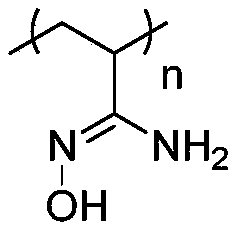
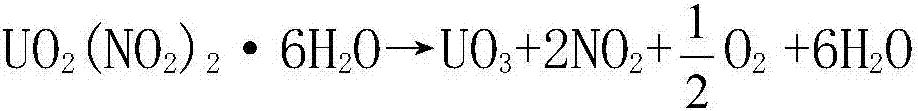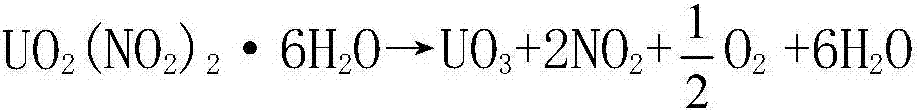Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
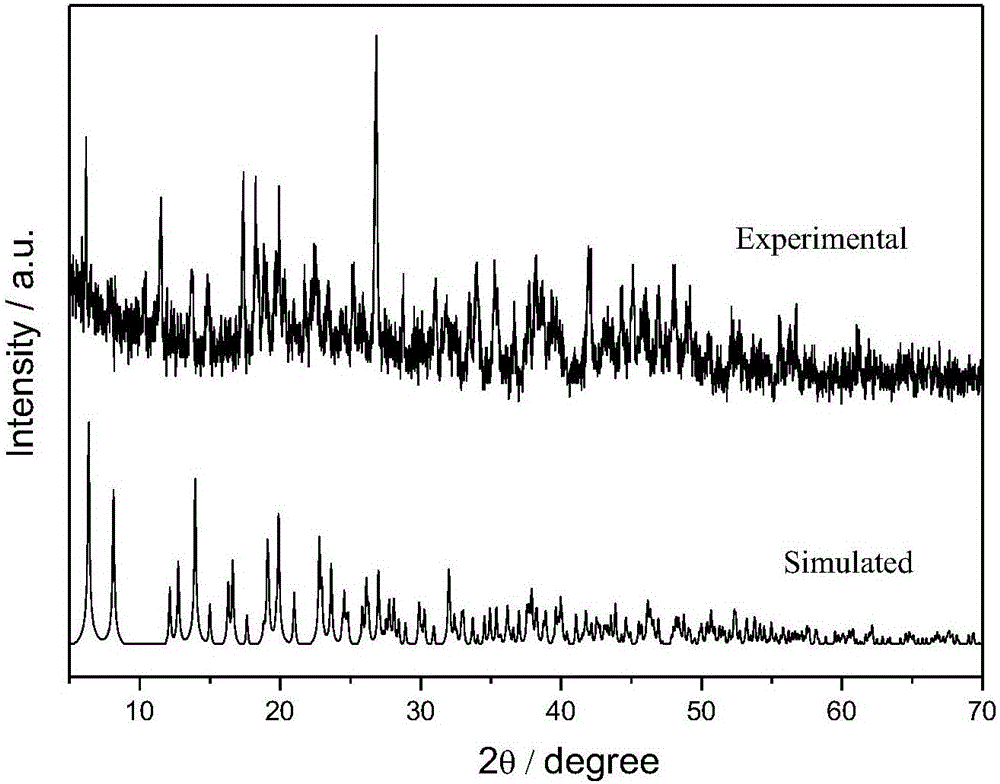
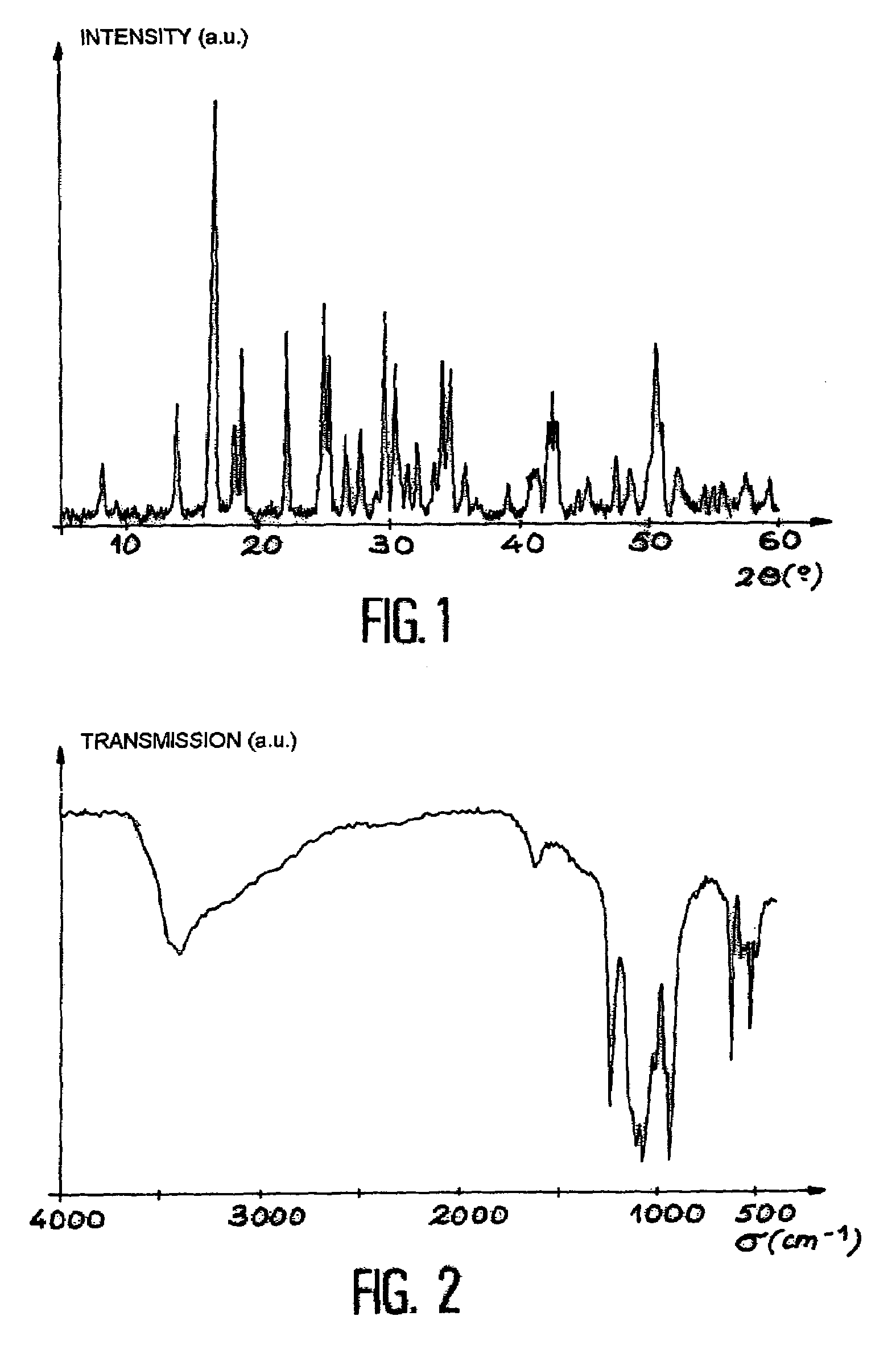
198 results about "Uranyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
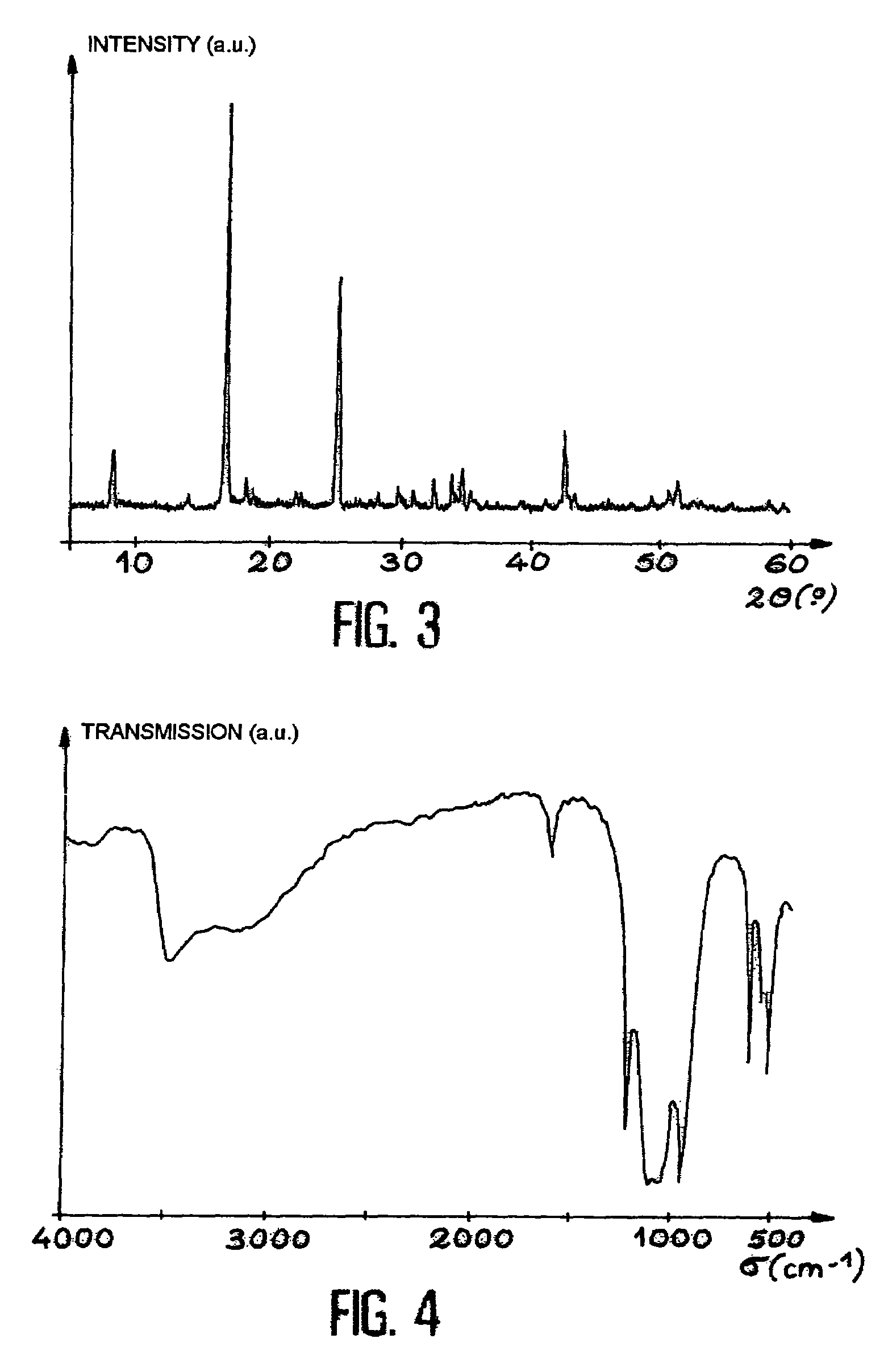
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO²⁺₂. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.
