Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Genetic vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-stochastic generation of genetic vaccines and enzymes
InactiveUS6713279B1Improve abilitiesPotent and direct effect on proliferation and Ig-synthesisAnimal cellsHydrolasesAntigenBiological property
This invention provides methods of obtaining novel polynucleotides and encoded polypeptides by use of non-stochastic methods of directed evolution (DirectEvolution(TM)). These methods include non-stochastic polynucleotide site-saturation mutagenesis (Gene Site Saturation Mutagenesis(TM)) and non-stochastic polynucleotide reassembly (GeneReassembly(TM)). Through use of the claimed methods, genetic vaccines, enzymes, and other desirable molecules can be evolved towards desirable properties. For example, vaccine vectors can be obtained that exhibit increased efficacy for use as genetic vaccines. Vectors obtained by using the methods can have, for example, enhanced antigen expression, increased uptake into a cell, increased stability in a cell, ability to tailor an immune response, and the like. This invention provides methods of obtaining novel enzymes that have optimized physical & / or biological properties. Furthermore, this invention provides methods of obtaining a variety of novel biologically active molecules, in the fields of antibiotics, pharmacotherapeutics, and transgenic traits.
Owner:BP CORP NORTH AMERICA INC
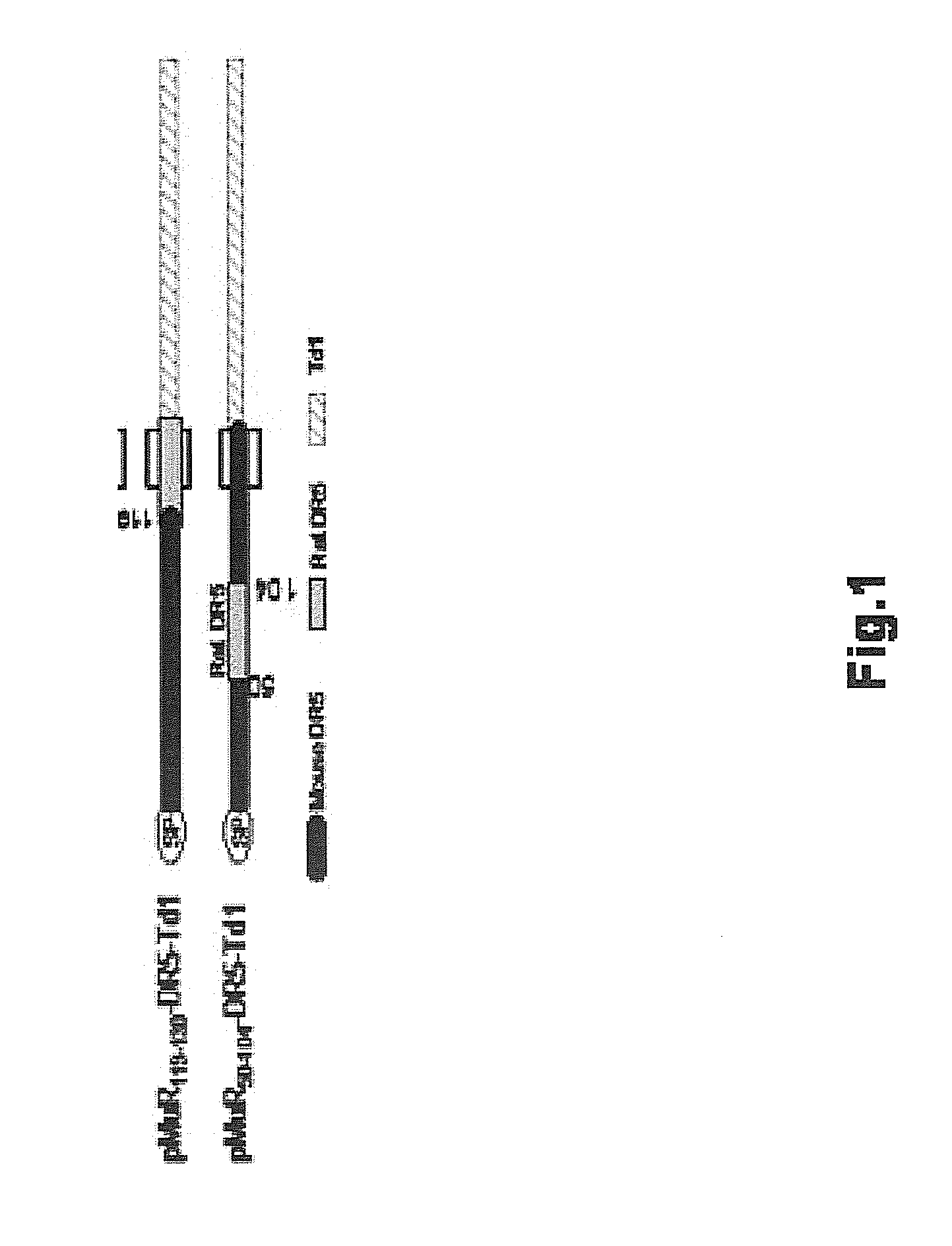
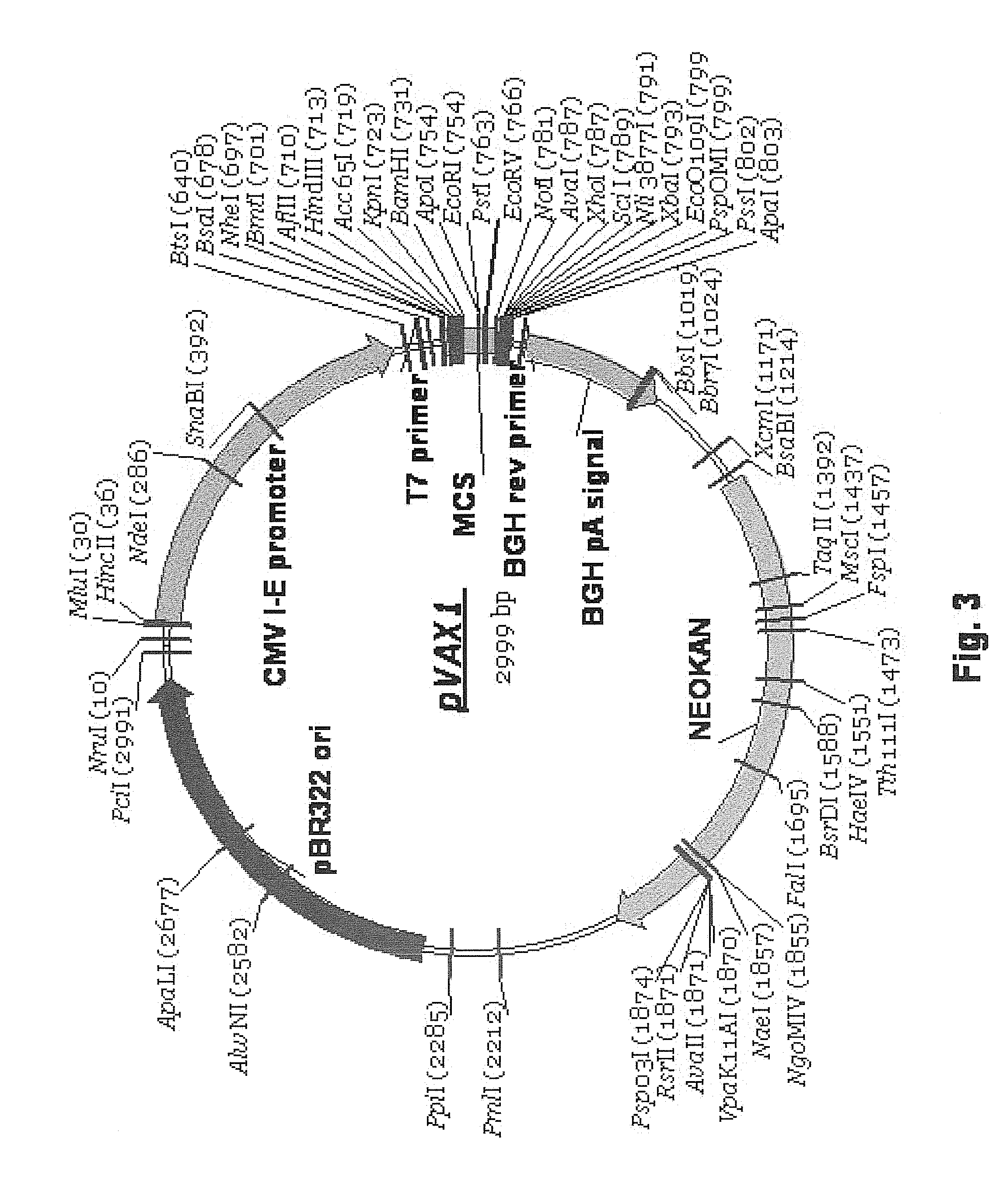
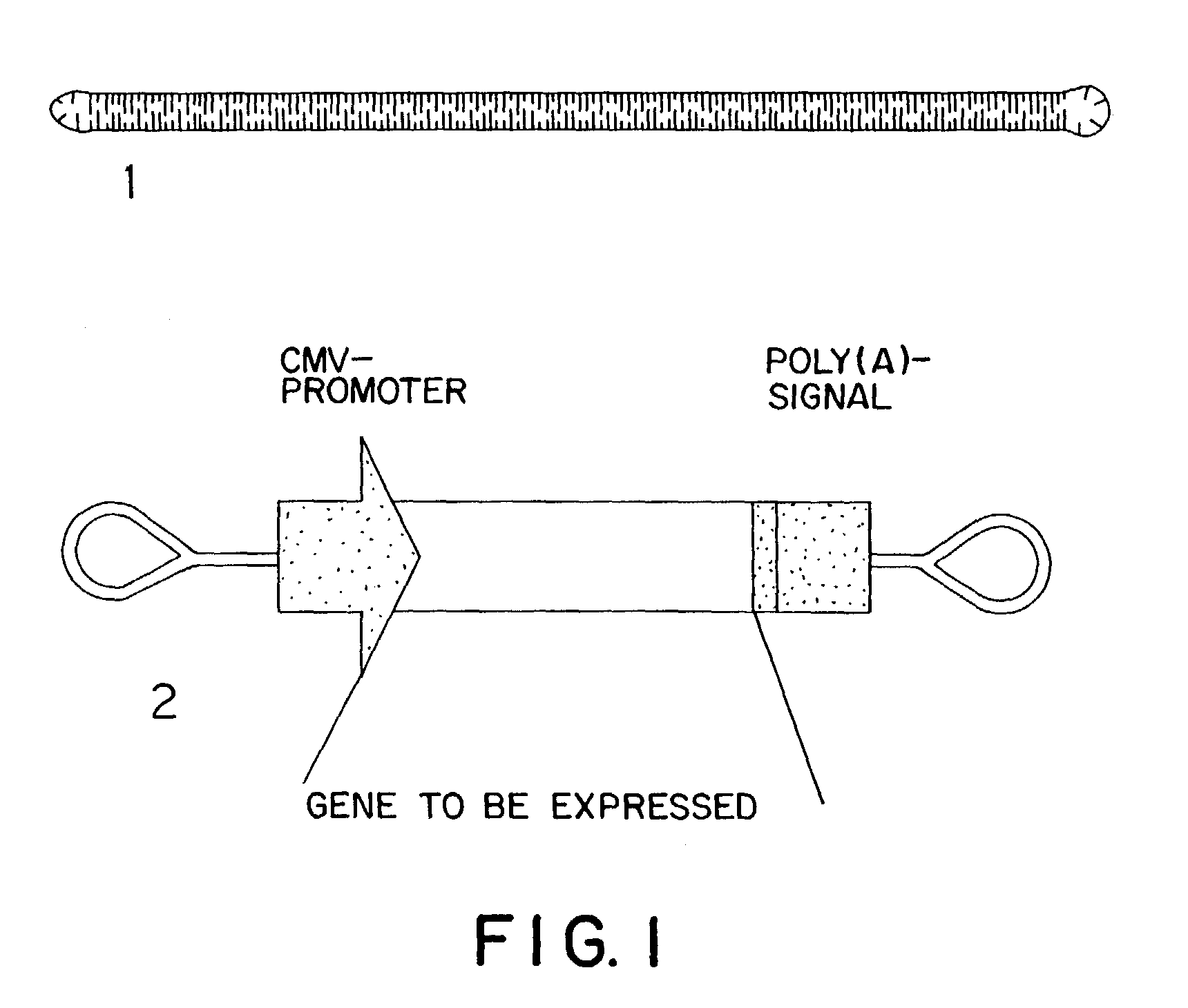
Design principle for construction of expression constructs for gene therapy
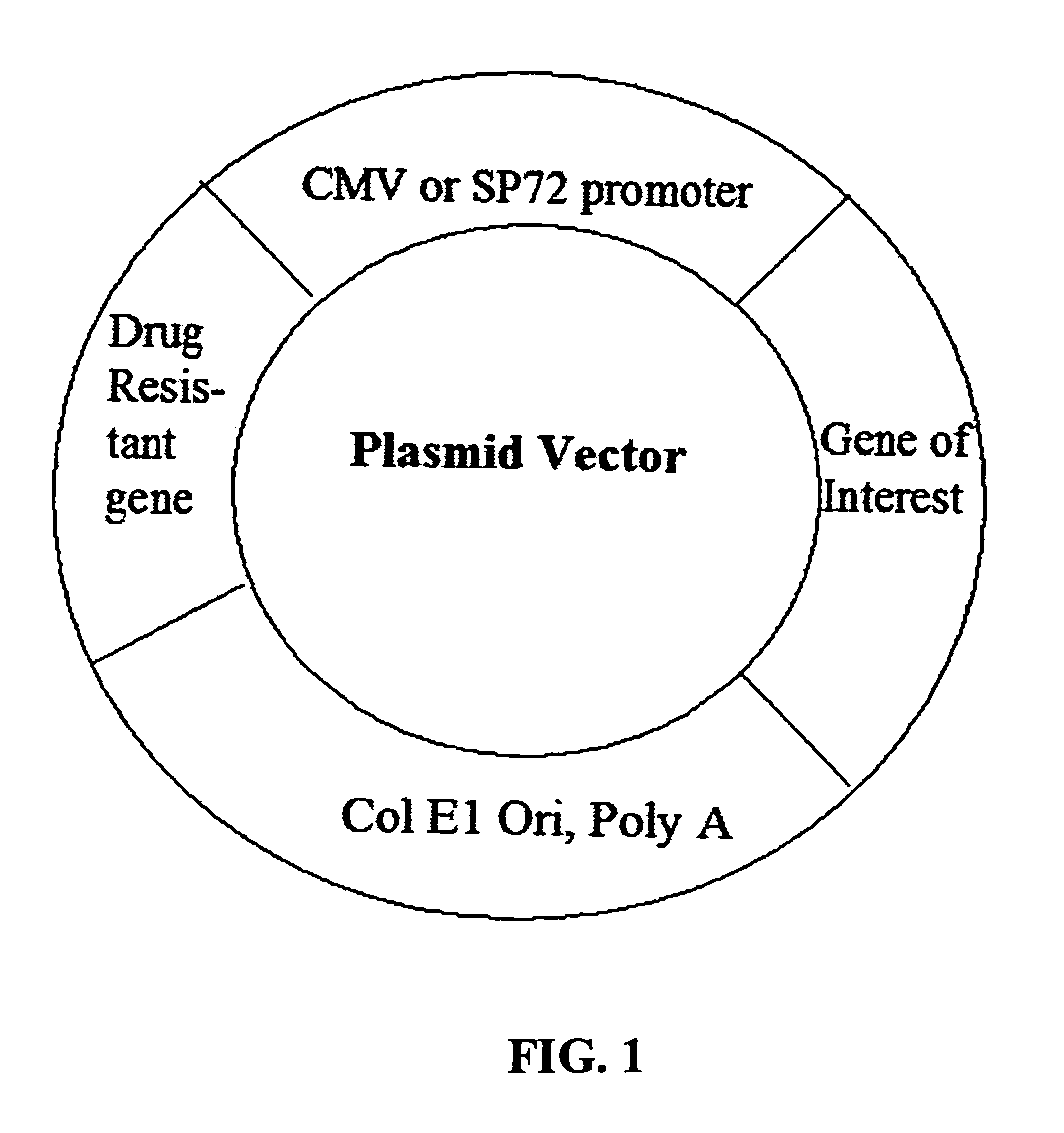
The invention concerns an expressible nucleic acid construct, which contains only the sequence information necessary for expressing a gene for RNA or protein synthesis. Expression constructs of this type can be used in gene therapy and genetic vaccination and avoid many of the risks associated with constructs today. The invention further concerns the possibility of improving the conveying of the construct into cells or tissue by covalent linkage of the construct, for example to particles or peptides.
