Antibodies, pharmaceutical compositions and methods
a technology of compositions and antibodies, applied in the field of antibodies, pharmaceutical compositions and methods, can solve the problems of breast cancer aggressiveness and poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fusion and Screening
[0376]A classical hybridoma fusion was performed. Mice received their first immunization with SSEA-4-DT-CRM197 (diphtheria toxin cross reacting material 197) conjugated with OBI-821 saponin adjuvant. Each mouse was administrated with designated treatment via subcutaneous (s.c.) injection at both left and right abdominal sites (100 μL / site). The mice were dosed four times (Day 0, Day 7, Day 14, and Day 23). Blood sample (approximately 0.1 mL whole blood / time point / animal) was obtained through facial vein without anticoagulant before Day 0 (pre-dose), Day 10, Day 17, Day 26 and on the sacrifice day (Day 35, volume of whole blood was collected through facial vein and heart punch). Coagulated blood samples were centrifuged (1500×g, 15 min, 4° C.), and serum was separated and transferred into eppendorf tubes. All serum samples were tested for titers of anti-SSEA-4 antibody. Five mice were found to produce high levels of anti-SSEA-4 IgG and IgM and were used for hybrid...
example 2
nt of the Anti-Tumor Activity of the Exemplary Antibody in Nude Mice
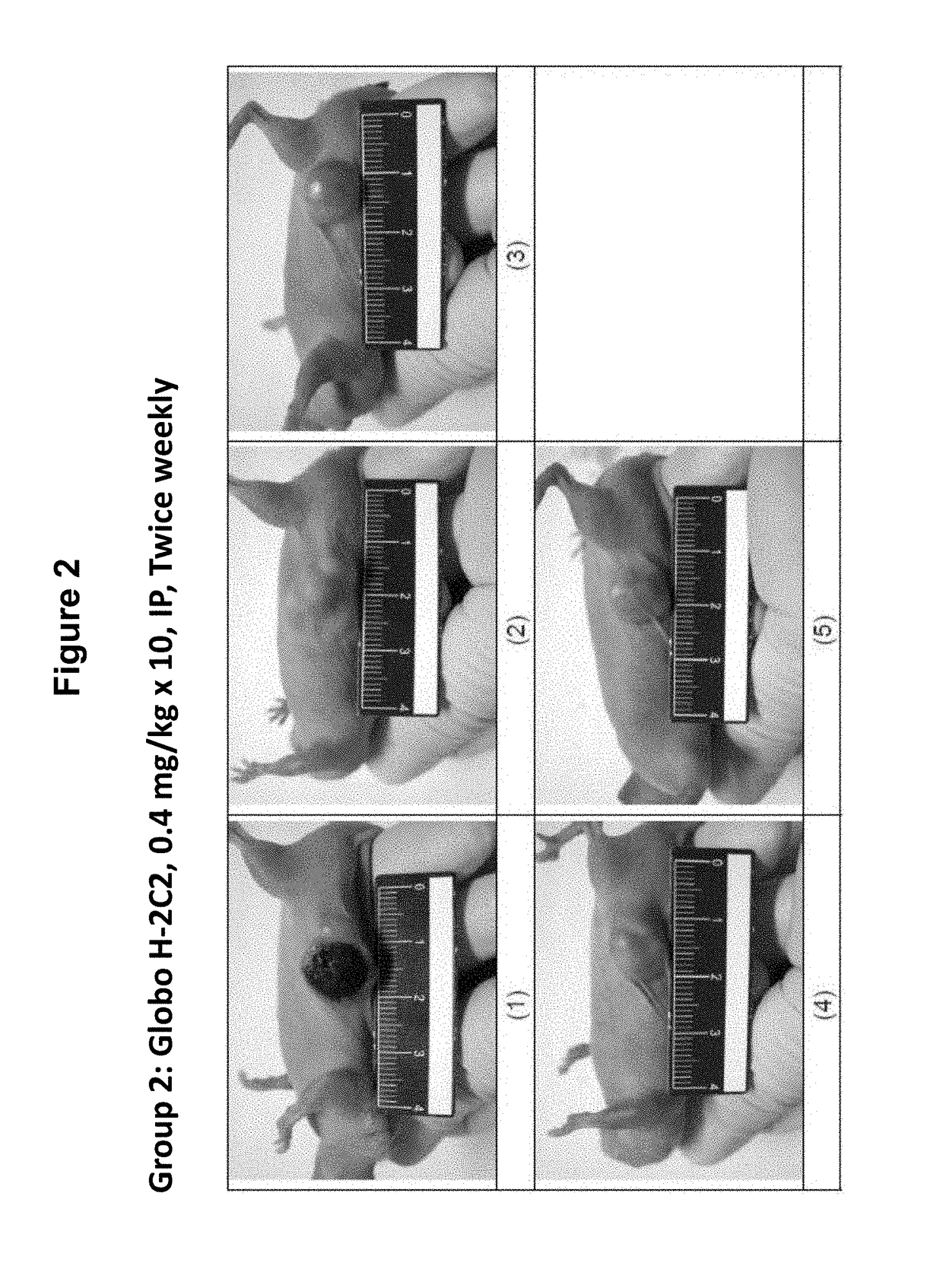
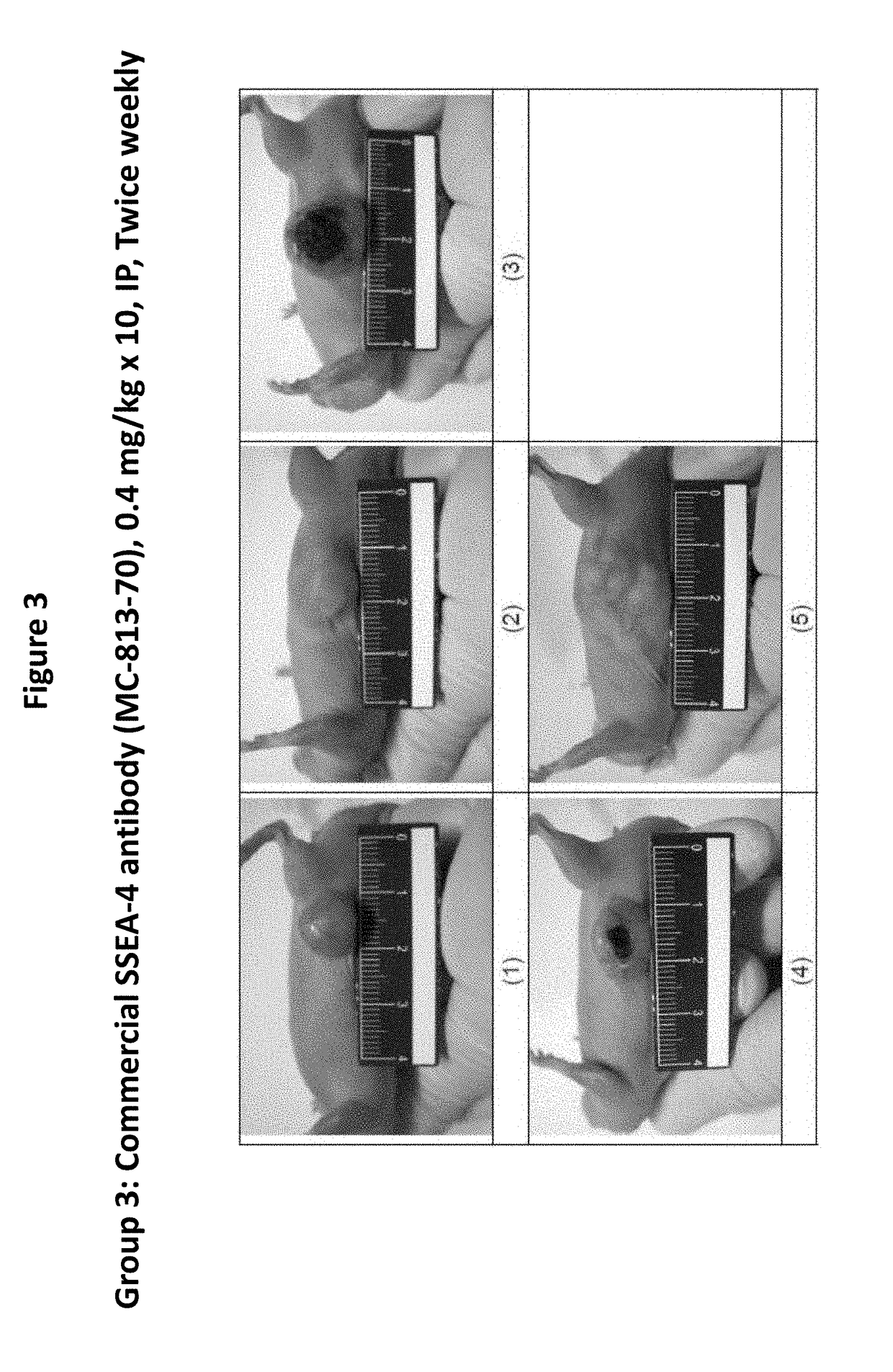
[0377]In a xenograft tumor model of human pancreatic adenocarcinoma, HPAC cells were subcutaneously (s.c.) implanted into BALB / c male nude mice and test articles were administered at 0.4 mg / kg by intraperitoneal (i.p.) injection twice weekly for 5 weeks starting