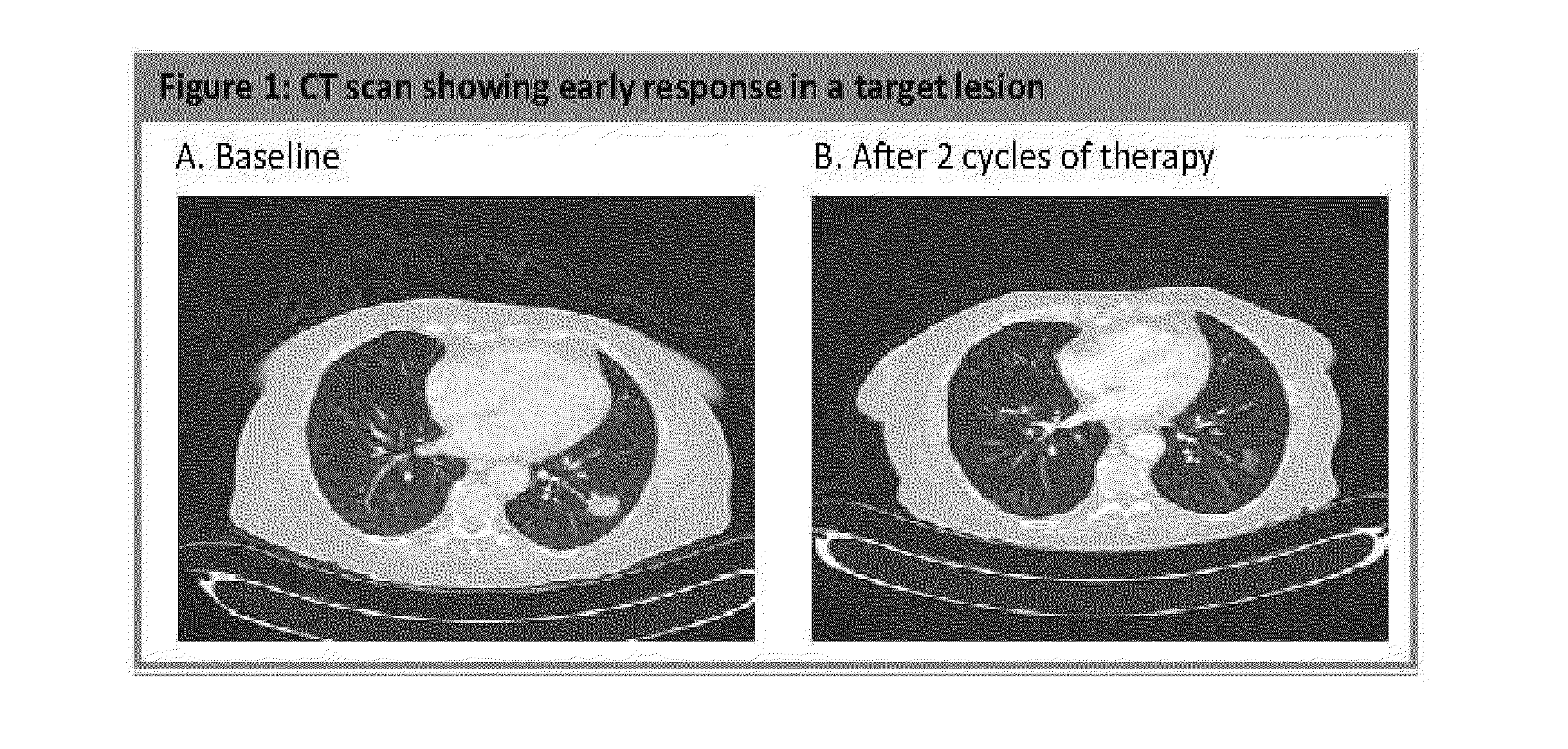
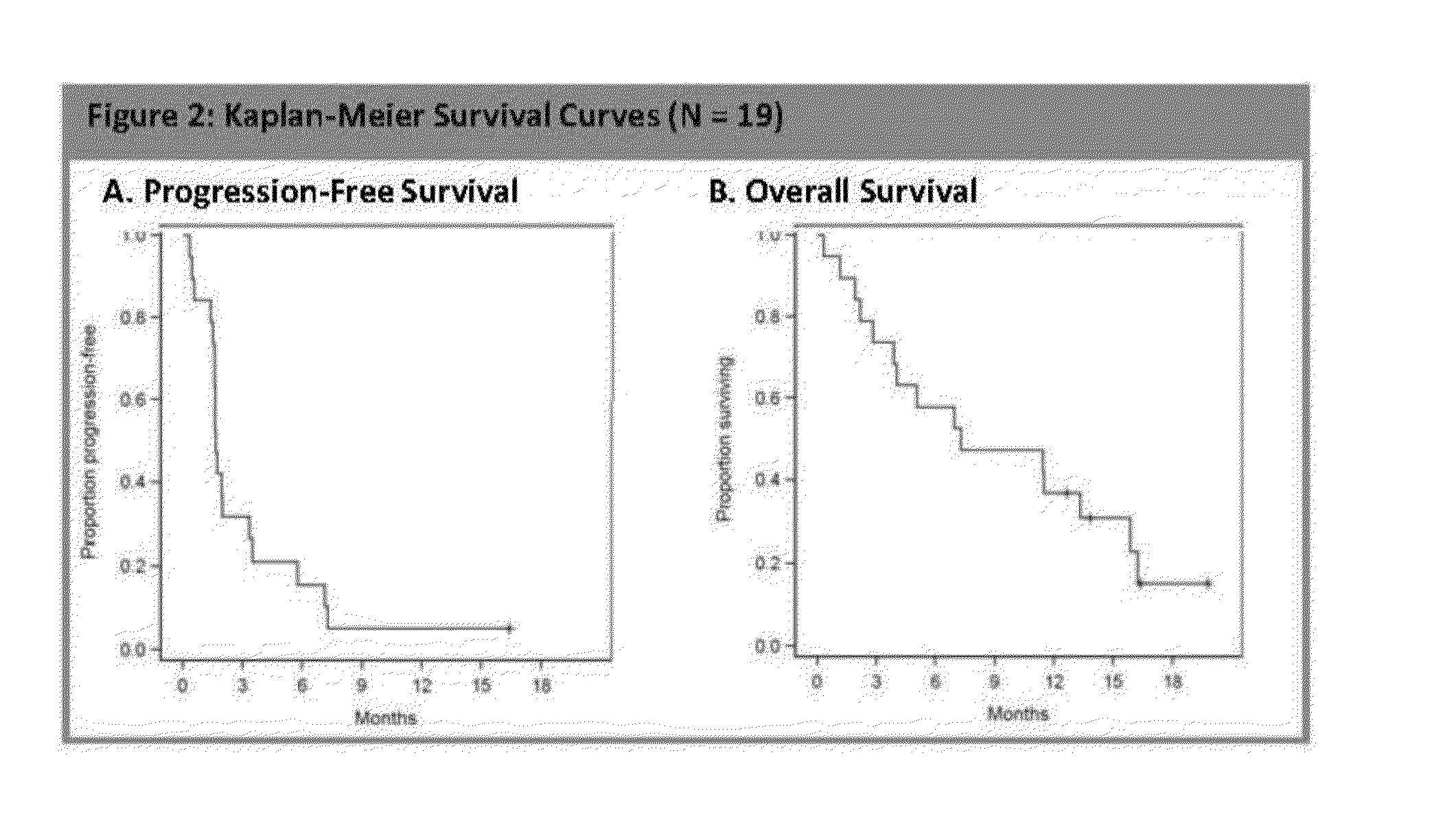
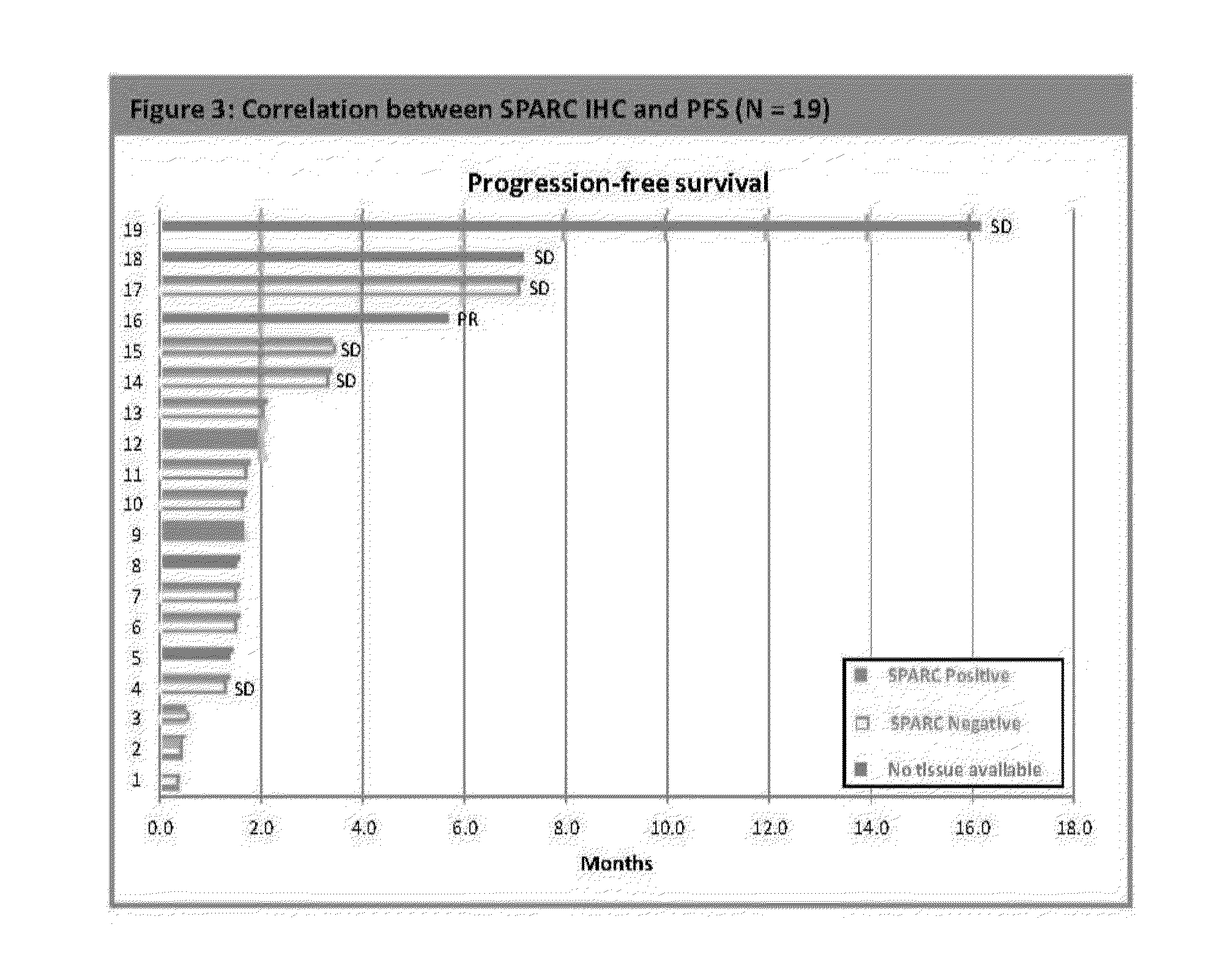
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
789 results about "Pancreas Cancers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pancreatic cancer occurs when a malignant tumour forms in the pancreas. There are two main types of pancreatic cancer: These make up the vast majority of all pancreatic cancers (around 90%) and come from the cells that line the ducts in the pancreas which carry digestive juices into the intestine.
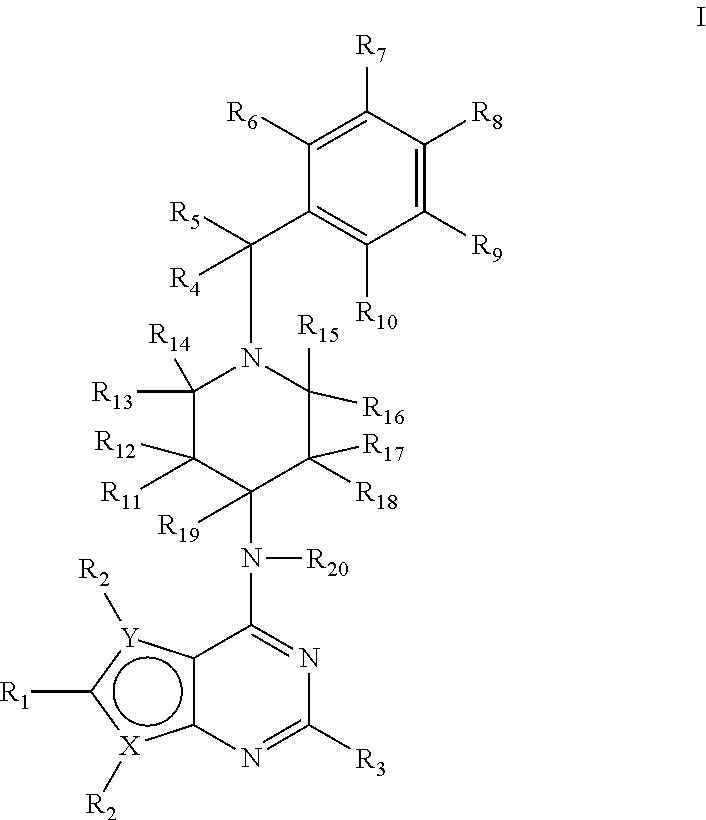
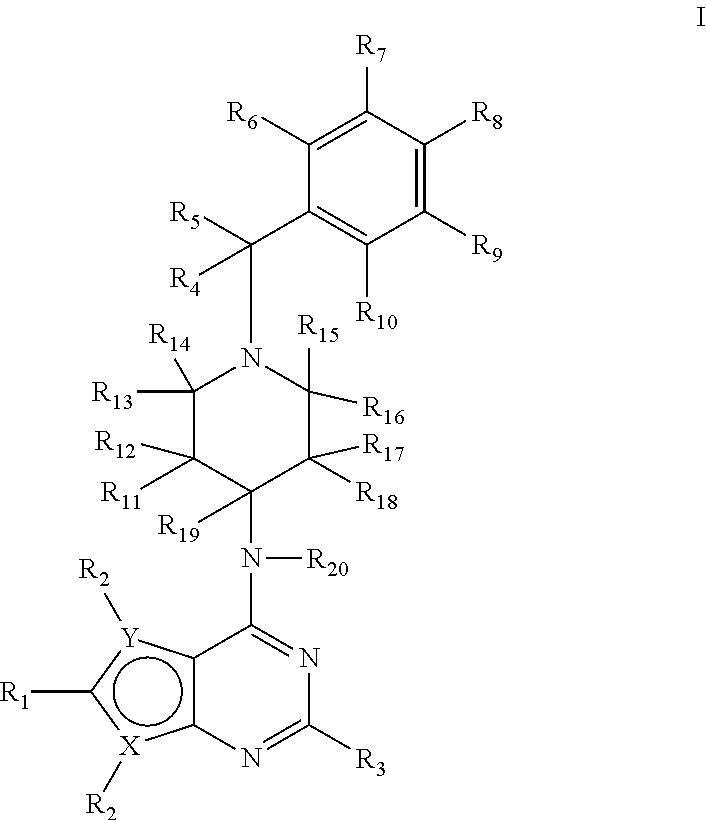
1H-pyrazolo[3,4-b]pyridines and therapeutic uses thereof
Provided herein are compounds according to Formula I and pharmaceutically acceptable salts thereof, and compositions comprising the same, for use in various methods, including treating cancers such as colon, ovarian, pancreatic, breast, liver, prostate and hematologic cancers:
Owner:BIOSPLICE THERAPEUTICS INC
Pancreatic cancer secreted targets and uses thereof
ActiveUS7358231B1Organic active ingredientsTumor rejection antigen precursorsDiseasePancreas Cancers
The present invention provides a method for diagnosing and detecting diseases associated with pancreas. The present invention provides one or more proteins or fragments thereof, peptides or nucleic acid molecules differentially expressed in pancreatic diseases (PCAST) and antibodies binds to PCAST. The present invention provides that PCAST is used as targets for screening agents that modulates the PCAST activities. Further the present invention provides methods for treating diseases associated with pancreas.
