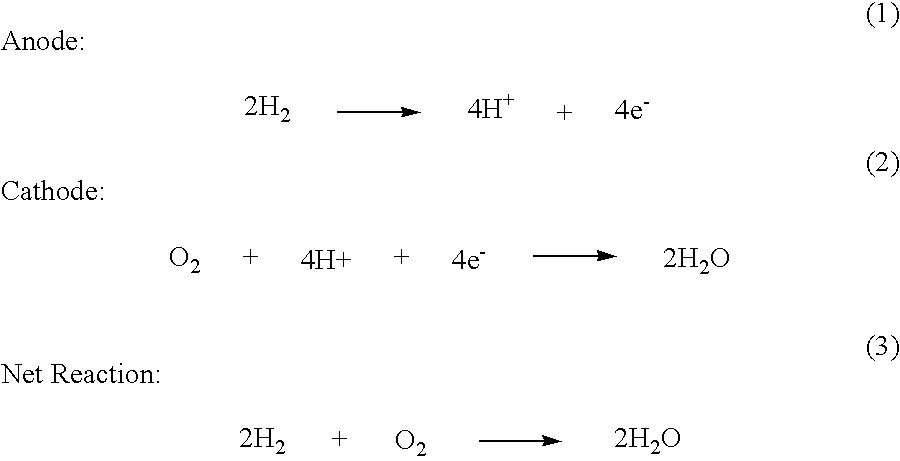Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
150results about "Mercury oxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
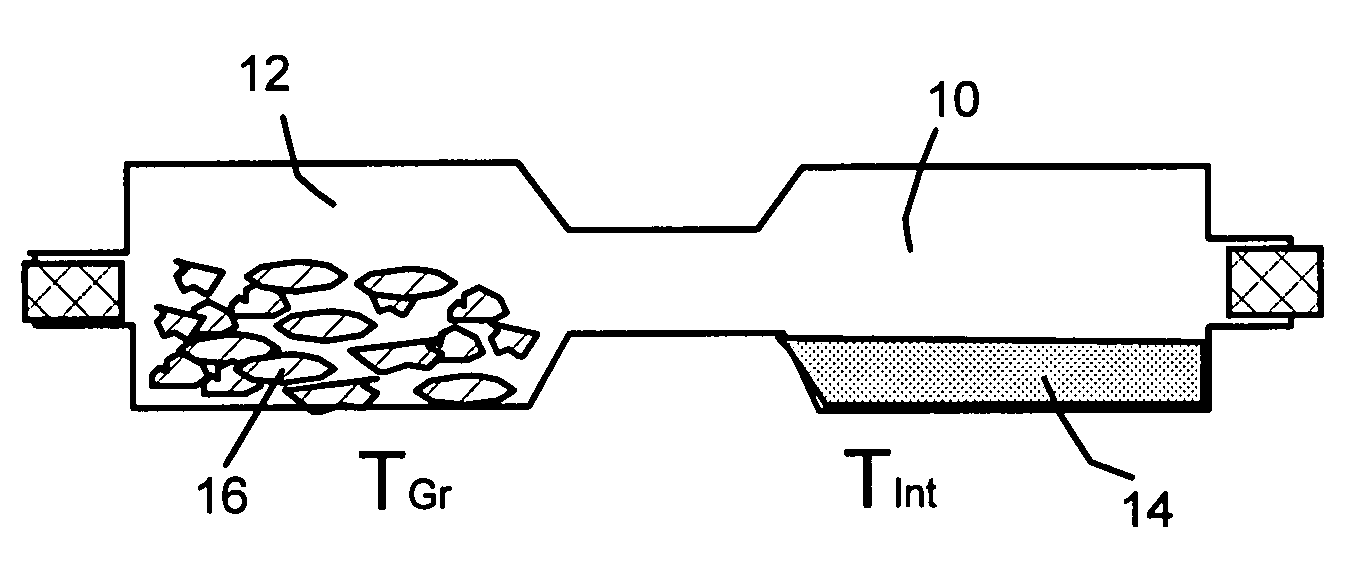
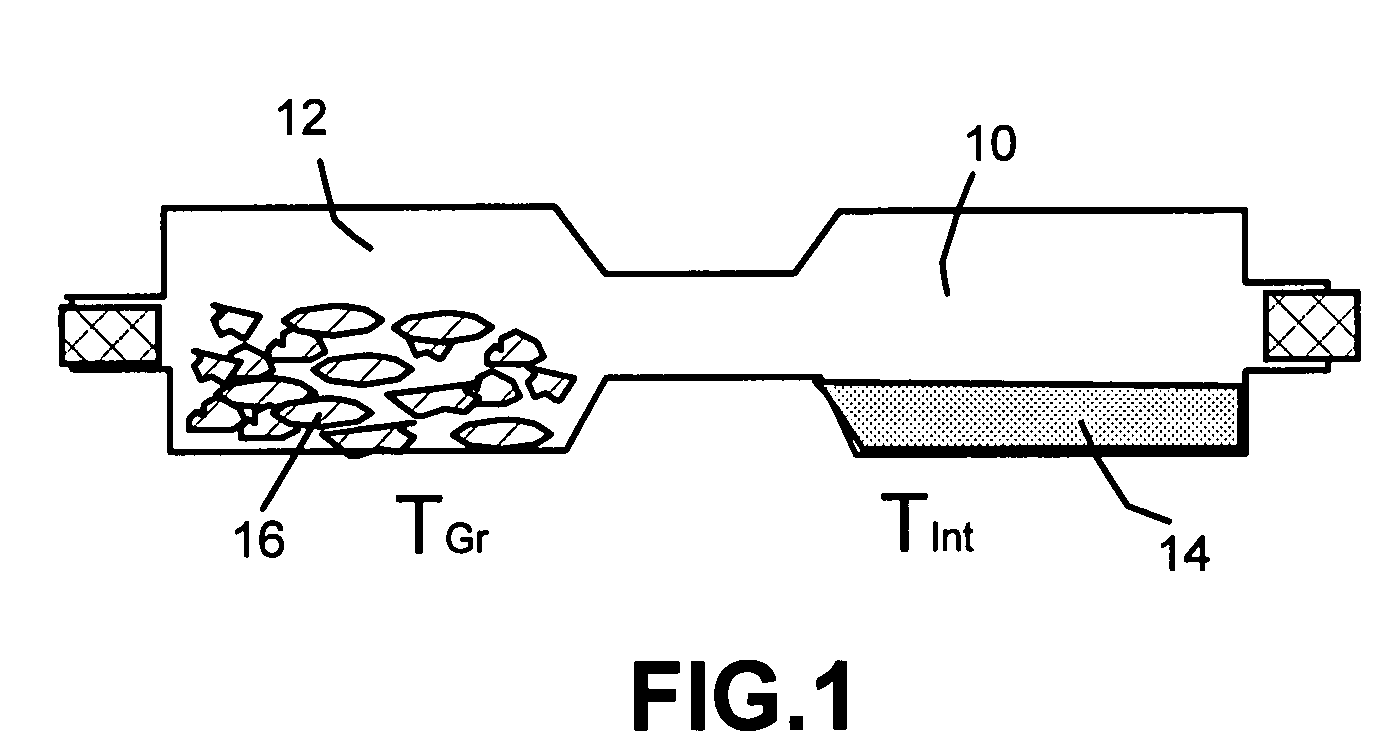
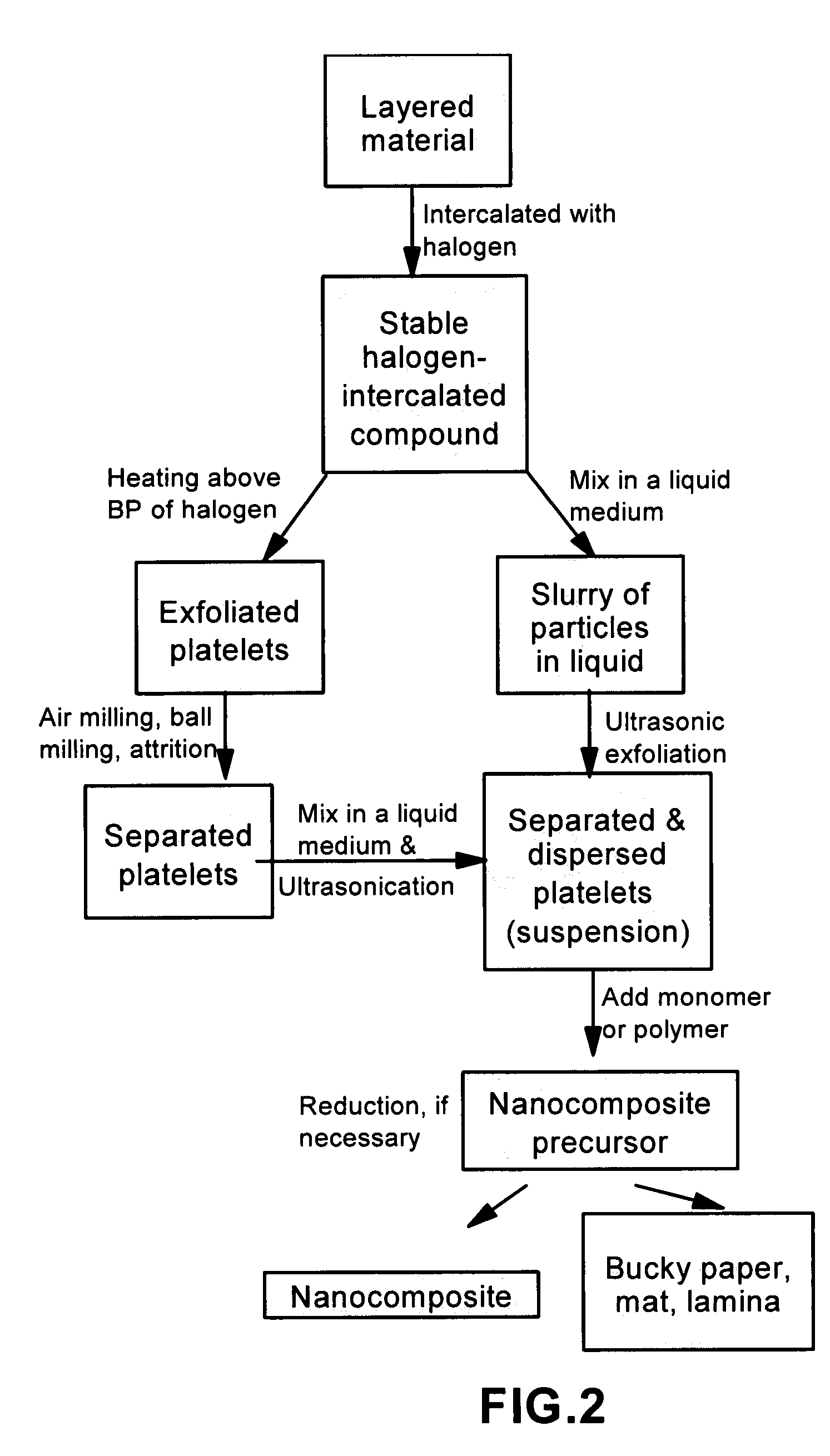
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS20080206124A1Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumPhysical chemistry
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
Doped, pyrogenically prepared oxides
InactiveUS6328944B1Germanium dioxidePipe protection by thermal insulationDoped oxideAqueous solution
Doped, pyrogenically prepared oxides of metals and / or non-metals which are doped with one or more doping components in an amount of 0.00001 to 20 wt. %. The doping component may be a metal and / or non-metal or an oxide and / or a salt of a metal and / or a non-metal. The BET surface area of the doped oxide may be between 5 and 600 m2 / g. The doped pyrogenically prepared oxides of metals and / or non-metals are prepared by adding an aerosol which contains an aqueous solution of a metal and / or non-metal to the gas mixture during the flame hydrolysis of vaporizable compounds of metals and / or non-metals.
