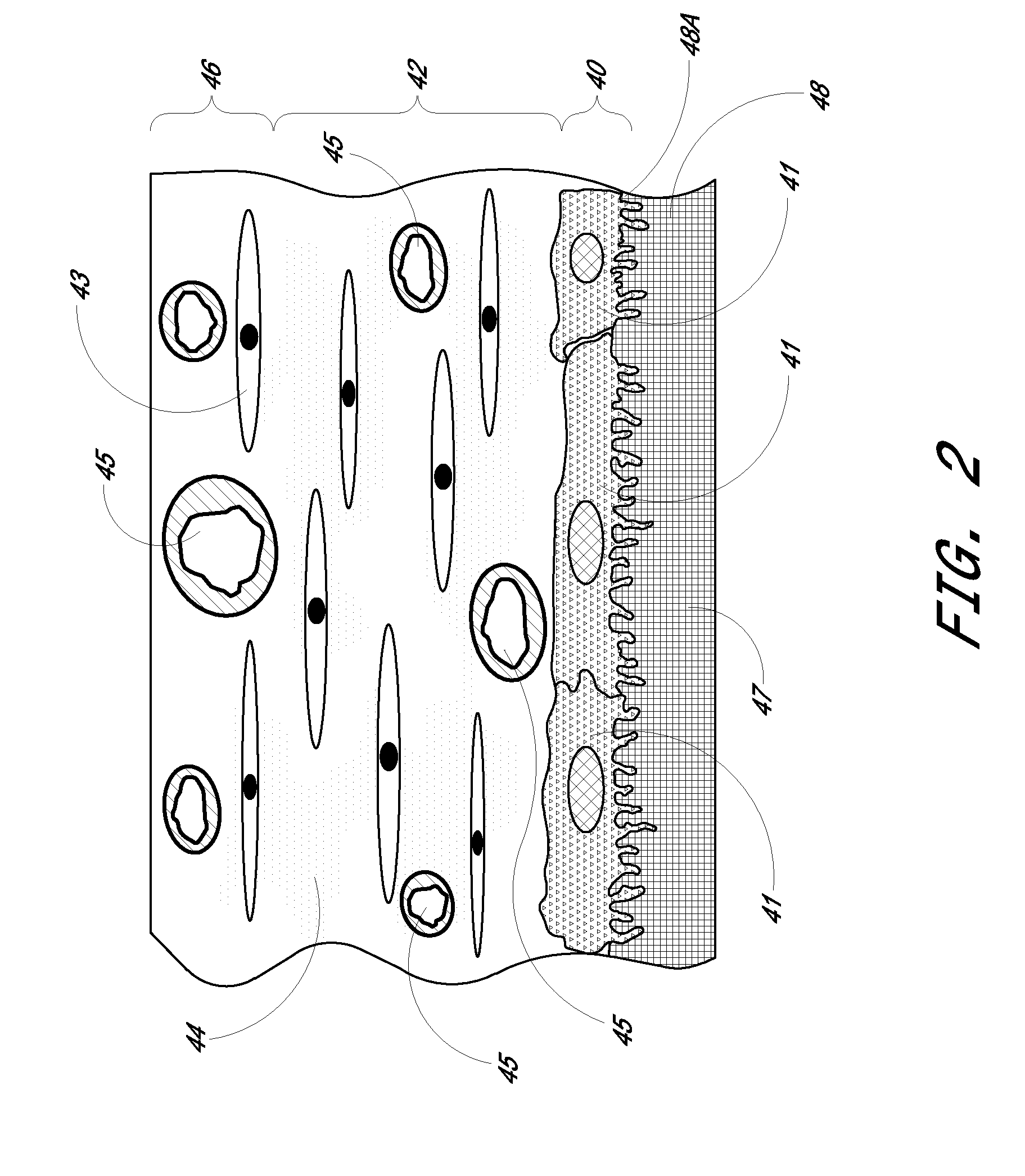
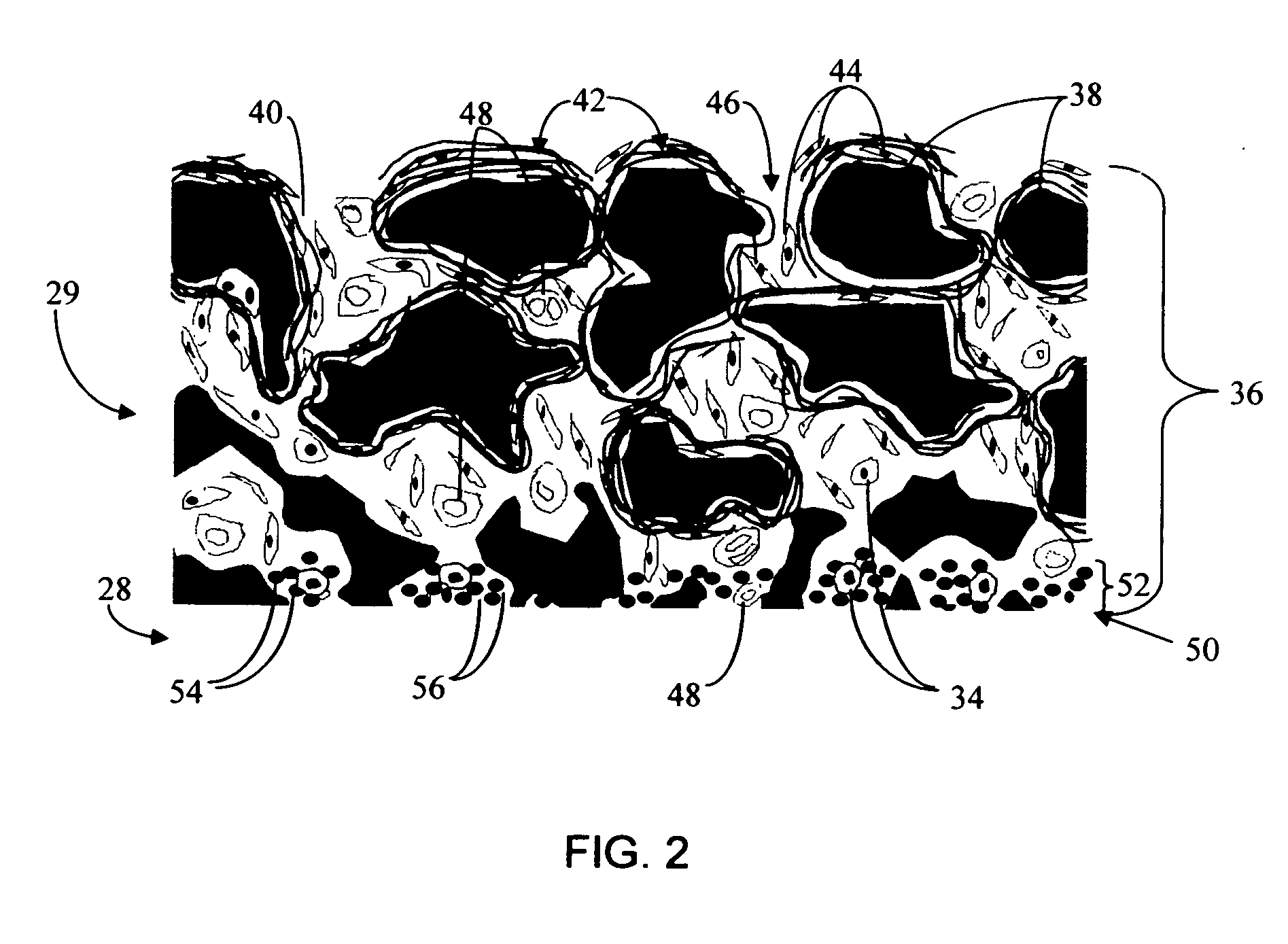
Biointerface membrane with macro-and micro-architecture
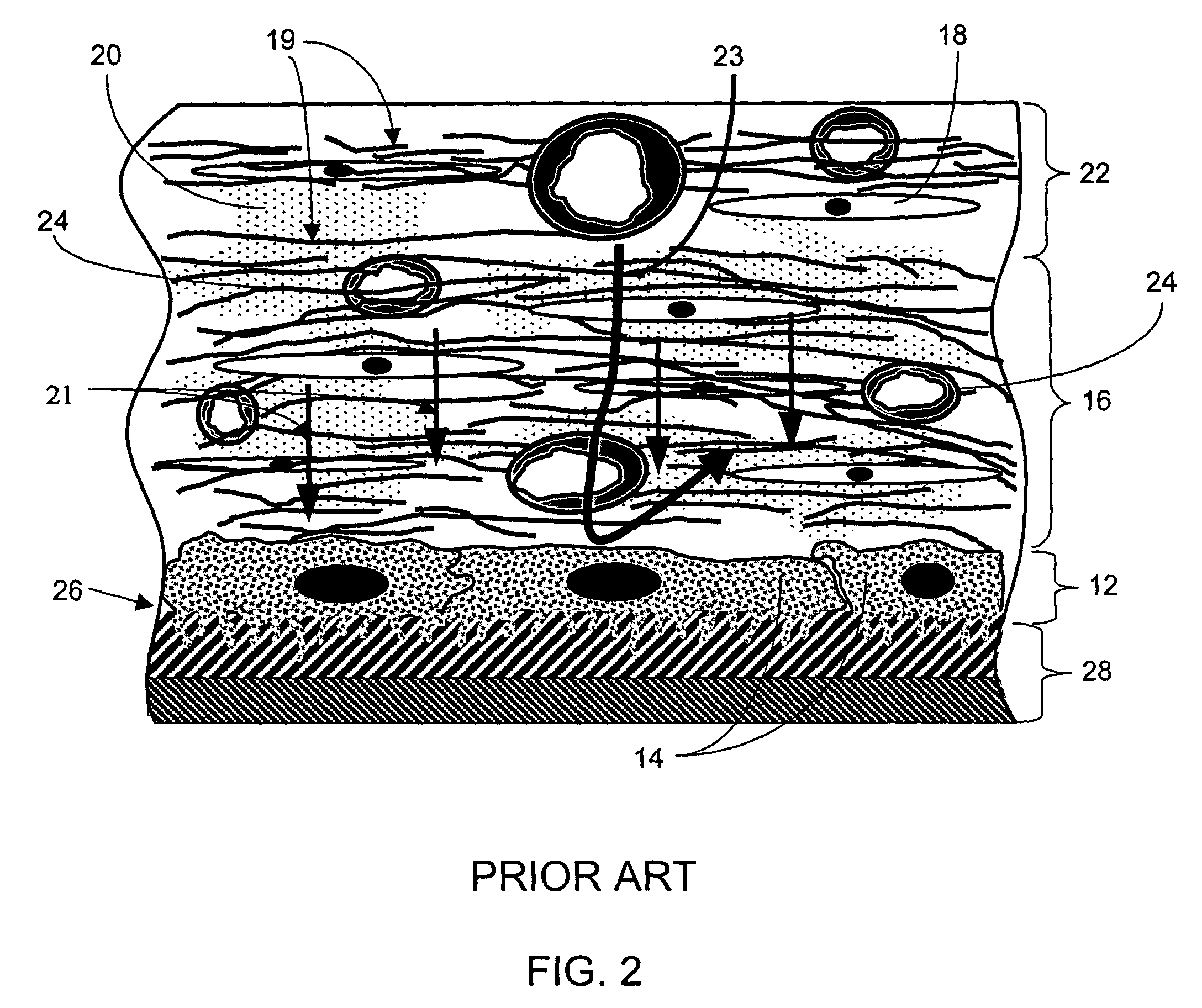
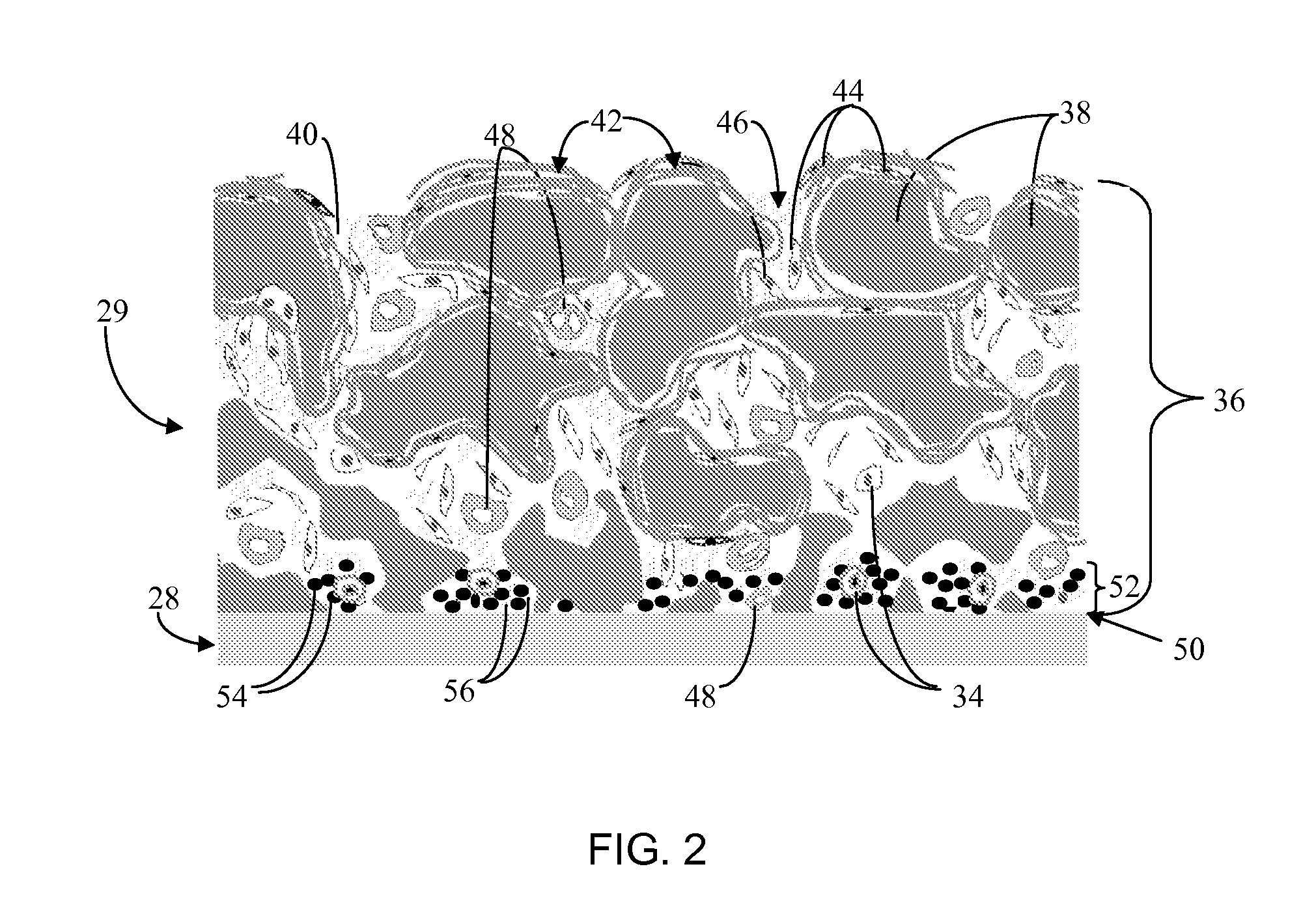
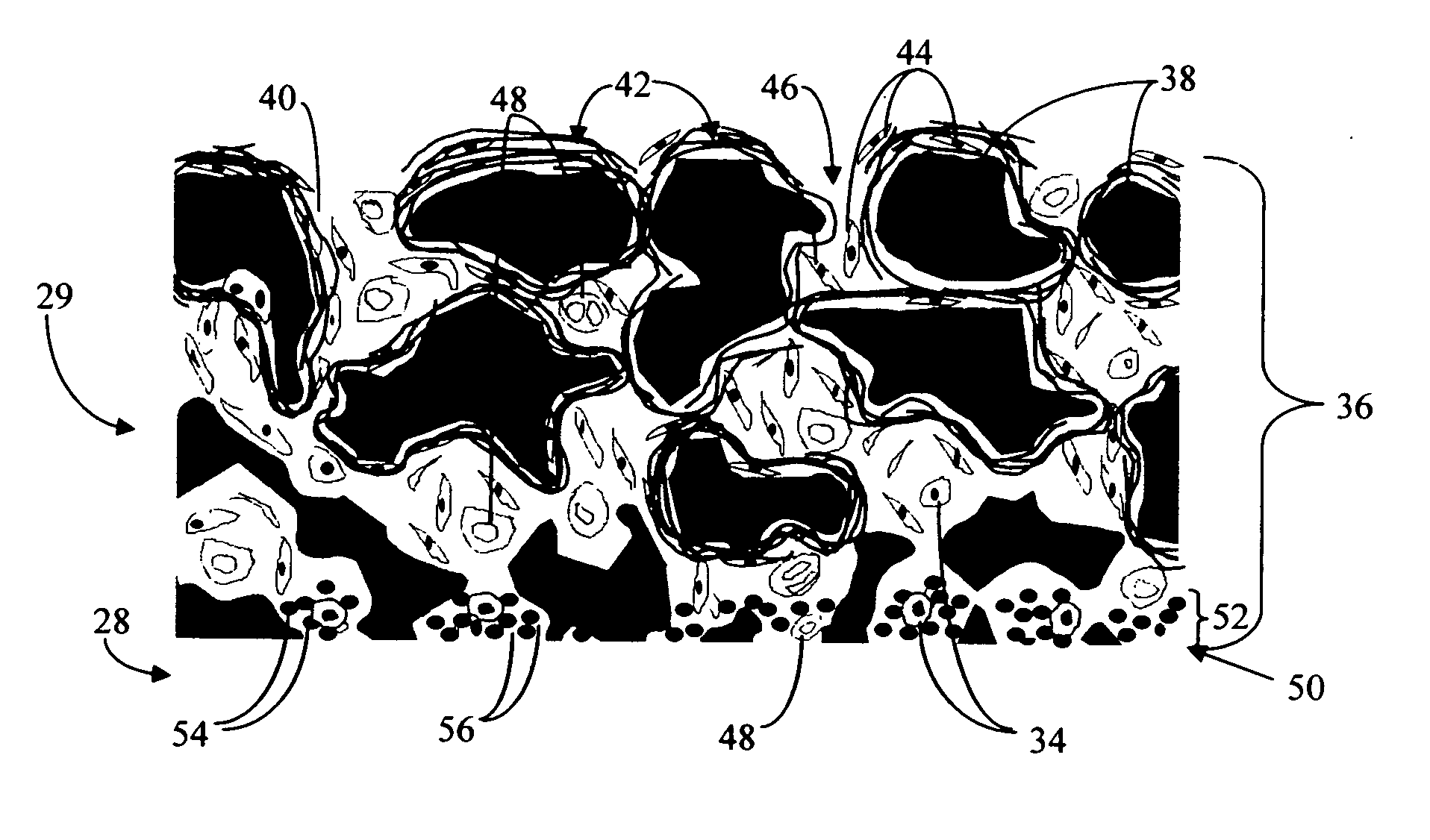
Disclosed herein are biointerface membranes including a macro-architecture and a micro-architecture co-continuous with and bonded to and / or located within at least a portion of the macro-architecture. The macro- and micro-architectures work together to manage and manipulate the high-level tissue organization and the low-level cellular organization of the foreign body response in vivo, thereby increasing neovascularization close to a device-tissue interface, interfering with barrier cell layer formation, and providing good tissue anchoring, while reducing the effects of motion artifact, and disrupting the organization and / or contracture of the FBC. The biointerface membranes of the preferred embodiments can be utilized with implantable devices such as devices for the detection of analyte concentrations in a biological sample (for example, from a body), cell transplantation devices, drug delivery devices, electrical signal delivering or measuring devices, and / or combinations thereof.
Owner:DEXCOM
Membrane for use with implantable devices
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM
Oxygen enhancing membrane systems for implantable devices
ActiveUS20050054909A1Immobilised enzymesBioreactor/fermenter combinationsImplanted deviceOxygen enhanced
The present invention relates generally to systems and methods for increasing oxygen availability to implantable devices. The preferred embodiments provide a membrane system configured to provide protection of the device from the biological environment and / or a catalyst for enabling an enzymatic reaction, wherein the membrane system includes a polymer formed from a high oxygen soluble material. The high oxygen soluble polymer material is disposed adjacent to an oxygen-utilizing source on the implantable device so as to dynamically retain high oxygen availability to the oxygen-utilizing source during oxygen deficits. Membrane systems of the preferred embodiments are useful for implantable devices with oxygen-utilizing sources and / or that function in low oxygen environments, such as enzyme-based electrochemical sensors and cell transplantation devices.
Owner:DEXCOM
