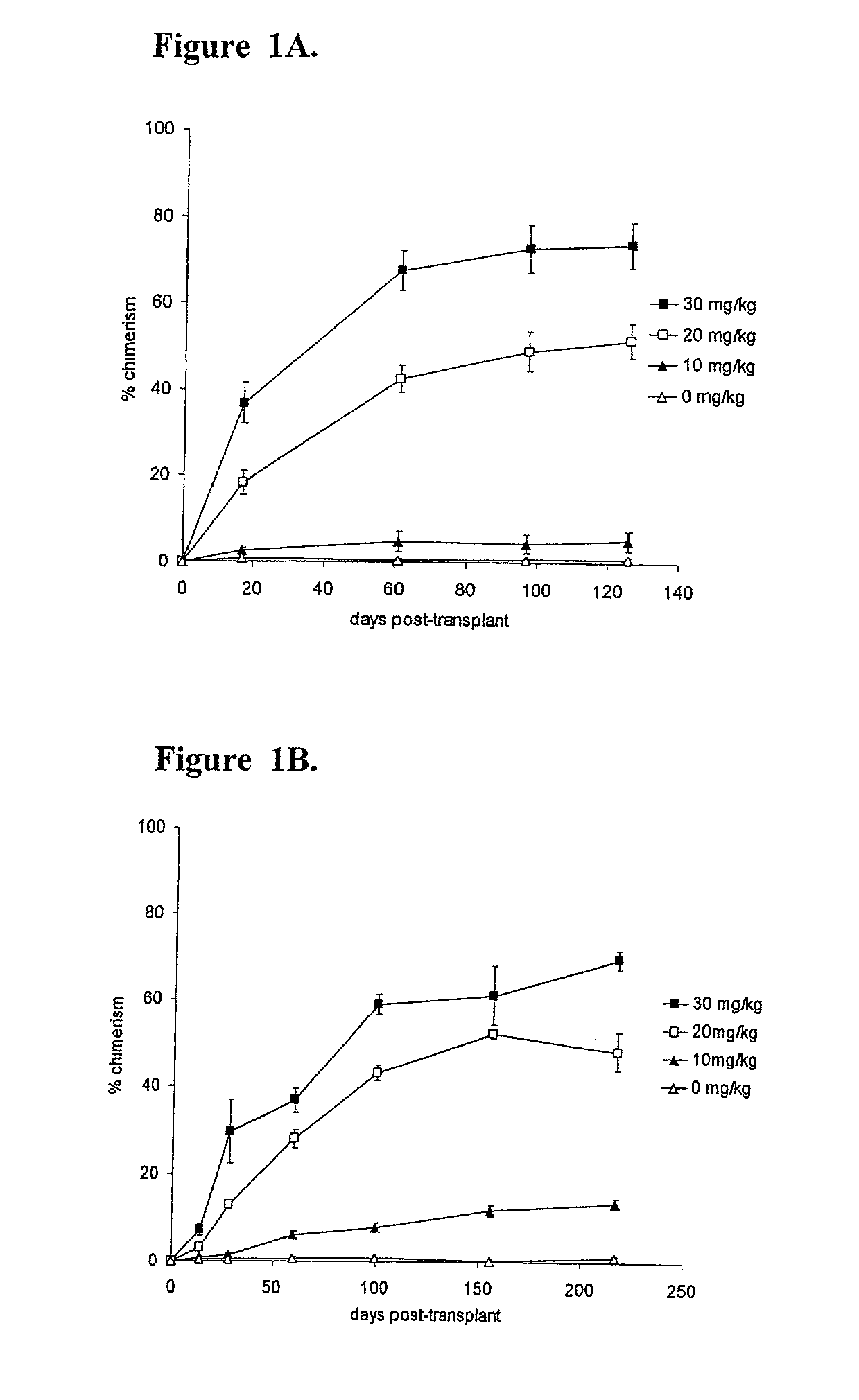
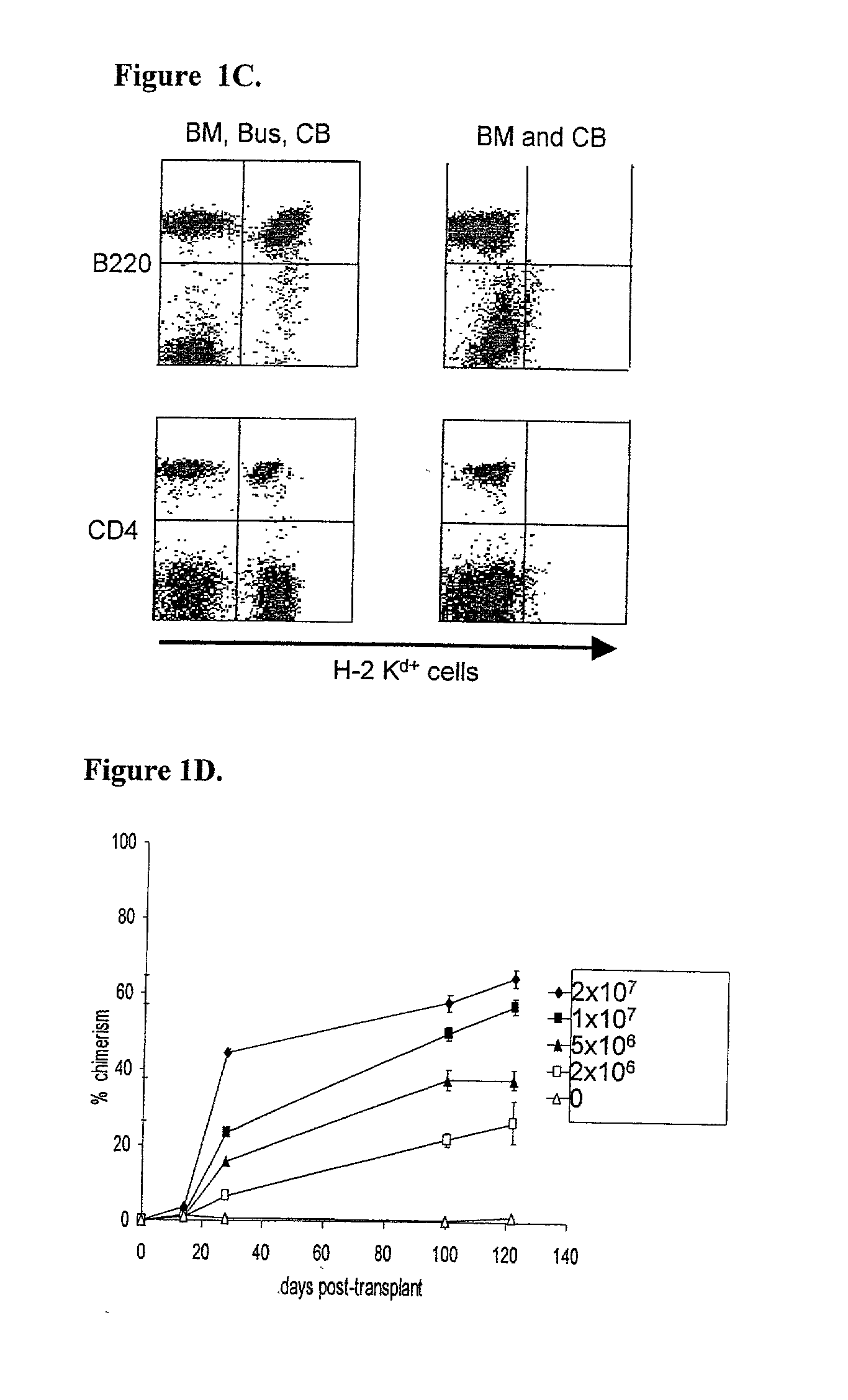
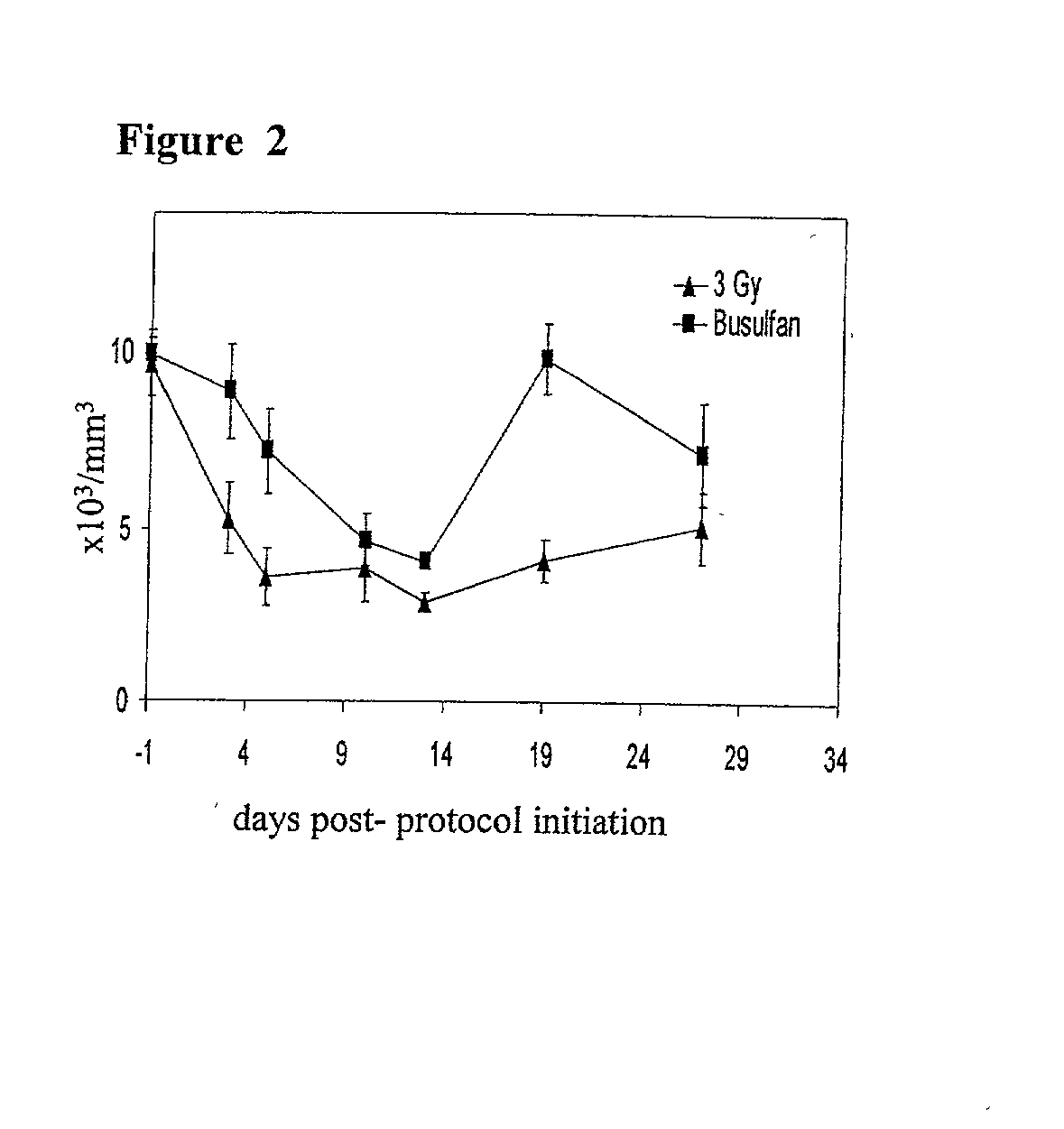
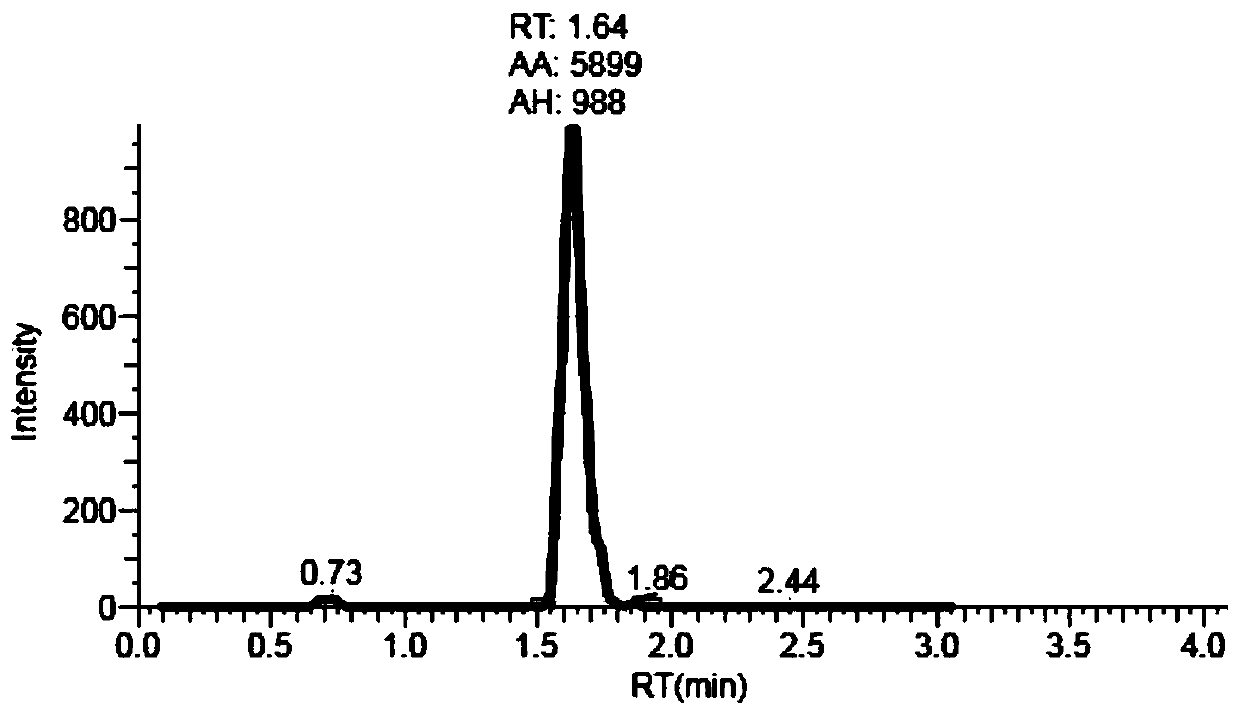
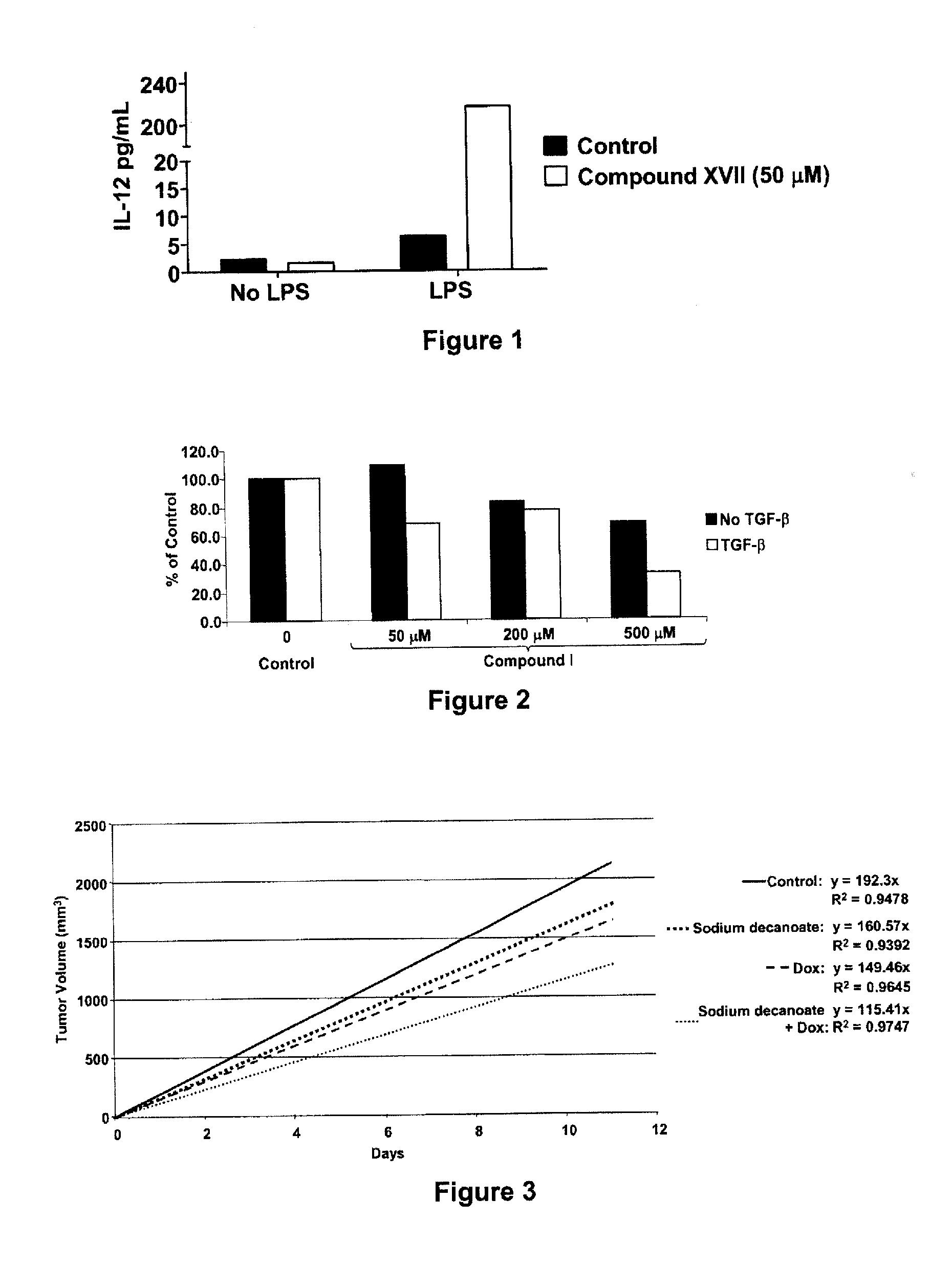
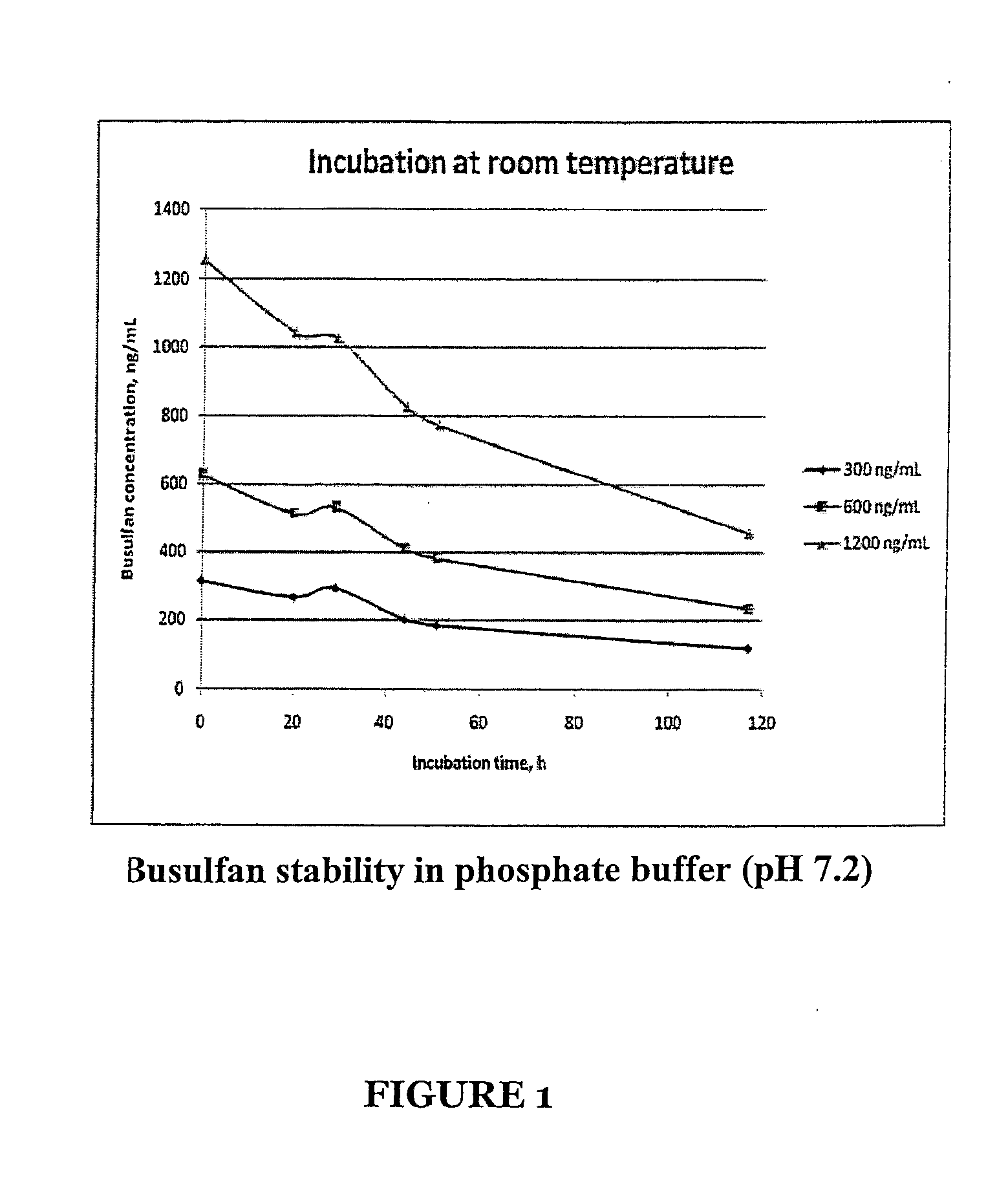
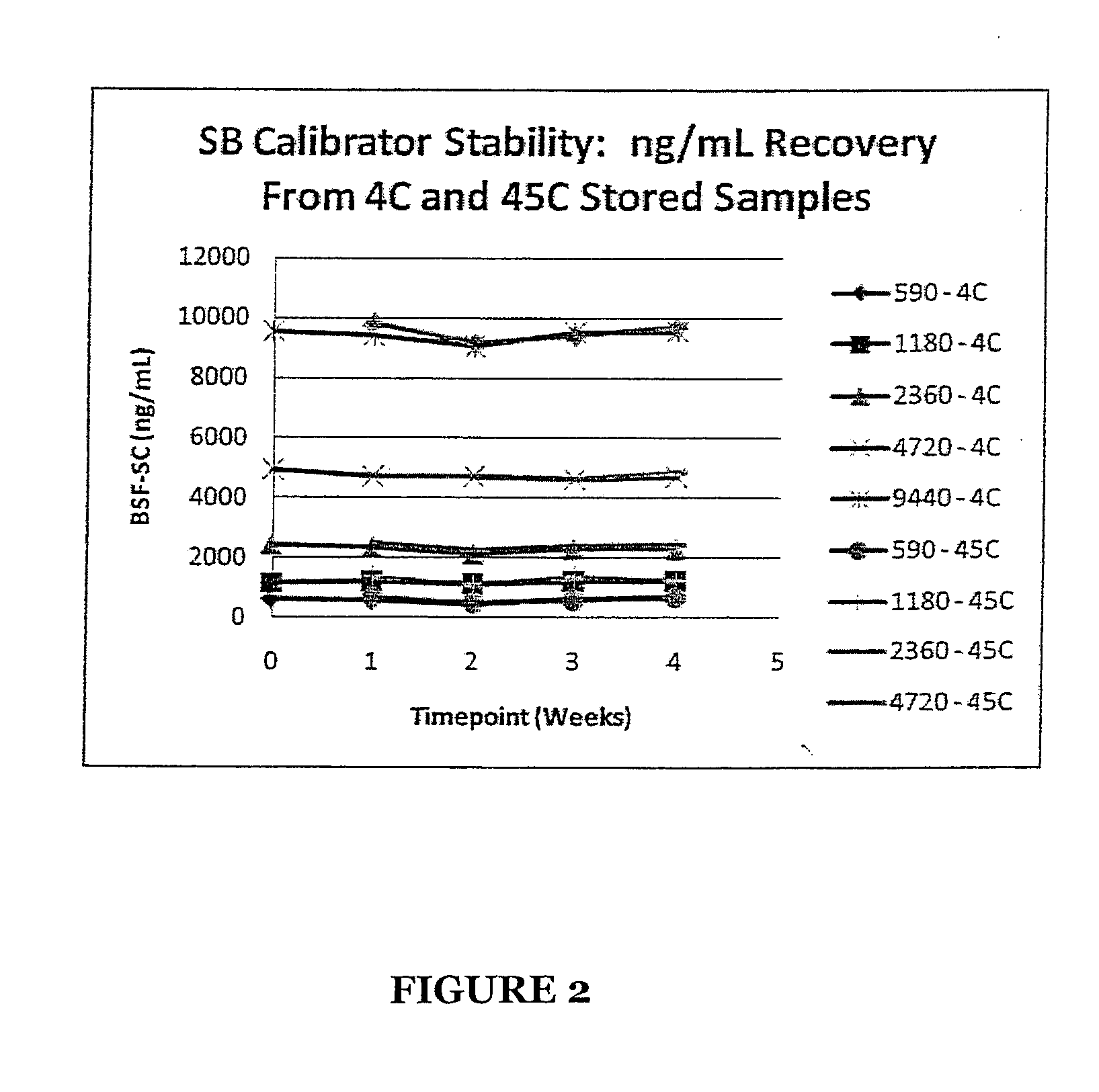
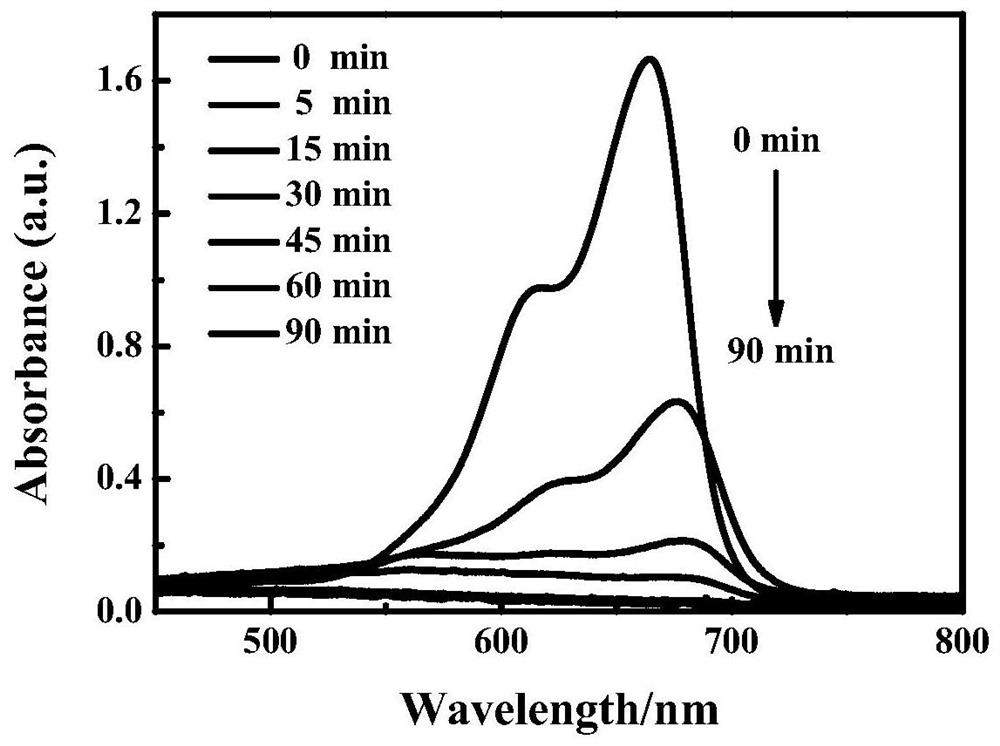
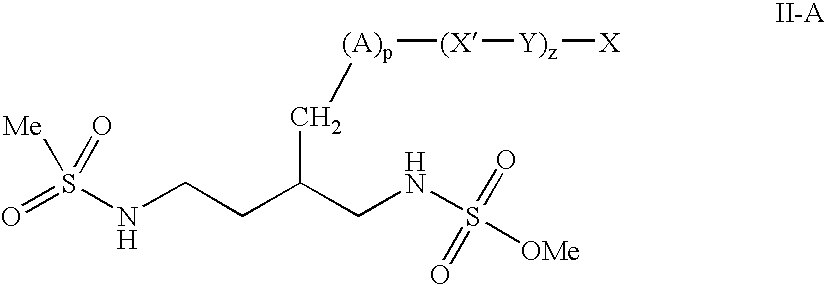
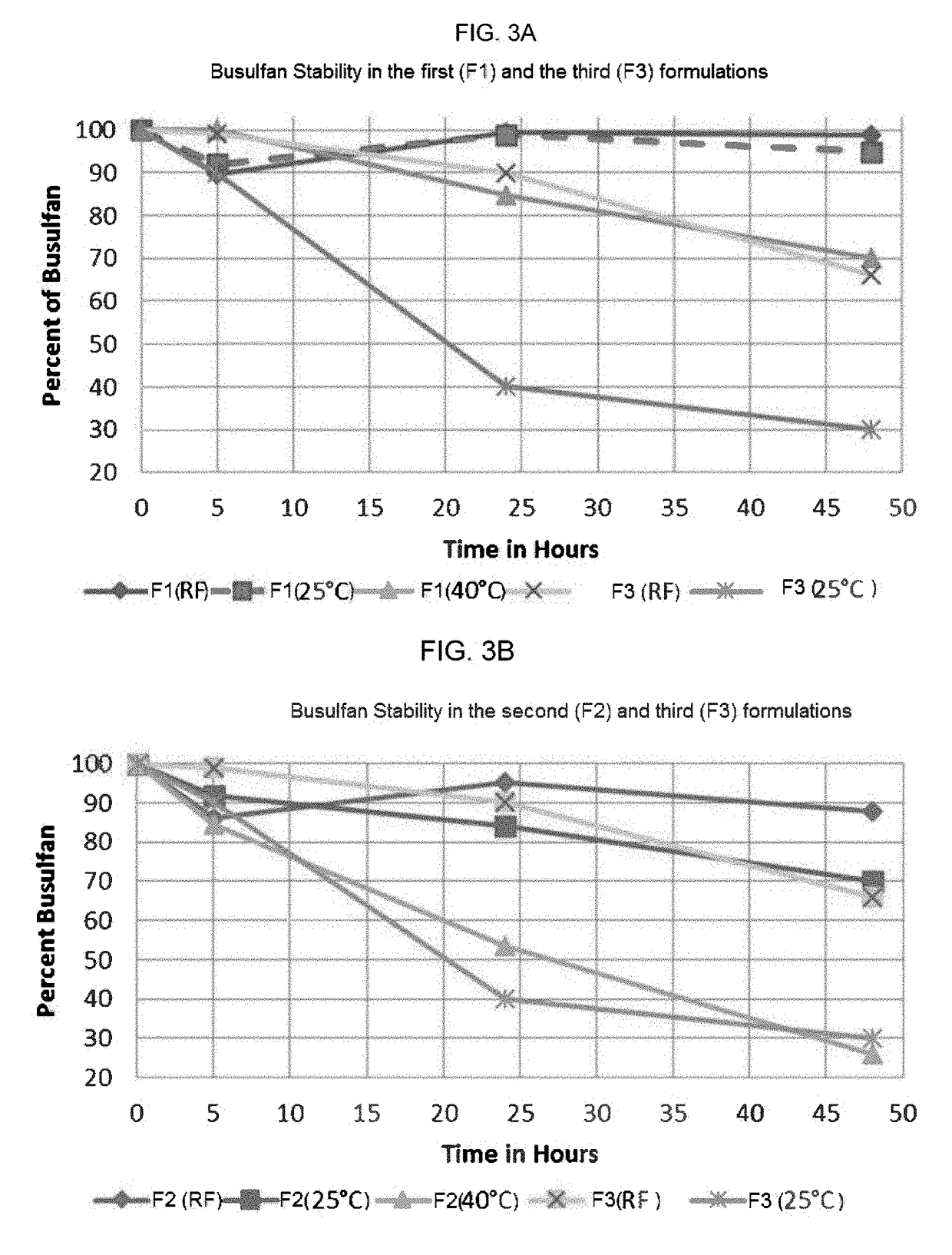
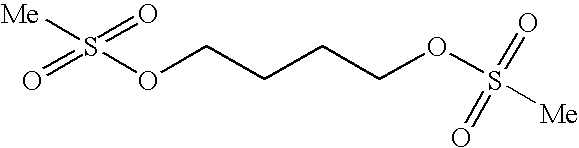
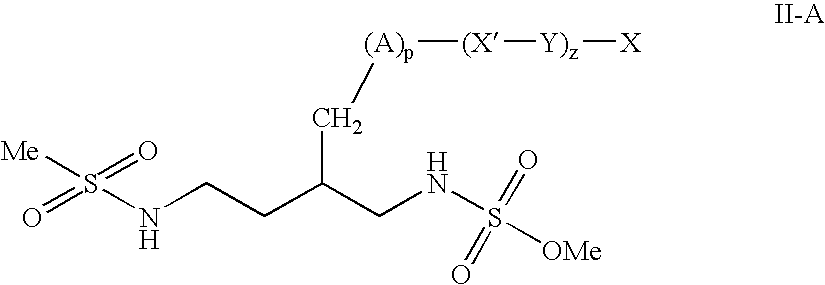
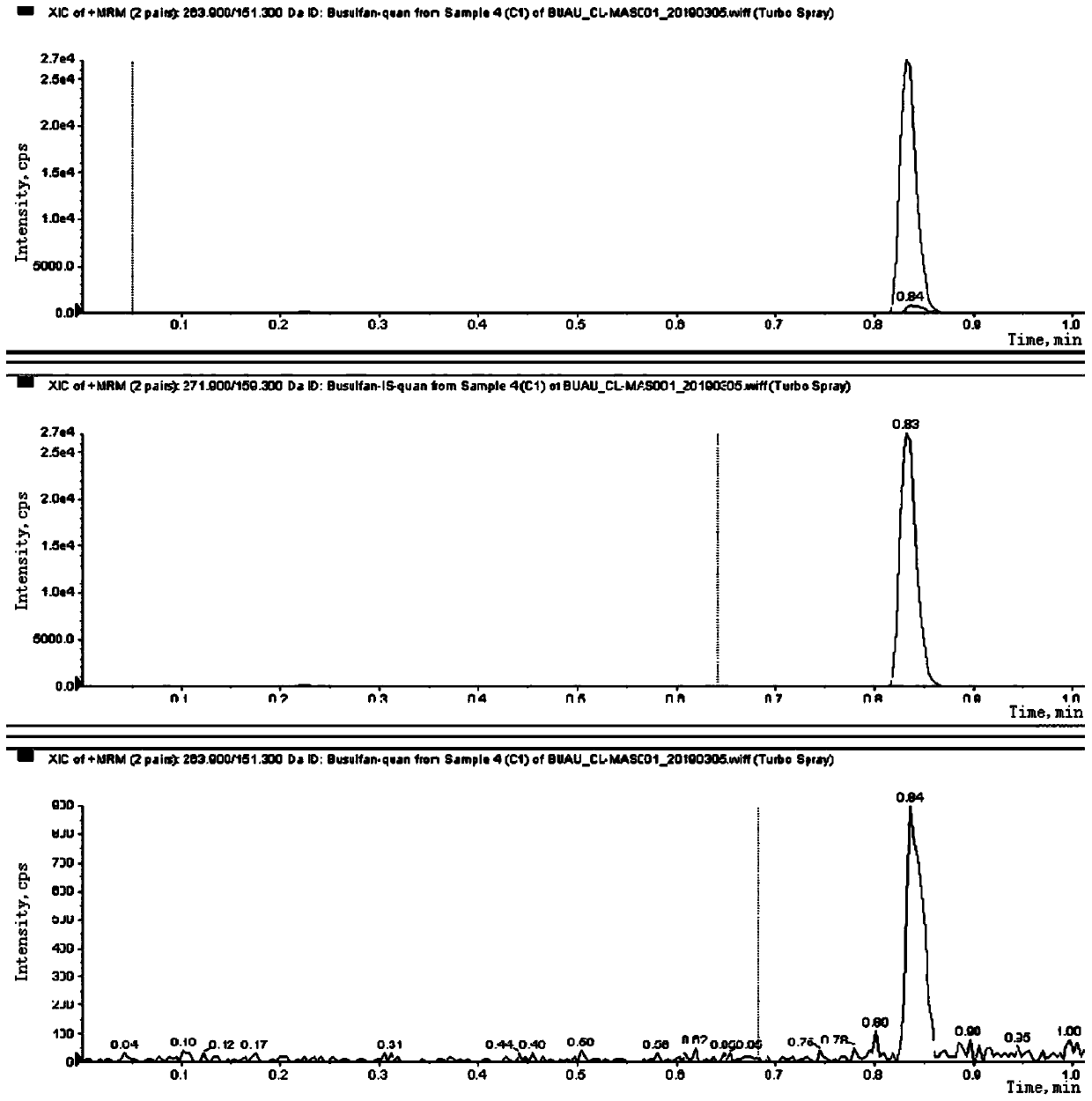
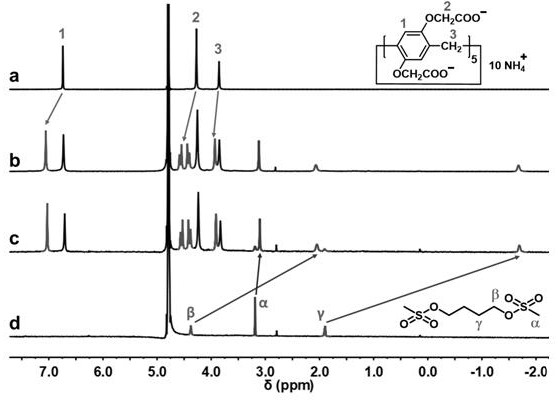
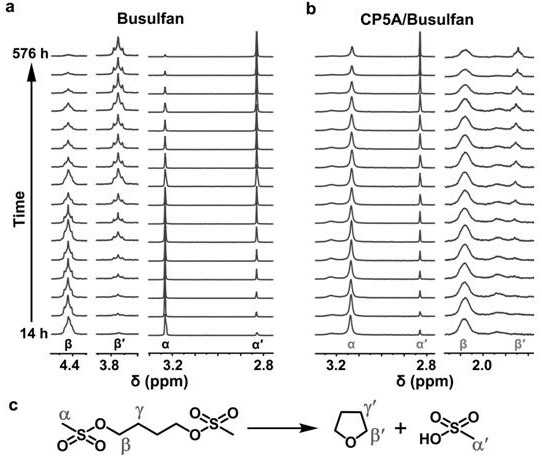
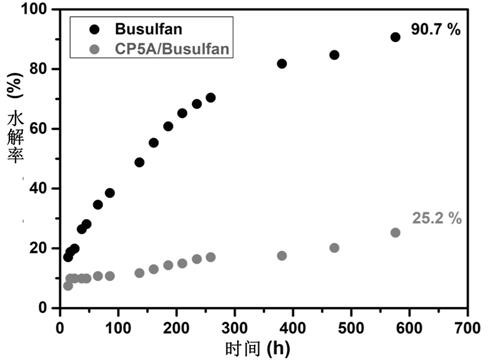
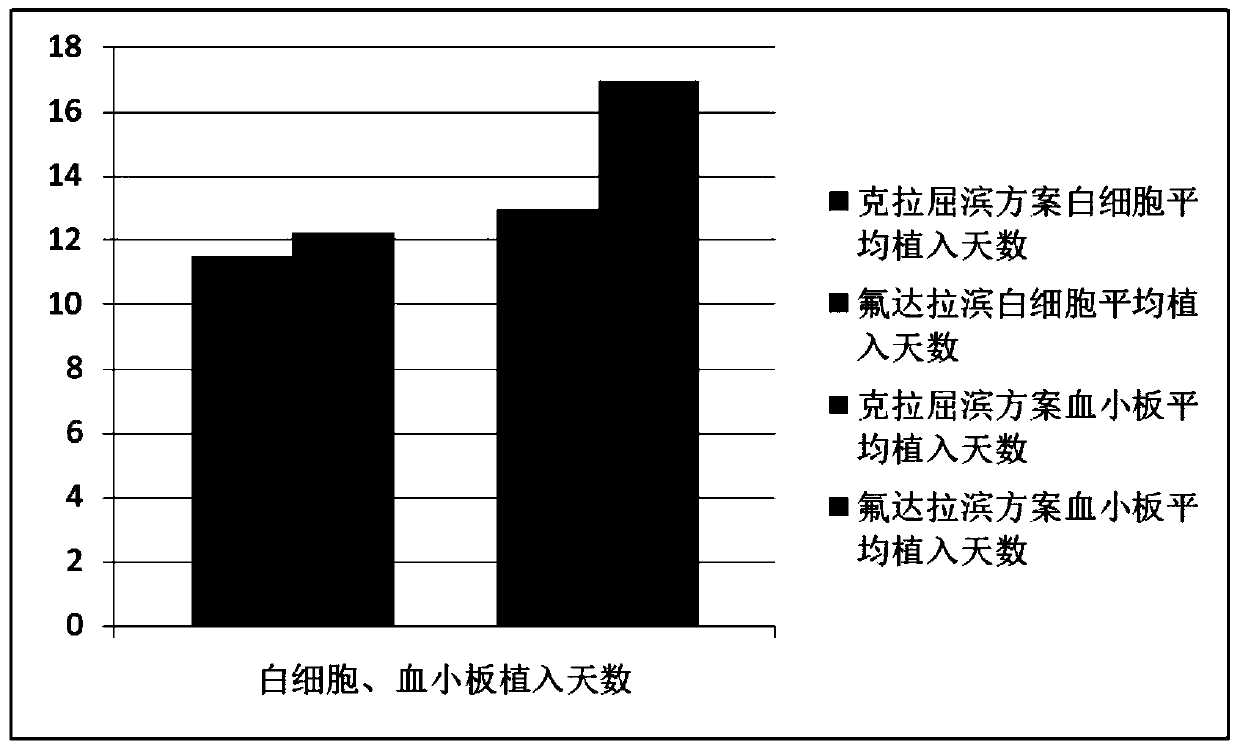
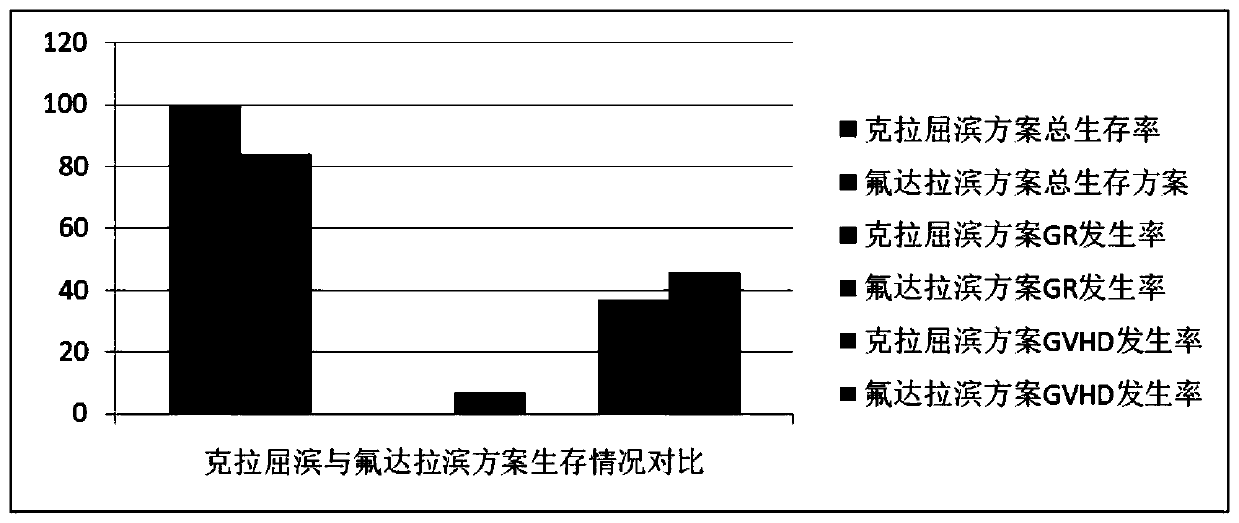
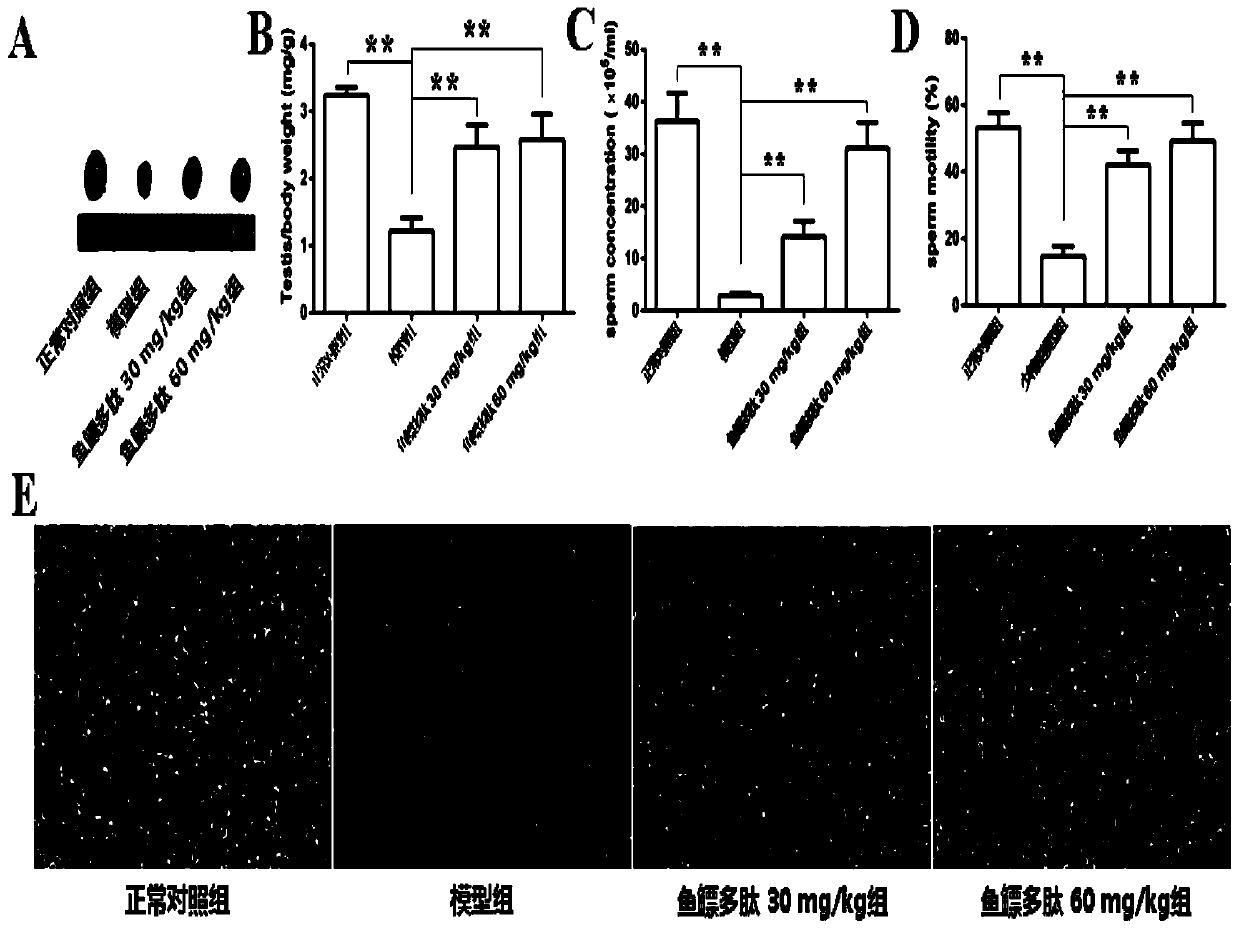
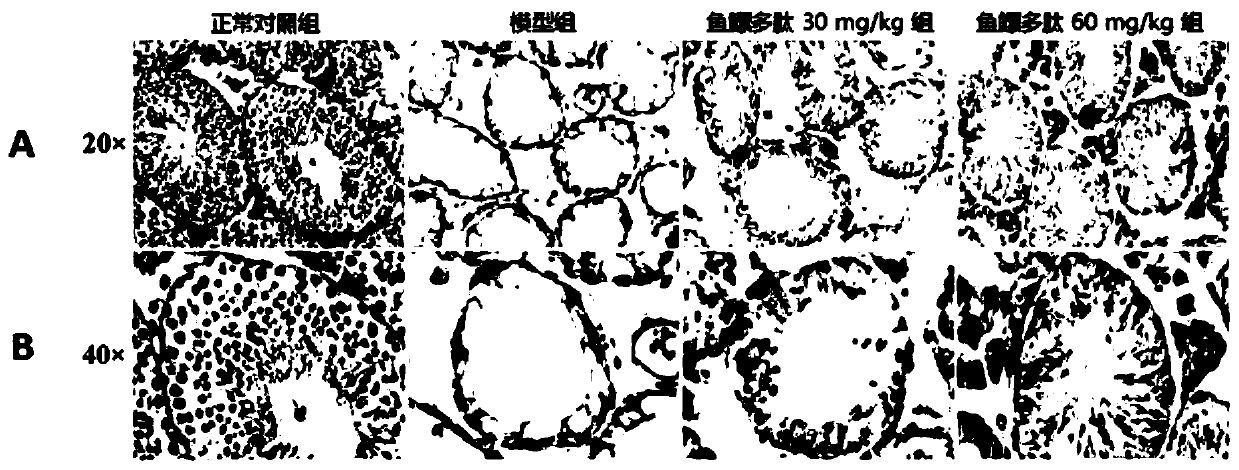
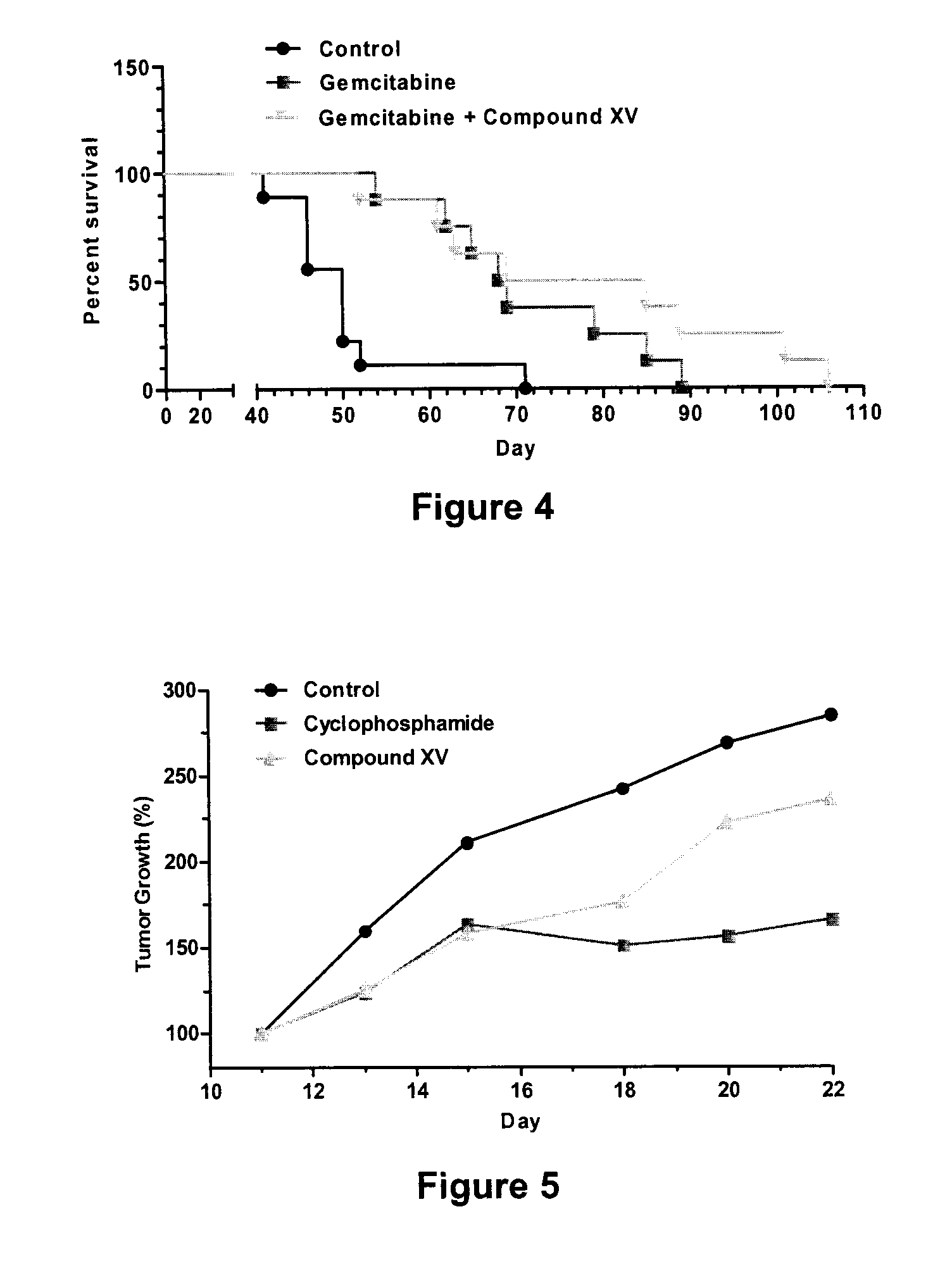
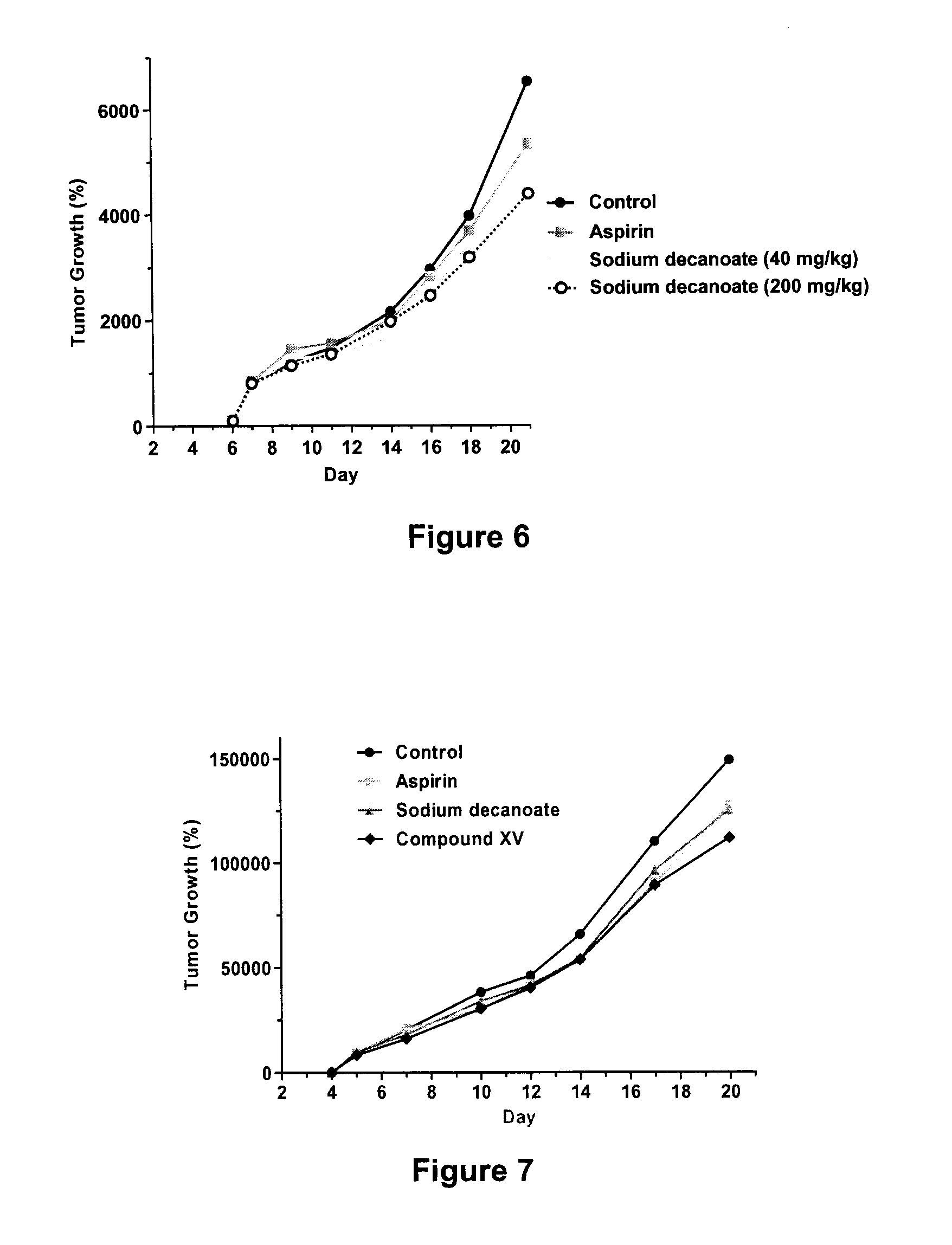
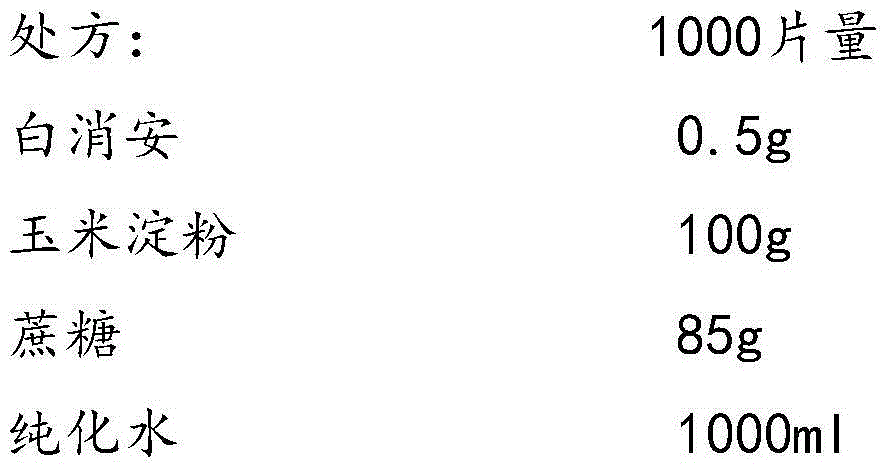Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Busulfan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Busulfan is used as a pretreatment for patients who are undergoing stem cell transplant for chronic myelogenous leukemia (CML)..
