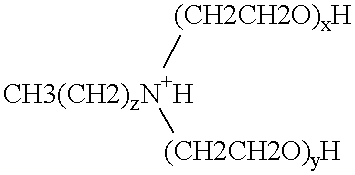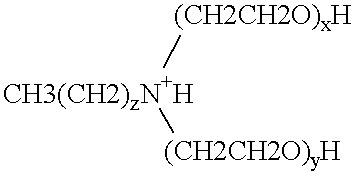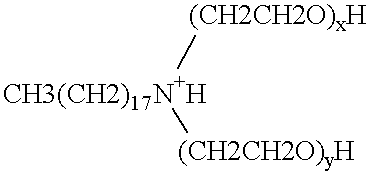Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Protein stabilization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polymerase stabilization by polyethoxylated amine surfactants
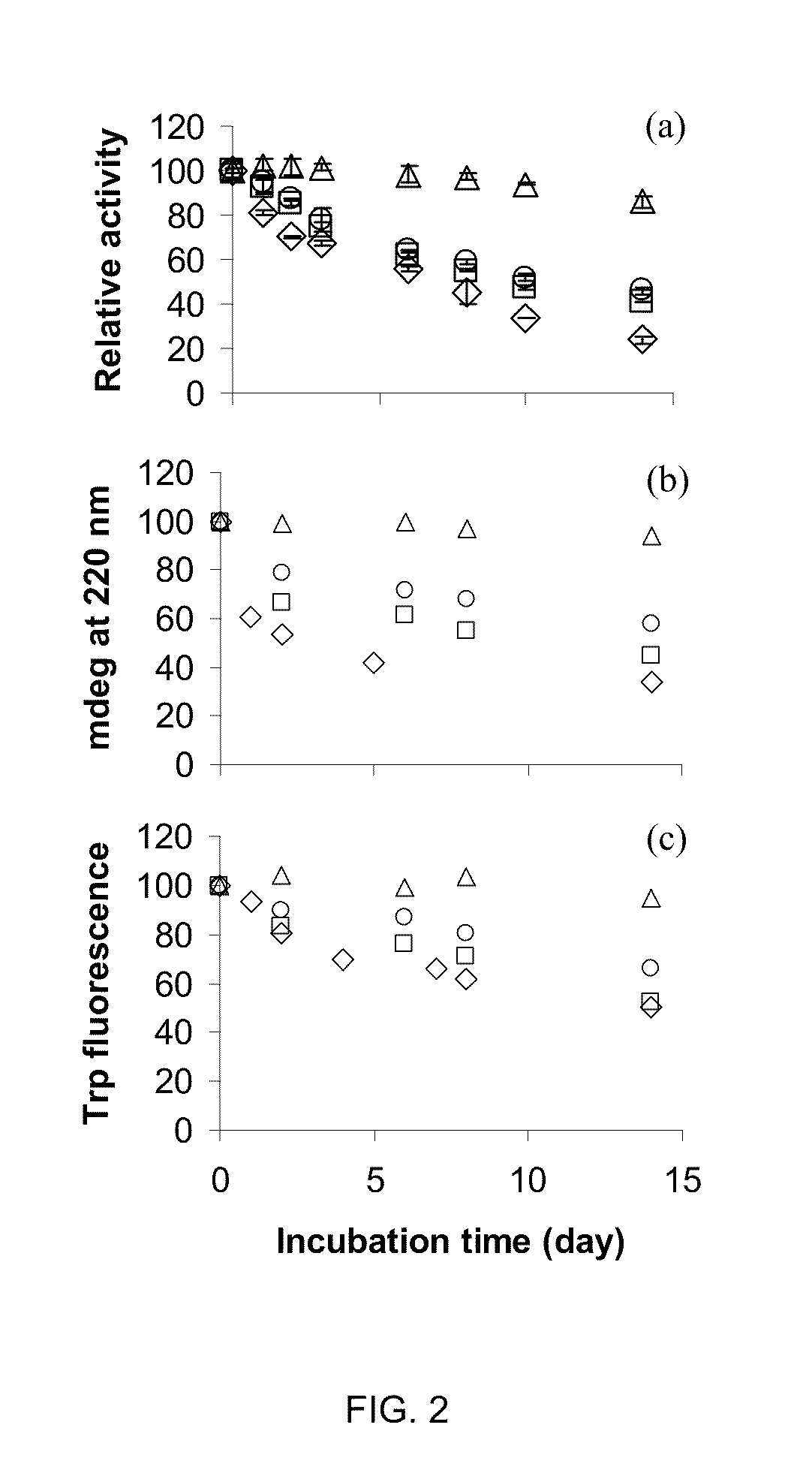
The present invention provides methods and compositions for protein stabilization, particularly the stabilization of polymerases in aqueous solutions with cationic surfactants. The present invention further provides cationic surfactants, including polyethoxylated amines, that stabilize thermostable and thermolabile enzymes in solution. These surfactants stabilize the activity of various enzymes, including thermostable DNA polymerases, thermolabile DNA polymerases and reverse transcriptases.
Owner:PROMEGA
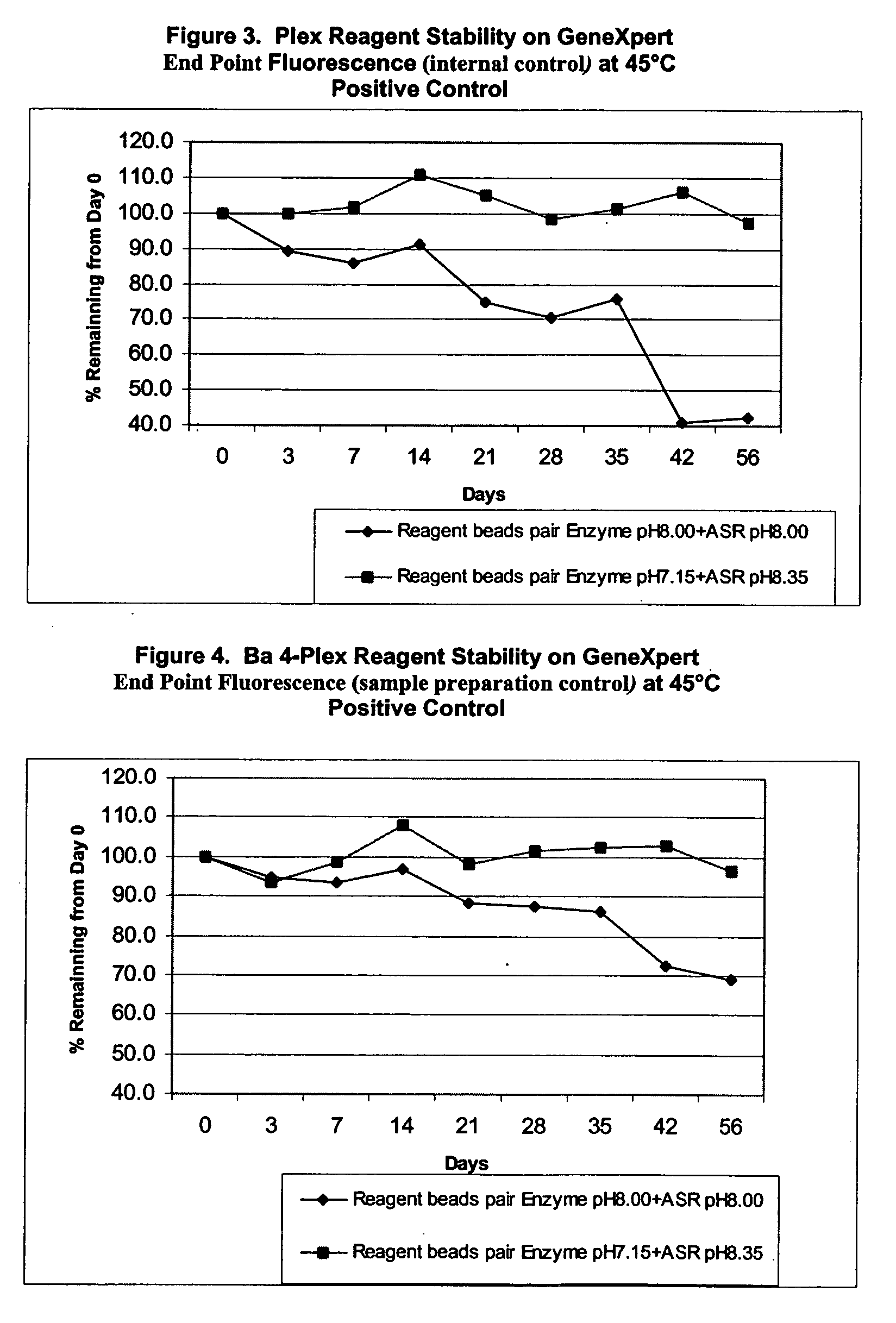
Multiple bead reagent system for protein based assays with optimized matrices
InactiveUS20060068399A1High activityMicrobiological testing/measurementBiological testingProtein stabilizationActivity level
The invention provides a multi-bead assay system for a protein based assay comprising at least two different beads. The first bead comprises protein and a protein stabilization matrix. The first bead forms a first solution when dissolved in liquid, and the first solution permits a first activity level for the assay. The second bead comprises a potentiation bead matrix that when dissolved in the first solution forms a second solution that potentiates the protein based assay to achieve a second activity level that is higher than the first activity level.
