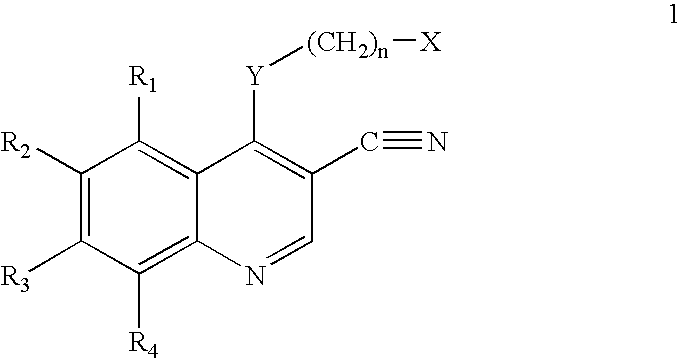
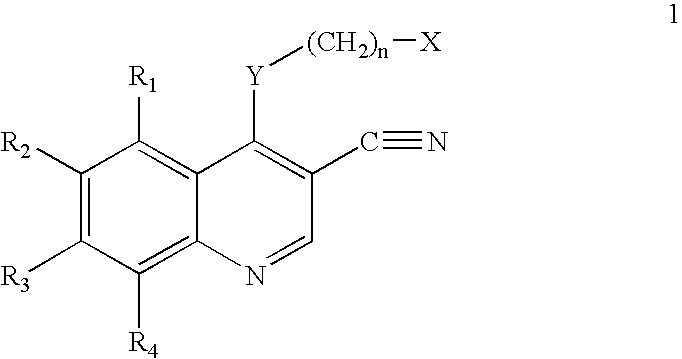
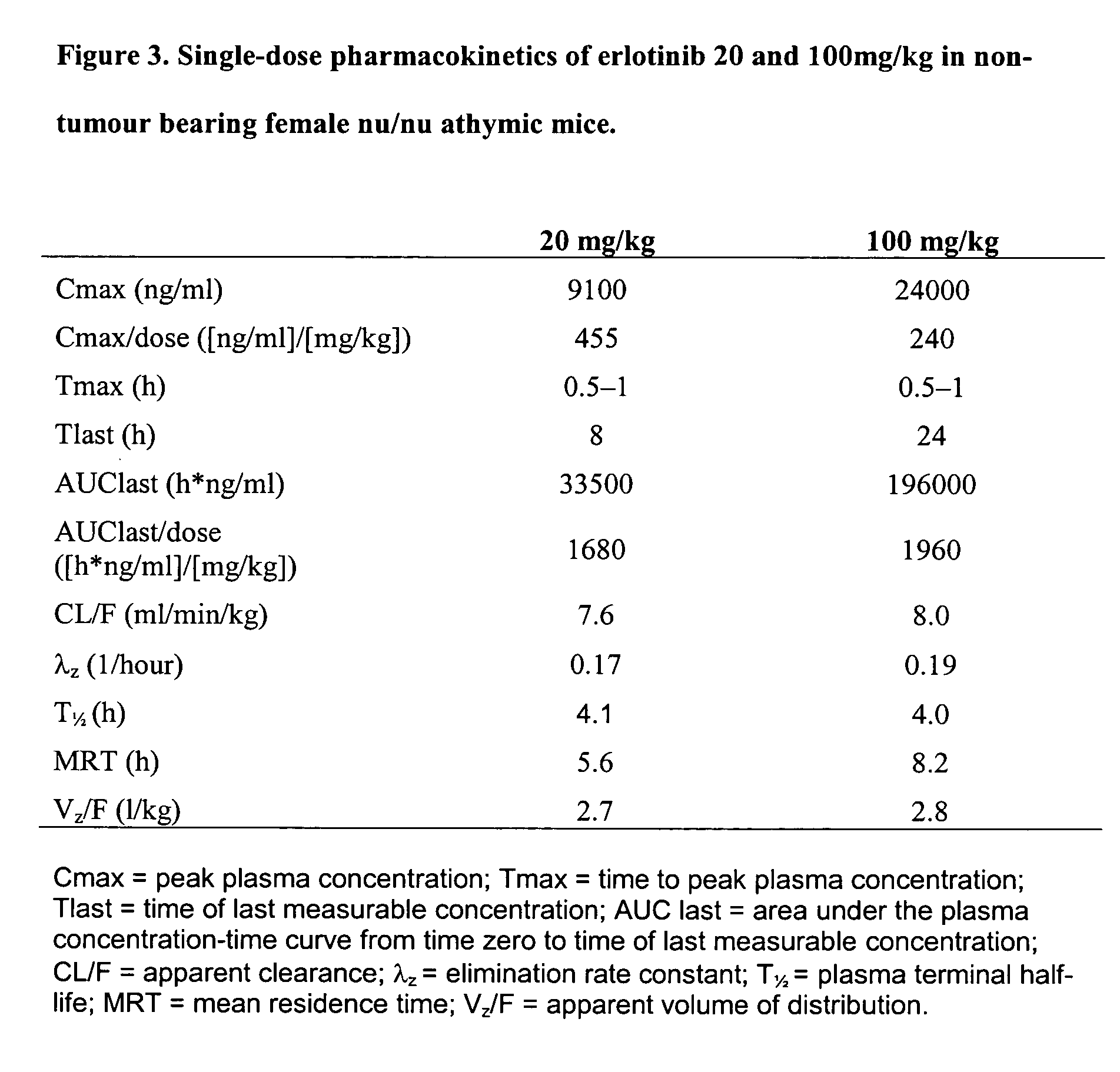
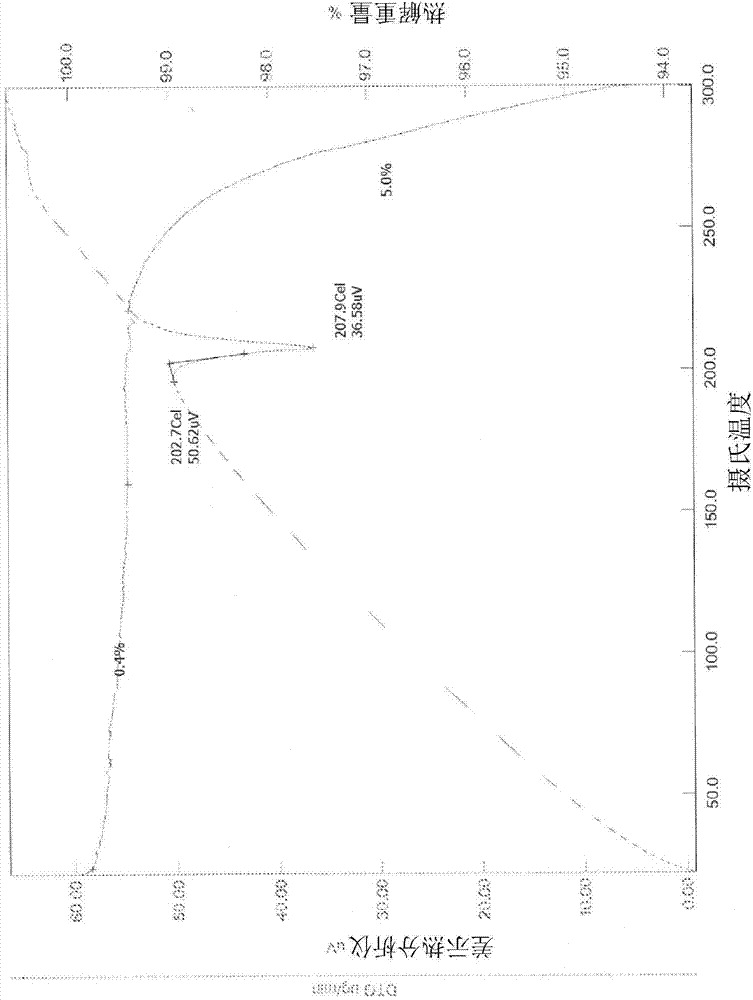
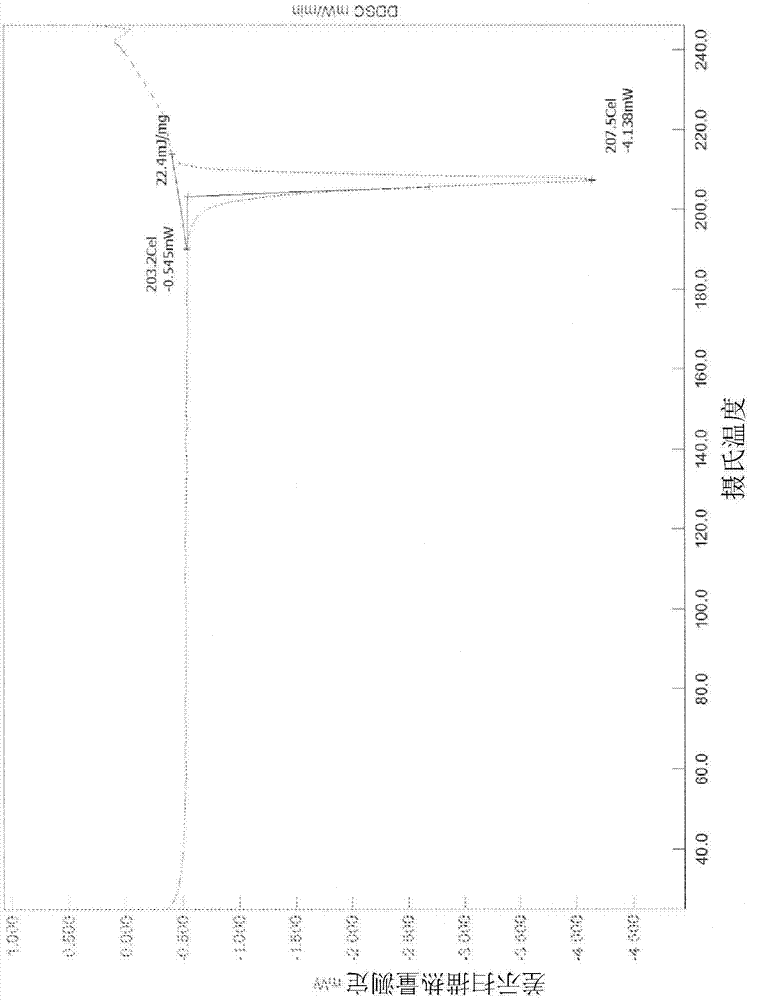
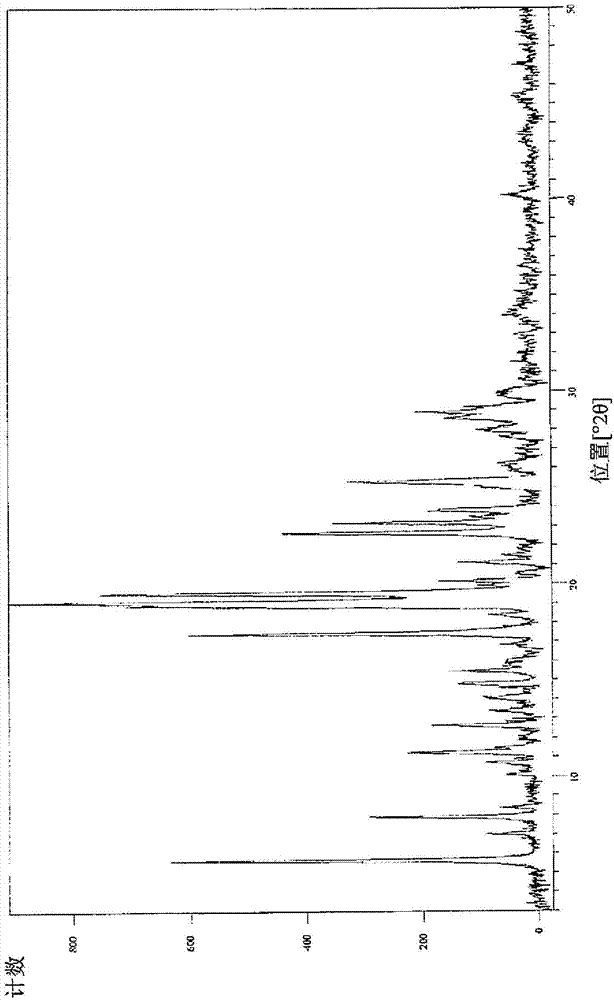
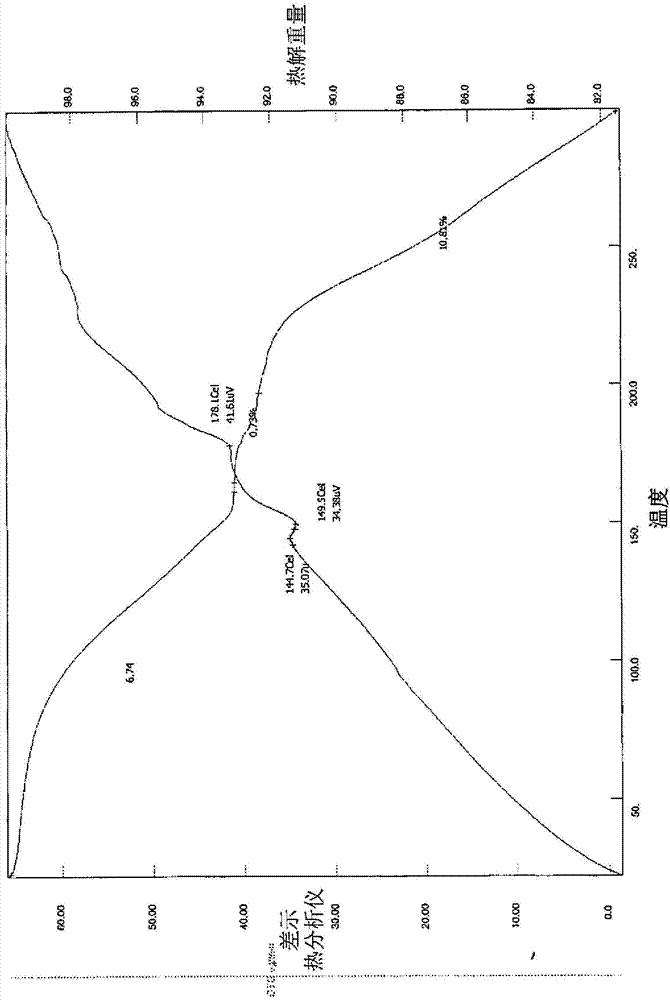
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Epidermal Growth Factor Receptor Kinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The protein encoded by this gene is a transmembrane glycoprotein that is a member of the protein kinase superfamily. This protein is a receptor for members of the epidermal growth factor family.
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20070212738A1Restore sensitivityHigh sensitivityDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseKinase
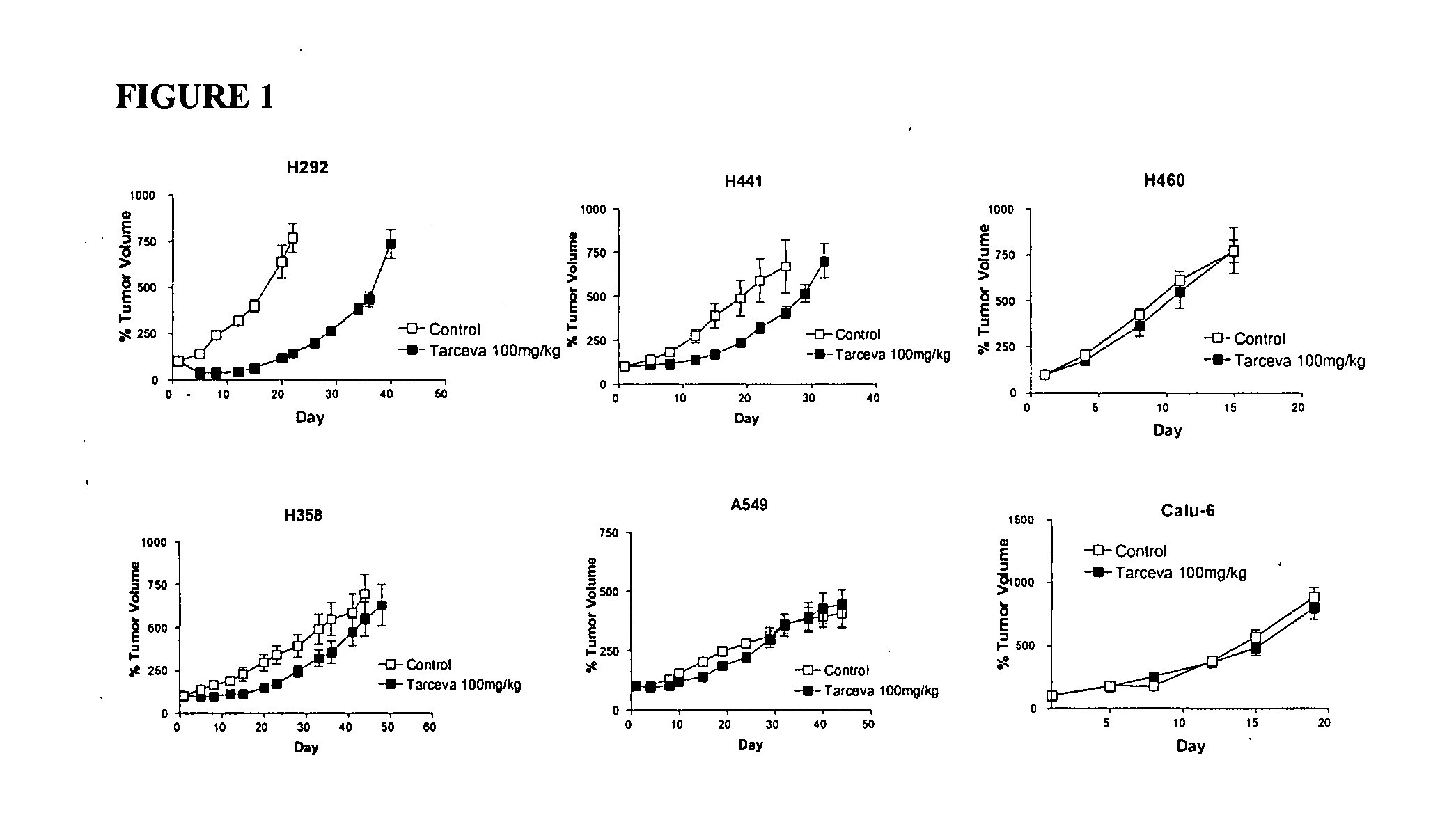
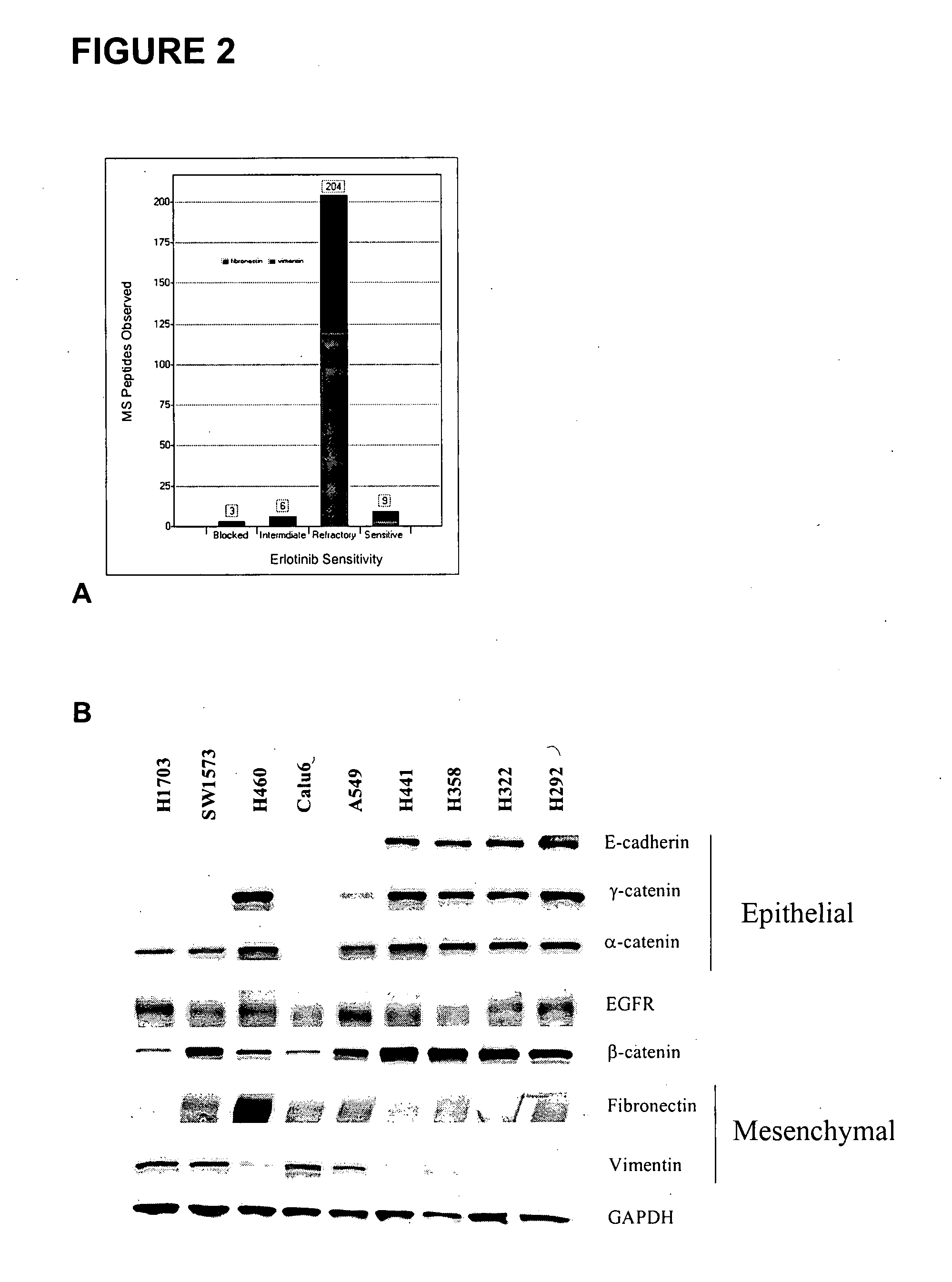
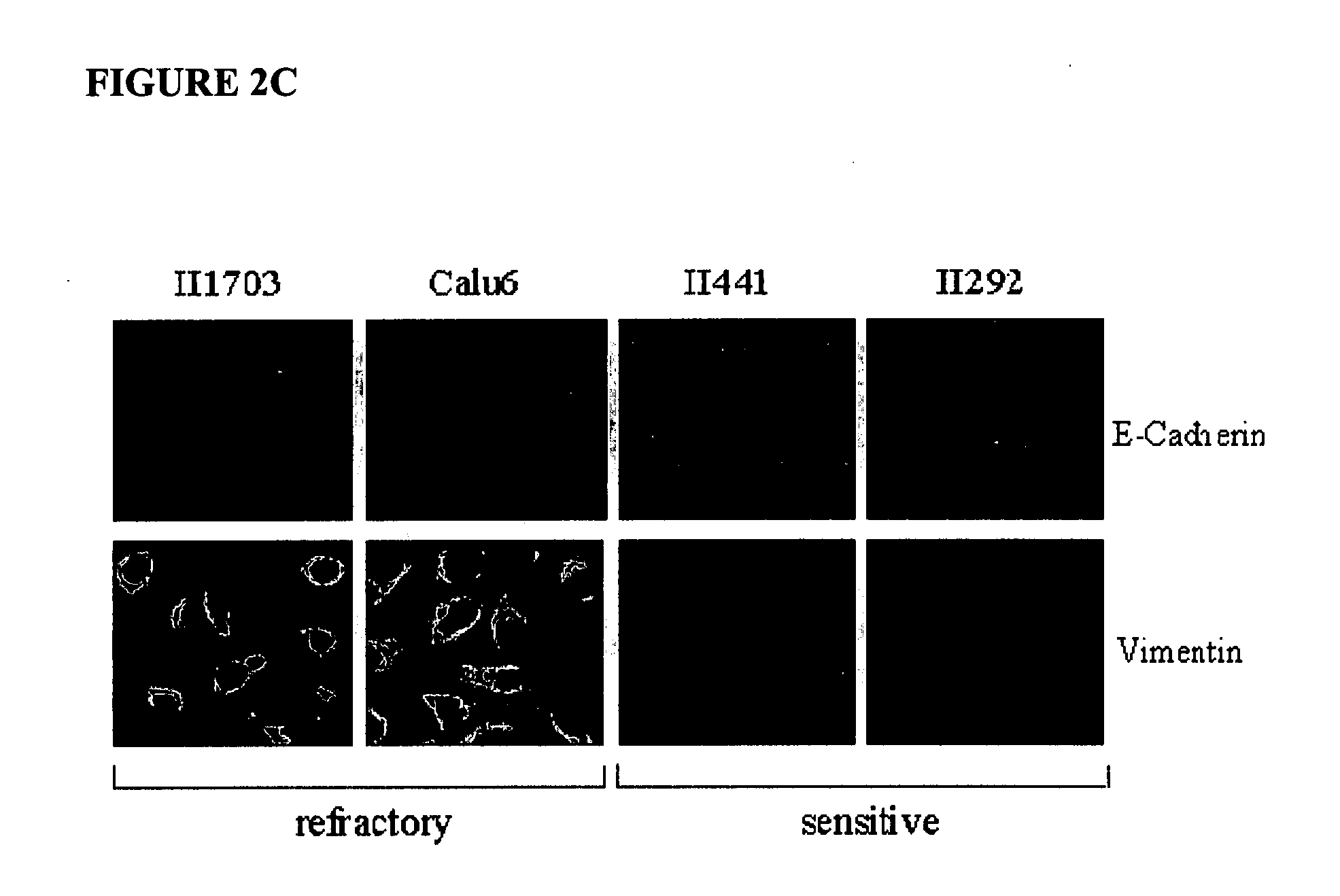
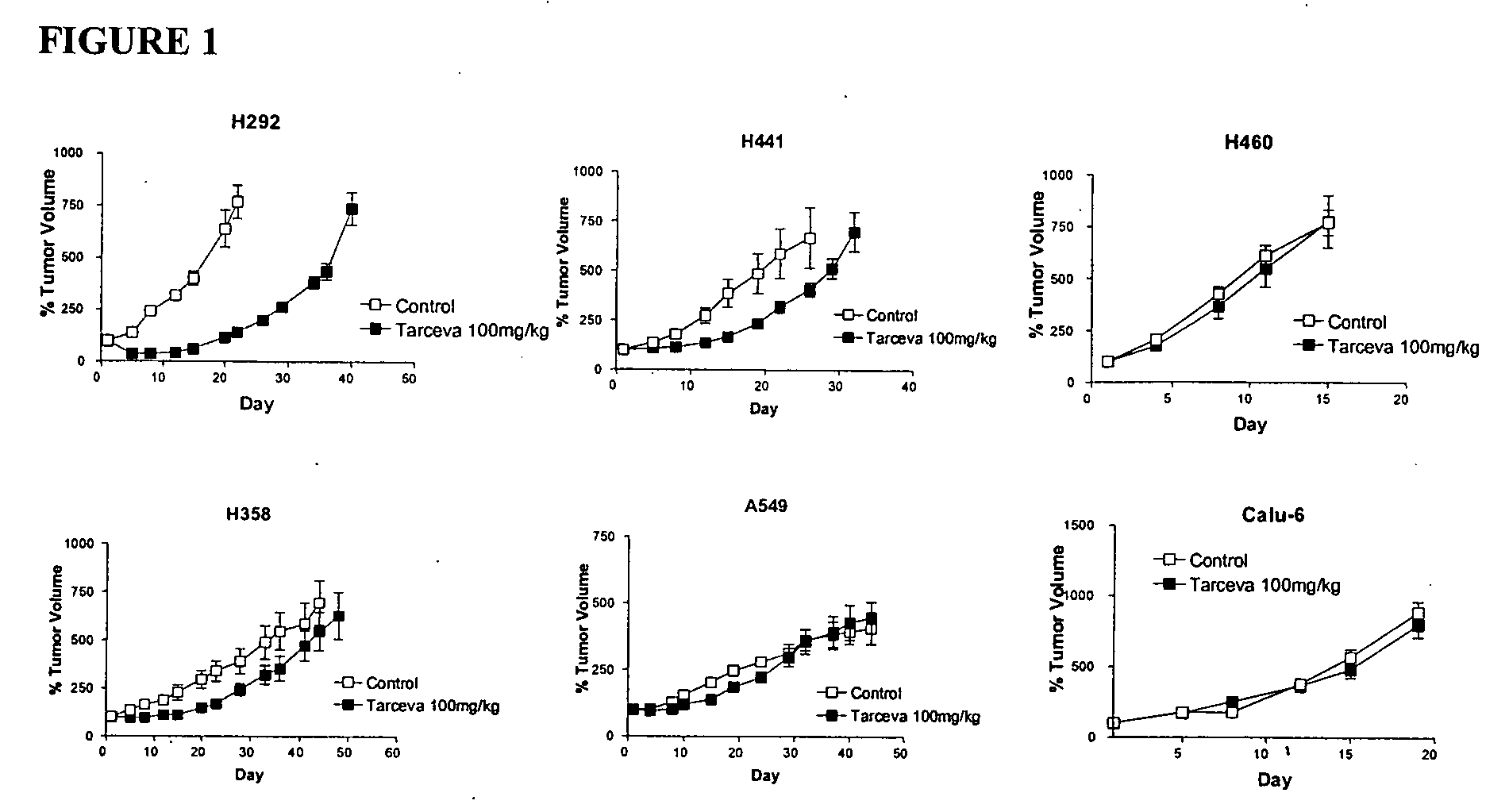
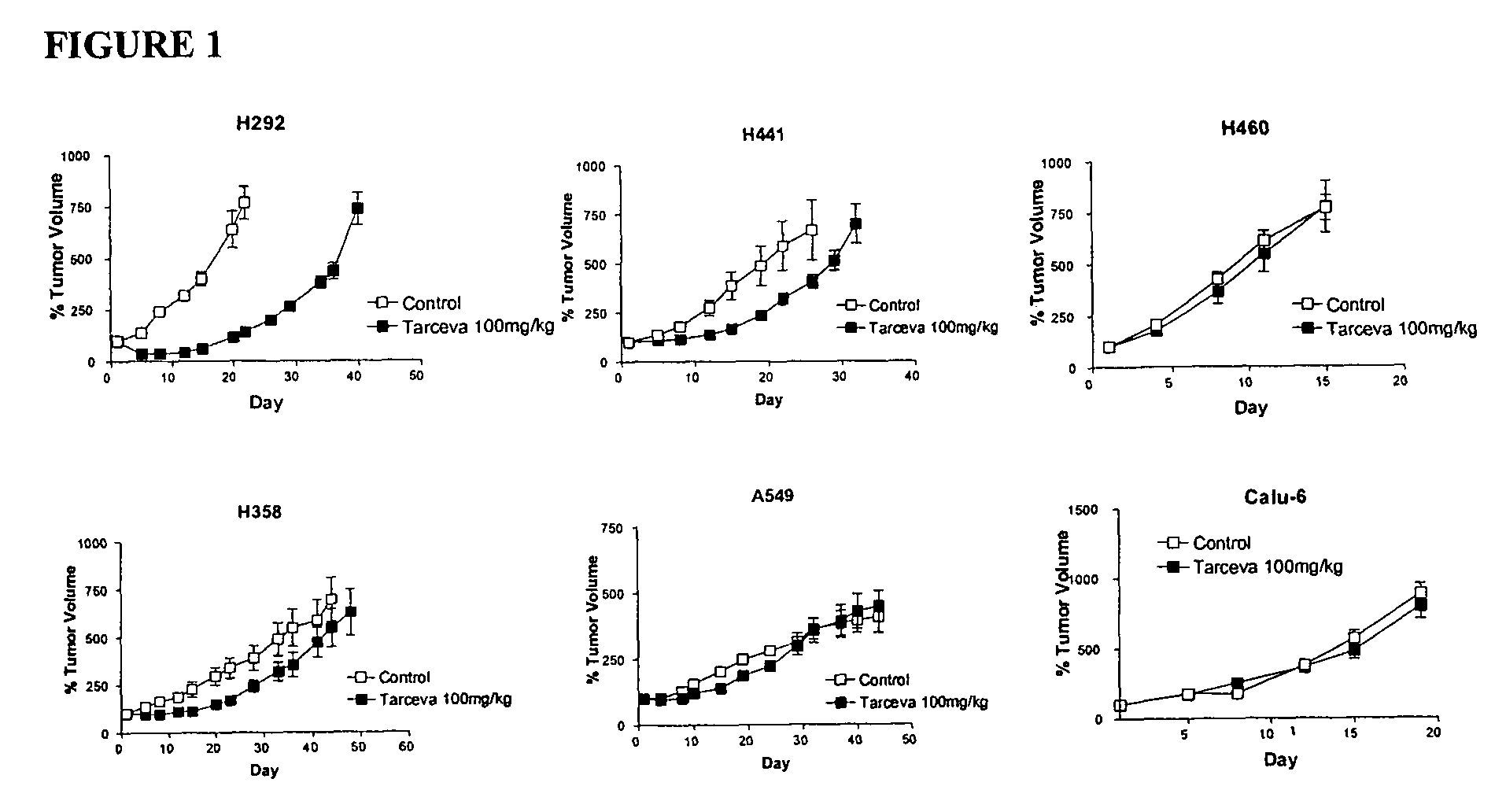
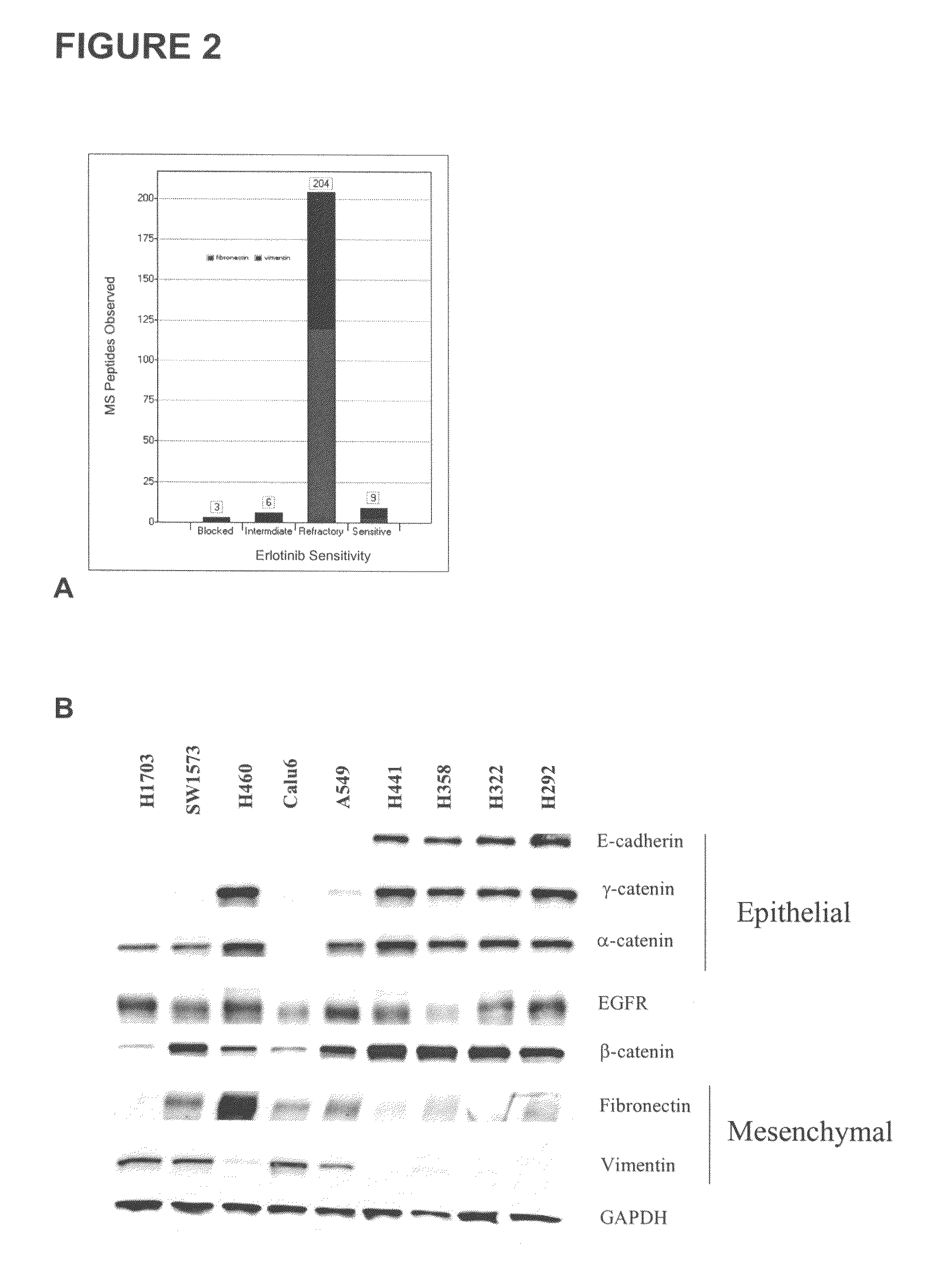
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA LLC
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
ActiveUS20060211060A1Restore sensitivityHigh sensitivityOrganic active ingredientsDisease diagnosisEpidermal Growth Factor Receptor KinaseKinase