Owner:MOLOGEN AG
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
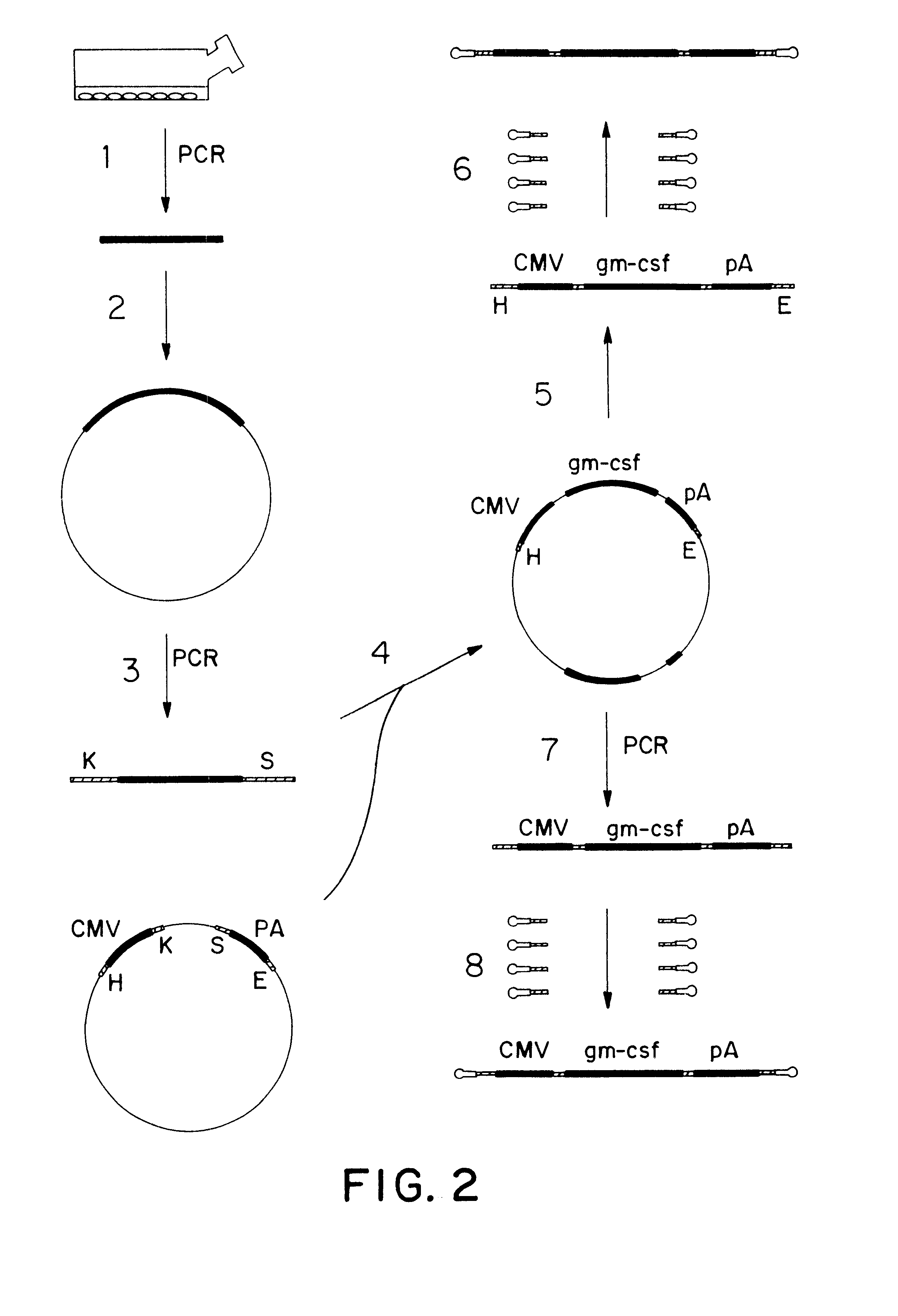
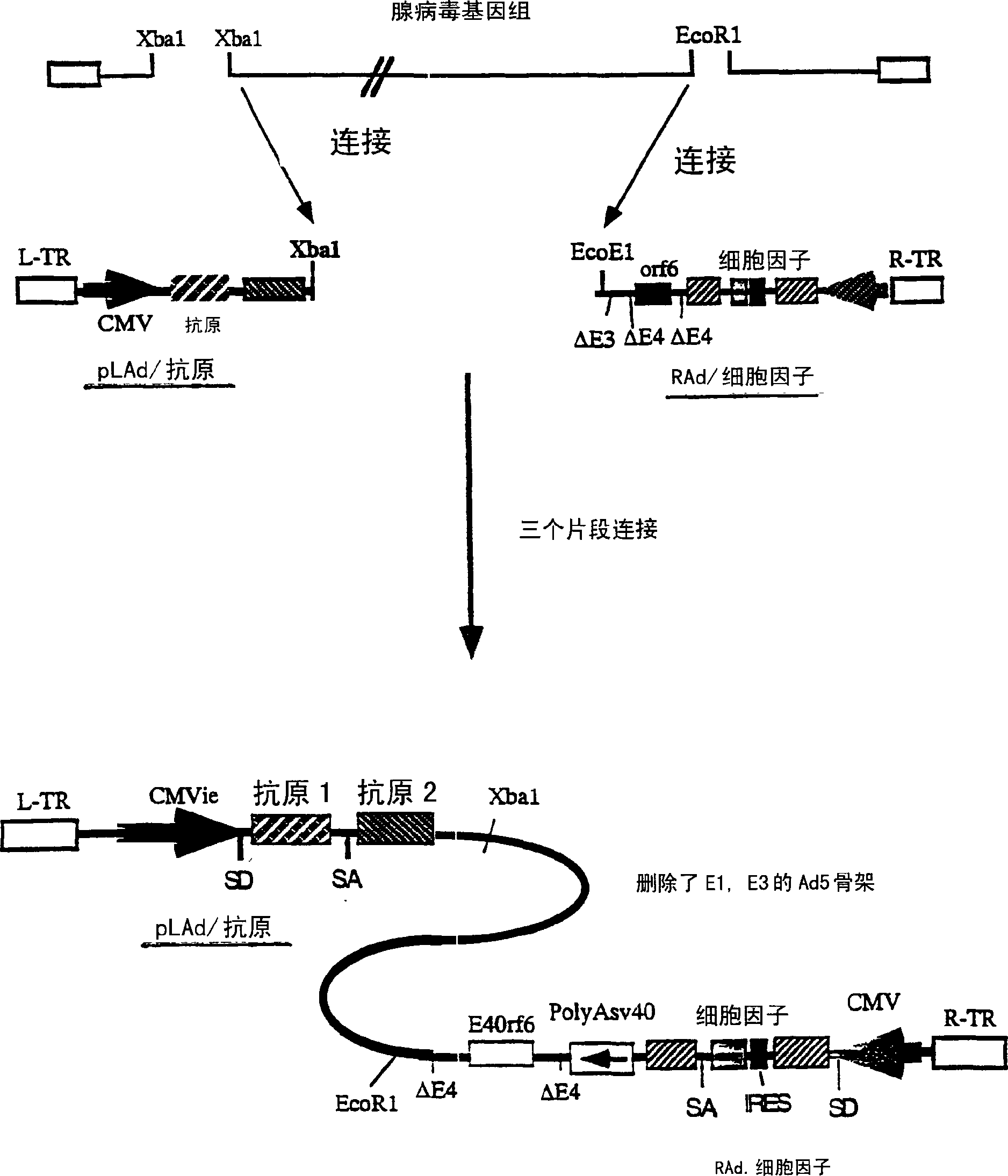
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
Adjuvanted genetic vaccines
InactiveUS7223739B1Good curative effectEasy to produceSsRNA viruses negative-senseHeavy metal active ingredientsAntigenAdjuvant
Reagents useful in nucleic acid immunization techniques are described. More particularly, adjuvanted genetic vaccine compositions are described, as are methods of using those compositions for inducing an enhanced immune response against a selected antigen.
Owner:POWDER JECT VACCINES INC
SARS vaccine of adenovirus carrier and preparation method, application of coronavirus S gene
InactiveCN1562365AEffective generationEffective induction ofGenetic material ingredientsAntiviralsGenetic engineeringOrganism
The invention pertains to biological genetic engineering field, specificly relating to SARS vaccine of adenovirus carrier and preparation method, application of related coronavirus S gene in preparation of the SARS vaccine for preventing SARS. Through a bioengineering means, the related coronary virus S gene and a defective adenovirus are recombined, which makes protective immunogen protein or polypeptide expressing in it. A genic vaccine that can arouse the mucosal immunogenicity is produced through amplifying training, purification, and preparation, which induces a immunity reaction in the respiratory mucosa, makes the organism produce the corresponding antibody, and prevents virus from infection. Compared with the traditional inactivated viral particles vaccine, the invention has a high safety; it is convenient to operate; it is not limited by the specific conditions such as intramuscular injection; and it has an extensive clinical application prospect.
