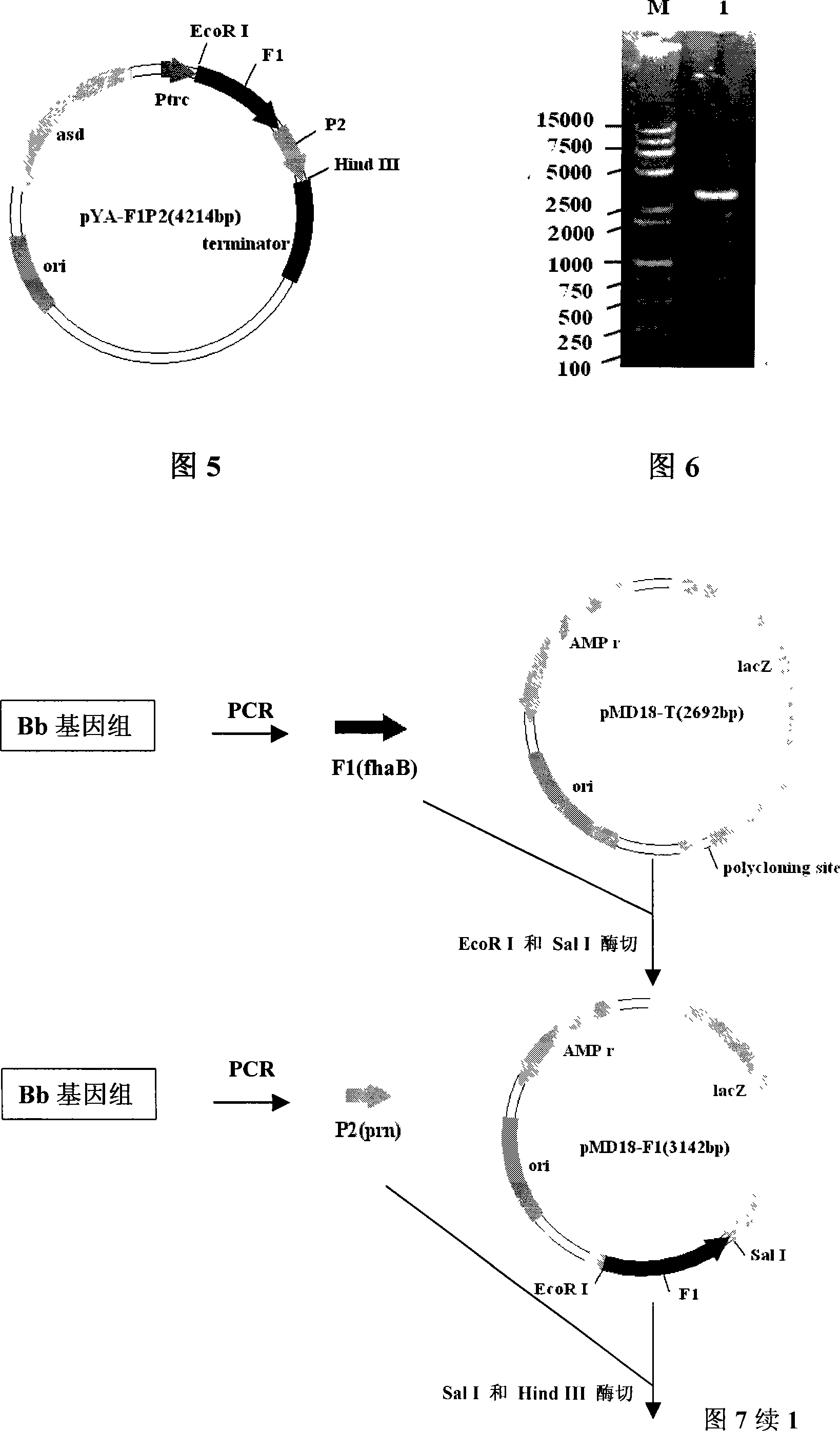
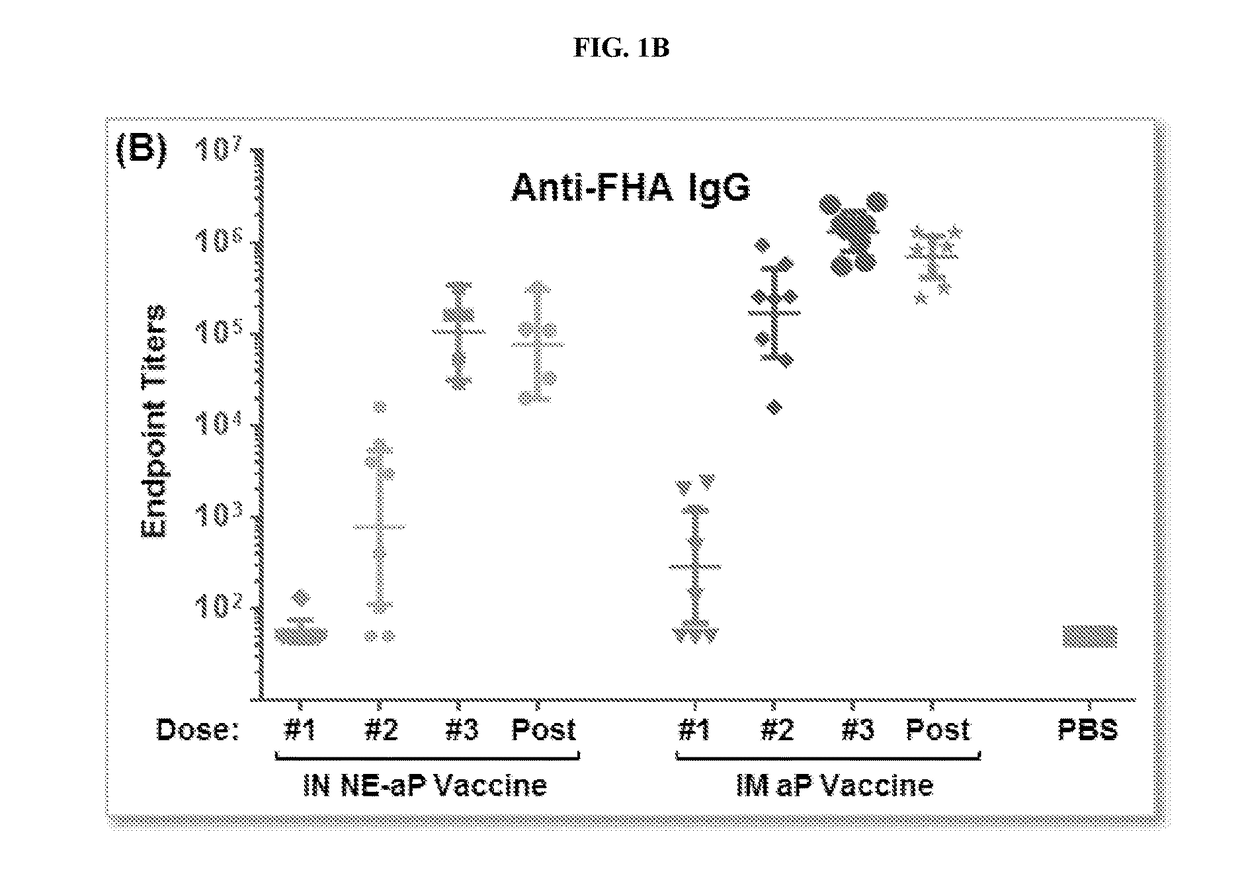
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Bordetella" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bordetella (/ˌbɔːrdəˈtɛlə/) is a genus of small (0.2 – 0.7 µm), Gram-negative coccobacilli of the phylum Proteobacteria. Bordetella species, with the exception of B. petrii, are obligate aerobes, as well as highly fastidious, or difficult to culture. All species can infect humans. The first three species to be described (B. pertussis, B. parapertussis, B. bronchiseptica); are sometimes referred to as the 'classical species'. Two of these (B. bronchiseptica and B. pertussis) are also motile.