Owner:APPL BIOSYSTEMS INC
Anti-Pancreatic Cancer Antibodies
InactiveUS20090304580A1Strong specificityHigh sensitivityPeptide/protein ingredientsNanomedicineAntigenPancreatic tissue
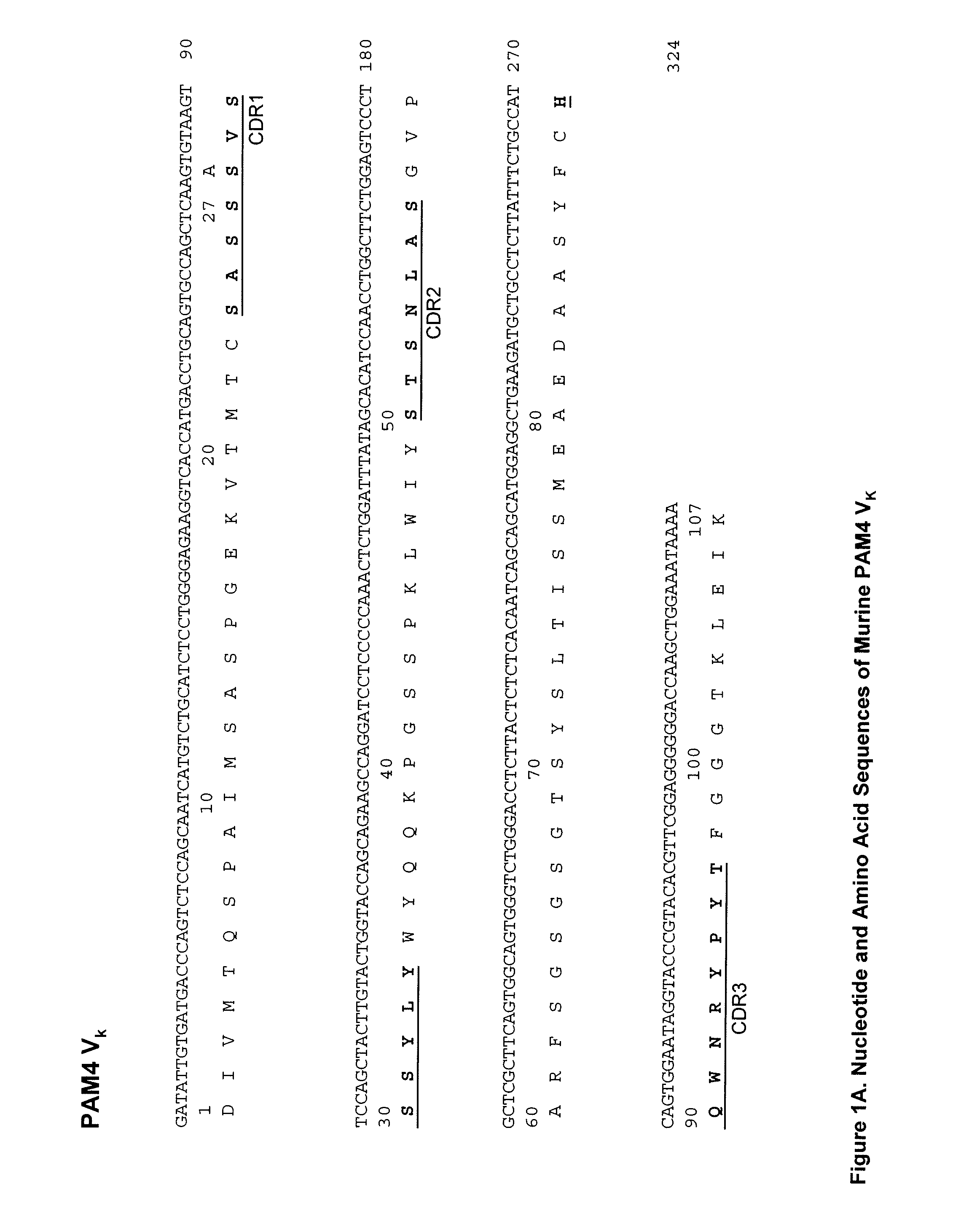
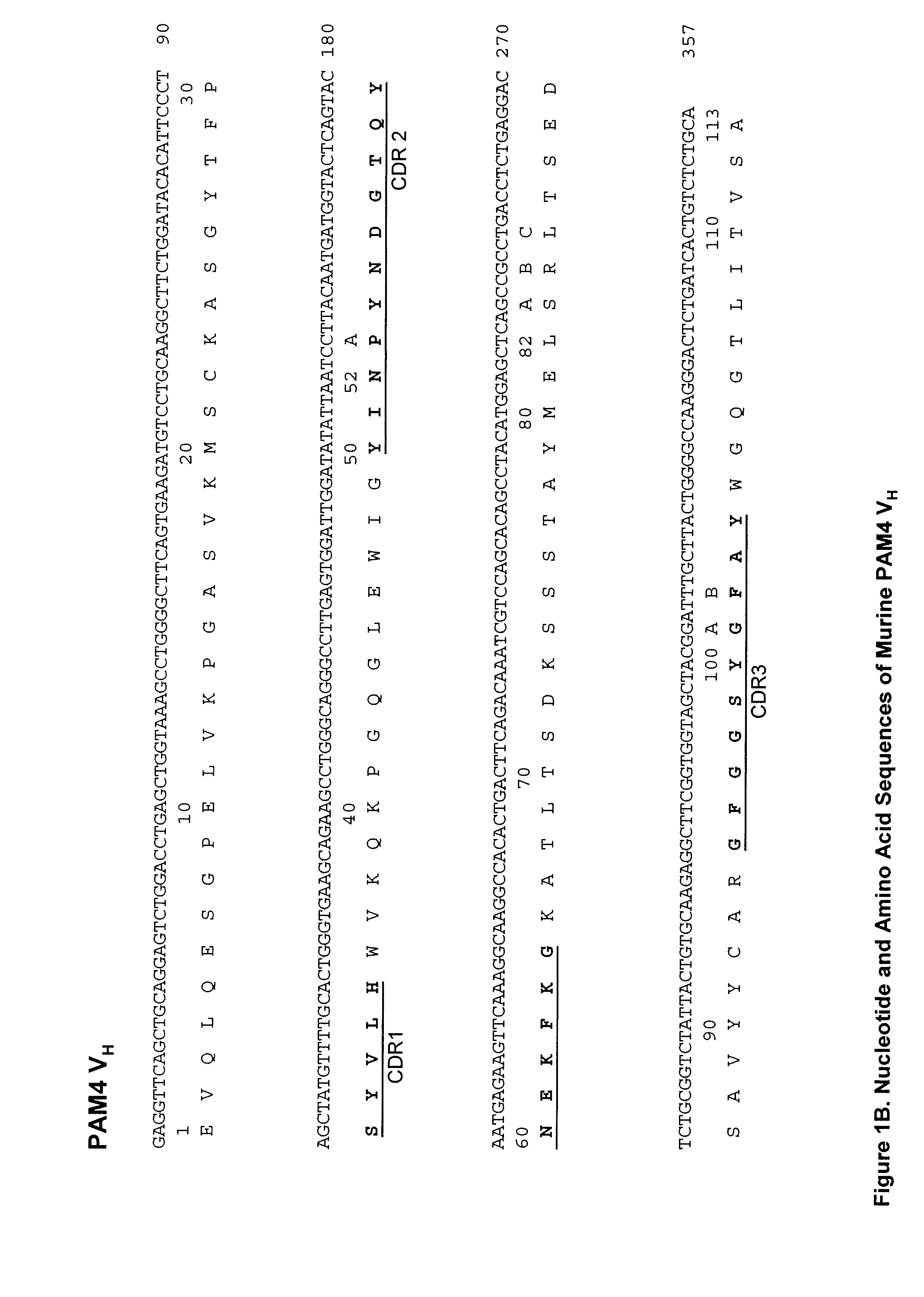
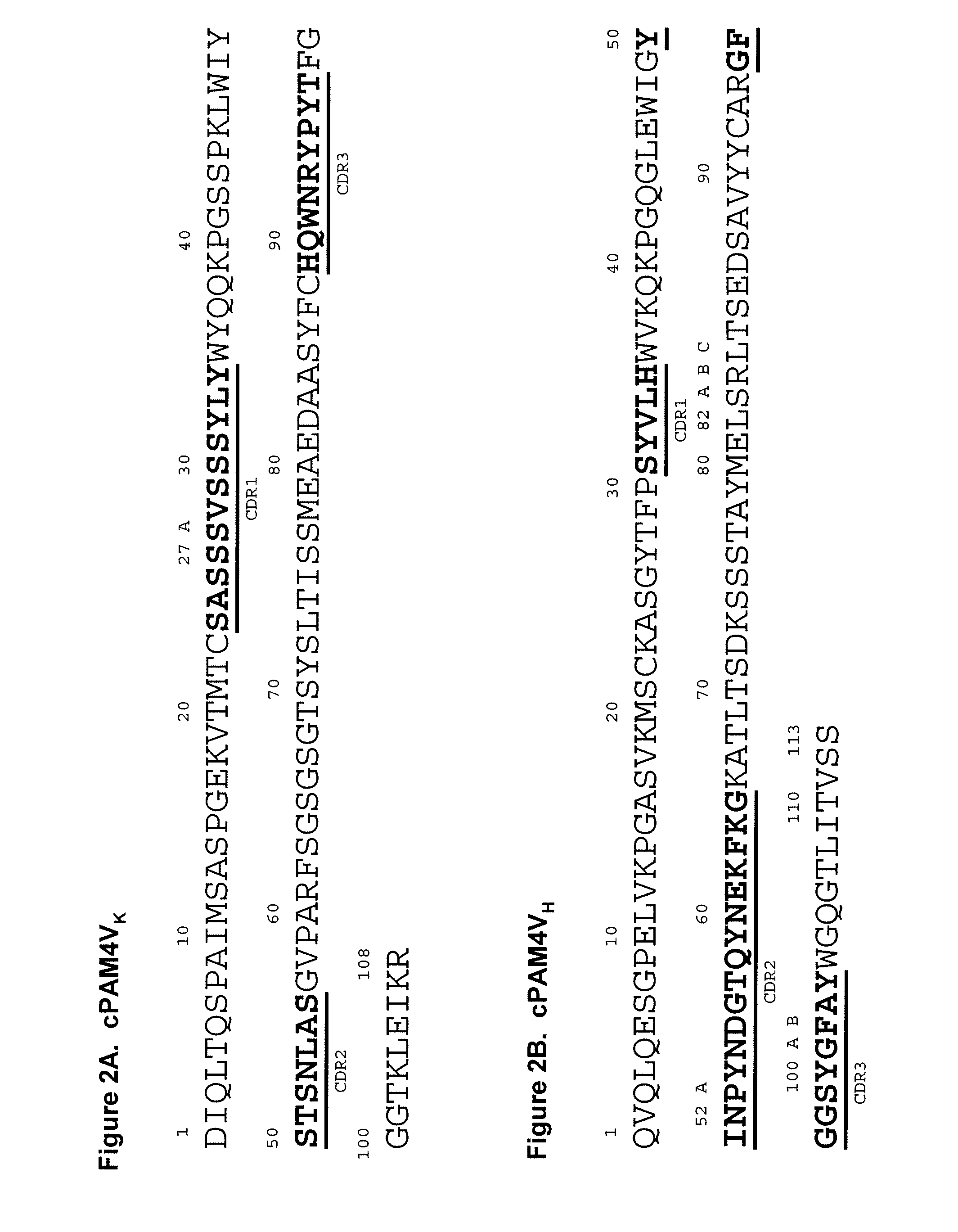
Described herein are compositions and methods of use of anti-pancreatic cancer antibodies or fragments thereof, such as murine, chimeric, humanized or human PAM4 antibodies. The subject antibodies show a number of novel and useful therapeutic characteristics, such as binding with high specificity to pancreatic and other cancers, but not to normal or benign pancreatic tissues and binding to a high percentage of early stage pancreatic cancers. In preferred embodiments, the antibodies bind to pancreatic cancer mucins. The antibodies and fragments are of use for the detection, diagnosis and / or treatment of cancer, such as pancreatic cancer. The antibodies, such as PAM4 antibodies, bind to a PAM4 antigen that shows unique cell and tissue distributions compared with other known antibodies such as CA19.9, DUPAN2, SPAN1, Nd2, B72.3, and Lea and Le(y) antibodies that bind to the Lewis antigens.
Owner:IMMUNOMEDICS INC
Compositions and methods for treatment and detection of cancers
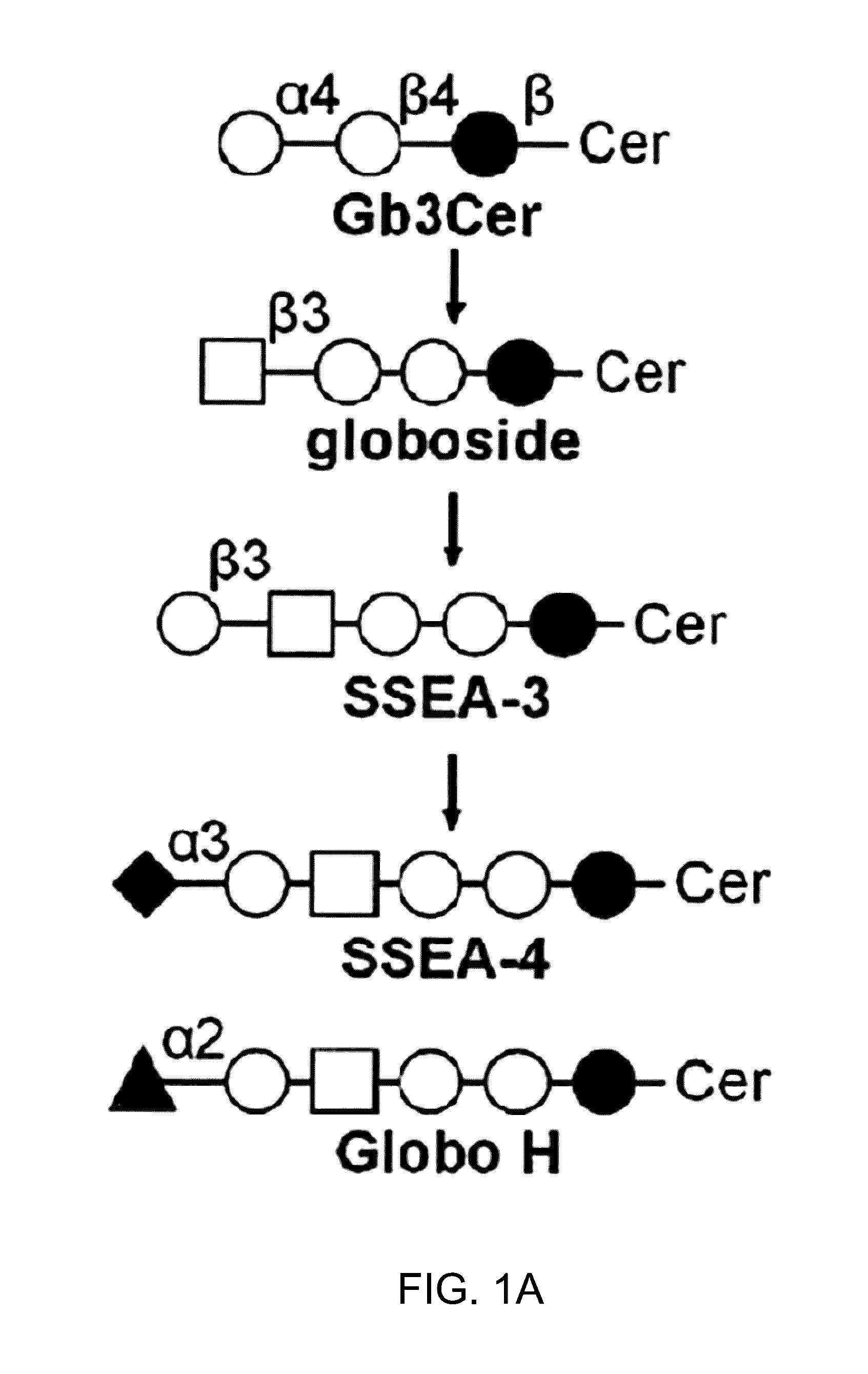
ActiveUS20160102151A1Shrink tumorElimination of malignant cellLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellAntigen Binding Fragment
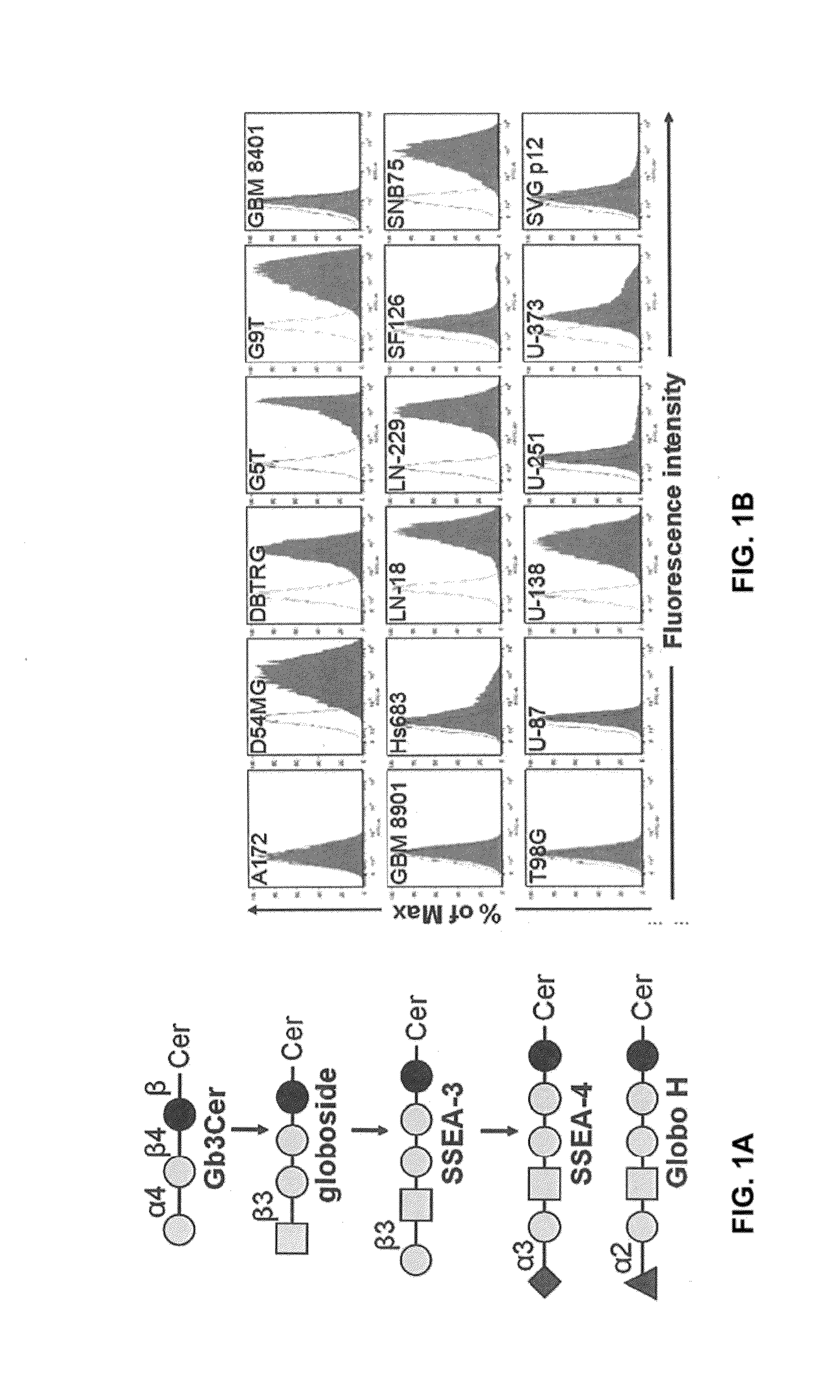
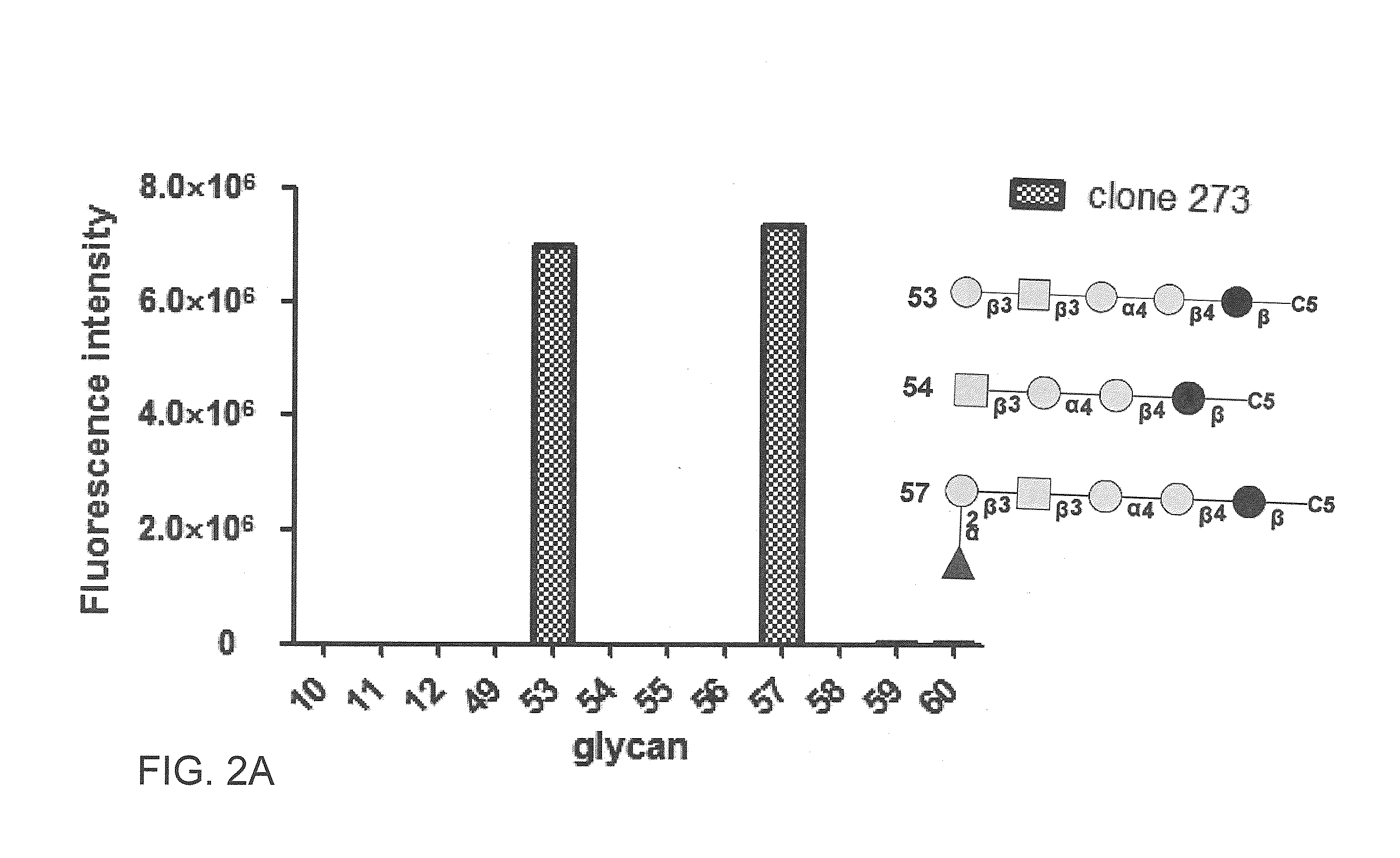
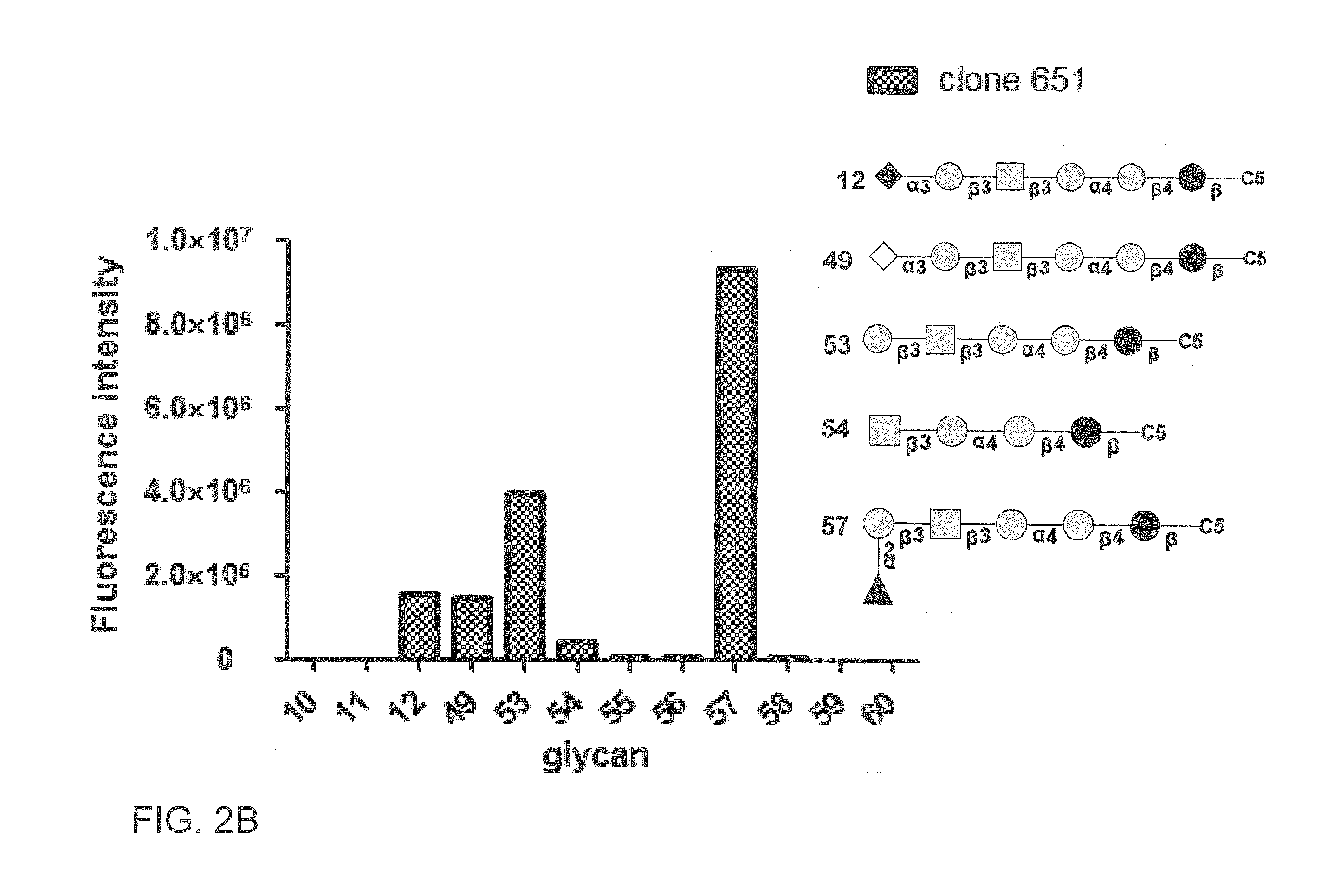
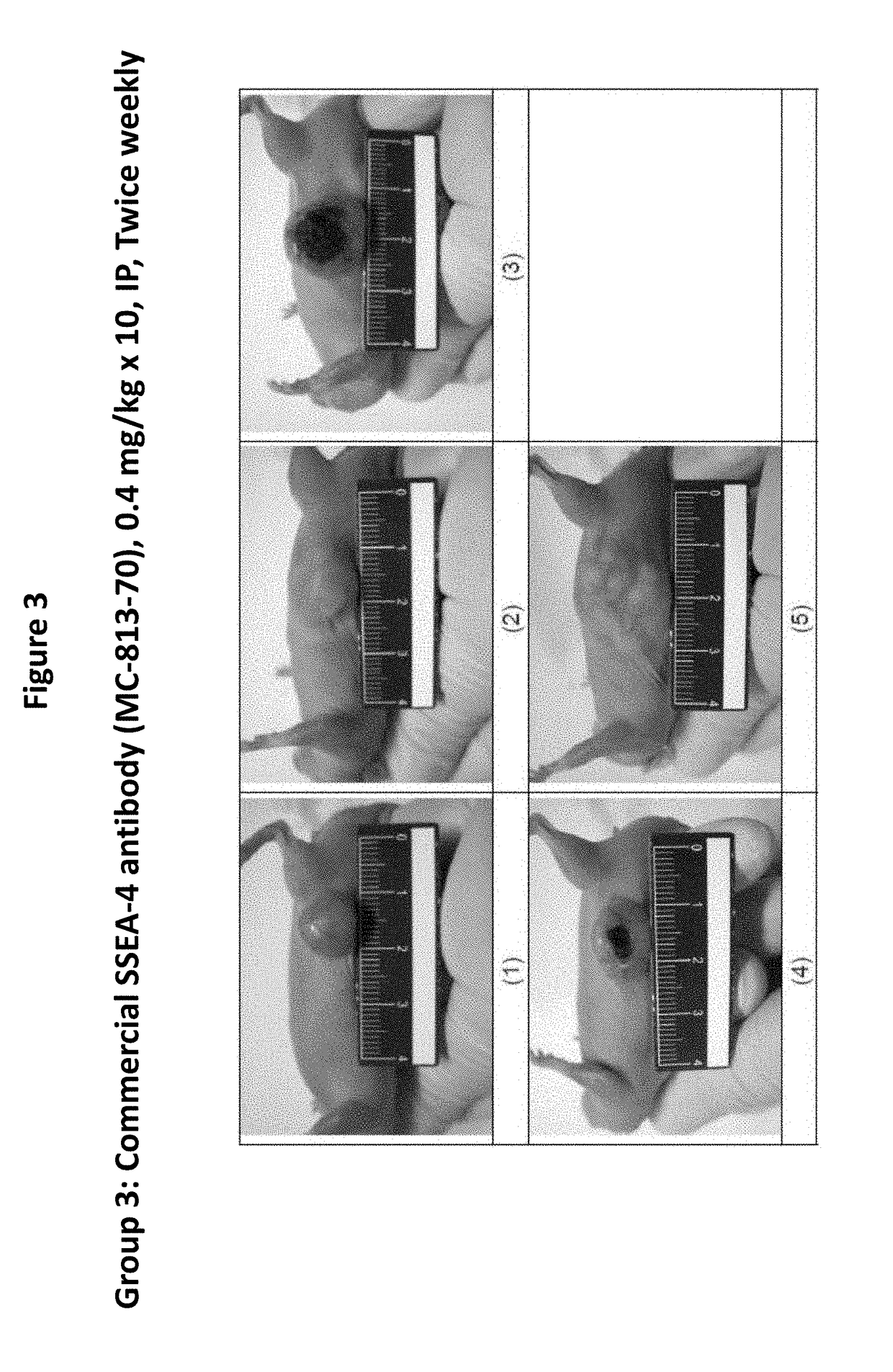
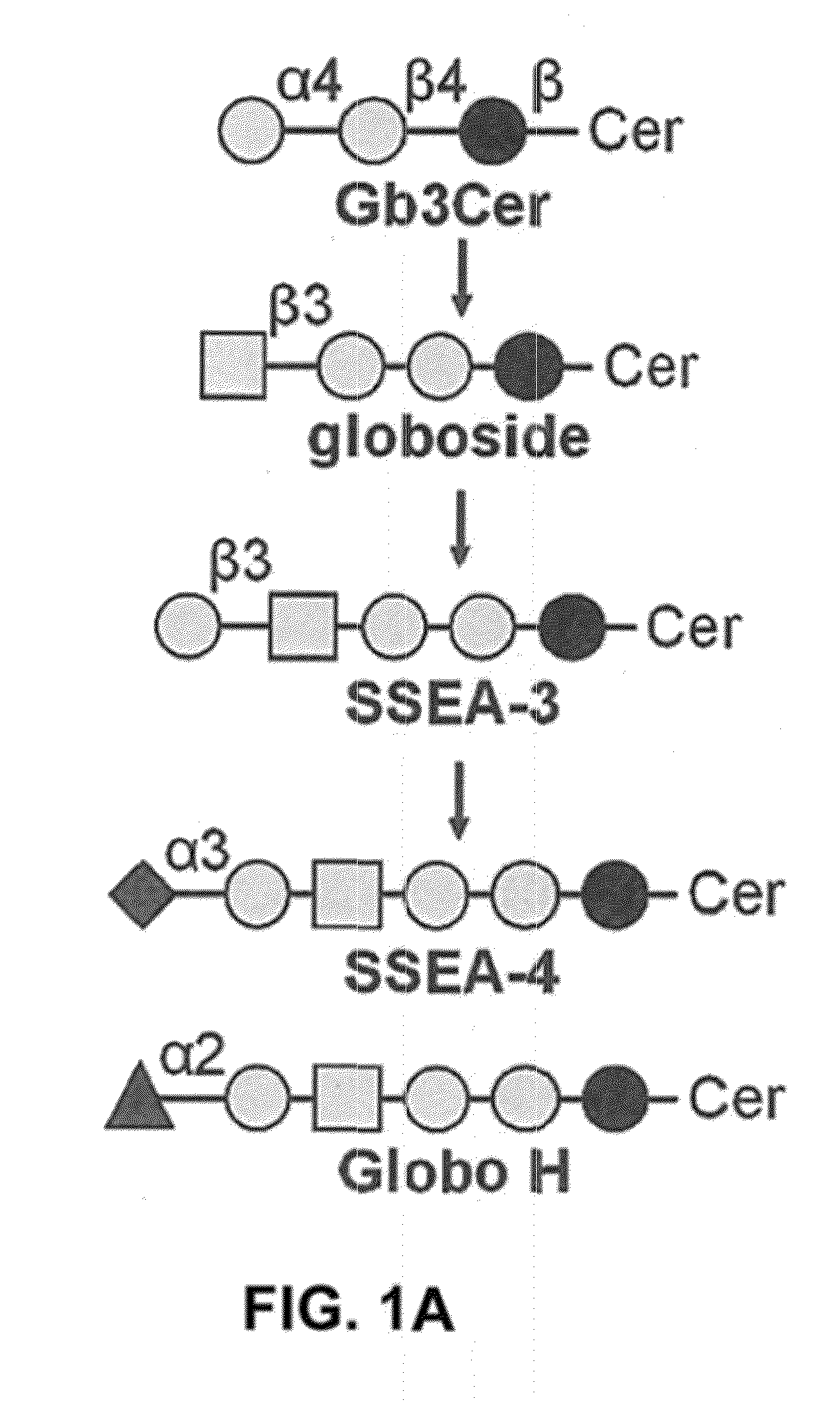
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to globo H, SSEA3, and SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, skin, bone, lungs, breast, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervical, ovarian, and / or prostate cancer.
Owner:ACAD SINIC
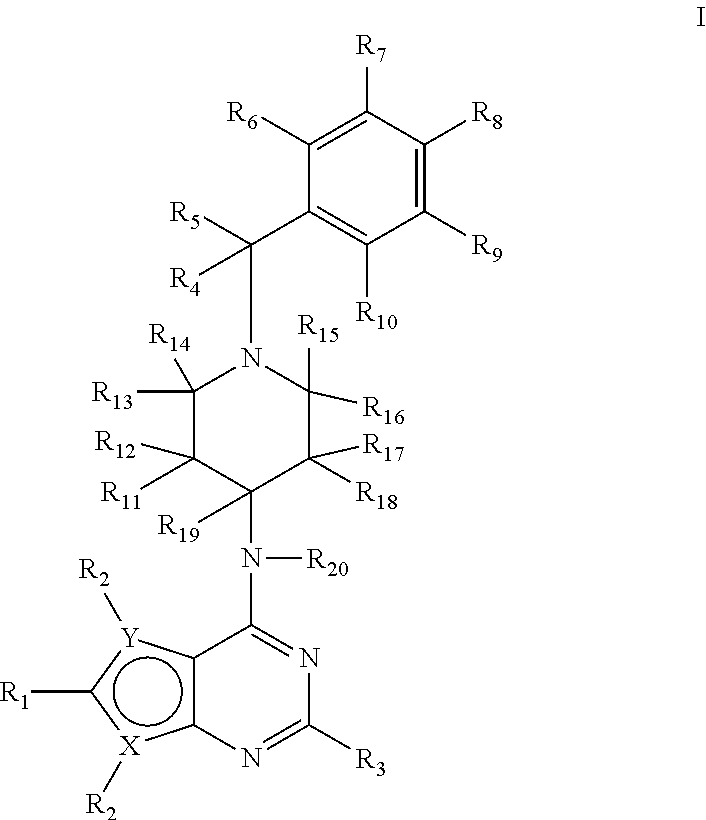
1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof
ActiveUS20110190290A1Antagonize activityBiocideSenses disorderPancreas CancersPharmaceutical medicine
Provided herein are compounds according to Formula I and pharmaceutically acceptable salts thereof, and compositions comprising the same, for use in various methods, including treating cancers such as colon, ovarian, pancreatic, breast, liver, prostate and hematologic cancers:
Owner:BIOSPLICE THERAPEUTICS INC
Colon and pancreas cancer specific antigens and antibodies
InactiveUS20130189268A1Slow tumor growthExcellent tumor regressionIn-vivo radioactive preparationsCell receptors/surface-antigens/surface-determinantsEpitopePancreas Cancers
