Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Viral immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inactivated or killed viral vaccines contain viruses, which have lost their ability to replicate and in order for it to bring about a response it contains more antigen than live vaccines. Attenuated or live vaccines contain the live form of the virus.
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS6884414B1Quick changeAvoid problemsSsRNA viruses negative-senseBiocideTumor reductionIn vivo
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS20040253273A1Prevent tumor formationQuick changeSsRNA viruses negative-senseAntibody mimetics/scaffoldsTumor reductionEpitope
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:PALESO PETER +2
Compositions containing recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS7015024B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses, also are provided.
Owner:HEALTH & HUMAN SERVICES DEPT OF REPRESENTED BY THE SEC OF UNITED STATES OF AMERICA THE
Nanoparticle vaccines with novel structural components
ActiveUS20200009244A1Treating and preventing HIV- infectionAvoid infectionSsRNA viruses negative-sensePowder deliveryEbola virusNanoparti cles
The present invention provides novel nanoparticle presented vaccine compositions that are stabilized with a locking domain. Various immunogens can be employed in the preparation of the vaccine compositions, including viral immunogens such as HIV-1 and Ebola viral immunogens, and non-viral immunogens such as immunogens derived from bacteria, parasites and mammalian species. The invention also provides methods of using such vaccine compositions in various therapeutic applications, e.g., for preventing or treating viral infections.
Owner:THE SCRIPPS RES INST
Method for preparing African swine fever virus P30 and P54 yeast vaccine
PendingCN111004330AIncrease the number ofGood antigenicityFungiViral antigen ingredientsHumoral immune reactionTGE VACCINE
The invention discloses a method for preparing an African swine fever virus P30 and P54 yeast vaccine. The vaccine contains African swine fever virus strong immunogen protein P30, membrane structure protein P54, Fc fragments of swine IgG1 and IgA1 (namely Fc gamma, Fc alpha), and His purification tags. The method comprises the following steps: S1, connecting ASFV immunogen protein genes p30 and p54 with Fc gamma and Fc alpha in series; S2, respectively constructing transcription units of p30-Fc gamma and p54-Fc alpha in fusion expression in the yeast; S3, realizing series connection of the twotranscription units through in-vitro enzyme linking, yeast in-vivo conversion and homologous recombination technologies, and stably integrating the two transcription units into surface display type saccharomyces cerevisiae; and S4, carrying out genome level and protein level verification, and confirming to obtain two recombinant yeast strains of which the independent surfaces show and express theP30-Fc gamma and P54-Fc alpha fusion proteins, and a tandem composite expression type recombinant yeast strain of which the surface shows and expresses the two fusion proteins at the same time in S1.The strain is mature in production technology, suitable for large-scale production, safe to use and capable of inducing remarkable mucous membrane and humoral immune response of target animals.
Owner:TIANJIN UNIV
Vectors expressing SARS immunogens, compositions containing such vectors or expression products thereof, methods and assays for making and using
SARS (severe acute respiratory syndrome virus, a coronavirus) immunogens, antigens, or epitopes, nucleic acid molecules encoding such immunogens, antigens, or epitopes; vectors containing such nucleic acid molecules, e.g., viral vectors such as baculovirus vectors, DNA vectors, such as DNA plasmid vectors, e.g., DNA plasmids that express a nucleic acid molecule in a mammalian cell, uses for such immunogens, antigens or epitopes and vectors, e.g., as an active component immunogenic, immunological or vaccine compositions, or to generate antibodies, such as monoclonal antibodies, and methods for making, and using such immunogens, antigens or epitopes, vectors, antibodies, including in methods for eliciting an immunological or immunogenic or vaccine response, as well as in assays or diagnostic kits or methods, are discussed, as well as a seamless fusion of sequences in a plasmid or vector, e.g., a sequence encoding a leader sequence and a sequence encoding a protein, epitope or immunogen or antigen.
