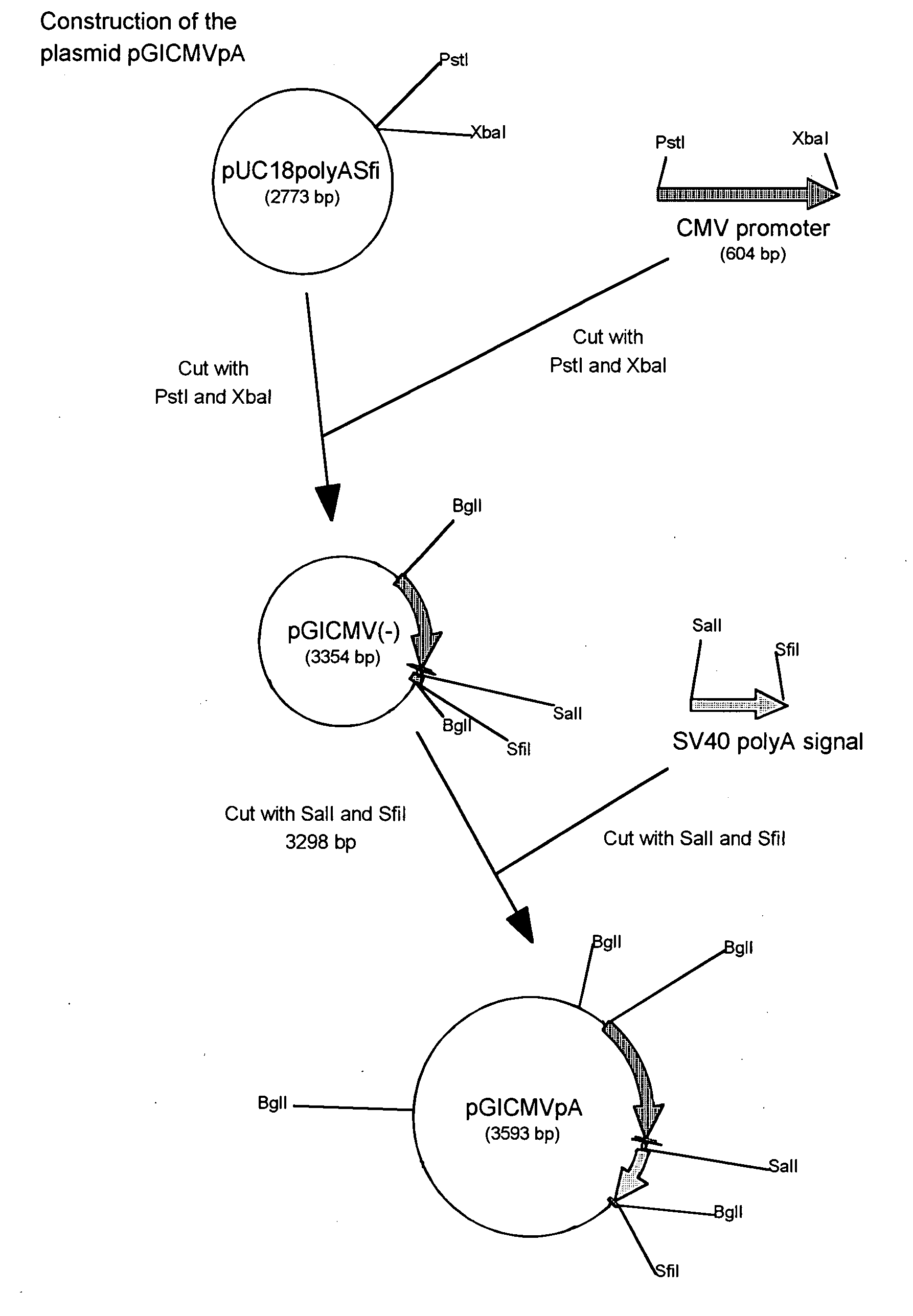
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Turkey Herpesvirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
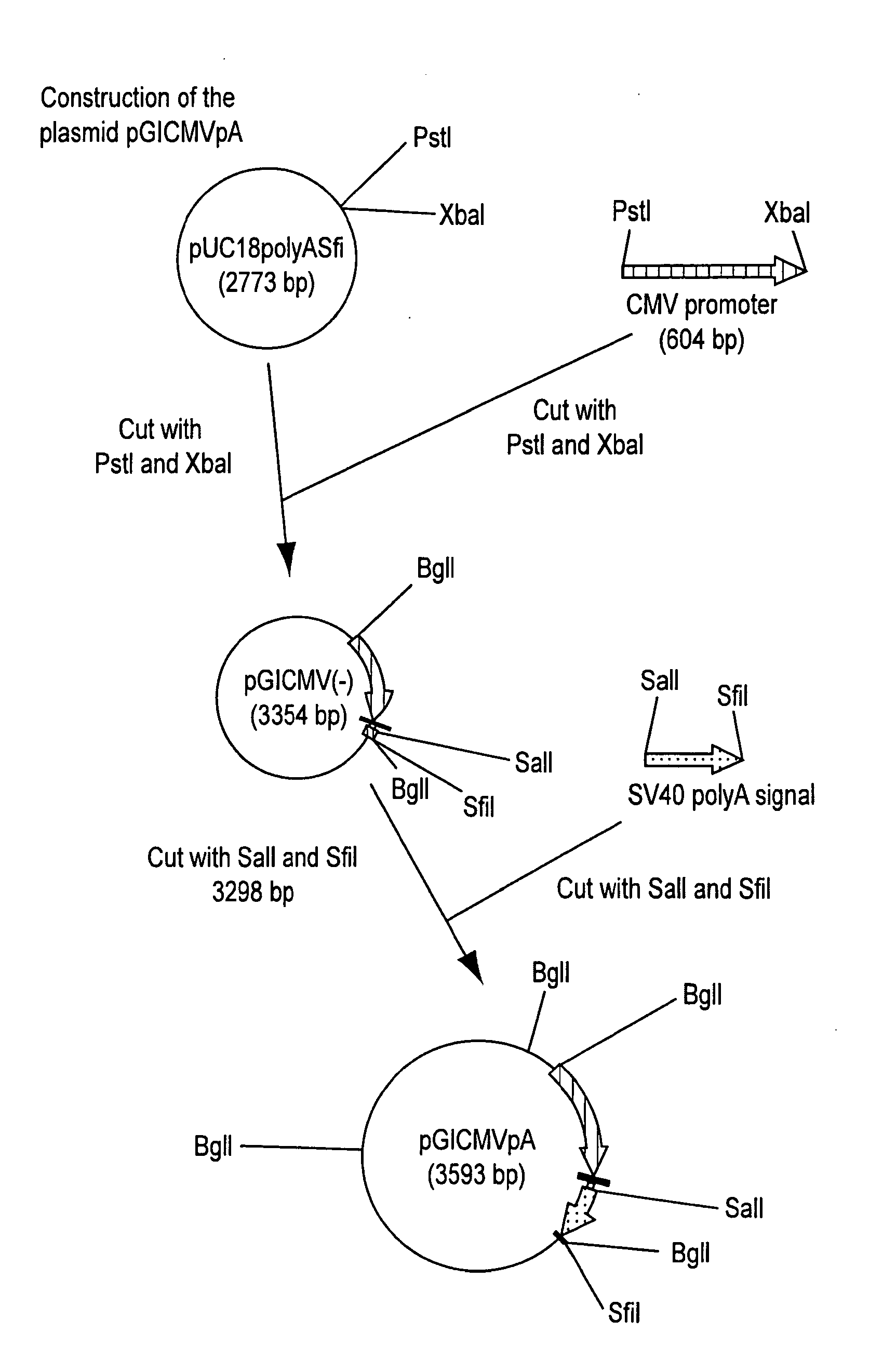
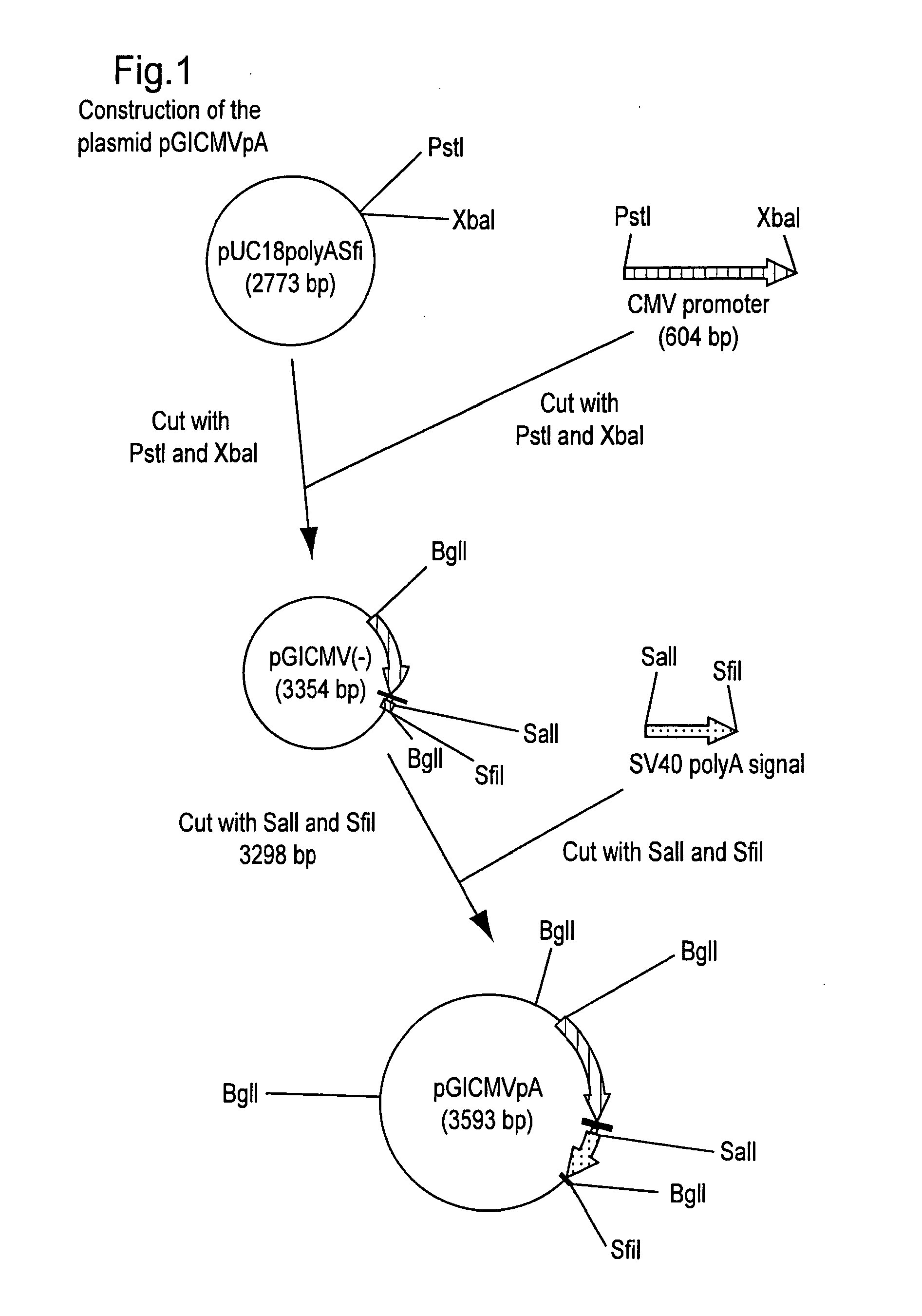
Turkey herpesvirus vectored recombinant containing avian influenza genes
InactiveUS20080241188A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsVaccinationElisa kit
The present invention provides a recombinant turkey herpesvirus modified by the presence of the cDNA encoding the hemagglutinin protein of avian influenza virus under a promoter. A poultry vaccine comprising the recombinant turkey herpesvirus described in the present invention can induce serological responses that may be easily detected by the hemagglutination inhibition assay but not by commercially available diagnostic ELISA kits; thus enabling easy differentiation between vaccination and field infection.
