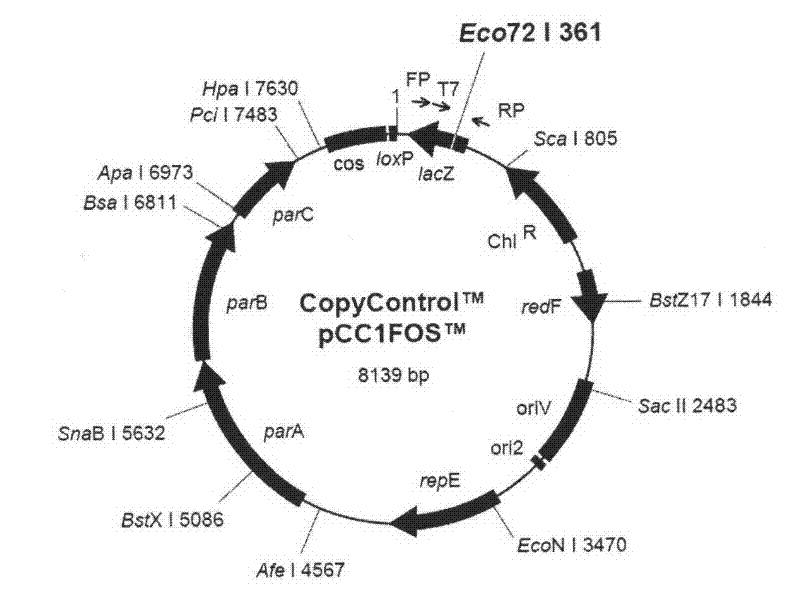
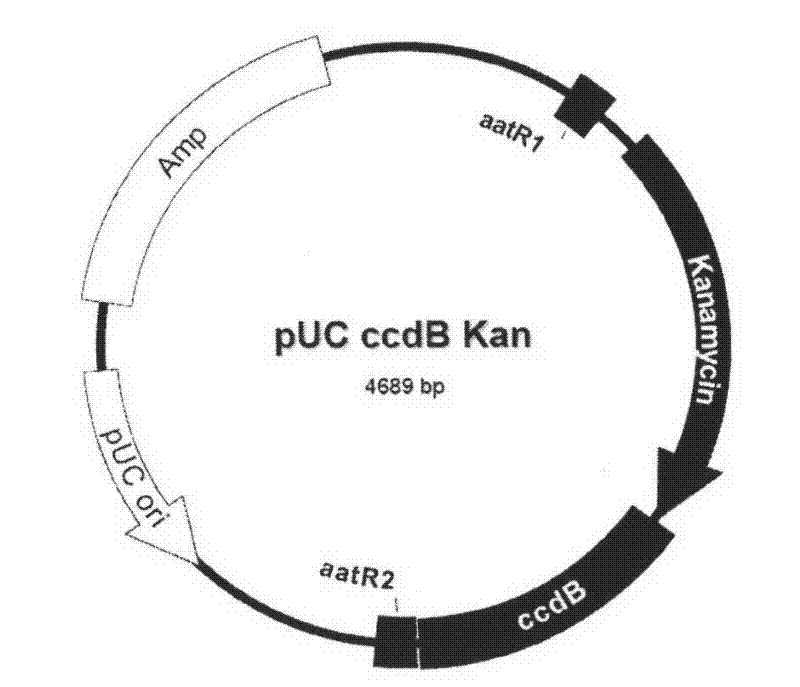
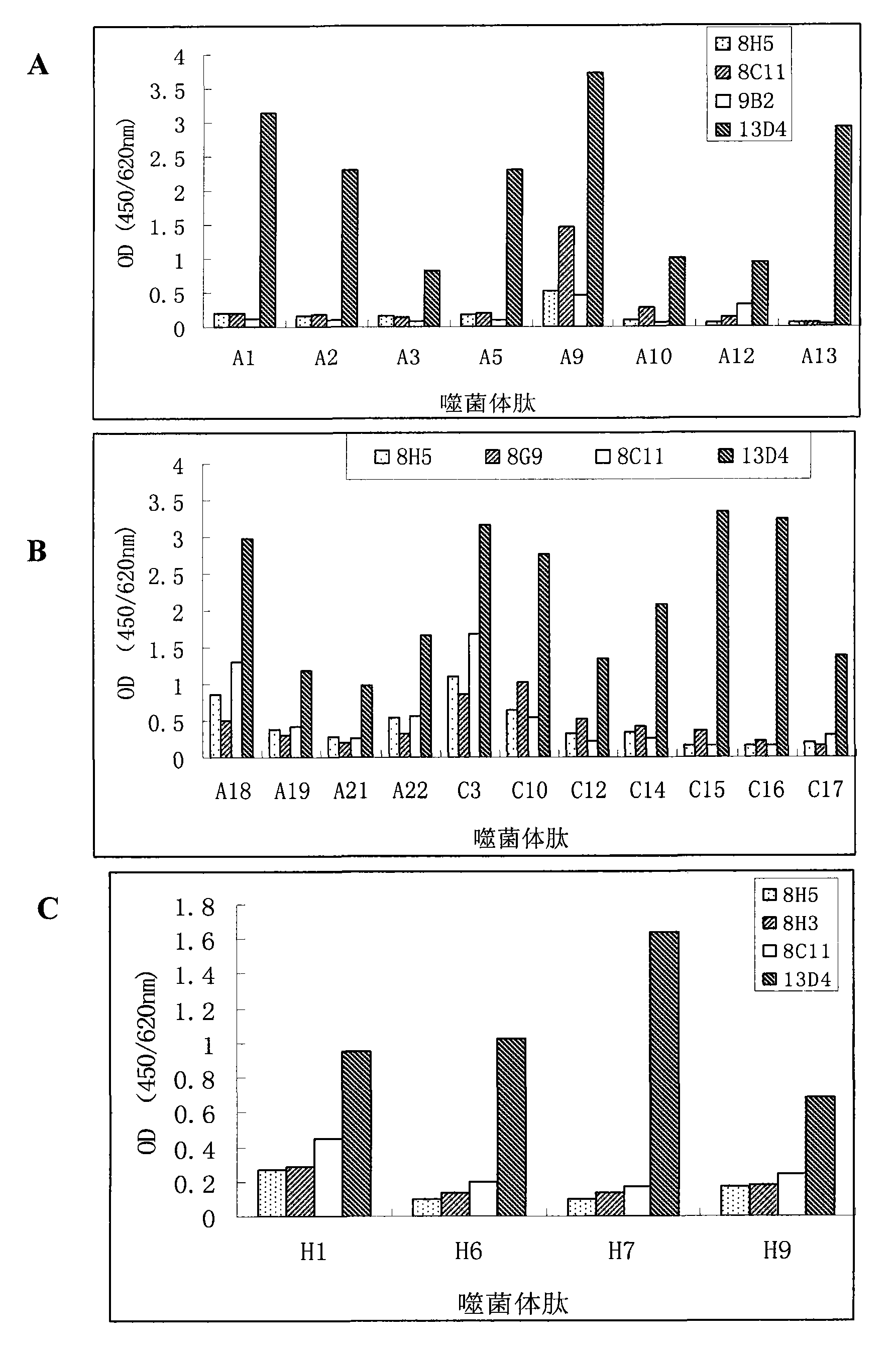
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
141 results about "Influenza virus A hemagglutinin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
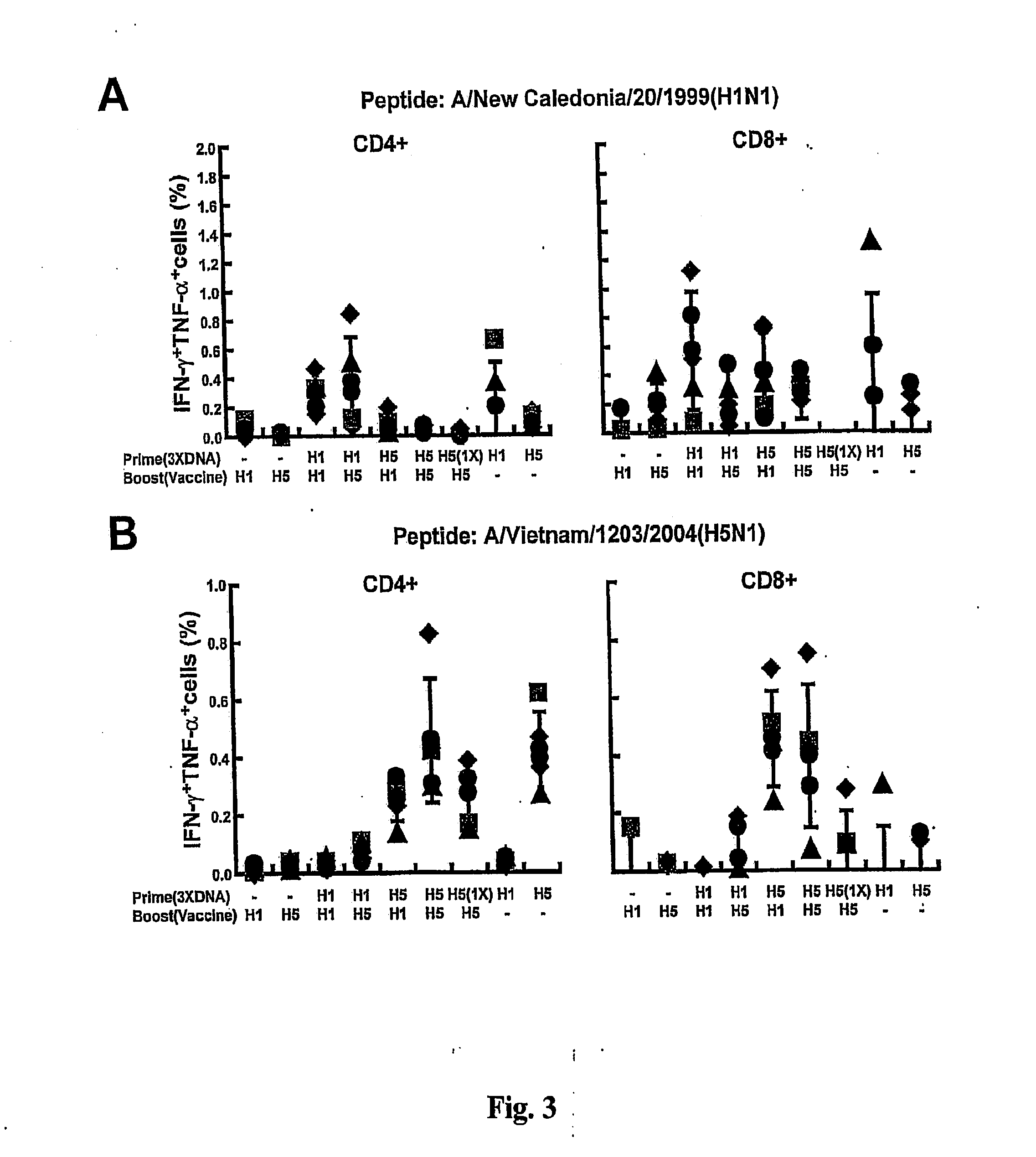
DNA prime/activated vaccine boost immunization to influenza virus
InactiveUS20110177122A1Enhance immune responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininEpitope
The present invention relates to a combination of a priming composition and a boosting composition to prime and boost an immune response in a subject whereby the immune response resulting from administration of the priming composition to the subject is capable of being boosted. The priming composition comprises a DNA plasmid that comprises a nucleic acid molecule encoding an influenza virus hemagglutinin (HA) or an epitope-bearing domain thereof. The boosting composition comprises an influenza vaccine. The present invention also relates to a method to use such a combination to vaccinate a subject and to enhance an immune response to an influenza vaccine administered alone. Such a combination can elicit an immune response not only against at least one influenza virus strain from which the priming composition or boosting composition is derived but also to at least one heterologous influenza virus strain.