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA INC
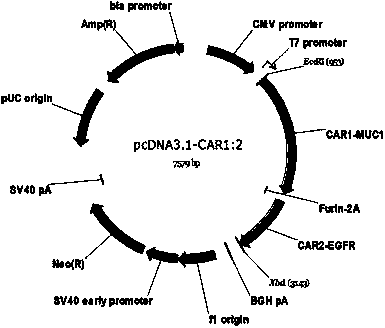
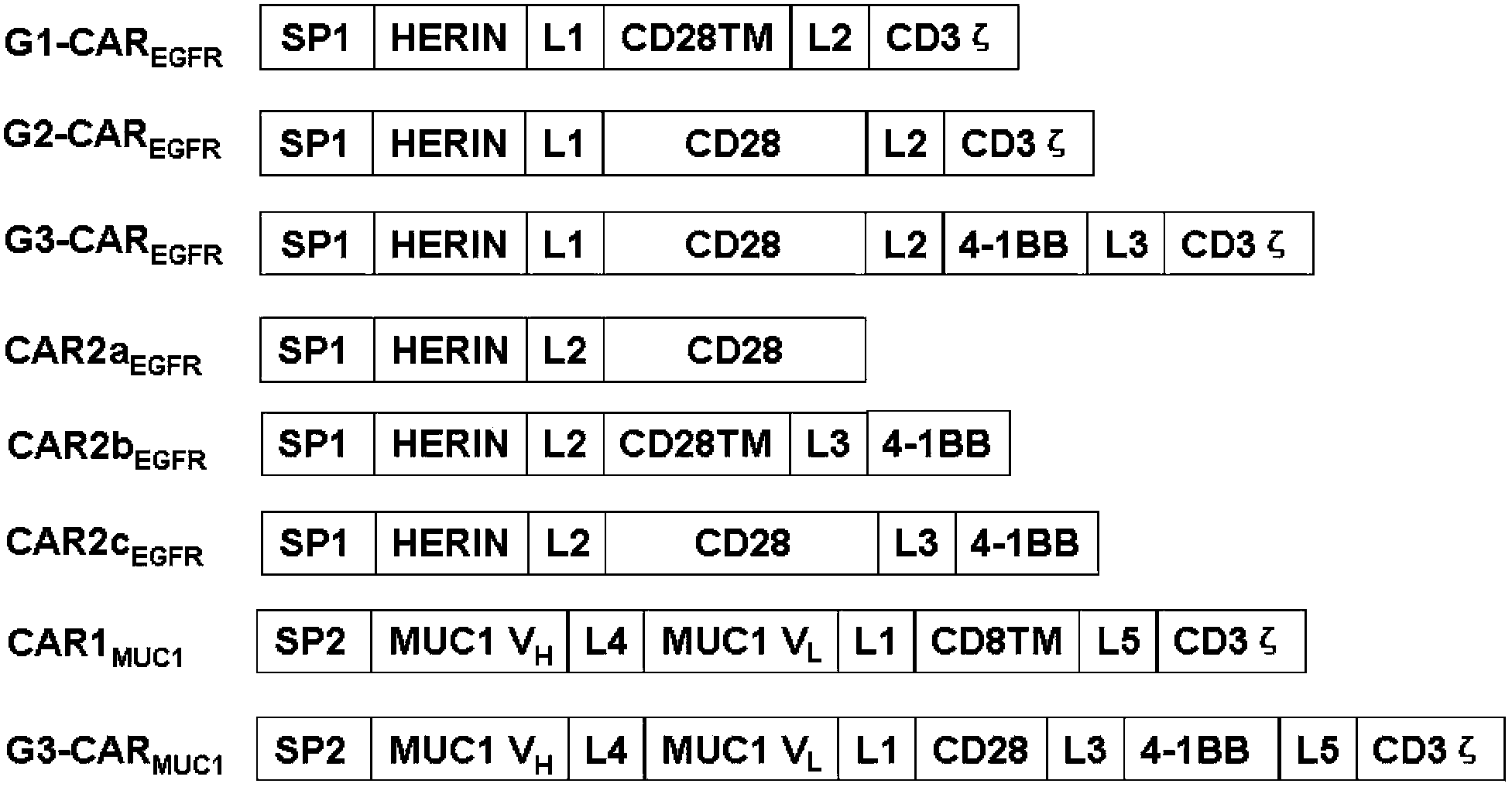
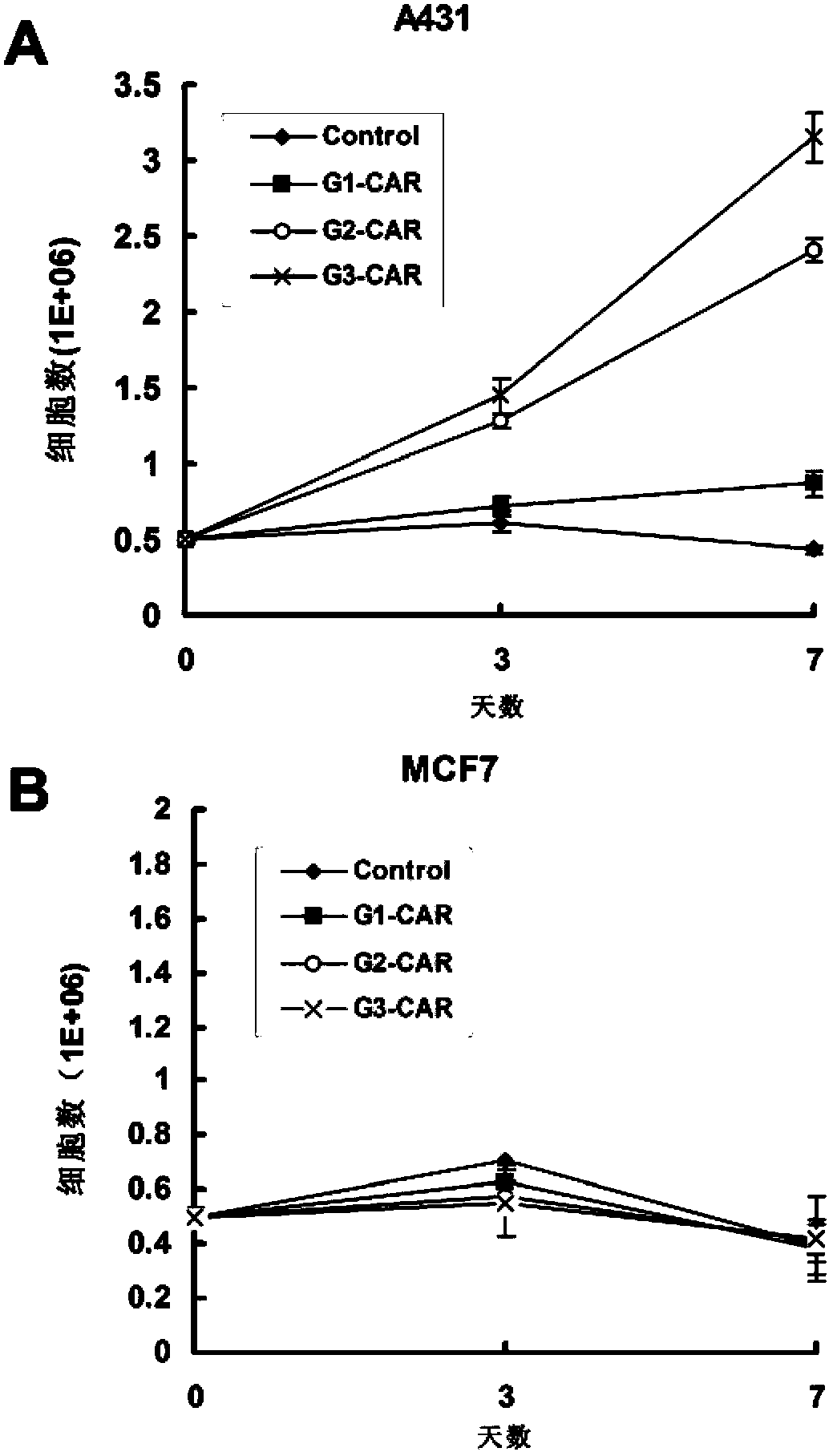
Chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and composition and uses thereof
ActiveCN103483453AFix security issuesImprove proliferative abilityPeptide/protein ingredientsGenetic material ingredientsEpidermal Growth Factor Receptor KinaseAntigen
The present invention belongs to the fields of molecular biology and immunology and relates to a chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and a composition and uses thereof The present invention particularly relates to the chimeric antigen receptor comprising polypeptides efficiently combining the EGFR family proteins and transmembrane domains, wherein the polypeptides efficiently combining EGFR family proteins comprise HERIN. The chimeric antigen receptor can promote the proliferation ability and killing effect of T cell at the premise of maintaining the killing specificity of the T cell.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
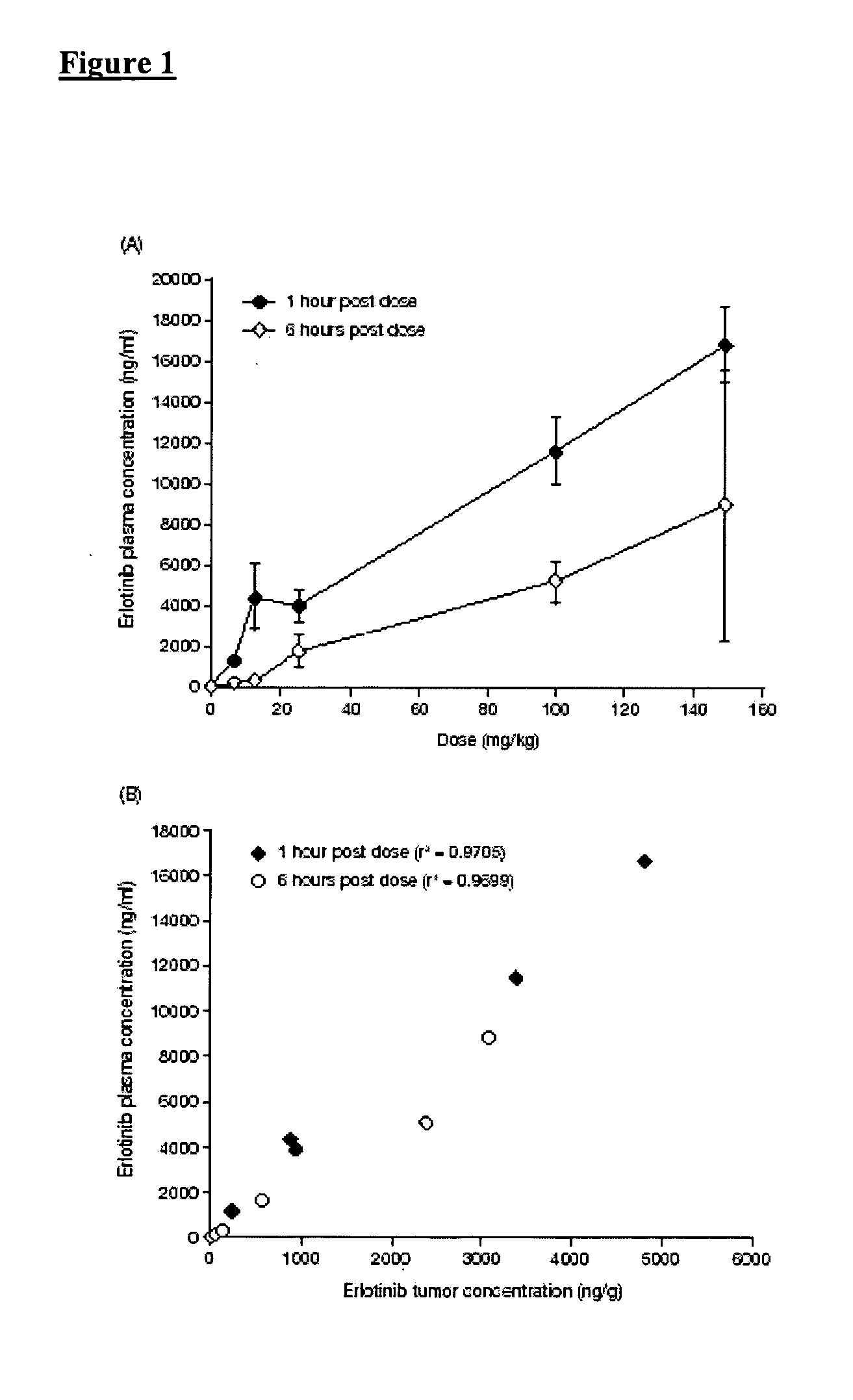
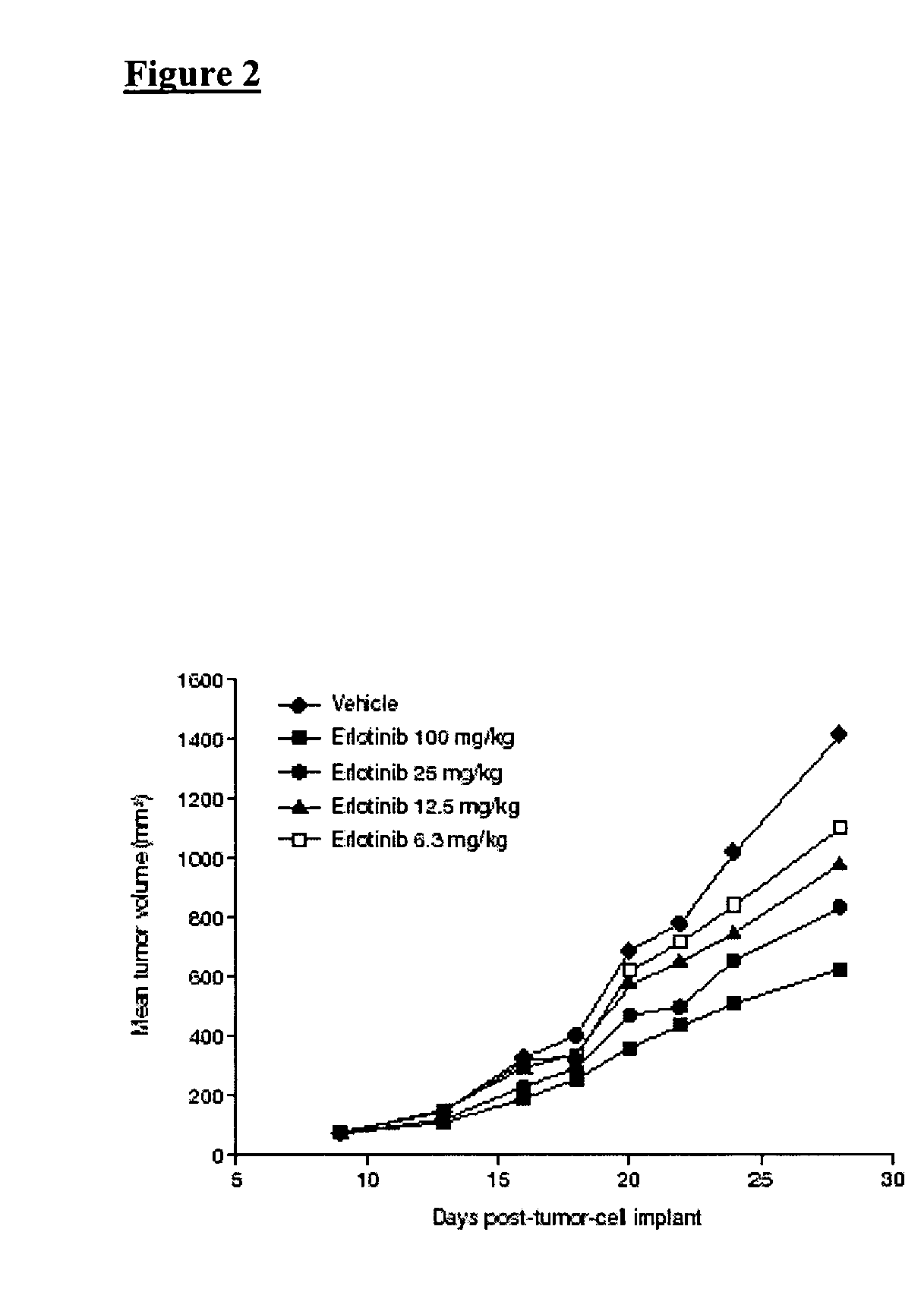
