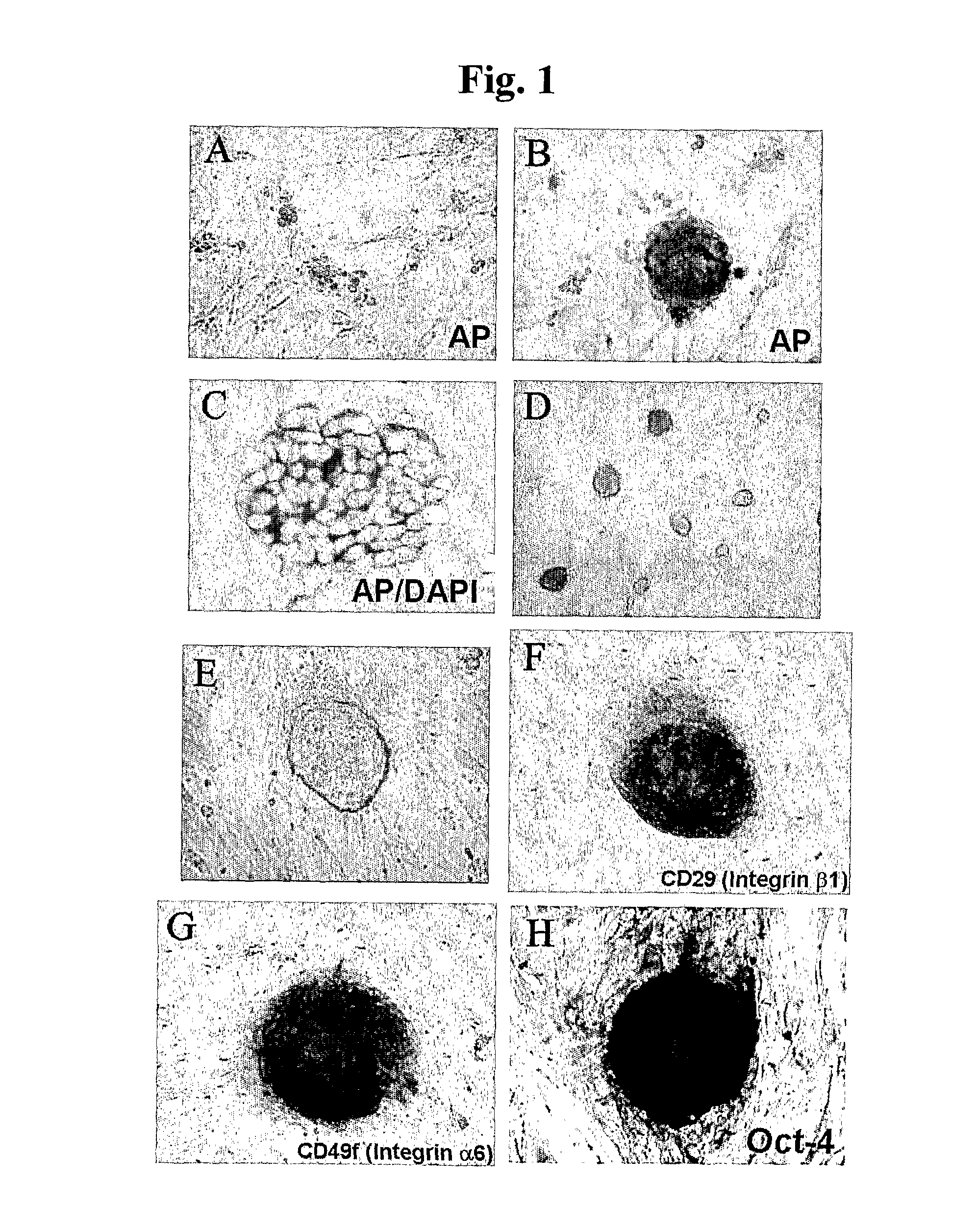
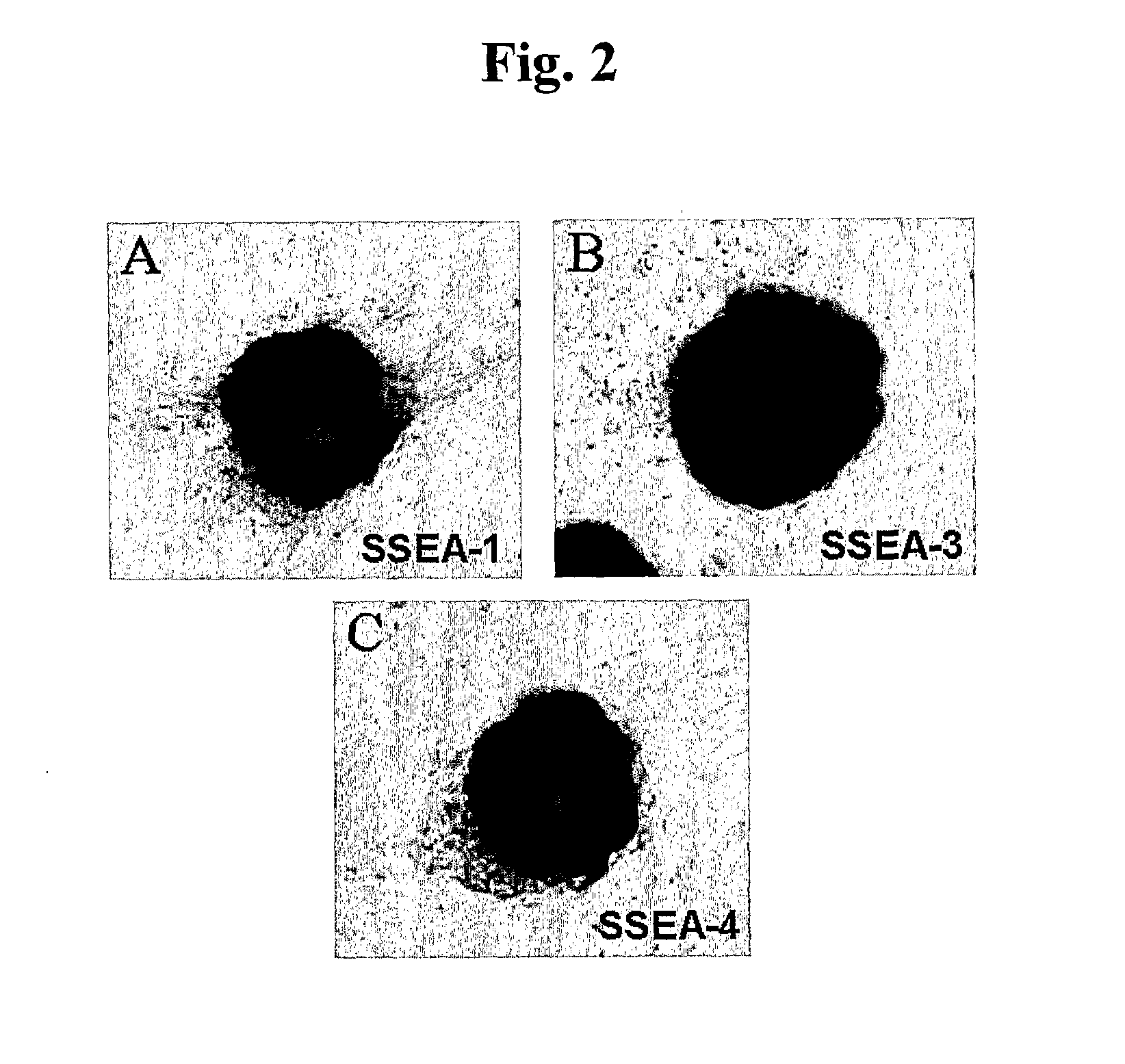
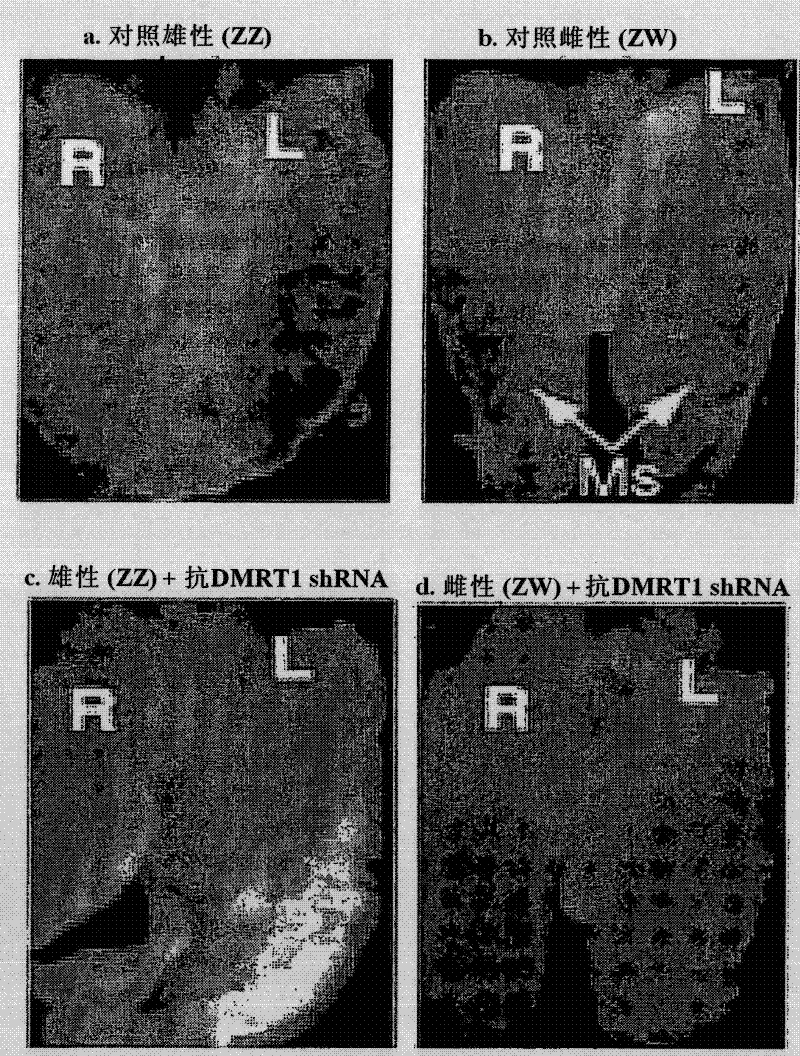
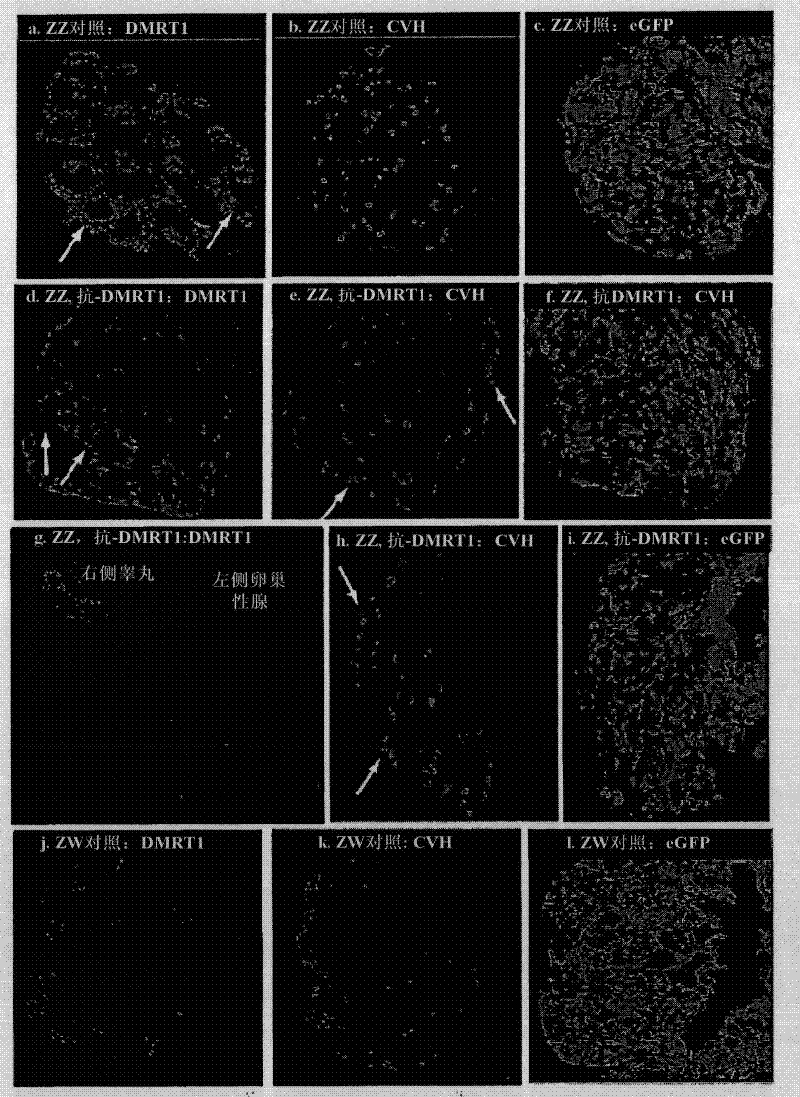
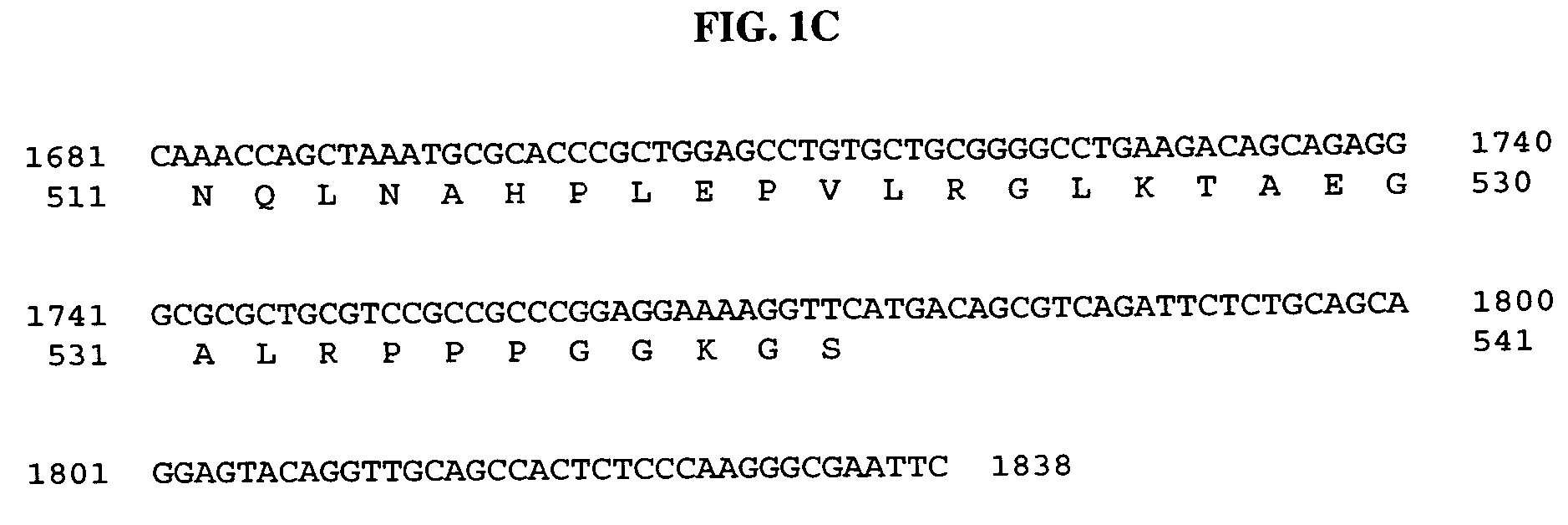
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
281 results about "Mouse Testis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Association of TSPYL polymorphisms with SIDDT syndrome
InactiveUS20050158754A1Cell receptors/surface-antigens/surface-determinantsSugar derivativesScreening methodTestis specific
The identification of a novel mutation in the testis specific Y-like gene and association of the mutation with SIDDT syndrome are disclosed. Methods for diagnosing SIDDT syndrome are disclosed. Methods for identifying compounds for use in the diagnosis and treatment of disorders associated with mutation in the TSPYL gene are also disclosed. The invention therefore provides nucleic acid sequences, genes, polypeptides, antibodies, vectors containing the gene, host cells transformed with vectors containing the gene, animal models for the disease, methods for expressing the polypeptide, genetic screening methods and kits, diagnostic methods and kits.
