Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
153results about "Cobalt carbonyls" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
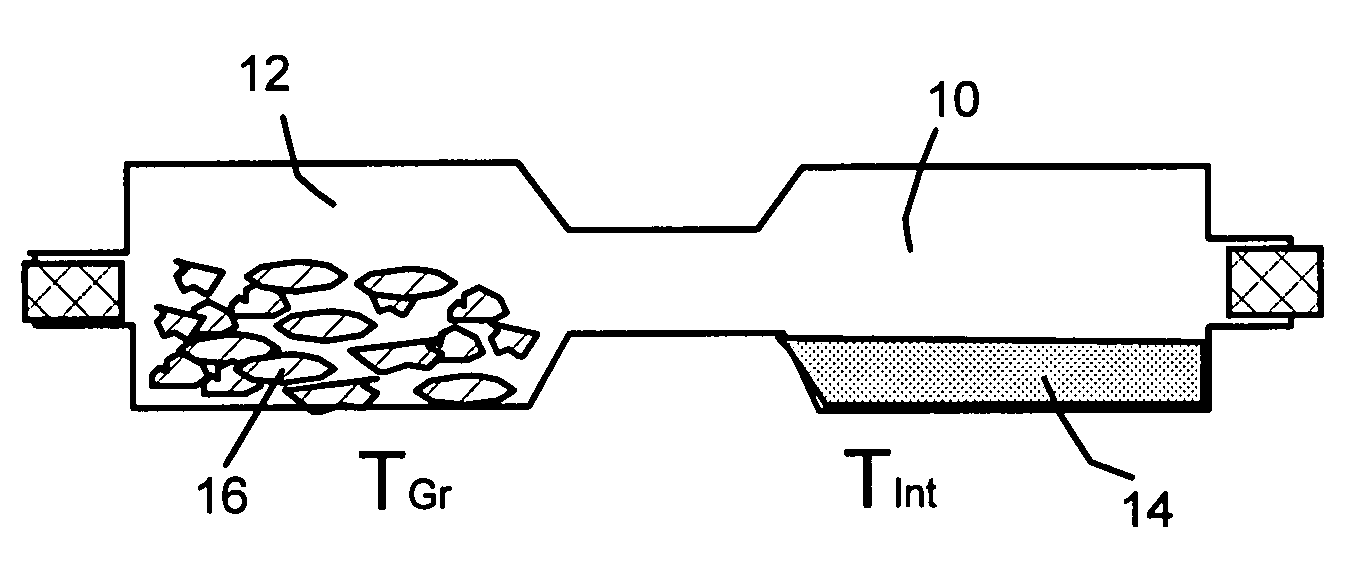
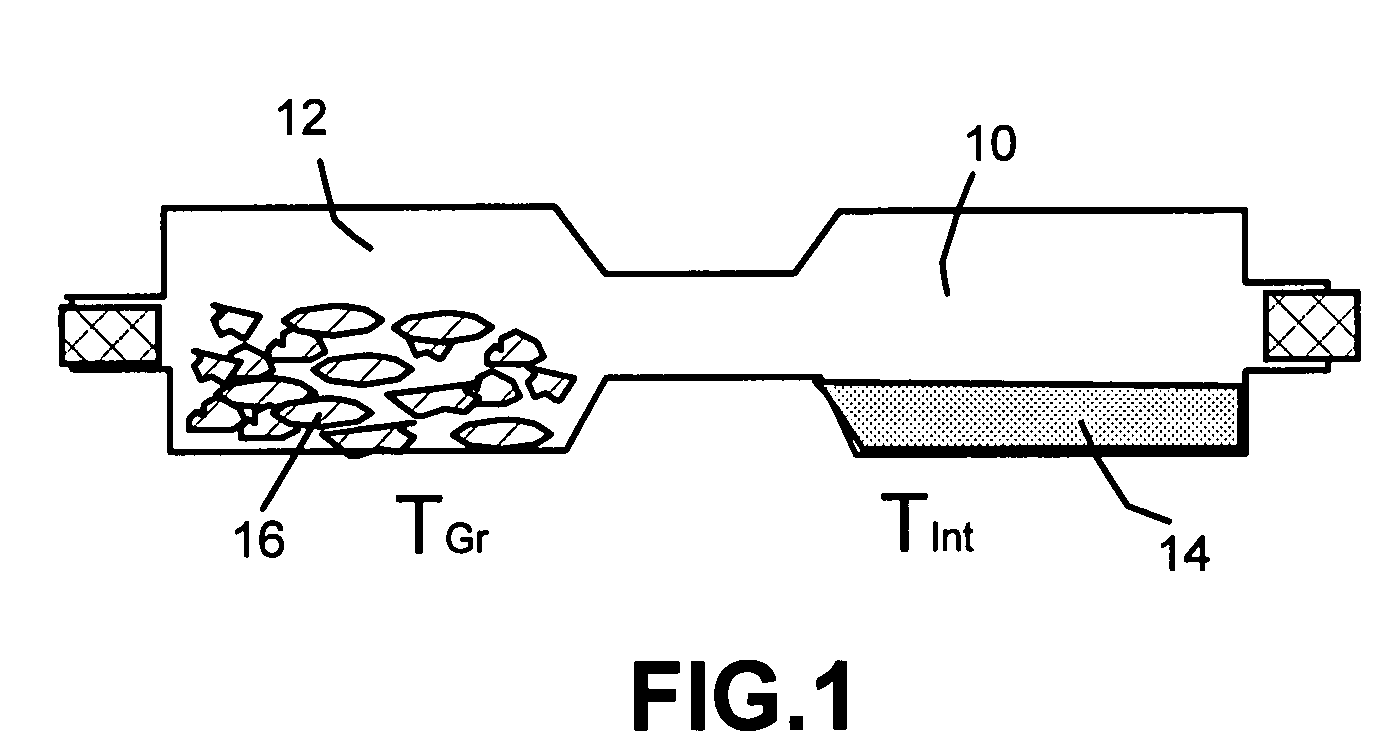
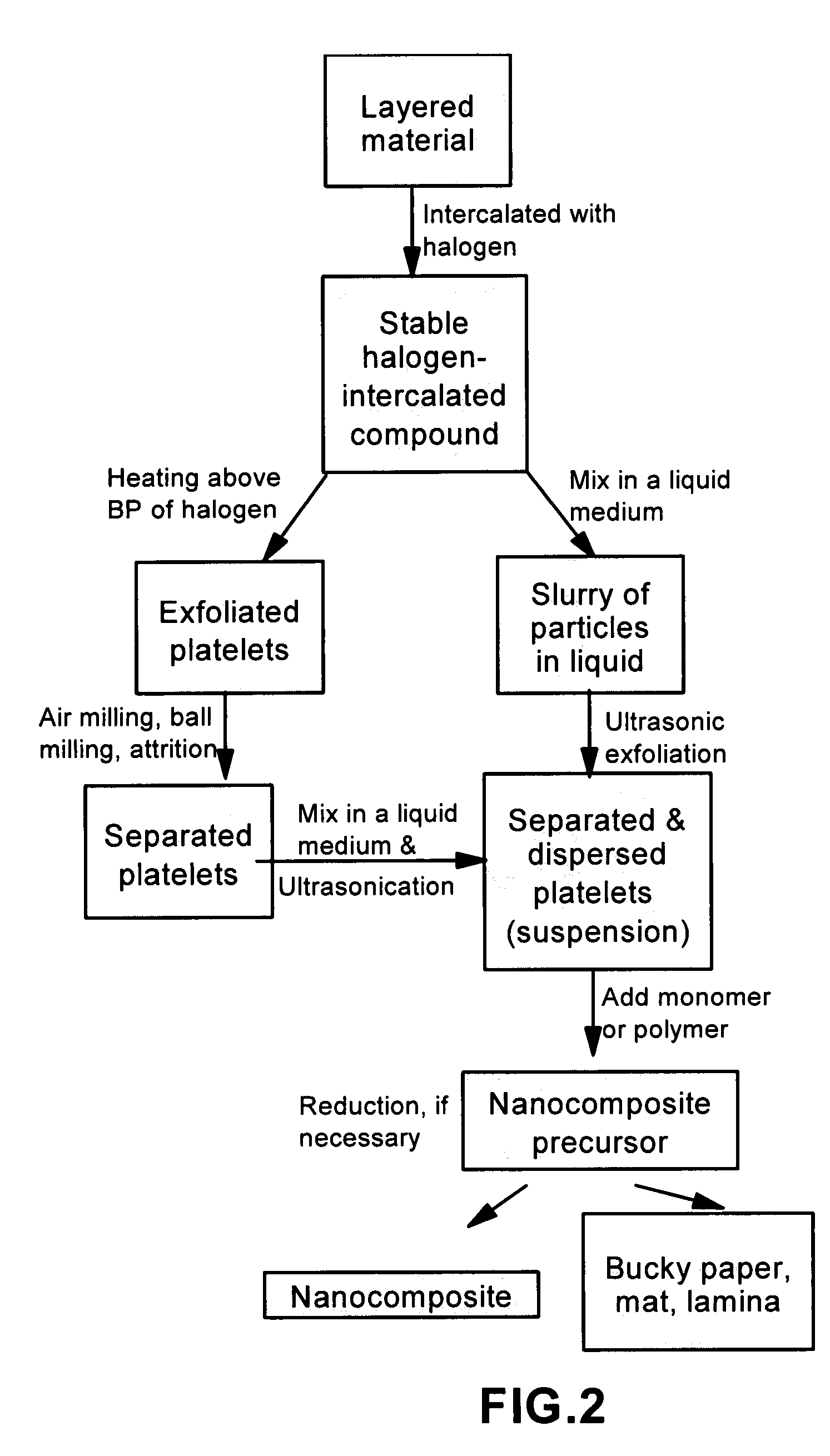
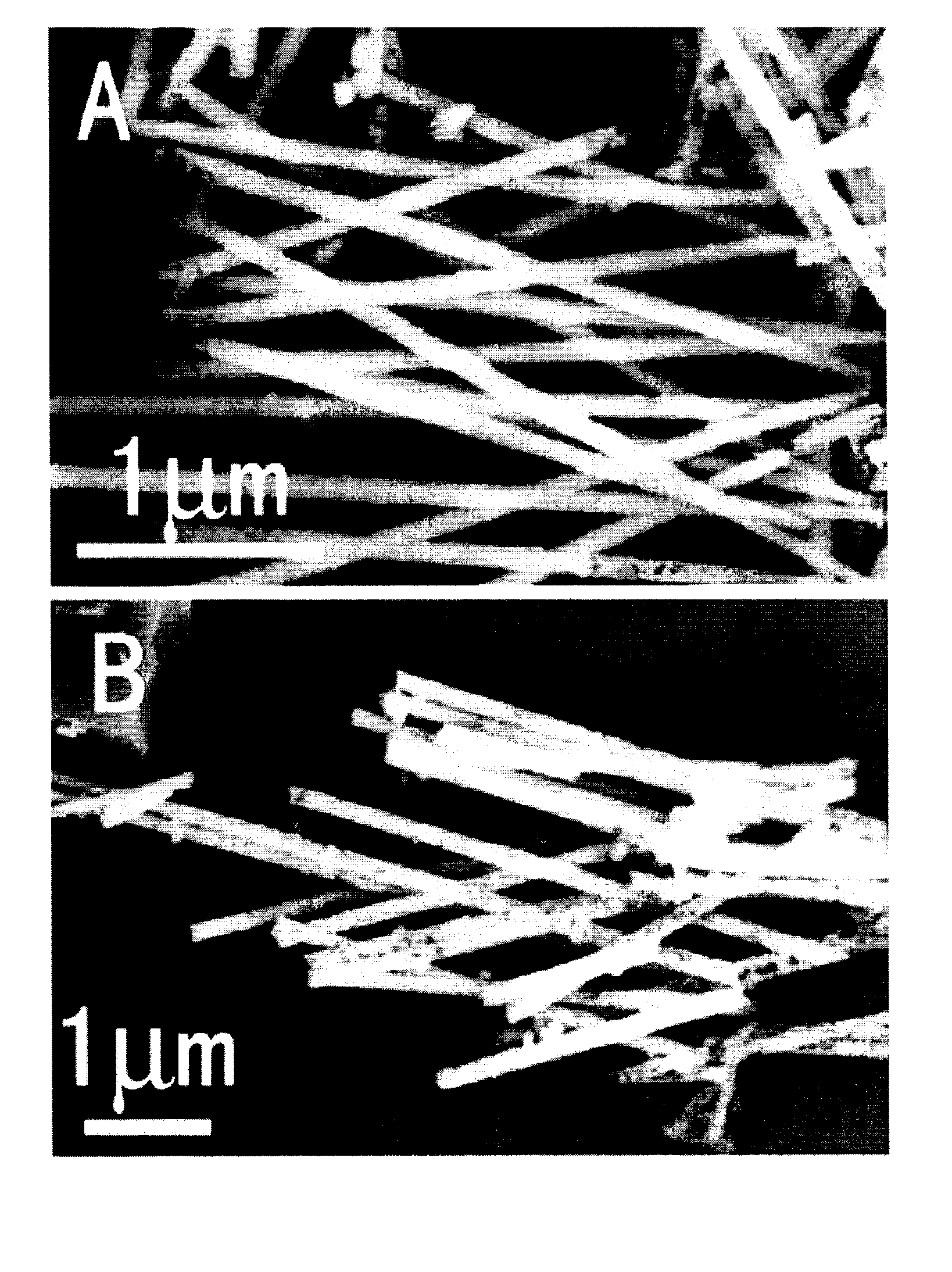
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS20080206124A1Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumPhysical chemistry
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
Process of making hydrophobic metal oxide nanoparticles
InactiveUS7081234B1Uniform coatingSulfur compoundsCopper oxides/halidesMetal oxide nanoparticlesTransport layer
A process of treating metal oxide nanoparticles that includes mixing metal oxide nanoparticles, a solvent, and a surface treatment agent that is preferably a silane or siloxane is described. The treated metal oxide nanoparticles are rendered hydrophobic by the surface treatment agent being surface attached thereto, and are preferably dispersed in a hydrophobic aromatic polymer binder of a charge transport layer of a photoreceptor, whereby π—π interactions can be formed between the organic moieties on the surface of the nanoparticles and the aromatic components of the binder polymer to achieve a stable dispersion of the nanoparticles in the polymer that is substantially free of large sized agglomerations.