Owner:CEPHEID INC
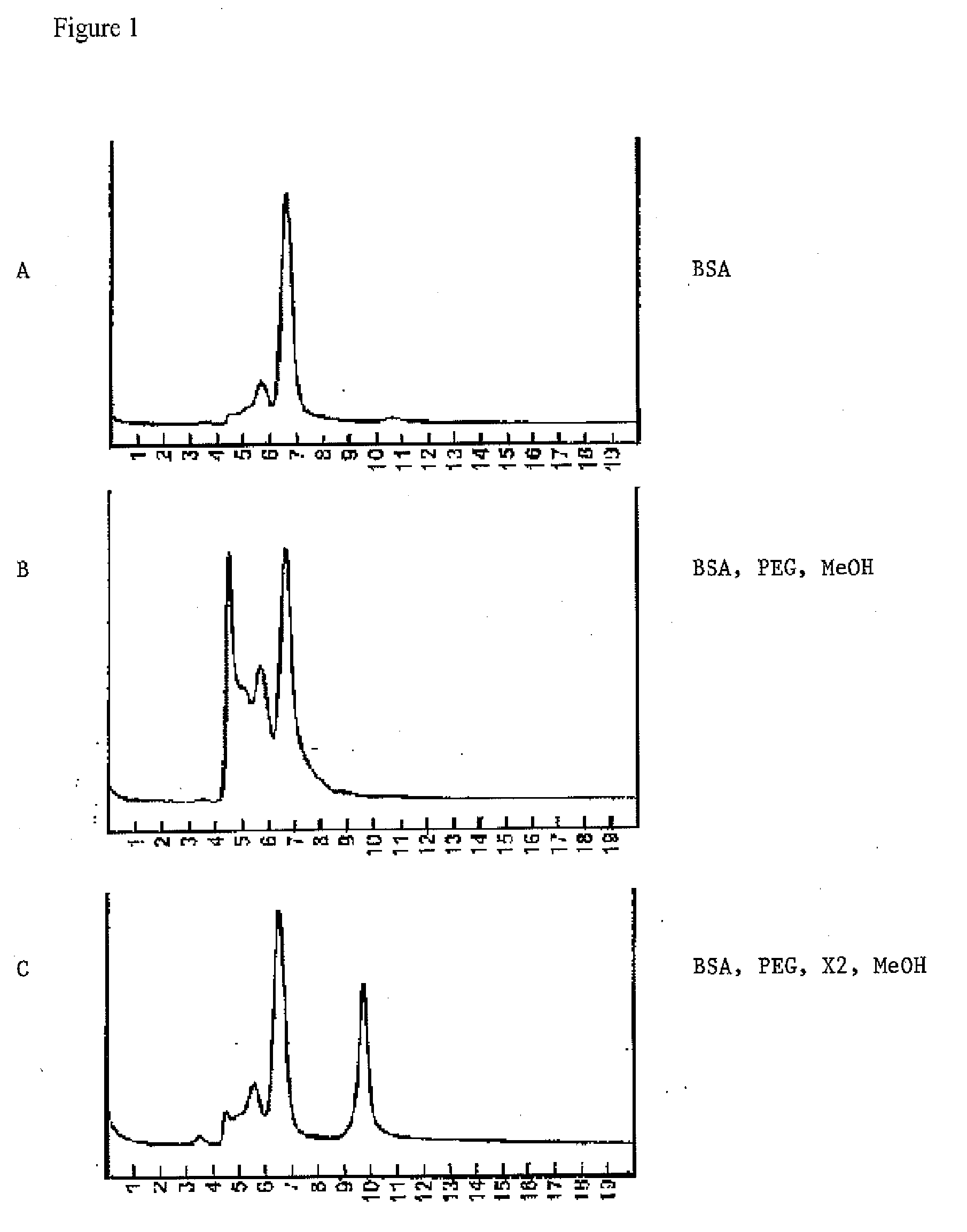
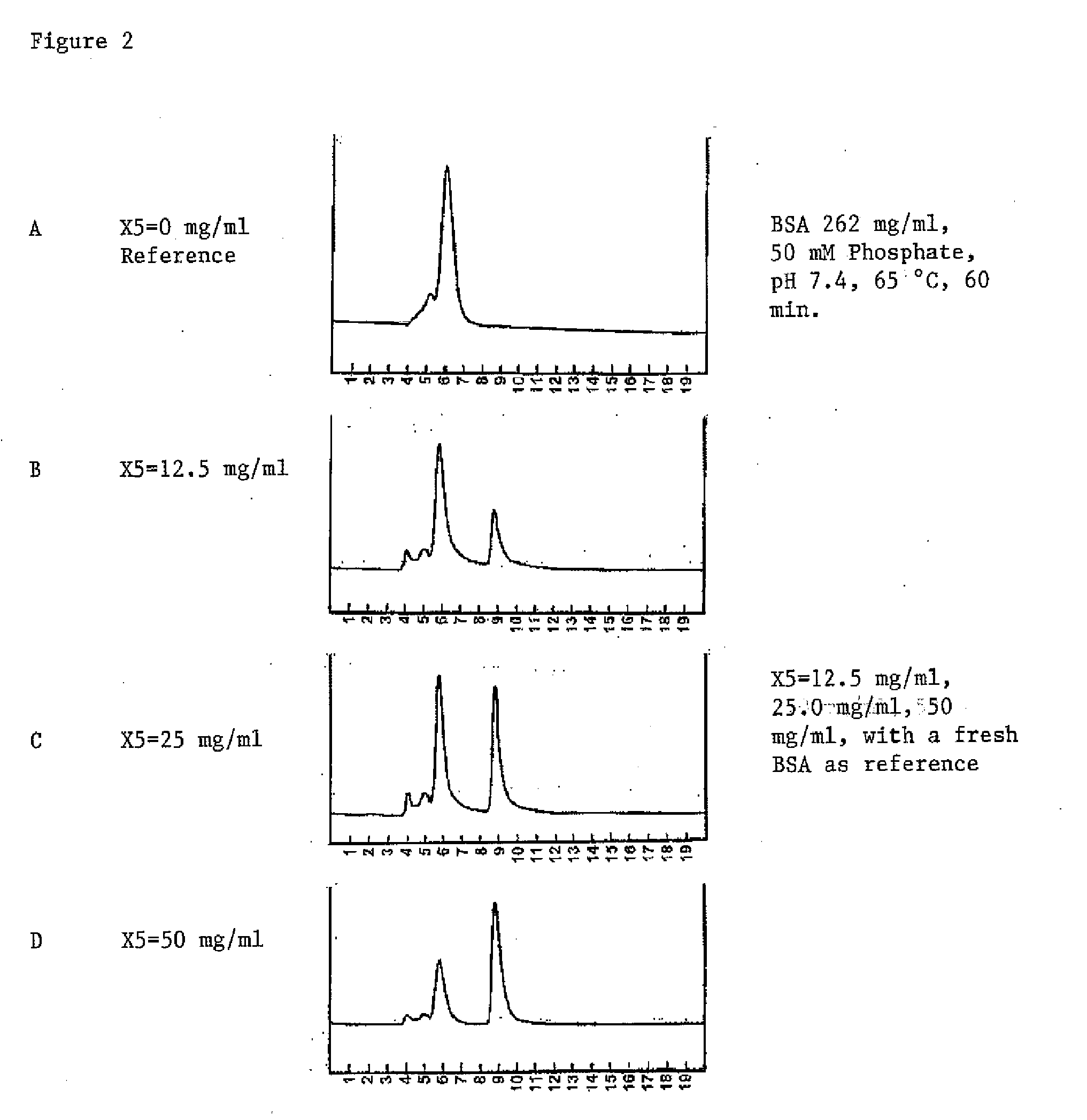
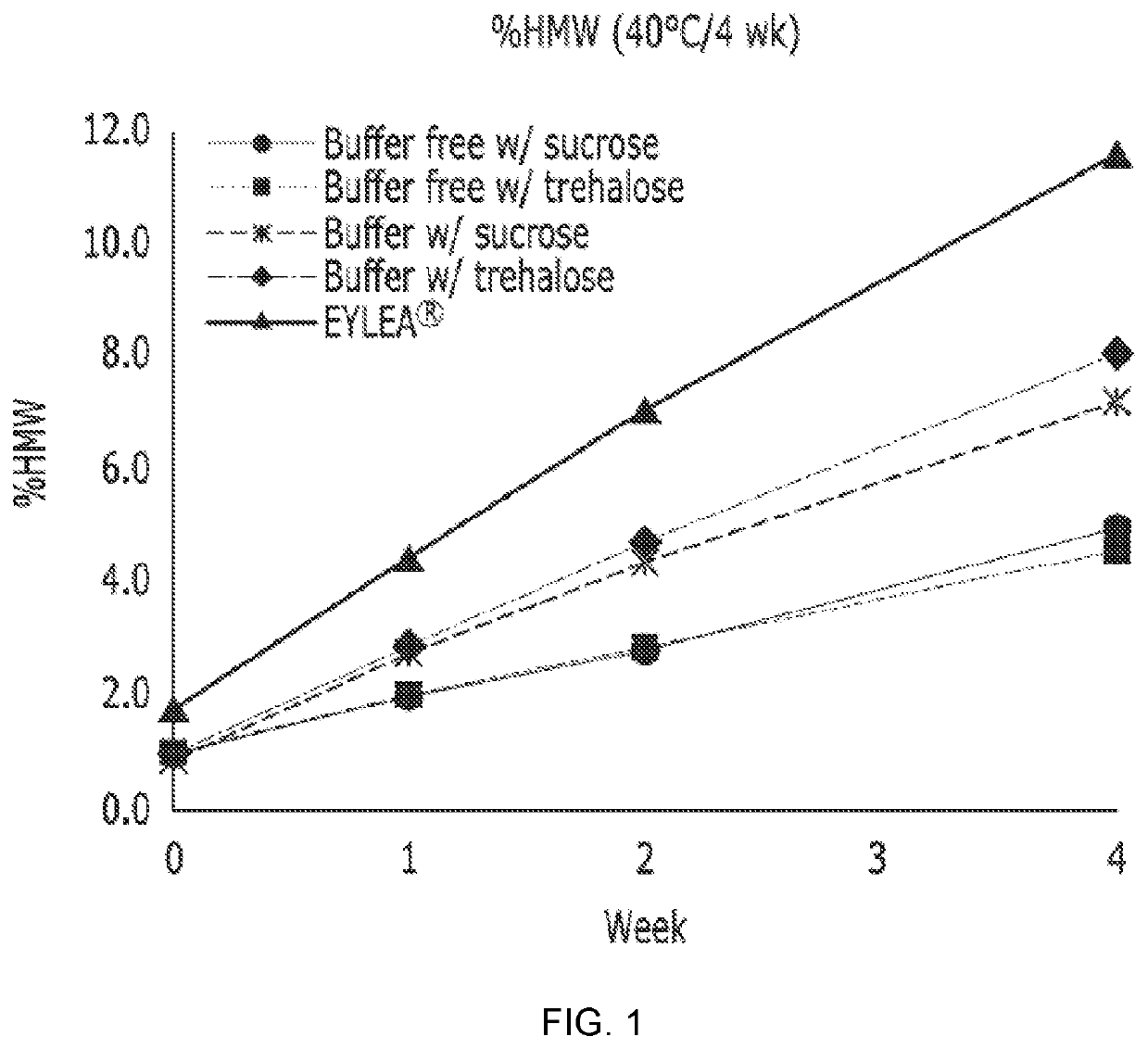
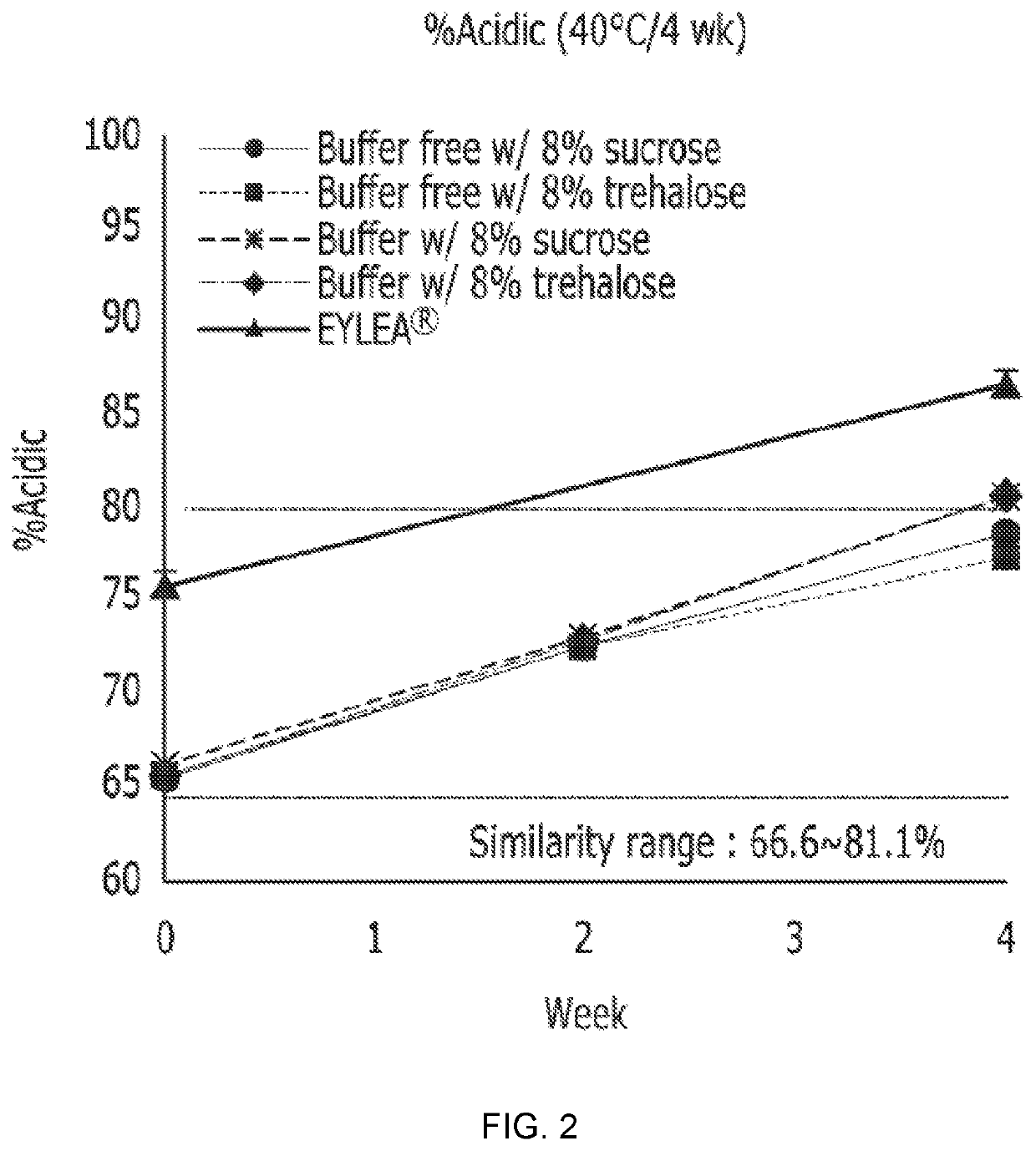
Excipients for Protein Stabilization
InactiveUS20110046052A1Minimize and eliminate protein aggregationImproving on-shelf storage stabilityPowder deliveryPeptide/protein ingredientsProtein moleculesProtein stabilization
Owner:YANG GUOHAN
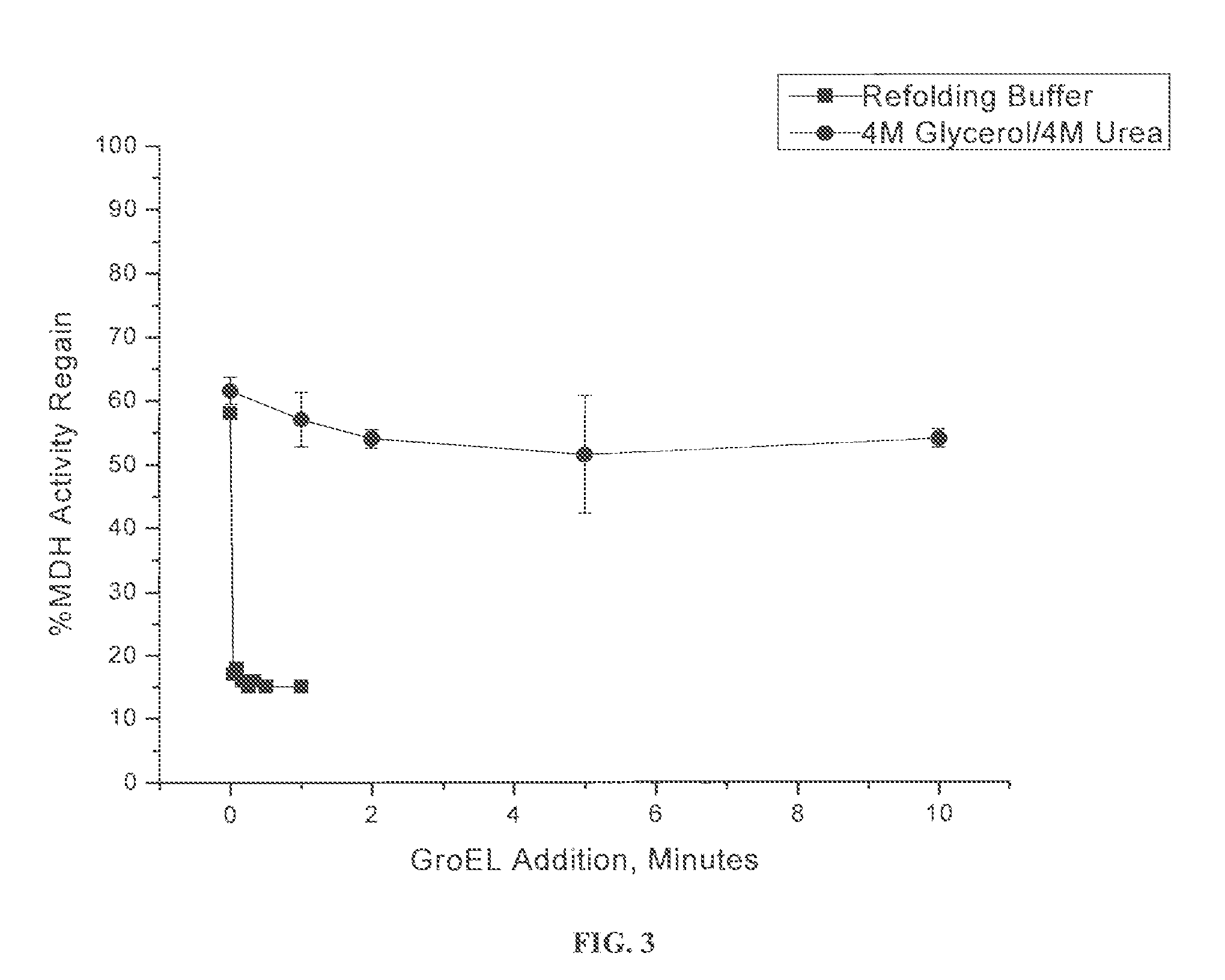
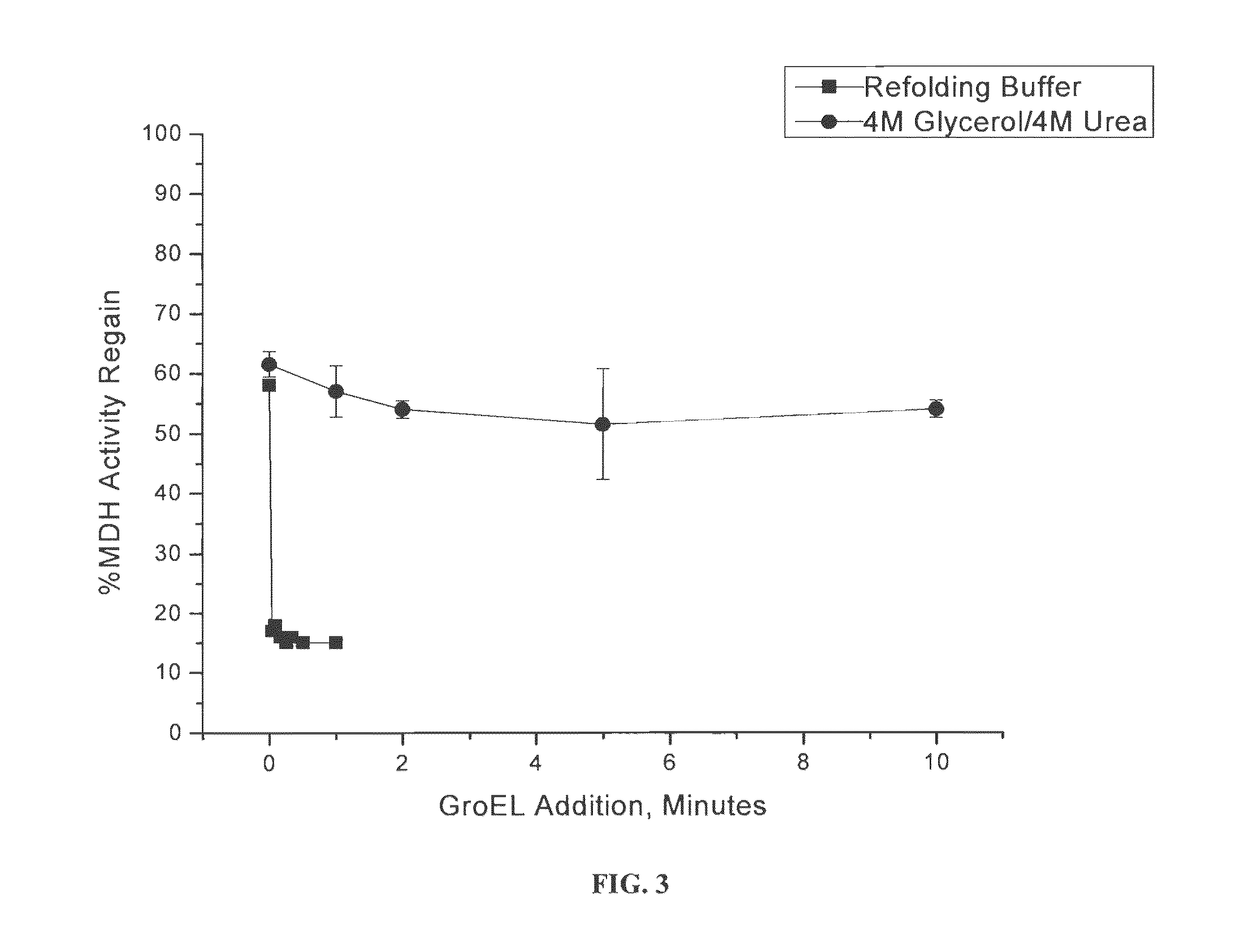
Osmolyte Mixture for Protein Stabilization
ActiveUS20110053795A1Speed up the processInhibit aggregationLibrary screeningEnzyme stabilisationProtein insertionOsmolyte
An osmolyte composition comprising 4 M glycerol and 4M urea for stabilizing previously transient protein folding intermediates as long-lived stable forms. A method to search for other possible stabilizing osmolyte mixtures using a screening array is also provided. These additional osmolyte mixtures may complement or augment the successful 4M glycerol / 4 M urea mixture.
