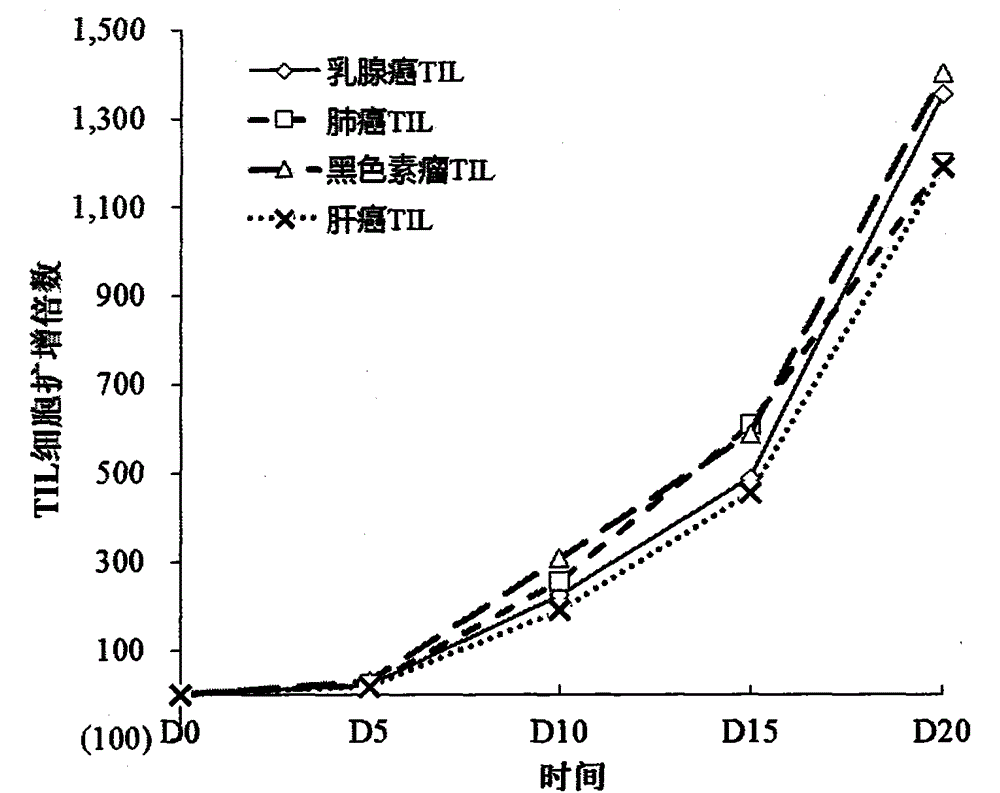
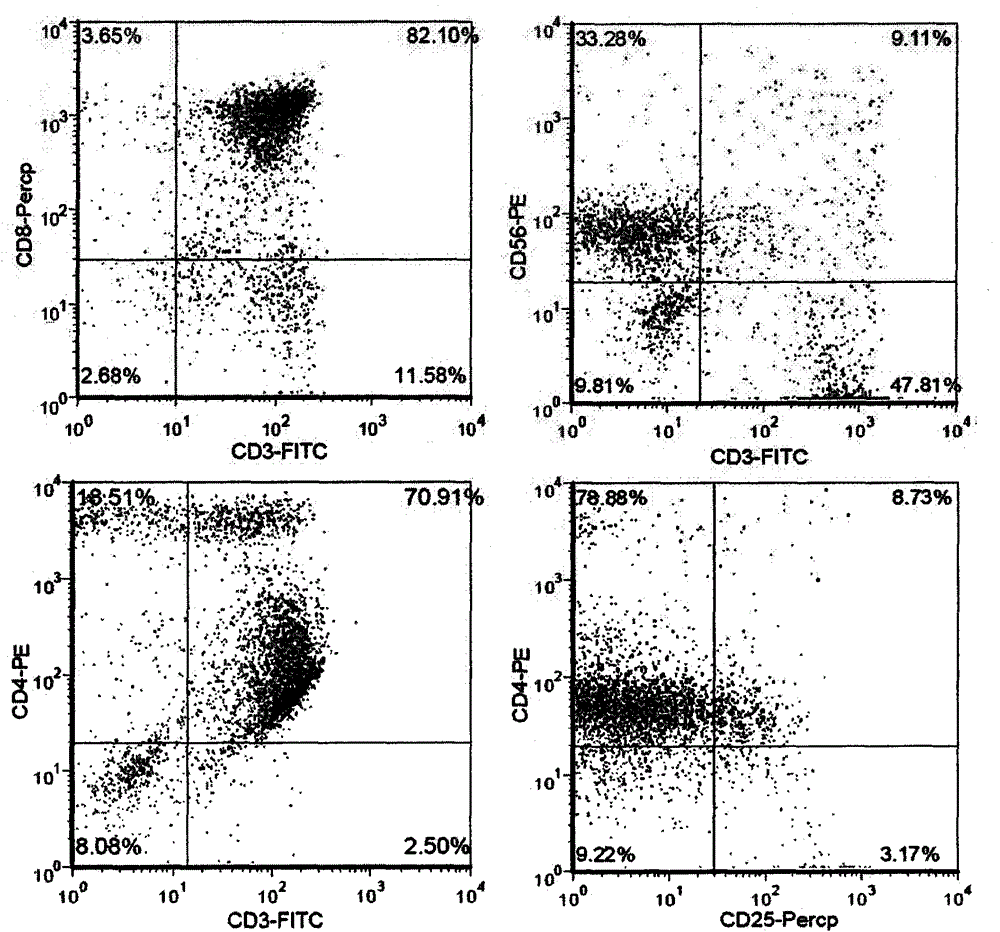
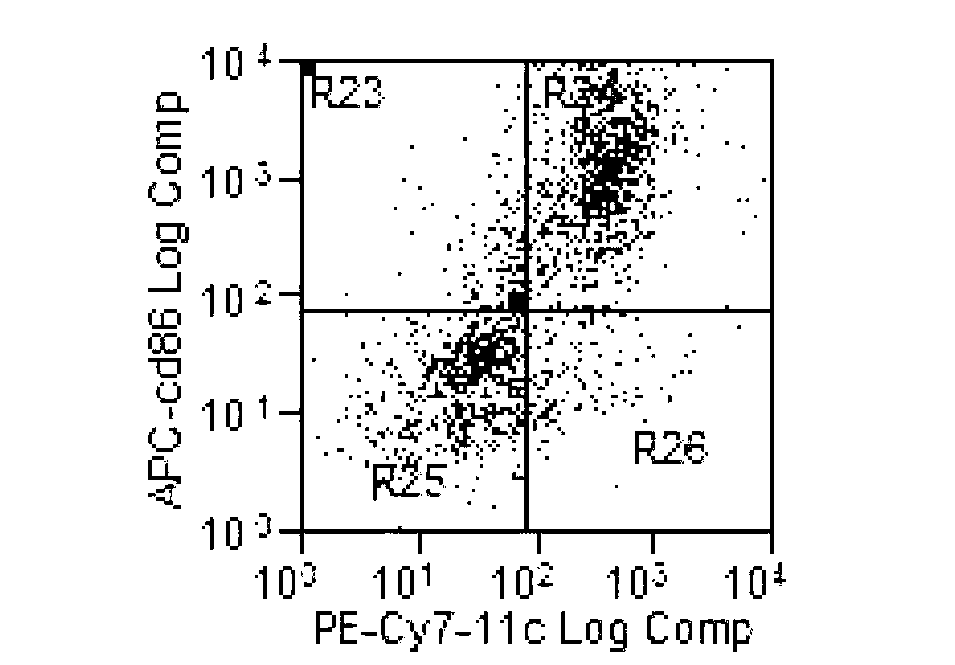
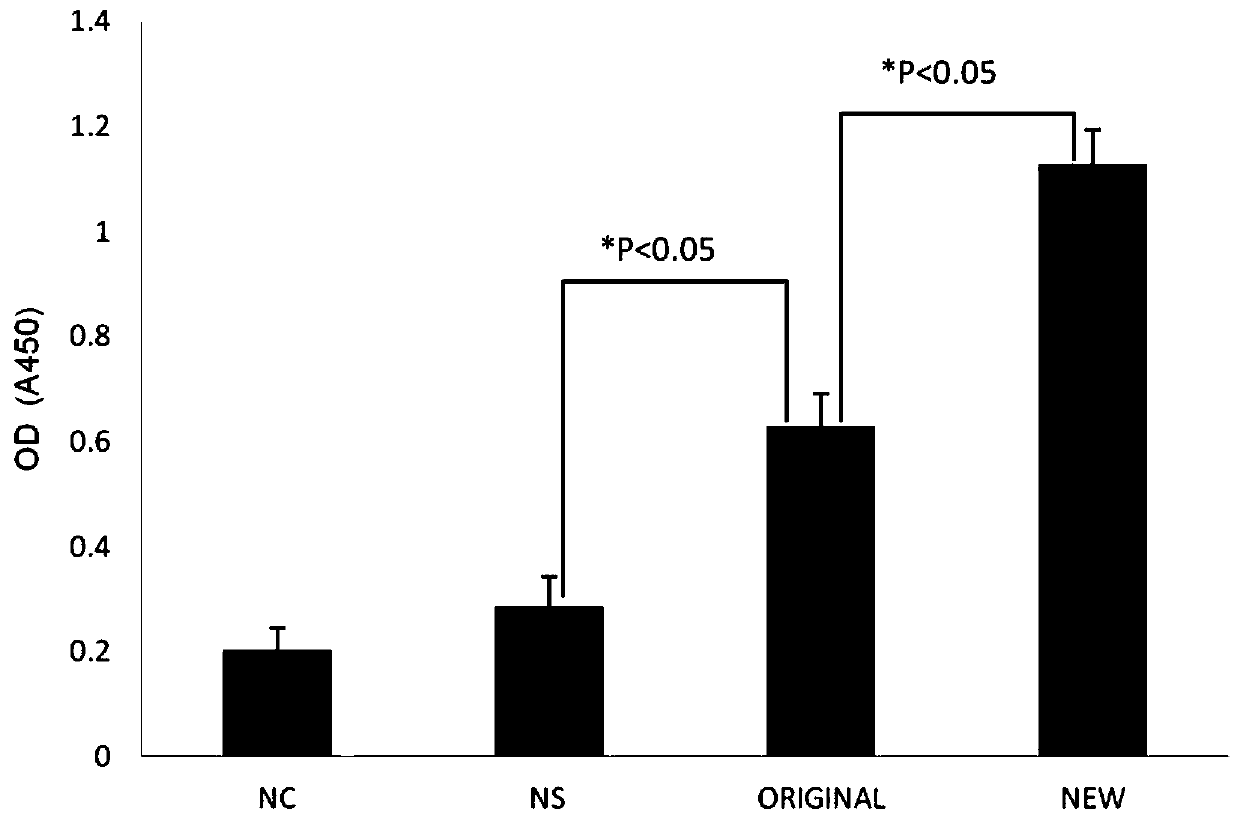
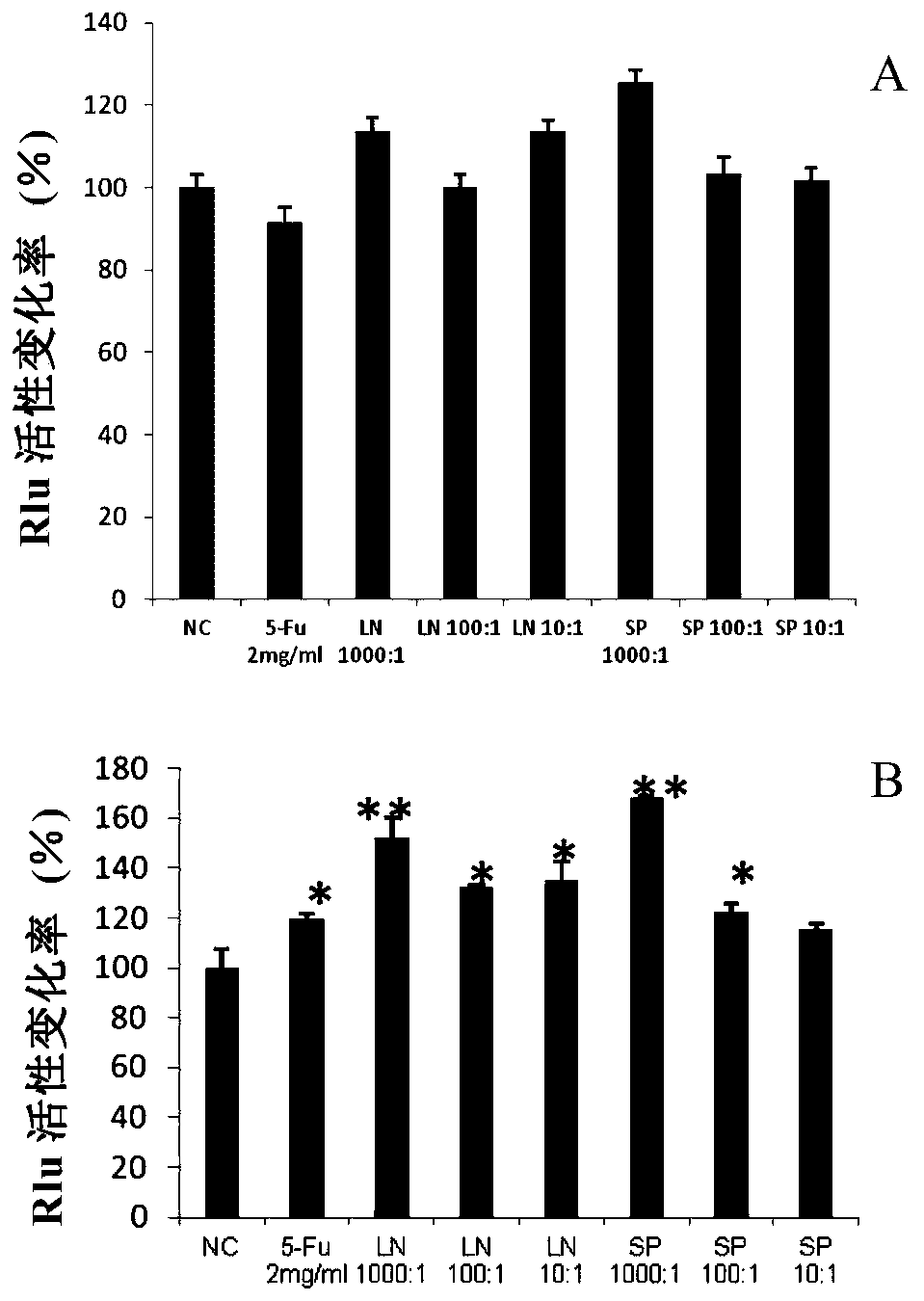
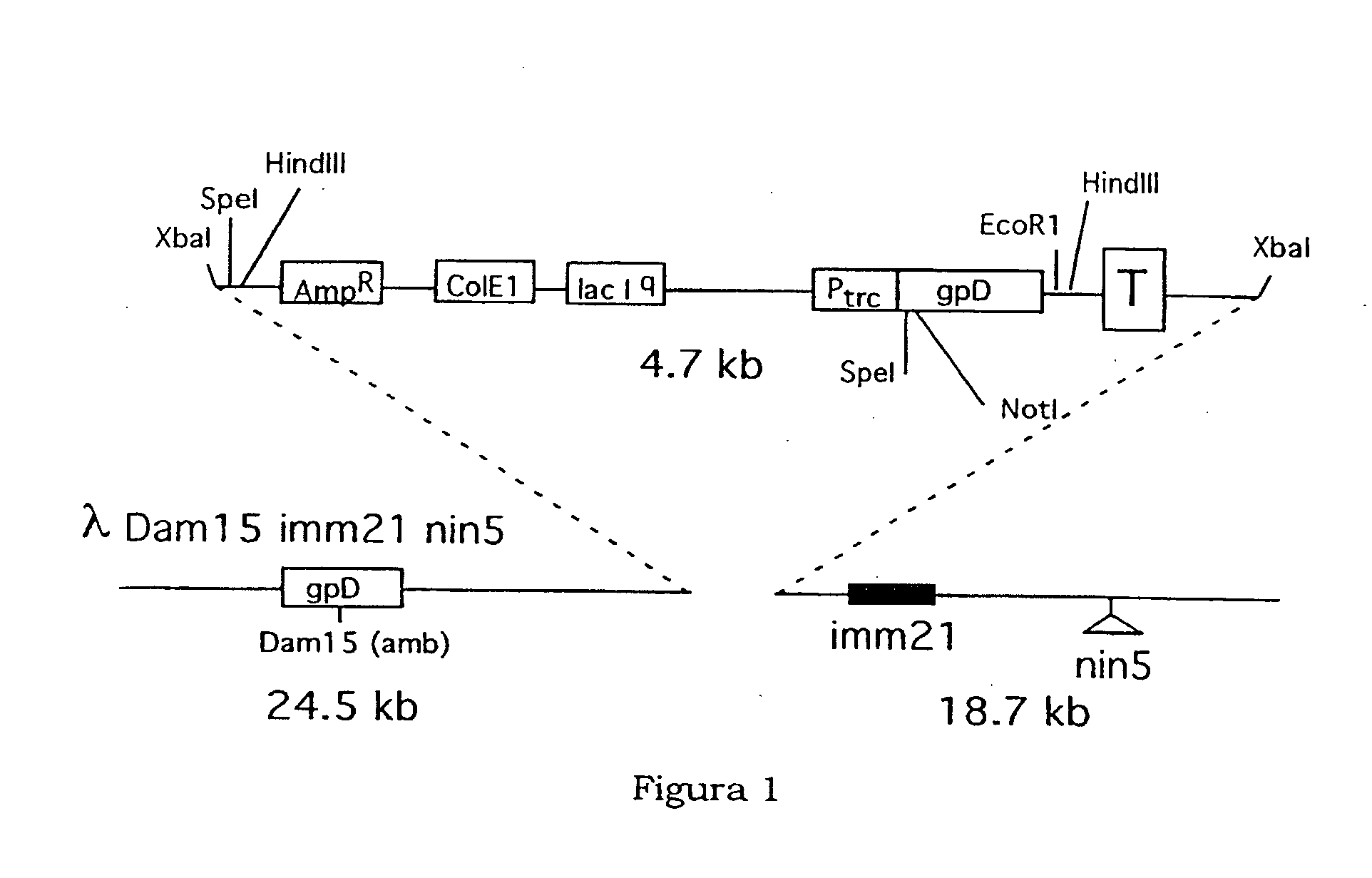
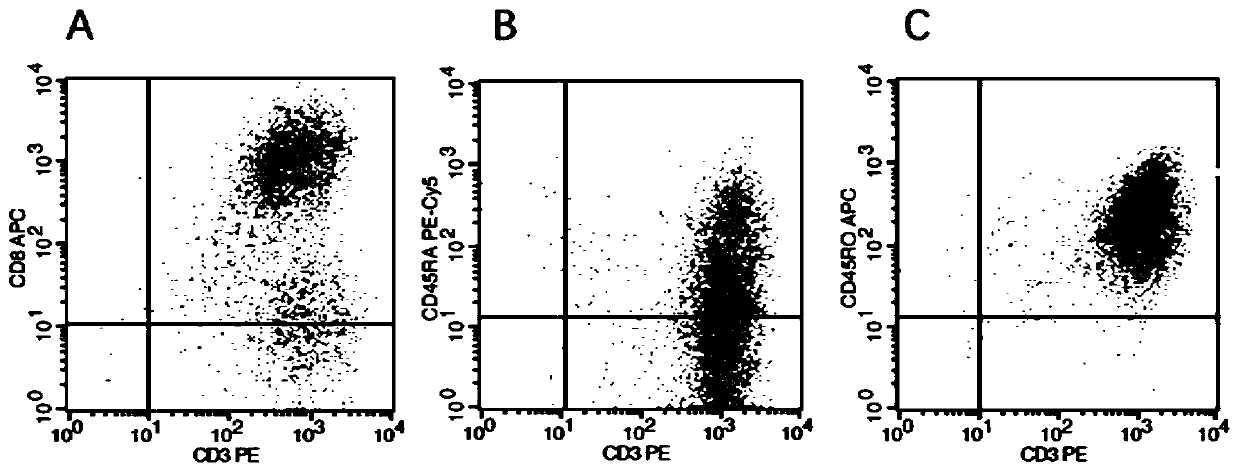
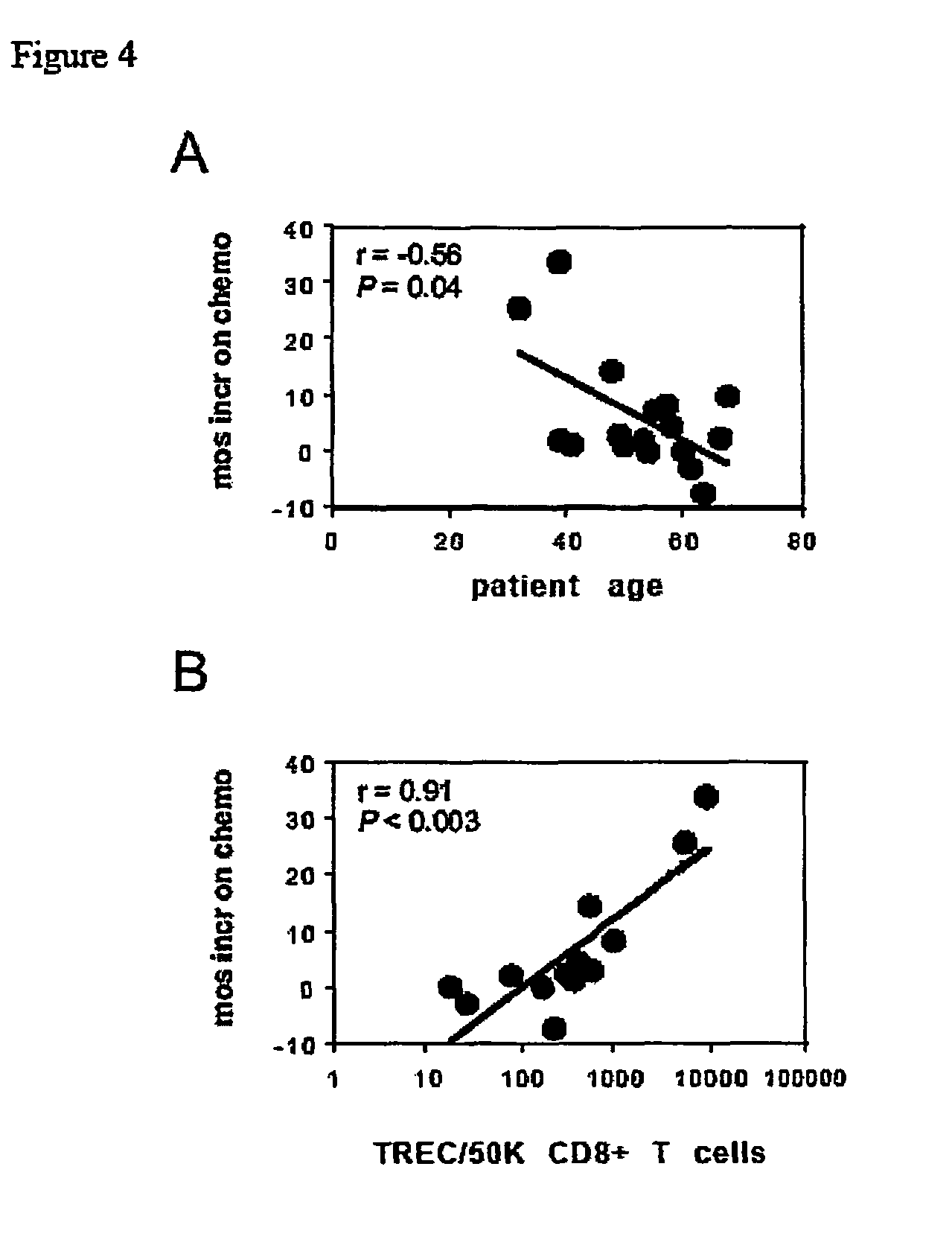