Owner:ZEON CORP +1
Promoter gene, recombinant turkey herpesvirus having the novel promoter gene, and poultry vaccine including the recombinant herpes virus of turkey
ActiveUS20080233146A1Increase gene expressionEasy to set upSsRNA viruses negative-senseVectorsTurkey HerpesvirusHerpes simplex virus DNA
Promoter genes that are derived from Marek's disease virus (MDV). These promoter genes can express two foreign genes when inserted in a recombinant turkey herpesvirus (HVT). A recombinant HVT having said novel promoter gene between two foreign genes. The poultry vaccine consisting of the recombinant turkey herpesvirus described in the present invention.
Owner:ZEON CORP
Recombinant turkey herpesvirus vaccine expressing infectious bursal disease virus VP2 protein and application thereof
InactiveCN104031889AViral antigen ingredientsMicroorganism based processesVp2 geneTurkey Herpesvirus
The invention provides a recombinant turkey herpesvirus vaccine expressing infectious bursal disease virus VP2 protein and application thereof. According to the invention, on the basis of a Fosmid library continuously covering the whole genome of herpesvirus of turkey (HVT), a VP2 gene carrying a Pec composite promoter is inserted into site HVT053 and site HVT054 in the nonessential region of HVT replication by using Red ET homologous recombination technology and Gateway LR clone technology so as to obtain recombinant HVT (rHVT-VP2). The recombinant HVT (rHVT-VP2) can provide a complete protecting force on the infectious bursal disease virus and is applicable to prevention of the infectious bursal disease virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant turkey herpesvirus strain rHMW for expressing H7N9 subtype avian influenza virus chimeric hemagglutinin and construction method of recombinant turkey herpesvirus strain rHMW
The invention belongs to recombinant viral vector vaccines in the technical fields of molecular biology and biology, and in particular relates to a recombinant turkey herpesvirus strain rHMW for expressing H7N9 subtype avian influenza virus chimeric hemagglutinin and a construction method of the recombinant turkey herpesvirus strain rHMW. The recombinant turkey herpesvirus strain rHMW has a collection number of CGMCC NO. 12984. The recombinant virus can be used for preparing vaccines capable of preventing H7N9 subtype avian influenza viruses. The recombinant turkey herpesvirus strain rHMW disclosed by the invention has obvious effects for preventing and controlling infectious diseases of poultry and achieves huge social benefits and economic benefits.
Owner:YANGZHOU UNIV
Recombinant turkey herpesvirus and application thereof
The invention relates to the field of animal virology, and provides a recombinant turkey herpesvirus (rHVT-VP2), which is actually a brand-new recombinant turkey herpesvirus rHVT-VP2 obtained by combining an expression box of an avian infectious bursal disease virus VP2 gene and a turkey herpesvirus HVT. The recombinant turkey herpesvirus is also a recombinant vaccine of Marek's disease and avianinfectious bursal disease. By inserting the VP2 gene into a gene complex of the HVT under control of promoter, the rHVT-VP2 not only has fine immune protection for avian Marek's disease, but also haspersistent protective immunity of induced resistance of IBDV (infectious bursal disease virus) for commercial chickens with high IBDV material antibody level.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Promoter gene, recombinant turkey herpesvirus having the novel promoter gene, and poultry vaccine including the recombinant herpes virus of turkey
ActiveUS7569365B2Increase gene expressionEasy to set upSsRNA viruses negative-senseVectorsTurkey HerpesvirusHerpes simplex virus DNA
Promoter genes that are derived from Marek's disease virus (MDV). These promoter genes can express two foreign genes when inserted in a recombinant turkey herpesvirus (HVT). A recombinant HVT having said novel promoter gene between two foreign genes. The poultry vaccine consisting of the recombinant turkey herpesvirus described in the present invention.
