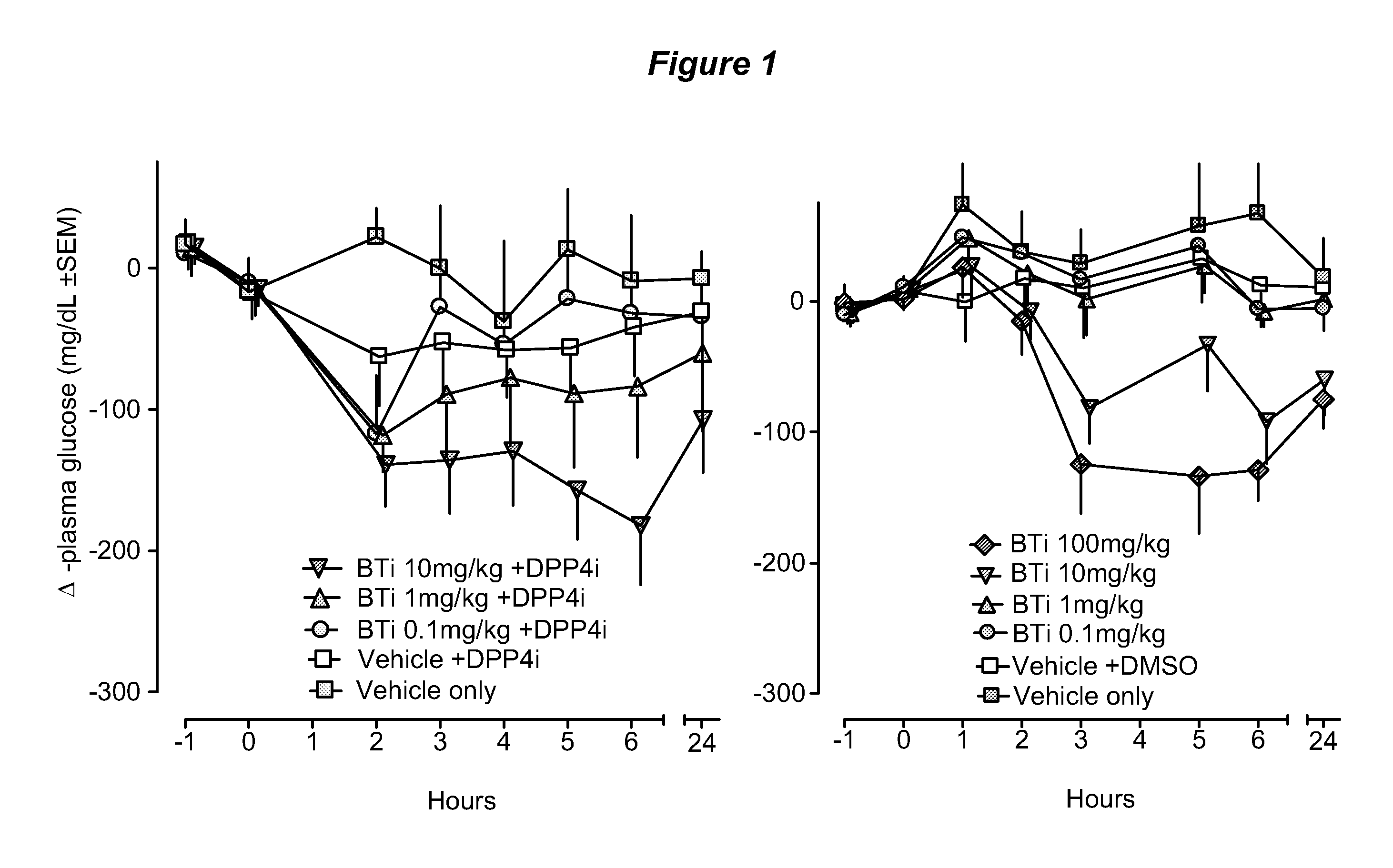
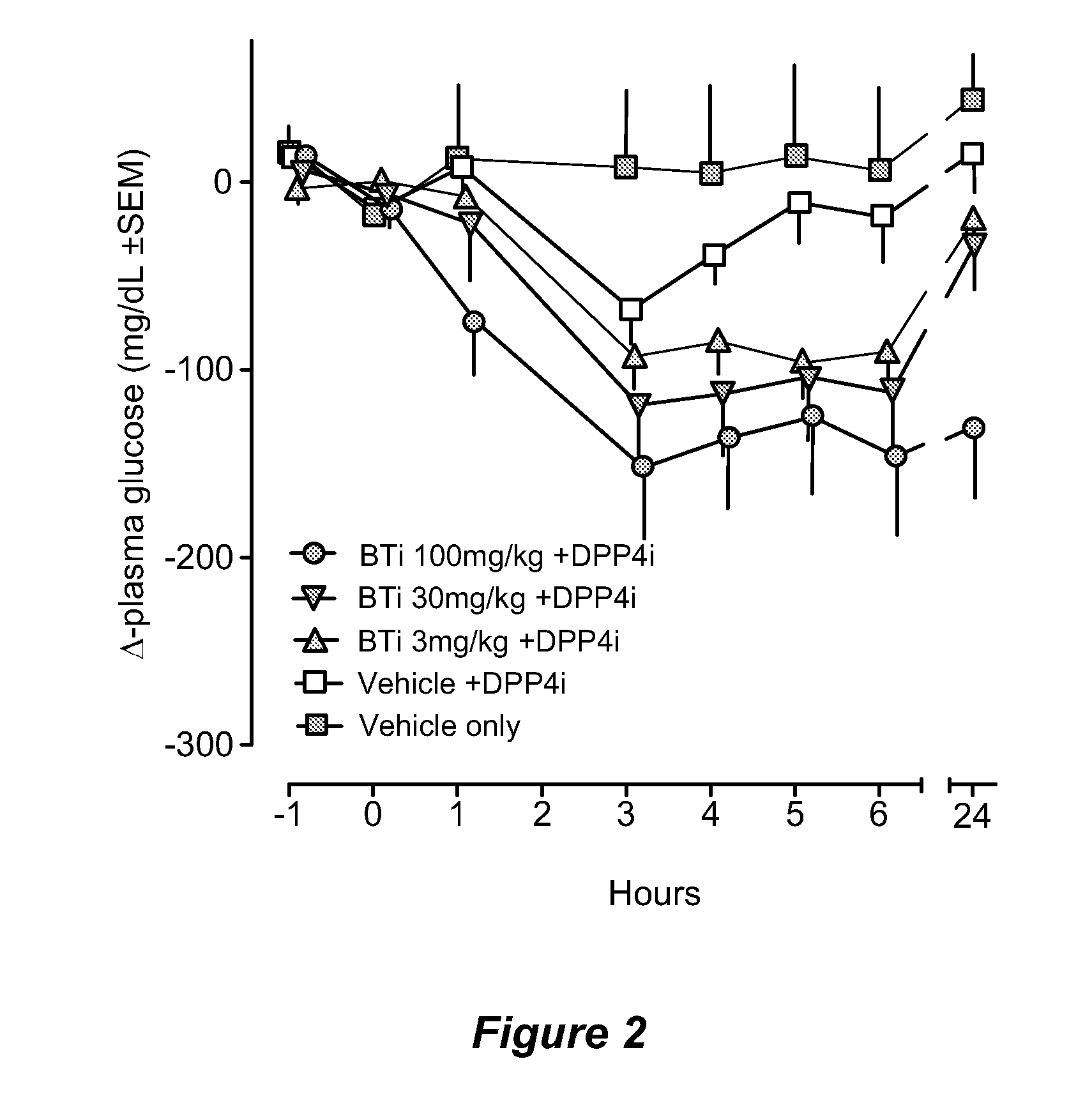
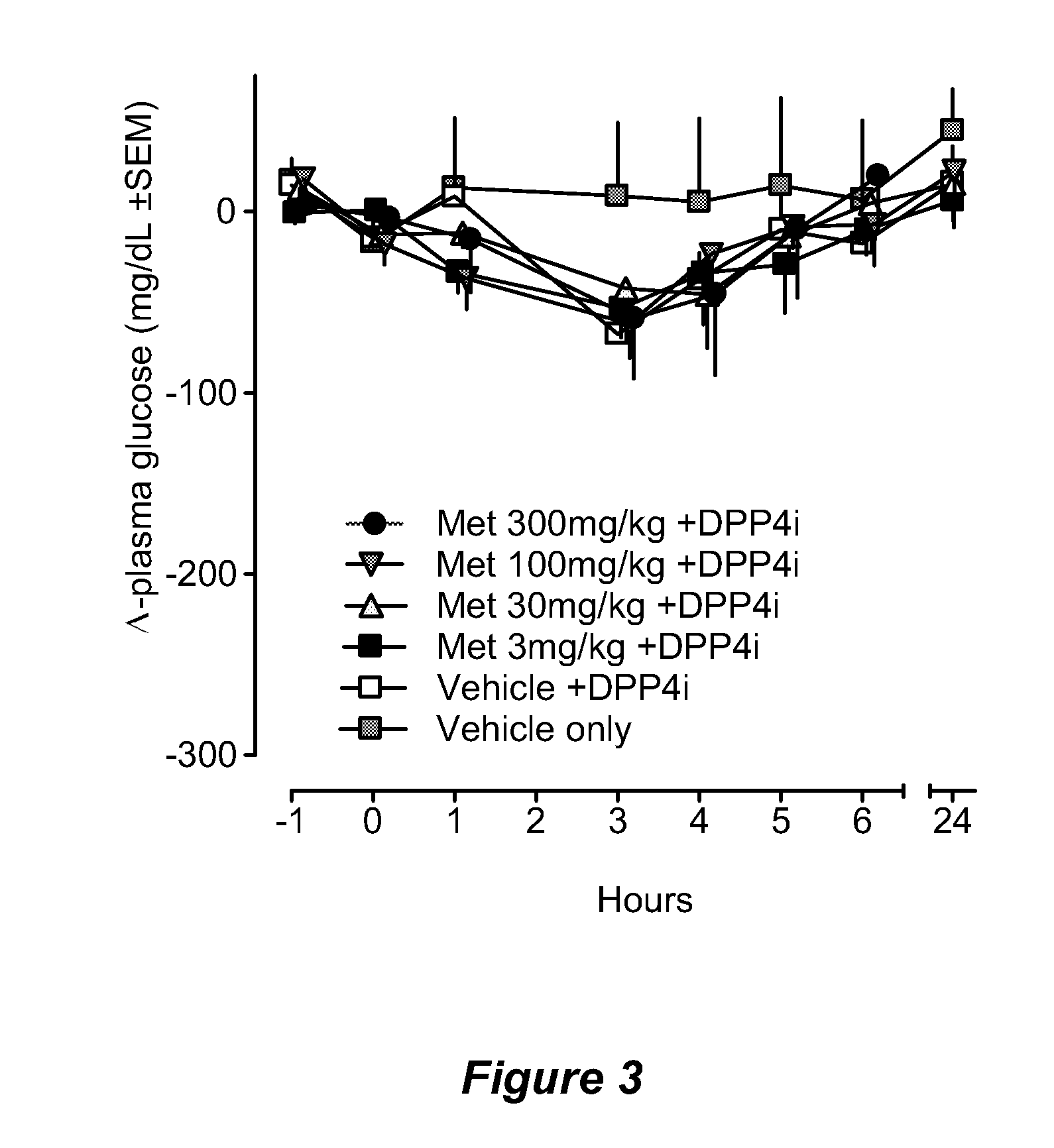
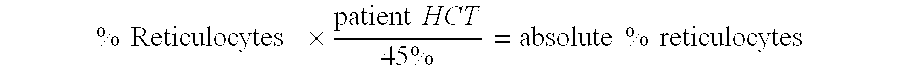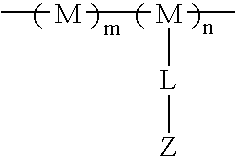Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4360 results about "Gastroenterology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gastroenterology is the branch of medicine focused on the digestive system and its disorders. Diseases affecting the gastrointestinal tract, which include the organs from mouth into anus, along the alimentary canal, are the focus of this speciality. Physicians practicing in this field are called gastroenterologists. They have usually completed about eight years of pre-medical and medical education, a year-long internship (if this is not a part of the residency), three years of an internal medicine residency, and two to three years in the gastroenterology fellowship. Gastroenterologists perform a number of diagnostic and therapeutic procedures including colonoscopy, endoscopy, endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound and liver biopsy. Some gastroenterology trainees will complete a "fourth-year" (although this is often their seventh year of graduate medical education) in transplant hepatology, advanced endoscopy, inflammatory bowel disease, motility or other topics.
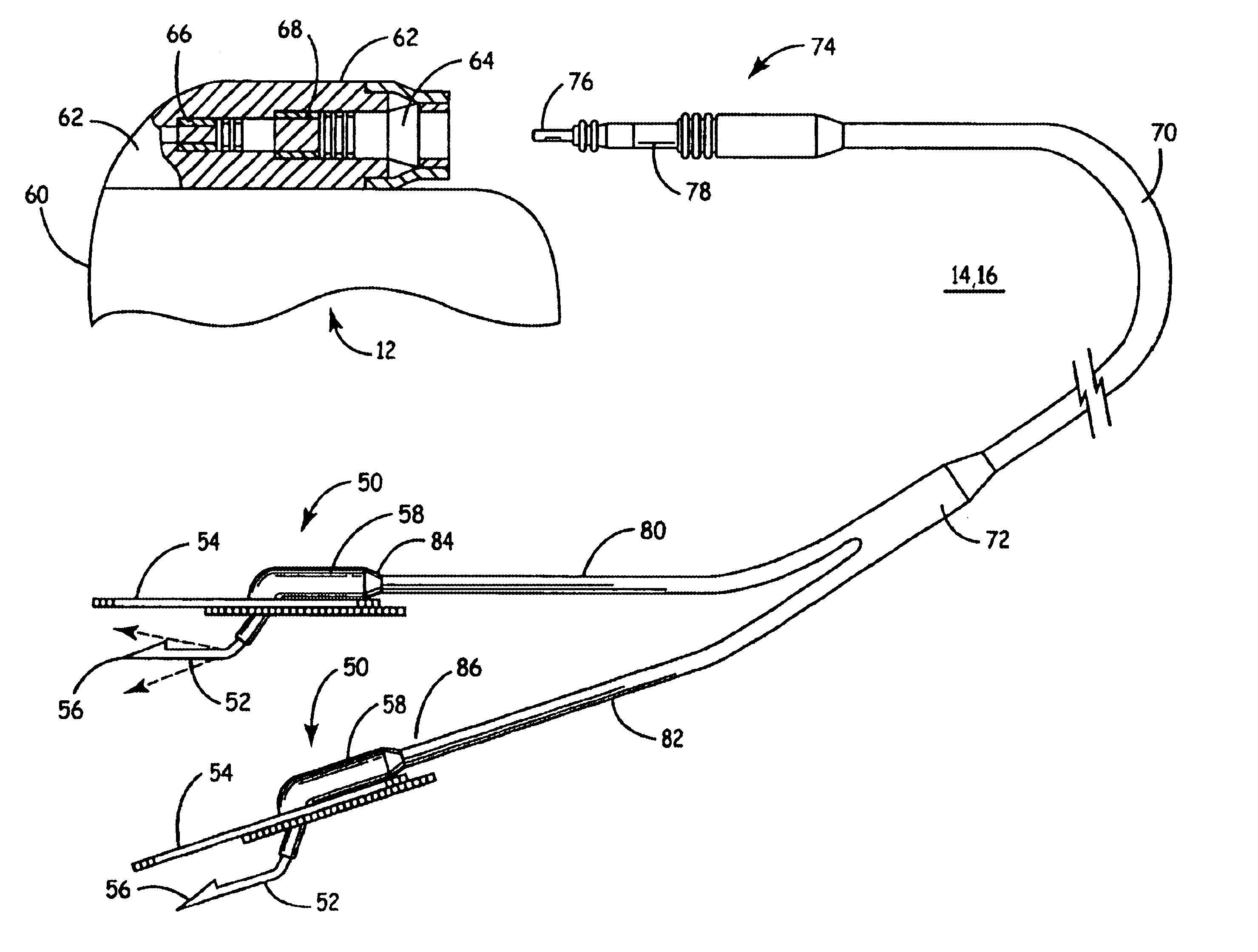
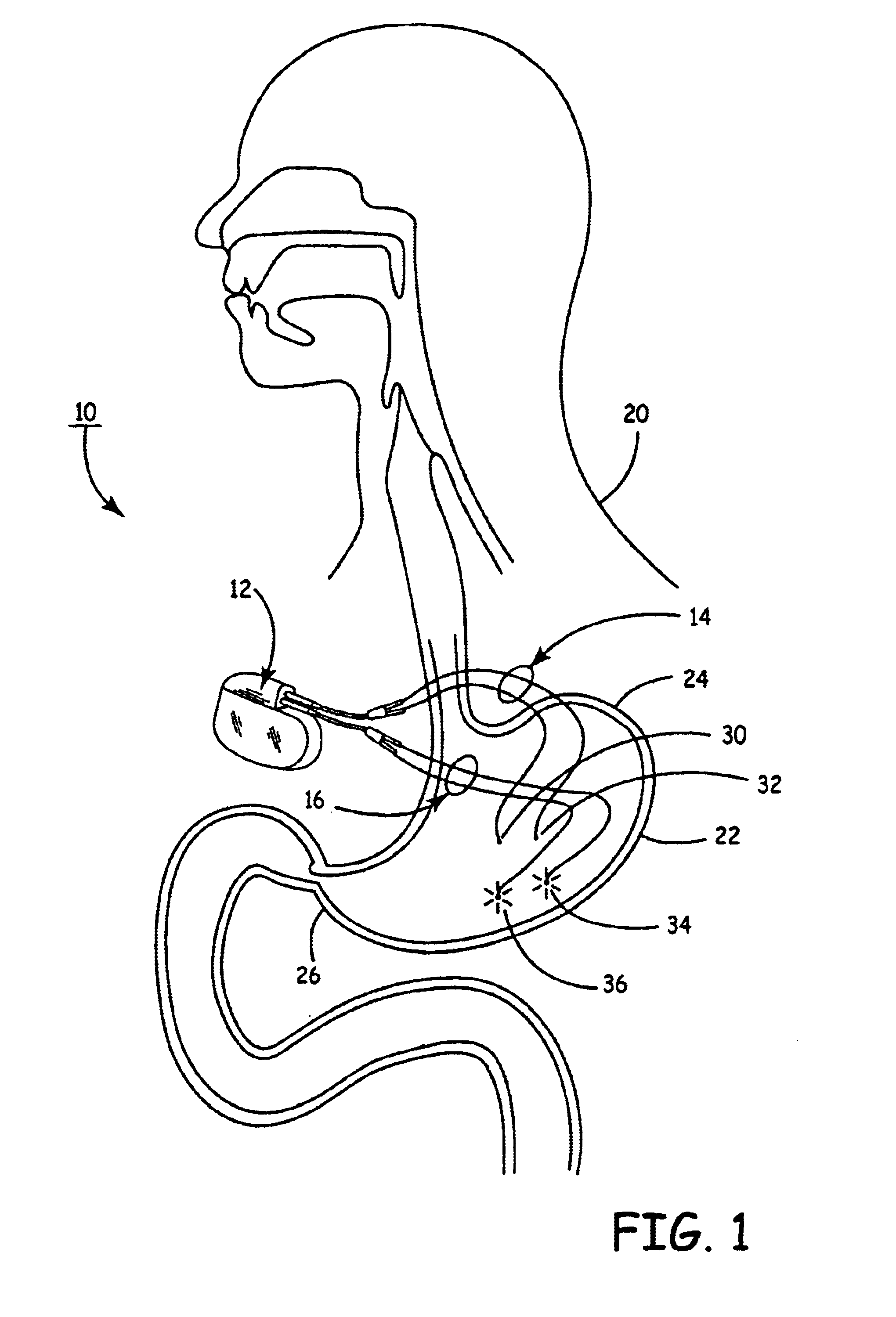
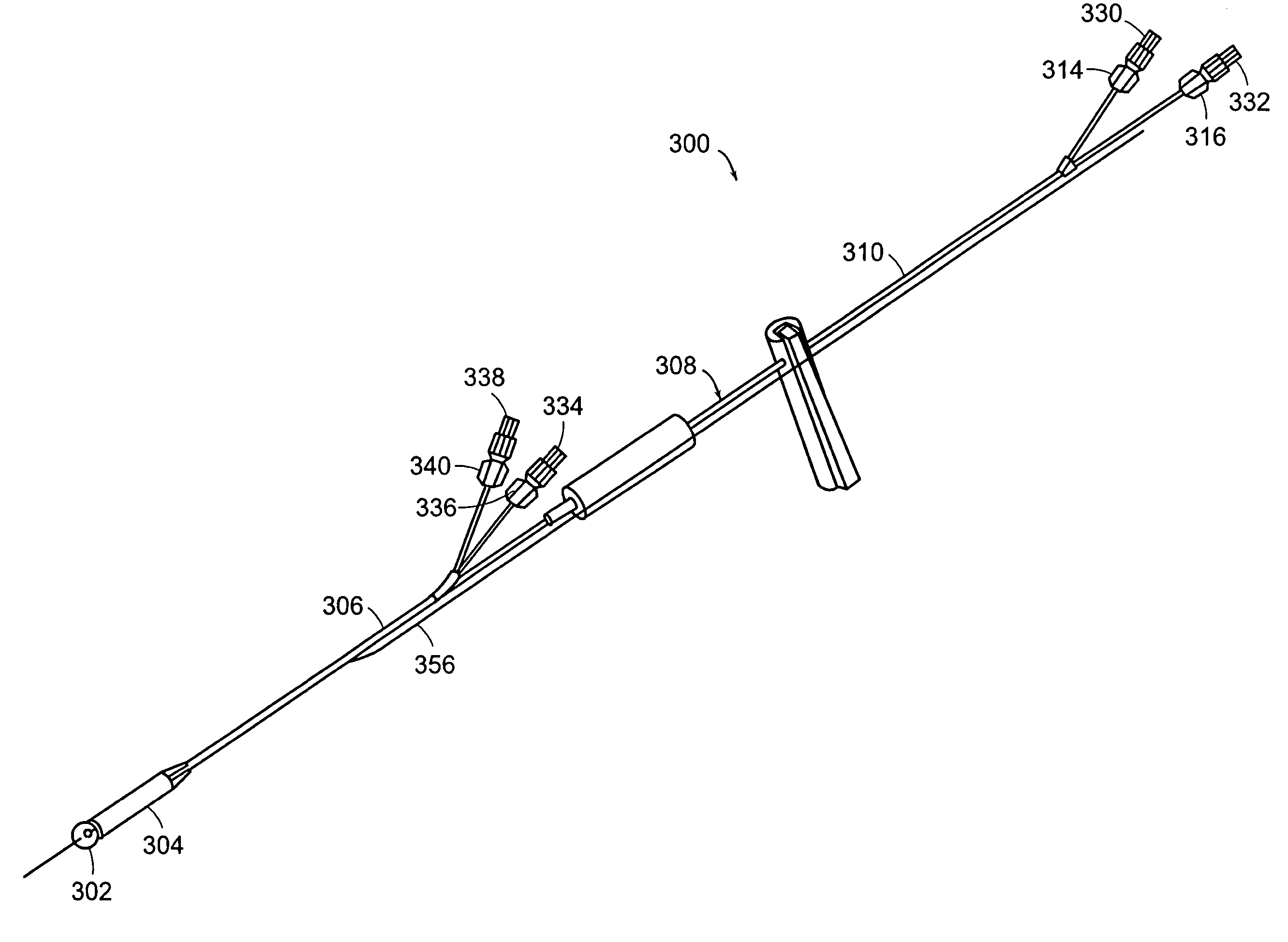
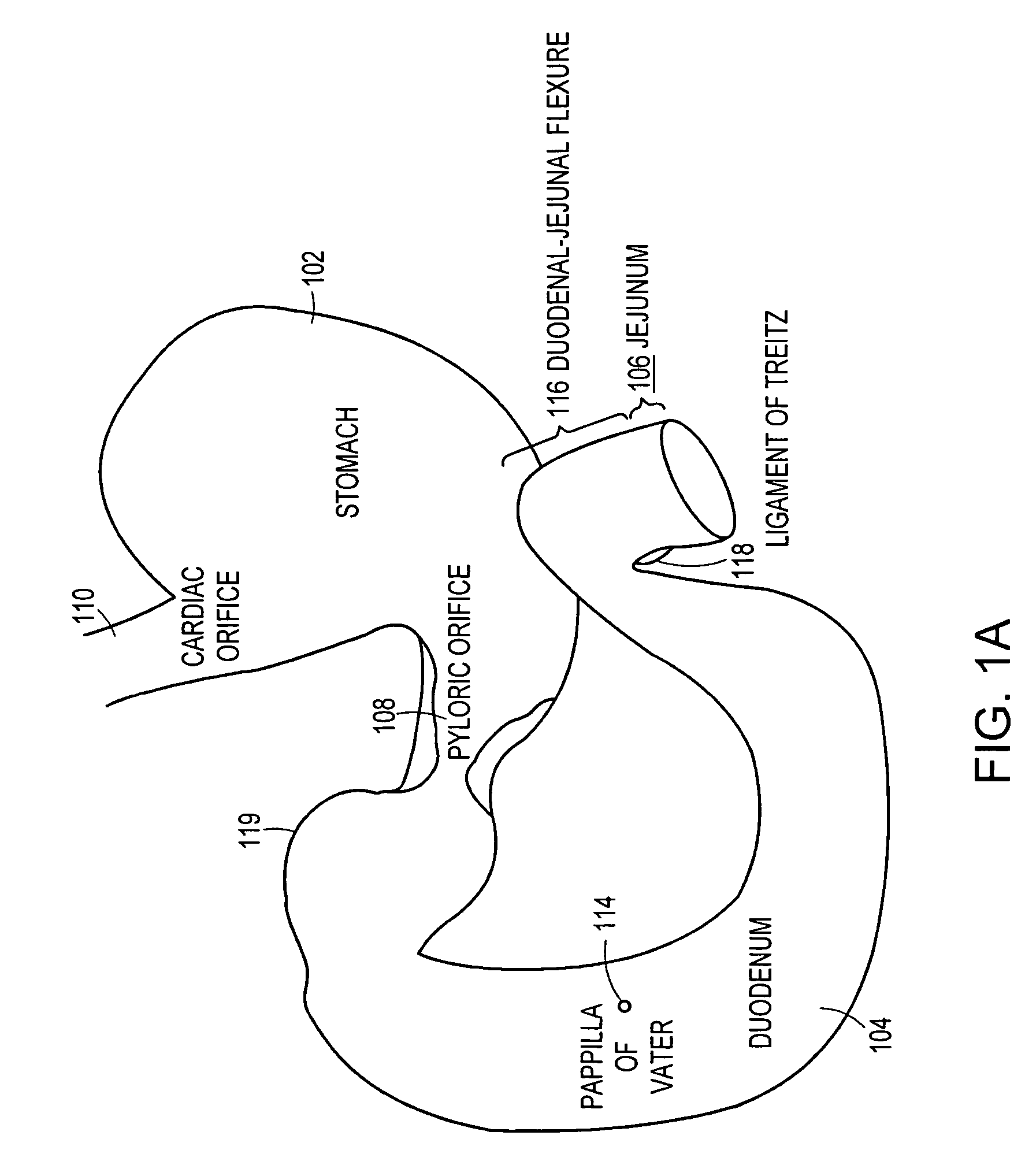
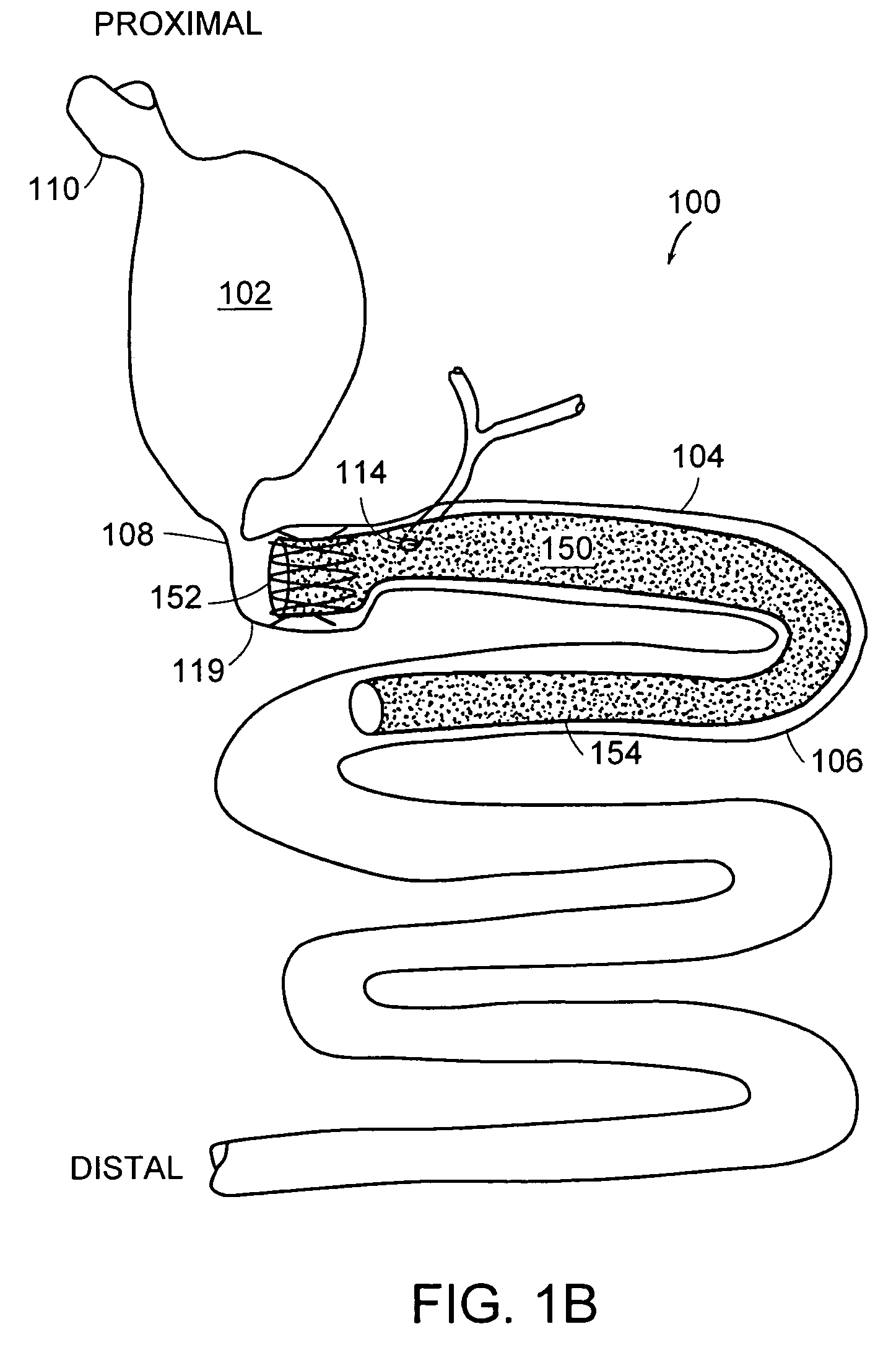
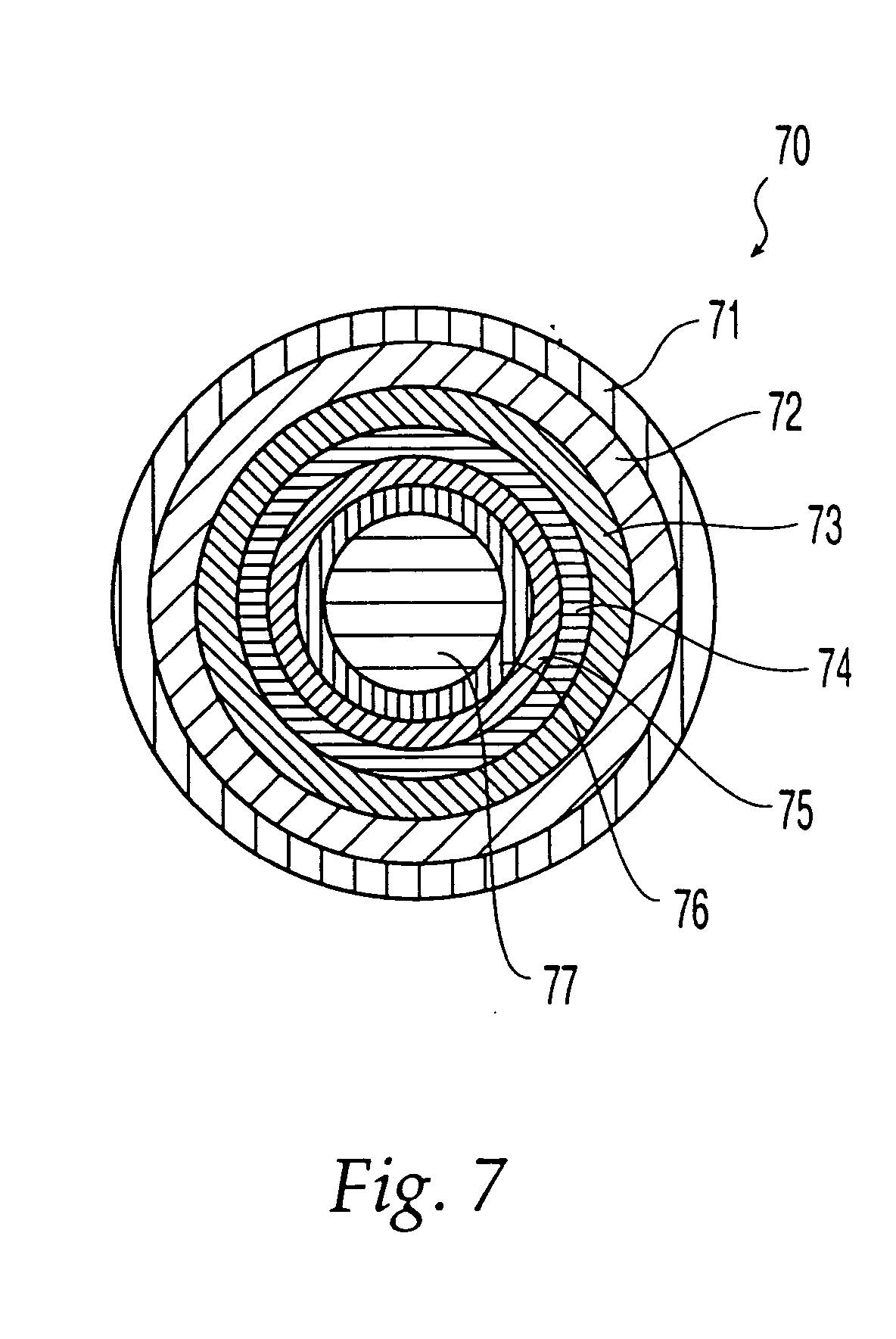
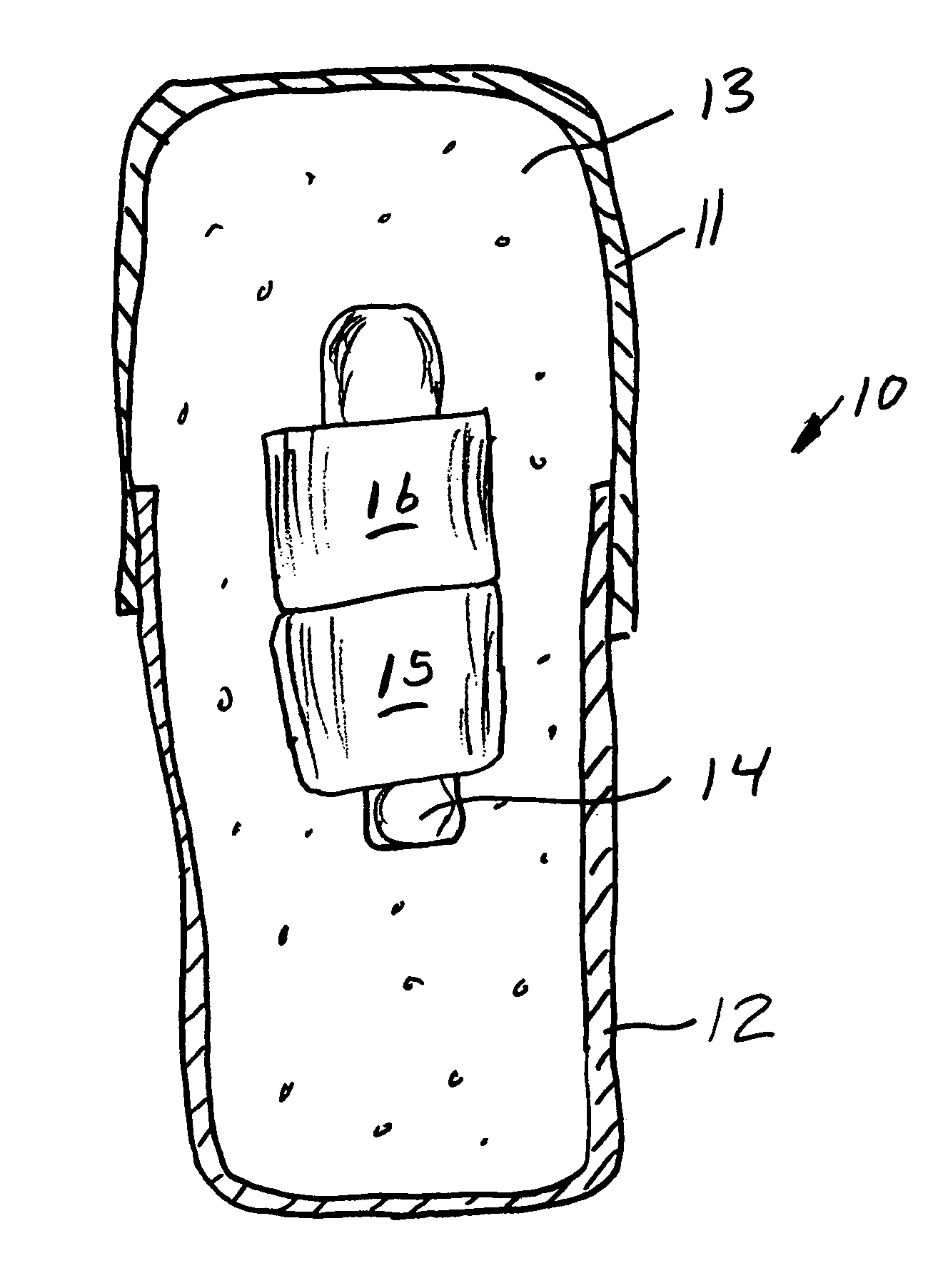
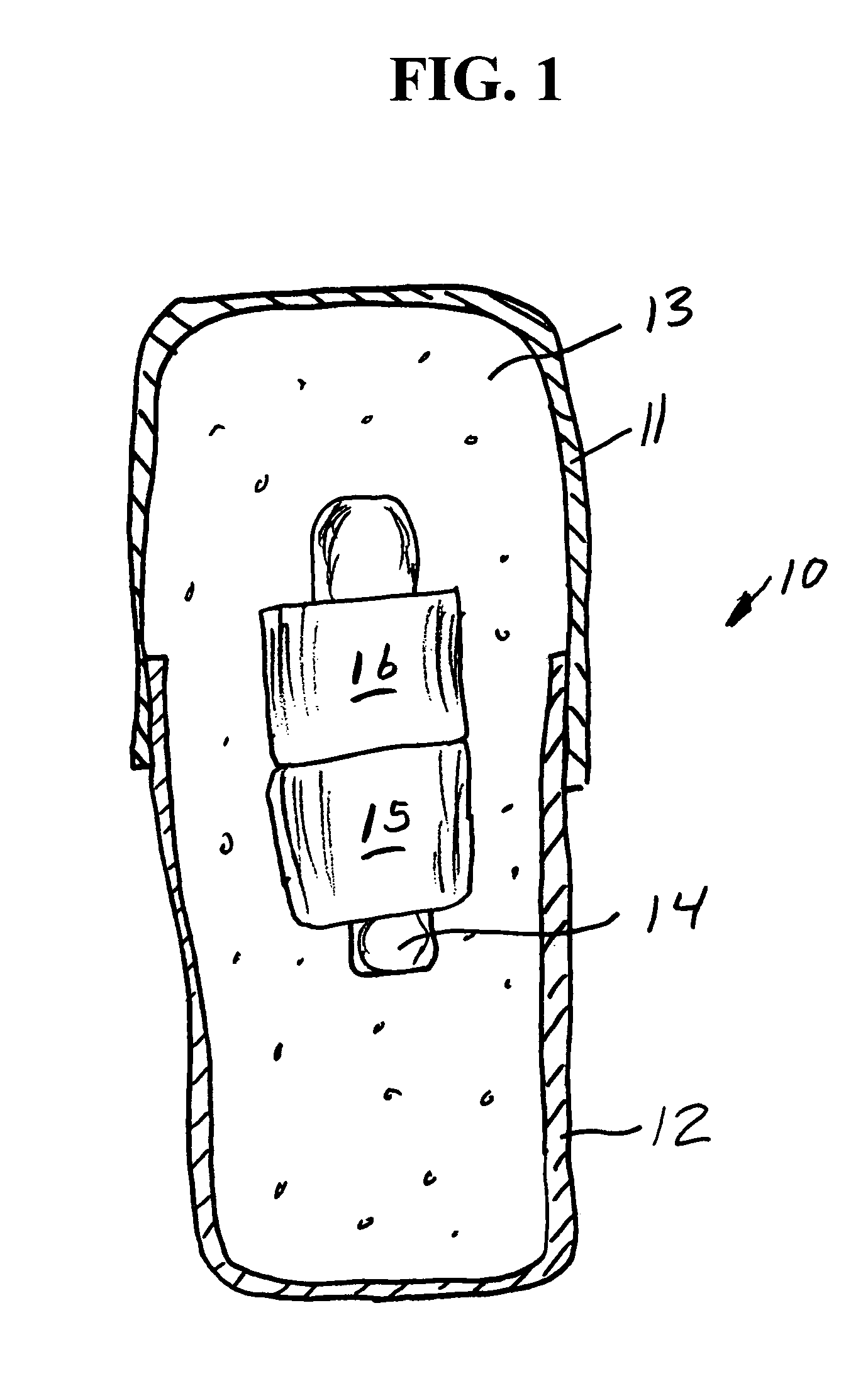
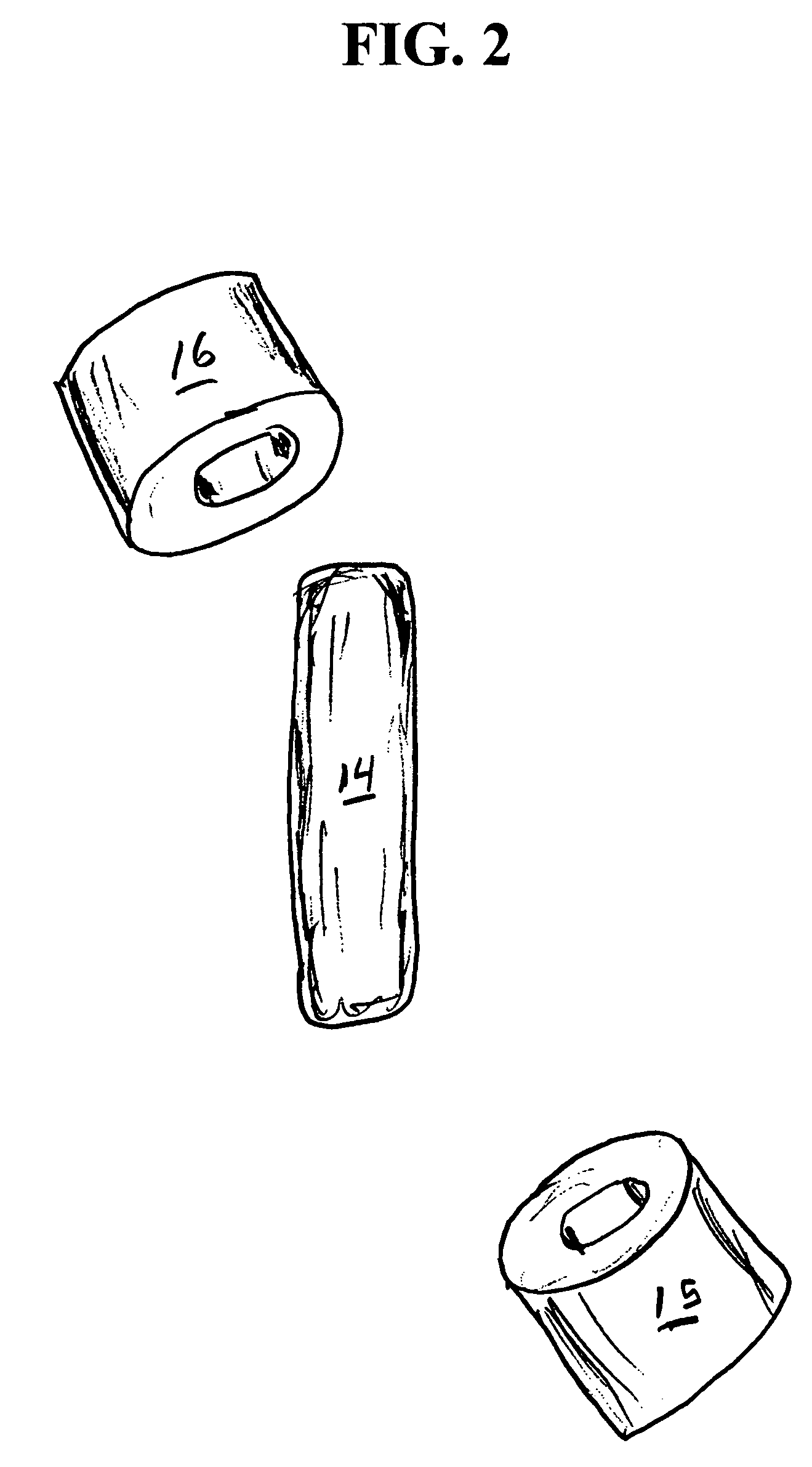
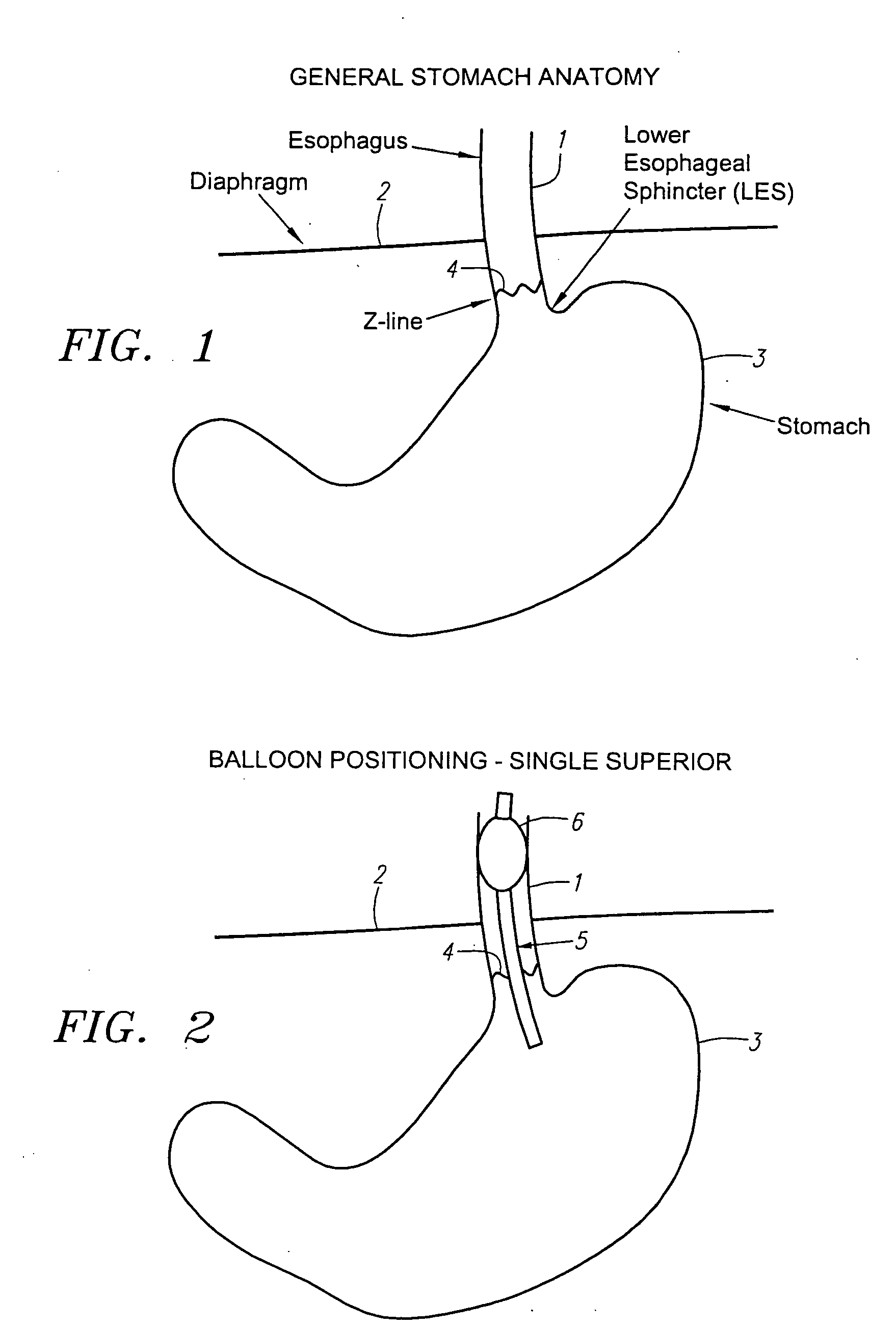
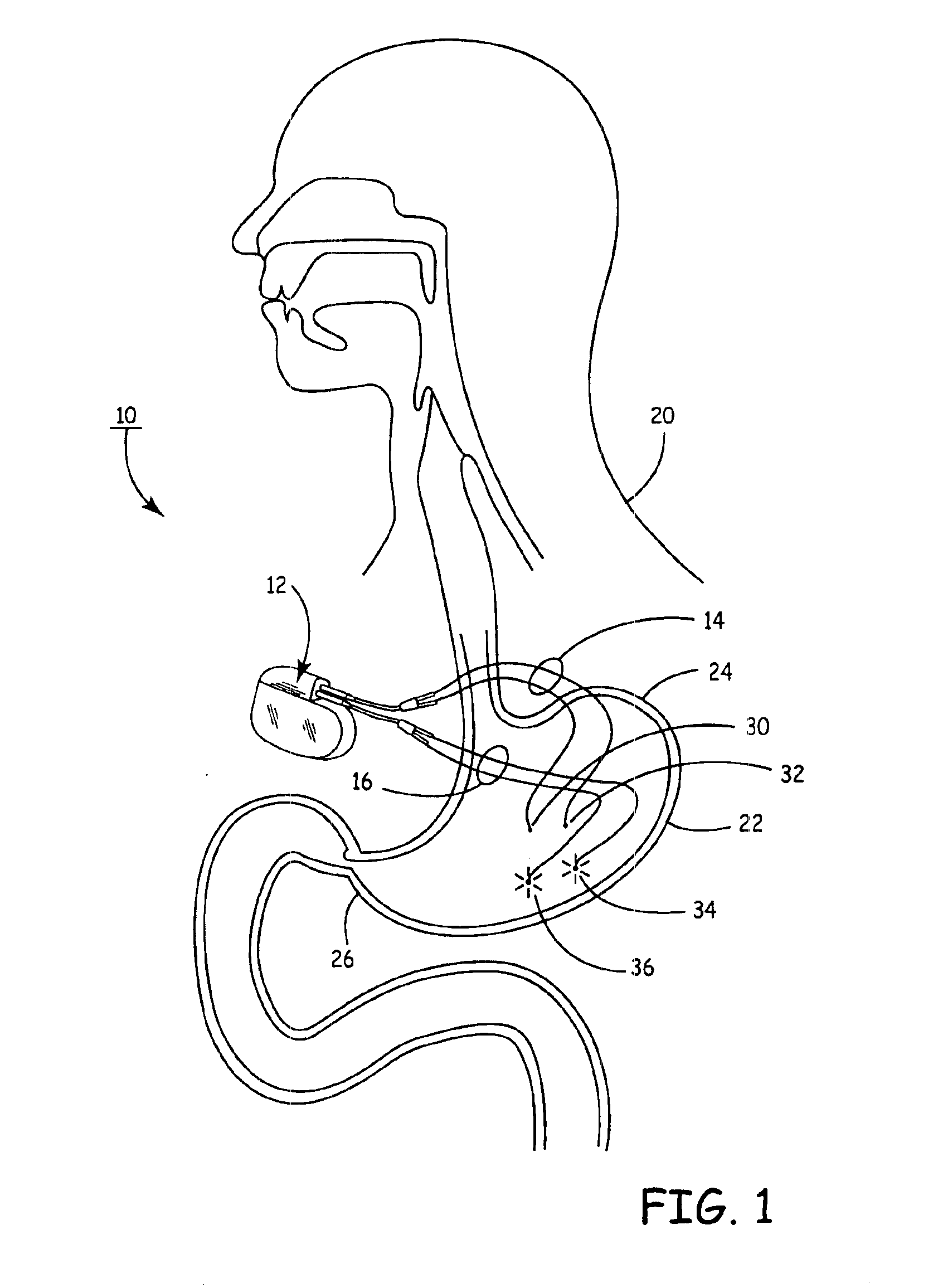
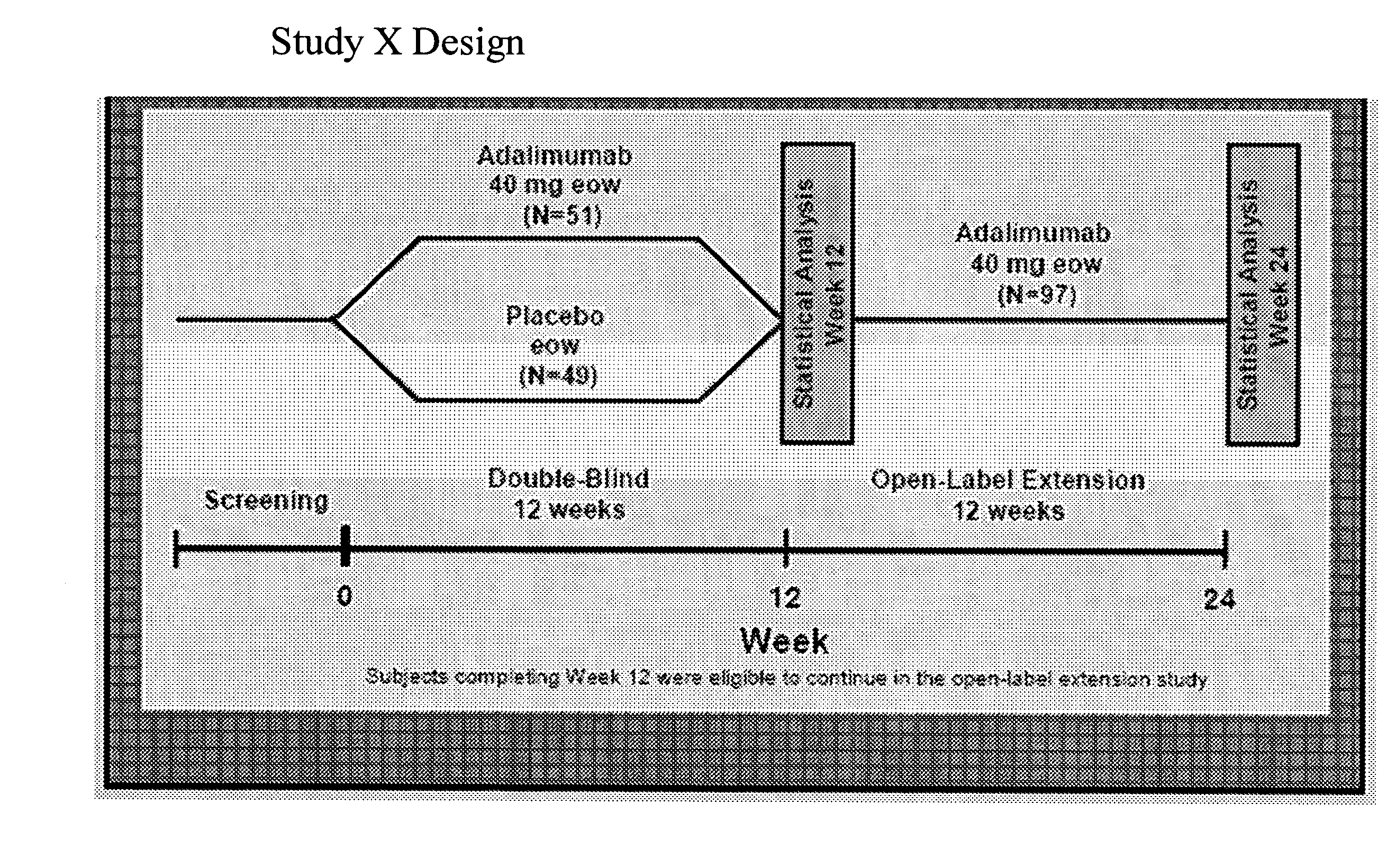
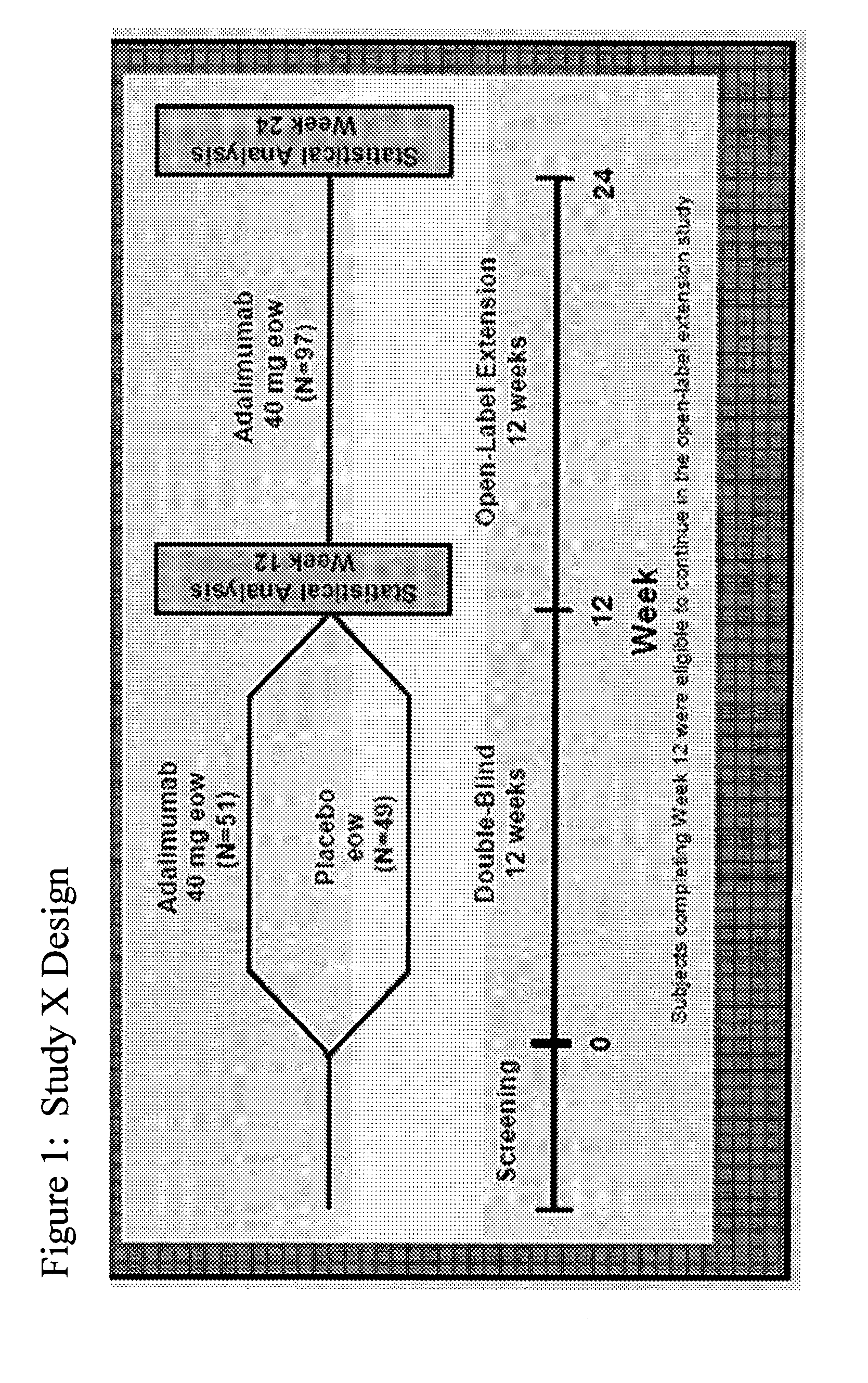
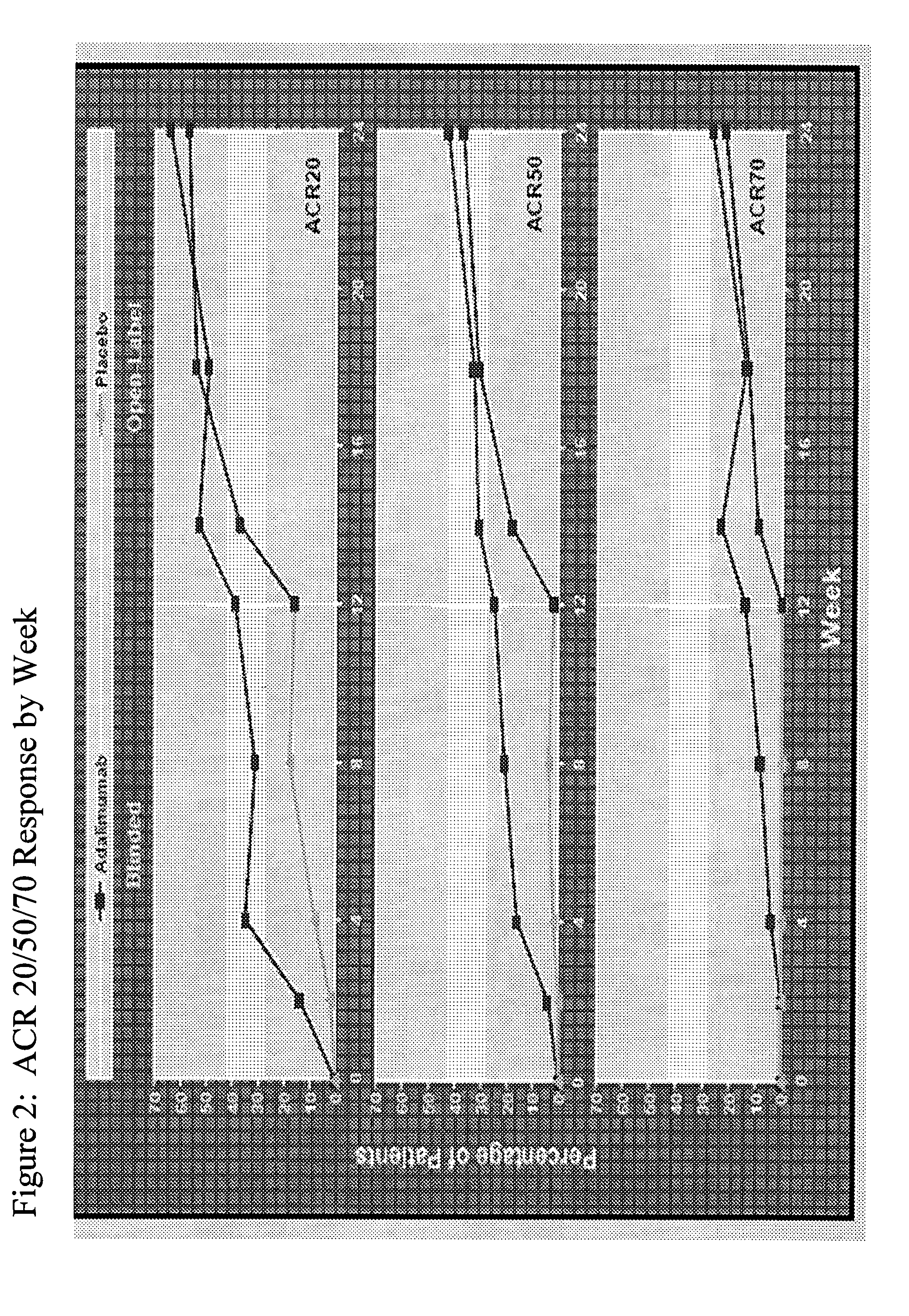
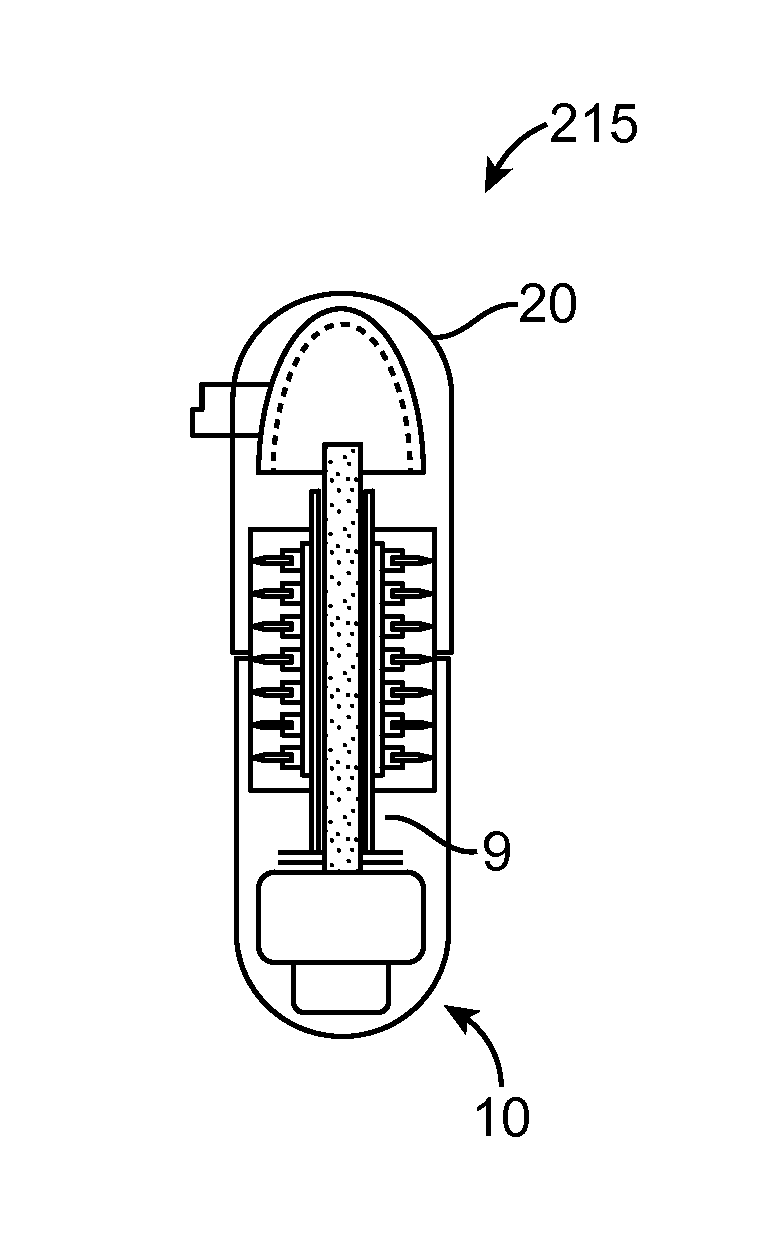
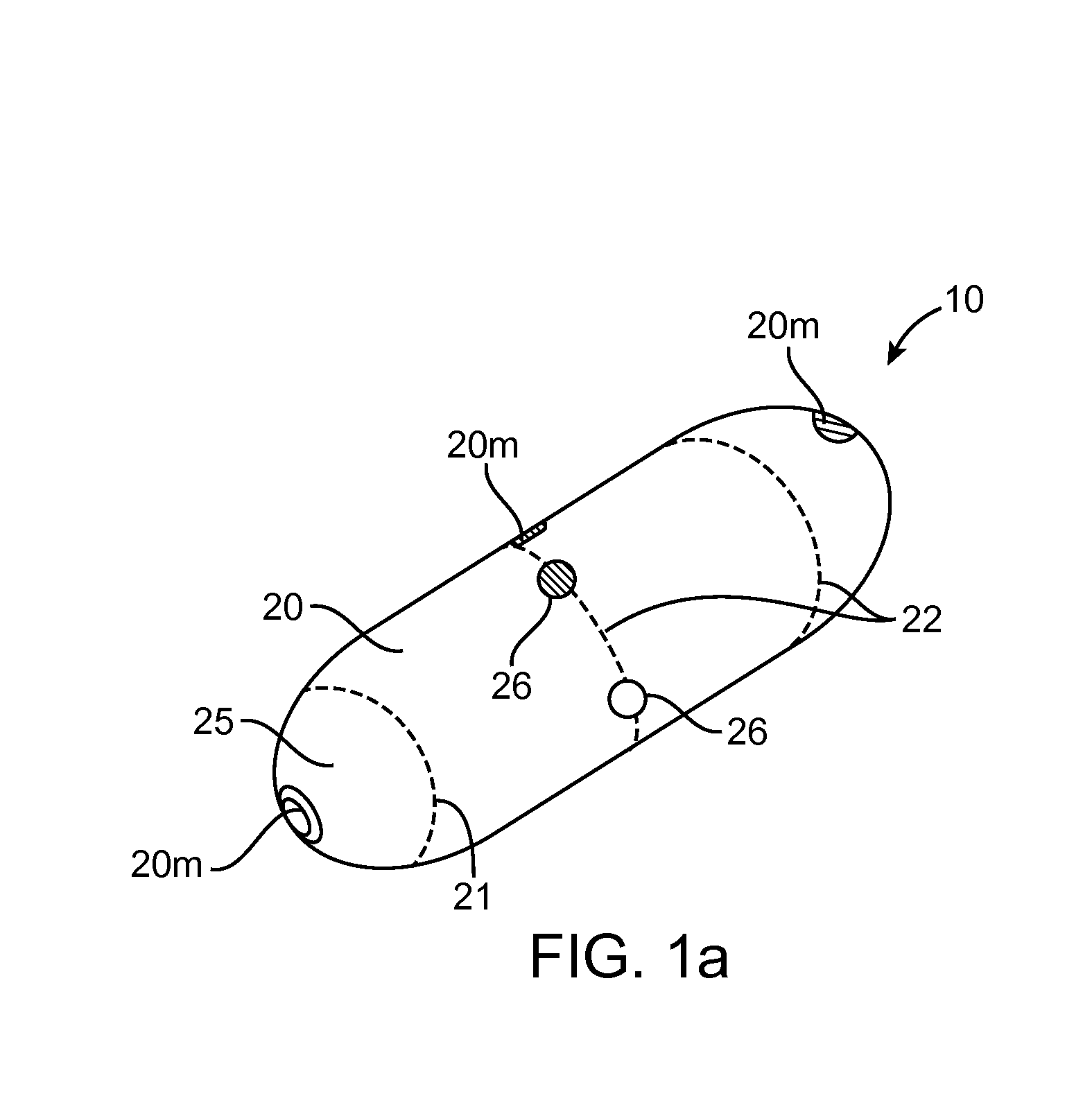
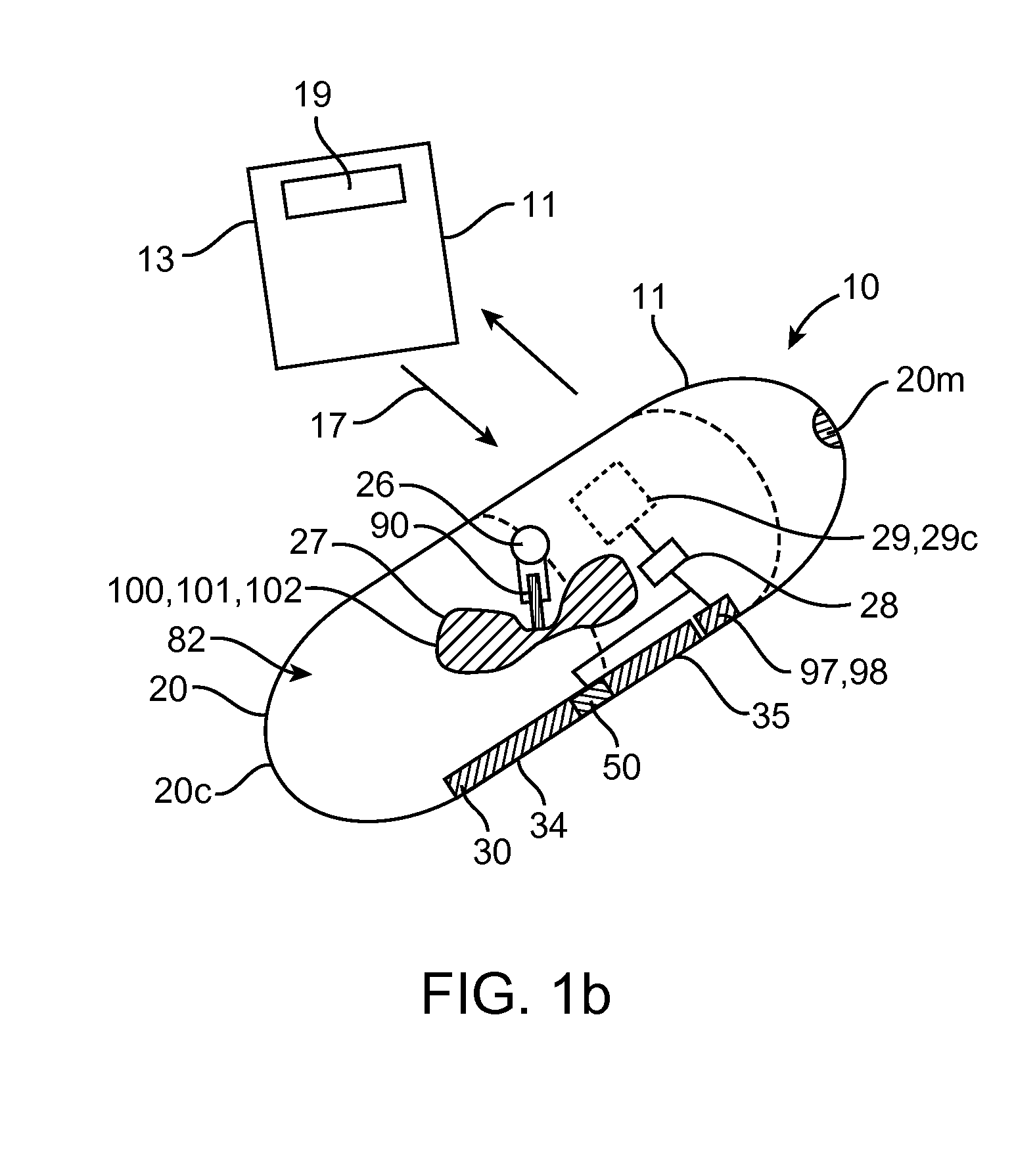
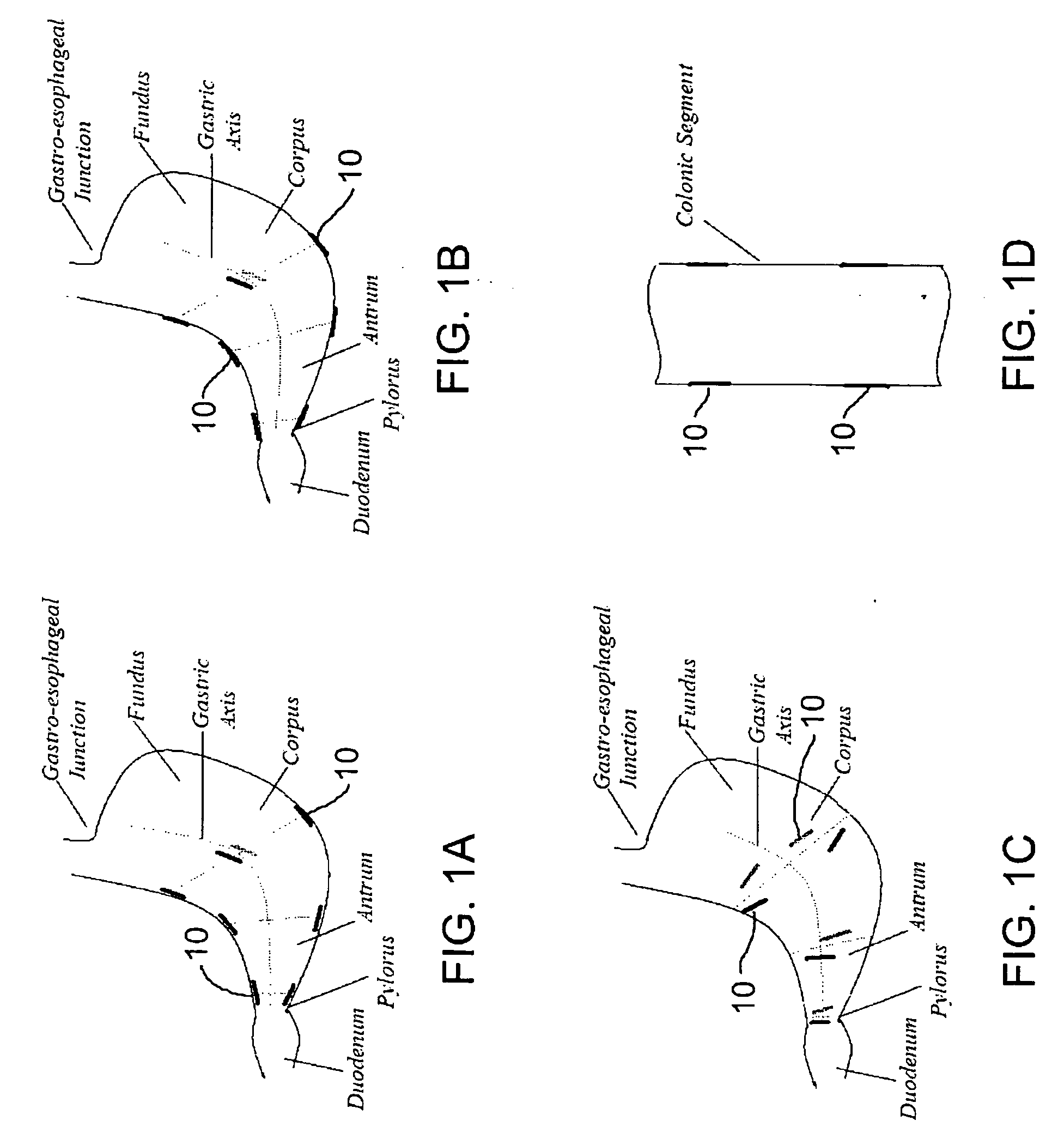
Implantable bifurcated gastrointestinal lead with active fixation
InactiveUS6876885B2Maximizing numberSpinal electrodesExternal electrodesTherapeutic DevicesActive fixation