Owner:THE CLINIC FOR SPECIAL CHILDREN
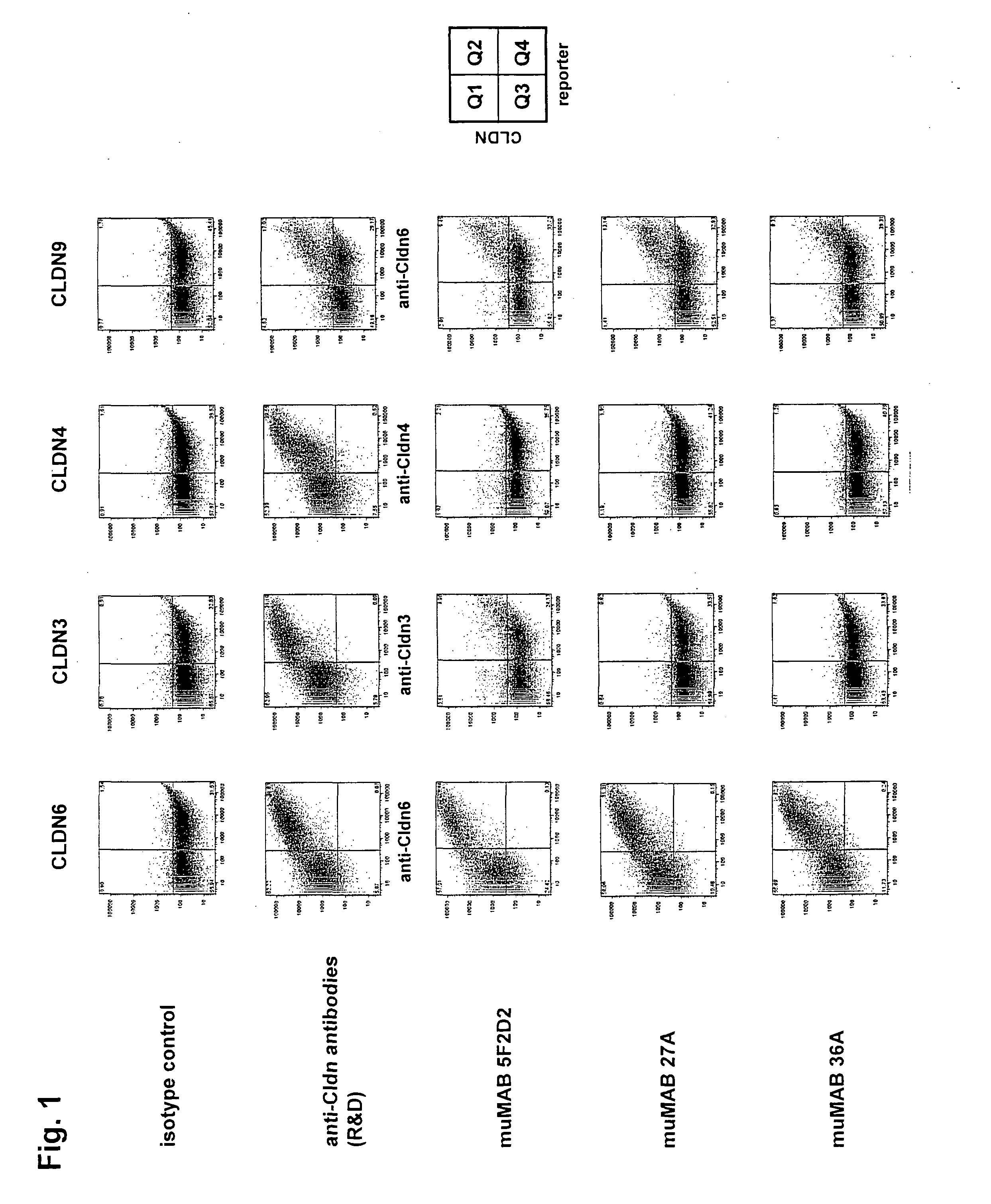
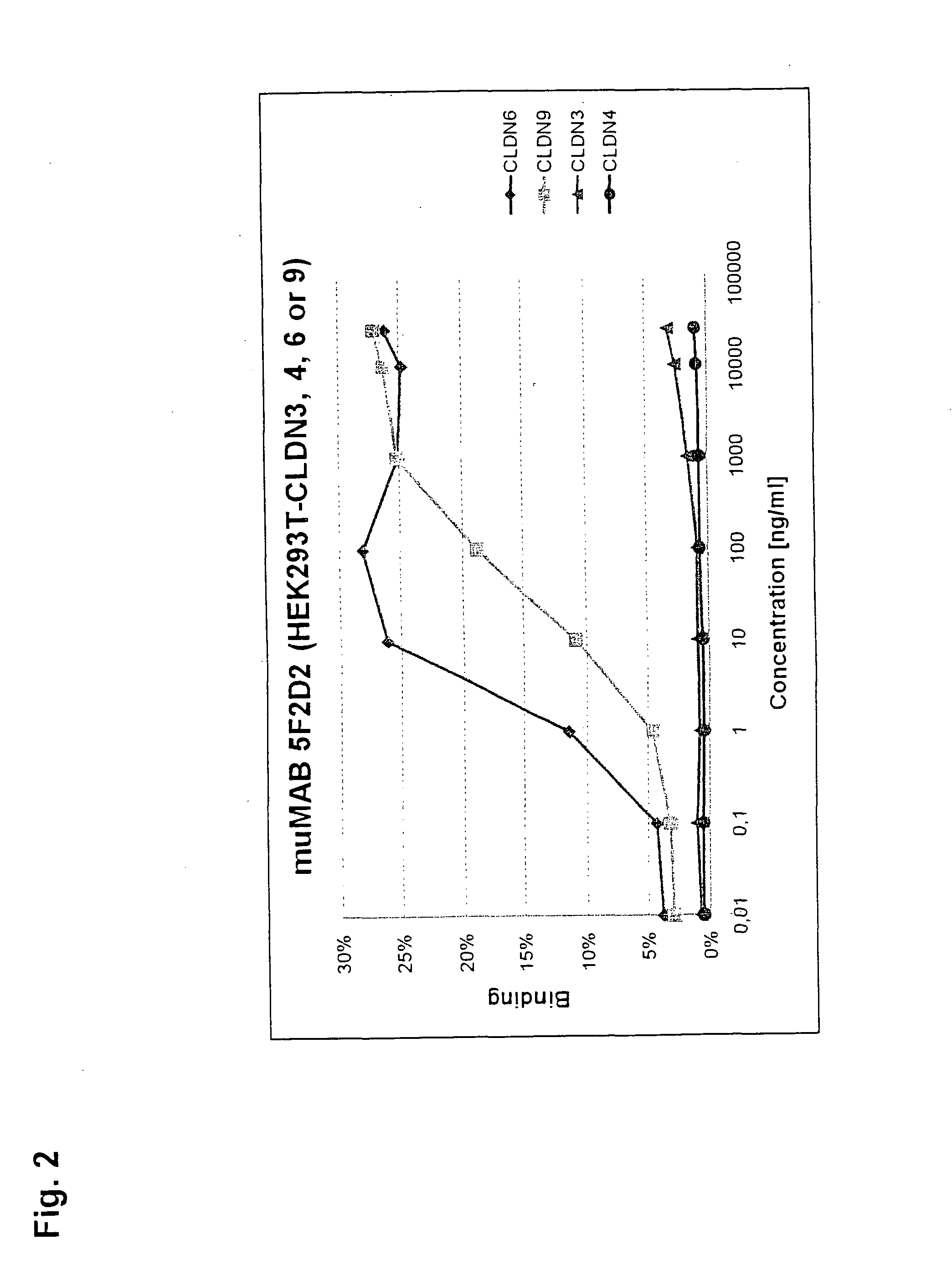
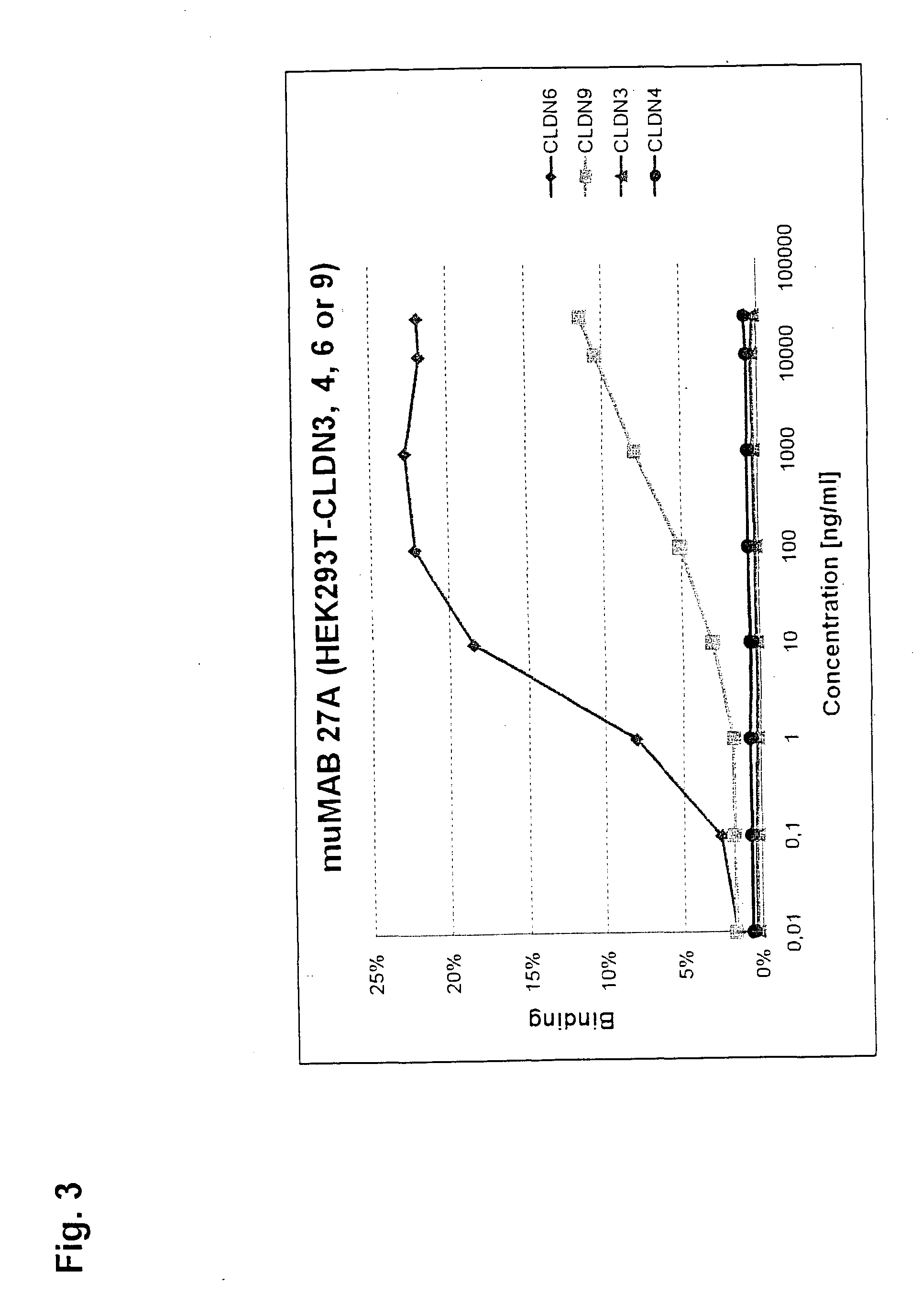
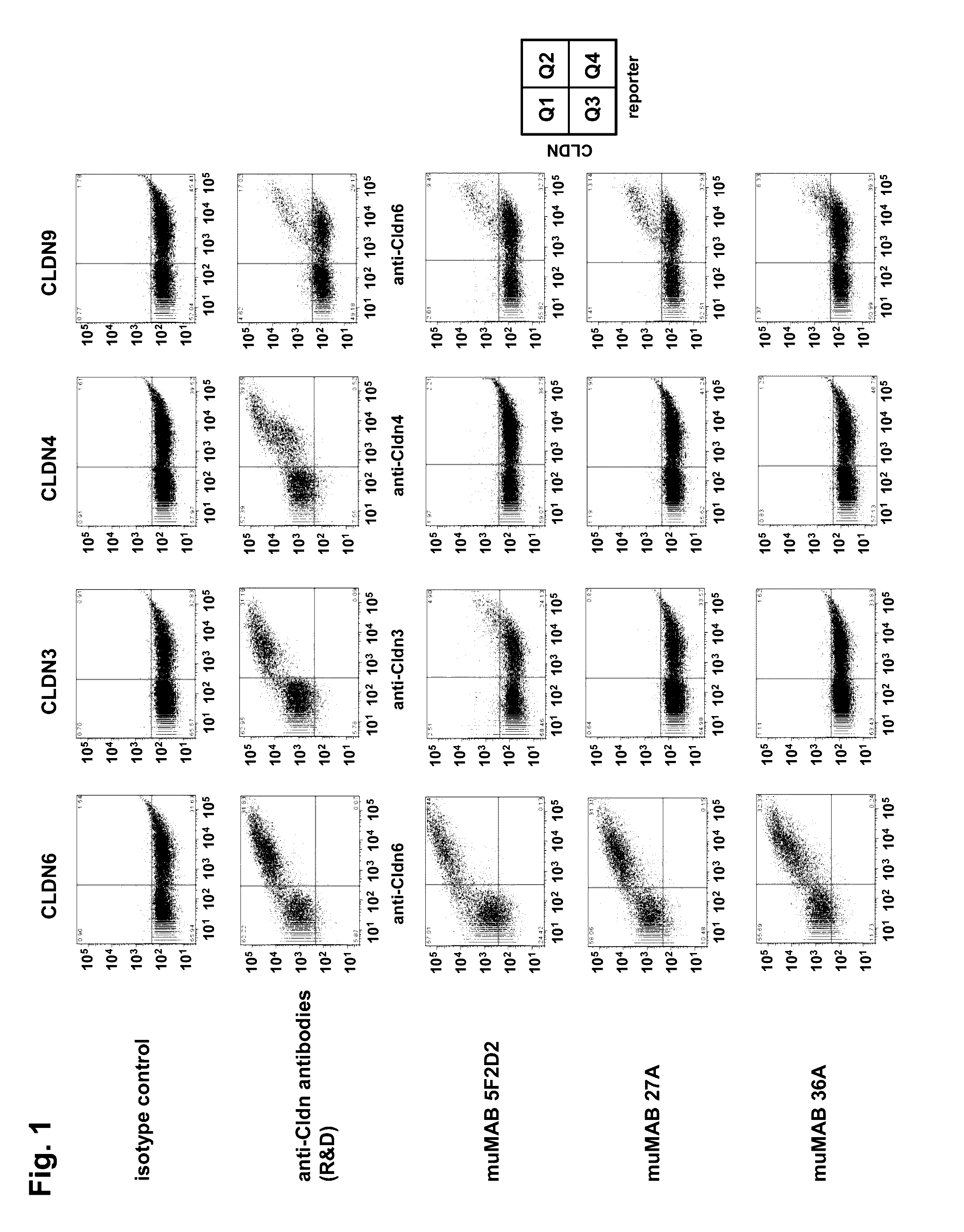
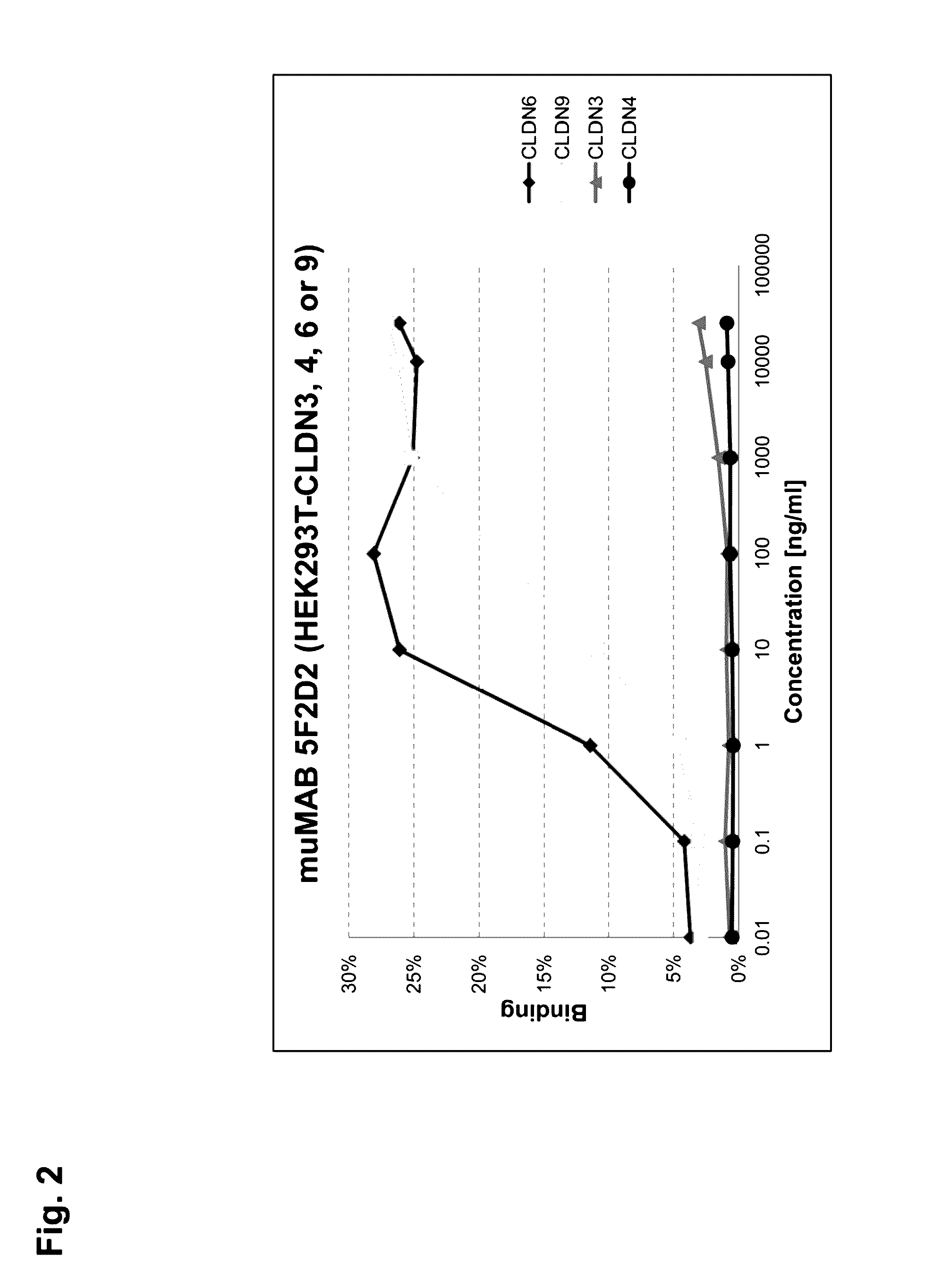
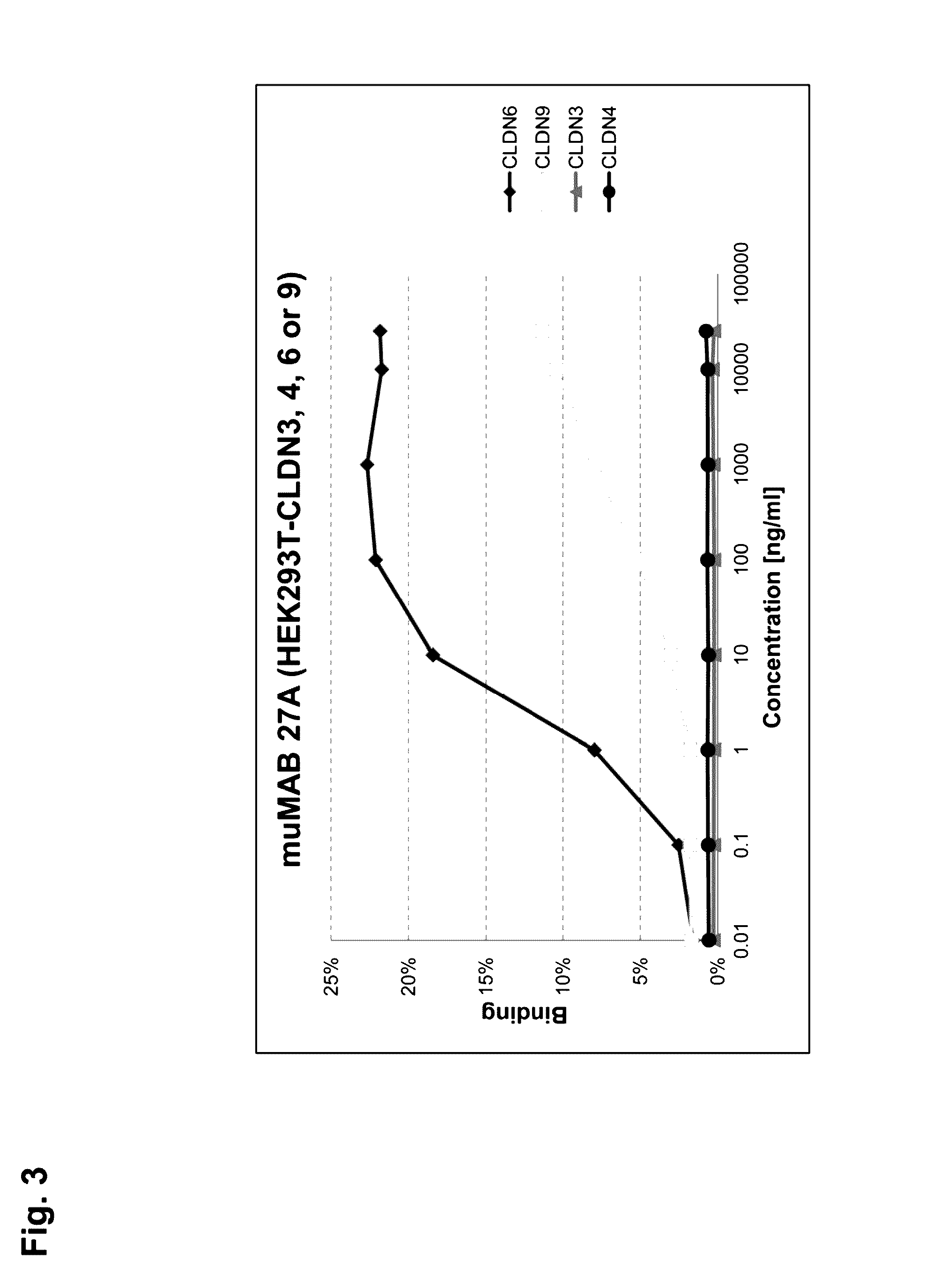
Cancer therapy using cldn6 target-directed antibodies in vivo
ActiveUS20130183305A1Improve survivalReduces tumor cell growthAnimal cellsCell receptors/surface-antigens/surface-determinantsAntibodyWilms tumour
The invention relates to the treatment and / or prevention of tumor diseases associated with cells expressing CLD-N6, in particular cancer and cancer metastasis using antibodies which bind to CLDN6. The present application demonstrates that the binding of antibodies to CLDN6 on the surface of tumor cells is sufficient to inhibit growth of the tumor and to prolong survival and extend the lifespan of tumor patients. Furthermore, binding of antibodies to CLDN6 is efficient in inhibiting growth of CLDN6 positive germ cell tumors such as teratocarcinomas or embryonal carcinomas, in particular germ cell tumors of the testis.
Owner:JOHANNES GUTENBERG UNIV +1
In Vitro Method for Isolating, Proliferating and Differentiating Germ-Line Stem Cells
InactiveUS20080044395A1Achieve isolationAchieve proliferationBiocideGenetic material ingredientsMale infertilityBiology
The present invention discloses methods for isolating, proliferating and differentiating germ-line stem cell in vitro. More specifically, the present invention discloses a method for the in vitro isolation and proliferation of mammalian germ-line stem cells, characterized in culturing cells isolated from mammalian testis in an embryonic stem cell culture medium, and for the in vitro differentiation, characterized in encapsulating mammalian germ-line stem cells and Sertoli cells with calcium alginate and co-culturing with peritubular cells. Also, the present invention discloses germ-line stem cells obtained by above methods, a composition for the treatment of male infertility, comprising the germ-line stem cells, and a method for the treatment of male infertility by the use of the germ-line stem cells.
Owner:COLLEGE OF MEDICINE POCHON CHA UNIV IND
Sex-determination and methods of specifying same
The present invention relates generally to the field of sex determination of animals. Provided are methods and agents to manipulate sex determination, particularly in avian animals such as chickens, through a male chromosome-linked testis (sex) regulatory gene. Expression or activity of the DMRT1 gene or protein is modulated to produce animals with displaying a phenotype sex that differs from their genotype.
Owner:UNIVERSITY OF MELBOURNE +1
Yolk antibody and antigen of pig's infective enterogastritis virus and its preparing process
InactiveCN1382736AGood treatment effectNo toxic side effectsEgg immunoglobulinsVirus peptidesYolkAntigen
A yolk antibody for pig's infective enterogastritis virus is prepared through reproducing the said virus on pig's testis cell, applying it as immunogen to the layer, collecting yolk and purifying. When it is applied to pig, the comparison between experimental and reference groups shows that it has high effect on preventing and cuting pig's infective enterogastritis.
Owner:INST OF ZOOTCHNICS & VERTERINARY SCI BEIJING ACAD OF AGRI & FORESTRY SCI
ST cell adapting to full-suspension culture, and application thereof, and vaccine virus culturing method
ActiveCN105112352ASolve the requirements of cultivating large-scale industrializationHigh degree of automationMicroorganism based processesVertebrate cellsVaccine virusEngineering
