Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
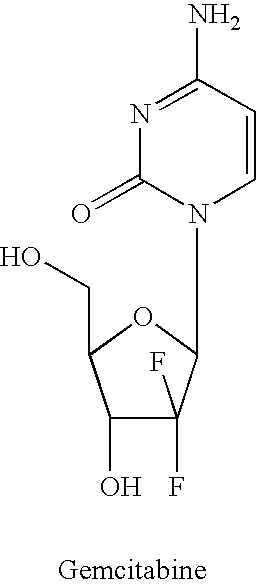
439 results about "Gemcitabine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gemcitabine is used to treat certain types of cancer (including breast, lung, ovarian, pancreatic).
Therapy of platinum-resistant cancer
The present invention concerns a method for treating platinum-resistant, ovarian cancer, primary peritoneal carcinoma or fallopian tube carcinoma with the combination of a HER2 antibody that effectively inhibits HER dimerization as well as gemcitabine.
Owner:GENENTECH INC
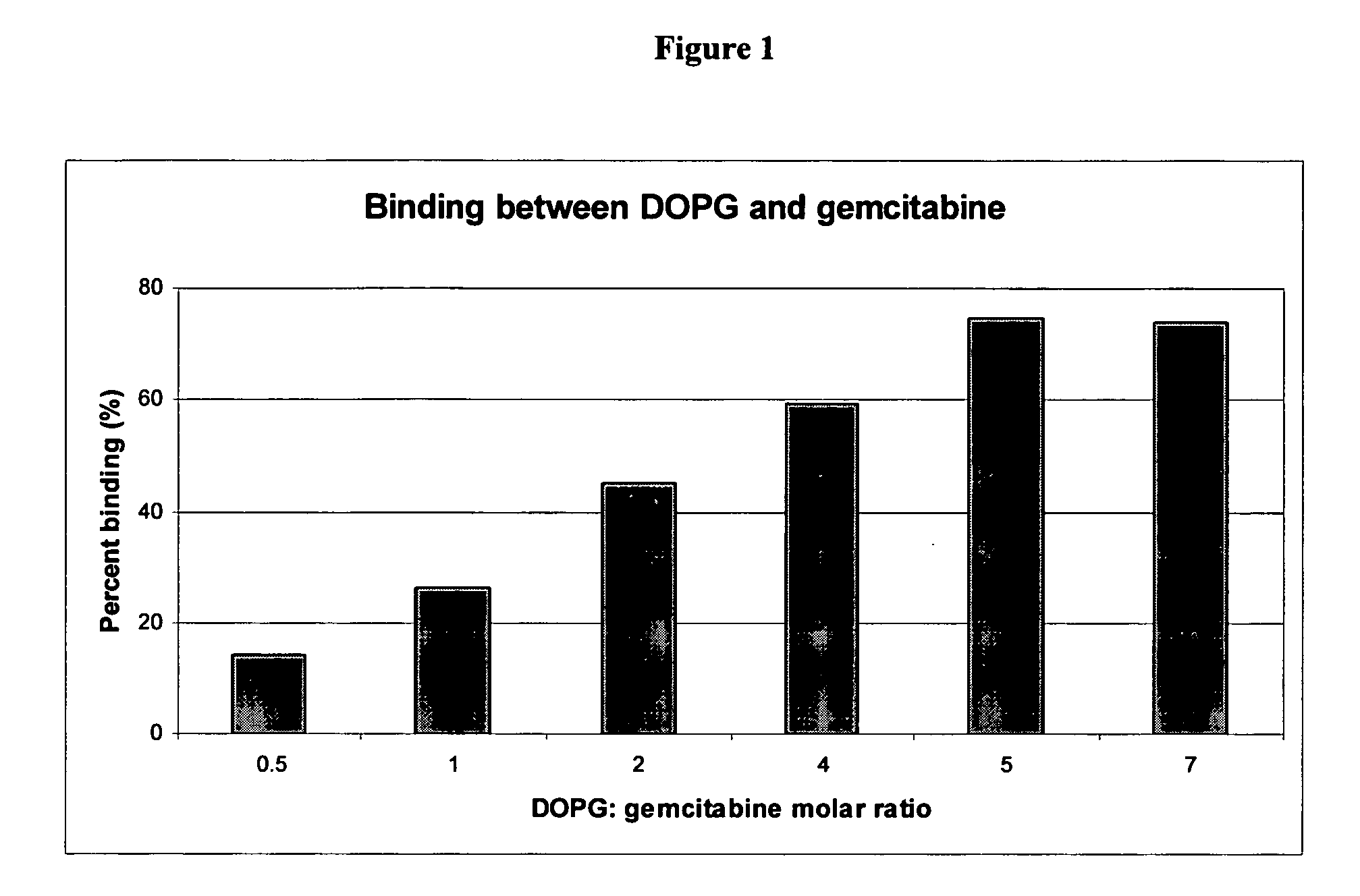
Gemcitabine compositions for better drug delivery
InactiveUS20050249795A1Inhibition of dissolutionHigh gemcitabineBiocidePhosphorous compound active ingredientsCompound (substance)Cardiolipin
Owner:NEOPHARMA INC
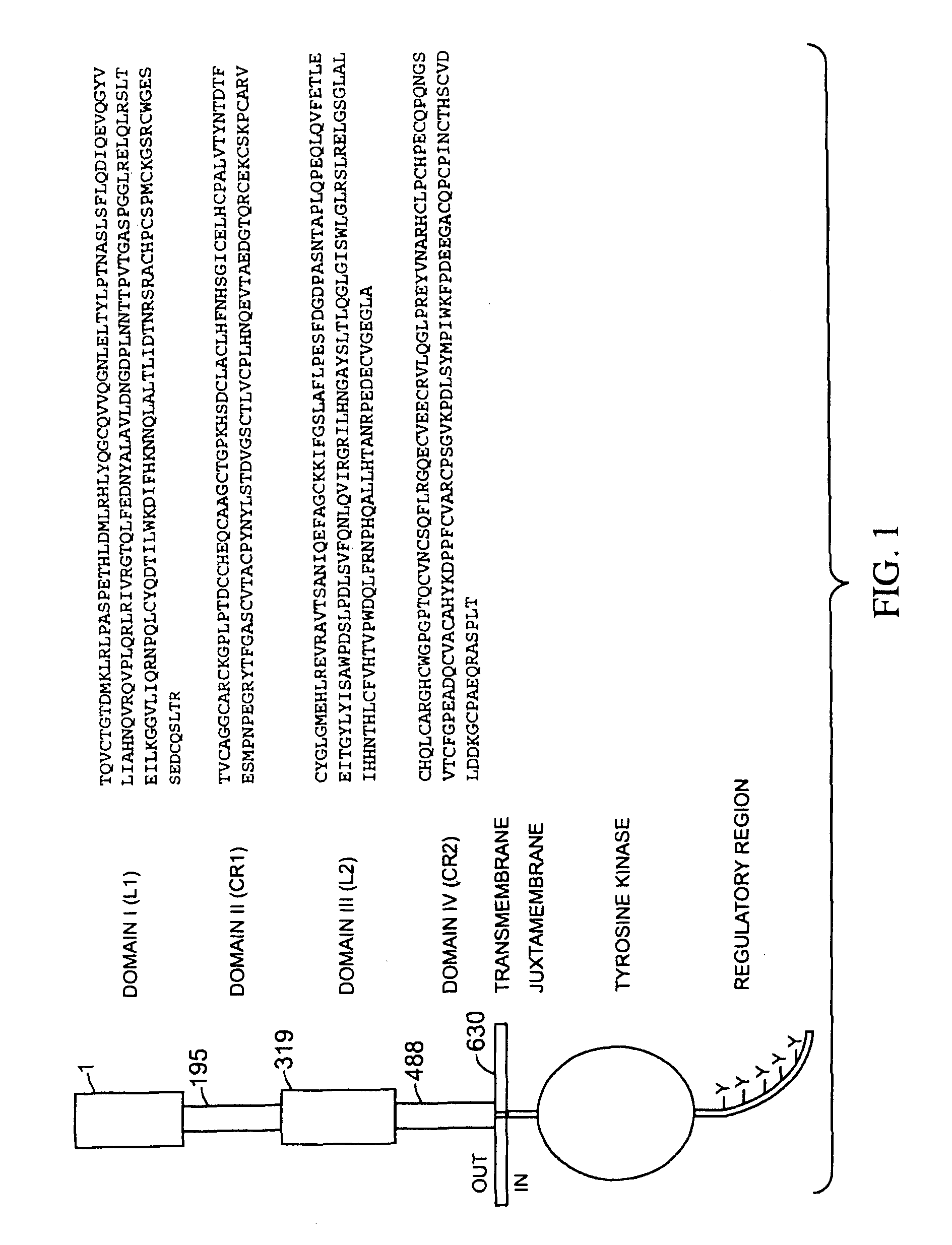
Predicting response to a HER inhibitor
InactiveUS7981418B2Improve responseOrganic active ingredientsData processing applicationsObstetricsSelection criterion
The present application describes the use of low HER3 as a selection criterion for treating patients with a HER inhibitor, such as pertuzumab.It also describes the use of high HER2:HER3 ratio as a selection criterion for treating cancer patients, such as ovarian cancer patients, with a HER inhibitor, such as pertuzumab.In addition, the application describes the use of high HER3 as a selection criterion for treating cancer patients with a chemotherapeutic agent, for instance gemcitabine.