Owner:UNKNOWN
Monoclonal antibody of immunoglobulin of anti lymphocyst vitos of Pacific fluke, and preparation method
ActiveCN101003572AInnovative designAchieve purificationImmunoglobulins against animals/humansBiological testingBALB/cCell engineering
This invention discloses monoclonal antibody against anti-LCDV immunoglobulin of Paralichthys olivaceus, which is excreted by hybridoma JF-lgM-H (CCTCC-C200631). The method comprises: immunizing Paralichthys olivaceus with LCDV inactivated by formalin to prepare antiserum, purifying Paralichthys olivaceus immunoglobulin, immunizing Balb / c mice as antigen, preparing hybridoma cells by cell engineering method, and screening the monoclonal antibody by immunoassay. Indirect ELISA and indirect immunofluorescent antibody assay show that this monoclonal antibody is located on the heavy chain (7-80 kDa) of the anti-LCDV immunoglobulin. The monoclonal antibody can be used for preparing reagents for detecting LCDV infection in early stage, and evaluating the immune effects of LCDV vaccine inactivated by formalin.
Owner:OCEAN UNIV OF CHINA
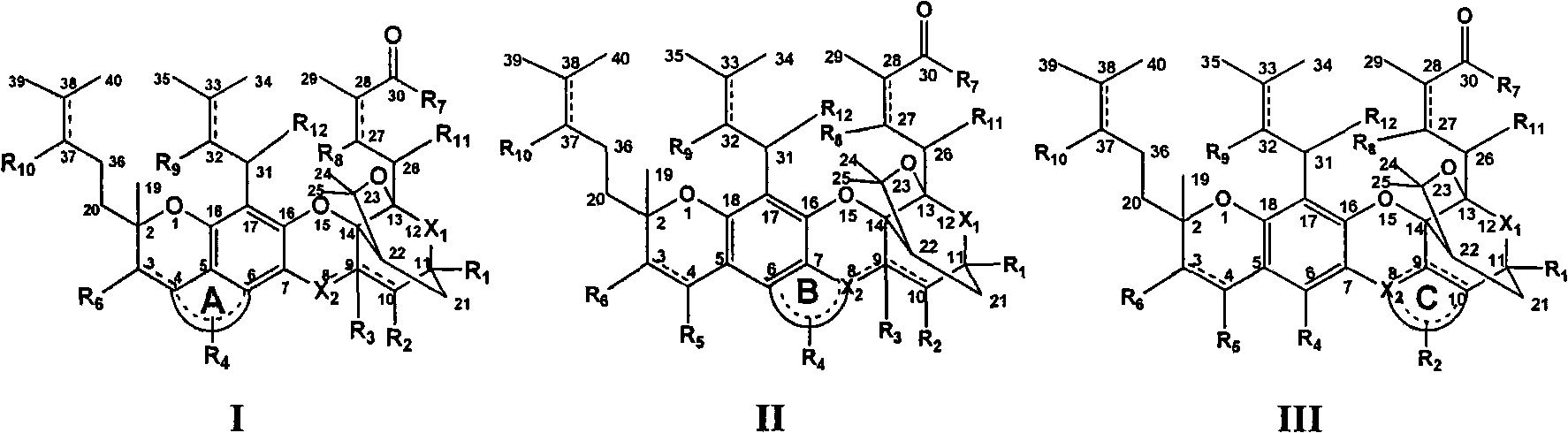
Gambogic acid cyclized analog, preparation method and application thereof
ActiveCN101613386ALow toxicityHigh activityAntibacterial agentsAntimycoticsAntifungalChemical synthesis
The invention discloses a gambogic acid cyclized analog, a preparation method and application thereof. The semi-synthesized gambogic acid cyclized analog is obtained by the extracted and purified gambogic acid through chemical synthesis and has a structural general formula I-III as the right formula, wherein substituent groups of A ring, B ring, C ring, R1, R2, R3, R4, R5, R6, R7, R7, R8, R9, R10, R11 or / and R12 contain glycosyl group, polyhydroxy, amino acid group, acyloxy group, phosphoric acid oxygen group, sulphonic acid oxygen group, alkoxyl group, aromatic oxygen group, heterocyclic oxygen group, mercapto group, substituted mercapto group, primary amine and secondary amines groups or / and substituted primary amine and secondary amines groups, and chain hydrocarbon or and cyclic group containing oxygen, sulphur, nitrogen, carbon or / and phosphor atoms, and one or combination of the substitution groups. The gambogic acid cyclized analog has anti-tumor, antivirus, antibacterial and antifungal pharmacology activities, can be used as anti-tumor, antivirus, immune, antibacterial and antifungal medicaments, and can be applied together with the known anti-tumor, antivirus, immune, antibacterial and antifungal medicaments.
