Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Bovine parvovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ungulate bocaparvovirus 1, formerly Bovine parvovirus (BPV), also known as Haemadsorbing Enteric Virus, is a member of the parvivirus group, with three significant sub-species: BPV1, 2 and 3. BPV most commonly causes diarrhoea in neonatal calves and respiratory and reproductive disease in adult cattle. The distribution of the virus is worldwide. Transmission is both vertical (transplacental route) and horizontal (oro-faecal route). The virus is very resistant to chemical and physical challenges.
Multiple real time fluorescence quantifying PCR method for detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and classical swine fever virus
InactiveCN101260442ALow costImprove application stabilityMicrobiological testing/measurementClassical swine fever virus CSFVRegression analysis
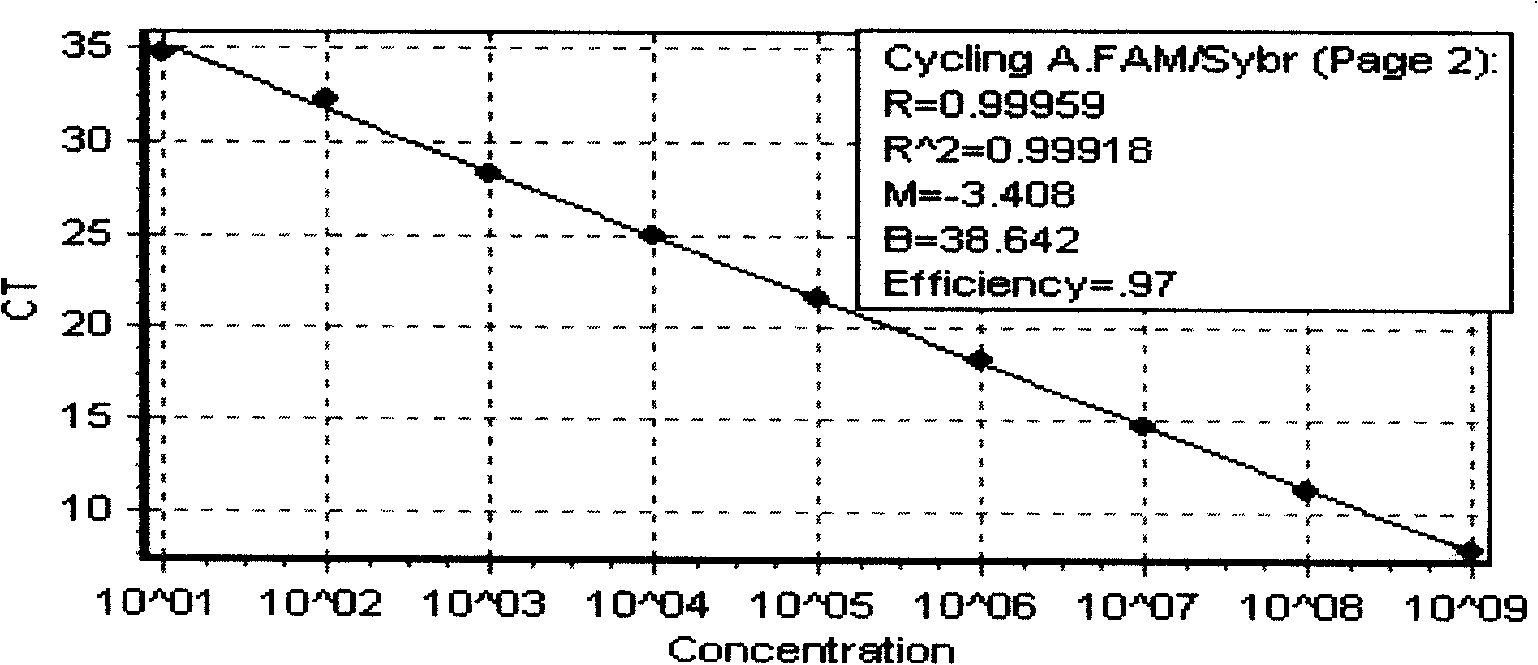
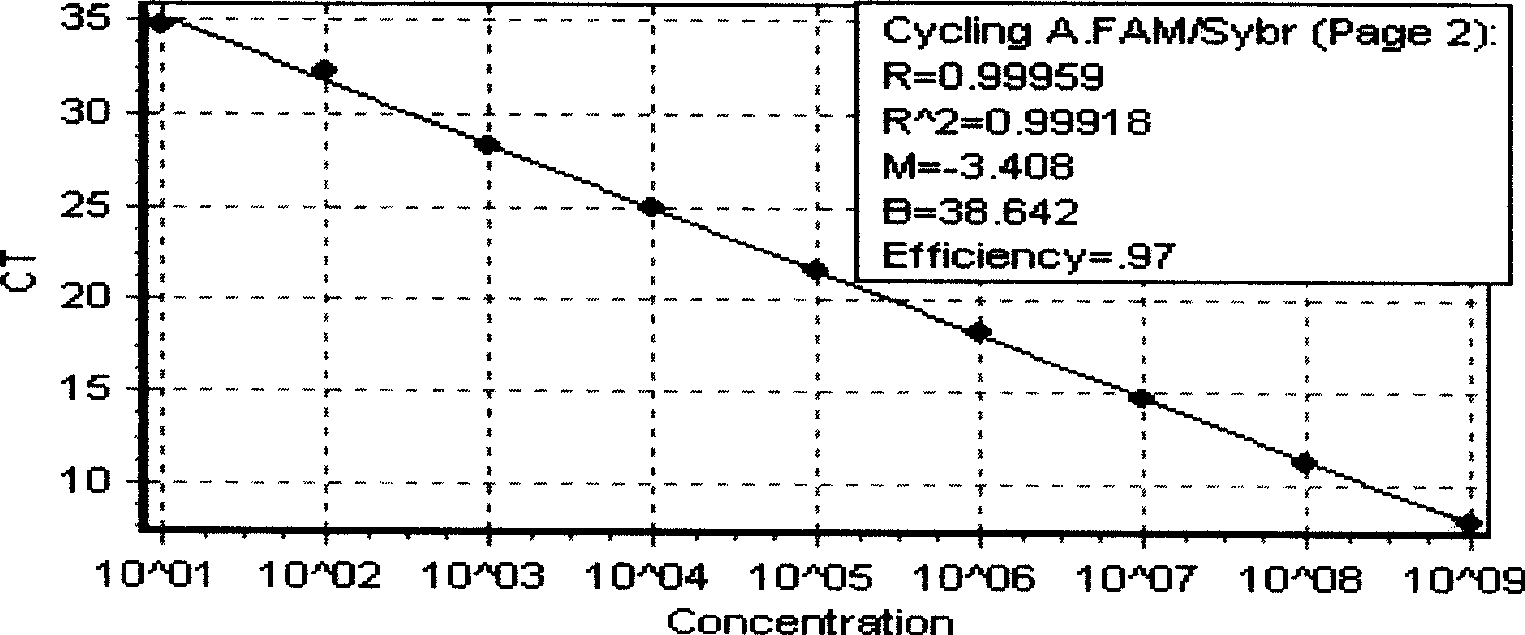
The invention discloses a multiplex real-time fluorescent quantitation PCR method capable of detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and hog cholera virus at the same time. The method of the invention detects that the four virus are all in good linear relations at the same time, all ten times serial dilution points of constructed normal plasmid are all in one straight line, CT value and copy number are in good linear relation, and regression analysis shows that the related coefficient of the CT value and the copy number is R<2> more than 0.99.The multiplex real-time fluorescent quantitation PCR method has the advantages of excellent specificity, sensibility and stability, which can rapidly, sensitively and differentially detect the four virus with serious harm to the economy and can be used for early diagnosis of virus infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triple virus-like particle vaccine and its preparation method
The purpose of the invention is to disclose a porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triplex virus-like particle vaccine and its preparation method. The triple virus-like particle vaccine (Triple VLP vaccine) of the invention contains VLP which is composed of PCV-2 major structural protein CAP protein, PPV VP2 protein epitope and PRRSV Gp5 protein epitope. It is proved by experiment that the vaccine can stimulate good double cellular and humoral immune response. It is shown by pharmacodynamic test that after immunization of different animal groups, the vaccine of injection, nose drops and water forms prepared by VLP antigen formed by the method with or without adjuvants can safely and effectively prevent the infection of PCV-2, PPV and PRRSV. The invention provides an ideal vaccine for the security of sows, piglets and fattening pigs to effectively prevent mixed infection of PCV-2, PPV and PRRSV.