This invention relates to NPC-1 antigen on the MUC5AC protein and 16C3 antigen on CEACAM5 and CEACAM6 proteins, and 31.1 epitope on the A33 protein are differentially expressed in cancers including, lung cancer, ovarian cancer, pancreas cancer, breast cancer, and colon cancer, and diagnostic and therapeutic usages. Further, NPC-1, 16C3, and / or 31.1 epitope specific antibodies and diagnostic and therapeutic methods of use.
Owner:PRECISION BIOLOGICS INC
Compositions and methods for treatment and detection of cancers
ActiveUS20150344551A1Shrink tumorEliminate the problemImmunoglobulins against animals/humansAntibody ingredientsCancer cellAntigen Binding Fragment
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, lung, breast, mouse, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervix, ovary, and / or prostate cancer.
Owner:ACAD SINIC
Methods of treatment of pancreatic cancer
ActiveUS20140079788A1Many symptomShorten the progressPeptide/protein ingredientsDigestive systemPancreas CancersNanoparticle
Owner:ABRAXIS BIOSCI LLC
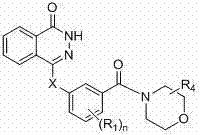
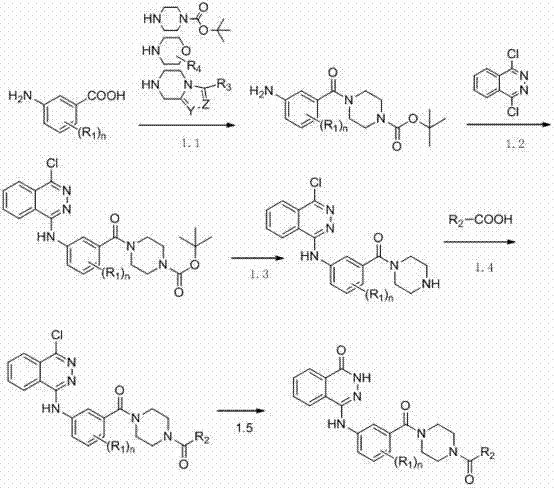
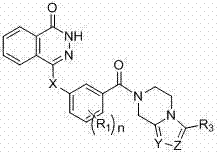
Novel phthalazinone derivatives and uses thereof
The present invention provides novel phthalazinone compounds and isomer thereof, pharmaceutically acceptable salts, solvates, chemically protected forms, and prodrugs; which can be used as PARP inhibitor and pharmaceutical compositions containing the novel phthalazinone compounds; wherein A, R1 and X are defined as shown. The medicine is used for the treatment of: vascular diseases, neurotoxicity, or diseases improved through the inhibition of PARP activity; or used as adjuvants for the treatment of cancers, or used for enhancing the therapeutic effect of radiation or chemotherapeutic agents on tumor cells, wherein the cancers includes breast cancer, ovarian cancer, colon cancer, melanoma, lung cancer, gastrointestinal stromal tumor, brain cancer, cervical cancer, pancreatic cancer, prostate cancer, gastric cancer, chronic myeloid leukocytes hypercytosis, liver canser, lymphoma, peritoneal cancer, soft tissue sarcoma, neuroendocrine tumors, advanced solid tumors, and glioblastoma.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Compositions and methods for treatment and detection of cancers
ActiveUS20160289340A1Useful in treatmentEnhanced ADCC activityImmunoglobulins against animals/humansAntibody ingredientsCancer cellAntigen Binding Fragment
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to globo H, SSEA3, and SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, skin, bone, lungs, breast, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervical, ovarian, and / or prostate cancer.