The invention relates to an ST cell adapting to full-suspension culture, and an application in vaccine virus culturing, and belongs to biotechnical field. The ST cell adapting to full-suspension culture is named as a swine testicle cell suspension adaptation strain ST-2014S, and is preserved in China Center for Type Culture Collection on May 13, 2015 with the preservation number of CCTCC C201567. The invention also discloses a method for culturing a vaccine virus through using the full-suspension adaptation ST cell. Compared with vaccine viruses in the prior art, the full-suspension adaptation ST cell cultured vaccine virus has the advantages of high automation degree, no need of vector intervention, realization of suspension culture in a serum-free or low-serum medium, meeting of large-scale industrial requirements of virus culture, development of a large-scale production method meeting GMP production technology requirements, and very good industrial application prospect.
Owner:郑州爱科生物科技有限公司
Compositions of very long chain polyunsaturated fatty acids and methods of use
The present invention relates to processes for production of Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFAs). The present invention also relates to compositions (e.g., nutritional supplements, food products, and pharmaceutic compositions) containing such VLC-PUFAs. In one embodiment, the present invention is directed to methods for biosynthesis and production of the VLC-PUFAs described herein (particularly C28-C40 PUFAs, also referred to herein as supraenes or supraenoics) by the expression, in a production host cell, of the full or partial sequence(s) of Elovl4 DNA / mRNA nucleic acids or ELOVL4 protein sequences encoded thereby, from any species (prokaryotic or eukaryotic) for use in the biosynthesis, production, purification and utilization of VLC-PUFAs in particular by the elongation of C18-C26 saturated fatty acids and PUFAs. The composition of the invention comprises, in various embodiments, a dietary supplement, a food product, a nutritional formulation, a pharmaceutical formulation, a humanized animal milk, an infant formula, and a cosmetic item and for example. A pharmaceutical formulation can include, but is not limited to: a composition for enhancing neural development and function, a drug for treatment of neurodegenerative disease, an ocular disorder, a retinal disorder, age related maculopathy, a fertility disorder, particularly regarding sperm or testes, or a skin disorder.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Method for separation and purification, long-term passage cultivation, cryopreservation and recovery of sheep spermatogonia stem cells
ActiveCN104004708AHigh puritySolve the problem of not finding antibodiesDead animal preservationArtificial cell constructsStem Cell IsolationSingle cell suspension
The invention relates to a method for separation and purification, long-term passage cultivation, cryopreservation and recovery of sheep spermatogonia stem cells. The method includes the steps that various solutions are prepared, 2-3 g of tissue blocks of the seminiferous tubule are taken out from the testis of a sheep of 3-4 mouths of age, single-cell suspension is prepared through enzymolysis and digestion of two steps, purified spermatogonia stem cells are separated according to the difference adherence method, the long-term passage cultivation of the sheep spermatogonia stem cells is achieved through an SSCM culture solution, and the sheep spermatogonia stem cells are cryopreserved and recovered after the long-term passage cultivation. By the technical scheme, the sheep spermatogonia stem cells with high purity can be obtained without using specific sheep spermatogonia stem cell antibodies, a long-term passage cultivation system of the sheep spermatogonia stem cells is built, and the method for preserving the sheep spermatogonia stem cells for a long term through a liquid nitrogen freezing method is successfully provided. The sheep spermatogonia stem cells achieving separation, purification and passage according to the method are cultivated at least for more than 40 generations, and the longest cultivation period reaches four years if the cryopreservation time is included.