Pertussis antigens and use thereof in vaccination
ActiveUS20070116711A1Readily apparentBacterial antigen ingredientsSugar derivativesVaccinationBacterial Adhesins
The invention provides BASB232 polypeptides and polynucleotides encoding BASB232 polypeptides and methods for producing such polypeptides by recombinant techniques. Also provided are diagnostic, prophylactic and therapeutic uses. The invention further provides immunogenic compositions comprising a plurality of antigens selected from at least two different categories of antigen, having different functions within Bordetella. Examples of such categories of antigen are autotransporter proteins, iron acquisition proteins, lipoproteins, adhesins and toxins / invasins.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
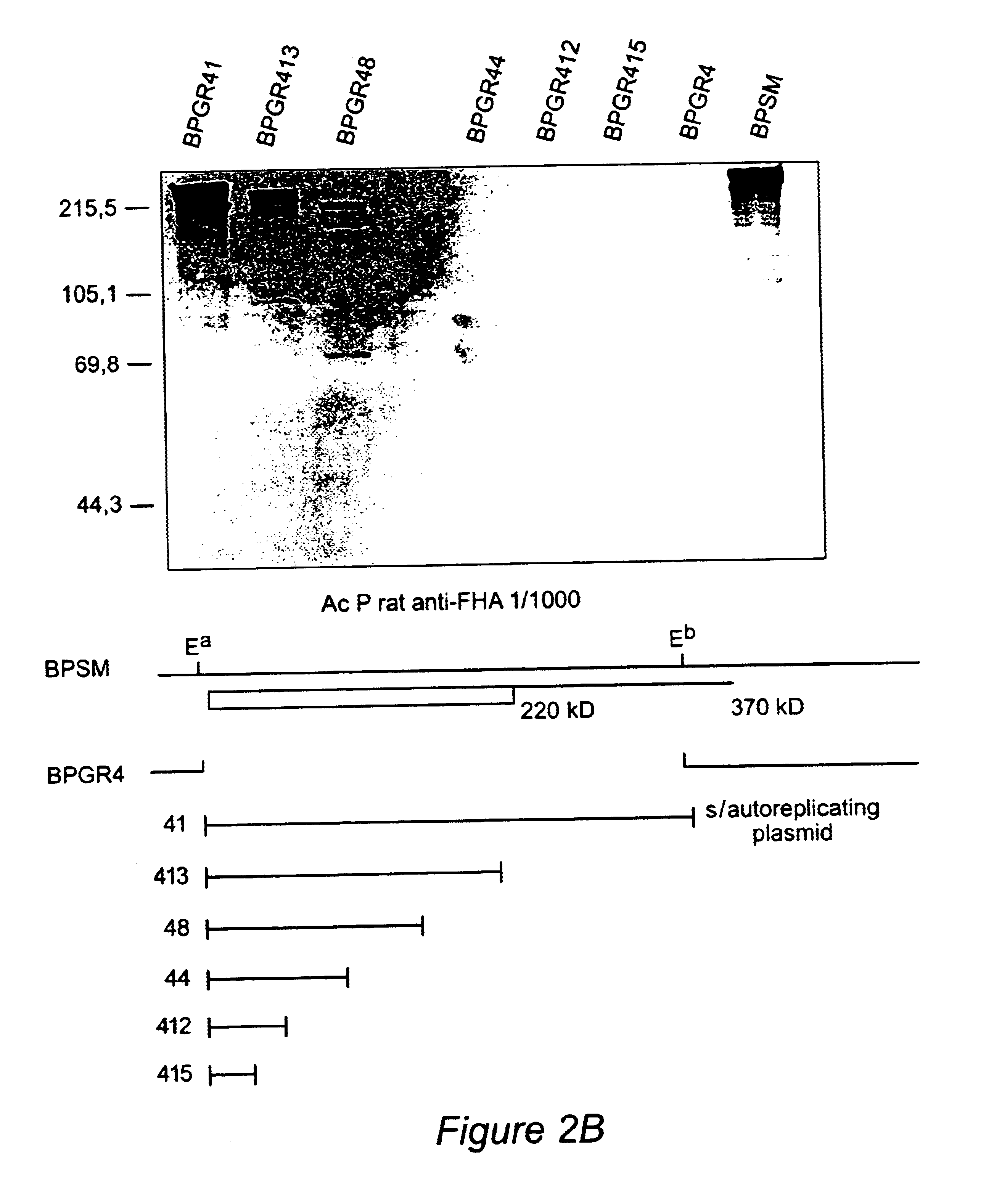
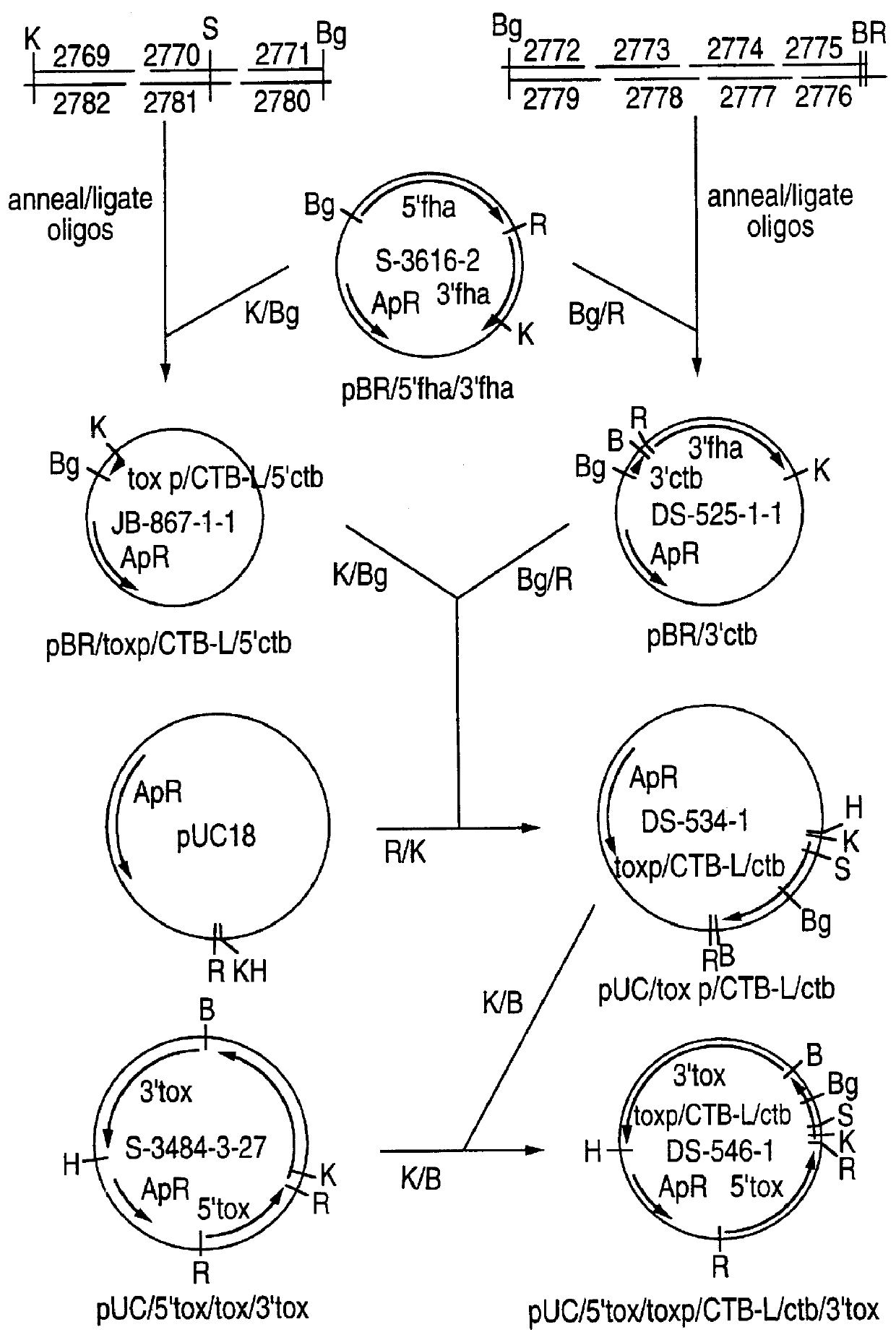
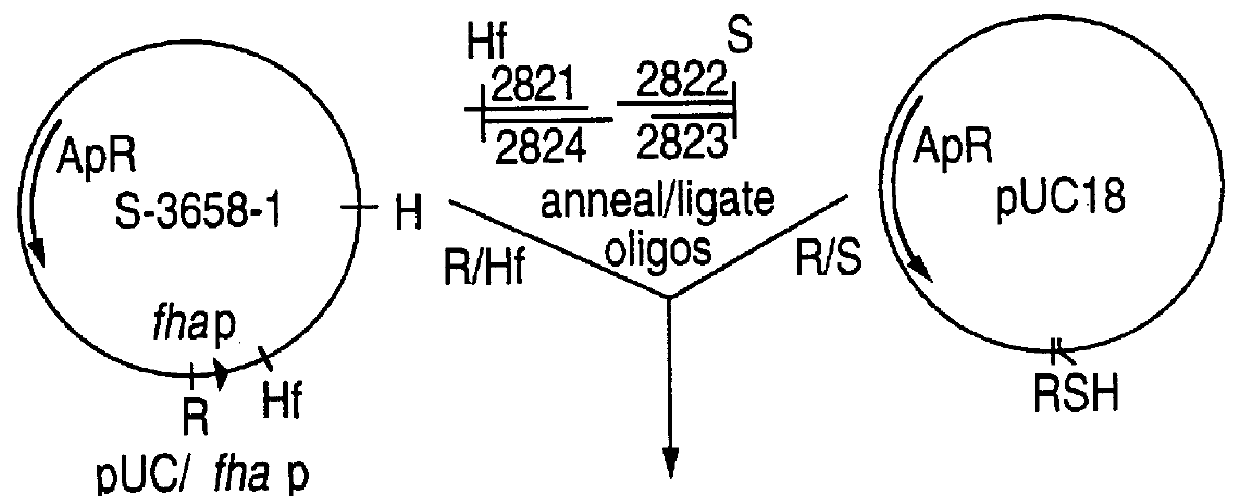
Recombinant proteins of filamentous haemagglutinin of bordetella, particularly bordetella pertussis, method for producing same, and uses thereof for producing foreign proteins of vaccinating active principles
InactiveUS6841358B1Easy to separateEasy to demonstrateBiocideBacterial antigen ingredientsHeterologousPolymerase L