Owner:CHONGQING UNIV
Canine combination vaccines
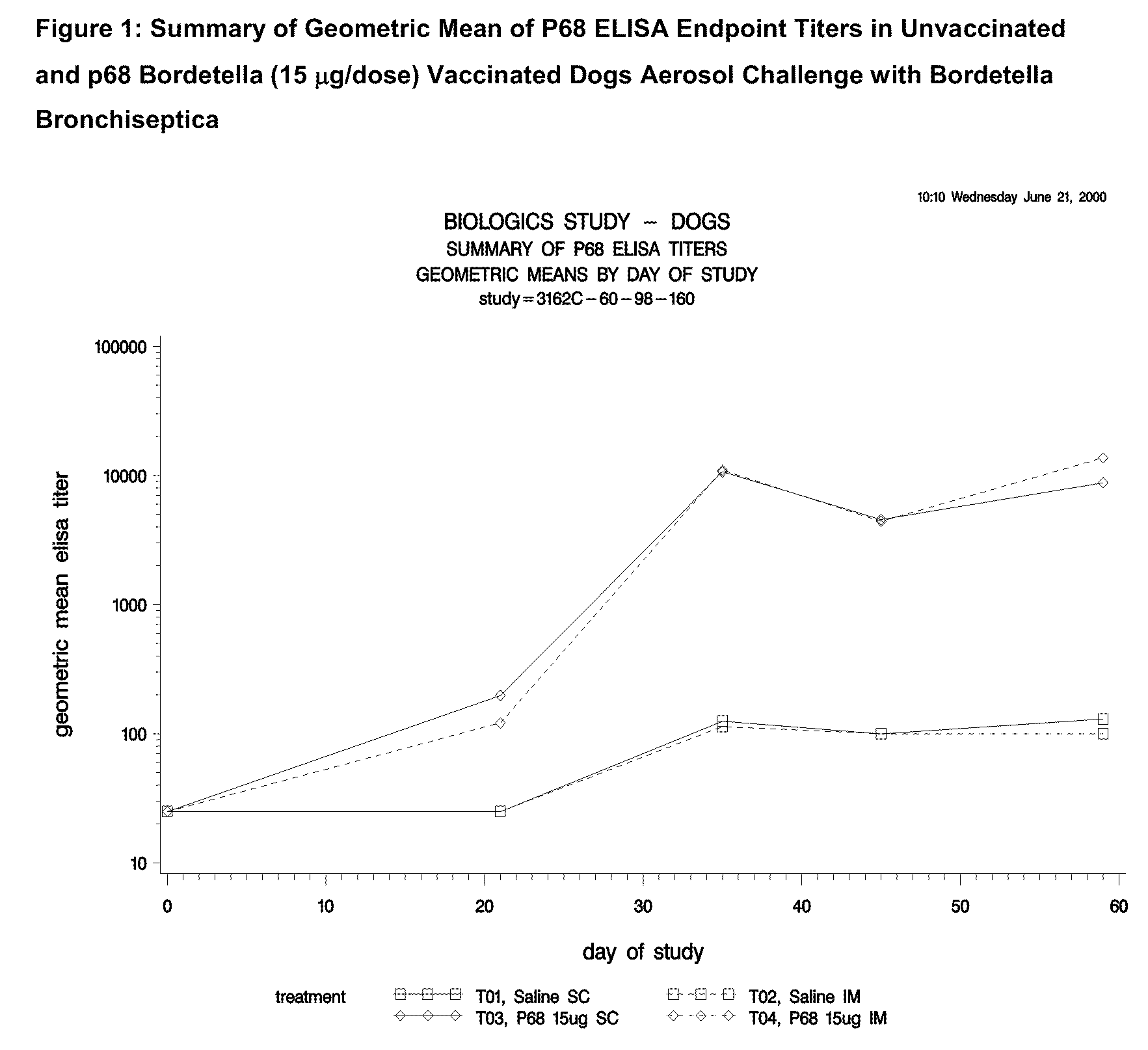
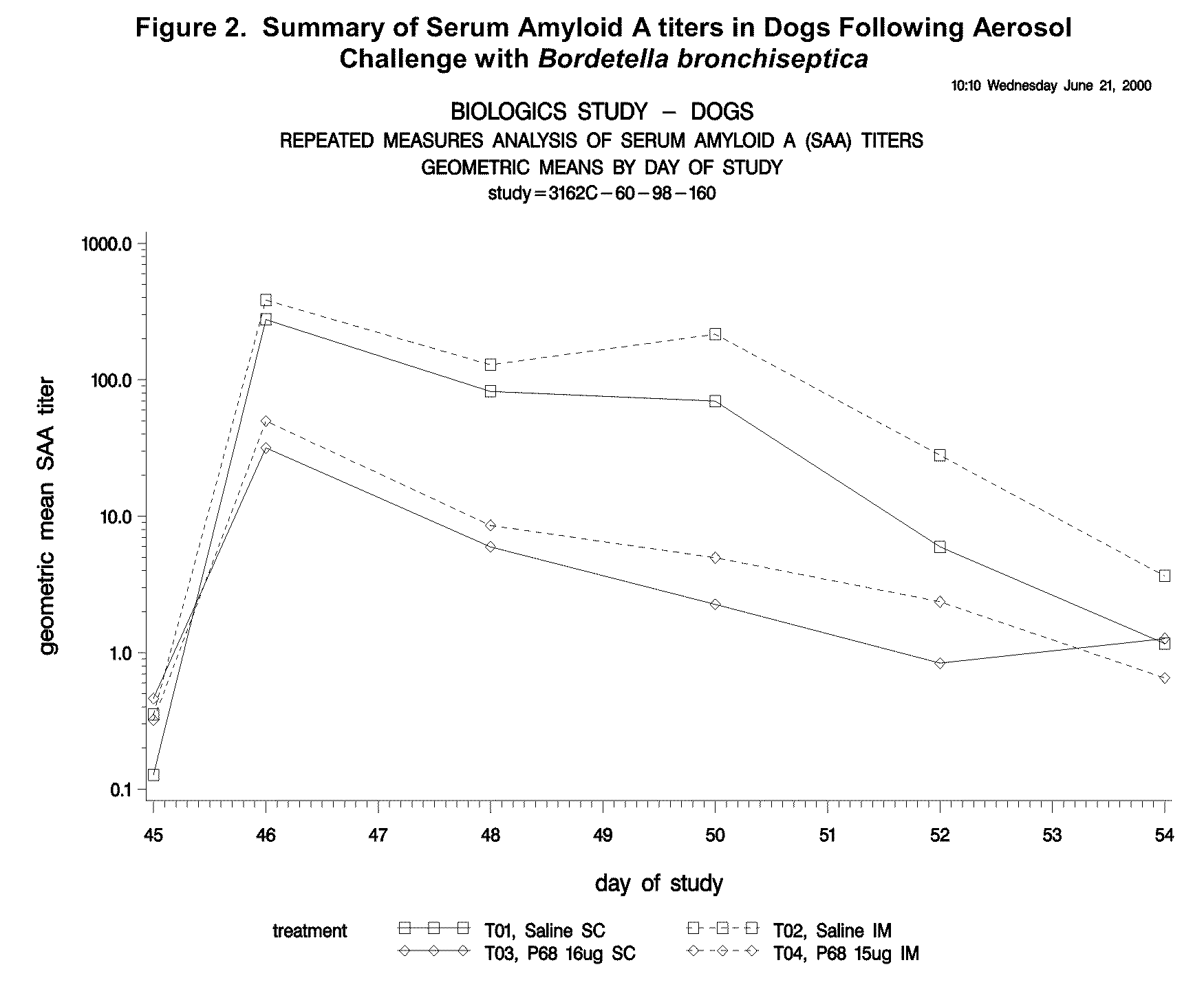
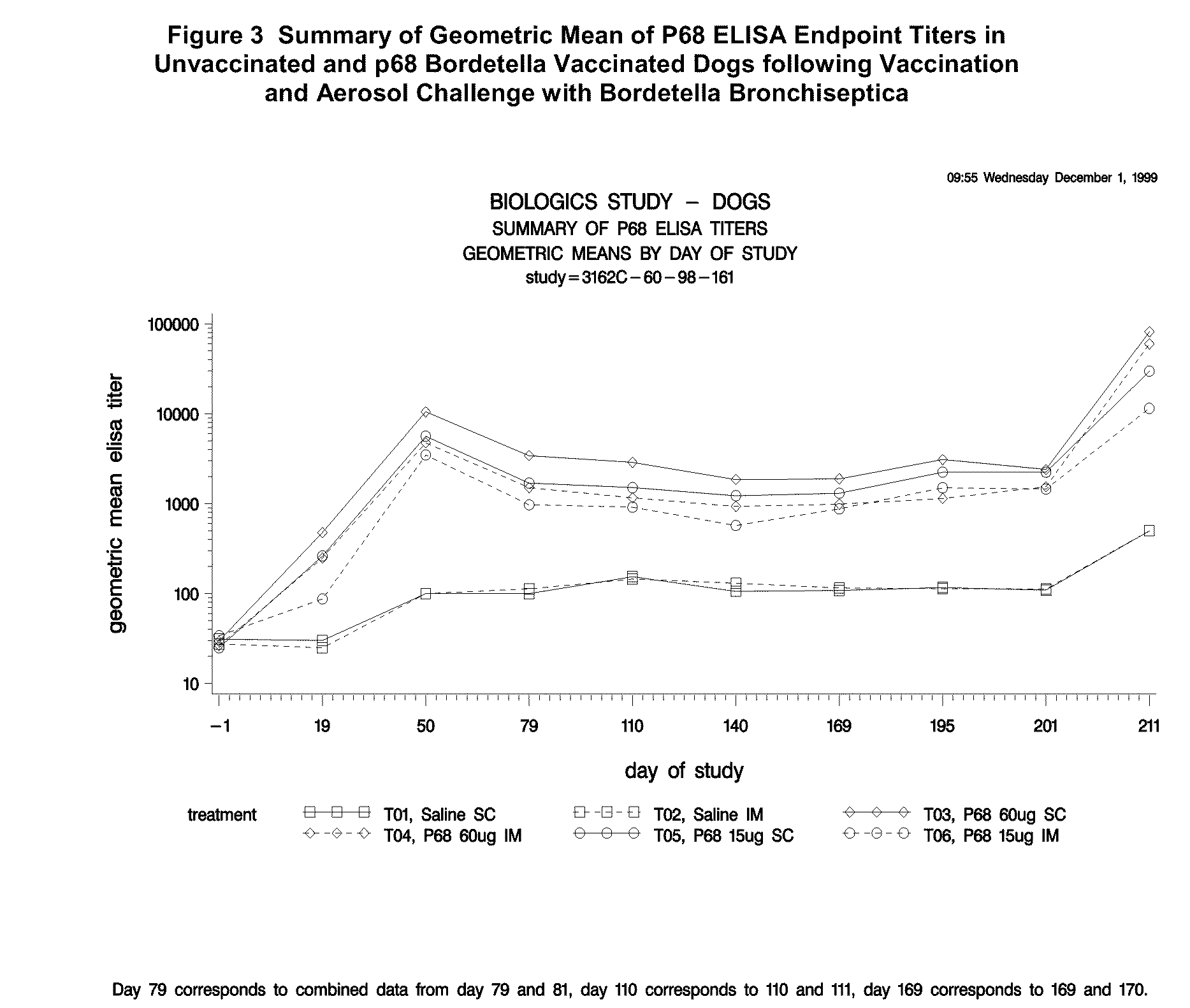
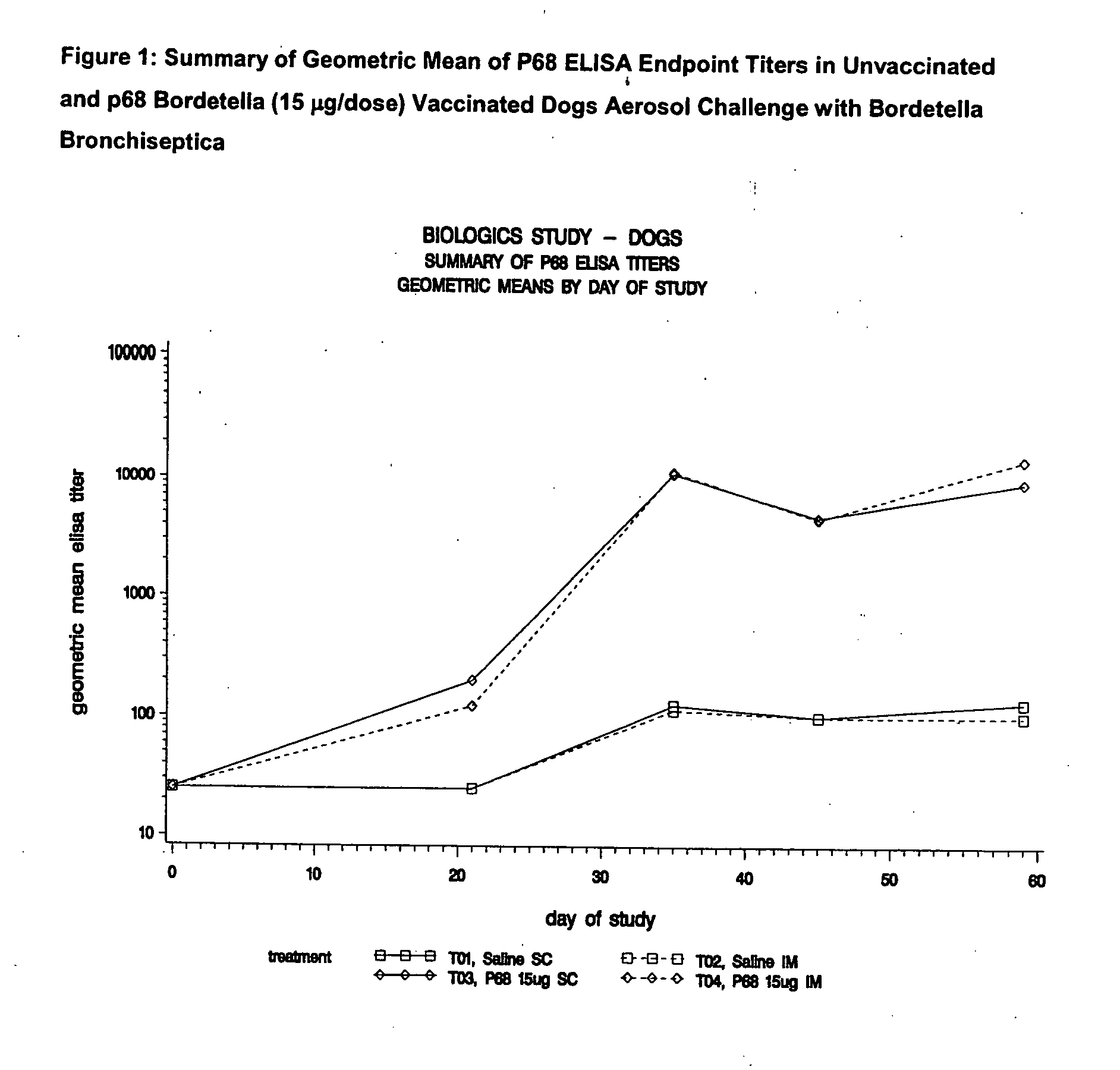
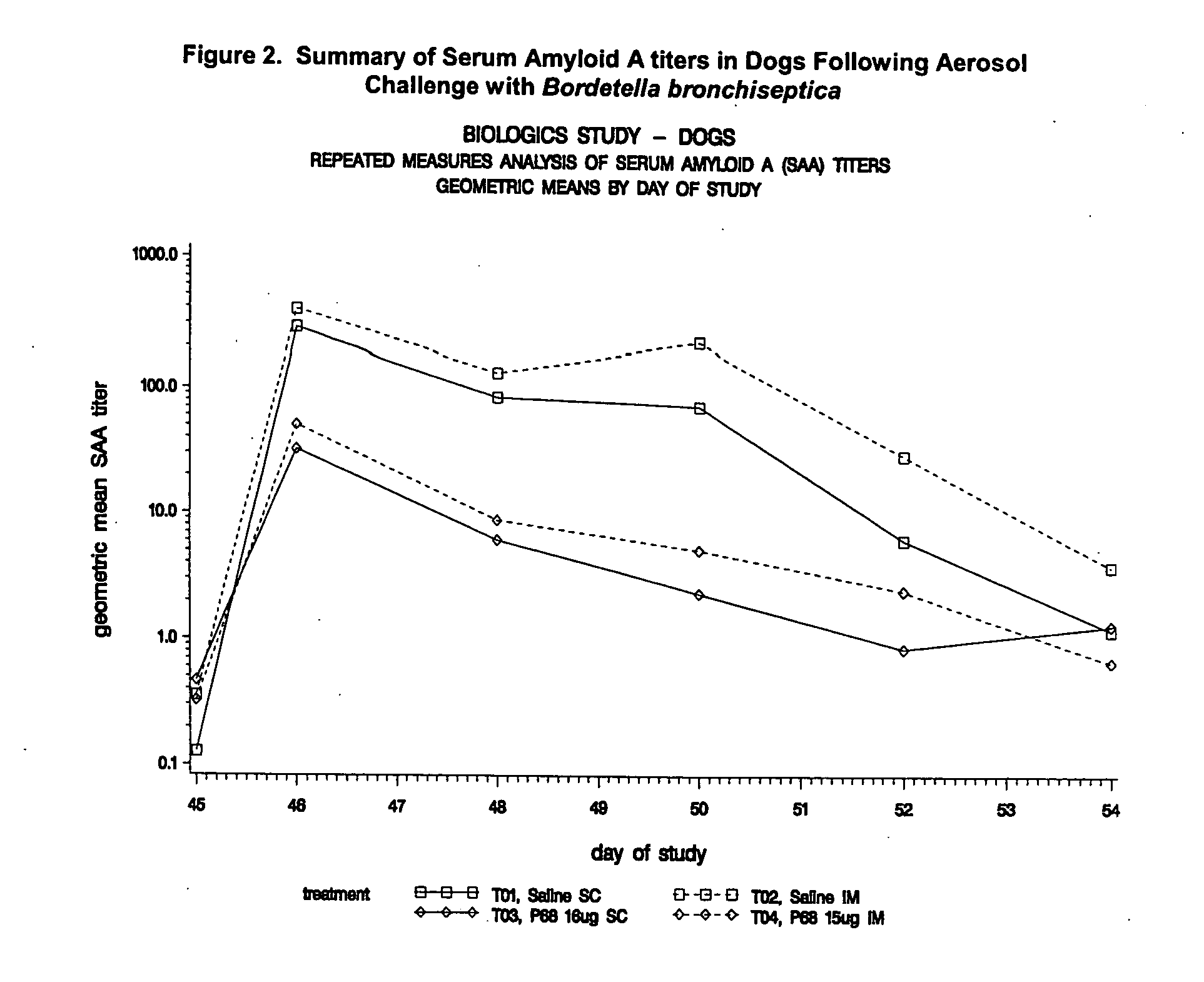
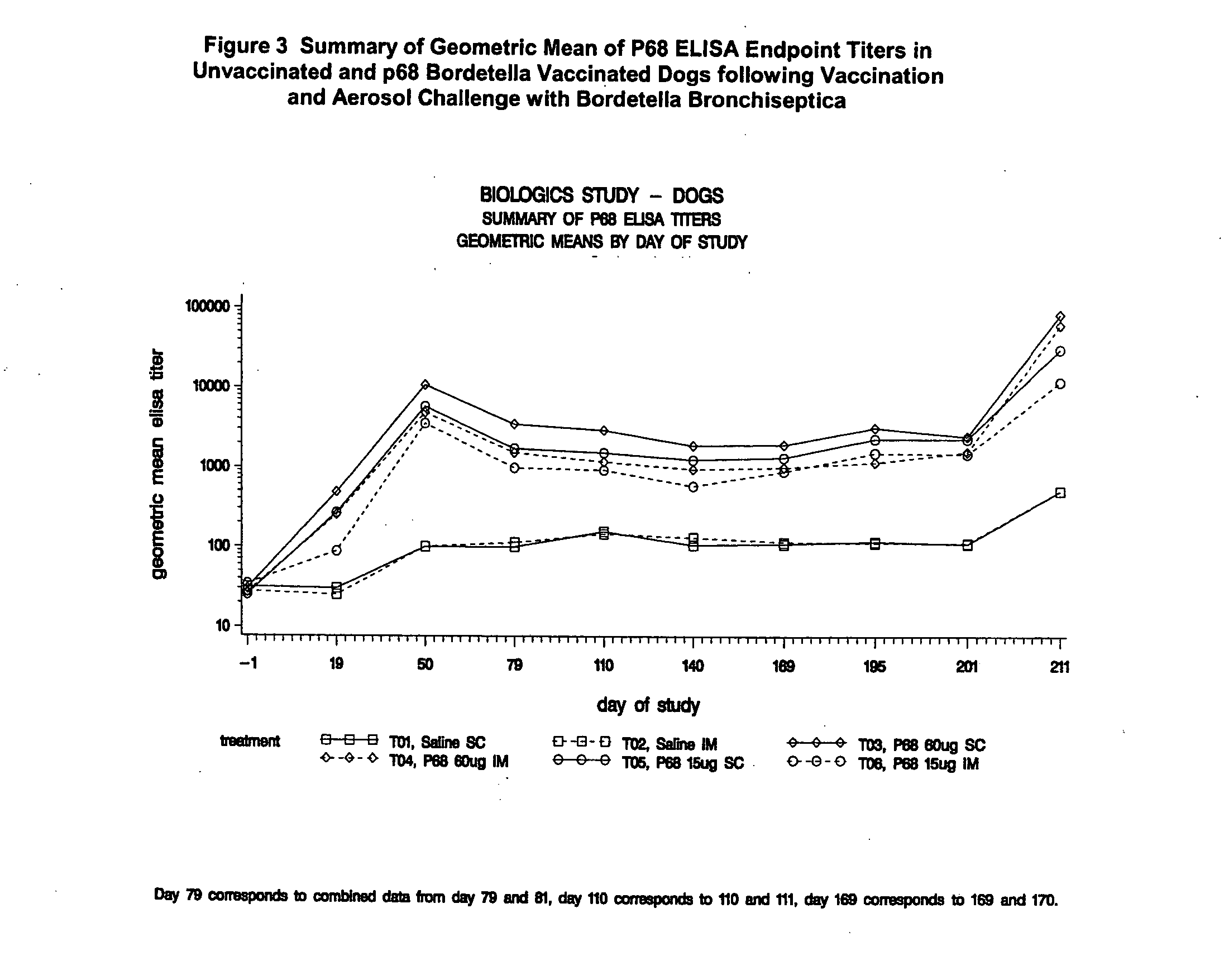
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
Multiplex real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2
InactiveCN102071259AEasy to identifyEasy diagnosisMicrobiological testing/measurementMicroorganism based processesAgricultural scienceFluorescence
The invention discloses a multiplex SYBR Green I real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2. The primer is obtained through synthesis according to design. The multiplex SYBR Green I real-time fluorescence PCR detection method for detecting porcine rabies virus, porcine parvovirus and porcine circovirus type 2 by utilizing the primer comprises the following steps: extracting the DNA of a sample, and then, detecting the sample by utilizing a SYBR Green I real-time fluorescence PCR reaction system and a SYBR Green I real-time fluorescence PCR amplification program. The invention has the beneficial effects that three types of viruses, namely the porcine rabies virus, the porcine parvovirus and the porcine circovirus type 2, can be simultaneously and effectively diagnosed and detected; non-specific swine fever virus, porcine reproductive and respiratory syndrome virus and swine influenza virus can not be detected; and the invention is beneficial to identification and diagnosis of the breeding disorder virus of a pregnant swine, and has better sensitivity, repeatability and stability.
Owner:HENAN AGRICULTURAL UNIVERSITY
Multiplex RT-PCR detection primer for porcine delta coronavirus, porcine epidemic diarrhea virus and porcine transmissible gastroenteritis virus
ActiveCN105483291ARapid differential diagnosisMicrobiological testing/measurementMicroorganism based processesPorcine parvovirusBovine parvovirus
The invention discloses a multiplex RT-PCR detection primer for a porcine delta coronavirus, a porcine epidemic diarrhea virus and a porcine transmissible gastroenteritis virus. The minimum detection capacity of the multiplex RT-PCR for the three viruses is 4.05*10<1> copies / microliter, 4.52*10<3> copies / microliter and 5.47*10<3> copies / microliter respectively. The amplification results for a porcine parvovirus (PPV) and a porcine pseudorabies virus (PRV) are both negative. The multiplex RT-PCR detection results of 57 clinical samples show that one sample is infected with the three viruses at the same time, 11 samples are infected with the PDCoV, 15 samples are infected with the PEDV, one sample is infected with the TGEV, five samples are infected with the PDCoV and the PEDV, and one sample is infected with the PDCoV and the TGEV.