Owner:ACAD SINIC
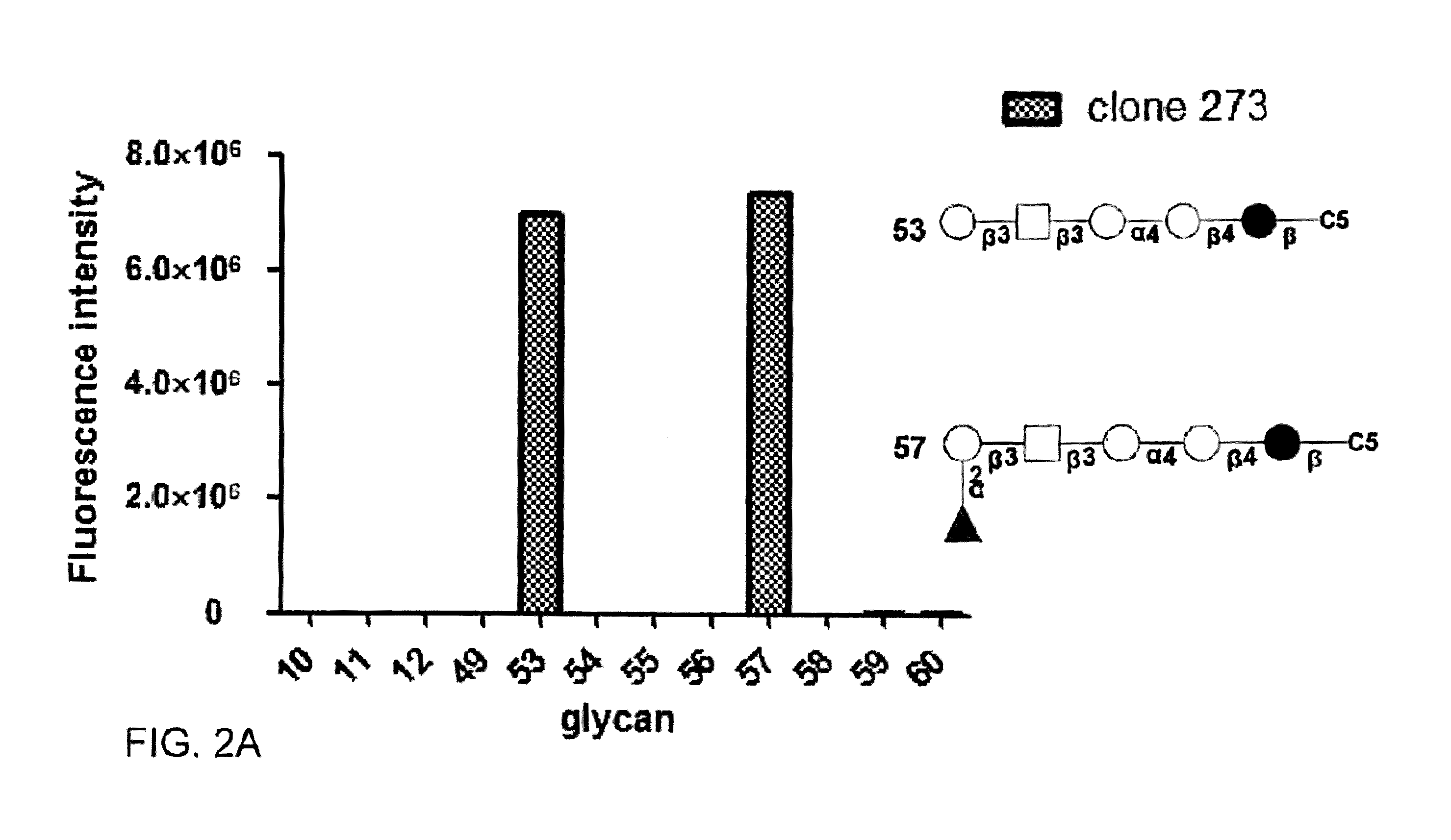
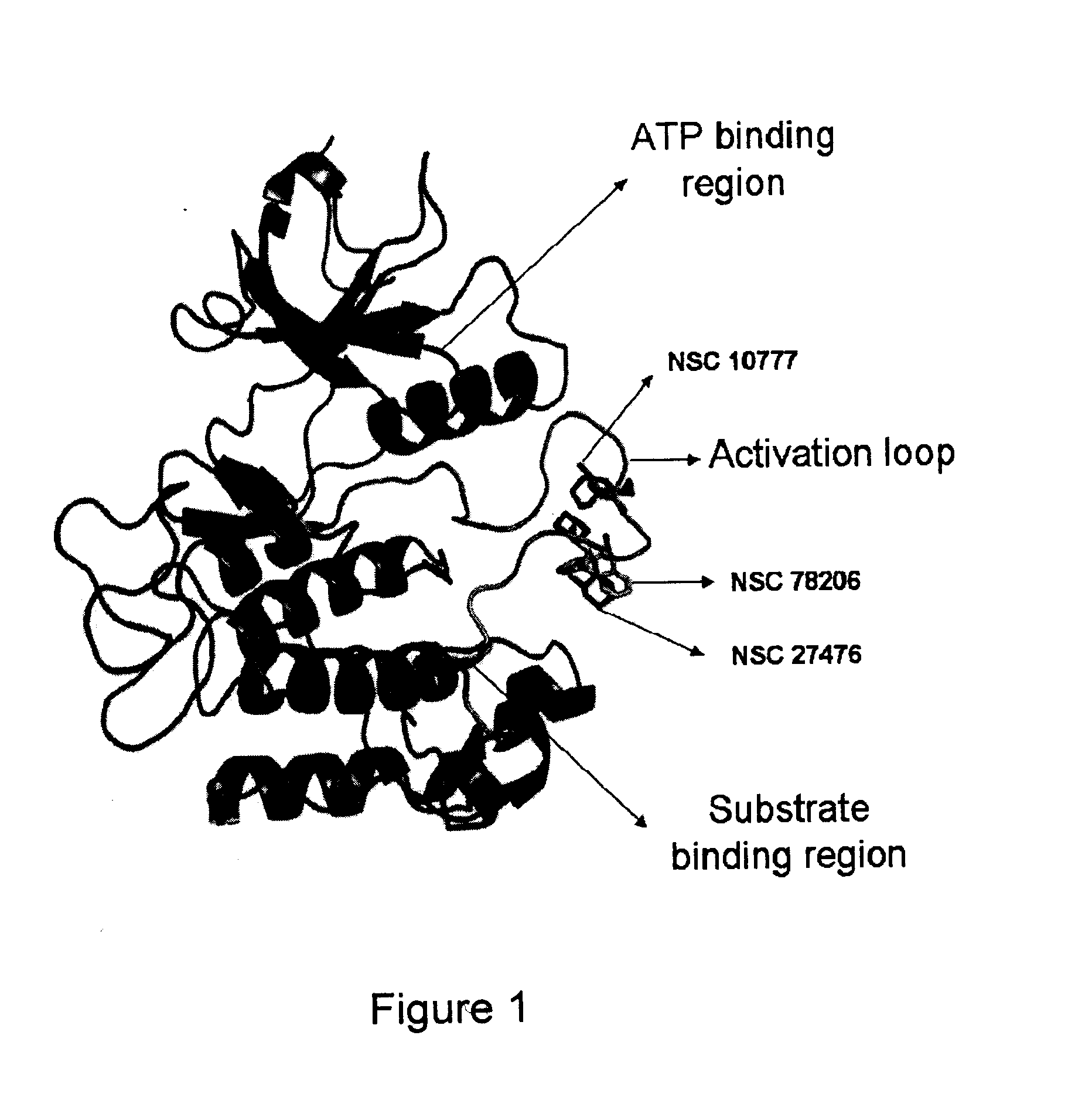
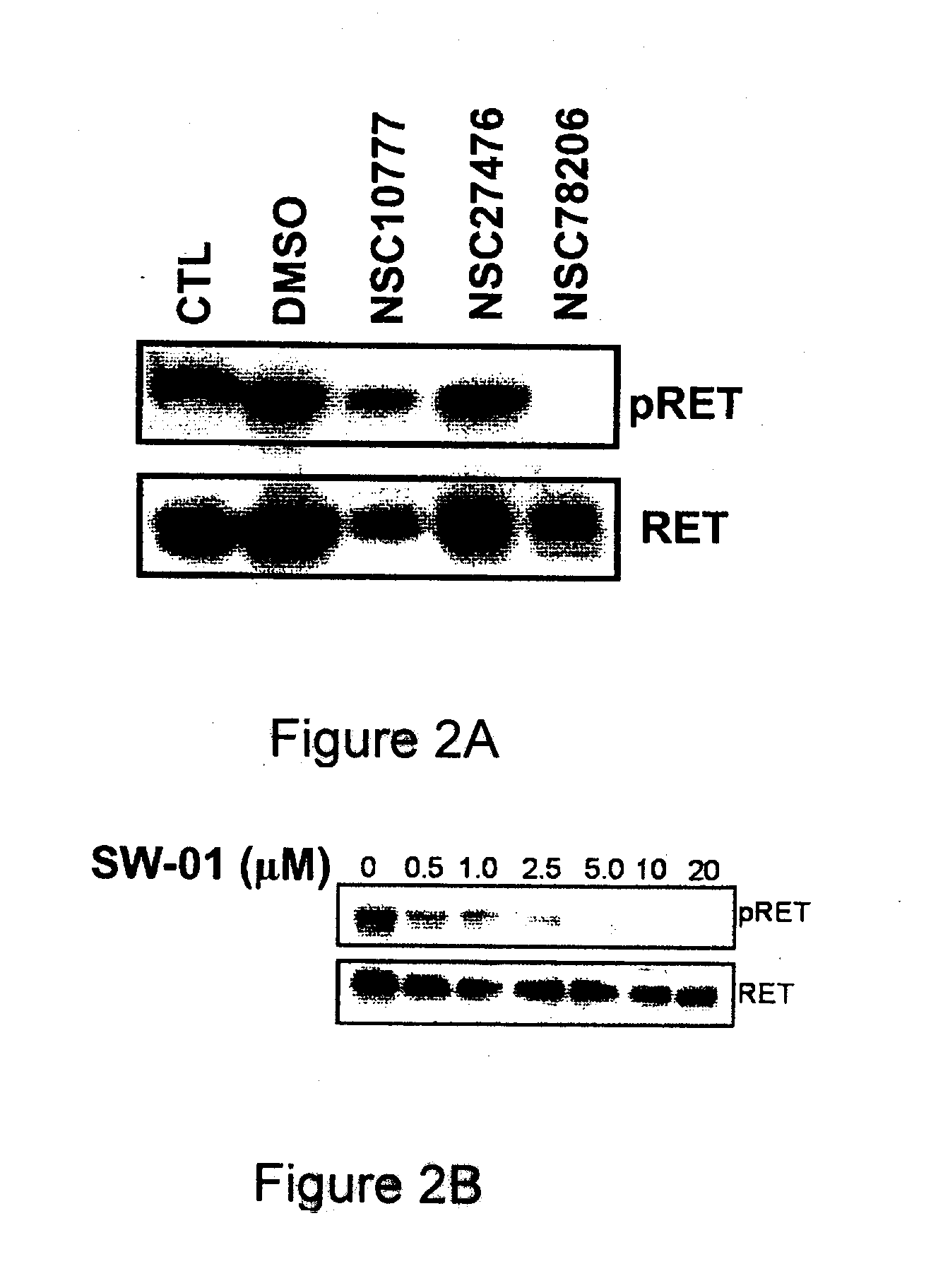
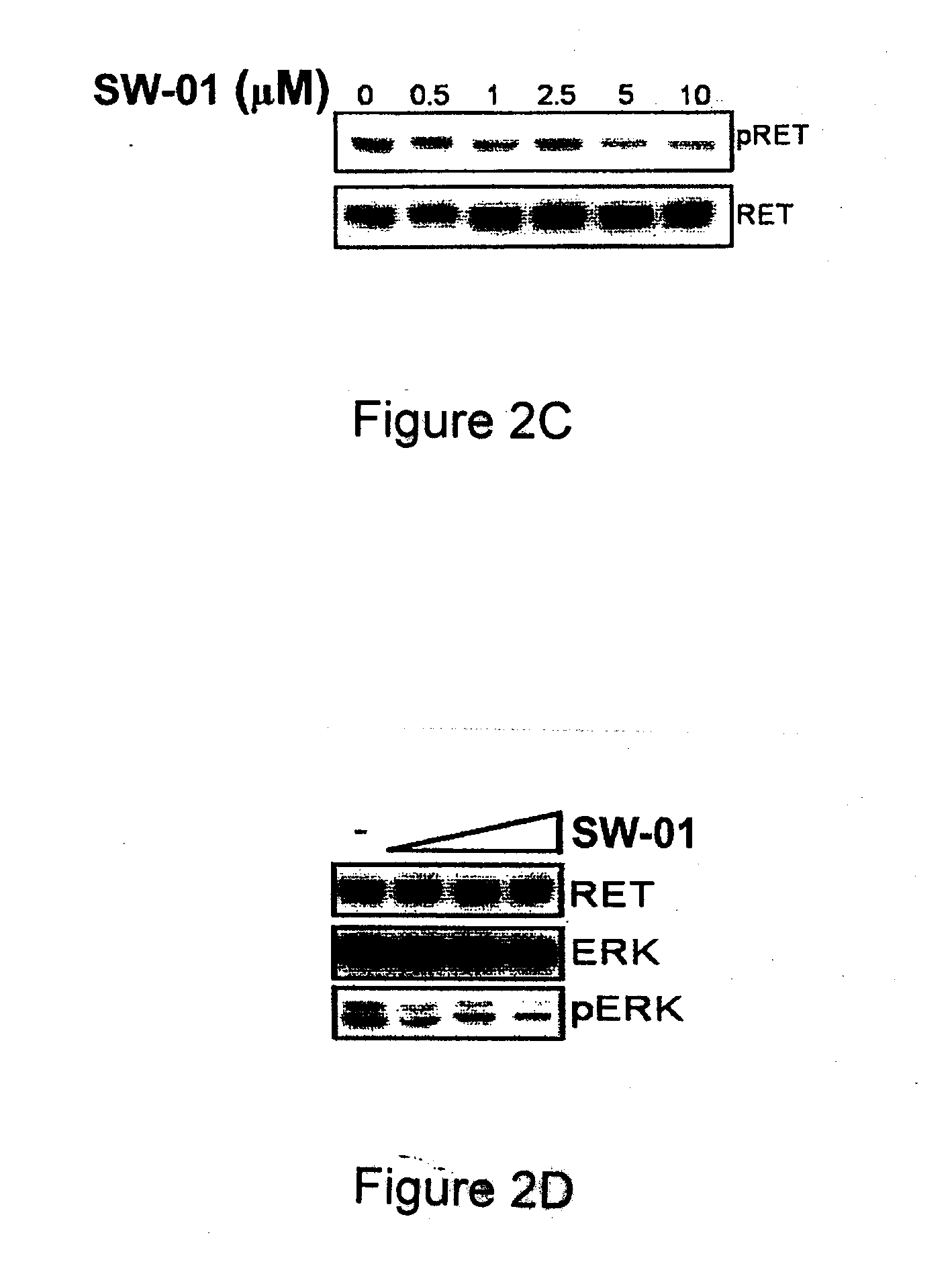
Pharmaceutical Compositions Comprising RET Inhibitors and Methods for the Treatment of Cancer
InactiveUS20110201598A1Inhibit tumor growthReduce transferBiocideOrganic compound preparationCancer preventionPancreas Cancers
A class of compounds useful in pharmaceutical compositions and methods for treating or preventing cancer is described. The compounds' ability to inhibit RET kinase is quantified, i.e., their respective RET IC50 and EC50 values are described. One such compound, known as cyclobenzaprine and herein as SW-01, has been identified as RET-specific with an IC50 of 300 nM. SW-01 inhibits RET autophosphorylation and blocks the growth and transformation of thyroid cancer cell lines. It has been further tested in pancreatic cancer, breast cancer, and SCLC cell lines. The compounds show utility for inhibition of survival and proliferation of tumour cells.
Owner:SINGH VINAY K +2
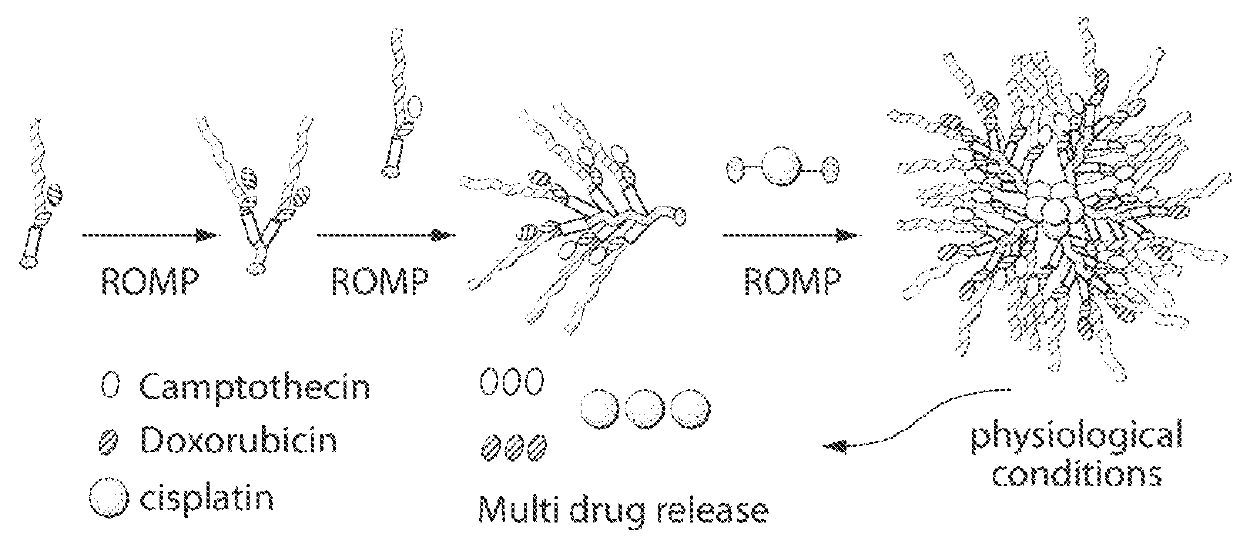
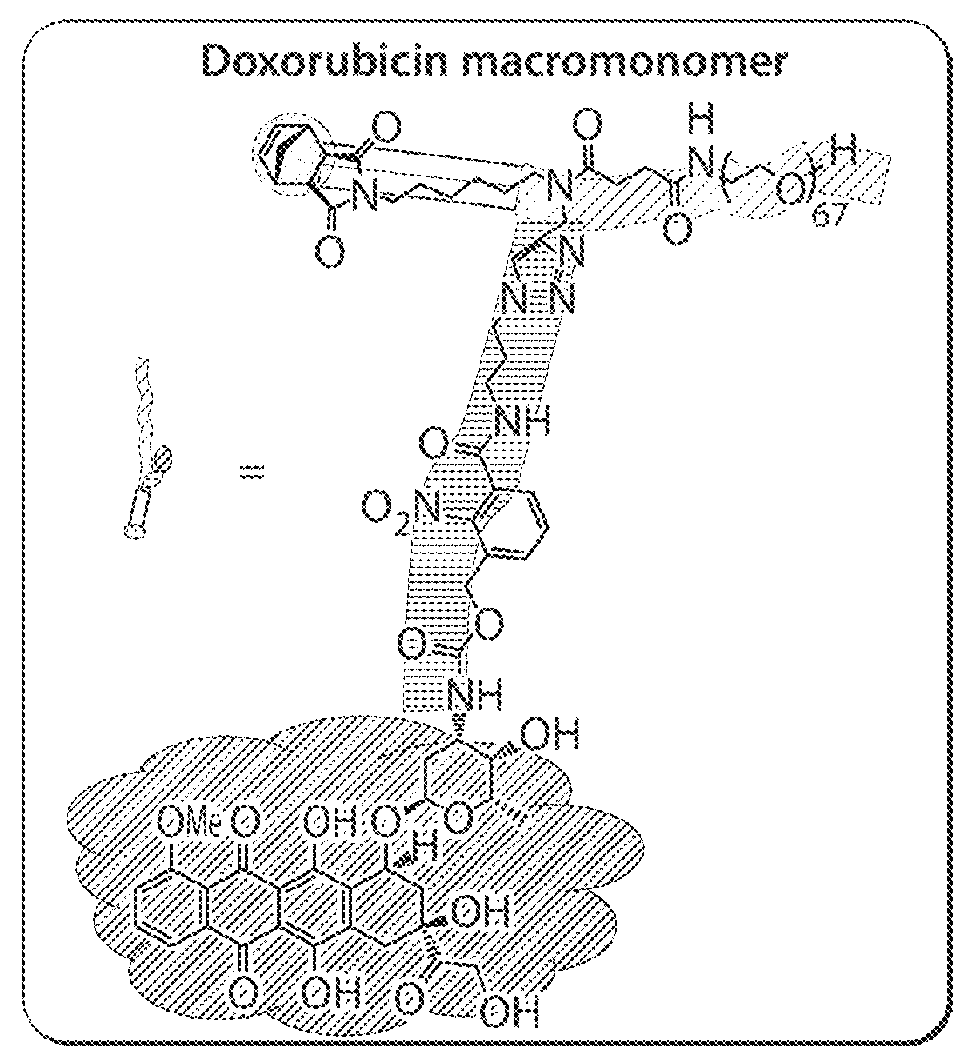
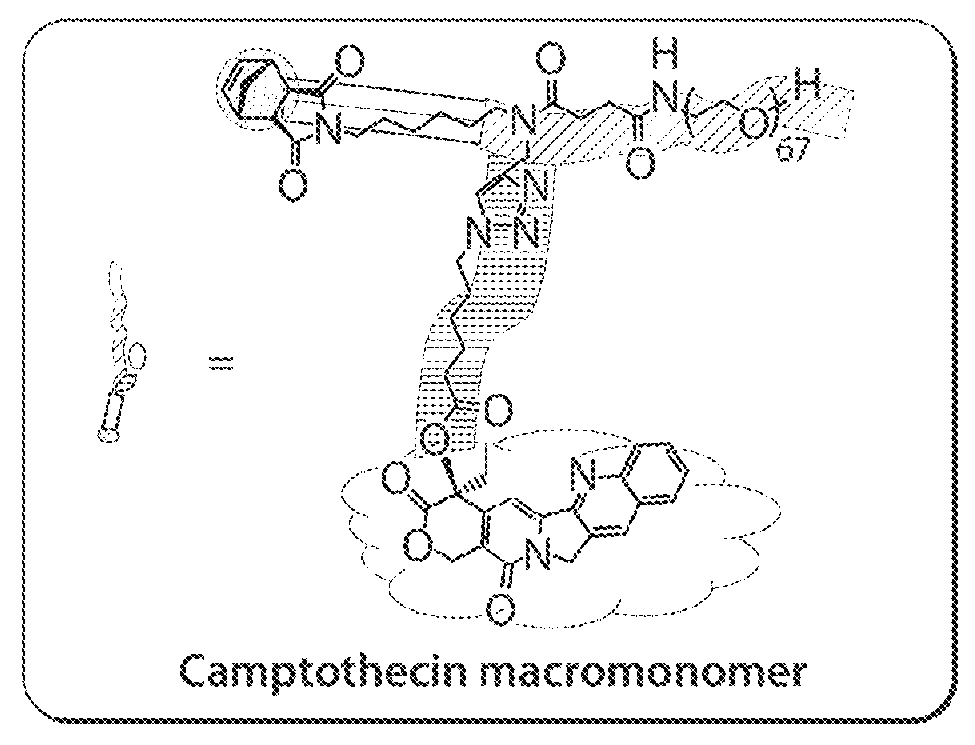
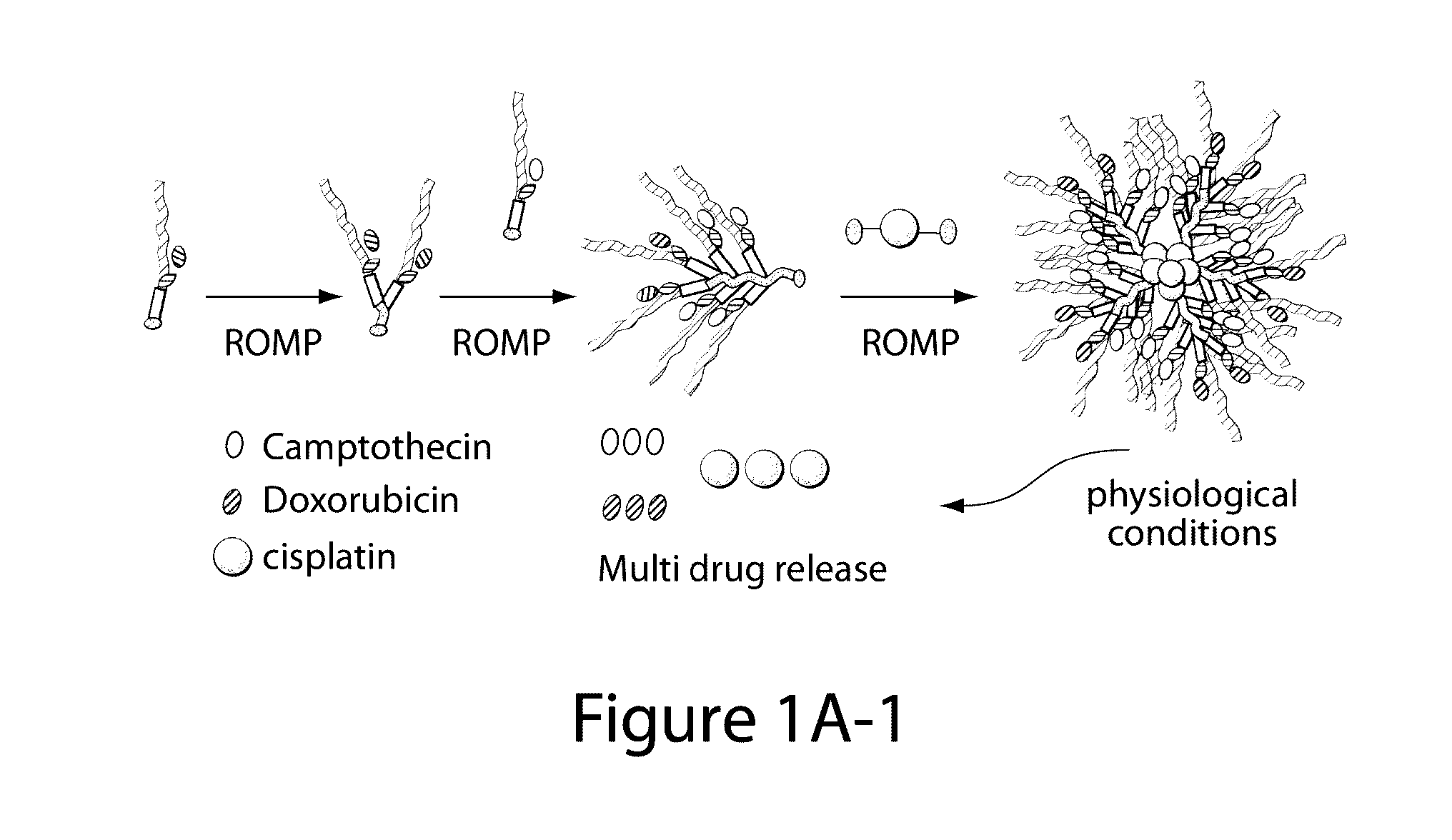
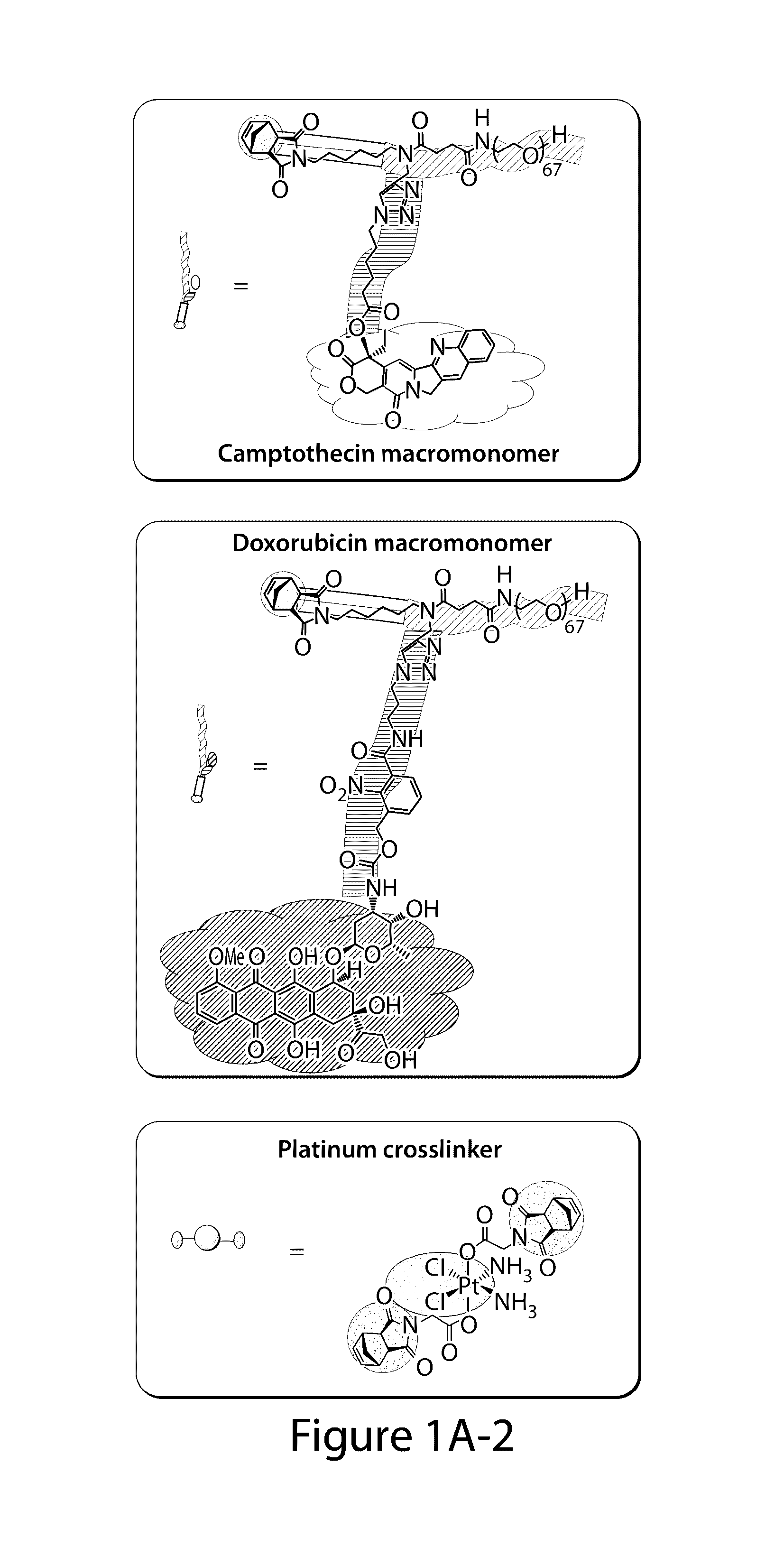
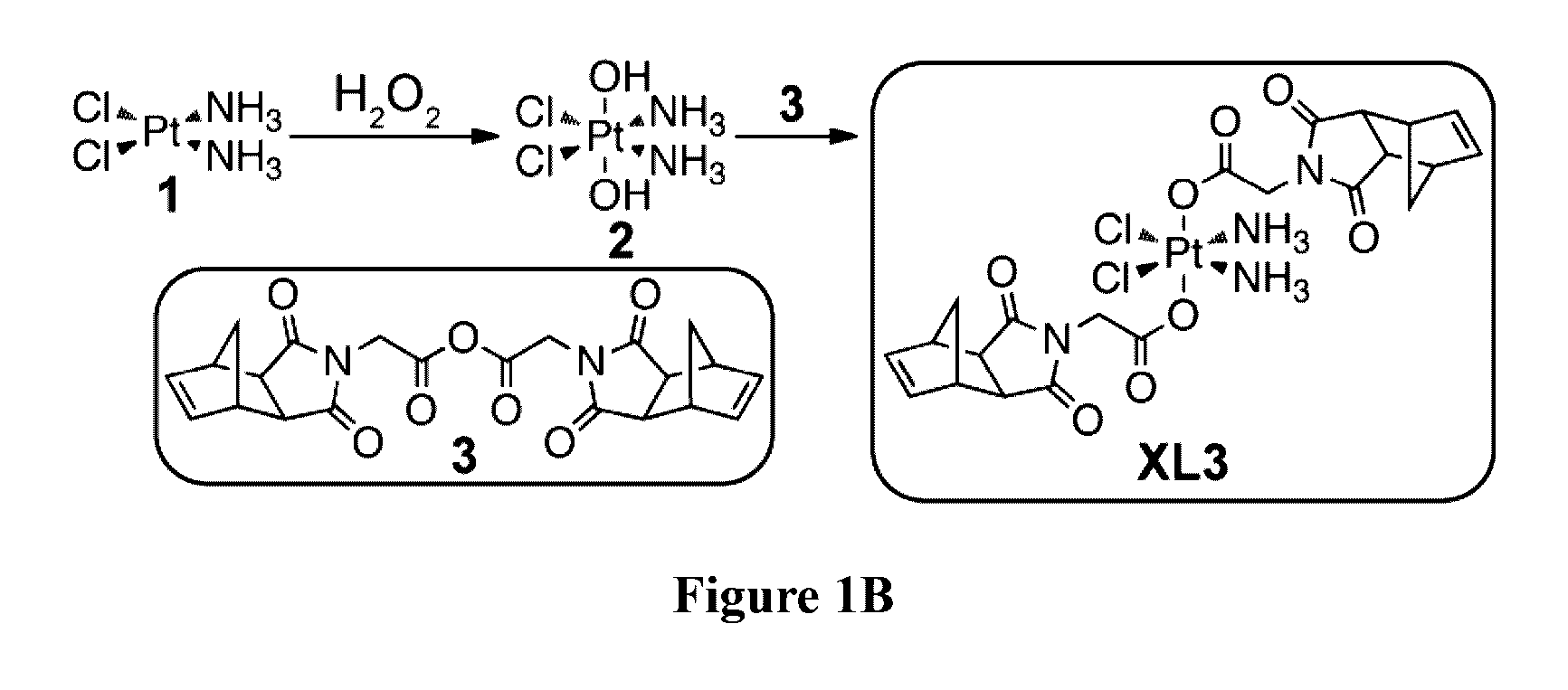
Drug delivery polymer and uses thereof
ActiveUS9381253B2Stop growthStop the spreadOrganic active ingredientsPlatinum organic compoundsPancreas CancersProstate cancer
Owner:MASSACHUSETTS INST OF TECH
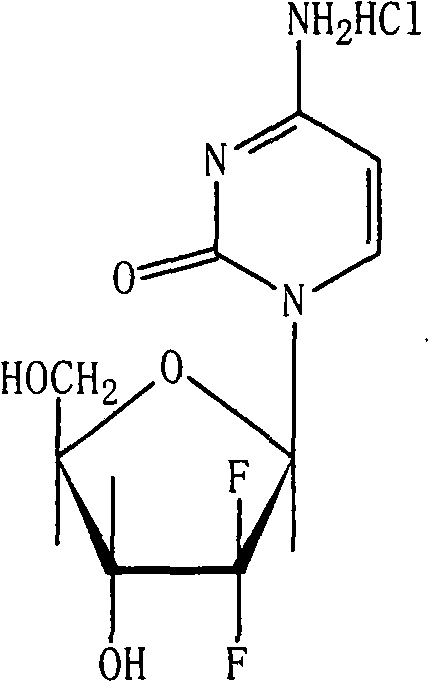
Gemcitabine hydrochloride lyophilized powder injection
ActiveCN101564381AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsPancreas CarcinomaDrug
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The gemcitabine hydrochloride lyophilized powder injection prepared by the method can be used as a therapeutic medicament for treating middle and late non-small cell lung cancer, pancreatic cancer and the like. The gemcitabine hydrochloride lyophilized powder injection is characterized by consisting of gemcitabine hydrochloride, mannitol and sodium acetate, wherein the weight ratio of the gemcitabine hydrochloride to the mannitol is 1:0.5-5, and the weight ratio of the gemcitabine hydrochloride to the sodium acetate is 1:0.01-0.1. The preparation method comprises the following steps: taking the mannitol and the sodium acetate; dissolving the mannitol and the sodium acetate by adding injection water; adding the gemcitabine hydrochloride to the mixture, stirring and dissolving the mixture, and adjusting the pH to between 2.7 and 3.3; fixing the volume; filtering the product by a 0.22 mu m microporous membrane; filling, dishing up, lyophilizing, and compressing; taking the product out of a box, and tying the product with an aluminum-plastic composite cover; and inspecting the quality, and packaging the product after passing the quality inspection to obtain the gemcitabine hydrochloride lyophilized powder injection.