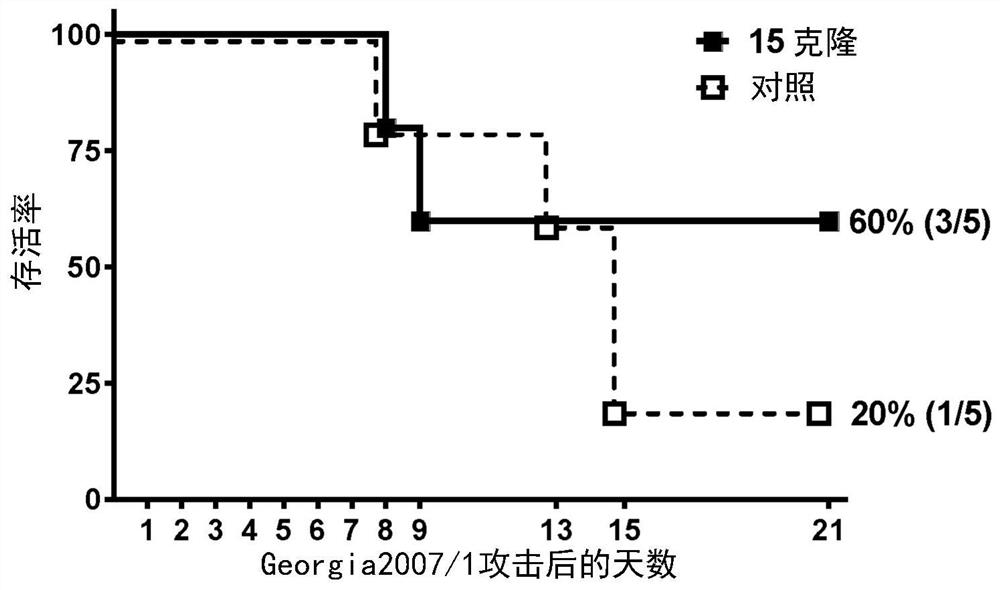
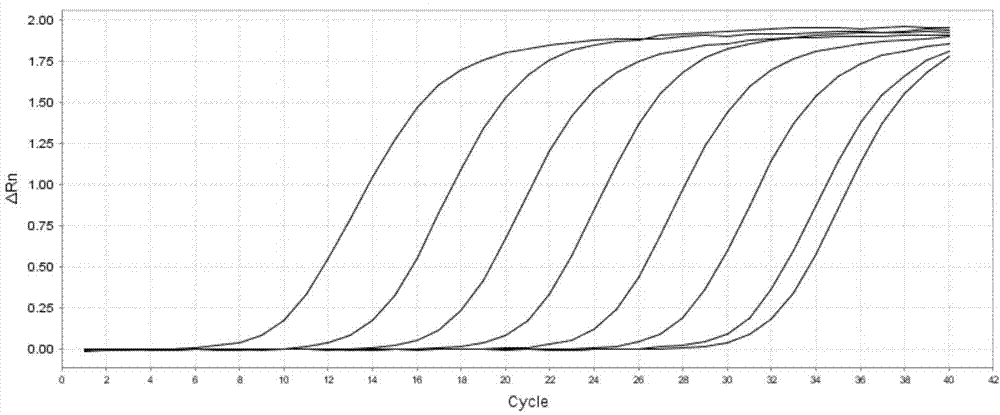
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
438 results about "Classical swine fever virus CSFV" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Four-gene-deletion weak-toxin strain for African swine fever viruses and application of four-gene-deletion weak-toxin strain
InactiveCN110551695AEasy to solveViral antigen ingredientsMicrobiological testing/measurementAfrican swine feverToxin
The invention discloses a four-gene-deletion weak-toxin strain for African swine fever viruses. The weak-toxin strain is the four-gene-deletion weak-toxin strain for an African swine fever virus SY18separation strain, and the following gene function protein is deleted: CD2v gene coding products and three multigene family genes( MGF360-12L, MGF360-13L and MGF360-14L ) coding products. The invention further discloses an application of the weak-toxin strain of the African swine fever viruses to preparation of vaccines for preventing or treating African swine fever. The weak-toxin strain of the African swine fever viruses can provide complete immunoprotection effect on attack of ASFV parent toxin strains, is high in safety, and is suitable for being used as vaccine candidate strains for preventing the African swine fever.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