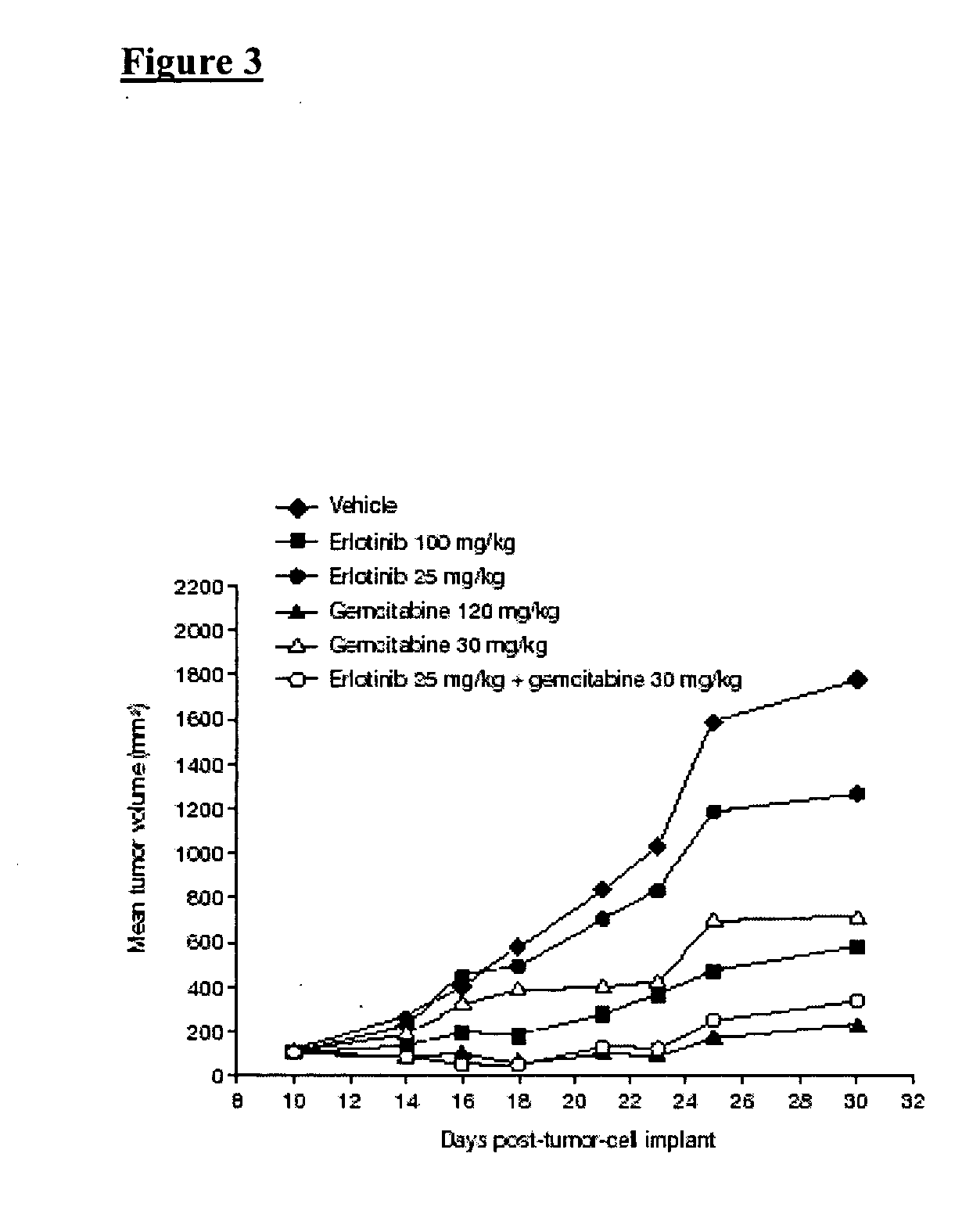
Combined treatment with gemcitabine and an epidermal growth factor receptor kinase inhibitor
InactiveUS20050272688A1BiocideCarbohydrate active ingredientsEpidermal Growth Factor Receptor KinaseLymphatic Spread
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and gemcitabine combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and gemcitabine combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as Tarceva™).
Owner:OSI PHARMA INC
Use of a combination of an epidermal growth factor receptor kinase inhibitor and cytotoxic agents for treatment and inhibition of cancer
Owner:WYETH HOLDINGS CORP
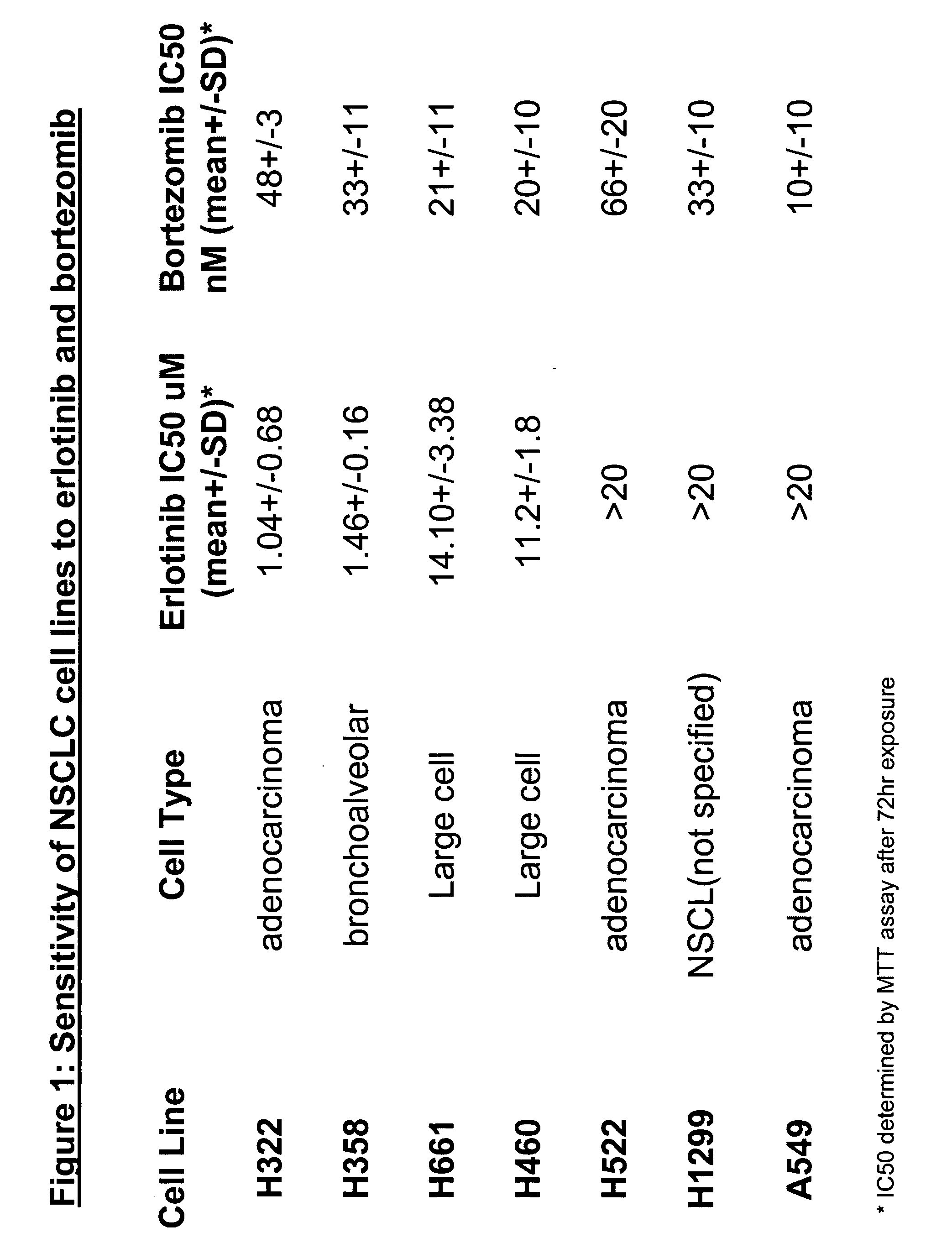
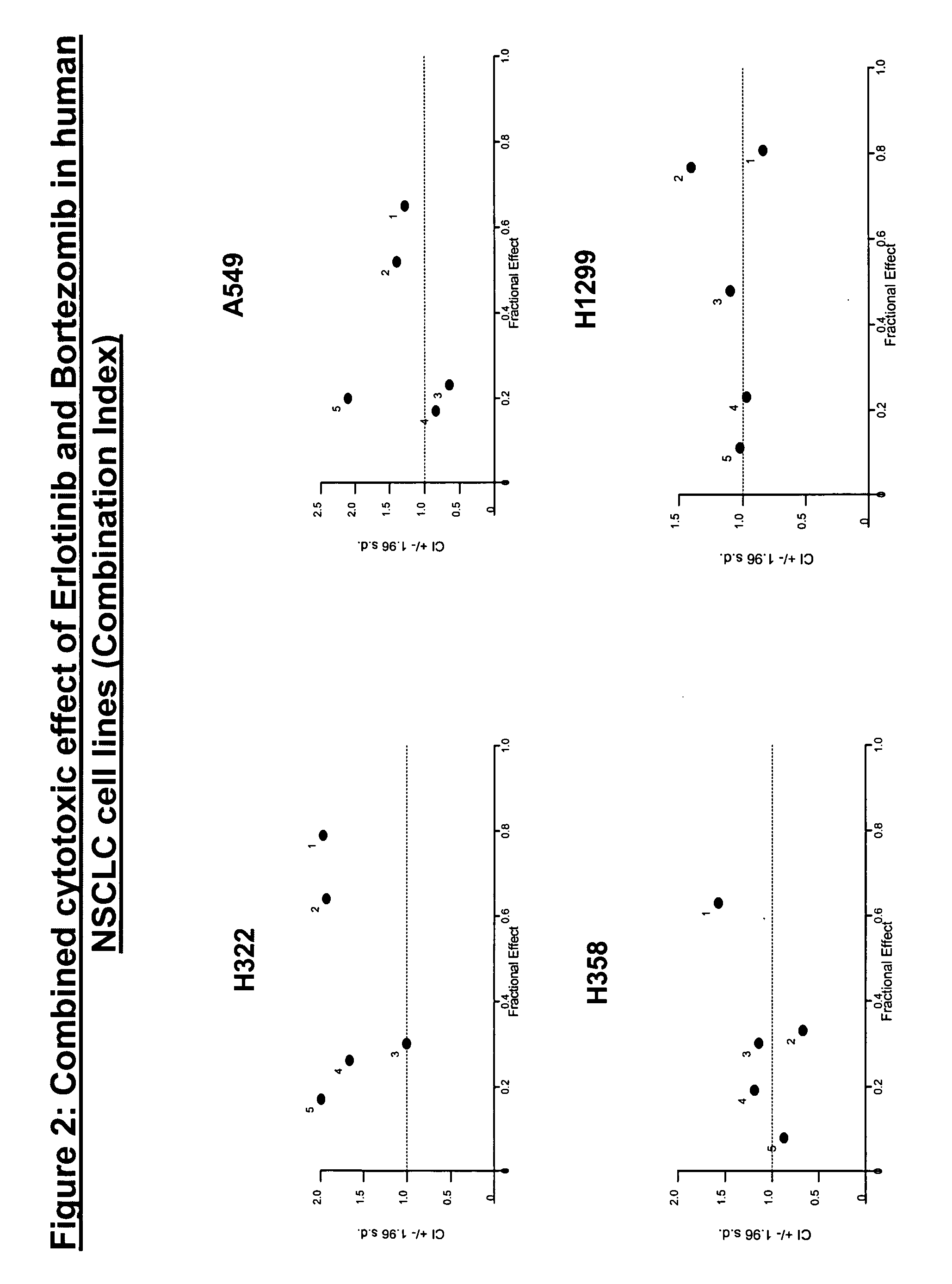
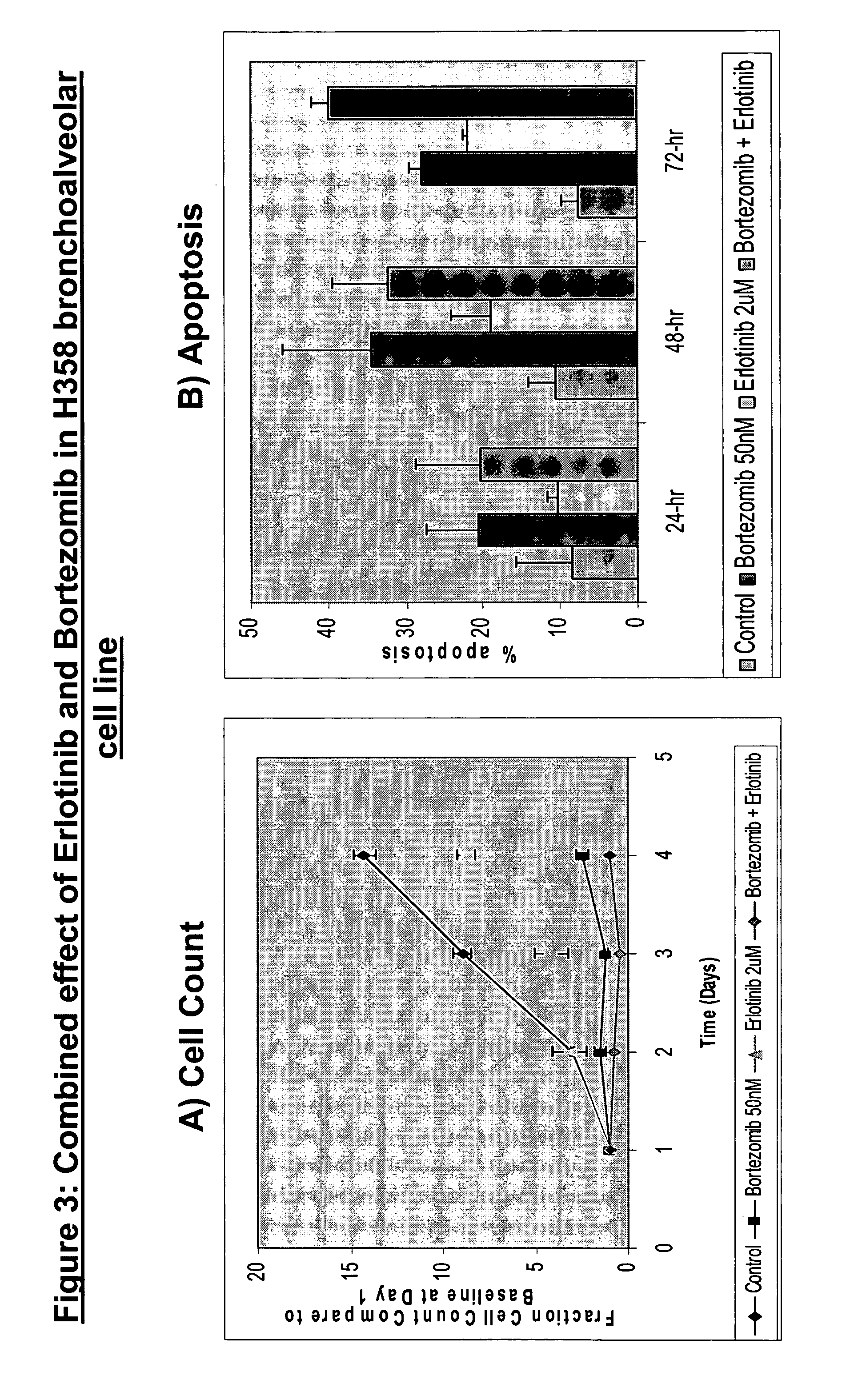
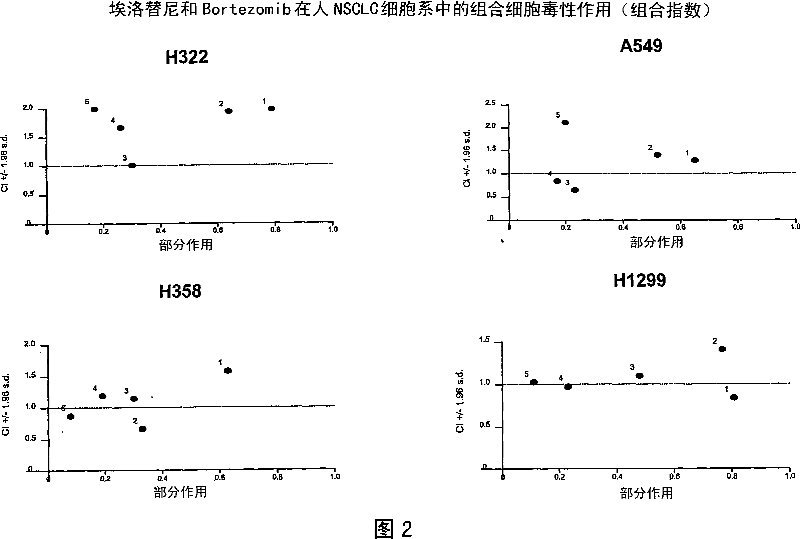
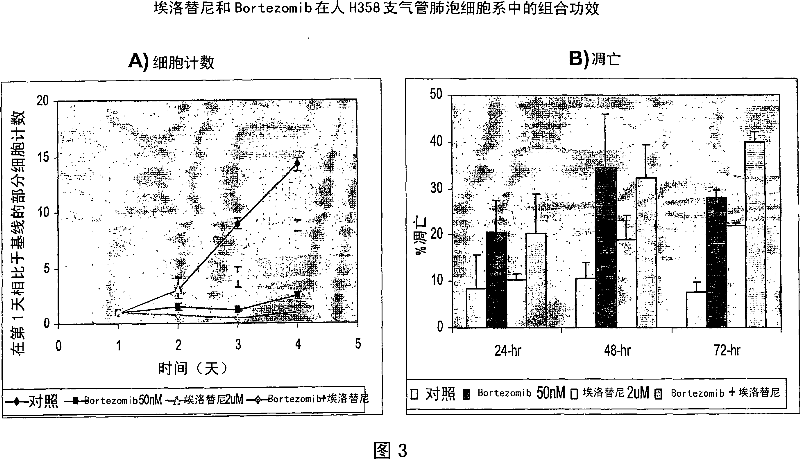
Combined treatment with bortezomib and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060084691A1BiocideBoron compound active ingredientsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and bortezomib combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and bortezomib combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlitinib HCl (also known as Tarceva™).
