Ectodomains of influenza matrix 2 protein, expression system, and uses thereof
A technology of influenza virus and ectodomain, applied in the direction of viruses, viral peptides, antiviral agents, etc., can solve problems such as insufficient efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0282] Embodiment 1 recombinant vector construction
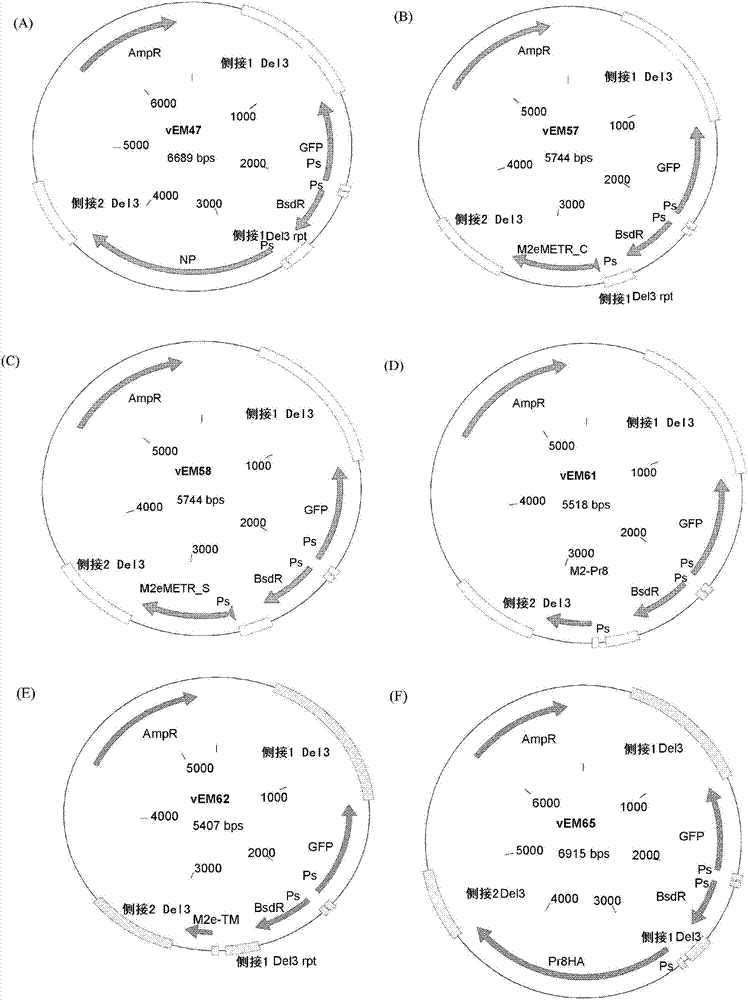
[0283] Type A influenza virus gene Pr8HA sequence (SEQ ID NO:51-52), NP consensus sequence (SEQ ID NO:19-20), Pr8M2 (SEQ ID NO:13-14), Pr8M2e_TML (SEQ ID NO:53-54) , METR_S (SEQ ID NO:17-18 and 56) and METR_C (SEQ ID NO:15-16 and 55) were cloned into the vEM11 recombinant vector ( figure 1 ). The resulting recombinant vectors vEM47 (encoding NP consensus sequence), vEM58 (encoding METR_S peptide), vEM57 (encoding METR_C peptide), vEM61 (encoding Pr8M2), vEM62 (encoding Pr8M2e-TML) and vEM65 (encoding Pr8HA) are shown in Figure 2A-F .
Embodiment 2
[0284] Example 2 Homologous recombination and recombinant virus isolation
[0285] For the modified MVA virus vector MVAtor TM (EBS company (Emergent Biosolutions)) into the influenza virus gene, with MVAtor to infect CEF cells and then with Figure 2A-F Transfection with recombinant vectors indicated in . 2-3 sets of parallel settings were carried out in each carrier. First, 5X10 5 CEF cells were incubated at 37°C and 5% CO 2 Incubate for 24 hours. The next day, the cell density was assumed to be 10 6 cell. The MVAtor standard was diluted in Opti-Pro SFM containing 4 mM L-glutamine and 2.5 μg gentamicin per ml so that 500 μl contained 5X10 4 TCID 50 , that is, the working concentration is 1X10 5 TCID 50 / ml and yielded a moi of 0.05. For infection, growth medium was removed from cells, 500 μl of diluted MVAtor standard was added to each well and incubated for one hour at room temperature with shaking. Virus inoculum was removed and cells were washed with Opti-Pro ...
Embodiment 3
[0292] Embodiment 3 is used for getting rid of the PCR of empty vector contamination
[0293] To confirm that residual non-recombinant MVAtor was completely excluded from the filled vials of the purified virus stock, samples from purified and filled MVAtor-NP for mEM10, MVAtor-METR_C for mEM18, MVAtor-METR_S for mEM19, MVAtor-METR_S for mEM19, DNA for MVAtor-Pr8M2 for mEM22, or MVAtor-Pr8M2e_TML for mEM23 was isolated and used for PCR. The recombinant vector vEM47 was used as a positive control for the recombinant virus (vEM47). DNA isolated from MVAtor was used as a positive control for empty vector (MVA). Use H 2 O and CEF cells were used as negative controls.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com