Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
548 results about "Humoral immune reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An incompatible blood transfusion causes a transfusion reaction, which is mediated by the humoral immune response. This type of reaction, called an acute hemolytic reaction, results in the rapid destruction (hemolysis) of the donor red blood cells by host antibodies.
Methods and compositions using immunomodulatory compounds for the treatment of immunodeficiency disorders
Methods of treating, preventing and / or managing an immunodeficiency disease or disorder are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active agent. Methods of boosting humoral immunity are also disclosed.
Owner:CELGENE CORP
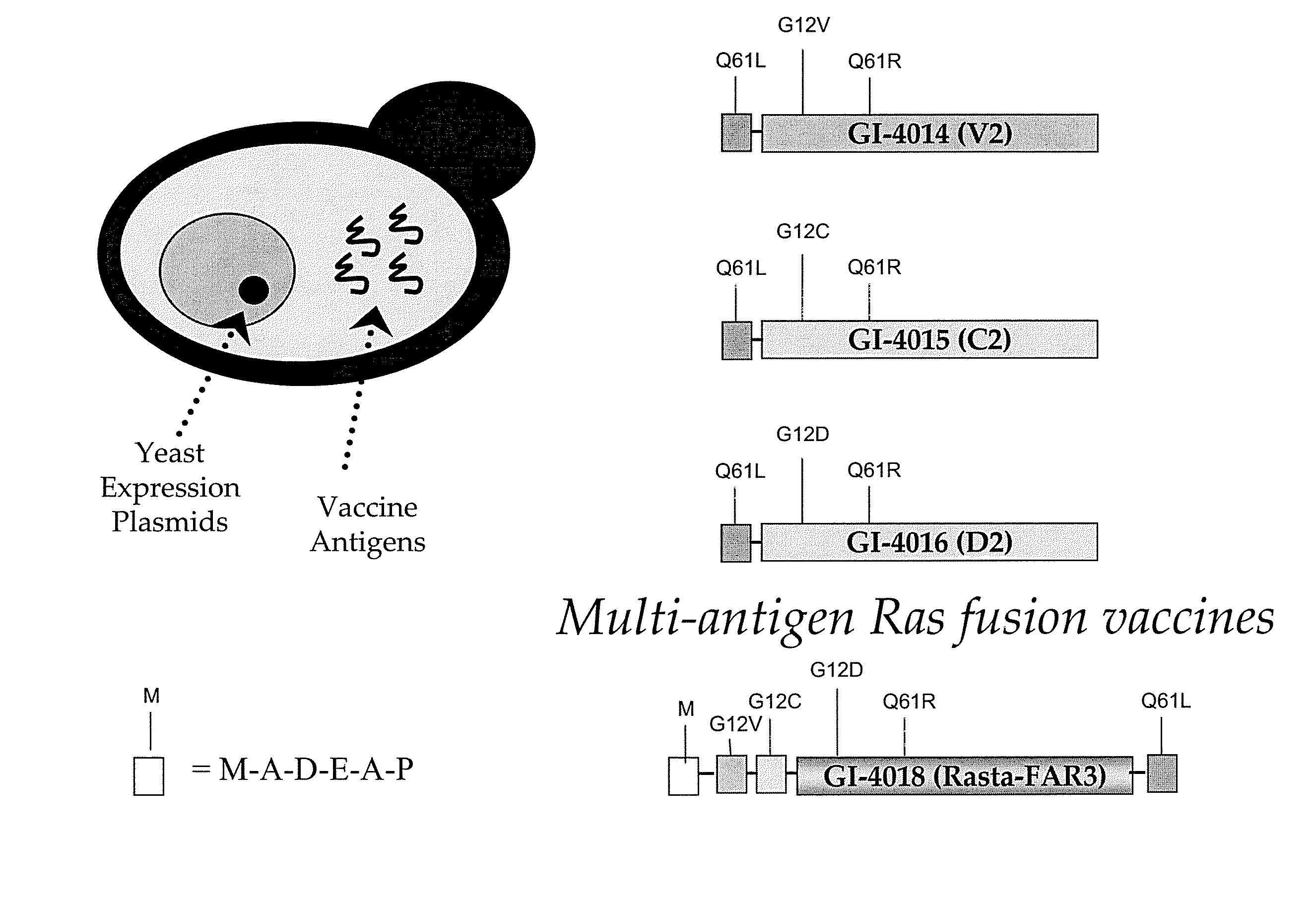
Yeast-based vaccines as immunotherapy
InactiveUS7465454B2Enhance immune responseExtended half-lifeBiocideAntibody mimetics/scaffoldsYeastDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
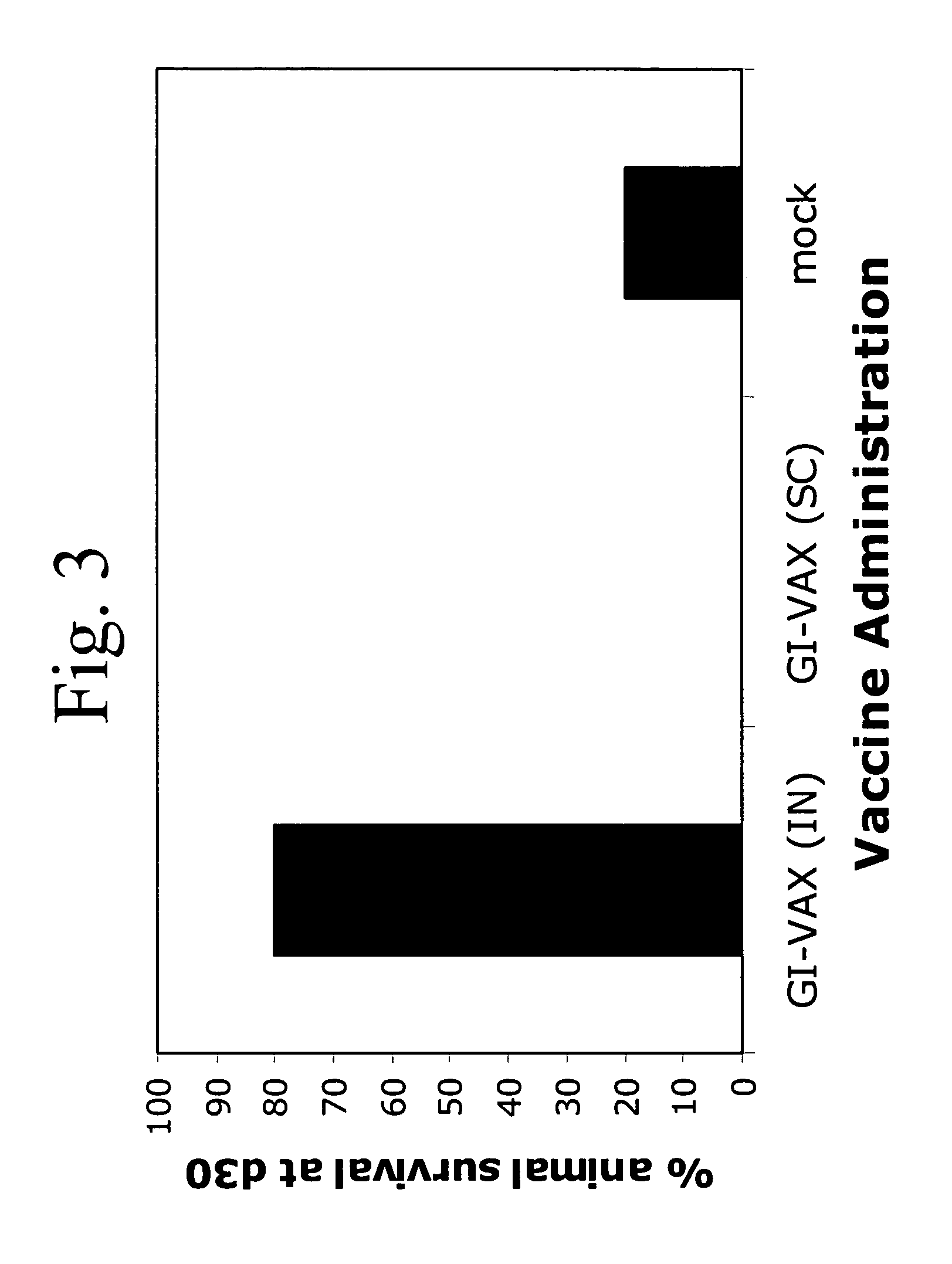
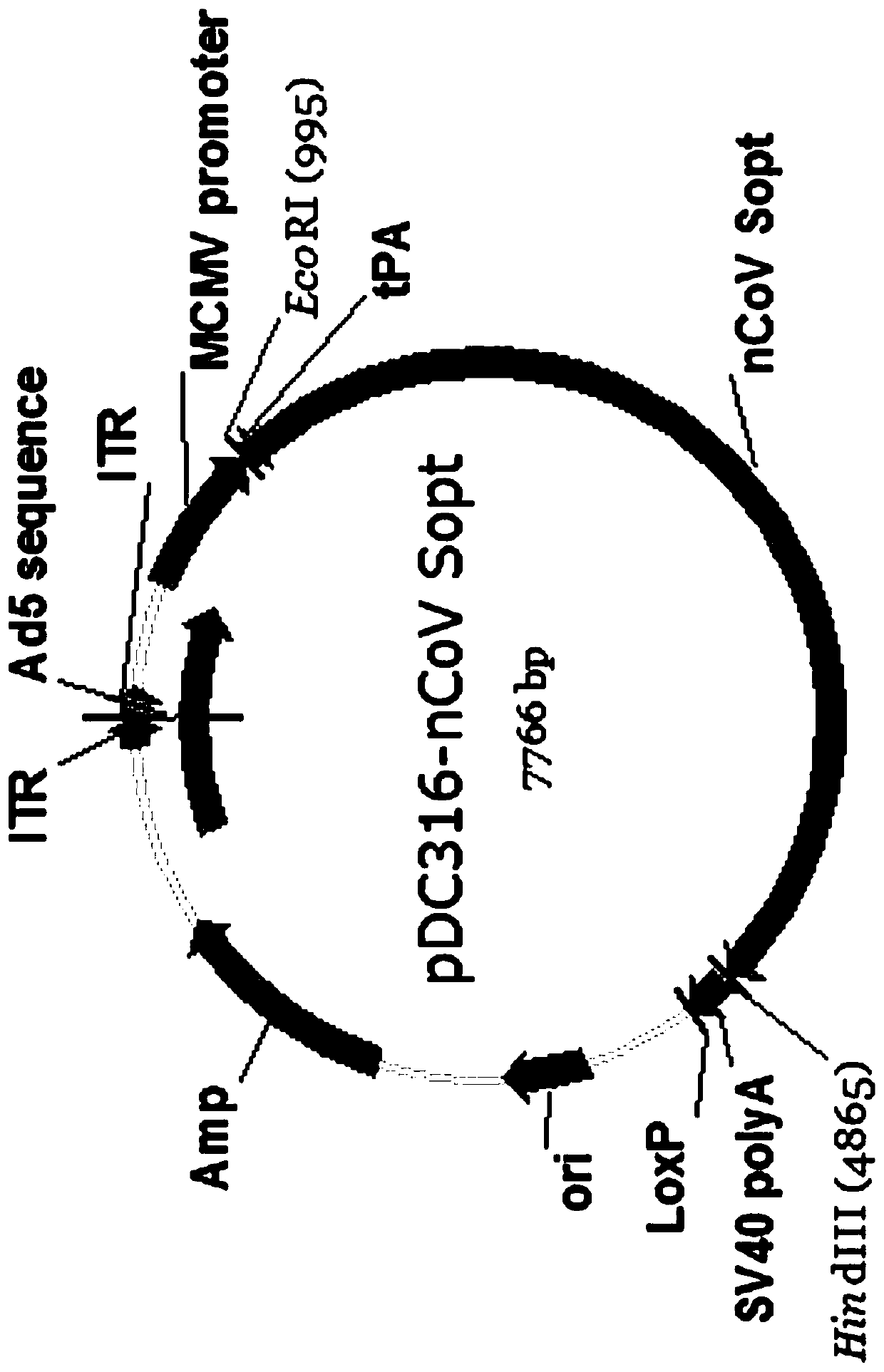
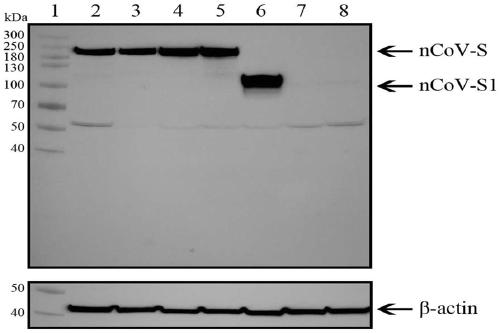
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Yeast-dendritic cell vaccines and uses thereof
InactiveUS20060104986A1Enhance immune responseExtended half-lifeBiocideViral antigen ingredientsBody fluidHumoral immune reaction
Disclosed is a vaccine that includes a dendritic cell loaded with a yeast vehicle and antigen. Also disclosed are methods of making the vaccine and using the vaccine to elicit cellular and humoral immune responses in a mammal. Additionally, a method to elicit an immune response by administration of a yeast vehicle and an antigen that is not complexed to the yeast vehicle is disclosed.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Immunization of infants
InactiveUS6204250B1Prevent side-effectsUndesirable effectSsRNA viruses negative-senseAntibacterial agentsTarget antigenImmunogenicity
The present invention relates to methods and compositions which may be used to immunize infant mammals against a target antigen, wherein an immunogeniclly effective amount of a nuclic acid encoding a relevant epitope of a desired target antigen is administered to the infant. It is based, at least in part, on the discovery that such genetic immunization of infant mammals could give rise to effective cellular and humoral immune responses against target antigens.