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Monoclonal antibodies for treatment of cancer
InactiveUS8946388B2Strong formationStrong proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
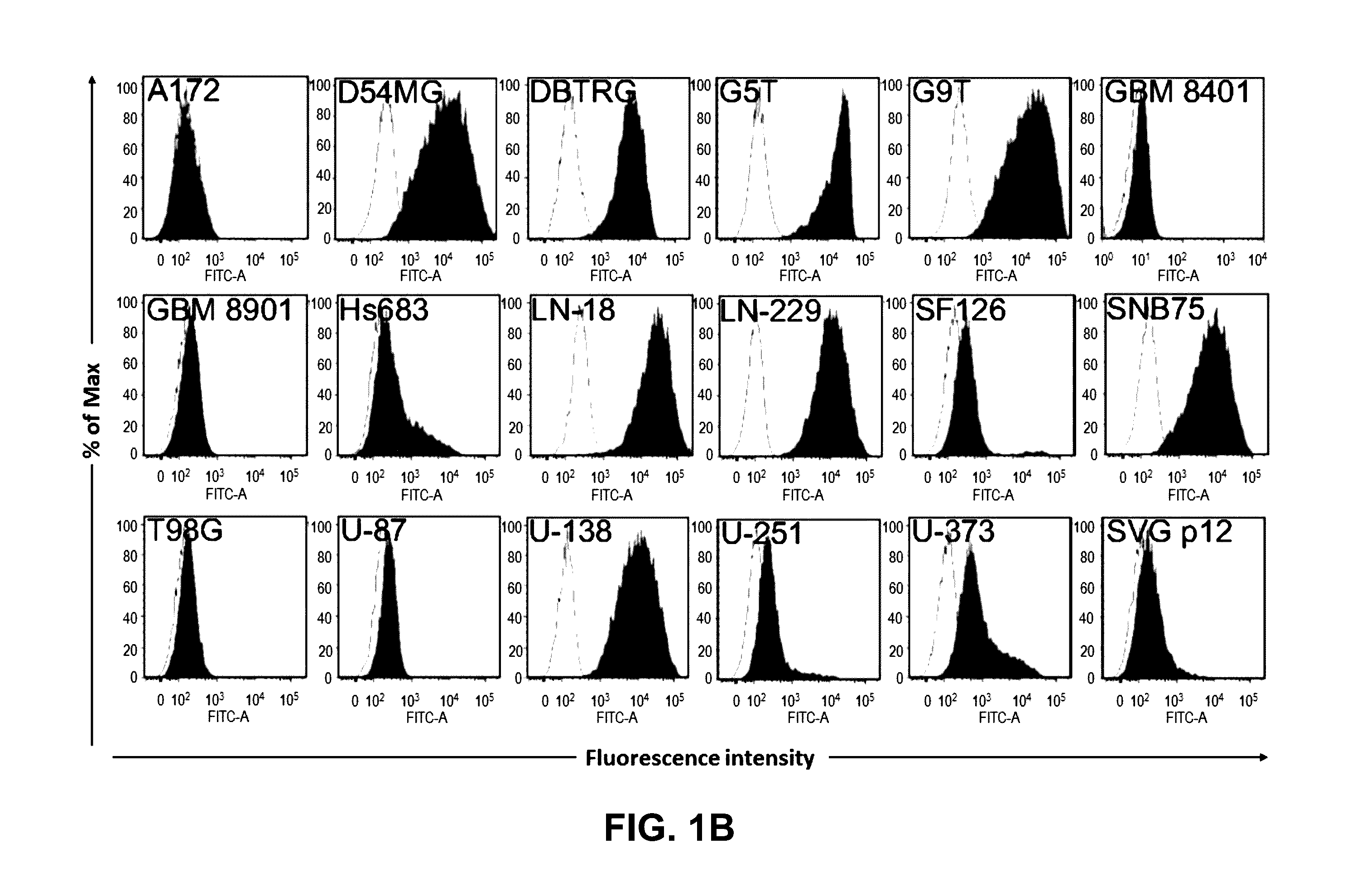
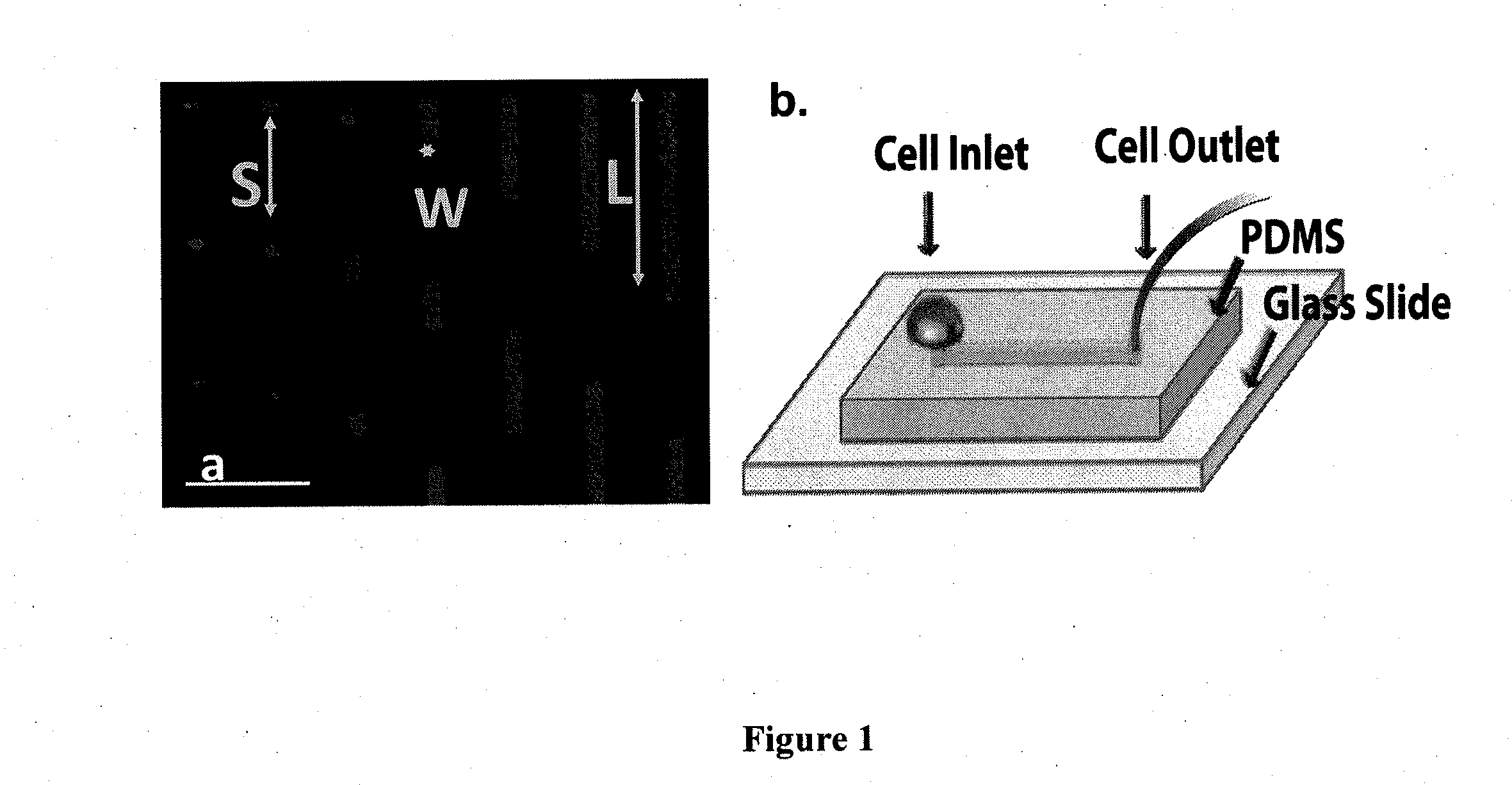
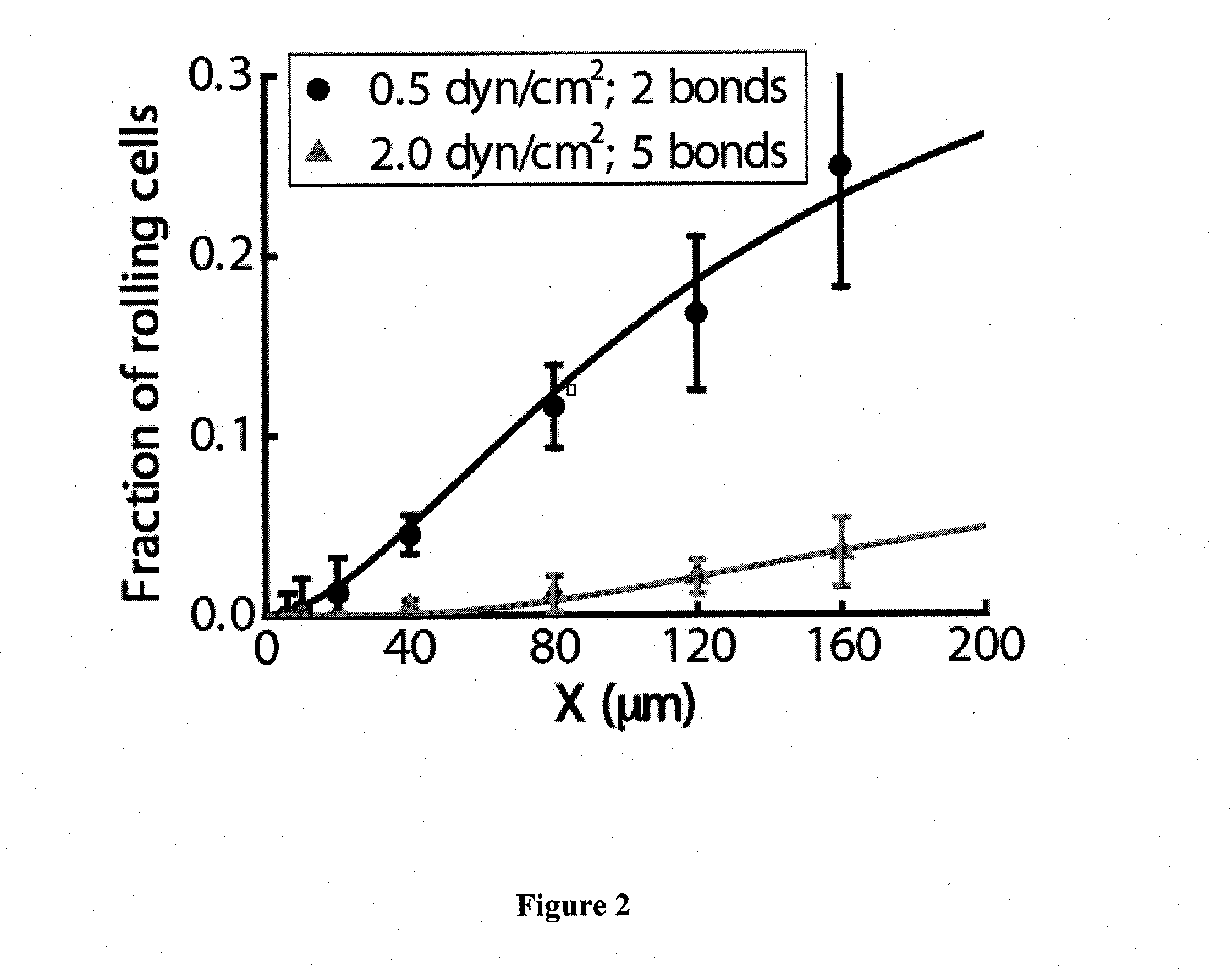
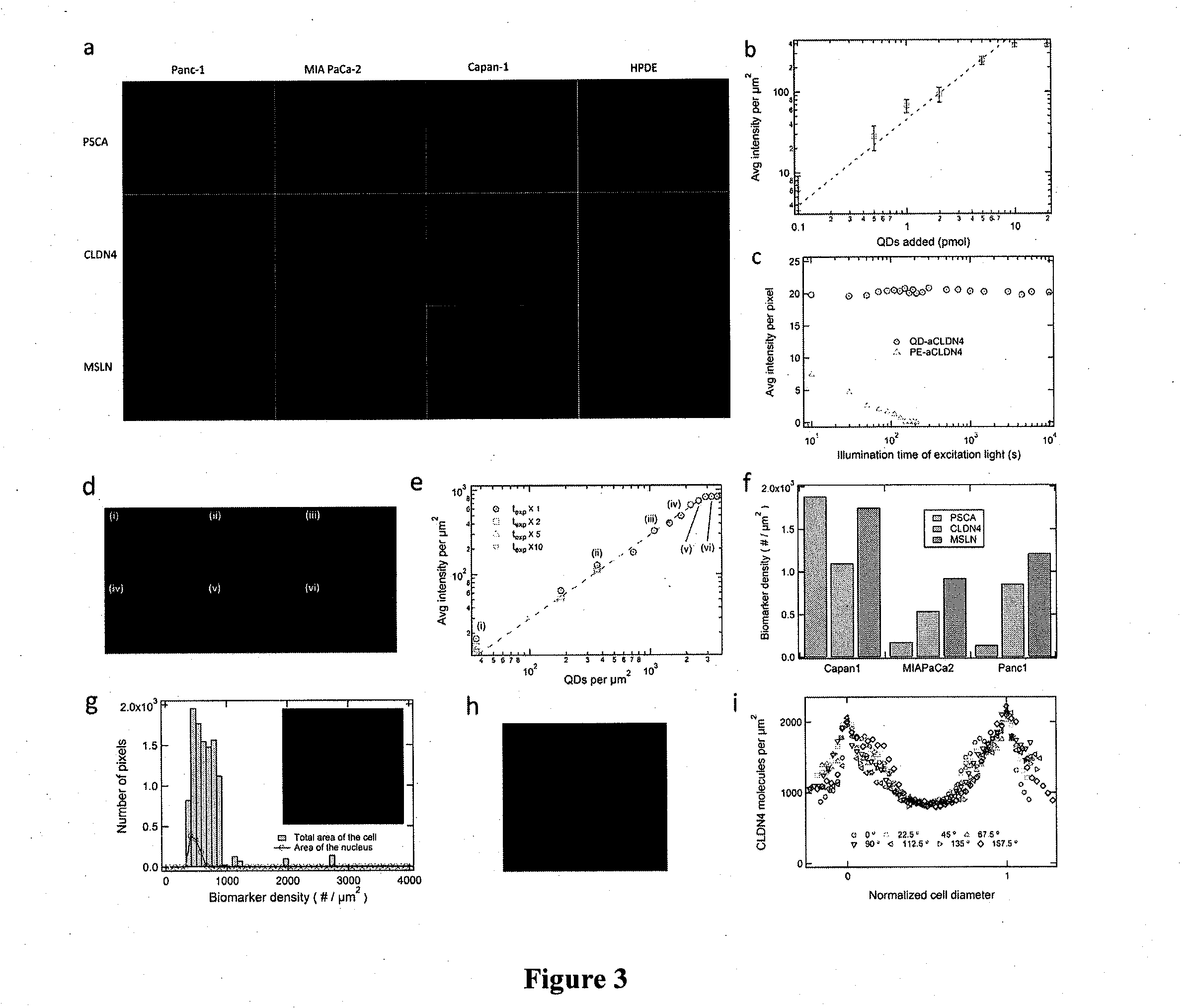
Device for capture, enumeration, and profiling of circulating tumor cells
InactiveUS20120100560A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSpatial mappingBiology
Applications in nanomedicine, such as diagnostics and targeted therapeutics, rely on the detection and targeting of membrane biomarkers. The present invention, in one embodiment, utilizes quantitative profiling, spatial mapping, and multiplexing of cancer biomarkers using functionalized quantum dots. This approach provides highly selective targeting molecular markers for pancreatic cancer with extremely low levels of non-specific binding and provides quantitative spatial information of biomarker distribution on a single cell, which is important since tumors cell populations are inherently heterogeneous. The quantitative measurements (number of molecules per square micron) is validated using flow cytometry and demonstrated using multiplexed quantitative profiling using color-coded quantum dots.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
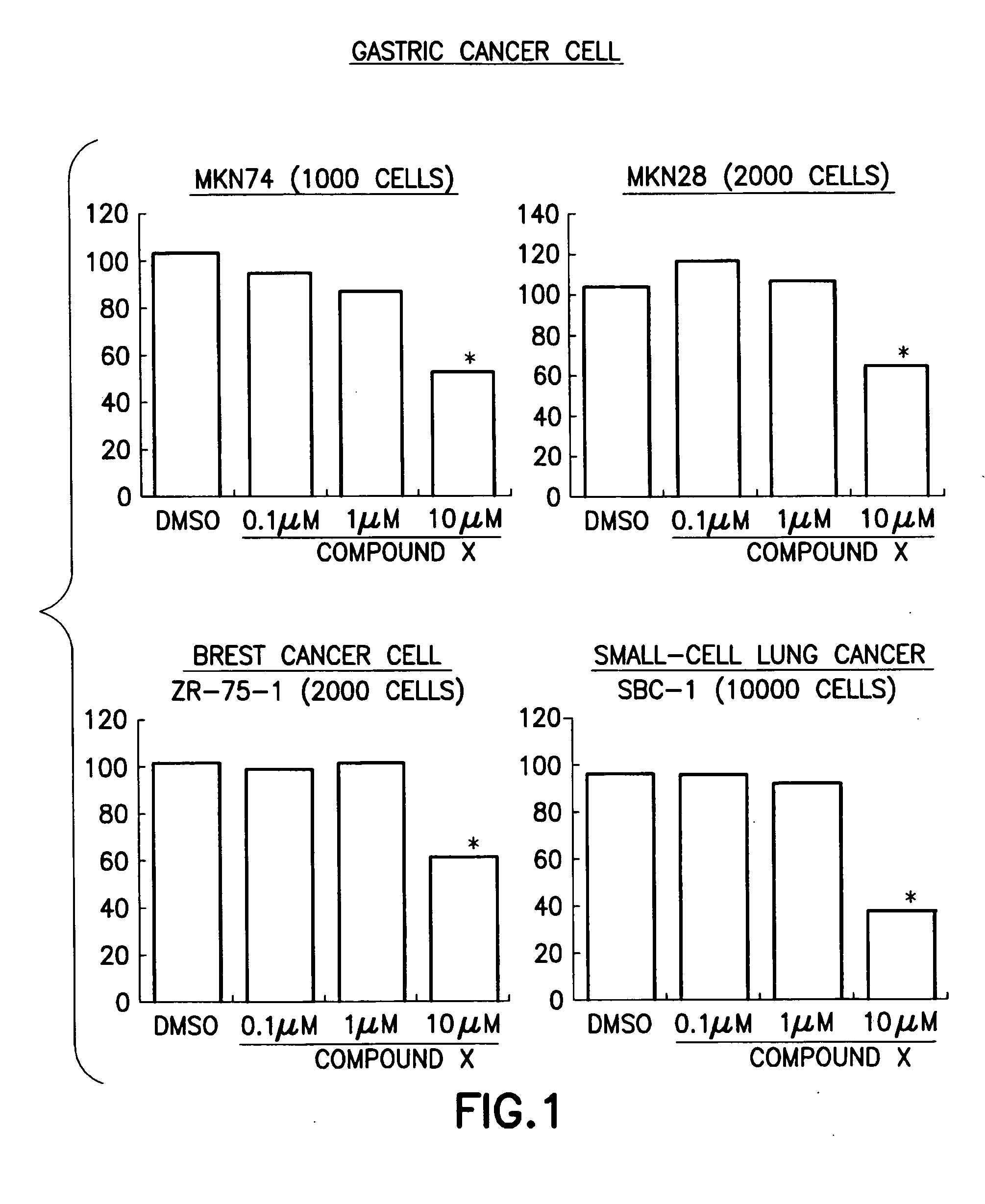
Anti-cancer pharmaceutical compositions and methods for treating patients with cancer
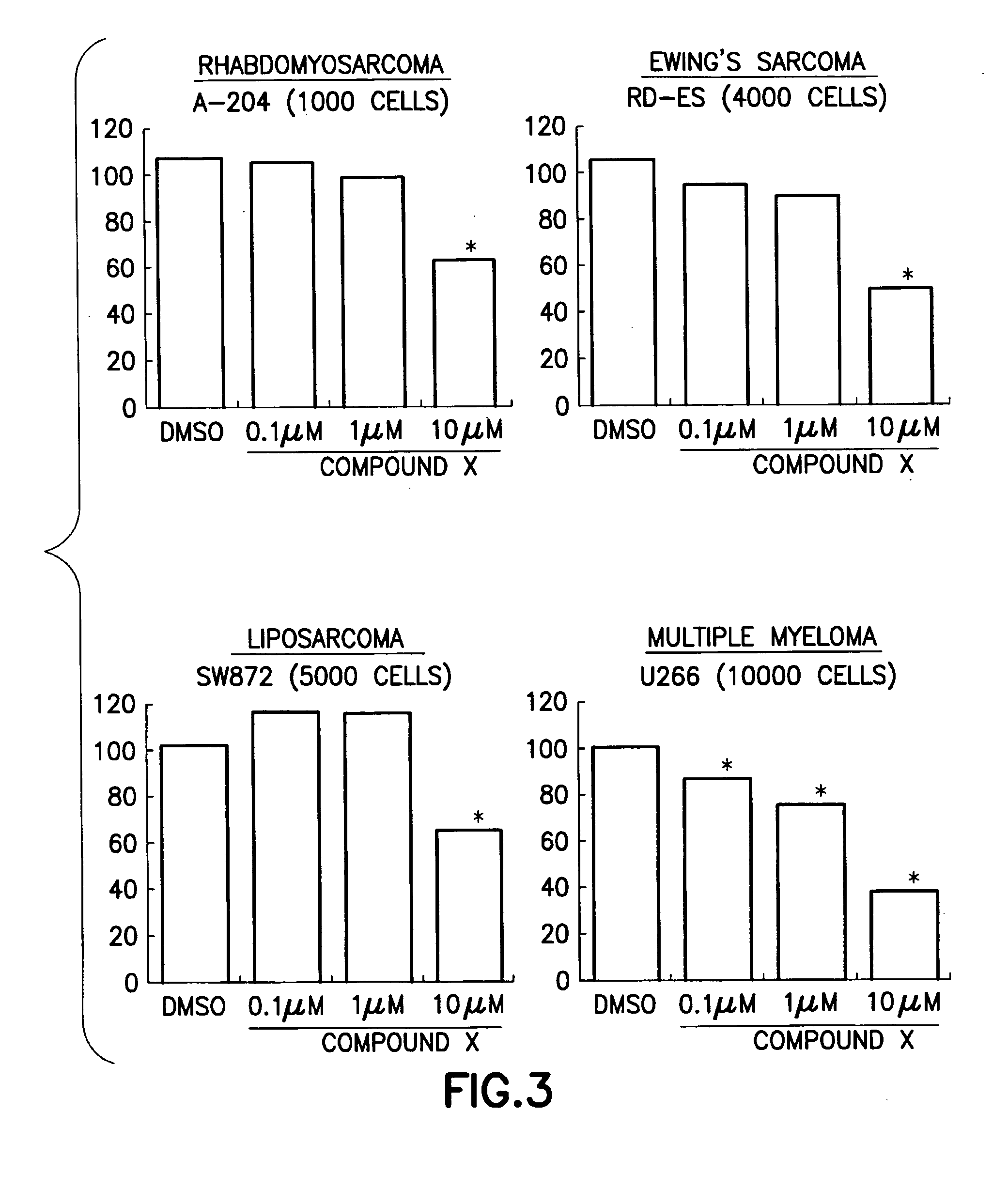
InactiveUS20090028868A1Agent for prophylaxis and treatmentBiocideOrganic chemistryPancreas CancersLiposarcoma
Method of treating persons having carcinoma, sarcoma or hematopoietic cancer by administering (i) a compound of the formula (I)and (ii) an epidermal growth factor receptor (EGFR) inhibitor, a vascular endothelial growth factor receptor (VEGFR) inhibitor and pharmaceutical compositions for use in said method. A method for treating gastric cancer, colon cancer, lung cancer, breast cancer, pancreas cancer, kidney cancer, prostate cancer, medulloblastoma, rhabdomyosarcoma, Ewing sarcoma, liposarcoma, multiple myeloma and leukemia by administering a compound of the formula (I).
Owner:DAIICHI SANKYO CO LTD