Methods of inducing organ transplant tolerance and correcting hemoglobinopathies
InactiveUS20030007968A1Easy to practiceOrganic active ingredientsSenses disorderBone marrow cellCostimulation blockade
Methods of establishing hematopoietic chimerism useful to correct hematological diseases and promote acceptance of organ transplants include administering busulfan, costimulation blockade, and readily attainable numbers of T-cell depleted bone marrow cells.
Owner:EMORY UNIVERSITY
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
Drug eluting stent coating with extended duration of drug release
A stent having a drug eluting formulation has three components: 1) Anti-neointimal hyperplasia or anti-restenosis agent 2) Main polymer 3) Additive polymer The anti-neointimal hyperplasia or anti-restenosis agent includes, but not limited to, Paclitaxel, Taxol, Rapamycin, Tacrolimus, Actinomycin D, Methotrexate, Doxorubicin, cyclophosphamide, and 5-fluorouracil, 6-mercapatopurine, 6-thioguanine, cytoxan, cyclosporine, cytarabinoside, cis-platin, chlorambucil, busulfan, and any other drug that can inhibit cell proliferation, and combinations thereof. The main polymer includes, but not limited to, polystyrene, parylene and polyurethane. The additive polymer includes, but not limited to, polyethylene glycol capped with diisocyanate moiety (NCO-PEG). TABLERatio between three components without solvent%ComponentformulationAgent1-10%Main polymer80-98% Additive1-19%polymer9.0 g of parylene, 0.6 g of tacrolimus, 0.4 g of NCO-PEG and 0.01 g of triethylene amine were dissolved in 90 g of tetrahydrofuran. The resulting mixture was heated at 40° C. for 30 minutes and cooled to room temperature. To the solution was added 0.1 g of pH 8.0 aqueous solution and mixed thoroughly. The resulting solution is applied to bare metal stents for coating.
Owner:HAHN SOONKAP
Compounds and compositions for the treatment of cancer