Owner:MOUNT SINAI MEDICAL CENT OF THE CITY OF NEW YORK THE
Recombinant adeno-associated virus vector for treatment of Alzheimer disease
ActiveUS8318687B2Highly safe treatmentReduce concentrationNervous disorderVirusesAdeno associate virusDna encoding
Owner:TABIRA TAKESHI +1
Immunogenic compositions and methods of using the compositions for inducing humoral and cellular immune responses
ActiveUS20120328655A1Reduce the possibilityAntibacterial agentsAntimycoticsInfectious DisorderLymphocyte
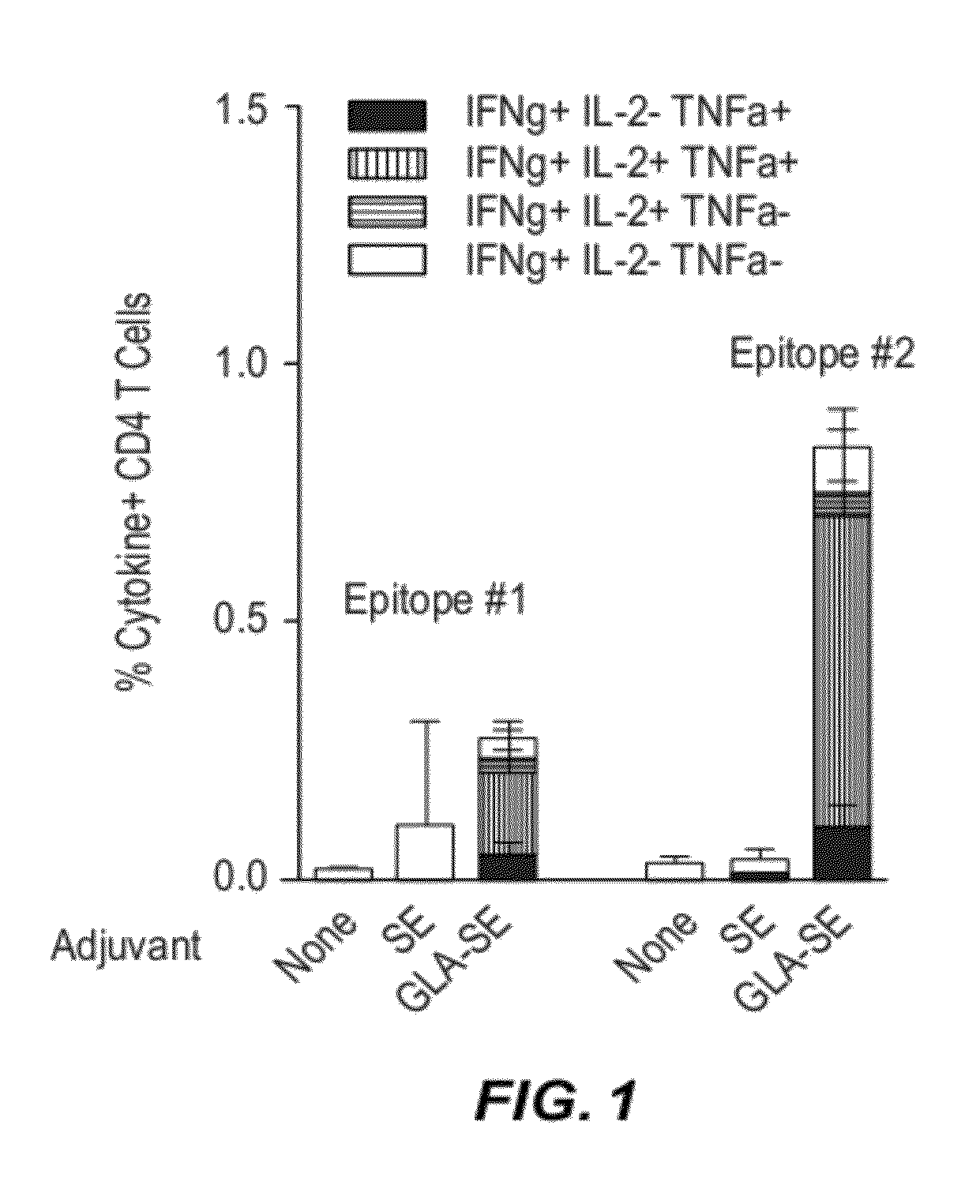
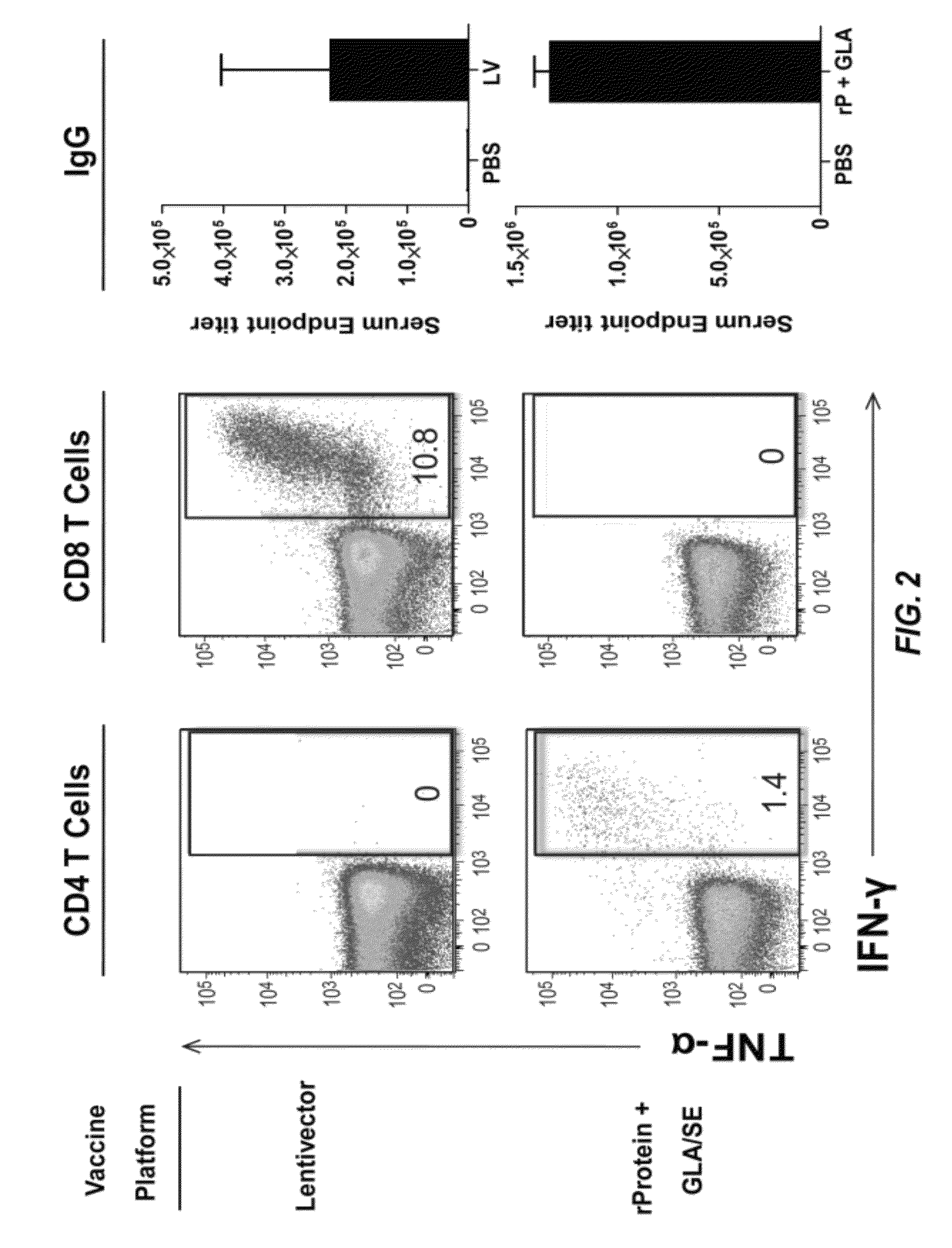
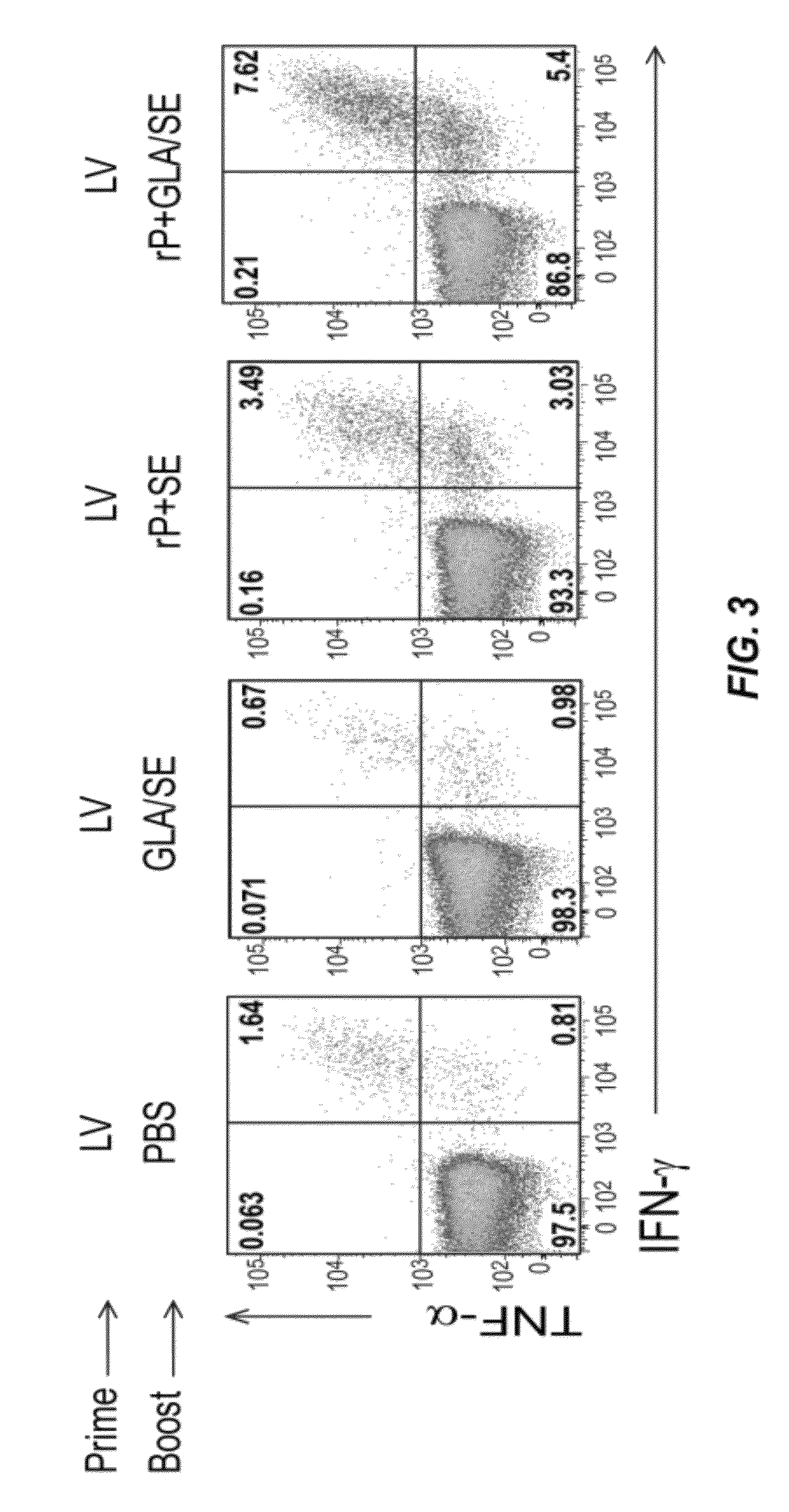
Compositions and methods are provided herein for improved dual immunization strategies that induce in a subject an immune response that includes a humoral immune response and cellular immune response, both CD4 and CD8 T lymphocyte immune responses, thereby providing a complete adaptive immune response to one or more antigens. The methods described are therefore useful for treating and / or preventing (i.e., reducing the likelihood or risk of occurrence) different diseases, disorders, and conditions such as cancers and infectious diseases for which induction of both a humoral immune response and cellular immune response is desired and beneficial.
Owner:IMMUNE DESIGN CORP
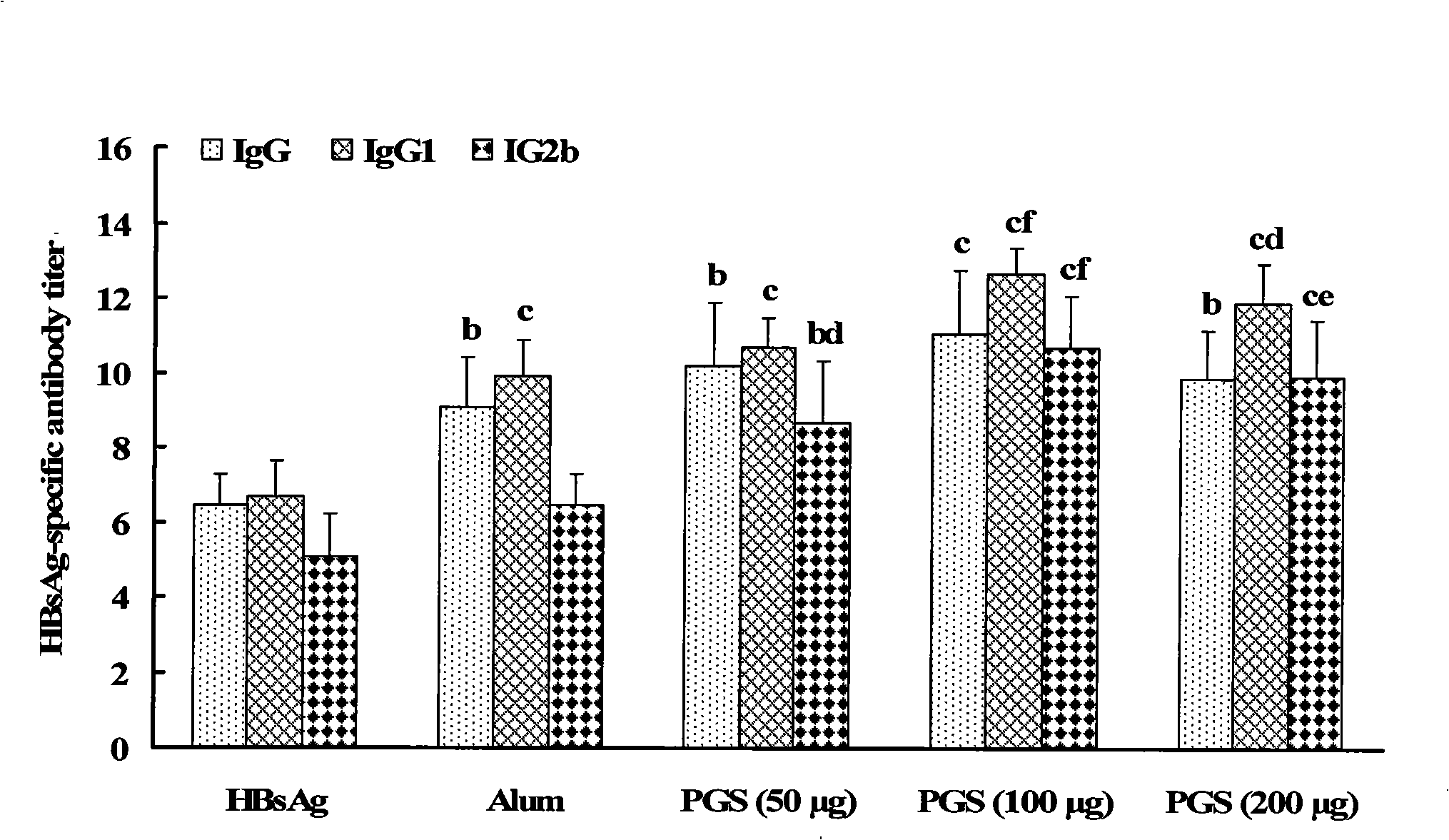
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
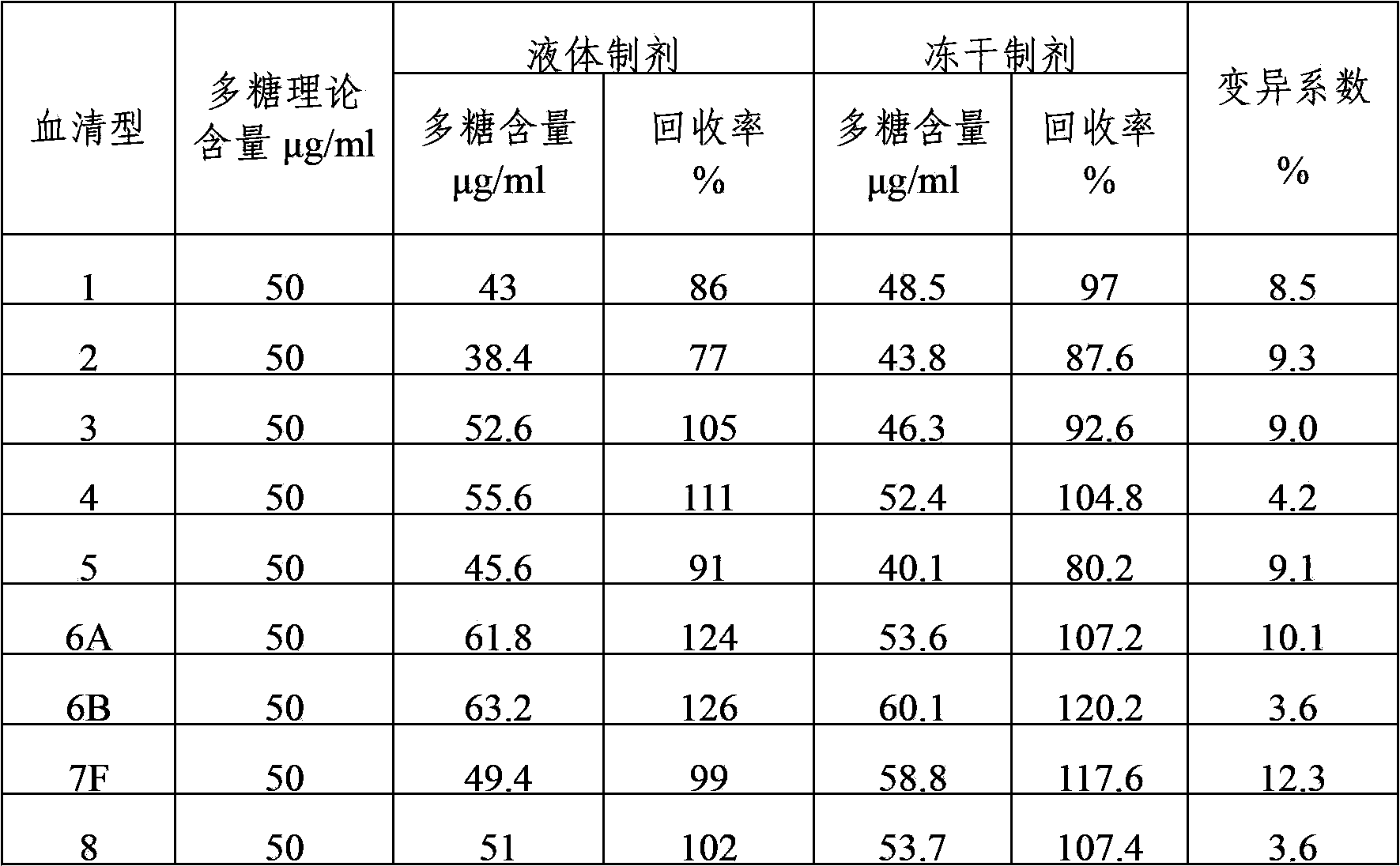
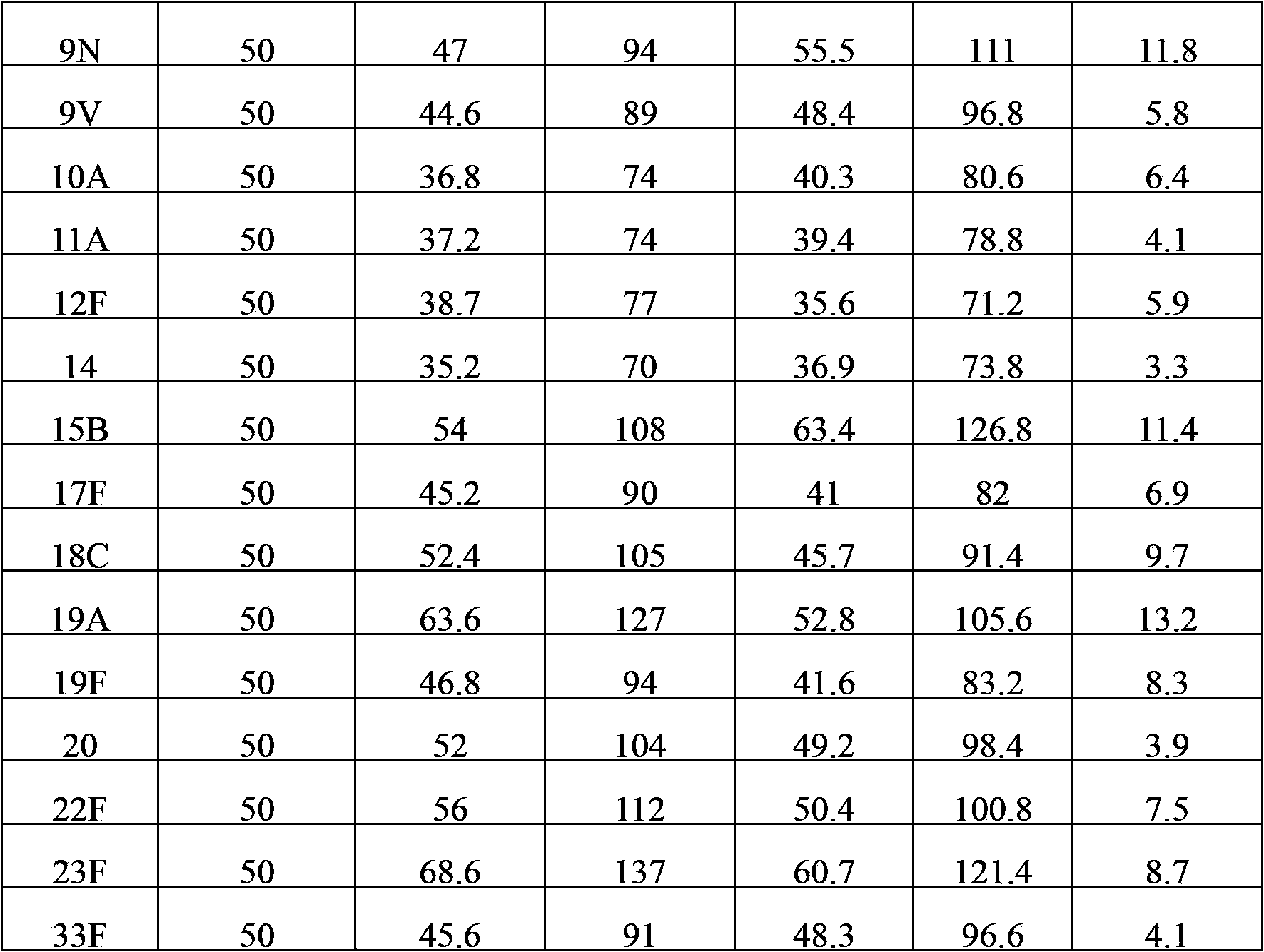
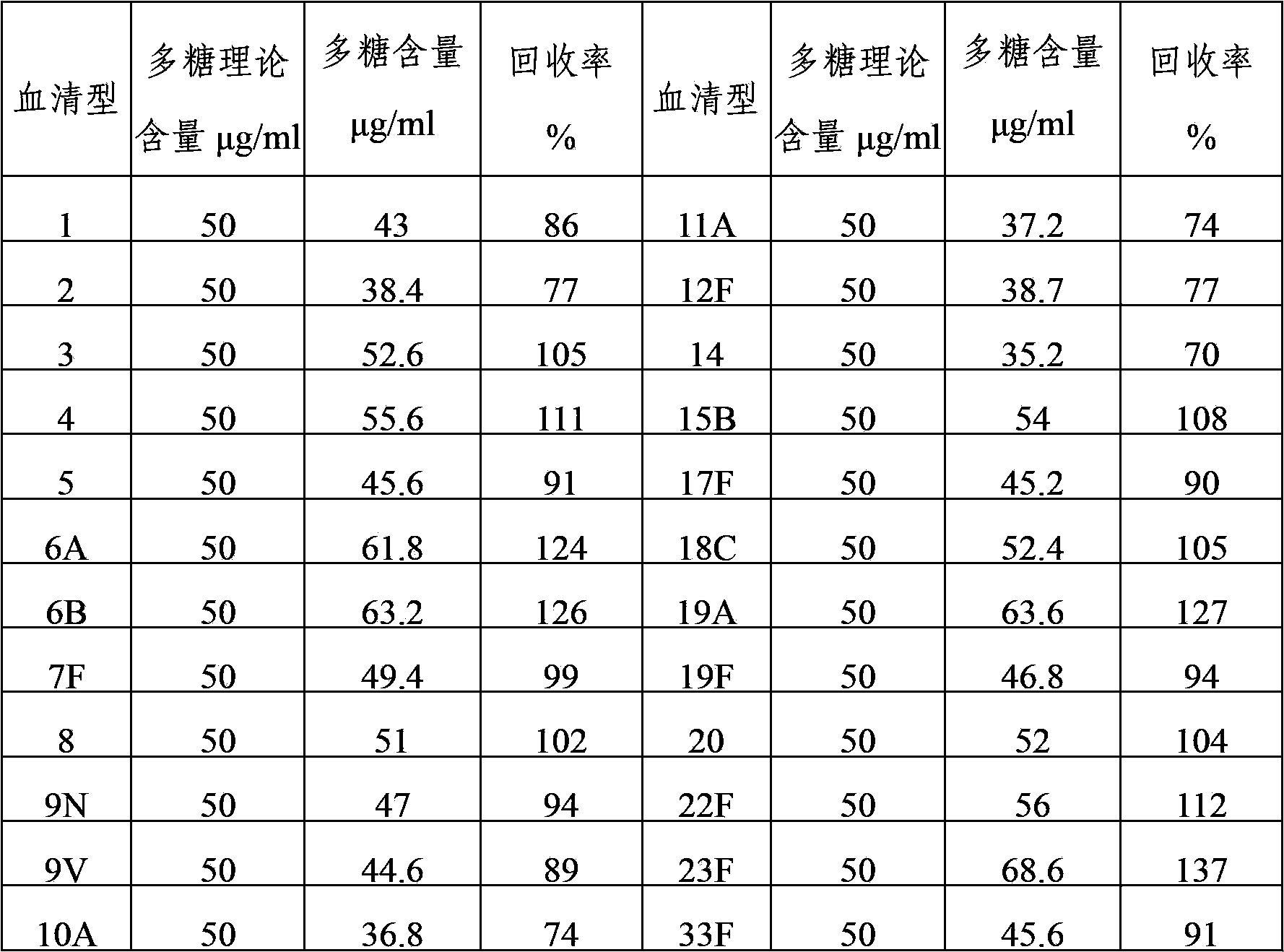
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Method for extracting ganoderma lucidum fruitbody polysaccharide using ultrahigh pressure
InactiveCN101357951AHigh yieldReduce extraction timeAntinoxious agentsImmunological disordersPhagocyteMicroorganism
The invention discloses a method for extracting ganoderma lucidum fruit body polysaccharides by using superpressure; the ganoderma lucidum fruit body polysaccharides are new endogenetic active materials extracted from the ganoderma lucidum fruit bodies. The ingredients of the ganoderma lucidum polysaccharides can improve the phagocytosis of phagocytes, the humoral immunity and cellular immune function, which can improve the SOD activity of superoxide dismutases in red blood cells in human bodies. However, the ganoderma lucidum cells are