Owner:UNITED STATES OF AMERICA
DNA vaccine expressing HA1 of equine-2 influenza virus
InactiveUS7244435B2Reduce riskReduce dosageSsRNA viruses negative-senseViral antigen ingredientsHemagglutininA-DNA
The invention is for a DNA vaccine expressing the hemagglutinin (HA1) gene of equine-2 influenza virus. By engineering a stop codon within HA1, expression of HA1 is ensured. By encapsulation of the DNA vaccine in liposome and by intranasal inoculation, it is sufficient to elicit protective immunity at a significantly lower dosage compared to a DNA vaccine expressing the full length HA gene. Lower dosage reduces the risk of induction of anti-DNA antibodies. Intranasal inoculation directly to the respiratory epithelial cells reduces the risk of DNA integration. The inventive vaccine is advantageous over current inactivated or live attenuated vaccines, as updating of the vaccine requires only the replacement of the encoding sequence with the new virus.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Lectin compositions and methods for modulating an immune response to an antigen
InactiveUS7629440B2Great and lesser in magnitudeResponse more and less-efficientSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus A hemagglutinin
The present invention relates to a fusion polypeptide comprising at least about 10 contiguous amino acid residues of an influenza virus hemagglutinin and at least about 5 contiguous amino acids of a naturally occurring GM-CSF molecule.
Owner:OPSANITX LLC
Vaccines for use in the prophylaxis and treatment of influenza virus disease
ActiveUS20130209499A1Promote multimerizationEasy to purifySsRNA viruses negative-senseBacteriaHemagglutininInfluenza virus A hemagglutinin
Provided herein are polypeptides comprising portions of the influenza virus hemagglutinin, compositions comprising such polypeptides that can be used as immunogens in vaccines and methods of their use to generate an immune response against multiple influenza subtypes in a subject.
Owner:MT SINAI SCHOOL OF MEDICINE
Peptide vaccine for influenza virus
The invention provides peptide epitopes for use in the prevention and / or treatment of influenza or for the development of such treatment or vaccine against influenza. The invention also relates to a method for evaluating the potential of a chemical entity, such as an antibody, to bind to a peptide epitope derived from the divalent sialoside binding site of hemagglutinin protein of influenza virus, and to conjugates containing one or more such peptide epitopes. The peptide epitopes of the invention are cyclic peptides comprising a 7-mer peptide derived from H1, H3 or H5 hemagglutinin of influenza virus. The 7-mer peptide has a sequence corresponding to the loop sequence at positions 220-226 of X31-hemagglutinin.
Owner:GLYKOS FINLAND
Turkey herpesvirus vectored recombinant containing avian influenza genes
InactiveUS20080241188A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsVaccinationElisa kit
The present invention provides a recombinant turkey herpesvirus modified by the presence of the cDNA encoding the hemagglutinin protein of avian influenza virus under a promoter. A poultry vaccine comprising the recombinant turkey herpesvirus described in the present invention can induce serological responses that may be easily detected by the hemagglutination inhibition assay but not by commercially available diagnostic ELISA kits; thus enabling easy differentiation between vaccination and field infection.
