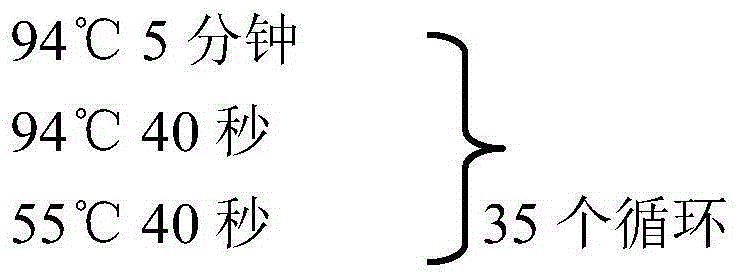Group I FAdV-4 (fowl adenovirus serotype 4) vaccine
A poultry adenovirus and virus technology, applied in antiviral agents, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve the problems of loopholes in the prevention and control of group I poultry adenoviruses, and achieve good commercial development prospects, The effect of effective immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Isolation and Identification of YBAV-4 Strain
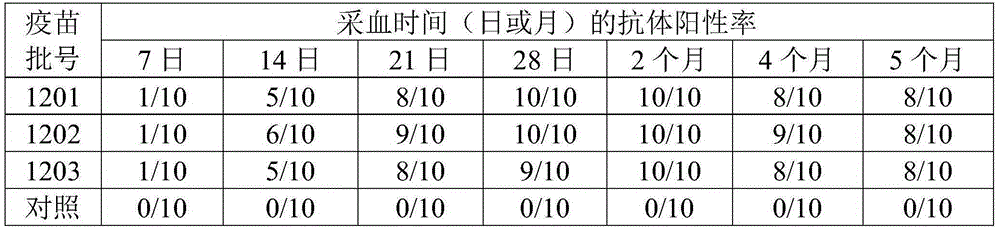
[0013] 1. Epidemiological survey Since 2010, some broiler breeders, laying hens and Ma chickens in Shandong, Jiangsu and other regions have experienced a disease characterized by high mortality and anatomical manifestations of hepatomegaly and hydropericardium. The disease was initially diagnosed as hydropericardium hepatitis syndrome caused by group I group C-4 avian adenovirus through clinical investigation and laboratory testing. In 2010, the inventor successfully isolated a virus strain from the liver of a dead chicken with typical symptoms of inclusion body hepatitis and hydropericardium in a farm in Zibo, Shandong.
[0014] 2. Virus isolation Take the liver of sick and dead chicken and grind it, add sterilized saline at a ratio of 1:5 to make a suspension; after repeated freezing and thawing 3 times, centrifuge at 3000r / min for 30min, take the supernatant; add penicillin and Streptomycin at 10,000 IU / ml each, overni...
Embodiment 2
[0036] Preparation of YBAV-4 Strain Virus Seeds
[0037] Select well-growing chicken hepatocytes, discard the original culture medium, add maintenance solution containing 1% virus seed (the third generation of the original virus seed of YBAV-4 strain), and culture at 37°C for 36-48 hours. % above, harvested, frozen and thawed twice, packed in sterilized containers, and sampled for identification. Indicate the date of harvest, generation of poisonous seeds, etc. According to this method, 15 generations were passed continuously, which were respectively marked as C4-C18 generations. Virus content was determined at each passage. Will test for sterility and virus content ≥10 7.0 TCID 50 The virus liquid is mixed, quantitatively divided, and stored in a freeze-dried manner. Indicate the date of harvest, generation of poisonous seeds, etc.
Embodiment 3
[0039] Preparation of virus solution for seedling production of YBAV-4 strain
[0040] 1 Seedling preparation material selection Group I Group 4 poultry adenovirus liquid preparation, select chicken liver cells.
[0041] 2 Cell preparation Take out the cryopreservation tube from the liquid nitrogen tank and put it in a 37°C water bath to thaw, transfer the cells into a centrifuge tube containing 10ml of culture medium, and centrifuge at 1000r / min for 5 minutes. Suspend cells in culture medium containing 20% newborn bovine serum, place at 37°C, 5% CO 2 Cultivate in an incubator, digest cells with trypsin-EDTA when they grow into a good monolayer, and subculture with cell nutrient solution.
[0042] 3. Cell monolayer culture Inoculate the expanded cultured seed cells into spinner bottles and culture at 37°C.
[0043] 4. Select chicken hepatocytes with good growth for poisoning, discard the original culture medium, add maintenance solution containing 1% virus species, and conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com