compact, and the polysaccharides are difficult to be extracted from ganoderma lucidum. The methods of extraction which are most used currently are defective. The method for extracting ganoderma lucidum fruit body polysaccharides comprises the steps of selecting ganoderma lucidum fruit bodies, adding extracting solution, sealing and immersing the ganoderma lucidum fruit bodies in the extracting solution, treating the ganoderma lucidum fruit bodies under superpressure, filtration and condensation, drying the condensed filtrate, getting the polysaccharides from the ganoderma lucidum fruit bodies. High temperature does not occur during the extraction; active ingredients in the ganoderma lucidum fruit bodies can be preserved; the extraction takes little time with high yield and low impurity content. In addition, some microbes can be killed through pressurization to complete extraction and sterilization at one step. The method for extracting ganoderma lucidum fruit body polysaccharides overwhelms the defects of the prior methods for extracting polysaccharides from ganoderma lucidum.
Owner:INFINITUS (CHINA) CO LTD +2
Yeast-based vaccines as immunotherapy
ActiveUS20080069833A1Prevents posttranslational modificationEnhance immune responseBiocideAntibody mimetics/scaffoldsBody fluidHumoral immune reaction
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
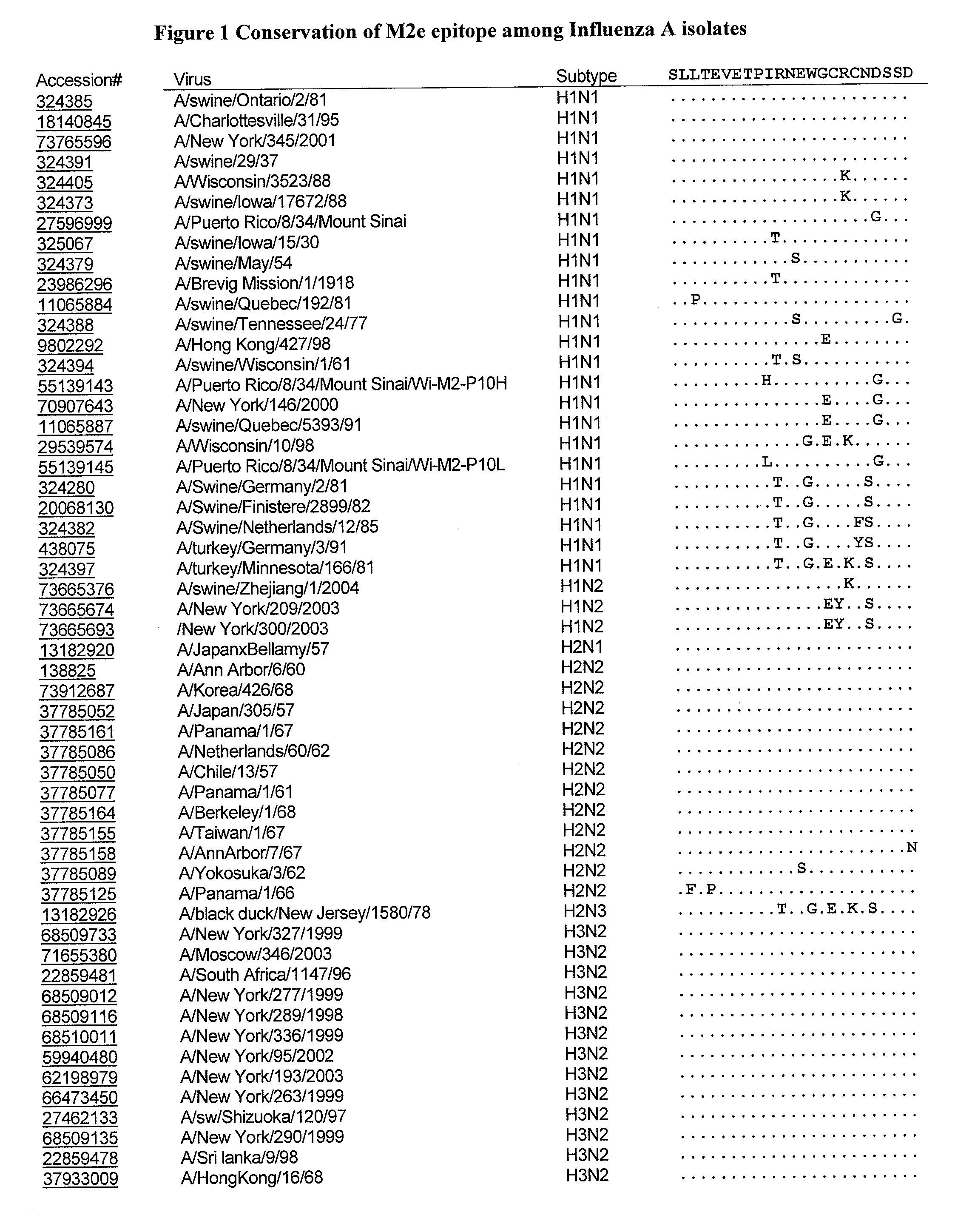
Composition and methods of making and using influenza proteins
InactiveUS20090196915A1Many symptomReduce the likelihood of infectionSsRNA viruses negative-sensePowder deliveryImmuno modulationBody fluid
The invention provides compositions of influenza proteins, such as matrix and nucleoprotein, that are presented to an individual's immune system as multimeric displays to induce an immune response. The compositions are optionally associated with any type of immunomodulatory compound (IMC) comprising an immunostimulatory sequences (ISS). The invention further provides compositions of influenza matrix and nucleoproteins that can induce cellular and / or humoral immune response. The invention also provides methods of making and using these compositions, e.g., as a vaccine, for ameliorating symptoms associated with infection with influenza virus or for reducing the risk of infection with influenza virus.