Owner:ZEON CORP +1
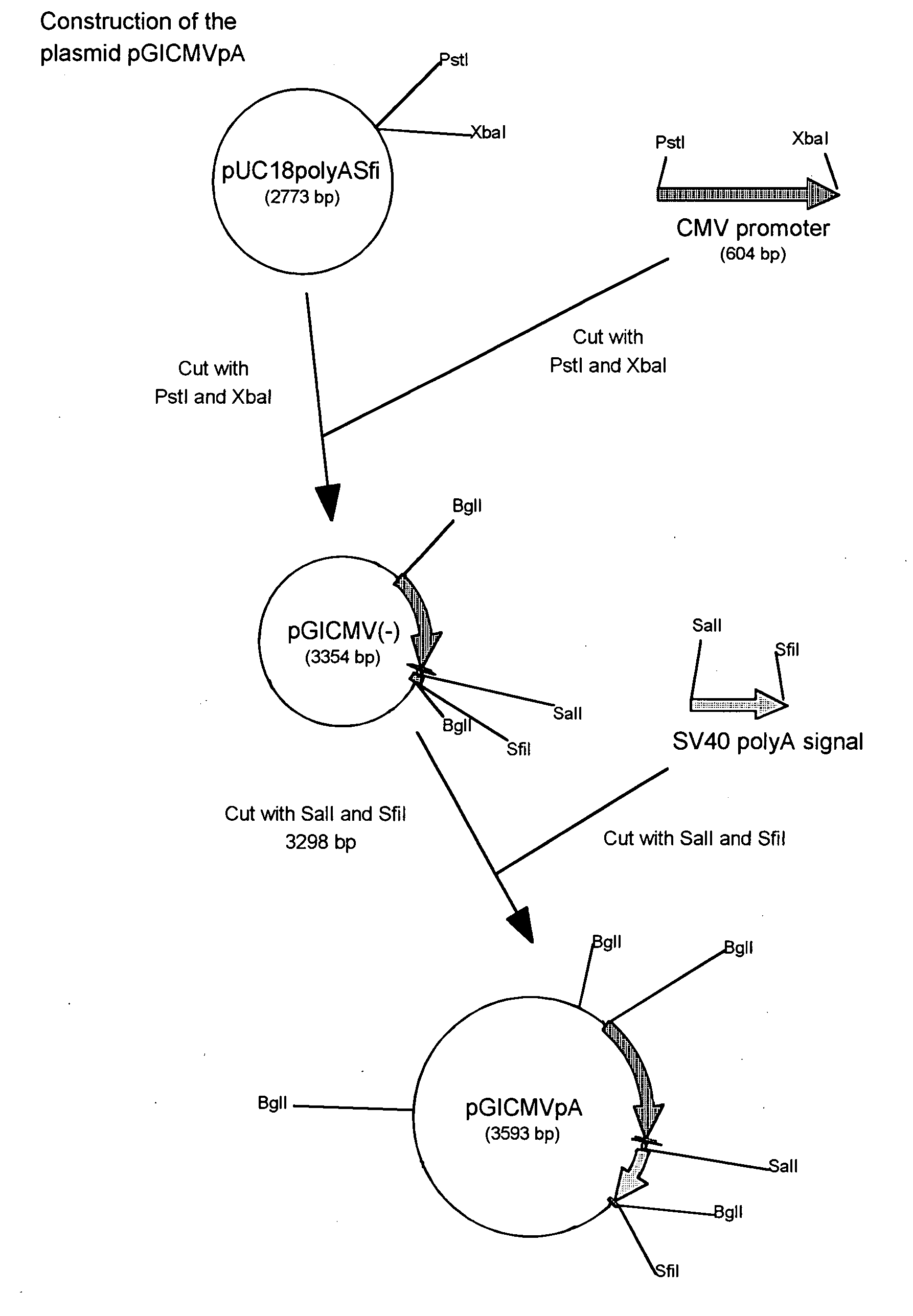
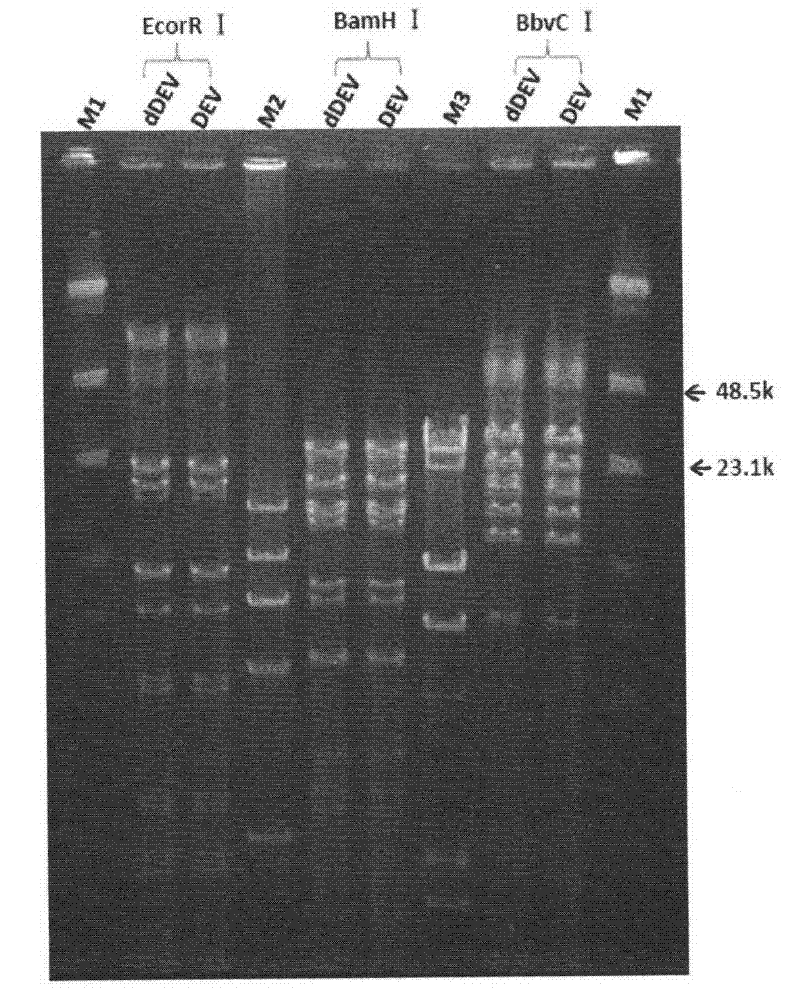
Recombinant duck enteritis virus (DEV) vaccine strain for expressing avian influenza virus haemagglutinin (HA) gene and constructing method and application thereof
ActiveCN102373180AMicroorganism based processesAntiviralsAvian influenza virusInfluenza virus A hemagglutinin
The invention provides a recombinant duck enteritis virus (DEV) vaccine strain CCTCC NO:V201125 named rDEVus78Ha-Re6 for expressing an avian influenza virus haemagglutinin (HA) gene, and a constructing method and application thereof. The vaccine strain is prepared by the following steps of: inserting a genetic fragment SV40-HA comprising the avian influenza virus HA gene and an SV40 promoter sequence into a spacer between a US7 gene and a US8 gene of a DEV by adopting a recombinant cloning technology; constructing a cosmid for inserting an SV40-HA expression frame between the US7 gene and theUS8 gene; and saving to obtain the recombinant DEV vaccine strain CCTCC NO:V201125 for expressing the avian influenza virus HA gene. The invention further relates to a method for constructing the recombinant DEV vaccine strain and application of the recombinant DEV vaccine strain to preparation of a vaccine for preventing duck viral enteritis and avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant duck virus enteritis virus vaccine strain (rDEVul41HA) for expressing avian influenza virus hemagglutinin (HA) genes and construction method as well as application thereof
The invention provides a recombinant duck virus enteritis virus vaccine strain CCTCC No.V201026, named as rDEVul41HA, for expressing avian influenza virus hemagglutinin (HA) genes and a construction method as well as application thereof. Specifically, gene segments SV40-HA containing avian influenza virus hemagglutinin HA genes and a SV40 promoter sequence are inserted into UL41 genes of duck virus enteritis virus to construct cosmids with SV40-HA expression cassette inserted into UL41 genes; and by the cosmids, the recombinant duck virus enteritis virus vaccine strain CCTCC V201026 for expressing the avian influenza virus hemagglutinin (HA) genes is saved and obtained. The invention also relates to a method for constructing the recombinant duck virus enteritis virus vaccine strain and application of the recombinant duck virus enteritis virus vaccine strain to preparing vaccines for preventing duck virus enteritis and avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Neutralizing Anti-influenza a antibodies and uses thereof
ActiveUS20160257732A1High activityWide coverageBiological material analysisImmunoglobulins against virusesHemagglutininInfluenza virus A hemagglutinin
The invention relates to antibodies and binding fragments thereof that are capable of binding to influenza A virus hemagglutinin and neutralizing at least one group 1 subtype and at least 1 group 2 subtype of influenza A virus. In one embodiment, an antibody or binding fragment according to the invention is capable of binding to and / or neutralizing one or more influenza A virus group 1 subtypes selected from H1, H2, H5, H6, H8, H9, H11, H12, H13, H16 and H17 and variants thereof and one or more influenza A virus group 2 subtype selected from H3, H4, H7, H1, 0, H14 and H15 and variants thereof.
Owner:MEDIMMUNE LLC +1
Conserved neutralizing epitope mimic peptide of H5 subtype avian influenza viruses and use thereof
The invention provides a hemagglutinin (HA) protein neutralizing epitope mimic peptide of H5 subtype avian influenza viruses, a conserved derivative or active fragment or mutant sequence thereof, related coding sequence thereof and the use of the mimic peptide or the conserved derivative or active fragment or mutant sequence thereof in prevention and diagnosis.
Owner:XIAMEN UNIV
Antibody neutralizing human infected H7N9 influenza A virus and use thereof
ActiveCN104892753AGenetic material ingredientsBiological material analysisAntibody fragmentsInfluenza virus A hemagglutinin
