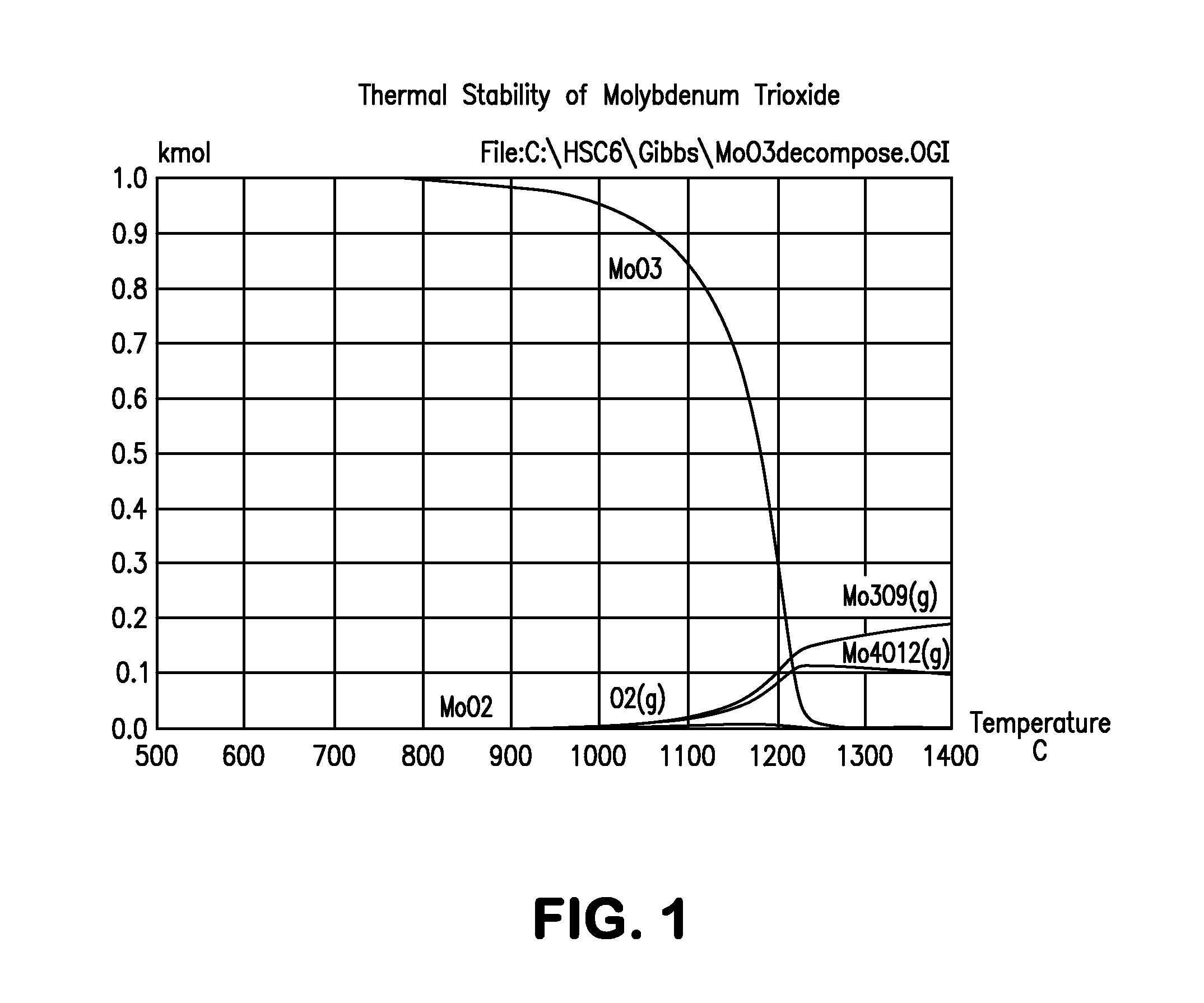
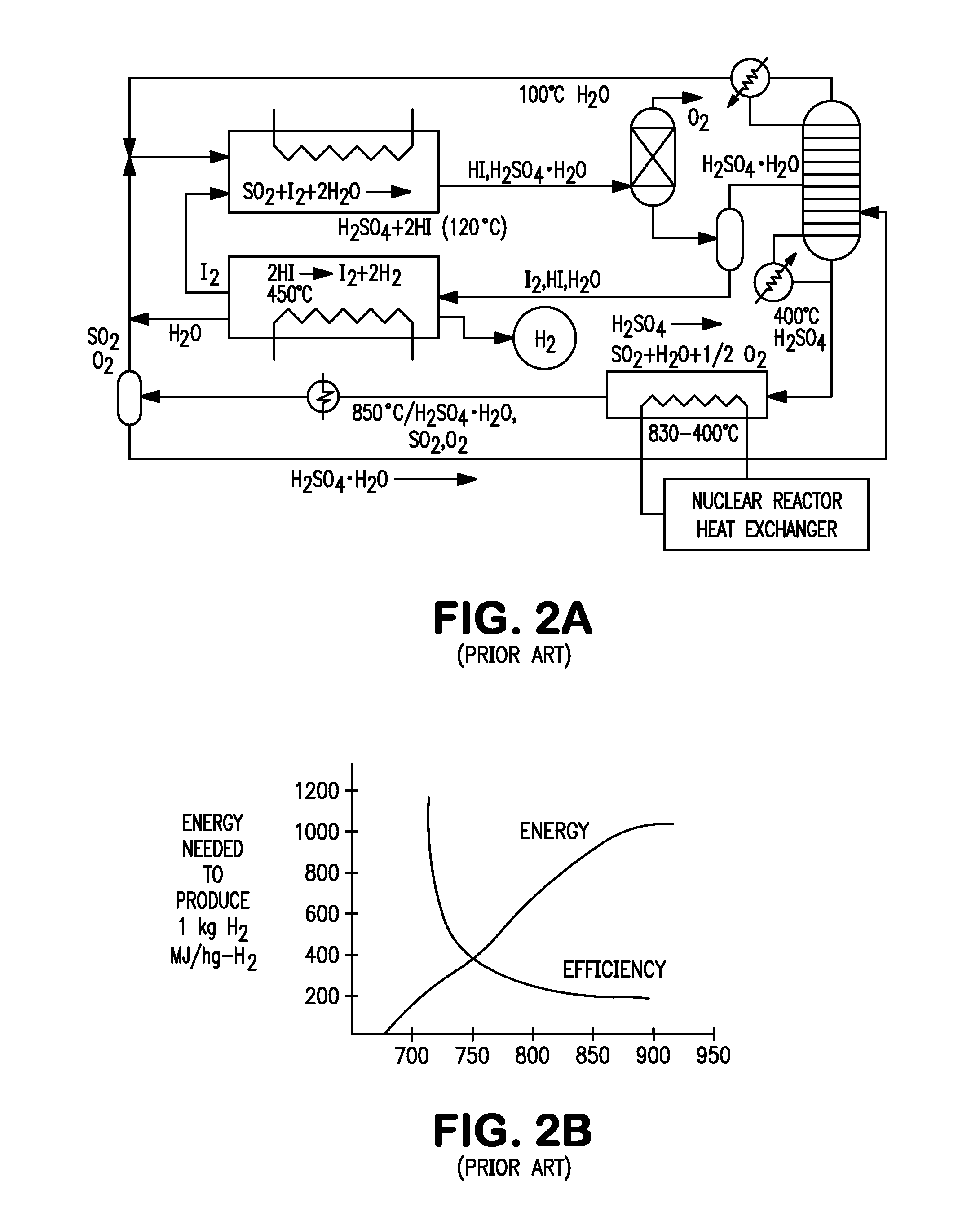
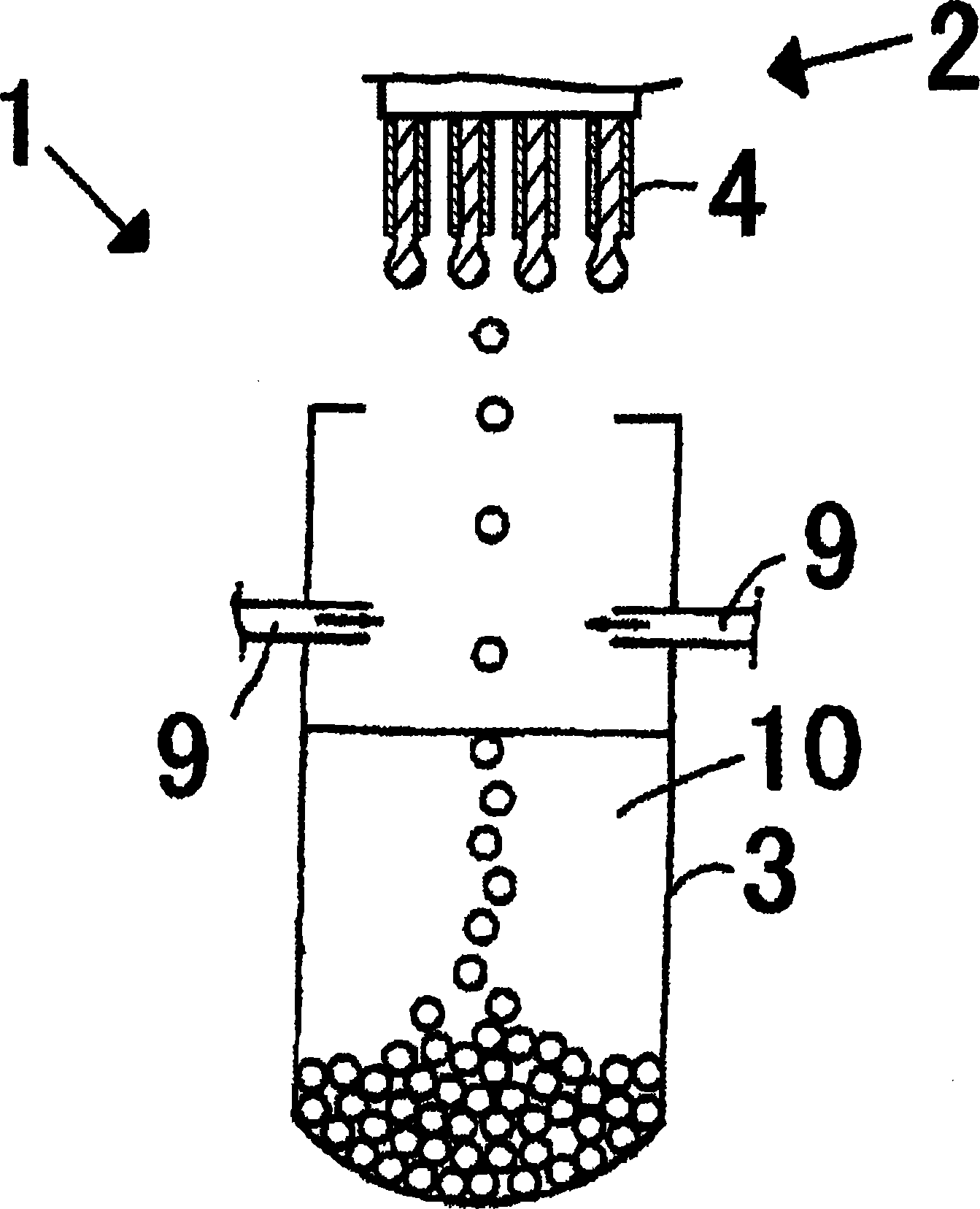
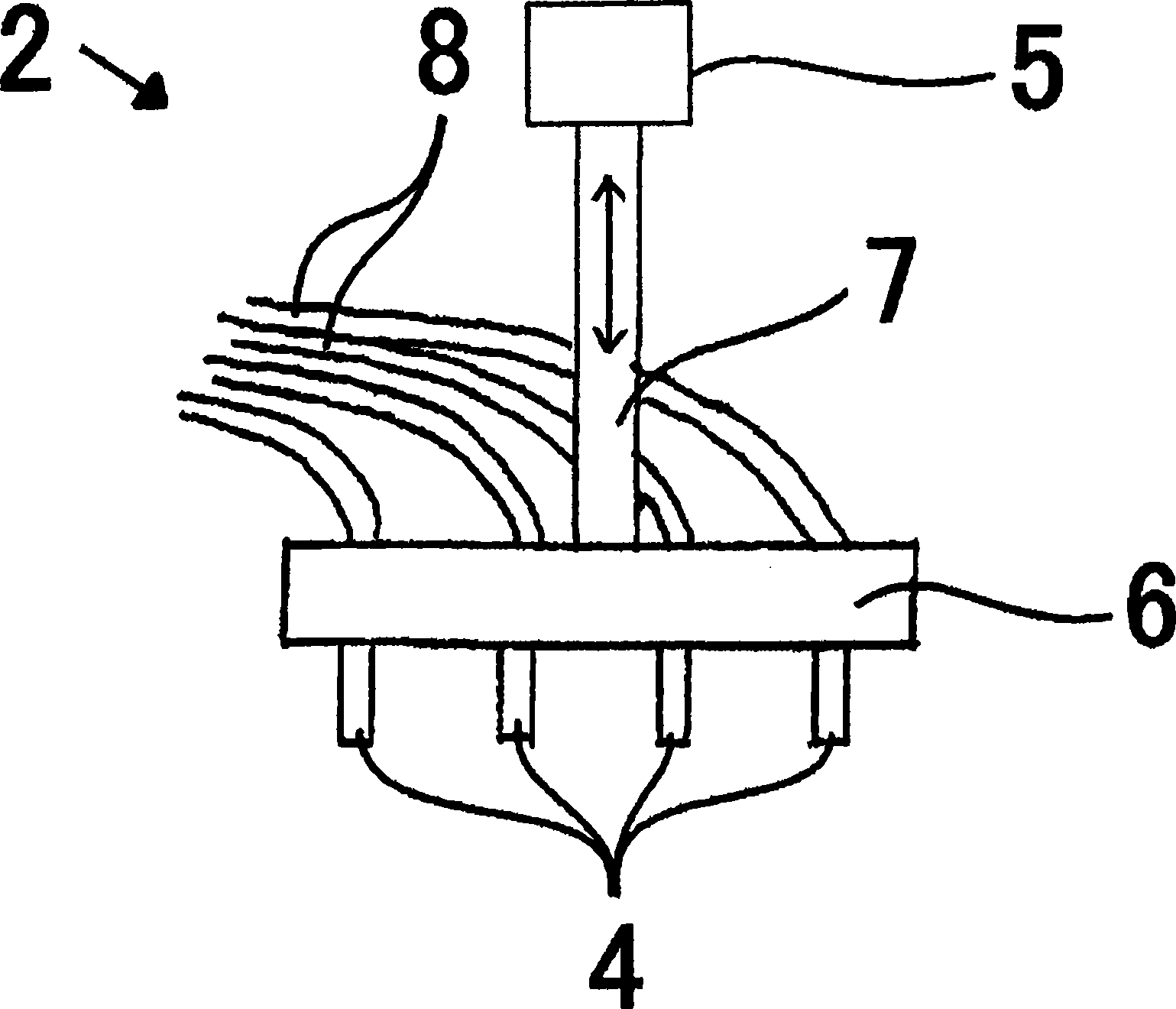
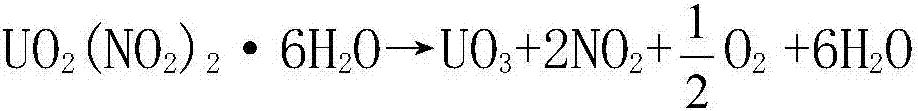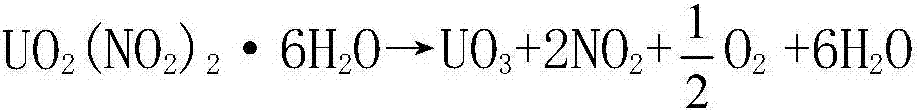Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81results about "Uranium oxides/hydroxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
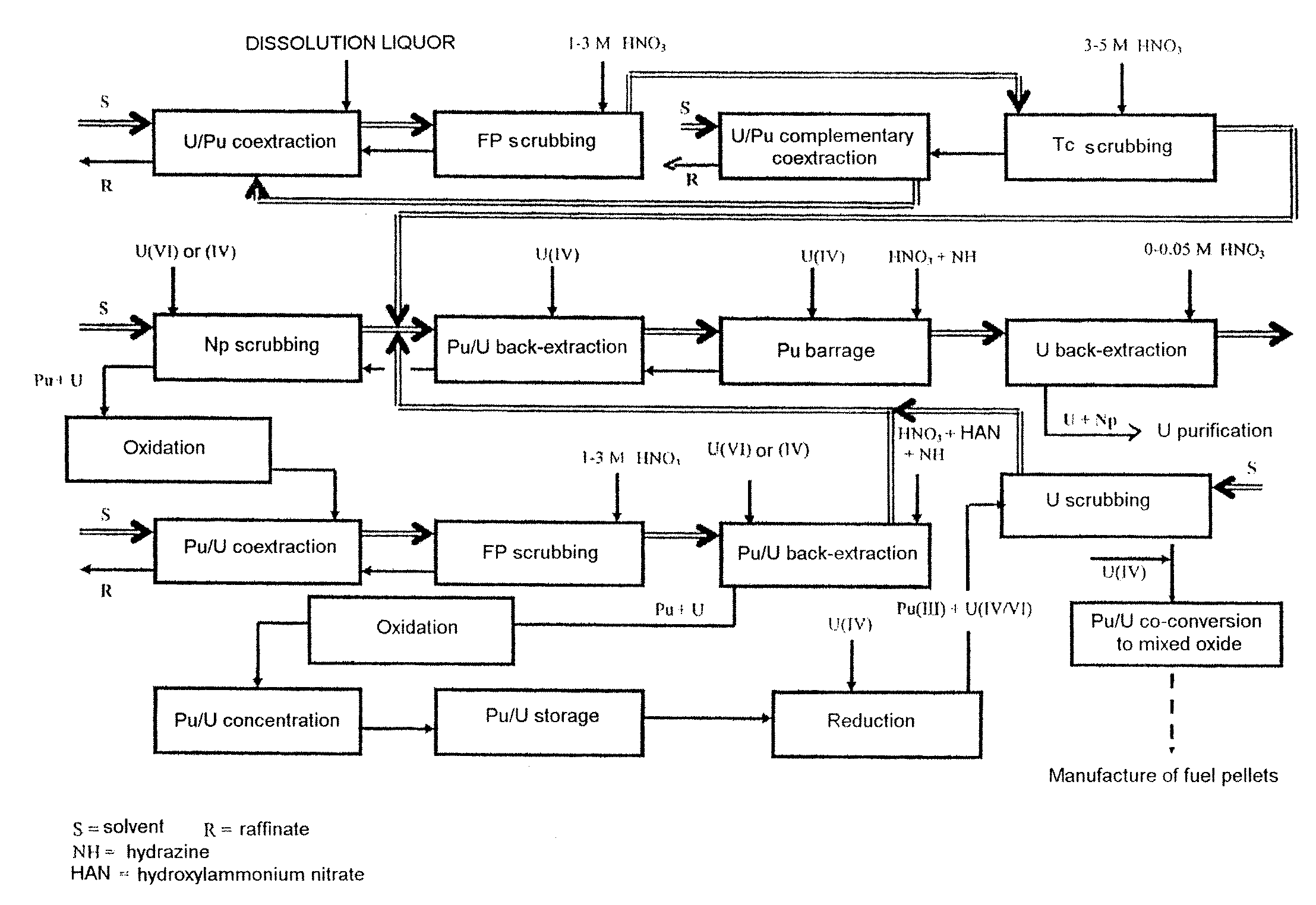
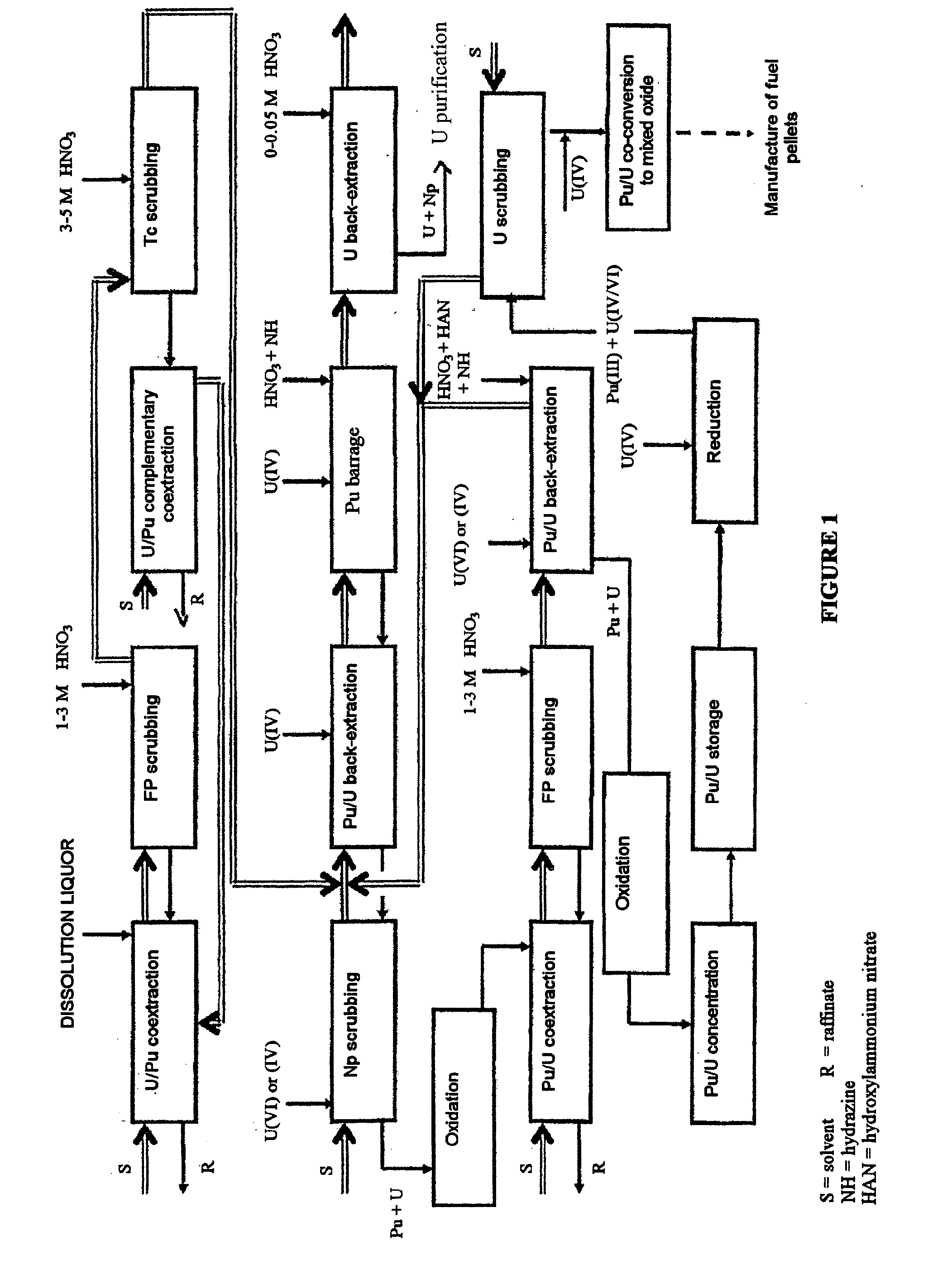
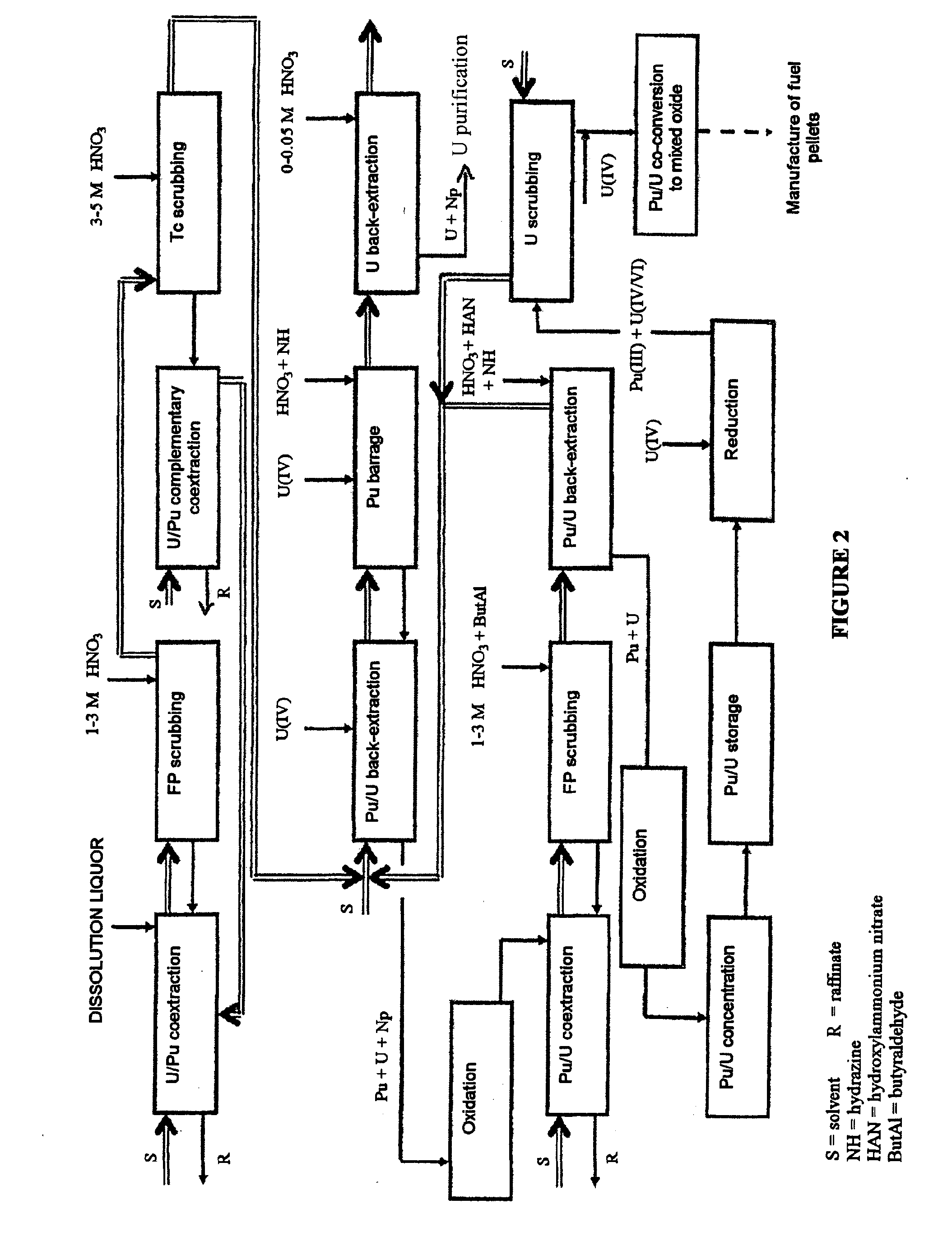
Process for reprocessing a spent nuclear fuel and of preparing a mixed uranium-plutonium oxide
ActiveUS20070290178A1Risk minimizationUranium compounds preparationSolvent extractionUranium oxideDissolution
The invention relates to a process for reprocessing a spent nuclear fuel and for preparing a mixed uranium-plutonium oxide, which process comprises: a) the separation of the uranium and plutonium from the fission products, the americium and the curium that are present in an aqueous nitric solution resulting from the dissolution of the fuel in nitric acid, this step including at least one operation of coextracting the uranium and plutonium from said solution by a solvent phase; b) the partition of the coextracted uranium and plutonium to a first aqueous phase containing plutonium and uranium, and a second aqueous phase containing uranium but no plutonium; c) the purification of the plutonium and uranium that are present in the first aqueous phase; and d) a step of coconverting the plutonium and uranium to a mixed uranium / plutonium oxide. Applications: reprocessing of nuclear fuels based on uranium oxide or on mixed uranium-plutonium oxide.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +2
Extraction of uranium from wet-process phosphoric acid
ActiveUS20100028226A1Lower iron levelsReduced valencyCobalt ammonia complexesPhotography auxillary processesFiltrationIon exchange
A process for the extraction of uranium compounds from wet-process phosphoric acid includes lowering the iron concentration of the wet-process phosphoric acid and reducing the valency of any remaining ferric iron in the wet-process phosphoric acid to ferrous iron, and then extracting uranium compounds from the wet-process phosphoric acid. The process can include separating a side stream from a feed stream of wet-process phosphoric acid, wherein the side stream has a greater concentration of the uranium compounds than the feed stream by filtration. Extracting uranium compounds from the wet-process phosphoric acid can be by ion exchange process or by solvent extraction.
