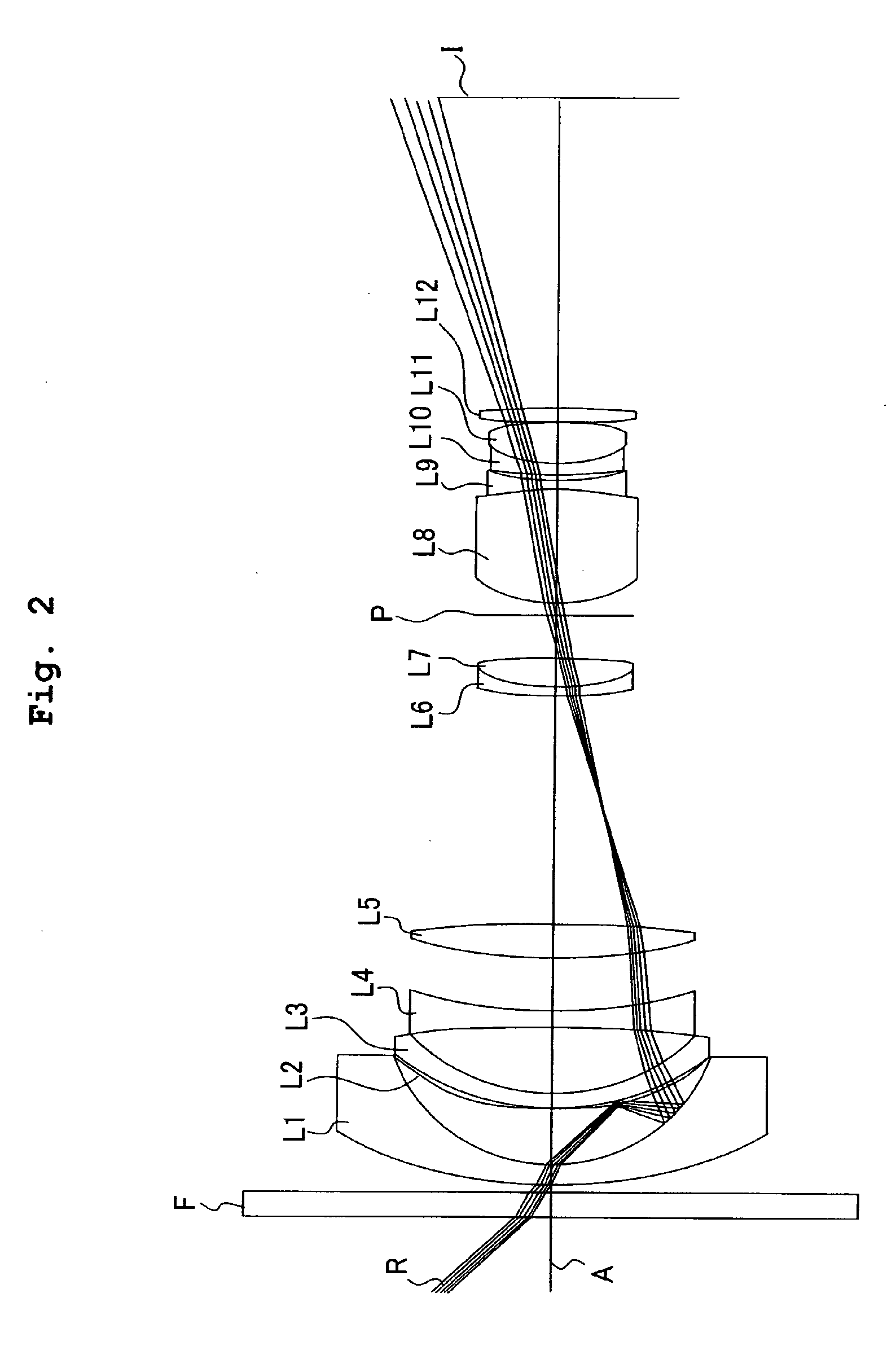
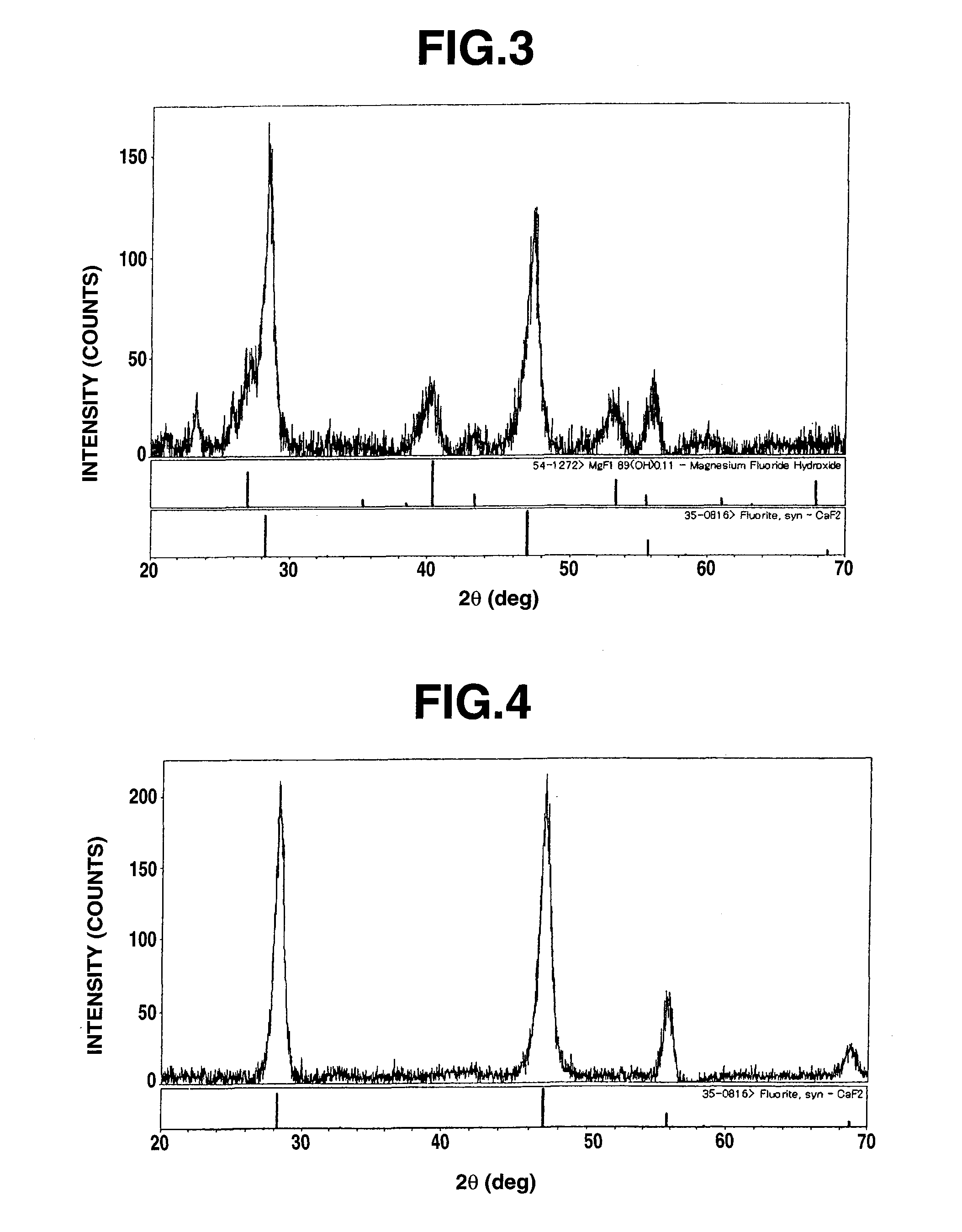
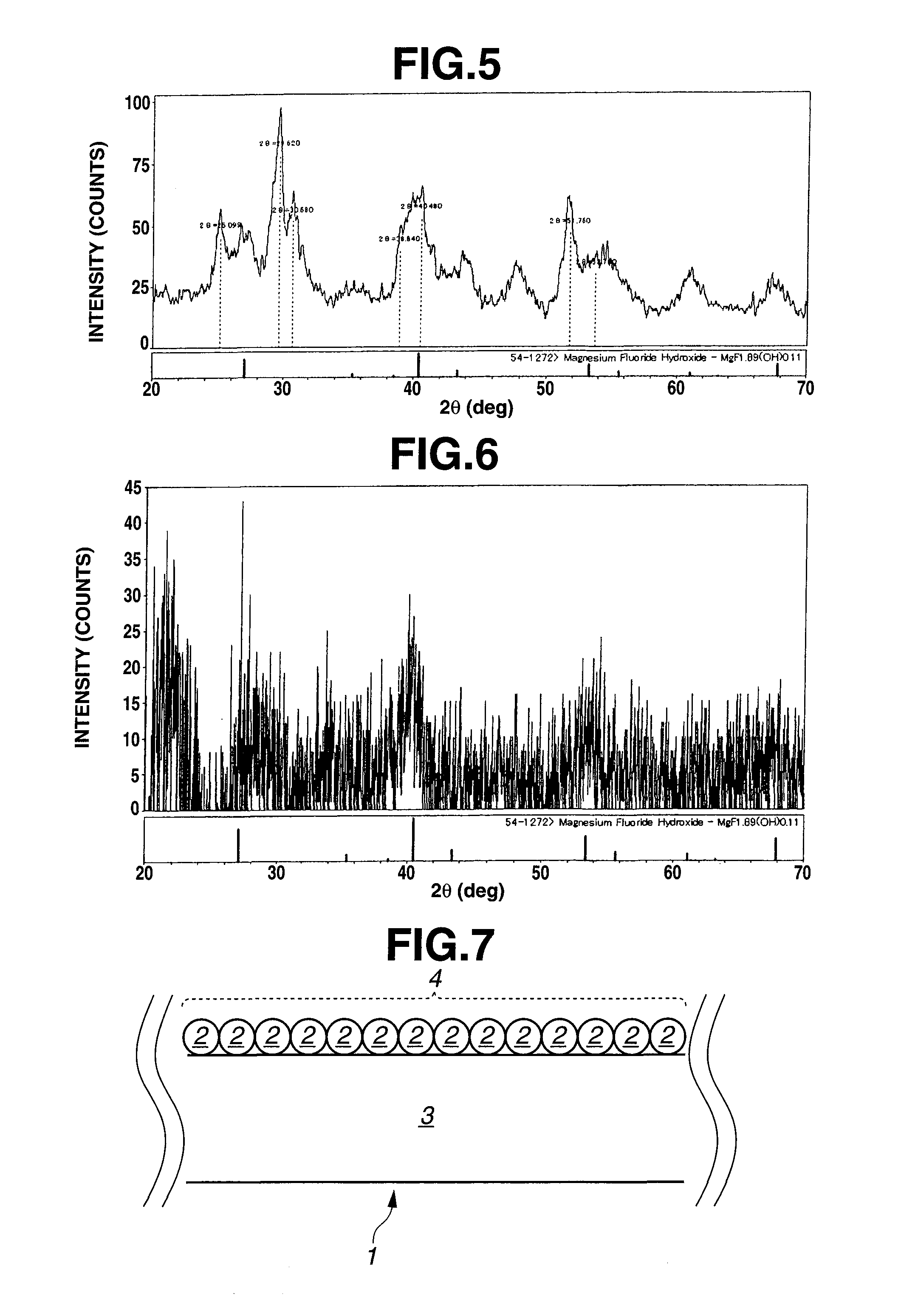
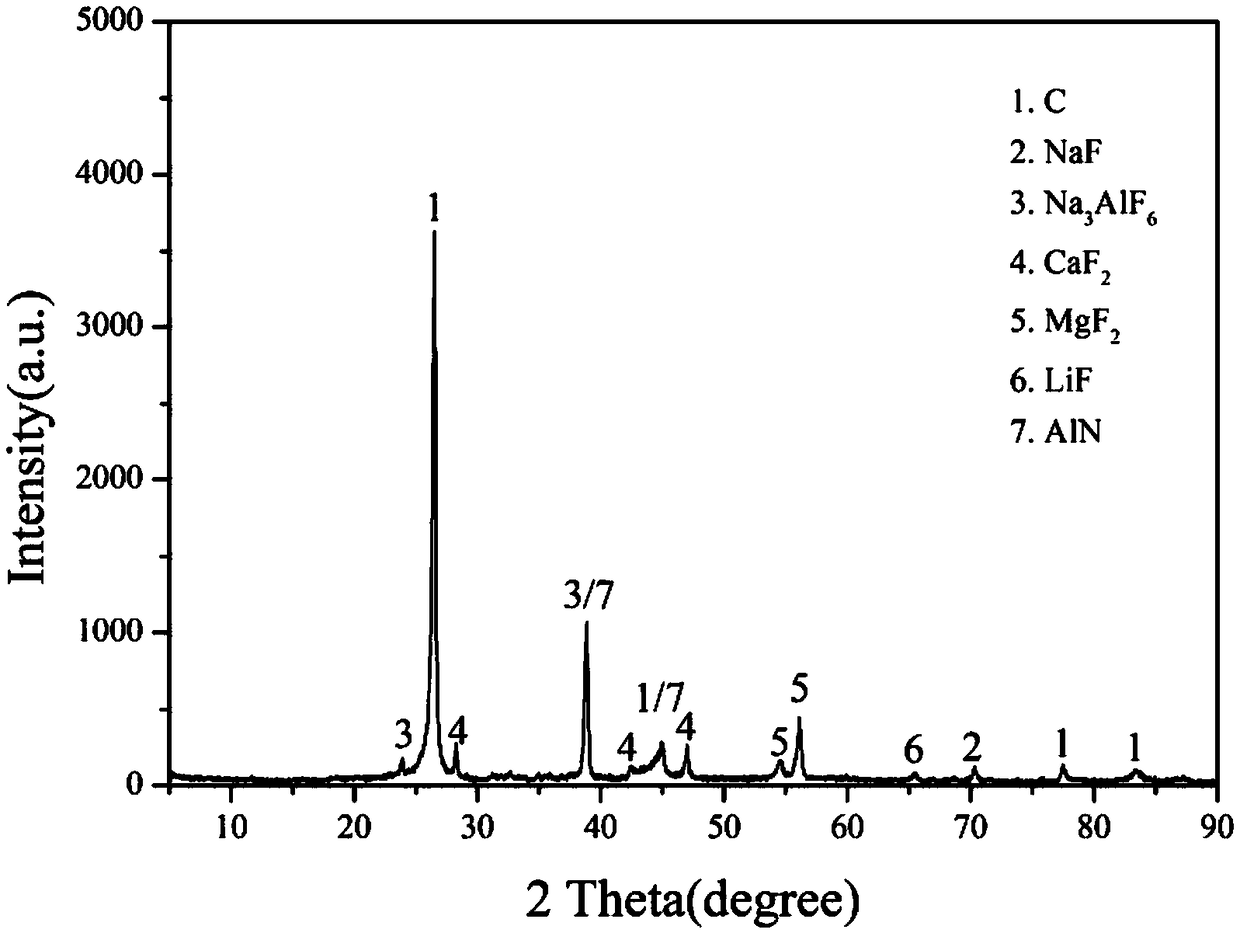
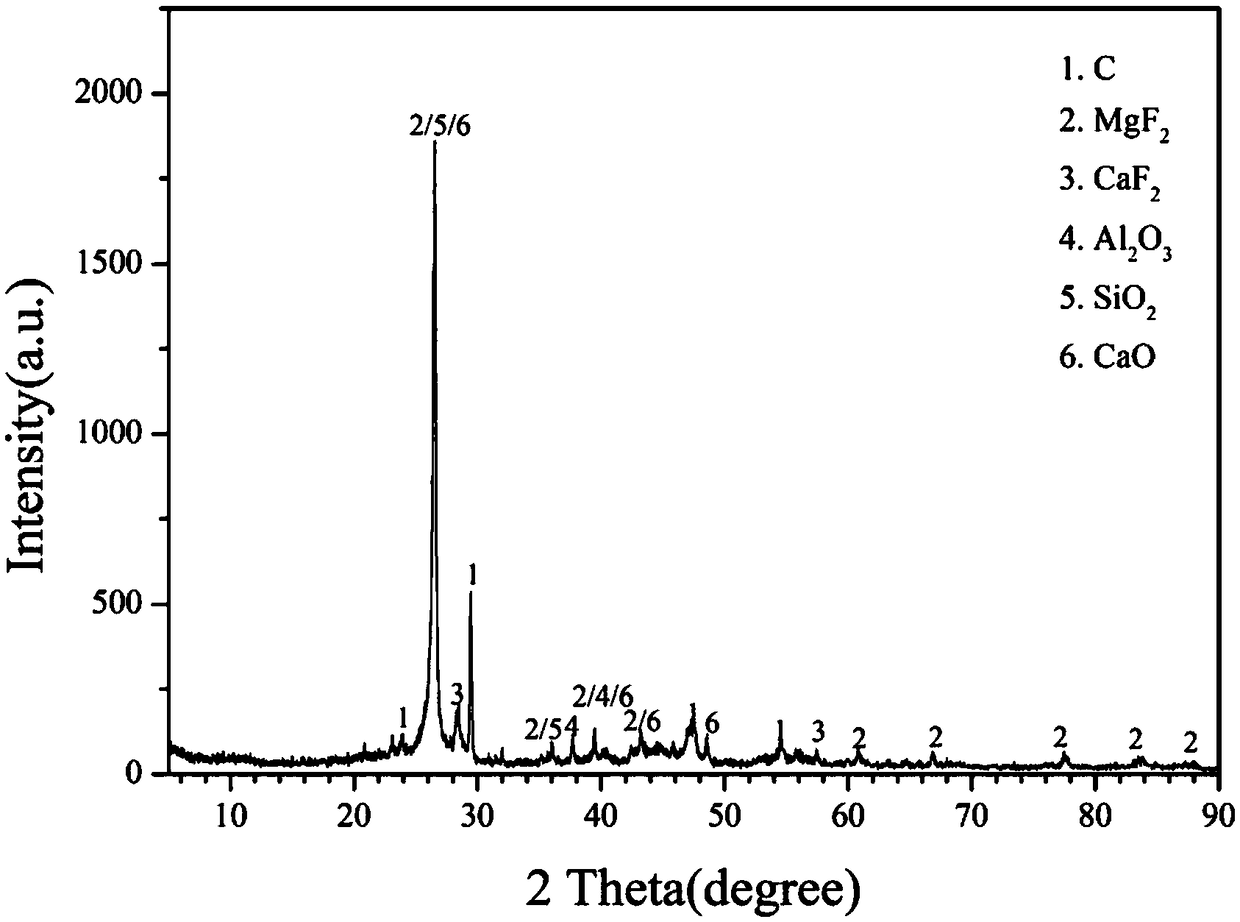
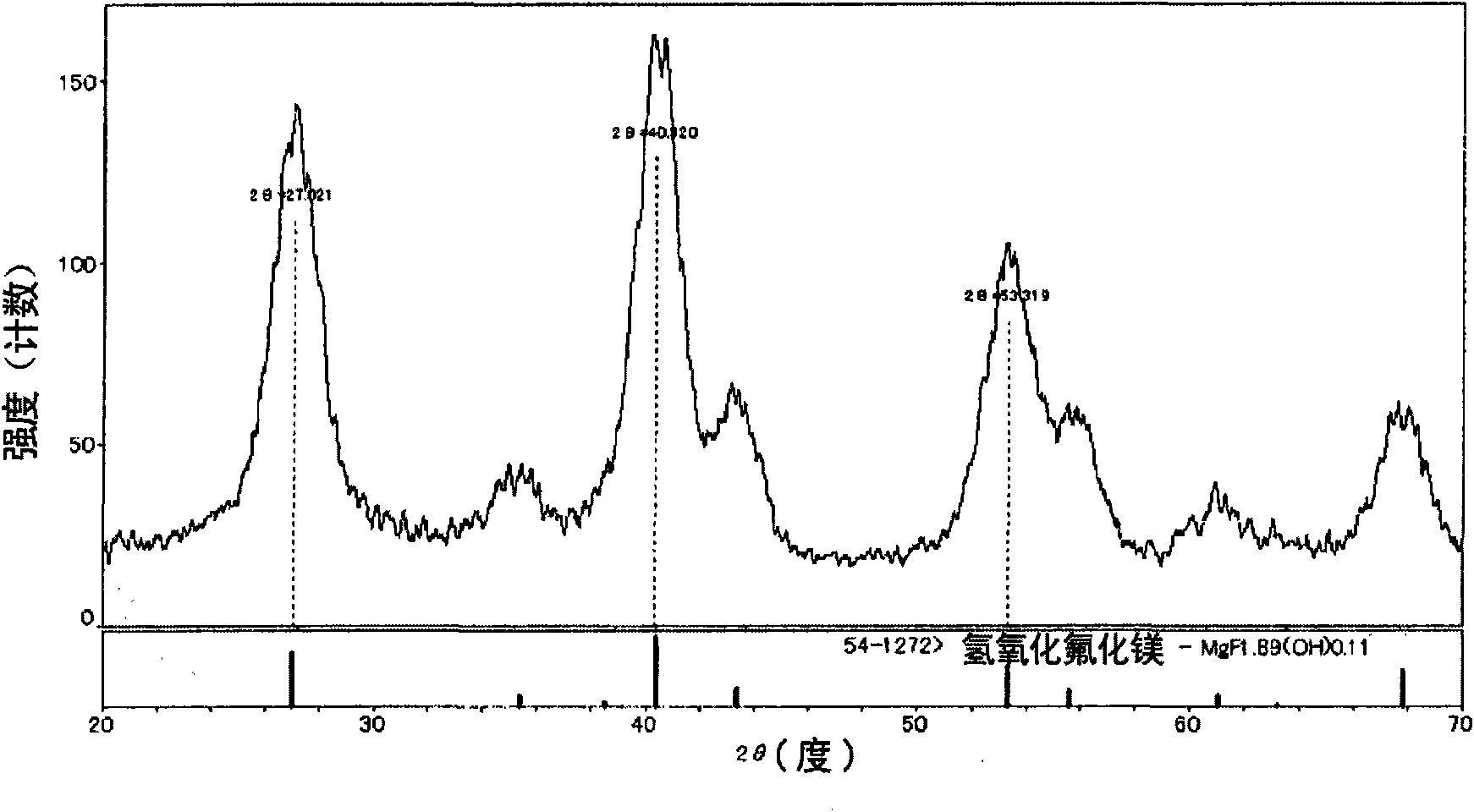
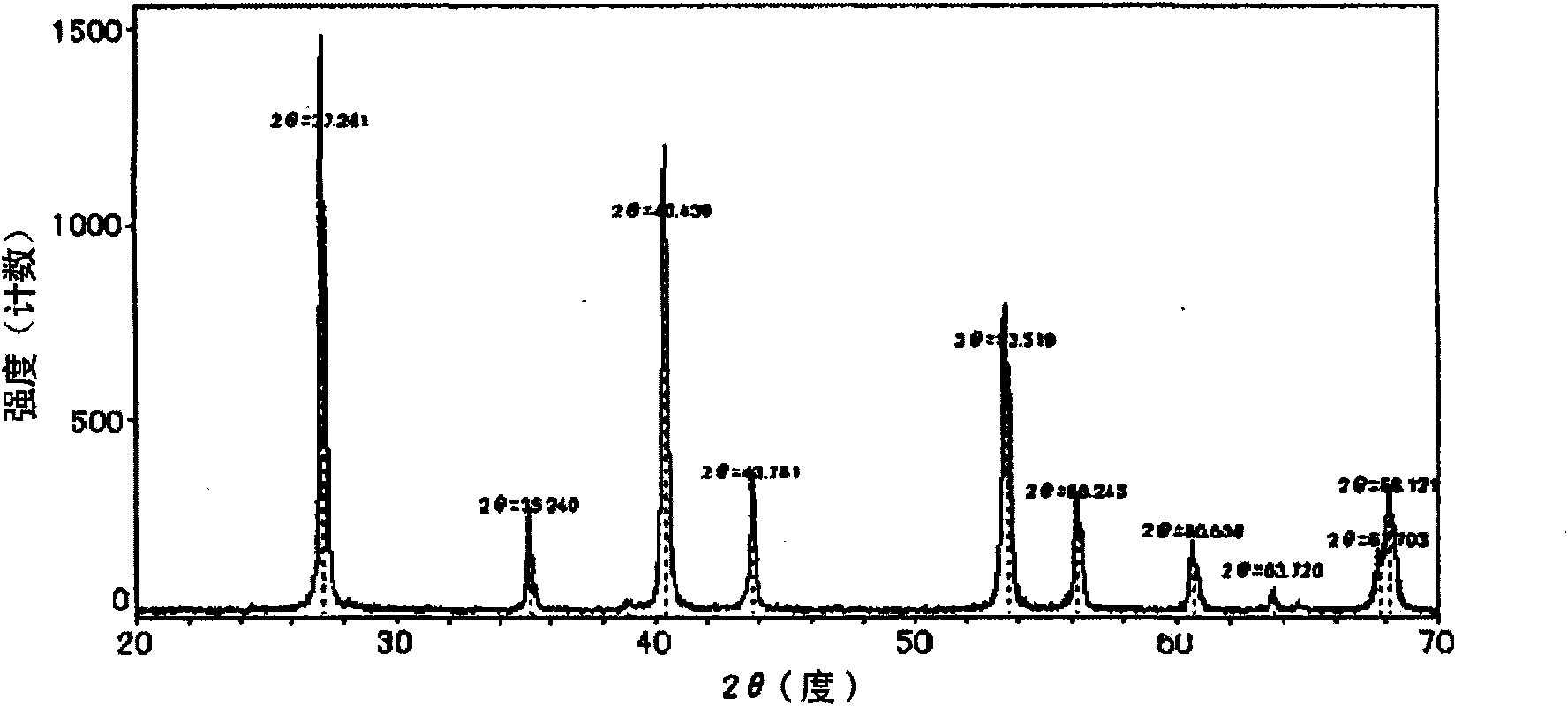
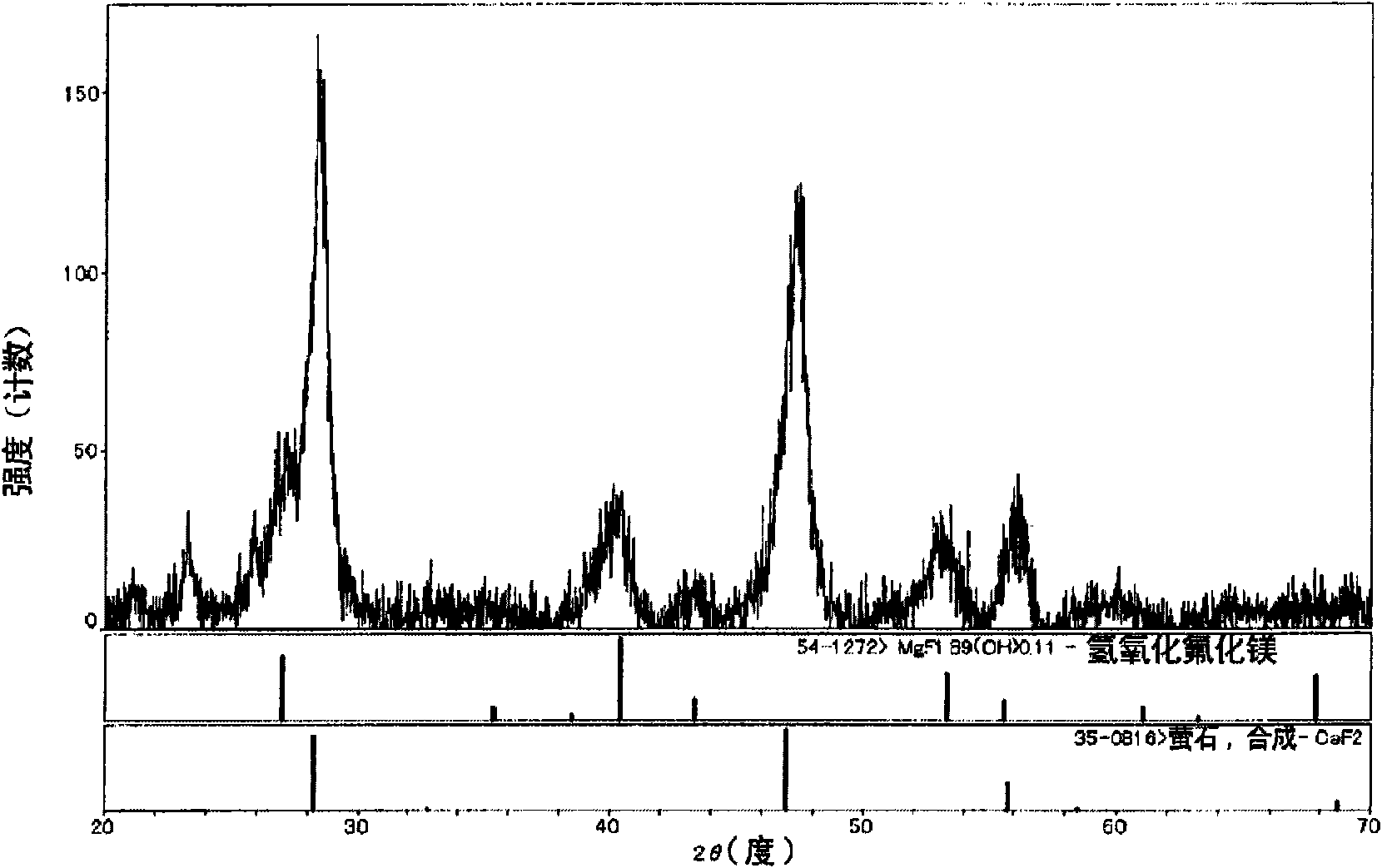
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
165results about "Magnesium fluorides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Single-crystal-like materials
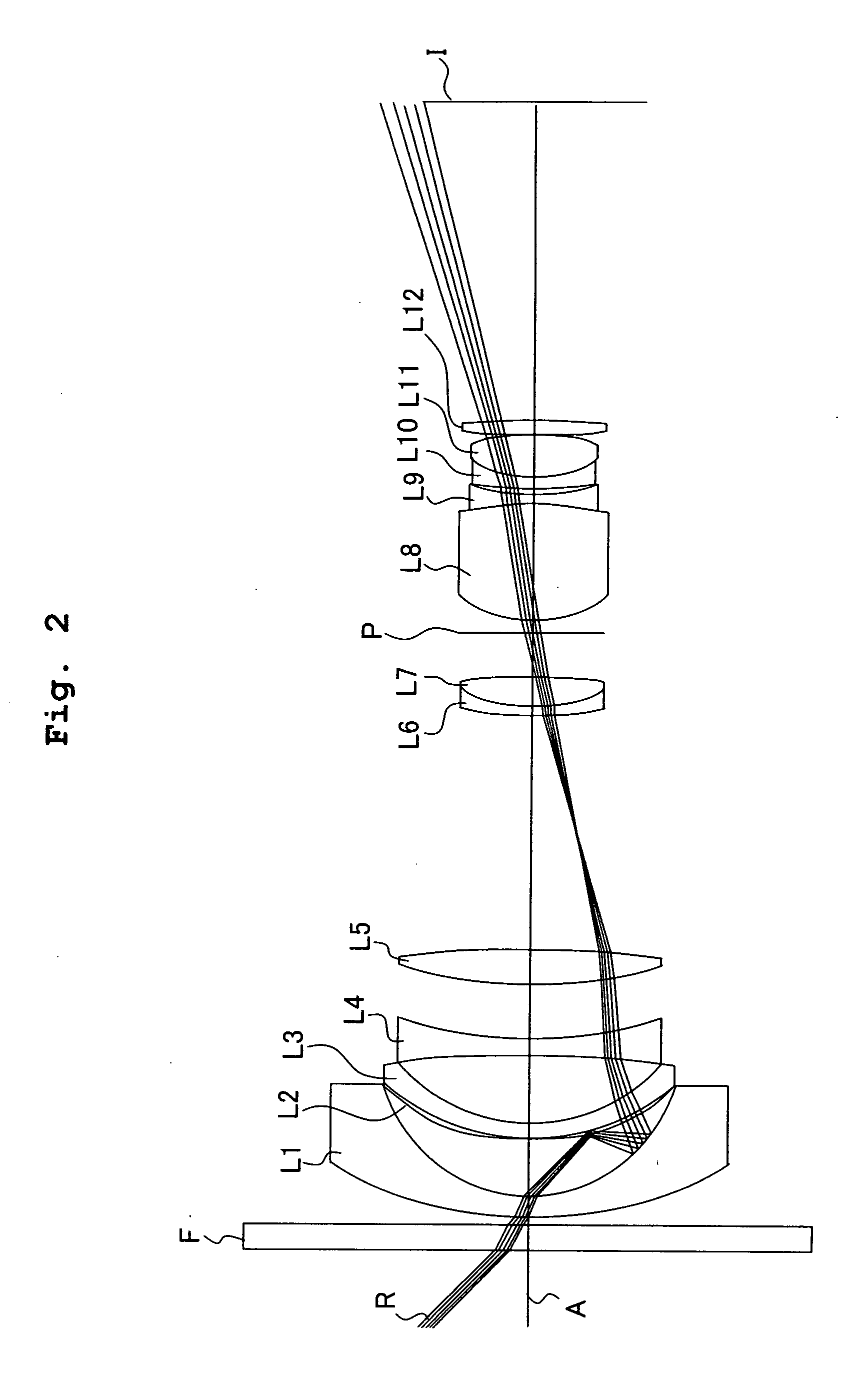
InactiveUS7022303B2Improve fracture toughnessReadily apparentMagnesium fluoridesCalcium/strontium/barium fluoridesMetallurgySingle crystal
Polycrystalline materials of macroscopic size exhibiting Single-Crystal-Like properties are formed from a plurality of Single-Crystal Particles, having Self-Aligning morphologies and optionally ling morphology, bonded together and aligned along at least one, and up to three, crystallographic directions.
Owner:RUTGERS THE STATE UNIV
Mgf2 Optical Thin Film Including Amorphous Silicon Oxide Binder, Optical Element Provided With the Same, and Method for Producing Mgf2 Optical Thin Film
InactiveUS20080002259A1Excellent in environment resistance (durability)Secure environment resistanceMagnesium fluoridesMaterial nanotechnologyRefractive indexAmorphous silicon
An MgF2 optical thin film is formed on an optical surface of a base material. The MgF2 optical thin film includes MgF2 particles and an amorphous silicon oxide-based binder which exists on the surfaces of the MgF2 particles and between the MgF2 particles. Owing to this amorphous silicon oxide-based binder, the optical thin film can have high mechanical strength and high adhesion to the base material, while having excellent environment resistance and a lower refractive index.
Owner:NIKON CORP
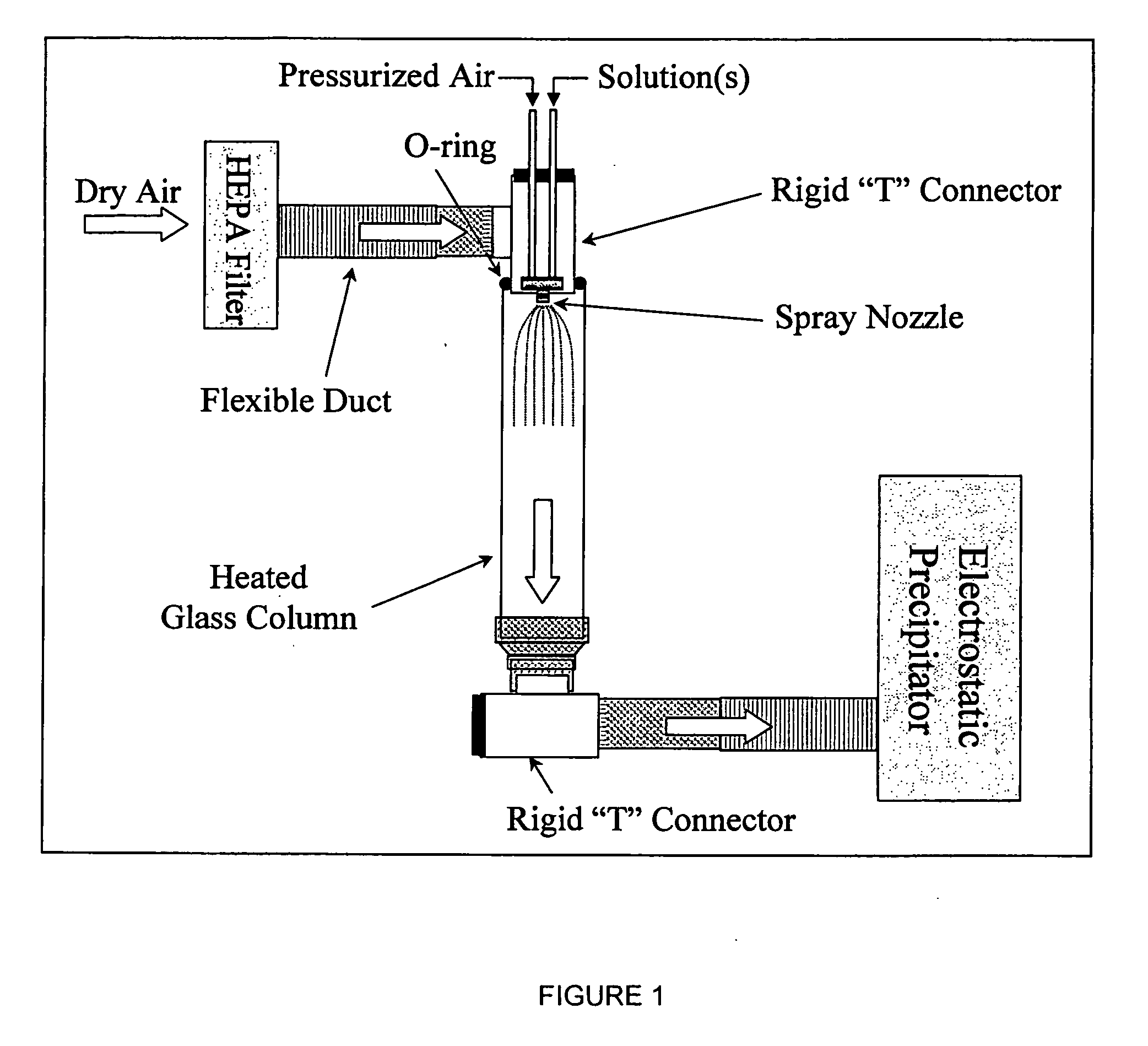
Nanostructured bioactive materials prepared by dual nozzle spray drying techniques
