Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
156 results about "Magnesium bicarbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Magnesium bicarbonate or magnesium hydrogen carbonate, Mg(HCO₃)₂, is the bicarbonate salt of magnesium. It can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide (milk of magnesia).
Process and appratus for use in preparing an aqueous magnesium bicarbonate solution
InactiveUS20050255174A1Rate of dissolutionFast dissolutionBiocideMagnesium carbonatesDissolutionMagnesium bicarbonate
A method and an apparatus for preparing a substantially clear aqueous solution containing magnesium bicarbonate are disclosed. One method of preparing a substantially clear aqueous solution containing magnesium bicarbonate, includes contacting, with species resulting from the dissolution of carbon dioxide in water, an aqueous suspension of magnesium carbonate, at suitable conditions of pressure and temperature, so as to obtain an aqueous solution of magnesium bicarbonate and controlling the pH of the said solution so that, after reaction of the said species with the magnesium carbonate, the final pH falls within a range of from about 8.0 to about 8.8. One apparatus suitable for preparing an aqueous solution of magnesium bicarbonate, comprises means for contacting, with species resulting from the dissolution of carbon dioxide in water, a suspension of powdered magnesium carbonate in water so as to form an aqueous solution of magnesium bicarbonate and means for controlling the pH of the solution between about 7 and about 9 by adjusting the amount of at least one of the said species and said powdered magnesium carbonate that is contacted with the other.
Owner:SHELLEY ARTHUR +2
Preparation method for zirconium-contained rare-earth composite oxide
ActiveCN102417352AExpand industrial applicationsHigh surface areaCatalyst carriersMetal/metal-oxides/metal-hydroxide catalystsChemical industryCalcium bicarbonate
The invention relates to a preparation method for a zirconium-contained rare-earth composite oxide. At a certain ratio, rare earth (cerium, yttrium, praseodymium or terbium) is mixed with zirconium to burden, or rare earth (cerium, yttrium, praseodymium or terbium) and zirconium are mixed with at least one of other metal ions (aluminum, barium, magnesium, strontium, titanium, manganese, ferrum, copper and hafnium) to burden. A magnesium bicarbonate or / and calcium bicarbonate aqueous solution prepared from raw materials of magnesium or / and calcium minerals or oxides and hydroxides by at least one working procedure of roasting, digesting, mixing size, carbonizing and the like can serve as a precipitator to carry out precipitation so as to obtain at least one of rare earth and zirconium composite carbonate and subcarbonate, and the at least one of rare earth and zirconium composite carbonate and subcarbonate is further roasted to obtain a zirconium-contained rare-earth composite oxide product. In the preparation method, cheap calcium or / and magnesium minerals or low-purity oxides and hydroxides can serve as initial raw materials to replace common chemical industry precipitators, such as ammonia water, ammonium bicarbonate, sodium carbonate, sodium hydroxide and the like, substances, such as magnesium, calcium, carbon dioxide and the like can be effectively circulated and utilized so as to greatly lower the production cost of the zirconium-contained rare-earth composite oxide, such as ceria-zirconia, yttrium zirconium, praseodymium zirconium, terbium zirconium and the like. In addition, in the production technology disclosed by the invention, no ammonia nitrogen wastewater, high-salinity wastewater and the like are generated, carbon dioxide greenhouse gas emission amount is reduced, the preparation technology is environmentally-friendly, and environment pollution is avoided.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Water curing reverse osmosis membrane chemical cleaning method
InactiveCN101224391AExtended service lifeEasy to cleanReverse osmosisCalcium bicarbonateReverse osmosis
The invention relates to a chemical cleaning method used for a water disposal reverse osmosis membrane, relating to a chemical cleaning method used for cleaning water disposal reverse osmosis membranes in the industrial water disposal process. The invention is characterized in that NaOH solution which contains EDTA is adopted to clean microorganism, organism and silicon dioxide, etc. pollutants on the reverse osmosis membrane; HCI solution is adopted to clean calcium bicarbonate and magnesium bicarbonate filth on the reverse osmosis membrane. By adopting the method of the invention, the reverse osmosis membrane used by captive power plant water disposal can be effectively cleaned in the alumina preparation process. The invention has better cleaning effect, reliable and practiced technique and convenient operation process.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
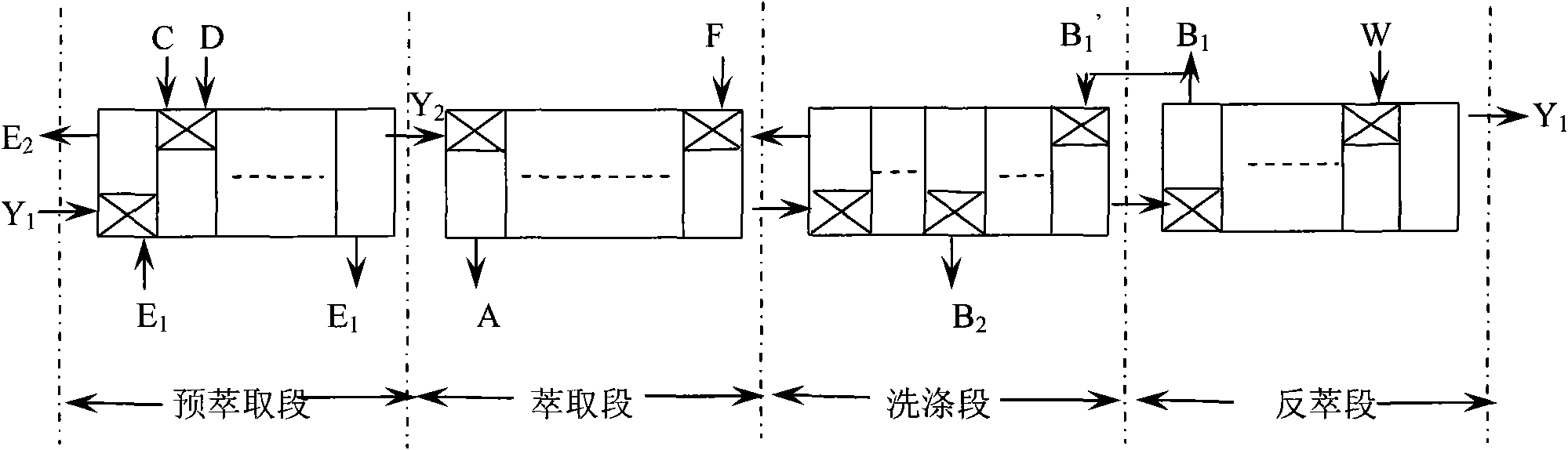
Process for separating rare-earth element by extraction
InactiveCN101781706ALow impurity contentEliminate pollutionProcess efficiency improvementRare-earth elementCalcium bicarbonate
The invention mixes and pre-extracts the mixed solution of acidic organic extractants such as P507, P204, C272, and naphthenic acid with magnesium bicarbonate and / or calcium bicarbonate solution and rare-earth solution. The rare-earth ions are extracted into the organic phase, then the loaded organic phase containing rare-earth ions are obtained through clarification, and can be used for the extract separation of the mixed rare-earth feed liquid. After a plurality of different levels of extraction, washing, stripping, single rare-earth compounds or rare-earth elements-containing enrichments can be obtained. The magnesium bicarbonate and / or calcium bicarbonate solution are prepared by roasting, digesting, carbonizing magnesite, limestone, calcite, dolomite and similar minerals, so that the content of impurities, such as silicon, iron, aluminum is lower. Ternary phase sediment is not produced in the pre-extraction and extraction separation process, so that the purity of the rare-earth products are not affected. The organic phase does not need ammonia saponification and does not produce ammonia-nitrogen wastewater. By adopting the invention, the production cost of rare-earth products is greatly lowered and the cost for three waste disposal is also greatly saved.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Method for preparing light magnesium carbonate and magnesium oxide from dolomite sea water bittern
InactiveCN101327942AReduce energy consumptionReduce manufacturing costMagnesium carbonatesMagnesiaHigh concentrationFiltration
The invention relates to a method for preparing light magnesium carbonate and magnesia using dolomite and sea water brine as materials. The dolomite is calcined to light fired dolomite and kiln gas, wherein the light fired dolomite reacts with the sea water brine to obtain magnesium hydroxide, the magnesium hydroxide is added into the magnesium-precipitation mother liquor to obtain a slurry of magnesium hydroxide, the kiln gas containing carbon dioxide of higher concentration is used for carbonizing the slurry of magnesium hydroxide. The magnesium bicarbonate solution is obtained by reacting the magnesium hydroxide slurry with compressed kiln gas which is cooled and purified in a carbonization tower. The basic magnesium carbonate is precipitated at room temperature by adding alkaline matters into the magnesium bicarbonate solution after pressure filtration and refine. The mixture solution is filtered or centrifugal separated to obtain wet basic magnesium carbonate and magnesium-precipitation mother liquor, the wet basic magnesium carbonate is dried to obtain light magnesium carbonate, the light magnesium carbonate is calcined to obtain light magnesium hydroxide. The method of the invention has advantages of low energy consumption, high resource utilization and obvious economic benefit without by product of calcium carbonate with low added value.