Owner:F HOFFMANN LA ROCHE & CO AG
Combination therapy with synthetic triterpenoids and gemcitabine
The present invention concerns methods for treating cancer, such as pancreatic cancer, using combination therapies, including the combination of a synthetic triterpenoid, e.g., CDDO-Me, and gemcitabine.
Owner:REATA PHARMA INC +1
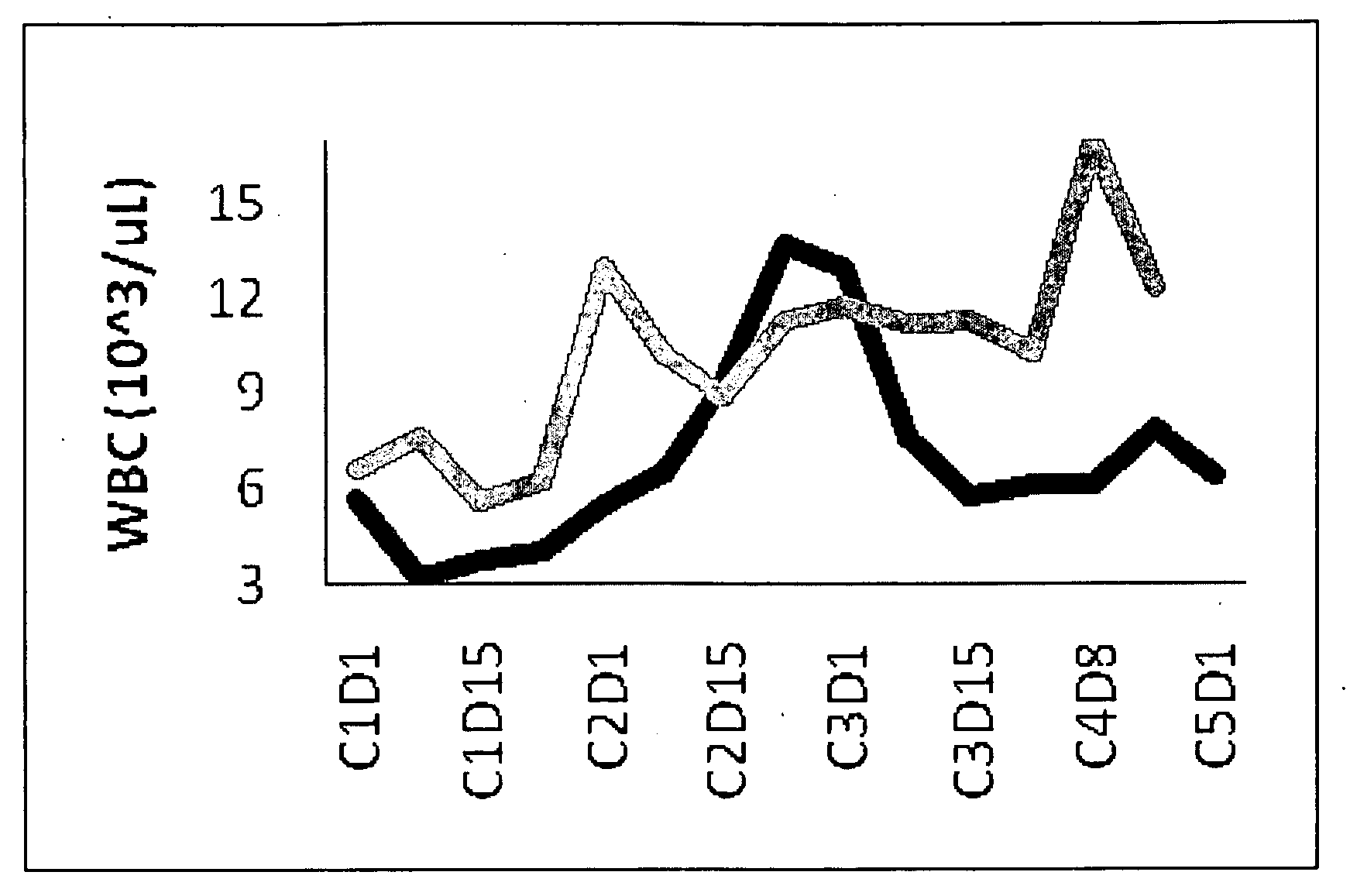
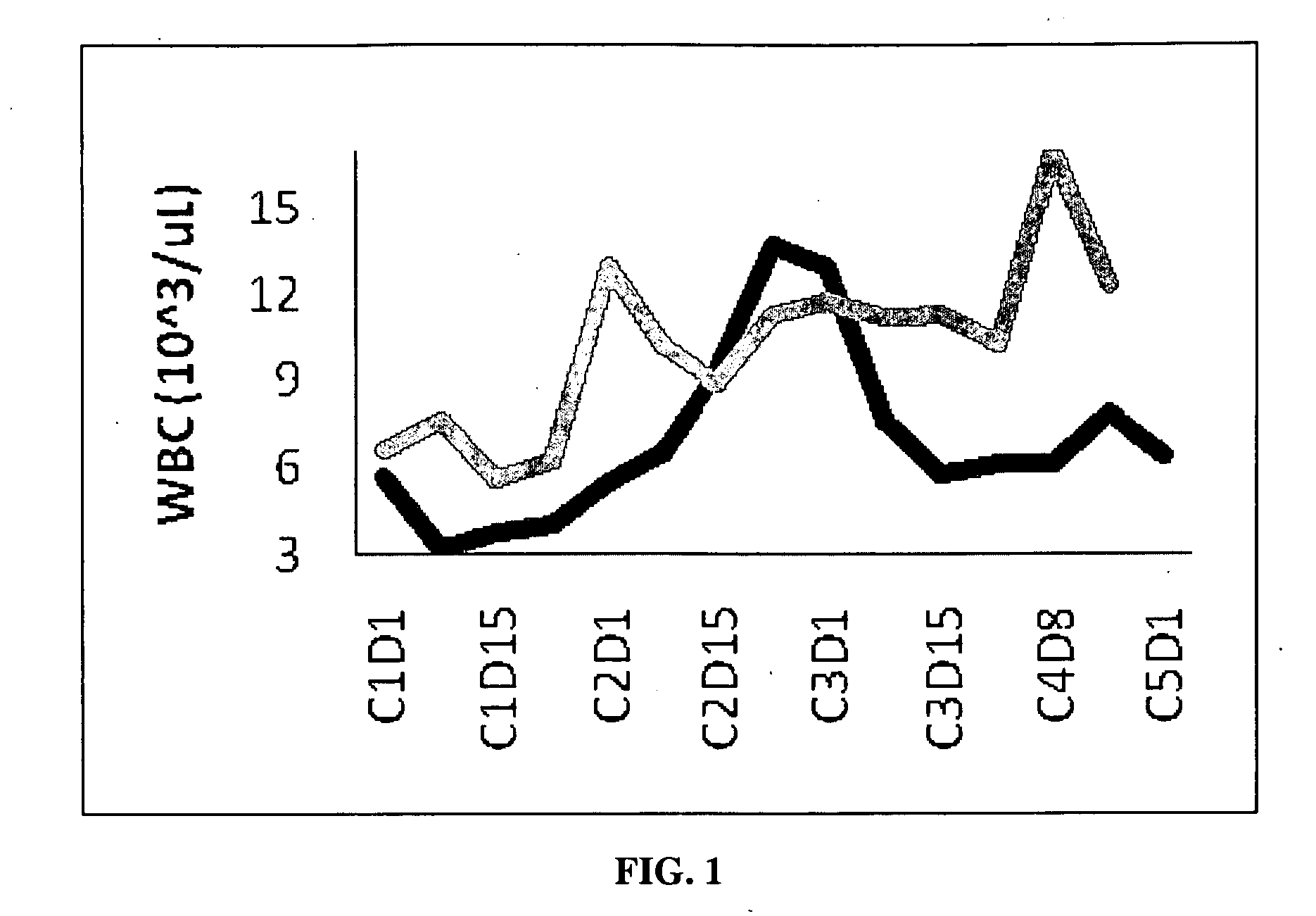
Methods of Treating Breast Cancer with Gemcitabine Therapy
The application describes methods for predicting overall survival in subjects with breast cancer. The application also describes for screening subjects with breast cancer to determine if the breast cancer will be responsive to a breast cancer therapy including gemcitabine. The application further describes methods for treating subjects with breast cancer by screening them for the likelihood of the effectiveness of treating the cancer with a therapy including gemcitabine and administering the therapy in subjects when it is found that gemcitabine is likely to be effective.
