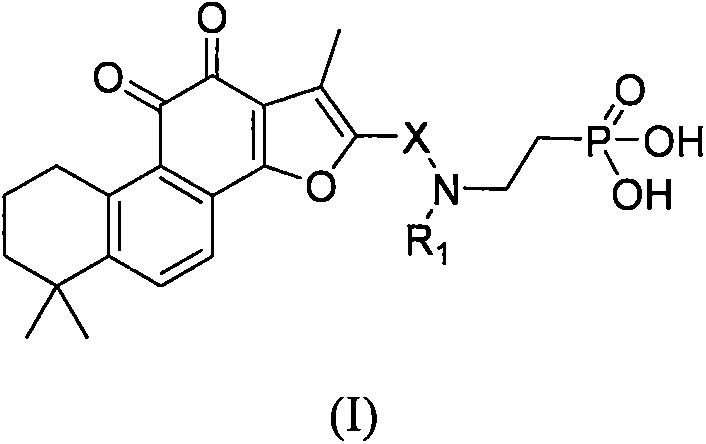
Tanshinone IIA phosphoric acid derivative and synthesis and use thereof as medicine
A technology of tanshinone and prodrug, applied in the field of medicine, can solve the problems of high irritation of products, pain of patients, low pH value of injections, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
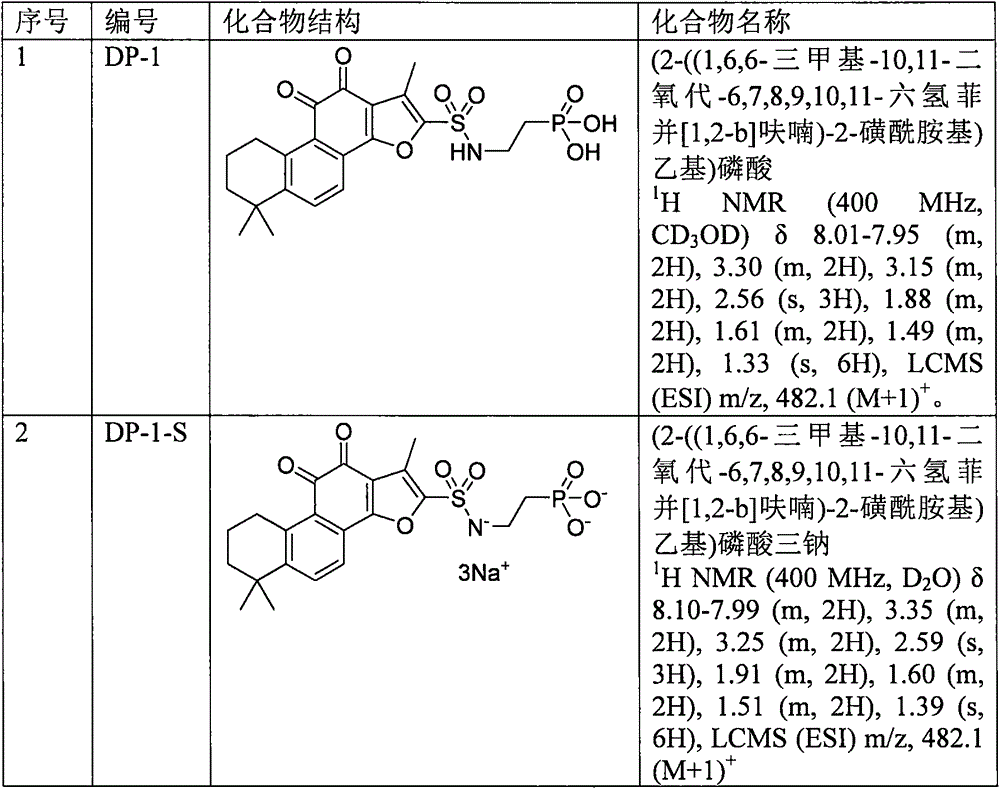
[0035] Example 1: (2-((1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2-b] Preparation of furan)-2-sulfonamido)ethyl)phosphoric acid (DP-1)
[0036]
[0037] step 1 : Tanshinone IIA Sulfonyl chloride ( intermediate IN-A) preparation of
[0038] Tanshinone IIA (10 g, 33 mmol) was added dropwise into 150 mL of sulfuryl chloride, and refluxed for 1 hour. The temperature of the reaction system was lowered to room temperature, and the unconsumed sulfuryl chloride was distilled off under reduced pressure. The remaining black oily liquid was dried under reduced pressure with an oil pump for 2 hours under the condition of avoiding light, and the obtained black oily liquid was directly used in the next reaction.
[0039] step 2 : DP-1 preparation of
[0040] Dissolve the intermediate IN-A (1g, about 2.55mmol) obtained in step 1 in 25mL of toluene, place the reaction bottle in an ice-water bath, and when the internal temperature drops to 5°...
Embodiment 2
[0042] Example 2: (2-((1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2-b] Preparation of furan)-2-sulfonamido)ethyl)trisodium phosphate (DP-1-S)
[0043]
[0044] DP-1 (100 mg, 0.20 mmol) was placed in 5 mL of methanol, sodium bicarbonate (50 mg) was added thereto, stirred at room temperature for 30 minutes, filtered, and the filtrate was evaporated to dryness to obtain DP-1-S, 110 mg, with a quantitative yield.
[0045] 1 H NMR (400MHz, D 2 O) δ8.10-7.99(m, 2H), 3.35(m, 2H), 3.25(m, 2H), 2.59(s, 3H), 1.91(m, 2H), 1.60(m, 2H), 1.51( m, 2H), 1.39 (s, 6H), LCMS (ESI) m / z, 482.1 (M+1) + .
Embodiment 3
[0046] Example 3: (2-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2-b]furan Preparation of -2-carbonylamide) ethyl) phosphoric acid (DP-2)
[0047]
[0048]
[0049] step 1 : Formyltanshinone IIA( intermediate IN-B) preparation of
[0050] Tanshinone IIA (30 g, 0.01 mol) was dissolved in 300 mL of DMF, 30 mL of phosphorus oxychloride was added dropwise at room temperature, and stirred at room temperature for 2 hours. After the reaction was completed, the reaction solution was poured into 1000 mL of ice-water mixture, stirred slowly, and a light yellow solid was precipitated, filtered with suction, and the filter cake was washed with cold water three times, 100 mL each time. The filter cake was collected and vacuum-dried to obtain 32 g of tanshinone IIA-2-carbaldehyde (light yellow solid), and the yield was quantitative.
[0051] 1 H NMR (400MHz, CDCl3), 9.85(s, 1H), 7.78-7.69(d and d, 2H), 3.19(m, 2H), 1.80(m, 2H), 1.67(m, 2H), 2.65(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com