ActiveUS20060110306A1Easy to evaporatePromote formationMagnesium fluoridesImpression capsNanoparticleNanostructure
Nano-particles of calcium and phosphorous compounds are made in a highly pure generally amorphous state by spray drying a weak acid solution of said compound and evaporating the liquid from the atomized spray in a heated colunm followed by collection of the precipitated particles. Hydroxyapatite (HA) particles formed by such apparatus and methods are examples of particle manufacture useful in bone and dental therapies. Dual nozzle spraying etechniques are utilized for generally insoluble compounds.
Owner:ADA FOUND
Silica-magnesium fluoride hydrate composite sols and process for their preparation
InactiveUS6291535B1Low refractive indexEasy to prepareMagnesium fluoridesMaterial nanotechnologyOrganic solventMagnesium salt
A sol comprising silica-magnesium fluoride hydrate composite colloidal particles used in an anti-reflection coating material for forming an anti-reflection coating and a process for its preparation are provided. A sol comprising silica-magnesium fluoride hydrate composite colloidal particles having a ratio of silica to magnesium fluoride hydrate MgF2.nH2O, n being in the range between 0.25 and 0.5, in terms of a SiO2 / MgF2 weight ratio of from 0.01 to 5 and a primary particle size of 5 to 50 nm. A process for the preparation of an aqueous sol comprising silica-magnesium fluoride hydrate composite colloidal particles which comprises the steps of adding an aqueous fluoride solution to a mixture liquid of a silica sol and an aqueous magnesium salt solution to produce a slurry of an agglomerate comprising silica-magnesium fluoride hydrate composite colloidal particles and removing the salts formed as by-products. A process for the preparation of an organosol further comprising the step of replacing water in the aqueous sol with an organic solvent.