Owner:NANOSTRING TECH INC +1
Combined treatment with gemcitabine and an epidermal growth factor receptor kinase inhibitor
InactiveUS20050272688A1BiocideCarbohydrate active ingredientsEpidermal Growth Factor Receptor KinaseLymphatic Spread
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and gemcitabine combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and gemcitabine combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as Tarceva™).
Owner:OSI PHARMA INC
Method of treating cancer, especially soft tissue sarcoma utilizing gemcitabine in combination with docetaxel and anti-VEGF therapy (bevacizumab)
InactiveUS20070065449A1Great likelihoodLong median survivalBiocideGenetic material ingredientsAbnormal tissue growthLymphatic Spread
The present invention relates to a pharmaceutical cocktail, in particular, effective amounts of gemcitabine, in combination with effective amounts of docetaxel and angiogenesis inhibitor, especially a vascular endothelial growth factor (VEGF) inhibitor, such as bevacizumab for the treatment of cancer, in particular sarcoma, especially soft tissue sarcoma. Pharmaceutical compositions and methods of treating cancer, including sarcoma, especially soft tissue sarcoma (prolonging the patient's life, eliminating the tumor, improving the patient's quality of life, shrinking the tumor, prolonging survival and / or preventing the tumor's metastases) are additional aspects of the present invention.
Owner:STC UNM
Dosing regimen for gemcitabine HCV therapy
InactiveUS20030225029A1Reduce viral loadRapid and large in viral loadBiocideSugar derivativesDosing regimenHepatitis c viral
A dosage regiment for the treatment of a Flaviviridae infection, including a hepatitis C viral infection, that includes administering gemcitabine (or its salt, prodrug or derivative, as described herein) in a dosage range of approximately 50 mg / m<2 >to about 1300 mg / m<2 >per day for between one and seven days (e.g. 1, 2, 3, 4, 5, 6, or 7 days) followed by cessation of therapy. Viral load is optionally monitored over time, and after cessation, viral rebound is monitored. Therapy is not resumed unless a significant viral load is again observed, and then therapy for 1-7 days and more preferred, 1, 2 or 3 days, is repeated. This therapy can be continued indefinitely to monitor and maintain the health of the patient.
Owner:PHARMASSET
Therapy of platinum-resistant cancer
The present invention concerns a method for treating platinum-resistant, ovarian cancer, primary peritoneal carcinoma or fallopian tube carcinoma with the combination of a HER2 antibody that effectively inhibits HER dimerization as well as gemcitabine.
Owner:GENENTECH INC
Methods of treatment of pancreatic cancer
ActiveUS20130202709A1Many symptomShorten the progressBiocidePeptide/protein ingredientsNanoparticlePreviously treated
The present invention provides methods and compositions for treating pancreatic cancer in an individual who has been previously treated for pancreatic cancer (e.g., gemcitabine-based therapy) by administering a composition comprising nanoparticles that comprise a taxane and an albumin. The invention also provides combination therapy methods of treating pancreatic cancer (for example, in an individual who has been previously treated for pancreatic cancer) comprising administering to an individual an effective amount of a composition comprising nanoparticles that comprise a taxane and an albumin and another agent.
Owner:ABRAXIS BIOSCI LLC
Methods of treatment of pancreatic cancer
ActiveUS20140079788A1Many symptomShorten the progressPeptide/protein ingredientsDigestive systemPancreas CancersNanoparticle
Owner:ABRAXIS BIOSCI LLC
Ready-to-use gemcitabine solutions and gemcitabin concentrates
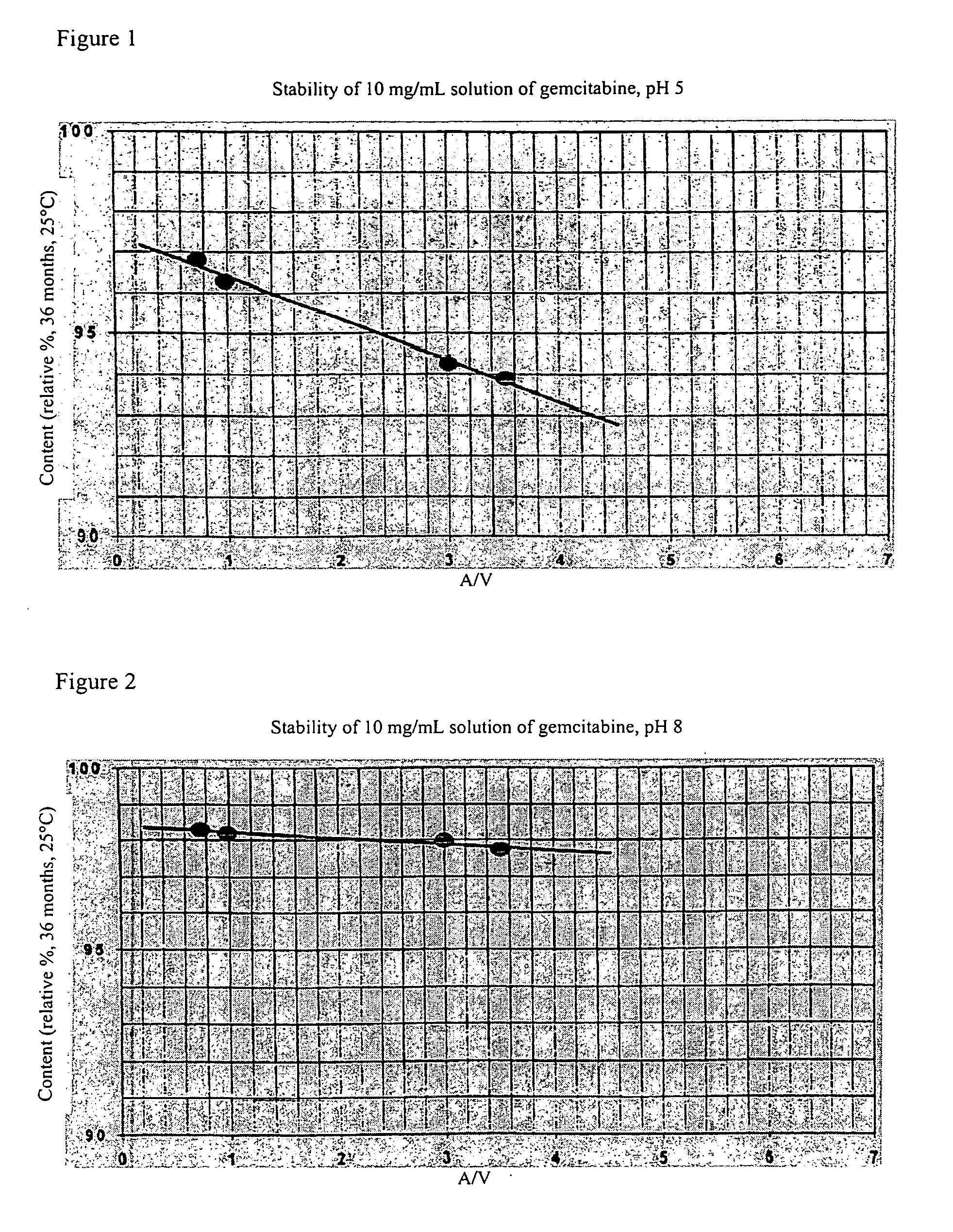
Pharmaceutical compositions in the form of ready-to-use preparations of gemcitabine in aqueous solutions in a glass containers having specified dimensional relationships demonstrate long shelf life over a wide range of solution pH values. The ratio of the surface area of the container wetted by the solution to the volume of the solution, expressed in cm2 to cm3, is less than 3.4.