Owner:SUN YAT SEN UNIV CANCER CENT
Biological product for preventing novel coronavirus
InactiveCN111228475ASsRNA viruses positive-senseViral antigen ingredientsGenetic vaccineProtein antigen
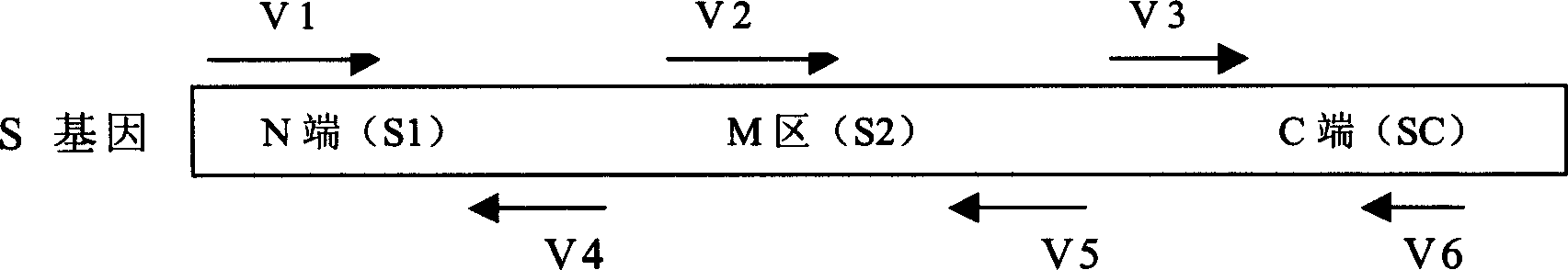
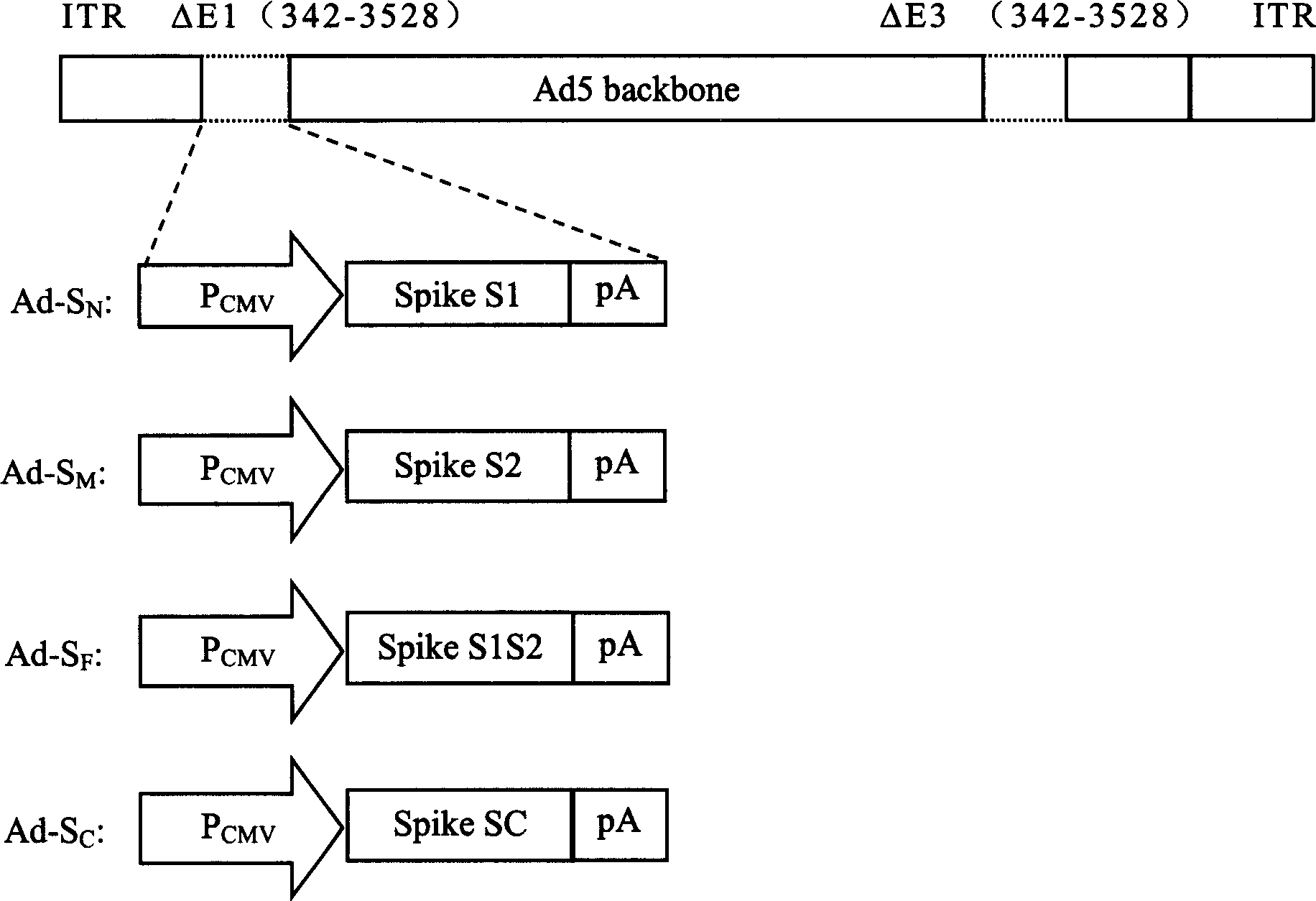
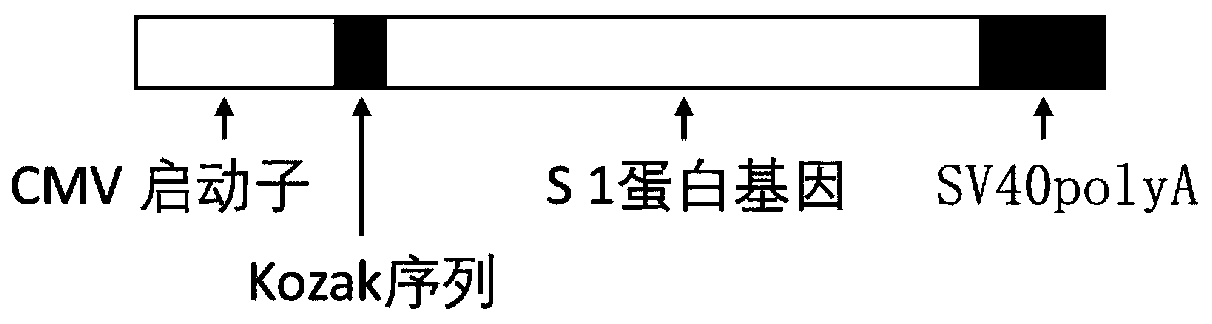
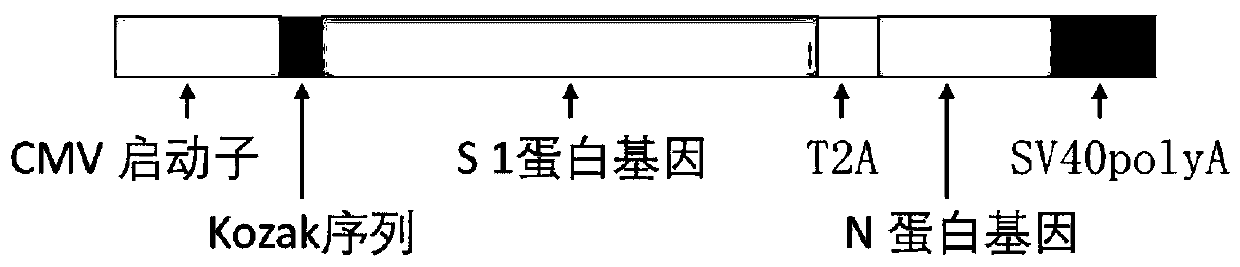
The invention discloses a biological product for preventing novel coronavirus (COVID-19). The biological product can be a gene vaccine or gene medicine, wherein the gene vaccine adopts a human type-5adenovirus with deletion of E1 and E3 genes as a vector for carrying an S1 protein antigen for expressing a Spike S1 subunit of the novel coronaviruses or simultaneously carrying an S1 expression protein antigen and N protein antigen. Through in-vivo expression of antigen proteins, the immune reaction of a body upon the novel coronavirus can be stimulated, and the effect of preventing infection and propagation of the novel coronavirus.
Owner:SYNO SHENZHEN BIOMEDICAL RES CO LTD
Epidermal mRNA vaccine
InactiveUS20200085852A1Easy to identifyFaster and strong attackOrganic active ingredientsWhole-cell/virus/DNA/RNA ingredientsDiseaseGenetic vaccine
The invention concerns the field of genetic vaccination, in particular RNA vaccines. The present invention provides an mRNA for use in the treatment and / or prevention of a disease, wherein the mRNA is administered to the epidermis. Furthermore, the invention provides compositions comprising the mRNA for epidermal administration or kits comprising the mRNA for epidermal administration. Moreover, the invention concerns the medical use of the mRNA or compositions comprising the mRNA, wherein the mRNA or compositions comprising the mRNA are administered to the epidermis.
Owner:CUREVAC AG
Attenuated vif DNA immunization cassettes for genetic vaccines
The present invention is directed to nucleic acid molecules encoding attenuated, non-functional virion infectivity factor (vif) proteins. The nucleic acid molecules of the invention are inserted into recombinant expression vectors and administered to mammals in order to induce a cellular and humoral immune response to the encoded protein product.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
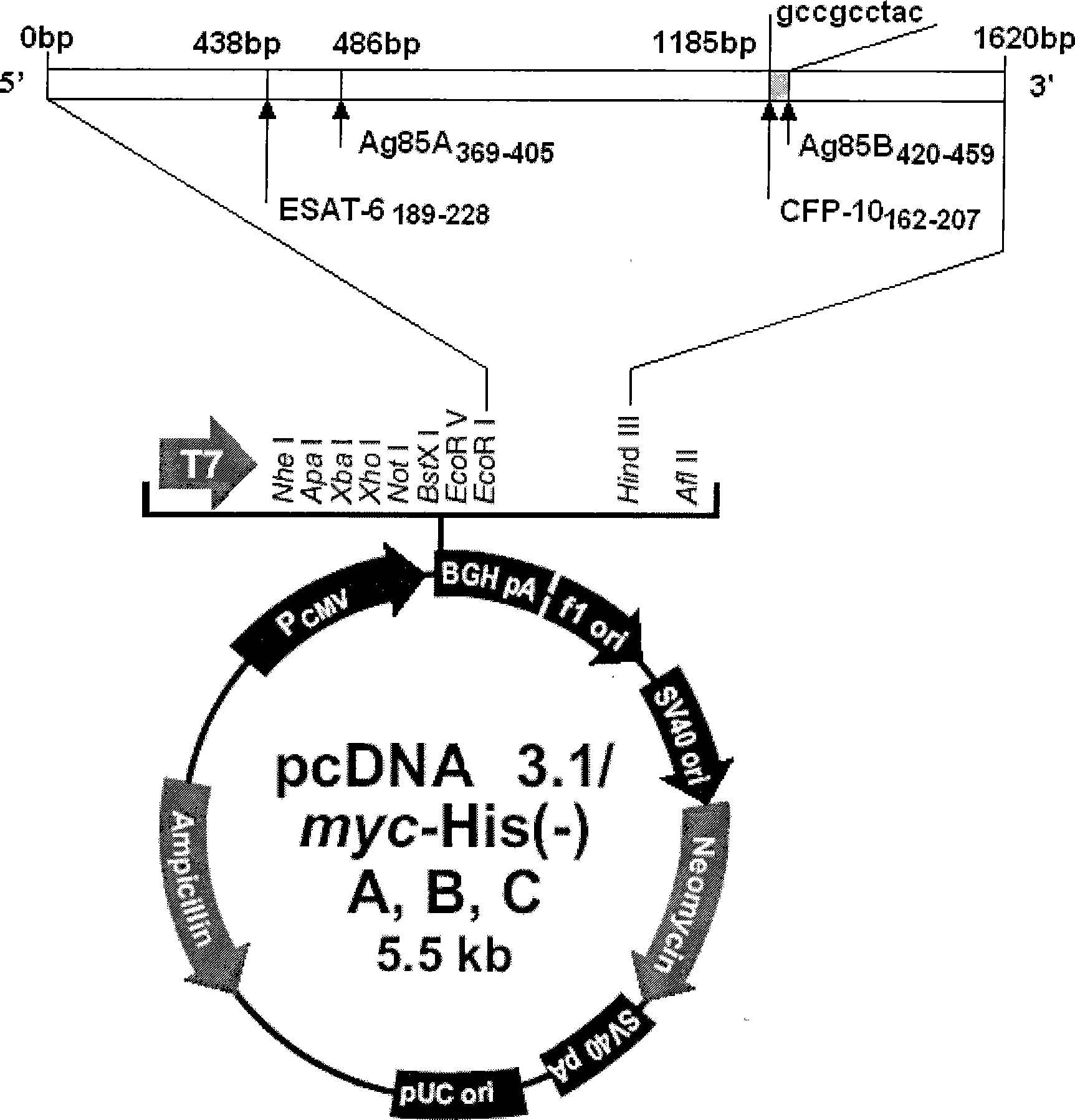
Tuberculosis gene vaccine based on T cell epitope as well as preparation method and use thereof
