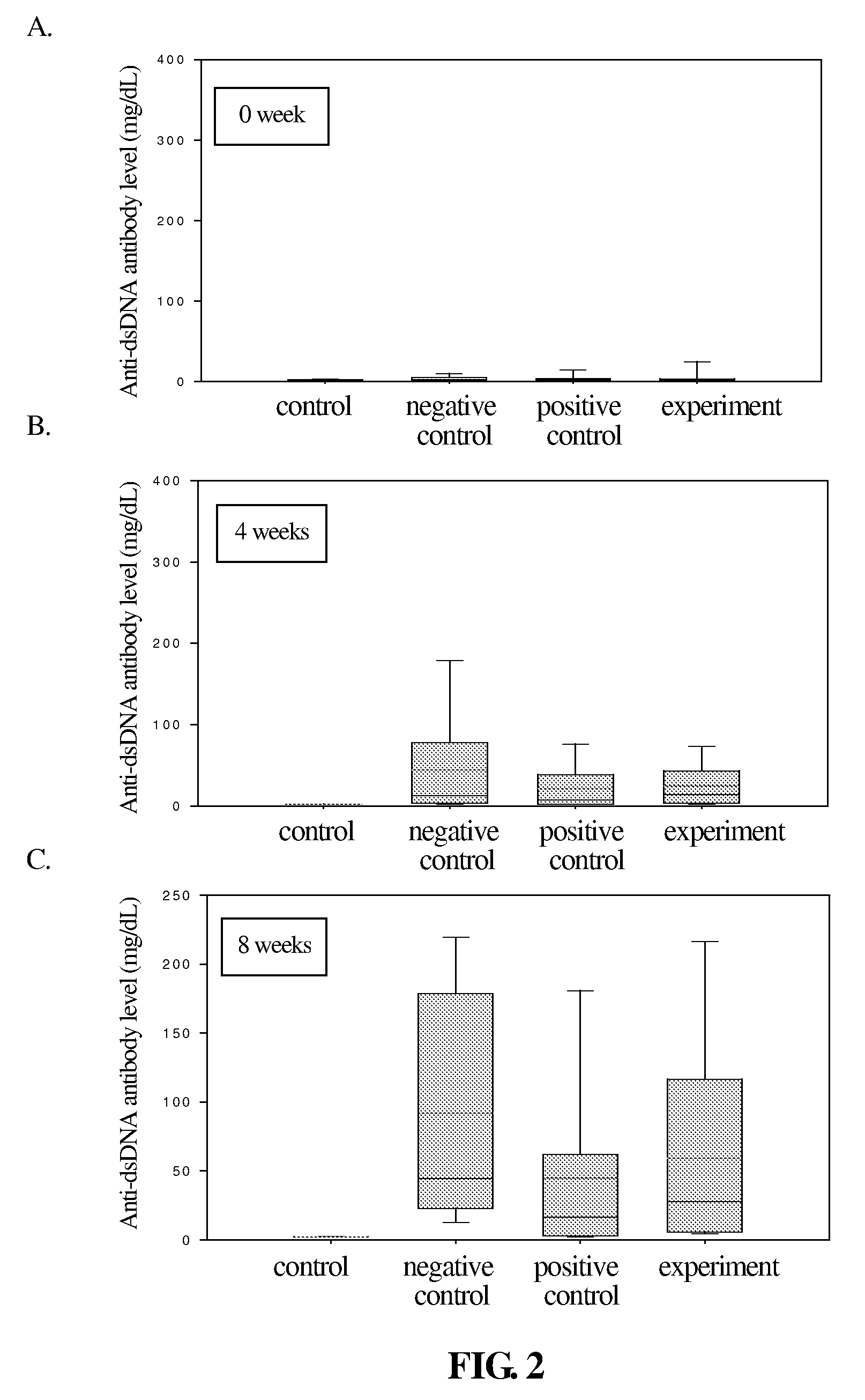
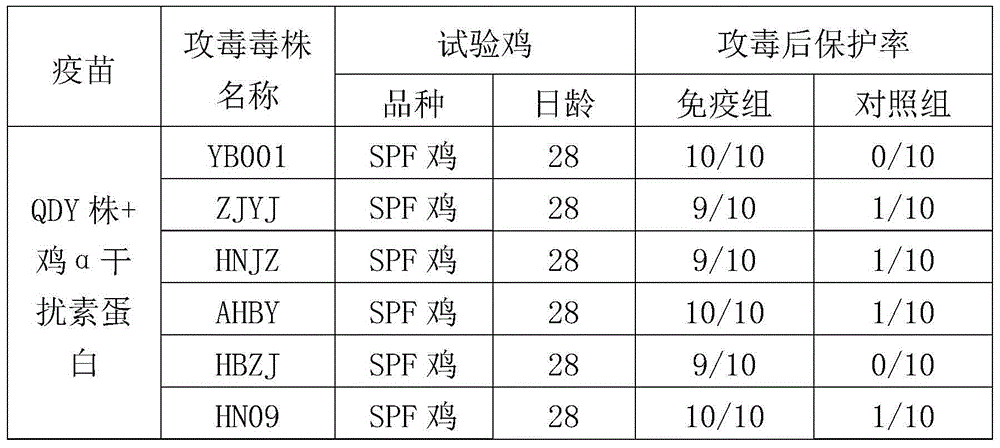
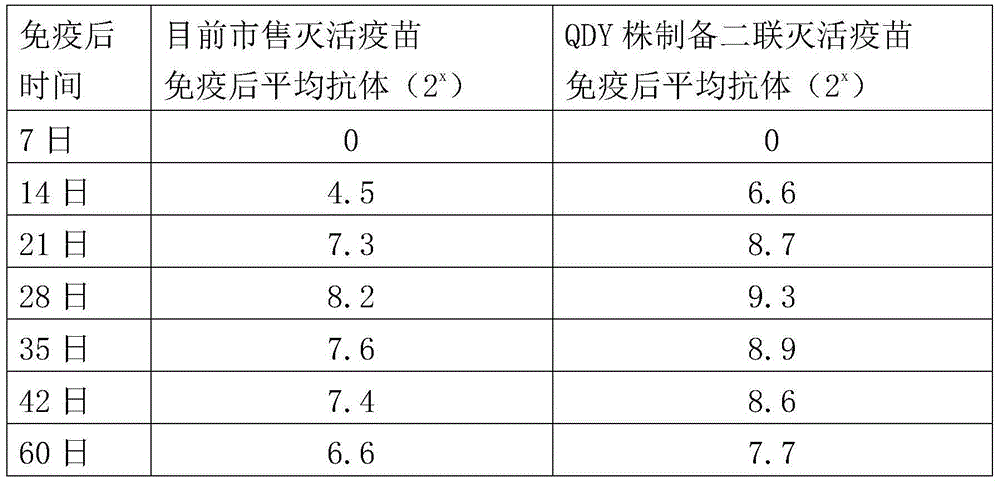
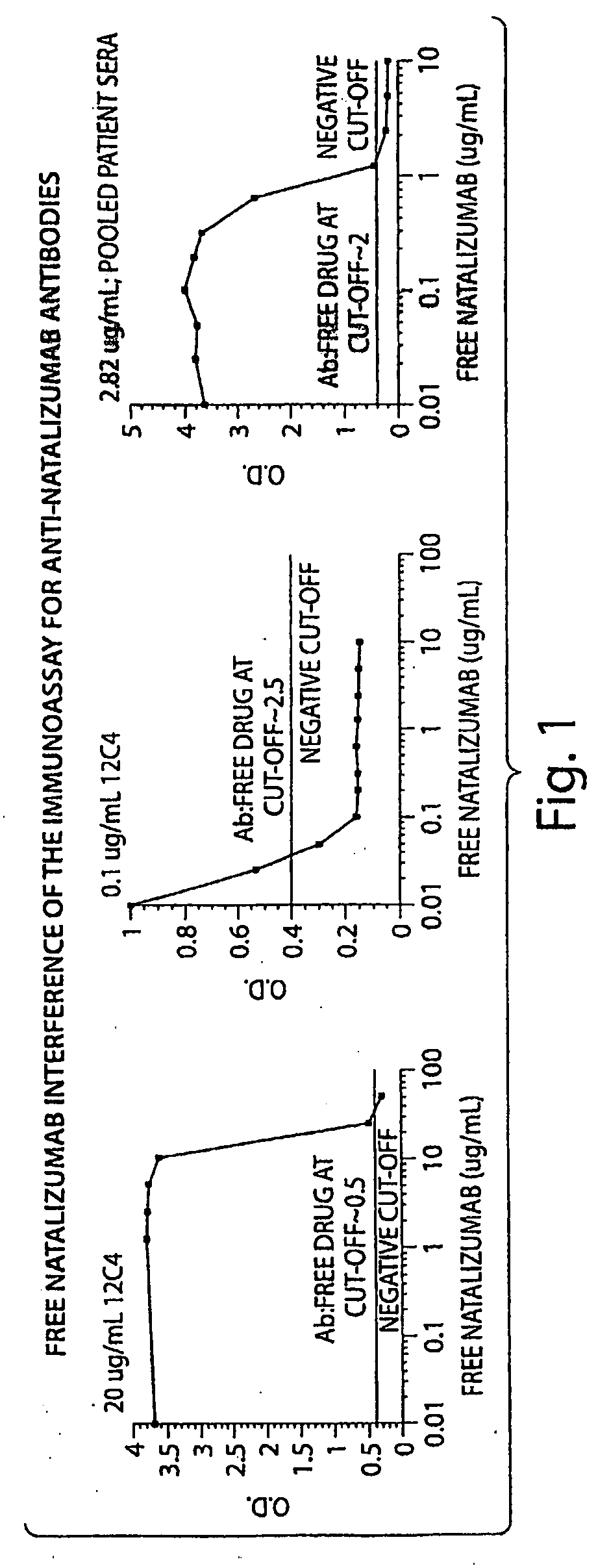
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
491 results about "Antibody titer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antibody titer is a measurement of how much antibody an organism has produced that recognizes a particular epitope, expressed as the inverse of the greatest dilution (in a serial dilution) that still gives a positive result. ELISA is a common means of determining antibody titers.
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Staphylococcal immunotherapeutics via donor selection and donor stimulation
InactiveUS20060222651A1Immunoglobulins against bacteriaPharmaceutical delivery mechanismPassive ImmunizationsCell-Extracellular Matrix
A method and composition for the passive immunization of patients infected with or susceptible to infection from Staphylococcus bacteria such as S. aureus and S. epidermidis infection is provided that includes the selection or preparation of a donor plasma pool with high antibody titers to carefully selected Staphylococcus adhesins or MSCRAMMs, or fragments or components thereof, or sequences with substantial homology thereto. The donor plasma pool can be prepared by combining individual blood or blood component samples which have higher than normal titers of antibodies to one or more of the selected adhesins or other proteins that bind to extracellular matrix proteins, or by administering carefully selected proteins or peptides to a host to induce the expression of desired antibodies, and subsequently recovering the enhanced high titer serum or plasma pool from the treated host.
Owner:PATTI JOSEPH +2
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic polypeptide
InactiveCN103509107AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansPenicillinKeyhole-limpet haemocyanin
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic polypeptide. The method comprises the following steps: synthesizing a "CGAGAGSGAGAGS" polypeptide sequence by utilizing an Fmoc method, coupling the polypeptide with keyhole limpet hemocyanin (KLH) through the cysteine on the N terminus of the polypeptide so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain a primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antibody titer in the rabbit blood sample reaches 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate.
Owner:ZHEJIANG UNIV +1
ELISA kit for detecting titer of novel coronavirus neutralizing antibody
ActiveCN111781354AThe pre-processing process is simpleShort timeImmunoassaysElisa kitHorseradish peroxidase
Owner:ACROBIOSYSTEMS INC
Myeloid derived suppressor cell inhibiting agents
ActiveUS20120156280A1Improve immune activityImproved therapeutic preparationSnake antigen ingredientsAntibody ingredientsAdjuvantCcr2 antagonist
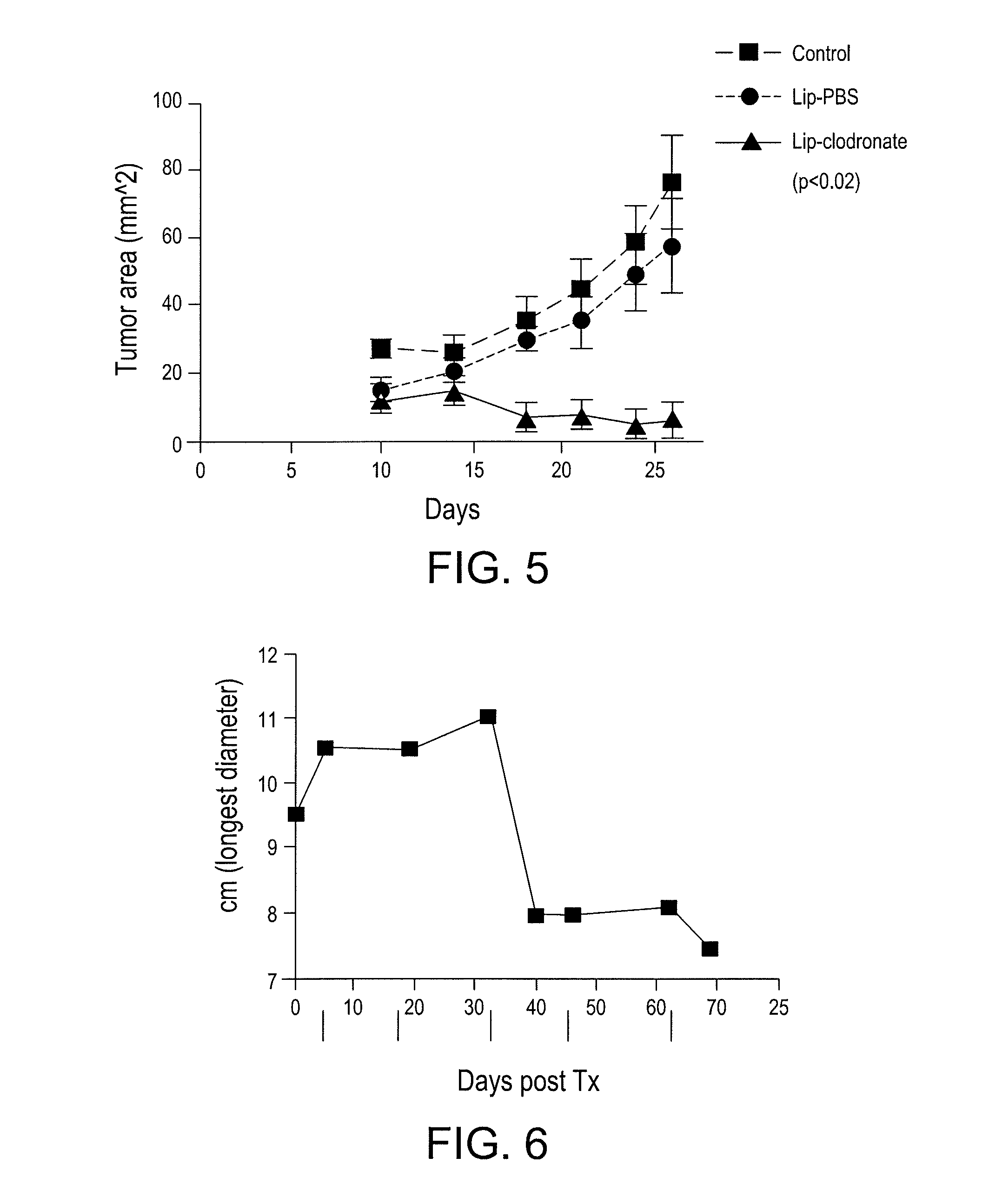
Myeloid derived suppressor cell (MDSC) inhibitory agents and vaccine and / or adjuvant enhancers are provided. Improved vaccine treatment regimens employing these agents are also provided. Cancer vaccines and methods for inhibiting tumor growth and cancer metastases are also presented. The myeloid derived suppressor cell (MDSC) inhibiting agents are described as bisphosphonates (such as liposomal clodronate) and CCR2 inhibitors and / or CCR2 antagonists. Methods for enhancing antibody titer levels in response to an antigen of interest are also provided.