Owner:DYNAVAX TECH CORP
Yeast-Based Vaccines As Immunotherapy
ActiveUS20110150909A1Reduce or prevent symptomPrevent modifyingAntibacterial agentsBiocideImmunotherapyDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
Bursa pentapeptide, deriving peptide thereof and use thereof
ActiveCN101434650ASimple structureSmall molecular weightCosmetic preparationsPeptide/protein ingredientsChemical synthesisT lymphocyte
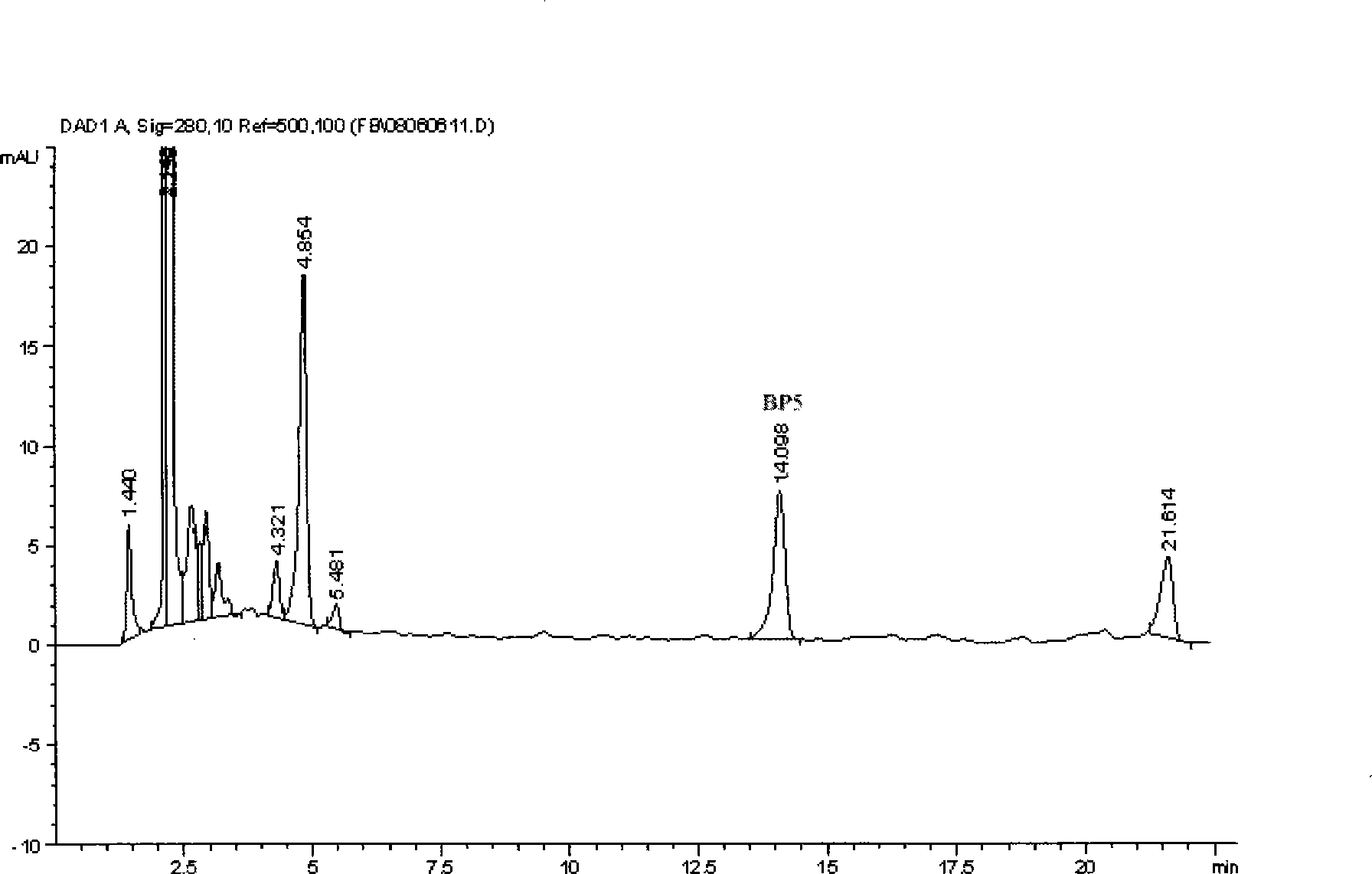
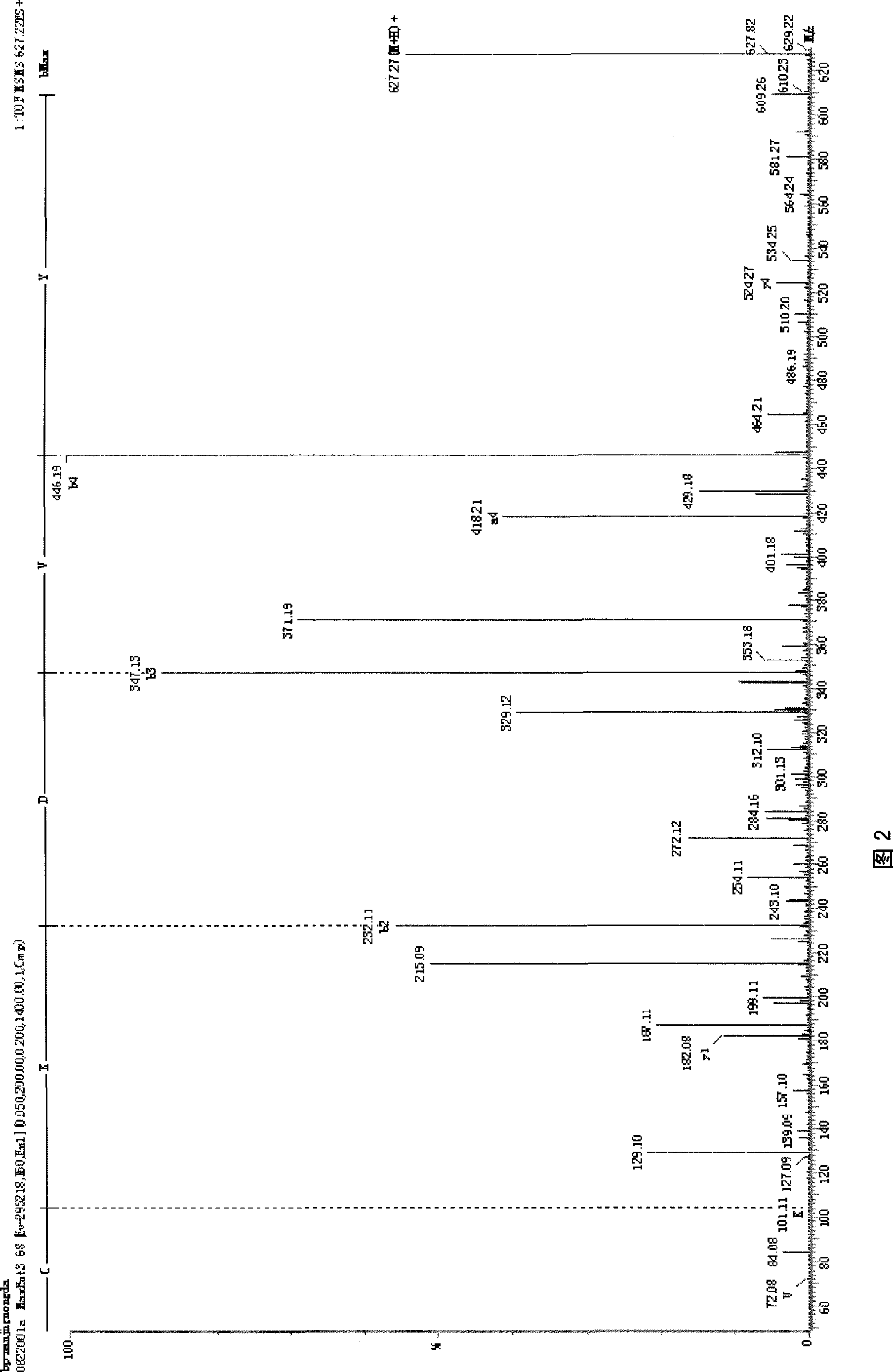
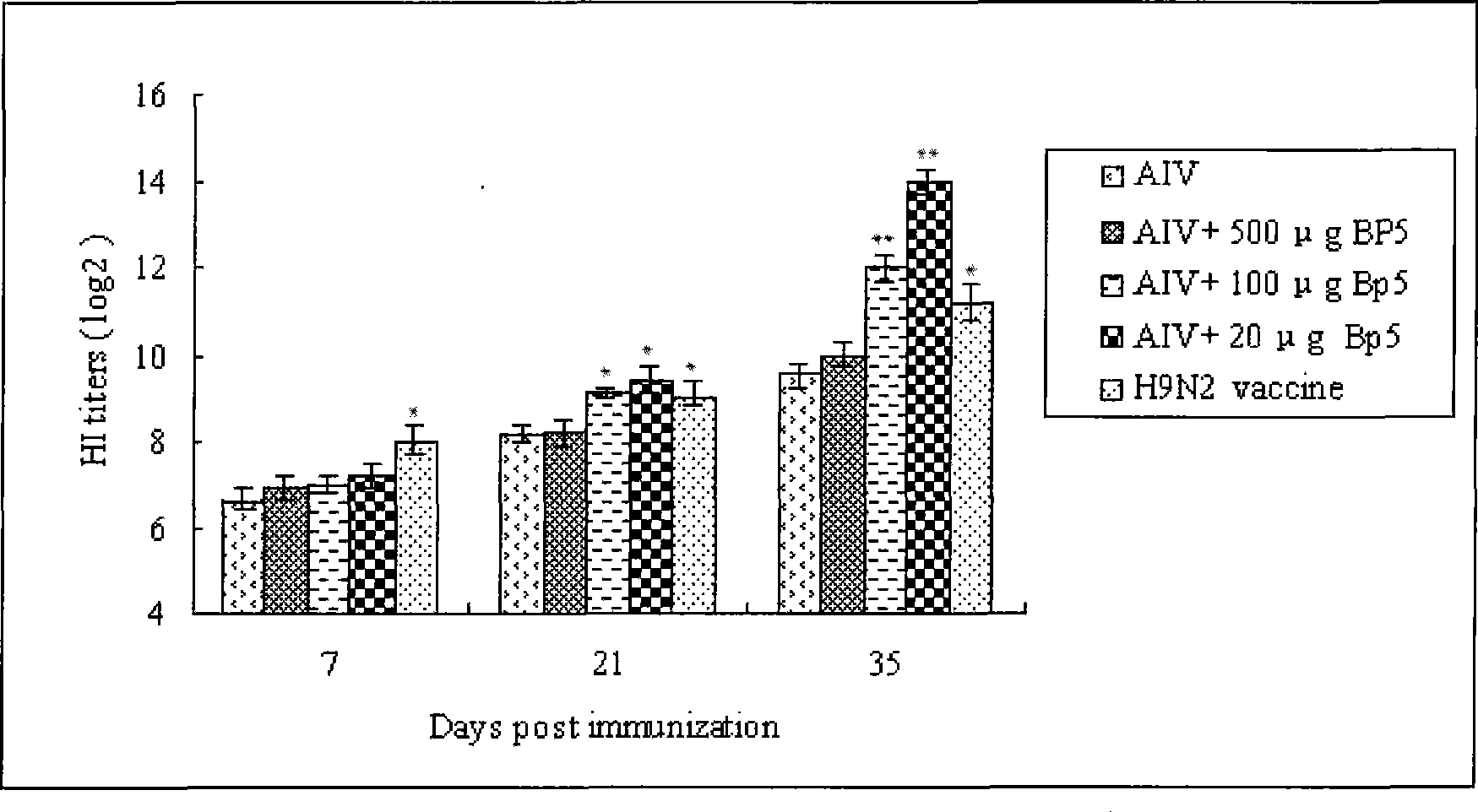
The invention relates to a bursal pentapeptide, and the structural sequence of amino acid of the bursal pentapeptide is Cys-Lys-Asp-Val-Tyr. The bursal pentapeptide (Bp5) has simple structure, small molecular weight, no chemical toxicity and no immunogenicity, is easy to be prepared, and can not only be extracted from the bursa of fabricius of chicken or other poultry, but also be prepared by chemosynthesis with quite low cost in large-scale. The bursal pentapeptide (Bp5) can promote the multiplication of T lymphocyte and B lymphocyte, improve the humoral immunity level and cellular immunity level of an organism as well as the capability of anti-peroxidation stress of the organism, is a medicament or preparation with wide application prospect and the functions of immunoregulation, immunotherapy and anti-peroxidation, and can be applied to the fields such as fundamental research, clinical treatment, nutrition and health care, cosmetics, and the like.
Owner:李德元
MntC recombinant protein of staphylococcus aureus and preparation method and application thereof
ActiveCN103694323AHigh expressionEasy to separate and purifyAntibacterial agentsBacteriaPurification methodsStaphylococcus aureus
The invention belongs to the field of biotechnology, and relates to a MntC recombinant protein of staphylococcus aureus (SA), a carrier comprising the recombinant protein, a host, a composition or a kit, application, preparation, fermentation and purification method of the protein. The MntC recombinant protein prepared by the method has strong immunogenicity, is safe and non-toxic, and is proved by animal tests to be able to effectively stimulate an organism to generate high efficient humoral immune response and good immune protection.
Owner:CHENGDU OLYMVAX BIOPHARM +1
Attenuated vif DNA immunization cassettes for genetic vaccines
The present invention is directed to nucleic acid molecules encoding attenuated, non-functional virion infectivity factor (vif) proteins. The nucleic acid molecules of the invention are inserted into recombinant expression vectors and administered to mammals in order to induce a cellular and humoral immune response to the encoded protein product.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Qs-21 and il-12 as an adjuvant combination
Adjuvant compositions comprising an effective amount IL-12 and QS-21 are disclosed. Immunogenic, vaccine and pharmaceutical compositions comprising a mixture of antigen and an adjuvant composition comprising an effective amount of IL-12 and QS-21 are also disclosed. These compositions elicit functional cell-mediated and humoral immune responses against at least one antigen. Methods of using the disclosed compositions are also disclosed.
Owner:WYETH HOLDINGS CORP
Fly maggot extractive as well as preparation method and application thereof
ActiveCN101991608AEnhance the body's humoral immunitySimple and efficient operationAnthropod material medical ingredientsAntineoplastic agentsMaggotWater soluble
The invention discloses a fly maggot extractive containing the principal components of water-soluble proteins, and the water-soluble proteins contained in the fly maggot extractive have the mass percentage of 50-70 percent and the molecular weight of 2-16 KDa. The fly maggot extractive not only has obvious inhibiting effect on human promyelocytic leukemia HL-60 cells, human erythroleukemia K562 cells, human liver cancers SMMC-7721, mouse leukemia P388 cells, human lung adenocarcinoma A549 cells, human nasopharyngeal darcinoma CNE cells, human prostatic carcinoma PC3 cells, human cervical carcinoma HeLa in vitro, but also has outstanding inhibiting effect on mouse S180 sarcomas and mouse Heps liver cancer solid tumors, and also has the effect on enhancing the humoral immunity of organisms. The invention also discloses a preparation method of the fly maggot extractive. The preparation method is easy and convenient for operation and control and low in cost and is suitable for industrialized production.