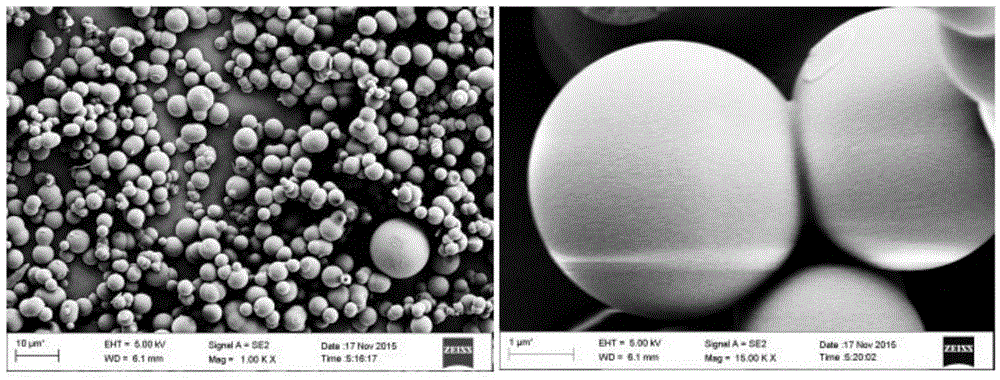
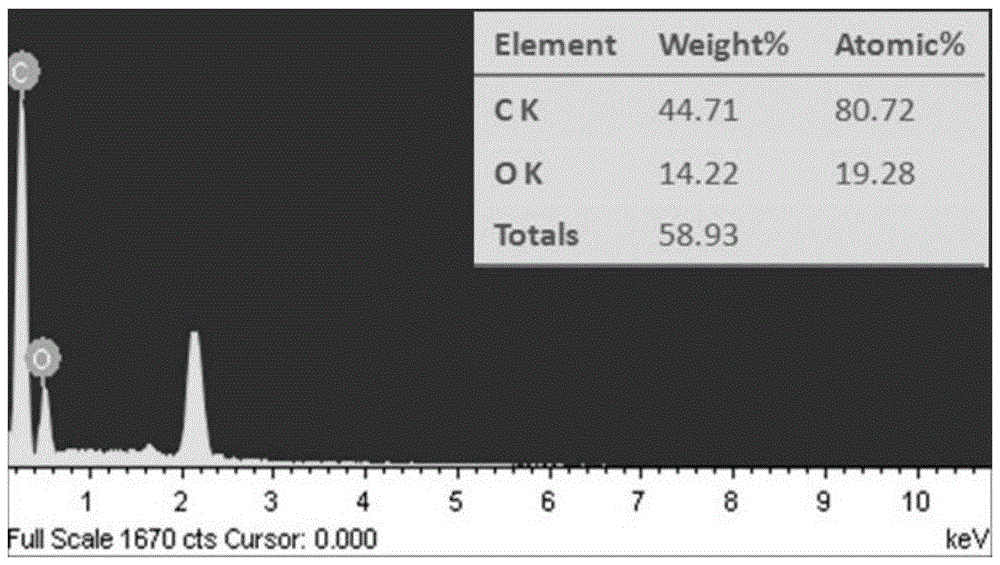
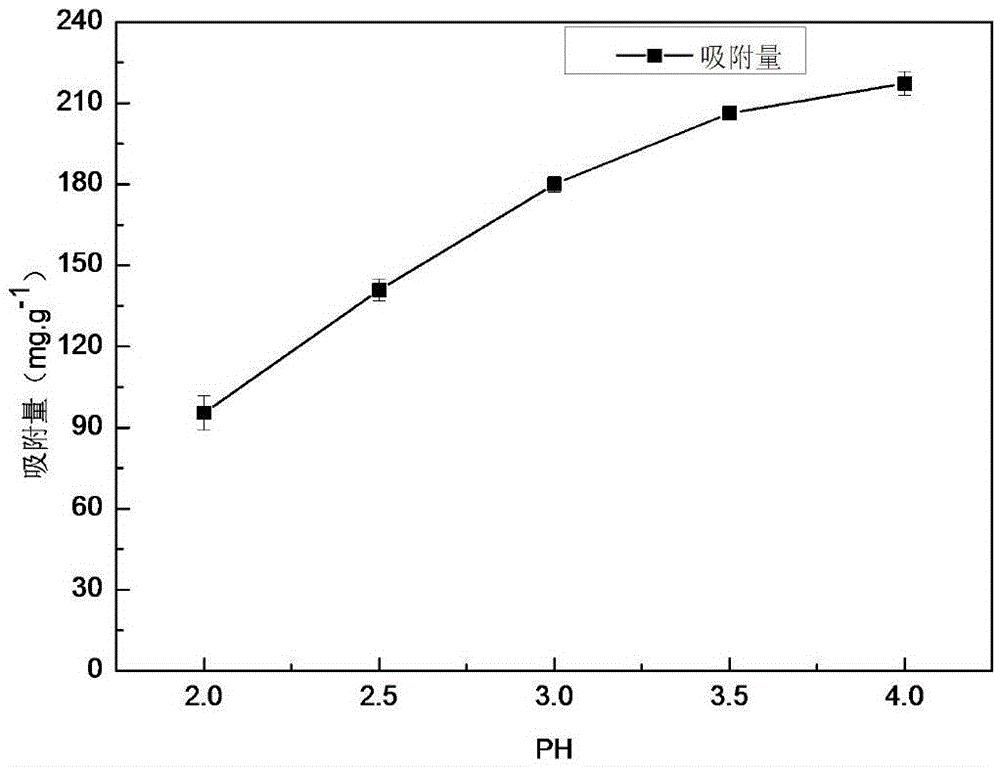
Method for preparing carbon microsphere adsorbent under catalysis of metal salt with low-temperature hydrothermal method
ActiveCN105582888AEasy disposalReduce energy consumptionOther chemical processesAlkali metal oxides/hydroxidesAir atmosphereSorbent
The invention discloses a method for preparing a carbon microsphere adsorbent under catalysis of metal salt with a low-temperature hydrothermal method. The method comprises steps as follows: (1) a biomass solution with the concentration being 2wt%-20wt% and a metal salt solution with the concentration being 0.5wt%-2wt% are prepared, then mixed in the mass ratio being (2-5):1 and stirred for 5-10 min, and a reaction solution is obtained; (2) the reaction solution is transferred to a stainless steel reaction kettle, the volume of the reaction solution accounts for 60%-80% of that of the reaction kettle, the reaction solution is heated to 100-160 DEG C at a certain heating speed, reacts at the constant temperature for 8-20 hours and is naturally cooled, separated and dried in vacuum, and hydrothermal carbon microspheres are obtained; (3) the hydrothermal carbon microspheres are calcined and carbonized at the air atmosphere, and the carbon microsphere adsorbent is obtained. The preparation process of the adsorbent is simple, energy consumption is low, the reaction time is shortened to be within 24 hours, and the adsorbent has high adsorption performance for part of positive ions, particularly, uranyl ions, has excellent radioresistance, thermal stability and acid stability and can be widely used for treatment of radioactive wastewater.
Owner:SOUTHWEAT UNIV OF SCI & TECH
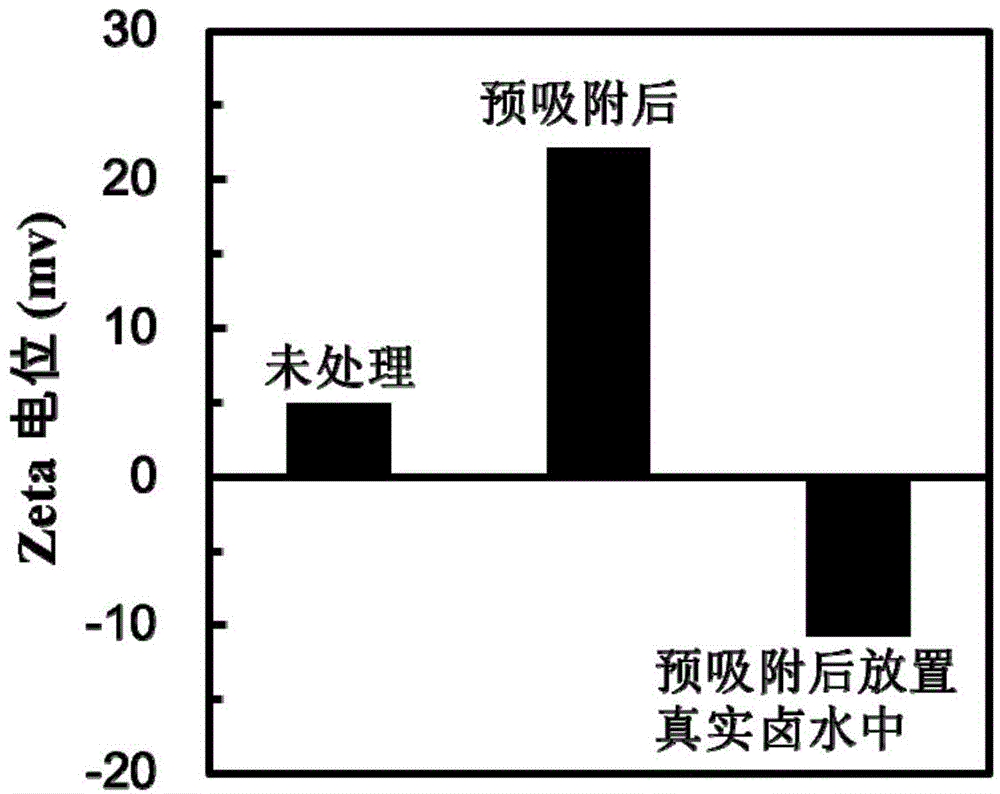
High-efficient method of extracting uranyl ions from water
ActiveCN104485148ASimple methodEasy to operateRadioactive decontaminationEnvironmental resistanceWastewater
The invention discloses a high-efficient method of extracting uranyl ions from water and belongs to the field of the water treatment technology; the method comprises the steps: absorbing the uranyl ions from the surface of an absorbing material in advance; throwing the absorbing material absorbed with the uranyl ions in advance to waste water containing the uranyl ions, stirring, filtering and finally collecting filter residues. The high-efficient method of extracting the uranyl ions from water has the advantages of simple method, easy operation at normal temperature and no other impurities introduced; the method is green, environmental-friendly, economic and effective; the secondary pollution caused by toxic pollutants is avoided; high-salt and complex ion environments in water can be overcome effectively and the selective extraction of the uranyl ions in water is realized.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Visualization method for rapidly detecting trace amount of uranyl ions in water environment
InactiveCN104964942ARealize visual quick detectionHigh sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsMagnetic beadHorse radish peroxidase
The invention belongs to the field of detecting a trace amount of ions in the water environment, and particularly relates to a visualization method for rapidly detecting a trace amount of uranyl ions in the water environment. The method mainly includes the steps that DNAzyme with the specific recognition function on UO2 <2+> is fixed to the surfaces of magnetic beads, and horse radish peroxidase is preassembled on the surface of nano-gold; then the magnetic beads are connected with the nano-gold through the cutting effect of the UO2<2+> on the DNAzyme and the hybridization reaction of DNA, after separation and collection are carried out through an external magnetic field, H2O2 oxidation tetramethyl benzidine is efficiently catalyzed through the horse radish peroxidase to enable a solution to be changed from the blank to the blue, and therefore sensitive and specific visualization rapid detection of the UO2<2+> ions is achieved. As the method has the advantages of being high in sensitivity, high in specificity, high in matrix interference resistance, simple, rapid, low in cost and the like, the method can be used for site rapid visualization detection of the trace amount of UO2<2+> ions in various water samples.
Owner:FUZHOU UNIV
Mesoporous chelate resin containing phosphorus-oxygen functional groups and method for separating and enriching uranium
InactiveCN105688844AEasy to prepareLow costOther chemical processesWater contaminantsOrganic solventSorbent
The invention relates to mesoporous chelate resin containing phosphorus-oxygen functional groups and a method for separating and enriching uranium. A preparation method of the mesoporous chelate resin includes: adding 1-5g of organic crosslinking agent, 1-5g of unsaturated compound containing the phosphorus-oxygen functional groups and 0.02-0.08g of azodiisobutyronitrile into 1-10mL of organic solvent, stirring under 25 DEG C for 1-3 hours, adding the solution into a hydrothermal reaction kettle, reacting under 100 DEG C for 24 hours, taking out, opening the reaction kettle, performing rotation evaporation to remove the organic solvent, and performing vacuum drying under 45 DEG C for 12-24 hours to obtain the mesoporous chelate resin containing the phosphorus-oxygen functional groups, wherein mass number and volume number are proportionally adjusted. The preparation method is simple in synthesizing path and easy to operate. When the mesoporous chelate resin serving as an absorbent is used to separate and enrich the uranium under high acidity, the mesoporous chelate resin is high in absorption ability, large in absorption quantity, fast in absorption, and the like, and the mesoporous chelate resin can be easily separated from an aqueous solution and can effectively absorb and recycle the uranyl ions in the aqueous solution.