Owner:ZEON CORP
Construction and application of recombinant turkey herpesvirus expressing H7N9 subtype highly pathogenic avian influenza virus HA protein
ActiveCN110218706AThoroughly purifiedShorten the timeSsRNA viruses negative-senseViral antigen ingredientsTurkey HerpesvirusNucleotide sequencing
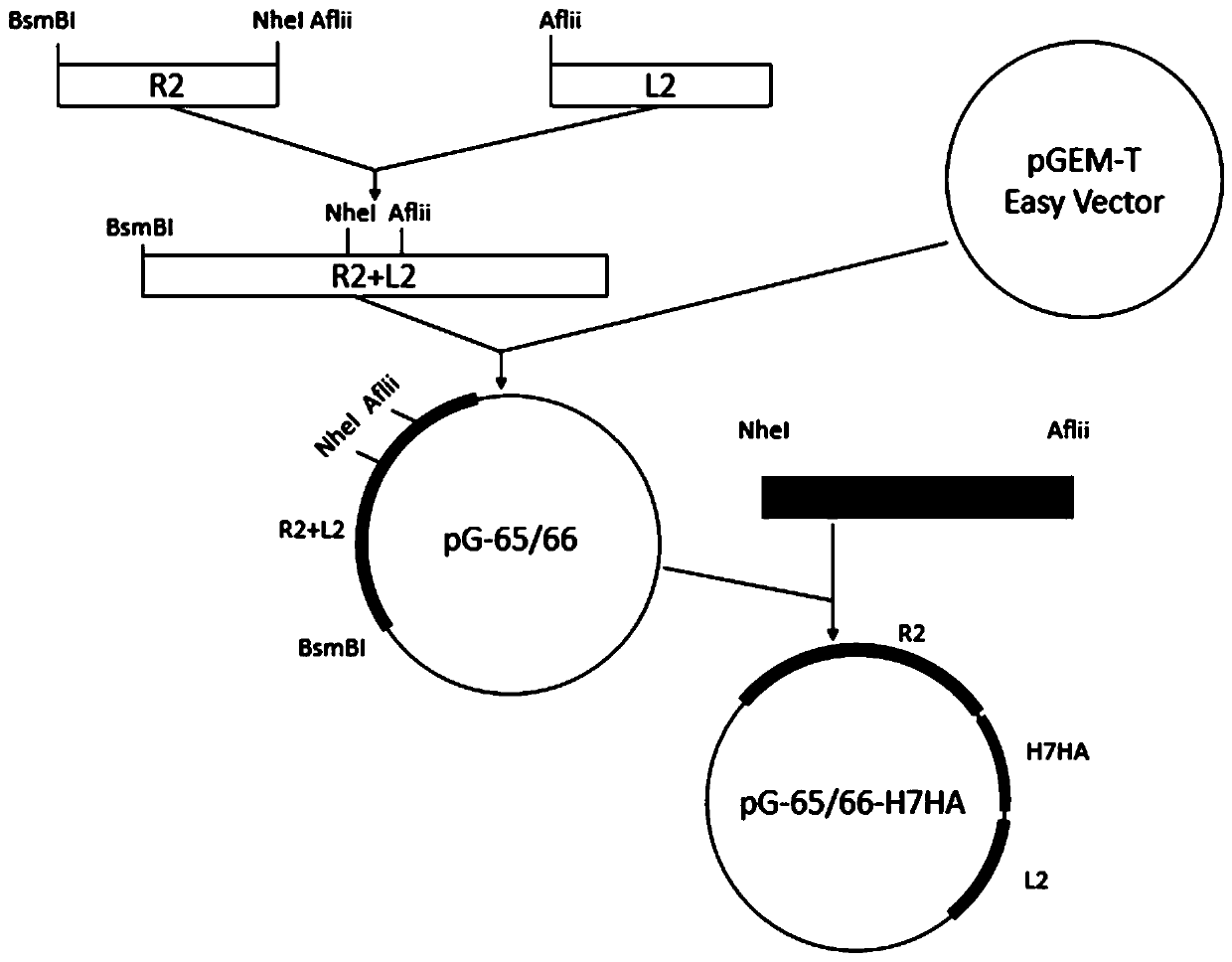
The invention discloses construction and application of a recombinant turkey herpesvirus expressing H7N9 subtype highly pathogenic avian influenza virus HA protein. The recombinant turkey herpesvirusis obtained by inserting an exogenous gene expression box between the HVT-065 and HVT-066 genes in a non-essential replication area of a coding sequence of a turkey herpesvirus (HVT), wherein the exogenous gene expression box is formed by connecting an MCMV promoter, an exogenous gene and SV40 poly A in sequence; the exogenous gene is the H7HA gene, and the nucleotide sequence of the exogenous gene is shown as SEQ ID NO:1. The construction method of the recombinant turkey herpesvirus is also provided. An ultrasonic cracking and breaking method is adopted for purification, the screening time isshortened, and the obtained recombinant turkey herpesvirus rHVT-H7HA provides good protection for the highly pathogenic H7N9 subtype avian influenza virus and can be used for subsequent development of new avian influenza virus vaccines.
Owner:SOUTH CHINA AGRI UNIV
Recombinant turkey herpesvirus virus strain rHOH expressing H7N9 subtype avian influenza virus haemagglutinin protein and construction method
InactiveCN106399267APrevention and Control of H7N9 OutbreakProtect public healthSsRNA viruses negative-senseVirus peptidesHeterologous AntigensChick embryos
The invention belongs to recombinant virus carrier vaccines in the field of molecular biology and biotechnology, and particularly relates to a recombinant turkey herpesvirus virus strain rHOH expressing H7N9 subtype avian influenza virus haemagglutinin protein and a construction method. The preservation number of the recombinant turkey herpesvirus virus strain rHOH is CGMCC NO.12985. A gB promoter of the recombinant turkey herpesvirus virus strain rHOH comes from a turkey herpesvirus virus carrier FC126 itself; an expressed heterologous antigen (haemagglutinin protein) gene is optimized through codon bias of Gallus Gallus, the nucleotide sequence changes, and no amino acid sequence changes. The recombinant turkey herpesvirus virus strain rHOH can be used for preparing vaccines for preventing H7N9 subtype avian influenza viruses; 18-day-old chick embryos obtained after hatch or 1-day-old chicks are inoculated, an H7N9 epidemic situation of poultry is prevented and controlled, and public health and safety are protected.
