Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
126 results about "Reactogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In clinical trials, the term reactogenicity refers to the property of a vaccine of being able to produce common, "expected" adverse reactions, especially excessive immunological responses and associated signs and symptoms—fever, sore arm at injection site, etc. (Much less frequently, the term has also been applied to therapeutic drug trials.) Other manifestations of reactogenicity typically assessed in such trials include bruising, redness, induration, and swelling.
Liposomal vaccine
InactiveUS20050169979A1High weight ratioHigh encapsulation efficiencyPeptide/protein ingredientsMicroencapsulation basedHormones regulationLiposome
The invention provides liposomal vehicles for encapsulating relatively high levels of immunogenic protein substances including immunogens directed against hormones and hormone receptors, such as gastrin and gonadotropin releasing hormone and their receptors. The liposome encapsulating large amounts of immunogens can be injected parenterally to induce effective immune responses without exhibiting significant adverse tissue reactogenicity. Methods for production of the liposomal vaccines and methods of their administration for treatment of diseases and conditions associated with the cognate hormones are also provided.
Owner:RECEPTOR BIOLOGIX +1
Liposomal vaccine
InactiveUS20070082043A1High weight ratioHigh encapsulation efficiencyPeptide/protein ingredientsMicroencapsulation basedWater solubleLiposome
The invention provides liposomal vehicles for encapsulating relatively high levels of water-soluble substances including immunogens directed against gastrin and gonadotropin releasing hormone. The liposome encapsulating large amounts of immunogens can be injected parentally to induce effective immune responses without exhibiting significant adverse tissue reactogenicity.
Owner:RECEPTOR BIOLOGIX
Acian Metapneumovirus antibody ELISA detection kit
ActiveCN103360472ASolve the difficulty of purificationReactogenicDepsipeptidesBiological testingAntigenHuman metapneumovirus
The invention provides an Acian Metapneumovirus antibody ELISA detection kit. The kit is the ELISA kit established for detecting the Acian Metapneumovirus antibody on the basis of treating the monomer protein of the A subgroup Acian Metapneumovirus F protein as a coating antigen. The invention also provides a preparation method of the monomer protein of the coating antigen F protein for making the kit. The F protein prepared through the method has a good reactionogenicity, and the established ELISA detection kit provides an effective method for the early-stage diagnosis and detection of the Acian Metapneumovirus infection, and plays a substantial promotion effect in the prevention researches of the Acian Metapneumovirus infected diseases.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Virus infection detection method
The invention discloses a virus infection detection method. The virus infection detection method comprises the following steps of: (1) coupling a viral reactogenicity polypeptide to a non-protein polymer to obtain a coupled polypeptide; and (2) adding the coupled polypeptide in a coating liquid, then adding a serum sample (to be detected) to perform the ELISA (enzyme-linked immunosorbent assay) detection, judging the virus and subtype infection thereof according to an immunoadsorption reaction. The detection method provided by the invention can obviously improve the detection sensitivity as well as obviously reduces the non-specific background readout, has high specificity, simplicity in operation, rapidness in diagnosis speed, and is economic and convenient in large scale detection; and the detection method is a good method easy for popularization and wide in application prospect. In the detection provided by the invention, the dosage of the coating antigen can be 10 ng / ml, and the less dosage is in favor of popularization and application.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
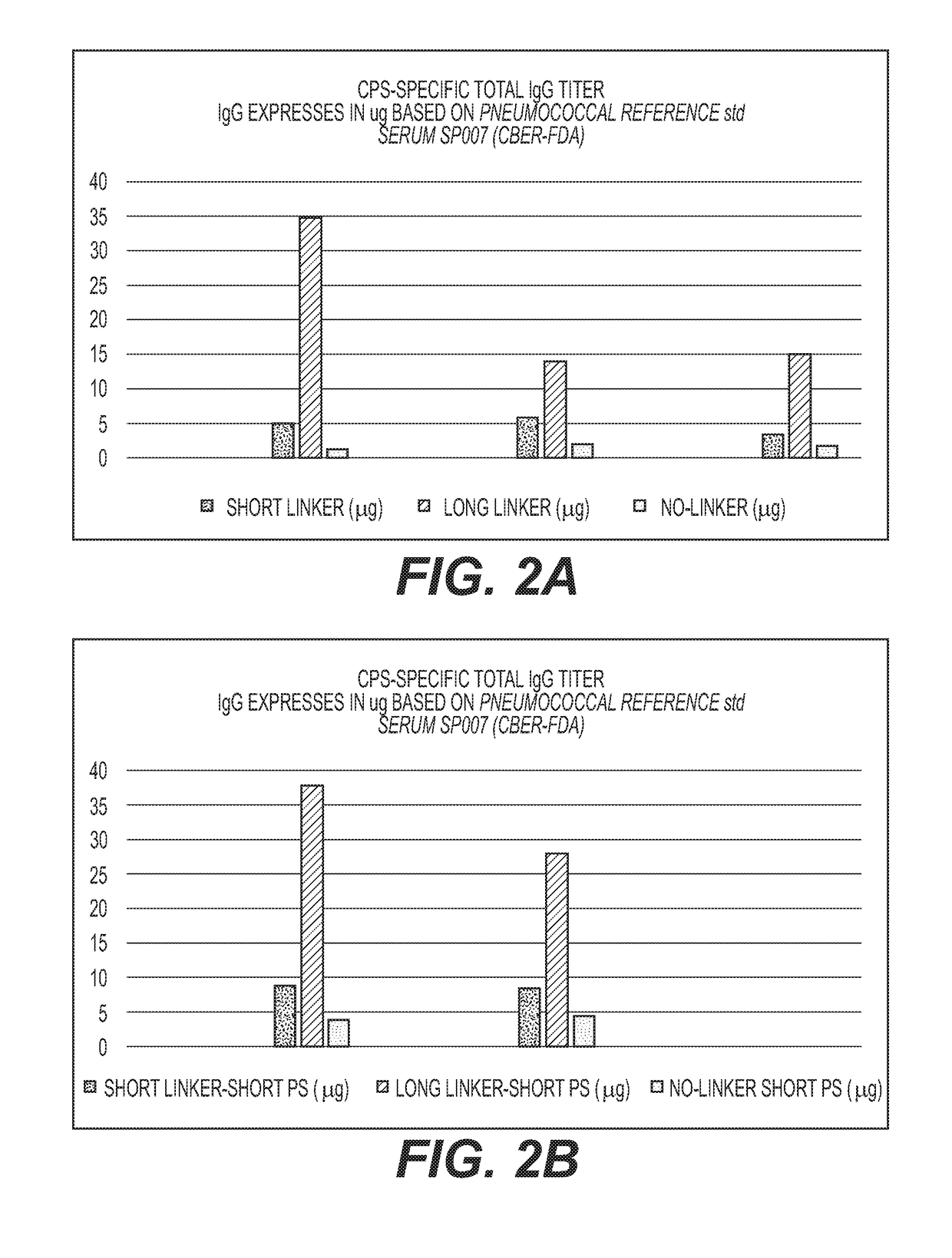
Multivalent Conjugate Vaccines with Bivalent or Multivalent Conjugate Polysaccharides that Provide Improved Immunogenicity and Avidity
ActiveUS20180353591A1Lower immune responseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineCarrier protein