Owner:TIANJIN SEA WATER DESALINATION & COMPLEX UTILIZATION INST STATE OCEANOGRAPHI
Method for precipitating rare earth from ionic rare earth ore magnesium sulfate leaching solution
ActiveCN104152693AIncrease sedimentationNo pollution in the processProcess efficiency improvementPregnant leach solutionRare earth ions
The invention discloses a method for precipitating rare earth from ionic rare earth ore magnesium sulfate leaching solution. The method specifically comprises the following steps: (1) leaching ionic adsorption type rare earth raw ore to obtain leaching solution by using magnesium sulfate solution; (2) adding a magnesium-containing precipitator into the leaching solution obtained in the step (1), so that rare earth ions in the leaching solution are precipitated out to obtain a magnesium-containing rare earth precipitate; and (3) introducing carbon dioxide gas into the magnesium-containing rare earth precipitate, wherein the aim of introducing carbon dioxide refers to accelerating the reaction and removing magnesium in the precipitate, converting the magnesium in the precipitate into easily dissoluble magnesium bicarbonate to enter the solution, and converting the rare earth into a rare earth carbonate precipitate. According to the method for precipitating rare earth from ionic rare earth ore magnesium sulfate leaching solution, ammonium bicarbonate solution is not used as a precipitator, ammonia nitrogen pollution is avoided, and the rare earth precipitation capacity is high.
Owner:JIANGXI UNIV OF SCI & TECH
Process for roasting chromite resources in ring kiln through pure oxygen by using low-temperature method and harmlessly and deeply utilizing chromium residue
InactiveCN101824530AImprove resource conversion rateMagnesium carbonatesChromium trioxideSodium bicarbonateSlag
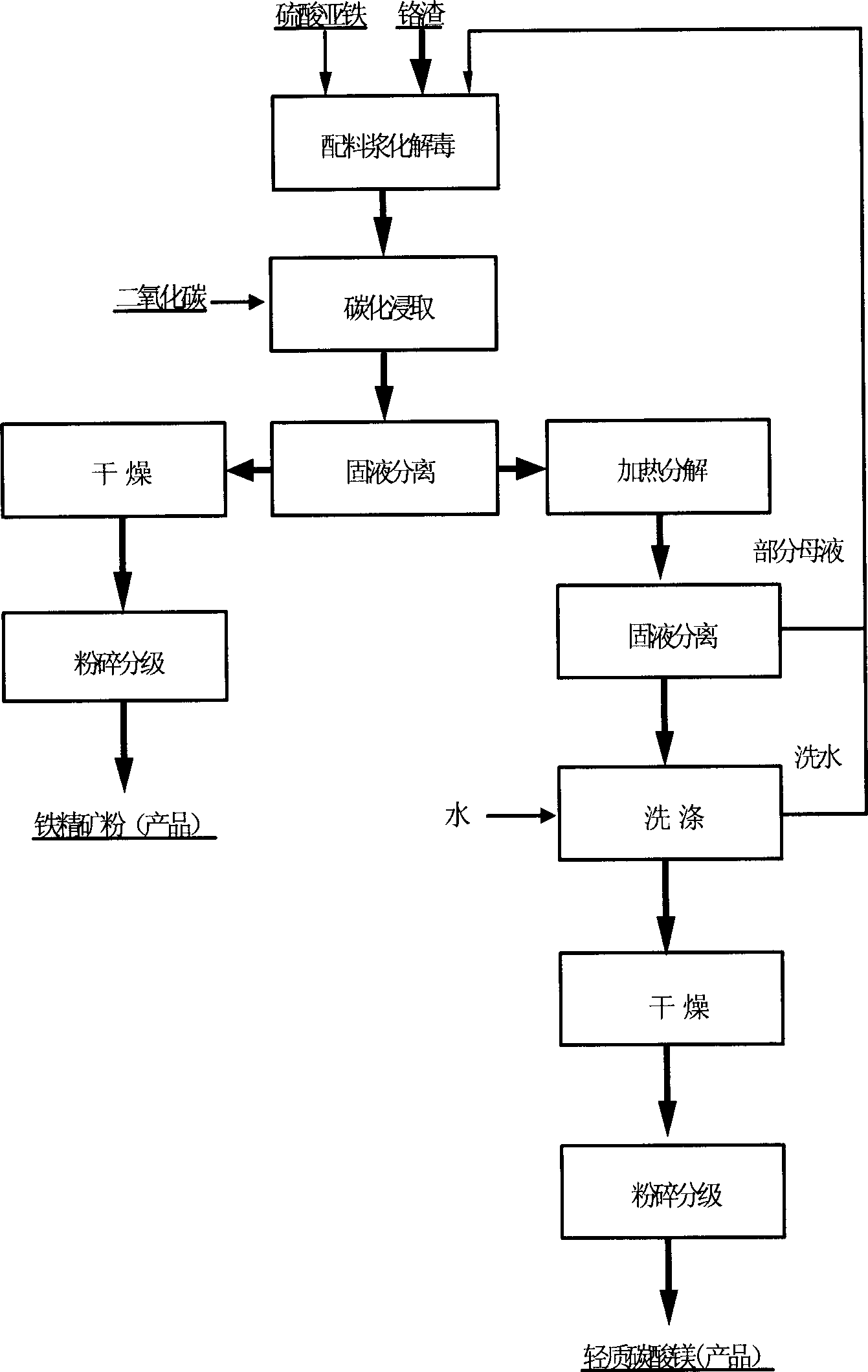
The invention belongs to the field of metallurgy and chemical engineering. The process comprises the following steps of: firstly, crushing chromite, adding sodium hydroxide and a catalyst to be oxidized and roasted by using a low-temperature pure oxygen method; diluting, cooling, extracting and filtering to obtain a sodium chromate crystal and ferrum-magnesium slag; adding an alkali washing solution into a sodium hydroxide solution to back extract to obtain the sodium hydroxide solution for recycling; adding water into the sodium chromate crystal and ferrum-magnesium slag to be dissolved and feeding filtrate into a carbonizer to decompose to extract aluminum; carbonizing, evaporating, condensing and crystallizing the extracted solution to obtain sodium chromate; and carbonizing ferrum-magnesium filter slag to generate sodium bicarbonate, reacting to generate a magnesium hydrogen carbonate solution, heating and cracking to generate a magnesium carbonate product and drying a filter cake to obtain ore refined powder; and secondly, crushing chromium residue, adding sodium bicarbonate in the ration of 1:8, adding a catalyst for calcination, cooling and adding water to soak; adding an aluminum hydroxide crystal into supernatant liquid, carbonizing and decomposing to remove aluminum in a reaction tank; filtering and washing an aluminum hydroxide product; adding a reducing agent into the filtrate to reduce hexavalent chromium to generate anhydrous chromium hydroxide and drying and roasting to obtain chromium sesquioxide; and returning the filtrate to a system for mixing after pyrolyzing and extracting to remove magnesium.
Owner:白向南 +2
Method for preparing light calcium carbonate and magnesium oxide from dolomite
ActiveCN104016393AHigh puritySimple processCalcium/strontium/barium carbonatesMagnesiaAtherion elymusCarbonization
The invention discloses a method for preparing light calcium carbonate and magnesium oxide from dolomite. The method comprises the following steps: firstly, calcining and slaking the dolomite to obtain a slaking liquid, converting calcium hydroxide in the slaking liquid to a soluble calcium ion solution through a phase-transfer reaction, meanwhile filtering out magnesium hydrate filter cakes containing impurities, feeding CO2 into the soluble calcium ion solution, precipitating and separating to obtain calcium carbonate and a filter liquor, pulping and carbonizing the magnesium hydrate filter cakes containing impurities to obtain a magnesium bicarbonate solution, then performing a pyrolytic reaction to obtain basic magnesium carbonate, and calcining to obtain light magnesium oxide. The method is simple in technical process and low in energy consumption without strong acid to leach and addition of an impurity removing precipitator, the calcium and magnesium separation effect is good, a common carbonization liquid-solid-gas three-phase reaction is avoided, the reaction condition is easily controlled, and prepared light calcium carbonate accords with demands of national corresponding product standard and lays research basis for industrial scale production.
Owner:HEFEI UNIV OF TECH
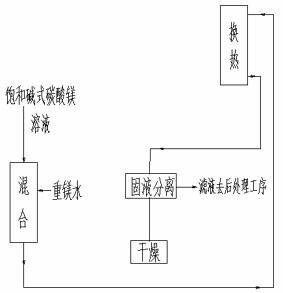
Method and device for preparing basic magnesium carbonate from heavy magnesium carbonate water by pyrolysis
ActiveCN102659147ALess investmentGuaranteed uptimeMagnesium carbonatesMagnesium bicarbonateAqueous solution
The invention relates to a method for preparing basic magnesium carbonate from heavy magnesium carbonate water by pyrolysis, which comprises the following steps: mixing a magnesium bicarbonate water solution, of which the temperature is 20-60 DEG C and the mass concentration is 3.5-100g / L, and a saturated basic magnesium carbonate or saturated basic magnesium carbonate solution containing small amount of solid crystals, of which the temperature is 40-90 DEG C, in a volume ratio of 1:(0.05-29); carrying out heat exchange in a 40-90 DEG C pyrolyzer for 10-240 minutes; carrying out solid-liquid separation on the reaction liquid to obtain solid basic magnesium carbonate crystals and a filtrate; and drying the solid basic magnesium carbonate crystals to obtain the heavy magnesium carbonate. The method provided by the invention can overcome the defects of high fuel consumption, high power consumption, high scaling tendency of the pyrolyzer or boiler, short operating cycle, incapability of long-cycle smooth operation, and the like in the existing methods.