Bifurcated, active fixation, gastrointestinal leads adapted to be implanted within the body at a site of the GI tract to conduct electrical stimulation and electrical signals of the GI tract between the gastrointestinal stimulator and the site are disclosed. The GI tract lead has a lead body comprising a common lead body trunk extending from a lead body trunk proximal end to a junction with a first plurality of lead body legs that extend from the junction to a like first plurality of lead body leg distal ends. An electrode head is formed at each lead body leg distal end having a plate and supporting at least one stimulation / sense electrode and an active fixation mechanism, whereby a plurality of active fixation attachment mechanisms are supported by a like plurality of electrode heads. The plurality of electrode heads can be affixed by the fixation mechanism at a plurality of spaced apart locations of the GI tract. The plurality of electrode heads can be affixed spaced apart an optimal distance for efficacious sensing and / or stimulation accommodating the physiology and any defects or surgical interventions of the physiology or other therapeutic equipment or IMDs that restrict full access to the GI tract.
Owner:MEDTRONIC INC
Methods and devices for placing a gastrointestinal sleeve
Methods and systems for delivering or placing a gastrointestinal implant device into a mammal. The gastrointestinal implant device can be used to limit absorption of food products in specific parts of the digestive system and can include a gastrointestinal sleeve having an anchor portion and a barrier or sleeve portion. The methods include endoluminal delivery of the device.
Owner:GI DYNAMICS
Treatment of metabolic disorders using TNFalpha inhibitors
Methods for treating metabolic disorders, including diabetes and obesity, using TNFalpha inhibitors are described.
Owner:ABBOTT BIOTECHNOLOGY LTD
Oral dosage form comprising a therapeutic agent and an adverse-effect agent
The present invention provides an oral dosage form comprising a first composition and a second composition. The first composition comprises an effective amount of a therapeutic agent and the second composition comprises an effective amount of an adverse-effect agent. The adverse-effect agent is covered with a coating that is substantially insoluble in the gastrointestinal tract. In one embodiment, the adverse-effect agent is coated with an outer base-soluble layer and an inner acid-soluble layer. The therapeutic agent can be uncoated or can be coated with a coating having an outer acid-soluble layer and an inner base-soluble layer. The dosage form discourages administration of the therapeutic agent by other than oral administration.