New uses for phenylketone carboxylate compounds and substituted aromatic compounds of Formula I, Formula I.1, Formula I.2, Formula IA, Formula IB, Formula IC and Formula II and their pharmaceutical acceptable salts for the treatment of cancer. The use of a combination of two of these compounds is described and the use of the combination of one of these compounds with an anticancer agent such as decarbazine, doxorubicin, daunorubicin, cyclophosphamide, busulfex, busulfan, vinblastine, vincristine, bleomycin, etoposide, topotecan, irinotecan, taxotere, taxol, 5-fluorouracil, methotrexate, gemcitabine, cisplatin, carboplatin and chlorambucil.
Owner:PROMETIC PHARMA SMT LTD
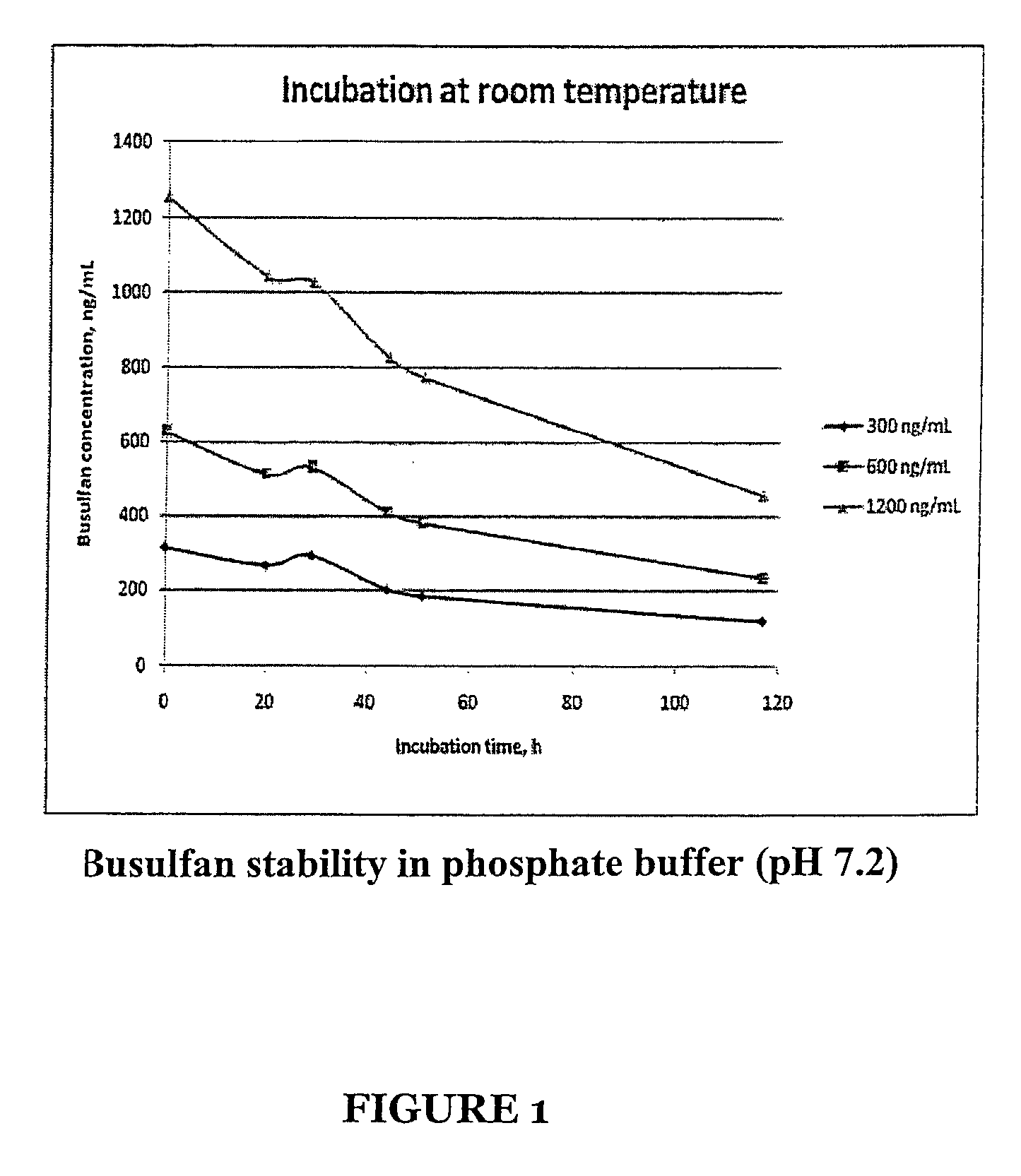
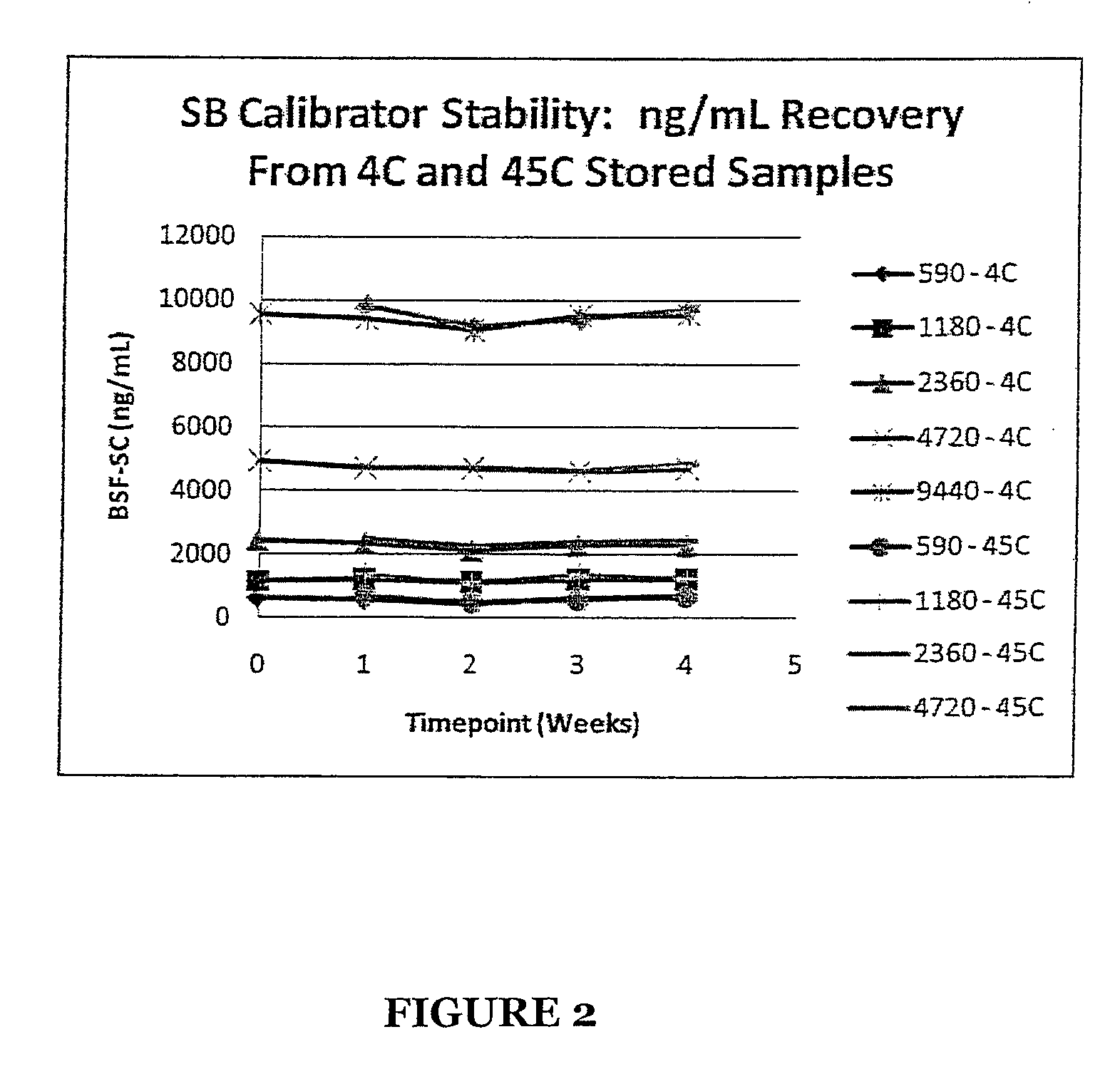
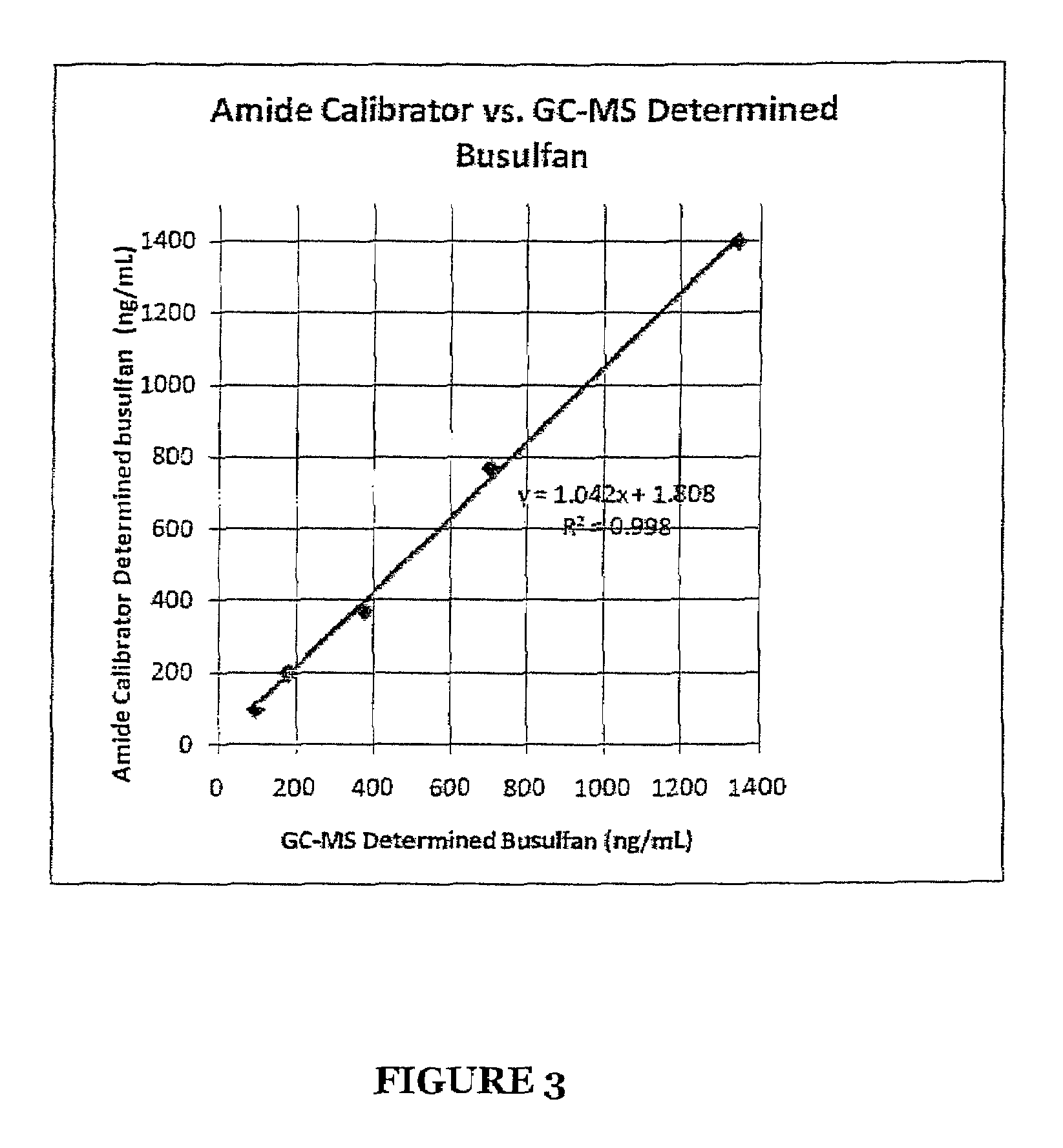
Stabilized Standards for Busulfan Immunoassay
ActiveUS20100068827A1Efficient methodEffective monitoringPeptide preparation methodsDepsipeptidesBusulfanBiological fluids
Use of busulfan amide as stabilized standards in immunoassays for quantifying the amount of busulfan in samples of human biological fluids, methods for carrying out said immunoassay and kits for use in said immunoassay.