Owner:EVONIK DEGUSSA GMBH
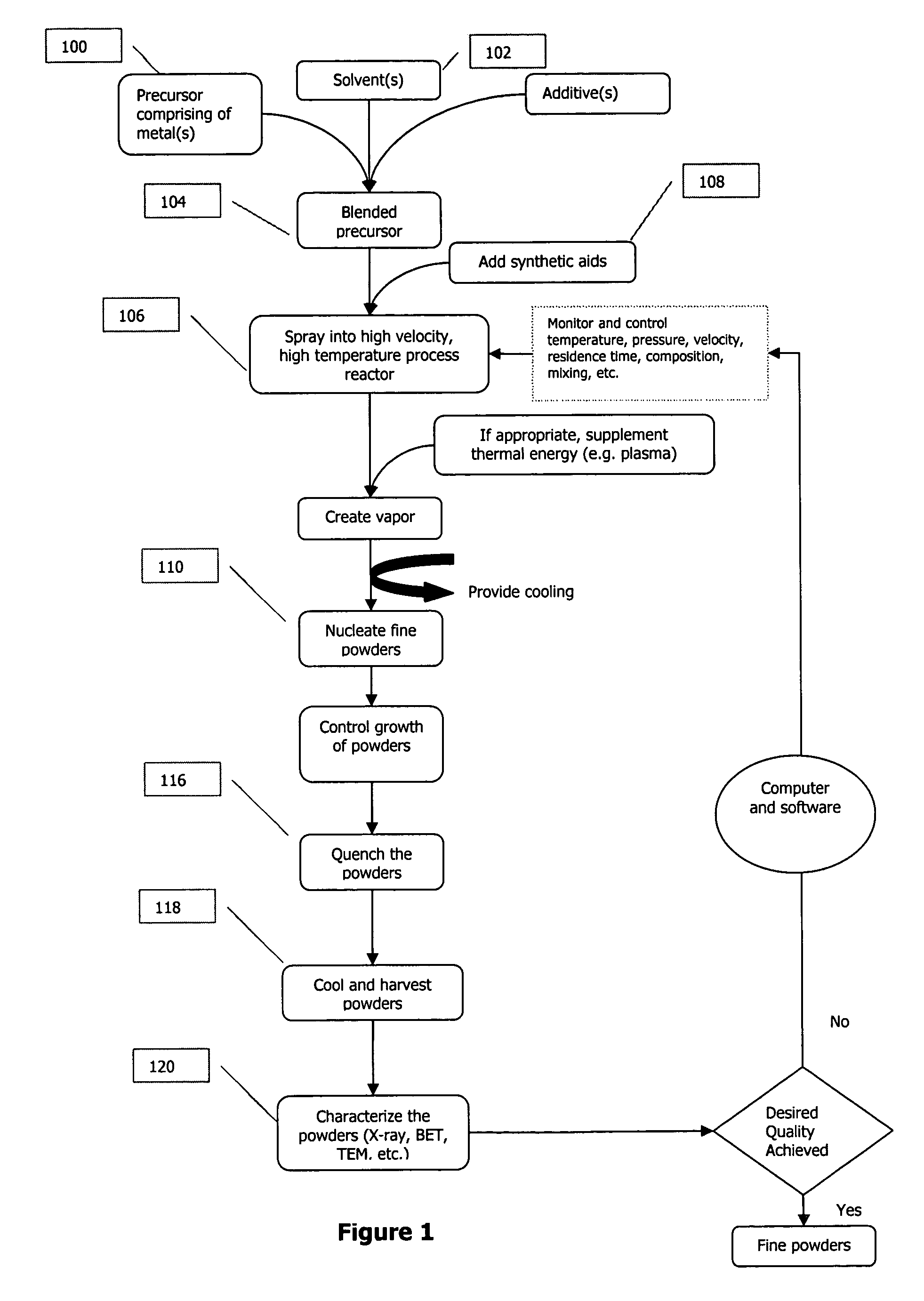
Metal oxide processing methods and systems
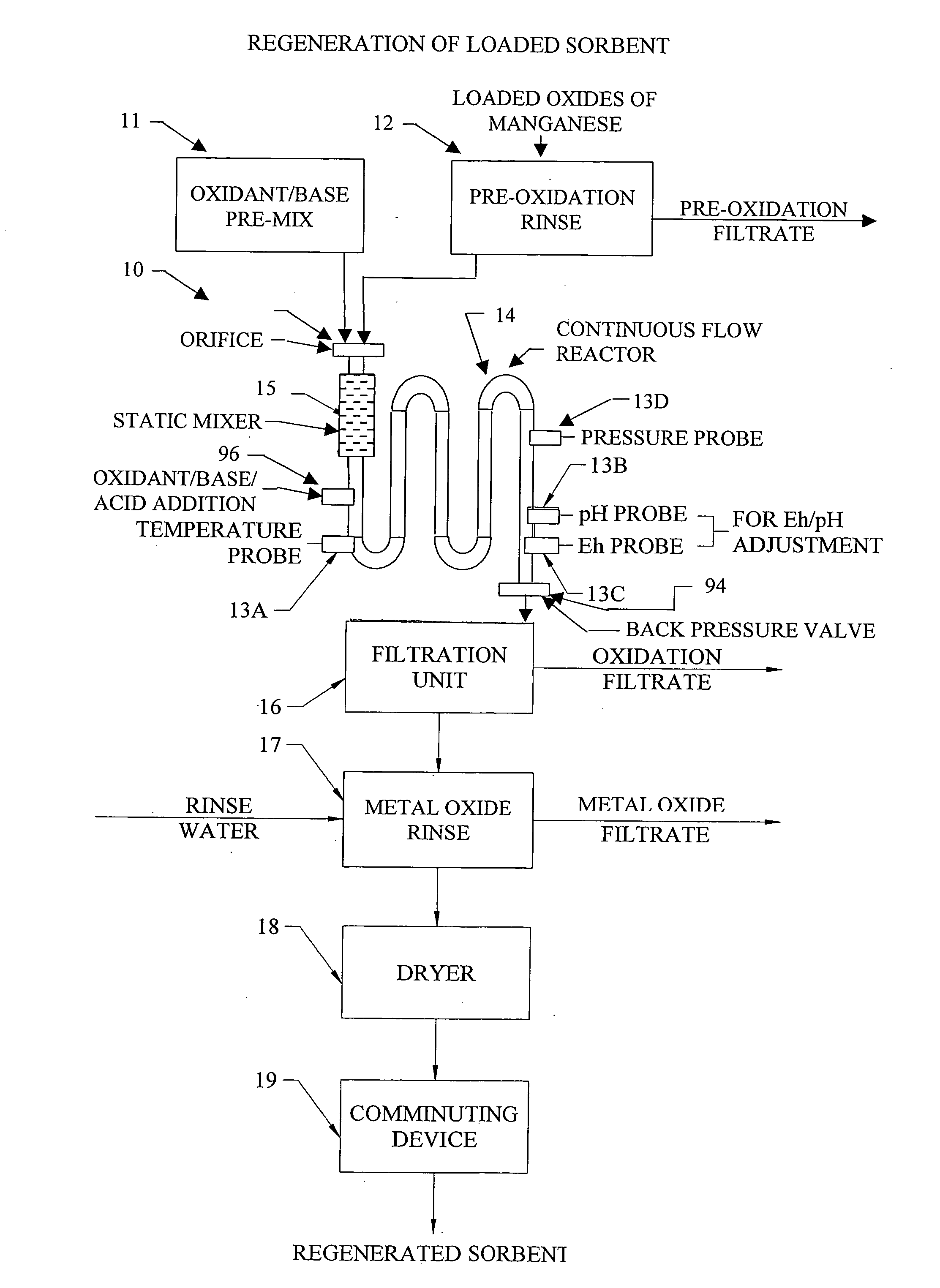
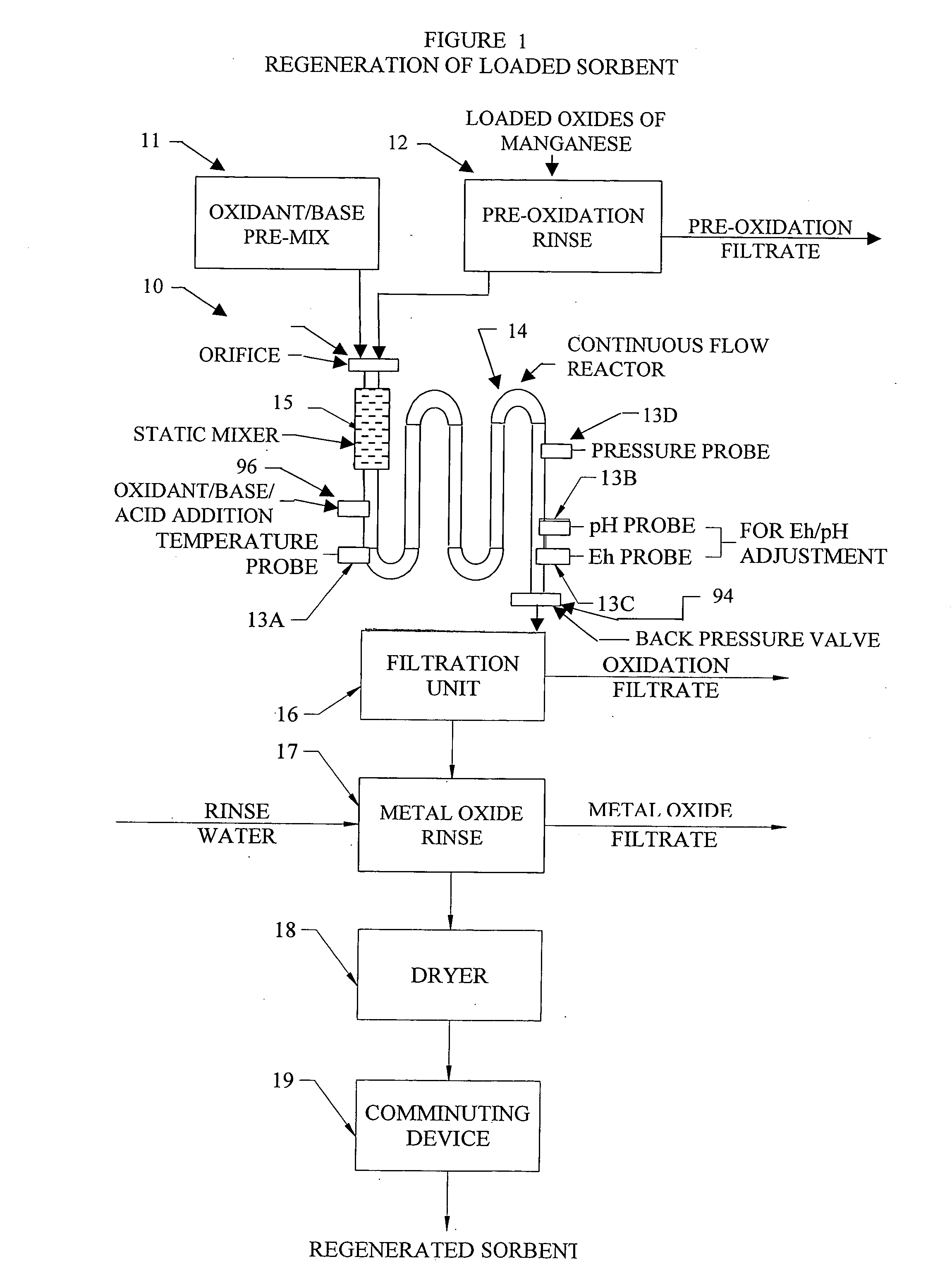
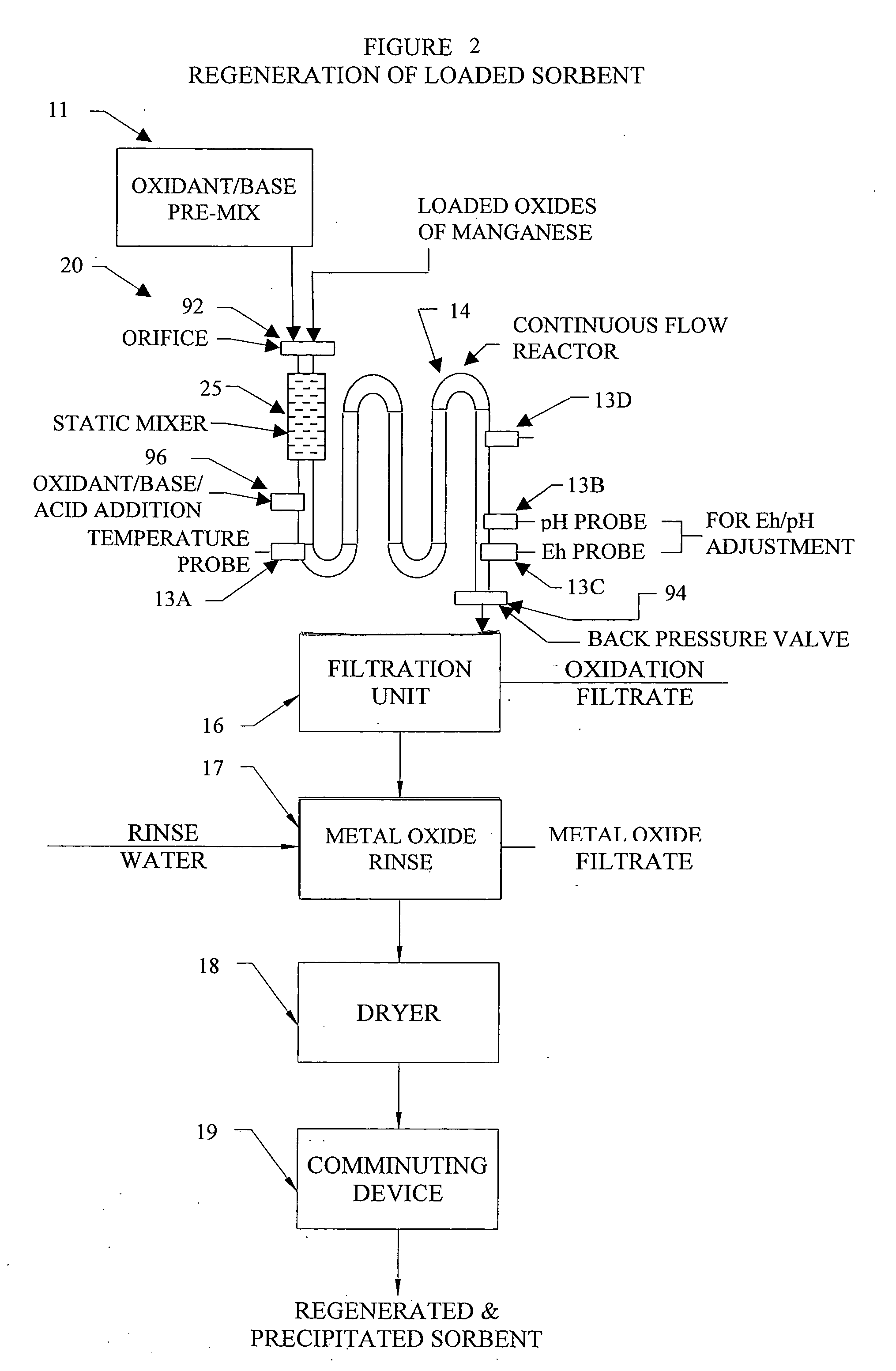
InactiveUS20050074380A1Move quicklyIncrease load capacityCombination devicesTemperatue controlIndustrial gasBatch processing
Methods and systems for processing metal oxides from metal containing solutions. Metal containing solutions are mixed with heated aqueous oxidizing solutions and processed in a continuous process reactor or batch processing system. Combinations of temperature, pressure, molarity, Eh value, and pH value of the mixed solution are monitored and adjusted so as to maintain solution conditions within a desired stability area during processing. This results in metal oxides having high or increased pollutant loading capacities and / or oxidation states. These metal oxides may be processed according to the invention to produce co-precipitated oxides of two or more metals, metal oxides incorporating foreign cations, metal oxides precipitated on active and inactive substrates, or combinations of any or all of these forms. Metal oxides thus produced are, amongst other uses; suitable for use as a sorbent for capturing or removing target pollutants from industrial gas streams or drinking water or aqueous streams or for personal protective respirators.
Owner:ENVIROSCRUB TECH CORP
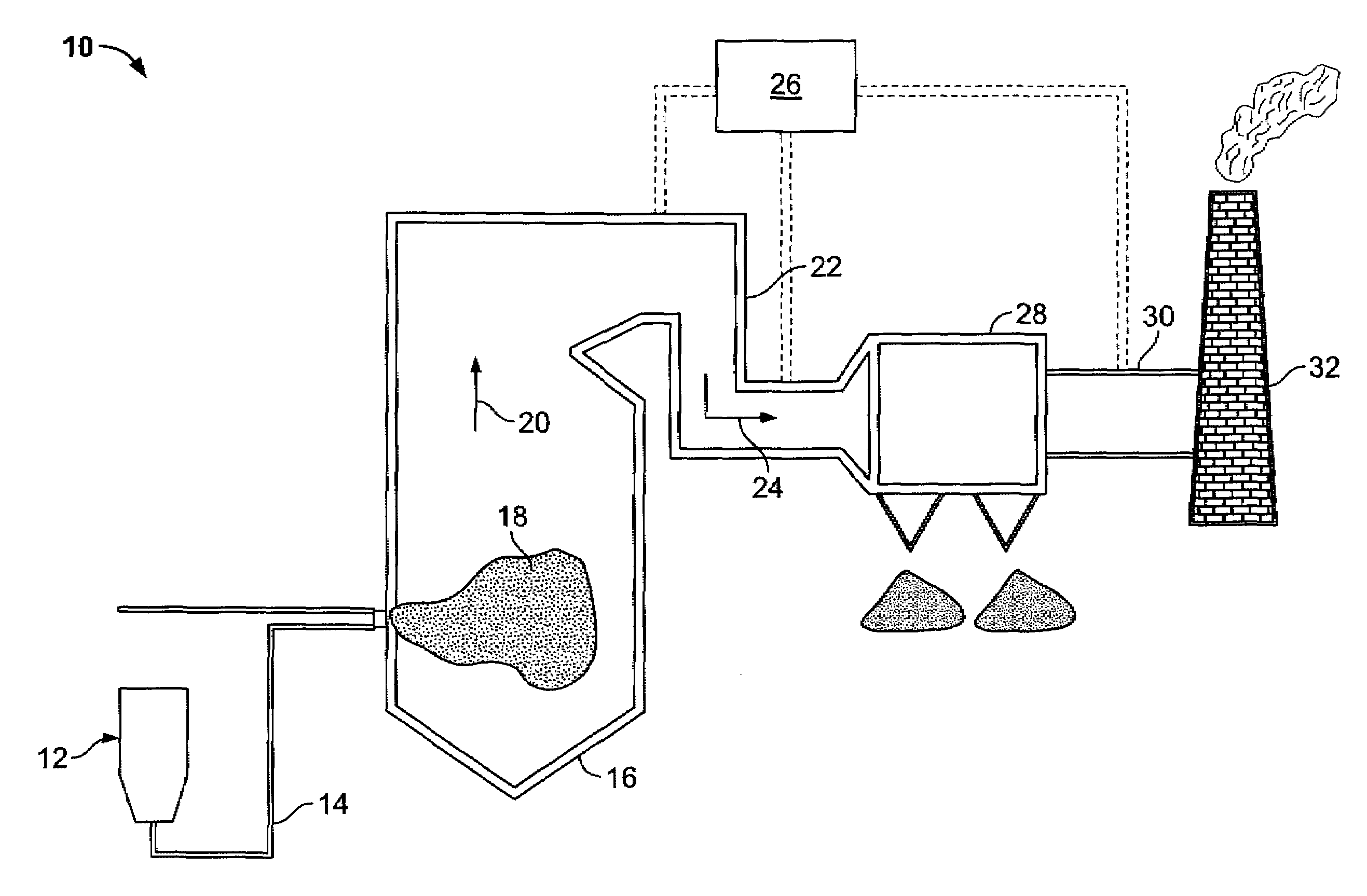
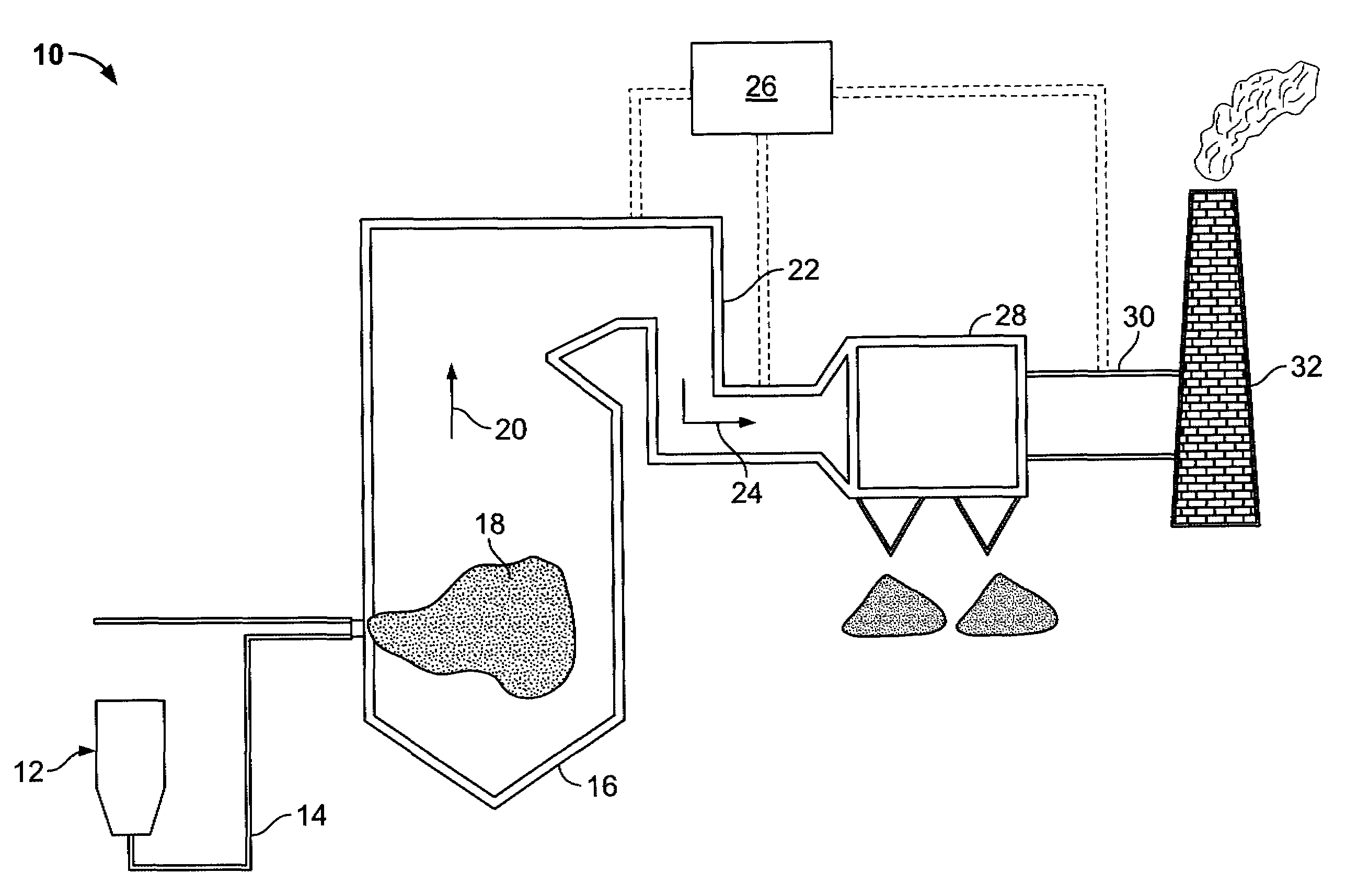
Sorbents and sorbent composition for mercury removal
A system for removing mercury from combustion gas. The system includes a combustion device, a stack, and a duct system that couples the combustion device to the stack. The system further comprises an injection system that is coupled to the duct system. The injection system injects sorbents including alkali-based sorbents and carbon-based sorbents into the duct system.