Owner:丁丽芳 +1
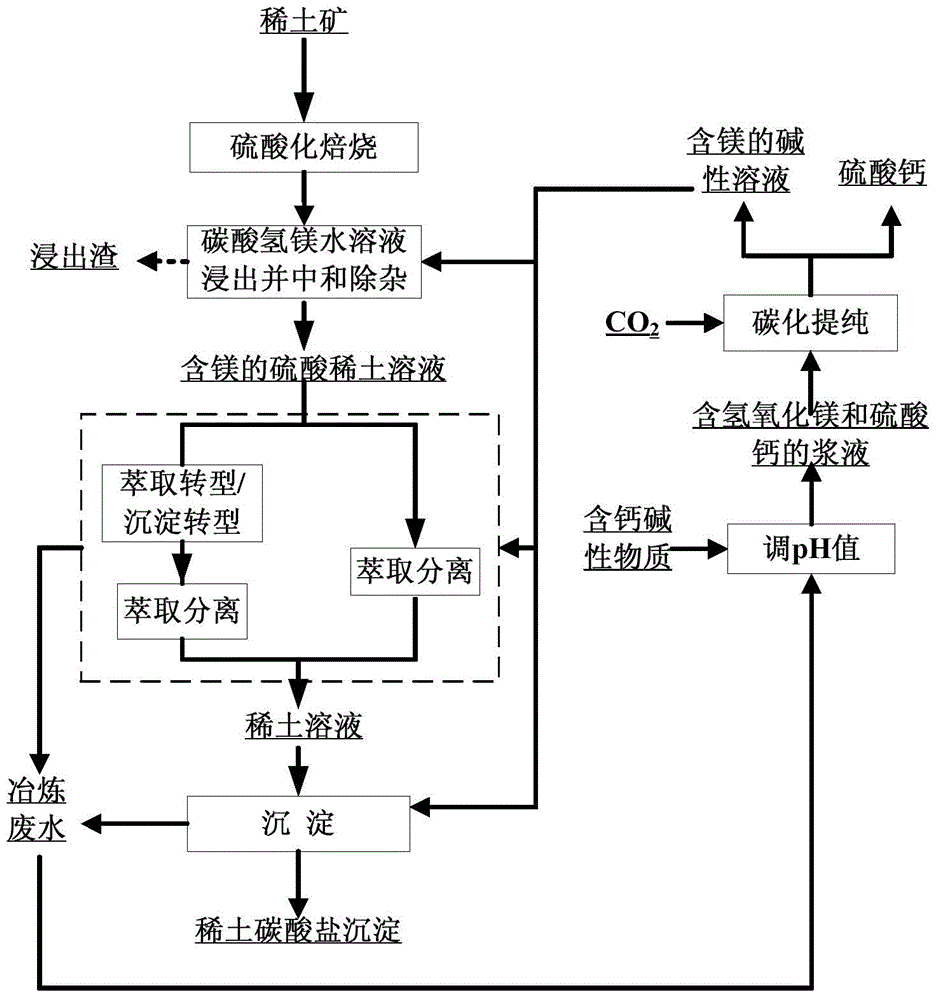
Smelting separation method of rare earth ores
ActiveCN106282553AHigh recovery rateTo achieve the purpose of removing impuritiesProcess efficiency improvementHigh concentrationCarbonization
The invention provides a smelting separation method of rare earth ores. The method comprises the following steps: carrying out leaching, neutralization and impurity removal on sulfuric acid roast ores by using an aqueous solution of magnesium bicarbonate, and carrying out solid-liquid separation to obtain a magnesium-containing rare earth sulfate solution; and carrying out aqueous magnesium bicarbonate solution saponification P507 or P204 extraction transformation or magnesium bicarbonate precipitation transformation enrichment to obtain a high-concentration mixed rare earth chloride solution, carrying out extraction separation, and recovering rare earth from the above obtained aqueous magnesium bicarbonate solution precipitate to obtain various rare earth compound products. Magnesium sulfate-containing wastewater generated in the above process undergoes alkali transformation by cheap alkaline compounds of calcium and magnesium, and CO2 recovered in the smelting separation process is introduced to carry out carbonization purification in order to obtain an aqueous magnesium bicarbonate solution which can be reused in rare earth leaching, transformation, extraction separation and precipitation processes. The method has the advantages of realization of recycling of magnesium and CO2 and zero discharge of ammonia nitrogen and wastewater, great reduction of the production cost, improvement of the recovery rate of rare earths, and realization of green, environmentally-friendly and high-efficient clean production of the rare earths.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Preparation and comprehensive utilization method of magnesium bicarbonate solution
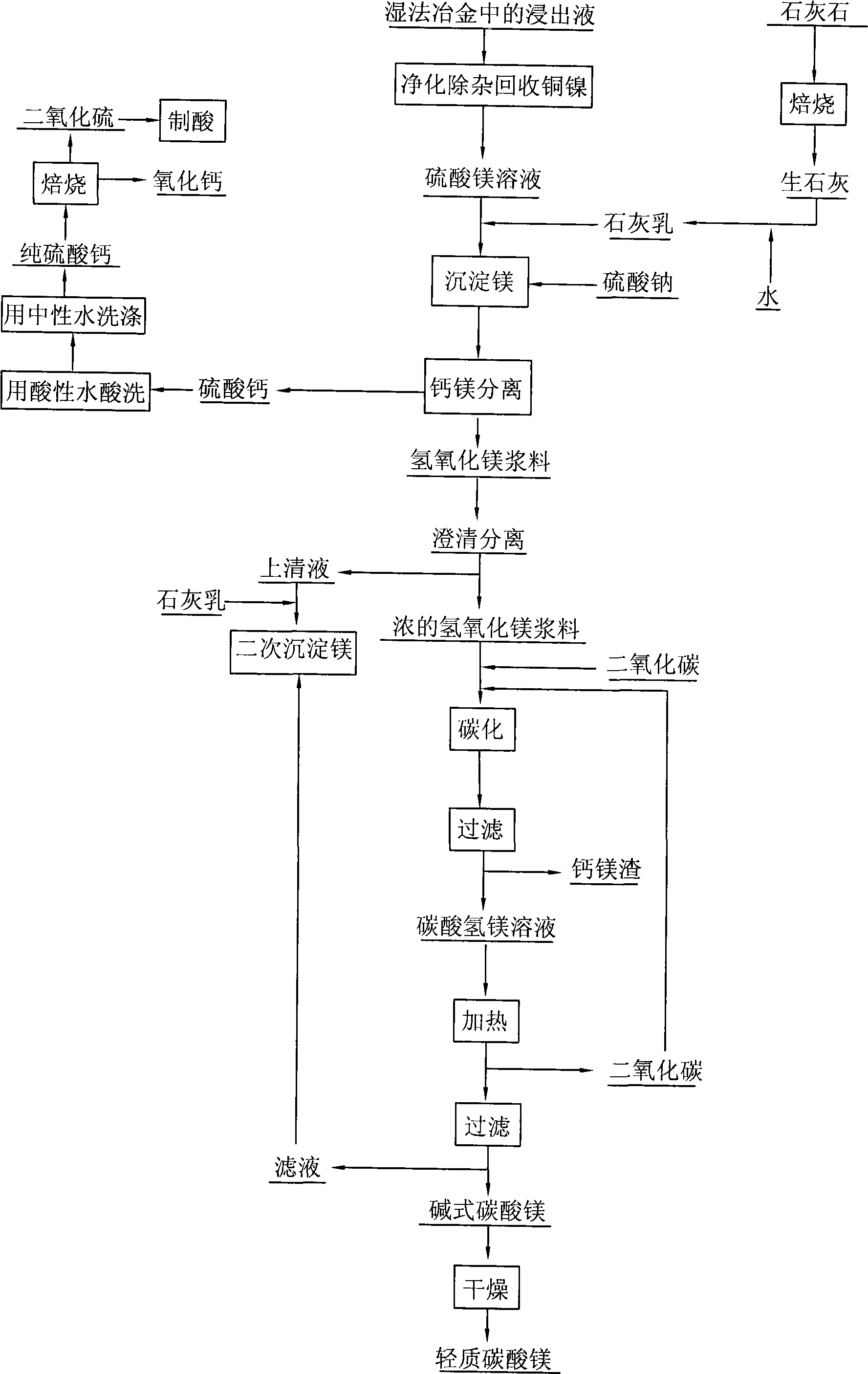
ActiveCN103382034ARealize comprehensive utilizationAchieve emissionsChlorine/hydrogen-chlorideCalcium/strontium/barium chloridesCarbonizationSlurry
The invention relates to a preparation and comprehensive utilization method of a magnesium bicarbonate solution. The method is used to prepare the magnesium bicarbonate solution used for deposition, crystallization and recovery of rare earth ions, and comprehensive recovery of calcium-compound products and magnesium-compound products by taking dolomite as a raw material. The method specifically comprises: roasting dolomite to obtain a powder containing calcium oxide and magnesium oxide, directly mixing with a magnesium chloride solution for digestion and alkali transformation at the same time to prepare a magnesium hydroxide secondary-product and a calcium compound secondary-product, and further to realize effective separation of calcium and magnesium; performing slurry mixing on magnesium hydroxide, introducing carbon dioxide gas for a carbonization reaction to prepare the magnesium bicarbonate solution; applying obtained magnesium bicarbonate to deposition and crystallization of the rare earth ions to produce rare-earth carbonate or rare-earth oxide products; and recycling one part of the filtrate to prepare magnesium bicarbonate, and evaporating one part of the filtrate for crystallization to produce the magnesium compound product.