Owner:UNIV KANSAS MEDICAL CENT
Protein stabilization formulations
InactiveUS7956028B2Preserving at least 60% of the biological activityImprove storage conditionsPeptide/protein ingredientsSkeletal disorderMedicineProtein stabilization
The present invention is directed to stabilizing Bone Morphogenetic Protein in various lyophilized formulations and compositions. The present invention comprises formulations primarily including trehalose as an excipient for lyophilized compositions and their subsequent storage and reconstitution, and can also optionally include other excipients, including buffers and surfactants.
Owner:DEPUY SYNTHES PROD INC
Protein stabilization in solution
InactiveUS20060160720A1Increase chanceSmall article dispensingPeptide/protein ingredientsCyclo olefin polymerSilicon dioxide
Provided are storage containers for proteinaceous pharmaceutical compositions which are characterized in, among other things, comprising (i) a wall portion, wherein an inner wall material thereof is selected from silica-coated glass, silicone-coated glass, polymers of non-cyclic olefins, cycloolefin polymers and cycloolefin / linear olefin copolymers and (ii) one or more closure portions not constituting part of the wall portion, and which contains a formulation of a protein. Also provided are new methods of storing proteinaceous compositions. In one aspect, the stored protein is characterized as having an amino-terminal γ-carboxyglutamic acid (Gla) domain with 9-12 Gla residues.
Owner:NOVO NORDISK AS
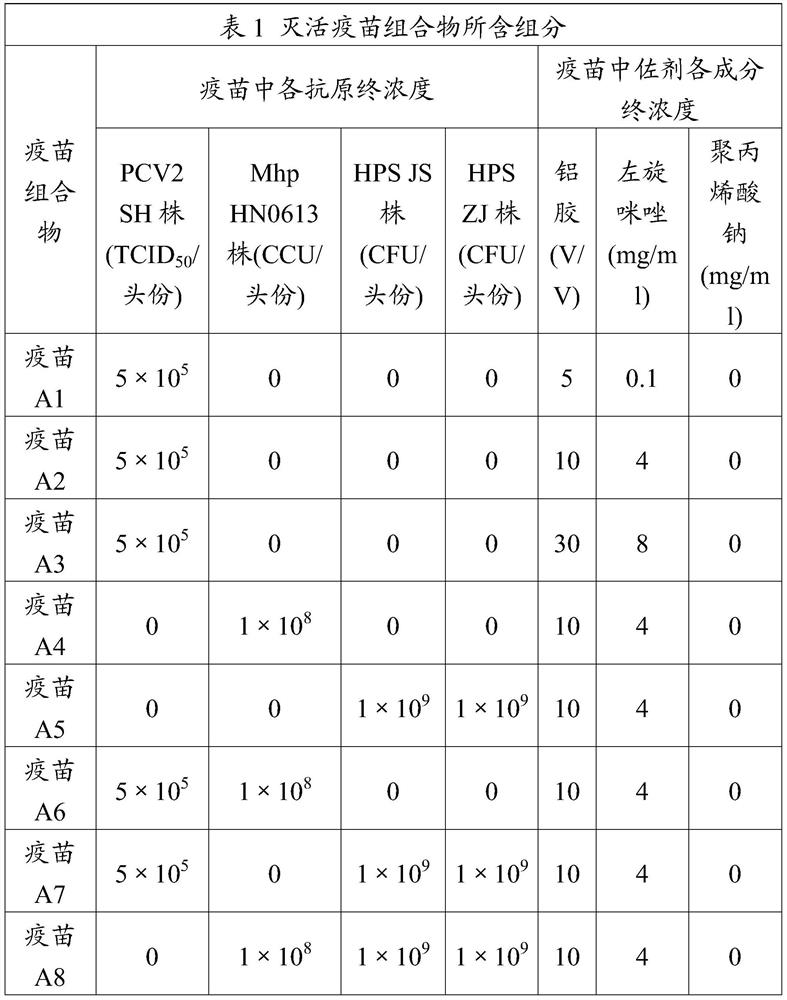
Adjuvant used for vaccine and application thereof
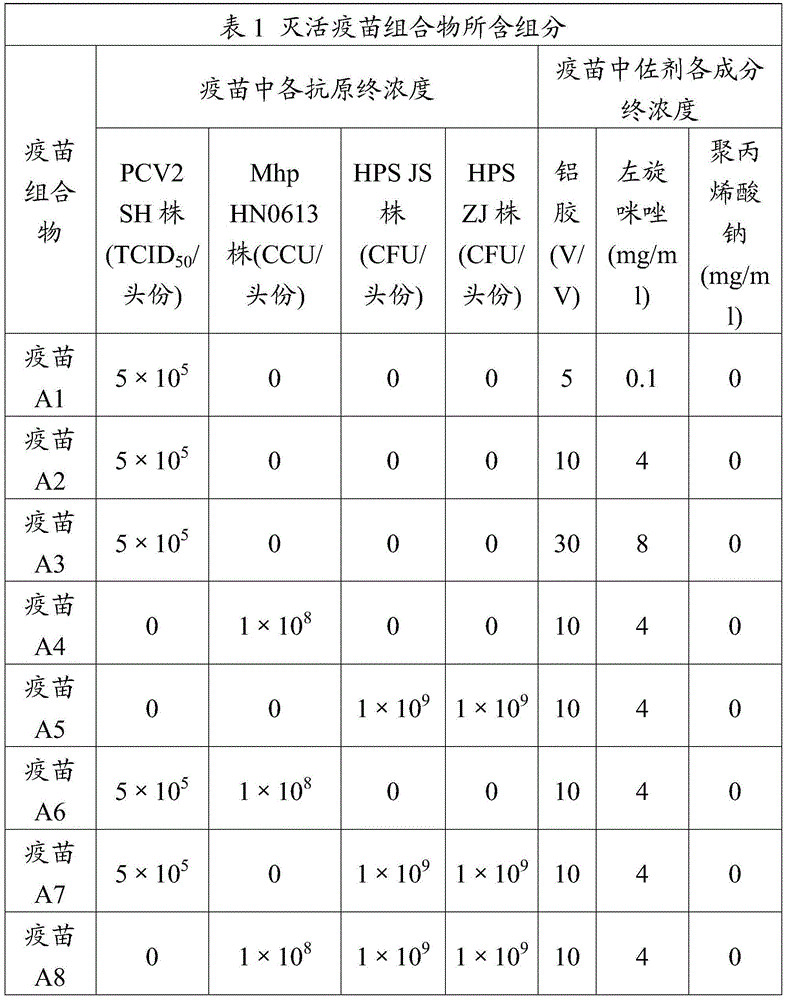
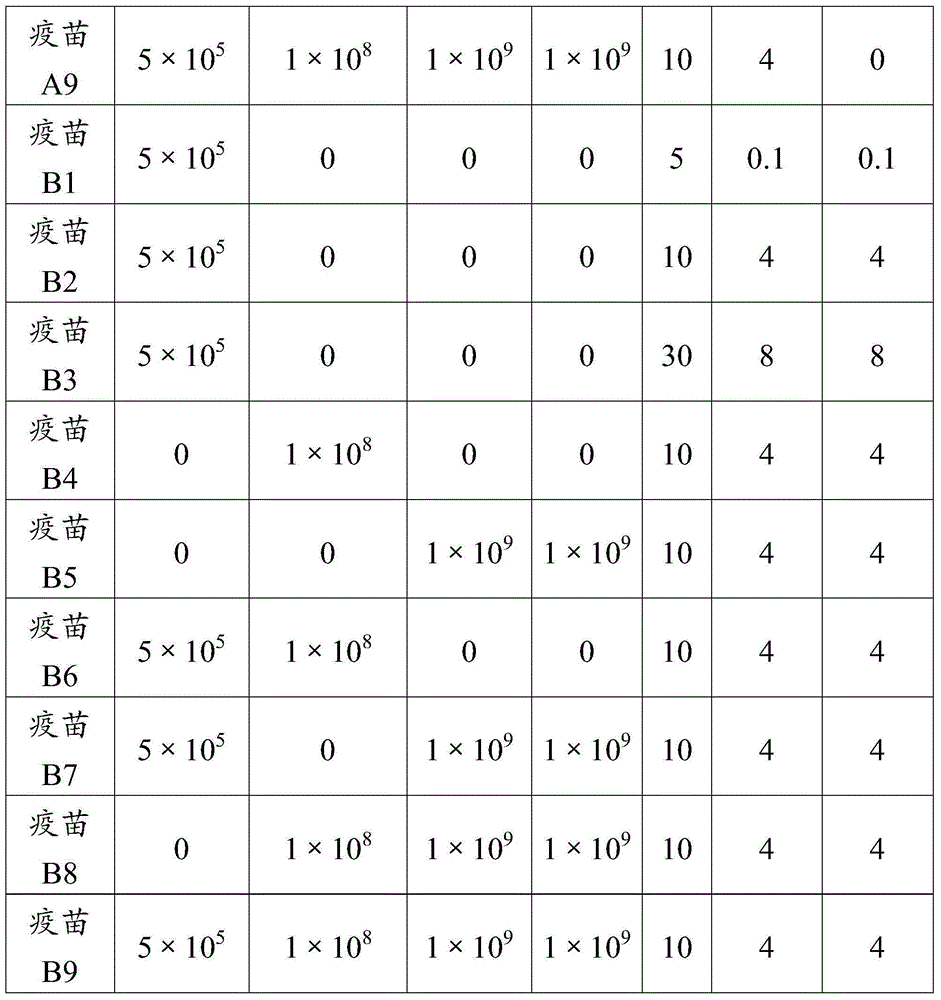
ActiveCN106177939ALow costEasy to usePharmaceutical non-active ingredientsImmunological disordersAdjuvantProtein stabilization
The invention provides an adjuvant used for vaccine. The adjuvant comprises alumina gel, levamisole or its derivative, and also polyacrylate. The invention also discloses a vaccine composition containing the adjuvant and an application thereof. The adjuvant can be used as the adjuvant for inactivated vaccine, and also can be used for preparing protein stabilization liquid. The vaccine composition containing the adjuvant can generate humoral immunity for human body and can generate cellular immunity, and can generate good immune response under condition of low antigen content and single immune, and can stabilize protein, degradation and irreversible change cannot be generated.