Owner:XEROX CORP
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
Organically modified fine particles
ActiveUS20070003463A1Easy transferManganese oxides/hydroxidesGermanium dioxideReaction fieldNanoparticle
A technique for bonding an organic group with the surface of fine particles such as nanoparticles through strong linkage is provided, whereas such fine particles are attracting attention as materials essential for development of high-tech products because of various unique excellent characteristics and functions thereof. Organically modified metal oxide fine particles can be obtained by adapting high-temperature, high-pressure water as a reaction field to bond an organic matter with the surface of metal oxide fine particles through strong linkage. The use of the same condition enables not only the formation of metal oxide fine particles but also the organic modification of the formed fine particles. The resulting organically modified metal oxide fine particles exhibit excellent properties, characteristics and functions.
Owner:SUPER NANO DESIGN CO LTD
Pure metal and ceramic nanofibers
InactiveUS20140332733A1Few voidFew defectOrganic active ingredientsGold compoundsFiberElectrospinning
Provided herein are nanofibers and processes of preparing nanofibers. In some instances, the nanofibers are metal and / or ceramic nanofibers. In some embodiments, the nanofibers are high quality, high performance nanofibers, highly coherent nanofibers, highly continuous nanofibers, or the like. In some embodiments, the nanofibers have increased coherence, increased length, few voids and / or defects, and / or other advantageous characteristics. In some instances, the nanofibers are produced by electrospinning a fluid stock having a high loading of nanofiber precursor in the fluid stock. In some instances, the fluid stock comprises well mixed and / or uniformly distributed precursor in the fluid stock. In some instances, the fluid stock is converted into a nanofiber comprising few voids, few defects, long or tunable length, and the like.
Owner:CORNELL UNIVERSITY
Transition metal/carbon nanotube composite and method of preparing the same
ActiveUS20100181200A1Good capacitance characteristicsImprove material removal rateElectrostatic separatorsMolten spray coatingCarbon nanotubeOxide coating
A transition metal / carbon nanotube composite includes a carbon nanotube and a transition metal oxide coating layer disposed on the carbon nanotube. The transition metal oxide coating layer includes a nickel-cobalt oxide
Owner:SAMSUNG ELECTRONICS CO LTD +1
Method for synthesis of colloidal nanoparticles
InactiveUS20060060998A1Large-scale, safe, convenient, reproducible, and energy-efficient productionHigh crystallinityDielectric heatingNanoinformaticsColloidal nanoparticlesContinuous flow
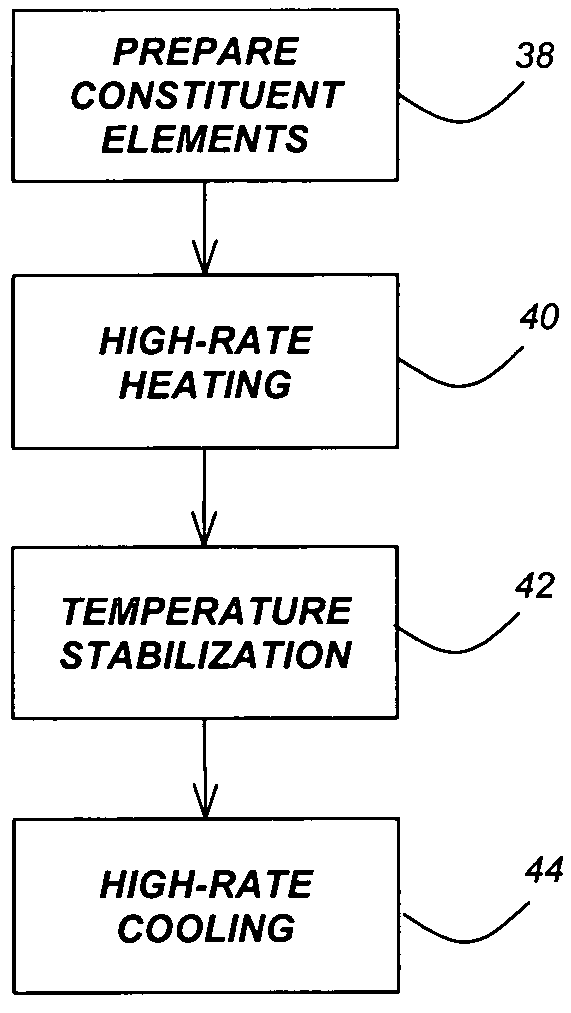
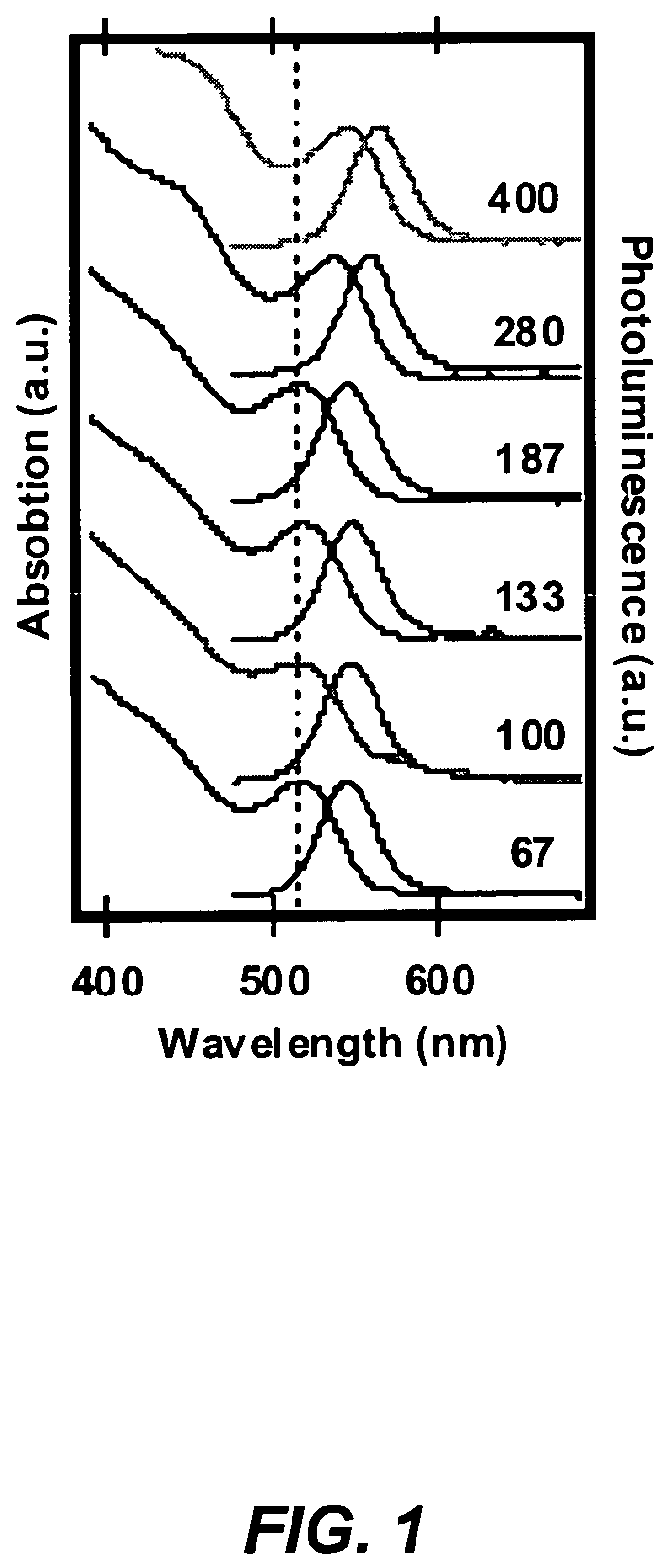
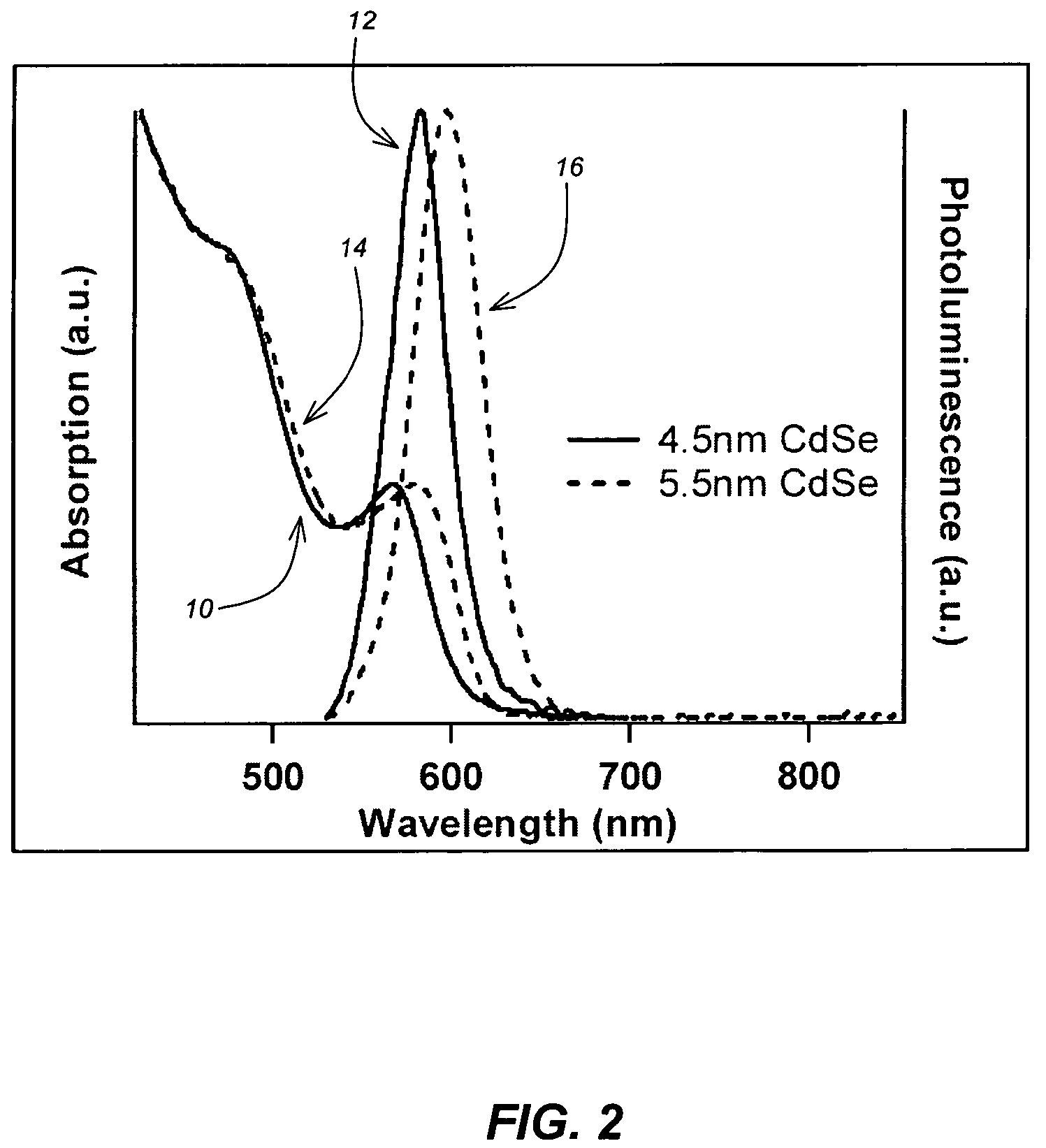
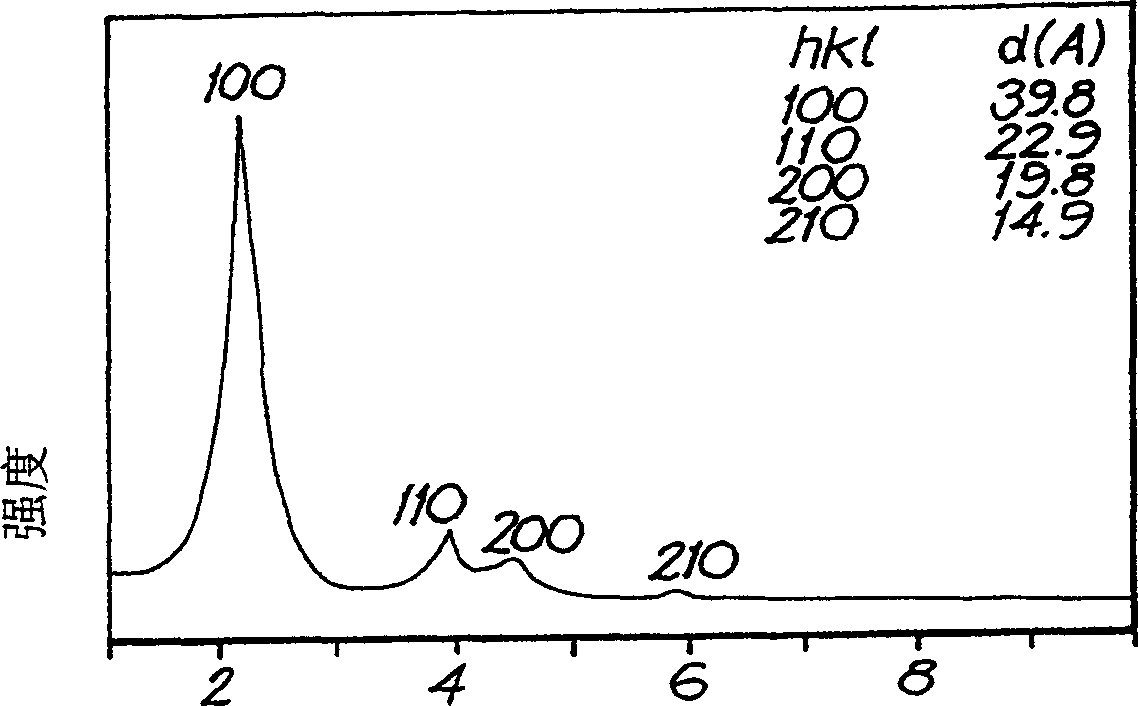
A method for synthesis of high quality colloidal nanoparticles using comprises a high heating rate process. Irradiation of single mode, high power, microwave is a particularly well suited technique to realize high quality semiconductor nanoparticles. The use of microwave radiation effectively automates the synthesis, and more importantly, permits the use of a continuous flow microwave reactor for commercial preparation of the high quality colloidal nanoparticles.