Owner:GENERAL ELECTRIC CO
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
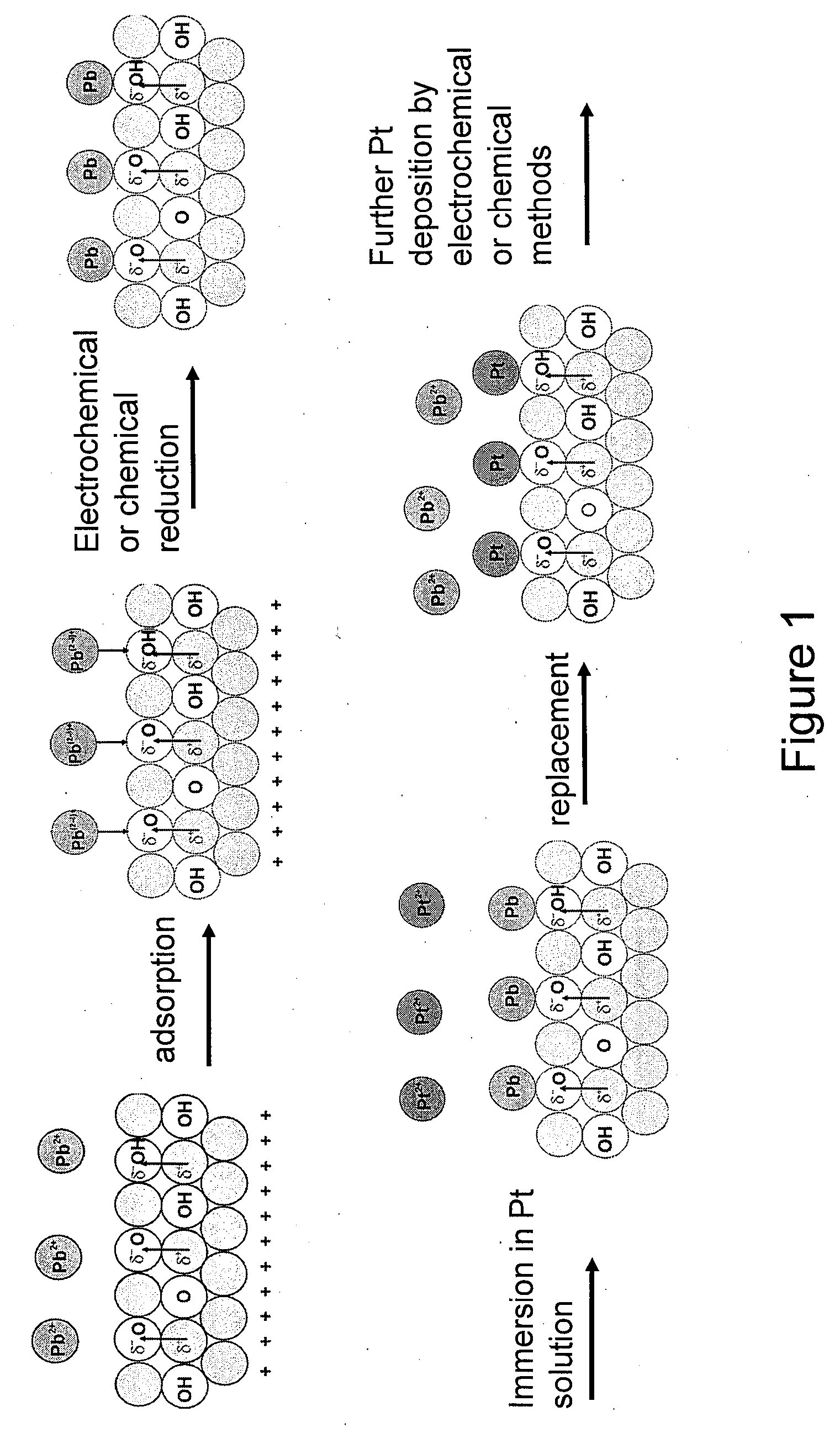
Synthesis of Metal-Metal Oxide Catalysts and Electrocatalysts Using a Metal Cation Adsorption/Reduction and Adatom Replacement by More Noble Ones
InactiveUS20070264189A1Improve stabilityReducing and preventing oxidationCell electrodesGold compoundsHydrogenFuel cells
The invention relates to platinum-metal oxide composite particles and their use as electrocatalysts in oxygen-reducing cathodes and fuel cells. The invention particularly relates to methods for preventing the oxidation of the platinum electrocatalyst in the cathodes of fuel cells by use of these platinum-metal oxide composite particles. The invention additionally relates to methods for producing electrical energy by supplying such a fuel cell with an oxidant, such as oxygen, and a fuel source, such as hydrogen. The invention also relates to methods of making the metal-metal oxide composites.
Owner:BROOKHAVEN SCI ASSOCS
Composition and method for oxidizing mercury in combustion processes
InactiveUS7413719B2Emission reductionExcessive emissionUsing liquid separation agentEmission preventionCombustion chamberCombustor
The invention can be summarized as follows. There is provided a method for oxidizing elemental mercury in a combustion process comprising, adding a composition comprising an aluminum silicate to a combustion chamber, boiler or kiln downstream from the burner region combustion zone. There is further provided a method for reducing the emission of one or more heavy metals in a combustion process by adding a composition comprising an aluminum silicate to a combustion chamber downstream from the burner region combustion zone. There is also provided a composition comprising an aluminum silicate that may be employed to oxidize elemental mercury generated in a combustion process. The composition also may be employed to reduce the emission of one or more heavy metals generated in a combustion process.
Owner:DIGDON WILLIAM TROY
Titanium comprising nanoparticles and related nanotechnology
ActiveUS7232556B2Increase volumeLow cost productionNitrogen compoundsGermanium dioxideNanoparticleTitanium metal
Owner:PPG IND OHIO INC
Titanium comprising nanoparticles and related nanotechnology
ActiveUS20050191492A1Increase volumeLow cost productionNitrogen compoundsGermanium dioxideNanoparticleTitanium metal
Owner:PPG IND OHIO INC
Hydrothermal synthesis of perovskite nanotubes
ActiveUS20050036939A1Reduce the amount requiredThe instrumentation is simpleDigital storageGermanium dioxideStrontium titanateBarium titanate
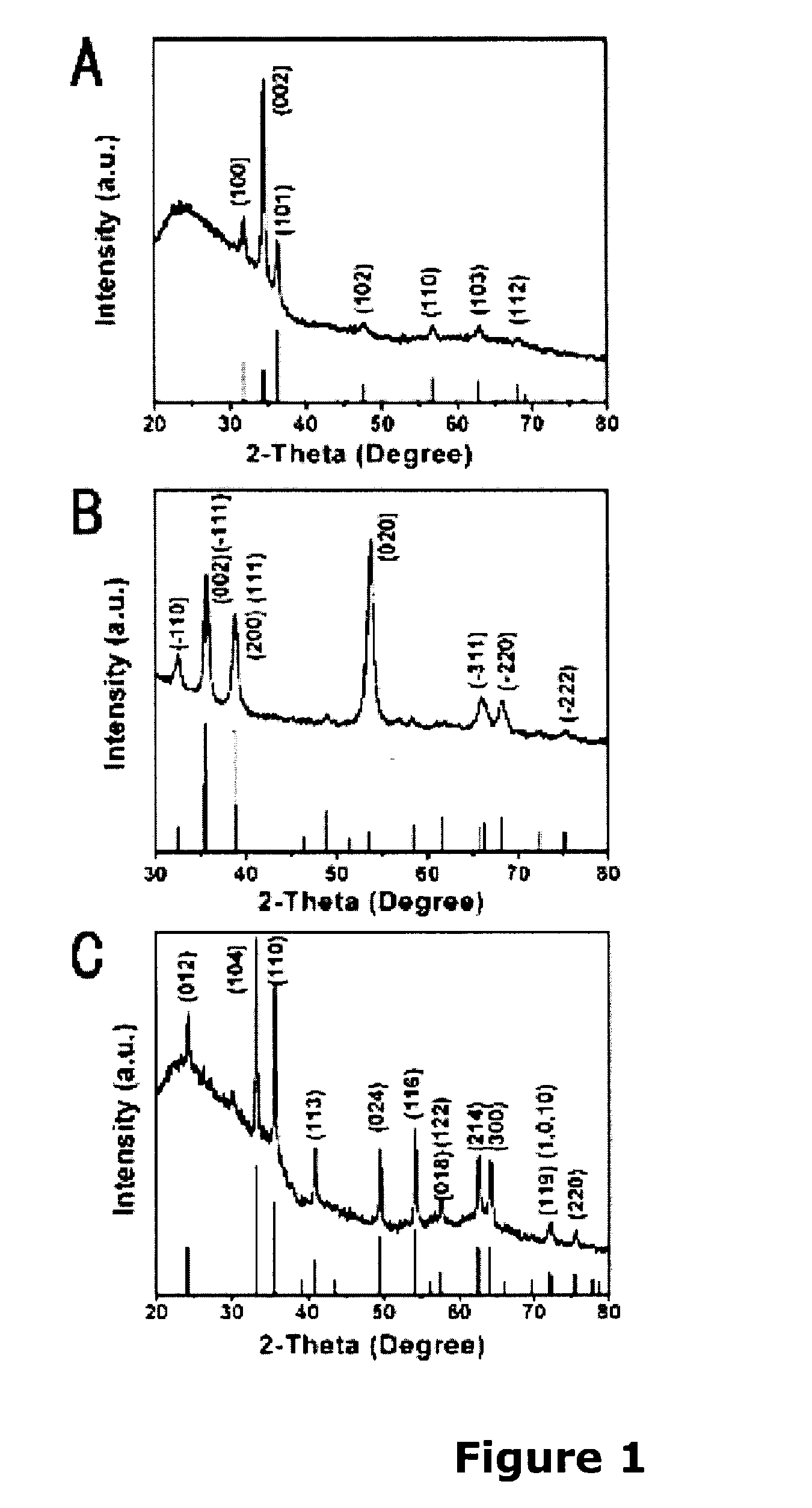
A low-temperature hydrothermal reaction is provided to generate crystalline perovskite nanotubes such as barium titanate (BaTiO3) and strontium titanate (SrTiO3) that have an outer diameter from about 1 nm to about 500 nm and a length from about 10 nm to about 10 micron. The low-temperature hydrothermal reaction includes the use of a metal oxide nanotube structural template, i.e., precursor. These titanate nanotubes have been characterized by means of X-ray diffraction and transmission electron microscopy, coupled with energy dispersive X-ray analysis and selected area electron diffraction (SAED).
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Process for preparing nano-sized metal oxide particles
InactiveUS20050260122A1Efficiently provideNanosized metal oxide particles more efficientlyNanostructure manufactureGold compoundsHigh concentrationAlcohol
The present invention is directed to novel sol-gel methods in which metal oxide precursor and an alcohol-based solution are mixed to form a reaction mixture that is then allowed to react to produce nanosized metal oxide particles. The methods of the present invention are more suitable for preparing nanosized metal oxide than are previously-described sol-gel methods. The present invention can provide for nanosized metal oxide particles more efficiently than the previously-described sol-gel methods by permitting higher concentrations of metal oxide precursor to be employed in the reaction mixture. The foregoing is provided by careful control of the pH conditions during synthesis and by ensuring that the pH is maintained at a value of about 7 or higher.