Owner:浙江佰科堂生物科技股份有限公司
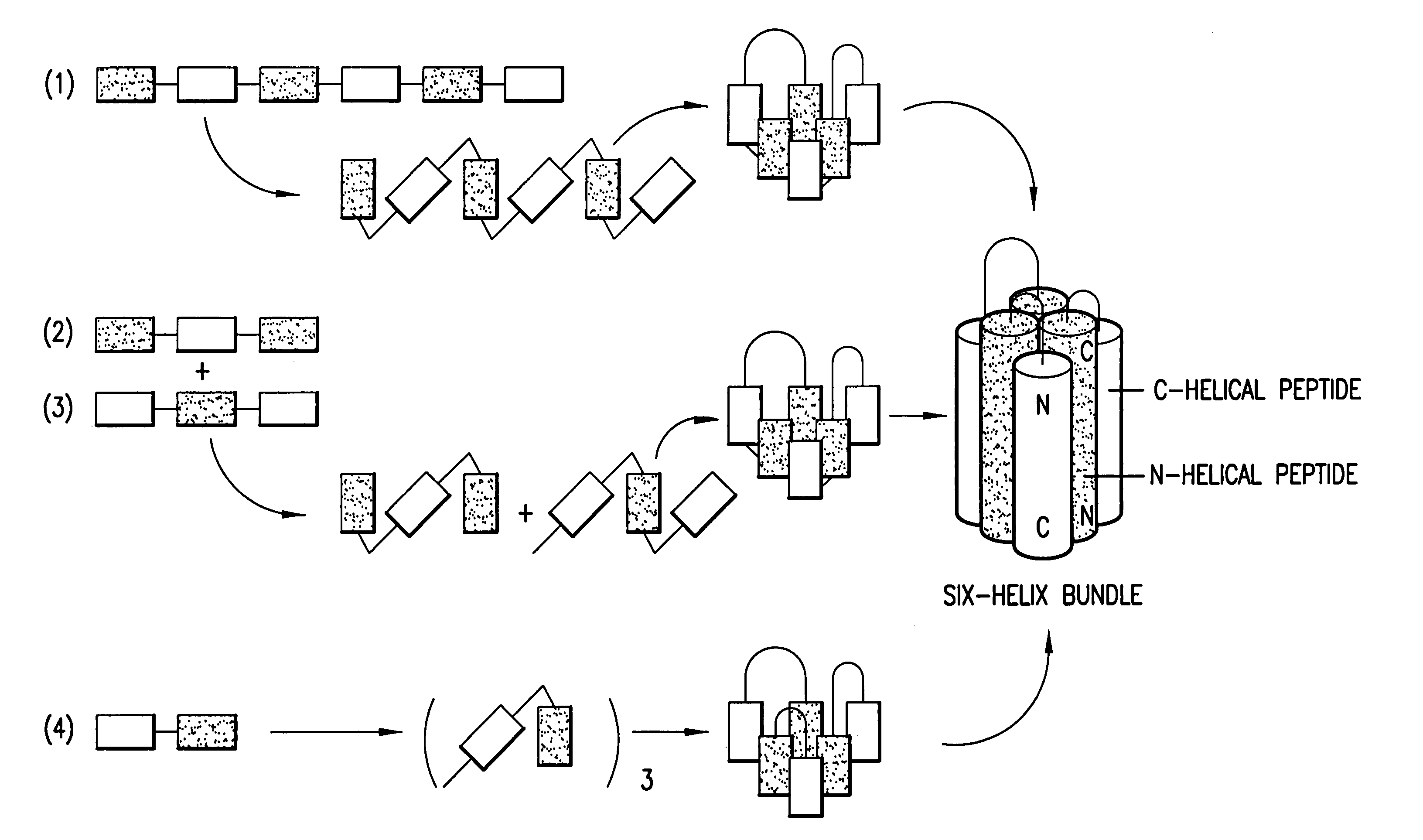
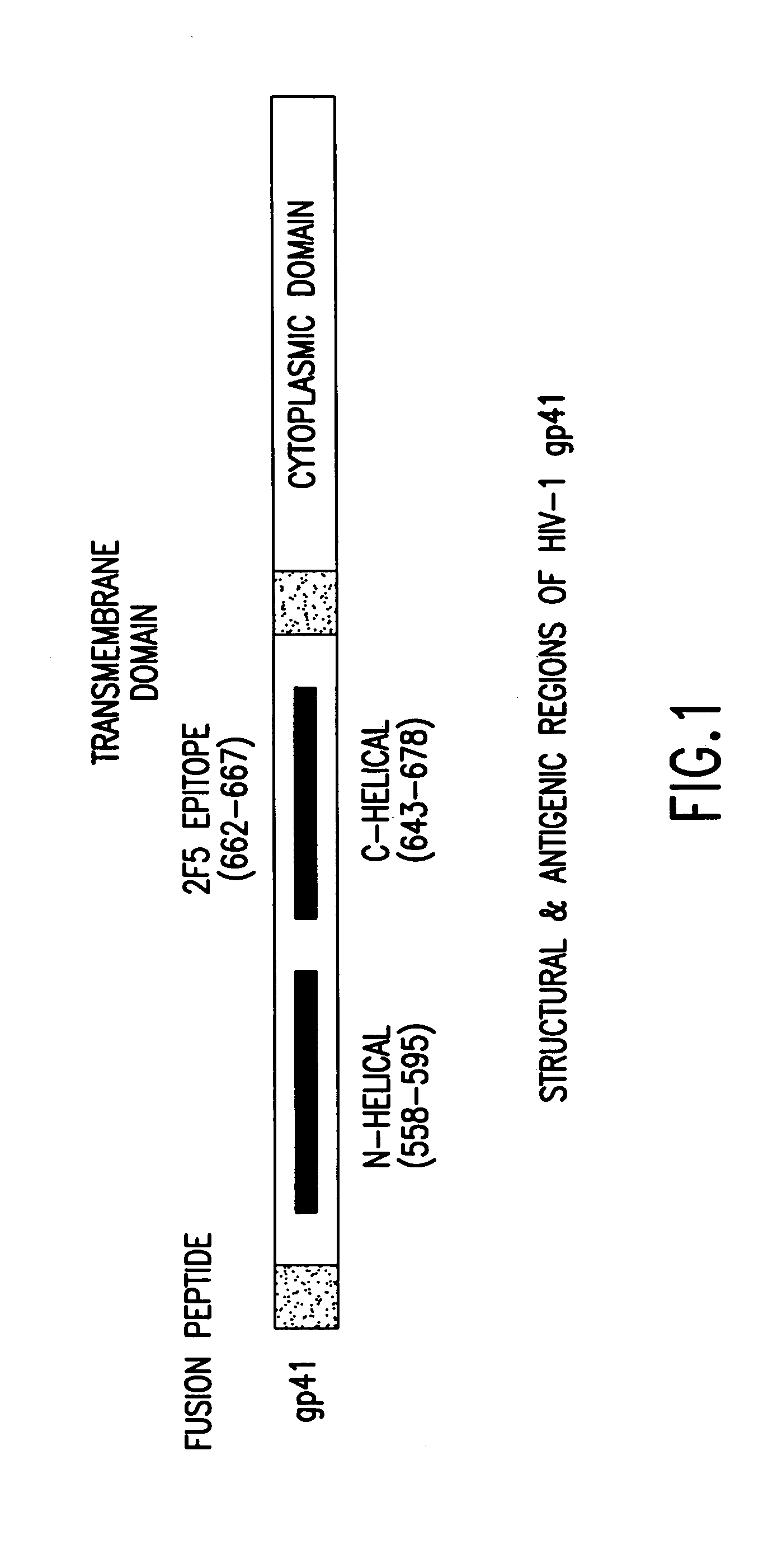
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting "entry-relevant" gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against "entry relevant" gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
QS-21 and IL-12 as an adjuvant combination
Adjuvant compositions comprising an effective amount IL-12 and QS-21 are disclosed. Immunogenic, vaccine and pharmaceutical compositions comprising a mixture of antigen and an adjuvant composition comprising an effective amount of IL-12 and QS-21 are also disclosed. These compositions elicit functional cell-mediated and humoral immune responses against at least one antigen. Methods of using the disclosed compositions are also disclosed.
Owner:WYETH HOLDINGS CORP
Methods and compositions for hybrid cell vaccines for the treatment and prevention of cancer
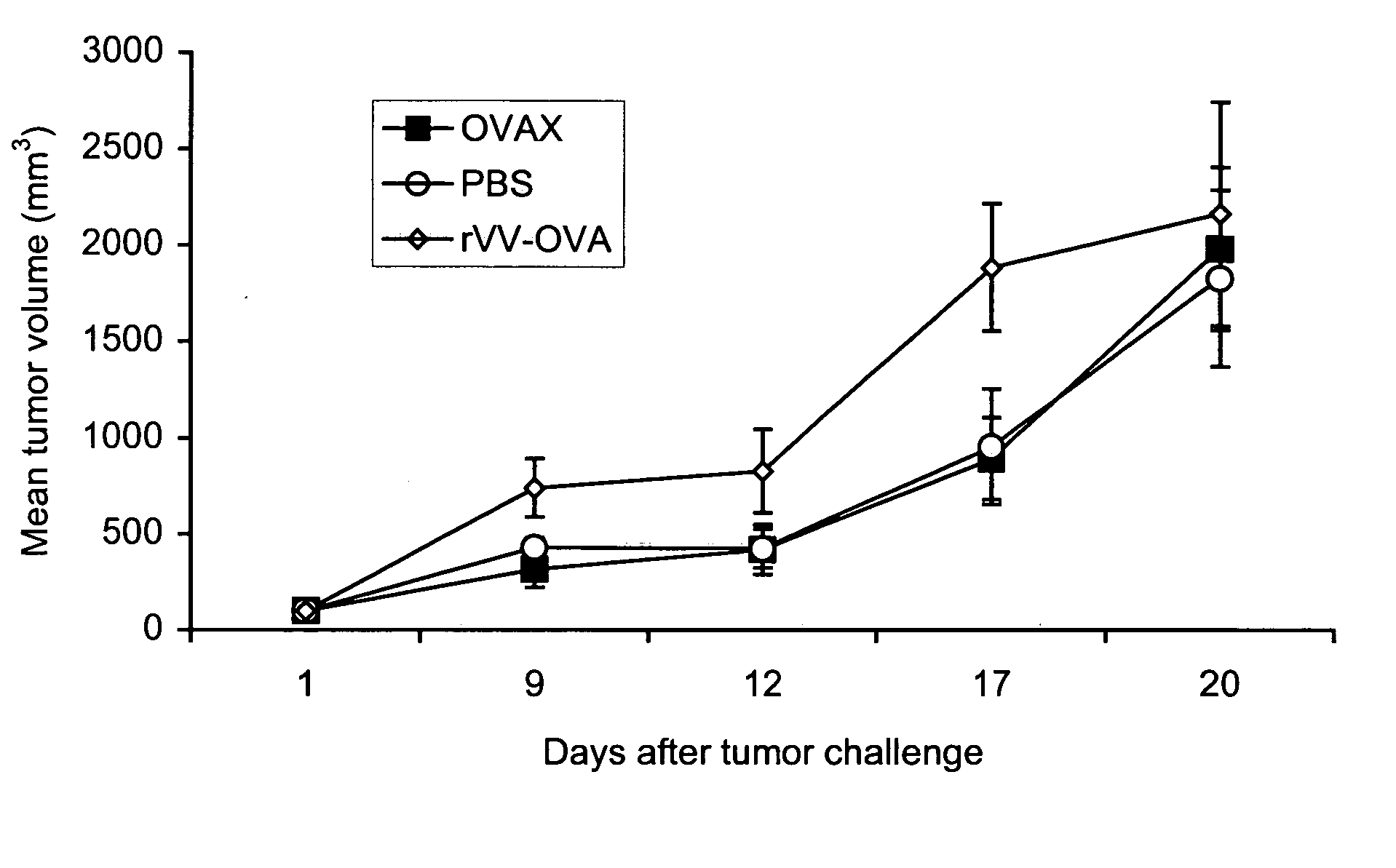
InactiveUS20050238627A1Strong immune responsePrevent cancerBiocideGenetic material ingredientsCancer preventionDendritic cell
The present invention relates to methods for treating and preventing cancer and for treating precancerous lesions by administering a therapeutically effective dose of a vaccine comprising fusion cells formed by fusion of antigen presenting cells and non-dendritic cells that contain genomic DNA or cDNA derived from a tumor cell or a pre-cancerous cell to a cancer patient or patient with a precancerous lesion. In certain embodiments, such vaccines are administered in combination with a cytokine or other molecule that stimulates a cytotoxic T cell (CTL) response and / or a humoral immune response. The present invention also relates to methods for treating and preventing an infectious disease by administering a therapeutically effective dose of a vaccine comprising fusion cells formed by fusion of antigen presenting cells and non-dendritic cells that contain genomic DNA or cDNA derived from the infectious agent that causes the infectious disease to be treated or prevented to a subject. The present invention also related to methods for producing the fusion cells to be used with the methods of the invention. The present invention also provides compositions comprising the fusion cells to be used with the methods of the invention.
Owner:OHNO +1
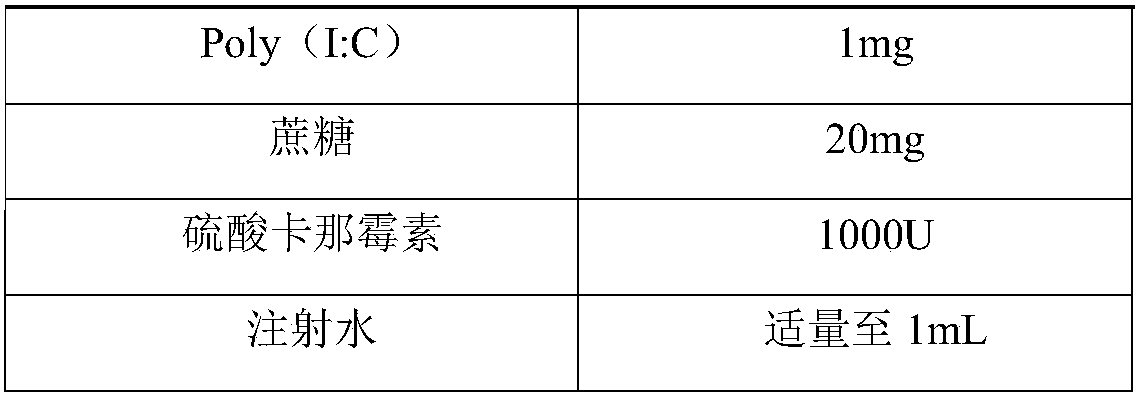
Herpes zoster vaccine, and preparation method and application thereof
InactiveCN108992667AImprove immunityEnhance humoral immune responseNervous disorderViral antigen ingredientsAdjuvantIn vivo
The invention discloses a herpes zoster vaccine, and a preparation method and an application thereof, and belongs to the field of vaccines. The herpes zoster vaccine is provided against the problem ofhigh safety risk of existing herpes zoster vaccines, and an adjuvant used by the herpes zoster vaccine is Poly (I:C). The Poly(I:C) is called polyinosinic-polycytidylic acid for short, and is double-stranded RNA, and the safety of the Poly(I:C) is confirmed during large-scale clinical application. The Poly(I:C) is a high-efficiency interferon inducer, can produce an immune reaction like viral infection in vivo, and can induce CD4<+> and CD8<+> T cells and enhance cellular and humoral immune responses.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting “entry-relevant” gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against “entry relevant” gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
Application of short-peptide serving as vaccine adjuvant and vaccine
The invention provides application of short-peptide serving as vaccine adjuvant and a vaccine taking the short-peptide as the vaccine adjuvant. The short-peptide is easy to prepare, and after the short-peptide is easily, conveniently and physically mixed with an antigen, the immune response capacity of the antigen can be effectively enhanced. In the application of the short-peptide in serving as the vaccine adjuvant, the short-peptide is subjected to end-capping through a group X, the short-peptide sequence is X-GFF, or the short-peptide sequence contains a sequence FFY. Preferably, the short-peptide sequence containing the sequence FFY is X-FFY or X-GFFY or X-GFFYK or X-GFFYE or X-GFFYG; preferably, the short-peptide has a D configuration. The short-peptide can serve as immune adjuvant to enhance immunogenicity of the antigen, a high cellular immunity and humoral immunity response of antigenic specificity of a main body occurs, and the short-peptide can be applicable to various antigens; the short-peptide is easy to prepare, and the component is single and controllable.