Owner:PIPERDI BILAL
Combined treatment with cisplatin and an epidermal growth factor receptor kinase inhibitor
ActiveUS20050271747A1BiocideHeavy metal active ingredientsEpidermal Growth Factor Receptor KinaseAbnormal tissue growth
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and cisplatin combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and cisplatin combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as Tarceva™).
Owner:OSI PHARMA INC
Protein tyrosine kinase modulators and methods of use
InactiveCN105377835AOrganic active ingredientsOrganic chemistryKinase activityTyrosine Protein Kinases
Heterocyclic pyrimidine compounds that modulate mutant-selective epidermal growth factor receptor (EGFR) and ALK kinase activity are disclosed. More specifically, the invention provides pyrimidines which inhibit, regulate and / or modulate kinase receptor, particularly in selectively modulation of various EGFR mutant activity and ALK kinase activity have been disclosed. Pharmaceutical compositions comprising the pyrimidine derivative,and methods of treatment for diseases associated with protein kinase enzymatic activity, particularly EGFR or ALK kinase activity including non-small cell lung cancer comprising administration of the pyrimidine derivative are disclosed.
Owner:BETTA PHARM CO LTD
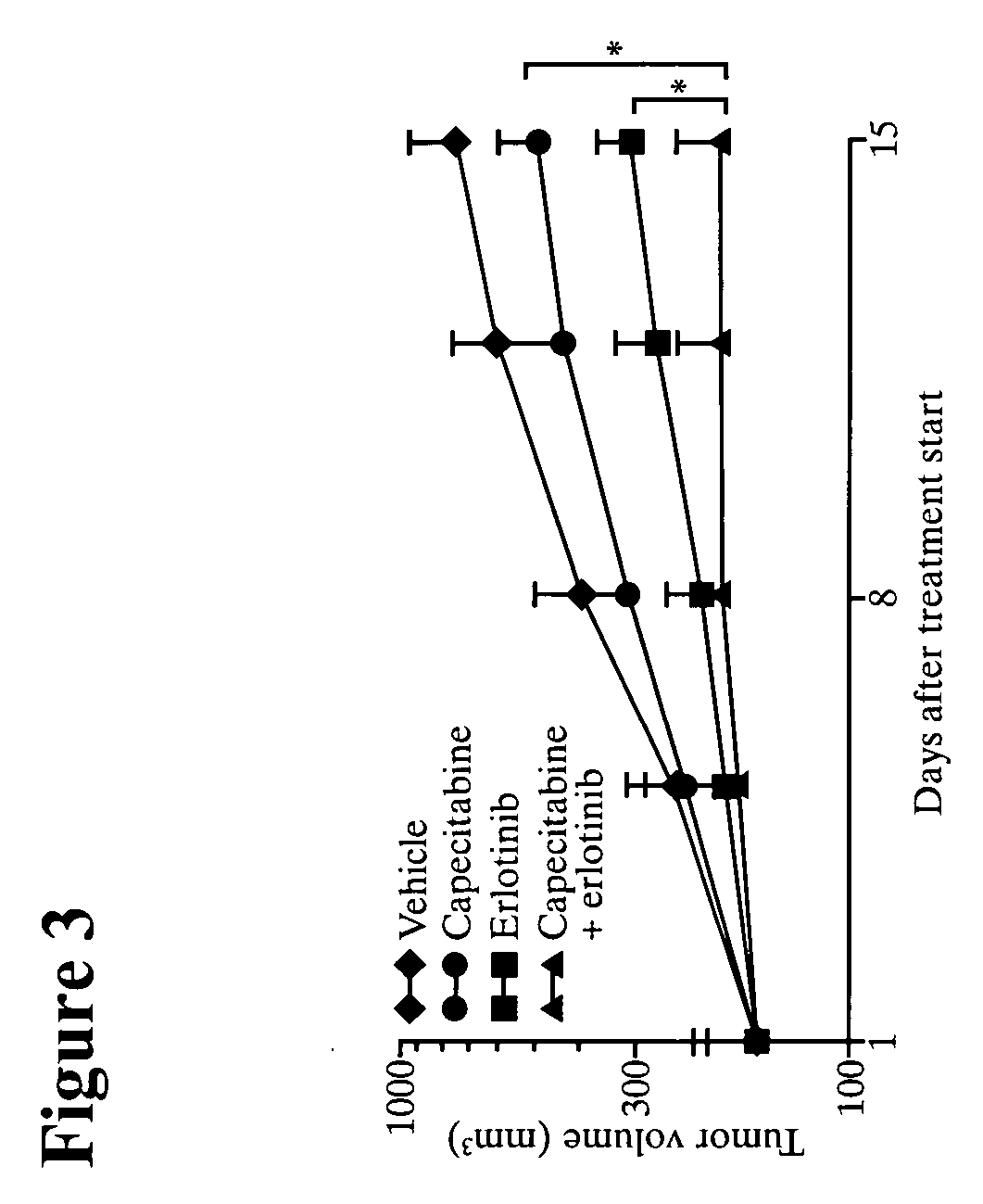
Combined treatment with capecitabine and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060178387A1Organic active ingredientsBiocideEpidermal Growth Factor Receptor KinaseLymphatic Spread
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and capecitabine combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and capecitabine combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as TARCEVA®).
Owner:FUJIMOTO OUCHI KAORI +3
Use of an epidermal growth factor receptor kinase inhibitor (EGFR) in gefitinib resistant patients
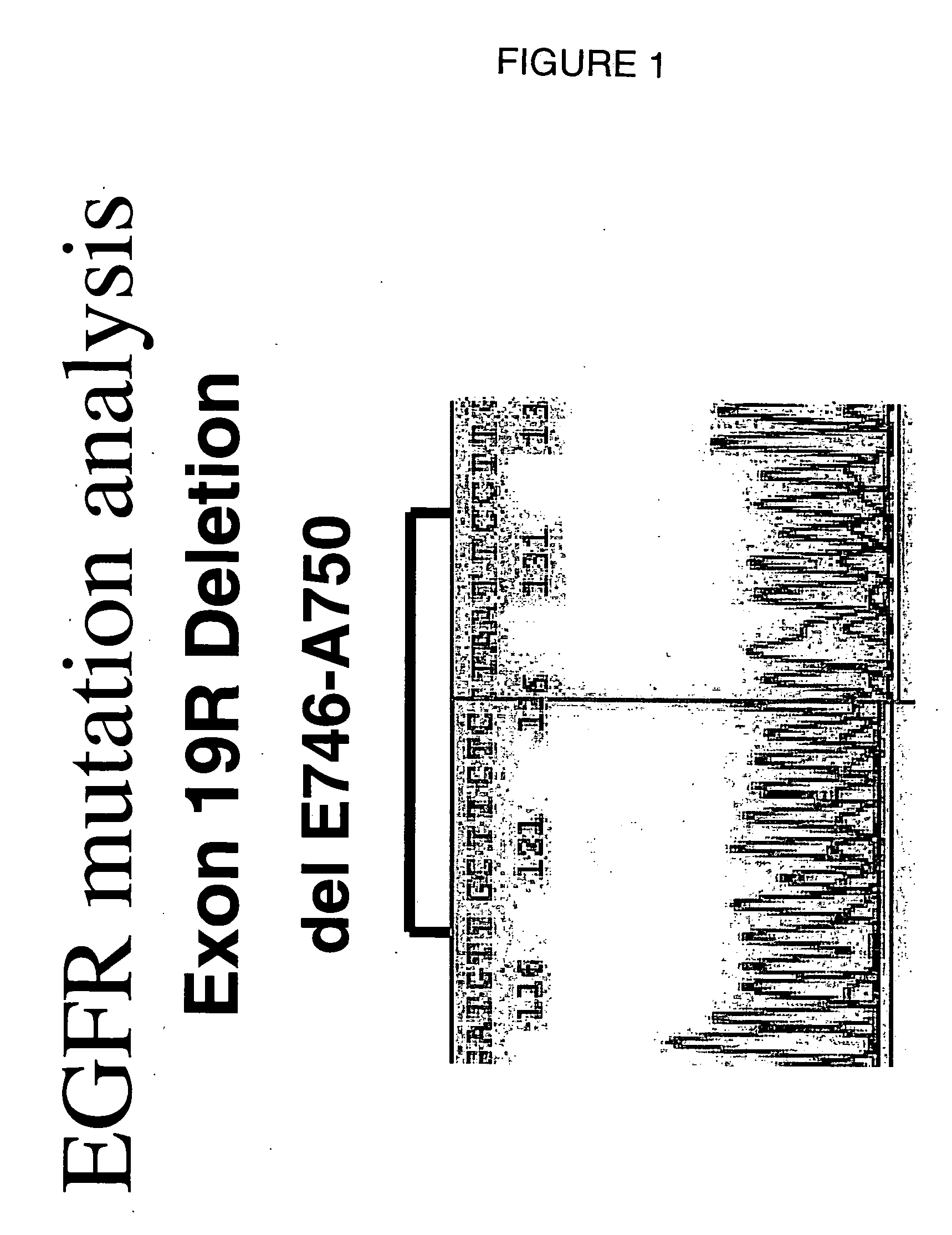
This invention discloses method of treating or inhibiting cancer in a human having at least one of an Exon 19 del E746-A750 and / or an Exon 21 point mutation comprising administering to said human gefitinib and / or iressa alone or in combination with other cytotoxic agents or chemotherapeutic agents and an effective amount of EGFR kinase inhibitor.
Owner:WYETH