InactiveCN101451145AActivate immune responseDoes not affect the spatial structureAntibacterial agentsGenetic material ingredientsIntramuscular injectionTreating tuberculosis
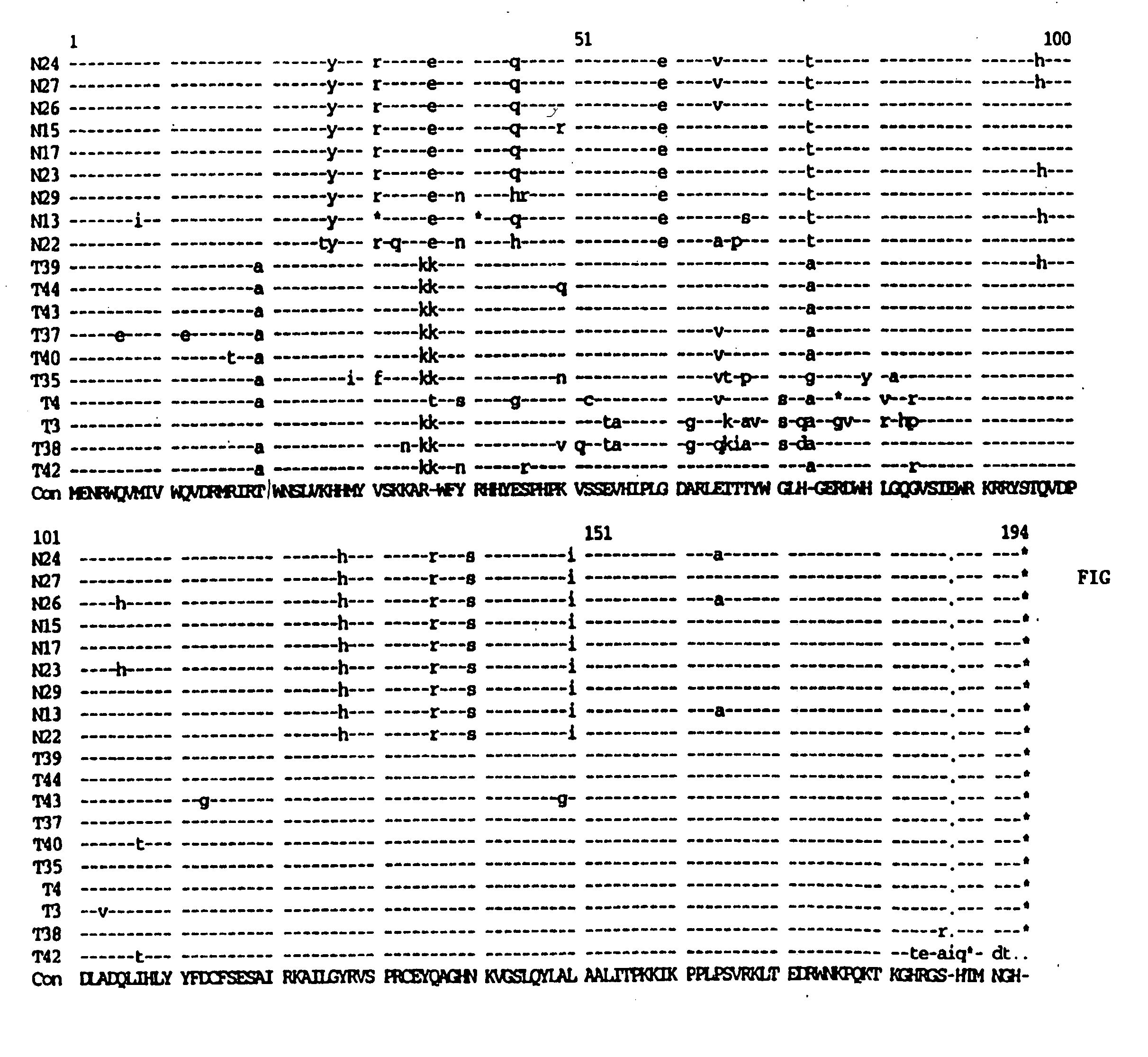
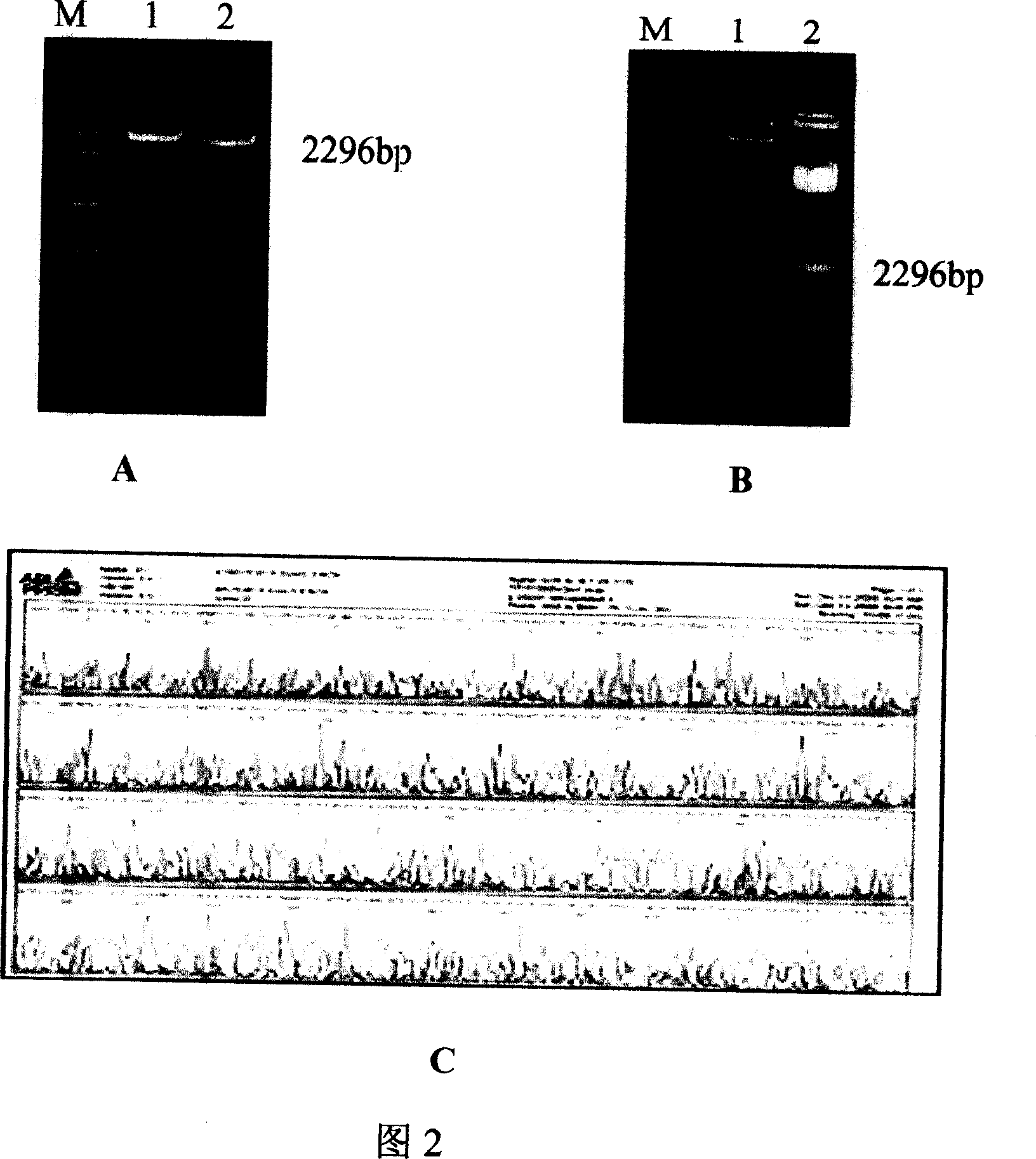
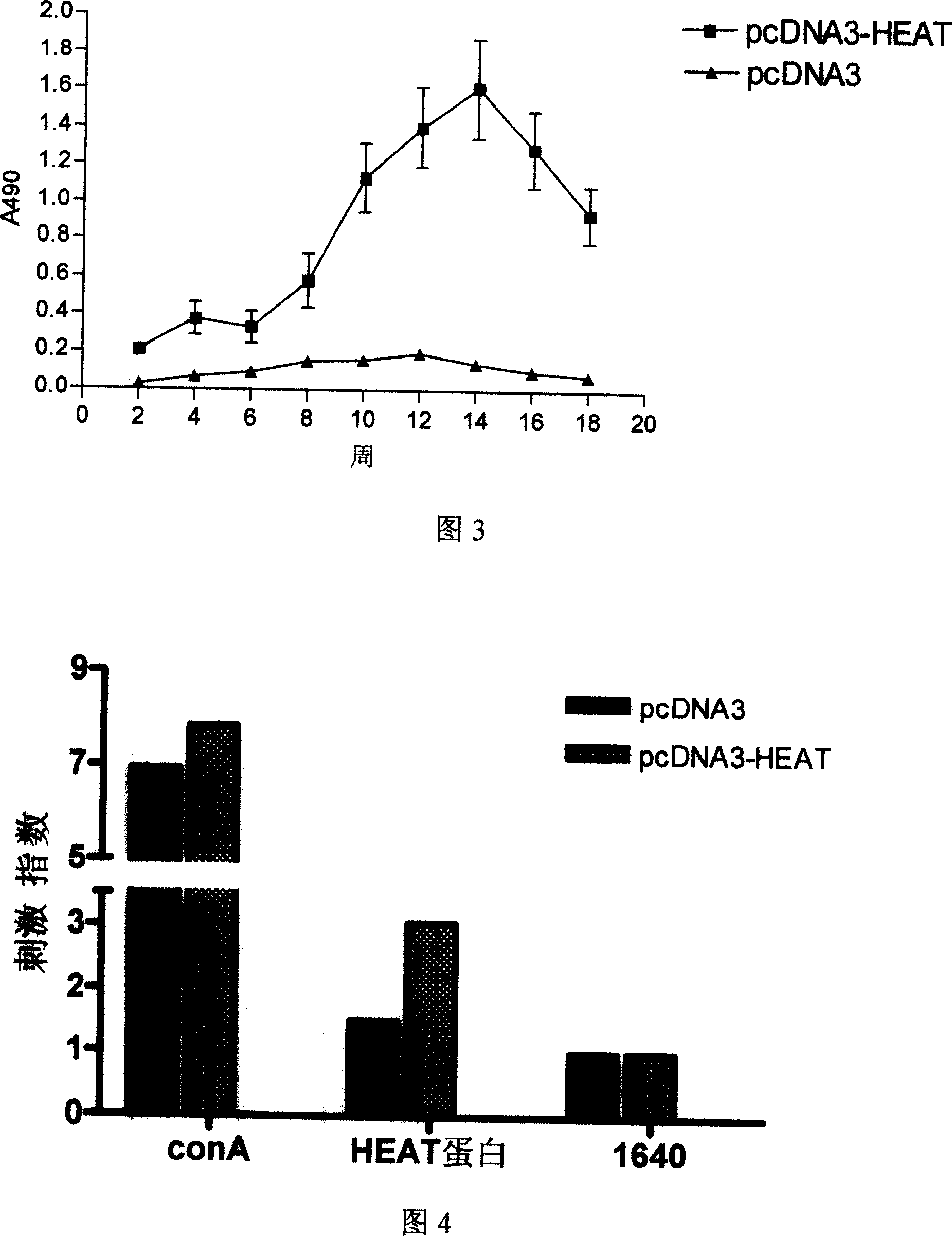
The invention discloses a tuberculosis gene vaccine based on T cell epitopes, wherein a full-length gene, embedded with four T cell epitope polypeptide genes which come from mycobacterium tuberculosis antigen, of mycobacterium tuberculosis heat shock protein is inserted into a vector. The invention also discloses a method for preparing the vaccine, which comprises the following steps: four T cell epitope genes, namely EAST-6189-228, Ag85A369-405, CFP10162-207 and Ag85B420-459 which come from the mycobacterium tuberculosis antigen are inserted into an HSP65 full-length gene. The invention also discloses application of an ECANS tuberculosis gene vaccine. Through the intramuscular injection of the gene vaccine into an immune mouse, the experiment proves that the vaccine can induce a specific antibody which aims at a plurality of tuberculosis antigens to response, can induce stronger tuberculosis specific killing response, can induce Th1 immune response at the same time, secrete high-level IFN gamma, and is a good vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
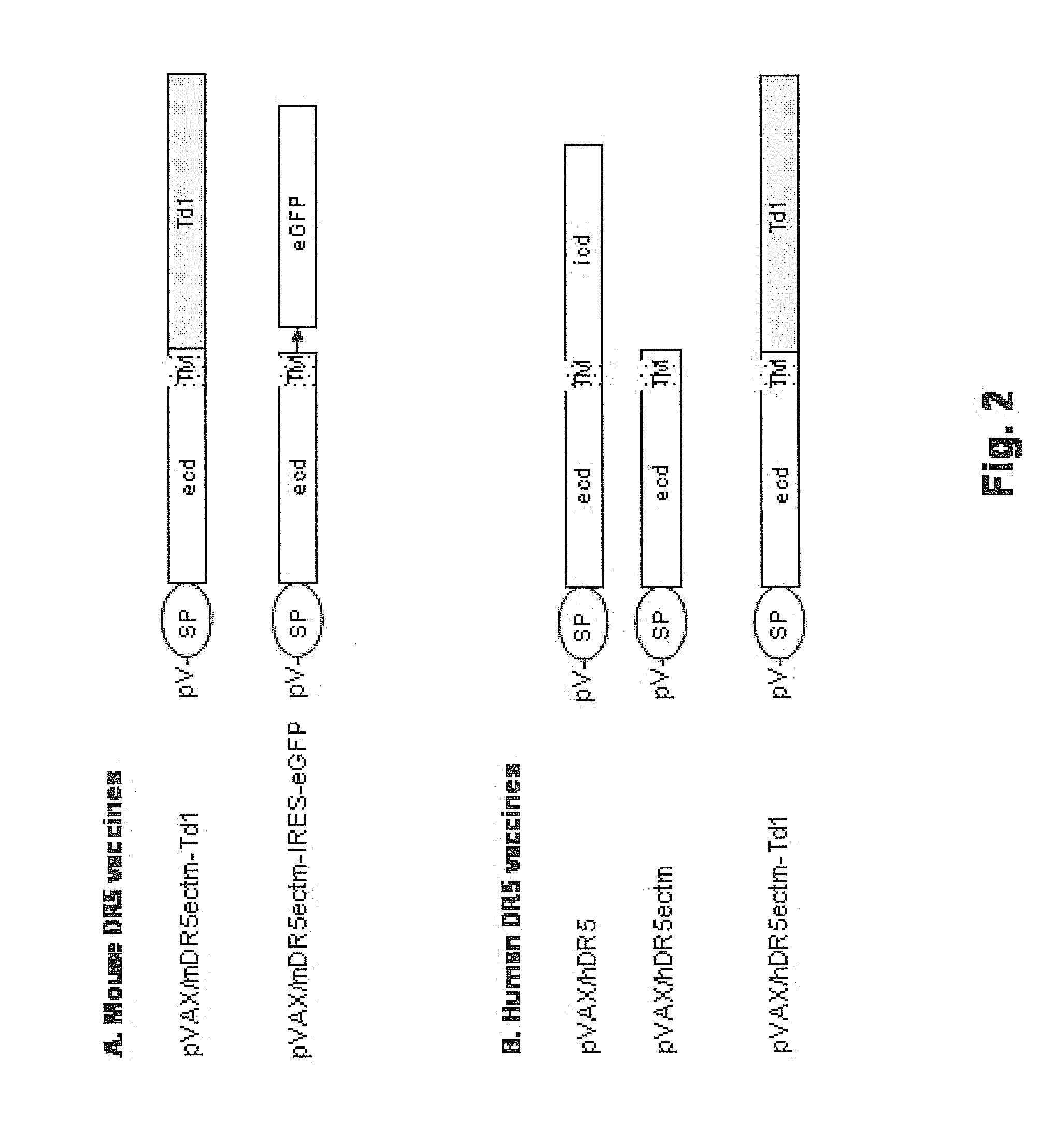
Vaccines targeting cellular death receptors
InactiveUS20140205538A1Improving immunogenicityTolerating abilityCompounds screening/testingPeptide/protein ingredientsCancer cellImmunocompetence
The invention provides therapeutics and methods to induce a mammalian host, including a human, to produce antibodies, which agonize death receptors and cause the apoptotic death of target cells within the host's body. The therapeutics are vaccine compositions, including genetic vaccines encoding death receptor antigens of the tumor necrosis factor receptor family. Also provided are means and methods for overcoming host immunological tolerance to death receptors. The vaccines are useful against cancer cells and other death receptor bearing target cells within the host, and can be used in both therapeutic and prophylactic settings. The vaccines are also useful for diagnostic testing of the immunocompetence of a host.