Owner:STADA ARZNEIMITTEL AG
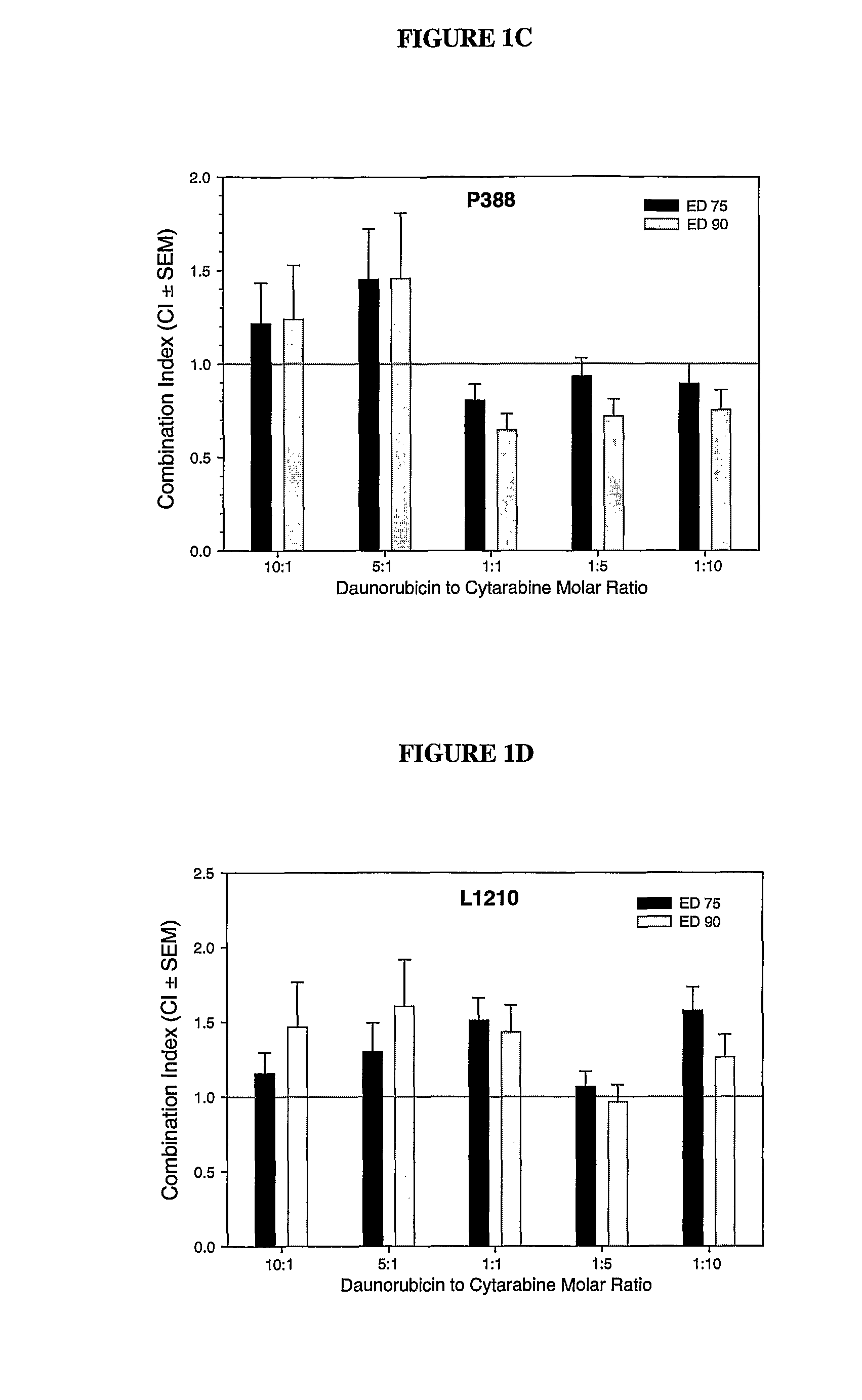
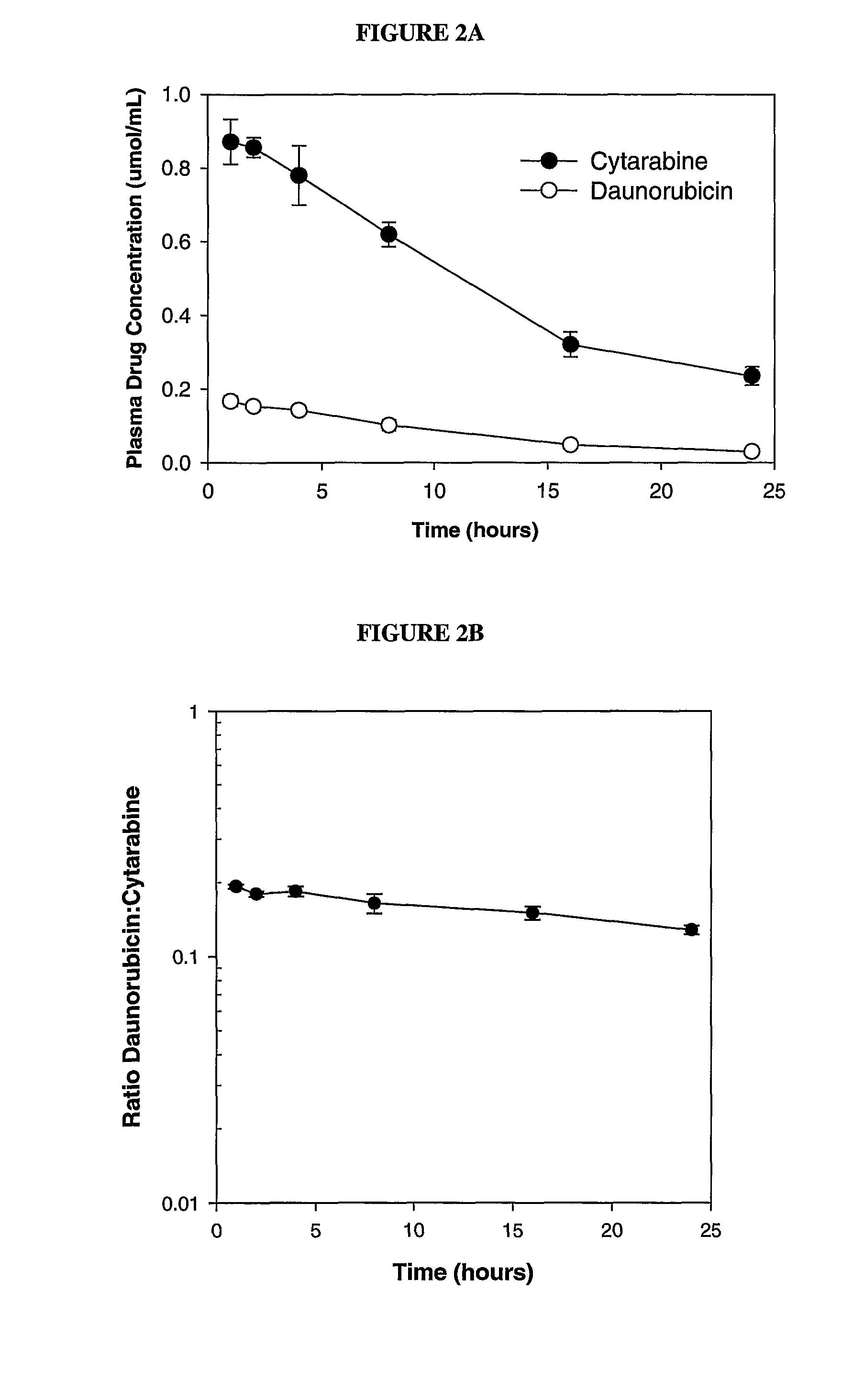
Liposomal formulations of anthracycline agents and cytidine analogs
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Method for treating cancer based on level of a nucleoside transporter
The present invention provides methods and compositions for treating cancer by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a nucleoside analog (e.g., gemcitabine) based upon levels of a nucleoside transporter (e.g., hENT1).