Owner:COLORADO STATE UNIVERSITY
Mature method for in vitro culture of porcine oocyte
The invention provides a method for in vitro culture of porcine oocytes, which comprises the steps of ovary acquisition, acquisition of oocytes from an ovary, screening of the oocytes, and maturation culture. The method is characterized in that the acquired ovary is stored in a container of physiological saline for preservation before the step of the acquisition of the oocytes; and the temperature in the container is maintained to between 32 and 34 DEG C; an egg washing liquid adopted in the step of the screening of the oocytes contains amphotericin B with a concentration of between 1 and 10mu g / mL, and serum with a volume percentage of between 2 and 20 percent v / v; and the maturation medium adopted in the step of the maturation culture of the oocytes contains a TCM-199[1X] basic medium with a volume percentage of between 50 and 90 percent v / v, hGG with an antibody titer of between 5 and 25IU / mL and PMSG with an antibody titer of between 5 and 20IU / mL. The method can solve the problems that the prior in vitro maturation technology of the porcine oocytes has long period, is not good for mass production, and has low maturation rate after the culture.
Owner:深圳华大基因农业控股有限公司
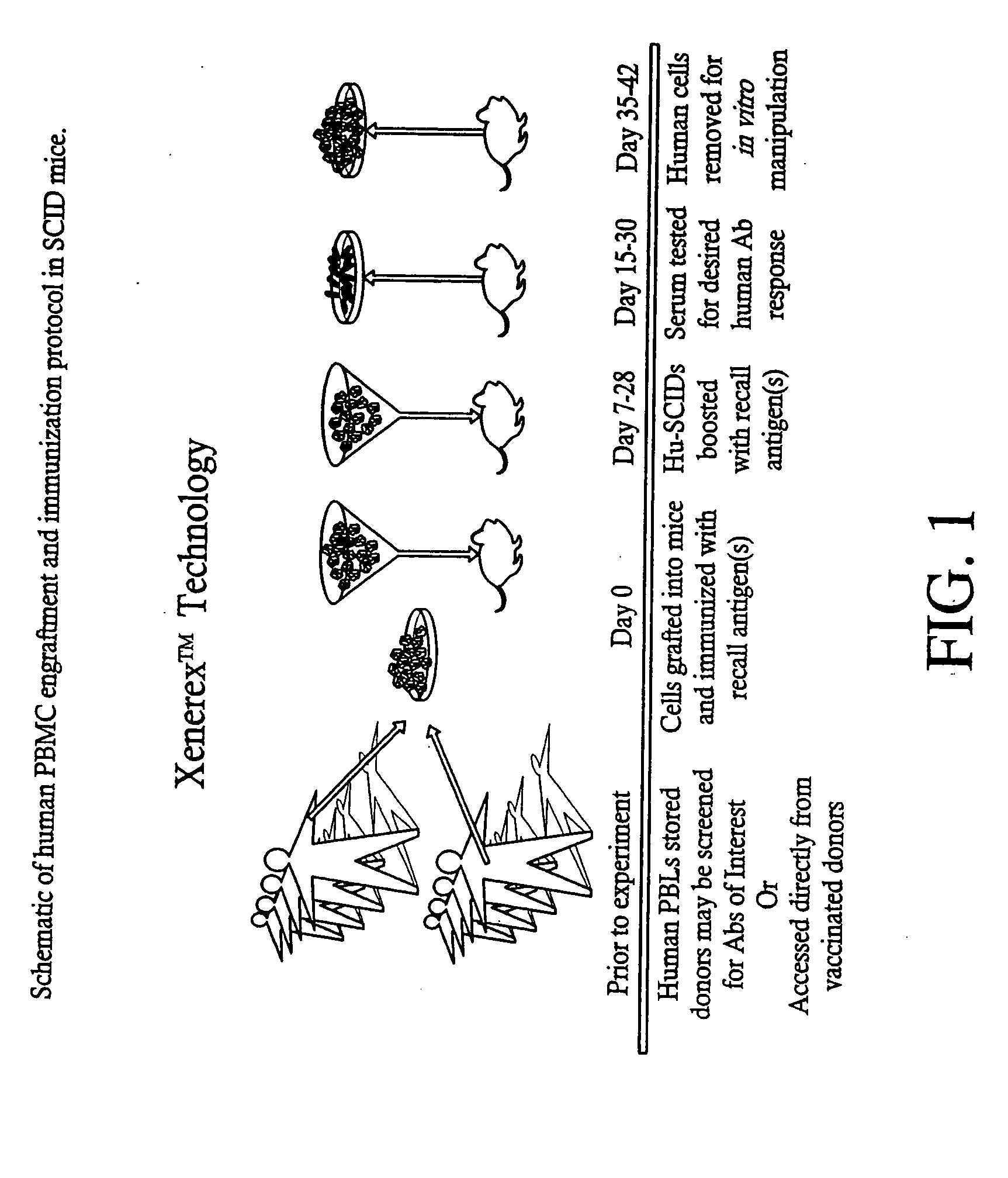
Neutralizing high affinity human monoclonal antibodies specific to RSV F-protein and methods for their manufacture and therapeutic use thereof
InactiveUS20020001798A1Simple methodPromote formationSsRNA viruses negative-sensePeptide/protein ingredientsF proteinRSV Infections
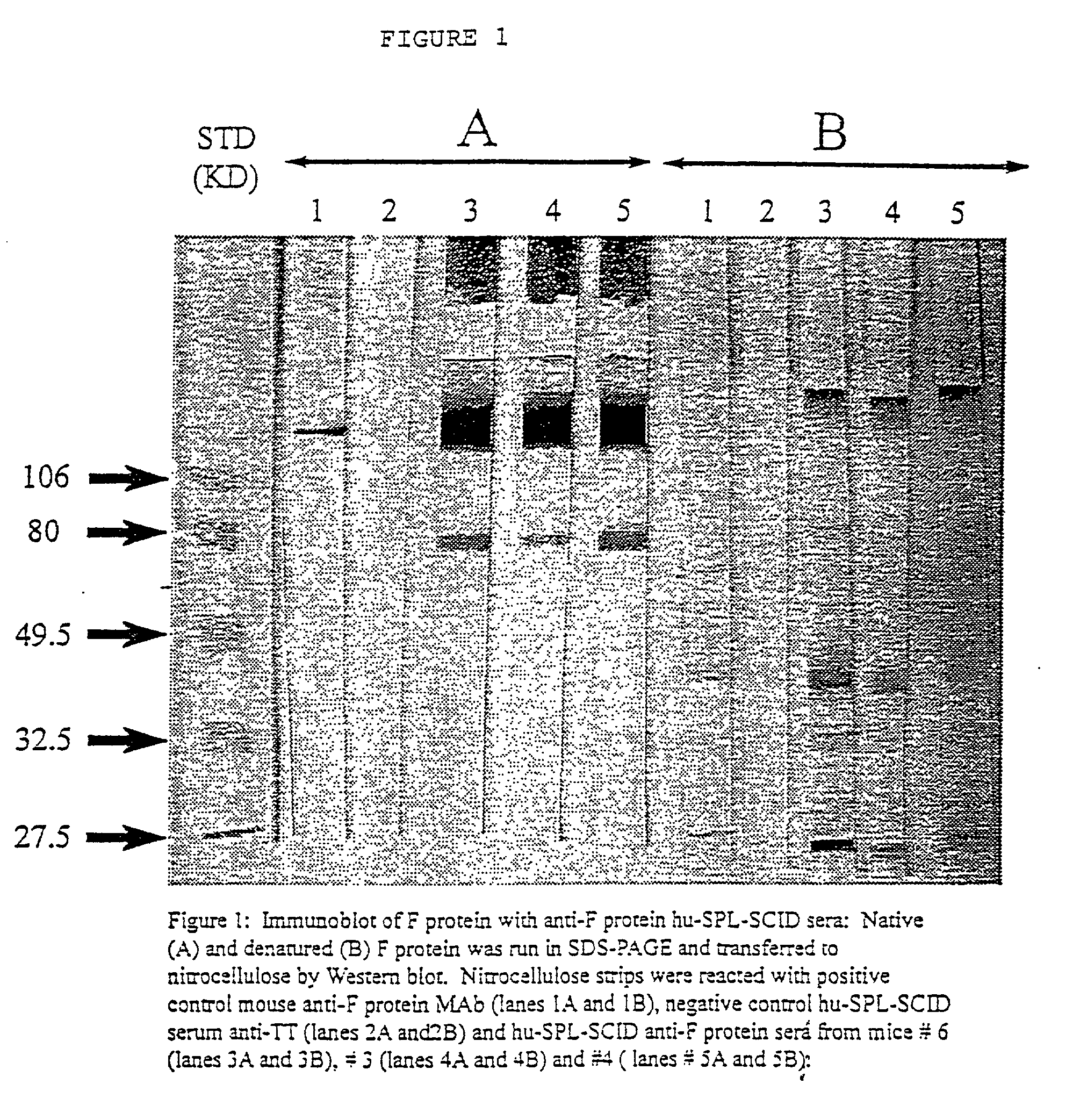
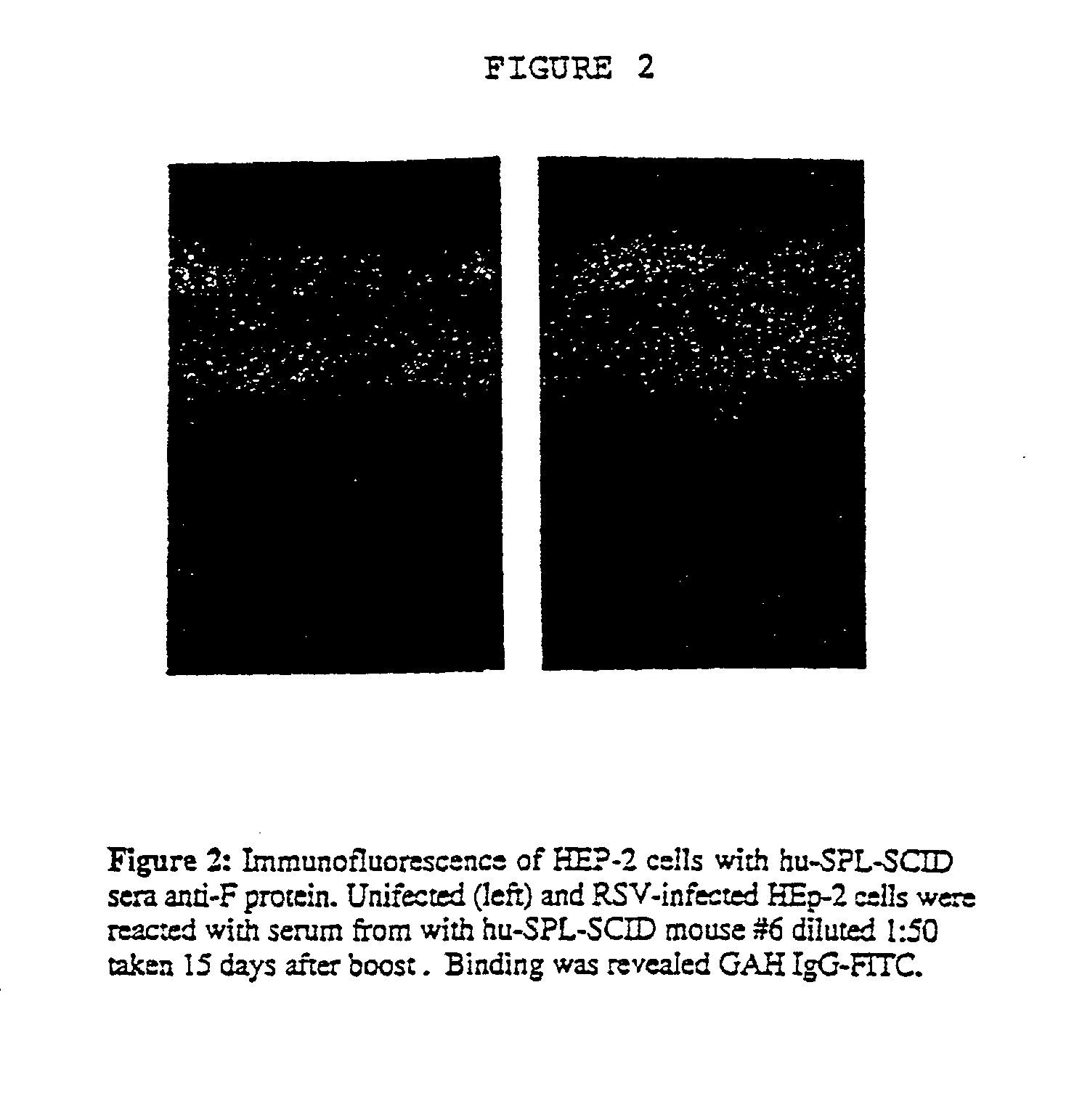
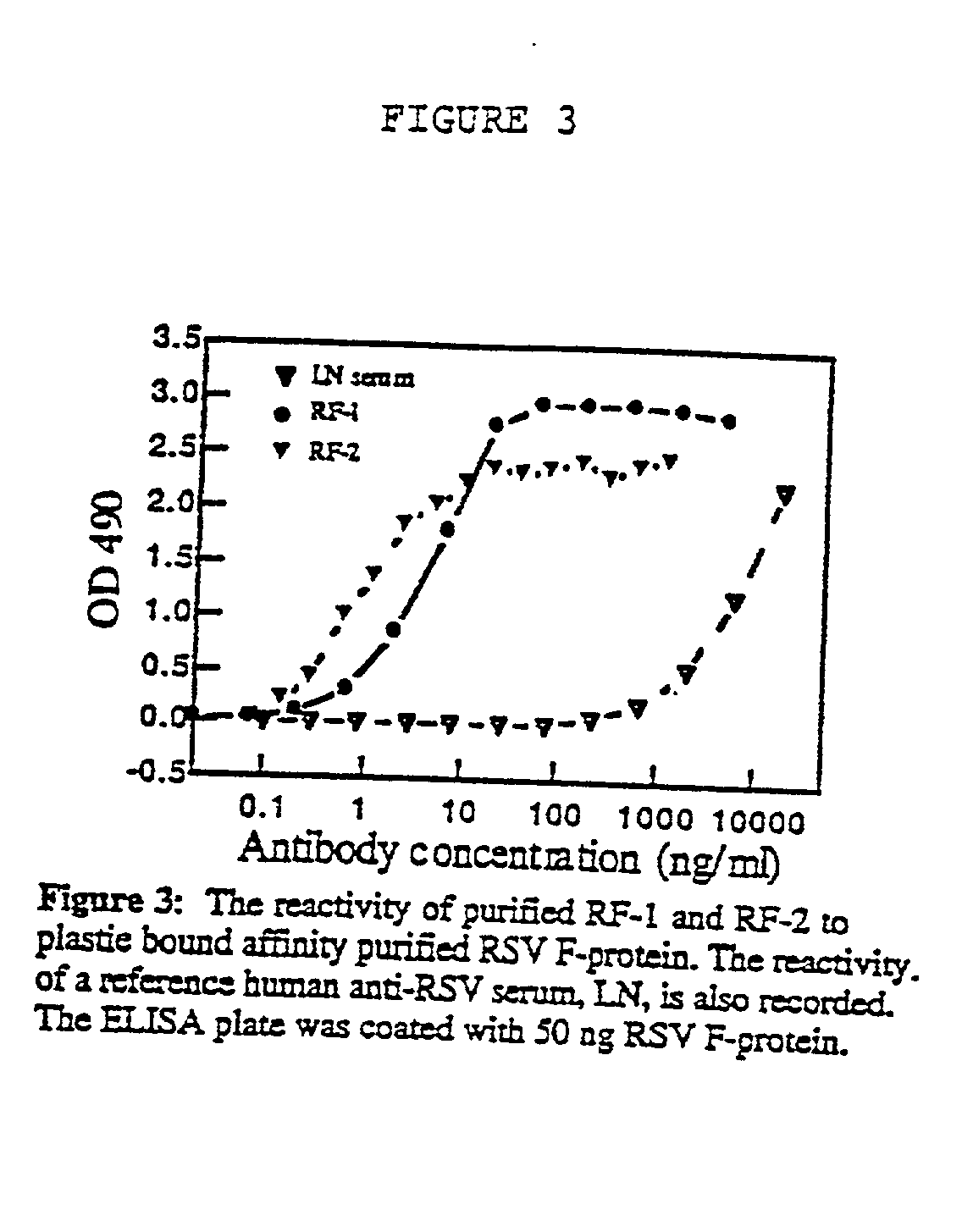
A highly efficient method for generating human antibodies in particular which are specific to be RSV fusion protein which combines in vitro primary of human spleen cells and antigen boosting in SCID mice is taught. This method provides for very high human antibody titers which are predominantly of the IgG isotype which contain antibodies of high specificity and affinity to desired antigens. This method is well suited for generating human monoclonal antibodies for therapeutic and diagnostic applications as well as for rescue of human cells for generation of combinational human antibody gene libraries. Two human monoclonal antibodies, RF-1 and RF-2 which each possess an affinity for RSV F-protein <=2x10--9 Molar are taught as well as their corresponding amino acid and DNA sequences. These antibodies are to be used therapeutically and prophylactically for treating or preventing RSV infection, as well as for diagnosis of RSV in analytes.