Owner:HENAN AGRICULTURAL UNIVERSITY
Canine vaccines against Bordetella bronchiseptica
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention relates to vaccines and methods for protecting dogs against disease caused by Leptospira bratislava. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona.
Owner:PFIZER INC +1
Porcine parvovirus L strain and use thereof in preparation of porcine parvovirus inactivated vaccines
The invention discloses a porcine parvovirus L strain and use thereof in preparation of porcine parvovirus inactivated vaccines. In the invention, microbial preservation number of the separated strain is CGMCC No. 3352. The strain is highly homologous with an NADL-2 strain and a China strain and keeps high reproduction, and the viruses are stable. Oil adjuvant is added into antigen liquid which inactivates the viruses by using binary ethylenimine to prepare the bidirectional oil-emulsion inactivated vaccines; and the prepared inactivated vaccines are applied in a vaccine inoculation experiment of 10,276 pigs (most of which are first farrowing sows), and in the experiment, an immune dosage of 2 millimeters is given to each pig through muscle injection, and each time of inactivated vaccine inoculation can obtain an immune period of over 6 months. Under the conditions that a 10 millimeter overdose vaccine is injected into a pig and that single dose inoculation is repeatedly carried out for three times, no abnormal reaction happens, which proves that the inactivated vaccines have high immunogenicity and safety.
Owner:哈药集团生物疫苗有限公司
Primer set, reagent kit and method for detecting canine distemper viruses, canine parvoviruses and canine coronaviruses
InactiveCN108374056AAchieve the expected purpose of the experimentIncreased sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesCanine distemper virus CDVNucleotide sequencing
The invention provides a primer set, a reagent kit and a method for detecting canine distemper viruses (CDV), canine parvoviruses (CPV) and canine coronaviruses (CCV), and belongs to the technical field of virus detection. The primer set comprises primers with nucleotide sequences shown as SEQ ID No.1-6. The primers correspond to three pairs of upstream and downstream primer pairs for the canine distemper viruses (CDV), the canine parvoviruses (CPV) and the canine coronaviruses (CCV). The reagent kit comprises the primer set. The method is based on the primer set and the reagent kit and is used for detecting the canine distemper viruses, the canine parvoviruses and the canine coronaviruses. The primer set, the reagent kit and the method have the advantages that the canine distemper viruses, the canine parvoviruses and the canine coronaviruses can be detected by the aid of the primer set, the reagent kit and / or the method, the primer set, the reagent kit and the method are good in specificity and accuracy and high in sensitivity and efficiency and are simple and speedy, reliable technical means can be provided to clinical detection, and the like.