Porcine parvovirus L strain and use thereof in preparation of porcine parvovirus inactivated vaccines
A parvovirus, inactivated vaccine technology, used in antiviral agents, viruses/phages, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
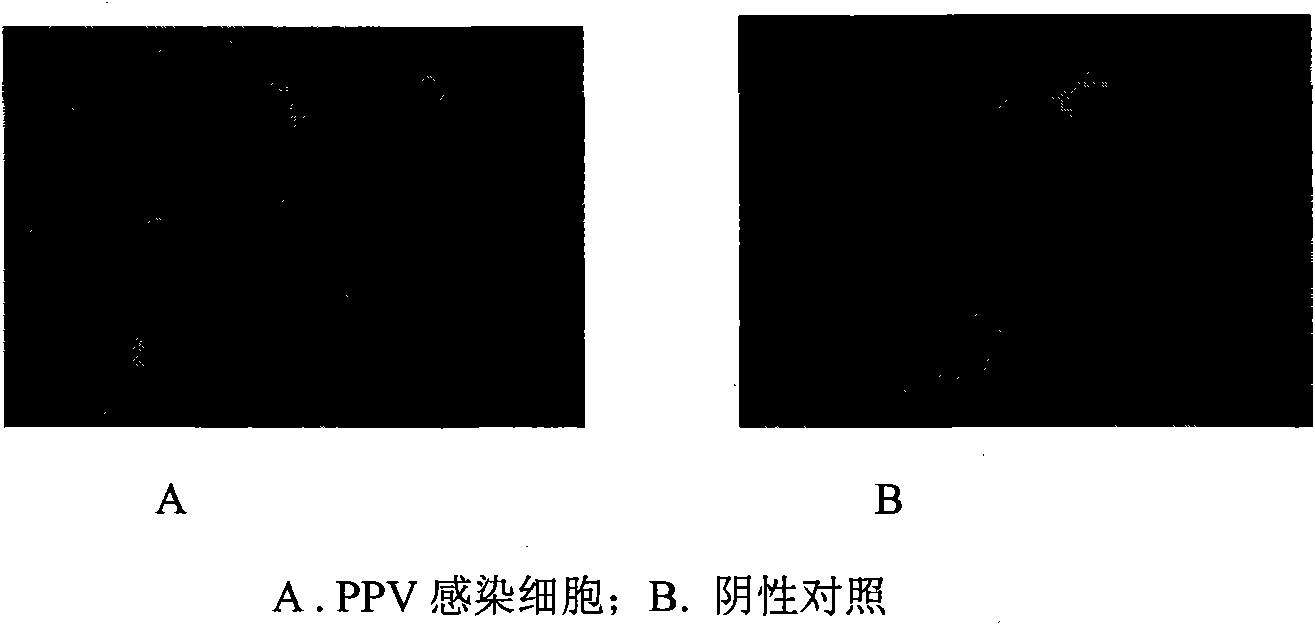
[0027] The present invention will be further described below in conjunction with specific embodiments, and the advantages and characteristics of the present invention will become clearer along with the description. However, these embodiments are only exemplary and do not constitute any limitation to the scope of the present invention. Those skilled in the art should understand that the details and forms of the technical solutions of the present invention can be modified or replaced without departing from the spirit and scope of the present invention, but these modifications and replacements all fall within the protection scope of the present invention. Example 1 Isolation, cultivation and identification of porcine parvovirus L strain
[0028] 1. Breeding method
[0029] 1. Collection of disease materials: Aseptically collect the liver, mesenteric lymph nodes, kidneys, brain and other tissues of stillborn sows. Add sterilized saline at 1:10, grind to prepare tissue suspension...
Embodiment 2
[0052] Example 2 Preparation of Porcine Parvovirus Inactivated Vaccine
[0053] 1. Preparation of virus liquid for seedling production: insert 0.5% of the virus culture liquid into the ST cell culture that has not yet formed a monolayer for the production of seed virus, put 37 ° C for rotary culture, and when the lesion reaches 70%, harvest the virus containing Toxic cell culture medium, after 3 times of freezing and thawing, the poison was collected.
[0054] 2. Inactivation: 0.02% of the total amount of virus liquid was added to the virus liquid with divinylimine solution, shaken fully, inactivated on a shaker at 30°C and 100r / min for 72 hours, and then added 1% sodium thiosulfate solution , to terminate the inactivation. And for sterility test.
[0055] 3. Concentration: Take the inactivated virus liquid, centrifuge continuously at 13000r / min, and then filter with a 0.2um membrane filter. The virus liquid after microfiltration was concentrated 5 times by ultrafiltration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com