A recombinant DNA containing a sequence (1) coding for a polypeptide heterologous to a filamentous haemagglutinin of Bordetella (Fha) fused within the reading frame to a sequence (2) located upstream from the first sequence. Sequence (2) codes for at least part of the Fha precursor, which part comprises at least the N-terminal region of a truncated mature Fha protein, which contains the interaction site of Fha and heparin and the secretion domain. This Fha protein is under the control of a promoter recognized by the cell polymerases of B. pertussis and is inserted into a B. pertussis cell culture, is expressed in the culture and excreted into the cell culture medium. The invention uses both the abilities of Bordetella and particularly B. pertussis to secrete or surface expose the heterologous polypeptide fused to the Fha portion corresponding to sequence (2), which does not appear to produce extracellular proteases, and the ease with which filamentous haemagglutinins can be isolated from other Bordetella proteins.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Canine combination vaccines
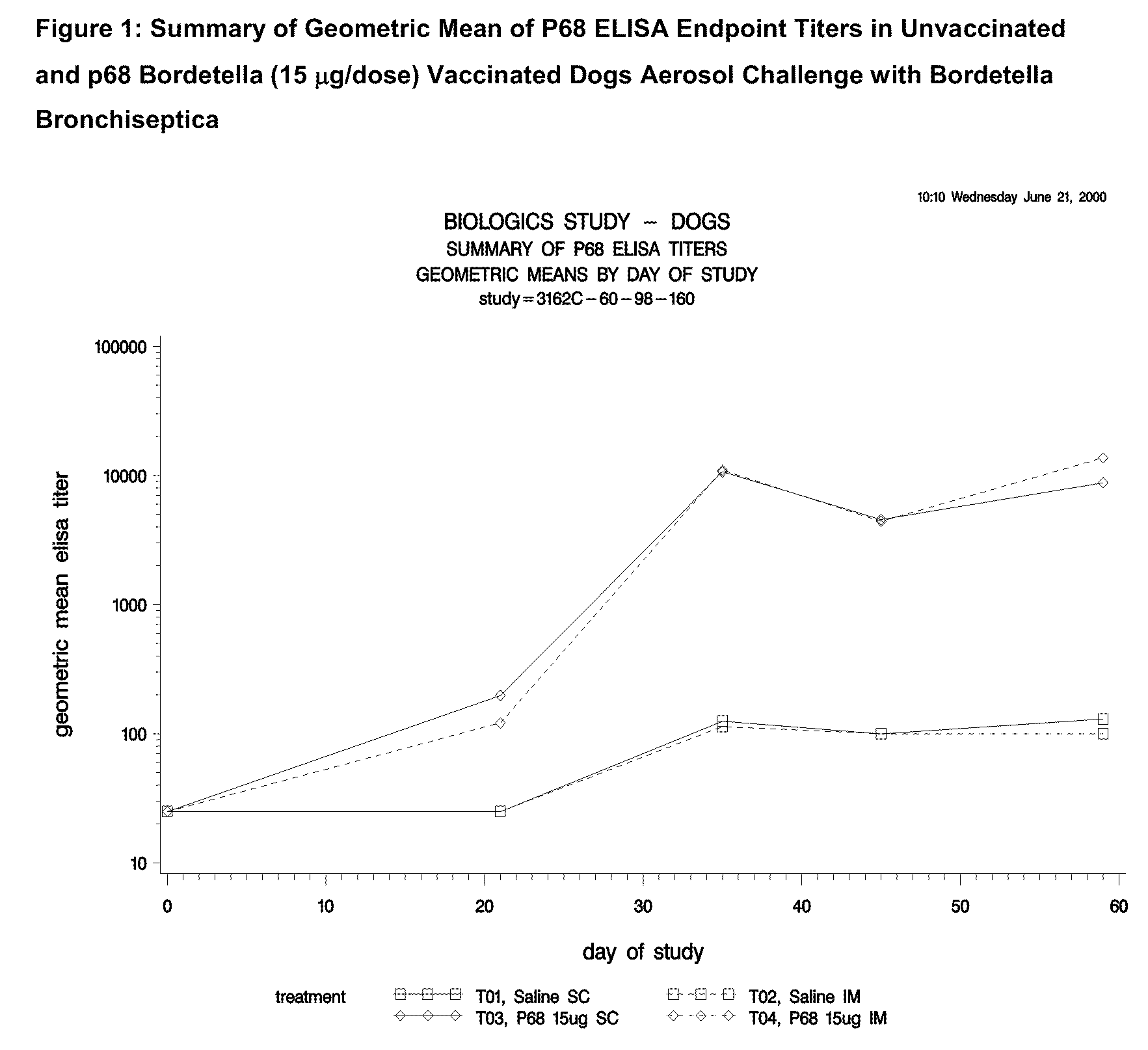
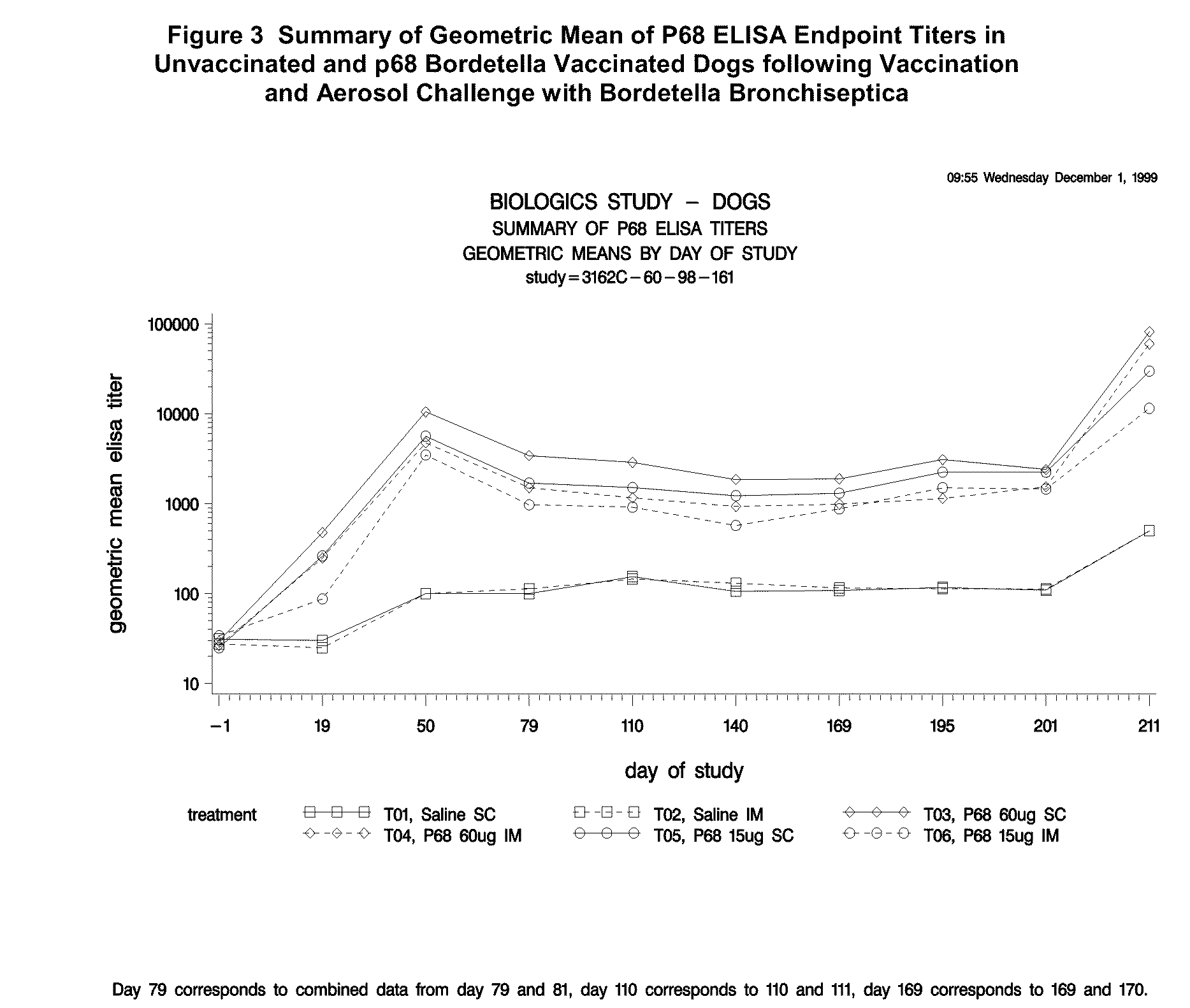