Biological markers predictive of Anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20120142028A1Long survival progression free survivalEffectiveness of treatmentDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseErlotinib
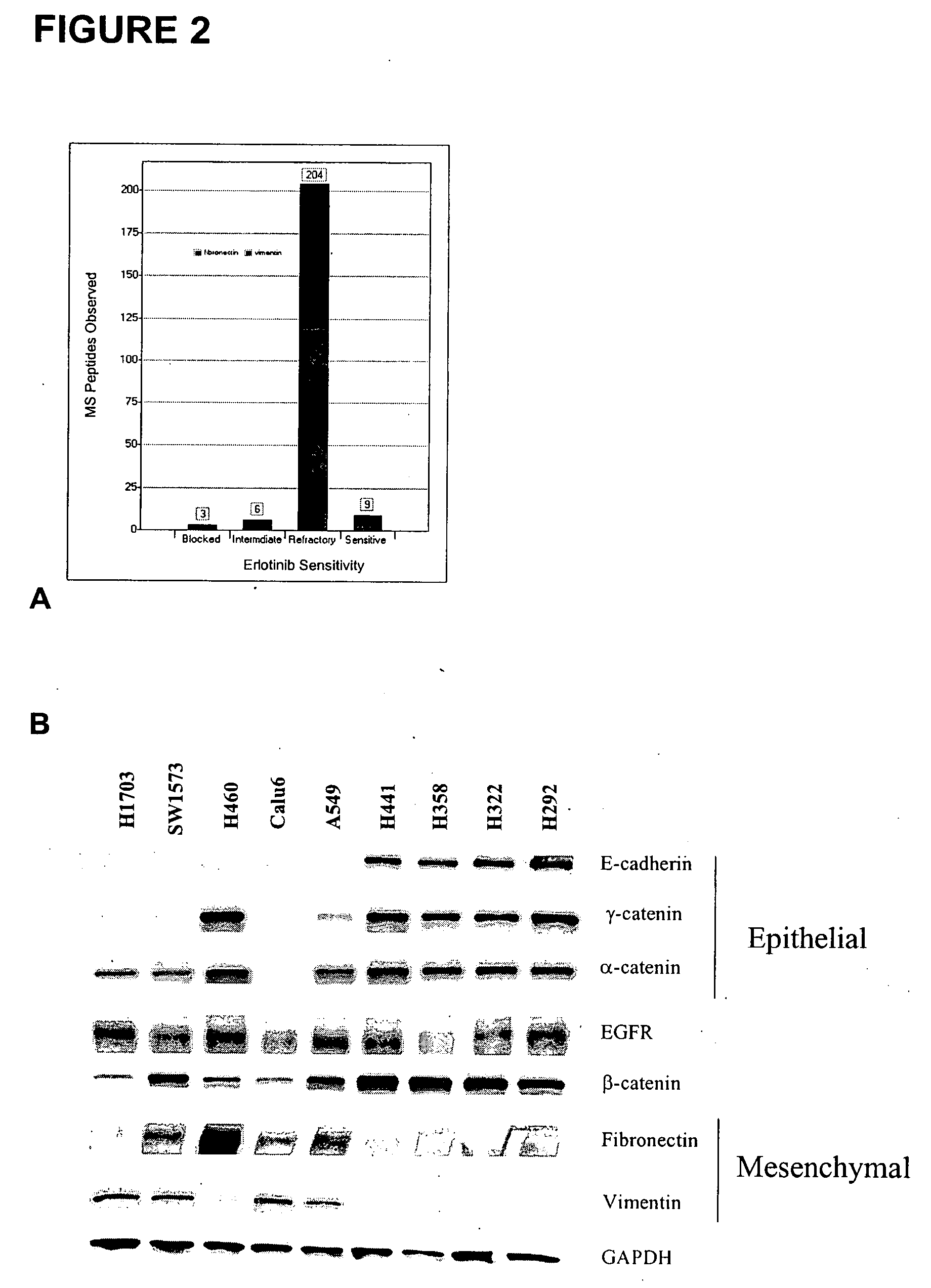
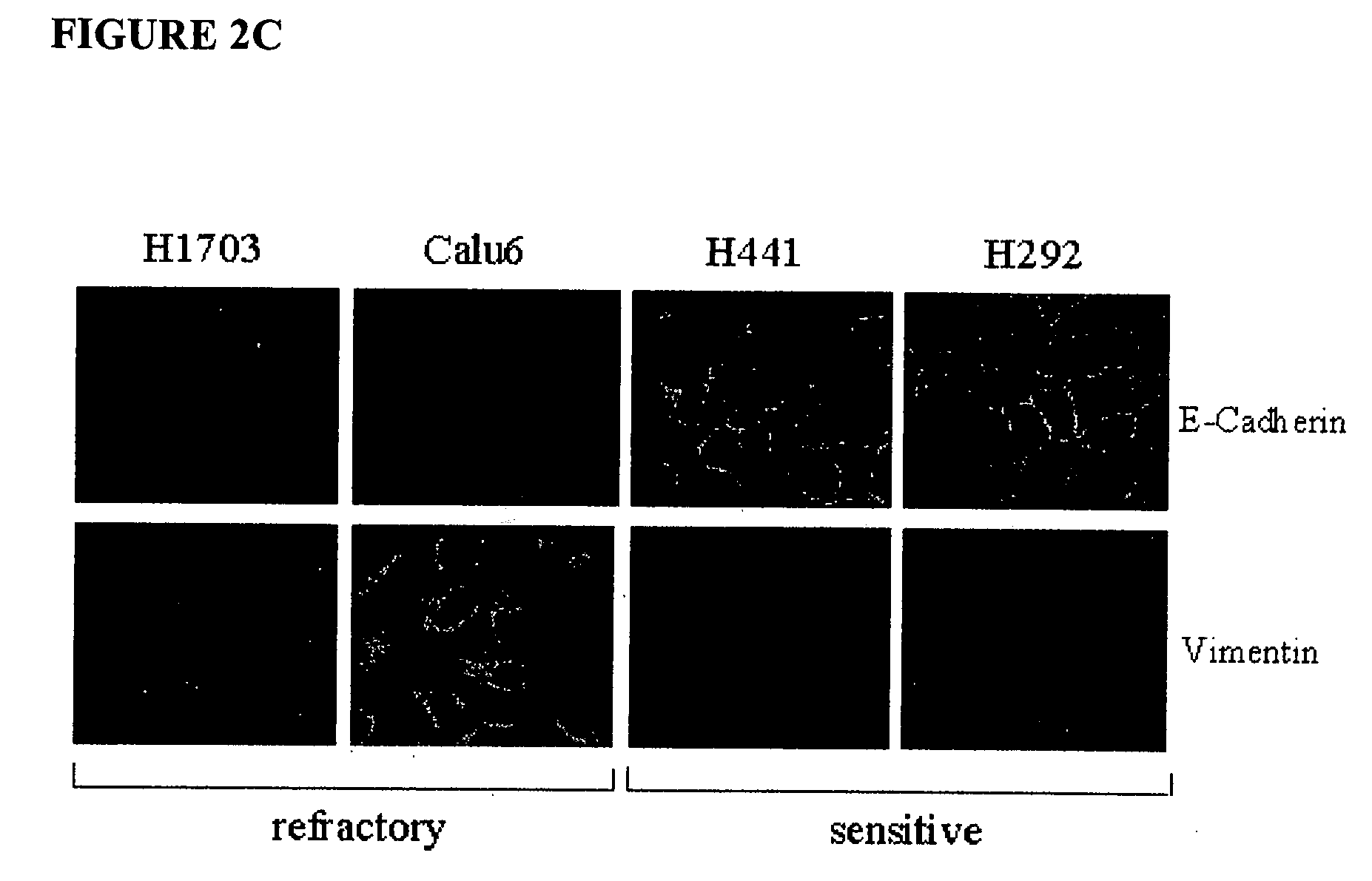
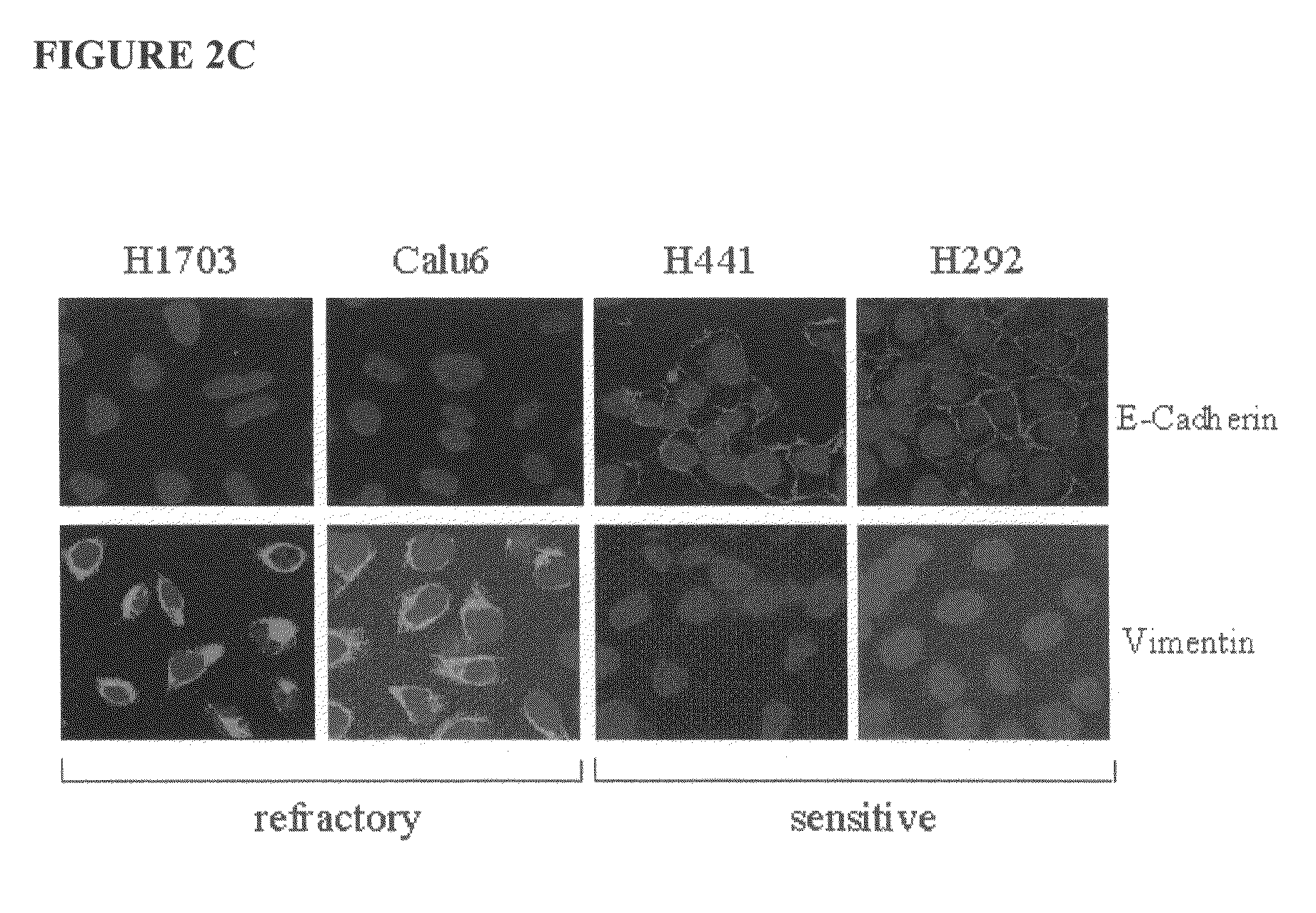
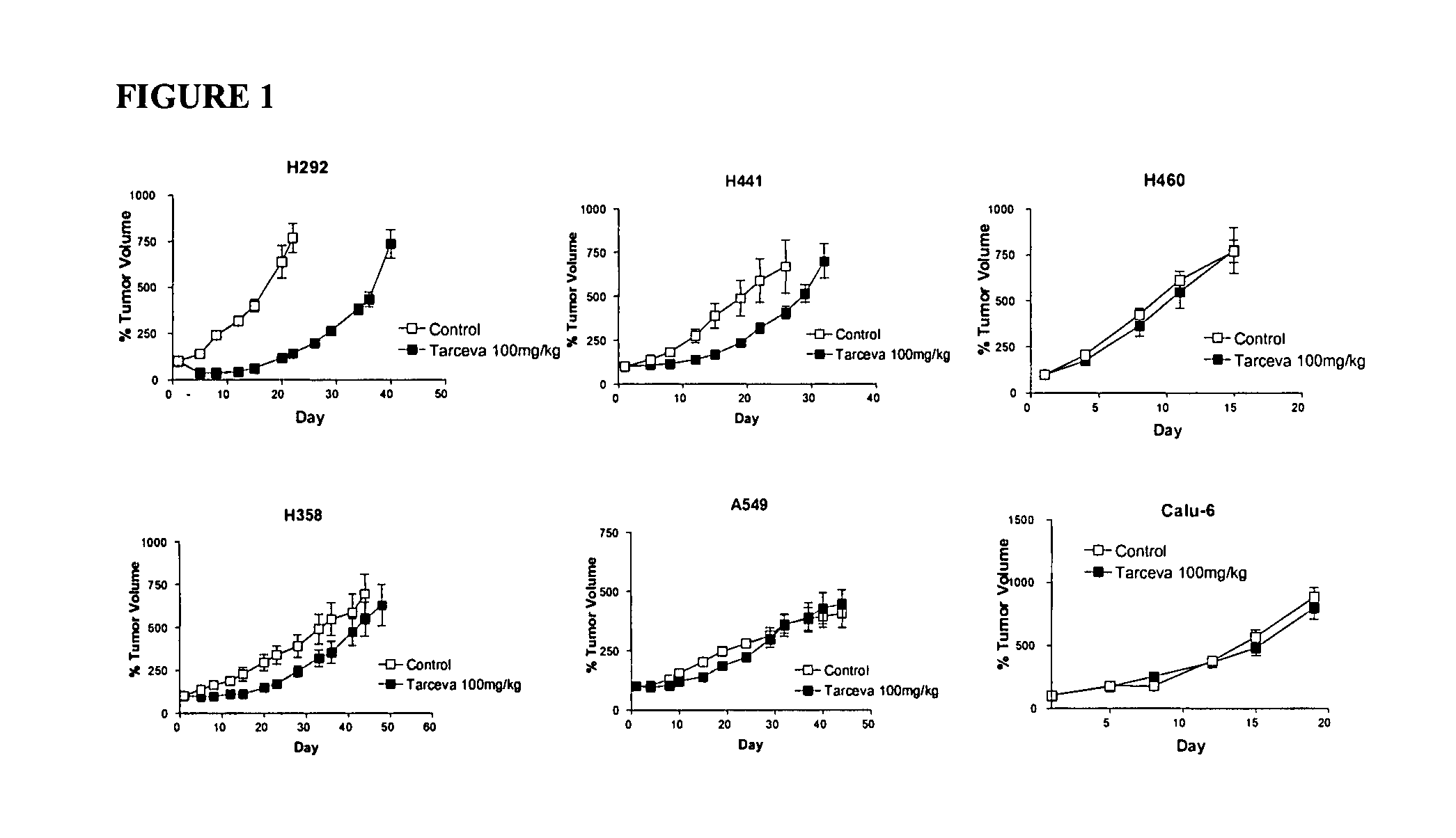
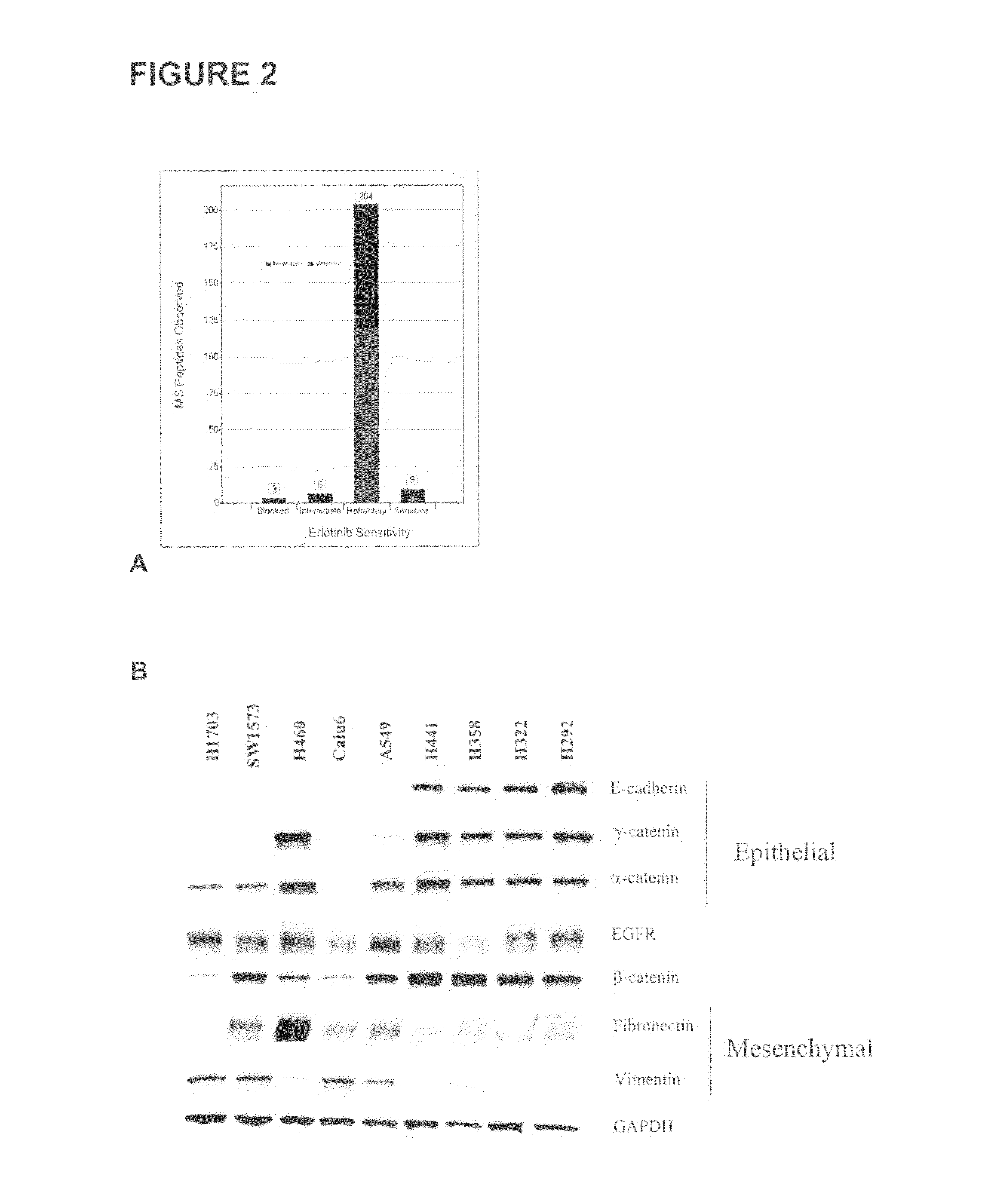
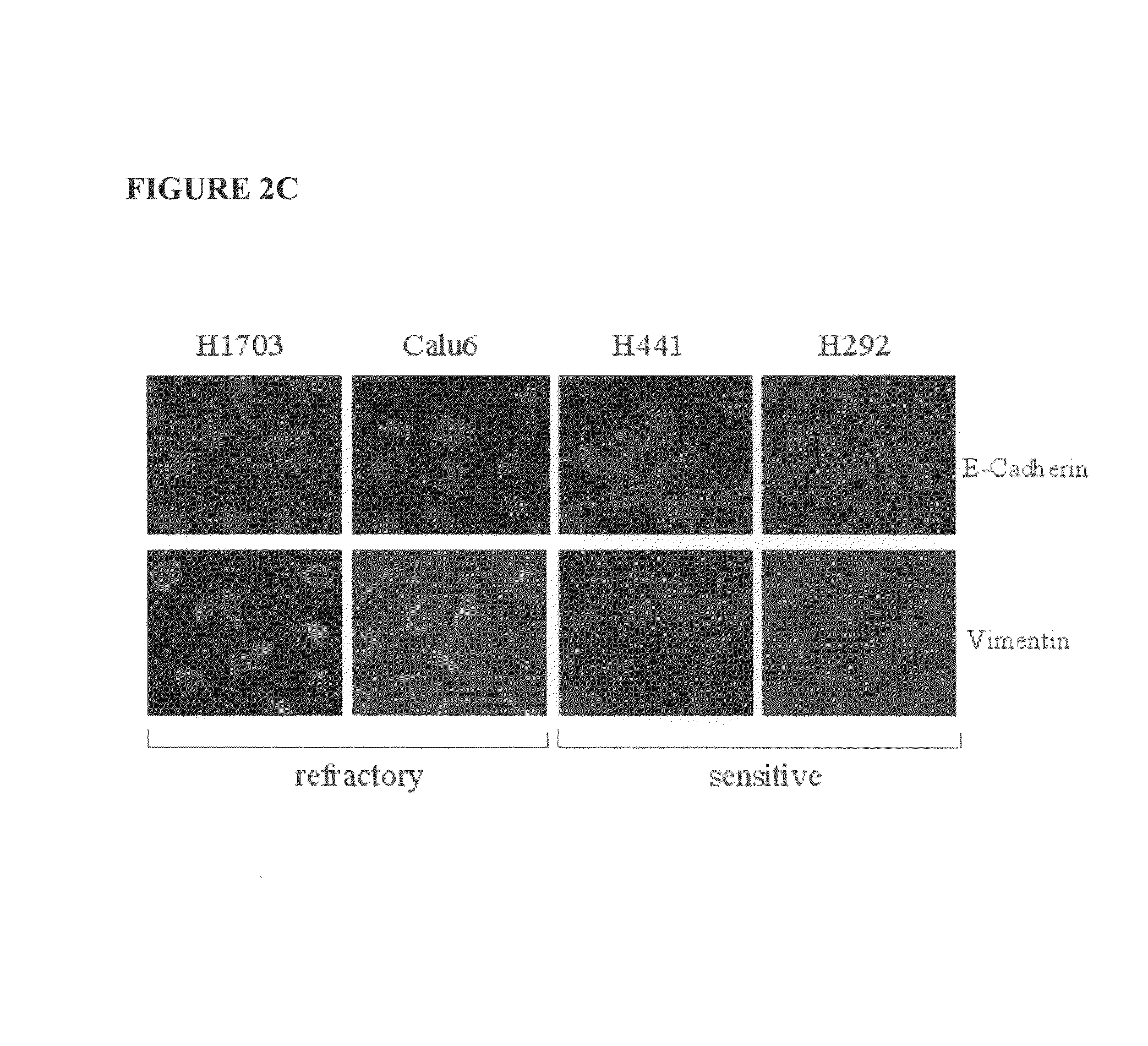
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. These methods are based on the surprising discovery that the effectiveness of treatment with an EGFR kinase inhibitor is predicted by whether a patient's tumor cells express a high or a low level of the biomarkers vimentin and E-cadherin, such that patients whose tumors express a high level of at least one of the biomarkers vimentin and E-cadherin have a longer overall survival and progression free survival than patients whose tumors express a low level of both vimentin and E-cadherin. The present invention further provides a method for treating tumors or tumor metastases in a patient, comprising the steps of diagnosing a patient's likely responsiveness to an EGFR kinase inhibitor by assessing whether tumor cells express a high level of at least one of the biomarkers vimentin and E-cadherin, and administering to said patient a therapeutically effective amount of an EGFR kinase inhibitor (e.g. erlotinib), particularly when effectiveness of the inhibitor is predicted.
Owner:OSI PHARMA LLC
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
ActiveUS8093011B2Organic active ingredientsDisease diagnosisEpidermal Growth Factor Receptor KinaseCell growth
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA INC
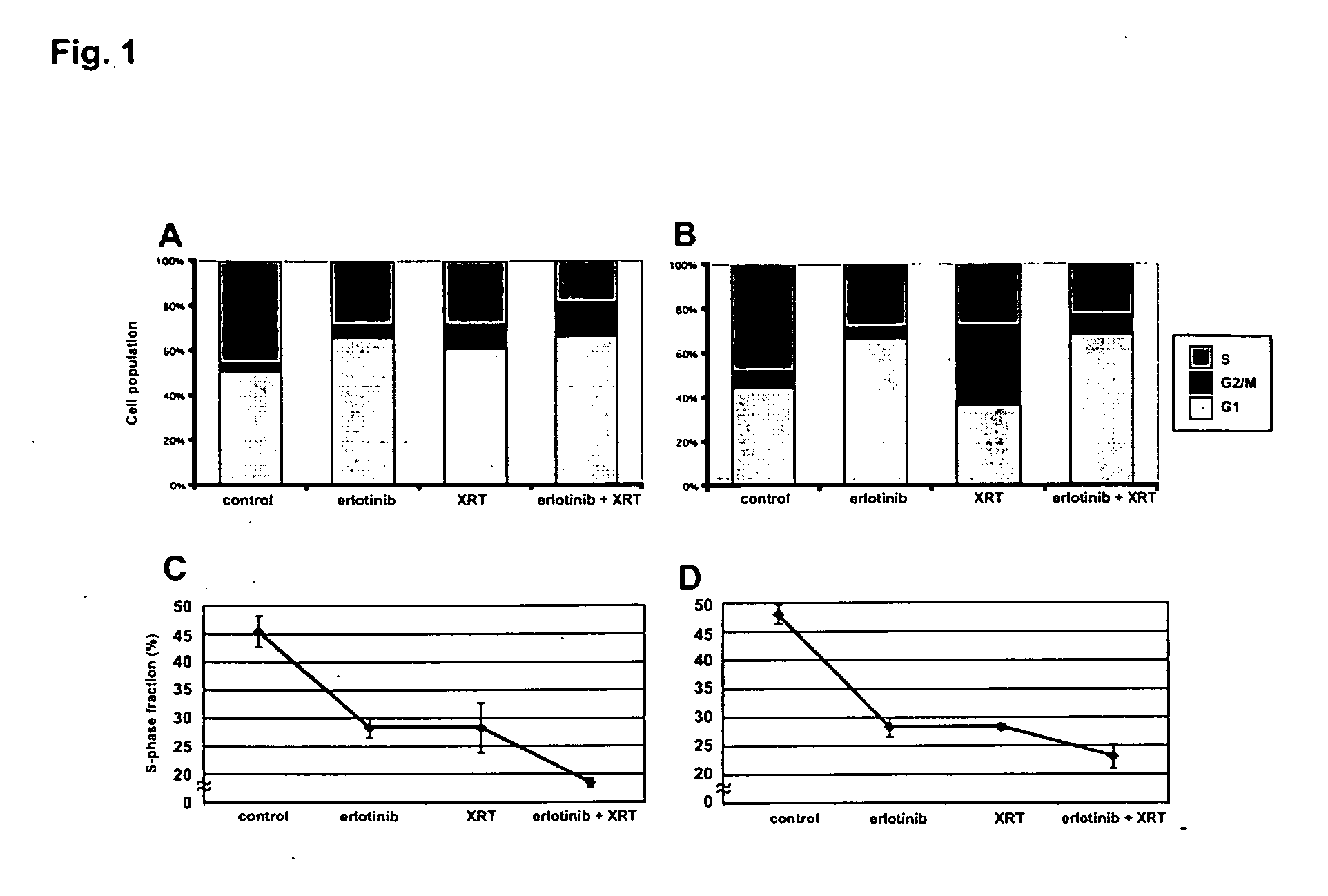
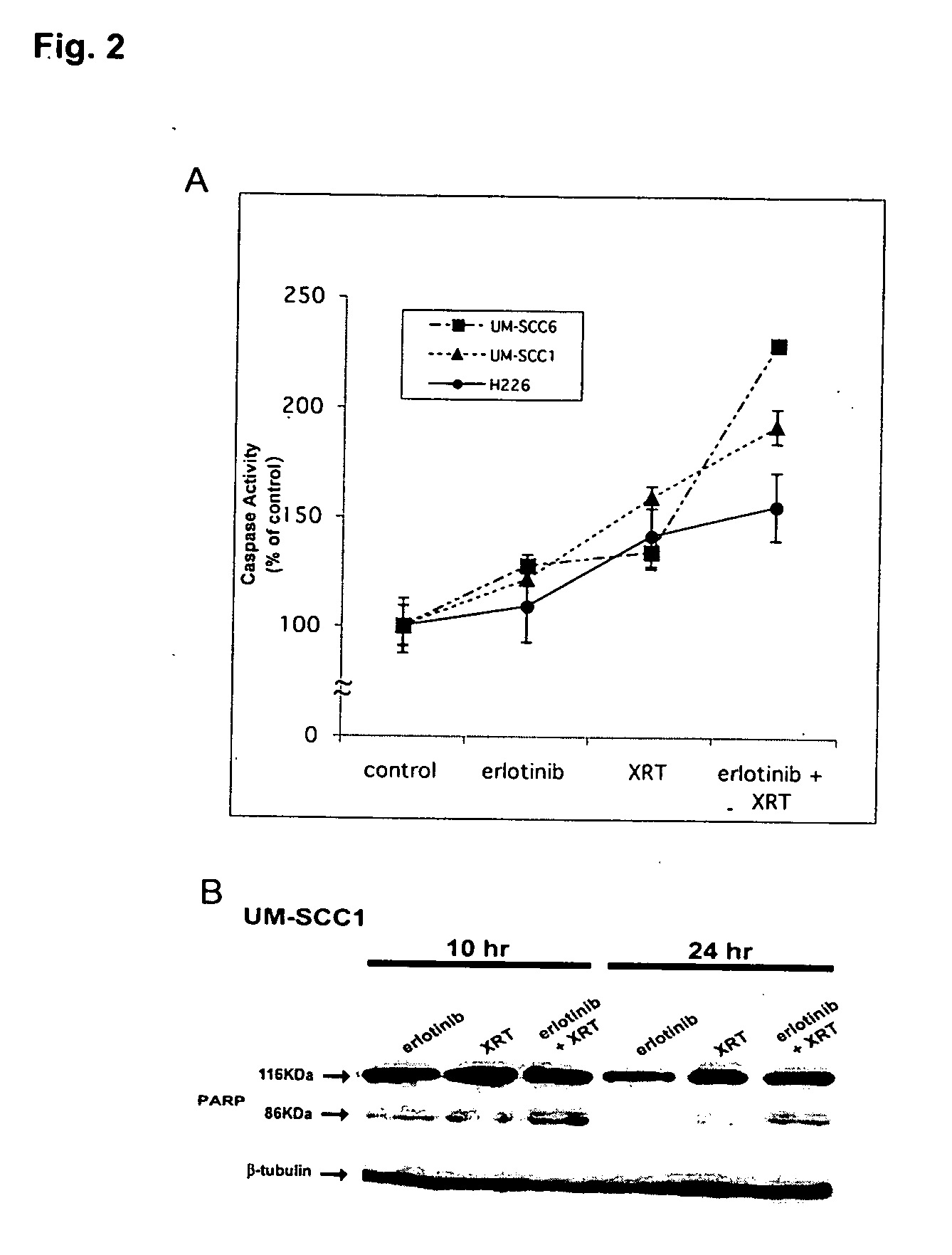
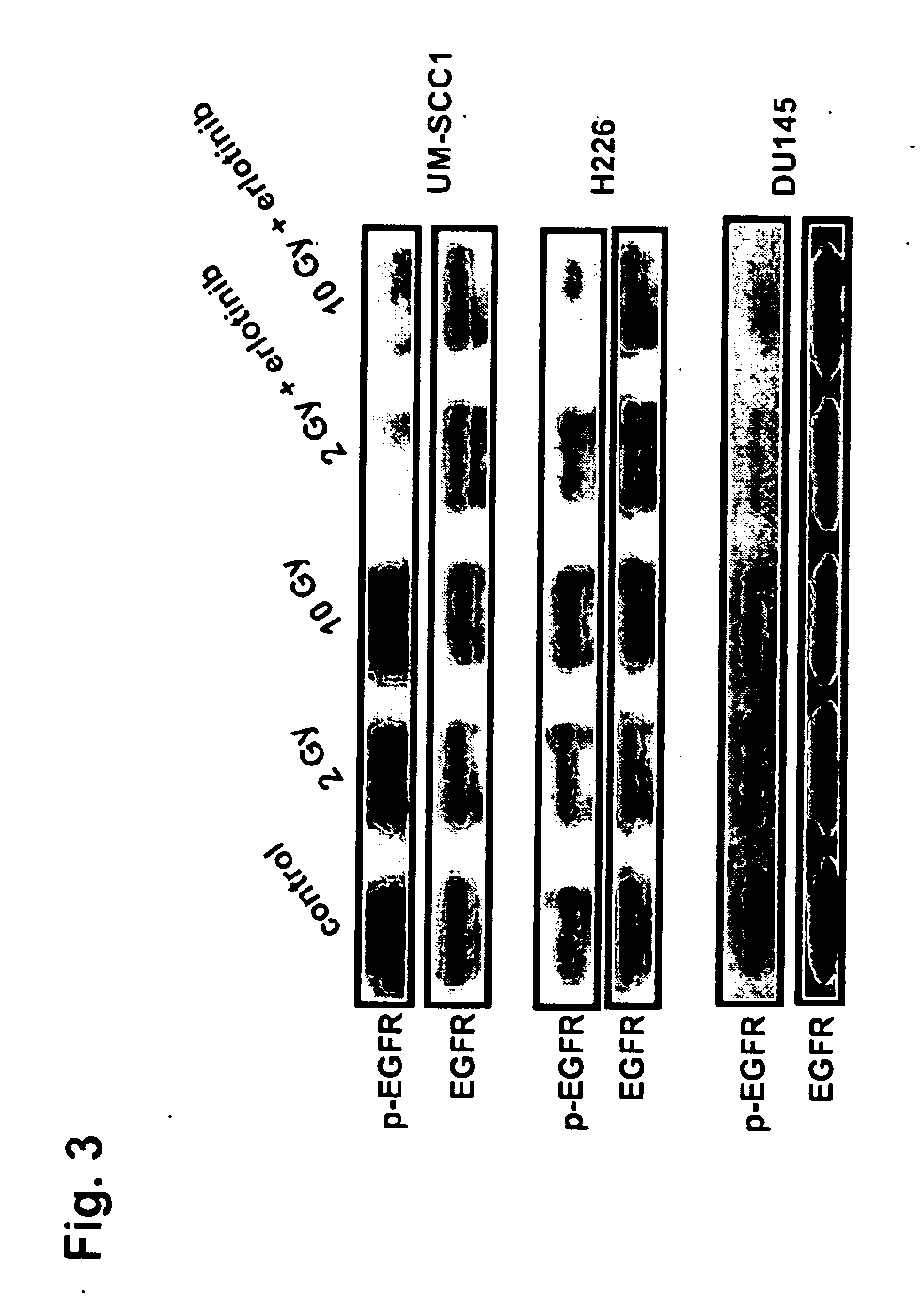
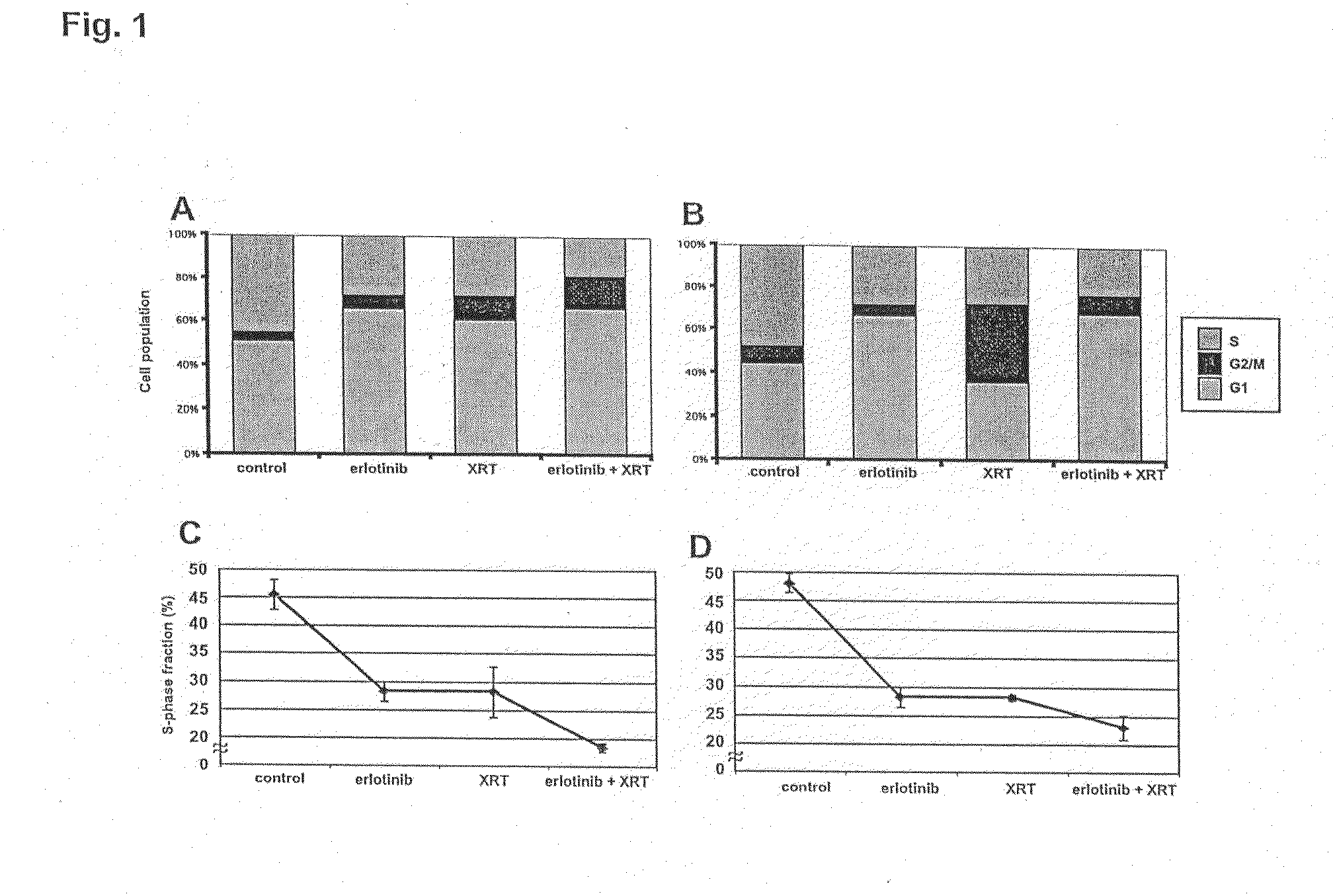
Combined treatment with radiation and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060084666A1BiocideEnergy modified materialsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient a therapeutically effective amount of an EGFR kinase inhibitor, combined with treating the patient simultaneously or sequentially with radiation therapy, with or without additional agents or treatments, such as other anti-cancer drugs. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlitinib HCl (also known as Tarceva™).