Owner:URTEK
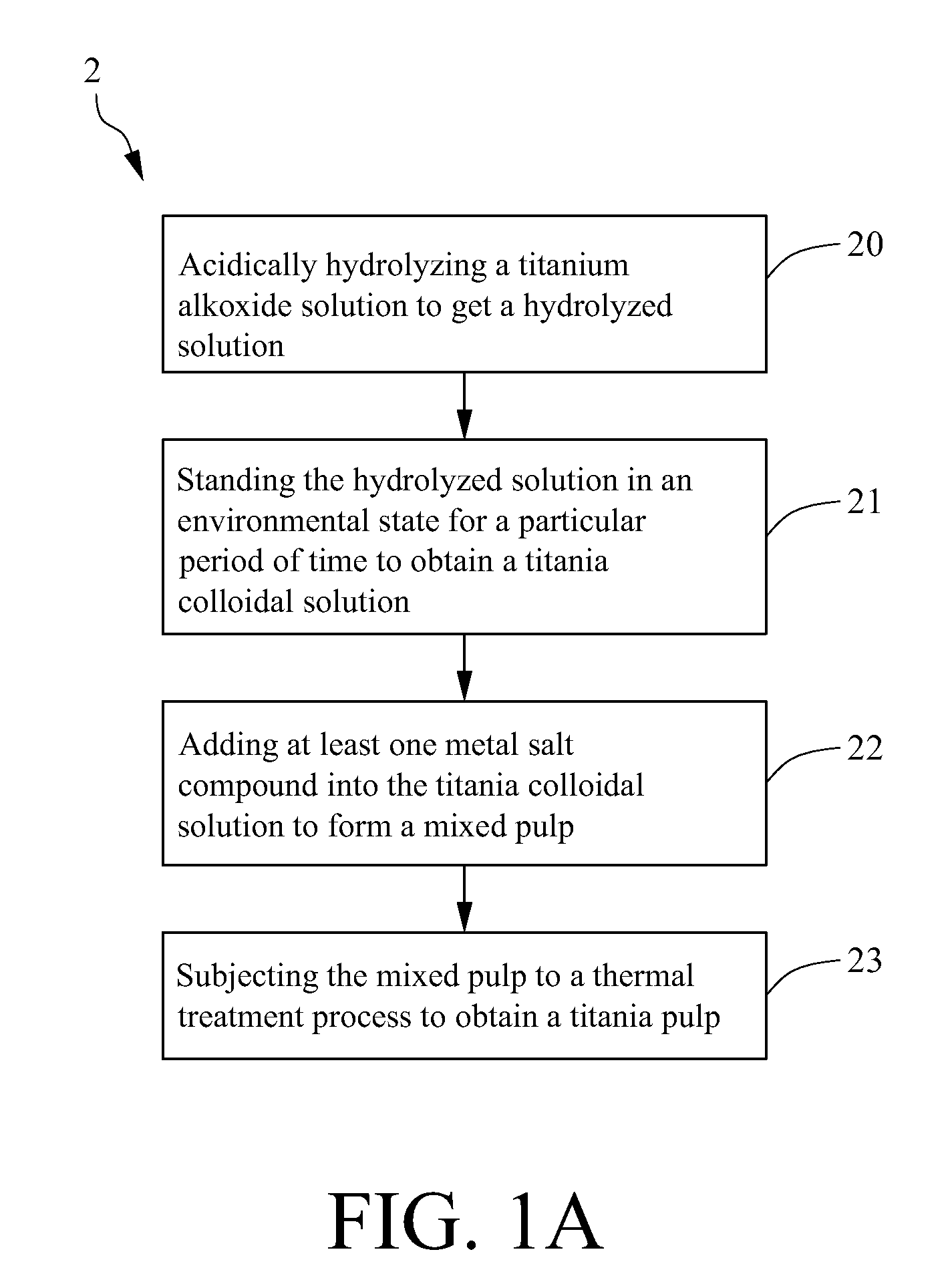
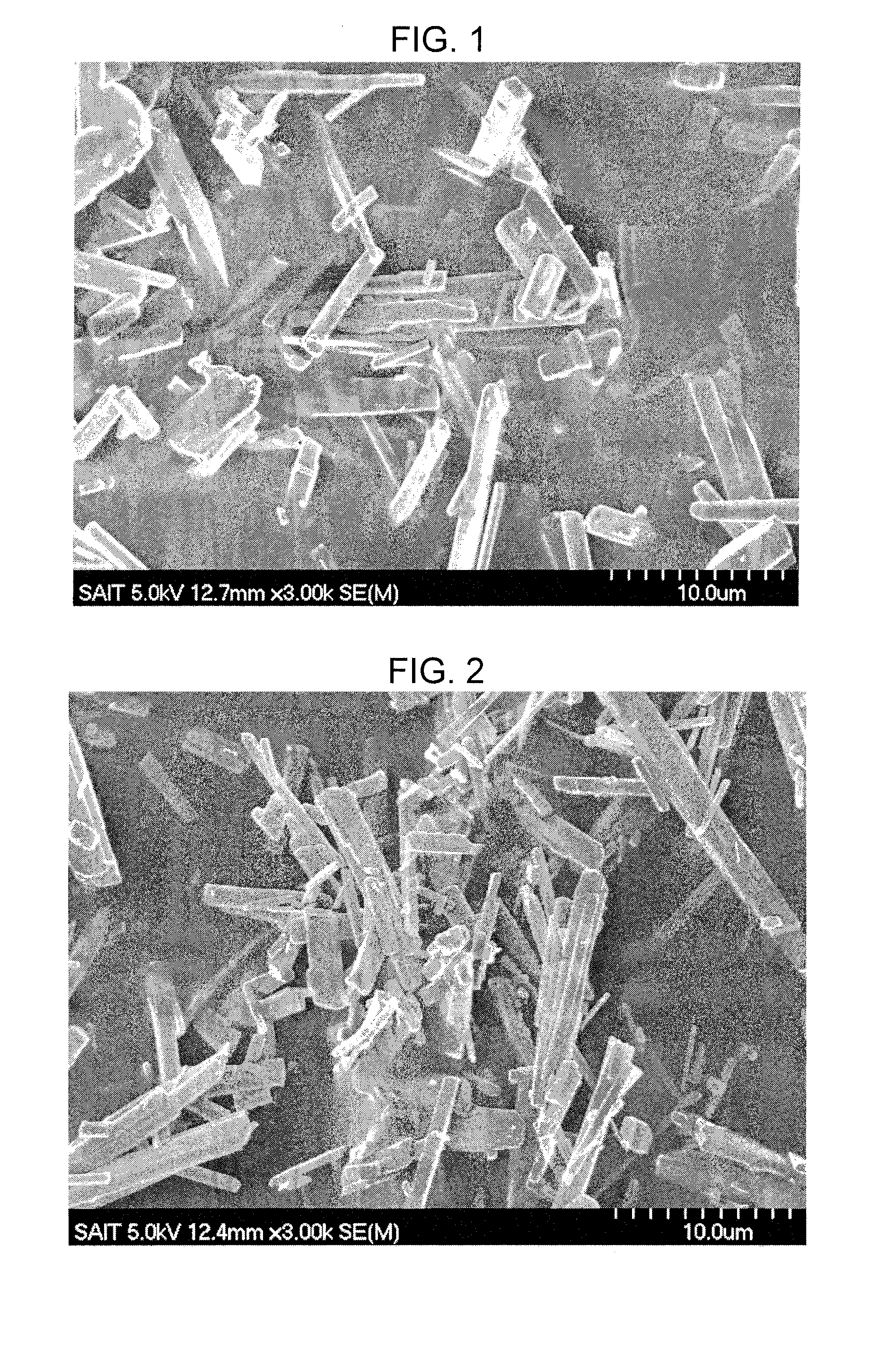
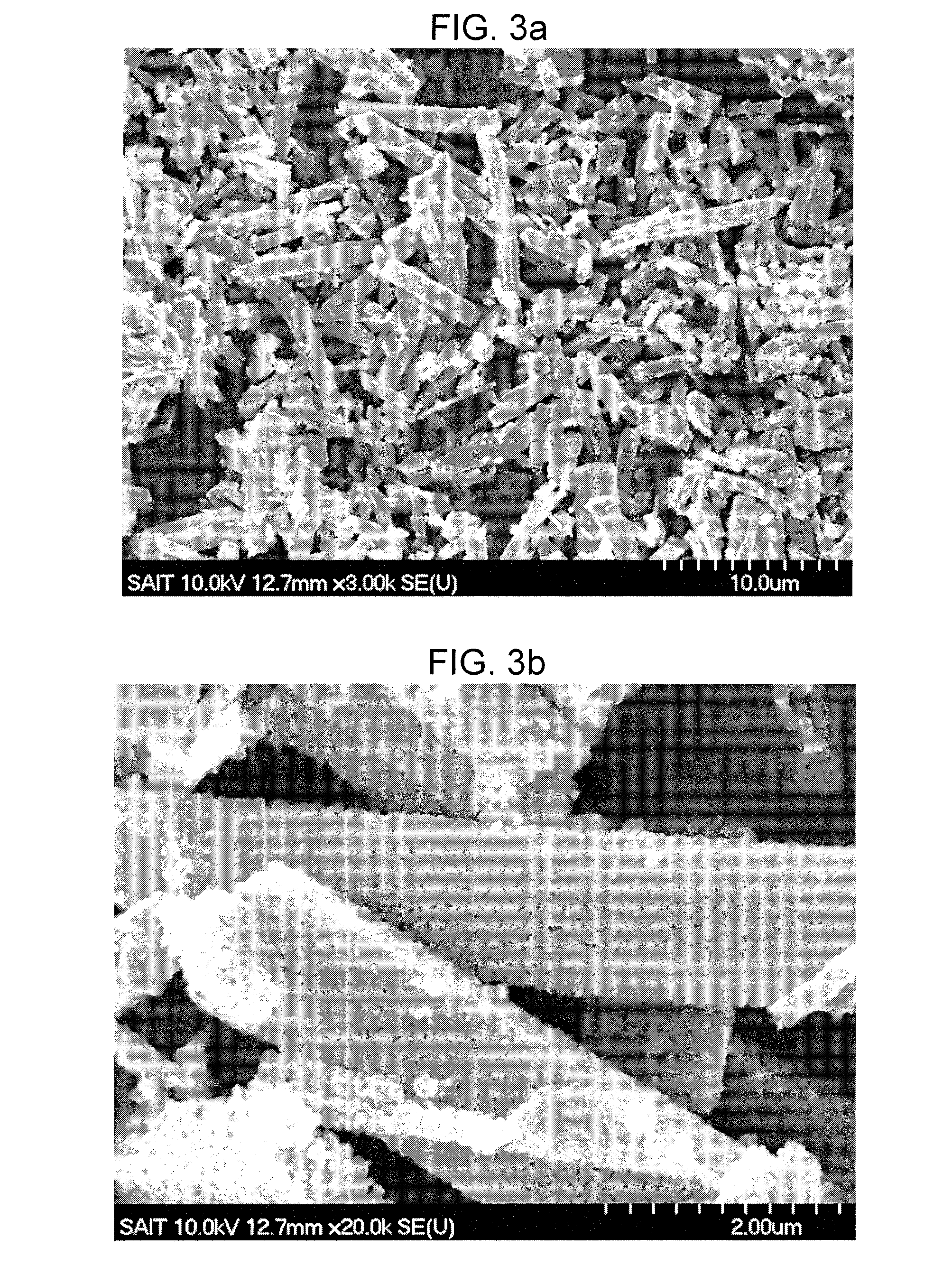
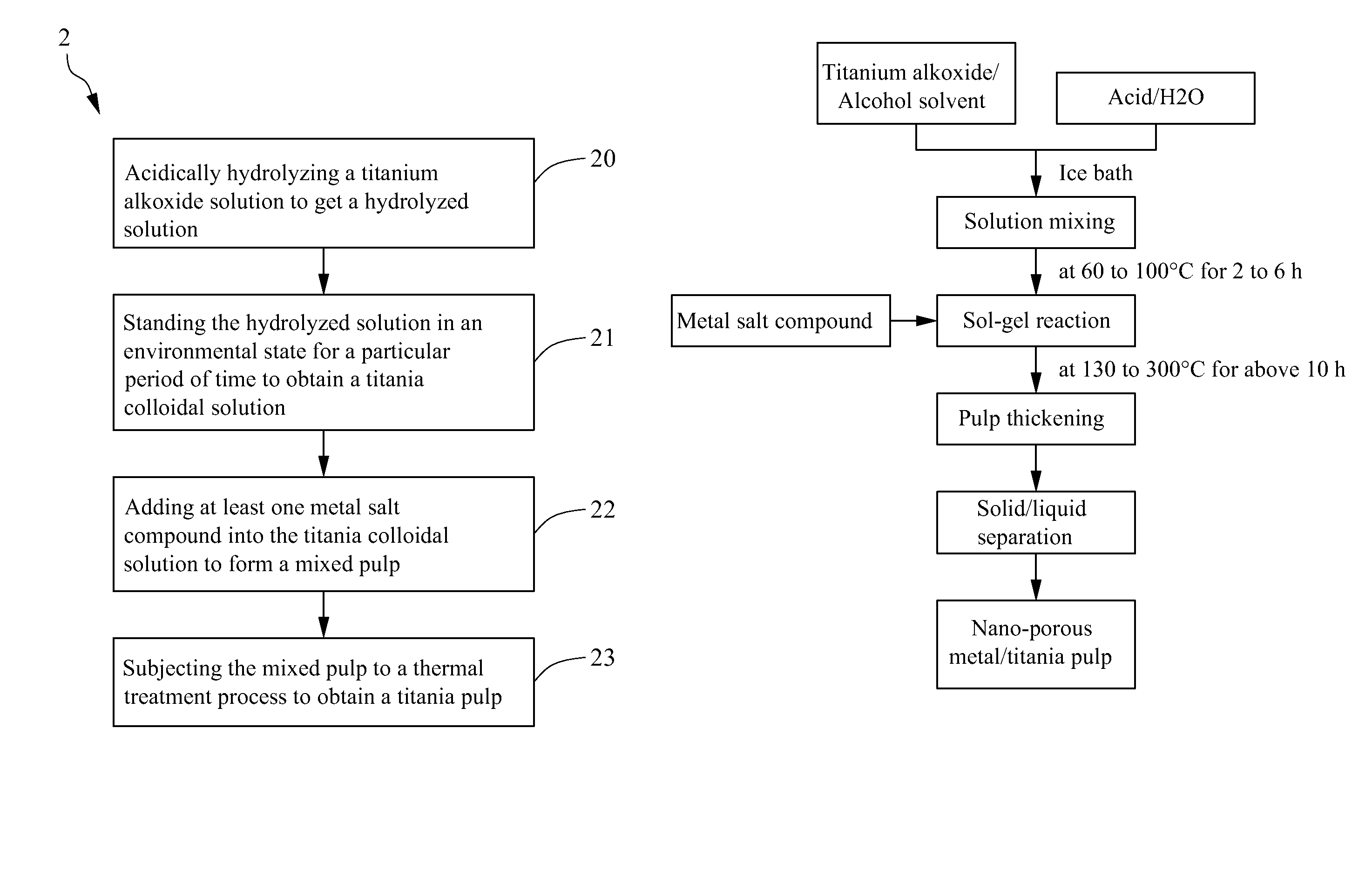
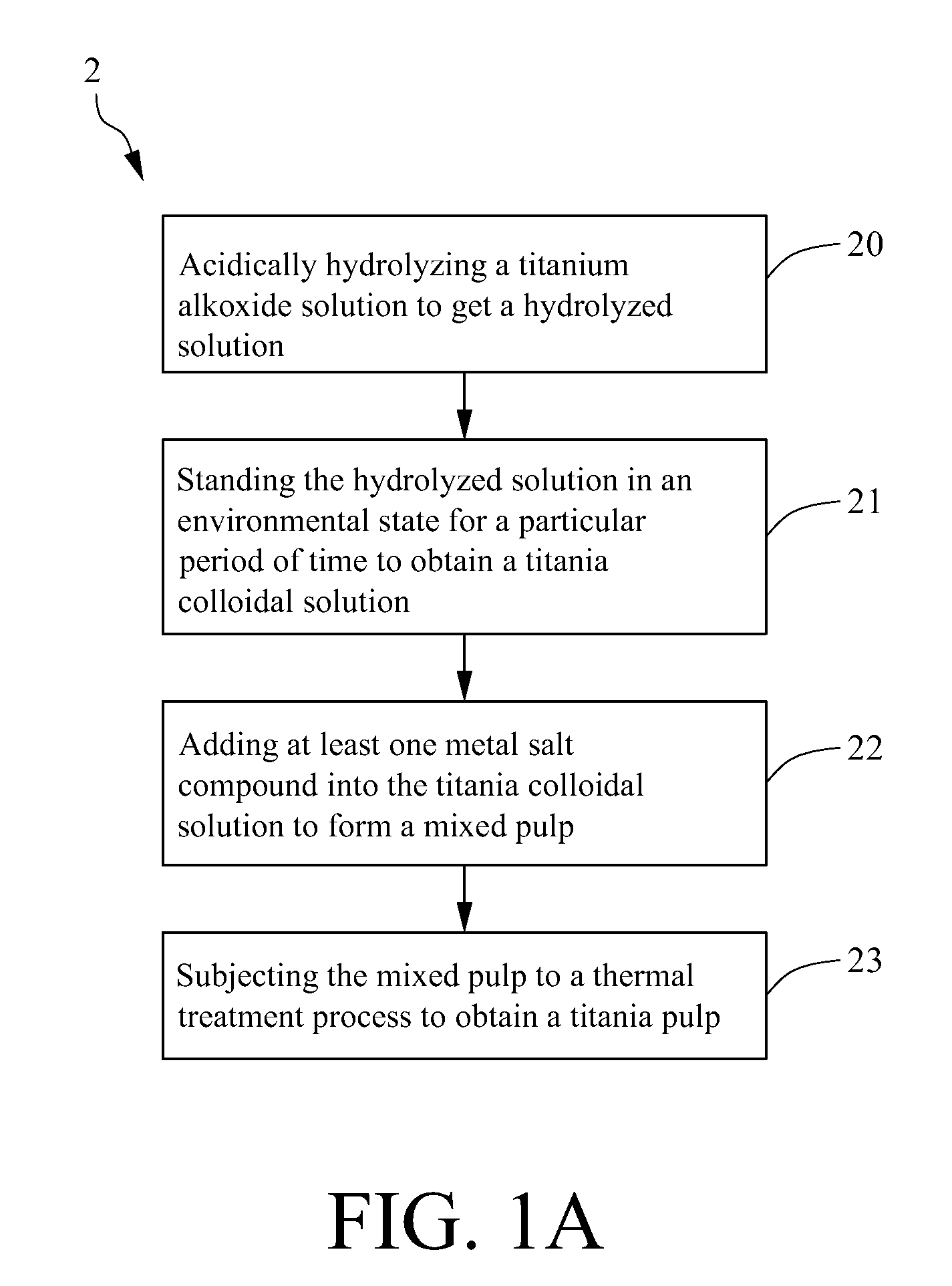
Method for making metal/titania pulp and photocatalyst
ActiveUS20100105549A1Convenient lightingIncrease production capacityUranium oxides/hydroxidesIncadescent envelopes/vesselsDecompositionSolvent
A method for making a metal-titania pulp and photocatalyst is provided, including firstly acidically hydrolyzing a titanium alkoxide solution in presence of an alcohol solvent to get a colloidal solution; then, adding at least one metal salt solution into the colloidal solution to produce a nano-porous metal / titania photocatalyst under appropriate conditions by appropriate reaction. The nano-porous metal / titania photocatalyst thus prepared has excellent optical activity and is applicable in research of water decomposition with light to improve production efficiency of hydrogen energy. In addition, the photocatalyst is further processed in the form of powder or film to facilitate industrial application in wastewater treatment.