Owner:SALADAX BIOMEDICAL INC
Yttrium-based metal-organic framework material and application thereof
ActiveCN112280054AGood biocompatibilityImprove water stabilityPowder deliveryPharmaceutical non-active ingredientsBenzoic acidMetal-organic framework
The invention discloses a yttrium-based metal organic framework material and application thereof. The chemical formula of the yttrium-based metal organic framework material (Y-MOF) is Y<4> (M)< 2>. (DMF)<3.5>. (H<2>O), and in the chemical formula, M is 2, 4, 6-tri (4-carboxyl phenyl) 1, 3, 5-triazine, 2, 4, 6-tri (4-pyridine) 1, 3, 5-triazine or 1, 3, 5-tribenzoic acid pyridine. The yttrium-basedmetal organic framework material (Y-MOF) prepared by the invention is of a porous structure, has better biocompatibility and water stability, can be used as a carrier material for simultaneously loading metal ions (Mn < 2 + >, Fe < 2 + > and the like) and drug molecules (methotrexate, busulfan, adriamycin and the like), and has the effects of magnetic resonance imaging, chemodynamic therapy and chemical drug therapy; the yttrium-based metal organic framework material (Y-MOF) is prepared by adopting a solvothermal method, so that the method is simple, convenient and safe to operate, the raw materials are easy to obtain, and the conditions are mild.
Owner:ZHEJIANG SCI-TECH UNIV
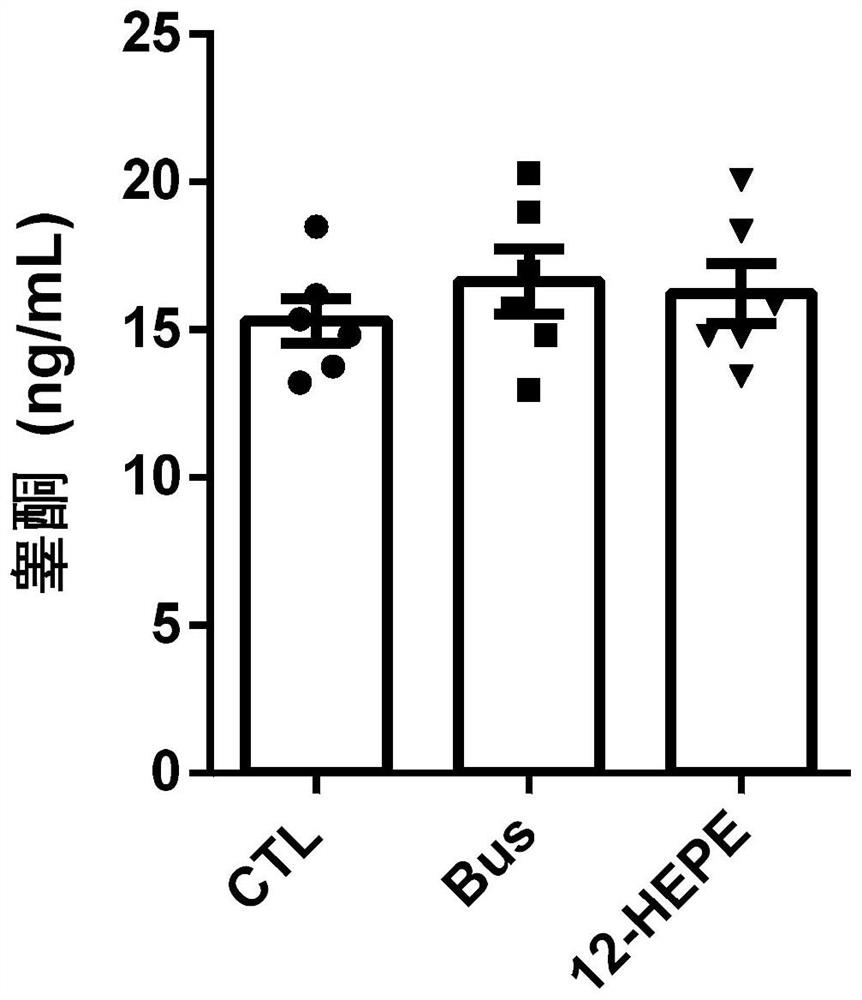
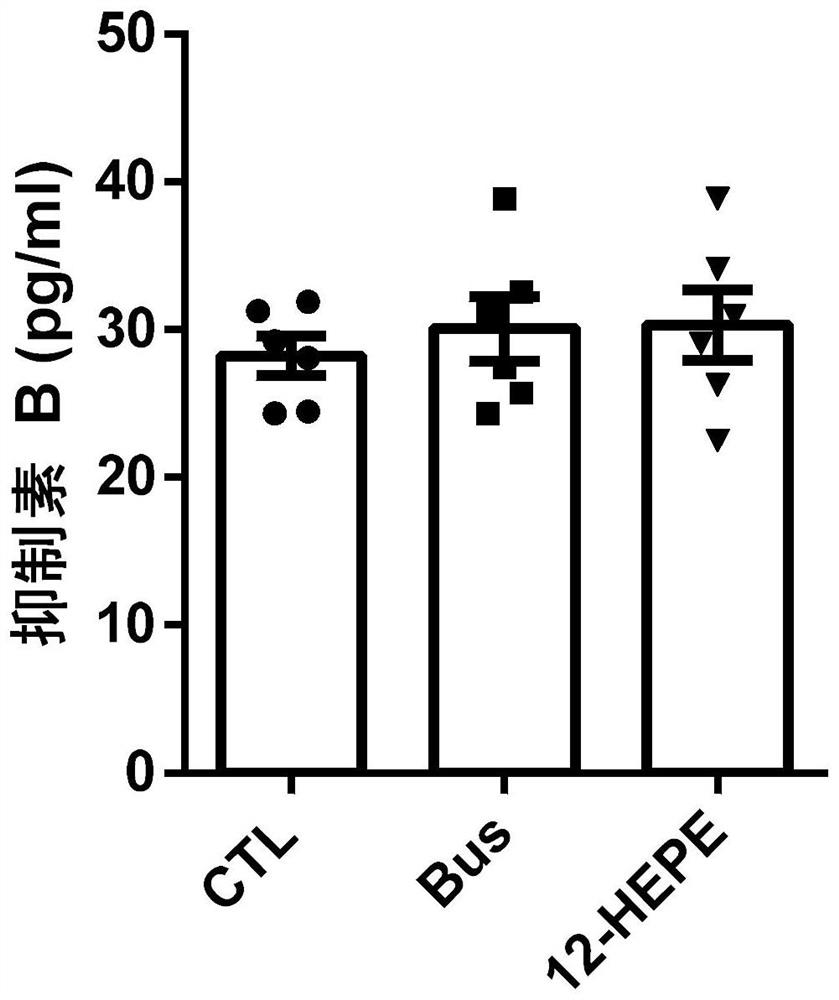
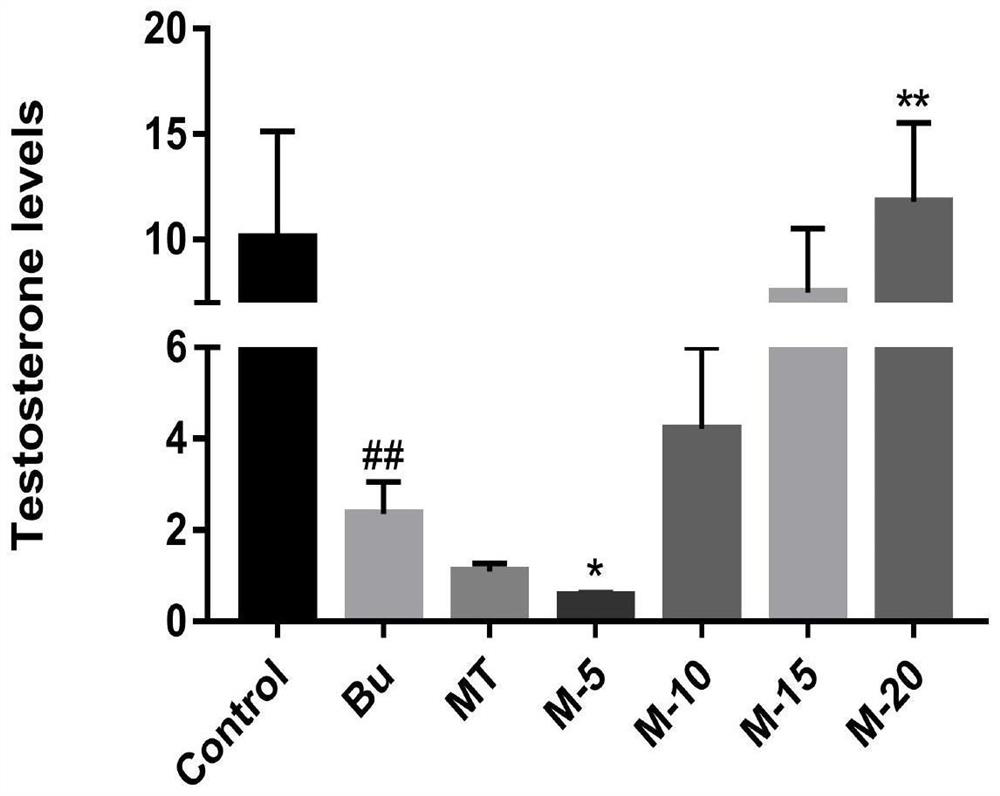
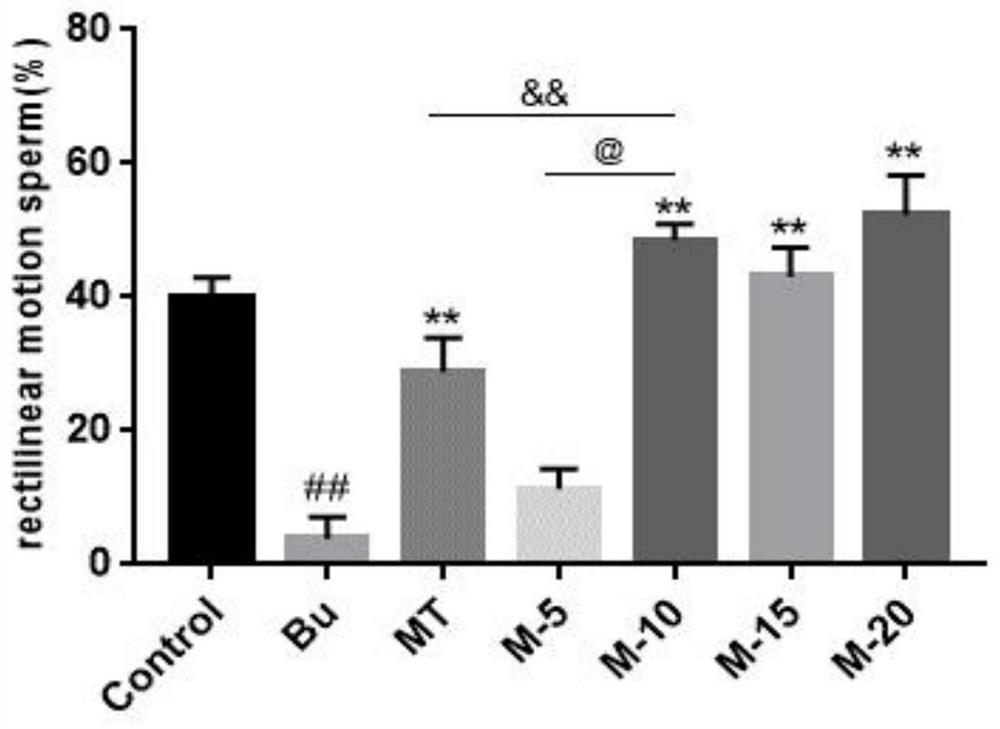
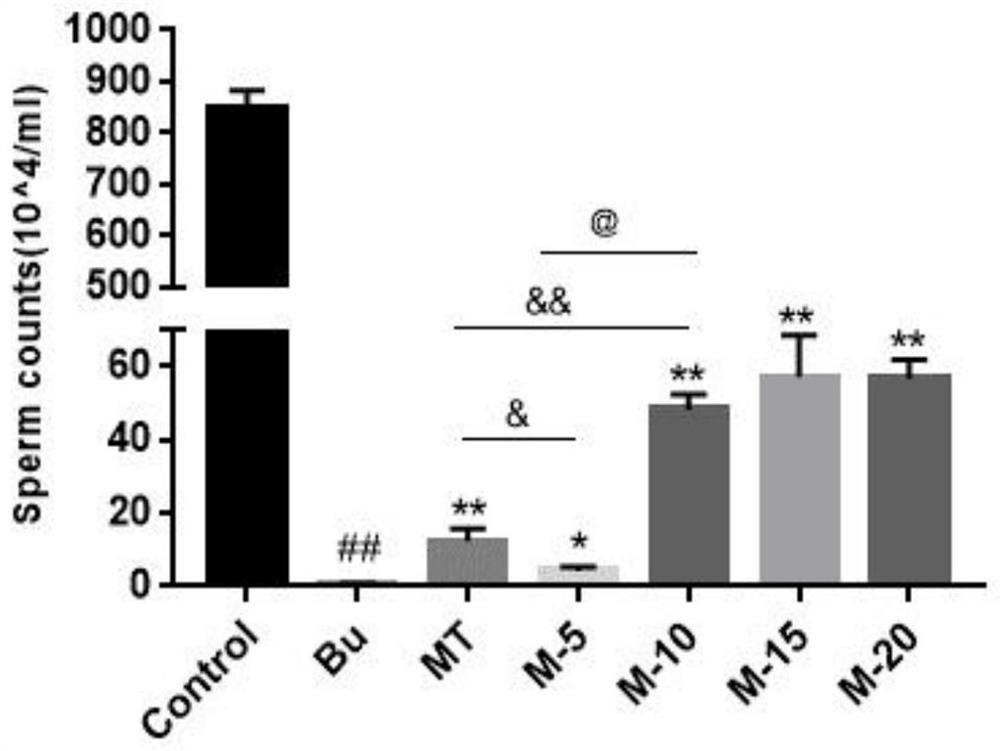
Application of 12-HEPE or pharmaceutically acceptable fatty acid thereof in alleviation of spermatogenesis disorder
ActiveCN113855659APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
The invention discloses novel application of 12-HEPE or pharmaceutically acceptable fatty acid thereof, and comprises the application of the 12-HEPE or the pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. The 12-HEPE or the pharmaceutically acceptable fatty acid thereof can play a therapeutic role by promoting proliferation and differentiation of the endogenous spermatogonium of a mouse damaged by busulfan, and experiments prove that recovery of the thickness and the integrity of the spermatogenic epithelium of the testis and the recovery of the sperm concentration of the tail of epididymiscan be observed through intragastric administration of the 12-HEPE into the NOA mouse induced by the busulfan, meanwhile, proliferation and differentiation of spermatogonium are promoted, expression of the paracrine factors of cells is supported, so that the 12-HEPE can be used as a new target for alleviating the spermatogenesis dysfunction of the testis of the NOA mouse, and a new method is provided for treating the non-obstructive azoospermia.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +3
Pharmaceutical composition, a method of preparing it and a method of treatment by use thereof
InactiveUS20080131498A1Overcomes insolubility problemEliminate side effectsBiocideTetrapeptide ingredientsSide effectOral medication
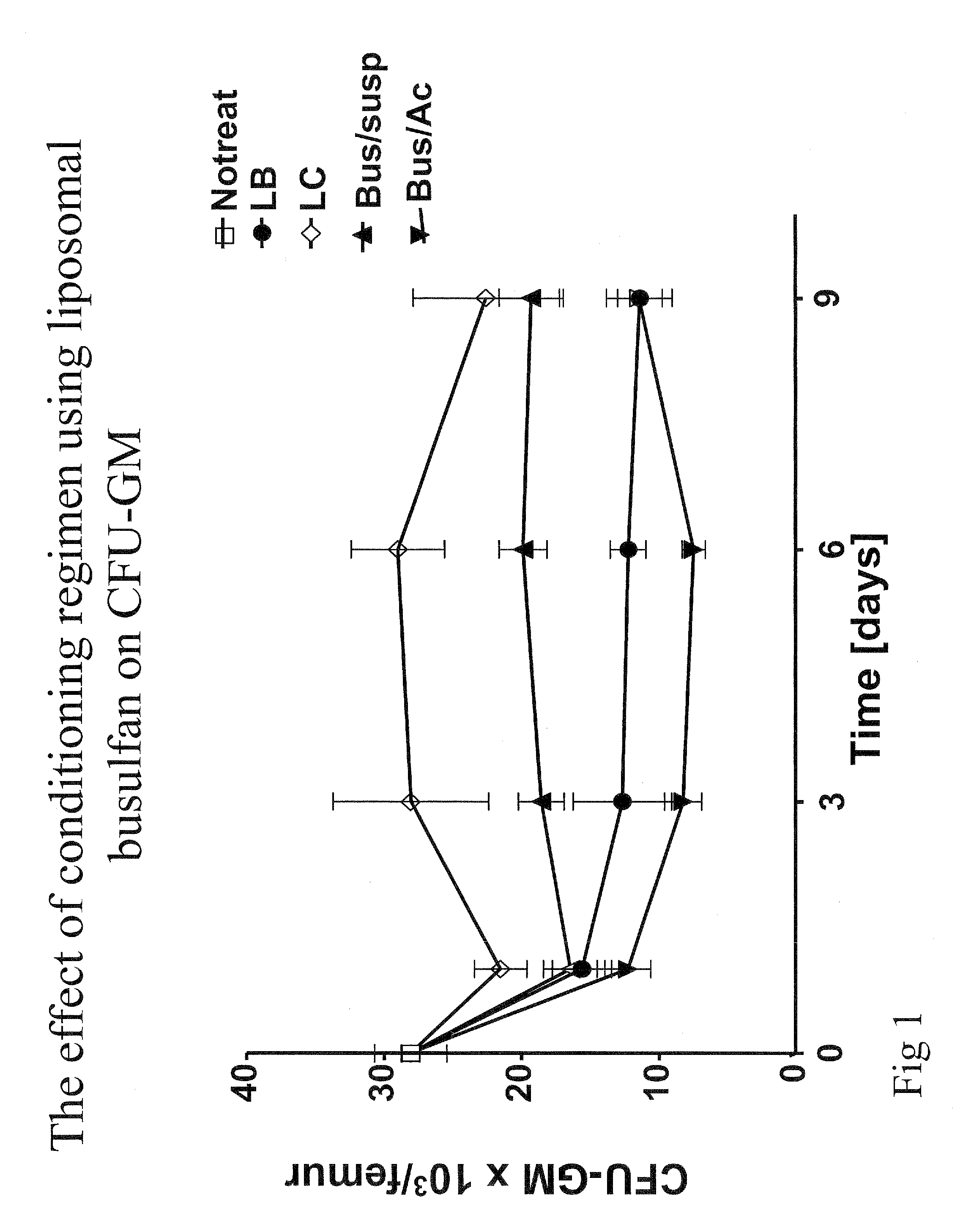
Pharmaceutically acceptable liposome-encapsulated busulphan formulations for parenteral administration are provided, as well as such formulations furthermore comprising glutathione and / or at least one glutathione precursor and a process for manufacture of the preparations. The formulations are stable, have improved biodistribution and significantly reduced side effects over those produced by oral administration or parenteral administration of free drug. The formulations are useful as part of stem cell and / or bone marrow transplant conditioning regimens. A method of treatment of a mammal by use of such formulations.
Owner:BUSULIPO
Combined medicine for treating leukemia, and application of combined medicine
InactiveCN108853506AReduce usageMitigate serious adverse reactionsOrganic active ingredientsAntineoplastic agentsTreatment effectMedicine