Provided is a neutralising antibody which binds to and neutralises an H7 type influenza A virus. The nucleotide sequences of the light and heavy chain variable regions of the antibody are at least 75% identical to the nucleotide sequence of any one of SEQ ID NO: 38-55, 58 or 59, and the antibody can neutralise a human infection H7N9 influenza A virus haemagglutinin protein. Also provided is an efficient expression method for integrating an antibody into a cell such as a CHO cell, and a use for the antibody, a related haemagglutinin protein antigen binding fragment and an epitope in the diagnosis, treatment and prevention of human infection H7N9 influenza A virus infection.
Owner:SINO CELL TECH INC
Recombined duck virus enteritis viral vaccine strain CCTCC for expressing bird flu virus hemagglutinin (HA) gene (rDEVus78Ha) as well as establishing method and application thereof
The invention provides a recombined duck virus enteritis viral vaccine strain CCTCC for expressing bird flu virus hemagglutinin HA gene with V201025, which is named as rDEVus78Ha, as well as an establishing method and application thereof. Particularly, by utilizing the recombination clone technology, the gene segment SV40-HA including the bird flu virus hemagglutinin HA gene and SV40 promoter sequence is inserted in the spacer between the US7 and US8 genes of the duck virus enteritis virus, so as to establish and obtain the cosmid inserted into the SV40-HA expression cassette between the US7 and US8 genes, as a result, the recombined duck virus enteritis viral vaccine strain CCTCC for expressing bird flu virus hemagglutinin HA gene with V201025 is saved and obtained. The invention also relates to a method for establishing the recombined duck virus enteritis viral vaccine strain and the application of the recombined duck virus enteritis viral vaccine strain to preparing the vaccine for preventing duck virus enteritis and bird flu.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Structure and application of influenza virus hemagglutinin protein binding polypeptide
InactiveCN102268072ACytopathic inhibitionNo obvious toxicityPeptide/protein ingredientsPeptidesDiseaseHemagglutinin
Belonging to the technical field of biomedicine, the invention relates to a sequence and structure of a polypeptide able to specifically bind with influenza virus hemagglutinin protein, and application of the polypeptide in anti-influenza viruses. By expressing purified influenza virus hemagglutinin protein and screening a random peptide library with a phage display technology, a polypeptide specifically bound with influenza virus hemagglutinin and equipped with sequences numbered 1-18 can be obtained. As a hemagglutinin-binding peptide can hinder the combination of hemagglutinin and a host cell receptor, so the influenza virus can be inhibited from infecting the host cell. Thus, the invention also conducts an anti-influenza virus activity study to the hemagglutinin-binding peptide selected from the phase peptide library, and finds that a polypeptide H17, with a sequence of NH2-SHGRITFAYFAN-COOH, can effectively inhibit the influenza virus from infecting the host cell and is of small toxicity. Therefore, the hemagglutinin-binding peptide of the invention and the H17 polypeptide therein with an anti-influenza virus activity are expected to become novel treatment medicaments for treating diseases caused by influenza virus infection and reducing the hazards of diseases caused by influenza viruses.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Broad spectrum monoclonal antibody for identification of influenza virus hemagglutinin protein HA1 structural domains
The invention relates to an antibody for identification of epitopes on influenza virus hemagglutinin protein HA1 structural domains, a cell strain generating the antibody and the use of the antibody and the cell strain. The antibody is can cross the HA subtype for specific binding of the hemagglutinin (HA) protein HA1 structural domains of H1 subtype (including seasonal H1N1 and new H1N1) and H5 subtype influenza virus. Therefore, the invention also relates vaccines or pharmaceutical compositions which comprise the antibody and are used for prevention and / or treatment of H1 subtype and H5 subtype influenza virus infection and / or diseases (such as the flu) induced by the H1 subtype and H5 subtype influenza virus.