Owner:PURDUE PHARMA LP
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device.
Owner:DOW GLOBAL TECH LLC
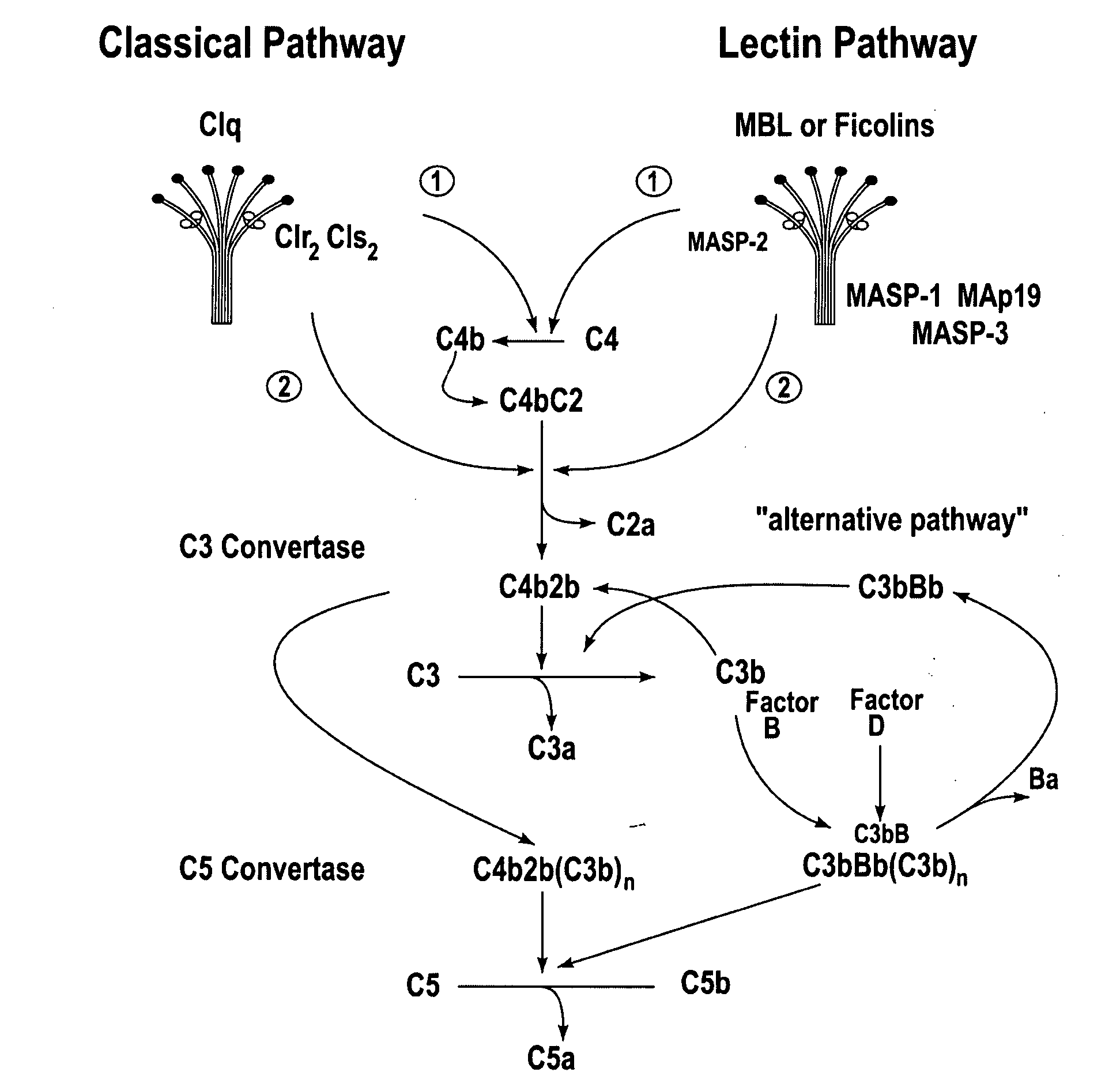
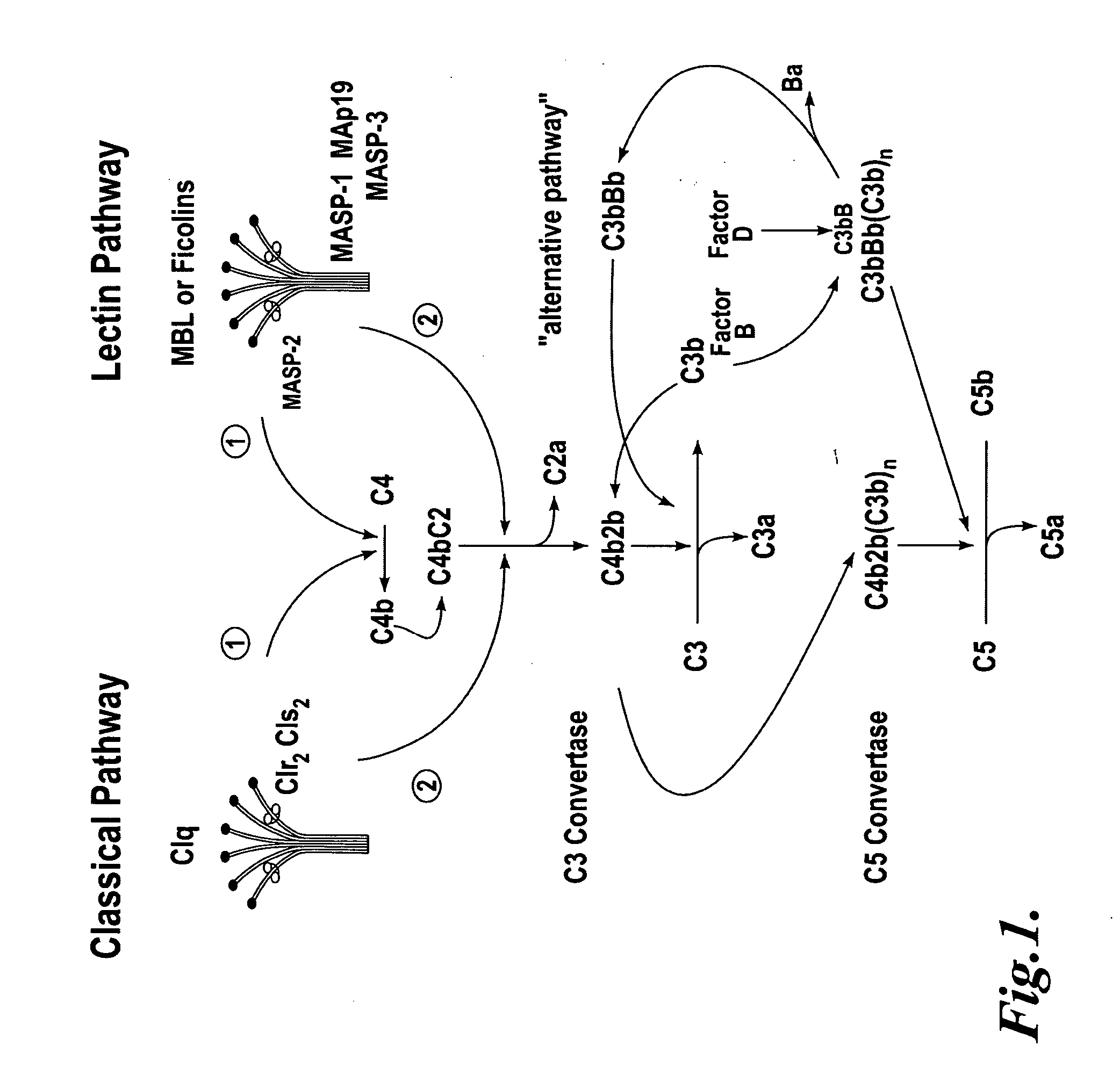
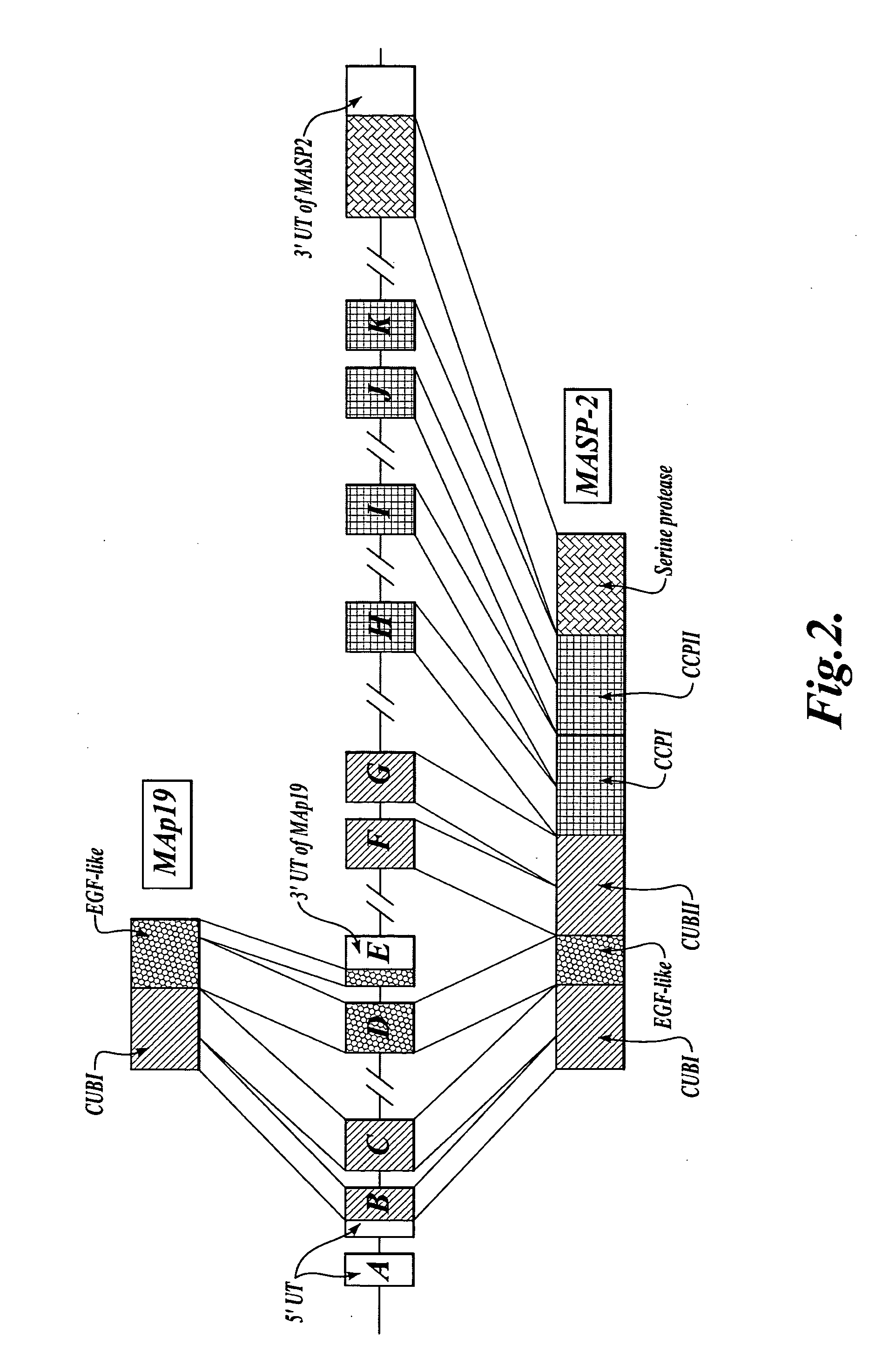
Methods for treating conditions associated with MASP-2 dependent complement activation
ActiveUS20070172483A1Eliminate side effectsInhibiting complement activationCompounds screening/testingAntibody ingredientsBiological activationAlternative complement pathway
In one aspect, the invention provides methods of inhibiting the effects of MASP-2-dependent complement activation in a living subject. The methods comprise the step of administering, to a subject in need thereof, an amount of a MASP-2 inhibitory agent effective to inhibit MASP-2-dependent complement activation. In some embodiments, the MASP-2 inhibitory agent inhibits cellular injury associated with MASP-2-mediated alternative complement pathway activation, while leaving the classical (C1q-dependent) pathway component of the immune system intact. In another aspect, the invention provides compositions for inhibiting the effects of lectin-dependent complement activation, comprising a therapeutically effective amount of a MASP-2 inhibitory agent and a pharmaceutically acceptable carrier.
Owner:OMEROS CORP +1
Method of using endoscopic truncal vagoscopy with gastric bypass, gastric banding and other procedures
Methods and devices for treating obesity by combining known bariatric surgeries with a method for disrupting the vagal nerve. The method for disrupting the vagal nerve comprises use of a high energy delivery device which is positioned within the esophagus to deliver transesophageal energy to interrupt the function of one or both branches of the vagal nerves. Combination of this method with known methods, such as gastric bypass and gastric banding, may increase weight loss by the patient in comparison to a single treatment on its own. In addition, methods and devices for disposing a restrictive structure such as a mesh around a selected segment of the gastrointestinal tract prevents enlargement of the segment.