Owner:NISSAN CHEM IND LTD
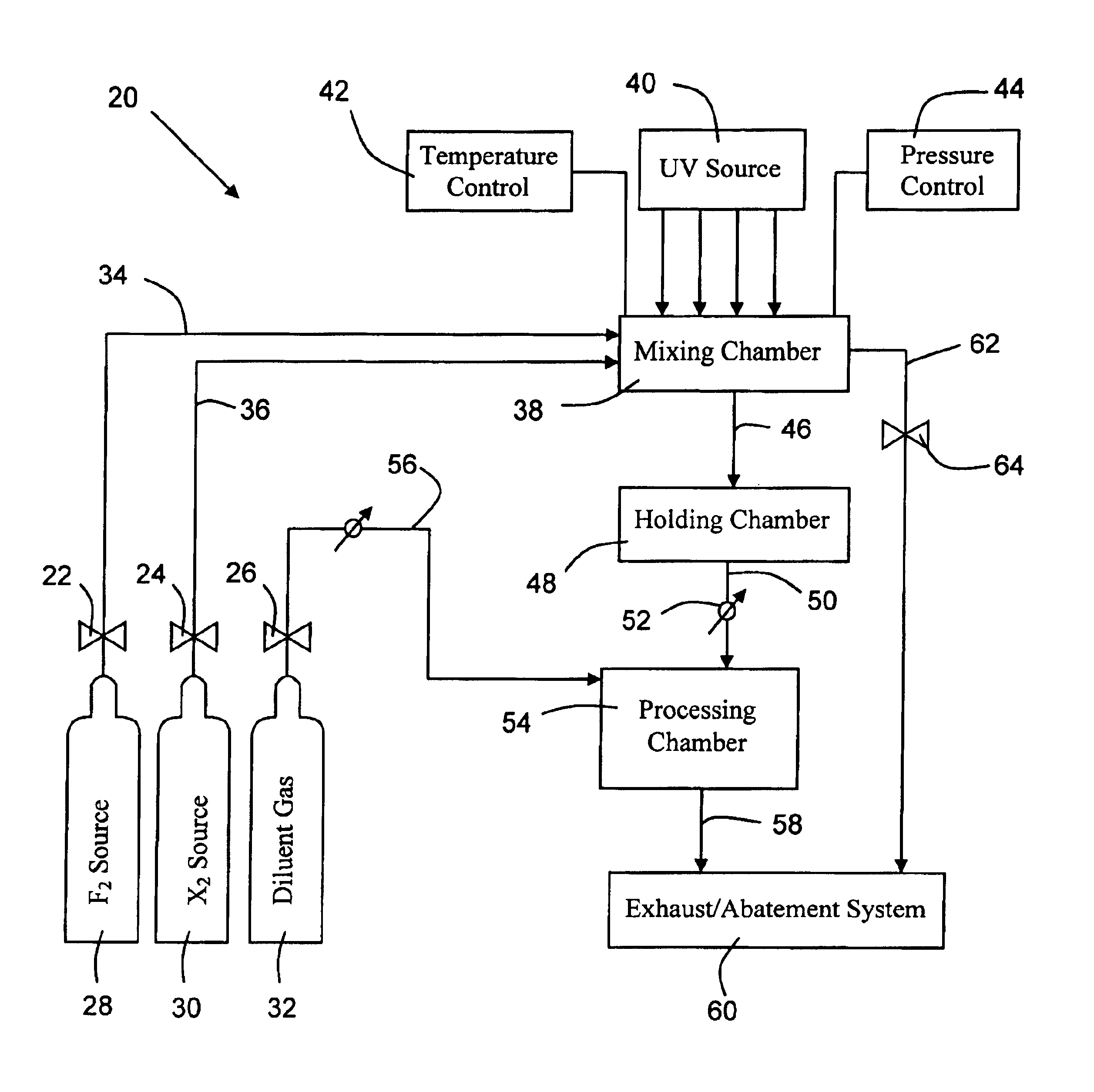
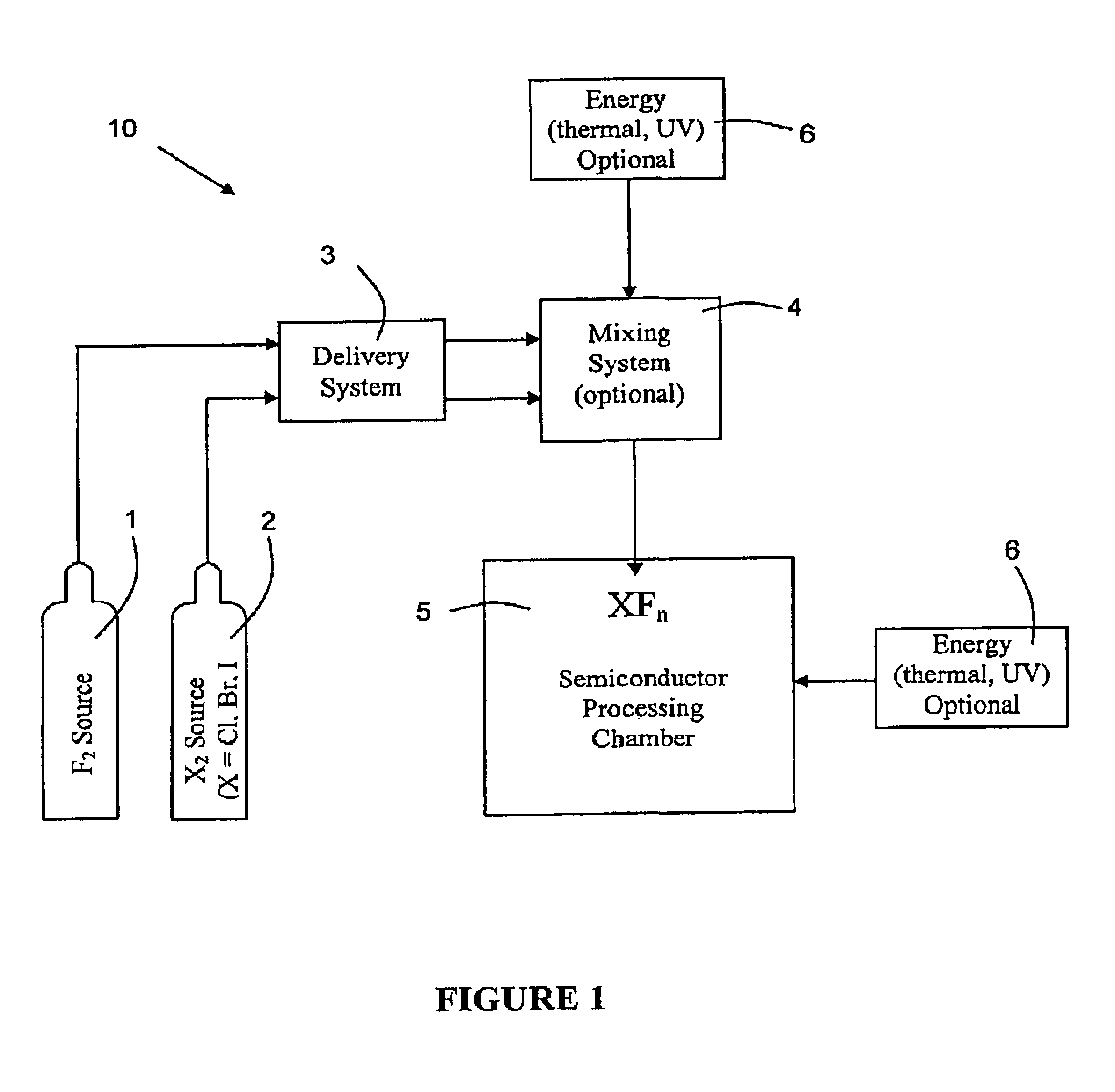
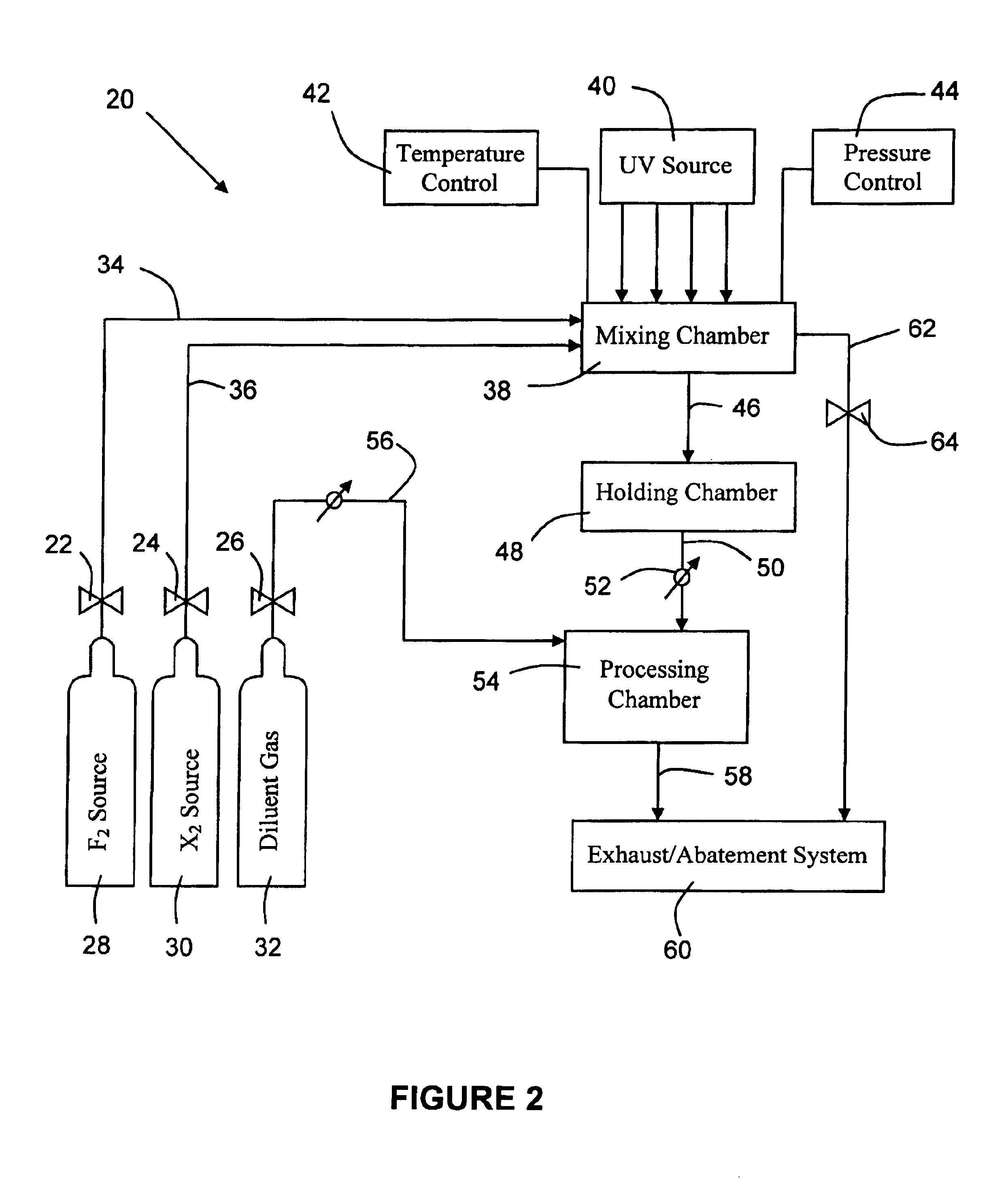
System for in-situ generation of fluorine radicals and/or fluorine-containing interhalogen (XFn) compounds for use in cleaning semiconductor processing chambers
InactiveUS6841141B2Shorten “ waiting-period ”Improve reaction speedMagnesium fluoridesCalcium/strontium/barium fluoridesChemistrySupply energy
A system for in-situ generation of fluorine radicals and / or fluorine-containing interhalogen compounds XFn (wherein X is Cl, Br, or I, and n is an odd integer). Such system comprises a fluorine source, a halogen source for supplying halogen species other than fluorine, a chamber for mixing fluorine with halogen species other than fluorine, and an energy source to supply energy to such chamber to facilitate reaction between fluorine and the halogen species other than fluorine. The chamber may be a semiconductor processing chamber, wherein the in situ generated fluorine radicals and / or fluorine-containing interhalogens are employed for cleaning the processing chamber.
Owner:ENTEGRIS INC
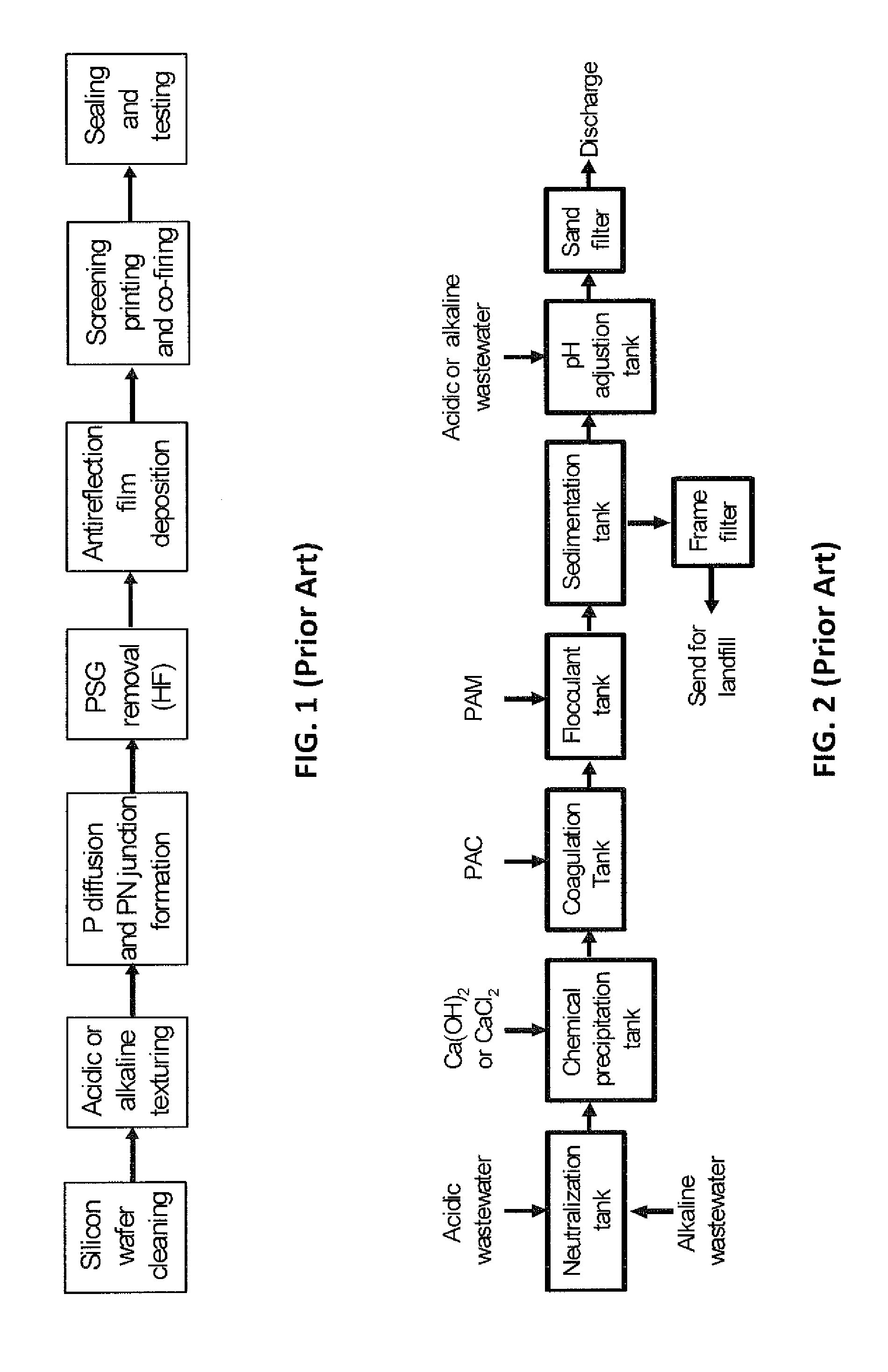
Environment-friendly separation and recovery method of fluorine in fluorine-containing waste liquid
InactiveCN105948083AAchieve separationAchieve recyclingMagnesium fluoridesHydrogen fluorideRecovery methodEnvironmental resistance
The invention discloses an environment-friendly separation and recovery method of fluorine in a fluorine-containing waste liquid. According to the invention, a magnesium-containing compound is added into the fluorine-containing waste liquid as a precipitation agent, such that fluorine in the waste liquid is selectively precipitated; filtering is carried out, and fluorine-removed liquid and magnesium fluoride precipitate are obtained; the fluorine-removed liquid is used in waste water recycling; the magnesium fluoride precipitate is decomposed with sulfuric acid, such that a series of compounds of fluorine are obtained; decomposition residue is subjected to a dissolution-crystallization treatment, such that magnesium sulfate crystals are obtained; the obtained magnesium sulfate crystals are returned and recycled in the fluorine selective precipitation process; the crystallization mother liquor of magnesium sulfate is returned to the dissolution-crystallization process or the magnesium fluoride precipitation decomposition process. The method has the advantages of simple process, simple operation, low production cost, and good fluorine-removing effect. With the method, fluorine resource utilization is realized. The method also has the advantages of no fluorine-containing waste production and no three-waste emission.
Owner:CENT SOUTH UNIV
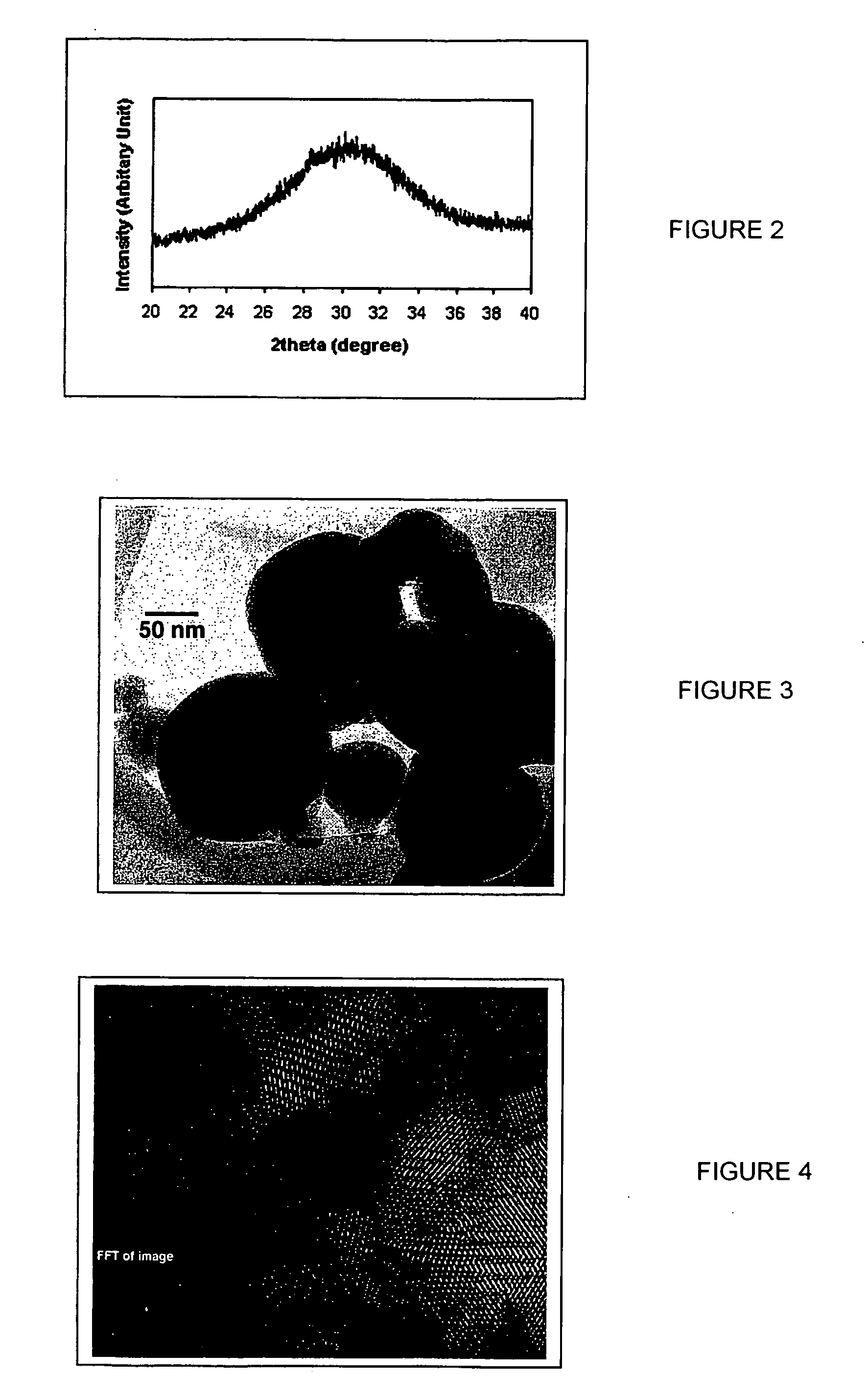
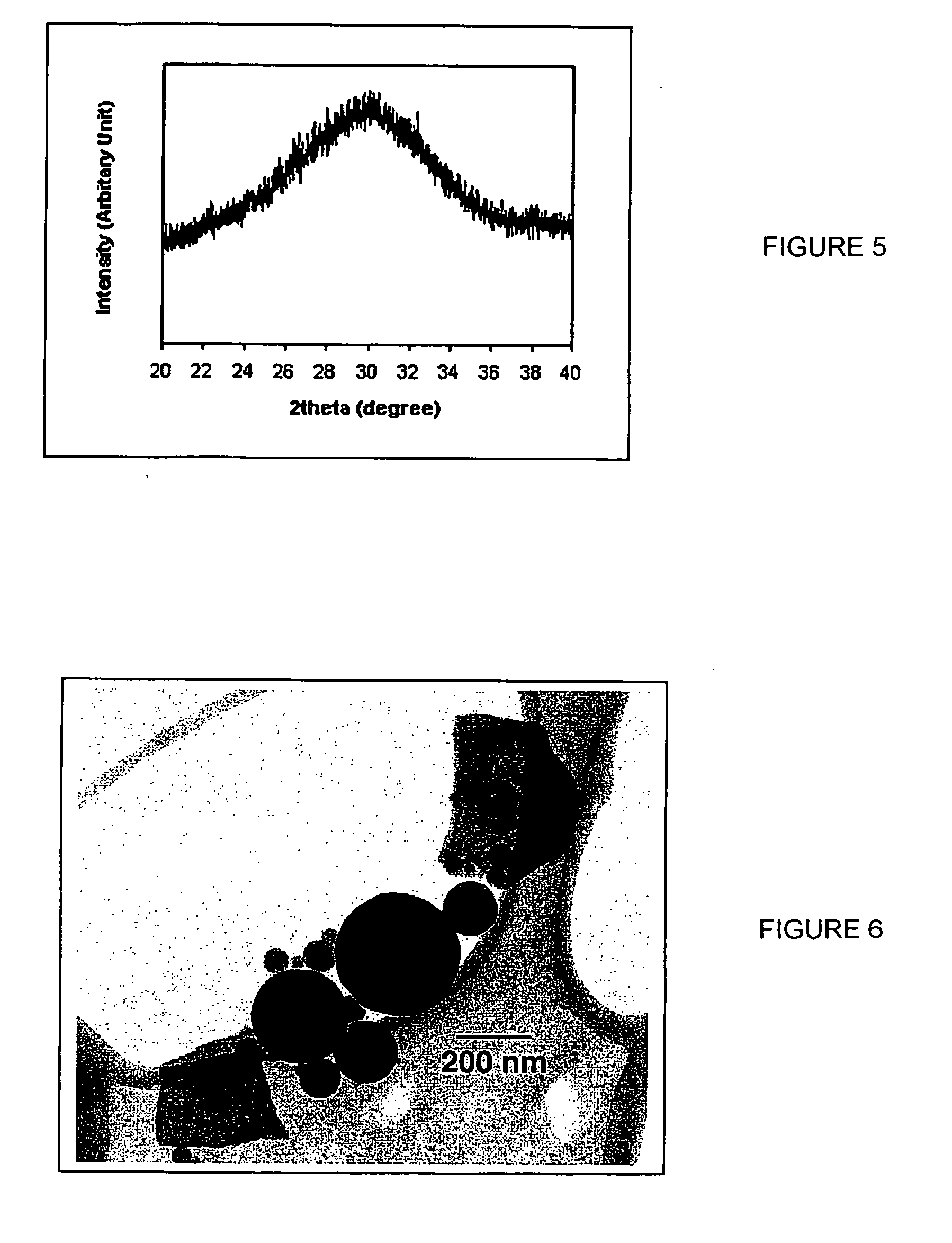
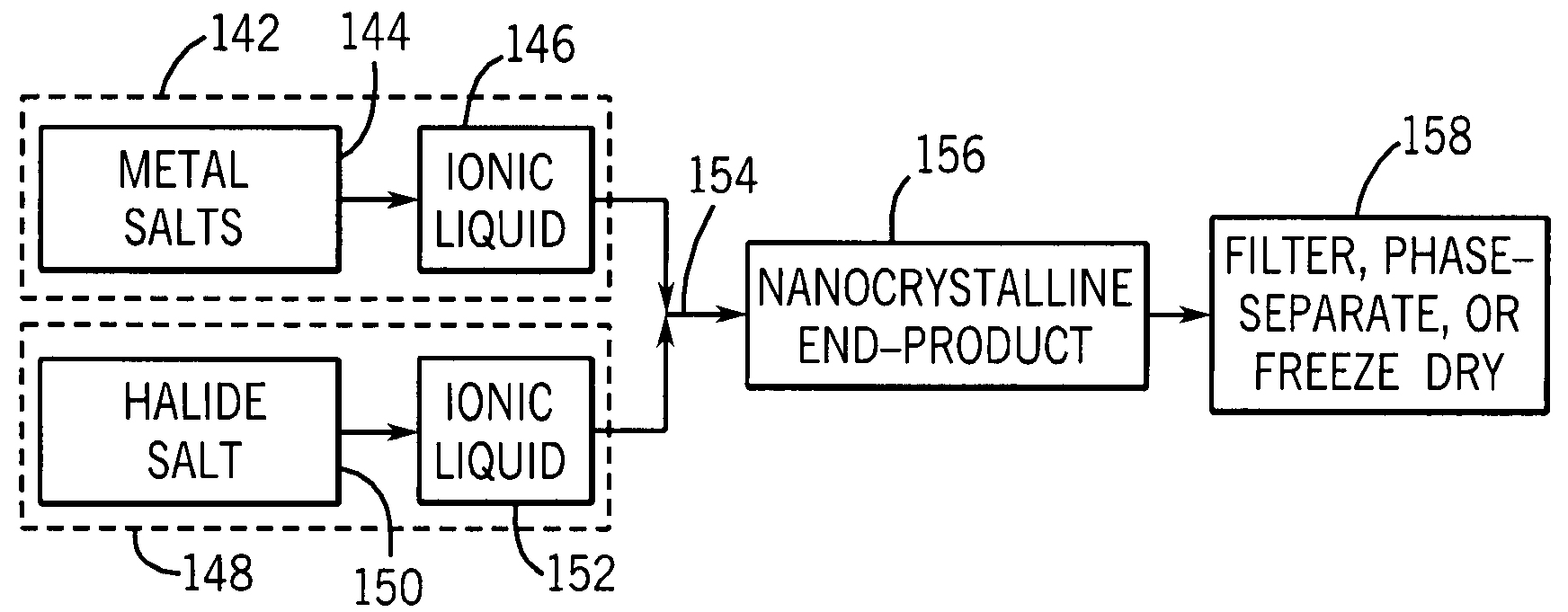
Nano-scale metal halide scintillation materials and methods for making same
Crystalline scintillator materials comprising nano-scale particles of metal halides are provided. The nano-scale particles are less than 100 nm in size. Methods are provided for preparing the particles. In these methods, ionic liquids are used in place of water to allow precipitation of the final product. In one method, the metal precursors and halide salts are dissolved in separate ionic liquids to form solutions, which are then combined to form the nano-crystalline end product. In the other methods, micro-emulsions are formed using ionic liquids to control particle size.