Owner:RGT UNIV OF CALIFORNIA
Making method of nickel hydroxide with coated gamma hydroxy cobalt oxide
ActiveCN101106193AAlkaline accumulator electrodesNickel oxides/hydroxidesHigh densityNickel oxide hydroxide
The invention relates to a preparation method for nickel hydroxide, which is used as a Ni-H storage battery positive material, coated with gamma hydroxy cobalt oxide. The preparation includes that the sphere surface of nickel hydroxide is coated with a layer of cobalt hydroxide firstly; then, under the conditions of high-density sodium hydroxide and oxygen, the cobalt hydroxide layer coated on the surface is oxidized the gamma hydroxy cobalt oxide, or the treatment of embedding doped metals is exerted on the gamma hydroxy cobalt oxide layer. By controlling the coating layer to be the cobalt hydroxide presenting a shape of flake, the method makes the resulted conducting network coated with the coating layer of gamma hydroxy cobalt oxide more uniform, more integrated and better conductive, and thus attributes the nickel hydroxide positive active material coated with gamma hydroxy cobalt oxide with some characteristics of high recovering rate of over-discharging capacity, good performance of big current discharging, low self-discharging, long lifetime of cycling use and so on.
Owner:JINCHI ENERGY MATERIALS CO LTD
Synthesis of cathode active materials
The present invention relates to a method for preparing an electroactive metal polyanion or a mixed metal polyanion comprising forming a slurry comprising a polymeric material, a solvent, a polyanion source or alkali metal polyanion source and at least one metal ion source; heating said slurry at a temperature and for a time sufficient to remove the solvent and form an essentially dried mixture; and heating said mixture at a temperature and for a time sufficient to produce an electroactive metal polyanion or electroactive mixed metal polyanion. In an alternative embodiment the present invention relates to a method for preparing a metal polyanion or a mixed metal polyanion which comprises mixing a polymeric material with a polyanion source or alternatively an alkali metal polyanion source and a source of at least one metal ion to produce a fine mixture and heating the mixture to a temperature higher than the melting point of the polymeric material, milling the resulting material and then heating the milled material. It is another object of the invention to provide electrochemically active materials produced by said methods. The electrochemically active materials so produced are useful in making electrodes and batteries.
Owner:LITHIUM WERKS TECH BV +1
High density cobalt-manganese coprecipitated nickel hydroxide and process for its production
The present invention provides high density cobalt-manganese coprecipitated nickel hydroxide, particularly having a tapping density of 1.5 g / cc or greater, and a process for its production characterized by continuous supply of an aqueous solution of a nickel salt which contains a cobalt salt and a manganese salt, of a complexing agent and of an alkali metal hydroxide, into a reactor either in an inert gas atmosphere or in the presence of a reducing agent, continuous crystal growth and continuous removal.