Owner:WAYNE STATE UNIV
Polyepitope tuberculosis gene vaccine and its prepn process
InactiveCN101088559ARaise the level of immune responseStimulate immune responseAntibacterial agentsBacterial antigen ingredientsEpitopeGenetic vaccine
The present invention discloses one kind of polyepitope tuberculosis gene vaccine comprising one kind of carrier and one inserted segment of target gene, which has in the 5' end one whole length HSP65 gene, and in the 3' end serially arranged selected epitope genes, including ESAT-6Á1-20 positions genes and 61-81 positions genes of ESAT-6, 62-84 positions genes of Ag85A, 121-155 positions genes of Ag85B, 143-166 positions genes of Ag85A, 234-256 positions genes of Ag85B and 177-228 positions genes of MPT64C. The present invention discloses the application of the polyepitope tuberculosis gene vaccine. Mouse immunizing experiment shows that the gene vaccine can well induce humoral immunity response. The gene vaccine of the present invention can well induce Th1 type immunity response to mycobacterium tuberculosis and enhance the anti-mycobacterium tuberculosis immunity response level.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
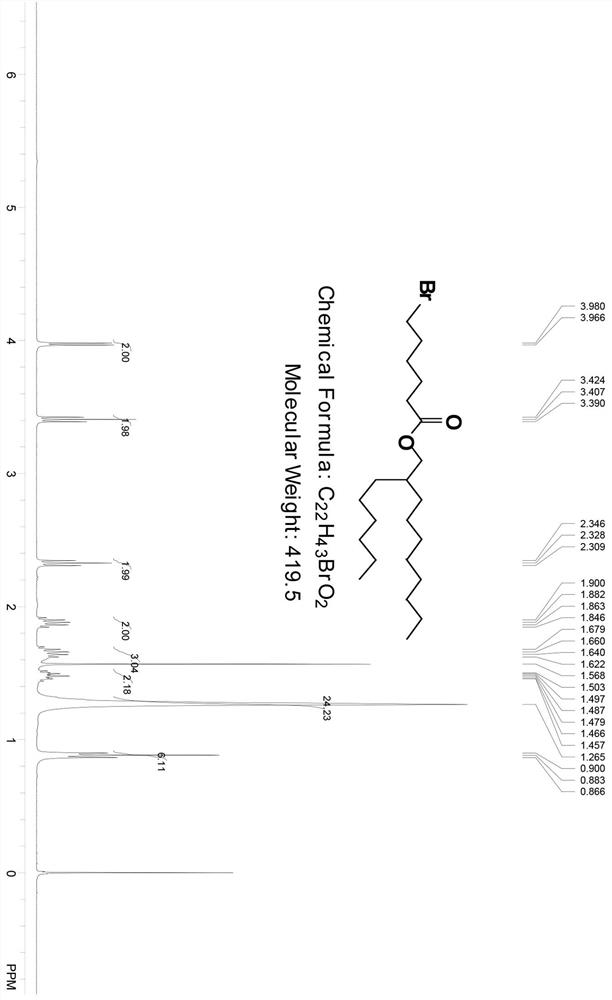
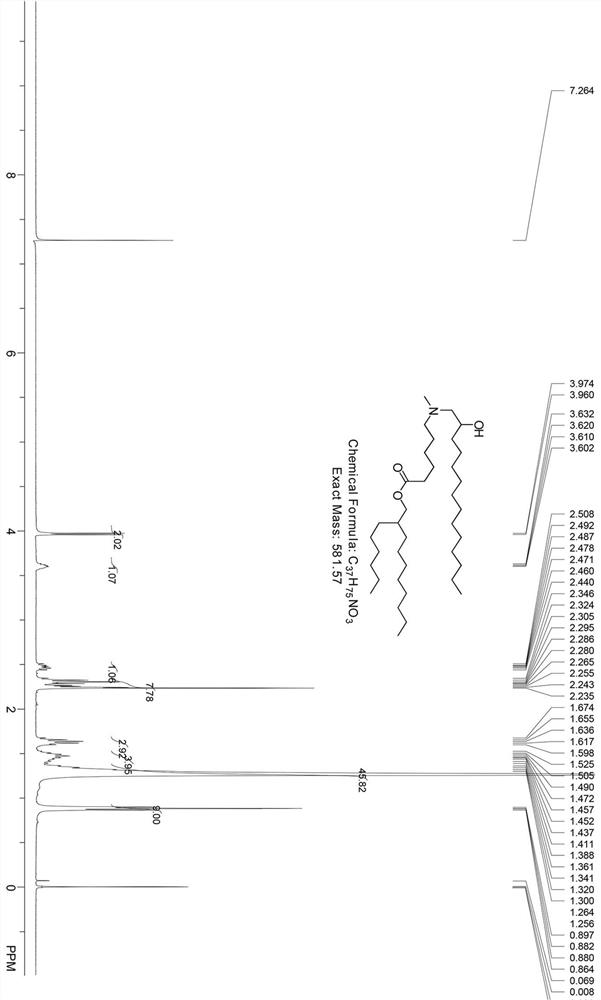
Cationic lipid compound, composition containing same and application
ActiveCN112979483ARich varietyOrganic active ingredientsOrganic compound preparationGenetic vaccineChemical compound
The invention provides a cationic lipid compound, a composition containing the cationic lipid compound and application of the cationic lipid compound. In order to provide more choices for delivery of preparations such as nucleic acid drugs, gene vaccines, small molecule drugs and the like, the invention provides a cationic lipid compound shown in the general formula or pharmaceutically available salts thereof. The cationic lipid compound provided by the invention can be used for delivering DNA, RNA or small molecule drugs, enriches the types of cationic lipid compounds, and has important significance for the development and application of nucleic acid preventive and therapeutic agents.
Owner:SUZHOU ABOGEN BIOSCIENCES CO LTD
Cationic lipid compound, composition containing same and application
The invention provides a cationic lipid compound, a composition containing the cationic lipid compound and application. In order to provide more choices for delivery of preparations such as nucleic acid drugs, gene vaccines, small molecule drugs and the like, the invention provides a cationic lipid compound shown in the general formula or pharmaceutically available salts thereof. The cationic lipid compound provided by the invention can be used for delivering DNA, RNA or small molecule drugs, enriches the types of cationic lipid compounds, and has important significance for the development and application of nucleic acid preventive and therapeutic agents.
Owner:SUZHOU ABOGEN BIOSCIENCES CO LTD
Vaccines targeting cellular death receptors
ActiveUS20120189572A1Reduce and eliminate cell populationPreventing initiationCompounds screening/testingPeptide/protein ingredientsCancer cellTolerability
The invention provides therapeutics and methods to induce a mammalian host, including a human, to produce antibodies, which agonize death receptors and cause the apoptotic death of target cells within the host's body. The therapeutics are vaccine compositions, including genetic vaccines encoding death receptor antigens of the tumor necrosis factor receptor family. Also provided are means and methods for overcoming host immunological tolerance to death receptors. The vaccines are useful against cancer cells and other death receptor bearing target cells within the host, and can be used in both therapeutic and prophylactic settings. The vaccines are also useful for diagnostic testing of the immunocompetence of a host.
Owner:WAYNE STATE UNIV
A kind of cationic lipid compound, its composition and application
ActiveCN112979483BRich varietyOrganic active ingredientsOrganic compound preparationGenetic vaccineChemical compound
The invention provides a cationic lipid compound, its composition and application. In order to provide more options for the delivery of preparations such as nucleic acid drugs, gene vaccines, and small molecule drugs, the present invention proposes cationic lipid compounds represented by the general formula, or pharmaceutically acceptable salts thereof. The cationic lipid compound of the present invention can be used to deliver DNA, RNA or small molecule drugs, enriches the category of cationic lipid compounds, and has important significance for the development and application of nucleic acid preventive and therapeutic agents.
Owner:SUZHOU ABOGEN BIOSCIENCES CO LTD
Design principle for the construction of expression constructs for gene therapy
InactiveUS7074772B2Facilitated releaseEasier chemical linkingBiocideNanotechGenetic vaccineCell biology
The invention concerns an expressible nucleic acid construct, which contains only the sequence information necessary for expressing a gene for RNA or protein synthesis. Expression constructs of this type can be used in gene therapy and genetic vaccination and avoid many of the risks associated with constructs today. The invention further concerns the possibility of improving the conveying of the construct into cells or tissue by covalent linkage of the construct, for example to particles of peptides.
Owner:MOLOGEN AG
Immunogenic peptides for use in the prevention and/or treatment of infectious diseases, autoimmune diseases, immune responses to allofactors, allergic diseases, tumors, graft rejection and immune responses against viral vectors used for gene therapy or gene vaccination
ActiveUS20130259885A1Enhance immune responseLimited degreeVirusesPeptide/protein ingredientsVaccinationAutoimmune responses
The invention describes new peptides containing epitopes recognized by CD4+ natural killer T (NKT) cells for increasing activity for use in infectious diseases, autoimmune diseases, immune reaction to administration of allofactors, allergic diseases, therapy of tumors, prevention of graft rejection and prevention of immunization against viral proteins used in gene therapy or gene vaccination.