Owner:INNER MONGOLIA UNIVERSITY
Compositions and methods of treating cancer
InactiveCN101796184AOrganic active ingredientsGenetic material ingredientsTesticular seminomaProstate cancer
Owner:ONCOTHERAPY SCI INC
Polynucleotides encoding a novel testis-specific tubulin tyrosine-ligase-like protein, BGS42
ActiveUS7115402B2Ameliorating and preventing and decreasing level of BGS-4Methylation capacity is decreasedBacteriaHydrolasesDiseaseAntiendomysial antibodies
Owner:BRISTOL MYERS SQUIBB CO
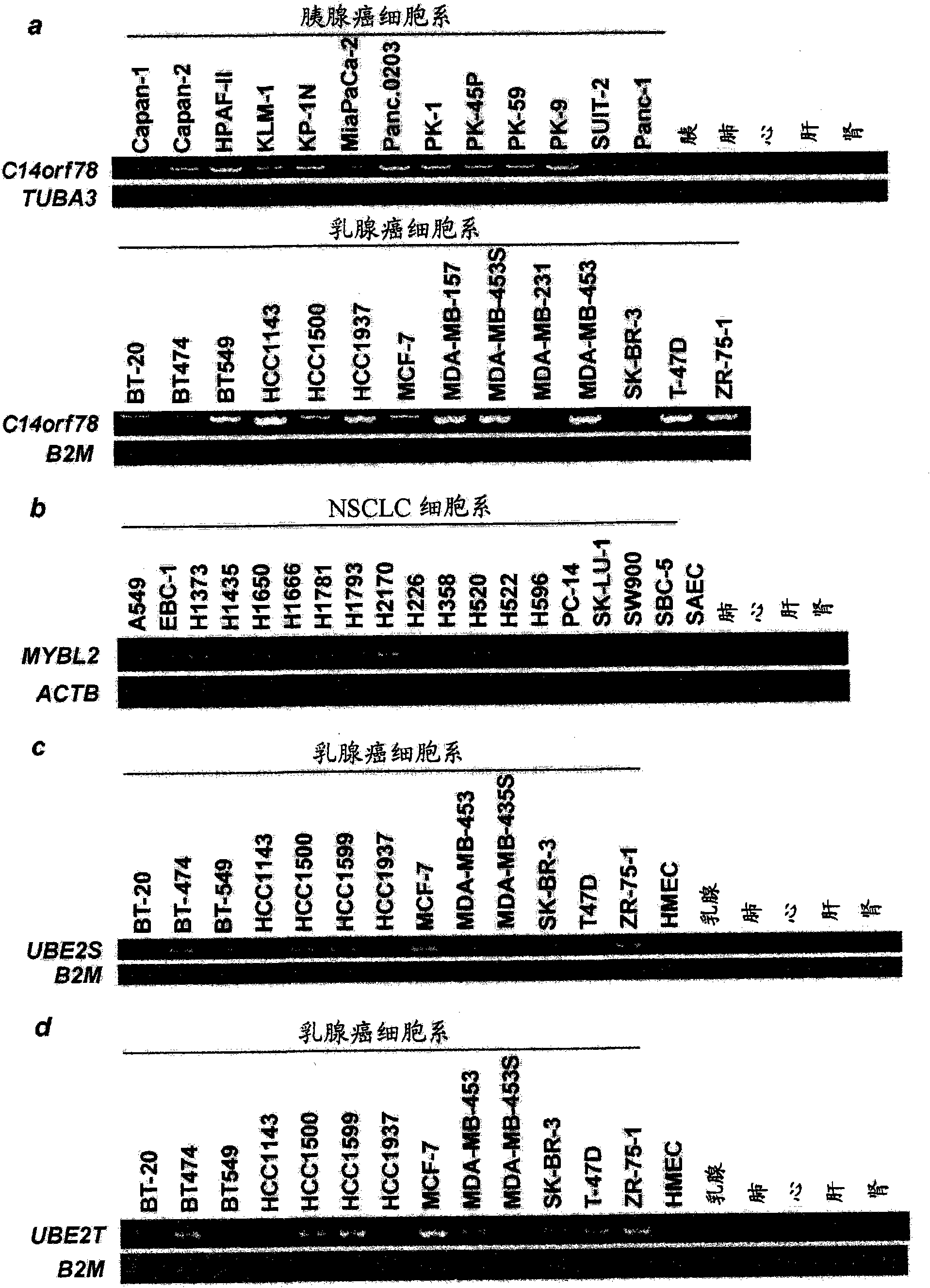
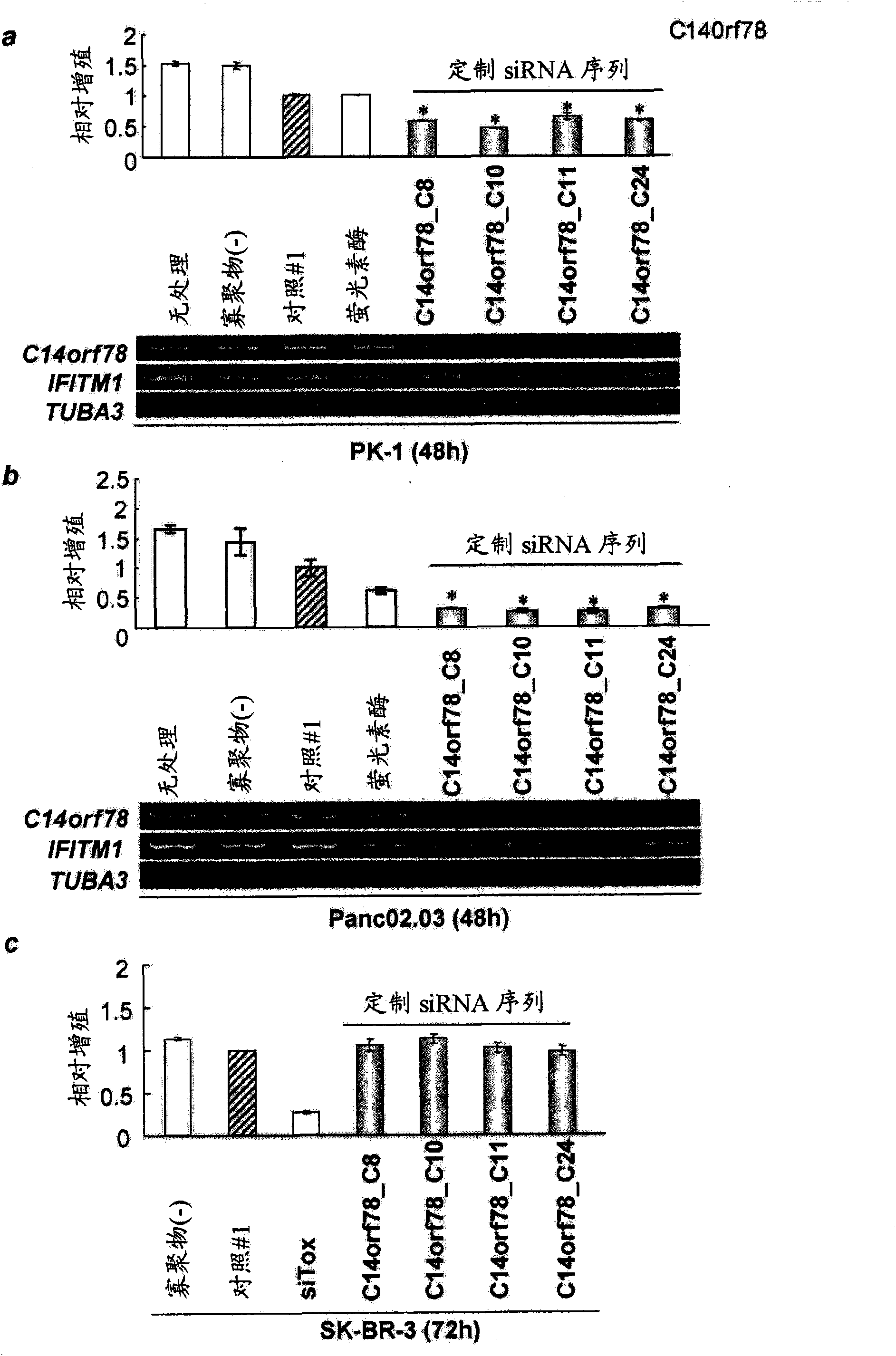
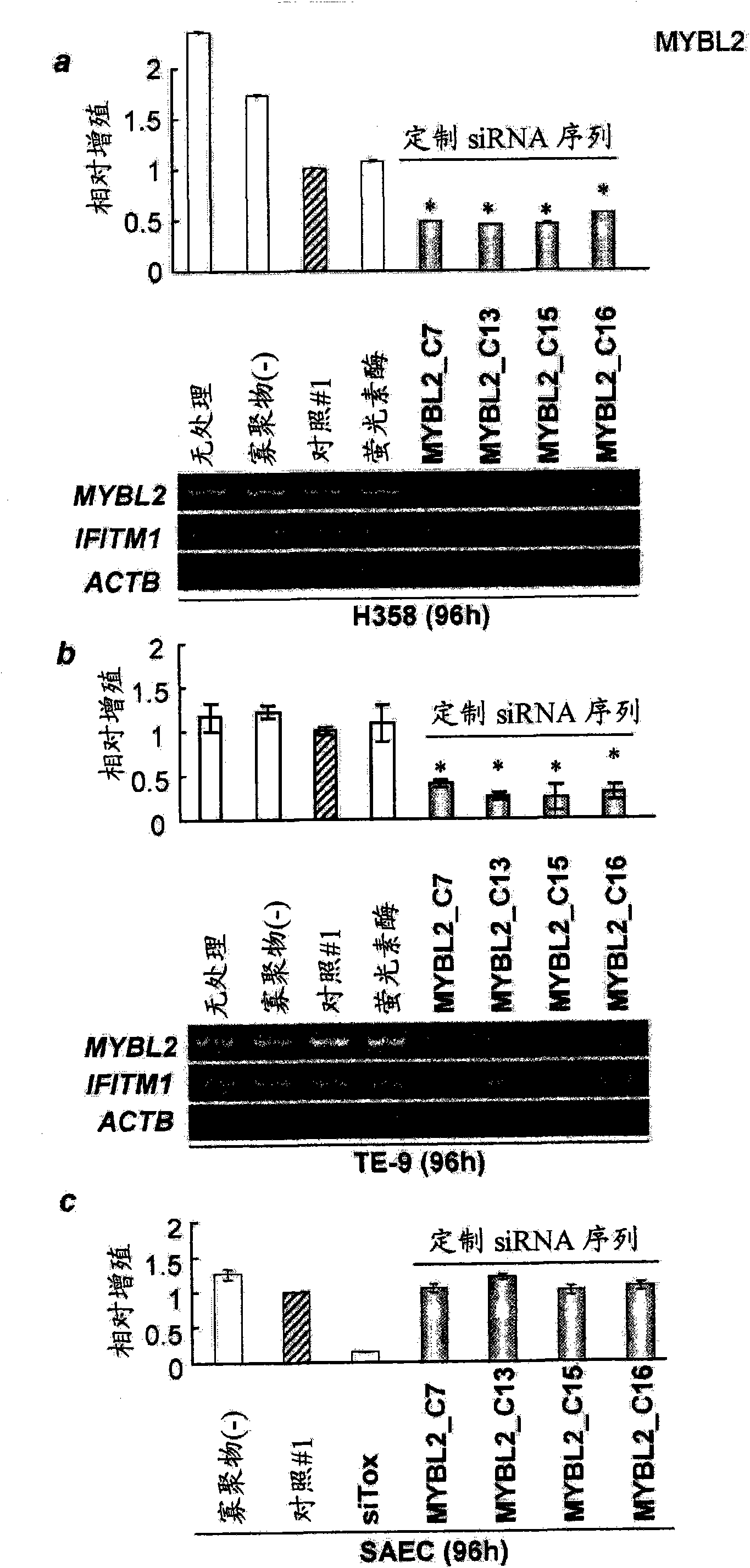
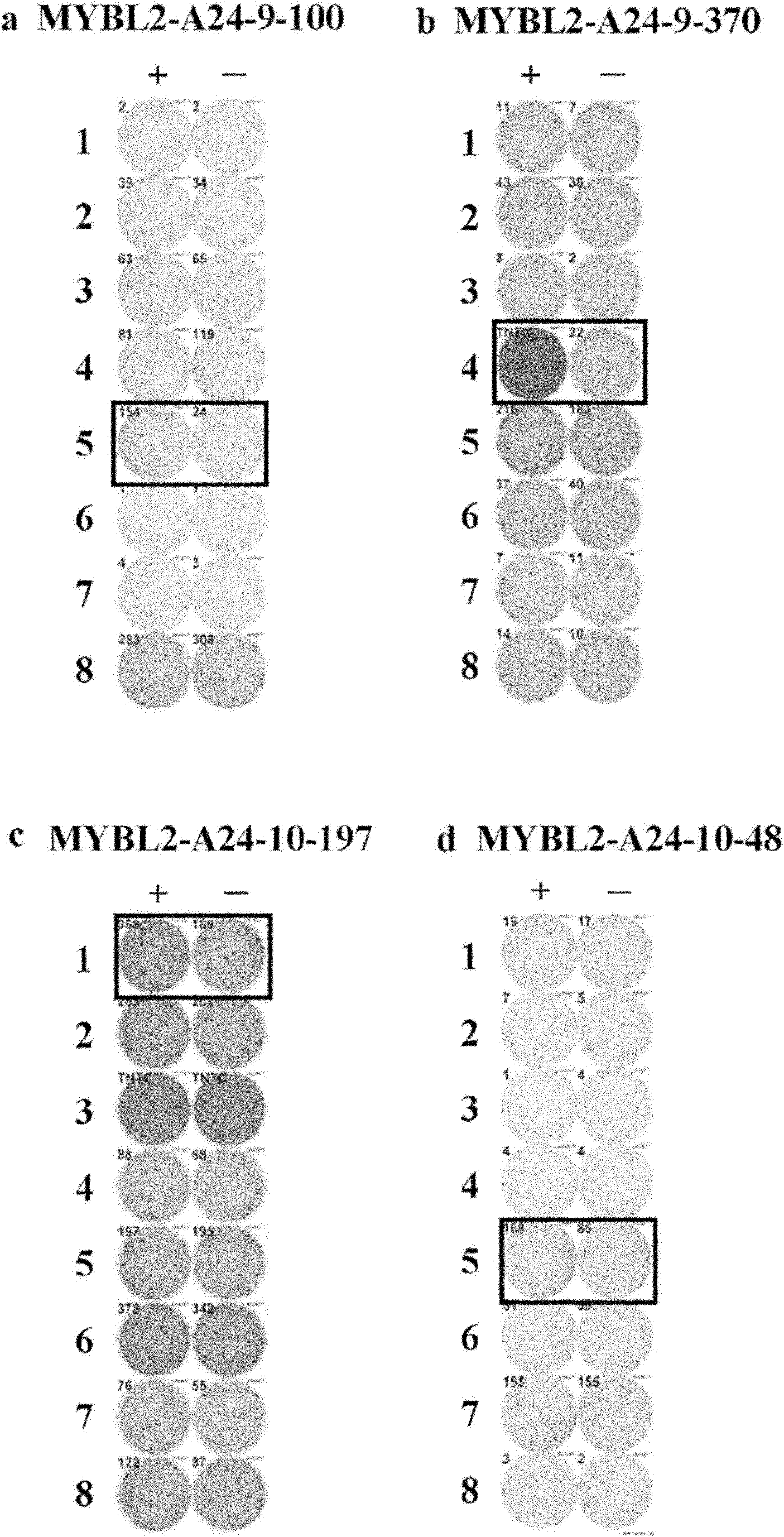
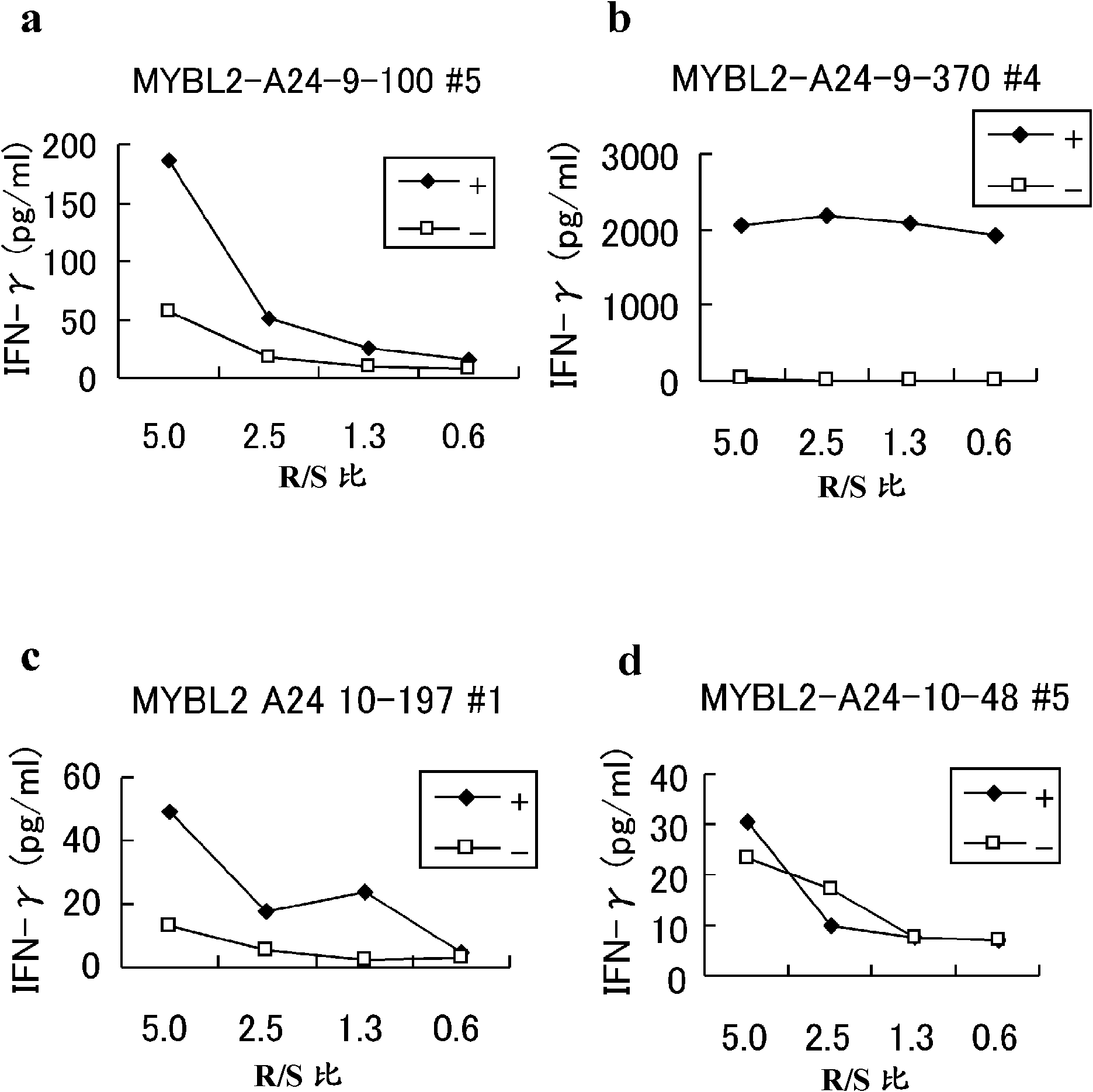
MYBL2 epitope peptides and vaccines containing the same
InactiveCN102119170APeptide/protein ingredientsVaccination/ovulation diagnosticsTesticular stromal tumorHLA-A24
Peptide vaccines against cancer are described herein. In particular, the present invention describes epitope peptides derived from MYBL2 that elicit CTLs. The present invention also provides established CTLs that specifically recognize HLA-A24 positive target cells pulsed with the peptides. Antigen-presenting cells and exosomes that present any of the peptides, as well as methods for inducing antigen-presenting cells are also provided. The present invention further provides pharmaceutical agents containing the MYBL2 polypeptides or polynucleotides encoding thereof, as well as exosomes and antigen-presenting cells as active ingredients. Furthermore, the present invention provides methods for treating and / or prophylaxis of (i.e., preventing) cancers (tumors), and / or prevention of postoperative recurrence thereof, as well as methods for inducing CTLs, methods for inducing anti-tumor immunity, using the MYBL2 polypeptides, polynucleotides encoding the polypeptides, exosomes or antigen-presenting cells presenting the polypeptides, or the pharmaceutical agents of the present invention. The cancers to be targeted include, but are not limited to, testicular tumor, pancreatic cancer, bladder cancer, non-small cell lung cancer, small cell lung cancer and esophageal cancer.
Owner:ONCOTHERAPY SCI INC
Methods for culturing swine testis cell in serum-free manner and producing classical swine fever cell vaccine by using swine testis cell
ActiveCN103602629AAvoid the risks of usingClear ingredientsArtificial cell constructsVertebrate cellsClassical swine fever virus CSFVSerum free
The invention discloses methods for culturing a swine testis cell in a serum-free manner and producing a classical swine fever cell vaccine by using the swine testis cell. The method for culturing the swine testis cell in the serum-free manner comprises the following steps: culturing the swine testis cell by a culture medium containing 10% calf serum; when the cell grows to 60%, changing a culture medium containing 5% calf serum to carry out adaptive culture; when the cell is dead