Membrane for use with implantable devices
ActiveUS20100087724A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteBiointerface
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM INC
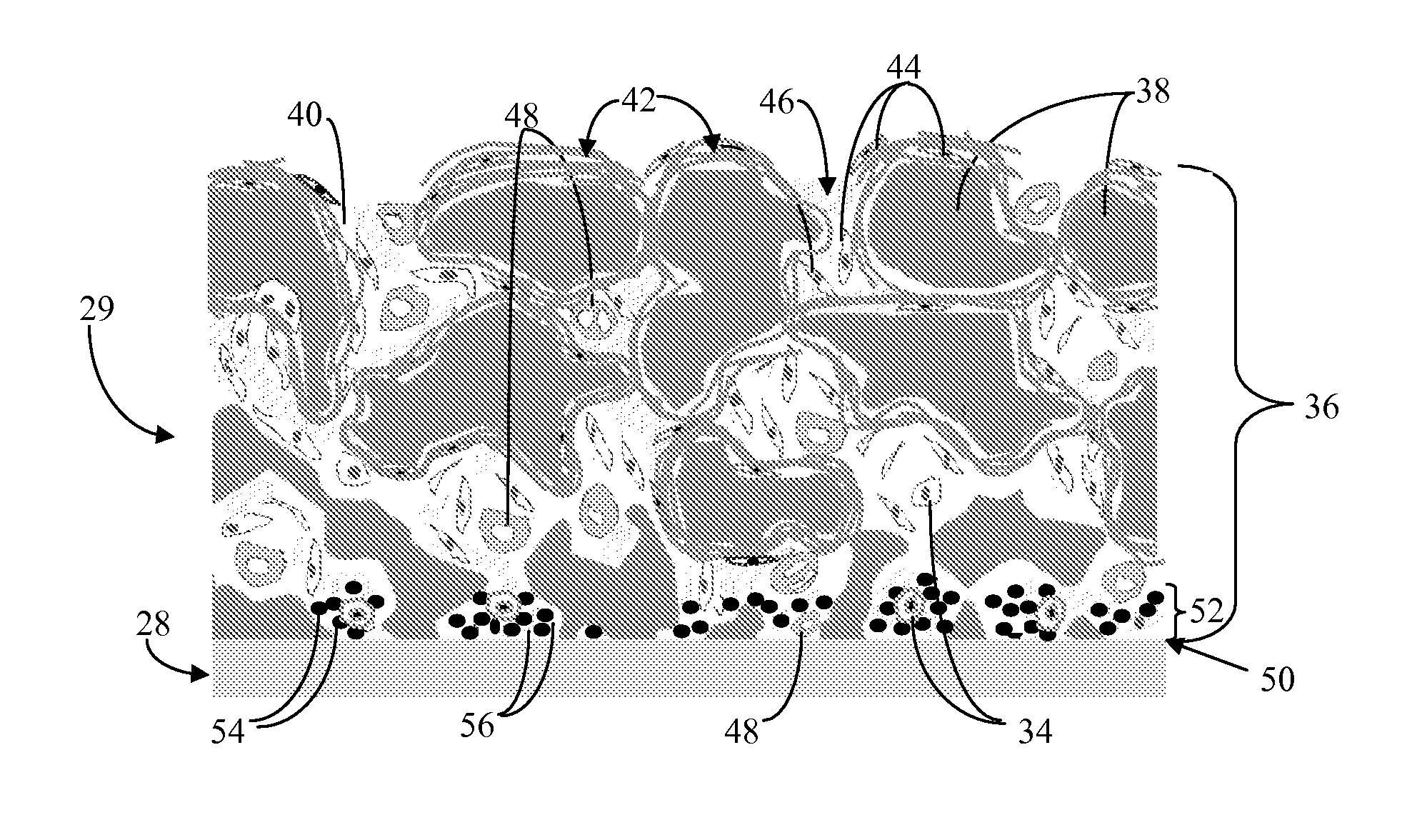
Biointerface with macro- and micro-architecture
Owner:DEXCOM INC
Biointerface with macro-and micro-architecture
Disclosed herein are biointerface membranes including a macro-architecture and a micro-architecture co-continuous with and bonded to and / or located within at least a portion of the macro-architecture. The macro- and micro-architectures work together to manage and manipulate the high-level tissue organization and the low-level cellular organization of the foreign body response in vivo, thereby increasing neovascularization close to a device-tissue interface, interfering with barrier cell layer formation, and providing good tissue anchoring, while reducing the effects of motion artifact, and disrupting the organization and / or contracture of the FBC. The biointerface membranes of the preferred embodiments can be utilized with implantable devices such as devices for the detection of analyte concentrations in a biological sample (for example, from a body), cell transplantation devices, drug delivery devices, electrical signal delivering or measuring devices, and / or combinations thereof.
Owner:DEXCOM
Oxygen enhancing membrane systems for implantable devices
ActiveUS20060189856A1Microbiological testing/measurementMaterial analysis by electric/magnetic meansImplanted deviceOxygen enhanced
The present invention relates generally to systems and methods for increasing oxygen availability to implantable devices. The preferred embodiments provide a membrane system configured to provide protection of the device from the biological environment and / or a catalyst for enabling an enzymatic reaction, wherein the membrane system includes a polymer formed from a high oxygen soluble material. The high oxygen soluble polymer material is disposed adjacent to an oxygen-utilizing source on the implantable device so as to dynamically retain high oxygen availability to the oxygen-utilizing source during oxygen deficits. Membrane systems of the preferred embodiments are useful for implantable devices with oxygen-utilizing sources and / or that function in low oxygen environments, such as enzyme-based electrochemical sensors and cell transplantation devices.