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Bivalent inactivated vaccine of porcine circovirus type 2 and porcine parvovirus and preparation method thereof
InactiveCN102961742AHigh titer contentImmunization is convenient and fastViral antigen ingredientsAntiviralsAntigenAdjuvant
The invention relates to polyvalent vaccines for prevention and treatment of porcine infectious diseases, especially to a combined vaccine for treatment and prevention of porcine circovirus type 2 (PCV2) and porcine parvovirus. By selecting PCV2 and porcine parvovirus, the preparation method provided in the invention consists of: culturing PCV2, and conducting inactivation and concentration; culturing a porcine parvovirus, and performing inactivation and concentration; mixing the two antigenic components in proportion, and supplement an adjuvant to prepare the vaccine. The bivalent vaccine prepared in the invention is easy to use, more secure, and has an immune effect superior to that of combined use of two single vaccines.
Owner:PU LIKE BIO ENG
Triple PCR primer set capable of simultaneously detecting porcine circovirus II, porcine pseudorabies virus and porcine parvovirus and application thereof
InactiveCN105400906AHigh sensitivityImprove detection efficiencyMicrobiological testing/measurementDNA/RNA fragmentationGene targetsCanine parvovirus
The invention discloses a triple PCR primer set capable of simultaneously detecting porcine circovirus II, porcine pseudorabies virus and porcine parvovirus and an application thereof. ORF2 encoding genes with porcine circovirus II characteristics (PCV2), gB (gII) encoding genes with porcine pseudorabies virus (PRV) characteristics and encoding genes for VP2 protein of porcine parvovirus (PPV) are screened out from published documents and serve as amplification primers of three gene targets for PCR detection. Three pairs of primers for amplifying the three gene segments are placed in a PCR system, various parameters such as annealing temperature and specificity are adjusted and optimized, and a triple PCR detection method for directly detecting porcine circovirus II, porcine pseudorabies virus and porcine parvovirus from a sample at a time is established. When the triple PCR detection primers are used for detection, specificity is high, sensitivity is high, and detection efficiency is improved.
