Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
96 results about "Human influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza virus. Human influenza viruses belong to the Orthomyxoviridae family, which consists of the genera influenza A, B, and C virus, thogotovirus (carried by ticks, and infecting humans), and isavirus (infecting fish), as well as of a few other, newly described arboviruses. Only influenza A and B viruses cause epidemics in human beings.
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Method and medicament for inhibiting the infection of influenza virus
InactiveUS8470771B2Limit scopeClear descriptionBiocidePeptide/protein ingredientsHuman influenzaPolynucleotide
The invention relates to a process for inhibiting the infection of influenza viruses and a polypeptide or protein medicine used therein. More particularly, the invention involves a process for inhibiting the highly pathogenic avian influenza virus (such as H5N1 subtype) infection and human influenza virus (such as H1N1 subtype and H3N2 subtype) infection, as well as the polypeptide or protein involved therein, and a polynucleotide encoding the polypeptide or protein and a vector or host cell expressing said polypeptide or protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Human Anti-Human Influenza Virus Antibody
InactiveUS20110319600A1Effect survival rateEffect weight lossSugar derivativesImmunoglobulins against virusesHemagglutininHuman Influenza A Virus
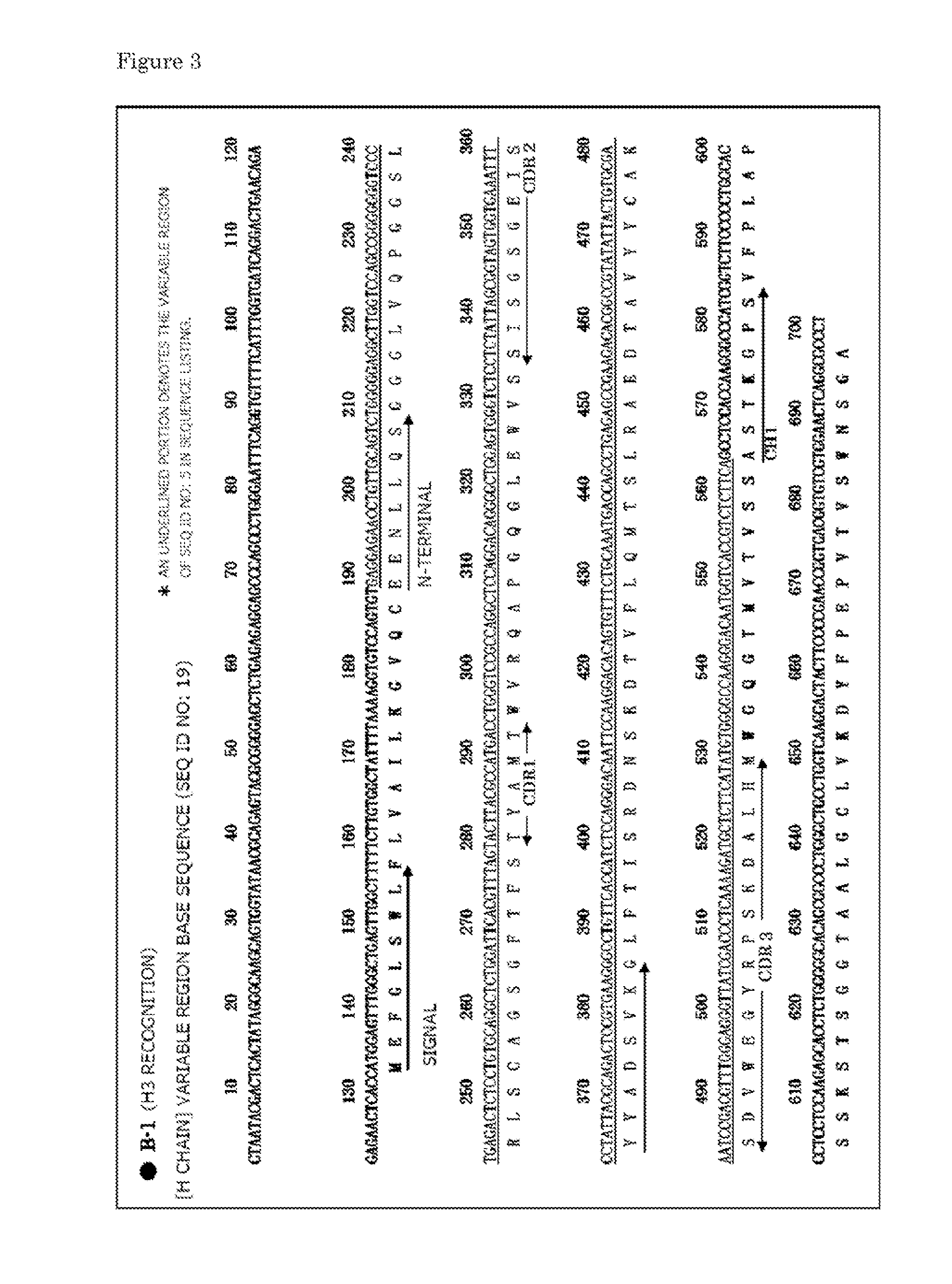
Provided is a human antibody having a neutralization activity against a human influenza virus. More specifically, provided is a human antibody which recognizes a highly conserved region in a human influenza A virus subtype H3N2 or a human influenza B virus and has a neutralization activity against the virus. The human antibody is a human anti-human influenza virus antibody, which has a neutralization activity against a human influenza A virus subtype H3N2 and binds to a hemagglutinin HA1 region of the human influenza A virus subtype H3N2, or which has a neutralization activity against a human influenza B virus, and includes, as a base sequence of a DNA encoding a variable region of the antibody, a sequence set forth in any one of SEQ ID NOS: 5 to 12.
Owner:OSAKA UNIV +1
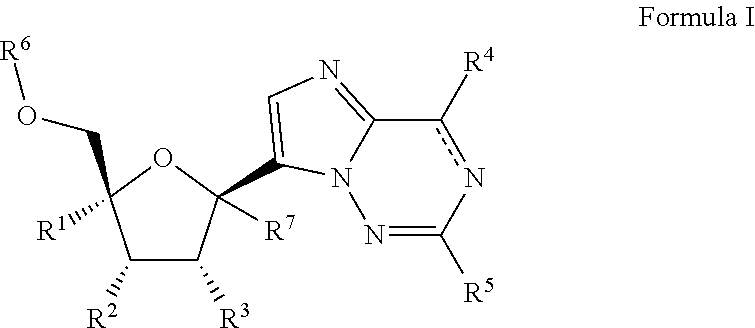
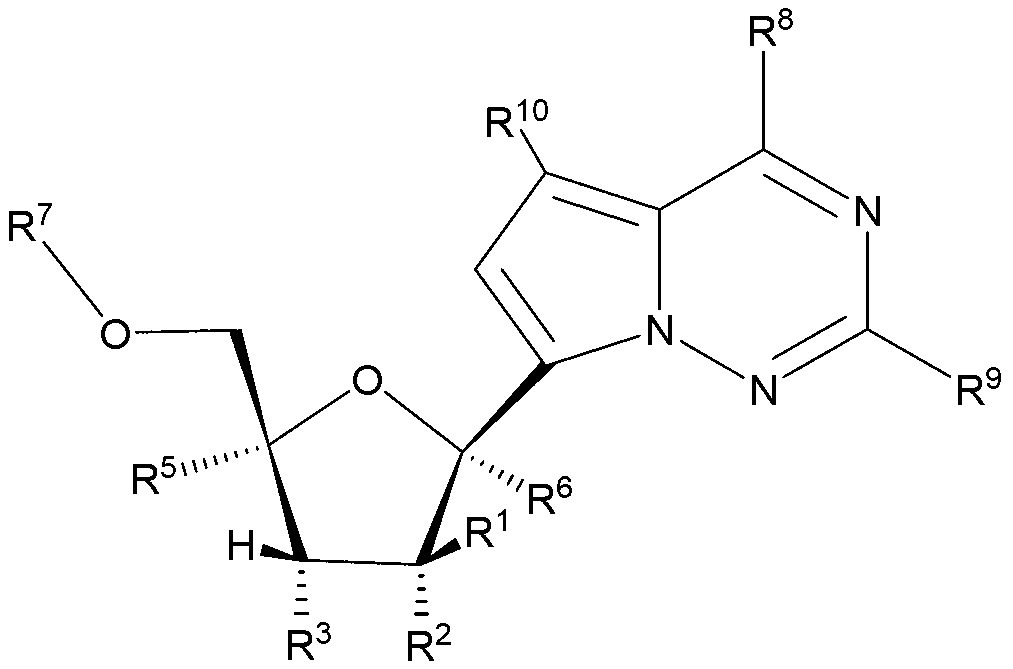
2'-substituted carba-nucleoside analogs for antiviral treatment
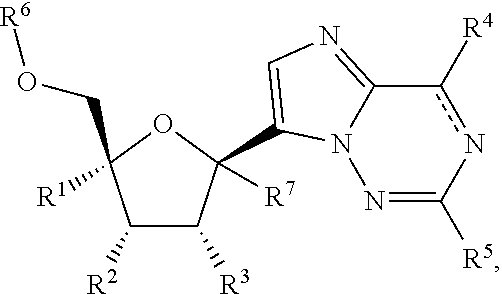
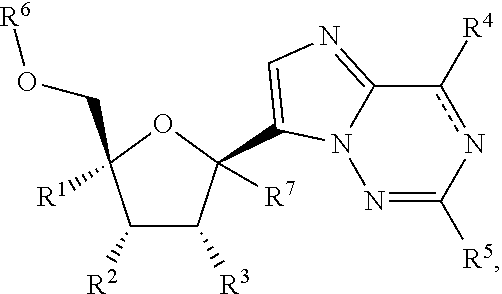
Provided are compounds of Formula I,as well as pharmaceutical compositions containing compounds of Formula I and methods for treating Orthomyxoviridae virus infections by administering these compounds. The compounds, compositions, and methods provided are particularly useful for the treatment of Human Influenza virus infections.