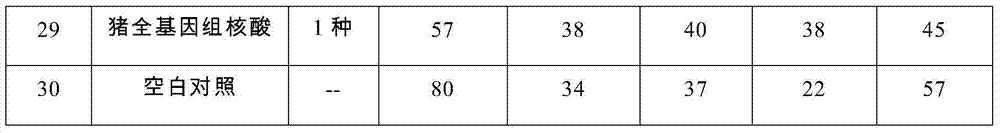
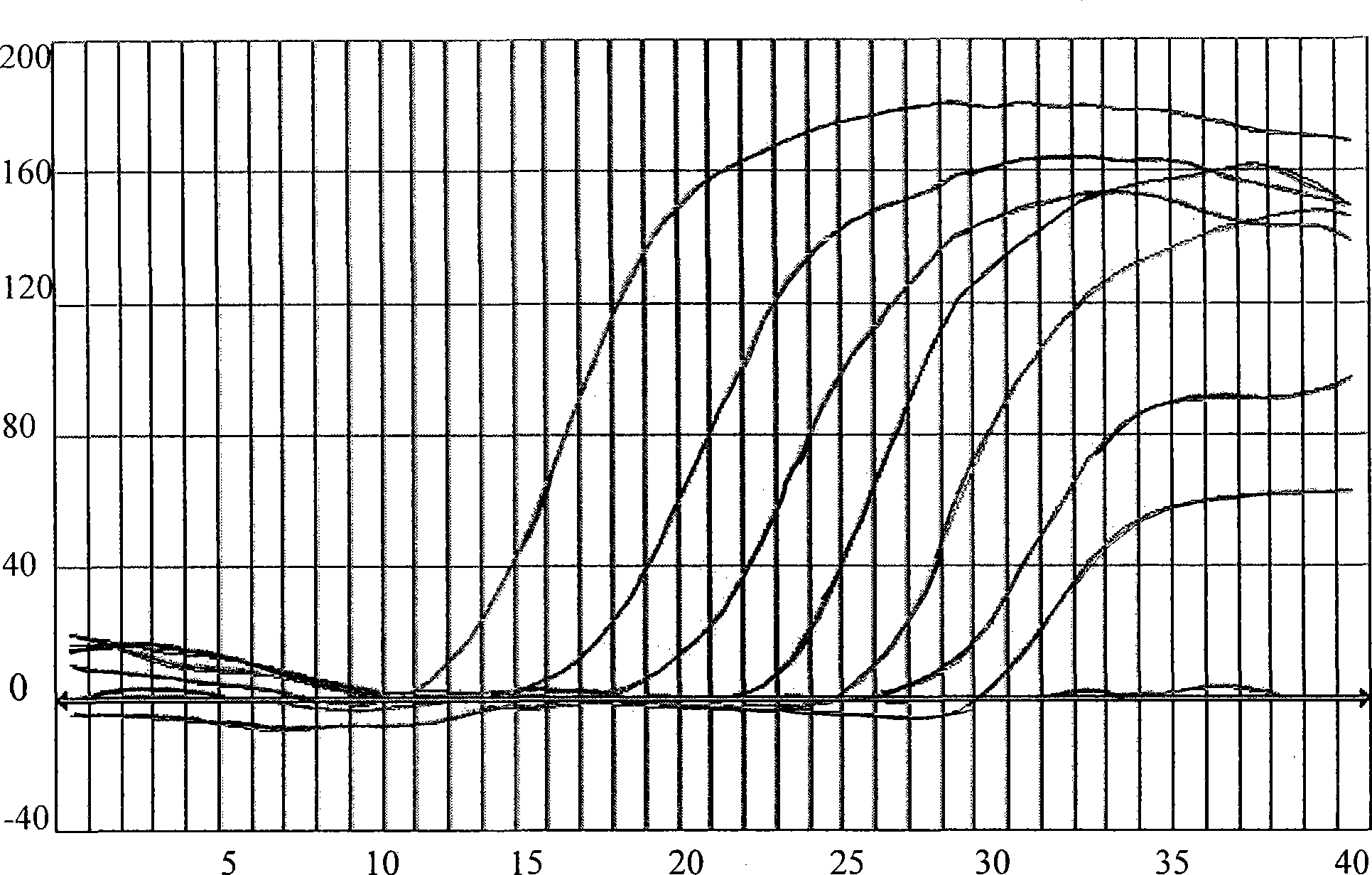
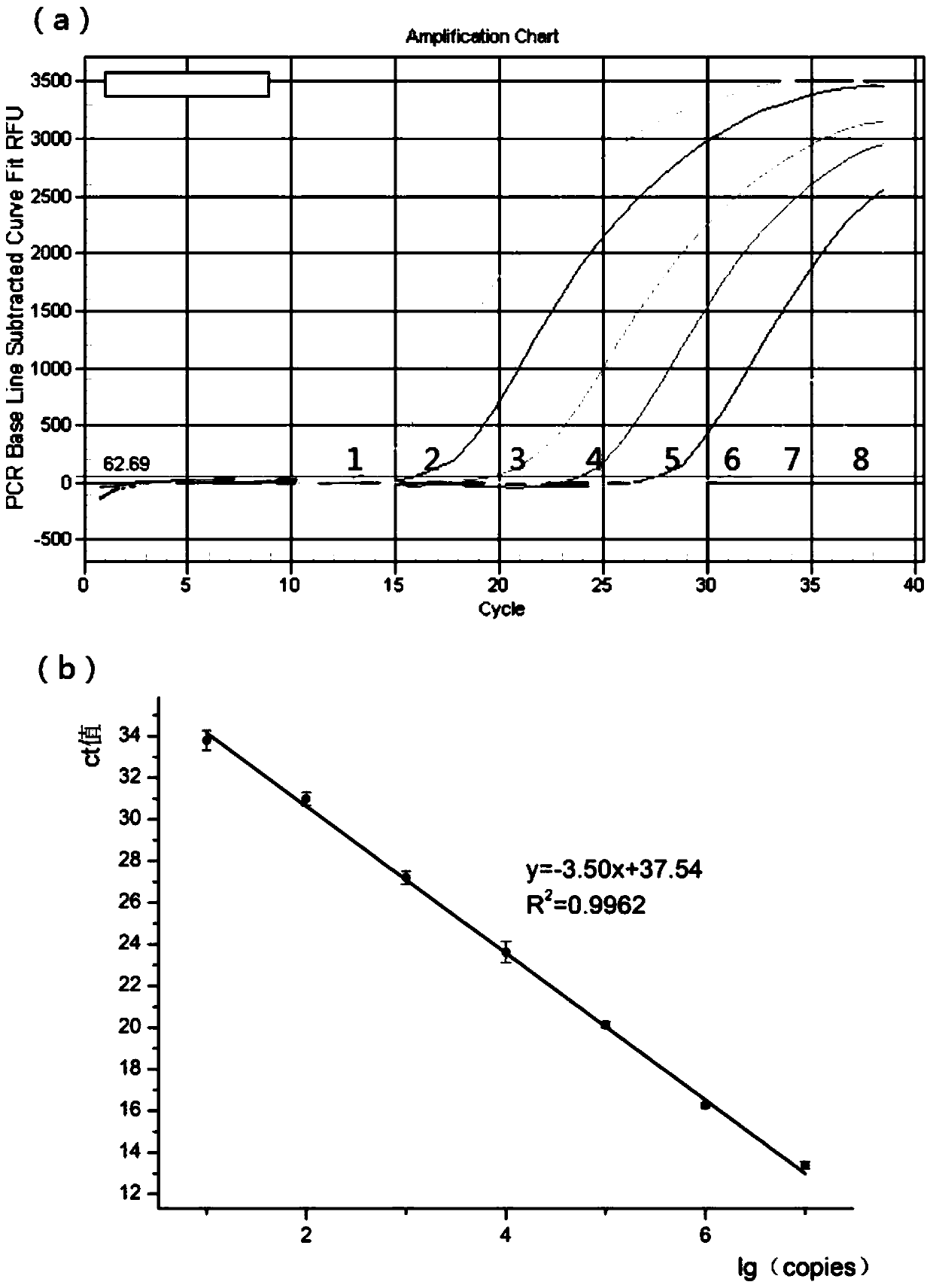
Multiple real time fluorescence quantifying PCR method for detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and classical swine fever virus
InactiveCN101260442ALow costImprove application stabilityMicrobiological testing/measurementClassical swine fever virus CSFVRegression analysis
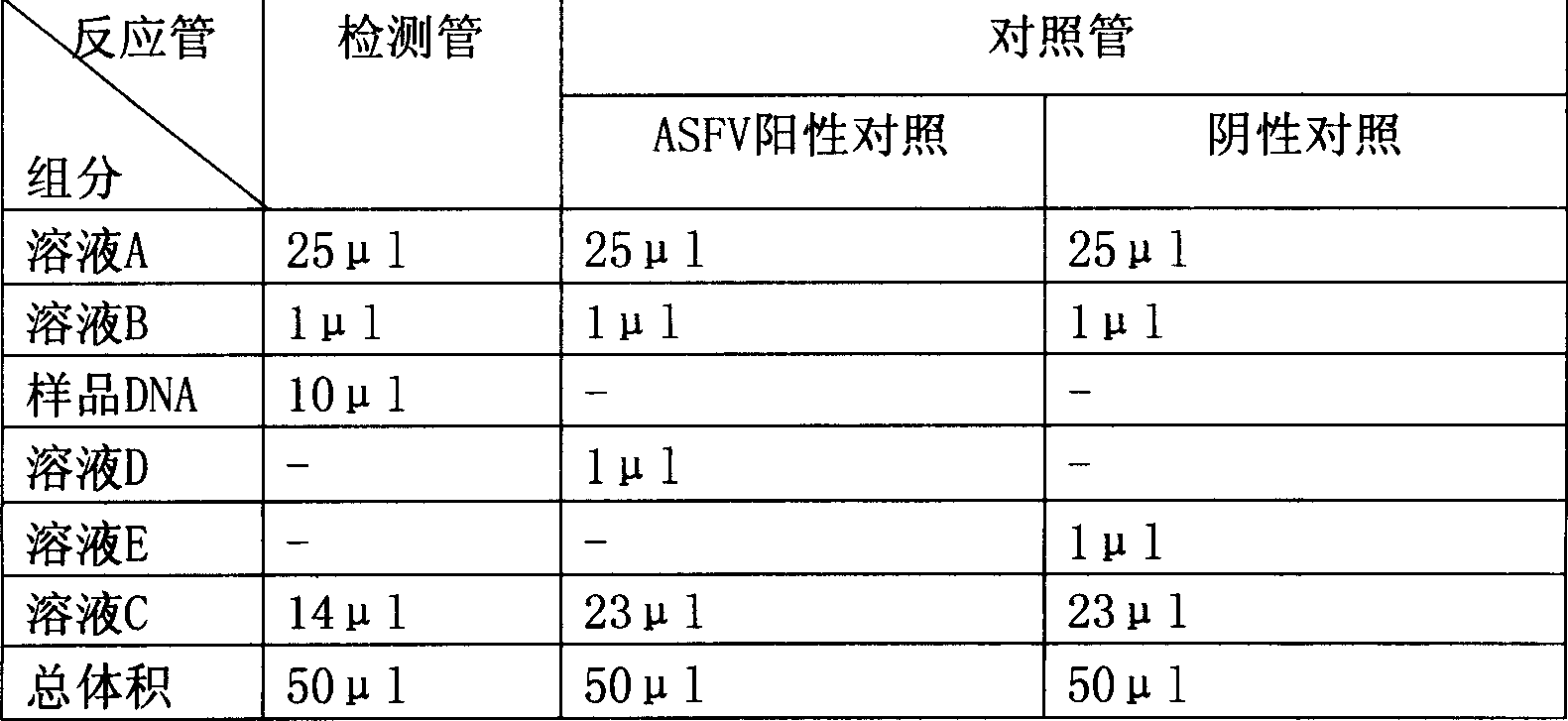
The invention discloses a multiplex real-time fluorescent quantitation PCR method capable of detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and hog cholera virus at the same time. The method of the invention detects that the four virus are all in good linear relations at the same time, all ten times serial dilution points of constructed normal plasmid are all in one straight line, CT value and copy number are in good linear relation, and regression analysis shows that the related coefficient of the CT value and the copy number is R<2> more than 0.99.The multiplex real-time fluorescent quantitation PCR method has the advantages of excellent specificity, sensibility and stability, which can rapidly, sensitively and differentially detect the four virus with serious harm to the economy and can be used for early diagnosis of virus infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Multi-epitope fusion diagnosis antigen for African swine fever virus as well as preparation method and application thereof
InactiveCN108148138AImprove featuresIncreased sensitivityAntibody mimetics/scaffoldsVirus peptidesAntigenBacillus coli
The invention discloses a multi-epitope fusion diagnosis antigen for African swine fever virus as well as a preparation method and application thereof. An ASFV (African swine fever virus) important structural protein gene encoding amino acid sequence is analyzed, screened and recombined through bioinformatics software, a multi-epitope fusion antigen gene is built and synthesized and is expressed in bacillus coli; through screening, the recombinant multi-epitope fusion antigen ASFV-meAg6 is obtained, so that diagnosis antigen protein with strong specificity and high sensitivity is provided foran ASFV serological diagnosis method.
