Viral myocarditis gene vaccine, its preparation method and application
A technology of viral myocarditis and gene vaccines, applied in the field of immunology, can solve the problems that naked DNA is difficult to retain, remove or degrade for a long time, and gene vaccines cannot effectively exert immune efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
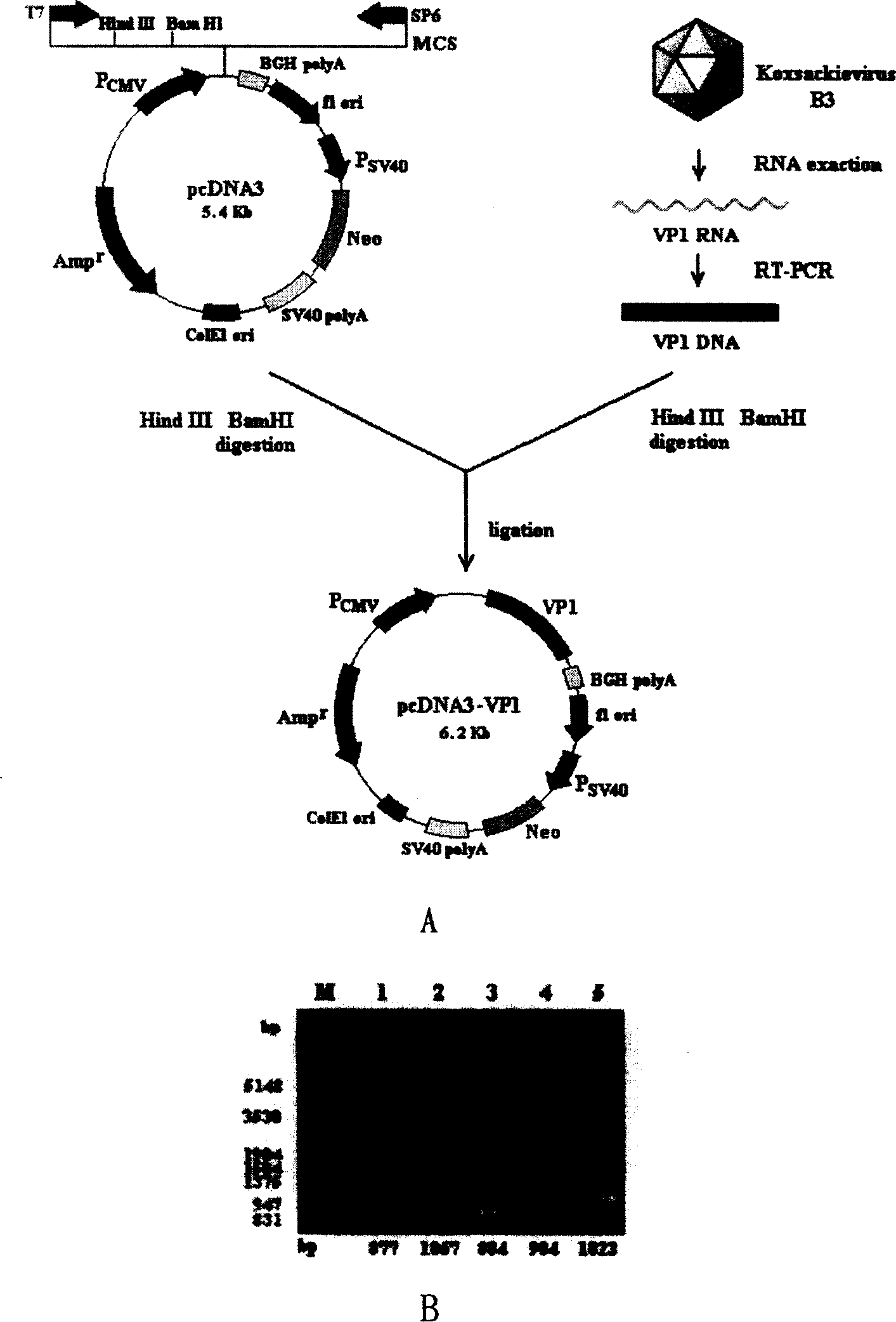
[0022] Construction of embodiment 1.pcDNA3-VP1
[0023] 1) First carry out the cultivation of Hela cells and the cultivation and passage of CVB3 virus, the method is as follows: the human cervical cancer cell line Hela cells are cultivated according to conventional methods, with 10% NBS, 2mM L-glutamine, 100U / ml penicillin and sulfuric acid Kanamycin-based RPMI-1640 medium was incubated at 37°C in 6% CO 2 Cultivate under the same conditions, subculture once every other day, gently digest the cells with 0.02% EDTA digestion solution at 37°C, and subculture at 1:2 to 1:4 after pipetting evenly. Infect approximately 5×10 6 For Hela cells, 80% of the cells were lysed by replicating virus after 40 hours of culture, and the liquid and cell fragments were centrifuged at 3000 rpm / min for 20 minutes, and the obtained supernatant was fresh CVB3 suspension.