Owner:EAST CHINA UNIV OF TECH
Fe/C composite material and applications thereof
ActiveCN106807325AEfficient fixationAvoid reunionOther chemical processesRadioactive decontaminationSorbentVacuum drying
The invention belongs to the technical field of material chemistry, and discloses a Fe / C composite material. The Fe / C composite material is prepared by adopting a carbon-thermal reduction method through the following steps: 1) dissolving and preparing ferric salt into an iron-containing solution, adding a carbon source into the iron-containing solution, oscillating and shaking well, controlling the pH value of the iron-containing solution to be about 8.0, stirring and heating until gel is generated, then transferring into a vacuum drying oven for drying moisture; and 2) putting a product obtained from the step I into a tubular resistance furnace with nitrogen protection, carbonizing for 4 h under the condition of 500-1000 DEG C, cooling and grinding and then cleaning with deionized water, removing water-soluble components, then filtering and drying, milling into powder, thereby obtaining a zero-valent Fe / C-loading composition material. The invention further provides applications of the Fe / C composite material for fixing uranium in waste water as an adsorbent. According to the invention, the technical problem that agglomeration is easily generated when zero-valent iron is synthesized by adopting a traditional NaBH4 liquid phase reduction method. The Fe / C composite material has a high-efficiency immobilization effect on uranyl ions in the waste water, and can be used for treating the mining uranium-containing waste water pollution.
Owner:GUANGZHOU UNIVERSITY
Technique for reagent-free in situ leaching uranium mining from sandstone type uranium deposit
InactiveCN101580900AEasy to extract and handleImprove product qualityProcess efficiency improvementWater insolubleUranyl
The invention relates to a technique for reagent-free in situ leaching uranium mining from a sandstone type uranium deposit, which comprises the following steps: performing preoxidation on ore beds by injecting air; injecting industrial oxygen into the ore beds in the processes of extracting and liquid charging; and forming hexavalent uranium into a stable soluble complex in bicarbonate type ground water. The technique achieves the oxidation of the metallic uranium in the ore beds under the condition of not introducing chemical reagents of acid, alkali and the like so that the water-insoluble tetravalent uranium is converted into water-soluble hexavalent uranyl ions for the convenience of performing extraction on the ground surface; besides, the technique has the advantages of stable product quality, low production cost, no pollution of an extraction-injection balanced closed cycle, easy decommissioning treatment and the like.
Owner:LIAOHE GASOLINEEUM EXPLORATION BUREAU
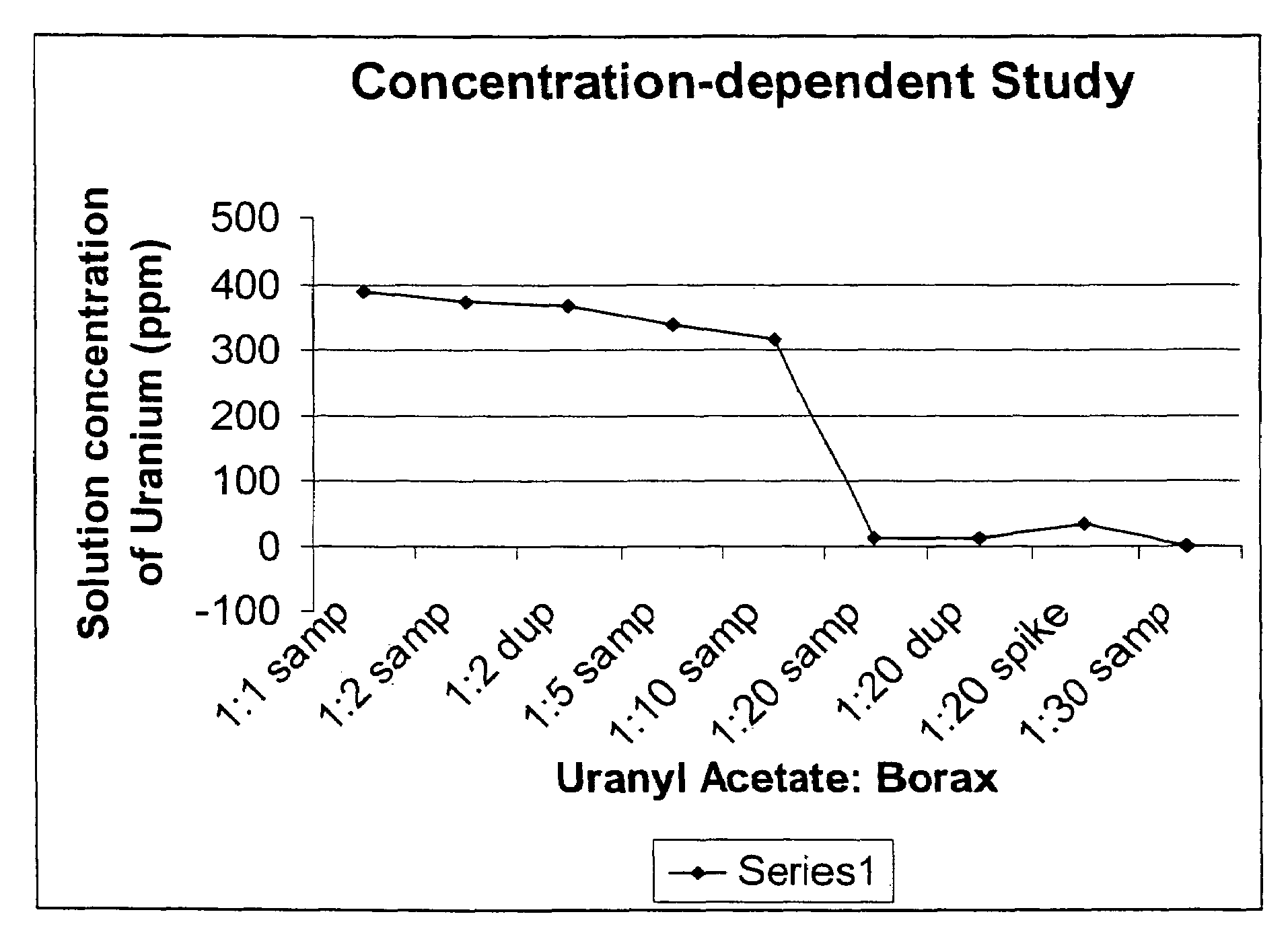
Use of boron compounds to precipitate uranium from water
InactiveUS7419604B1Improve effectivenessIncrease supplyTransuranic element compoundsContaminated soil reclamationUranylBoron
Owner:UNIV OF KENTUCKY RES FOUND
Magnetic cucurbit urils/grapheme oxide composite material and preparation method thereof
InactiveCN104722276AUniform sizeGood dispersionOther chemical processesAlkali metal oxides/hydroxidesHydrogenOxide composite
The invention discloses a magnetic cucurbit urils / grapheme oxide composite material and a preparation method thereof with an aim to solve problems that an existing cucurbit urils solid loading method is tedious in preparation steps, low in yield and high in cost. The magnetic cucurbit urils / grapheme oxide composite material is made of components comprising, by weight, 1%-20% of cucurbit urils, 1%-30% of grapheme oxide and 50%-80% of ferroferric oxide nanoparticles; the cucurbit urils and the grapheme oxide are mutually connected through hydrogen bonds, and the cucurbit urils and the ferroferric oxide nanoparticles are evenly dispersed on the surface of the grapheme oxide. The advantages of the cucurbit urils, the grapheme oxide and the ferroferric oxide nanoparticles are combined in the magnetic cucurbit urils / grapheme oxide composite material which has excellent uranyl ion adsorption performance and cyclic utilization capability, and potential application values are achieved. Meanwhile, the preparation method is simple in operation, low in production cost, high in yield, capable of meeting requirements on large-scale industrialization application, good in application prospect and worthy of large-scale promotion and application.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
Method for concentrating uranium from water solution with uranyl ions
ActiveCN104916342AReduce adsorptionImprove adsorption capacityRadioactive decontaminationCapacity lossAcrylonitrile
The invention discloses a method for concentrating uranium from a water solution with uranyl ions. The method for concentrating uranium from the water solution with the uranyl ions comprises the following steps that a uranyl ion absorbing material is used for conducting adsorption on the water solution with the uranyl ions; the uranyl ion absorbing material is a material containing a group (please see the formula in the instruction) or (please see the formula in the instruction). According to the method for concentrating uranium from the water solution with the uranyl ions, the low-production-cost uranyl ion absorbing material is used, the uranium element is concentrated from the low concentration uranyl ion water solution, the use of poisonous compound acrylonitrile is avoided, in the production process, the pollution to the environment is small, the adsorption capacity on vanadium is low, the adsorption capacity and the repeating use efficiency of the material can not be lowered even if vanadiumism happens to the material, the adsorption rate is high, the maximum adsorption capacity on the uranium can be as high as 7.57 mg / g, the stability is good, the repeating use efficiency is high, the average capacity loss is only 5% after recycling use for ten times, and the adsorption capacity on the uranium is still very high after the uranyl ion absorbing material is used for one hundred times.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