Pancreatic cancer biomarkers
The present invention provides a method of diagnosing, prognosing or screening for pancreatic cancer in a subject. The method may be carried out on a sample such as a blood or tissue sample collected from the subject. The is carried out by (a) detecting one or more markers in a biological sample of said subject, said markers selected from the markers set forth in Table 1 (e.g. one or more markers selected from the group set forth in Table 2, and / or the group consisting of ALCAM, TIMP-1, ICAM1, LCN2, REG1A, REG3, IGFBP4, TNFRSF1A and WFDC2); and (b) determining an altered level of said marker(s), said altered level indicating said subject may be afflicted with or at risk of developing pancreatic cancer. Kits useful for carrying out the methods are also described.
Owner:THE GENERAL HOSPITAL CORP +2
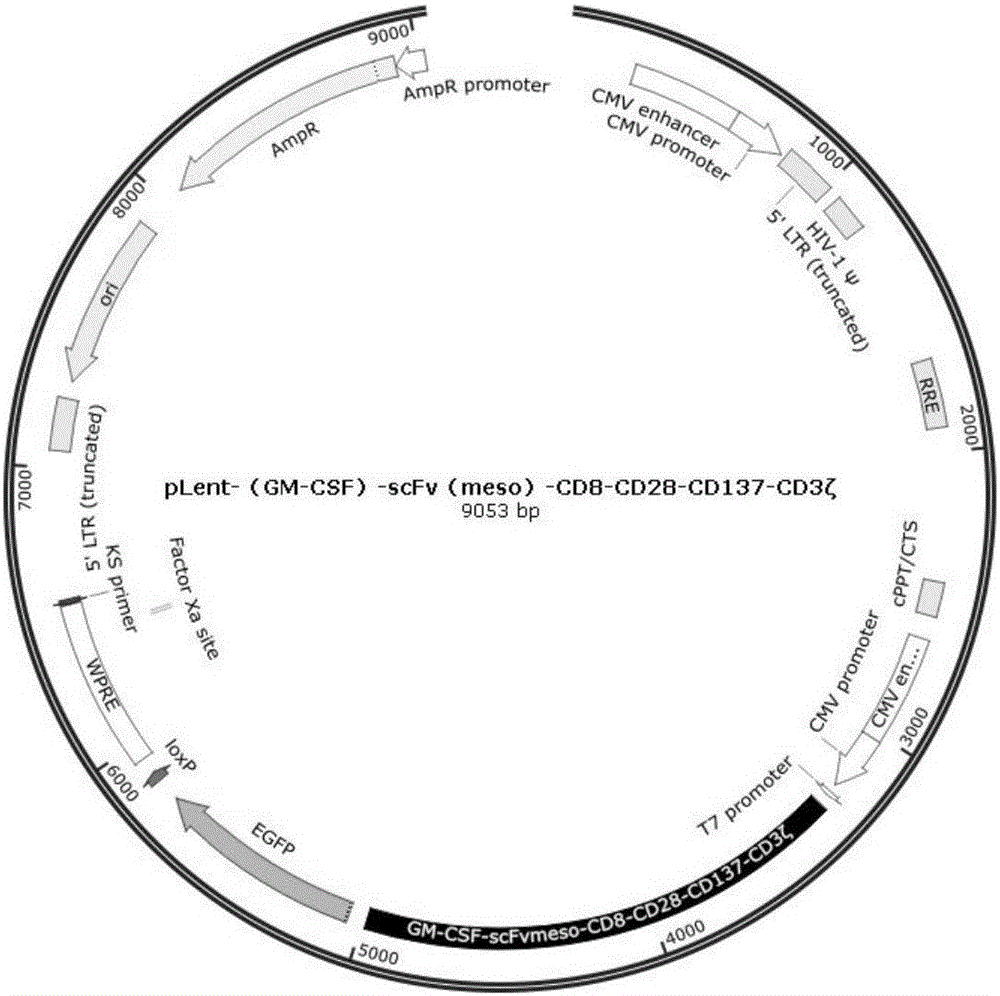
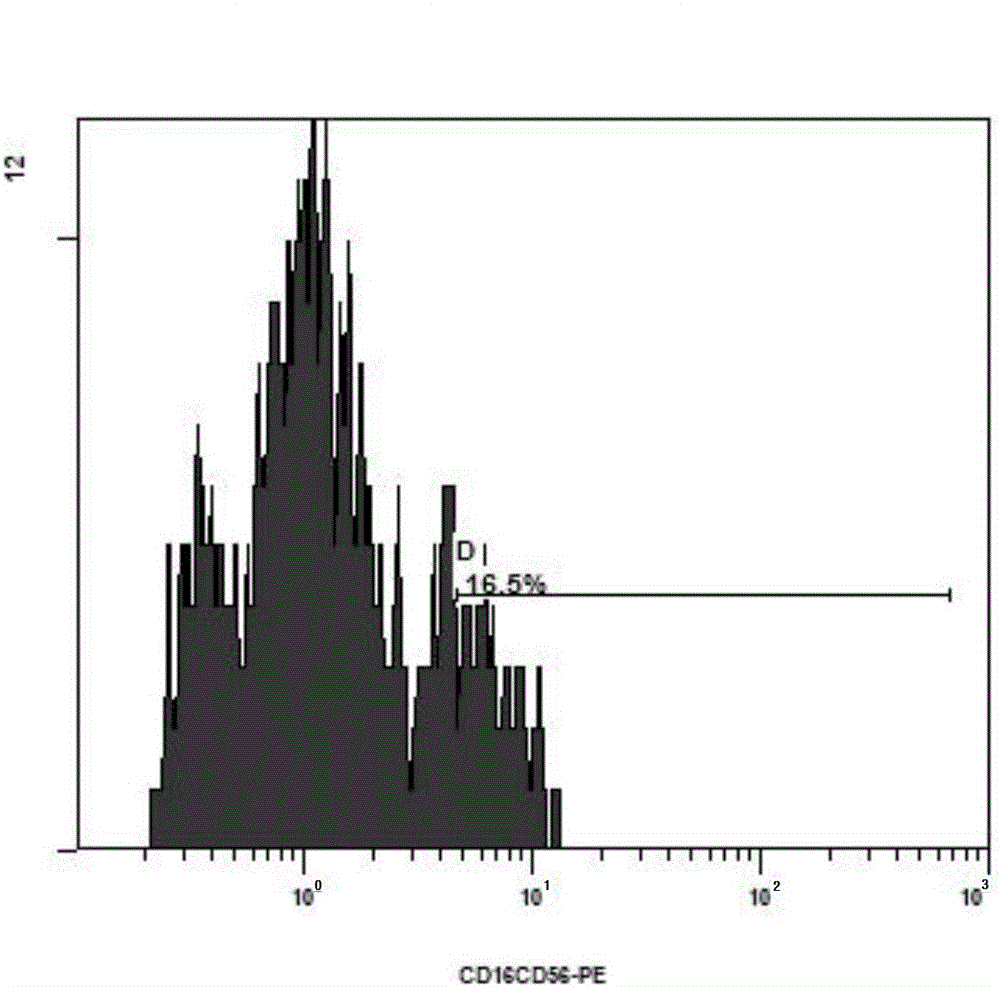
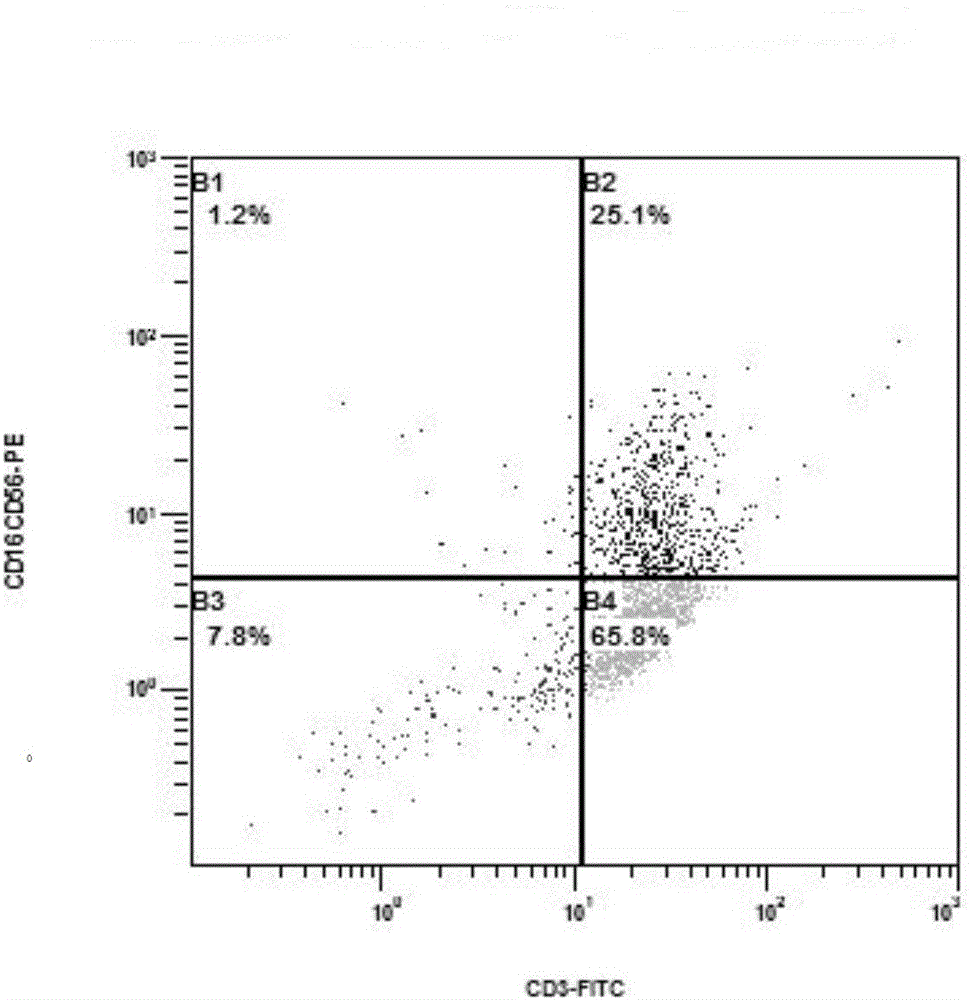
Preparation of mesothelin chimeric antigen receptor modified T cells and application of T cells in pancreatic cancer treatment
InactiveCN106543288AAntibody mimetics/scaffoldsMammal material medical ingredientsPancreas CarcinomaDrug
The invention provides preparation of mesothelin chimeric antigen receptor modified T cells and application of the T cells in pancreatic cancer treatment, discloses a chimeric antigen receptor, particularly relates to a chimeric antigen receptor which serves as a guiding sequence by combining a granulocyte-macrophage cluster stimulating factor receptor, and further relates to the T cells expressing the chimeric antigen receptor. The T cells can kill tumor cells highly expressing mesothelin in a targeted mode and have the application of preparing drugs for treating pancreatic cancer malignant tumors and virus infective diseases.
Owner:SHANDONG XINRUI BIOTECH CO LTD
Antibodies, pharmaceutical compositions and methods
ActiveUS20170283488A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsURINARY BLADDER CARCINOMASquamous Carcinomas
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA INC
Ras inhibitors and uses thereof
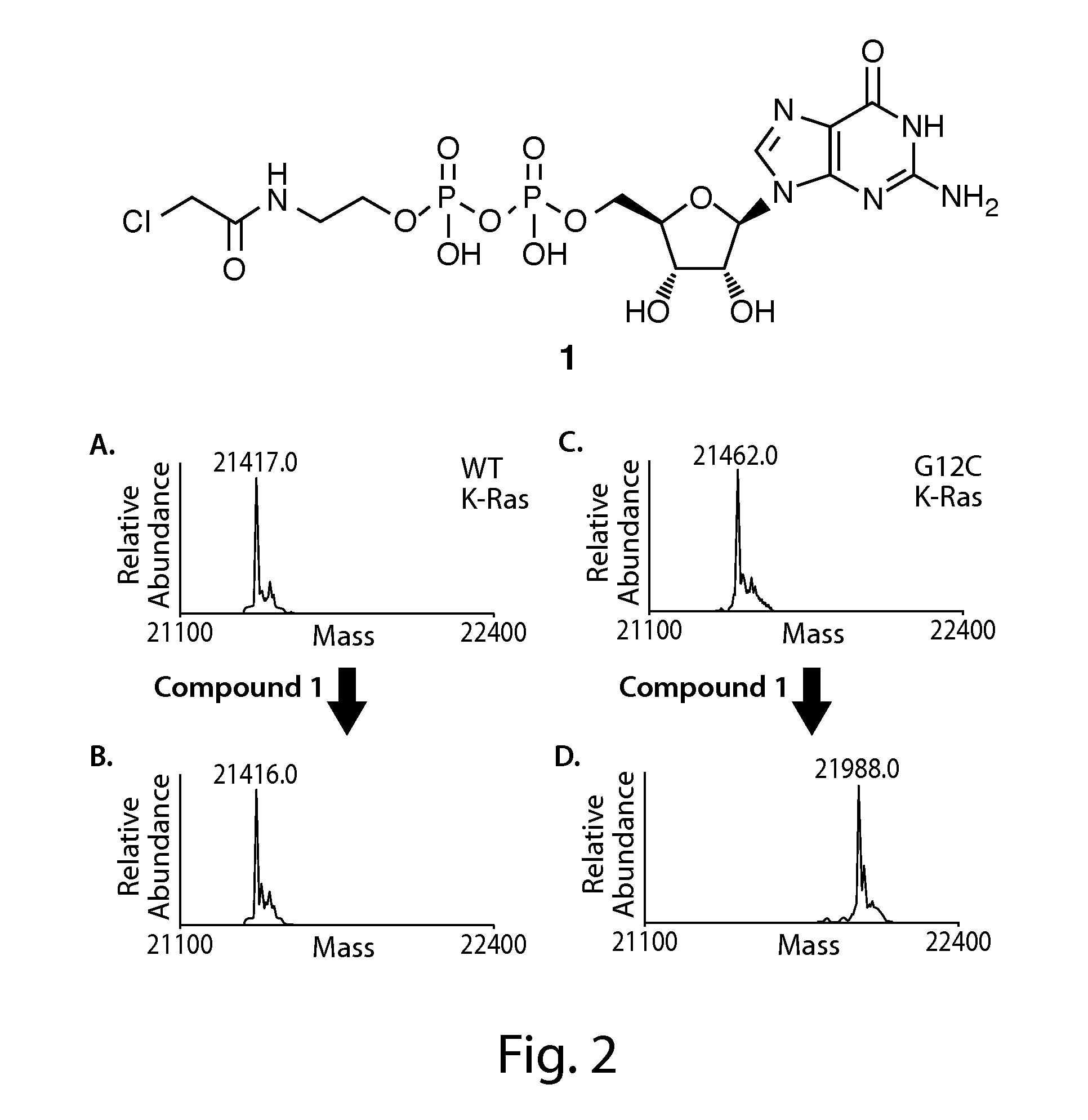
Described herein are compounds of Formulae (I)-(II), and pharmaceutically acceptable salts, and pharmaceutical compositions thereof. Also provided are methods and kits involving the inventive compounds or compositions for treating or preventing proliferative diseases such as cancers (e.g., lung cancer, large bowel cancer, pancreas cancer, biliary tract cancer, or endometrial cancer), benign neoplasms, angiogenesis, inflammatory diseases, autoinflammatory diseases, and autoimmune diseases in a subject.
