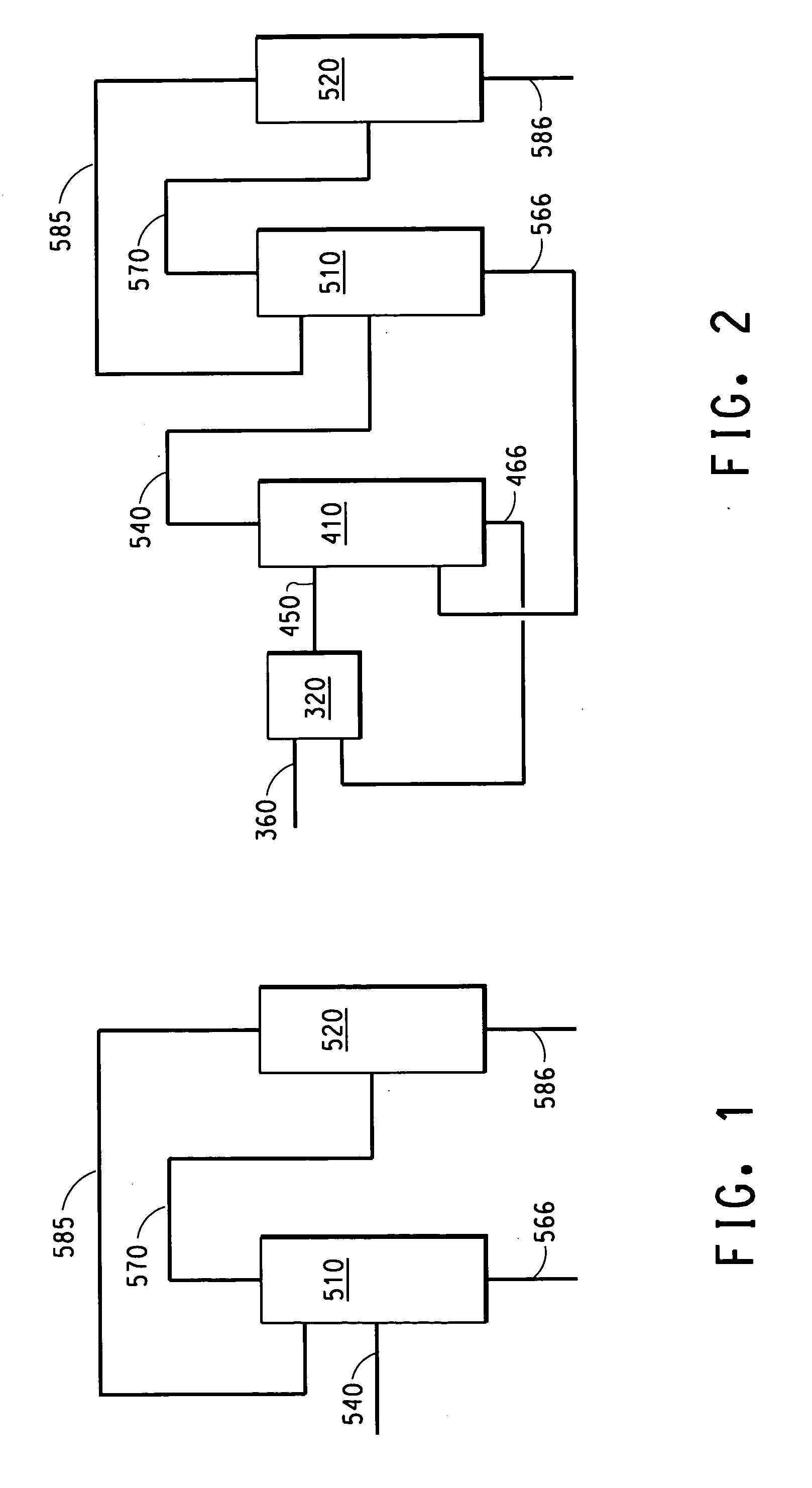
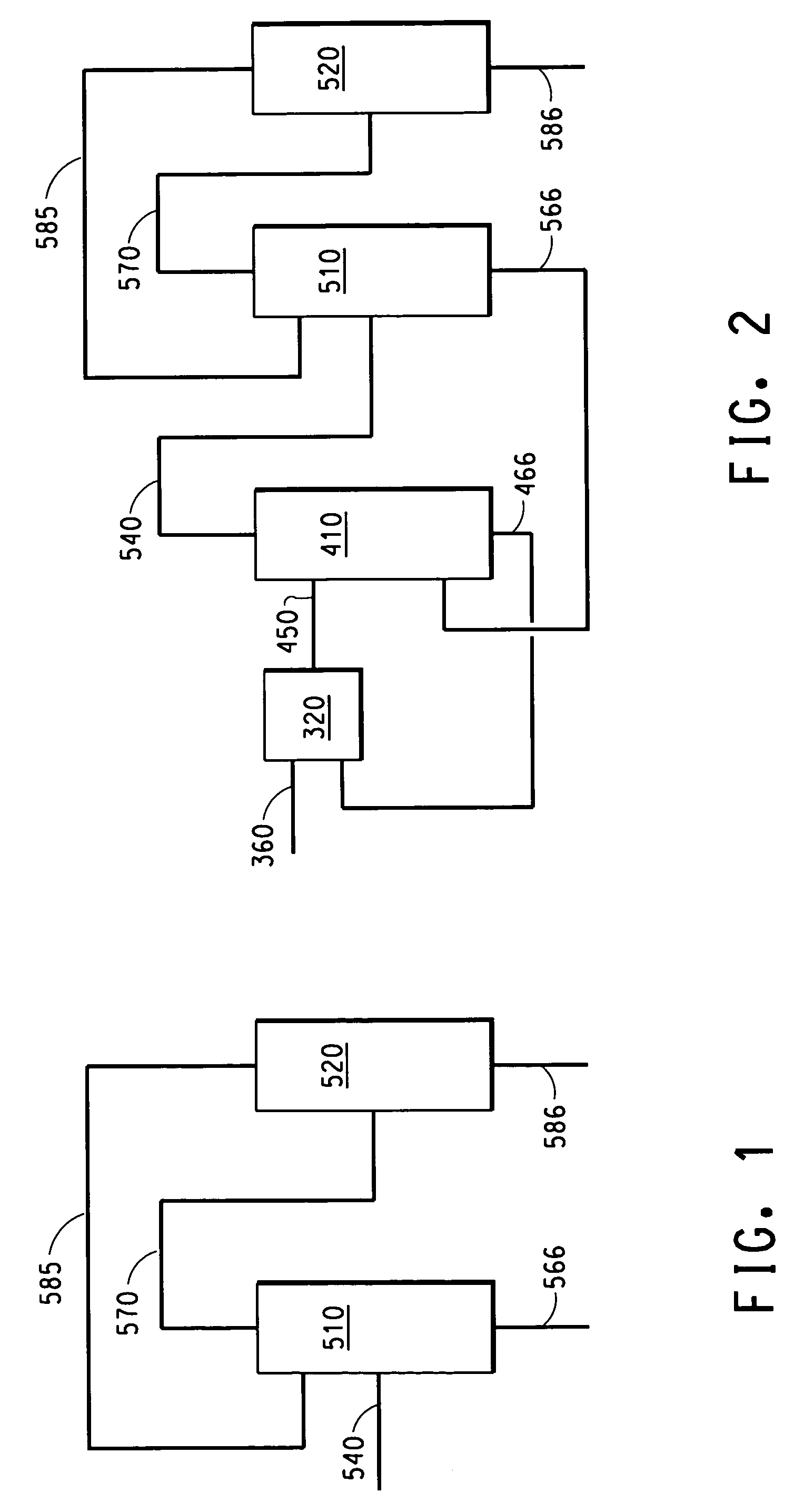
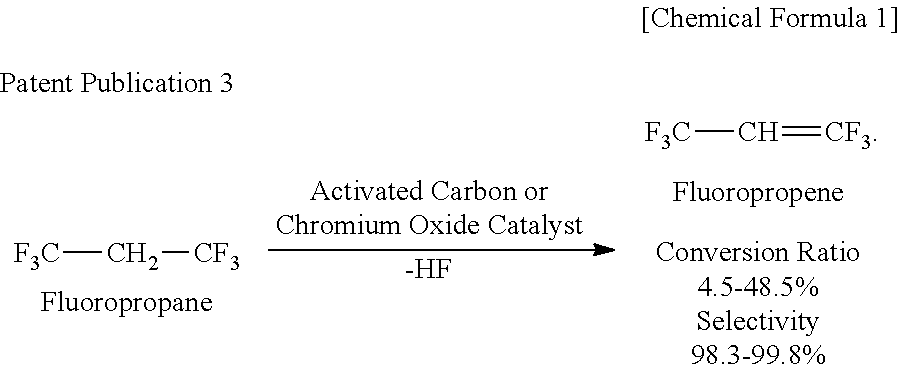
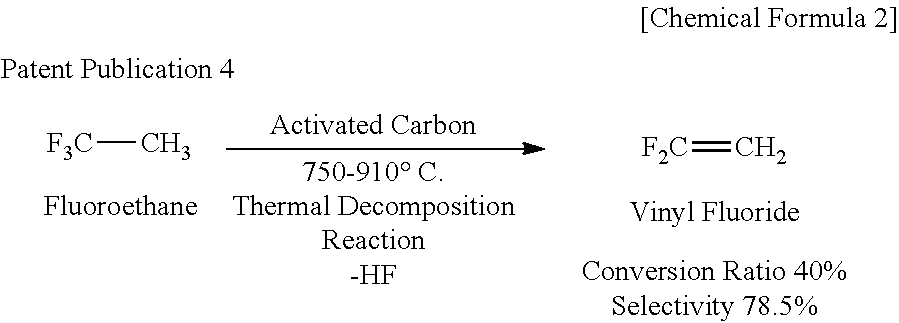
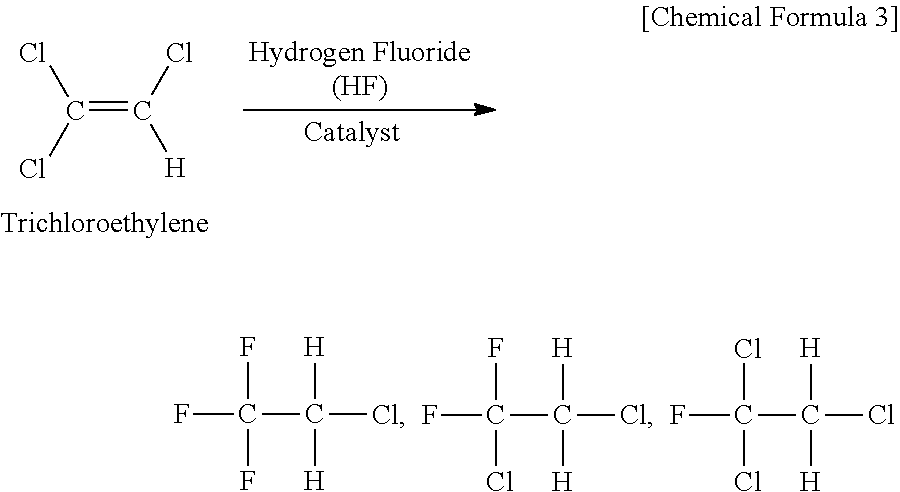
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
528results about "Hydrogen fluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
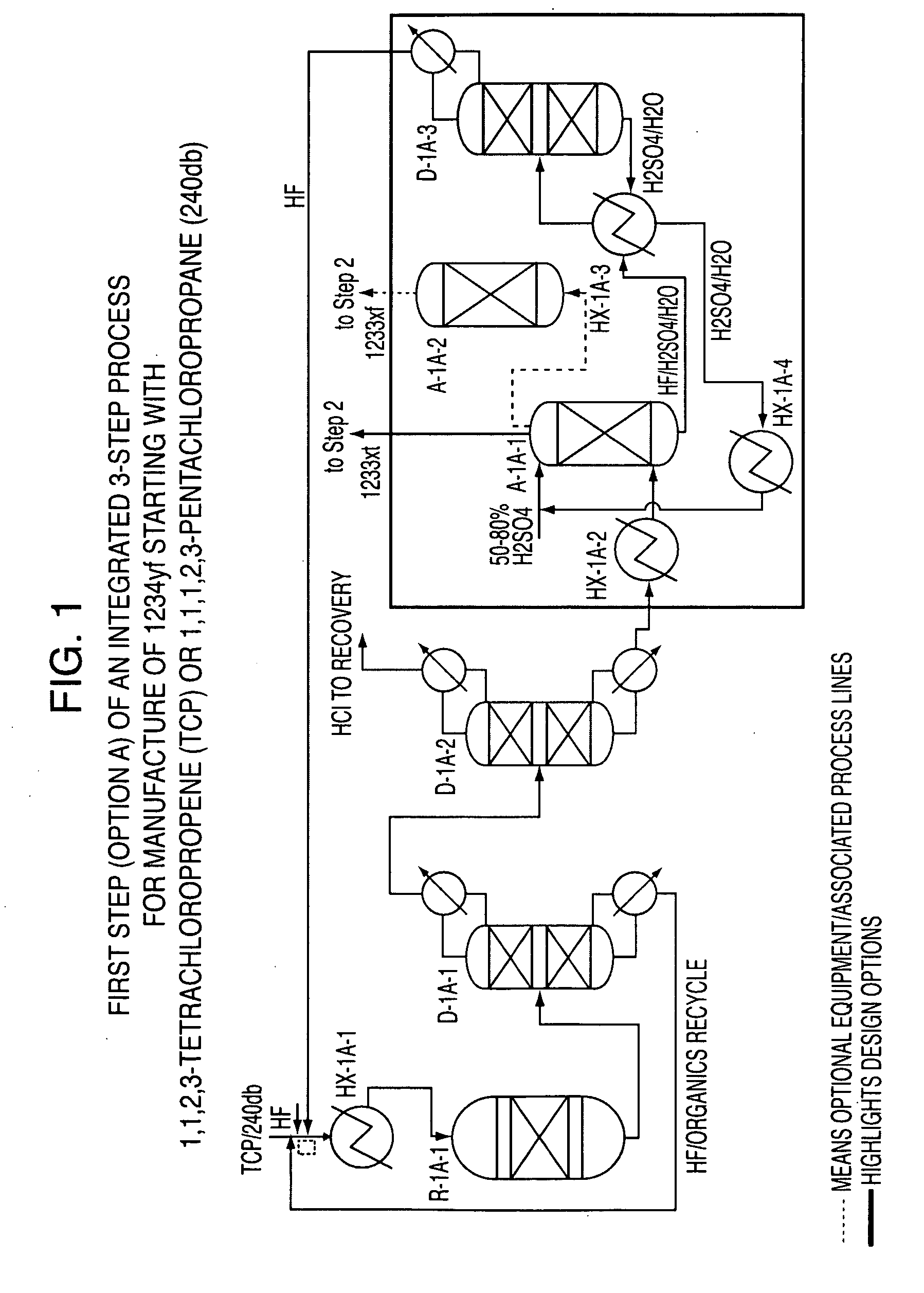
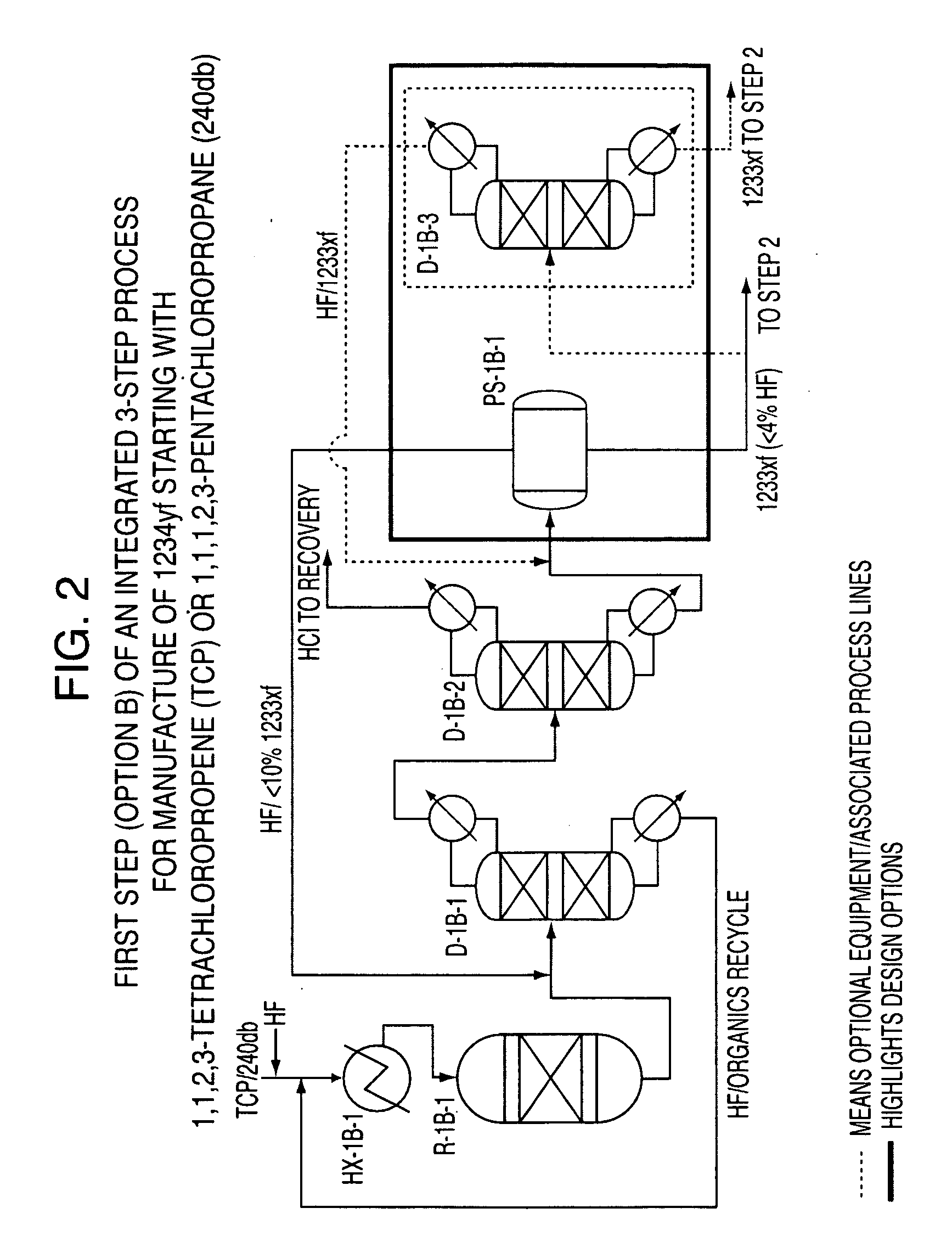
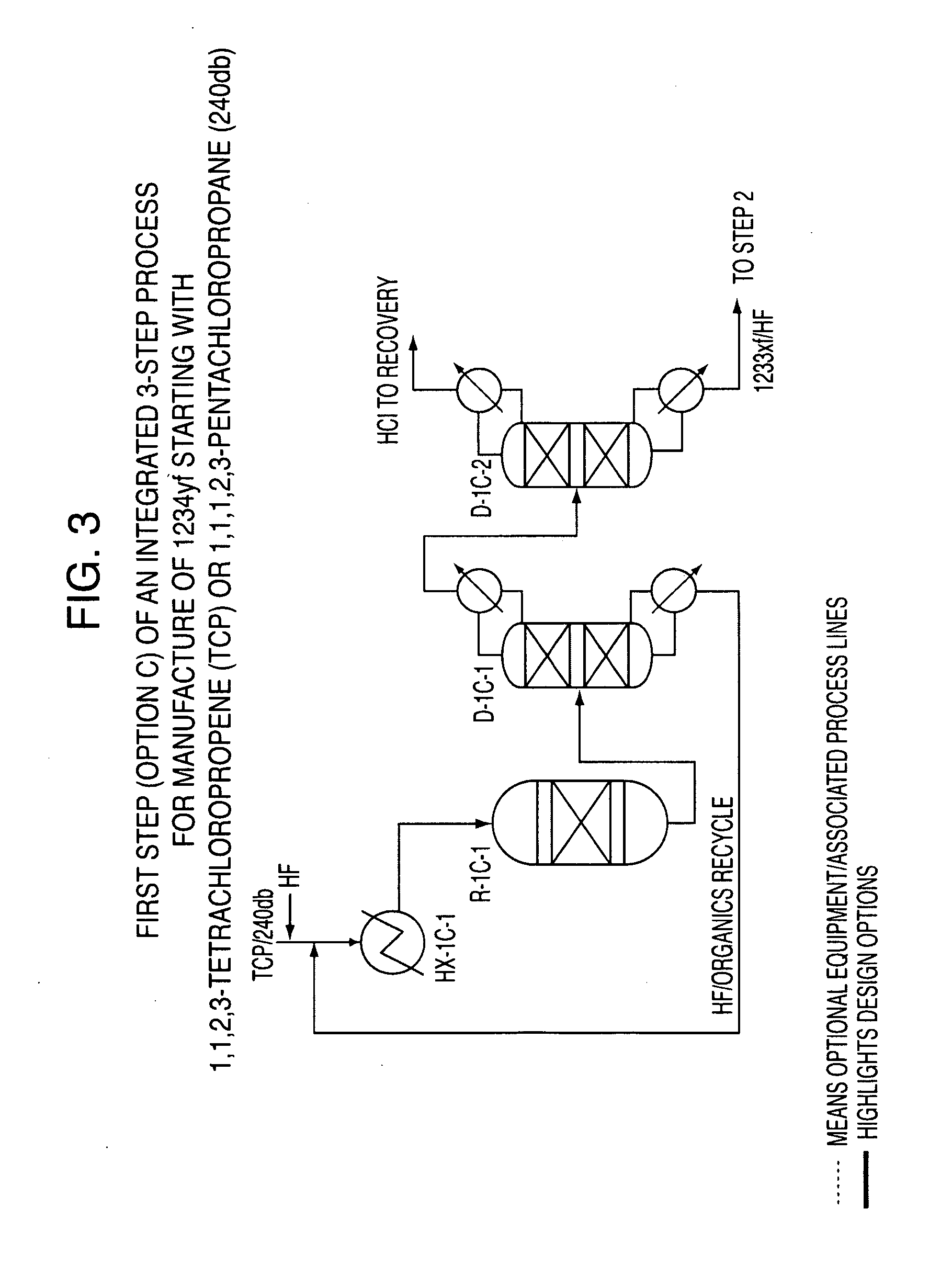
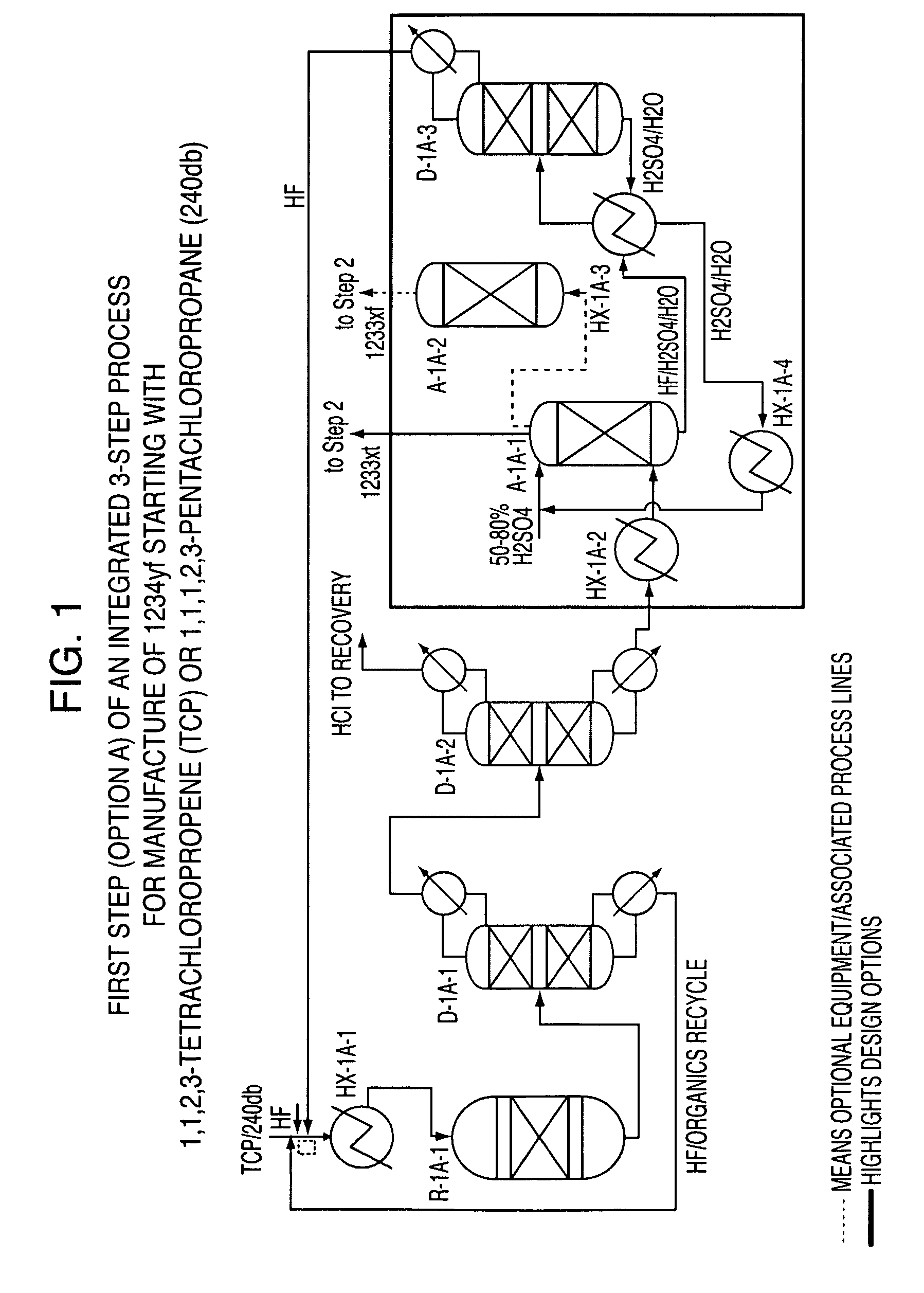
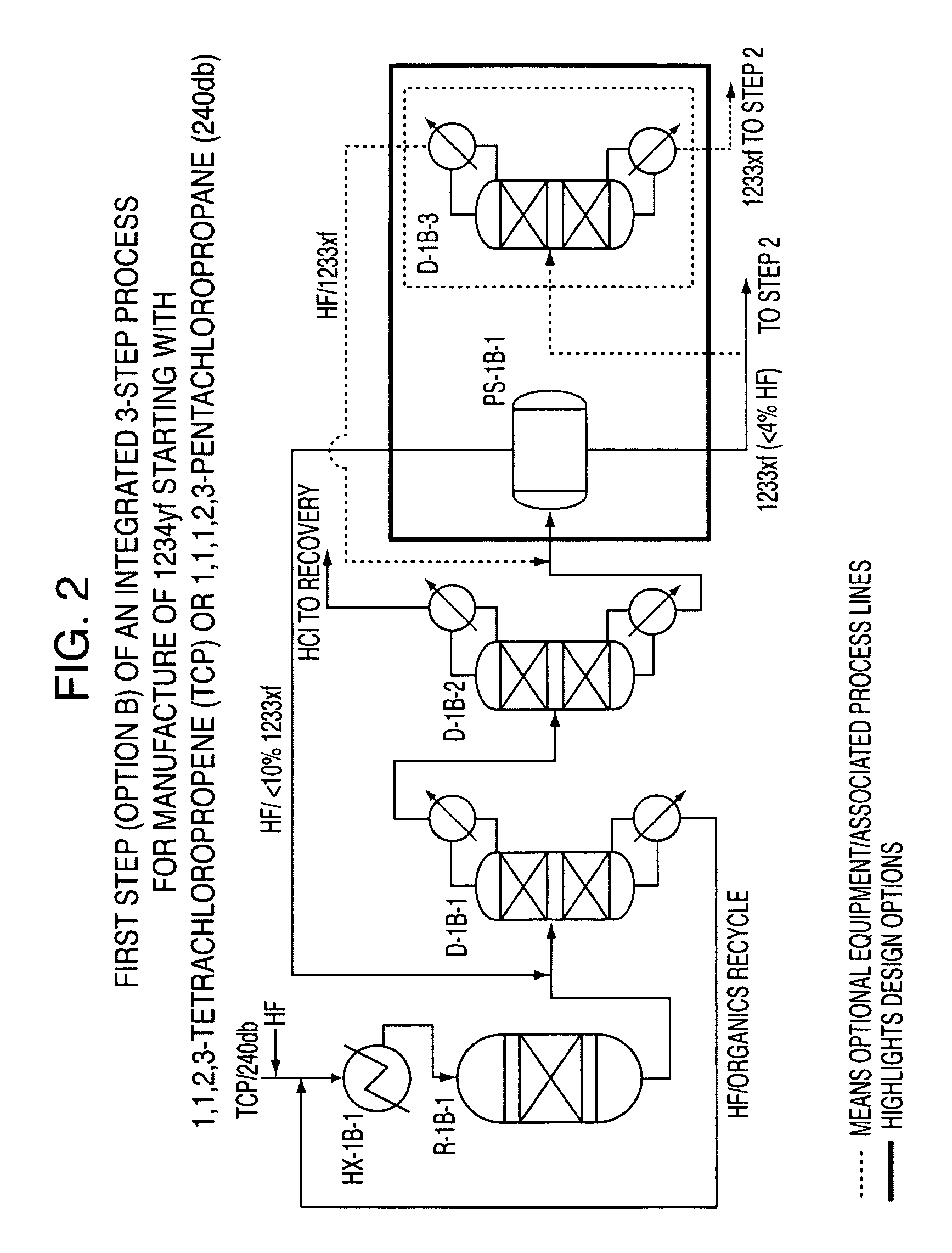
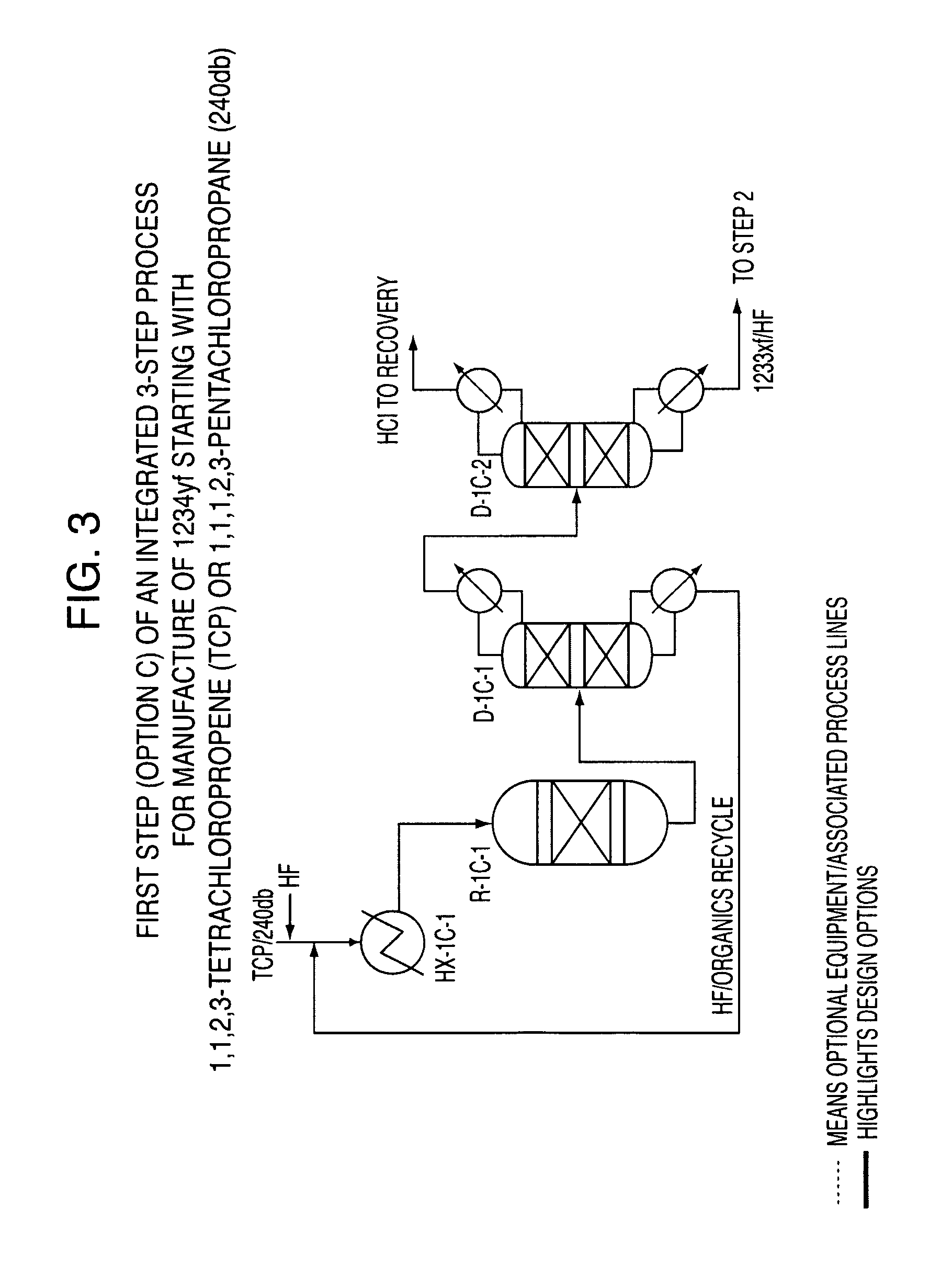
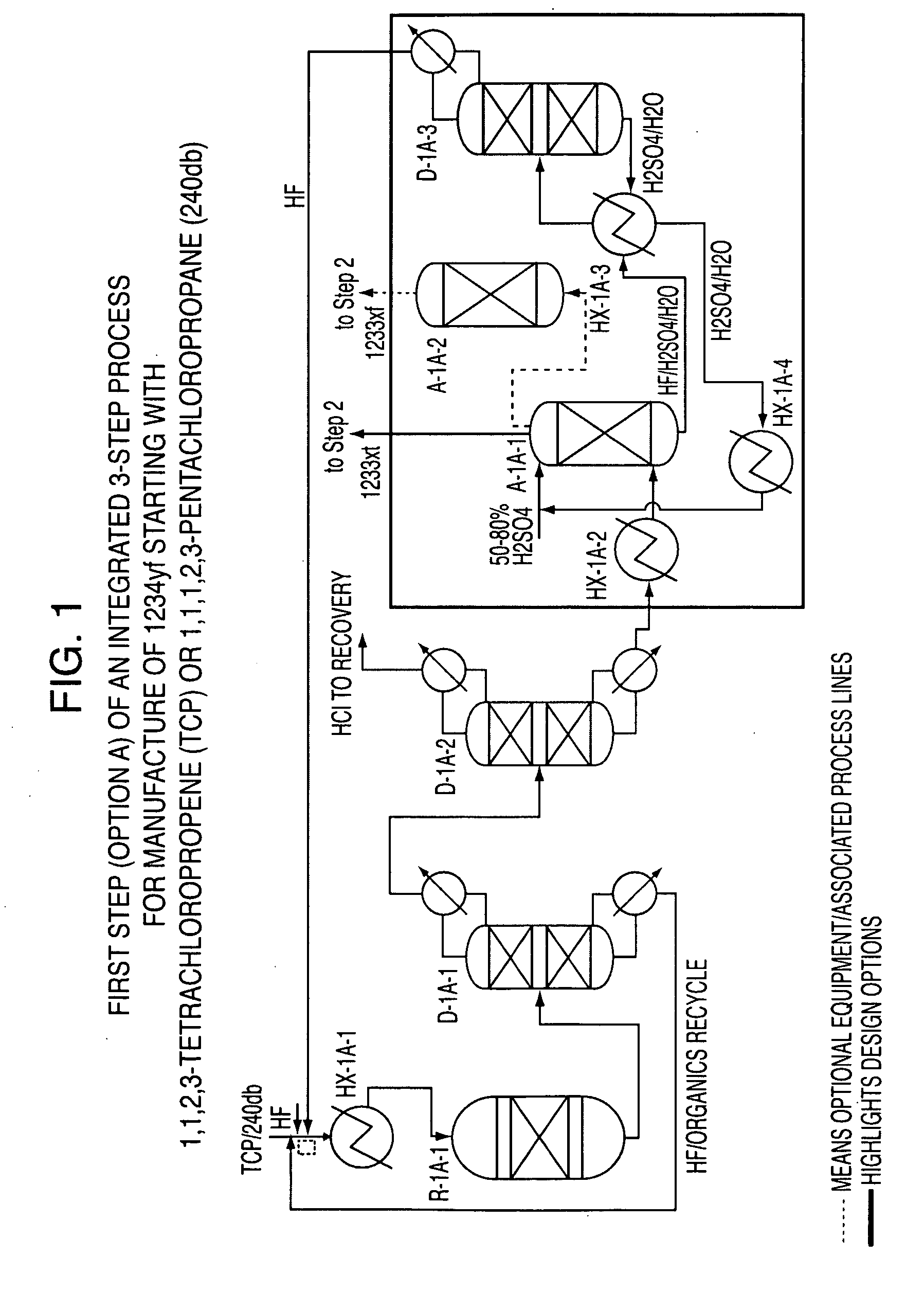
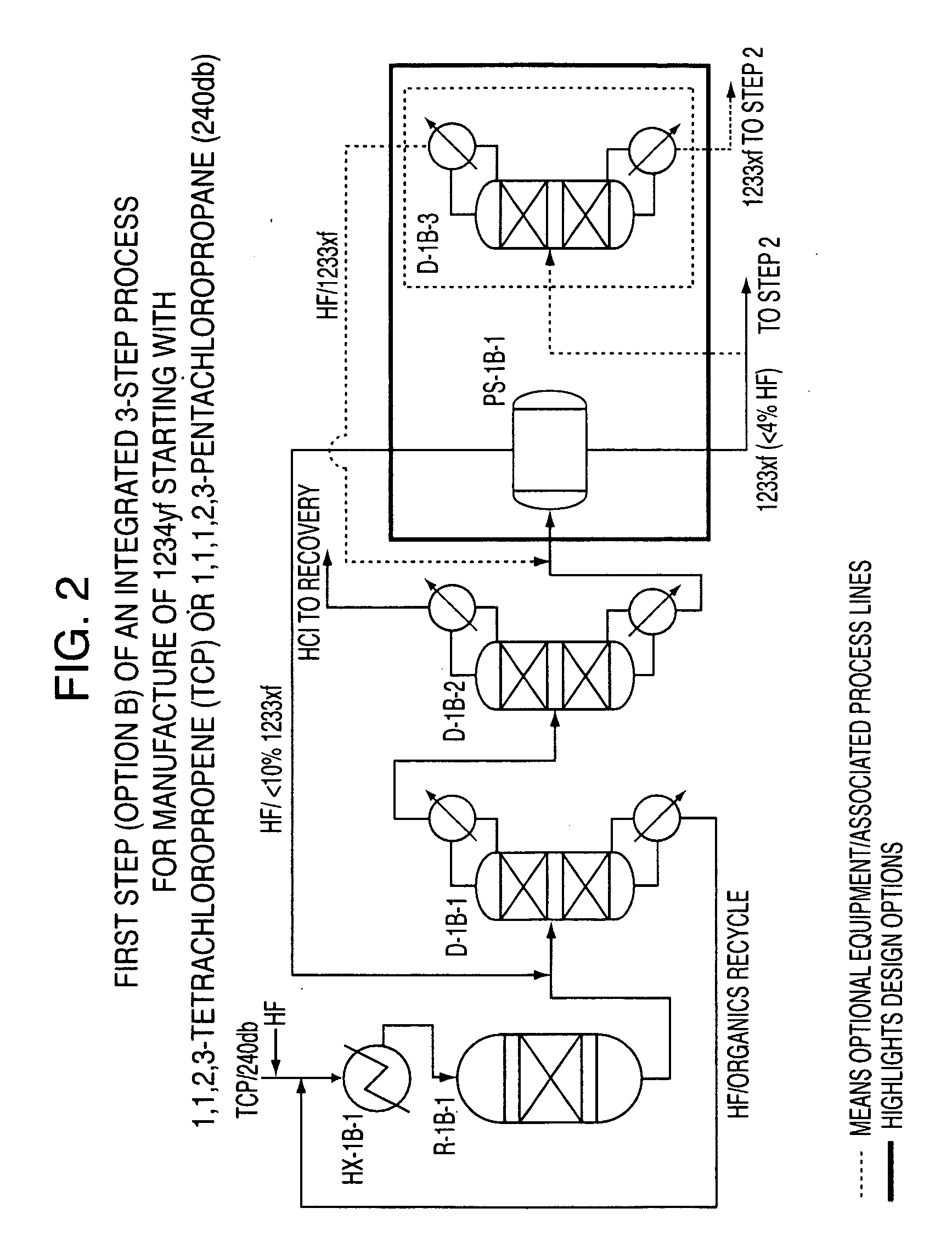
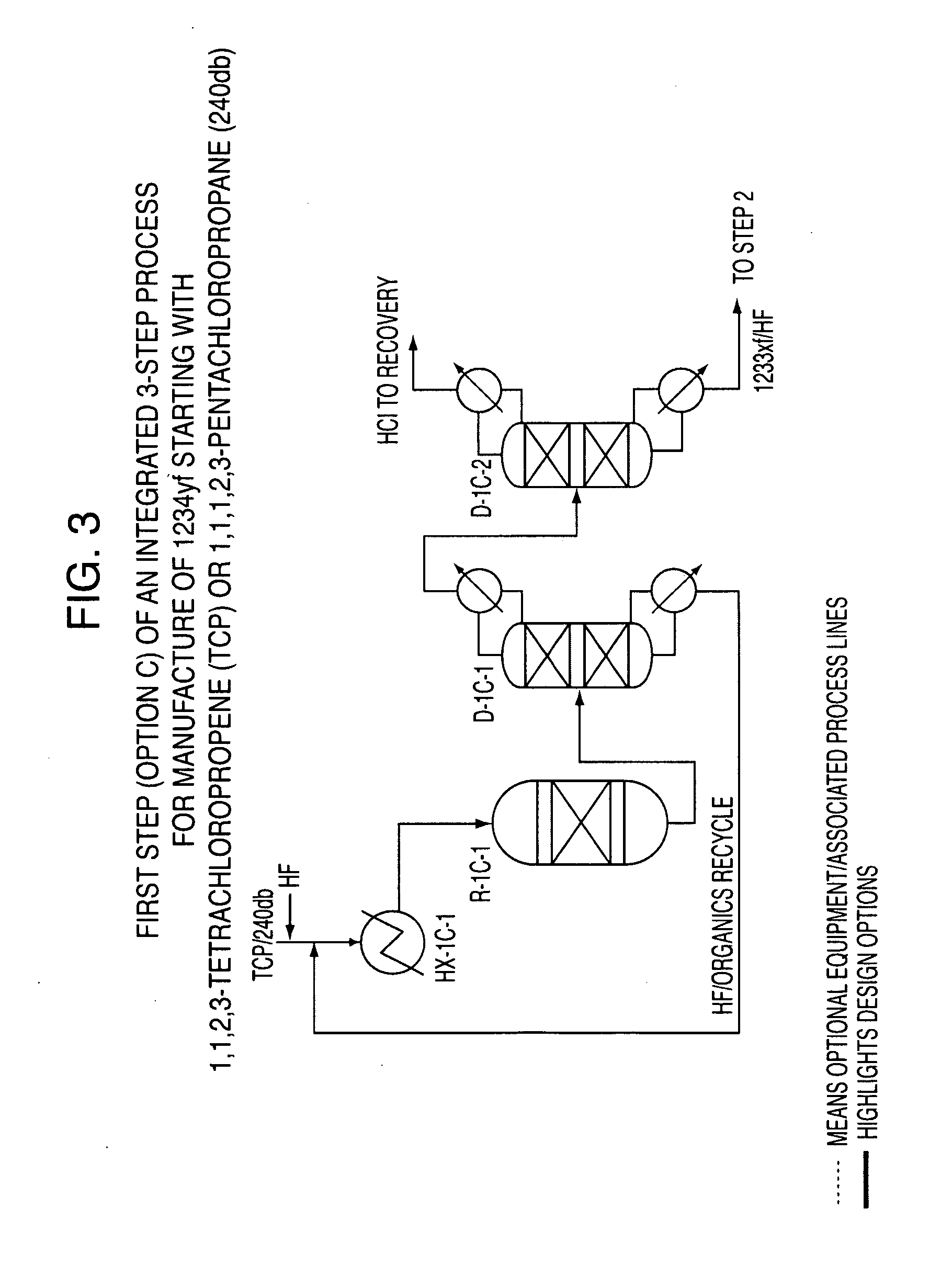
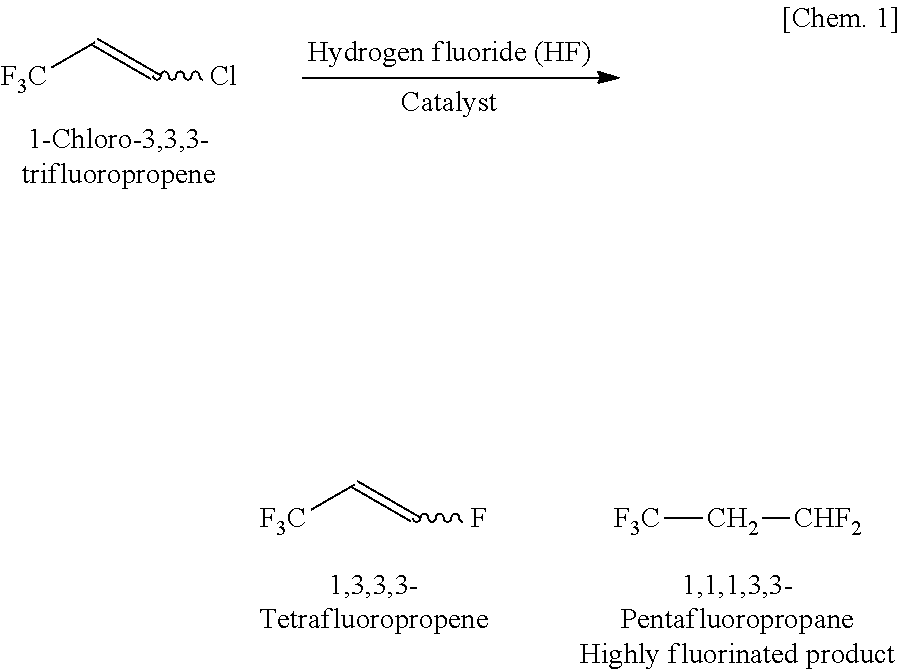
Integrated process to produce 2,3,3,3-tetrafluoropropene
ActiveUS20090240090A1Maximize raw material utilizationMaximize product yieldPreparation by hydrogen halide split-offPreparation by halogen halide additionChromium2,3,3,3-Tetrafluoropropene