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Autologous tumor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
System and method for the treatment of cancer, including cancers of the central nervous system
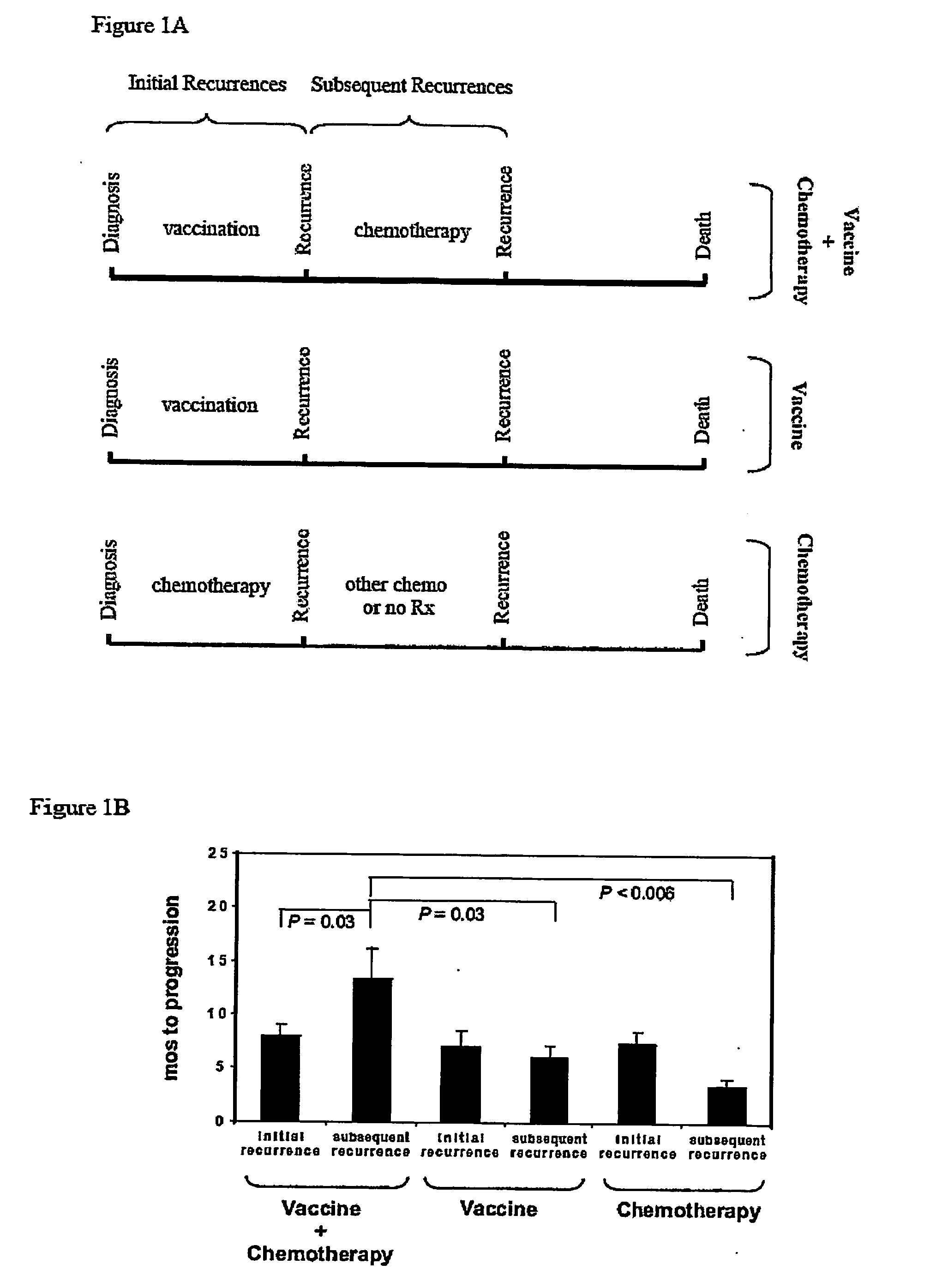
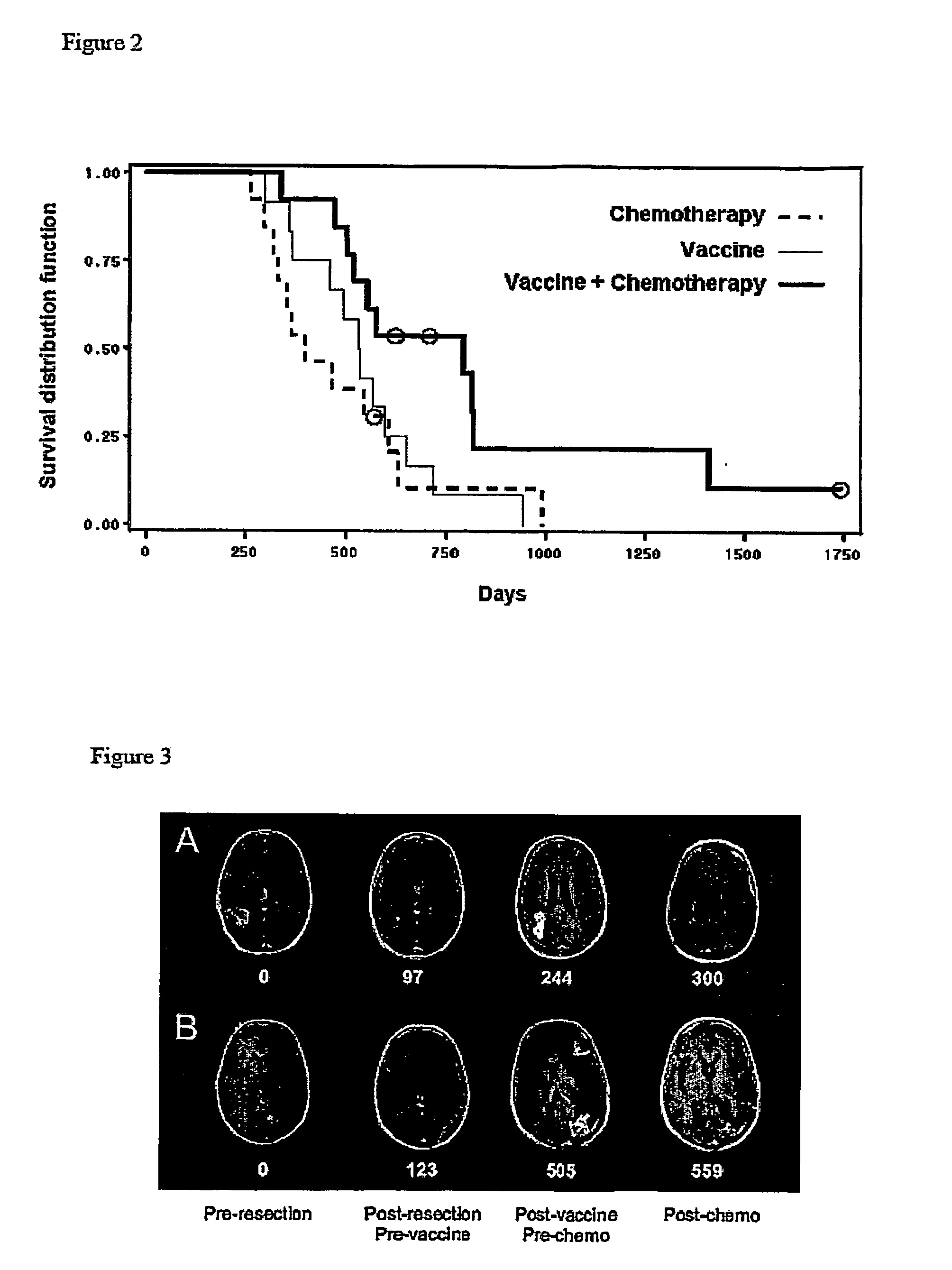
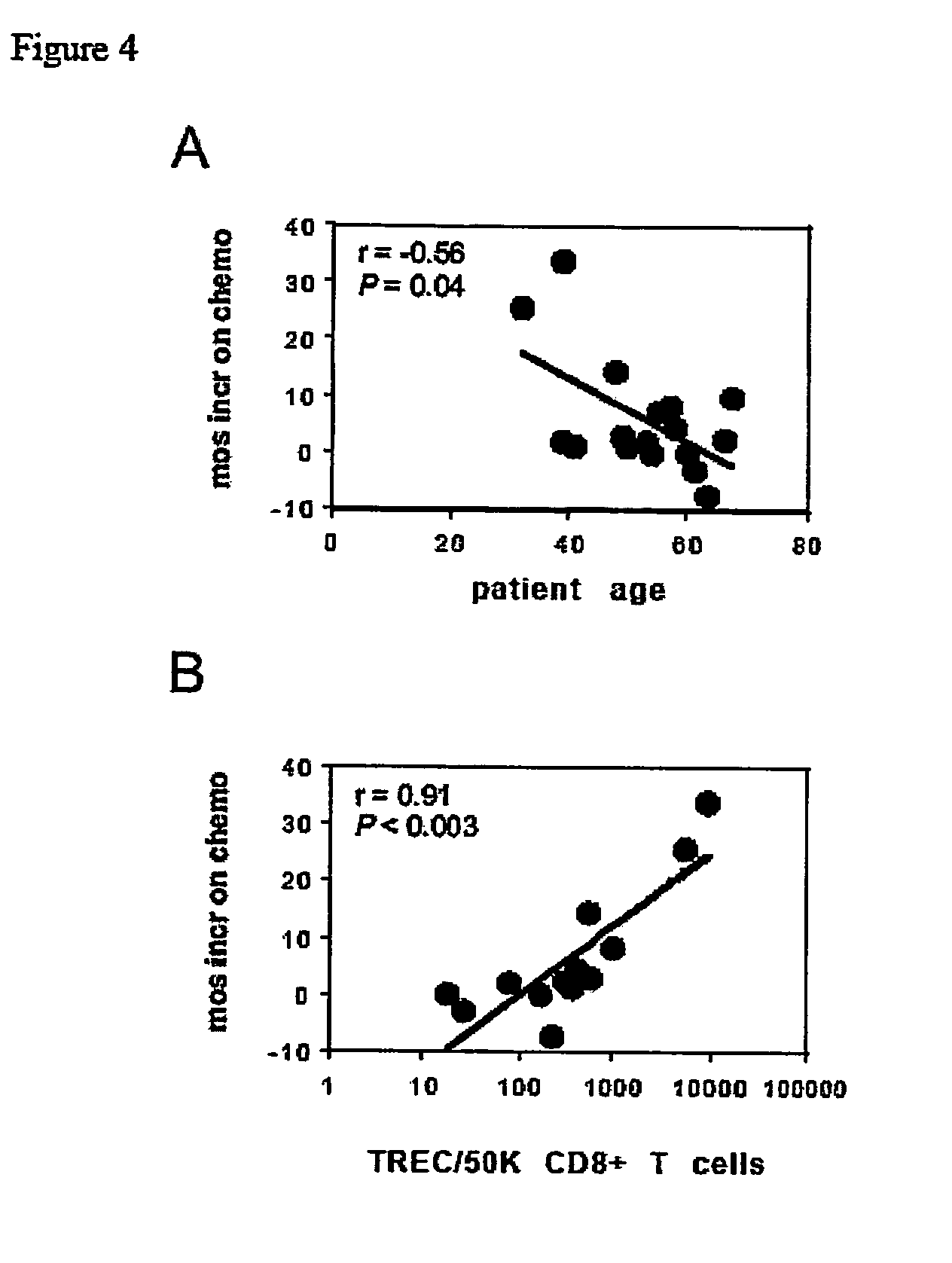
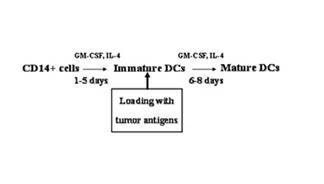
The invention relates to the treatment of cancer, and particularly to the treatment of cancers of the central nervous system, such as glioblastoma multiforme. A dual therapeutic approach is provided, including the administration of a dendritic cell-based cancer vaccine and a regimen of chemotherapy. The two therapies may be administered concurrently with one another and / or with an initial vaccination preceding chemotherapy. In various embodiments, the dendritic cell-based cancer vaccine includes either primed or unprimed dendritic cells; for instance, the dendritic cells may be autologous tumor antigen-presented dendritic cells. The dual therapeutic approach of the instant invention beneficially influences the chemosensitivity of a mammal with cancer.
