Protective agent for foot-and-mouth disease inactivated virus and preparation method of microcapsule vaccine
A technology of foot-and-mouth disease virus and foot-and-mouth disease, which is applied in the direction of antiviral agents, microcapsules, and capsule delivery, can solve problems such as lack of methods, and achieve the effects of simple formula, improved stability, and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of foot-and-mouth disease microencapsulated vaccine of the present invention
[0038] 1 Preparation of inactivated antigen
[0039] 1.1 Preparation of virus liquid
[0040] 1.1.1 Preparation of type A foot-and-mouth disease virus solution
[0041] 10000L bioreactor full suspension culture of BHK-21 cells, the cell density reaches 3-5×10 6 Inoculate the cell-adapted strain of foot-and-mouth disease virus (bovine foot-and-mouth disease virus A / AKT-Ⅲ strain, prepared by Inner Mongolia Biwei Antai Biotechnology Co., Ltd.) according to the virus multiplicity of infection MOI 0.01-0.1, prepare the virus stock solution, and the stirring speed does not exceed 40rpm , culturing for 8-12h to harvest virus fluid.
[0042] 1.1.2 Preparation of O-type foot-and-mouth disease virus solution
[0043] 10000L bioreactor full suspension culture of BHK-21 cells, the cell density reaches 3-5×10 6 1 / ml, inoculate a foot-and-mouth disease virus cell-adapted strain (...
Embodiment 2
[0068] Embodiment 2 Immune effect monitoring of foot-and-mouth disease microcapsule vaccine of the present invention
[0069]The oil adjuvant vaccine prepared in Example 3.1 and the foot-and-mouth disease microcapsule vaccine prepared in Example 2.1 were immunized with a single dose of 30-45 kg, respectively, and 8 pigs were negative for FMDV antibody. 4 weeks, 8 weeks, 10 weeks, 12 weeks, 14 weeks, 18 weeks, and 20 weeks after immunization, respectively. FMDV antibody titers were determined by ELISA.