Owner:KANEKA CORP +1
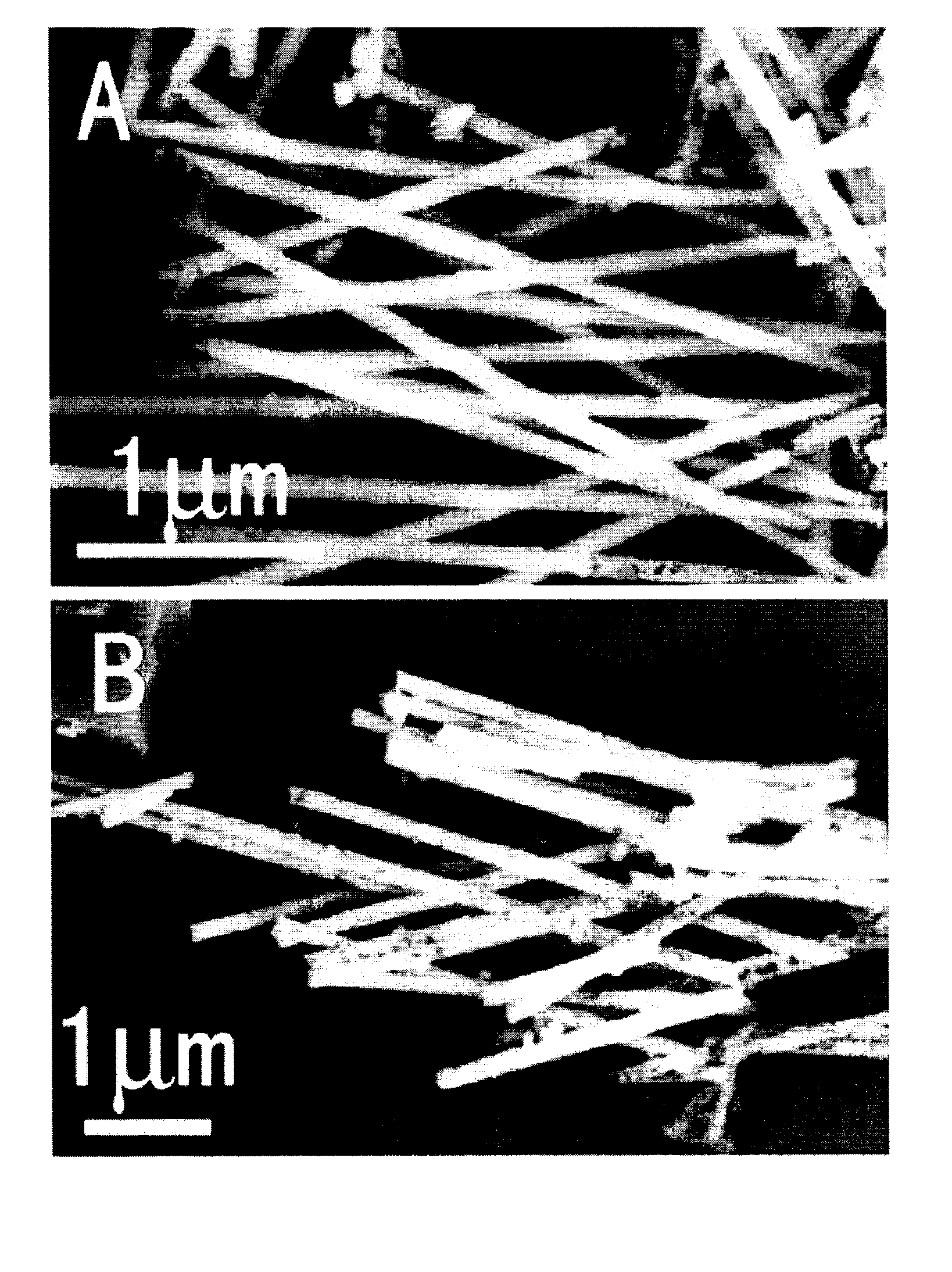
Pure metal and ceramic nanofibers
InactiveUS20140332733A1Few voidFew defectOrganic active ingredientsGold compoundsFiberElectrospinning
Provided herein are nanofibers and processes of preparing nanofibers. In some instances, the nanofibers are metal and / or ceramic nanofibers. In some embodiments, the nanofibers are high quality, high performance nanofibers, highly coherent nanofibers, highly continuous nanofibers, or the like. In some embodiments, the nanofibers have increased coherence, increased length, few voids and / or defects, and / or other advantageous characteristics. In some instances, the nanofibers are produced by electrospinning a fluid stock having a high loading of nanofiber precursor in the fluid stock. In some instances, the fluid stock comprises well mixed and / or uniformly distributed precursor in the fluid stock. In some instances, the fluid stock is converted into a nanofiber comprising few voids, few defects, long or tunable length, and the like.
Owner:CORNELL UNIVERSITY
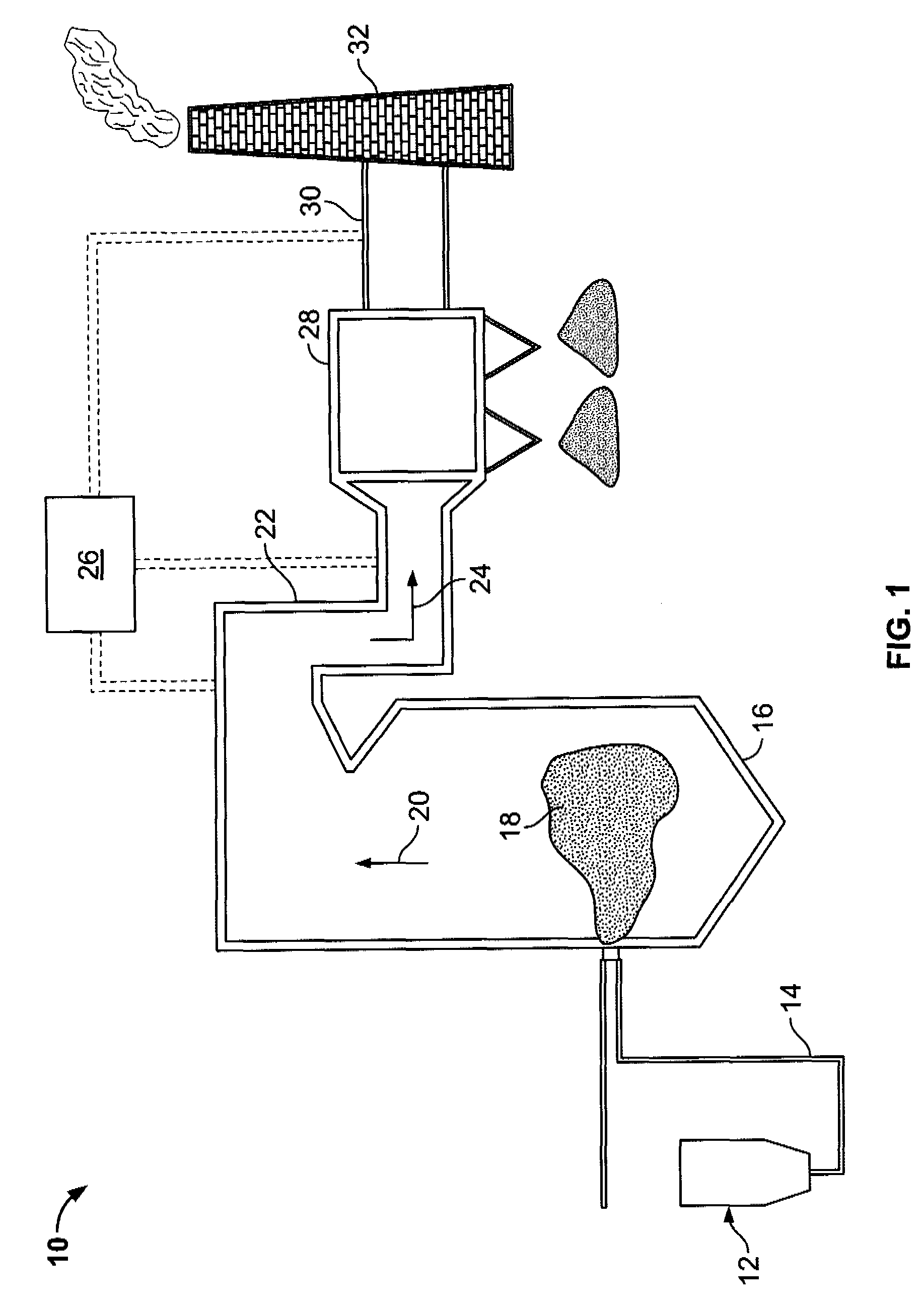
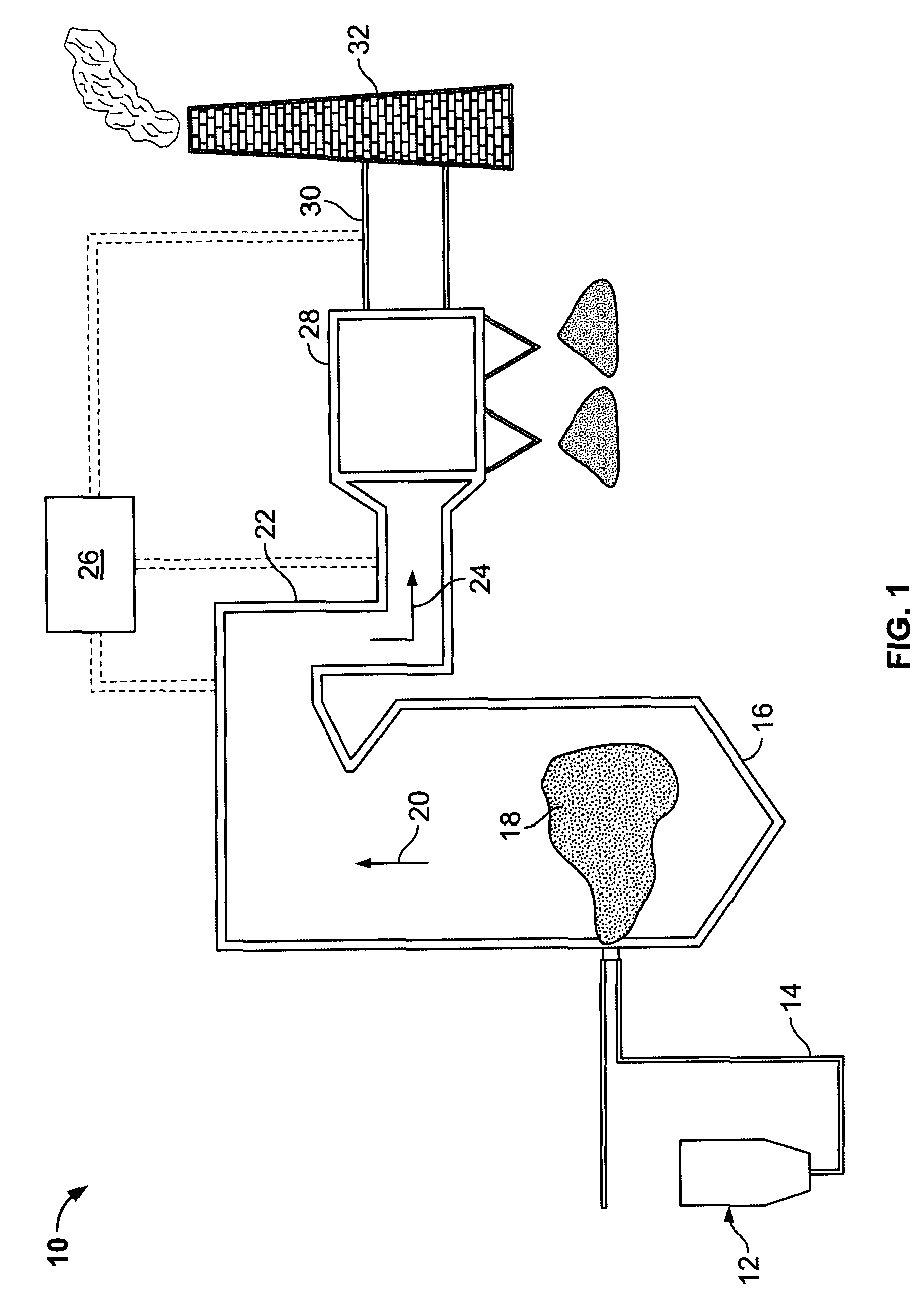
Sorbents and sorbent composition for mercury removal
A system for removing mercury from combustion gas. The system includes a combustion device, a stack, and a duct system that couples the combustion device to the stack. The system further comprises an injection system that is coupled to the duct system. The injection system injects sorbents including alkali-based sorbents and carbon-based sorbents into the duct system.
Owner:GENERAL ELECTRIC CO
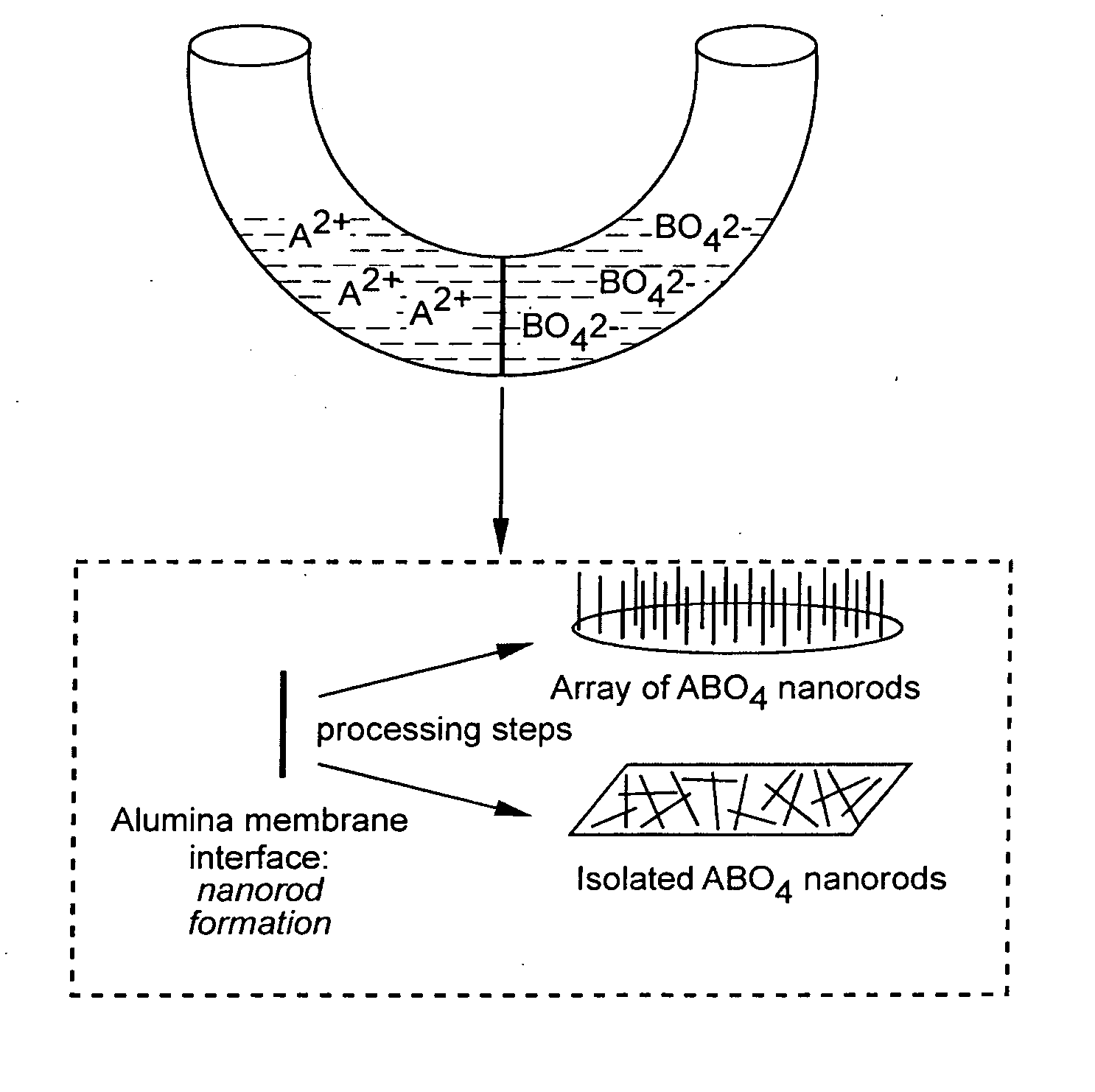
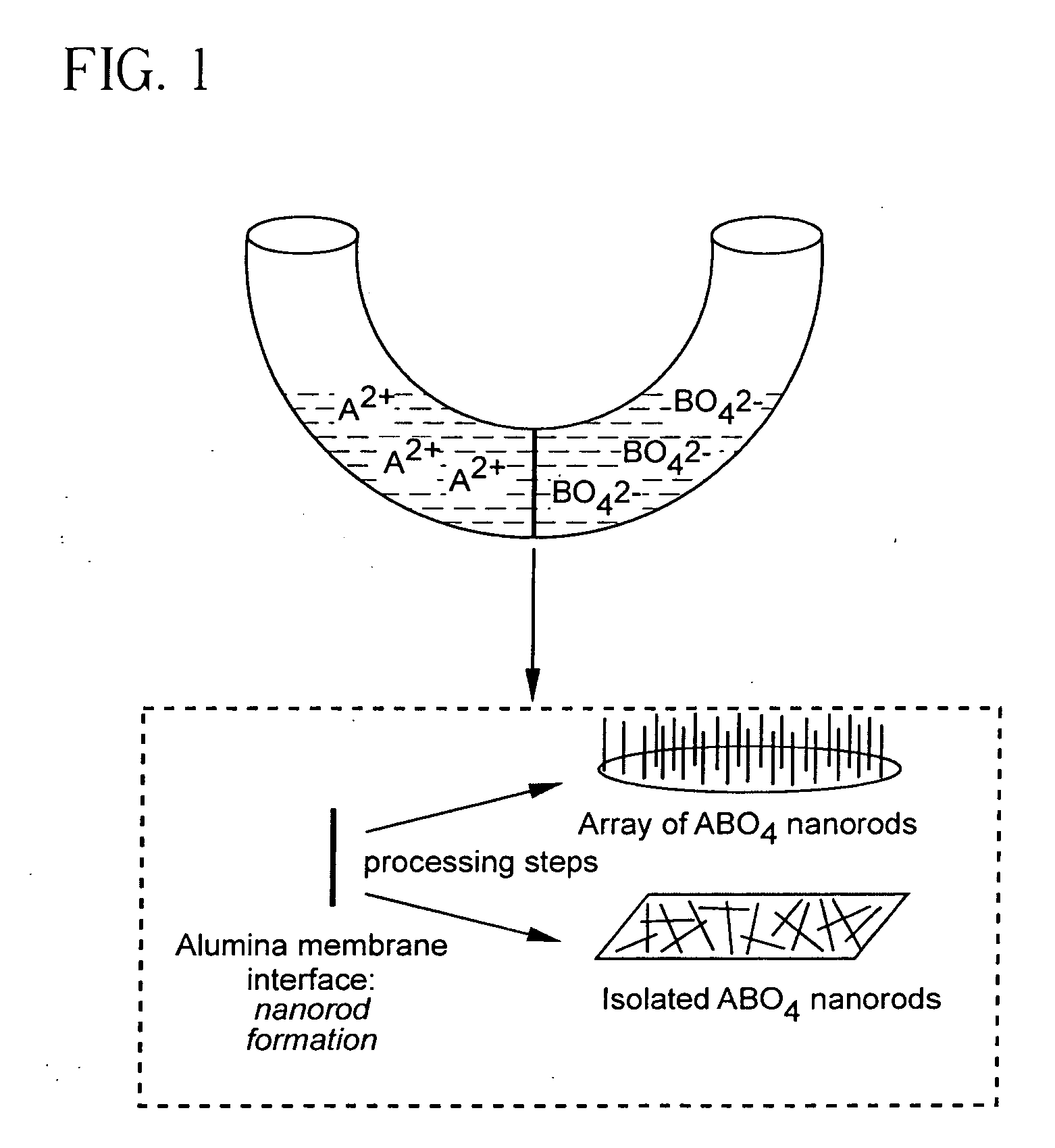
Metal oxide and metal fluoride nanostructures and methods of making same
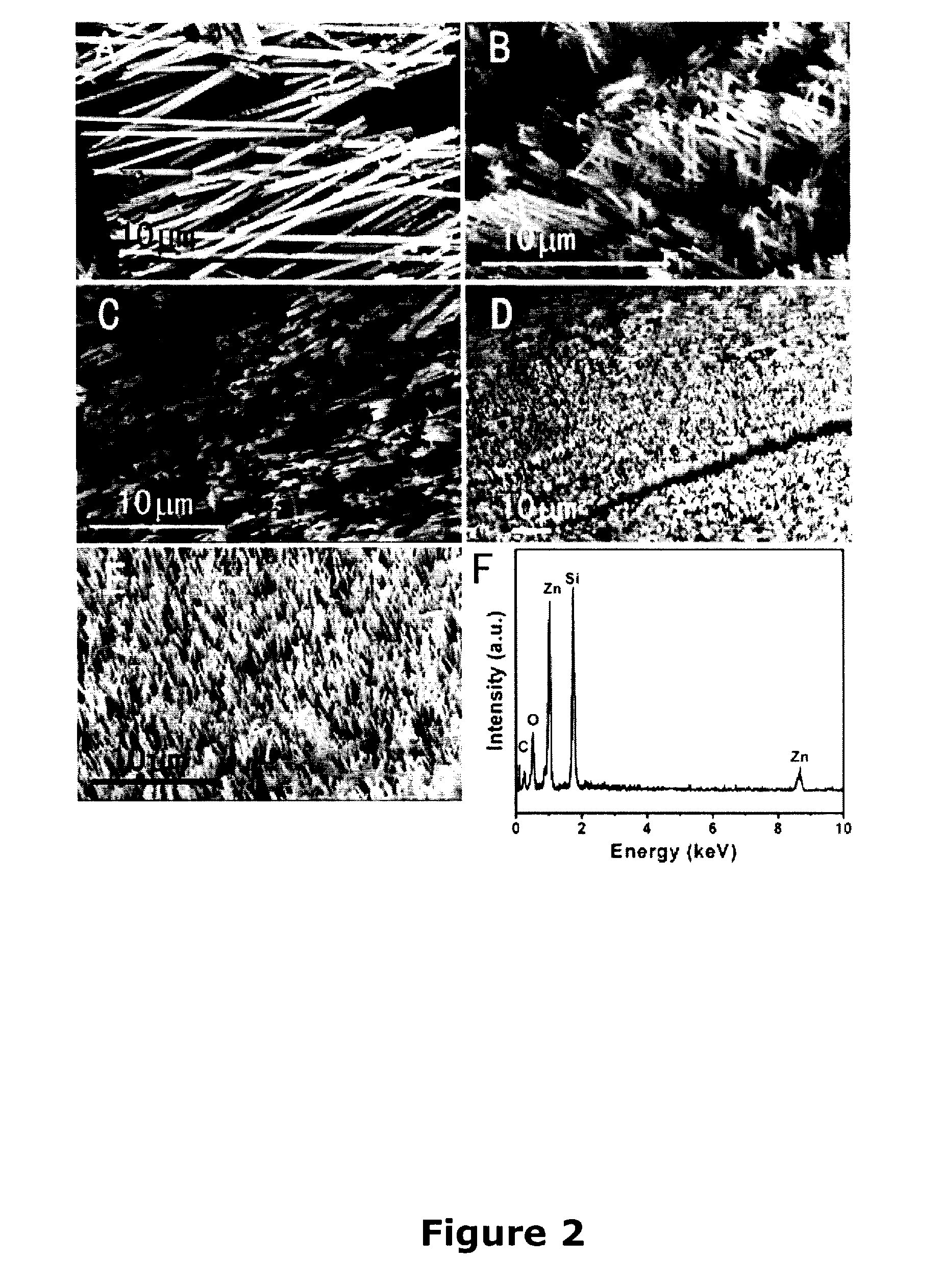
InactiveUS20070113779A1Easy to controlOvercomes shortcomingMercury oxidesAlkali metal oxides/hydroxidesSingle crystalNanostructure
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Hydrothermal synthesis of perovskite nanotubes
ActiveUS7147834B2The instrumentation is simpleReduce the amount requiredNanoinformaticsDigital storageStrontium titanateBarium titanate
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Cathode active material for lithium rechargeable battery, manufacturing method thereof and lithium rechargeable battery
ActiveUS20080118428A1Inhibition of attachmentExcellent cycle characteristicsCell electrodesLithium compoundsHydrogen phosphatePhosphate