Owner:GILEAD SCI INC
Influenza Virus Vaccines
InactiveUS20080254065A1SsRNA viruses negative-senseViral antigen ingredientsFowlInfluenza virus vaccine
The invention provides a vaccine for protecting a human patient against infection by a human influenza virus strain, wherein the vaccine comprises an antigen from an avian influenza virus strain that can cause highly pathogenic avian influenza. The antigen can invoke an antibody response in the patient that is capable of neutralising said human influenza virus strain. Whereas the prior art used known non-pathogenic avian strains to generate antibodies in humans against known pathogenic avian strains, the invention uses known pathogenic avian strains to protect against emerging pathogenic human strains. Furthermore, whereas the prior art focused on achieving a close antigenic match between the vaccine strain and the target strain, the invention selects vaccine strains based on their pathogenicity, regardless of any perceived close antigenic relationship to the target strain. As the invention does not require detailed knowledge of an emerging strain, a vaccine can be provided further in advance to reduce the risk and potential effects of a human pandemic outbreak.
Owner:SEQIRUS UK LTD
Method for inhibiting influenza virus infection and medicament thereof
InactiveCN101186637APeptide/protein ingredientsGenetic material ingredientsHighly pathogenicHuman influenza
The invention relates to a method for restraining enveloped virus infection, relative polypeptide and protein drug, belonging to biological medicine technical field. The invention comprises a method for restraining influenza virus, particularly for restraining highly pathogenic influenza virus and human influenza virus (as H1N1 hypotype and H3N2 hypotype), relative polypeptide and protein, relative nucleic acid encoding the polypeptide and protein, and relative carriers and cells for representing the polypeptide and protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Method and medicament for inhibiting the infection of influenza virus
InactiveUS20100249021A1Limit scopeClear descriptionBiocidePeptide/protein ingredientsHuman influenzaMedicine use
The invention relates to a process for inhibiting the infection of influenza viruses and a polypeptide or protein medicine used therein. More particularly, the invention involves a process for inhibiting the highly pathogenic avian influenza virus (such as H5N1 subtype) infection and human influenza virus (such as H1N1 subtype and H3N2 subtype) infection, as well as the polypeptide or protein involved therein, and a polynucleotide encoding the polypeptide or protein and a vector or host cell expressing said polypeptide or protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
2′-substituted carba-nucleoside analogs for antiviral treatment
Provided are compounds of Formula I,as well as pharmaceutical compositions containing compounds of Formula I and methods for treating Orthomyxoviridae virus infections by administering these compounds. The compounds, compositions, and methods provided are particularly useful for the treatment of Human Influenza virus infections.
Owner:GILEAD SCI INC
Method and kit for performing quick co-detection on anti-human Hi (Haemophilus influenzae) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling
ActiveCN104181301AFast separationIt has the effect of synergistic amplification of multiple signalsFluorescence/phosphorescenceSerum igeMagnetic bead
The invention discloses a method and a kit for performing quick co-detection on anti-human Hi (Haemophilus influenzae) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling. The kit consists of anti-human Hi antibody capturing nano magnetic beads with an anti-human Hi IgM and IgG antibody gathering function, anti-human IgM and IgG antibody nano probes labeled by multi-color quantum dots, quality control substances and a PBST buffering solution, wherein the quality control substances comprise a positive quality control substance and a negative quality control substance; the positive quality control substance is serum, in which anti-human Hi IgM and IgG antibodies of human Hi infected people are respectively positive; the negative quality control substance is serum, in which anti-human Hi IgM and IgG are respectively negative. The kit and the method have the advantages of simplicity, quickness and high sensitivity, and can be used for carrying out synchronous detection on the anti-human Hi IgM and IgG antibodies.
Owner:HUBEI UNIV OF TECH +1
Polypeptide or derivative thereof and application of polypeptide or derivative in influenza virus infection
ActiveCN104151403AGood anti-influenza activityShort peptide chain lengthSsRNA viruses negative-senseGenetic material ingredientsHemagglutininInfluenza virus A hemagglutinin
The invention relates to polypeptide, protein or peptide-like medicine from influenza virus hemagglutinin, and a method of the polypeptide, belongs to the technical field of biological medicines, and in particular relates to eight influenza virus hemagglutinin fragment peptides which can block influenza virus infection and have the serial numbers SEQ ID NO.1 to SEQ ID NO.8. The fragment peptides can inhibit and block infection of different species and influenza viruses of different subtypes to a host, including multiple influenza virus strains such as highly pathogenic avian influenza virus, seasonal human influenza virus and the like. The invention provides the peptide sequence (including amino acid sequences of peptides and polynucleotide sequences of encoded peptides), derivative peptides (including amino acid sequences of peptides and polynucleotide sequences of encoded peptides), peptide compositions and independent or united applications of peptides in preventing or treating influenza virus, such as medicine combination of peptides provided by the invention and other anti-influenza medicines.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