The disclosure describes compositions containing conjugates using novel linkers, bivalent polysaccharide conjugates, and methods of bivalent polysaccharide conjugation in the development of multivalent conjugate vaccines. Conjugation of capsular polysaccharides to carrier proteins is carried out using homo-bifunctional and / or hetero-bifunctional linkers of specific lengths. Incorporation of the linkers and their use in bifunctional linkers induces higher titers of functional antibodies with high avidity, eliciting higher immunologic memory, and reduced carrier protein effect. This provides immunochemically cross-reactive capsular polysaccharides wherein one or more cross-reactive capsular polysaccharides are conjugated sequentially or concurrently to carrier protein using bifunctional linkers bearing the same or different functional groups. Such a linker and the size of the capsular polysaccharides provides an effective multivalent conjugate vaccine with high antibody titers and a reduced carrier effect and results in reduction in the content of the capsular polysaccharide and protein per dose of vaccine which reduces reactogenicity.
Owner:INVENTPRISE INC
Epitope peptide H362 of HN protein in peste des petits ruminants virus (PPRV), and determination, preparation method and application thereof
ActiveCN107216372AStrong green fluorescenceGood reactogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinHN Protein
The invention relates to an epitope peptide H362 of an HN protein in PPRV, and determination, a preparation method and application thereof. The amino acid sequence of the epitope peptide is H362: <362>EANWVVPSTDVRDL<375>. The invention detects reactogenicity of a monoclonal antibody and PPRV and specificity of the monoclonal antibody; according to detection results, the monoclonal antibody has good reactogenicity to rPPRV-HN-F protein; immunoinformatic technology is cooperatively used for predicating the B cell epitope of the HN protein; an aminated ELISA plate is employed for detecting candidate epitopes and the monoclonal antibody 10E3, and the epitope peptide H362 corresponding to 10E3 is determined; and determination of the epitope peptide lays a theoretical foundation for preparation of epitope vaccine antigens and diagnostic reagent antigens for PPRV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant swine fever virus E2 protein swine source monoclonal antibody and preparation method and application thereof
ActiveCN107973850AEasy to prepareImprove responseImmunoglobulins against virusesAntiviralsHeavy chainSwine Fever Virus
The invention discloses a recombinant swine fever virus E2 protein swine source monoclonal antibody and a preparation method and application thereof. Firstly, the recombinant swine fever virus E2 protein swine source monoclonal antibody is disclosed, an amino acid sequence of a heavy chain is shown in SEQ ID NO.1, and an amino acid sequence of a light chain is shown in SEQ ID NO.2. The invention also discloses a suspension HEK293 cell line which can stably express the recombinant swine fever virus E2 protein swine source monoclonal antibody. The recombinant swine fever virus E2 protein swine source monoclonal antibody has the advantages that the coding genes of the heavy chain and light chain are cloned into an eukaryotic expression vector, and the stable and high-efficiency expression ofthe recombinant swine fever virus E2 protein swine source monoclonal antibody is realized by the suspension HEK293 cell line; the reactogenicity and neutralizing activity are good, and the important development value is realized in development of novel swine fever virus diagnosing and treating preparations.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation and use of giant panda Ascaris schroederi antigen
InactiveCN101348787AGood reactogenicityImprove diagnostic toolsGenetic material ingredientsAntiparasitic agentsBacteroidesAntigen
The invention discloses preparation and application of a giant panda Ascaris schroederi Mcintosh antigen, which belong to the giant panda Ascaris schroederi Mcintosh prevention, treatment and detection field. The method comprises the following steps: a Ascaris schroederi Mcintosh antigen gene primer is designed; total RNA of ascarids undergoes reverse transcription by the RT-PCR method to synthesize cDNA, and then the cDNA is taken as a template for PCR amplification of a target product; the purified target product is connected with a pMD18-T vector and then converted into DH5 alpha competent bacteria; positive recombinant clone is selected through flat screening and culture of the bacteria, and the antigen genes have a Bs-Ag1gene, a Bs-Ag2 gene and a Bs-Ag3 gene after culture and sequencing; and then recombinant plasmids which are accurately sequenced are converted into Escherichia coli BL21 competent cells for mass expression of proteins after construction of the recombinant plasmids, induction expression and purification of the recombinant proteins. After detection and immunologic tests of the product and ELISA detection and analysis of a test animal antibody IgG, the Bs-Ag1gene, the Bs-Ag2 gene and the Bs-Ag3 gene of the giant panda Ascaris schroederi Mcintosh antigen can be taken as candidate genes for preparing genetic engineering vaccines through the sascarids; and the recombinant proteins Bs-Ag1, Bs-Ag2 and Bs-Ag3 have good reactionogenicity and can be used for detecting infection of giant panda Ascaris by the ELISA method.