Owner:ABRAXIS BIOSCI LLC
Composition for treatment of pancreatic cancer
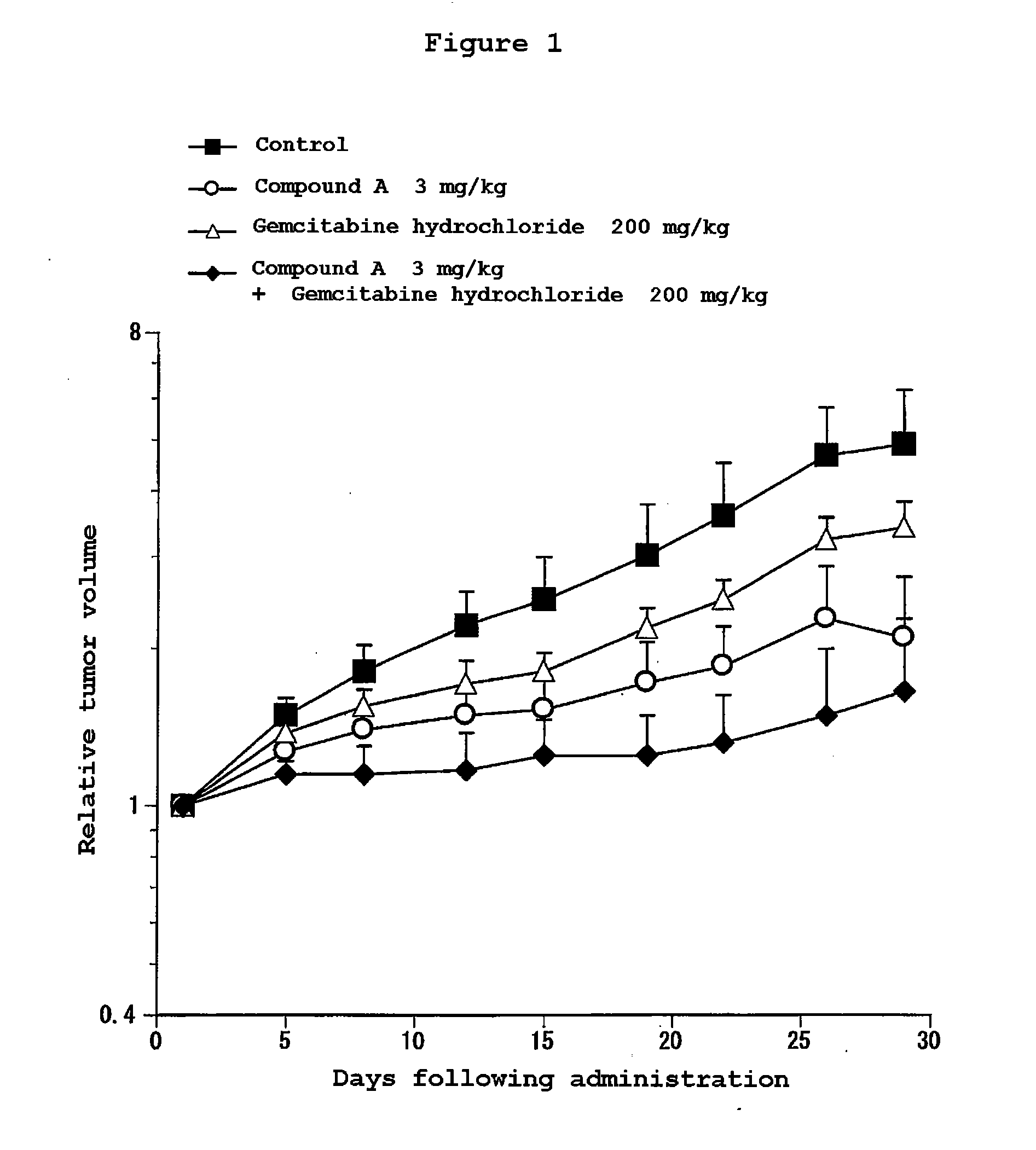
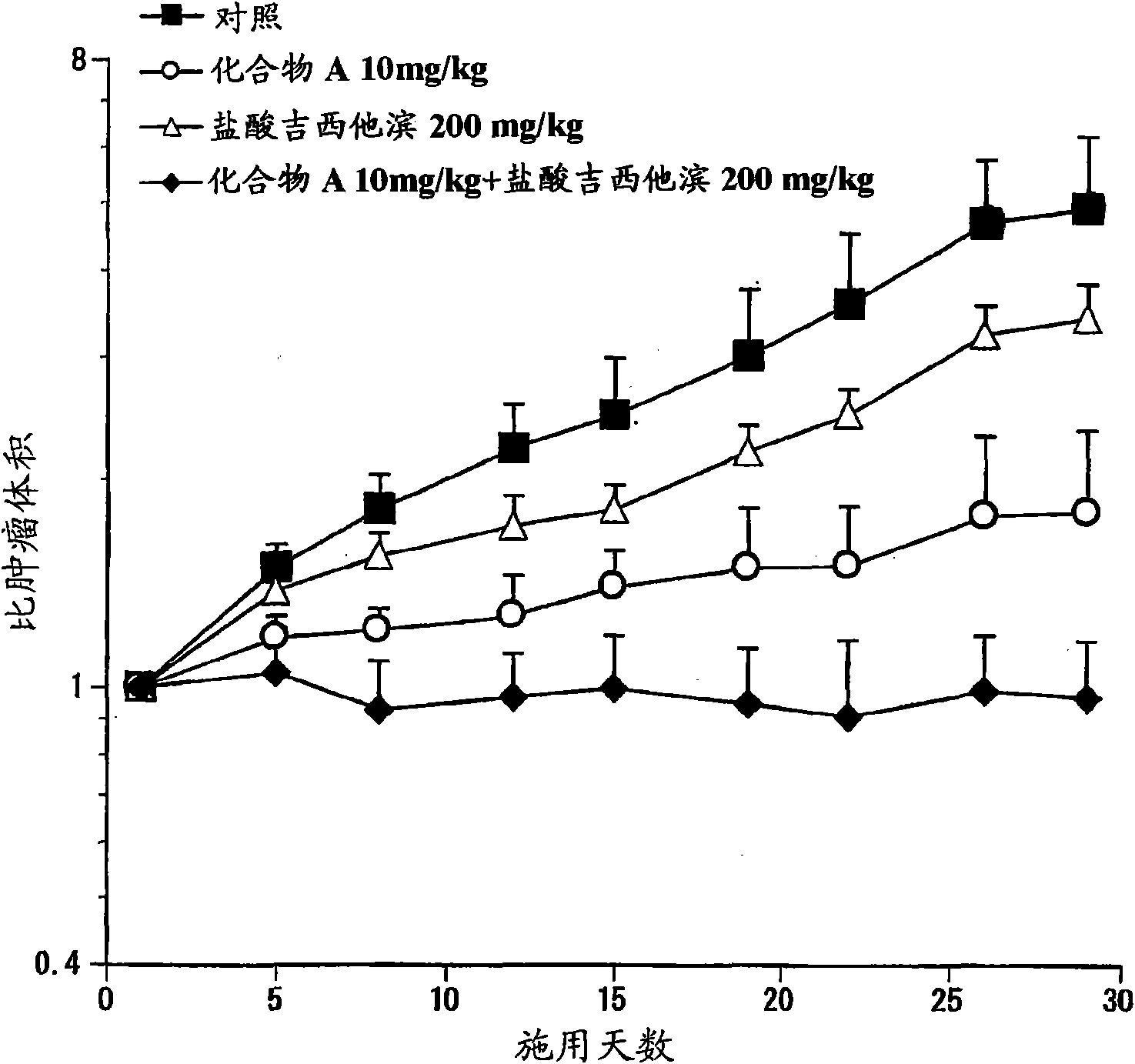
Disclosed are a pharmaceutical composition having excellent antitumor activity, and a method for treating a cancer. Specifically, excellent antitumor activity is achieved when 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide or an analogous compound thereof, a pharmacologically acceptable salt thereof or a solvate thereof is used in combination with gemcitabine or erlotinib, a pharmacologically acceptable salt thereof or a solvate of any of them.
Owner:EISIA R&D MANAGEMENT CO LTD
Method for treating cancer based on mutation status of k-ras
The present invention provides methods and compositions for treating cancer by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and / or b) a therapeutic agent (e.g., gemcitabine) based upon K-ras mutation status.
Owner:ABRAXIS BIOSCI LLC
Composition for treatment of pancreatic cancer
Disclosed are a pharmaceutical composition having excellent antitumor activity, and a method for treating a cancer. Specifically, excellent antitumor activity is achieved when 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7- methoxy-6-quinolinecarboxamide or an analogous compound thereof, a pharmacologically acceptable salt thereof or a solvate of any of them is used in combination with gemcitabine or erlotinib, a pharmacologically acceptable salt thereof or a solvate of any of them.
Owner:EISIA R&D MANAGEMENT CO LTD
Antitumor T Cell Response Enhancer
ActiveUS20130202588A1Improve anti-tumor effectEnhance immune functionBiocideSugar derivativesCancer therapyOrganism
An objective of the present invention is to provide novel therapeutic agents for cancer, which have an excellent antitumor effect in cancer patients by enhancing their immune function. The present inventors discovered that the administration of at IL-6 inhibitor and / or gemcitabine or a salt thereof to tumor-bearing organisms yields an excellent antitumor T cell response-enhancing effect, and completed the present invention. In addition, the present inventors discovered that the T cell response-enhancing effect produces an excellent antitumor effect.