A method for manufacturing a cathode active material for a lithium rechargeable battery, including: selecting a first metal compound from a group consisting of a halide, a phosphate, a hydrogen phosphate and a sulfate of Mg or Al; selecting a second metal compound from a group consisting of an oxide, a hydroxide and a carbonate of Mg or Al; combining the first metal compound and the second metal compound to obtain a metal compound, the metal compound containing either Mg or Al atoms; mixing a lithium compound, a transition metal compound and the metal compound to obtain a mixture; and sintering the mixture.
Owner:NIPPON CHECMICAL IND CO LTD
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS20060154146A1Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
It is to provide a positive electrode active material for a lithium secondary battery, which has a large volume capacity density and high safety, is excellent in uniform coating properties and is excellent in the charge and discharge cyclic durability and low temperature characteristics even at a high charge voltage. A process for producing a lithium-containing composite oxide represented by the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than the Q element and the N element, 0.9≦p≦1.1, 0<q≦0.03, 0.97≦x<1.00, 0≦y<0.03, 1.9≦z≦2.1, q+x+y=1 and 0≦a≦0.02) from a lithium source, an Q element source and an N element source, and if necessary, at least one source selected from the group consisting of an M element source and a fluorine source, characterized by using as the Q element source an Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
NOx, Hg, and SO2 removal using ammonia
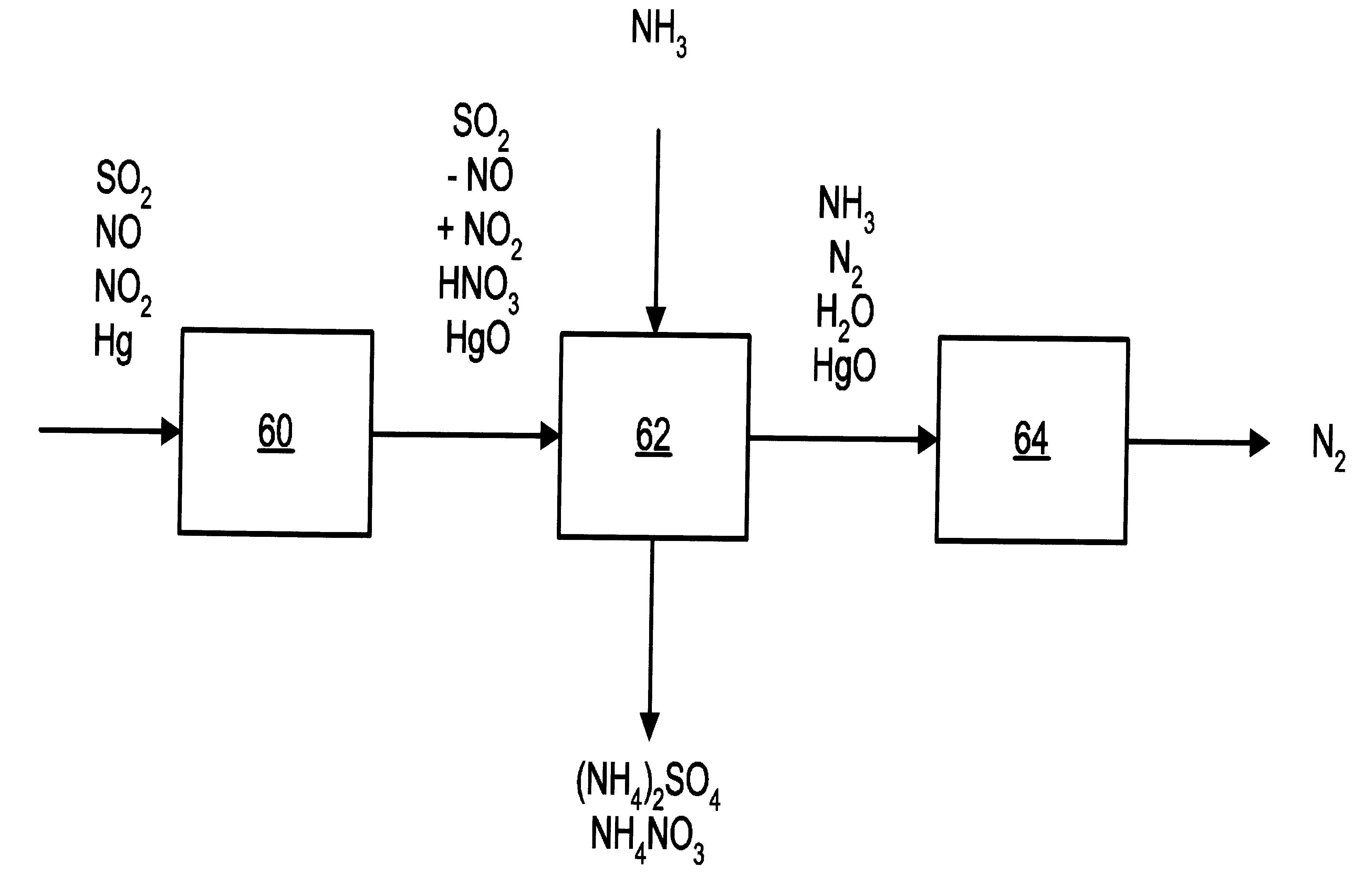
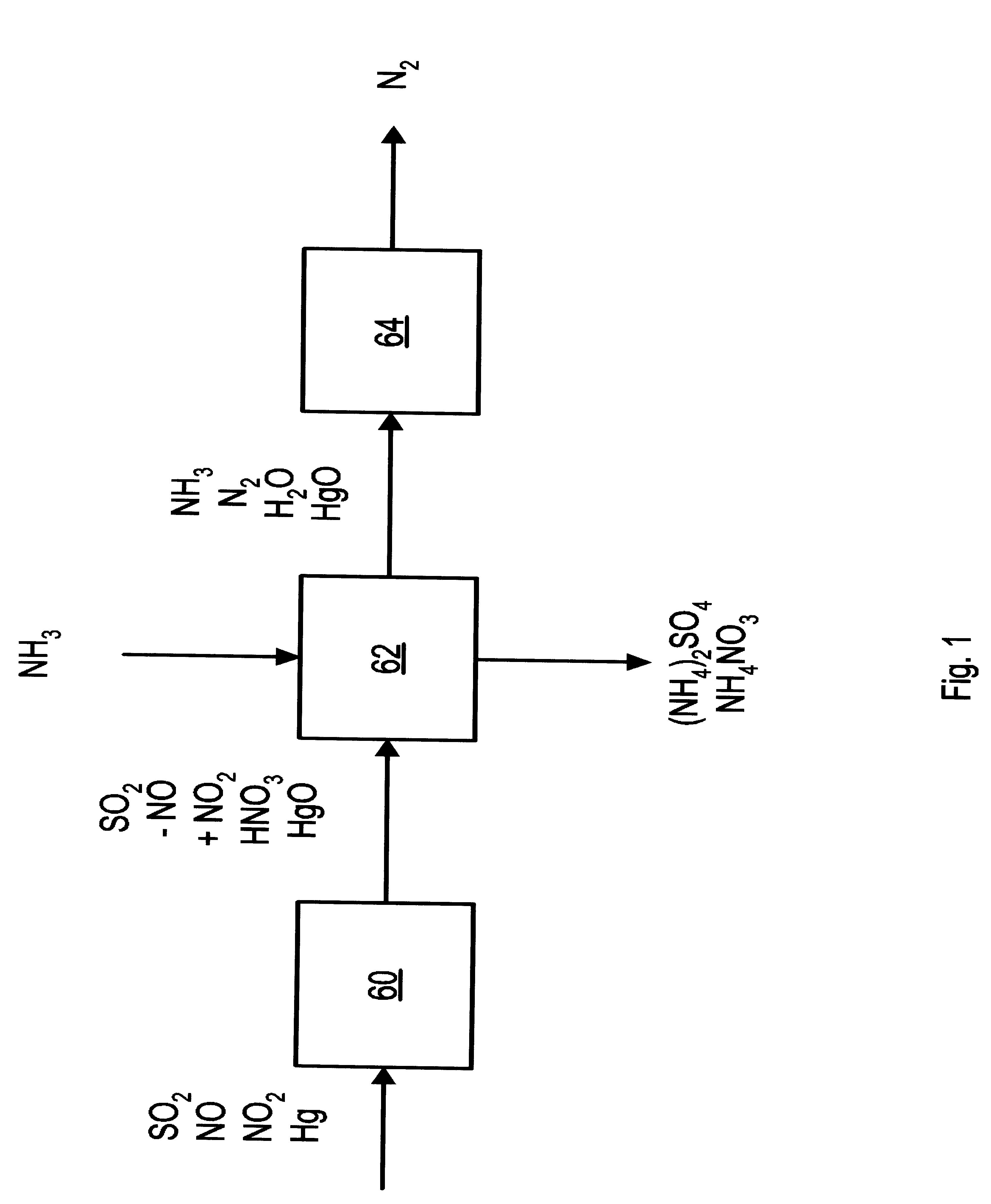
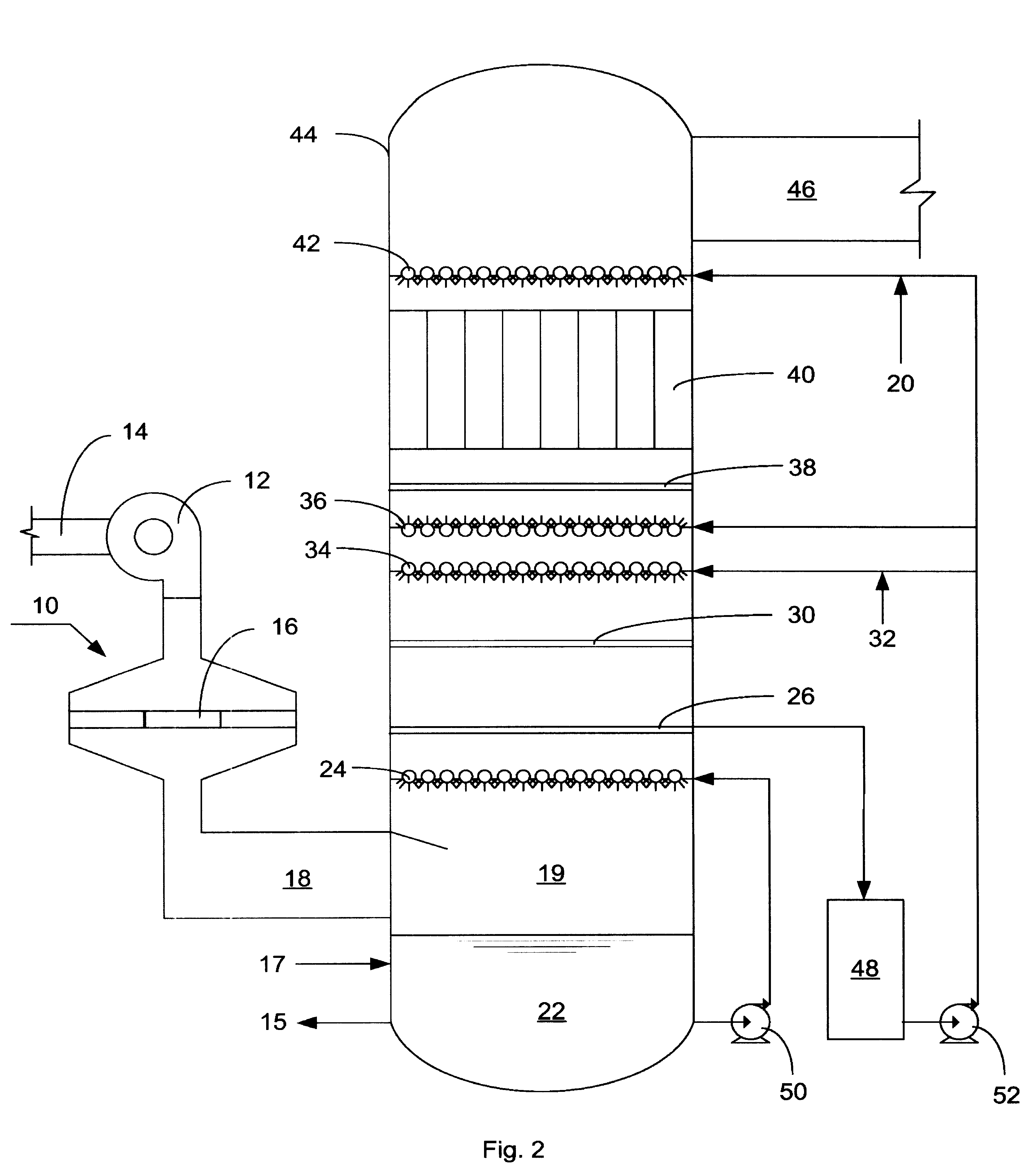
InactiveUS6991771B2Raise the ratioSmall sizeInternal combustion piston enginesExhaust apparatusFlue gasAmmonia
A process and apparatus for removing SO2, NO, and NO2 from a gas stream having the steps of oxidizing a portion of the NO in the flue gas stream to NO2, scrubbing the SO2, NO, and NO2 with an ammonia scrubbing solution, and removing any ammonia aerosols generated by the scrubbing in a wet electrostatic precipitator. The process can also remove Hg by oxidizing it to HgO and removing it in the wet electrostatic precipitator. Ammonium sulfate, a valuable fertilizer, can be withdrawn from the scrubbing solution.