A method for preparing 2,3,3,3-tetrafluoroprop-1-ene comprising (a) providing a starting composition comprising at least one compound having a structure selected from Formulae I, II and III:CX2═CCl—CH2X (Formula I)CX3—CCl═CH2 (Formula II)CX3—CHCl—CH2X (Formula III)wherein X is independently selected from F, Cl, Br, and I, provided that at least one X is not fluorine;(b) contacting said starting composition with a first fluorinating agent to produce a first intermediate composition comprising 2-chloro-3,3,3-trifluoropropene and a first chlorine-containing byproduct; (c) contacting said first intermediate composition with a second fluorinating agent to produce a second intermediate composition comprising 2-chloro-1,1,1,2-tetrafluoropropane and a second chlorine-containing byproduct; and (d) catalytically dehydrochlorinating at least a portion of said 2-chloro-1,1,1,2-tetrafluoropropane to produce a reaction product comprising 2,3,3,3-tetrafluoroprop-1-ene.
Owner:HONEYWELL INT INC
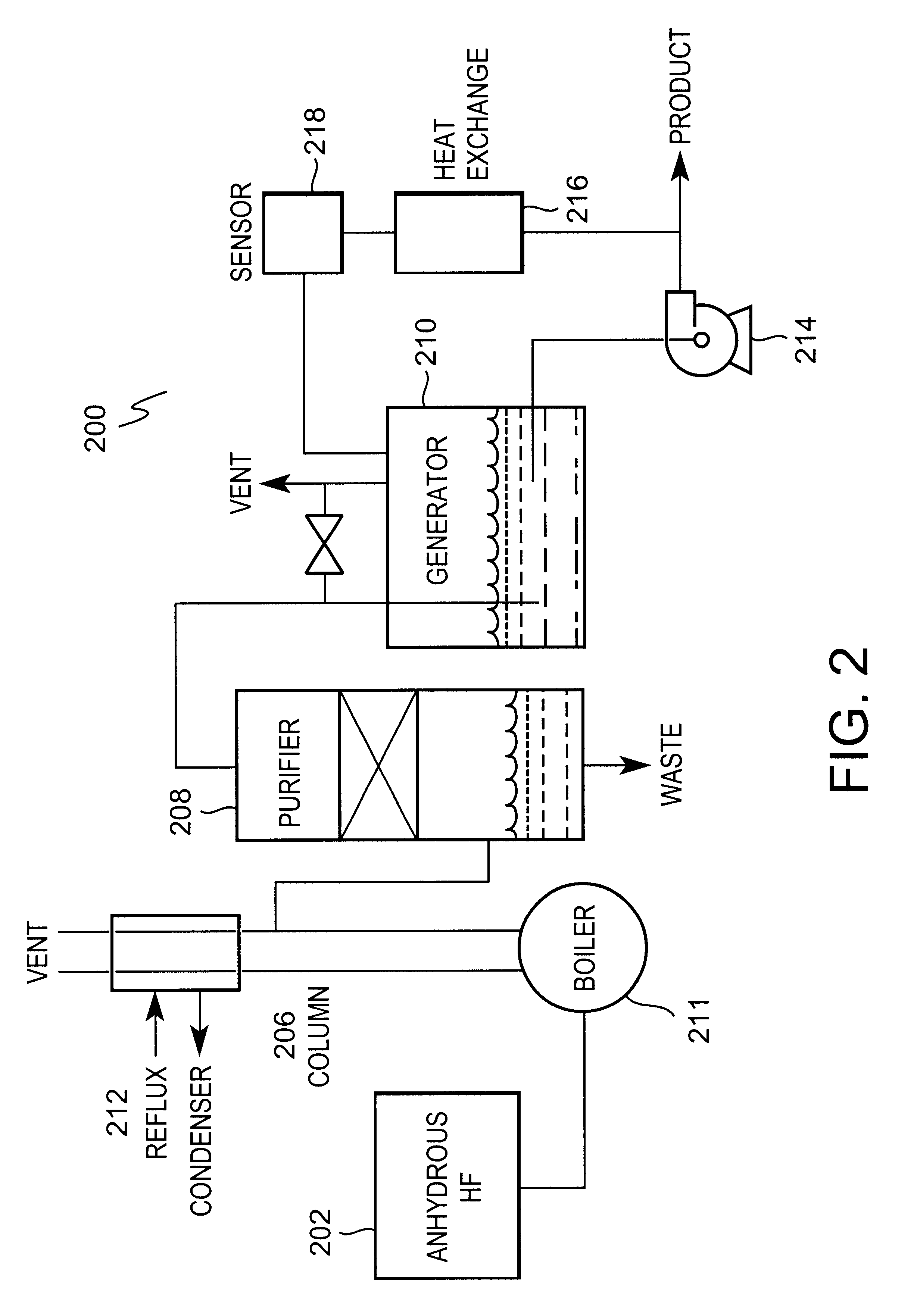
Processes for production and purification of hydrofluoroolefins
ActiveUS20060106263A1High selectivityPreparation by hydrogen halide split-offHydrogen fluorideHydrogen fluoridePurification methods
Owner:THE CHEMOURS CO FC LLC
Integrated process to produce 2,3,3,3-tetrafluoropropene
ActiveUS8058486B2Maximize utilizationYield maximizationPreparation by hydrogen halide split-offPreparation by halogen halide additionChromium2,3,3,3-Tetrafluoropropene
A method for preparing 2,3,3,3-tetrafluoroprop-1-ene comprising (a) providing a starting composition comprising at least one compound having a structure selected from Formulae I, II and III:CX2═CCl—CH2X (Formula I)CX3—CCl═CH2 (Formula II)CX3—CHCl—CH2X (Formula III)wherein X is independently selected from F, Cl, Br, and I, provided that at least one X is not fluorine;(b) contacting said starting composition with a first fluorinating agent to produce a first intermediate composition comprising 2-chloro-3,3,3-trifluoropropene and a first chlorine-containing byproduct; (c) contacting said first intermediate composition with a second fluorinating agent to produce a second intermediate composition comprising 2-chloro-1,1,1,2-tetrafluoropropane and a second chlorine-containing byproduct; and (d) catalytically dehydrochlorinating at least a portion of said 2-chloro-1,1,1,2-tetrafluoropropane to produce a reaction product comprising 2,3,3,3-tetrafluoroprop-1-ene.