Owner:SICHUAN AGRI UNIV
1-type duck hepatitis A virus VP2 recombinant protein, ELISA kit and preparation method thereof
ActiveCN105273065AGood reactogenicitySsRNA viruses positive-senseVirus peptidesDuck hepatitis A virusInclusion bodies
The invention belongs to the technical field of bioengineering and particularly relates to a 1-type duck hepatitis A virus VP2 recombinant protein, an ELISA kit and a preparation method thereof. The amino acid sequence of the 1-type duck hepatitis A virus VP2 recombinant protein is shown as SEQ ID NO:1. The preparation method comprises the following steps of: acquiring a VP2 target segment; constructing recombinant expression plasmid pProEx-HTb-VP2; preparing VP2 recombinant protein. The successfully-obtained VP2 recombinant protein is expressed in an insoluble inclusion body, the VP2 recombinant protein has good reactogenicity with rabbit-anti-DHAV-1 serum, and proved by the above, prokaryotic expression is successfully obtained by VP2 protein of DHAV-1; the ELISA kit for detecting 1-type duck hepatitis A virus antibody is established by the expressed VP2 recombinant protein, and provides test data and basic materials for detecting the DHAV-1 antibody and further performing relevant study of the DHAV-1.
Owner:SICHUAN AGRI UNIV
HCMV pp65 prokaryotically-expressed recombinant strain and construction method thereof
The invention discloses HCMV pp65 gene fragment-containing engineering bacteria. An expressed recombinant pp65 protein secreted by the HCMV pp65 gene fragment-containing engineering bacteria can be used as an antigen, has good reactogenicity, can be used for preparation of corresponding antigen-diagnosis monoclonal antibodies for animal immunization, and provides a basis for development of an ELISA rapid diagnosis antibody kit. The recombinant antigen can be used as an excellent material for preparation of a pp65 subunit vaccine.
Owner:HUNAN NORMAL UNIVERSITY
Immunologic Constructs and Methods
InactiveUS20140235836A1Easily refoldedAmenable to manufactureSsRNA viruses negative-senseAntibody mimetics/scaffoldsSpatial OrientationsFlagellin
The present invention relates to improved vaccines and the design and making of such vaccines that enhance immunogenicity of the vaccine and / or reduce reactogenicity to the vaccine when administered. In particular the vaccines and immunogenic compositions of the present invention relate to flagellin-antigen fusion proteins in which the spatial orientation of the flagellin to antigen and the charge distribution of the antigen is optimized to enhance immunogenicity and / or reduce reactogenicity and / or improve folding of the protein.
Owner:VAXINNATE
Method for preparing gosling plague virus-like granules with escherichia coli system
ActiveCN106754981AGood reactogenicityVirus peptidesInactivation/attenuationEscherichia coliDigestion
The invention relates to a method for preparing gosling plague virus-like granules with an escherichia coli system for soluble expression of gosling plague virus VP2 protein. The method for soluble expression of gosling plague virus VP2 protein comprises the following steps: performing codon optimization on a gosling plague virus VP2 gene, performing site-specific mutagenesis, namely, mutating a codon AGA into CGC and mutating GGA into GGT, cloning to a pET-Sumo vector, establishing a recombinant expression vector pET-Sumo-VP2, transforming the pET-Sumo-VP2 into a prokaryotic expression bacterium, and inducing with IPTG (isopropyl beta-D-1-Thiogalactopyranoside) at 37 DEG C so as to obtain soluble recombinant VP2 recombinant protein; and performing digestion on the recombinant protein with a ULP enzyme, and purifying with a Ni column, thereby obtaining purified VP2 protein. Electron microscope results show that the gosling plague virus-like granules can be prepared from VP2 protein after digestion, and moreover, the purified VP2 protein has good reactogenicity and can be applied to preparation of subunit vaccines of gosling plague virus genetic engineering.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Mycobacterium tuberculosis ESAT-6 recombinant dipolymer, preparation method and application thereof