Owner:XENEREX BIOSCI +1
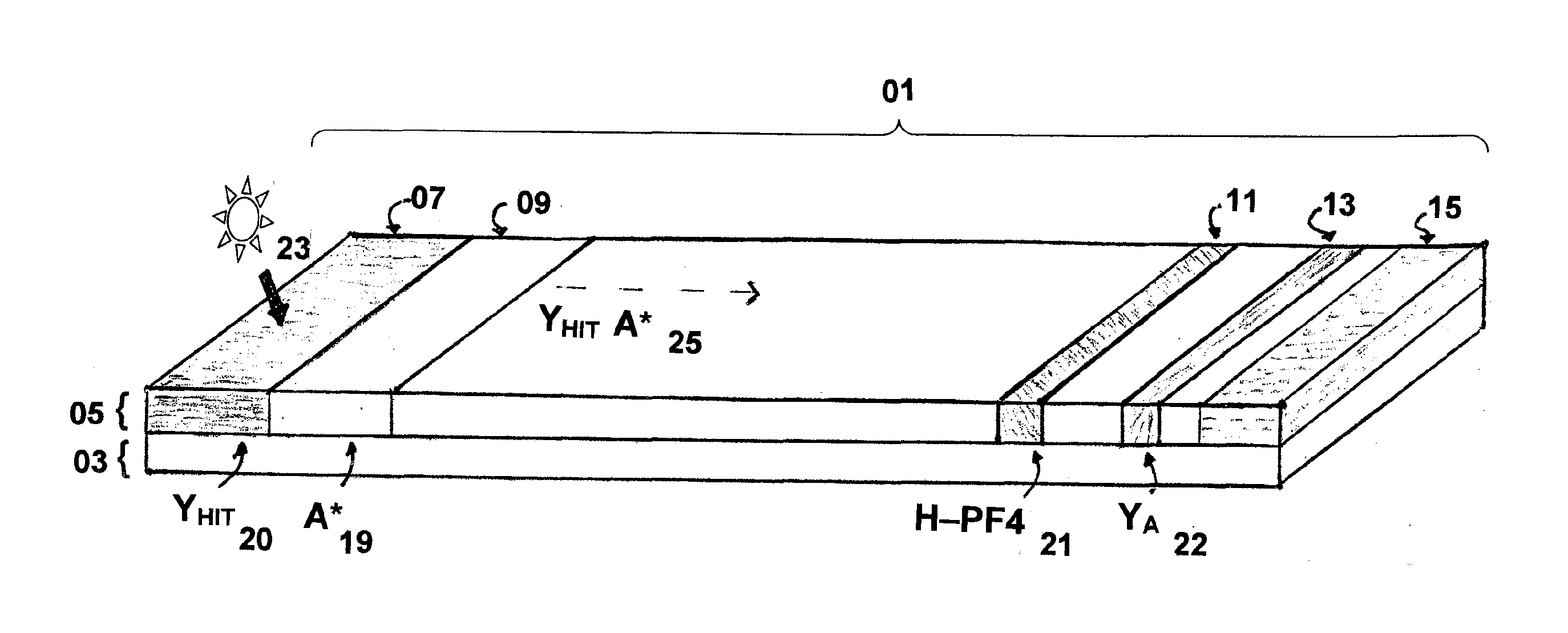
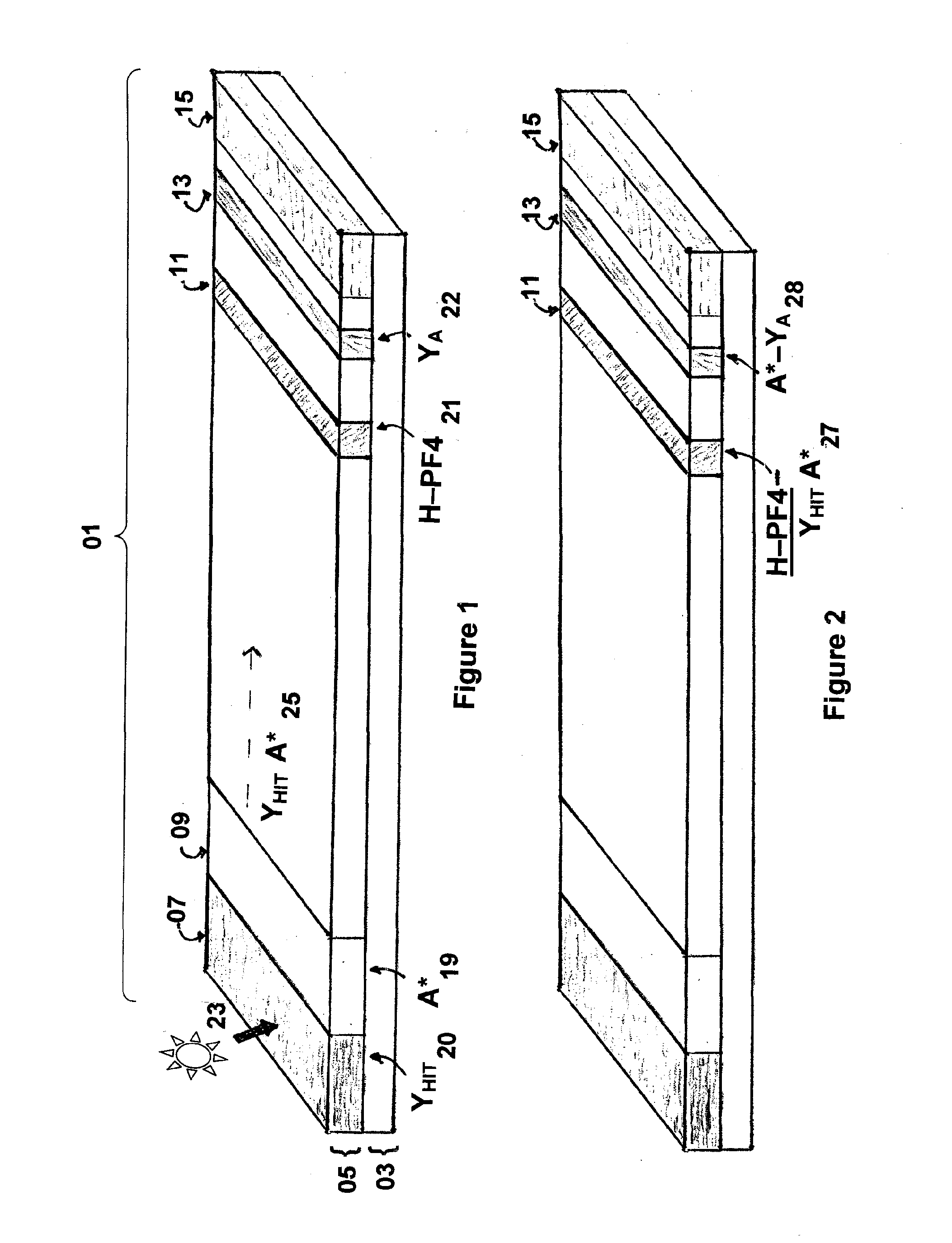
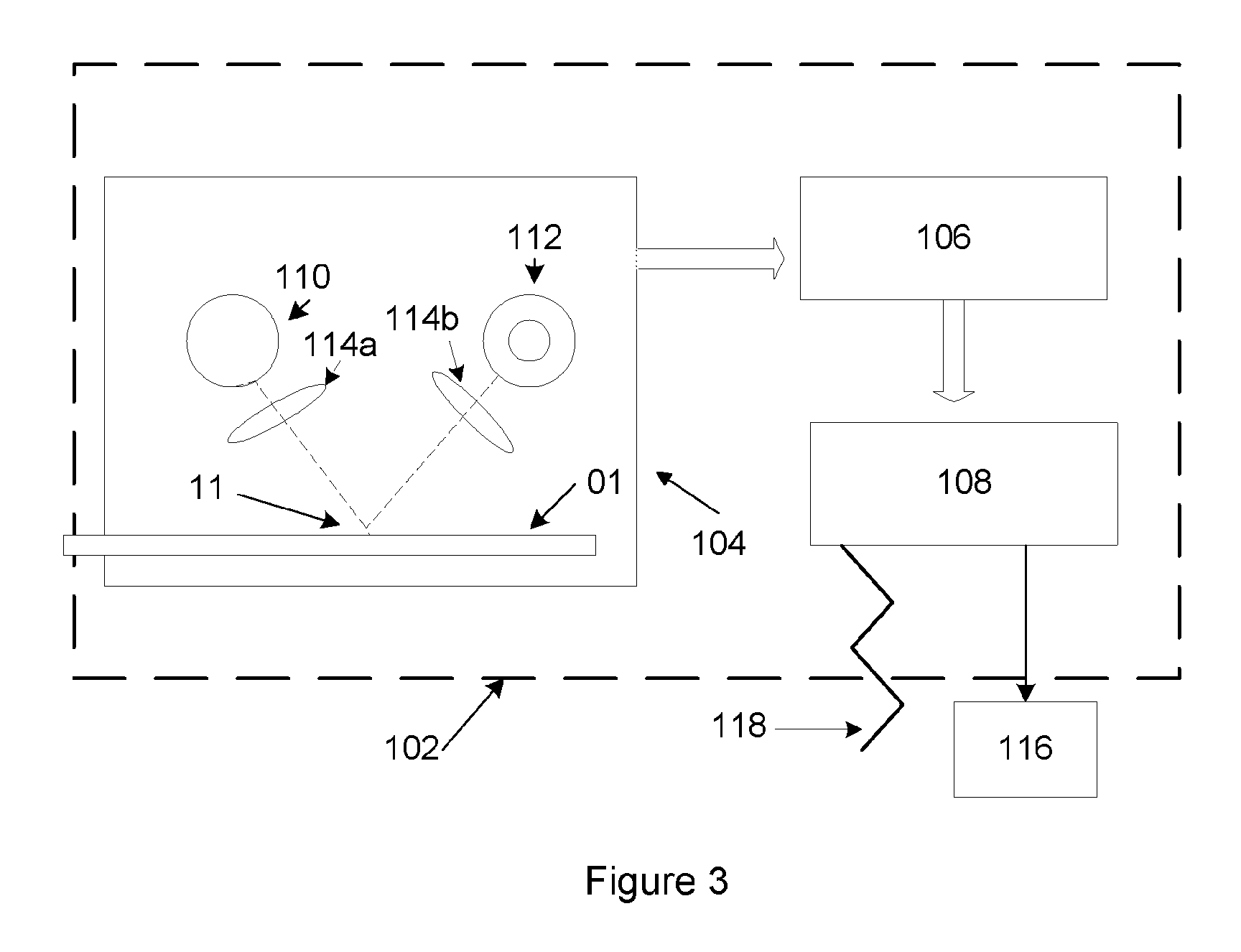
Rapid and sensitive method for quantitative determination of the level of heparin - pf4 complex induced immunoglobulin antibodies
ActiveUS20100255510A1Bioreactor/fermenter combinationsBiological substance pretreatmentsImmuno assayQuantitative determination
Disclosed herein is a lateral flow immuno-assay system capable of rapidly, cost effectively, and quantitatively detecting and assessing the level of HIT antibodies in body fluids of a patient. Also taught are methods for employing the system to assist in diagnosis of HIT, and for screening or detecting a changing titer of HIT antibodies in the body fluids of a patient to determine susceptibility toward HIT.