Owner:HONEYWELL INT INC
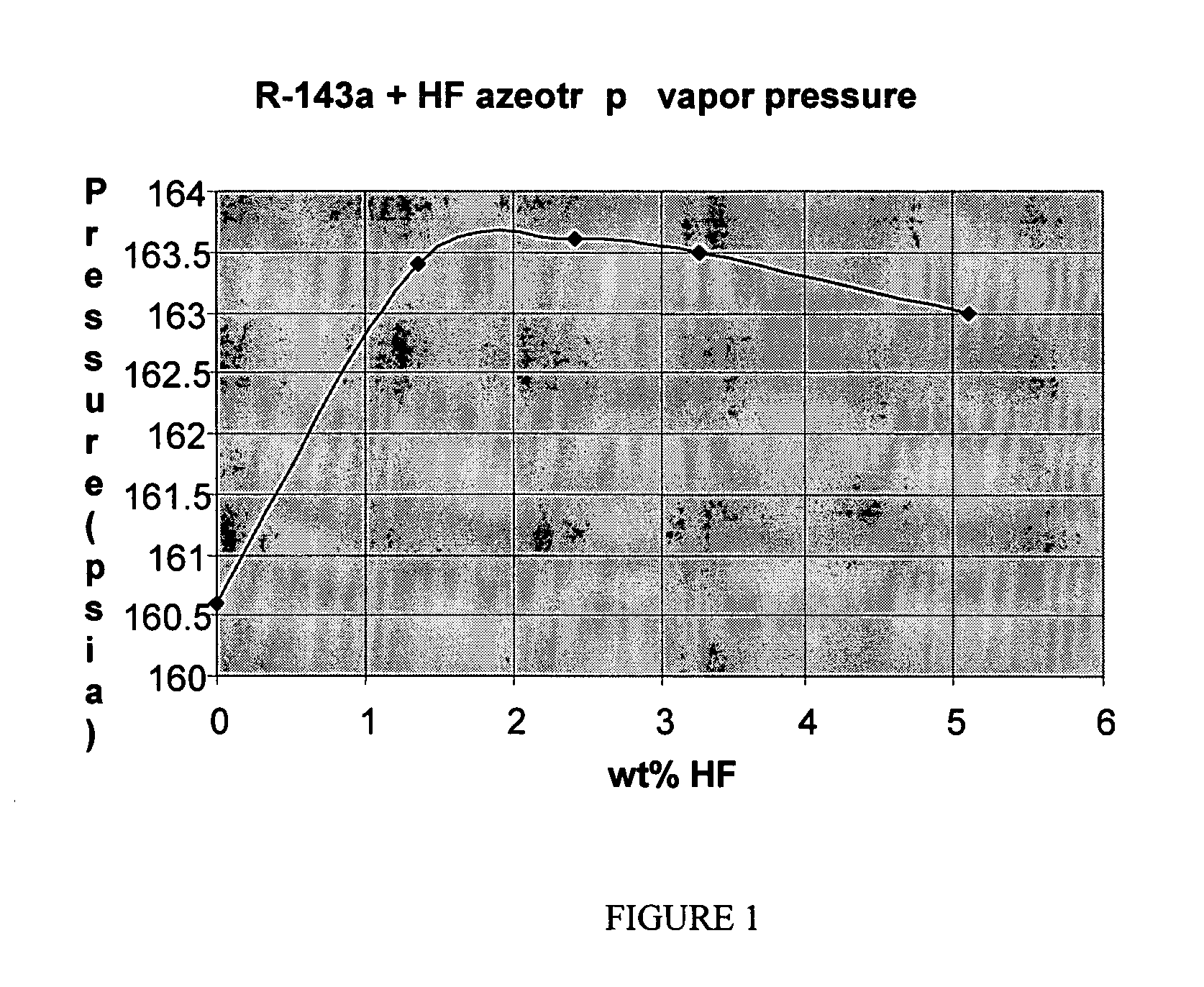
Azeotrope compositions comprising 2,3,3,3-tetrafluoropropene and hydrogen fluoride and uses thereof
ActiveUS20070100175A1Preparation by hydrogen halide split-offHydrogen fluorideHydrogen fluoridePhysical chemistry
Owner:THE CHEMOURS CO FC LLC
Azeotrope compositions comprising E-1,3,3,3-tetrafluoropropene and hydrogen fluoride and uses thereof
ActiveUS20070100173A1Preparation by hydrogen halide split-offHydrogen fluorideHydrogen fluoride1,3,3,3-Tetrafluoropropene
Disclosed herein are azeotrope and near-azeotrope compositions comprising E-1,3,3,3-tetrafluoropropene and hydrogen fluoride. These azeotrope and near-azeotrope compositions are useful in processes to produce E-1,3,3,3-tetrafluoropropene and in processes to purify E-1,3,3,3-tetrafluoropropene from mixtures of E-1,3,3,3-tetrafluoropropene with 1,1,1,3,3-pentafluoropropane and / or with hydrogen fluoride.
Owner:THE CHEMOURS CO FC LLC
Integrated HFC trans-1234ze manufacture process
ActiveUS7485760B2Preparation by hydrogen halide split-offPreparation by halogen replacementHydrogen fluoride1,3,3,3-Tetrafluoropropene
An integrated process for the manufacture of HFO trans-1,3,3,3-tetrafluoropropene (HFO trans-1234ze) by first catalytically dehydrofluorinating 1,1,1,3,3-pentafluoropropane to thereby produce a mixture of cis-1,3,3,3-tetrafluoropropene, trans-1,3,3,3-tetrafluoropropene and hydrogen fluoride. Then optionally recovering hydrogen fluoride, catalytically isomerizing cis-1234ze into trans-1234ze, and recovering trans-1,3,3,3-tetrafluoropropene.
Owner:HONEYWELL INT INC
Azeotrope compositions comprising E-1,3,3,3-tetrafluoropropene and hydrogen fluoride and uses thereof
ActiveUS7423188B2Preparation by hydrogen halide split-offHydrogen fluorideHydrogen fluorideMedicinal chemistry
Disclosed herein are azeotrope and near-azeotrope compositions comprising E-1,3,3,3-tetrafluoropropene and hydrogen fluoride. These azeotrope and near-azeotrope compositions are useful in processes to produce E-1,3,3,3-tetrafluoropropene and in processes to purify E-1,3,3,3-tetrafluoropropene from mixtures of E-1,3,3,3-tetrafluoropropene with 1,1,1,3,3-pentafluoropropane and / or with hydrogen fluoride.
Owner:THE CHEMOURS CO FC LLC
Noncatalytic manufacture of 1,1,3,3,3-pentafluoropropene from 1,1,1,3,3,3-hexafluoropropane
InactiveUS20060094911A1High selectivityPreparation by hydrogen halide split-offHydrogen fluorideHydrogen fluorideTube reactor
1,1,3,3,3-Pentafluoropropene (CF3CH═CF2, HFC-1225zc) can be produced by pyrolyzing 1,1,1,3,3,3-hexafluoropropane (CF3CH2CF3, HFC-236fa) in the absence of dehydrofluorination catalyst at temperatures of from about 700° C. to about 1000° C. and total pressures of about atmosphere pressure in an empty, tubular reactor, the interior surfaces of which comprise materials of construction resistant to hydrogen fluoride.
Owner:EI DU PONT DE NEMOURS & CO
Integrated HFC trans-1234ZE manufacture process
ActiveUS20080051610A1Preparation by hydrogen halide split-offPreparation by halogen replacementHydrogen fluoride1,3,3,3-Tetrafluoropropene
An integrated process for the manufacture of HFO trans-1,3,3,3-tetrafluoropropene (HFO trans-1234ze) by first catalytically dehydrofluorinating 1,1,1,3,3-pentafluoropropane to thereby produce a mixture of cis-1,3,3,3-tetrafluoropropene, trans-1,3,3,3-tetrafluoropropene and hydrogen fluoride. Then optionally recovering hydrogen fluoride, catalytically isomerizing cis-1234ze into trans-1234ze, and recovering trans-1,3,3,3-tetrafluoropropene.
Owner:HONEYWELL INT INC
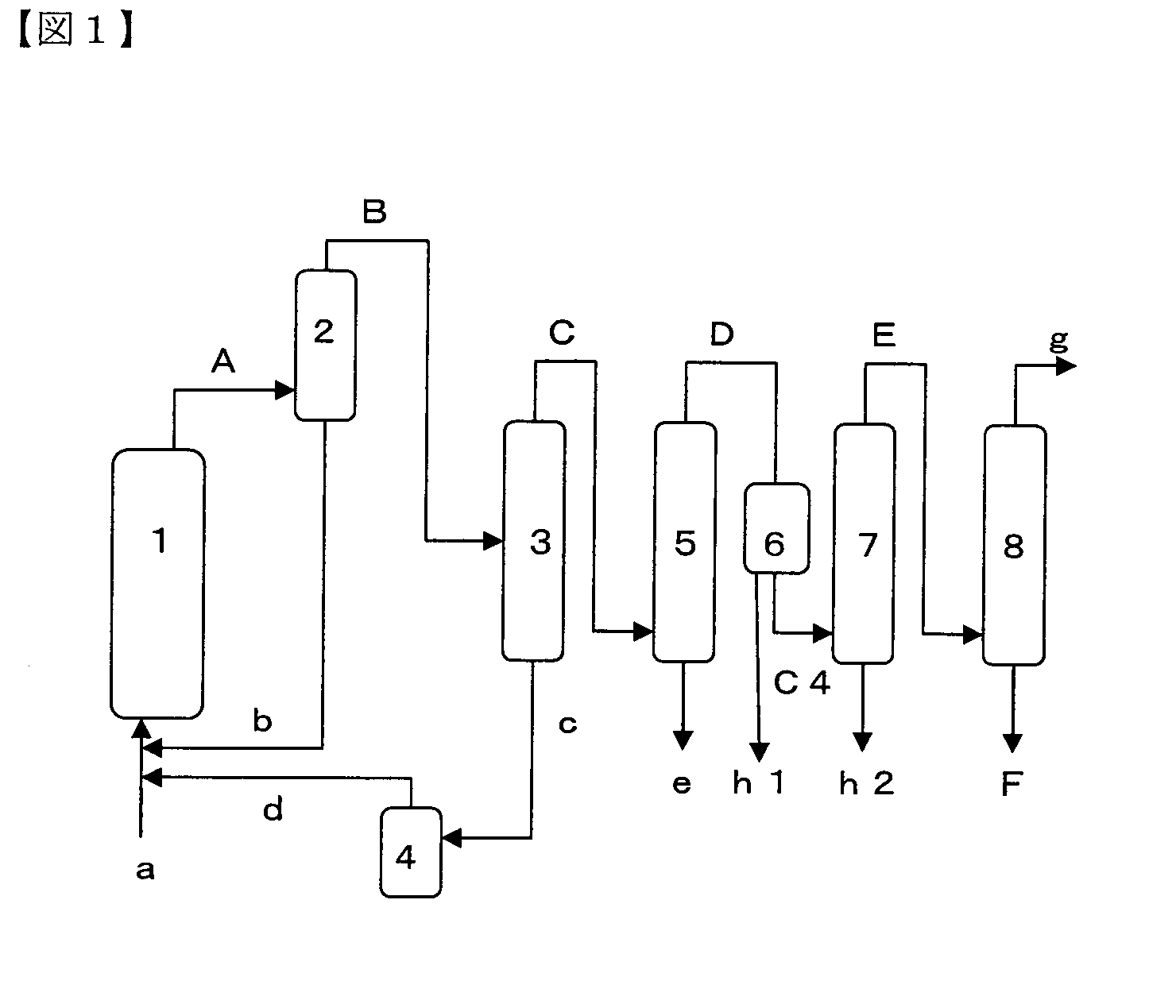
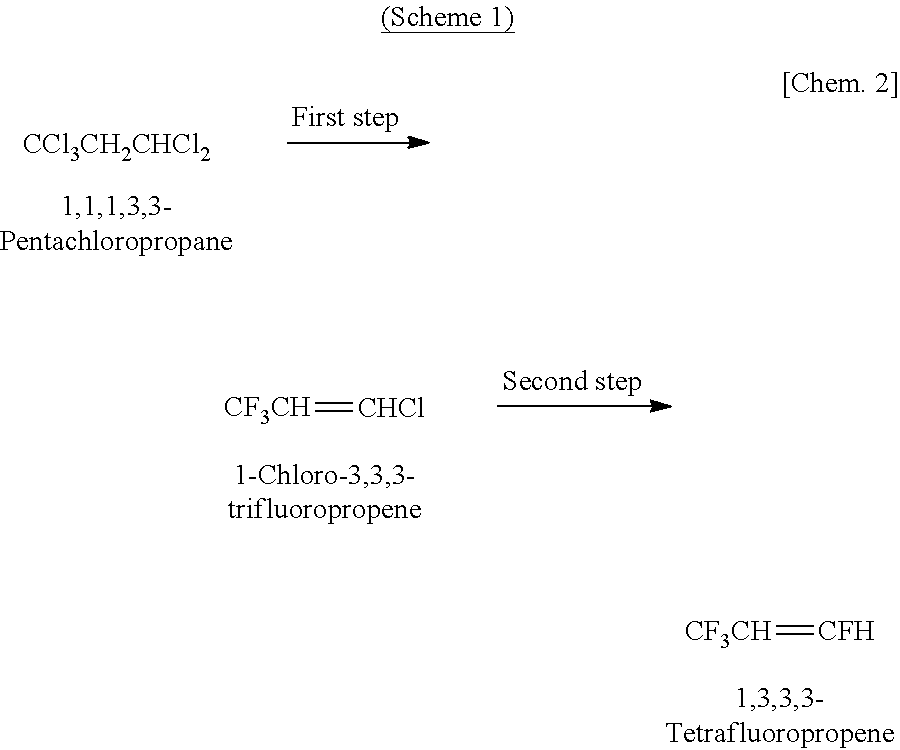
Process for Producing 1,3,3,3-Tetrafluoropropene
InactiveUS20110172472A1High selectivityHigh yieldChlorine/hydrogen-chloride purificationPreparation by hydrogen halide split-offHydrogen fluoride1,3,3,3-Tetrafluoropropene
According to the first characteristic of the present invention, there is provided a production process for 1,3,3,3-tetrafluoropropene including: the first step of reacting 1,1,1,3,3-pentachloropropane with hydrogen fluoride thereby obtaining 1-chloro-3,3,3-trifluoropropene; and the second step of reacting 1-chloro-3,3,3-trifluoropropene obtained in the first step with hydrogen fluoride in a gaseous phase in the presence of a fluorination catalyst. According to the second characteristic of the present invention, there is provided a dehydration process including bringing 1,3,3,3-tetrafluoropropene containing at least water into contact with zeolite.