Owner:DEXCOM INC
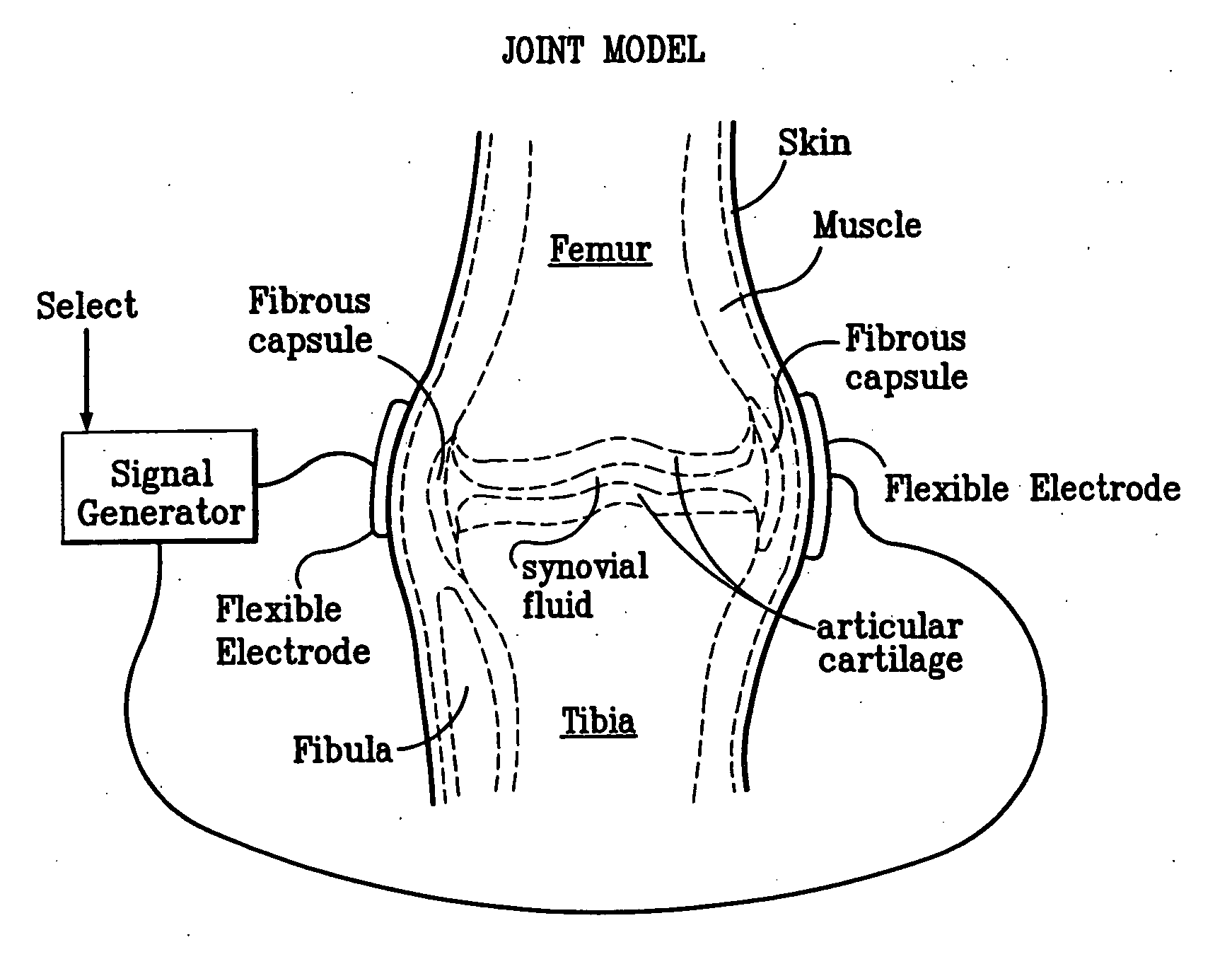
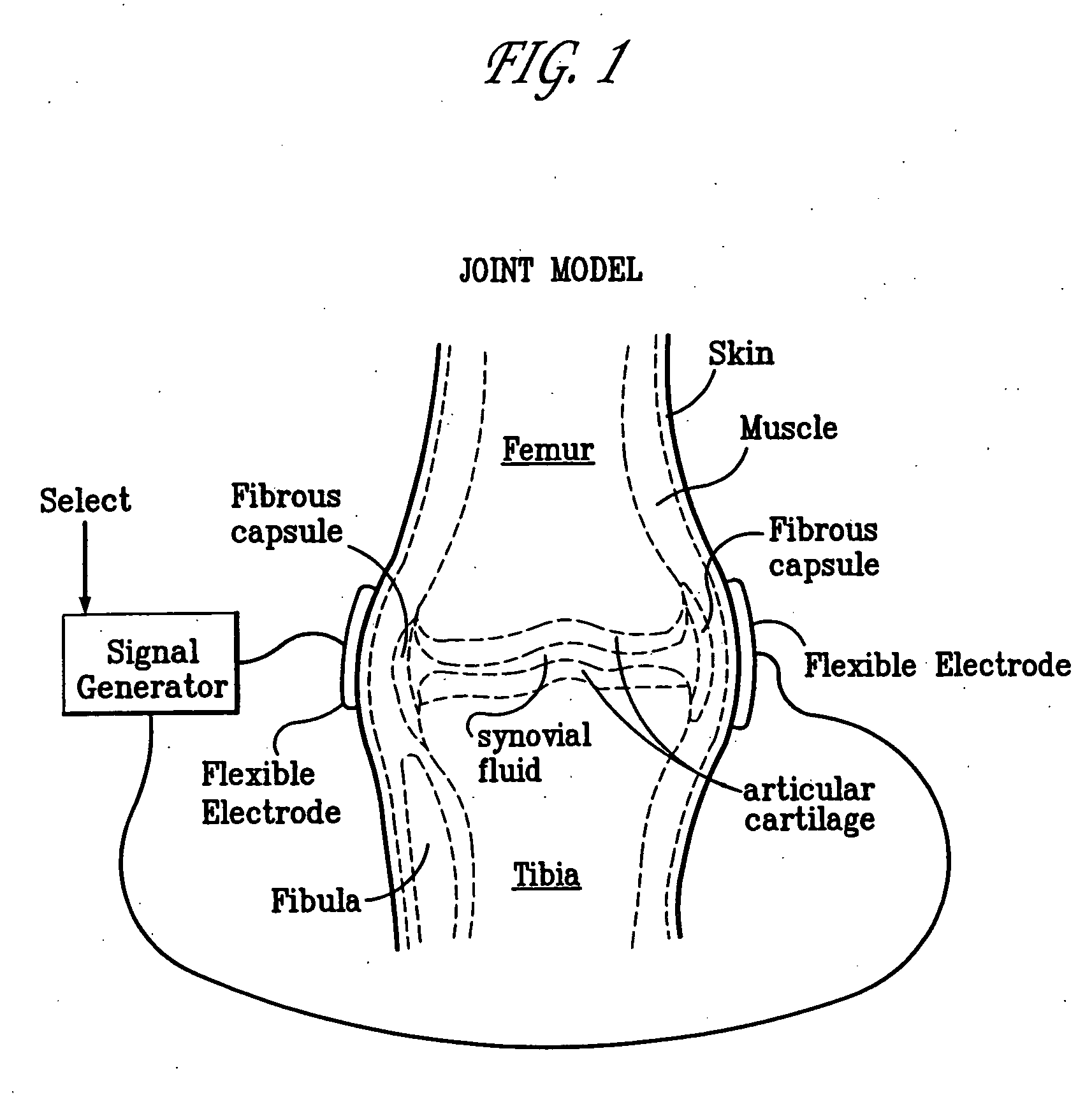
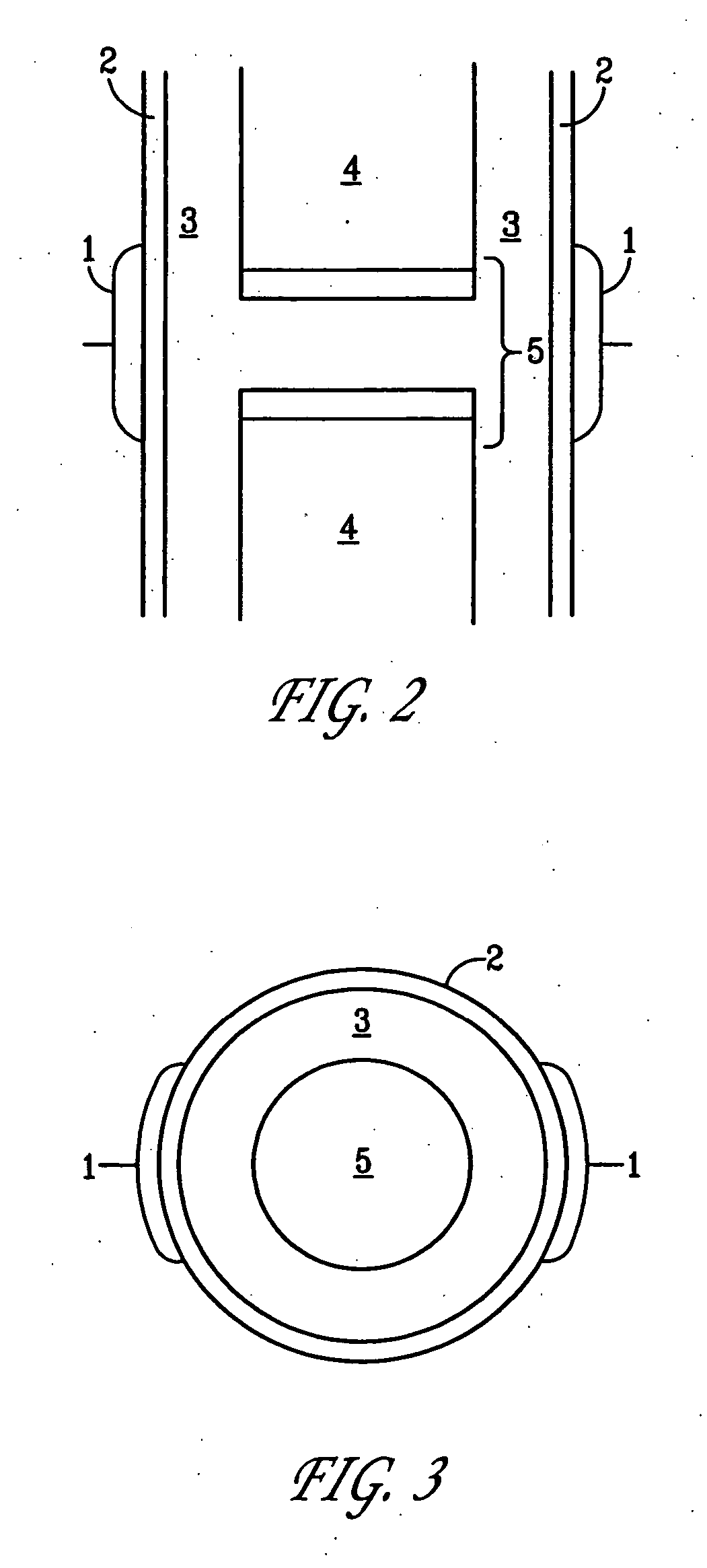
Method and device for treating osteoarthritis, cartilage disease, defects and injuries in the human knee
InactiveUS20060190043A1ElectrotherapyMagnetotherapy using coils/electromagnetsHuman useArticular cartilage
A method of determining the voltage and current output required for the application of specific and selective electric and electromagnetic signals to diseased articular cartilage in the treatment of osteoarthritis, cartilage defects due to trauma or sports injury, or used as an adjunct with other therapies (cell transplantation, tissue-engineered scaffolds, growth factors, etc.) for treating cartilage defects in the human knee joint and a device for delivering such signals to a patient's knee. An analytical model of the human knee is developed whereby the total tissue volume in the human knee may be determined for comparison to the total tissue volume of the diseased tissue in the animal model using electric field and current density histograms. The voltage and current output used in the animal model is scaled based on the ratio of the total tissue volume of the diseased tissue of the human to the total tissue volume of the diseased tissue in the animal model and the resulting field is applied to the diseased tissue of the human using at least two electrodes applied to the knee or a coil or solenoid placed around the knee. The voltage of the signal applied to the electrodes, coil or solenoid is varied based on the size of the knee joint; larger knee joints require larger voltages to generate the effective electric field.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Scaffolds For Cell Transplantation
ActiveUS20120134967A1Enhance cell viabilityIncrease successBiocideSpecial deliveryBiologyBiological activity
A device that includes a scaffold composition and a bioactive composition with the bioactive composition being incorporated into or coated onto the scaffold composition such that the scaffold composition and / or a bioactive composition controls egress of a resident cell or progeny thereof. The devices mediate active recruitment, modification, and release of host cells from the material.
Owner:RGT UNIV OF MICHIGAN +1
Preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of allogenic mesenchymal stem cells in treating myocardial infarction
ActiveCN105985985AImprove anti-apoptotic abilityPromote homingUnknown materialsFermentationAntigenInflammatory factors
The invention belongs to the field of allogenic mesenchymal stem cells, and particularly relates to a preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of the allogenic mesenchymal stem cells in treating myocardial infarction. The preparation method comprises the following steps: carrying out separation by density gradient centrifugation to obtain allogenic single karyocytes, and carrying out adherent culture to obtain mesenchymal stem cells; designing a mesenchymal stem cell surface antigen B2M-gRNA and an inflammatory factor TNF-alpha-gRNA; establishing recombinant slow virus particles, and transfecting the mesenchymal stem cells; optimizing the mesenchymal stem cells by using IGF-1; and preparing drugs for treating myocardial infarctions by using the modified and optimized mesenchymal stem cells. The CRISPR / Cas9 technique is utilized to remove the antigens capable of causing immunological rejection and the inflammatory factors capable of causing inflammatory reaction on the mesenchymal stem cell surface, and the IGF-1 is utilized to enhance the apoptosis resistance of the mesenchymal stem cells and promote the homing of the mesenchymal stem cells, thereby providing a new technical scheme for preparing drugs for treating cardiovascular diseases in clinic. The prepared allogenic mesenchymal stem cells can not cause immunological rejection after cell transplantation.