Owner:NANKAI UNIV
Saponin with immunoadjuvant function, preparation method, vaccine preparation containing the saponin as adjuvant and uses thereof
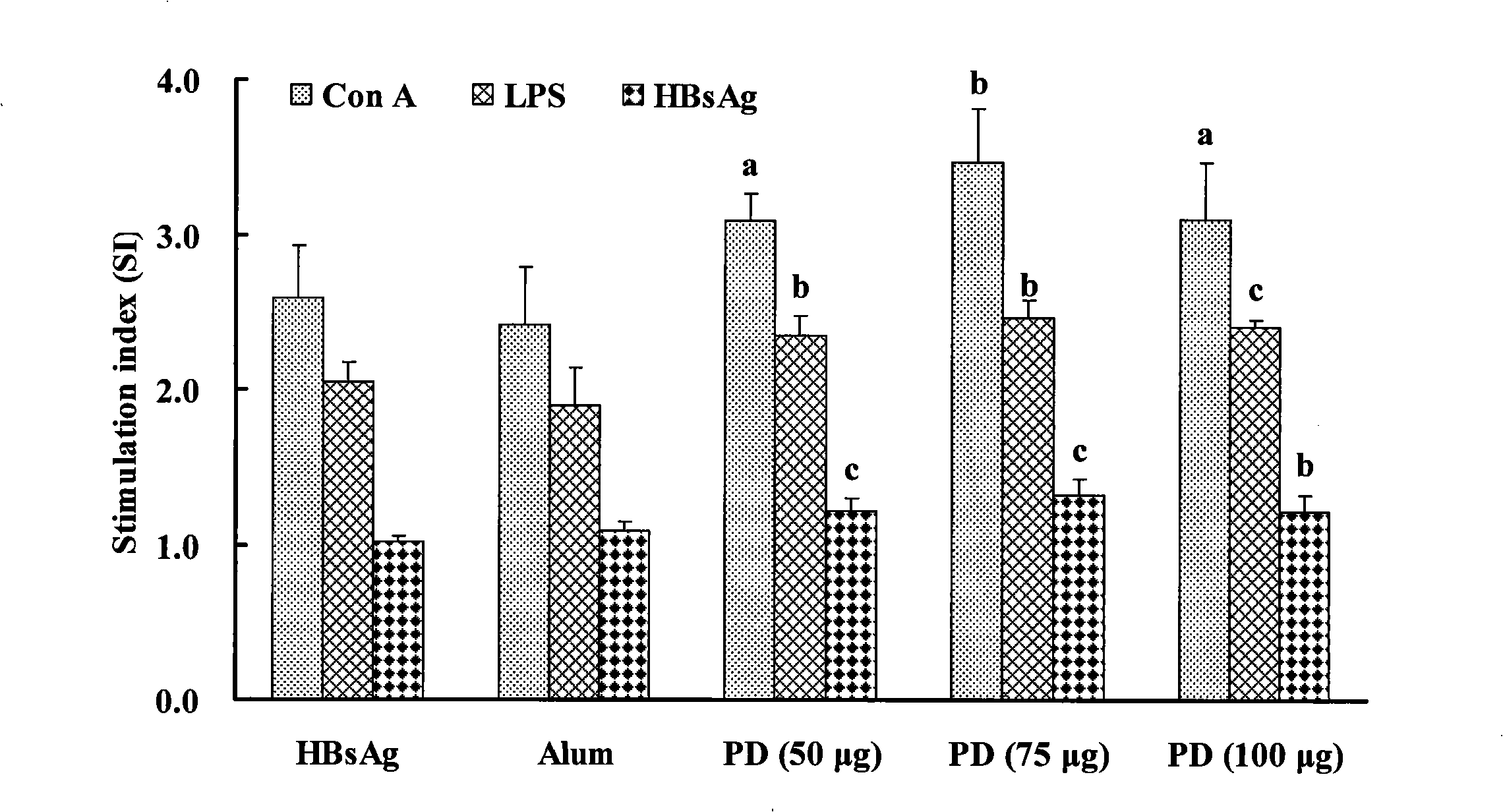
InactiveCN101402666ASimple preparation processSimple methodAntiinfectivesSteroidsDiseaseCell immune response
The invention relates to a saponin with immune adjuvant function and a preparation method thereof, a vaccine preparation containing the saponin as an adjuvant, and applications of the saponin and the vaccine preparation in the prevention and treatment of infectious diseases and cancers of human and animals. The saponin is platycodin D, platycodin D2, or a total-saponin containing the two saponin compounds. The platycodin D and platycodin D2 are both extracted and separated from balloonflower, a Chinese medicine. The saponin can induce an organism to generate Th1-type and Th2-type immune responses, show the capability of inducing the organism to generate stronger cell immune response and humoral immune response to a vaccine than the alhydrogel adjuvant known in the prior art, and can be taken as the immune adjuvant for a plurality of vaccines and achieve an ideal immunity effect. The vaccine which takes the saponin as the adjuvant has simple preparation technology and simple and convenient method, and the quality is easy to control and the saponin can be reserved by freezing.
Owner:ZHEJIANG UNIV
Nano-element traditional Chinese medicinal fabric antibacterial underwear for preventing and treating psoriasis
InactiveCN102078029AAmphibian material medical ingredientsElectrotherapyHigh morbidityHumoral immune reaction
The invention discloses nano-element traditional Chinese medicinal fabric antibacterial underwear for preventing and treating psoriasis, which belongs to the technical field of functional healthy fabric underwear production. Psoriasis is a skin disease which has high morbidity and stubborn disease process, is easy to relapse, is difficult to cure and does serious harm to the physical and psychological health of people. Far infrared heat effect takes effect in deep subcutaneous tissues through medical stone, tourmaline and a nano silver (Ag) antimicrobial agent, so the underwear expands blood vessels, quickens blood flow, increases cell activity and blood oxygen content, makes human bodies absorb more negative ions and trace elements, promotes body metabolism, enhances cellular immunity and humoral immunity of the human bodies, promotes the balance of elements in the human bodies, strengths the regeneration activity of cells and resists bacteria, cancers and inflammation. The underwear prevents and treats psoriasis when a user wears the underwear.
Owner:成进学
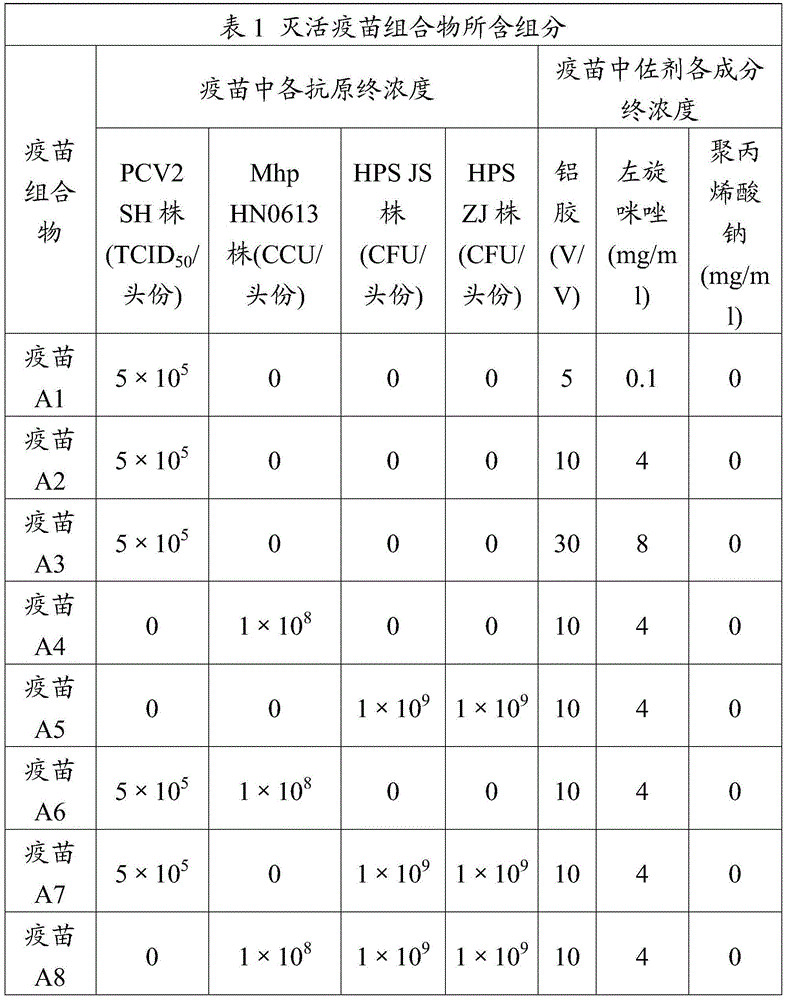
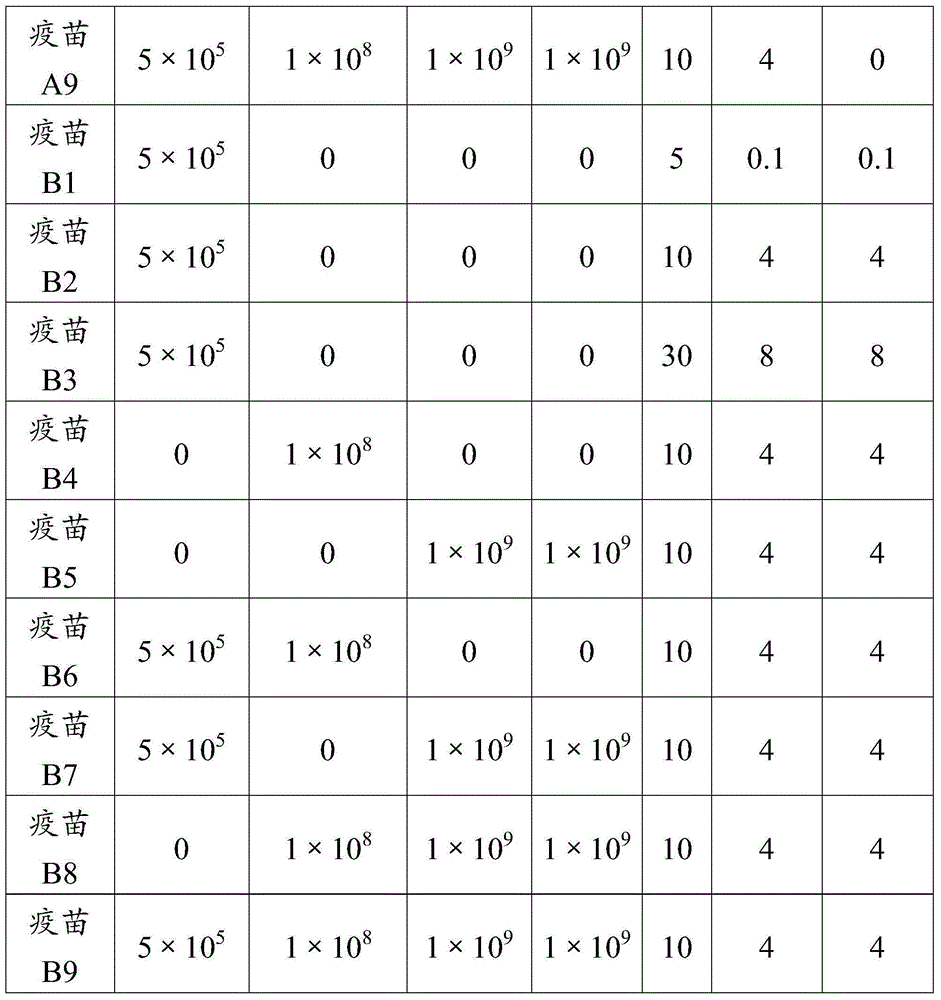
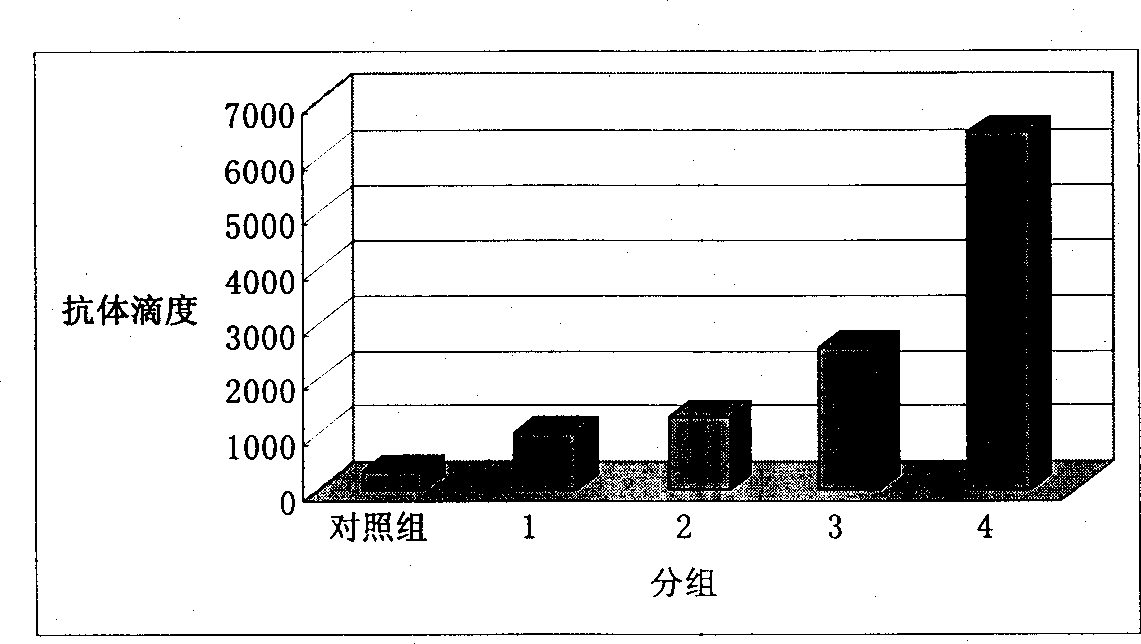
Adjuvant used for vaccine and application thereof
ActiveCN106177939ALow costEasy to usePharmaceutical non-active ingredientsImmunological disordersAdjuvantProtein stabilization
The invention provides an adjuvant used for vaccine. The adjuvant comprises alumina gel, levamisole or its derivative, and also polyacrylate. The invention also discloses a vaccine composition containing the adjuvant and an application thereof. The adjuvant can be used as the adjuvant for inactivated vaccine, and also can be used for preparing protein stabilization liquid. The vaccine composition containing the adjuvant can generate humoral immunity for human body and can generate cellular immunity, and can generate good immune response under condition of low antigen content and single immune, and can stabilize protein, degradation and irreversible change cannot be generated.
Owner:PU LIKE BIO ENG
Ginseng total saponin or monomer saponin RbI vaccine immunological adjuvant application
InactiveCN1367022ALong validity periodShort validity periodAntibody medical ingredientsSide effectAluminium hydroxide
The present invention discloses an application of ginseng total saponin or monomer saponin Rb1 as vaccine immunologic adjuvant. The ginseng total saponin or monomer saponin Rb1 and aluminium hydroxide are mixed and used as vaccine immunologic adjuvant, and as compared with traditional aluminium hydroxide adjuvant said invention possesses the following advantages: (1) its side effect is less; (2) said vaccine can be stored under the condition of frozen low-temp. and it can prolong effective period of vaccine; (3) its method is simple and convenient, its quality is easy to be controlled; and (4) it can induce body to produce higher humoral immunity response.