Owner:SHIHEZI UNIVERSITY
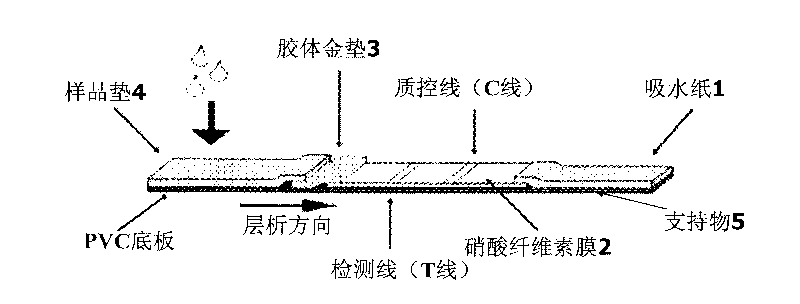
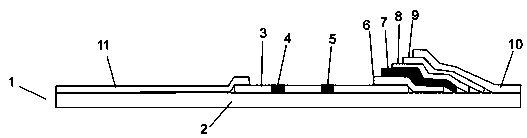
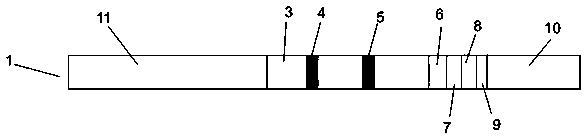
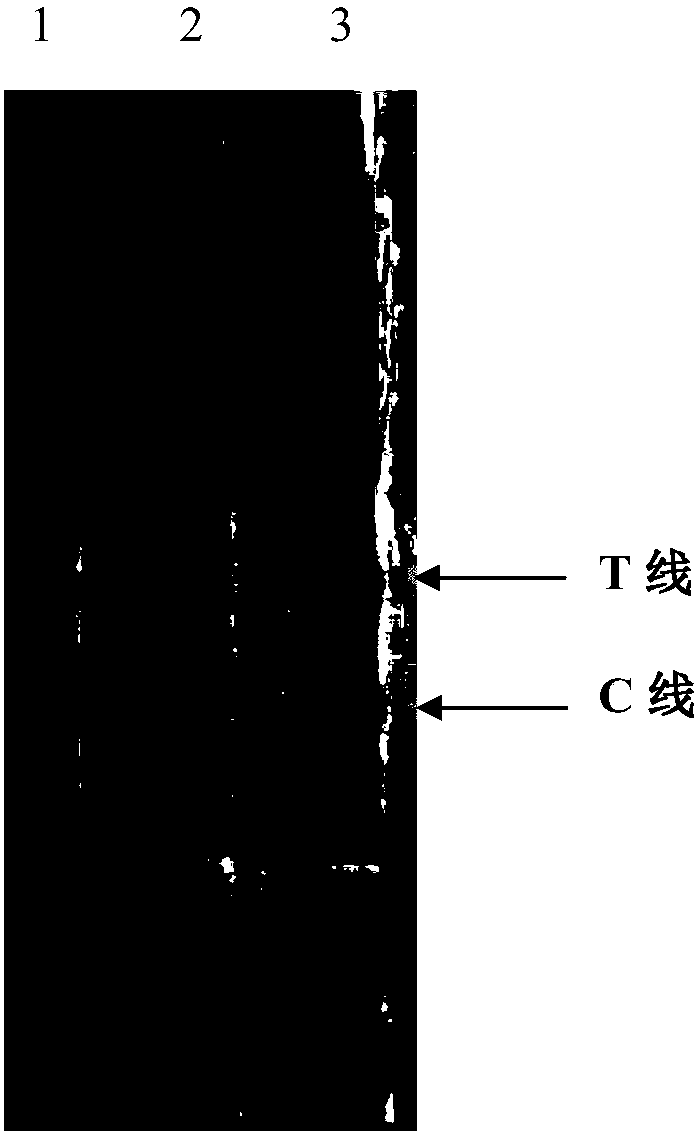
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Nucleic acids of liquid-phase gene chip for synchronously detecting five porcine viruses and detection method thereof
ActiveCN104328218AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVMultiplex
The invention provides a set of nucleic acids of a liquid-phase gene chip for synchronously detecting five porcine viruses, which comprise forward and reverse primers and hybrid probes for porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), porcine pseudorabies virus (PRV), classical swine fever virus (CSFV) and porcine parvovirus (PPV). The invention also provides a multiplex liquid-phase chip high-flux molecular biology detection method of the five porcine viruses. According to the method, porcine virus nucleic acids in the sample to be detected are extracted to perform multiplex unsymmetric nucleic acid amplification / multiplex liquid-phase gene chip (suspension chip) combined detection, thereby synchronously and accurately detecting and identifying the five porcine viruses in the sample to be detected. The method has the advantages of high specificity, high sensitivity, high stability, high flux and high detection speed, and is simple to operate.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and preparation method thereof
InactiveCN101900731AConvenient prevention and controlImprove purification effectMaterial analysisElisa kitStructural protein
The invention relates to an ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and a preparation method thereof. An indirect ELISA kit or a blockage ELISA kit is formed by expressing and purifying classical swine fever virus (CSFV) non-structural protein NS3, coating a solid-phase carrier and assembling with other matched reagents. The kit has the characteristic of distinctively detecting different antibodies generated by the CSF vaccine immunity and the wild virus infection. The kit can distinctively diagnose the CSF vaccine immunity and the wild virus infection.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
African hog cholera virus fluorescent quantitative PCR detecting reagent and preparation and use thereof
InactiveCN101463396AFast detection methodSensitive highMicrobiological testing/measurementLower limitFluorescence
The invention discloses a fluorescence quantitative PCR detection reagent for African swine fever virus, and a preparation method and the application thereof. A set of specific primers and Taqman probes are designed and synthesized to be used for detecting ASFV P54 in relevant porcine products. A standard curve drawn in the invention provides a standard for the quantitative detection of ASFV P54. The invention establishes a fast and simple real-time fluorescence quantitative PCR detection system with strong specificity and high flexibility. The detection time is only several hours, and the detection lower limit can be 15 copies. The invention can be applied to the diagnosis and quarantine technology towards the imported relevant porcine products at port, and the invention provides reliable and effective technical condition for the import quarantine work of the country without ASF.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
African swine fever virus p72 recombinant protein, monoclonal antibody and test paper
ActiveCN110642926AImproving immunogenicityStrong specificityVirus peptidesBiological material analysisClassical swine fever virus CSFVNucleotide
The invention provides an African swine fever virus p72 recombinant protein, a monoclonal antibody and test paper. An amino acid sequence of the African swine fever virus p72 recombinant protein is obtained by linking amino acid sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2 through flexible amino acid fragments, and has strong immunogenicity and specificity. The invention also provides a nucleotide sequence for encoding the recombinant protein. The invention also provides the monoclonal antibody against the African swine fever virus p72 recombinant protein obtained by immunizing animals byusing the recombinant protein. The invention also provides hybridoma cells 7A7 and 3E5 with preservation numbers of CGMCC No. 18540 and CGMCC No. 18539, respectively, and the monoclonal antibody secreted by the hybridoma cells has high sensitivity and specificity to African swine fever virus p72. The invention also provides the latex microsphere test paper containing the recombinant protein antibody and the colloidal gold test paper containing the recombinant protein antibody, and the test paper has the advantages of small batch difference, high detection sensitivity and simple operation.