Owner:POWERSPAN CORP
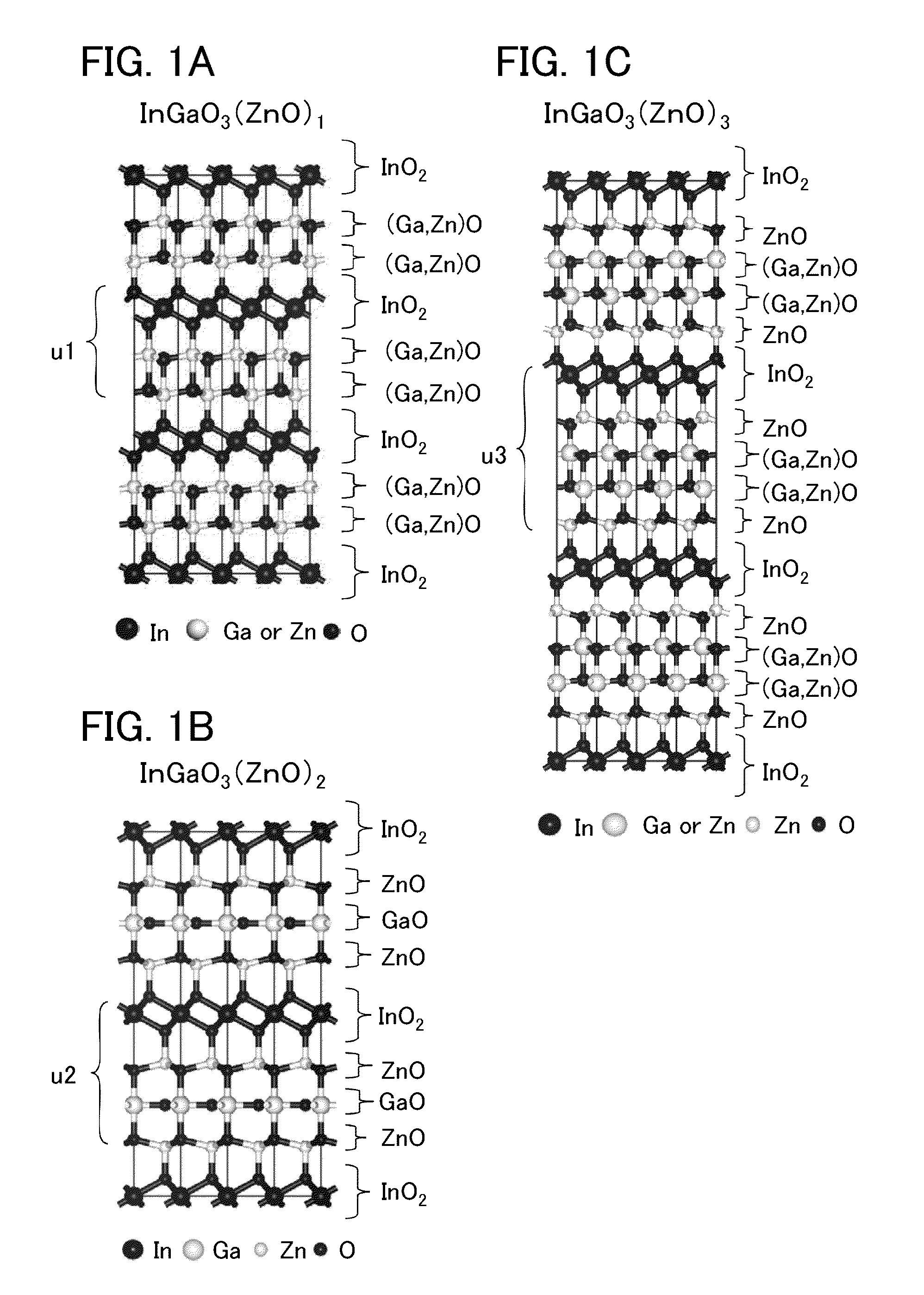
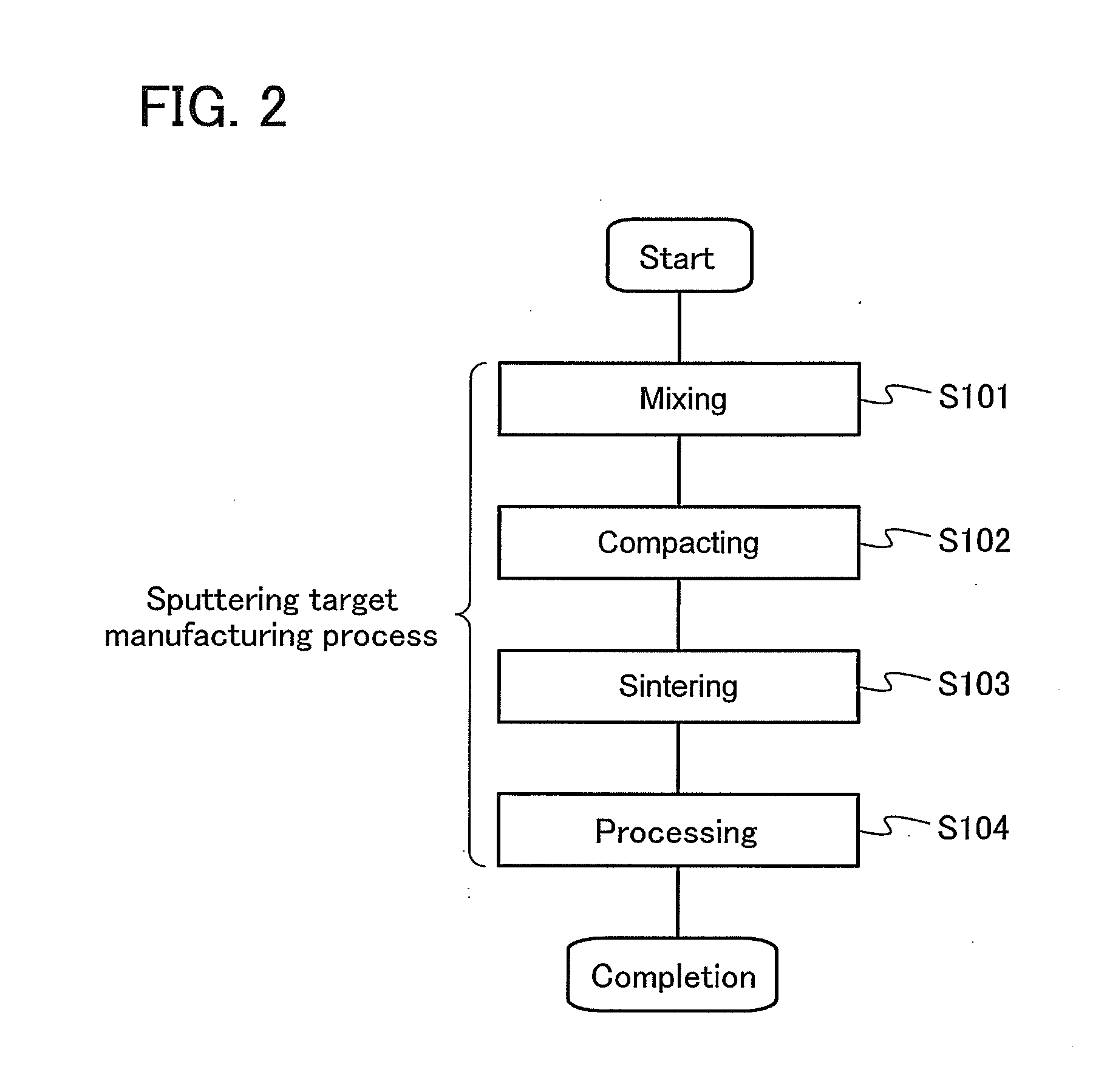
Method for manufacturing sputtering target, method for forming oxide film, and transistor
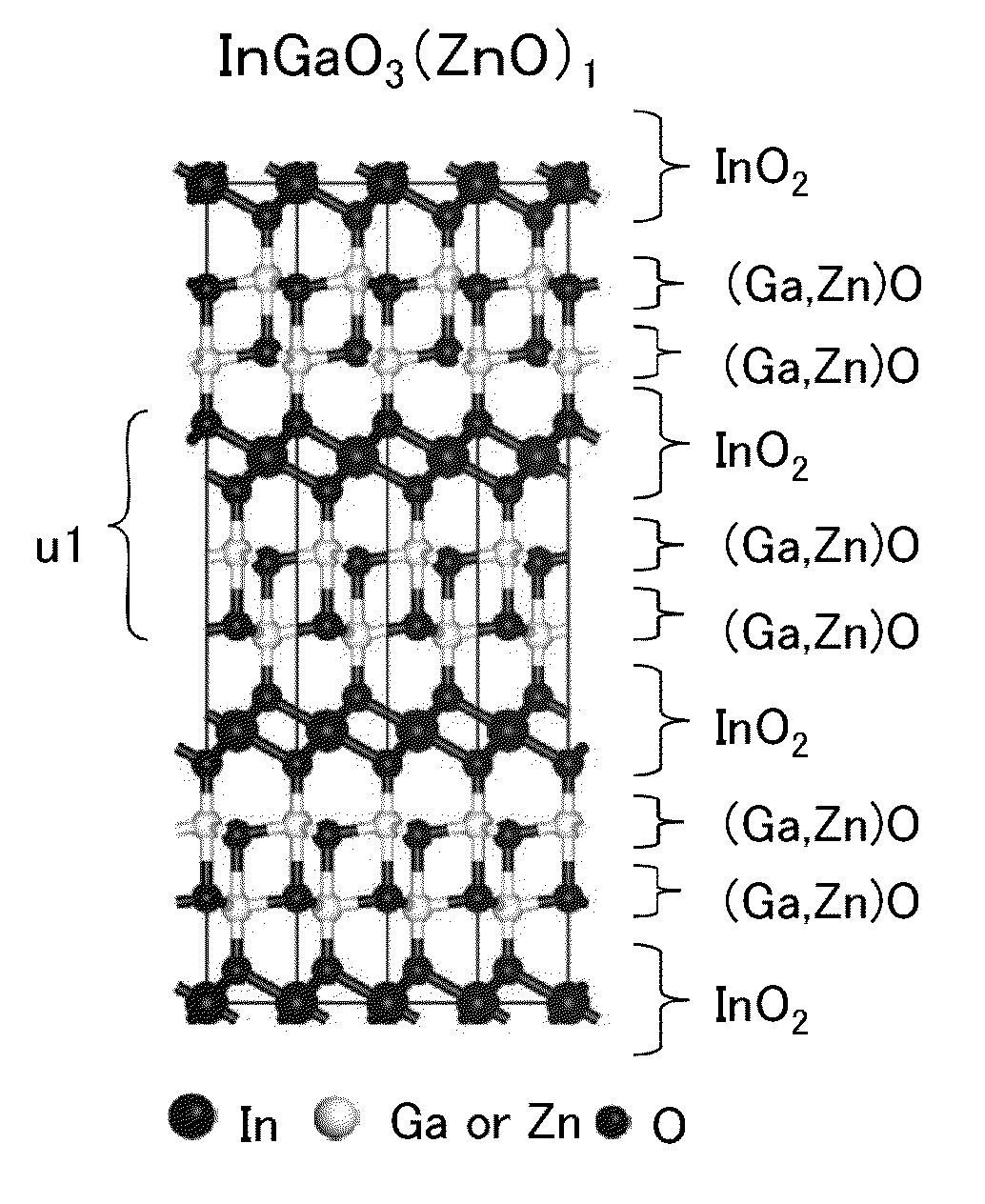
ActiveUS20140241978A1Improve featuresImprove reliabilityMaterial nanotechnologyFrom solid stateIndiumHafnium
A method for manufacturing a sputtering target with which an oxide semiconductor film with a small amount of defects can be formed is provided. Alternatively, an oxide semiconductor film with a small amount of defects is formed. A method for manufacturing a sputtering target is provided, which includes the steps of: forming a polycrystalline In-M-Zn oxide (M represents a metal chosen among aluminum, titanium, gallium, yttrium, zirconium, lanthanum, cesium, neodymium, and hafnium) powder by mixing, sintering, and grinding indium oxide, an oxide of the metal, and zinc oxide; forming a mixture by mixing the polycrystalline In-M-Zn oxide powder and a zinc oxide powder; forming a compact by compacting the mixture; and sintering the compact.
