Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
482 results about "Serology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serology is the scientific study of serum and other bodily fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection (against a given microorganism), against other foreign proteins (in response, for example, to a mismatched blood transfusion), or to one's own proteins (in instances of autoimmune disease).
Meningococcal antigens
InactiveUS6709660B1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsCoccidiaNucleotide
The invention provides proteins from Neisseria meningitidis (strains A & B), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics.
Owner:NOVARTIS AG
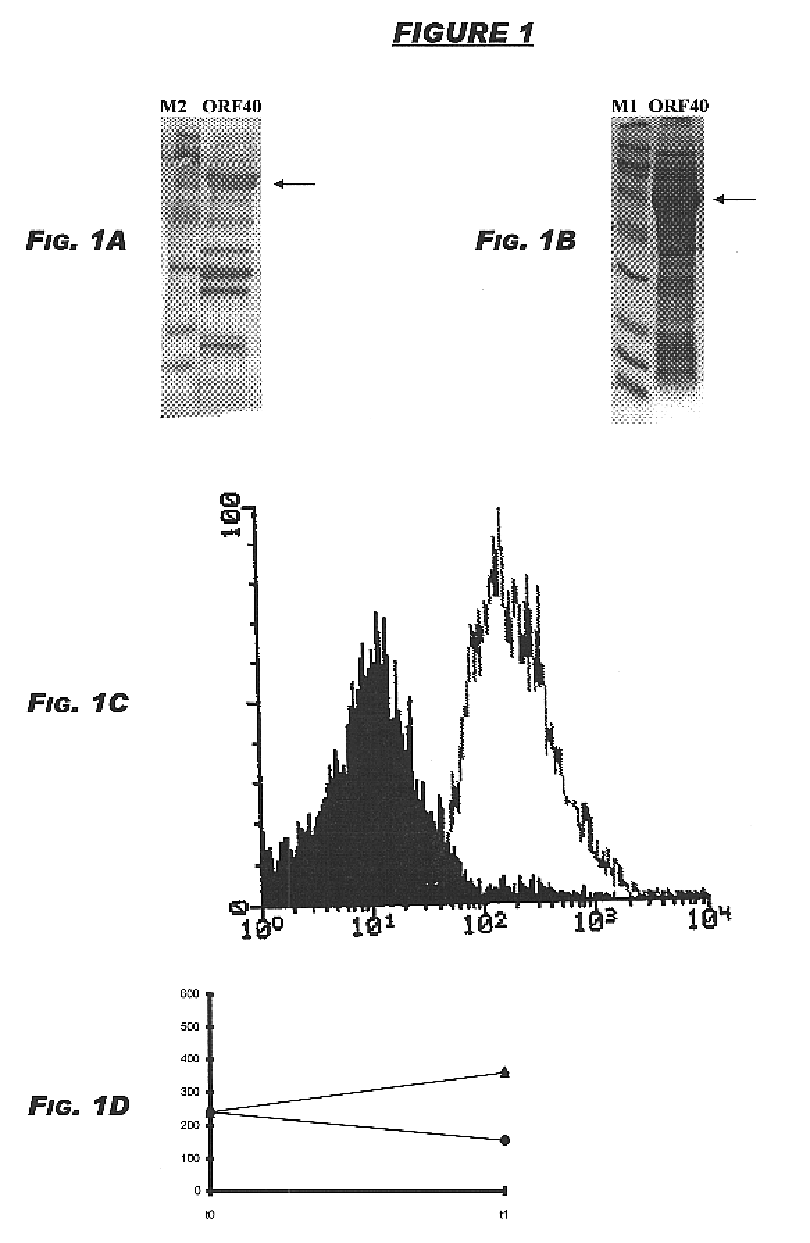
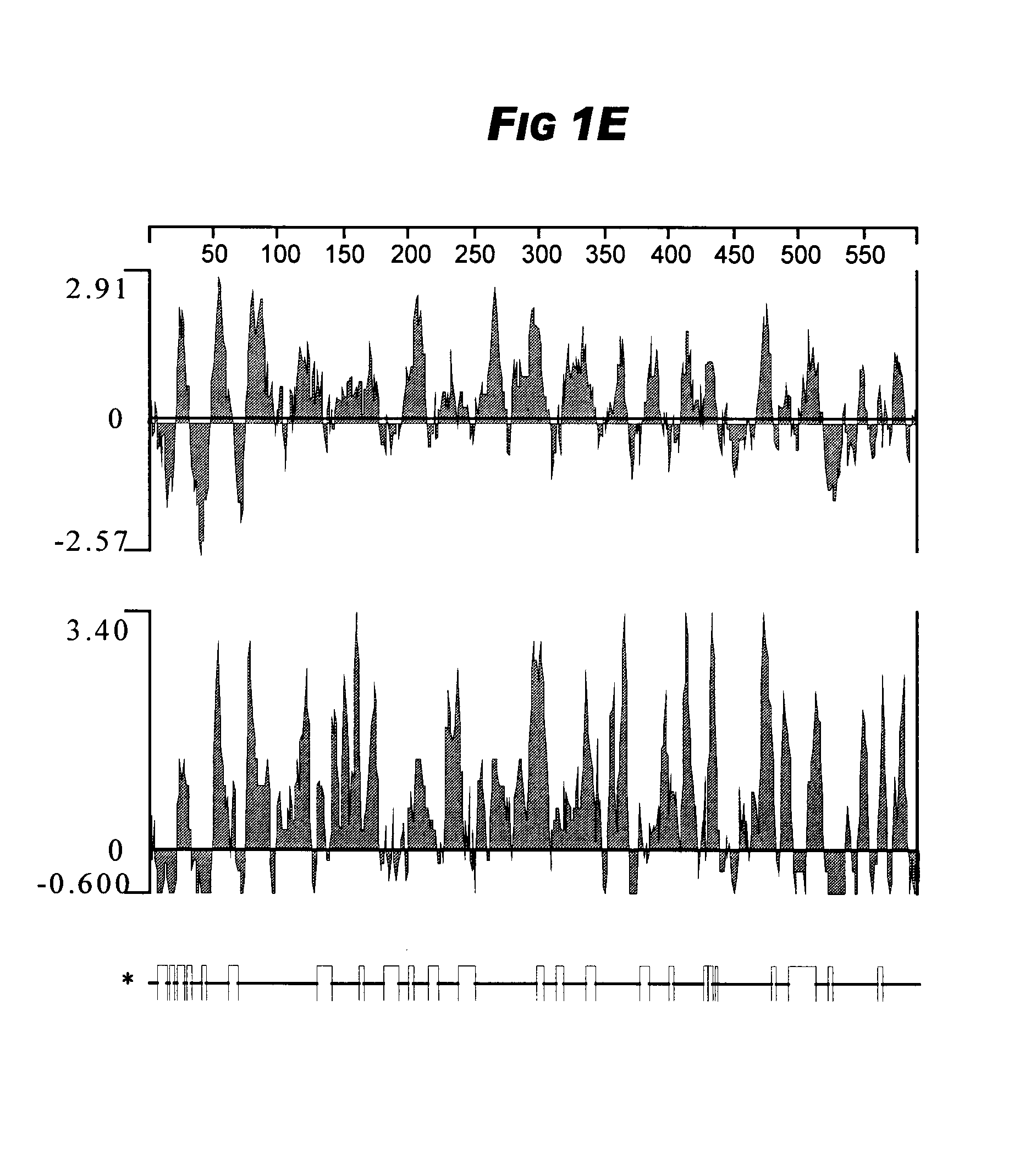
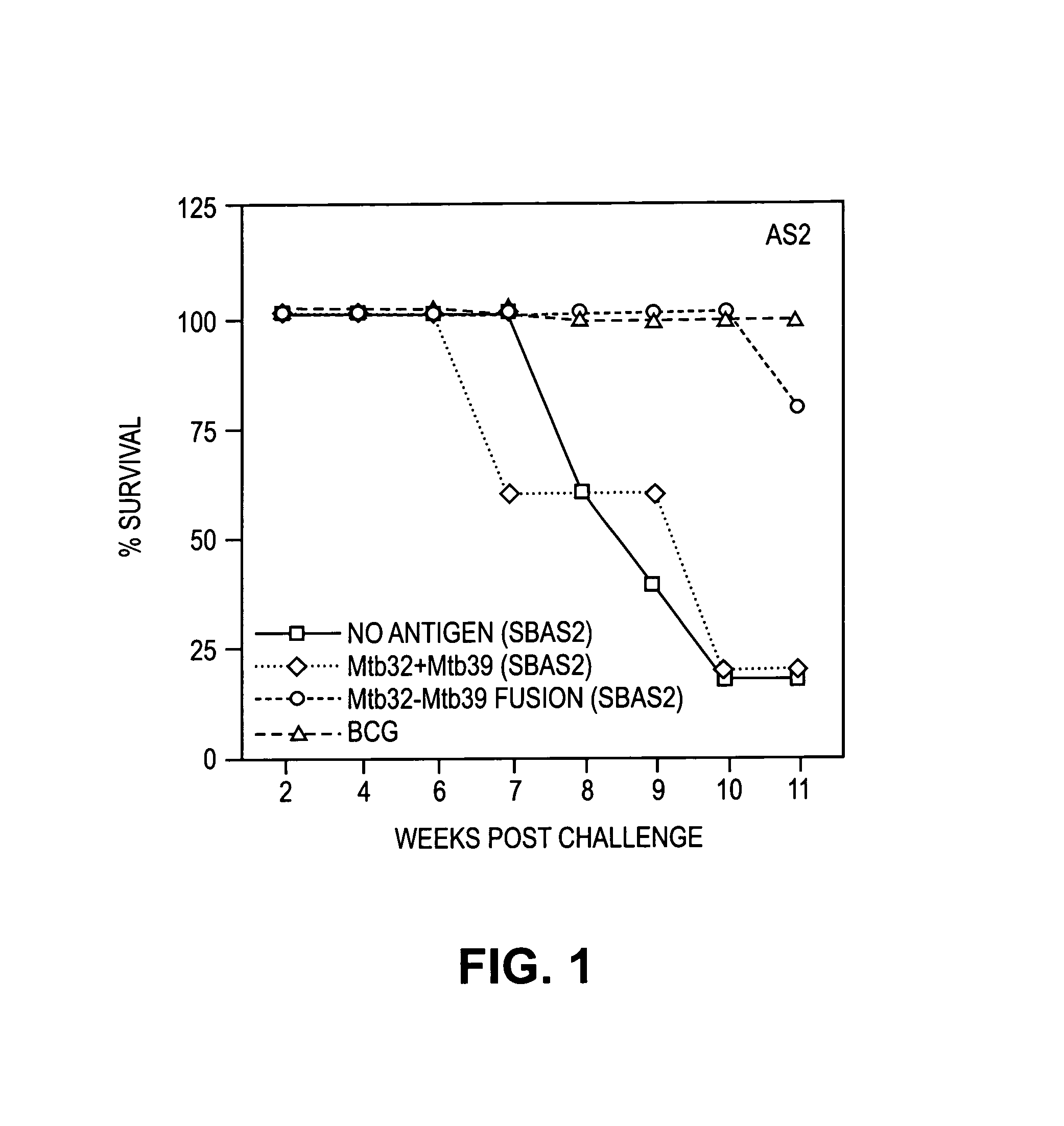
Fusion proteins of Mycobacterium tuberculosis
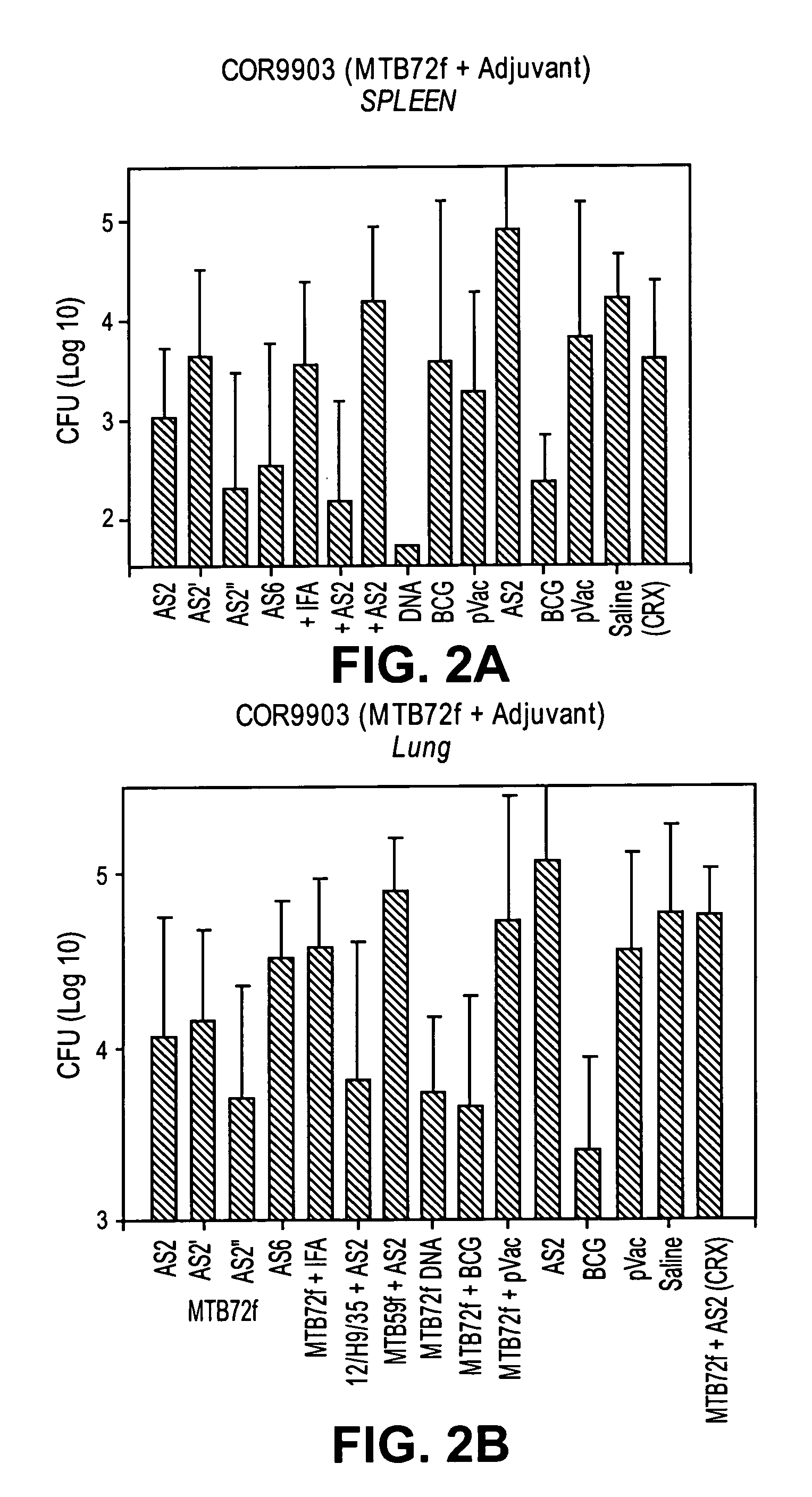
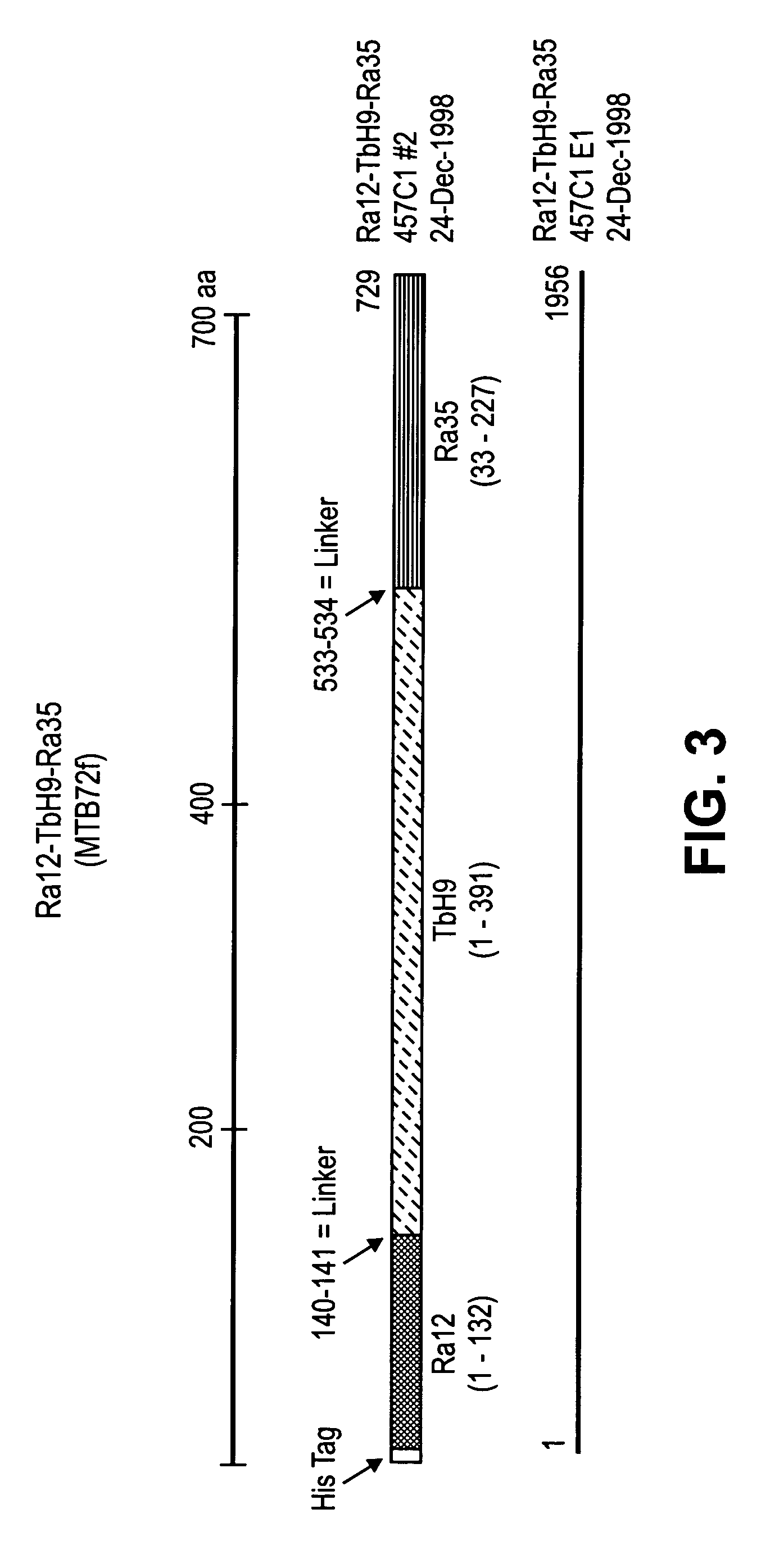
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Fusion proteins of mycobacterium tuberculosis
InactiveUS7311922B1Good antigenicityHigh sensitivityAntibacterial agentsPeptide/protein ingredientsAntigenSerum ige
The present invention relates to fusion proteins containing at least two Mycobacterium species antigens. In particular, it relates to nucleic acids encoding fusion proteins that include two or more individual M. tuberculosis antigens, which increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Gonococcal proteins and nucleic acids
InactiveUS7504111B2Stable maintenanceAntibacterial agentsOrganic active ingredientsAntigenSalmonella serotype typhi