Owner:CENT GLASS CO LTD
Azeotrope compositions comprising 1,1,1,2,3- pentafluoropropene and hydrogen fluoride and uses thereof
ActiveUS20070100174A1Preparation by hydrogen halide split-offHydrogen fluorideHydrogen fluoridePhysical chemistry
Owner:THE CHEMOURS CO FC LLC
Azeotrope compositions comprising 2,3,3,3-tetrafluoropropene and hydrogen fluoride and uses thereof
ActiveUS7476771B2Preparation by hydrogen halide split-offHydrogen fluorideHydrogen fluoridePhysical chemistry
Owner:THE CHEMOURS CO FC LLC
Process for production of azeotrope compositions comprising hydrofluoroolefin and hydrogen fluoride and uses of said azeotrope compositions in separation processes
ActiveUS7897823B2High selectivityPreparation by hydrogen halide split-offPreparation by halogen halide additionHydrogen fluorideHydrogen
Disclosed herein is a process to produce an azeotrope composition comprising a hydrofluoroolefin and hydrogen fluoride, said process comprising, dehydrofluorinating a hydrofluorocarbon containing at least one hydrogen and at least one fluorine on adjacent carbons, thereby forming a mixture comprising said hydrofluoroolefin, unreacted hydrofluorocarbon and hydrogen fluoride, and distilling the mixture to produce a distillate composition comprising an azeotrope composition containing said hydrofluoroolefin and hydrogen fluoride and a column bottoms composition comprising said hydrofluorocarbon essentially free of hydrogen fluoride. Also disclosed herein are processes for separation of hydrofluoroolefins from hydrofluorocarbons and from hydrogen fluoride.
Owner:THE CHEMOURS CO FC LLC
Azeotrope-like compositions of trifluoroethane and hydrogen fluoride
The invention relates to azeotropic and azeotrope-like mixtures of 1,1,1-trifluoroethane (HFC-143a) and hydrogen fluoride and a process for separating the azeotrope-like mixtures. The compositions of the invention are useful as an intermediate in the production of HFC-143a. The latter is useful as a nontoxic, zero ozone depleting fluorocarbon useful as a solvent, blowing agent, refrigerant, cleaning agent and aerosol propellant.
Owner:HONEYWELL INT INC
Process for the manufacture of halocarbons and selected compounds and azeotropes with HF
InactiveUS20050080302A1Preparation by hydrogen halide split-offPreparation by halogen halide additionAlkaneHalocarbon
A liquid phase process is disclosed for producing halogenated alkane adducts of the formula CAR1R2CBR3R4 (where A, B, R1, R2, R3, and R4 are as defined in the specification) which involves contacting a corresponding halogenated alkane, AB, with a corresponding olefin, CR1R2═CR3R4 in a dinitrile or cyclic carbonate ester solvent which divides the reaction mixture into two liquid phases and in the presence of a catalyst system containing (i) at least one catalyst selected from monovalent and divalent copper; and optionally (ii) a promoter selected from aromatic or aliphatic heterocyclic compounds which contain at least one carbon-nitrogen double bond in the heterocyclic ring. When hydrochlorofluorocarbons are formed, the chlorine content may be reduced by reacting the hydrochlorofluorocarbons with HF. New compounds disclosed include CF3CF2CCl2CH2CCl3, CF3CCl2CH2CH2Cl and CF3CCl2CH2CHClF. These compounds are useful as intermediates for producing hydrofluorocarbons. Azeotropes of CClF2CH2CF3 with HF and azeotropes of CF3CH2CHF2 with HF are also disclosed; as are process for producing such azeotropes. A process for purification of certain hydrofluorocarbons and / or chloro-precursors thereof from mixtures of such compounds with HF is also disclosed.
Owner:THE CHEMOURS CO FC LLC
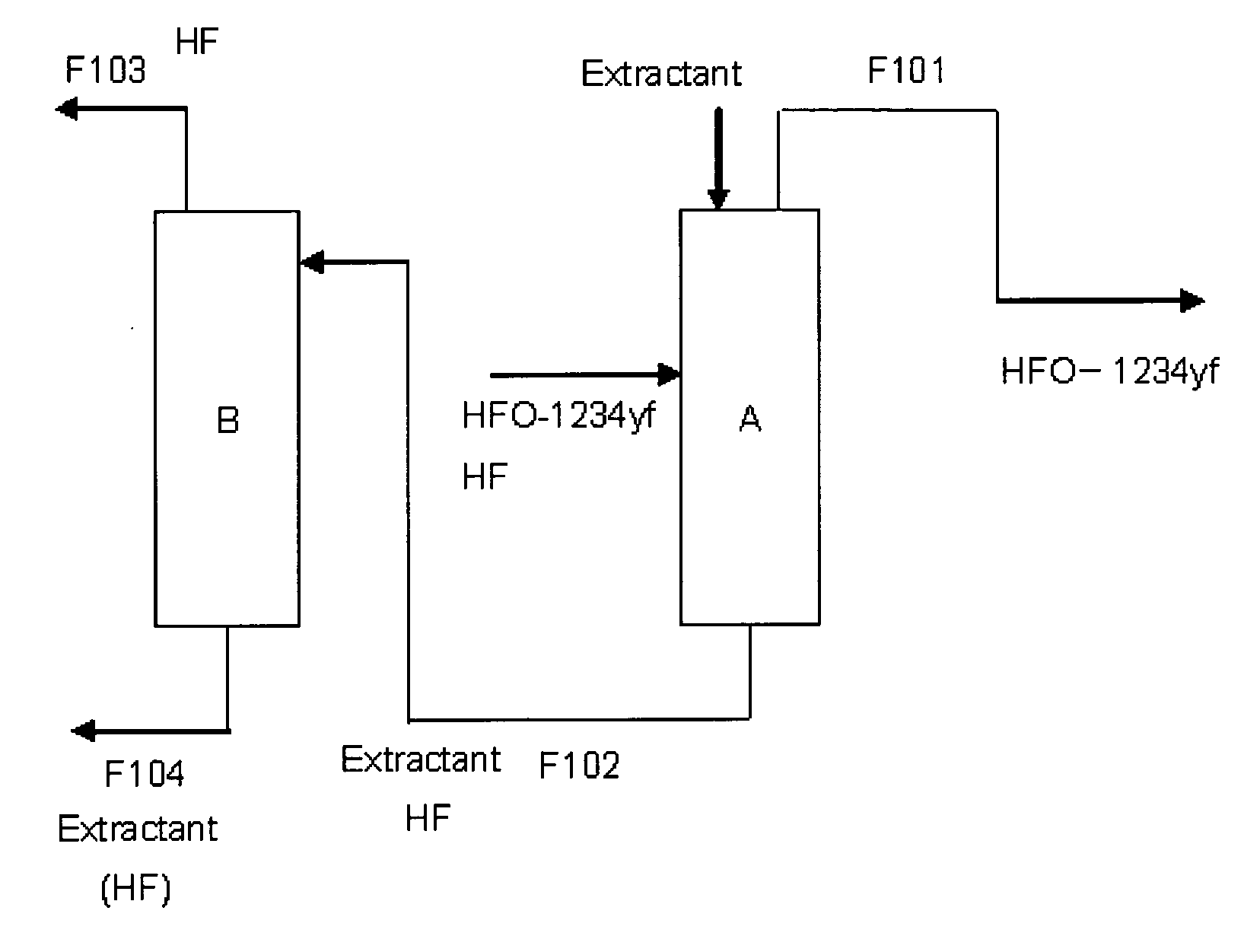
Purification method of 2,3,3,3-tetrafluoropropene
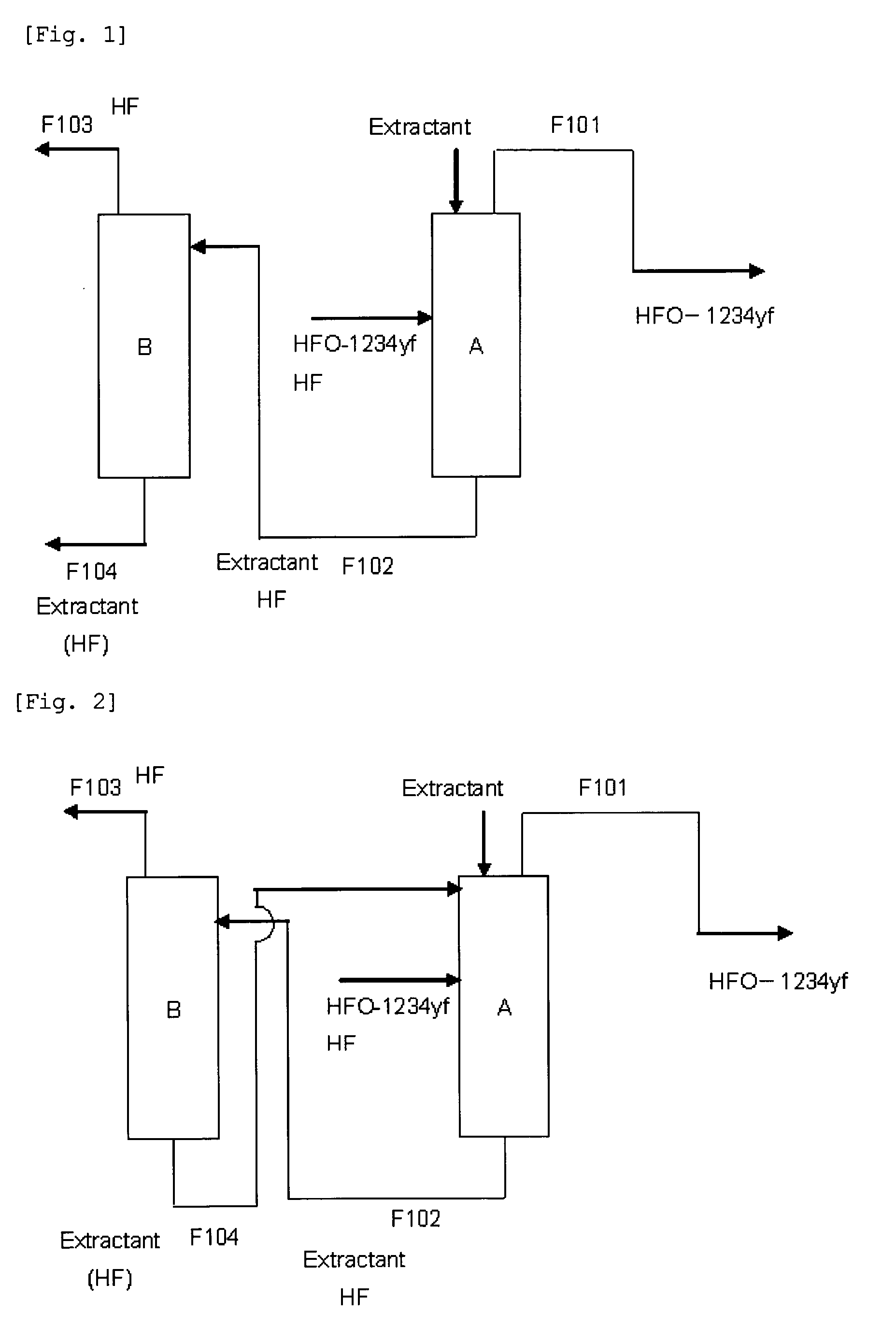
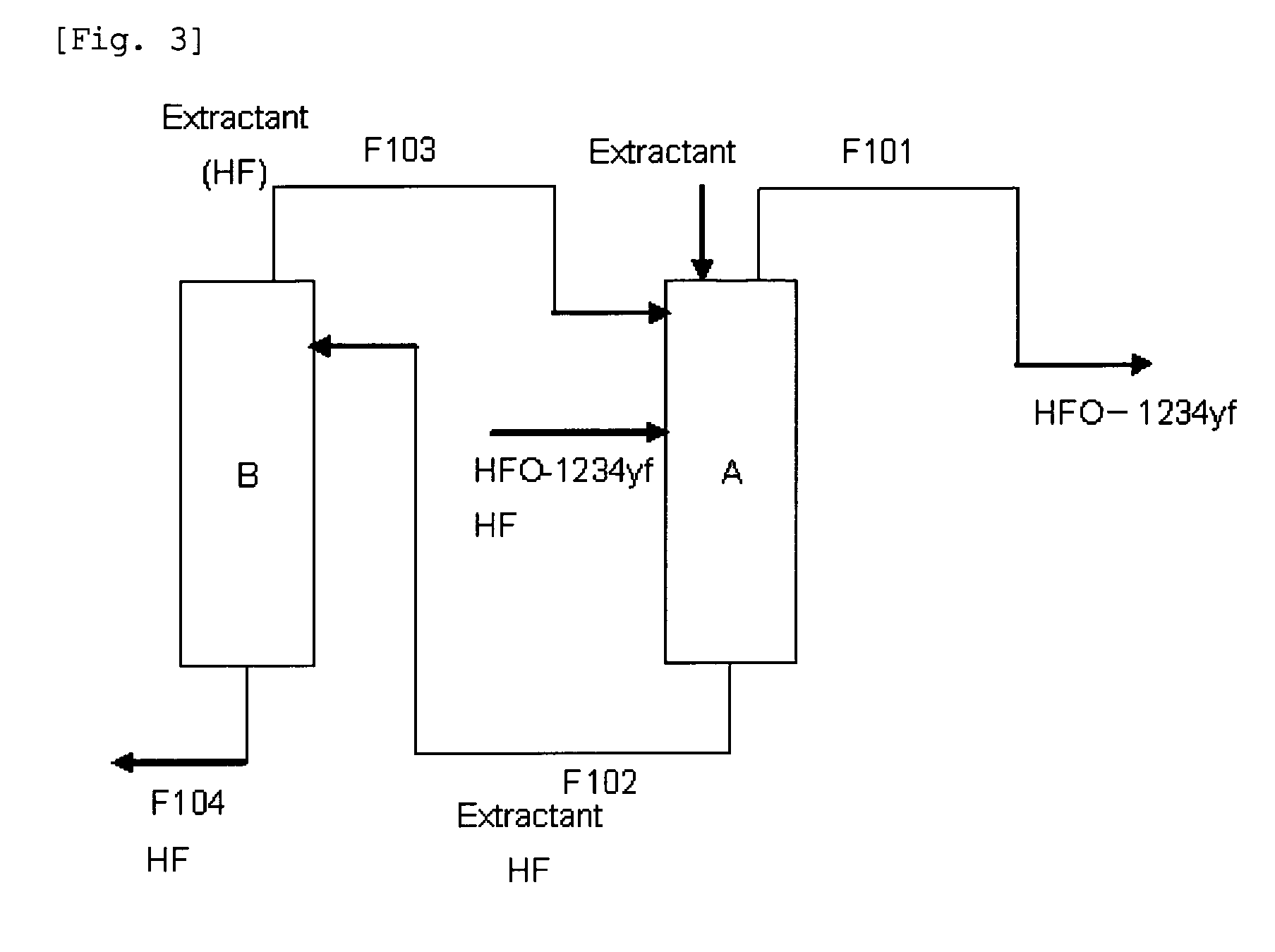
ActiveUS20130105296A1Efficient methodReduce the amount of wasteHydrogen fluorideHalogenated hydrocarbon separation/purificationPurification methodsExtractive distillation
This invention provides a method for purifying HFO-1234yf by removing HF from a mixture of HFO-1234yf and HF under simple and economically advantageous conditions. According to the present invention, this is a purification method for 2,3,3,3-tetrafluoropropene, (1) the purification method comprising the step of subjecting a mixture comprising 2,3,3,3-tetrafluoropropene and hydrogen fluoride to extractive distillation in a distillation column A using an extractant, thereby obtaining a fraction I that contains 2,3,3,3-tetrafluoropropene and has a lower ratio of hydrogen fluoride to 2,3,3,3-tetrafluoropropene than that of the mixture, while obtaining a fraction II that contains hydrogen fluoride and has a lower ratio of 2,3,3,3-tetrafluoropropene to hydrogen fluoride than that of the mixture; (2) the extractant comprising at least one member selected from the group consisting of: (i) alcohols represented by ROH, wherein R is a C1-5 alkyl group, (ii) ethers represented by ROR′, wherein R and R′ are the same or different, and each is a C1-4 alkyl group, (iii) fluorinated alcohols represented by RfOH, wherein Rf is a C1-3 fluoroalkyl group, (iv) ketones represented by RCOR′, wherein R and R′ are the same or different, and each is a C1-4 alkyl group, (v) esters represented by RCOOR′, wherein R and R′ are the same or different, and each is a C1-4 alkyl group, (vi) polyols represented by R(OH)n, wherein R is a C1-4 alkyl group, and n is an integer of 2 to 3, and (vii) ethylene glycols represented by R1O(CH2CH2O)nR2, wherein R1 and R2 are the same or different, and each is hydrogen or a C1-4 alkyl group, and n is an integer of 1 to 3.