Owner:TANAKA CHEM
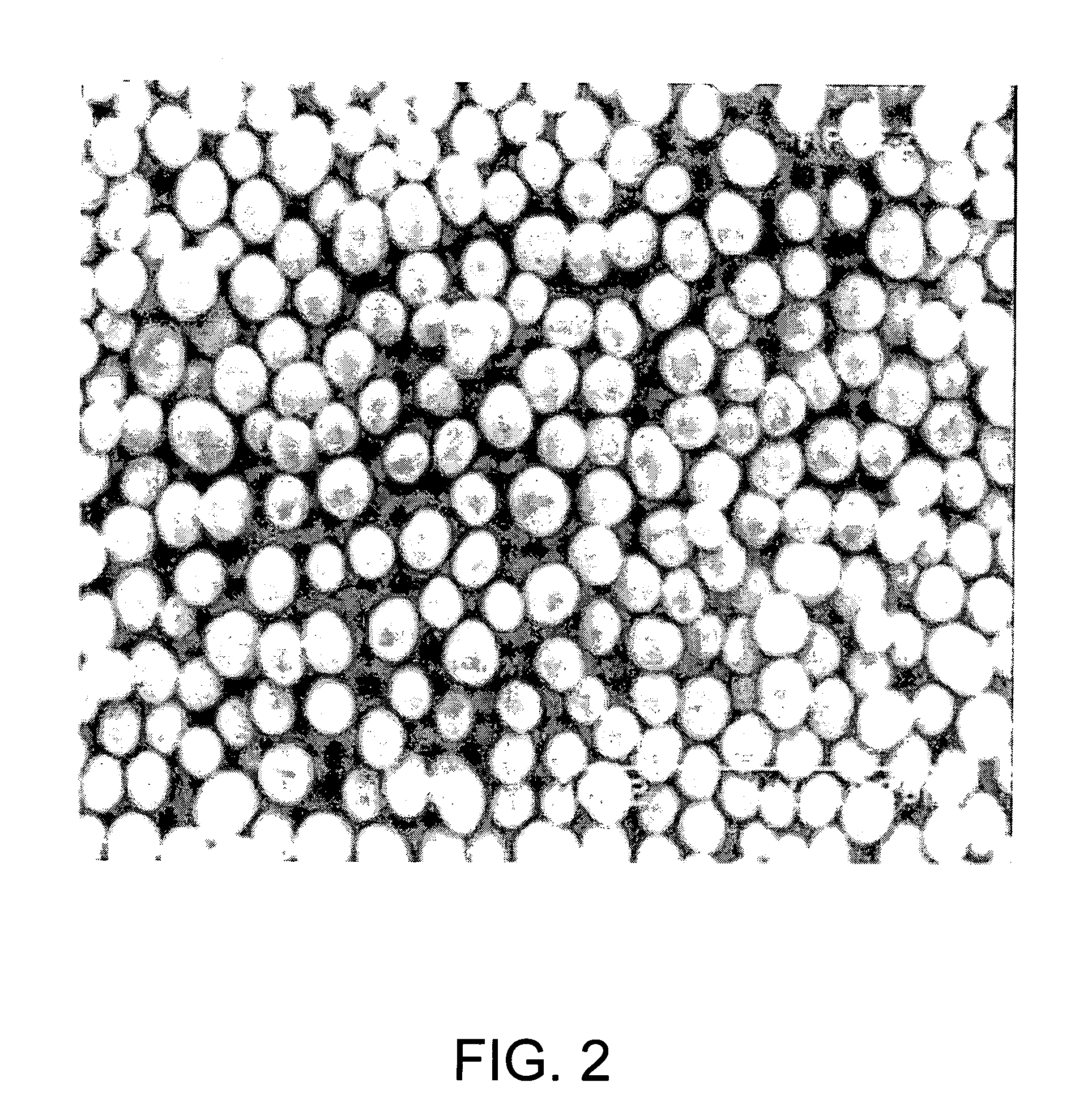
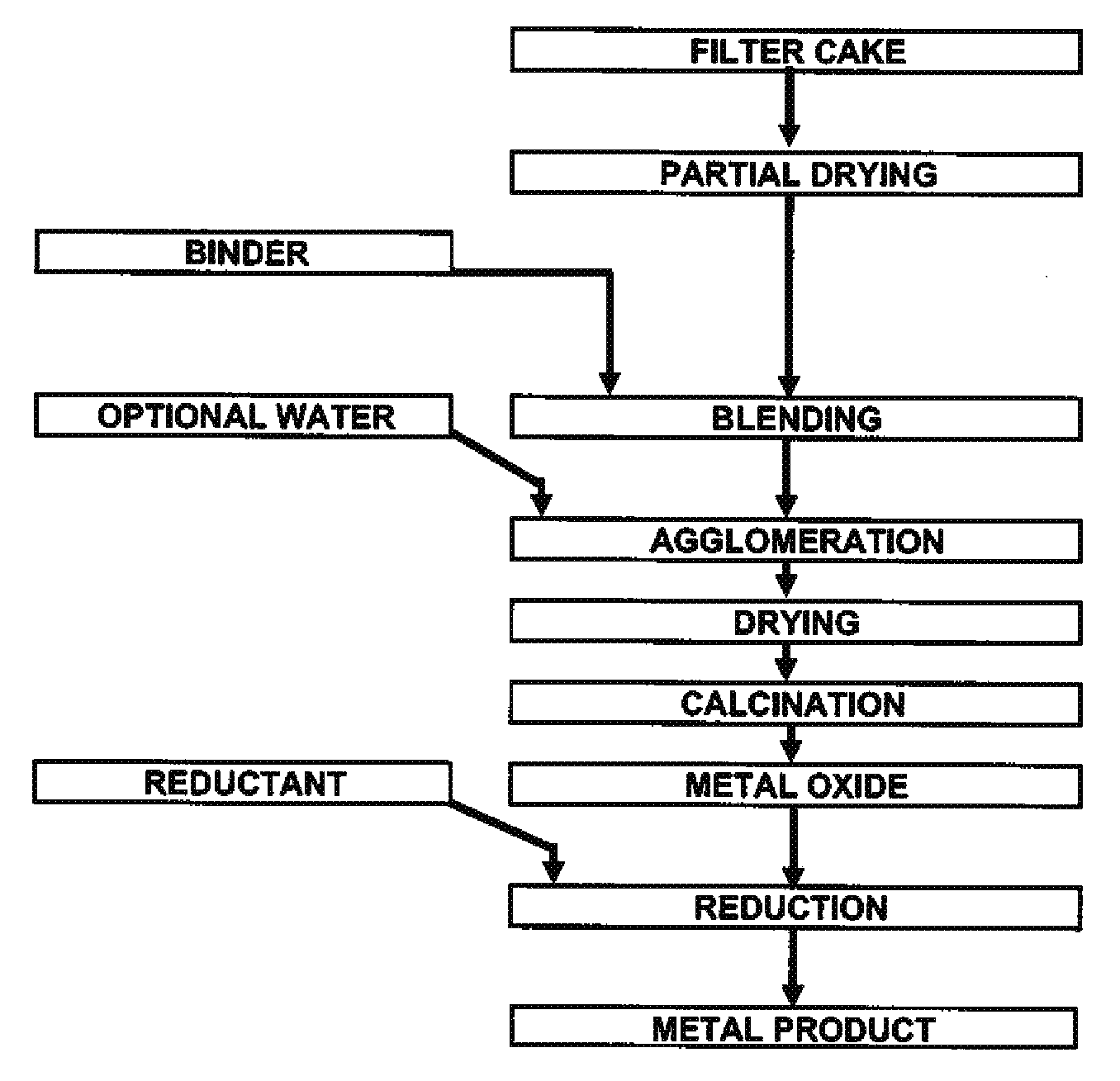
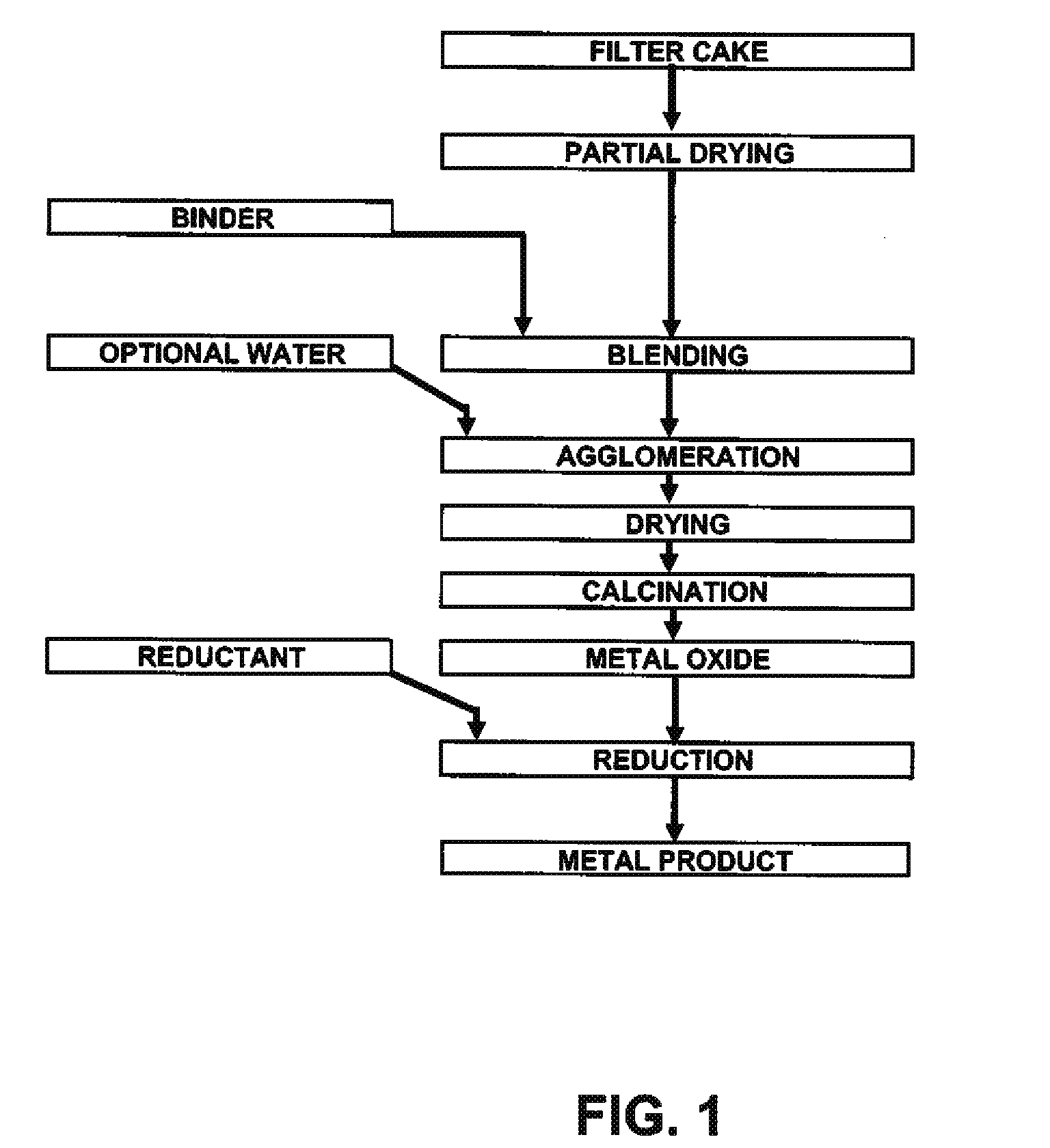
Process for production of nickel and cobalt using metal hydroxide, metal oxide and/or metal carbonate
A method for producing metal oxide from a metal salt selected from nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate, mixed nickel-cobalt carbonate and combinations thereof includes providing a mixture of the metal salt, mixing the metal salt with a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof, forming the mixture into agglomerates, and calcining the agglomerates to produce metal oxide. A method for making metallic nickel or cobalt includes providing a metal salt selected from the group consisting of nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate and combinations thereof, mixing the metal salt with a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof to form a mixture, optionally adding water, forming the mixture into agglomerates, drying the agglomerates, adding an effective reducing amount of coke and / or coal and directly reducing the dried agglomerates with an effective amount of heat to produce metallic nickel and / or cobalt. Coke particles may be added to the mixture prior to agglomeration. An agglomerate includes a metal salt selected from the group consisting of nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate, mixed nickel-cobalt carbonate and combinations thereof; and a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof.
Owner:VALE CANADA
Bioprocesses enhanced by magnetic nanoparticles
InactiveUS20060040388A1Increase volumeEnhances gas transferMaterial nanotechnologyNanomagnetismMagnetite NanoparticlesOxygen
One aspect of the present invention relates to magnetic nanoparticles colloidally stabilized in aqueous milieu by association with an organic phase. The organic phase may be either a fluorinated polymer or an organic hydrocarbon bilayer, wherein the two layers are chemically bonded to each other. The stabilized particles are further non-toxic and provide useful enhancements in bioprocesses. Another aspect of the present invention relates to compositions comprising an oxygen-dissolving fluid vehicle and surface modified, nanometer-sized magnetic particles. The inventive compositions have utility in a wide range of applications, but are particularly suitable for use as recyclable oxygen carriers, separation and purification vehicles, and bioprocessing media, including fermentation processes.
Owner:MASSACHUSETTS INST OF TECH
Spherical tricobalt tetraoxide and method of preparing the same
ActiveUS20100135897A1Stable structureMaintain good propertiesIron oxides/hydroxidesCell electrodesCobalt(II,III) oxideHigh activity
A method of preparation of spherical tricobalt tetraoxide, including at least oxidizing a bivalent cobalt salt in a wet environment and in the presence of a precipitant, a complexing agent, and an oxidant to yield spherical cobalt oxyhydroxide.cobalt hydroxide according to the following equation Co2++3OH−+O→CoOOH.Co(OH)2; oxidizing the spherical hydroxy cobalt oxyhydroxide.cobalt hydroxide to yield spherical tricobalt tetraoxide according to the following equation 6 CoOOH.Co(OH)2+O→4 Co3O4+9 H2O; and roasting the spherical tricobalt tetraoxide at low or intermediate temperature to yield a black powder. The method is easily practiced and suitable for mass production, and the resultant spherical tricobalt tetraoxide has stable structure, reliable properties, and high activity.
Owner:NINGBO RONBAY LITHIUM BATTERY MATERIAL CO LTD
Methods of forming a nanocrystal
InactiveUS20110033368A1Low pour pointMaterial nanotechnologyFrom normal temperature solutionsBoiling pointOxygen donor
Methods of forming a nanocrystal are provided. The nanocrystal may be a binary nanocrystal of general formula M1A or of general formula M1O, a ternary nanocrystal of general formula M1M2A, of general formula M1AB or of general formula M1M2O or a quaternary nanocrystal of general formula M1M2AB. M1 is a metal of Groups II-IV, Group VII or Group VIII of the PSE. A is an element of Group VI or Group V of the PSE. O is oxygen. A homogenous reaction mixture in a non-polar solvent of low boiling point is formed, that includes a metal precursor containing the metal M1 and, where applicable M2. For an oxygen containing nanocrystal the metal precursor contains an oxygen donor. Where applicable, A is also included in the homogenous reaction mixture. The homogenous reaction mixture is under elevated pressure brought to an elevated temperature that is suitable for forming a nanocrystal.
