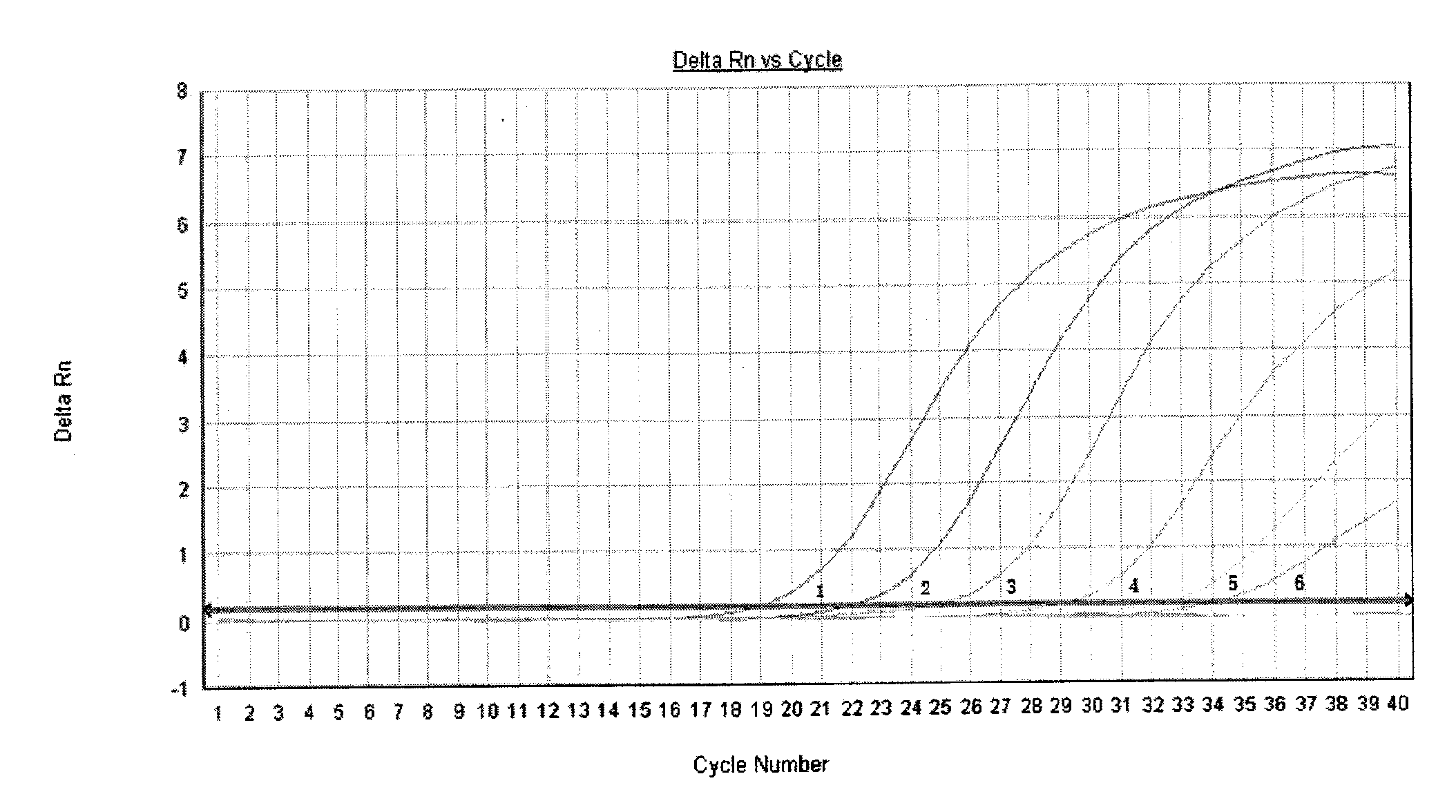
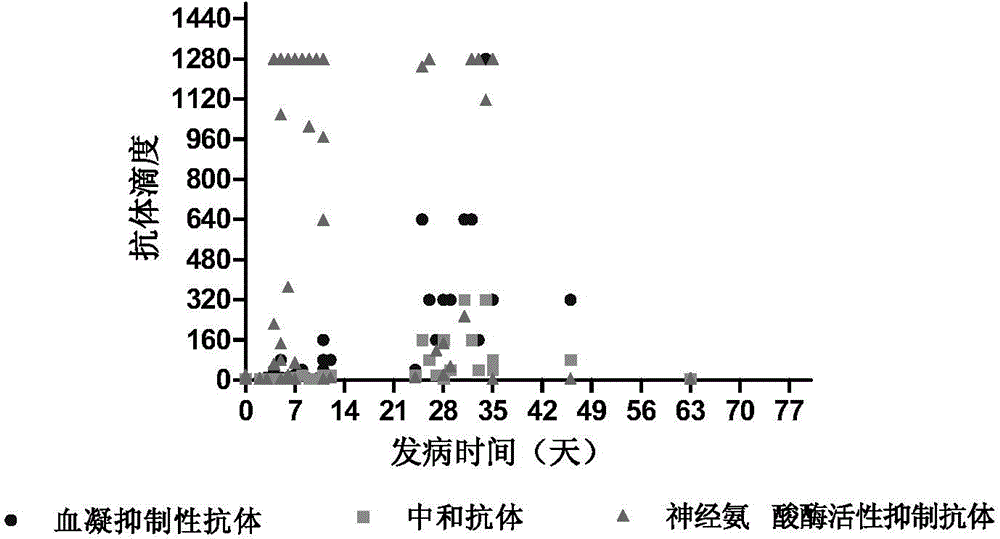
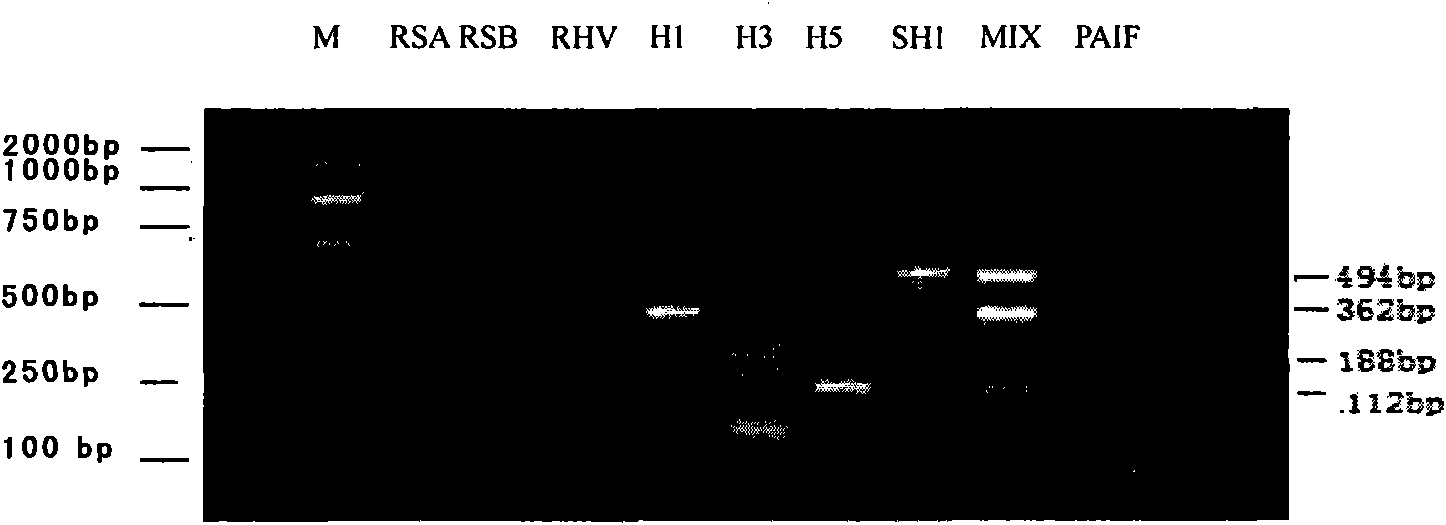
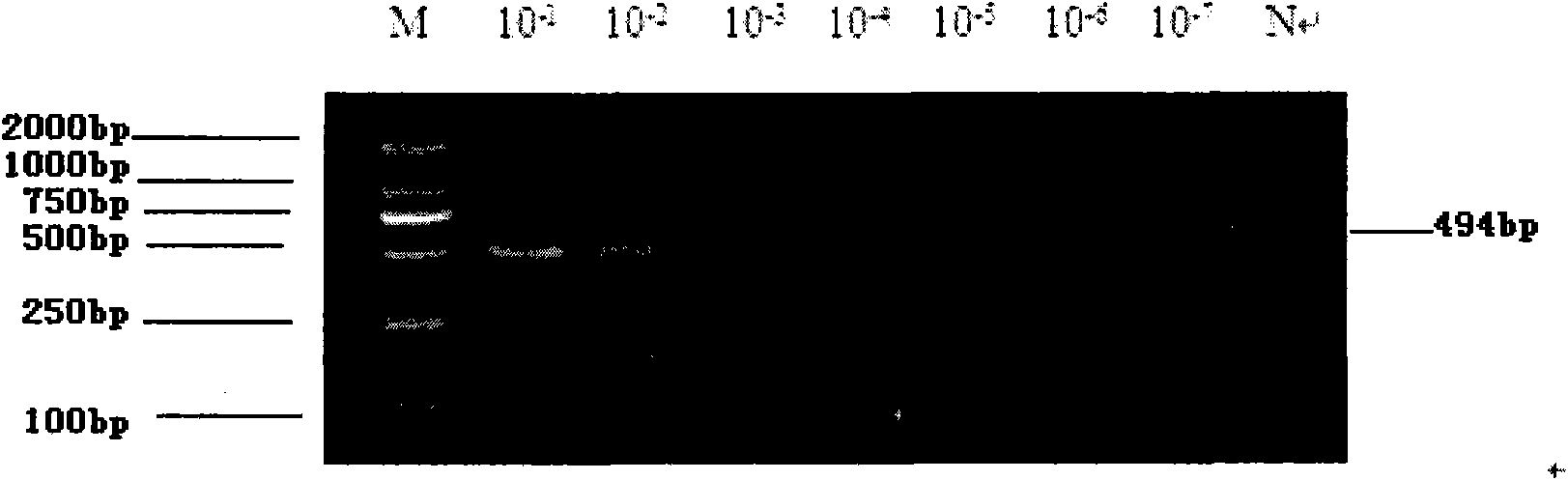
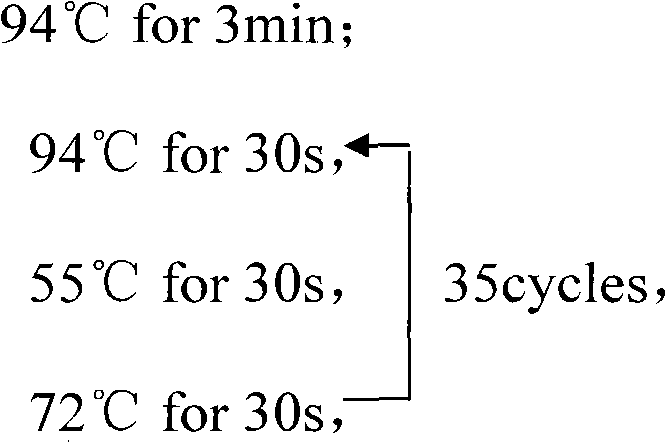
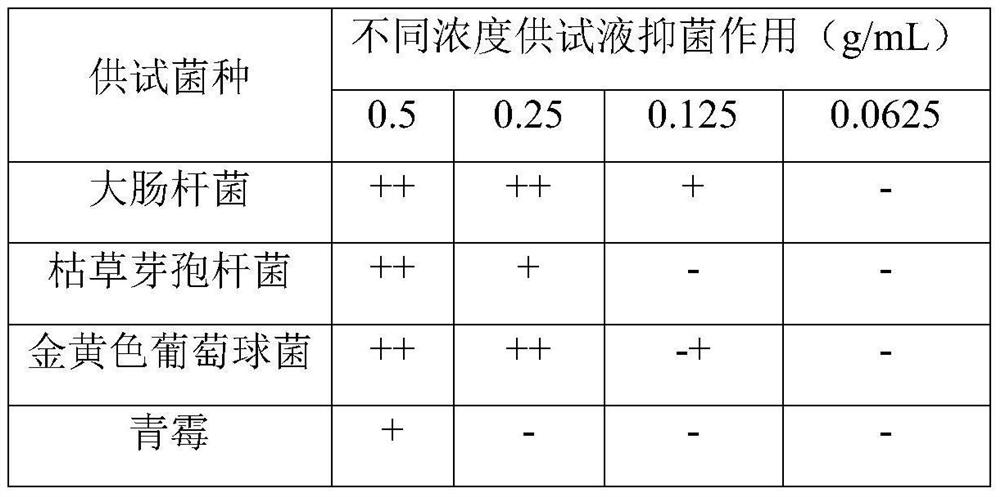
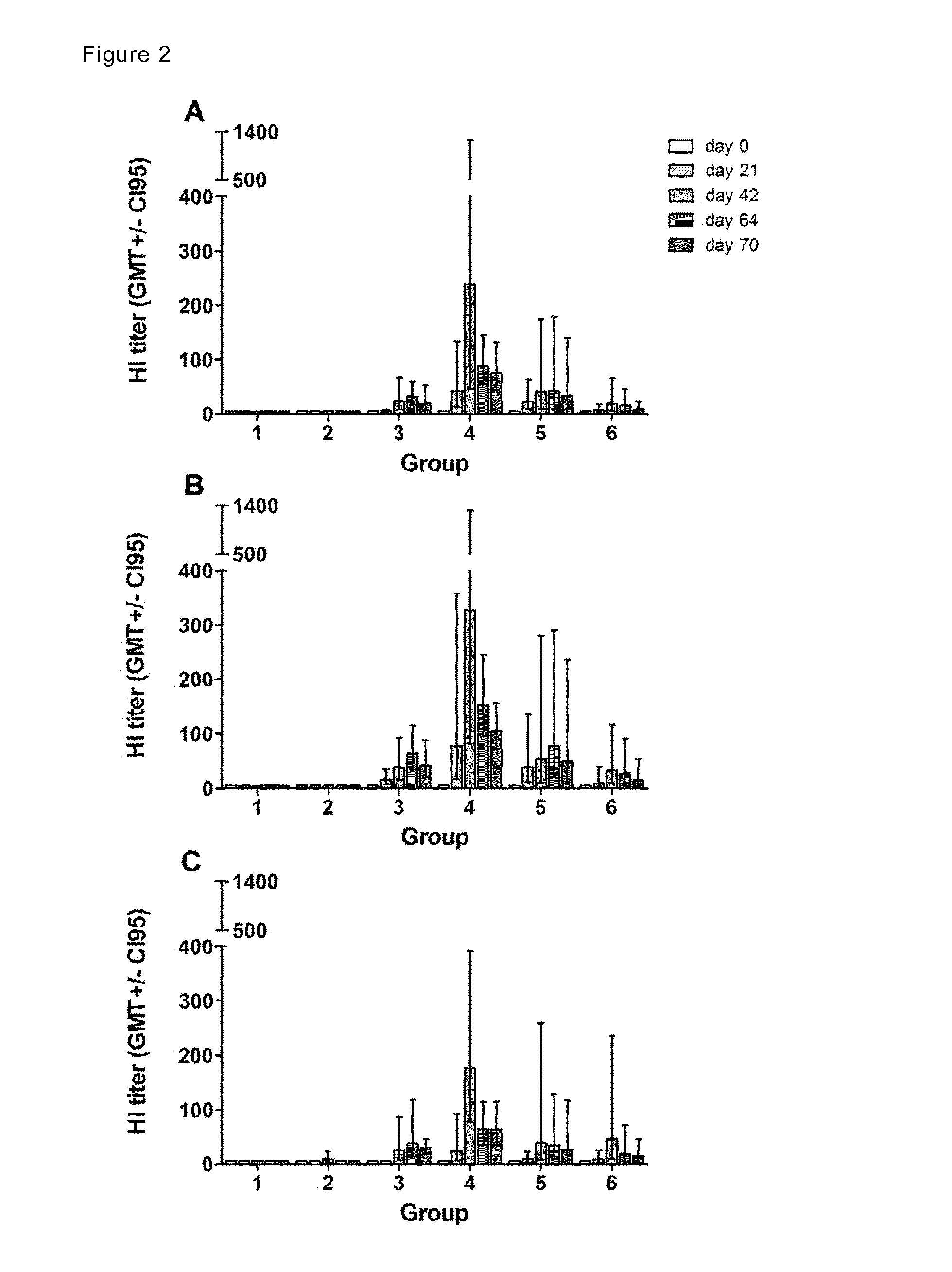
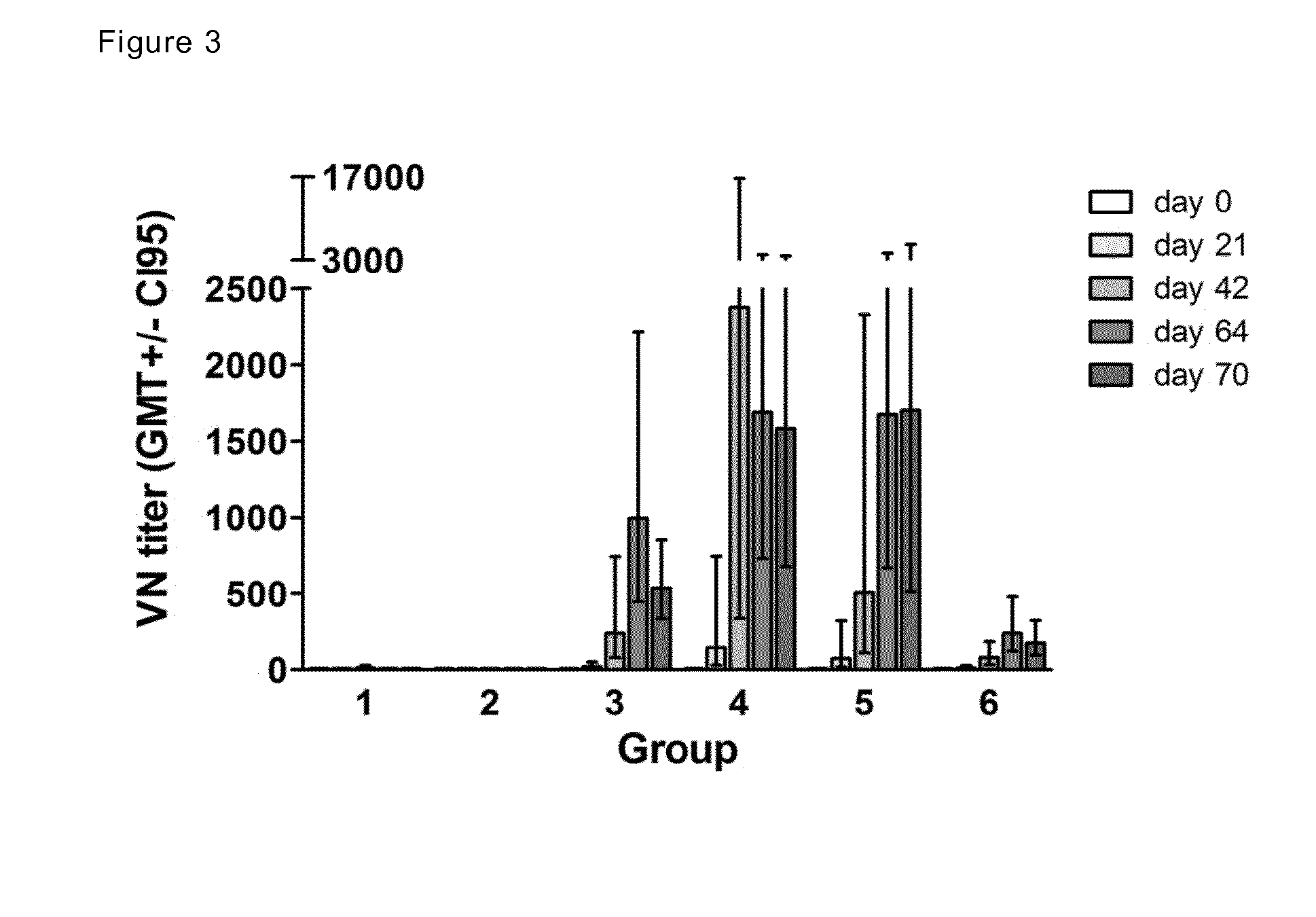
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Seasonal influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Functional influenza virus like particles (VLPs)
ActiveUS8080255B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Functional influenza virus like particles (VLPs)
ActiveUS8506967B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
Owner:NOVAVAX
Functional influenza virus-like particles (VLPS)
InactiveUS20120207786A1SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Detection of influenza virus
InactiveUS20070224594A1Compound screeningBioreactor/fermenter combinationsPathogenicitySpecific antibody
The present application describes methods for detecting influenza A and / or influenza B and / or distinguishing between pathogenic and seasonal influenza A subtypes. Many of these preferred formats employ pan-specific antibodies (i.e., that react with all or at least multiple strains within an influenza type) to detect presence of influenza A and / or influenza B and PDZ domains in combination with panspecific antibodies to influenza A to distinguish pathogenic and seasonal influenza A subtypes.
Owner:AVC ROYALTY FUND I
Bead Array Reader Based-Hemagglutination and Hemagglutination Inhibition Assay
InactiveUS20090325148A1High sensitivityMicrobiological testing/measurementBiological material analysisViral VaccineAnti viral immunity