Owner:WISCONSIN ALUMNI RES FOUND
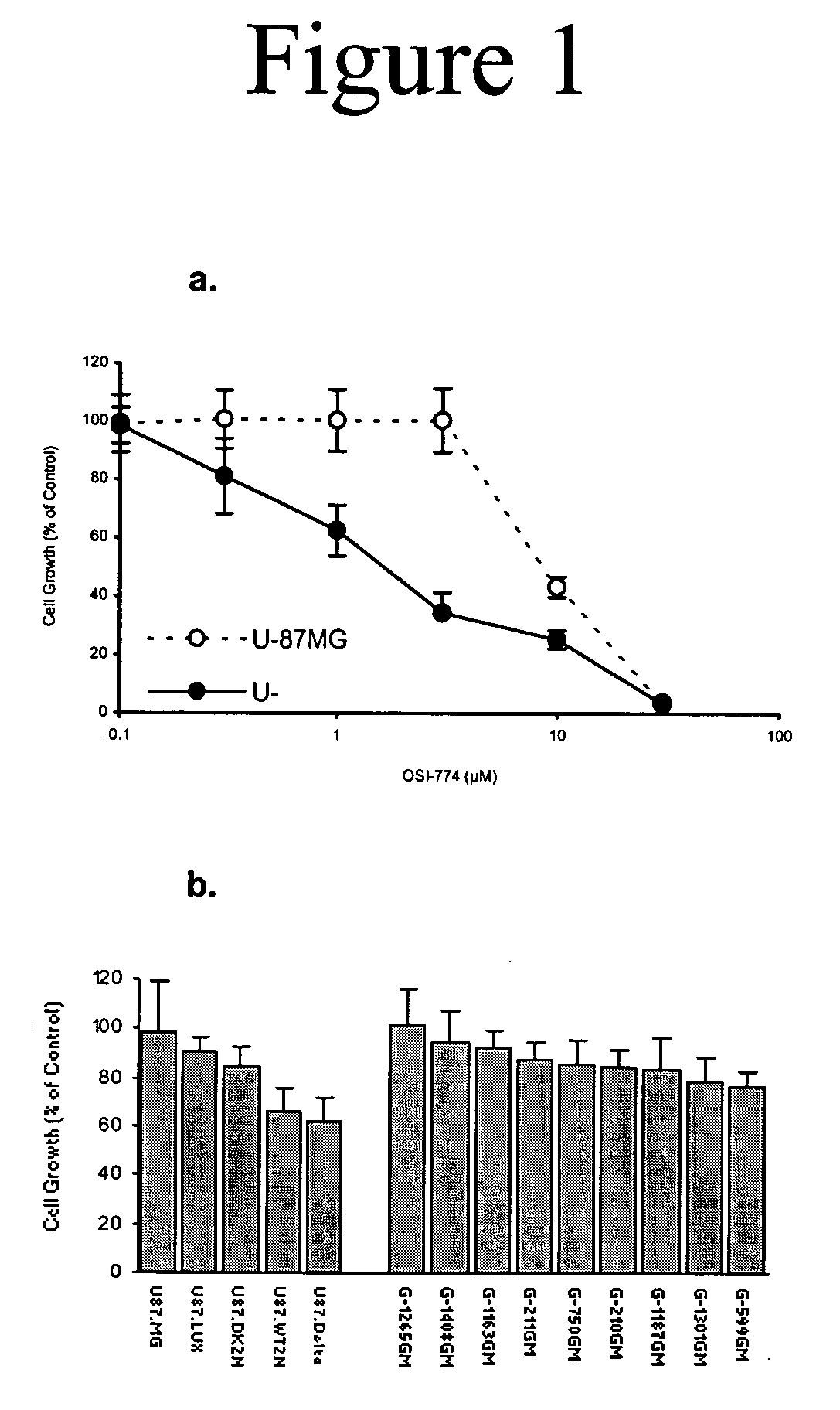
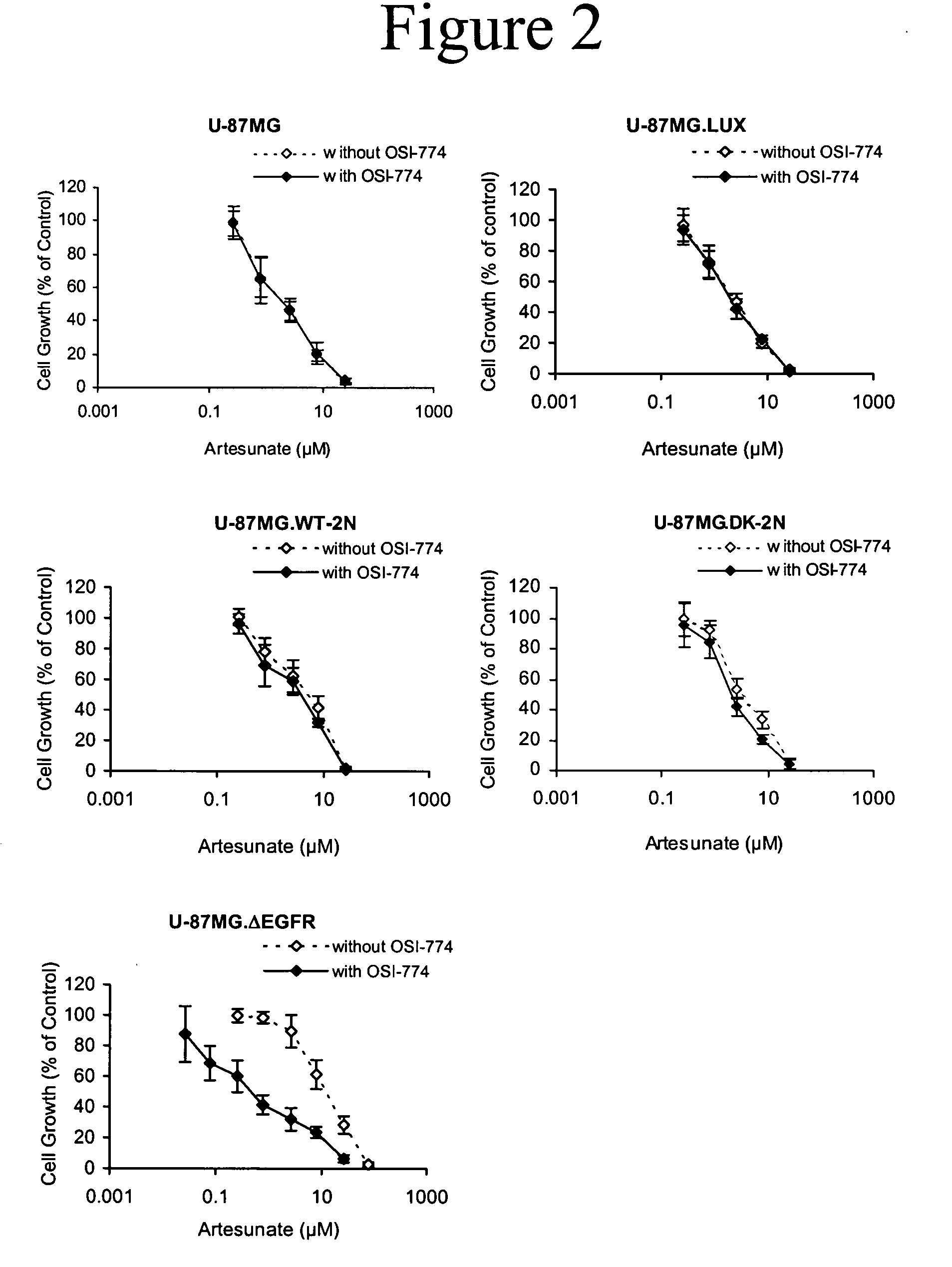
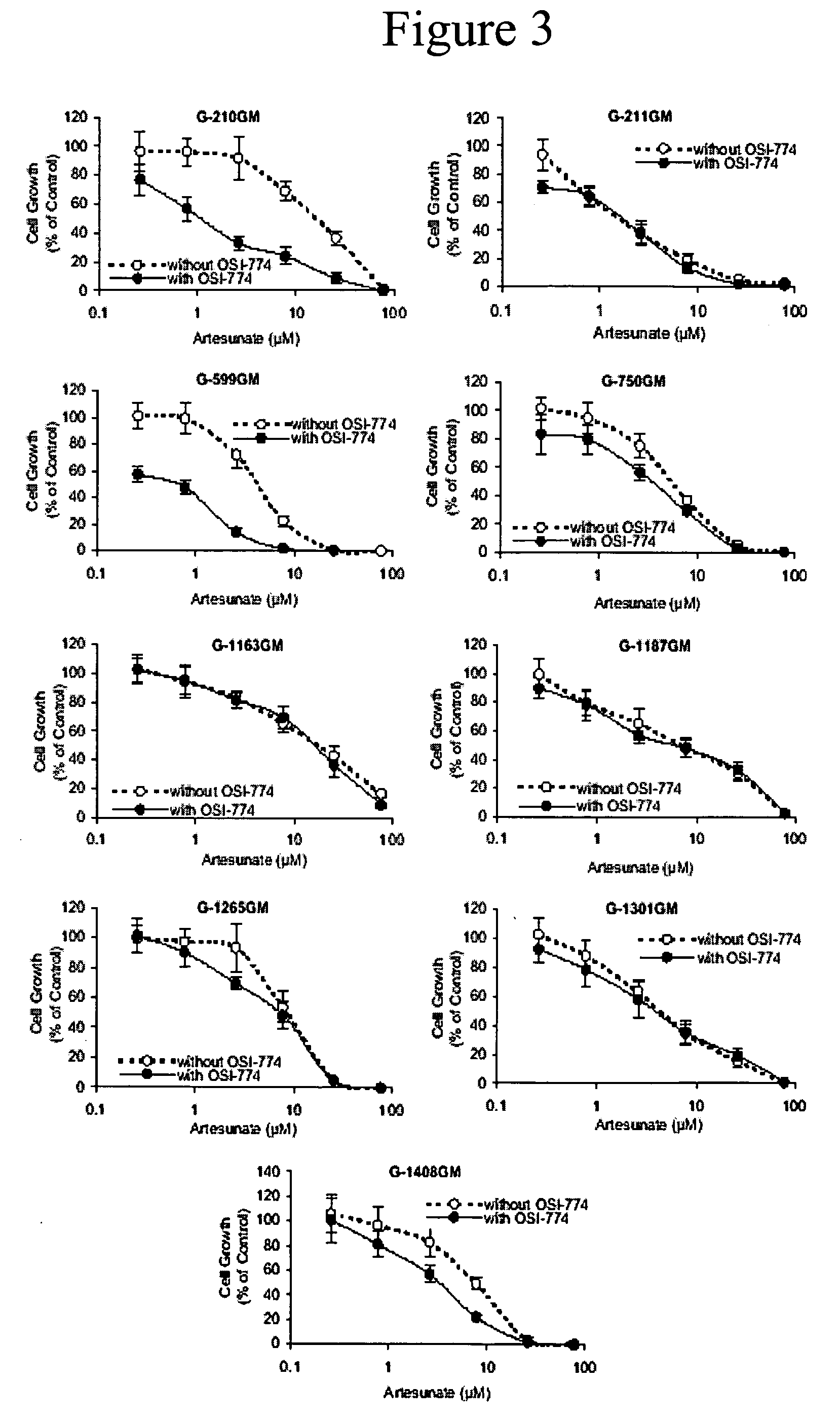
Combined treatment with artesunate and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060084675A1BiocideAnimal repellantsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and artesunate combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and artesunate combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as Tarceva™).
Owner:EFFERTH THOMAS +1
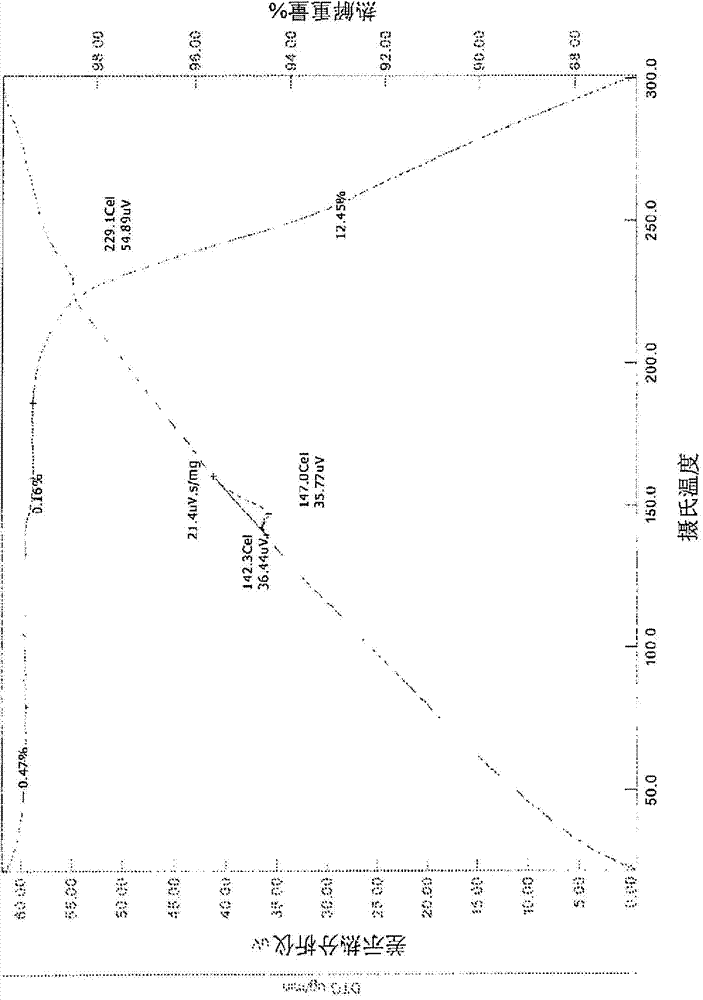
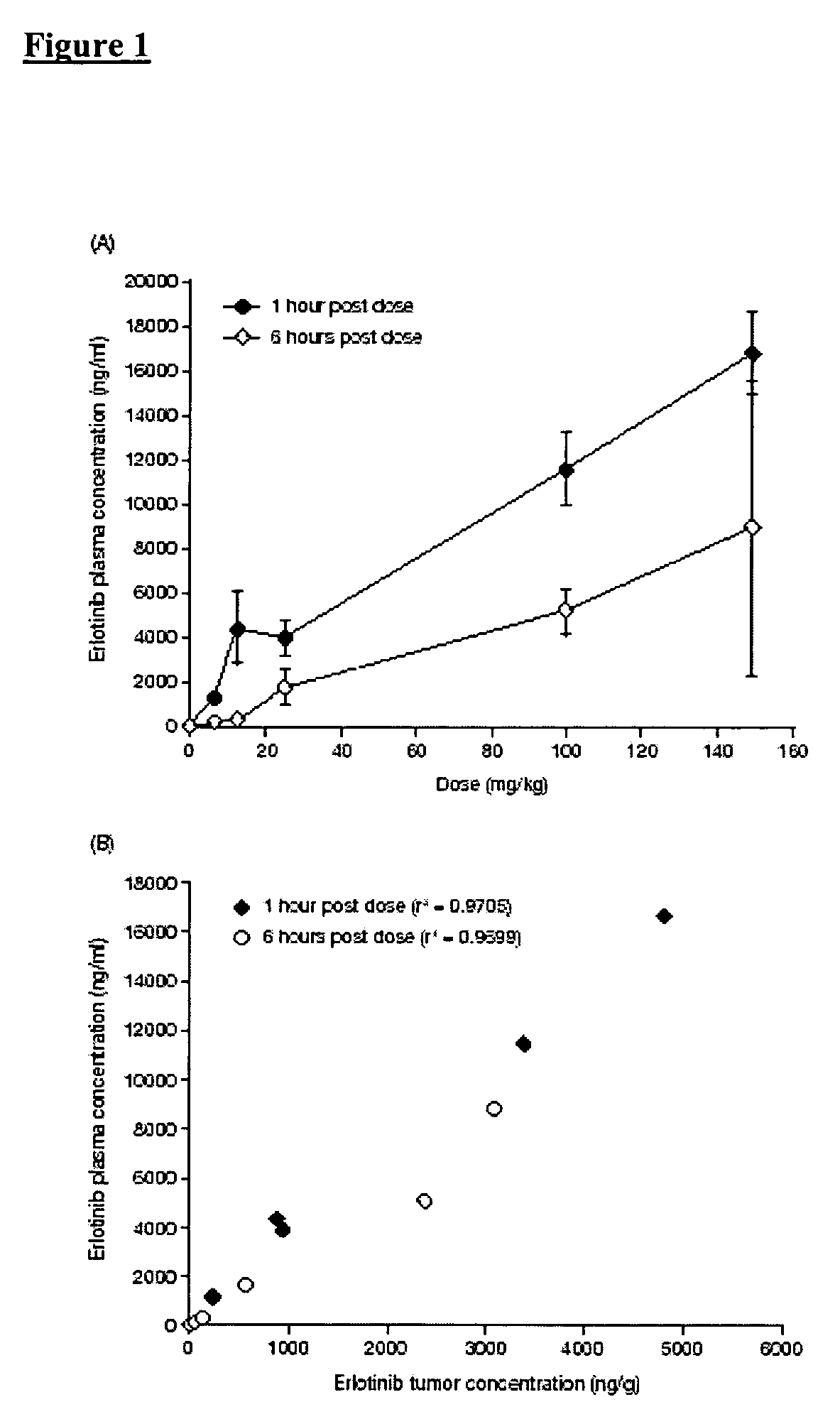
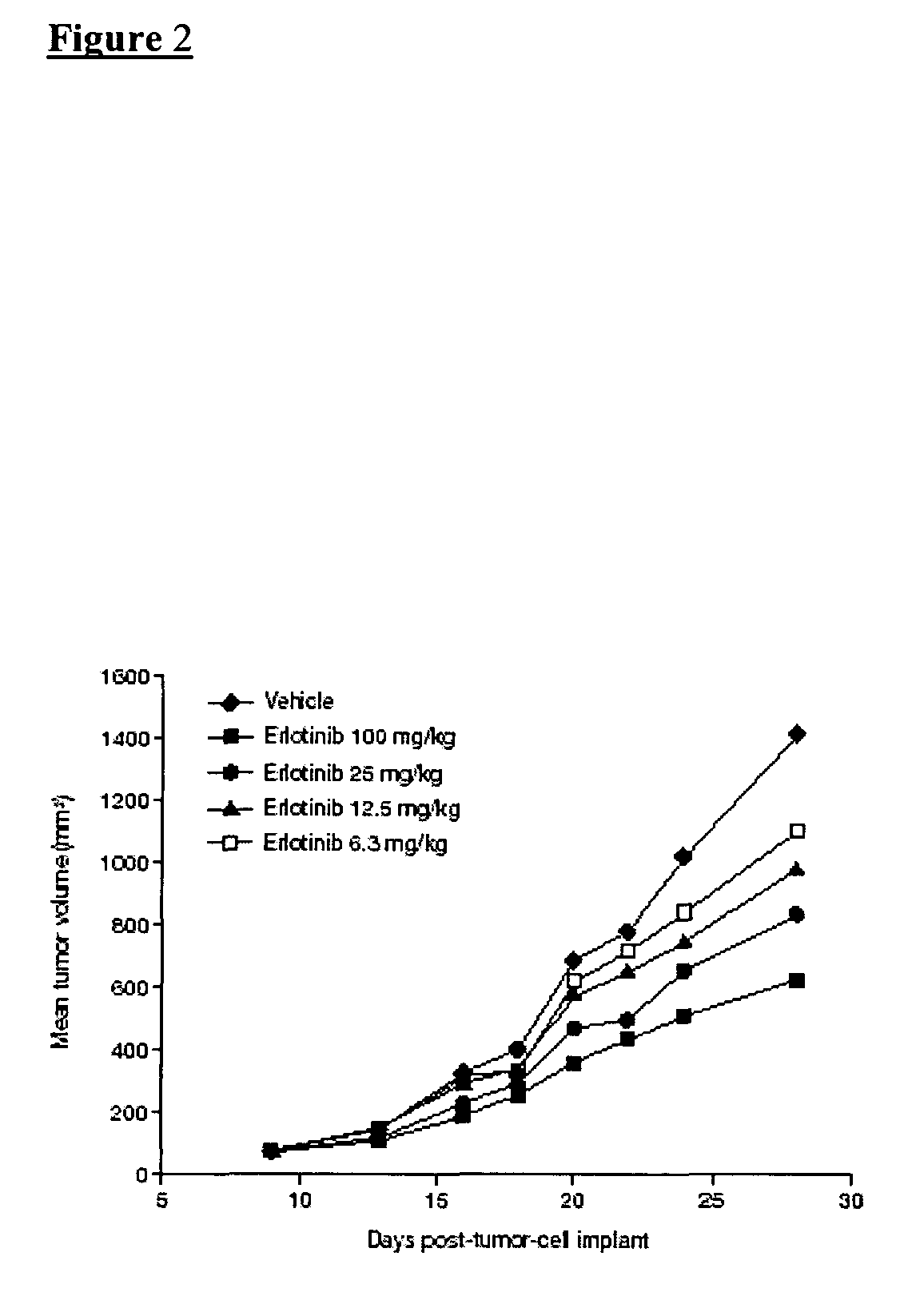
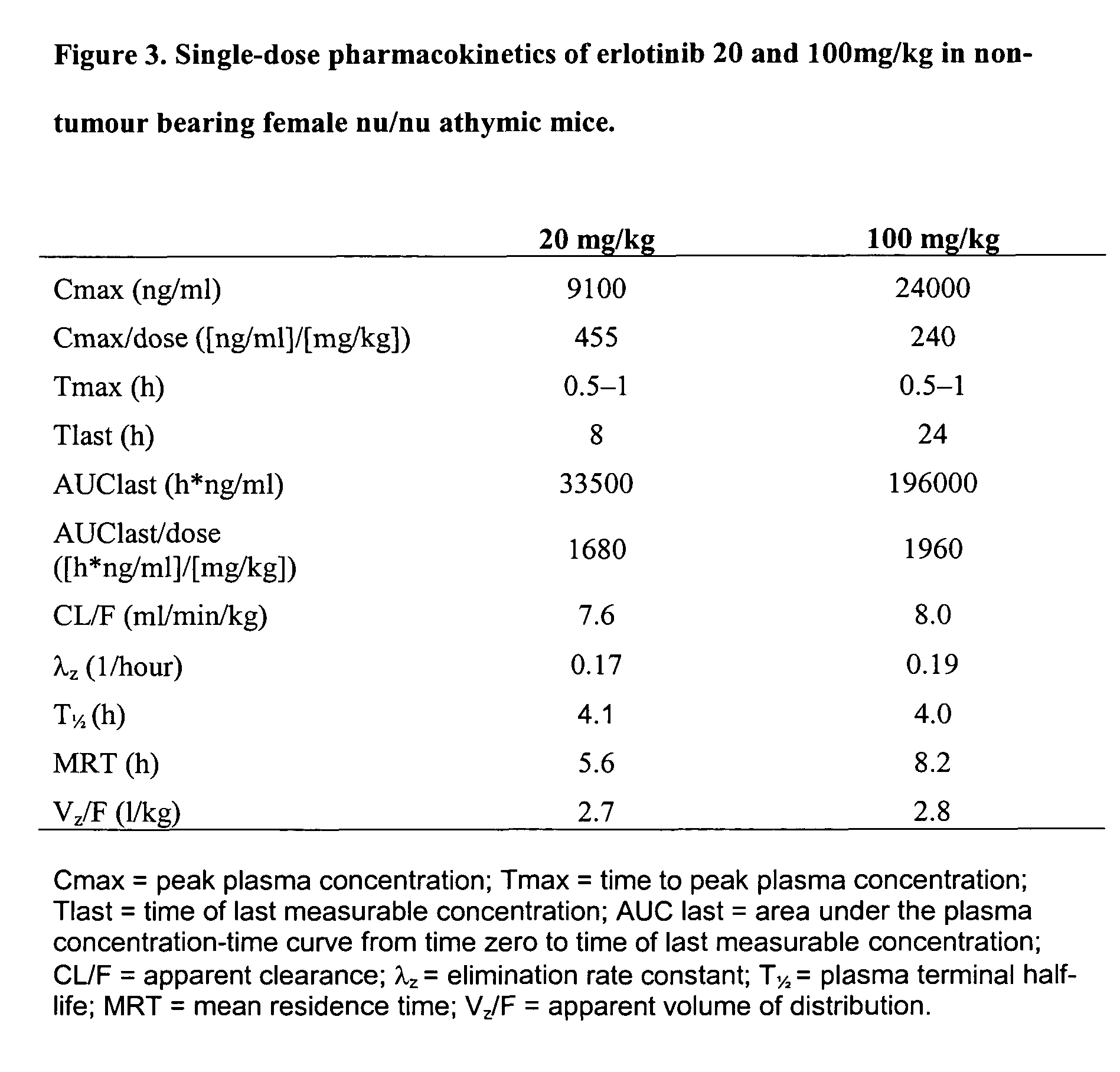
Solid forms of an epidermal growth factor receptor kinase inhibitor
ActiveCN104302178AReduce severityBiocideOrganic active ingredientsEpidermal Growth Factor Receptor KinaseKinase
The present invention provides a solid form and compositions thereof, which are useful as an inhibitor of EGFR kinases and which exhibit desirable characteristics for the same.