Owner:ENDOVX
Treatment of Anemia Using TNFalpha Inhibitors
Owner:ABBOTT BIOTECHNOLOGY LTD
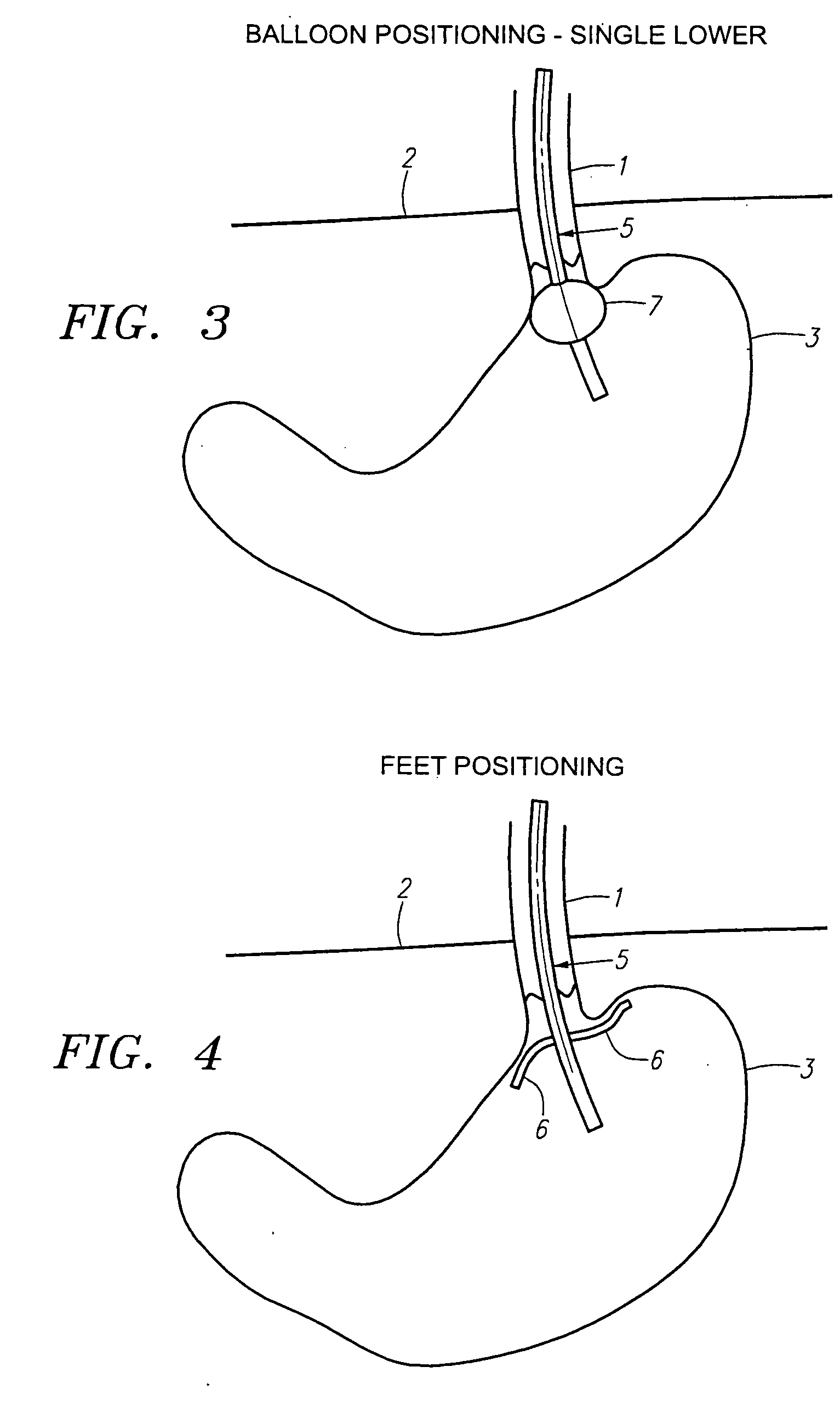
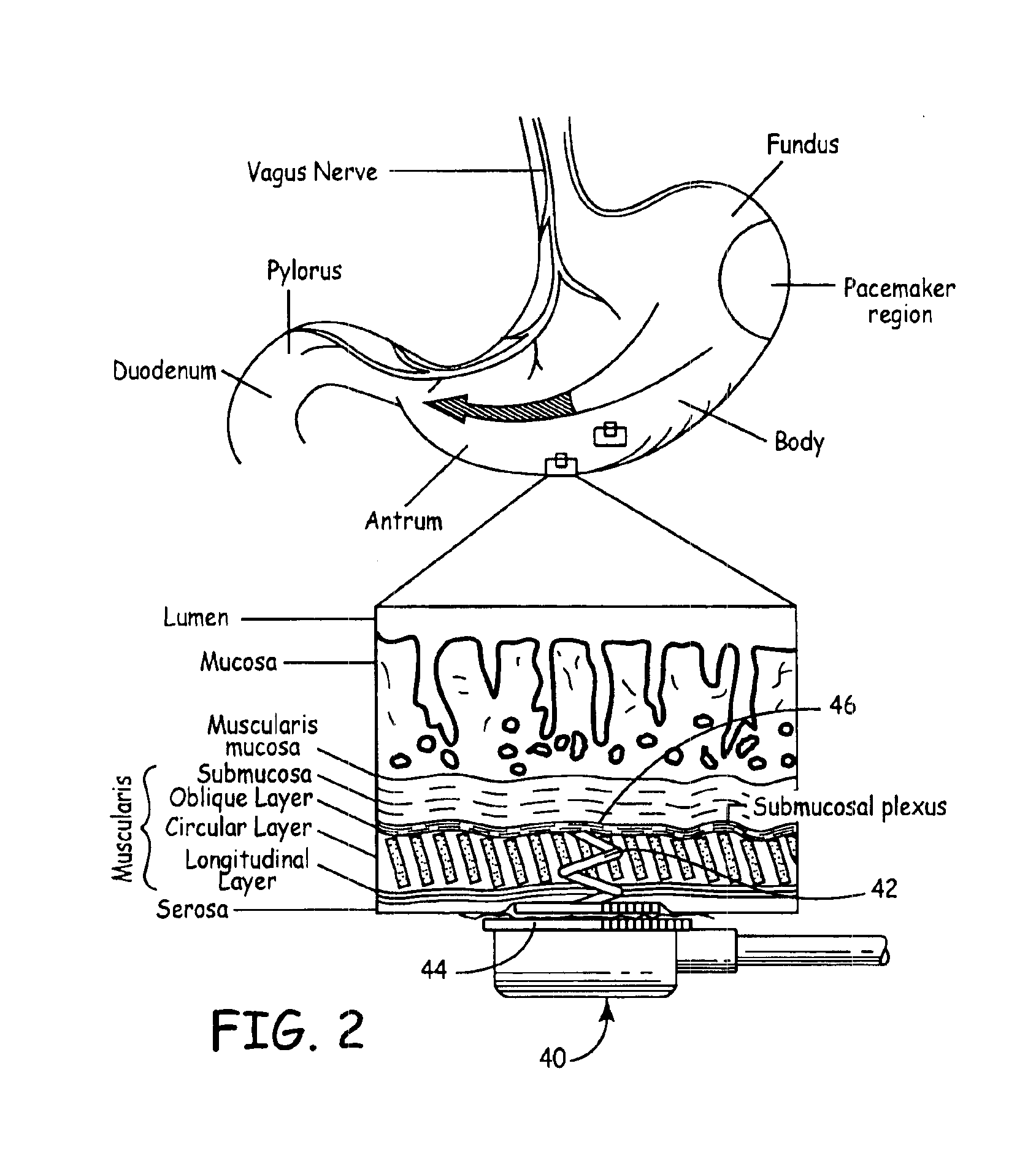
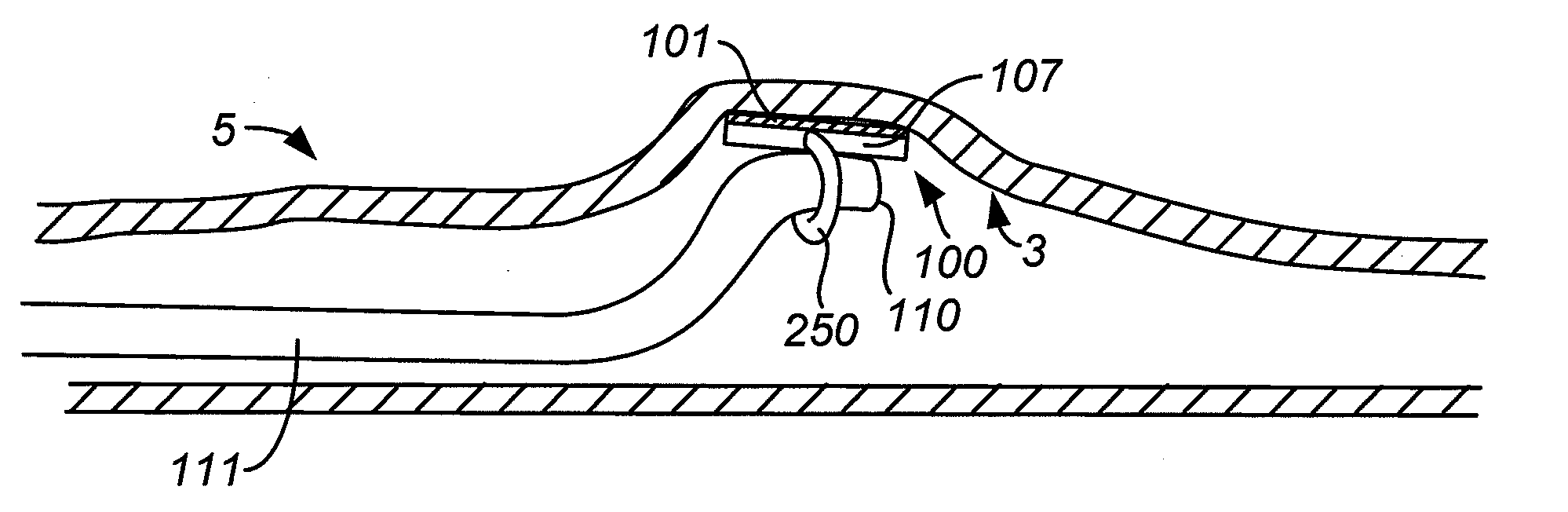
Implantable gastrointestinal lead with active fixation
InactiveUS6952613B2Avoid displacementReduce polarizationSpinal electrodesExternal electrodesActive fixationCatheter
Active fixation, gastrointestinal leads adapted to be implanted within the body at a site of the GI tract to conduct electrical stimulation from an implantable or external gastrointestinal stimulator to the site and to conduct electrical signals of the GI tract from the site to the implantable or external gastrointestinal stimulator are disclosed. Disclosed active fixation mechanisms include one or more of hooks, and helixes extending from stops, e.g. plates, of an electrode head and functioning as stimulation / sense electrodes in unipolar and bipolar configurations or simply as fixation mechanisms. The active fixation mechanisms are coated to reduce inflammation and polarization effects.
Owner:MEDTRONIC INC
Methods and compositions using immunomodulatory compounds for the treatment of immunodeficiency disorders
Methods of treating, preventing and / or managing an immunodeficiency disease or disorder are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active agent. Methods of boosting humoral immunity are also disclosed.
Owner:CELGENE CORP
Uses and compositions for treatment of psoriatic arthritis
InactiveUS20080311043A1Improve the quality of lifeTreatment safetyCompounds screening/testingPeptide/protein ingredientsAntigen bindingPsoriasis arthropathy
The invention provides methods, uses and compositions for the treatment of psoriatic arthritis. The invention describes methods and uses for treating psoriatic arthritis, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to psoriatic arthritis in a subject. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of psoriatic arthritis in a subject.