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Enhanced diagnostic multimarker serological profiling
InactiveUS20070042405A1Improve predictive performanceRapid and early diagnosisMicrobiological testing/measurementNanoinformaticsDiagnosis earlyBlood markers
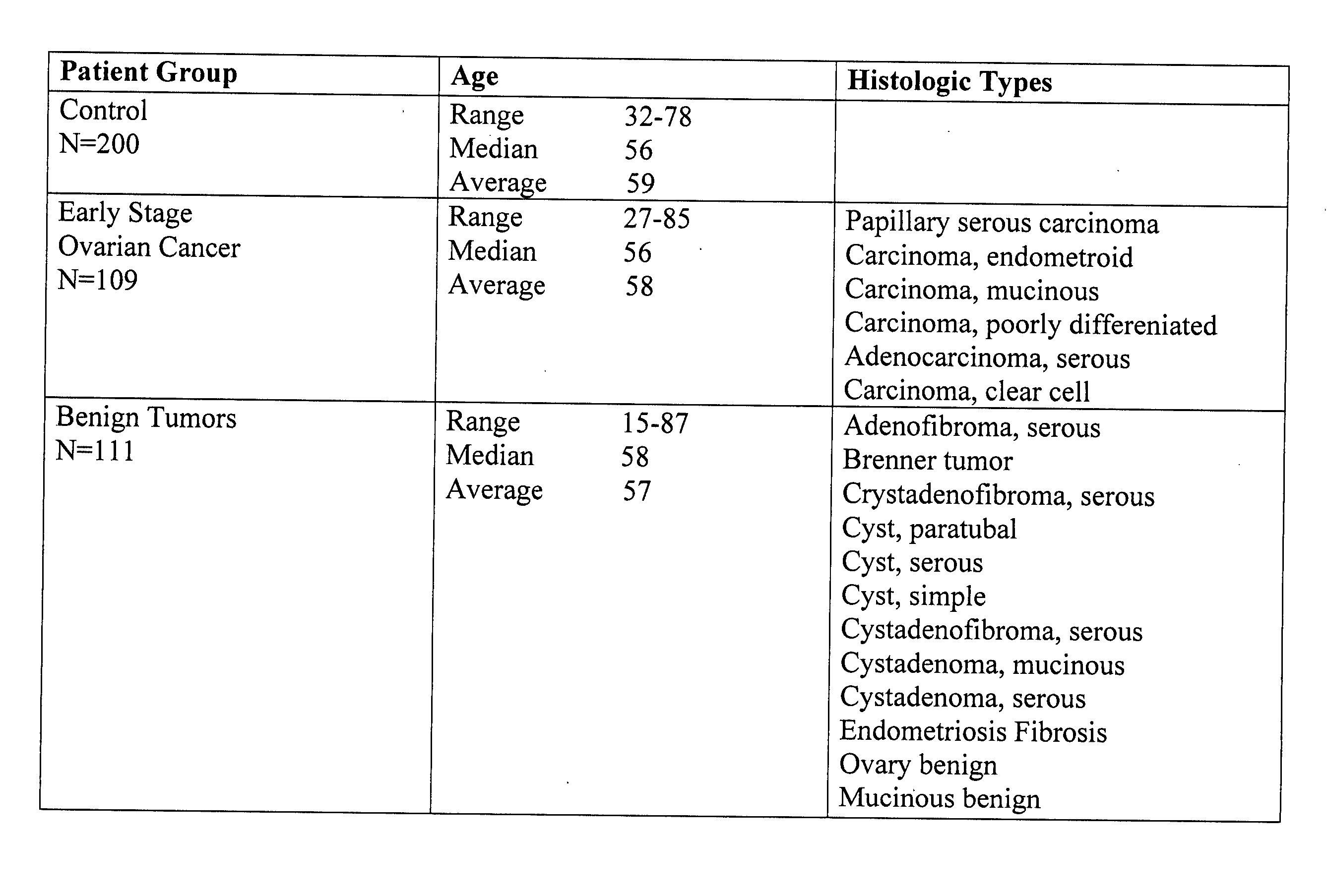
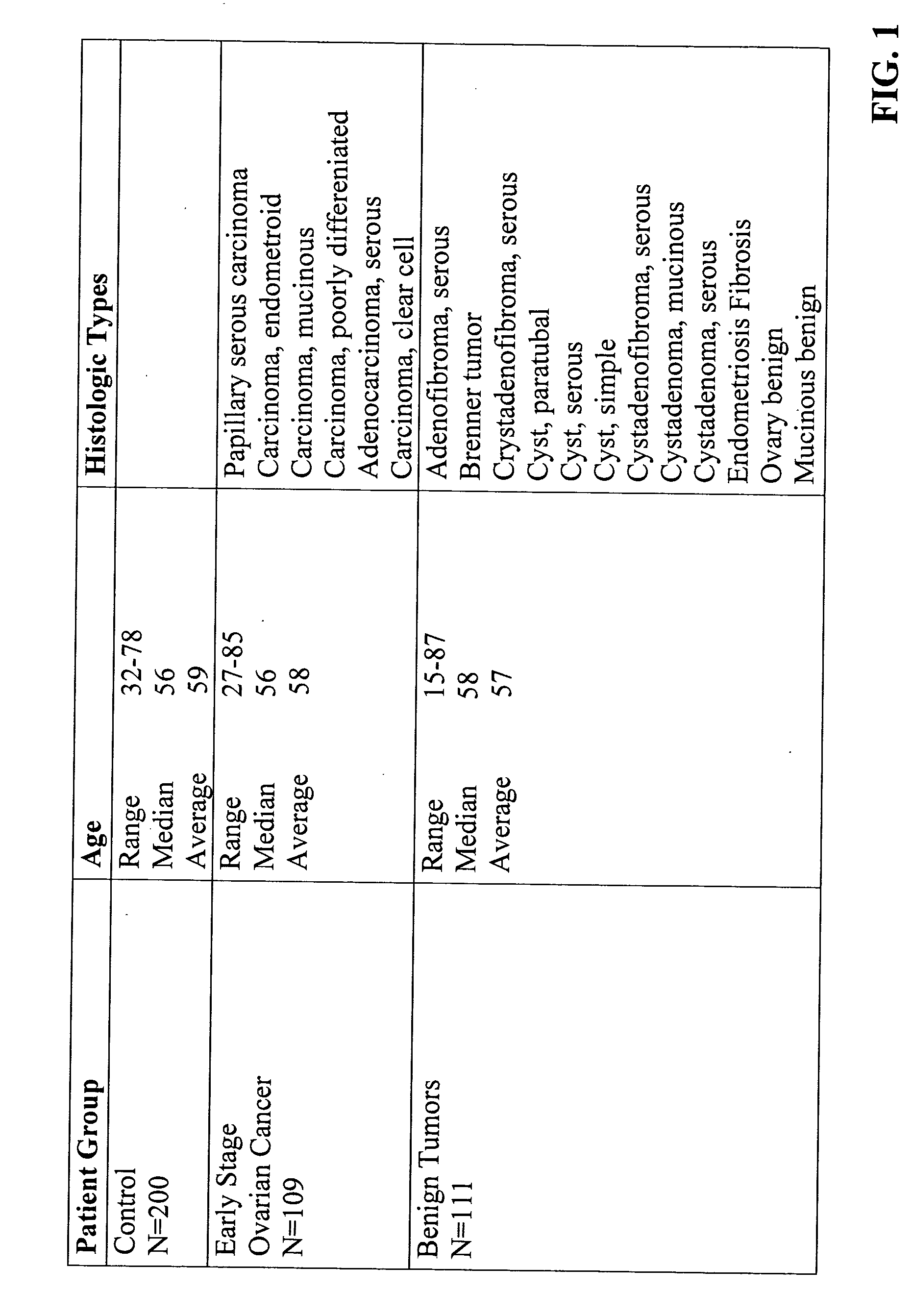
The present invention is related to methods of early diagnosis of ovarian cancer in a patient by determining serum levels of blood markers using a novel LabMAP™ technology (Luminex Corp., Austin, Tex.), which allows for simultaneous measurement of the blood markers in serum. The panel of blood markers offers extremely high predictive power for discrimination of ovarian cancer from both healthy control patients and from patients with benign pelvic / ovarian tumors. The methods of the present invention allow for rapid, early diagnosis of ovarian cancer with extremely high sensitivity and specificity to be clinically useful in disease diagnosis.
Owner:UNIVERSITY OF PITTSBURGH
P153 and P156 antigens for the immunodiagnosis of canine and human ehrlichioses and uses thereof
Sequences encoding two immunoreactive glycoproteins were cloned from Ehrlichia canis (p153 gene) and Ehrlichia chaffeensis (p156 gene). These two glycoproteins are species-specific immunoreactive orthologs that are useful as subunit vaccines and for serologic and molecular diagnostics for E. canis and E. chaffeensis.
Owner:RES DEVMENT FOUND
Fusion proteins of mycobacterium tuberculosis
InactiveUS20080269151A1Antibacterial agentsOrganic active ingredientsAntigenTuberculosis mycobacterium