Owner:INST NUCLEAR ENERGY RES ROCAEC
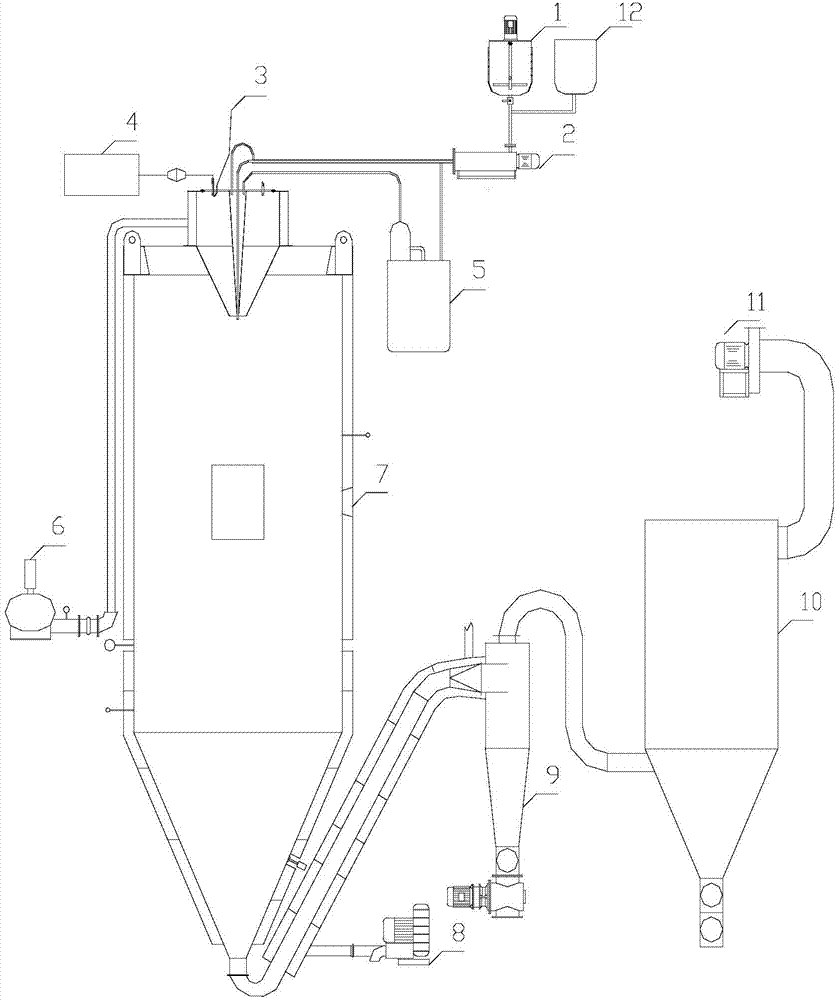
Method and device for declogging filter
A method of declogging at least one filter of a plant for manufacturing uranium oxide from uranium hexafluoride, including separating, from the wall of the filter, uranium oxyfluoride particles deposited, by a stream of inert gas such as nitrogen, injected into the filter, in a counter-currentwise direction to the flow of hydrofluoric acid.
Owner:AREVA NP SAS
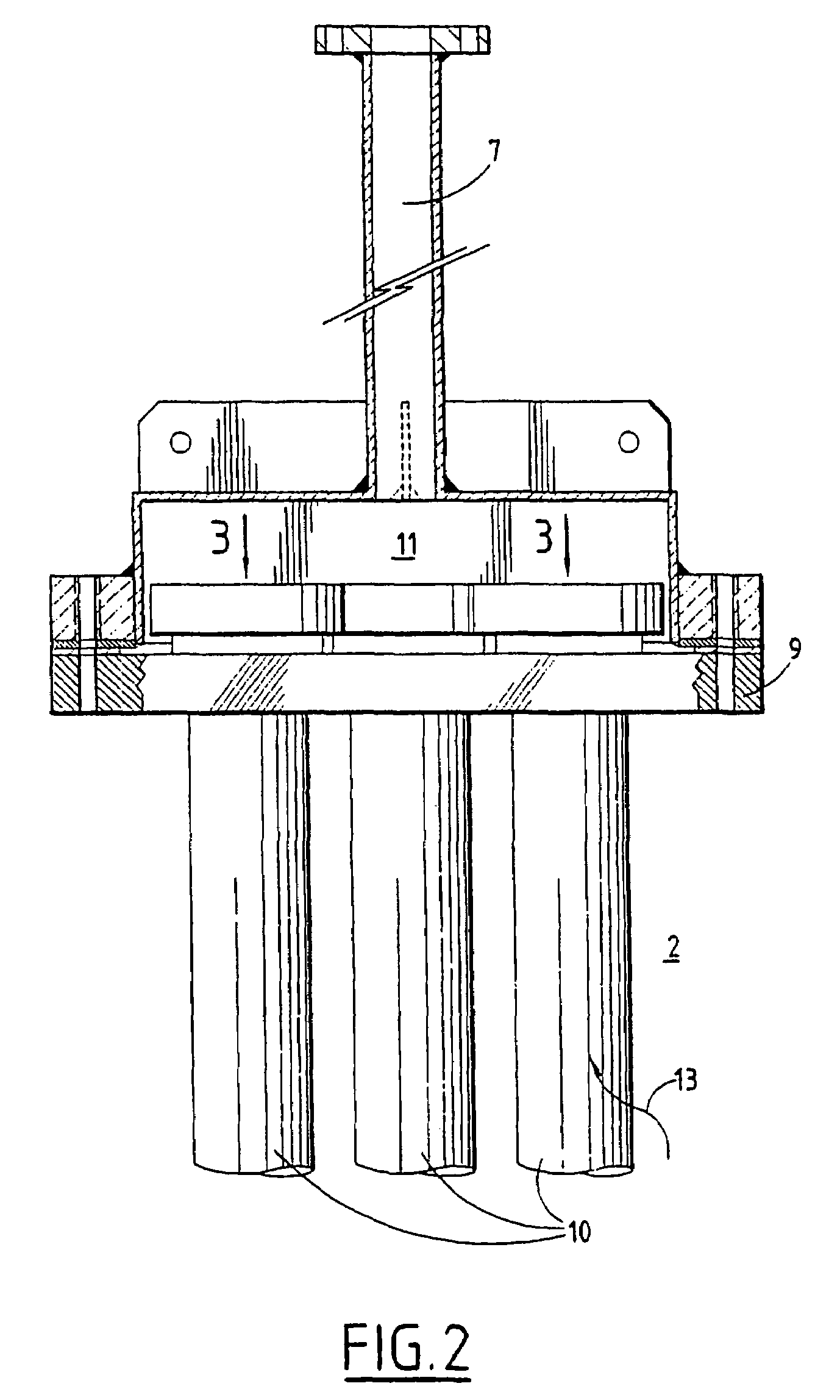
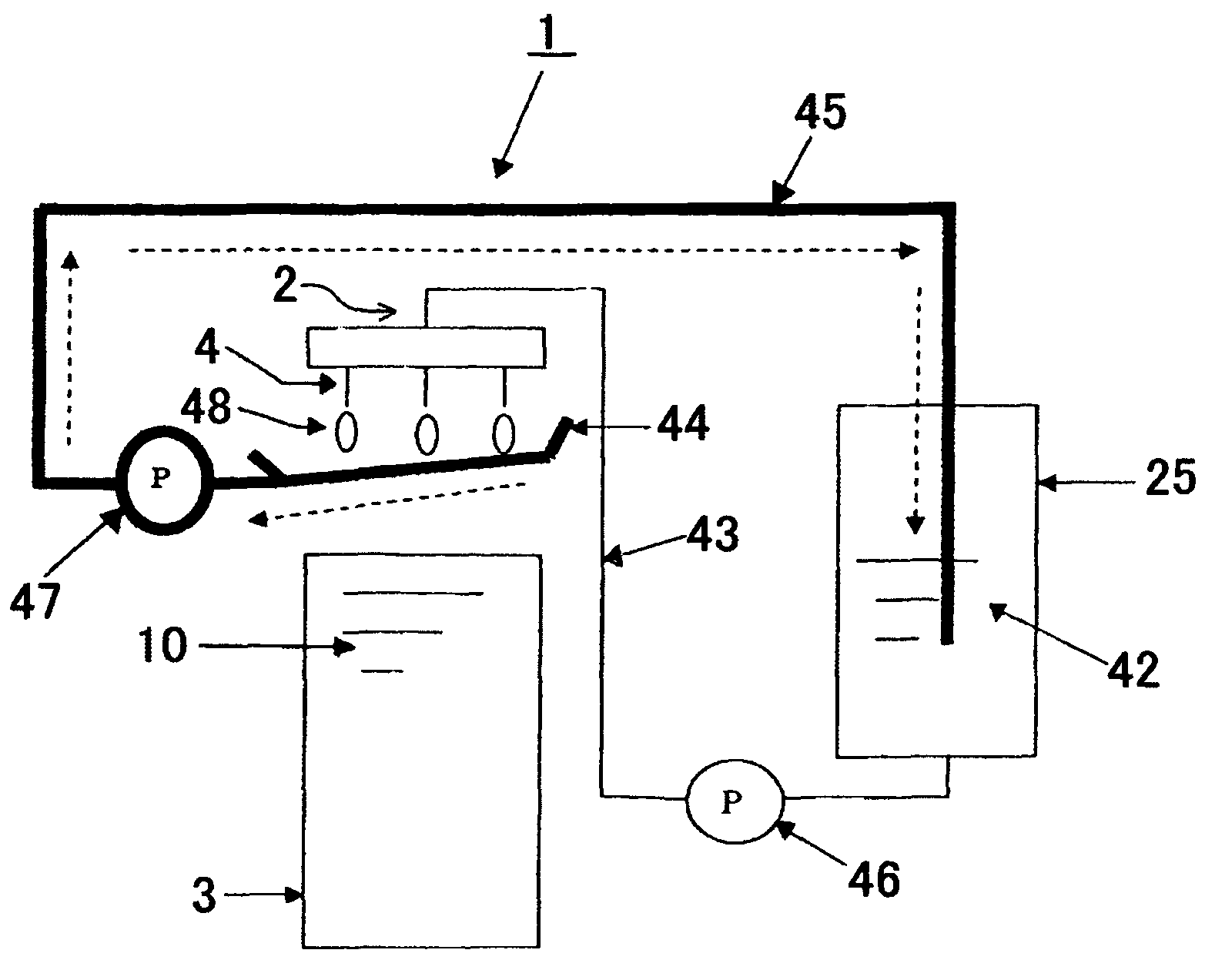
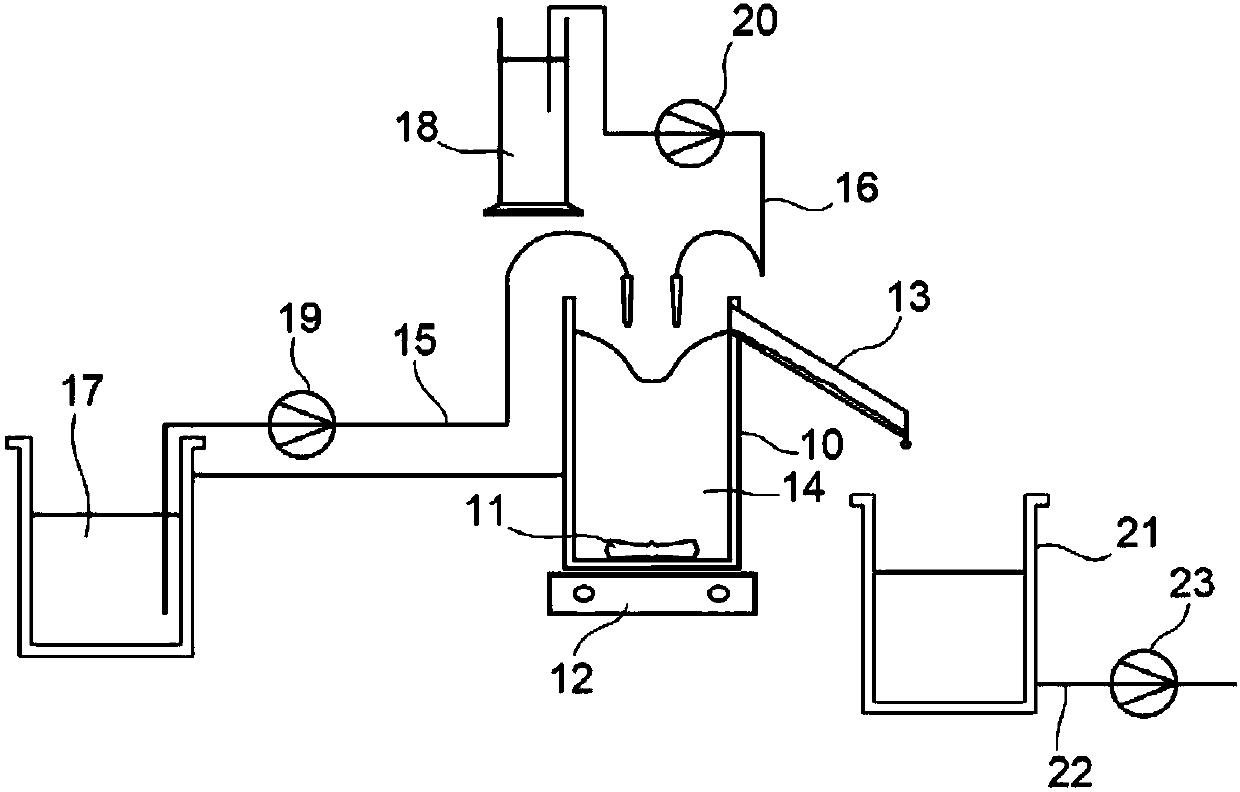
Dripping nozzle device, device for recovering feedstock liquid, device for supplying a feedstock liquid, device for solidifying the surfaces of drops, device for circulation aqueous ammonia solution, and apparatus for droducing ammonium diuranate particles
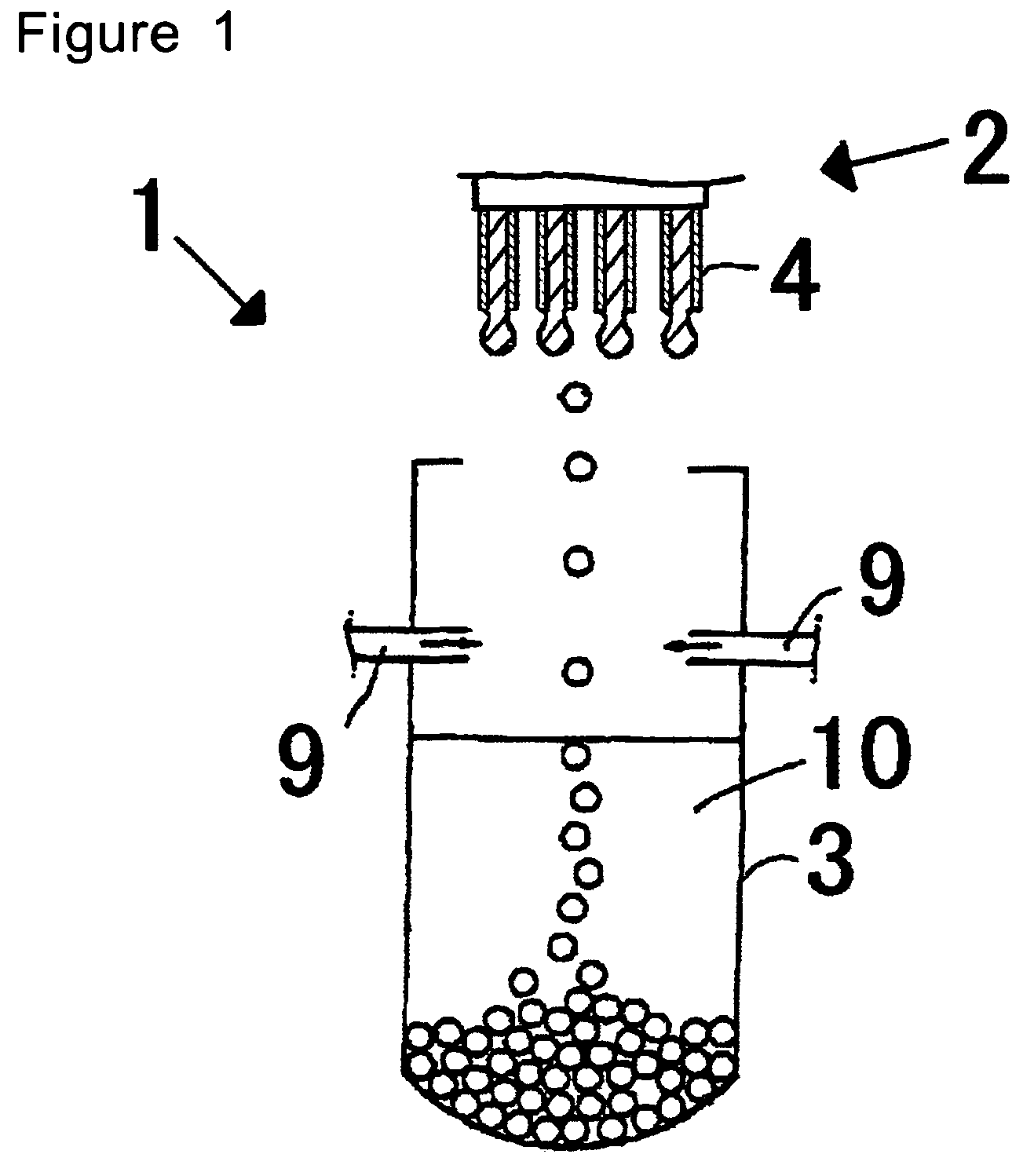
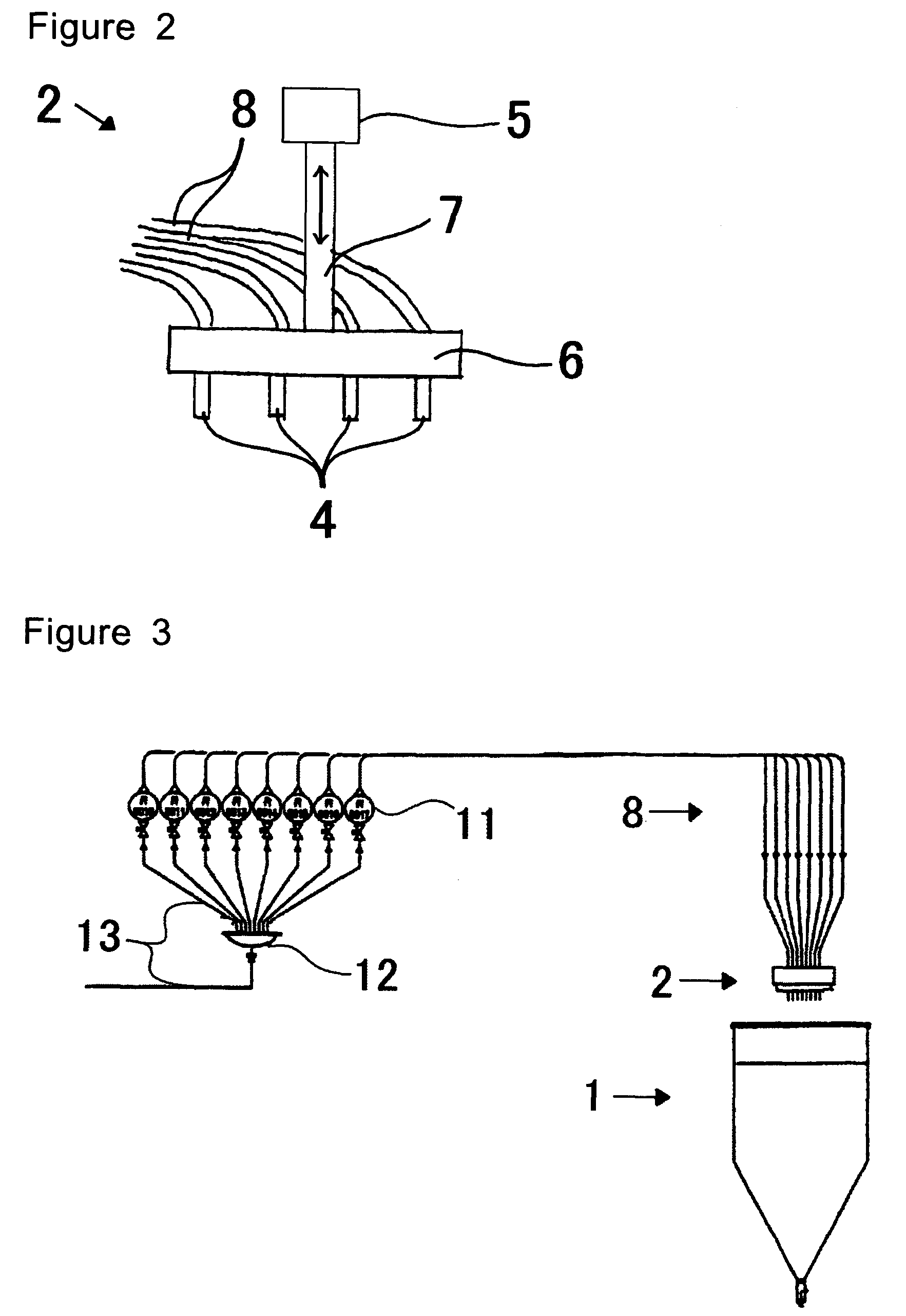
InactiveUS20070056637A1Particle diameters of the drops can be controlled easilyEliminate resonanceShaking/oscillating/vibrating mixersGranulation by liquid drop formationAmmonium hydroxideAmmonium diuranate
This invention provides a dripping nozzle device to produce ADU particles with good sphericity, a device for recovering a feedstock liquid to prepare a uniform feedstock liquid, a device for supplying a feedstock liquid to form drops with a uniform volume, a device for solidifying the surfaces of drops so that the drops will not deform easily when they fall onto and hit the surface of an aqueous ammonia solution, a device for circulating an aqueous ammonia solution so that the uranyl nitrate in the drops can be changed to ammonium diuranate completely, to such an extent that uranyl nitrate in the center of each drop is changed to ammonium diuranate, and an apparatus for producing ammonium diuranate particles with good sphericity. The dripping nozzle device is provided with a single vibrator to vibrate nozzles simultaneously. The device for recovering a feedstock liquid recovers the feedstock liquid remaining in the nozzles and mixes it with a fresh feedstock liquid. The device for supplying a feedstock liquid is provided with a light irradiator for irradiating falling drops with light. The device for solidifying the surfaces of drops sprays ammonia gas over each of the paths along which the drops dripping from the nozzles fall. The device for circulating an aqueous ammonia solution enables drops to flow upward in the aqueous ammonia solution in the aqueous ammonia solution reservoir. The apparatus for producing ammonium diuranate utilizes these devices.