Owner:LIAONING LIFENG SCI & TECH DEV
Use of a varicellovirus tap-inhibitor for the induction of tumor-or virus-specific immunity against teipp
InactiveUS20100092435A1Reduce transportationEffective blockingBiocidePeptide/protein ingredientsAntigenAbnormal tissue growth
The present invention provides a novel approach to the modulation of the immune response, directing it towards specific antigens, away from antigens against which no response is desired. The invention is based on the use of viral immune evasion proteins, such as UL49.5, which block antigen presentation to CD8+ T cells. The viral immune evasion proteins are used for: 1) the induction of tumor-specific or virus-specific immunity in cases where a conventional immune response is absent due to antigen processing defects; 2) the induction of empty MHC class I molecules at the cell surface that can be loaded with peptides of a desired specificity; 3) the inhibition of unwanted immune responses against transplanted tissues or organs, e.g. against islets of Langerhans in type 1 diabetes or allogeneic stem cells, or against self antigens in the case of autoimmunity.
Owner:PUBLIEKRECHTELIJKE RECHTSPERSOON ACADEMISCH ZIEKENHUIS LEIDEN H O D N LEIDS UNIVIR MEDISCH CENT
Immunity enhancing reagent
ActiveCN104887717APromote activationIncreased proliferationViral/bacteriophage medical ingredientsAntibody ingredientsReceptorT lymphocyte
The invention discloses an immunity enhancing reagent comprising AAVs carrying molecule encoding genes of antibodies at immunodetection points, wherein the immunodetection points are one or several from PD-1, PD-L1 and CTLA-4. The AAVs carrying the molecule encoding genes of the antibodies at different immunodetection points are combined with each other, T cells, natural killer cells, cytotoxic T lymphocytes or cell factor induced killer cells and other immune cells are infected in vitro, and the antibodies at the immunodetection points are expressed and secreted after the infected immune cells are fed back to bodies of patients suffering from tumors, so that the combination of receptors at the immunodetection points and ligands of the receptors is blocked, the transfer of an immunosuppression signal is effectively blocked, the immune tumor suppression microenviroment is destroyed, the immune cell killing capacity is improved, and furthermore the curative effect of adoptive immune therapy is improved.
Owner:ICARTAB BIOMEDICAL
Arrayed detector system for measurement of Anti-viral immune response
ActiveUS20100075300A1High sensitivityMaintain robustnessBioreactor/fermenter combinationsBiological substance pretreatmentsVirus-like particleAnti viral immunity
A sensor chip for detecting an immune response against a virus, the sensor chip including a substrate having a surface and a plurality of virus-like particles or capsid fragments bound to discrete locations on the surface of the substrate. Detection devices containing the sensor chip and methods of detecting anti-viral immune responses are also described herein.