Owner:CEDARS SINAI MEDICAL CENT
Preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen
ActiveCN102091327ASignificant technological progressConvenient for clinical operationBlood/immune system cellsAntibody medical ingredientsCytotoxicityT lymphocyte
The invention belongs to preparation of biological cell formulations, and in particular relates to a preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen. The preparation method comprises the following steps: preparing the autologous tumor associated holoantigen, collecting and separately culturing DCs, impacting the DCs by the autologous tumor associated holoantigen, maturing the DCs and preparing autologous tumor antigen specific DC vaccine. The invention solves the problems in the prior art that the immunogenicity of tumor antigen is not strong enough, the antigen target spots are incomplete, the tumor antigens of most tumor patients are difficult to acquire, and the like. The DC vaccine provided by the invention has the advantages of effectively inducing tumor antigen specific cytotoxic T lymphocyte (CTL) in vitro and in vivo, efficiently generating specific cytotoxicity on tumors, having high overall effective rate and no obvious toxic side effects in clinical application, and the like.
Owner:玥特农生物科技河北有限责任公司
Tumor lesion regression and conversion in situ into autologous tumor vaccines by compositions that result in anti-Gal Antibody Binding
InactiveUS20060251661A1Reduce the overall heightBiocideSugar derivativesAntigenAbnormal tissue growth
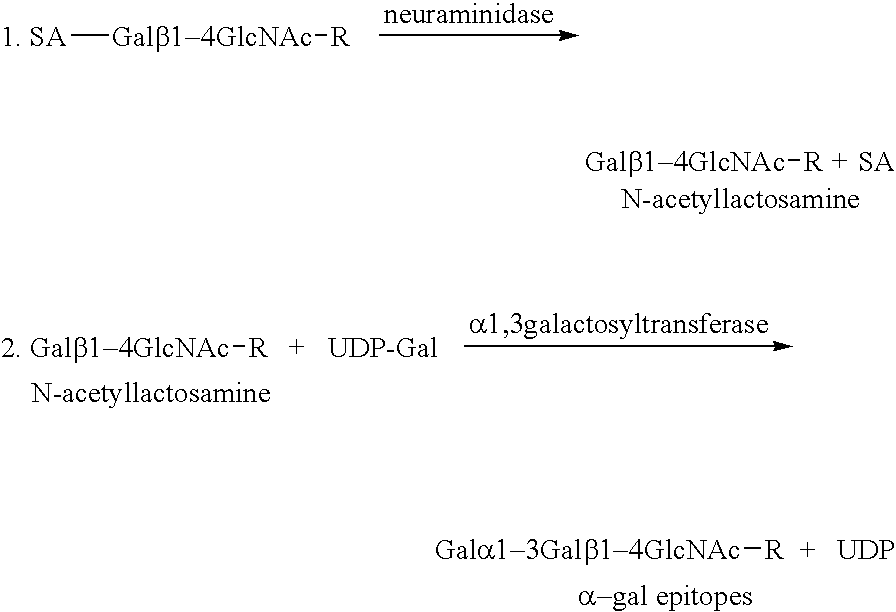
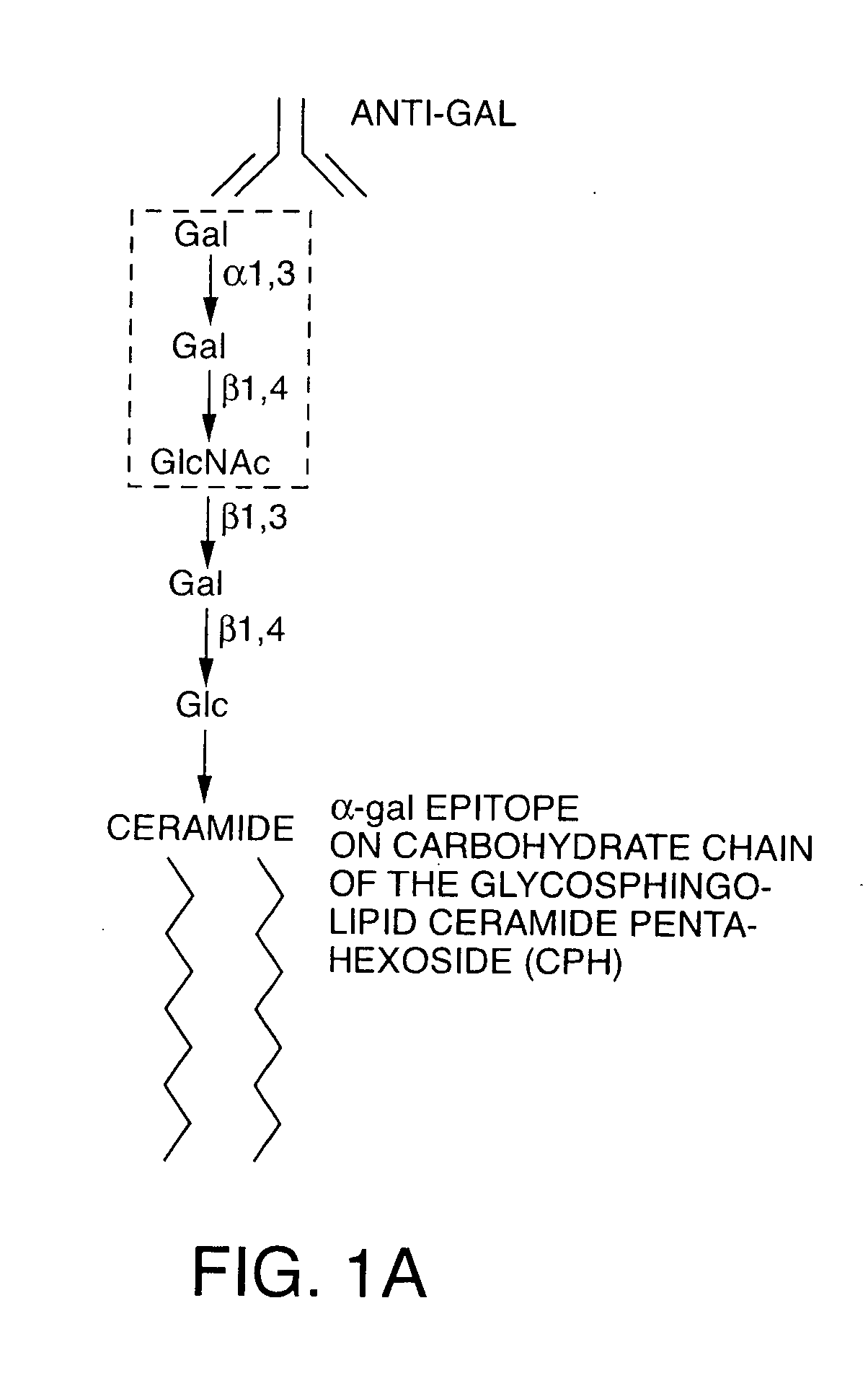
The present invention discloses that an intratumoral injection of: i) glycolipids with α-gal epitope; ii) gene vectors comprising an α1,3galactosyltransferase gene; or iii) a mixture of α1,3galactosyltransferase, neuraminidase, and uridine diphosphate galactose results in tumor regression and / or destruction. Binding of the natural anti-Gal antibody to de novo expressed tumoral α-gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Binding agents and their use in targeting tumor cells
InactiveUS20050260208A1Reduce spreadReduce in quantityPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsDendritic cellTumor antigen
The present invention concerns methods and compositions for administering a binding agent to a patient wherein the patient generates a response to autologous tumor. The binding agents target apoptotic tumor cells and facilitates the uptake of these apoptotic tumor cell are taken up by dendritic cells or other antigen presenting cells for processing and presentation to the immune system without the expression of circulating tumor-associated antigen (or without the need of circulating tumor antigen).