Owner:NUCLEAR FUEL INDS
Ceramicrete stabilization of U-and Pu-bearing materials
InactiveUS7294291B2Magnesium fluoridesTransuranic element compoundsHydrocotyle bowlesioidesRadioactive agent
A method of stabilizing nuclear material is disclosed. Oxides or halides of actinides and / or transuranics (TRUs) and / or hydrocarbons and / or acids contaminated with actinides and / or TRUs are treated by adjusting the pH of the nuclear material to not less than about 5 and adding sufficient MgO to convert fluorides present to MgF2; alumina is added in an amount sufficient to absorb substantially all hydrocarbon liquid present, after which a binder including MgO and KH2PO4 is added to the treated nuclear material to form a slurry. Additional MgO may be added. A crystalline radioactive material is also disclosed having a binder of the reaction product of calcined MgO and KH2PO4 and a radioactive material of the oxides and / or halides of actinides and / or transuranics (TRUs). Acids contaminated with actinides and / or TRUs, and / or actinides and / or TRUs with or without oils and / or greases may be encapsulated and stabilized by the binder.
Owner:UCHICAGO ARGONNE LLC +2
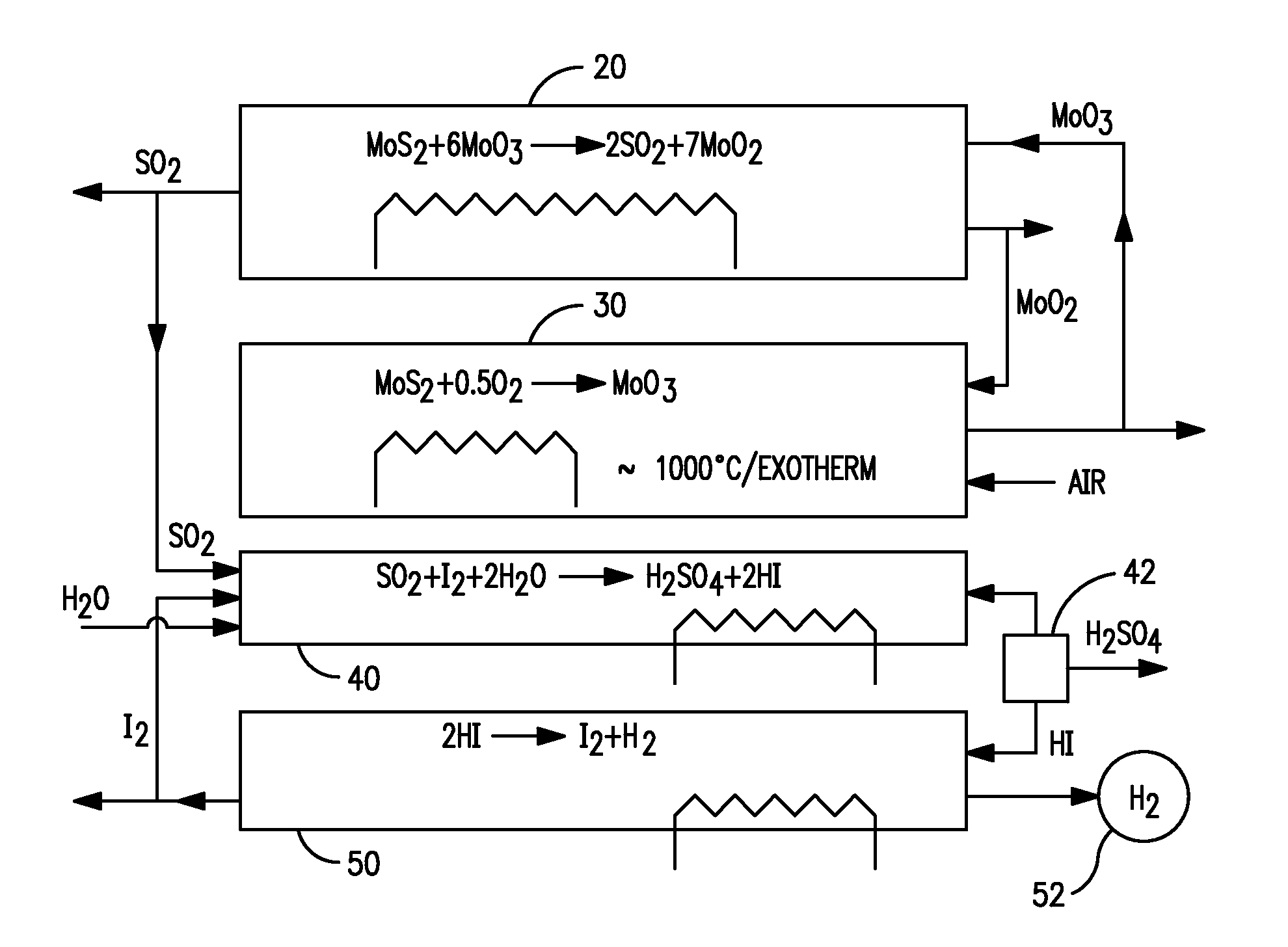
Production of hydrogen through oxidation of metal sulfides
InactiveUS20120034154A1Good benefitEfficiency benefitSulfur-dioxide/sulfurous-acidHydrogen productionMetallic sulfideTwo step
Utilization of process and equipment for oxidation of metal sulfides, preferably two step metal sulfide oxidation reactions, and more preferably with looping back of second step oxide to the first step as an oxidizing agent, to generate sulfur dioxide and a useful metal or metal oxide, and react the sulfur dioxide with halogen (iodine or bromine) and water to produce sulfuric and halogen acid under moderate process conditions and equipment requirements and then dissociating the halogen acids (HI or HBr) to halogen and hydrogen as an overall environmentally and cost efficient and otherwise acceptable safe process for producing hydrogen and other useful products.