Owner:PU LIKE BIO ENG
Preparation method of low-fluorine Euphasia superb protein base stock
ActiveCN102524510AIncrease autolysisHigh yieldProtein composition from fishProtein stabilizationSlurry
The invention discloses a preparation method of low-fluorine Euphasia superb protein base stock. The preparation method comprises the following steps of: 1, low-temperature autolysis: homogenizing fresh and alive Euphasia superb and treating through ultraviolet irradiation to realize autolysis; 2, protein extraction: adding water into homogenate, stirring and extracting, carrying out centrifugal separation to obtain an upper-layer liquid phase and precipitates; 3, protein stabilization: refrigerating the upper-layer liquid for storage or drying the upper-layer liquid before refrigerating the upper-layer liquid for storage; 4, protein preparation: thawing the refrigerated upper-layer liquid; adjusting pH value to 4.8-5.8 or heating for 10-30 minutes at 80-100 DEG C and then centrifuging to obtain precipitate products and carrying out spray drying to finally obtain the Euphasia superb protein base stock. The preparation method is simple to operate and is quite suitable for field operation on a fishing ship; through adoption of the preparation method, the protein recovery rate is up to 46%-51% and the fat recovery rate is up to 54%-60%; and in the prepared low-fluorine Euphasia superb protein base stock, the protein content is 70-78% / 100g of dry base stock, the fat content is 25-31g / 100g of dry base stock and the fluorine content is lower than 1.5 mcg. / g of dry base stock.
Owner:DALIAN POLYTECHNIC UNIVERSITY
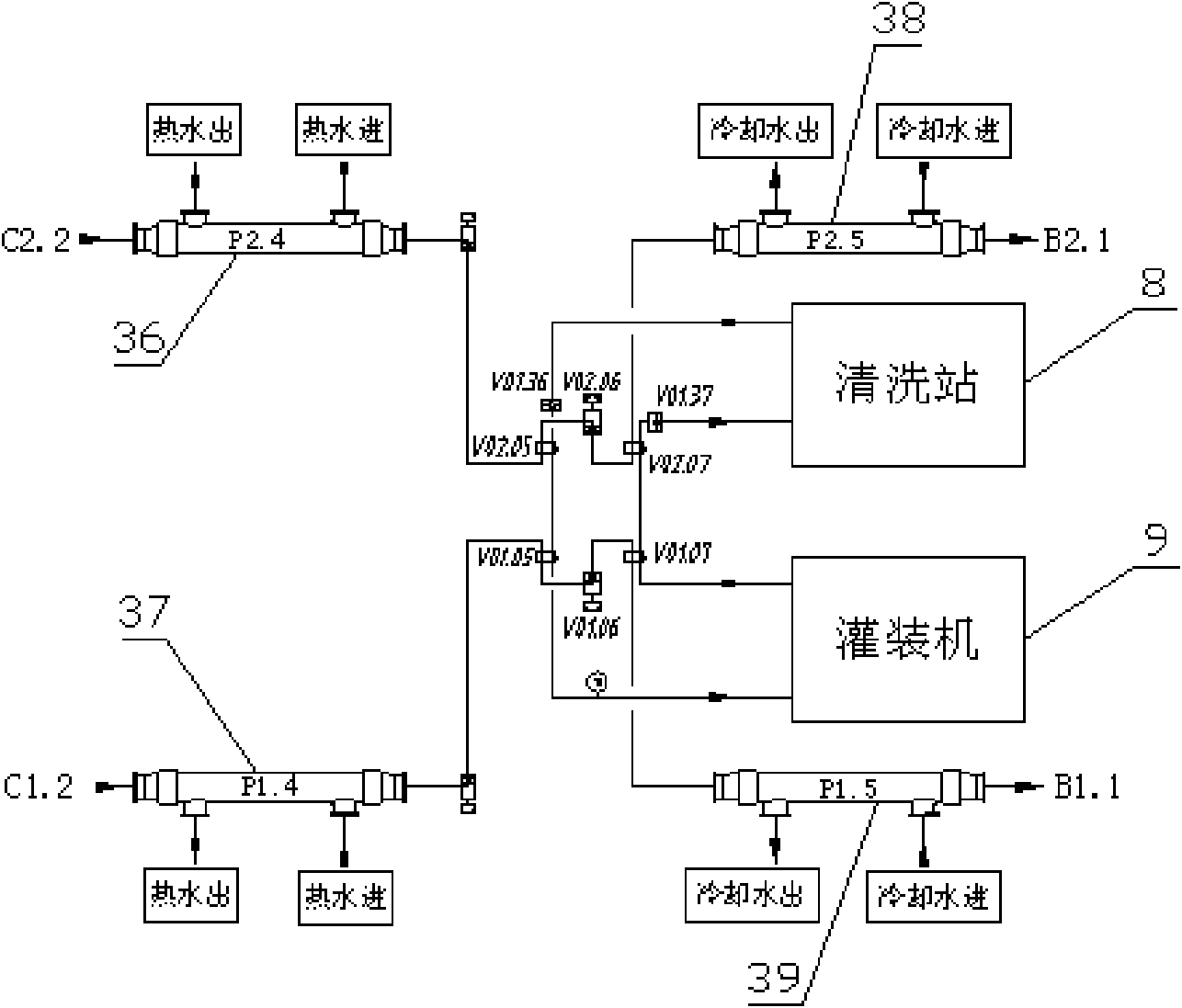
Double tube type superhigh temperature sterilization machine system and sterilization technology
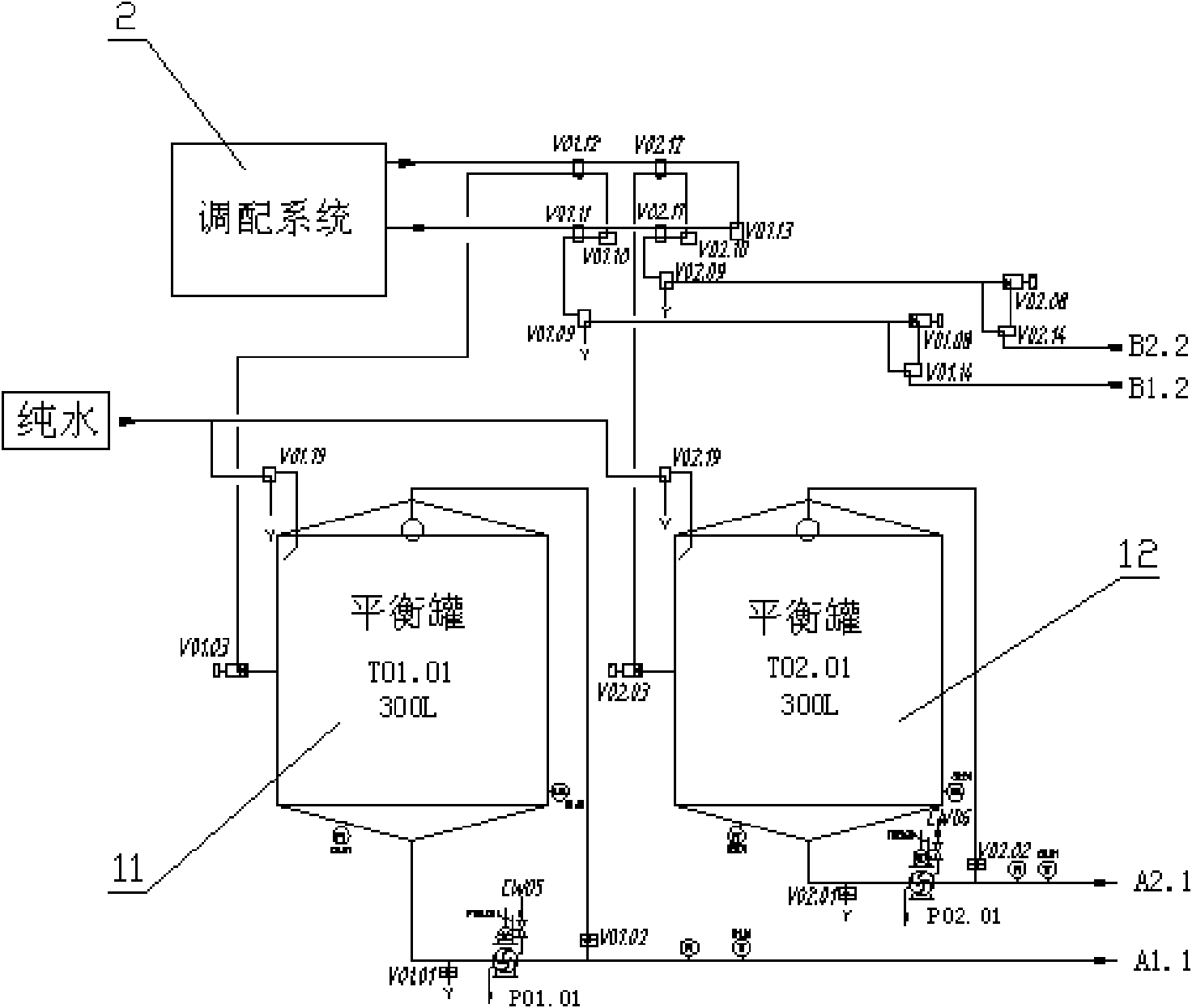
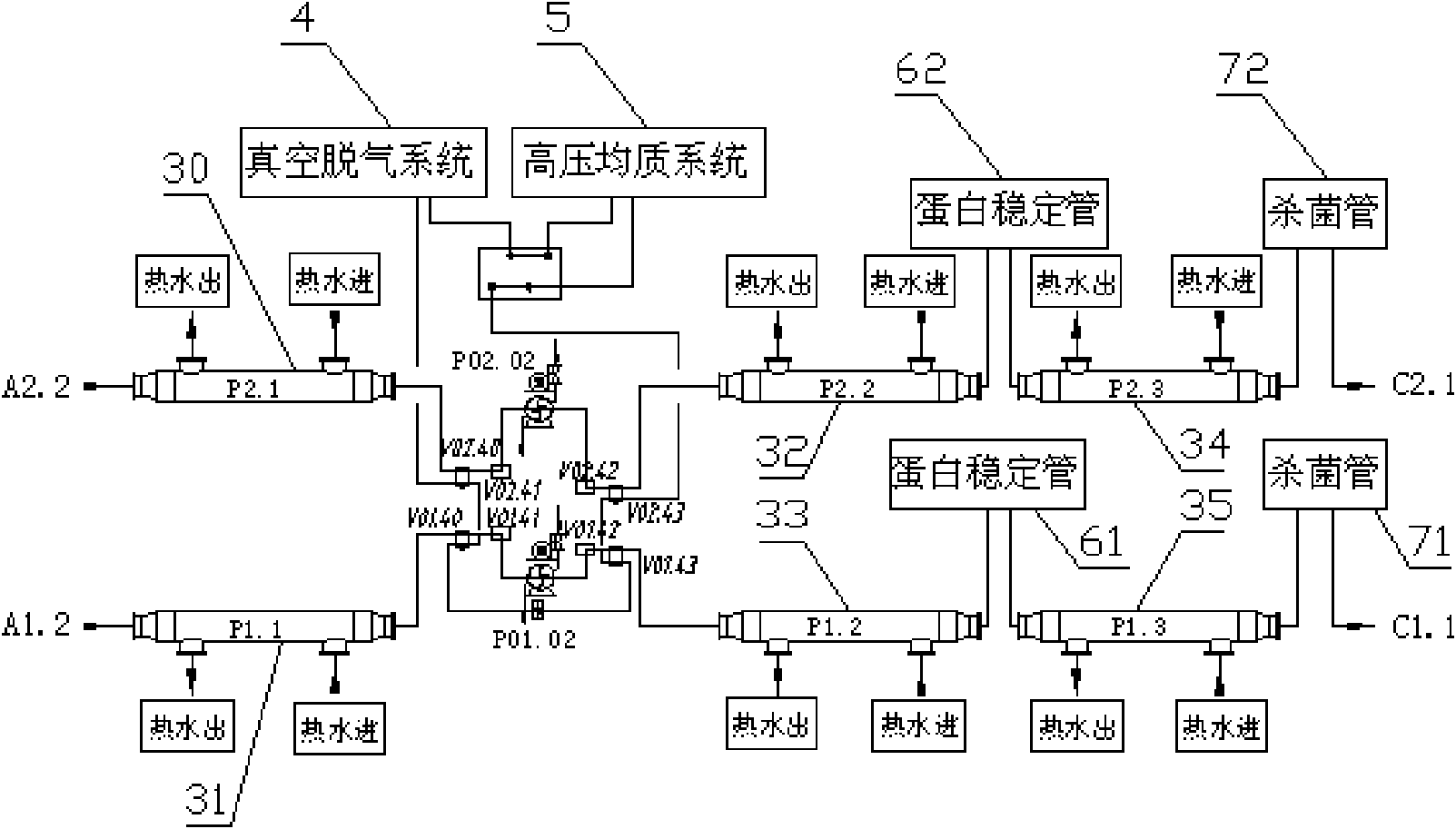
ActiveCN101653170AGuaranteed continuityGood technical effectMilk preservationFood scienceProduction lineDouble tube
The invention relates to a sterilization machine system for dairy and beverage and a sterilization technology and discloses a double tube type superhigh temperature sterilization machine system and asterilization technology. The invention comprises two sets of completely independent tubular type sterilization machine systems which share a set of vacuumizing