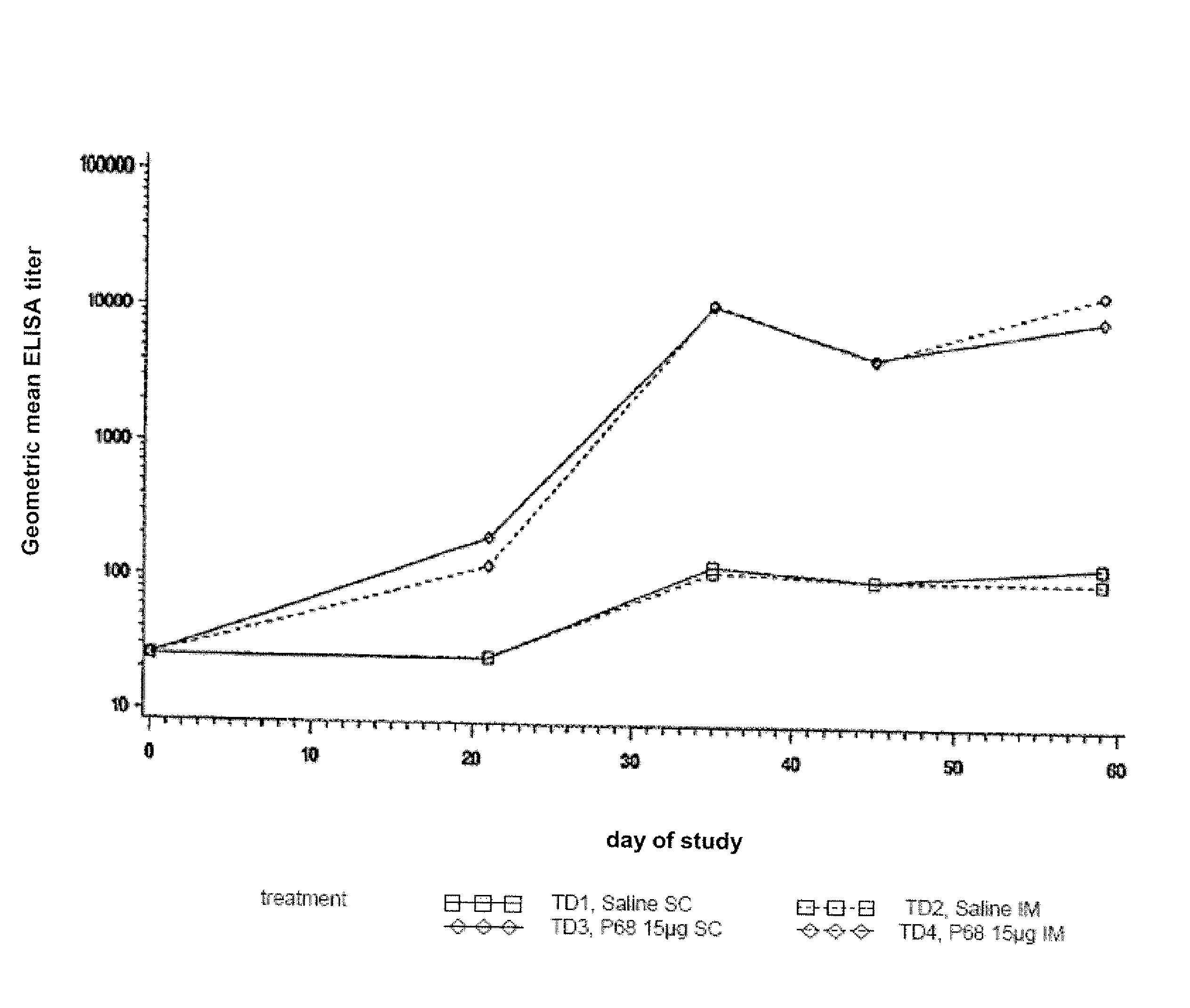
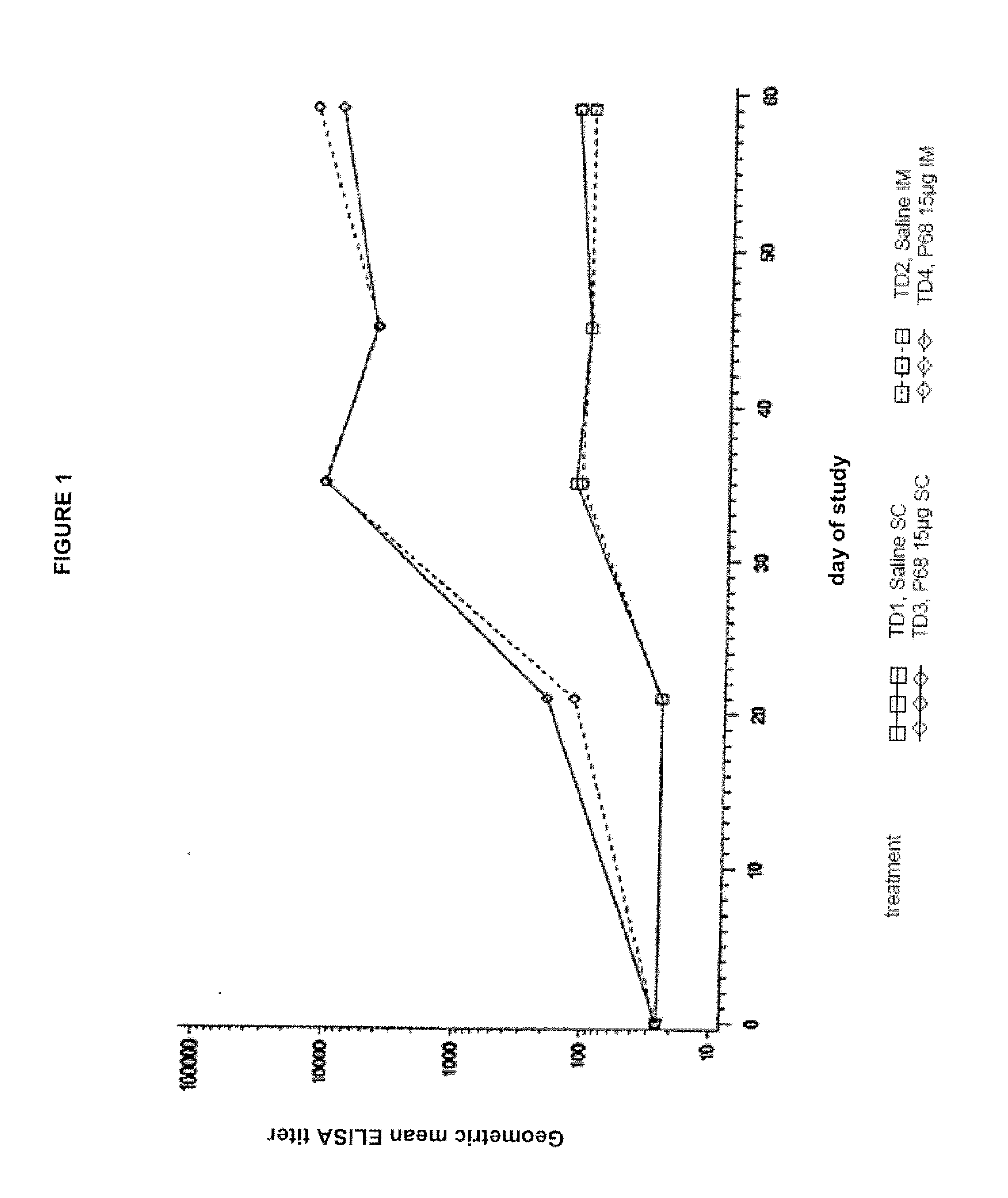
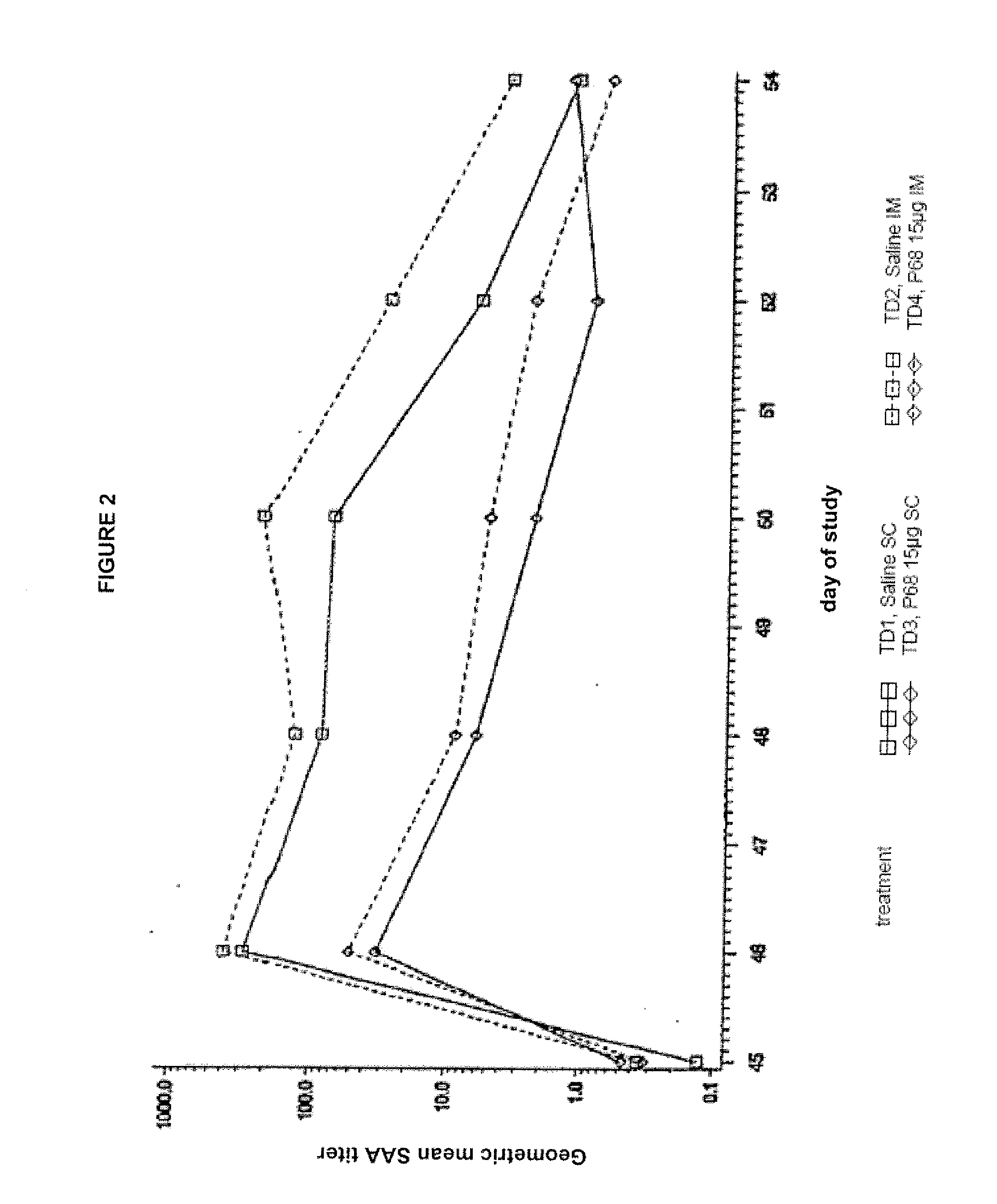
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
Acellular antibordetella vaccine
The invention relates to an immunogenic composition, characterized in that it comprises an adenyl cyclase-hemolysin (AC-Hly) protein, or an immunogenic portion of this AC-Hly, of a strain of Bordetella chosen from B. Tertussis, B. parapertussis or B. bronchiseptica, and in that it comprises, in addition, a bacterial extract containing the expression products of the vrg genes of a strain of Bordetella chosen from B. pertussis, B. parapertussis or B. bronchiseptica, or a portion of these expression products which is sufficient to induce an immune response in a host to which the extract might be administered.
Owner:INST PASTEUR
Immunogenic Bordetella Bronchiseptica Compositions
PendingUS20130302369A1Avoid infectionAntibacterial agentsSsRNA viruses negative-senseRespiratory infectionBordetella
Provided herein are compositions, combinations, and methods comprising Bordetella bronchiseptica and isolated pertactin, which are effective in treating or preventing respiratory infections, such as kennel cough, in animals.