Owner:IMCYSE
Vectors for the treatment of Alzheimer's disease
InactiveCN102272301AImprove quality of lifeConvenient careSsRNA viruses negative-senseNervous disorderDiseaseAntiendomysial antibodies
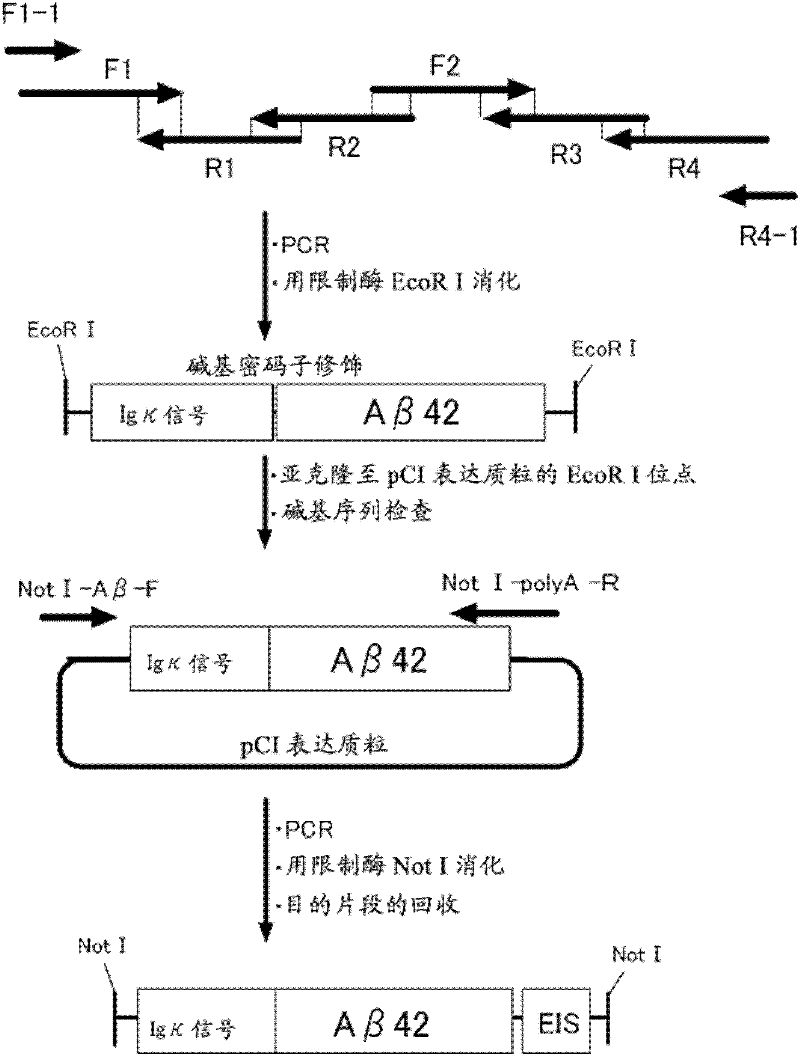
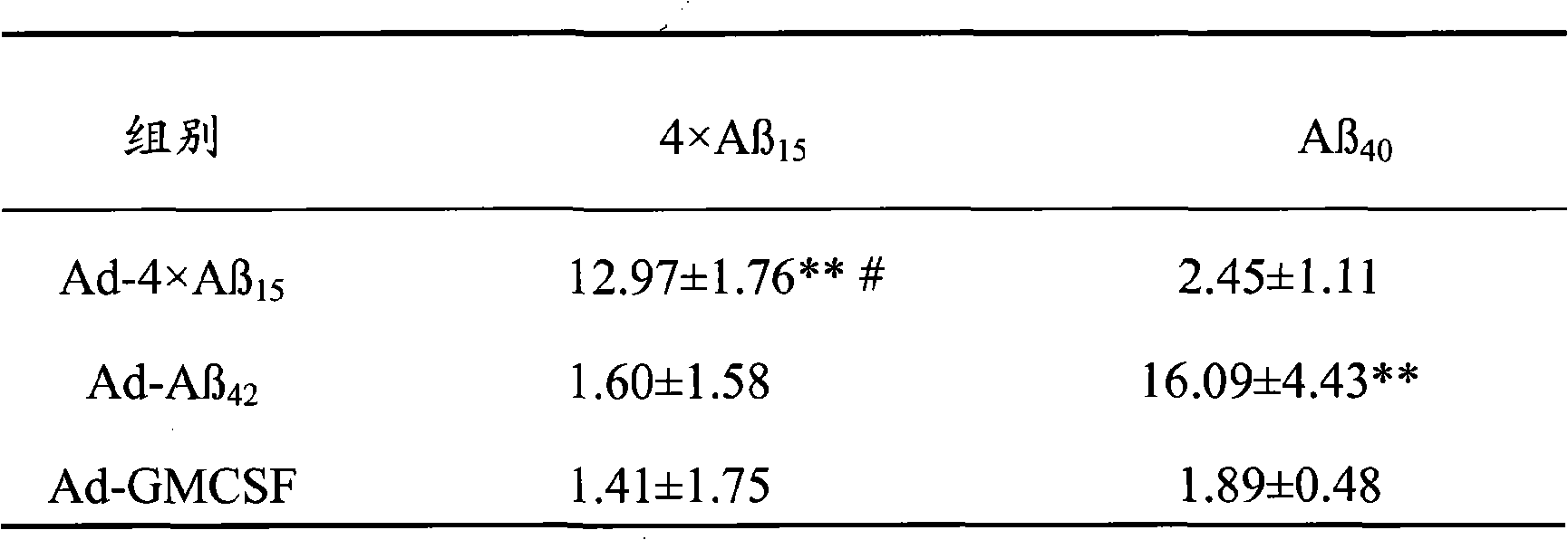
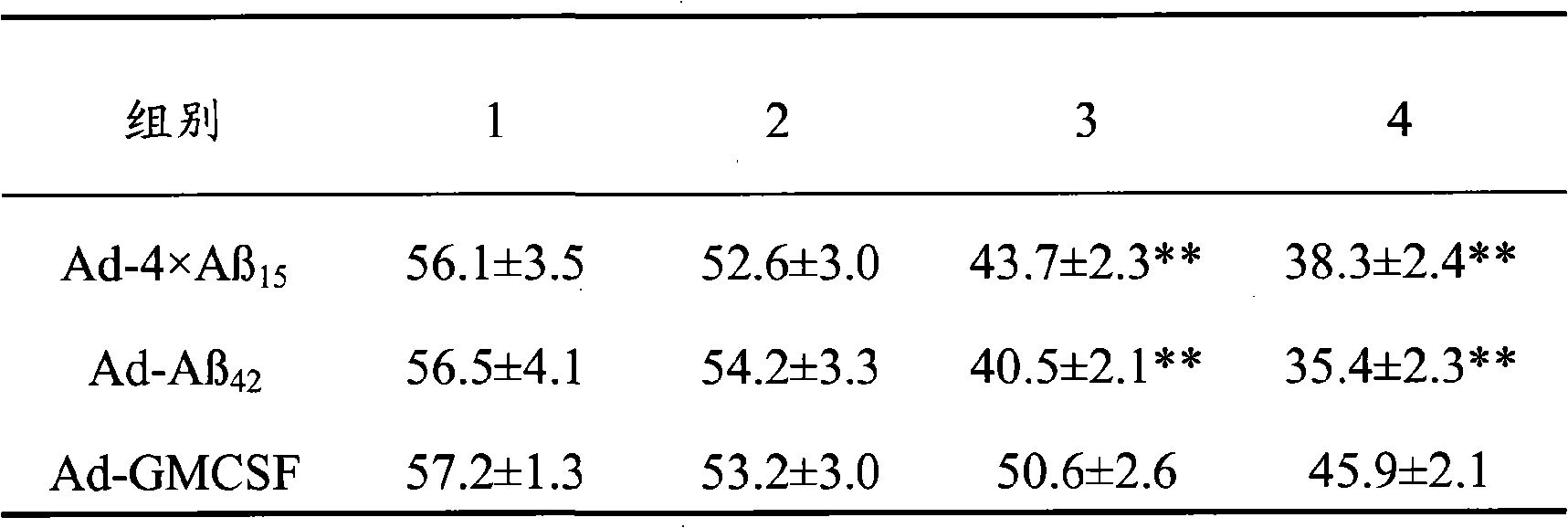
The present invention provides methods for efficiently inducing anti-Aβ antibody and methods for preventing and treating Alzheimer's disease. The present inventors successfully induced anti-Aβ antibody in a highly efficient manner by administering an RNA viral vector that expresses a fusion protein between an AB5 toxin B subunit and an Aβ antigen peptide. Administration of the vector resulted in a significant increase of anti-Aβ antibody in plasma, and decrease in the Aβ level in brain tissues and decrease in the anti-Aβ antibody-positive area. The present invention enables more efficient vaccine gene therapy for preventing and treating Alzheimer's disease.
Owner:DNAVEC CORP
Gene vaccine for anti SARS coronal virus and use thereof
InactiveCN1449826AAdvantage preventionAdvantageous therapeuticGenetic material ingredientsAntiviralsNucleotideIn vivo
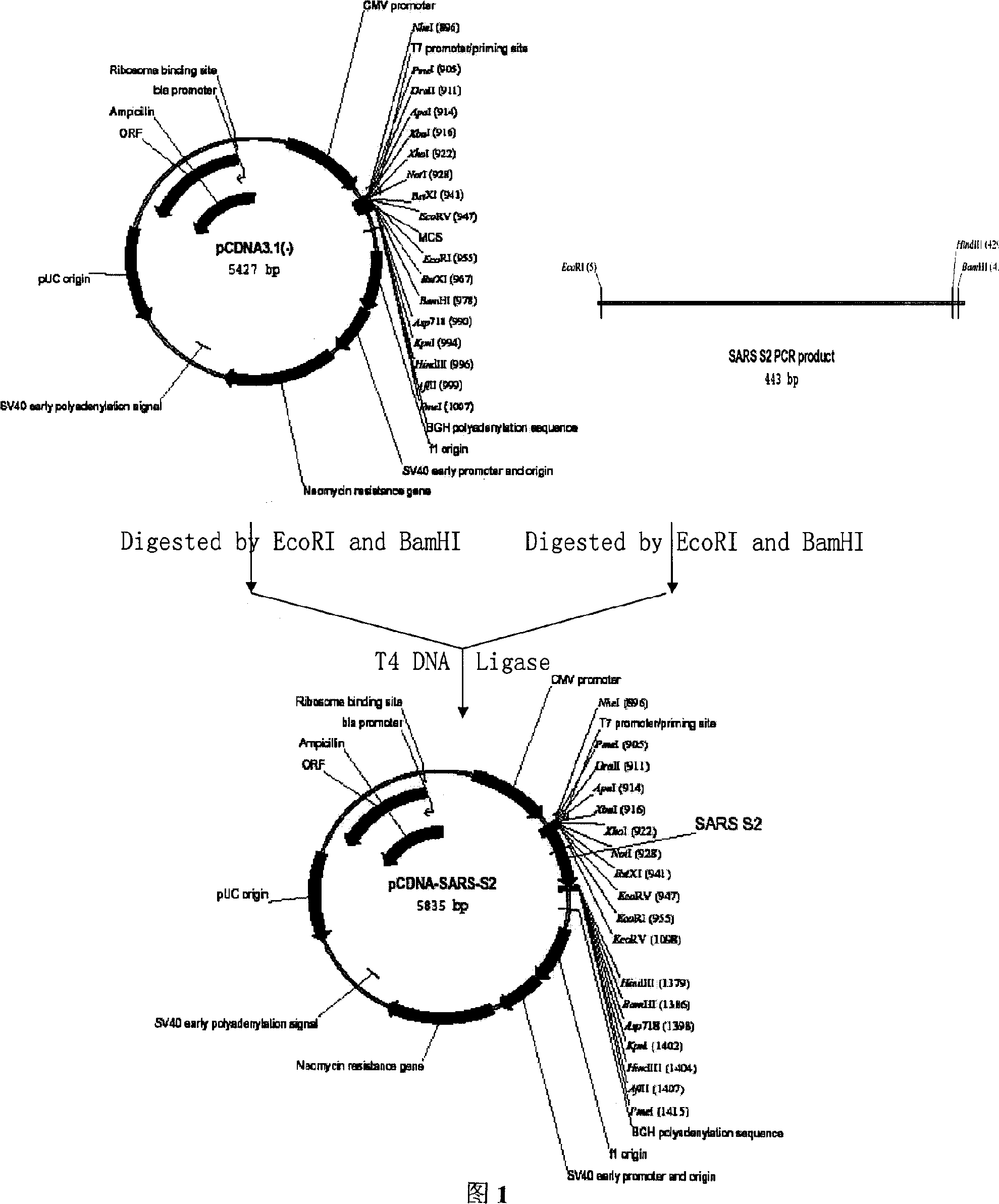
The present invention discloses a gene vaccine for resisting SARS coronavirus and its application, including eucaryotic expressino plasmid containing total or partial gene fragment of total-length gene sequence of SARS coronavirus S protein. The nucleotide sequence of coded SARS coronavirus S protein can be operatively connected with a promotor, then it can express the complete or partial polypeptide of the coded SARS coronavirus S protein in vivo. The described gene vaccine can be used for immunizing host including human body and rodent, for example mouse to make it produce specific protective body fluid immune and cell immune to resist the infection of SARS coronavirus. Said invention is good in stability, convenient in production and low in cost.
Owner:WUHAN UNIV
Follicle stimulating hormone receptor(FSHR) specific binding region polypeptide, its expression vector, preparation method and application in drug preparation
The invention discloses the FSH receptor special bonding area polypeptide, expression vector, the preparation method and their pharmaceutical application. Wherein, the polypeptide has amino acid sequence shown as SEQ ID No.2, SEQ ID No.4 or SEQ ID No.6, and can restrain spermatogenesis safely without effect to androgen and as the gene vaccine for male contraceptive.