Owner:UNIVERSITY OF ROCHESTER
Expression and application of bovine viral diarrhea virus (BVDV) type I E2 protein
InactiveCN105949286AStrong specificityIncreased sensitivityPolypeptide with localisation/targeting motifSsRNA viruses positive-sensePurification methodsBovine Viral Diarrhea Viruses
The invention relates to expression and application of bovine viral diarrhea virus (BVDV) type I E2 protein. The expression and the application have the advantages that (1) an expression and purification method for efficiently obtaining BVDV E2 protein is established and can be used for preparing the pure BVDV E2 protein; (2) a rabbit is immunized by the BVDV E2 protein to prepare an antibody; compared with an antibody prepared by whole-virus immune cattle, the antibody has good specificity and shallow background and is easy to determine; a secondary antibody is marked by FITC (Fluorescein Isothiocyanate) and an indirect fluorescence method is established, so that the sensitivity is better; the operation is simple: a pre-mixed solution is adopted so that operation time is shortened; (3) the pure BVDV E2 protein is used for covering, sealing conditions, blood serum and antibody preserving fluid are optimized, and a BVDV antibody indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) method is established; a BVDV antibody indirect ELISA kit, which is sensitive and low in price, is assembled and a situation of dependence on imported BVDV antibody ELISA kits for a long period can be changed.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Immunity detection chip of prawn white spot syndrome virus (WSSV) and preparation method thereof and application
ActiveCN101629954ARealize simultaneous parallel detectionSimple requirementsFluorescence/phosphorescenceFluorescencePrawn
The invention discloses an immunity detection chip of prawn white spot syndrome virus (WSSV), comprising a chip carrier and an agarose gel layer which is paved on the chip carrier, wherein, the agarose gel layer is fixed with a plurality of antibody microarrays of 4*4, and chip-dedicated fences or Super PAP Pen scribings are used for separating the microarrays from each other. The invention comprises the following steps: adopting the sandwich method to detect the antigen; fixing the pathogenic polyclonal antibody (PcAb) on the chip slice base; taking the target organ tissue of the individual to be detected to prepare to-be-detected sample liquid; incubating directly the to-be-detected sample liquid with the chip which is fixed with PcAb to capture the antigen and lead the antigen to combine on the chip; adding a specific monoclonal antibody probe marked by fluorescence; and reading results through a CCD chip scanner. The invention has the advantage of detecting white spot syndrome virus (WSSV) in multiple samples simultaneously, and is applicable to the rapid and accurate detection of white spot syndrome virus (WSSV) of prawns / crabs in breeding production and the quarantine inspection of WSSV in import and export prawns / crabs.
Owner:OCEAN UNIV OF CHINA
Adjuvanted formulations of rabies virus immunogens
InactiveUS20150030630A1Higher immune titersImprove protectionSsRNA viruses negative-senseViral antigen ingredientsAdjuvantTlr agonists
The efficacy of rabies vaccines can be enhanced by adjuvanting rabies virus immunogens with a mixture of a TLR agonist (preferably a TLR7 agonist) and an insoluble metal salt (preferably an aluminium salt). The TLR agonist is typically adsorbed to the metal salt. The rabies virus immunogen can also be adsorbed to the metal salt.
Owner:NOVARTIS AG
Sabin strain poliovirus type III monoclonal antibody and application thereof
ActiveCN104371980AHigh titer reactivityHigh School and ValenceImmunoglobulins against virusesMicroorganism based processesEnterovirusPoliomyelitis
The invention provides a Sabin strain poliovirus type III monoclonal antibody and an application thereof, and belongs to the field of immunology. After a mouse is immunized and inoculated with Sabin strain poliovirus type III, mouse spleen cells are fused with mouse myeloma cells, a hybridoma cell strain producing the anti-Sabin strain poliovirus type III specific monoclonal antibody is screened and has the preservation number of CGMCC No.9233, and the secreted monoclonal antibody has high titer, has strong neutralizing activity, and can effectively block infection of the poliovirus type III; at the same time, the antibody can specifically distinguish the poliovirus type III from poliovirus type I, poliovirus type II and other various enteroviruses, has good specificity, and can be used for preparing poliovirus type III antigen content detection kits and antibody detection kits, also can be used for identification detection of the poliovirus type III, and has the broad application prospect.