Owner:北京纳百生物科技有限公司 +1
Recombinase polymerase application (RPA) primer for rapidly detecting African swine fever virus (ASFV) nucleic acid, preparation method of RPA primer, and kit
InactiveCN107937624AQuick checkStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyWater baths
The invention discloses a recombinase polymerase application (RPA) primer for rapidly detecting African swine fever virus (ASFV) nucleic acid, a preparation method of the RPA primer, and a kit, belonging to the technical field of biology. A pair of RPA primers, i.e., an upstream primer <210>2 and a downstream primer <210>3 which have high specificity and strong sensibility are screened out; an RPAdetection system for rapidly detecting the ASFV nucleic acid is further established by means of the primers. Compared with the common polymerase chain reaction (PCR) method, RPA-lateral flow assay (LFA) has the advantages that firstly, the RPA-LFA belongs to an isothermal amplification technology and is low in requirements for instruments and equipment, and reactions can be completed only by means of a constant temperature water bath kettle; secondly, the detection speed of the RPA-LFA is fast, and the reaction time is 40 minutes and is shorter than the conventional PCR reaction time; thirdly, the visualization of detection results can be realized. Due to the characteristics, the RPA-LFA method established by the invention can be used for rapid detection and differential diagnosis of ASFVin common laboratories of grassroots units.
Owner:SHIHEZI UNIVERSITY
African swine fever virus nucleic acid amplification primer, detection method and kit
InactiveCN101921878AQuick checkEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationGold particlesHybrid compound
The invention relates to an African swine fever virus nucleic acid amplification primer, a detection method and a kit. The primer pair and the specific nucleic acid probe are selected from SEQ ID NO: 1-12. The detection method comprises the following steps: (a) amplifying nucleic acid in the sample to be detected by using a PCR primer; (b) labeling the detection probe labeled by alkyl sulfydryl group on nano gold particles; (c) hybridizing the capture probe labeled by biotin and the detection probe labeled by nano gold in step (b) with a metamorphic PCR product; (d) adding the hybrid system in step (c) to a Streptavidin-coated ELISA plate to capture the hybrid compound; (e) carrying out silver enhancement to capture nano gold; (f) and stoping the silver enhancement reaction, and visually inspecting the grey scale judgment result. The invention has the characteristics of high detection sensitivity, strong detection specificity and low detection cost, can effectively eliminate false positive or false negative result when the PCR method is used for detecting African swine fever virus, and quickly detect the African swine fever virus nucleic acid.
Owner:YANGZHOU UNIV
Immunogenic compositions and vaccines comprising african swine fever virus peptides and proteins and uses thereof
PendingCN113543801AViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVPolynucleotide
The present invention relates to African swine fever virus (ASFV) peptides and / or polypeptides as well as immunogenic fragments thereof, corresponding encoding AFSV oligonucleotides and / or polynucleotides as well as immunogenic fragments thereof, immunogenic compositions, vaccines and uses thereof.