InactiveCN101805397AIncreased antigen specificityIncreased sensitivityMicroorganism based processesDepsipeptidesSerodiagnosesSkin test
The invention belongs to the field of biological engineering and diagnostic medicine, in particular to a mycobacterium tuberculosis ESAT-6 recombinant dipolymer, a preparation method thereof, an antigenicity analysis and an application thereof in the serodiagnosis of tuberculosis. The invention uses the DNA of a chimeric gene nucleic acid vaccine HG856A plasmid containing 2 * esat-6 copies as the template, obtains 2 * esat-6 gene segments by PCR amplification, and clones, expresses and purifies an ESAT-6 recombinant dipolymer protein in E.coli. The protein is connected with the two ESAT-6 monomers through amino acid Tyr and Val which are coded by the AccI restriction enzyme cutting site, i.e. GTC TAC, carries six* His tags at the N end, and can be purified by nickel-chelate affinity chromatography. Experiments confirm that the purified recombinant dipolymer has favorable reactogenicity and antigenic specificity. The recombinant dipolymer can be used as an antigen for the early diagnosis of the tuberculosis, including the serodiagnosis of ELISA, ELISPOT or the like, or can be used as an antigen for a skin test.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
ELISA kit for rapidly testing melamine content and method thereof
The invention relates to the preparation of an antigen and an antibody of a small-molecule addictive melamine or analogues thereof related to food security, and a method for semi-quantifying or quantifying samples potentially containing the melamine or the analogues thereof. Based on the principle of specific reaction of the antigen and the antibody, the invention utilizes a specific antibody of the melamine to test the concentration of the melamine or the analogues thereof in the samples with a blocking-ELISA method. The antigen is an artificially prepared immunogen and a coatingen of the melamine with reactionogenicity and immunogenicity, which are prepared by using immunogen immunoassay animals, and the blocking-ELISA theory is utilized to establish the melamine testing method. The melamine antigen provided by the invention owns good immunogenicity and the coatingen obtained owns good activity. The testing method established is capable of testing the melamine content in milk, dairy products and feedstuff without non-specific reaction with other components in milk, dairy products and feedstuff.
Owner:CUSABIO TECH LLC
African swine fever virus CD2v extracellular domain recombinant protein and application thereof
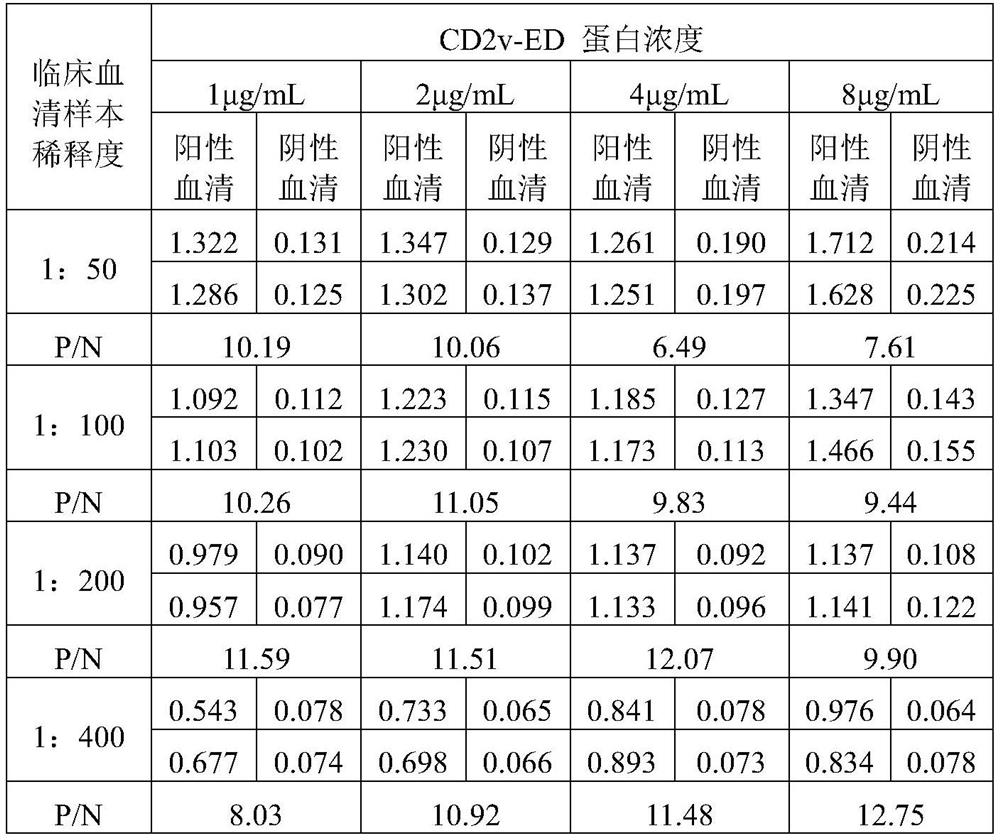
ActiveCN112877348ASet accurate and scientificReduce false positive rateVirus peptidesMicroorganism based processesClassical swine fever virus CSFVAfrican swine fever virus Antibody
The invention belongs to the technical field of biology, particularly relates to an African swine fever virus CD2v extracellular domain recombinant protein, and further discloses the application of the African swine fever virus CD2v extracellular domain recombinant protein to construction of ELISA (enzyme-linked immuno sorbent assay) and an ELISA detection kit prepared from the African swine fever virus CD2v extracellular domain recombinant protein and used for detecting the African swine fever virus. According to the scheme, prokaryotic expression CD2v extracellular domain recombinant protein is constructed based on the characteristics of strains popular in China at present, the recombinant protein has high acquisition amount, high purity and good reactogenicity and can induce an organism to generate a neutralizing antibody, and an ELISA kit for detecting the African swine fever virus can be further constructed based on the recombinant protein. The kit has high specificity, sensitivity and repeatability, and can be used as a reliable detection method for monitoring the African swine fever virus antibody or recognizing the virus.