Hemagglutination assays and hemagglutination inhibition assays were introduced in medical and virology practice more than 60 years ago. Since then, these assays have become important tools for measuring concentrations and strengths of viral cultures, the efficacy of the anti-viral immunization, and for studying the neutralizing capacity of virus-specific antibodies. The present invention comprises an improved hemagglutination inhibition assay (HAI), with at least about a 10-fold increase in sensitivity versus the traditional the HAI, to provide more accurate measurements of components in, for example, fluids from the in vitro MIMIC® system when assessing the effects of anti-viral vaccines (e.g., for seasonal influenza).
Owner:SANOFI PASTEUR VAX DESIGN
Functional influenza virus like particles (VLP)
ActiveCN101325966ASsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Novel Kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses and detecting method therefor
InactiveCN101665842AAccurate detectionHigh detection specificityMicrobiological testing/measurementMicroorganism based processesH1n1 virusInfluenza A (H1N1) virus
The invention discloses a novel kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses, containing a primer, a probe, Taqman 2*universal PRC master mix, 40*multi scribe<TM> and RNase inhibitor mix and nuclease-free water. The primer and the probe have the advantages of good detecting specificity and high sensitivity so that the kit is particularly suitable for the influenza AH1N1 which breaks out at present and has no cross reaction with the Avian flu, swine flu and common seasonal flu and has lower cost.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Recombinant influenza virus and application thereof
InactiveCN104312983AAvoid interferenceIncrease productionAntiviralsViruses/bacteriophagesHemagglutininNeuraminidase
The invention discloses a recombinant influenza virus and application thereof and provides a preparation method of the recombinant virus. The preparation method comprises the following steps: guiding an HA6 subtype influenza virus hemagglutinin encoding gene, an influenza virus neuraminidase (NA) gene and six genes including