Owner:ZHEJIANG UNIV
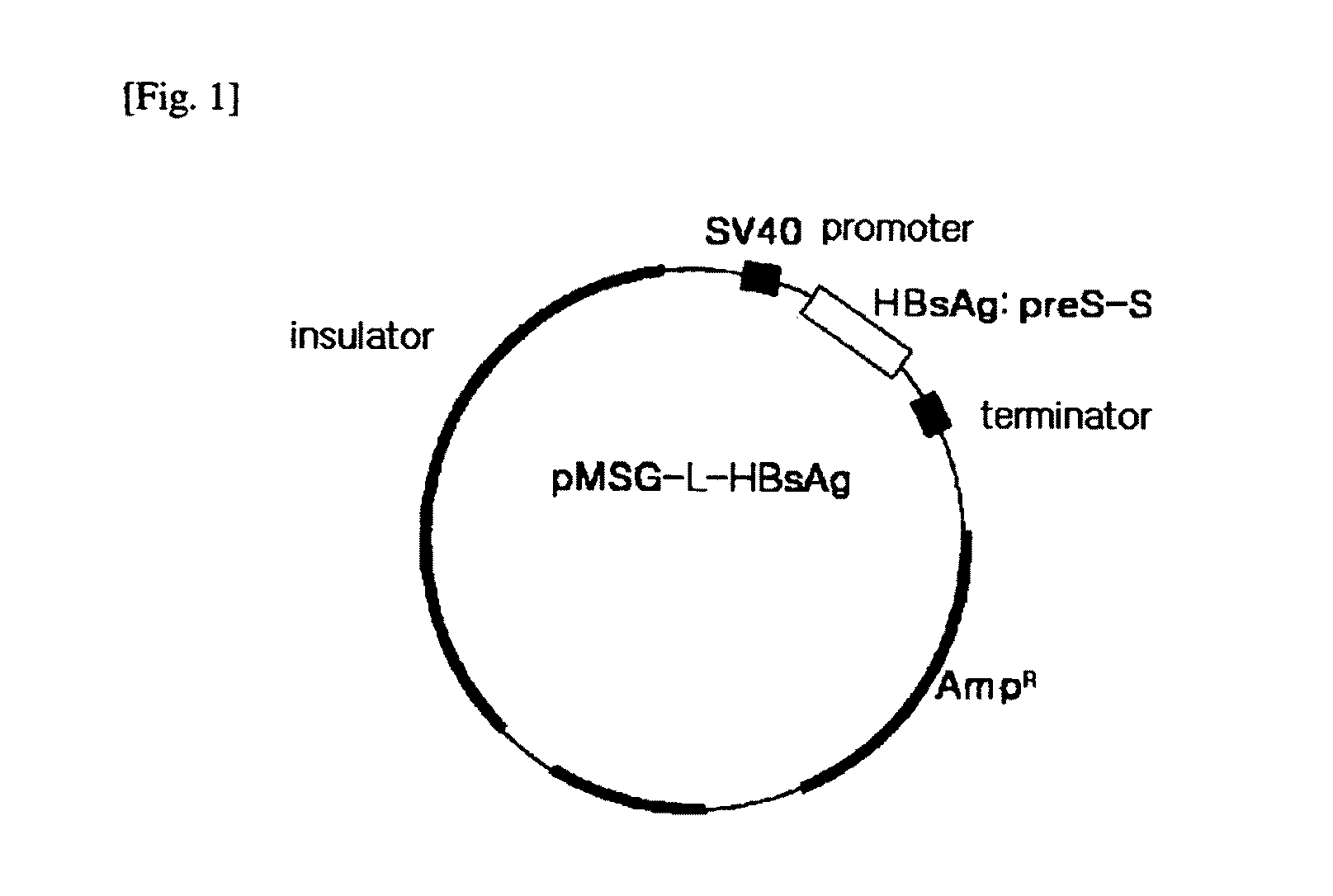
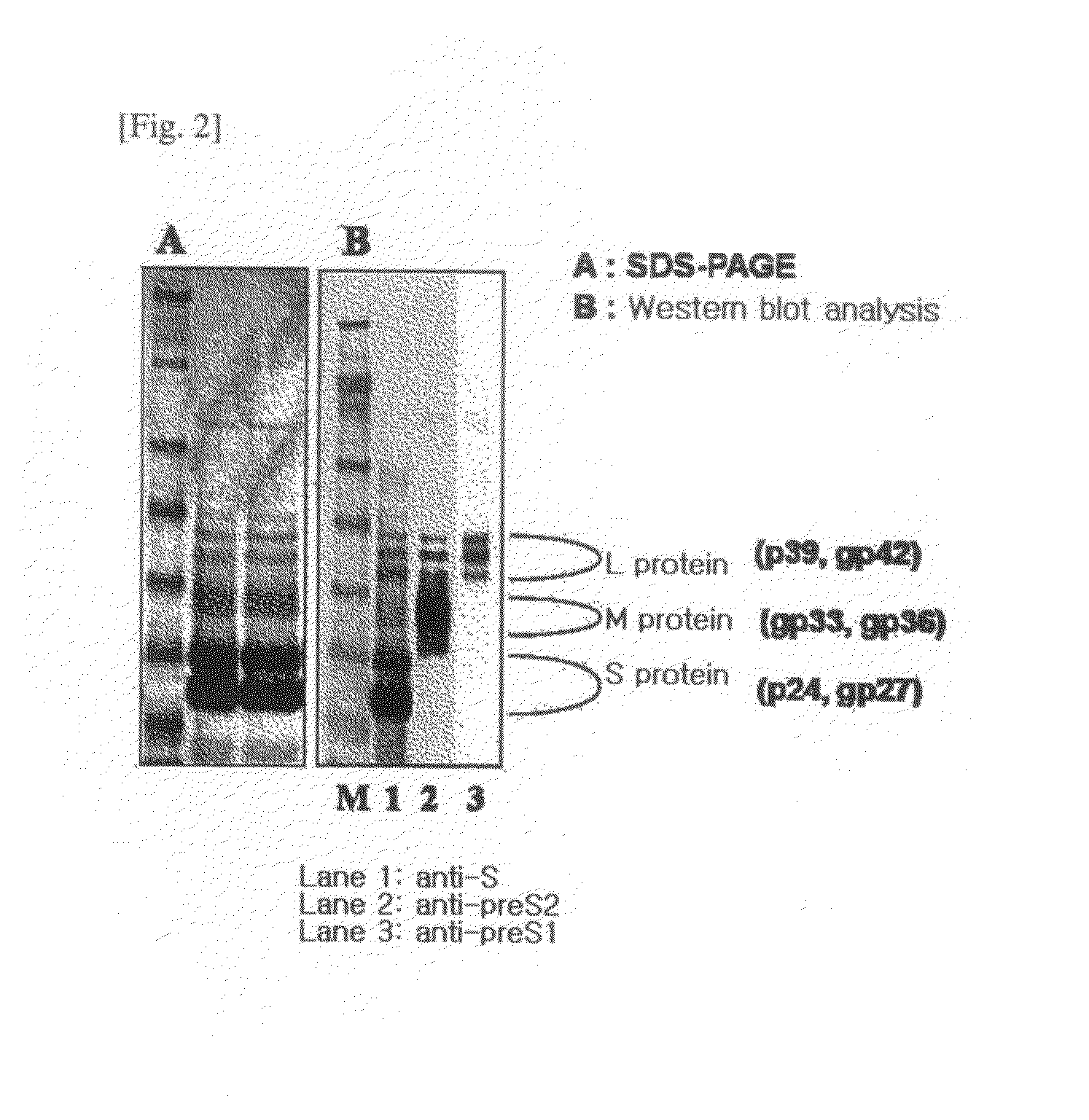
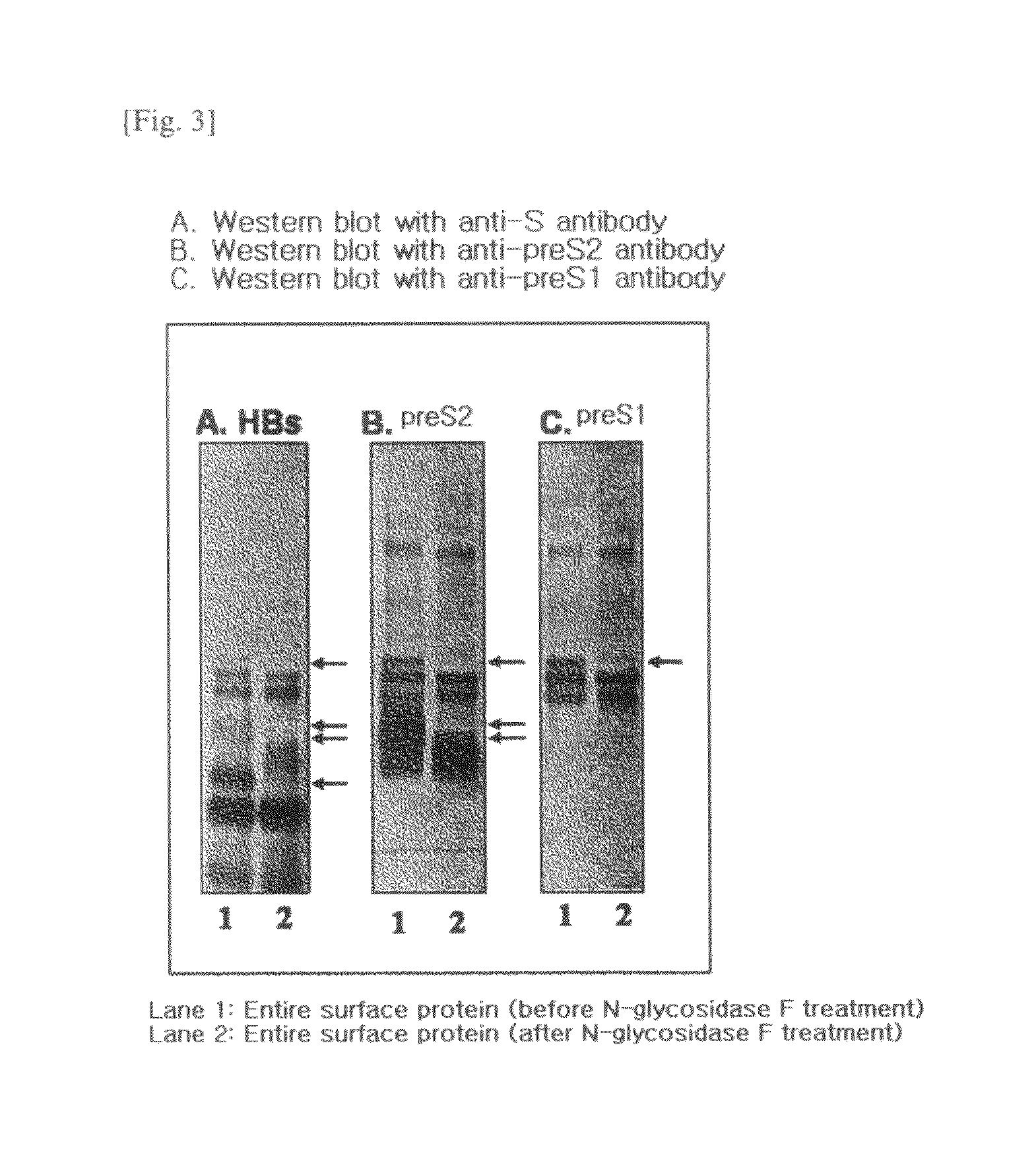
HBV vaccine and a process of preparing the same
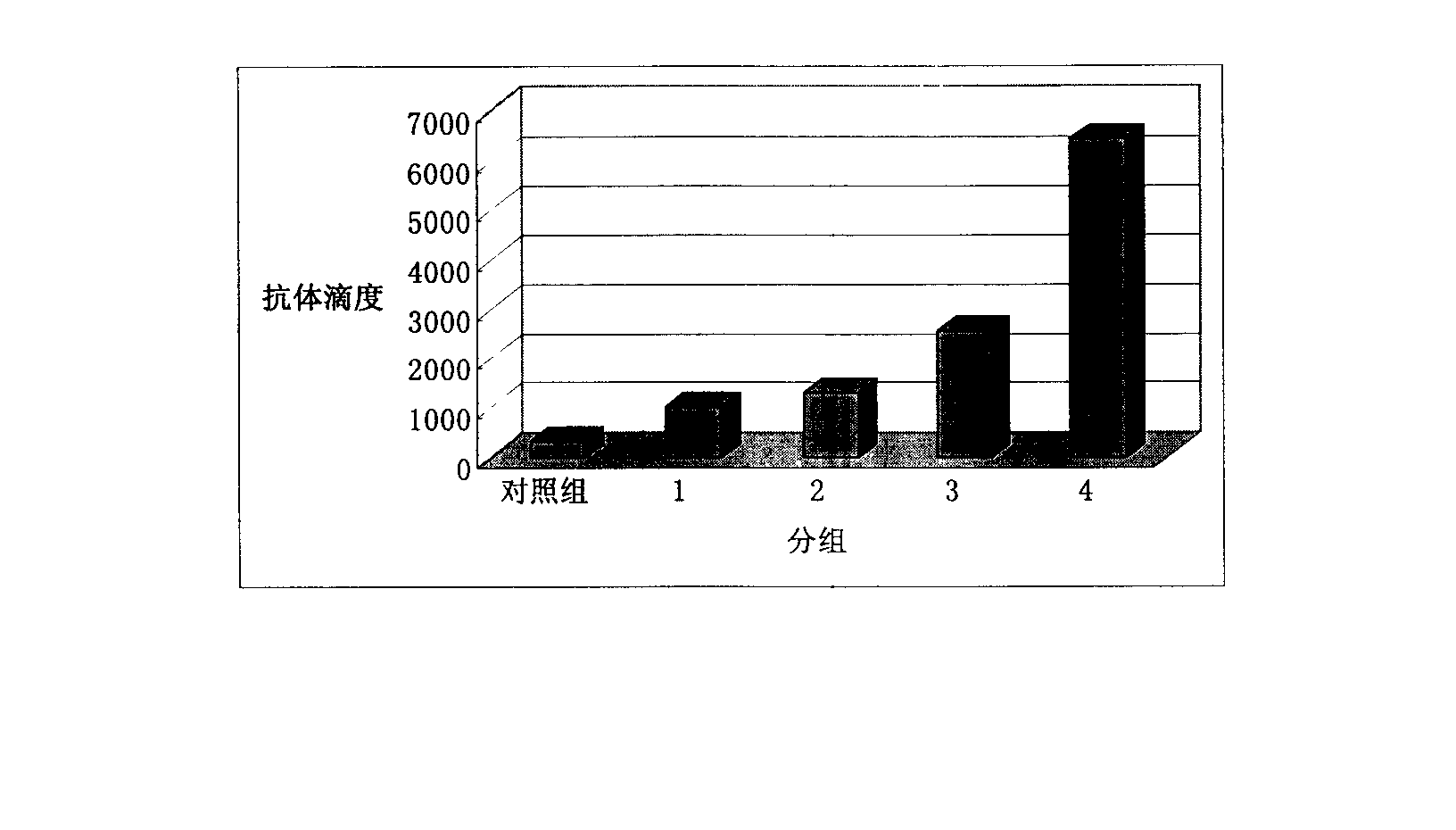
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
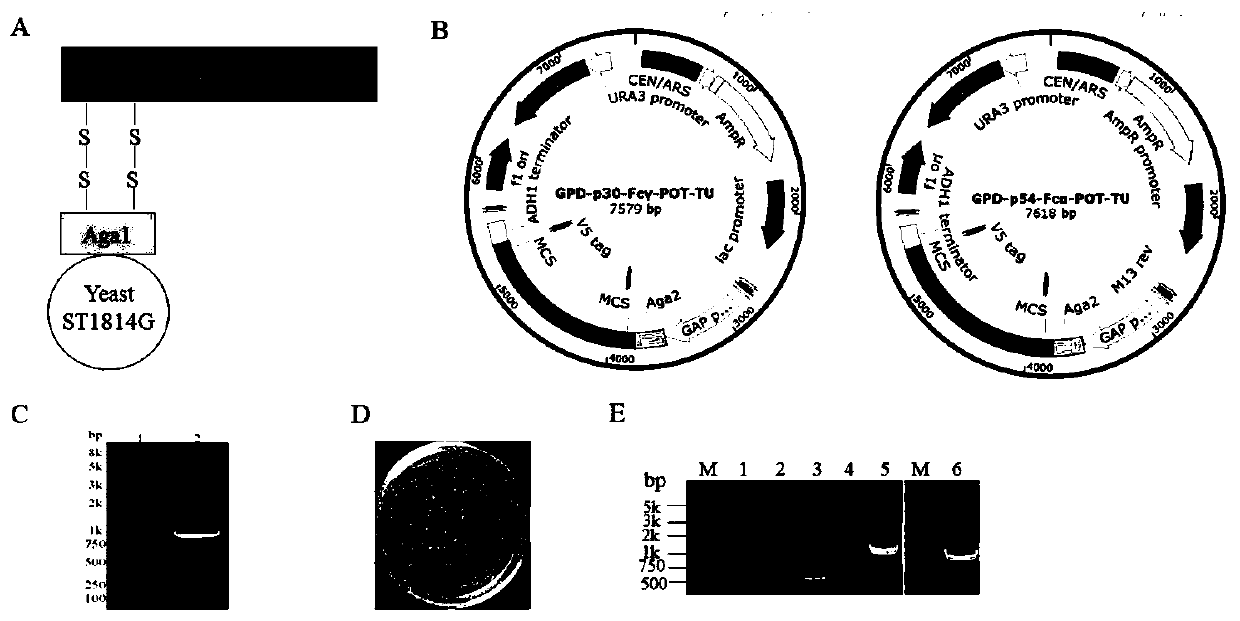
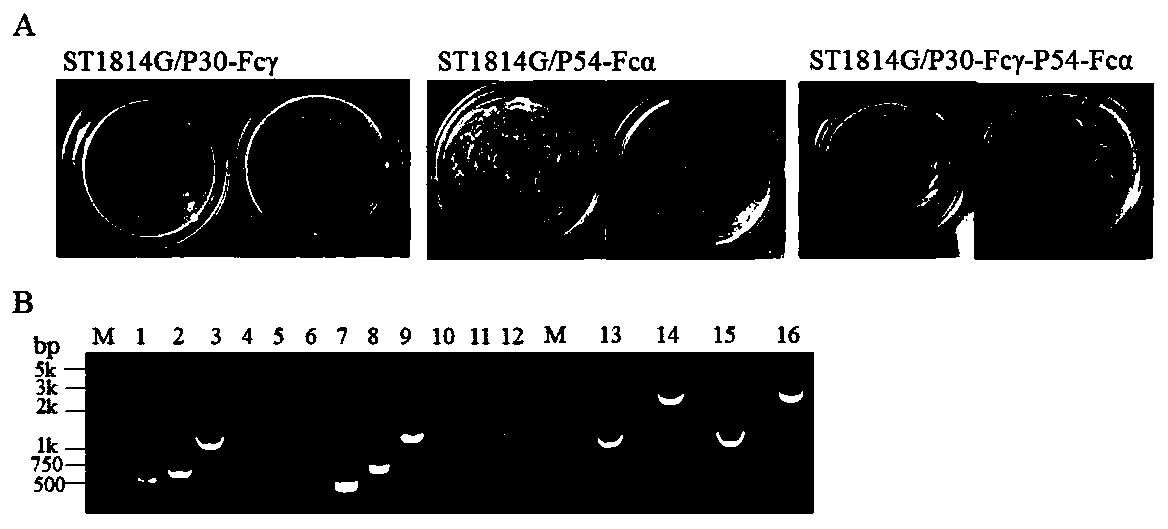
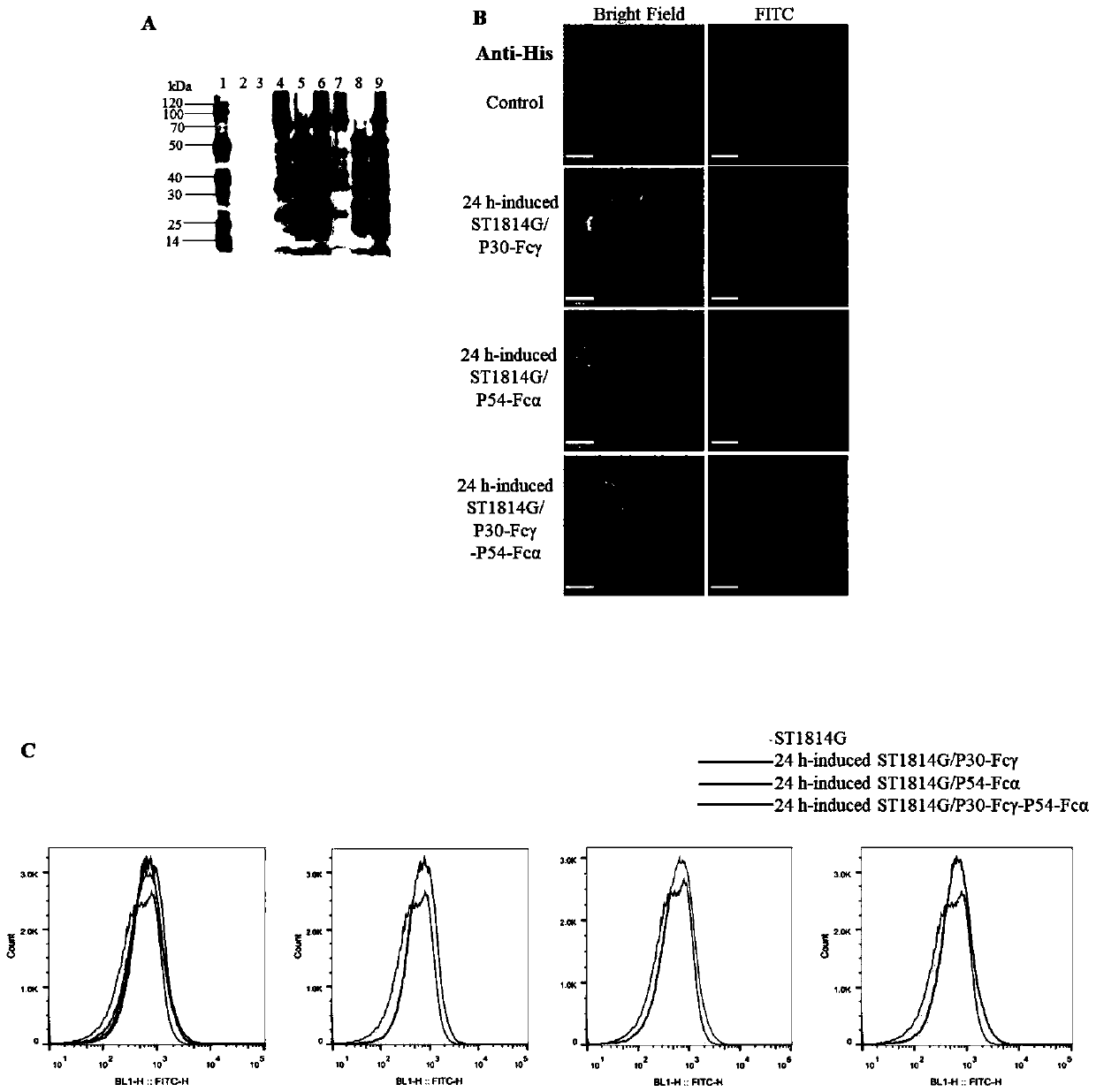
Method for preparing African swine fever virus P30 and P54 yeast vaccine
PendingCN111004330AIncrease the number ofGood antigenicityFungiViral antigen ingredientsHumoral immune reactionTGE VACCINE
The invention discloses a method for preparing an African swine fever virus P30 and P54 yeast vaccine. The vaccine contains African swine fever virus strong immunogen protein P30, membrane structure protein P54, Fc fragments of swine IgG1 and IgA1 (namely Fc gamma, Fc alpha), and His purification tags. The method comprises the following steps: S1, connecting ASFV immunogen protein genes p30 and p54 with Fc gamma and Fc alpha in series; S2, respectively constructing transcription units of p30-Fc gamma and p54-Fc alpha in fusion expression in the yeast; S3, realizing series connection of the twotranscription units through in-vitro enzyme linking, yeast in-vivo conversion and homologous recombination technologies, and stably integrating the two transcription units