The present invention relates to fusion proteins containing at least two Mycobacterium species antigens. In particular, it relates to nucleic acids encoding fusion proteins that include two or more individual M. tuberculosis antigens, which increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
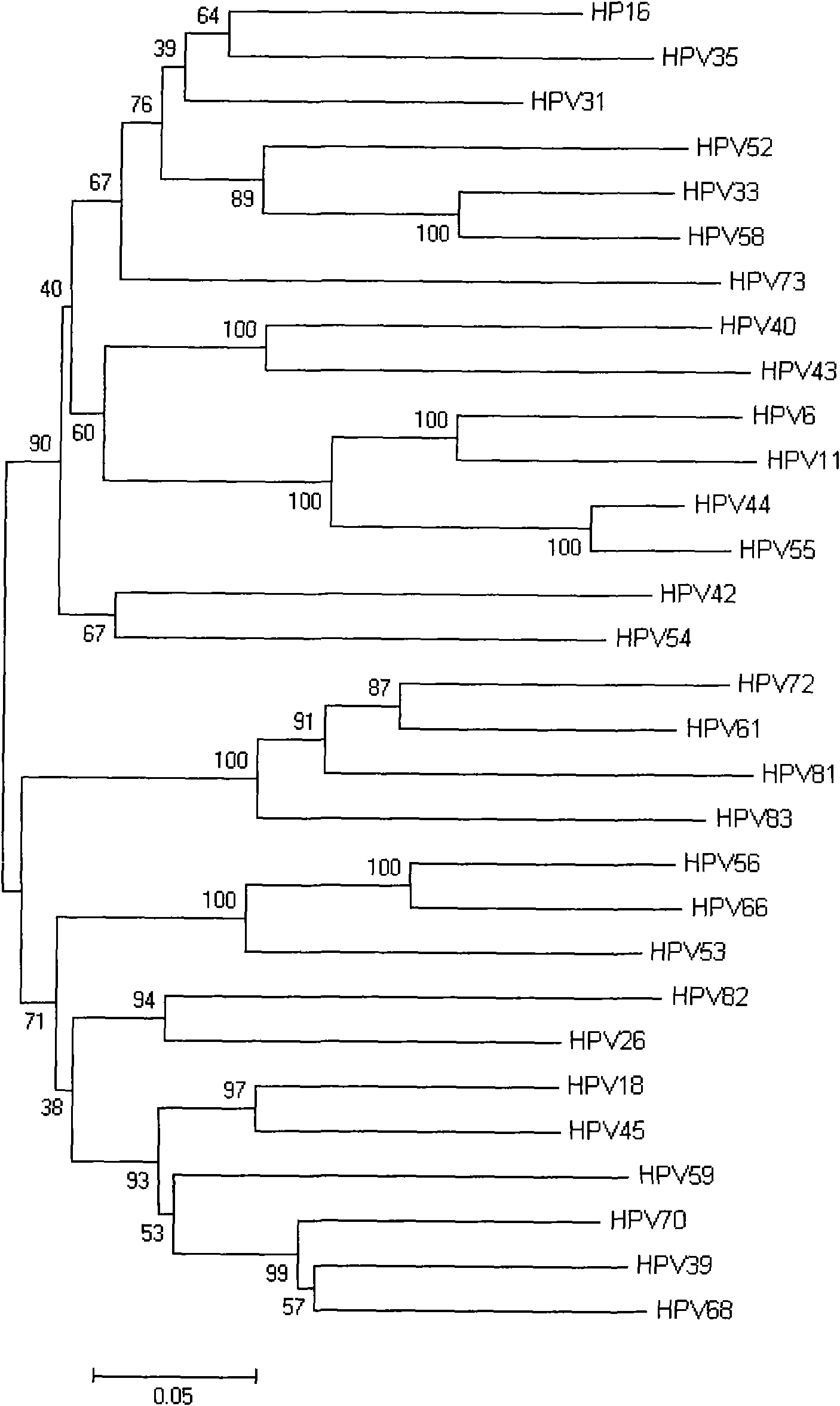
Method for detecting and typing 26 human papillomaviruses
InactiveCN1814796AImprove detection efficiencyShorten detection timeMicrobiological testing/measurementFluorescence/phosphorescenceHuman papillomavirusType specific
This invention relates to a suspension chip technology for the gene test to human papillomavirus used in testing and typing 26 kinds of papillomaviruses, in which, specific probes of which are crosslinked on 26 kinds of fluorescent microspheres to be reacted with the tested specimens then reacted with the report molecules labeled by the fluorescein to test the type specific nucleic acid of the virus by a fluorescent test device, which can test 26 kinds of ordinary HPV once and increases the test efficiency greatly and overcomes the shortcoming of missing testing the potential infections in the serology and immunity test method to realize early diagnosis to HPA diseases.
Owner:ZHEJIANG UNIV
P153 and P156 antigens for the immunodiagnosis of canine and human ehrlichioses and uses thereof
Sequences encoding two immunoreactive glycoproteins were cloned from Ehrlichia canis (p153 gene) and Ehrlichia chaffeensis (p156 gene). These two glycoproteins are species-specific immunoreactive orthologs that are useful as subunit vaccines and for serologic and molecular diagnostics for E. canis and E. chaffeensis.