[0024] 2) Obtain CVB3 VP1 gene from freshly cultured CVB3 by RT-PCR. The method is as follows: According to the CVB3 VP1 cDNA s...
Embodiment 2
[0029] Example 2. Preparation of novel viral myocarditis gene vaccine chitosan-pcDNA3-VP1
[0030] 1) Prepare chitosan solution and pcDNA3-VP1 solution respectively, the method is as follows: firstly prepare 0.02% chitosan solution with pH 5.7, weigh 0.02g chitosan (Sigma, MW=39000), and first mix with 500 μl ~ 1ml 1% HAc solution It dissolved slowly and was left at 37°C for 1 hr. Then weigh 0.042gNaAc, with 80ml H 2 O is dissolved, add the above-mentioned completely dissolved chitosan solution, mix well, and adjust the pH value to 5.7 with 1N NaOH, which is 0.02% chitosan solution with pH 5.7. PcDNA3 plasmid with 50mM Na 2 SO 4 Dissolve, the DNA concentration is 1mg / ml, and store at -20°C for later use.
[0031] 2) The chitosan-pcDNA3-VP1 complex was prepared by the complex co-precipitation method as follows: in a 55°C water bath, a 0.02% chitosan solution with a pH of 5.7 was dropped into the 80ug / ml plasmid DNA solution, and at the same time Shake for 20 seconds to for...
Embodiment 3
[0032] 3) Observe the freshly prepared chitosan-pcDNA3-VP1 complex with a transmission electron microscope, the method is as follows: drop the freshly prepared chitosan-pcDNA3-VP1 complex on a copper grid, room temperature for 5 minutes, negatively stain with uranyl acetate, and observe with a transmission electron microscope And take pictures. It was found that the freshly prepared solution contained spherical or elliptical particles with uniform shape, with a diameter of about 80-100nm ( figure 2 ). Example 3. In vitro expression of novel viral myocarditis gene vaccine chitosan-pcDNA3-VP1
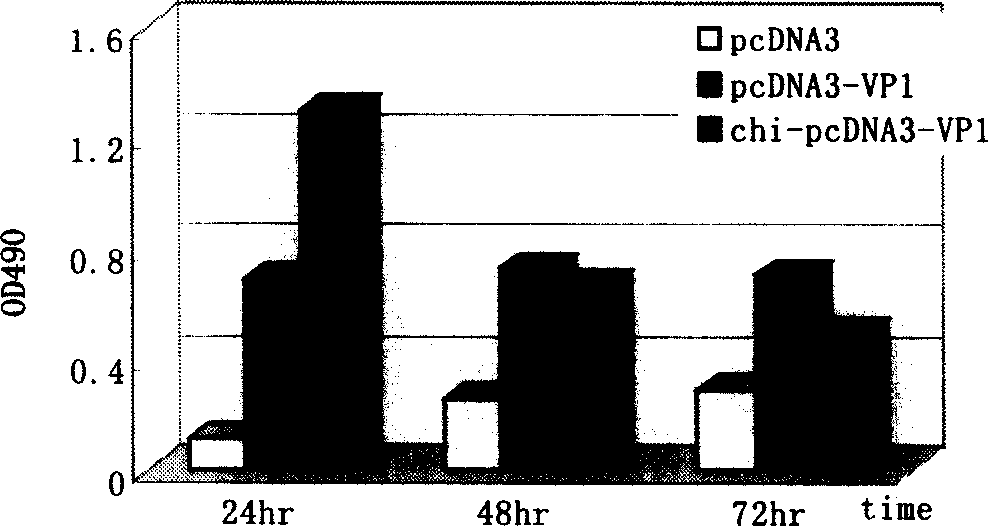
[0033] Add 100ul of chitosan-DNA solution containing 1μg pcDNA3-VP1 DNA directly to 2×10 5 On the surface of Hela cells, add 2 μl Lipofectamine to 1 μg pcDNA3-VP1 DNA TM -2000 transfection complex was used as a control (1-3×10 5 The cells were seeded into 24-well plates, and 2ml of fresh antibiotic-free culture medium was replaced in the first 4 hours, and the cell density was prefer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com