Owner:BIOMEDOMICS
Detection, prevention, and treatment systems for anthrax
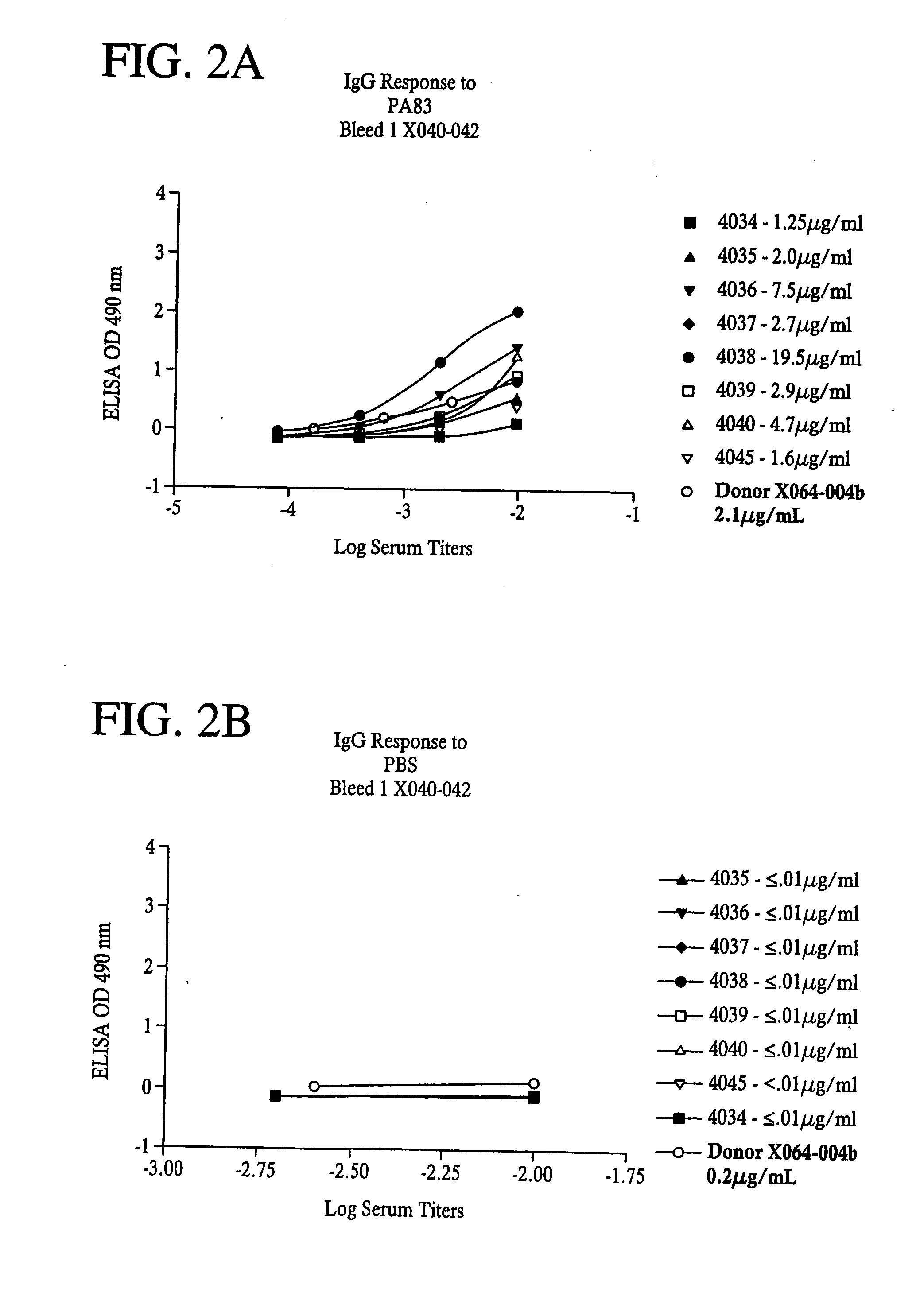
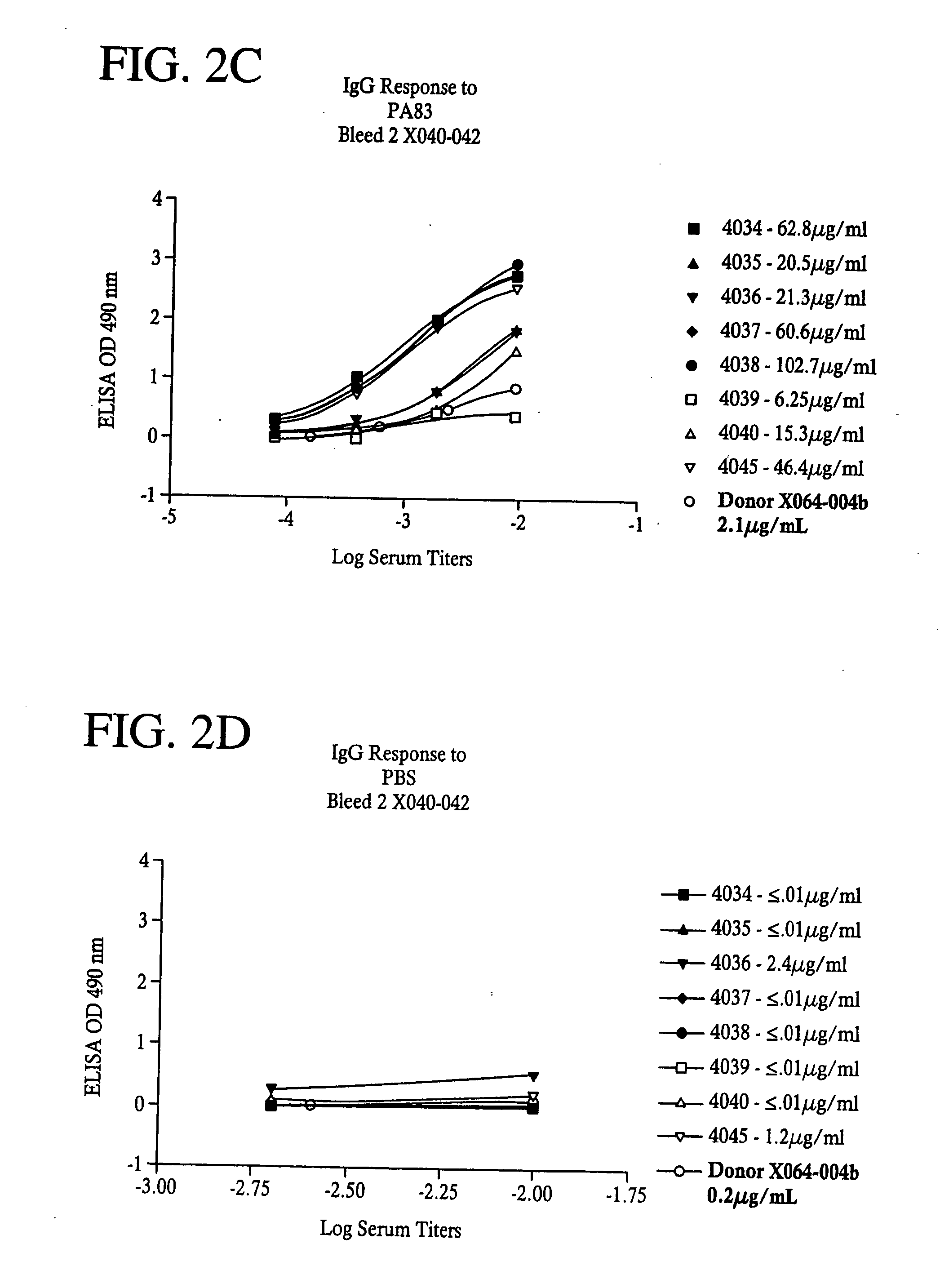
InactiveUS20050281830A1Avoid toxicityReduce severityBiological material analysisAntibody ingredientsAntigenMammal
A highly efficient method for generating human antibodies using recall technology is provided. In one aspect, human antibodies which are specific to the anthrax toxin are provided. In one aspect, human peripheral blood cells that have been pre-exposed to anthrax toxin are used in the SCID mouse model. This method results in high human antibody titers which are primarily of the IgG isotype and which contain antibodies of high specificity and affinity to desired antigens. The antibodies generated by this method can be used therapeutically and prophylactically for preventing or treating mammals exposed to anthrax. Thus, in one embodiment, a prophylactic or therapeutic agent used to counter the effects of anthrax toxin, released as a mechanism of bioterrorism, is provided. In one embodiment, a formulation and method for preventing and / or treating anthrax infection comprising a binding agent that prevents the assembly of the PA63 heptamer is also provided. Methods for diagnosis and methods to determine anthrax contamination are also described.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
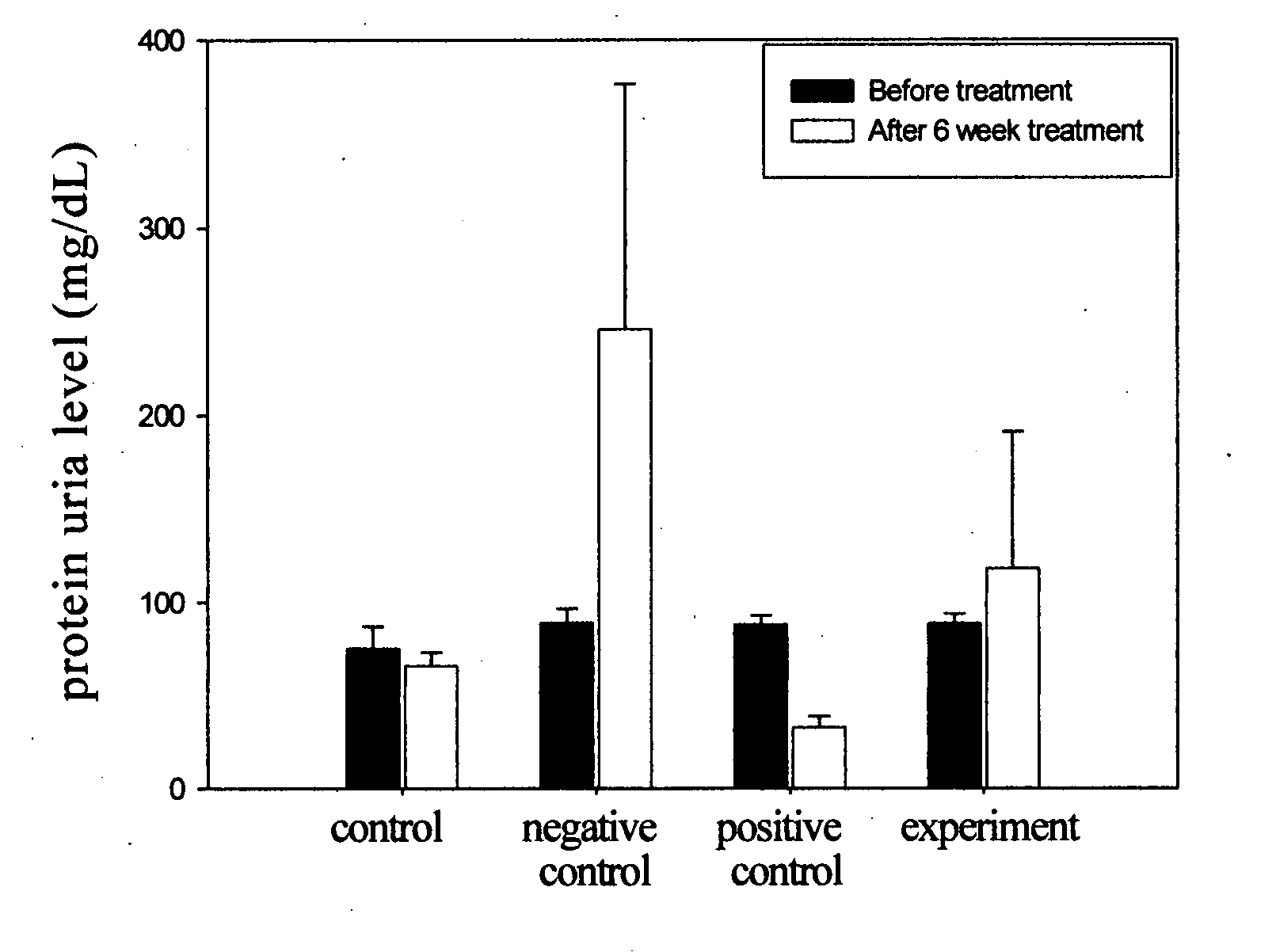
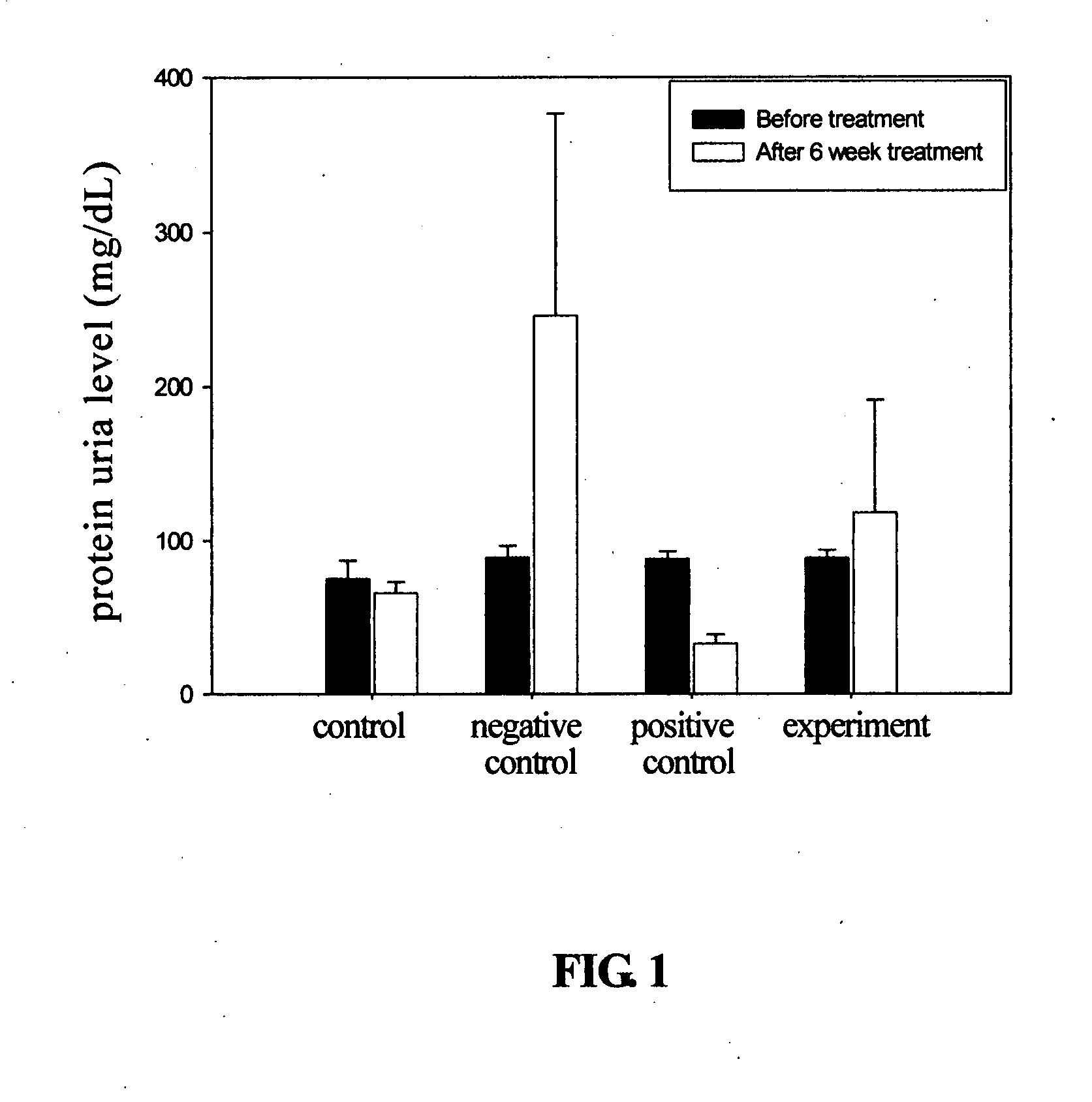
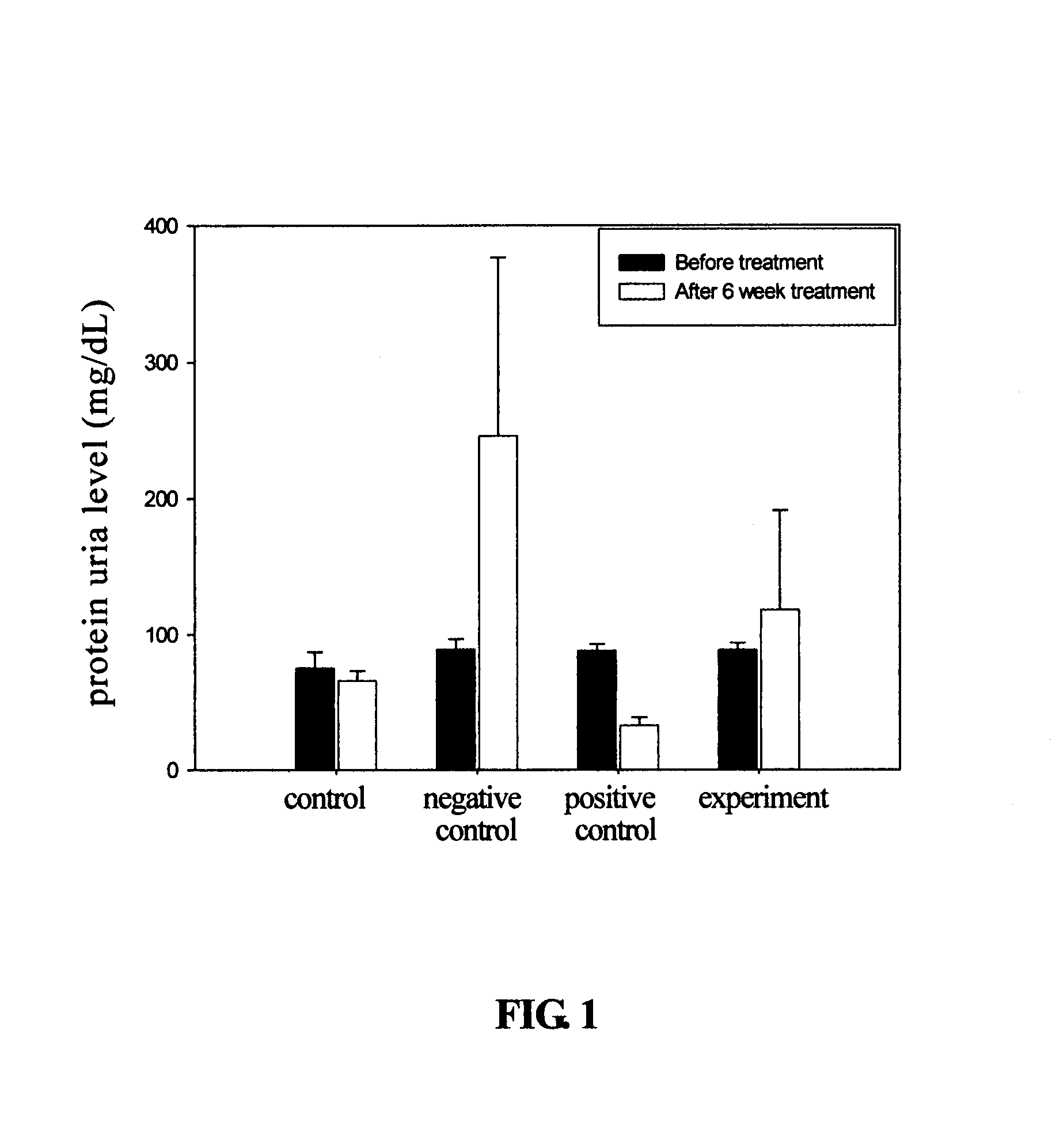
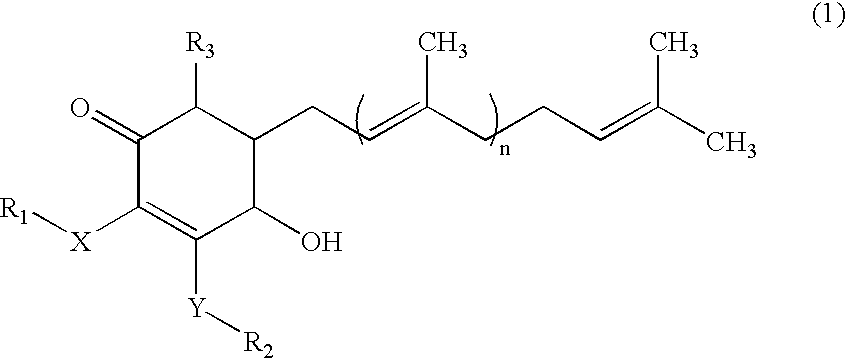
Cyclohexenone compounds from antrodia camphorata to treat autoimmune diseases
ActiveUS20080312334A1Avoid complicationsAvoid side effectsBiocideOrganic chemistryDiseaseImmunologic disorders
The present invention relates to a compound of Antrodia camphorata used to treat autoimmune diseases, in particular to an extract, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone, isolated from Antrodia camphorata, and its use in alleviating symptoms of autoimmune diseases such as systemic lupus erythematosus (SLE). The cyclohexenone compound according to the present invention helps to decrease proteinuria levels and antinuclear antibody titers in SLE mammals in order to alleviate kidney inflammation and disease, as well as the self-damage caused by antinuclear antibodies. The purpose for prevention and treatment of autoimmune diseases and kidney diseases by the natural, side-effect free substance can then be accomplished.
Owner:GOLDEN BIOTECH