Owner:RES DEVMENT FOUND
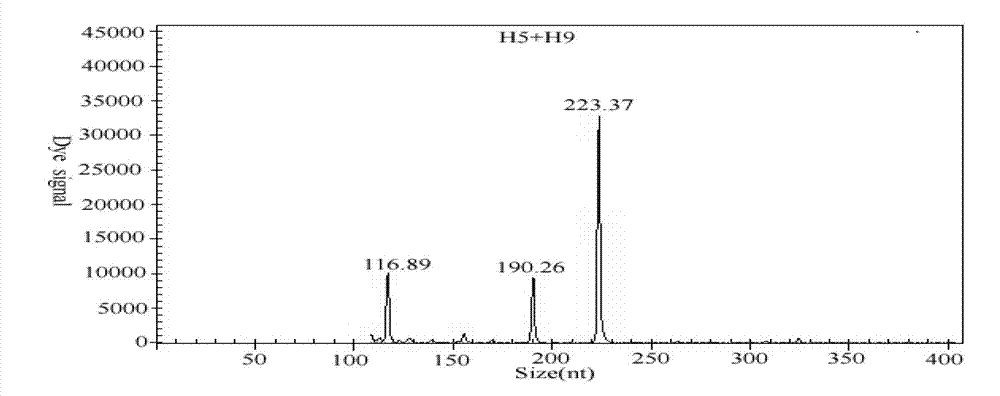
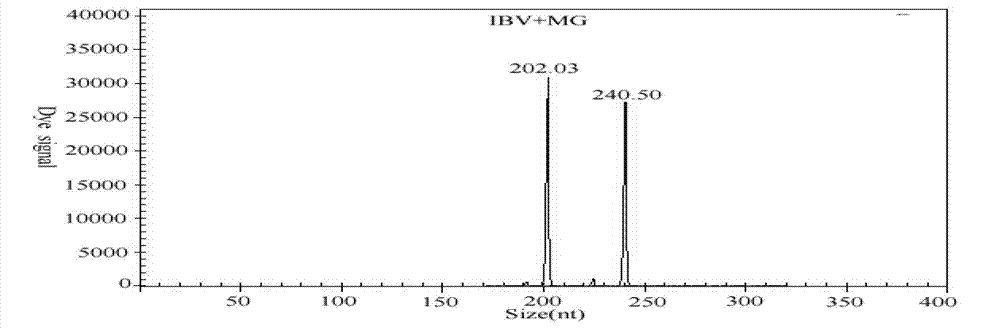
GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases
ActiveCN102899424AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationInfectious laryngotracheitisRespiratory tract disease
The invention discloses a GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases. The kit is used based on a GeXP system and comprises ten polymerase chain reaction (PCR) primer pairs; the kit is used for identifying and detecting avian influenza virus, H5, H7 and H9 subtype avian influenza virus, newcastle disease virus, infectious bronchitis, infectious laryngotracheitis, mycoplasma gallisepticum, bursa synovialis mycoplasma and haemophilus paragallinarum; and the kit is good in specificity, high in sensitivity and can detect 100 copy / mu l. Compared with an identifying result of the conventional experiment method of a pathogen separation and hemagglutination inhibition experiment or a serology experiment and the like, the GeXP rapid detection kit has the advantage that the coincidence rate reaches 100 percent. The kit is generally used for detecting the main chicken respiratory tract diseases and the pathogens thereof, so that a simple and high-flux detection kit and a detection system are provided, an actual requirement is met, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Fusion proteins of Mycobacterium tuberculosis
InactiveUS20060193876A1Antibacterial agentsBacterial antigen ingredientsMycobacterial antigenSerology
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Genetic engineering marked attenuated vaccine strain of porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN102250843AMeet the differential diagnosisEasy to solveViral antigen ingredientsAntiviralsNucleotideGenetic engineering
The invention discloses a genetic engineering marked attenuated vaccine strain of a porcine reproductive and respiratory syndrome virus (PRRSV). The attenuated vaccine strain comprises a genomic nucleic acid of a porcine reproductive and respiratory syndrome virus attenuated vaccine strain HuN4-F112; the HuN4-F112 genome includes a mutation in a genetic region for coding an Nsp2 protein, and the mutation is as follows: a nucleotide sequence for coding a Newcastle disease virus NP protein is inserted to a lacking region of a nucleotide sequence for coding 480-532-site amino acid of the Nsp2 protein; or the nucleotide sequence for coding the Newcastle disease virus NP protein is inserted to the lacking region of a nucleotide sequence for coding 508-532-site amino acid of the Nsp2 protein. The invention also discloses an application of the genetic engineering marked attenuated vaccine strain. The genetic engineering marked attenuated vaccine strain of the porcine reproductive and respiratory syndrome virus provided by the invention not only can provide completely safe immune protection to resist high-pathogenicity PRRSV after the porcine is immunized, but also can effectively distinguish the immunized porcine of the porcine reproductive and respiratory syndrome vaccine with the naturally infected porcine of the field virus.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
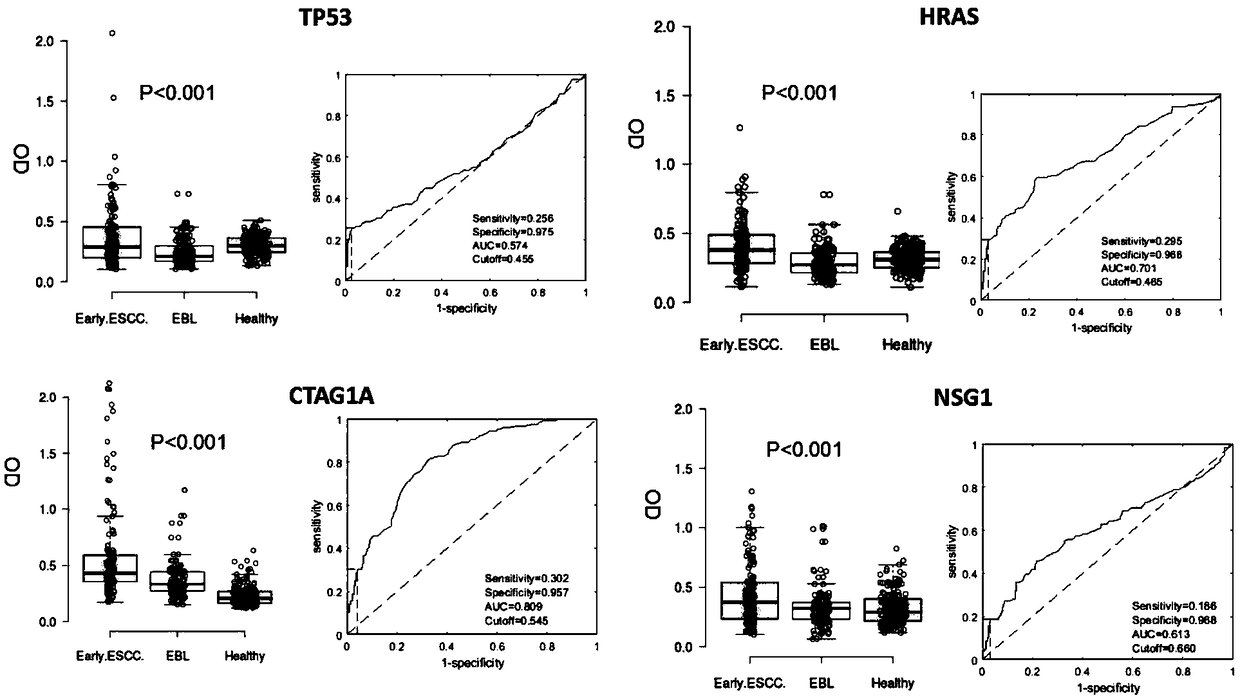
Four-autoantibody combined detection kit for diagnosing early esophageal squamous cancer and application thereof
The invention provides a four-autoantibody combined detection kit for diagnosing an early esophageal squamous cancer and application thereof. The four-autoantibody combined detection kit for diagnosing the early esophageal squamous cancer comprises a solid substrate and antigen protein which coats the solid substrate; and the antigen protein comprises TP53, HRAS, CTAG1A and NSG1. The four-autoantibody combined detection kit for diagnosing the early esophageal squamous cancer, provided by the invention, has the advantages that the TP53, the HRAS, the CTAG1A and the NSG1 are firstly adopted as acombination to be used for early diagnosis of ESCC, higher sensibility and higher specificity are realized, the detection kit is conducive to further improvement of serologic screening quality of ESCC early diagnosis, and therefore, the cure rate and survival rate of patients are further promoted.
Owner:FUJIAN PROVINCIAL HOSPITAL
Gonococcal proteins and nucleic acids
InactiveUS20050260581A1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiVaccine Immunogenicity
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Influenza virus compositions and methods for universal vaccines
InactiveUS20120014972A1Reducing or preventing efficient viral infection and diseaseReducing or inhibiting membrane fusion eventsSsRNA viruses negative-senseSsRNA viruses positive-senseInfluenza vaccineSerology
The disclosure relates at least in part to embodiments of compositions and methods including vaccines for protection against multiple serologically distinct strains of influenza virus. This disclosure provides significant advances and addresses important needs in the influenza vaccine field.
Owner:UNIV OF COLORADO THE REGENTS OF
Indirect ELISA (enzyme linked immunosorbent assay) kit for detecting porcine epidemic diarrhea virus antibody
ActiveCN103675274AStrong specificityIncreased sensitivityBiological material analysisSerum igeEpidemic diarrhea
The invention discloses an indirect ELISA (enzyme linked immunosorbent assay) kit for detecting a porcine epidemic diarrhea virus antibody. The indirect ELISA kit comprises a coated ELISA plate, negative control serum, positive control serum, ELISA secondary antibody, a concentrated cleaning solution, a sample diluent, a developing liquid and a stop buffer, wherein the coated ELISA plate utilizes recombinant protein Nh as a coating antigen. Research and practice show that the indirect ELISA kit has the characteristics of high specificity and sensitiveness, simplicity in operation and the like, is easy to popularize and use in a large range, has a broad market prospect, and can be used for the fields of serological investigation, antibody monitoring and vaccine immunity effect evaluation and the like of epidemic diarrhea infection condition.
Owner:GUANGXI UNIV
Chimeric sindbis-western equine encephalitis virus and uses thereof
The present invention discloses a chimeric alphavirus comprising a Sindbis virus cDNA fragment, an Eastern equine encephalitis virus cDNA fragment, a Western equine encephalitis virus cDNA fragment or a combination thereof. The present also discloses the use of this chimeric alphavirus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Human papillomavirus HPV DNA fragment, specific primer and application thereof
InactiveCN101851630ASimple methodMature technologyMicrobiological testing/measurementMicroorganism based processesDiseaseHuman papillomavirus DNA
The invention provides a human papillomavirus HPV DNA fragment, a mix primer for human papillomavirus HPV DNA detection and / or parting and application thereof. The mixed primer can specifically amplify to obtain one or a plurality of DNA sequences from HPV6, HPV 11, HPV 16, HPV 18, HPV 26, HPV 31, HPV 33, HPV 35, HPV 39, HPV 40, HPV 42, HPV 43, HPV 44, HPV45, HPV 51, HPV 52, HPV 53, HPV 54, HPV 55, HPV 56, HPV 58, HPV 59, HPV 61, HPV 66, HPV 68, HPV 70, HPV 72, HPV 73, HPV 81, HPV 82 and HPV 83, can be used for the human papillomavirus HPV DNA detection and / or parting and prepared into a PCR kit. The invention can detect common types of HPV at a time, greatly improve the HPV detection efficiency, overcome the defect of missed detection of latent infection by a serology and IHC detection method, realize early diagnosis of HPV diseases, and is suitable for clinical HPV gene detection and parting and guides prevention and cure of cervical carcinoma.