Owner:BRISTOL MYERS SQUIBB CO
Salts of epidermal growth factor receptor kinase inhibitor
ActiveCN104284584AReduce severityOrganic active ingredientsBiocideEpidermal Growth Factor Receptor KinaseKinase
Owner:CELGENE CAR LLC
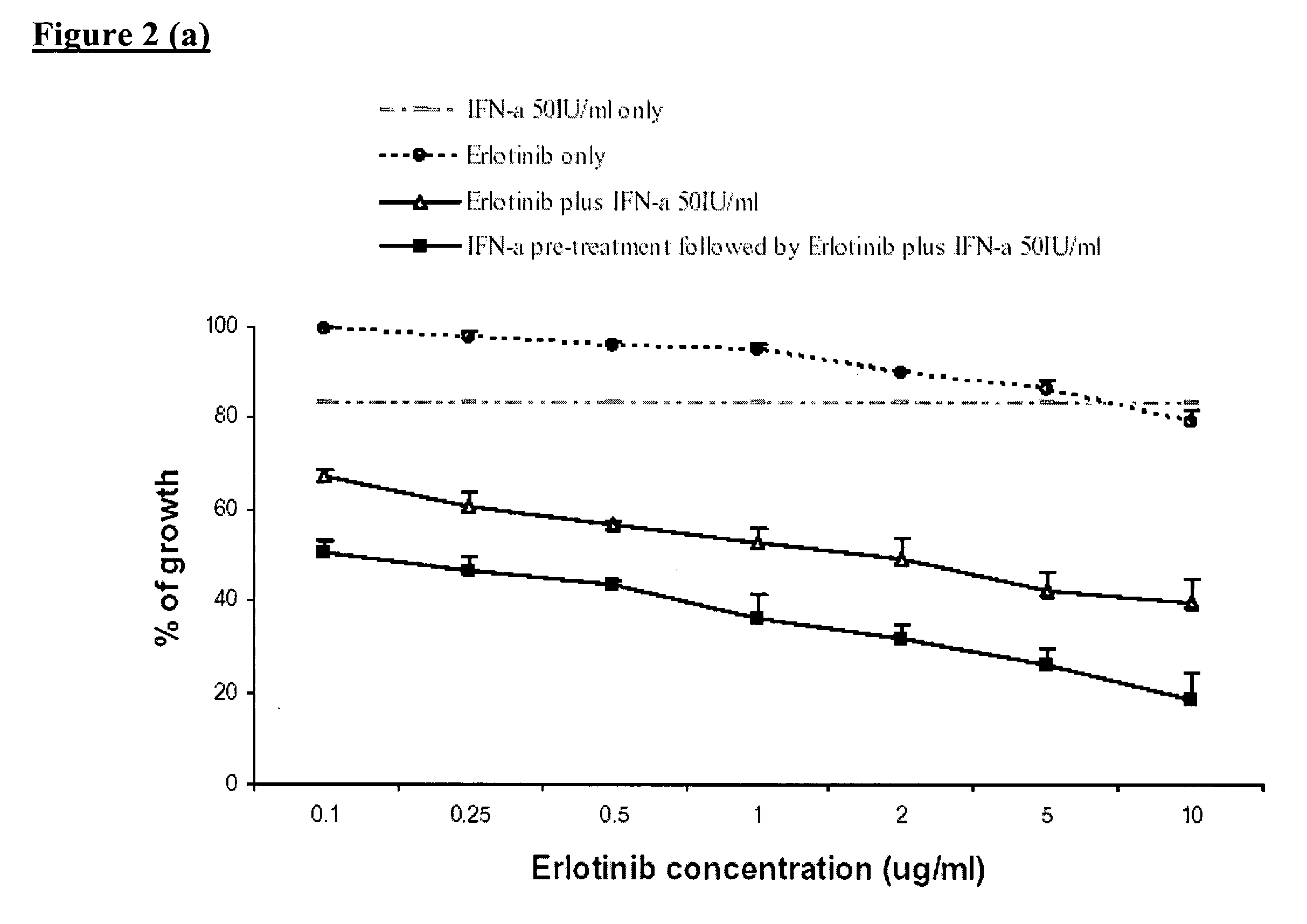
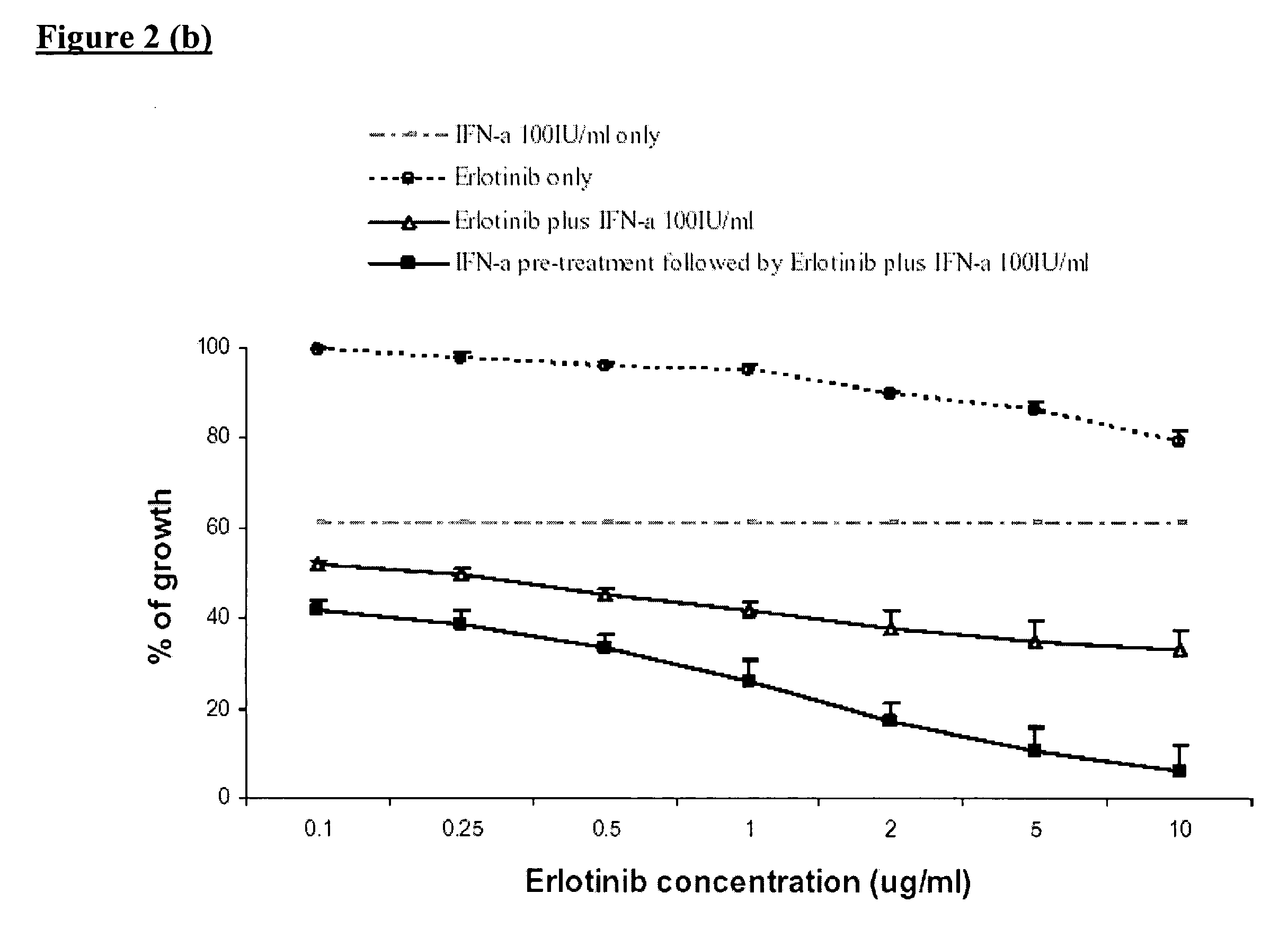
Combined treatment with interferon-alpha and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060134064A1BiocidePeptide/protein ingredientsEpidermal Growth Factor Receptor KinaseAbnormal tissue growth
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and IFNα combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and IFNα combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as TARCEVA®).
Owner:GOLDSTEIN DAVID +3
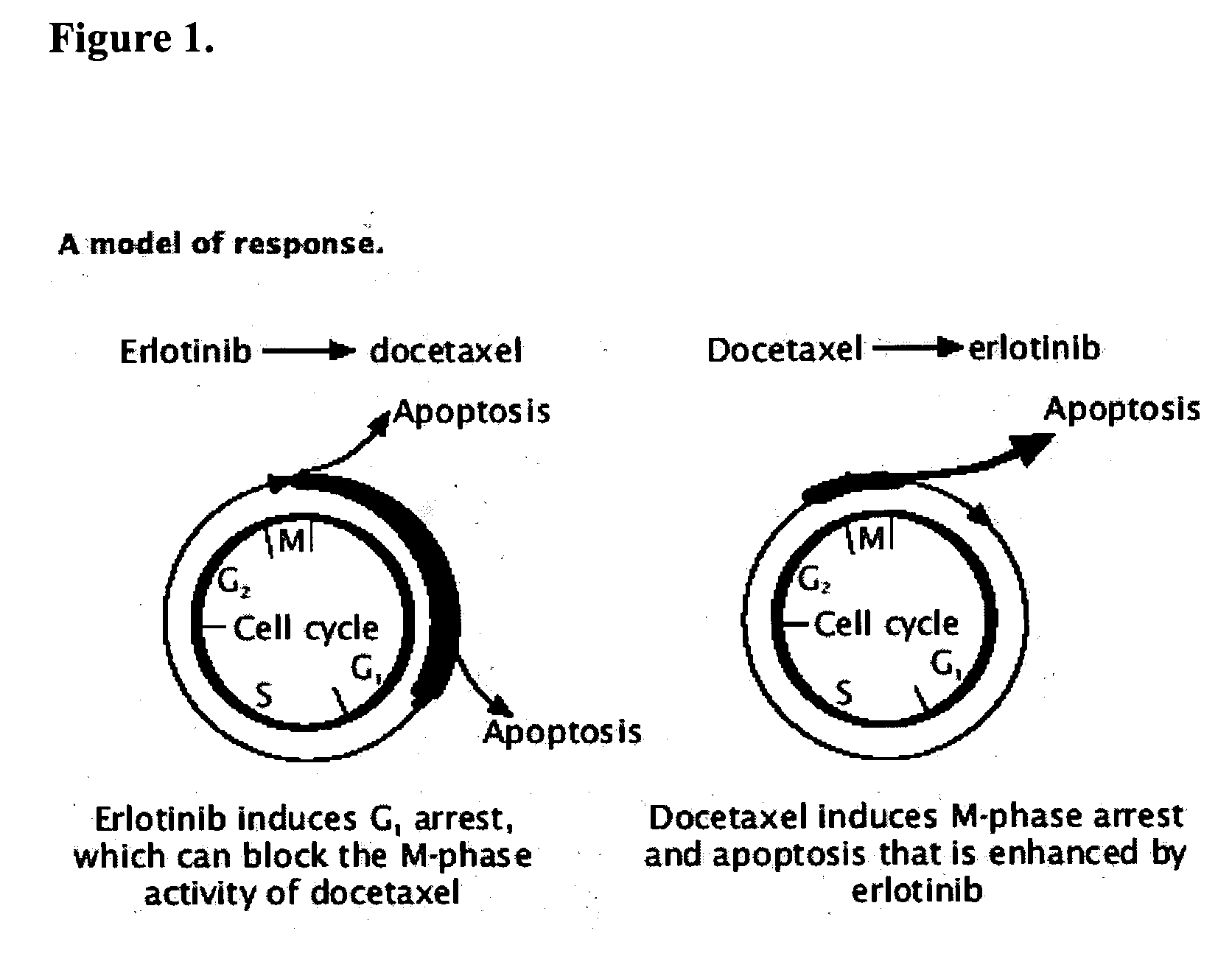
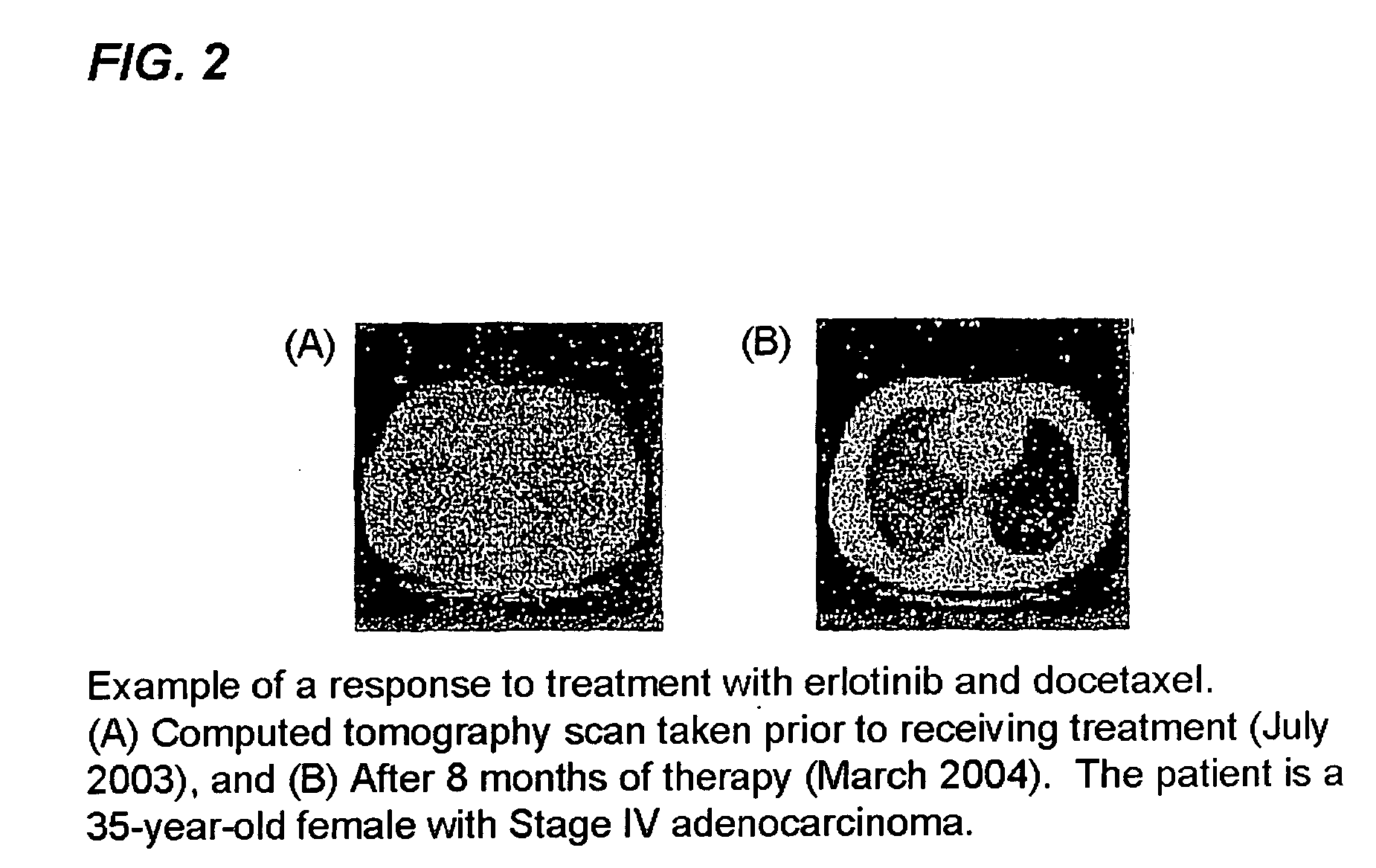
Combined treatment with docetaxel and an epidermal growth factor receptor kinase inhibitor using an intermittent dosing regimen
InactiveUS20070099856A1BiocideCarbohydrate active ingredientsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient a therapeutically effective amount of an EGFR kinase inhibitor and docetaxel combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy, wherein the EGFR kinase inhibitor is administered intermittently after administration of a dose of docetaxel. Multiple cycles of this treatment can be administered until an effective result is obtained. Other anti-cancer agents that, like docetaxel, induce M-phase arrest, can be also used in the practice of this invention, e.g. vinblastine, paclitaxel. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as TARCEVA™).
Owner:RGT UNIV OF CALIFORNIA
Novel Complex Mutations in the Epidermal Growth Factor Receptor Kinase Domain
ActiveUS20130121996A1BiocideOrganic active ingredientsEpidermal Growth Factor Receptor KinaseAbnormal tissue growth
Six new mutations were found in exon 19 of the EGFR gene, the exon that is often mutated in tumors. The invention comprises methods of detecting the mutations, methods of prognosis and methods of predicting response to treatment based on the presence of absence of the mutations.