Owner:GENERAL ELECTRIC CO
Method for separating lithium carbonate from electrolyte acidic leachate
InactiveCN107935015AEfficient extractionReduce energy consumptionMagnesium fluoridesCalcium/strontium/barium fluoridesEvaporationCarbonate
The invention discloses a method for separating lithium carbonate from electrolyte acidic leachate. The method comprises the following steps of: S1, adding a soluble salt solution to aluminum electrolyte acidic leachate while stirring and heating are performed, monitoring the acidity and the concentration of fluoride ions, and stopping the addition when the pH value is greater than 5 and the concentration of the fluoride ions is less than 0.01 g / L, wherein the soluble salt is one or more selected from MeSO4, MeNO3 and MeCl, and Me is metal which can produce precipitate with F<->; S2, performing filtration on reactants, performing washing and drying on filter residues to obtain a fluoride salt of the metal Me, adding a soluble carbonate solution into the obtained filtrate with stirring andheating, and terminating the reaction when the concentration of lithium ions is less than 0.08 g / L; S3, filter the filtrate, performing washing and drying on the filter cake to obtain lithium carbonate, and performing evaporation, crystallization, washing and drying on the filtrate to obtain inorganic salts. According to the method for separating lithium carbonate from the electrolyte acidic leachate, the reaction process is controlled by controlling the concentration of the fluoride ions and the acidity, so that the lithium ions are separated from other ions, lithium carbonate with high recovery rate is obtained, and meanwhile high-purity fluoride and inorganic salt products are obtained.
Owner:NORTHEASTERN UNIV
Making a dispersion managing crystal
InactiveUS6630117B2Magnesium fluoridesCalcium/strontium/barium fluoridesLithography processCrystalline materials
The present invention provides fluoride lens crystals for VUV optical lithography systems and processes. The invention provides a barium fluoride optical lithography crystal for utilization in 157 nm optical microlithography elements which manipulate below 193 nm optical lithography photons. The invention includes a barium fluoride crystalline material for use in dispersion management of below 160 nm optical lithography processes.
Owner:CORNING INC
Mgf2 optical thin film including amorphous silicon oxide binder, optical element provided with the same, and method for producing mgf2 optical thin film
InactiveUS20110122497A1Improve environmental resistanceAverage forceMagnesium fluoridesMaterial nanotechnologyRefractive indexAmorphous silicon
An MgF2 optical thin film is formed on an optical surface of a base material. The MgF2 optical thin film includes MgF2 particles and an amorphous silicon oxide-based binder which exists on the surfaces of the MgF2 particles and between the MgF2 particles. Owing to this amorphous silicon oxide-based binder, the optical thin film can have high mechanical strength and high adhesion to the base material, while having excellent environment resistance and a lower refractive index.
Owner:NIKON CORP
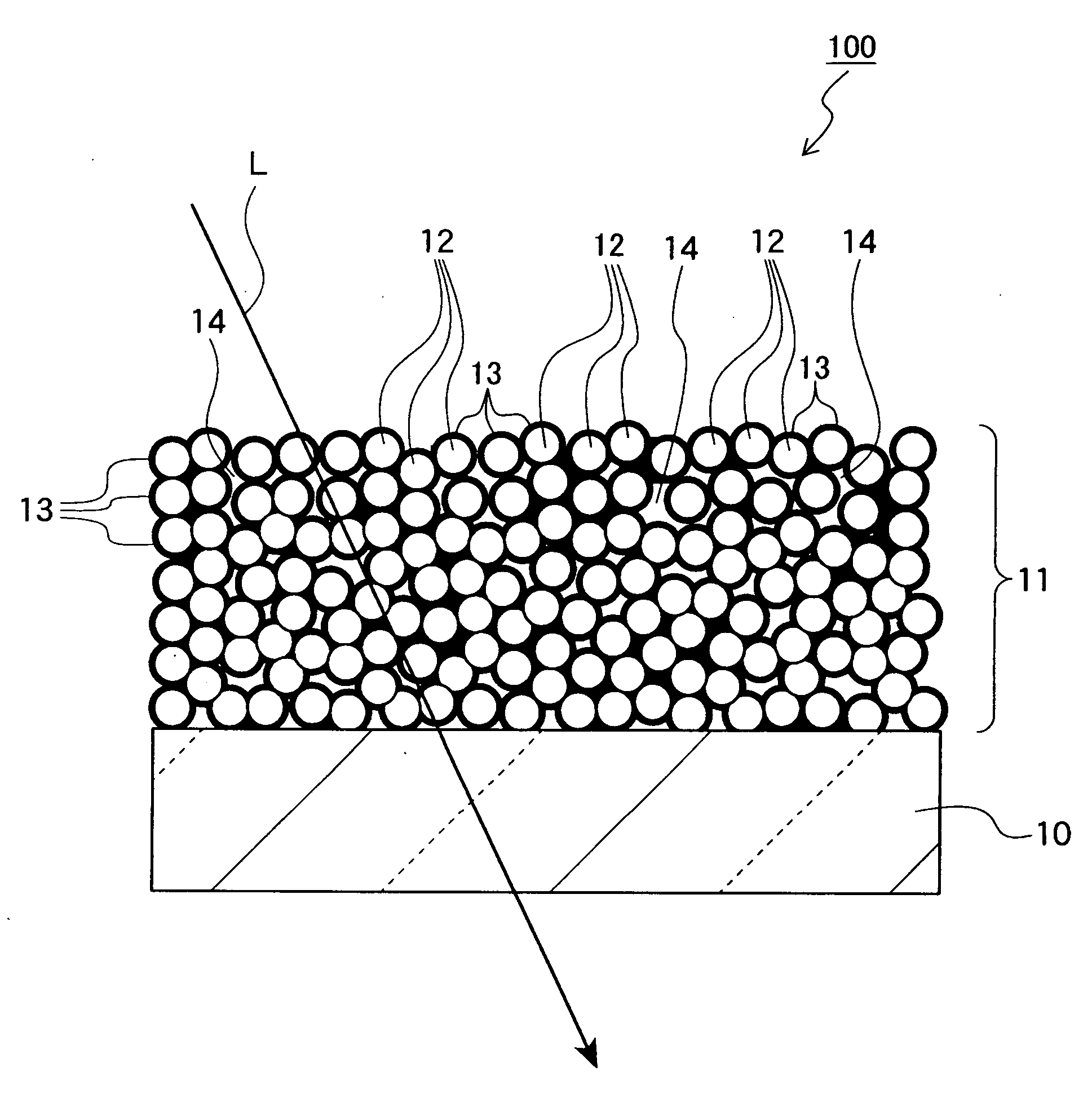
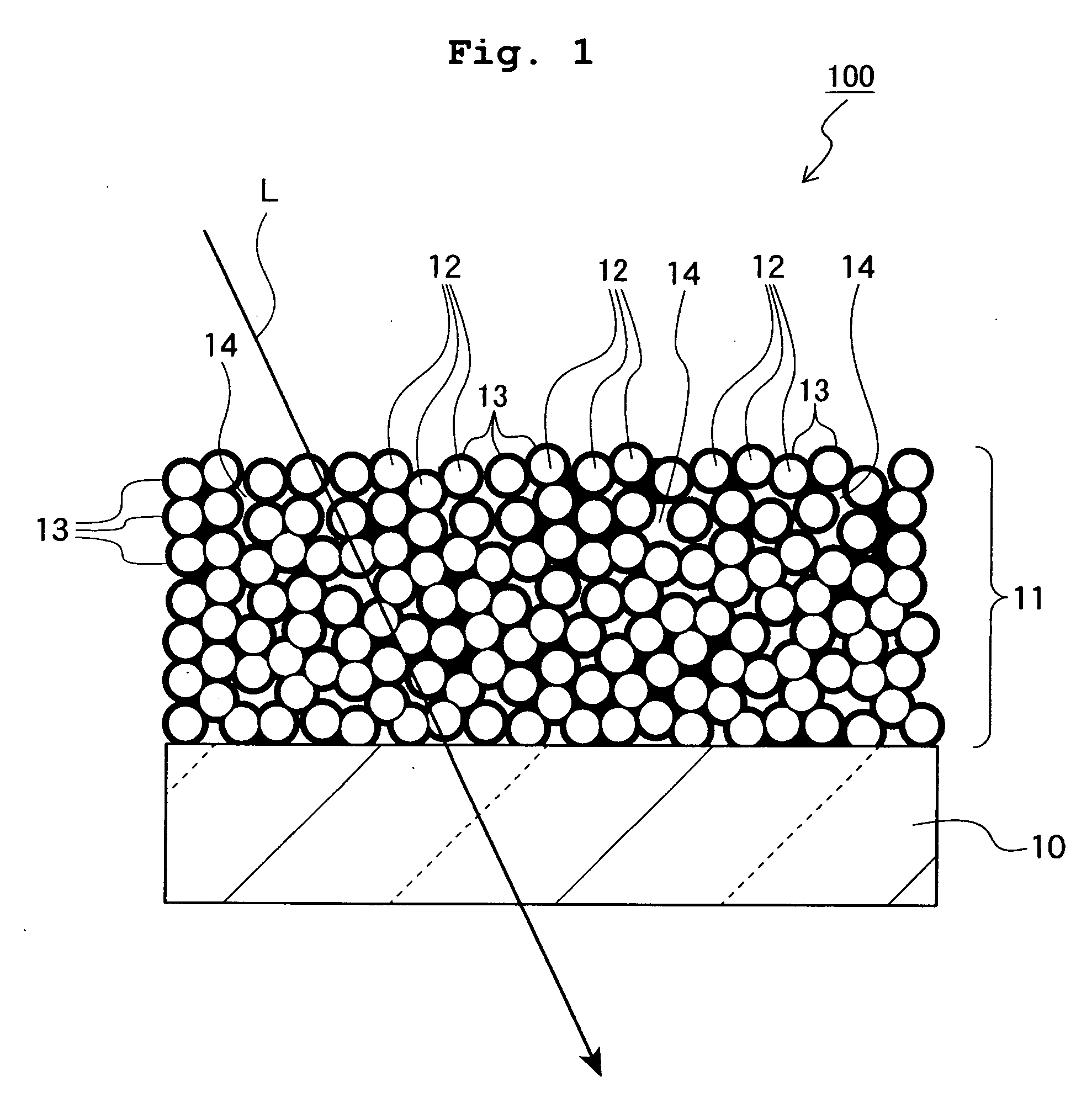
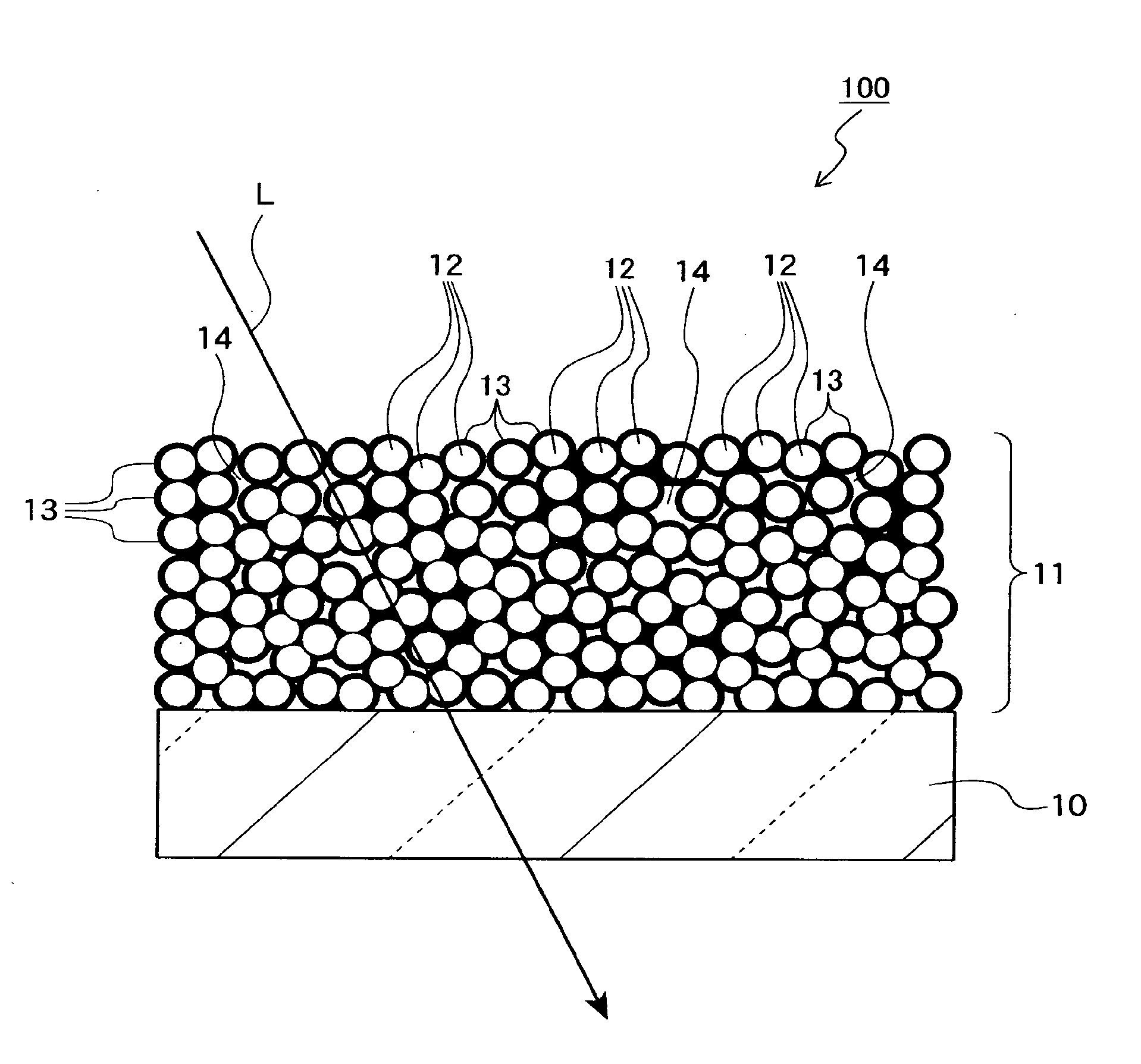
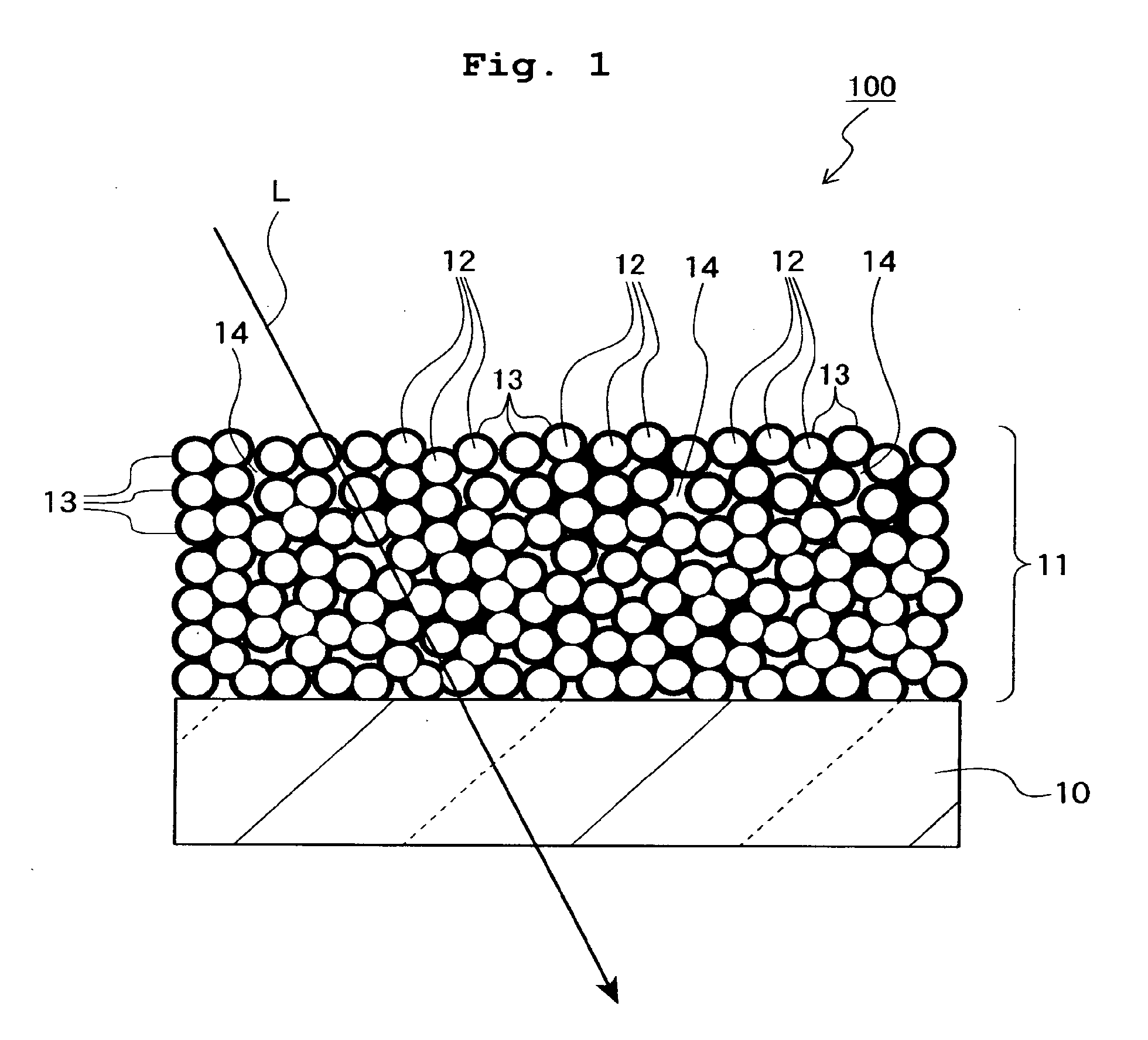
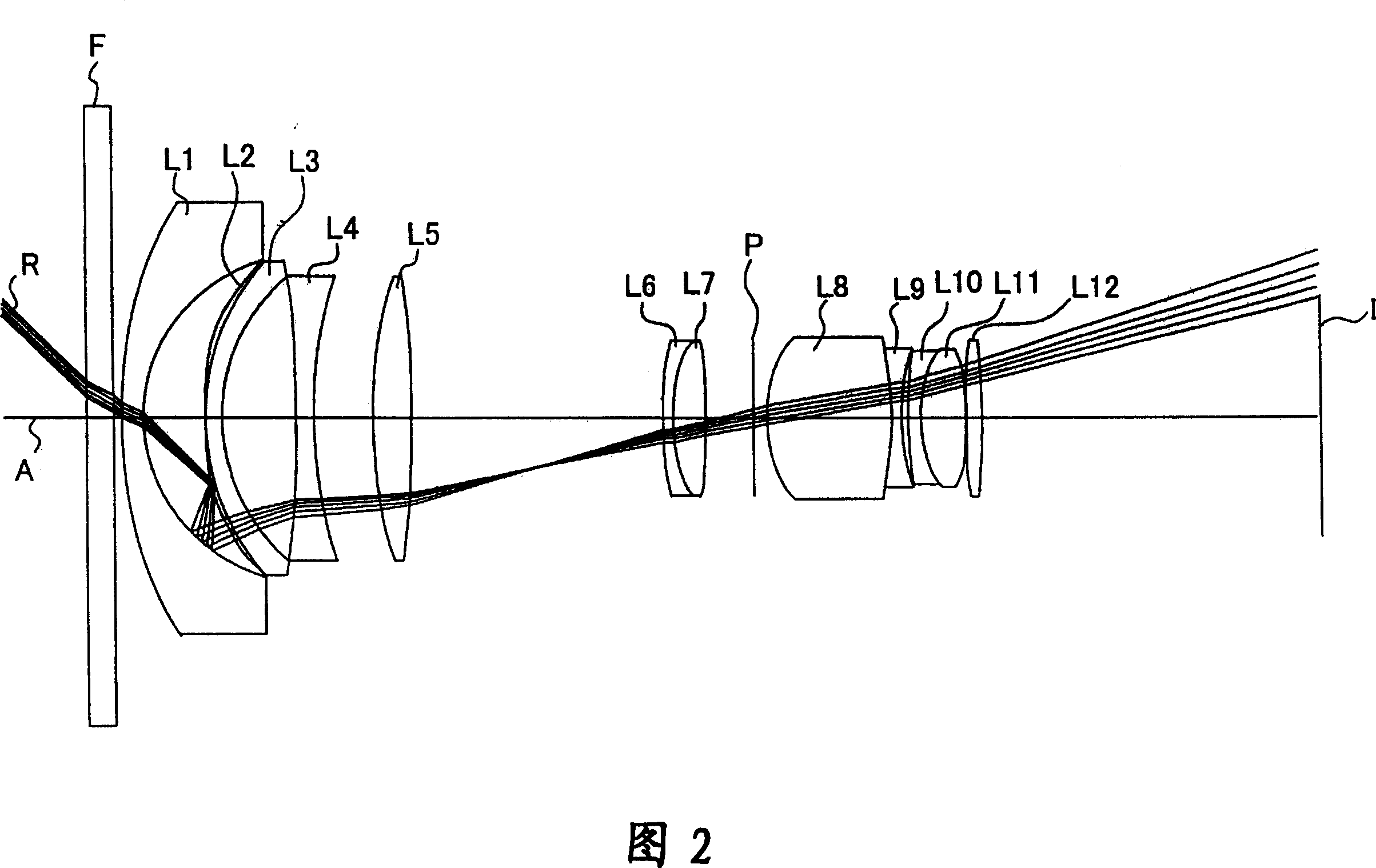
Mgf2 optical thin film containing amorphous silicon oxide binder, optical device having same, and method for producing such mgf2 optical thin film
An MgF 2 optical thin film 100 is formed on an optical surface of a base material 10. The MgF 2 optical thin film 100 includes MgF 2 particles 12 and an amorphous silicon oxide-based binder 13 which exists on the surfaces of the MgF 2 particles and between the MgF 2 particles. Owing to this amorphous silicon oxide-based binder 13, the optical thin film 100 can have high mechanical strength and high adhesion to the base material 10, while having excellent environment resistance and a lower refractive index.