The invention relates to a combined medicine for treating leukemia, and application of the combined medicine. The combined medicine is prepared from 5-HT1A receptor agonist and busulfan. The combinedmedicine has a remarkable treatment effect in treatment of the leukemia, especially treatment of chronic myelogenous leukemia; compared with the singly used busulfan, the combined medicine is better in treatment effect; the combined medicine has a synergistic effect.
Owner:SICHUAN CREDIT PHARMA
Busulfan sustained-release implantation agent for curing entity tumour
InactiveCN101181229APrevent postoperative recurrenceOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a busulfan sustained-release implant for the treatment of solid tumors, which is characterized in that the sustained-release implant contains anti-cancer effective amount of busulfan, sustained-release excipient and certain amount of sustained-release regulator. The sustained-release excipient mainly comprises one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan and poly (L-lactide-co-propyl phosphate). The invention can slowly release the busulfan in the local part of the tumor during the degradation and absorption process, so the invention can maintain effective drug concentration at the local part of the tumor at the same time of significantly reducing systemic toxic reaction. The sustained-release implant is arranged at the local part of the tumor, which can not only reduce the systemic toxic reaction of busulfan, but can also selectively improve the drug concentration at the local part of the tumor and strengthen the treatment effects of chemotherapy drugs, radiation therapy and other non-surgical therapies. The solid tumors include pancreatic cancer, lung cancer, liver cancer, breast cancer, brain tumor, ovarian cancer, prostate cancer, esophageal cancer, lymphoma, osteosarcoma, colorectal cancer and so on.
Owner:SHANDONG LANJIN PHARMA +1
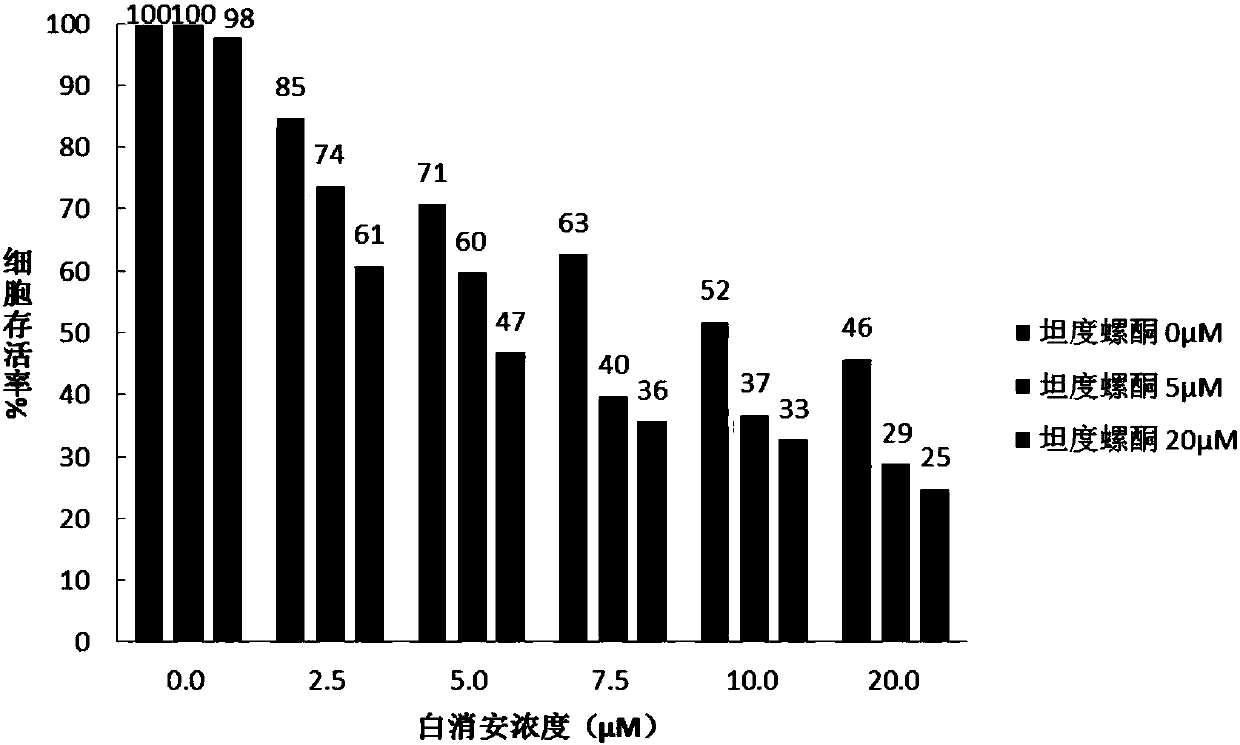
Busulfan composition and preparation method and application thereof
ActiveCN109078005AReduce riskNot worried about DEHP riskOrganic active ingredientsPill deliveryOrganic solventFreeze-drying
Owner:JIANGSU LINGHANG BIOLOGICAL TECH CO LTD
Busulfan immunoassay
Novel conjugates of busulfan and novel busulfan immunogens derived from α-substituted derivatives of busulfan and antibodies generated by these busulfan linked immunogens are useful in immunoassays for the quantification and monitoring of busulfan in biological fluids.
Owner:SALADAX BIOMEDICAL INC
Application of SP in preparation of drug for treating male infertility
ActiveCN110732017AImprove spermatogenic functionTachykinin ingredientsSexual disorderPhysiologyAzoospermia
The invention discloses application of a P substance in preparation of a drug for treating male infertility. Treatment of an azoospermia mouse model by the P substance finds that the P substance can effectively improve spermatogenic functions of the azoospermia mouse model caused by busulfan and further proves that the P substance improves the spermatogenic functions by promoting proliferation ofspermatogenic cells. The invention provides a new thought and theoretical support for treatment of male infertility.
Owner:SUN YAT SEN UNIV
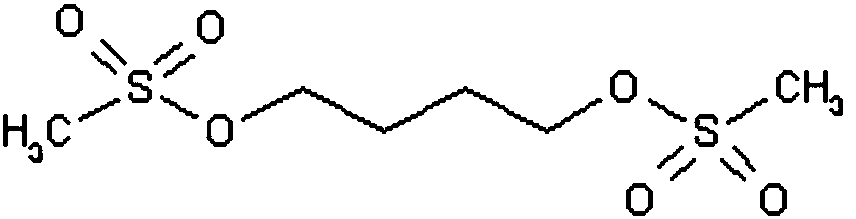
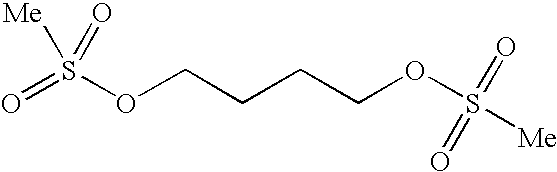
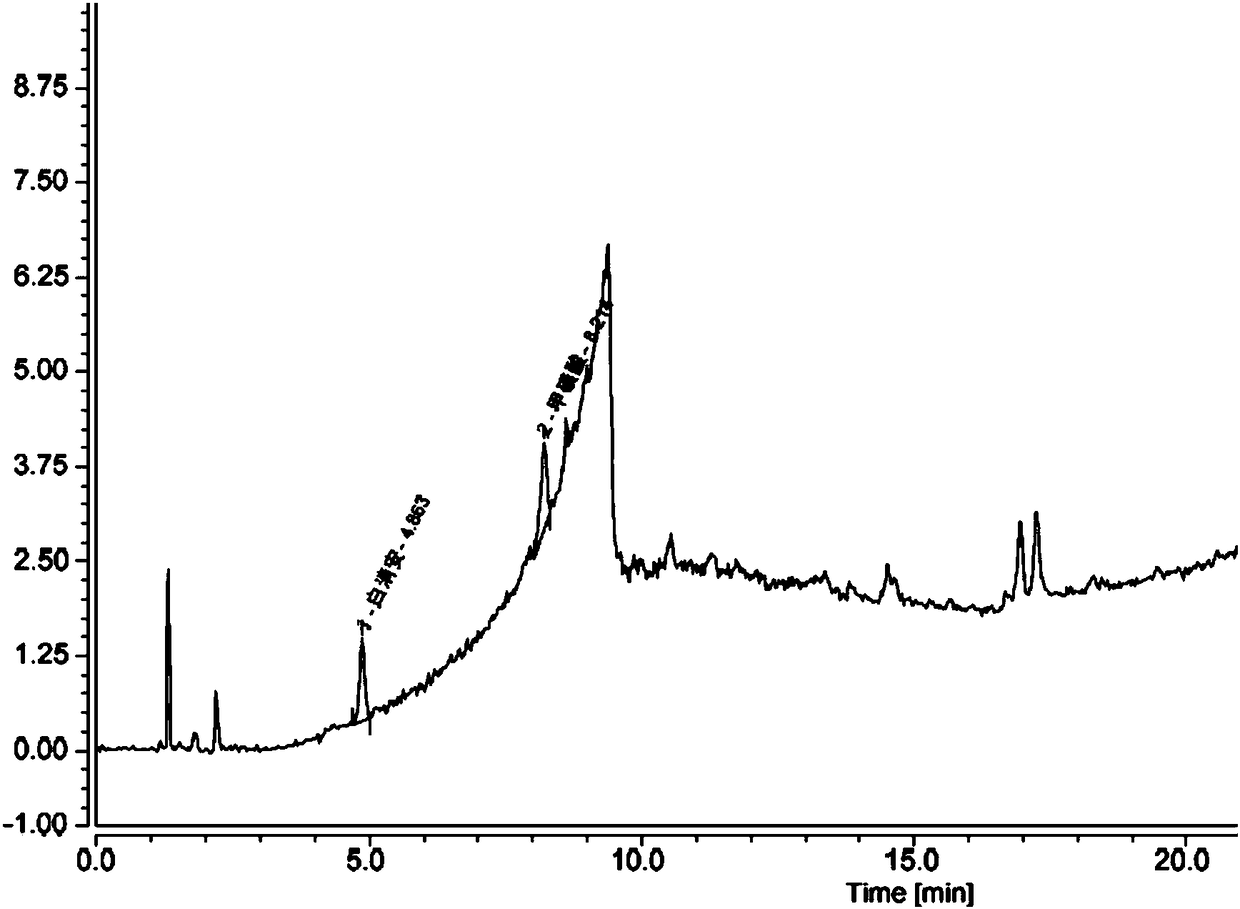
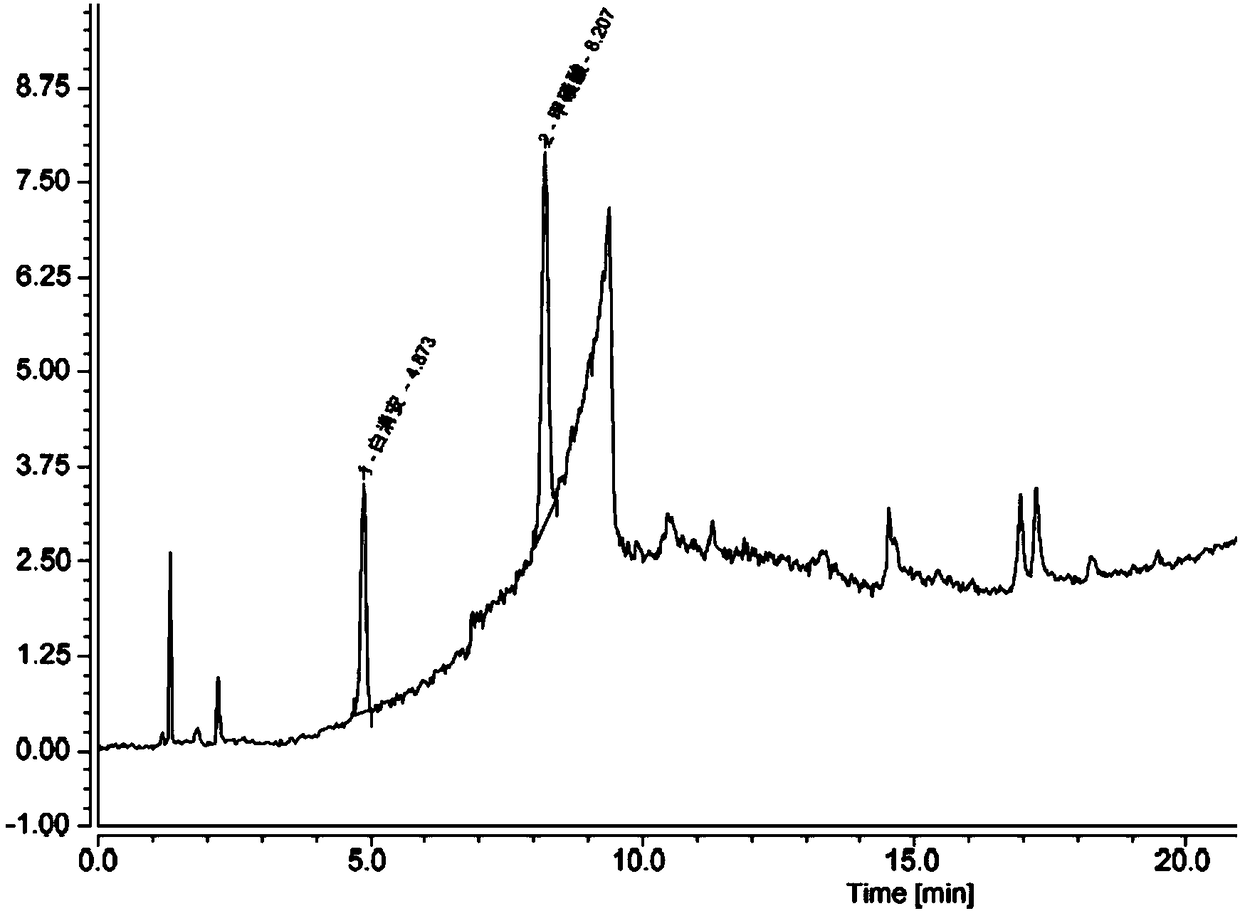
Method for determining content of methanesulfonic acid in busulfan
The invention discloses a method for determining the content of methanesulfonic acid in busulfan. According to the method, the content of methanesulfonic acid is directly determined through the combination of a high performance liquid chromatography and a charged aerosol detector (hereinafter CAD for short). Compared with the prior art, the analysis method has the characteristics of simplicity inreproducibility, good in specificity, high in sensitivity and the like.