and is suspended, replacing a new culture medium; when the cell completely grows, carrying out subculture by using a culture medium containing 10% calf serum for 3-5 generations; repeating the steps, and gradually reducing the serum content to 5%, 2% and 1% from 10% till realizing serum-free culture; and finally, screening a pure cell line by using a disappearing dilution method. According to the methods, the swine testis cell can be obtained by serum-free habituated culture; the swine testis cell can enable classical swine fever viruses to be multiplied and is applied to the production of a classical swine cell vaccine, thus not only is the risk caused by use of animal serum avoided, but also the components of the culture mediums are clear, the identity among batches is improved and the downstream purifying process is simplified.
Owner:CHONGQING AULEON BIOLOGICALS
Cancer therapy using cldn6 target-directed antibodies in vivo
ActiveUS20160355604A1Extend your lifeImprove survivalCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsParanasal Sinus CarcinomaDisease
The invention relates to the treatment and / or prevention of tumor diseases associated with cells expressing CLDN6, in particular cancer and cancer metastasis using antibodies which bind to CLDN6. The present application demonstrates that the binding of antibodies to CLDN6 on the surface of tumor cells is sufficient to inhibit growth of the tumor and to prolong survival and extend the lifespan of tumor patients. Furthermore, binding of antibodies to CLDN6 is efficient in inhibiting growth of CLDN6 positive germ cell tumors such as teratocarcinomas or embryonal carcinomas, in particular germ cell tumors of the testis.
Owner:GANYMED PHARMA AG +1
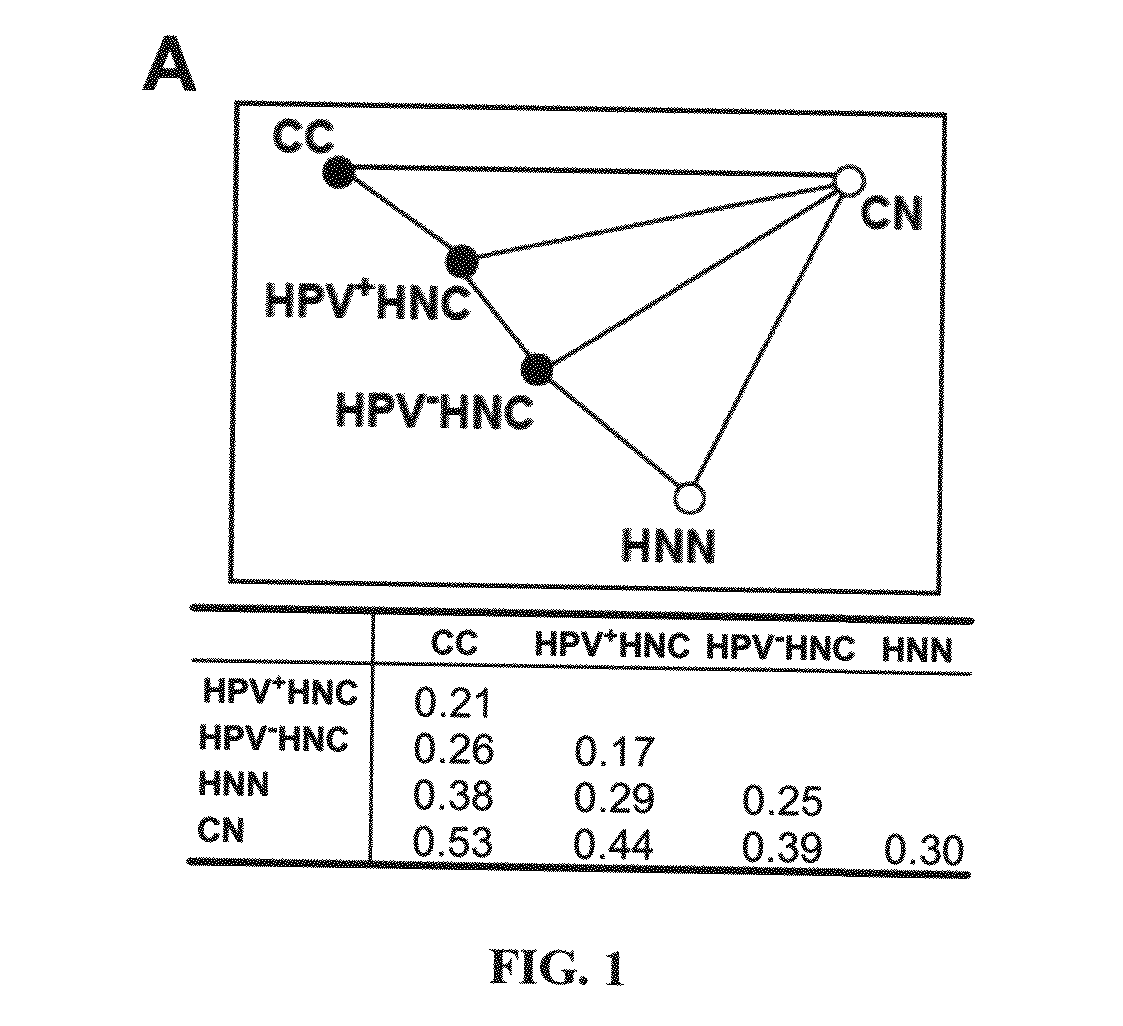
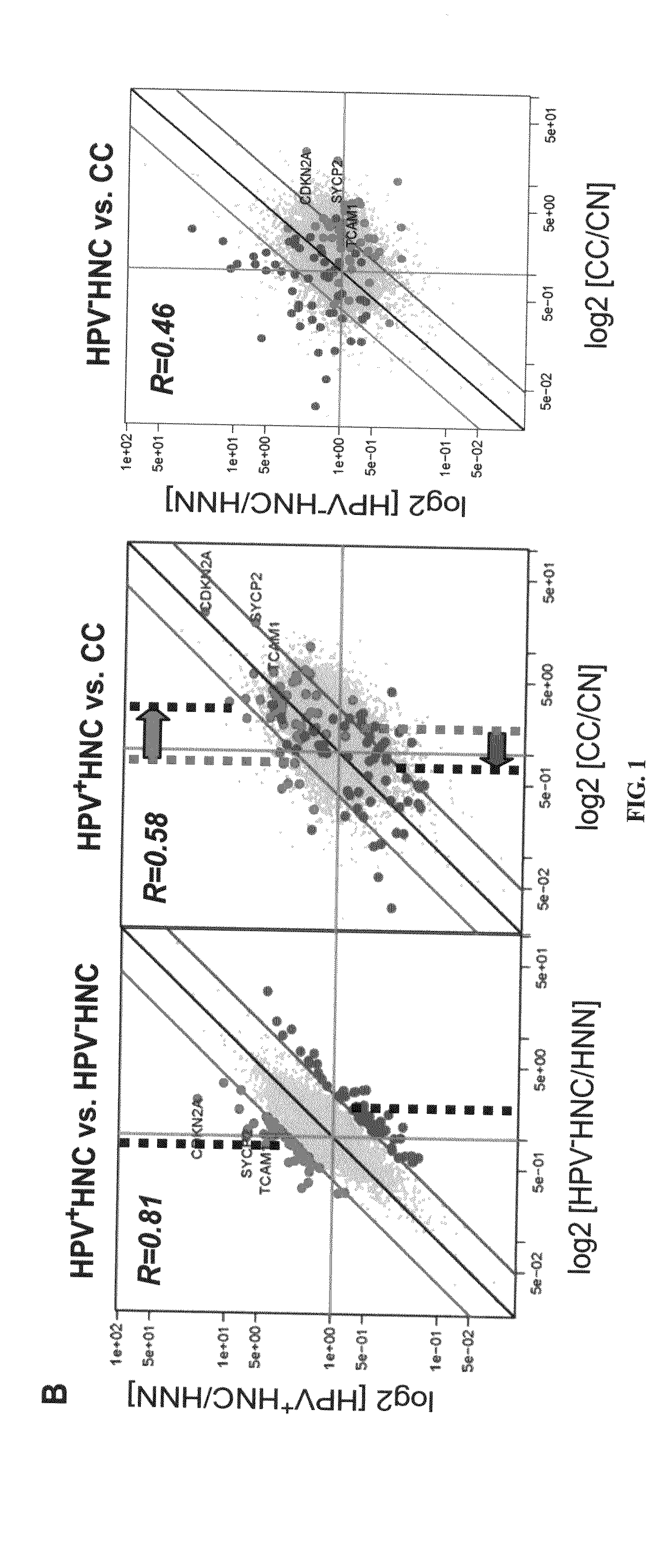
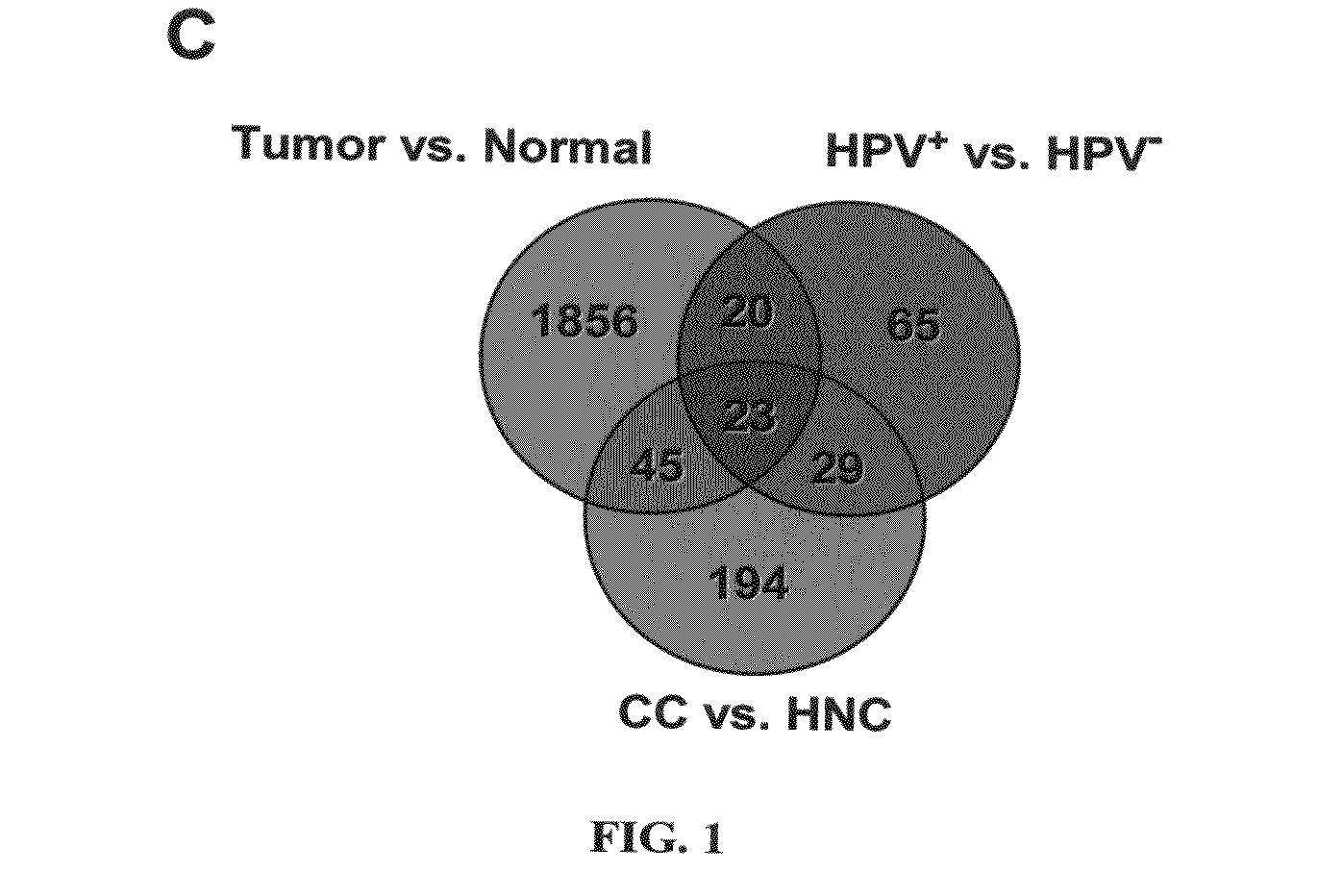
Biomarkers for human papilloma virus-associated cancer
ActiveUS20090136486A1Microbiological testing/measurementAntibody ingredientsSynaptonemal complex protein 2Antigen
Cervical cancer cells and HPV+ head and neck cancer cells express three testis-specific genes not normally expressed in somatic cells: testicular cell adhesion molecule 1 (TCAM1), synaptonemal complex protein 2 (SYCP2) and stromal antigen 3 (STAG3). Among the three markers, TCAM1 and SYCP2 are early detection markers. Various methods for identifying a human or non-human animal as a candidate for further examination for cervical cancer, preneoplastic lesion for cervical cancer, head and neck cancer, or preneoplastic lesion for head and neck cancer are disclosed. Methods of detecting said cancers and preneoplastic lesions, methods of screening for drugs for treating said cancers and preneoplastic lesions, methods for monitoring the effectiveness of a treatment for said cancers, and methods of treating said cancers are also disclosed. Further disclosed are kits that can be used to practice the above methods.