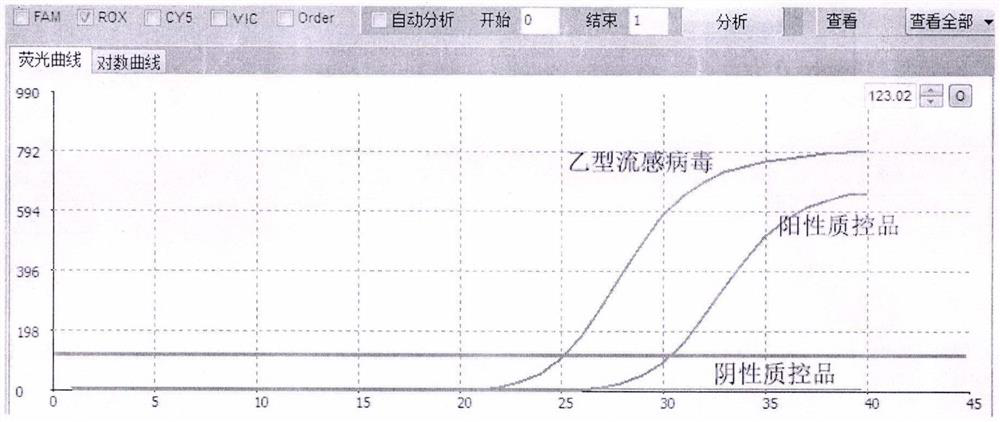
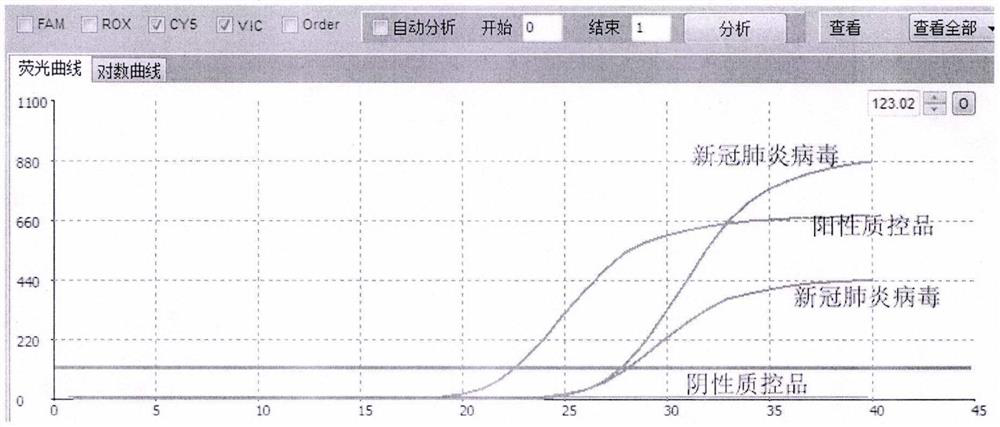
Fluorescent quantitative detection kit for simultaneously detecting human influenza virus and novel coronavirus
InactiveCN111748649ASolve problems that require multiple retestsHigh detection sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesDisease monitoringHuman Influenza A Virus
The invention relates to a fluorescent quantitative detection kit for simultaneously detecting human influenza A virus, human influenza B virus and novel coronavirus, and belongs to the technical field of nucleic acid detection. The detection kit specifically comprises four groups of specific primer pairs and probes, a negative quality control material, a positive quality control material and a fluorescent quantitative PCR reaction system, wherein the four groups of specific primer pairs and probes are respectively a pair of specific primers and a probe for detecting influenza A virus and influenza B virus, and two pairs of specific primers and two probes for detecting novel coronavirus; wherein the positive quality control material is an artificially synthesized target sequence, and the negative quality control material is deionized water; wherein the fluorescent quantitative PCR reaction system is composed of components for PCR reaction and reaction conditions. According to the kit disclosed by the invention, the specific primer pair and the probe are designed at a conservative site of a virus sequence; the kit can realize simultaneous detection of influenza A virus, influenza Bvirus and novel coronavirus in a single tube, has strong detection specificity and high sensitivity, and can accurately and rapidly distinguish influenza from new coronapneumonia patients from people,so as to realize early discovery, early isolation and early treatment. The kit has important application value in the fields of disease monitoring, clinical diagnosis and the like.
Owner:上海同进基因科技有限公司
Novel severe acute respiratory syndrome coronavirus 2 N proteantigen variant and application thereof to detection of novel severe acute respiratory syndrome coronavirus 2 antibody
PendingCN112028977AEfficient captureReduce false positive problemsSsRNA viruses positive-senseVirus peptidesViral antibodySevere acute respiratory syndrome
The invention provides a novel severe acute respiratory syndrome coronavirus 2 N proteantigen variant and an application thereof to detection of the novel severe acute respiratory syndrome coronavirus2 antibody, and relates to variants of N protein important epitope polypeptide. The variants reduce the non-specific reaction of the novel severe acute respiratory syndrome coronavirus 2 protein as acapture antigen with other coronavirus antibodies such as SARS and human influenza coronavirus antibodies. The polypeptide has coronavirus N protein epitope peptide activity, and cysteine residues are added to the C end of the polypeptide. Amino acid residue sites of the mutation are selected from: lysine at the 342rd site, lysine at the 347 site, lysine at the 387 site and lysine atthe 388 site are mutated into amino acids with relatively low homology. The sequence of the N protein 340-419 polypeptide is shown as SEQ ID NO 7. The invention further discloses an antigen composition, use and method of the variant.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Recombinant tryptophan mutants of influenza
InactiveUS6974686B2Lower Level RequirementsGenetic stabilitySsRNA viruses negative-senseSugar derivativesHuman influenzaTryptophan
Recombinant PB2 tryptophan variant influenza viruses, RNA, cDNA and vectors are provided. Also provided are immunogenic compositions containing the variant viruses, methods of producing such viruses and methods for the prophylactic treatment of influenza in humans.
Owner:MEDIMMUNE VACCINES
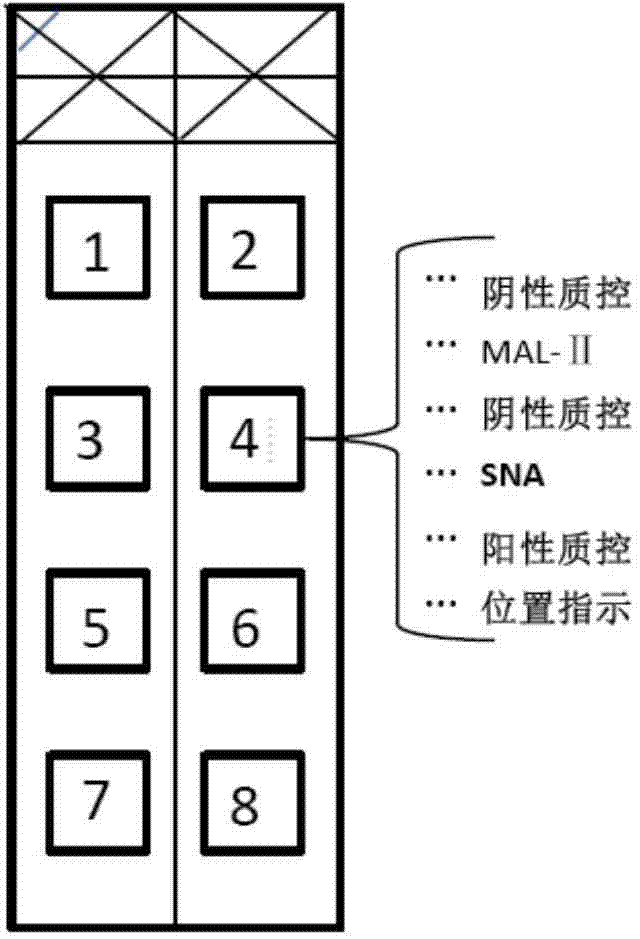
Detection tool for screening susceptible population of bird influenza and human influenza viruses
ActiveCN103884834AQuick checkEasy to detectBiological material analysisBiological testingSaliva sampleSusceptible population
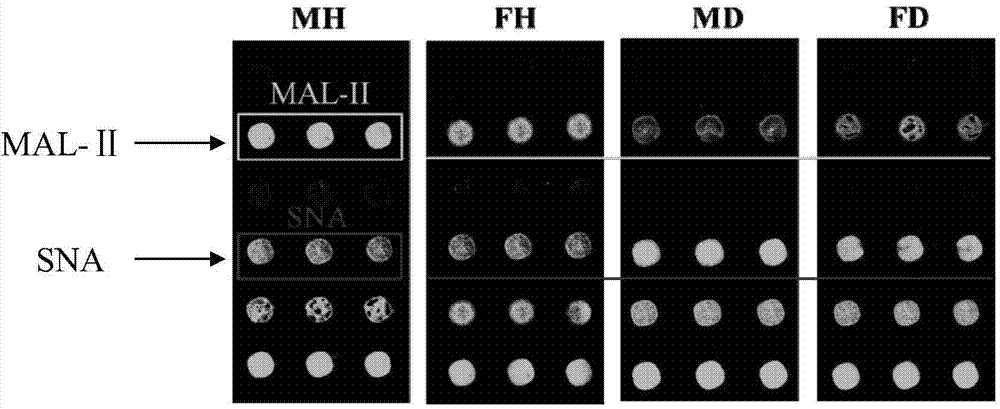
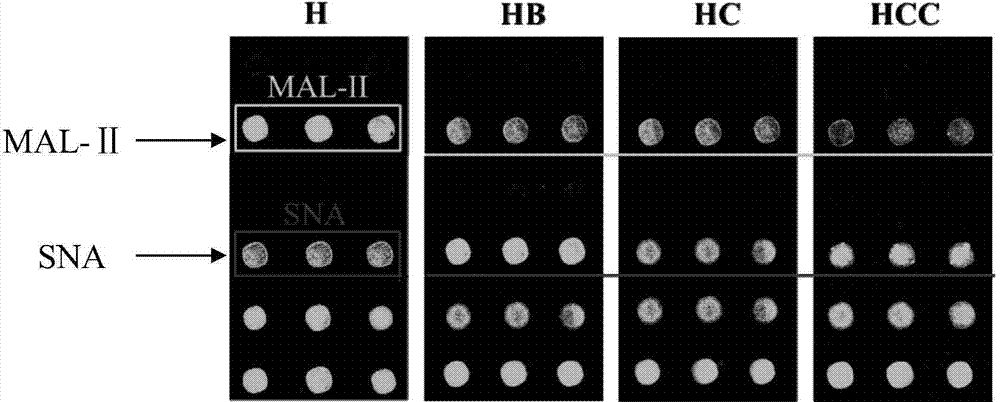
The invention provides a special detection tool capable of screening susceptible population of bird influenza and human influenza viruses. The detection tool is a lectin testing chip aiming at a saliva sample and adopts an epoxidation film base; two types of lectins including MAL-II (Maackia Amurensis Leukoagglutinin-II) and SNA (Sambucus Nigra Agglutinin) are fixed on the epoxidation film base by sample application. A corresponding lectin chip kit comprises the lectin testing chip, confining liquid, washing liquid and an incubation buffer solution. The invention further optimizes a preparation and treatment method of the lectin testing chip so as to establish an optimal reaction system for detecting saliva glycoprotein carbohydrate chain end sialic acid alpha2-3 and alpha2-6 carbohydrate chain structures in the saliva sample. The product provided by the invention is low in production cost and strong in testing pertinence; the inherent advantages of the lectin chip are combined to rapidly, simply and efficiently detect information of an evaluating result of the susceptible population of the bird influenza and human influenza viruses without damages.