New castle disease and H9 subtype bird flu bivalent vaccine
ActiveCN104922663AImproving immunogenicitySmall dose of immunizationViral antigen ingredientsAntiviralsDiseaseOil adjuvant
The invention aims at providing a new castle disease and H9 subtype bird flu bivalent vaccine. The new castle disease and H9 subtype bird flu bivalent vaccine contains antigens and adjuvant. The antigens are inactivated H9 subtype bird flu viruses and new castle disease viruses. The H9 subtype bird flu viruses are QDY strains, and the preservation number of the H9 subtype bird flu viruses is CCTCC v201517. The QDY strains of the H9 subtype bird flu viruses and a Lasota strain of the new castle disease viruses are inoculated to chick embryos respectively, and then virus liquid is collected; the virus liquid and the oil adjuvant are mixed and emulsified into the vaccine after the virus liquid is inactivated through a formaldehyde solution. The new castle disease and bird flu bivalent inactivated vaccine is good in immunogenicity, antibody production is fast after immunity, the produced antibody titer is high, the produced antibody holding time is long, the retention period is long, the immunizing dose is small, the selected adjuvant is easy to inject, and two kinds of diseases can be prevented through one-time injection. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
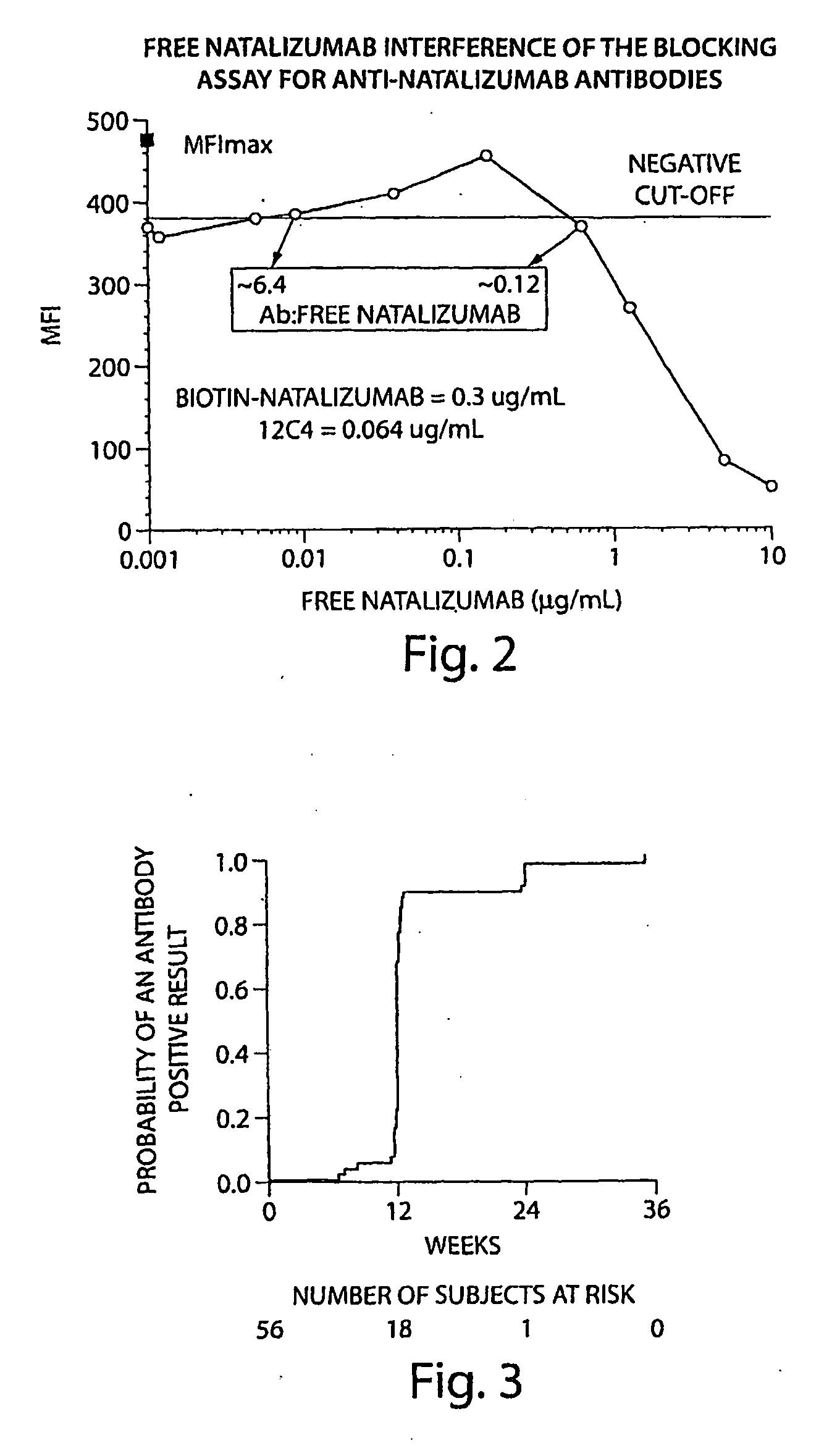
Methods and Products for Evaluating an Immune Response to a Therapeutic Protein
ActiveUS20090176256A1Reduced clinical efficacyHigh threshold levelNervous disorderAntipyreticNatalizumab AntibodyTherapeutic protein
The invention relates to methods and products for the identification of a clinically significant immune response in subjects treated with a therapeutic protein. A first aspect of the invention relates to methods and compositions for identifying a clinically significant immune response in patients treated with therapeutic amounts of a VLA4 binding antibody (e.g., natalizumab). A second aspect of the invention concerns the chronological details of sample collection for determining the titre of antibodies against the therapeutic protein, e.g. the collection of at least two samples at two different time points. A third aspect of the invention relates to the selection of the critical threshold level, which corresponds to the antibody titre of untreated patients increased by the double of the standard deviation of this control antibody titre.