Owner:SHANGHAI JIAO TONG UNIV
Molecular design of porcine parvovirus-like particle B cell epitope insertion site
InactiveCN102417912ANon-pathogenicImprove replication efficiencyGenetic material ingredientsViruses/bacteriophagesVaccinationDelta-v
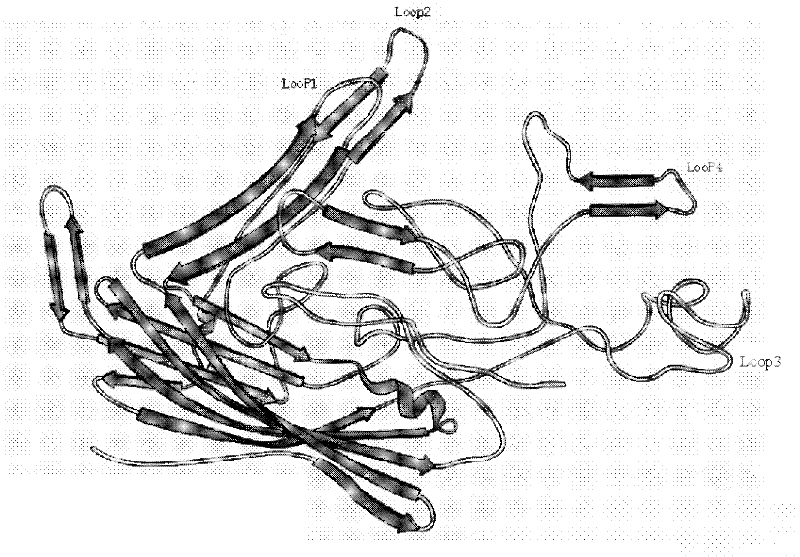
The invention relates to a molecular design of porcine parvovirus-like particle B cell epitope insertion site and belongs to the field of genetic engineering vaccine. Structure modeling of porcine parvovirus (PPV) capsid protein VP2 is performed by using bioinformatics software and the location of four extrusive Loop structures is determined by three-dimensional structure analysis. Firstly, on the basis of molecular simulation and literature support, we infer that loop 2,4 region can act as insertion sites of exogenous epitope gene. As is shown through experiments, after respective deletion of corresponding genes in PPV VP2 Loop2 (212aa-245aa), Loop (413aa-424aa) and then expression by adenovirus expression system, all recombinant virus with deletion mutations of Loop 2,4 can assemble regular virus-like particles [PPV: delta V LPs]. The invention also relates to an application of recombinant PPV delta V P2 virus-like particles of exogenous gene expressed by the recombinant virus in vaccination and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Canine combination vaccines
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
Vaccine against porcine parvovirus and porcine reproductive and respiratory syndrome virus and methods of production thereof
ActiveUS20180147278A1Broad protection spectrumAvoid infectionSsRNA viruses positive-senseViral antigen ingredientsDiseaseCanine parvovirus
The present invention relates to a porcine parvovirus and porcine reproductive and respiratory syndrome virus vaccine for protecting a subject, preferably swine, against diseases associated with porcine parvovirus and porcine reproductive and respiratory syndrome virus. The present invention further relates to methods of producing immunogenic compositions as well as such immunogenic compositions exhibiting reduced virucidal activity.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Full-length infectious DNA (deoxyribonucleic acid) clone of porcine parvoviruses, method for constructing full-length infectious DNA and application thereof
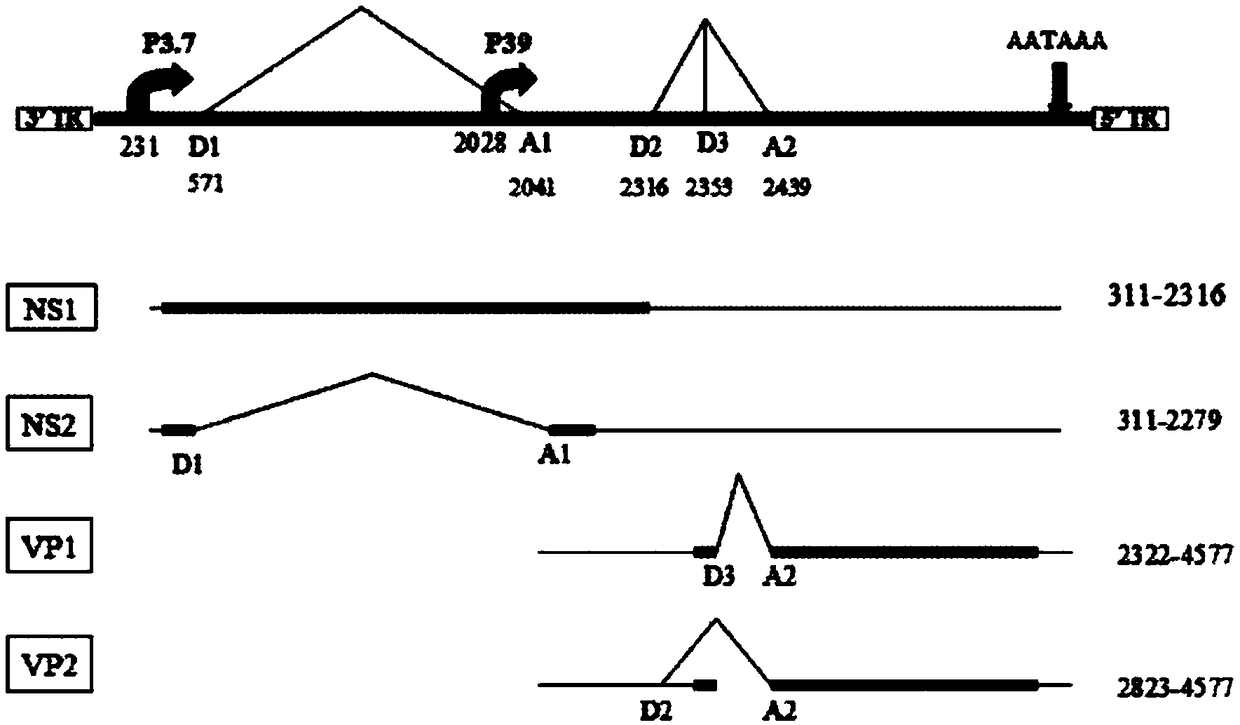
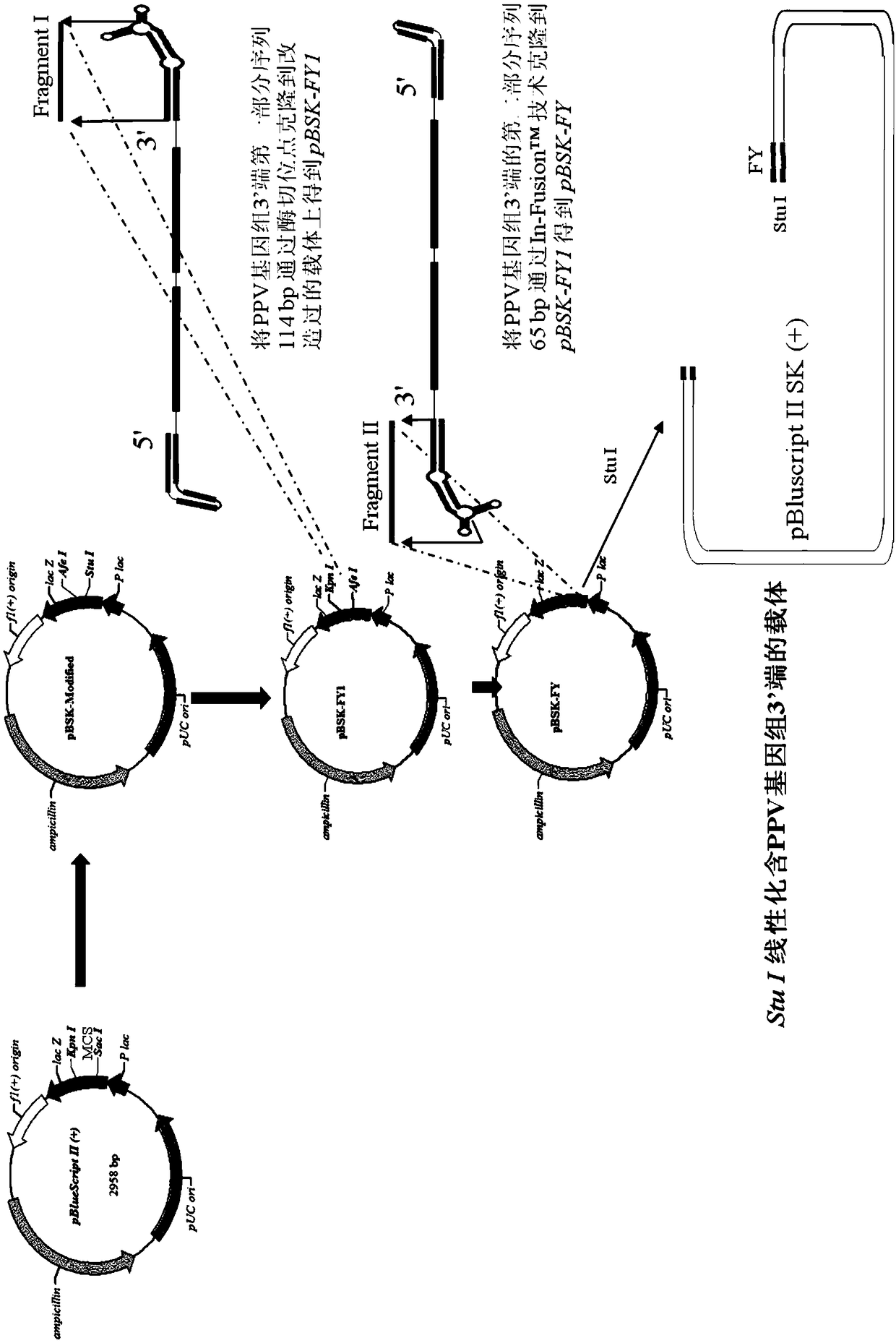
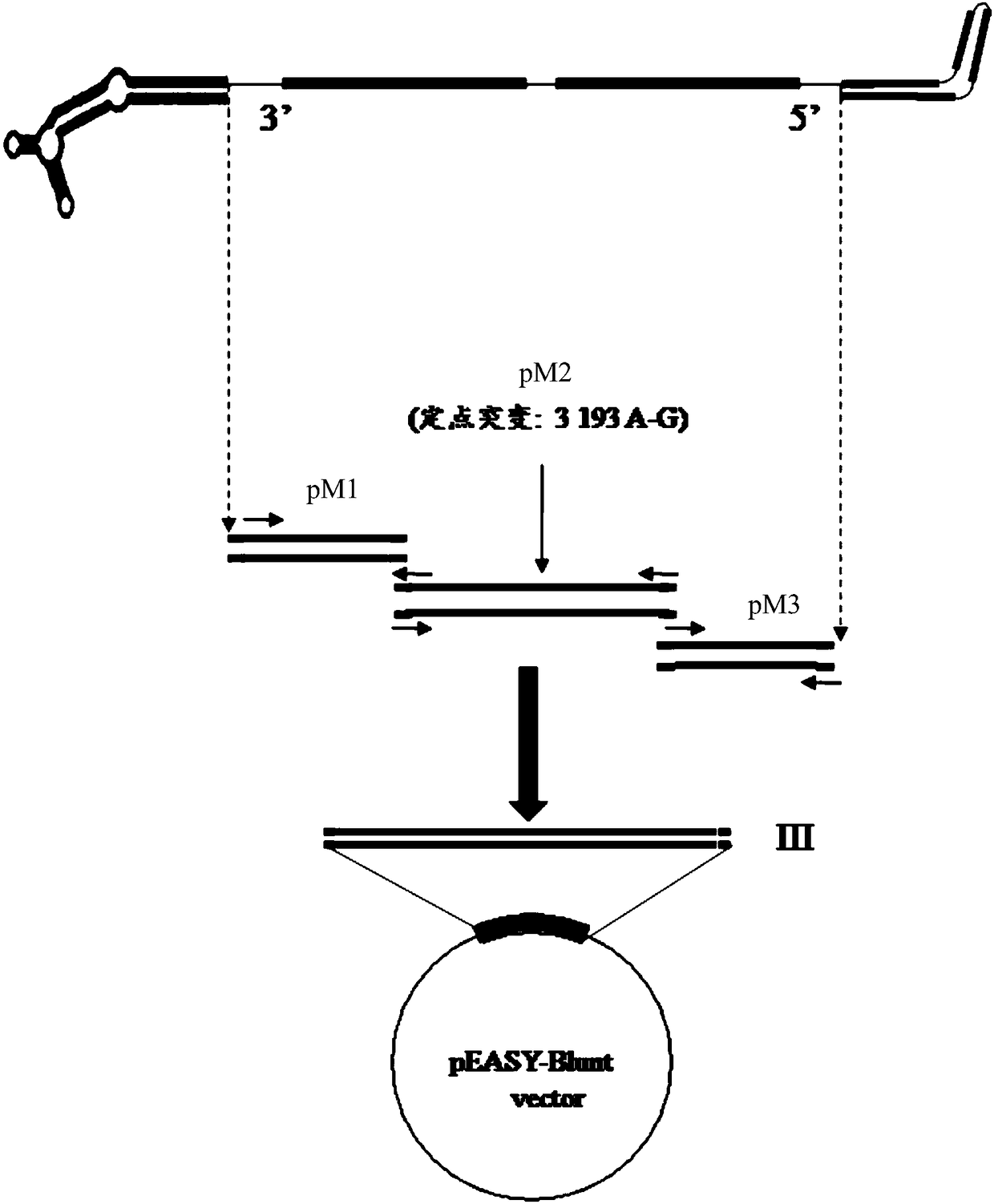
The invention discloses full-length infectious DNA (deoxyribonucleic acid) clone of porcine parvoviruses, a method for constructing the full-length infectious DNA and application thereof. Nucleotide sequences of the full-length infectious DNA of the porcine parvoviruses are shown as SEQ ID NO.1. The full-length infectious DNA clone, the method and the application have the advantages that rescue viruses with growth characteristics similar to growth characteristics of wild PPV (porcine parvoviruses) can be rescued by the aid of the full-length infectious DNA, and genetic markers EcoRv are introduced during full-length infectious clone, still can stably exist even after passage is carried out, and can be used as reliable genetic markers for identifying the wild porcine parvoviruses and the rescue viruses; a porcine parvovirus reverse genetics operating system is further established and can be used for analyzing the virulence of porcine parvovirus proteins, and novel inactivated vaccine orattenuated vaccine can be correspondingly prepared; platforms can be provided to the aspects such as research on porcine parvovirus gene structures and functions and research on the pathogenicity, and a foundation can be laid for research on vaccine and the like for porcine parvovirus diseases.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Canine parvovirus proliferation method
InactiveCN105039264APromote proliferationMaintain growth characteristicsMicroorganism based processesViruses/bacteriophagesHigh densityCanine parvovirus
The invention provides a canine parvovirus proliferation method. According to the method, F81 cells are creatively taken as host cells of canine parvovirus, so that virus proliferation is facilitated especially; a riptide-type bioreactor is adopted, so that compared with conventional spinner bottle modes, processes are easier to control, and products are more stable in quality; a microcarrier culture mode is adopted, so that a culture solution can be utilized fully, growing characteristics of adherent cells are maintained, and high-density culture effect is realized. In addition, aiming at own characteristics of the F81 cells and the canine parvovirus and culture environment of the bioreactor, specific culture conditions are matched, and components of the culture solution, inoculation amount and condition parameters are designed in an optimized manner, so that canine parvovirus proliferation efficiency is improved remarkably. Protruding technological effect is realized through an ingenious technological concept, and the method is low in cost and easy in realization and has outstanding popularization prospect.
Owner:TIANJIN RINGPU BIO TECH
Mink parvovirus attenuated vaccine strain and application of mink parvovirus attenuated vaccine strain in preparation of mink parvovirus attenuated vaccine