Owner:GRIREM ADVANCED MATERIALS CO LTD
New method for preparing magnesium-aluminum hydrotalcite
The invention relates to a method for preparing magnesium-aluminum hydrotalcite, comprising the following steps of: (a) adding aluminium hydroxide gel and magnesium hydroxide together to a reactor; (b) adding magnesium hydrogen carbonate to the reactor; (c) adjusting the temperature of the reactor at 60-90 DEG C and heating and stirring for 1-2 hours; (d) obtaining a white precipitate, i.e., hydrotalcite, and drying by a spray dryer. The invention has the following advantages: the generated hydrotalcite has no impurities, does not need to be washed; the particle is fine and does not need follow-up smashing; the obtained product is dried by the mist spray and the loss during the washing and drying operation in the traditional method is reduced.
Owner:ZHEJIANG HAIHONG HLDG
Manufacturing process for light magnesium carbonate
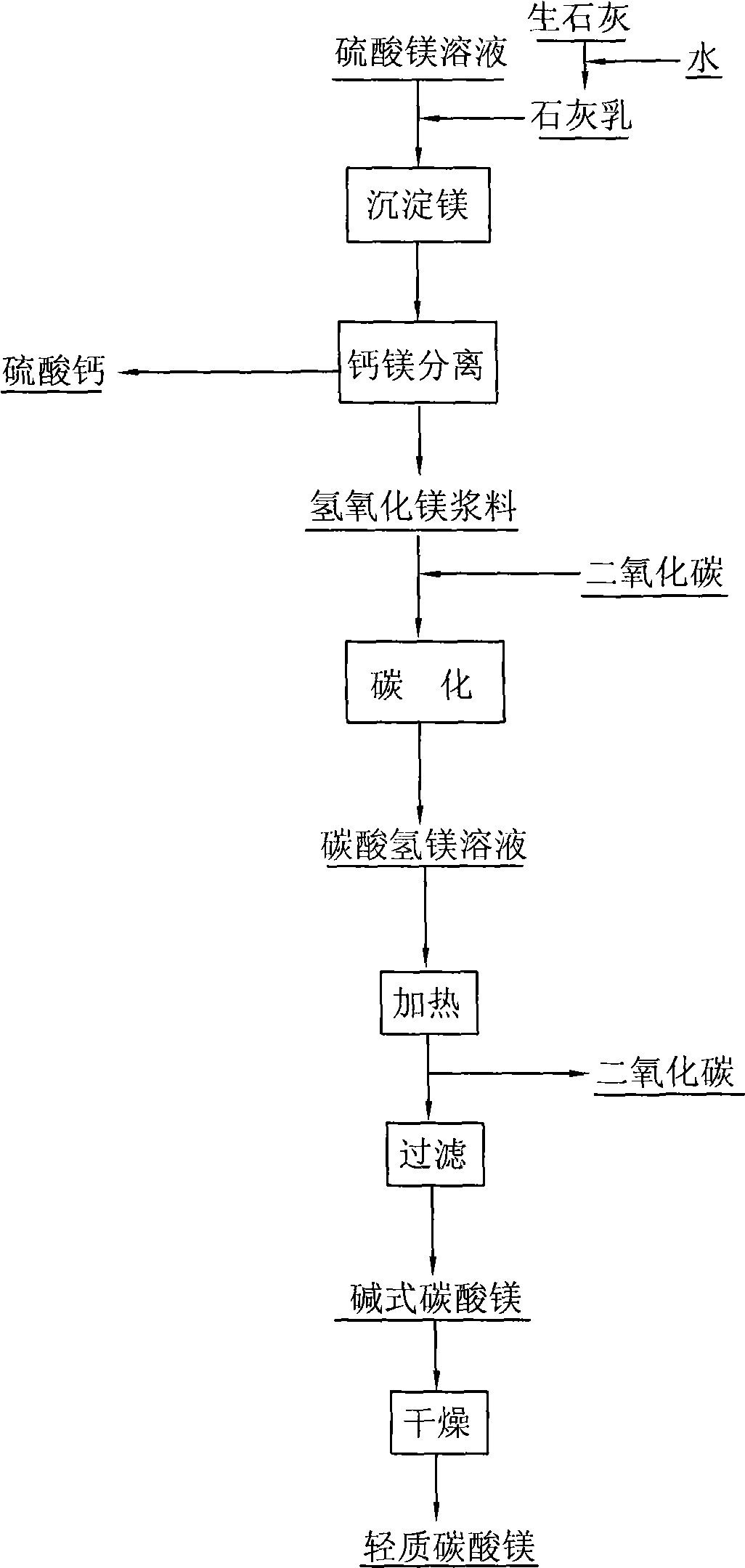
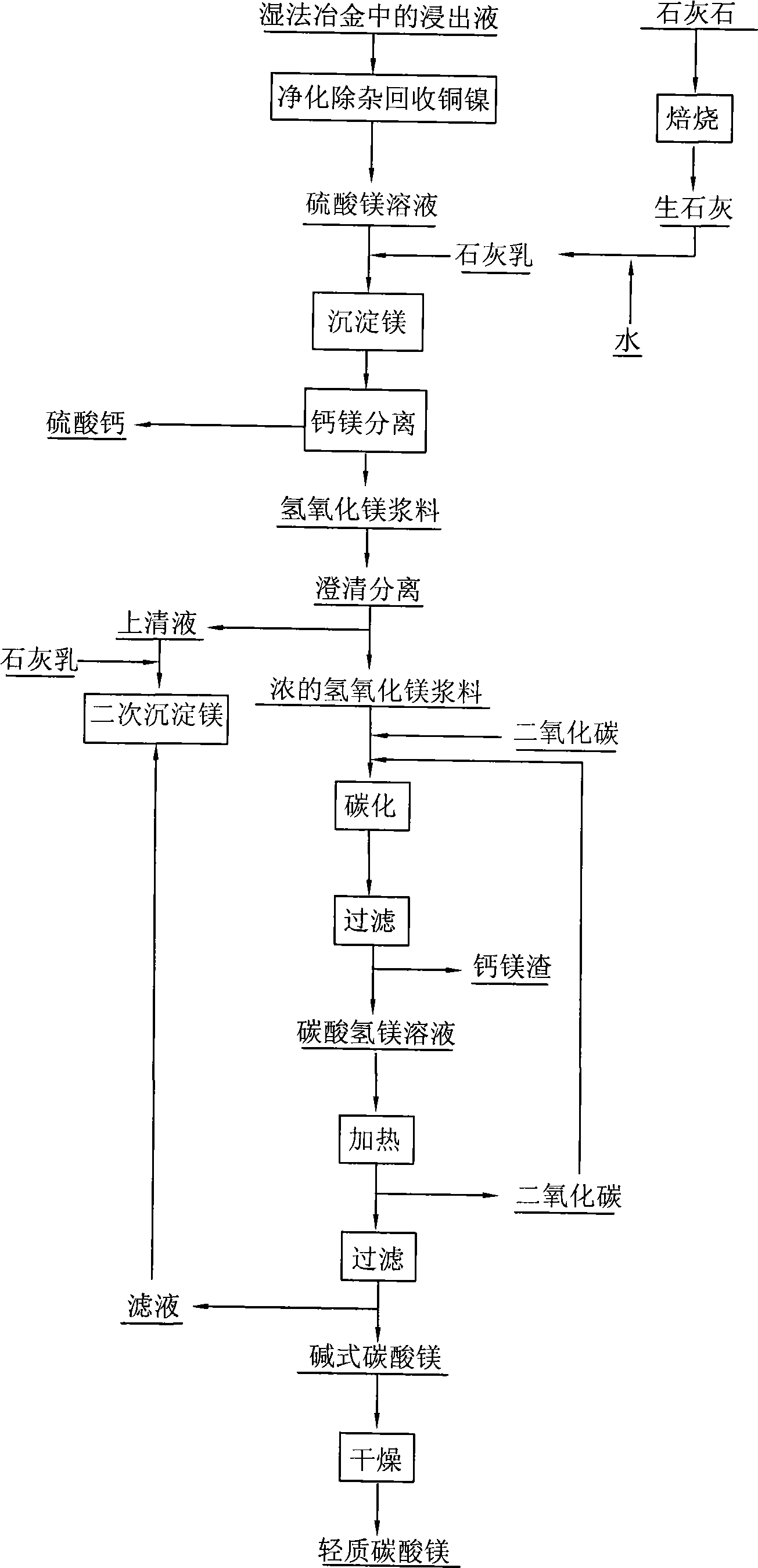
The invention relates to a manufacturing process for light magnesium carbonate, in particular to a manufacturing process for light magnesium carbonate by adopting calcium lime and magnesium sulfate solution produced during the wet process metallurgy. The process comprises the following steps of: slaking calcium lime to obtain lime cream; using the lime cream for precipitating magnesium to ensure that crystalline calcium sulphate and gelatinous magnesium hydroxide are precipitated out; separating the calcium sulphate from the magnesium hydroxide to obtain calcium sulphate pulp and magnesium hydroxide pulp; feeding carbon dioxide into the magnesium hydroxide pulp to obtain magnesium bicarbonate solution; heating the magnesium bicarbonate solution to ensure that magnesium bicarbonate is decomposed to generate basic magnesium carbonate and release carbon dioxide; and filtering the basic magnesium carbonate out, washing and drying to obtain the light magnesium carbonate. The manufacturing process has the advantages that the manufacturing process is simple, the cost is low, and the waste liquor produced during the wet process metallurgy can be treated.