Owner:XIAMEN UNIV
Recombinant influenza virus and application thereof
InactiveCN104312983AAvoid interferenceIncrease productionAntiviralsViruses/bacteriophagesHemagglutininNeuraminidase
The invention discloses a recombinant influenza virus and application thereof and provides a preparation method of the recombinant virus. The preparation method comprises the following steps: guiding an HA6 subtype influenza virus hemagglutinin encoding gene, an influenza virus neuraminidase (NA) gene and six genes including PB2, PB1, PA, NP, M and NS in an influenza virus into a host cell together, and packing to obtain the recombinant virus. The invention also discloses a detection method for detecting antibodies for inhibiting activities of seasonal influenza N1, N2, 2009H1N1N1 and 2013H7N9N9 enzymes by virtue of the recombinant virus. The detection method comprises the steps of sample treatment and result analysis. According to the invention, the immune response to NA of people can be rapidly and effectively measured, the operation is simple, the application is convenient, and viable technical support is provided for clinical detection and vaccine evaluation.
Owner:中国疾病预防控制中心病毒病预防控制所
Avianinflu virus H5 subtype emulsion agglutination kit and its use
ActiveCN1978634AStrong specificityHigh sensitivityImmunoglobulins against virusesFused cellsPositive controlHemagglutinin protein
This invention discloses a latex agglutination kit to quickly detect H5 subtype of avian influenza virus. The anti monoclonal antibodies of H5 subtype of avian influenza virus hemagglutinin protein were coupled to the surface of carboxyl polystyrene latex particles using water-soluble carbodiimide, the methods about detection of H5 subtype of avian influenza virus were established, the latex agglutination detection kits of H5 subtype of avian influenza virus were prepared supplemented by other matching reagents. This kit was made up of box body and the body latex diagnostic reagents in the box, sample handling liquid A, sample handling liquid B, the positive control samples and negative control samples. This invention kit can directly detect the H5 subtype avian flu virus, with high specificity, high sensitivity, simple and rapid diagnosis, and other significant advantages.
Owner:HUAZHONG AGRI UNIV
Polypeptide or derivative thereof and application of polypeptide or derivative in influenza virus infection
ActiveCN104151403AGood anti-influenza activityShort peptide chain lengthSsRNA viruses negative-senseGenetic material ingredientsHemagglutininInfluenza virus A hemagglutinin
The invention relates to polypeptide, protein or peptide-like medicine from influenza virus hemagglutinin, and a method of the polypeptide, belongs to the technical field of biological medicines, and in particular relates to eight influenza virus hemagglutinin fragment peptides which can block influenza virus infection and have the serial numbers SEQ ID NO.1 to SEQ ID NO.8. The fragment peptides can inhibit and block infection of different species and influenza viruses of different subtypes to a host, including multiple influenza virus strains such as highly pathogenic avian influenza virus, seasonal human influenza virus and the like. The invention provides the peptide sequence (including amino acid sequences of peptides and polynucleotide sequences of encoded peptides), derivative peptides (including amino acid sequences of peptides and polynucleotide sequences of encoded peptides), peptide compositions and independent or united applications of peptides in preventing or treating influenza virus, such as medicine combination of peptides provided by the invention and other anti-influenza medicines.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Compositions and methods for increasing immunogenicity of glycoprotein vaccines
ActiveUS20100145015A1Improve targetingHeightened humoral and cellular immune responseSsRNA viruses negative-senseFusion with post-translational modification motifHemagglutininInfluenza virus A hemagglutinin
The present invention relates to the microbial immunogens engineered to bear α-gal epitope(s) for induction of potent humoral and cellular immune responses when administered to subjects having anti-Gal antibodies. In one embodiment, the present invention provides compositions and methods for propagating influenza virus in human, ape, Old World monkey or bird cells that have been engineered to express an α1,3galactosyltransferase (α 1,3GT) gene to produce virions bearing hemagglutinin molecules containing α-gal epitopes, to increase the immunogenicity of the influenza virus. In another embodiment, the present invention provides fusion proteins between influenza virus hemagglutinin and a microbial peptide or protein of interest, and enzymatic processing of this fusion protein to carry α-gal epitopes, to increase the immunogenicity of the microbial peptide or protein of interest.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
H1-subtype influenza A virus double-antibody sandwich ELISA kit and application
ActiveCN102633878AStrong specificityHigh sensitivityMicroorganism based processesImmunoglobulins against virusesHemagglutininElisa kit
The invention belongs to the fields of detection technology of animal virology and epizootiology. The invention particularly relates to an H1-subtype influenza A virus double-antibody sandwich ELISA kit and an application. The kit of the invention comprises an enzyme label plate which contains anti-H1-subtype influenza A virus hemagglutinin, has a preservation number of CCTCC NO: C201106, and is coated by a monoclonal antibody, and a horseradish peroxidase labeled H1-subtype influenza A virus hemagglutinin monoclonal antibody is used as a second antibody. The invention discloses separation, amplification, inactivation, and purification methods of the H1-subtype influenza A virus, and preparation and purification methods of the H1-subtype influenza A virus hemagglutinin monoclonal antibody. The invention also discloses an H1-subtype influenza A virus double-antibody sandwich ELISA detection method.