Owner:BIOGEN IDEC MA INC +1
Compositions and methods for the treatment of immunodeficiency
ActiveUS9107906B1Antibacterial agentsSerum immunoglobulinsPassive ImmunizationsPrimary immunodeficiency
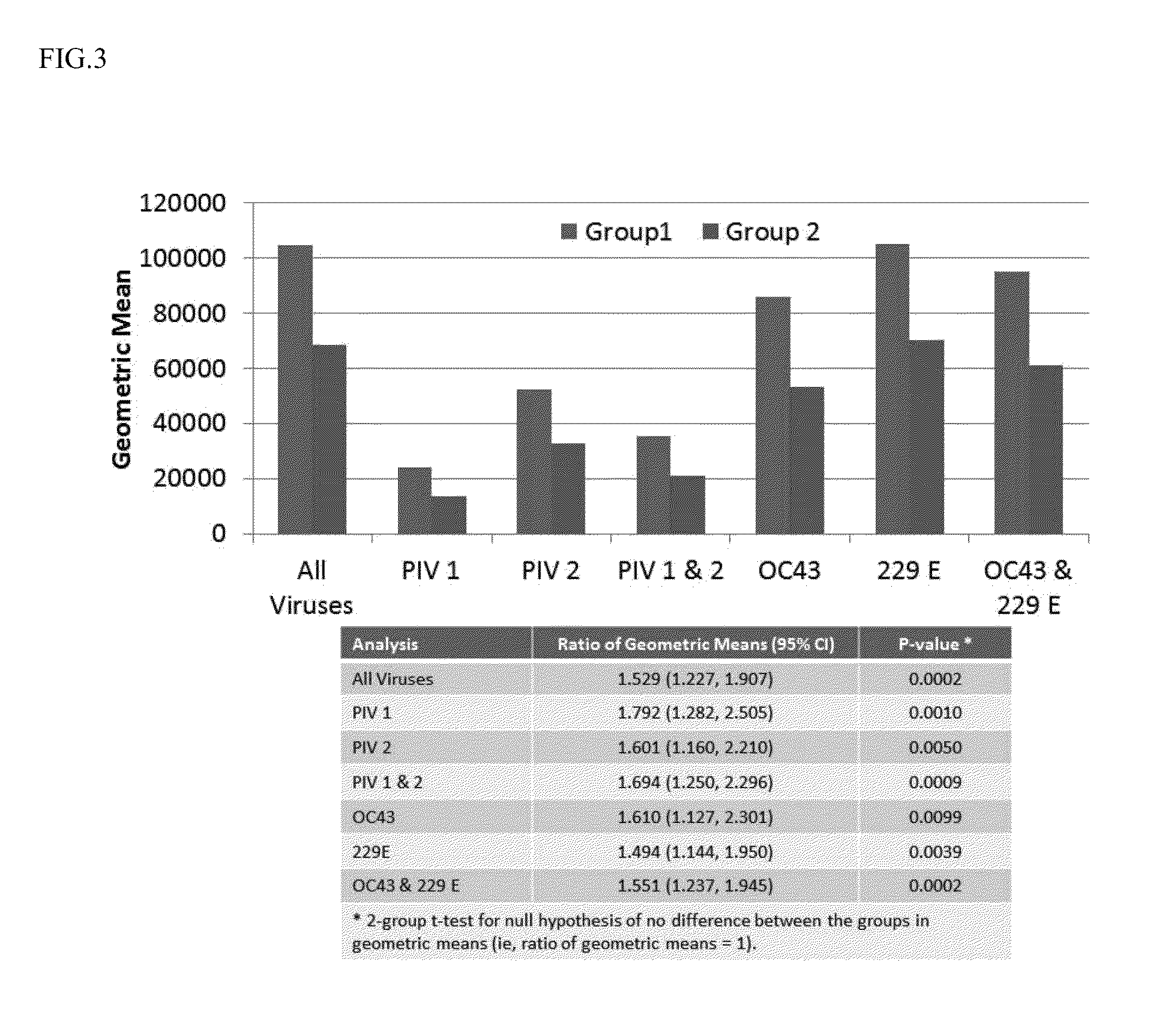
The present invention relates to compositions and methods for the treatment of immunodeficiency (e.g., primary immunodeficiency disease). In particular, the invention provides human plasma immunoglobulin compositions containing select antibody titers specific for a plurality of respiratory pathogens, methods of identifying human donors and donor samples for use in the compositions, methods of manufacturing the compositions, and methods of utilizing the compositions (e.g., for prophylactic administration and / or therapeutic treatment (e.g., passive immunization (e.g., immune-prophylaxis))).
Owner:ADMA BIOMANUFACTURING LLC
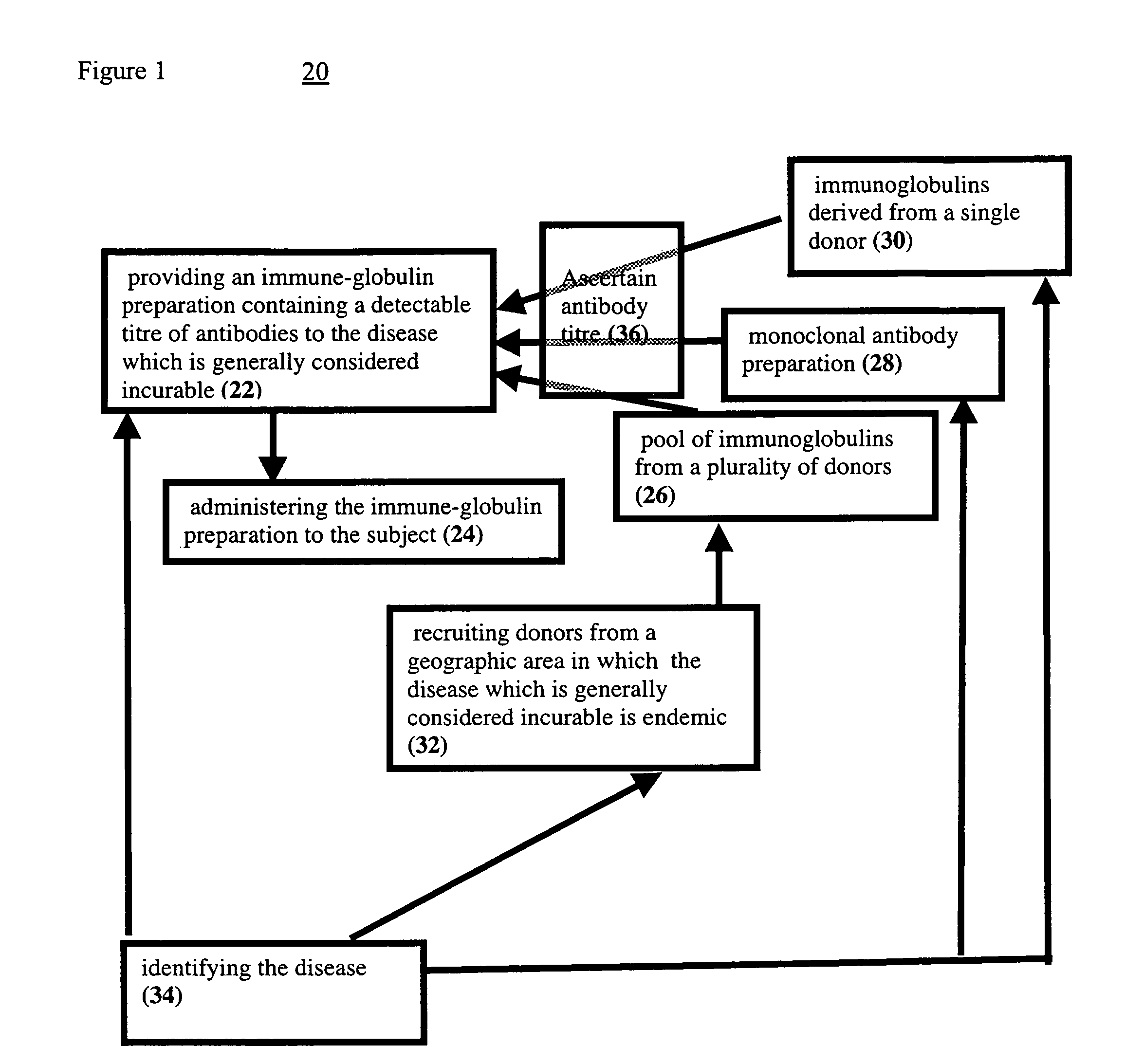
Pharmaceutical compositions and articles of manufacture useful in reversal of a clinical epiosode of an incurable disease and methods of use thereof
InactiveUS7488486B2Viral antigen ingredientsImmunoglobulins against virusesIncurable diseasesDisease
A method of reversing a clinical episode of a disease which is generally considered incurable in a subject. The method includes providing an immune-globulin preparation containing a detectable titre of antibodies to the disease which is generally considered incurable and administering the immune-globulin preparation to the subject. Preferably, the immune globulin preparation is a pool of immune globulin fractions gathered from blood of donors living in an area where the disease is endemic. Further disclosed are pharmaceutical compositions and articles of manufacture suited for use in practice of the method.
Owner:SANZ MEDICAL CENT LANIADO HOSPITAL
Cyclohexenone compounds from Antrodia camphorata to treat autoimmune diseases
ActiveUS7501454B2Lower Level RequirementsDelay progressBiocideOrganic chemistryImmunologic disordersCyclohexenone
The present invention relates to a compound of Antrodia camphorata used to treat autoimmune diseases, in particular to an extract, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone, isolated from Antrodia camphorata, and its use in alleviating symptoms of autoimmune diseases such as systemic lupus erythematosus (SLE). The cyclohexenone compound according to the present invention helps to decrease proteinuria levels and antinuclear antibody titers in SLE mammals in order to alleviate kidney inflammation and disease, as well as the self-damage caused by antinuclear antibodies. The purpose for prevention and treatment of autoimmune diseases and kidney diseases by the natural, side-effect free substance can then be accomplished.
Owner:GOLDEN BIOTECH
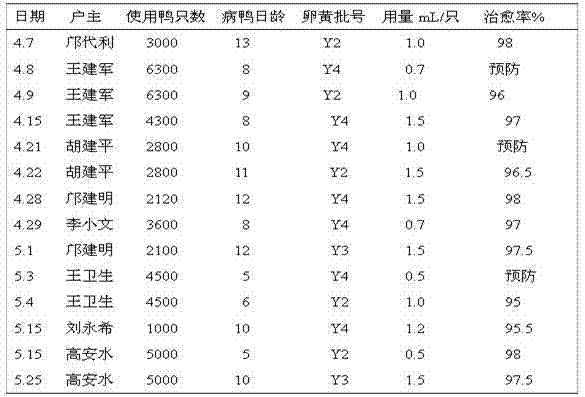
Duck egg yolk antibody for preventing and treating virus hepatitis of duckling, and preparation method thereof
ActiveCN102241771AImprove the level ofHigh potencyEgg immunoglobulinsImmunoglobulins against virusesDuck hepatitis A virusDuck viral hepatitis
The invention discloses a duck egg yolk antibody for preventing and treating virus hepatitis of ducklings, and a preparation method thereof. The duck egg yolk antibody is extracted from the primiparous eggs of a high-immunity homebred breed duck starting laying for one month. The preparation method comprises the following steps of: (a) highly immunizing the breed duck before laying; (b) collecting high-immunity primiparous breed duck eggs; (c) cleaning and sterilizing; (d) separating egg yolks; (e) diluting; (f) stirring and filtering; and (g) packaging and preserving. After the egg yolks are continuously diluted for 2 times by using normal saline, the DHV (Duck Hepatitis Virus) precipitating antibody is detected by a conventional method, the antibody titer is more than 1:64, and the HI titer of H5 is more than 1:512. 0.5mL of the duck egg yolk antibody is subcutaneously injected to a 3-day-old duckling for preventing virus hepatitis; and 1mL of egg yolk antibody to which antibiotics are added is subcutaneously injected to a duck for treating virus hepatitis, and the egg yolk antibody is re-injected to a seriously diseased duck every other day.