Owner:HOFFMAN REBECCA S +7
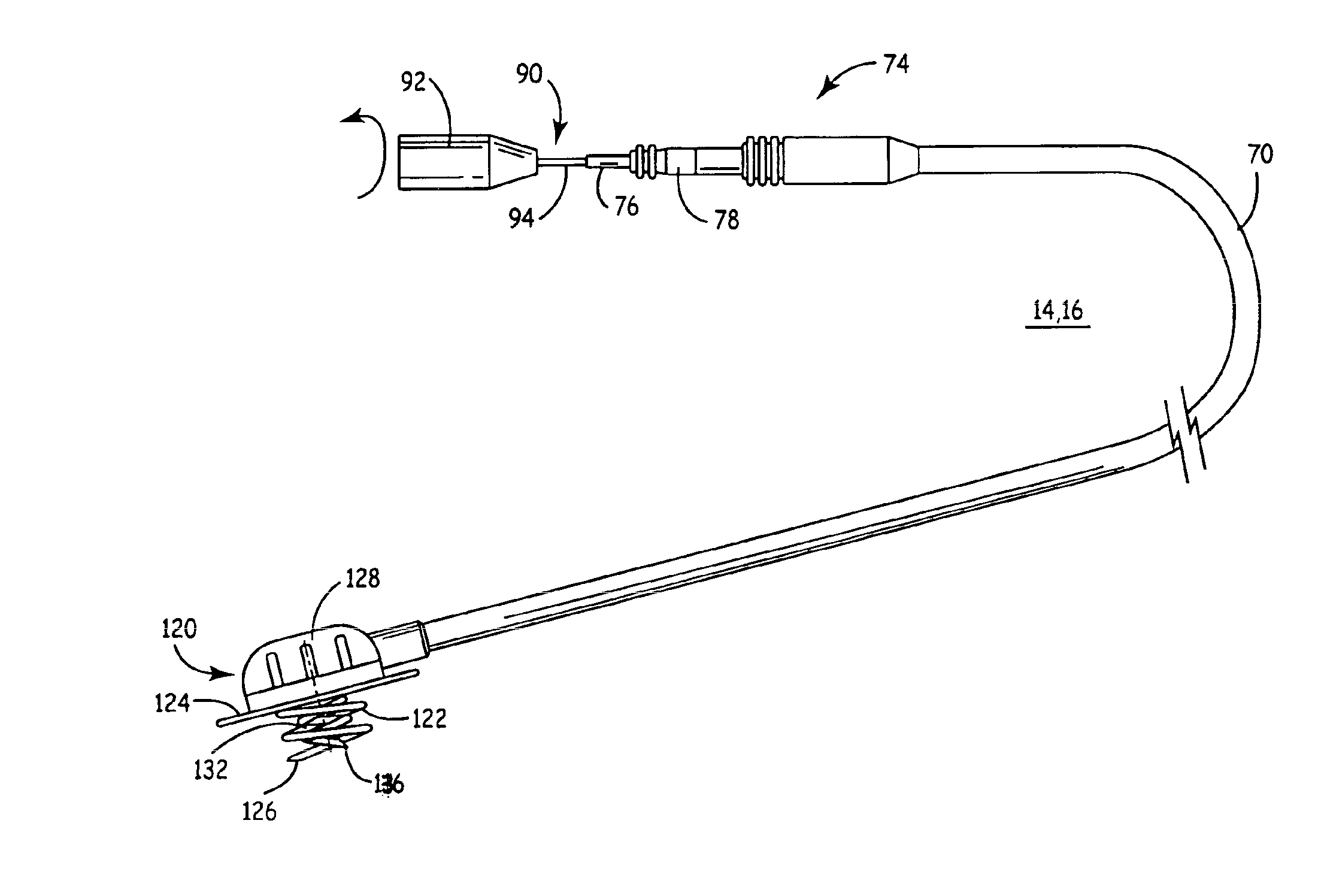
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS8734429B2Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsDrugs preparations
Owner:RANI THERAPEUTICS
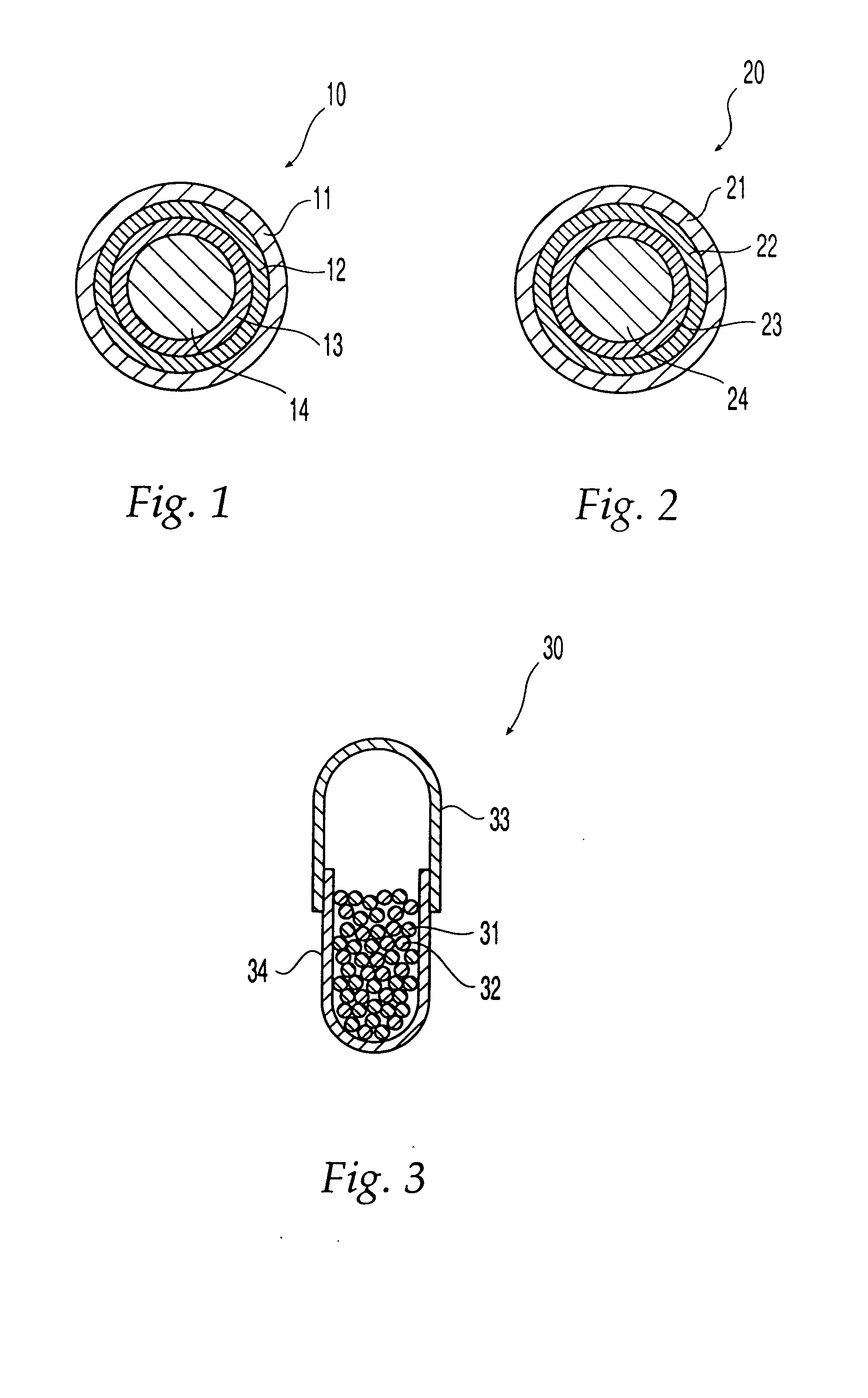
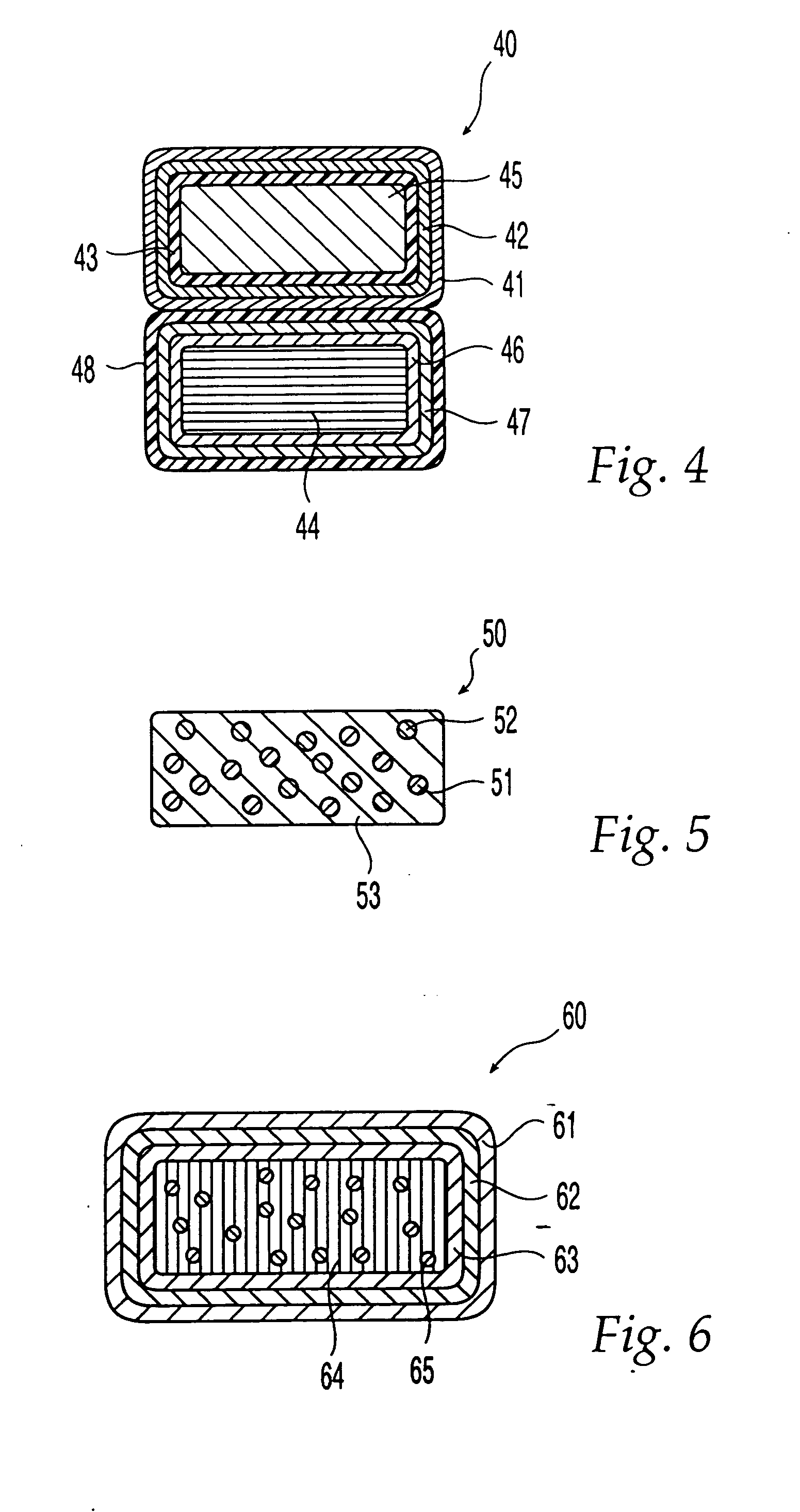
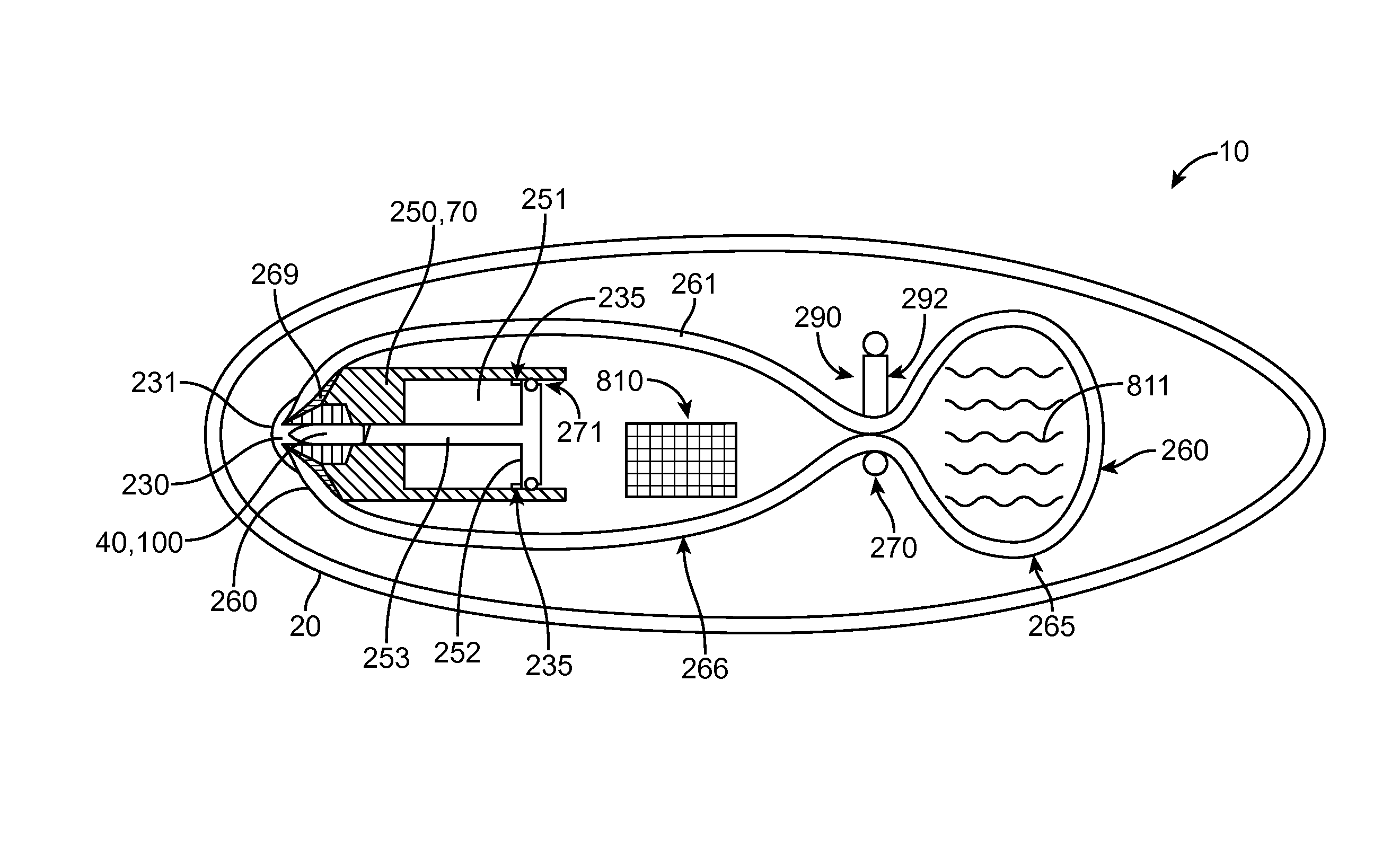
System and method of evaluating a subject with an ingestible capsule
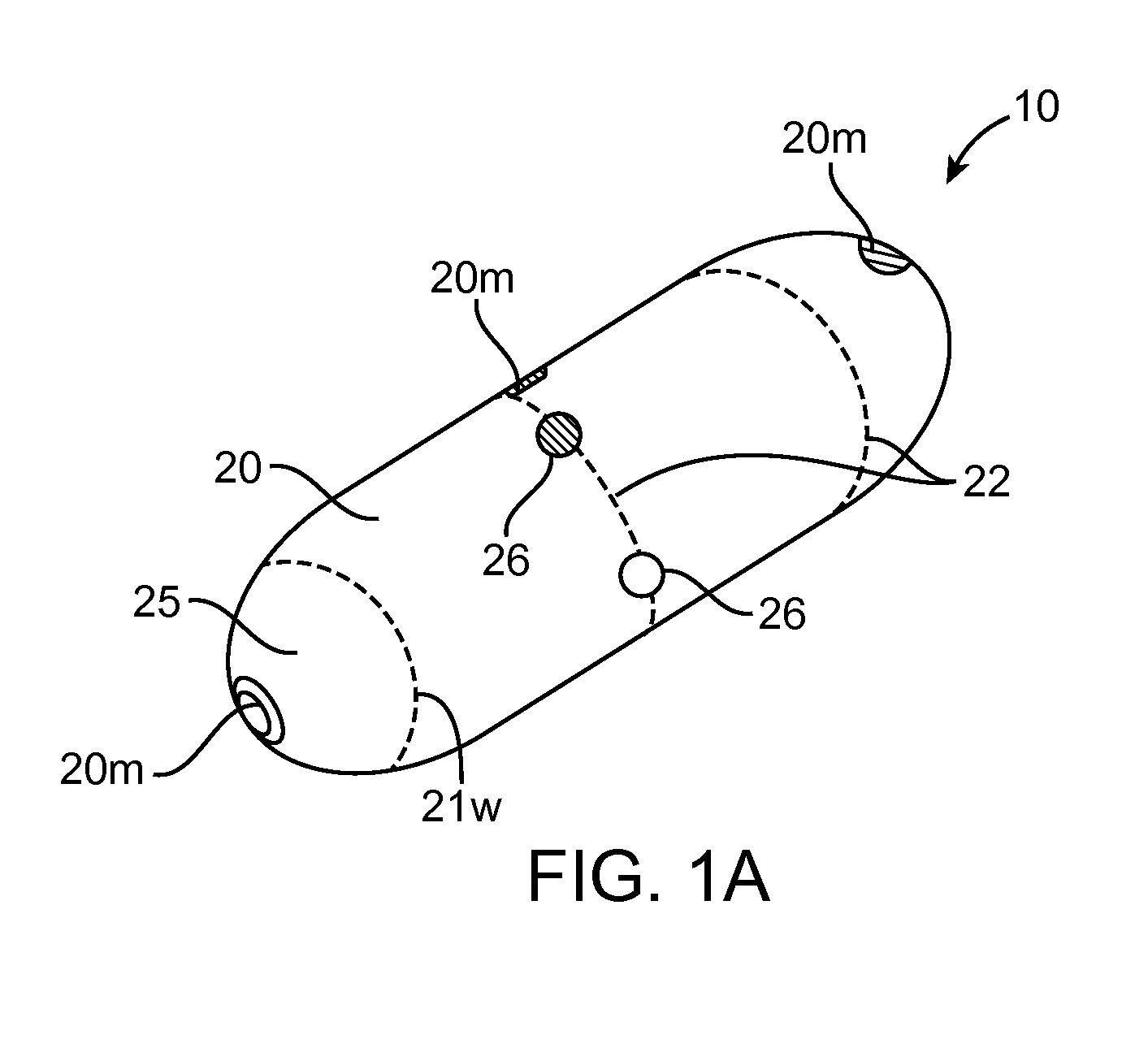
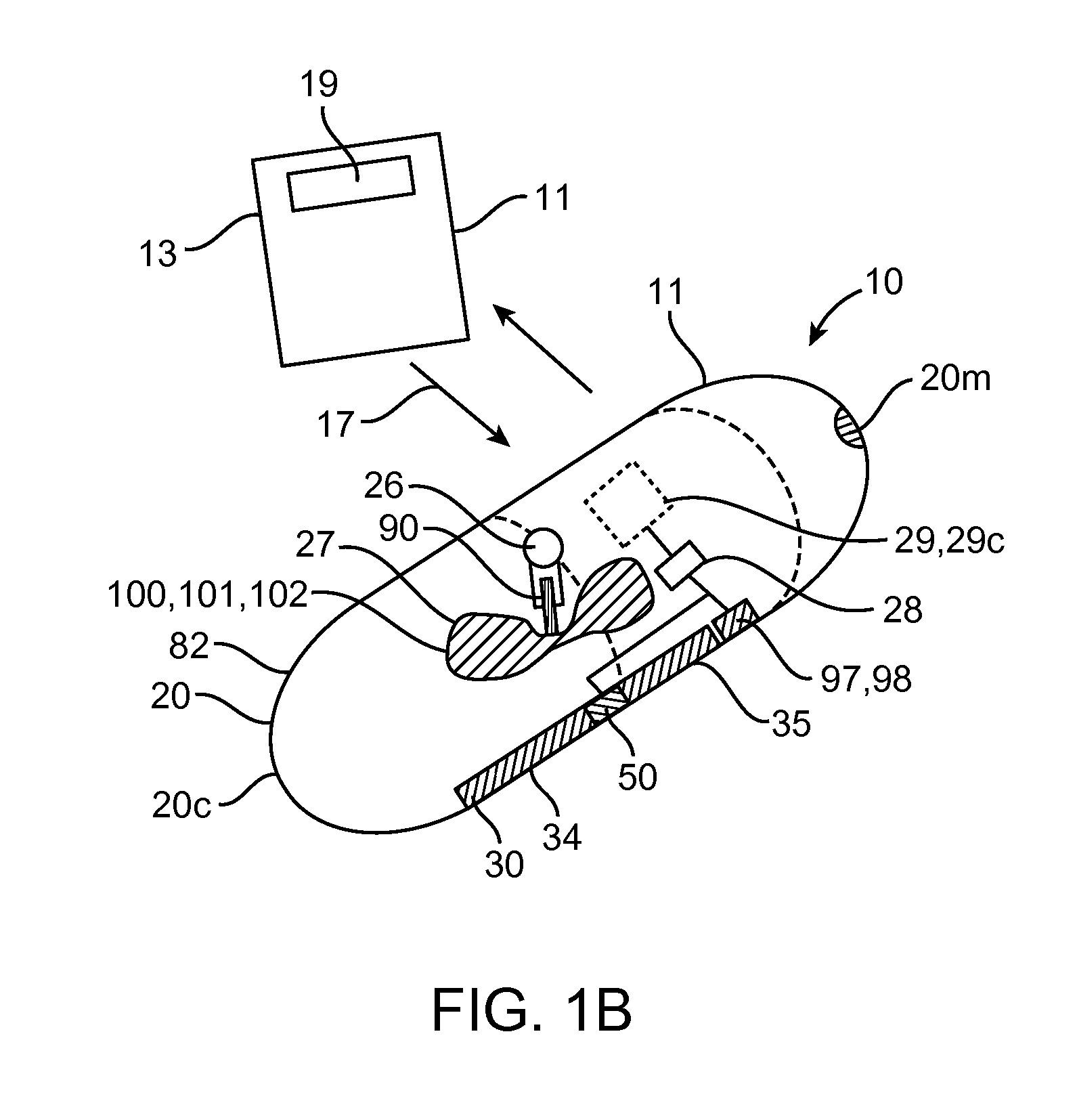
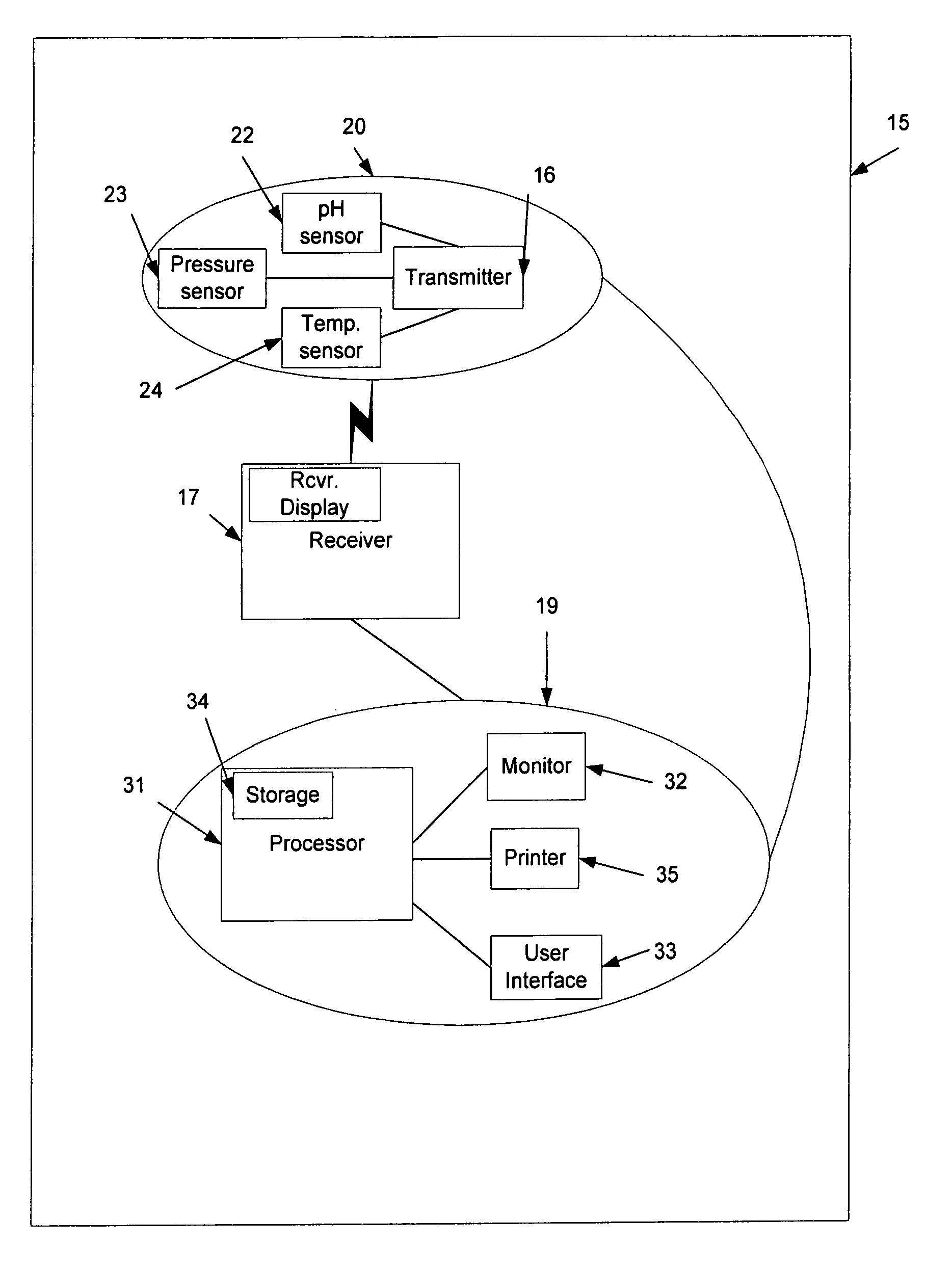
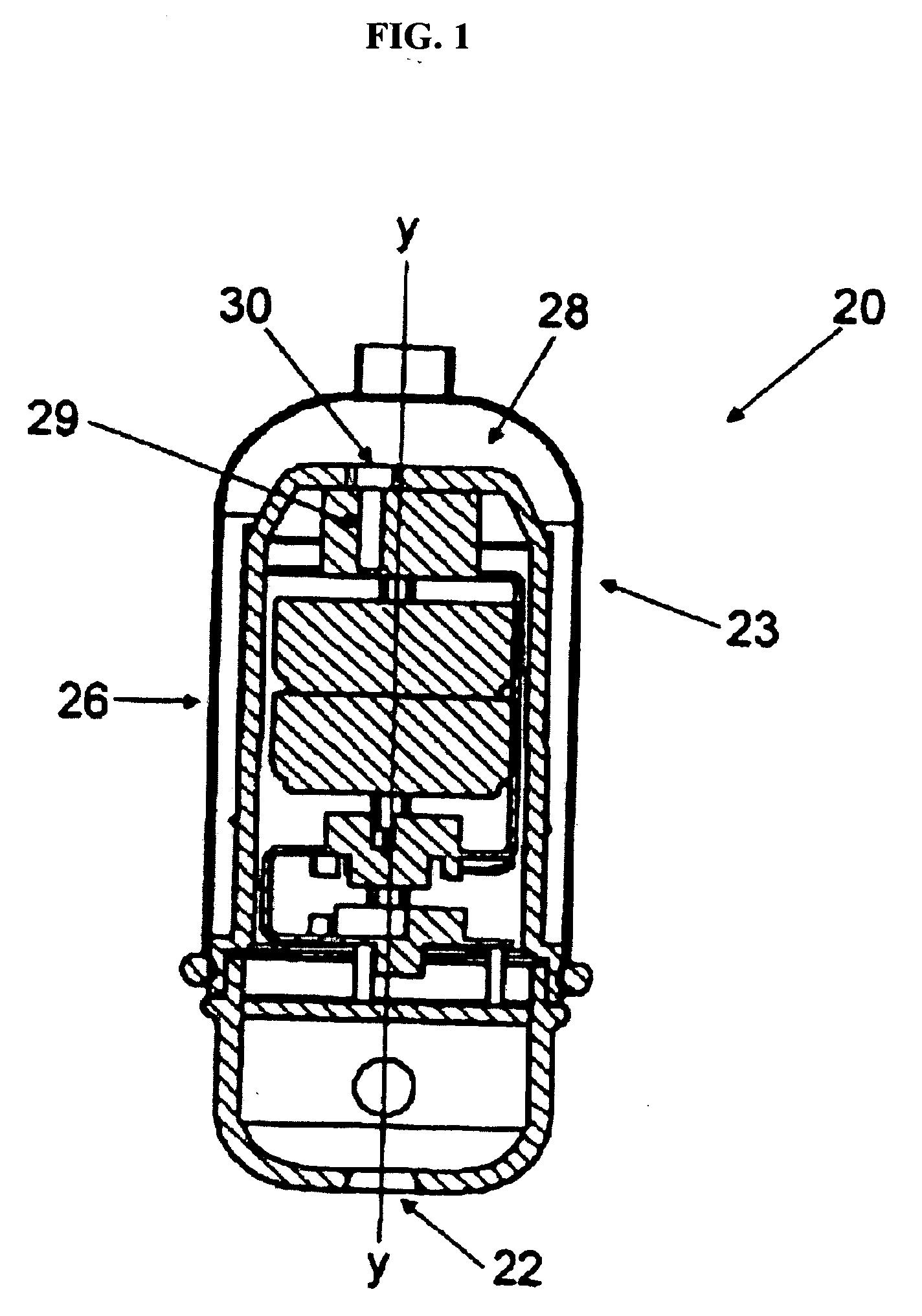
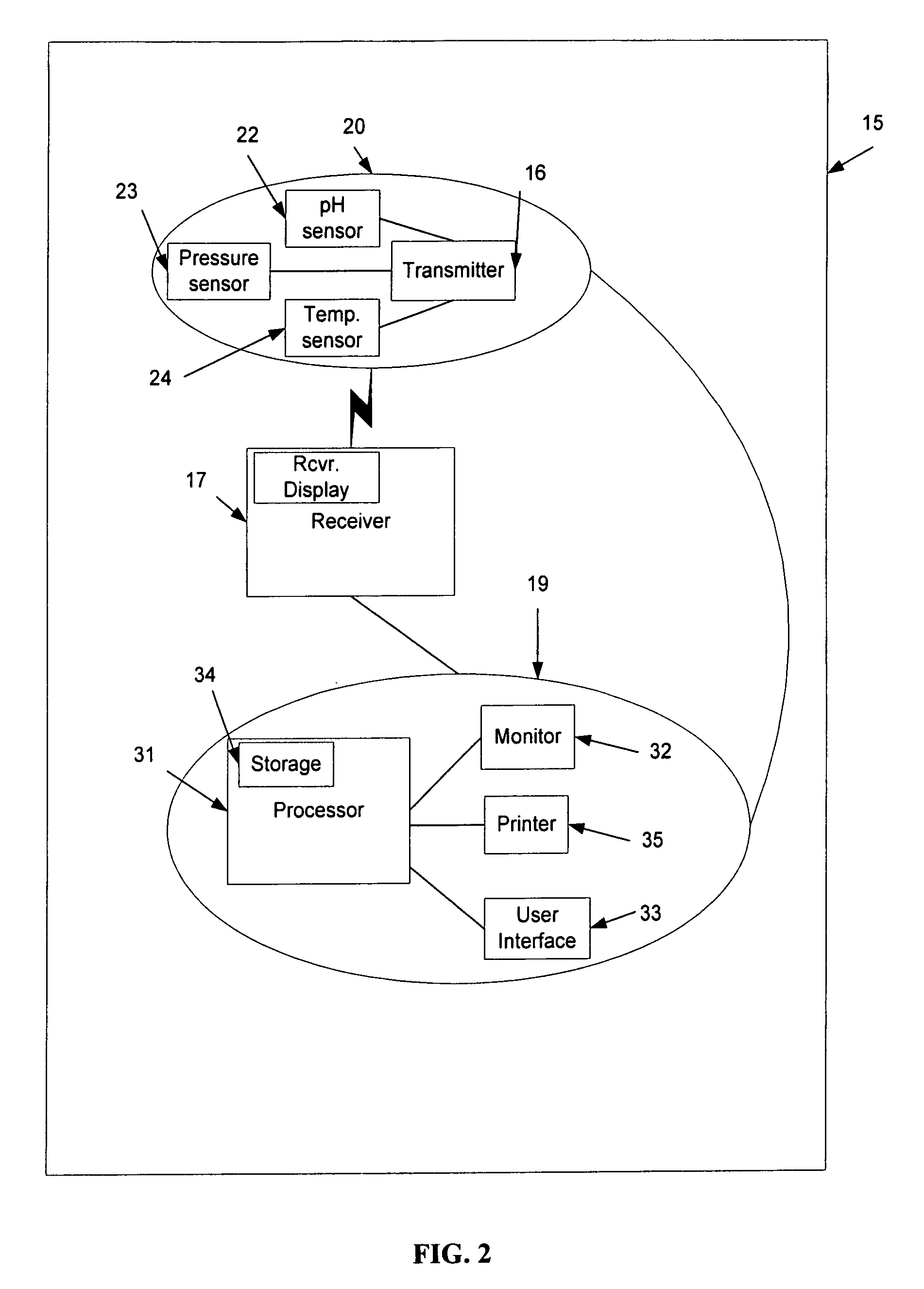
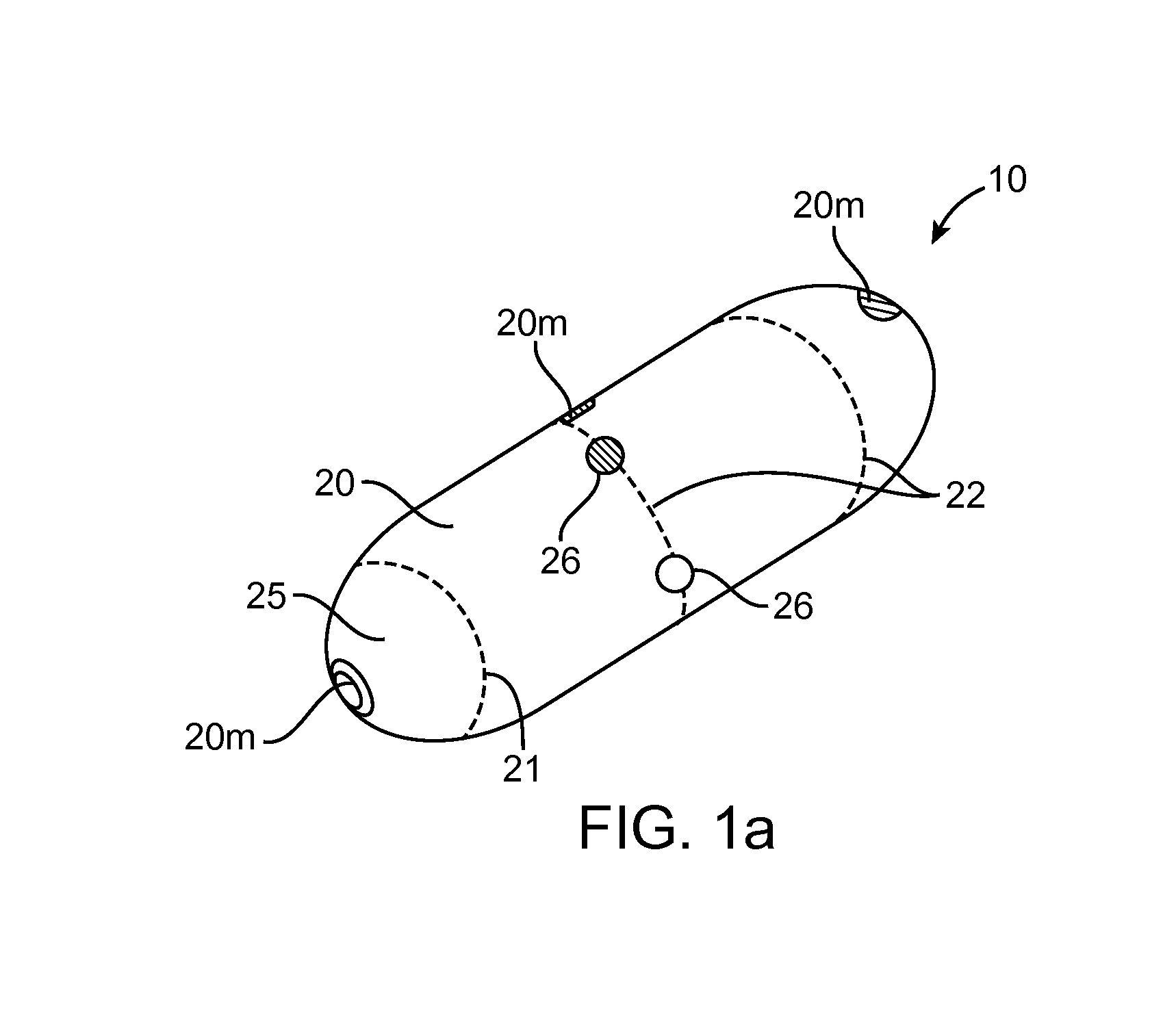
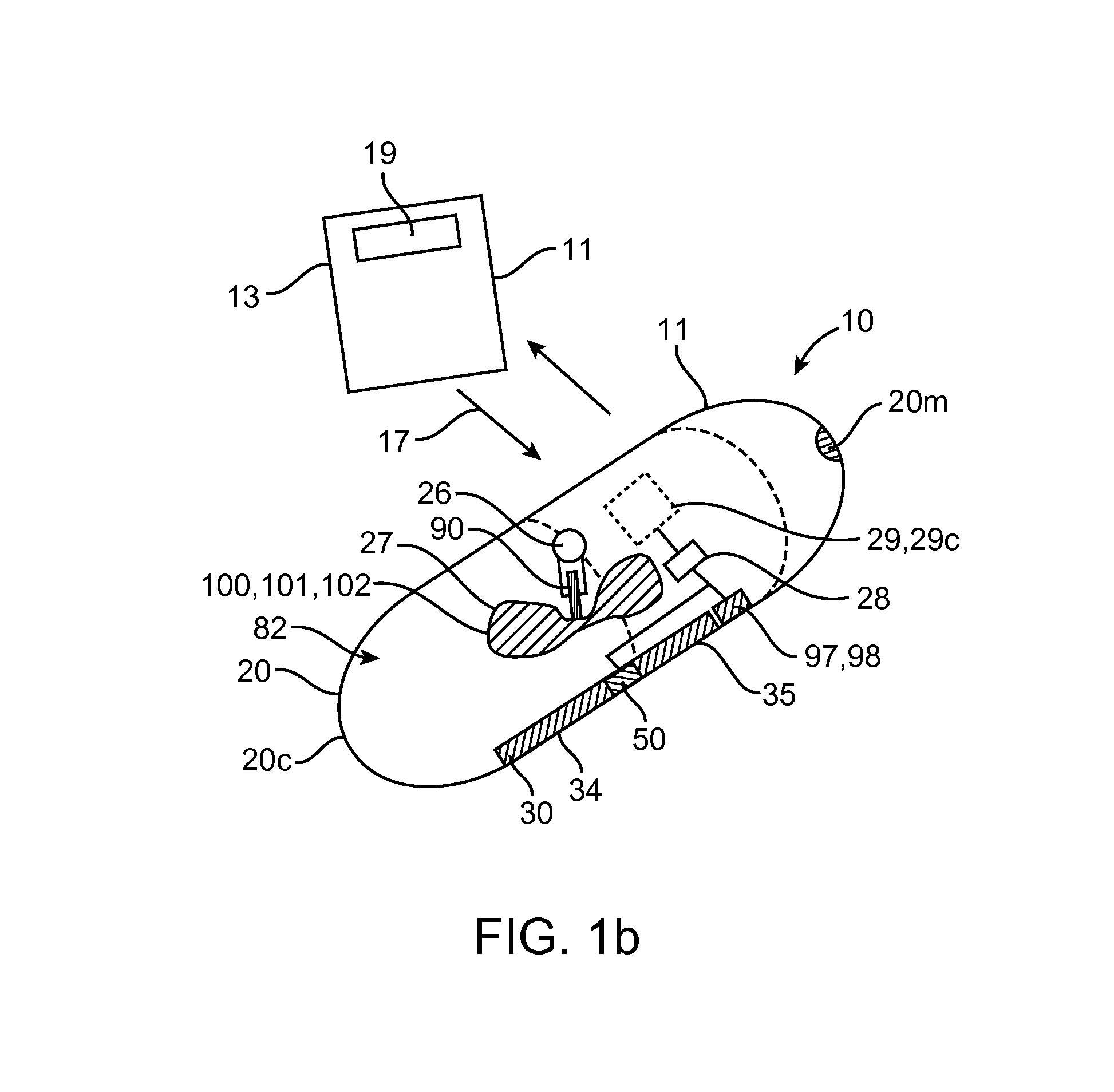
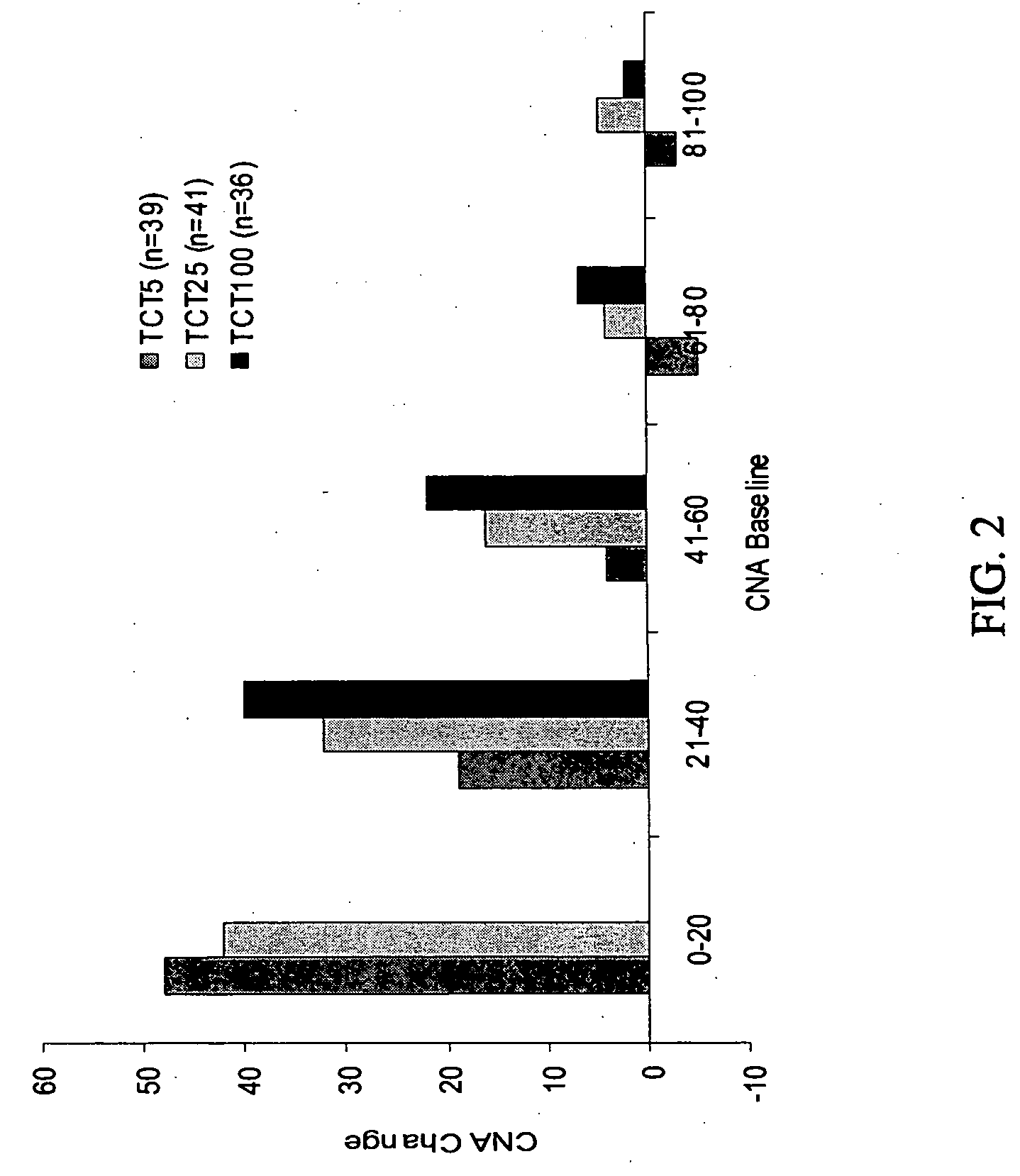
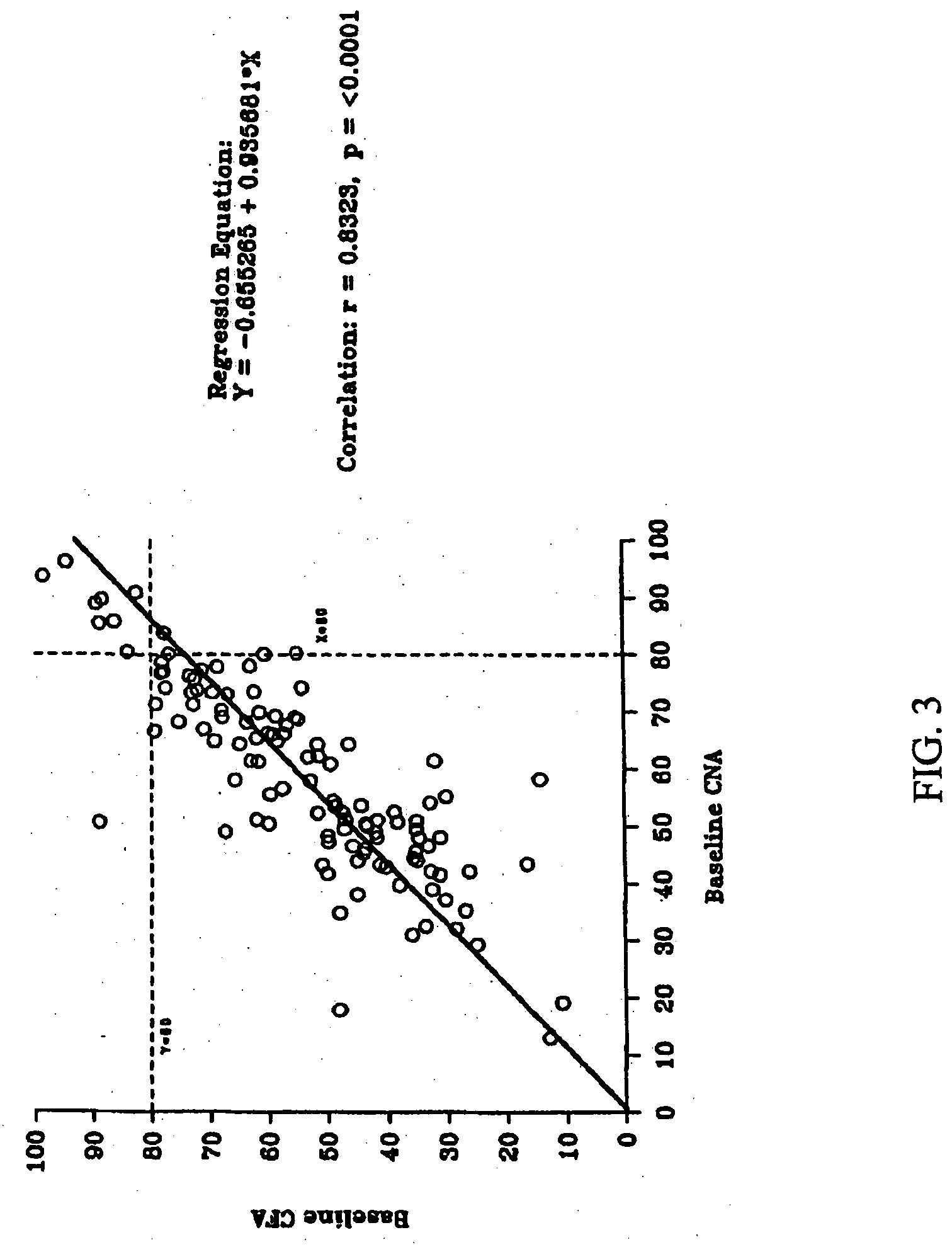
A computerized method of analyzing measurements obtained from the gastrointestinal tract of subject comprising the steps of providing an ingestible capsule (20) having a sensor (22, 23, 24) for measuring a parameter of the gastrointestinal tract of a subject, having a subject ingest (118) the capsule, recording (130) measurements from the sensor as the capsule passes through the gastrointestinal tract of the subject, transmitting (131, 122) the measurements to a processor (31), conditioning (132) the measurements to provide data as a function of a time interval, plotting (133) the data on a display (32), providing a query (205, 216, 234), on the display, receiving input (207, 218, 237) from a user in response to the query, setting a marker (50-53) on the plot (40) at a location as a function of the input, and determining (238) a capsule transit time for a selected portion of the gastrointestinal tract as a function of the location of the marker.