Owner:SUZHOU UNIV
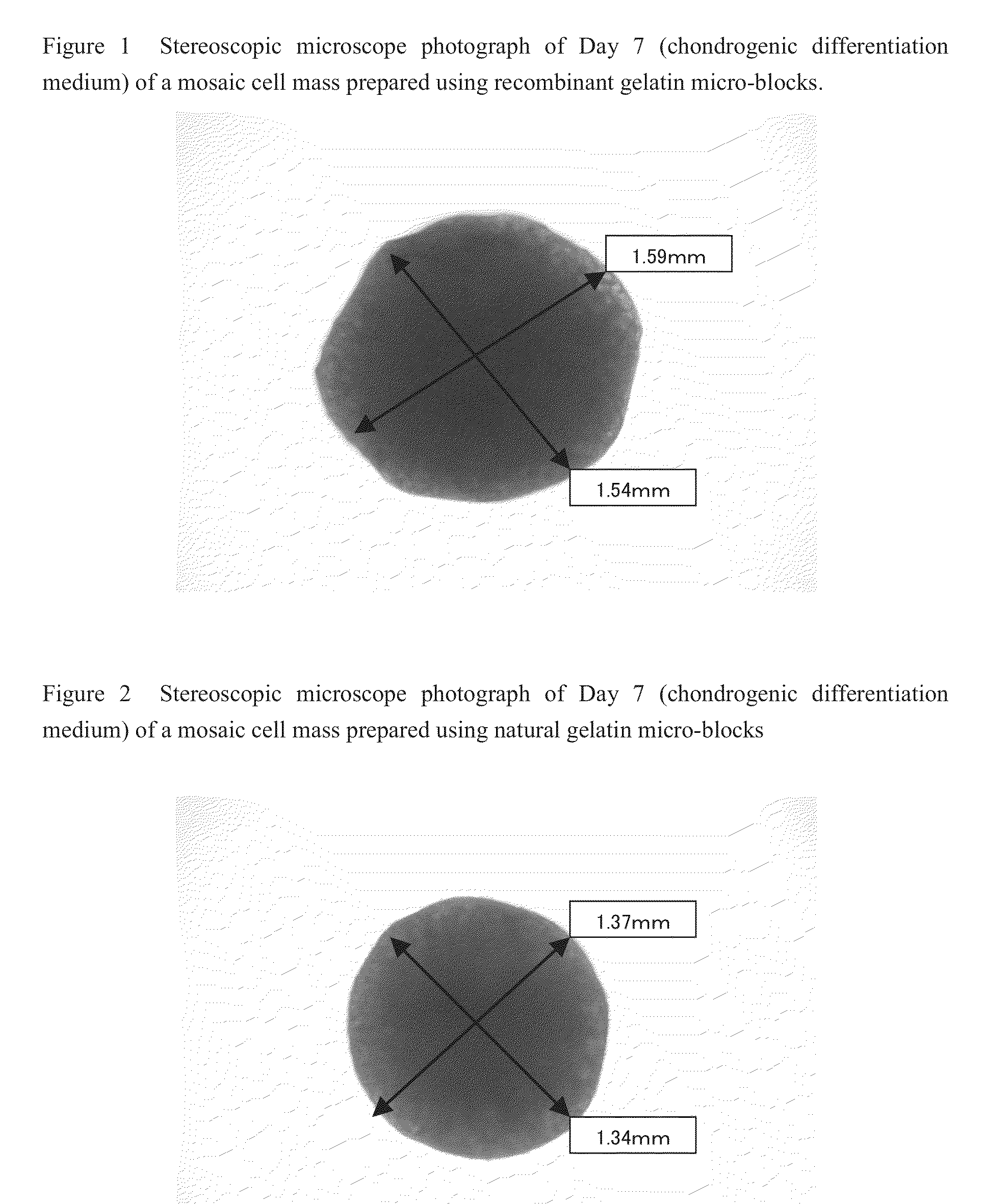
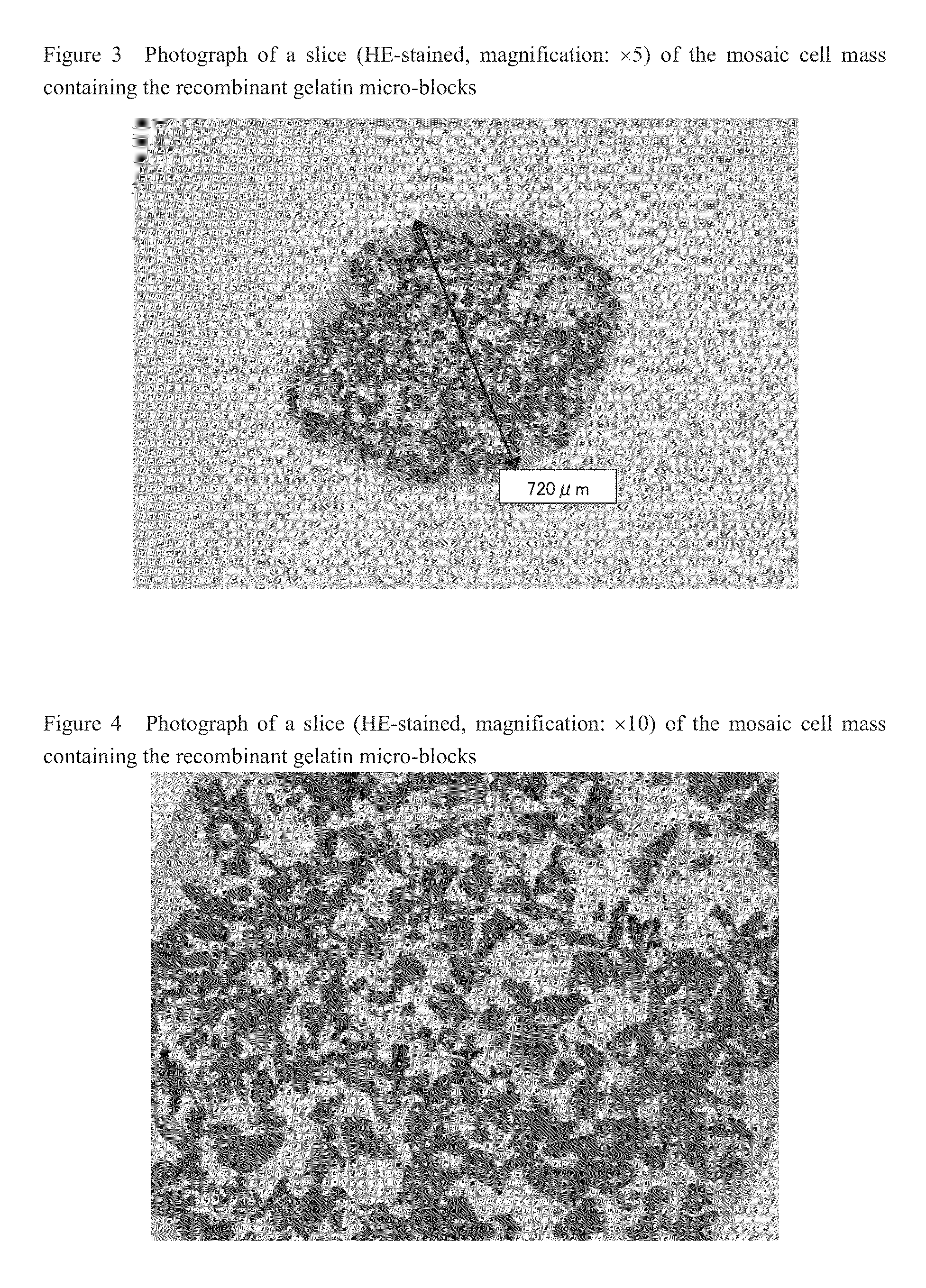
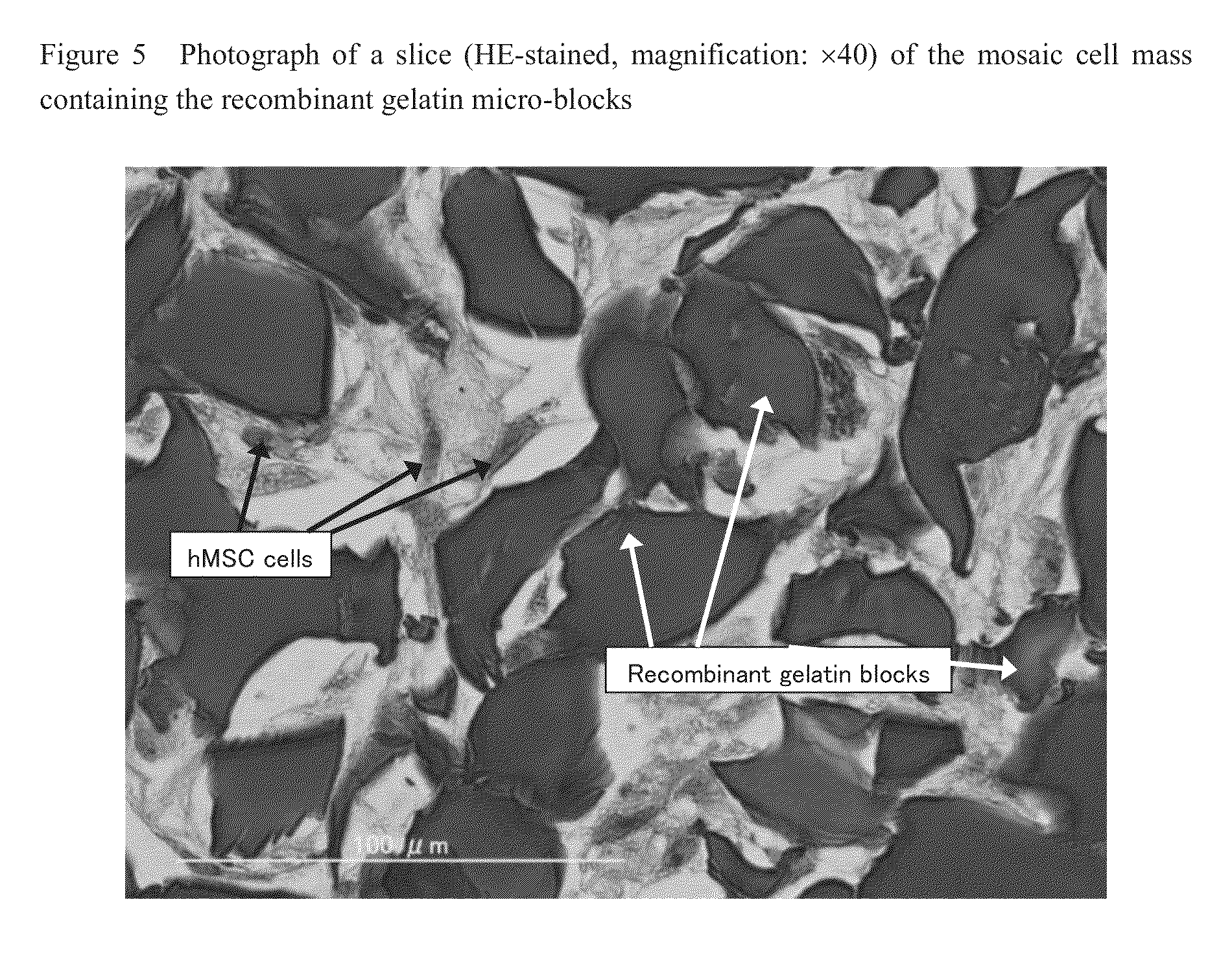
Cell construct for cell transplantation and cell aggregate for cell transplantation
An object of the present invention is to provide a cell construct for cell transplantation capable of having a thickness suitable for cell transplantation, preventing the necrosis of transplanted cells, and forming blood vessels in the transplantation site after transplantation. The present invention provides a cell construct for cell transplantation which comprises polymer blocks having biocompatibility and cells of at least one type, wherein the plural polymer blocks are arranged in spaces between the plural cells.
Owner:FUJIFILM CORP
Catheter-based delivery of Skeletal Myoblasts to the Myocardium of Damaged Hearts
InactiveUS20060263338A1Avoiding tissue rejection problemMinimally invasiveBiocideMammal material medical ingredientsDiseaseTissue defect
The present invention provides improved systems and methods for the minimally invasive treatment of heart tissue deficiency, damage and / or loss, especially in patients suffering from disorders characterized by insufficient cardiac function, such as congestive heart failure or myocardial infarction. In certain embodiments, a cell composition comprising autologous skeletal myoblasts and, optionally, fibroblasts, cardiomyocytes and / or stem cells, is delivered to a subject's myocardium at or near the site of tissue deficiency, damage or loss, using an intravascular catheter with a deployable needle. Preferably, the cell transplantation is performed after identifying a region of the subject's myocardium in need of treatment. The inventive procedure, which can be repeated several times over time, results in improved structural and / or functional properties of the region treated, as well as in improved overall cardiac function. In particular, the inventive therapeutic methods may be performed on patients that have previously undergone CABG or LVAD implantation.
Owner:MYTOGEN
Embryonic or stem-like cell lines produced by cross species nuclear transplantation and methods for enhancing embryonic development by genetic alteration of donor cells or by tissue culture conditions
InactiveUS20030229908A1Good curative effectIncrease productionNew breed animal cellsPeptide/protein ingredientsHeterologousEmbryo
An improved method of nuclear transfer involving the transplantation of differentiated donor cell nuclei into enucleated oocytes of a species different from the donor cell is provided. The resultant nuclear transfer units are useful for the production of isogenic embryonic stem cells, in particular human isogenic embryonic or stem cells. These embryonic or stem-like cells are useful for producing desired differentiated cells and for introduction, removal or modification, of desired genes, e.g., at specific sites of the genome of such cells by homologous recombination. These cells, which may contain a heterologous gene, are especially useful in cell transplantation therapies and for in vitro study of cell differentiation. Also, methods for improving nuclear transfer efficiency by genetically altering donor cells to inhibit apoptosis, select for a specific cell cycle and / or enhance embryonic growth and development are provided.