Owner:白向阳 +1
Chimeric antigen receptor targeting of tumor endothelium
InactiveUS20160228547A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCell membraneAntigen binding
Disclosed are methods, protocols, and compositions of matter related to utilization of chimeric antigen receptor (CAR) expressing cells for the targeting of tumor endothelium utilizing chimeric antigen receptor expressing stem cells. In one embodiment tumor endothelium specific antigens are utilized as targets of the antigen binding domain of a CAR, which is attached to an extracellular hinge domain, a domain that transverses the T cell membrane and an intracellular domain associated with T cell signaling. Suitable antigens for the practice of the invention include TEM-1, ROBO-4, surviving, and FasL. In other aspects of the invention antigens are identified through serological analysis of recombinant cDNA expression libraries (SEREX) using plasma from a patient immunized with placental endothelial cells.
Owner:BATU BIOLOGICS
Immunogenic protein in schistosoma japonicum soluble egg antigen as well as screening method and application thereof
InactiveCN102079781ADiagnostic valueBiological testingAnimals/human peptidesElectrophoresisScreening method
The invention discloses an immunogenic protein in a schistosoma japonicum soluble egg antigen. The immunogenic protein is selected from amino acid sequences shown in SEQ ID NO:2-SEQ ID NO:14. In addition, the invention also discloses a screening method and application of the immunogenic protein in the schistosoma japonicum soluble egg antigen and is mainly characterized by screening the immunogenic protein in the schistosoma japonicum soluble egg antigen through a combined proteomics technology and a serological method, particularly analyzing the immunogenic protein in the schistosoma japonicum soluble egg antigen by utilizing bidirectional electrophoresis, Westernblot (2D-WB) and a mass spectrum method and using the immunogenic protein as a potential target spot for schistosomiasis diagnosis.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Method for obtaining sogatella furcifera carrying SRBSDV (southern rice black-streaked dwarf virus) and application of sogatella furcifera
InactiveCN102630640AOvercome technical bottlenecks that are difficult to preserve for a long timeRapid identificationHorticulture methodsRice cultivationDiseaseDiseased plant
The invention discloses a method for obtaining sogatella furcifera carrying SRBSDV (southern rice black-streaked dwarf virus) and an application sogatella furcifera. The method for obtaining the sogatella furcifera carrying the SRBSDV, comprises the following steps of: feeding the sogatella furcifera by utilizing rice infecting the SRBSDV or cryopreserved rice diseased plant so that the sogatella furcifera obtains the SRBSDV, transferring the infected sogatella furcifera to a healthy rice to be fed till passing through a circulation period of virus, and detecting the sogatella furcifera by utilizing an RT-PCR (reverse transcription-polymerase chain reaction) technology and a serology method to obtain the sogatella furcifera carrying the SRBSDV, wherein the carrier rate of the sogatella furcifera can reach more than 50% through a manual feeding manner based on the experiment. Through utilizing the method for obtaining the sogatella furcifera carrying the SRBSDV, the obtained sogatella furcifera carrying the SRBSDV can be applied to screening of SRBSDV-resisting monoclonal antibody to carry out a rice inoculation experiment to identify the resistance of the rice, screen disease-resistant variety and provide a service of the breeding for disease resistance. The invention can be further applied to researching mutual relation between the SRBSDV and a vector insect sogatella furcifera and provides powerful theoretical evidence for prevention and treatment on the viruses.
Owner:ZHEJIANG UNIV
Indirect ELISA kit for detecting avian infectious bronchitis virus antibody
InactiveCN102093999AGood securityGood antigenicityRecombinant DNA-technologyMaterial analysisProtein servingsSorbent