Owner:NIKON CORP
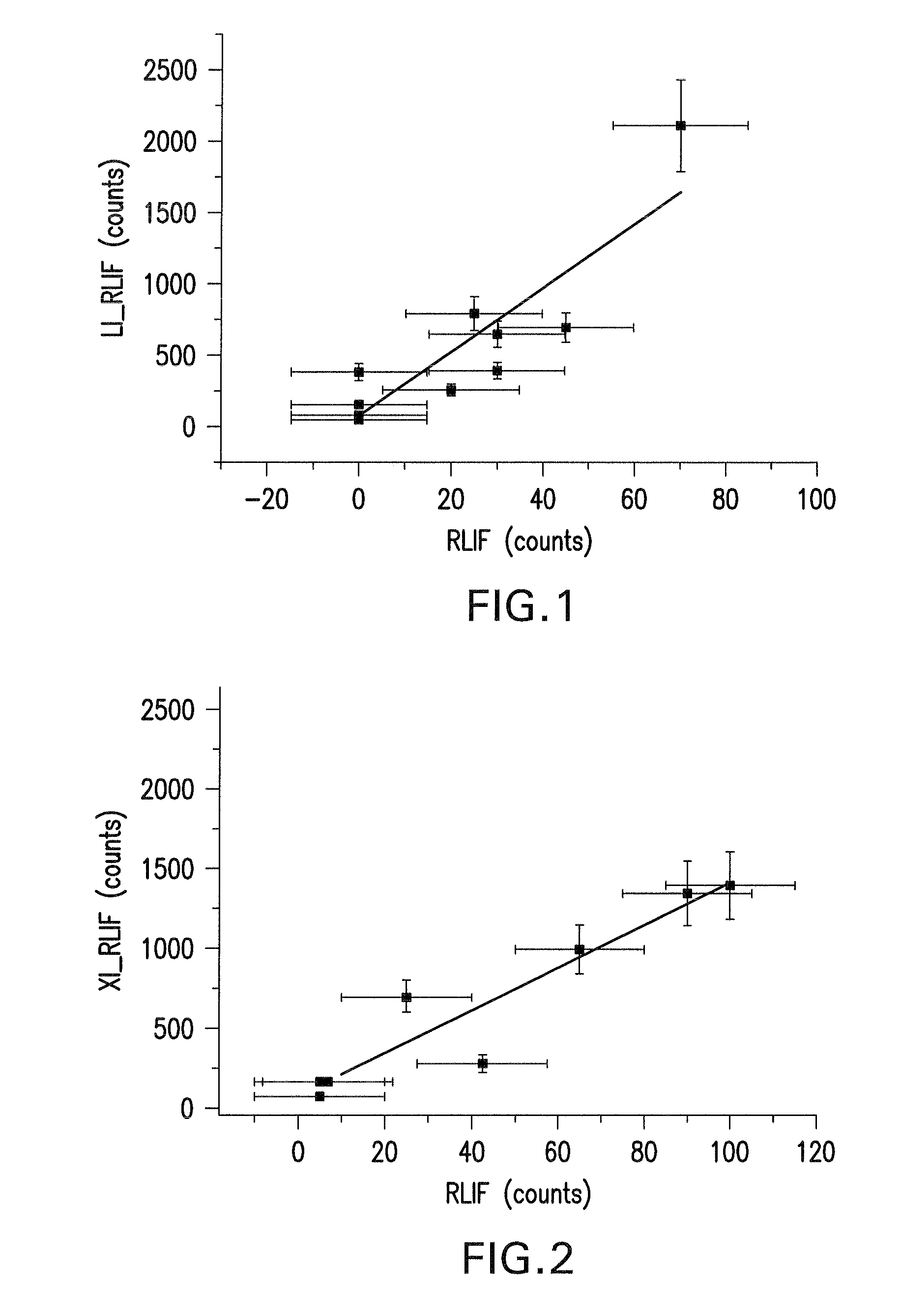
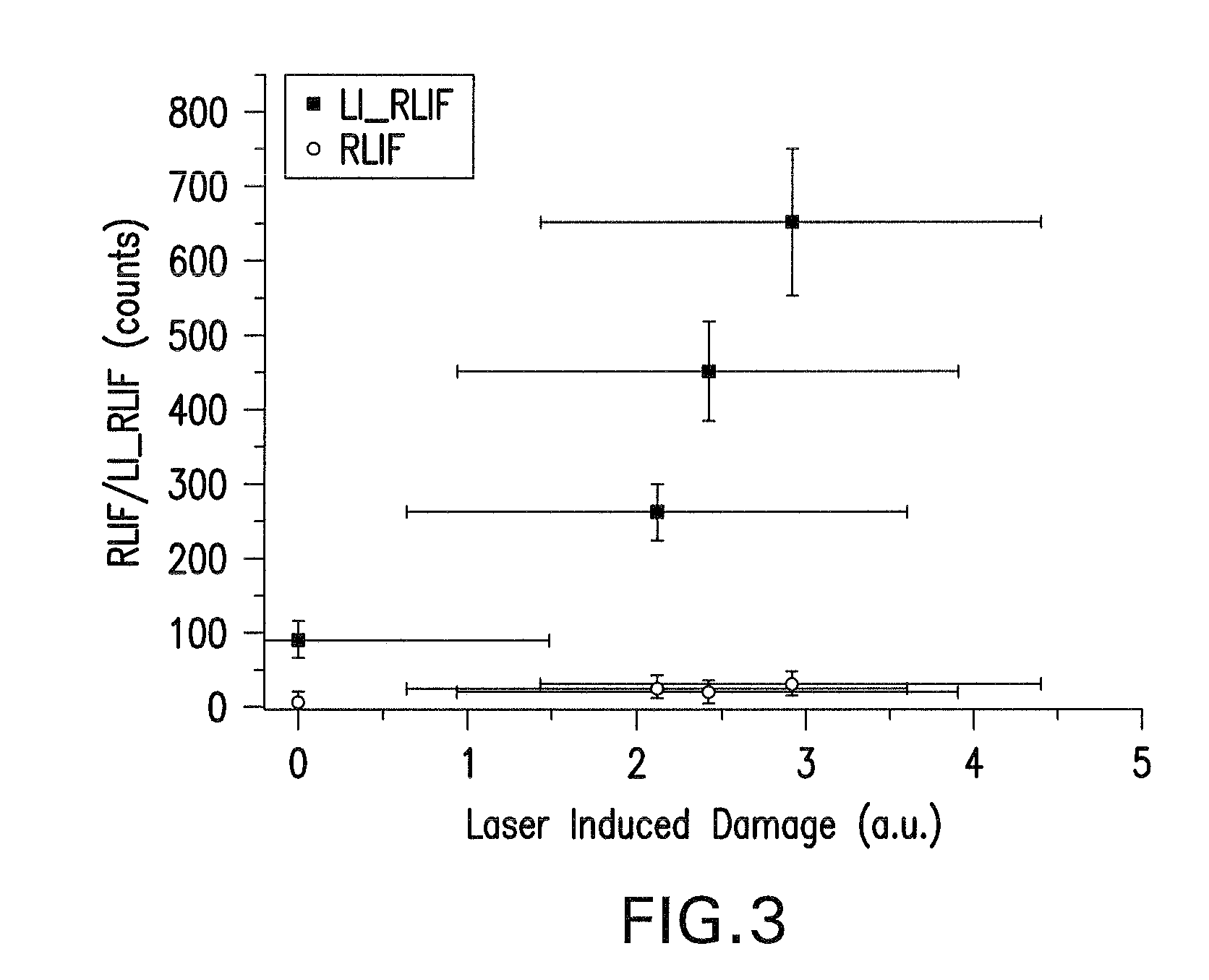
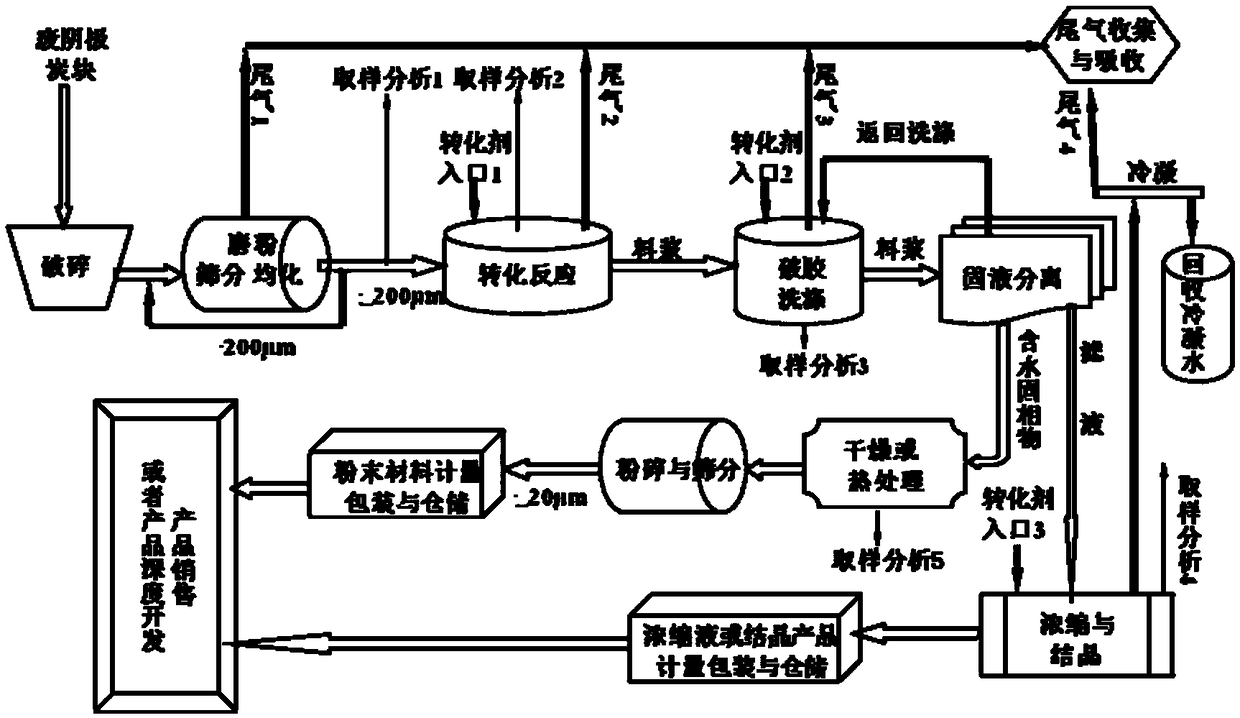
Method of determining laser stabilities of optical material, crystals obtained with said method, and uses of said crystals
InactiveUS20100111820A1Simple methodLong durationMagnesium fluoridesCalcium/strontium/barium fluoridesPre irradiationHigh energy
A method of selecting suitable laser-stable optical material for making an optical element, especially for transmission at wavelengths under 200 nm, is described. It includes a first pre-irradiation to produce radiation damage, subsequent excitation of induced fluorescence with light at between 350 to 700 nm at least ten minutes after the first pre-irradiation and measurement of induced fluorescence intensities at one or more wavelengths between 550 nm and 810 nm. After the fluorescence intensity measurement a second pre-irradiation is performed with an at least 1000-fold higher energy than in the first pre-irradiation and then induced fluorescence intensities are again measured to determine the increase in the fluorescence intensities. The materials determined to have suitable laser stability are used for making lenses, prisms, light-conducting rods, optical windows and optical devices for DUV lithography, especially steppers and excimer lasers, integrated circuits, computer chips as well as other electronic devices.
Owner:HELLMA MATERIALS
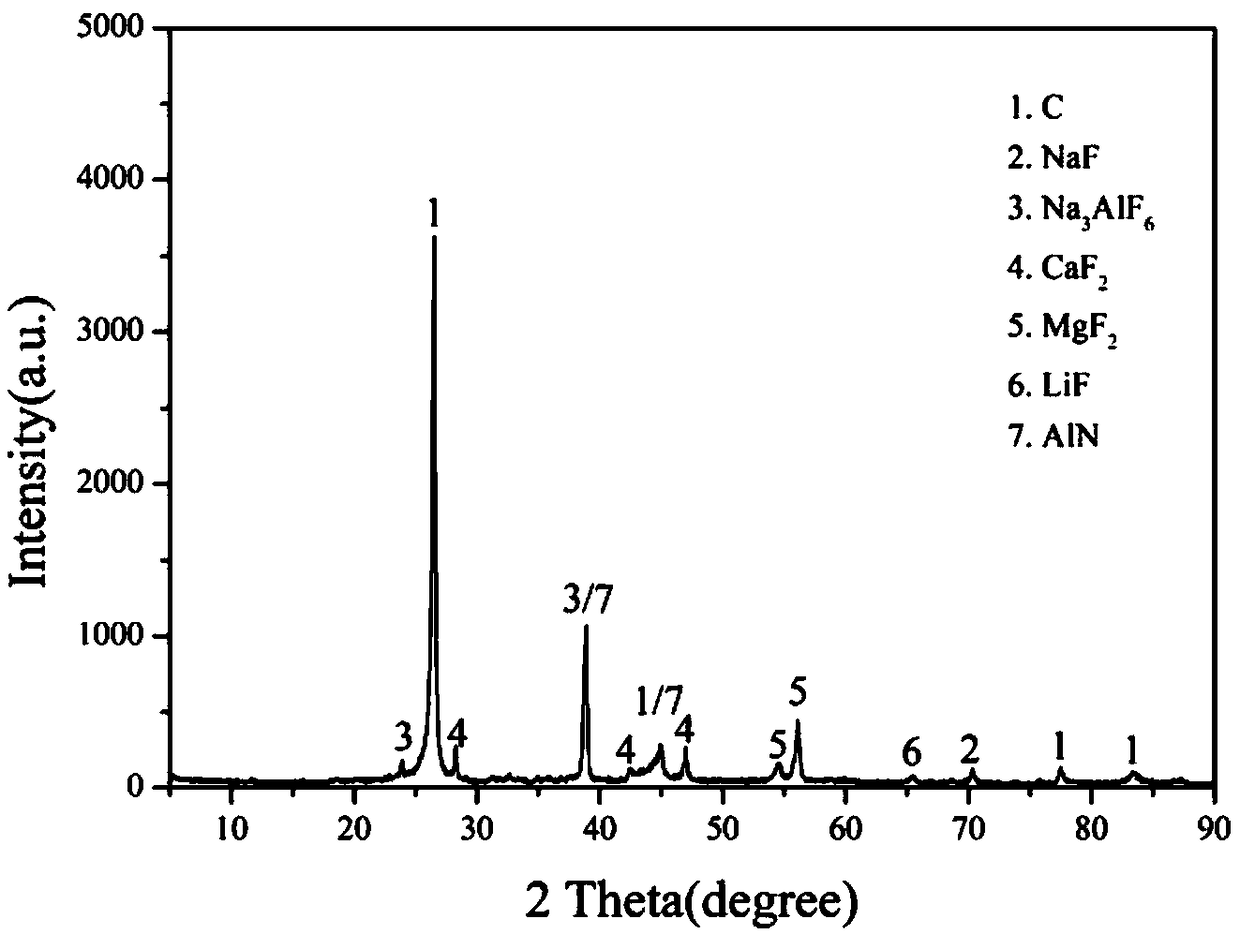
Method and system for converting sodium-containing and fluorine-containing compounds in waste cathode carbon block of aluminum electrolytic cell
ActiveCN109047285AEasy to controlEasy to industrializeMagnesium fluoridesTransportation and packagingPollutionHeat treated
The invention relates to a method and system for converting sodium-containing and fluorine-containing compounds in a waste cathode carbon block of an aluminum electrolytic cell. The conversion methodcomprises the step that a conversion agent is used for converting the sodium-containing compound in the waste cathode carbon block of the aluminum electrolytic cell into a fluorine-free soluble sodiumcompound, converting the fluorine-containing compound into an insoluble and harmless mineral fluorine compound and converting a cyanide-containing compound into harmless N2 or NH3 and CO2 in an oxidized manner through a mechanochemical conversion reaction in a conversion mill, and therefore the hazards of fluoride and cyanide in the waste cathode carbon block of the aluminum electrolytic cell arecompletely eliminated. The conversion system comprises a waste cathode carbon block crushing device, a milling device, the conversion mill, a stirred reactor and a solid-liquid separation device which are sequentially connected in series, and the solid-liquid separation device is then directly connected with a concentration or crystallization device and a drying or heat treatment device, and thedrying or heat treatment device is connected to the crushing device. By means of the method and system, the process is simple, mass production is easy, the production cost is low, pollution of three wastes is avoided, and the method and system are environmentally friendly.
Owner:XIANGTAN UNIV
Fluoride sintered compact for neutron moderator, and method for producing said fluoride sintered compact
A fluoride sintered body suitable for a moderator which moderates high-energy neutrons so as to generate neutrons for medical care with which an affected part of the deep part of the body is irradiated to make a tumor extinct comprises MgF 2 of a compact polycrystalline structure having a bulk density of 2.90g / cm 3 or more and as regards mechanical strengths, a bending strength of 10MPa or more and a Vickers hardness of 71 or more.
Owner:UNIV OF TSUKUBA +1
Organosol Containing Magnesium Fluoride Hydroxide, and Manufacturing Method Therefor
InactiveUS20110003143A1Highly preventive effectImprove hydrophilicityMagnesium fluoridesMaterial nanotechnologyHydrogen fluorideOrganic solvent
An object of the present invention is to provide an organosol for forming a coating that can remain hydrophilic for a long time even in a situation with no exposure to light, and a manufacturing method thereof. As a means for solving this problem, the organosol is created by dispersing magnesium fluoride compound obtained by reacting at least a magnesium compound (the main raw material) with a solution containing hydrogen fluoride, in an organic solvent. The organosol is characterized by containing fine particles of the magnesium fluoride compound which particles have an average particle diameter of from 5 to 500 nm.
Owner:CENT GLASS CO LTD
Mechano-chemical conversion and recovery method for sodium-containing compound and fluorine-containing compound in aluminum electrolysis cell waste cathode carbon blocks
ActiveCN108941167AEasy to controlEasy to industrializeMagnesium fluoridesSolid waste disposalRecovery methodElectrolysis
The invention relates to a mechano-chemical conversion and recovery method for a sodium-containing compound and a fluorine-containing compound in aluminum electrolysis cell waste cathode carbon blocks. The method comprises the steps that the aluminum electrolysis cell waste cathode carbon blocks are crushed, ground and homogenized at first, so that waste cathode carbon powder with particles beingsmaller than or equal to 200 [mu]m is obtained; the waste cathode carbon powder, a sodium compound and fluorine compound conversion agent, a cyanide conversion agent, a grinding aid and water are added into a conversion mill; and under synchronous action of high-energy mechanical force, the sodium-containing compound in the waste cathode carbon powder is converted into a fluorine-free soluble sodium compound, the fluorine-containing compound is converted into an insoluble and harmless mineral substance fluorine compound, a cyanogen-containing compound is oxidized and converted into harmless N2or NH3 and CO2, and therefore harm of fluoride and cyanide in the aluminum electrolysis waste cathode carbon blocks are removed thoroughly, and harmless resource recycling of the aluminum electrolysis waste cathode carbon blocks is achieved. The mechano-chemical conversion and recovery method is simple in process, low in production cost, free of three-waste pollution and environmentally friendly,and large-scale production is easy to implement.