Owner:CHINA ENFI ENGINEERING CORPORATION
Process for preparing magnesium carbonate whisker
The present invention is the process of preparing magnesium carbonate crystal whisker from magnesium bicarbonate solution. Into magnesium bicarbonate solution, proper amount of additive is added, and through controlling the crystallization condition, magnesium carbonate crystal whisker in different sizes is prepared. Thus obtained magnesium carbonate crystal whisker has diameter of 0.1-5 microns and length of 10-2000 microns, may be used as reinforcing material for paint, plastic, rubber, etc. and may be used also as the intermediate product for preparing high-purity magnesia and other magnesium salt. The present invention opens new way for the comprehensive utilization of magnesium salt and has excellent application foreground.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing alkaline type magnesium carbonate by low temperature pyrogenation of Mg(HCO3)2 water and coproducing magnesium silicate
InactiveCN1970451AHigh yieldLow pyrolysis temperatureMagnesium silicatesMagnesium carbonatesMagnesium bicarbonateSodium silicate
The invention discloses a recycling method of magnesium in the heavy magnesium water solution, which comprises the following steps: aerating air in the heat decomposing reactor bottom under indoor temperature; reducing partial pressure of carbon dioxide through extracting into vacuum; transmitting 80-90% magnesium bicarbonate in the heavy magnesium solution into the sediment of basic magnesium carbonate sediment; filtering; drying to obtain the basic magnesium carbonate; adding sodium silicate in the filtrate; reacting residual 10-20% magnesium bicarbonate and sodium silicate to obtain magnesium silicate; recycling magnesium in the heavy magnesium solution completely.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Technology for recovering magnesium from magnesium sulfate solution
ActiveCN101760641AEfficient recyclingReduce processing costsCarbon compoundsMagnesium carbonatesSlurryCarbonate
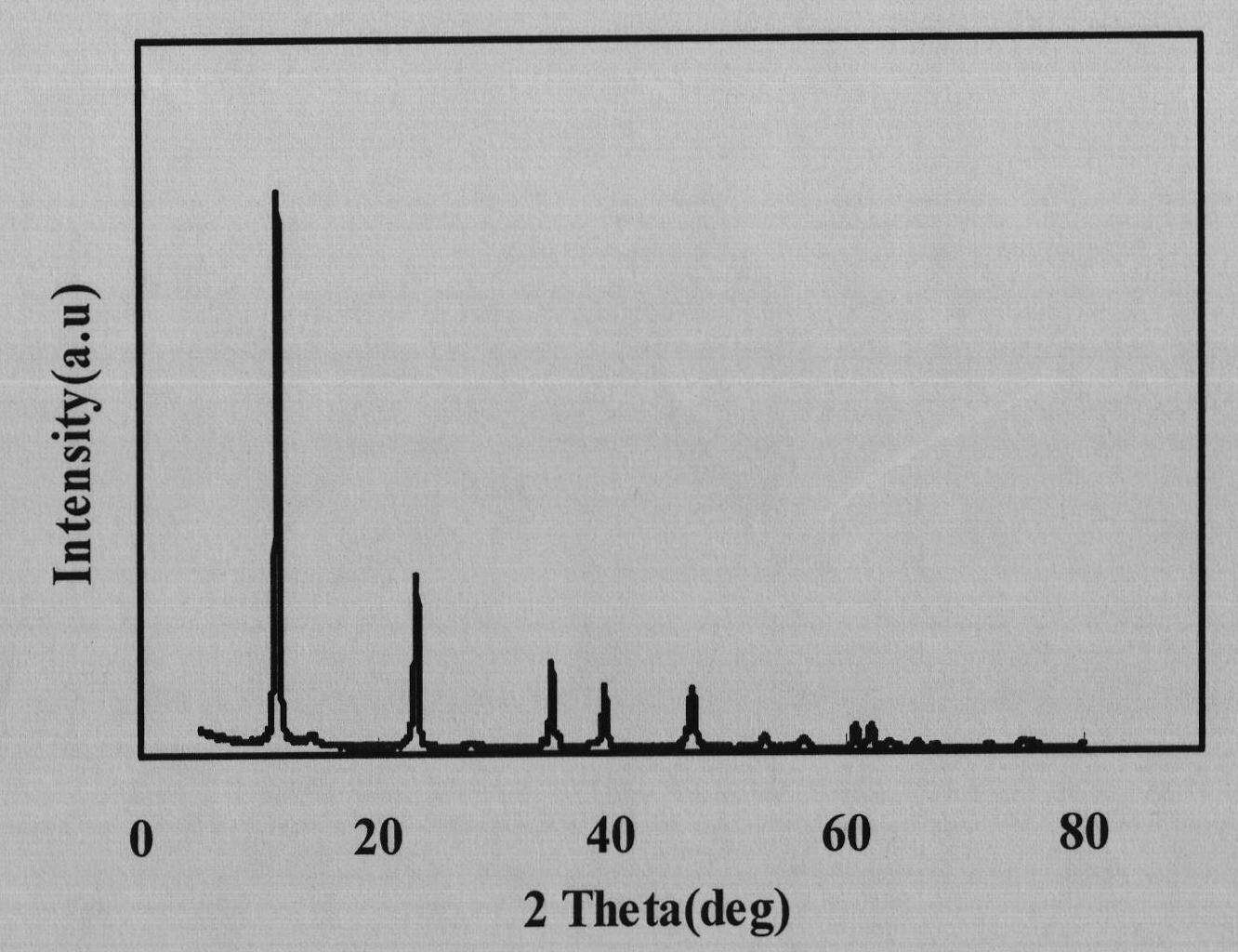
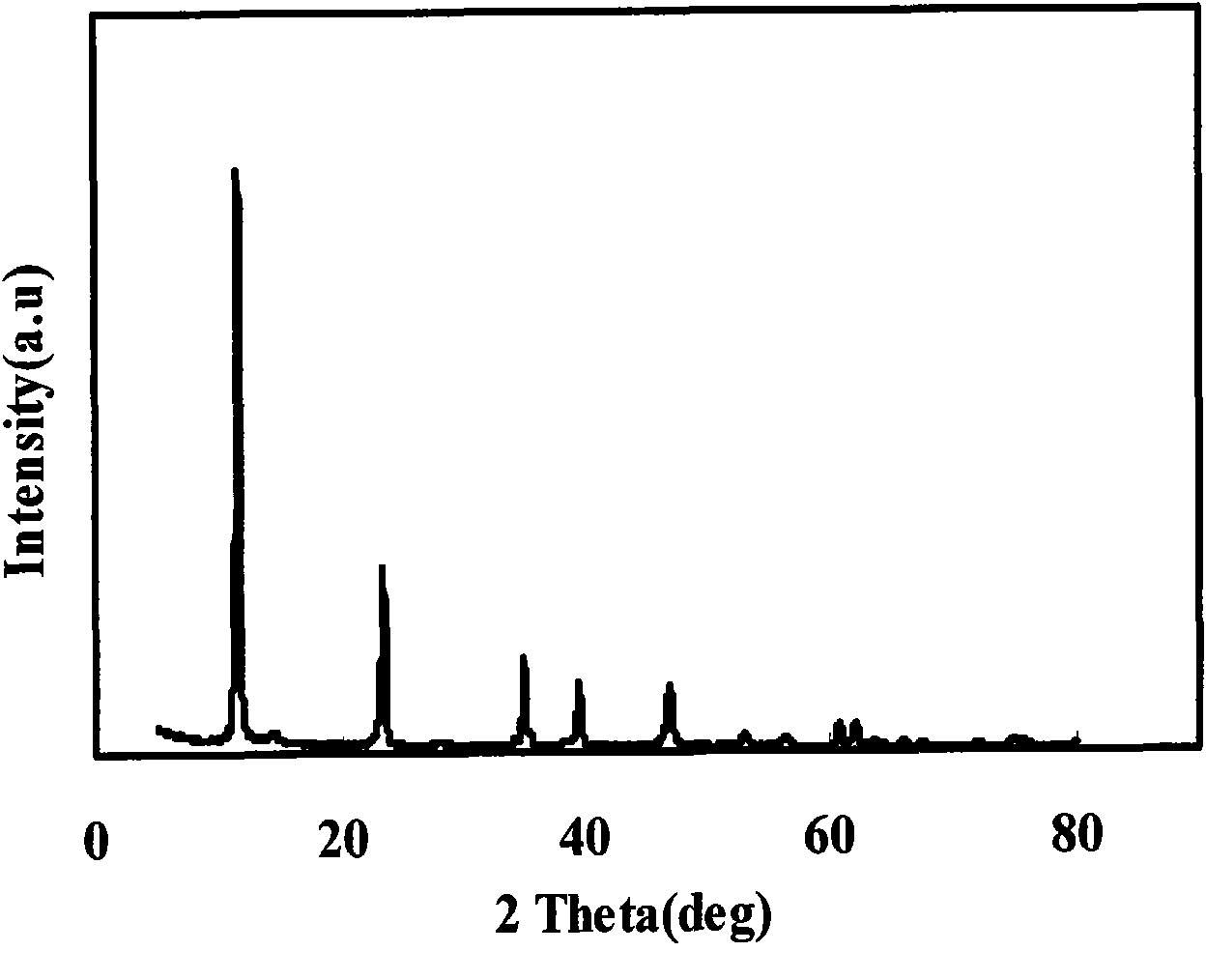
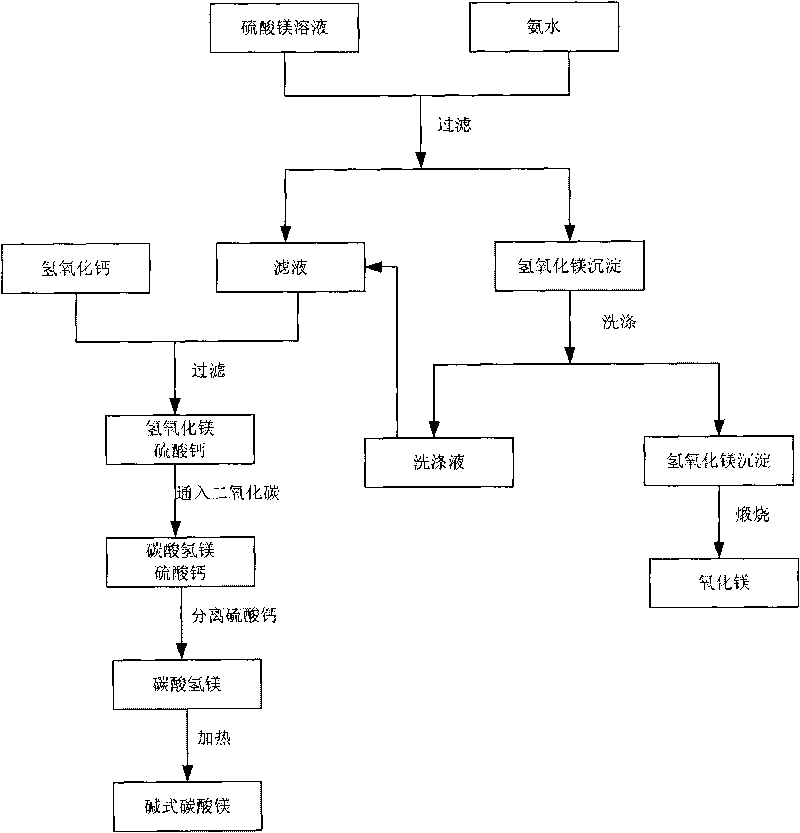
The invention relates to a technology for recovering magnesium from a magnesium sulfate solution, comprising the following steps of: (A) mixing the magnesium sulfate solution with ammonia water to obtain slurry containing a magnesium hydroxide sediment and residual magnesium sulfate; (B) filtering the slurry containing the magnesium hydroxide sediment and the residual magnesium sulfate to respectively obtain the magnesium hydroxide sediment and a filtrate; (C) causticizing the filtrate by using calcium hydroxide and / or calcium oxide to obtain slurry containing the magnesium hydroxide sediment and calcium sulfate and generate ammonia; (D) introducing carbon dioxide in the slurry containing the magnesium hydroxide sediment and the calcium sulfate so that magnesium hydroxide is carbonized to generate magnesium hydrogen carbonate and the calcium sulfate is separated; and (E) heating a magnesium hydrogen carbonate solution separated from the calcium sulfate so that the magnesium hydrogen carbonate is decomposed to generate a basic magnesium carbonate sediment and the carbon dioxide. The technology can be used for efficiently recovering the magnesium hydrogen carbonate and the basic magnesium carbonate from the magnesium sulfate solution.
Owner:CHINA ENFI ENGINEERING CORPORATION
Comprehensive utilization method of converting chromium slag totally into light magnesium carbonate and fine iron breeze
InactiveCN1410352AReduce corrosionSimple processMagnesium carbonatesSolid waste disposalHydrogenSlag
A process for converting all the chromium slags to light-wt. magnesium carbonate and powdered iron ore concentrate includes such steps as proportionally mixing Cr-slag with water, adding FeSO4.7H2O, reaction at 15-50 deg.C for 5-30 min, carbonizing at 15-50 deg.c for 0.5-4 hr in a high-pressure reactor by introducing CO2 while stirring, press-filtering to obtain cake of iron ore concentrate and aqueous solution of magnesium hydrogen carbonate, drying the cake at 100-200 deg.C for 1-4 hr, pulverizing to obtain the powdered iron core concentrate, thermolyzing the said aqueous solution at 95-105deg.C for 10-60 min by introducing steam, washing, drying and pulverizing to obtain light-wt. magnesium carbonate.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Cleaning method of reverse osmosis membrane of steel recycled water membran method desalting system