The invention discloses an indirect enzyme-linked immuno sorbent assay (ELISA) kit for detecting avian infectious bronchitis virus (IBV) antibody. The kit contains an ELISA plate enveloped by IBV-N recombinant protein serving as antigen. The IBV-N recombinant protein is obtained by the following method: designing a pair of specific primers according to an IBV-N gene sequence; amplifying the N gene of IBV by using a reverse transcription polymerase chain reaction (RT-PCR) method, directionally inserting the N gene into a pET-32a(+) expression vector, and screening to obtain a positive recombinant expression plasmid of pET-32a(+)-IBV-N; and transferring the plasmid to a BL21 competent cell, and performing isopropyl thiogalactoside (ITPG) induction expression to obtain the IBV-N recombinant protein. The kit is low in cost, easy, convenient and quick to operate, and particularly suitable for detecting batch samples, and greatly improves the efficiency of serodiagnosis of the avian infectious bronchitis.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Turkey herpesvirus vectored recombinant containing avian influenza genes
InactiveUS20080241188A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsVaccinationElisa kit
The present invention provides a recombinant turkey herpesvirus modified by the presence of the cDNA encoding the hemagglutinin protein of avian influenza virus under a promoter. A poultry vaccine comprising the recombinant turkey herpesvirus described in the present invention can induce serological responses that may be easily detected by the hemagglutination inhibition assay but not by commercially available diagnostic ELISA kits; thus enabling easy differentiation between vaccination and field infection.
Owner:ZEON CORP +1
Reagent box for enzyme linked immunosorbent assay of EB virus protease and its preparation
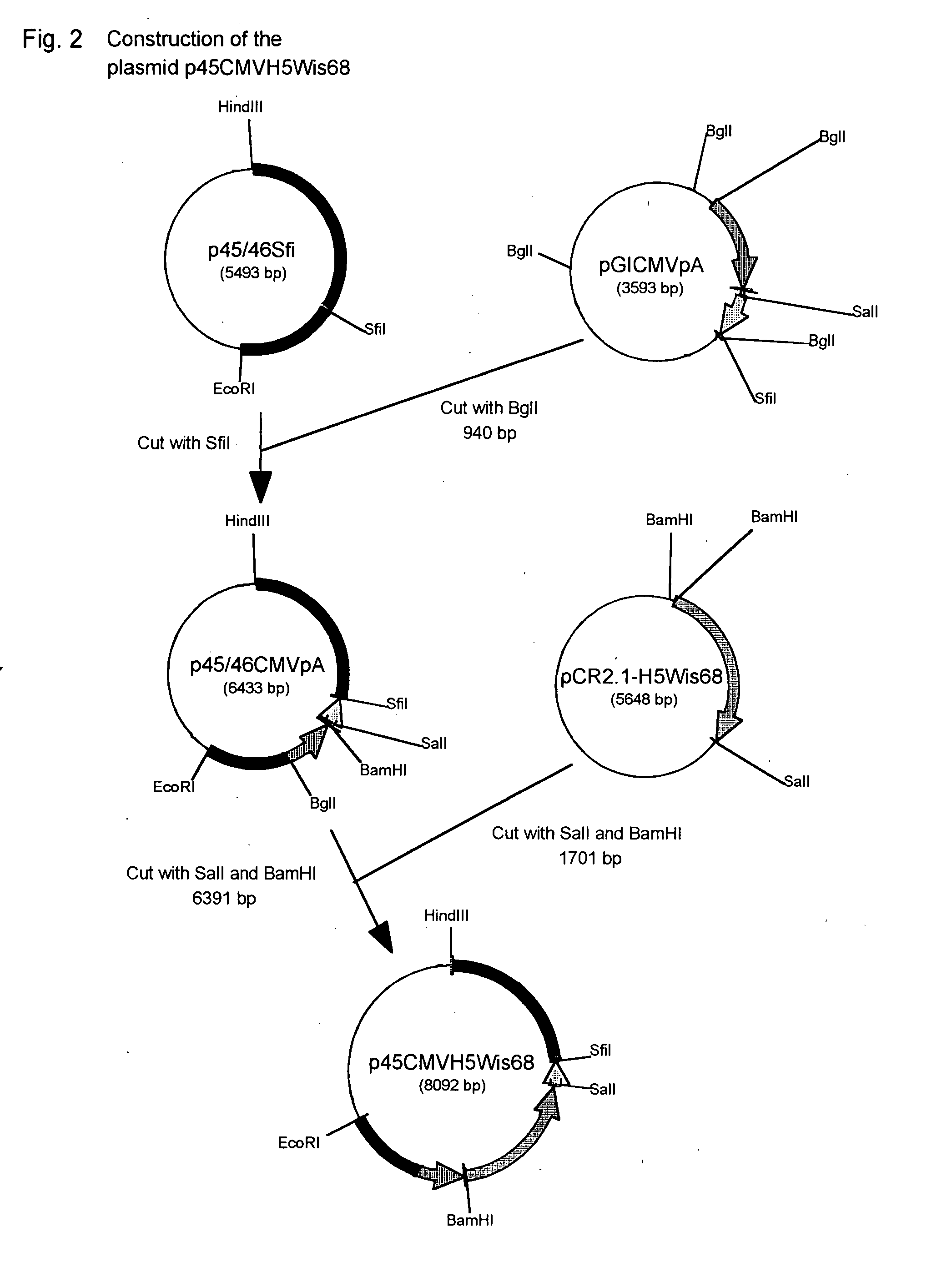
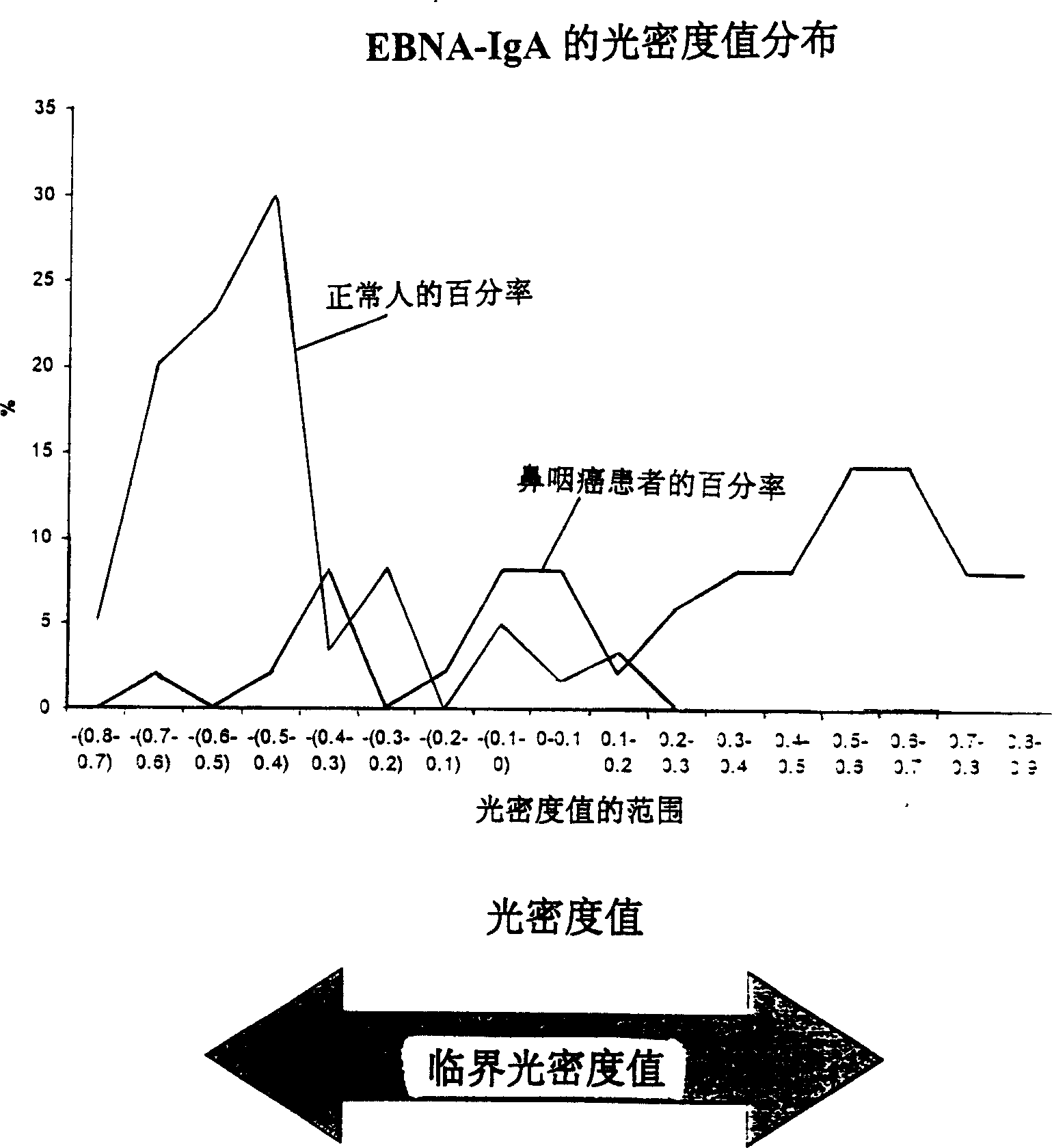
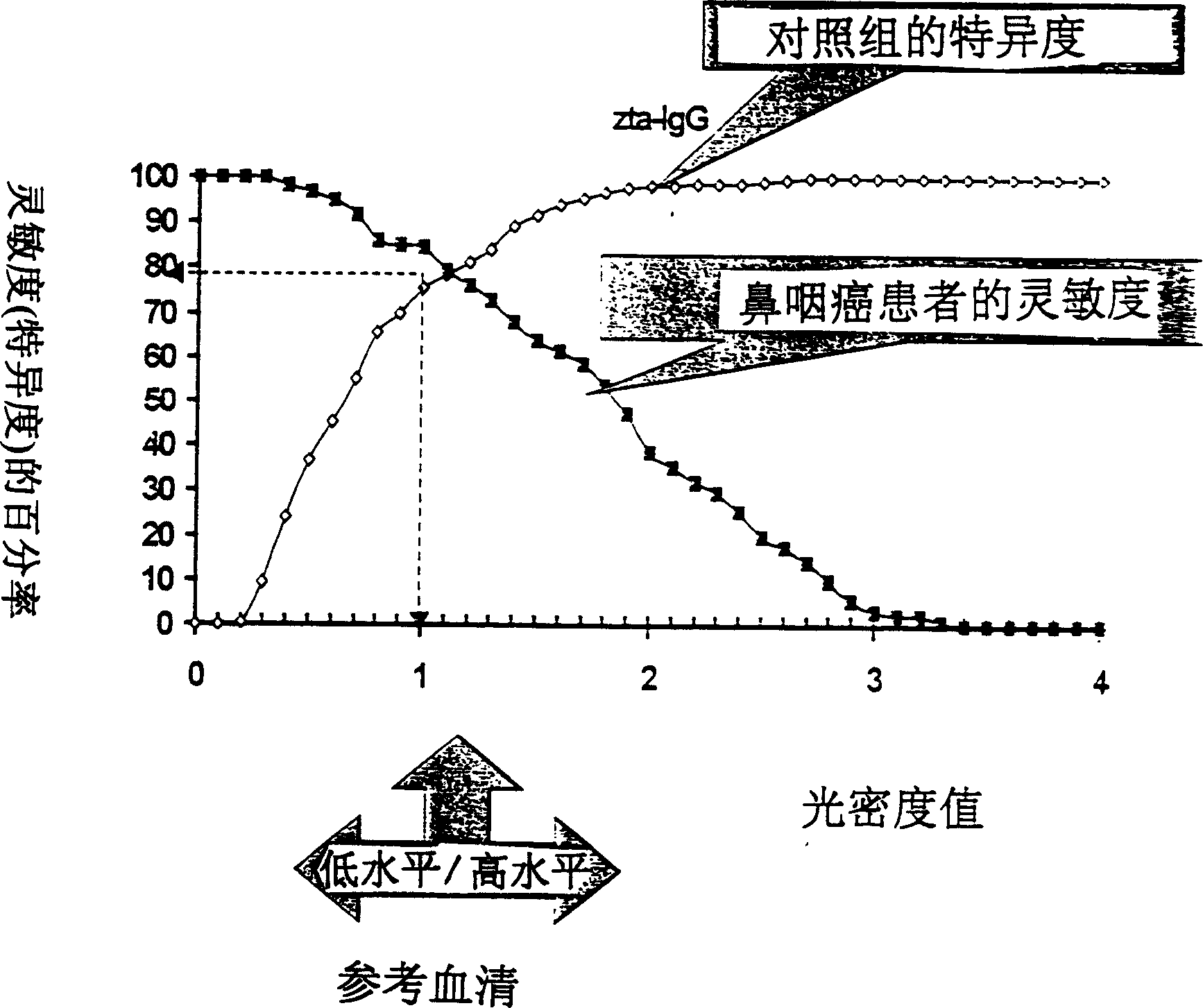
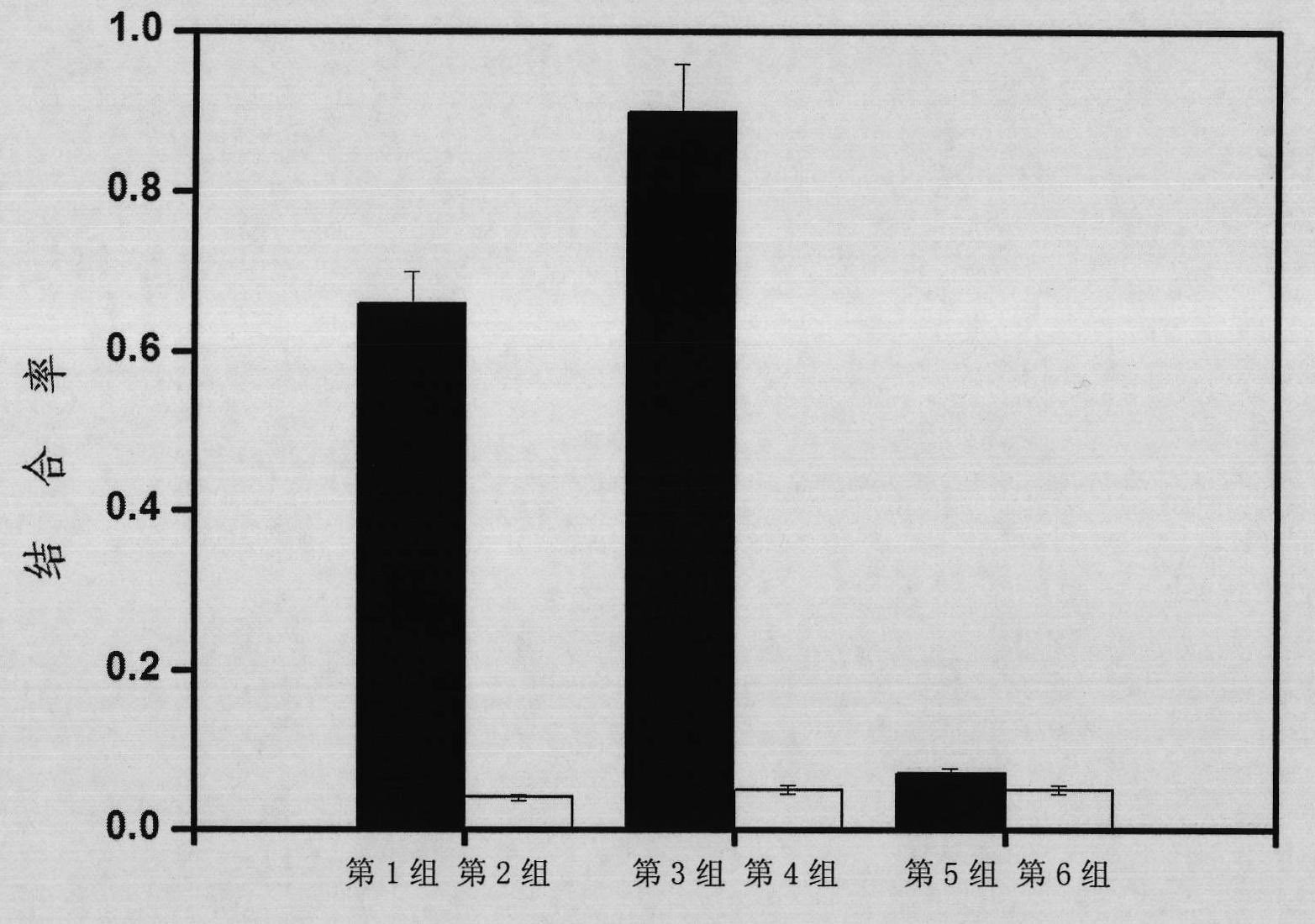
A method for preparing EB viral protein enzyme-linked immunosorbent diagnostic kit includes selectng EBNAl (BKRF1) prote, Zta (BZLF1) protein and VCA-p18 protin in EB viral protein for diagnosing nasopharyngeal carcinoma of serodiagnosis as target antigen for detecting antibody level in blood serum, using glutathione-transferase gene fusion system to carry on clone, presentation and purification of EB viral protein for creating diagnostic kit.
Owner:SINOCLONE LTD
Nucleic acid aptamer specifically combined with hepatitis C virus core protein and application thereof
ActiveCN102121009ASensitive highLow costMicrobiological testing/measurementDNA/RNA fragmentationAptamerMonoclonal antibody
The invention discloses a nucleic acid aptamer specifically combined with a hepatitis C virus core protein and application thereof. The nucleic acid aptamer is a single stranded DNA shown in a sequence 2 of a sequence table or single stranded DNA containing the nucleic acid aptamer. The nucleic acid aptamer has better affinity with a hepatitis C virus core protein. By using the nucleic acid aptamer disclosed by the invention, the hepatitis C virus core protein in a solution can be captured and can be also detected, and hepatitis C serodiagnosis and blood screening can be realized. Part of thenucleic acid aptamer disclosed by the invention can be used to replace a monoclonal antibody to capture the core protein for hepatitis C detection. The invention has the advantages of high sensitivity, low cost, easiness of preparation and storage, and high application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method for African swine fever virus antibody detection colloidal gold immunochromatography test paper strip
InactiveCN103293306AEasy to manufactureShorten detection timeMaterial analysisAfrican swine fever virus AntibodyTrue positive rate
The invention relates to a preparation method for an African swine fever virus (ASFV) antibody detection colloidal gold immunochromatography test paper strip, belonging to the field of bioengineering and animal epidemic disease diagnosis. The preparation method comprises the following steps of: expressing an ASFV recombinant antigen p54, purifying, preparing an antibody, preparing a colloidal gold immunochromatography test paper strip, and detecting the sensitivity and specificity of the ASFV antibody. Compared with the other conventional serological methods, the colloidal gold immunochromatography test paper strip prepared by the method has the advantages of convenience, quickness, sensitivity, specificity and the like, can be used for obtaining a definite diagnosis result within 5 minutes and directly detecting a suspicious swine serum (or anticoagulation) virus antibody, and is particularly suitable for field ASFV serological diagnosis, epidemiological survey and swine international trade quarantine and inspection.