Owner:SINOVAC BIOTECH
Preparation method for goose-origin reovirus inactivated vaccine
InactiveCN106540250AInfection Prevention and ControlViral antigen ingredientsAntiviralsReovirus RNAOil phase
The invention provides a preparation method for a goose-origin reovirus inactivated vaccine. The preparation method comprises the following steps that a goose embryo is inoculated against reoviruses through an allantoic cavity, dead embryo allantoic fluids or live embryo allantoic fluids are collected within 24-144 h after incubation, and reovirus fluids are obtained; the reovirus fluids are inactivated through formaldehyde, Tween-80 is added to be mixed to serve as a water phase, and white oil, Span-80 and aluminium stearate are mixed to serve as an oil phase; and the water phase is added into the oil phase to be mixed uniformly, and the inactivated vaccine is obtained. According to the preparation method, the prepared goose-origin reovirus inactivated vaccine has high protective effects on a gosling, and is suitable for virus immunity of the gosling.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Multi-virus immunodetection device and method based on smart phone and micro-fluidic chip
ActiveCN111693694AReal-time displayReduce testing costsLaboratory glasswaresBiological testingAntigenSample purification
The invention discloses a multi-virus immunodetection device and method based on a smart phone and a micro-fluidic chip. The device is used for carrying out multi-virus immunodetection on a blood sample infected with viruses of a human body, a horizontally arranged mobile phone slot is formed in the middle of the upper surface of the shell, a horizontally arranged detection device, a reaction chipand a reagent chip are sequentially arranged above the mobile phone slot, and the reagent chip is driven by a lifting device to vertically move up and down. Reagent bottles with different depths andpenetrable adhesive films at the bottoms are designed, and separation and coupling of the reagent bottles and a sharp tube on the reaction chip is realized, and sample purification, incubation, colordevelopment and the like are integrated on one chip. The square air cavity is controlled to contract and expand by the electromagnetic clamp, so that the requirement of driving liquid to flow in different reaction steps is met, and the existing microplate reader can be replaced by only one smart phone for absorbance detection. The reaction chip is independent of other structures, different antibodies or antigens can be coated in reaction tubes in different reaction chips, and multi-virus detection is realized.
Owner:JIANGSU UNIV
Recombinant porcine pseudorabies virus strain used for expression of porcine circovirus type II (PCV2) ORF2 gene, and preparation method thereof
ActiveCN103509761AHigh antibody titerResist attackMicroorganism based processesViruses/bacteriophagesCircovirusMouse Lymphocyte
The invention discloses a recombinant porcine pseudorabies virus (Herpesviridae) strain used for expression of porcine circovirus type II ORF2 gene. Preservation number of the recombinant porcine pseudorabies virus strain is CCTCC No.V201315. According to the preparation method, PCV2ORF2 gene is inserted into common carrier PG so as to construct transferring plasmid PGO; monolayer ST cells are inoculated with PRVTK<-> / gG<-> / gE<-> virus by 2h of adsorption; ST cells are transfected with plasmid PGO, wherein fusion degree of the monolayer ST cells is 80 to 90%; the recombinant porcine pseudorabies virus PGO strain is obtained by plaque purification, and is used for immunization of mouse. It is shown by results that commercial PCV2 inactivated vaccine and a group immunized by the recombinant porcine pseudorabies virus PGO strain are both capable of inducing specific humoral immune response of mouse, antibody titers of both the commercial PCV2 inactivated vaccine and the group are obviously higher than that of a group immunized by DMEM medium, and difference is significant (p<0.05). It is shown by the results of mouse lymphocyte proliferation test that specific ceullar immune response caused by the group immunized by the recombinant porcine pseudorabies virus PGO strain is more obvious than that caused by the commercial PCV2 inactivated vaccine and the group immunized by DMEM medium, and difference is obvious (p<0.05). In addition, the group immunized by the recombinant porcine pseudorabies virus PGO strain is capable of resisting severe attack by PCV2. Therefore application of the recombinant porcine pseudorabies virus strain in development of a novel PCV2 vaccine is possible.