degassing system and high pressure homogenization system. Each set of sterilization system includes a charging balance cylinder system, a pre-heating section, a protein stabilization section, a high temperature sterilization section, a cooling filling section, a cooling backflow section and a hot water circulation system. Two sets of sterilization machine systems are connected through a plurality of reversing valves and aseptic valvesto realize the seamless switching operation between two sets of systems, thus, achieving the uninterrupted production of the sterilization machine system so as to substantially improve the productionefficiency of the heat filling production line.
Owner:SONGYUAN MACHINERY MFG
Use of l-carnitine as stabilizing agent of proteins
The present invention relates to the technical field of stabilizing proteins, in particular to the therapeutic aspects of protein stabilization. L-carnitine is a useful agent for stabilizing proteins, and in a particularly favourable aspect in proteins used in the medical field. In a preferred aspect, L-carnitine is used for protecting chaperone activity, and in the medical field for preserving the activity of altered chaperone proteins. In connection with this invention L-carnitine is used for the preparation of a medicament for the treatment of diseases due to altered chaperone proteins, such as eye diseases, in particular cataract.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
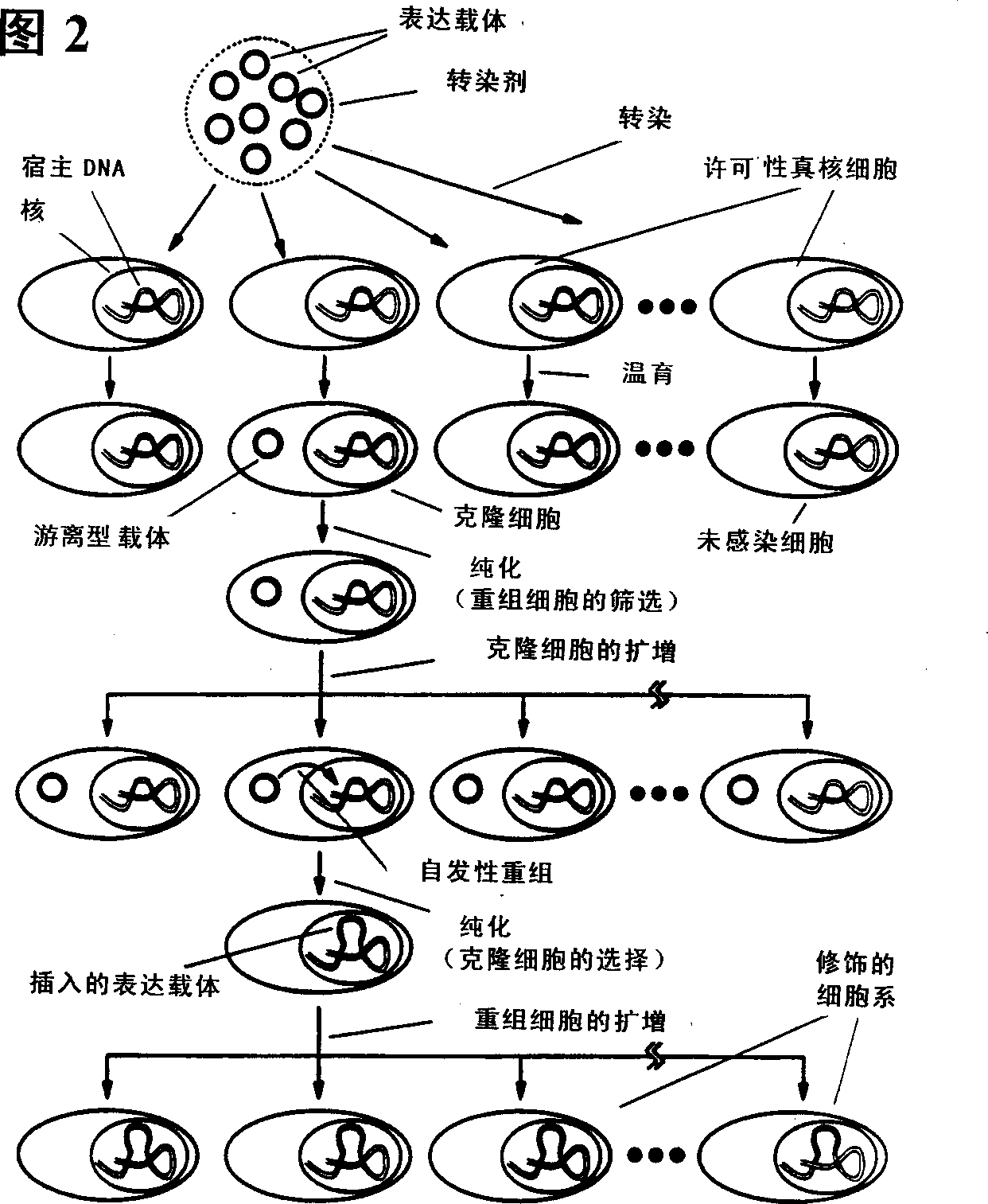
Method for enhanced protein stabilization and production of cell liell lines useful for production of such stabilized proteins
A method for increasing production yield of viruses, viral proteins, and other related biological materials through enhanced control and stabilization of protein production via stress proteins and the resultant protein products. The present invention is also directed to methods for selection or engineering of cell lines yielding such enhanced stabilized products. More specifically, example embodiments of the present invention are directed to methods for enhancing production of a viral agent, production of cell lines exhibiting permanent genetic modification, production of permissive eucaryotic cell lines, enhancing functional recombinant product yield, and the products of such methods.