Owner:BOEHRINGER INGELHEIM VETMEDICA GMBH
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN105567871AShorten test timeLow reaction temperatureMicrobiological testing/measurementMicroorganism based processesBiologyDifferential diagnosis
The invention discloses an RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting a high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof. The kit comprises a pair of primers and a probe, the sequences of the primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe is shown as SEQ ID NO.3. It is proved through experiments that the kit can detect adverse effects of the high-pathogenicity porcine reproductive and respiratory syndrome virus (HP-PRRSV), a hog cholera virus, a C-type porcine reproductive and respiratory syndrome virus, a porcine circovirus type II, a porcine pseudorabies virus and a foot and mouth disease virus in a specificity mode. It is proved through experiments that the kit can detect out templates of at least 70 copies at the temperature of 40 DEG C on the condition of 20 min amplification, and the conformity between the kit and RT-qPCR is high. This shows that the kit can detect HP-PRRSV fast, efficiently and sensitively and provides an effective technological means for differential diagnosis of HP-PRRSV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
LAMP kit for detecting hogcholera virus and preparation method thereof
InactiveCN101358246AEasy to operateJudging whether to expand or notMicrobiological testing/measurementFood safetyQuarantine
The invention belongs to the field of sanitary examination, and relates to a LAMP kit for testing classical swine fever virus and an establishing method and an application thereof. The kit contains a test system which is composed of the LAMP reaction liquid of six LAMP primers. The tests prove that the kit of the invention has good specificity and sensitivity, fast amplification speed, high efficiency and simple and convenient identification. The test system of the invention can rapidly and conveniently test the classical swine fever virus in high-efficiency, high-specificity and high-sensitivity under the isothermal condition of 65 DEG C without complicate instruments, can better satisfy the spot tests for the classical swine fever virus, provides a novel technical platform for food safety testing, can better meet the urgent requirements for the spot testing of foot and mouth diseases at present, is used for the spot testing of import and export quarantine, food sanitary departments, animal breeding farms, etc, and is easy to be popularized in a wide range.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and preparation method and application thereof
InactiveCN104745731AMicrobiological testing/measurementMicroorganism based processesPositive controlAfrican swine fever
The invention discloses a triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and a preparation method and application thereof. Three sets of specific primers and Taqman probes as well as positive controls specific to African swine fever virus CP530R genes, swine fever virus 5 minute-UTR genes and swine respiratory syndrome virus NSP2 genes respectively are designed and synthesized, and a rapid, easy and convenient triple fluorescent RT-PCR detection system with high specificity and high sensitivity is established by using the three sets of primers and probes, so that nucleic acids of the African swine fever viruses, the swine fever viruses and the respiratory syndrome viruses can be detected synchronously from a detected sample within 3-4 hours in a rapid, accurate, specific, safe, easy and convenient way. The detection reagent can be applied to synchronous detection of the nucleic acids of trace African swine fever viruses, swine fever viruses and respiratory syndrome viruses in hogs and relevant samples thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Triple real-time fluorescent quantitative PCR kit for detecting African swine fever wild strains and gene deletion strain
InactiveCN111020062AHigh detection sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVAfrican swine fever
The invention provides a triple real-time fluorescent quantitative PCR detection primer for detecting an African swine fever wild strain and a gene deletion strain, and a kit and a detection method thereof. The triple fluorescent quantitative detection kit is developed and researched for three genes CD2V, VP72 and MGF-360 14L of the African swine fever virus by utilizing a multiple fluorescent PCRtest means, and whether a sample is infected with the African swine fever virus and whether gene deletion exists in the infected virus or not can be determined at the same time. The method can detecta large number of samples at the same time, provides an effective tool for scientifically and reasonably preventing and controlling African swine fever, guarantees the healthy development of the pigindustry, and has the advantages of convenience in operation, high sensitivity, strong specificity, short detection time and the like.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Dual-fluorescence PCR detection reagent, kit and detection method for classical swine fever virus and African swine fever virus
PendingCN110760620AReduce workloadLow costMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVSwine Fever Virus
The invention discloses a dual fluorescence PCR detection reagent, a kit and a detection method for swine fever virus and African swine fever virus, and belongs to the field of animal pathogen detection. Aiming at a 5'-UTR gene of the swine fever virus and a P72 gene of the African swine fever virus, primers and probes capable of covering all strains and suitable for double detection are separately designed and screened, including two pairs of specific primers and two specific probes; the invention also describes a kit containing the primers and the probes and a PCR detection method using theprimers and the probes; the invention, through the design of the primers and the adjustment of each component of PCR, realizes purposes of one-time analysis and simultaneous detection and differentiation of the swine fever virus and the African swine fever virus on the premise of no reduction in sensitivity and specificity, which not only reduces the workload and cost of detection, but also greatly saves the detection time, thus gaining valuable time for epidemic disease prevention and treatment.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY +1
Gene chip and method for detecting classical swine fever virus (CSFV), porcine circovirus virus type 2 (PCV-2) and porcine reproductive and respiratory syndrome virus (PRRSV)
InactiveCN102234693AStrong specificityEnsure consistencyMicrobiological testing/measurementFluorescence/phosphorescenceClassical swine fever virus CSFVDisease
The invention provides a gene chip detection method for a classical swine fever virus (CSFV), a porcine circovirus virus type 2 (PCV-2), a porcine reproductive and respiratory syndrome virus Europe type (PRRSVE) and a porcine reproductive and respiratory syndrome virus America type (PRRSVA). A gene chip is shown as a probe sequence in the table of the specification. The invention discloses a method for simultaneously detecting three diseases, namely classical swine fever (CSF), a porcine circovirus type 2 (PCV-2) and a porcine reproductive and respiratory syndrome (PRRS) by using the gene chip. By the method, the problems that the conventional detection technology is time-consuming and labor-consuming and has low specificity, or only can be used for detecting a single disease are solved. All probes provided by the invention are synthesized into a connecting amino group at a 5' end, a poly T connecting arm with the length of 15 bp is provided, and a result shows that fixing efficiency is relatively high. By a polymerase chain reaction (PCR) amplification technology with mark primers, various kinds of fluorescent mark deoxyribonucleic acid (DNA) complementary to corresponding probescan be amplified at a time, and the high specificity and sensitivity of nucleic acid hybridization in a solid-liquid phase can be ensured. In addition, by a mark method, detection time is greatly shortened, the cost of chip detection is lowered, and the detection method is more suitable to be popularized in clinical application.
Owner:CHONGQING UNIV OF TECH
Condon optimized African swine fever virus P54 gene, nucleic acid vaccine and application thereof
InactiveCN103805615ATo solve the immune prevention and controlSame immune effectViral antigen ingredientsGenetic material ingredientsImmune effectsAfrican swine fever
The invention relates to a condon optimized African swine fever virus P54 gene and a nucleic acid vaccine based on the gene, wherein the nucleic acid vaccine consists of an eukaryotic expression vector and the condon optimized African swine fever virus P54 gene. The nucleic acid vaccine is constructed through the following steps of optimizing the condon of P54protein, designing a specific primer, transferring sequence of the artificially synthesized African swine fever virus P54 gene into a cloning vector and recombining the purified P54 gene into the eukaryotic expression vector. The nucleic acid vaccine can be used for preventing the occurrence of African swine fever and is a powerful tool for solving the immune prevention and control of the African swine fever. Compared with the traditional vaccines, the nucleic acid vaccine has the advantages of small quantity, security, long-term effect, stability and convenience in operation when reaching same immune effect.