Owner:TYCO HEALTHCARE GRP LP
IBD-associated microbial antigens and methods of using same
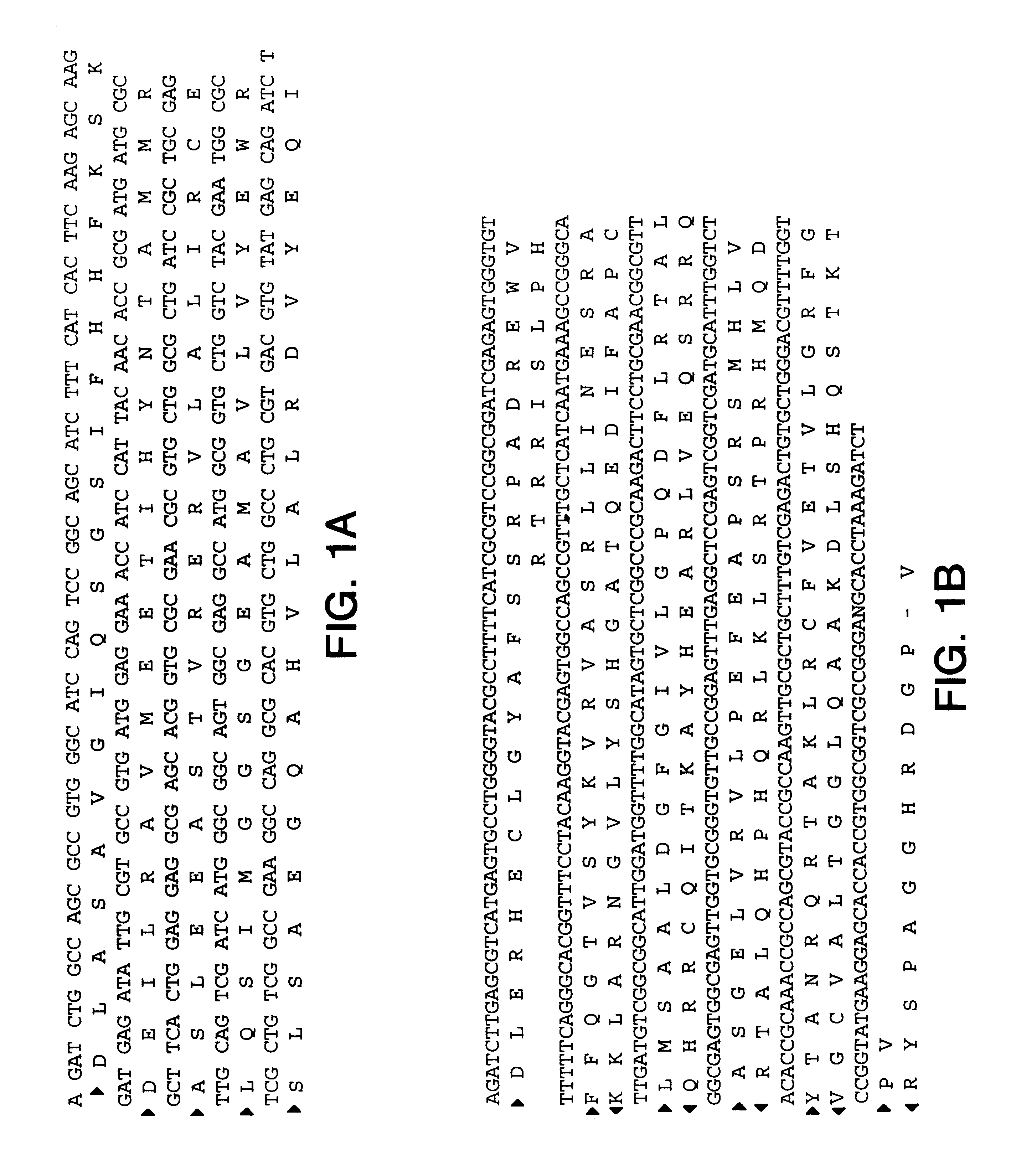
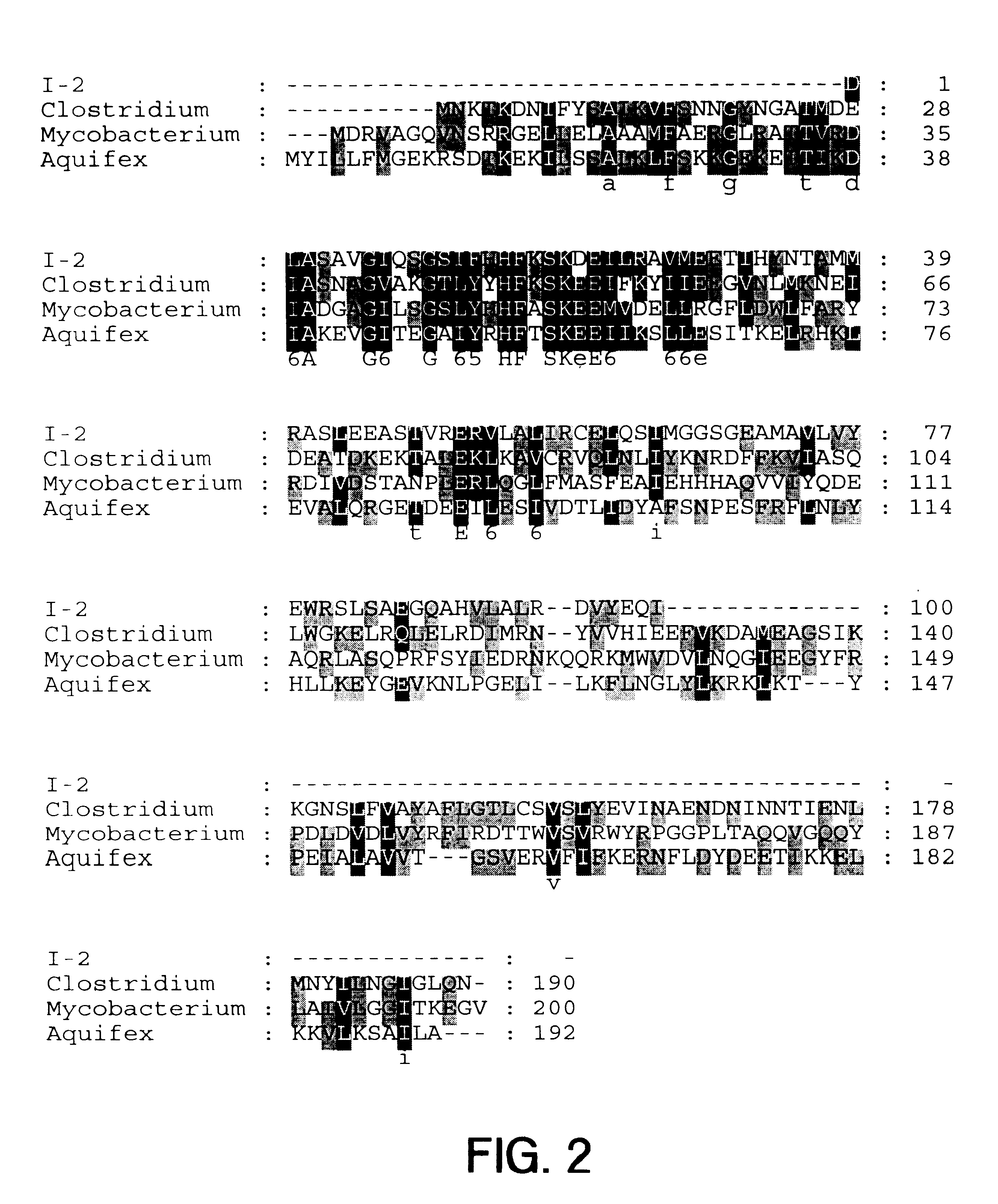
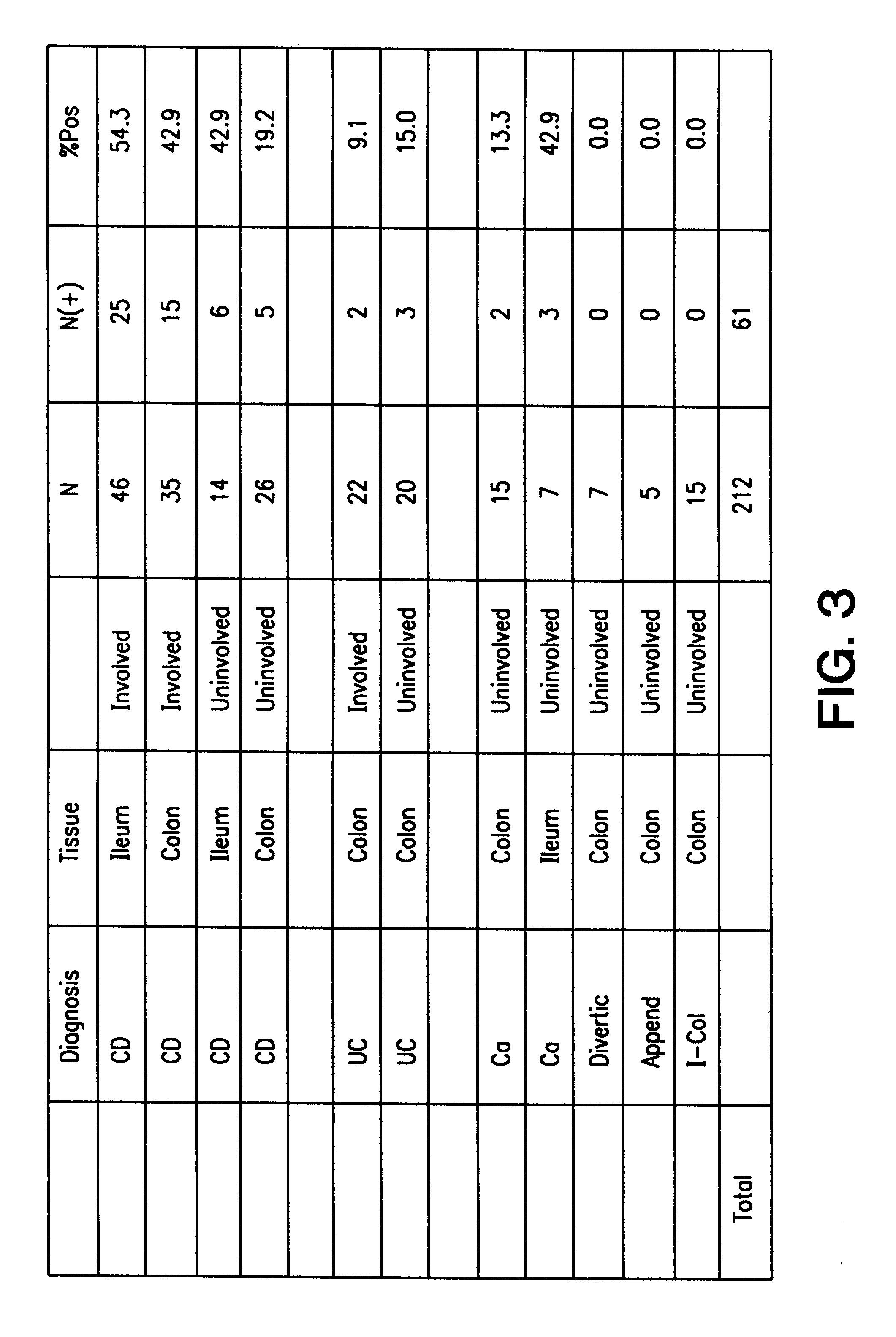
The present invention provides nucleic acid and amino acid sequence of the novel I-1 and I-2 polypeptides, which are associated with human inflammatory bowel disease (IBD). Methods of diagnosing and treating inflammatory bowel disease using the IBD-associated I-1 and I-2 antigens also are provided.
Owner:RGT UNIV OF CALIFORNIA
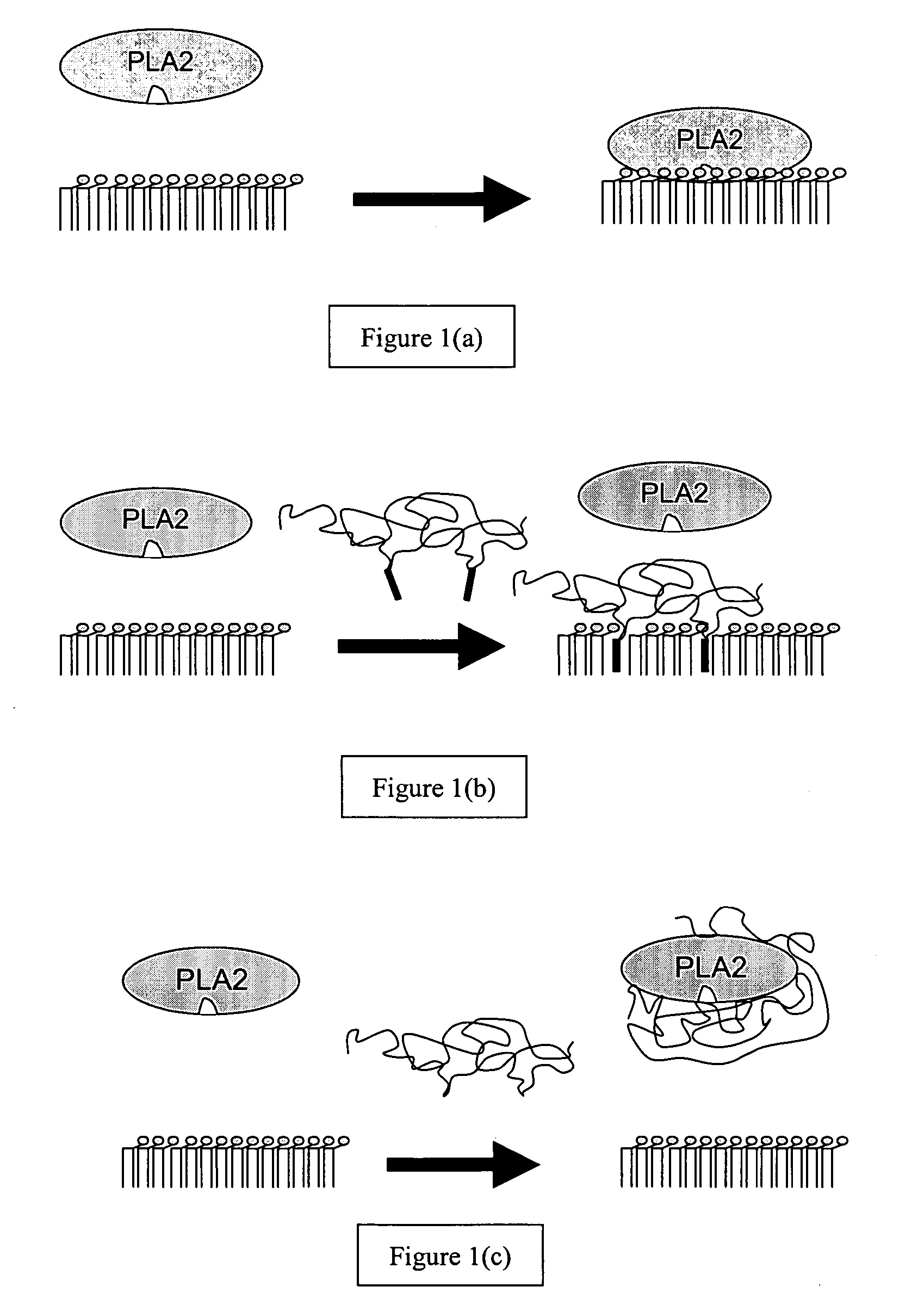
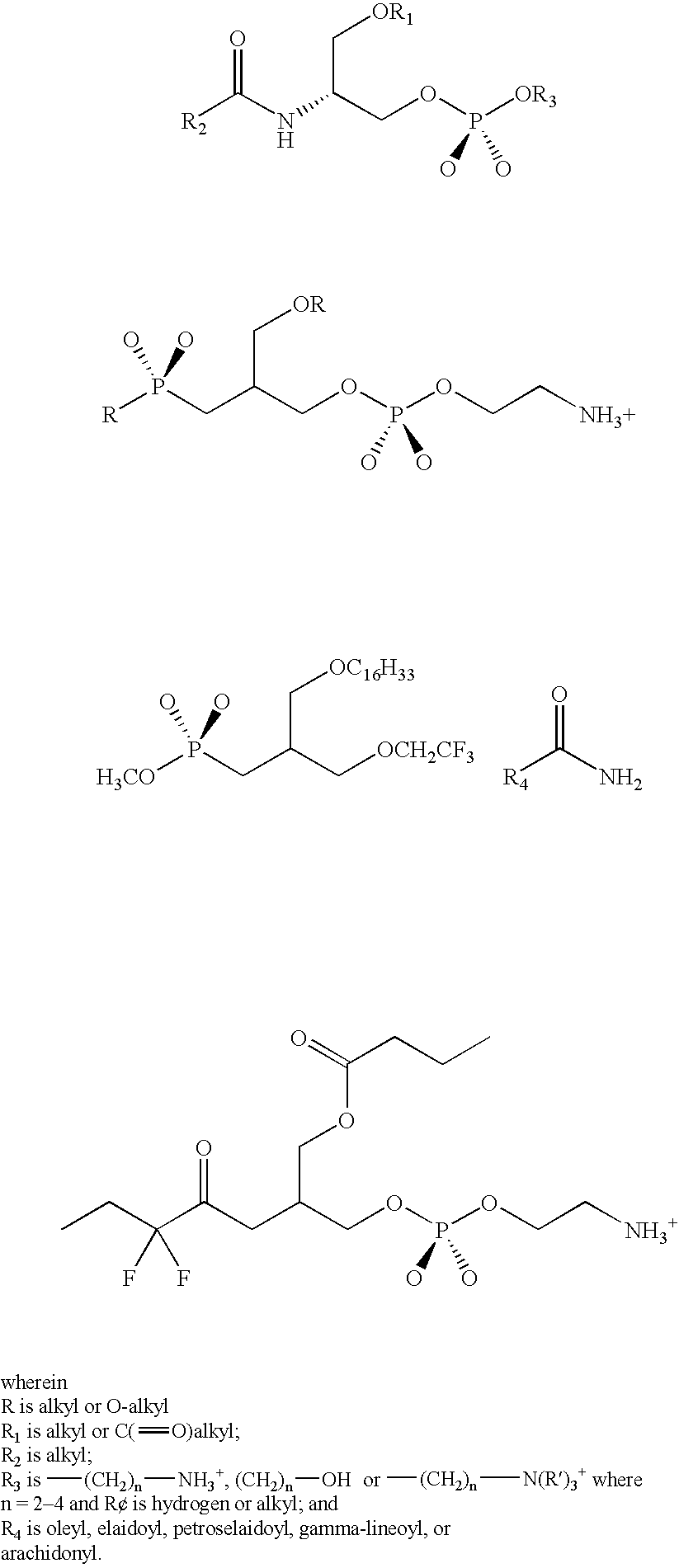
Phospholipase inhibitors localized in the gastrointestinal lumen
The present invention provides methods and compositions for the treatment of phospholipase-related conditions. In particular, the invention provides a method of treating insulin-related, weight-related conditions and / or cholesterol-related conditions in an animal subject. The method generally involves the administration of a non-absorbed and / or effluxed phospholipase A2 inhibitor that is localized in a gastrointestinal lumen.
Owner:ILYPSA
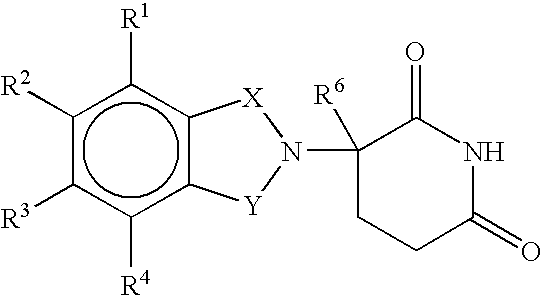
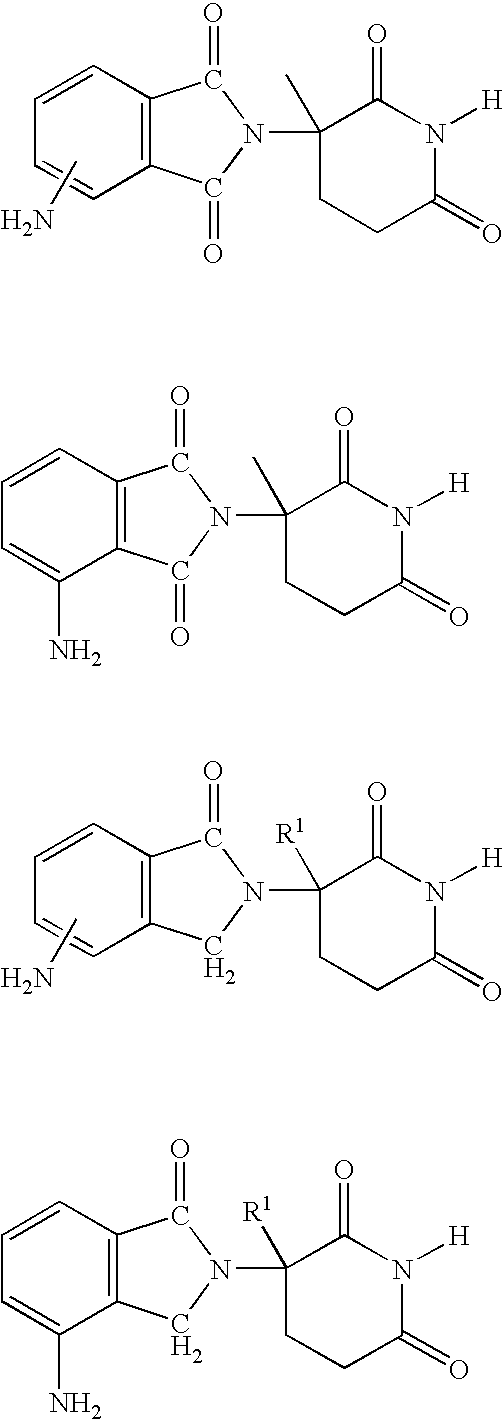
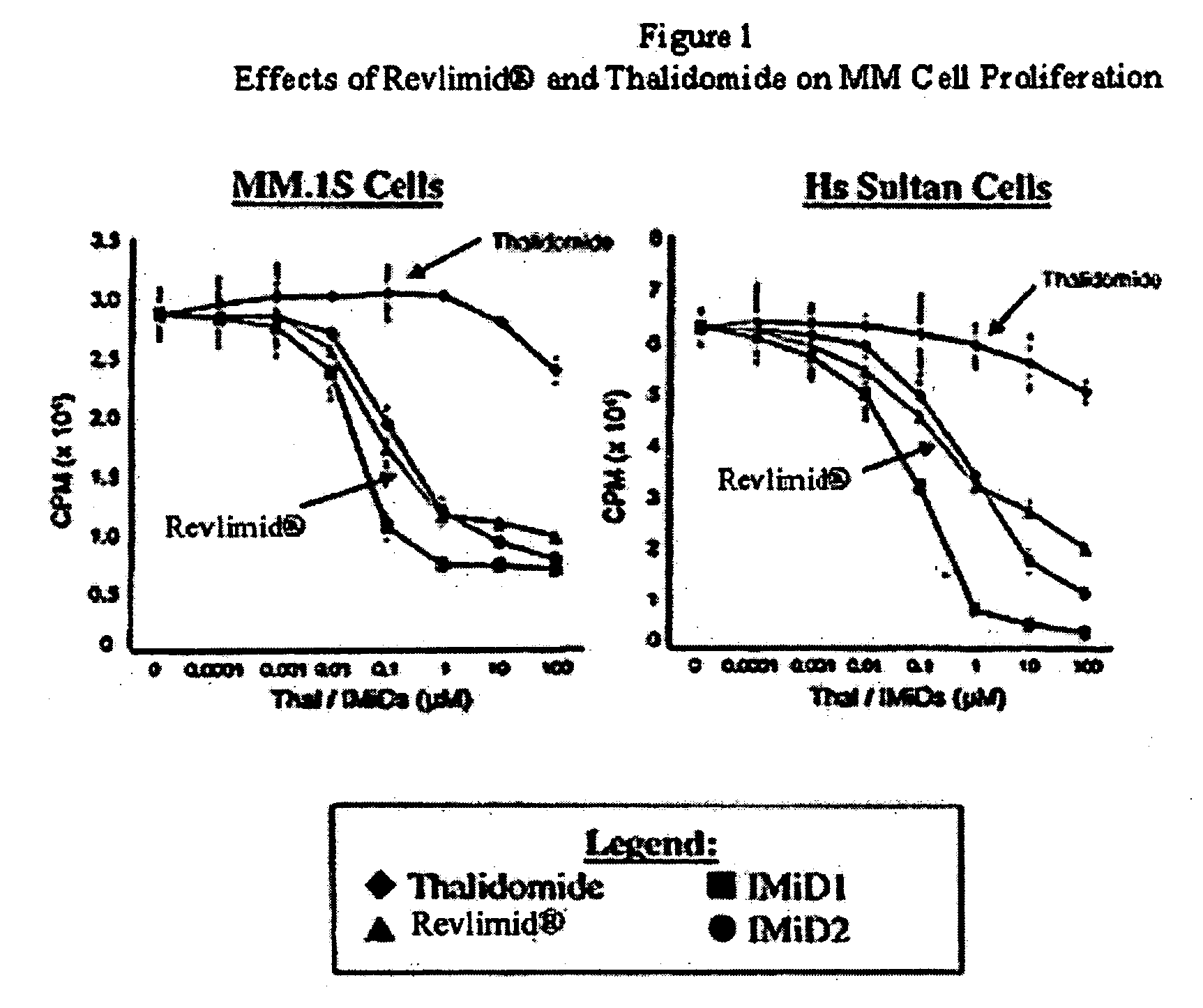
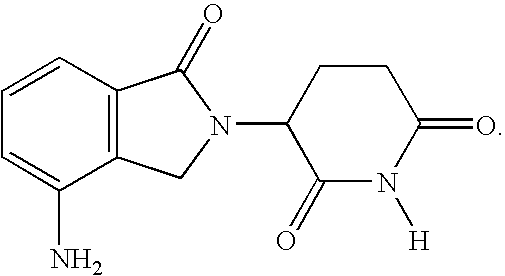
Method using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias
InactiveUS20060030594A1Prevent proliferationBiocidePeptide/protein ingredientsCompound (substance)Radiation therapy
Methods of treating, preventing or managing leukemias are disclosed. The methods encompass the administration of an immunomodulatory compound of the invention known as Revlimid® or lenalidomide. The invention further relates to methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Methods and devices for placing a gastrointestinal sleeve
Methods and systems for delivering or placing a gastrointestinal implant device into a mammal. The gastrointestinal implant device can be used to limit absorption of food products in specific parts of the digestive system and can include a gastrointestinal sleeve having an anchor portion and a barrier or sleeve portion. The methods include endoluminal delivery of the device.
Owner:GI DYNAMICS
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS9149617B2Rapid drug releasePoor absorptionPeptide/protein ingredientsMedical devicesIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Compositions and methods for treating pancreatic insufficiency
InactiveUS20060121017A1Stable enzyme componentEffective low dose treatment regimenPeptide/protein ingredientsHydrolasesProteinase activityPancreas
The present invention relates to compositions for the treatment of conditions, including pancreatic insufficiency. The compositions of the present invention comprise lipase, protease and amylase in a particular ratio that provides beneficial results in patients, such as those afflicted with pancreatic insufficiency. This invention also relates to methods using such compositions for the treatment of pancreatic insufficiency.
Owner:ELI LILLY & CO
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20130165859A1Rapid drug releasePoor absorptionPeptide/protein ingredientsSurgeryIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
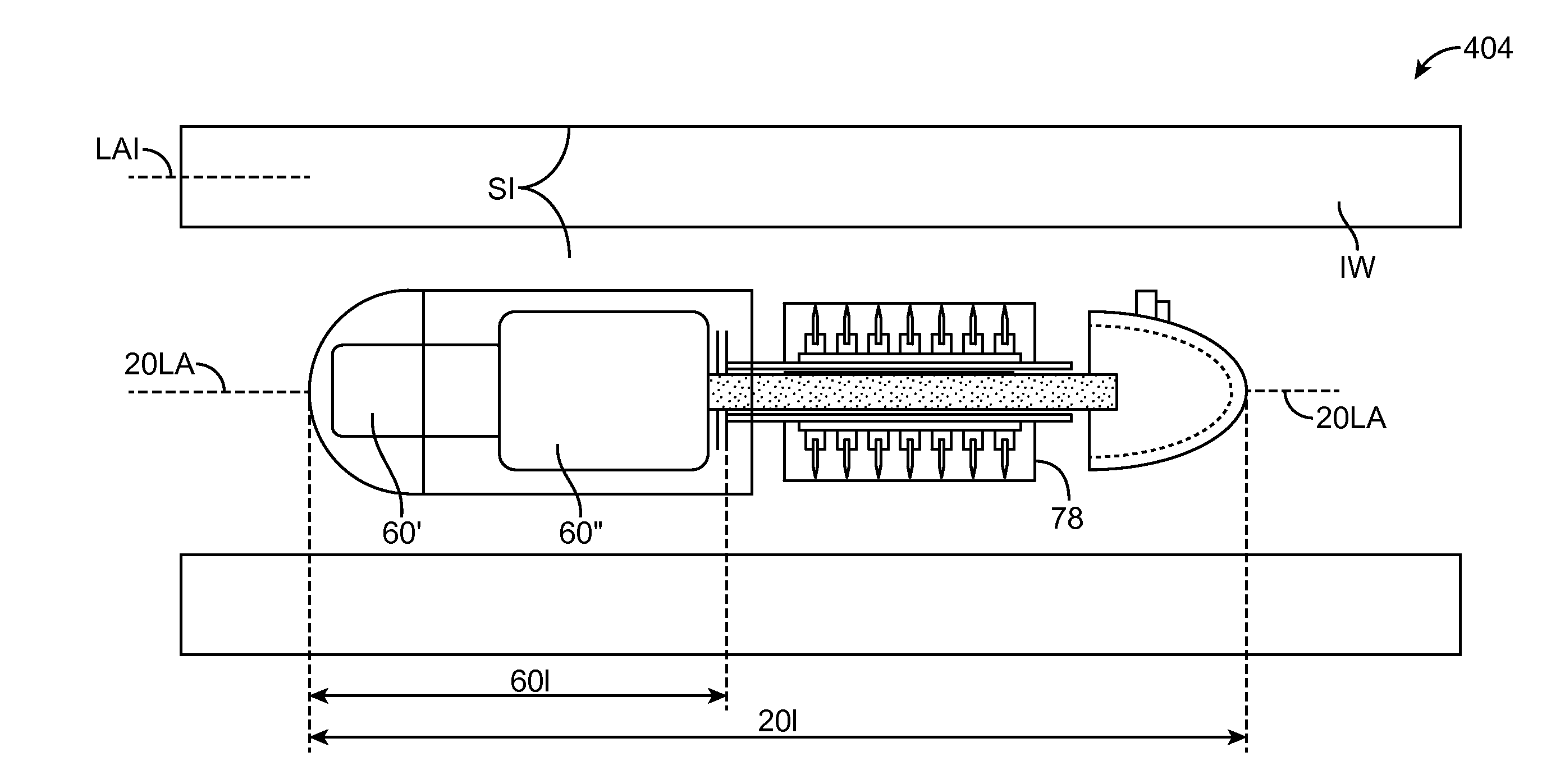
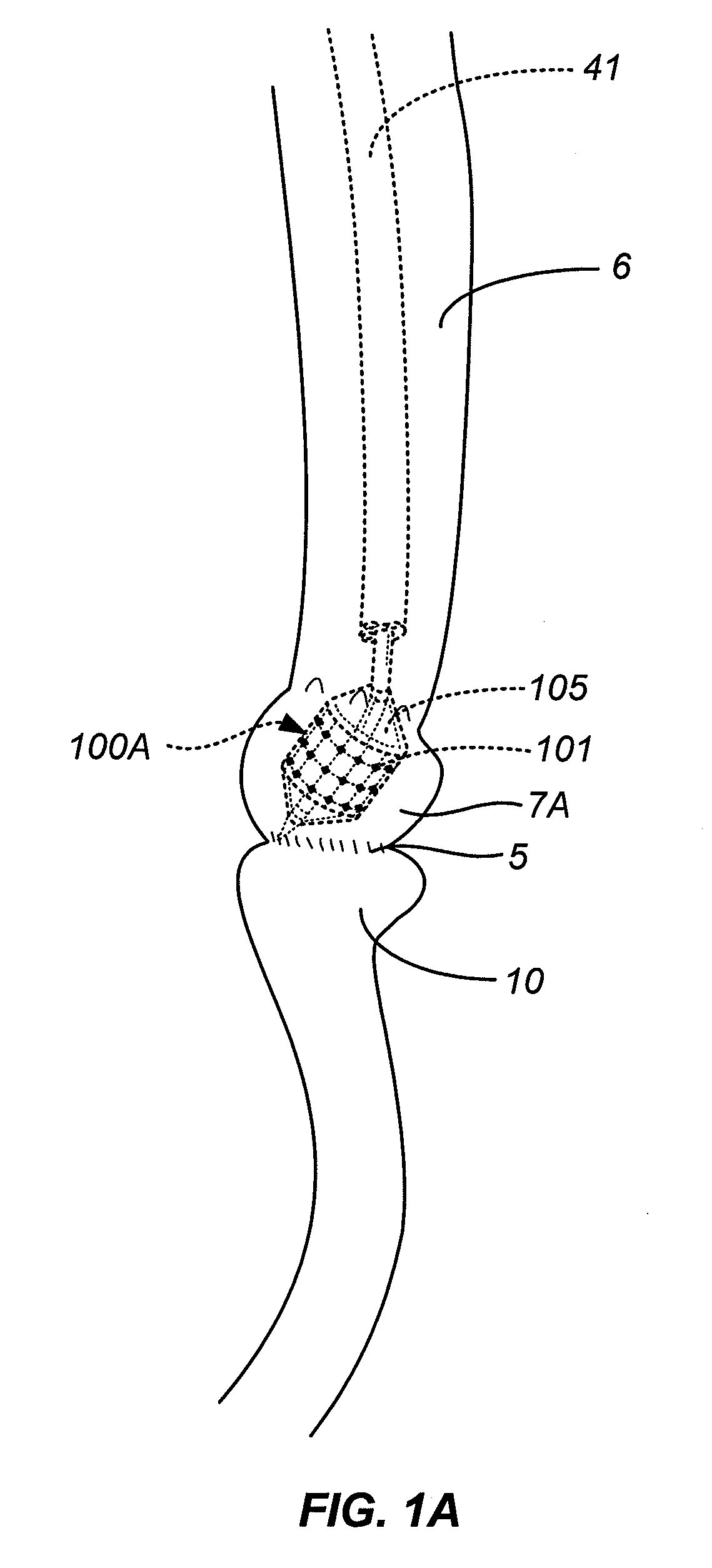
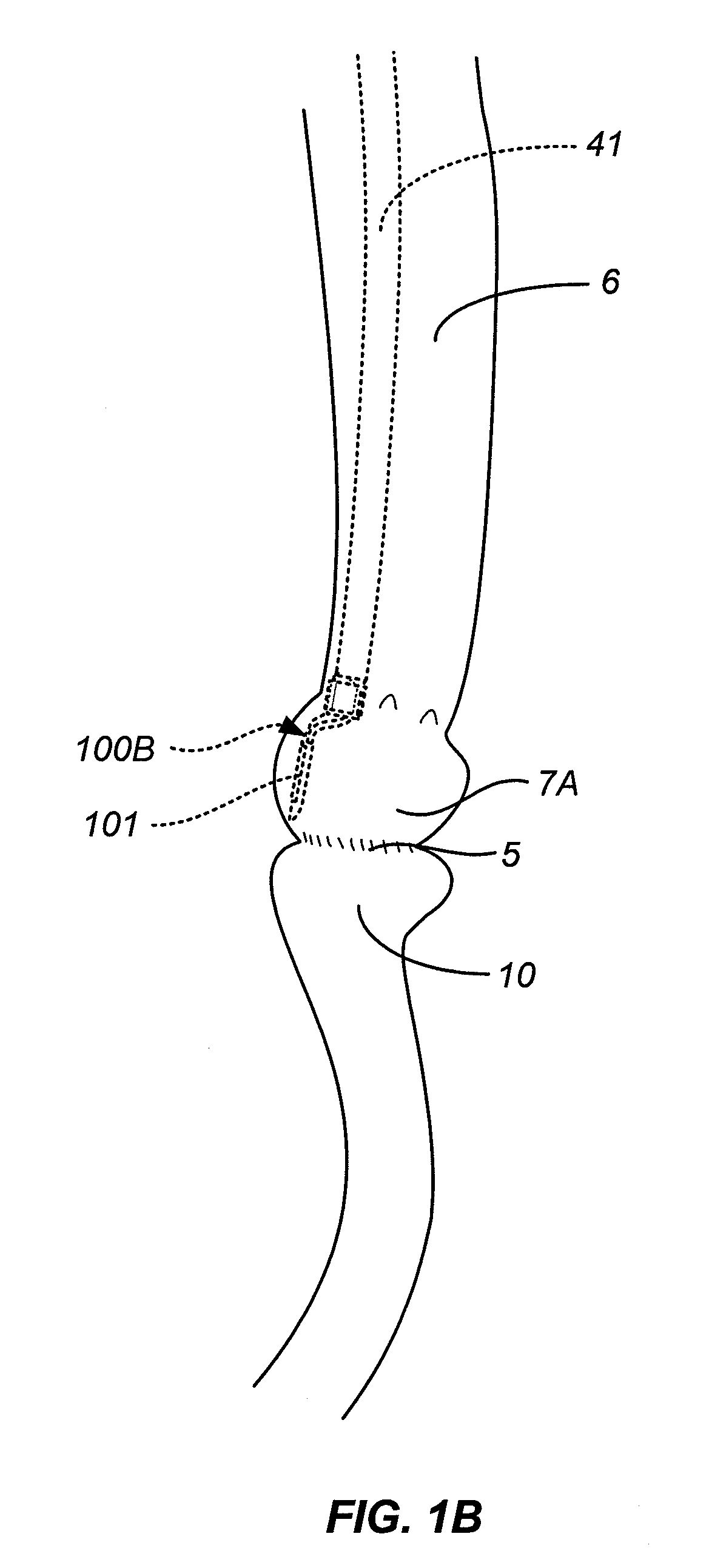
Method and Apparatus for Gastrointestinal Tract Ablation to Achieve Loss of Persistent and/or Recurrent Excess Body Weight Following a Weight-Loss Operation
ActiveUS20090012512A1Reduce complianceSmall sizeSurgical instruments for heatingSurgical instruments for coolingStomaGastrectomy
Devices and methods are provided for ablational treatment of regions of the digestive tract in post-bariatric surgery patients who fail to achieve or maintain the desired weight loss. Bariatric procedures include Roux-en-Y gastric bypass, biliopancreatic diversion, and sleeve gastrectomy. These procedures reconstruct gastrointestinal tract features, creating pouches, stoma, and tubes that restrict and / or divert the digestive flow. Post-surgical dilation of altered structures is common and diminishes their bariatric effectiveness. Ablation of compromised structures can reduce their size and compliance, restoring bariatric effectiveness. Ablation, as provided the invention, starts at the mucosa and penetrates deeper into the gastrointestinal wall in a controlled manner. Control may also be provided by a fractional ablation that ablates some tissue within a target region and leaves a portion substantially unaffected. Embodiments of the device include an ablational electrode array that spans 360 degrees and an array that spans an arc of less than 360 degrees.