Owner:CHINA AGRI UNIV
Kit for detecting avian onfectious synovitis antibody and preparation method
The invention provides an ELISA kit for detecting avian onfectious synovitis antibody. The kit adopts the ELISA kit for detecting the avian onfectious synovitis antibody, based on avian onfectious synovitis pyruvate kinase (PYK) recombinant protein as envelope antigen. The invention further provides a preparation method of the envelope antigen of the kit, namely PYK protein. The PYK protein prepared through the method has excellent reactogenicity, and the ELISA detection kit provides an effective method for the diagnosis of avian onfectious synovitis and the detection of an antibody level after immunization, and has an obvious promotion effect on the prevention research on avian onfectious synovitis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
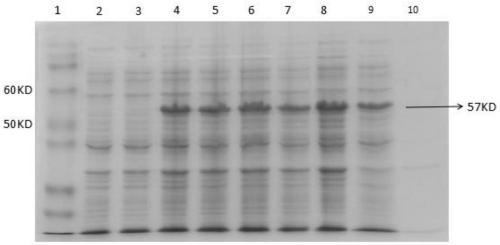
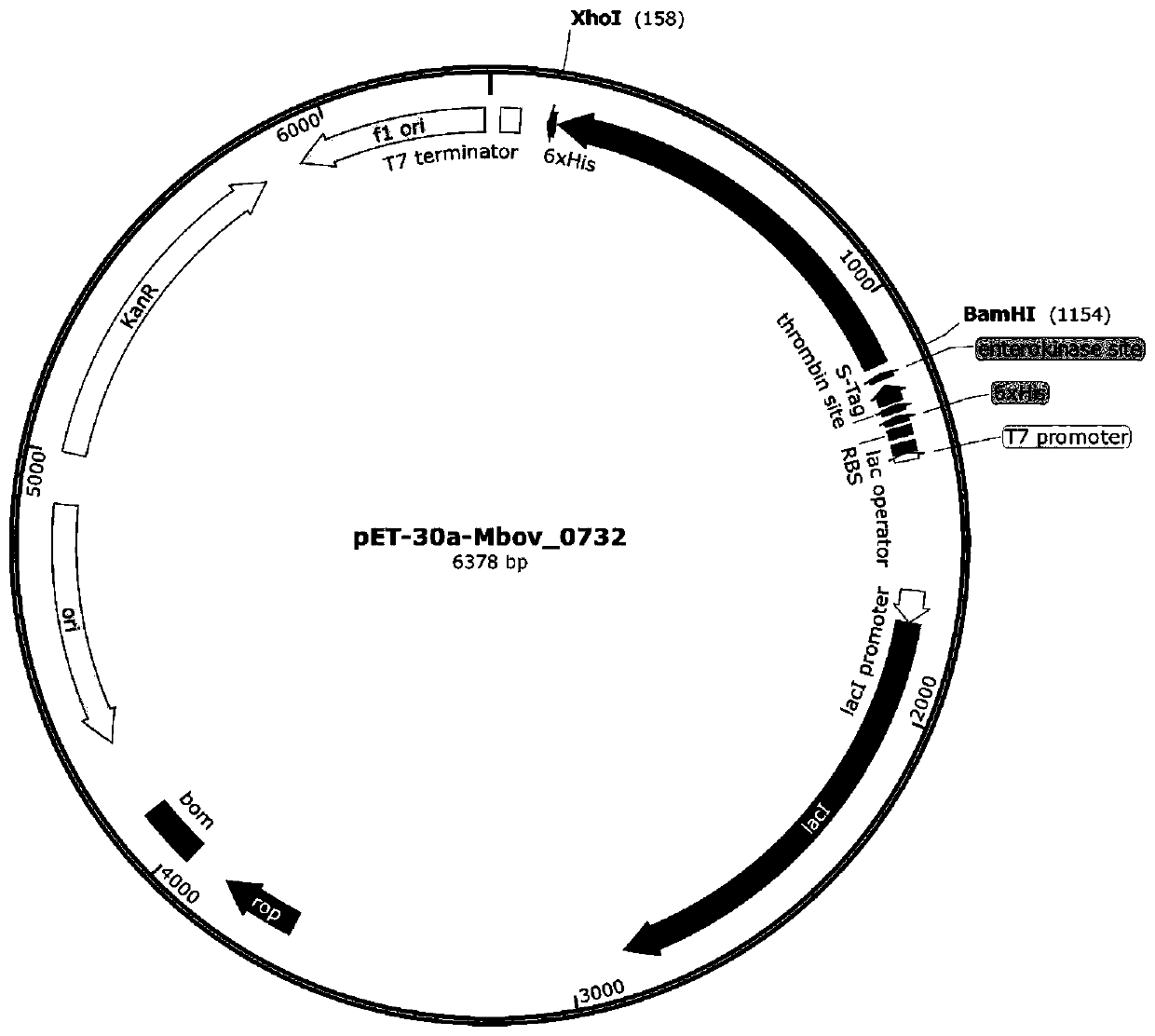
Bovine mycoplasma hypothetical protein MbovP732 and application thereof
InactiveCN110483625AValid differential diagnosisBacteriaImmunoglobulins against bacteriaNucleotide sequencingFhit gene
The invention discloses a bovine mycoplasma hypothetical protein MbovP732 and application thereof. The bovine mycoplasma hypothetical protein MbovP732 has an amino acid sequence as shown in the sequence table SEQ ID NO:1, and a gene encoding the protein has a nucleotide sequence as shown in the sequence table SEQ ID NO:2. The bovine mycoplasma hypothetical protein MbovP732 has reactogenicity and immunogenicity, can specifically react with bovine mycoplasma wild strains, and cannot react with vaccine strains, therefore, the bovine mycoplasma hypothetical protein MbovP732 can be used for distinguishing infection of the bovine mycoplasma wild strains from immunization of the vaccine strains.