Owner:PHOTOGEN INC
Protein stabilization by domain insertion into a thermophilic protein
ActiveUS8592192B2Improve dynamic stabilityCompromise in its activityEnzyme stabilisationImmunoglobulinsProtein targetADAMTS Proteins
A strategy to improve protein stability by domain insertion. TEM 1 beta-lactamase (BLA) and exo-inulinase, as model target enzymes, are inserted into a hyperthermophilic maltose binding protein from Pyrococcus furiosus (PfMBP). Unlike conventional protein stabilization methods that employ mutations and recombinations, the inventive approach does not require any modification on a target protein except for its connection with a hyperthermophilic protein scaffold. For that reason, target protein substrate specificity was largely maintained, which is often modified through conventional protein stabilization methods. The insertion was achieved through gene fusion by recombinant DNA techniques.
Owner:POLYTECHNIC INSTITUTE OF NEW YORK UNIVERSITY
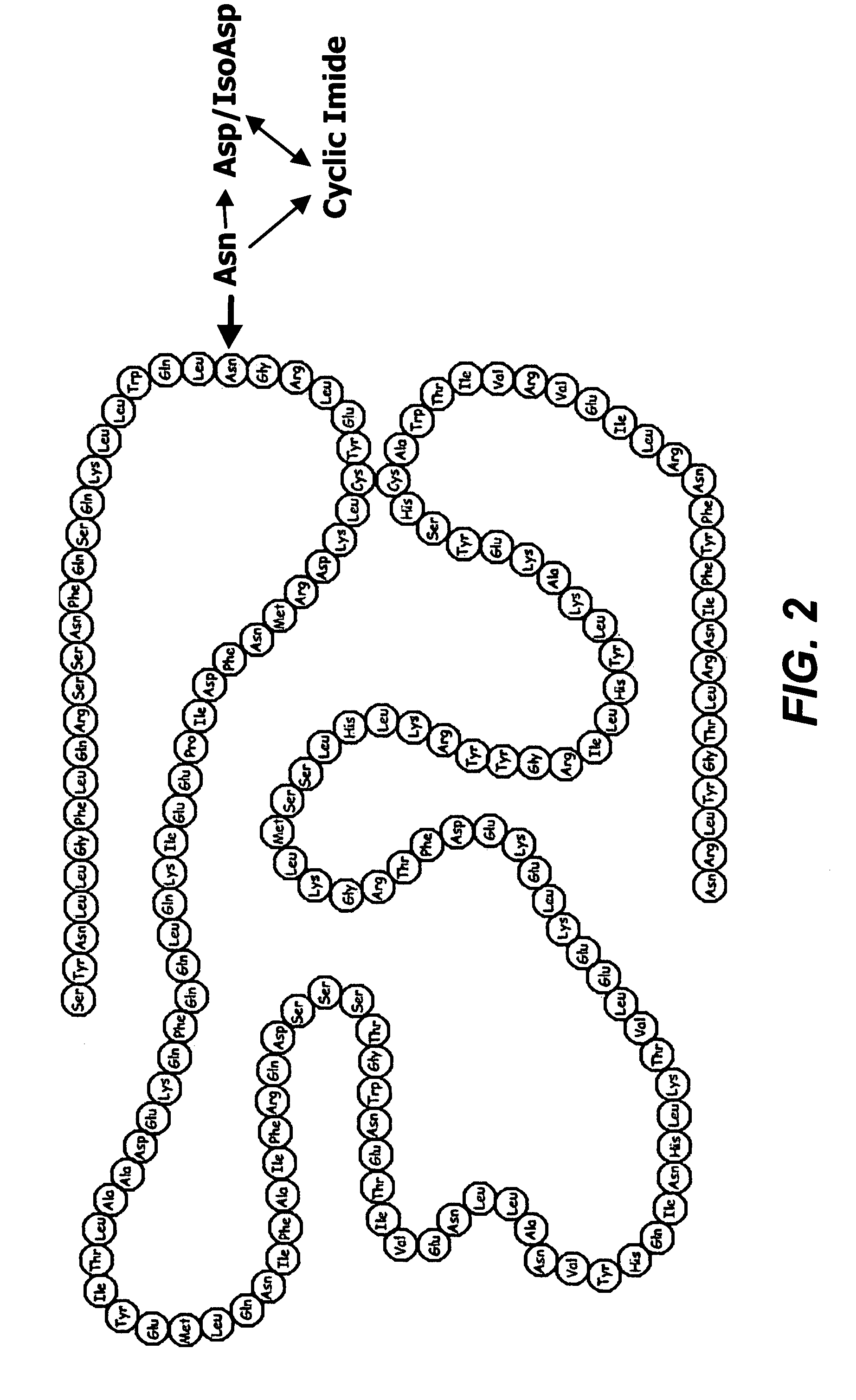
Deamidated interferon-beta
InactiveUS7595040B2Reduce frequencyImprove biological activityNervous disorderPeptide/protein ingredientsDiseaseInterferon alpha
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Protein stabilization by domain insertion into a thermophilic protein
ActiveUS20100227374A1Improve dynamic stabilityImprove stabilityEnzyme stabilisationImmunoglobulinsProtein targetProtein insertion
A strategy to improve protein stability by domain insertion. TEM 1 beta-lactamase (BLA) and exo-inulinase, as model target enzymes, are inserted into a hyperthermophilic maltose binding protein from Pyrococcus furiosus (PfMBP). Unlike conventional protein stabilization methods that employ mutations and recombinations, the inventive approach does not require any modification on a target protein except for its connection with a hyperthermophilic protein scaffold. For that reason, target protein substrate specificity was largely maintained, which is often modified through conventional protein stabilization methods. The insertion was achieved through gene fusion by recombinant DNA techniques
Owner:POLYTECHNIC INSTITUTE OF NEW YORK UNIVERSITY
Preparation method of low-fluorine Euphasia superb protein base stock
Owner:DALIAN POLYTECHNIC UNIVERSITY
Enzyme stabilization by cationic surfactants
The present invention provides methods and compositions for protein stabilization, particularly the stabilization of polymerases in aqueous solutions with cationic surfactants. The present invention further provides cationic surfactants, including polyethoxylated amines, that stabilize thermostable and thermolabile enzymes in solution. These surfactants stabilize the activity of various enzymes, including thermostable DNA polymerases, thermolabile DNA polymerases and reverse transcriptases.
Owner:PROMEGA CORP
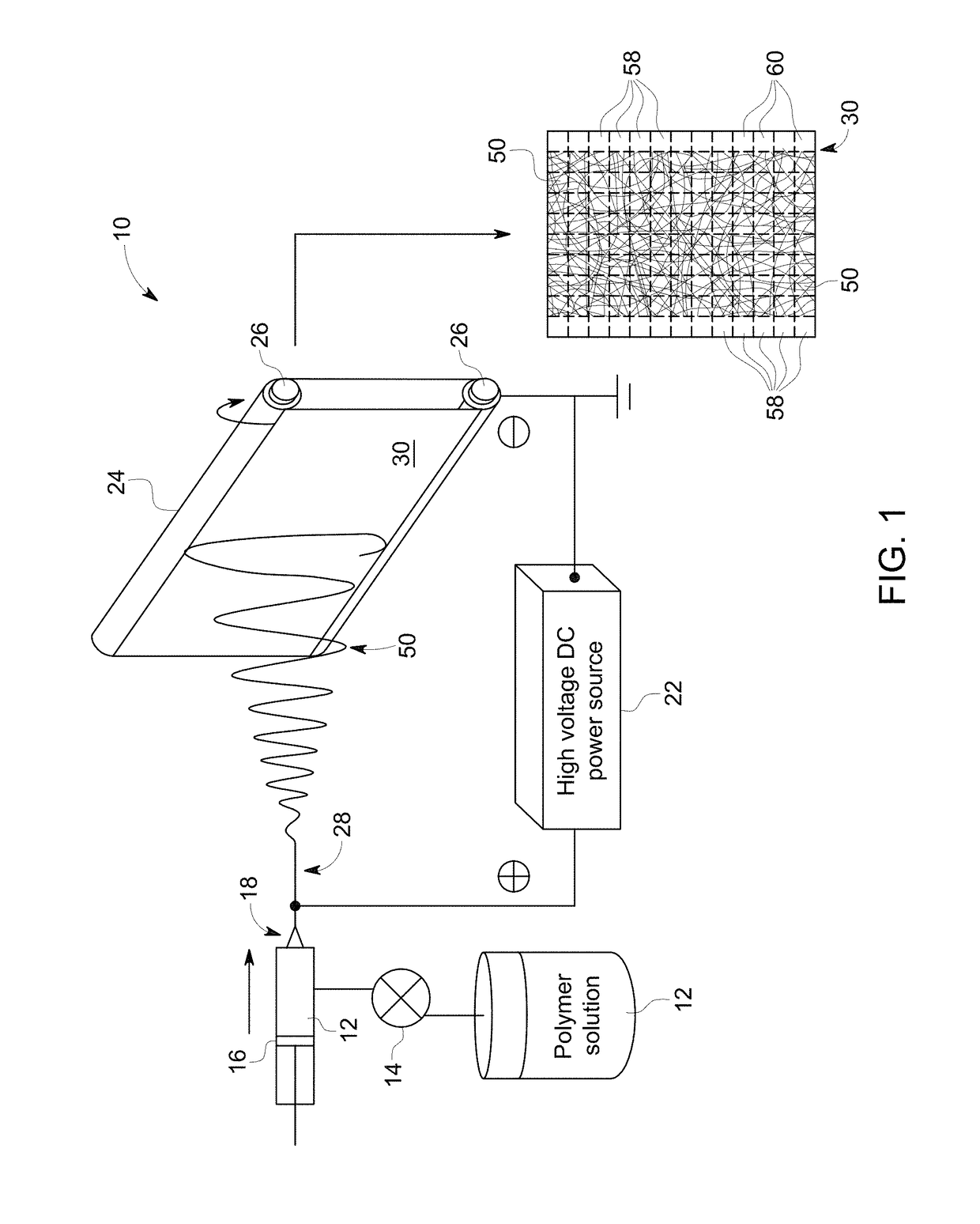
Electrospun fibers for protein stabilization and storage
An electrospinning approach is disclosed for generating a dissolvable formulation of a reagent of interest in a nanoscale fiber medium. In one embodiment, the nanoscale fibers can incorporate and stabilize biological agents of interest, such as for storage at room temperature for extended periods. In one implementation, the fibers can be produced in a continuous manner and dissolve rapidly.