PB2, PB1, PA, NP, M and NS in an influenza virus into a host cell together, and packing to obtain the recombinant virus. The invention also discloses a detection method for detecting antibodies for inhibiting activities of seasonal influenza N1, N2, 2009H1N1N1 and 2013H7N9N9 enzymes by virtue of the recombinant virus. The detection method comprises the steps of sample treatment and result analysis. According to the invention, the immune response to NA of people can be rapidly and effectively measured, the operation is simple, the application is convenient, and viable technical support is provided for clinical detection and vaccine evaluation.
Owner:中国疾病预防控制中心病毒病预防控制所
Method for preparing seasonal influenza virus split vaccine
InactiveCN102406930ALess impuritiesHigh content of HAAntiviralsAntibody medical ingredientsEmbryoTGE VACCINE
The invention provides a method for preparing a seasonal influenza virus split vaccine and a vaccine prepared by using the method, belonging to the technical field of vaccine preparation processes. The preparation method comprises the following steps of: preparing an original liquid from influenza A1, A3 and B virus strain verified as WHO (World Health Organization) recommended virus strain or similar strain through the procedures of working seed bank creation through subculture, chick embryo inoculation, virus liquid culture and harvesting, virus inactivation, ultrafilter concentration, gradient centrifugation, ultrafilter sugar removal, column chromatography, virus splitting, secondary purification and filtration sterilization; and carrying out semi-finished product configuration and finished product packaging to obtain the seasonal influenza virus split vaccine. Because gradient centrifugation purification and molecular sieve chromatography column purification are combined and secondary purification including gradient centrifugation purification after virus splitting is carried out, the method provided