Owner:TYCO HEALTHCARE GRP LP
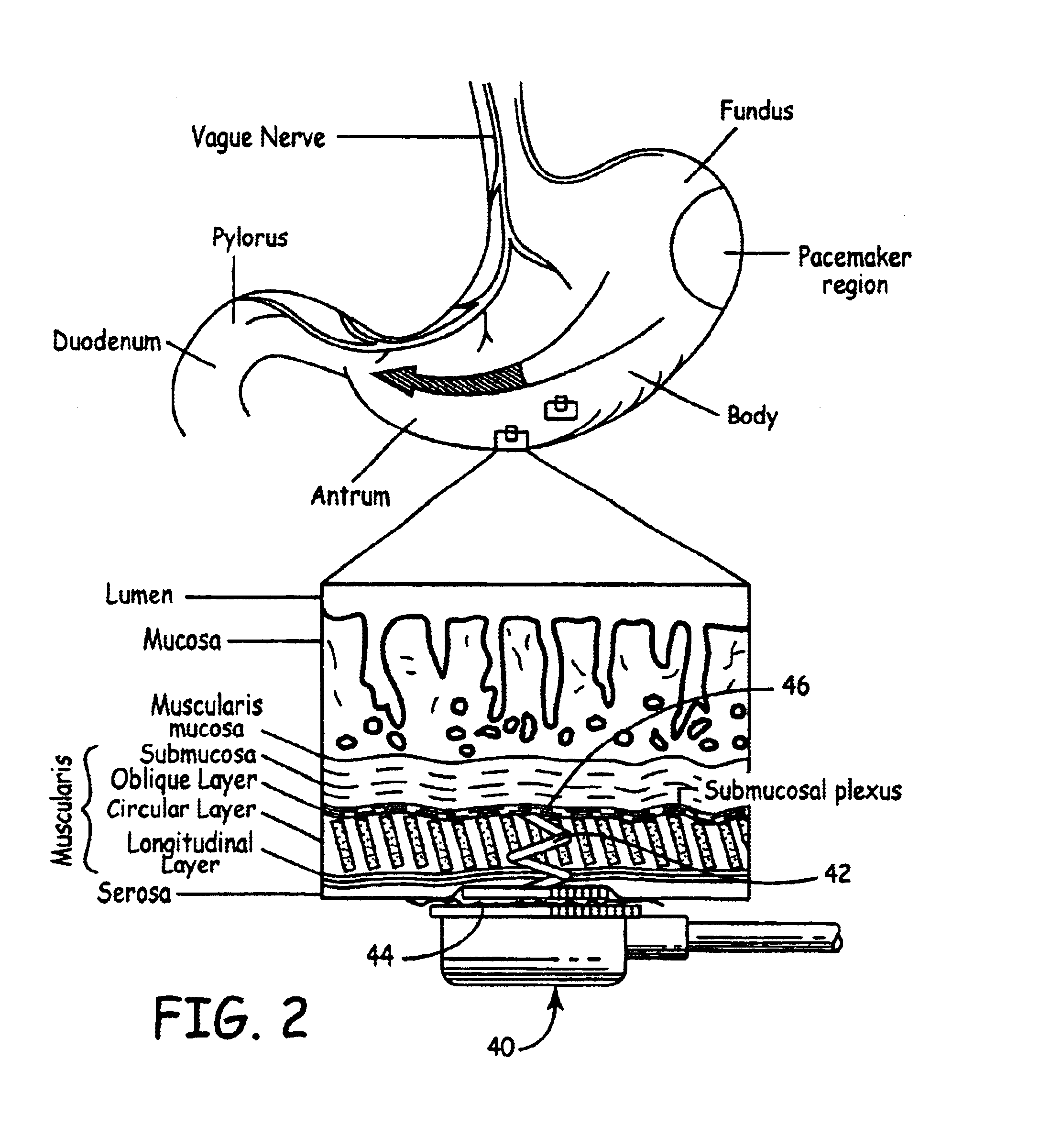
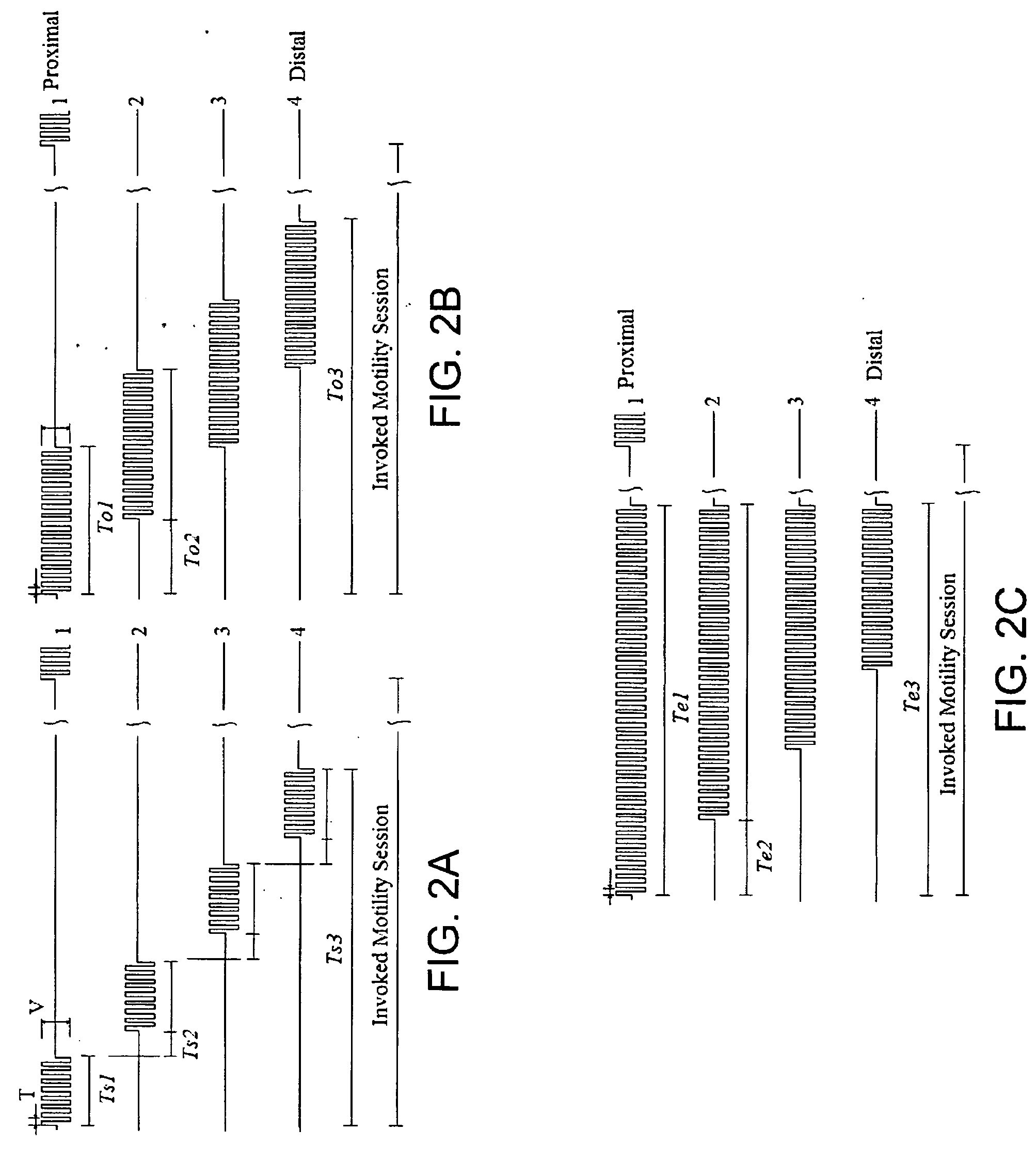
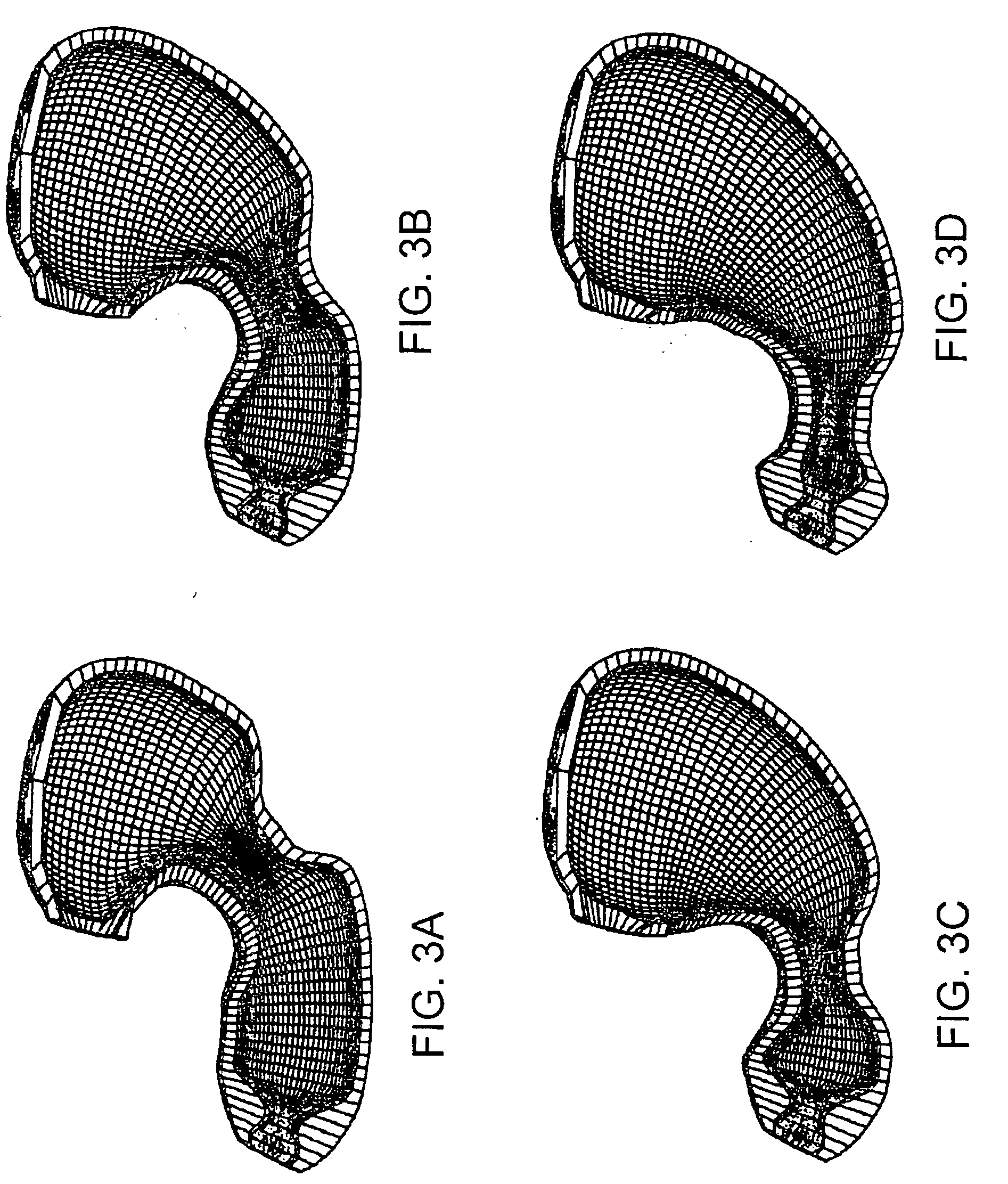
Gastrointestinal motility control
A method and a multichannel implantable device are described for partial or complete restoration of impaired gastrointestinal motility, or for disturbing and / or partially or completely blocking normal gastrointestinal motility using one or multiple microsystem-controlled channels of circumferentially arranged sets of two or more electrodes which provide externally-invoked synchronized electrical signals to the smooth muscles via the neural pathways.
Owner:UTI LLP
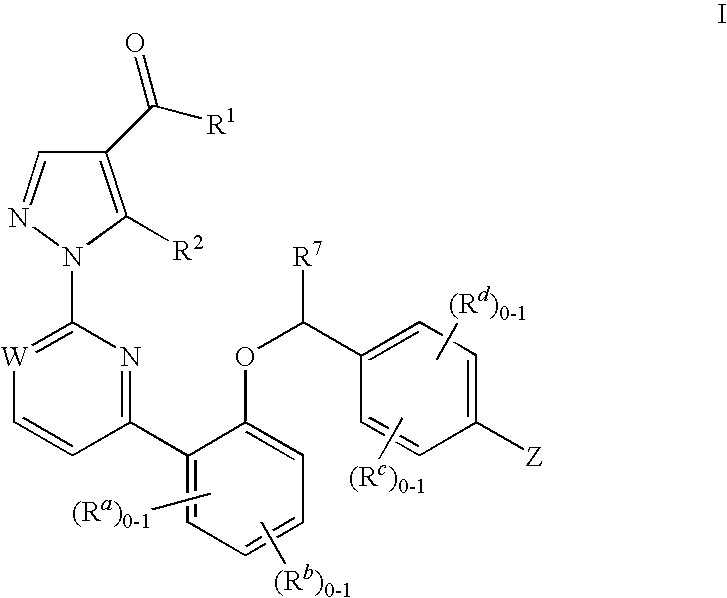
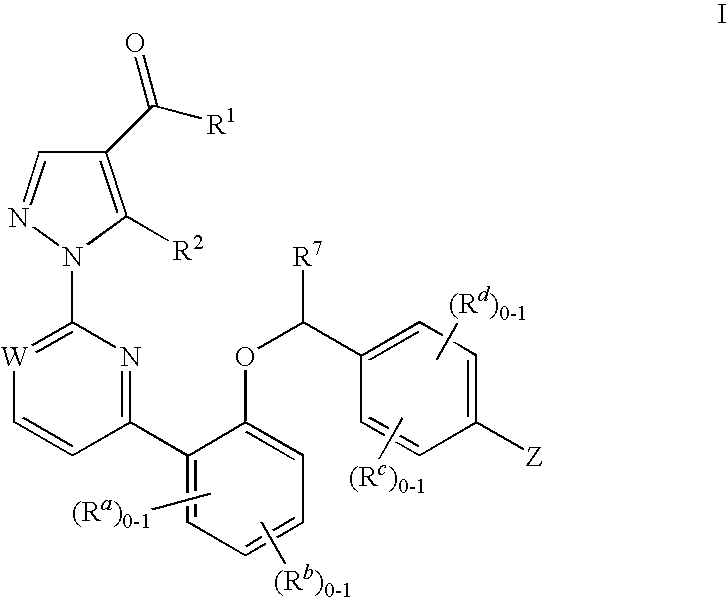
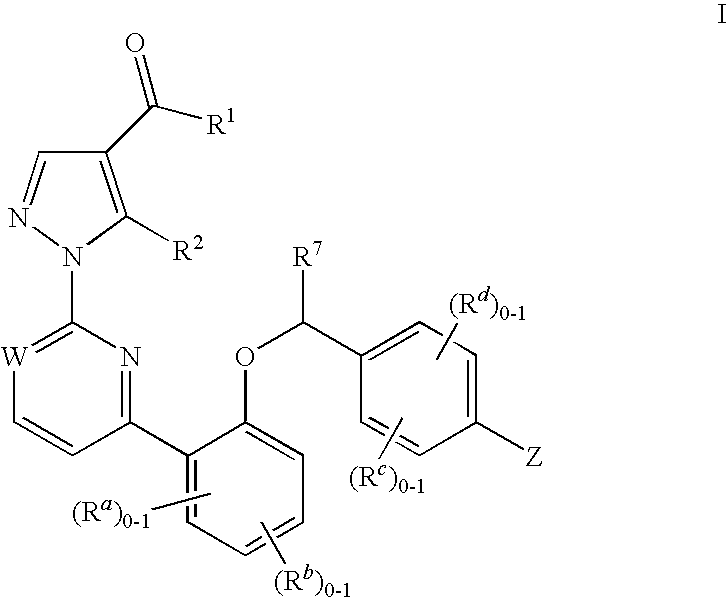
Soluble Guanylate Cyclase Activators
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
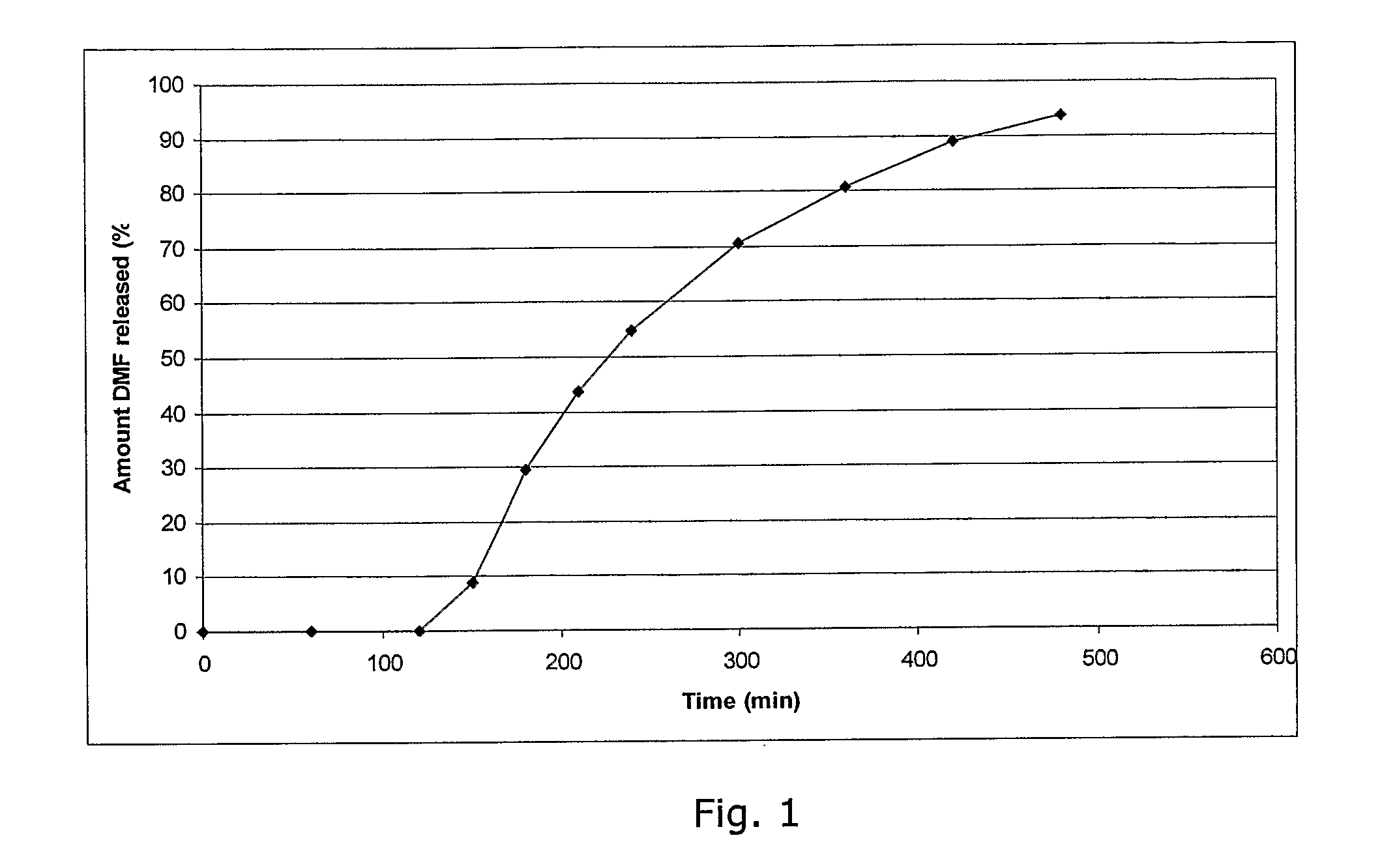
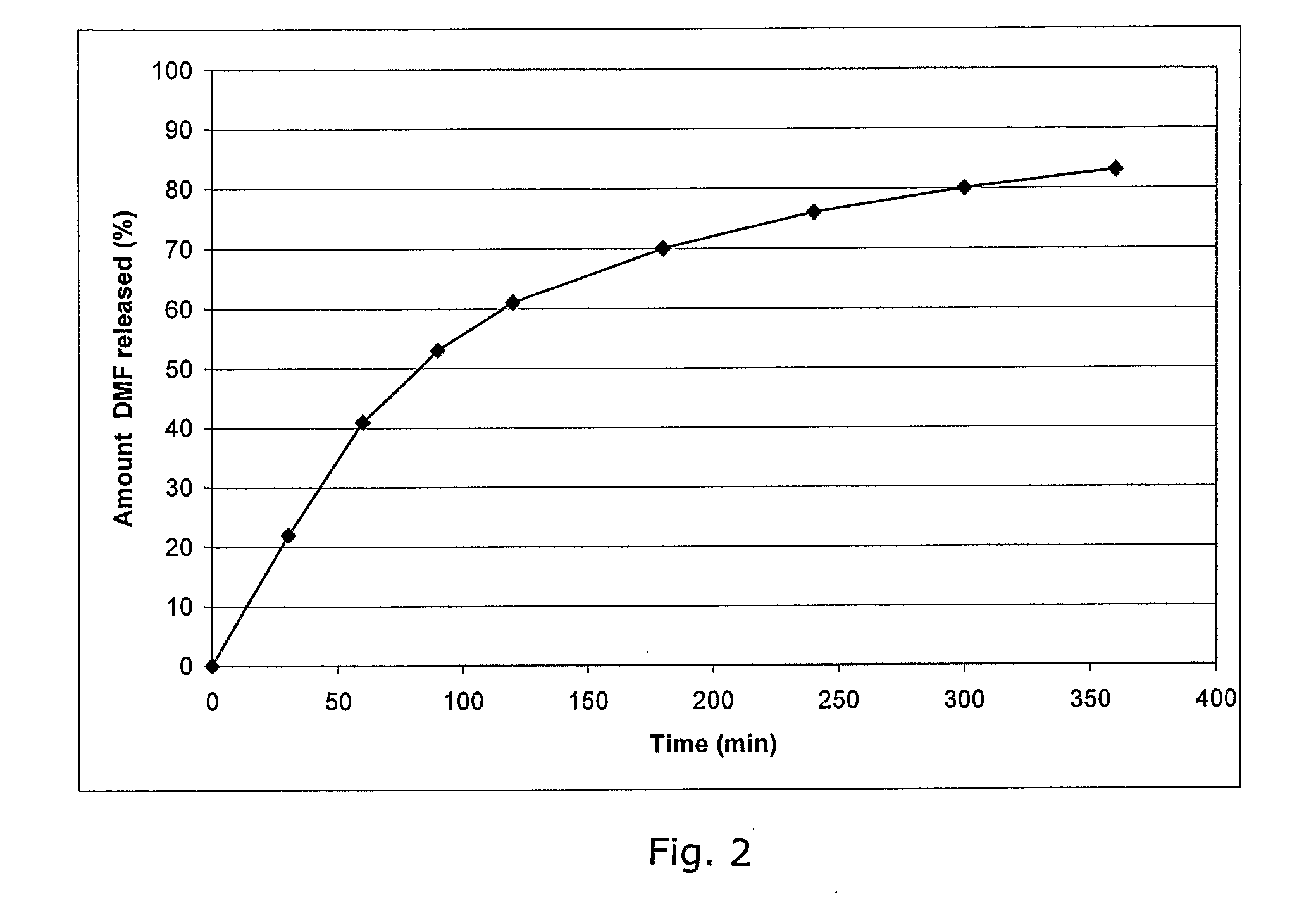
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
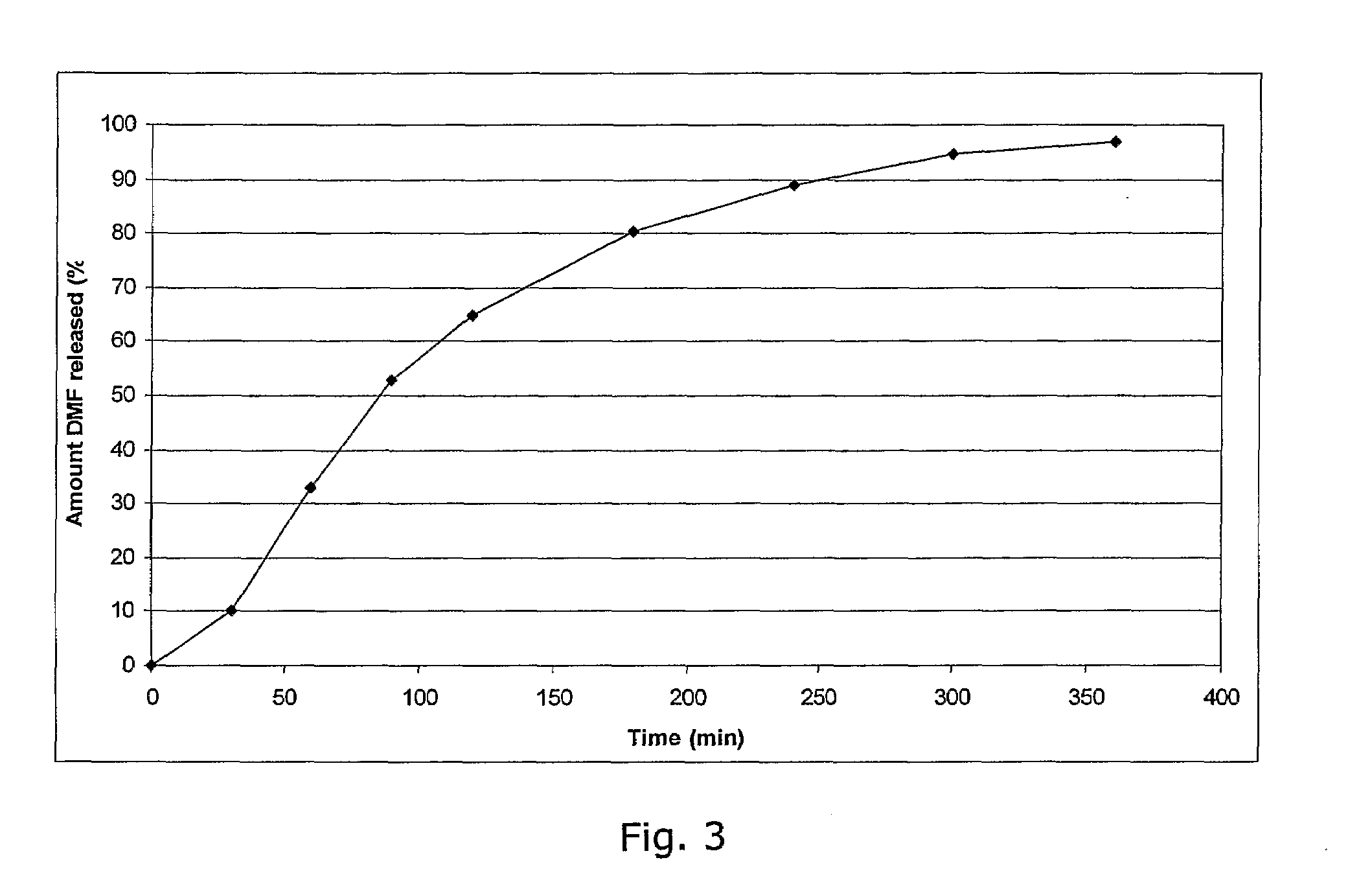
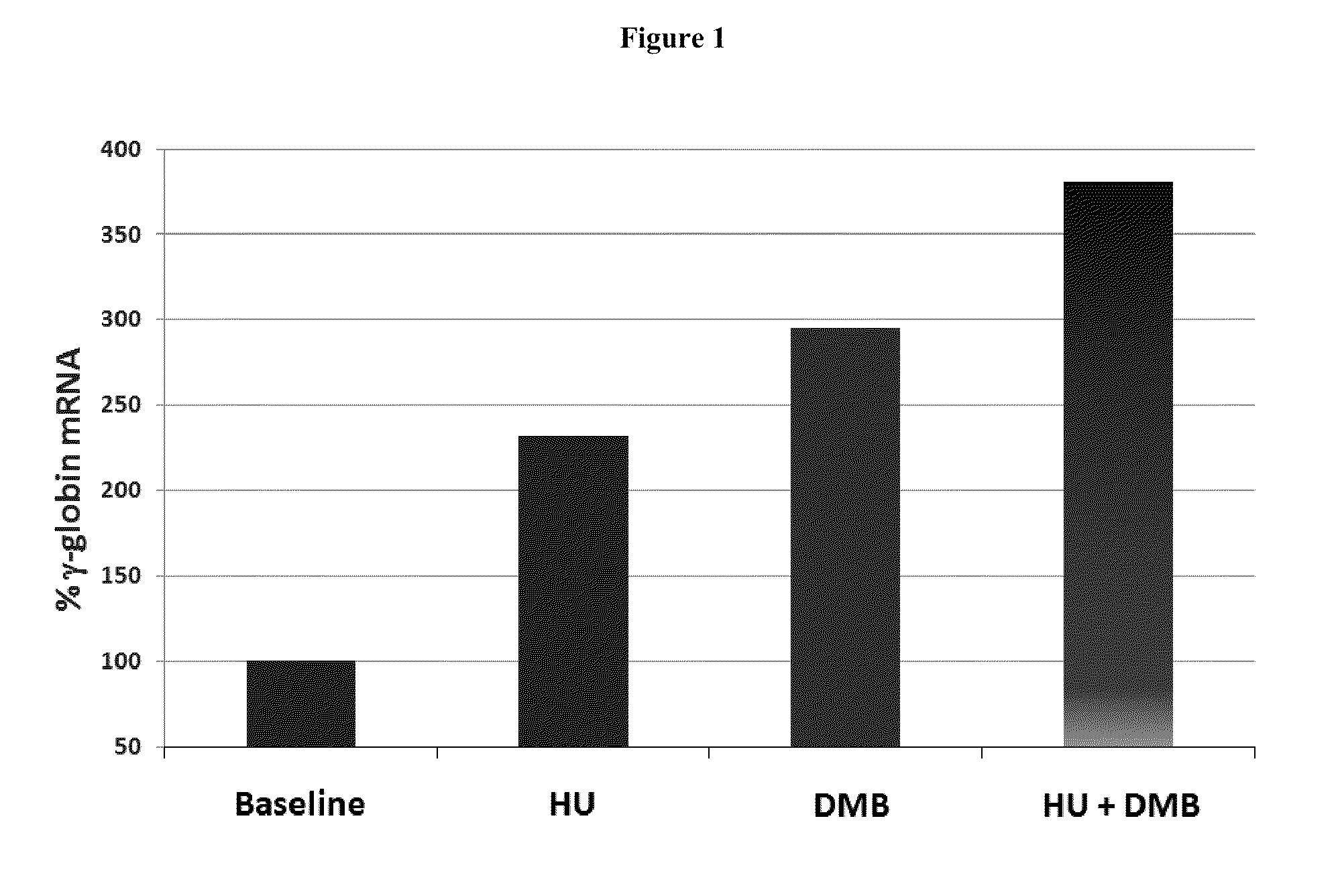
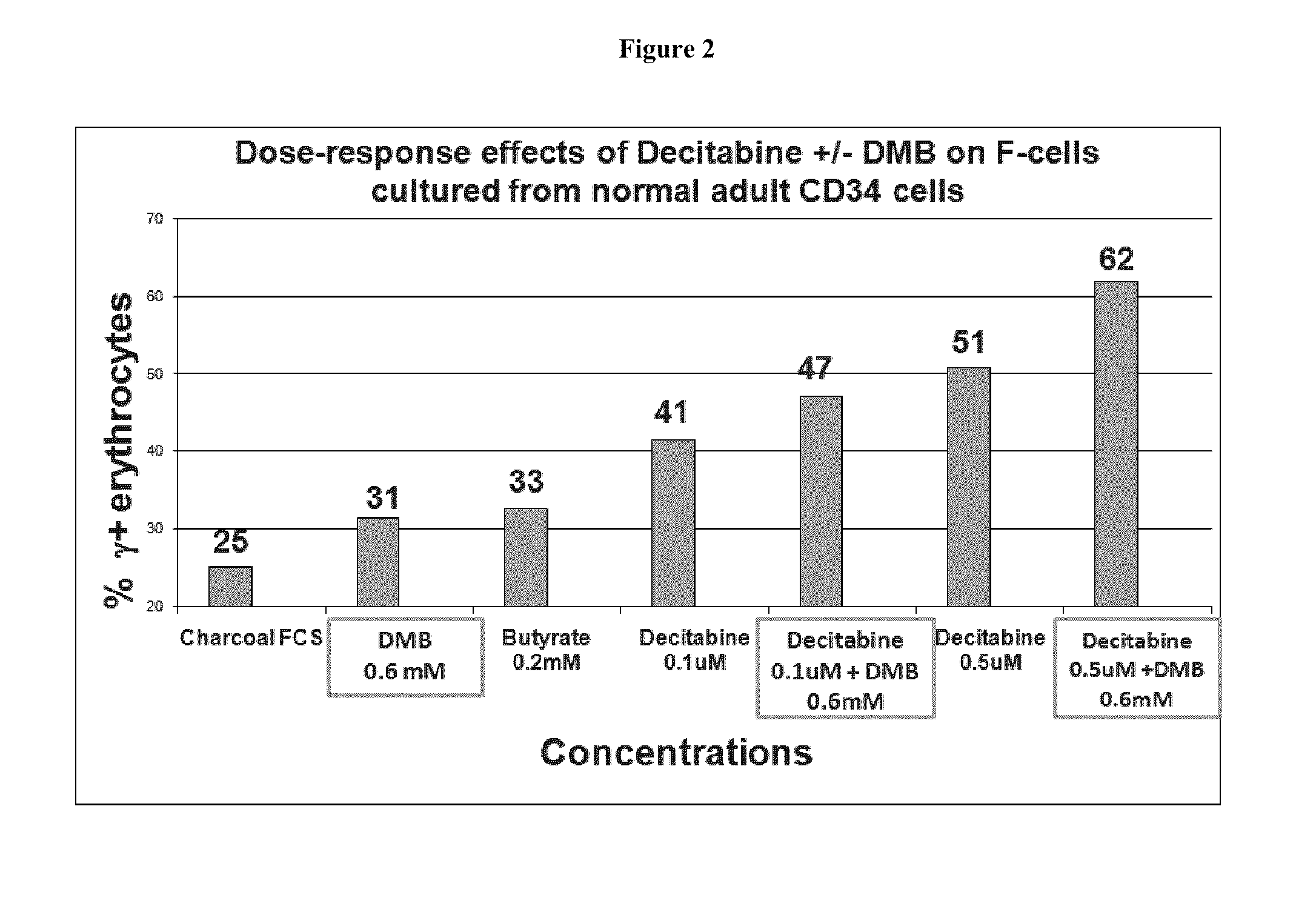
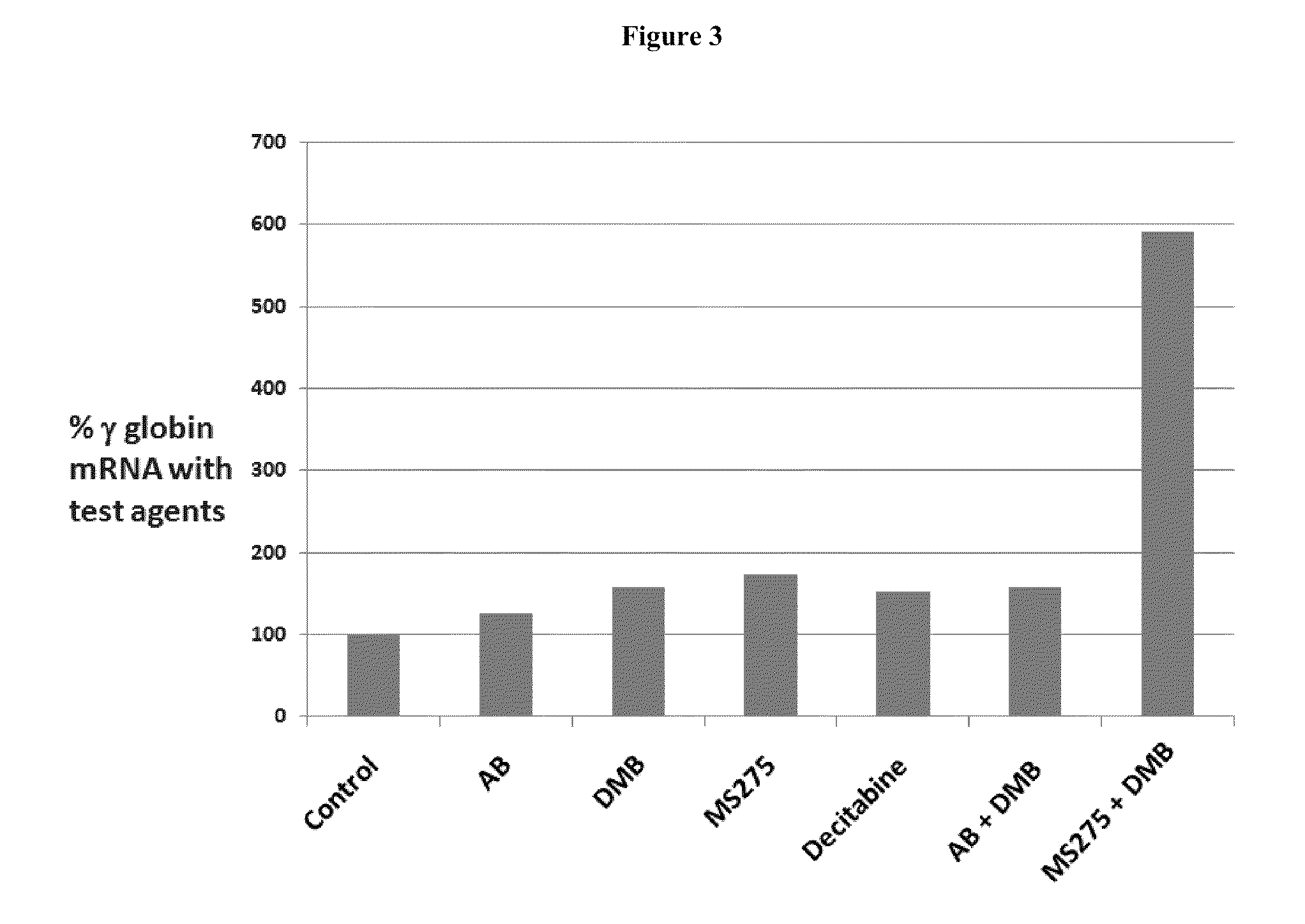
Methods and low dose regimens for treating red blood cell disorders
ActiveUS20110251149A1Increase volumeIncrease the number ofBiocideCarbohydrate active ingredientsBeta thalassemiaRegimen
Disclosed herein are methods and low dose regimens for increasing fetal hemoglobin levels in patients with red blood cell disorders, such as beta thalassemia, sickle cell disease, other anemias, or blood loss. Fetal and total hemoglobin levels and red blood cell counts are increased by administering 2,2-dimethylbutyrate (DMB) alone or in combination with hydroxyurea, decitabine or an HDAC inhibitor. Treatment can be continued for at least two weeks.
Owner:HEMAQUEST PHARMA INC +1
Therapy of rituximab-refractory rheumatoid arthritis patients
A method is disclosed of treating a rituximab-refractory rheumatoid arthritis (RA) patient comprising administering an anti-CD20 antibody other than rituximab to the patient in an amount effective to treat the RA.