Owner:ADVANCED CELL TECH INC
Induced hepatocytes
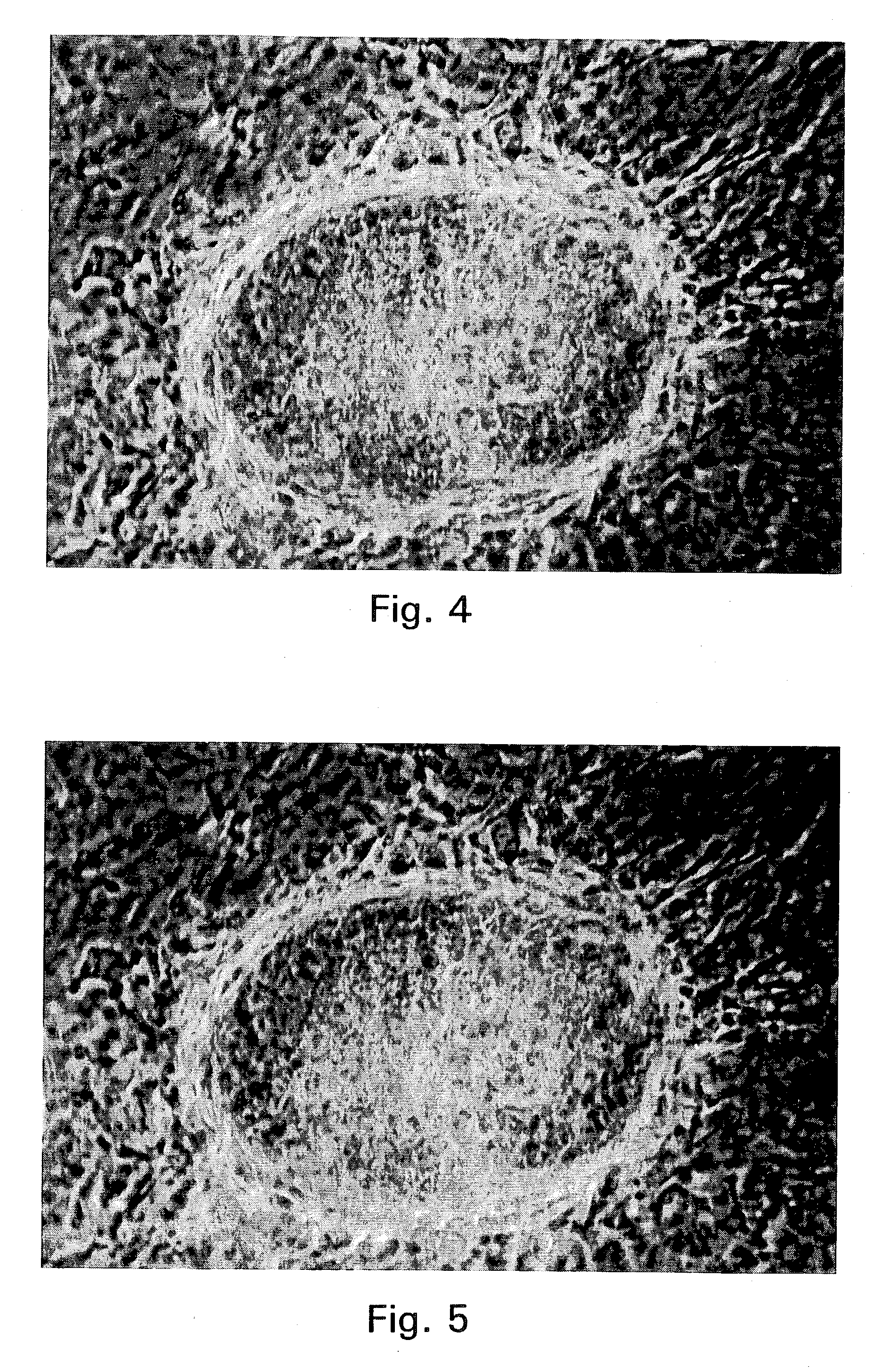
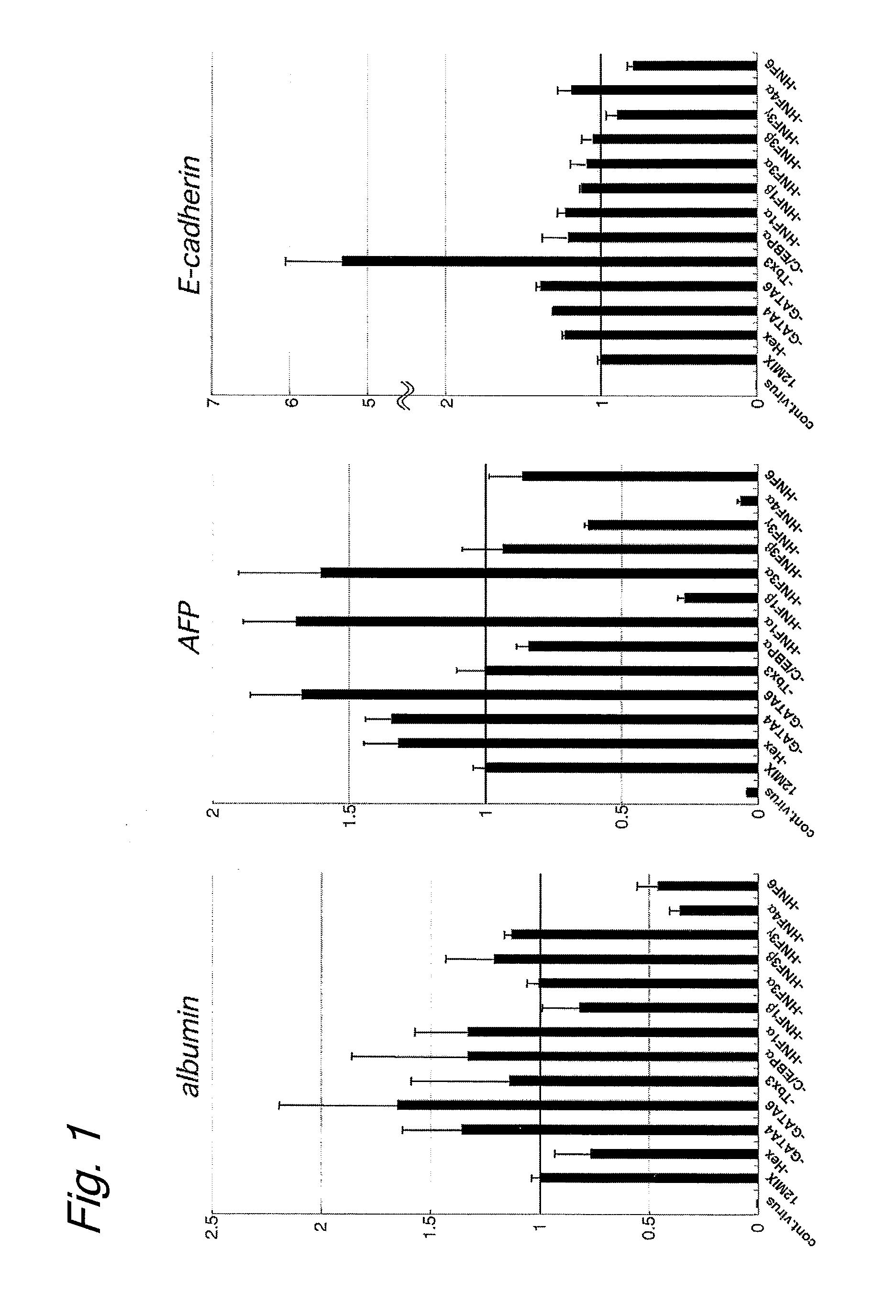
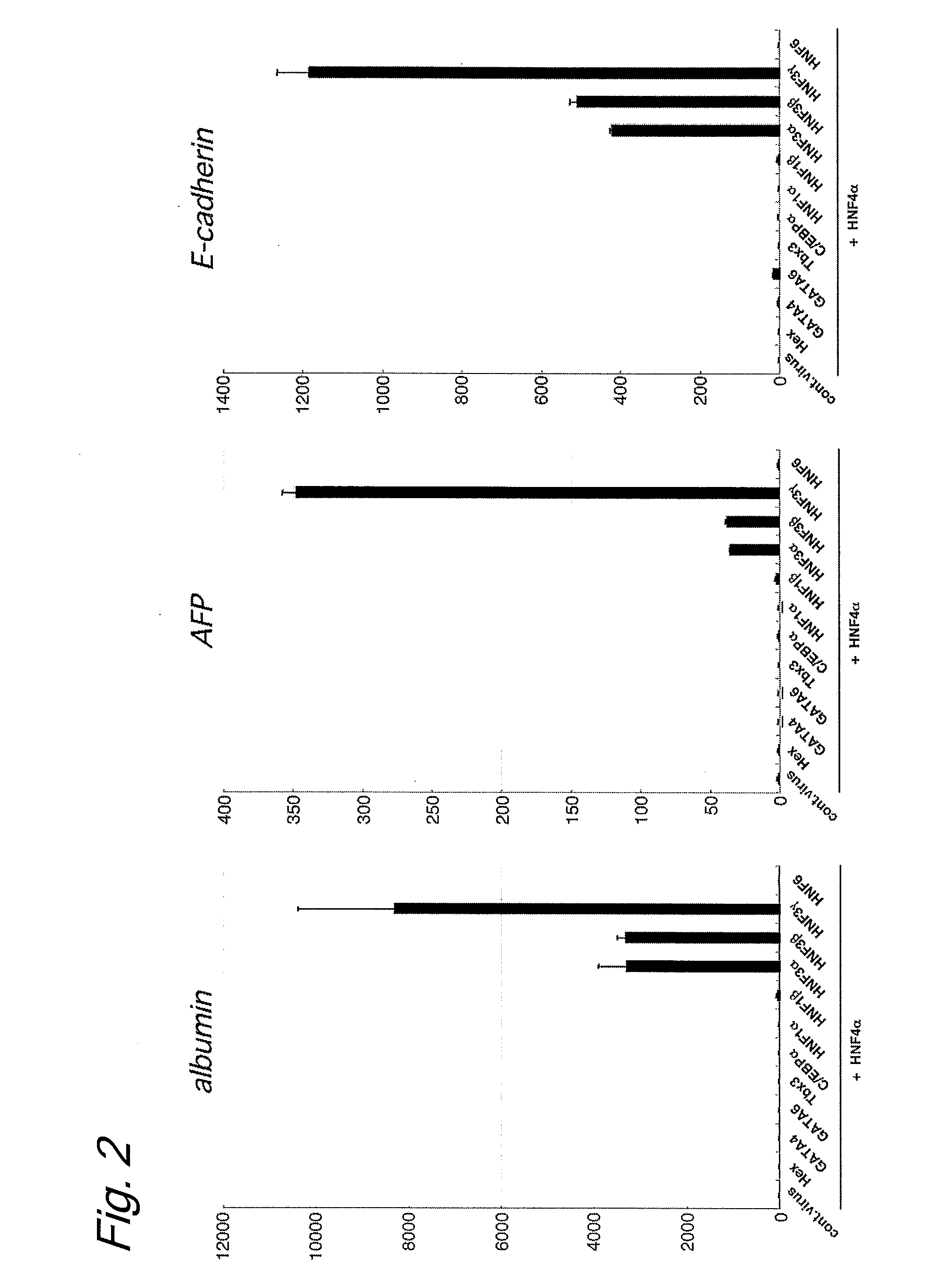
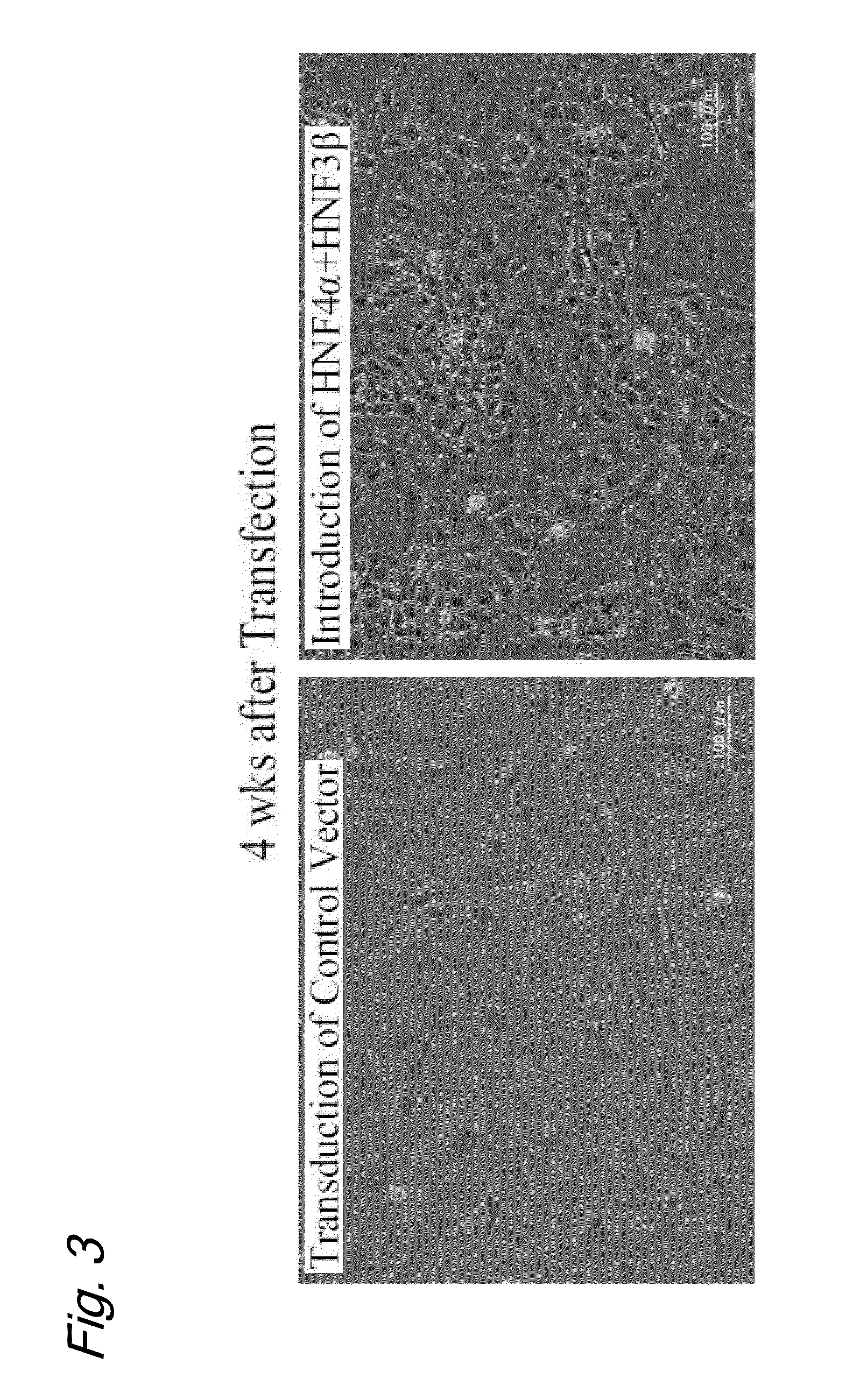
An object of the present invention is to produce hepatic cells from non-hepatic cells that can be obtained less invasively and at low cost. The gene of HNF3α, HNF3β, or HNF3γ and the gene of HNF4α are transduced into non-hepatic cells. The present invention enables the production of induced hepatocytes from somatic cells that are non-hepatic cells, such as skin cells. The use of the induced hepatocytes obtained by the present invention can establish and develop, as general medical treatment, cell transplantation into the liver, artificial livers, and drug response tests, which are the areas that have not been generalized because of the difficulty of procuring hepatocytes.