by the invention has the advantages of good concentration efficiency and recovery rate, low cost, high efficiency and easiness for operation. The finally prepared product has low impurity content and high HA content, and the standards of endotoxin and ovalbumin are far better than the requirements of Chinese Pharmacopoeia 2010 and European Pharmacopoeia. Moreover, the product provided by the invention does not contain thiomersal, inoculation is safer, and inoculated population is larger.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
ELISA (Enzyme-linked Immuno Sorbent Assay) kit and detection method for avian influenza H7N9 hemagglutinin HA antigen
InactiveCN106771181AStrong specificityEasy to distinguishBiological testingImmunoassaysHemagglutininSorbent
The invention relates to an ELISA (Enzyme-linked Immuno Sorbent Assay) kit and detection method for an avian influenza H7N9 hemagglutinin HA antigen. The kit comprises an H7N9 hemagglutinin HA specific antibody pre-coated ELISA plate, a standard protein, a detection antibody, a horseradish peroxidase enzyme-labeled rabbit anti-mouse IgG antibody, an antibody diluting solution, a color developing solution A, a color developing solution B, a stopping solution and a washing solution, wherein the standard protein is a purified recombinant H7N9 hemagglutinin HA; and the detection antibody is a biotin-labeled H7N9 hemagglutinin HA specific antibody. The kit provided by the invention can be used for rapidly and effectively distinguishing H7N9 infection patients from common seasonal influenza patients at the early period of virus infection and the kit is low in price, simple and high in sensitivity; and the kit can be widely applied to front-line hospitals and is used for detecting the H7N9 antigen in a blood serum sample of the influenza infected patients and isolating H7N9 antigen positive patients as soon as possible so as to effectively control epidemic situations.