Owner:CHUGAI PHARMA CO LTD
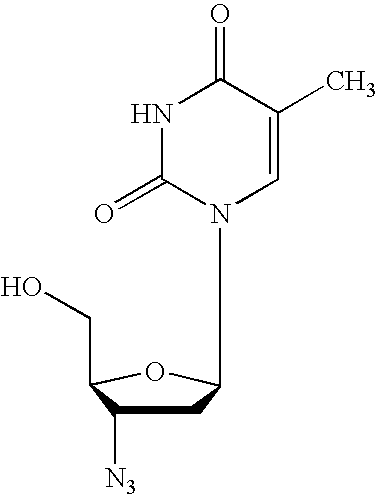
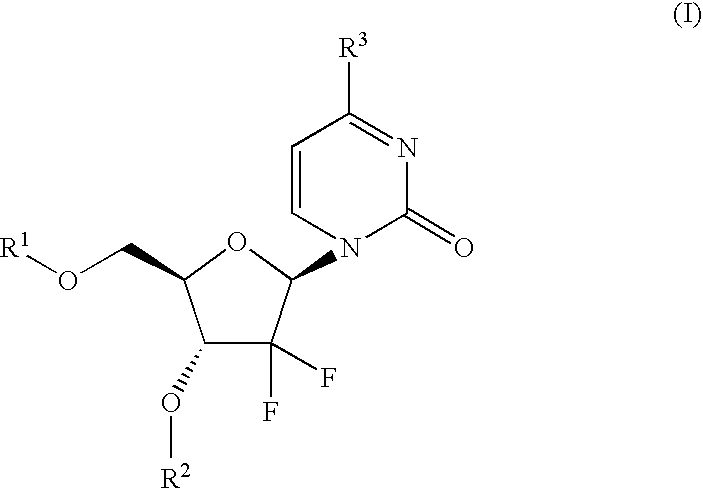
Gemcitabine prodrugs, pharmaceutical compositions and uses thereof
The present invention provides gemcitabine prodrugs, methods of making gemcitabine prodrugs, pharmaceutical compositions of gemcitabine prodrugs and methods of using gemcitabine prodrugs and pharmaceutical compositions thereof to treat or prevent diseases or disorders such as cancer or viral infections.
Owner:XENOPORT
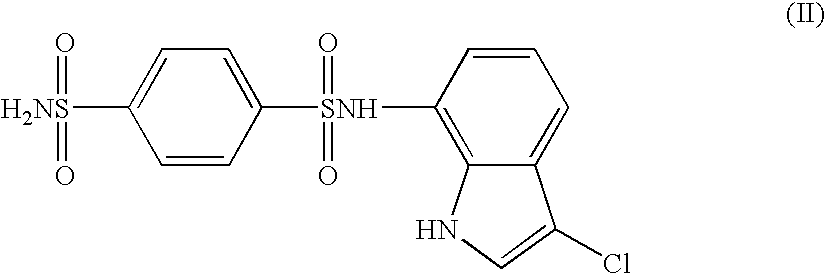
Medicinal compositions for cocominant use as anticancer agent
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl) -4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabinehydrochloride; (6) doxorubicin; (7) taxol; and (8) a salt of the above-mentioned (1) to (7).
Owner:EISIA R&D MANAGEMENT CO LTD
Formulation comprising a gemcitabine-prodrug
This invention relates to pharmaceutical formulations of gemcitabine-[phenyl-benzoxy-L-alaninyl)]-phosphate, a monophosphate derivative of the well-known oncology drug gemcitabine. In particular, the invention relates to formulations which comprise a polar aprotic solvent, preferably dimethyl acetamide (DMA). Formulations comprising these solvent provide therapeutically effective treatments of gemcitabine-[phenyl-benzoxy-L-alaninyl)]-phosphate. The invention also relates to methods of using said formulations and kits comprising said formulations.
Owner:NUCANA PLC
Lipophilic monophosphorylated derivatives and nanoparticles
There are provided, inter alia, lipophilic monophosphorylated derivatives of gemcitabine. There are further provided nanoparticles compositions incorporating lipophilic monophosphorylated derivatives of gemcitabine, pharmaceutical compositions thereof, and a method of treating cancer or a viral infection in a subject in need thereof, which method includes administration of a pharmaceutical composition disclosed herein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Pharmaceutical composition for treating cancer, comprising interferon alpha conjugate
ActiveUS20140219961A1Longer in vivo half-lifeHigh anticancer activityOrganic active ingredientsPeptide/protein ingredientsSide effectAnticarcinogen
A method for preventing or treating a cancer includes administering an anti-cancer pharmaceutical composition including an interferon alpha or a polymer conjugate thereof. The pharmaceutical composition can be co-administered with anti-cancer agents. The interferon alpha conjugate shows a longer in vivo half-life and a more excellent anti-cancer activity than the conventional interferon alpha, and in particular, its co-administration with an anti-cancer agent such as gemcitabine has synergistic inhibitory effects on cancer cell growth and proliferation so as to exhibit a remarkably excellent anti-cancer activity. Further, the anti-cancer pharmaceutical composition has excellent in vivo half-life and anti-cancer activity to greatly reduce administration frequency. Co-administration of an anti-cancer agent and the interferon alpha conjugate having excellent anti-cancer activity reduces administration dose of anti-cancer agent so as to reduce side effects of anti-cancer agent and increase treatment compliance of patient.
Owner:HANMI SCI CO LTD +1
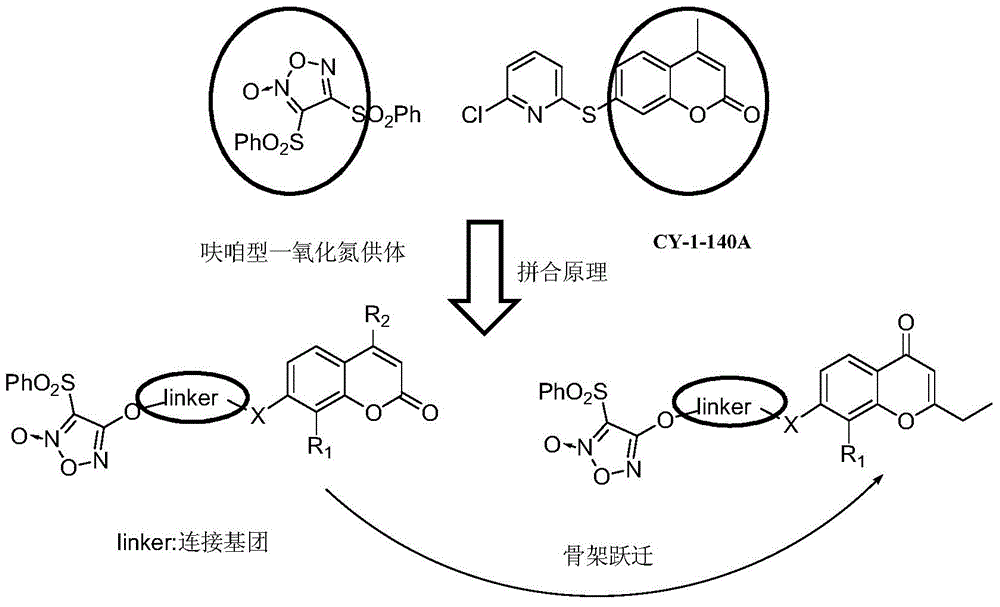
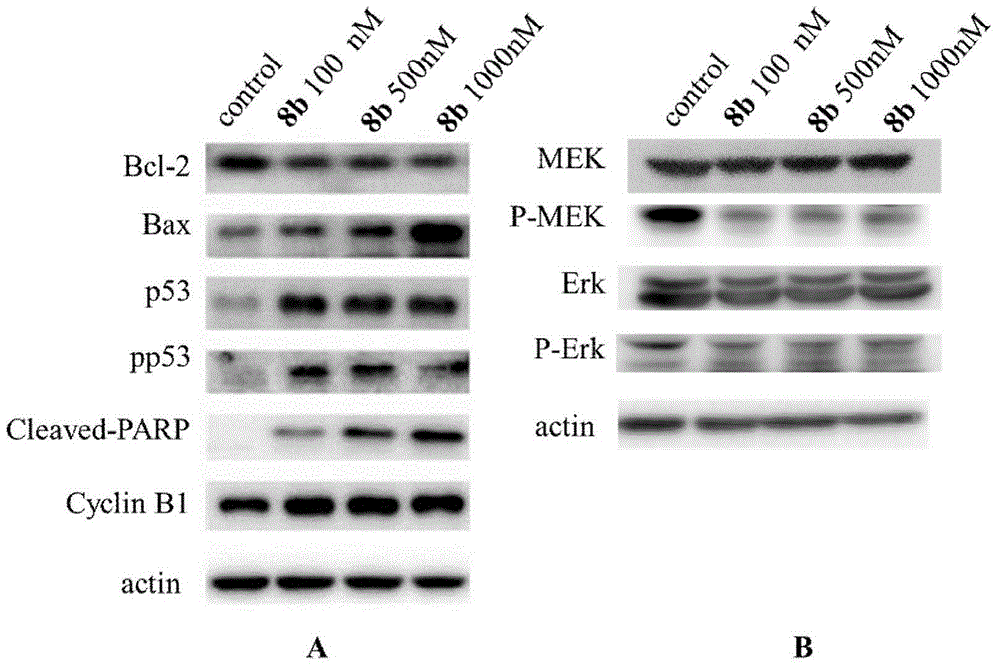
Furazan derivative of coumarin parent nucleus and antineoplastic activity
ActiveCN105153142ANovel structureInhibitory activityOrganic active ingredientsOrganic chemistryHigh concentrationCancer cell
Owner:FUDAN UNIV
Methods of treatment of pancreatic cancer
ActiveUS9399071B2Many symptomShorten the progressBiocidePeptide/protein ingredientsNanoparticlePreviously treated
The present invention provides methods and compositions for treating pancreatic cancer in an individual who has been previously treated for pancreatic cancer (e.g., gemcitabine-based therapy) by administering a composition comprising nanoparticles that comprise a taxane and an albumin. The invention also provides combination therapy methods of treating pancreatic cancer (for example, in an individual who has been previously treated for pancreatic cancer) comprising administering to an individual an effective amount of a composition comprising nanoparticles that comprise a taxane and an albumin and another agent.