Owner:ORCHARD MATERIAL TECH
Dropping nozzle device, device for recovering dropping undiluted solution, device for supplying dropping undiluted solution, device for solidifying surface of droplet, device for circulating aqueous a
InactiveCN1867516AEasy to manufactureHigh degree of sphericityNuclear energy generationReactors manufactureEngineeringAmmonium diuranate
The object of the present invention is to provide the following devices: a dripping nozzle device that forms substantially spherical ADU particles, a dripping stock solution recovery device that prepares a homogeneous dripping stock solution, a dripping stock solution supply device that can drop liquid droplets of a uniform volume, and even liquid droplets Droplet surface solidification device that does not deform when it collides with the surface of ammonia solution when dropped, ammonia solution circulation device that can fully turn uranyl nitrate into ADU from the center of the droplet, and ADU with good sphericity The ADU particle manufacturing device for particles, the dripping nozzle device has an exciter that vibrates multiple nozzles at the same time, the dripping stock solution recovery device recovers the bottom fine stock solution in the nozzle and mixes it into the dripping stock solution, and the dripping stock solution supply device has a mechanism for falling The light irradiation mechanism that irradiates the droplet of the original solution with light, the droplet surface curing device sprays ammonia gas toward the drop path of the droplet of the original solution dripped from the nozzle, and the ammonia solution circulation device can make the droplets in the ammonia solution storage tank The ammonium diuranate particle manufacturing device uses the above-mentioned device for ascending flow in the ammonia solution in the interior.
Owner:NUCLEAR FUEL INDS
Metal oxide powder and method for the production of the same
InactiveUS20040219087A1Industrially advantageousRare earth metal oxides/hydroxidesManganese oxides/hydroxidesTitanium oxideRutile
Owner:MOHRI MASAHIDE +6
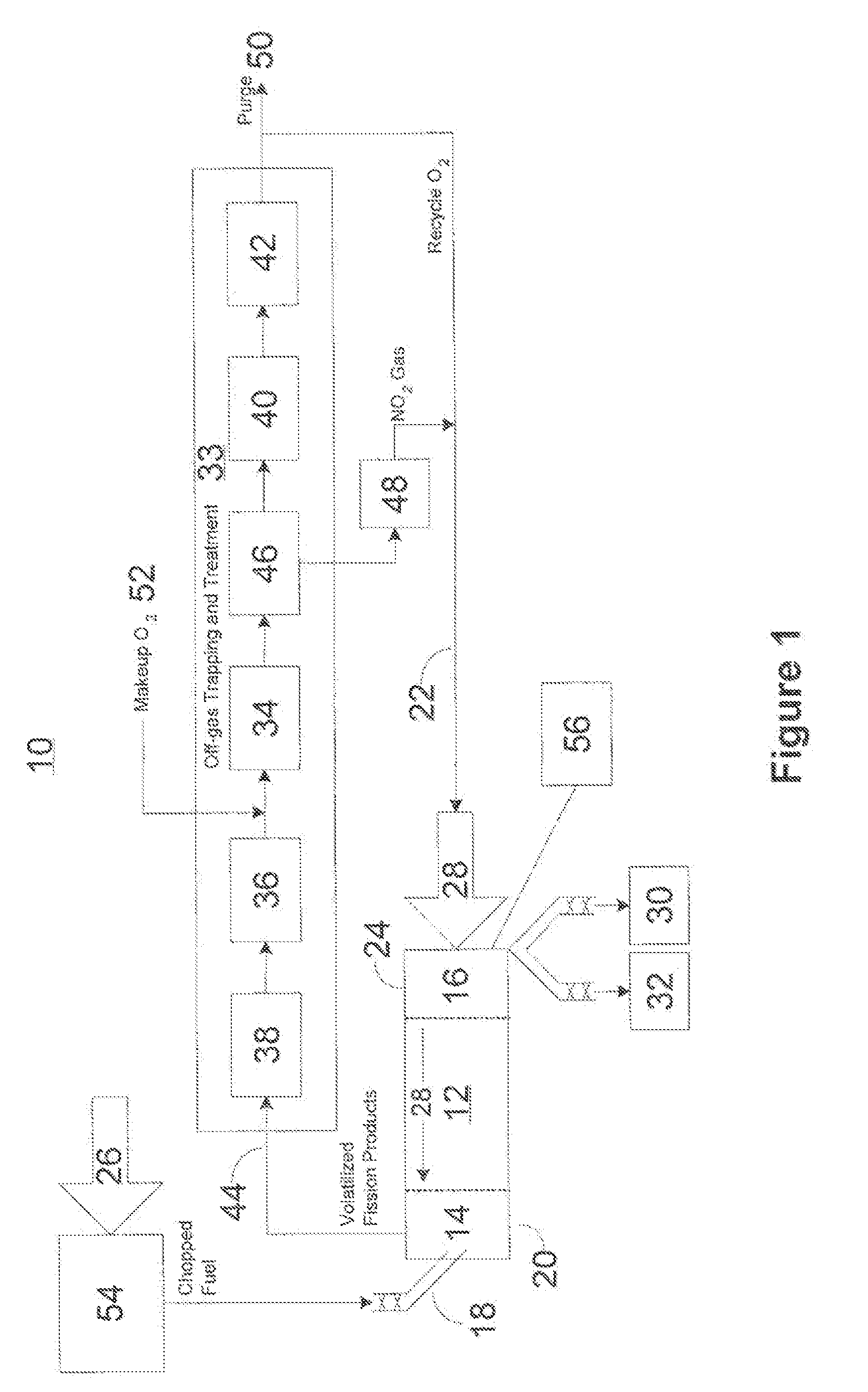
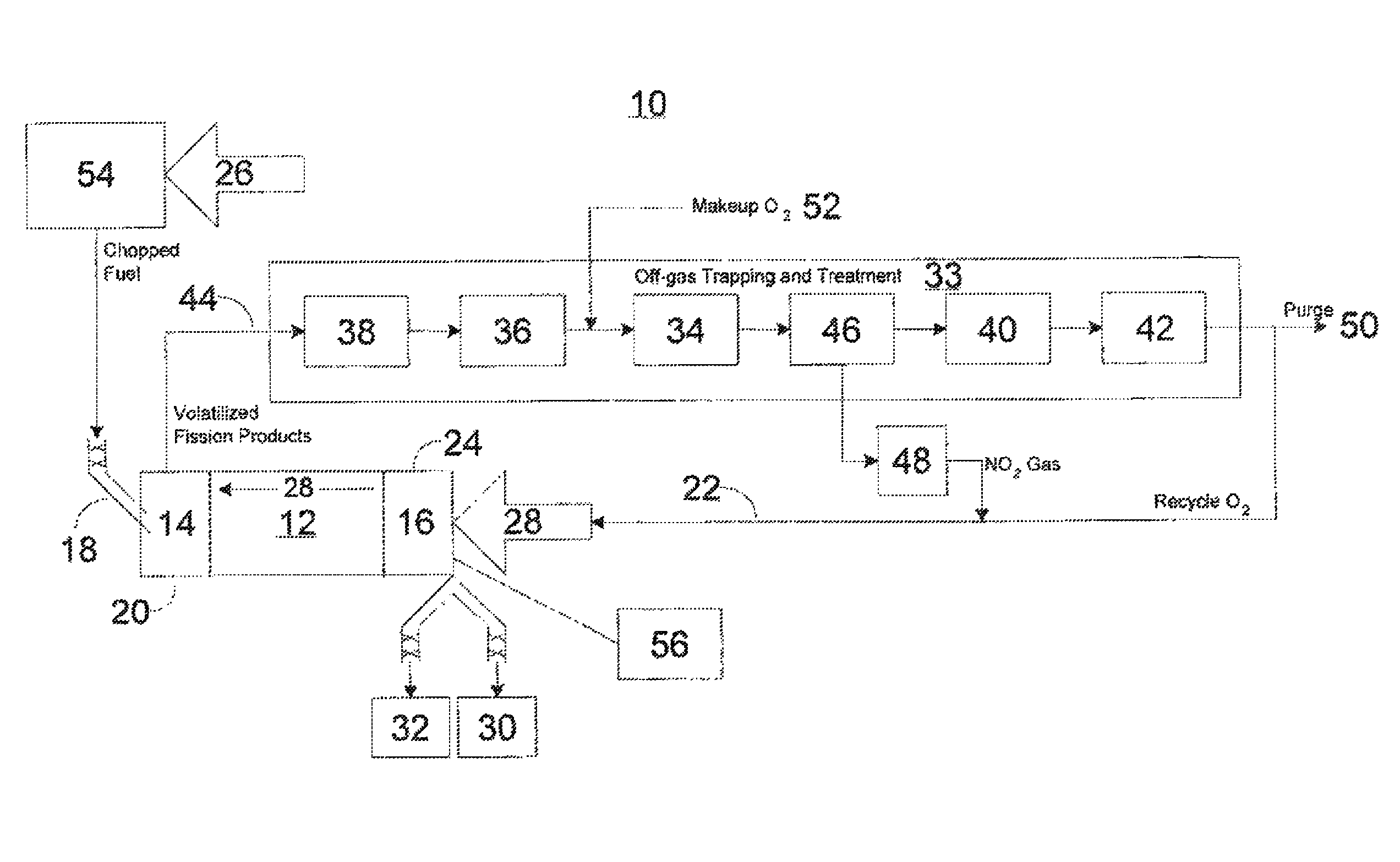
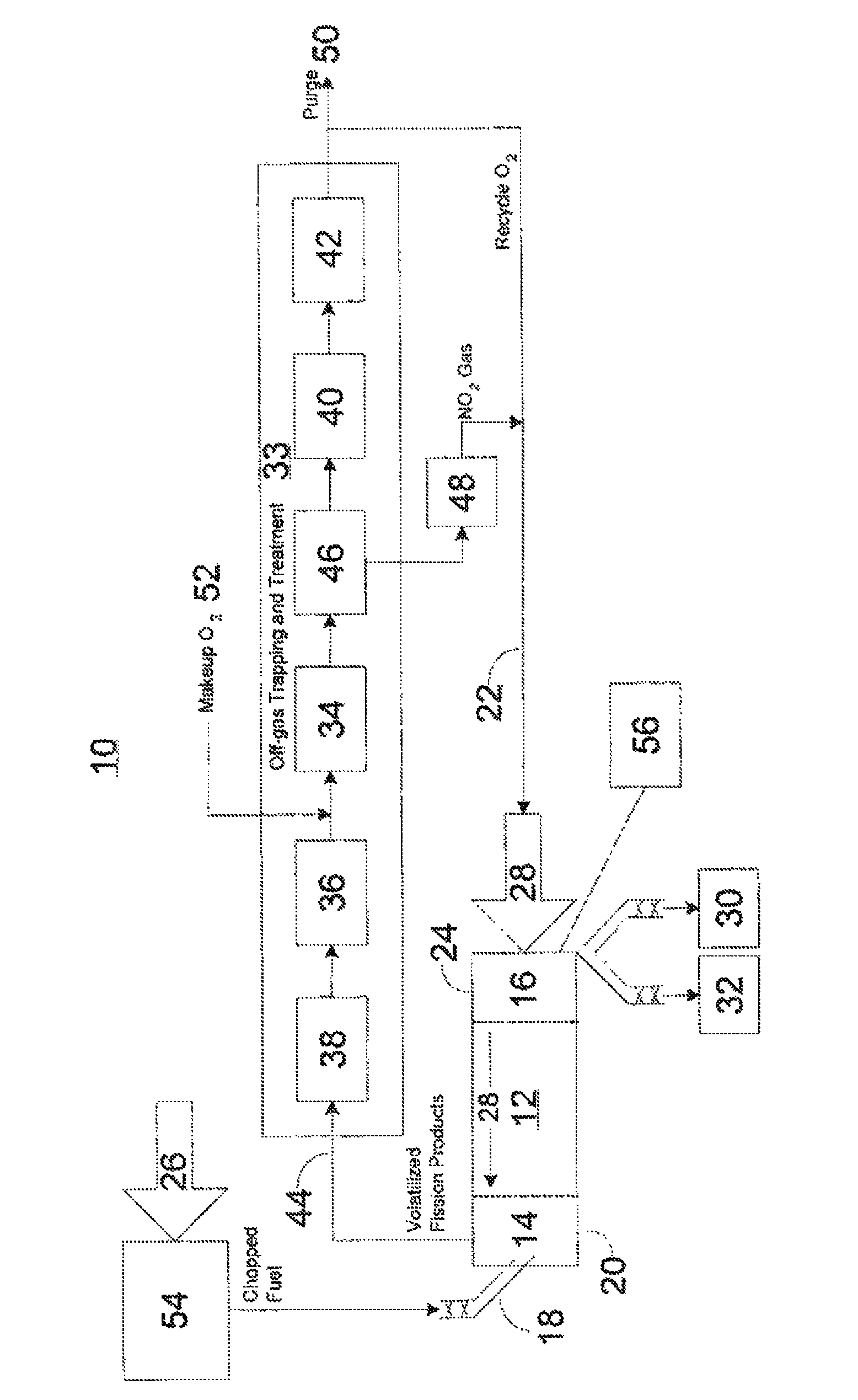
Advanced dry head-end reprocessing of light water reactor spent nuclear fuel
ActiveUS20110250108A1Solvent extractionTransuranic element compoundsNitrogen monooxideNitrogen dioxide
A method for reprocessing spent nuclear fuel from a light water reactor includes the step of reacting spent nuclear fuel in a voloxidation vessel with an oxidizing gas having nitrogen dioxide and oxygen for a period sufficient to generate a solid oxidation product of the spent nuclear fuel. The reacting step includes the step of reacting, in a first zone of the voloxidation vessel, spent nuclear fuel with the oxidizing gas at a temperature ranging from 200-450° C. to form an oxidized reaction product, and regenerating nitrogen dioxide, in a second zone of the voloxidation vessel, by reacting oxidizing gas comprising nitrogen monoxide and oxygen at a temperature ranging from 0-80° C. The first zone and the second zone can be separate. A voloxidation system is also disclosed.
Owner:UT BATTELLE LLC
Method for producing triuranium octoxide by microwave calcination of ammonium diuranate
InactiveCN101633521AEvenly heatedUniform compositionUranium oxides/hydroxidesMicrowaveRoom temperature
The invention relates to a method for producing triuranium octoxide by microwave calcination of ammonium diuranate, comprising the steps: evenly mixing ammonium diuranate and triuranium octoxide by weight percent of (10 to 2): 1 to obtain a mixed material; then putting the mixed material into a microwave reactor; regulating microwave output power to be 10 to 80 kW; room temperature of the material is raised to 350 DEG C to 800 DEG C at the rate of 20 to 100 DEG C / min; keeping the temperature for 10 to 40 min; and obtaining a triuranium octoxide product. In the method, a calcination product with strong wave absorbing performance is added to a calcination raw material with relatively weak wave absorbing performance; the microwave calcination of substances with weak wave absorbing performance is realized; and moreover, the method has short production period, low energy consumption and easy industrial application.