Owner:DAIKIN IND LTD
1,3,3,3-tetrafluoropropene process azeotropes with hf
The present invention pertains to azeotropic and azeotrope-like compositions of the following three blends:1. Trans-1,3,3,3-tetrafluoropropene (HFO-1234ze(E)), cis-1,3,3,3-tetrafluoropropene (HFO-1234ze(Z)) and hydrogen fluoride (HF);2. HFO-1234ze(E), 1,1,1,3,3-pentafluoropropane (HFC-245fa) and HF; and3. HFO-1234ze(Z), HFC-245fa and HF.These azeotropic and azeotrope-like compositions are useful as intermediates in the production of HFO-1234ze(E).
Owner:HONEYWELL INT INC
Environment-friendly separation and recovery method of fluorine in fluorine-containing waste liquid
InactiveCN105948083AAchieve separationAchieve recyclingMagnesium fluoridesHydrogen fluorideRecovery methodEnvironmental resistance
The invention discloses an environment-friendly separation and recovery method of fluorine in a fluorine-containing waste liquid. According to the invention, a magnesium-containing compound is added into the fluorine-containing waste liquid as a precipitation agent, such that fluorine in the waste liquid is selectively precipitated; filtering is carried out, and fluorine-removed liquid and magnesium fluoride precipitate are obtained; the fluorine-removed liquid is used in waste water recycling; the magnesium fluoride precipitate is decomposed with sulfuric acid, such that a series of compounds of fluorine are obtained; decomposition residue is subjected to a dissolution-crystallization treatment, such that magnesium sulfate crystals are obtained; the obtained magnesium sulfate crystals are returned and recycled in the fluorine selective precipitation process; the crystallization mother liquor of magnesium sulfate is returned to the dissolution-crystallization process or the magnesium fluoride precipitation decomposition process. The method has the advantages of simple process, simple operation, low production cost, and good fluorine-removing effect. With the method, fluorine resource utilization is realized. The method also has the advantages of no fluorine-containing waste production and no three-waste emission.
Owner:CENT SOUTH UNIV
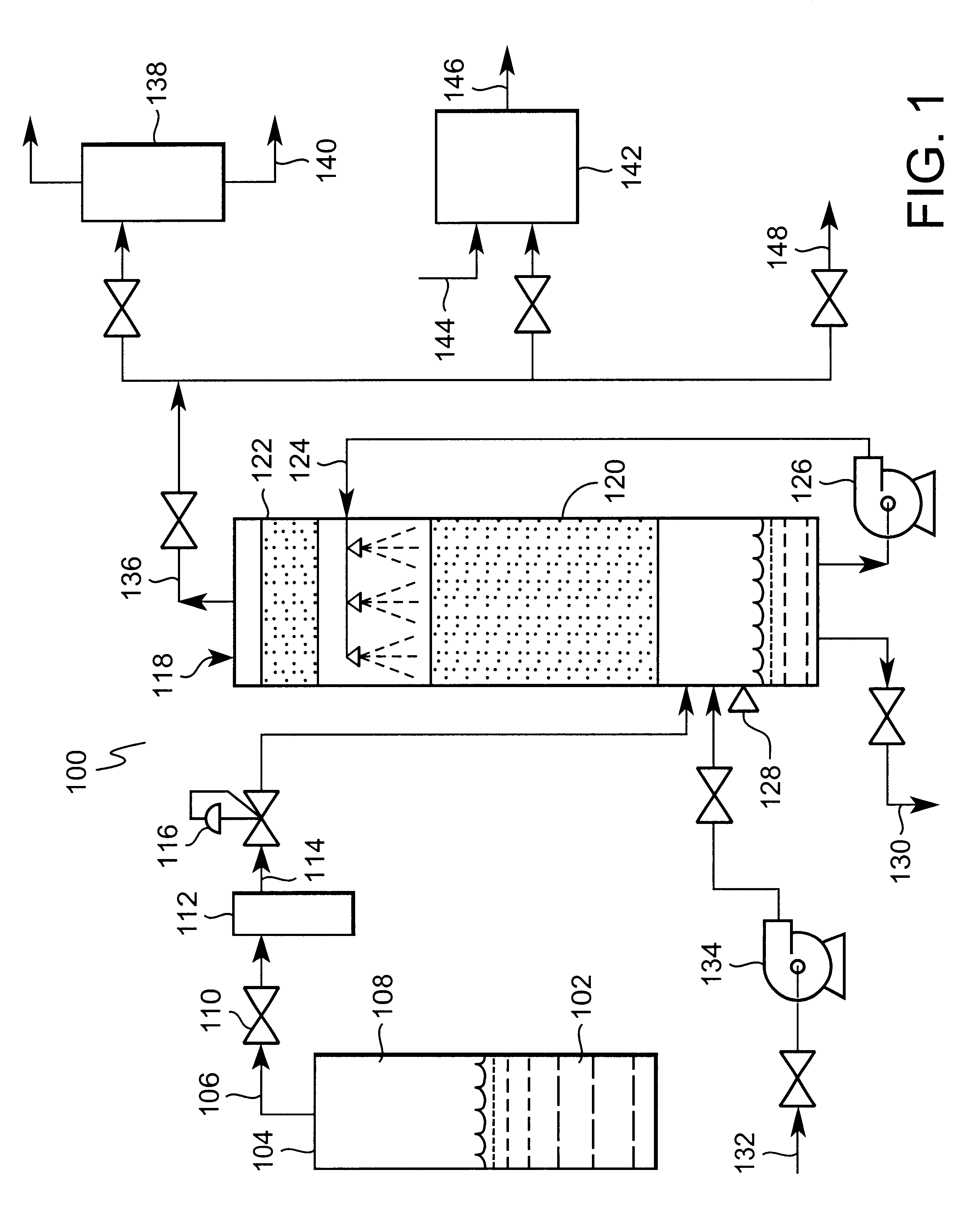
Catalyst composition and method of controlling PFC and HFC emissions
The present invention relates to a catalytic process for the destruction of PFC's and HFC's using a catalyst which comprises aluminum oxide that has preferably been stabilized through the addition of a stabilizing agent (such as, titanium, zirconium, or cobalt, or mixtures of these elements). The addition of these elements to the aluminum oxide unexpectedly enhances the catalyst's stability without significantly altering its reactivity. The total amount of stabilizing agent added to the catalyst can be as low as 0.005 parts (by weight) stabilizing agent per part (by weight) aluminum oxide (Al2O3) or as great as 2 or more parts (by weight) stabilizing agent per part (by weight) aluminum oxide; so long as there is sufficient aluminum oxide available to effectively catalyze the destruction of the target PFC's and / or HFC's. An oxidizing agent, such as, for example, platinum, palladium, rhodium, iridium, silver, nickel, copper, iron, vanadium, and / or cerium, may be added to the catalyst to effectively convert any carbon monoxide to carbon dioxide.
Owner:GUILD ASSOCS
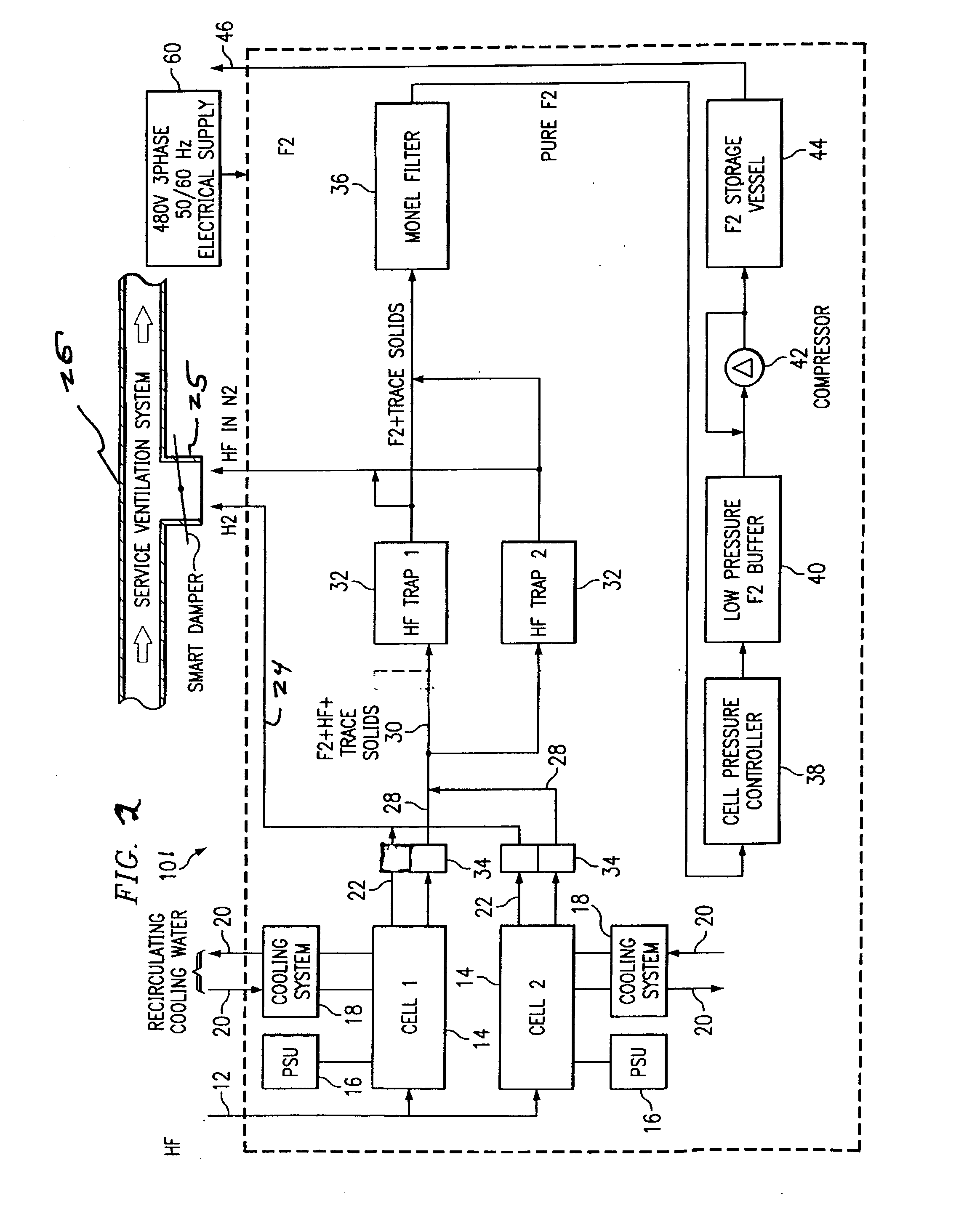
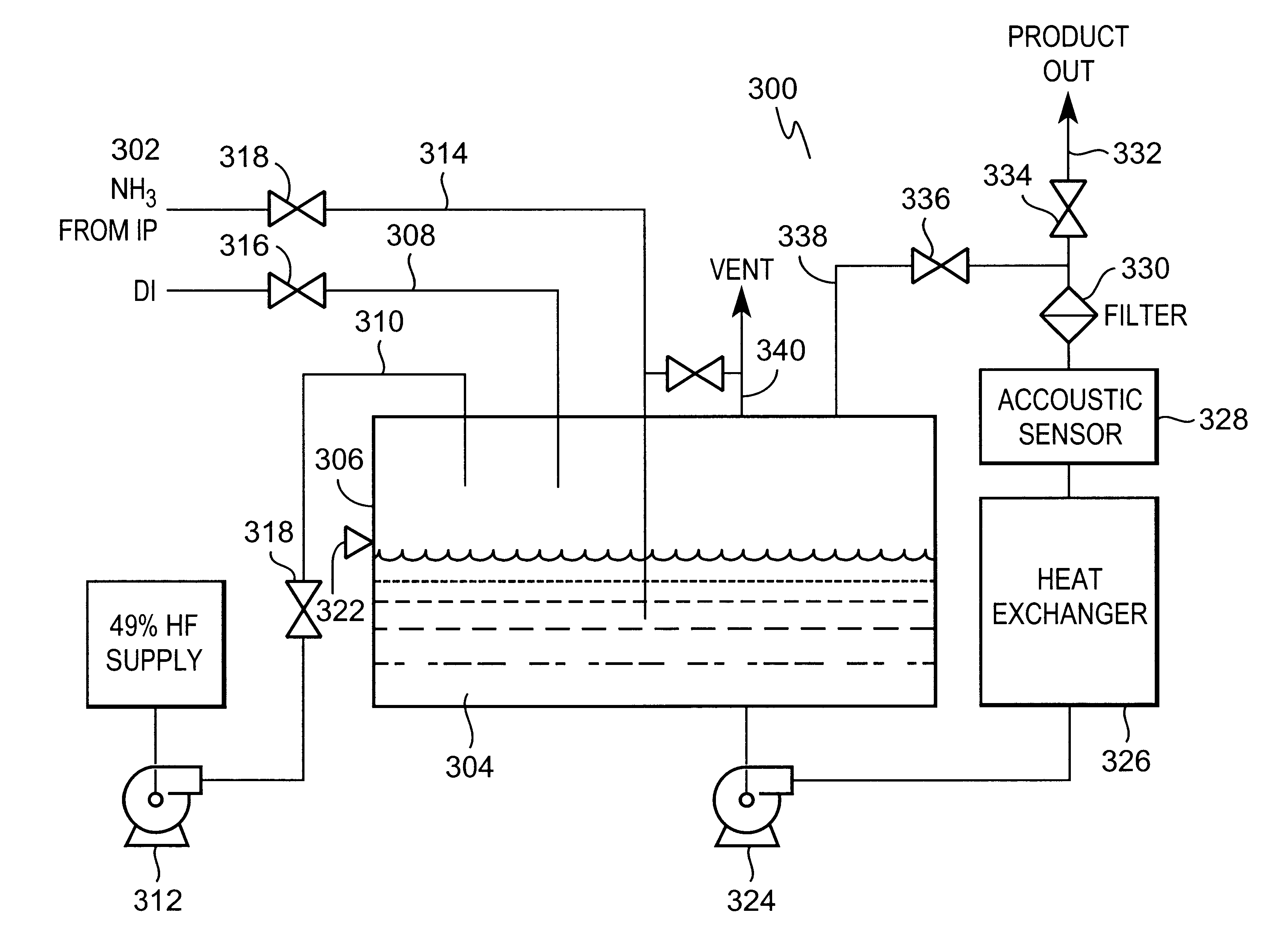
On-site generation of ultra-high-purity buffered-hf and ammonium fluoride
InactiveUS20010051128A1Low impurity contentImprove device characteristicsChlorine/hydrogen-chloride purificationControlling ratio of multiple fluid flowsHydrofluoric acidUltra high purity