Owner:ABRAXIS BIOSCI LLC
Parenteral formulations of gemcitabine derivatives
Owner:CLAVIS PHARMA ASA
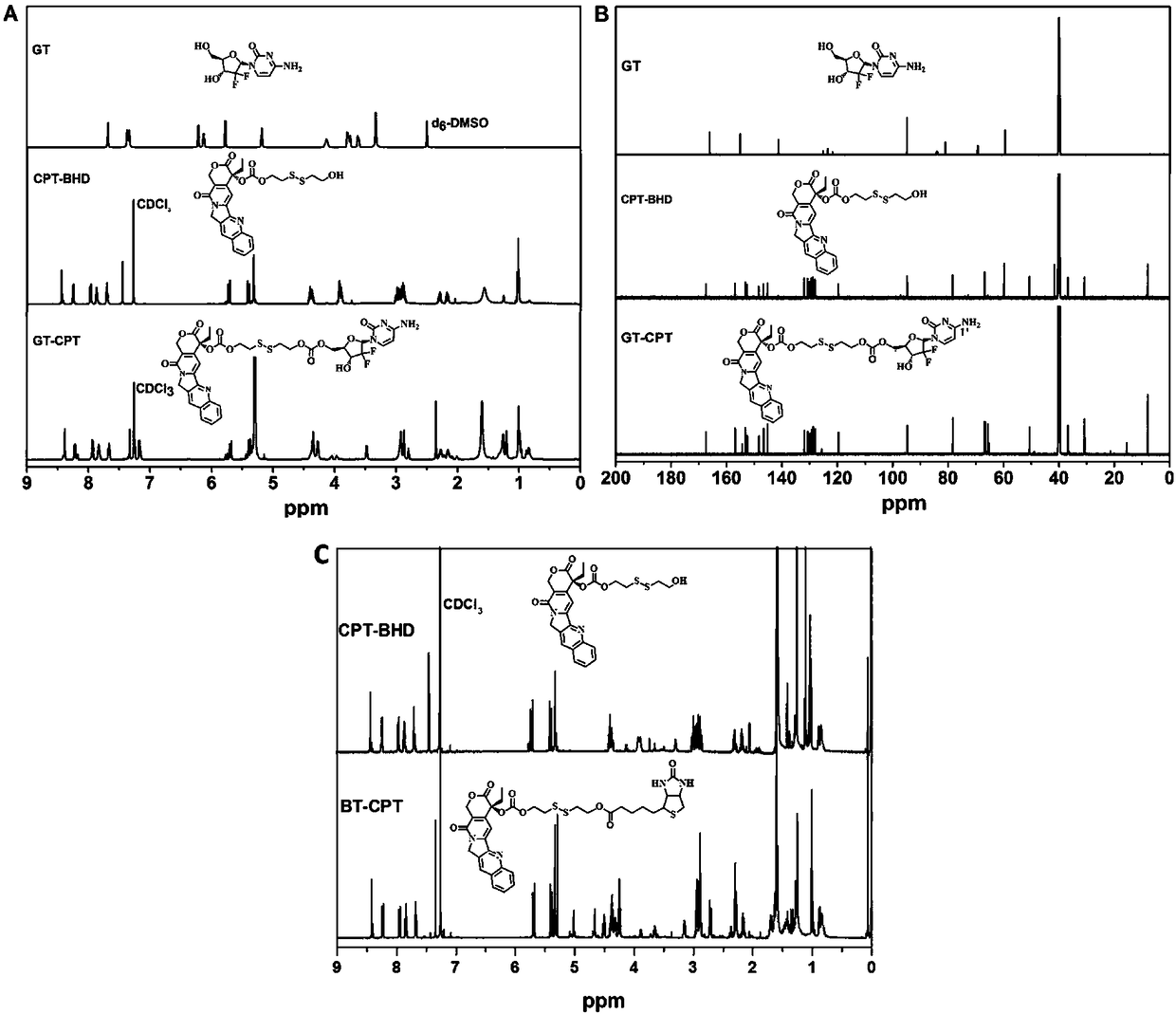
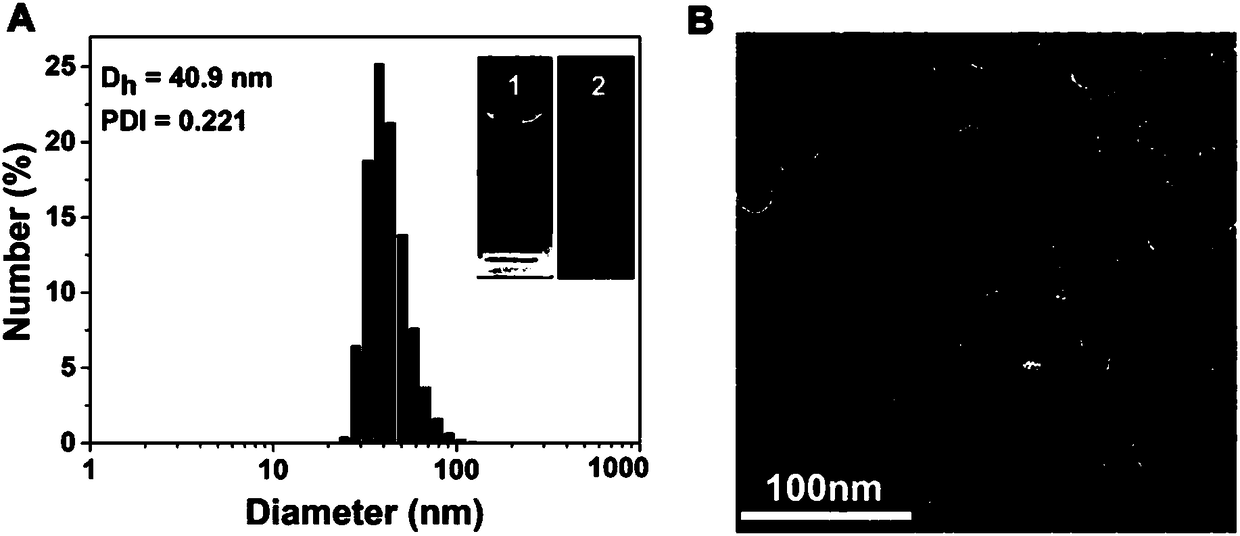
Reduced responsive drug-drug conjugate preparation method
InactiveCN108785683AIncrease upload volumeAddress targeted deliveryPowder deliveryOrganic active ingredientsCancer cellFluorescence
The invention discloses a reduced responsive drug-drug conjugate preparation method which is characterized in that the method includes the steps: preparing a camptothecin intermediate (CPT-SS) containing a reduced responsive bond; preparing a reduced responsive drug-drug conjugate (GT-CPT) or (BT-CPT). The reduced responsive drug-drug conjugate has advantages of a small-molecule prodrug and a nano-drug, the uploading capability of the reduced responsive drug-drug conjugate can be effectively improved, the reduced responsive drug-drug conjugate solves the problems of targeting delivery of a current small-molecule prodrug delivery system and controlled release of drug selectivity, and accurate diagnosis and efficient treatment of tumors are facilitated. Gemcitabine (drug) and biotin (target)molecules are introduced, fluorescence of drug molecules can be temporarily shielded, the conjugate is combined with specificities of cancer cells, so that nano-micelles are disassembled and assembled, the fluorescence of the drug molecules can be reduced, and a drug releases drug properties under stimulation response, so that accurate diagnosis and treatment of cancers are combined.