Owner:SHANGHAI AOBO PHARMTECH INC LTD +1
Compounds and compositions for the treatment of cancer
New uses for phenylketone carboxylate compounds and substituted aromatic compounds of Formula I, Formula I.1, Formula I.2, Formula IA, Formula IB, Formula IC and Formula II and their pharmaceutical acceptable salts for the treatment of cancer. The use of a combination of two of these compounds is described and the use of the combination of one of these compounds with an anticancer agent such as decarbazine, doxorubicin, daunorubicin, cyclophosphamide, busulfex, busulfan, vinblastine, vincristine, bleomycin, etoposide, topotecan, irinotecan, taxotere, taxol, 5-fluorouracil, methotrexate, gemcitabine, cisplatin, carboplatin and chlorambucil.
Owner:PROMETIC PHARMA SMT LTD
Composition containing cyclodextrin and busulfan
Pharmaceutical formulations containing busulfan and cyclodextrin are described. The formulation can include busulfan and cyclodextrin in a clear aqueous solution. A process for preparing the busulfan formulation and method of using the formulation are also described.
Owner:CYDEX PHARMACEUTICALS INC
Busulfan immunoassay
Novel conjugates of busulfan and novel busulfan immunogens derived from α-substituted derivatives of busulfan and antibodies generated by these busulfan linked immunogens are useful in immunoassays for the quantification and monitoring of busulfan in biological fluids.
Owner:SALADAX BIOMEDICAL INC
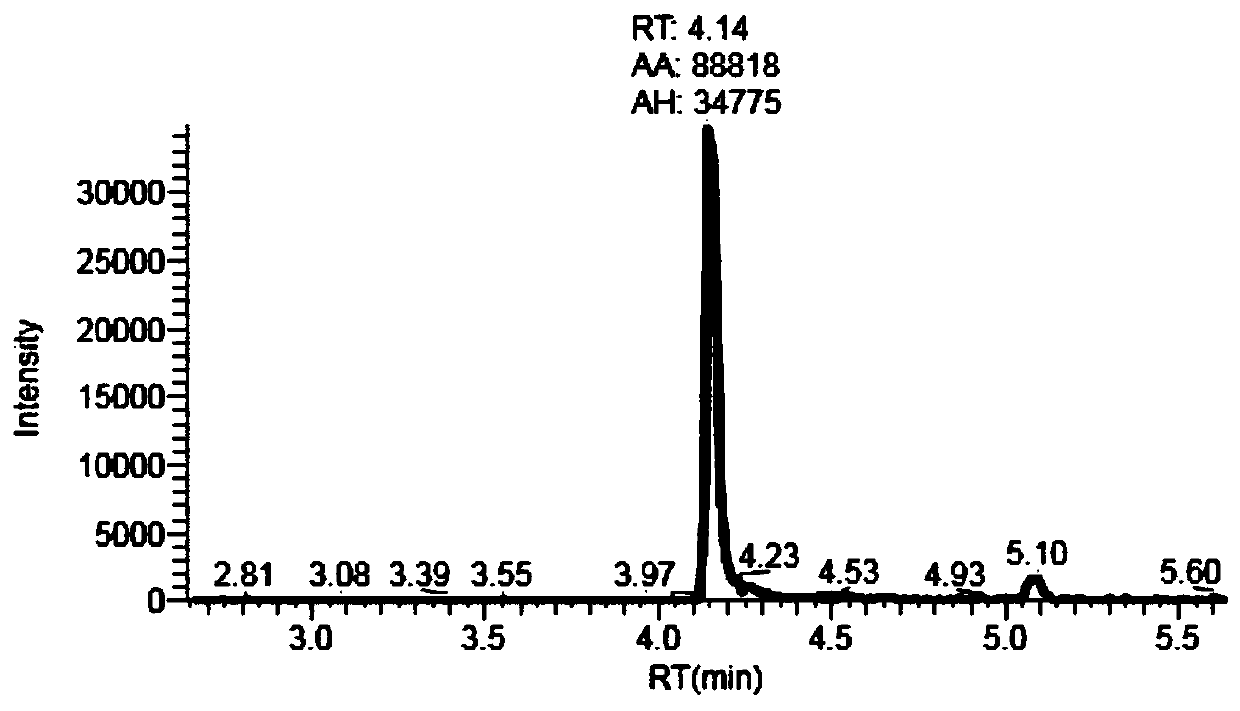
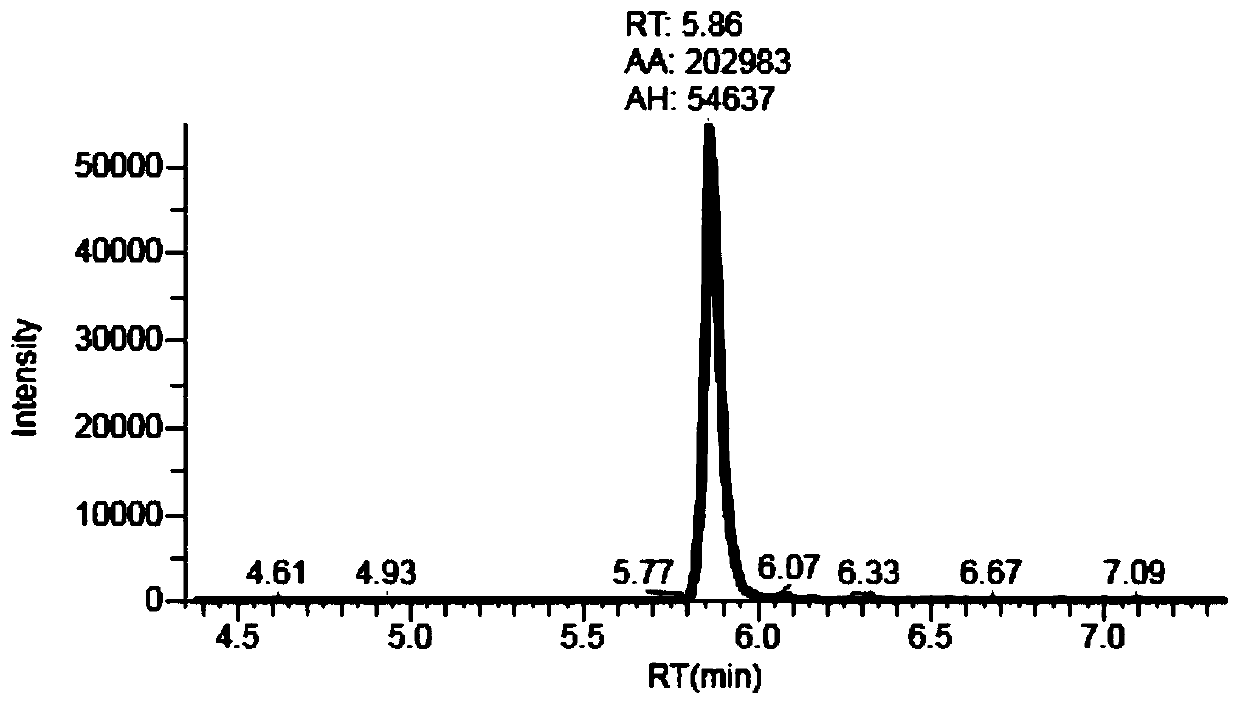
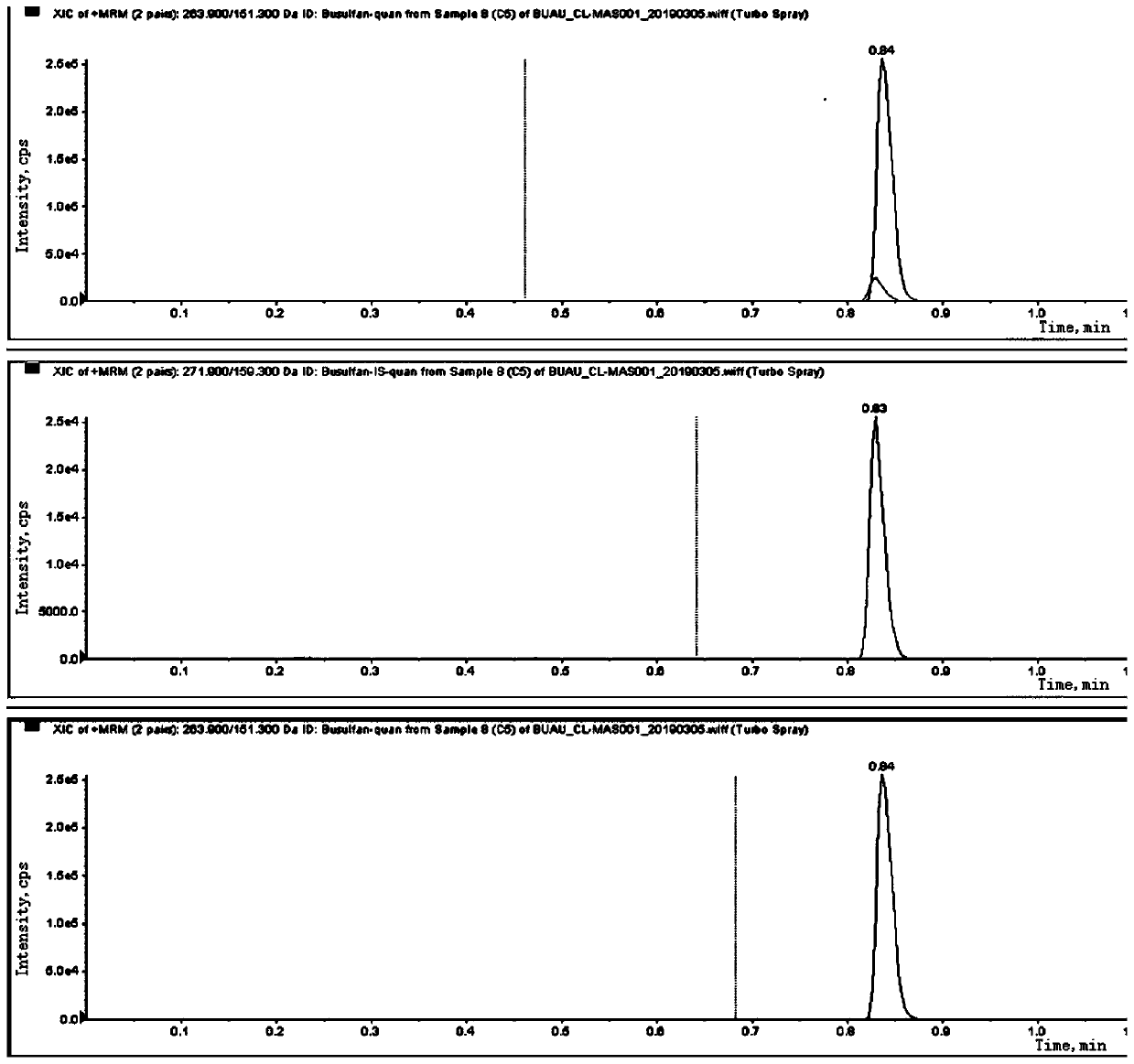
Liquid chromatography tandem mass spectrometry (LC-MS/MS) method for detecting busulfan in plasma
InactiveCN110749666AInjection volume is smallHigh precisionComponent separationFluid phasePhysical chemistry
The invention provides a liquid chromatography tandem mass spectrometry (LC- MS / MS) method for detecting busulfan in plasma. The method comprises the following steps: preparing a working solution; performing pretreatment; performing LC-MS / MS analysis; extracting a chromatogram, and fitting a calibration curve; and establishing a drug time curve graph. The LC-MS / MS method provided by the inventionoptimizes the method for detecting busulfan in human plasma, so that the analysis time is shortened to 2 minutes, and the analysis accuracy and precision and the data stability are ensured; and moreover, the method is simple in pretreatment process, short in time and easy to operate. The detection time is saved, the detection accuracy and reliability are ensured, and more accurate, stable, and high-precision and high-sensitivity detection data can be provided within shorter time.
Owner:上海药明傲喆医学检验所有限公司
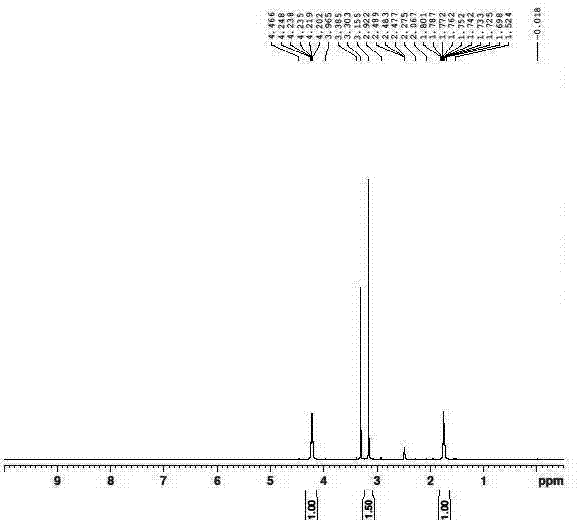
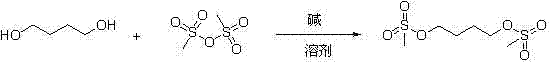
Method for synthesizing busulfan
ActiveCN102408363AAvoid it happening againControl contentSulfonic acid esters preparationSodium bicarbonateButanediol
The invention discloses a method for synthesizing busulfan, which is characterized in that: 1,4-butanediol and methanesulfonic anhydride is taken as main materials; at least one of triethylamine, pyridine, diethylisopropyl amine or sodium bicarbonate is taken as alkali; acetone, tetrahydrofuran, acetonitrile or diethyl ether is taken as solvent; and the whole reaction process is carried out underthe protection of nitrogen gas. The invention also further discloses a method for re-crystallizing busulfan. The methanesulfonic anhydride is used to replace methanesulfonyl chloride so that chlorine-containing product is avoided being produced in the synthesis technology; excessive methanesulfonic anhydride can be reacted with the alkali to form salt, and the salt can be ensured to be all removed after being repeatedly washed by large amounts of water, so that types and content of impurities in the busulfan can be effectively controlled. The method disclosed by the invention is simple, reasonable and easy to operate. The busulfan prepared by re-crystallization can be directly used for preparing injection.