ActiveCN101596410AShort cleaning timeDoes not affect productionSemi-permeable membranesGeneral water supply conservationAluminium chlorideAluminum Ion
The invention is a cleaning method of reverse osmosis membrane of a steel recycled water membran method desalting system, belonging to the technical field of steel recycled water desalting. The method comprises the following steps: automatically adding alkaline washing liquid in a running membran method desalting system to wash the organism, silicon dioxide, polymer of aluminum sulfate or aluminum chloride and pollutant of calcium, magnesium, iron, aluminum ion and the like on the reverse osmosis membrane at regular time, then washing rapidly by reverse osmosis producing water; or adding acid washing liquid to clear up calcium bicarbonate, magnesium bicarbonate incrustation, aluminate or aluminium hydroxide floc, metallic oxide and microorganism, then washing rapidly by the reverse osmosis producing water. One time washing is carried out when the desalting system runs 6-24h, and acid washing and alkali washing are carried out alternately. The method provided by the invention has the advantages that the reverse osmosis water flux and differential pressure can be restored without affecting production with less drug consumption, convenience and effectiveness, due to the short washing time in each time.
Owner:SHOUGANG CORPORATION
Method of precipitation of metal ions
ActiveUS20110280778A1Low production costLow priceAluminium hydroxide preparationMercury oxidesCalcium bicarbonateIndium
The present invention relates to a method of precipitation of metal ions. Mineral(s), oxide(s), hydroxide(s) of magnesium and / or calcium are adopted as raw materials, and the raw material(s) is processed through at least one step of calcination, slaking, or carbonization to produce aqueous solution(s) of magnesium bicarbonate and / or calcium bicarbonate, and then the solution(s) is used as precipitant(s) to deposit rare earth, such as nickel, cobalt, iron, aluminum, gallium, indium, manganese, cadmium, zirconium, hafnium, strontium, barium, copper and zinc ions. And at least one of metal carbonates, hydroxides or basic carbonates is obtained, or furthermore the obtained products are calcined to produce metal oxides. The invention takes the cheap calcium and / or magnesium minerals or their oxides, hydroxides with low purity as raw materials to instead common precipitants such as ammonium bicarbonate and sodium carbonate etc. The calcium, magnesium, carbon dioxide etc are efficiently and circularly used, and the environment pollution by ammonium-nitrogen wastewater, high concentration salts wastewater is avoided, and both of the discharge of greenhouse gas carbon dioxide and the production cost of metal are decreased.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Method for preparing magnesium oxide for high-performance silicon steel through dolomite
ActiveCN105271845ANo three wastes pollutionSimple production processMagnesium bicarbonateChemical combination
The invention relates to the field of orientation silicon steel manufacturing, in particular to a method for preparing magnesium oxide for high-performance silicon steel through dolomite. The problems that in the existing orientation silicon steel manufacturing process, magnesium oxide for silicon steel is prepared through a bittern method, adhesiveness is poor, the magnetic flux density is low, insulation film bottom layer concession is high are solved. The method comprises the steps that firstly, dolomite is roasted, subjected to chemical combination with water and then carbonized in a carbonizing tower; secondly, solid calcium carbonate and a magnesium bicarbonate aqueous solution are filtered and separated, the obtained magnesium bicarbonate aqueous solution is pyrolyzed and filtered, magnesium carbonate is obtained, then subjected to multi-step calcination, smashed through air flow and packaged, and magnesium oxide for the high-performance silicon steel is obtained. According to the method, the production process is unique, cost is low, three wastes are not generated, the adhesiveness, electromagnetic performance and grade rate of the orientation silicon steel can be remarkably improved, and the method can be applied and popularized in orientation silicon steel factories on a large scale.