Owner:孙洁
Recombination baculovirus for expressing Africa swine fever CD2V protein in SF9 cell
InactiveCN110157737AHigh protein expressionEasy to purifyViral antigen ingredientsVirus peptidesSf9Amino acid
The invention provides a recombination baculovirus for expressing Africa swine fever CD2V protein in an SF9 cell. The CD2V protein can be recombined and expressed in the SF9 cell; the amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; the sequence of the nucleotide fragment is shown in SEQ ID NO:3. The recombination baculovirus is used for preparing the Africa swine fever CD2V protein in an insect cell. The insect cell Sf9 is used for recombining and expressing the Africa swine fever CD2V protein, the protein expression amount is high, purification is easy, the recombination baculovirus is used for preparing and identifying a diagnosis product, and a solid foundation is laid for producing Africa swine fever subunit vaccines and diagnosis reagents.
Owner:YEBIO BIOENG OF QINGDAO
African swine fever virus ASFV-LAMP detection primer set and reagent kit
PendingCN110093457AGuaranteed accuracyAvoid missing detectionMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionAfrican swine fever
The invention relates to an African swine fever virus ASFV-LAMP detection primer set, reagent kit and detection method. In order to cope with the epidemic situation of African swine fever in Chin at 2018, an African swine fever virus P72 gene is used as an LAMP detection primer set. The primer set specially detects the African swine fever virus gene P72, ASFV can be effectively detected, a new technique means is provided for prevention and control of the African swine fever, and toxin strain detection and entry and exit quick screening are facilitated. The LAMP detection method can realize quick detection of the African swine fever viruses, detection results can be directly tested visually, and the entire reaction can be realized only needing heating. The relying of a traditional nucleic acid detection technique on a PCR instrument can be shaken off. The method is high in detection sensitivity, high in specificity, good in repeatability and high in detection speed, and can be used as an effectively on-field detection means for African swine fever.
Owner:陕西诺威利华生物科技有限公司
African swine fever virus synthetic peptide ELISA antibody detection kit
ActiveCN110642925AIncreased sensitivityStrong specificityVirus peptidesBiological material analysisClassical swine fever virus CSFVEpitope
The invention discloses an African swine fever virus synthetic peptide ELISA antibody detection kit. The kit includes an enzyme-labeled plate, a positive control serum, a negative control serum, an enzyme-labeled secondary antibody, a sample dilution solution, a 20-fold concentrated washing solution, a substrate solution A, a substrate solution B and a stop solution. The enzyme-labeled plate is coated with African swine fever virus epitope polypeptide composition. The epitope polypeptide composition is any combination of one or two or more of a polypeptide shown as a sequence 1 in a sequence listing, a polypeptide shown in a sequence 2 in the sequence listing and a polypeptide shown as a sequence 3 in the sequence listing. The kit uses a chemically synthesized antigen peptide-coated enzyme-labeled plate with low antigen consumption, high sensitivity and specificity, and can efficiently detect whether African swine fever virus antibodies exist or not. The kit is high in sensitivity, good in specificity, convenient to operate and good in market prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Recombined new castle disease virus vaccine strain for expressing African swine fever virus p72 proteins
ActiveCN104962581AImprove protectionEffective protectionViral antigen ingredientsMicroorganism based processesDiseaseNewcastle disease virus NDV
The invention provides a preparation method of a recombined new castle disease virus vaccine strain which expresses African swine fever virus p72 proteins and a recombined new castle disease virus vaccine strain. The method provided by the invention comprises the following steps: constructing a recombinant transcriptional plasmid which is inserted with a p72 gene of African swine fever virus (ASFV); constructing a transcriptional helper plasmid system; carrying out a contransfection for the transcriptional plasmids and the transcriptional helper plasmids into host cells BHK-21 which can be duplicated in new castle disease attenuate strains; and saving and obtaining the recombined virus stain. The vaccine strain for expressing the African swine fever virus p72 proteins provided by the invention has important preservation and strategy meaning in the aspect of animal epidemic disease prevention and control, and can be applied to the treatment and prevention of African swine fever virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for identifying wild strain and vaccine strain of hog cholera virus
ActiveCN103320535AStrong specificityPromote amplificationMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVTGE VACCINE
The invention discloses a method for identifying a wild strain and a vaccine strain of a hog cholera virus. According to the method, a primer specially used for identifying the wild strain and the vaccine strain of the hog cholera virus is designed in a conservative region shared by the wild strain and the vaccine strain. The primer is high in specificity and can well multiply the wild strain and the vaccine low virulent strain; according to a reverse transcription-polymerase chain reaction technology and a high resolution melting (HRM) technology, the wild strain and the vaccine strain can be obviously identified. The method is a simple, quick and practical novel technology for identifying the wild strain and the vaccine strain of the hog cholera virus.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Classical swine fever virus recombinant E2 protein and IgM (immune globulin M) antibody ELISA (enzyme-linked immunosorbent assay) test kit thereof
ActiveCN102532281AGood antigenicityGood repeatabilityBacteriaVirus peptidesEscherichia coliClassical swine fever virus CSFV
The invention belongs to the field of molecular biology and relates to a classical swine fever virus recombinant E2 protein and an IgM (immune globulin M) antibody ELISA (enzyme-linked immunosorbent assay) test kit thereof. The classical swine fever virus E2 protein expressed by recombinant Escherichia coli is obtained by cloning the main antigen region of E2 protein into a pronucleus expression vector to obtain a recombinant expression vector, transforming the recombinant expression vector to Escherichia coli BL21 (DE3) and expressing and purifying with the recombinant Escherichia coli. A Westernblot test indicates that the protein has good antigenicity. According to the invention, an elisa plate is coated the protein; an ELISA method is established through optimization of antigen coating concentration, serum dilution and action time, secondary antibody concentration and action time as well as developing time for the purpose of detecting negative serum so as to determine a critical value and a judgment standard. According to the invention, the expression of a recombinant strain constructed by the recombinant E2 protein on a heterologous protein is stable, and the recombinant strain is good in antigenicity; and on the basis, the recombinant E2 protein disclosed by the invention is used for establishing a CSFV (classical swine fever virus) IgM antibody ELISA test kit for the first time.