Owner:HUAZHONG AGRI UNIV
Loop-mediated isothermal amplification detection kit of influenza A3 viruses and detecting method
InactiveCN101376912AThe result judgment method is flexible and simpleRealize accumulationMicrobiological testing/measurementHemagglutininInfluenza virus A hemagglutinin
The invention provides a loop-mediated isothermal amplification test method of an influenza A3 virus. The method comprises the following steps: a specificity primer sequence is designed according to an influenza A3 virus hemagglutinin gene, a sample RNA to be tested is extracted as the template to have LAMP reaction; the LAMP reaction result is analyzed; if the LAMP amplification result is positive, the sample to be tested contains the influenza A3 virus. The LAMP influenza A3 virus test method is characterized by convenience, economy, fastness, sensitivity and specificity with flexible and convenient result judgment, is applicable in clinical or laboratory diagnosis, and has broad application prospect.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Modified influenza virus for monitoring and improving vaccine efficiency
ActiveUS7871626B2Alter antigenicityAlter immunogenicityAnimal cellsVirusesHemagglutininInfluenza virus A hemagglutinin
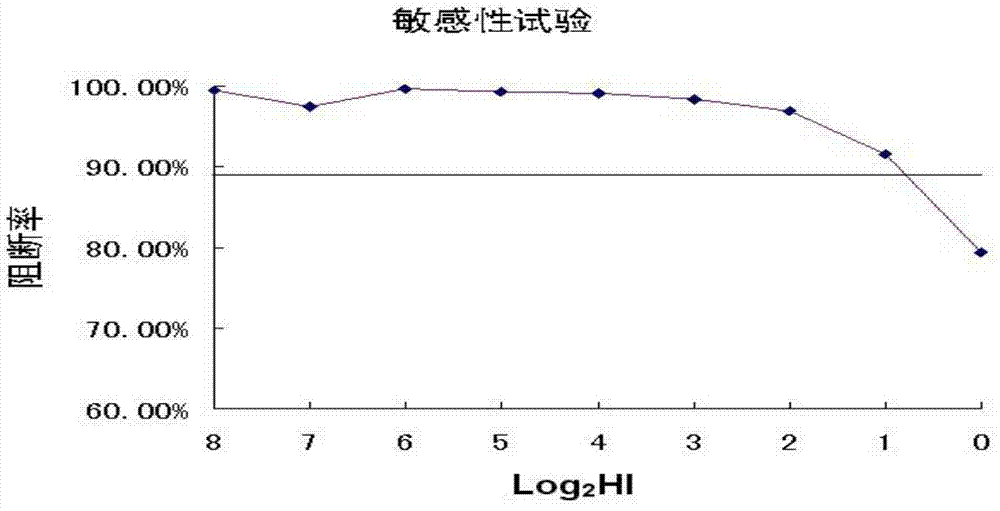
The immunogenicity of the influenza virus hemagglutinin (HA) molecule may be increased by substitutions of amino acids in the HA sequence. The substitution of specific HA residues, such as asparagine at position 223 of H5 HA, increase the sensitivity of the hemagglutinin inhibition (HI) assay by altering receptor specificity and / or antibody-antigen binding. HA molecules containing such substitutions will be useful in the development of diagnostic reference viruses and improved influenza vaccines.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
A-type H1 subtype influenza virus antibody blocking ELISA kit and applications thereof
InactiveCN103592436AStrong specificityHigh sensitivityImmunoglobulins against virusesMaterial analysisElisa kitPositive control
The invention discloses an A-type H1 subtype influenza virus antibody blocking ELISA kit. The kit comprises: a) an A-type H1 subtype influenza virus A-Influ / JML-F9; b) a monoclonal antibody with strong specificity of an A-type H1 subtype influenza virus-resistant hemagglutinin protein; c) an antibody blocking ELISA core kit prepared from the monoclonal antibody of the A-type H1 subtype influenza virus-resistant hemagglutinin protein; and d) a sample diluting liquid, 10 times of a washing liquid, a substrate liquid A, a substrate liquid B, a reaction stop solution, a positive control sample and a negative control sample in the kit. The invention also discloses applications of the A-type H1 subtype influenza virus antibody blocking ELISA kit in A-type H1 subtype influenza virus antibody detection. The kit has strong specificity, high sensitivity, short detection time, easy and practical operation, and no need of being operated by a professional person. The kit is good in stability and long in retention period, and solves a problem of the A-type H1 subtype influenza virus antibody detection.