Owner:AGENCY FOR SCI TECH & RES
Production of fine-grained particles
InactiveUS20050025698A1Heating fastMaterial nanotechnologyNanostructure manufactureMaterials scienceCrystallization
Particles of mixed metal oxide include at least two metal species. The particles have a grain size within the range of 1-100 nm. The particles are substantially crystalline. The particles contain only small or negligible amounts of amorphous material. The at least two metal species are uniformly dispersed in the particles.
Owner:VERY SMALL PARTICLE CO LTD
Synthesis of cathode active materials
Owner:LITHIUM WERKS TECH BV +1
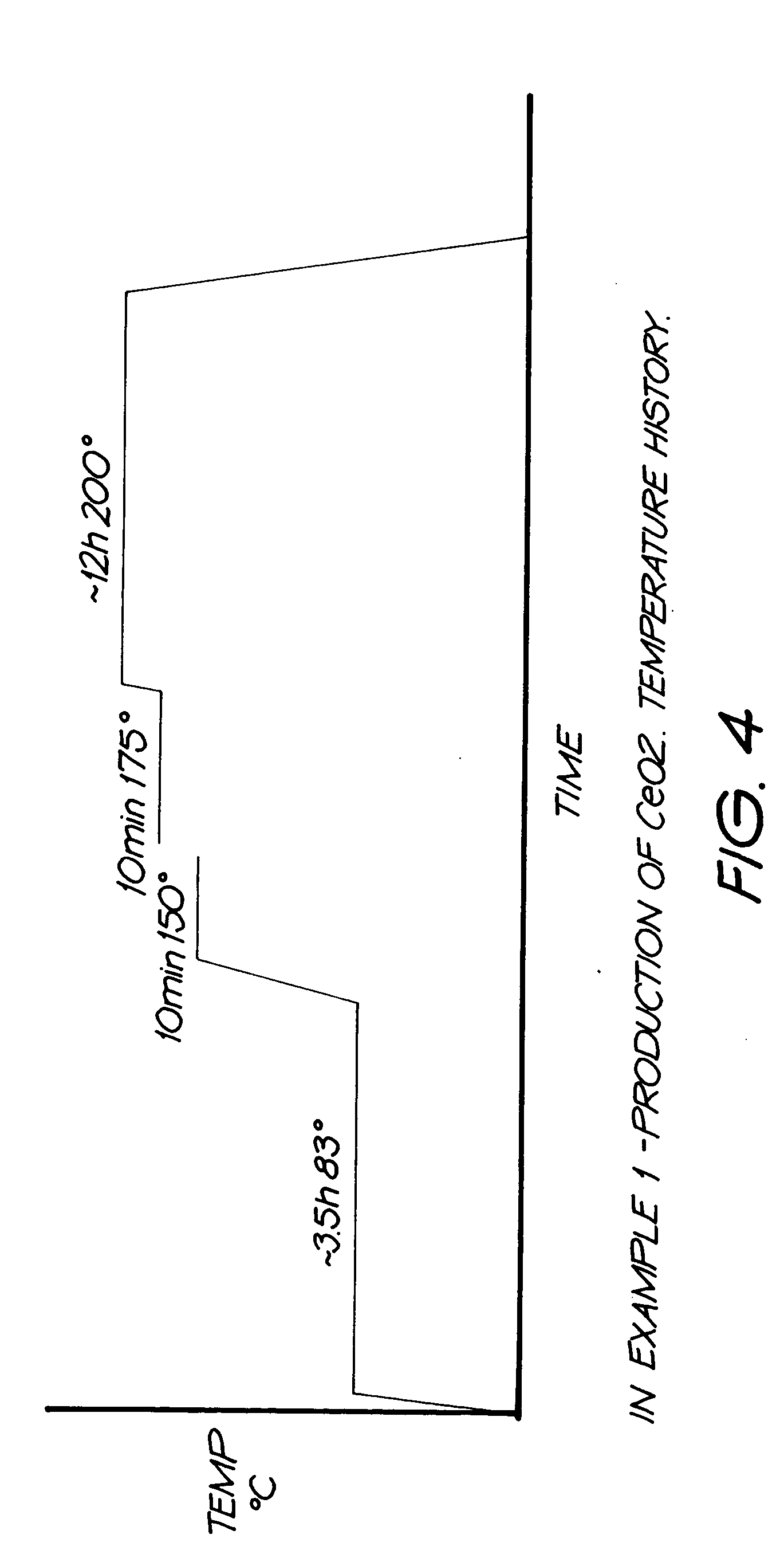
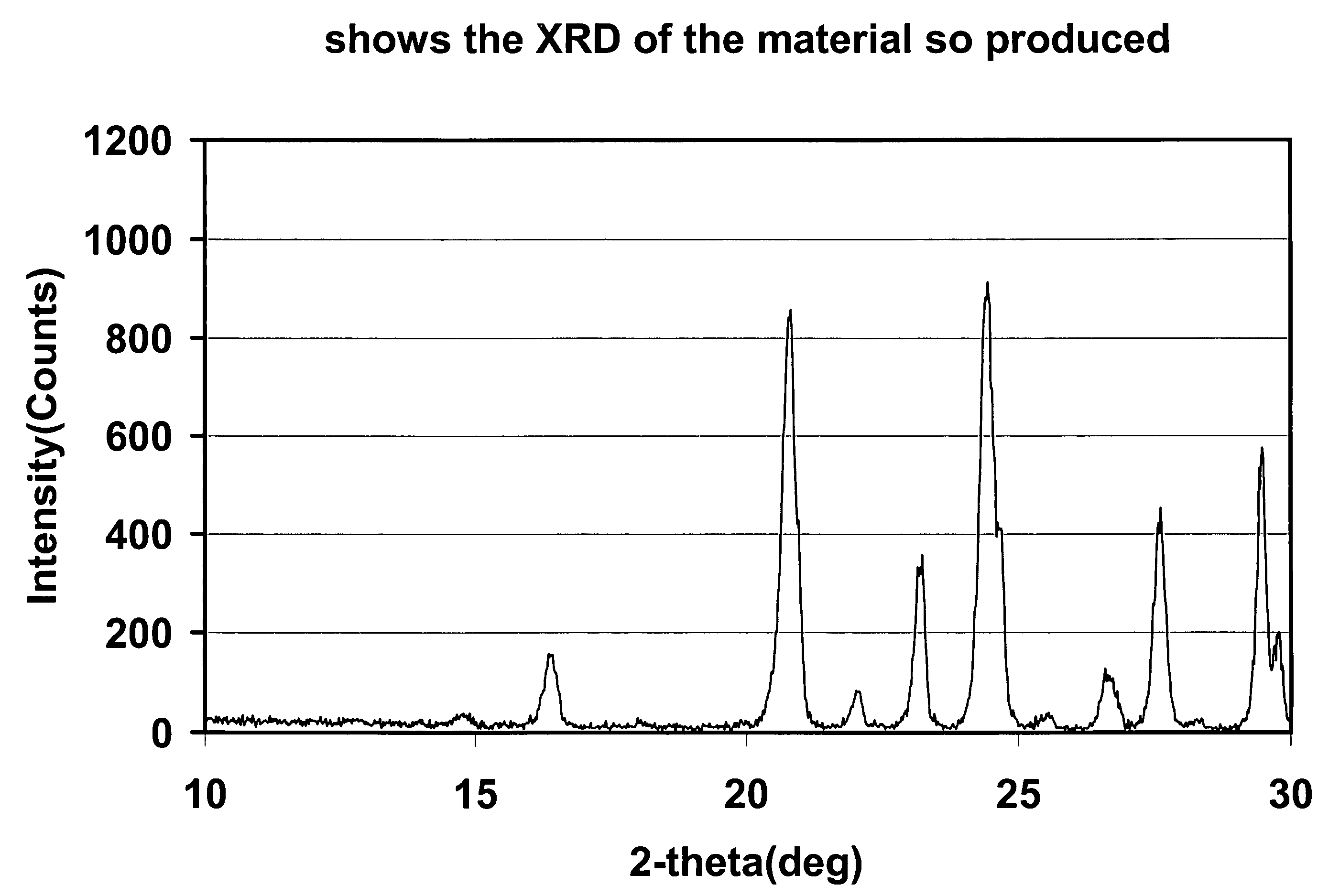
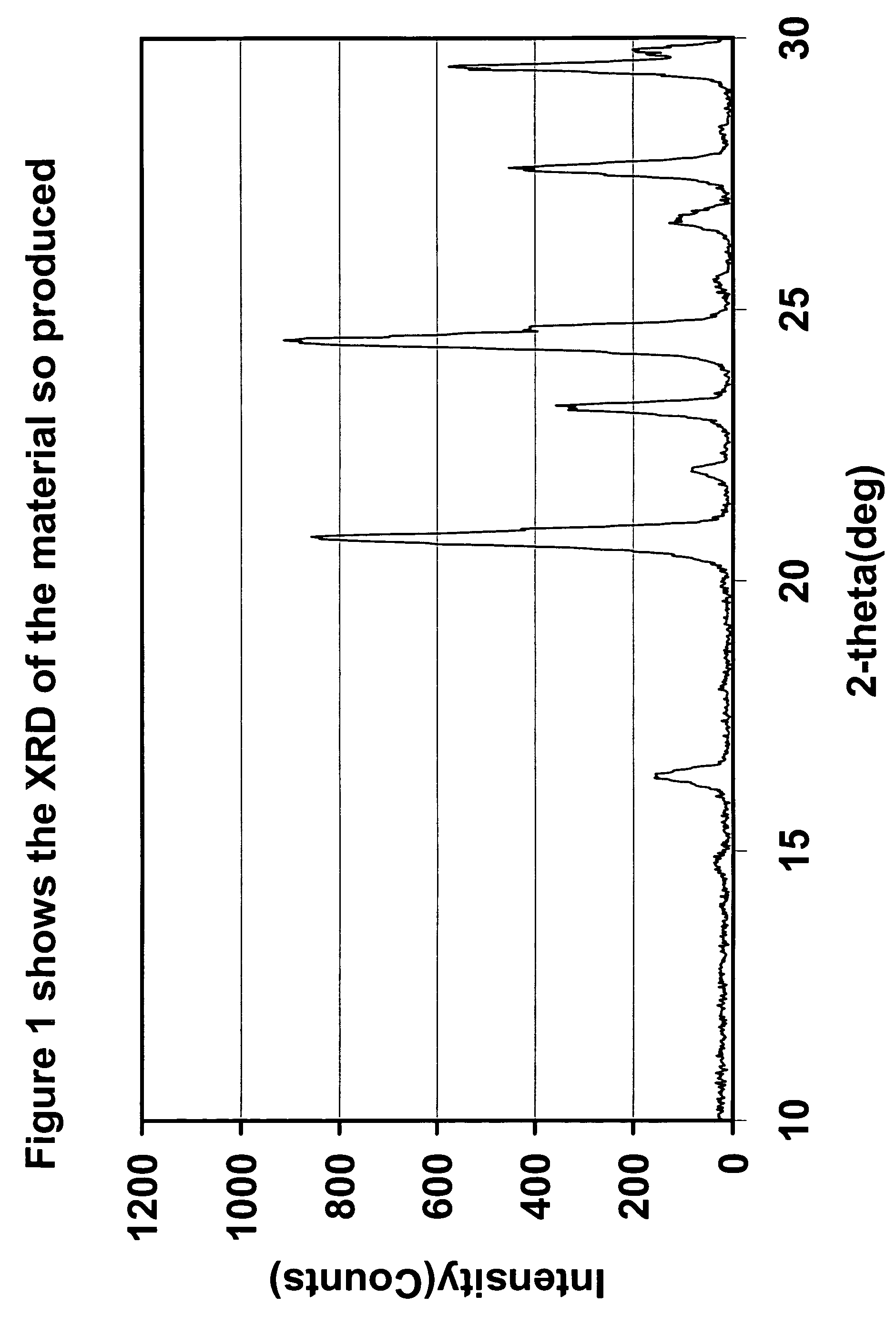
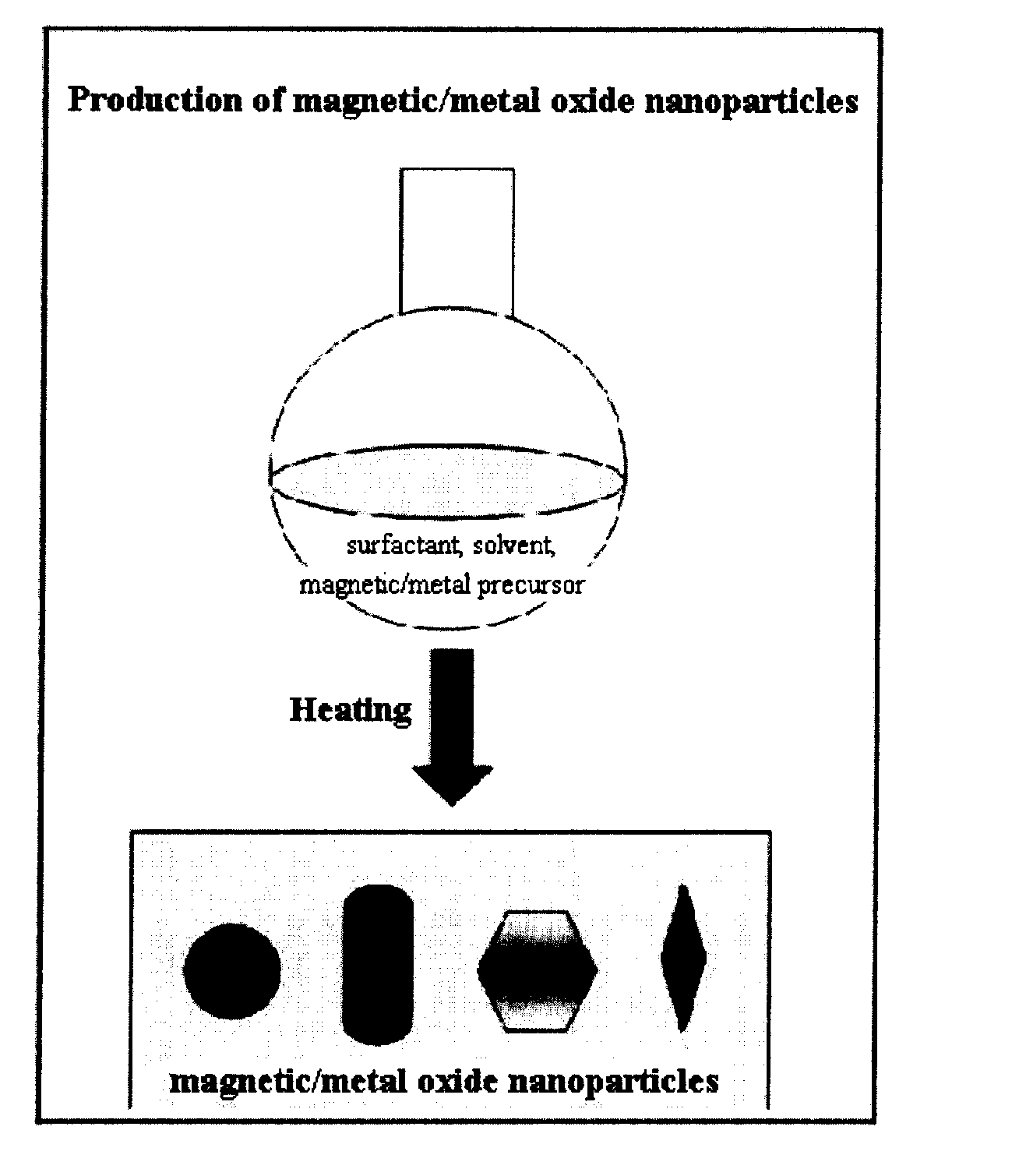
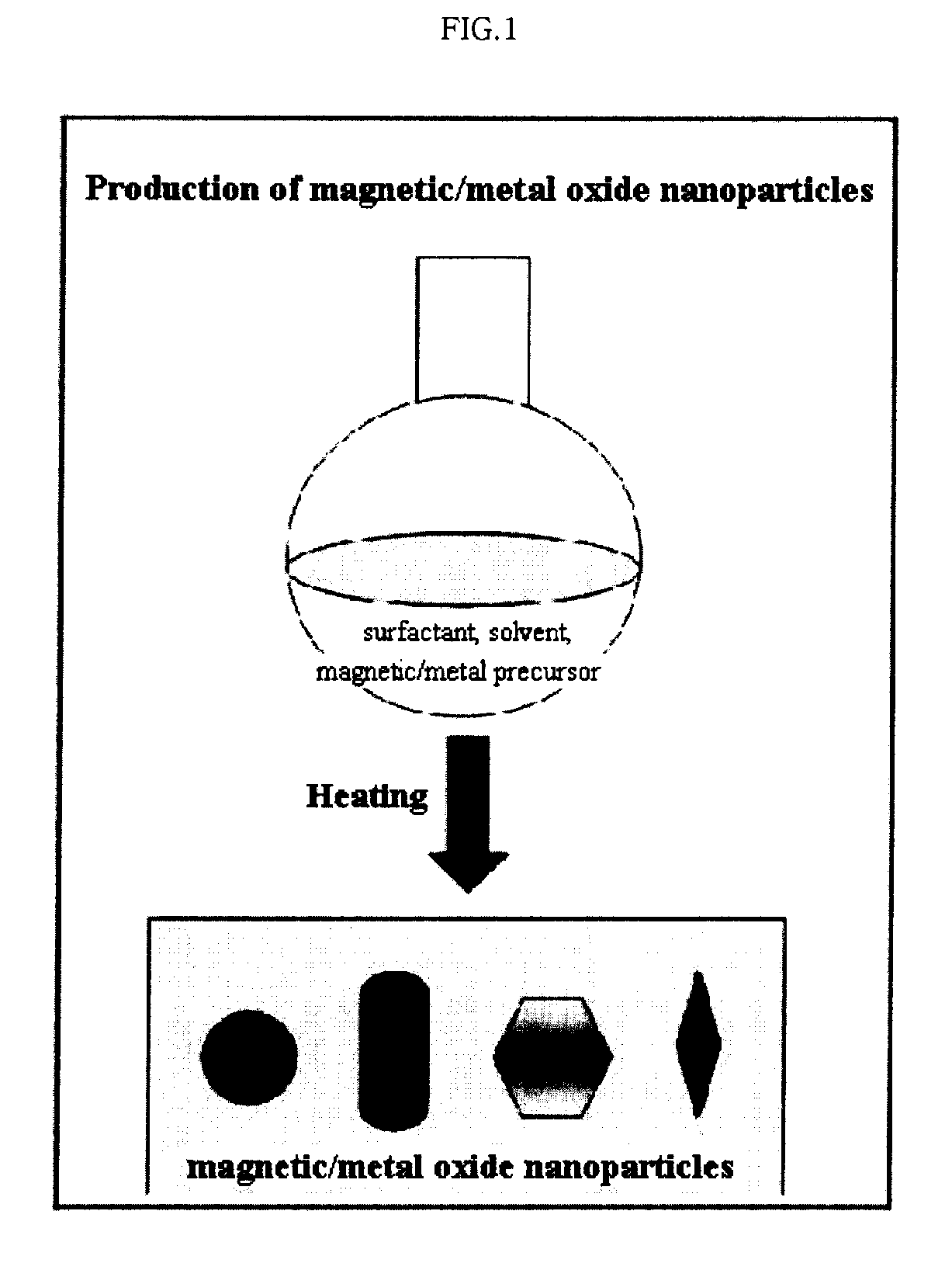
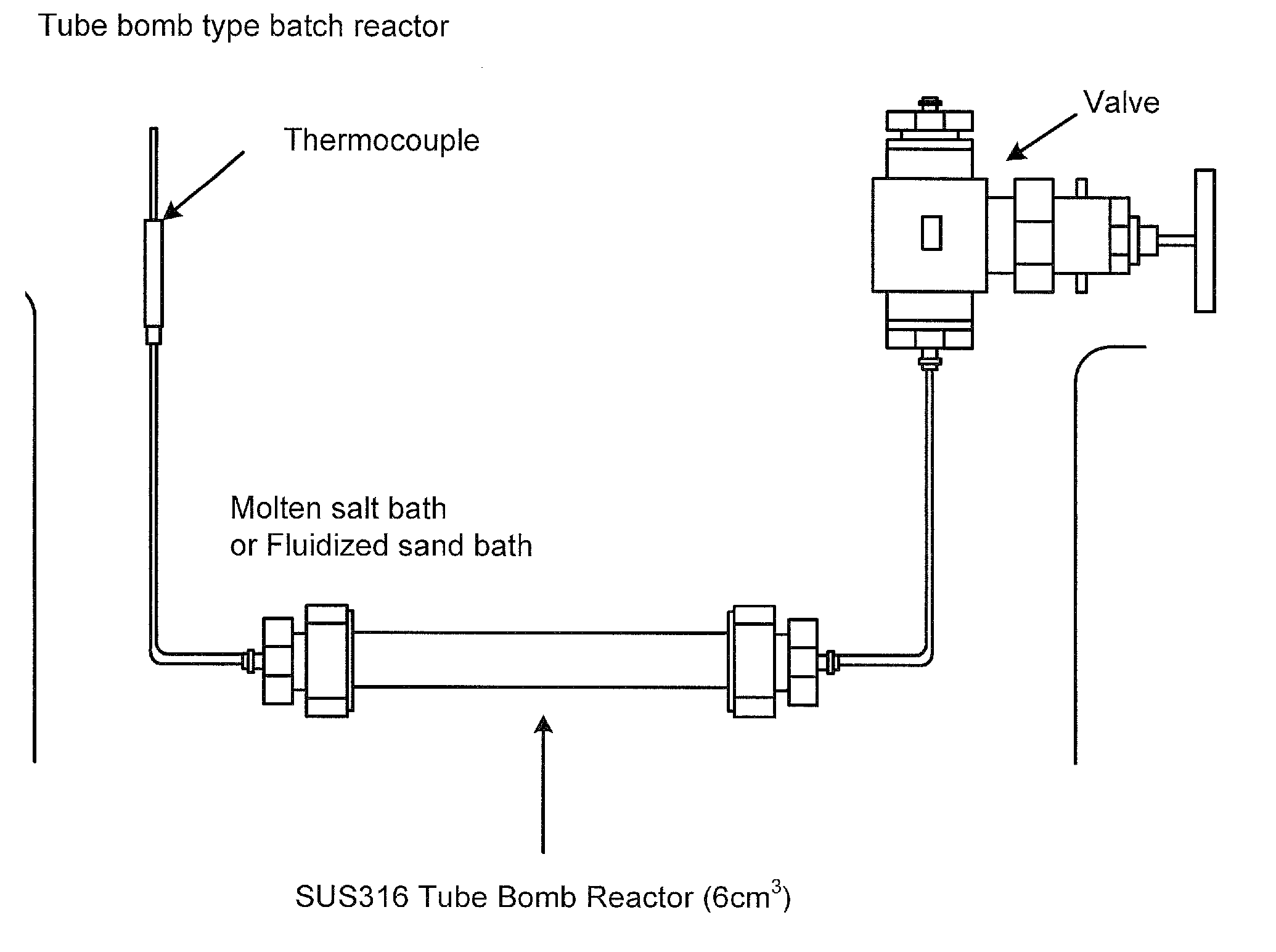
Preparation Method of Magnetic and Metal Oxide Nanoparticles
ActiveUS20080003159A1Efficient mass productionUniform shapeCopper oxides/halidesManganese oxides/hydroxidesMetal oxide nanoparticlesMagnetic oxide
This invention relates, in general, to a method of producing magnetic oxide nanoparticles or metal oxide nanoparticles and, more particularly, to a method of producing magnetic or metal oxide nanoparticles, which comprises (1) adding a magnetic or metal precursor to a surfactant or a solvent containing the surfactant to produce a mixed solution, (2) heating the mixed solution to 50-6001 C to decompose the magnetic or metal precursor by heating so as to form the magnetic or metal oxide nanoparticles, and (3) separating the magnetic or metal oxide nanoparticles. Since the method is achieved through a simple process without using an oxidizing agent or a reducing agent, it is possible to simply mass-produce uniform magnetic or metal oxide nanoparticles having desired sizes compared to the conventional method.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Biologically inspired synthesis of thin films and materials
InactiveUS20070254141A1Easy to adjustMaximize absorption efficiencyCell electrodesVacuum evaporation coatingVolumetric Mass DensityNanostructure
A method for the fabrication of nanostructured semiconducting, photoconductive, photovoltaic, optoelectronic and electrical battery thin films and materials at low temperature, with no molecular template and no organic contaminants. High-quality metal oxide semiconductor, photovoltaic and optoelectronic materials can be fabricated with nanometer-scale dimensions and high dopant densities through the use of low-temperature biologically inspired synthesis routes, without the use of any biological or biochemical templates.