Owner:SHUNHUA DUCK INDAL DEVMET LINWU COUNTY HUNAN
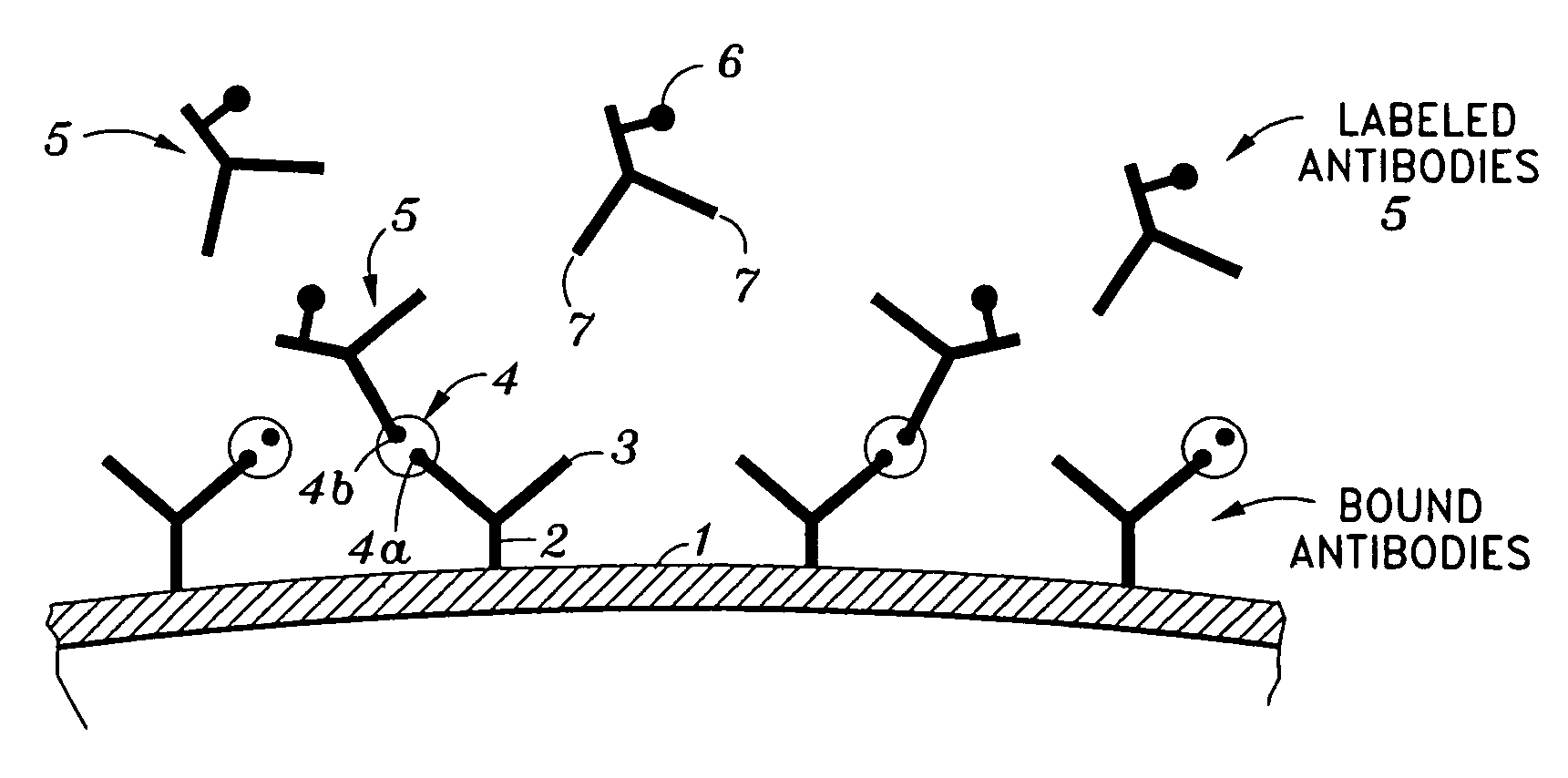
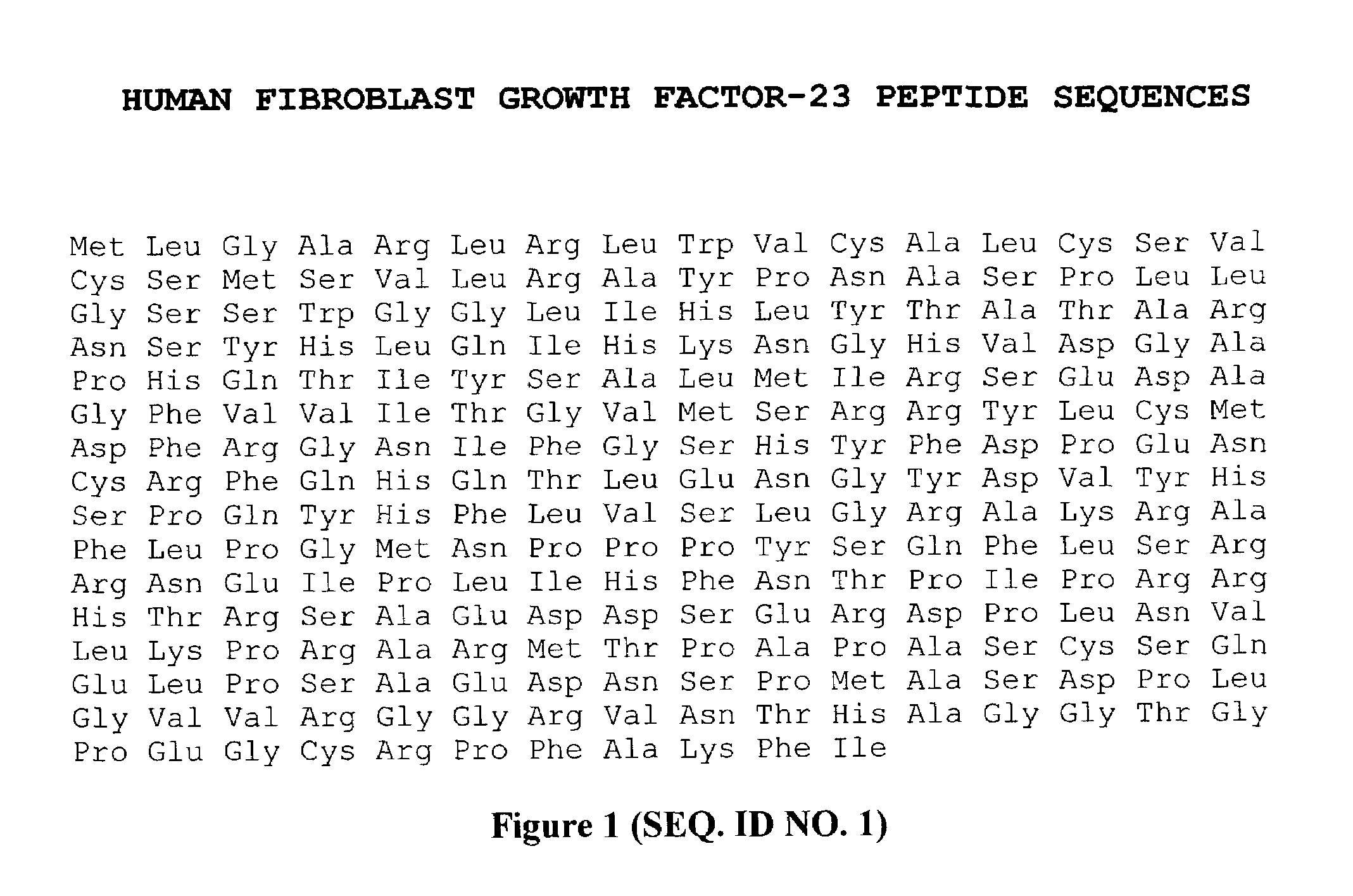
Immunoassays, assay methods, antibodies and method of creating antibodies for detecting FGF-23
ActiveUS7094551B2Readily and accurately determiningEasy to detectImmunoglobulins against growth factorsBiological testingSerum igeAntigen binding
Owner:IMMUTOPICS
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Self-calibrating gradient dilution in a constituent assay and gradient dilution apparatus performed in a thin film sample
InactiveUS20090252399A1Material analysis by optical meansCharacter and pattern recognitionAnalyteAssay
A method and apparatus for measuring antibody titers in a thin film sample in an automated system which does not require multiple dilutions. The system provides a simple method for creating an in-situ dilution within a sample analysis chamber without the use of any precision fluid-handling components, and further, to use the same principles to provide a wide range of sample dilutions within the chamber so as to obviate the need for additional dilution steps when dealing with samples possibly containing wide ranges of analyte concentrations.
Owner:ABBOTT POINT CARE
Preparation method of inactivated newcastle disease vaccine
ActiveCN103110942AReduce in quantityEnhance immune functionViral antigen ingredientsAntiviralsOil phaseAntibody titer
The invention relates to a preparation method of an inactivated newcastle disease vaccine. The preparation method comprises the steps of virus liquid inactivation, preparation of a compound traditional Chinese medicine extract, preparation of an aqueous phase, preparation of an oil phase and emulsification. According to the preparation method, the compound traditional Chinese medicine extract serving as an immunopotentiator is added to the inactivated newcastle disease vaccine so as to well cause cellular immunologic response and enhance the immunologic function of an organism, so that an antibody is generated in advance, and the antibody titer is obviously improved.
Owner:山东滨州沃华生物工程有限公司
Compound immunoenhancement agent, vaccine for birds and method for preparing compound immunoenhancement agent
ActiveCN102743750AShorten the immune window periodShort immune windowAntiviralsImmunological disordersBird fluDipeptide
The invention relates to a compound immunoenhancement agent and an application thereof on preparing a vaccine for birds. The compound immunoenhancement agent contains 5ng-10mg / mL of poly IC, 10ng-10mg / mL of muramyl dipeptide, 10ng-5mg / mL of levamisole, 10ng-5mg / mL of resiquimod and 10ng-5mg / mL of imiquimod. After the immunoenhancement agent and H5 hypotype bird flu inactivated vaccine are mixed together, antibody can be produced one week earlier, and the antibody titer is improved above 1.8log2. After chicken are immunized through H5 hypotype bird flu inactivated vaccine, H9 hypotype bird flu vaccine or infectious bronchitis vaccine which contains the compound vaccine immunoenhancement agent, the window phases of the antibody production are shortened, the antibody titer of the vaccine is improved, the immunization duration is prolonged, and the probability of infection disease is reduced.
Owner:南京国创生物技术研究院有限公司
Preparation method of duck hepatitis antibody
ActiveCN101704890AEasy to get materialsThe separation and purification method is simpleEgg immunoglobulinsImmunoglobulins against virusesYolkPurification methods
The invention relates to the field of immunology, in particular a preparation method of duck hepatitis antibody. The preparation method comprises the following steps: (1) feeding healthy laying hens by poplar bark lipoid at a dosage of 0.08 to 0.1g per hen each day; carrying out duck hepatitis inactivated vaccine intensive immunity for healthy laying hens fed by poplar bark lipoid after 20 to 30 days; collecting hen blood serum to measure anti-DHV neutralizing antibody titer; and collecting eggs laid by qualified flocks; and (2) separating egg white under aseptic conditions, mashing yolk and evenly mixing the yolk liquid with aseptic physiological saline to prepare yolk antibody. Compared with the antibody from mammals, antibody prepared through the preparation method has the advantages of convenient material sources, simple separation and purification method, high yield, high specificity and better stability.
Owner:烟台爱士津动物保健品有限公司
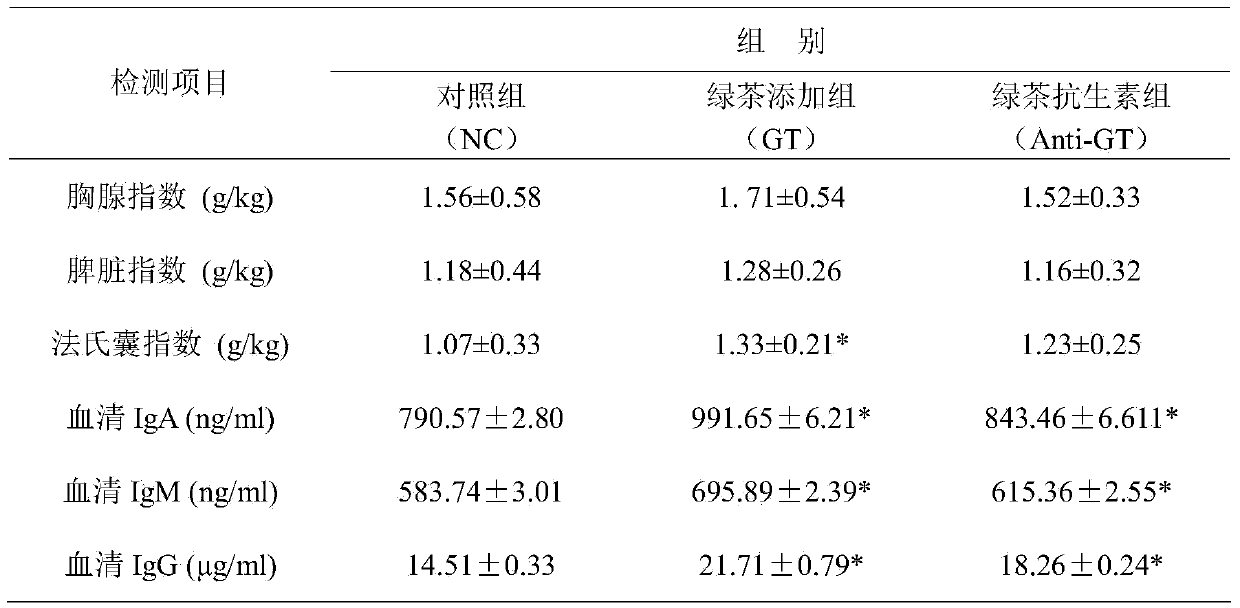
Green tea composite chicken feed additive capable of enhancing immunity of chickens
Owner:ANHUI AGRICULTURAL UNIVERSITY
Foot and mouth disease virus-like particle vaccine and preparation method thereof
InactiveCN105126096AEasy to manufactureEasy to purifyAntiviralsGranular deliveryVaccine ProductionNeutralizing antibody
The invention discloses a foot and mouth disease virus-like particle vaccine and a preparation method thereof. By expressing P12A (three amino acid site mutation, VP1K210E, VP1E83K and VP1C134S) and serum-free suspension culture, a virus-like particle (VLP) standard similar to natural 75S FMDV is built, and VLP yield is increased. The invention further provides serum-free suspension cultured and high pH tamed insect cells, the insect cells can stably and efficiently produce FMDV VLP, the size and structure of the VLP produced by the insect cells is similar to those of the VLP produced by standard BHK-21, and the yield of the VLP produced by the insect cells is 11 times of that of the VLP produced by female parent cells Sf21. The vaccine prepared by the VLP produced by the insect cells can immunize animals to generate IgG antibody response. The IgG antibody titer, foot and mouth disease virus neutralizing antibody titer and antibody titer of immunization of the VLP vaccine produced by the insect cells are not evidently different from those of immunization of the VLP vaccine prepared by the BHK-21, so that the high-pH-adaptive insect cells are applicable to FMDV VLP vaccine production.