Owner:YANGZHOU UNIV
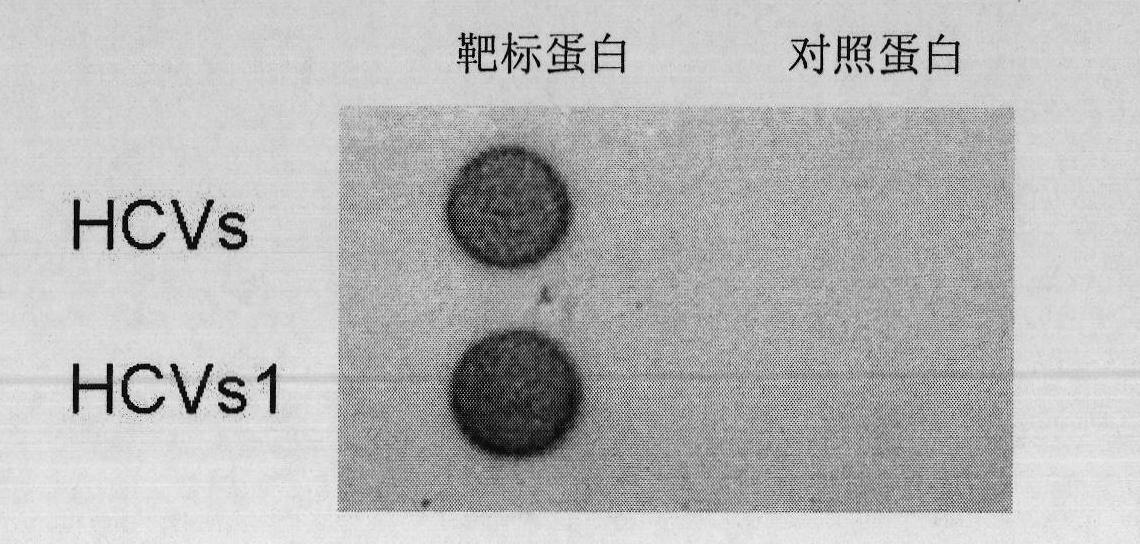
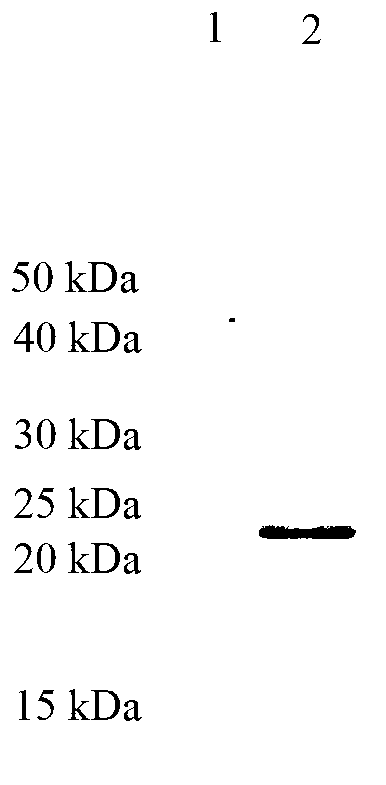
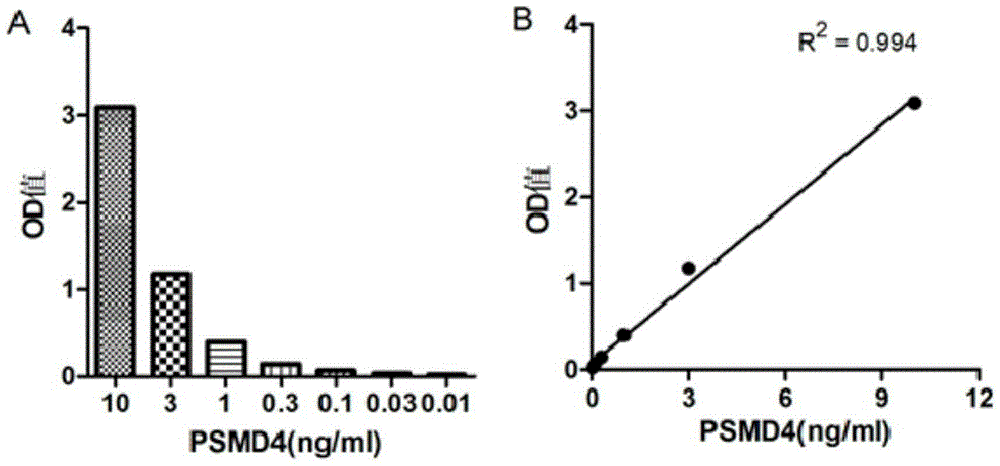
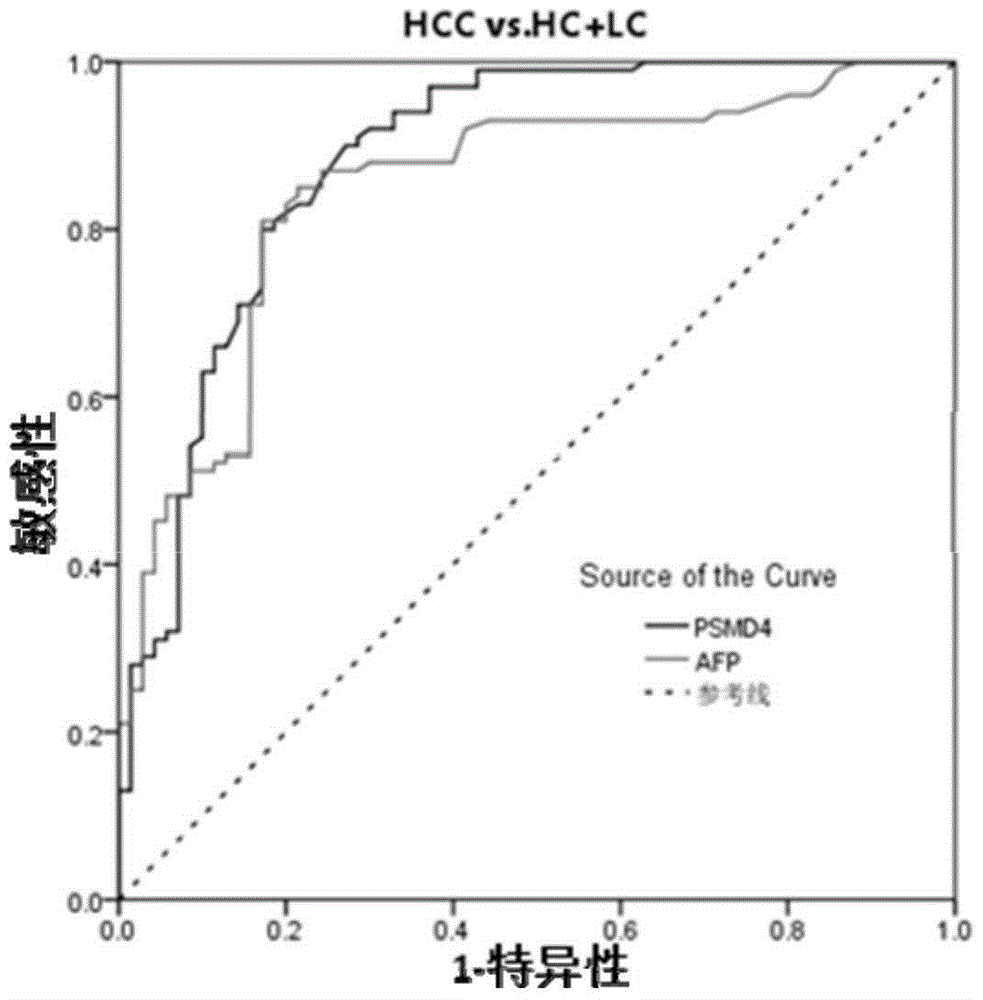
PSMD4 protein ELISA detection kit as well as detection method and application thereof
The invention belongs to the fields of immunology and biotechnology, and particularly relates to a PSMD4 protein ELISA detection kit as well as a detection method and application to serological diagnosis for liver cancer of the kit. According to extensive and deep research of the inventor, the expression level of PSMD4 protein in the serum of liver cancer patients can be detected according to an enzyme-linked immunosorbent assay (ELISA) method, and the PSMD4 protein is proved for the first time in the serum of the liver cancer patients. The kit comprises an ELISA plate coated with a PSMD4 antibody, a PSMD4 antigen detection antibody, an ELIAS secondary antibody, standard protein and the like. The invention further provides the detection method and application of the kit. The kit is simple and convenient to operate, can detect the content of the PSMD4 protein in the serum of a liver cancer patient correctly and highly sensitively, and provides a novel method for clinical examination and basic research.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Epitope antigens of human infection with H7N9 avian influenza and applications of epitope antigens in immune detection reagent
InactiveCN103450350AImmunoassay technology is simple and fastShort durationVirus peptidesMaterial analysisHemagglutininEpitope
The invention discloses dominant epitope antigens of hemagglutinin (HA) and neuraminidase (NA) of human infection with H7N9 avian influenza, and further discloses the applications of the antigens in an antibody detection reagent of human infection with H7N9 avian influenza. According to the epitope antigens of human infection with H7N9 avian influenza and the applications of epitope antigens in immune detection reagent, bioinformatics technology is employed to analyze the existing HA and NA sequences of H7N9, four antigen sections are screened to be cloned and expressed, the sensitivity and sensitivity of the antigens are screened by serological tests, and the third antigen is finally determined to have the highest diagnostic value so as to be applicable to detect the antibody of human infection with H7N9 avian influenza.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Group I FAdV-4 (fowl adenovirus serotype 4) vaccine
ActiveCN105412921AEffective immune protectionGood prospects for commercial developmentAntiviralsAntibody medical ingredientsImmune effectsAntigen
The invention provides a group I FAdV-4 (fowl adenovirus serotype 4) vaccine. An antigen of the group I FAdV-4 vaccine is an inactivated YBAV-4 virus. According to the prepared group I FAdV-4 inactivated vaccine, the immune effect of the vaccine is evaluated with a serology method and a vaccinated challenge method, a result shows that the prepared fowl adenovirus inactivated vaccine can provide effective immune protection for fowls, and the group I FAdV-4 inactivated vaccine has a good commercial development prospect.