Nano-composite material and preparation method thereof
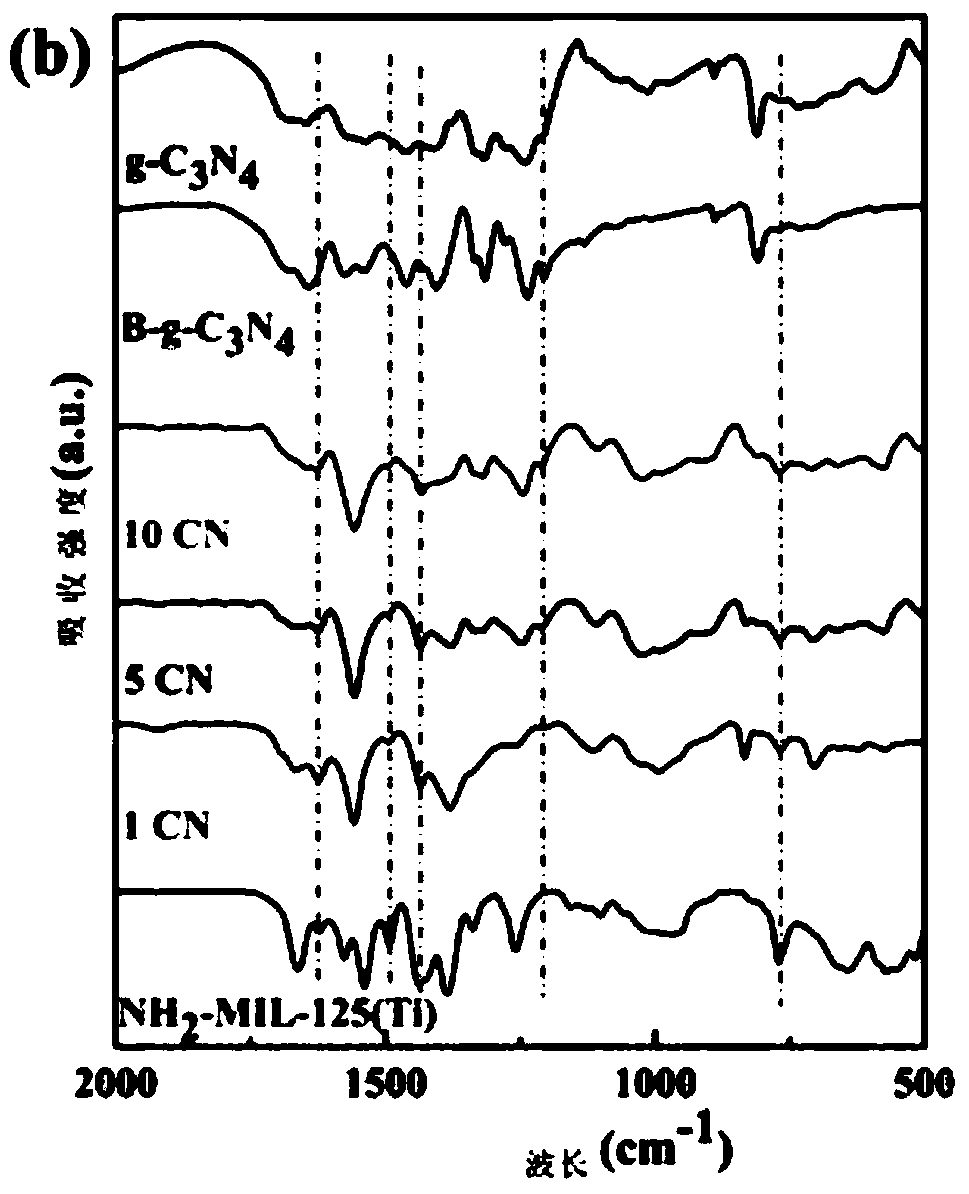
InactiveCN109012607AEfficient separationImprove photocatalytic reduction of U(VI) to U(IV) performanceOther chemical processesOrganic-compounds/hydrides/coordination-complexes catalystsHeterojunctionUranyl
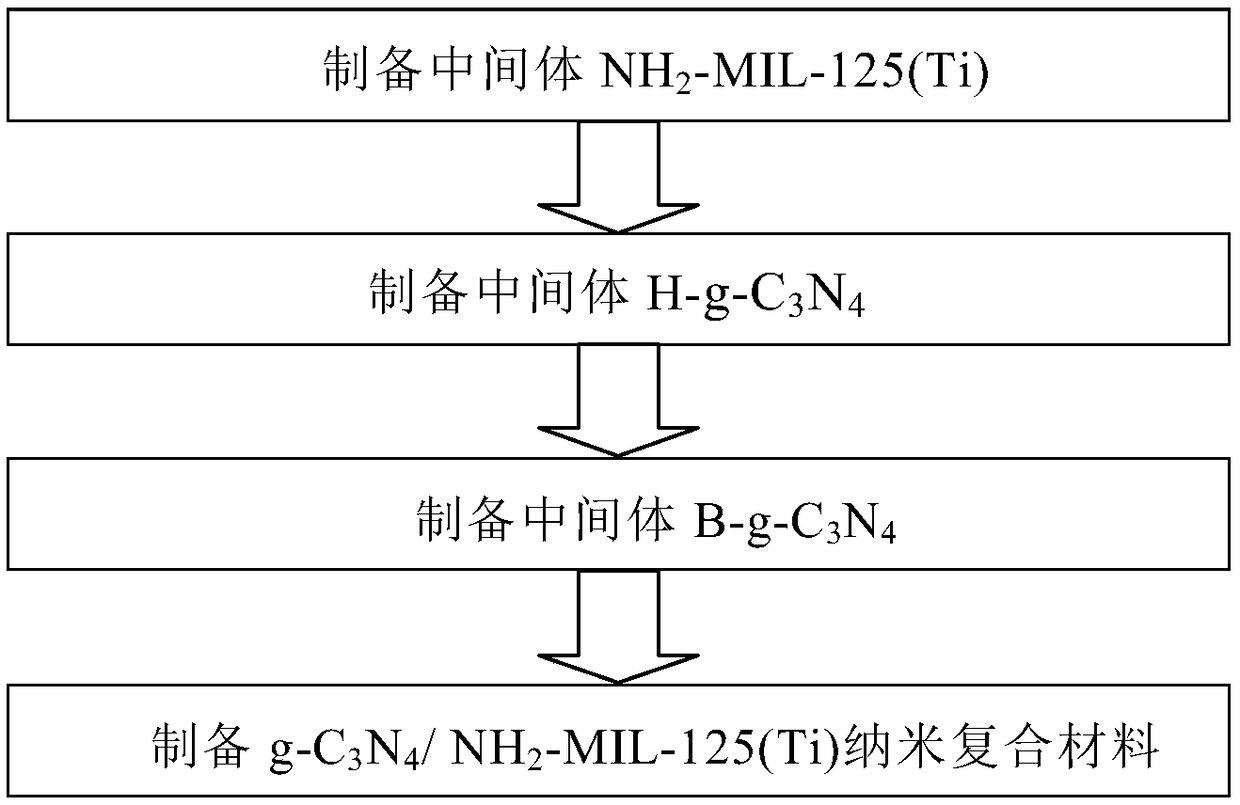
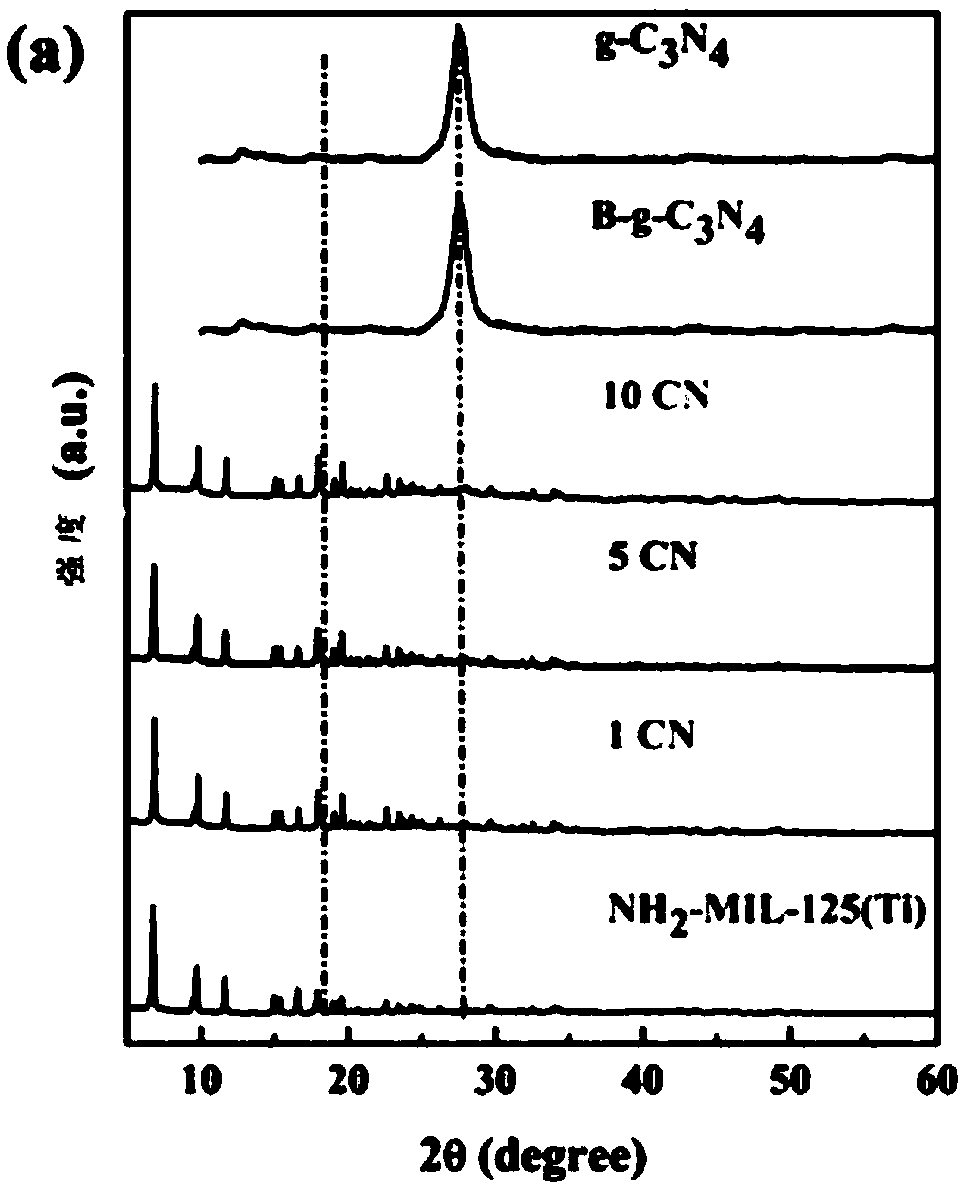
The invention discloses a g-C3N4 / NH2-MIL-125(Ti) nano-composite material and a preparation method thereof, and relates to the field of preparation of uranyl ion removal materials. The preparation method includes the following steps: preparing an intermediate NH2-MIL-125(Ti), preparing an intermediate H-g-C3N4, preparing an intermediate B-g-C3N4, and finally preparing the g-C3N4 / NH2-MIL-125(Ti) nano-composite material. The B-g-C3N4 is used for the first time, and the B-g-C3N4 and an aminated NH2-MIL-125(Ti) material undergo amido bond formation to synthesizes the heterojunction photocatalytic composite material, and photo-induced electrons and holes are effectively separated, so the performance for catalytic reduction of U (VI) into U(IV) is improved; and when the mass proportion of the B-g-C3N4 is 1%, the composite material can effectively remove radioactive uranyl ions through the synergistic mechanism of adsorption and photocatalysis, so the U (VI) removal rate is improved.
Owner:南通寰宇博新化工环保科技有限公司
Method for preparing uranous solution
InactiveCN102249331AIncrease concentrationSimple and fast operationUranium compoundsUranylReducing agent
The invention discloses a method for preparing uranous solution, which comprises the following steps of: adding an organic reducing agent and a catalyst into acidic solution of uranyl ions, and reacting under the action of the catalyst to reduce the uranyl ions into uranous ions. The method for preparing the uranous solution used in the nuclear fuel reprocessing industry is high in uranium conversion rate, easy and convenient to operate, mild in reaction conditions and high in prepared uranous concentration.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Method for efficiently removing and recovering uranium in water by utilizing titanium-based titanium dioxide nanotube array electrode
ActiveCN109867333AEfficient and fast removalEfficient and fast recyclingWater/sewage treatment using germicide/oligodynamic-processRadioactive decontaminationHigh concentrationOxygen
The invention discloses a method for efficiently removing and recovering uranium in water by utilizing a titanium-based titanium dioxide nanotube array electrode. The method adopts an anatase phase titanium dioxide nanotube array electrode and a stable good-conductivity material as a cathode and an anode respectively to construct an electrochemical reduction system, hexavalent uranium in a solution is subjected to electrochemical reduction to form tetravalent uranium by utilizing a stronger coordination effect of anatase phase titanium dioxide and uranyl ions in the absence of external additives, and the tetravalent uranium adheres to the electrode surfaces; and after electrochemical reduction enrichment is completed, a tetravalent uranium-rich anatase phase titanium dioxide nanotube arrayelectrode is taken out from the solution, so that high-efficiency reduction removal of the uranium in the wastewater, the groundwater and / or the seawater can be realized. The method provided by the invention has a wide application range, and can realize high-efficiency removal and recover of the uranium for the wastewater, the groundwater and the seawater containing a high concentration of dissolved oxygen, a high concentration of a carbonate and a low concentration of the uranium.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Method for extracting and separating uranyl ions from aqueous phase
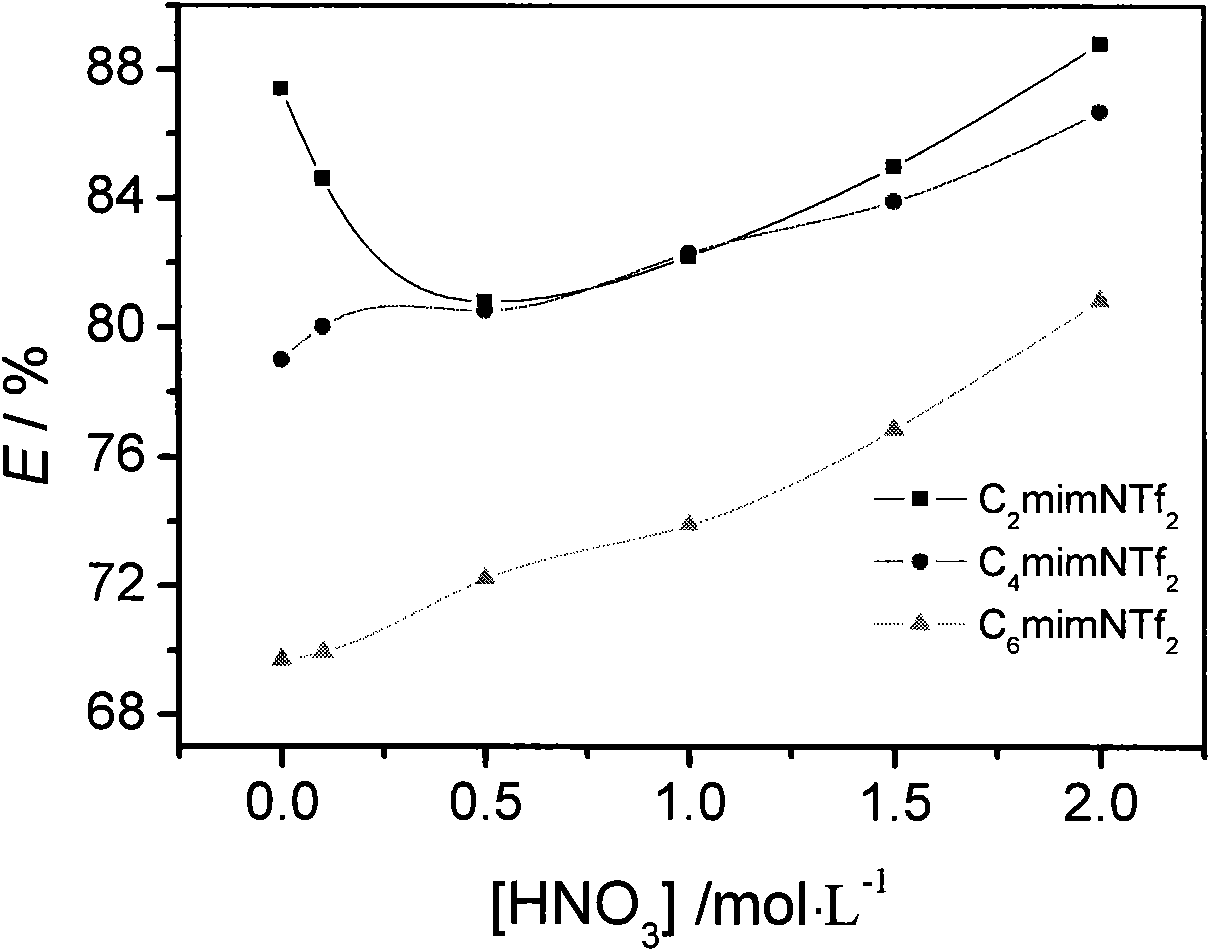
InactiveCN101638722AEfficient extraction performanceEasy extractionProcess efficiency improvementDiluentUranyl
The invention discloses a method for extracting and separating uranyl ions from an aqueous phase. In the method, hydrophobic ion liquid serves as a diluting agent and octyl phenyl group-N, N-diisobutylamine formacyl methylphosphineoxide serves as an extracting agent so as to extract uranyl ions from an aqueous solution containing the uranyl ions. The method can be used for efficiently extracting the cesium ions from the aqueous phase, and the extracting distribution ratio can reach more than 10<3> at most. The method has a wide applicable range to different aqueous phase acidity conditions, and can be used for extracting the cesium ions from neutral conditions to 3MHNO3; ion liquid serves as a diluting agent, and phenomena which are disadvantageous to technology such as emulsification in the process of extracting the cesium ions do not exist. Ion liquid almost has no volatility, thus the system is environmental-friendly. To sum up, the method of the invention has favorable applicationprospect in the field of nuclear fuel recycling.