Owner:JAPAN SCI & TECH CORP
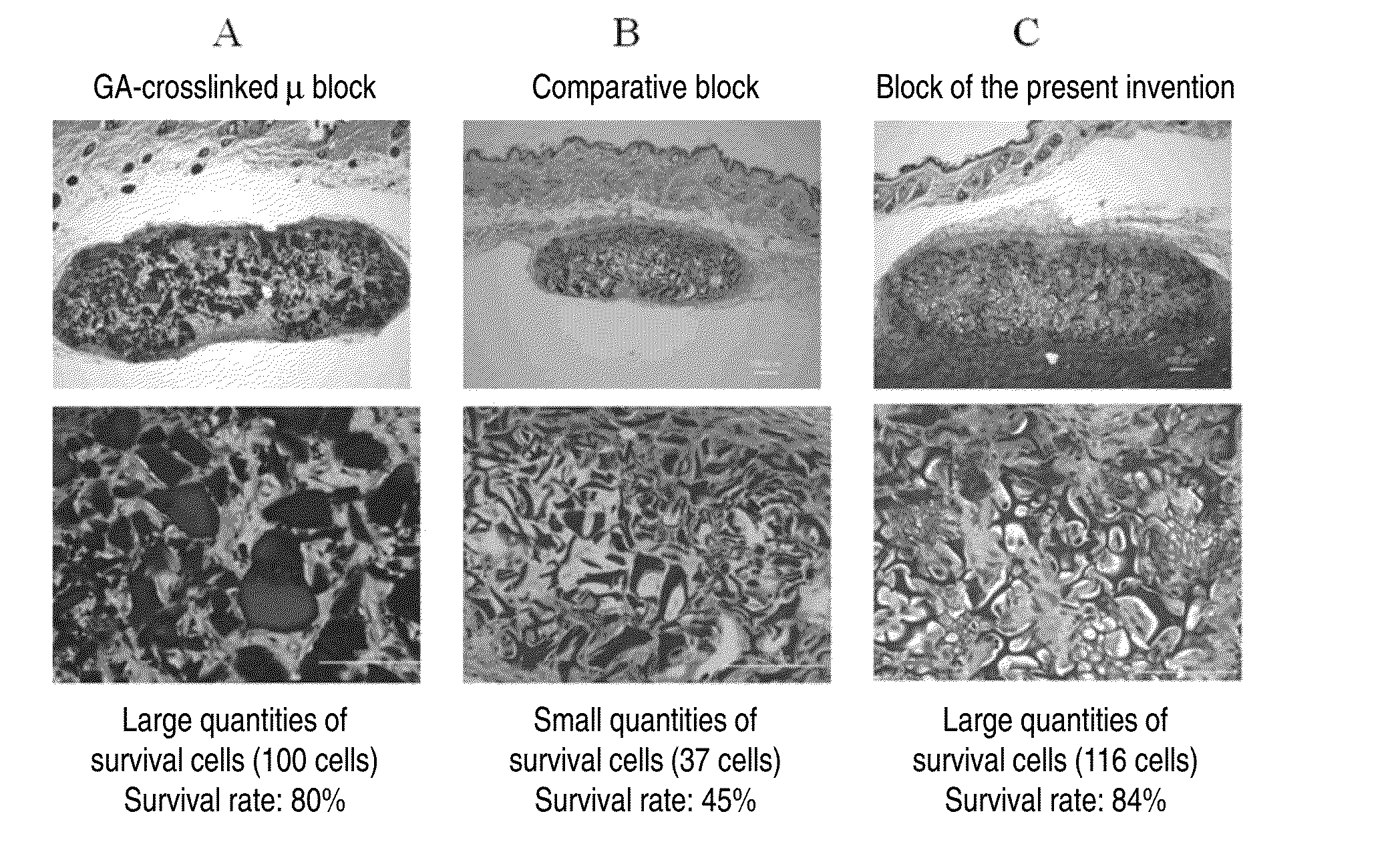
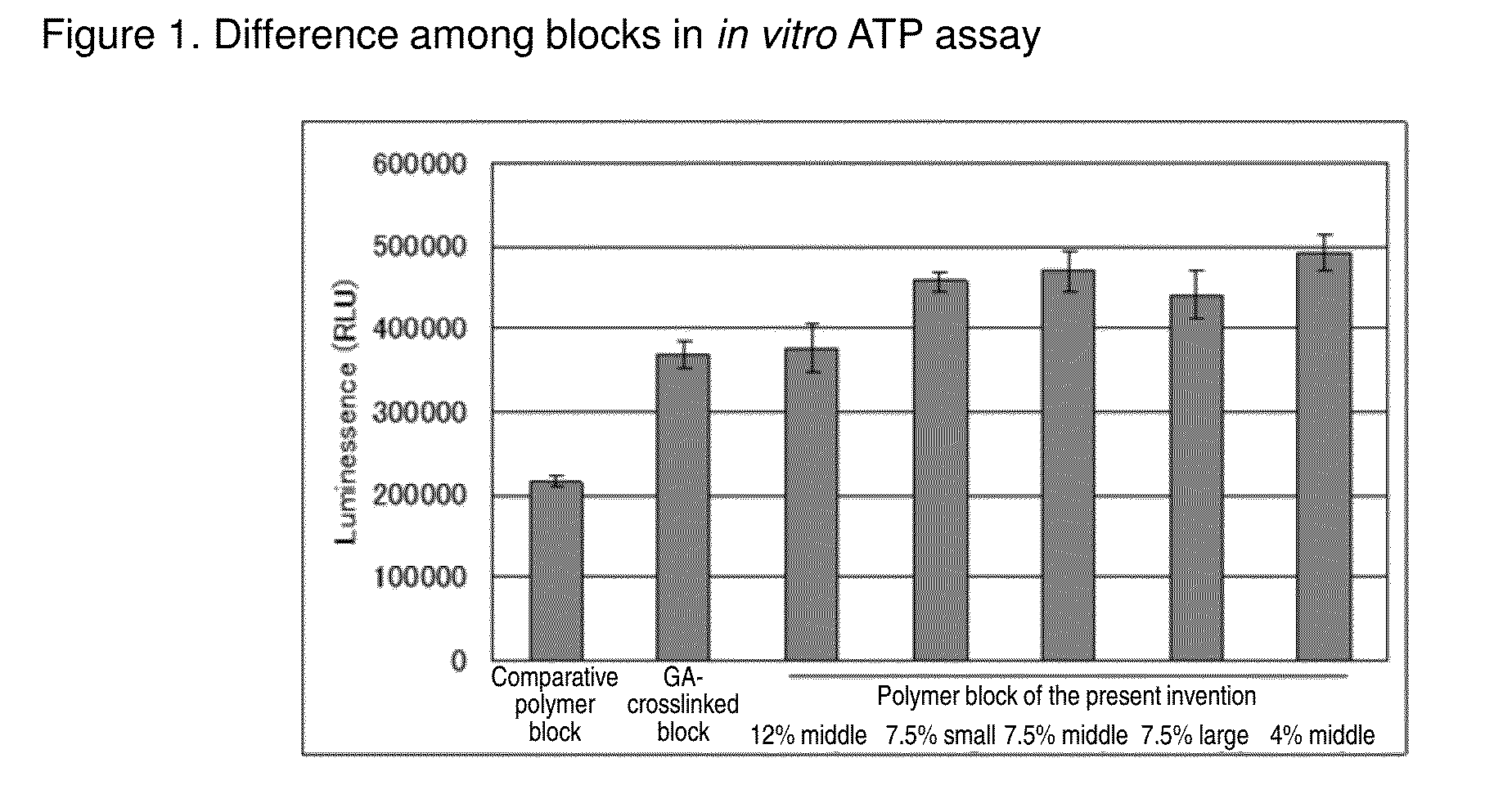
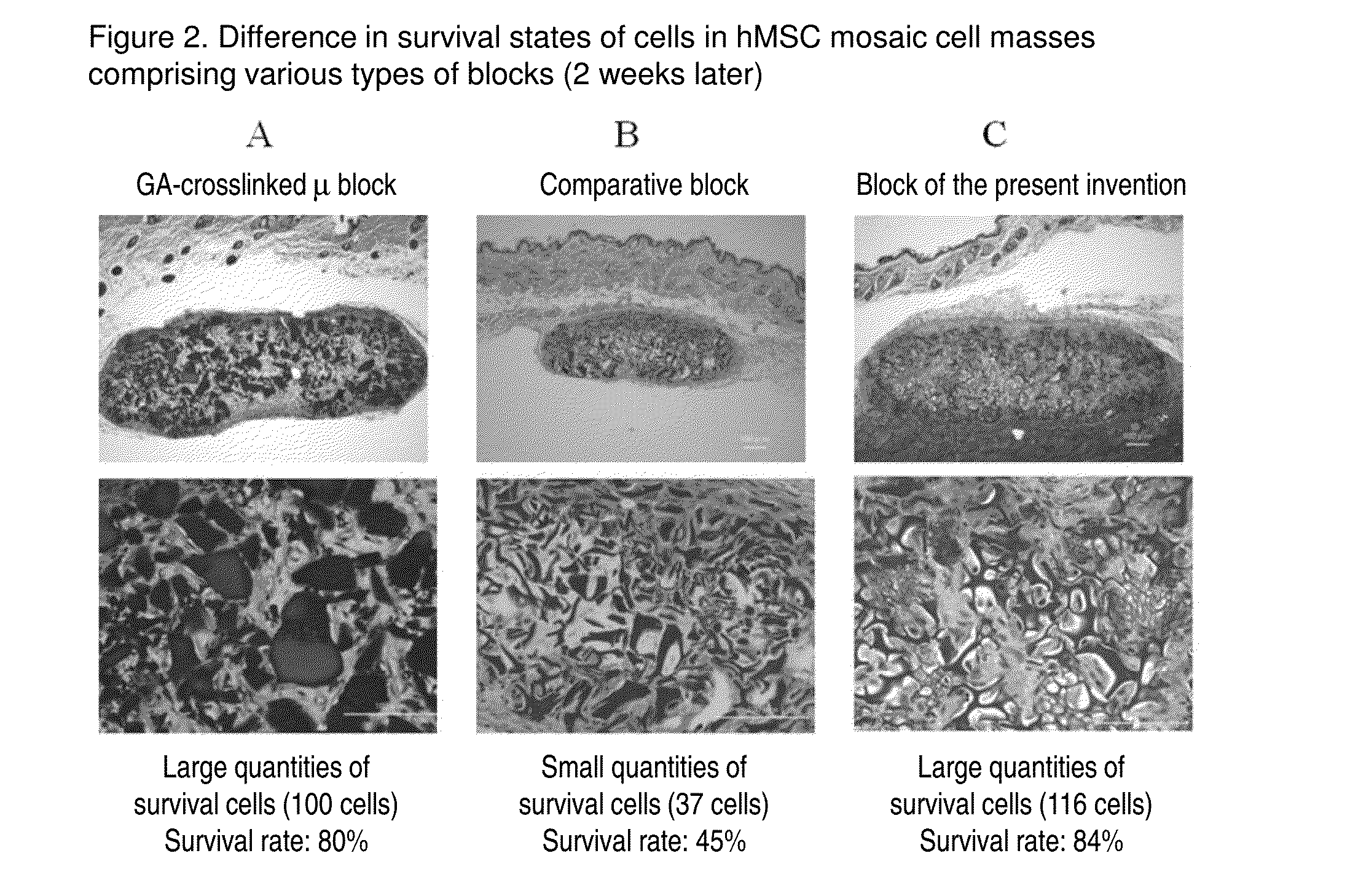
Cell construct for cell transplantation, biocompatible polymer block, and method for producing the same
InactiveUS20150352252A1Suppress the necrosis of the transplanted cellsConducive to survivalBiocidePeptide/protein ingredientsHigh cellCytotoxicity
It is an object of the present invention to provide a cell construct for cell transplantation that does not contain a substance having cytotoxicity, such as glutaraldehyde, and suppresses the necrosis of the transplanted cells in the construct (namely, having a high cell survival rate). The present invention provides a cell construct for cell transplantation comprising biocompatible polymer blocks that do not contain glutaraldehyde and at least one type of cells, wherein a plurality of biocompatible polymer blocks are disposed in gaps among a plurality of cells, and wherein the biocompatible polymer blocks have a tap density of 10 mg / cm3 or more and 500 mg / cm3 or less, or the value of the square root of the cross-sectional area / boundary length in the two-dimensional sectional image of the polymer block is 0.01 or more and 0.13 or less.
Owner:FUJIFILM CORP
Method for the isolation and expansion of cardiac stem cells from biopsy
ActiveUS20070020758A1Convenient and growth factorImprove adhesionArtificial cell constructsMammal material medical ingredientsCardiologyCardiac Stem Cell
Method for the isolation, expansion and preservation of cardiac stem cells from human or animal tissue biopsy samples to be employed in cell transplantation and functional repair of the myocardium or other organs. Cells may also be used in gene therapy for treating genetic cardiomyopathies, for treating ischemic heart diseases and for setting in vitro models to study drugs.