Owner:剑药(苏州)生物技术有限公司
Simultaneous detection of new H1N1 A influenza virus and human seasonal H1N1 and H3N2 influenza viruses by multi-fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR)
InactiveCN102086476AMicrobiological testing/measurementMicroorganism based processesReference genesConserved sequence
The invention belongs to the field of application of biotechnology, and relates to simultaneous detecting and monitoring of HA genes in human new H1N1 A influenza viruses and human seasonal H1N1 and H3N2 influenza viruses of specimens such as nasopharyngeal swabs of fever patients and the like by disease prevention and control mechanisms of all levels, influenza detection network laboratories, sentinel point hospitals and the like. Conserved sequences of HA genes in human new H1N1 A influenza viruses and human seasonal H1N1 and H3N2 influenza viruses are specifically analyzed by computer software, two corresponding primers and probes are designed respectively, single tube multi (quadruple)-fluorescence quantitative RT-PCR (comprising internal reference genes) is performed, and an entire reaction lasts for no more than 2 hours. The defects of complex operation, long time and high cost existing in the conventional multi-tube multi-fluorescence quantitative RT-PCR method are overcome, a good tool for tracing possible coinfection and recombination of new H1N1 A influenza and seasonal influenza is provided, and powerful technical support is provided for quick and correct screening of a large number of influenza suspected cases by the characteristics of high specificity, high sensitivity and high speed.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Detection of influenza virus
The present application describes methods for detecting influenza A and / or influenza B and / or distinguishing between pathogenic and seasonal influenza A subtypes. Many of these preferred formats employ pan-specific antibodies (i.e., that react with all or at least multiple strains within an influenza type) to detect presence of influenza A and / or influenza B and PDZ domains in combination with panspecific antibodies to influenza A to distinguish pathogenic and seasonal influenza A subtypes.
Owner:AVC ROYALTY FUND I
Typing detection kit for human seasonal influenza viruses and application method thereof
InactiveCN105219876ALow costImprove throughputMicrobiological testing/measurementBiotin-streptavidin complexMicro plate
The invention belongs to the field of biotechnologies, and particularly relates to a multi-PCR-ELISA detection kit for human seasonal influenza viruses and an application method thereof. The detection kit disclosed by the invention is composed of a RT-PCR reaction system, an ELISA detection system, four target-gene (an influenza-A-virus M gene, a H1 subtype virus HA gene, a H3 subtype virus HA gene, and a B type virus NS gene) positive plasmids (Pa-m, Ph1-ha, Ph3-ha and Pb-ns), a negative quality control specimen and four target-gene specific primer probes. Specific amplification is performed by using specific primers labeled by using four sets of biotins through RT-PCR, an amplified product after being denatured is hybridized with a specific probe labeled by using digoxin, a hybridized product is enveloped with a streptavidin-enveloped 96-hole micro-plate, and an anti-digoxin antibody labeled by using horse radish peroxidase is added for carrying out detection through an ELISA method, so that a rapid, sensitive and specific typing detection kit for seasonal influenza viruses such as H1 and H3 subtypes and hepatitis B viruses is established. The invention relates to the application of four sets of specific primer probes in the clinical differential diagnosis of influenza virus infection and the typing authentication of influenza virus isolates.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Composition for treating and/or preventing influenza, method and application thereof
ActiveCN109675042AHigh biosecurityHigh internal and external protection effectPeptide/protein ingredientsAntiviralsTherapeutic effectInfluenza a
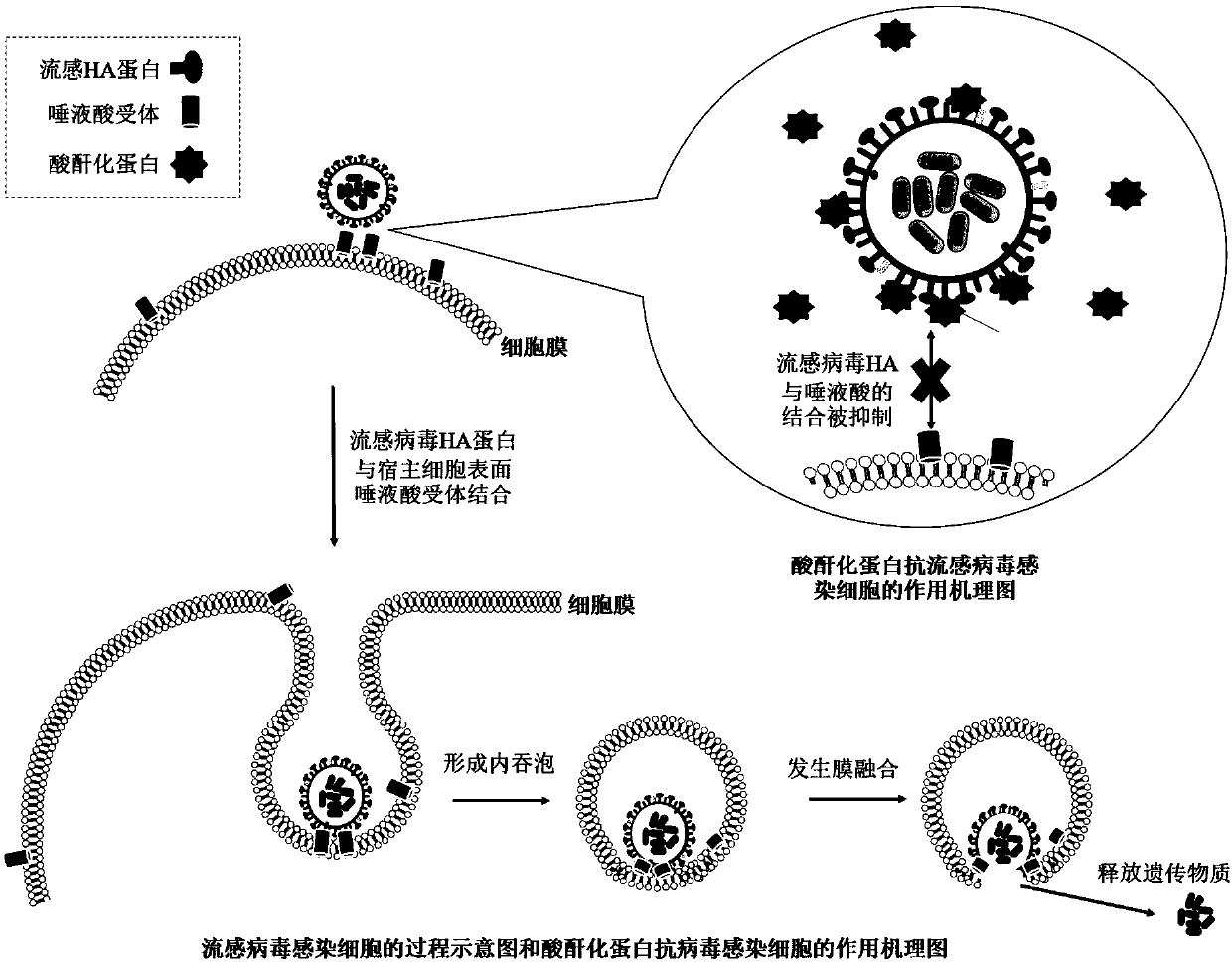
The present invention provides a composition for treating and / or preventing influenza, a method and an application thereof. The composition contains protein treated with acid anhydride and another drug for treating and / or preventing influenza. The acid anhydride-treated protein prepared by the invention is acid anhydride-treated lactoglobulin. An in vitro experiment shows that the protein can broadly inhibit influenza virus infected cells of various subtypes. In animal in vivo experiments, the protein has a significant prevention and treatment effect on influenza virus infection. Influenza viruses have many subtypes and high mutation rates, so that the development of broad-spectrum anti-influenza preparations is very important. The acid anhydride-treated protein can not only inhibits the infection of many seasonal influenza viruses, but also significantly inhibits the highly pathogenic avian influenza virus H7N9, and the influenza virus H1N1 (2009) which caused a pandemic, suggesting that the protein can be developed as a highly effective and broad-spectrum biological preparation for the prevention and treatment of influenza viruses.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Influenza A virus genotype detection kit