Provided is a novel method and system for preparing ultra-high-purity buffered-hydrofluoric acid or ammonium fluoride controlled concentration. The method comprises bubbling purified ammonia vapor into ultra-pure hydrofluoric acid. The inventive method and system can be used as an on-site subsystem in a semiconductor device fabrication facility for supplying the buffered-hydrofluoric acid and ammonium fluoride to points of use in the semiconductor device fabrication facility.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
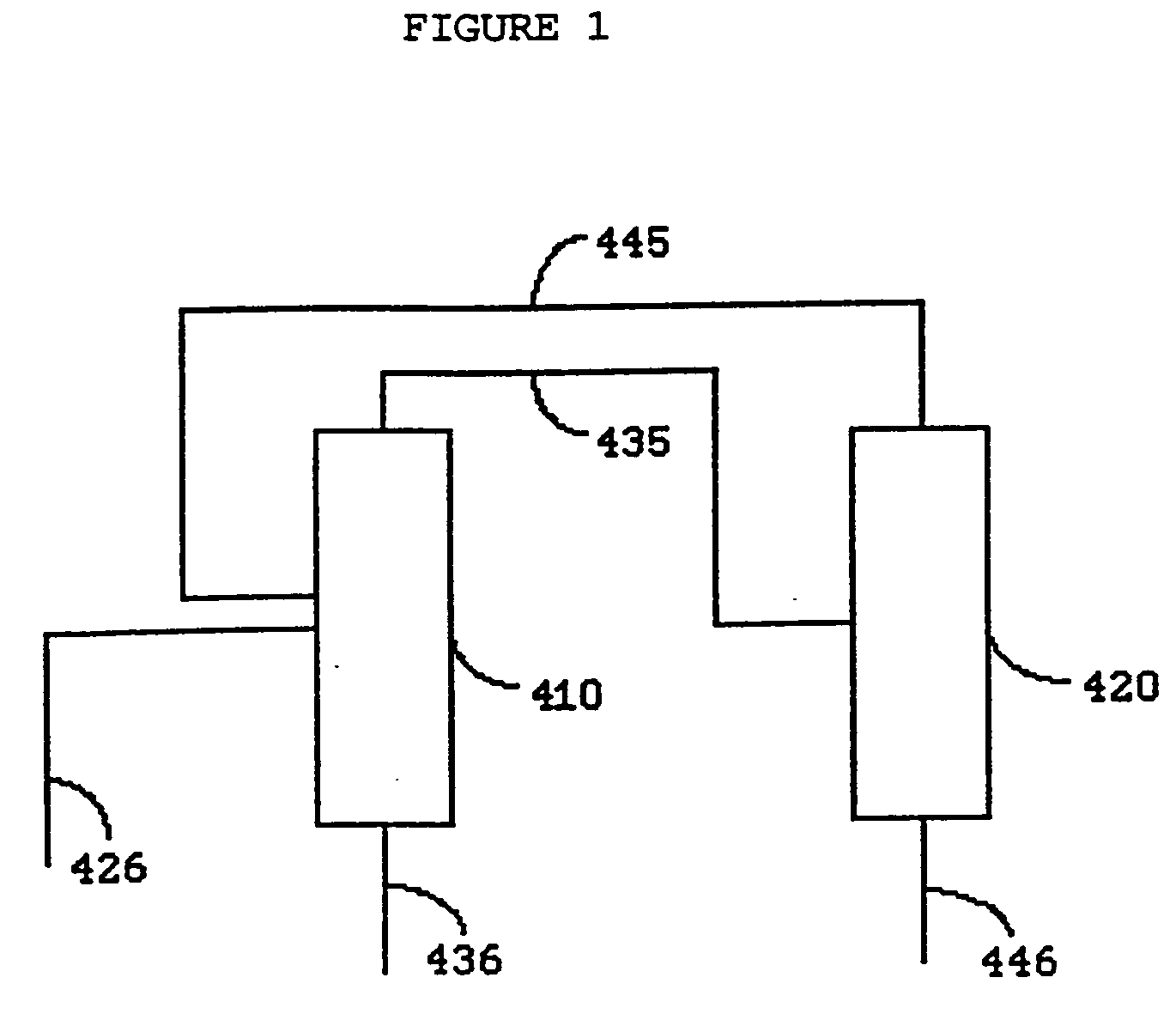
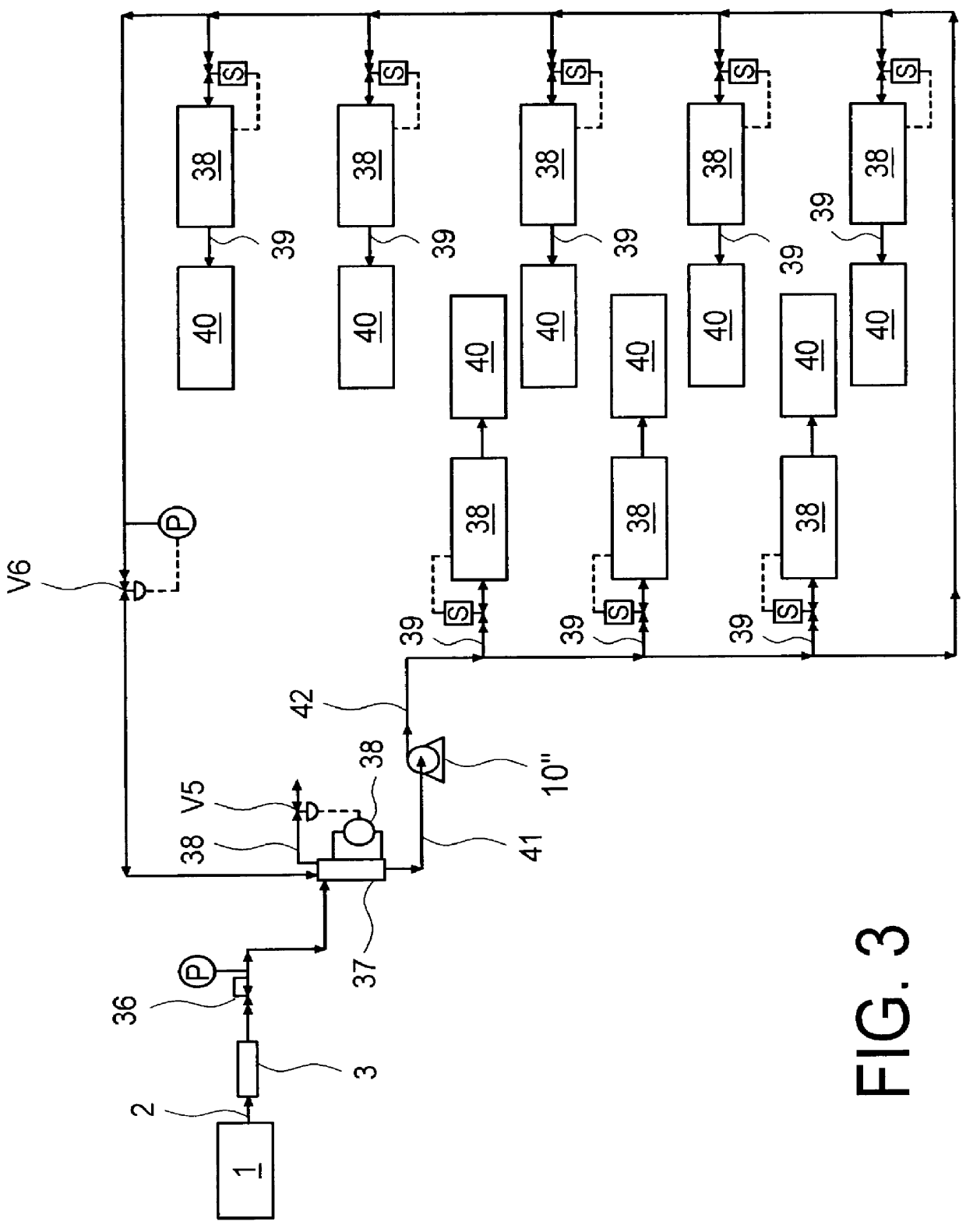
System and method for delivery of a vapor phase product to a point of use
Provided are a novel system and method for delivery of a vapor phase product to a point of use, as well as a novel on-site chemical distribution system and method. The system for delivery of a vapor phase product includes a storage vessel containing a liquid chemical under its own vapor pressure, a column connected to receive the chemical in liquified state from the storage vessel, wherein the chemical is fractionated into a contaminated liquid heavy fraction and a purified light vapor fraction and a conduit connected to the column for removing the purified light vapor fraction therefrom. The system is connected to the point of use for introducing the purified vapor fraction thereto. Particular applicability is found in semiconductor manufacturing in the delivery of electronic specialty gases to one or more semiconductor processing tools.
Owner:AIR LIQUIDE AMERICA INC
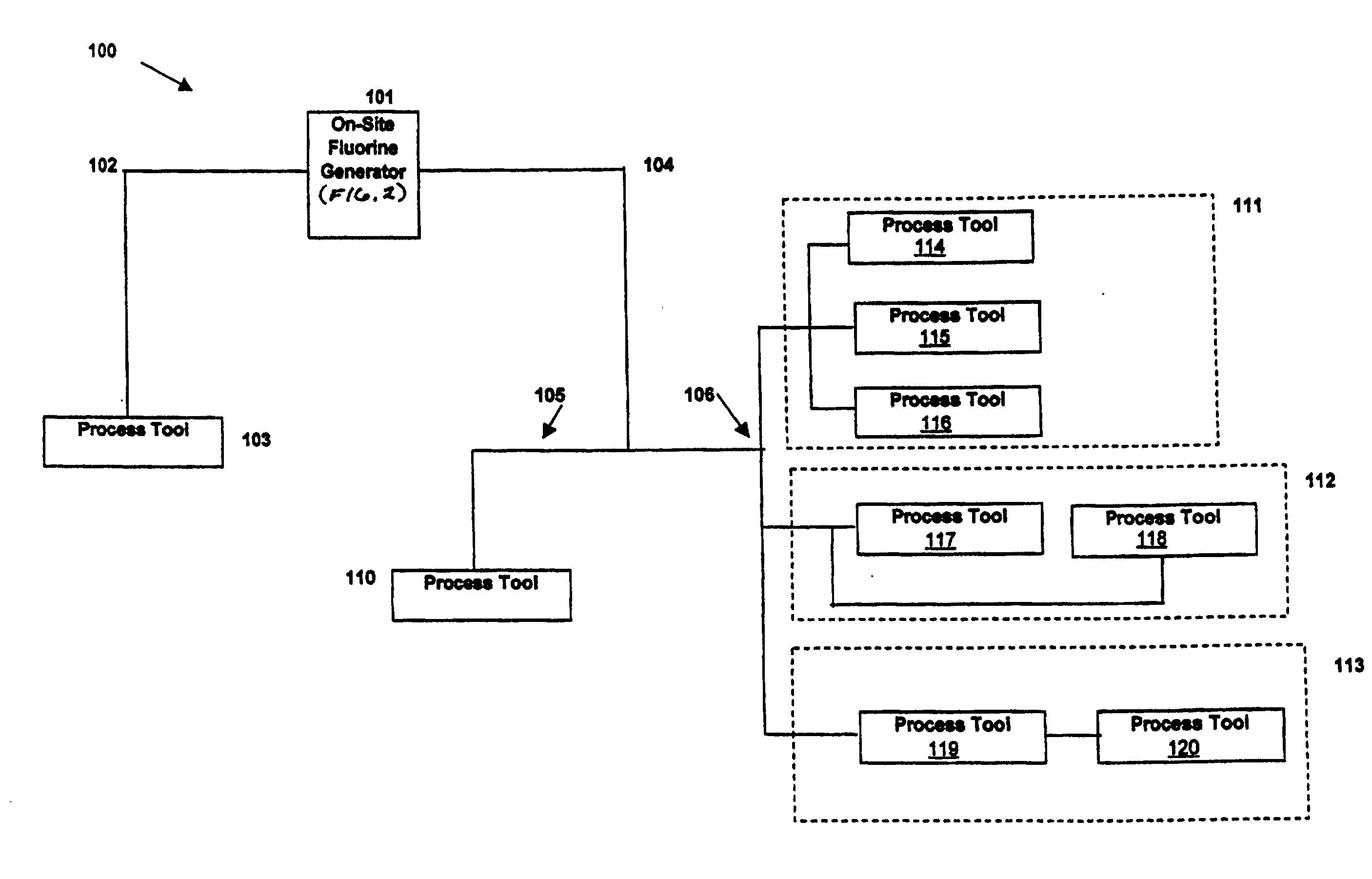
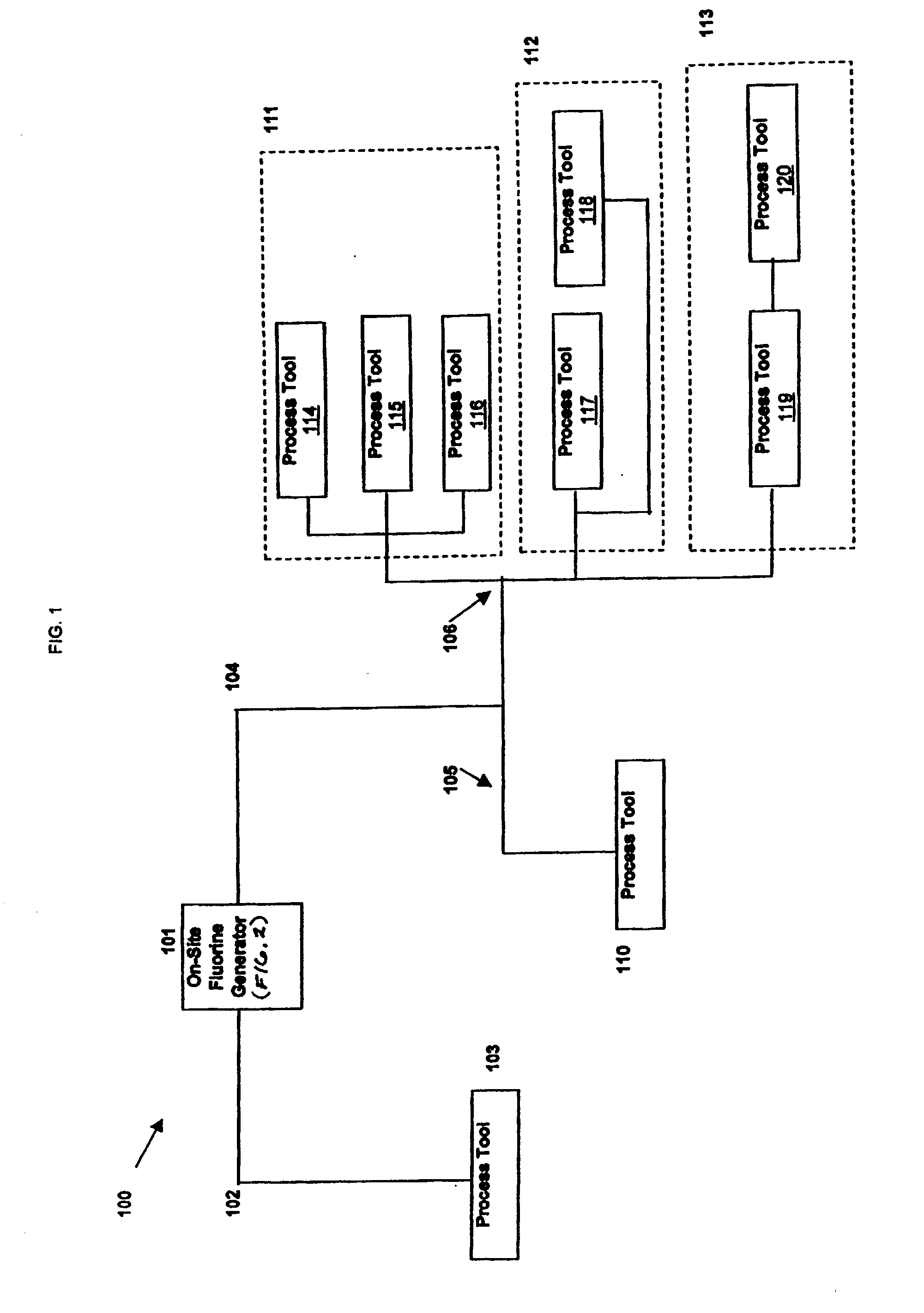
Generation and distribution of molecular fluorine within a fabrication facility
Molecular fluorine may be generated and distributed on-site at a fabrication facility. A molecular fluorine generator may come in a variety of sizes to fit better the needs of the particular fabrication facility. The generator may service one process tool, a plurality of process tool along a process bay, the entire fabrication facility, or nearly any other configuration within the facility. The process can obviate the need and inherent risks with transporting or handling gas cylinders. The process can be used in conjunction with a cleaning or fabrication operation used in the electronics fabrication industry.
Owner:FLUORINE ON CALL
Integrated process to produce 2,3,3,3-tetrafluoropropene
ActiveUS20110207975A9Maximize utilizationYield maximizationPreparation by hydrogen halide split-offPreparation by halogen halide additionChromium2,3,3,3-Tetrafluoropropene
A method for preparing 2,3,3,3-tetrafluoroprop-1-ene comprising (a) providing a starting composition comprising at least one compound having a structure selected from Formulae I, II and III:CX2═CCl—CH2X (Formula I)CX3—CCl═CH2 (Formula II)CX3—CHCl—CH2X (Formula III)wherein X is independently selected from F, Cl, Br, and I, provided that at least one X is not fluorine;(b) contacting said starting composition with a first fluorinating agent to produce a first intermediate composition comprising 2-chloro-3,3,3-trifluoropropene and a first chlorine-containing byproduct; (c) contacting said first intermediate composition with a second fluorinating agent to produce a second intermediate composition comprising 2-chloro-1,1,1,2-tetrafluoropropane and a second chlorine-containing byproduct; and (d) catalytically dehydrochlorinating at least a portion of said 2-chloro-1,1,1,2-tetrafluoropropane to produce a reaction product comprising 2,3,3,3-tetrafluoroprop-1-ene.