Owner:ZOETIS SERVICE LLC
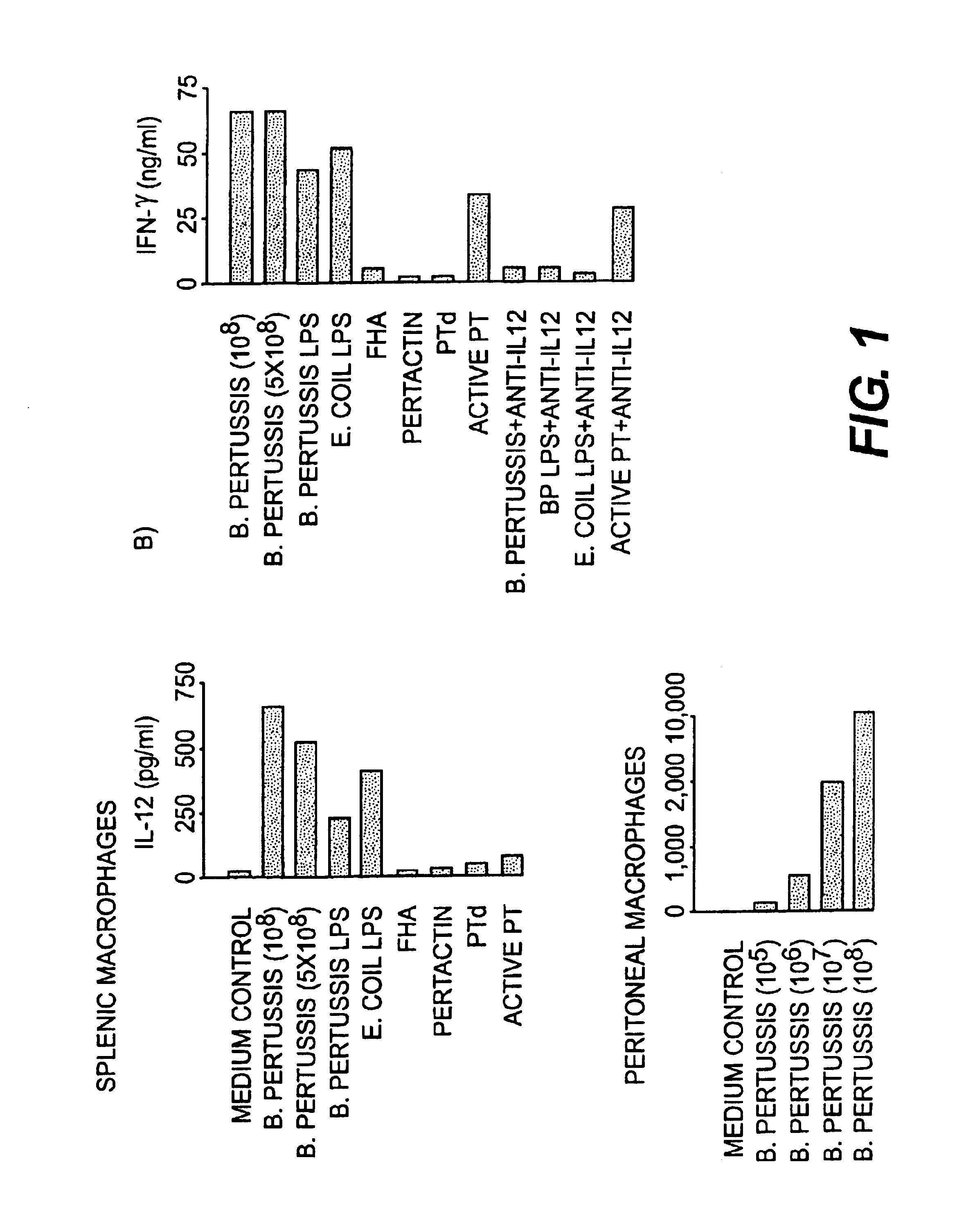
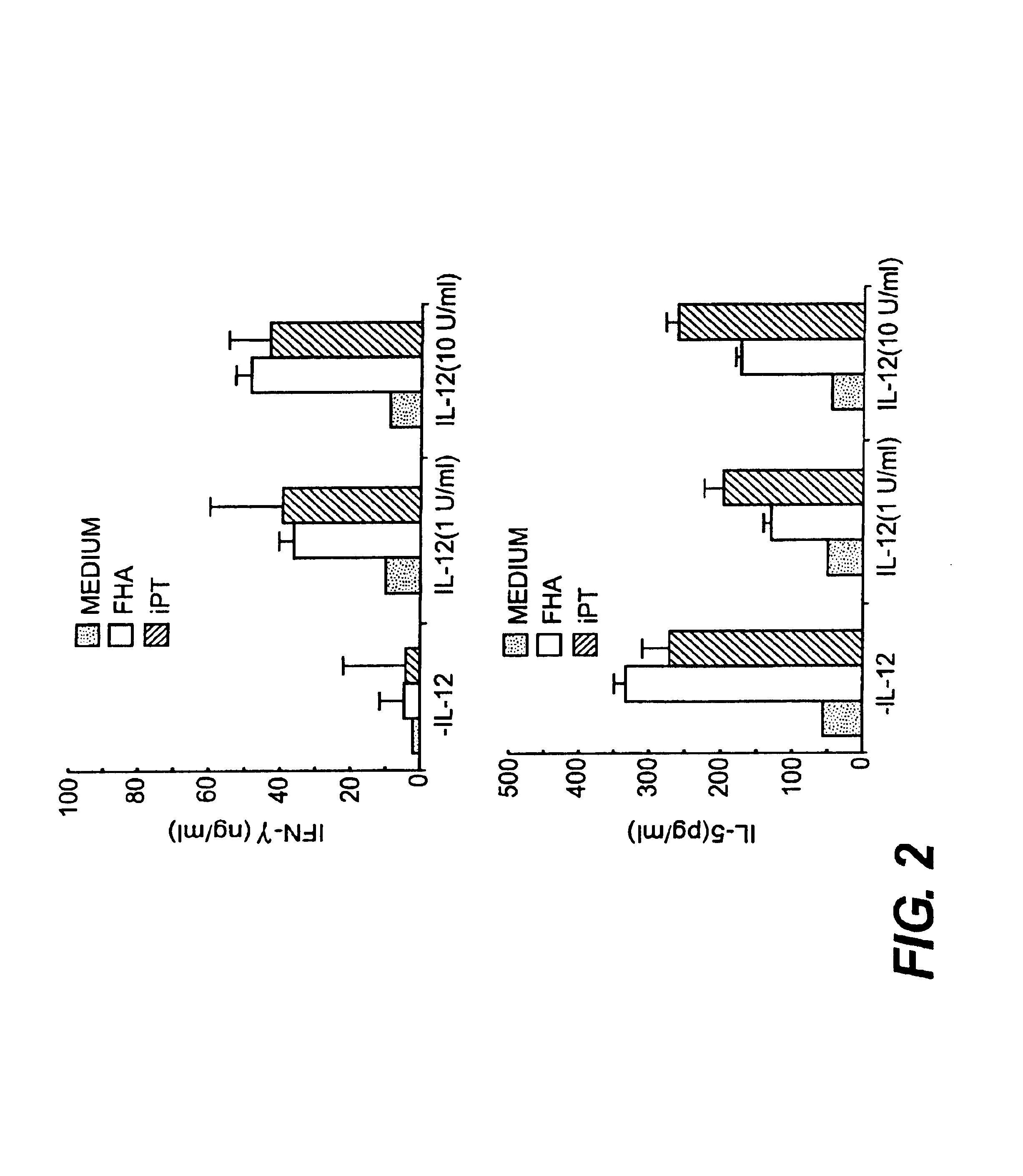
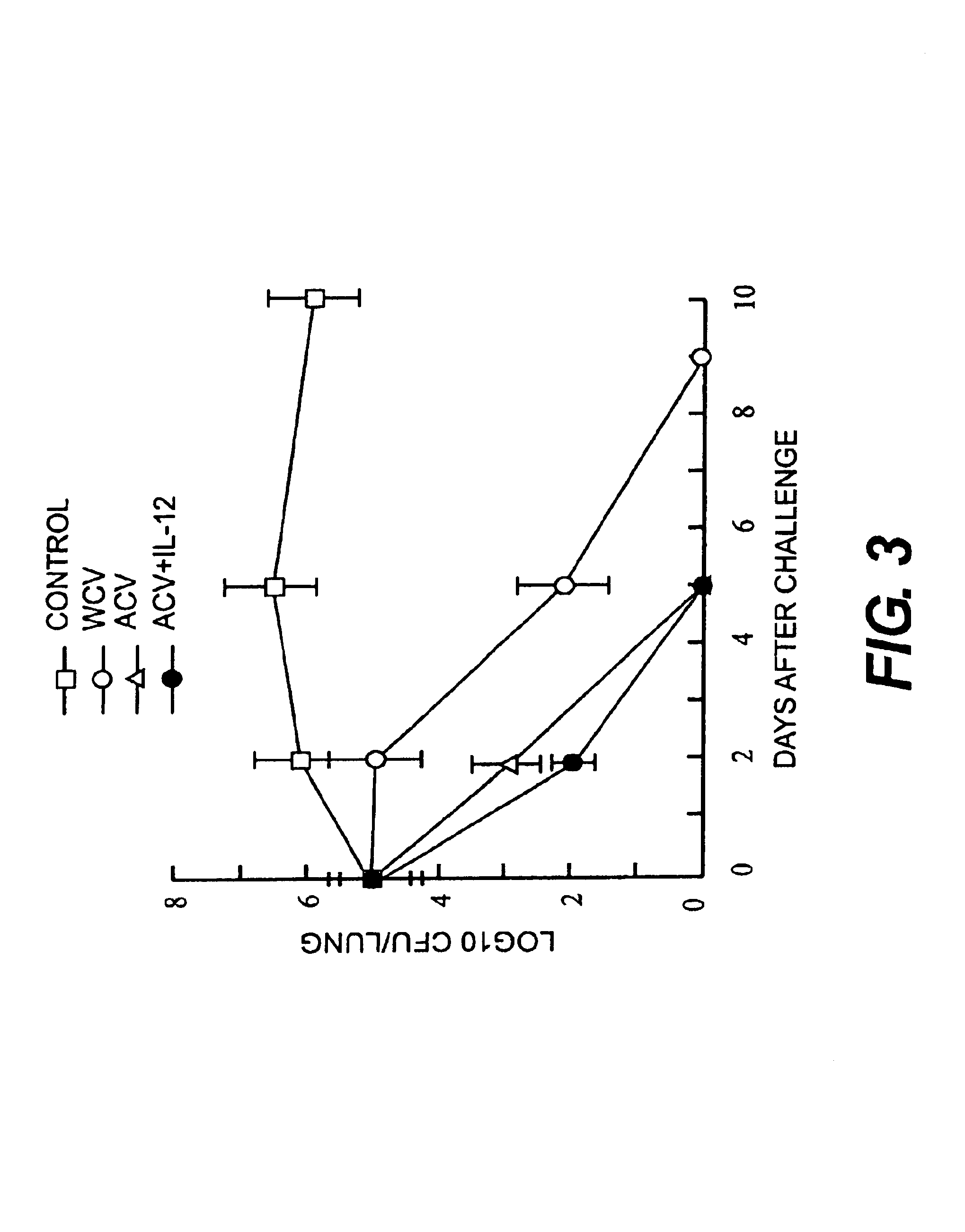
IL-12 as an adjuvant for Bordetella pertussis vaccines
This invention provides a composition of at least one Bordetella antigen and an effective adjuvant amount of interleukin-12 (IL-12), and uses thereof as a vaccine against Bordetella infection. Methods for using IL-12 as an adjuvant in combination with vaccines against Bordetella are also provided.
Owner:NATIONAL UNIVERSITY OF IRELAND
Recombinant salmonella choleraesuis strain for expression of pig origin bordetella bronchisepatica fhaB and prn gene segment, bacterin and uses thereof
InactiveCN101157907AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesBordetella
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Immunogenic compositions for use in vaccination against bordetella
InactiveUS20180071380A1Efficient combinationAntibacterial agentsBacterial antigen ingredientsVaccinationVirulent characteristics
The present application relates to immunogenic compositions comprising a mixture of Bordetella (e.g., B. pertussis) antigens and an oil in water nanoemulsion. In particular, the invention provides immunogenic compositions comprising nanoemulsion and a combination of Bordetella (e.g., B. pertussis) antigens that have different functions, for example, combinations including B. pertussis adherence factors (adhesins), B. pertussis toxins or B. pertussis virulence factors. Vaccines, methods of treatment, uses of and processes to make a pertussis or whooping cough vaccine are also described. Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications.
Owner:NANOBIO CORP +1
Influenza vaccine, composition, and methods of use
ActiveUS20120121647A1Elicit immune responseSsRNA viruses negative-senseBacterial antigen ingredientsMammalBordetella
The invention relates to compositions and vaccines that include a mutated Bordetella strain for treating or preventing an influenza infection in a mammal. In addition, the invention further provides methods for protecting a mammal against infection by influenza and / or eliciting an immune response against an influenza virus in a mammal using the composition or vaccine.