Owner:DANA FARBER CANCER INST INC
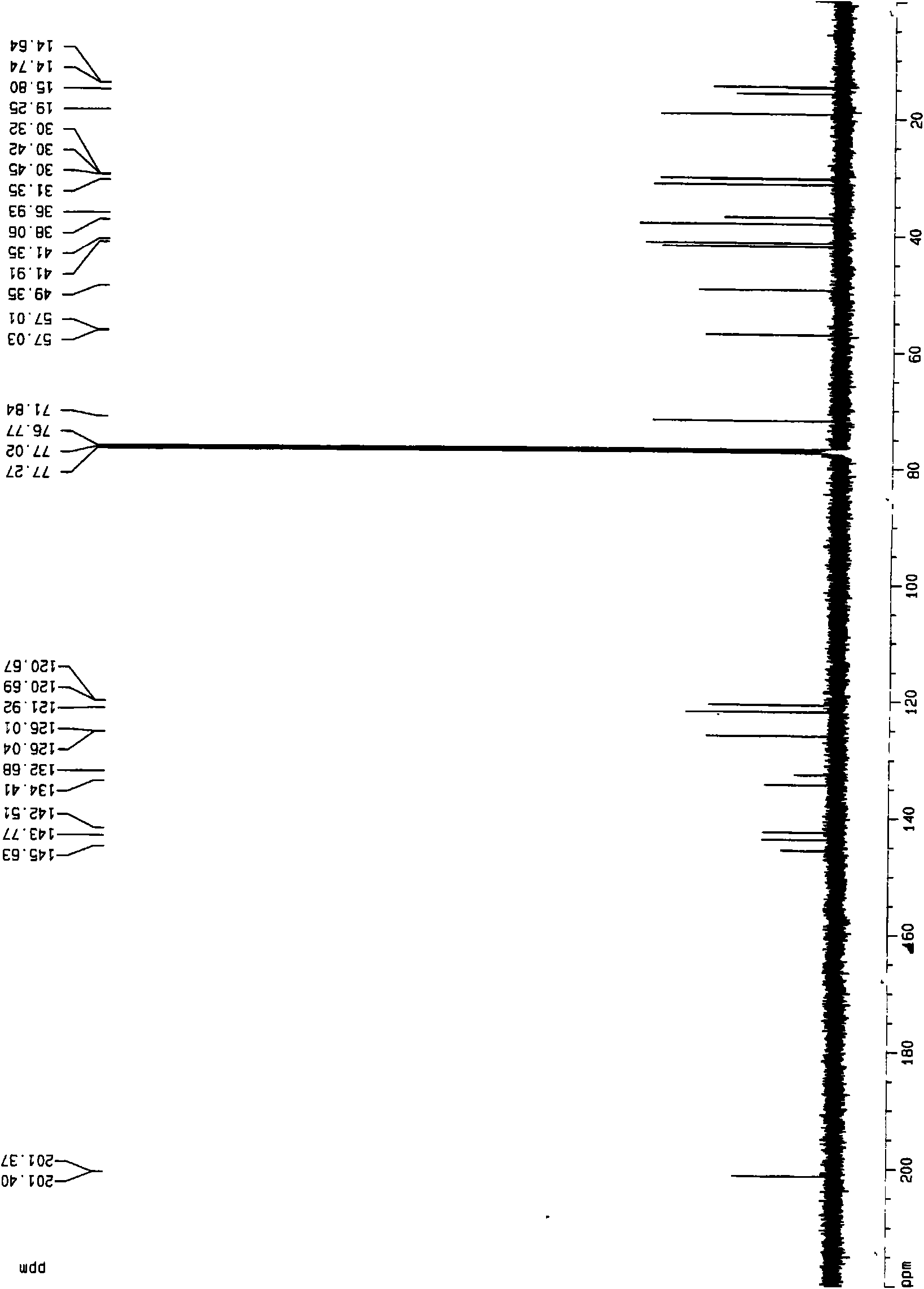
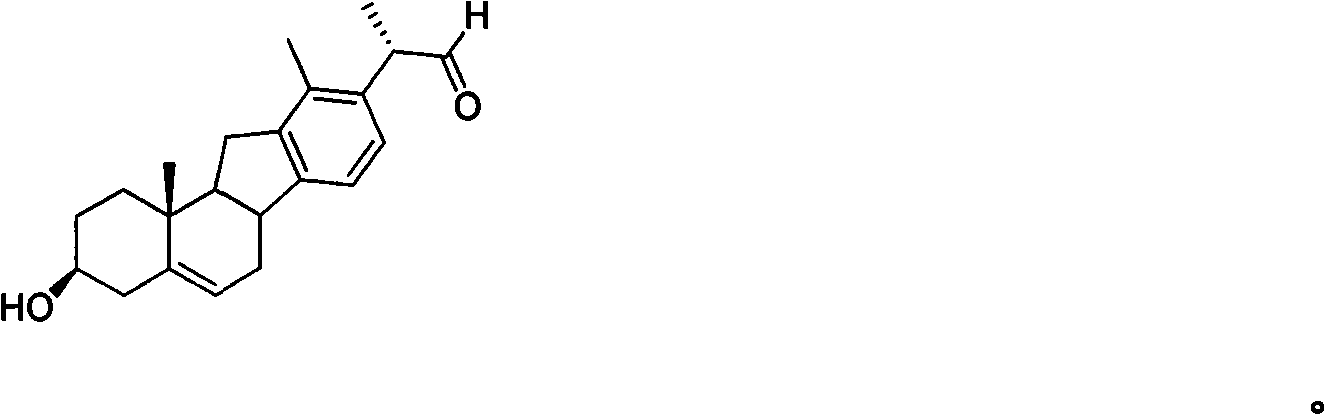
Veratramine degradation product veratrum fluorene aldehyde and the derivatives thereof, as well as the preparation and application thereof
InactiveCN101565446AEnhanced inhibitory effectOrganic active ingredientsSteroidsCancer cellSteroidal alkaloid
The invention provides a veratramine degradation product veratrum fluorene aldehyde 1 and derivatives thereof. The invention uses steroidal alkaloid veratramine (A) as raw material and prepares veratrum fluorene aldehyde by oxidative degradation of m-chloroperoxybenzoic acid to obtain 20 derivative compounds of the veratrum fluorene aldehyde 1 after further oxidation, reduction and condensation reaction. The 20 compounds of the invention have good inhibitory activity on cancer cells, wherein the compounds 1, 2, 5, 8, 13 and 17 have good inhibitory activity on cells of human pancreatic cancer cells BxPC-3 and SW1990, small-cell lung cancer cells NCI-H446, human colorectal cancer LOVO and the like, and the inhibitory activity of the compound veratrum fluorene aldehyde 1 is the most significant.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
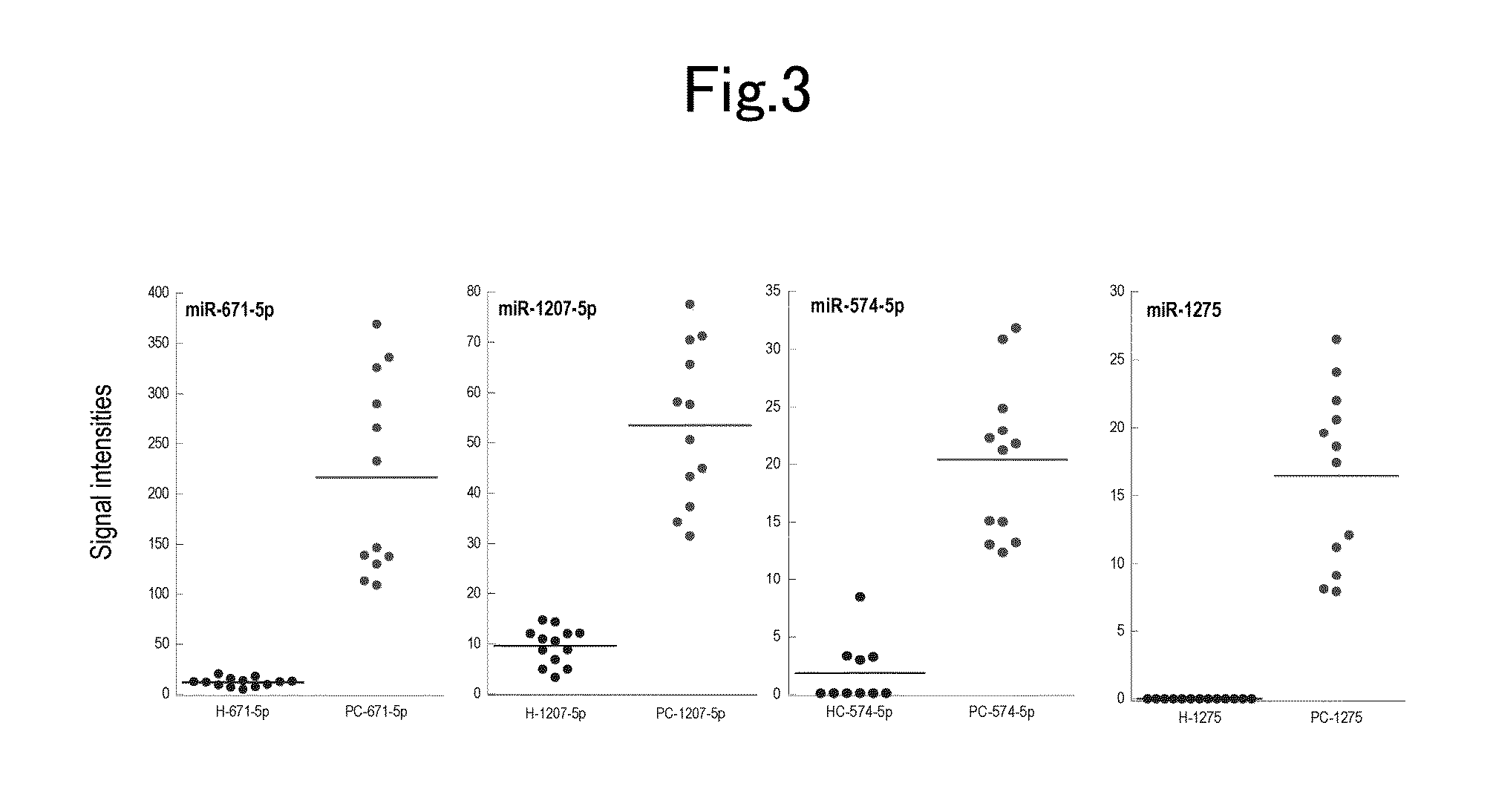
Method for detecting pancreatic cancer and detection kit
InactiveUS20150184248A1High sensitivityStrong specificityNucleotide librariesMicrobiological testing/measurementPancreas CancersOncology
An object of the invention is to provide a simple and easy method for detecting pancreatic cancer having high sensitivity and specificity or risk of the pancreatic cancer.The present invention provides a method of detecting pancreatic cancer including a step of measuring, in a sample derived from a subject, at least one miRNA selected from the following (i) and (ii):(i) an miRNA having a sequence represented by any of SEQ ID NO: 1 to 120; and(ii) an miRNA that hybridizes, under stringent conditions, with a nucleic acid having a sequence complementary to the miRNA (i) and having from 17 to 30 bases.
Owner:NAT CANCER CENT
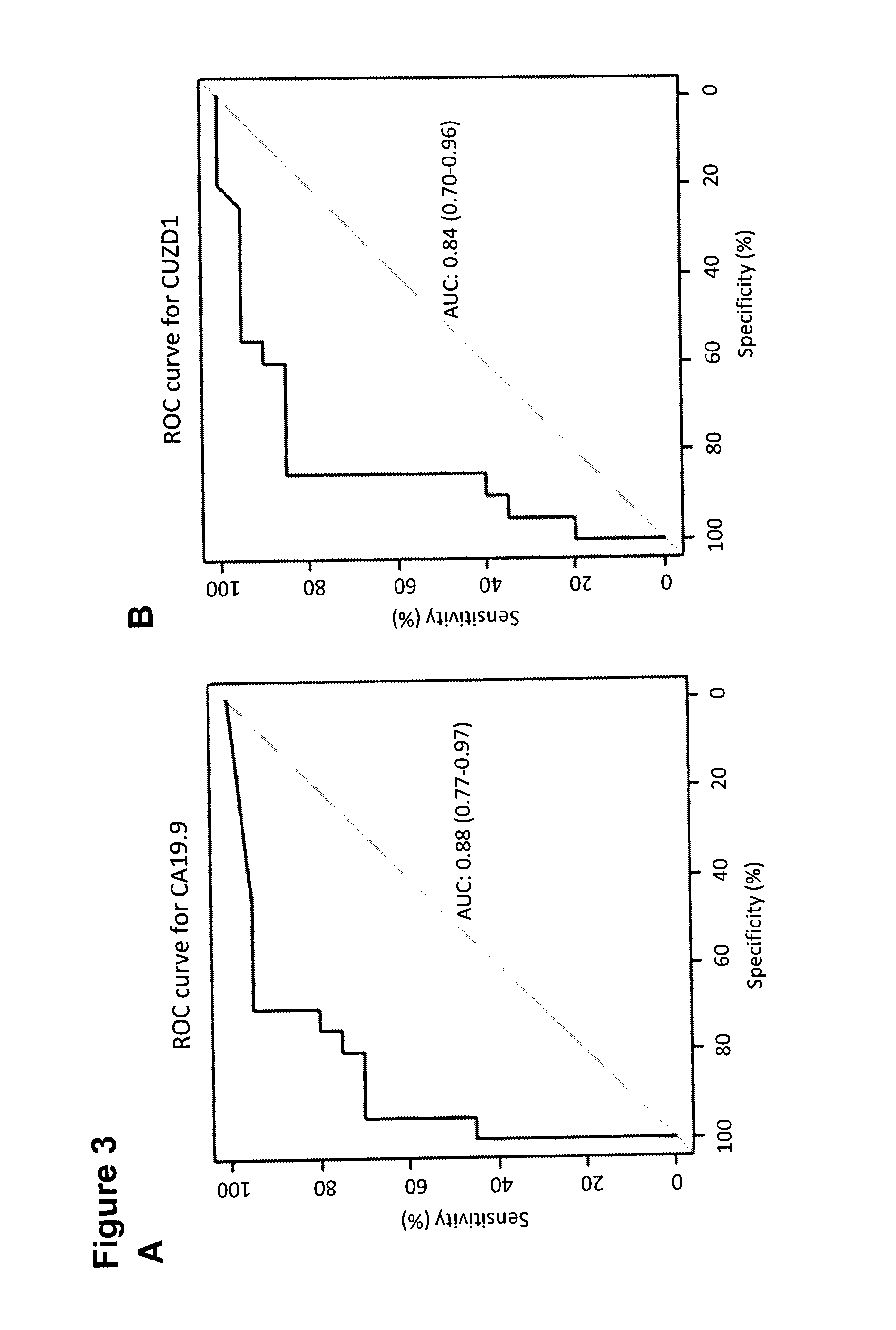
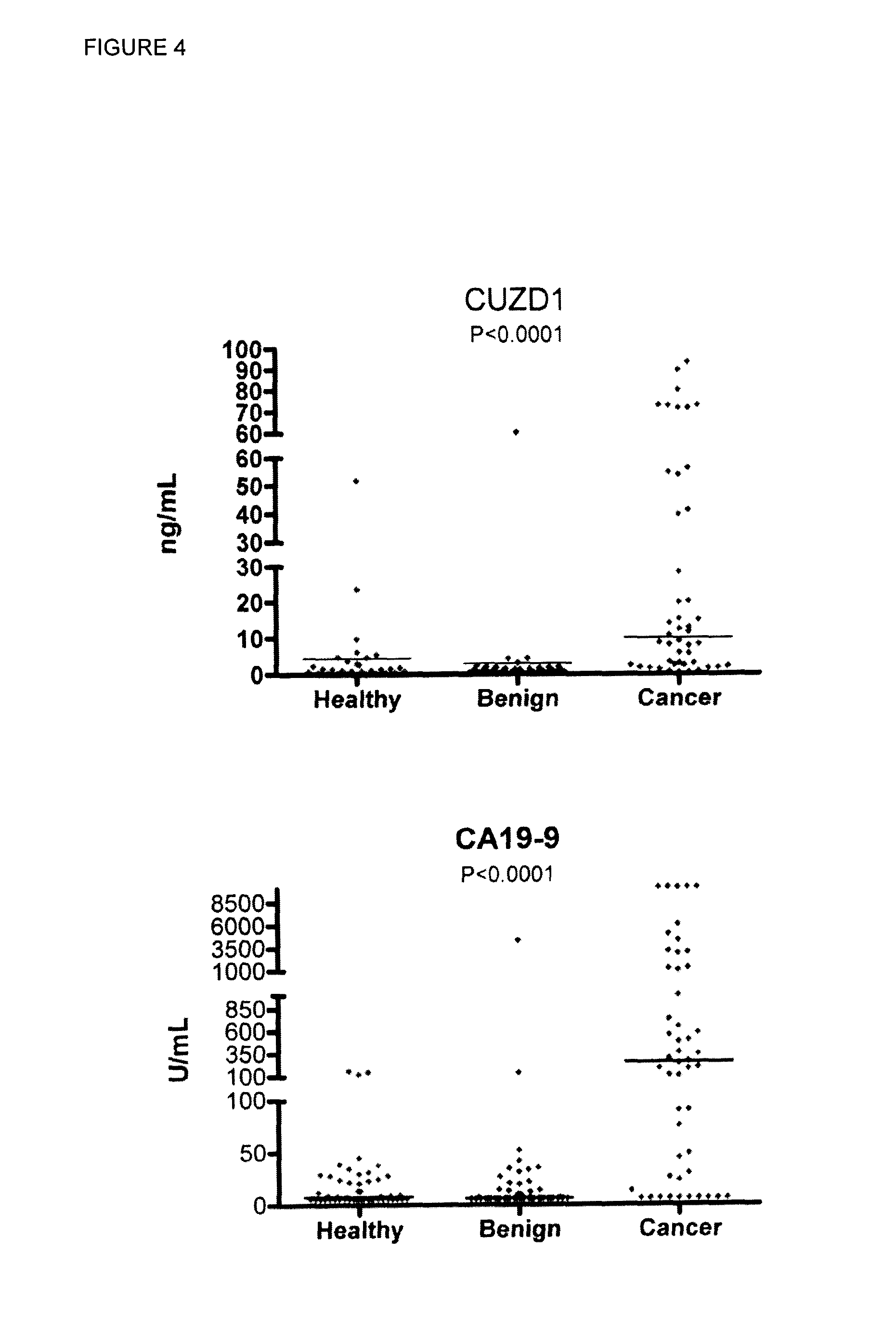
Cancer Biomarkers and Methods of Use
InactiveUS20150072349A1Increase probabilityMicrobiological testing/measurementBiological material analysisPancreas CancersTumor Biomarkers
A method of evaluating a probability a subject has a cancer, diagnosing a cancer and / or monitoring cancer progression comprising: a. measuring an amount of a biomarker selected from the group consisting of CUZD1 and / or LAMC2 and / or the group CUZD1, LAMC2, AQP8, CELA2B, CELA3B, CTRB1, CTRB2, GCG, IAPP, INS, KLK1, PNLIPRP1, PNLIPRP2, PPY, PRSS3, REG3G, SLC30A8, KLK3, NPY, PSCA, RLN1, SLC45A3, DSP, GP73, DSG2, CEACAM7, CLCA1, GPA33, LEFTY1, ZG16, IRX5, LAMP3, MFAP4, SCGB1A1, SFTPC, TMEM100, NPY, PSCA, RLN1 and / or SLC45A3 in a test sample from a subject with cancer; wherein the cancer is pancreas cancer if CUZD1, LAMC2, AQP8, CELA2B, CELA3B, CTRB1, CTRB2, GCG, LAPP, INS, KLK1, PNLIPRP1, PNLIPRP2, PPY, PRSS3, REG3G, SLC30A8, DSP, GP73 and / or DSG2 is selected; the cancer is colon cancer if CEACAM7, CLCA1, GPA33, LEFTY1 and / or ZG16 is selected, the cancer is lung cancer if IRX5, LAMP3, MFAP4, SCGB1A1, SFTPC and / or TMEM100 is selected; or the cancer is prostate cancer if NPY, PSCA, RLN1 and / or SLC45A3 is selected; b. comparing the measured amount to a control and detecting an increase in the amount of the biomarker compared to control; and c. identifying the subject as having or having an increased probability of having the cancer when an increase in the biomarker compared to control is detected.