Owner:KUNMING UNIV OF SCI & TECH
Method for preparing oriented tungstic trioxide nano-film
InactiveCN101318705AThickness is easy to controlGood orientationNanostructure manufactureUranium oxides/hydroxidesRoom temperatureSolvent
The invention relates to a method for preparing an orientated tungsten oxide nano film. The method takes an ethanol suspension of monocrystal tungsten trioxide (WO3) nano-plates as a precursor, and utilizes the habit that the nano-plate with high diameter-thickness ratio tends to be parallel to a substrate and the principle that solvent volatilization causes the oriented self-assembly of the nano-plate to prepare the tungsten oxide (WO3) nano film which is formed by overlapping the tungsten trioxide (WO3) nano-plates and oriented along the [002] crystallographic direction. The method is completed at a room temperature, and does not require subsequent heating treatment; and the tungsten trioxide (WO3) nano film prepared is strongly oriented along the [002] crystallographic direction, namely the WO3 nano film is spread along an ab plane and a c- shaft is the film thickness direction. The method has the characteristics of simple technological process, convenient operation, low requirements on equipment conditions and strong adaptability; and WO3 nano film materials have the characteristics of high crystallinity and good orientation.
Owner:ZHENGZHOU UNIV
Process for preparing UO3 through uranyl nitrate air-blast atomization dry pyrolysis denitration
InactiveCN107162060AImprove conversion rateShort processUranium oxides/hydroxidesChemical reactionCombustion chamber
The invention discloses a process for preparing UO3 through uranyl nitrate air-blast atomization dry pyrolysis denitration. According to the process, a designed and processed combustion chamber and a denitration reactor are adopted as main body equipment of uranyl nitrate pyrolysis denitration, a high-temperature high-speed gas generated from combustion of propane and excessive air is adopted as a heat source and an atomization gas source, and a uranyl nitrate liquid is subjected to drying dehydration and pyrolysis denitration. The process has the advantages of being short in process procedure, simple in equipment structure, stable in operation and high in UNH (Uranyl-Nitrate Hexahydrate) conversion rate, the prepared UO3 is uniform in granularity distribution and good in chemical reaction activity, and the like.
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
Extraction of uranium from wet-process phosphoric acid
ActiveUS8226910B2Reduced valencyLower iron levelsCobalt ammonia complexesPhotography auxillary processesFiltrationPhosphoric acid
Owner:URTEK
Preparation method of monodisperse micron-sized uranium oxide particle
ActiveCN101891253AShould be operatedEnsure safetyUranium oxides/hydroxidesUranium oxideThermal decomposition method
The invention discloses a method for preparing a monodisperse micron-sized uranium oxide particle by using a sol-spray thermal decomposition method. The method comprises the following steps of: forming a monodisperse aerosol by a uranyl nitrate solution by using a vibrating orifice aerosol generator; drying and carrying out static removal to form a uranyl nitrate solid particle; carrying out high-temperature thermal decomposition to form a monodisperse uranium oxide particle; and collecting after cooling, wherein carrier gas is preheated at the back end of a neutralizer to a temperature of 70-80 DEG C or so, the flow of the carrier gas is 35-45 L / min, and the high-temperature thermal decomposition is carried out by the direct heating of a muffle furnace. The technical scheme of the invention is simpler and more convenient and ensures the safety in the operation process.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Uranium oxide micro-sphere and preparation method thereof
ActiveCN103936076AHigh capacity for storing fission gasStrong radiation resistanceUranium oxides/hydroxidesUranium oxideNanoparticle
The invention discloses an uranium oxide micro-sphere and a preparation method, a porous structure is provided in the uranium oxide micro-sphere, and the porous structure is composed of nano particles. The invention also comprises a preparation method of the uranium oxide micro-sphere, which comprises the following steps: 1)performing a hydro-thermal reaction on a mixing aqueous solution to obtain a sea urchin-state uranium-containing micro-sphere precursor, wherein the mixing aqueous solution comprises a uranyl nitrate aqueous solution, glycerol and urea; 3)washing and drying the uranium-containing micro-sphere precursor; and 3)placing the dried uranium-containing micro-sphere precursor in a tubular furnace for pyrolysis to obtain the uranium oxide micro-sphere. The preparation method has the advantages of simple process and easy control, and the prepared uranium oxide micro-sphere has the characteristics of small size and has porous structure, and has latent application value in the relative fields of catalysis and nuclear energy.
Owner:INST OF HIGH ENERGY PHYSICS CHINESE ACADEMY OF SCI
Method for producing uranium trioxide by heating uranyl nitrate solution in microwave manner
The invention discloses a method for producing uranium trioxide by heating uranyl nitrate solution in a microwave manner. The method comprises the following steps: delivering the uranyl nitrate solution as a raw material; and carrying out explosive evaporation of the uranyl nitrate solution, concentrating, decomposing uranyl nitrate to remove nitrate, stopping micro-wave heating,, placing materials in a muffle furnace, and ball-milling to obtain powder so as to obtain uranium trioxide powder. In the concentrating process, the temperature is controlled by adjusting micro-wave power, and the solution is prevented from splashing and overflowing; in a nitrate removing process, the temperature and the heating time are controlled, and the circumstance that triuranium octaoxide is generated due to over-high temperature is avoided; nitrate removing products are stable in a muffle furnace at the temperature of 350 DEG C, and the uranium trioxide conversion rate reaches 100%. By the method of ball-milling to obtain the powder, the powdery uranium trioxide is prepared, meanwhile, materials adhered to walls can be ground, and therefore, the retention amount of the materials in a container is reduced.
Owner:RES INST OF PHYSICAL & CHEM ENG OF NUCLEAR IND
Method of preparing high-activity uranium trioxide by thermal denitration of uranyl nitrate
The invention belongs to the technical field of preparation of uranium trioxide, and particularly relates to a method of preparing high-activity uranium trioxide by thermal denitration of uranyl nitrate. The method comprises the following steps: step (1) evaporating and concentrating an uranyl nitrate solution to obtain a concentrated uranyl nitrate solution; step (2) conveying the concentrated uranyl nitrate solution to a cooling crystallizer, cooling the concentrated uranyl nitrate solution to a temperature below 40 DEG C, carrying out cooling crystallization to form UO2(NO3)2.6H2O crystals,and returning a saturated solution to an evaporation and concentration device in step (1); step (3) dehydrating the UO2(NO3)2.6H2O crystals obtained in step (2) by microwave drying, and converting the UO2(NO3)2.6H2O crystals obtained in step (3) into UO2(NO3)2 powder; and step (4) generating the UO3 product in a fixed bed by using the UO2(NO3)2 powder obtained in step (3) in a vacuum environmentthrough microwave heating. The method has the advantages of short technological process, no generation of radioactive ammonia-nitrogen wastewater, good product activity and the like, increases the integral level of a uranium purification and conversion technology in the nuclear fuel cycle, and has obvious social and economic benefits.
Owner:中核二七二铀业有限责任公司
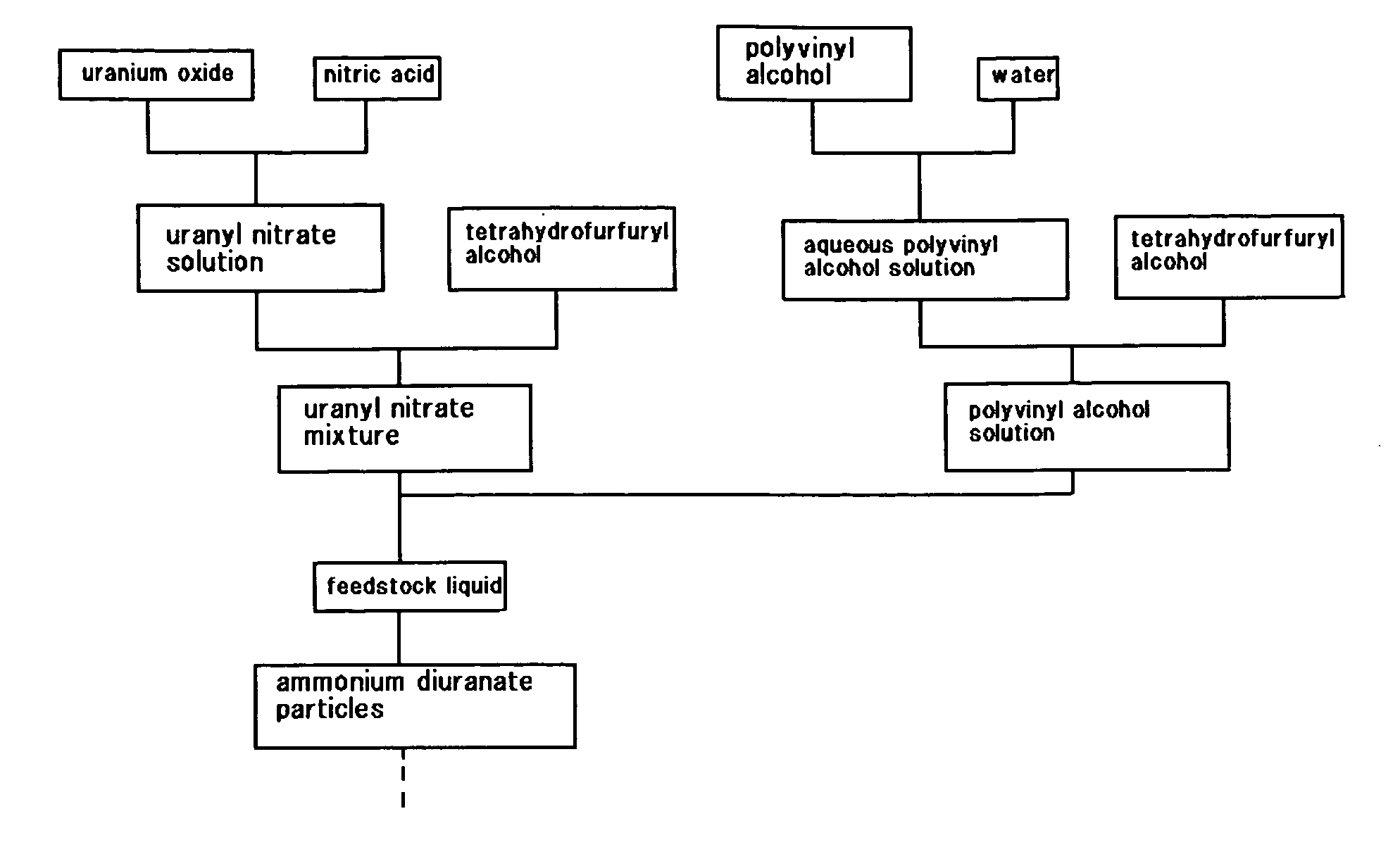
Method of preparing feedstock liquid, method of preparing uranyl nitrate solution, and method of preparing polyvinyl alcohol solution
InactiveUS20070178036A1Good spherical shapeSimple internal structureNuclear energy generationReactors manufacturePolyvinyl alcoholAmmonium diuranate
The object of the present invention is to provide a feedstock liquid, from which fuel kernels with good quality can be produced, and a method of preparing the feedstock liquid. The present invention provides a feedstock liquid with a viscosity from 4.0×10−2 to 6.5×10−2 Pa·s at 15° C., for the production of ammonium diuranate particles. The present invention also provides a method of preparing a feedstock liquid used for the production of ammonium diuranate particles, which includes mixing a uranyl nitrate solution and tetrahydrofurfuryl alcohol to produce a uranyl nitrate mixture, dissolving polyvinyl alcohol in water to produce an aqueous polyvinyl alcohol solution, mixing the aqueous polyvinyl alcohol solution with tetrahydrofurfuryl alcohol to produce a polyvinyl alcohol solution, and mixing the uranyl nitrate mixture with the polyvinyl alcohol solution.
Owner:NUCLEAR FUEL INDS
Method for producing triuranium octoxide by calcining ammonium uranyl tricarbonate with microwaves
The invention discloses a method for producing triuranium octoxide by calcining ammonium uranyl tricarbonate with microwaves, which comprises the following steps: putting ammonium uranyl tricarbonate into a microwave reactor, turning on a microwave heating switch, adjusting the microwave output power to 5-60kW, heating the materials to 400-700 DEG C from room temperature at the speed of 20-100 DEG C / minute, preserving heat for 5-30 minutes, and then naturally cooling to the room temperature to acquire a triuranium octoxide product. The production method has low energy consumption as well as uniform product ingredients and granularity, and the calcining time in the whole technical process is only one third of a conventional method.
Owner:KUNMING UNIV OF SCI & TECH
Process for reprocessing a spent nuclear fuel and of preparing a mixed uranium-plutonium oxide
InactiveUS20090184298A1Risk minimizationUranium compounds preparationNuclear energy generationDissolutionSolvent
A process for reprocessing a spent nuclear fuel and for preparing a mixed uranium-plutonium oxide. The process: a) separates the uranium and plutonium from fission products, americium, and curium that are present in an aqueous nitric solution resulting from dissolution of the fuel in nitric acid, the separating including at least one operation of coextracting the uranium and plutonium from the solution by a solvent phase; b) partitions the coextracted uranium and plutonium to a first aqueous phase containing plutonium and uranium, and a second aqueous phase containing uranium but no plutonium; c) purifies the plutonium and uranium that are present in the first aqueous phase; and d) coconverts the plutonium and uranium to a mixed uranium / plutonium oxide.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
Device for recovering feedstock liquid, device for supplying a feedstock liquid, device for solidifying the surfaces of drops, and apparatus for producing ammonium diuranate particles
InactiveUS7811526B2Particle diameters of the drops can be controlled easilyEliminate resonanceShaking/oscillating/vibrating mixersGranulation by liquid drop formationAmmonium hydroxideAmmonium diuranate
This invention provides a dripping nozzle device to produce ADU particles with good sphericity, a device for recovering a feedstock liquid to prepare a uniform feedstock liquid, a device for supplying a feedstock liquid to form drops with a uniform volume, a device for solidifying the surfaces of drops so that the drops will not deform easily when they fall onto and hit the surface of an aqueous ammonia solution, a device for circulating an aqueous ammonia solution so that the uranyl nitrate in the drops can be changed to ammonium diuranate completely, to such an extent that uranyl nitrate in the center of each drop is changed to ammonium diuranate, and an apparatus for producing ammonium diuranate particles with good sphericity. The dripping nozzle device is provided with a single vibrator to vibrate nozzles simultaneously. The device for recovering a feedstock liquid recovers the feedstock liquid remaining in the nozzles and mixes it with a fresh feedstock liquid. The device for supplying a feedstock liquid is provided with a light irradiator for irradiating falling drops with light. The device for solidifying the surfaces of drops sprays ammonia gas over each of the paths along which the drops dripping from the nozzles fall. The device for circulating an aqueous ammonia solution enables drops to flow upward in the aqueous ammonia solution in the aqueous ammonia solution reservoir. The apparatus for producing ammonium diuranate utilizes these devices.