Owner:XIANGTAN UNIV
Calcium fluoride crystal and method and apparatus for using the same
InactiveUS6989060B2Decrease of refractive index homogeneityLess residueMagnesium fluoridesPolycrystalline material growthTransition densityCrystal growth
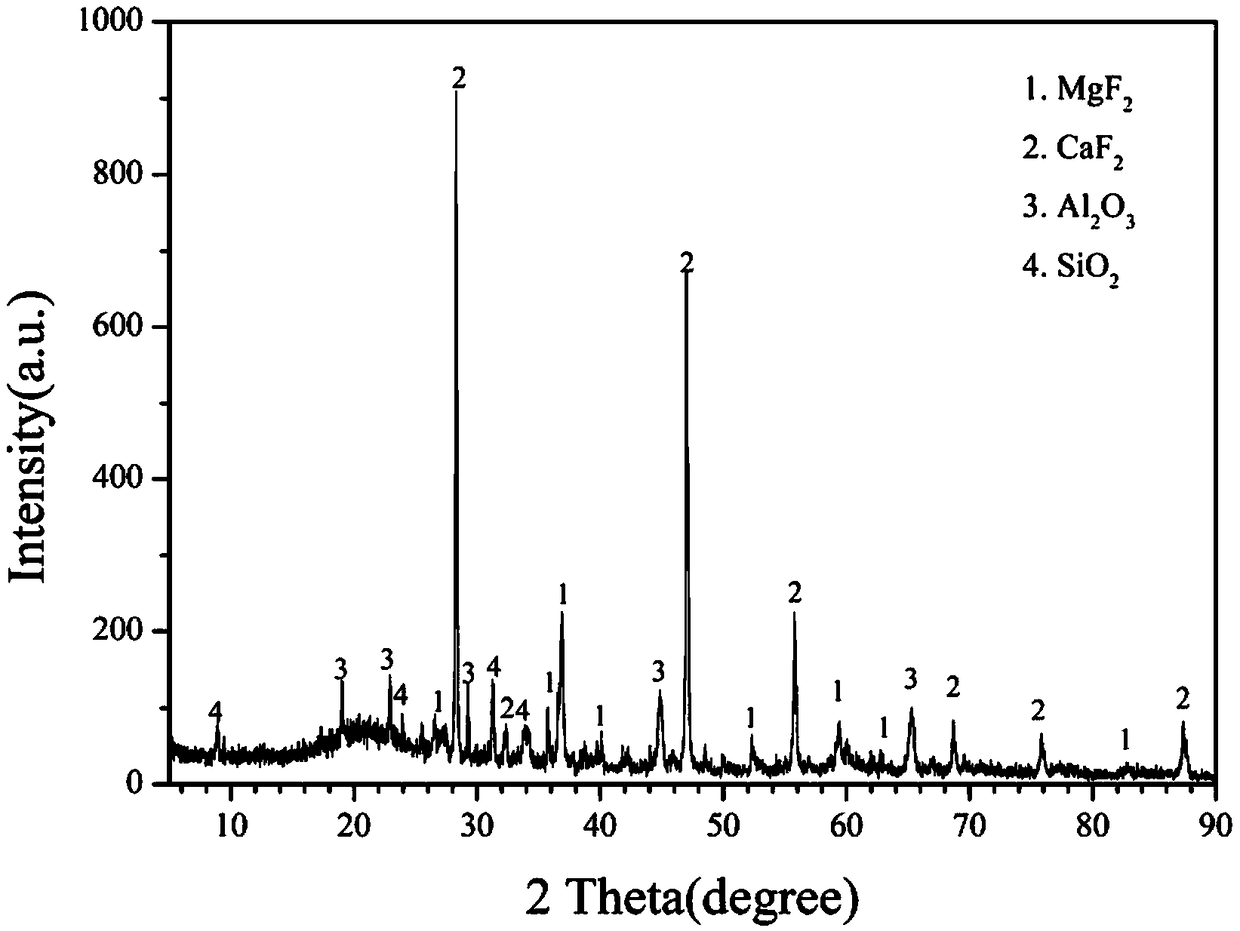
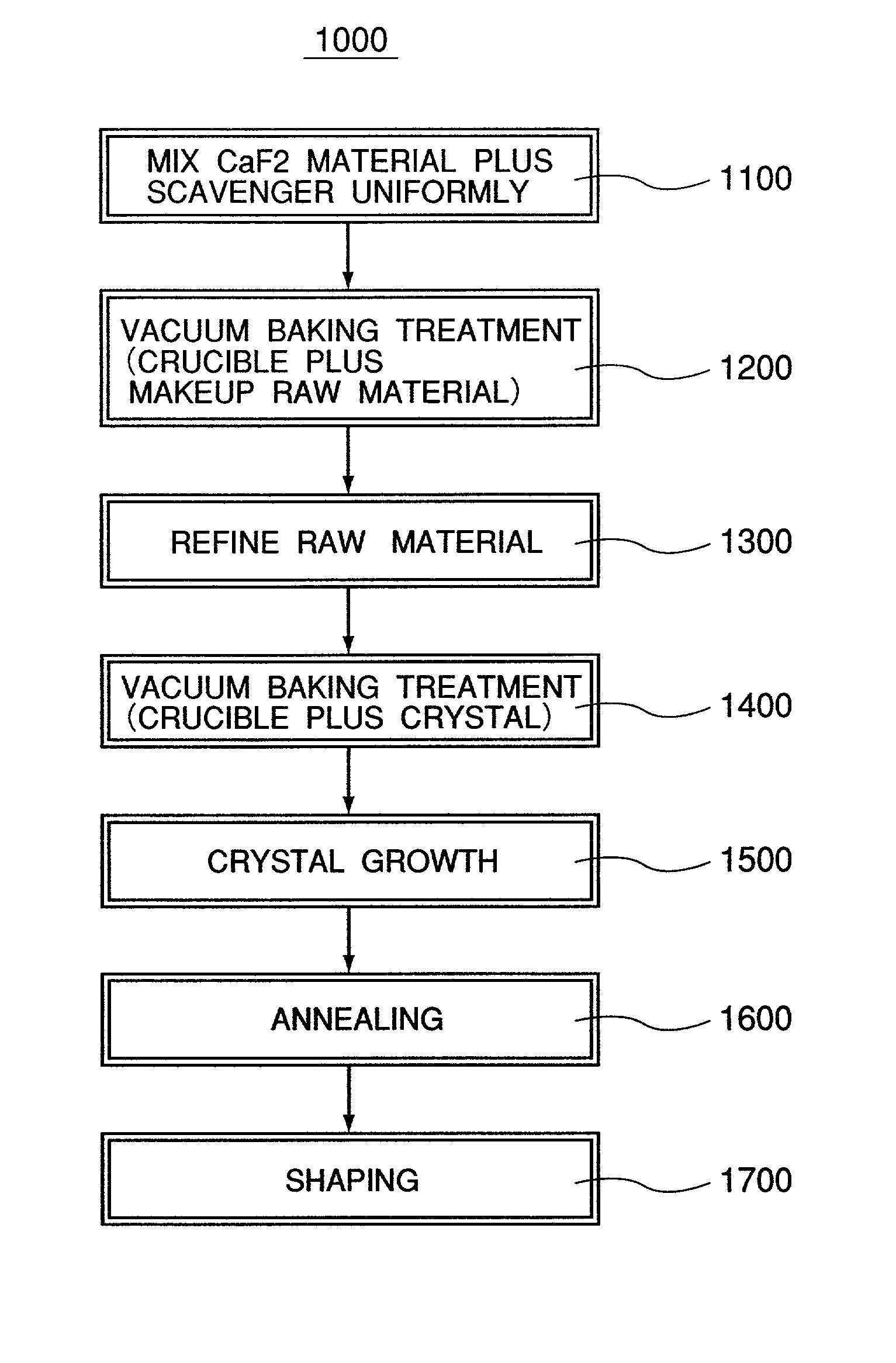
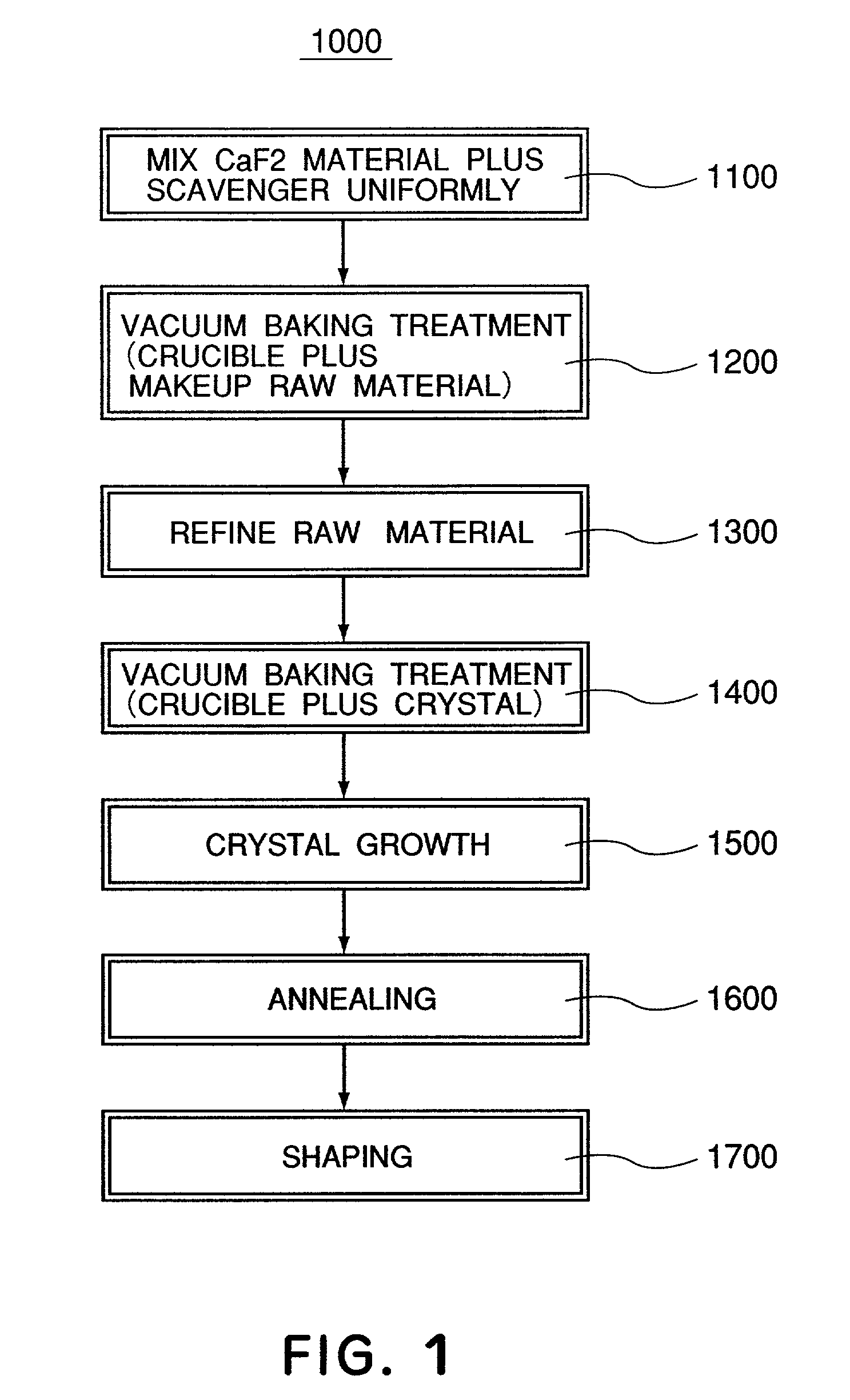
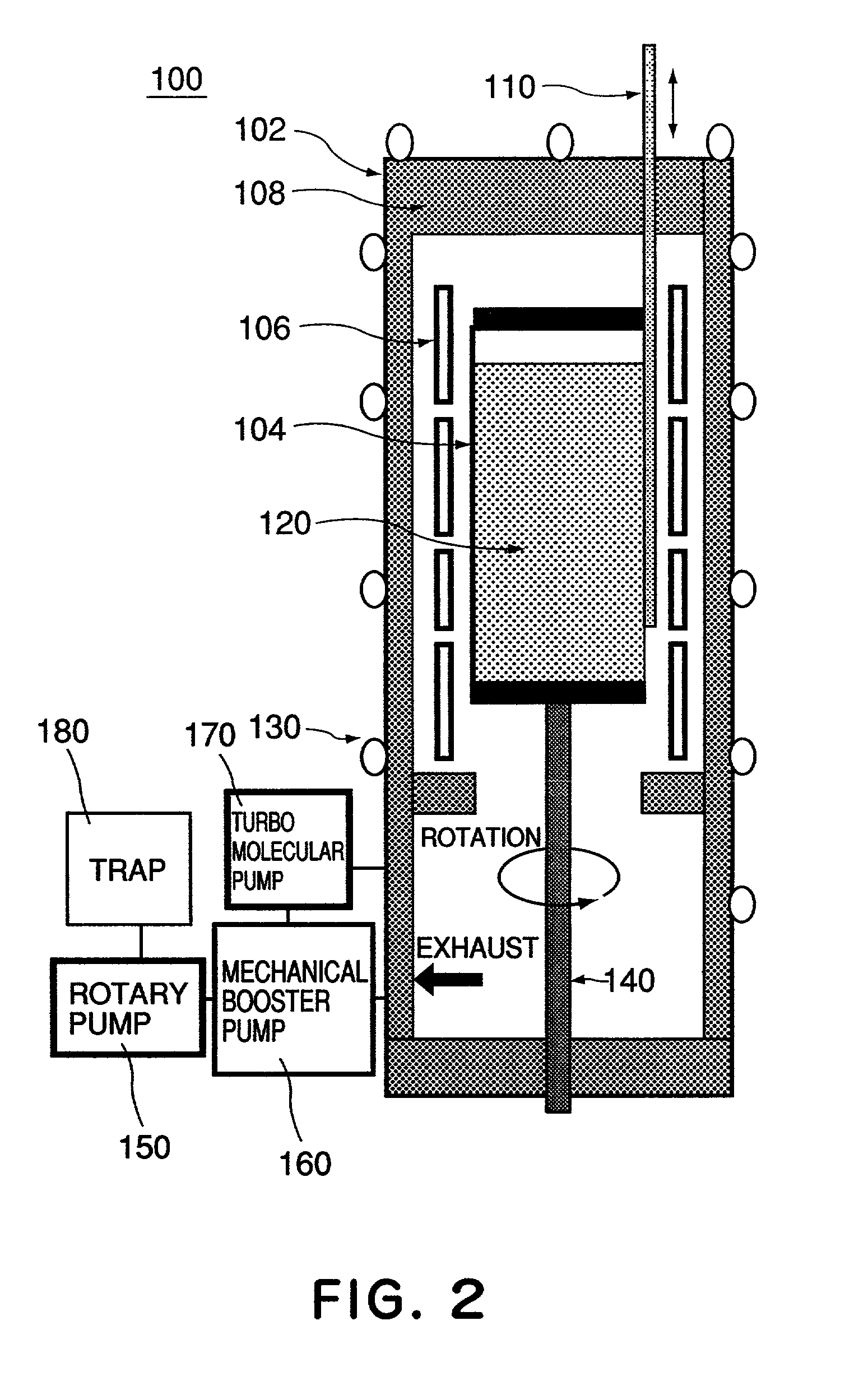
A calcium fluoride crystal produced in accordance with a method for producing calcium fluoride crystal on the basis of refining a raw material of calcium fluoride and causing crystal growth of the refined calcium fluoride, the method including a process of raising a purity of the calcium fluoride to complement the refining, wherein a transition density in crystal is not greater than 1×105 / cm2, and that dispersion of transition density inside an effective portion in crystal is in a range of ±5×104 / cm2. Also disclosed is an optical element to be manufactured by use of such CaF2 crystal.
Owner:CANON KK
Composite wavelength conversion powder, resin composition containing composite wavelength conversion powder, and light emitting device
ActiveCN104471729AImprove utilization efficiencyImprove light utilization efficiencyMagnesium fluoridesCalcium/strontium/barium fluoridesInorganic particlePhosphor
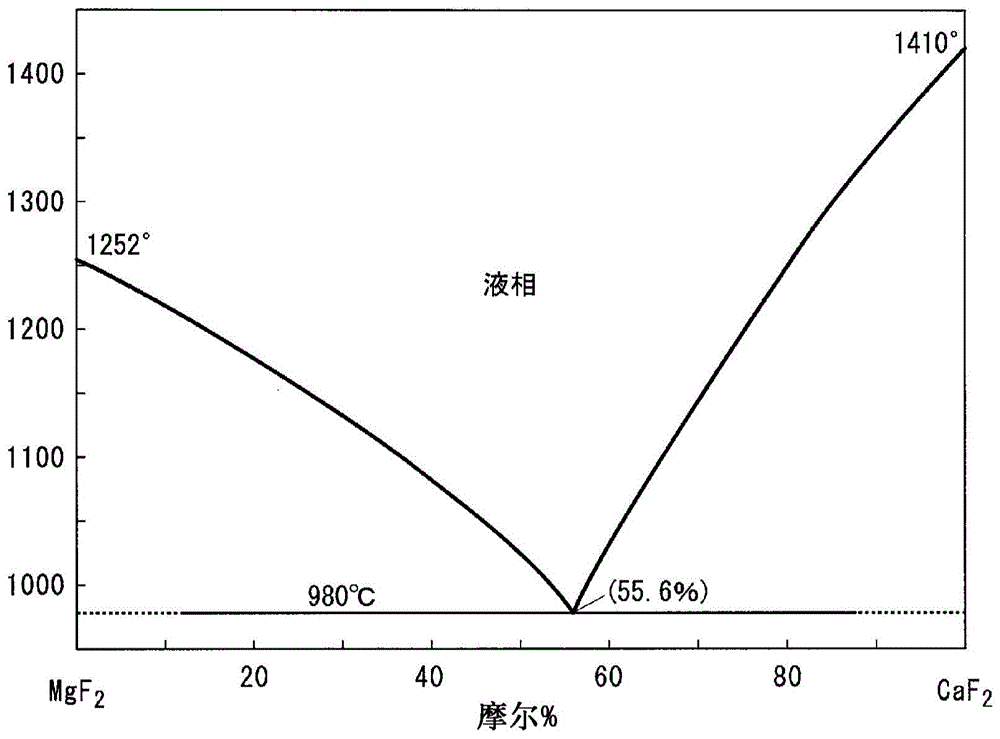
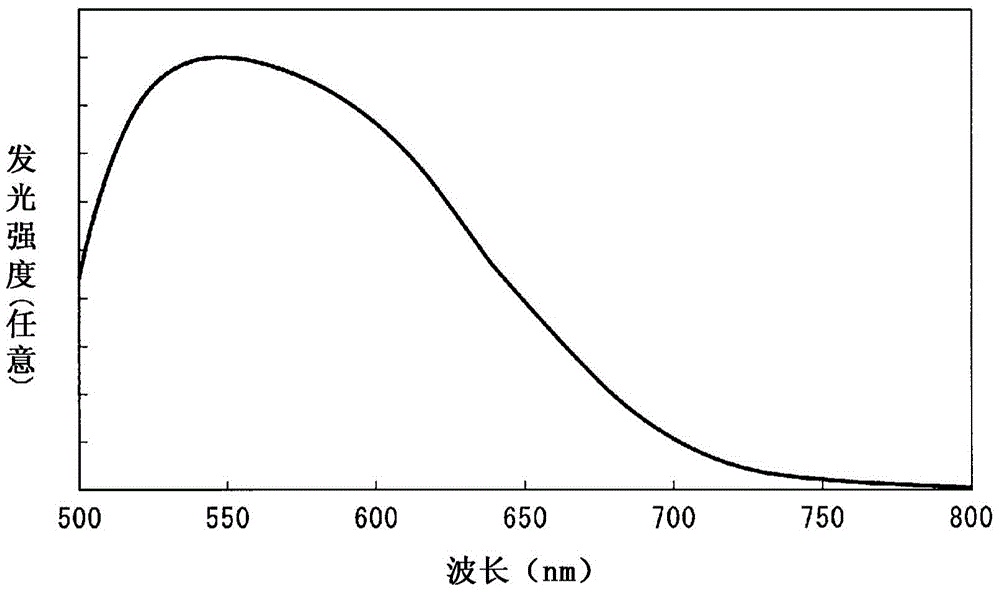
Provided are: a composite wavelength conversion powder which has high utilization efficiency of light and high utilization efficiency of a constituent material, and which is capable of achieving a good balance between highly efficient light emission and high reliability; and a resin composition which contains this composite wavelength conversion powder. This composite wavelength conversion powder is obtained by dispersing phosphor particles having a refractive index of 1.6 or more in a matrix particle which contains fine magnesium fluoride particles or fine calcium fluoride particles. Also provided is a light emitting device which is capable of improving the utilization efficiency of light produced by phosphor particles that are excited by primary irradiation light irradiated from a light emitting element, and which is capable of improving the optical output of light emission by increasing the amount of secondary irradiation light produced by the phosphor particles, while suppressing the occurrence of color unevenness or color variation of the light discharged to the outside of the device. A light emitting device (1) of the present invention is provided with a substrate (2), a light emitting element (3) that is mounted on the surface of the substrate (2), and a light transmitting member (4) that covers the light emitting element (3). This light transmitting member (4) contains composite wavelength conversion particles (12) which are composed of phosphor particles that have an average particle diameter of 500 nm or less and inorganic particles that are transparent to visible light and have an average particle diameter of 500 nm or less.
Owner:SUMITOMO OSAKA CEMENT CO LTD
Ceramicrete stabilization of u-and pu-bearing materials
InactiveUS20070235702A1Magnesium fluoridesTransuranic element compoundsRadioactive agentHydrocotyle bowlesioides
A method of stabilizing nuclear material is disclosed. Oxides or halides of actinides and / or transuranics (TRUS) and / or hydrocarbons and / or acids contaminated with actinides and / or TRUs are treated by adjusting the pH of the nuclear material to not less than about 5 and adding sufficient MgO to convert fluorides present to MgF2: alumina is added in an amount sufficient to absorb substantially all hydrocarbon liquid present, after which a binder including MgO and KH2PO4 is added to the treated nuclear material to form a slurry. Additional MgO may be added. A crystalline radioactive material is also disclosed having a binder of the reaction product of calcined MgO and KH2PO4 and a radioactive material of the oxides and / or halides of actinides and / or transuranics (TRUs). Acids contaminated with actinides and / or TRUs, and / or actinides and / or TRUs with or without oils and / or greases may be encapsulated and stabilized by the binder.
Owner:UCHICAGO ARGONNE LLC +2
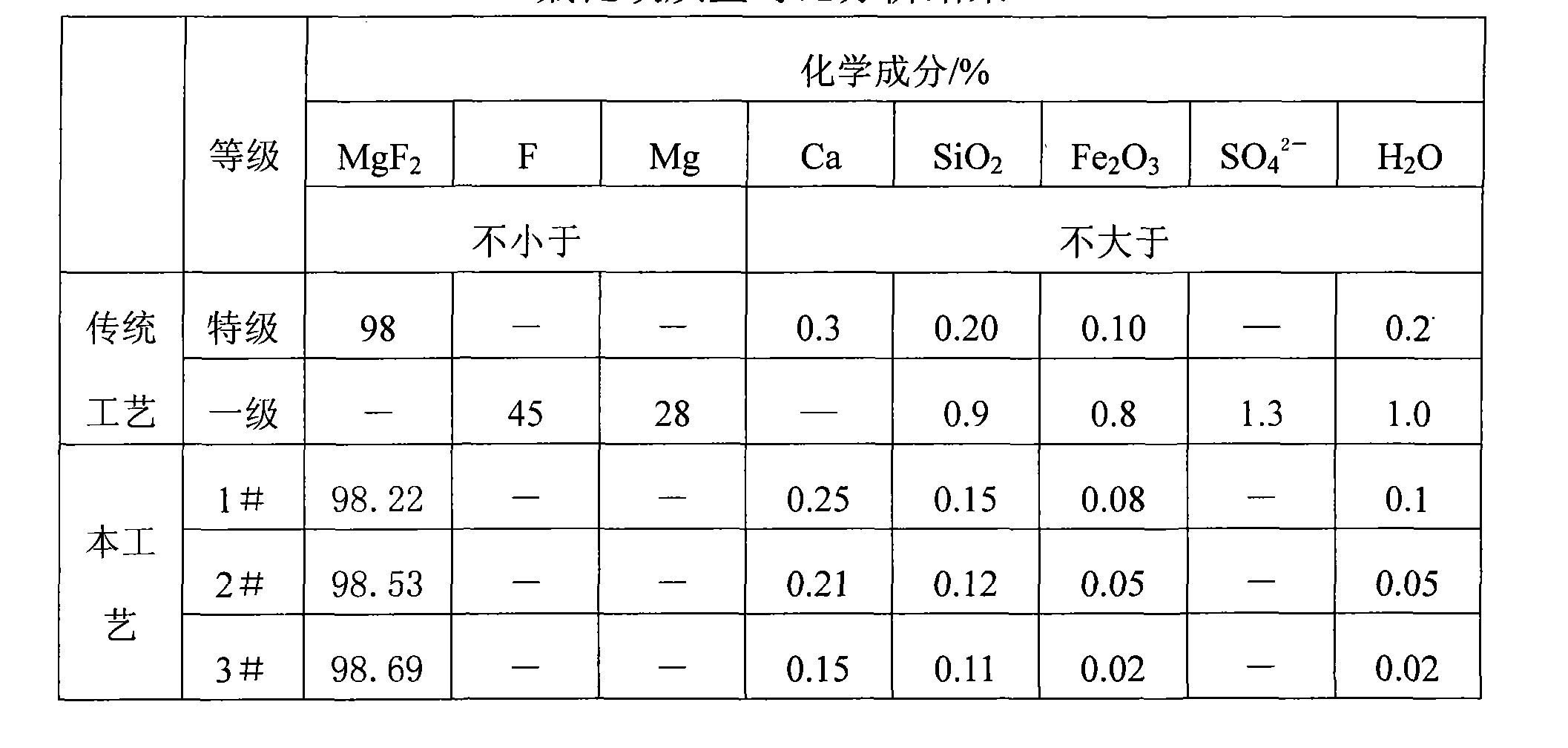
Preparation of magnesium fluoride
The invention relates to a manufacturing method of magnesium fluoride, which takes ammonium fluoride and magnesium chloride as raw materials and comprises the steps as follows: (1) an ammonium fluoride solution whose concentration is 30 to 45 percent and a magnesium chloride solution whose concentration is 25 to 36 percent are added in a reaction kettle at the same time for reaction, and a magnesium fluoride ground-paste is generated; (2) the obtained magnesium fluoride ground-paste is filtered to prepare a magnesium fluoride paste which is cleaned by using hot water the temperature of which ranges from 60 to 70 DEG C; the magnesium fluoride paste is dried for 1 to 2 hours at the temperature ranging from 250 to 400 DEG C after washing, and the finished product of the magnesium fluoride is obtained. The manufacturing method adopts the ammonium fluoride instead of hydrofluoric acid, wherein, the ammonium fluoride can be obtained through the ammonolysis by adding ammonia water into fluosilicic acid that is a by-product in the phosphate fertilizer industry; the fluosilicic acid is the deleterious-waste which is the by-product during the phosphate fertilizer production process, has very little purpose, and badly influences the environmental protection; in the manufacturing method, the development and the utilization of the fluosilicic acid greatly relieve the environmental protection pressure of the phosphate fertilizer production and the influences on the surrounding environment; and the cost is lower and the material is easy to get because the fluosilicic acid is the by-product in a phosphate fertilizer factory, thereby lowering the manufacturing cost of the magnesium fluoride.