Owner:UNIV DEGLI STUDI DI ROMA LA SAPIENZA
Method of preventing fat graft resorption by administering fat-derived cells and poloxamer P 188
Owner:THE GENERAL HOSPITAL CORP
Vascularized human skin equivalent
InactiveUS20070207125A1Accelerated rate of vascularizationImprove clinical utilityBiocideEpidermal cells/skin cellsSkin equivalentIn vivo
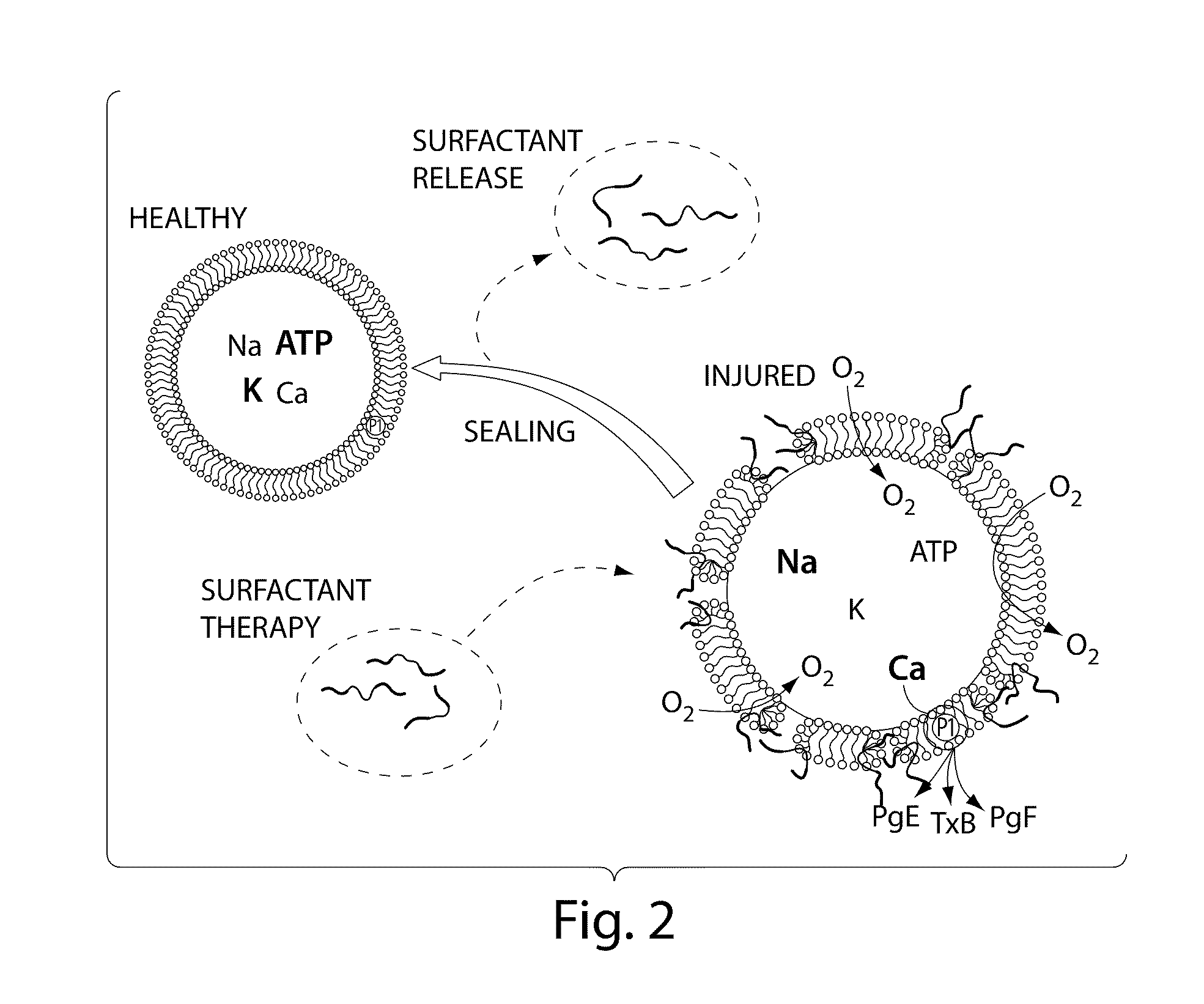
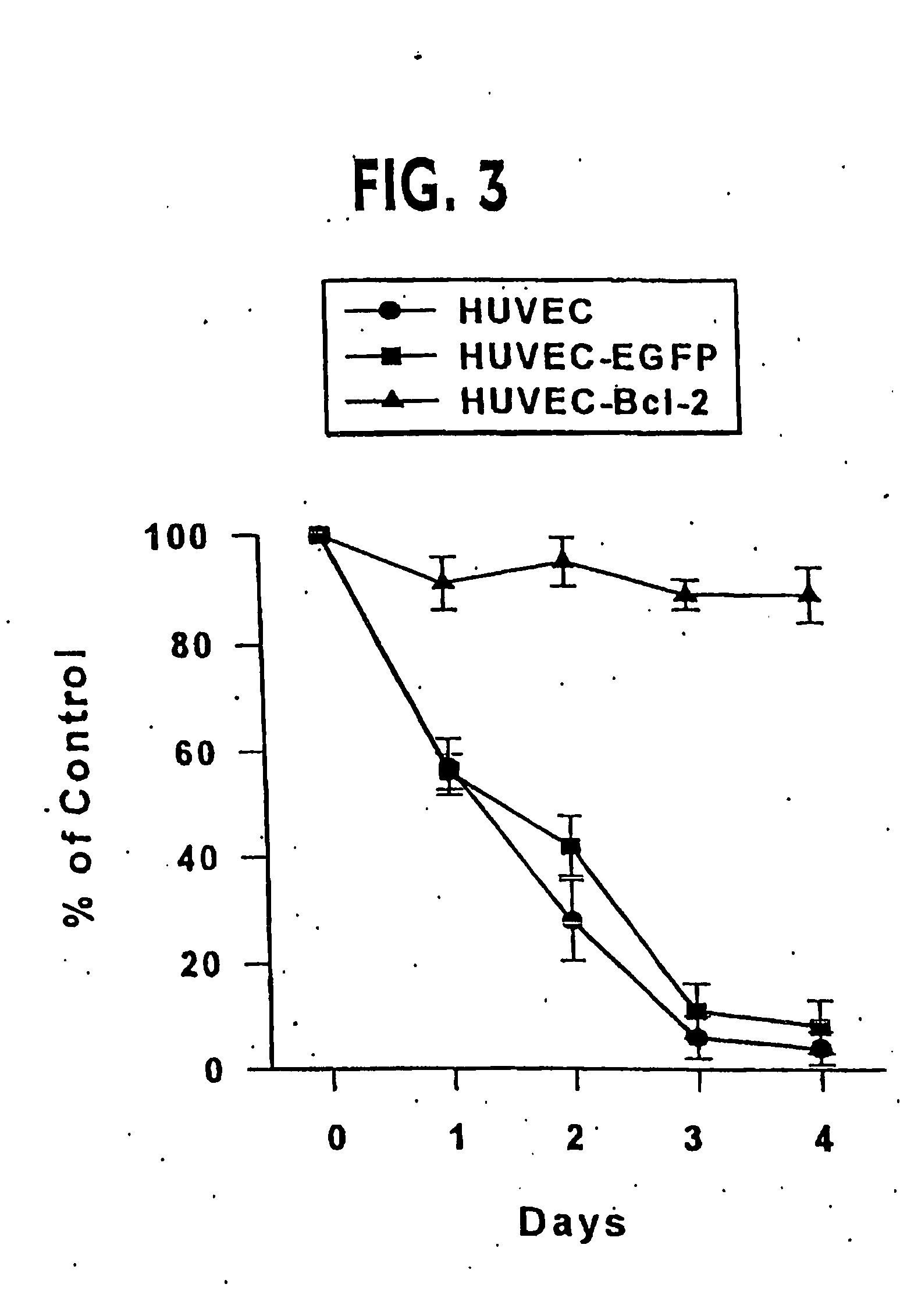
Clinical performance of currently available human skin equivalents is limited by failure to develop perfusion. To address this problem we have developed a method of endothelial cell transplantation that promotes vascularization of human skin equivalents in vivo. Living skin equivalents were constructed by sequentially seeding the apical and basal surfaces of acellular dermis with cultured human keratinocytes and Bcl-2 transduced HUVEC or umbilical cord cells sequentially. After orthotopic implantation of grafts comprising cultured human keratinocytes and Bcl-2 transduced HUVEC cells onto mice, the grafts displayed both a differentiated human epidermis and perfusion through the HUVEC-lined microvessels. These vessels, which showed evidence of progressive maturation, accelerated the rate of graft vascularization. Successful transplantation of such vascularized human skin equivalents should enhance clinical utility, especially in recipients with impaired angiogenesis.
Owner:YALE UNIV
Non-disruptive three-dimensional culture and harvest system for anchorage-dependent cells
InactiveUS6905875B2Easy to useEasily brokenHepatocytesDead animal preservationCell-Extracellular MatrixECM Protein
A non-disruptive three-dimensional culture system allows cell growth and proliferation in three dimensions, permitting cell splitting without subjecting cells to disruptive conditions that affect cell structure and functions. An extracellular matrix provides a good environment for culturing or co-culturing anchorage-dependent cells. The cells cultured this manner can be readily used in such applications as cell transplantation, tissue engineering seeding of cells on scaffolds, and other applications that require immediate availability of functioning cells.
Owner:AGENCY FOR SCI TECH & RES +1
Preparation for treating heart disease used in cell therapy
ActiveUS20100303909A1Improve survivalSufficient degreeBiocidePowder deliveryInduced pluripotent stem cellCardiac muscle
An object of the present invention is to establish a cell transplantation method which can markedly improve the survival of grafted pluripotent stem cells and the efficiency of cardiomyocyte regeneration in cell therapy using pluripotent stem cells derived from heart tissue and can treat heart disease further effectively. Specifically, according to the present invention, enhancement in the survival of grafted pluripotent stem cells and significant improvement in the efficiency of cardiomyocyte regeneration are achieved by using, in combination, pluripotent stem cells derived from heart tissue and a hydrogel containing a basic fibroblast growth factor (bFGF) in cell therapy for heart disease.
Owner:KYOTO UNIV
Compositions Containing Resveratrol and Nucleotides
A resveratrol-containing composition capable of providing a therapeutic benefit to a subject such as modulation of a biological activity, improving cell transplantation therapy, or improving macular degeneration or dystrophy treatments. The compositions comprise trans-resveratrol, a metal chelator, one or more additional antioxidants such as quercetin, gamma-tocotrienol, or apple polyphenols, allicin, and nucleotides.
Owner:RESVERATROL PARTNERS
Preparation method of alginate/epsilon-polylysine/alginate biological microcapsule
ActiveCN102101037AConducive to the maintenance of activityMild reaction conditionsUnknown materialsPharmaceutical non-active ingredientsEpsilon-PolylysineDrug release
The invention provides a biological microcapsule, in particular relating to a preparation method of an alginate / epsilon-polylysine / alginate biological microcapsule (epsilon-APA microcapsule). Based on the characteristic that the highly polymerized multivalence-cationic epsilon-polylysine has a high electrostatic interaction with a substance with negative ions, the epsilon-polylysine is taken as amicrocapsule film material, and the alginate microcapsule is externally made into a coating film with slow-release performance to change the permeability and immune isolation of the biological microcapsule, so that the epsilon-APA microcapsule is used for the biological and medical fields such as the cell transplantation, the cell culture, the drug release, and the like.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
Polymer composite micro-bead and preparation method thereof
InactiveCN102327761AEasy to operateFew stepsGranular deliveryChemical/physical/physico-chemical processesDisplay deviceDrug carrier
The invention relates to a polymer composite micro-bead and a preparation method thereof. The micro-bead which is in a core-shell structure comprises 1-3 inner cores and an outer shell. Each inner core is composed of two hemispherical polymer droplets; the outer shell is composed of polymer droplets that are incompatible with the shell; the diameter of each inner core is in a range from 50 microns to 1 millimeter; and the diameter of the outer shell is in a range from 100 microns to 1.5 millimeters. The preparation method comprises the steps of: introducing a shell layer polymer solution and two core layer polymer solutions into a micro-tube to form a micro-sphere; then introducing water into the micro-tube; and finally solidifying the shell layer to obtain the micro-bead. The composite micro-bead disclosed by the invention can be prepared through a multi-level micro-tube reactor; and the diameter and the structure of the micro-bead can be controlled by the structure of the micro-tubereactor and preparation parameters. The composite micro-bead disclosed by the invention can be applied to the fields, such as drug carriers and release, cell transplantation and culture, micro-reactors, display devices and the like. The preparation method disclosed by the invention is simple in operation, less in procedures and low in cost and has low requirements on apparatuses.
Owner:DONGHUA UNIV
Cell differentiation of adipose-derived precursor cells
The present invention provides a simple method for controlled differentiation of adipose-derived precursor cells. A method is provided for preparing a differentiated cell. The method comprises A) obtaining a mixture by mixing a) an adipose-derived precursor cell and b) a differentiated cell corresponding to a desired site; and B) culturing the mixture under sufficient conditions which allow the adipose-derived precursor cell to differentiate. The present invention also provides a composition for cell implantation comprising a) an adipose-derived precursor cell and b) a differentiated cell corresponding to a desired site.