Owner:ROCHE MOLECULAR SYST INC
Methods of assessing therapeutic benefits of patients having cancers resistant to epidermal growth factor receptor kinase inhibitors
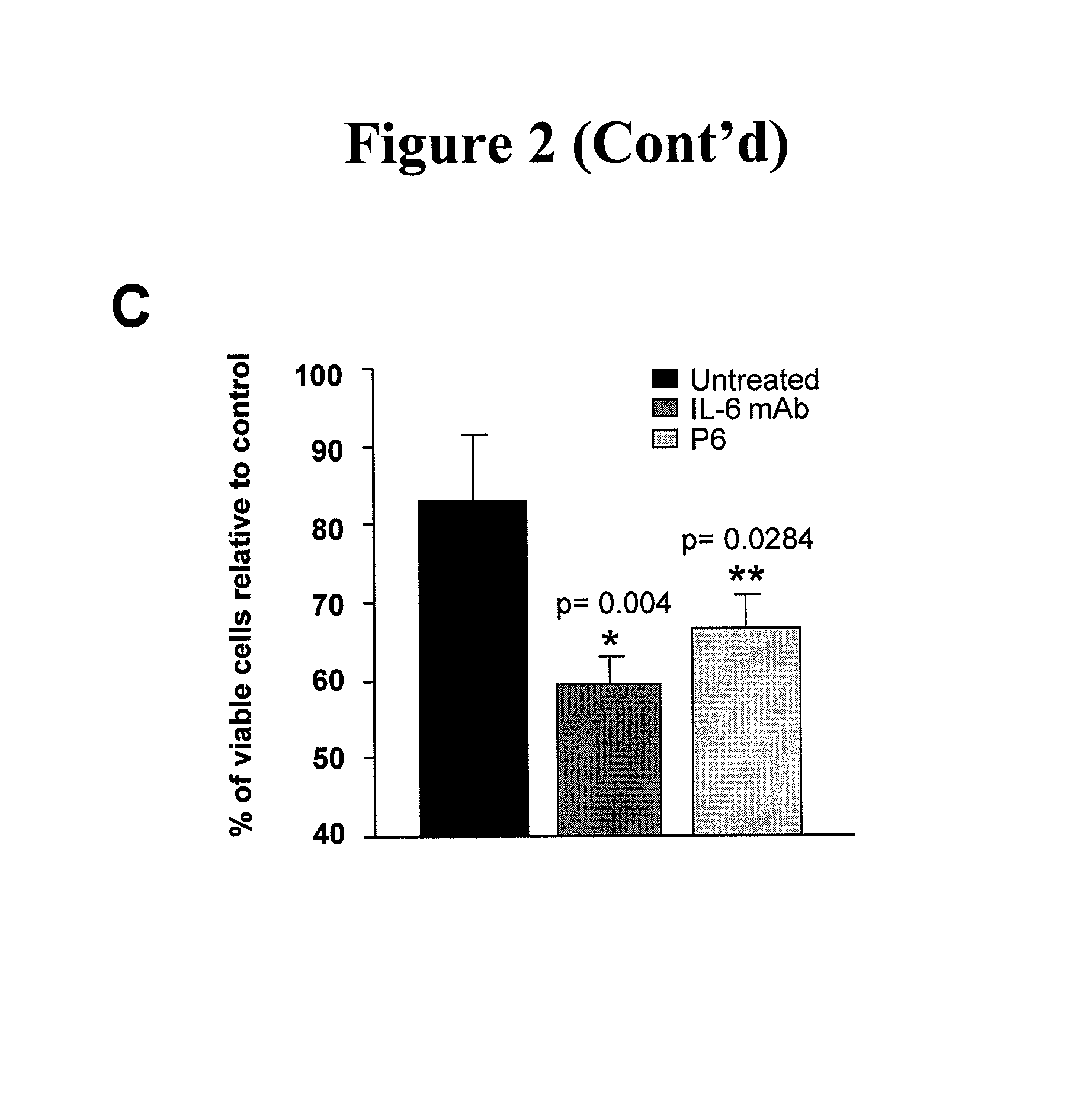
InactiveUS8323883B1Prevent further growthReduce tumor volumeMicrobiological testing/measurementDisease diagnosisEpidermal Growth Factor Receptor KinaseInterleukin 6
Cancer treatment with a combination of an Epidermal Growth Factor Receptor tyrosine kinase inhibitor and an Interleukin-6 inhibitor.
Owner:COLD SPRING HARBOR LAB INC
Application of epidermal growth factor receptor kinase substrate 8-type protein 3 to enhancement of curative effect of multi-target kinase inhibitor
ActiveCN111617247AMicrobiological testing/measurementAntineoplastic agentsEpidermal Growth Factor Receptor KinaseProteinoid
The invention relates to an application of epidermal growth factor receptor kinase substrate 8-type protein 3 to enhancement of the curative effect of a multi-target kinase inhibitor. The epidermal growth factor receptor kinase substrate 8-type protein 3 (EPS8L3) is a novel oncogene for HCC. In-vivo and in-vitro experiments prove that the growth potential of HCC tumor cells can be reduced by knocking down the EPS8L3. In addition, cells knocked down in EPS8L3 are more sensitive to treatment of sorafenib. The technical scheme provided by the invention not only provides important factors for HCCprogress, but also provides a potential treatment target for late HCC treatment.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS8383357B2Disease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseCell growth
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an EGFR kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by EGFR kinase inhibitors. Improved methods for treating cancer patients with EGFR kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to EGFR kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by EGFR kinase inhibitors are also provided.
Owner:OSI PHARMA LLC
Combined treatment with bortezomib and an epidermal growth factor receptor kinase inhibitor
InactiveCN101043892ABoron compound active ingredientsAntineoplastic agentsEpidermal Growth Factor Receptor KinaseErlotinib
The present invention provides a method for method for manufacturing a medicament intended for treating tumors or tumor metastases in a patient, characterized in that a therapeutically effective amount of an EGFR kinase inhibitor and bortezomib is used, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and bortezomib combination in a combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCI (also known as Tarceva TM).
Owner:比拉勒·皮佩蒂
Combined treatment with cisplatin and an epidermal growth factor receptor kinase inhibitor
ActiveUS7951405B2BiocideHeavy metal active ingredientsEpidermal Growth Factor Receptor KinaseLymphatic Spread
Owner:OSI PHARMA INC
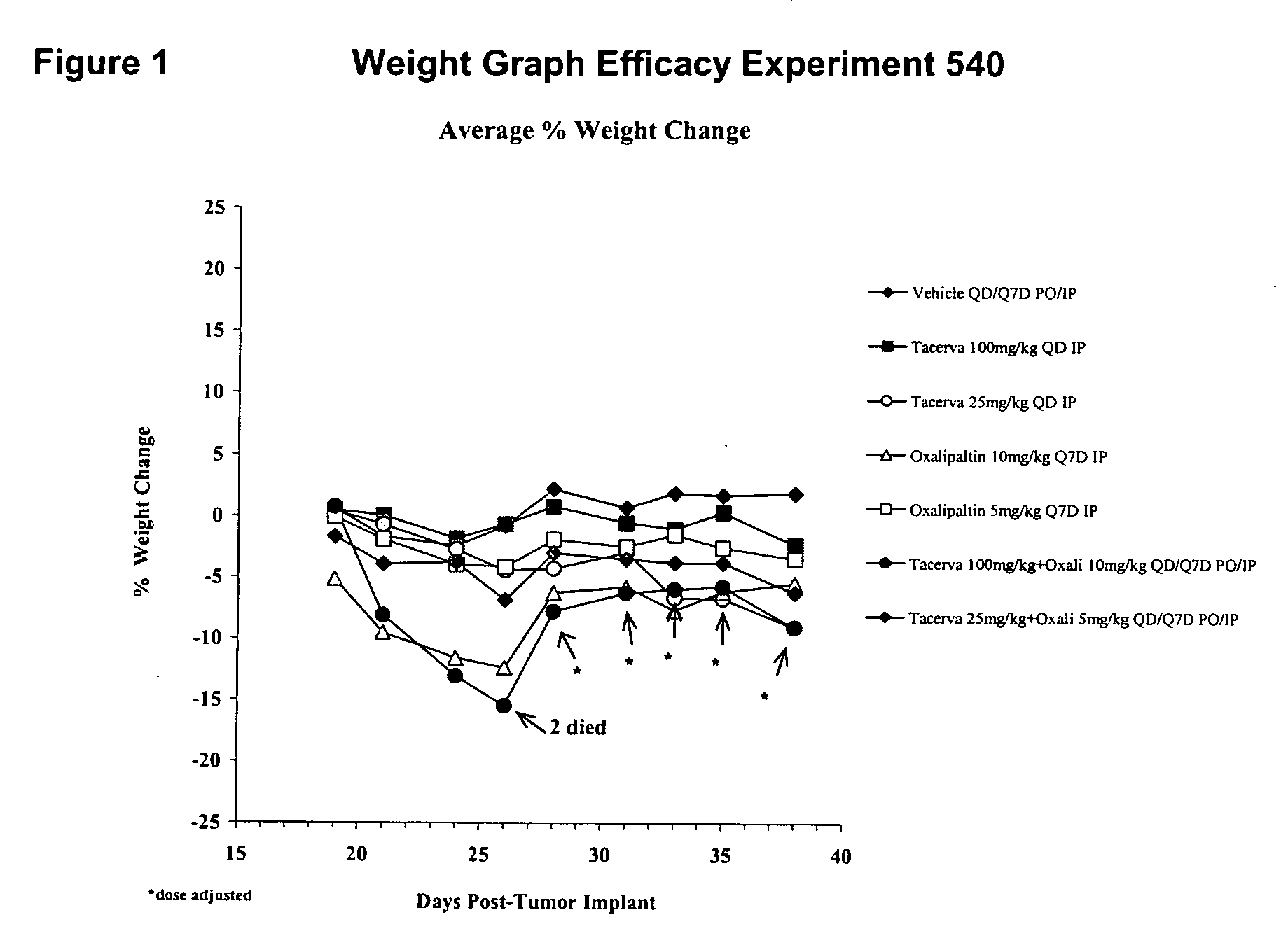
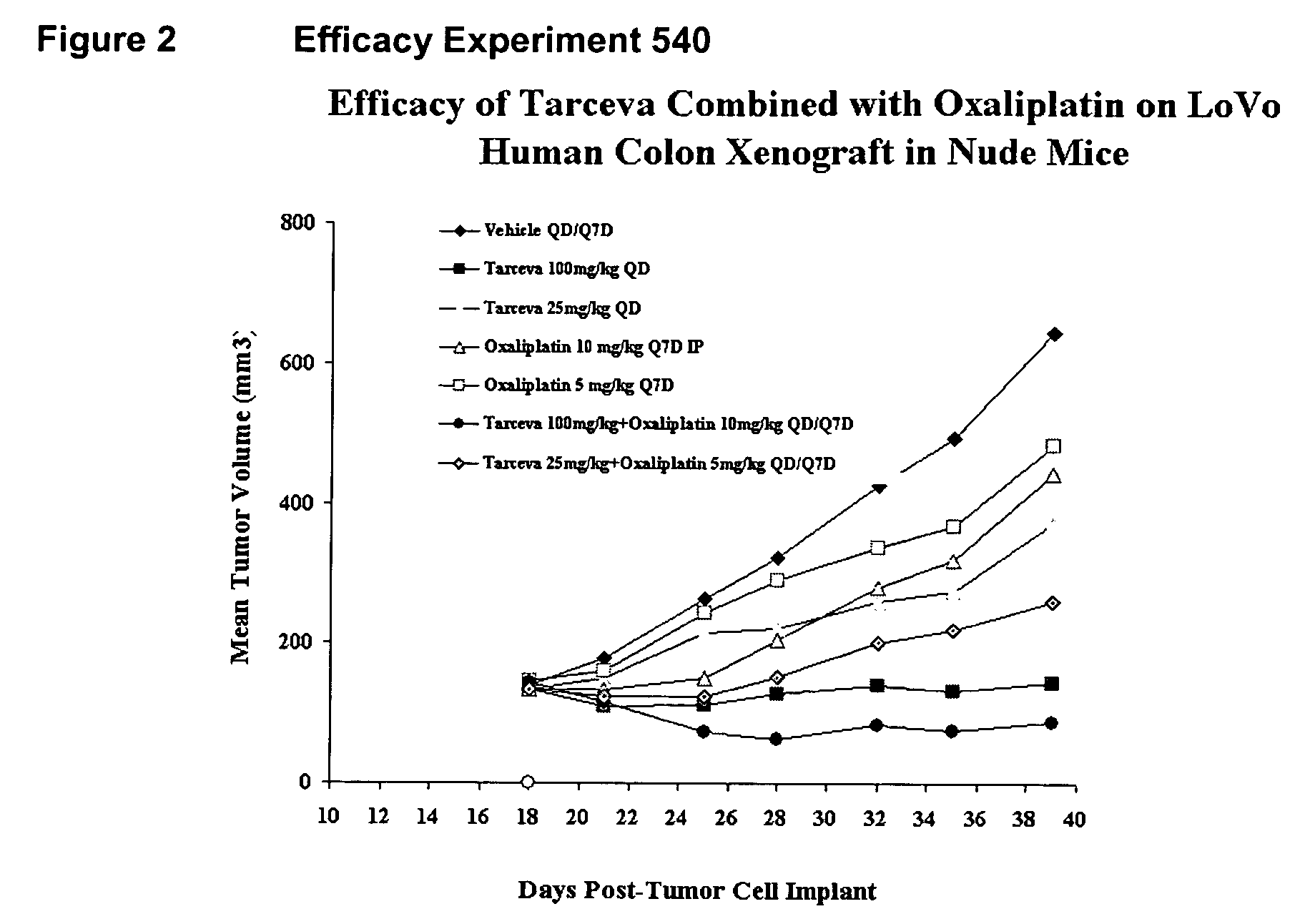
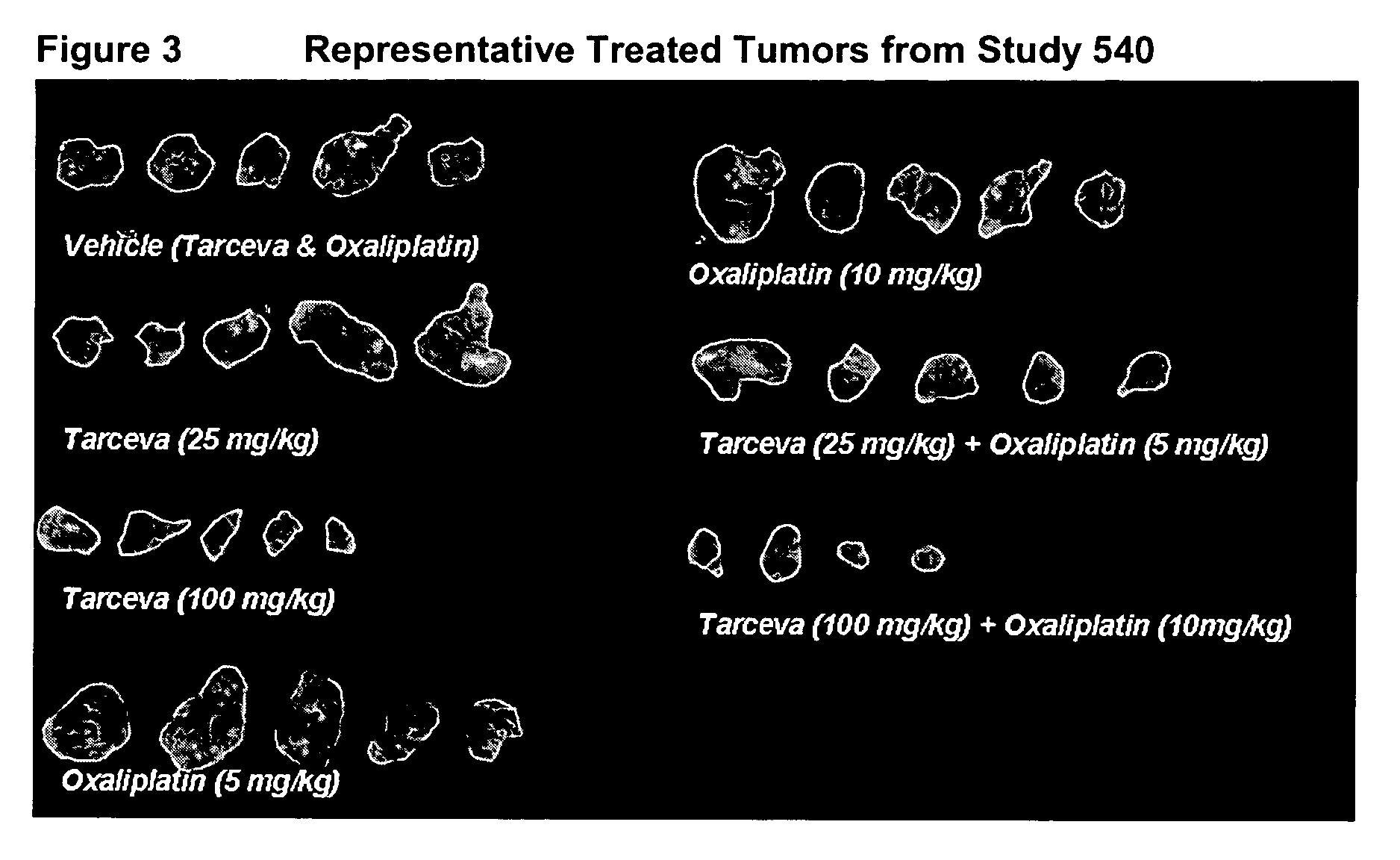
Combined treatment with oxaliplatin and an epidermal growth factor receptor kinase inhibitor
InactiveUS20050272738A1Heavy metal active ingredientsBiocideEpidermal Growth Factor Receptor KinaseLymphatic Spread
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and oxaliplatin combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and oxaliplatin combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as Tarceva™).
Owner:OSI PHARMA INC
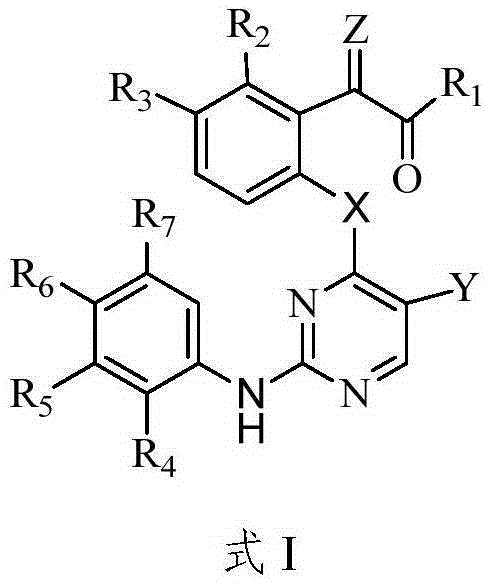
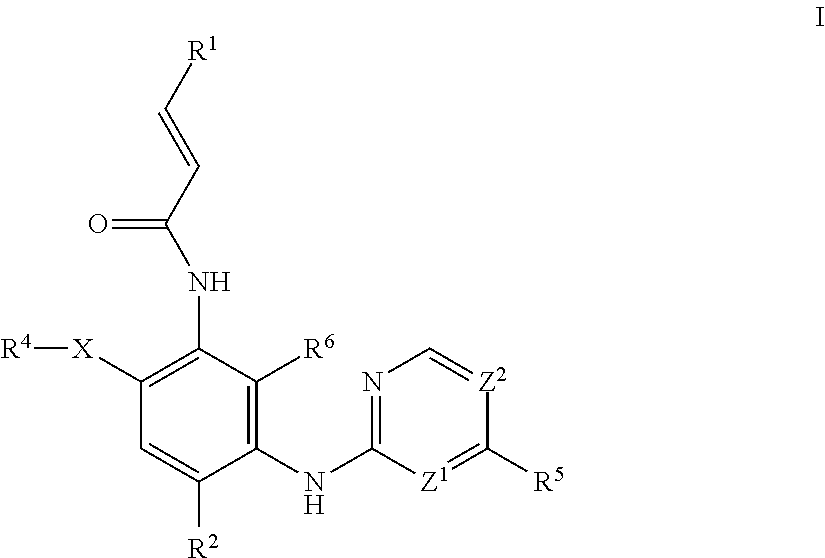
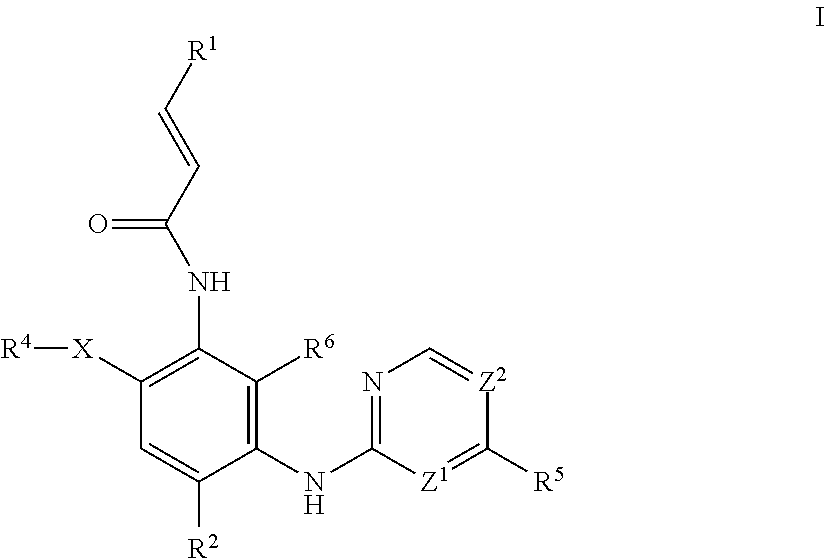
Aminopyrimidine compound, preparation method therefor and use thereof
ActiveUS20200087296A1Good hygroscopicityImprove stabilityOrganic active ingredientsOrganic chemistryEpidermal Growth Factor Receptor KinaseKinase
The present invention relates to an aminopyrimidine compound, a preparation method therefor and use thereof. The aminopyrimidine compound has the structure as shown in formula I:the compound is an inhibitor of an epidermal growth factor receptor (EGFR) kinase. The present invention also relates to a pharmaceutical composition containing the compounds, a method for preparing same and the use of same in preparation of anti-tumor drugs.
Owner:BEIJING ADAMADLE BIOTECHNOLOGY LLC
Novel mutation in the epidermal growth factor receptor kinase domain
ActiveUS20170067114A1Organic active ingredientsMicrobiological testing/measurementEpidermal Growth Factor Receptor KinaseMedicine
Owner:ROCHE MOLECULAR SYST INC
Combined treatment with radiation and an epidermal growth factor receptor kinase inhibitor
InactiveUS20090253721A1Organic active ingredientsBiocideEpidermal Growth Factor Receptor KinaseLymphatic Spread
Owner:HARARI PAUL M +2
Phosphate of epidermal growth factor receptor kinase inhibitor and preparation method thereof
InactiveCN104961688AReduce humidityForm A has high solubilityOrganic active ingredientsOrganic chemistry methodsEpidermal Growth Factor Receptor KinaseMeth-
The invention relates to phosphate of N-(3-(2-(4-(4-acetylpiperazine-1-yl)-2-methoxyphenylamino)-5-(trifluoromethyl)pyrimidine-4-yl-amino)phenyl)acrylamide), and a crystal form and a preparation method thereof. The phosphate of the compound as shown in a formula (I) which is described in the specification has favorable performance like good stability, low hygroscopicity and process developability and manageability; and the preparation method is simple and has low cost, so the method has an important value to optimization and development of the phosphate of the compound as a drug in the future.
Owner:CRYSTAL PHARMATECH CO LTD +1
Solid forms of epidermal growth factor receptor kinase inhibitors
ActiveCN104302178BReduce severityOrganic chemistryAmide active ingredientsEpidermal Growth Factor Receptor KinaseIsrapafant
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com