Owner:吕宏亮 +1
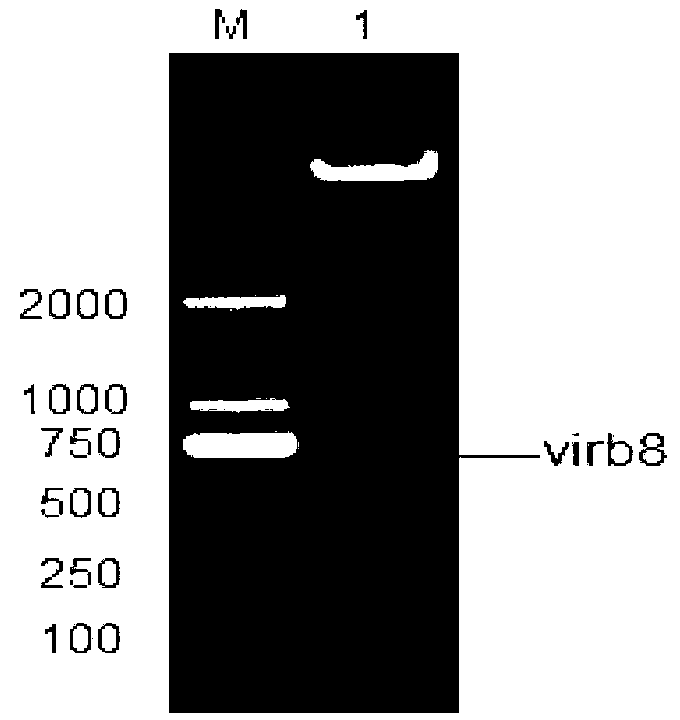
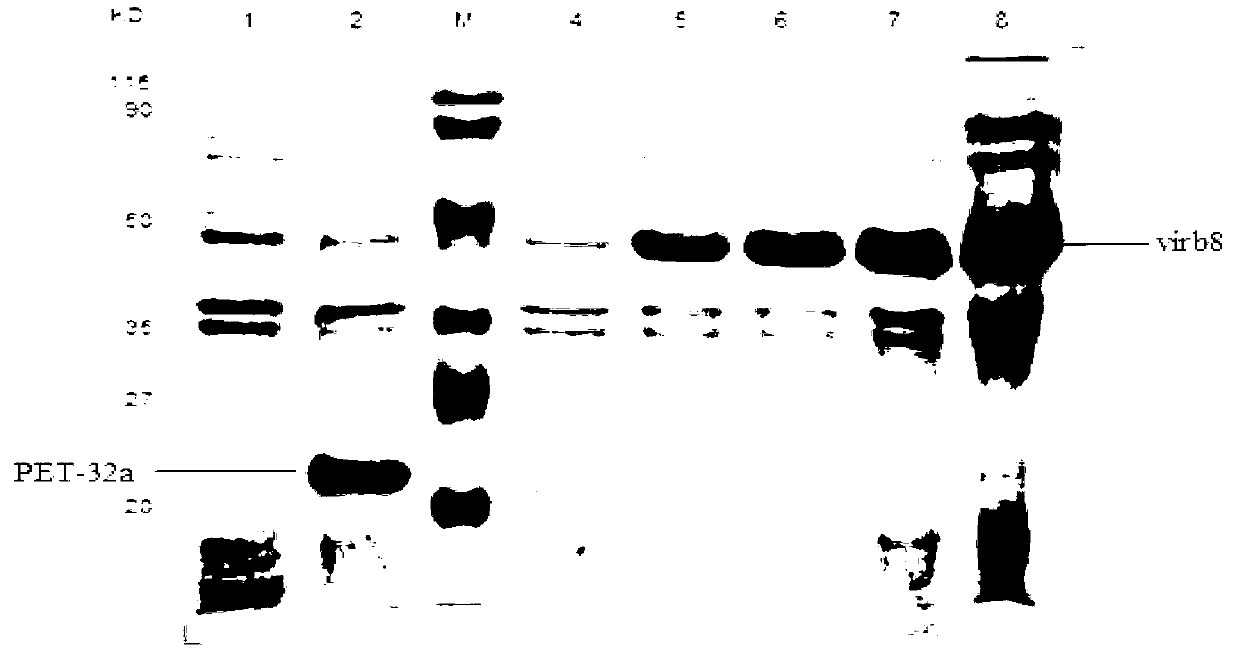
Brucella abortus indirect enzyme linked immunosorbent assay (ELISA) detection kit
InactiveCN103344770ASensitive and accurate detectionThe pre-processing process is simpleBiological testingELISA unitStearate
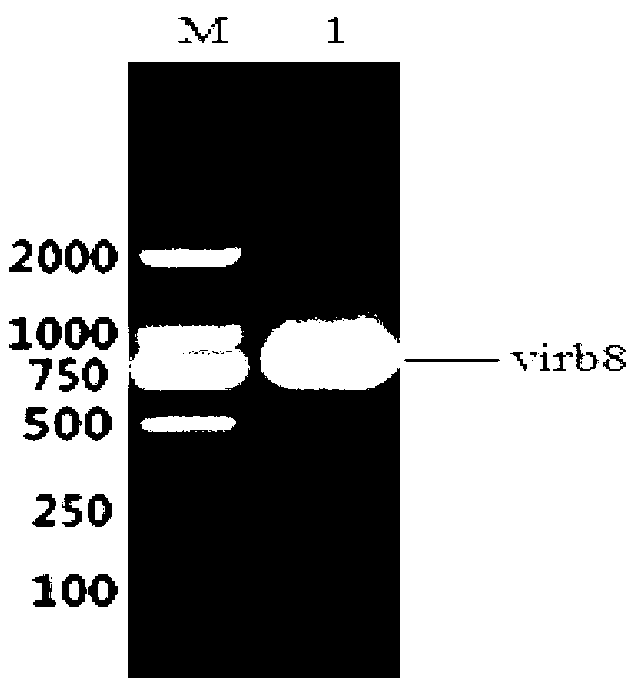
The invention discloses a brucella abortus indirect enzyme linked immunosorbent assay (ELISA) detection kit which consists of the following reagents: (1) a pre-enveloped ELISA reaction plate comprising enveloping liquid, an enveloping antigen and closing liquid, (2) plumbous stearate (PBST) washing liquid, (3) IgG-HRP, (4) ending liquid, and (5) color developing liquid, wherein the enveloping antigen is a Virb8 protein. The Virb8 protein related by the invention can be only specifically expressed in the early stage of the brucella abortus infection; an antibody correspondingly produced by the protein is in the early stage of the infection; the produced antibody can live for a long time, so that whether an antibody titer caused by the brucella abortus infection and the quantitative infection exists can be specifically and sensitively judged by cloning and expressing the brucella abortus Virb8 protein and constructing a corresponding indirect ELISA detection method; a quick and accurate method can be supplied to early serologic diagnosis of the brucella abortus disease; the brucella abortus indirect ELISA detection kit has a great practical significance for a brucella abortus site detection technology for a large batch of samples.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
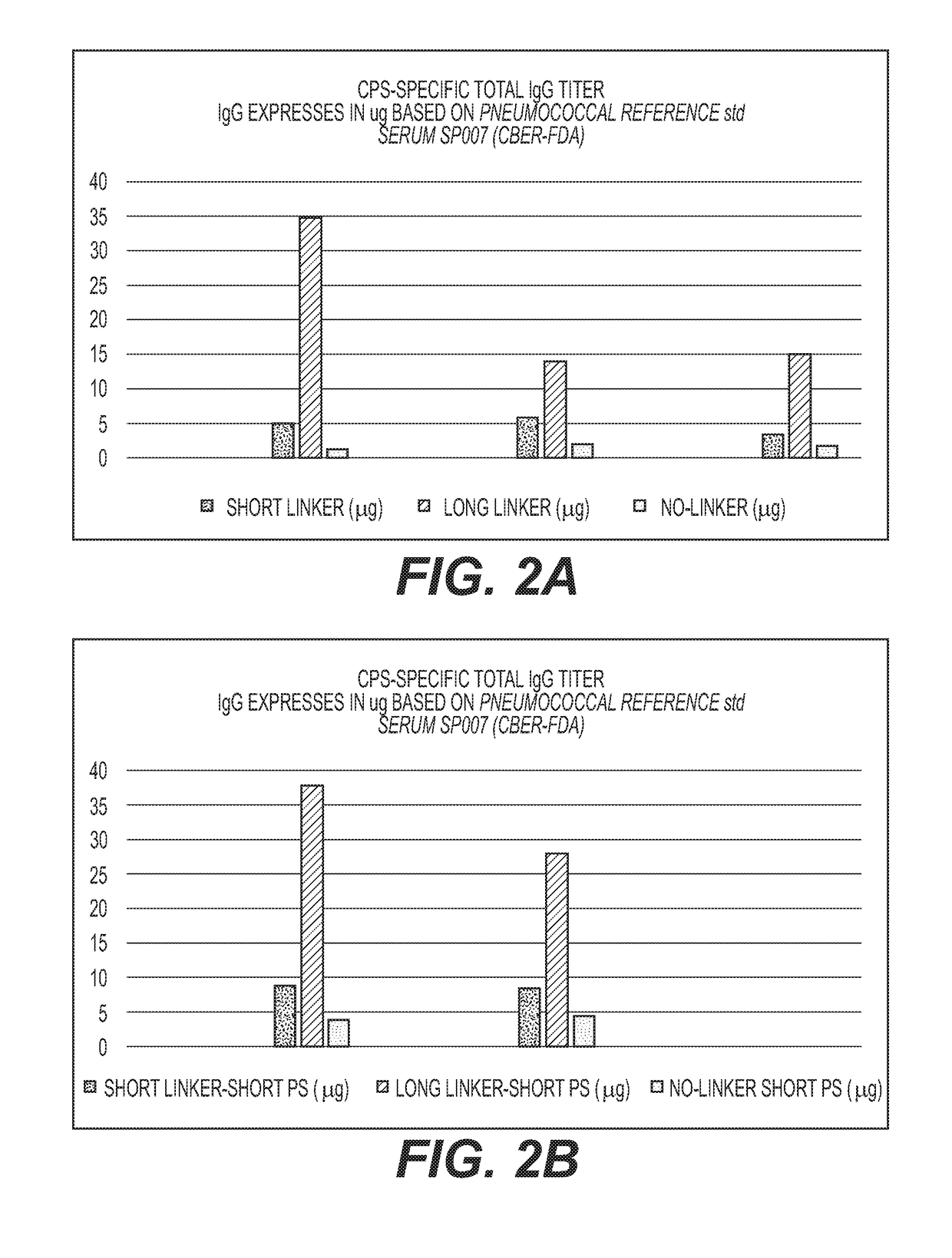
Multivalent Conjugate Vaccines with Bivalent or Multivalent Conjugate Polysaccharides that Provide Improved Immunogenicity and Avidity
ActiveUS20180353591A1Lower immune responseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineCarrier protein
The disclosure describes compositions containing conjugates using novel linkers, bivalent polysaccharide conjugates, and methods of bivalent polysaccharide conjugation in the development of multivalent conjugate vaccines. Conjugation of capsular polysaccharides to carrier proteins is carried out using homo-bifunctional and / or hetero-bifunctional linkers of specific lengths. Incorporation of the linkers and their use in bifunctional linkers induces higher titers of functional antibodies with high avidity, eliciting higher immunologic memory, and reduced carrier protein effect. This provides immunochemically cross-reactive capsular polysaccharides wherein one or more cross-reactive capsular polysaccharides are conjugated sequentially or concurrently to carrier protein using bifunctional linkers bearing the same or different functional groups. Such a linker and the size of the capsular polysaccharides provides an effective multivalent conjugate vaccine with high antibody titers and a reduced carrier effect and results in reduction in the content of the capsular polysaccharide and protein per dose of vaccine which reduces reactogenicity.
Owner:INVENTPRISE INC
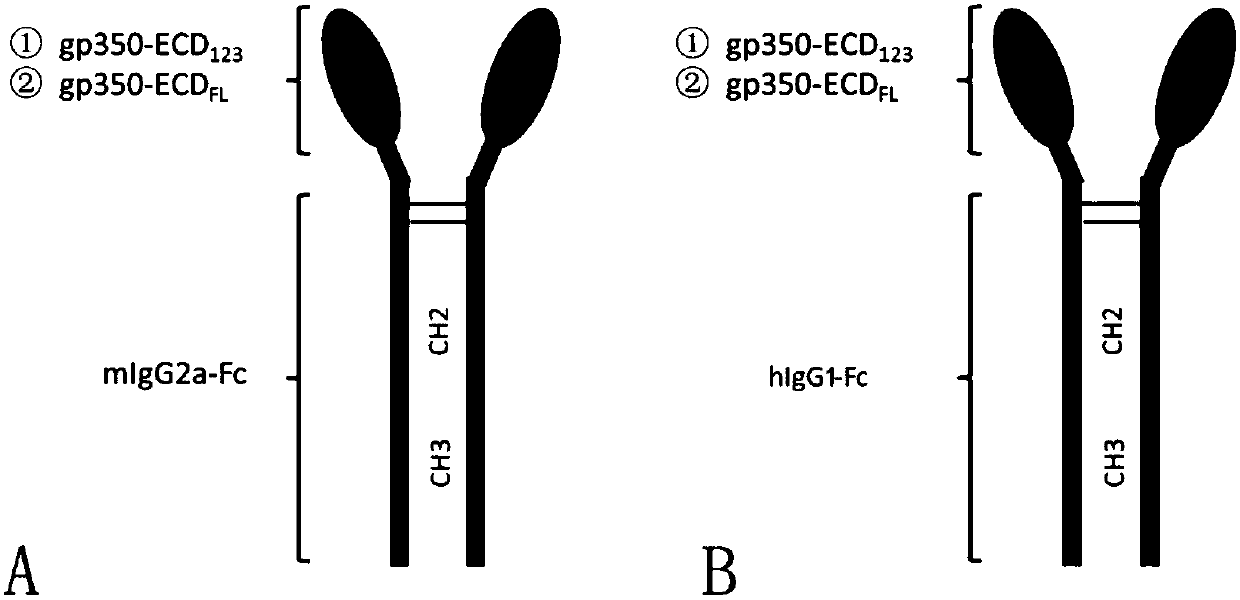
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
ActiveCN109824779AImproving immunogenicityHigh infection blocking efficiencyAntiviralsAntibody ingredientsDiseaseImmunogenicity
The invention discloses a fusion protein comprising an Fc domain of IgG and an extracellular domain of an EB virus envelope glycoprotein. The fusion protein is represented by the following formula: P-E-F, wherein P represents a secretion signal peptide, E represents an amino acid sequence of the extracellular domain of the EB virus envelope glycoprotein gp350, and F represents the amino acid sequence of the Fc domain of the IgG. It is found for the first time that after the fusion of the Fc domain of the immunoglobulin IgG with the envelope glycoprotein gp350 from the surface of the EB virus,the immunogenicity in vivo is significantly improved. After immunization with the fusion protein, the total serum titer an immunized animal, the serum specific neutralizing antibody titer and the serum in vitro viral infection blocking efficiency are significantly higher than that of a non-fused control protein. The fusion protein using the Fc domain of the immunoglobulin IgG and the EB virus membrane glycoprotein is adopted as an evidence for the efficacy of an EB virus vaccine and has important practical and theoretical significance and application prospects for prevention and treatment of EB virus-related diseases.
Owner:SUN YAT SEN UNIV +1
Novel vaccine adjuvant and application thereof in novel coronavirus pneumonia vaccine and other vaccines
ActiveCN111956797ASsRNA viruses positive-senseViral antigen ingredientsAntiendomysial antibodiesCoronavirus vaccination
The invention relates to the field of biological medicines, in particular to a novel vaccine adjuvant and an application thereof in a novel coronavirus pneumonia vaccine and other vaccines. Chemicallymodified cyclic dinucleotide, namely an SF compound, is used as the vaccine adjuvant and is used in cooperation with the novel coronavirus vaccine, so that the SARS-CoV-2 virus antigen specific antibody titer and the generation of T cells can be remarkably improved; and the SF compound as the vaccine adjuvant is obviously superior to an aluminum adjuvant in immunopotentiation effect.
Owner:TSINGHUA UNIV
Fusion protein of SVV and FMDV, encoding gene of fusion protein, expression vector, cell line, engineering bacteria, vaccine and application
The invention relates to the technical field of biomedicine, and particularly provides a fusion protein of an SVV and an FMDV, an encoding gene of the fusion protein, an expression vector, a cell line, engineering bacteria, a vaccine and application. The fusion protein is obtained by replacing an epitope capable of inducing the body to produce a neutralizing antibody with a decoy epitope and comprises SVV VP1 protein fragments, FMDV VP1 protein fragments and complement C3d protein fragments. The fusion protein combines antigens for inducing the body to produce the neutralizing antibody, of twopathogens of SVV and FMDV, and the safety of the antigens is improved while the antigenic epitopes of the SVV and the FMDV are reserved. The complement C3d molecules can excite nonspecific body fluidand cellular immune reactions in the body, and play an important role in increasing the antibody titer of the vaccine, and activating the cellular immune response of the body. The vaccine prepared bymeans of the fusion protein is safe and effective, and hydroa and foot-and-mouth diseases can be effectively prevented.
Owner:天康生物制药有限公司
Compound astragalus polysaccharides and echinacea purpurea herb nanoemulsion adjuvant and preparation method thereof
InactiveCN101869706AReduce viscosityEasy injectionEmulsion deliveryAntibody medical ingredientsAntigenAdjuvant
The invention discloses a compound astragalus polysaccharides and echinacea purpurea herb nanoemulsion adjuvant, the partical size of which is between 1nm and 100nm, and is composed by echinacea purpurea herb, astragalus polysaccharides, surface active agent, cosurfactant, oil and distilled water. The product presents clear light yellow liquid, has the partical size smaller than 100nm, has lower viscosity and better stability, does not need special emulation when seedling, and has low requirements on equipment and easy controlled product quality. By taking albumin egg OVA as model antigen, tests show that the compound astragalus polysaccharides and echinacea purpurea herb nanoemulsion adjuvant of the invention has better immunoreaction than using aluminium adjuvant in a certain dosage, has higher antibody titer, reduces the dosage of oil or antigen in the adjuvant, stimulates the reaction of Th1 and Th2, increases the immunization of the liquid and the cell of the an organism, and plays a good role of immune protection of the organism.
Owner:NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com