Owner:JIANGSU ACAD OF AGRI SCI
P72 fusion protein of African swine fever virus , and preparation method and application thereof
ActiveCN111234036AImproving immunogenicityImprove biological activityVirus peptidesImmunoglobulins against virusesClassical swine fever virus CSFVImmunogenicity
The invention provides a p72 fusion protein of an African swine fever virus, and a preparation method and an application thereof, and relates to the technical field of biology. The p72 fusion proteinof the African swine fever virus contains a p72 protein fragment and a GCN4 fragment of the African swine fever virus, wherein the GCN4 fragment has an amino acid sequence of SEQ ID NO. 1. The p72 fusion protein of the African swine fever virus can form a trimer and has good immunogenicity.
Owner:天康制药股份有限公司
Pig virus gene chip and detection method thereof
ActiveCN102605099ARich varietyAvoid false positivesNucleotide librariesMicrobiological testing/measurementClassical swine fever virus CSFVMultiplex
The invention provides a pig virus gene chip and a detection method thereof. The gene chip comprises a probe fixedly arranged on a substrate carrier, wherein the probe is selected from the characteristic segments of the following viruses: a classical swine fever virus (CSFV), a porcine reproductive and respiratory comprehensive virus (PRRSV), a pseudorabies virus (PRV), a porcine circovirus (PCV) and a porcine parvovirus (PPV). The types of the viruses detected by the method are various and the viruses cover common porcine viruses substantially. Random primer polymerase chain reaction (PCR) and multiplex-PCR are adopted to mark, so that false positive which is easy to cause when a PCR result is detected by gel electrophoresis is avoided, and high-flux accurate detection with short time is realized. The substrate carrier is a glass sheet which is subjected to aldehyde treatment, so that the combination between the probe and a target is facilitated, and higher noise is not brought to detection.
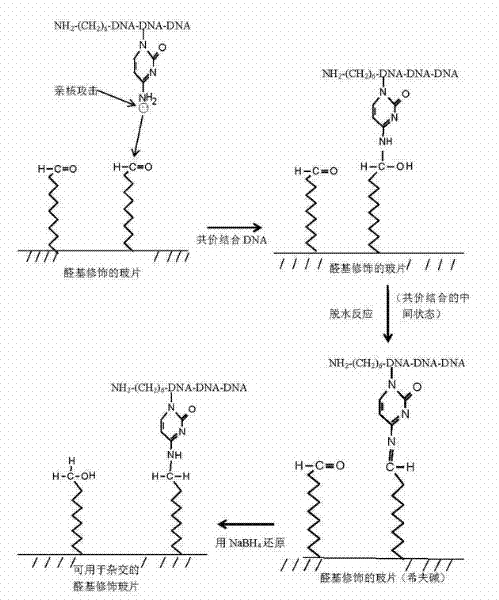
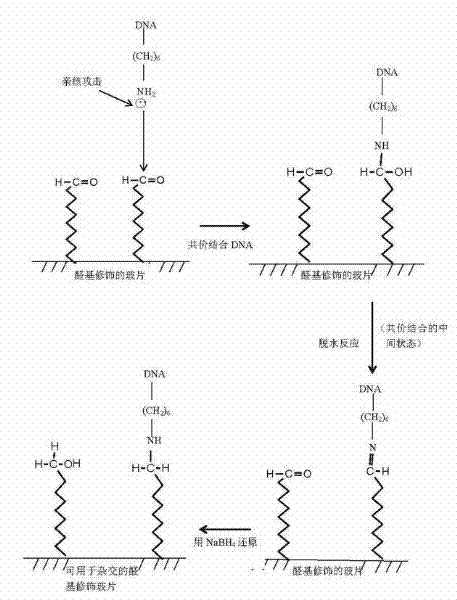
Owner:BEIJING UNIV OF AGRI
Monoclonal antibody for African swine fever virus gene II type strain as well as preparation method and application thereof
InactiveCN104497136AGuaranteed testingAccurate detectionImmunoglobulins against virusesTissue cultureSpleen cellIn vivo
The invention discloses a monoclonal antibody for an African swine fever virus gene II type strain as well as a preparation method and application thereof. The preparation method of the monoclonal antibody for the African swine fever virus gene II type strain comprises the following steps: immunizing a mouse by adopting a purified p54 immune protein, fusing spleen cells of the immunized mouse with myeloma cells to prepare hybridoma cells, screening with three screening antigens so as to obtain the hybridoma cells which can stably secrete a monoclonal antibody, and then preparing the monoclonal antibody by adopting an in vivo or in vitro method, wherein the three screening antigens comprise a prokaryotically expressed p54 recombinant protein, an eukaryotically expressed p54 recombinant protein and an artificially synthesized polypeptide shown in Seq ID No.1. The preparation method of the monoclonal antibody for the African swine fever virus gene II type strain has the advantages that the three screening antigens are adopted for screening the hybridoma cells, and especially a special artificially synthesized polypeptide for the gene II type strain is adopted as the screening antigen, so that the prepared monoclonal antibody can guarantee the detection rate of the gene II type strain, the misdetection rate is reduced, and the quarantine working quality and efficiency are improved.
Owner:SHENZHEN AUDAQUE DATA TECH
Fluorescence quantitative PCR detection reagent for Asf virus and preparation method and use thereof
InactiveCN1840698AThe detection method is accurateFast detection methodMicrobiological testing/measurementFluorescence/phosphorescenceClassical swine fever virus CSFVAfrican swine fever
The related bio-reagent is designed by: with conservative African swine fever virus VP73 gene fragment as target object, designing and composing carefully for large quantities of primers and probes, taking optimal pairing screen test on different conditions to obtain the most proper primer and probe. The fluorescence quantitative RT-PCR testing reagent includes one couple of specific primers and one specific fluorescent probe, and the amplification fragment length is 66bp. This invention is fit to fast testing for African swine fever.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com