ActiveCN104388394APrevention of parvovirus enteritisMicroorganism based processesAntiviralsMicroorganismMink
The invention discloses a mink parvovirus attenuated vaccine strain and application of the mink parvovirus attenuated vaccine strain in preparation of a mink parvovirus attenuated vaccine. The mink parvovirus attenuated vaccine strain is named as MEVB-F61 and preserved in the China General Microbiological Culture Collection Center, and the preservation number of the mink parvovirus attenuated vaccine strain is CGMCC No.9560. A test result shows that when used as a vaccine, the vaccine strain is safe and effective, and prevents the mink parvovirus enteritis effectively, and no reversion to virulence of the vaccine strain is realized through inoculation experiments of continuous five generations of animals. Therefore, the mink parvovirus attenuated vaccine strain has a wide application prospect in the field of preventing mink parvovirus enteritis.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Culture method of porcine parvovirus
InactiveCN103865885AHigh poison priceSimple processViruses/bacteriophagesSocial benefitsMicrobiology
The invention discloses a culture method of porcine parvovirus; porcine testis passage cells (ST) are used as a cell source for culturing the porcine parvovirus, and while cell passage, the virus is inoculated, so that multiplication culture of the virus is realized. The method has the characteristics of simple technology, high increment, high yield and low cost, and a vaccine prepared by use of the porcine parvovirus cultured by the method has a complete preventive effect on the porcine parvovirus, and has good social benefits and application prospects.
Owner:QINGDAO ZHONGREN PHARMA
Bivalent inactivated vaccine for porcine circovirus type 2 and porcine parvovirus and preparation method thereof
InactiveCN105749273AHigh titer contentImmunization is convenient and fastViral antigen ingredientsAntiviralsImmune effectsAdjuvant
The invention relates to a multivalent vaccine for preventing and treating the infectious diseases of a pig, in particular to a combined vaccine for treating and preventing porcine circovirus type 2 and porcine parvovirus and a preparation method thereof. The multivalent vaccine selects and uses the porcine circovirus type 2 and the porcine parvovirus. The preparation method comprises the following steps of: culturing the porcine circovirus type 2, inactivating and concentrating; culturing the porcine parvovirus, inactivating and concentrating; mixing the two antigen components by proportion, and adding adjuvants to prepare into the vaccine. The bivalent vaccine prepared by the invention is convenient in use and is safer, and the immune effect is superior to that ofcombination of two single vaccines.
Owner:PU LIKE BIO ENG
Subunit vaccine of PPV (porcine parvovirus) disease and preparation method of subunit vaccine
The invention provides a subunit vaccine of PPV (porcine parvovirus) disease and a preparation method of the subunit vaccine. PPV virus-like particles are provided firstly and produced by steps as follows: optimized VP2 gene and IL-15 (interleukin-15) gene are subjected to fusion expression, and the PPV virus-like particles are produced by a baculovirus / insect cell expression system. The PPV virus-like particles are mixed with a preservative and an adjuvant, and the PPV disease subunit vaccine is prepared. The PPV disease vaccine has the advantages of high yield, low production cost, high immunogenicity, good safety and the like, facilitates large-scale production, and has good immunization and prevention effects on PPV disease.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Visible gene chip and kit for pig pseudorabies virus, porcine parvovirus and porcine circovirus type-II
ActiveCN104862423AObserve intuitivelyGrasp the infection situation quicklyMicrobiological testing/measurementMicroorganism based processesLivestock diseasePorcine parvovirus
The invention discloses a visible gene chip for a pig pseudorabies virus, porcine parvovirus and porcine circovirus type-II. The visible gene chip comprises a solid phase carrier and a probe fixed on the solid phase carrier, wherein the probe comprises gene segments as shown in SEQ ID NO:1, 3, and 4; and the gene segments are used for respectively detecting the pig pseudorabies virus, the porcine parvovirus and the porcine circovirus type-II. The invention further discloses a kit for detecting the pig pseudorabies virus, the porcine parvovirus and the porcine circovirus type-II. By adopting the gene chip and the kit disclosed by the invention, pathogens of the pig pseudorabies virus, the porcine parvovirus and the porcine circovirus type-II can be effectively detected, infection caused by vaccine strains and wild strains of the pig pseudorabies virus can be distinguished, the gene chip and the kit have the characteristics of good specificity, high sensitivity and long preservation period, are short in time, rapid in detection, visible in detection result observation and good in clinical application prospect, no expensive equipment is needed, and the infection situation of livestock diseases can be rapidly handled.