Owner:INSTITUT PASTEUR DE LILLE +2
Expression of gene products from genetically manipulated strains of bordetella
InactiveUS6140082AHigh expressionPolypeptide with localisation/targeting motifBacteriaHeterologousGene product
An expression system for expressing gene products from recombinant Bordetella strains and specific nucleic acid molecules useful in transforming Bordetella strains for such expression are described. A nucleic acid molecule may comprise a Bordetella promoter operatively coupled to a heterologous gene encoding a non-Bordetella gene product with the heterologous gene transcriptionally regulated by the Bordetella promoter. The nucleic acid molecule may further comprise a further nucleic acid molecule encoding a leader sequence for secretion of the non-Bordetella gene product. Another nucleic acid molecule may comprise a Bordetella promoter coupled to a nucleic acid sequence encoding a non-Bordetella leader sequence for secretion of a gene product, which may be a Bordetella gene product or a non-Bordetella gene product.
Owner:CONNAUGHT LAB
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
Canine combination vaccines
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
Modified pertussis toxin
The development of subunits and subunit analogs of the Bordetella exotoxin by recombinant DNA techniques provides vaccine products that retain their biological activity, are highly immunogenic, and can confer protection against disease challenge. Genetically-engineered modifications of the subunits can result in products that retain immunogenicity, yet are free of enzymatic activity associated with toxin of reactogenicity.
Owner:AMGEN INC
Triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and preparation method of vaccine
ActiveCN110201153AProduced fastHigh immune attack protection rateAntibacterial agentsBacterial antigen ingredientsDiseaseBordetella
The invention relates to a triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and a preparation method of the vaccine. The effective components of the vaccine comprise VP60 protein antigen of rabbit viral hemorrhagic disease virus, inactivated pasteurella multocida QLT-1 strain and bordetella bronchiseptica JN01 strain antigen. The triple inactivated vaccine has fast generation of protective antibody after immunization and high immune attack protection rate, with the attack protection rate for rabbit viral hemorrhagic disease virus AV-34 strain, pasteurella multocida QLT-1 strain and bordetella bronchiseptica JN01 strain all reaching above 80%. The results show that the vaccine is safe and reliable, and can be used for preventing the occurrence of rabbit viral hemorrhagic disease (rabbit plague), rabbit pasteurella multocida disease (type A) and rabbit bronchisepticaemia Bordetella disease.
Owner:QILU ANIMAL HEALTH PROD
Method of separating protective components of Bordetella pertussis
InactiveUS6051240AImprove efficiencyHigh recovery rateAntibacterial agentsBacterial antigen ingredientsHemagglutininCalcium biphosphate
PCT No. PCT / JP95 / 00830 Sec. 371 Date Oct. 13, 1995 Sec. 102(e) Date Oct. 13, 1995 PCT Filed Apr. 26, 1995 PCT Pub. No. WO95 / 29934 PCT Pub. Date Nov. 9, 1995To provide a method of efficiently separate protective components of Bordetella pertussis. On the basis of differences in adsorbability to calcium phosphate gel formed by adding calcium ions to a Bordetella pertussis culture in the presence of excess phosphate ions, protective components of Bordetella pertussis are separated from the Bordetella pertussis culture. Traditionally, protective components of Bordetella pertussis have been separated using different purification methods for the respective components. According to the present invention, the use of the same means of purification for all subject components makes it possible to purify each component with high efficiency and high recovery rate, an aspect very advantageous for industrial production. It is also possible to efficiently produce an improved purified pertussis component vaccine comprising an effective combination of pertussis filamentous hemagglutinin (FHA), pertactin (PRN, 69K-OMP), pertussis fimbriae (FIM) and pertussis toxin (PT).
Owner:TAKEDA PHARMA CO LTD
Compound microbial inoculum resistant to high-temperature decomposition for crop straws and preparation method thereof
InactiveCN109504633AShorten the maturity timeGuaranteed to return to the field after decayBio-organic fraction processingFungiBacillus licheniformisDecomposition
The invention relates to a fermentation microbial inoculum, in particular to a compound microbial inoculum resistant to high-temperature decomposition for crop straws and a preparation method thereof.The composite microbial inoculum comprises YM bacteria, bacillus licheniformis, alcaligenes, agaricus bisporus, bordetella, streptomyces, coccidioides immitis, aspergillus flavus and trichoderma koningii. The components are evenly mixed in proportion to obtain a final product. The compound microbial inoculum provided by the invention can rapidly decompose crop straws and shorten the time for decomposing the straws; in the prior art, the time for decomposing the straws is generally more than 10 days; through the treatment with the compound microbial inoculum resistant to ultra-high temperaturedecomposition, straws can be decomposed and returned to the field within 7 days, and the time interval between postharvest and planting is shortened; and a fermentation process has no odor and fullykills pathogenic bacteria and pathogenic microorganisms.
Owner:ZHENGZHOU INST OF TECH
Method of separating protective components of bordetella pertussis
InactiveUS20020119161A1Improve efficiencyOperating efficiency greatAntibacterial agentsDepsipeptidesHemagglutininCalcium biphosphate
On the basis of differences in adsorbability to calcium phosphate gel formed by adding calcium ions to a Bordetella pertussis culture in the presence of excess phosphate ions, protective components of Bordetella pertussis are separated from the Bordetella pertussis culture. Traditionally, protective components of Bordetella pertussis have been separated using different purification methods for the respective components. According to the present invention, the use of the same means of purification for all subject components makes it possible to purify each component with high efficiency and high recovery rate, an aspect very advantageous for industrial production. It is also possible to efficiently produce an improved purified pertussis component vaccine comprising an effective combination of pertussis filamentous hemagglutinin (FHA), pertactin (PRN, 69K-OMP), pertussis fimbriae (FIM) and pertussis toxin (PT).
Owner:TAKEDA PHARMA CO LTD
Influenza vaccine, composition, and methods of use
ActiveUS9526778B2SsRNA viruses negative-senseBacterial antigen ingredientsBordetellaInfluenza vaccine
The invention relates to compositions and vaccines that include a mutated Bordetella strain for treating or preventing an influenza infection in a mammal. In addition, the invention further provides methods for protecting a mammal against infection by influenza and / or eliciting an immune response against an influenza virus in a mammal using the composition or vaccine.
Owner:INSTITUT PASTEUR DE LILLE +2
Uses of Phyllanthus emblica, and Phyllanthus emblica composition and uses thereof
InactiveCN109276602AExpand the scope of antibacterial applicationGrowth inhibitionAntibacterial agentsDigestive systemEscherichia coliBacteroides
The invention discloses applications of Phyllanthus emblica or Phyllanthus emblica and a Phyllanthus emblica composition in preparation of products for preventing and / or treating at least one of mastitis, diarrhea and porcine respiratory diseases, and further provides antibacterial effects of Phyllanthus emblica or Phyllanthus emblica extract and the Phyllanthus emblica composition, wherein the Phyllanthus emblica or Phyllanthus emblica extract and the Phyllanthus emblica composition can effectively inhibit the growth of one or a variety of bacteria selected from Staphylococcus aureus, Streptococcus agalactiae, Pseudomonas, Salmonella choleraesuis, Escherichia coli, Group A beta-hemolytic streptococcus, Streptococcus suis, Bordetella, Pasteurella multocida, Salmonella enterica, Staphylococcus haemolyticus, Enterococcus faecalis and Erysipelothrix rhusiopathiae, and the effectiveness of the uses are proved through experiments so as to expanded the applications of Phyllanthus emblica andthe Phyllanthus emblica composition.
Owner:SICHUAN ANIMAL SCI ACAD
Defective entities and uses therefor
InactiveUS20050238657A1Efficient inactivationBacterial antigen ingredientsBacteriaBordetellaPathogenicity
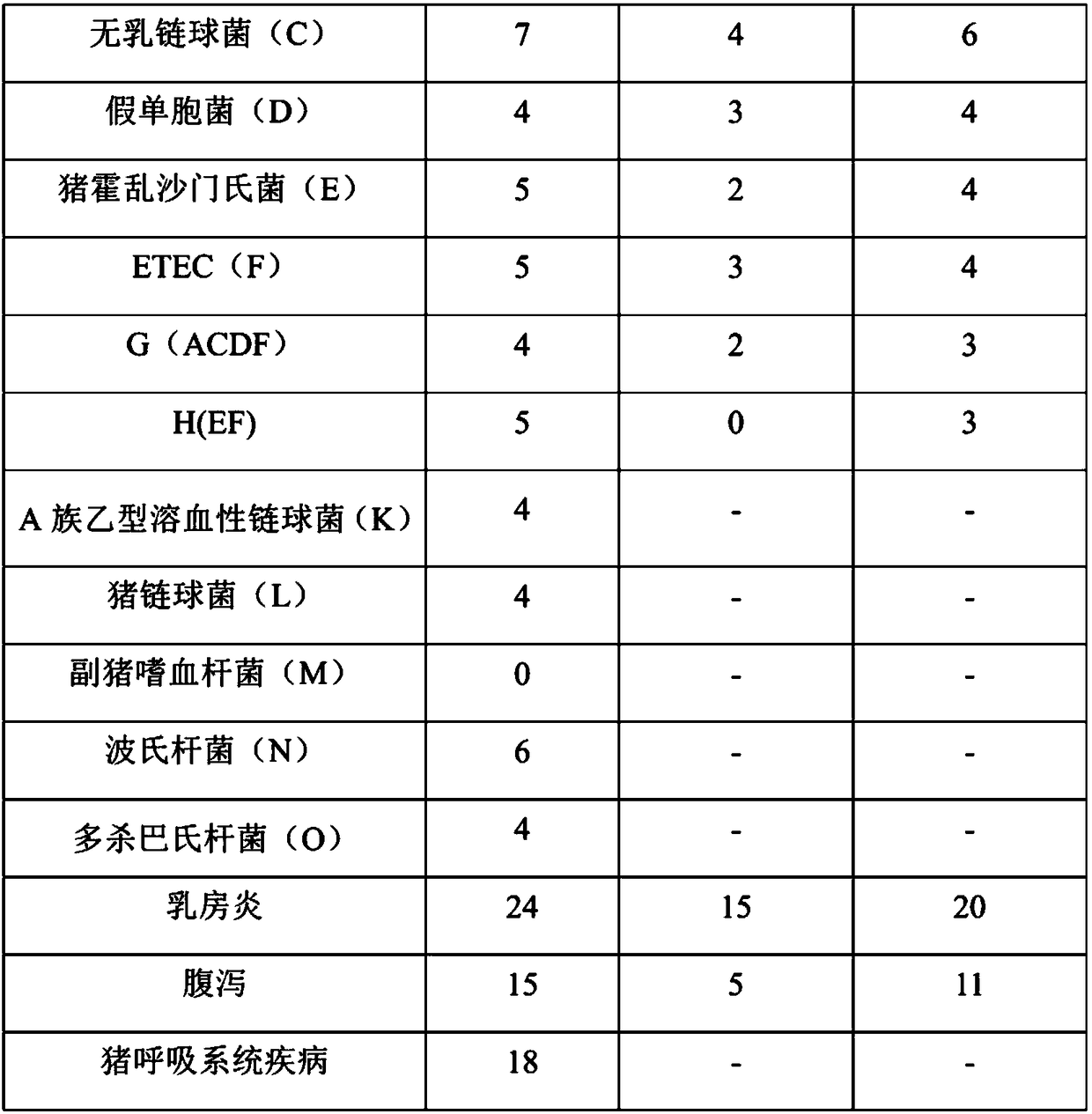
The present invention discloses the structure and sequence of aroQ from Bordetella pertussis, which are useful inter alia for the production of the genetically modified attenuated Bordetella strains of the present invention and for detecting and isolating variant aroQ genes and expression products. The present invention also discloses attenuated Bordetella strains of pathogenic origin, and more particularly genetically modified Bordetella strains, which have been attenuated by disruption or inactivation of the aroQ gene. The genetically modified Bordetella strain of the present invention has a reduced capacity to propagate in a mammalian host, but remains viable in the host for a period of time sufficient to induce a protective immune response against the natural pathogenic Bordetella counterpart. The present invention is also directed to the use of such genetically modified Bordetella strains in immunopotentiating compositions for treating and / or preventing inter alia Bordetella infections, and particularly pathogenic infections, caused by Bordetella.