[0070] As a result, single-dose (conventional 146s content of 2-6 μg / mL) immunized pigs with foot-and-mouth disease microencapsulated vaccine, and 4-fold dose (ie, 4 times the single dose) orally immunized pigs had FMDV antibody efficacy after 8 weeks. The titer of the FMDV antibody can reach 1:1024, and the oil adjuvant vaccine can not achieve the FMDV antibody titer until 12 weeks and 16 weeks. Therefore, the foot-and-mouth disease microcapsule vaccine of the present inventi...
Embodiment 3
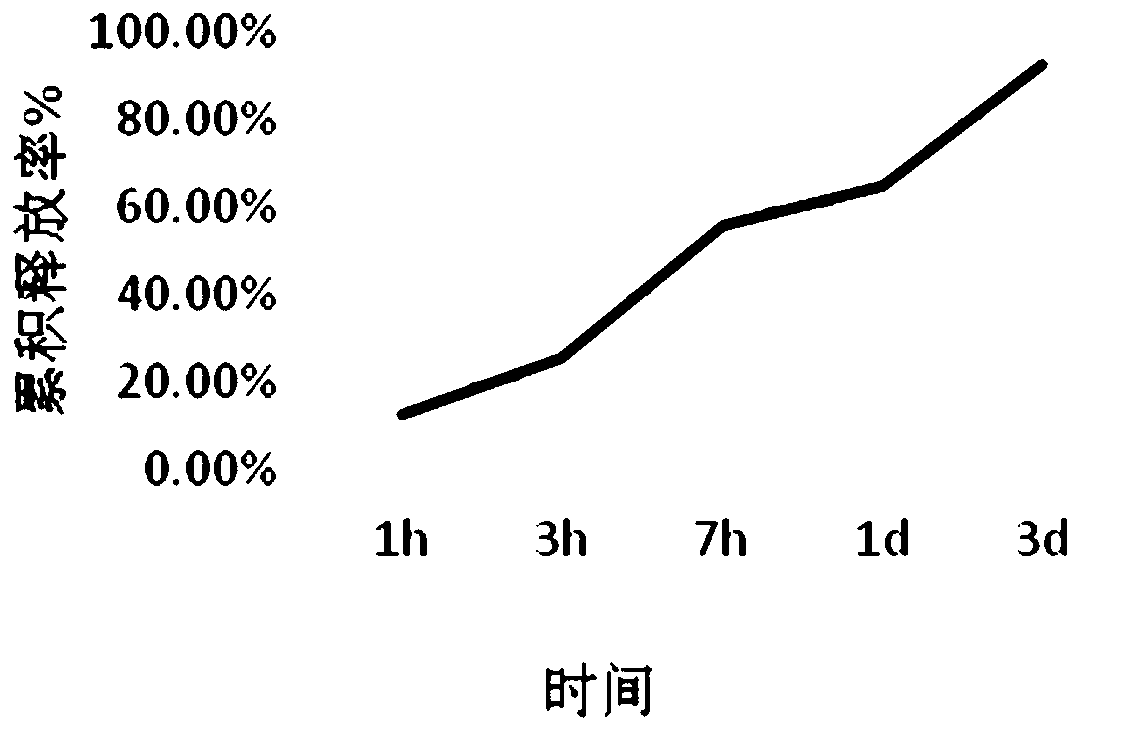
[0083] Embodiment 3 Embedding rate and release effect monitoring of foot-and-mouth disease microcapsules
[0084] 3.1 Determination of the encapsulation rate of foot-and-mouth disease microcapsule vaccine
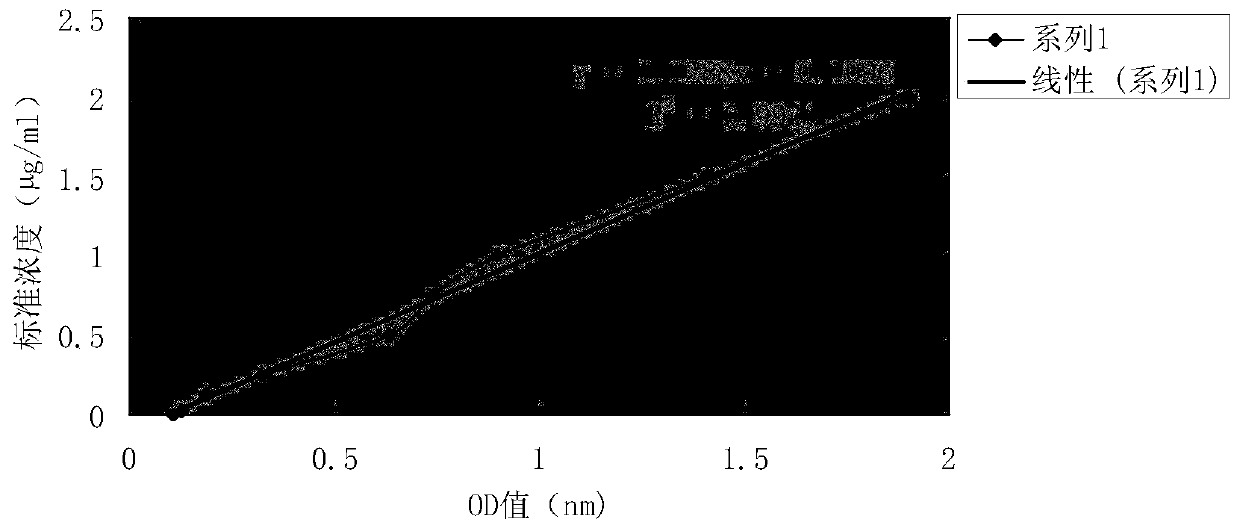
[0085] Accurately weigh 0.01 g of the foot-and-mouth microcapsule vaccine prepared in Example 1, dissolve it in sterilized water filtered by a Φ0.22 μm membrane, centrifuge at a low speed of 800 rpm for 10 min, discard the supernatant, and wash away the uncoated antigen; the remaining product Precise volume into a 25mL volumetric flask. The protein content was determined by the standard curve method with the BCA protein kit (refer to the kit instructions for specific operation steps), and the encapsulation rate of the microcapsules was calculated. Calculated as follows:
[0086] Microcapsule entrapment rate (%) = (total protein content - unencapsulated protein content) / total protein content × 100%
[0087] see standard curve figure 1 , after calculation, the embedding e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| embedding rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com