Owner:HUAZHONG AGRI UNIV
Porcine lawsonia intracellularis IPMA antigen detection method and application thereof
PendingCN111830257AGood antigenicityImprove hydrophilic abilitySerum immunoglobulinsMaterial analysis by observing effect on chemical indicatorInfected cellStaining
The invention relates to a porcine lawsonia intracellularis immunoperoxidase monolayer assay (IPMA) antigen detection method. The rabbit anti-lawsonia intracellularis SodC protein polyclonal antibodyis prepared by taking purified recombinant SodC protein as immunogen, and the titer and the reactogenicity are detected. The lawsonia intracellularis IPMA antigen detection method is established by taking lawsonia intracellularis positive bacteria as a coating antigen and taking a prepared polyclonal antibody as a primary antibody. The method has good specificity, sensitivity and repeatability, expensive instruments such as a fluorescence inversion microscope, a microplate reader and a PCR instrument are not needed, operation is easy and convenient, judgment is more visual, the dyed microporous plate can be stored for a long time, the method is suitable for screening of a large number of samples, and a new means is provided for positioning and detection of lawsonia intracellularis in infected cells.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Prokaryotic soluble expression method of VP3 gene of South African type 2 foot-and-mouth disease virus
PendingCN110951757AEasy to purifyGood reactogenicitySsRNA viruses positive-senseVirus peptidesEscherichia coliDisease
The invention discloses a prokaryotic soluble expression method of VP3 gene of a South African type 2 foot-and-mouth disease virus. The method comprises the following steps: according to a gene sequence of the South African type 2 foot-and-mouth disease virus, directly coupling an SUMO solubilization expression tag coding gene to the upstream of a VP3 protein coding gene of FMDV SAT2; introducingsix His tag coding genes into the upstream of SUMO; then, according to escherichia coli codon tropism, optimizing the HisSUMO-SAT2-VP3 gene; recombining the gene to a PUC57 plasmid; taking a HisSUMO-SAT2-VP3-PUC57 plasmid as a template; designing a specific primer for amplifying the South African type 2 foot-and-mouth disease virus VP3 gene; carrying out PCR amplification, and carrying out enzymedigestion on a target fragment and an expression vector by using the same restriction enzyme; cloning a target gene into a pET32a (+) prokaryotic expression vector; constructing a recombinant plasmidpET32a-HisSUMO-VP3; transferring the recombinant plasmid into escherichia coli for expression; and purifying recombinant protein. According to the invention, the pET32a-HisSUMO-VP3 prokaryotic expression vector is successfully constructed, expression in the escherichia coli is achieved, the purified target protein has good reactogenicity, and a foundation is laid for subsequent assembly of a SouthAfrican type 2 foot-and-mouth disease virus subunit vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Fusion protein SUMO1-PLA2, and preparation method and medical use thereof
InactiveCN105542012AOvercoming low protein expressionSolving technical challenges of insoluble expressionHydrolasesMicrobiological testing/measurementInclusion bodiesChemistry
The invention provides a fusion protein SUMO1-PLA2, and a preparation method thereof. The invention also provides a medical use of the SUMO1 fusion protein to solve the technical problem of insoluble expression of PLA2 in Escherichia coli. The fusion protein SUMO1-PLA2 is efficiently prepared by using a pColdTF cold-induced expression vector. The defects of low PLA2 protein expression level, subsequent purifying inconvenience brought by existence of an expression product in an inclusion body form, and lower reactogenicity and immunogenicity of the expression product than soluble proteins in the prior art are overcome in the invention.
Owner:JILIN UNIV
Multi-epitope antigen of porcine epidemic diarrhea virus, and encoding gene, preparation method and application thereof
ActiveCN110093357AAvoid reactionAvoid problems such as virulence reversionSsRNA viruses positive-senseViral antigen ingredientsStructural proteinAntigenic protein
The invention provides a gene encoding a multi-epitope antigen of porcine epidemic diarrhea virus, the multi-epitope antigen of the porcine epidemic diarrhea virus, and a preparation method of the multi-epitope antigen of the porcine epidemic diarrhea virus. The invention has the following advantages and beneficial effects: 1) the multi-epitope antigen of the porcine epidemic diarrhea virus is a multi-epitope antigen using norovirus P particles chimeric with the porcine epidemic diarrhea virus, natural structural proteins of pathogens are fully reduced, only main viral surface antigen proteinsare expressed, and nonspecific immunoreaction, strong virulence reversion and other problems induced by many irrelevant antigens are solved obviously; 2) the preparation method is low in preparationcost, short in time consuming and safety; and 3) the prepared multi-epitope antigen of the porcine epidemic diarrhea virus has good bioactivity and reactogenicity.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Modified pertussis toxin
The development of subunits and subunit analogs of the Bordetella exotoxin by recombinant DNA techniques provides vaccine products that retain their biological activity, are highly immunogenic, and can confer protection against disease challenge. Genetically-engineered modifications of the subunits can result in products that retain immunogenicity, yet are free of enzymatic activity associated with toxin of reactogenicity.