Owner:ALTAREX MEDICAL
Tumor lesion regression and conversion in situ into autologous tumor vaccines by compositions that result in anti-Gal antibody binding
The present invention discloses that an intratumoral injection of: i) glycolipids with α-gal epitope; ii) gene vectors comprising an α1,3galactosyltransferase gene; or iii) a mixture of α1,3galactosyltransferase, neuraminidase, and uridine diphosphate galactose results in tumor regression and / or destruction. Binding of the natural anti-Gal antibody to de novo expressed tumoral α-gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Method for preparing tumor-specific DC vaccine by applying CD34+ cells of umbilical cord blood
ActiveCN103405759ABreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
The invention discloses a method for preparing a tumor-specific DC vaccine by applying CD34+ cells in umbilical cord blood. The method comprises (1) a step of preparing autologous tumor-related holoantigen; (2) a step of obtaining the umbilical cord blood; (3) a step of obtaining mononuclear cells derived from the umbilical cord blood; (4) a step of purifying CD34+ cells in the mononuclear cells derived from the umbilical cord blood; (5) performing induction culture for a precursor DC; (6) a step of performing amplification and culture of an immature DC; and (7) a step of preparing the DC vaccine.
Owner:玥特农生物科技河北有限责任公司
Composite siRNA nanocarrier and preparation method and application thereof
ActiveCN109125291AHighly specific targetingImprove transfection efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsNanocarriersSerum protein albumin
The invention discloses a composite siRNA nanocarrier, comprising exosome lipid membrane from autologous tumor cells and siRNA-carrying cationic serum albumin covered in the exosome lipid membrane. The invention also discloses a preparation method and application of the composite siRNA nanocarrier. The composite siRNA nanocarrier has the advantages of high transfection, specific targeting propertyof cancer metastatic tissues, good biocompatibility, good stability, zero immunogenicity and the like.
Owner:NANTONG UNIVERSITY
Methods of preparing lymphocytes that express interleukin-2 and their use in the treatment of cancer
A method of preparing autologus T-lymphocytes for re-introduction into a patient having cancer, which method comprises obtaining peripheral blood mononuclear cells (PBMCs) from a patient immunized with an antigen of the cancer, stimulating the PBMCs with the antigen of the cancer in vitro, transducing the PBMCs with a retrovial vector, which (a) comprises and expresses a human interleukin-2 (IL-2) coding sequence operably linked to a retroviral promoter, (b) does not comprise an exogenously introduced gene that enables phenotypic selection, and (c) comprises a viral envelope that efficiently transduces CD8+ T-lymphocytes; a method of preparing autologous tumor-infiltrating lymphocytes (TILs) for reintroduction into a patient having cancer, which method comprises obtaining TILs from a tumor of the patient, optionally immunized with an antigen of the cancer, optionally stimulating the TILs with the antigen of the cancer in vitro, and transducing the TILs with the aforementioned retroviral vector, compositions comprising cells obtained in accordance with such methods; and methods of treating a patient having cancer by administering to the patient cells obtained in accordance with such methods or compositions comprising same.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Autologous dendritic cell activated tumor-infiltrating T-lymphocyte preparation method and application of T-lymphocyte
The invention provides an autologous dendritic cell activated tumor-infiltrating T-lymphocyte preparation method. The method has the following characteristics that: a tumor-infiltrating T-lymphocyte from a cancer patient is activated and amplified in a large quantity by using a dendritic cell loaded by an autologous tumor antigen as a trophoblast cell and interleukin-2, and has a highly active anti-tumor cell toxic response. The invention further provides an autologous tumor-infiltrating T-lymphocyte kit prepared according to the method, and the kit has an efficient anti-tumor function.
Owner:YANTAI BOYU BIOTECH CO LTD
Method for preparing dendritic cell vaccine
InactiveCN102847145AIncrease multipleStrong specific lethalityBlood/immune system cellsAntibody medical ingredientsLiquid ChangeHuman lymphocyte
The invention relates to the technical field of biology, in particular to a method for preparing dendritic cell vaccine. The method is characterized by comprising the following preparation steps: (1) taking autologous blood of a patient, and separating red blood cells, mononuclear cells and plasma from separating medium of human lymphocyte; (2) adjusting concentration of the mononuclear cells; (3) adding the solution into a six-pore plate and performing adherence for 16 hours; (4) sucking and discharging non-adherent cells on the upper layer of each pore of the six-pore plate; (5) adding 3ml of dendritic cell (DC) culture medium, placing the solution in a carbon dioxide cultivating box with the concentration of carbon dioxide of 5% at the temperature of 37 DEG C to perform cultivation for 2-3 days; (6) performing half-quantity liquid changing; (7) enabling the concentration of the autologous tumor antigen in each pore of the six-pore plate to be 20 mu g / ml by loading the autologous tumor antigen in the sixth day; and (8) detecting the quantity and maturity of the matured DC cells after 24 hours. Compared with the prior art, the number of the matured DC cells at least reaches 1*107, and the maturity is larger than 85%.
Owner:FUDAN UNIV
Preparation method and application of autologous tumor vaccine
InactiveCN109876137ATo achieve the goal of individualized tumor immunotherapyImprove immunityCancer antigen ingredientsAntineoplastic agentsAdjuvantAutologous tumor cell
The invention relates to a preparation method and application of an autologous tumor vaccine. The method comprises the following specific steps: radiating and / or heating autologous tumor cells to greatly excite the antigenicity of the autologous tumor cells and enhance the presentation property of the antigen by using heat shock proteins; and inactivating the tumor cells and adding an adjuvant toobtain the vaccine. The vaccine prepared by using the method has the special advantages of high presentation property and high tumor immunogenicity, and can be used for greatly exciting the body fluidand cellular immune response of tumor patients to autologous tumors, monitoring immune response, and timely adjusting a usage method of the novel vaccine and adjuvant treatment, so that the aims of improving the antigenicity and metastatic tumor effect and prolonging the survival time of tumor patients are fulfilled.
Owner:XIAMEN LUJIA BIOTECH
Preparation method of nano aluminum adjuvant/ autologous tumor vaccine
InactiveCN103948921AUniform particle sizeSmall particle sizeAntibody medical ingredientsAntineoplastic agentsAdjuvantBiocompatibility Testing
The invention discloses a preparation method of a nano aluminum adjuvant / autologous tumor vaccine. The preparation method comprises the following steps: firstly, preparing a nano aluminum adjuvant by use of a micro-emulsion method, namely after adding and stirring benzalkonium bromide, n-octyl alcohol and cyclohexane in equal proportion at a high speed, adding a proper amount of AlCl3 liquor and then stirring; slowly dropwise adding ammonia water, reacting for 2 hours by keeping PH of a reaction system greater than 10, then adding acetone, performing demulsification and centrifugation, centrifuging and washing precipitates for three times, drying the centrifuged precipitates in a drying oven and staying overnight to obtain a fluffy nano Al(OH)3 adjuvant; then, co-incubating the H22 liver cancer cell suspension liquid with the nano aluminum adjuvant to obtain the nano aluminum adjuvant / autologous tumor vaccine. The nano aluminum adjuvant / autologous tumor vaccine prepared by the preparation method disclosed by the invention is small in particle size, good in dispersion, better in stability and good in biocompatibility. Animal experiment results show that the anti-tumor effect of the nano aluminum adjuvant / autologous tumor vaccine is obviously superior to that of the common aluminum adjuvant.
Owner:SOUTHEAST UNIV
Identification of specific tumour antigens by means of the selection of cdna libraries with sera and the use of said antigens in diagnostic imaging techniques
InactiveUS20050084857A1Compound screeningTumor rejection antigen precursorsCDNA libraryAbnormal tissue growth
A method is described for the identification of specific tumor antigens by means of the selection of cDNA display libraries by using sera, characterised in that said selection is accomplished with the phage display technique, and in particular said selection is accomplished by means of the SEREX technique (serological analysis of autologous tumor antigens through the expression of recombinant cDNA). The method according to the invention described herein advantageously combines the SEREX approach with the potency of the phage display technique defined above, at the same time avoiding the drawbacks characteristic of the SEREX technique. The so identified antigens are useful for the preparation of medicaments for the treatment of tumors.
Owner:KENTON
Peripheral blood memory T cell culture method
ActiveCN110093315AEfficient amplificationRaise the ratioBlood/immune system cellsCell culture active agentsAntigenCytokine
The invention relates to a peripheral blood memory T cell culture method. The method comprises: a) magnetically separating the PBMC isolated from the peripheral blood to obtain CD8<+> T cells; and inoculating the CD8<+> T cells in a culture container coated with a CD3 antibody and a CD28 antibody for culture; wherein a culture system contains autologous plasma, and the cytokine mainly includes IFN-gamma and IL-2; b) changing liquid for amplification culture, wherein a novel culture system contains autologous plasma and the cytokines mainly include IL-2, IL-1a, IL-7, and IL-15; and c) co-culturing the expanded T cells with DC cells; wherein the DC cells are previously co-cultured with an autologous tumor antigen. The method can effectively amplify memory T cells in vitro and effectively increase the proportion of active memory T cells.