Owner:山西银圣科技有限公司
Method for removing calcium and magnesium ions in high-purity manganese sulfate production
ActiveCN111908511AAchieve removalGood removal effectManganese sulfatesManganese sulphateCalcium bicarbonate
The invention relates to the technical field of lithium battery positive electrode materials, and provides a method for removing calcium and magnesium ions in high-purity manganese sulfate production.The method provided by the invention comprises the following steps: firstly, adding soluble carbonate to convert a high-purity manganese sulfate precursor (crude manganese carbonate and / or crude manganese hydroxide) into manganese carbonate, simultaneously converting calcium and magnesium ions in the manganese carbonate into calcium carbonate and magnesium carbonate, and then introducing carbon dioxide to convert the calcium carbonate and the magnesium carbonate into calcium bicarbonate and magnesium bicarbonate; calcium bicarbonate and magnesium bicarbonate are easily dissolved in water andcan be separated from manganese carbonate through solid-liquid separation, so that calcium and magnesium ions are removed. The method provided by the invention is simple in steps, easy to operate, lowin cost and good in calcium and magnesium ion removal effect.
Owner:TSINGHUA UNIV
Method for utilizing high-magnesium phosphate tailings to produce magnesium fluoride and by-product calcium carbonate
InactiveCN102923739ASolve the occupation of land resourcesSolve the pollution of the environmentCalcium/strontium/barium carbonatesMagnesium fluoridesMagnesium phosphateCarbonization
The invention discloses a method for utilizing high-magnesium phosphate tailings to produce magnesium fluoride and by-product calcium carbonate. The method comprises the following steps: calcinating the high-magnesium phosphate tailings at high temperature to form calcined dolomite, adding water to the calcined dolomite to perform digestion to obtain digestion solution, supplementing water to the digestion solution and then leading carbon dioxide to perform a carbonization reaction to obtain carbonized liquid, separating bottom phosphate ore sediments of the carbonized liquid after the reaction stops, filtering, and obtaining filtrate which is magnesium bicarbonate aqueous solution, and obtaining a calcium carbonate product after drying a filter cake; adding hydrofluoric acid in the obtained magnesium bicarbonate aqueous solution, filtering after the reaction, washing, and obtaining a magnesium fluoride product after drying the filter cake, The industrial waste high-magnesium phosphate tailings serve as raw materials, waste is turned into wealth, abundant magnesium calcium resources in the high-magnesium phosphate tailings are fully utilized to produce the magnesium fluoride and the by-product calcium carbonate, and the method has the advantages of being simple in process, low in production cost and basically free of three wastes.
Owner:WENGFU (GRP) CO LTD
Manufacturing method of active magnesium oxide
InactiveCN106976895AHigh activityEasy accessMaterial nanotechnologyOther chemical processesEnvironmental resistanceEmulsion
The invention discloses a manufacturing method of active magnesium oxide. The manufacturing method comprises the following steps: I, putting a primary magnesium oxide into a ball mill for ball milling, digesting with water, removing residues, and preparing into an ash emulsion; II, carbonizing the ash emulsion with CO-, to obtain a high-purity magnesium bicarbonate solution; III, pyrolyzating the high-purity magnesium bicarbonate solution at the temperature of 35-40DEG C, separating and drying for 2.5-3.5h, to obtain high-purity alkaline type magnesium carbonate; and IV, performing ball-milling to the high-purity alkaline type magnesium carbonate for 30-40 minutes through the ball mill, and then calcinating for 30-45 minutes at 620-810DEG C, to obtain active magnesium oxide. The active magnesium oxide manufactured by adopting the manufacturing method of active magnesium oxide has high activity, the raw materials needed during preparation is cheap and easily available, the production cost is low, no toxicity and harmfulness exist, the production process is environment-friendly, the manufacturing method is simple and efficient and easy to control, and applicable to large-scale production and application. The active magnesium oxide manufactured by adopting the manufacturing method of active magnesium oxide has high activity, thus being capable of adsorbing toxic substances.
Owner:广州峰华化工科技有限公司
Novel process for innocent treatment and resource regeneration of chromic slag
InactiveCN101318188AHigh extraction rateSimple processSolid waste disposalSuspended particlesFiltration
The invention discloses a chromium slag harmless treatment and resource recycling new technology, which belongs to the chemical and metallurgical field, and includes the following main technological processes: the extracting technological process of a petroleum aided reagent, which follows that the chromium slag is ground and then pulpified by hot water, hexavalent chromium solution is obtained by pressure filtration, hydroxyl chromium oxide is generated by adding in sodium sulfide, chromium acetate and chromium lactate are generated by adding in acetic acid and lactic acid, and the petroleum aided reagent is obtained by adding in a flocculant; the extracting technological process of magnesium oxide, which follows that the chromium slag is pulpified by adding water, carbon dioxide is pressed in, and magnesium bicarbonate solution is obtained, then the magnesium bicarbonate solution is decomposed by heating to a temperature of 90 DEG C to prepare crystal magnesium carbonate, and the magnesium oxide is obtained under a high temperature of 900 DEG C; and the extracting technological process of a chromite sand, which follows that the chromium slag is washed by water so as to remove suspended particles, and a concentrate ore is obtained by removing particle impurities with smaller specific gravity through water floatation. Compared with the present chromium slag treatment technology, the technology of the invention has the advantages of simple process, low cost, high chromium extraction rate, and being capable of extracting a plurality of products, etc.
Owner:白向南 +2
Salvianolic acid A magnesium salt, preparation method and use of the salvianolic acid A magnesium salt, and salvianolic acid A magnesium salt-containing freeze-dried powder injection composition
InactiveCN102432467AImprove solubilityLess irritatingOrganic active ingredientsPowder deliveryMagnesium saltFreeze-drying
The invention discloses a salvianolic acid A magnesium salt, specially, relates to the salvianolic acid A magnesium salt, a preparation method and a use of the salvianolic acid A magnesium salt, and a salvianolic acid A magnesium salt-containing freeze-dried powder injection composition, and belongs to the technical field of medicines. The preparation method of the salvianolic acid A magnesium salt comprises the following steps of adding magnesium hydroxide into water, feeding CO2 into the magnesium hydroxide solution until the magnesium hydroxide solution is clarified, removing the CO2 in the magnesium hydroxide solution by ultrasonic waves to obtain magnesium hydrogen carbonate, adding salvianolic acid A into the obtained magnesium hydrogen carbonate so that the salvianolic acid A and the obtained magnesium hydrogen undergo a reaction at a temperature of 20 to 40 DEG C for 20 to 40 minutes to produce a reaction product solution, adding ethyl acetate into the reaction product solution, wherein the volume of the added ethyl acetate is equal to that of the reaction product solution, carrying out vortexing, and then carrying out centrifugal separation and freeze drying to obtain the salvianolic acid A magnesium salt. The salvianolic acid A magnesium salt has strong stability in an aqueous solution, high solubility and small irritation. The preparation method of the salvianolic acid A magnesium salt adopts a good preparation technology and has the simple steps. The salvianolic acid A magnesium salt-containing freeze-dried powder injection composition has good stability and high uniformity, and satisfies medicine rehydration capability requirements. The salvianolic acid A magnesium salt can be utilized for preparation of medicines for treating ischemic stroke.
Owner:吴谢军 +2
High-hardness pressure-resistant terrace material
The invention relates to a high-hardness pressure-resistant terrace material which is composed of the following components in parts by weight: 26-28 parts of polypropylene, 9-13 parts of furan resin, 11-12 parts of magnesium bicarbonate, 7-9 parts of calcium pyrophosphate, 11-13 parts of acrylonitrile, 5-6 parts of diphenyl ether and 3-5 parts of methylethylketone. The prepared terrace material has the characteristics of high hardness and pressure resistance.
Owner:江苏悠谷未来科技有限公司
Process for preparing light magnesium carbonate from boron mud
The invention discloses a process for preparing light magnesium carbonate from boron mud. The process comprises the following steps of (A), digesting: mixing boron mud, calcium oxide and water and carrying out reaction to prepare digesting slurry; (B), carbonizing: supplementing quantitative water into digesting slurry, regulating concentration of the slurry, lowering the temperature to 20 DEG C-30 DEG C, continuously ventilating carbon dioxide, stirring and reaching a carbonizing final point when pH of the liquor is 7.2; (C), pyrolyzing: filtering the carbonizing liquor to obtain filtrate and slag body, adding alkaline into the filtrate and pyrolyzing; and (D), purifying: filtering, washing and drying the pyrolyzed mixed liquor to obtain the light magnesium carbonate. In the process, the alkaline is added into the magnesium bicarbonate liquor for lowering a pyrolyzing temperature to 45 DEG C-55 DEG C which is greatly lowered in comparison with the pyrolyzing temperature of 95 DEG C-105 DEG C when alkaline is not added. The process realizes energy-saving effect, and prepares the high-purity light magnesium carbonate at the same time. The carbonized slag body is used for preparing bricks, so that zero emission is realized.