Owner:AMGEN INC
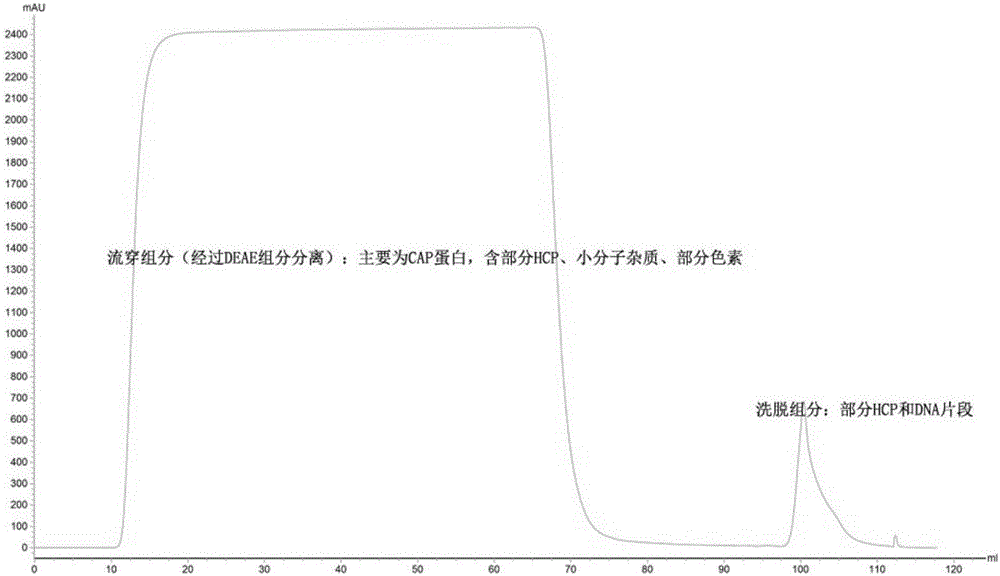
Chromatographic method for separating and purifying high-purity porcine circovirus Cap proteins
InactiveCN106432432AImproving immunogenicityImprove responseVirus peptidesPeptide preparation methodsProtein solutionHigh concentration
The invention relates to a chromatographic method for separating and purifying high-purity porcine circovirus Cap proteins. The chromatographic method comprises the following steps: (S1) grinding and breaking insect cells expressing the porcine circovirus Cap proteins, extracting supernatant, and filtering by virtue of a filter membrane with a pore diameter of 0.45 micron, so as to obtain an original sample solution containing the porcine circovirus Cap proteins; (S2) carrying out weak anion exchange chromatography on the original sample solution, so as to obtain a first purified solution; (S3) carrying out strong cation exchange exchange chromatography on the first purified solution, so as to obtain a second purified solution; and (S4) carrying out gel filtration chromatography on the second purified solution, so as to obtain a high-purity porcine circovirus Cap protein solution. By virtue of the chromatographic method, DNA, pigments and host cell proteins in a rough original sample solution obtained from the insect cells expressing the porcine circovirus Cap proteins can be removed, and the porcine circovirus Cap proteins are greatly concentrated so as to obtain the high-purity and high-concentration porcine circovirus Cap proteins, so that the immunogenicity and reactogenicity of the Cap proteins are improved, and side effects are reduced.
Owner:WUHAN HUIYAN BIOTECH
Recombined expression plamid vector, expression antige of N gene of bubble cell stomatitis virus and preparation method
This invention relates to a diagnostic reagent used in preparing VSV N protein recombination antigen and its preparation method. The reagent includes its N gene recombination expression plasmid vector and its antigen. The preparation method includes 1) cloning the VSV coding group specific antigen N gene fragment to pMD18-T plasmid vectors to make up of N gene clone recombination plasmid, 2) sub-cloning plugging into the pBAD / Thio TOPO expression vector, 3, converting TOP10 cells, 4) screening positive clones getting SVS N reading code frame.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR
Feline coronavirus S recombinant protein and preparation method thereof
ActiveCN113072626AImprove responseImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsTGE VACCINEAmino acid
The invention provides a feline coronavirus S recombinant protein, the amino acid sequence of which is shown as SEQ ID NO: 1. The invention also provides a preparation method of the feline coronavirus S recombinant protein, which comprises the following steps: (a) carrying out PCR (Polymerase Chain Reaction) amplification by taking the gene of the feline coronavirus S protein as a template, and cloning an S recombinant protein fragment; (b) carrying out electrophoretic separation on the S recombinant protein fragment, recovering and purifying, connecting to a vector, and tranforming to competent cells; (c) ligating the plasmid with the prokaryotic expression vector to obtain the recombinant plasmid. and (d) transforming the recombinant plasmid to BL21 for prokaryotic expression to obtain the feline coronavirus S recombinant protein. The inventor finds that the protein fragment in the FCoV S2 protein has good reactogenicity and immunogenicity for the first time, and can be used as a basis for subsequent research and development of FCoV vaccines and related diagnosis and detection kits.