Owner:新疆西部赛澳生物科技有限责任公司
Binding agents and their use in targeting tumor cells
InactiveUS20090291075A1Powder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsDendritic cellTumor antigen
The present invention concerns methods and compositions for administering a binding agent to a patient wherein the patient generates a response to autologous tumor. The binding agents target apoptotic tumor cells and facilitates the uptake of these apoptotic tumor cell are taken up by dendritic cells or other antigen presenting cells for processing and presentation to the immune system without the expression of circulating tumor-associated antigen (or without the need of circulating tumor antigen).
Owner:ALTAREX MEDICAL
Autologous tumor vaccines and methods
ActiveUS20180008686A1Preserving heterogeneityEfficient responseEnergy modified materialsVaccinesImmune compromisedVaccine Production
Autologous anti-cancer vaccines and methods of manufacture and treatment are provided, including expansion of individual patient-derived tumor cells in an immune-compromised animal(s) to attain, quantitatively and qualitatively, sufficient material for efficacious vaccine production and utilization, to elicit an immune response against micrometastases and / or recurrence in the individual patient following tumor excision.
Owner:VACCINOGEN
Autologous tumor vaccines and methods
InactiveUS20150093416A1Minimize cytotoxicityEliminate tumorigenicityEnergy modified materialsVaccinesImmune compromisedVaccine Production
Autologous anti-cancer vaccines and methods of manufacture and treatment are provided, including expansion of individual patient-derived tumor cells in an immune-compromised animal(s) to attain, quantitatively and qualitatively, sufficient material for efficacious vaccine production and utilization, to elicit an immune response against micrometastases and / or recurrence in the individual patient following tumor excision.
Owner:VACCINOGEN
System and method for the treatment of cancer, including cancers of the central nervous system
The invention relates to the treatment of cancer, and particularly to the treatment of cancers of the central nervous system, such as glioblastoma multiforme. A dual therapeutic approach is provided, including the administration of a dendritic cell-based cancer vaccine and a regimen of chemotherapy. The two therapies may be administered concurrently with one another and / or with an initial vaccination preceding chemotherapy. In various embodiments, the dendritic cell-based cancer vaccine includes either primed or unprimed dendritic cells; for instance, the dendritic cells may be autologous tumor antigen-presented dendritic cells. The dual therapeutic approach of the instant invention beneficially influences the chemosensitivity of a mammal with cancer.
Owner:CEDARS SINAI MEDICAL CENT
Process for preparing xenomal or autogenous tumor vaccine of liposome
InactiveCN1141975COvercoming limited difficultiesLasting effectAntibody medical ingredientsAntineoplastic agentsFreeze thawingLipid formation
A process for preparing xenogenic or autologous tumor vaccine of liposome includes such steps as taking the fresh specimen of human tumor, preparing suspension of tumor cells; freeze-thaw step to break off and deactivate tumor cells; n-butanol suspending to remove lipid; centrifugal step to remove karyon; lowry's test to test qualification of supernatant; and coating with liposome as carrier and wrapping with adjuvant to make vaccine. Its advantages include advanced and efficient extraction process, no toxic by-effect, and durable and slow release.
Owner:广州市肿瘤医院 +1
Method for individually culturing tumor organoid by using hydrogel prepared from autologous chest and ascites of tumor patient
PendingCN114231491AGood biocompatibilityAvoid contamination riskCell dissociation methodsHepatocytesCellular componentHeterologous
The invention discloses a method for individually culturing tumor organs by using hydrogel prepared from autologous chest and ascites of a tumor patient. The invention provides a method for culturing autologous tumor organoid of a tumor patient, which comprises the following steps: mixing tumor cells to be cultured from the tumor patient with a matrix to enable the final concentration of the cells to be (5-10) * 10 < 3 > / mL; the matrix is in-vitro autologous body cavity effusion of a tumor patient without cell components; adding a protein cross-linking agent, then adding a hydrogel solution obtained by mixing to the bottom of a cell culture container, and flatly standing at 37 DEG C until the hydrogel solution is solidified; and adding a tumor organoid culture solution to the upper part of the hydrogel, and culturing to obtain the tumor organoid. The method has the advantages of good biocompatibility, avoidance of heterologous component pollution and low culture cost.
Owner:SUZHOU GENOARRAY
Tumor neoantigen specific tumor infiltration lymphocyte co-culture method
InactiveCN113106062AEfficient killingHas immune memory effectBlood/immune system cellsCell culture active agentsPancreas CancersMelanoma
The invention relates to the technical field of cell culture, in particular to a tumor neoantigen specific tumor infiltration lymphocyte co-culture method which is characterized by comprising the following steps: S1, tumor neoantigen preparation: respectively collecting the tissues of lung cancer (N1), pancreatic cancer (N2), bile duct cancer (N3), triple-negative breast cancer (N4) and melanoma tumor (N5), separating tumor cells, andcollecting normal tissues of corresponding individuals; comparing the all-exon sequencing and normal tissue and tumor tissue sequencing results to determine all amino acid mutations, then designing a gene construct containing all mutant peptide sequences and synthesizing a long-chain polypeptide according to a neoantigen coded by a mutant gene. The TIL cells harvested by designing a co-culture method of tumor neoantigen specific tumor infiltration lymphocytes can effectively kill autologous tumor target cells, can induce immune cells to have multiple target spots, are free of limitation of cancer species, and have an immune memory effect.
Owner:赜誉(上海)生物科技有限公司
Method for preparing tumor-specific DC vaccine by applying mononuclear cells in umbilical cord blood
ActiveCN103405758ABreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
The invention discloses a method for preparing a tumor-specific DC vaccine by applying mononuclear cells of umbilical cord blood. The method comprises (1) a step of preparing autologous tumor-related holoantigen; (2) a step of obtaining the umbilical cord blood; (3) a step of obtaining the mononuclear cells derived from the umbilical cord blood; (4) a step of performing induction culture for a precursor DC of the mononuclear cells derived from the umbilical cord blood; (5) a step of performing amplification and culture of an immature DC; and (6) a step of preparing the DC vaccine.
Owner:玥特农生物科技河北有限责任公司
Gm-csf/cd40l vaccine and checkpoint inhibitor combination therapy
A method is disclosed for treating a cancer in a subject. The method comprises administering to the subject a composition comprising a therapeutically effective amount of a checkpoint inhibitor and a therapeutically effective amount of a tumor vaccine. In some embodiments, the tumor vaccine comprises radiated autologous tumor cells and a cell line engineered to express granulocyte- macrophage colony-stimulating factor (GM-CSF) and cluster of differentiation 40 (CD40) ligand. In some embodiments, the checkpoint inhibitor comprises an anti-programmed death-1 (anti-PD-1) antibody (e.g., BMS 936558), anti-programmed death ligand-1 (anti-PD-L1) antibody (e.g., cloneMIHI), anti-cytotoxic T lymphocyte antigen-4 (anti-CTLA-4) antibody (e.g., Ipilimumab, BMS), or any combination thereof.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen
ActiveCN102091327BSignificant technological progressConvenient for clinical operationBlood/immune system cellsAntibody medical ingredientsCytotoxicityT lymphocyte
The invention belongs to preparation of biological cell formulations, and in particular relates to a preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen. The preparation method comprises the following steps: preparing the autologous tumor associated holoantigen, collecting and separately culturing DCs, impacting the DCs by the autologous tumor associated holoantigen, maturing the DCs and preparing autologous tumor antigen specific DC vaccine. The invention solves the problems in the prior art that the immunogenicity of tumor antigen is not strong enough, the antigen target spots are incomplete, the tumor antigens of most tumor patients are difficult to acquire, and the like. The DC vaccine provided by the invention has the advantages of effectively inducing tumor antigen specific cytotoxic T lymphocyte (CTL) in vitro and in vivo, efficiently generating specific cytotoxicity on tumors, having high overall effective rate and no obvious toxic side effects in clinical application, and the like.