Owner:SOUTHERN QUEENSLAND UNIV OF
Bordetella outer-membrane protein antigens and methods of making and using the same
ActiveUS8877201B2Raised anti-seraAntibacterial agentsPeptide/protein ingredientsBordetellaADAMTS Proteins
Owner:WAKE FOREST UNIV HEALTH SCI INC
Application of phage depolymerase ORF38 protein in pasteurella multocida capsule typing identification
PendingCN114015746ALysis does notEasy to identifyMicrobiological testing/measurementMicroorganism based processesNucleotideBordetella
The invention discloses application of phage depolymerase ORF38 protein in pasteurella multocida capsule typing identification, wherein the amino acid sequence of the protein is shown as SEQ ID No.2, and the nucleotide sequence for coding the protein is shown as SEQ ID No.1. According to the invention, it is found for the first time that the phage depolymerase ORF38 protein only has a splitting effect on PmD and does not have a splitting effect on pasteurella multocida (including A, B, E and F) of other capsule serotypes and other bacteria such as escherichia coli, salmonella, haemophilus parasuis, bordetella bronchii, streptococcus, staphylococcus aureus and the like, so that the phage depolymerase ORF38 protein can be used for PmD capsule typing identification; and the phage depolymerase ORF38 protein has the advantages of simplicity, rapidness, accuracy and the like.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Recombinant adenylate cyclase toxin of bordetella induces T cell responses against tumoral antigens
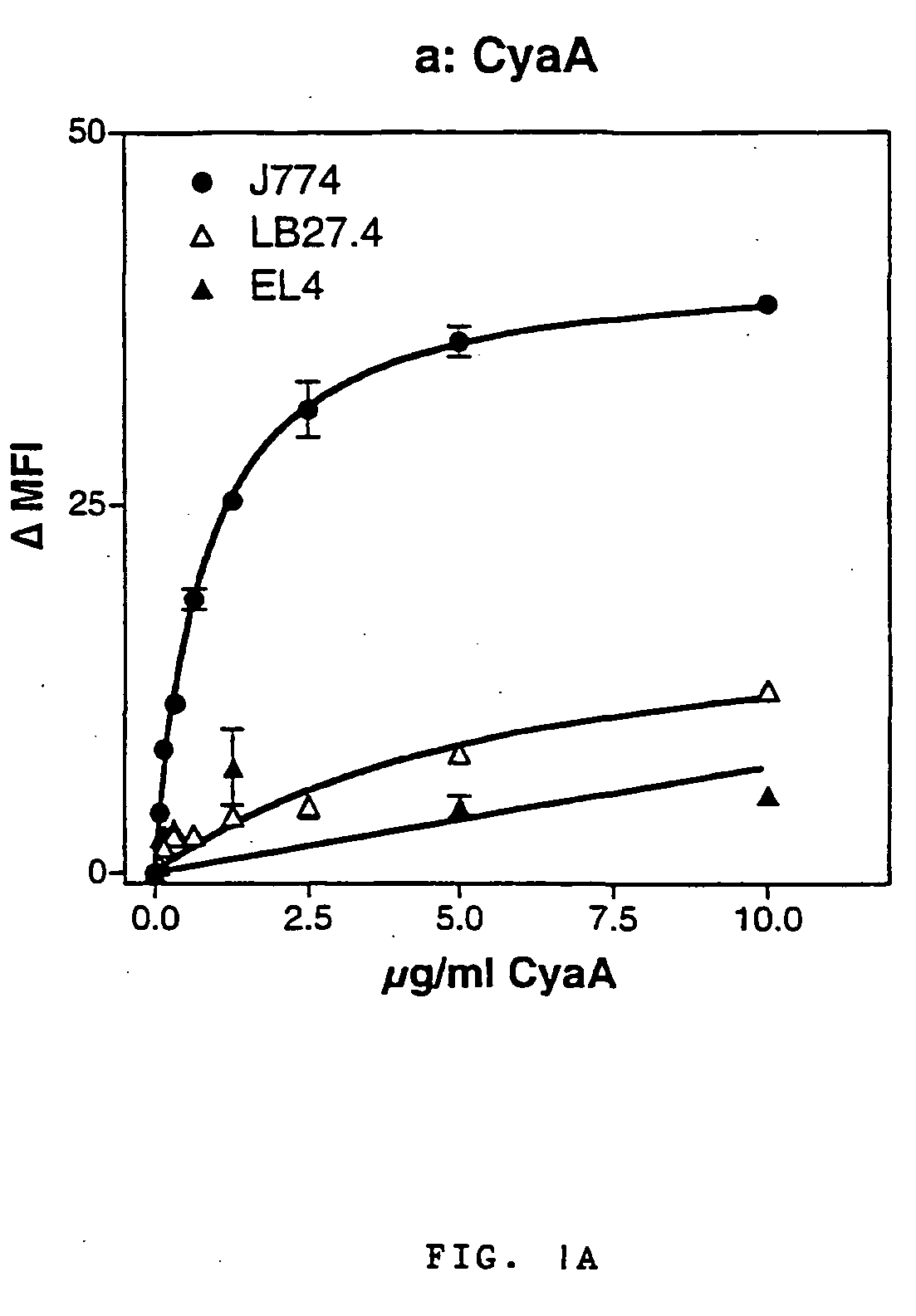
An immunogenic composition comprising a recombinant protein comprising a Bordetella CyaA, or a fragment thereof, and a peptide that corresponds to a tumor antigen is provided as a cancer treatment. Methods of treatment with this immunogenic composition are also provided. In an embodiment, the therapeutic composition is a treatment for melanoma, and comprises epitopes from the HLA*0201 epitope. These epitopes include Tyr or GnT-V, and are present in the recombinant proteins CyaA-E5-Tyr and CyaA-E5-GnT-V.
Owner:INST PASTEUR +3
Use of garlic volatile oil in preparing medicine for preventing and treating septicemic bordetella bacilli
The invention discloses a novel purpose of garlic volatile oil, in particular to an application of the garlic volatile oil in the preparation of a medicine for preventing and curing septica Bordetella. The experiments show that the minimum inhibitory concentration of the garlic volatile oil to the septica Bordetella is 125 mug / ml and the minimum bactericidal concentration is 250 Mug / mL.
Owner:CHINA AGRI UNIV +2
Monoclonal antibody of hemophilus parasuis resistant OMP5 (outer membrane protein), hybridoma cell strain HPS1E2 and application
InactiveCN102876636ANo cross reactionStrong specificityImmunoglobulins against bacteriaTissue cultureEscherichia coliBordetella
The invention discloses a monoclonal antibody of hemophilus parasuis resistant OMP5 (outer membrane protein), a hybridoma cell strain HPS1E2 and application. The hybridoma cell strain is preserved in a Chinese typical culture preservation center, and the preservation number is CCTCCC2012138. The monoclonal antibody prepared with the hybridoma cell strain is fine in specificity, high in valence and high in universality, is not in cross reaction with swine Escherichia coli, swine pasteurella, actinobcillus pleuropneumoniae, streptococcus suis and swine bordetella, the valence of the purified ELISA (enzyme-linked immuno sorbent assay) antibody can reach 1:409600, hemophilus parasuis with different serotypes can be detected, and the monoclonal antibody can be widely used for etiology diagnosis, serological test, immunological test, prevention and treatment and the like of hemophflussuis.
Owner:广东省农业科学院兽医研究所
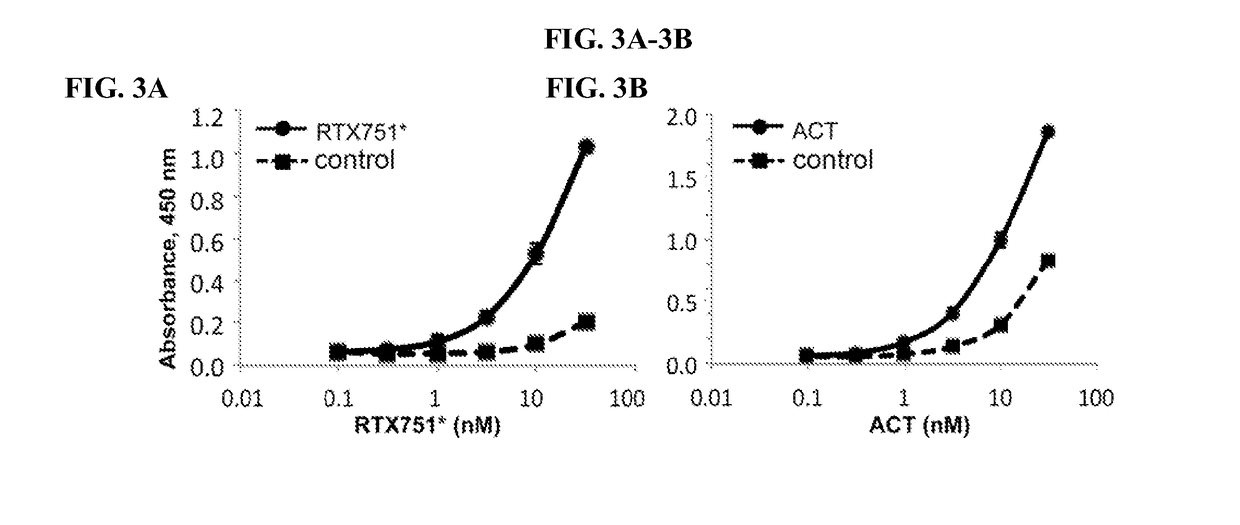
Bordetella adenylate cyclase toxin vaccines and neutralizing antibodies
InactiveUS20180256699A1Avoid interactionSimilar neutralizing activityBacterial antigen ingredientsPhosphorus-oxygen lyasesBordetellaImmunogenicity
The present disclosure describes immunogenic portions of Bordetella adenylate cyclase toxin (ACT), and neutralizing antibodies specific for such polypeptides. The antibodies can be used for diagnosis and anti-Bordetella therapies. Further provided are vaccine compositions including recombinant Bordetella adenylate cyclase toxin polypeptides.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Canine vaccines
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention relates to vaccines and methods for protecting dogs against disease caused by Leptospira bratislava. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona.