into surface display type saccharomyces cerevisiae; and S4, carrying out genome level and protein level verification, and confirming to obtain two recombinant yeast strains of which the independent surfaces show and express theP30-Fc gamma and P54-Fc alpha fusion proteins, and a tandem composite expression type recombinant yeast strain of which the surface shows and expresses the two fusion proteins at the same time in S1.The strain is mature in production technology, suitable for large-scale production, safe to use and capable of inducing remarkable mucous membrane and humoral immune response of target animals.
Owner:TIANJIN UNIV
Fusion cells and cytokine compositions for treatment of disease
InactiveUS20020168351A1Enhance immune responseImprove responseBiocideGenetic material ingredientsCancer preventionT cell
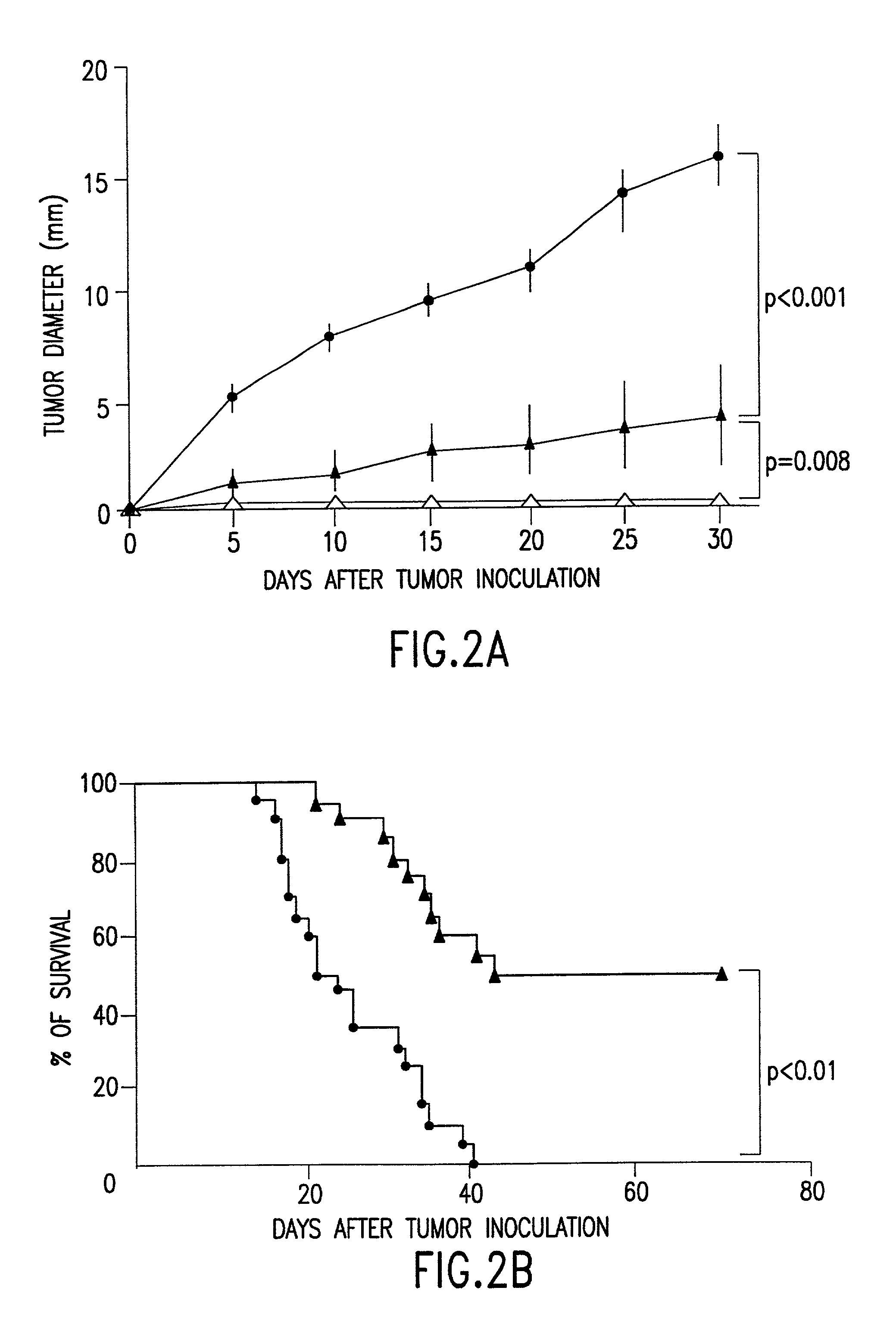
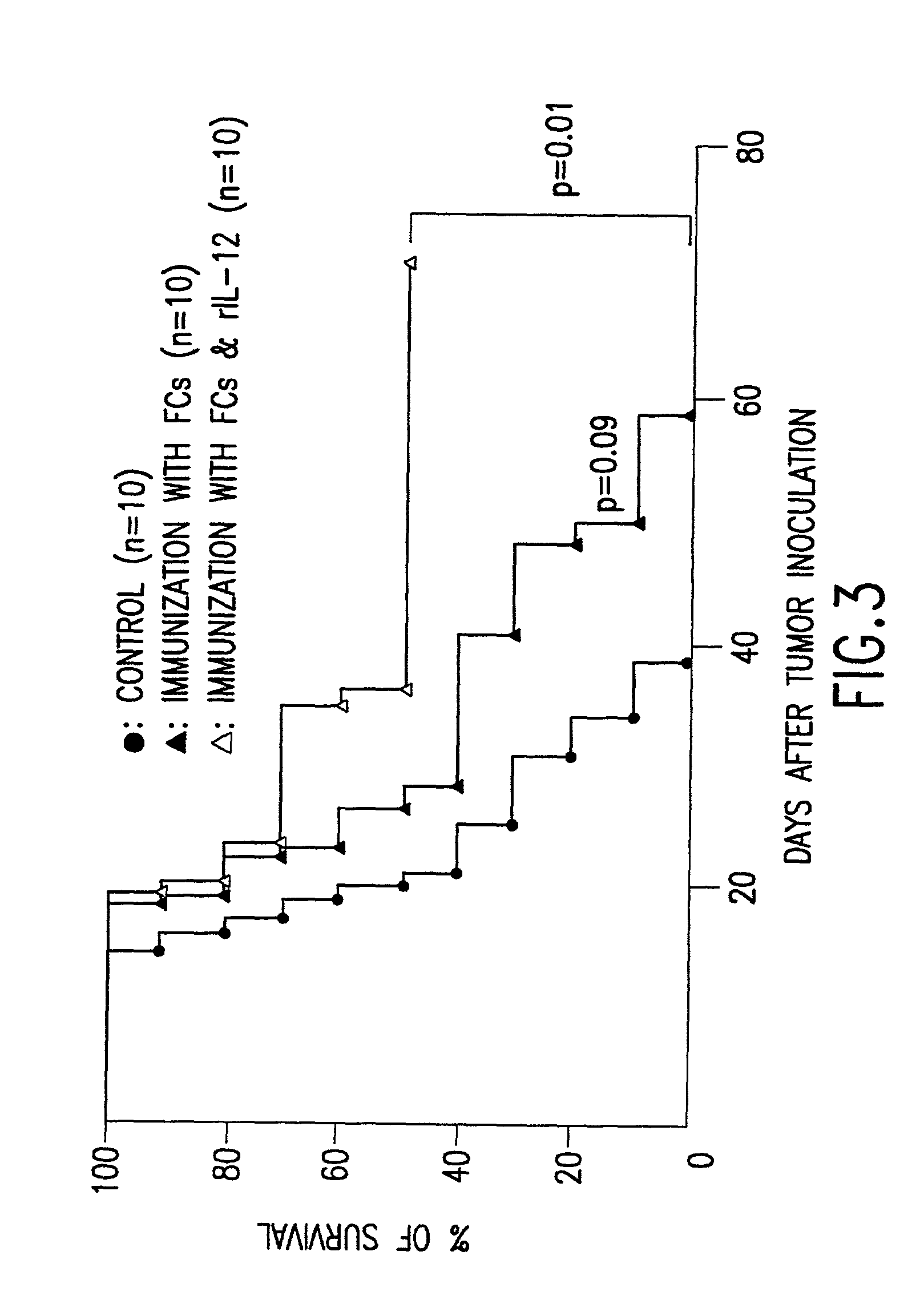
The present invention relates to methods and compositions for treating and preventing cancer and infectious disease by administering a therapeutically effective dose of fusion cells formed by fusion of autologous dendritic cells and autologous non-dendritic cells, in combination with a cytokine or other molecule which stimulates or induces a cytotoxic T cell response and / or a humoral immune response.
Owner:OHNO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com