Owner:RGT UNIV OF CALIFORNIA
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS7892514B2Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumHalogen
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
Organically modified fine particles
A technique for bonding an organic group with the surface of fine particles such as nanoparticles through strong linkage is provided, whereas such fine particles are attracting attention as materials essential for development of high-tech products because of various unique excellent characteristics and functions thereof. Organically modified metal oxide fine particles can be obtained by adapting high-temperature, high-pressure water as a reaction field to bond an organic matter with the surface of metal oxide fine particles through strong linkage. The use of the same condition enables not only the formation of metal oxide fine particles but also the organic modification of the formed fine particles. The resulting organically modified metal oxide fine particles exhibit excellent properties, characteristics and functions.
Owner:SUPER NANO DESIGN CO LTD
Method and system for capturing carbon dioxide in an oxyfiring process where oxygen is supplied by regenerable metal oxide sorbents
An oxyfiring system and method for capturing carbon dioxide in a combustion process is disclosed. The oxyfiring system comprises (a) an oxidation reactor for oxidizing a reduced metal oxide; (b) a decomposition reactor wherein a decomposition fuel is combusted and oxidized metal oxide sorbents are reduced with oxygen being released and a flue gas with an oxygen enriched carbon dioxide stream is produced; (c) a fuel combustion reactor for combusting a primary fuel and the oxygen enriched carbon dioxide stream into a primary flue gas; and (d) separation apparatus for separating a portion of the primary flue gas so that a carbon dioxide enriched stream can be prepared. The method comprises providing a primary fuel and an oxygen enriched carbon dioxide stream to a fuel combustion reactor. The primary fuel and oxygen enriched carbon dioxide stream are combusted into a primary flue gas stream which is split into a first flue gas portion and a second flue gas portion. The first flue gas portion is processed to produce a high purity carbon dioxide stream suitable for sequestration. The second flue gas portion is sent to the decomposition reactor to provide heat and serve as a fluid stream. The primary flue gas may include oxygen or else synthesis gas depending on whether the oxygen enriched carbon dioxide stream contains a stoichiometric excess or deficit of oxygen needed for complete combustion.
Owner:CHEVROU USA INC
Method for synthesis of colloidal nanoparticles
InactiveUS7575699B2Large-scale, safe, convenient, reproducible, and energy-efficient productionHigh crystallinityDielectric heatingNanoinformaticsColloidal nanoparticlesContinuous flow
A method for synthesis of high quality colloidal nanoparticles using comprises a high heating rate process. Irradiation of single mode, high power, microwave is a particularly well suited technique to realize high quality semiconductor nanoparticles. The use of microwave radiation effectively automates the synthesis, and more importantly, permits the use of a continuous flow microwave reactor for commercial preparation of the high quality colloidal nanoparticles.
Owner:RGT UNIV OF CALIFORNIA
Method of making oxide particles
The present invention provides a process for producing particles, such as oxide nanoparticles, in a substantially water-free environment. The process involves mixing at least one metal compound of the formula MX(m−n) with at least one surfactant and at least one solvent, wherein M is an electropositive element of Groups 1–15; each X is independently selected from the group consisting of O1 / 2, F, Cl, Br, I, OR, O2CR, NR2, and R; each R is independently a hydrocarbyl group; n is equal to ½ the oxidation state of the metal M in the product particle; and m is equal to the oxidation state of the element M. The components are typically combined to form a mixture which is thermally treated for a time period sufficient to convert the metal compound into particles of the corresponding oxide, having sizes in a range between about 0.5 nanometer and about 1000 nanometers. Examples of metal compounds employed in this process include materials such as Si(OR)4, Ti(OR)4 (where R is as described above), (Zr(OiPr)2)(OAc)2, and the like. Illustrative oxide materials which can be prepared by this process include TiO2, ZrO2, SiO2, and B2O3.
Owner:GENERAL ELECTRIC CO
Bioprocesses enhanced by magnetic nanoparticles
One aspect of the present invention relates to magnetic nanoparticles colloidally stabilized in aqueous milieu by association with an organic phase. The organic phase may be either a fluorinated polymer or an organic hydrocarbon bilayer, wherein the two layers are chemically bonded to each other. The stabilized particles are further non-toxic and provide useful enhancements in bioprocesses. Another aspect of the present invention relates to compositions comprising an oxygen-dissolving fluid vehicle and surface modified, nanometer-sized magnetic particles. The inventive compositions have utility in a wide range of applications, but are particularly suitable for use as recyclable oxygen carriers, separation and purification vehicles, and bioprocessing media, including fermentation processes.
Owner:MASSACHUSETTS INST OF TECH
Method of making NiO and Ni nanostructures
ActiveUS20080019901A1Restore charge neutralityObvious advantagesMaterial nanotechnologyHydrogenFiberGlycol synthesis
The alpha form of nickel (II) hydroxide is formed by dissolving a compound of nickel (II), such as nickel acetate, in a water miscible dihydric alcohol (diol), such as ethylene glycol, propylene glycol and suitable oligomers, and adding a suitable base such as sodium carbonate. The α-Ni(OH)2 precipitate is separated from the diol-based mother liquor and dried. This stable α-Ni(OH)2 can be calcined at temperatures in the range of about 573K to about 1073K to form nanometer-size particles of NiO having, for example, fibrous shapes. And the small particles of NiO can be reduced with hydrogen to form small, fibrous nickel particles. Both the NiO particles and Ni particles have utility as catalysts and offer utility in applications requiring electronic and / or magnetic properties.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Production of fine-grained particles
InactiveCN1476413AImprove physical propertiesMaterial nanotechnologyNanostructure manufactureActive agentPhysical chemistry
Particles of mixed metal oxide include at least two metal species. The particles have a grain size within the range of 1-100 nm. The particles are substantially crystalline. The particles contain only small or negligible amounts of amorphous material. The at least two metal species are uniformly dispersed in the particles.
Owner:VERY SMALL PARTICLE CO LTD
Process for producing micro-mesoporous metal oxide having regulated pores formed by novel template removal method
InactiveUS7223377B2Copper oxides/halidesManganese oxides/hydroxidesPolymeric surfaceAlkaline earth metal
The present invention is the method for preparation of transition metal oxide having micro-mesoporous structure whose average fine pores size is not less than 1 nm and not more than 2 nm comprising, adding and dissolving transition metal salt which is a precursor of transition metal oxide and / or metal alkoxide in the solution prepared by dissolving polymer surfactant in organic solvent, hydrolyzing said transition metal salt and / or metal alkoxide and preparing sol solution which is polymerized and self organized, then obtaining gel whose organization is stabilized from said sol solution and removing said polymer surfactant by using water of room temperature or water to which alkali metal or alkaline earth metal ion is added.
Owner:JAPAN SCI & TECH CORP
Nickel electrode material,and production method therefor, and nickel electrode and alkaline battery
ActiveUS20040241545A1Improved cycle life characteristicsHigh degreeElectrochemical processing of electrodesLayered productsInternal pressureNickel oxide hydroxide
Subjects for the invention are to provide a nickel electrode material having a satisfactory tap density and capable of attaining a sufficient reduction in discharge reserve and a process for producing the nickel electrode material, and to provide a nickel electrode and an alkaline storage battery having a high capacity and excellent internal-pressure characteristics. For accomplishing the subjects, the invention provides a nickel electrode material for use in a nickel electrode, wherein the positive-electrode material constituting the electrode material comprises: positive active material particles which comprise as the main component either a nickel hydroxide or a solid solution in a nickel hydroxide of one or more other elements and in which part of the nickel hydroxide has been oxidized; and a coating layer formed on the surface of the positive active material particles and comprising as the main component a high-order cobalt compound in which the cobalt has an oxidation number larger than 2, the average oxidation number of the nickel in the positive active material particles and the cobalt in the coating layer being from 2.04 to 2.40, and the positive-electrode material having a tap density of 2.0 g / cm<3 >or higher. The invention further provides a process for producing the electrode material, a nickel electrode employing the electrode material, and an alkaline storage battery having the nickel electrode.
Owner:GS YUASA INT LTD
Clean method for preparing layered double hydroxides
ActiveUS20080170978A1Efficient responseProtect environmentLithium compoundsManganese oxides/hydroxidesFiltrationCleaning methods
Disclosed is a clean method for preparing layered double hydroxides (LDHs), in which hydroxides of different metals are used as starting materials for production of LDHs by atom-economical reactions. The atom efficiency of the reaction is 100% in each case because all the atoms of the reactants are converted into the target product since only M2+(OH)2, M3+(OH)3, and CO2 or HnAn− are used, without any NaOH or other materials. Since there is no by-product, filtration or washing process is unnecessary. The consequent reduction in water consumption is also beneficial to the environment.