Owner:HENAN AGRICULTURAL UNIVERSITY
Viral immunotherapy drug compound and purpose thereof
InactiveCN104338132AEnhance immune responseAvoid unresponsiveness andOrganic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention involves a new viral immunotherapy drug complex, and in particular, a viral immunotherapy drug complex for persistent hepatitis B infection. The present drug consists of antiviral drugs, immunoregulating drugs and a recombinant hepatitis B vaccine, for use in treating hepatitis B and in particular chronic hepatitis B infections. Antiviral drugs of the described drug complex are selected among α-IFN and nucleosides; the immunoregulating drugs are selected from among GM-CSF and similar.
Owner:FUDAN UNIV
New coronavirus SARS-CoV-2 broad-spectrum polypeptide antigen, specific neutralizing antibody thereof and application
ActiveCN113354717ASsRNA viruses positive-senseAntibody mimetics/scaffoldsPeptide antigenViral antibody
The invention provides a new coronavirus SARS-CoV-2 broad-spectrum polypeptide antigen, a specific neutralizing antibody thereof and application, and belongs to the technical field of virus immunodetection. The new coronavirus SARS-CoV-2 broad-spectrum polypeptide antigen has an amino acid sequence shown as SEQ ID NO: 1, and can be specifically combined with a new coronavirus antibody through reaction with SARS-CoV-2 human positive serum. Based on the polypeptide sequence, triple SARS-CoV-2 broad-spectrum polypeptide tandem fusion protein is prepared by utilizing PCR, prokaryotic expression and protein purification technologies, a trimer mode of SARS-CoV-2S protein in a natural state is simulated, the fusion protein is used as an antigen to immunize mice, the SARS-CoV-2 specific neutralizing antibody can be generated, and the neutralizing antibody has good application prospects in SARS-CoV-2 anti-infection treatment, vaccine research and development and detection kit development.
Owner:YANGZHOU UNIV
Method of enhancing virus-resistance in plants and producing virus-immune plants
InactiveUS20040068764A1Lower Level RequirementsDelayed and reduced spreadSsRNA viruses positive-senseMicrobiological testing/measurementBiotechnologyNucleotide
The present invention provides a method of enhancing resistance of plants to one or multiple viruses, comprising introducing to a plant cell, and preferably expressing therein, a nucleotide sequence encoding a virus-encoded polypeptide. The present invention provides a method of enhancing the proportion of virus-resistant or virus-immune lines obtained from a single transformation experiment comprising introducing to a plant cell, a nucleotide sequence encoding a virus-encoded polypeptide operably in connection with a strong promoter sequence. The present invention provides novel gene sequences encoding the coat proteins of a virus and novel dysfunctional replicase sequences as well as gene constructs comprising same, in particular binary vector constructs suitable for introducing into plants and expressing the genes therein. The present invention provides and methods using same to enhance viral resistance in plants. The present invention provides novel methods of testing transgenic plants for the presence of a transgene.
Owner:DAIRY AUSTRALIA +2
Viral Immunogenic Compositions
Disclosed herein are immunogenic compositions for producing immediate and sustained immunity to infectious viral and bacteriological pathogens. A univalent immunogenic composition is disclosed comprising an isolated antigen and a polynucleotide formulated into a nanoparticle or liposome. Furthermore, multivalent immunogenic compositions are disclosed comprising multiple univalent immunogenic compositions. Also disclosed, are methods of inducing protective or therapeutic immune responses in individuals comprising administering one or more univalent immunogenic compositions.