Owner:NORTHWEST UNIV
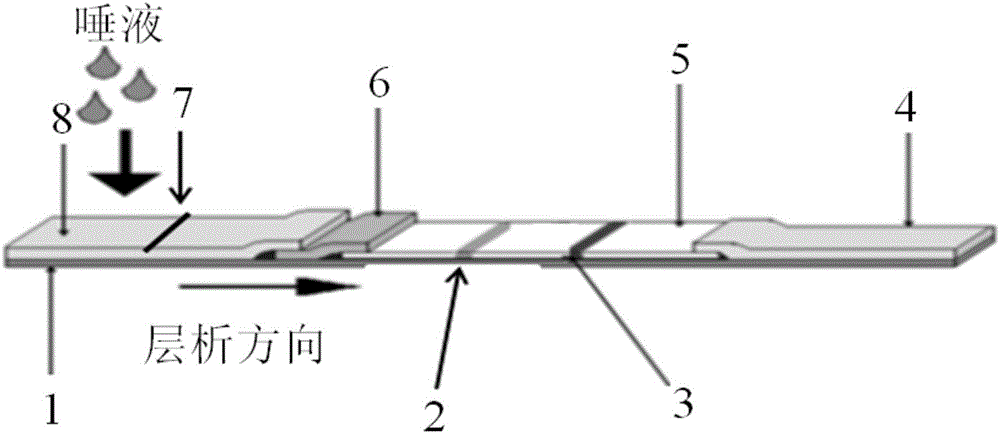
Method and test strip for rapidly detecting people susceptible to avian flu and human influenza
The invention discloses a method and test strip for rapidly detecting people susceptible to avian flu and human influenza. The test strip orderly comprises a sample pad, a gold labeled pad, a detection control zone and a water absorption pad for absorbing the excess liquid in a detected sample along a chromatography direction. The detection control zone orderly comprises a detection band and a quality control line. The gold labeled pad comprises colloidal gold labeled lectin MAL-II or SNA. The detection band comprises stable internal reference lectin which is not bonded to the lectin MAL-II or a stable internal reference lectin which is not bonded to the lectin SNA. One end of the sample pad of the test strip is inserted into a saliva sample or is contained by the oral cavity, after 10 to 15 minutes, the test strip is taken out and the color development states of the detection band and the quality control line are observed so that the detection result is obtained. The method and test strip realize non-invasive, safe and simple detection of people susceptible to avian flu and human influenza.
Owner:陕西中药研究所
Hybrid influenza seed viruses, compositions thereof, and use thereof in the diagnosis or therapy of influenza
ActiveUS20160289304A1Good reproducibilityImprove efficiencySsRNA viruses negative-senseImmunoglobulins against virusesHemagglutininHuman influenza
The invention provides antibody reagents for screening for seed viruses, in particular, antibodies that bind to one or more discontinuous epitopes in hemagglutinin (HA) polypeptide of human influenza virus (H1N1 strain). Additionally, the invention relates to compositions, kits, supports, and biologicals comprising the antibodies or fragments thereof. Also provided are nucleic acids encoding such antibodies or fragments, including, cells and / or hybridomas which generate such molecules. Additional embodiments relate to the immunogens useful in generating the antibodies, including nucleic acids encoding such immunogens, and compositions comprising such immunogens. Further embodiments relate to methods for screening for seed viruses, including human influenza type A virus seed viruses, using the antibodies or fragments thereof. Embodiments of the invention also provide for the prevention, reduction of incidence of, or therapy of subjects having influenza, via administration of the seed viruses, or vaccines and / or pharmaceutical compositions containing the seed viruses.
Owner:VIRO DYNAMICS CORP
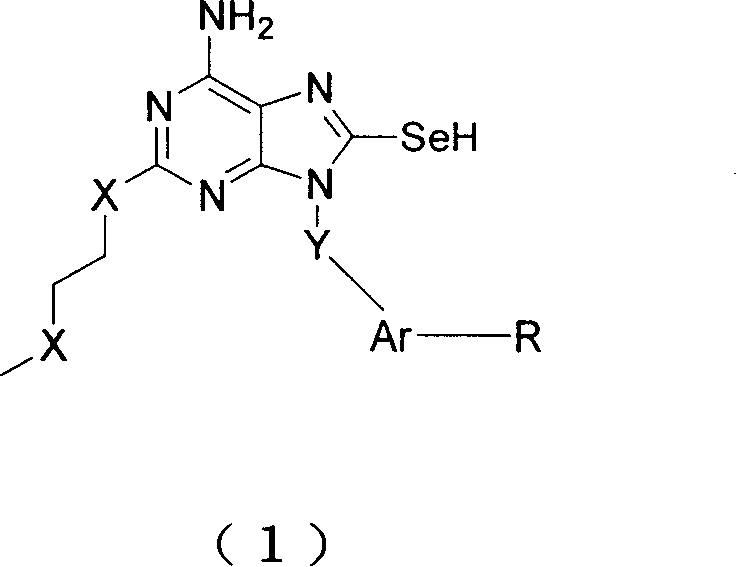
Immunity excitant containing selenium
The invention relates to an immune agonist containing element selenium for improving the immune function, in particular to a purine compound 8-substituted derivative. The immune agonist containing selenium element of the invention is a compound showed in formula (1). Owing to the immune-competence of the selenium element, the immune agonist containing selenium element of the invention leads to stronger immune improving activity of the compound than the corresponding compound analog (the representative compound 9) of the invention, which is applied in resisting viral diseases such as Hepatitis B Virus and Hepatitis C Virus, AIDS, SARS, human influenza and bird flu, and the treatment and assistance of various cancers, with good efficacy.