Owner:NANJING MEDICAL UNIV
Senile dementia recombinant adenovirus gene vaccine and preparation method thereof
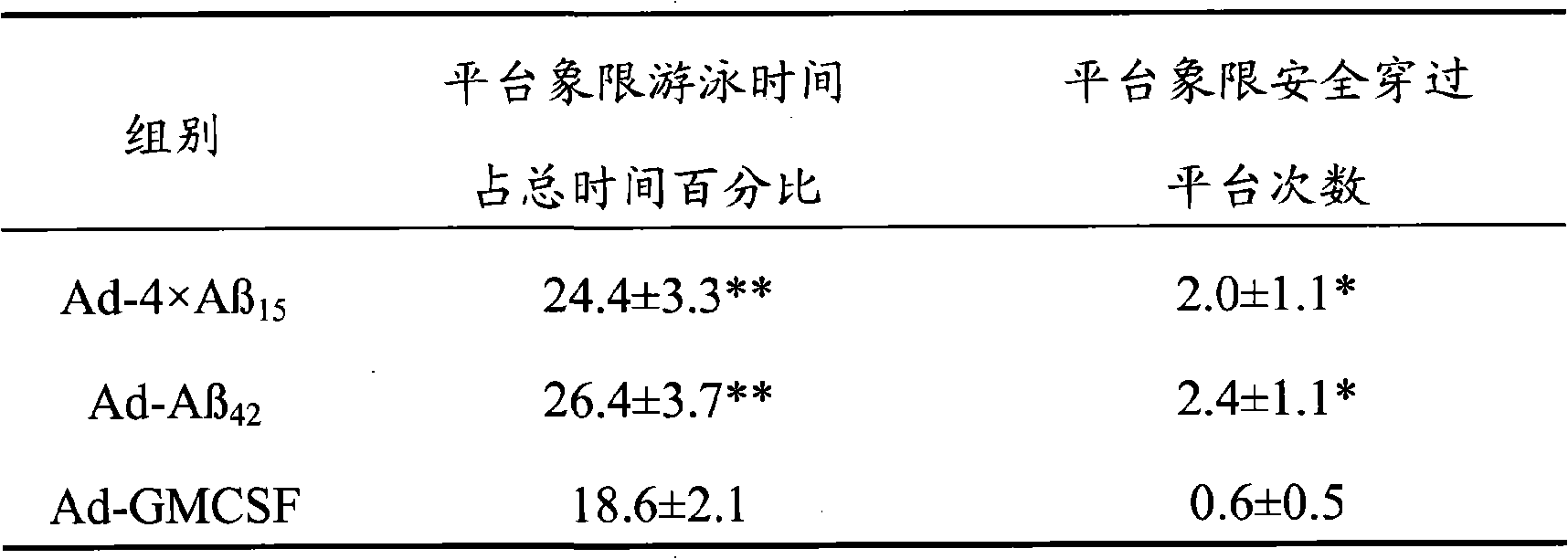
ActiveCN101357227AHigh molecular weightAvoid spatially rigid structuresNervous disorderPeptide/protein ingredientsChemical synthesisAmyloid
The invention discloses senile dementia Ad-GFP gene vaccine and a preparation method theerof and is Ad-GFP vaccine of flexible foldable multivalence Beta- amyloid 1-15 (ABeta1-15) of secretion co-expression and genetic adjuvant, which a flexible connecting peptide is used for connecting multivalence. The invention selects foldable multivalence ABeta1-15 as immunogen, which avoids the space rigid structure of multiple copy ABetaB cell epitopes, also ensures that ABeta15 epitopes are more easily exposed, thus enhancing immunogenicity and also increasing immunogenic molecular weight and reducing the possibility of degradation; a antigen delivery system that takes adenovirus as a carrier is selected, which avoids the defects of high challenge and high cost of chemosynthesis.
Owner:LIVZON PHARM GRP INC
HPV58 (Human Papilloma Virus) type therapeutic composite genetic vaccine and construction method thereof
InactiveCN102228698APreserve antigenicityGood antigenicityViral antigen ingredientsGenetic material ingredientsGlycineE6 protein
The invention discloses an HPV58 (Human Papilloma Virus) type therapeutic composite genetic vaccine and a construction method thereof. In the vaccine, an HPV58 type E6 / E7 fusion gene is used as a target antigen, and GM / B7 is used as an antigen adjuvant, wherein stop codes of HPV58 type E6 and E7 genes are removed from the HPV58 type E6 / E7 fusion gene; and meanwhile, amino acids of 50, 63 and 106 on E6 protein and the amino acids 24, 26 and 92 on E7 protein are mutated into glycines. The HPV58 type therapeutic composite genetic vaccine provided by the invention has high safety, strong immunity and an ideal anti-tumor effect, provides a better choice for treating cervical carcinoma and precancerous lesion related to the HPV58 type infection, and is particularly suitable for HPV58 type highly-infective cervical carcinoma patients in China and Asia.
Owner:广西医科大学附属肿瘤医院
Method of accelerated vaccination against Ebola viruses
The present invention relates to genetic vaccines for stimulating cellular and humoral immune responses in humans and other hosts, and, in particular, relates to recombinant viruses that express heterologous antigens of pathogenic viruses, in single dose form.
Owner:UNITED STATES OF AMERICA +1
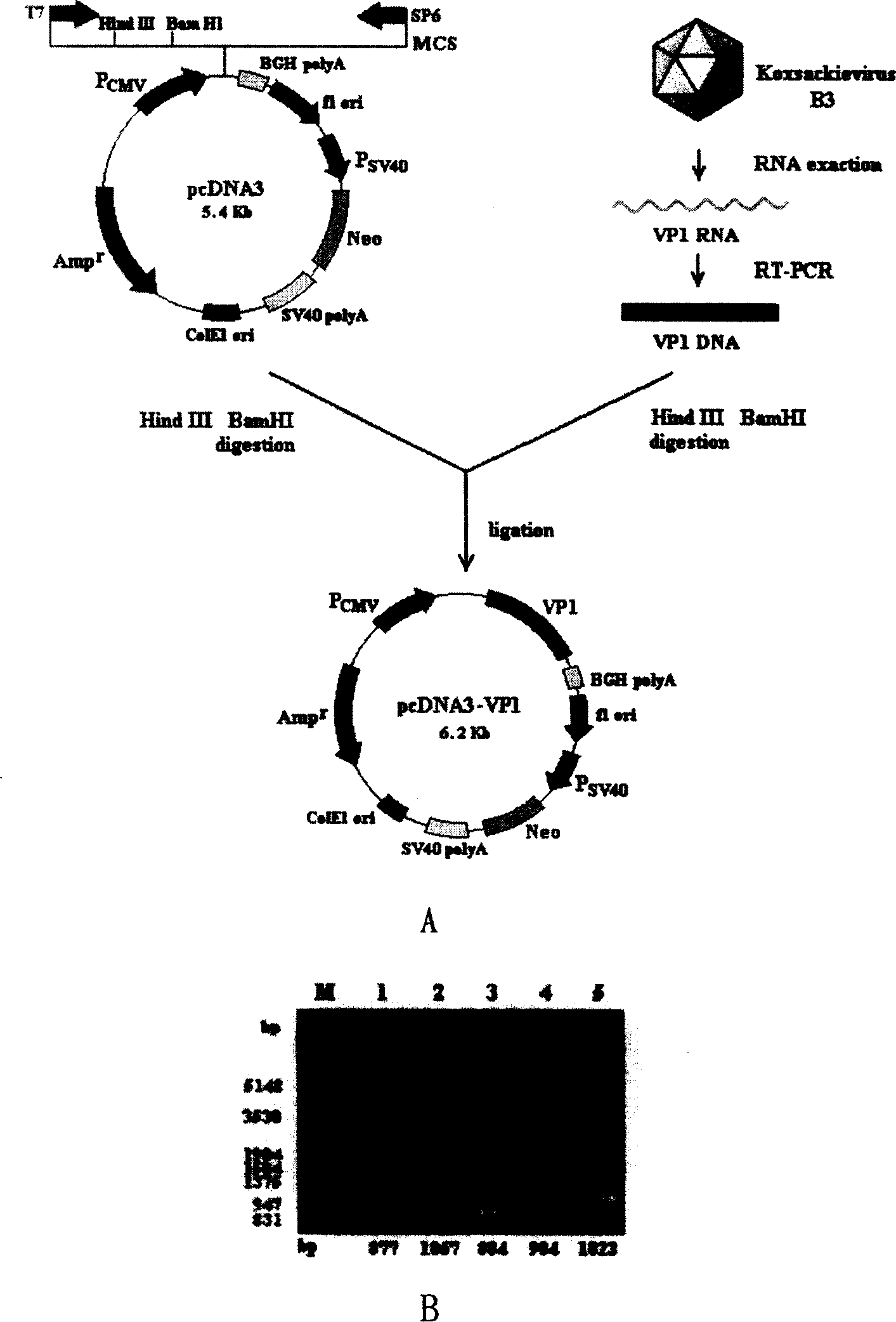
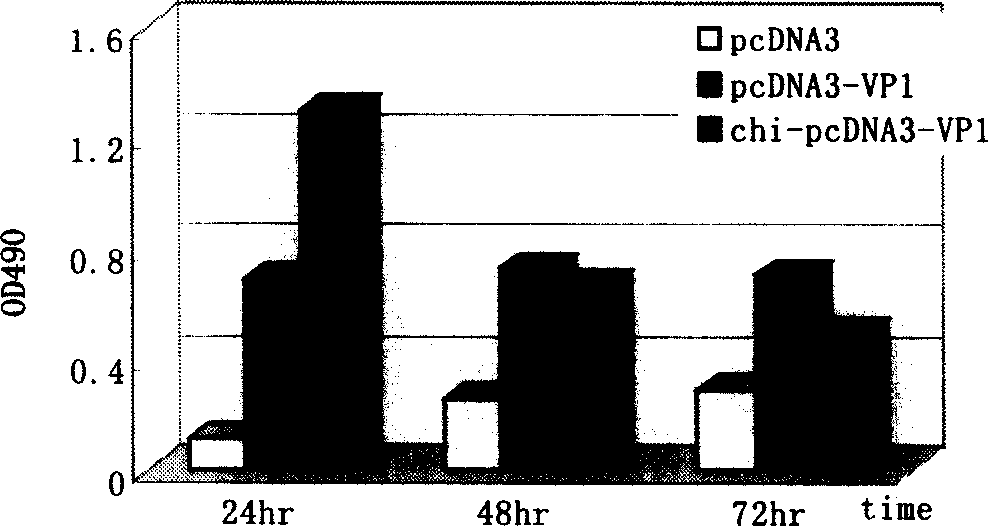
Viral myocarditis gene vaccine, its preparation method and application
The present invention discloses a viral myocarditis gene vaccine, Said vaccine is constructed by using B3 type Coxsackie virus structural protein VPI gene and pcDNA carrier together, and its exterior is covered with a biological polysaccharide. It also discloses a method for preparing said gene vaccine and the application of said gene vaccine in preparation of medicine for preventing viral myocarditis.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
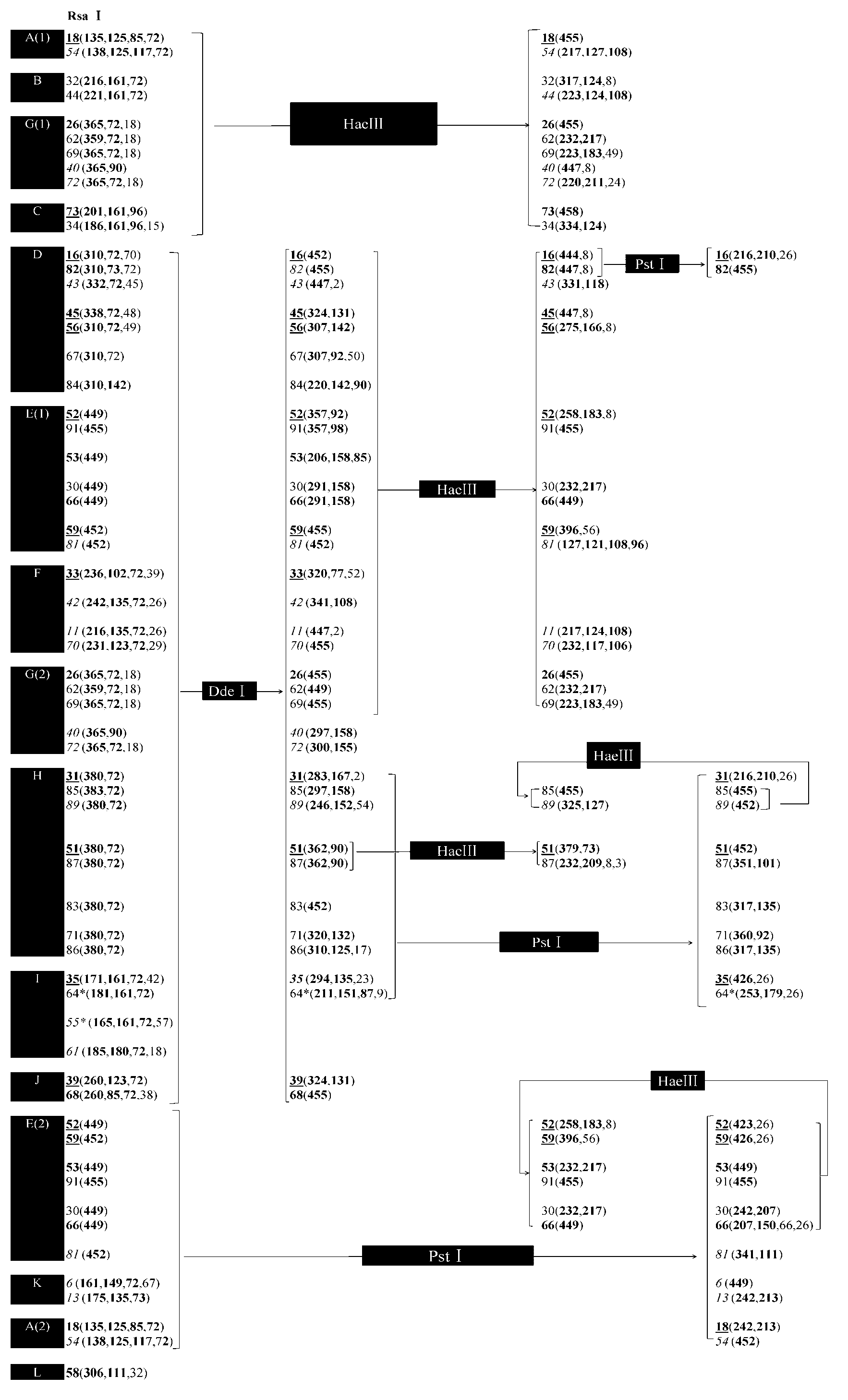
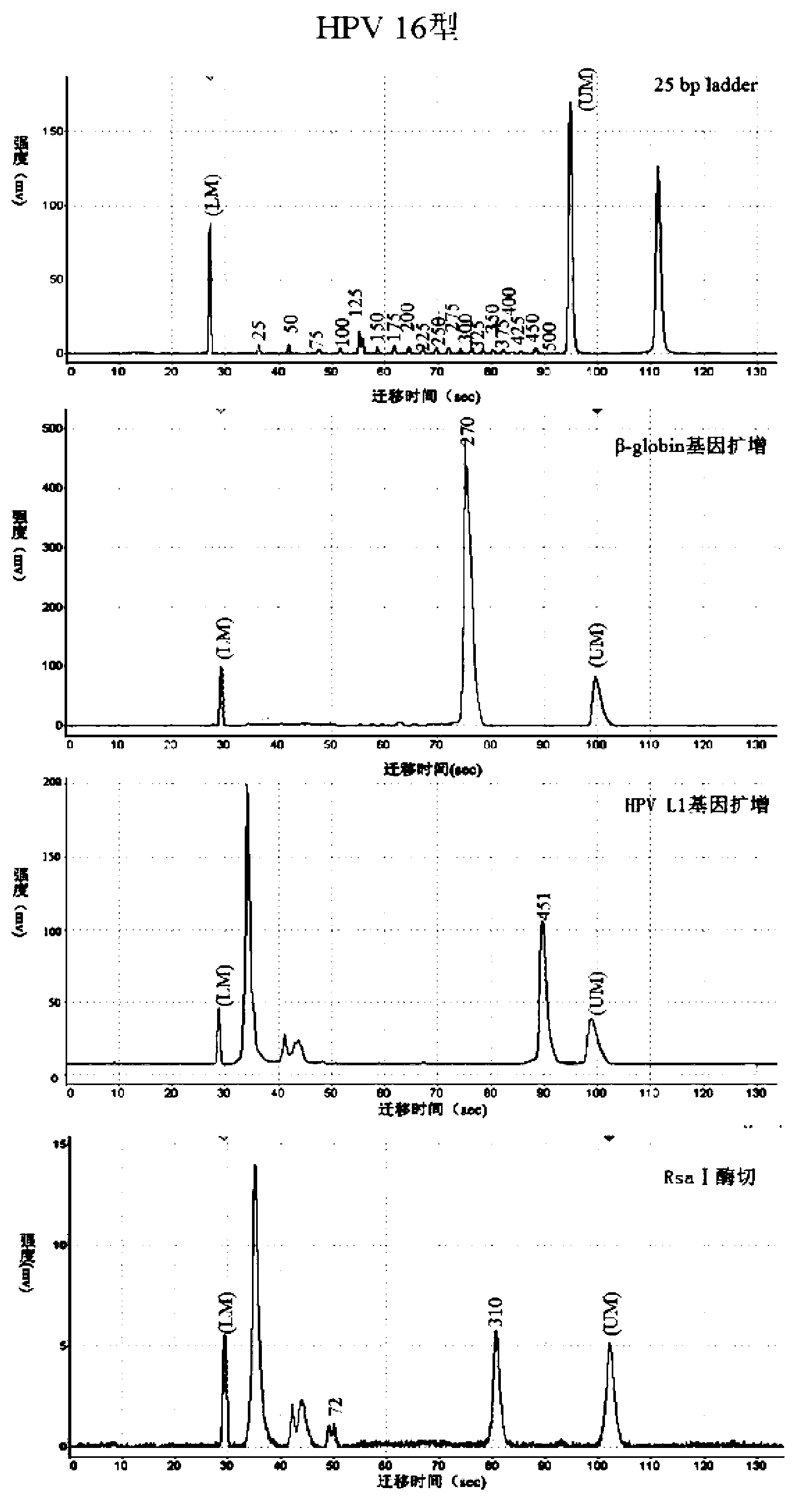
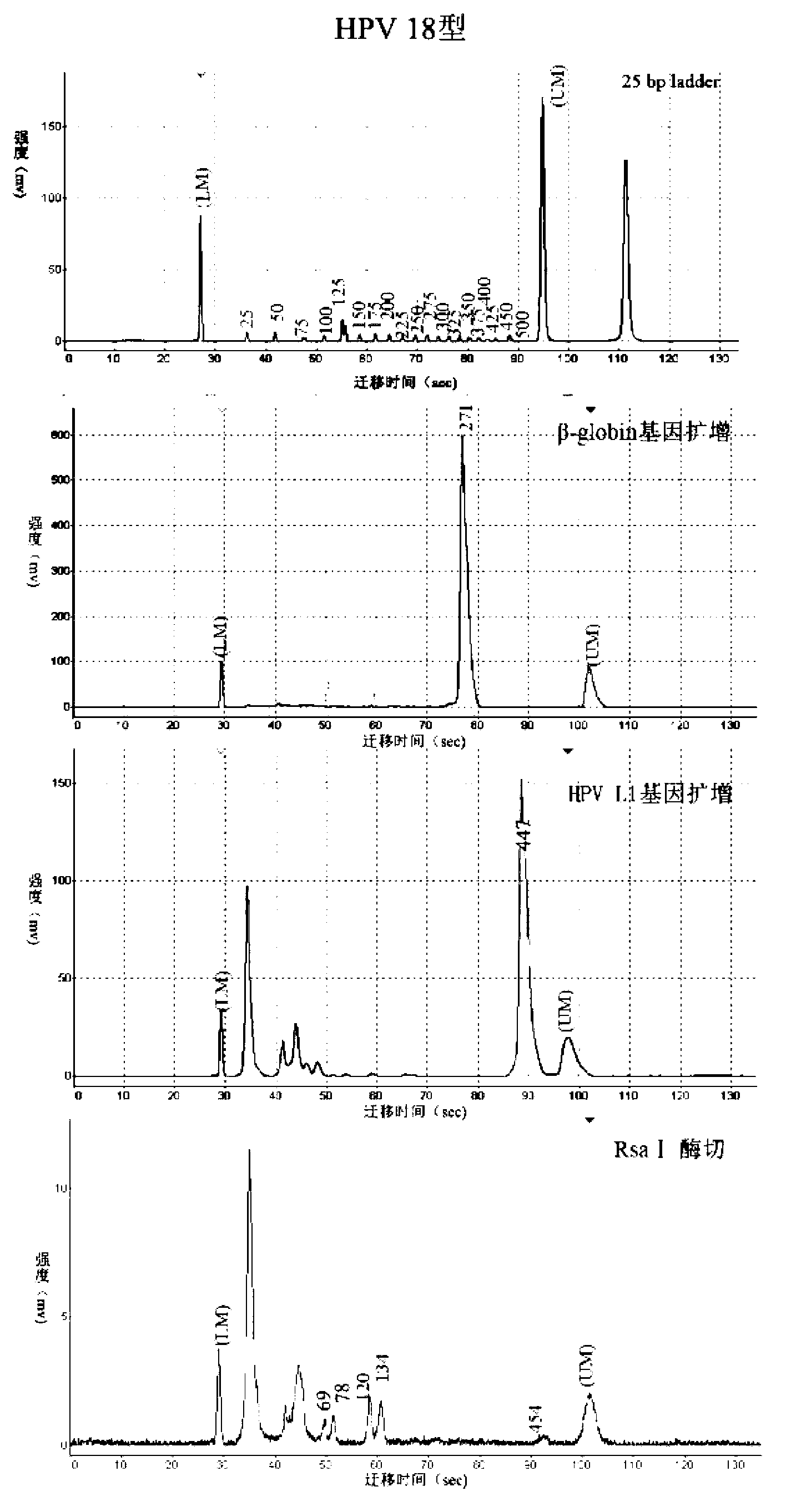
HPV (human papillomavirus) DNA (deoxyribonucleic acid) genetic typing method and kit thereof
InactiveCN103320533AHelp in early preventionEasy to operateMicrobiological testing/measurementMicroorganism based processesHuman papillomavirusTyping methods
The invention discloses an HPV(human papillomavirus) DNA (deoxyribonucleic acid) genetic typing method and a kit thereof. The method disclosed by the invention can be used for typing DNA of 46 types of HPVs, is simple to operate, is economical and practical and fast and efficient, and is combined with chip capillary electrophoresis testing to analyze the HPV DNA type with high throughput. When applied in the medical domain, the HPV / DNA genetic typing method is combined with the existing lamina ThinPrep cytology morphology diagnosis and is benefit to the early-stage prevention, prognosis and research of target gene vaccines for cervical cancer; and the method can be used for detecting multiple types and finding unknown types, thereby being especially suitable for research of pathogenesis and epidemiology of HPV-related diseases, such as hypopharyngeal carcinoma and head and neck cancer.