Owner:SMITH HENRY J +1
Virus immune computer system and method
ActiveUS10592697B1Disabling spreadAvoid spendingDigital data protectionInternal/peripheral component protectionSoftware engineeringHacker
A method and apparatus prevents hacker code from infecting an application program by requiring decryption of the application program prior to running the application program on a computer. The method includes steps of: providing a security device that is a separate unit from components necessary to operate the computer; storing a symmetric private key on the security device; using the device symmetric private key to produce an encrypted application program upon first installation; thereafter decrypting that part of the encrypted application program needed implement a command to run the application program; and, decrypting, on the fly, only those follow-on parts of the encrypted application program needed to perform functions called for during operation of the application program.
Owner:ATENSE INC
Method for preparing avian influenza virus and inactivated vaccine thereof with Vero passage cells
ActiveCN102586195AReduce manufacturing costAntiviralsViruses/bacteriophagesHemagglutininVaccine Production
The invention discloses a method for preparing an avian influenza virus and an inactivated vaccine thereof with Vero passage cells. According to the method disclosed by the invention, the Vero passage cells are adopted to replace chicken embryo to culture influenza virus, so that the problems of chicken embryo remnant and exogenous virus inflection are solved and the immunogenicity of the cultured virus is more stable. On the other hand, for avoiding the phenomenon that the cracking of HA is influenced because protease is acted by an inhibitor in a maintaining liquid to inactivate, pancreatinis coated with chitosan and then is added in a cell maintaining liquid, so that the pancreatin is slowly released in the maintaining liquid and the defect that the cracking of hemagglutinin is influenced because the pancreatin is inactivated in the reproduction process of viruses is overcome. In addition, the probability that the cells are polluted since the pancreatin is added for multiple timesis avoided. The virus cultured by the method disclosed by the invention is high in titer and favorable in stability and is suitable for large-scale vaccine production.
Owner:哈药集团生物疫苗有限公司
Monoclonal antibody for duck tembusu virus and application
ActiveCN104749368AStrong specificityHigh sensitivityBiological material analysisImmunoglobulins against virusesBALB/cTembusu virus
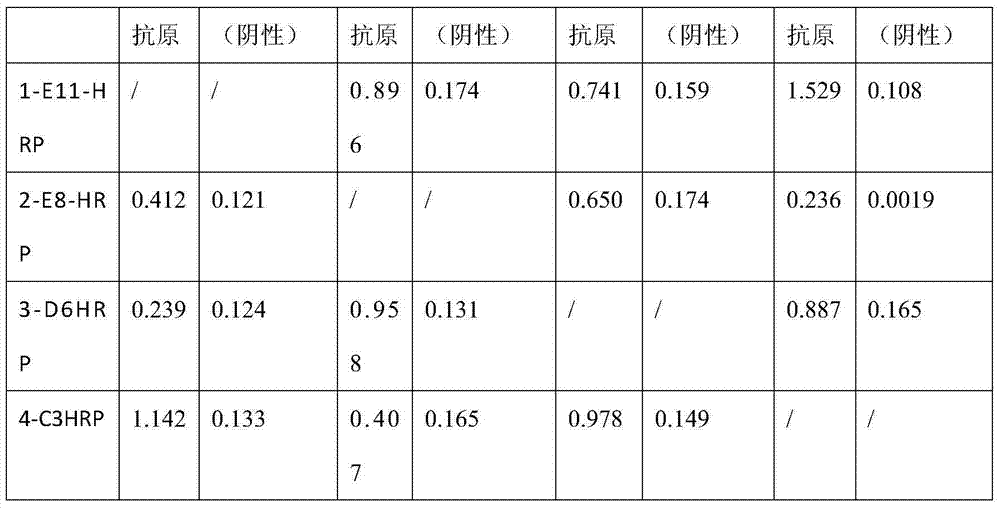
The invention provides a monoclonal antibody for a duck tembusu virus and application. A hybridoma cell strain 1-E11 with the collection number CCTCCNO being C2014219 and a hybridoma cell strain 4-C3 with the collection number CCTCCNO being C2014220 of the monoclonal antibody resisting the duck tembusu virus are obtained by using a purified duck-tembusu-virus immunized Balb / c mouse; a colloidal-gold test strip is prepared by utilizing the monoclonal antibody secreted by the cell strains, the 1-E11 is adopted as a labeled monoclonal antibody, 4-C3 is used as a coated monoclonal antibody, and the obtained test strip has the characteristics of fast and sensitive detection and good specificity. A result is clear and the judgment is easy; the carry and the use are convenient, and the detection cost for disease is saved.