Owner:HUAZHONG AGRI UNIV
Modified influenza virus for monitoring and improving vaccine efficiency
ActiveUS20070031453A1Alter antigenicityAlter immunogenicityAnimal cellsVirusesHemagglutininInfluenza virus A hemagglutinin
The immunogenicity of the influenza virus hemagglutinin (HA) molecule may be increased by substitutions of amino acids in the HA sequence. The substitution of specific HA residues, such as asparagine at position 223 of H5 HA, increase the sensitivity of the hemagglutinin inhibition (HI) assay by altering receptor specificity and / or antibody-antigen binding. HA molecules containing such substitutions will be useful in the development of diagnostic reference viruses and improved influenza vaccines.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Composition comprising at least two influenza a virus-neutralizing-binding molecules
ActiveUS20160052997A1Prevention and treatmentEfficiently neutralizedPeptide/protein ingredientsMicrobiological testing/measurementHemagglutininDisease
The present invention provides a composition comprising at least two influenza A virus-neutralizing binding molecules that bind to an epitope in the stem region of influenza A virus hemagglutinin (HA) protein, the method comprising: i) a first binding molecule capable of neutralizing at least one influenza A virus subtype selected from the group consisting of H1, H2, H5 and H9; ii) a second binding molecule capable of neutralizing at least one influenza A virus subtype selected from the group consisting of H1, H3, H5, H7 and H9. The mixed composition of the present invention can effectively neutralize the multiple influenza subtypes of both phylogenetic group 1 and phylogenetic group 2 and can be used in combination with a chemical compound. Thus, the composition of the present invention is very useful for the prevention and treatment of a disease by influenza virus.
Owner:CELLTRION INC
Monoclonal Antibodies Specific to the Fusion Peptide From Hemagglutinin From Influenza A Viruses and Uses Thereof
ActiveUS20110256141A1Effective protectionMicrobiological testing/measurementBiological material analysisHemagglutininHighly pathogenic
This invention relates to methods and products for the diagnosis, surveillance, prevention, and treatment of influenza A virus infections in animals and humans. More particularly, the invention relates to antibodies and related binding proteins for the detection, prevention and treatment of influenza A viruses. The monoclonal antibodies and related binding proteins of the invention are useful for the treatment of the highly pathogenic H5 subtypes of avian influenza virus (AIV).
Owner:TEMASEK LIFE SCIENCES LABORATORY
Monoclonal antibody of anti-H9 subtype flu virus haemagglutinin protein and application thereof
InactiveCN103059132AStrong specificityHigh sensitivityImmunoglobulins against virusesMaterial analysisPurification methodsInfluenza virus A hemagglutinin
The invention discloses a monoclonal antibody of anti-H9 subtype flu virus haemagglutinin protein, which is secreted by hybridoma cell strain 4D10 with the preserving number of CCTCC No: C2012152. The invention further discloses a double antibody sandwich ELISA test kit of H9 subtype flue virus and a detection method. The monoclonal antibody of anti-H9 subtype flu virus haemagglutinin protein is used as a primary antibody, a monoclonal antibody marked by horseradish peroxidase is used as a second antibody, and a separation, augmentation, inactivation and purification method of H9 subtype flue virus and a preparation and purification method of anti-H9 subtype flu virus haemagglutinin monoclonal antibody are disclosed. According to the test kit and the detection method disclosed by the invention, the H9 subtype flue virus can be detected directly, and the monoclonal antibody has the characteristics of being high in specificity, high in sensitivity, short in detection time, wide in detection sample range and the like.
Owner:HUAZHONG AGRI UNIV
Antibodies against influenza virus hemagglutinin and uses thereof
ActiveUS20160137721A1Reduce in quantityLower titerBacteriaAntibody mimetics/scaffoldsHemagglutininDisease
Provided herein are antibodies that cross-react with hemagglutinin from strains of influenza virus of the same subtype or different subtypes, host cells for producing such antibodies, and kits comprising such antibodies. Also provided herein are compositions comprising antibodies that cross-react with hemagglutinin from strains of influenza virus of the same subtype or different subtypes and methods of using such antibodies to diagnose, prevent or treat influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
H1N1 swine influenza virus-resistant hemagglutinin protein monoclonal antibody, hybridoma cell line and antigen-capture ELISA kit
InactiveCN101988050AImprove featuresHigh sensitivitySerum immunoglobulinsImmunoglobulins against virusesElisa kitInfluenza virus A hemagglutinin
The invention discloses an H1N1 swine influenza virus-resistant hemagglutinin protein monoclonal antibody, a hybridoma cell line and an antigen-capture enzyme linked immunosorbent assay (ELISA) kit. The monoclonal antibody is secreted by a hybridoma cell of which the collection number is CCTCC C2010121, and has high titer and specificity. The developed antigen-capture ELISA kit comprises a solid-phase vector coated with the monoclonal antibody, the H1N1 swine influenza virus-resistant hemagglutinin protein monoclonal antibody labeled by a horse radish peroxidase, substrate reaction solution of an enzyme, positive and negative controls, cleaning solution and reaction stop solution. The kit has high specificity and sensitivity, is easy to operate, can be used for the clinical and laboratory detection of large-scale H1N1 swine influenza viruses and has wide application prospect.
Owner:湖南出入境检验检疫局检验检疫技术中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com