Owner:SICHUAN AGRI UNIV
Multi-nano-PCR primer group for detecting bovine rotavirus (BRV), bovine parvovirus (BPV) and bovine viral diarrhea virus (BVDV) and application of multi-nano-PCR primer group
ActiveCN110423845AShort stayAvoid crossbreedingMicrobiological testing/measurementAgainst vector-borne diseasesBovine rotavirusRotavirus RNA
The invention discloses a multi-nano-PCR primer group for detecting bovine rotavirus (BRV), bovine parvovirus (BPV) and a bovine viral diarrhea virus (BVDV) and application of the multi-nano-PCR primer group. The primer group contains dual-priming oligonucleotide (DPO) primer pairs used for detecting the BRV, the BPV and the BVDV correspondingly. A multi-DPO-nano-PCR detection method capable of simultaneously detecting the BRV, the BPV and the BVDV is established by combining DPO primers with a nano PCR technology. Compared with a conventional PCR method, the multi-DPO-nano-PCR detection method is time-saving and labor-saving, can quickly, accurately and specifically detect pathogens, and achieves high sensitivity on the basis of ensuring specificity. By providing the multi-nano PCR primergroup, the new method is provided for diagnosis of early infection and inapparent infection of the BRV, the BPV and the BVDV, a reliable technical means is provided for detection of clinical mixed infection of the BRV, the BPV and the BVD, and technical support is provided for epidemic disease testing, screening purification and comprehensive prevention and control.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
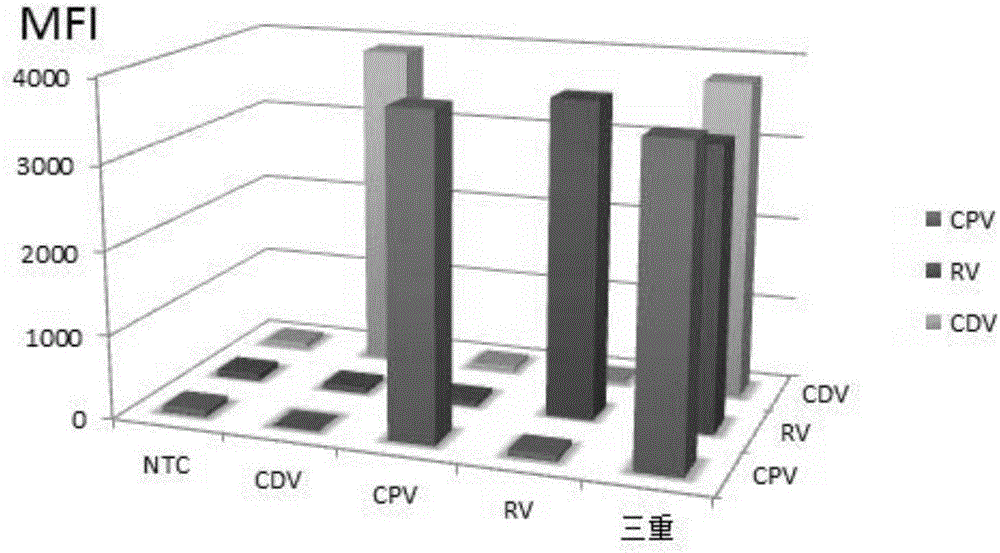
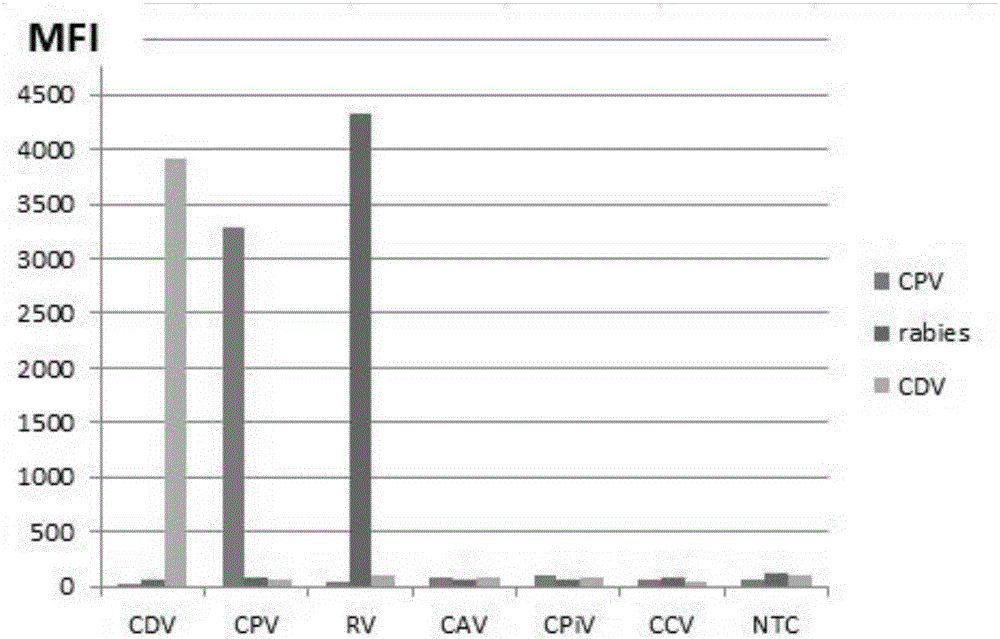
Multiple-FIA (fluorescence immunoassay) method and kit for quickly distinguishing CDV (canine distemper virus), CPV (canine parvovirus) and RV (rabies virus)
ActiveCN106319091AAccurate detectionReduce dosageMicrobiological testing/measurementMicroorganism based processesMicrosphereFluorescence
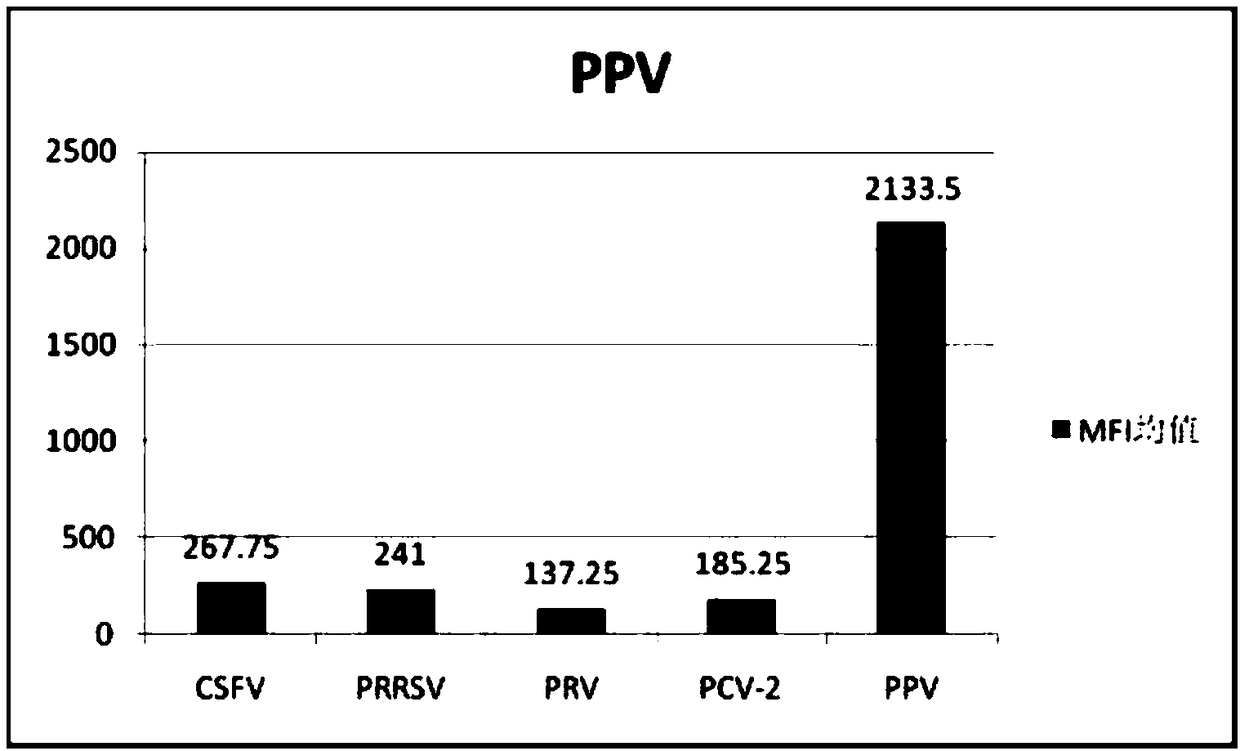
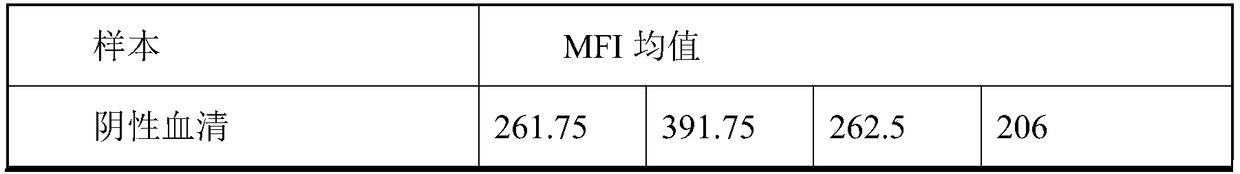
The invention discloses a multiple-FIA (fluorescence immunoassay) method and kit for quickly distinguishing CDV (canine distemper virus), CPV (canine parvovirus) and RV (rabies virus). The operation is simple, a target amplified fragment is obtained through a PCR (polymerase chain reaction), then an amplification product, fluorescence coded microspheres and streptavidin-phycoerythrin are hybridized, the MFI (mean fluorescence intensity) value is read through a detector, and different types of pathogens are distinguished. The method can be used for detecting and distinguishing the CDV, CPV and RV simultaneously, has the advantages of high specificity, high sensitivity, good repeatability and the like and can realize simultaneous detection of various different target molecules in the same sample. The method is good in flexibility, and the type of detected pathogens can be increased or decreased on the basis as required.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
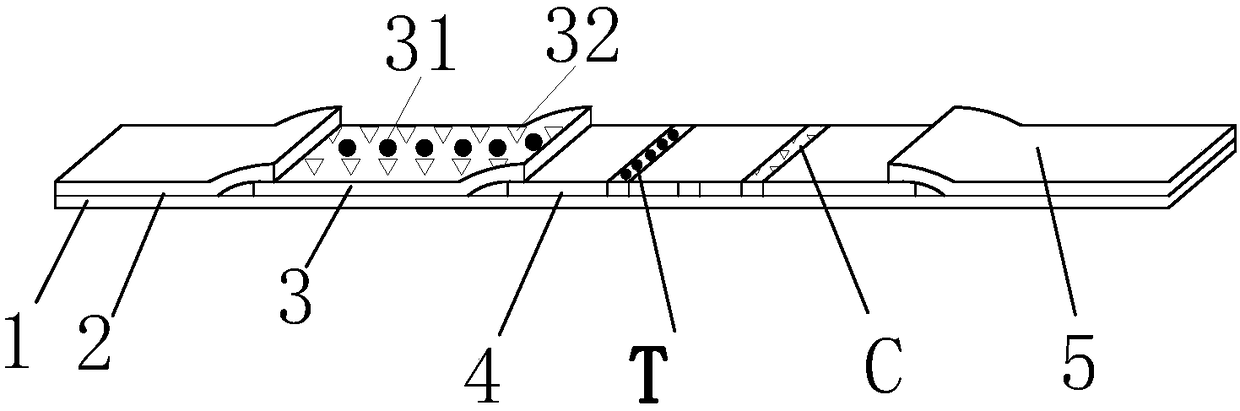
Immunofluorescence tomography detection card for detecting antigen of CPV (canine parvovirus) and preparation method
PendingCN108414753APreprocessing method optimizationEasy to operateMaterial analysisAntigenHigh density
The invention discloses an immunofluorescence tomography detection card for detecting antigen of CPV (canine parvovirus) and a preparation method, aims at providing the immunofluorescence tomography detection card for detecting the antigen of the CPV with the advantages of high density and reliable detection results, and belongs to the field of detection of animal epidemic diseases. The immunofluorescence tomography detection card is technically characterized by comprising a bottom board, wherein the bottom board is sequentially connected with a sample pad, a bonding pad, a nitrocellulose membrane and a water absorbing pad; a fluorescent microsphere-marked CPV monoclonal antibody and fluorescent microsphere-marked goat anti-chicken IgY are arranged on the bonding pad; a chicken IgY-coatedquality control line C, and a CPV monoclonal antibody-coated detection line T are arranged on the nitrocellulose membrane.