Owner:YANGZHOU UNIV
Recombinant turkey herpesvirus expressing H9N2 subtype avian influenza virus (AIV) H9 proteins
ActiveCN109402071AGood immune protectionSsRNA viruses negative-senseViral antigen ingredientsNasal cavityVisceral organ
The invention discloses a recombinant turkey herpesvirus expressing H9N2 subtype avian influenza virus (AIV) H9 proteins. The recombinant turkey herpesvirus is obtained in the mode that a genome of the recombinant turkey herpesvirus is subjected to following transformation: DNA molecules containing coding genes of H9N2 subtype AIV H9 proteins are added; and the amino acid sequence of the H9N2 subtype AIV H9 proteins is shown as the sequence 2 in a sequence table. Through an experiment, it is proved that at the first day of the experiment, the necks of SPF chickens with one day age is subjectedto subcutaneous inoculation of the recombinant turkey herpesvirus; at the thirty-fifth day of the experiment, nasal cavities are inoculated with BJ / 15 virus strains; at the thirty-eighth day and thefortieth day, neither oral cavities nor cloacas of the SPF chickens are subjected to toxin expelling, and the BJ / 15 virus strains cannot be effectively duplicated in visceral organs (such as the internal organs, tracheas, lungs, spleens and kidneys) of the SPF chickens. Thus, it can be seen that the recombinant turkey herpesvirus has an excellent immune protection effect on an H9N2 subtype AIV, and the recombinant turkey herpesvirus has important application value.
Owner:CHINA AGRI UNIV
Construction of recombinant turkey herpesvirus used for expressing F gene of chicken Newcastle disease virus and application thereof
ActiveCN106929483ASsRNA viruses negative-senseViral antigen ingredientsRecombinant vaccinesTurkey Herpesvirus
The invention relates to construction of a recombinant turkey herpesvirus used for expressing an F gene of a chicken Newcastle disease virus and an application of the construction. The recombinant turkey herpesvirus used for expressing the F gene of the chicken Newcastle disease virus is named as recombinant turkey herpesvirus rHVT-F strain; the virus strain combines an expression cassette containing the F gene of the chicken Newcastle disease virus with the turkey herpesvirus (HVT), that is, and the F gene controlled by a CMV promoter is inserted into an HVT gene to construct to obtain the brand new promoter rHVT-F strain. The rHVT-F virus strain can induce a chicken to produce lasting protective immunity on MD and ND resistance. The virus strain produces a strain to prepare a bivalent recombinant vaccine for the fowl paralysis and the chicken Newcastle disease.
Owner:北京邦卓生物科技有限公司
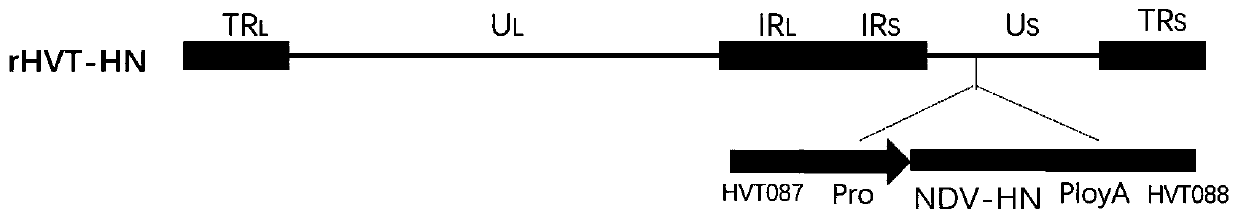
Construction method and application of HVT co-expressing NDV HN and IBDV VP2 genes
The invention relates to a construction method of a recombinant herpesvirus of turkey rHVT-HN strain expressing Newcastle disease virus (HN) gene. The recombinant virus can be used for preparing vector vaccines to prevent Marek's disease and Newcastle disease. The invention further relates to a construction method and application of a recombinant herpesvirus of turkey co-expressing the Newcastle disease HN gene and infectious bursa fabricius VP2 gene. The recombinant herpesvirus of turkey rHVT-HN strain is a recombinant virus obtained by recombining an expression box of the Newcastle disease HN gene and the infectious bursa fabricius VP2 gene into the HVT. The recombinant virus can serve as a production virus seed to be used for preparing virus live vector vaccines which can prevent both the Marek's disease and the Newcastle disease as well as infectious bursa fabricius.
Owner:北京邦卓生物科技有限公司
Recombinant turkey herpesvirus candidate vaccine strain for expressing gene VII-type Newcastle disease virus fusion protein and preparation method thereof
InactiveCN110331135ASsRNA viruses negative-senseViral antigen ingredientsNewcastle disease virus NDVTurkey Herpesvirus
The invention belongs to the technical field of genetic engineering vaccines, and particularly relates to a recombinant turkey herpesvirus candidate vaccine strain for expressing gene VII-type Newcastle disease virus fusion protein and a preparation method thereof. The recombinant turkey herpesvirus candidate vaccine strain for expressing the gene VII-type Newcastle disease virus fusion protein isnamed as rHVT-NDV-VII-F, and has a preservation number of CCTCC NO: V201904. The recombinant virus can express a gene VII-type NDV F protein, is completely matched with the genotype of a currently domestically popular NDV wild strain, and can be used as a gene engineering vaccine candidate strain for preventing NDV infection.