Owner:PEKING UNIV
Method for extracting and separating uranyl ions from water phase containing zirconium ions and lanthanide ions
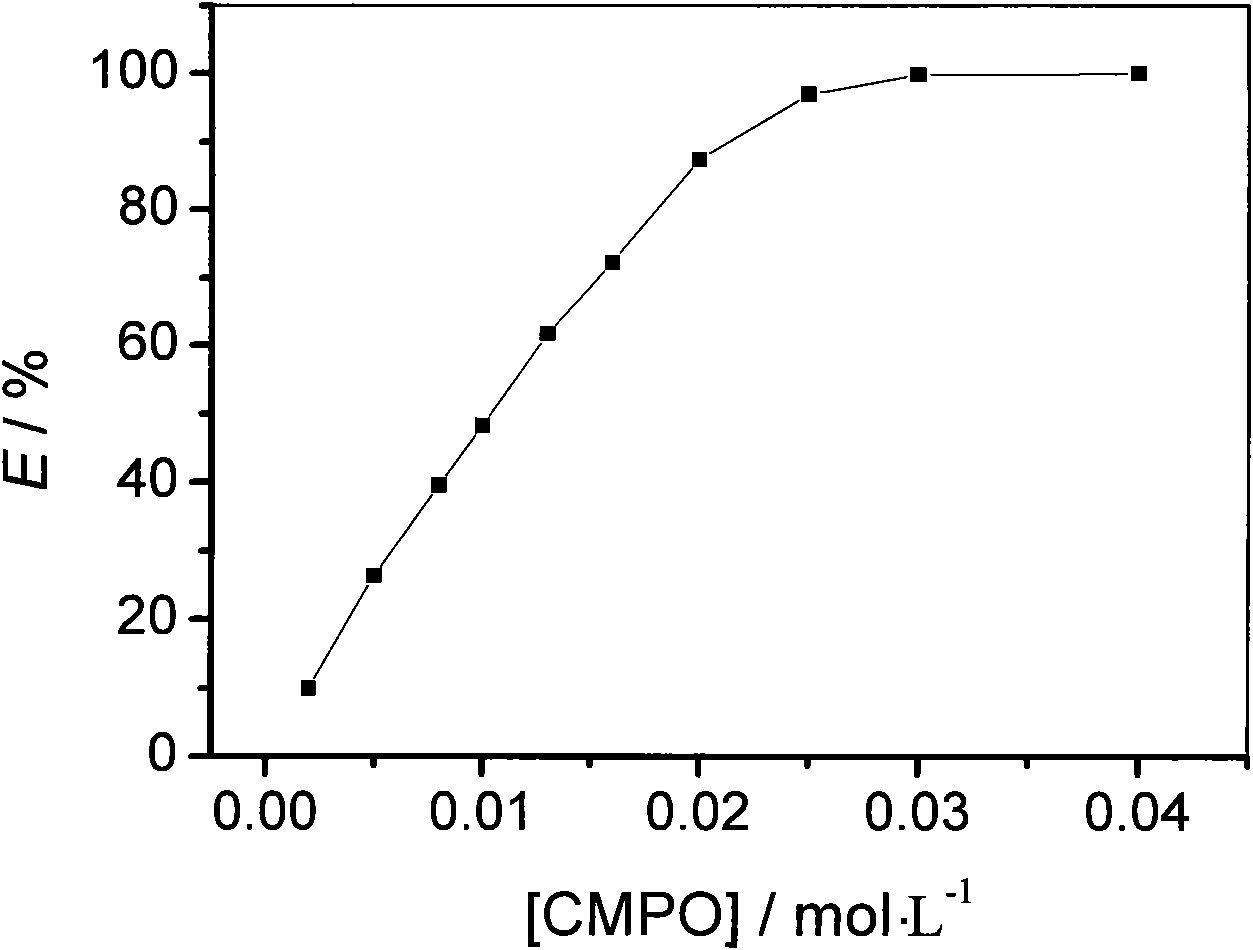
InactiveCN102776373AGreat selective extraction performanceEasy to separateProcess efficiency improvementLanthanideUranyl
The invention discloses a method for extracting and separating uranyl ions from a water phase containing zirconium ions and lanthanide ions. The method taking a hydrophobic ionic liquid as a diluent and taking octyl (phenyl)-N, N-diisobutyl carbonoyl methyl-phosphine oxide (CMPO) as an extracting agent comprises the following steps of: extracting and separating out the uranyl ions efficiently from the water phase containing the zirconium ion and / or the lanthanide ions, wherein the zirconium ions and the lanthanide ions are not extracted or are low in extracted ratio. The method is suitable for a large water phase acid degree range, and the uranyl ions can be selectively extracted from the moderate condition to 4mol / L of HNO3. The ion liquid nearly has volatility, and the using process is environment-friendly. The method provided by the invention has wide application prospect in the field of nuclear fuel cycle.
Owner:PEKING UNIV
Luminous metal organic framework material for uranyl ion detection in water and preparation method thereof
ActiveCN106632433ARealize quantitative detectionEasy to manufactureGroup 3/13 organic compounds without C-metal linkagesFluorescence/phosphorescenceFluorescenceMetal-organic framework
The invention provides a luminous metal organic framework material for uranyl ion detection in water and a preparation method thereof. Under the hydrothermal condition or solvent heat condition, the terbium metal iron and organic ligand molecular self-assembly is performed in an auxiliary way through auxiliary agents and pH regulators; a luminous terbium metal organic framework material is synthesized. A terbium metal organic framework provided by the invention has strong green fluorescence; the 491, 545, 585 and 621nm emitting peak intensity is gradually reduced along with the increase of uranyl ion concentration in the water solution; the emitting peak intensity in a position being 338nm can be gradually enhanced along the concentration increase of uranyl irons in the water solution; further, the fast, simple, convenient and high-selectivity detection on the uranyl irons can be realized.
Owner:DALIAN UNIV OF TECH
Pre-embedded bag for multi-stage remediation of uranium-contaminated soil and use method thereof
ActiveCN110369481AHigh organic contentImprove fertilityContaminated soil reclamationOrganic fertilisersEngineeringContamination
The invention discloses a pre-embedded bag for multi-stage remediation of uranium-contaminated soil and a use method thereof, and belongs to the technical field of uranium-contaminated soil remediation. The pre-embedded bag for multi-stage remediation of uranium-contaminated soil can be pre-embedded by a soil cleaning and per-embedding manner, on the one hand, uranyl ions in the soil are transferred through a strict water-conducting network, a large amount of uranyl ions are actively and quickly collected into the pre-embedded bag to facilitate fixed-point remediation, on the other hand, a special soil remediation agent is used to greatly improve adsorption and accumulation for uranyl ions, agglomeration of uranyl ions is improved based on nano porous properties, and uranyl ions are filledin micro-pores to avoid contamination and diffusion. After the active primary remediation, uranyl ions in the uranium-contaminated soil are reduced to a certain value, then natural secondary remediation, namely heavy metal uranium enrichment plant remediation, is followed, the soil organic matter content and soil fertility are increased, a soil surface structure is improved and maintained, and asolid foundation for direct use of the soil after uranium pollution remediation is laid.
Owner:绍兴市上虞区武汉理工大学高等研究院
Method of preparing a product based on phosphate of thorium and/or actinide(s)
The invention relates to a process for the preparation of a product based on a phosphate of at least one element M(IV), for example of thorium and / or of actinide(IV)(s).This process comprises the following stages:a) mixing a solution of thorium(IV) and / or of at least one actinide(IV) with a phosphoric acid solution in amounts such that the molar ratioPO4M(IV) is from 1.4 to 2,b) heating the mixture of the solutions in a closed container at a temperature of 50 to 250° C. in order to precipitate a product comprising a phosphate of at least one element M chosen from thorium(IV) and actinide(IV)s having a P / M molar ratio of 1.5, andc) separating the precipitated product from the solution.The precipitate can be converted to phosphate / diphosphate of thorium and of actinide(s). The process also applies to the separation of uranyl ions from other cations.
Owner:CENT NAT DE LA RECHERCHE SCI
Preparation method and application of carbon dot/mesoporous silica composite material
ActiveCN106669602AReduce energy consumptionExcellent fluorescence propertiesOther chemical processesFluorescence/phosphorescenceEthylenediamineMesoporous silica
The invention discloses a preparation method and application of a carbon dot / mesoporous silica composite material and belongs to the field of synthesis and application of fluorescent carbon nanomaterials and mesoporous composite materials. The method comprises the following steps: blending citric acid, ethanediamine, mesoporous silica and sodium nitrate into deionized water; carrying out ultrasonic dispersion and then putting in a reactor; carrying out normal pressure microplasma discharge processing; after discharging is ended, centrifugally separating, and washing and drying a solid to obtain the carbon dot / mesoporous silica composite material. The composite material is used for aspects of uranyl ion adsorption and adsorption process monitoring. The method has the advantages of quickness, simplicity and low energy consumption; the prepared carbon dot / mesoporous silica composite material has good fluorescent property and porous structure, and good adsorptive property to uranyl ions; in addition, the fluorescent property of the composite material is used for monitoring the adsorption process, and the visualization of the adsorption process is realized.