Owner:靳广毅
Combined nucleic acid real-time fluorescent detection method for influenza A H1N1 virus and influenza A virus and kit
InactiveCN101942524ASensitivity is not affectedDoes not affect accuracyMicrobiological testing/measurementMicroorganism based processesFluorescenceInfluenza A (H1N1) virus
The invention provides a combined nucleic acid real-time fluorescent detection method for simultaneously detecting an influenza A H1N1 virus and an influenza A virus and a kit. The combined nucleic acid real-time fluorescent detection method for the influenza A H1N1 virus and the influenza A virus comprises the following steps of: (1) extracting virus RNA; (2) carrying out fluorescent quantitative PCR (Polymerase Chain Reaction) detection; and (3) judging a detecting result. By carrying out multiple sequence comparison and aiming at a conserved gene fragment of the influenza A virus and the influenza A H1N1 virus (infecting in 2009), a primer and a probe with high specificity are designed and used for real-time fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection. The invention can be used for detecting influenza A virus RNA of human influenza, swine influenza, avian influenza, and other influenza viruses, meanwhile specifically detecting the influenza A H1N1 virus (infecting in 2009) RNA and carrying out double analysis so that a detecting result is more reliable.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
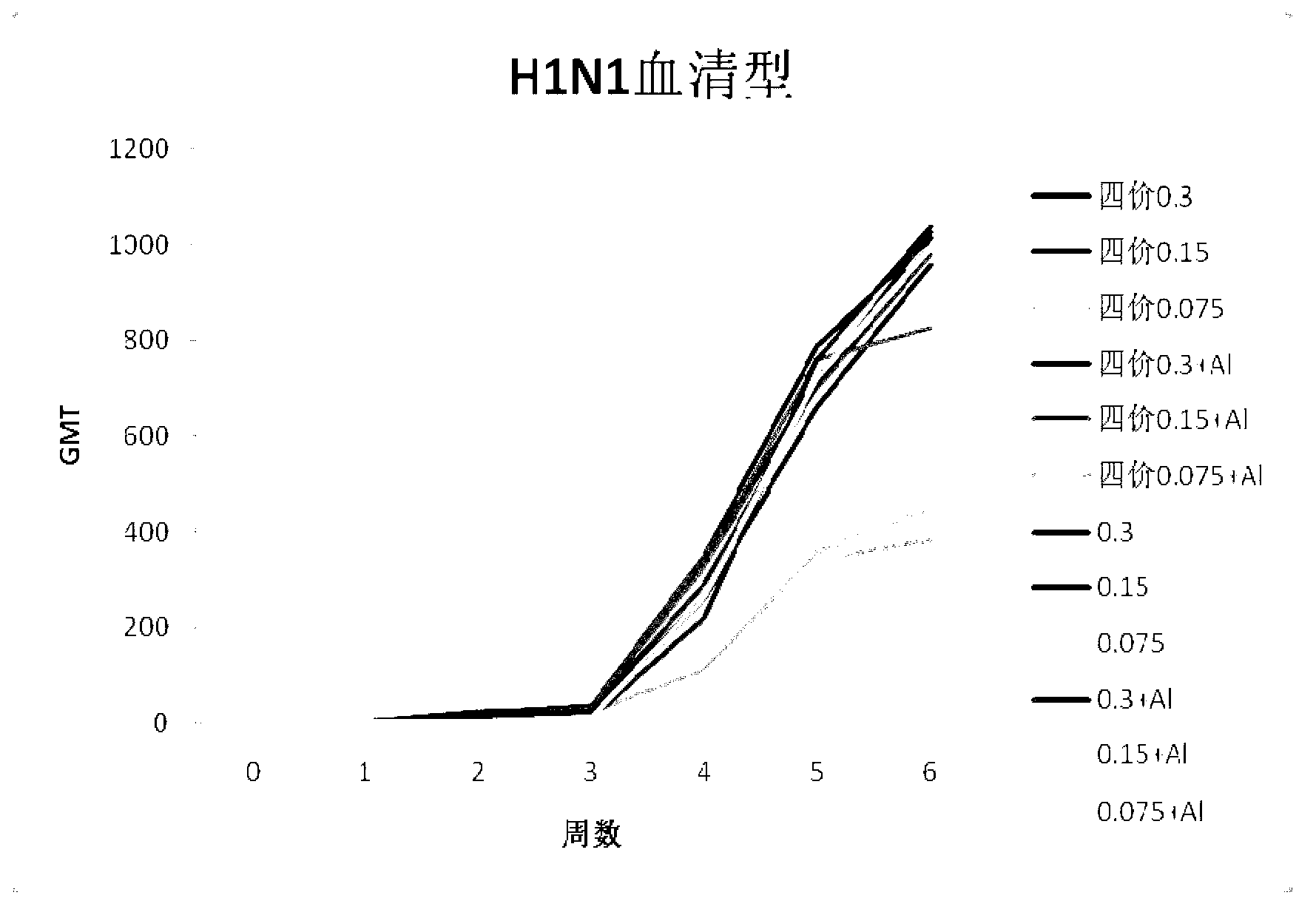
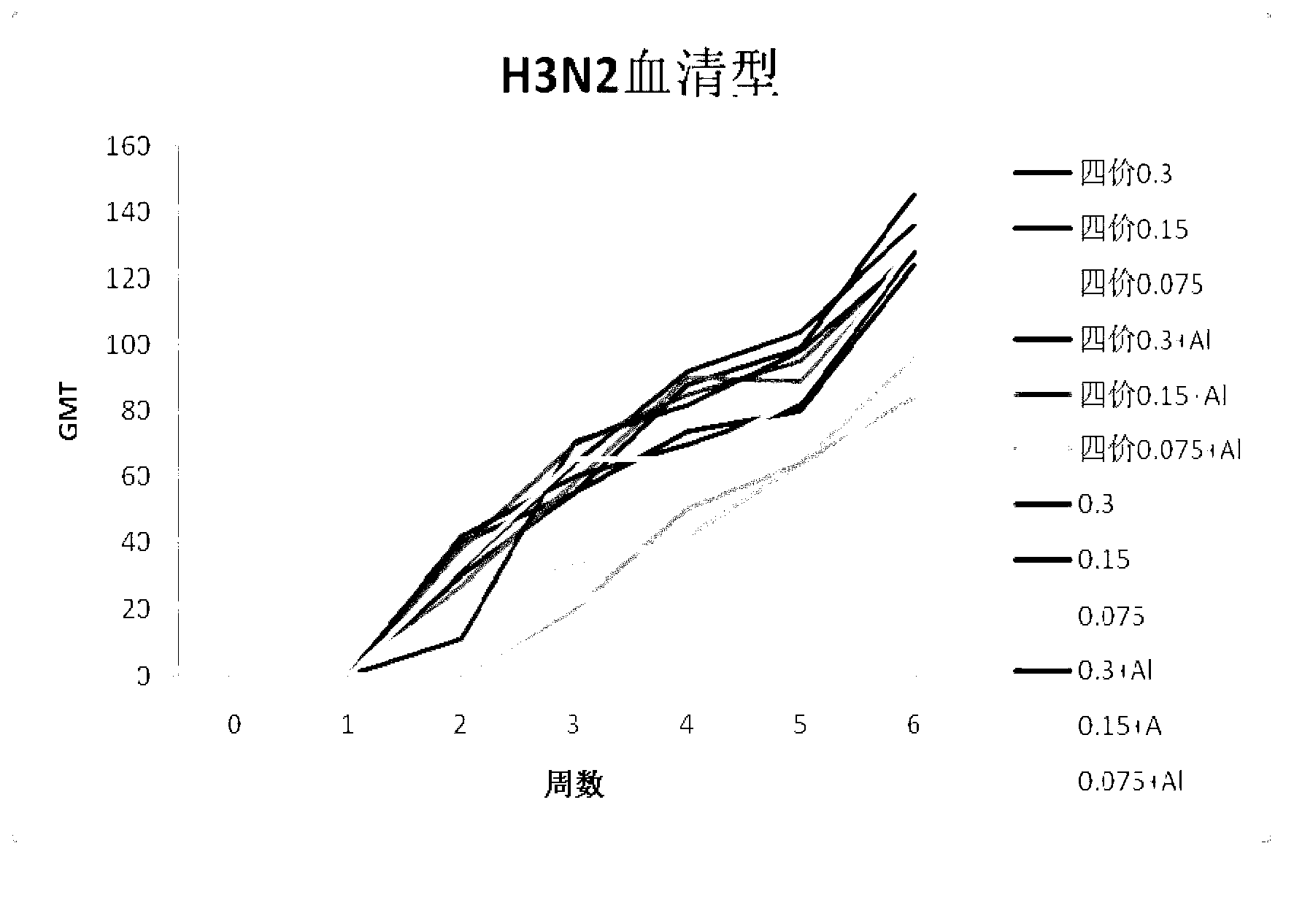
Combined vaccine of seasonal influenza and pandemic influenza for people and preparation method
ActiveCN103285391ANo local irritationSolve the difficult problem of large-scale vaccinationAntiviralsAntibody medical ingredientsHemagglutininHuman influenza
The invention discloses a combined vaccine of seasonal influenza and pandemic influenza for people. The hemagglutinin concentration of each vaccine is 10-60 mug / ml of H1N1 human influenza vaccine,10-60 mug / ml of H3N2 human influenza vaccine, 10-60 mug / ml of B human influenza vaccine, and 10-60 mug / ml of H5N1 human influenza vaccine respectively. The invention also discloses a preparation method of the combined vaccine. The combined vaccine disclosed by the invention has high safety; the problem that the traditional avian influenza vaccine is difficultly vaccinated at a large scale is solved; explosive epidemics of the H5N1 human influenza vaccine is effectively prevented and controlled when the seasonal influenza is prevented; the combined vaccine has great social and economic benefits.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Conjugate of influenza A virus conservative peptides M2e and virus-like particles, and application thereof
InactiveCN103083656AShort preparation timeEase of mass productionAntiviralsCarrier-bound antigen/hapten ingredientsHuman influenzaVirus-like particle
The invention relates to a conjugate of virus-like particles (VLP) of oligopeptide vector Q[beta] bacteriophage with target immunotherapy and conservative peptides M2e of human influenza virus M2 protein. The conjugate is mainly used for preventing human influenza A. The conjugate is coupled by a Sulfo-SMCC method which comprises a first step of preparing the virus-like particles (VLP) of the Q[beta] bacteriophage; a second step of preparing the virus-like particles (VLP) activated by Sulfo-SMCC; and a third step of preparing the conjugate of M2e-VLP. The conjugate can produce high titer protective antibody in mice and cross-protect the attack of various subtypes of the influenza A viruses.