Owner:TSINGHUA UNIV +1
HBx and human IL-12 double-gene recombinant vector and liver caner-resistant vaccine
ActiveCN102660579AGood treatment effectGenetic material ingredientsGenetic engineeringHBxLiver cancer
The invention belongs to the field of gene therapy and aims at providing a novel targeted gene vaccine for treating HBV (Hepatitis B Virus)-relevant liver cancer and application of the novel targeted gene vaccine in preparation of a targeted gene immunotherapeutic drug for the HBV-relevant liver cancer. The main active component of the liver caner-resistant vaccine disclosed by the invention is a recombinant vector of a gene coded with an HBx protein and a gene for coding a human IL-12 protein; and the recombinant vector can be used for simultaneously expressing the HBx protein and the human IL-12 protein in a eukaryotic cell. Provided by the experiment, a recombinant adenovirus vaccine disclosed by the invention can be used for selectively killing HBx-electropositive liver cancer cell and expresses a favorable liver cancer-resisting effect. The recombinant adenovirus vaccine provides a new selection for targeted gene immunization therapy of the HBV-relevant liver cancer and has a favorable application prospect.
Owner:SICHUAN UNIV
Genetic vaccine that mimics natural viral infection and induces long-lasting immunity to pathogens
Owner:GENPHAR INC
Rationally designed and chemically synthesized promoter for genetic vaccine and gene therapy
ActiveUS7312202B2High activityEnhance immune responseBiocideGenetic material ingredientsChemical synthesisIntramuscular injection
The present invention relates to chemically synthesized promoters that circumvent the disadvantages of the universal CMV promoter / enhancer elements. The promoter may be used in a variety of applications, particularly in genetic immunization. The chemically synthesized promoter overcomes the common problems of the CMV promoter element such as: low transgene expression levels, transient expression, and the large amount of plasmid DNA needed for intramuscular injection in subjects.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Trichinella Spiralis larva ES antigen gene vaccine and preparation method
ActiveCN103352047AAvoid infectionImprove insect reduction rateGenetic material ingredientsAntiparasitic agentsAntigenGenetic vaccine
The invention discloses a Trichinella Spiralis larva ES antigen gene vaccine and a preparation method thereof. Trichinella Spiralis larva 43ku and 45ku ES protein genes are respectively inserted to a pVAXI eukaryotic expression vector to obtain recombinant plasmids, and the recombinant plasmids are mixed according to a ratio of 1:1 to prepare a combined recombined DNA vaccine for preventing Trichinella Spiralis infection and improving the worm reduction rate of bodies. After the injection immunization of hindlimb muscles of mice for three weeks, 2000 worms are killed, the worm reduction rate can reach 76.65%, and the quantity of eggs at the tongue tissues of the mice is obviously reduced.
Owner:JILIN AGRICULTURAL UNIV
Multi-T cell epitope tuberculosis gene vaccine with HSP65 as epitope scaffold
InactiveCN104127883AEfficient removalImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsTreating tuberculosisTGE VACCINE
The invention discloses a multi-T cell epitope tuberculosis gene vaccine with HSP65 as an epitope scaffold. The full-length gene of HSP65 protein in which 5 T cell epitope polypeptide genes originated from mycobacterium tuberculosis antigen are embedded is inserted into a vector. A preparation method for the vaccine comprises a step of inserting the 5 T cell epitope genes originated from mycobacterium tuberculosis antigen into the HSP65 full-length gene, wherein the 5 T cell epitope genes comprise EAST-61-60, Ag85B721-765, MTB10.47-33, PPE25721-765 and PE1910-54. It is proved that the tuberculosis gene vaccine can induce response of serum IgG and lung SIgA to the HSP65 vector and induce cellular immunologic response of specific strong secretion of high-level IFN gamma to multiple tuberculosis antigen epitopes through intranasal immunization of mice with a nanometer granular compound formed by the gene vaccine and deacetylated chitosan, so the tuberculosis gene vaccine is a potential vaccine for prevention and treatment of tuberculosis.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com