Owner:广州敏捷生物技术有限公司
Porcine parvovirus, porcine epizootic diarrhea and Escherichia coli triple-antigen vaccine
ActiveCN106039304AImproving immunogenicityPassive immunity is goodAntibacterial agentsSsRNA viruses positive-senseEscherichia coliMating
The invention provides porcine parvovirus, porcine epizootic diarrhea and Escherichia coli triple-antigen vaccine. The porcine parvovirus, porcine epizootic diarrhea and Escherichia coli triple-antigen vaccine contains antigens and vaccine adjuvants. The antigens are porcine parvovirus VP2 proteins and fimbrial antigens of porcine epizootic diarrhea viruses and Escherichia coli K88, K99 and 987P, the porcine parvovirus VP2 proteins are expressed by strains X33-VP2 with a preservation number of CCTCC M 2016098, and a preservation number of the porcine epizootic diarrhea viruses and the Escherichia coli K88, K99 and 987P is CGMCC No.8503. The porcine parvovirus, porcine epizootic diarrhea and Escherichia coli triple-antigen vaccine has the advantages that the porcine parvovirus, porcine epizootic diarrhea and Escherichia coli triple-antigen vaccine is good in immunogenicity, high in post-immunization antibody production rate, long in storage life and low in immunizing dose, produced antibodies are high in titer and long in hold time, the selected adjuvants are easy to inject, and three types of diseases can be prevented and treated by means of injection at one step; immune injection can be carried out prior to mating, accordingly, piglets laid by pregnant sow are excellent in passive immunity and firm in immunity and can resist virulent virus infection, and the survival rate of the piglets can be increased.
Owner:YEBIO BIOENG OF QINGDAO
Canine parvovirus resistant refined antibody and preparation method thereof
InactiveCN107304230AIncrease concentrationHigh puritySerum immunoglobulinsDigestive systemSide effectUltrafiltration
The invention discloses a canine parvovirus resistant refined antibody and a preparation method thereof. The preparation method comprises the following steps: S1, preparing canine parvovirus inactivated vaccines by taking canine parvovirus CPV-S5 and CPV-1401 separated and identified in Guangdong regions as basic strains; S2, preparing canine parvovirus hyper-immune serum from vaccine immunity healthy dogs; S3, separating canine parvovirus resistant immune globulin in the serum prepared in the S2 through saturated ammonium sulfate stepped salting-out; S4, purifying, namely desalting the canine parvovirus resistant immune globulin extracted in the step S3 through dextrangel; S5, performing ultrafiltration concentration on the canine parvovirus resistant immune globulin subjected to desalting treatment in the step S4, filtering to remove bacteria, and performing split charging, thereby obtaining the product. According to the canine parvovirus resistant refined antibody prepared by the invention, the protein content reaches 134mg / ml, and the hemagglutination inhibition titer is not lower than 1:10240; and the canine parvovirus resistant refined antibody can be applied to treatment and emergency prevention when dogs of different varieties are in contact infection with the canine parvovirus, is capable of increasing the organism immunity and resistance of ill dogs, does not cause any adverse reaction, does not have any clinical side effect, has high safety and has excellent popularization and application prospects.
Owner:SOUTH CHINA AGRI UNIV +1
Kit capable of quickly detecting classical swine fever virus/porcine reproductive and respiratory syndrome virus/pseudorabies virus/porcine parvovirus
ActiveCN105734172AEasy to operateShort timeMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
The invention relates to a kit capable of quickly detecting classical swine fever virus / porcine reproductive and respiratory syndrome virus / pseudorabies virus / porcine parvovirus, in particular to a multiplex real-time fluorescence polymerase chain reaction technology capable of detecting CSFV (classical swine fever virus), PRRSV (porcine reproductive and respiratory syndrome virus), PRV (pseudorabies virus) and PPV (porcine parvovirus) simultaneously.The kit mainly comprises a RT-PCR (reverse transcription-polymerase chain reaction) mixture, primer probe mixed liquor, an RT-PCR enzyme system and DEPC (diethyl pyrocarbonate) H2O as well as packaging boxes for packaging reagent bottles or tubes separately and in a centralized manner.Through multiple fluorescence channels for separate detection, the kit applying a one-step real-time fluorescence PCR mode is capable of detecting and identifying the classical swine fever virus, the porcine reproductive and respiratory syndrome virus, the pseudorabies virus and the porcine parvovirus quickly and accurately and can be widely applied to multiple fields such as early clinical diagnosis of porcine reproductive disturbance diseases, port inspection and quarantine, plague prevention and scientific research.
Owner:DAAN GENE CO LTD
Purification method of virus-like particles of porcine parvovirus and application of purification method
ActiveCN110283236AHigh purityHigh recovery rateViral antigen ingredientsVirus peptidesPurification methodsActive protein
The invention discloses a purification method of virus-like particles of porcine parvovirus and an application of the purification method, and belongs to the field of separation and purification technology of biological products. The method comprises the following steps: clarifying a culture of expressed virus-like particles of the porcine parvovirus, collecting supernatant, and performing inactivation with pyrrole to obtain a semi-finished product; regulating the pH value of the semi-finished product to be smaller than 7; adding the regulated semi-finished product to a weak anion exchanging column balanced by a first buffer solution, eluting the weak anion exchanging column with a second buffer solution, and collecting peak components; determining active protein components of each peak by polyacrylamide gel electrophoresis. The purity of the virus-like particles of the porcine parvovirus obtained with the purification method is up to 90%, and recovery rate is up to 99%. The method is conductive to production and operation, and a safer and more reliable way is provided for preparing inactivated vaccine for rhabdovirus vectors of the porcine parvovirus.
Owner:扬州优邦生物药品有限公司
Liquid phase chip detection method for porcine parvovirus
InactiveCN108918870ASave time and costHigh sensitivityBiological material analysisBiological testingBiotin-streptavidin complexAntigen
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Single-chain antibody used for detecting canine parvovirus (CPV)
InactiveCN108623678AImprove detection accuracyEasy to getBiological material analysisImmunoglobulins against virusesSingle-Chain AntibodiesVirus-like particle
Disclosed is a single-chip antibody used for detecting canine parvovirus (CPV). The single-chip antibody is characterized by establishment of a CPV-VLP (virus like particles) resistant single-chain antibody library, panning of a specific CPV-VLP resistant single-chain antibody and expression of the specific CPV-VLP resistant single-chain antibody. Compared with the prior art, the single-chain antibody has the advantages of high detection accuracy, simple acquisition and the like.
Owner:NORTHWEST A & F UNIV
Multiple real time fluorescence quantifying PCR method for detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and classical swine fever virus
InactiveCN101260442BSimple and fast operationHigh sensitivityMicrobiological testing/measurementClassical swine fever virus CSFVFluorescence
The invention discloses a multiplex real-time fluorescent quantitation PCR method capable of detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and hog cholera virus at the same time. The method of the invention detects that the four virus are all in good linear relations at the same time, all ten times serial dilution points of constructed normal plasmid are all in one straight line, CT value and copy number are in good linear relation, and regression analysis shows that the related coefficient of the CT value and the copy number is R<2> more than 0.99.The multiplexreal-time fluorescent quantitation PCR method has the advantages of excellent specificity, sensibility and stability, which can rapidly, sensitively and differentially detect the four virus with serious harm to the economy and can be used for early diagnosis of virus infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com