Owner:TSINGHUA UNIV
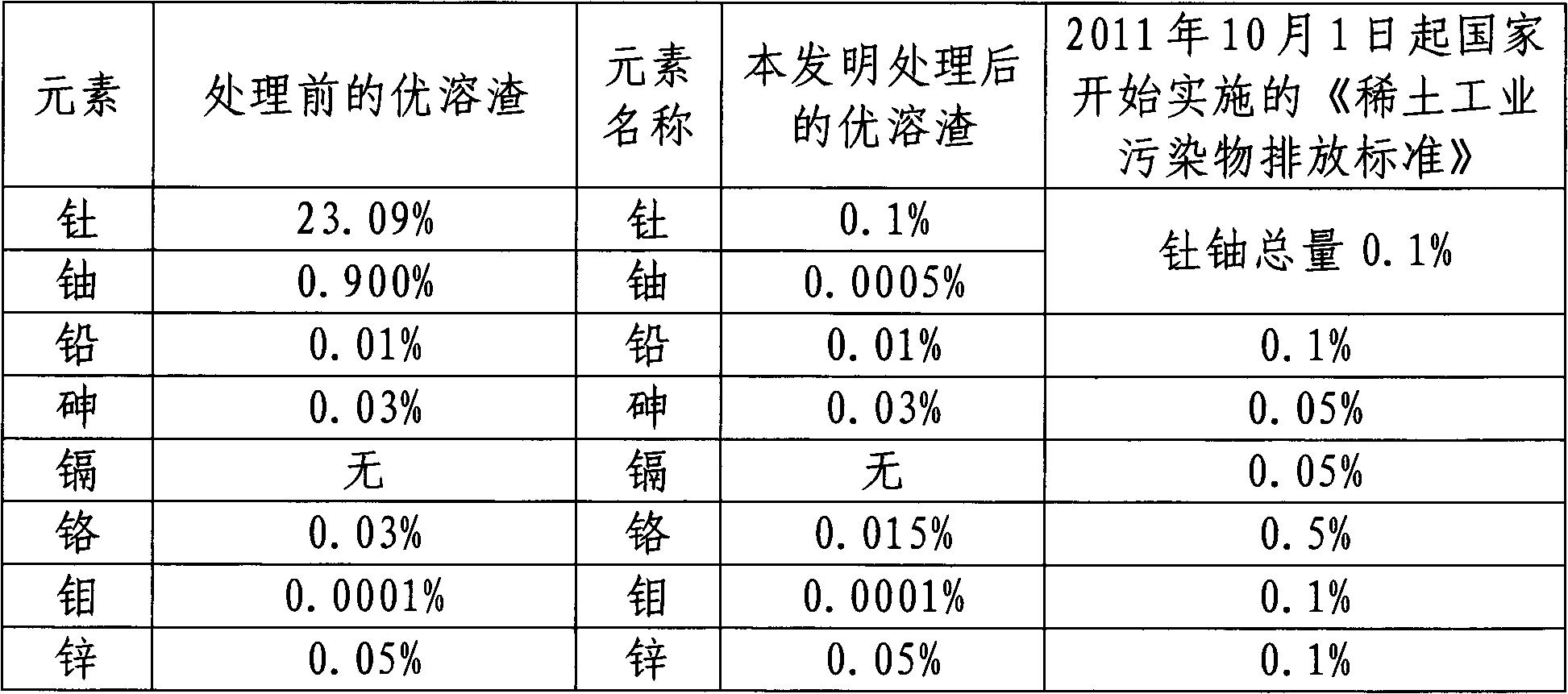
Method for separating and extracting uranium and thorium from superior molten slag
InactiveCN102154560ASimple processEasy to operateProcess efficiency improvementSeparation technologyRare earth
The invention relates to the field of hydrometallurgy, in particular to a method for separating enriched uranium and thorium from rare earth superior molten slag. In the method for separating the enriched uranium and thorium from the superior molten slag, disclosed by the invention, the technical scheme for performing alkaline leaching on the superior molten slag, performing acid leaching on precipitated uranium and separating precipitated thorium is adopted, so that the precipitate of uranyl and precipitate of thorium are obtained respectively. Due to the adoption of a separation technology of stepwise leaching and precipitating, complete separation of thorium and uranium is realized, the equipment and process flow are simple, the operation is convenient, raw material are readily available, and the environmental pollution is reduced.
Owner:王北华
Synthesis method and application of uranyl adsorbent material
ActiveCN107349919ALarge specific surface areaHigh physisorption poresOther chemical processesSeawater treatmentCross-linkSynthesis methods
The invention belongs to the field of adsorbent materials, and particularly relates to a synthesis method and an application of a uranyl adsorbent material. The provided synthesis method comprises the following steps: a) fibers, a polymer, a double-bond monomer and water are mixed and subjected to electron beam irradiation, and a gel body is obtained, wherein the polymer comprises one or more of polyvinyl alcohol, polyvinylpyrrolidone, polystyrene and polyacrylonitrile; the double-bond monomer comprises one or more of acrylonitrile, divinyl benzene, styrene, acrylic acid, acrylamide, butadiene, pentadiene and 1,3,5-tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione; b) the gel is mixed with acrylonitrile for electron beam irradiation, and a graft cross-linked body is obtained; c) the graft cross-linked body is mixed with the gel body for a reaction, and the uranyl adsorbent material is obtained. The provided synthesis method has the advantages that the cost is low, the adsorption capacity of the synthesized uranyl adsorbent material is high, the adsorptive selectivity for uranyl is good, and good use stability is realized.
Owner:CGN DASHENG ELECTRON ACCELERATOR TECH
Preparation of amidoxime modified magnetic nano biological adsorbent and method for adsorbing low-concentration uranium by utilizing amidoxime modified magnetic nano biological adsorbent
ActiveCN107537455AHigh adsorption rateThe adsorption rate is stableOther chemical processesAlkali metal oxides/hydroxidesCross-linkSorbent
The invention relates to preparation of an amidoxime modified magnetic nano biological adsorbent and a method for adsorbing low-concentration uranium by utilizing the amidoxime modified magnetic nanobiological adsorbent. The preparation comprises the following steps: modifying magnetic nano Fe3O4 by utilizing amidoxime; grafting the amidoxime modified nano Fe3O4 on aspergillus niger through cross-linking reaction to obtain an amidoxime modified magnetic nano Fe3O4-aspergillus niger biological adsorbent. Lone pair electrons on an electron-donating group of the amidoxime based adsorbent and uranyl ions can form a coordination bond or a stable structure, so that the adsorption speed and the adsorption capacity of the material on the uranyl ions can be effectively improved. The uranium compatible property of the aspergillus niger, the magnetic property of the nano Fe3O4 and the high selectivity and affinity of an amidoxime group on the uranium are combined and the aspergillus niger is a biological material, so that the amidoxime modified magnetic nano biological adsorbent has the advantages of good adsorption property, simplicity in operation, low production cost, low energy consumption, easiness for solid-liquid separation and the like; the product has a good application prospect in the fields of prevention and control of radioactive pollution and uranium cyclic utilization.
Owner:NANHUA UNIV
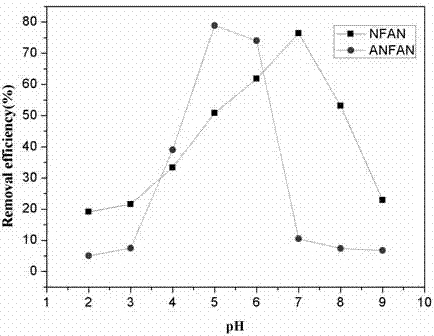
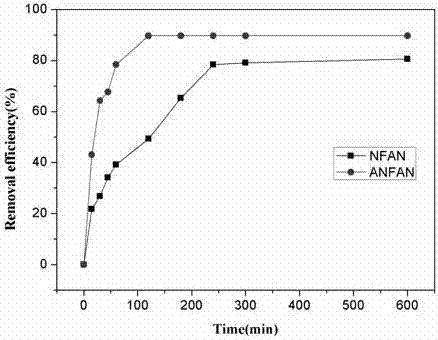
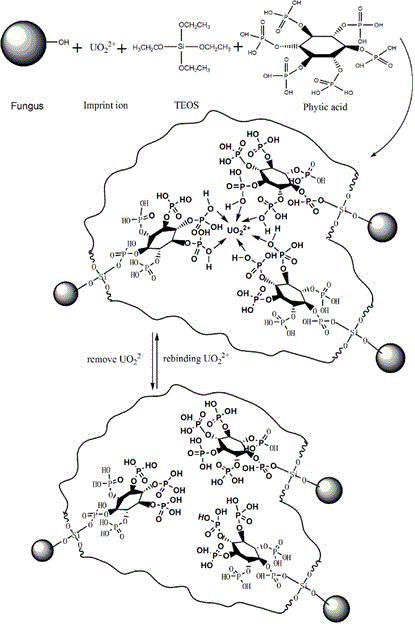
Method for remediating uranium contaminated water with imprinted material prepared from facultative marine fungus as matrix and phytic acid as functional monomer
InactiveCN106340337ASimple stepsSimple and fast operationOther chemical processesRadioactive decontaminationFunctional monomerMangrove
The invention relates to a method for remediating uranium contaminated water with an imprinted fungal material having high affinity and high recognition capability to uranyl ions. An uranium ion imprinted material is prepared by means of the sol-gel technique from a typical facultative marine mangrove endophytic fungus Fusarium sp.#ZZF51 as a matrix, phytic acid as a functional monomer, UO2<2+> as a template ion, and tetraethoxysilane (TEOS) as a cross-linking agent; then, the obtained material is thrown in uranium contaminated water to remediate the uranium contaminated water; and after a period of time, the material is retrieved and subjected to centralized treatment. The material proposed provided by the invention has high affinity and high recognition capability to uranyl ions, and may lead to a removal rate of uranyl ions of 90% or above 50-70 min later after being thrown in water. The imprinted fungal material is in the form of spherical granules, and is low in cost, high in remediation efficiency, environmentally friendly without secondary pollution, and relatively simple and convenient in retrieval and centralized treatment.
Owner:NANHUA UNIV
Meso-porous silica particle supported amidoxime polymer uranium-absorbing material and preparation method thereof
ActiveCN109954484ARegular porous structureLarge specific surface areaUranium compounds preparationOther chemical processesSilica particleHydroxylamine Hydrochloride
The invention provides a meso-porous silica particle supported amidoxime polymer uranium-absorbing material and a preparation method thereof. The method comprises the following steps: 1, dissolving polyacrylonitrile and a pore forming agent in a solvent to prepare a polyacrylonitrile solution, coating the surface of meso-porous silica gel particles with the polyacrylonitrile solution by a negativepressure osmosis process, and carrying out phase separation and drying to prepare meso-porous silica particles with polyacrylonitrile supported on the surface; and 2, placing and sealing the meso-porous silica particles prepared in step 1 in a hydroxylamine hydrochloride solution, carrying out an amidoximation reaction, and washing and drying the obtained reaction product after the reaction is completed in order to obtain the meso-porous silica particle supported amidoxime polymer uranium-absorbing material. The meso-porous silica particle supported amidoxime polymer uranium-absorbing material has the advantages of regular porous structure, large specific surface area and good structural stability, and can effectively adsorb uranyl ions in water. The preparation method of the material hasthe advantages of simplicity, low cost, realization of large-scale production, and broad application prospect.
Owner:HARBIN ENG UNIV
Method for removing and recovering uranium in water and achieving synchronous electricity generation by utilizing microbial fuel cell
ActiveCN109942076AReduce consumptionCan be reused for a long timeSeawater treatmentBiochemical fuel cellsClean energySeawater
The method discloses a method for removing and recovering uranium in water and achieving synchronous electricity generation by utilizing a microbial fuel cell. The method comprises the steps that an array electrode of a ti-based titanium dioxide nanotube is taken as a negative electrode, a carbon material where microorganisms grow is taken as a positive electrode, hexavalent uranyl ions can obtainthe characteristic that electrons are subjected to reduction to obtain uranium dioxide, and the uranium dioxide is deposited on the surfaces of the electrodes, and the characteristic is utilized formaking hexavalent uranium subjected to reduction to obtain uranium dioxide gathering on the surfaces of the electrodes. After microbial electrochemical reduction and gathering are completed, the electrodes with enrichment of the uranium dioxide are taken from a solution, and efficient reduction removal of the uranium in waste water, underground water and seawater can be achieved. In the process ofreduction removal, chemical energy contained in organic matter in a positive electrode chamber and water containing the uranium in a negative electrode chamber can be converted into electric energy,and clean energy production is achieved. The method is wide in application range and can achieve efficient removal and recovery of the uranium in the waste water containing the uranium of different concentrations and carbonate of different concentrations, the underground water and the seawater and synchronous electricity generation.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Method for adsorbing uranyl in waste water with silicon dioxide composite
ActiveCN106710659AImprove adsorption capacityEasy to recycleRadioactive decontaminationSorbentUranyl
The invention discloses a method for adsorbing uranyl in waste water with a silicon dioxide composite. The method comprises the following steps that 1, a silicon dioxide material is utilized for carrying out normal pressure micro plasma discharging treatment, and therefore an adsorbent is prepared; 2, the waste water containing uranyl is adjusted till the pH ranges from 4 to 6.8, the adsorbent is put in the waste water, stirring and adsorbing are carried out for 36 h to 48 h, after filtering, filtrate is centrifugally treated to further separate solids in the waste water, and the solid particles are collected. The method is easy to operate, high in adsorption efficiency and good in waste water treatment effect, the adsorption effect can be monitored in real time, and good industrial practical prospects are achieved.