Owner:GENENTECH INC
Bile acid recycling inhibitors for treatment of obesity and diabetes
ActiveUS20100130472A1Reduces and inhibits recyclingIncreased L-cell secretionBiocideOrganic chemistryDiabetes mellitusObesity
Owner:SATIOGEN PHARMA
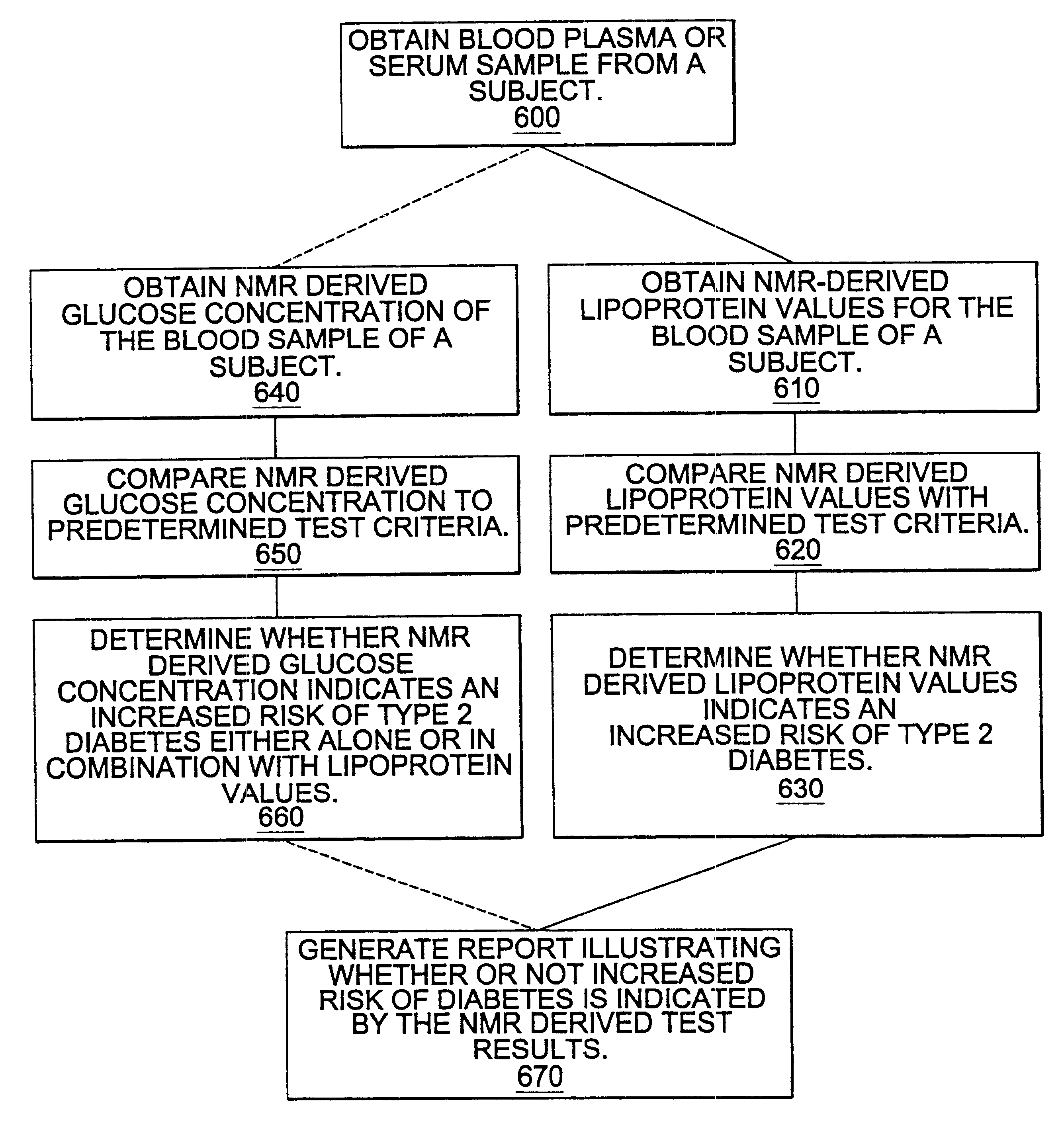
Methods and computer program products for determining risk of developing type 2 diabetes and other insulin resistance related disorders
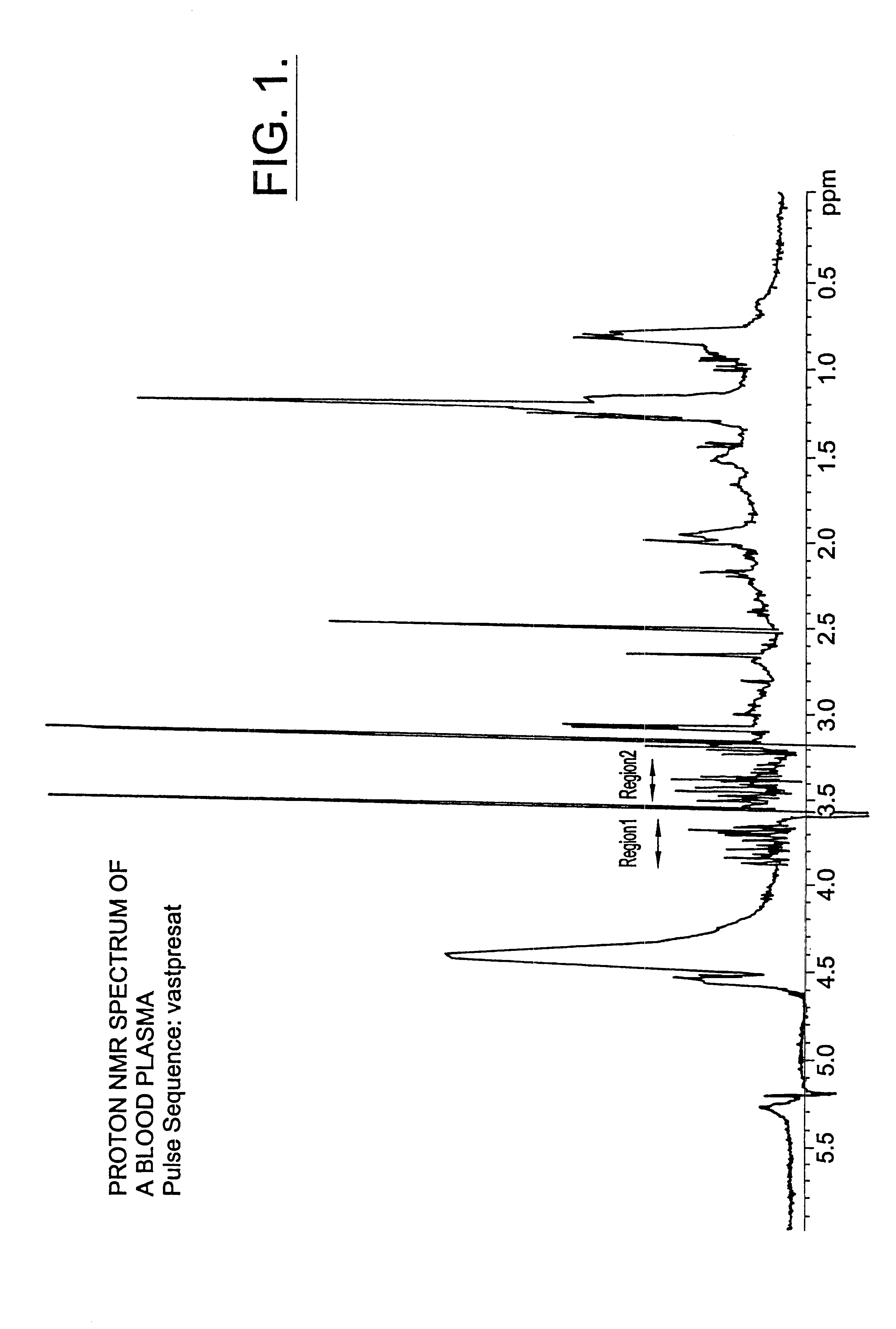
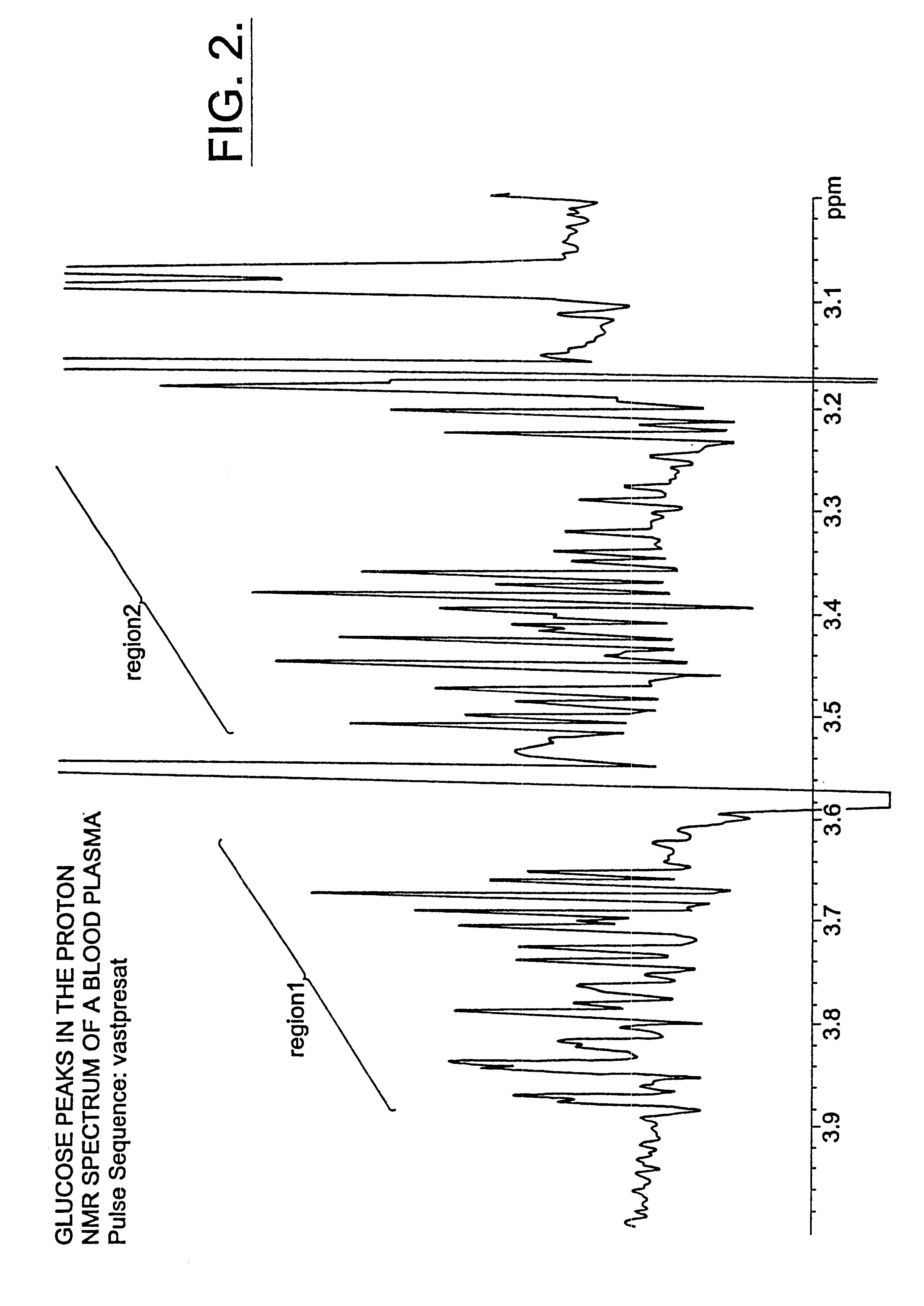
InactiveUS6518069B1Facilitate early detectionAvoid spendingDisease diagnosisAnalysis using nuclear magnetic resonanceDiseaseData set
Methods for assessing the risk of developing Type 2 diabetes and other related disorders include obtaining an NMR derived reference spectrum for a known glucose concentration sample and storing this information as a reference standard. A patient blood sample is collected and NMR derived patient spectrums for the blood sample are obtained. The two NMR data sets (the reference and the patient) are compared and a glucose concentration is determined for the patient sample. The glucose concentration can be evaluated with a blood sample undergoing lipoprotein cholesterol evaluation. The NMR based test can be used to concurrently provide a glucose concentration and lipoprotein constituent values based on a single testing event. The disclosure also includes a multi-purpose test, i.e., a test which concurrently provides lipoprotein screening and coronary heart disease risk evaluation along with a diabetes screening and risk assessment for developing Type 2 diabetes. A method for assessing diabetes includes identifying the presence of diabetic dyslipidemia based on the values of predetermined NMR measured lipoprotein constituents.
Owner:LIPOSCI +1
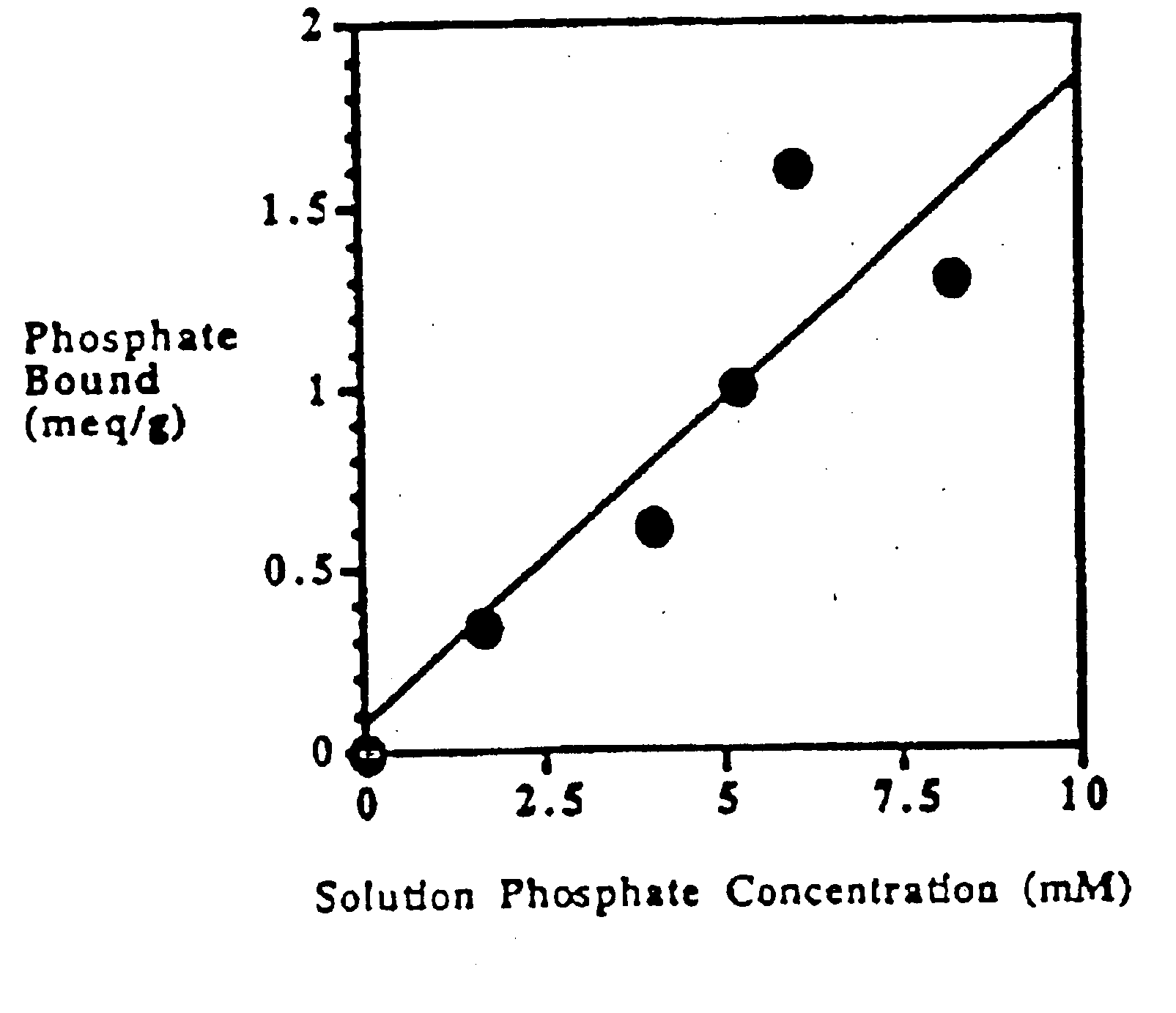
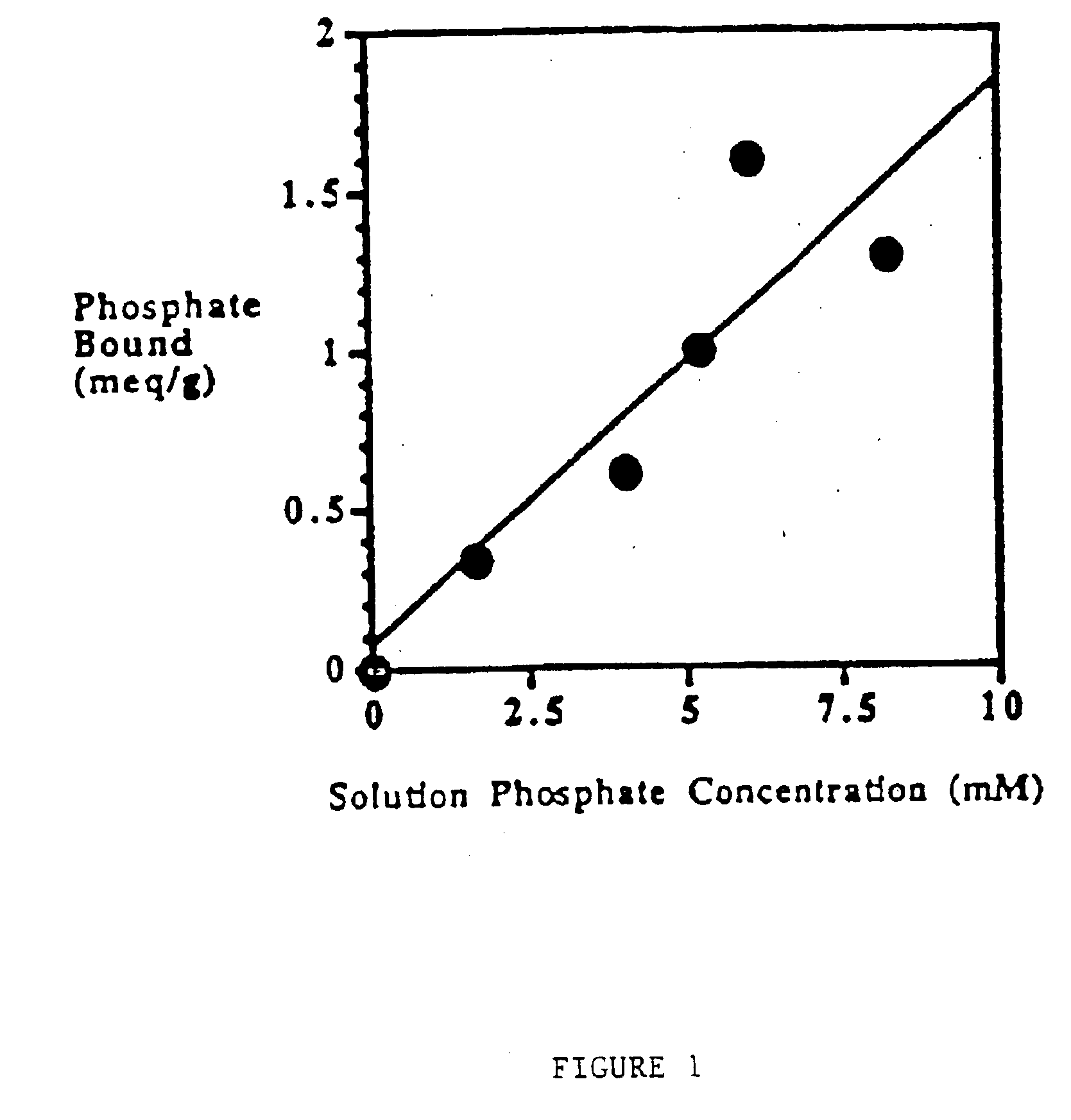
Method of making phosphate-binding polymers for oral administration
InactiveUS6858203B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com