Owner:YEBIO BIOENG OF QINGDAO
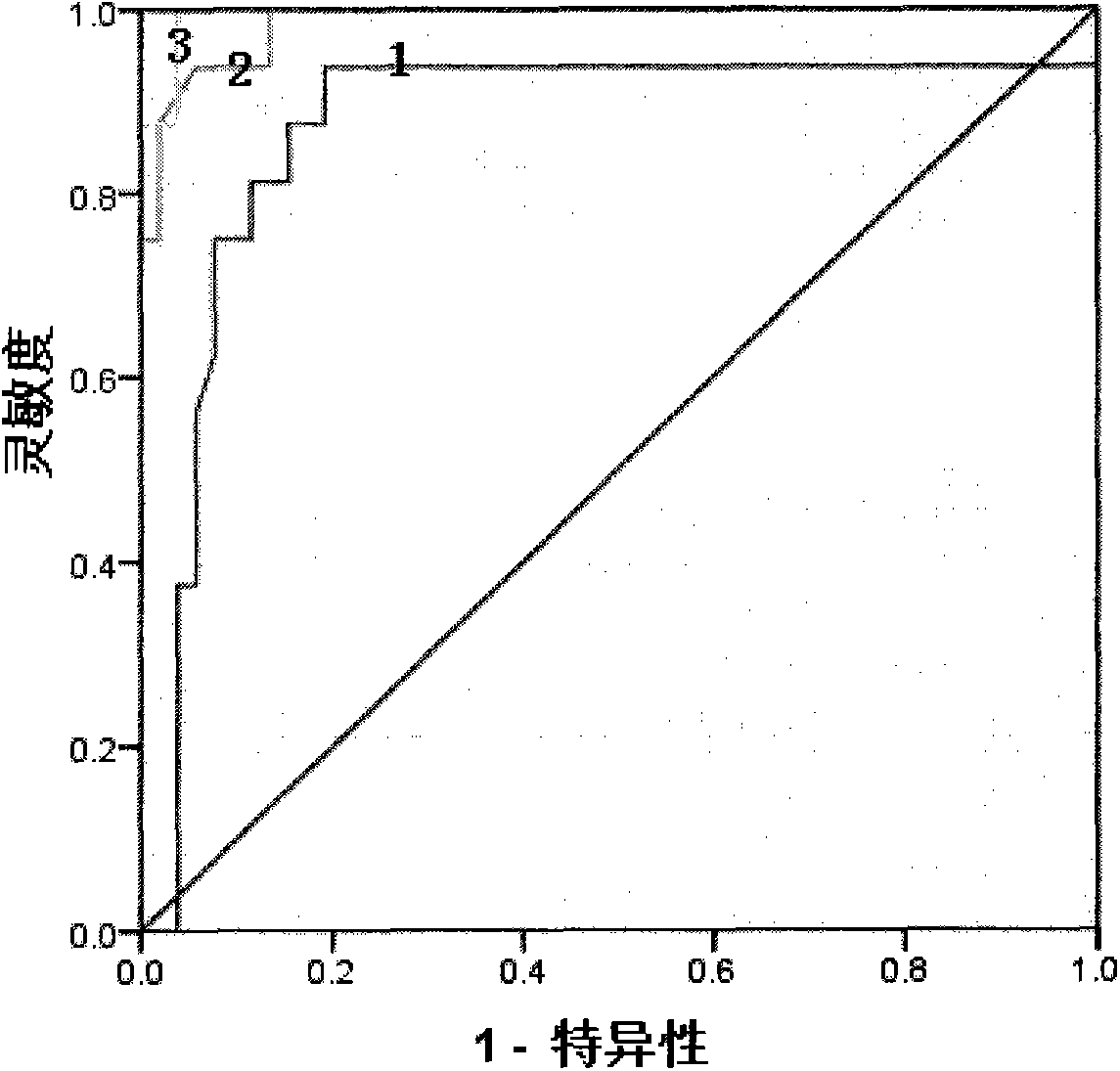
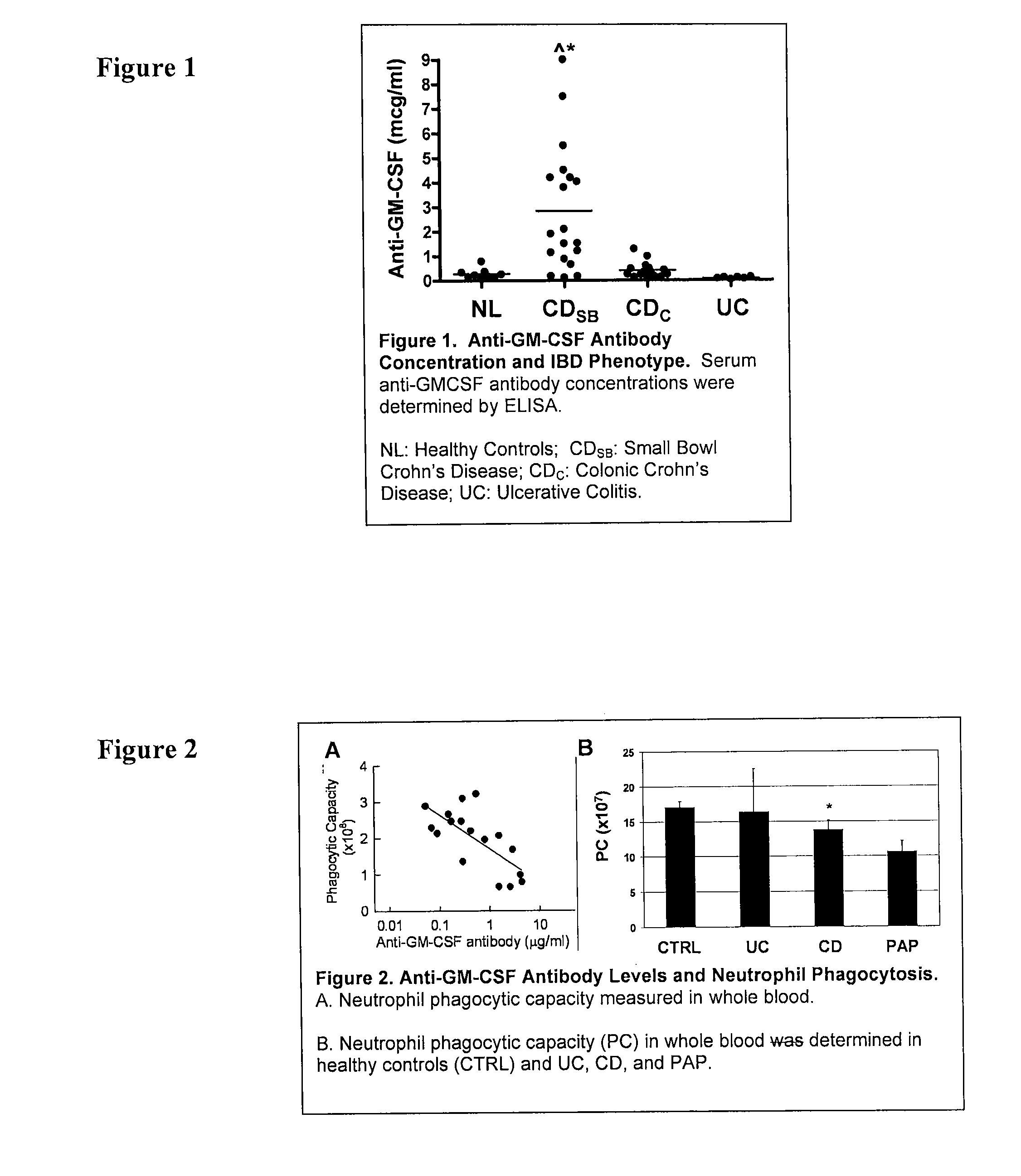
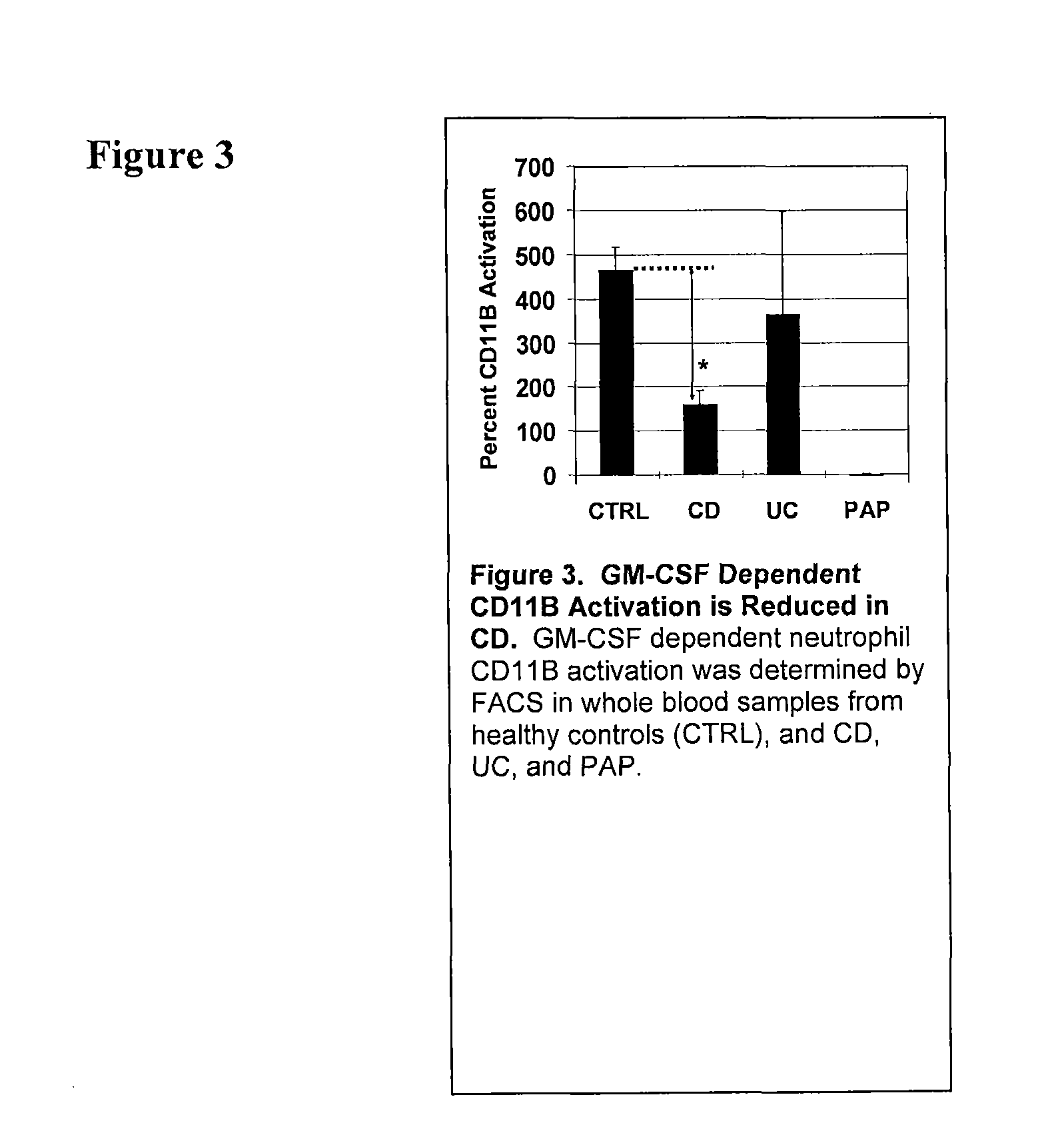
Serological markers of inflammatory bowel disease phenotype and disease progression
InactiveUS20100255513A1Peptide/protein ingredientsDisease diagnosisDiagnostic testUlcerative colitis
Disclosed are novel biomarkers and methods related to diagnostic tests for the detection and characterization of inflammatory bowel diseases, such as Crohn's disease and ulcerative colitis. In particular, the instant invention relates to novel biomarkers and methods of using such biomarkers to predict disease behavior and severity, to differentiate among disease types, and to optimize selection of treatment options in individuals suspected of having an inflammatory bowel disease.
Owner:DENSON LEE A +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com