Owner:UNIV HEALTH NETWORK
Drug delivery polymer and uses thereof
ActiveUS20160296631A1Stop growthStop the spreadOrganic active ingredientsPlatinum organic compoundsPancreas CancersProstate cancer
Described herein are platinum-based brush-arm star polymers (Pt-BASPs), or a pharmaceutical composition thereof, for delivery of platinum-based agents, such as cisplatin. Also provided are methods and kits involving the Pt-BASPs, or a pharmaceutical composition thereof, for treating proliferative diseases such as cancers (e.g., lung cancer, head-and-neck cancer, esophagus cancer, stomach cancer, breast cancer, pancreas cancer, liver cancer, kidney cancer, or prostate cancer) in a subject.
Owner:MASSACHUSETTS INST OF TECH
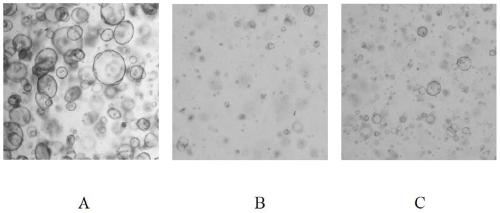
Method for separation and in-vitro culture of pancreatic cancer tissue organs of human
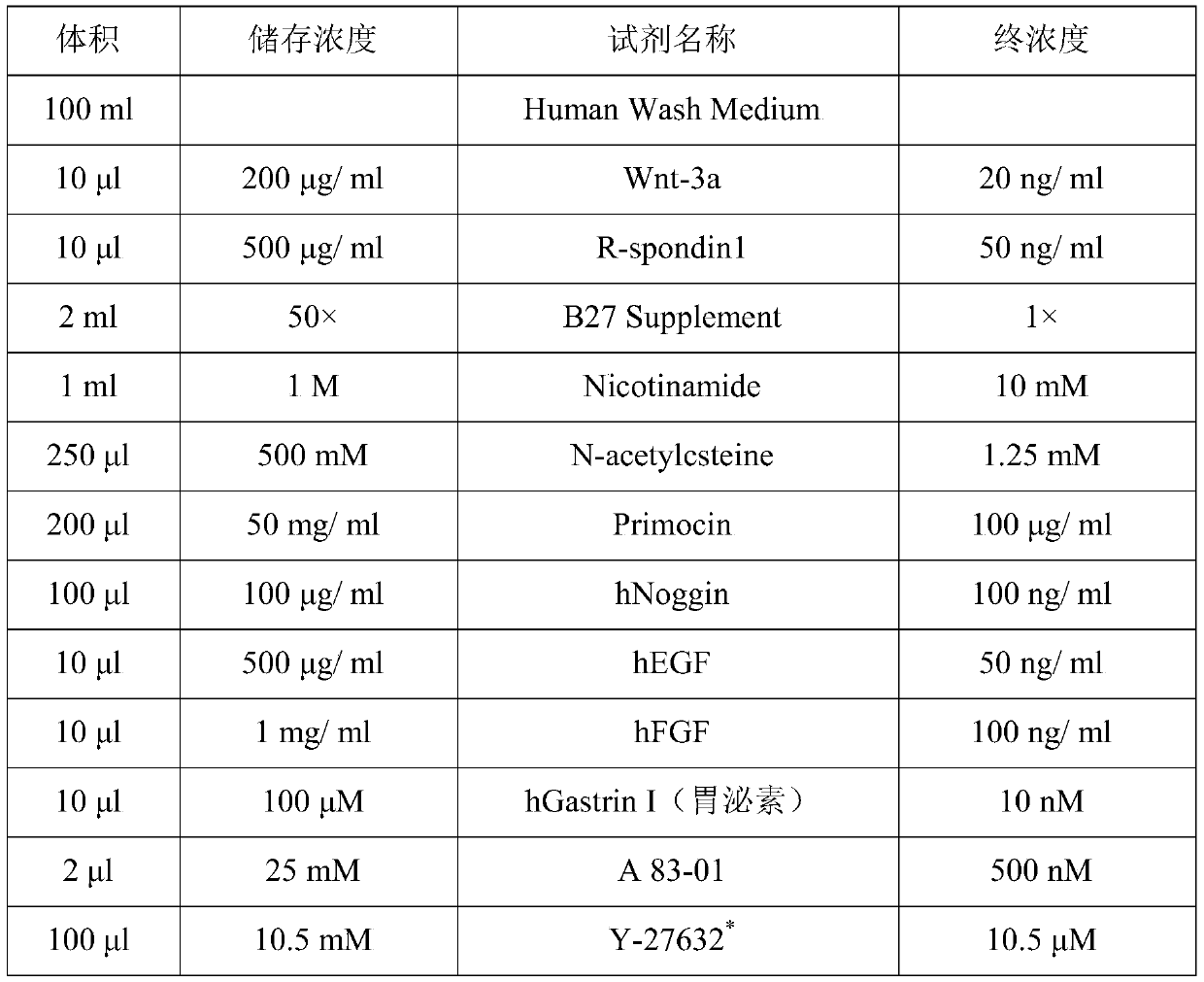
ActiveCN110317790AMaintain genetic heterogeneityImprove performanceCell dissociation methodsCulture processMatrigelDigestion
The invention discloses a method for separation and in-vitro culture of pancreatic cancer tissue organs of human. The method comprises the following steps that (1) after the pancreatic cancer tissue of human is cut, enzymic digestion is repeatedly carried out for 10-20 minutes at 37 DEG C in a rotation speed of 30-40 rpm three times, and a pancreatic cancer single cell is obtained; (2) the pancreatic cancer single cell and matrigel are uniformly mixed, then a culture medium containing a growth factor PEG2 and gastrin I are added for culture, and the pancreatic cancer tissue organs of human areobtained. The method for separation and in-vitro culture of the pancreatic cancer tissue organs of human has the advantages that the tumor tissue is digested by adopting a short-time repeated digestion method, which can effectively improve the cell yield and cell viability; the culture medium containing the growth factor PEG2 and the gastrin I is adopted for culture, which can effectively promotethe growth of the pancreatic cancer organs and increase the survival rate of the pancreatic cancer organs.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Methods for damaging cells using effector functions of Anti-cdh3 antibodies
InactiveUS20090169572A1Strong cytotoxicityInduce cytotoxicitySnake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCytotoxicityProstate cancer
The present invention relates to the use of cytotoxicity based on the effector function of anti-CDH3 antibodies. Specifically, the present invention provides methods and pharmaceutical compositions that comprise an anti-CDH3 antibody as an active ingredient for damaging CDH3-expressing cells using antibody effector function. Since CDH3 is strongly expressed in pancreatic, lung, colon, prostate, breast, gastric or liver cancer cells, the present invention is useful in pancreatic, lung, colon, prostate, breast, gastric or liver cancer therapies.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD +1
Blood serum/blood plasma micro ribonucleic acid (miRNA) marker relevant with pancreatic cancer and application thereof
ActiveCN102876676AAids in diagnosisEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationBlood plasmaGenetic engineering
The invention belongs to the fields of genetic engineering and oncology, and discloses a blood serum / blood plasma micro ribonucleic acid (miRNA) marker relevant with pancreatic cancer and application thereof. The marker is formed by combining miR-451 and miR-490-3p. The marker and primers of the marker can be used for preparing a diagnostic kit and are used for the auxiliary diagnosis of pancreatic cancer.
Owner:NANJING MEDICAL UNIV
Detection of micro metastasis of melanoma and breast cancer in paraffin-embedded tumor draining lymph nodes by multimarker quantitative RT-PCR
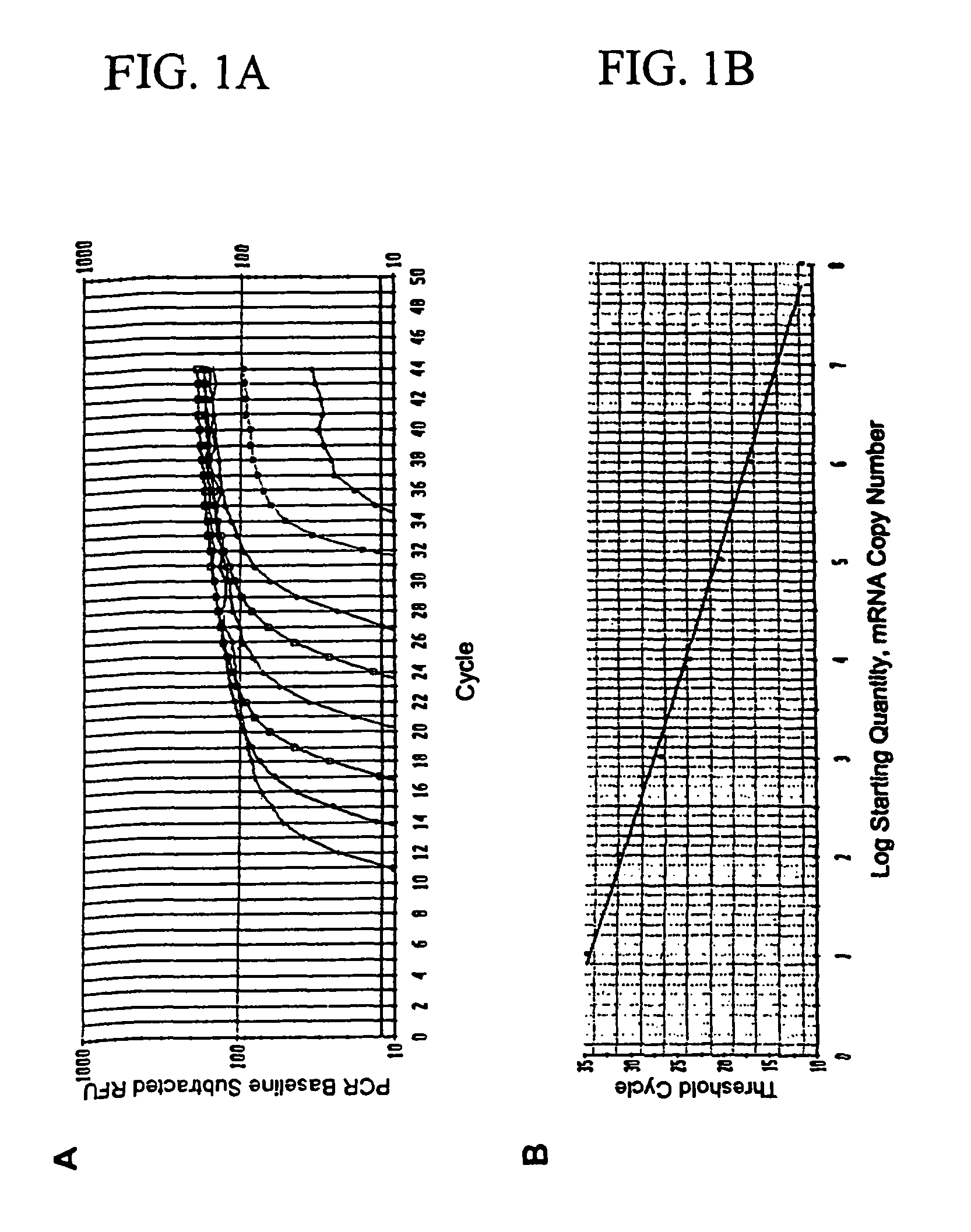
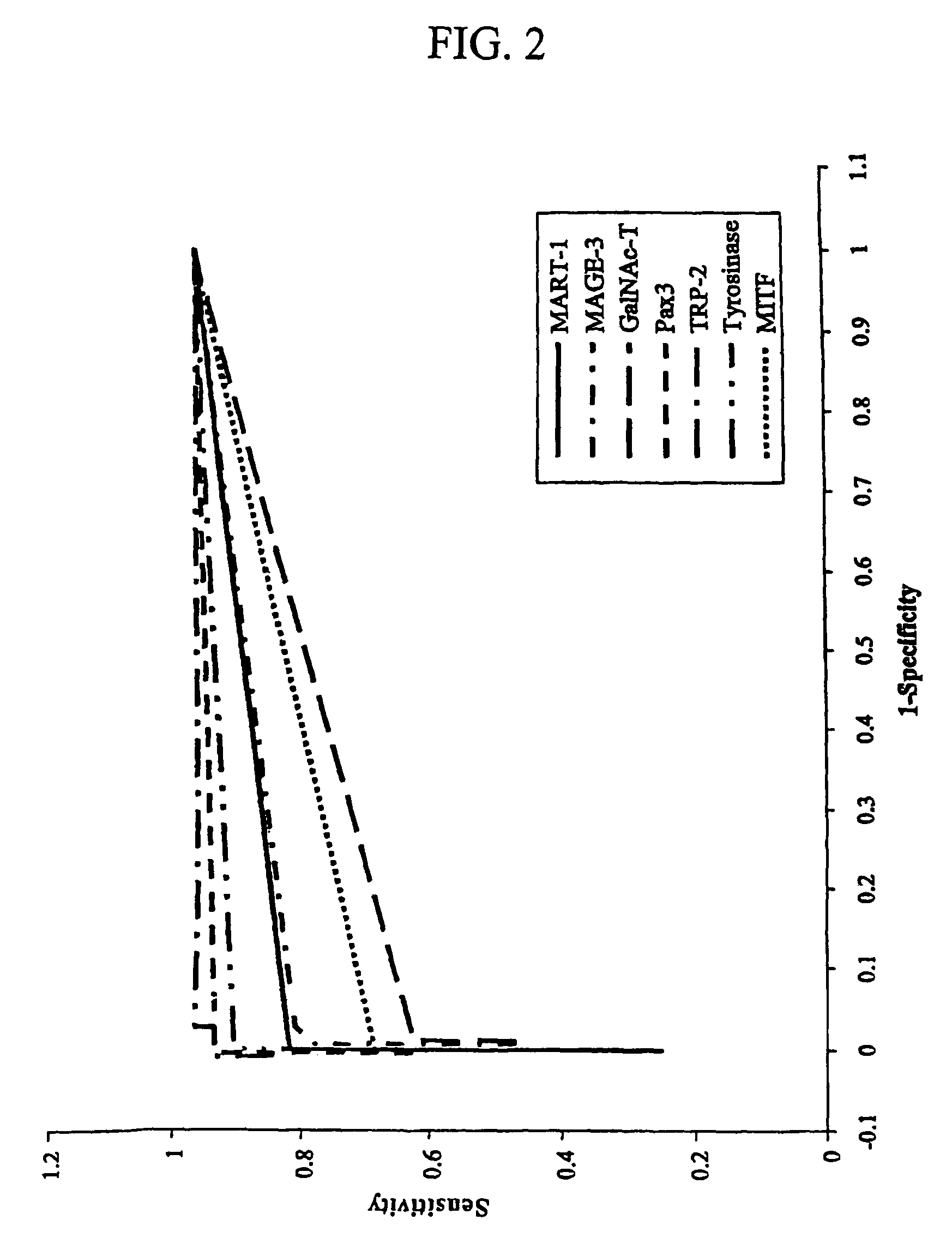
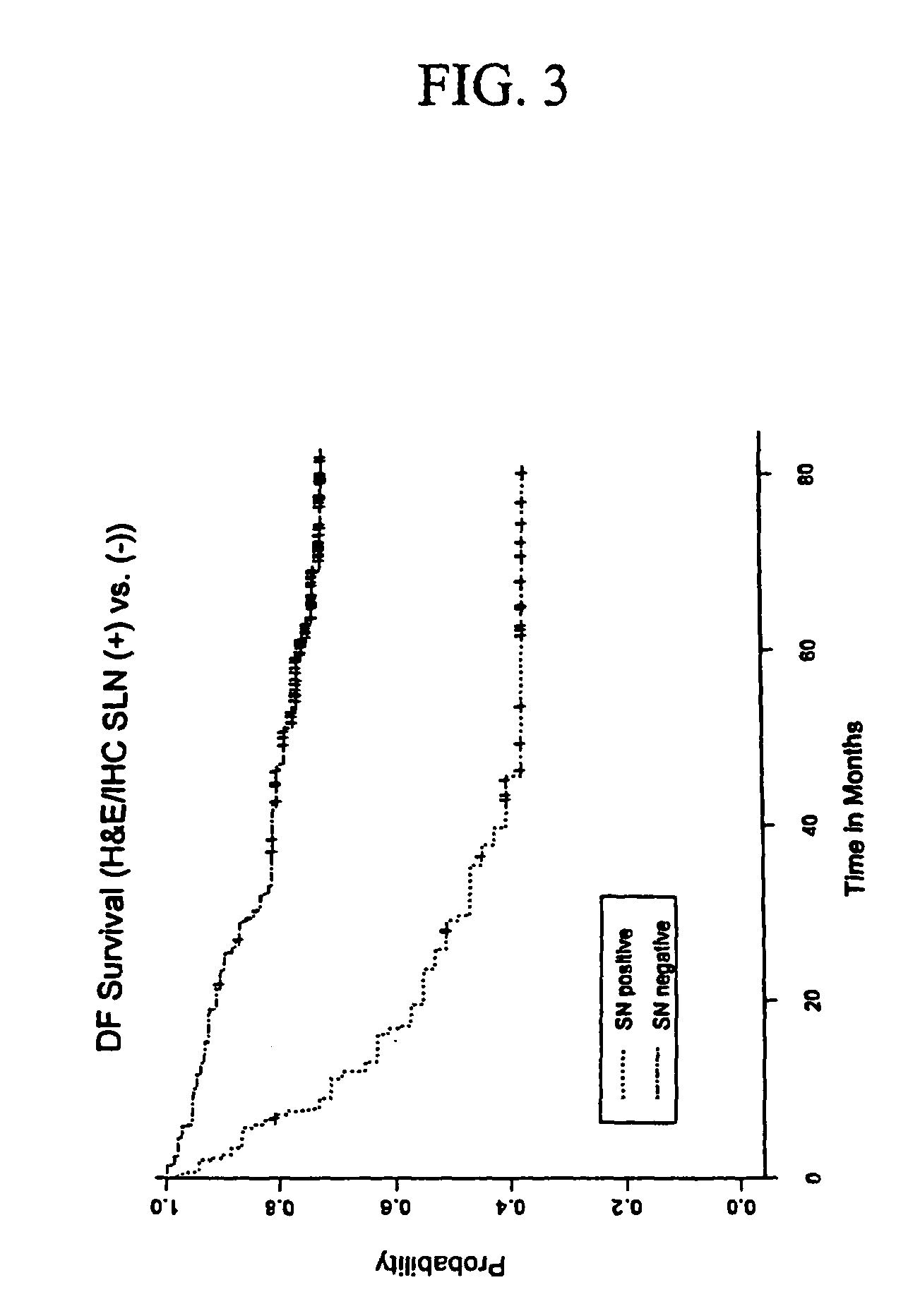
InactiveUS7910295B2High sensitivityStrong specificityMicrobiological testing/measurementBiological testingAbnormal tissue growthMetastatic melanoma
The invention provides a quantitative realtime RT-PCR assay for detection of metastatic breast, gastric, pancreas or colon cancer cells or metastatic melanoma. The assay allows to predict disease recurrence and survival in patients with AJCC stage I and II, and III disease using multimarker panels. The method for detecting metastatic melanoma cells utilizes panels of markers selected from a group consisting of MAGE-A3, GalNAcT, MART-1, PAX3, Mitf, TRP-2, and Tyrosinase. The method for detecting metastatic breast, gastric, pancreas or colon cancer cells in paraffin-embedded samples utilizes panels of markers selected from a group consisting of C-Met, MAGE-A3, Stanniocalcin-1, mammoglobin, HSP27, GalNAcT, CK20, and β-HCG.
Owner:JOHN WAYNE CANCER INST
Deuterium-Enriched Pyrimidine Compounds and Derivatives
The present invention is concerned with deuterium-enriched pyrimidine compounds of formula I, their derivatives, enantiomers, diastereomers, solvates and pharmaceutical salts thereof,and their uses in the treatment, prevention and modulation of various diseases including chronic liver diseases, liver cirrhosis, liver fibrosis, hepatocellular carcinoma, liver cancer, renal cell carcinoma, kidney cancer, colorectal cancer, brain cancer, breast cancer, blood cancer, lung cancer, thyroid cancer, ovarian cancer, pancreas cancer, prostate cancer, stomach cancer, testicular cancer, uterus cancer, intestinal cancer, skin cancer, and other forms of cancer, carcinoid tumors, teratocarcinoma, tumor progression, metastasis and fibrosis in the neuroendocrine neoplasia, fibrotic processes as well as a disease state modulated directly or indirectly with 5-HT receptors, 5-HT1, 5-HT1A, 5-HT2 receptors, 5-HT2A and 5-HT2B receptors, dopamine receptors and multiple kinase pathways.
Owner:DHANOA DALJIT SINGH
Anti-mucin antibodies for early detection and treatment of pancreatic cancer
InactiveUS20140227179A1Strong specificityHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsEpitopeSerum samples
Described herein are compositions and methods of use of anti-pancreatic cancer antibodies or fragments thereof, such as murine, chimeric, humanized or human PAM4 antibodies. The antibodies show novel and useful diagnostic characteristics, such as binding with high specificity to pancreatic and other cancers, but not to normal or benign pancreatic tissues and binding to a high percentage of early stage pancreatic cancers. Preferably, the antibodies bind to an epitope located within the second to fourth cysteine-rich domains of MUC5ac (amino acid residues 1575-2052) and are of use for the detection and diagnosis of early stage pancreatic cancer. In more preferred embodiments, the anti-pancreatic cancer antibodies can be used for immunoassay of serum samples, wherein the immunoassay detects a marker for early stage pancreatic cancer in serum. Most preferably, the serum is extracted with an organic phase, such as butanol, before immunoassay.
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a208a788-d164-4d46-bdfe-b503be2f9d03/US08450340-20130528-C00001.png)
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a208a788-d164-4d46-bdfe-b503be2f9d03/US08450340-20130528-C00002.png)
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a208a788-d164-4d46-bdfe-b503be2f9d03/US08450340-20130528-C00003.png)

![1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof 1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b447f0f5-f312-4b8c-a534-1c9837ef1f72/US20110190290A1-20110804-C00001.png)
![1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof 1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b447f0f5-f312-4b8c-a534-1c9837ef1f72/US20110190290A1-20110804-C00002.png)
![1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof 1h-pyrazolo[3,4-b]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b447f0f5-f312-4b8c-a534-1c9837ef1f72/US20110190290A1-20110804-C00003.png)