Owner:NUCLEAR FUEL INDS
Method for preparing triuranium octaoxide from uranium hexafluoride
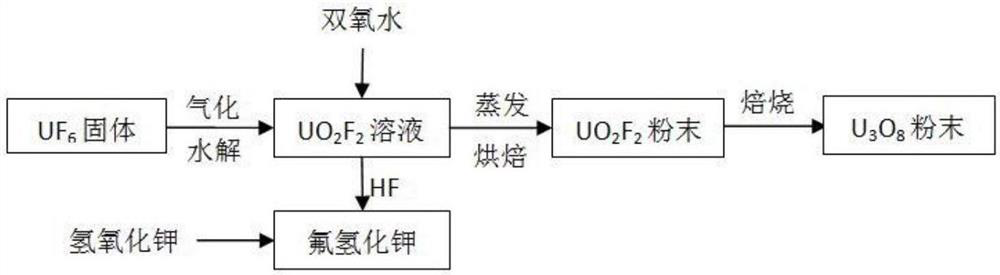
PendingCN112607780AAvoid joiningReduce generationAlkali metal fluoridesUranium oxides/hydroxidesHydrolysatePotassium hydroxide
The invention belonging to the technical field of nuclear fuel circulation, and particularly relates to a method for preparing triuranium octaoxide from uranium hexafluoride. The method comprises the following three steps: step 1, preparation of a UO2F2 hydrolysate; step 2, preparation of UO2F2 powder; step 3, roasting for preparation of U3O8. According to the method, the process of preparing U3O8 through conventional UF6 reduction is shortened, and the addition of ammonia water in the process is avoided, so ammonia nitrogen wastewater generated in the process is reduced, a preparation process is simple, cost is low, and industrialization is easy to realize. According to the method, a large amount of fluorine in UF6 is converted into HF acid through water, the HF acid reacts with potassium hydroxide to finally prepare a byproduct potassium hydrogen fluoride, and the byproduct potassium hydrogen fluoride can be returned to an electrolytic fluorine production unit in a uranium conversion system so as to realize recycling. The U3O8 prepared by the method is more uniform in granularity, good in powder flowability, low in radioactivity, low in fluorine content and convenient for long-term stable storage.
Owner:中核二七二铀业有限责任公司
Method for converting UO3 or u3o8 into hydrated UO4
ActiveUS20130280157A1Improve responseQuick conversionPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesUranium fluoridesHydration reactionWater of crystallization
A method for converting UO3 and / or U3O8 into hydrated UO4 of formula UO4.nH2O wherein n is 2 or 4, comprising the following successive steps:a) preparing an aqueous suspension of a UO3 powder and / or a U3O8 powder;b) adding hydrogen peroxide H2O2 to the aqueous suspension of a UO3 and / or U3O8 powder, converting the UO3 and / or U3O8 into hydrated UO4 and precipitating, crystallizing the hydrated UO4 in the suspension;g) recovering the precipitate, crystals of UO4 hydrate;h) optionally, washing the recovered UO4 hydrate precipitate, crystal(s);i) optionally, repeating step d);j) optionally, drying the precipitate, the crystals;wherein the addition of H2O2 to the aqueous suspension is carried out so that the suspension contains a stoichiometric excess of H2O2 relatively to the stoichiometry of the reaction from UO3:UO3+nH2O+H2O2→UO4.nH2O+H2O (1)or of the reaction from U3O8 UO2.67+1.33H2O2+nH2O→UO4.nH2O+H2O (2)and the pH of the suspension is maintained in steps a) and b) at a value comprised between 2 and 3.
Owner:ORANO CYCLE
Porous metal oxide and method of preparing the same
InactiveUS8617510B2Easy to controlEasy to adjustCell electrodesCopper oxides/halidesMaterials scienceHeat treating
Porous metal oxides are provided. The porous metal oxides are prepared by heat treating a coordination polymer. A method of preparing the porous metal oxide is also provided. According to the method, the shape of the particles of the metal oxide can be easily controlled, and the shape and distribution of pores of the porous metal oxide can be adjusted.
Owner:SAMSUNG SDI CO LTD
Method for making metal/titania pulp and photocatalyst
ActiveUS8241604B2Convenient lightingIncrease production capacityIncadescent envelopes/vesselsUranium oxides/hydroxidesHydrogenDecomposition
A method for making a metal-titania pulp and photocatalyst is provided, including firstly acidically hydrolyzing a titanium alkoxide solution in presence of an alcohol solvent to get a colloidal solution; then, adding at least one metal salt solution into the colloidal solution to produce a nano-porous metal / titania photocatalyst under appropriate conditions by appropriate reaction. The nano-porous metal / titania photocatalyst thus prepared has excellent optical activity and is applicable in research of water decomposition with light to improve production efficiency of hydrogen energy. In addition, the photocatalyst is further processed in the form of powder or film to facilitate industrial application in wastewater treatment.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Advanced dry head-end reprocessing of light water reactor spent nuclear fuel
A method for reprocessing spent nuclear fuel from a light water reactor includes the step of reacting spent nuclear fuel in a voloxidation vessel with an oxidizing gas having nitrogen dioxide and oxygen for a period sufficient to generate a solid oxidation product of the spent nuclear fuel. The reacting step includes the step of reacting, in a first zone of the voloxidation vessel, spent nuclear fuel with the oxidizing gas at a temperature ranging from 200-450° C. to form an oxidized reaction product, and regenerating nitrogen dioxide, in a second zone of the voloxidation vessel, by reacting oxidizing gas comprising nitrogen monoxide and oxygen at a temperature ranging from 0-80° C. The first zone and the second zone can be separate. A voloxidation system is also disclosed.
Owner:UT BATTELLE LLC
Method for preparing powder comprising particles of triuranium octoxide and particles of plutonium dioxide
PendingCN109896540AEnsure co-managementEasy to manageNuclear energy generationPlutonium oxides/hydroxidesOxalatePlutonium dioxide
The invention relates to a method for preparing a powder comprising an intimate mixture of U3O8 particles and PuO2 particles and which may further comprise particles of ThO2 or NpO2. The method comprises: a) preparing, by oxalic precipitations, an aqueous suspension Si of particles of uranium(IV) oxalate and an aqueous suspension S2 of particles of plutonium(IV) oxalate; b) mixing the aqueous suspension Si with the aqueous suspension S2 to obtain an aqueous suspension S1+2 comprising particles of uranium(IV) oxalate and particles of plutonium(IV) oxalate; c) separating the aqueous suspension S1+2 into an aqueous phase and a solid phase comprising the particles of uranium(IV) oxalate and the particles of plutonium(IV) oxalate; and d) calcining the solid phase to convert (1) the particles ofuranium(IV) oxalate to particles of triuranium octoxide and (2) the particles of plutonium(IV) oxalate to particles of plutonium(IV) dioxide, whereby the powder is obtained; and is characterized in that steps b) and c) are performed simultaneously or successively. The invention further relates to the fabrication of nuclear fuels of MOX type, e.g. for LWR or FNR reactors.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
Treatment method of superior molten slag of monazite
ActiveCN111945001AEasy to separateReduce consumptionRadium compoundsProcess efficiency improvementMischmetalPhysical chemistry
The invention relates to a treatment method of superior molten slag of monazite. Specifically, the treatment method is a method for extracting and separating iron, uranium, thorium and mischmetal in the superior molten slag of the monazite in a mixed acid solution of hydrochloric acid and nitric acid, and comprises the following steps : thermally dissolving the superior molten slag using hydrochloric acid; separating iron, uranium-thorium and rare earth; separating iron and uranium; preparing mixed feed liquor of hydrochloric acid and nitric acid for thorium and rare earth; separating thoriumand rare earth; and extracting mischmetal. The treatment method is good in uranium, thorium and rare earth separation effect and high in recovery rate, uranium and thorium products meeting nuclear fuel requirements can be produced, cyclic utilization of acid can be achieved, and the treatment method is environmentally friendly.
Owner:永州市湘江稀土有限责任公司
Method for preparing U3O8 from uranium-containing sodium carbonate solution
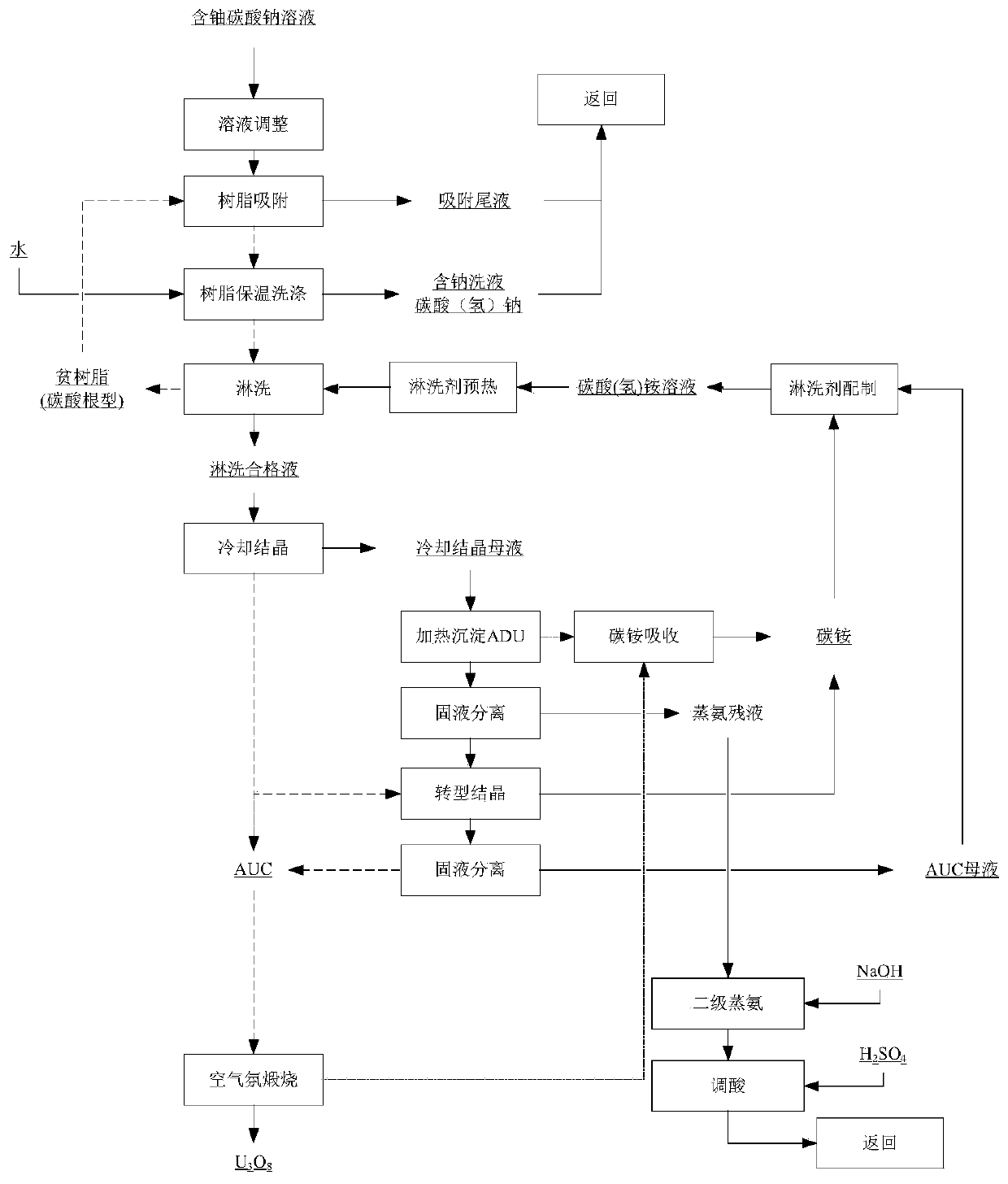
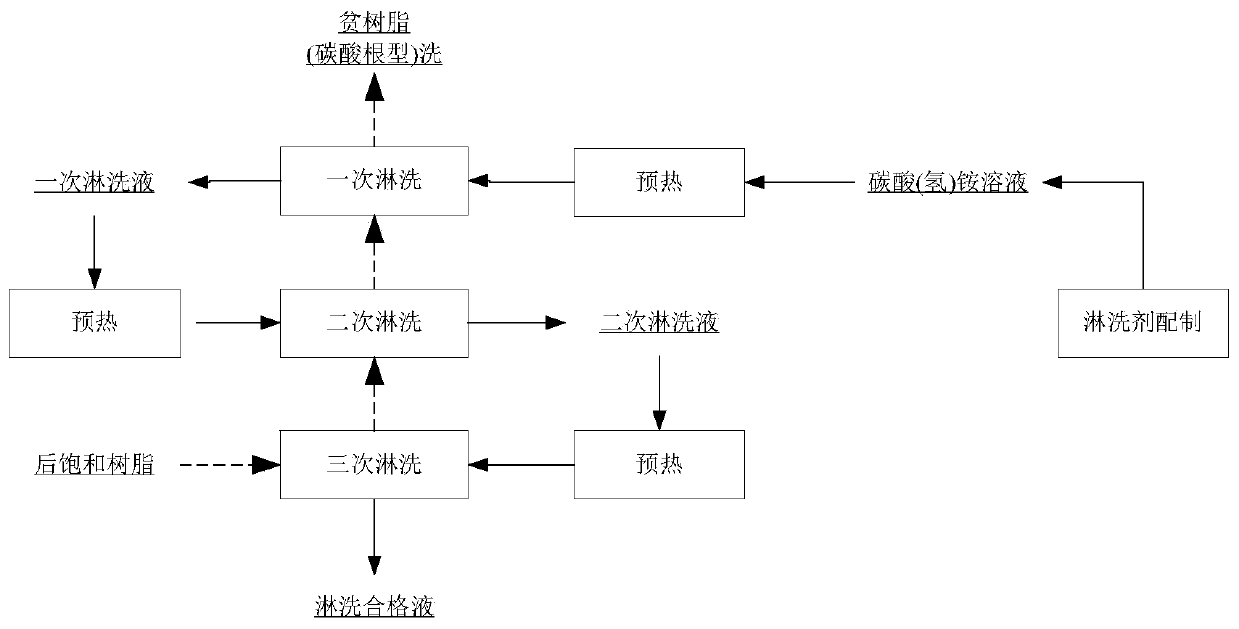
ActiveCN111547773AImprove adsorption capacityIncrease concentrationUranium compounds preparationUranium oxides/hydroxidesDistillationPhysical chemistry
The invention belongs to the technical field of uranium ore hydrometallurgy, and particularly relates to a method for preparing U3O8 from a uranium-containing sodium carbonate solution, which comprises the following steps: step 1, adsorbing a sodium carbonate system by using high-capacity resin; step 2, leaching non-crystalline ammonium bicarbonate; 3, carrying out double-part crystallization; step 4, performing ammonia distillation circulation technology; by adopting the technical process, the qualified U3O8 product is prepared. PH adjustment is adopted, so that the resin adsorption capacityis improved, the uranium concentration is improved, and the ammonia distillation treatment capacity is reduced; by adopting the recommended process parameters and equipment, the problems of bed blockage and pipeline blockage in the ammonium bicarbonate leaching process are avoided. A double-part crystallization process is adopted, so that the crystallization efficiency is improved, the crystal form is good, and the crystallization and impurity removal effects are improved. By adopting the process, ammonia-free and chlorine-free emission is realized, and ammonia is completely circulated.
Owner:BEIJING RESEARCH INSTITUTE OF CHEMICAL ENGINEERING AND METALLURGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com