Owner:YANGZHOU UNIV
Turkey herpesvirus vectored recombinant containing avian influenza genes
ActiveUS20100092510A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsElisa kitHemagglutinin protein
Owner:BIOMUNE
Avian vaccines possessing a positive marker gene
InactiveUS20110311994A1Easy to identifyEasy to trackSsRNA viruses negative-senseViral antigen ingredientsFowlGene product
The present invention provides a poultry vaccine containing a positive marker gene. More specifically, recombinant turkey herpesvirus modified by the presence of an extraneous antigen gene that may be used as a positive marker to identify and track vaccinated animals is provided. When inoculated into host animals, a poultry vaccine comprising the recombinant turkey herpesvirus provided in the present invention can elicit serological immune responses to the marker gene product that may be detected by serological assays such as enzyme-linked immunosorbent assay and serum agglutination test, thus enabling easy identification and tracking of vaccinated animals.
Owner:ESAKI MOTOYUKI +2
Recombinant turkey herpesvirus vaccines and uses thereof
The present disclosure provides a recombinant viral vector comprising at least one transgene inserted into a Marek's disease viral genome for treatment of diseases in poultry. Also provided are immunogenic compositions comprising such recombinant viral vectors and methods for preventing or inhibiting Marek's disease in combination with at least a second disease in poultry.
Owner:TEXAS A&M UNIVERSITY
Cell Line for Production of Marek's Disease Virus Vaccine and Methods of Making and Using the Same
PendingUS20210386854A1HydrolasesArtificial cell constructsTurkey HerpesvirusHerpes simplex virus DNA
The present application relates to an avian cell line capable of supporting viral growth of Marek's Disease Virus (MDV), including Herpes Virus of Turkeys (HVT), methods of producing such cell lines, and therapeutic uses of the cell lines and resulting vaccines.
Owner:ZOETIS SERVICE LLC
Recombinant turkey herpesvirus strain for expressing chicken infectious bursal disease virus VP2 gene
PendingCN110684744AViral antigen ingredientsVirus peptidesInfectious bursal disease virus IBDVVariant strain
The invention provides a VP2 gene recombinant turkey herpesvirus strain for expressing a chicken infectious bursal disease virus variant. The VP2 gene recombinant turkey herpesvirus strain is preparedby inserting an IBDV VP2 gene into a turkey herpesvirus genome. The recombinant virus rHVT-vVP2 strain is obtained by inserting an expression cassette containing a VP2 gene into the turkey herpesvirus genome by taking a turkey herpesvirus (HVT) FC-126 vaccine strain as a carrier and taking a spacer region of UL45-UL46 of the turkey herpesvirus (HVT) FC-126 vaccine strain as an insertion site. Theimmune protection evaluation results show that after chickens are immunized by the rHVT-vVP2 strain, 100% virus attacking protection can be provided for attacks of IBDV virulent strains and variant strains.
Owner:YEBIO BIOENG OF QINGDAO
Recombinant turkey herpesvirus vaccine strain coexpressing H5 subtype avian influenza HA protein and infectious bursal VP2 protein
InactiveCN108543067ASsRNA viruses negative-senseViral antigen ingredientsVp2 geneAvian influenza virus
The invention relates to a recombinant turkey herpesvirus vaccine strain coexpressing an H5 subtype avian influenza HA protein and an infectious bursal VP2 protein, and an application thereof. The preservation number of the recombinant turkey herpesvirus vaccine strain coexpressing H5 subtype avian influenza HA gene and infectious bursal VP2 gene is CCTCC NO:V201738. The invention also provides atriple vaccine for preventing avian influenza virus, infectious bursal disease virus and Marek virus. The triple vaccine comprises an effective amount of the recombinant turkey herpesvirus vaccine strain coexpressing the H5 subtype avian influenza HA gene and the infectious bursal VP2 gene.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant turkey herpesvirus expressing HA gene and application thereof
PendingCN110205308ABroad immunogenicityRecombination is fast and accurateSsRNA viruses negative-senseViral antigen ingredientsFowlNucleotide
The invention provides a recombinant turkey herpesvirus expressing HA gene and an application thereof. Based on a viral vector HVT- BAC as a platform, combined with a RED / ET recombination technology and a ccdB reverse screening technology, and the recombinant turkey herpesvirus expressing HA gene is prepared by a two-step recombination method, wherein, a nucleotide sequence of the HA gene is shownas SEQ ID No. 1. The beneficial effect is that the HA gene used in the present invention is derived from a H9N2 toxic strain widely prevalent in recent years in China, and has broad immunogenicity tobirds. A preparation method of the invention realizes rapid and accurate recombination of the exogenous gene, and a used screening marker is beneficial to reduce a screening step, and the obtained recombinant virus strain can be stably passaged. A vaccine prepared by the recombinant virus strain can stimulate the latent infection of the chicken body, causes lifelong immunity, provides technical support for effectively preventing and controlling H9N2 avian influenza and Marek's disease, and has important production guiding significance.
Owner:SOUTH CHINA AGRI UNIV
Avian vaccines possessing a positive marker gene
InactiveUS20090246226A1Easy to identifyEasy to trackSsRNA viruses negative-senseViral antigen ingredientsFowlAssay
The present invention provides a poultry vaccine containing a positive marker gene. More specifically, recombinant turkey herpesvirus modified by the presence of an extraneous antigen gene that may be used as a positive marker to identify and track vaccinated animals is provided. When inoculated into host animals, a poultry vaccine comprising the recombinant turkey herpesvirus provided in the present invention can elicit serological immune responses to the marker gene product that may be detected by serological assays such as enzyme-linked immunosorbent assay and serum agglutination test, thus enabling easy identification and tracking of vaccinated animals.