Owner:WISCONSIN ALUMNI RES FOUND
Immune response inducer
ActiveUS20100297071A1Therapy and prophylaxis of a cancer(s)Peptide/protein ingredientsImmunoglobulinsAbnormal tissue growthCDNA library
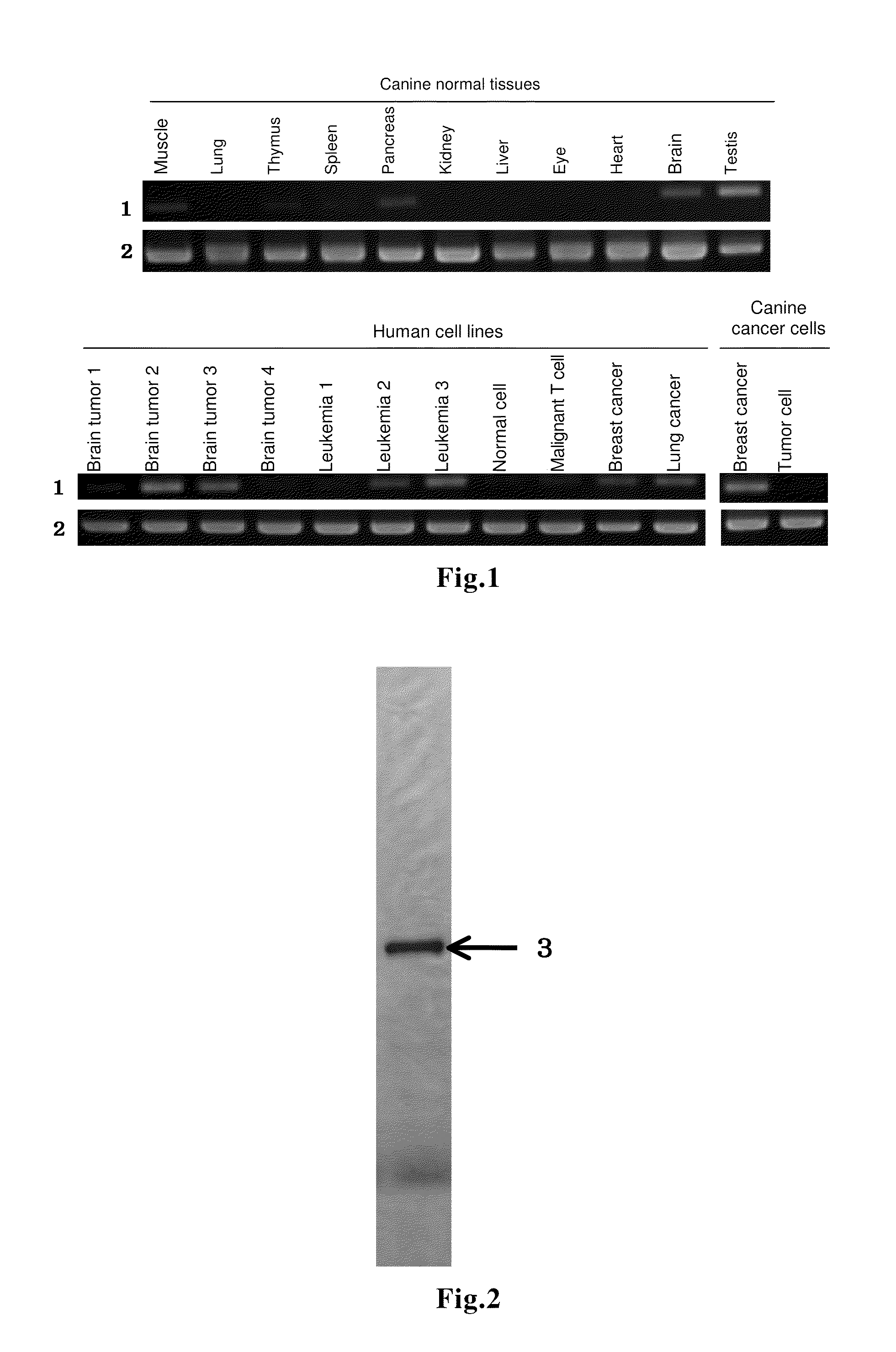
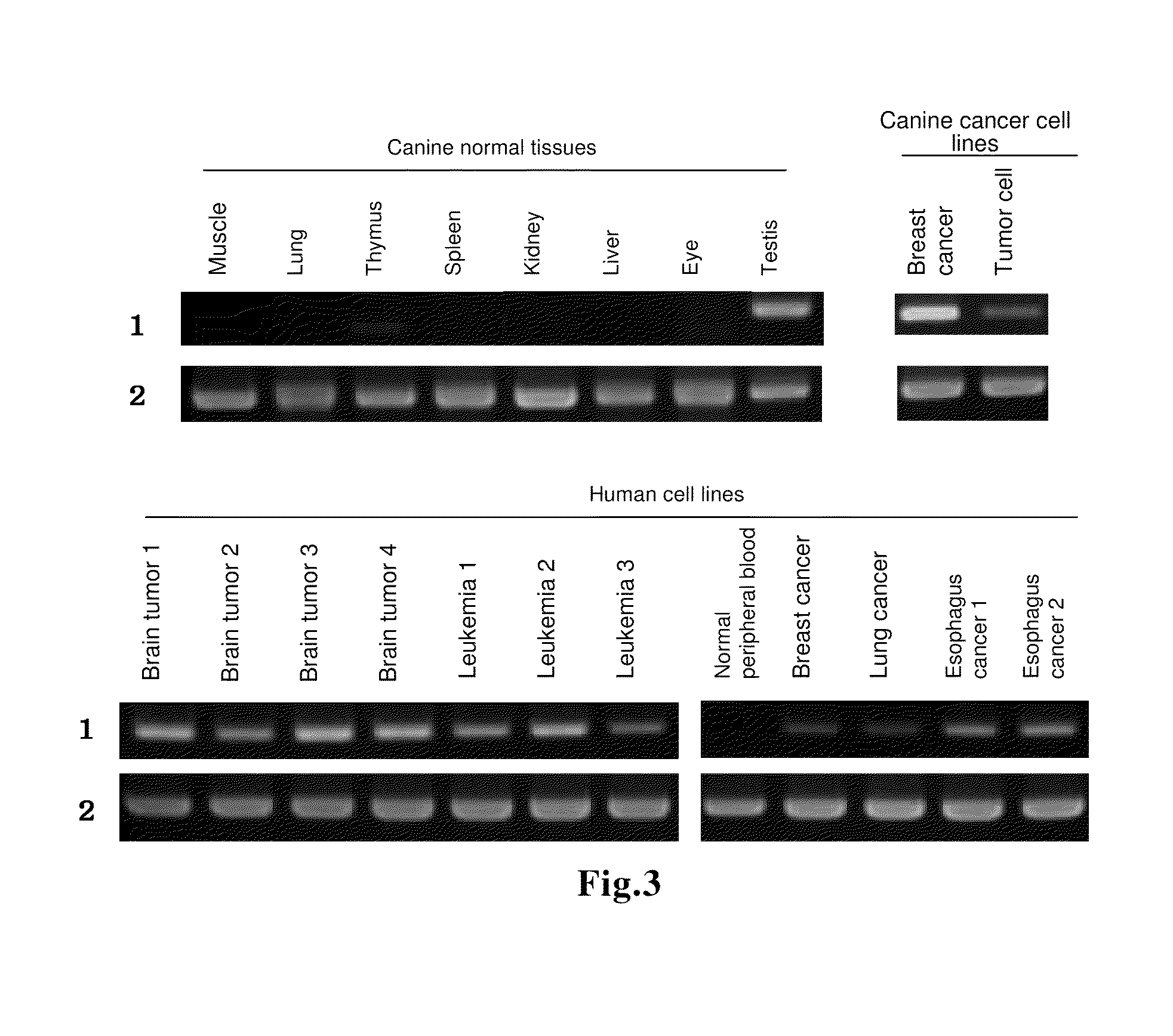
An immunity-inducing agent comprising as an effective ingredient a specific polypeptide is disclosed. These polypeptides were isolated, by the SEREX method using a cDNA library derived from canine testis and serum from a cancer-bearing dog, as a polypeptide which binds to an antibody existing specifically in serum derived from a cancer-bearing living body. The polypeptides can induce immunity in a living body and cause regression of a tumor in a cancer-bearing living body. Therefore, these polypeptides are especially effective as a therapeutic and / or prophylactic agent for a cancer(s).
Owner:TORAY IND INC
Guanylate cyclase receptor agonists for the treatment of tissue inflammation and carcinogenesis
A method of treatment of inflamed, pre-cancerous or cancerous tissue or polyps in a mammalian subject is disclosed. The treatment involves administration of a composition of at least one peptide agonist of a guanylate cyclase receptor and / or other small molecules that enhance intracellular production of cGMP. The at least one peptide agonist of a guanylate cyclase receptor may be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The inhibitor may be a small molecule, peptide, protein or other compound that inhibits the degradation of cGMP. Without requiring a particular mechanism of action, this treatment may restore a healthy balance between proliferation and apoptosis in the subject's population of epithelial cells, and also suppress carcinogenesis. Thus, the method may be used to treat, inter alia, inflammation, including gastrointestinal inflammatory disorders, general organ inflammation and asthma, and carcinogenesis of the lung, gastrointestinal tract, bladder, testis, prostate and pancreas, or polyps.
Owner:SYNERGY PHARMA
Health-care food with function of reducing fat
ActiveCN102334688AStrong Antioxidant PropertiesGood anticancer effectFood preparationFucoxanthinSoftgel
The invention relates to a health-care food containing natural fucoxanthin, which is a soft capsule. Moreover, an obesity preventing model method is adopted, and the fucoxanthin is used as a functional factor for carrying out a fat reducing experiment on a rat. By the display of data analysis, the weight and the perinephric spermary fat weight of the rat fed with the fucoxanthin are obviously lowered in comparison with those of a model set. Furthermore, the fat cell area is obviously reduced in comparison with that of the model set and a blank set. The result shows that the fucoxanthin has a very good fat reducing effect, and a favorable basis is provided to the application of substances containing the fucoxanthin in the field of health-care foods.