Owner:暨明医药科技(苏州)有限公司
Water-soluble host-guest compound as well as preparation and application thereof
ActiveCN113679852AGood water solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSide effectAqueous solubility
The invention discloses a water-soluble host-guest compound as well as a preparation and application thereof. Water-soluble pillararene is used as a macrocyclic carrier to act with an anticancer drug busulfan, so that the stability of the water-soluble host-guest compound is greatly improved, the hydrolysis rate of the water-soluble host-guest compound is reduced, and the water solubility of the water-soluble host-guest compound is improved. The water-soluble host-guest compound as well as the preparation and application thereof has the advantages that the raw materials of the water-soluble pillararene can be commercially purchased, the synthesis is simple and convenient, the yield is high, the operation of stabilizing anticancer drugs is simple and convenient, the stabilizing effect is good, the hydrolysis rate is greatly inhibited, the water solubility is improved, the problem of hydrolysis of busulfan drugs is expected to be solved. The application prospect in the aspects of treating leukemia and reducing toxic and side effects and the like is wide.
Owner:TIANJIN NORMAL UNIVERSITY
Method for building mouse severe aplastic anemia model
InactiveCN103182071AOrganic active ingredientsPeptide/protein ingredientsBusulfanAplastic bone marrow
The invention discloses a method for building a mouse severe aplastic anemia model, wherein the mouse severe aplastic anemia model is built through combination of an interferon-gama with a busulfan administration method, and the mouse severe aplastic anemia model induced by the invention is simple and convenient in method and high in success rate of induction and accords with the characteristics of human aplastic anemia.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Combined chemotherapeutic agent for transplantation pretreatment of thalassemia stem cells in children
InactiveCN109771643AImprove complianceImprove toleranceOrganic active ingredientsAntibody ingredientsLarge doseThalassemia
The invention discloses a combined chemotherapeutic agent for transplantation pretreatment of thalassemia stem cells in children, which comprises the following components: 5 mg / m<2>*5 days of cladrobin, 1.8 g / m<2>*2 days of cyclophosphamide, 4-4.8 mg / kg*3 days of busulfan and 2.5 mg / kg*2 days of anti-human thymus globulin. The combined chemotherapeutic agent is based on the cladrobin, and the cladrobin is combined with the cyclophosphamide, the busulfan and anti-human thymus globulin to perform pre-treatment large-dose chemotherapy before transplantation of Mediterranean stem cells of children, so that the compliance and tolerance of the chemotherapy are improved, the immunosuppression strength is increased, the transplantation success rate of transplantation of stem cells of a patient isimproved, the transplantation-related complications are reduced, the transplantation curative effect is improved, the survival time of the patient is prolonged, and the combined chemotherapeutic agenthas good social benefit and economic benefit and wide clinical application prospect. The efficient and safe pretreatment scheme can be used for transplantation of thalassemia stem cells in children,and the transplantation survival rate is improved.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hair removal methods and compositions
InactiveUS20180177701A1Avoid typingImprove permeabilityCosmetic preparationsHair removalTransdermal patchHair removal
Compositions and methods for selectively and permanently removing hair from the body. The compositions are formulated to be topically applied upon discrete areas of the body and contain an agent present in an amount sufficient to cause permanent alopecia about the area upon which the composition is applied. Exemplary of such agents include taxanes, such as docetaxel and paclitaxel, and busulfan and other agents known in the art to cause permanent chemotherapy-induced alopecia (CIA). The compositions and methods of applying the same are limited exclusively to transdermal application and operative to induce a localized CIA, and thus avoid systemic distribution of the CIA agent throughout the body. The compositions may further be deployed through known transdermal application mechanisms, such as transdermal patches and the like.
Owner:ROTUNDA ADAM M
Application of swim bladder polypeptide
PendingCN110201140AAntioxidantWith blood pressure loweringHydrolysed protein ingredientsAntinoxious agentsPlant Germ CellsSwim bladder
The invention belongs to the field of biochemical pharmacy and provides application of a swim bladder polypeptide to medicines for inhibiting oxidative damage and cell apoptosis in human bodies or animal bodies. The oxidative damage refer to testicle oxidative damage, the cell apoptosis refers to testicular spermatogenic cell apoptosis, the oxidative damage and the cell apoptosis are caused by antitumor drugs including busulfan, cyclophosphamide and thiotepa, and busulfan is used as the antitumor drug in an embodiment. The swim bladder polypeptide is capable of raising the expression level ofa transcription factor Nrf2, reducing an apoptosis promoting factor Bax and raising the expression level of an anti-apoptosis factor Bcl2; the swim bladder polypeptide is capable of relieving busulfaninduced germ cell vacuolization, spermatogenic cell apoptosis, seminiferous tubule degradation and sperm amount and viability reduction. By adoption of the swim bladder polypeptide for preparation ofthe medicines for inhibiting oxidative damage and cell apoptosis, medicines for recovering fertility of men or male animals can be prepared, and a direction is provided for treatment of fertility ofmen or male animals.
Owner:上海拜汶生物医药股份有限公司
Compounds and Compositions for the Treatment of Cancer
New uses for phenylketone carboxylate compounds and substituted aromatic compounds of Formula I, Formula I.1, Formula I.2, Formula IA, Formula IB, Formula IC and Formula II and their pharmaceutical acceptable salts for the treatment of cancer. The use of a combination of two of these compounds is described and the use of the combination of one of these compounds with an anticancer agent such as decarbazine, doxorubicin, daunorubicin, cyclophosphamide, busulfex, busulfan, vinblastine, vincristine, bleomycin, etoposide, topotecan, irinotecan, taxotere, taxol, 5-fluorouracil, methotrexate, gemcitabine, cisplatin, carboplatin and chlorambucil.
Owner:PROMETIC PHARMA SMT LTD
Application of Omega-3 or pharmaceutically acceptable fatty acid thereof to improvement of spermatogenesis disorder
PendingCN114146076APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
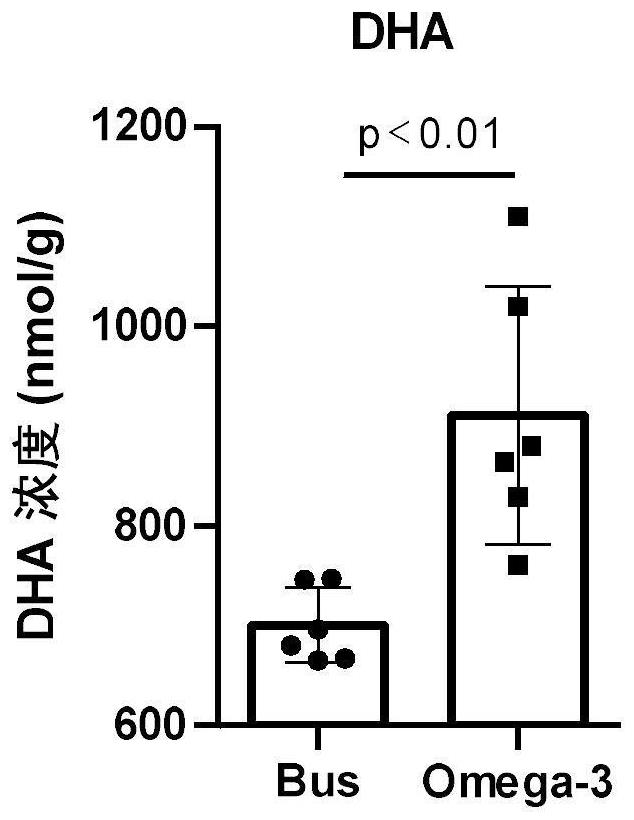
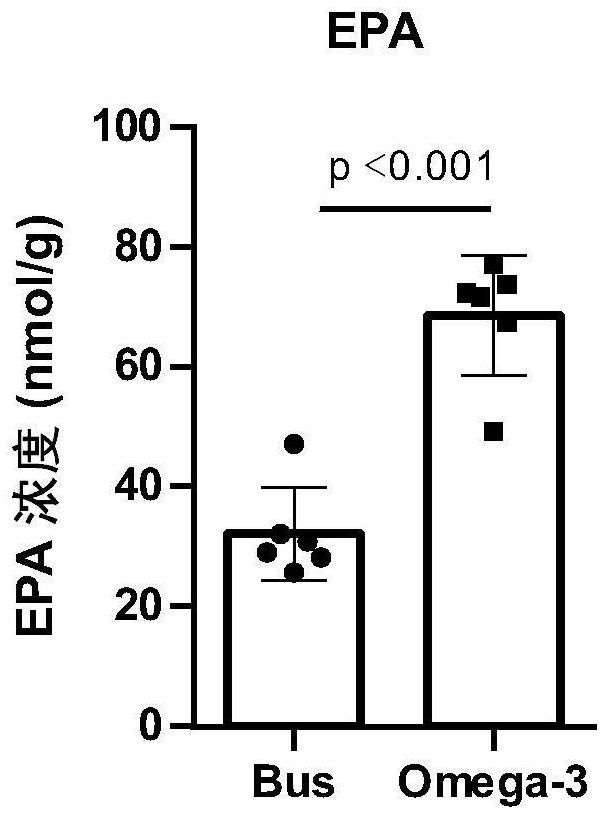
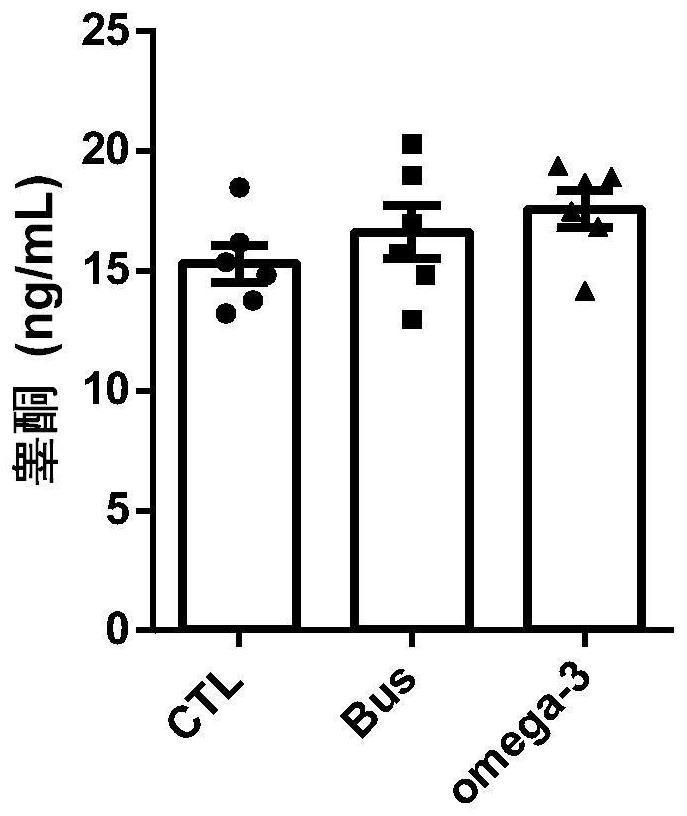
The invention discloses a novel application of Omega-3 or pharmaceutically acceptable fatty acid thereof, and the novel application comprises an application of Omega-3 or pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. Through eicosanoic acid targeted metabonomics, it is screened that DHA and EPA in testicular tissue of a polyunsaturated fatty acid Omega-3 gavage male mouse are obviously up-regulated. Experiments prove that Omega-3 is injected into the stomach of a busulfan-induced NOA mouse, the thickness and integrity of testis recovery spermatogenic epithelium and the concentration of epididymis tail sperms can be observed, and meanwhile, proliferation and differentiation of spermatogonium and expression of supporting cell paracrine factors are promoted, so that the Omega-3 can be used as a new target for improving the NOA mouse testis spermatogenic dysfunction; a novel method is provided for treating the non-obstructive azoospermia.
Owner:NANJING MEDICAL UNIV +1
Hair removal compositions and methods
InactiveUS20180177700A1Avoid typingImprove permeabilityCosmetic preparationsHair removalTransdermal patchDocetaxel-PNP
Compositions and methods for selectively and permanently removing hair from the body. The compositions are formulated to be topically applied upon discrete areas of the body and contain an agent present in an amount sufficient to cause permanent alopecia about the area upon which the composition is applied. Exemplary of such agents include taxanes, such as docetaxel and paclitaxel, and busulfan and other agents known in the art to cause permanent chemotherapy-induced alopecia (CIA). The compositions and methods of applying the same are limited exclusively to transdermal application and operative to induce a localized CIA, and thus avoid systemic distribution of the CIA agent throughout the body. The compositions may further be deployed through known transdermal application mechanisms, such as transdermal patches and the like.
Owner:ROTUNDA ADAM M
Busulfan composition freeze-dried tablet and preparation method thereof
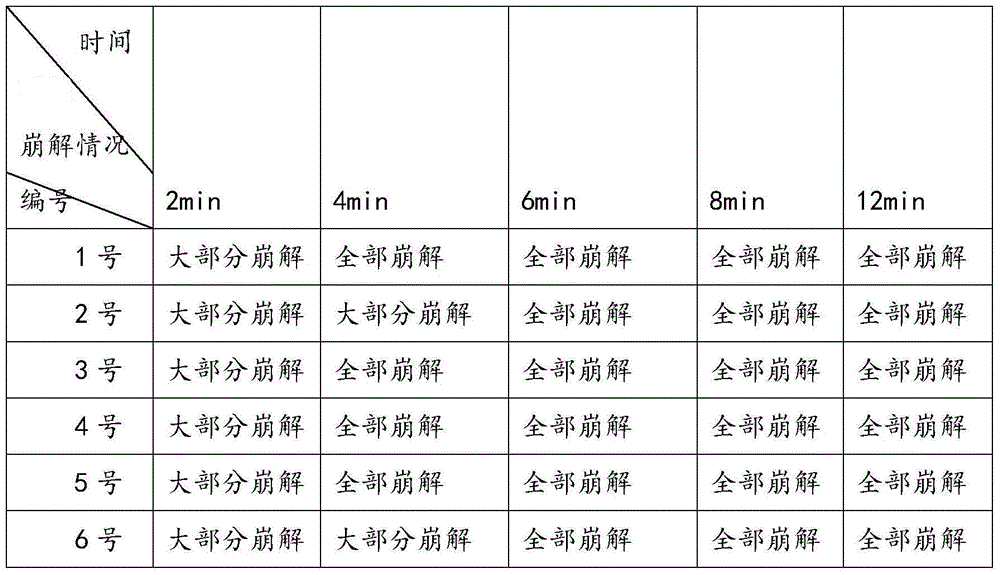
The invention provides a busulfan composition freeze-dried tablet and a preparation method thereof, and relates to the technical field of medicines and medicine production. The busulfan composition freeze-dried tablet comprises busulfan, starch and cane sugar, wherein the starch and the cane sugar are taken as auxiliary materials. Common corn starch is treated by a heating process, so that the bonding and disintegration functions of the starch in the tablet can be improved, and the formability of the tablet is improved. The busulfan composition freeze-dried tablet only needs two auxiliary materials namely the starch and the cane sugar. The busulfan composition freeze-dried tablet adopts the freeze-drying process that temperature is reduced and increased for two times respectively, and the process that temperature is reduced and increased for two times enables the formability of the tablet to be better and increases the dissolution rate of the tablet so as to improve the biological availability of the tablet; the tablet overcomes the defects of a common busulfan tablet, the types and dosage of auxiliary materials in the busulfan tablet are reduced, the dissolution rate of the tablet is high, the biological availability of the tablet is high, and the curative effect and the safety of clinical medication are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Application of maca extract and maca polysaccharide in preparation of product for treating spermatogenesis dysfunction diseases
InactiveCN112156121AHas a therapeutic effectOrganic active ingredientsSexual disorderDiseaseMicrobiology
The invention discloses application of maca and maca polysaccharide in treating testis spermatogenesis disorders, and particularly relates to the application of the maca and the maca polysaccharide intreating the spermatogenesis disorders including oligospermia, asthenozoospermia and azoospermia, especially azoospermia caused by medicine. According to the application, an azoospermia model of a busulfan-induced mouse is used for verifying the improvement effect of the maca and the maca polysaccharide on the busulfan-induced azoospermia mouse.
Owner:CHINA PHARM UNIV
Stabilized standards for busulfan immunoassay
ActiveUS8039220B2Efficient methodEffective monitoringPeptide preparation methodsDepsipeptidesBusulfanBiological fluids
Use of busulfan amide as stabilized standards in immunoassays for quantifying the amount of busulfan in samples of human biological fluids, methods for carrying out said immunoassay and kits for use in said immunoassay.
Owner:SALADAX BIOMEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com