Owner:江西城宏实业有限公司
Preparation method of magnetic core-shell type nanoparticle surface uranyl molecularly imprinted polymer
InactiveCN106750316AImprove hydrophilicityImprove extraction efficiencyOther chemical processesRadioactive contaminantsSuperparamagnetismUranyl
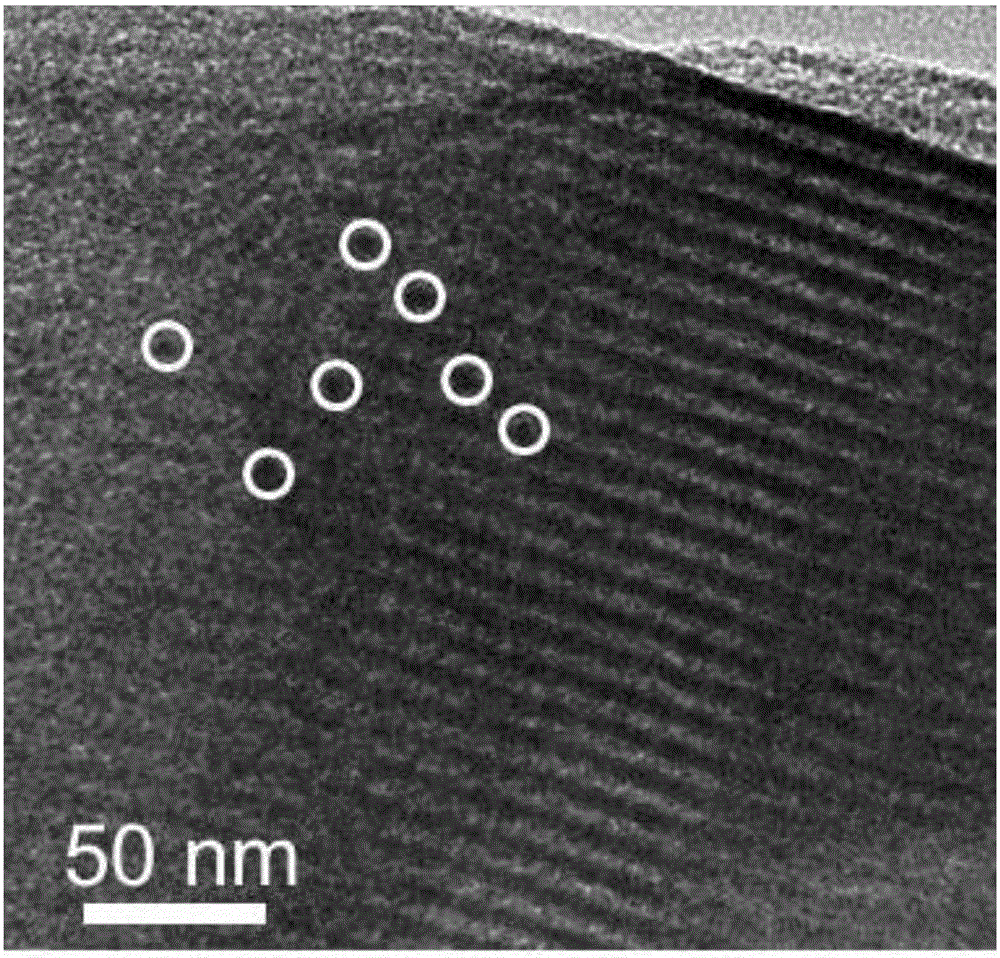
The invention relates to a preparation method of a magnetic core-shell type surface uranyl molecularly imprinted polymer nanoparticle. The preparation method comprises the following steps of firstly, using a precipitation method to prepare magnetic Fe3O4 nanoparticles; polymerizing an SO2 outer shell on the surface of a magnetic particle, so as to obtain an Fe3O4 core and SO2 shell nanoparticle; using UO22+-Salophen as a template molecule, and adopting a sol-gel method to prepare the uranyl molecularly imprinted polymer particle product. The uranyl molecularly imprinted polymer particle product has the advantages that the water dispersion property is good, and the magnetic response is strong; the diameter of the nanoparticles is 30 to 108mm, and the superparamagnetism is realized; the good identification, adsorption, separation, enriching and purification functions on uranium in complicated samples are realized; the separation efficiency is high, the selectivity is good, and the operation is simple and convenient; the application prospect is broad in the fields of detection of uranium in water, treatment of uranium-polluted water, extraction of uranium in seawater, and the like.
Owner:NANHUA UNIV
Intelligent photonic crystal material for simultaneously monitoring and removing uranyl ions
ActiveCN107262079AHigh sensitivityGood choiceOther chemical processesAlkali metal oxides/hydroxidesPhotonic crystalSorbent
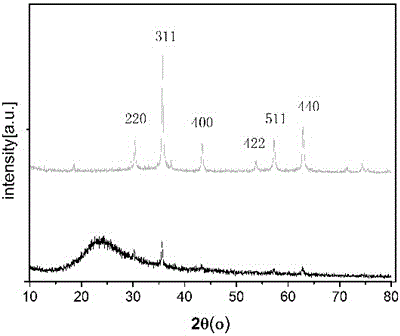
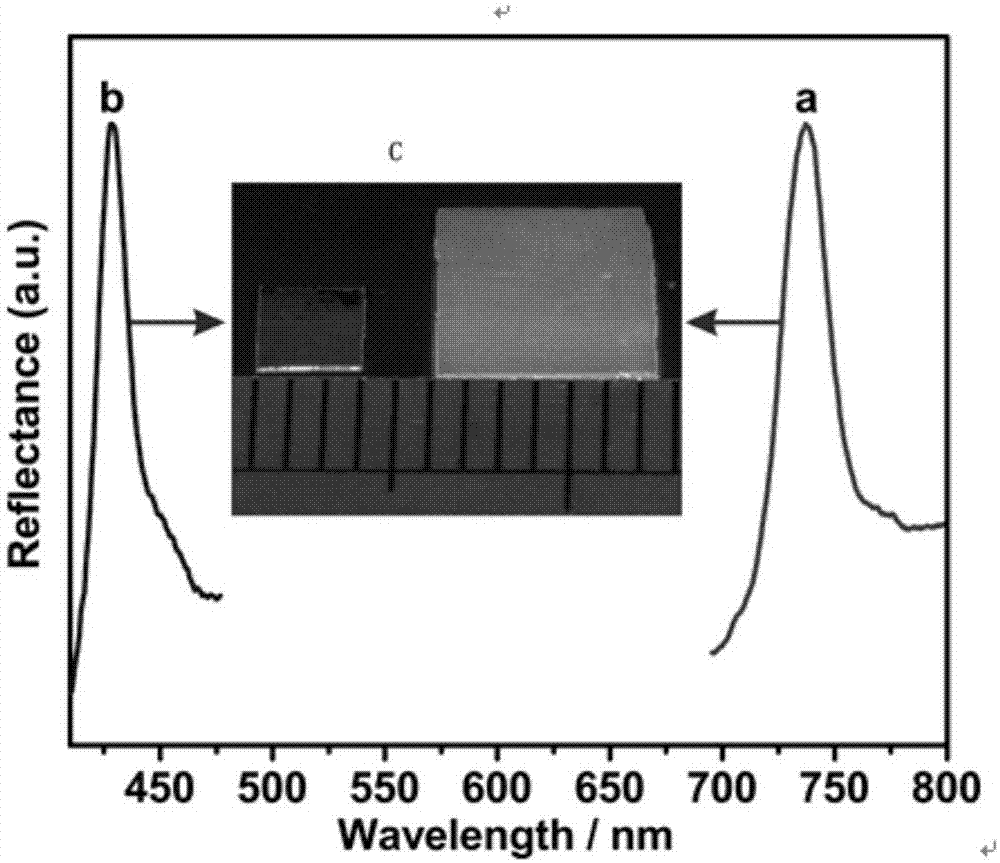
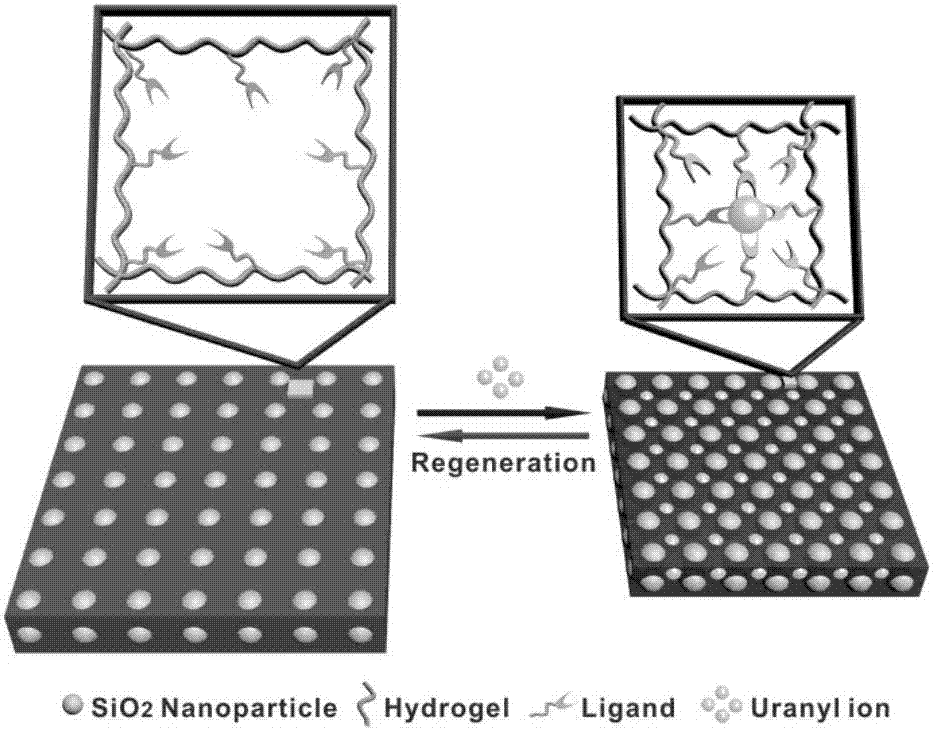
The invention provides an intelligent photonic crystal material for simultaneously monitoring and removing uranyl ions. The material is synthesized by steps of synthesis and purification of monodisperse SiO2 nanoparticles, preparation of a photonic crystal precursor solution, manufacturing of a photonic crystal hydrogel material forming mold and preparation of hydrogel, wherein the prepared intelligent photonic crystal materials can be used for monitoring and removing uranyl ions in water. A signal indicating function of the intelligent photonic crystal material and an adsorption and removal performance of the hydrogel on the uranyl ions of the invention successfully solve the problems that a traditional adsorption material cannot detect the concentration of UO2<2+> in the solution in situ and display an adsorption degree of an adsorbent in real time, show high adsorption capacity on the removal of UO2<2+>, has the lowest detection concentration of UO2<2+> of 10 nM, and meet requirements on detection sensitivity in practical application.