Owner:LONGYAN UNIV
Multivalent huma-bovine retavirus vaccine
The present invention provides vaccine compositions for protection against human rotaviral disease without significant reactogenicity. Human×bovine reassortant rotavirus comprising each of the four clinically most important VP7 serotypes of human rotavirus are combined in a multivalent formulation which provides a high degree of infectivity and immunogenicity without producing a transient febrile condition. Methods for producing an immunogenic response without producing a transient febrile condition are also provided.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF HEALTH & HUMAN SERVICES THE
Mycoplasma bovis alcohol dehydrogenase gene and coded protein and application thereof
ActiveCN110257405AHas alcohol dehydrogenase activityAvoid infectionAntibacterial agentsBacteriaVirulent characteristicsNucleotide
The invention discloses a Mycoplasma bovis alcohol dehydrogenase gene, having a nucleotide sequence shown as SEQ ID NO: 1. The invention also discloses a protein coded by the Mycoplasma bovis alcohol dehydrogenase gene; the protein has an amino acid sequence shown as SEQ ID NO: 2 and belongs to the technical field of prevention and treatment of animal infectious diseases and biology. The protein, as a recombinant protein herein, has alcohol dehydrogenase activity, can attach to EBLs (embryonic bovine lung cells), can combine with bovine Fn (fibronectin), has good immunogenicity and reactogenicity, is a virulence-related protein of Mycoplasma bovis, and has a good application prospect in the research on pathogenesis of Mycoplasma bovis, and the research and development of vaccines and diagnostic reagents.
Owner:HUAZHONG AGRI UNIV
E2 recombinant protein and application thereof
PendingCN110746495AImprove purification effectStrong neutralizing activitySsRNA viruses positive-senseVirus peptidesBovine Viral Diarrhea VirusesBlot
The invention provides an E2 recombinant protein. The E2 recombinant protein has an amino acid sequence of a sequence table SEQ.ID.No.1, and the E2 recombinant protein has a base sequence of a sequence table SEQ.ID.No.2. The invention also provides application to an indirect ELISA kit for detecting bovine viral diarrhea virus antibodies. The E2 recombinant protein provided by the invention has high accuracy, not only is the neutrality of the protein ensured, but also the trouble of high mutation rate is avoided, and the recombinant protein is more beneficial to the development of a BVDV antibody detection technology. The E2 recombinant protein uses a traditional protein prokaryotic expression technology, the cost is low, the expression quantity is large, and after the successfully expressed E2 recombinant protein is subjected to urea gradient renaturation, the West blot detection reactionogenicity is strong. The E2 recombinant protein is applied to the indirect ELISA kit for detectingbovine viral diarrhea virus antibodies, and an established BVDV antibody ELISA detection method is high in sensitivity and good in specificity, is more beneficial to popularization and application ingrass-roots farmers, and is more beneficial to purification of BVDV in cattle herds in China.
Owner:SHIHEZI UNIVERSITY
gad gene of porcine globular adiponectin and protein encoded by gad gene and application
InactiveCN102181437AAdjustment functionRegulate balanceHormone peptidesBacteriaEscherichia coliBiotechnology
The invention discloses a gAd gene of porcine globular adiponectin, a protein encoded by the gAd gene and application. A method comprises the following steps of: designing primers, namely P3 and P4 on the basis of a sequence of the gAd, adding six His labels at a terminal 5'of the gAd gene, cloning a gene fragment to a cloning host, namely Escherichia coli, identifying and amplifying a recombinant plasmid, performing electroporation on an expression host, namely Lactococcus lactis by using the plasmid, further screening to obtain a modified Lactococcus lactis strain which can efficiently express the gAd, preparing an antiserum specifically resisting the gAd, and determining that the gAd protein expressed in vitro has the reactogenicity. A lactic acid bacteria microecological preparation rich in the porcine globular adiponectin is prepared; and an important basis is laid for regulating fat metabolism and fat deposition of pigs by expressing adiponectin by recombinant Lactococcus lactis NZ9000.
Owner:东莞市畜牧科学研究所 +1
Recombinant S1 protein of novel mutant strain of porcine epidemic diarrhea virus and subunit vaccine of recombinant S1 protein
ActiveCN104046637AGood reactogenicityAntiviralsAntibody medical ingredientsPseudomonas aeruginosa exotoxin ASeroconversion
The invention discloses a recombinant S1 protein of a novel mutant strain of a porcine epidemic diarrhea virus and a subunit vaccine of the recombinant S1 protein. The protein is obtained by the steps of cloning a fusion gene fragment PE(delta III)-S1(m)-KDEL3 containing a KDEL3 gene sequence, a gene sequence of III-region-deleted pseudomonas aeruginosa exotoxin A and an S1(m) gene sequence to a baculovirus vector to obtain a recombinant vector; carrying out recombinant vector site specificity transposition on a seroconversion DH10Bac competent cell of the recombinant vector to obtain a recombinant bacmid; transfecting the recombinant bacmid to an Sf9 cell under the mediation of a liposome to obtain a recombinant Sf9 cell; expressing by using the recombinant Sf9 cell. According to the invention, a PE(delta III)-S1(m)-KDEL3 fusion protein is firstly expressed by using a baculovirus expression system on the basis of optimizing the predilection of a codon of mammalian with S1 genes, so that the recombinant baculovirus for efficiently and accurately expressing the fusion protein is obtained. Proved by IFA and Western blot, the recombinant baculovirus can be used for accurately expressing the fusion protein, and the fusion protein has favorable reactionogenicity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com