Owner:ZEON CORP
Recombinant herpesvirus turkey live vector vaccine capable of simultaneously expressing classical strain and variant infectious bursal disease virus VP2 protein
ActiveCN114107227AGuaranteed immune effectImprove securityViral antigen ingredientsVirus peptidesVector vaccineVariant strain
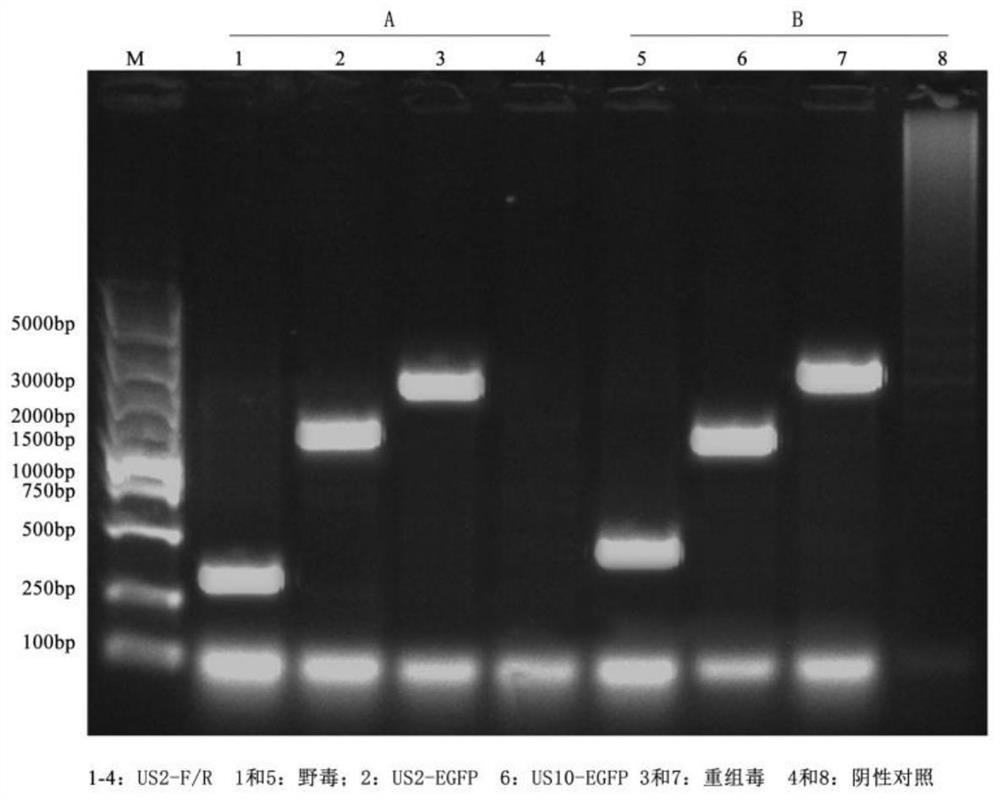
The invention discloses a recombinant herpesvirus turkey live vector vaccine capable of simultaneously expressing classical strain and variant strain infectious bursal disease virus VP2 protein, and belongs to the field of veterinary biological products. According to the invention, a VP2 protein and LTB fusion expression cassette containing an infectious bursal disease virus classical strain is knocked into a US2 virus replication non-essential region of a turkey herpesvirus, and a VP2 protein and LTB fusion expression cassette of a variant strain is inserted into a US10 virus replication non-essential region. The finally obtained recombinant virus simultaneously and efficiently expresses LTB-VP2 fusion antigen protein of the IBDV classic strain and the variant strain in CEF cells. The vaccine prepared by the invention can improve the antibody level after immunization, improve the uniformity of the antibody after immunization and ensure the immunization effect of the vaccine, has the advantages of high efficiency, good safety and lifelong immunization after one-time inoculation, does not cause clinical reaction and pathological damage to chicks, and has the vaccine protection rate of 100%.
Owner:扬州优邦生物药品有限公司
A recombinant turkey herpes virus expressing h9 protein of h9n2 subtype avian influenza virus
ActiveCN109402071BGood immune protectionSsRNA viruses negative-senseViral antigen ingredientsVirus strainSpleen
Owner:CHINA AGRI UNIV
Construction and application of recombinant turkey herpes virus expressing h7n9 subtype highly pathogenic avian influenza virus ha protein
ActiveCN110218706BThoroughly purifiedShorten the timeSsRNA viruses negative-senseViral antigen ingredientsNucleotideTGE VACCINE
The invention discloses construction and application of a recombinant turkey herpesvirus expressing H7N9 subtype highly pathogenic avian influenza virus HA protein. The recombinant turkey herpesvirusis obtained by inserting an exogenous gene expression box between the HVT-065 and HVT-066 genes in a non-essential replication area of a coding sequence of a turkey herpesvirus (HVT), wherein the exogenous gene expression box is formed by connecting an MCMV promoter, an exogenous gene and SV40 poly A in sequence; the exogenous gene is the H7HA gene, and the nucleotide sequence of the exogenous gene is shown as SEQ ID NO:1. The construction method of the recombinant turkey herpesvirus is also provided. An ultrasonic cracking and breaking method is adopted for purification, the screening time isshortened, and the obtained recombinant turkey herpesvirus rHVT-H7HA provides good protection for the highly pathogenic H7N9 subtype avian influenza virus and can be used for subsequent development of new avian influenza virus vaccines.