Owner:DO FLUORIDE CHEM CO LTD
Method for preparing thermocompressed polycrystalline magnesium fluoride powder
The invention discloses a method for preparing thermocompressed polycrystalline magnesium fluoride powder. The method comprises the steps of: taking industrial grade magnesium sulfate and industrial grade sodium carbonate as initial raw materials, preparing basic magnesium carbonate by purifying the raw materials, carbonating the basic magnesium carbonate, and finally, performing a reaction of the carbonated basic magnesium carbonate and anhydrous hydrofluoric acid to obtain the thermocompressed polycrystalline magnesium fluoride powder. The method takes the industrial grade magnesium sulfate and the industrial grade sodium carbonate as the raw materials to synthesize the basic magnesium carbonate, and has the advantages of cheap raw materials, low production cost, and remarkable economic benefit; the magnesium fluoride powder prepared by the reaction of the basic magnesium carbonate and the anhydrous hydrofluoric acid has high purity and uniform particle size; the utilization rate of fluorine and magnesium are high and can reach over 98 percent; the method has no discharge of three wastes during preparation, makes all mother solutions entirely in closed circulation, and has remarkable environmental protection benefit; the method has the advantages of less investment, simple equipment, and easy operation; and an infrared optical element obtained by thermocompressing the magnesium fluoride powder prepared by the preparation method has high transmittance on infrared bands, and particularly the transmittance on infrared bands with wavelengths of between 1 and 7mu m reaches 95 percent.
Owner:BAIYIN ZHONGTIAN CHEM
Nano-scale metal halide scintillation materials and methods for making same
Crystalline scintillator materials comprising nano-scale particles of metal halides are provided. The nano-scale particles are less than 100 nm in size. Methods are provided for preparing the particles. In these methods, ionic liquids are used in place of water to allow precipitation of the final product. In one method, the metal precursors and halide salts are dissolved in separate ionic liquids to form solutions, which are then combined to form the nano-crystalline end product. In the other methods, micro-emulsions are formed using ionic liquids to control particle size.
Owner:GENERAL ELECTRIC CO
Ceramicrete stabilization of U-and Pu-bearing materials
InactiveUS7294291B2Magnesium fluoridesTransuranic element compoundsHydrocotyle bowlesioidesRadioactive agent
A method of stabilizing nuclear material is disclosed. Oxides or halides of actinides and / or transuranics (TRUs) and / or hydrocarbons and / or acids contaminated with actinides and / or TRUs are treated by adjusting the pH of the nuclear material to not less than about 5 and adding sufficient MgO to convert fluorides present to MgF2; alumina is added in an amount sufficient to absorb substantially all hydrocarbon liquid present, after which a binder including MgO and KH2PO4 is added to the treated nuclear material to form a slurry. Additional MgO may be added. A crystalline radioactive material is also disclosed having a binder of the reaction product of calcined MgO and KH2PO4 and a radioactive material of the oxides and / or halides of actinides and / or transuranics (TRUs). Acids contaminated with actinides and / or TRUs, and / or actinides and / or TRUs with or without oils and / or greases may be encapsulated and stabilized by the binder.
Owner:UCHICAGO ARGONNE LLC +2
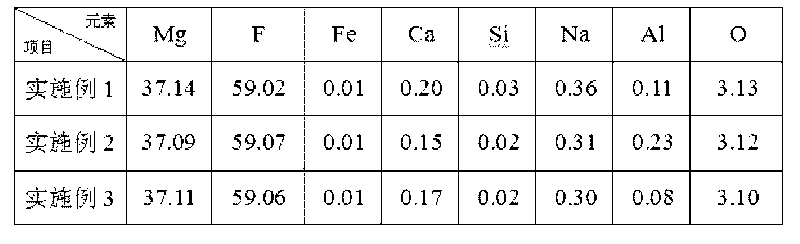
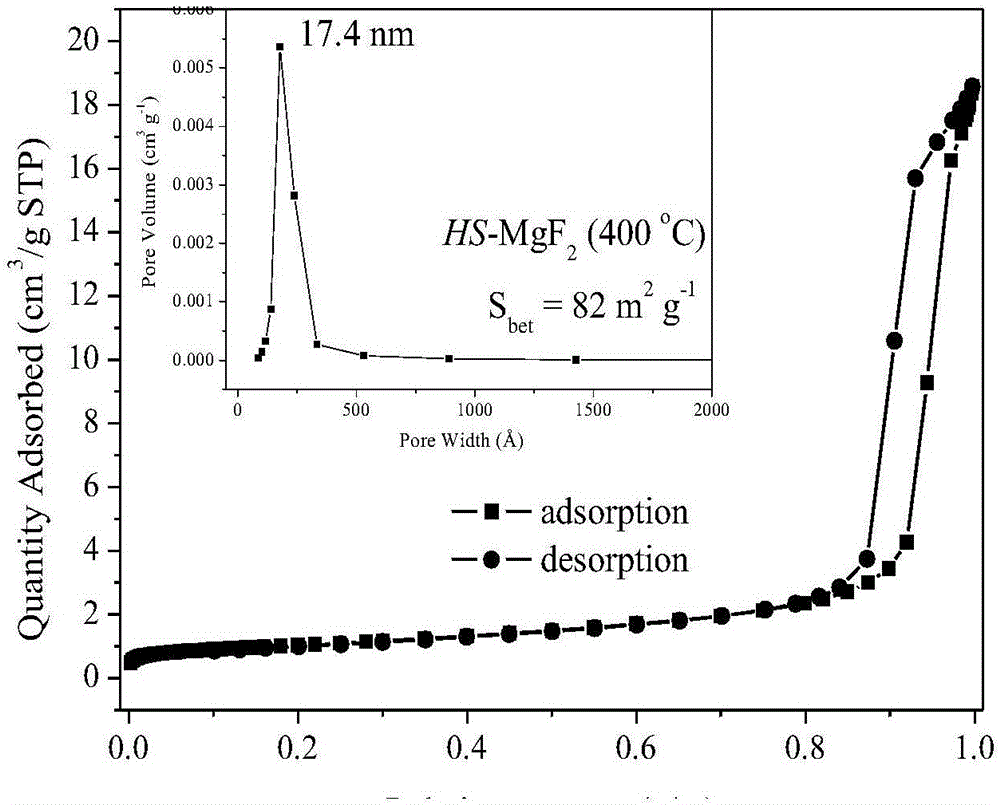
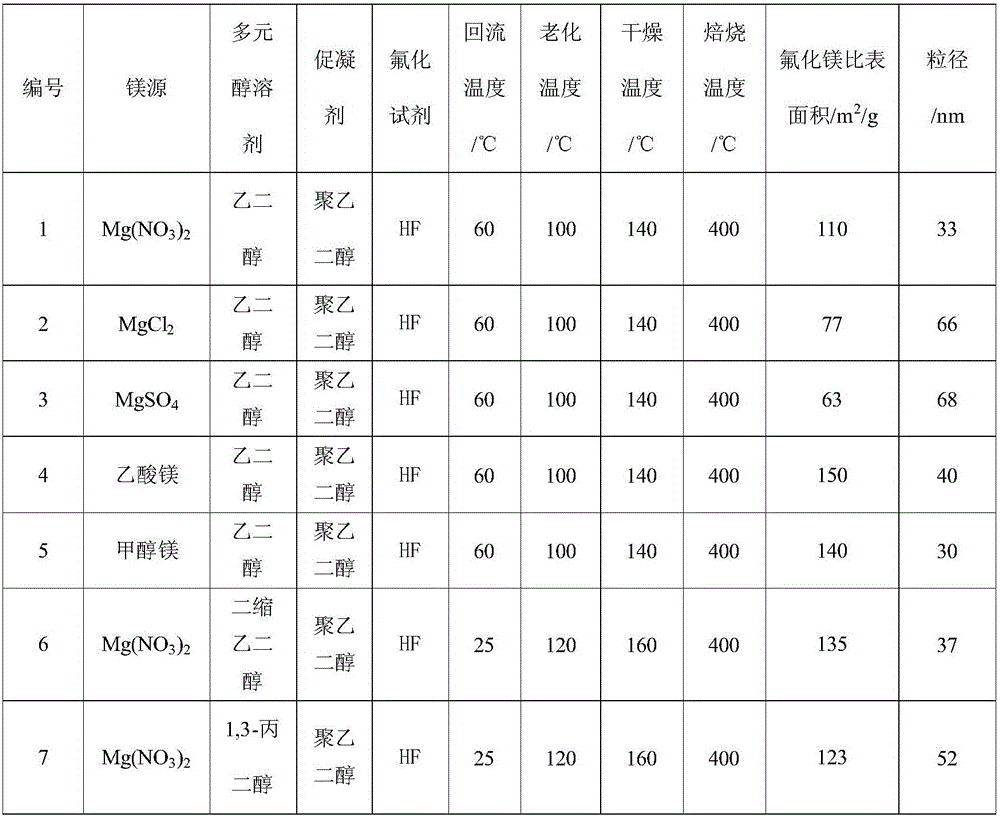
Method for preparing nano-crystal magnesium fluoride with high specific surface area
ActiveCN106745111ALess corrosiveRaw materials are easy to getMagnesium fluoridesMaterial nanotechnologyAlcoholMagnesium fluoride
The invention discloses a method for preparing nano-crystal magnesium fluoride with a high specific surface area, which aims at solving the problems that magnesium fluoride preparation has small high-temperature specific surface area and poor stability and an existing preparation method is expensive and complicated and has heavy pollution. The method comprises the following steps: (1) preparing a solution containing magnesium-source precursor, polyhydric alcohol and coagulation accelerator, and refluxing at 20-80 DEG C; (2) fluoridizing the solution obtained in the step (1) with a fluoridizing reagent, and aging at 100-160 DEG C to obtain liquid sol; and (3) drying the liquid sol at 140-200 DEG C to obtain solid gel, and roasting at 350-450 DEG C to obtain nano-magnesium fluoride.
Owner:XIAN MODERN CHEM RES INST
Single crystal of magnesium fluoride, optical member and optical element comprising the same
InactiveUS20120057222A1Excellent internal transmittanceGood for long termMagnesium fluoridesPolycrystalline material growthTransmittanceEnergy density
A single crystal of magnesium fluoride having a large diameter and excellent optical properties such as internal transmittance and long term laser durability, and suited for use as optical elements for exposing apparatus. The single crystal of magnesium fluoride is of a cylindrical shape having a straight body portion of a diameter of not smaller than 10 cm, has an internal transmittance of at least 85% / cm at 120 nm and at least 98% / cm at 193 nm and has, desirably, an induced absorption of not larger than 0.0030 absorption / cm at 255 nm and, particularly desirably, not larger than 0.0010 absorption / cm. at 255 nm immediately after the irradiation with 2 million shorts of an ArF excimer laser of an energy density of 30 mJ / cm2 and 2000 Hz. The invention further provides an optical element for optical lithography comprising the single crystal and an optical member for vacuum ultraviolet ray transmission comprising the single crystal.
Owner:TOKUYAMA CORP
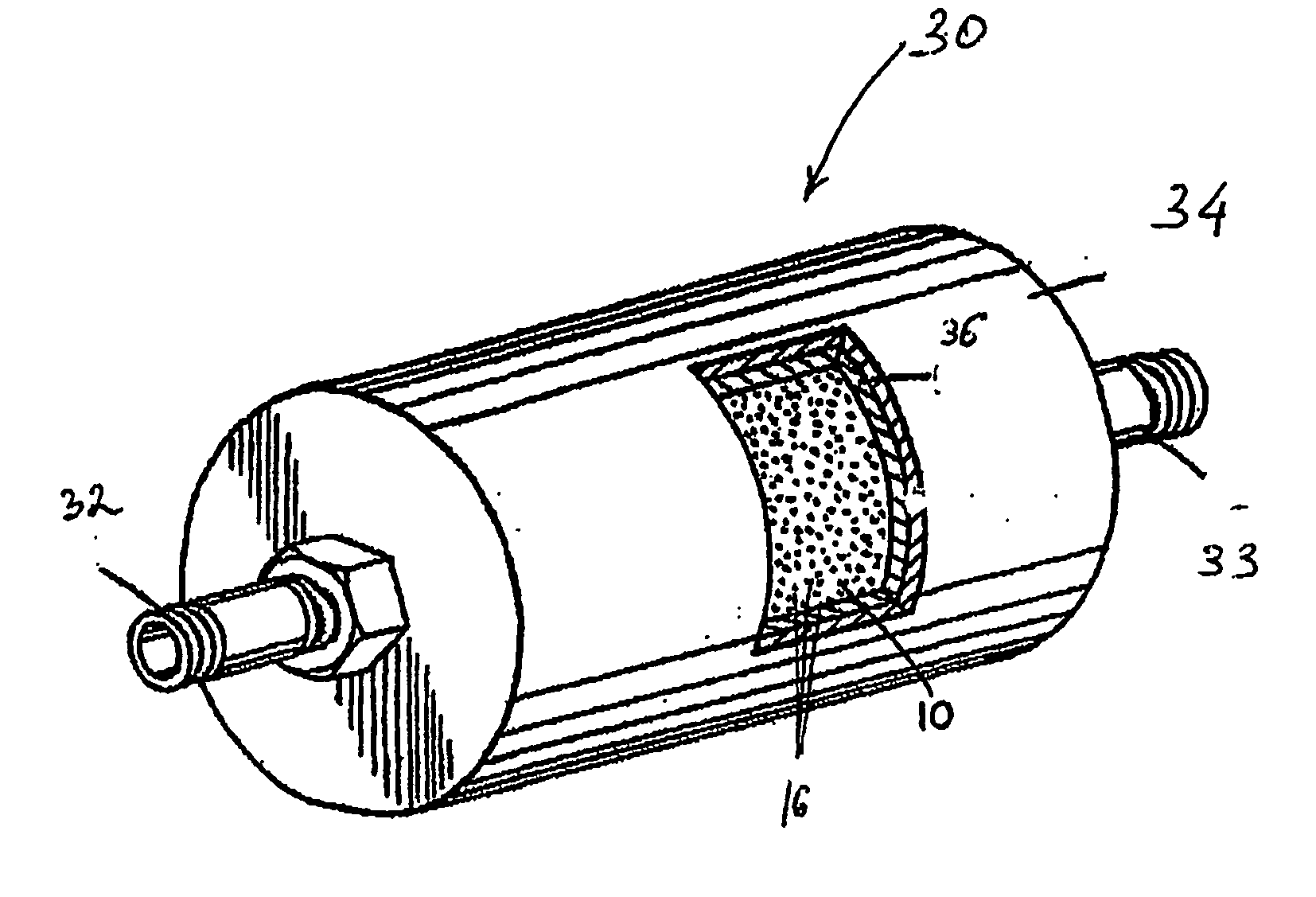
Apparatus and method for purification of corrosive gas streams
InactiveUS20070031321A1Reduce moistureWater contentMagnesium fluoridesHydrogen bromideParticulatesHalogen
A process, composition and apparatus for the removal of impurities from corrosive gases, particularly halogen-containing gases, down to about 100 ppb concentration are described. The critical component is zirconia (ZrO2), which in a variety of physical forms is capable of dehydrating such gases. The zirconia can be in the form of a coating on a substrate, as a granular bulk material, or deposited within the pores of a porous body. The zirconia is retained in a simple container which is easily installed in a gas supply line, such as to a gas- or vapor-deposition manufacturing unit. The purification process can be operated for long periods of time in the presence of these gases. The invention provides final purification to gas streams intended for gas- or vapor-deposition formation of high purity electronic, prosthetic or similar products, and can be used in combination with a preliminary dehydration process or a solid particulate removal unit upstream.
Owner:MYKROLIS CORP +1
Method for utilizing high-magnesium phosphate tailings to produce magnesium fluoride and by-product calcium carbonate
InactiveCN102923739ASolve the occupation of land resourcesSolve the pollution of the environmentCalcium/strontium/barium carbonatesMagnesium fluoridesMagnesium phosphateCarbonization
The invention discloses a method for utilizing high-magnesium phosphate tailings to produce magnesium fluoride and by-product calcium carbonate. The method comprises the following steps: calcinating the high-magnesium phosphate tailings at high temperature to form calcined dolomite, adding water to the calcined dolomite to perform digestion to obtain digestion solution, supplementing water to the digestion solution and then leading carbon dioxide to perform a carbonization reaction to obtain carbonized liquid, separating bottom phosphate ore sediments of the carbonized liquid after the reaction stops, filtering, and obtaining filtrate which is magnesium bicarbonate aqueous solution, and obtaining a calcium carbonate product after drying a filter cake; adding hydrofluoric acid in the obtained magnesium bicarbonate aqueous solution, filtering after the reaction, washing, and obtaining a magnesium fluoride product after drying the filter cake, The industrial waste high-magnesium phosphate tailings serve as raw materials, waste is turned into wealth, abundant magnesium calcium resources in the high-magnesium phosphate tailings are fully utilized to produce the magnesium fluoride and the by-product calcium carbonate, and the method has the advantages of being simple in process, low in production cost and basically free of three wastes.