InactiveCN108239677ARapid identificationAccurate identificationMicrobiological testing/measurementMicroorganism based processesReference genesPositive control
The invention provides an influenza A virus genotype detection kit. The kit comprises RT-PCR reaction liquid, an RT-PCR enzyme mixture, an influenza A virus typing primer probe, an internal reference,a negative control, a critical positive control and a strongly positive control. The kit can directly carry out one-step RT-PCR on extracted influenza A virus RNA, can carry out fluorescent typing detection on influenza A viruses in a sample, takes an internal reference gene sequence as the internal reference and prevents pollution by utilizing UNG enzyme. The kit provided by the invention is simple in an amplification method, short in procedure and easy to operate, pollution is prevented, detection result specificity is strong, sensitivity is high, result is clear, reliability is high, and the kit provided by the invention can be applied to fluorescent type identification and detection on seasonal influenza virus H1 subtype and H3 subtype and influenza A virus subtype H1N1 (2009).
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Combined vaccine of seasonal influenza and pandemic influenza for people and preparation method
ActiveCN103285391ANo local irritationSolve the difficult problem of large-scale vaccinationAntiviralsAntibody medical ingredientsHemagglutininHuman influenza
The invention discloses a combined vaccine of seasonal influenza and pandemic influenza for people. The hemagglutinin concentration of each vaccine is 10-60 mug / ml of H1N1 human influenza vaccine,10-60 mug / ml of H3N2 human influenza vaccine, 10-60 mug / ml of B human influenza vaccine, and 10-60 mug / ml of H5N1 human influenza vaccine respectively. The invention also discloses a preparation method of the combined vaccine. The combined vaccine disclosed by the invention has high safety; the problem that the traditional avian influenza vaccine is difficultly vaccinated at a large scale is solved; explosive epidemics of the H5N1 human influenza vaccine is effectively prevented and controlled when the seasonal influenza is prevented; the combined vaccine has great social and economic benefits.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Primer group and probe group capable of simultaneously detecting various influenza viruses and kit capable of detecting various influenza viruses
InactiveCN107034310AQuick checkEnables direct RT-PCRMicrobiological testing/measurementDNA/RNA fragmentationThroat swab sampleThroat swab
The invention provides a primer group and a probe group capable of simultaneously detecting various influenza viruses. Sequences of the primer group are shown as SEQ ID NO:1-8, and sequences of the probe group are shown as SEQ ID NO:9-12; and the influenza viruses include influenza A virus, influenza B virus, seasonal influenza virus H1 sub-type and H3 sub-type. On the basis, a kit, which is capable of simultaneously detecting the various influenza viruses, is provided. According to the prime group, the probe group and the kit, direct amplification and multiplex PCR are combined; direct RT-PCR can be conducted on suspected influenza cases or epidemiological investigation throat swab samples; and in comparison with a conventional method, a nucleic acid extraction step is avoided, so that it is conducive to implementation of automatic integrated detection and to improvement of efficiency. The prime group, the probe group and the kit have obvious advantages in aspect of being applied to rapid discrimination and detection of people infected by the influenza viruses, and the invention is simple and convenient to operate, short in detection time and relatively low in detection cost. The prime group, the probe group and the kit are applicable to rapid detection at a site of epidemic situation; and the prime group, the probe group and the kit have a broad and practical application value.
Owner:SOUTHERN MEDICAL UNIVERSITY
Attenuated influenza virus and a live vaccine comprising the same
The present invention relates to an isolated attenuated influenza virus strain and a live vaccine comprising the same. The isolated attenuated influenza virus strain is prepared by cold-adaptation of a mother strain which carries 6 internal genomes of A / PR / 8 / 34(H1N1) and two surface antigens HA and NA of A / Aichi / 2 / 68(H3N2). The attenuated influenza virus strain and the live vaccine of the present invention are useful for prevention of seasonal influenza episodes and sudden outbreak of influenza pandemics of predicted or unknown identity, since they have safety, efficacy, high production yield, immediate protection against variety of influenza subtypes and prolonged protection against specific influenza subtype.