Owner:HONEYWELL INT INC
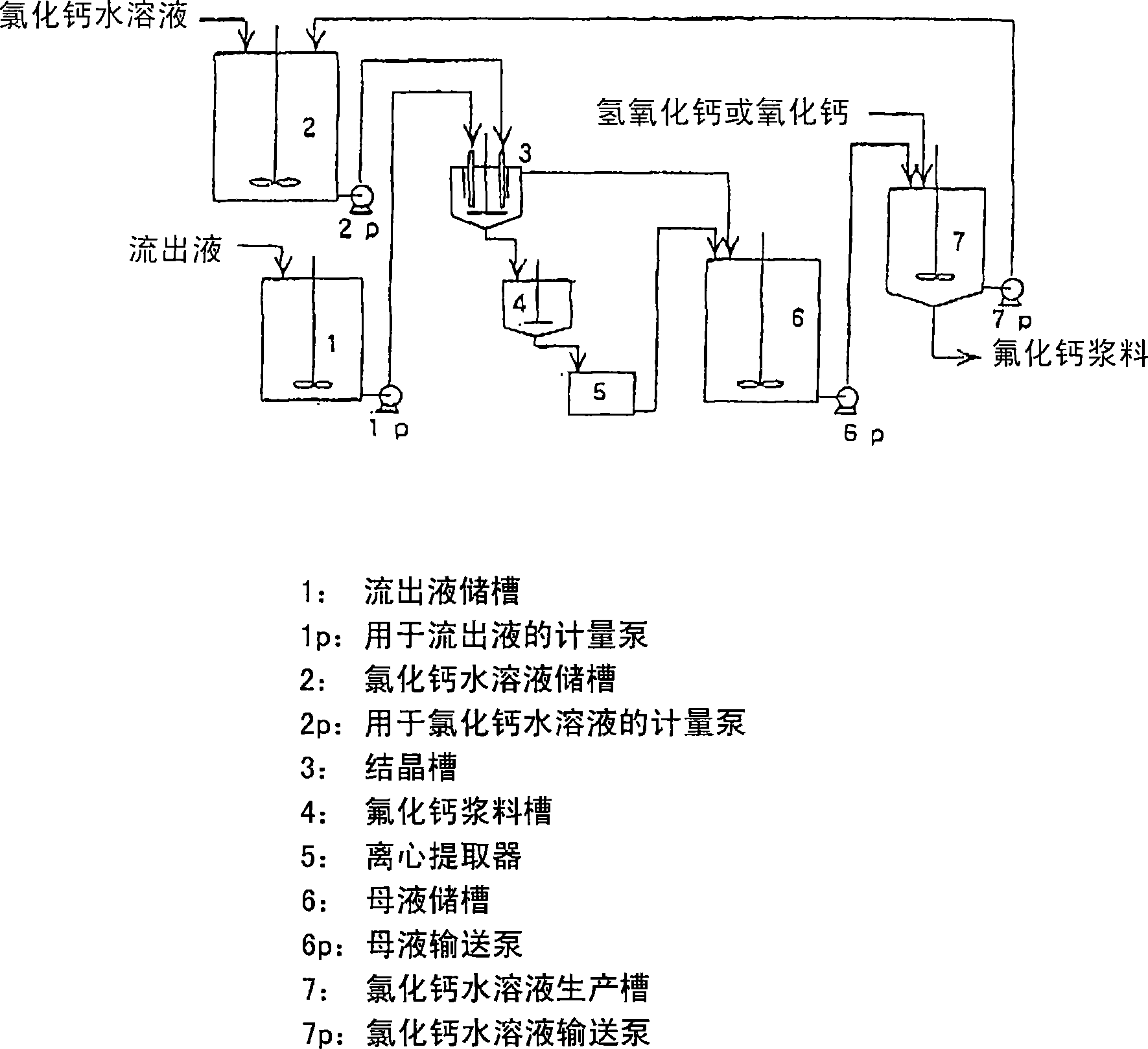
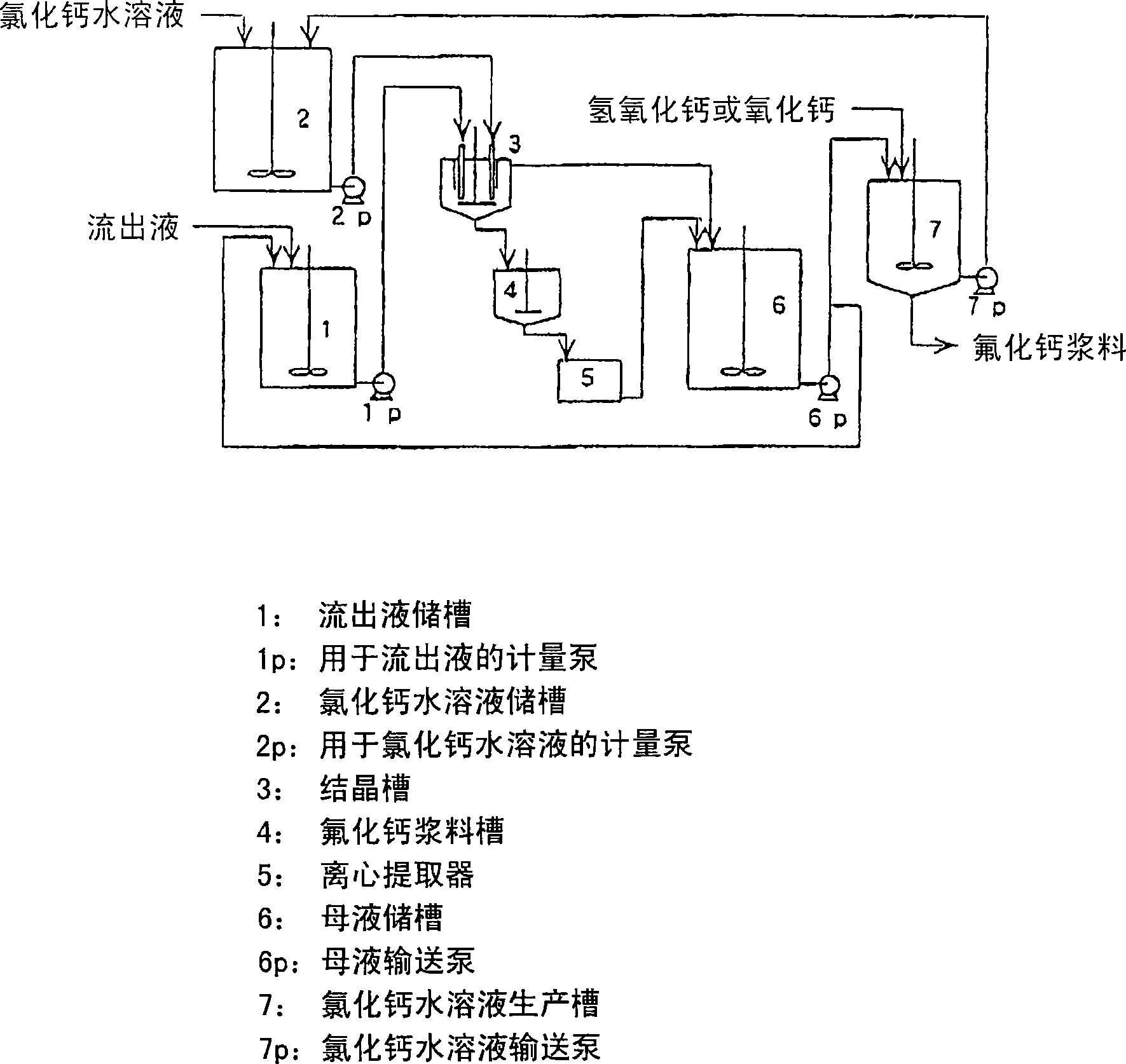
Method for producing calcium fluoride, reusing method and recycling method thereof
ActiveCN1906129AWater treatment parameter controlSpecific water treatment objectivesSolubilityLarge particle
In order to recycle the fluoride, calcium fluoride having a particle size and purity suitable for the production of hydrogen fluoride is recovered from the fluoride-containing effluent or the hydrofluoric acid-containing effluent. The method involves reacting a fluoride-containing effluent or a hydrofluoric acid-containing effluent with an aqueous solution of calcium chloride under acidic conditions formed by hydrochloric acid, under which calcium fluoride has a relatively high solubility. Calcium fluoride can be precipitated with high purity and large particle size. The hydrochloric acid residue from or formed by the reaction reacts with inexpensive calcium compounds such as calcium hydroxide, calcium oxide, and calcium carbonate to produce an aqueous calcium chloride solution that is reused to treat effluents containing hydrofluoric acid . The obtained calcium fluoride can be used as a raw material for producing hydrogen fluoride, and the remaining calcium chloride aqueous solution can be provided for other industrial purposes.
Owner:MORITA CHEMICAL IND CO LTD
Radiation shielding materials and containers incorporating same
InactiveUS6960311B1Improve shielding effectMaximum flexibilityOther chemical processesTransuranic element compoundsMicrosphereUranium carbide
An improved radiation shielding material and storage systems for radioactive materials incorporating the same. The PYRolytic Uranium Compound (“PYRUC”) shielding material is preferably formed by heat and / or pressure treatment of a precursor material comprising microspheres of a uranium compound, such as uranium dioxide or uranium carbide, and a suitable binder. The PYRUC shielding material provides improved radiation shielding, thermal characteristic, cost and ease of use in comparison with other shielding materials. The shielding material can be used to form containment systems, container vessels, shielding structures, and containment storage areas, all of which can be used to house radioactive waste. The preferred shielding system is in the form of a container for storage, transportation, and disposal of radioactive waste. In addition, improved methods for preparing uranium dioxide and uranium carbide microspheres for use in the radiation shielding materials are also provided.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
System and method for delivery of a vapor phase product to a point of use
Provided are a novel system and method for delivery of a vapor phase product to a point of use, as well as a novel on-site chemical distribution system and method. The system for delivery of a vapor phase product includes a storage vessel containing a liquid chemical under its own vapor pressure, a column connected to receive the chemical in liquified state from the storage vessel, wherein the chemical is fractionated into a contaminated liquid heavy fraction and a purified light vapor fraction and a conduit connected to the column for removing the purified light vapor fraction therefrom. The system is connected to the point of use for introducing the purified vapor fraction thereto. Particular applicability is found in semiconductor manufacturing in the delivery of electronics specialty gases to one or more semiconductor processing tools.
Owner:AIR LIQUIDE AMERICA INC
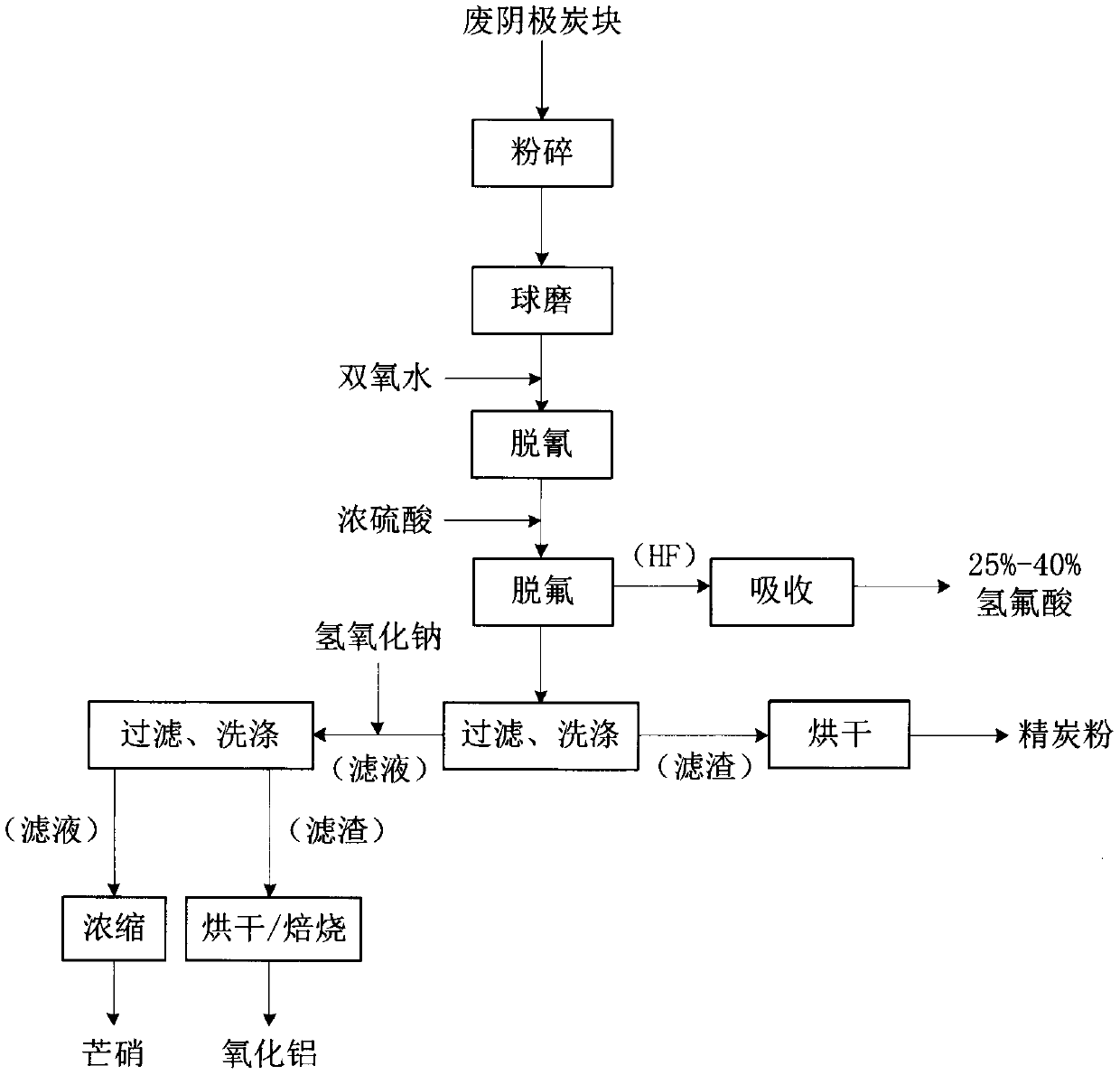
Recycling method for waste cathode carbon blocks of electrolytic aluminum electrolysis cell
ActiveCN110127649AAchieve recyclingAdvanced technologyHydrogen fluorideSulfate/bisulfate preparationElectrolysisBall mill
The invention discloses a recycling method of waste cathode carbon blocks of an aluminum electrolysis cell. The method comprises the following steps: breaking, crushing and ball milling the waste cathode carbon block raw material of the aluminum electrolysis cell to form powder, and then adding hydrogen peroxide to remove cyanide; adding concentrated sulfuric acid to react to generate hydrogen fluoride gas, introducing the hydrogen fluoride gas into a condensation absorption tower, and circularly absorbing the hydrogen fluoride gas with deionized water or a low-concentration hydrofluoric acidsolution to obtain a 25-40% hydrofluoric acid product; neutralizing carbon powder by adding NaOH, filtering and drying the carbon powder to use as a raw material for processing a new cathode for recycling; finally, concentrating a large amount of sodium sulfate contained in filtrate to obtain sodium sulfate crystals. The recycling method has the advantages of advanced technology and obvious advantages, and realizes zero emission of environmental pollutants in the recycling process. The treated waste cathode carbon blocks can be reused to process new cathode carbon blocks, and the main harmfulcomponent fluorine in the original waste cathode carbon blocks is converted into high side addition hydrogen fluoride to realize the recycling of fluorine.
Owner:广西田东曙光科技有限公司
On-site generation of ultra-high-purity buffered-HF and ammonium fluoride
InactiveUS6350425B2Reduce processingLow impurity contentChlorine/hydrogen-chloride purificationControlling ratio of multiple fluid flowsHydrofluoric acidUltra high purity
Provided is a novel method and system for preparing ultra-high-purity buffered-hydrofluoric acid or ammonium fluoride controlled concentration The method comprises bubbling purified ammonia vapor into ultra-pure hydrofluoric acid. The inventive method and system can be used as an on-site subsystem in a semiconductor device fabrication facility for supplying the buffered-hydrofluoric acid and ammonium fluoride to points of use in the semiconductor device fabrication facility.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Catalyst composition and method of controlling PFC and HFC emissions
The present invention relates to a catalyst composition and a catalytic process for the destruction of PFC's and HFC's using a catalyst which comprises aluminum oxide that has preferably been stabilized through the addition of a stabilizing agent (such as titanium, zirconium, or cobalt, or mixtures of these elements). The addition of these elements to the aluminum oxide unexpectedly enhances the catalyst's stability without significantly altering the reactivity of the catalyst. The total amount of stabilizing agent added to the catalyst can be as low as 0.005 parts (by weight) stabilizing agent per part (by weight) aluminum oxide (Al2O3) or as great as 2 or more parts (by weight) stabilizing agent per part (by weight) aluminum oxide; so long as there is sufficient aluminum oxide available to effectively catalyze the destruction of the target PFC's and / or HFC±.
Owner:GUILD ASSOCS
Production Method Of Trans-1,3,3,3-Tetrafluoropropene
InactiveUS20120123172A1Improve productivityHigh yieldPreparation by halogen replacementHydrogen fluorideHydrogen fluorideDistillation
Production of trans-1,3,3,3-tetrafluoropropene by reacting 1-chloro-3,3,3-trifluoropropene with hydrogen fluoride to obtain a reaction product A containing formed trans-1,3,3,3-tetrafluoropropene, unreacted 1-chloro-3,3,3-trifloropropene and hydrogen fluoride, and by-product cis-1,3,3,3-tetrafluoropropene, 1,1,1,3,3-pentafluoropropane and hydrogen chloride; distilling reaction product A to recover a distillation bottom product containing 1-chloro-3,3,3-trifloropropene and hydrogen fluoride and supplying recovered distillation bottom product to the reacting step; recovering hydrogen fluoride from a residue B remaining after recovery of the distillation bottom product and supplying recovered hydrogen fluoride to the reacting step; contacting a residue C remaining after recovery of hydrogen fluoride with water or aqueous sodium hydroxide solution to separate hydrogen chloride; dehydrating a residue D remaining after separation of hydrogen chloride; and distilling a residue E remaining after the dehydration to obtain trans-1,3,3,3-tetrafluoropropene. The method reuses unreacted reactants and produces the target compound efficiently.
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com