Owner:秦皇岛惠恩生物技术有限公司
Degradation of PA200 and acetylation-mediated core histones through proteasome
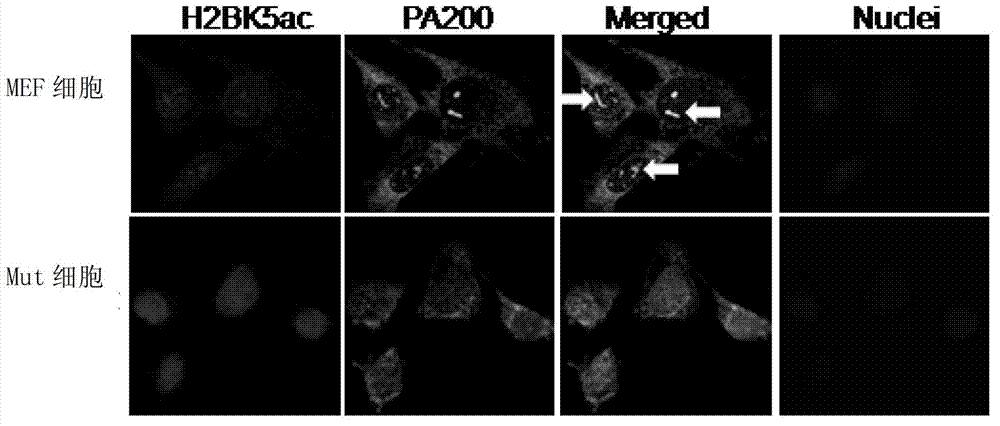
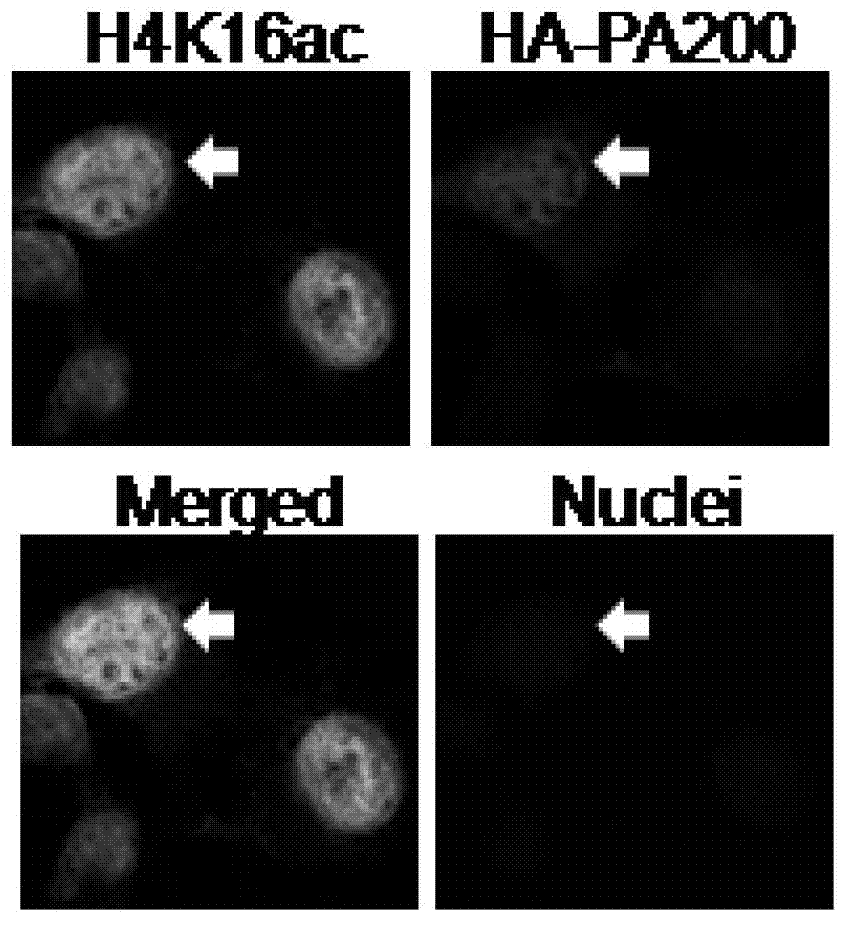
ActiveCN103191412APromote degradationIncreased sensitivityPeptide/protein ingredientsHydrolasesDNA repairTestis specific
The invention discloses an application of PA200 proteins in preparation of products. The products have at least one of the following functions from (1) to (4): (1) combining with acetylated proteins; (2) promoting the degradation of the acetylated proteins; (3) participating in repair of DNA (deoxyribonucleic acid) damages of somatic cells; and (4) participating in the formation of sperms. The invention has breakthroughs in four following aspects: (1) finding a mechanism of regulation and control of degradation of the histones, the sperm formation and the DNA repair by acetylation; (2) revealing the degradation of the histones medicated by acetylation rather than ubiquitination through PA200 / Blm10 proteasome; (3) finding novel testis-specific proteasome containing PA200 and alpha 4s, and revealing the core histones as a first type biological substrate for the proteasome; and (4) revealing that a histone deacetylase inhibitor can promote the degradation of the histones which are induced by DNA double-strand breaks and medicated by acetylation, enhance the sensitivity of cells to DNA damages and easily lead to the death of the cells.
Owner:BEIJING NORMAL UNIVERSITY
Testis-specific tubulin tyrosine-ligase-like protein, BGS42
ActiveUS7122358B2Ameliorating and preventing and decreasing level of BGS-4Methylation capacity is decreasedBacteriaPeptide/protein ingredientsDiseaseNucleotide
Owner:BRISTOL MYERS SQUIBB CO
Separation and culture method and application of human testis mesenchymal stem cells
InactiveCN105331579AIncrease serum testosterone levelsMammal material medical ingredientsSkeletal/connective tissue cellsDiseaseTissue biopsy
The invention discloses a separation and culture method and application of human testis mesenchymal stem cells. The separation method comprises the steps that an obstructive azoospermia patient testis specimen which is processed through tissue biopsy separation is digested with type IV collagenase, digested cell suspension is filtered to remove tissue clumps, and human testis cells are obtained; the human testis cells are marked with a CD51 antibody, separation is performed with a flow cytometer, the cells expressing the CD51 are collected, and then the human testis mesenchymal stem cells with the positive CD51 are separated. The invention further provides the human testis mesenchymal stem cells which are obtained through the separation method and have the positive CD51, the culture method of the stem cells and application of the stem cells in preparation of medicine for treating related diseases caused by low testosterone level.
Owner:SUN YAT SEN UNIV
Method for diagnosing testicular seminomas
InactiveCN1703522AMicrobiological testing/measurementAntineoplastic agentsTesticular seminomaDiagnostic methods
Owner:ONCOTHERAPY SCI INC
Method for detecting expression of 305 sperm localization proteins in fertility-related human testis and epididymis
The invention relates to a method for detecting 305 kinds of semen positioning protein of human testicle and epididymis expression related to human procreation. More specifically, the invention provides an important protein group related to sterility caused by human semen maturation and also provides a qualitative and quantitative method for detecting human semen positioning protein on semen as well as in seminal plasma, thereby providing a new way for definitely diagnosing the causes of male sterility.
Owner:山东艾胚康生物科技有限公司
Epididymal protease inhibitor protein monoclonal antibody and application thereof
InactiveCN101519448AStrong specificityMicroorganism based processesAntibody ingredientsEpididymal protease inhibitorMale infertility
The invention discloses an epididymal protease inhibitor protein monoclonal antibody and application thereof, which belong to the field of biological engineering. The monoclonal antibody is secreted by hybridoma preserved in China Center for Type Culture Collection (CCTCC) with the preservation number of C200849. The monoclonal antibody can be combined to the epididymal protease inhibitor protein Eppin protein of specific expression in a testis and epididymis tissue by specificity. The monoclonal antibody can be used for preparing male infertility diagnostic reagents, male contraceptives and anti-fertility study biological products.
Owner:NANJING MEDICAL UNIV
Method for predicting whether sperms exist in testis of azoospermia patient or not according to seminal plasma mRNA
PendingCN111518889AImprove the success rate of sperm extractionSolve the problem of infertilityMicrobiological testing/measurementDNA/RNA fragmentationBiologySeminal fluid specimen
The invention discloses a method for predicting whether sperms exist in the testis of an azoospermia patient or not according to seminal plasma mRNA. The method comprises the following steps of dividing seminal fluid specimens of the azoospermia patient into a testicular sperm group and a testicular azoospermia group by taking a testicular biopsy as a gold standard, determining the difference of exRNA expression profiles of two seminal fluid groups, carrying out comparison with an existing spermatogenic cell transcriptome database, and searching for differentially expressed exRNA capable of judging spermatogenic cell types, which is used for judging whether haploid sperm cells exist in the testis of the azoospermia patient or not. The clinical application of the scheme provided by the invention can greatly improve the sperm taking success rate of the azoospermia patient, and is also helpful for guiding the azoospermia patient to select an economical, painless and effective treatment method to solve the problem of infertility of the azoospermia patient.
Owner:广东省计划生育科学技术研究所
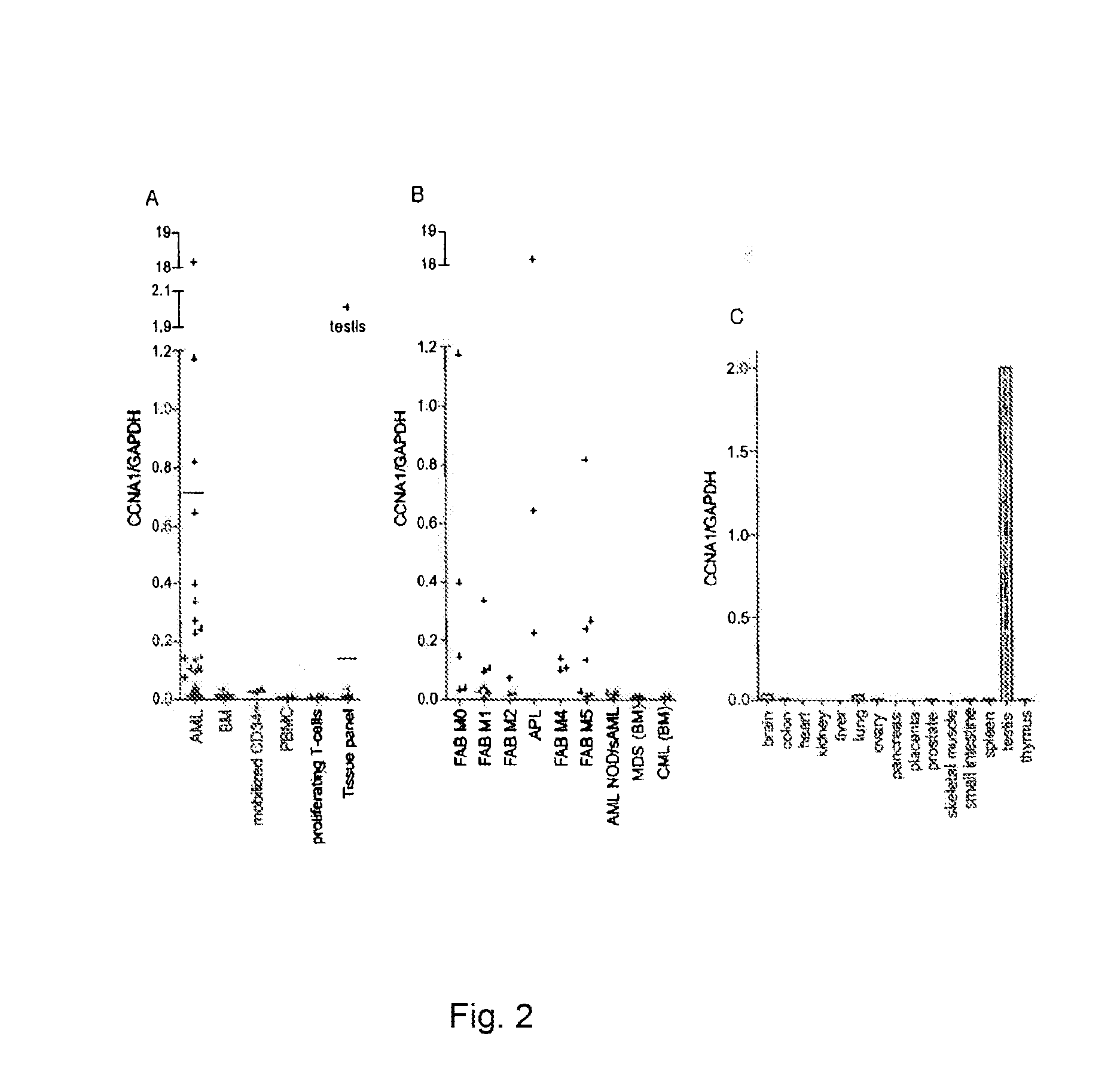
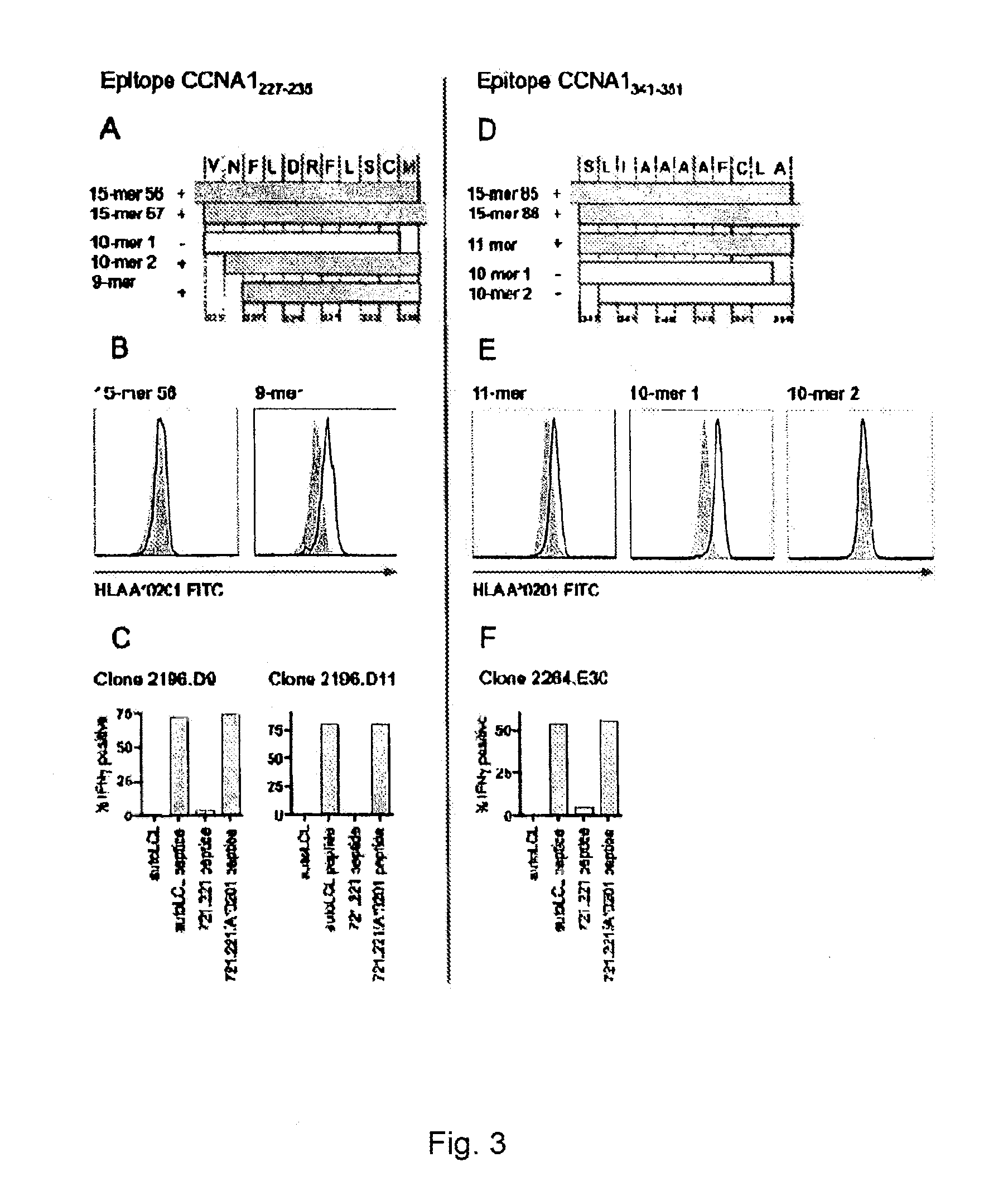
Cyclin a1-targeted t-cell immunotherapy for cancer
Compositions and methods are provided for eliciting antigen-specific T-cell responses against human cyclin A1 (CCNA1), which is herein identified as a leukemia-associated antigen based on its overexpression in acute myeloid leukemia (AML) including leukemia stem cells (LSC) and in immunologically privileged testis cells, but not in other normal cell types. CCNA1-derived peptide epitopes that are immunogenic for T-cells including CTL are disclosed, as are immunotherapeutic approaches using such peptides for vaccines and generation of adoptive transfer therapeutic cells.