Owner:PFIZER INC +1
Vectors for molecule delivery to CD11b expressing cells
InactiveUS20050238637A1Efficient targetingEasy maintenancePeptide/protein ingredientsAntipyreticBordetellaIn vivo
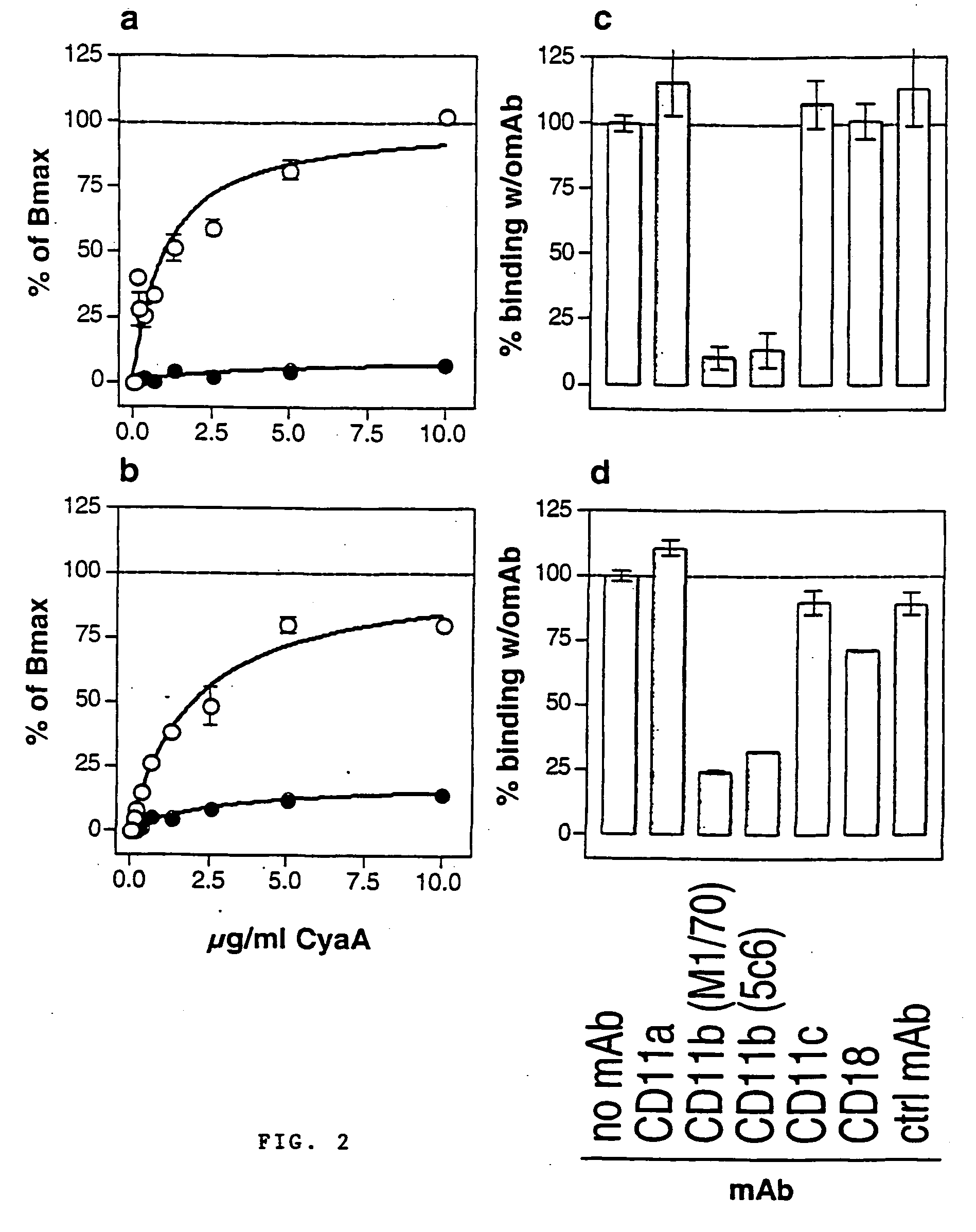
The invention relates to a novel use of a Bordetella adenylcyclase toxin in the manufacturing of vectors for targeting in vivo a molecule of interest, specifically to CD11b expressing cells. The invention also relates to an immunogenic composition that primes immune responses, to pharmaceutical compositions, and to a new vector for molecule delivery to CD11b expressing cells.
Owner:INST PASTEUR +1
Vaccines for the prevention of infections with bordetella
ActiveUS20160038582A1Bacterial antigen ingredientsDiphtheria-tetanus-pertussis combination vaccineBacteroidesAdjuvant
A vaccine for the prevention of infections with Bordetella, comprising at least outer membrane vesicles (OMVs) of B. parapertussis, excipients and / or adjuvants. Bordetella may be, for example, B. pertussis or B. parapertussis. The vaccine can comprise adjuvants, for example, aluminum hydroxide and other immunogens such as tetanus toxoid, diphtheria toxoid, or combinations thereof.In another preferred embodiment, the vaccine for the prevention of infections with Bordetella comprises at least outer membrane vesicles (OMVs) of B. pertussis and the lipopolysaccharide of B. parapertussis, excipients and / or adjuvants. The vaccine can comprise between 3 to 20 μg per dose of OMVs from B. pertussis and between the amount equivalent to 107 and 1010 bacteria per dose of lipopolysaccharide of B. parapertussis. The adjuvant can be aluminum hydroxide and other immunogens such as tetanus toxoid, diphtheria toxoid, or combinations thereof. The Tdap vaccine exhibits cross activity.
Owner:CONSEJO NAT DE INVESTIGACIONES CIENTIFICAS Y TECH CONICET +2
Fermentation process
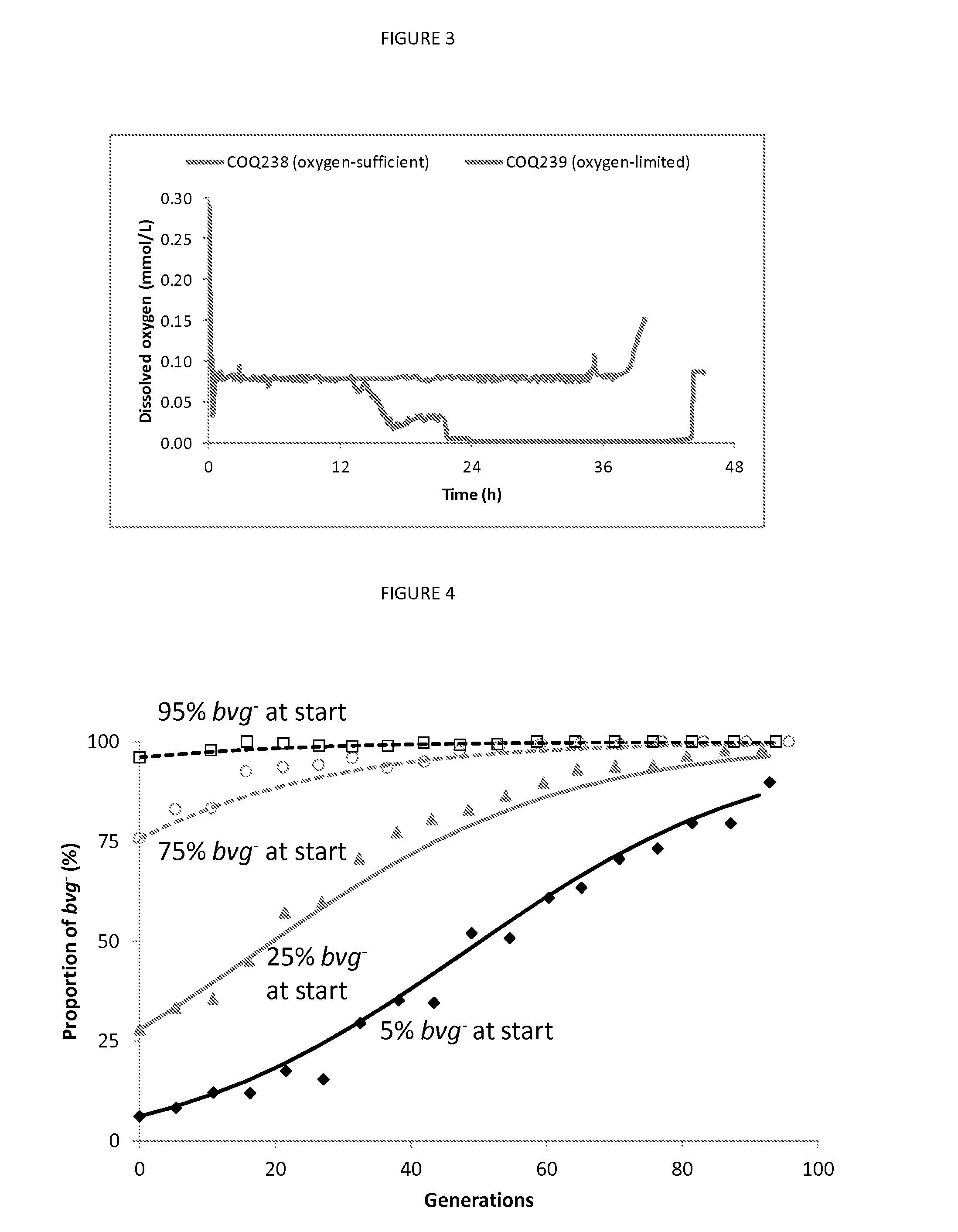
A process for fermenting Bordetella species comprising incubating a sample of bacteria of a Bordetella species in a first environment under at least one bvg (Bordetella virulence genes) modulating condition, for at least 5 generations, to produce a mature culture, and then incubating the mature culture in a second environment in the absence of the at least one bvg modulating condition.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com