Owner:苏州科贝生物技术有限公司
Rapid diagnosis method specific to avian influenza virus
ActiveUS20090311667A1Simple wayMicrobiological testing/measurementBiological material analysisHuman influenzaAvian influenza virus
The present invention relates to a method for detecting an avian influenza virus by an immunological assay using an anti-influenza virus antibody being unreactive to human influenza type-A virus subtypes H1, H2 and H3 and a human influenza type-B virus and being reactive to plural subtypes of avian influenza viruses, and an immunochromatographic test tool for use in the method. According to the present invention, an avian influenza virus can be detected specifically, rapidly and in a simple manner, as distinguishing an avian influenza virus from a human influenza virus.
Owner:OSAKA PREFECTURE +1
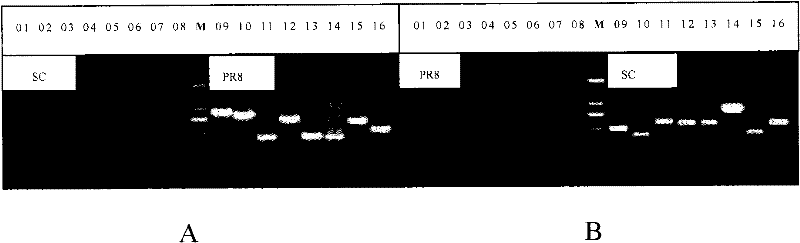
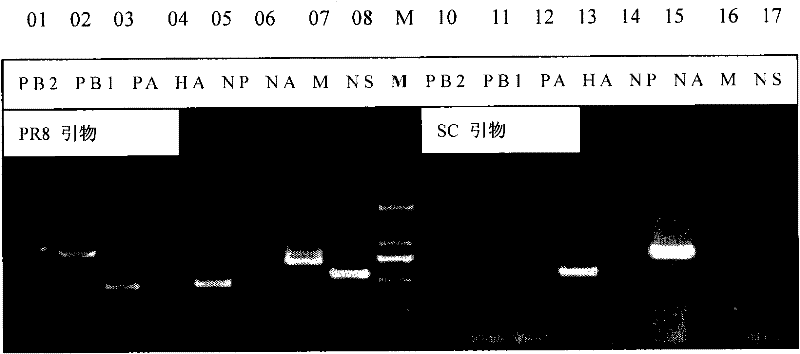
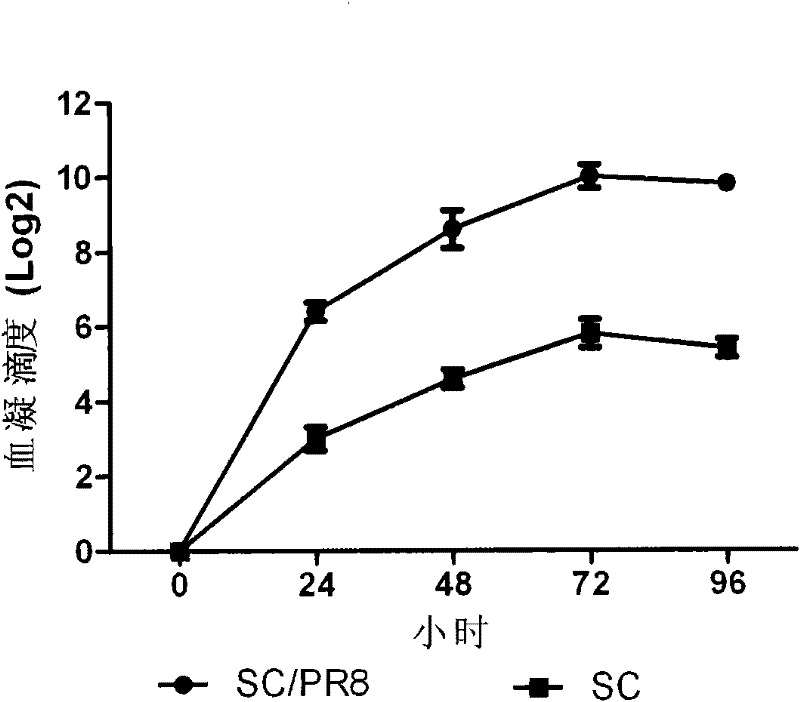
Preparation for reconstruction influenza A H1N1 virus inactivated vaccine strain (SC/PR8), and use thereof
InactiveCN102234637AQuality improvementReduce manufacturing costInactivation/attenuationMicroorganism based processesInfluenza A (H1N1) virusHuman influenza
The invention relates to a reconstruction influenza A H1N1 virus inactivated vaccine strain. The reconstruction influenza A H1N1 virus inactivated vaccine strain is characterized by expressing HA protein and NA protein of the influenza A H1N1 virus. In particular, the reconstruction influenza A H1N1 virus inactivated vaccine strain is SC / PR8. The invention further discloses a method for preparing the reconstruction influenza A H1N1 virus inactivated vaccine strain (SC / PR8), and a use of the reconstruction influenza A H1N1 virus inactivated vaccine strain (SC / PR8) in prevention of animal influenza A H1N1 or human influenza A H1N1.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Human influenza-poultry influenza combined vaccine and preparation method thereof
InactiveCN101450207ASolve the problem of difficult vaccinationImprove securityAntiviralsAntibody medical ingredientsHemagglutininSide effect
The invention provides a quadrivalence combined vaccine comprising H1N1, H3N2, B types flu and H5N1 type avian influenza and a preparing method. Concentration of each vaccine hemagglutinin is calculated with hemagglutinin: H1N1 type flu vaccine 20-40 mcg / mL, H3N2 type flu vaccine 20-40 mcg / mL, B type flu vaccine 20-40 mcg / mL and H5N1 type flu avian influenza 20-40 mcg / mL. The vaccine combining with human flu and avian influenza for human can solves problem of difficult to vaccination avian influenza vaccine, prevents avian influenza at the same time prevents huamn flu. Four indications (egg albumen, endotoxin, total protein contents, rate of total protein and hemagglutinin) The combined vaccine closly-related with side effect in the combined vaccine has low flu preparing regulation than national regulation, and has higher safety.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
Buccal tablets for preventing influenza virus and application thereof
InactiveCN106215169AIncreased glycoprotein concentrationAvoid infectionPeptide/protein ingredientsAntiviralsThroatHuman influenza
The invention discloses buccal tablets for preventing influenza virus and application of the buccal tablets. The buccal tablets comprise glycoprotein of a SAalpha2-3Gal sugar chain structure and glycoprotein of a SAalpha2-6Gal sugar chain structure. According to the technical scheme, the buccal tablets have the advantages of directly acting on the mouth cavity and the throat, increasing the concentration of glycoprotein of the SAalpha2-3Gal sugar chain structure and the SAalpha2-6Gal sugar chain structure, neutralizing and inhibiting recognition of avian and human influenza virus on the human body upper respiratory tract cell surface specific receptor, and achieving the effect of preventing avian and human influenza virus infection; due to slow mouth dissolving, medicine can stay on the surface of the mouth cavity and the throat for a long time, and the medicine effect is continuously achieved.