Owner:李广凡
Leaching method of magnesium-containing ore
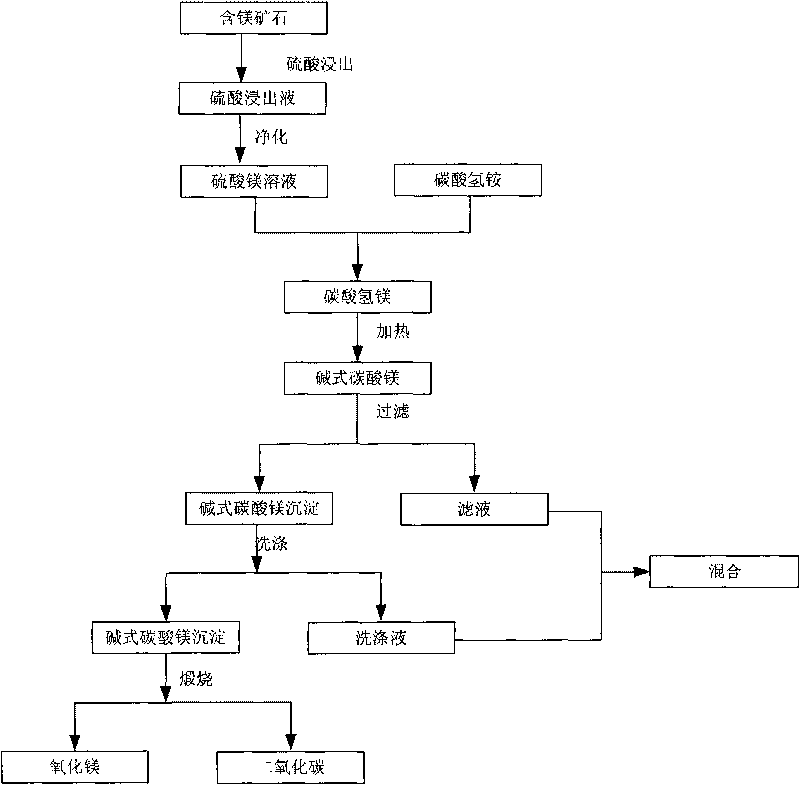
InactiveCN101760646AReduce processing costsImprove recycling efficiencyMagnesium carbonatesAmmonia preparation/separationPregnant leach solutionHydrogen
The invention provides a leaching method of a magnesium-containing ore. The method comprises the following steps: mixing the magnesium-containing ore with sulfuric acid solution and performing acid leaching to obtain leaching solution containing magnesium sulfate; purifying the leaching solution to obtain magnesium sulfate solution; mixing the magnesium sulfate solution with ammonium bicarbonate to obtain solution which contains magnesium hydrogen carbonate and residual magnesium sulfate; heating the solution and disintegrating the magnesium hydrogen carbonate to produce basic magnesium carbonate precipitation and carbon dioxide; and heating serous fluid which contains the basic magnesium carbonate precipitation and then performing filtering separation on the serous fluid to obtain the basic magnesium carbonate precipitation and filter liquor. By utilizing the leaching method of the magnesium-containing ore of the invention, not only the metal in the ore can be recovered, but also the magnesium in the ore can be efficiently recovered and the pollution is reduced.
Owner:CHINA ENFI ENGINEERING CORPORATION
Method for mineralization storage of CO2 through magnesium resources in serpentine
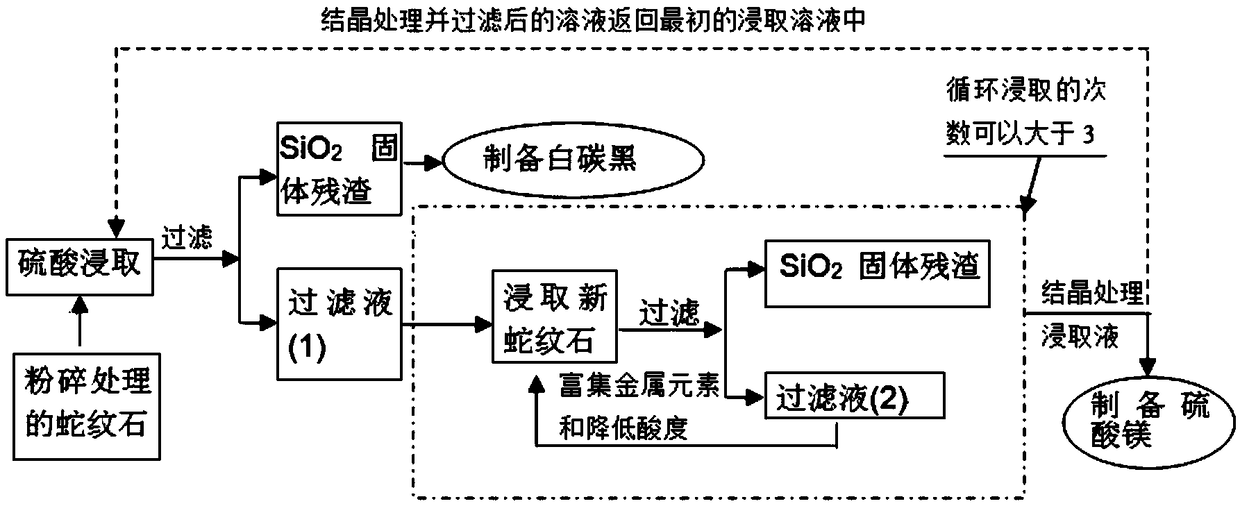
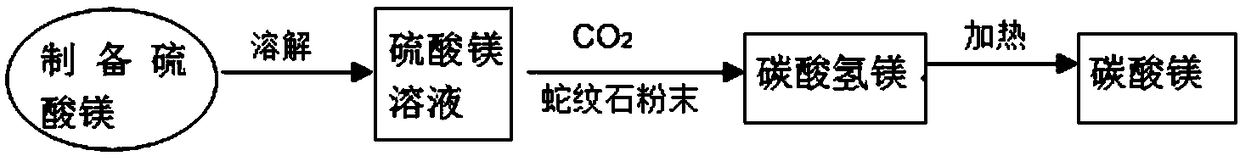
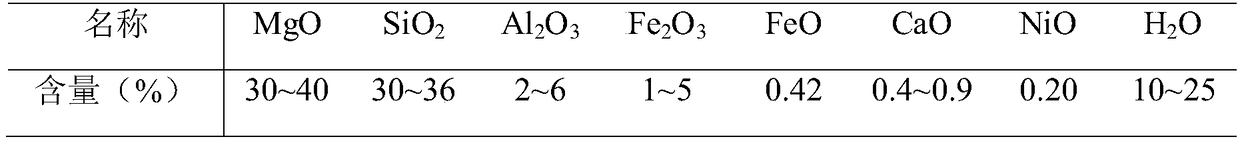
ActiveCN109399675AImprove resource processing capabilitiesReduce acidityMagnesium carbonatesChemical industryCarbonate
The invention discloses a method for mineralization storage of CO2 through magnesium resources in serpentine, and belongs to the fields of mines, metallurgy, chemical industry and environments. The serpentine is subjected to Mg leaching, and the CO2 is subjected to mineralization storage through the serpentine and leached Mg. The method comprises the steps: the pretreated serpentine and sulfuric acid are subjected to crossflow leaching, and a filtered leaching solution is crystallized to obtain magnesium sulfate heptahydrate; the solution after crystal filtering returns into a first-time serpentine leaching reaction system; then magnesium sulfate heptahydrate crystals are dissolved in water, serpentine powder is added, the CO2 gas is introduced, and a magnesium bicarbonate solution is prepared; then the magnesium bicarbonate solution is heated to obtain magnesium carbonate; and finally, mineralization storage of the magnesium to the greenhouse gas and enrichment of metal nickel and iron are achieved.
Owner:SHANGHAI UNIV
Simple nanometer magnesium oxide preparation method
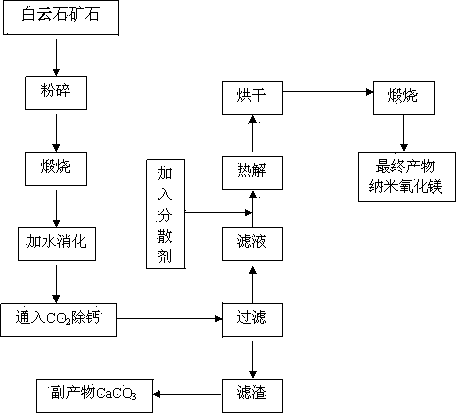
The present invention relates to a simple nanometer magnesium oxide preparation method, which comprises: crushing dolomite, calcining at a temperature of 950-1200 DEG C, digesting the product at a temperature of 50-90 DEG C after calcining, introducing CO2 to the digested product at a room temperature to carry out a carbonization reaction, stopping CO2 introduction when the pH value of the system is 7-9, carrying out solid-liquid separation on the reaction product to obtain a magnesium bicarbonate solution (Mg(HCO3)2 solution), adding a dispersant to the magnesium bicarbonate solution according to a certain ratio, completely and uniformly stirring for 20-60 min, carrying out pyrolysis for 30-90 min at a temperature of 80-100 DEG C until the solid basic magnesium carbonate is precipitated, and calcining the solid basic magnesium carbonate for 30-60 min at a temperature of 450-750 DEG C to obtain the nanometer magnesium oxide with an average particle size of 50 nm. According to the present invention, the used raw materials are abundant, the price is low, the process is simple, characteristics of safety and no pollution are provided, the process is easy to control, the nanometer magnesium oxide prepared by using the method has a characteristic of uniform particle size, and the method is suitable for industrial development of nanometer magnesium oxide production.
Owner:CHANGCHUN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com