Owner:RAPAVAX INC
Isothermal amplification assay kit for human seasonal influenza H1N1 and detection method thereof
InactiveCN101864497AGood repeatabilityComparableMicrobiological testing/measurementSingle sampleRNA extraction
The invention relates to an isothermal amplification assay kit for human seasonal influenza H1N1 and a detection method thereof. The assay kit comprises RNA extract, human seasonal influenza virus H1N1 nucleic acid isothermal amplification reaction liquid, and human seasonal influenza virus H1N1 positive control and negative control. The assay kit has the advantages of high specificity, high sensitivity, and high response speed. Only 2 hours are needed from processing to detection of a single sample; the assay kit can meet high-throughput and low-throughput sample detection simultaneously; and the whole reaction process does not need any complex instrument.
Owner:上海国际旅行卫生保健中心 +1
Kit for detecting multiple influenza viruses by polymerase chain reaction (PCR) microarray
ActiveCN102337352AEasy to operateShort timeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceInfluenza A (H1N1) virus
The invention relates to a kit for detecting multiple influenza viruses by a polymerase chain reaction (PCR) microarray. The kit comprises mainly a PCR microarray reaction plate, a PCR reaction solution, and a packaging box for centralized and partitioned packaging of reagent bottles or reagent tubes. Through the combination of a real-time fluorescence PCR technology and a microarray technology, on the single PCR microarray reaction plate, the kit can detect eight influenza viruses comprising influenza A virus, influenza B virus, seasonal influenza H1 subtype virus, seasonal influenza H3 subtype virus, influenza A H1N1 virus, highly pathogenic avian influenza H5 subtype virus, highly pathogenic avian influenza H7 subtype virus, and highly pathogenic avian influenza H9 subtype virus simultaneously. A result of detection adopting the kit can be utilized for differential diagnosis and monitoring of pathogens capable of causing influenza.
Owner:DAAN GENE CO LTD
Attenuated influenza virus and a live vaccine comprising the same
The present invention relates to an isolated attenuated influenza virus strain and a live vaccine comprising the same. The isolated attenuated influenza virus strain is prepared by cold-adaptation of a mother strain which carries 6 internal genomes of A / PR / 8 / 34(H1N1) and two surface antigens HA and NA of A / Aichi / 2 / 68(H3N2). The attenuated influenza virus strain and the live vaccine of the present invention are useful for prevention of seasonal influenza episodes and sudden outbreak of influenza pandemics of predicted or unknown identity, since they have safety, efficacy, high production yield, immediate protection against variety of influenza subtypes and prolonged protection against specific influenza subtype.
Owner:RAPAVAX INC
Fluorescent quantitative pcr detection kit and non-diagnostic detection method for novel type A h1n1 virus
InactiveCN102296127AAccurate detectionHigh detection specificityMicrobiological testing/measurementMicroorganism based processesH1n1 virusBird flu
The invention discloses a fluorescent quantitative PCR kit for detecting novel type A influenza virus and a non-diagnostic detection method. The kit includes primers and probes of specific sequences. The primers and probes have good detection specificity and high sensitivity, and are especially suitable for The H1N1 flu that broke out this time has no cross-reaction with bird flu, swine flu and common seasonal flu, and the cost is also greatly reduced.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Antibodies useful in passive influenza immunization
InactiveCN104302321ASugar derivativesPeptide/protein ingredientsMonoclonal antibodyBispecific antibody
Specific monoclonal antibodies and fragments including bispecific antibodies thereof that are crossreactive with multiple clades of influenza virus including both Group 1 and Group 2 representatives are disclosed. These antibodies are useful in controlling influenza epidemics and pandemics as well as in providing prophylactic or therapeutic protection against seasonal influenza.
Owner:TRELLIS BIOSCIENCE LLC
Real-time fluorescent nucleic acid constant-temperature amplification and detection kit for human seasonal influenza viruses H1N1 (HuH1N1)
ActiveCN103525946AReduce pollutionEfficient captureMicrobiological testing/measurementFluorescence/phosphorescenceT7 RNA polymeraseFluorescence
The invention discloses a real-time fluorescent nucleic acid constant-temperature amplification and detection kit for human seasonal influenza viruses H1N1 (HuH1N1). The real-time fluorescent nucleic acid constant-temperature amplification and detection kit comprises a capturing probe, a HuH1N1 detection primer T7, a primer nT7, a HuH1N1 detection probe, an M-MLV reverse transcriptase, T7 RNA (ribonucleic acid) polymerase and the like. The kit disclosed by the invention can detect HuH1N1 RNA in a swab, has the characteristics of high specificity, high sensitivity (which is up to 100copies / reaction), low pollution (due to easy degradation of the RNA of amplification products under a natural environment) and quickness in detection ( due to detection normally implemented within 50 minutes), plays an important role in clinical diagnosis of early infection of the human seasonal influenza and has a large application prospect.
Owner:SHANGHAI RENDU BIOTECH
Vaccine composition for use in immuno-compromised populations
InactiveUS20150306204A1Reduce severitySsRNA viruses negative-senseViral antigen ingredientsVirus strainSeasonal influenza
The invention relates to nasally-administered vaccine compositions effective against infection in immuno-compromised populations. One aspect of the invention is directed to the pediatric use of the vaccine of the invention including a vaccine effective in children against seasonal influenza virus strains. A further aspect of the invention is directed to subjects of all age groups when the composition is for pandemic use.