Owner:HUNAN UNIV
Non-reagent ground dipping uranium extracting process flow
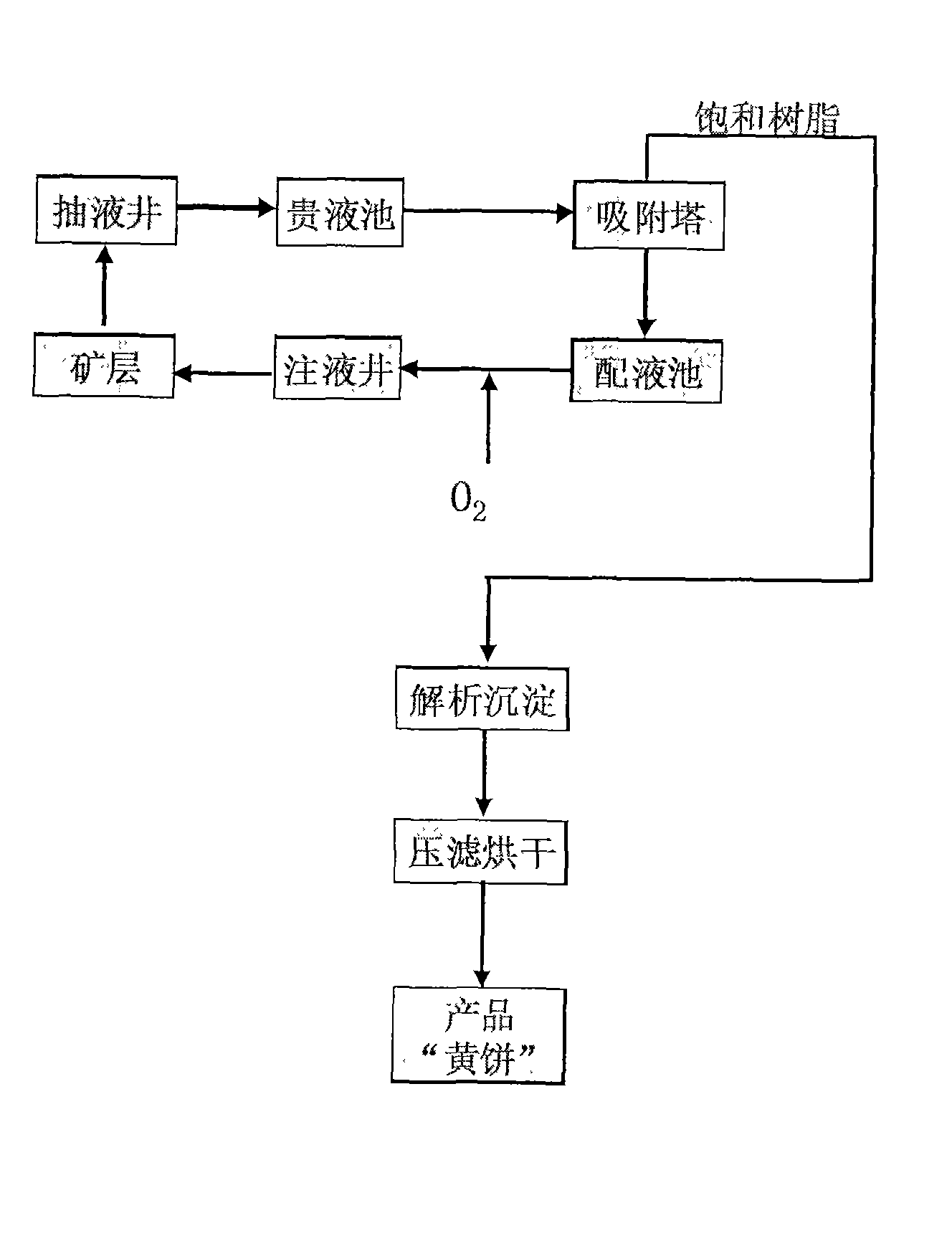
InactiveCN101435320AGuarantee continuous and stable operationReduce supplementationFluid removalIon exchangeMaterials science
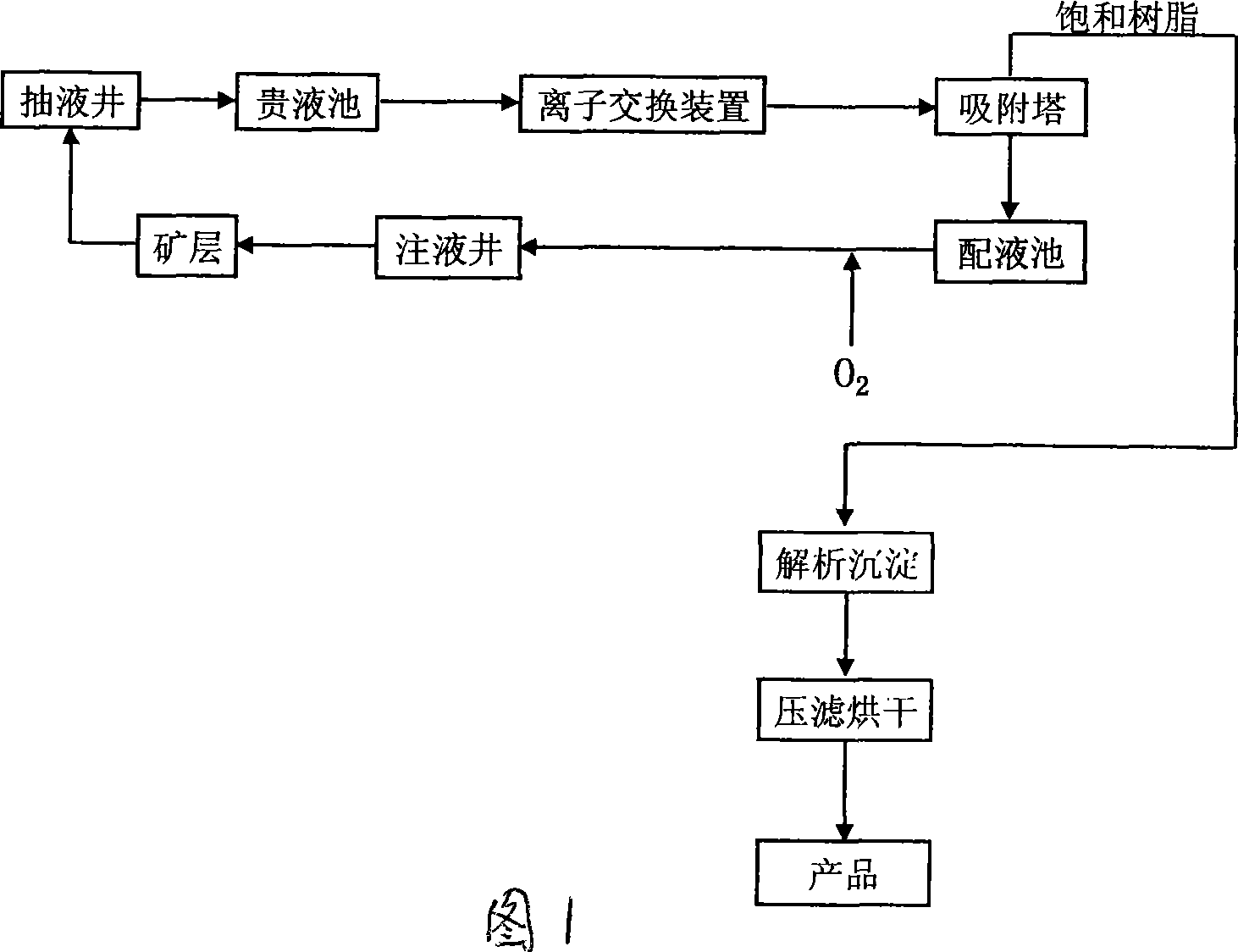
The invention relates to a reagent-free in-situ leaching uranium mining technological process which is additionally provided with a set of ion interchange unit consisting of an ion exchanger, a blast blower, a sand filtering tank, a regenerant tank and a waste liquid pool before a medium entering an absorption tower on the basis of the conventional reagent-free in-situ leaching uranium mining technological process. Leaching solution containing an oxidizing agent is injected into an ore bed through an injection hole so that the uranium in the ore bed is changed into the form of uranyl ions or other complex icons which are dissolved in leaching solution groundwater, then leaching solution containing uranium components is extracted to ground through an extracting hole, the leaching solution is injected back to the ore bed for recycling after carrying out sedimentation, icon exchange treatment and adsorption, and a uranate product is obtained after carrying out the procedures of analyzing, sedimentation, filter pressing and the like to saturated resin containing uranium. The method has the advantages of little investment, mature operative technique, obvious anti-blocking effect and the like. The utilizing of the process method can guarantee the continuous and stable running of uranium mining wells and a production system, and improve the production time efficiency and the product output.
Owner:LIAOHE GASOLINEEUM EXPLORATION BUREAU
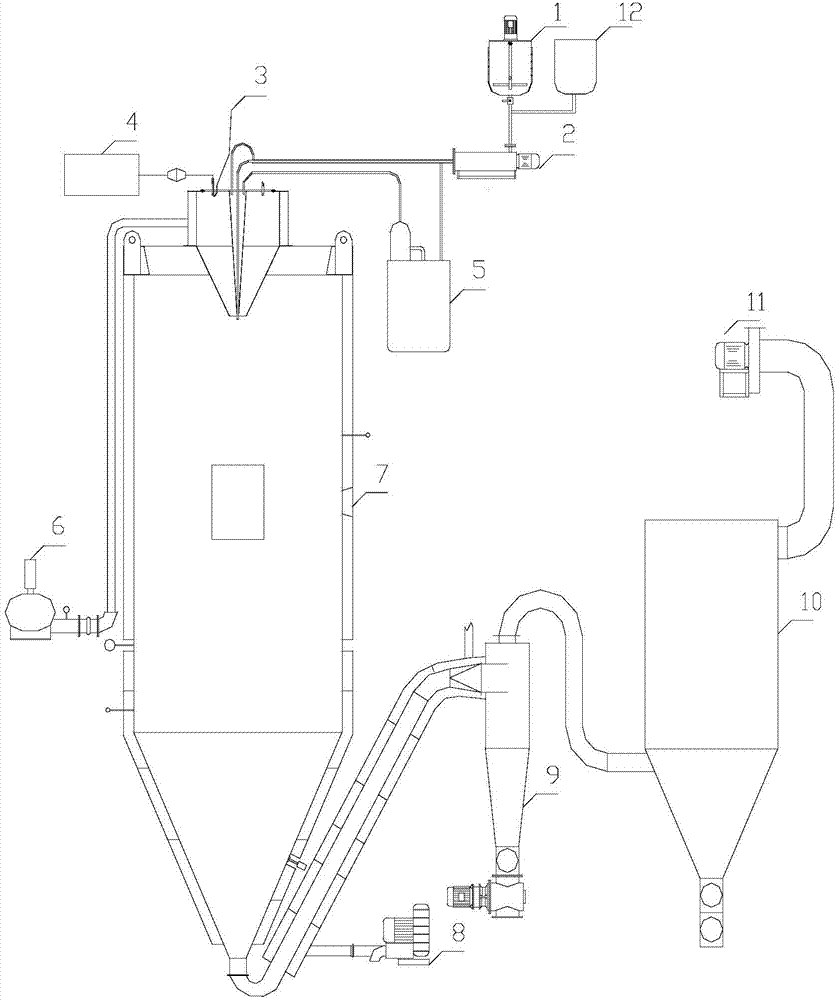
Process for preparing UO3 through uranyl nitrate air-blast atomization dry pyrolysis denitration
InactiveCN107162060AImprove conversion rateShort processUranium oxides/hydroxidesChemical reactionCombustion chamber
The invention discloses a process for preparing UO3 through uranyl nitrate air-blast atomization dry pyrolysis denitration. According to the process, a designed and processed combustion chamber and a denitration reactor are adopted as main body equipment of uranyl nitrate pyrolysis denitration, a high-temperature high-speed gas generated from combustion of propane and excessive air is adopted as a heat source and an atomization gas source, and a uranyl nitrate liquid is subjected to drying dehydration and pyrolysis denitration. The process has the advantages of being short in process procedure, simple in equipment structure, stable in operation and high in UNH (Uranyl-Nitrate Hexahydrate) conversion rate, the prepared UO3 is uniform in granularity distribution and good in chemical reaction activity, and the like.
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
Preparation method of nuclear power (U, Ti) O2 mixing ceramic fuel pellet
ActiveCN103474114ALower sintering temperatureMicroorganizedNuclear energy generationReactors manufactureNuclear powerTitanium oxide
The invention discloses a preparation method of nuclear power (U, Ti) O2 mixing ceramic fuel pellet, wherein acid-lacking nitric acid uranyl solution, nanometer titanium oxide, urea and polypropylene glycol are taken as raw materials; sol preparation, heating and solidification in a crucible, drying, calcining, powdering, reduction and sintering are performed to obtain the (U, Ti) O2 nuclear fuel pellet. The prepared (U, Ti) O2 nuclear fuel pellet has the advantages of low sintering temperature, excellent microstructure, higher mechanical strength density and the like. The technological process comprises solution mixture preparation, heating and solidification in the crucible, drying, calcining, powdering, reduction, sintering and metallographic erosion.
Owner:NUCLEAR POWER INSTITUTE OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com