Owner:SEMICON ENERGY LAB CO LTD
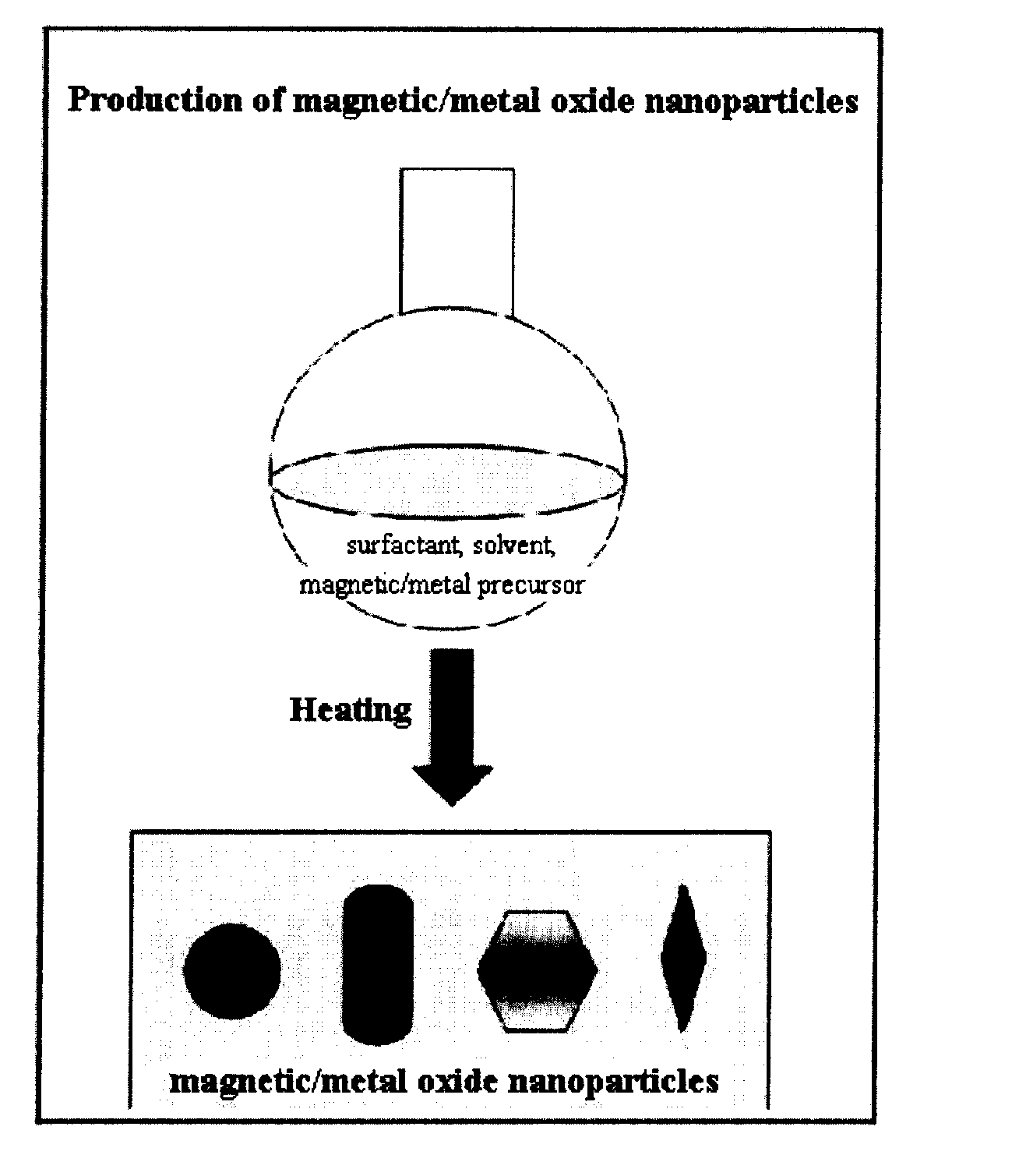
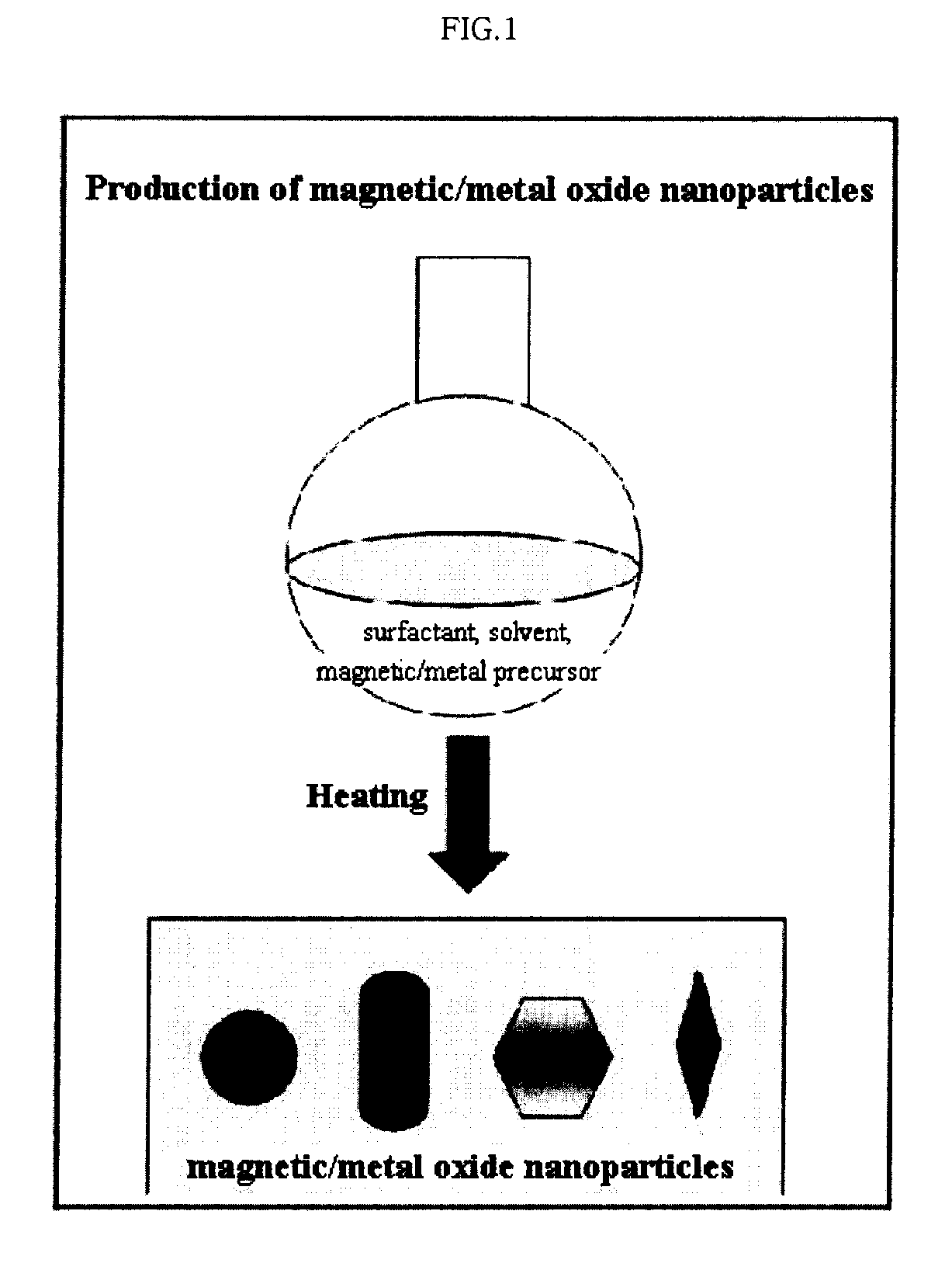
Preparation Method of Magnetic and Metal Oxide Nanoparticles
ActiveUS20080003159A1Efficient mass productionUniform shapeCopper oxides/halidesManganese oxides/hydroxidesMetal oxide nanoparticlesMagnetic oxide
This invention relates, in general, to a method of producing magnetic oxide nanoparticles or metal oxide nanoparticles and, more particularly, to a method of producing magnetic or metal oxide nanoparticles, which comprises (1) adding a magnetic or metal precursor to a surfactant or a solvent containing the surfactant to produce a mixed solution, (2) heating the mixed solution to 50-6001 C to decompose the magnetic or metal precursor by heating so as to form the magnetic or metal oxide nanoparticles, and (3) separating the magnetic or metal oxide nanoparticles. Since the method is achieved through a simple process without using an oxidizing agent or a reducing agent, it is possible to simply mass-produce uniform magnetic or metal oxide nanoparticles having desired sizes compared to the conventional method.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
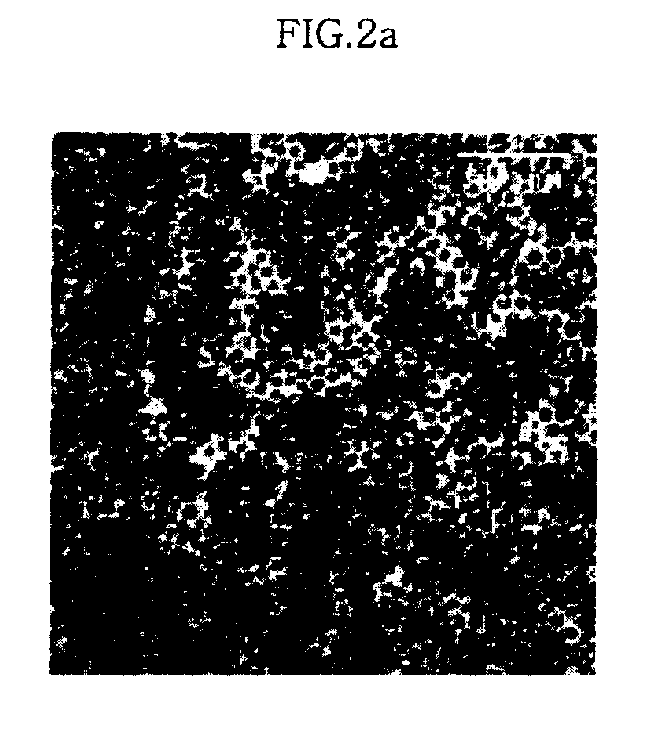
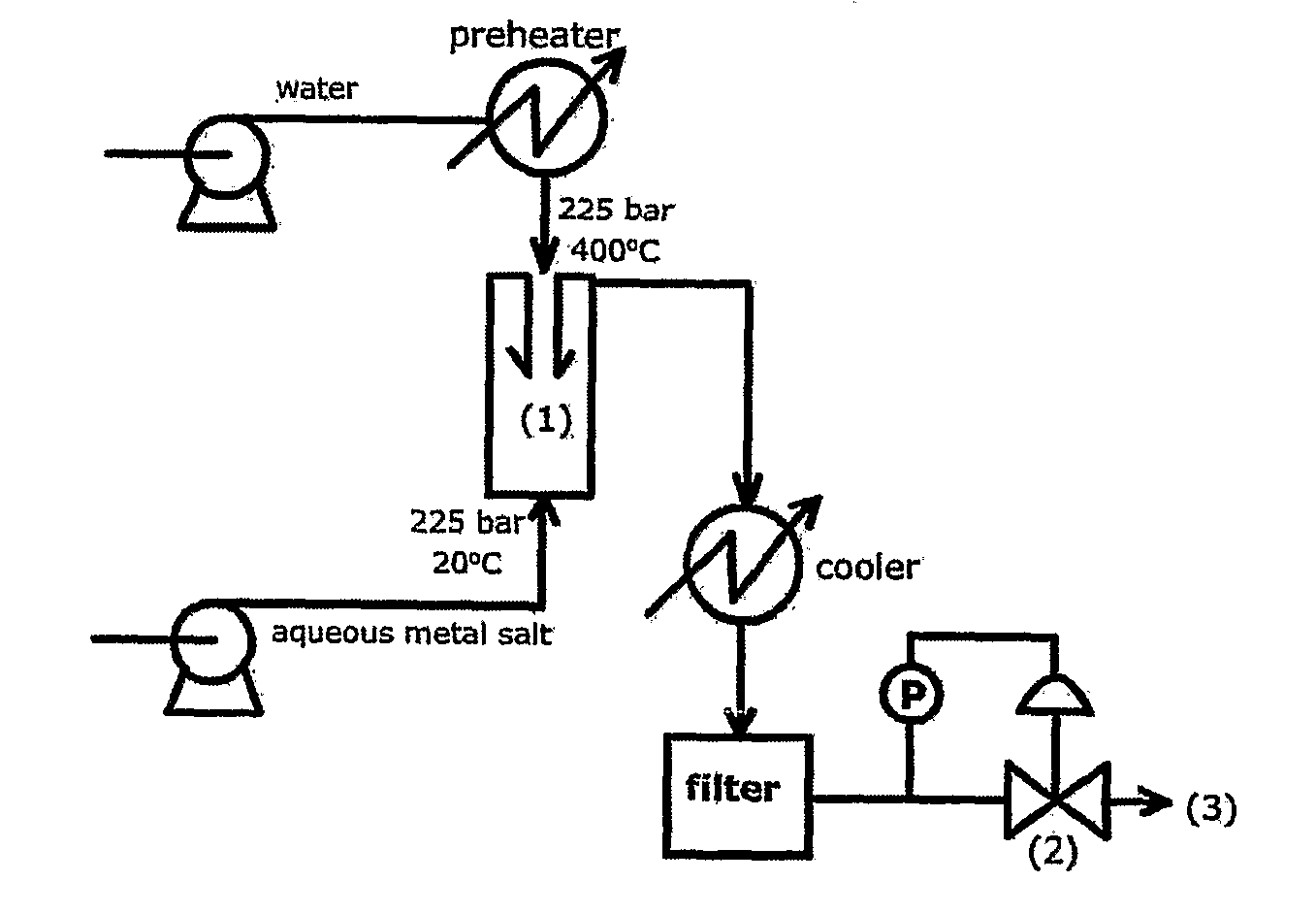
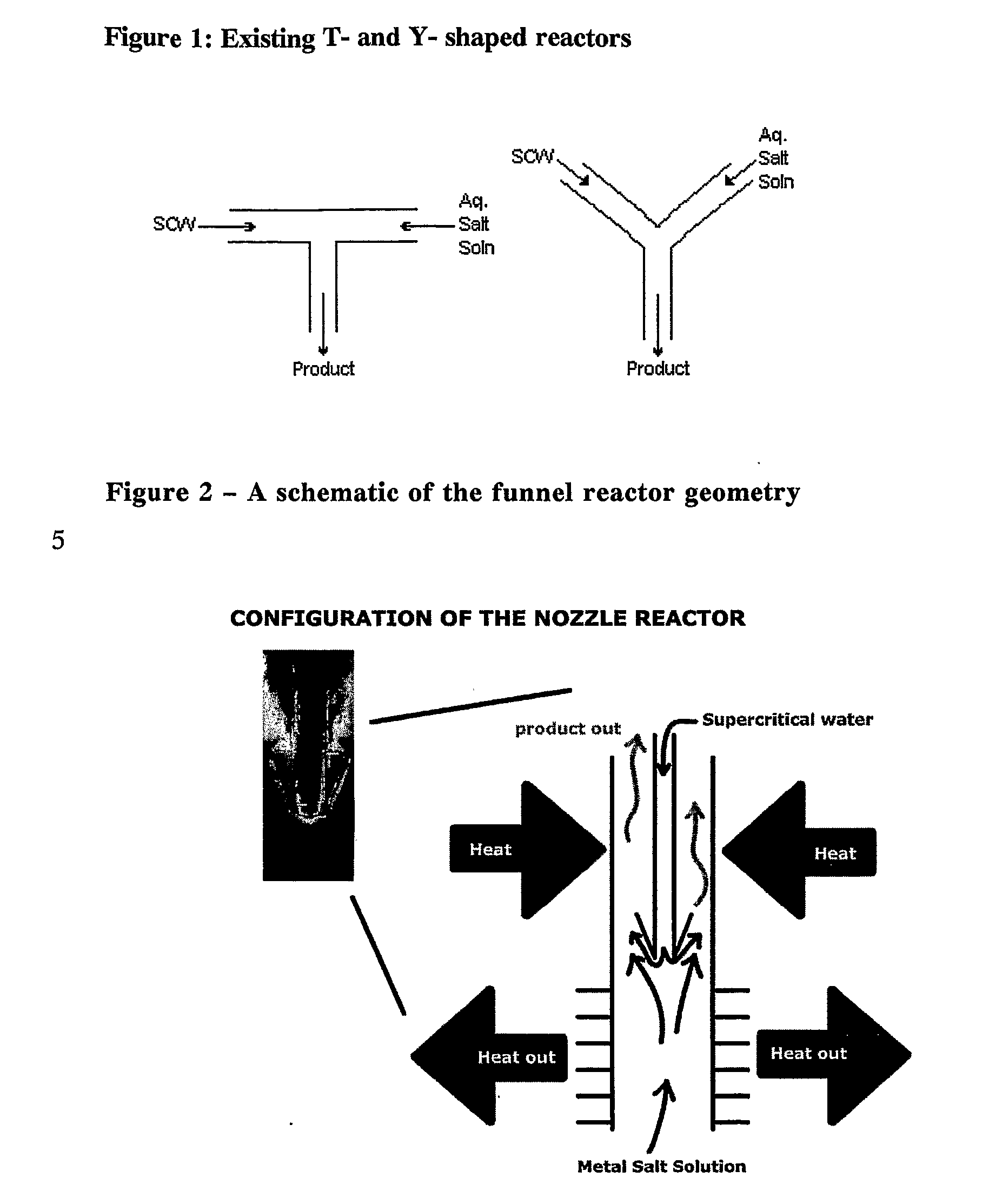
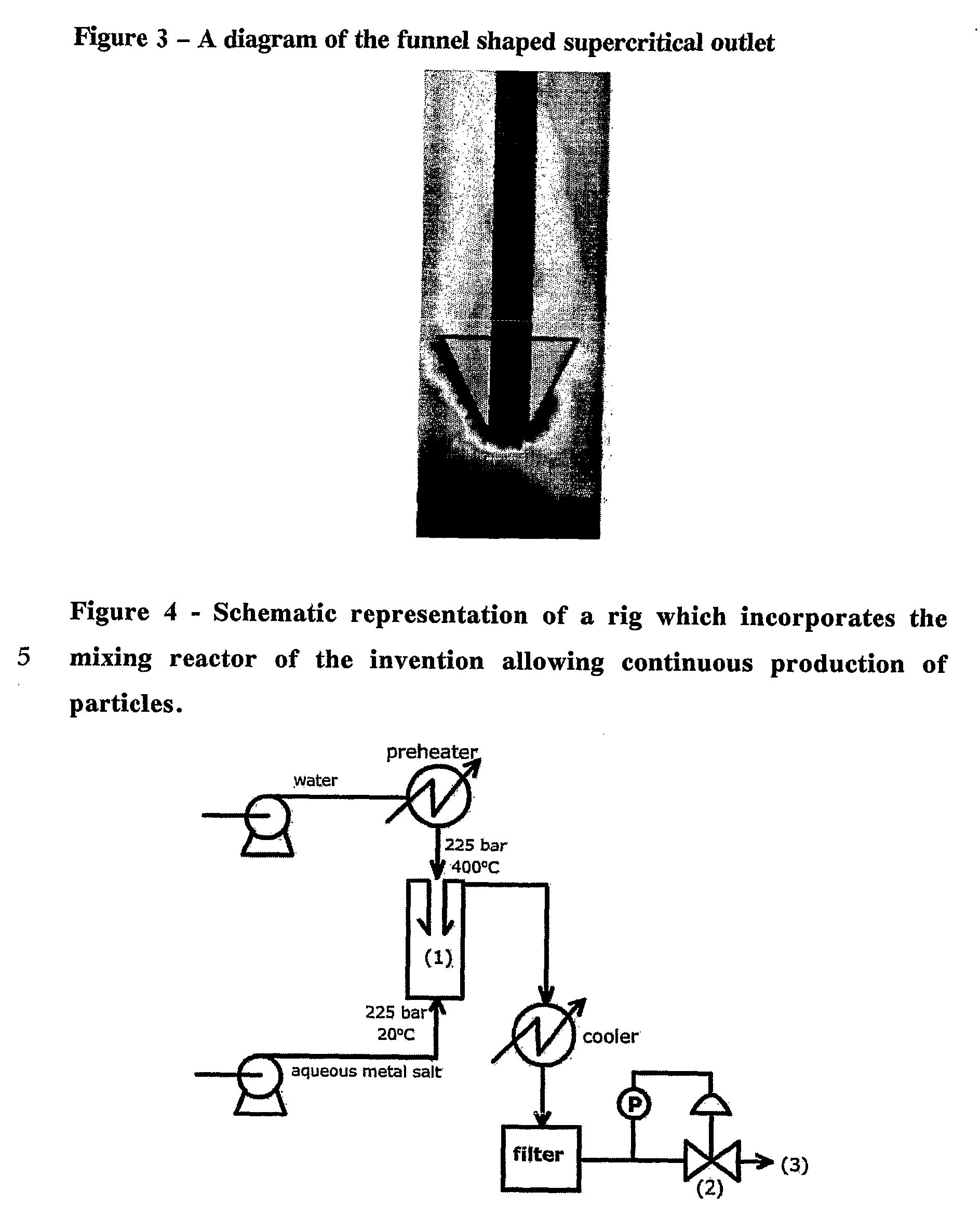
Counter Current Mixing Reactor
ActiveUS20070206435A1Minimize blockingEliminate mixingRare earth metal oxides/hydroxidesFlow mixersMetal oxide nanoparticlesReactor design
A mixing reactor for mixing efficiently streams of fluids of differing densities. In a preferred embodiment, one of the fluids is supercritical water, and the other is an aqueous salt solution. Thus, the reactor enables the production of metal oxide nanoparticles as a continuous process, without any risk of the reactor blocking due to the inefficient mixing inherent in existing reactor designs.
Owner:PROMETHEAN PARTICLES
Mesoporous mixed oxide materials as a new class of SO2 resistant catalysts for hydrocarbon oxidation
InactiveUS7132093B2Large specific surface areaGood dispersionGold compoundsMercury oxidesCeriumMesoporous silica
The oxide materials are of the class of ternary mesoporous mixed oxide materials including lanthanum, a metal M selected from the group consisting of Cr, Mn, Fe, Co, Ni, Cu and Zn, and zirconium or cerium such a mesoporous La—Co—Zr mixed oxide material designated as Meso LCZ[x] where x is the atomic ratio (La+Co) / La+Co+Zr. They are useful as catalysts since they show high activities for hydrocarbon oxidation and good resistance against poisoning agents. These highly ordered mesoporous mixed oxides are synthesized by: preparing an amorphous solution of a La-M precursor and adding a salt of zirconium or cerium thereto; acidifying the amorphous solution in the presence of a surfactant under conditions to obtain a clear homogeneous solution; adjusting pH of the solution under conditions to form a solid precipitate; separating the solution and surfactant from the precipitate; and calcinating the precipitate.
Owner:UNIV LAVAL
Nano-scale metal oxyhalide and oxysulfide scintillation materials and methods for making same
InactiveUS20080241041A1Rare earth metal oxides/hydroxidesMaterial nanotechnologyEmulsionNanoparticle
Crystalline scintillator materials comprising nano-scale particles of metal oxides, metal oxyhalides and metal oxysulfides are provided. The nano-scale particles are less than 100 nm in size. Methods are provided for preparing the particles. In one method, used to form oxyhalides and oxysulfides, metal salts are dissolved in water, and then precipitated out as fine particles using an aqueous base. After the particles are separated from the solution, they are annealed under a flow of a water saturated hydrogen anion gas, such as HCl or H2S, to form the crystalline scintillator particles. The other methods take advantage of the characteristics of microemulsion solutions to control droplet size, and, thus, the particle size of the final nano-particles. For example, in one method, a first micro-emulsion containing metal salts if formed. The first micro-emulsion is mixed with an aqueous base in a second micro-emulsion to form the final nano-scale particles.
Owner:GENERAL ELECTRIC CO
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS7892514B2Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumHalogen
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
Preparation of new layered double hydroxides exchanged with osmate for asymmetric dihydroxylation of olefins to vicinal diols
InactiveUS6387033B1Facilitates oxygen transferHigh activityPreparation by oxidation reactionsCarboxylic acid esters preparationDiolAlkene
LDH-osmate of the formula [MII(1-x)MIIIx(OH)2][OsO42-]x / 2.zH2O wherein MII is a divalent cation selected from the group consisting of Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+ and Ca2+ and MIII is a trivalent ion selected from the group consisting of Al3+, Cr3+, Mn3+, Fe3+ and Co3+, and x is the mole fraction having integral value ranging from 0.2 to 0.33, and z is the number of water molecules and ranges from 1 to 4, useful as, a catalyst, and a process for the preparation thereof and use thereof to manufacture vicinal diols.