Owner:HUAZHONG AGRI UNIV +1
Ectodomains of influenza matrix 2 protein, expression system, and uses thereof
The present invention provides a polynucleotide, polypeptide, recombinant modified vaccinia virus Ankara (rMVA) and related vaccine compositions and methods useful in the prevention and treatment of an influenza viral infection. Provided is an isolated polynucleotide encoding multiple copies of M2 infuenza ectodomain peptides or rMVA comprising the polynucleotide. Also provided are methods for inducing an immune response to a subject against an influenza virus or for treating a disease or symptom caused by or resulting from infection with an influenza virus.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Sabin strain poliovirus type II monoclonal antibody and application thereof
ActiveCN104371979AHigh titer reactivityHigh School and ValenceMicroorganism based processesImmunoglobulins against virusesEnterovirusMicrobiology
The invention provides a Sabin strain poliovirus type II monoclonal antibody and an application thereof, and belongs to the immunology field. After a mouse is immunized and inoculated with Sabin strain poliovirus type II, mouse spleen cells are fused with mouse myeloma cells, a hybridoma cell strain producing the anti-Sabin strain poliovirus type II specific monoclonal antibody is screened and has the preservation number of CGMCC No.9232, and the secreted monoclonal antibody has high titer, has strong neutralizing activity, and can effectively block infection of the poliovirus type II; at the same time, the antibody can specifically distinguish the poliovirus type II from poliovirus type I, poliovirus type III and other various enteroviruses, and can be used for preparing type II antigen content detection kits and antibody detection kits of the poliovirus type II and a poliomyelitis inactivated vaccine, also can be used for identification detection of the poliovirus type II, and has the broad application prospect.
Owner:SINOVAC BIOTECH
Methods and compositions for combination immunotherapy
ActiveUS20180187211A1Permit immunizationOvercome obstaclesOrganic active ingredientsTumor rejection antigen precursorsVaccinationPreexisting immunity
Methods for generating immune responses using adenovirus vectors that allow multiple vaccinations with the same adenovirus vector and vaccinations in individuals with preexisting immunity to adenovirus are provided.
Owner:ETUBICS CORP
Novel coronapneumonia virus receptor ACE2 affinity adsorbent as well as preparation method and application thereof
PendingCN111992186ASimple structureRaw materials are easy to getOther chemical processesDialysis systemsAptamerCarboxyl radical
The invention provides a novel coronapneumonia virus receptor ACE2 affinity adsorbent as well as a preparation method and application thereof. The novel coronapneumonia virus receptor ACE2 affinity adsorbent comprises a carrier microsphere and a novel coronapneumonia virus receptor ACE2 aptamer combined on the surface of the carrier microsphere. The surface of the carrier microsphere is modified by amino, the surface of the novel coronapneumonia virus receptor ACE2 aptamer is modified by carboxyl, and the amino on the surface of the carrier microsphere and the carboxyl on the surface of the novel coronapneumonia virus receptor ACE2 aptamer form a stable amido bond. The virus adsorbent has the advantages of simple structure, easily available raw materials, high coupling efficiency and stability, simple and controllable preparation method, one-step synthesis, high yield and good development prospect. The aptamer can be recycled after being recovered and enriched, so that the cost is greatly reduced. The novel coronavirus receptor ACE2 virus adsorbent has a huge application prospect in the field of novel coronavirus immune adsorption.
Owner:WUHAN RUIFA MEDICAL DEVICES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com