Owner:BIOMASTER
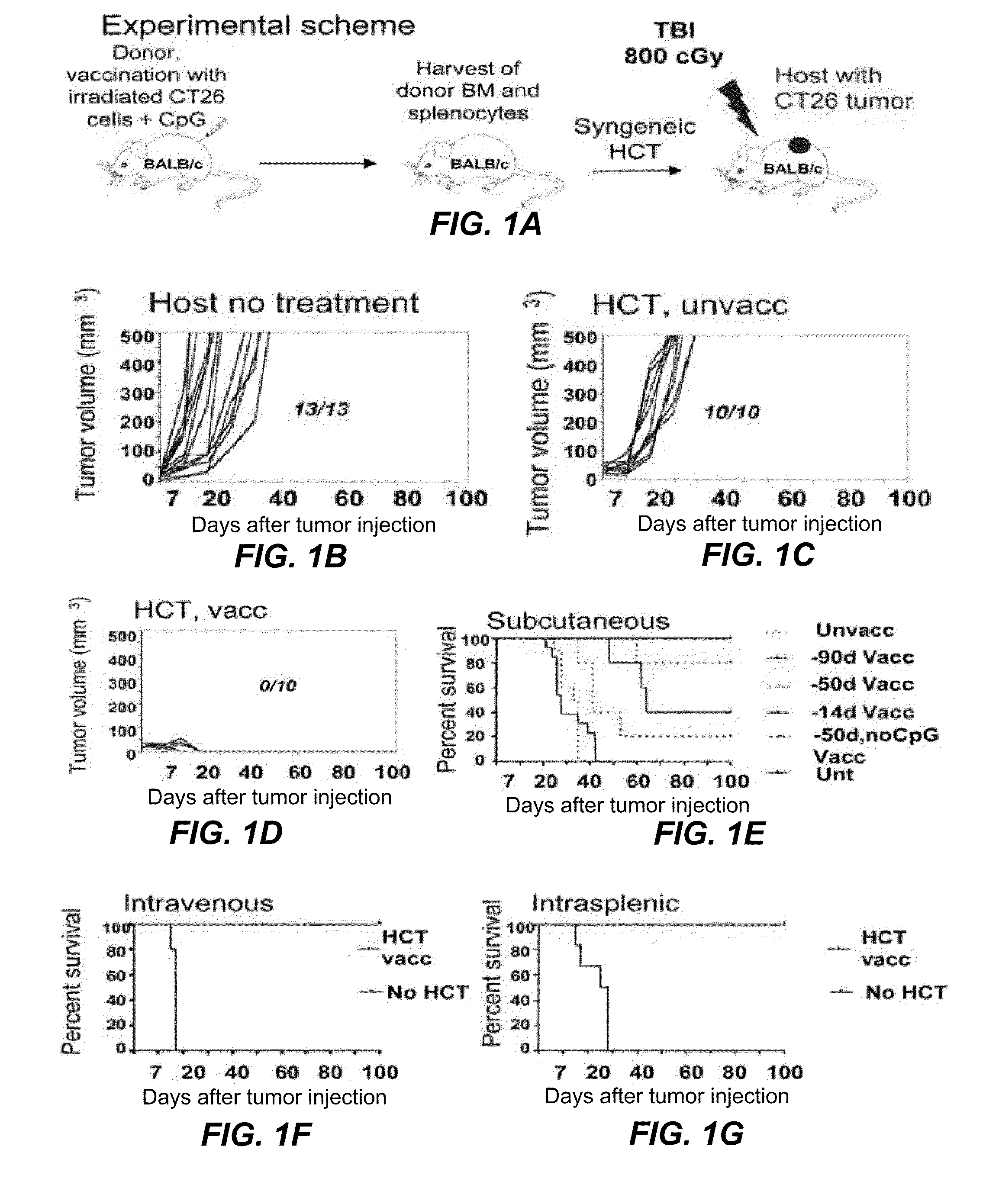
Tumor vaccination in combination with hematopoietic cell transplantation for cancer therapy
ActiveUS20110129503A1Eliminate side effectsBiocideEnergy modified materialsAbnormal tissue growthVaccination
In one aspect, the present invention provides a method for treating cancer comprising tumor cell vaccination in combination with hematopoietic and immune cell transplantation. In some embodiments, the method involves autologous tumor cell vaccination prior to autologous hematopoietic and immune cell transplantation. In another aspect, the present invention provides a method of purifying tumor cells from a subject in preparation for vaccination.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Cell sheet for tissue repair and bio-artificial tissue engineering, method of producing the same and method of using the same
ActiveCN102614546AEasy to transplantPromote healingMuscular disorderSkeletal disorderCell-Extracellular MatrixTissue repair
Disclosed is a cell sheet for tissue repair and bio-artificial tissue engineering. The cell sheet comprises treated stem cell embedded in its self-secreted extracellular matrix (ECM) and formed a cell sheet. The cell sheet is formed by isolating the stem cell, expanding the stem cell and treating the stem cell with biological factors or factors leading to the production of biological factors, to induce its differentiation, production of extracellular matrix and formation of a cell sheet in vitro. The cell sheet is used as a bioactive material or as an acellular material for the promotion of tissue repairs or used to form a bio-artificial organ for tissue replacement. The cell sheet of the present invention eliminates the need to use scaffolds for cell delivery. The cell sheet facilitates in vivo cell transplantation and provides some tensile mechanical strength for bearing early mechanical load during tissue repair.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
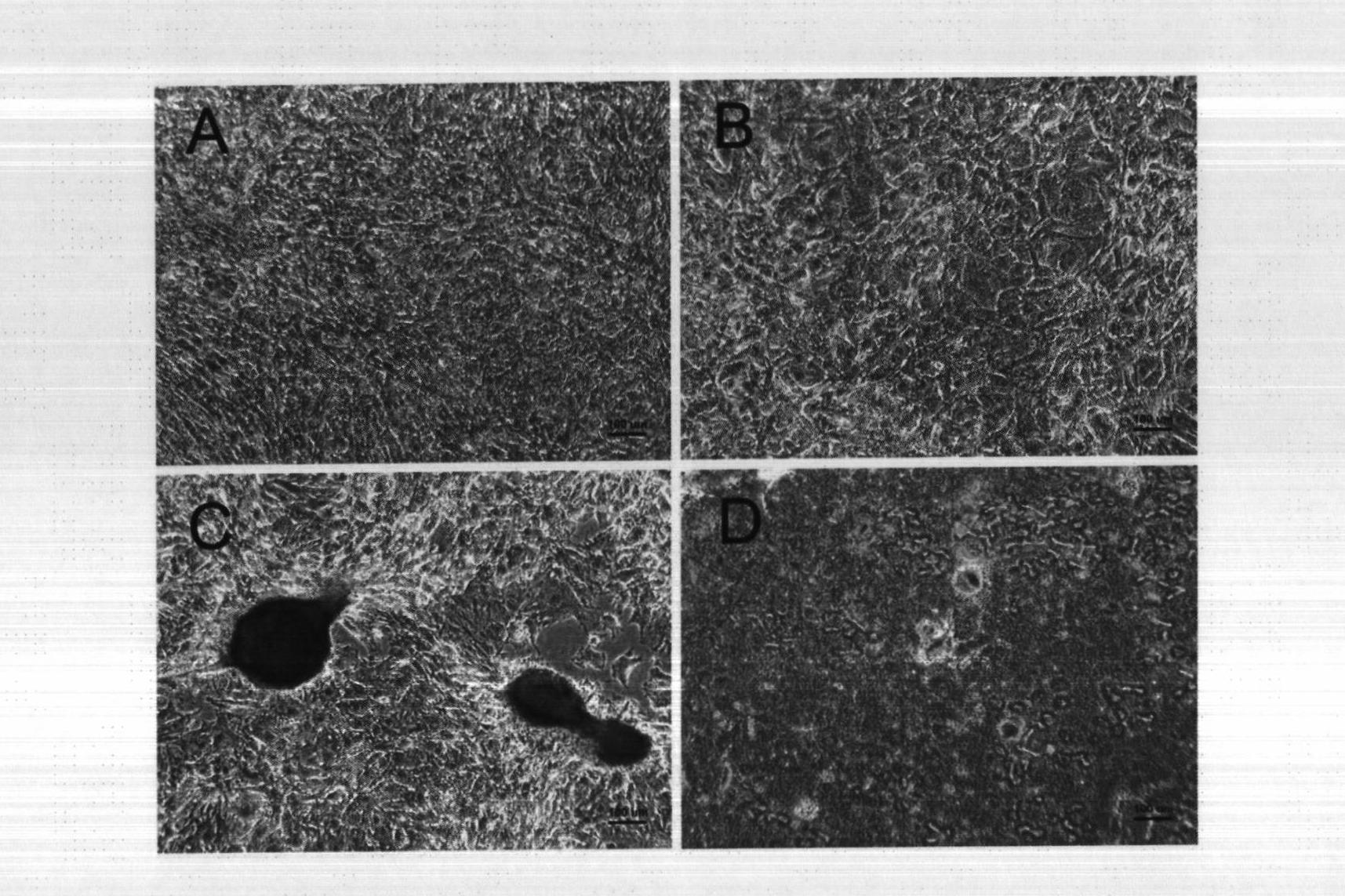
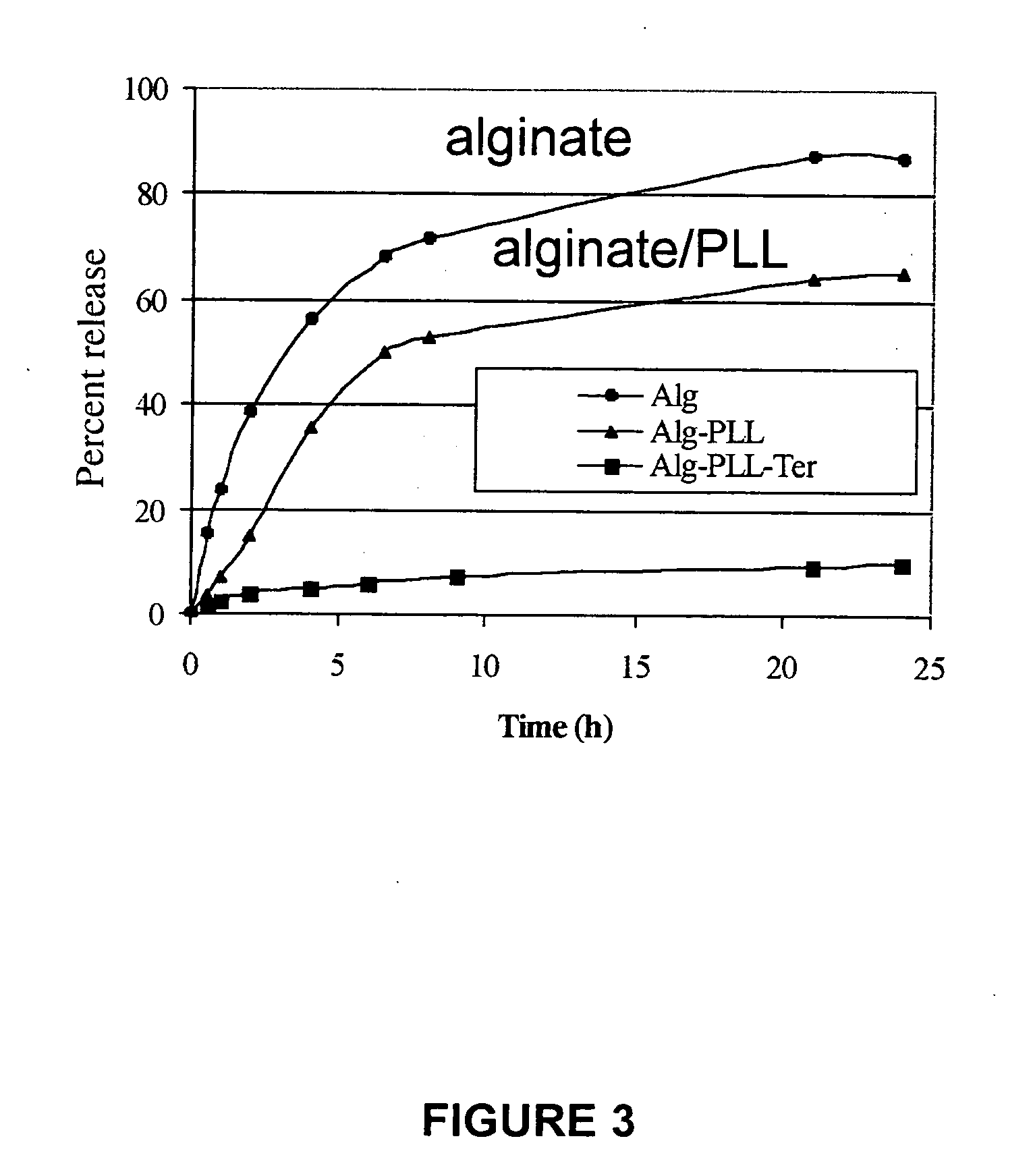
Anti-inflammatory conformal barriers for cell transplantation
ActiveUS20060002903A1Reduces primary islet non-functionImproved long-term graft survivalBiocideTissue cultureMedicineAnticoagulant
The invention comprises anti-inflammatory conformal barriers with controllable permeability properties that can be applied to living cells prior to transplant, and methods for coating living cells with conformal barriers. The coatings comprise polymer layers deposited on a cell surface by layer-by-layer polymer assembly, wherein each layer contains a positive and a negative polymer pair. The barriers can be actively anti-inflammatory through incorporation of anticoagulant and / or anti-inflammatory agents into the barrier.
Owner:EMORY UNIVERSITY
Porous biodegradable polymeric materials for cell transplantation
InactiveUS20060141000A1Enhanced cell attachmentDiffuse fullyBiocidePharmaceutical delivery mechanismPorosityMetabolite
Polymeric materials are used to make a pliable, non-toxic, injectable porous template for vascular ingrowth. The pore size, usually between approximately 100 and 300 microns, allows vascular and connective tissue ingrowth throughout approximately 10 to 90% of the matrix following implantation, and the injection of cells uniformly throughout the implanted matrix without damage to the cells or patient. The introduced cells attach to the connective tissue within the matrix and are fed by the blood vessels. The preferred material for forming the matrix or support structure is a biocompatible synthetic polymer which degrades in a controlled manner by hydrolysis into harmless metabolites, for example, polyglycolic acid, polylactic acid, polyorthoester, polyanhydride, or copolymers thereof. The rate of tissue ingrowth increases as the porosity and / or the pore size of the implanted devices increases. The time required for the tissue to fill the device depends on the polymer crystallinity and is less for amorphous polymers versus semicrystalline polymers. The vascularity of the advancing tissue is consistent with time and independent of the biomaterial composition and morphology.
Owner:CHILDRENS MEDICAL CENT CORP +1
Mesenchymal stem cell for expressing related gene of neurotrophin family and application thereof
The invention relates to a mesenchymal stem cell of marrow, umbilical cord or bleeding of the umbilicus and purpose in the field of cell transplantation, stem cell culture, expansion and oriented differentiation. The mesenchymal stem cell for expressing a related gene of a neurotrophin family is prepared by the following steps of extracting a mesenchymal stem cell from the marrow, the umbilical cord or the bleeding of the umbilicus; recombining the related gene of the neurotrophin family to an AAV (Adeno-Associated Virus) used as a carrier; preparing the AAV expressing the related gene of the neurotrophin family; and infecting separated and purified mesenchymal stem cell through AAV particles to obtain the mesenchymal stem cell expressing the related gene of the neurotrophin family. The invention is used as a culture layer cell to provide a manually controlled microenvironment for stem cell culture, expansion and oriented differentiation and promote the proliferation and the oriented differentiation of the stem cells so as to provide a new curing measure for human diseases.
Owner:广州吉帝生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com