Owner:GLOBAL LIFE SCI SOLUTIONS OPERATIONS UK LTD
Deamidated interferon-beta
InactiveUS20060120998A1Reduce frequencyImprove biological activityNervous disorderPeptide/protein ingredientsC-peptideOrganism
Interferon-β protein analogs in which the asparagine at position 25, numbered in accordance with native interferon-β, is deamidated exhibit a biological activity of native human interferon-β at an increased level and do not require HA for protein stabilization. The deamidated product is suitable for large scale manufacturing for incorporation in HA-containing or HA-free therapeutics for treatment of diseases including multiple sclerosis. An endoproteinase-C peptide map technique that produces a fingerprint profile for proteins using an enzymatic digest followed by RP-HPLC is also useful in quality control as an ID and / or quantitative test for the deamidated products.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Protein stabilization in solution
Provided are storage containers for proteinaceous pharmaceutical compositions which are characterized in, among other things, comprising (i) a wall portion, wherein an inner wall material thereof is selected from silica-coated glass, silicone-coated glass, polymers of non-cyclic olefins, cycloolefin polymers and cycloolefin / linear olefin copolymers and (ii) one or more closure portions not constituting part of the wall portion, and which contains a formulation of a protein. Also provided are new methods of storing proteinaceous compositions. In one aspect, the stored protein is characterized as having an amino-terminal gamma-carboxyglutamic acid (Gla) domain with 9-12 Gla residues.
Owner:NOVO NORDISK HEALTH CARE AG
Protein stabilization in solution
Provided are storage containers for proteinaceous pharmaceutical compositions which are characterized in, among other things, comprising (i) a wall portion, wherein an inner wall material thereof is selected from silica-coated glass, silicone-coated glass, polymers of non-cyclic olefins, cycloolefin polymers and cycloolefin / linear olefin copolymers and (ii) one or more closure portions not constituting part of the wall portion, and which contains a formulation of a protein. Also provided are new methods of storing proteinaceous compositions. In one aspect, the stored protein is characterized as having an amino-terminal γ-carboxyglutamic acid (Gla) domain with 9-12 Gla residues.
Owner:NOVO NORDISK HEALTH CARE AG
Deamidated interferon-beta
Owner:CHIRON CORP
Excipients for protein stabilization
InactiveUS8642532B2Improving on-shelf storage stabilityImprove solubilityOrganic active ingredientsBiocideCell AggregationsProtein molecules
Owner:YANG GUOHAN
A kind of vaccine adjuvant and its application
ActiveCN106177939BLow costEasy to usePharmaceutical non-active ingredientsImmunological disordersAdjuvantLevamisole
The invention provides an adjuvant for vaccines, which includes aluminum colloid, levamisole or derivatives thereof, and polyacrylate. The invention also discloses a vaccine composition containing the adjuvant and its application. The adjuvant can not only be used as an adjuvant for inactivated vaccines, but can also be used to prepare protein stabilizing solutions. Vaccine compositions containing this adjuvant can not only cause the body to produce humoral immunity but also cellular immunity. It can also produce a better immune response when the antigen content is low and a single immunization is performed; it can also stabilize the protein without degradation or irreversibility. Variety.
Owner:PU LIKE BIO ENG
Protein stabilization
InactiveUS20050148058A1High activityReduced activitySenses disorderFungiProtein stabilizationHybrid protein
The present invention relates to protein chaperones, such as hybrid chaperones and methods for stabilizing proteins and protein activities comprising adding said protein chaperone to the protein. The present invention also provides a stabilized protein formulation comprising said protein chaperone associated with a protein and further relates to the enhancement of native chaperone activity by making hybrid protein chaperones.
Owner:UNIV COURT OF THE UNIV OF DUNDEE
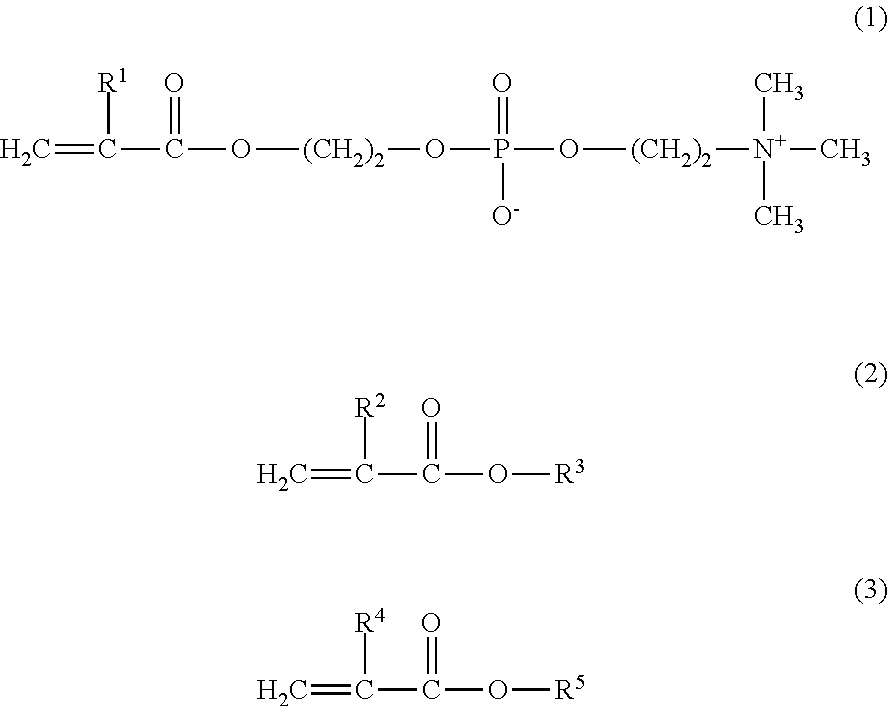
Protein stabilizer and protein stabilization reagent
ActiveUS20200102415A1Stable storageLow viscosityPharmaceutical delivery mechanismPeptide preparation methodsAbzymeAntiendomysial antibodies
There is provided a protein stabilizer for stably storing a protein in a solution. The protein may be an enzyme, an antibody, an enzyme-labeled antibody, or the like for a biochemical assay, etc. There is further provided a protein stabilization reagent comprising a protein-containing solution and the protein stabilizer dissolved therein. The protein stabilizer comprises a copolymer prepared by copolymerizing a monomer (a) of the following formula (1), a monomer (b) of the following formula (2), and a monomer (c) of the following formula (3).
Owner:NOF CORP
Protein stabilizer and protein stabilization reagent
ActiveUS11261275B2Stable storageLow viscosityPharmaceutical delivery mechanismPeptide preparation methodsAbzymeAntiendomysial antibodies
There is provided a protein stabilizer for stably storing a protein in a solution. The protein may be an enzyme, an antibody, an enzyme-labeled antibody, or the like for a biochemical assay, etc. There is further provided a protein stabilization reagent comprising a protein-containing solution and the protein stabilizer dissolved therein. The protein stabilizer comprises a copolymer prepared by copolymerizing a monomer (a) of the following formula (1), a monomer (b) of the following formula (2), and a monomer (c) of the following formula (3).
Owner:NOF CORP
Use of L-Carnitine as stabilizing agent of proteins
The present invention relates to the technical field of stabilizing proteins, in particular to the therapeutic aspects of protein stabilization. L-carnitine is a useful agent for stabilizing proteins, and in a particularly favourable aspect in proteins used in the medical field. In a preferred aspect, L-carnitine is used for protecting chaperone activity, and in the medical field for preserving the activity of altered chaperone proteins. In connection with this invention L-carnitine is used for the preparation of a medicament for the treatment of diseases due to altered chaperone proteins, such as eye diseases, in particular cataract.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Osmolyte mixture for protein stabilization
ActiveUS9052323B2Speed up the processInhibit aggregationLibrary screeningEnzyme stabilisationOsmolyteGlycerol
An osmolyte composition comprising 4 M glycerol and 4M urea for stabilizing previously transient protein folding intermediates as long-lived stable forms. A method to search for other possible stabilizing osmolyte mixtures using a screening array is also provided. These additional osmolyte mixtures may complement or augment the successful 4M glycerol / 4 M urea mixture.
Owner:UNIV KANSAS MEDICAL CENT
Liquid composition comprising protein
PendingUS20220054586A1Reduce physical activityIncreased riskPeptide/protein ingredientsPharmaceutical delivery mechanismActive agentProtein stabilization
The present invention relates to a protein-stabilized liquid formulation and provides: a composition for protein stabilization, the composition including a stabilizer and a surfactant and not including a buffer; a protein liquid composition including the composition for stabilization and a protein; and a method of producing a protein-stabilized liquid composition which uses the composition for stabilization.
Owner:SAMSUNG BIOEPIS CO LTD
Compound containing disulfide bond and its use and preparation method
ActiveCN108997447BImprove stabilityAchieve in situ degradationSugar derivativesPeptide preparation methodsDisulfide bondingActive agent
The invention provides a compound containing a disulfide bond and its use and preparation method. The general structural formula of the compound is: . Wherein, said A is a substituted or unsubstituted hydrophilic monosaccharide group, oligosaccharide group, or ammonium ammonium salt group, and said B is a hydrophobic aliphatic group within 40 carbon atoms. In the present invention, by introducing a disulfide bond at the corresponding position of the small molecule, it not only retains the film dissolution and protein stabilization effect equivalent to or even better than that of the original detergent, but also can completely realize the In situ degradation, degraded into two thiol fragments, losing the properties of surfactants. With the aid of the compound, it can be used to prepare and study membrane proteins.
Owner:SHANGHAI TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com