Owner:BEIJING UNIV OF CHEM TECH
Active material for rechargeable batteries
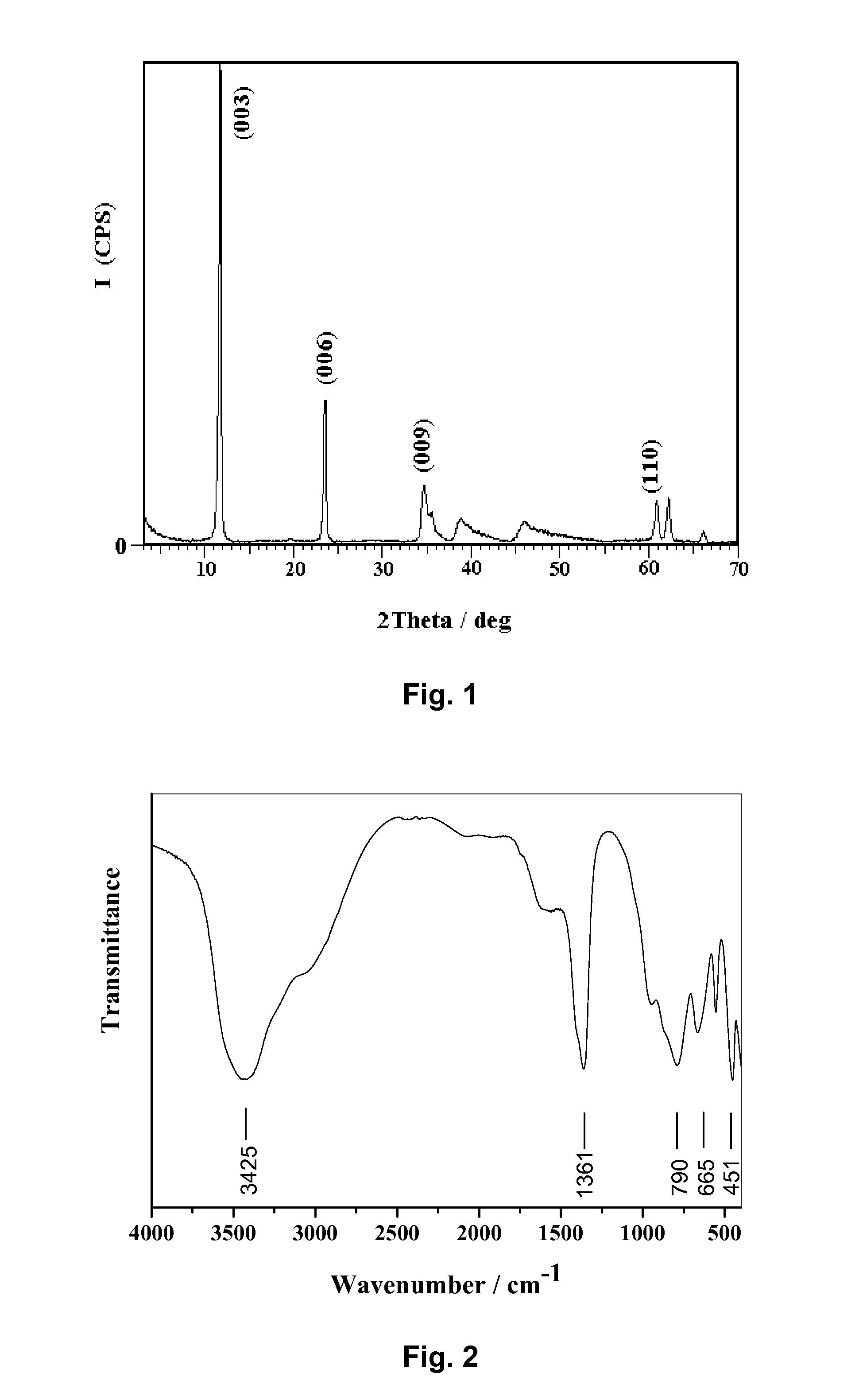
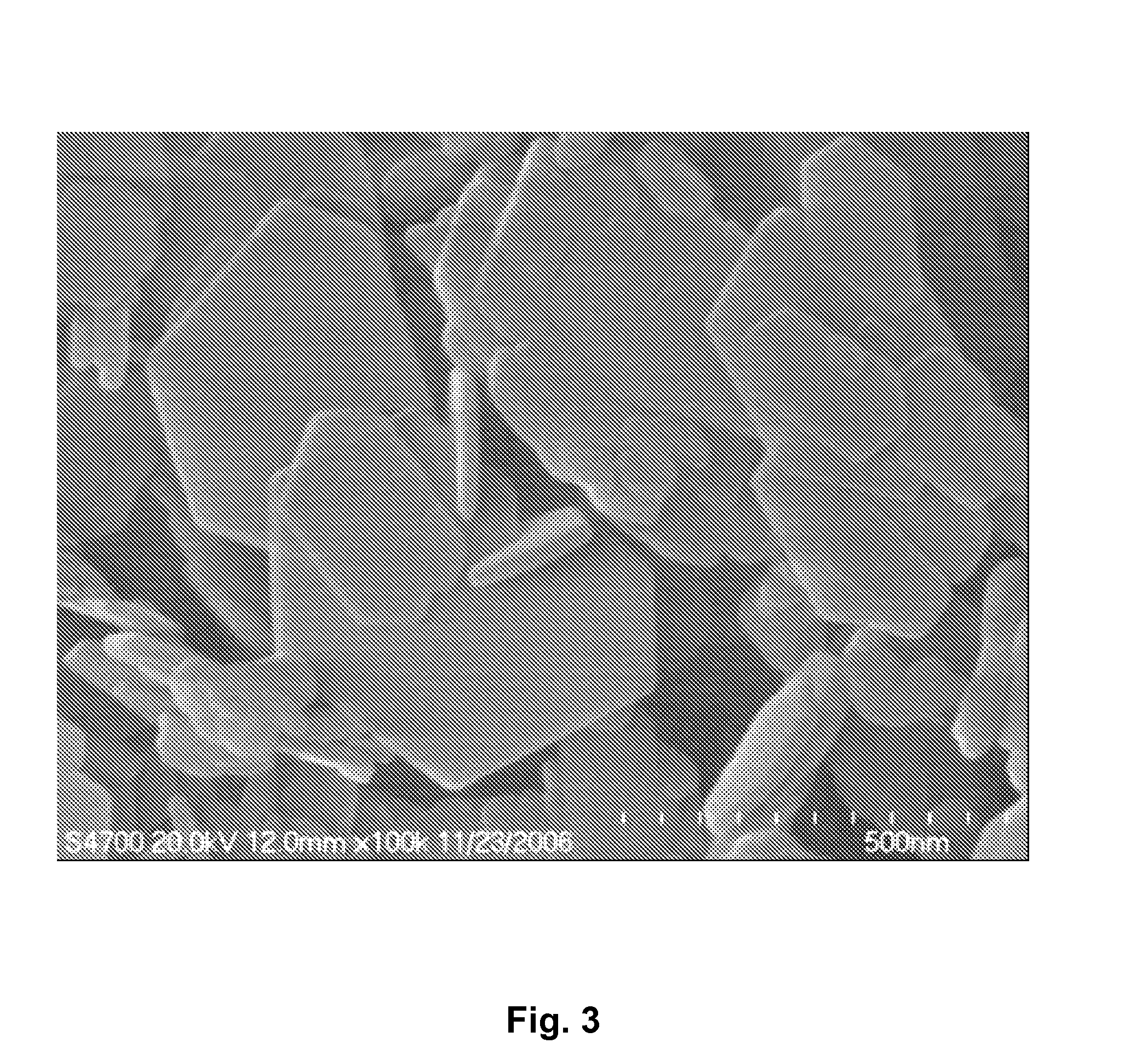
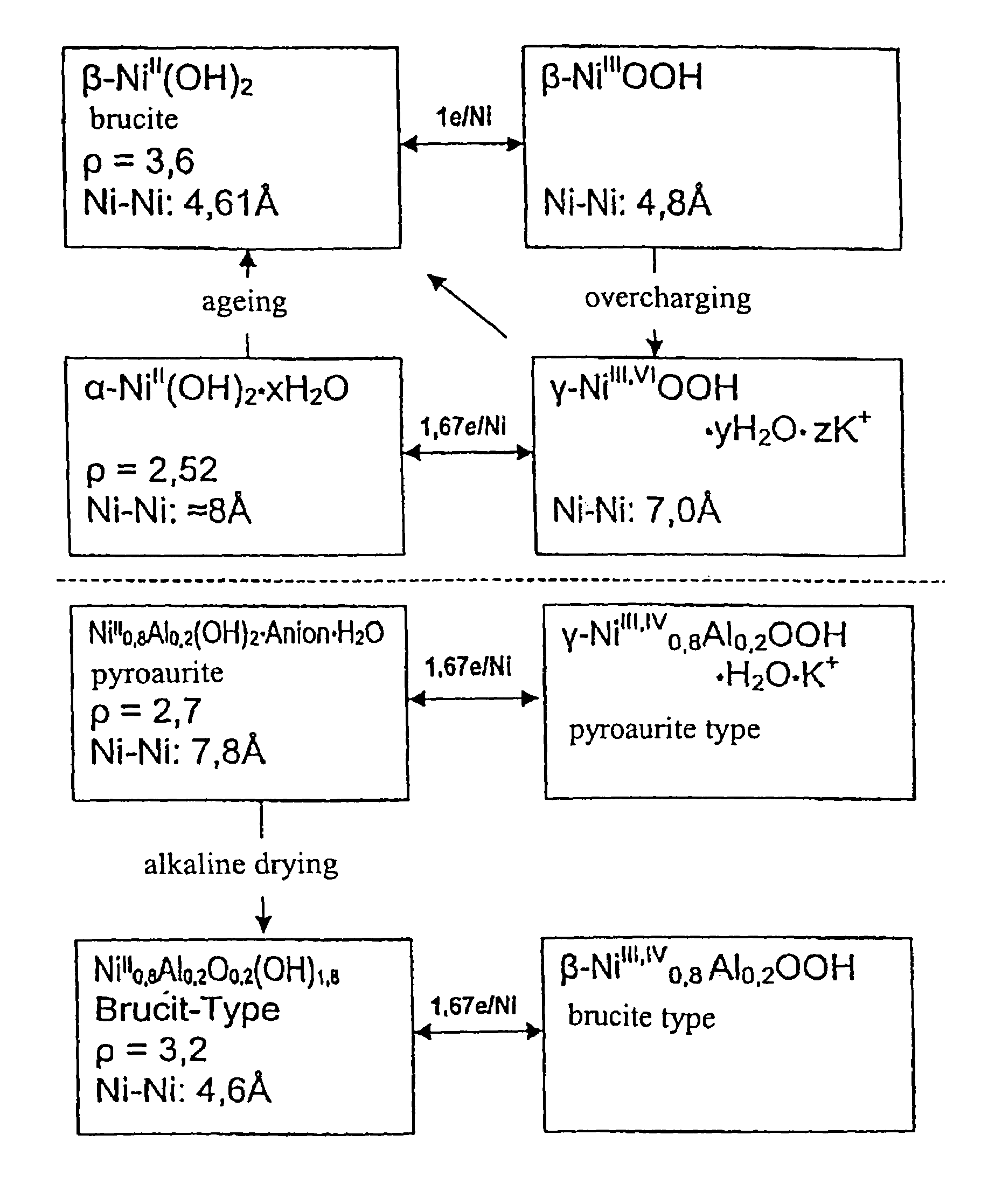
InactiveUS6958139B1Reduce spacingImprove storage densityNickel carbonylsAlkaline accumulator electrodesElectrical batteryNickel oxide hydroxide
A mixed metal hydroxide with brucite structure is described, which contains nickel hydroxide as its main component and at least one trivalent metal selected from the group consisting of Co, Fe, Al, Ga, In, Sc, Y and La in an amount of from about 12 to about 30 atom % relative to the sum of the metal components including Ni. The invention also relates to a rechargeable battery containing a mixed metal hydroxide according to the invention as an electrochemically active material as well as a secondary batter containing the mixed metal hydroxide. The invention also relates to a process for producing a mixed metal hydroxide having a brucite structure.
Owner:TODA IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com