Owner:SOUTHWEST UNIVERSITY
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
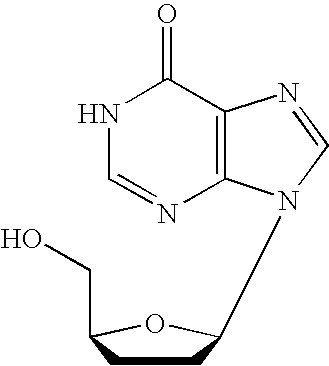
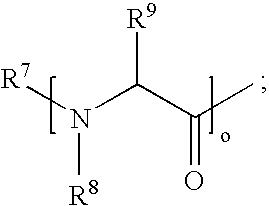
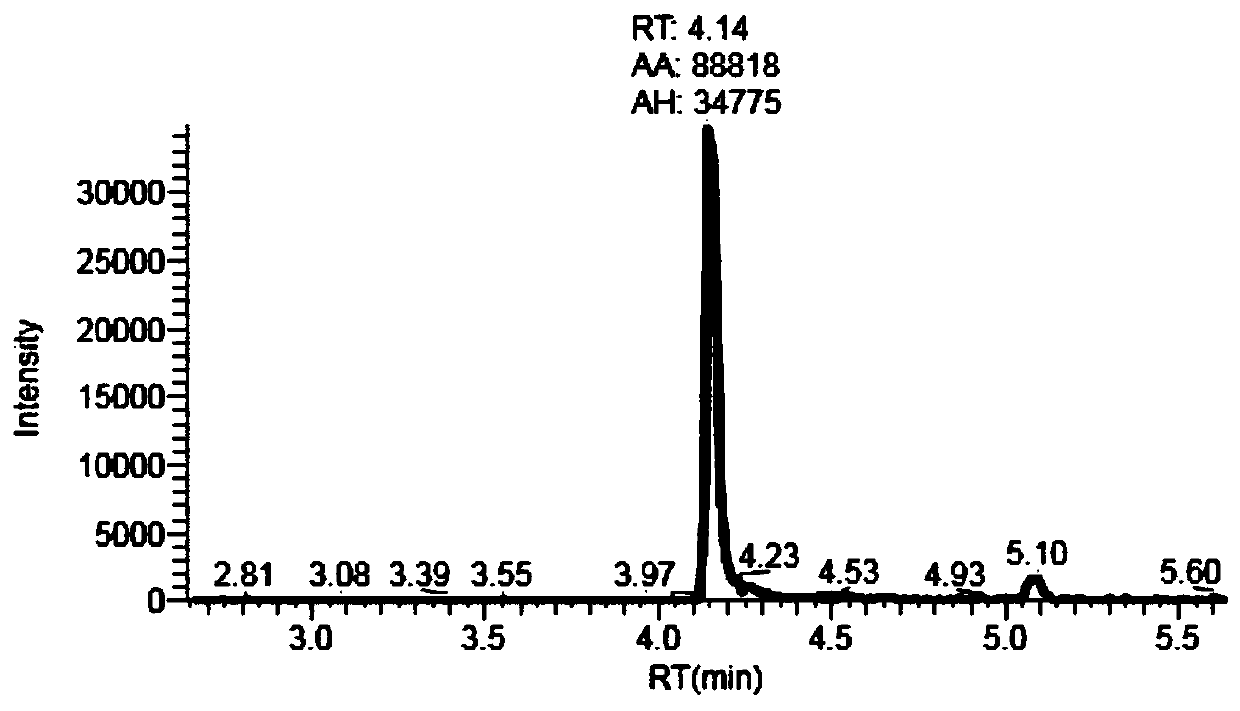
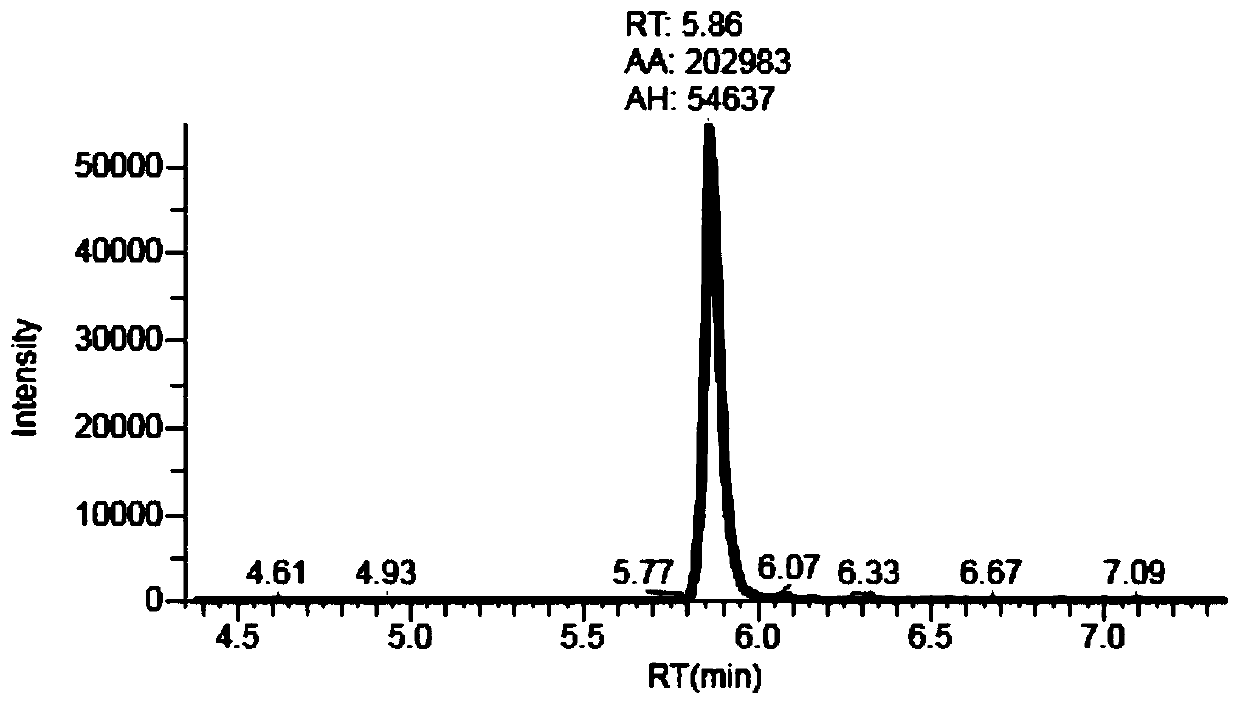
Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate
ActiveUS9834577B2High purityHigh yieldSugar derivativesSugar derivatives preparationPhosphateGemcitabine
The present invention provides a process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate of Formula I in high yield and purity.
Owner:NUCANA PLC
Drug delivery systems and methods for treatment of bladder cancer with gemcitabine
InactiveUS20150250717A1Efficient ConcentrationEnhance and otherwise alter solubilizationBiocideSugar derivativesBladder cancerBacteriuria
Drug delivery devices and methods are provided for administering gemcitabine to a patient in need of treatment of bladder cancer by intravesically administering gemcitabine into the bladder of the patient to achieve a sustained concentration of the gemcitabine in urine in the bladder sufficient to produce a therapeutically effective concentration of the gemcitabine in the tissues of the bladder. In embodiments, the local administration into the patient's bladder is at a mean average amount of from 1 mg / day to about 300 mg / day of the gemcitabine (FBE).
Owner:TARIS BIOMEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com














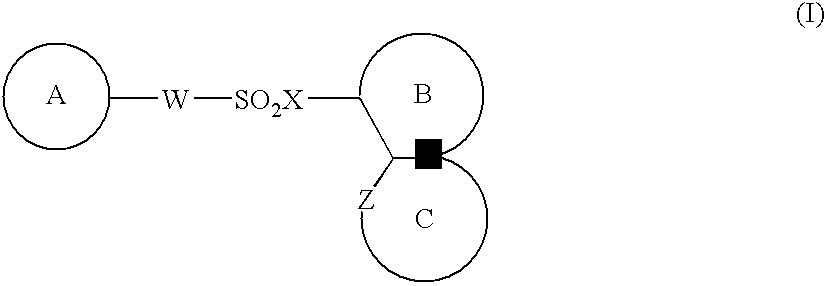
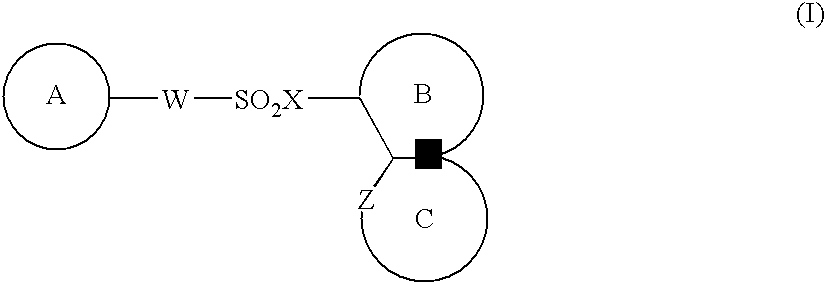














![Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7227d321-24ef-4bed-8f6a-7f4adba306e2/US09834577-20171205-C00001.png)
![Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7227d321-24ef-4bed-8f6a-7f4adba306e2/US09834577-20171205-C00002.png)
![Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate Process for the preparation of gemcitabine-[phenyl(benzoxy-L-alaninyl)] phosphate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7227d321-24ef-4bed-8f6a-7f4adba306e2/US09834577-20171205-C00003.png)