Owner:WENGFU (GRP) CO LTD
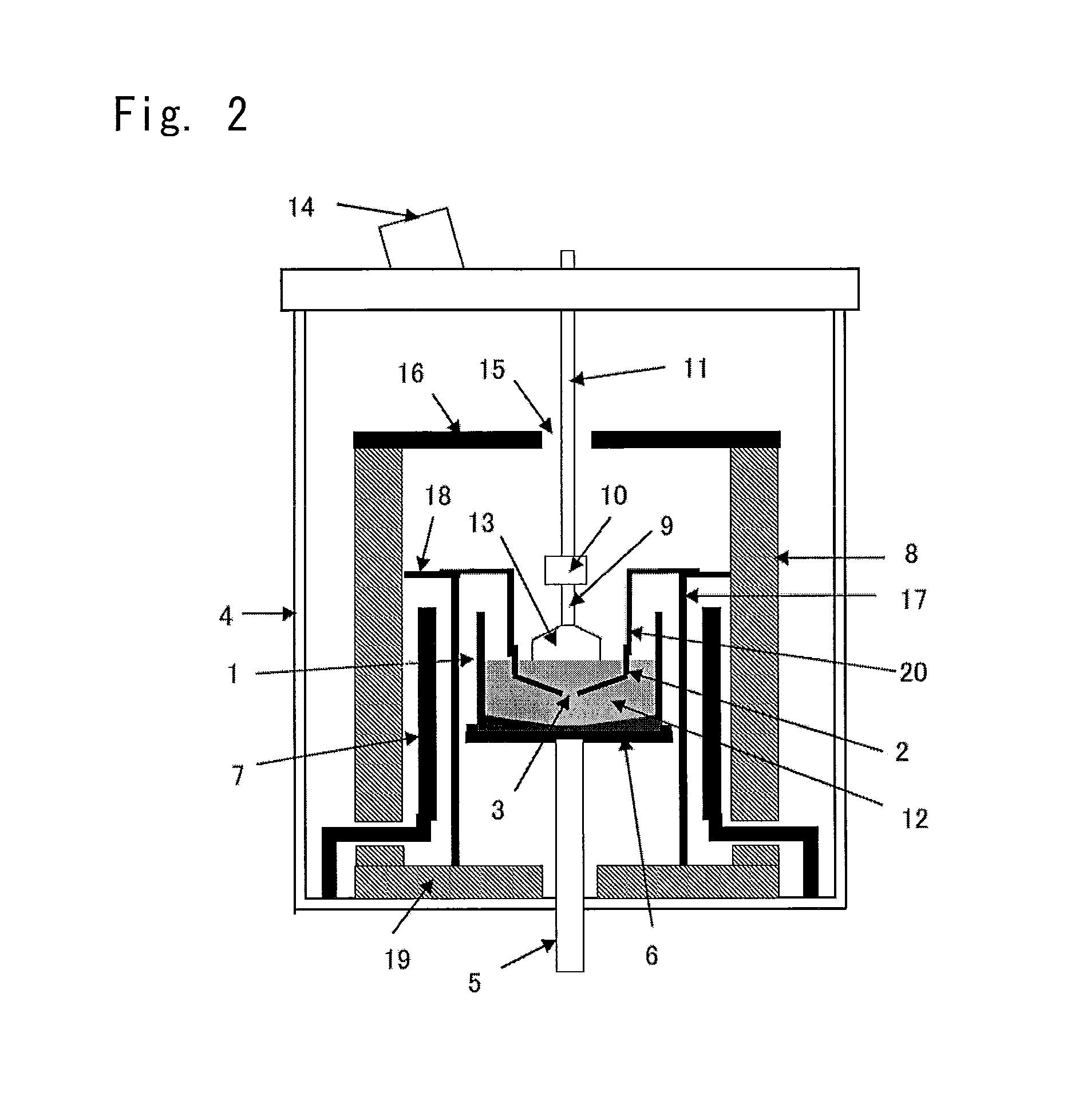
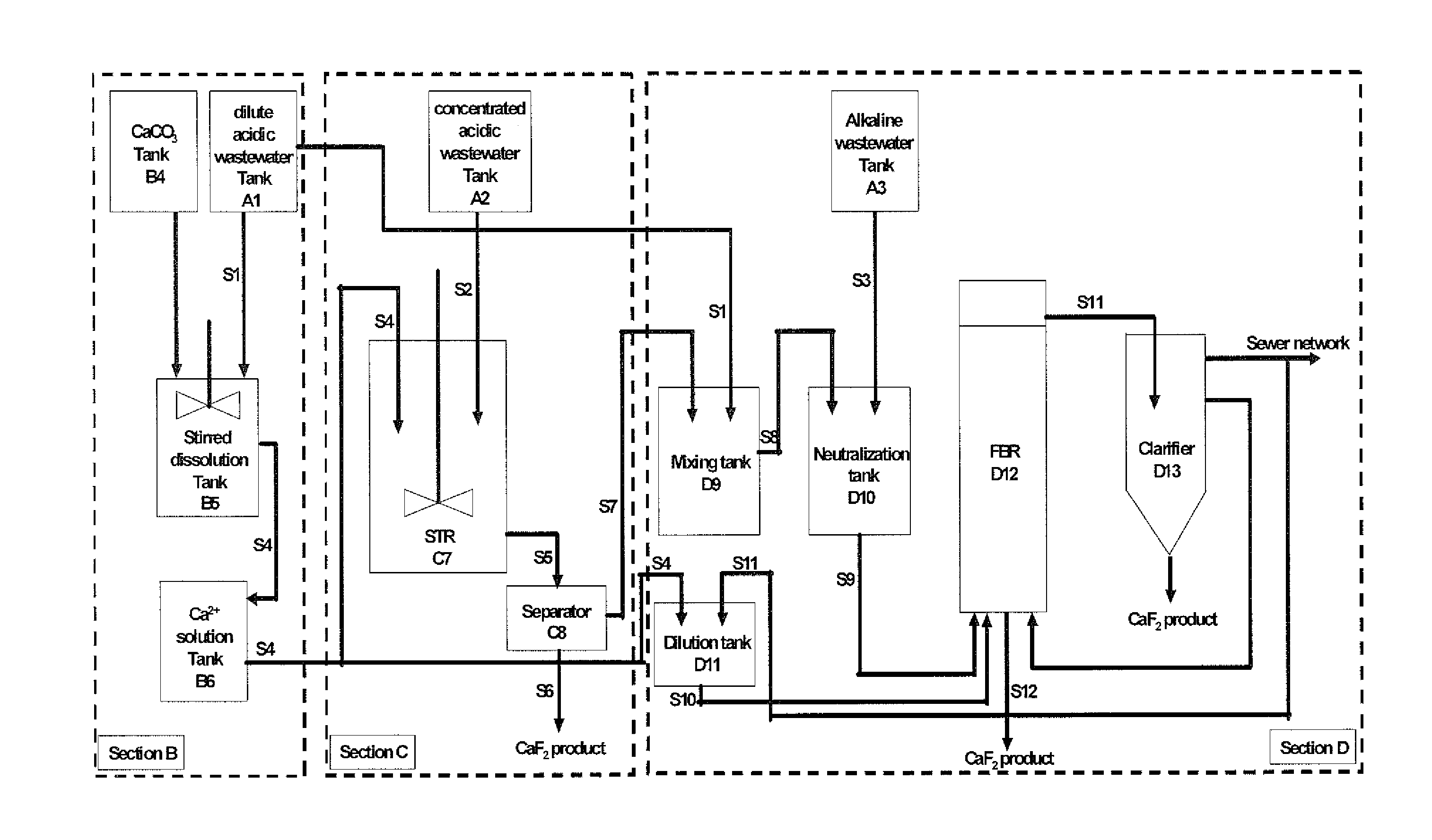
Process for recovery of fluoride from wastewater produced in crystalline silicon solar cell manufacturing
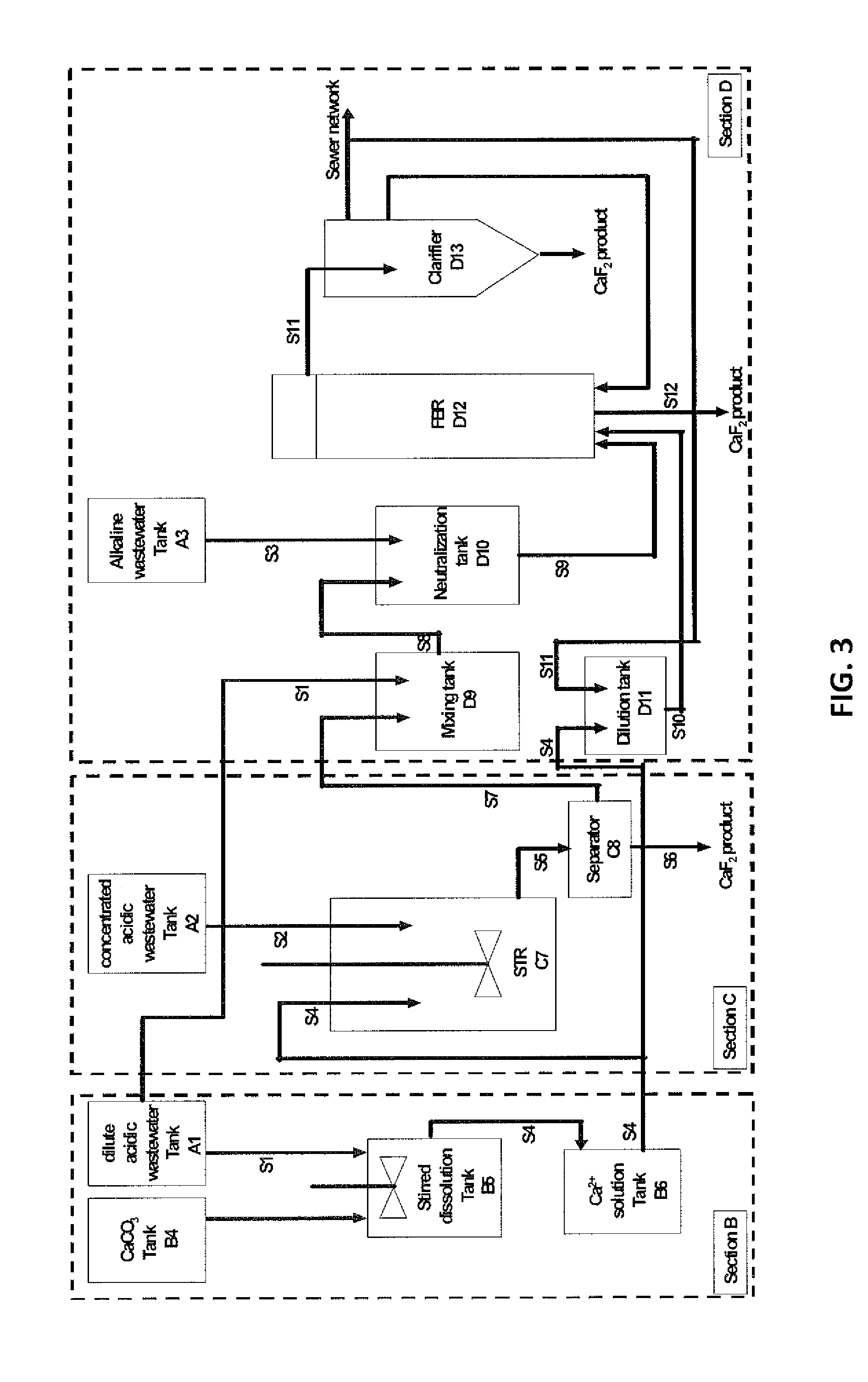
InactiveUS20140161714A1Improve efficiencyReduce the amount requiredMagnesium fluoridesCalcium/strontium/barium fluoridesHigh fluorideFluidized bed
A method for processing fluoride-containing wastewaters from a factory, which includes the following steps: Step 1: collecting the fluoride-containing wastewaters into three pools: an acidic high-fluoride wastewater, an acidic low-fluoride wastewater, and an alkaline wastewater; Step 2: adding a calcium compound to the acidic low-fluoride wastewater to produce a calcium-containing solution; Step 3: reacting a portion of the calcium-containing solution with the acidic high-fluoride wastewater at a calcium-to-fluoride molar ratio of from about 0.5:1 to about 1.5:1 to produce a mixture comprising calcium fluoride particles suspended in a mother liquor; Step 4: separately collecting the calcium fluoride particles and the mother liquor; Step 5: diluting the mother liquor with a diluent to produce a mixed solution; and Step 6: introducing the mixed solution, the calcium-containing solution, and the alkaline wastewater into a fluidized bed reactor, which contains a calcium fluoride crystallization seed material, to form calcium fluoride crystals.
Owner:BEIJING GUOHUAN TSINGHUA ENVIRONMENT ENG DESIGN & RES INST CO LTD BEIJING CHINA
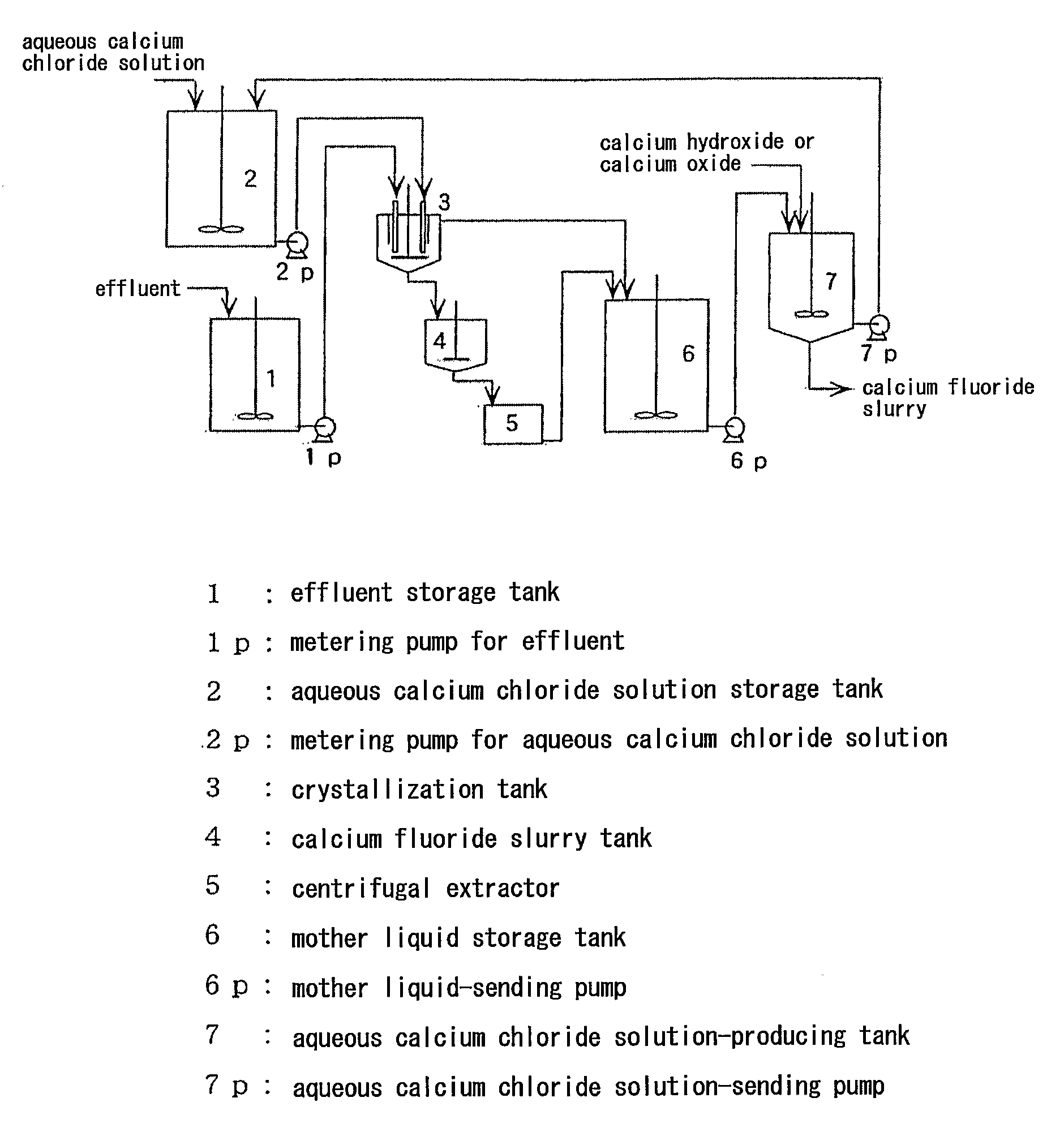
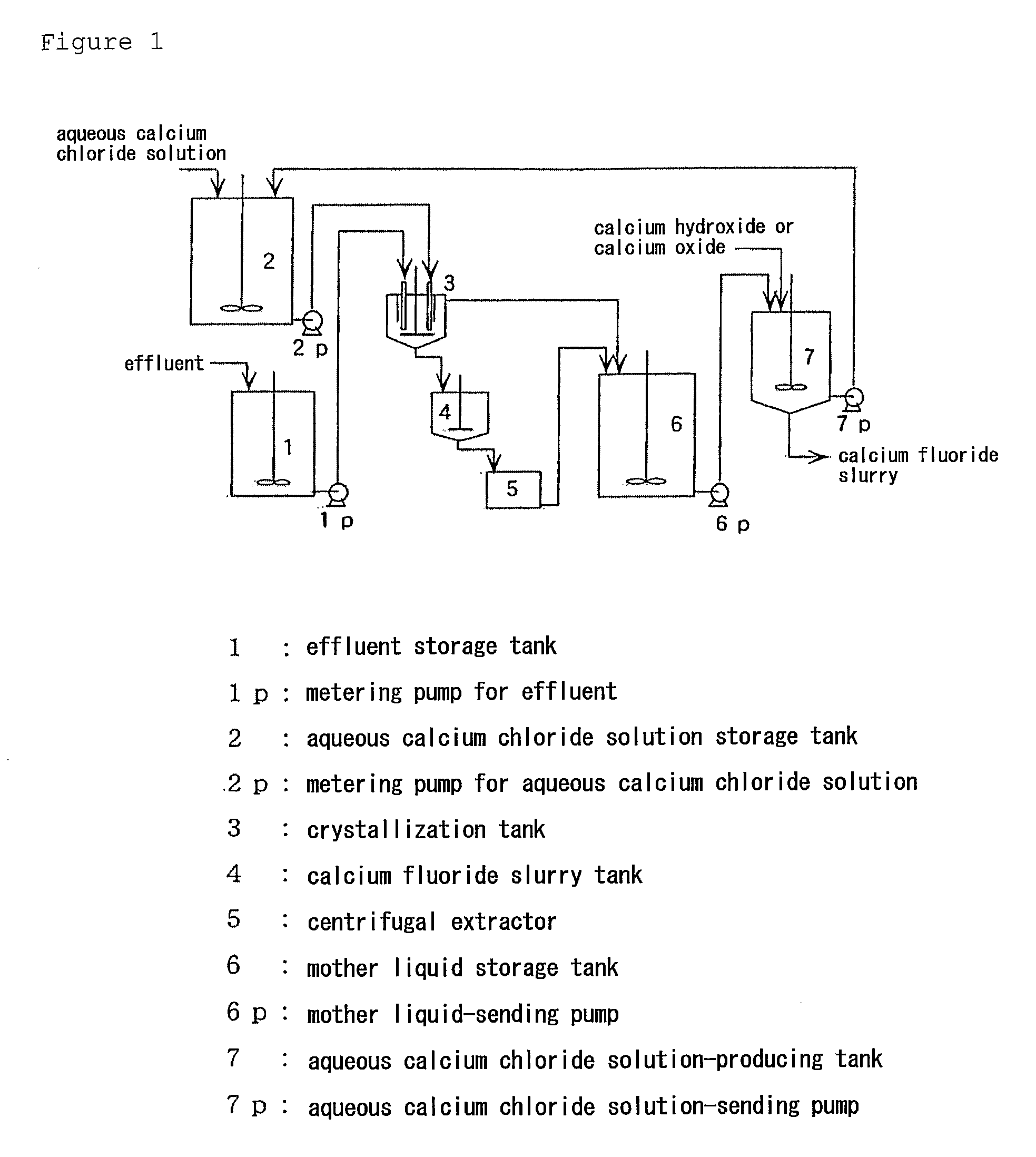
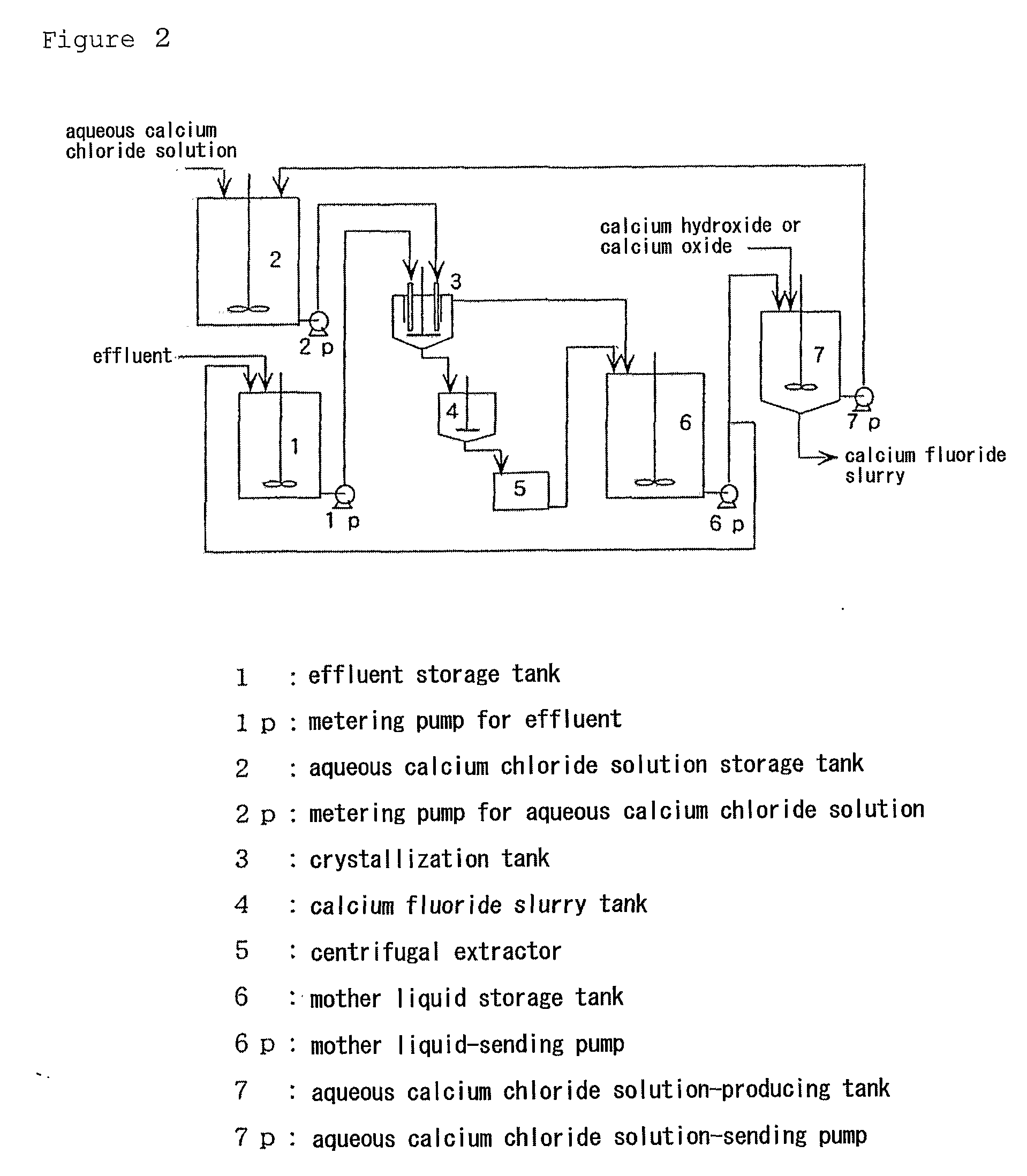
Method For Producing Calcium Fluoride, Reusing Method And Recycling Method Thereof
InactiveUS20090180947A1Large particle sizeHigh purityMagnesium fluoridesWater treatment parameter controlRecovery methodSolubility
To recycle fluoride by recovering calcium fluoride having a particle size and purity suitable for production of hydrogen fluoride, from a fluoride-containing effluent or a hydrofluoric acid-containing effluent. This method comprises reacting the fluoride-containing effluent or the hydrofluoric acid-containing effluent with an aqueous calcium chloride solution, under an acidic condition with hydrochloric acid where calcium fluoride has a comparatively high solubility. Calcium fluoride having a high purity and a large particle size can be deposited. The hydrochloric acid residues from the reaction or formed through the reaction is reacted with an inexpensive calcium compound such as calcium hydroxide, calcium oxide and calcium carbonate to produce an aqueous calcium chloride solution, and the aqueous calcium chloride solution is reused for the treatment of the hydrofluoric acid-containing effluent. The calcium fluoride obtained can be used as a raw material as it is for producing hydrogen fluoride, and the surplus aqueous calcium chloride solution can be supplied for other industrial uses.
Owner:MORITA CHEMICAL IND CO LTD
Organosol containing magnesium fluoride hydroxide, and manufacturing method therefor
InactiveCN101959798AHigh transmittance of visible lightLow refractive indexMagnesium fluoridesMaterial nanotechnologyHydrogen fluorideOrganic solvent
Provided are an organosol for forming a coating that can remain hydrophilic for a long time even in a situation with no exposure to light, and a manufacturing method thereof. The organosol is created by dispersing a magnesium fluoride compound obtained by reacting, at a minimum, a magnesium compound (the main raw material) with a solution containing hydrogen fluoride, in an organic solvent. The organosol is characterized by containing minute particles of a magnesium fluoride compound, with mean particle diameter from 5 to 500 nm.
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com