Owner:FRED HUTCHINSON CANCER CENT
Methods and materials relating to stem cell growth factor-like polypeptides and polynucleotides
InactiveUS20030211987A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCDNA librarySpinal cord
The invention provides novel polynucleotides and polypeptides encoded by such polynucleotides and mutants or variants thereof that correspond to a novel human secreted stem cell growth factor-like polypeptide. These polynucleotides comprise nucleic acid sequences isolated from cDNA libraries prepared from human fetal liver spleen, ovary, adult brain, lung tumor, spinal cord, cervix, ovary, endothelial cells, umbilical cord, lymphocyte, lung fibroblast, fetal brain, and testis. Other aspects of the invention include vectors containing processes for producing novel human secreted stem cell growth factor-like polypeptides, and antibodies specific for such polypeptides.
Owner:KIRIN PHARMA
Bovine testicle cell line as well as establishment method and application thereof
ActiveCN103849602AHigh purityQuality improvementMicroorganism based processesVector-based foreign material introductionBiotechnologyLipofectamine
The invention provides a bovine testicle cell line and an establishment method thereof. The establishment method comprises the following main steps: (1) primary culture, namely collecting a bovine testicle of a newborn calf in a sterile manner, cleaning adhesive tissues, separating by virtue of a trypsinization method so as to obtain bovine testicle cells with high vitality, culturing the separated bovine testicle cells with high vitality in a DMEM (Dulbecco modified eagle medium), and culturing at 37 DEG C under the condition of 5% CO2 to obtain primary bovine testicle cells; (2) transfection and screening, namely extracting and purifying eukaryotic expression plasmids pCI-neo-hTERT containing hTERT genes, leading the extracted and purified eukaryotic expression plasmids pCI-neo-hTERT to the primarily cultured bovine testicle cells in the step (1) to perform transfection by adopting a lipidosome transfection method; (3) screening and enlarged culture. The bovine testicle cells provided by the invention are easy in culture and relatively fast in growth speed, can be stably inherited, are high in motility rate and purity, and can be well preserved; the method provided by the invention is simple and convenient in operation and convenient in popularization and application. The cell line can be used for producing hog cholera vaccines, sheep poxes, ecthyma vaccines and the like.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
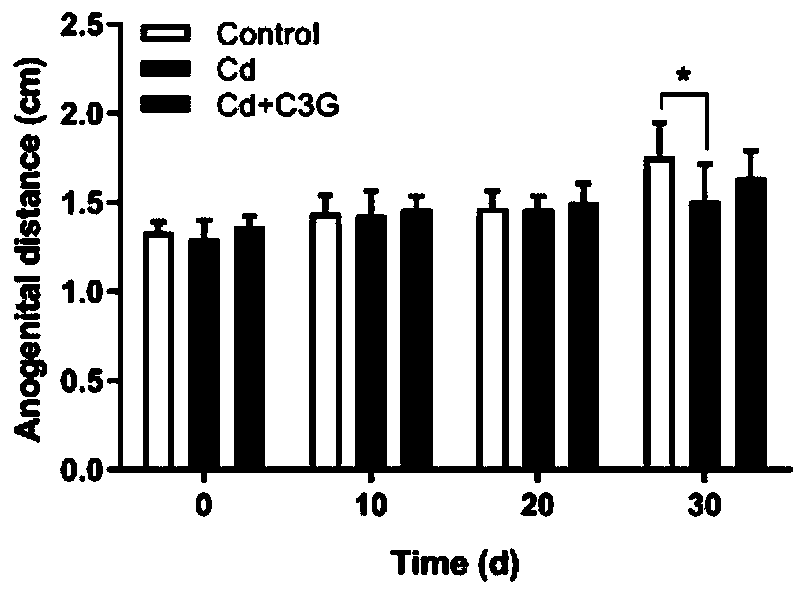
Application of cyanidin-3-O-glucoside in preparing drugs for treating and/or preventing sperm quality reduction caused by cadmium
PendingCN110755442AImproves Antioxidant SystemGuaranteed normal generationOrganic active ingredientsAntinoxious agentsOxidative stressPharmaceutical drug
The invention discloses application of cyanidin-3-O-glucoside in preparing drugs for treating and / or preventing sperm quality reduction caused by cadmium. A phenomenon is found that the cyanidin-3-O-glucoside (C3G) can alleviate sperm quality reduction caused by Cd exposure during adolescence. C3G interference can slow a histone-protamine replacement process during a sperm deformation stage through histone modification and improve a testis antioxidant system to prevent sperms from apoptosis induced by Cd oxidative stress, therefore, a normal spermatogenesis process is maintained, and normal spermatogenesis is ensured.
Owner:JINAN UNIVERSITY
Method for efficiently separating mouse spermatogonial stem cells
InactiveCN105969723AHigh separation purityIncrease the number ofGerm cellsEnzyme digestionMale infertility
The invention discloses a method for efficiently separating mouse spermatogonial stem cells. The method includes the following steps that firstly, mouse testis are collected, and a testicular cell suspension is prepared through a two-step enzyme digestion method; secondly, the testicular cell suspension is inoculated in a porous plate according to a certain density, and a spermatogonial stem cell culture solution is added for in-vitro culture; thirdly, the culture solution is removed, testis cells of different times are cultured through tryptic digestion, and spermatogonial stem cells are sorted from the testis cells through the magnetic activated cell sorting; fourthly, the sorted spermatogonial stem cells are inoculated to an STO trophoblast, and a spermatogonial stem cell culture solution is added for primary culture and subculture of the spermatogonial stem cells. A mouse spermatogonial stem cell system is built through the method including the steps of separation, culture, re-separation and re-culture. The method can achieve the aim of efficiently enriching the spermatogonial stem cells in vitro, and is economical and easy to implement, so that a direction is provided for protection of rare animal varieties and treatment of male infertility.
Owner:INNER MONGOLIA UNIVERSITY
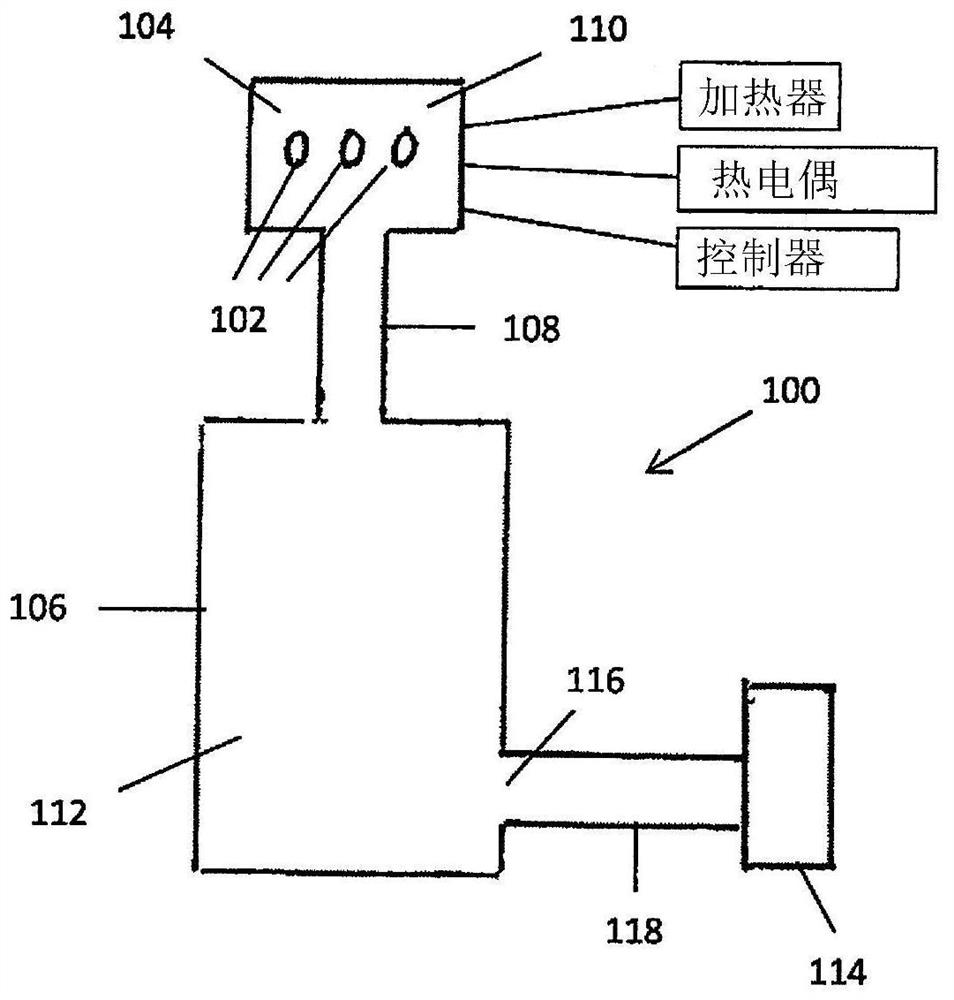
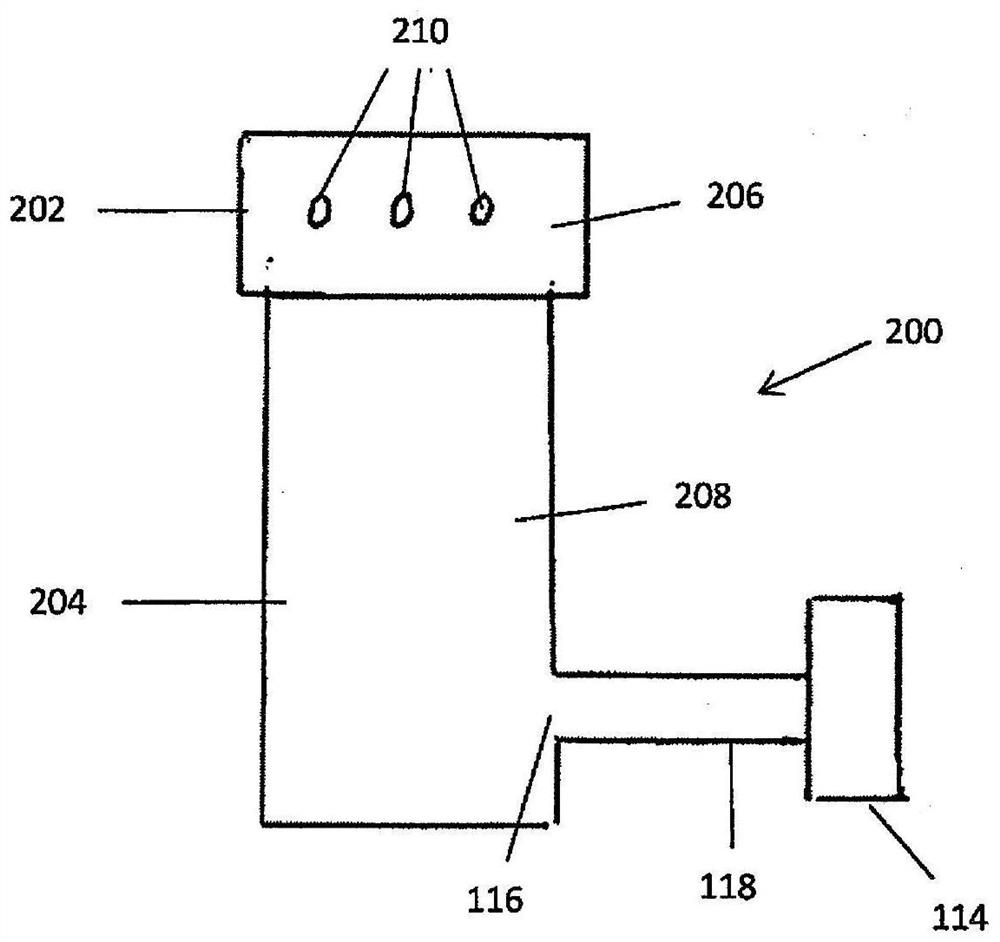
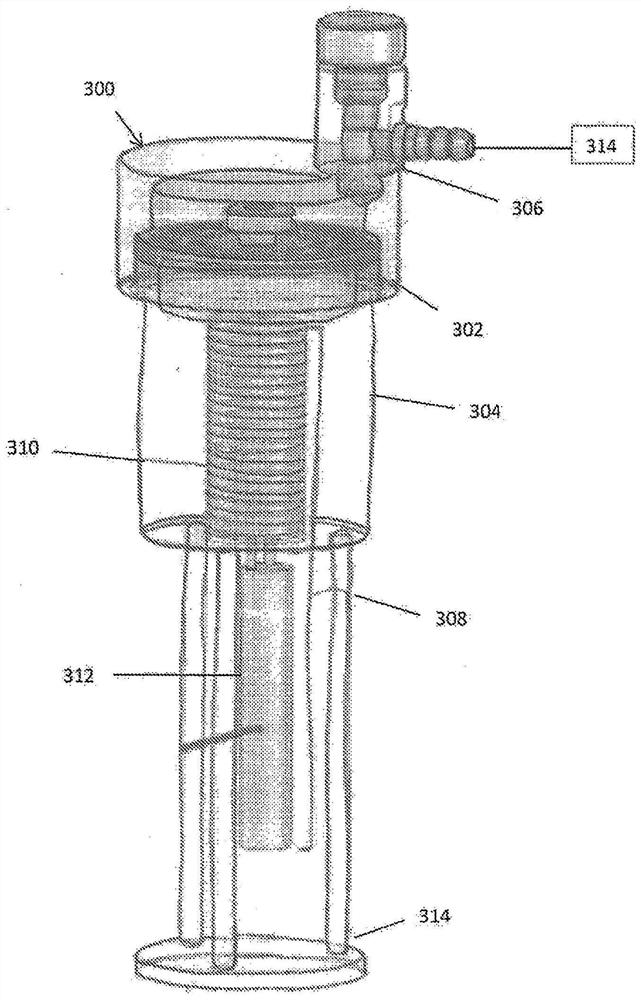
Device and method for freeze drying biological samples
A method and device for freeze-drying a biological sample of mammalian cells or tissue are disclosed. The method includes placing a biological sample on or in a structure to increase a temperature ofthe biological sample and with the biological sample in a closed chamber applying a vacuum to the chamber to lower a pressure within the chamber, cooling the chamber to lower a temperature within thechamber and applying heat to the biological sample within the chamber. The biological sample can include one or more of stem cells, hematopoietic stem cells, mesenchymal stem cells, embryonic stem cells, induced pluripotent stem cells, sperm, oocytes, embryos, ovarian tissue, uterine tissue or testicular tissue. A method for rehydration of a sample is further disclosed.
Owner:安全生殖公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com