Owner:COUNCIL OF SCI & IND RES
Organically modified fine particles
A technique for bonding an organic group with the surface of fine particles such as nanoparticles through strong linkage is provided, whereas such fine particles are attracting attention as materials essential for development of high-tech products because of various unique excellent characteristics and functions thereof. Organically modified metal oxide fine particles can be obtained by adapting high-temperature, high-pressure water as a reaction field to bond an organic matter with the surface of metal oxide fine particles through strong linkage. The use of the same condition enables not only the formation of metal oxide fine particles but also the organic modification of the formed fine particles. The resulting organically modified metal oxide fine particles exhibit excellent properties, characteristics and functions.
Owner:SUPER NANO DESIGN CO LTD
Method of making oxide particles
The present invention provides a process for producing particles, such as oxide nanoparticles, in a substantially water-free environment. The process involves mixing at least one metal compound of the formula MX(m−n) with at least one surfactant and at least one solvent, wherein M is an electropositive element of Groups 1–15; each X is independently selected from the group consisting of O1 / 2, F, Cl, Br, I, OR, O2CR, NR2, and R; each R is independently a hydrocarbyl group; n is equal to ½ the oxidation state of the metal M in the product particle; and m is equal to the oxidation state of the element M. The components are typically combined to form a mixture which is thermally treated for a time period sufficient to convert the metal compound into particles of the corresponding oxide, having sizes in a range between about 0.5 nanometer and about 1000 nanometers. Examples of metal compounds employed in this process include materials such as Si(OR)4, Ti(OR)4 (where R is as described above), (Zr(OiPr)2)(OAc)2, and the like. Illustrative oxide materials which can be prepared by this process include TiO2, ZrO2, SiO2, and B2O3.
Owner:GENERAL ELECTRIC CO
Method for producing perovskite-type composite oxide
InactiveUS20050249653A1Efficient heat treatmentImprove securityInternal combustion piston enginesMercury oxidesComposite oxideOxide
To provide a highly safe and hygienic method for industrially efficiently producing a perovskite-type composite oxide at low temperatures in heat treatment, in which the resulting perovskite-type composite oxide can maintain the catalytic activity of a noble metal at a high level over a long time, in a method for producing a perovskite-type composite oxide, a perovskite-type composite oxide is produced by mixing organometal salts of all elementary components constituting the perovskite-type composite oxide to prepare a precursor of the perovskite-type composite oxide, or mixing one or more organometal salts of part of the elementary components constituting the perovskite-type composite oxide with the other elementary components prepared as alkoxides of the respective elements, a coprecipitate of salts of the respective elements or a citrate complex of the respective elements to prepare a precursor of the perovskite-type composite oxide, and heat-treating the precursor of the perovskite-type composite oxide.
Owner:DAIHATSU MOTOR CO LTD +1
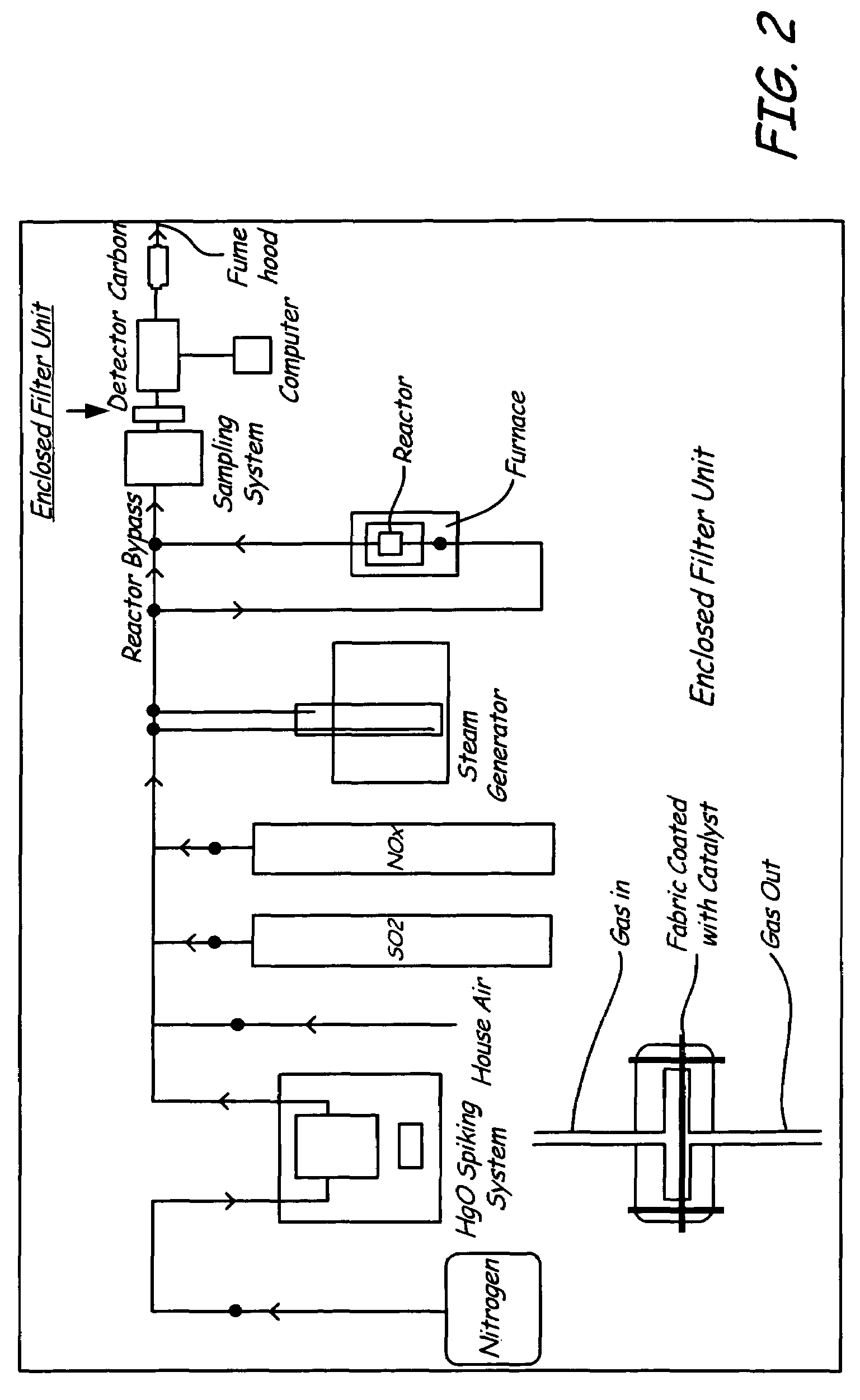
Mercury oxidation of flue gas using catalytic barrier filters
InactiveUS7618603B2Facilitates simultaneous removalCombination devicesGas treatmentOxidizing agentMercury oxidation
A method for oxidizing elemental mercury contained in flue gas uses a catalytic barrier filter. The method comprises directing the flue gas towards the catalytic barrier filter; passing the flue gas through the catalytic barrier filter in the presence of an oxidant; and outletting the flue gas from the catalytic barrier filter, wherein about 50 percent to about 99 percent of the elemental mercury is oxidized.
Owner:NORTH DAKOTA THE UNIV OF
Process for producing micro-mesoporous metal oxide having regulated pores formed by novel template removal method
InactiveUS7223377B2Copper oxides/halidesManganese oxides/hydroxidesPolymeric surfaceAlkaline earth metal
The present invention is the method for preparation of transition metal oxide having micro-mesoporous structure whose average fine pores size is not less than 1 nm and not more than 2 nm comprising, adding and dissolving transition metal salt which is a precursor of transition metal oxide and / or metal alkoxide in the solution prepared by dissolving polymer surfactant in organic solvent, hydrolyzing said transition metal salt and / or metal alkoxide and preparing sol solution which is polymerized and self organized, then obtaining gel whose organization is stabilized from said sol solution and removing said polymer surfactant by using water of room temperature or water to which alkali metal or alkaline earth metal ion is added.
Owner:JAPAN SCI & TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com