Owner:海安葛莱森生物科技有限公司
Technology for preparing compound human inter feron spray agent capable of preventing polytypic influenza
The present invention provides a novel human compound interferon spray for preventing and curing various types of influenza virus infection and its production preparation method. The described human compound interferon spray composition includes various types of human interferon alpha or beta or gamma or omega or delta or epsi or kappa or lambda etc. human interleukin-2, natural polysaccharide, human alhumin and preservative substance. The described influenza virus includes human influenza virus and avian influenza virus type A and type B. The invented human compound interferon spray can obtain obvious effect for preventing and curing various types of influenza virus infection.
Owner:北京远策药业有限责任公司 +1
Method and kit for human influenza virus typing detection based on magnetic resolution and colorful quantum dot labelling
ActiveCN105203764AFast separationIt has the effect of synergistic amplification of multiple signalsMaterial analysisMagnetic beadHuman influenza
The invention discloses a method and a kit for human influenza virus typing detection based on magnetic resolution and colorful quantum dot labelling. The kit comprises anti-human influenza virus immune nano-magnetic beads, anti-human influenza virus nanoprobes, quality control samples, a sample treating liquid and a PBST buffer solution, wherein the anti-human influenza virus immune nano-magnetic beads are rich in human influenza virus antigen; the anti-human influenza virus nanoprobes are labelled by colorful quantum dots; the quality control samples are a positive quality control sample and a negative quality control sample; the positive quality control sample is obtained by drying an inactived A-type human influenza virus and an inactived B-type human influenza virus, and combining the dried human influenza viruses on a swab; the negative quality control sample is a pharynx swab of a person whose detection results for the A-type human influenza virus and the B-type human influenza virus are both negative through clinical confirmation. The method and the kit are simple, convenient, fast, and high in sensitivity. The invention further provides a preparation method and a use method of the kit.
Owner:湖北诺美华抗体药物技术有限公司
Virus split deactivation method for human influenza virus split vaccine