Owner:玥特农生物科技河北有限责任公司
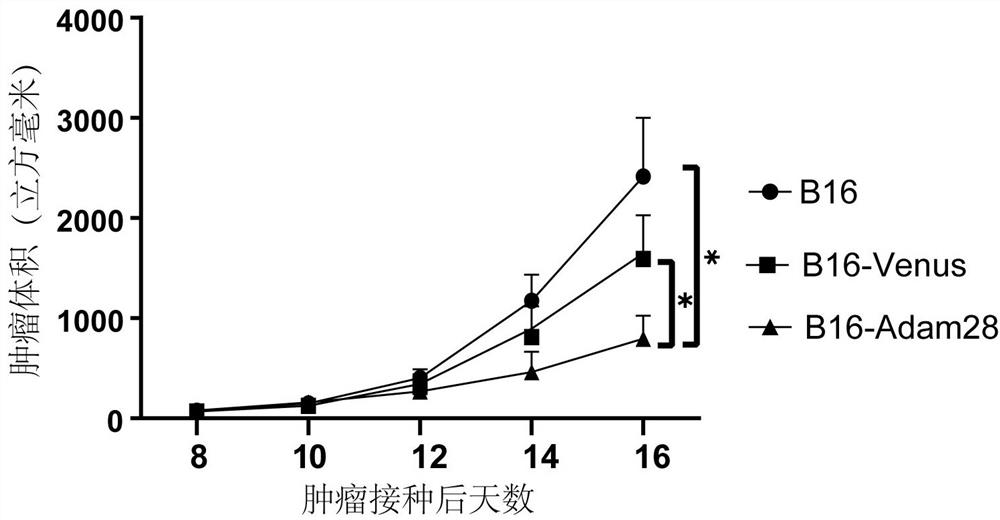
Preparation method and application of melanoma autologous tumor vaccine for high expression of ADAM-28
ActiveCN114569709AHas tumor immunopreventive effectQuick responseGenetically modified cellsSkin cancer vaccineStage melanomaCD8
The invention provides a preparation method and application of a melanoma autologous tumor vaccine with high expression of ADAM-28. ADAM-28 genes are amplified from B16 cells through a PCR method, the ADAM-28 genes are cloned into a lentiviral vector to construct recombinant lentivirus, B16-F10 cells are infected with the recombinant lentivirus to obtain a melanoma cell line with high expression of ADAM-28, and after irradiation inactivation, the melanoma autologous tumor vaccine with high expression of ADAM-28 is obtained. The melanoma autologous tumor vaccine is obtained. According to the invention, the melanoma cell line with high expression of ADAM-28 is prepared for the first time, and the melanoma autologous tumor vaccine is prepared from the melanoma cell line. A mouse tumor model verifies that the vaccine efficiently activates CD8 + T cell immune response and has an obvious tumor immunoprophylaxis effect, so that the vaccine can be applied to the field of preparation of tumor immunoprophylaxis drugs, especially melanoma.
Owner:SUZHOU HEALIRNA BIOTECHNOLOGY CO LTD +1
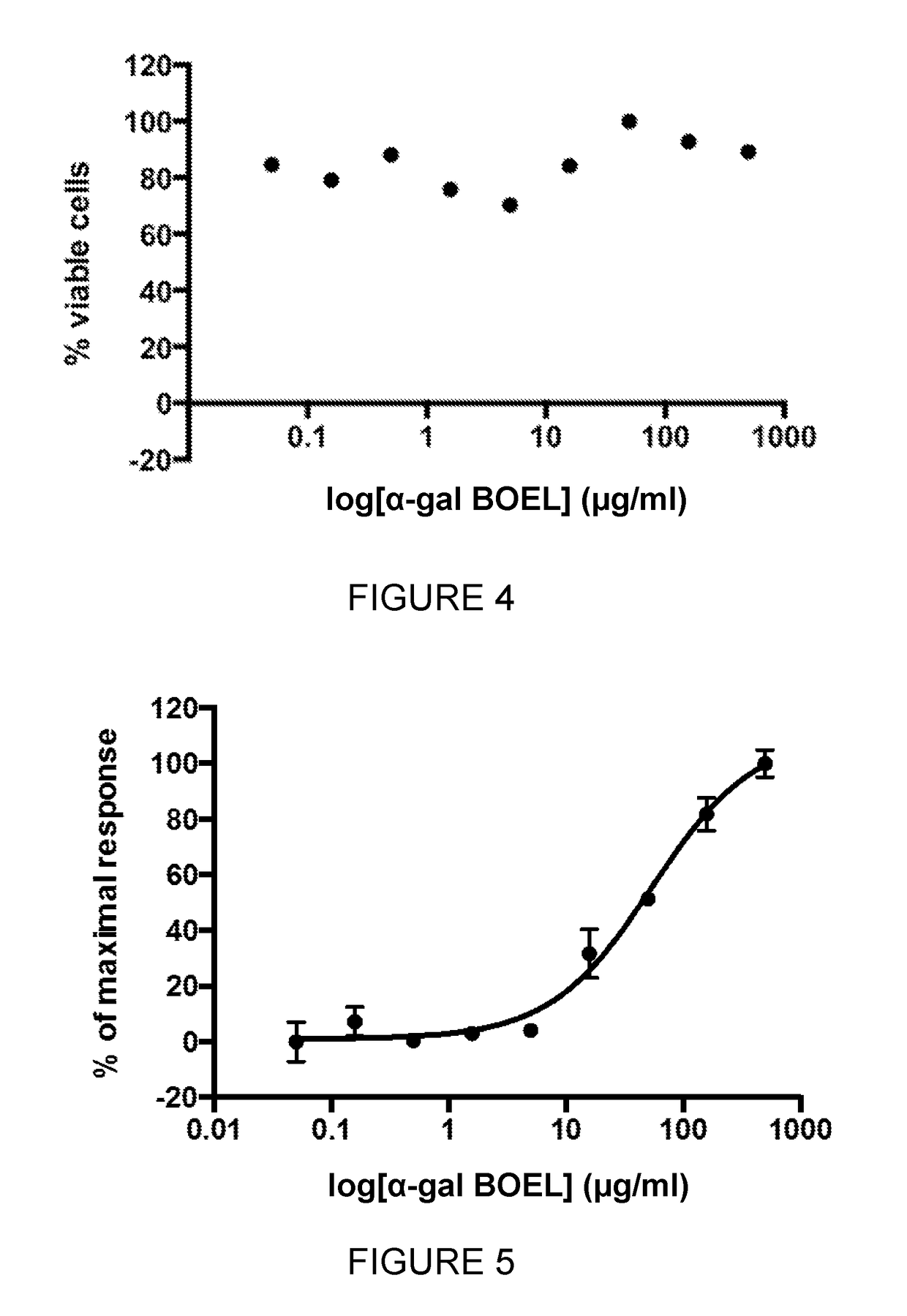
Glycolipid containing compositions for use in the treatment of tumors
ActiveUS10092586B2Ease of administrationBenefits of easeOrganic active ingredientsPharmaceutical delivery mechanismAntigenDrainage lymph nodes
The invention relates to pharmaceutical compositions comprising α-Gal BOEL for use in treating patients with tumors. The invention also relates to methods of treating tumors using said compositions. The invention discloses that following intratumoral injection of α-Gal BOEL, binding of the natural anti-Gal antibody to de novo expressed tumoral α-Gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor. The invention further teaches the enhancement of anti-tumor α-Gal BOEL treatment by the use of antibodies that inhibit the activity of immunological checkpoints molecules.
Owner:AGALIMMUNE
Method for preparing tumor-specific DC vaccine by applying CD34+ cells of umbilical cord blood
ActiveCN103405759BBreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
Owner:玥特农生物科技河北有限责任公司
A method for screening tumor-specific TCRs
ActiveCN114134221BNucleic acid vectorBlood/immune system cellsSingle cell transcriptomeAutologous tumor cell
The invention relates to a method for screening tumor-specific TCR, which belongs to the technical field of tumor immunity. In the present invention, after co-incubating tumor cells of tumor patients and tumor infiltrating T cells (TILs) corresponding to autologous tumor cells in vitro, these cells are subjected to single-cell transcriptome and TCR group sequencing to obtain the TCR sequence of each TILs cell, for For all TILs cells expressing the same TCR, the average value of the expression values of the 10 T cell markers was used as the activation score of the TCR marker. According to the activation score, the one with the higher score was selected as the TCR corresponding to the tumor-specific T cell.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Vaccination with immuno-isolated cells producing an immunomodulator
Provided herein are vaccine compositions containing at least one retrievable biocompatible macrocapsule containing immuno-isolated allogeneic cells that secrete an immunomodulator such as GM-CSF (granulocyte-macrophage colony stimulating factor) and an antigenic component such as autologous tumor cells or infectious agents. Also provided are kits and pharmaceutical compositions containing the vaccine compositions as well as methods of use thereof for therapeutic or preventative vaccination against tumors or infectious agents.
Owner:MAXIVAX SA
Autologous tumor vaccines and methods
ActiveUS11351235B2Preserving heterogeneityEfficient responseEnergy modified materialsVaccinesImmune compromisedVaccine Production
Autologous anti-cancer vaccines and methods of manufacture and treatment are provided, including expansion of individual patient-derived tumor cells in an immune-compromised animal(s) to attain, quantitatively and qualitatively, sufficient material for efficacious vaccine production and utilization, to elicit an immune response against micrometastases and / or recurrence in the individual patient following tumor excision.
Owner:VACCINOGEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com