Owner:SOUTH CHINA AGRI UNIV
Construction and application of a kind of HVT co-expressing ndv HN and IBDV VP2 genes
The invention relates to a construction method of a recombinant herpesvirus of turkey rHVT-HN strain expressing Newcastle disease virus (HN) gene. The recombinant virus can be used for preparing vector vaccines to prevent Marek's disease and Newcastle disease. The invention further relates to a construction method and application of a recombinant herpesvirus of turkey co-expressing the Newcastle disease HN gene and infectious bursa fabricius VP2 gene. The recombinant herpesvirus of turkey rHVT-HN strain is a recombinant virus obtained by recombining an expression box of the Newcastle disease HN gene and the infectious bursa fabricius VP2 gene into the HVT. The recombinant virus can serve as a production virus seed to be used for preparing virus live vector vaccines which can prevent both the Marek's disease and the Newcastle disease as well as infectious bursa fabricius.
Owner:北京邦卓生物科技有限公司
Method for measuring viral titer of Marek's disease (MD) turkey herpesvirus vaccine, live
ActiveCN102399911AIncrease profitGood energyMicrobiological testing/measurementMicroorganism based processesFibroblastTurkey Herpesvirus
The invention discloses a method for measuring viral titer of a Marek's disease (MD) turkey herpesvirus vaccine, live. The method comprises the following steps of: (1) preparing chick embryo fibroblast suspension; (2) performing cell culture; (3) diluting a virus solution to be measured; (4) performing virus inoculation and culture; (5) counting plaques; and (6) calculating the viral titer. The method is easy to impellent, uniform in measurement conditions, strong in controllability and small in batch errors, and shortens the period of measuring the viral titer of the MD turkey herpesvirus vaccine, live.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Method for measuring viral titer of Marek's disease (MD) turkey herpesvirus live vaccine
ActiveCN102399911BIncrease profitGood energyMicrobiological testing/measurementMicroorganism based processesFibroblastTurkey Herpesvirus
The invention discloses a method for measuring viral titer of a Marek's disease (MD) turkey herpesvirus vaccine, live. The method comprises the following steps of: (1) preparing chick embryo fibroblast suspension; (2) performing cell culture; (3) diluting a virus solution to be measured; (4) performing virus inoculation and culture; (5) counting plaques; and (6) calculating the viral titer. The method is easy to impellent, uniform in measurement conditions, strong in controllability and small in batch errors, and shortens the period of measuring the viral titer of the MD turkey herpesvirus vaccine, live.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Construction and Application of Recombinant Turkey Herpes Virus Expressing Chicken Newcastle Disease Virus f Gene
ActiveCN106929483BSsRNA viruses negative-senseViral antigen ingredientsRecombinant vaccinesTGE VACCINE
The invention relates to construction of a recombinant turkey herpesvirus used for expressing an F gene of a chicken Newcastle disease virus and an application of the construction. The recombinant turkey herpesvirus used for expressing the F gene of the chicken Newcastle disease virus is named as recombinant turkey herpesvirus rHVT-F strain; the virus strain combines an expression cassette containing the F gene of the chicken Newcastle disease virus with the turkey herpesvirus (HVT), that is, and the F gene controlled by a CMV promoter is inserted into an HVT gene to construct to obtain the brand new promoter rHVT-F strain. The rHVT-F virus strain can induce a chicken to produce lasting protective immunity on MD and ND resistance. The virus strain produces a strain to prepare a bivalent recombinant vaccine for the fowl paralysis and the chicken Newcastle disease.
Owner:北京邦卓生物科技有限公司
Construction and application of recombinant virus vector for expressing infectious bursal disease virus VP2 protein
InactiveCN111909965AViral antigen ingredientsVirus peptidesInfectious bursal disease virus IBDVRecombinant vaccines
The invention constructs a recombinant turkey herpesvirus expressing chicken infectious bursal disease virus VP2 gene; the recombinant virus strain is prepared into a live vaccine, so that Marek's diseases and infectious bursal diseases can be prevented; due to a homologous recombination method, the vector is used to express exogenous gene; and a foundation is laid for constructing a novel recombinant vaccine for resisting Marek's disease and other pathogens by taking the turkey herpesvirus as the vector.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Recombinant turkey herpesvirus as well as preparation method and application thereof
PendingCN114134124AQuick buildEfficient insertionSsRNA viruses negative-senseHydrolasesGenomic intervalNucleotide
The invention provides a recombinant turkey herpesvirus as well as a preparation method and application thereof, and particularly provides a recombinant turkey herpesvirus, an exogenous gene is inserted into a spacer region between an HVT005 region and an HVT006 region of a turkey herpesvirus genome, and the exogenous gene is selected from genes from a Newcastle disease virus, an avian influenza virus or an avian infectious bursal disease virus; the spacer region between the HVT005 region and the HVT006 region of the turkey herpesvirus genome is between 8867nt and 9319nt of the turkey herpesvirus genome, and the nucleotide sequence of the spacer region is SEQ ID NO: 1. The invention discovers that the HVT005-HVT006 spacer region can be used as a site for inserting an HVT exogenous gene for the first time. An exogenous gene is inserted into a spacer region HVT005-HVT006 of an HVT genome by utilizing a CRISPR / Cas9 technology, and the vaccine strain can be used as a vaccine candidate strain for preventing the Newcastle disease and the avian influenza.
Owner:YANGZHOU UNIV
Recombinant turkey herpesvirus vaccines and uses thereof
The present disclosure provides a recombinant viral vector comprising at least one transgene inserted into a Marek's disease viral genome for treatment of diseases in poultry. Also provided are immunogenic compositions comprising such recombinant viral vectors and methods for preventing or inhibiting Marek's disease in combination with at least a second disease in poultry.
Owner:TEXAS A&M UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com