ActiveCN102614508ASolve the aggregation problemImprove uniformityInactivation/attenuationAntiviralsHuman influenzaVirus
The invention relates to a virus split deactivation method for a human influenza virus split vaccine, and belongs to the field of biological pharmacy. A method of firstly purifying and cracking H1N1 type, H3N2 type or B type monovalent influenza totivirus after gradient density centrifugal purification and then deactivating is adopted, so that the virus cracking effect can be improved, the deactivation time can be shortened, the deactivation efficiency can be increased, the using amount of a deactivator can be obviously reduced, deactivation operation steps can be simplified, the contamination risk can be reduced, factory buildings, facilities and energy consumption required for deactivation can be reduced, and the uniformity and the stability of deactivation can be increased.
Owner:长春生物制品研究所有限责任公司
2' -fluoro substituted carba-nucleoside analogs for antiviral treatment
InactiveCN103153314ASaccharide with heterocyclic radicalsOrganic active ingredientsHuman influenzaViral infection
Owner:GILEAD SCI INC
Method for inhibiting influenza virus infection and medicament thereof
The invention relates to a method for restraining enveloped virus infection, relative polypeptide and protein drug, belonging to biological medicine technical field. The invention comprises a method for restraining influenza virus, particularly for restraining highly pathogenic influenza virus and human influenza virus (as H1N1 hypotype and H3N2 hypotype), relative polypeptide and protein, relative nucleic acid encoding the polypeptide and protein, and relative carriers and cells for representing the polypeptide and protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
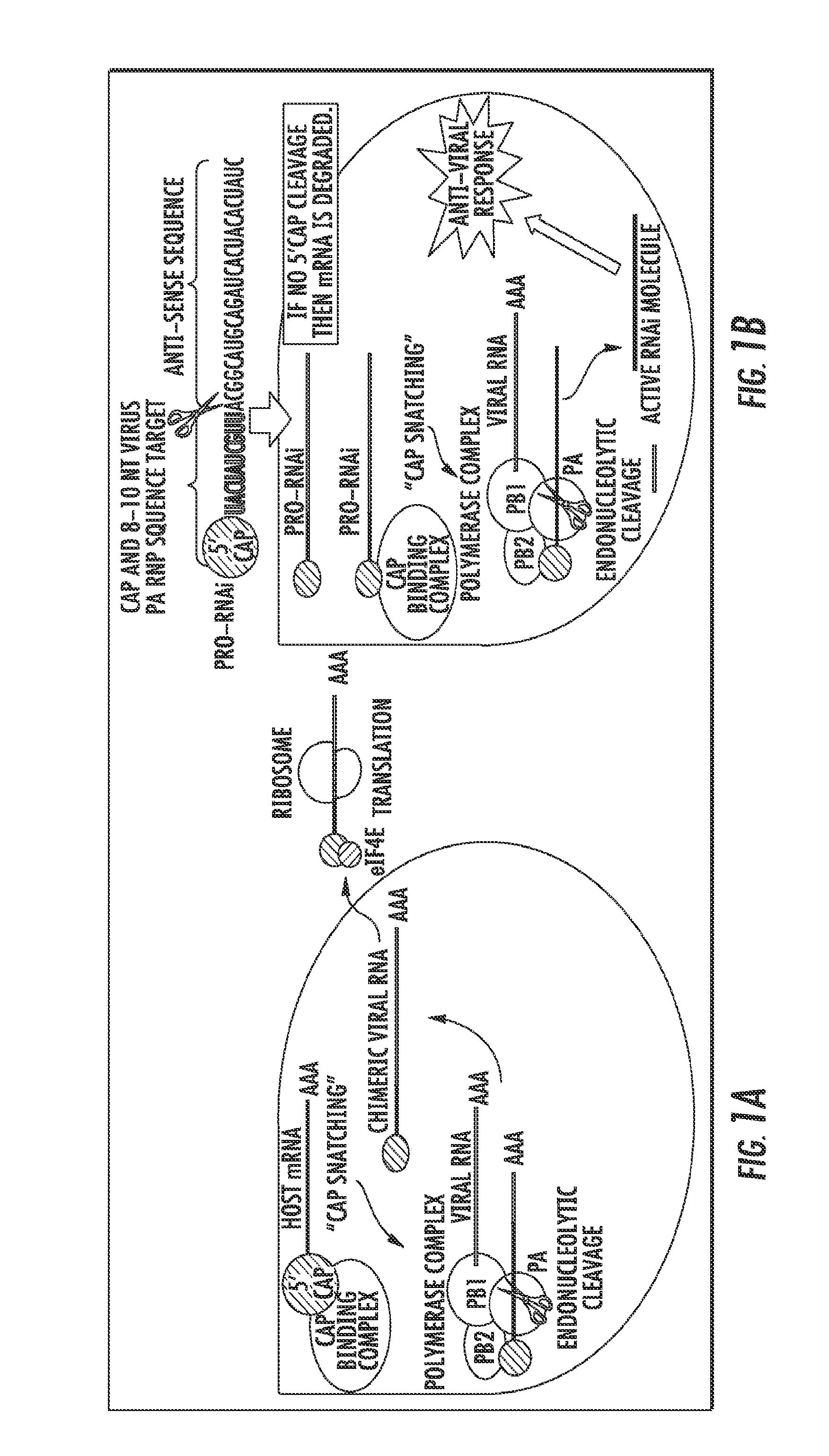
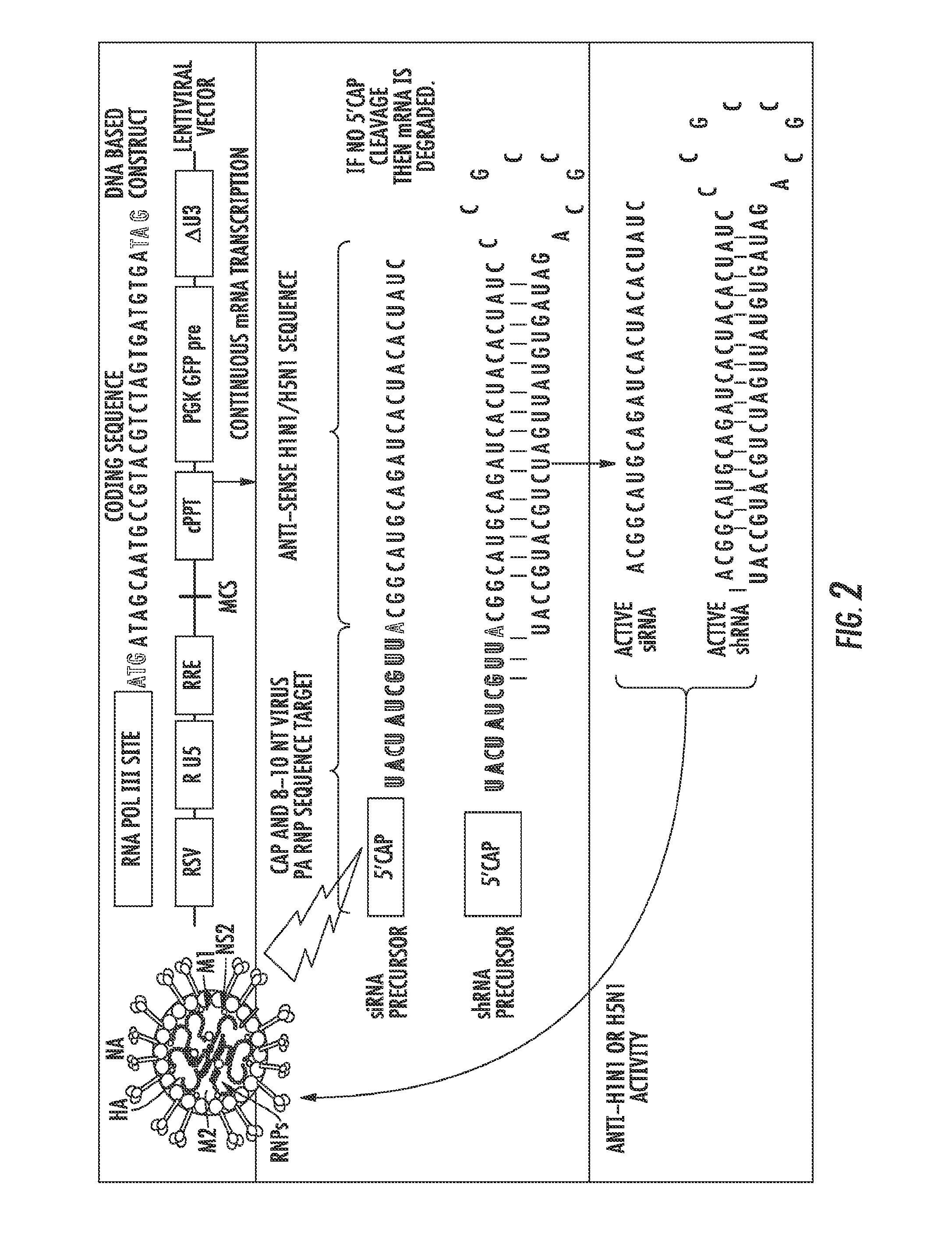
Influenza-activated constructs and methods of use thereof
ActiveUS20150376622A1Prevent sepsisPrevent cytokine stormMicrobiological testing/measurementFermentationHuman influenzaSubject matter
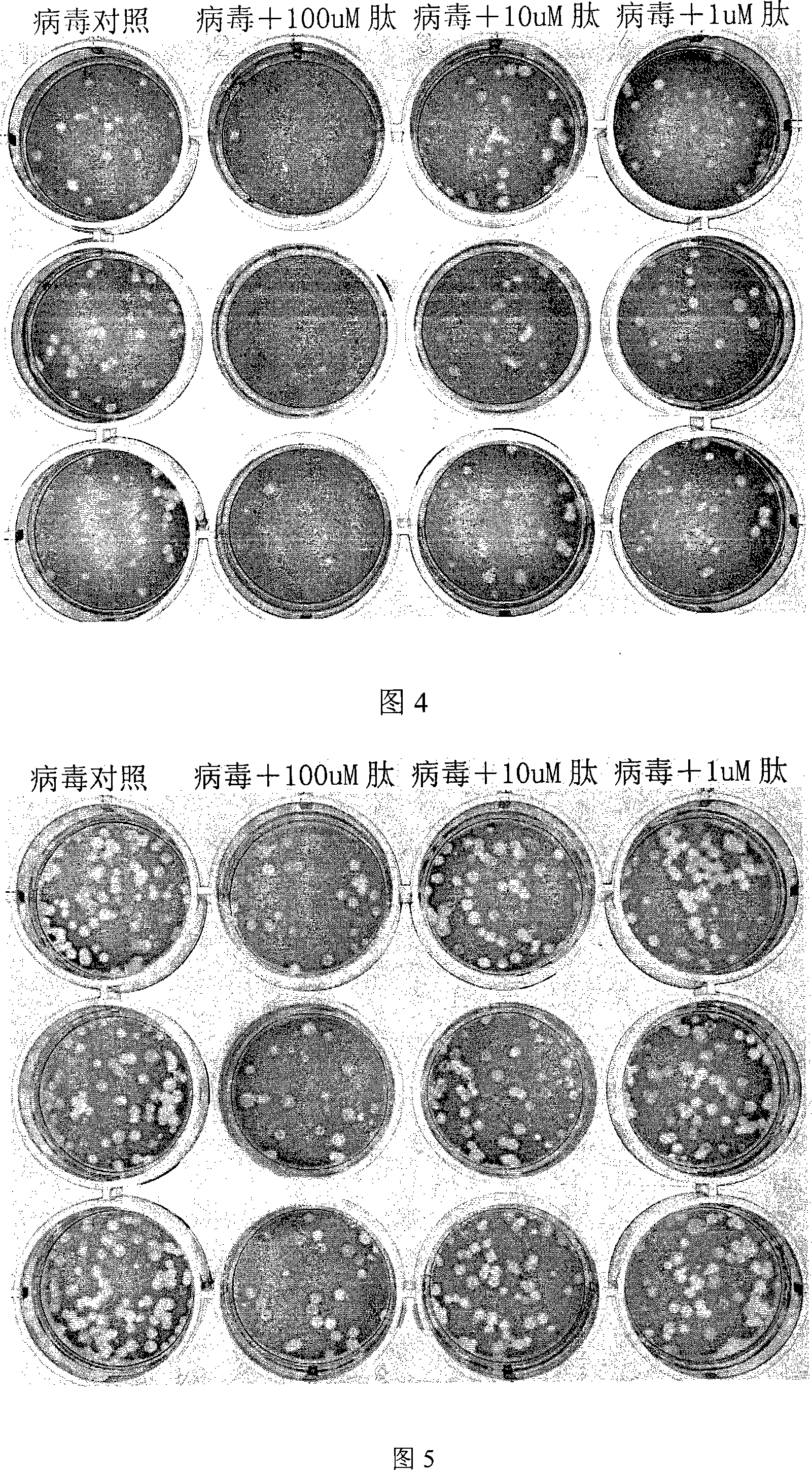
The presently disclosed subject matter provides a novel approach for the treatment, prevention, and diagnosis of Cap-Snatching virus infections, particularly all classes of human influenza, including pandemic influenza. The methods involve the use of constructs for RNA-interference (RNAi).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com