Owner:EUROCINE VACCINES
Kit for identifying subtypes of influenza A virus
InactiveCN101875933AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationInfluenza A (H5N1) VirusHuman Influenza A Virus
The invention discloses a kit for identifying subtypes of influenza A virus. The kit comprises the following primer pairs: one primer of which the sequence is shown in a sequence 5 in a sequence table and the other primer of which the sequence is shown in a sequence 6 in the sequence table; and the subtypes of influenza A virus are subtypes of new influenza A H1N1 virus, influenza H1N1 virus, influenza A H5N1 virus or influenza A H3N2 virus. Experiments prove that the method has the advantages of reliable detection results, high sensitivity (capable of detecting 103 copy number of the new influenza A H1N1 virus to the lowest), and good specificity; and particularly, the method can also separate the subtypes of the new influenza A H1N1 virus from the subtypes of common seasonal H1N1 influenza virus, which cannot be realized by the conventional technology. Besides, the method has the advantages of no need of precious equipment, simple and quick operation, and capacity of realizing high-flux quick detection. The kit and the PCR reagent have the advantages of high sensitivity, good specificity, wide sources and low cost. The invention provides a simple, feasible and effective method for early diagnosis of the infections of human influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Kit for detecting seasonal influenza virus H1N1 through real-time PCR
InactiveCN101948934AEasy to operateExcellent specific detection primersMicrobiological testing/measurementFluorescence/phosphorescenceDiagnosis methodsVirus
The invention discloses a kit for detecting seasonal influenza virus H1N1 through real-time polymerase chain reaction (PCR) and also discloses methods for treating samples to be detected and analyzing Real-time PCR system and conditions and results. The kit comprises forward and reverse specific primers, standard products to be detected, etc. The kit can rapidly and quantitatively detect the seasonal influenza virus H1N1, has high sensitivity, can be used as the assistant diagnosis method of seasonal influenza virus H1N1 infection and the monitoring means of the clinical effects in the basic and clinical laboratories, has relatively low requirement for the experiment operators and has practical clinical application value.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Chinese herbal medicine bacteriostatic effervescent tablet containing ligusticum wallichii extract and preparation method thereof
PendingCN112999177AReduce transmissionAchieve synergyAntibacterial agentsAntisepticsDiseasePhenolic content in tea
The invention discloses a Chinese herbal medicine bacteriostatic effervescent tablet containing ligusticum wallichii extract and a preparation method thereof. The effervescent tablet mainly comprises active ingredients and auxiliary materials, the active ingredients comprise 15-30 parts by mass of ligusticum wallichii, 10-20 parts by mass of fructus gardeniae, 5-15 parts by mass of szechwan chinaberry fruit and 5-15 parts by mass of fructus. The auxiliary materials comprise an acid source, an alkali source, a lubricant, lactose and tea polyphenol. The effervescent tablet disclosed by the invention has a good inhibition effect on common bacteria which are easily contacted by daily exposure of skin. The product is convenient to carry and easy to store, and exposed skin parts such as hands and the like can be soaked and cleaned at any time after the hand-washing liquid is soaked in water, so that the cleaning and antibacterial effects are achieved. Many seasonal influenza viruses are transmitted through a hand-mouth way, and the purpose of reducing epidemic disease transmission can be achieved by using the effervescent tablets disclosed by the invention.
Owner:CHENGDU UNIV
Vaccine composition for naive subjects
ActiveUS20150306205A1Reduce severitySsRNA viruses negative-senseViral antigen ingredientsPandemicVirus strain
The invention relates to nasally-administered vaccine compositions effective in naive subjects such as children. Further, the vaccine composition is suitable for vaccinating the general population during a pandemic. One aspect of the invention is directed to the paediatric use of the vaccine of the invention including a vaccine effective in children against seasonal influenza virus strains. A further aspect of the invention is directed to subjects of all age groups when the composition is for pandemic use.
Owner:EUROCINE VACCINES
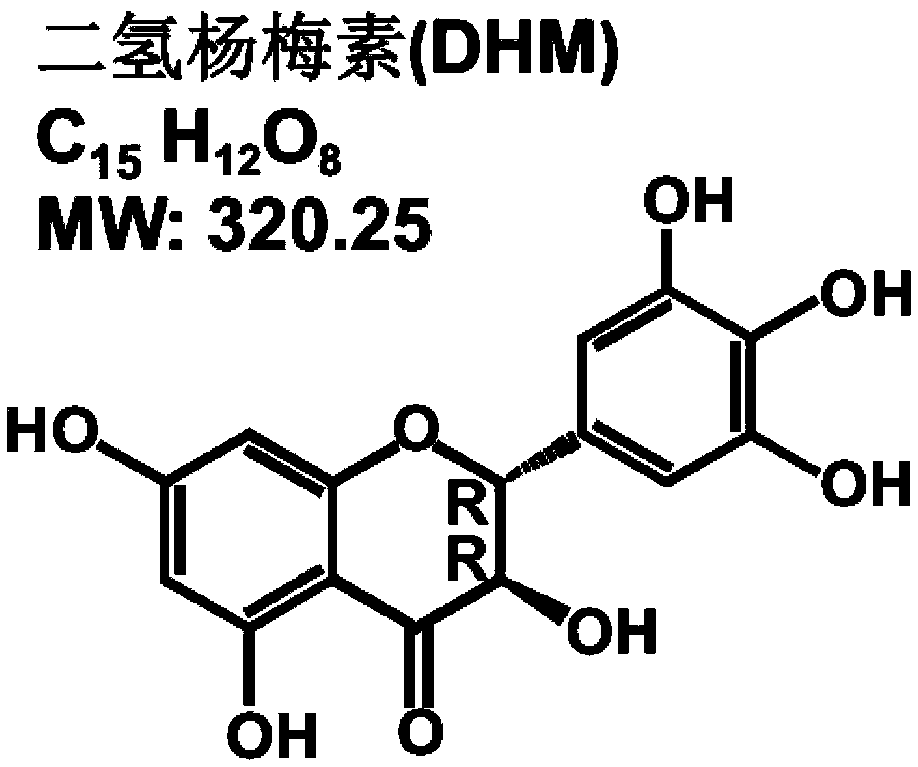
Anti-haze drug, anti-haze mask of anti-haze drug and application of dihydromyricetin in control of haze and treatment of seasonal influenza
InactiveCN109512814APrevent and/or mitigate damageAvoid damageAntibacterial agentsOrganic active ingredientsHuman Influenza A VirusEffective treatment
The invention provides an anti-haze drug, an anti-haze mask of the anti-haze drug and application of dihydromyricetin in control of haze and treatment of seasonal influenza. A product used for haze resistance is prepared from dihydromyricetin. The anti-haze product is a drug, and the drug is capable of obviously preventing and / or alleviating damage of haze to lung organs. Furthermore, the anti-haze product is an anti-haze mask. By using the anti-haze mask, PM10-2.5 particles and / or harmful gases can be filtered, and the filtering rate reaches 95% or higher. Furthermore, a product for treatinginfluenza is prepared with the dihydromyricetin. Since the effect of the dihydromyricetin for inhibiting a human influenza A virus ultravirus H1N1 exceedds 100 times that of other flavonoids compounds, and meanwhile, the damage of infection to the respiratory system can be obviously prevented and alleviated, the product for treating influenza prepared by using the dihydromyricetin is capable of effectively treating various influenzas, and is used for key treatment of patients suffering from influenza A virus H1N1 and / or streptococcus.
Owner:李定文
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com