when the average tumor size reached 50-120 mm3. The tumor size was monitored and recorded twice weekly for 36 days. Mortality, body weight, and signs of overt animal toxicity were also recorded twice weekly for 36 days. Tumor growth was calculated as T / C (treatment / control)×100%.
Test Substances and Dosing Pattern
[0378]Test substances were prepared at 1.45, 1, or 0.5 mg / mL in liquid form. Test substances were freshly prepared each day before dosing by diluting stocks solutions with phosphate buffer saline (PBS, pH7.4) to obtain the designated dosing concentration (0.04 mg / mL). Test substances were administered intraperitoneally (i.p.) at a dosing volume at 10...
example 3
tion of Cell Binding Ability of the Exemplary Antibody with MCF7 by FACS
Cell Culture
[0387]MCF-7 cell were cultured in Minimum essential medium (Invitrogen, Cat #10370021) with 2 mM L-glutamine (Invitrogen, Cat #25030081) and 1 mM sodium pyruvate (Invitrogen, Cat #11360070) and supplemented with 0.01 mg / mL insulin (Sigma, Cat #SI-I9278-5 mL), 10% fetal bovine serum (Invitrogen, Cat #16000044).
[0388]Test cells were suspended by discarding media from the test sample flask containing monolayer cells. Cells were rinsed with 5 mL PBS twice. 1 mL of 0.05% trypsin was added to flask, swirled to cover all surface area, and put in a 37° C. CO2 incubator for 5-10 minutes. 5 mL of complete growth medium was added. Cells were aspirated by pipetting gently. Cell suspension was transferred to a 15 mL conical tube. The tube was centrifuged for 5 minutes at 200 g. Following centrifugation, the supernatant was removed, and the cell pellet was agitated.
[0389]1-2 mL FACS buffe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com